- 1Gynecological Endocrinology Unit, Division of Endocrinology, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil
- 2Post-graduate Program in Endocrinology, Medicine School, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil
- 3Department of Physiology, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil
Background: Polycystic ovary syndrome (PCOS) is an endocrine disorder that commonly affects women of childbearing age and has been associated with metabolic and reproductive abnormalities. Only a few studies have investigated metabolic traits in women with PCOS in Latin America. Therefore, we conducted a systematic review to provide an overview of the available evidence on the metabolic profile of Latin American women with PCOS.
Methods: We searched PubMed, Cochrane Central Register of Controlled Trials, and Embase databases for cross-sectional, case-control, or cohort studies focusing on populations of countries in South and Central America and Mexico, published until October 31, 2019. We selected studies that reported the diagnostic criteria for PCOS. In the absence of a control group, we included studies if they reported relevant metabolic data.
Results: The initial search yielded 4878 records, of which 41 studies were included in the systematic review. Sample sizes ranged from 10 to 288 in PCOS groups and from 10 to 1500 in control groups. The prevalence of phenotypes A and B (classic PCOS) ranged from 65.8% to 87.5% as reported in studies from Argentina, Brazil, and Chile. Metabolic syndrome ranged from 33.3% to 44.0% for phenotype A, from 15.0% to 58.0% for phenotype B, from 11.9% to 36.0% for phenotype C, and from 14.2% to 66.0% for phenotype D. Women with PCOS had higher body mass index, waist circumference, blood pressure, glucose, and homeostasis model assessment index as well as a more adverse lipid profile than those without PCOS.
Conclusions: Evidence from the present systematic review suggests that anthropometric and metabolic profiles are worse in women with PCOS who live in different Latin American countries than in women without PCOS living in the same region. Additional studies assessing metabolic comorbidities, such as diabetes, and distinct PCOS phenotypes in different Latin American countries are warranted and may produce invaluable information for primary and secondary prevention of PCOS in the region. This systematic review was registered with PROSPERO under number CRD42016038537.
Systematic Review Registration: PROSPERO, identifier CRD42016038537.
Introduction
Polycystic ovary syndrome (PCOS) is an endocrine condition that commonly affects women of childbearing age. The etiology of PCOS is uncertain, but the available evidence strongly suggests that its onset is triggered by environmental, genetic, and behavioral factors that interact in a complex manner (1–3).
Obesity affects the majority of women with PCOS, placing them at increased risk for impaired glucose tolerance, metabolic abnormalities, and type 2 diabetes (4–7), and possibly for cardiovascular and cerebrovascular events and venous thromboembolism (2, 8–11). Insulin resistance with compensatory hyperinsulinemia affects approximately 65% to 70% of women with PCOS (12). An estimated 30%–40% of patients with PCOS have impaired glucose tolerance, and 7.5%–10% have type 2 diabetes (13–15). While the prevalence of insulin resistance is high in both lean and obese women with PCOS (16), the presence of obesity may exacerbate the development of metabolic comorbidities and cardiovascular risk factors (17–19).
Many studies have investigated the prevalence of PCOS and related metabolic abnormalities in different continents. A recent meta-analysis showed a lower prevalence of PCOS in Chinese women than in white (Caucasian), Middle Eastern (Iranian and Turkish), and black (African American and Afro-Brazilian) women (20). However, the prevalence of PCOS and metabolic profile has not yet been described in several ethnic groups, especially in Latin American populations (6, 17, 21, 22), except for a recent meta-analysis of metabolic disturbances in Brazilian women with PCOS (23). Therefore, we conducted the present systematic review to provide an overview of the available evidence on the metabolic profile of Latin American women with PCOS, as well as the frequency of different PCOS phenotypes in this population.
Methods
Search Strategy and Study Selection
A systematic review was designed and described in agreement with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. This systematic review was registered with PROSPERO under number CRD42016038537. We searched PubMed, Cochrane Central Register of Controlled Trials, and Embase databases for cohort, case-control, cross-sectional, and prevalence studies with populations of South and Central America and Mexico, published until October 31, 2019. We set no language or publication date restrictions. To identify eligible studies, we used medical subject headings (MeSH) for PubMed and Ovid Tree terms for Embase. We used the following search strategy for PubMed, with equivalent terms being used in the other databases: “Polycystic Ovary Syndrome” [MeSH] OR “Ovary Syndrome, Polycystic” OR “Syndrome, Polycystic Ovary” OR “PCOS” OR “Polycystic Ovarian Syndrome” OR “Ovarian Syndrome, Polycystic” OR “Polycystic Ovary Syndrome 1” AND “Body Mass Index” [MeSH] OR “Metabolic Syndrome” OR “Glucose Intolerance” [MeSH] OR “Intolerance, Glucose” OR “Intolerances, Glucose” OR “Diabetes Mellitus, Type 2” [MeSH]. We performed additional searches in review articles and research articles focusing on PCOS.
We selected only studies that clearly defined the diagnostic criteria for PCOS and that included at least one of the following variables in the analysis: waist circumference (WC), body mass index (BMI), glucose levels, lipid profile, homeostasis model assessment of insulin resistance (HOMA-IR), blood pressure, diabetes mellitus, metabolic syndrome (MetS), PCOS prevalence, and milder PCOS phenotypes. Eligibility assessment was done by screening the titles and abstracts of all articles selected, and when abstracts did not provide the necessary information, the full text of the article was reviewed. This was performed independently, in a standardized manner, by two investigators (RBR and LBM). Disagreements between reviewers were resolved with discussion. If a consensus was not reached, a third investigator (PMS) was consulted. When articles had missing information, we contacted the authors for further information. In the case of duplicate data that had been published more than once, we opted to include the most complete study. In addition, the reference lists of all articles fulfilling the eligibility criteria were hand searched to identify other essential citations.
Data Extraction and Quality Control Assessment
Data were individually extracted by two researchers (LBM and RBR), and agreement was pursued in all extracted items. When an agreement could not be achieved, data extraction discrepancies were resolved by referring to the original publication or by consulting a third reviewer (PMS). Data extracted from each study included: name of the authors, country, publication year, type of study, characteristics of the population, diagnostic criteria, total sample size, and outcomes of interest in the PCOS and control groups. We assessed the quality of observational studies included in this systematic review using the Newcastle-Ottawa Scale (NOS). The NOS uses a “star system” to judge the quality of the studies in three broad perspectives: selection of the study groups, comparability of the groups, and ascertainment of the outcome of interest. Each item contains a sequence of alternative questions to be answered by the investigators. Then, a star rating system allows the semi-quantitative analysis of article quality. No statistical quantitative meta-analysis was performed due to study heterogeneity.
Results
Flowchart of Study Selection
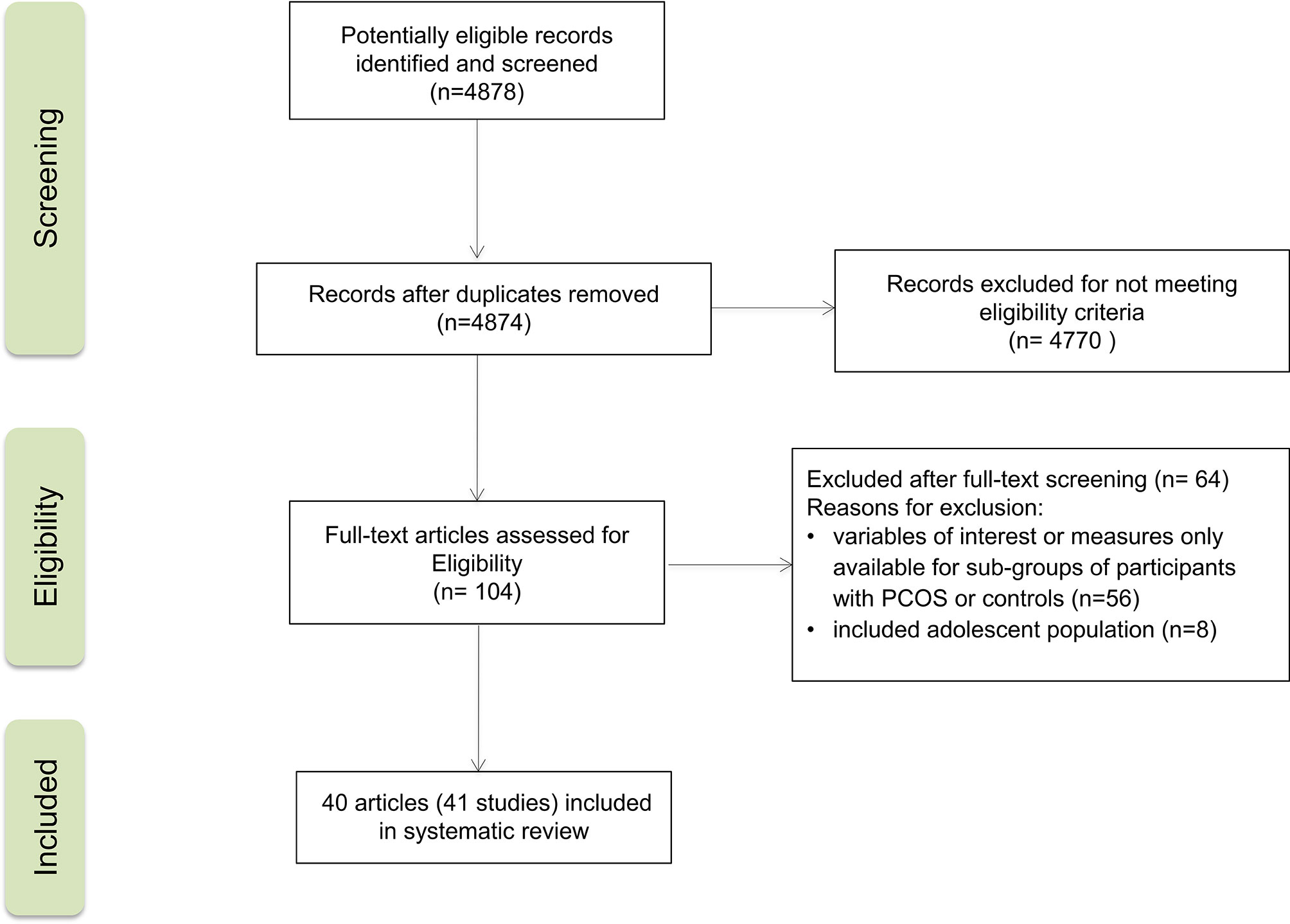
Figure 1 provides a flowchart summarizing the study selection process. The initial search yielded 4878 records. Of these, 41 studies from 40 reports were included in the systematic review. All of them were observational studies: 24 cross-sectional studies, 16 case-control studies, and one cohort study. Publication years ranged from 2004 to 2019. PCOS group size ranged from 10 to 288 participants, and control group size ranged from 10 to 1500 participants. Age ranged from 20.6 to 31.1 years for women with PCOS and from 22.7 to 34.5 years for non-PCOS controls.
Characteristics of Included Studies
Table 1 presents the characteristics of studies, which included populations from Argentina (n=3) (24–26), Brazil (n=27) (27–53), Chile (n=8) (26, 54–60), Venezuela (n=2) (62, 63), and Mexico (n=1) (61). Most studies used the Rotterdam criteria to diagnose PCOS, except for one study conducted in Argentina (25), one in Brazil (27), and three in Chile (54, 58, 60), all of which used the National Institutes of Health (NIH) criteria. The two studies from Venezuela (62, 63) used criteria defined by the authors. Sixteen studies had no control group for comparison (24, 26, 27, 35, 38, 39, 43–45, 47, 51, 53, 57, 59, 63), and six had BMI-matched controls without PCOS for comparison (28, 29, 34, 40, 50, 55). NOS score was 7-9 in 33 studies and ≤ 6 in 7 (Table 2).
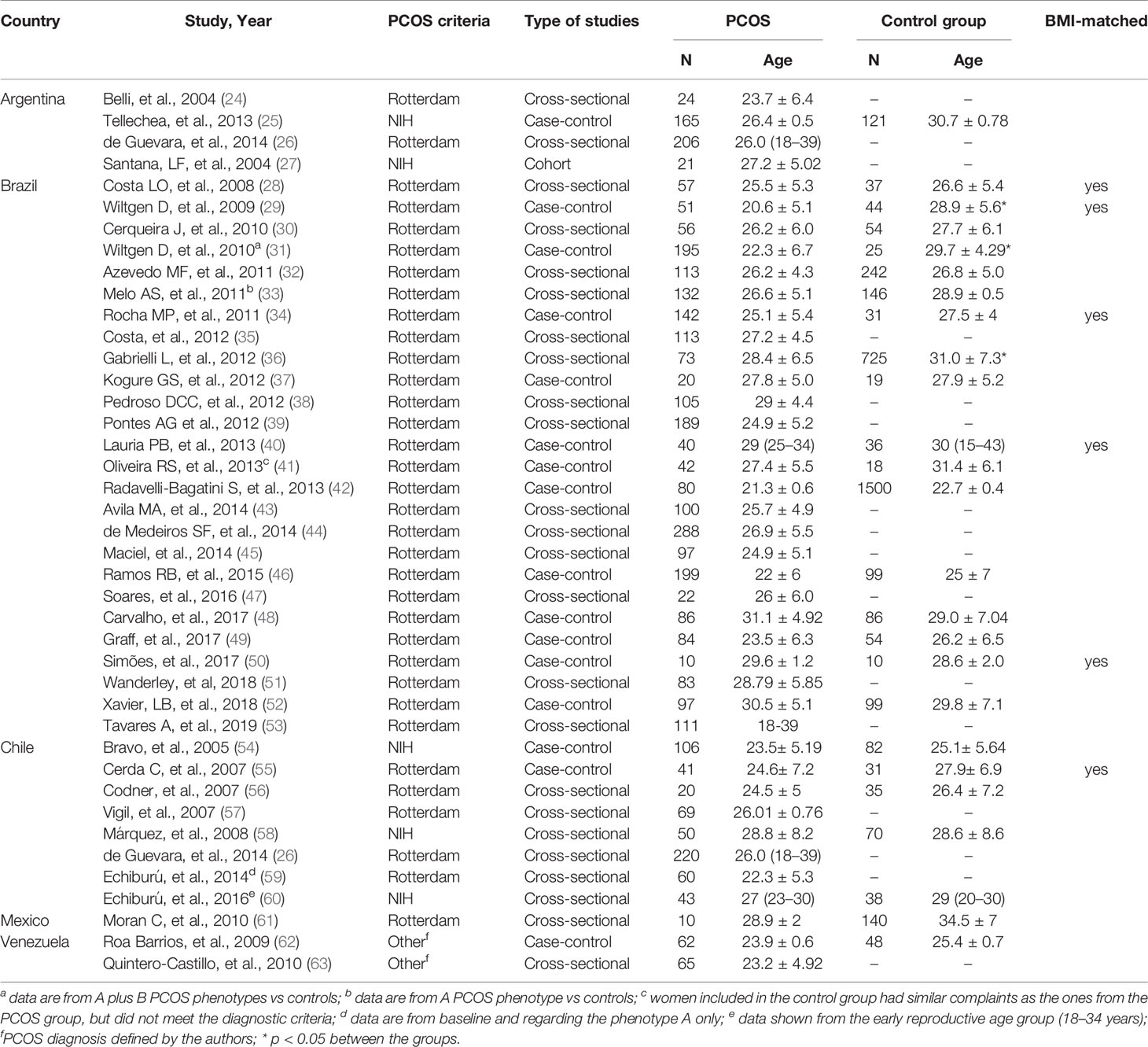
Table 1 Characteristics of the studies from Latin America included in the systematic review about women with PCOS.
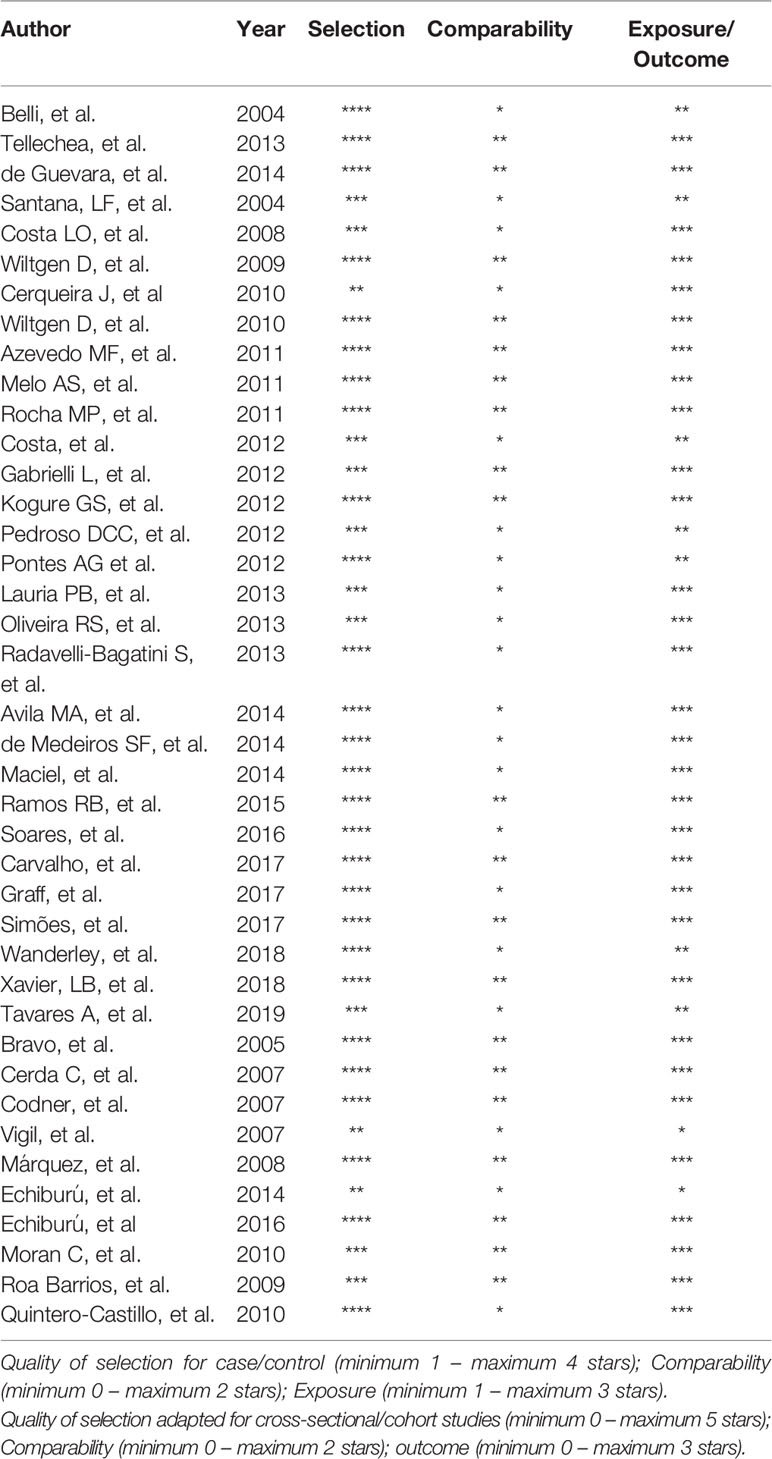
Table 2 Newcastle-Ottawa quality (NOS) assessment scale for studies included in the systematic review.
Qualitative Data
Overweight (BMI 25-29.9 kg/m²) or obesity (BMI ≥ 30 kg/m²) was prevalent among Latin American women with PCOS (Figure 2). BMI ranged from 24.2 to 33.3 kg/m² in women with PCOS. Most studies comparing women with PCOS versus BMI-unmatched controls showed higher BMI in PCOS groups (25, 30–33, 42, 46, 48, 49, 52, 54, 56, 58, 60). Several studies also assessed HOMA-IR, a marker of insulin resistance, in women with PCOS (Figure 3). HOMA-IR was > 2.5 in women with PCOS in 16 studies, six of them with obese participants (24, 31, 33, 41, 48, 58) and the others with overweight women (25, 28–30, 34, 45, 49, 52, 59, 62). In six studies HOMA-IR was ≤ 2.5 (37, 40, 44, 47, 60, 61), all of them with overweight participants. Seventeen studies compared HOMA-IR between women with PCOS and non-PCOS controls. HOMA-IR was higher in women with PCOS than in controls in 13 studies, 10 BMI-unmatched (25, 30, 31, 33, 37, 48, 49, 52, 58, 62) and 3 BMI-matched (28, 29, 34). While HOMA-IR was > 2.5 in most studies from Argentina, Brazil, Chile, and Venezuela, it was < 2.5 in the only included study from Mexico (61) (Figure 3).
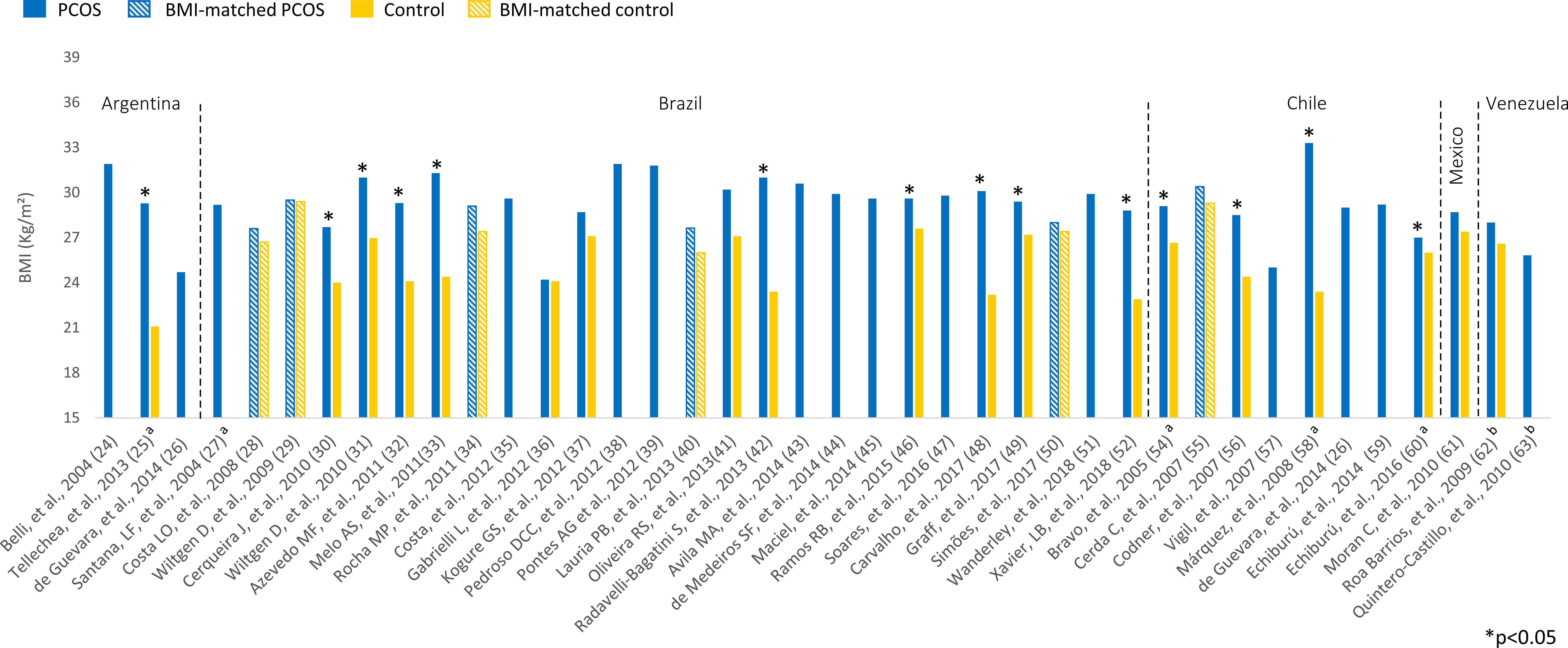
Figure 2 BMI (kg/m²) among Latin American women with PCOS and controls. Mean values. The “x” axis shows the name of studies and reference numbers (refer to the text). a PCOS diagnosis according to NIH criteria; b PCOS diagnosis defined by the authors.
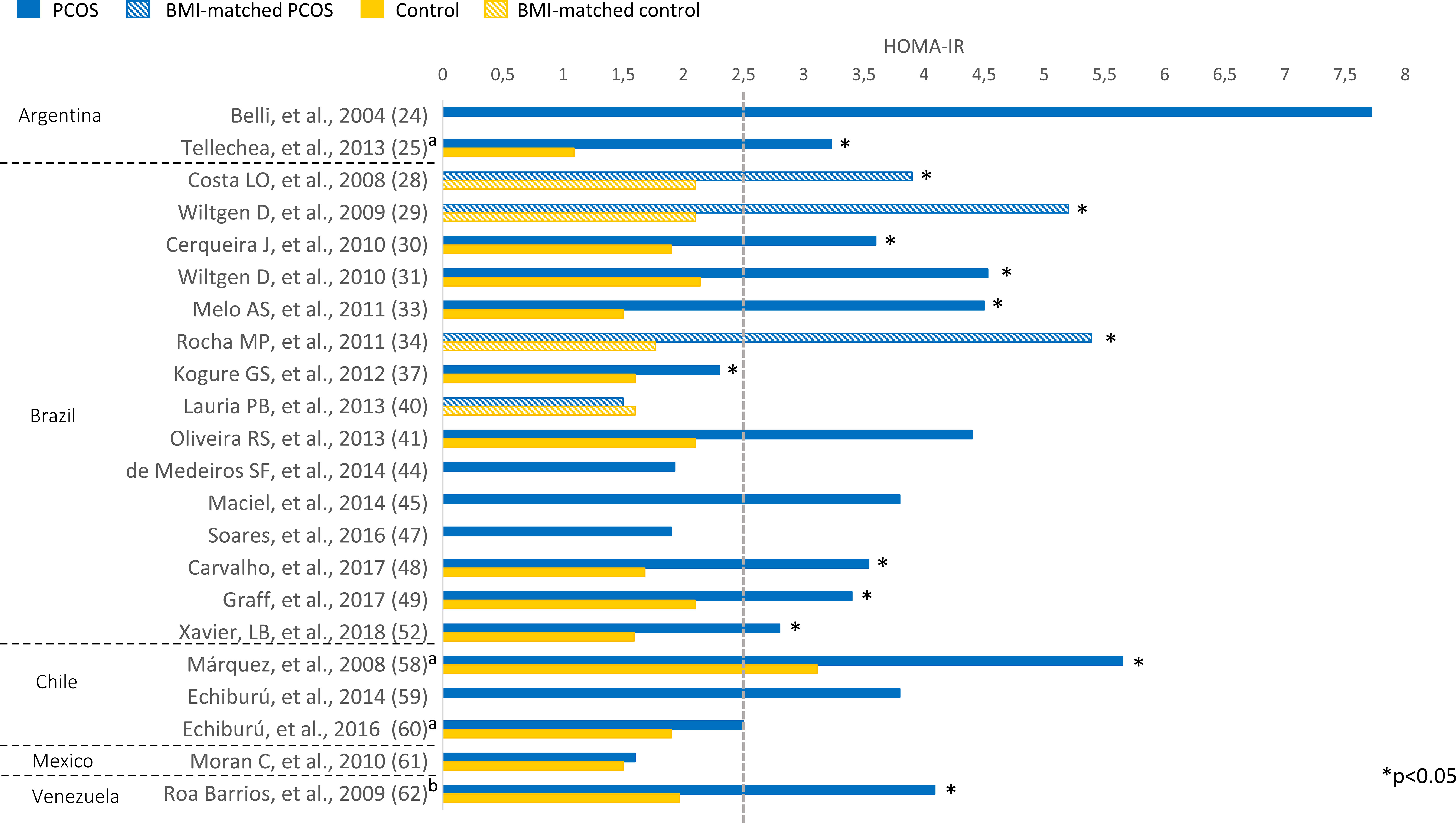
Figure 3 HOMA-IR among Latin American women with PCOS and controls. Mean values. The “x” axis shows the name of studies and reference numbers (refer to the text). a PCOS diagnosis according to NIH criteria; b PCOS diagnosis defined by the authors.
Figure 4 summarizes the variation of MetS components among studies of women with PCOS in Latin American countries. Central obesity (WC ≥ 88 cm) was prevalent among women with PCOS, who had higher WC values than controls in 13 of the 20 studies that reported this information (Supplementary Table 1).
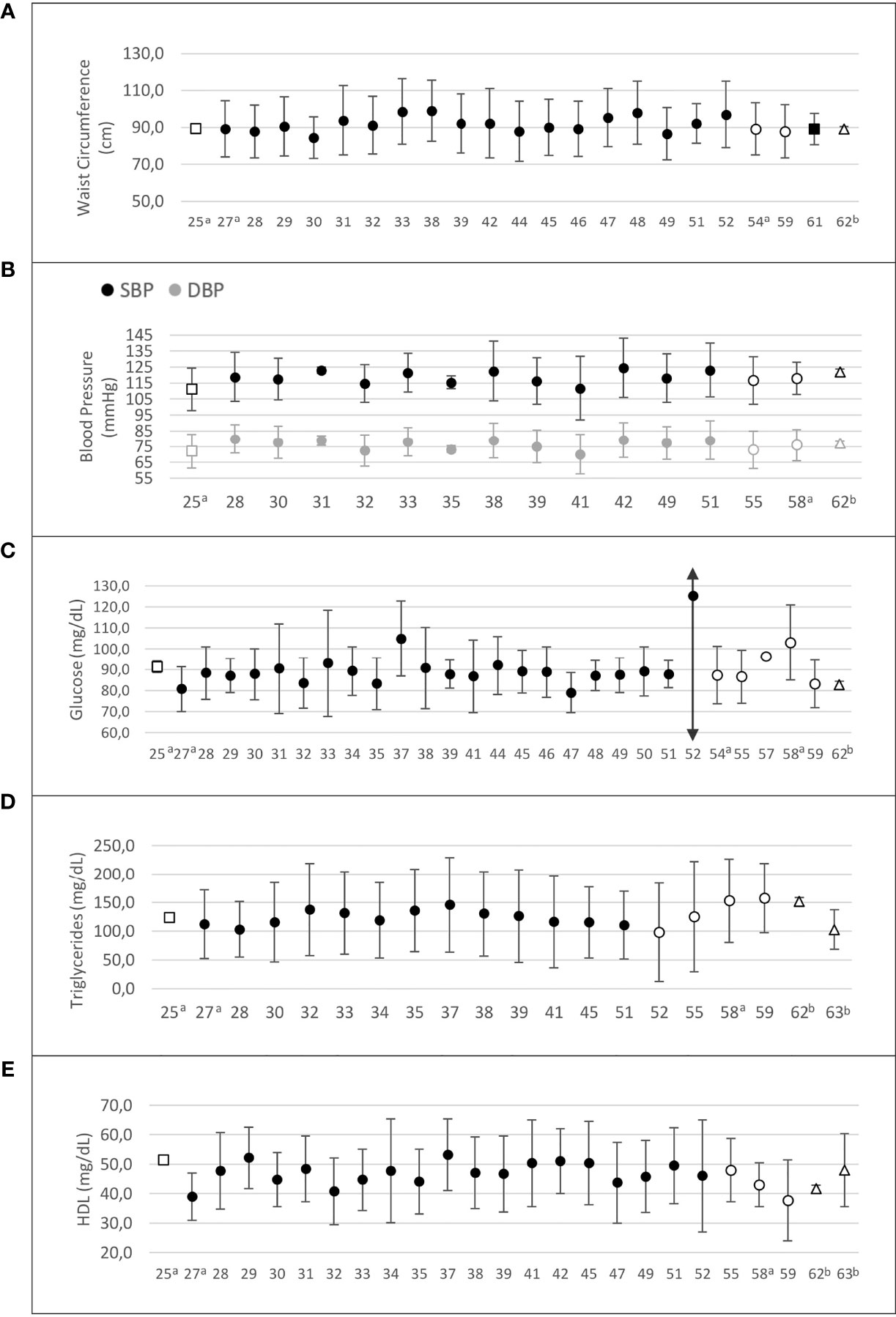
Figure 4 Risk factors composing the metabolic syndrome in Latin American women with PCOS. (A) Waist circumference (cm); (B) systolic and diastolic blood pressure (mm Hg); (C) fasting glucose (mg/dL); (D) triglycerides (mg/dL); (E) HDL-cholesterol (mg/dL). Values are expressed as mean and standard deviation. The “x” axis shows the reference number of studies (refer to the text). □ Argentina; •Brazil; ○ Chile; ∆ Venezuela. a PCOS diagnosis according to NIH criteria; b PCOS diagnosis defined by the authors.
Fifteen studies reported blood pressure data for PCOS and control groups (25, 28, 30–33, 40–42, 46, 49, 55, 58, 60, 62) (Figure 4). In nine of these studies, women with PCOS had higher systolic (SBP) and/or diastolic blood pressure (DBP) than controls (28, 30–33, 42, 46, 49, 58). One study evaluated blood pressure as a MetS component and found a higher prevalence of this criterion in the PCOS group, considering a 130/85 mm Hg cutoff point (35.1% vs. 7.1%, p=0.005, PCOS vs. controls) (46). Another study found higher SBP and DBP only in late reproductive-age (35–40 years) women with PCOS (60). Blood pressure levels were homogeneously distributed across countries. However, in all four studies from Chile, where these data were available, the mean SBP and DBP would be classified as “normal” according to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) definition of high blood pressure (64) (Supplementary Table 1).
Fasting glucose was measured in 31 studies (25, 27–35, 37–41, 44–52, 54, 55, 57–60, 62). Glucose levels ranged from 79 to 125.2 mg/dL in women with PCOS. In six of 21 studies (25, 30, 32, 33, 54, 58), women with PCOS had higher glucose levels than controls (Supplementary Table 2). Mean fasting glucose was homogeneously distributed across countries, and in most of them mean glucose levels were within the reference range. However, in two studies from Brazil (37, 52) and in one from Chile (58), mean fasting glucose was within the prediabetes range in patients with PCOS (Figure 4).
Regarding lipid profile, 26 studies showed triglyceride levels ranging from 81 to 157.8 mg/dL (Supplementary Table 2). Triglyceride levels were higher in women with PCOS than in controls in 11 of 17 studies (25, 29–33, 37, 52, 58, 60, 62). One BMI-matched study (29) also found higher triglyceride levels in the PCOS group. Whereas Brazilian and Argentinian studies showed mean triglyceride levels within the reference range, two studies from Chile (58, 59) and one from Venezuela (62) reported mean triglyceride levels > 150 mg/dL in patients with PCOS (Figure 4).
Twenty-seven studies assessed high-density lipoprotein cholesterol (HDL-C), and 18 of them compared HDL-C levels between PCOS and control groups (25, 28–34, 37, 40–42, 46, 49, 55, 58, 60, 62). In 10 of these studies, HDL-C was significantly lower in women with PCOS than in controls (25, 28, 30, 32–34, 42, 46, 49, 58). In the remaining studies, HDL-C levels did not differ between PCOS and control groups (Supplementary Table 2). In most studies, patients with PCOS had HDL-C < 50 mg/dL (27, 28, 30–35, 38–40, 47, 49, 51, 52, 55, 58–60, 62, 63). One study of women with PCOS conducted in Argentina reported HDL-C > 50 mg/dL (25), and studies of Brazilian women with PCOS showed variable HDL-C results, but mostly below the cutoff point of 50 mg/dL (27, 28, 30–35, 38–40, 47, 49, 51, 52). Studies from Chile and Venezuela reported mean HDL-C levels below this cutoff point (55, 58–60, 62, 63) (Figure 4).
Low-density lipoprotein cholesterol (LDL-C) levels ranged from 88.6 to 127.3 mg/dL in women with PCOS in 24 studies. Six of 15 studies comparing data between women with PCOS and controls reported higher LDL-C levels for PCOS (28, 29, 40, 42, 52, 58). LDL-C was within the reference range in control groups (Supplementary Table 2).
In 25 studies, mean total cholesterol levels ranged from 167 to 209.7 mg/dL in PCOS groups. Eight of 17 studies showed higher total cholesterol levels for women with PCOS than controls (25, 29, 30, 40, 42, 52, 58, 62) (Supplementary Table 2).
The prevalence of PCOS was estimated in only two studies. One study was conducted in Mexico (61) with a convenience sample of 150 female Mexican volunteers aged 20 to 45 years, and the authors found a prevalence of 6.6% (95% confidence interval, 2.3%–10.9%) according to the Rotterdam criteria. The other study was conducted in the city of Salvador, Brazil (36), and estimated a prevalence of 8.5% using the Rotterdam criteria in a probability sample of 859 women aged 18 to 45 years.
Six studies reported prevalence data on PCOS phenotypes and on MetS stratified by phenotype (26, 31, 33, 53, 59) for Brazilian, Chilean, and Argentinian populations. Phenotypes A+B were more prevalent in all studies, with rates ranging from 65.8% to 87.5%. The prevalence of MetS ranged from 33.3% to 44.0% for phenotype A, from 15.0% to 58.0% for phenotype B, from 11.9% to 36.0% for phenotype C, and from 14.2% to 66.0% for phenotype D (Table 3).
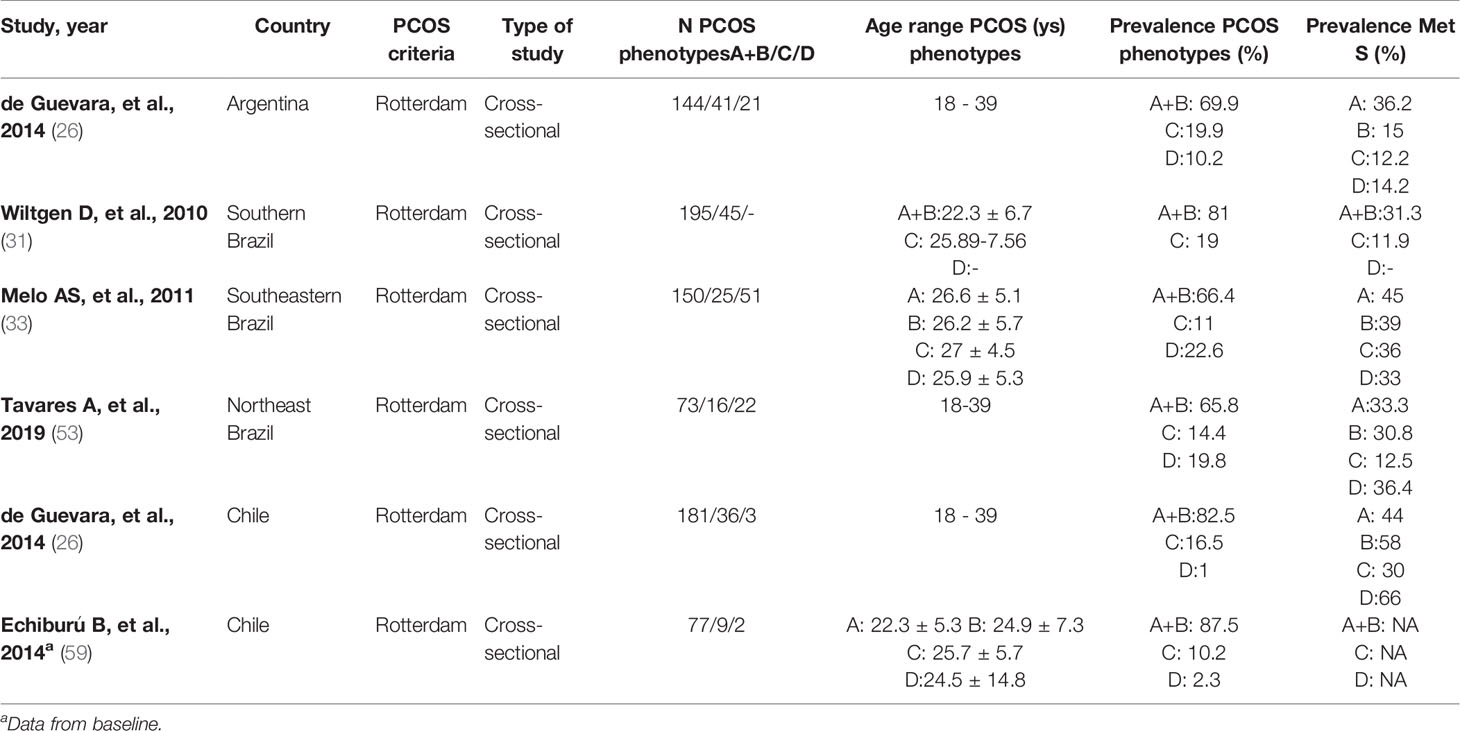
Table 3 Prevalence of PCOS phenotypes and of Metabolic syndrome in the studies included in the systematic review.
Discussion
PCOS is a complex disorder affecting metabolic and reproductive functions. This systematic review, which included 24 cross-sectional studies, 16 case-control studies, and one cohort study conducted in Latin America, found that women with PCOS had a more adverse metabolic profile than non-PCOS controls across different countries. In most studies, BMI was within the overweight or obesity range for women with PCOS, reinforcing its contribution to the disease phenotype. In addition, MetS components, such as central obesity (measured by WC), low HDL-C, and hypertension, were prevalent in women with PCOS from different Latin American countries.
Although efforts have long been made to assess the impact of different sociocultural and ethnic backgrounds on PCOS-related metabolic abnormalities, few data are available for Latin America. This region is known to have populations of different ancestry. In Brazil, pooled ancestry contributions have been listed as 0.62 European, 0.21 African, and 0.17 Amerindian (65), whereas Pacific Latin American countries are predominantly Amerindian. Argentina and Chile are particular cases that show similar European and Amerindian ancestry contributions but lower African ancestry contribution compared with Brazil (65, 66). It is reasonable to assume that different genetic backgrounds may influence the phenotypic heterogeneity of PCOS, but evidence from the present systematic review rather suggests that Latin American countries are similar in terms of metabolic traits. This information may be potentially useful to public health systems in developing PCOS prevention programs and policies.
Metabolic abnormalities are considered common in women with PCOS, especially those linked to the MetS cluster, as shown in this study. However, controversy exists as to whether these features are directly related to PCOS itself or dependent on obesity—mainly on abdominal adiposity, a well-known cardiometabolic risk factor (7, 67, 68). In this respect, the finding of decreased insulin sensitivity in Latin American women with PCOS, as opposed to controls, is in line with current evidence from other regions (6, 15) and has been associated with low-grade chronic inflammation, linked to increasing BMI (68, 69). Besides, in meta-analyses of different populations, women with PCOS were more likely to have MetS (4, 17, 70). However, these studies provide relatively few data from Latin American populations. Insulin resistance may actually drive most of the alterations observed in PCOS, even in nonobese women. While not universally present in patients with PCOS, the presence of insulin resistance has been considered an intrinsic factor independent of obesity (71, 72). Recently, we have also observed an association of insulin resistance with hypertension, regardless of BMI, in Brazilian women with PCOS, with hypertension being associated with other MetS components (18). Data from the present systematic review add support to this notion by showing that Latin American women with PCOS had higher HOMA-IR than controls in most studies.
Although patients with PCOS consistently show a more unfavorable metabolic profile than controls in different regions of the world, there are discrepancies between PCOS populations. In China, the prevalence of MetS in PCOS ranged from 18.2% in community-dwelling patients in one study (73) to 53.3% in women older than 40 years in another study (74). In a prospective cohort of 479 women with PCOS from Vietnam (Southeast Asia), patients were lean, had no increase in metabolic disease and Rotterdam phenotype D was the most prevalent (67.6%) (75). Current evidence also indicates a lower prevalence of hyperandrogenemia in women with PCOS from Asian countries (76). In Latin America, we found a predominance of Rotterdam phenotypes A and B, similar to what has been reported in most of the available studies across the world (76). A recent meta-analysis reported that, compared with controls, patients with PCOS from North America had a higher risk of MetS than those from Asia and Europe (17). Likewise, in the present systematic review, we also found a high prevalence of MetS in Latin American women with PCOS. In addition to the ethnic composition of the population, dietary habits may also influence the expression of metabolic traits in different populations. Indeed, adherence to the Mediterranean diet (77) or a low-glycemic-index diet (78) has been associated with a better metabolic profile in PCOS. Regarding the dietary pattern in Latin America, the Latin American Study of Nutrition and Health (ELANS) (79) reported low consumption of vegetables, nuts, whole grains, fish, and yogurt according to the recommendations of the World Health Organization. This may explain, at least in part, the similarities in the adverse metabolic profile between Latin American countries and other countries with high consumption of processed foods (80).
Despite the paucity of research undertaken to date, the results of the present systematic review provide a broad overview of the evidence on metabolic and anthropometric parameters in women with PCOS living in Latin American countries. The comprehensive search strategy can be seen as a strength of this study, as it covered the major electronic databases in order not to miss any relevant articles and included an active search for publications without language restrictions. Limitations include the relatively few studies found despite the vast size of the region, possible heterogeneity between studies, small sample sizes, and a lack of studies in some countries of the region, which hindered a proper comparison between women with PCOS from different Latin American countries. Nevertheless, no similar analysis has yet been undertaken. The present study is the first to provide evidence that allows us to characterize the metabolic profile of women with PCOS from an array of sociocultural and ethnic backgrounds in Latin American countries.
Conclusions
The results of the present systematic review suggest that anthropometric and metabolic profiles are worse in women with PCOS who live in different Latin American countries than in women without PCOS living in the same region. These findings are similar to those from North America but differ from the milder phenotype seen in Asia and Europe. Further studies assessing the prevalence of cardiometabolic comorbidities, such as diabetes and hypertension, in Latin American countries are needed, which could positively impact the prevention and management strategies for PCOS.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
LM contributed to study design, was involved with data collection and analysis, drafted the article and final review. RR contributed to study design, was involved with data collection and analysis, drafted the article and final review. PS was involved in the conception and design of the study, data collection and analysis, drafted the article and final review. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (grant number INCT/CNPq 465482/2014–7) and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (grant number INCT/FAPERGS: 17/2551–0000519-8). The funding source had no role in the collection, analysis, interpretation of data and in the writing of the report or in the decision to submit the article for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.759835/full#supplementary-material
References
1. Rosenfield RL, Ehrmann DA. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr Rev (2016) 37(5):467–520. doi: 10.1210/er.2015-1104
2. Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, et al. Polycystic Ovary Syndrome. Nat Rev Dis Primers (2016) 2:16057. doi: 10.1038/nrdp.2016.57
3. March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The Prevalence of Polycystic Ovary Syndrome in a Community Sample Assessed Under Contrasting Diagnostic Criteria. Hum Reprod (2010) 25(2):544–51. doi: 10.1093/humrep/dep399
4. Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired Glucose Tolerance, Type 2 Diabetes and Metabolic Syndrome in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Hum Reprod Update (2010) 16(4):347–63. doi: 10.1093/humupd/dmq001
5. Rubin KH, Glintborg D, Nybo M, Abrahamsen B, Andersen M. Development and Risk Factors of Type 2 Diabetes in a Nationwide Population of Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab (2017) 102(10):3848–57. doi: 10.1210/jc.2017-01354
6. Kakoly NS, Earnest A, Teede HJ, Moran LJ, Joham AE. The Impact of Obesity on the Incidence of Type 2 Diabetes Among Women With Polycystic Ovary Syndrome. Diabetes Care (2019) 42(4):560–7. doi: 10.2337/dc18-1738
7. Diamanti-Kandarakis E, Spritzer PM, Sir-Petermann T, Motta AB. Insulin Resistance and Polycystic Ovary Syndrome Through Life. Curr Pharm Des (2012) 18(34):5569–76. doi: 10.2174/138161212803307590
8. Osibogun O, Ogunmoroti O, Michos ED. Polycystic Ovary Syndrome and Cardiometabolic Risk: Opportunities for Cardiovascular Disease Prevention. Trends Cardiovasc Med (2019) 30(7):399–404. doi: 10.1016/j.tcm.2019.08.010
9. Zhao L, Zhu Z, Lou H, Zhu G, Huang W, Zhang S, et al. Polycystic Ovary Syndrome (PCOS) and the Risk of Coronary Heart Disease (CHD): A Meta-Analysis. Oncotarget (2016) 7(23):33715–21. doi: 10.18632/oncotarget.9553
10. de Groot PC, Dekkers OM, Romijn JA, Dieben SW, Helmerhorst FM. PCOS, Coronary Heart Disease, Stroke and the Influence of Obesity: A Systematic Review and Meta-Analysis. Hum Reprod Update (2011) 17(4):495–500. doi: 10.1093/humupd/dmr001
11. Cooney LG, Dokras A. Cardiometabolic Risk in Polycystic Ovary Syndrome: Current Guidelines. Endocrinol Metab Clin North Am (2021) 50(1):83–95. doi: 10.1016/j.ecl.2020.11.001
12. DeUgarte CM, Bartolucci AA, Azziz R. Prevalence of Insulin Resistance in the Polycystic Ovary Syndrome Using the Homeostasis Model Assessment. Fertil Steril (2005) 83(5):1454–60. doi: 10.1016/j.fertnstert.2004.11.070
13. Salley KE, Wickham EP, Cheang KI, Essah PA, Karjane NW, Nestler JE. Glucose Intolerance in Polycystic Ovary Syndrome–a Position Statement of the Androgen Excess Society. J Clin Endocrinol Metab (2007) 92(12):4546–56. doi: 10.1210/jc.2007-1549
14. Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of Impaired Glucose Tolerance and Diabetes in Women With Polycystic Ovary Syndrome. Diabetes Care (1999) 22(1):141–6. doi: 10.2337/diacare.22.1.141
15. Ollila MM, West S, Keinanen-Kiukaaniemi S, Jokelainen J, Auvinen J, Puukka K, et al. Overweight and Obese But Not Normal Weight Women With PCOS are at Increased Risk of Type 2 Diabetes Mellitus-a Prospective Population-Based Cohort Study. Hum Reprod (2017) 32(4):968. doi: 10.1093/humrep/dex030
16. Diamanti-Kandarakis E, Dunaif A. Insulin Resistance and the Polycystic Ovary Syndrome Revisited: An Update on Mechanisms and Implications. Endocr Rev (2012) 33(6):981–1030. doi: 10.1210/er.2011-1034
17. Lim SS, Kakoly NS, Tan JWJ, Fitzgerald G, Bahri Khomami M, Joham AE, et al. Metabolic Syndrome in Polycystic Ovary Syndrome: A Systematic Review, Meta-Analysis and Meta-Regression. Obes Rev (2019) 20(2):339–52. doi: 10.1111/obr.12762
18. Marchesan LB, Spritzer PM. ACC/AHA 2017 Definition of High Blood Pressure: Implications for Women With Polycystic Ovary Syndrome. Fertil Steril (2019) 111(3):579–87.e1. doi: 10.1016/j.fertnstert.2018.11.034
19. Wild RA, Rizzo M, Clifton S, Carmina E. Lipid Levels in Polycystic Ovary Syndrome: Systematic Review and Meta-Analysis. Fertil Steril (2011) 95(3):1073–9.e1-11. doi: 10.1016/j.fertnstert.2010.12.027
20. Ding T, Hardiman PJ, Petersen I, Wang FF, Qu F, Baio G. The Prevalence of Polycystic Ovary Syndrome in Reproductive-Aged Women of Different Ethnicity: A Systematic Review and Meta-Analysis. Oncotarget (2017) 8(56):96351–8. doi: 10.18632/oncotarget.19180
21. Diamanti-Kandarakis E, Papavassiliou AG, Kandarakis SA, Chrousos GP. Pathophysiology and Types of Dyslipidemia in PCOS. Trends Endocrinol Metab (2007) 18(7):280–5. doi: 10.1016/j.tem.2007.07.004
22. Carmina E, Nasrallah MP, Guastella E, Lobo RA. Characterization of Metabolic Changes in the Phenotypes of Women With Polycystic Ovary Syndrome in a Large Mediterranean Population From Sicily. Clin Endocrinol (Oxf) (2019) 91(4):553–60. doi: 10.1111/cen.14063
23. Spritzer PM, Ramos RB, Marchesan LB, de Oliveira M, Carmina E. Metabolic Profile of Women With PCOS in Brazil: A Systematic Review and Meta-Analysis. Diabetol Metab Syndr (2021) 13(1):18. doi: 10.1186/s13098-021-00636-5
24. Belli SH, Graffigna MN, Oneto A, Otero P, Schurman L, Levalle OA. Effect of Rosiglitazone on Insulin Resistance, Growth Factors, and Reproductive Disturbances in Women With Polycystic Ovary Syndrome. Fertil Steril (2004) 81(3):624–9. doi: 10.1016/j.fertnstert.2003.08.024
25. Tellechea ML, Muzzio DO, Iglesias Molli AE, Belli SH, Graffigna MN, Levalle OA, et al. Association Between Beta2-Adrenoceptor (ADRB2) Haplotypes and Insulin Resistance in PCOS. Clin Endocrinol (Oxf) (2013) 78(4):600–6. doi: 10.1111/cen.12019
26. Ladron de Guevara A, Fux-Otta C, Crisosto N, Szafryk de Mereshian P, Echiburu B, Iraci G, et al. Metabolic Profile of the Different Phenotypes of Polycystic Ovary Syndrome in Two Latin American Populations. Fertil Steril (2014) 101(6):1732–9.e1-2. doi: 10.1016/j.fertnstert.2014.02.020
27. Santana LF, de Sá MF, Ferriani RA, de Moura MD, Foss MC, dos Reis RM. Effect of Metformin on the Clinical and Metabolic Assessment of Women With Polycystic Ovary Syndrome. Gynecol Endocrinol (2004) 19(2):88–96. doi: 10.1080/09513590400002342
28. Costa LO, dos Santos MP, Oliveira M, Viana A. Low-Grade Chronic Inflammation is Not Accompanied by Structural Arterial Injury in Polycystic Ovary Syndrome. Diabetes Res Clin Pract (2008) 81(2):179–83. doi: 10.1016/j.diabres.2008.04.005
29. Wiltgen D, Benedetto IG, Mastella LS, Spritzer PM. Lipid Accumulation Product Index: A Reliable Marker of Cardiovascular Risk in Polycystic Ovary Syndrome. Hum Reprod (2009) 24(7):1726–31. doi: 10.1093/humrep/dep072
30. Cerqueira JM, Costa LO, Nogueira Ade A, Silva DC, Torres Dde O, Santos AC. [Homocysteinemia in Polycystic Ovary Syndrome Women]. Rev Bras Ginecol Obstet (2010) 32(3):126–32. doi: 10.1590/S0100-72032010000300005
31. Wiltgen D, Spritzer PM. Variation in Metabolic and Cardiovascular Risk in Women With Different Polycystic Ovary Syndrome Phenotypes. Fertil Steril (2010) 94(6):2493–6. doi: 10.1016/j.fertnstert.2010.02.015
32. Azevedo MF, Costa EC, Oliveira AI, Silva IB, Marinho JC, Rodrigues JA, et al. [Elevated Blood Pressure in Women With Polycystic Ovary Syndrome: Prevalence and Associated Risk Factors]. Rev Bras Ginecol Obstet (2011) 33(1):31–6. doi: 10.1590/S0100-72032011000100005
33. Melo AS, Vieira CS, Romano LG, Ferriani RA, Navarro PA. The Frequency of Metabolic Syndrome is Higher Among PCOS Brazilian Women With Menstrual Irregularity Plus Hyperandrogenism. Reprod Sci (2011) 18(12):1230–6. doi: 10.1177/1933719111414205
34. Rocha MP, Marcondes JA, Barcellos CR, Hayashida SA, Curi DD, da Fonseca AM, et al. Dyslipidemia in Women With Polycystic Ovary Syndrome: Incidence, Pattern and Predictors. Gynecol Endocrinol (2011) 27(10):814–9. doi: 10.3109/09513590.2010.508852
35. Costa EC, Sa JC, Soares EM, Lemos TM, Maranhao TM, Azevedo GD. Anthropometric Indices of Central Obesity How Discriminators of Metabolic Syndrome in Brazilian Women With Polycystic Ovary Syndrome. Gynecol Endocrinol (2012) 28(1):12–5. doi: 10.3109/09513590.2011.583956
36. Gabrielli L, Aquino EM. Polycystic Ovary Syndrome in Salvador, Brazil: A Prevalence Study in Primary Healthcare. Reprod Biol Endocrinol (2012) 10:96. doi: 10.1186/1477-7827-10-96
37. Kogure GS, Piccki FK, Vieira CS, Martins Wde P, dos Reis RM. Analysis of Muscle Strength and Body Composition of Women With Polycystic Ovary Syndrome. Rev Bras Ginecol Obstet (2012) 34(7):316–22. doi: 10.1590/S0100-72032012000700005
38. Pedroso DC, Melo AS, Carolo AL, Vieira CS, Rosa e Silva AC, dos Reis RM. [Frequency and Risk Factors for Metabolic Syndrome in Adolescents and Adults Women With Polycystic Ovary Syndrome]. Rev Bras Ginecol Obstet (2012) 34(8):357–61. doi: 10.1590/s0100-72032012000800003
39. Pontes AG, Rehme MF, Martins AM, Micussi MT, Maranhão TM, Pimenta WdeP, et al. Insulin Resistance in Women With Polycystic Ovary Syndrome: Relationship With Anthropometric and Biochemical Variables. Rev Bras Ginecol Obstet (2012) 34(2):74–9. doi: 10.1590/s0100-72032012000200006
40. Lauria PB, Del Puerto HL, Reis AM, Candido AL, Reis FM. Low Plasma Atrial Natriuretic Peptide: A New Piece in the Puzzle of Polycystic Ovary Syndrome. J Clin Endocrinol Metab (2013) 98(12):4882–9. doi: 10.1210/jc.2013-2141
41. de Oliveira R, Redorat RG, Ziehe GH, Mansur VA, Conceição FL. Arterial Hypertension and Metabolic Profile in Patients With Polycystic Ovary Syndrome. Rev Bras Ginecol e Obstetrícia (2013) 35:21–6. doi: 10.1590/S0100-72032013000100005
42. Radavelli-Bagatini S, de Oliveira IO, Ramos RB, Santos BR, Wagner MS, Lecke SB, et al. Haplotype TGTG From SNP 45t/G and 276G/T of the Adiponectin Gene Contributes to Risk of Polycystic Ovary Syndrome. J Endocrinol Invest (2013) 36(7):497–502. doi: 10.3275/8966
43. Avila MA, Bruno RV, Barbosa FC, Andrade FC, Silva AC, Nardi AE. Polycystic Ovary Syndrome: Implications of Metabolic Dysfunction. Rev Col Bras Cir (2014) 41(2):106–10. doi: 10.1590/s0100-69912014000200006
44. de Medeiros SF, Yamamoto MM, Bueno HB, Belizario D, Barbosa JS. Prevalence of Elevated Glycated Hemoglobin Concentrations in the Polycystic Ovary Syndrome: Anthropometrical and Metabolic Relationship in Amazonian Women. J Clin Med Res (2014) 6(4):278–86. doi: 10.14740/jocmr1829w
45. Maciel GA, Moreira RP, Bugano DD, Hayashida SA, Marcondes JA, Gomes LG, et al. Association of Glucocorticoid Receptor Polymorphisms With Clinical and Metabolic Profiles in Polycystic Ovary Syndrome. Clinics (Sao Paulo) (2014) 69(3):179–84. doi: 10.6061/clinics/2014(03)06
46. Ramos RB, Spritzer PM. FTO Gene Variants are Not Associated With Polycystic Ovary Syndrome in Women From Southern Brazil. Gene (2015) 560(1):25–9. doi: 10.1016/j.gene.2015.01.012
47. Soares NP, Santos AC, Costa EC, Azevedo GD, Damasceno DC, Fayh AP, et al. Diet-Induced Weight Loss Reduces DNA Damage and Cardiometabolic Risk Factors in Overweight/Obese Women With Polycystic Ovary Syndrome. Ann Nutr Metab (2016) 68(3):220–7. doi: 10.1159/000444130
48. Carvalho LML, Ferreira CN, de Oliveira DKD, Rodrigues KF, Duarte RCF, Teixeira MFA, et al. Haptoglobin Levels, But Not Hp1-Hp2 Polymorphism, are Associated With Polycystic Ovary Syndrome. J Assist Reprod Genet (2017) 34(12):1691–8. doi: 10.1007/s10815-017-1030-3
49. Graff SK, Mario FM, Magalhaes JA, Moraes RS, Spritzer PM. Saturated Fat Intake Is Related to Heart Rate Variability in Women With Polycystic Ovary Syndrome. Ann Nutr Metab (2017) 71(3-4):224–33. doi: 10.1159/000484325
50. Simoes RS, Soares JM Jr., Simoes MJ, Nader HB, Baracat MCP, Maciel GAR, et al. Small Leucine-Rich Proteoglycans (SLRPs) in the Endometrium of Polycystic Ovary Syndrome Women: A Pilot Study. J Ovarian Res (2017) 10(1):54. doi: 10.1186/s13048-017-0349-9
51. Wanderley MDS, Pereira LCR, Santos CB, Cunha VSD, Neves MVJ. Association Between Insulin Resistance and Cardiovascular Risk Factors in Polycystic Ovary Syndrome Patients. Rev Bras Ginecol Obstet (2018) 40(4):188–95. doi: 10.1055/s-0038-1642634
52. Xavier LB, Sóter MO, Sales MF, Oliveira DK, Reis HJ, Candido AL, et al. Evaluation of PCSK9 Levels and its Genetic Polymorphisms in Women With Polycystic Ovary Syndrome. Gene (2018) 644:129–36. doi: 10.1016/j.gene.2017.11.006
53. Tavares A, Rêgo Barros RC. The Prevalence of Metabolic Syndrome in the Different Phenotypes of Polycystic Ovarian Syndrome. Rev Bras Ginecol Obstet (2019) 41(1):37–43. doi: 10.1055/s-0038-1676568
54. Perez-Bravo F, Echiburu B, Maliqueo M, Santos JL, Sir-Petermann T. Tryptophan 64 –> Arginine Polymorphism of Beta-3-Adrenergic Receptor in Chilean Women With Polycystic Ovary Syndrome. Clin Endocrinol (Oxf) (2005) 62(2):126–31. doi: 10.1111/j.1365-2265.2004.02183.x
55. Cerda C, Pérez-Ayuso RM, Riquelme A, Soza A, Villaseca P, Sir-Petermann T, et al. Nonalcoholic Fatty Liver Disease in Women With Polycystic Ovary Syndrome. J Hepatol (2007) 47(3):412–7. doi: 10.1016/j.jhep.2007.04.012
56. Codner E, Iniguez G, Villarroel C, Lopez P, Soto N, Sir-Petermann T, et al. Hormonal Profile in Women With Polycystic Ovarian Syndrome With or Without Type 1 Diabetes Mellitus. J Clin Endocrinol Metab (2007) 92(12):4742–6. doi: 10.1210/jc.2007-1252
57. Vigil P, Contreras P, Alvarado JL, Godoy A, Salgado AM, Cortes ME. Evidence of Subpopulations With Different Levels of Insulin Resistance in Women With Polycystic Ovary Syndrome. Hum Reprod (2007) 22(11):2974–80. doi: 10.1093/humrep/dem302
58. Marquez JL, Pacheco A, Valdes P, Salazar LA. Association Between CAPN10 UCSNP-43 Gene Polymorphism and Polycystic Ovary Syndrome in Chilean Women. Clin Chim Acta (2008) 398(1-2):5–9. doi: 10.1016/j.cca.2008.07.028
59. Echiburu B, Ladron de Guevara A, Pereira C, Perez C, Michael P, Crisosto N, et al. Effects of Pregnancy and Changes in Body Weight on Polycystic Ovary Syndrome Phenotypes According to the Rotterdam Criteria. Rev Med Chil (2014) 142(8):966–74. doi: 10.4067/s0034-98872014000800003
60. Echiburu B, Crisosto N, Maliqueo M, Perez-Bravo F, de Guevara AL, Hernandez P, et al. Metabolic Profile in Women With Polycystic Ovary Syndrome Across Adult Life. Metabolism (2016) 65(5):776–82. doi: 10.1016/j.metabol.2016.01.006
61. Moran C, Tena G, Moran S, Ruiz P, Reyna R, Duque X. Prevalence of Polycystic Ovary Syndrome and Related Disorders in Mexican Women. Gynecol Obstet Invest (2010) 69(4):274–80. doi: 10.1159/000277640
62. Roa Barrios M, Arata-Bellabarba G, Valeri L, Velazquez-Maldonado E. [Relationship Between the Triglyceride/High-Density Lipoprotein-Cholesterol Ratio, Insulin Resistance Index and Cardiometabolic Risk Factors in Women With Polycystic Ovary Syndrome]. Endocrinol Nutr (2009) 56(2):59–65. doi: 10.1016/s1575-0922(09)70553-4
63. Quintero-Castillo D, Luz-Araujo H, Guerra-Velazquez M, Reyna-Villasmil E, Santos Bolivar J, Torres-Cepeda D, et al. Lipid Profile in Obese and non-Obese Women With Polycystic Ovary Syndrome Treated With Metformin. Endocrinol Nutr (2010) 57(6):262–7. doi: 10.1016/j.endonu.2010.04.001
64. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension (2018) 71(6):1269–1324. doi: 10.1161/HYP.0000000000000065
65. Homburger JR, Moreno-Estrada A, Gignoux CR, Nelson D, Sanchez E, Ortiz-Tello P, et al. Genomic Insights Into the Ancestry and Demographic History of South America. PloS Genet (2015) 11(12):e1005602. doi: 10.1371/journal.pgen.1005602
66. Moura RR, Coelho AV, Balbino Vde Q, Crovella S, Brandao LA. Meta-Analysis of Brazilian Genetic Admixture and Comparison With Other Latin America Countries. Am J Hum Biol (2015) 27(5):674–80. doi: 10.1002/ajhb.22714
67. Couto Alves A, Valcarcel B, Mäkinen VP, Morin-Papunen L, Sebert S, Kangas AJ, et al. Metabolic Profiling of Polycystic Ovary Syndrome Reveals Interactions With Abdominal Obesity. Int J Obes (Lond) (2017) 41(9):1331–40. doi: 10.1038/ijo.2017.126
68. Spritzer PM, Lecke SB, Satler F, Morsch DM. Adipose Tissue Dysfunction, Adipokines, and Low-Grade Chronic Inflammation in Polycystic Ovary Syndrome. Reproduction (2015) 149(5):R219–27. doi: 10.1530/rep-14-0435
69. Kelly CC, Lyall H, Petrie JR, Gould GW, Connell JM, Sattar N. Low Grade Chronic Inflammation in Women With Polycystic Ovarian Syndrome. J Clin Endocrinol Metab (2001) 86(6):2453–5. doi: 10.1210/jcem.86.6.7580
70. Behboudi-Gandevani S, Amiri M, Bidhendi Yarandi R, Noroozzadeh M, Farahmand M, Rostami Dovom M, et al. The Risk of Metabolic Syndrome in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Clin Endocrinol (Oxf) (2018) 88(2):169–84. doi: 10.1111/cen.13477
71. Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound Peripheral Insulin Resistance, Independent of Obesity, in Polycystic Ovary Syndrome. Diabetes (1989) 38(9):1165–74. doi: 10.2337/diab.38.9.1165
72. Manco M, Castagneto-Gissey L, Arrighi E, Carnicelli A, Brufani C, Luciano R, et al. Insulin Dynamics in Young Women With Polycystic Ovary Syndrome and Normal Glucose Tolerance Across Categories of Body Mass Index. PloS One (2014) 9(4):e92995. doi: 10.1371/journal.pone.0092995
73. Li R, Yu G, Yang D, Li S, Lu S, Wu X, et al. Prevalence and Predictors of Metabolic Abnormalities in Chinese Women With PCOS: A Cross- Sectional Study. BMC Endocr Disord (2014) 14:76. doi: 10.1186/1472-6823-14-76
74. Cheung LP, Ma RCW, Lam PM, Lok IH, Haines CJ, So WY, et al. Cardiovascular Risks and Metabolic Syndrome in Hong Kong Chinese Women With Polycystic Ovary Syndrome. Hum Reprod (2008) 23(6):1431–8. doi: 10.1093/humrep/den090
75. Cao NT, Le MT, Nguyen VQH, Pilgrim J, Le VNS, Le DD, et al. Defining Polycystic Ovary Syndrome Phenotype in Vietnamese Women. J Obstet Gynaecol Res (2019) 45(11):2209–19. doi: 10.1111/jog.14097
76. Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The Prevalence and Phenotypic Features of Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Hum Reprod (2016) 31(12):2841–55. doi: 10.1093/humrep/dew218
77. Barrea L, Arnone A, Annunziata G, Muscogiuri G, Laudisio D, Salzano C, et al. Adherence to the Mediterranean Diet, Dietary Patterns and Body Composition in Women With Polycystic Ovary Syndrome (PCOS). Nutrients (2019) 11(10):2278. doi: 10.3390/nu11102278
78. Graff SK, Mário FM, Alves BC, Spritzer PM. Dietary Glycemic Index is Associated With Less Favorable Anthropometric and Metabolic Profiles in Polycystic Ovary Syndrome Women With Different Phenotypes. Fertil Steril (2013) 100(4):1081–8. doi: 10.1016/j.fertnstert.2013.06.005
79. Kovalskys I, Rigotti A, Koletzko B, Fisberg M, Gómez G, Herrera-Cuenca M, et al. Latin American Consumption of Major Food Groups: Results From the ELANS Study. PloS One (2019) 14(12):e0225101. doi: 10.1371/journal.pone.0225101
80. Chan JL, Kar S, Vanky E, Morin-Papunen L, Piltonen T, Puurunen J, et al. Racial and Ethnic Differences in the Prevalence of Metabolic Syndrome and its Components of Metabolic Syndrome in Women With Polycystic Ovary Syndrome: A Regional Cross-Sectional Study. Am J Obstet Gynecol (2017) 217(2):189.e1–.e8. doi: 10.1016/j.ajog.2017.04.007
Keywords: obesity, metabolic syndrome, insulin resistance, PCOS (polycystic ovary syndrome), Latin America
Citation: Marchesan LB, Ramos RB and Spritzer PM (2021) Metabolic Features of Women With Polycystic Ovary Syndrome in Latin America: A Systematic Review. Front. Endocrinol. 12:759835. doi: 10.3389/fendo.2021.759835
Received: 17 August 2021; Accepted: 04 October 2021;
Published: 19 October 2021.
Edited by:
Giulia Rastrelli, University of Florence, ItalyReviewed by:
Irene Scavello, Careggi University Hospital, ItalyNunzia Verde, University of Naples Federico II, Italy
Copyright © 2021 Marchesan, Ramos and Spritzer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Poli Mara Spritzer, spritzer@ufrgs.br
†Present address: Ramon Bossardi Ramos, Department of Molecular and Cellular Physiology, Albany Medical College, Albany, NY, United States
 Lucas Bandeira Marchesan
Lucas Bandeira Marchesan Ramon Bossardi Ramos
Ramon Bossardi Ramos Poli Mara Spritzer
Poli Mara Spritzer