- 1Department of Pancreatic Surgery, West China Hospital, Sichuan University, Chengdu, China
- 2The First Clinical College, Chongqing Medical University, Chongqing, China
Background: Small non-functional neuroendocrine tumors (NF-PNETs) are a heterogeneous subset of tumors with controversy regarding their optimal management. We aimed to analyze the prognostic factors of patients with small NF-PNETs and create a risk score for lymph node metastasis (LNM).
Methods: Data of 751 patients with NF-PNETs ≤ 2 cm were obtained from the Surveillance, Epidemiology, and End Results (SEER) database. Multivariate survival analysis was performed to analyze the prognostic factors. Logistic regression was used to identify risk factors for LNM.
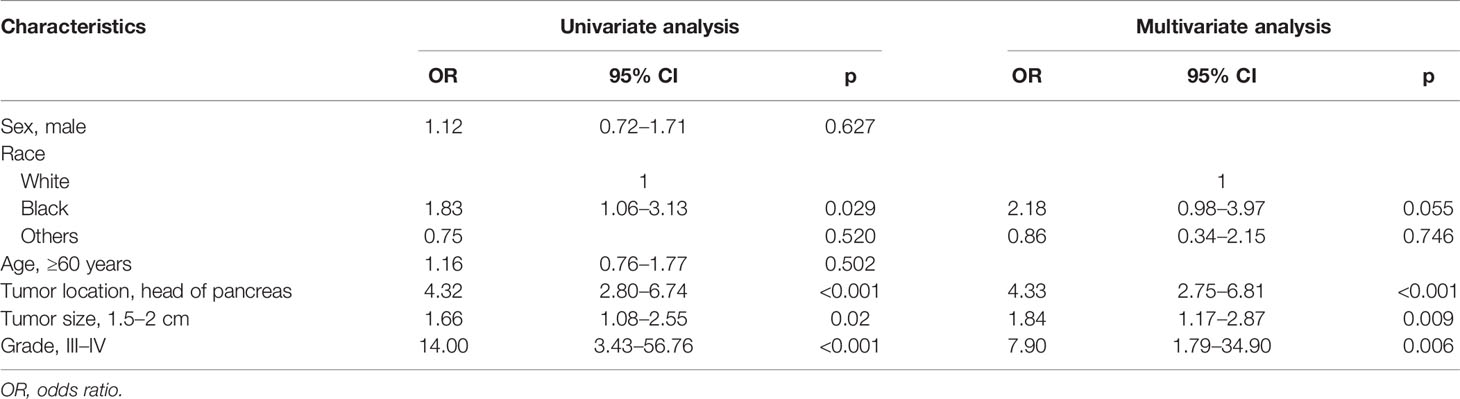
Results: Of the 751 patients, 99 (13.2%) were confirmed to have LNM. In multivariate survival analysis, LNM (hazard ratio [HR], 2.12; 95% CI, 1.04–4.32, p = 0.040) was independently associated with disease-specific survival. Logistic regression identified that tumor location in the head of the pancreas (odds ratio [OR], 4.33; 95% CI, 2.75–6.81; p < 0.001), size ≥ 1.5–2 cm (OR, 1.84; 95% CI, 1.17–2.87; p = 0.009), and grade III–IV (OR, 7.90; 95% CI, 1.79–34.90; p = 0.006) were independent risk factors of LNM. According to the OR value, the risk of LNM was scored as follows: a score of 1 for tumors located in the body/tail of the pancreas and 4 for those located in the head; a score of 1 for tumors <1 cm and 2 for those ≥1.5–2 cm; and a score of 1 for tumors with grade I–II and 8 for those with grade III–IV. Finally, the median score for this cohort was 4, with an interquartile range of 3–6. Therefore, patients were classified as three groups based on the risk score system: a total score of 1–3 for low risk, 4–6 for intermediate risk (OR, 2.98; 95% CI, 1.59–5.60; p = 0.001), and 7–14 for high risk (OR, 8.94; 95% CI, 4.50–17.7; p < 0.001), with an incidence of LNM 5.0%, 13.5%, and 31.8%, respectively (p < 0.001).
Conclusion: Surgical resection with regional lymphadenectomy is recommended for small NF-PNETs with malignant potential of LNM. A risk score for LNM based on tumor grade, location, and size may preoperatively predict LNM of small NF-PNETs and guide clinical practice.
Introduction
Pancreatic neuroendocrine tumors (PNETs) are a group of rare tumors with a variety of clinical manifestations and biological behaviors (1). PNETs can be divided into functional and non-functional tumors. Non-functional PNETs (NF-PNETs) are defined as those without a clinical symptom of hormone overproduction (2). In the past few decades, because of the widespread use of high revolution image technology and elevated attention from both clinicians and radiologists, there is an increased detection of NF-PNETs, especially for small NF-PNETs (≤2 cm) (3, 4).
Surgical resection is the mainstay treatment for PNETs; however, the management of small NF-PNETs remains controversial. Several studies have demonstrated the safety and feasibility of conservative management for asymptomatic sporadic small NF-PNETs (5–7). Considering the relatively high risk of morbidity and mortality in pancreatic surgery as well as the indolent course of small NF-PNETs, both National Comprehensive Cancer Network (NCCN) and European Neuroendocrine Tumor Society (ENETS) guidelines suggest that patients with an asymptomatic small tumor may be selectively observed (2, 8). Nevertheless, results from some surgical cohorts showed that 10%–15% of small NF-PNETs had the malignant potential with regional and distant metastases for which surgery is recommended (9–12). Moreover, although patients with small NF-PNETs generally have a good prognosis after surgery, 4%–6% of recurrences or tumor-related deaths have been observed (9, 13–15). Due to the overall rarity and heterogeneity, the prognostic factors of small NF-PNETs are not well defined.
Lymph node metastasis (LNM) has been proved to be a robust prognostic factor for PNETs. However, data regarding the prognostic value of lymph node status in small NF-PNETs were limited and with contrasting results. Vega et al. found that LNM was an independent predictor of poor disease-specific survival (DSS) and overall survival (9). Data from the National Cancer Database (NCDB) showed that LNM significantly decreased the disease-free survival (DFS) in patients with small NF-PNETs (mean survival: 115 vs. 95 months, p < 0.0001) (14). Conversely, in a European study with 210 resected small NF-PNETs, the presence of positive lymph nodes was not associated with DFS (15). In addition, the indication for regional lymphadenectomy for small NF-PNETs has also been debated. NCCN guidelines recommend that lymphadenectomy may be performed in tumors with a size >1 cm (8). According to the ENETS guideline, lymphadenectomy is confined within tumors larger than 2 cm (2). Recently, a multicenter study reported that patients with NF‐PNETs measuring 1.5–2.0 cm had a much higher risk of LNM than patients with tumors < 1.5 cm (17.9% vs. 8.7%, p = 0.013) for whom lymphadenectomy should be considered (13). To date, tumor size is a major determinant of lymphadenectomy. In terms of the present controversy, more factors are needed to predict LNM and help choose an appropriate strategy for these patients.
Therefore, the primary aim of this study was to analyze the prognostic factor in patients with small NF-PNETs without distant metastasis. The second aim was to explore the risk of LNM and create a risk score based on preoperative factors.
Methods
Study Population and Materials
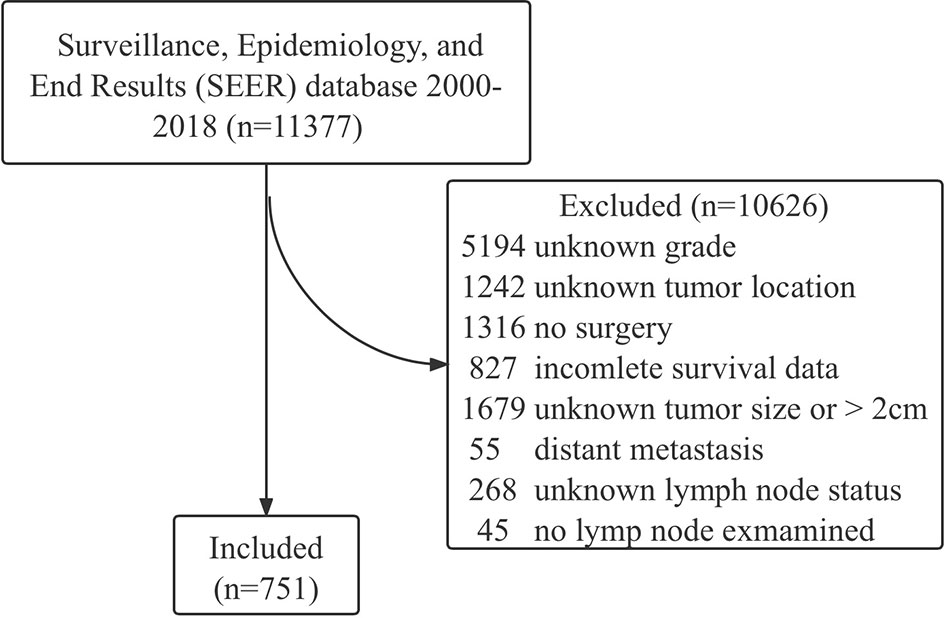
The data of patients with NF-PNETs were obtained from the Surveillance, Epidemiology, and End Results (SEER) database between 2000 and 2018 by SEER stat software (version 8.3.9), and the reference number was 18464-Nov2020. NF-PNETs were classified according to the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) codes: 8150 (pancreatic endocrine tumor), 8240 (carcinoid tumor), 8241 (enterochromaffin cell carcinoid), 8242 (enterochromaffin-like cell tumor), 8243 (goblet cell carcinoid), 8246 (neuroendocrine carcinoma), and 8249 (atypical carcinoid tumor). The inclusion criteria were known grade, known location (head, body, tail), surgery performed, tumor size ≤2 cm, known lymph node status, at least one lymph node were examined, known death cause, and complete survival data. The exclusion criteria were synchronous distant metastasis (M1). The flowchart of patient selection is shown in Figure 1. Variables analyzed in this study included age, sex, race, year of diagnosis, tumor location, tumor grade (I, well differentiated; II, moderately differentiated; III, poorly differentiated; and IV, undifferentiated), tumor size, lymph node status, number of positive and examined lymph nodes, and type of surgery.
This international database study is exempt from institutional review board (local ethics committee of the Sichuan University, West China Hospital) approval.
Statistical Analysis
Categorical variables were summarized using counts and percentages and compared using Pearson’s χ2 test or Fisher’s exact test, as appropriate. DSS was evaluated using the Kaplan–Meier method. Univariate survival analysis was performed by log-rank test. Multivariate survival analysis was conducted by Cox proportional hazards model, with results expressed as hazard ratio (HR) and 95% CI. Univariate and multivariate logistic regression analyses were used to identify preoperatively available factors associated with LNM, with results expressed as odds ratio (OR) and 95% CI. Based on the OR value of multivariate logistic regression analysis, a risk score for preoperatively predicting LNM was established. All statistical analyses were performed by SPSS 26.0 software (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism (version 8.2.1). A p-value <0.05 was considered statistically significant.
Results
Patient Characteristics
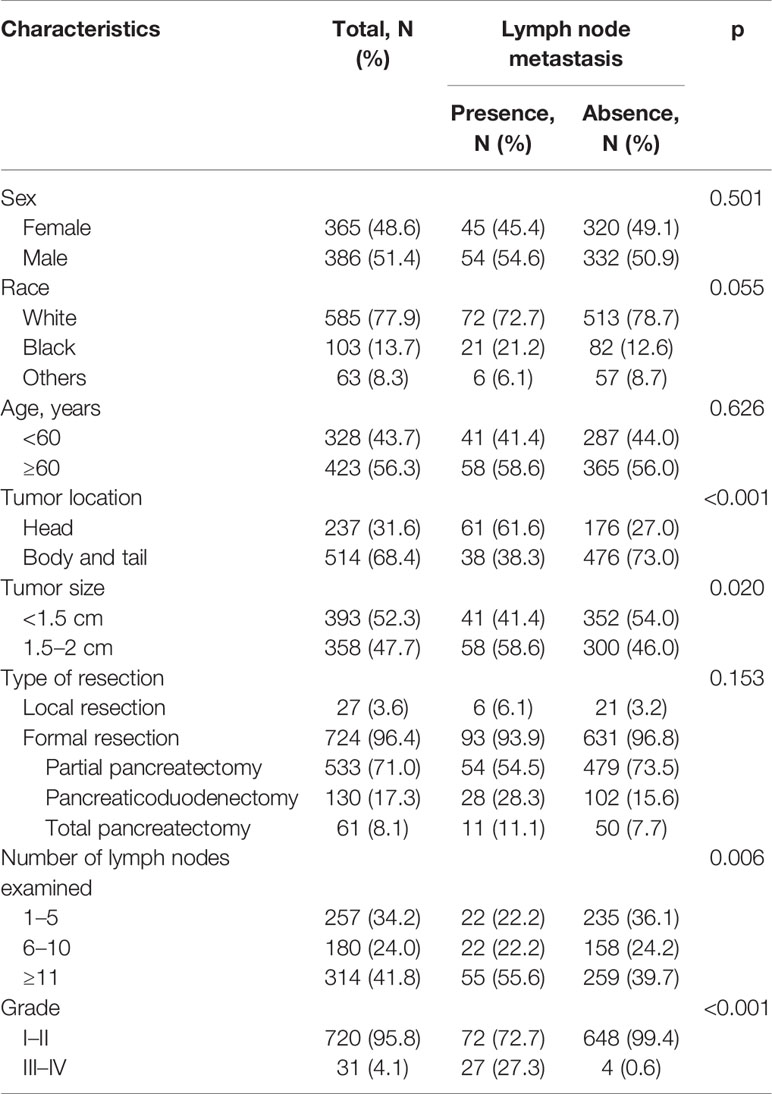
In the SEER database, a total of 751 patients with small NF-PNETs without distant metastasis were identified for analysis (Table 1). There was no sexual difference, and most of the patients were white. Of these, 423 patients (56.3%) with age ≥ 60 years were classified as old people. The majority of patients (68.4%) had a tumor located in the body/tail of the pancreas. The median tumor size was 1.4 cm; thus, the cutoff value was defined as 1.5 cm. The most common surgery type was partial pancreatectomy (71%), followed by pancreaticoduodenectomy (17.3%), total pancreatectomy (8.1%), and local resection (3.6%). The percentages of patients with 1–5, 6–10, and ≥11 examined lymph nodes were 34.2%, 24.0%, and 41.8%, respectively. Most of the patients (95.8%) had a well or moderately differentiated (grade I–II) tumor, while 31 patients (4.1%) had a poorly differentiated or undifferentiated (grade III–IV) tumor.
Of the 751 patients, 99 (13.2%) were confirmed to have LNM. Baseline characteristics for patients with and without LNM were compared (Table 1). The proportion of tumors located in the head of the pancreas was significantly higher in patients with LNM. Patients with LNM were more likely to have a large tumor size (p = 0.020), advanced tumor grade (p < 0.001), and large numbers of lymph nodes examined (p = 0.006). Patients with tumor size measuring 1.5–2 cm had a much higher prevalence of LNM compared with those with tumor size <1.5 cm (16.2% vs. 10.4%; p = 0.020). However, no difference was found in the incidence of LNM between patients with tumor size <1 and 1–1.5 cm (14/154, 9.1% vs. 27/239, 11.2%; p = 0.485).
Survival Analysis
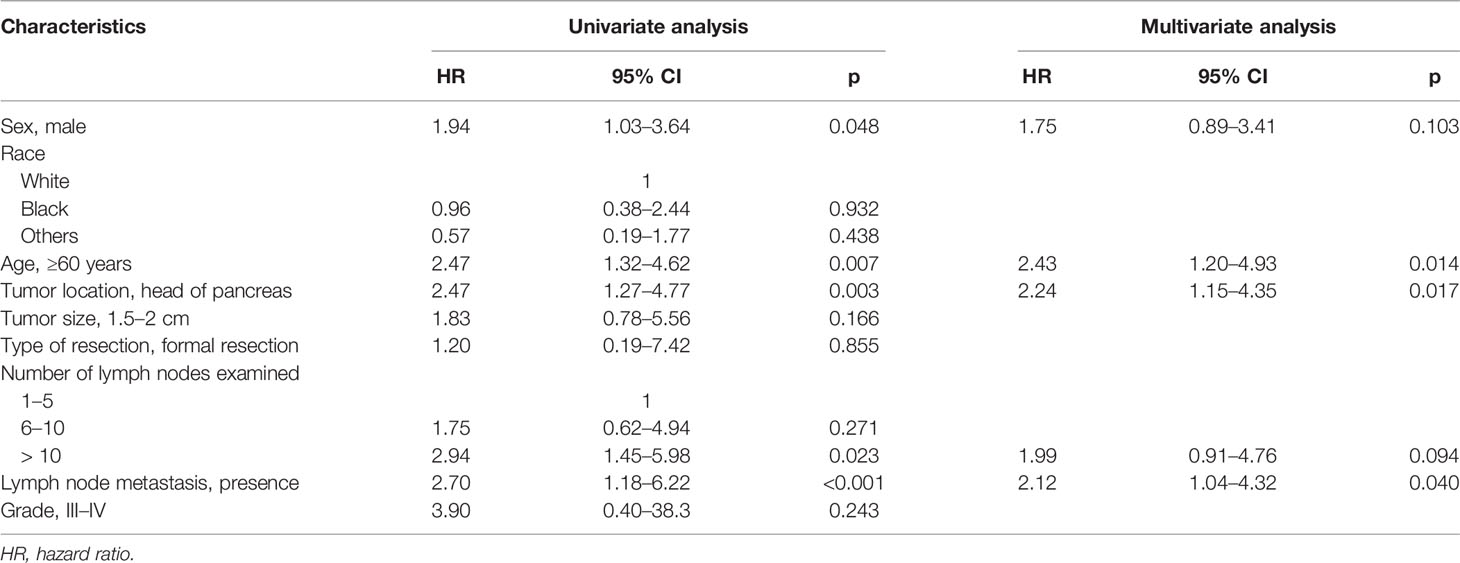
In the 751 patients with small NF-PNETs, 41 disease-specific deaths (5.5%) were observed. The 1-, 5-, and 10-year DSS rates were 97.3%, 94.7%, and 87.1%, respectively. The 5-year DSS rate for patients with and without LNM was 86.7% and 93.4% (p < 0.001, Figure 2A). Moreover, tumor location in the head of the pancreas and old age (≥ 60 years) were associated with a poor DSS (p = 0.003 and p = 0.007, respectively; Figures 2B, C). Univariate survival analysis identified that sex, age, tumor location, number of lymph nodes examined, and LNM were associated with DSS (Table 2). In multivariate survival analysis, LNM (HR, 2.12; 95% CI, 1.04–4.32, p = 0.040) combined with age (HR, 2.43; 95% CI, 1.20–4.93; p = 0. 014) and tumor location (HR, 2.24; 95% CI, 1.15–4.35; p = 0.017) were independently associated with DSS in patients with small NF-PNETs.
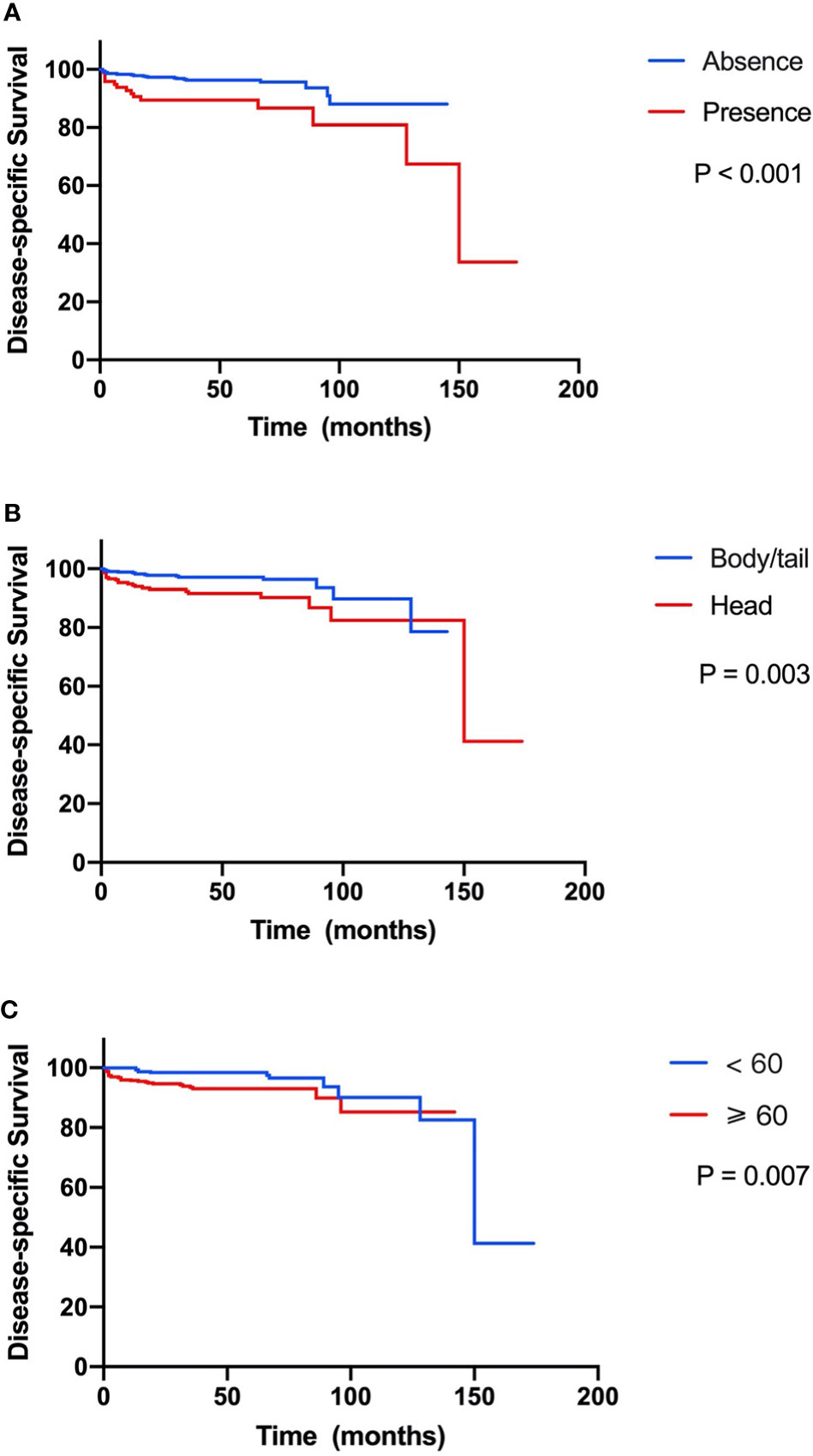
Figure 2 Kaplan–Meier curve of disease-specific survival stratified by prognostic factors. (A) Presence versus absence of lymph node metastasis. (B) Tumor located in head versus body/tail. (C) Age ≥60 versus <60 years.
Risk Factors for Lymph Node Metastasis
In a univariate logistic regression analysis (Table 3), factors including race, tumor location, tumor size, and tumor grade were associated with LNM. Multivariate analysis identified that tumor location in the head of the pancreas (OR, 4.33; 95% CI, 2.75–6.81; p < 0.001), size ≥ 1.5–2 cm (OR, 1.84; 95% CI, 1.17–2.87; p = 0.009), and grade III–IV (OR, 7.90; 95% CI, 1.79–34.90; p = 0.006) were independent risk factors of LNM. According to the OR value of multivariate analysis, the risk of LNM was scored as follows: a score of 1 for tumors located in the body/tail of the pancreas and 4 for those located in the head; a score of 1 for tumors < 1.5 cm and 2 for those ≥ 1.5–2 cm; and a score of 1 for tumors with grade I–II and 8 for those with grade III–IV. Finally, the median score for this cohort was 4, with an interquartile range of 3–6. Therefore, patients were classified into three groups based on the risk score system (Table 4): a total score of 1–3 for low risk, 4–6 for intermediate risk (OR, 2.98; 95% CI, 1.59–5.60; p = 0.001), and 7–14 for high risk (OR, 8.94; 95% CI, 4.50–17.7; p < 0.001). Patients in the high-risk and intermediate-risk groups were nearly 9 and 3 times more likely to develop LNM compared with those in the low-risk group (Figure 3A, p < 0.001). As shown in Table 4, the influence of the number of examined lymph nodes on LNM was also evaluated. Only patients with ≥11 examined lymph nodes were associated with LNM. For patients with 1–5, 6–10, and ≥11 examined lymph nodes, the incidence of LNM was 8.6%, 12.2%, and 17.5% (Figure 3B, p = 0.006), respectively.
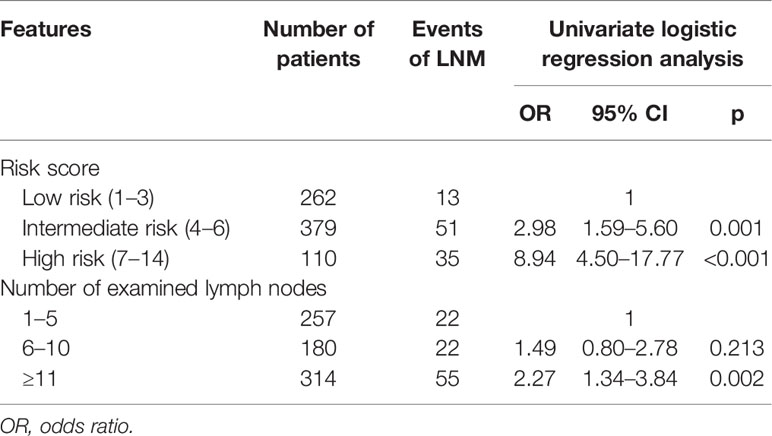
Table 4 The risk for lymph node metastasis (LNM) stratified by risk score and number of examined lymph nodes.
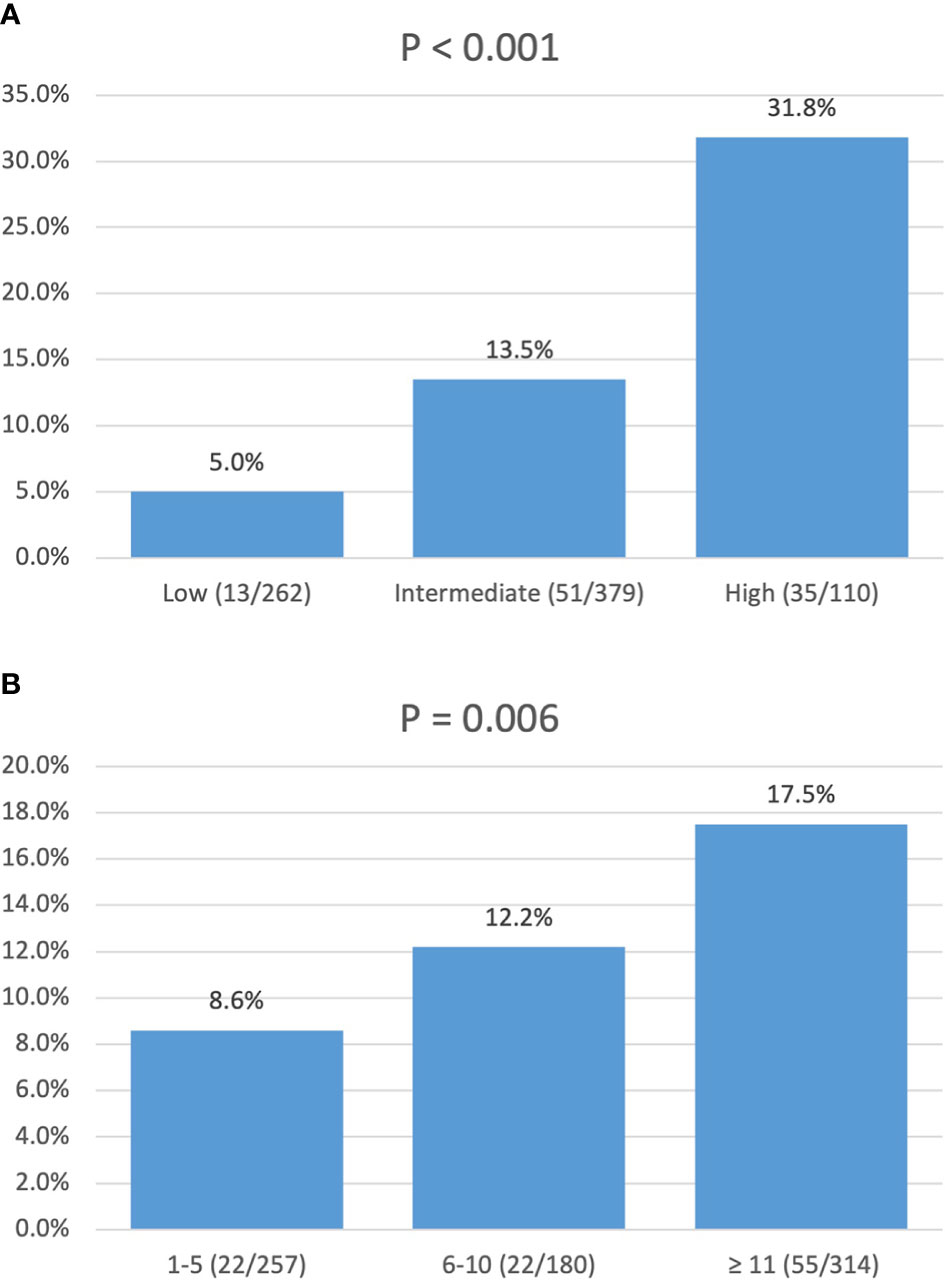
Figure 3 Incidence of lymph node metastasis: (A) stratified by risk score; (B) stratified by number of lymph nodes examined.
Discussion
In this population-level study of 751 patients with resected NF-PNETs less than 2 cm, the overall 1-, 5-, and 10-year DSS rates were 97.3%, 94.7%, and 87.1%, respectively. The incidence of LNM was 13.2 (99/751). Multivariate survival analysis demonstrated that LNM combined with age and tumor location were independently associated with DSS. In a further logistic regression analysis, tumor location in the head of the pancreas, size 1.5–2 cm, and grade III–IV were independent risk factors for LNM. We further created a risk score for LNM of small NF-PNETs based on the OR values from logistic regression analysis and divided all patients into low-, intermediate-, and high-risk groups. The incidence of LNM in low-, intermediate-, and high-risk groups was 5.0%, 13.5%, and 31.8%, which was significantly different and increased with the risk level (Figure 3A, p < 0.001).
Small NF-PNETs are a heterogeneous subset of tumors with controversy regarding their optimal management. On the one hand, surgery has been considered a cornerstone for the management of NF-PNETs. A retrospective analysis from NCDB reported a significantly elevated 5-year overall survival of 82.2% in patients who underwent curative resection compared with a 5-year survival of 27.6% in patients who did not undergo surgery (11). Results from a meta-analysis also showed that an aggressive surgical policy for small NF-PNETs was associated with longer survival, while a watch-and-wait policy did not provide a benefit (16). On the other hand, in terms of the severe and relatively high incidence of complications in pancreatic surgery and the indolent course of small NF-PNET, conservative management has been recently proposed as a possible option. Two meta-analyses (5, 6) and several retrospective studies (7, 17, 18) have shown that a surveillance approach can be safely applied to selective patients with small asymptomatic NF-PNETs. Currently, the main conundrum for the management of small NF-PNETs is to identify patients with a high risk of malignancy.
The standard of selecting patients for surgery versus surveillance needs to be carefully evaluated. A retrospective study has demonstrated that small asymptomatic NF-PNETs have an unpredictable clinical course, and a subset of them may show aggressive behavior (12). A European multicenter study found that the presence of biliary or pancreatic duct dilatation and WHO grade 2–3 was associated with the recurrence of small NF-PNETs, and surgical resection was advocated in patients with these signs (15). In order to ensure the safety of conservative management, the NCCN guideline recommends that observation can be considered for small (<1 cm), low-grade, incidentally discovered tumors (8). Consistently, surveillance is preferred for low-grade, asymptomatic tumors with no suspicious malignancy in ENETS guidelines (2). In the present study, we found that lymph node status, age, and tumor location were independently associated with DSS in patients with small NF-PNETs, which implies that patients with a high risk of LNM, old age, and tumor located in the head of the pancreas may not be good candidates for observation. Nevertheless, for patients with old age or tumors located in the head of the pancreas, the morbidity of surgery should be taken into consideration. Enucleation (and regional lymphadenectomy), which has been proved to achieve the completable oncological outcomes compared with formal resections by previous studies (19–21) and our present data (p = 0.885), may be applied to these patients, when appropriate.
The reported incidence of LNM in small NF-PNETs varies from 2.6% to 27.5% (13–15, 22, 23). In our data, after the exclusion of patients with distant metastasis, the incidence of LNM was 9.9% in 751 patients with at least one examined lymph node. In line with most previous studies (9, 13, 14, 23), LNM was associated with the poor prognosis of small NF-PNETs in our study, suggesting the necessity of surgical resection and regional lymphadenectomy for patients with a high risk of LNM. Factors including age (9), tumor size (13), Ki-67 index (23), and lymphovascular invasion (14) have been considered predictors of LNM. In the present study, we focused on the factors that are available preoperatively. Of all the assessed factors, tumor grade III–IV was most highly associated with LNM (OR 7.90), although the proportion of these patients was relatively low. Tumor grade has been considered the main determinant of the malignancy of NF-PNETs, which is associated with not only the metastasis potential (9, 14) but also the long-term survival (15, 24). With the support of developed image technology, fine-needle aspiration biopsy (guided by endoscopic ultrasound (EUS), US, or CT) is recommended to evaluate the tumor grade preoperatively. Tumor location is another associated factor for LNM, with an OR of 4.33. Similar to our finding, Mei et al. found that tumors located in the pancreatic head were more likely to have LNM compared with those in the body/tail (42.8% vs. 30.9%, p < 0.001) and were associated with poor survival (25). The different embryological origins of the head and body/tail of the pancreas may partly contribute to these differences. As proved by previous studies (13, 14), tumor size was also related to the LNM of small NF-PNETs in this study. There was a significant difference in incidence of LNM (10.4% vs. 16.2%, p = 0.02) between tumors <1.5 cm and ≥1.5–2 cm. However, no difference was found in the incidence of LNM between patients with tumor size <1 and 1–1.5 cm. These results may give a reference to determine the therapeutic management of tumors between 1 and 2 cm. Based on three preoperatively available factors including tumor grade, location, and size, we created a risk score for LNM, which may be utilized to guide clinical practice and help choose an optimal strategy for the management of small NF-PNETs.
Although previous studies (26, 27) and our present data have shown that the detection rate of LNM is increasing with the number of examined lymph nodes, the minimal number of lymph nodes to be harvested for accurate nodal staging remains unclear. During pancreaticoduodenectomy, the number of harvested lymph nodes is generally adequate for an appropriate nodal staging. For distal pancreatectomy, Lopez-Aguiar et al. found that 7 or more lymph nodes should be examined for accurate staging (28), while a minimal number of 12 lymph nodes was suggested by Guarneri et al. (29). Moreover, the role of lymphadenectomy in organ-preserving surgeries such as enucleation and central pancreatectomy is undefined. In this study, we found that patients with ≥11 examined lymph nodes were more likely to have at least one positive node. However, more high-quality prospective studies are needed to determine the minimal number of lymph nodes in surgery for PNETs.
The strength of our findings is the population-level and long-term survival outcomes. However, there were several limitations in our study. The main limitations were the retrospective nature and the possible selection bias. All patients enrolled in this study have undergone surgery, which may lead to an overestimate of the malignant potential of small NF-PNETs. Furthermore, the SEER database does not collect information on recurrence; therefore, the primary end-point was DSS in our study. In addition, some tumor-related variables such as the Ki-67 index, vascular and perineural invasion, and postoperative complications are not available.
Conclusion
Patients with small NF-PNETs have a favorable prognosis after surgery; however, a subset of them may show the malignant potential of LNM. Lymph node status combined with age and tumor location was associated with DSS in patients with small NF-PNETs. Surgical resection with regional lymphadenectomy is recommended for small NF-PNETs with malignant potential of LNM. A risk score for LNM based on tumor grade, location, and size may preoperatively predict LNM of small NF-PNETs and guide clinical practice.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. The ethics committee waived the requirement of written informed consent for participation.
Author Contributions
QT: conception, data collection, manuscript drafting, and editing. XW: conception, data analysis, manuscript drafting, and editing. YCL: data collection and analysis, and manuscript editing. YYL: data collection and analysis, and manuscript editing. XL: resources, supervision, and manuscript review and editing. NK: conception, resources, and manuscript review and editing. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This research was supported by Sichuan Province Science and Technology Planning Project (2020YFS0262), West China Hospital Clinical Research Incubation Project (21HXFH058), and the 1·3·5 Project for Disciplines of Excellence–Clinical Research Incubation Project of West China Hospital, Sichuan University (ZY2017302 and ZYJC21037).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.907415/full#supplementary-material
References
1. Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic Neuroendocrine Tumors (PNETs): Incidence, Prognosis and Recent Trend Toward Improved Survival. Ann Oncol (2008) 19:1727–33. doi: 10.1093/annonc/mdn351
2. Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, et al. ENETS Consensus Guidelines Update for the Management of Patients With Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology (2016) 103:153–71. doi: 10.1159/000443171
3. Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol (2017) 3:1335–42. doi: 10.1001/jamaoncol.2017.0589
4. Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the Rising Incidence of Neuroendocrine Tumors: A Population-Based Analysis of Epidemiology, Metastatic Presentation, and Outcomes. Cancer (2015) 121:589–97. doi: 10.1002/cncr.29099
5. Partelli S, Cirocchi R, Crippa S, Cardinali L, Fendrich V, Bartsch DK, et al. Systematic Review of Active Surveillance Versus Surgical Management of Asymptomatic Small Non-Functioning Pancreatic Neuroendocrine Neoplasms. Br J Surg (2017) 104:34–41. doi: 10.1002/bjs.10312
6. Sallinen V, Le Large TY, Galeev S, Kovalenko Z, Tieftrunk E, Araujo R, et al. Surveillance Strategy for Small Asymptomatic Non-Functional Pancreatic Neuroendocrine Tumors - A Systematic Review and Meta-Analysis. HPB (Oxford) (2017) 19:310–20. doi: 10.1016/j.hpb.2016.12.010
7. Kurita Y, Hara K, Kuwahara T, Mizuno N, Okuno N, Haba S, et al. Comparison of Prognosis Between Observation and Surgical Resection Groups With Small Sporadic Non-Functional Pancreatic Neuroendocrine Neoplasms Without Distant Metastasis. J Gastroenterol (2020) 55:543–52. doi: 10.1007/s00535-019-01655-w
8. Shah MH, Goldner WS, Halfdanarson TR, Bergsland E, Berlin JD, Halperin D, et al. NCCN Guidelines Insights: Neuroendocrine and Adrenal Tumors, Version 2.2018. J Natl Compr Canc Netw (2018) 16:693–702. doi: 10.6004/jnccn.2018.0056
9. Vega EA, Kutlu OC, Alarcon SV, Salehi O, Kazakova V, Kozyreva O, et al. Clinical Prognosticators of Metastatic Potential in Patients With Small Pancreatic Neuroendocrine Tumors. J Gastrointest Surg (2021) 25:2593–9. doi: 10.1007/s11605-021-04946-x
10. Sharpe SM, In H, Winchester DJ, Talamonti MS, Baker MS. Surgical Resection Provides an Overall Survival Benefit for Patients With Small Pancreatic Neuroendocrine Tumors. J Gastrointest Surg (2015) 19:117–23. doi: 10.1007/s11605-014-2615-0
11. Gratian L, Pura J, Dinan M, Roman S, Reed S, Sosa JA. Impact of Extent of Surgery on Survival in Patients With Small Nonfunctional Pancreatic Neuroendocrine Tumors in the United States. Ann Surg Oncol (2014) 21:3515–21. doi: 10.1245/s10434-014-3769-4
12. Haynes AB, Deshpande V, Ingkakul T, Vagefi PA, Szymonifka J, Thayer SP, et al. Implications of Incidentally Discovered, Nonfunctioning Pancreatic Endocrine Tumors: Short-Term and Long-Term Patient Outcomes. Arch Surg (2011) 146:534–8. doi: 10.1001/archsurg.2011.102
13. Dong DH, Zhang XF, Poultsides G, Rocha F, Weber S, Fields R, et al. Impact of Tumor Size and Nodal Status on Recurrence of Nonfunctional Pancreatic Neuroendocrine Tumors </=2 Cm After Curative Resection: A Multi-Institutional Study of 392 Cases. J Surg Oncol (2019) 120:1071–9. doi: 10.1002/jso.25716
14. Blakely AM, Lafaro KJ, Li D, Kessler J, Chang S, Ituarte PHG, et al. Lymphovascular Invasion Predicts Lymph Node Involvement in Small Pancreatic Neuroendocrine Tumors. Neuroendocrinology (2020) 110:384–92. doi: 10.1159/000502581
15. Sallinen VJ, Le Large TYS, Tieftrunk E, Galeev S, Kovalenko Z, Haugvik SP, et al. Prognosis of Sporadic Resected Small (</=2 Cm) Nonfunctional Pancreatic Neuroendocrine Tumors - A Multi-Institutional Study. HPB (Oxford) (2018) 20:251–9. doi: 10.1016/j.hpb.2017.08.034
16. Ricci C, Casadei R, Taffurelli G, Pacilio CA, Campana D, Ambrosini V, et al. Sporadic Small (</=20 Mm) Nonfunctioning Pancreatic Neuroendocrine Neoplasm: Is the Risk of Malignancy Negligible When Adopting a More Conservative Strategy? A Systematic Review and Meta-Analysis. Ann Surg Oncol (2017) 24:2603–10. doi: 10.1245/s10434-017-5946-8
17. Sadot E, Reidy-Lagunes DL, Tang LH, Do RK, Gonen M, D'Angelica MI, et al. Observation Versus Resection for Small Asymptomatic Pancreatic Neuroendocrine Tumors: A Matched Case-Control Study. Ann Surg Oncol (2016) 23:1361–70. doi: 10.1245/s10434-015-4986-1
18. Yohanathan L, Dossa F, St Germain AT, Golbafian F, Moulton CA, McGilvray ID, et al. Management and Surveillance of Non-Functional Pancreatic Neuroendocrine Tumours: Retrospective Review. Pancreatology (2019) 19:360–6. doi: 10.1016/j.pan.2019.02.008
19. Huttner FJ, Koessler-Ebs J, Hackert T, Ulrich A, Buchler MW, Diener MK. Meta-Analysis of Surgical Outcome After Enucleation Versus Standard Resection for Pancreatic Neoplasms. Br J Surg (2015) 102:1026–36. doi: 10.1002/bjs.9819
20. Altimari M, Abad J, Chawla A. The Role of Oncologic Resection and Enucleation for Small Pancreatic Neuroendocrine Tumors. HPB (Oxford) (2021) 23(10):1533–1540. doi: 10.1016/j.hpb.2021.03.005
21. Faitot F, Gaujoux S, Barbier L, Novaes M, Dokmak S, Aussilhou B, et al. Reappraisal of Pancreatic Enucleations: A Single-Center Experience of 126 Procedures. Surgery (2015) 158:201–10. doi: 10.1016/j.surg.2015.03.023
22. Ha S, Song KB, Hong S, Shin D, Park Y, Kwon J, et al. The Clinicopathologic and Operative Characteristics of Patients With Small Nonfunctioning Pancreatic Neuroendocrine Tumors. ANZ J Surg (2021) 91:E484–92. doi: 10.1111/ans.17055
23. Masui T, Sato A, Nakano K, Uchida Y, Yogo A, Anazawa T, et al. Predictive Value of the Ki67 Index for Lymph Node Metastasis of Small Non-Functioning Pancreatic Neuroendocrine Neoplasms. Surg Today (2019) 49:593–600. doi: 10.1007/s00595-019-01779-9
24. Tan QQ, Wang X, Yang L, Chen YH, Tan CL, Ke NW, et al. Predicting Survival in Non-Functional Pancreatic Neuroendocrine Tumours. ANZ J Surg (2020) 90:2026–31. doi: 10.1111/ans.16072
25. Mei W, Ding Y, Wang S, Jia Y, Cao F, Li F. Head and Body/Tail Pancreatic Neuroendocrine Tumors Have Different Biological Characteristics and Clinical Outcomes. J Cancer Res Clin Oncol (2020) 146:3049–61. doi: 10.1007/s00432-020-03303-w
26. Zhang XF, Xue F, Dong DH, Lopez-Aguiar AG, Poultsides G, Makris E, et al. New Nodal Staging for Primary Pancreatic Neuroendocrine Tumors: A Multi-Institutional and National Data Analysis. Ann Surg (2021) 274:e28–35. doi: 10.1097/SLA.0000000000003478
27. Zhang X, Lu L, Liu P, Cao F, Wei Y, Ma L, et al. Predictive Effect of the Total Number of Examined Lymph Nodes on N Staging and Survival in Pancreatic Neuroendocrine Neoplasms. Pancreas (2018) 47:183–9. doi: 10.1097/MPA.0000000000000987
28. Lopez-Aguiar AG, Zaidi MY, Beal EW, Dillhoff M, Cannon JGD, Poultsides GA, et al. Defining the Role of Lymphadenectomy for Pancreatic Neuroendocrine Tumors: An Eight-Institution Study of 695 Patients From the US Neuroendocrine Tumor Study Group. Ann Surg Oncol (2019) 26:2517–24. doi: 10.1245/s10434-019-07367-y
Keywords: small tumors, non-functional, pancreatic neuroendocrine tumors, lymph node metastasis, prognosis, SEER
Citation: Tan Q, Wang X, Li Y, Liu Y, Liu X and Ke N (2022) Prognostic Factors of Small Non-Functional Pancreatic Neuroendocrine Tumors and the Risk of Lymph Node Metastasis: A Population-Level Study. Front. Endocrinol. 13:907415. doi: 10.3389/fendo.2022.907415
Received: 29 March 2022; Accepted: 04 May 2022;
Published: 06 July 2022.
Edited by:
D. Mark Pritchard, University of Liverpool, United KingdomReviewed by:
Krystallenia I. Alexandraki, National and Kapodistrian University of Athens, GreeceAntonio Bianchi, Agostino Gemelli University Polyclinic (IRCCS), Italy
Copyright © 2022 Tan, Wang, Li, Liu, Liu and Ke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xubao Liu, lxb_td@163.com; Nengwen Ke, kenengwen@scu.edu.cn
†These authors have contributed equally to this work and share first authorship
 Qingquan Tan
Qingquan Tan Xing Wang
Xing Wang Yichen Li1
Yichen Li1 Nengwen Ke
Nengwen Ke