- 1Department of Orthopedics, The First People’s Hospital of Yunnan Province, The Affiliated Hospital of Kunming University of Science and Technology, The Key Laboratory of Digital Orthopedics of Yunnan Province, Kunming, China
- 2School of Basic Medical Sciences, Yunnan University of Chinese Medicine, Kunming, China
Background: Although some studies have found that nitrates were beneficial for bone health, the findings are inconsistent. To assess the efficacy of nitrates for bone health, we conducted a meta-analysis.
Methods: PubMed, EMBASE databases, Cochrane Library for relevant articles published before December 2021 were searched. All observational and randomized controlled studies that reporting bone mineral density (BMD), fractures with nitrates use were included. A meta-analysis was performed to calculate risk ratios (RRs) for fractures, change differences for bone mineral density.
Results: Four cohort studies and two case-control studies examining the association between nitrates use and fractures were identified. The nitrates use was not associated with any fracture risk (RR = 0.97; 95% CI, 0.94–1.01; I2 = 31.5%) and hip fracture (RR = 0.88; 95% CI, 0.76–1.02; I2 = 74.5%). Subgroup analyses revealed no differences in fracture risk, whereas two cohort studies revealed a reduced risk of hip fracture (RR = 0.71, 95% CI, 0.58–0.86, I2 = 0.0%). There were no statistically significant differences in BMD percent changes at lumbar spine (WMD = -0.07, 95% CI,-0.78–0.65; I2 = 0.0%), total hip (WMD = -0.42, 95% CI,-0.88–0.04; I2 = 0.0%), femoral neck (WMD = -0.38, 95% CI,-1.02–0.25; I2 = 0.0%), or total body (WMD = -0.17, 95% CI,-0.51–0.17; I2 = 0.0%) in two randomized controlled trials (RCTs) compared with a placebo. Another two RCTs compared nitrates with alendronate. Nitrates were comparable to alendronate in increasing bone mineral density at lumbar spine (WMD = 0.00, 95% CI,-0.01–0.02; I2 = 0.0%). Besides, the most common adverse effect was headache, contributing to low adherence to therapy.
Conclusion: Our meta-analysis showed no association between nitrates use and fractures in observational studies. The results of RCTs on the usage of nitrates and their effects on BMD were inconsistent. High-quality, long-term studies are needed to clarify the efficacy of nitrates for bone health.
Introduction
Osteoporosis, defined as a decrease in bone mineral density (BMD) and an increase in bone fragility, is a major public health issue that affects both men and women around the world (1, 2). The population aged 50 or more who are at high risk of osteoporotic fracture was predicted to be 158 million in 2010, and this number is expected to double by 2040 (3). Bone fractures are connected with significant disability and morbidity, as well as a significant financial burden on injured individuals (4).
Nitrates (isosorbide mononitrate, isosorbide dinitrate, nitroglycerin), which are a type of angina medicine (5), appear to have beneficial effects on bone. These drugs, which act as nitric oxide donors, uncouple bone resorption and formation, resulting in improved bone metabolism (6). Nitric oxide has been shown to regulate osteoclasts, which are responsible for bone resorption (7). Besides, low NO levels have been shown to improve osteogenic proliferation, differentiation, and survival (8). However, higher concentrations inhibit osteoclast differentiation and survival (9). Animal studies have suggested that nitric oxide donors may increase bone mass by regulating osteoblast and osteoclast functions in ovariectomized mice (10). According to two epidemiological studies (11, 12), people who use nitrates had higher BMD and lower rates of bone turnover. However, one cohort study found no evidence that nitrate use was related to a decreased incidence of fractures or a higher BMD (13). Furthermore, the results of two randomized controlled trials (RCTs) that examined the effects of nitroglycerin ointment on BMD were contradictory (14–16).
Recently, several clinical trials evaluating the efficacy of nitrates for bone health have been reported. To our knowledge, no comprehensive meta-analysis on this topic has been performed. To determine the effect of nitrates on bone health, a comprehensive systematic review and meta-analysis based on an extensive search of observational and randomized controlled trials is required.
Methods
Search Strategy
The Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines were used for randomized controlled trials (RCTs) (17), and the Meta-Analyses and Systematic Reviews of Observational Studies guidelines were used for observational studies (18). Two independent reviewers (Liu and Wang) systematically searched PubMed, EMBASE database, Cochrane Library for relevant articles published before December 2021. An experienced librarian was consulted to generate a list of keywords and MeSH terms to conduct the search. The detailed search strategies are described in the Supplementary Table 1. Additional researches were discovered by searching the references of relevant research and review publications.
Selection Criteria
Eligible studies were included if they fulfilled the following criteria (1): cohort studies, case-control studies, or randomized controlled trials (2), reported on bone mineral density (BMD), incident fractures with nitrates use (3), the reference group were non- nitrates users (3), studies provided adequate data for the efficacy estimates. The exclusion criteria were as follows (1): duplicate articles (2), molecular biology or animal research, and (3) reviews, case reports, letters, editorials, and meta-analyses. Two investigators (Liu and Wang) independently screened the articles by title and abstract after removing duplicate articles. Then, the full texts were obtained to identify the eligible studies. Disagreements in the study selection process were fully discussed and resolved through consultation with Meng.
Data Extraction and Quality Assessment
The following information was extracted from each study: the first author’s name, the year of publication, the study design, the country, the interventions and co-interventions, the sample size, age, BMD, the duration of follow-up, and reported outcomes, including effect sizes (risk ratios (RRs), odds ratios (ORs), hazard ratios (HRs), BMD percent change, or BMD change) and adverse events. We extract the reported outcomes of the final time point for RCTs. If standard deviations were not reported, we used the confidence intervals to calculate the standard deviation. We used image extraction software (Engauge Digitizer) to extract data presented only in figures without corresponding numerical data.
We evaluated the quality of included RCTs using the Cochrane Risk of Bias tool (19), the quality of included observational studies was evaluated using the Newcastle–Ottawa Scale (NOS) (20). The data extraction and quality assessments were conducted independently by two authors (Liu and Wang).
Data Analysis
The Stata 12.0 software was used to conduct the analysis. ORs were used as approximations of RRs since the incidence of fracture is so low (less than 5% per year). HRs, ORs, and RRs were extracted from the included studies. The pooled risk ratios (RRs) with 95% confidence intervals (CIs) from HRs and ORs were calculated using a random-effects model. Because most RCTs provided within-group changes in BMD outcomes, we used the reported or computed difference between the nitrates and reference groups as the effect size measure in the meta-analysis for BMD outcomes. We conducted meta-analyses when data from at least two trials were sufficiently homogenous in terms. To measure heterogeneity across trials, the I2 and Q statistics were used. I2 > 50% and P < 0.05 showed high heterogeneity across the studies examined. When significant heterogeneity was detected, subgroup analyses were performed to investigate the reasons for the heterogeneity. The Begger and Egger test was used to assess the publication bias of the studies included in the final analysis.
Results
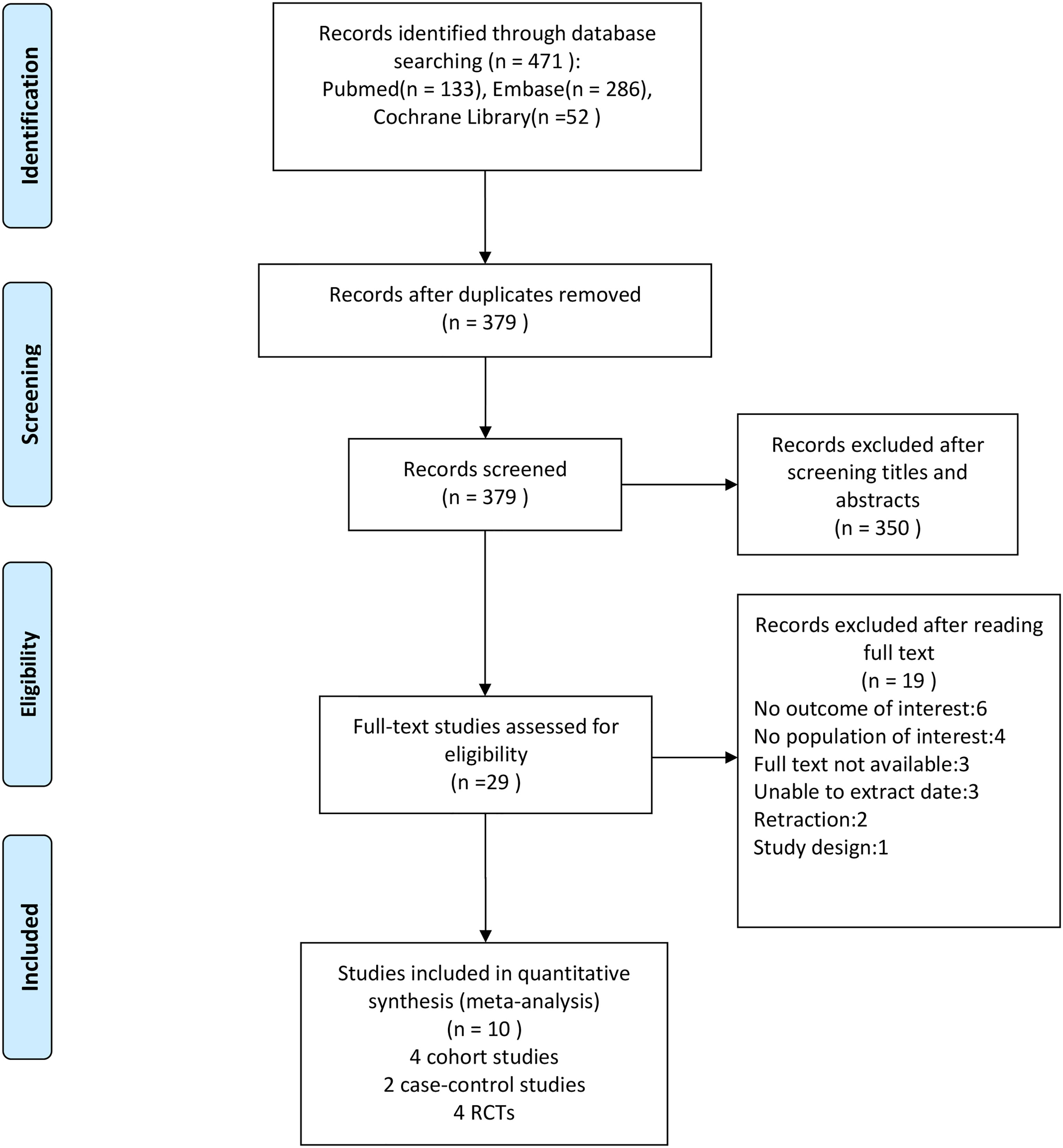
After conducting a literature search, we discovered 471 possibly eligible studies. After removing duplicates from the 471 papers retrieved, 379 were left, with 29 of them being chosen as potentially suitable after reviewing the titles and abstracts. After examining full texts, 10 were included for data extraction in our meta-analysis (four cohort studies, two case-control studies, and four RCTs). The literature search process is illustrated in Figure 1.
Study Characteristics
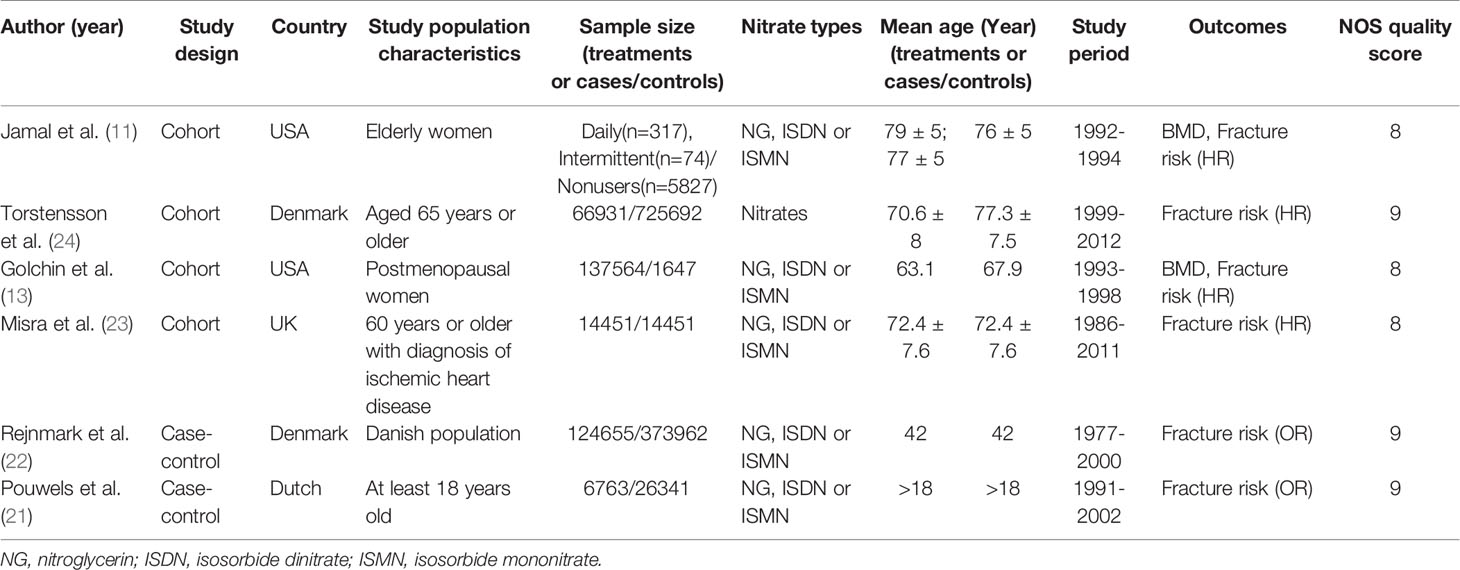
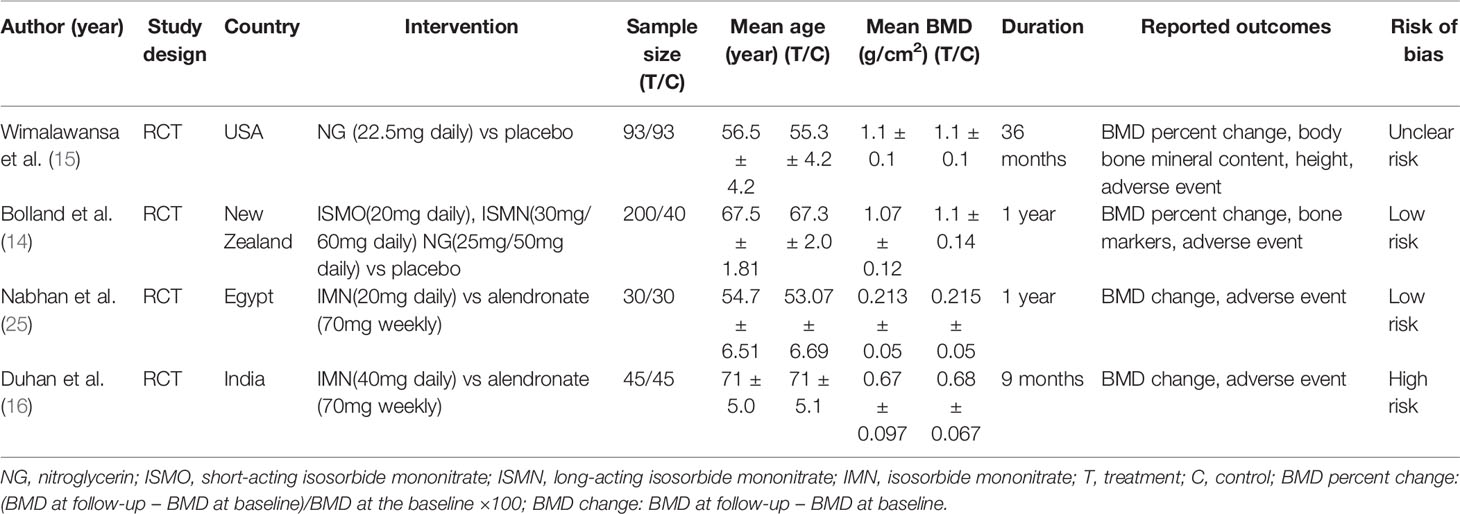
There were 10 (11, 13–16, 21–25) studies included in our meta-analysis. Detailed characteristics of the included studies are presented in Tables 1 and 2. They were published between 1998 and 2020, including four cohort studies, two case-control studies, and four randomized controlled trials. Three studies were conducted in North America, four in Europe, one in Oceania, one in Asia, and one in Africa. Six studies reported BMD, and six studies reported fractures. Besides, two studies compared nitrates with a placebo, and two studies compared nitrates with alendronate. As indicated in Table 1, the NOS scores ranged from eight to nine points, indicating that all the observational studies chosen were of good quality. We classified RCT studies as having a low, uncertain, or high risk of bias (Table 3). There are two studies with a low risk of bias, one study with an uncertain risk of bias, and one study with a high risk of bias.
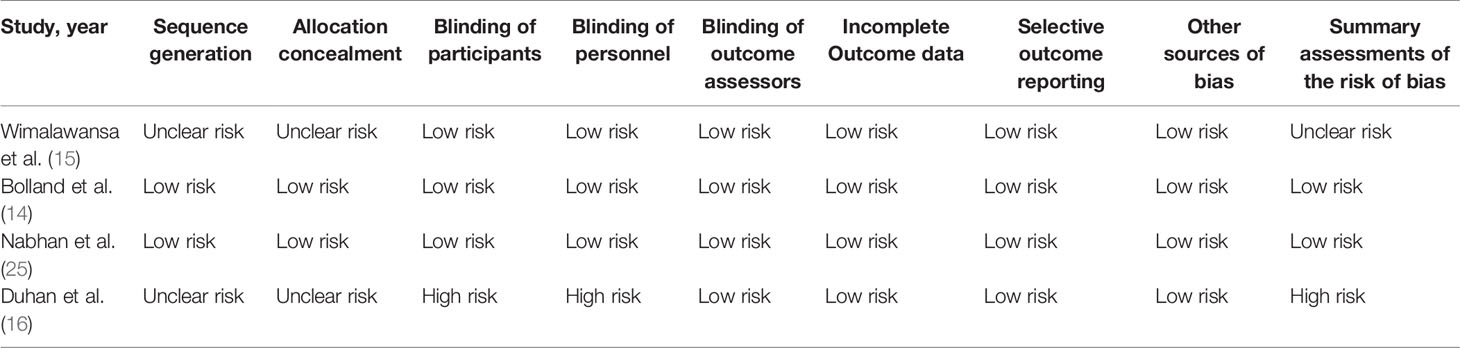
Table 3 Risk of bias of randomized controlled trials evaluating the efficacy of nitrates for bone health.
Main Analysis
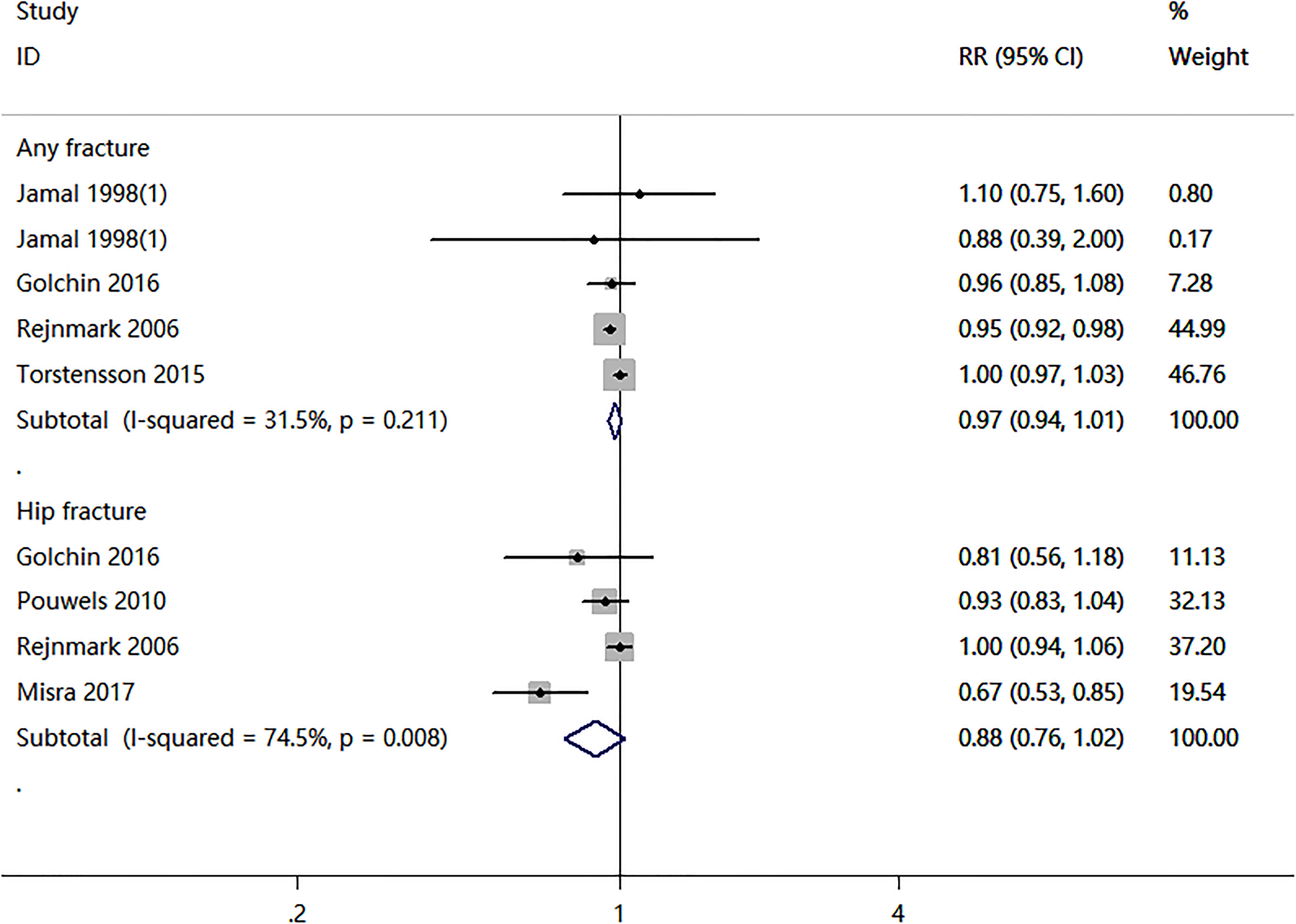
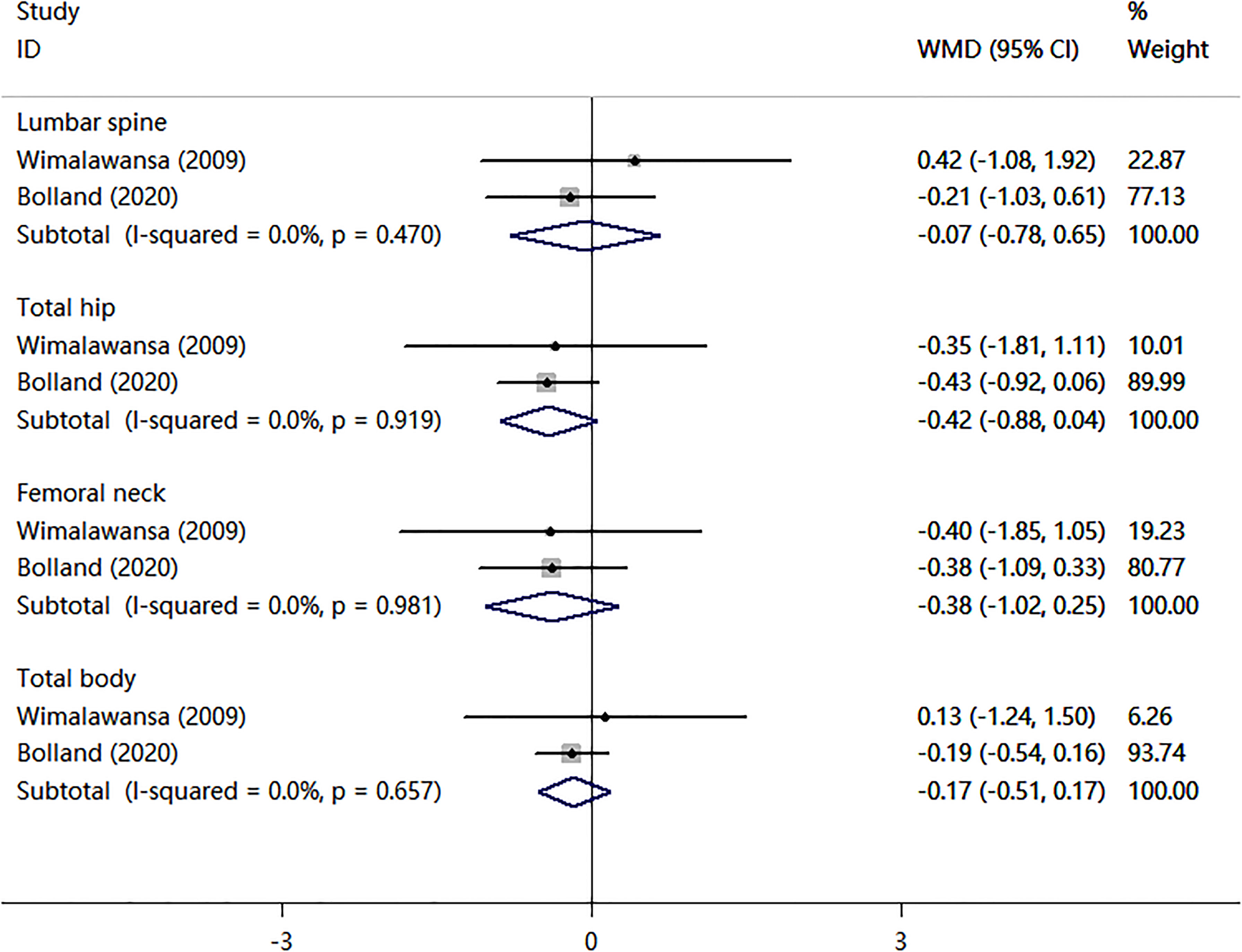
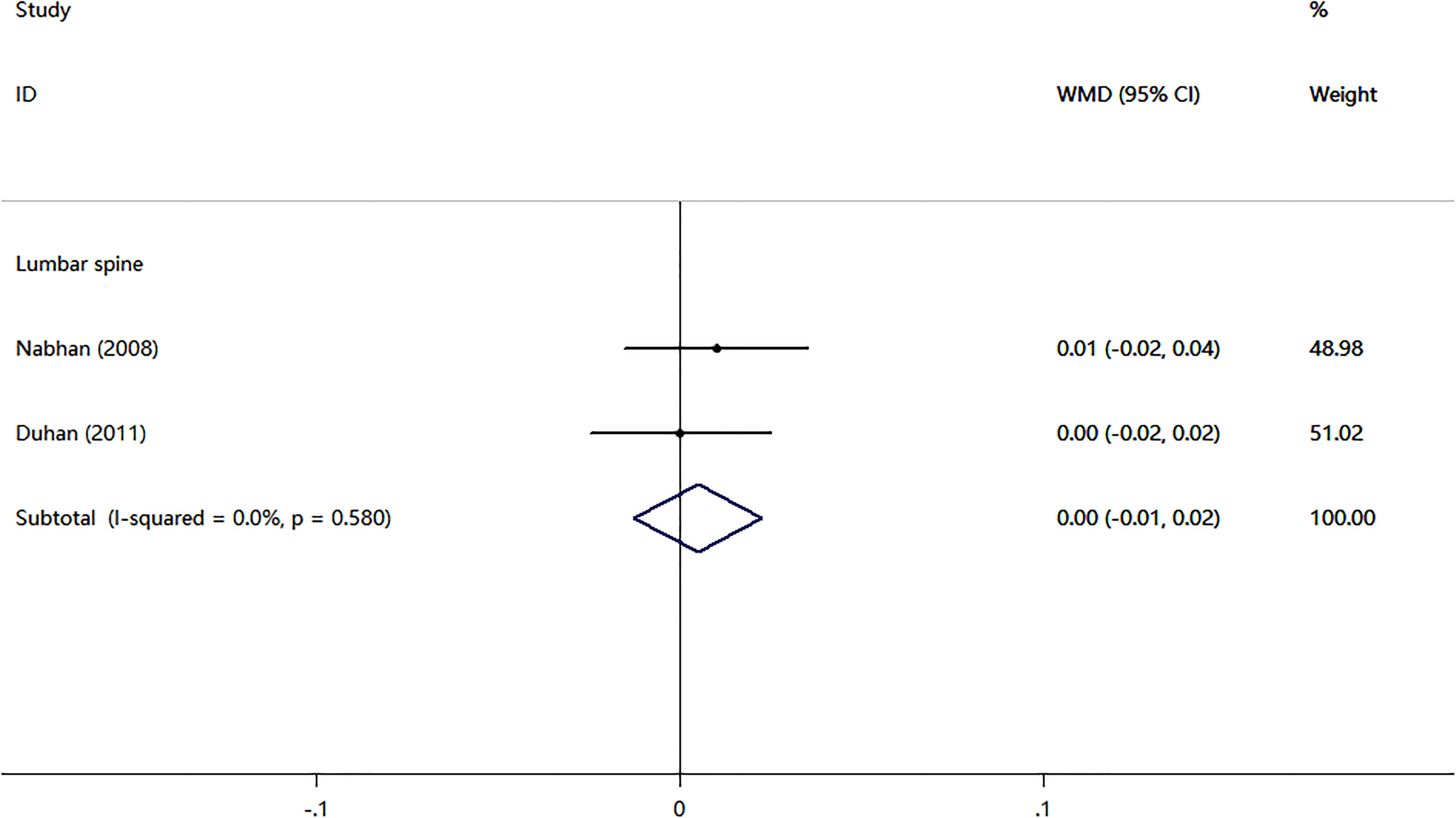
Four cohort studies and two case-control studies examining the association between nitrates use and fractures were identified. As shown in Figure 2, the nitrates use was not associated with any fracture risk (RR = 0.97; 95% CI, 0.94–1.01; I2 = 31.5%) and hip fracture (RR = 0.88; 95% CI, 0.76–1.02; I2 = 74.5%). Two RCTs compared nitrates with a placebo. As shown in Figure 3, there were no statistically significant differences in BMD percent change at lumbar spine (WMD = -0.07, 95% CI,-0.78–0.65; I2 = 0.0%), total hip (WMD=-0.42, 95% CI,-0.88–0.04; I2 = 0.0%), femoral neck (WMD=-0.38, 95% CI,-1.02–0.25; I2 = 0.0%), or total body (WMD = -0.17, 95% CI,-0.51–0.17; I2 = 0.0%). Two randomized controlled trials (RCTs) compared nitrates with alendronate. As shown in Figure 4, nitrates were comparable to alendronate in increasing bone mineral density at lumbar spine (WMD = 0.00, 95% CI,-0.01–0.02; I2 = 0.0%). Four RCTs reported on the adverse events of nitrates use (Table 2). The most common adverse effect was headache (14%–31.1% incidence), contributing to low adherence to therapy. Other adverse effects included palpitations, nausea, flushing, and diaphoresis.
Subgroup Meta-Analyses
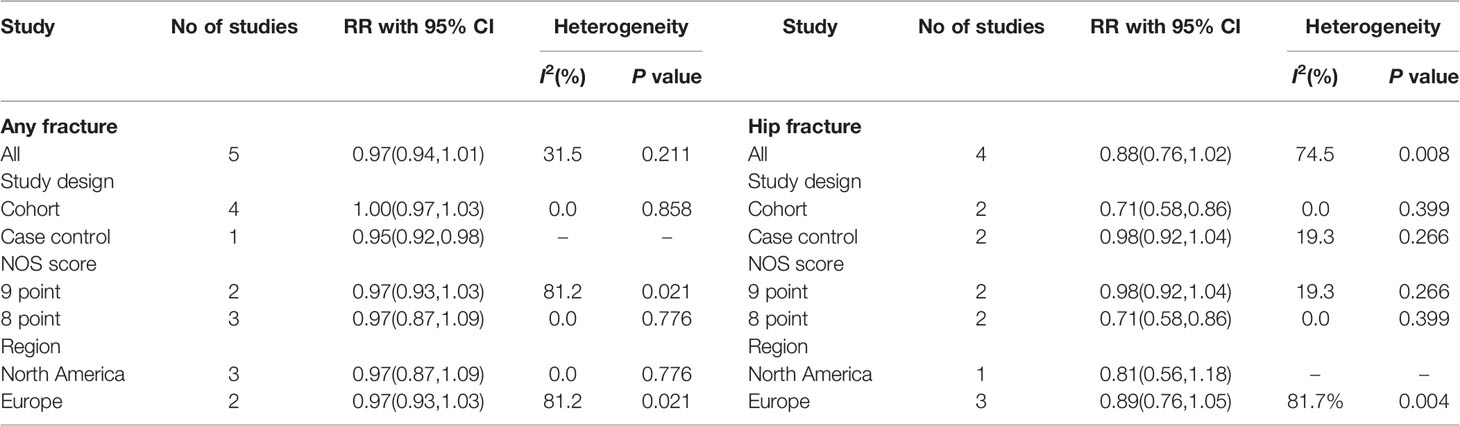
In the subgroup meta-analyses, the risk of fracture is shown in Table 4. When the selected studies for any fracture were grouped by study design, no significant association was seen in the three cohort studies (RR = 1.00; 95% CI, 0.97–1.03; I2 = 0.0%). However, a negative association between the use of nitrates and any fracture risk was found only in one case-control study (RR = 0.95; 95% CI, 0.92–0.98). Two cohort and two case-control studies evaluated the association between nitrates use and hip fracture risk. The overall pooled RR for cohort studies was 0.71 (95%CI: 0.58–0.86, I2 = 0.0%), while the pooled RR for case-control studies was 0.98 (95%CI: 0.92–1.04, I2 = 19.3%).
Grouping of studies by NOS score revealed no significant association between the nitrates use and the any fracture risk in both the 9 point groups (RR = 0.97; 95% CI, 0.93–1.03; I2 = 81.2%) and 8 point groups (RR =0.97; 95% CI, 0.87–1.09; I2 = 0.0%). However, there was a significant association of nitrates with hip fracture in 8 point groups (RR, 0.71; 95% CI, 0.58–0.89; I2 = 0.0%), but no significant association in 9 point groups (RR, 0.98; 95% CI, 0.92–1.04; I2 = 19.3%)
When we grouped studies by region, we found no significant association between the nitrates use and the any fracture risk in North America (RR = 0.97; 95% CI, 0.87–1.09; I2 = 0.0%) and Europe (RR = 0.97; 95% CI, 0.93–1.03; I2 = 81.2%). The pooled RR for the hip fracture risk of North American people with nitrates was 0.81 (95%CI: 0.56–1.18), and the pooled RR for the hip fracture risk of European people with nitrates was 0.89 (95%CI: 0.76–1.05, I2 = 81.7%).
Sensitivity Analysis and Publication Bias
The results of the sensitivity analysis demonstrated the stability of outcomes in meta-analyses(Supplementary Figures 1, 2). No indication of publication bias was found for studies that reported any fracture risk (Begg P = 1.000; Egger P = 0.983) and hip fracture (Begg P = 0.139; Egger P = 0.308) (Supplementary Figures 3, 4).
Discussion
In this meta-analysis of 10 studies, we found that nitrates use was not associated with a reduced risk of any fracture or hip fracture in observational studies. The results of four randomized controlled trials on the effects of nitrates on BMD were inconsistent. There were no statistically significant differences in BMD percent change at any sites in these two RCTs compared with a placebo (14, 15). In contrast, nitrates and alendronate had similar effects in increasing bone BMD in another two RCTs (16, 25).
NO is a short-lived free radical that regulates a variety of physiological processes, including bone remodeling (26). In the acid environment of the stomach, NO can be created nonenzymatically from nitrites. Organic nitrates (nitroglycerin, isosorbide mononitrate, isosorbide dinitrate) can operate as NO donors (27). Intermediate dosages of NO have been demonstrated to improve skeletal health in several studies. However, the benefits of NO supplements on bone mass have been controversial. Numerous in vivo animal studies have demonstrated that NO donors help to decrease bone resorption while also improving bone growth (10, 28, 29). NO appears to have a biphasic effect on bone-forming cells, promoting bone growth at low doses while inhibiting bone formation at higher concentrations (30). Because nitroglycerin has a somewhat narrow therapeutic window for osteoporosis treatment, the proper dose must be employed to get positive BMD results (15). Continuous exposure to nitrates may promote tachyphylaxis in bone, just as it does with angina symptom management. Once-daily treatment of nitroglycerin ointment enhanced BMD in ovariectimized rats, but more frequent application had little effect (31). Based on this potential for tachyphylaxis, randomized controlled trials using once-daily dosing of nitroglycerin ointment would not achieve satisfactory results for bone health. The most well-known study on nitrates found that nitroglycerin improved BMD by 6% to 7% at all sites over 24 months, with significant increases in markers of bone formation and decreases in markers of bone resorption, but the study was retracted five years later (32). Another observational study (33) reported that nitrate use was associated with increased BMD at the hip and spine in men and women. It was also retracted. Two articles about the results of nitrates and alendronate have similar effects in increasing bone BMD and should be carefully considered. More randomized control trials are needed to determine the effects of nitrates on bone health.
Our meta-analysis has several strengths. This meta-review was the first to review the efficacy of nitrates for bone health. In addition, it examined the associations stratified by the type of fracture, the study design, NOS score, and region. However, our meta-analysis has some limitations as well. First, due to the small number of RCT studies, the results of our meta-analysis of RCTs are highly heterogeneous. Second, we may have missed unpublished studies and those that were not in English, resulting in an overestimation of the efficacy of these treatments. Third, we were unable to conduct a meta-analysis on adverse events, because many studies failed to report different adverse events.
Conclusion
This meta-analysis of observational data found no association between nitrate use and fracture risk. The results of RCTs on the usage of nitrates and their effects on BMD are contradictory. Further well-designed trials confirming their benefit for bone health are required before it can be recommended for routine use.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
WL and GW designed the study and collected the data. WL drafted the manuscript. ZM contributed to the writing. GW provided critical feedback and contributed to the review of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This study received no particular support from governmental, private, or non-profit funding agencies.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.833932/full#supplementary-material
References
1. Cauley JA. Public Health Impact of Osteoporosis. J Gerontol A Biol Sci Med Sci (2013) 68(10):1243–51. doi: 10.1093/gerona/glt093
2. Kanis JA, Melton LJ 3rd, Christiansen C, Johnston CC, Khaltaev N. The Diagnosis of Osteoporosis. J Bone Miner Res (1994) 9(8):1137–41. doi: 10.1002/jbmr.5650090802
3. Oden A, McCloskey EV, Kanis JA, Harvey NC, Johansson H. Burden of High Fracture Probability Worldwide: Secular Increases 2010-2040. Osteoporos Int (2015) 26(9):2243–8. doi: 10.1007/s00198-015-3154-6
4. Johnell O, Kanis JA. An Estimate of the Worldwide Prevalence and Disability Associated With Osteoporotic Fractures. Osteoporos Int (2006) 17(12):1726–33. doi: 10.1007/s00198-006-0172-4
5. Divakaran S, Loscalzo J. The Role of Nitroglycerin and Other Nitrogen Oxides in Cardiovascular Therapeutics. J Am Coll Cardiol (2017) 70(19):2393–410. doi: 10.1016/j.jacc.2017.09.1064
6. Wimalawansa SJ. Nitric Oxide and Bone. Ann NY Acad Sci (2010) 1192:391–403. doi: 10.1111/j.1749-6632.2009.05230.x
7. Dong SS, Williams JP, Jordan SE, Cornwell T, Blair HC. Nitric Oxide Regulation of cGMP Production in Osteoclasts. J Cell Biochem (1999) 73(4):478–87. doi: 10.1002/(SICI)1097-4644(19990615)73:4<478::AID-JCB6>3.0.CO;2-T
8. Kalyanaraman H, Schall N, Pilz RB. Nitric Oxide and Cyclic GMP Functions in Bone. Nitric Oxide (2018) 76:62–70. doi: 10.1016/j.niox.2018.03.007
9. Mancini L, Moradi-Bidhendi N, Becherini L, Martineti V, MacIntyre I. The Biphasic Effects of Nitric Oxide in Primary Rat Osteoblasts are cGMP Dependent. Biochem Biophys Res Commun (2000) 274(2):477–81. doi: 10.1006/bbrc.2000.3164
10. Kalyanaraman H, Ramdani G, Joshua J, Schall N, Boss GR, Cory E, et al. A Novel, Direct NO Donor Regulates Osteoblast and Osteoclast Functions and Increases Bone Mass in Ovariectomized Mice. J Bone Miner Res (2017) 32(1):46–59. doi: 10.1002/jbmr.2909
11. Jamal SA, Browner WS, Bauer DC, Cummings SR. Intermittent Use of Nitrates Increases Bone Mineral Density: The Study of Osteoporotic Fractures. J Bone Miner Res Off J Am Soc Bone Miner Res (1998) 13(11):1755–9. doi: 10.1359/jbmr.1998.13.11.1755
12. Jamal SA, Cummings SR, Hawker GA. Isosorbide Mononitrate Increases Bone Formation and Decreases Bone Resorption in Postmenopausal Women: A Randomized Trial. J Bone Miner Res Off J Am Soc Bone Miner Res (2004) 19(9):1512–7. doi: 10.1359/JBMR.040716
13. Golchin N, Hohensee C, LaCroix A, Gray SL. Nitrate Medications, Fractures, and Change in Bone Mineral Density in Postmenopausal Women: Results From the Women's Health Initiative. J Bone Miner Res Off J Am Soc Bone Miner Res (2016) 31(9):1760–6. doi: 10.1002/jbmr.2838
14. Bolland MJ, House ME, Horne AM, Pinel V, Gamble GD, Grey A, et al. Nitrates Do Not Affect Bone Density or Bone Turnover in Postmenopausal Women: A Randomized Controlled Trial. J Bone Miner Res Off J Am Soc Bone Miner Res (2020) 35(6):1040–7. doi: 10.1002/jbmr.3982
15. Wimalawansa SJ, Grimes JP, Wilson AC, Hoover DR. Transdermal Nitroglycerin Therapy may Not Prevent Early Postmenopausal Bone Loss. J Clin Endocrinol Metab (2009) 94(9):3356–64. doi: 10.1210/jc.2008-2225
16. Duhan N, Siwach RC, Yadav K, Dahiya K, Nanda S, Sirohiwal D. Comparative Evaluation of Isosorbide Mononitrate and Alendronate in Management of Postmenopausal Osteoporosis. Arch Gynecol Obstet (2012) 285(4):1019–23. doi: 10.1007/s00404-011-2095-3
17. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ (2009) 339:b2700. doi: 10.1136/bmj.b2700
18. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, et al. Meta-Analysis of Observational Studies in Epidemiology: A Proposal for Reporting. Meta-Analysis Of Observational Studies in Epidemiology (MOOSE) Group. Jama (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
19. Higgins J, Thomas J. Cochrane Handbook for Systemic Reviews of Interventions. Version 6.2. The Cochrane Collaboration (2021). Available from: http://handbook.cochrane.org/.
20. Stang A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
21. Pouwels S, Lalmohamed A, van Staa T, Cooper C, Souverein P, Leufkens HG, et al. Use of Organic Nitrates and the Risk of Hip Fracture: A Population-Based Case-Control Study. J Clin Endocrinol Metab (2010) 95(4):1924–31. doi: 10.1210/jc.2009-2342
22. Rejnmark L, Vestergaard P, Mosekilde L. Decreased Fracture Risk in Users of Organic Nitrates: A Nationwide Case-Control Study. J Bone Miner Res Off J Am Soc Bone Miner Res (2006) 21(11):1811–7. doi: 10.1359/jbmr.060804
23. Misra D, Peloquin C, Kiel DP, Neogi T, Lu N, Zhang Y. Intermittent Nitrate Use and Risk of Hip Fracture. Am J Med (2017) 130(2):229.e15– e20. doi: 10.1016/j.amjmed.2016.09.006
24. Torstensson M, Hansen AH, Leth-Moller K, Jorgensen TS, Sahlberg M, Andersson C, et al. Danish Register-Based Study on the Association Between Specific Cardiovascular Drugs and Fragility Fractures. BMJ Open (2015) 5(12):e009522. doi: 10.1136/bmjopen-2015-009522
25. Nabhan AF, Rabie NH. Isosorbide Mononitrate Versus Alendronate for Postmenopausal Osteoporosis. Int J Gynaecol Obstetrics: Off Organ Int Fed Gynaecol Obstet (2008) 103(3):213–6. doi: 10.1016/j.ijgo.2008.07.011
26. Chae HJ, Park RK, Chung HT, Kang JS, Kim MS, Choi DY, et al. Nitric Oxide Is a Regulator of Bone Remodelling. J Pharm Pharmacol (1997) 49(9):897–902. doi: 10.1111/j.2042-7158.1997.tb06132.x
27. Thatcher GR, Nicolescu AC, Bennett BM, Toader V. Nitrates and NO Release: Contemporary Aspects in Biological and Medicinal Chemistry. Free Radic Biol Med (2004) 37(8):1122–43. doi: 10.1016/j.freeradbiomed.2004.06.013
28. Wimalawansa SJ, De Marco G, Gangula P, Yallampalli C. Nitric Oxide Donor Alleviates Ovariectomy-Induced Bone Loss. Bone (1996) 18(4):301–4. doi: 10.1016/8756-3282(96)00005-1
29. Wimalawansa SJ. Restoration of Ovariectomy-Induced Osteopenia by Nitroglycerin. Calcif Tissue Int (2000) 66(1):56–60. doi: 10.1007/s002230050011
30. Wimalawansa SJ. Rationale for Using Nitric Oxide Donor Therapy for Prevention of Bone Loss and Treatment of Osteoporosis in Humans. Ann NY Acad Sci (2007) 1117:283–97. doi: 10.1196/annals.1402.066
31. Wimalawansa S, Chapa T, Fang L, Yallampalli C, Simmons D, Wimalawansa S. Frequency-Dependent Effect of Nitric Oxide Donor Nitroglycerin on Bone. J Bone Miner Res Off J Am Soc Bone Miner Res (2000) 15(6):1119–25. doi: 10.1359/jbmr.2000.15.6.1119
32. Jamal SA, Hamilton CJ, Eastell R, Cummings SR. Effect of Nitroglycerin Ointment on Bone Density and Strength in Postmenopausal Women: A Randomized Trial. JAMA (2011) 305(8):800–7. Notice of Retraction. doi: 10.1001/jama.2011.176
33. Jamal SA, Goltzman D, Hanley DA, Papaioannou A, Prior JC, Josse RG. Nitrate Use and Changes in Bone Mineral Density: The Canadian Multicentre Osteoporosis Study. Osteoporosis Int J Established as Result Cooperation Between Eur Found Osteoporosis Natl Osteoporosis Found USA (2009) 20(5):737–44. doi: 10.1007/s00198-008-0727-7
Keywords: nitrates, bone health, fracture, bone mineral density, meta-analysis
Citation: Liu W, Meng Z and Wang G (2022) The Efficacy of Nitrates for Bone Health: A Systematic Review and Meta-Analysis of Observational and Randomized Controlled Studies. Front. Endocrinol. 13:833932. doi: 10.3389/fendo.2022.833932
Received: 12 December 2021; Accepted: 06 January 2022;
Published: 10 February 2022.
Edited by:
Zhi-Feng Sheng, Central South University, ChinaReviewed by:
Fei Xing, Sichuan University, ChinaFei-Long Wei, Fourth Military Medical University (Air Force Medical University), China
Connie M Weaver, Purdue University, United States
Copyright © 2022 Liu, Meng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ge Wang, 13888563080@139.com
 Weibing Liu
Weibing Liu Zhuoran Meng2
Zhuoran Meng2