- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, South Korea
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, South Korea
- 3Department of Pediatrics, Seoul National University Children’s Hospital, Seoul National University College of Medicine, Seoul, South Korea
- 4Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, South Korea
- 5Department of Internal Medicine, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul, South Korea
There is a lack of studies regarding the long-term outcomes of Asian adults with classic congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency. We hypothesized that adults with CAH are at higher metabolic risk than their age-, and sex-matched controls. We further investigated the long-term health outcome-related factors in adults with CAH. We compared metabolic risk between adults with CAH (71 men, 93 women) and age-, and sex-matched controls (190 men, 261 women) from the Korean National Health and Nutrition Examination Survey data. The presence of obesity, testicular adrenal rest tumors (TARTs), and menstrual irregularity was assessed. Hormone status and treatment regimens were compared according to the presence of adverse outcomes. The median age was 27.0 y and 28.0 y for men and women, respectively. Adults with CAH had a higher waist circumference (88.0 vs. 82.3 cm in men, and 83.5 vs. 72.3 cm in women), and blood pressure (125.0 vs. 113.0 mmHg in men, and 120.0 vs. 104.0 mmHg in women) than age- and sex-matched controls (P<0.05 for all). The 2.7-fold increased risk for hypertension (men) and 2.0-fold increased risk for obesity (women) was significant in patients with CAH (P<0.05 for both). Obese adults with CAH showed significantly higher adrenal limb thicknesses (men) and 17-hydroxyprogesterone and dehydroepiandrosterone sulfate levels (women) (P<0.05 for both). TARTs occurred in 58.1% of men and did not differ by hormone or treatment regimen. Irregular menstruation was observed in 57.1% of women, with higher dehydroepiandrosterone sulfate levels in those with irregular periods. Adults with CAH had a higher metabolic risk than the general population. Poor disease control may increase their risk of metabolic morbidity and menstrual irregularity.
Introduction
Congenital adrenal hyperplasia (CAH) refers to a group of genetic disorders characterized by defective steroidogenesis due to enzyme deficiency. The most common form of CAH, 21-hydroxylase deficiency, affects approximately 1:15,000 live births (1, 2). A 21-hydroxylase deficiency results in glucocorticoid deficiency and an increase in pituitary adrenocorticotropin (ACTH) secretion, leading to adrenal androgen excess and adrenal hyperplasia. Depending on disease severity, CAH due to 21-hydroxylase deficiency is classified into classic (salt-wasting and simple virilizing) and non-classic forms (1). The early diagnosis of 21-hydroxylase deficiency through newborn screening test using 17-hydroxyprogesterone (17-OHP) has decreased mortality and morbidity rates, leading to increased interest in improving long-term health outcomes in adulthood (1, 2).
Several studies have reported adverse outcomes in adults with CAH. In the United Kingdom Congenital Adrenal Hyperplasia Adult Study Executive (CaHASE), 203 adults with CAH were significantly shorter and had a higher body mass index (BMI) compared to the health survey data (3). In a Swedish study, based on disease codes, 360 adults with CAH had an approximately four-fold higher risk of having any cardiovascular and metabolic disorders than matched controls (4). In a cross-sectional study of the National Institutes of Health (NIH) and French cohort, the prevalence of obesity was common, ranging from 35% to 44% (5, 6), although the latter two studies did not compare the patients’ metabolic risk with that of healthy controls. In a European multicenter study, 226 adults with CAH were a higher risk for hypertension, dyslipidemia, and cardiovascular disease but not type 2 diabetes (7). A recent meta-analysis including children and adults with CAH demonstrated that these patients had a high prevalence of cardiovascular and metabolic risk factors (8).
The two main goals for CAH management are to replace the deficient hormones for adrenal insufficiency and control androgen excess. However, it is challenging to balance glucocorticoid doses because supraphysiologic doses of glucocorticoids are usually required to suppress androgen excess. It remains controversial whether over- or undertreatment is more harmful to the metabolic and cardiovascular health of patients with CAH (3, 5, 6, 9–11). Moreover, the relationship between testicular adrenal rest tumors (TARTs) and disease control remains unclear (5, 6, 12).
Most related studies have not performed a sex-stratified analysis and rarely evaluated adrenal morphology such as hyperplasia or thinning (6, 13). In Asia, few studies have examined the determining factors for adverse outcomes in adults with CAH. In this context, we hypothesized that adults with CAH are at a higher risk of metabolic morbidity than their age- and sex-matched controls in the Asian general population. Furthermore, we aimed to investigate sex-specific indicators related to adverse health outcomes by focusing on metabolic morbidity, TARTs, and menstrual irregularity in adults with CAH.
Materials and Methods
Study Subjects
Among the 233 adults with CAH aged over 20 years at the last follow-up visit between 2000 and 2020 at Seoul National University Hospital, those with non-classic CAH (n = 19) or for whom laboratory findings or an appropriate medical history was lacking (n = 50) were excluded. Finally, we included 164 adults with classic 21-hydroxylase deficiency. Of the 71 men and 93 women included in this study 42 (59.2%) men and 34 (36.6%) women had salt-wasting form. We diagnosed patients with CAH based on clinical and biochemical data since the genetic testing for CYP21A2 mutation was only done in 34 of 164 patients. All patients were diagnosed clinically since neonatal screening was first introduced in 2006 in Korea. For comparison, age- and sex-matched controls (190 men, 261 women) were included in this study, with a 1:3 case to control ratio from the nationwide representative survey database (Korean National Health and Nutrition Examination Survey [KNHANES] 2015) (14).
Clinical, Imaging, and Biochemical Data
We retrospectively reviewed the patients’ electronic medical records and retrieved the data from the last follow-up visit. BMI was calculated as body weight divided by height squared (kg/m2). Waist circumference was measured at the level of the umbilicus with the subject standing and breathing normally while wearing light clothing. Blood pressure was measured twice on different days using an automated technique while the subjects were in a seated position after a 20-min rest. Body composition data, such as lean mass and fat mass, were obtained from a bioimpedance analyzer (Inbody720®; Inbody Co. Korea).
Obesity was defined as a BMI > 25 kg/m2 (15). Dyslipidemia was defined as the use of lipid-lowering agents or having an abnormal lipid panel (total cholesterol ≥ 240 mg/dL, LDL cholesterol ≥ 160 mg/dL, triglycerides ≥ 200 mg/dL, or HDL cholesterol < 40 mg/dL). The presence of hyperglycemia included diabetes mellitus and prediabetes. Diabetes mellitus was defined as an HbA1c ≥ 6.5% or the use of any oral anti-diabetic drugs or insulin therapy. Prediabetes was determined as an HbA1c value between 5.7% and 6.4% or a fasting plasma glucose ≥100 mg/dL. Subjects taking any antihypertensive medications or systolic blood pressure ≥ 130 mmHg and/or diastolic blood pressure ≥ 85 mmHg in repeated measurements were considered to have hypertension (16). In men, TARTs were identified using testicular sonography. In women, the regularity of menstruation was classified into two categories: regular (21–35 days) or irregular (oligomenorrhea/amenorrhea).
Computed tomography was performed to evaluate the morphology of the adrenal glands. The examiner was blinded to the patients’ history. The widths of the medial and lateral adrenal limbs were measured as the maximum width of the limbs perpendicular to the long axis (17). The thickness of the adrenal limb was defined as the mean medial and lateral limb widths. The current glucocorticoid and mineralocorticoid (fludrocortisone) regimen was identified for each patient. The daily glucocorticoid dose was calculated based on glucocorticoid type and daily dose. Glucocorticoid doses were calculated based on the anti-inflammatory equivalent dose compared to hydrocortisone (30 mg hydrocortisone = 7.5 mg prednisolone).
Morning fasting blood samples were taken before steroid medications were administered. Laboratory tests included hemoglobin A1c (HbA1c), plasma glucose, total cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, 17-hydroxyprogesterone (17-OHP), total testosterone, dehydroepiandrosterone sulfate (DHEAS), plasma renin activity, and adrenocorticotropin (ACTH).
Biochemical assays
Serum 17-OHP and DHEAS levels were measured using a radioimmunoassay (RIA) CT kit (Asbach Medical Products GmbH, Germany), with intra- and inter-assay CVs of 4.6–6.8% and 7.7–8.8% for 17-OHP (reference range, 0.11-5.00 ng/mL) and 3.6–5.9% and 6.5% for DHEAS (reference range, 1187-4289 ng/mL), respectively. Serum total testosterone was measured using a TESTO-CT2 kit (Cisbio Bioassays, Saclay, France) with intra- and inter-assay CVs of 3.1–8.9% and 5.2–11.6%, respectively (reference range, 2.7-10.7 ng/mL in men, and 0-1.0 ng/mL in women). Plasma renin activity was measured using a PRA RIA kit (TFB, Inc.), with intra- and inter-assay CVs of 3.8% and 6.7%, respectively (reference range, 0.32-1.84 ng/mL/hr). Plasma ACTH was measured using an immunoradiometric assay (Cisbio Bioassays) with a reference range of 10.0–60.0 pg/mL. The intra- and inter-assay CVs of the ACTH used were 3.7% and 3.8%, respectively.
Statistical Analysis
Data are shown as number (percentage) for categorical variables and mean ± standard deviation or median (interquartile range) for continuous variables based on the results of the normality test. The normality test was performed using the Shapiro-Wilk test. Categorical variables were analyzed using the chi-squared test. Continuous variables were compared using Student’s t-test or the Mann-Whitney U test according to the distribution of normality. Logistic regression models were constructed to assess the metabolic risk of patients with CAH by sex. To adjust for the effect of age and BMI, we performed a multivariate logistic regression analysis, and age and BMI-adjusted odds ratio (OR) and 95% confidence interval (95% CI) are presented. Statistical significance was set at P < 0.05. All statistical analyses were performed using R Statistical Software (version 4.0.3).
Results
Comparison of Metabolic Risk Between Patients with CAH and Healthy Controls
Table 1 compares the clinical and biochemical characteristics of patients with CAH and age- and sex-matched controls by sex. The median age for men and women was 27.0 and 28.0 years, respectively. The men with CAH (n = 71) exhibited a shorter stature (P < 0.001), higher weight, and greater waist circumference (P = 0.005 for both) than the control men (n = 190) without a BMI difference. Fasting plasma glucose and blood pressure were higher in the men with CAH (P < 0.05), but HbA1c was similar between the two groups. Although total cholesterol, triglyceride, and LDL cholesterol levels were similar between the two groups, HDL cholesterol was higher in the men with CAH than in the control men (P < 0.001).
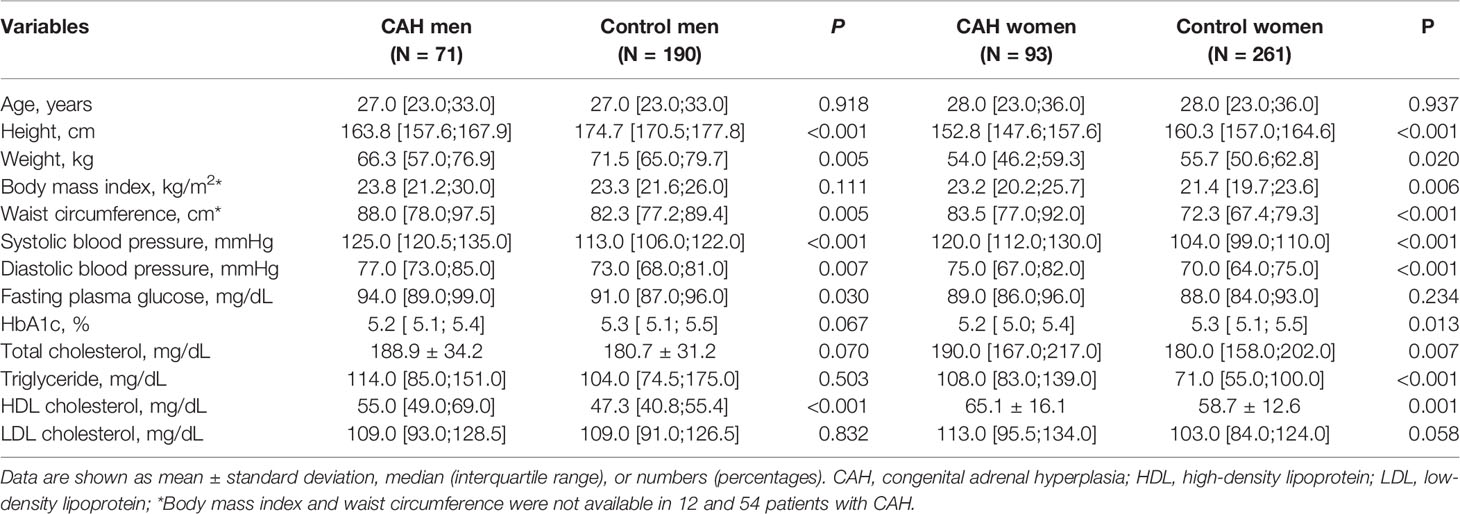
Table 1 Comparison of clinical characteristics between CAH patients and age- and sex-matched control.
The women with CAH (n = 93) showed a shorter stature, higher BMI, and higher waist circumference (P < 0.05) than the control women (n = 261). Blood pressure was also higher in the women with CAH than in the control women (P < 0.001). Fasting plasma glucose levels were similar between the two groups, but HbA1c was lower in women with CAH (P < 0.013). Total cholesterol, triglyceride, and HDL cholesterol levels were higher in the women with CAH than in the control women (P < 0.05).
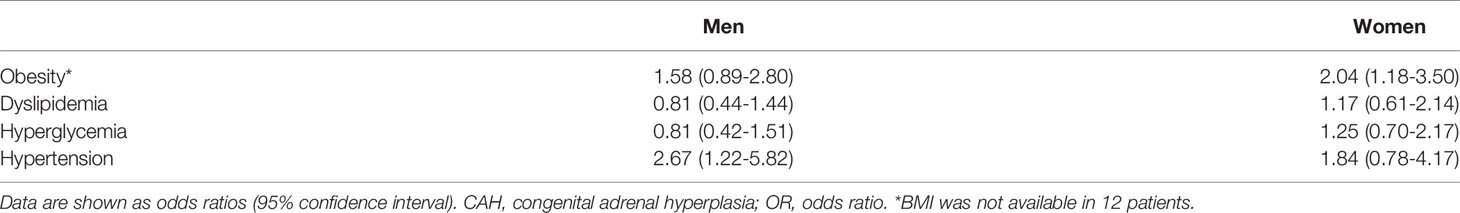
We analyzed the metabolic outcomes of the CAH group versus the control group (Table 2). The CAH group had a two-fold higher risk of obesity than the control group among the women (OR [95% CI], 2.04 [1.18-3.50]), but the difference was not significant in the men. The increased risk of hypertension was found in CAH men (OR [95% CI], 2.67 [1.22-5.82]) but not in women. The risk of dyslipidemia and hyperglycemia did not differ between the two groups in either sex.
Comparison According to Whether CAH Patients Have Metabolic Comorbidities
Figure 1 shows the prevalence of comorbidities in adults with CAH. The prevalence of any metabolic comorbidity was significantly higher in men than in women (70.4% vs. 52.7%, P = 0.032) without sex differences for each component. The prevalence of obesity, dyslipidemia, hyperglycemia, and hypertension was 43.9%, 29.6%, 23.9%, 19.7% in men and 33.7%, 18.3%, 23.7%, 10.8% in women, respectively. The number of patients with diabetes was only 6 in women and 2 in men with CAH. There was no history of gestational diabetes, cardiovascular or cerebrovascular disease.
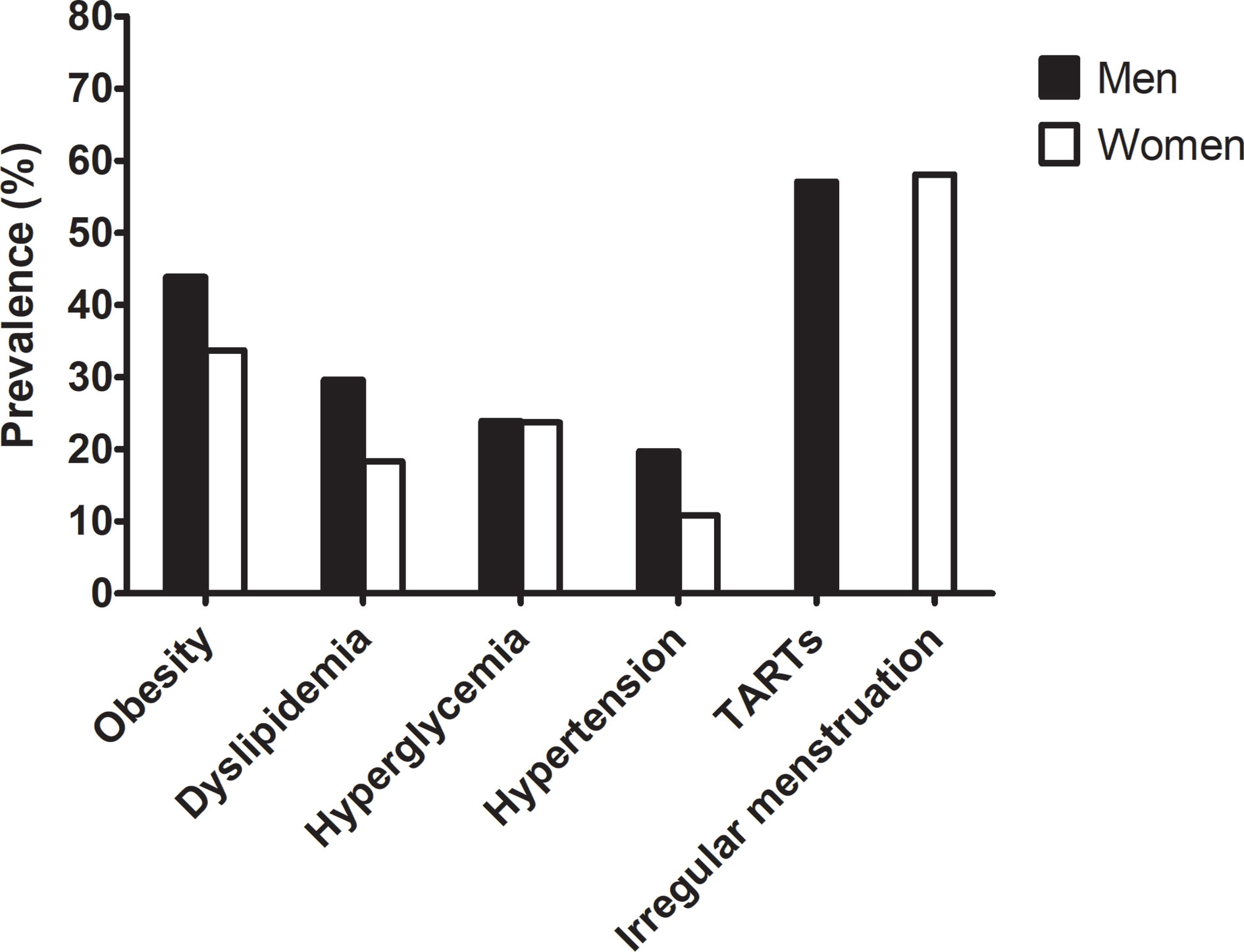
Figure 1 Prevalence of metabolic risk and gonadal dysfunction in the adult CAH patients according to sex.
We further analyzed the differences in hormone status and treatment regimen according to whether CAH patients were obese (Table 3). Neither age at diagnosis nor proportion of subtypes differed between obese and non-obese patients. Obese adults with CAH had a higher waist circumference and percentage of fat mass in both sexes but a lower percentage of lean mass than those without (P < 0.05 for all for both sexes). Adrenal limb thickness was significantly higher in obese men compared to non-obese men (P< 0.001). Although the difference of adrenal limb thickness was marginally significant between obese and non-obese women, adrenal tumors was more frequently found in obese women compared to non-obese women (P=0.019). Among the hormones, 17-OHP and DHEAS levels were significantly higher in obese women than in non-obese ones (P< 0.001, and P=0.009, respectively). All obese women had a 17-OHP level ≥ of 10 ng/mL. However, the glucocorticoid regimen and dose were not related to obesity in either sex. The number of patients with 0, 1, and ≥2 metabolic comorbidities was 29.6% (n=21), 35.2% (n=25), and 35.2% (n=25) in men with CAH, and 47.3% (n=44), 26.8% (n=25), and 25.8% (n=24) in women with CAH, respectively. No significant relationships of age at diagnosis, proportion of subtypes, and glucocorticoid doses with number of metabolic comorbidities were found in both sex (data not shown).
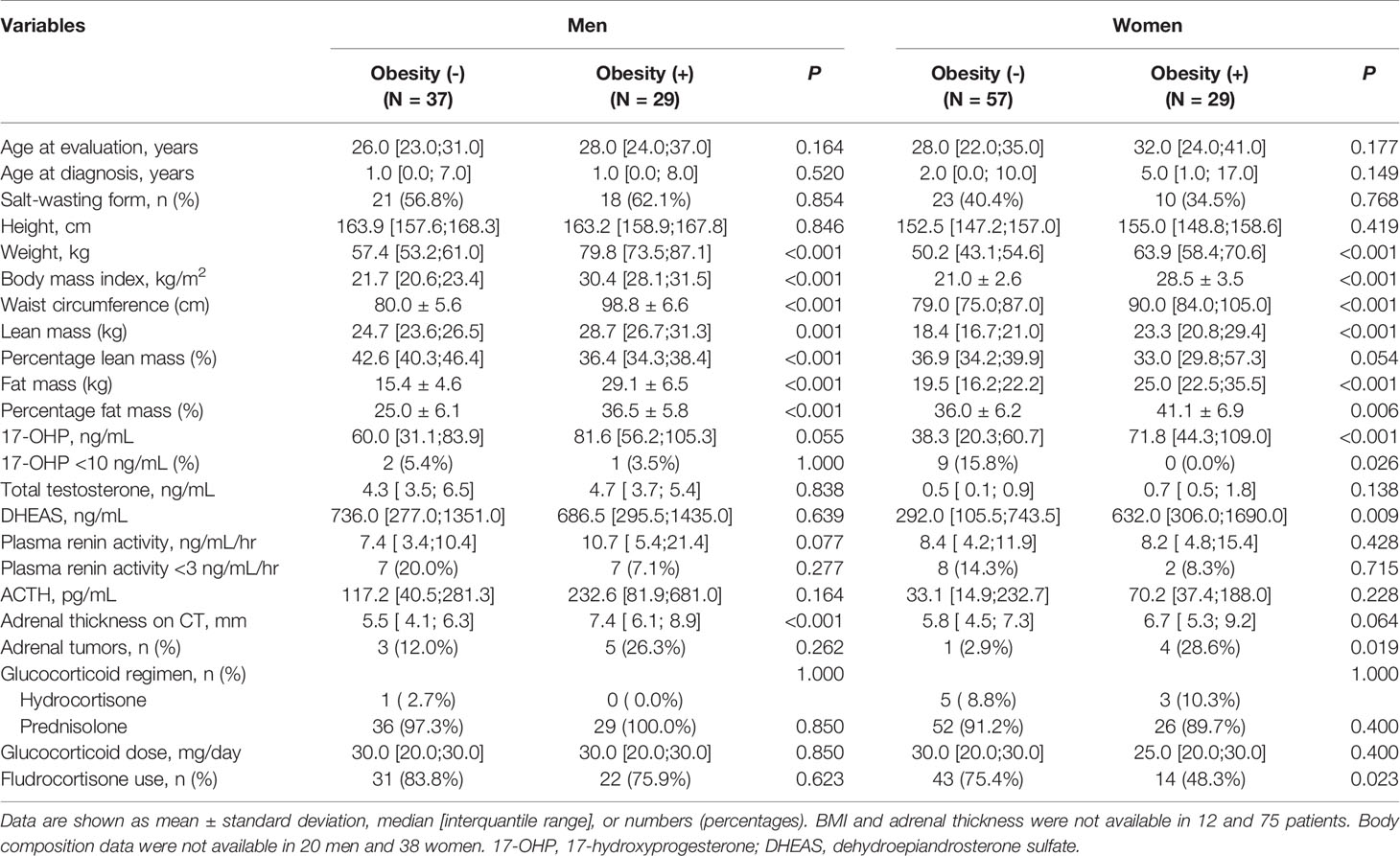
Table 3 Hormone status and treatment regimens in CAH men and women according to the presence of obesity.
Comparison of Whether CAH Patients Had TARTs or Menstrual Irregularities
The prevalence of TARTs in men was 58.1% (Figure 1 and Table 4). The presence of TARTs was not related to disease control, hormone status, glucocorticoid regimen, or adrenal limb thickness in men. Among the women, 57.1% exhibited menstrual irregularities (Figure 1 and Table 4). Women with irregular periods showed a shorter stature and a higher DHEAS level than those with regular periods (P< 0.05 for both). Other hormones, steroid regimens, and adrenal limb thickness did not differ according to menstrual regularity.

Table 4 Hormone status and treatment regimen in men with CAH according to the presence of testicular adrenal rest tumors (n = 70) and women with CAH according to menstruation (n = 86).
Discussion
The present study suggested that waist circumference and blood pressure were higher in adults with CAH compared with age- and BMI-matched controls from the KNHANES database. Moreover, there was an increased risk of hypertension in men and obesity in women with CAH than controls. Obese men with CAH showed higher adrenal limb thicknesses than non-obese ones, while obese women had higher 17-OHP and DHEAS levels than non-obese ones. In addition, more than half of the adults with CAH exhibited TARTs or menstrual irregularities. The presence of TARTs in men with CAH was not related to disease control, while women with CAH who had irregular periods were shorter and had higher DHEAS levels than those with regular periods.
The prevalence of obesity or central obesity in adults with classic CAH is reportedly 20–50%, higher than that in healthy controls (3–6). In the present study, obesity was more prevalent in women with CAH, and waist circumference was obviously higher in both sexes than in healthy controls, consistent with the increased abdominal obesity and higher visceral to subcutaneous fat ratio linked to insulin resistance and inflammation, suggesting an unhealthy metabolic phenotype among adults with CAH (18).
It remains controversial whether hypertension, dyslipidemia, and hyperglycemia are more prevalent in adults with CAH than in healthy controls. The prevalence of hypertension was higher in the NIH and Swedish nationwide CAH cohorts (4, 5, 8) and similar or lower in the CaHASE and French cohorts (3, 19). In our study, men with CAH but not women with CAH showed a higher prevalence of hypertension than the controls, which can be related to the relatively high proportion of fludrocortisone use and central obesity among men with CAH and the protective effect of estrogen on blood pressure among women with CAH. The prevalence of dyslipidemia and hyperglycemia was similar between adults with CAH and controls in this study. However, fasting glucose and HDL cholesterol levels in men with CAH and total cholesterol, triglyceride, and HDL cholesterol levels in women with CAH were higher than the values in controls, although a recent meta-analysis including 300 pediatric and 137 adults showed no difference in glucose and lipid levels (8). The result of higher HDL-cholesterol levels in our patients with CAH remains to be reproduced in further studies, and the underlying mechanism also remains to be determined. However, Falhammar et al. suggested that older women with CAH had also higher lean mass which may explain the favorable lipid profile (20). Our study population mainly consisted of young adult patients who showed elevated androgen levels, suggesting poor disease control. Inconsistency in reporting the prevalence of metabolic comorbidities may have resulted from differences in patient age, glucocorticoid and mineralocorticoid doses, treatment target goals, disease control status, and 21-hydroxylase activity.
We further analyzed factors that differed according to whether patients with CAH had more metabolic risk factors. Previous studies identified that adults with CAH are at risk for hyperandrogenism and iatrogenic hypercortisolism, both of which lead to obesity, insulin resistance, and cardiometabolic comorbidities (5, 6, 9, 10). In addition to glucocorticoid overtreatment, suppressed 17-OHP levels were related to obesity or metabolic morbidity (3, 11) as well as androgen excess, suggesting that poor disease control contributed to higher metabolic risk factors as shown in our study.
Intriguingly, obese men with CAH presented with thick adrenal limbs, while obese women had high 17-OHP and DHEAS levels. Few studies have examined the adrenal morphology of adults with CAH (6, 21). Adrenal nodules were more frequently detected in poorly controlled patients with CAH, and regressed in size after high-dose steroids (13). Adrenal hyperplasia and/or nodules have been reported in 29.3– 45% of patients with CAH (22), with significant correlation with high levels of ACTH, 17-OHP, and plasma renin activity (6). Adrenal tumors were detected in 15% (14/93) among our adult patients evaluated by CT. Despite the limitation of a single hormonal measurement in the morning due to the cross-sectional nature of this study, adrenal limb thickness or the presence of adrenal tumors could be a long-term disease control marker in adults with CAH.
Obese women with CAH showed higher 17-OHP levels than non-obese ones, suggesting the contribution of androgen excess. A similar phenomenon was demonstrated in polycystic ovary syndrome in postmenopausal women (23–25). Androgen excess drives visceral fat accumulation and induces the android phenotype in women with CAH (26, 27). Even with continued treatment, patients may respond poorly to glucocorticoid treatment (6, 28) related to glucocorticoid receptor gene polymorphism in addition to low adherence to glucocorticoid therapy regimens (29). Further studies are warranted to determine optimal targets and androgen metabolites that reflect adequate hormonal control and ideal glucocorticoid regimens to minimize cardiometabolic comorbidities.
The presence of TART is the main factor of male infertility since it blocks the seminiferous tubules and leads to Leydig cell failure, which has a reported prevalence of around 37% (14–86%) depending on age and genotype (5, 6, 19, 30). In our study, TARTs were found in 57.1% of men with CAH who underwent testicular sonography. Since TARTs express adrenal-specific enzymes and ACTH and angiotensin II receptors (31), poor disease control and high ACTH or renin activity can lead to adrenocortical hyperplasia and TARTs (5, 32, 33), although the lack of a correlation between 17-OHP levels and TARTs has been reported (6, 12, 34). Although the present study failed to demonstrate a relationship between disease control and the presence of TARTs, in our previous study, TART size was related to the undertreatment percentage (35).
The prevalence of menstrual irregularity was up to 58.1% in the women with CAH in our study, which is consistent with that of a previous report of 30–75% depending on genotype (3). Women with CAH and irregular periods exhibited shorter stature and higher DHEAS levels, reflecting hyperandrogenism. Patients with CAH exposed to high androgens in childhood can experience earlier epiphyseal closure and short stature. Hyperandrogenism suppresses serum progesterone, which reduces luteinizing hormone pulsatility during the follicular phase and induces endometrial thinning, leading to oligo- or anovulation and oligo- or amenorrhea (36–38).
This cross-sectional study had several limitations. There were several missing values due to the retrospective study design. We could not assess the total cumulative doses of glucocorticoid or perform a longitudinal hormone assessment to determine disease control due to a lack of childhood data before the electronic medical record era. Since most patients were diagnosed before the genetic testing era, we could not analyze the patients’ clinical or hormone characteristics according to genotype. When we assessed single hormone measurements in the morning at the last visit, only 7.3% of patients had well-controlled disease based on the early-morning 17-OHP levels. Since undertreatment still affected most of our patients compared to those in previous reports (3), we could not compare the effect of undertreatment versus overtreatment on long-term outcomes. Androstenedione, which was known to be the reliable marker for disease control (1), was not checked due to the cost and unavailability. Although this cross-sectional design could not prove the causal relationship of disease itself or treatment effect with metabolic complications, our study had several strengths. First, it is the largest single-center Asian study of adults with CAH. We also compared age-, sex-, and ethnicity-matched controls to assess metabolic risk. We separately analyzed sex-specific indicators according to health outcomes and obtained different findings by sex. Adrenal thickness assessed by CT was incorporated into the variables to reflect long-term glucocorticoid exposure. Most patients had taken the same dosage of prednisolone in adulthood, thereby allowing us to compare their hormonal effects in patients with CAH without glucocorticoid dose interference.
Conclusion
The present study confirmed the higher prevalence of metabolic morbidities in young adults with CAH than in healthy controls. Moreover, adverse metabolic outcomes and menstrual irregularity were associated with poor disease control in our study subjects. Considering the harmful effects of poor disease control on long-term outcomes, compliance should be guaranteed. Early attention should be paid to identifying at-risk patients and regular follow-up provided to minimize cardiovascular risk in patients with CAH. Further studies are needed to elucidate the mechanisms leading to metabolic morbidity and the respective roles of androgen excess and glucocorticoid exposure.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary files. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Seoul National University Hospital Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
SL and JK contributed in the conception of the work, participated in the study design, and wrote the manuscript. YL contributed in the conception of the work, participated in the study design, and critically revised the manuscript. HJ, SK, CA, SK, and CS contributed in the conception of the work, performed statistical analyses, and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by a donation from one CAH patient.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to Sei Won Yang, the emeritus professor of pediatrics at Seoul National University College of Medicine, and Seong Yeon Kim, the emeritus professor of internal medicine at Seoul National University College of Medicine. We also thank Serena Park for building up the database.
References
1. Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, et al. Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2018) 103(11):4043–88. doi: 10.1210/jc.2018-01865
2. Claahsen-van der Grinten HL, Speiser PW, Ahmed SF, Arlt W, Auchus RJ, Falhammar H, et al. Congenital Adrenal Hyperplasia - Current Insights in Pathophysiology, Diagnostics and Management. Endocr Rev (2021). doi: 10.1210/endrev/bnab016
3. Arlt W, Willis DS, Wild SH, Krone N, Doherty EJ, Hahner S, et al. Health Status of Adults With Congenital Adrenal Hyperplasia: A Cohort Study of 203 Patients. J Clin Endocrinol Metab (2010) 95(11):5110–21. doi: 10.1210/jc.2010-0917
4. Falhammar H, Frisen L, Hirschberg AL, Norrby C, Almqvist C, Nordenskjold A, et al. Increased Cardiovascular and Metabolic Morbidity in Patients With 21-Hydroxylase Deficiency: A Swedish Population-Based National Cohort Study. J Clin Endocrinol Metab (2015) 100(9):3520–8. doi: 10.1210/JC.2015-2093
5. Finkielstain GP, Kim MS, Sinaii N, Nishitani M, Van Ryzin C, Hill SC, et al. Clinical Characteristics of a Cohort of 244 Patients With Congenital Adrenal Hyperplasia. J Clin Endocrinol Metab (2012) 97(12):4429–38. doi: 10.1210/jc.2012-2102
6. Bachelot A, Golmard JL, Dulon J, Dahmoune N, Leban M, Bouvattier C, et al. Determining Clinical and Biological Indicators for Health Outcomes in Adult Patients With Childhood Onset of Congenital Adrenal Hyperplasia. Eur J Endocrinol (2015) 173(2):175–84. doi: 10.1530/EJE-14-0978
7. Falhammar H, Claahsen-van der Grinten H, Reisch N, Slowikowska-Hilczer J, Nordenstrom A, Roehle R, et al. Health Status in 1040 Adults With Disorders of Sex Development (DSD): A European Multicenter Study. Endocr Connect (2018) 7(3):466–78. doi: 10.1530/EC-18-0031
8. Tamhane S, Rodriguez-Gutierrez R, Iqbal AM, Prokop LJ, Bancos I, Speiser PW, et al. Cardiovascular and Metabolic Outcomes in Congenital Adrenal Hyperplasia: A Systematic Review and Meta-Analysis. J Clin Endocrinol Metab (2018) 103(11):4097–103. doi: 10.1210/jc.2018-01862
9. Zhang HJ, Yang J, Zhang MN, Liu CQ, Xu M, Li XJ, et al. Metabolic Disorders in Newly Diagnosed Young Adult Female Patients With Simple Virilizing 21-Hydroxylase Deficiency. Endocrine (2010) 38(2):260–5. doi: 10.1007/s12020-010-9382-9
10. Paizoni L, Auer MK, Schmidt H, Hubner A, Bidlingmaier M, Reisch N. Effect of Androgen Excess and Glucocorticoid Exposure on Metabolic Risk Profiles in Patients With Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency. J Steroid Biochem Mol Biol (2020) 197:105540. doi: 10.1016/j.jsbmb.2019.105540
11. Torky A, Sinaii N, Jha S, Desai J, El-Maouche D, Mallappa A, et al. Cardiovascular Disease Risk Factors and Metabolic Morbidity in a Longitudinal Study of Congenital Adrenal Hyperplasia. J Clin Endocrinol Metab (2021). doi: 10.1210/clinem/dgab133
12. Reisch N, Rottenkolber M, Greifenstein A, Krone N, Schmidt H, Reincke M, et al. Testicular Adrenal Rest Tumors Develop Independently of Long-Term Disease Control: A Longitudinal Analysis of 50 Adult Men With Congenital Adrenal Hyperplasia Due to Classic 21-Hydroxylase Deficiency. J Clin Endocrinol Metab (2013) 98(11):E1820–6. doi: 10.1210/jc.2012-3181
13. Giacaglia LR, Mendonca BB, Madureira G, Melo KF, Suslik CA, Arnhold IJ, et al. Adrenal Nodules in Patients With Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency: Regression After Adequate Hormonal Control. J Pediatr Endocrinol Metab (2001) 14(4):415–9. doi: 10.1515/JPEM.2001.14.4.415
14. Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, et al. Data Resource Profile: The Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol (2014) 43(1):69–77. doi: 10.1093/ije/dyt228
15. Seo MH, Lee WY, Kim SS, Kang JH, Kang JH, Kim KK, et al. 2018 Korean Society for the Study of Obesity Guideline for the Management of Obesity in Korea. J Obes Metab Syndr (2019) 28(1):40–5. doi: 10.7570/jomes.2019.28.1.40
16. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation (2009) 120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
17. Kong SH, Kim JH, Shin CS. Contralateral Adrenal Thinning as a Distinctive Feature of Mild Autonomous Cortisol Excess of the Adrenal Tumors. Eur J Endocrinol (2020) 183(3):325–33. doi: 10.1530/EJE-20-0301
18. Kim MS, Ryabets-Lienhard A, Dao-Tran A, Mittelman SD, Gilsanz V, Schrager SM, et al. Increased Abdominal Adiposity in Adolescents and Young Adults With Classical Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency. J Clin Endocrinol Metab (2015) 100(8):E1153–59. doi: 10.1210/jc.2014-4033
19. Bouvattier C, Esterle L, Renoult-Pierre P, de la Perriere AB, Illouz F, Kerlan V, et al. Clinical Outcome, Hormonal Status, Gonadotrope Axis, and Testicular Function in 219 Adult Men Born With Classic 21-Hydroxylase Deficiency. A French National Survey. J Clin Endocrinol Metab (2015) 100(6):2303–13. doi: 10.1210/jc.2014-4124
20. Falhammar H, Filipsson H, Holmdahl G, Janson PO, Nordenskjold A, Hagenfeldt K, et al. Metabolic Profile and Body Composition in Adult Women With Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency. J Clin Endocrinol Metab (2007) 92(1):110–6. doi: 10.1210/jc.2006-1350
21. Reisch N, Scherr M, Flade L, Bidlingmaier M, Schwarz HP, Muller-Lisse U, et al. Total Adrenal Volume But Not Testicular Adrenal Rest Tumor Volume Is Associated With Hormonal Control in Patients With 21-Hydroxylase Deficiency. J Clin Endocrinol Metab (2010) 95(5):2065–72. doi: 10.1210/jc.2009-1929
22. Nermoen I, Falhammar H. Prevalence and Characteristics of Adrenal Tumors and Myelolipomas in Congenital Adrenal Hyperplasia: A Systematic Review and Meta-Analysis. Endocr Pract (2020) 26(11):1351–65. doi: 10.4158/EP-2020-0058
23. Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, et al. Assessment of Cardiovascular Risk and Prevention of Cardiovascular Disease in Women With the Polycystic Ovary Syndrome: A Consensus Statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab (2010) 95(5):2038–49. doi: 10.1210/jc.2009-2724
24. Janssen I, Powell LH, Kazlauskaite R, Dugan SA. Testosterone and Visceral Fat in Midlife Women: The Study of Women's Health Across the Nation (SWAN) Fat Patterning Study. Obes (Silver Spring) (2010) 18(3):604–10. doi: 10.1038/oby.2009.251
25. Diamanti-Kandarakis E, Dunaif A. Insulin Resistance and the Polycystic Ovary Syndrome Revisited: An Update on Mechanisms and Implications. Endocr Rev (2012) 33(6):981–1030. doi: 10.1210/er.2011-1034
26. Rosenfield RL, Ehrmann DA. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr Rev (2016) 37(5):467–520. doi: 10.1210/er.2015-1104
27. Pasquali R, Vicennati V, Gambineri A, Pagotto U. Sex-Dependent Role of Glucocorticoids and Androgens in the Pathophysiology of Human Obesity. Int J Obes (Lond) (2008) 32(12):1764–79. doi: 10.1038/ijo.2008.129
28. Han TS, Conway GS, Willis DS, Krone N, Rees DA, Stimson RH, et al. Relationship Between Final Height and Health Outcomes in Adults With Congenital Adrenal Hyperplasia: United Kingdom Congenital Adrenal Hyperplasia Adult Study Executive (CaHASE). J Clin Endocrinol Metab (2014) 99(8):E1547–55. doi: 10.1210/jc.2014-1486
29. Moreira RP, Gomes LG, Mendonca BB, Bachega TA. Impact of Glucocorticoid Receptor Gene Polymorphisms on the Metabolic Profile of Adult Patients With the Classical Form of 21-Hydroxylase Deficiency. PloS One (2012) 7(9):e44893. doi: 10.1371/journal.pone.0044893
30. Engels M, Span PN, van Herwaarden AE, Sweep F, Stikkelbroeck N, Claahsen-van der Grinten HL. Testicular Adrenal Rest Tumors: Current Insights on Prevalence, Characteristics, Origin, and Treatment. Endocr Rev (2019) 40(4):973–87. doi: 10.1210/er.2018-00258
31. Claahsen-van der Grinten HL, Otten BJ, Sweep FC, Span PN, Ross HA, Meuleman EJ, et al. Testicular Tumors in Patients With Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency Show Functional Features of Adrenocortical Tissue. J Clin Endocrinol Metab (2007) 92(9):3674–80. doi: 10.1210/jc.2007-0337
32. Martinez-Aguayo A, Rocha A, Rojas N, Garcia C, Parra R, Lagos M, et al. Testicular Adrenal Rest Tumors and Leydig and Sertoli Cell Function in Boys With Classical Congenital Adrenal Hyperplasia. J Clin Endocrinol Metab (2007) 92(12):4583–9. doi: 10.1210/jc.2007-0383
33. Yu MK, Jung MK, Kim KE, Kwon AR, Chae HW, Kim DH, et al. Clinical Manifestations of Testicular Adrenal Rest Tumor in Males With Congenital Adrenal Hyperplasia. Ann Pediatr Endocrinol Metab (2015) 20(3):155–61. doi: 10.6065/apem.2015.20.3.155
34. Delfino M, Elia J, Imbrogno N, Argese N, Mazzilli R, Toscano V, et al. Testicular Adrenal Rest Tumors in Patients With Congenital Adrenal Hyperplasia: Prevalence and Sonographic, Hormonal, and Seminal Characteristics. J Ultrasound Med (2012) 31(3):383–8. doi: 10.7863/jum.2012.31.3.383
35. Kang MJ, Kim JH, Lee SH, Lee YA, Shin CH, Yang SW. The Prevalence of Testicular Adrenal Rest Tumors and Associated Factors in Postpubertal Patients With Congenital Adrenal Hyperplasia Caused by 21-Hydroxylase Deficiency. Endocr J (2011) 58(6):501–8. doi: 10.1507/endocrj.K11E-034
36. Bachelot A, Chakhtoura Z, Plu-Bureau G, Coudert M, Coussieu C, Badachi Y, et al. Influence of Hormonal Control on LH Pulsatility and Secretion in Women With Classical Congenital Adrenal Hyperplasia. Eur J Endocrinol (2012) 167(4):499–505. doi: 10.1530/EJE-12-0454
37. Mulaikal RM, Migeon CJ, Rock JA. Fertility Rates in Female Patients With Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency. N Engl J Med (1987) 316(4):178–82. doi: 10.1056/NEJM198701223160402
Keywords: adrenal hyperplasia, congenital, dyslipidemia, hyperglycemia, hypertension, obesity
Citation: Lim SG, Lee YA, Jang HN, Kong SH, Ahn CH, Kim SW, Shin CH and Kim JH (2021) Long-Term Health Outcomes of Korean Adults With Classic Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency. Front. Endocrinol. 12:761258. doi: 10.3389/fendo.2021.761258
Received: 19 August 2021; Accepted: 27 September 2021;
Published: 12 October 2021.
Edited by:
Giuseppe Reimondo, University of Turin, ItalyReviewed by:
Henrik Falhammar, Karolinska Institutet (KI), SwedenMiguel Debono, Royal Hallamshire Hospital, United Kingdom
Copyright © 2021 Lim, Lee, Jang, Kong, Ahn, Kim, Shin and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jung Hee Kim, jhee1@snu.ac.kr; jhkxingfu@gmail.com; Young Ah Lee, nina337@snu.ac.kr
 Seung Gyun Lim1,2
Seung Gyun Lim1,2 Young Ah Lee
Young Ah Lee Sang Wan Kim
Sang Wan Kim Jung Hee Kim
Jung Hee Kim