- 1Department of Pharmacy, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan
- 2School of Pharmacy, Kaohsiung Medical University, Kaohsiung, Taiwan
- 3Department of Pediatrics, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan
- 4Institute for Translational Research in Biomedicine, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan
Chronic kidney disease (CKD) and hypertension are becoming a global health challenge, despite developments in pharmacotherapy. Both diseases can begin in early life by so-called “developmental origins of health and disease” (DOHaD). Environmental chemical exposure during pregnancy can affect kidney development, resulting in renal programming. Here, we focus on environmental chemicals that pregnant mothers are likely to be exposed, including dioxins, bisphenol A (BPA), phthalates, per- and polyfluoroalkyl substances (PFAS), polycyclic aromatic hydrocarbons (PAH), heavy metals, and air pollution. We summarize current human evidence and animal models that supports the link between prenatal exposure to environmental chemicals and developmental origins of kidney disease and hypertension, with an emphasis on common mechanisms. These include oxidative stress, renin-angiotensin system, reduced nephron numbers, and aryl hydrocarbon receptor signaling pathway. Urgent action is required to identify toxic chemicals in the environment, avoid harmful chemicals exposure during pregnancy and lactation, and continue to discover other potentially harmful chemicals. Innovation is also needed to identify kidney disease and hypertension in the earliest stage, as well as translating effective reprogramming interventions from animal studies into clinical practice. Toward DOHaD approach, prohibiting toxic chemical exposure and better understanding of underlying mechanisms, we have the potential to reduce global burden of kidney disease and hypertension.
1 Introduction
The association between maternal exposure to environmental risk factors and the increased risk for developing adult disease has received increasing recognition in recent decades. This phenomenon is referred to as “developmental programming” or “developmental origins of health and disease” (DOHaD) (1, 2). The DOHaD hypothesis gained attention after the emergence of observational studies from the famine cohorts combined with several subsequent epidemiologic investigations (3–5), illuminating events before birth can predispose offspring towards non-communicable diseases (NCDs) in later life. Considering the increasing burden of global NCDs, therefore, the WHO informed the public about NCD prevention and control policies (6). So much so, in fact, that the DOHaD concept becomes a key prevention strategy to limit the passage of NCD risks to the next generation (7).
Kidney disease and hypertension are highly prevalent NCDs worldwide (8). About 10% of the global population is affected by chronic kidney disease (CKD) (8). Despite hypertension prevalence is highest in older populations, up to 20% of young adults are hypertensive (9). Kidney disease and hypertension have a bidirectional relationship (10), such that CKD is a complication of uncontrolled hypertension and hypertension is a frequent finding in kidney disease. Both kidney disease and hypertension can take their origins in early life (11). During critical period of development, the fetal kidney is particularly vulnerable to adverse impacts of gestational events, leading to functional and structural modifications, known as renal programming (12). A wide range of maternal insults can induce renal programming, giving rise to kidney disease and hypertension in later life. These include maternal malnutrition, maternal illness, substance abuse or medication use during pregnancy, exposure to environmental chemicals, etc (13–16). Numerous studies have reported the adverse renal effects that occur following exposure to a broad spectrum of environmental chemicals (17–20). However, little is known about the long-term adverse consequences on the offspring from maternal exposure to environmental chemicals in pregnancy. Of note, emerging evidence supports a “two-hit” hypothesis that explains the developmental programming of adult diseases (21). Hypertension and kidney disease may develop with two sequential hits: the first hit being the prenatal environmental chemical exposure, followed by the second hit in response to postnatal insult. CKD is characterized by a progressive loss of nephrons. There is a ten-fold variation in nephron number at birth (22), and a further decrease over the life cycle. Reduced nephron number can stimulate hypertrophy of remaining nephrons, resulting in glomerulosclerosis and more nephron loss. From an evolutionary perspective, the transition of hypertrophied nephrons to fibrosis is considered to be maladaptive (23). Accordingly, the recognition of the contribution of environmental chemicals to the changing nephron formation and numbers from embryo through senescence could provide new insight into the prevention of CKD.
In this Review, we focus on environmental chemicals that pregnant mothers are likely to be exposed as a consequence of normal consumer activities, that is, dioxins, bisphenol A (BPA), phthalates, per- and polyfluoroalkyl substances (PFAS), polycyclic aromatic hydrocarbons (PAH), heavy metals, and air pollution. We aim to provide an overview of maternal exposure to environmental chemicals implicated in developmental origins of kidney disease and hypertension. The mechanisms mediating renal programming will be a special focus, and their interrelationships to individual chemicals will be discussed. Furthermore, the potential of preventive approach to protect offspring against developmental origins of kidney disease and hypertension will be summarized. A drawing schematic summarizing the sources of environmental chemicals, adverse impact of maternal exposure on kidney disease and hypertension on adult offspring, and common mechanisms underlying renal programming are depicted in Figure 1.
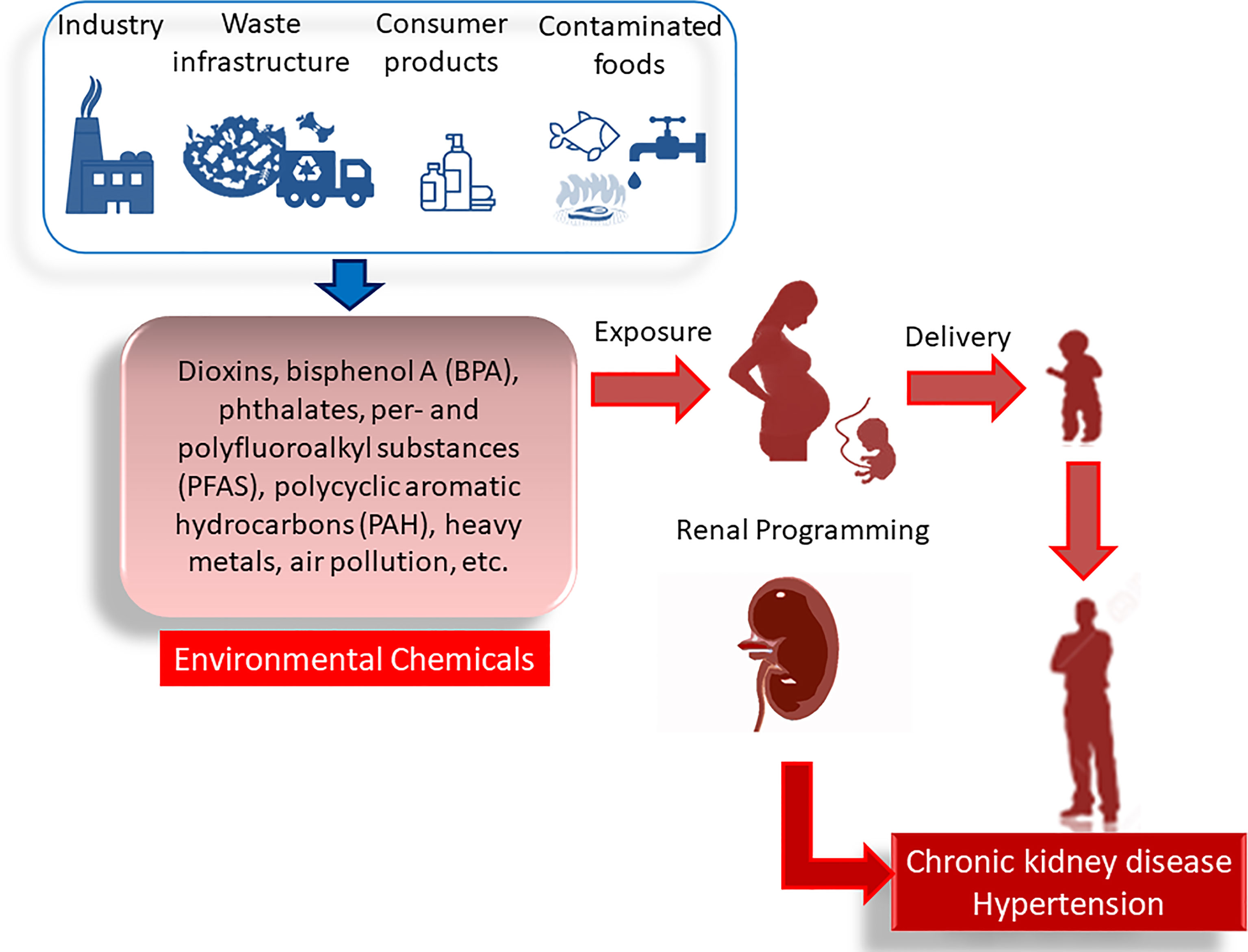
Figure 1 Adverse impact of maternal environmental chemical exposure on developmental origins of kidney disease and hypertension. In pregnancy, exposure to various environmental chemicals occurs through daily consumer activity. There are many sources of contamination like industry, waste infrastructure, consumer products, contaminated foods, etc. These environmental chemicals cause renal programming, resulting in chronic kidney disease and hypertension in adulthood.
The PubMed/MEDLINE database was searched for English-language and full-text articles published from 1980 to June 2021 using the following search terms: “bisphenol A”, “polychlorinated dibenzo-p-dioxins”, “dioxins”, “polychlorinated biphenyls”, “polychlorinated biphenyl”, “perfluoroalkyl acid”, “perfluoroalkyl”, “perfluoroalkyl compound”, “phthalates”, “phthalic acids”, “polycyclic aromatic hydrocarbons”, “heavy metal”, “lead”, “mercury”, “cadmium”, “air pollution”, “particulate matter”, “renal function”, “kidney”, “nephrogenesis”, “blood pressure”, “albuminuria”, “hypertension”, “developmental programming”, “DOHaD”, “mother”, “maternal”, “pregnancy”, “gestation”, “offspring”, “progeny”, and “prenatal”. Additional studies were then selected and assessed based on appropriate references in eligible papers.
2 Sources and Adverse Renal Effects of Environmental Chemicals
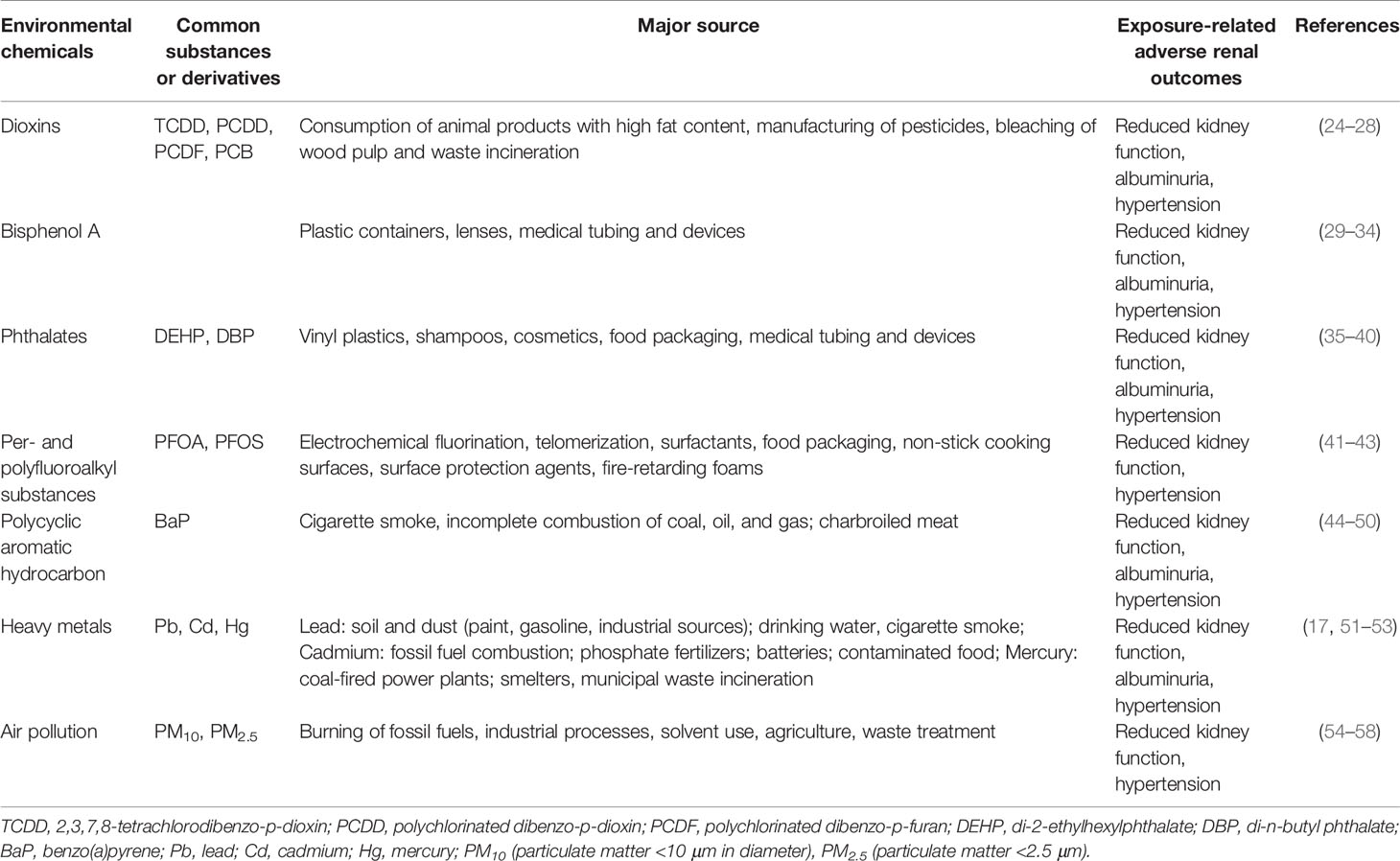
Various environmental chemicals pose a broad range of adverse effects on the kidney. Table 1 illustrates the major source and reported adverse renal effects for environmental chemicals that individuals are likely to be exposed during normal consumer activity. Each of these chemicals will be discussed in turn.
2.1 Dioxins
The chemical name for dioxin is 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), the most extensively studied and toxic dioxin. While the name “dioxins” is habitually used for the family of structurally and chemically related polychlorinated dibenzo-p-dioxins (PCDD), polychlorinated dibenzo-p-furans (PCDFs), and dioxin-like polychlorinated biphenyl (PCB). Dioxins are synthetic halogenated aromatic hydrocarbons, emitted mostly from anthropogenic sources like manufacturing of pesticides, bleaching of wood pulp and waste incineration (24) (Table 1). The presence of dioxins in the environment and the risk of exposure for human health has raised great concern. The half-lives of PCDDs and PCDFs range from 2–15 years (25); as such, dioxins last a long time in fat tissue of the body. Dioxins tend to accumulate in the food chain in the environment. Accordingly, pregnant mothers can be exposed to these chemicals by eating diet high in animal fat or occupational exposure. A high-level exposure to dioxins is associated with decreased kidney function and hypertension in adults (26, 27). Additionally, the prevalence of hypertension was correlated with circulating PCDD and PCDF concentrations in adults with dioxin exposure (28). Nevertheless, the association between dioxins on kidney function and blood pressure (BP) in children remains largely unknown. The effects of dioxins are mainly mediated by the aryl hydrocarbon receptor (AHR)—a ligand-activated transcription factor that contribute to the pathogenesis of CKD and hypertension (59, 60).
2.2 Bisphenol A
Bisphenol A (BPA) was initially designed as a synthetic estrogen. It is now widely used for lining metal cans and in polycarbonate plastics, such as baby bottles, intravenous tubing, and dialysis circuits (29). Incomplete polymerization and polymer degradation of BPA causes it to leach out of food and beverage containers. BPA can be absorbed through ingestion, respiration, and the skin contact (30). As human exposure to BPA is frequent and widespread, more than 90% of individuals have detectable amounts of BPA in their urine (31). In humans, free BPA is rapidly metabolized in the liver and eliminated by renal excretion (32). High BPA concentrations have been reported in uremic patients received hemodialysis or peritoneal dialysis (32). Additionally, urinary BPA level was associated negatively with the estimated glomerular filtration rate (eGFR) and positively with BP (33, 34).
At concentrations lower than that reported in toxicological studies, BPA could provoke different endocrine-disrupting effects (30). BPA acts as an endogenous estrogen by interacting with estrogen receptors. Also, BPA is a ligand for the AHR. Thus, taking into account that endocrine disruption chemical (EDC) function as environmental signals and can be passed on to subsequent generations (61), there will be a growing need to understand the mechanisms of BPA action in order to decipher the association between maternal BPA exposure and kidney health in adult offspring.
2.3 Phthalates
Phthalates are a family of EDCs generally used as plasticizers in various industrial commodities (35). Low-molecular weight (LMW) phthalates have 3–6 carbon atoms in the backbone of their structure, whereas high-molecular weight (HMW) phthalates have 7–13 backbone carbons. LMH phthalates are frequently added to cosmetics, shampoos, and other personal hygiene products. HMW phthalates are commonly used to make vinyl plastics in applications in flooring, food packaging and intravenous tubing (35). Phthalates can be delivered to the human body through diet, inhalation, and skin contact. Di-2-ethylhexylphthalate (DEHP) and di-n-butyl phthalate (DBP) are the primary phthalate ester pollutants in the environment (36). The metabolites of phthalates can cross the placenta and be transferred to the fetus (37). Epidemiological studies demonstrated that high urinary DEHP levels are associated with high BP, low eGFR and albuminuria (38–40). As phthalates have estrogenic or antiandrogenic properties, emerging evidence suggests the associations between prenatal phthalate exposure and adverse offspring outcomes (37). Following these findings, steps should be taken to explore the effect of phthalate exposure during pregnancy on offspring kidneys.
2.4 Per- and Polyfluoroalkyl Substances
Per- and polyfluoroalkyl substances (PFAS) are a diverse group of human-made chemicals used in a broad range of consumer and industrial products (41). PFAS exposure is ubiquitous with perfluoorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) detectable in >90% of the population (42). For pregnant women, contaminated diet, drinking water, and air are the main sources of exposure. PFAS can be transferred from mother to fetus in utero and through breastfeeding to neonates (42). In adults, high PFOA or PFOS levels are associated with CKD (43). Likewise, elevated PFOA levels are associated with reduced kidney function in children and adolescents (62). Nevertheless, the association between blood PFOA and PFOS levels and hypertension was not identified in a pediatric cohort (63).
Several mechanisms have been linked to PFAS-induced kidney disease, including oxidative stress, peroxisome proliferators-activated receptor (PPAR) pathways, NF-E2–related factor 2 (NRF2) pathways, enhanced endothelial permeability, and epithelial mesenchymal transition (64). Of note, these mechanisms are also linked to developmental origins of kidney disease and hypertension (12–16). Despite emerging evidence portends PFAS are environmental threats to renal outcome; yet there is a gap in our understanding of whether maternal PFAS exposure affects offspring’s kidney health.
2.5 Polycyclic Aromatic Hydrocarbons
Polycyclic aromatic hydrocarbons (PAHs) are organic pollutants and composed of two or more fused aromatic rings of carbon and hydrogen atoms, which come from industrial, mobile, domestic, and agricultural emission (44). PAHs are highly lipophilic and can easily accumulate in fat tissue of living organisms. Many PAHs are mutagenic, carcinogenic, teratogenic, and immunotoxic to humans (45). In pregnancy, comparable amounts of PAHs in maternal blood and cord blood, whereas low levels in placental tissue were found (44). These data indicates that PAHs can cross the placenta and transfer to the fetus. Another report illustrated that up to 30–95% of infants have exposure to PAHs by breastfeeding (45). Current evidence supports gestational exposure of PAHs is responsible for adverse birth outcomes like low birth weight and premature delivery (46). It has also been shown that benzo(a)pyrene (BaP) and other PAHs can increase stillbirths and congenital abnormalities (47). Regarding the kidney, studies in adults have identified increases in urinary PAH metabolites were associated with a decrease in eGFR (48), an elevation in BP (49), and the presence of albuminuria (50). Similar to many environmental chemicals, PAHs are known AHR ligands. Activation of PAH/AHR signaling can alter the toxicokinetic profile of many nephrotoxic drugs, like aminoglycosides, to mediate kidney injury (65).
2.6 Heavy Metals
Heavy metals constitute an ill-defined group of inorganic chemical hazards, and those most commonly found at contaminated sites related to nephrotoxicity are lead (Pb), cadmium (Cd), and mercury (Hg) (17, 51). The general population is mainly exposed to lead from air and food, as lead in foodstuff originated from pots used for cooking and over 50% of lead emissions originating from petrol. Cadmium compounds are currently used as stabilizers and in re-chargeable nickel–cadmium batteries. Accordingly, cadmium exposure is generally from contaminated household waste and food; and cigarette smoking. Regarding mercury, the major source of exposure comes from contaminated food (i.e., fish) and dental amalgam. During pregnancy, there were greater accumulations of lead, cadmium, and mercury in the fetal kidney than in brain (52). Chronic exposure to lead has been linked to the development of lead nephropathy (53). Likewise, cadmium can cause nephrotoxicity via entering the renal epithelial cells (66). Mercury exposure has also been shown to elicit nephrotoxic effects like acute kidney injury and proximal tubule damage (17). In children, chronic relatively low-level exposure to various heavy metals may also increase the risk for CKD and hypertension (19, 20, 67). Owing to heavy metals remain the most important occupational and environmental pollutants, especially their nephrotoxic effects, there will be a growing need to understand whether maternal exposure to heavy metals impact renal outcomes in adult progeny.
2.7 Air Pollution
Epidemiological studies have obviously established that air pollution contributes to cardiovascular morbidity and mortality (54). Air pollutants include gaseous pollutants (e.g., carbon mono oxide, oxides of nitrogen, ozone and sulfur dioxide) and particulate matters (PMs). The coarse fraction contains the particles with a size ranging from PM10 (<10 μm in diameter), PM2.5 (<2.5 μm) to ultrafine particle (PM0.1). A meta-analysis study suggested that BP was positively related to PM2.5 exposure with an elevation of 1.393 mmHg, 95% CI (0.874-1.912) and 0.895 mmHg, 95% CI (0.49-1.299) per 10 μg/m increase for systolic and diastolic BP, respectively (55). Additionally, there are several studies showing association with various PMs and CKD (56–58). Despite the association between maternal air pollution exposure and birth defects has been addressed (68, 69), how early exposure to particulate matters may increase the risk of adverse renal outcome in offspring is still largely unknown.
3 Prenatal Environmental Chemical Exposure on Renal Programming
All of the above-mentioned epidemiological evidence linking environmental chemical pollutants to kidney diseases and hypertension are from studies established in direct but not maternal exposure. Certain chemicals can impair nephrogenesis, resulting in low nephron endowment and a spectrum of defects in the kidney and urinary tract (70). Accordingly, developmental nephrotoxic effects can be expected during environmental chemical exposure of pregnant women. Any of these anomalies coinciding with reduced nephron number may have long-term sequelae such as kidney disease and hypertension in later life (70, 71). Although infants can be an increased risk of nephrotoxicity to elemental (e.g., mercury) or organic contaminants (e.g., melamine) (19, 72, 73), studies focusing on association for postnatal environmental chemical exposures (a time after completion of nephrogenesis) and adverse renal outcomes were excluded. Here, we summarize clinical and experimental studies regarding environmental chemical exposure in pregnancy related to adverse renal outcomes and hypertension in offspring.
3.1 Epidemiological Evidence
As shown in Table 2, very few human observational studies addressed maternal environmental chemical exposure implicating in offspring’s BP and renal outcome (74–84). All epidemiological evidence are mother-child cohort studies and none of them have been observed until adulthood. Prior prospective studies on the associations of maternal exposure to BPA and phthalates with childhood BP showed inconsistent results (74–78). Some studies did not show any association of fetal exposure to BPA with childhood BP, while others showed fetal exposure to BPA was associated with higher diastolic blood pressure (DBP) (74, 75). Another study showed higher second trimester maternal urine BPA levels were associated with higher systolic blood pressure (SBP) in boys at the mean age of 9.7 years (76). In the same cohort study of 1,064 mother-child pairs, maternal urine phthalate concentrations were not associated with BP in boys but were associated with lower BP in girls (76). A study of 500 children, found that participants born to mothers had high urinary phthalate metabolite concentrations was associated with low SBP and DBP at age 4 (77). Another study similarly showed that maternal urinary phthalate metabolite levels were negatively associated with SBP z-scores in girls (77). These studies investigating the associations of maternal phthalate exposure with childhood BP reported sex specific effects (76–78).
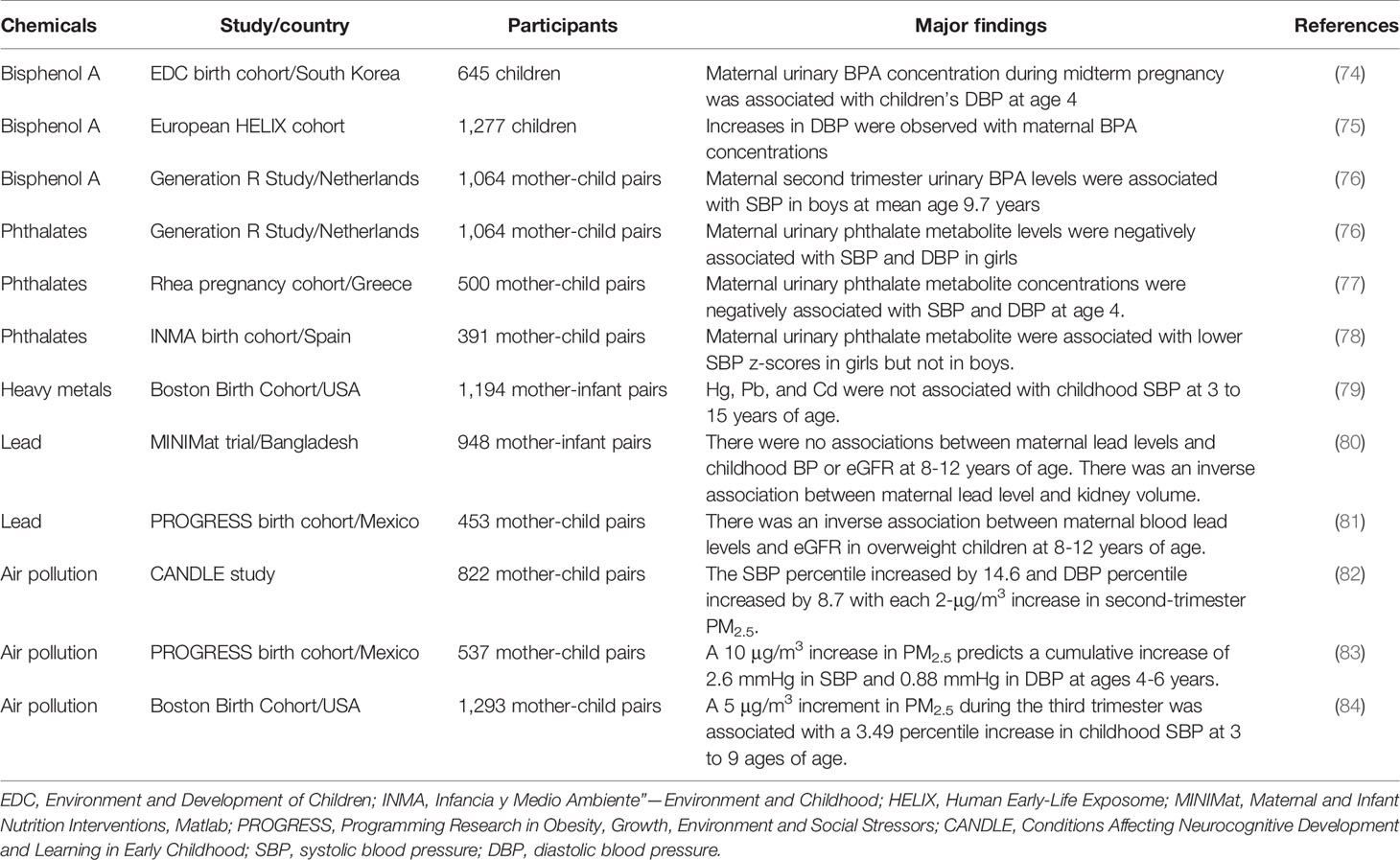
Table 2 Effects of maternal environmental chemical exposure on blood pressure and renal outcomes in children.
Table 2 illustrates that maternal heavy metal exposure, especially lead, is related to adverse renal effects on children. One study of 1,194 mother-infant pairs has evaluated the effect of prenatal exposure to heavy metals and trace elements on childhood BP (79). Hg and Pb were not associated with childhood SBP at 3 to 15 years of age. Although Cd was not associated with childhood systolic BP overall, the inverse association between manganese and childhood SBP was stronger at higher levels of Cd (79). Two studies investigated the associations between maternal lead levels and renal outcomes in offspring (80, 81). One study found there were no associations between maternal lead levels and childhood BP or eGFR at 8-12 years of age. However, they observed maternal lead level was negatively associated with kidney volume in children (80). Another study reported there was an inverse association between maternal blood lead levels and eGFR in overweight children at 8-12 years of age (81).
Regarding air pollution, one report demonstrated that higher prenatal PM2.5 exposure, particularly in the second trimester, was associated with elevated childhood BP at 4-6 years of age (82). Another report similarly showed that second and third trimester PM2.5 exposure may increase children’s BP at 4-6 years of age (83). Analysis of one study of 1,293 mother-child pairs indicated that a 5-μg/m3 increment in PM2.5 during the third trimester was associated with a 3.49 percentile increase in childhood systolic BP at 3 to 9 ages of age (84) (Table 2).
So far, there is lack of information about the BP and renal outcomes in children born to mothers exposed to PFOA, PFNA, or PAHs. However, maternal exposure to these chemicals have been linked to preterm birth, low birth weight (LBW), and intrauterine growth retardation (IUGR) (85–87). It is noteworthy that these risk factors related to reduced nephron number (70, 71) as well as kidney disease and hypertension in later life (11, 72, 88, 89). Likewise, prenatal PM2.5/PM10 or phthalate exposure were related with IUGR and LBW (76, 90). Since developmental origins of kidney disease can be attributed to multiple hits, a programmed low nephron endowment likely constitutes a first-hit to the kidney which makes the remaining glomeruli more vulnerable to environmental influences and increases the risk for developing CKD when facing other chemical pollutants in later life.
3.2 Evidence from Animal Models
To establish a causal relationship between prenatal exposure to environment chemicals and kidney disease and hypertension, animal models are valuable tools for establishing the dose–response relationship, understanding the mechanisms of developmental programming, and developing therapeutic interventions (15).
Table 3 summarizes animal studies demonstrating the association between maternal environmental chemical exposure and subsequent kidney disease and hypertension in progeny (91–105). The current review is solely restricted to chemical exposures happening during the duration of kidney development, with a focus on reporting offspring outcomes starting after birth. As shown in Table 3, rats have been the dominant animal species used. However, using large animals to study similar exposures are not applied as of today. The programming effects of environmental chemicals have been reported in rats ranging from 2 to 21 weeks of age, which is roughly equivalent to human ages from infancy to young adulthood (106).
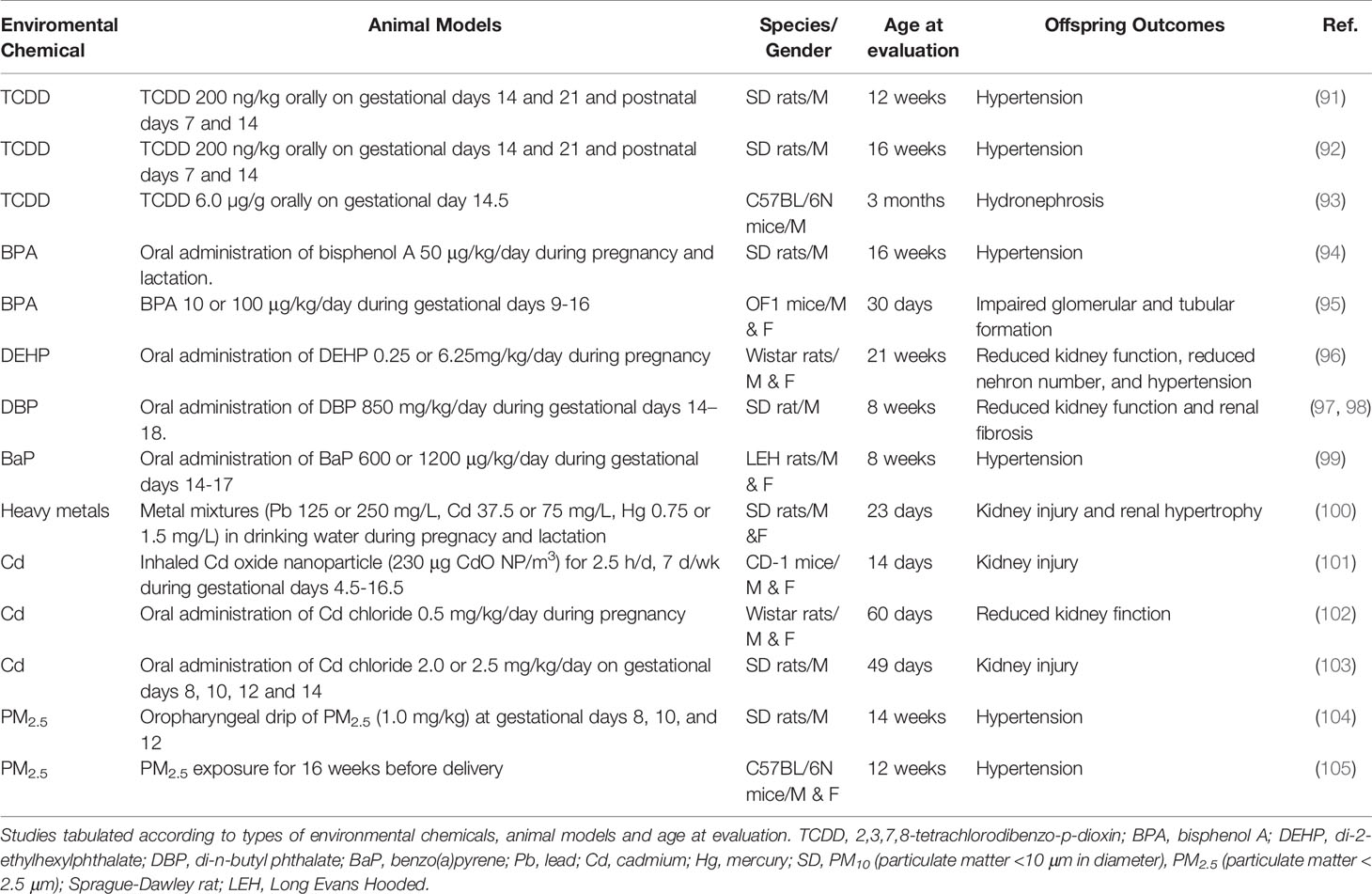
Table 3 Summary of animal models of developmental programming of kidney disease and hypertension categorized according to environmental chemical exposures.
Several types of chemicals have been evaluated, including TCDD (88–90), BPA (94, 95), DEHP (96), DBP (97, 98), BaP (99), heavy metal mixture (100), Cd (101–103), and PM2.5 (104, 105). Maternal exposure to TCDD or BPA causes the rise of BP in adult rat offspring (91, 92, 94), which was relevant to dysregulated AHR signaling pathway. Besides, hydronephrosis was described in rat offspring prenatally exposed to TCDD (93). EDC exposure during pregnancy induced kidney disease and hypertension in adult offspring was observed in three studies where BPA, DEHP, and DBP were orally administered in mother rats (95–98). Another environmental chemical that has been investigated is BaP (99). Oral doses of BaP exposure (600 or 1200 μg/kg/day) were administered to dams during gestational days 14-17 and showed hypertension in rat offspring of both sexes at 8 weeks of age (99). Animal studies of maternal heavy metal exposure implicating in the offspring kidney suggested that Cd is the main cause of adverse renal outcomes programmed by early-life heavy metal exposure (100–103). A combined metal mixtures (Pb, Cd, and Hg) in drinking water administered to mother rats during pregnancy and lactation study in rats resulted in kidney injury and renal hypertrophy in their offspring (100). Additionally, prenatally Cd-exposed offspring rats presented the features of kidney injury in other three studies (101–103), while no prior studies have addressed the effects of Pb or Hg. Furthermore, air pollution was shown to lead to hypertension in rats or mice prenatally exposed to PM2.5 (104, 105).
3.3 Mechanisms behind Developmental Origins of Kidney Disease and Hypertension
Taking all these evidences in consideration, various environmental chemical exposures in pregnancy can increase the risk of kidney disease and hypertension later in life. Considering that diverse maternal chemical exposures induce similar offspring renal outcomes, there might be some common mechanisms behind renal programming. Up to the present, a number of mechanisms of renal programming have been identified and some of them are linked to the pathogenesis underlying environmental chemical-induced kidney disease and hypertension (12–15, 72, 107–110). Several mechanisms have been considered, including oxidative stress, aberrant activation of the renin-angiotensin system (RAS), reduced nephron numbers, and dysregulated AHR signaling pathway, as illustrated below (Figure 2). These mechanisms are discussed in the following sections.
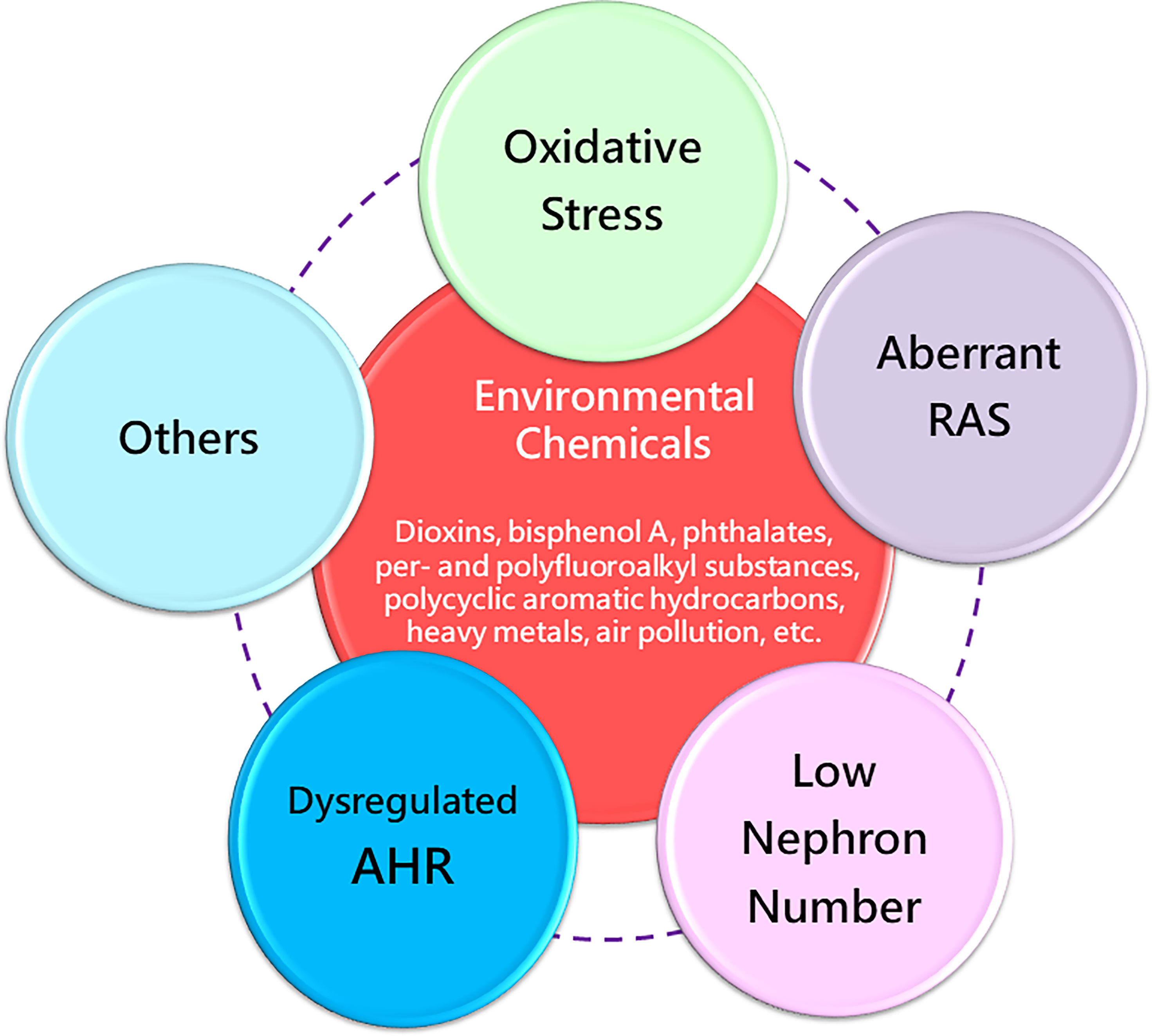
Figure 2 Overview of the common mechanisms of renal programming in response to various environmental chemicals in early life. RAS, renin-angiotensin system; AHR, aryl hydrocarbon receptor.
3.3.1 Oxidative Stress
Oxidative stress is referred to overproduction of reactive oxygen and nitrogen species (ROS/RNS) prevails over the defensive antioxidant system, resulting in oxidative stress damage (111). ROS/RNS play a dual role in pregnancy; such as moderate ROS/RNS levels contribute to normal organogenesis, whereas their overproduction adversely affects fetal outcomes (112). There are several models of maternal chemical exposure tied up with oxidative stress in mediating kidney disease and hypertension of developmental origins, comprising TCDD (91, 92), BPA (94), and PM2.5 (104). Increased ROS generation, decreased antioxidant capacity, and impaired nitric oxide (NO) signaling pathway all contribute to oxidative stress-induced renal programming, as we reviewed elsewhere (108). A marker of oxidative DNA damage, 8-hydroxydeoxyguanosine (8-OHdG), was increased in offspring kidneys prenatally exposed to TCDD (91) or BPA (94). Conversely, various antioxidants have been used as a therapeutic strategy to prevent developmental origins of kidney disease and hypertension (113). In a prenatal PM2.5 exposure rat model (104), offspring developed hypertension coinciding with oxidative stress, which was prevented by tempol, a synthetic antioxidant. These findings support the notion that kidney disease and hypertension programmed by maternal chemical exposure might be attributed to oxidative stress. In view to oxidative stress is proposed as one of the main mechanisms of chemical-induced pathology in humans (114), its role in kidney disease and hypertension of developmental origins, especially in response to various prenatal chemical pollutants, awaits further exploration.
3.3.2 Reduced Nephron Number
Nephron number is a major determinant of kidney health in later life. In general, nephron number is approximately 1 million per human kidney, with a huge individual differences ranging from 0.2 to 2.5 million (115). As we mentioned earlier, prior research has demonstrated that reduced nephron number, in relation to LBW and preterm birth, may result in hypertension and kidney disease in later life (11, 72, 88, 89). Epidemiological studies demonstrated that maternal exposure to PFOA, PFNA, PAHs, phthalates, and PM2.5/PM10 associated with preterm birth and LBW (76, 85–87, 90), both are risk factors related to reduced nephron number. Therefore, the role of these chemicals on nephron number in kidney disease and hypertension of developmental origins is still awaiting discovery but is certainly a subject of great interest.
Reduced nephron number can cause compensatory glomerular hyperfiltration and glomerular hypertension, consequently resulting in further nephron loss later in life. Accordingly, reduced nephron number has been found to be a key mechanism behind renal programming (72). In a maternal DEHP exposure model, adult offspring displayed reduced kidney function and hypertension coinciding with dysregulation of several nephrogenesis gene expression (96). These data suggest that maternal DEHP exposure impaired nephrogenesis, resulting in a nephron deficit, and subsequently kidney disease and hypertension later in life (96). Moreover, the severity of adverse nephrotoxic effects and the extent of renal involvement may be modified by the stage of kidney development (20). Thus, whether nephron number can be influenced by various chemical exposures in a dose- and stage-specific manner are required for further evaluation.
3.3.3 Aberrant Activation of RAS
The kidney is a major target for the multiple elements of the RAS (116). Blockers of the RAS have been the cornerstones of pharmacologic treatment for patients with hypertension and CKD (116). During nephrogenesis, constituents of the RAS are highly expressed and play key roles in mediating proper renal morphology and physiological function (117). As reviewed elsewhere (110), a transient biphasic response with downregulation of classical RAS axis in neonatal stage that becomes normalized with age. Thus, varied maternal insults can disturb this normalization in adulthood, insomuch that the classical RAS axis is inappropriately activated resulting in adult kidney disease and hypertension. While RAS blocker fetopathy, which presents renal malformation, appears when pregnant women taking angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) during the nephrogenesis stage (118). Table 3 shows several environmental chemicals can program the kidney and RAS concurrently—TCDD (92), DEHP (96), and BaP (99)—giving rise to hypertension in adult offspring. Currently, several early-life interventions targeting the RAS to prevent kidney disease and hypertension have been employed in animal models (110). To what extent the RAS are interconnected with various environmental chemicals towards kidney disease and hypertension of developmental origins are issues that await further clarification.
3.3.4 Dysregulated AHR Signaling Pathway
Quite a few environmental chemicals are ligands for AHR, such as TCDD, PCDD, PCDF, PCB, BPA, BaP (119). In addition to exogenous ligands (i.e., environmental chemicals), AHR signaling can be activated by endogenous ligands like tryptophan metabolites (119). In patients with kidney disease, the most important AHR ligands are uremic toxins, especially those gut microbiota-derived from tryptophan metabolism. These tryptophan-derived uremic toxins have proinflammatory, prooxidant, procoagulant, and pro-apoptotic effects, all of which are involved in the pathogenesis of hypertension and CKD (120). In a maternal BPA exposure model, adult offspring developed hypertension coinciding with increased AHR protein level as well as the mRNA expression of AHR target gene Ahrr, Cyp1a1, and Arnt (94). Similarly, maternal TCDD-induced programmed hypertension was associated with mediation of the AhR signaling pathway (91, 92). Conversely, antagonizing AHR signaling by resveratrol has been reported to protect adult offspring against hypertension programmed by environmental chemicals like TCDD (92) and BPA (94). Moreover, AHR signaling can modulate pro-inflammatory T helper 17 (TH17) axis and trigger inflammation, by which environmental chemicals may link to the development of hypertension and kidney disease (121, 122). Hopefully, elucidation of the role of AHR in chemical-induced programmed kidney disease and hypertension will aid in the development of novel therapies.
3.3.5 Others
Other molecular mechanisms relevant to the development of kidney disease and hypertension are identified in different animal models of developmental origins, such as dysbiotic gut microbiota (123), dysregulated nutrient-sensing signaling (124), impaired sodium transport (12), and epigenetic regulation (125). Since these mechanisms are more or less related to environmental chemicals (126–128), there might be considerable interplay among these mechanisms behind kidney disease and hypertension of developmental origins, even though this remains speculative.
4 Therapeutic Strategies Targeting on Environmental Chemicals
Taking into account the fact that our advanced understanding of the DOHaD research recently, it turns out therapeutic interventions can be shifted from adulthood to early life before disease occurs, by so-called reprogramming (129). So far, reprogramming strategies to reverse the programming processes that have been investigated include lifestyle modification, nutritional intervention, and pharmacological therapy. Concerning environmental chemical pollutants, there is no doubt that reprogramming strategies should focus on avoiding exposure to theoretically harmful chemicals prenatally and promoting a healthy lifestyle. As mentioned earlier, several chemicals induced programmed kidney disease and hypertension are associated with oxidative stress (91, 92, 94, 104). Several natural antioxidants have been used as nutritional interventions in pregnancy to prevent kidney disease and hypertension in a number of animal models, as we reviewed elsewhere (113, 130). Additionally, early-life interventions targeting specific signaling pathways might be of benefit in the prevention of chemical pollutant-induced renal programming. An example of therapeutic target is the RAS. Several RAS-based interventions have also shown benefits in protecting against programmed hypertension, such as renin inhibitor, ACE inhibitor, ARB, and ACE2 activator (110). In view of that aberrant RAS signaling contributes to maternal chemical exposure-induced renal programming (91, 96, 99), RAS-based interventions might be an ideal reprogramming strategy. Furthermore, resveratrol acting like an AHR antagonist benefits kidney disease and hypertension of developmental origins (131, 132). Although various reprogramming interventions that show tremendous advances with regard to renal programming, their protective benefits against kidney disease and hypertension programmed by maternal environmental chemical exposure remain still a long way off.
5 Conclusions and Future Perspectives
Previous studies have indicated the adverse impact of environmental chemicals on public health. This review sought to highlight the risks of environmental chemicals are communicable to the future generations and the value of DOHaD approach will aid in prevention rather than treatment of kidney disease and hypertension. At face value, it would be logical to consider early prohibiting exposure to hazardous chemicals. However, there are many aspects still unsolved. Limited environmental chemicals have been evaluated in humans and animal models of kidney disease and hypertension, not to mention that only a few of them have been studied in the DOHaD filed. Global chemicals production is expected to double by 2030, and the already widespread use of chemicals is likely to also increase, including in consumer products (133). At a deeper level, little reliable information currently exists regarding the long-term effects of environmental chemical exposure in human cohorts and animal studies. Most epidemiological evidence are mother-child cohorts, which are hard to proceed to adulthood. Considering certain chemicals like EDCs have shown transgenerational epigenetic effects on endocrine function, future work in animal studies is needed to better understand various environmental chemicals can induce kidney disease and hypertension in future generations to which extent. Moreover, reprogramming interventions targeting common mechanisms to prevent kidney disease and hypertension are still missing in the literature.
Peace, dignity and equality on a healthy planet — these are the ultimate goals stated by the United Nations in 2015, to be achieved by 2030 (134). While much remains to be done to tackle the challenging of NCDs, kidney disease in particular (135, 136). In 2020, the World Kidney Day informed the public about the importance of preventive interventions – be it primary, secondary or tertiary (137). Seeing the prevention strategy from a DOHaD perspective, primary and secondary prevention seems our best strategy to improve global kidney health. First, primary prevention aims to prevent kidney disease before it ever occurs. There is an urgent need for multidisciplinary efforts to perform investigations that identify toxic chemicals in the environment. During pregnancy through early childhood, avoiding harmful chemicals and toxins exposure at home, at work, and at play are essential for supporting kidney health. Although various environmental chemicals have been identified so far, preventive efforts should continue to discover other potentially harmful chemicals. Secondary prevention is early screening to identify and prompt treatment of kidney disease in the earliest stages. Although early detection CKD has the potential to yield marked public health benefits, most countries had inadequate CKD detection and surveillance systems to achieve this goal (136). Additionally, there will be a growing need to translate effective reprogramming interventions from animal studies into clinical practice as the process moves far slower than expected.
In conclusion, maternal environmental chemical exposure is a considerably pathogenetic link in kidney disease and hypertension of developmental origins. Further advances in the DOHaD field, aimed at the pregnant mothers and their offspring, hence have the potential to combat the burden of kidney disease and hypertension, which represent major global health challenges.
Author Contributions
C-NH contributed to concept generation, data interpretation, methodology, drafting of the manuscript, critical revision of the manuscript, and approval of the article. Y-LT contributed to concept generation, methodology, drafting of the manuscript, critical revision of the manuscript, and approval of the article. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Grants CMRPG8J0253, CORPG8L0301, CORPG8L0261, and CORPG8L0121 from Chang Gung Memorial Hospital, Kaohsiung, Taiwan.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Barker DJ. The Origins of the Developmental Origins Theory. J Intern Med (2007) 261:412–7. doi: 10.1111/j.1365-2796.2007.01809.x
3. Roseboom T, de Rooij S, Painter R. The Dutch Famine and its Long-Term Consequences for Adult Health. Early Hum Dev (2006) 82:485–91. doi: 10.1016/j.earlhumdev.2006.07.001
4. Hanson M, Gluckman P. Developmental Origins of Noncommunicable Disease: Population and Public Health Implications. Am J Clin Nutr (2011) 94:1754S–8S. doi: 10.3945/ajcn.110.001206
5. Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of In Utero and Early-Life Conditions on Adult Health and Disease. N Engl J Med (2008) 359:61–73. doi: 10.1056/NEJMra0708473
6. World Health Organization. Tackling Ncds: ‘Best Buys’ and Other Recommended Interventions for the Prevention and Control of Noncommunicable Diseases. Switzerland: World Health Organization (2017).
7. Baird J, Jacob C, Barker M, Fall CH, Hanson M, Harvey NC, et al. Developmental Origins of Health and Disease: A Lifecourse Approach to the Prevention of Non-Communicable Diseases. Healthcare (Basel) (2017) 5:14. doi: 10.3390/healthcare5010014
8. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and Regional Mortality From 235 Causes of Death for 20 Age Groups in 1990 and 2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet (2012) 380:2095–128. doi: 10.1016/S0140-6736(12)61728-0
9. Nguyen QC, Tabor JW, Entzel PP, Lau Y, Suchindran C, Hussey JM, et al. Discordance in National Estimates of Hypertension Among Young Adults. Epidemiology (2011) 22:532–41. doi: 10.1097/EDE.0b013e31821c79d2
10. Weir MR. Hypertension and the Kidney: Perspectives on the Relationship of Kidney Disease and Cardiovascular Disease. Clin J Am Soc Nephrol (2009) 4:2045–50. doi: 10.2215/CJN.03050509
11. Luyckx VA, Bertram JF, Brenner BM, Fall C, Hoy WE, Ozanne SE, et al. Effect of Fetal and Child Health on Kidney Development and Long-Term Risk of Hypertension and Kidney Disease. Lancet (2013) 382:273–83. doi: 10.1016/S0140-6736(13)60311-6
12. Kett MM, Denton KM. Renal Programming: Cause for Concern? Am J Physiol Integr Comp Physiol (2011) 300:R791–803. doi: 10.1152/ajpregu.00791.2010
13. Chong E, Yosypiv IV. Developmental Programming of Hypertension and Kidney Disease. Int J Nephrol (2012) 2012:760580. doi: 10.1155/2012/760580
14. Paixão AD, Alexander BT. How the Kidney Is Impacted by the Perinatal Maternal Environment to Develop Hypertension. Biol Reprod (2013) 89:144. doi: 10.1095/biolreprod.113.111823
15. Hsu CN, Tain YL. Animal Models for Dohad Research: Focus on Hypertension of Developmental Origins. Biomedicines (2021) 9:623. doi: 10.3390/biomedicines9060623
16. Nüsken E, Dötsch J, Weber LT, Nüsken KD. Developmental Programming of Renal Function and Re-Programming Approaches. Front Pediatr (2018) 6:36. doi: 10.3389/fped.2018.00036
17. Xu X, Nie S, Ding H, Hou FF. Environmental Pollution and Kidney Diseases. Nat Rev Nephrol (2018) 14:313–24. doi: 10.1038/nrneph.2018.11
18. Kataria A, Trasande L, Trachtman H. The Effects of Environmental Chemicals on Renal Function. Nat Rev Nephrol (2015) 11:610–25. doi: 10.1038/nrneph.2015.94
19. Weidemann DK, Weaver VM, Fadrowski JJ. Toxic Environmental Exposures and Kidney Health in Children. Pediatr Nephrol (2016) 31:2043–54. doi: 10.1007/s00467-015-3222-3
20. Solhaug MJ, Bolger PM, Jose PA. The Developing Kidney and Environmental Toxins. Pediatrics (2004) 113:1084–91.
21. McMullen S, Mostyn A. Animal Models for the Study of the Developmental Origins of Health and Disease. Proc Nutr Soc (2009) 68:306–20. doi: 10.1017/S0029665109001396
22. Luyckx VA, Brenner BM. The Clinical Importance of Nephron Mass. J Am Soc Nephrol (2010) 21:898–910. doi: 10.1681/ASN.2009121248
23. Chevalier RL. Evolution, Kidney Development, and Chronic Kidney Disease. Semin Cell Dev Biol (2019) 91:119–31. doi: 10.1016/j.semcdb.2018.05.024
24. Dopico M, Gómez A. Review of the Current State and Main Sources of Dioxins Around the World. J Air Waste Manag Assoc (2015) 65:1033–49. doi: 10.1080/10962247.2015.1058869
25. Milbrath MO, Wenger Y, Chang CW, Emond C, Garabrant D, Gillespie BW, et al. Apparent Half-Lives of Dioxins, Furans, and Polychlorinated Biphenyls as a Function of Age, Body Fat, Smoking Status, and Breast-Feeding. Environ Health Perspect (2009) 117:417–25. doi: 10.1289/ehp.11781
26. Chang JW, Ou HY, Chen HL, Su HJ, Lee CC. Hyperuricemia After Exposure to Polychlorinated Dibenzo-P-Dioxins and Dibenzofurans Near a Highly Contaminated Area. Epidemiology (2013) 24:582–9. doi: 10.1097/EDE.0b013e318294ef68
27. Uemura H, Arisawa K, Hiyoshi M, Kitayama A, Takami H, Sawachika F, et al. Prevalence of Metabolic Syndrome Associated With Body Burden Levels of Dioxin and Related Compounds Among Japan’s General Population. Environ Health Perspect (2009) 117:568–73. doi: 10.1289/ehp.0800012
28. Karouna-Renier NK, Rao KR, Lanza JJ, Davis DA, Wilson PA. Serum Profiles of Pcdds and Pcdfs, in Individuals Near the Escambia Wood Treating Company Superfund Site in Pensacola, FL. Chemosphere (2007) 69:1312–9. doi: 10.1016/j.chemosphere.2007.05.028
29. Schecter A, Malik N, Haffner D, Smith S, Harris TR, Paepke O, et al. Bisphenol a (BPA) in U.S. Food. Environ Sci Technol (2010) 44:9425–30. doi: 10.1021/es102785d
30. Acconcia F, Pallottini V, Marino M. Molecular Mechanisms of Action of BPA. Dose Response (2015) 13:1559325815610582. doi: 10.1177/1559325815610582
31. Koch HM, Kolossa-Gehring M, Schröter-Kermani C, Angerer J, Brüning T. Bisphenol a in 24 H Urine and Plasma Samples of the German Environmental Specimen Bank From 1995 to 2009: A Retrospective Exposure Evaluation. J Expo Sci Environ Epidemiol (2012) 22:610–6. doi: 10.1038/jes.2012.39
32. Kanno Y, Okada H, Kobayashi T, Takenaka T, Suzuki H. Effects of Endocrine Disrupting Substance on Estrogen Receptor Gene Transcription in Dialysis Patients. Ther Apher Dial (2007) 11:262–5. doi: 10.1111/j.1744-9987.2007.00472.x
33. You L, Zhu X, Shrubsole MJ, Fan H, Chen J, Dong J, et al. Renal Function, Bisphenol a, and Alkylphenols: Results From the National Health and Nutrition Examination Survey (NHANES 2003-2006). Environ Health Perspect (2011) 119:527–33. doi: 10.1289/ehp.1002572
34. Bae S, Kim JH, Lim YH, Park HY, Hong YC. Associations of Bisphenol a Exposure With Heart Rate Variability and Blood Pressure. Hypertension (2012) 60:786–93. doi: 10.1161/HYPERTENSIONAHA.112.197715
35. Katsikantami I, Sifakis S, Tzatzarakis MN, Vakonaki E, Kalantzi OI, Tsatsakis AM, et al. A Global Assessment of Phthalates Burden and Related Links to Health Effects. Environ Int (2016) 97:212–36. doi: 10.1016/j.envint.2016.09.0132
36. Gao D-W, Wen Z-D. Phthalate Esters in the Environment: A Critical Review of Their Occurrence, Biodegradation, and Removal During Wastewater Treatment Processes. Sci Total Environ (2016) 541:986–1001. doi: 10.1016/j.scitotenv.2015.09.148
37. Qian Y, Shao H, Ying X, Huang W, Hua Y. The Endocrine Disruption of Prenatal Phthalate Exposure in Mother and Offspring. Front Public Health (2020) 8:366. doi: 10.3389/fpubh.2020.00366
38. Trasande L, Sathyanarayana S, Spanier AJ, Trachtman H, Attina TM, Urbina EM. Urinary Phthalates Are Associated With Higher Blood Pressure in Childhood. J Pediatr (2013) 163:747–53. doi: 10.1016/j.jpeds.2013.03.072
39. Lee I, Park JY, Kim S, An JN, Lee J, Park H, et al. Association of Exposure to Phthalates and Environmental Phenolics With Markers of Kidney Function: Korean National Environmental Health Survey (Konehs) 2015-2017. Environ Int (2020) 143:105877. doi: 10.1016/j.envint.2020.105877
40. Kang H, Lee JP, Choi K. Exposure to Phthalates and Environmental Phenols in Association With Chronic Kidney Disease (CKD) Among the General US Population Participating in Multi-Cycle NHANES (2005-2016). Sci Total Environ (2021) 791:148343. doi: 10.1016/j.scitotenv.2021
41. Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. A Review of the Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (Pfass) and Present Understanding of Health Effects. J Expo Sci Environ Epidemiol (2019) 29:131–47. doi: 10.1038/s41370-018-0094-1
42. Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in Exposure to Polyfluoroalkyl Chemicals in the U.S. Population: 1999-2008. Environ Sci Technol (2011) 45:8037–45. doi: 10.1021/es1043613
43. Shankar A, Xiao J, Ducatman A. Perfluoroalkyl Chemicals and Chronic Kidney Disease in US Adults. Am J Epidemiol (2011) 174:893–900. doi: 10.1093/aje/kwr171
44. Patel AB, Shaikh S, Jain KR, Desai C, Madamwar D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front Microbiol (2020) 11:562813. doi: 10.3389/fmicb.2020.562813
45. Pulkrabova J, Stupak M, Svarcova A, Rossner P, Rossnerova A, Ambroz A, et al. Relationship Between Atmospheric Pollution in the Residential Area and Concentrations of Polycyclic Aromatic Hydrocarbons (Pahs) in Human Breast Milk. Sci Total Environ (2016) 562:640–7. doi: 10.1016/j.scitotenv.2016.04.013
46. Drwal E, Rak A, Gregoraszczuk EL. Review: Polycyclic Aromatic Hydrocarbons (Pahs)-Action on Placental Function and Health Risks in Future Life of Newborns. Toxicology (2019) 411:133–42. doi: 10.1016/j.tox.2018.10.003
47. Perera FP, Rauh V, Whyatt RM, Tang D, Tsai WY, Bernert JT, et al. A Summary of Recent Findings on Birth Outcomes and Developmental Effects of Prenatal ETS, PAH, and Pesticide Exposures. Neurotoxicology (2005) 26:573–87. doi: 10.1016/j.neuro.2004.07.007
48. Farzan SF, Chen Y, Trachtman H, Trasande L. Urinary Polycyclic Aromatic Hydrocarbons and Measures of Oxidative Stress, Inflammation and Renal Function in Adolescents: NHANES 2003-2008. Environ Res (2016) 144:149–57. doi: 10.1016/j.envres.2015.11.012
49. Jacobs L, Buczynska A, Walgraeve C, Delcloo A, Potgieter-Vermaak S, Van Grieken R, et al. Acute Changes in Pulse Pressure in Relation to Constituents of Particulate Air Pollution in Elderly Persons. Environ Res (2012) 117:60–7. doi: 10.1016/j.envres.2012.05.003
50. Li J, Fan H, Liu K, Li X, Fan D, Lu X, et al. Associations of Urinary Polycyclic Aromatic Hydrocarbons With Albuminuria in U.S. Adults, NHANES 2003-2014. Ecotoxicol Environ Saf (2020) 195:110445. doi: 10.1016/j.ecoenv.2020.110445
51. Järup L. Hazards of Heavy Metal Contamination. Br Med Bull (2003) . 68:167–82. doi: 10.1093/bmb/ldg032
52. Lutz E, Lind B, Herin P, Krakau I, Bui TH, Vahter M. Concentrations of Mercury, Cadmium and Lead in Brain and Kidney of Second Trimester Fetuses and Infants. J Trace Elem Med Biol (1996) 10:61–7. doi: 10.1016/S0946-672X(96)80013-7
53. Wedeen RP, D’Haese P, Van de Vyver FL, Verpooten GA, De Broe ME. Lead Nephropathy. Am J Kidney Dis (1986) 8:380–3. doi: 10.1016/s0272-6386(86)80113-5
54. Du Y, Xu X, Chu M, Guo Y, Wang J. Air Particulate Matter and Cardiovascular Disease: The Epidemiological, Biomedical and Clinical Evidence. J Thorac Dis (2016) 8:E8–E19. doi: 10.3978/j.issn.2072-1439.2015.11.37
55. Liang R, Zhang B, Zhao X, Ruan Y, Lian H, Fan Z. Effect of Exposure to PM2.5 on Blood Pressure: A Systematic Review and Meta-Analysis. J Hypertens (2014) 32:2130–40. doi: 10.1097/HJH.0000000000000342
56. Afsar B, Elsurer Afsar R, Kanbay A, Covic A, Ortiz A, Kanbay M. Air Pollution and Kidney Disease: Review of Current Evidence. Clin Kidney J (2019) 12:19–32. doi: 10.1093/ckj/sfy111
57. Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z. Particulate Matter Air Pollution and the Risk of Incident CKD and Progression to ESRD. J Am Soc Nephrol (2018) 29:218–30. doi: 10.1681/ASN.2017030253
58. Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z. Associations of Ambient Coarse Particulate Matter, Nitrogen Dioxide, and Carbon Monoxide With the Risk of Kidney Disease: A Cohort Study. Lancet Planet Health (2017) 1:e267–76. doi: 10.1016/S2542-5196(17)30117-1
59. Brito JS, Borges NA, Esgalhado M, Magliano DC, Soulage CO, Mafra D. Aryl Hydrocarbon Receptor Activation in Chronic Kidney Disease: Role of Uremic Toxins. Nephron (2017) 137:1–7. doi: 10.1159/000476074
60. Zhang N. The Role of Endogenous Aryl Hydrocarbon Receptor Signaling in Cardiovascular Physiology. J Cardiovasc Dis Res (2011) 2:91–5. doi: 10.4103/0975-3583.83033
61. McLachlan JA. Environmental Signaling: From Environmental Estrogens to Endocrine-Disrupting Chemicals and Beyond. Andrology (2016) 4:684–94. doi: 10.1111/andr.12206
62. Watkins DJ, Josson J, Elston B, Bartell SM, Shin HM, Vieira VM, et al. Exposure to Perfluoroalkyl Acids and Markers of Kidney Function Among Children and Adolescents Living Near a Chemical Plant. Environ Health Perspect (2013) 121:625–30. doi: 10.1289/ehp.1205838
63. Geiger SD, Xiao J, Shankar A. No Association Between Perfluoroalkyl Chemicals and Hypertension in Children. Integr Blood Press Control (2014) 7:1–7. doi: 10.2147/IBPC.S47660
64. Stanifer JW, Stapleton HM, Souma T, Wittmer A, Zhao X, Boulware LE. Perfluorinated Chemicals as Emerging Environmental Threats to Kidney Health: A Scoping Review. Clin J Am Soc Nephrol (2018) 13:1479–92. doi: 10.2215/CJN.04670418
65. Mokhtar MM, Khidr EG, Shaban HM, Allam S, Elsadek BEM, Salama SA, et al. The Effect of Aryl Hydrocarbon Receptor Ligands on Gentamicin-Induced Nephrotoxicity in Rats. Environ Sci Pollut Res Int (2020) 27:16189–202. doi: 10.1007/s11356-020-08073-z
66. Yang H, Shu Y. Cadmium Transporters in the Kidney and Cadmium-Induced Nephrotoxicity. Int J Mol Sci (2015) 16(1):1484–94. doi: 10.3390/ijms16011484FowlerBA
67. Skroder H, Hawkesworth S, Kippler M, El Arifeen S, Wagatsuma Y, Moore SE, et al. Kidney Function and Blood Pressure in Preschool-Aged Children Exposed to Cadmium and Arsenic—Potential Alleviation by Selenium. Environ Res (2015) 140:205–13. doi: 10.1016/j.envres.2015.03.038
68. Hu CY, Huang K, Fang Y, Yang XJ, Ding K, Jiang W, et al. Maternal Air Pollution Exposure and Congenital Heart Defects in Offspring: A Systematic Review and Meta-Analysis. Chemosphere (2020) 253:126668. doi: 10.1016/j.chemosphere.2020.126668
69. Lin HC, Guo JM, Ge P, Ou P. Association Between Prenatal Exposure to Ambient Particulate Matter and Risk of Hypospadias in Offspring: A Systematic Review and Meta-Analysis. Environ Res (2021) 192:110190. doi: 10.1016/j.envres.2020.110190
70. Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. Human Nephron Number: Implications for Health and Disease. Pediatr Nephrol (2011) 26:1529–33. doi: 10.1007/s00467-011-1843-8
71. Wang X, Garrett MR. Nephron Number, Hypertension, and CKD: Physiological and Genetic Insight From Humans and Animal Models. Physiol Genomics (2017) 49:180–92. doi: 10.1152/physiolgenomics.00098.2016
72. Tain YL, Hsu CN. Developmental Origins of Chronic Kidney Disease: Should We Focus on Early Life? Int J Mol Sci (2017) 18:381. doi: 10.3390/ijms18020381
73. Dalal RP, Goldfarb DS. Melamine-Related Kidney Stones and Renal Toxicity. Nat Rev Nephrol (2011) 7:267–74. doi: 10.1038/nrneph.2011.24
74. Bae S, Lim YH, Lee YA, Shin CH, Oh SY, Hong YC. Maternal Urinary Bisphenol a Concentration During Midterm Pregnancy and Children’s Blood Pressure at Age 4. Hypertension (2017) 69:367–74. doi: 10.1161/HYPERTENSIONAHA.116.08281
75. Warembourg C, Maitre L, Tamayo-Uria I, Fossati S, Roumeliotaki T, Aasvang GM, et al. Early-Life Environmental Exposures and Blood Pressure in Children. J Am Coll Cardiol (2019) 74:1317–28. doi: 10.1016/j.jacc.2019.06.069
76. Sol CM, Santos S, Asimakopoulos AG, Martinez-Moral MP, Duijts L, Kannan K, et al. Associations of Maternal Phthalate and Bisphenol Urine Concentrations During Pregnancy With Childhood Blood Pressure in a Population-Based Prospective Cohort Study. Environ Int (2020) 138:105677. doi: 10.1016/j.envint.2020.105677
77. Vafeiadi M, Myridakis A, Roumeliotaki T, Margetaki K, Chalkiadaki G, Dermitzaki E, et al. Association of Early Life Exposure to Phthalates With Obesity and Cardiometabolic Traits in Childhood: Sex Specific Associations. Front Public Health (2018) 6:327. doi: 10.3389/fpubh.2018.00327
78. Valvi D, Casas M, Romaguera D, Monfort N, Ventura R, Martinez D, et al. Prenatal Phthalate Exposure and Childhood Growth and Blood Pressure: Evidence From the Spanish INMA-Sabadell Birth Cohort Study. Environ Health Perspect (2015) 123:1022–9. doi: 10.1289/ehp.1408887
79. Zhang M, Liu T, Wang G, Buckley JP, Guallar E, Hong X, et al. In Utero Exposure to Heavy Metals and Trace Elements and Childhood Blood Pressure in a U.S. Urban, Low-Income, Minority Birth Cohort. Environ Health Perspect (2021) 129:67005. doi: 10.1289/EHP8325
80. Skröder H, Hawkesworth S, Moore SE, Wagatsuma Y, Kippler M, Vahter M. Prenatal Lead Exposure and Childhood Blood Pressure and Kidney Function. Environ Res (2016) 151:628–34. doi: 10.1016/j.envres.2016.08.028
81. Saylor C, Tamayo-Ortiz M, Pantic I, Amarasiriwardena C, McRae N, Estrada-Gutierrez G, et al. Prenatal Blood Lead Levels and Reduced Preadolescent Glomerular Filtration Rate: Modification by Body Mass Index. Environ Int (2021) 154:106414. doi: 10.1016/j.envint.2021.106414
82. Ni Y, Szpiro AA, Young MT, Loftus CT, Bush NR, LeWinn KZ, et al. Associations of Pre- and Postnatal Air Pollution Exposures With Child Blood Pressure and Modification by Maternal Nutrition: A Prospective Study in the CANDLE Cohort. Environ Health Perspect (2021) 129:47004. doi: 10.1289/EHP7486
83. Zhang M, Mueller NT, Wang H, Hong X, Appel LJ, Wang X. Maternal Exposure to Ambient Particulate Matter ≤2.5 µm During Pregnancy and the Risk for High Blood Pressure in Childhood. Hypertension (2018) 72:194–201. doi: 10.1161/HYPERTENSIONAHA.117.10944
84. Rosa MJ, Hair GM, Just AC, Kloog I, Svensson K, Pizano-Zárate ML, et al. Identifying Critical Windows of Prenatal Particulate Matter (PM2.5) Exposure and Early Childhood Blood Pressure. Environ Res (2020) 182:109073. doi: 10.1016/j.envres.2019.109073
85. Gao X, Ni W, Zhu S, Wu Y, Cui Y, Ma J, et al. Per- and Polyfluoroalkyl Substances Exposure During Pregnancy and Adverse Pregnancy and Birth Outcomes: A Systematic Review and Meta-Analysis. Environ Res (2021) 201:111632. doi: 10.1016/j.envres.2021.111632
86. Choi H, Wang L, Lin X, Spengler JD, Perera FP. Fetal Window of Vulnerability to Airborne Polycyclic Aromatic Hydrocarbons on Proportional Intrauterine Growth Restriction. PloS One (2012) 7:e35464. doi: 10.1371/journal.pone.0035464
87. Kumar SN, Saxena P, Patel R, Sharma A, Pradhan D, Singh H, et al. Predicting Risk of Low Birth Weight Offspring From Maternal Features and Blood Polycyclic Aromatic Hydrocarbon Concentration. Reprod Toxicol (2020) 94:92–100. doi: 10.1016/j.reprotox.2020.03.009
88. Hsu CW, Yamamoto KT, Henry RK, De Roos AJ, Flynn JT. Prenatal Risk Factors for Childhood CKD. J Am Soc Nephrol (2014) 25:2105–11. doi: 10.1681/ASN.2013060582
89. Tain YL, Luh H, Lin CY, Hsu CN. Incidence and Risks of Congenital Anomalies of Kidney and Urinary Tract in Newborns: A Population-Based Case-Control Study in Taiwan. Med (Baltimore) (2016) 95:e2659. doi: 10.1097/MD.0000000000002659
90. Uwak I, Olson N, Fuentes A, Moriarty M, Pulczinski J, Lam J, et al. Application of the Navigation Guide Systematic Review Methodology to Evaluate Prenatal Exposure to Particulate Matter Air Pollution and Infant Birth Weight. Environ Int (2021) 148:106378. doi: 10.1016/j.envint.2021.106378
91. Hsu CN, Chan JYH, Yu HR, Lee WC, Wu KLH, Chang-Chien GP, et al. Targeting on Gut Microbiota-Derived Metabolite Trimethylamine to Protect Adult Male Rat Offspring Against Hypertension Programmed by Combined Maternal High-Fructose Intake and Dioxin Exposure. Int J Mol Sci (2020) 21:5488. doi: 10.3390/ijms21155488
92. Hsu CN, Lin YJ, Lu PC, Tain YL. Maternal Resveratrol Therapy Protects Male Rat Offspring Against Programmed Hypertension Induced by TCDD and Dexamethasone Exposures: Is it Relevant to Aryl Hydrocarbon Receptor? Int J Mol Sci (2018) 19:2459. doi: 10.3390/ijms19082459
93. Aragon AC, Kopf PG, Campen MJ, Huwe JK, Walker MK. In Utero and Lactational 2,3,7,8-Tetrachlorodibenzo-P-Dioxin Exposure: Effects on Fetal and Adult Cardiac Gene Expression and Adult Cardiac and Renal Morphology. Toxicol Sci (2008) 101:321–30. doi: 10.1093/toxsci/kfm272
94. Hsu CN, Lin YJ, Tain YL. Maternal Exposure to Bisphenol a Combined With High-Fat Diet-Induced Programmed Hypertension in Adult Male Rat Offspring: Effects of Resveratrol. Int J Mol Sci (2019) 20:4382. doi: 10.3390/ijms20184382
95. Nuñez P, Fernandez T, García-Arévalo M, Alonso-Magdalena P, Nadal A, Perillan C, et al. Effects of Bisphenol a Treatment During Pregnancy on Kidney Development in Mice: A Stereological and Histopathological Study. J Dev Orig Health Dis (2018) 9:208–14. doi: 10.1017/S2040174417000939
96. Wei Z, Song L, Wei J, Chen T, Chen J, Lin Y, et al. Maternal Exposure to Di-(2-Ethylhexyl)Phthalate Alters Kidney Development Through the Renin-Angiotensin System in Offspring. Toxicol Lett (2012) 212:212–21. doi: 10.1016/j.toxlet.2012.05.023
97. Sun WL, Zhu YP, Ni XS, Jing DD, Yao YT, Ding W, et al. Potential Involvement of Fgf10/Fgfr2 and Androgen Receptor (AR) in Renal Fibrosis in Adult Male Rat Offspring Subjected to Prenatal Exposure to Di-N-Butyl Phthalate (DBP). Toxicol Lett (2018) 282:37–42. doi: 10.1016/j.toxlet.2017.09.009
98. Ye Q, Zhao S, Zhang Y, Su YM, Chen M, Zhao J, et al. Activation of the Rhoa/ROCK Pathway Contributes to Renal Fibrosis in Offspring Rats Induced by Maternal Exposure to Di-N-Butyl Phthalate. Toxicology (2020) 443:152573. doi: 10.1016/j.tox.2020.152573
99. Jules GE, Pratap S, Ramesh A, Hood DB. In Utero Exposure to Benzo(a)Pyrene Predisposes Offspring to Cardiovascular Dysfunction in Later-Life. Toxicology (2012) 295:56–67. doi: 10.1016/j.tox.2012.01.017
100. Zhou F, Yin G, Gao Y, Liu D, Xie J, Ouyang L, et al. Toxicity Assessment Due to Prenatal and Lactational Exposure to Lead, Cadmium and Mercury Mixtures. Environ Int (2019) 133:105192. doi: 10.1016/j.envint.2019.105192
101. Blum JL, Edwards JR, Prozialeck WC, Xiong JQ, Zelikoff JT. Effects of Maternal Exposure to Cadmium Oxide Nanoparticles During Pregnancy on Maternal and Offspring Kidney Injury Markers Using a Murine Model. J Toxicol Environ Health A (2015) 8:711–24. doi: 10.1080/15287394.2015.1026622
102. Jacquillet G, Barbier O, Rubera I, Tauc M, Borderie A, Namorado MC, et al. Cadmium Causes Delayed Effects on Renal Function in the Offspring of Cadmium-Contaminated Pregnant Female Rats. Am J Physiol Renal Physiol (2007) 293:F1450–60. doi: 10.1152/ajprenal.00223.2007
103. Saillenfait AM, Payan JP, Brondeau MT, Zissu D, de Ceaurriz J. Changes in Urinary Proximal Tubule Parameters in Neonatal Rats Exposed to Cadmium Chloride During Pregnancy. J Appl Toxicol (1991) 11:23–7. doi: 10.1002/jat.2550110105
104. Ye Z, Lu X, Deng Y, Wang X, Zheng S, Ren H, et al. In Utero Exposure to Fine Particulate Matter Causes Hypertension Due to Impaired Renal Dopamine D1 Receptor in Offspring. Cell Physiol Biochem (2018) 46:148–59. doi: 10.1159/000488418
105. Pan K, Jiang S, Du X, Zeng X, Zhang J, Song L, et al. Parental PM2.5 Exposure Changes Th17/Treg Cells in Offspring, Is Associated With the Elevation of Blood Pressure. Environ Toxicol (2021) 36:1152–61. doi: 10.1002/tox.23114
107. Hsu CN, Tain YL. Regulation of Nitric Oxide Production in the Developmental Programming of Hypertension and Kidney Disease. Int J Mol Sci (2019) 20:681. doi: 10.3390/ijms20030681
108. Hsu CN, Tain YL. Developmental Origins of Kidney Disease: Why Oxidative Stress Matters? Antioxidants (Basel) (2020) 10:33. doi: 10.3390/antiox10010033
109. Hsu CN, Tain YL. Early-Life Programming and Reprogramming of Adult Kidney Disease and Hypertension: The Interplay Between Maternal Nutrition and Oxidative Stress. Int J Mol Sci (2020) 21:3572. doi: 10.3390/ijms21103572
110. Hsu CN, Tain YL. Targeting the Renin-Angiotensin-Aldosterone System to Prevent Hypertension and Kidney Disease of Developmental Origins. Int J Mol Sci (2021) 22(5):2298. doi: 10.3390/ijms22052298
111. Dennery PA. Oxidative Stress in Development: Nature or Nurture? Free Radic Biol Med (2010) 49:1147–51. doi: 10.1016/j.freeradbiomed.2010.07.011
112. Thompson LP, Al-Hasan Y. Impact of Oxidative Stress in Fetal Programming. J Pregnancy (2012) 2012:582748. doi: 10.1155/2012/582748
113. Hsu CN, Tain YL. Early Origins of Hypertension: Should Prevention Start Before Birth Using Natural Antioxidants? Antioxidants (Basel) (2020) 9:1034. doi: 10.3390/antiox9111034
114. Zheng F, Gonçalves FM, Abiko Y, Li H, Kumagai Y, Aschner M. Redox Toxicology of Environmental Chemicals Causing Oxidative Stress. Redox Biol (2020) 34:101475. doi: 10.1016/j.redox.2020.101475
115. Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. Human Nephron Number: Implications for Health and Disease. Pediatr Nephrol (2011) 26:1529–33. doi: 10.1007/s00467-011-1843-8
116. Te Riet L, van Esch JH, Roks AJ, van den Meiracker AH, Danser AH. Hypertension: Renin-Angiotensin-Aldosterone System Alterations. Circ Res (2015) 116:960–75. doi: 10.1161/CIRCRESAHA.116.303587
117. Gubler MC, Antignac C. Renin-Angiotensin System in Kidney Development: Renal Tubular Dysgenesis. Kidney Int (2010) 77:400–6. doi: 10.1038/ki.2009.423
118. Cragan JD, Young BA, Correa A. Renin-Angiotensin System Blocker Fetopathy. J Pediatr (2015) 167:792–4. doi: 10.1016/j.jpeds.2015.07.024
119. Mo Y, Lu Z, Wang L, Ji C, Zou C, Liu X. The Aryl Hydrocarbon Receptor in Chronic Kidney Disease: Friend or Foe? Front Cell Dev Biol (2020) 8:589752. doi: 10.3389/fcell.2020.589752
120. Sallée M, Dou L, Cerini C, Poitevin S, Brunet P, Burtey S. The Aryl Hydrocarbon Receptor-Activating Effect of Uremic Toxins From Tryptophan Metabolism: A New Concept to Understand Cardiovascular Complications of Chronic Kidney Disease. Toxins (Basel) (2014) 6(3):934–49. doi: 10.3390/toxins6030934
121. Baricza E, Tamási V, Marton N, Buzás EI, Nagy G. The Emerging Role of Aryl Hydrocarbon Receptor in the Activation and Differentiation of Th17 Cells. Cell Mol Life Sci (2016) 73:95–117. doi: 10.1007/s00018-015-2056-2
122. Zhang N. The Role of Endogenous Aryl Hydrocarbon Receptor Signaling in Cardiovascular Physiology. J Cardiovasc Dis Res (2011) 2:91–5. doi: 10.4103/0975-3583.83033
123. Hsu CN, Hou CY, Hsu WH, Tain YL. Cardiovascular Diseases of Developmental Origins: Preventive Aspects of Gut Microbiota-Targeted Therapy. Nutrients (2021) 13:2290. doi: 10.3390/nu13072290
124. Tain YL, Hsu CN. Interplay Between Oxidative Stress and Nutrient Sensing Signaling in the Developmental Origins of Cardiovascular Disease. Int J Mol Sci (2017) 18:841. doi: 10.3390/ijms18040841
125. Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental Origins of Health and Disease: Brief History of the Approach and Current Focus on Epigenetic Mechanisms. Semin Reprod Med (2009) 27:358–68. doi: 10.1055/s-0029-1237424
126. Rosenfeld CS. Gut Dysbiosis in Animals Due to Environmental Chemical Exposures. Front Cell Infect Microbiol (2017) 7:396. doi: 10.3389/fcimb.2017.00396
127. Belkin S. Microbial Whole-Cell Sensing Systems of Environmental Pollutants. Curr Opin Microbiol (2003) 6:206–12. doi: 10.1016/s1369-5274(03)00059-6
128. Chappell G, Pogribny IP, Guyton KZ, Rusyn I. Epigenetic Alterations Induced by Genotoxic Occupational and Environmental Human Chemical Carcinogens: A Systematic Literature Review. Mutat Res Rev Mutat Res (2016) 768:27–45. doi: 10.1016/j.mrrev.2016.03.004
129. Tain YL, Joles JA. Reprogramming: A Preventive Strategy in Hypertension Focusing on the Kidney. Int J Mol Sci (2015) 17:23. doi: 10.3390/ijms17010023
130. Hsu CN, Tain YL. The Good, the Bad, and the Ugly of Pregnancy Nutrients and Developmental Programming of Adult Disease. Nutrients (2019) 11:894. doi: 10.3390/nu11040894
131. Revel A, Raanani H, Younglai E, Xu J, Rogers I, Han R, et al. Resveratrol, a Natural Aryl Hydrocarbon Receptor Antagonist, Protects Lung From DNA Damage and Apoptosis Caused by Benzo[a]Pyrene. J Appl Toxicol (2003) 23:255–61. doi: 10.1002/jat.916
132. Hsu CN, Hou CY, Tain YL. Preventive Aspects of Early Resveratrol Supplementation in Cardiovascular and Kidney Disease of Developmental Origins. Int J Mol Sci (2021) 22:4210. doi: 10.3390/ijms22084210
133. European Commission. Green Deal: Commission Adopts New Chemicals Strategy Towards a Toxic-Free Environment (2020). Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_20_1839 (Accessed July 22, 2021).
134. United Nations. Report of the Secretary-General on SDG Progress 2019. Special Edition. New York: United Nations (2019).
135. Levin A, Tonelli M, Bonventre J, Coresh J, Donner JA, Fogo AB, et al. ISN Global Kidney Health Summit Participants. Global Kidney Health 2017 and Beyond: A Roadmap for Closing Gaps in Care, Research, and Policy. Lancet (2017) 390:1888–917. doi: 10.1016/S0140-6736(17)30788-2
136. Htay H, Alrukhaimi M, Ashuntantang GE, Bello AK, Bellorin-Font E, Benghanem Gharbi M, et al. Global Access of Patients With Kidney Disease to Health Technologies and Medications: Findings From the Global Kidney Health Atlas Project. Kidney Int Suppl (2018) 8:64–73. doi: 10.1016/j.kisu.2017.10.010
Keywords: chronic kidney disease, hypertension, DOHaD (developmental origins of health and disease), environmental chemical, oxidative stress, endocrine disruption chemical, renin-angiotensin system
Citation: Hsu C-N and Tain Y-L (2021) Adverse Impact of Environmental Chemicals on Developmental Origins of Kidney Disease and Hypertension. Front. Endocrinol. 12:745716. doi: 10.3389/fendo.2021.745716
Received: 22 July 2021; Accepted: 27 September 2021;
Published: 14 October 2021.
Edited by:
Kunal Sharan, Central Food Technological Research Institute (CSIR), IndiaReviewed by:
Wojciech Hanke, Nofer Institute of Occupational Medicine, PolandHunasanahally Puttaswamygowda Gurushankara, Central University of Kerala, India
Copyright © 2021 Hsu and Tain. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: You-Lin Tain, tainyl@cgmh.org.tw
 Chien-Ning Hsu
Chien-Ning Hsu You-Lin Tain
You-Lin Tain