- 1Chair and Department of Endocrinology, Jagiellonian University Medical College, Cracow, Poland
- 2Department of Endocrinology, Oncological Endocrinology and Nuclear Medicine, University Hospital, Cracow, Poland
- 3Department of Radiology, University Hospital, Cracow, Poland
- 4Department of Pathomorphology, Jagiellonian University Medical College, Cracow, Poland
- 5Nuclear Medicine Unit, Department of Endocrinology, Oncological Endocrinology and Nuclear Medicine, University Hospital, Cracow, Poland
Background: Adrenal hemorrhage is a rare, usually life-threating complication. The most common neoplasm resulting in spontaneous adrenal bleeding is pheochromocytoma and it accounts for nearly 50% of cases. Currently, the recommendations for the diagnosis and management of patients with adrenal bleeding due to pheochromocytoma are unavailable.
Materials and methods: We performed a database search for all pheochromocytoma patients, diagnosed and treated from 2005 to 2021 in tertiary endocrinology center. 206 patients were identified, 183 with complete data were included in the analysis. We investigated clinicopathological characteristics, treatment and outcomes of hemorrhagic pheochromocytoma cases and characterize our approach to perioperative diagnosis and medical management. Finally our experiences and data from previously published articles concerning adrenal hemorrhage were analyzed to propose a diagnostic and therapeutic algorithm for hemorrhagic pheochromocytomas.
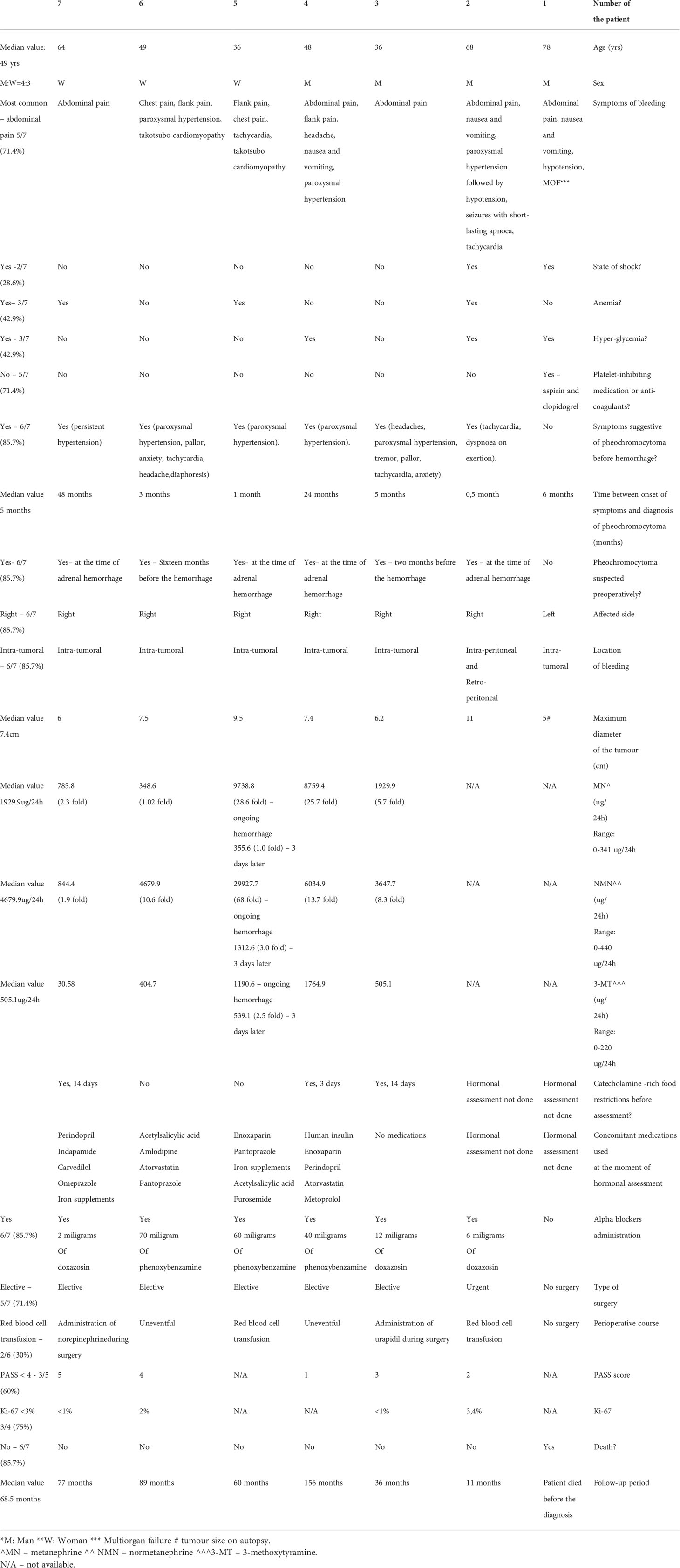
Results: In the whole group, seven patients (4 men and 3 women) with adrenal bleeding were found, (3.8%). Median patient’s age was 49 years (range: 36-78 years). The most common manifestation of adrenal bleeding was acute abdominal pain (5/7). Two patients developed shock. Hormonal assessment was performed in five patients, based on 24-hour urinary fractionated metanephrines with urinary 3-methoxytyramine. Normetanephrine was elevated in all patients, metanephrine and 3-methoxytyramine - in four cases (4/5). Most patients (6/7) had symptoms suggesting pheochromocytoma before hemorrhage – most commonly paroxysmal hypertension (4/7). One patient died, before the diagnosis of adrenal bleeding was made. Diagnostic imaging performed in six out of seven patients revealed adrenal tumor, with median largest diameter equal to 7.4 cm (range: 5-11 cm). Five patients had elective surgery, in one case an urgent surgery was performed. In all cases the diagnosis of pheochromocytoma was confirmed in postoperative histopathology or in autopsy. The perioperative survival rate was 85.7%.
Conclusions: Diagnosis of pheochromocytoma should be always considered in patients with adrenal bleeding, especially with accompanying abdominal pain, hemodynamic shock and previous history of pheochromocytoma-associated symptoms. Lack of proper diagnosis of pheochromocytoma before surgery is associated with an additional perioperative risk. To improve the decision making in this life-threatening clinical situation, based on our results and literature data, we proposed a diagnostic and treatment algorithm.
Introduction
Spontaneous adrenal hemorrhage is a rare, potentially life-threatening condition. According to literature, the most common neoplasm resulting in spontaneous adrenal bleeding is pheochromocytoma, which accounts for nearly 50% of cases (1). The mortality rate of a ruptured pheochromocytoma is reported to be about 30% (2, 3). Hypovolemia from hemorrhagic shock, heart failure as a result of catecholamine excess, postoperative hypotension or pulmonary oedema are leading causes of perioperative mortality (2, 4). Although most cases of hemorrhage within a pheochromocytoma are spontaneous, sometimes episodes of bleeding could be precipitated by preceding anticoagulant therapy (5), trauma (6) or thrombolysis (7). Lately, the first case of hemorrhage in a pheochromocytoma in the course of SARS-CoV-2 infection was described (8). Moreover, adrenal hematoma could sometimes mimic neoplasm of the adrenal gland (1, 9, 10).
Most publications dedicated to a hemorrhagic pheochromocytoma are case reports, with only several series published to date, including four literature reviews and one case series (1–3, 11, 12).
Therefore we reviewed the records of patients with hemorrhagic pheochromocytoma treated in our department. Our objective was to investigate clinicopathological characteristics, treatment and outcomes of hemorrhagic pheochromocytoma cases and characterize our approach to perioperative diagnosis and medical management. Based on our results and literature data we proposed a diagnostic and treatment algorithm.
Materials and methods
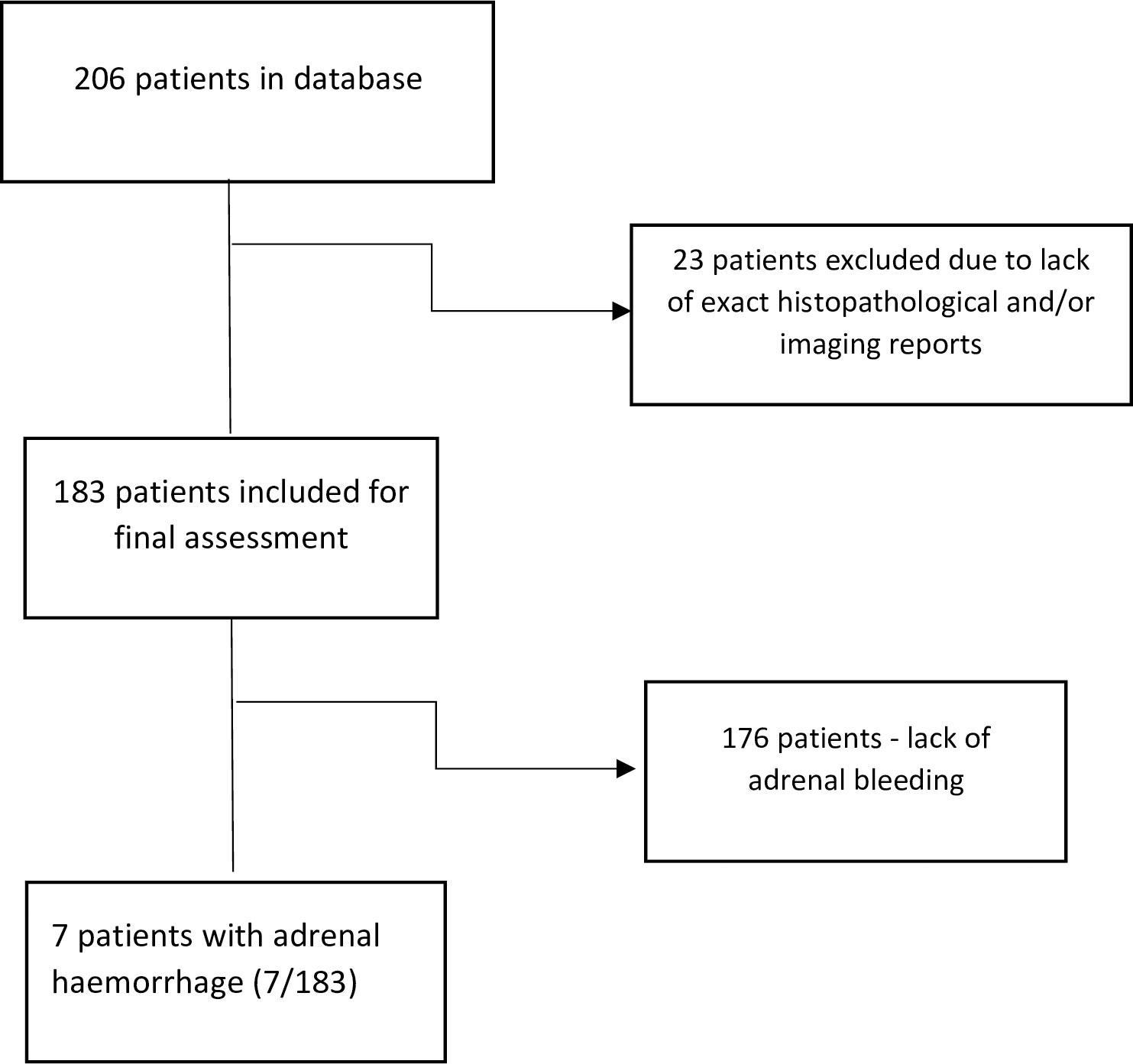
We performed a database search for pheochromocytoma patients, diagnosed and treated in tertiary endocrinology unit from 2005 to 2021. 206 consecutive patients with pheochromocytoma were identified. Subsequently, 23 cases were excluded due to incomplete medical data (histopathological or imaging) necessary to rule out potential adrenal bleeding. Of the remaining 183 patients with histologically confirmed pheochromocytoma, seven cases with adrenal bleeding confirmed in histopathological examination were found (3.8% of cases). Clinical manifestation, imaging, hormonal status and histopathological results were analyzed. All imaging records of patients suspected for adrenal bleeding were reassessed by one radiologist, skilled in adrenal gland pathology (AG).
24-hour urinary fractionated metanephrines and 3-metoxytyramine were measured using high performance liquid chromatography with electrochemical detection.
Due to the need of urgent hormonal assessment in most cases, only 3 patients have followed the proper dietary restrictions regarding catecholamine-rich products withdrawal before and during 24-hour urine collection.
The summary of patient’s concomitant medications and diet are summarized in Table 1.
Statistical analysis
Demographic and clinical characteristics were analyzed by the use of frequency tables for categorical variables and by calculation of the median and range for continuous variables. Length of follow-up was presented as range and median value in months, from the time of curative surgery until the last follow-up appointment. The mean values were calculated with standard deviation. The software Statistica 13 made by StatSoft Polska in 2017 was used.
Consort diagram for the study population is presented on Figure 1.
Results
Clinical presentation
In the whole cohort, seven patients with adrenal bleeding were found, comprising 3.8% of pheochromocytoma cases (four men and three women). Median patient age was 49 years (range: 36-78 years). All patients had spontaneous adrenal hemorrhage in the absence of recent abdominal trauma. One patient had a history of platelet-inhibiting treatment. The most common manifestation of adrenal bleeding was acute abdominal pain (5 out of 7 patients).
Other symptoms accompanying ongoing hemorrhage were: nausea and vomiting (3/7 patients), paroxysmal hypertension (3/7 patients), flank pain (3/7 patients), chest pain (2/7 patients), tachycardia (2/7 patients), seizures with short-lasting apnoea (1/7 patients), headache (1/7 patients). Two patients developed shock, in one case resulted in multiple organ failure (MOF). In both patients with severe chest pain, takotsubo cardiomyopathy was diagnosed.
Most patients (6/7 cases) had symptoms suggestive of a pheochromocytoma prior to adrenal hemorrhage – most commonly paroxysmal hypertension (4/7). The median time period between onset of symptoms and diagnosis of pheochromocytoma was 5 months (range: 0.5-48 months).
Nevertheless, in four patients diagnosis of pheochromocytoma was made at the time of adrenal hemorrhage, based on severe clinical manifestation, hormonal status and/or imaging. One patient died, before the diagnosis of adrenal bleeding was established. In two patients pheochromocytoma was suspected before the episode of hemorrhage: two months and sixteen months, respectively.
Imaging
Six out of seven patients had diagnostic imaging: All patients underwent contrast – enhancement computed tomography (CT), with one angio-CT scan. One patient had additional magnetic resonance imaging (MRI) done. Median largest diameter of the lesions was 7.4 cm (range: 5-11 cm). The images revealed different stages and severity of bleeding. The most common features on CT scans were solid-cystic appearance of the lesions, with strong enhancement of the solid component (four patients) and forms of thick-walled hemorrhagic cysts (four patients).
The summary of CT and MRI results are presented in Table 2. The CT and MRI images are shown on Figures 2–5.
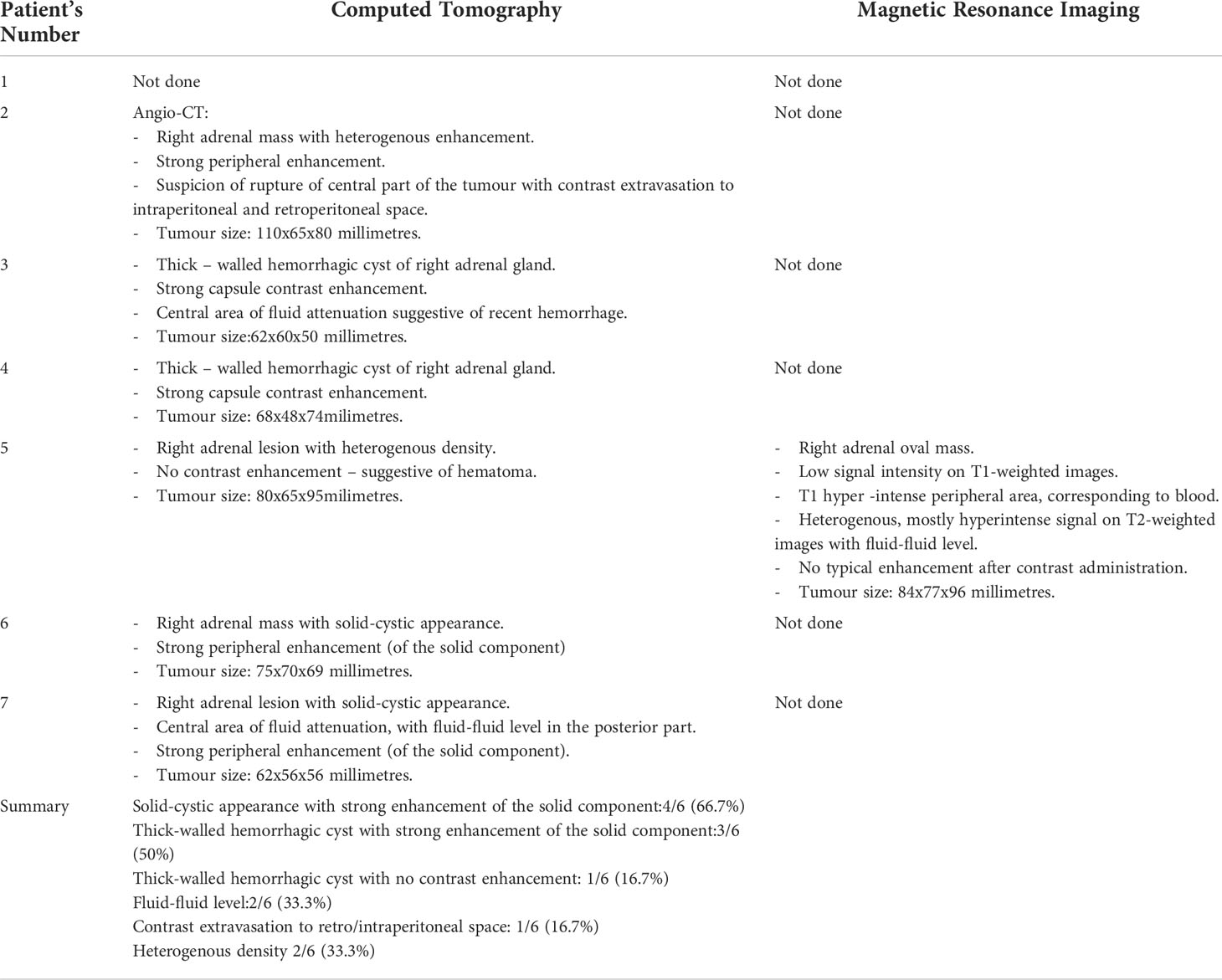
Table 2 Summary of the results of Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) in six out of seven patients.
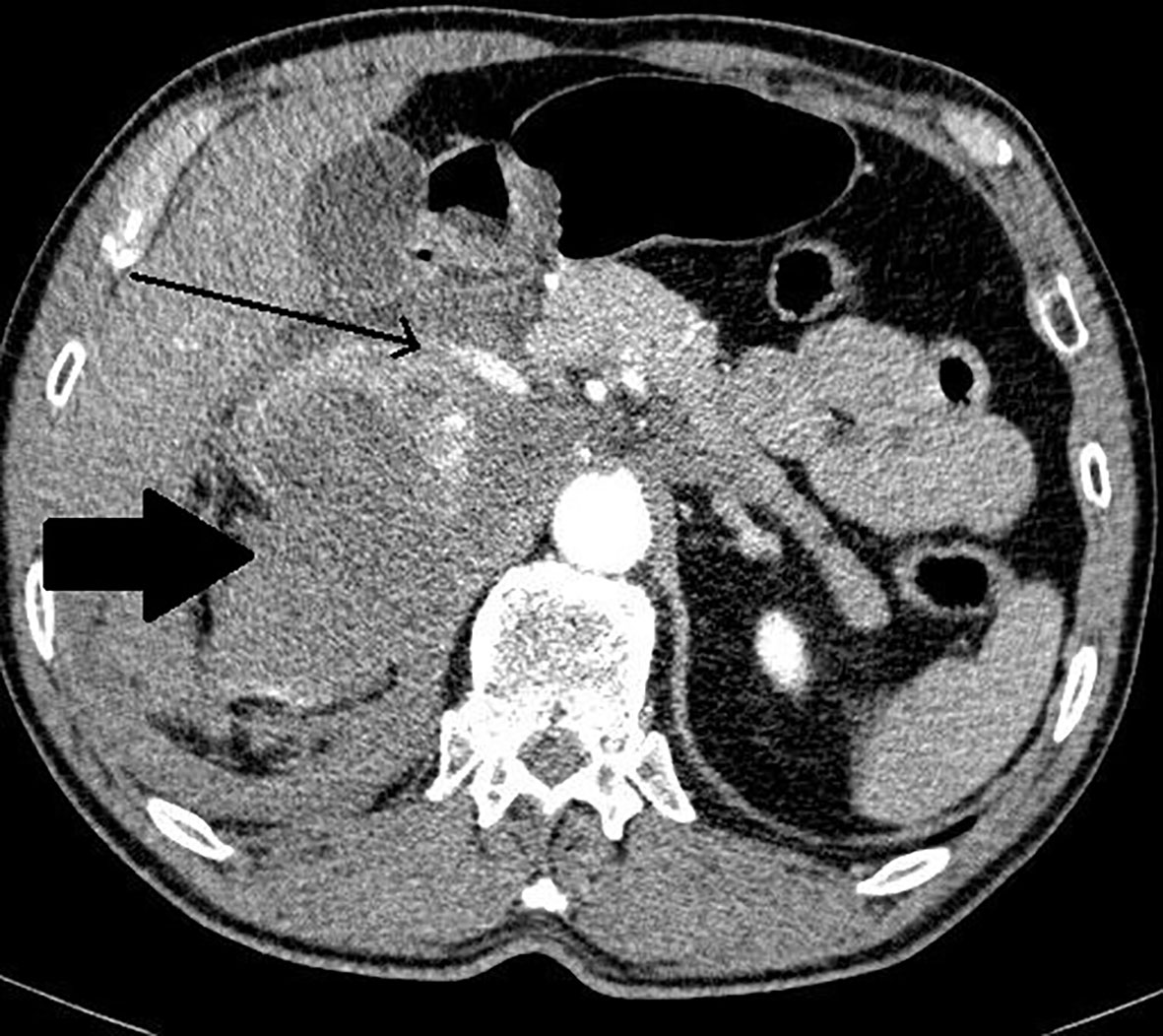
Figure 2 Patient number 2: angio-CT, arterial phase, axial image- right adrenal mass with heterogenous enhancement (thick arrow), suspicion of rupture of central part of the tumour with contrast extravasation (thin arrow). Right adrenal gland is not separately visualized. Left adrenal gland visible, with physiological contrast enhancement.
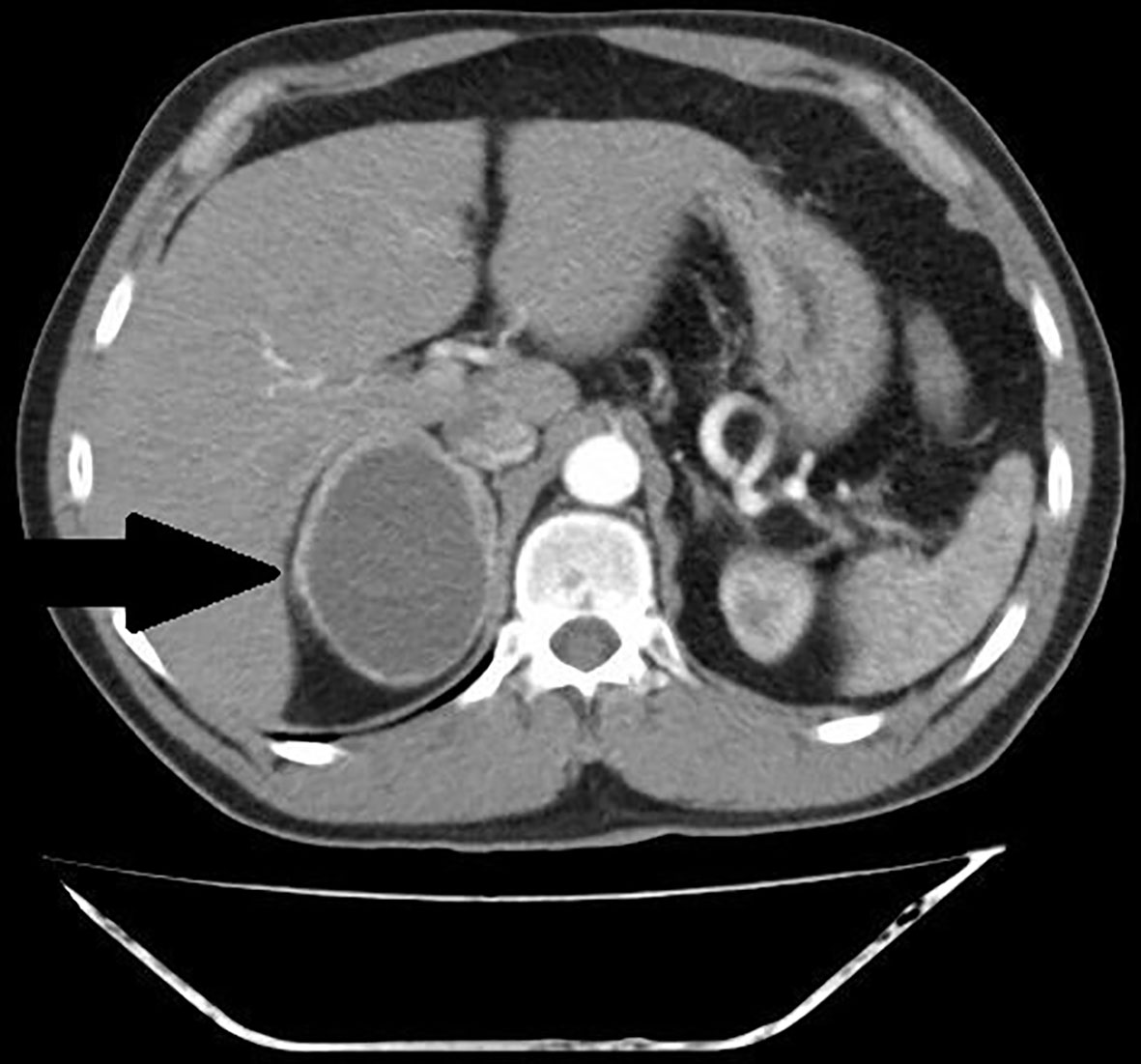
Figure 3 Patient number 4: CT of the abdomen, arterial phase, axial image- thick – walled hemorrhagic cyst of right adrenal gland with strong capsule-contrast enhancement (thick arrow).
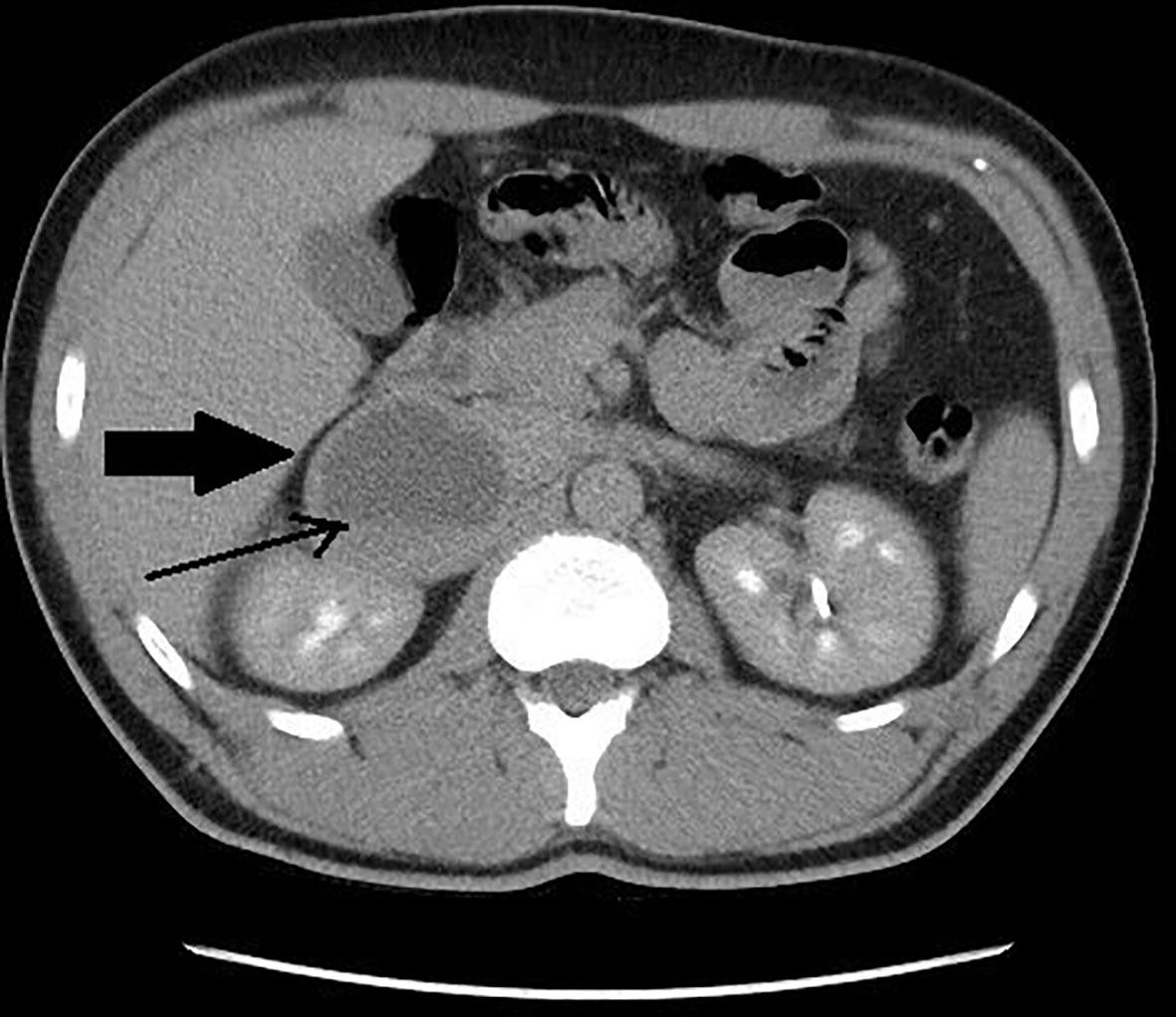
Figure 4 Patient number 7: CT of the abdomen, venous phase, axial image – right adrenal lesion with solid-cystic appearance (thick arrow), central area of fluid attenuation, with fluid-fluid level (thin arrow).
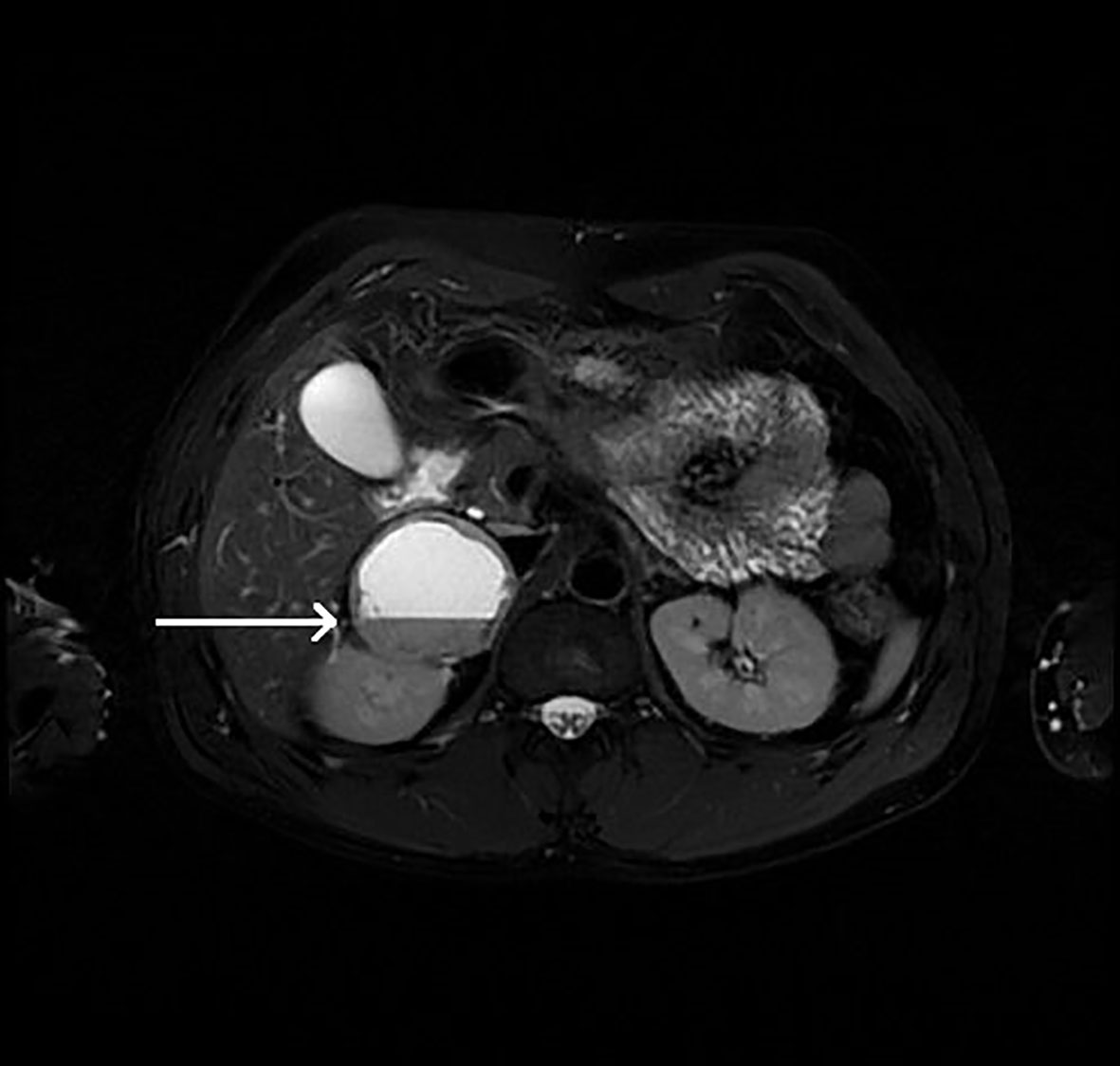
Figure 5 Patient number 5: MRI of the abdomen, T2-weighted axial image – right adrenal lesion with mostly hyperintense signal with fluid-fluid level (thin arrow).
Laboratory assessment
Hormonal assessment concerning pheochromocytoma was performed in five patients, based on 24-hour urinary fractionated metanephrines with urinary 3-methoxytyramine.Normetanephrine was elevated in all patients (from 1.9 to 68-fold above the upper limit). Metanephrine concentration was substantially increased in 4 cases (from 2.3 to 28.6-fold above the upper limit) - in one case it only slightly exceeded the normal range. In all patients, at least one metabolite of catecholamines – metanephrine or normetanephrine - was significantly elevated (more than twice the upper reference limit). Both metanephrines were increased in 4 out of 5 patients subjected to hormonal assessment. 3-metoxythyramine was elevated in 4 patients. The median levels of metanephrine, normetanephrine and 3-methoxytyramine were 1929.9 ug/24h (range: 348.6-9738.8), 4679.7 ug/24h (range: 844.4-29927.7) and 505.1 ug/24h (range: 30.58-1764.9), respectively. Two patients had no hormonal tests – one died before the diagnosis of adrenal hemorrhage was made, in the other, an urgent surgery was not preceded by evaluation of hormonal activity. However, the radiological images suggested pheochromocytoma and typical pharmacological treatment was implemented. Anemia was observed in three out of seven cases, similar to hyperglycemia, which was noted also in 3/7 patients.
Treatment
In six out of seven patients pheochromocytoma was suspected preoperatively, based on clinical manifestation, hormonal status or imaging. One patient had been admitted to hospital in hemorrhagic shock and multiple organ failure and had died before an etiology of adrenal bleeding was revealed. The diagnosis of hemorrhagic pheochromocytoma was made on autopsy. In five cases, the elective surgery was performed, preceded by a two- week pharmacological treatment with alpha-receptor blockers. In one case, due to patient’s prolonged hemodynamic instability despite supportive care, accompanied by rapid decrease of hemoglobin level and radiological suspicion of intraperitoneal hemorrhage, four-day alpha-receptor blockage was administered, followed by the urgent surgery. None of the patients has transcatheter arterial embolization (TAE) procedure performed before the surgery. In perioperative care two patients had indications to red blood cell transfusion. In one case administration of urapidil was necessary due to significant hypertension during surgery. One patient required intravenous infusion of norepinephrine intraoperatively.
Histopathological examination
In all cases the diagnosis of pheochromocytoma was histopathologically confirmed by immunohistochemistry - typical positive staining: synaptophysin and chromogranin and the presence of S100+ sustentacular cells in neoplasm. In all patients massive hemorrhagic changes in the tumor’s tissue was confirmed. PASS score was defined precisely in 5 patients. In three cases, it was no higher than 3. In two remaining patients, PASS score was 5 and 4, respectively. Because of massive hemorrhage, in two patients it couldn’t be determined. Histopathological images are shown on Figures 6–8.
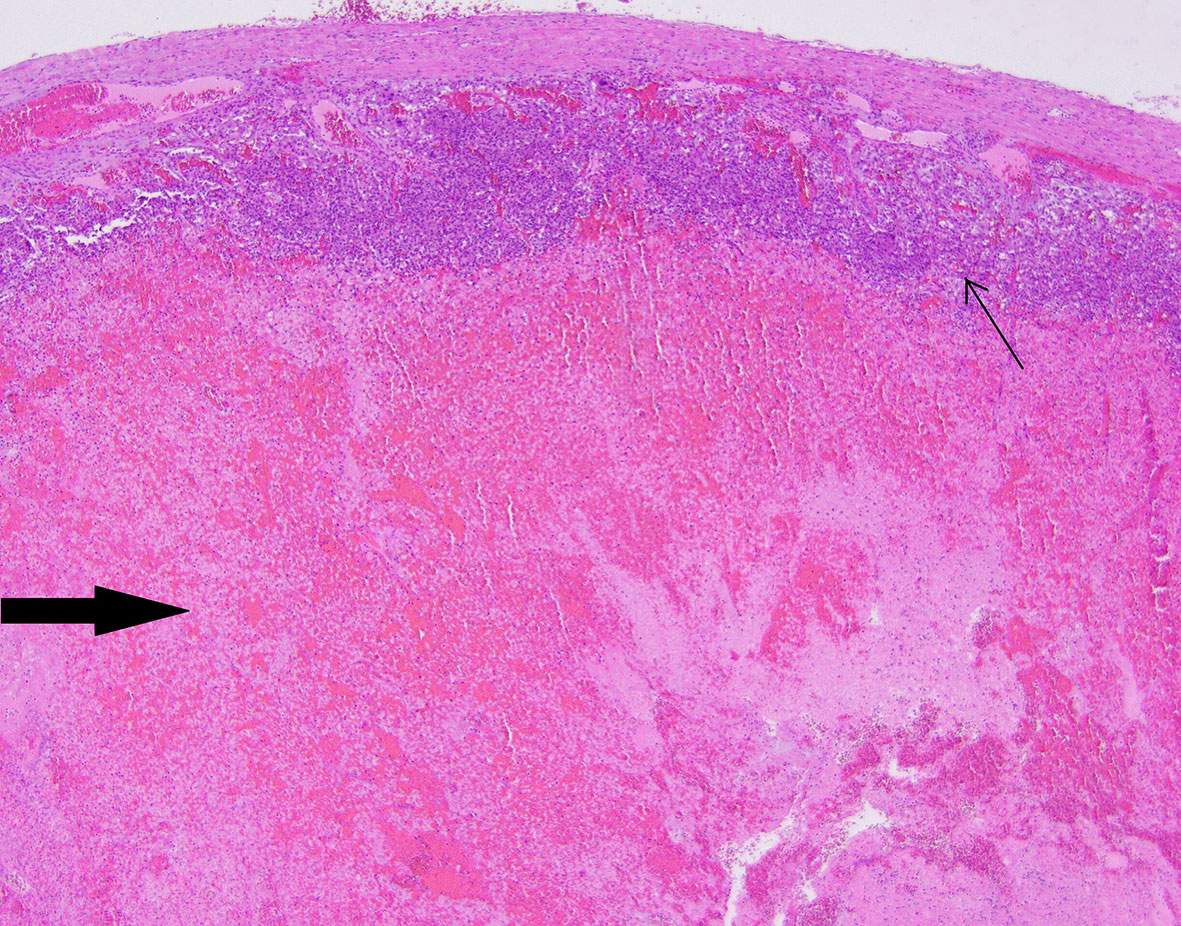
Figure 6 Patient number 4: extensive, diffuse hemorrhage in the central part of the pheochromocytoma (thick arrow), the neoplasm’s tissue is present as subcapsular narrow rim (thin arrow).
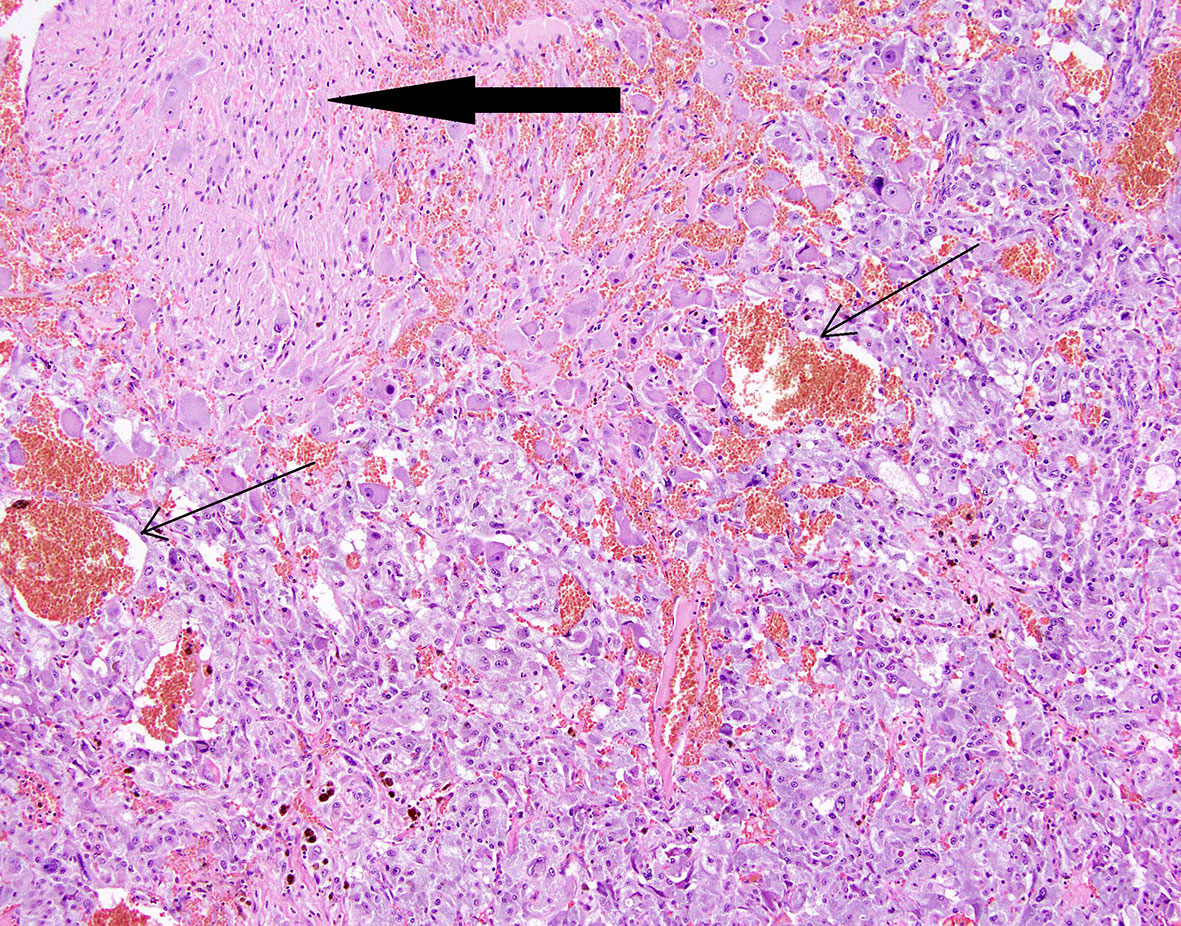
Figure 7 Patient number 3: composite pheochromocytoma and ganglioneuroma (ganglioneuroma component - thick arrow). Hemorrhages are present within pheochromocytoma tissue (thin arrows).
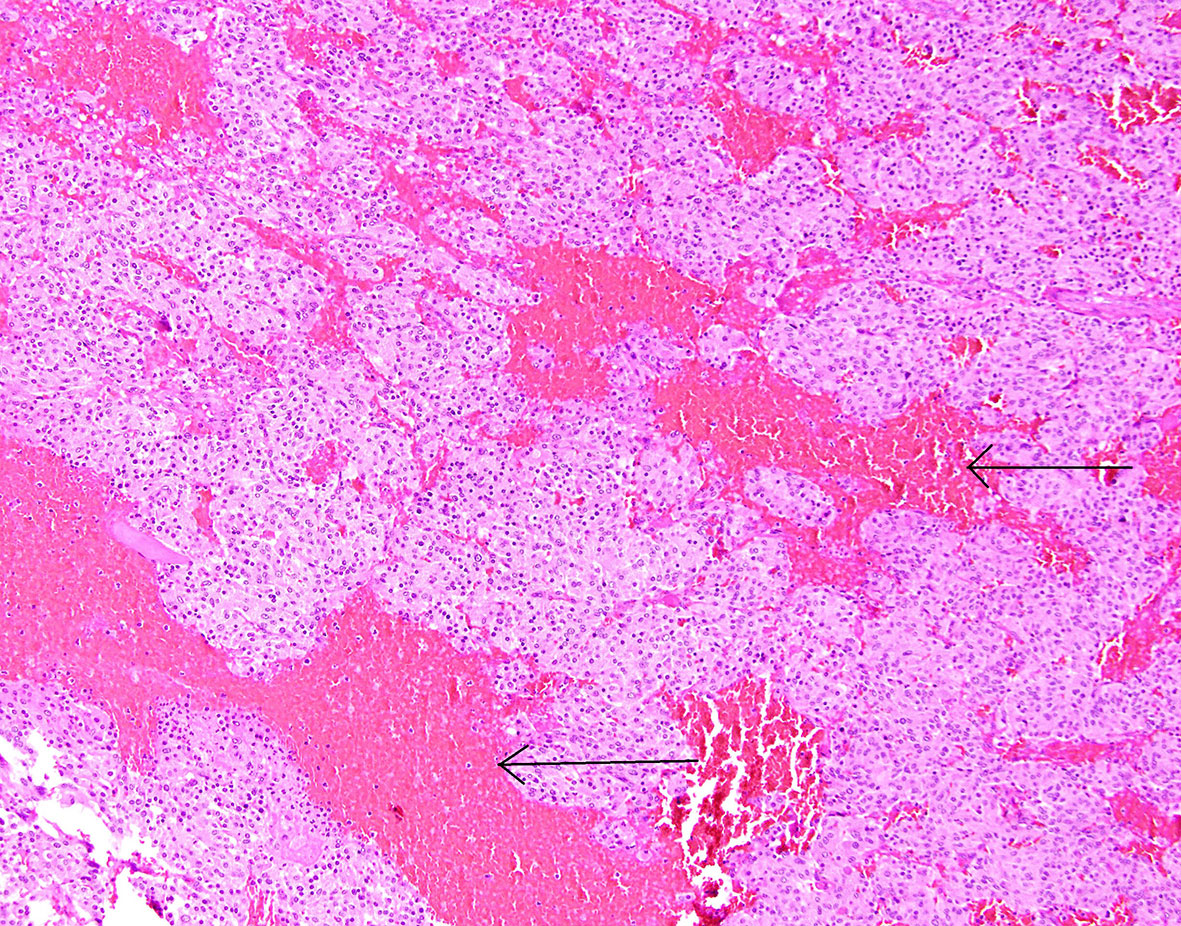
Figure 8 Patient number 2: central part of the pheochromocytoma with irregular, diffuse hemorrhages between tumour nests (thin arrows).
Outcome and follow – up
In our group, the survival rate was 85.7%. Only one patient died, before the initial diagnosis of adrenal bleeding, shortly after admission to the department. The median follow up period was 68.5 months (Range: 11-156 months). All six patients who survived, remained at good condition, without any evidence of recurrence on last follow-up visit.
The clinicopathological characteristics of the patients are summarized in Table 1.
Discussion
Pheochromocytoma is the most common neoplasm responsible for spontaneous adrenal hemorrhage (1). In our data we found the clinically significant hemorrhage in 3.8% of patients with pheochromocytoma.
According to the literature, the most common symptom of hemorrhagic pheochromocytoma is abdominal pain of acute onset, which is in accordance with our work (2, 3, 11). The pain is presumably connected with local compression of surrounding viscera and adjacent structures by the lesion but it might be also caused by catecholamine over-secretion, accompanied by stimulation of alpha-adrenergic receptors, constriction of intestinal vascular smooth muscle and contraction of the ileocolic sphincter (3, 10, 13).
The status of shock in patients with hemorrhagic pheochromocytoma can be the result of massive bleeding, but also a sudden fall in the blood catecholamine level or excessive release of catecholamines, followed by cardiogenic shock (2, 4, 14). In our group, two out of seven patients developed shock. In one case it was accompanied by massive intra – and retro-peritoneal bleeding and anemia, which suggested a hemorrhagic shock as an important element of patient’s hemodynamic destabilization. In second case, multiorgan failure, hyperglyaemia and acidosis without evident anemia raised suspicion that catecholamine crisis could play a key role in patient’s deterioration.
Catecholamines oversecretion often leads to tachyarrhytmias, of which severe and refractory sinus tachycardia, atrial fibrillation and ventricular tachycardia seem to be the most common (15).
The rare complication of catecholaminergic crisis, which could accompany hemorrhagic pheochromocytoma is Takotsubo cardiomyopathy, characterized by transient left ventricular systolic dysfunction (16, 17). In our group, Takotsubo cardiomyopathy was diagnosed in two patients.
As spontaneous adrenal hemorrhage is an insidious medical condition, it might remain unrecognized with lethal consequences (18, 19). Similar course of the disease was reported in one of our patients, who died few hours after admission, without a diagnostic statement.
On CT and MRI scans, the attenuation value or signal intensity of an adrenal hematoma depends on the stage of the bleeding. On CT, adrenal bleeding presents heterogenous, hyperdense appearance, which decreases over time (20–23). Contrast-enhanced CT may demonstrate active contrast extravasation in the setting of acute bleeding (21). A homogenous density greater than 50 Hounsfield Units (HU) is characteristic for hematoma, especially in cases with acute hemorrhage (22).
On MRI, the acute stage of adrenal bleeding is characterized by isointense or slightly low signal intensity on T1-weighted images and markedly low signal intensity on T2-weighted images, whereas in the early subacute phase hyperintense T1- and hypointense T2- weighted images are observed (20, 21). The high signal intensity appears at the periphery of the hematoma on T1-weighted images about 7 days after onset of the hemorrhage (23). Moreover, in the late subacute stage T2-weighted images become hyperintense (20, 21). In comparison to that, hyperintense signal on T2-weighted images with avid enhancement after contrast administration is characteristic for pheochromocytoma (20, 21, 24).
The radiographic findings similar to hemorrhagic adrenal neoplasm can be observed in patients with adrenal pseudocysts (hematomas), thus making the differential diagnosis notably difficult, especially in terms of a clot in a hemorrhagic cyst mimicking a solid component or a cyst presenting thick and irregular walls. Undoubtedly, it should be taken into consideration in biochemically negative patients (9, 23). Intravenous contrast injection on MRI or CT could help in stating a correct diagnosis since the presence of enhancement in adrenal mass raises the suspicion for an underlying tumor (20, 21, 23, 24). Interval imaging to assess possible resolution of hematoma could be helpful in patients without biochemical evidence of a functional tumor to exclude neoplasm (1). In some cases, sedimentation level could be seen in adrenal lesions as a sign of a previous hemorrhage (25).
In one of our patients, on MRI examination, low-signal intensity with peripheral hyperintense signal on T1-weighted images and heterogenous, mostly hyperintense signal on T2- weighted images were shown. Uncharacteristically, there was no typical contrast enhancement after both iodinated contrast (on CT) and gadolinium contrast (on MRI) administration in this patient. It could be explained by massive hemorrhage filling in and damaging the tumor’s tissue, which was confirmed histopathologically. In this regard, the appearance of the non-contrast enhancing pheochromocytoma in our series was quite different from the cases of hemorrhagic pheochromocytomas reported by the others (20, 21, 23).
All patients with hemorrhagic adrenal mass should undergo hormonal assessment to exclude hormonally active tumors. Both catecholamine and cortisol levels should be evaluated (1, 26).
The preferred tests for biochemical diagnosis of pheochromocytoma is measurement of plasma or urinary free metanephrines with the use of high performance liquid chromatography, especially with mass spectrometry. In patients at low risk for a PPGL, the assessment of plasma free metanephrines has similar diagnostic accuracy as those in urine, however in patients, for whom biochemical proof of PPGL is the objective, the measurement of plasma free metanephrines is recommended. The measurement of urinary fractionated metanephrines might have higher ratio of false-positive results, since their levels are more affected by the diet (27–32). Increments in any plasma metabolite in excess of twice the upper reference limit or increases in two metabolites indicate a high likelihood of pheochromocytoma (27). To optimize the diagnostic performance, use of personalized age-specific reference intervals for plasma normetanephrine and gender-specific reference intervals for urinary metanephrines is recommended (31) .
Due to physiological stress, levels of catecholamines can be elevated in patients with adrenal hemorrhage, even without pathologically confirmed pheochromocytoma, which may lead to misdiagnosis.
Previous work showed that in cases of adrenal bleeding other than underlying pheochromocytoma, concentrations of urinary fractionated metanephrines only slightly exceeded normal laboratory range (50-100% above the upper limit) in about 30% of cases, with predominant normetanephrine level increase – moreover, the patients did not present symptoms indicative of an excess of catecholamines (33). Kyoda et al. revealed normal or slight elevation to no more than 3-fold the upper limit of catecholamines or their metabolites in the plasma and 24-hour urine in patients with hemorrhagic pseudotumor (9).
We also reviewed all the articles published from 2005 to 2022 in PubMed, dedicated to adrenal bleeding with known results of urinary or plasma catecholamines or metanephrines (8, 10, 12, 14, 16, 17, 24, 34–61). The analysis showed that catecholamines or metanephrines (plasma or urine) were elevated in 38.9% of patients with adrenal bleeding without pheochromocytoma. In most cases only normetanephrine/noradrenaline exceeded the normal range. In one patient slight increase in urinary adrenaline was observed. The urinary or plasma normetanephrine levels were increased to less than 3-fold the normal upper limit (maximum 2.1-fold the upper normal limit for plasma normetanephrine, 2.9-fold for urinary normetanephrine), meanwhile urinary or plasma noradrenaline levels were increased no higher than 1.6 -fold the upper limit of normal range. Contrarily to that, in patients with hemorrhage due to a pheochromocytoma, in most cases both urinary or plasma catecholamines/metanephrines were elevated (21/24, 87.5%). In patients with assessed levels of urinary or plasma metanephrines, at least one metabolite exceeded 2-fold the upper normal limit. Based on that, we might conclude, that adrenal bleeding in non-pheochromocytoma patients results in slightly, non-specific elevation of normetanephrine/noradrenaline to no more than 3-fold the upper limit of normal in about 30-40% of cases. In patients with hemorrhagic pheochromocytomas, most often both catecholamines (or their derivatives) are elevated.
The results are shown in Table 3 and on Figures 9, 10.
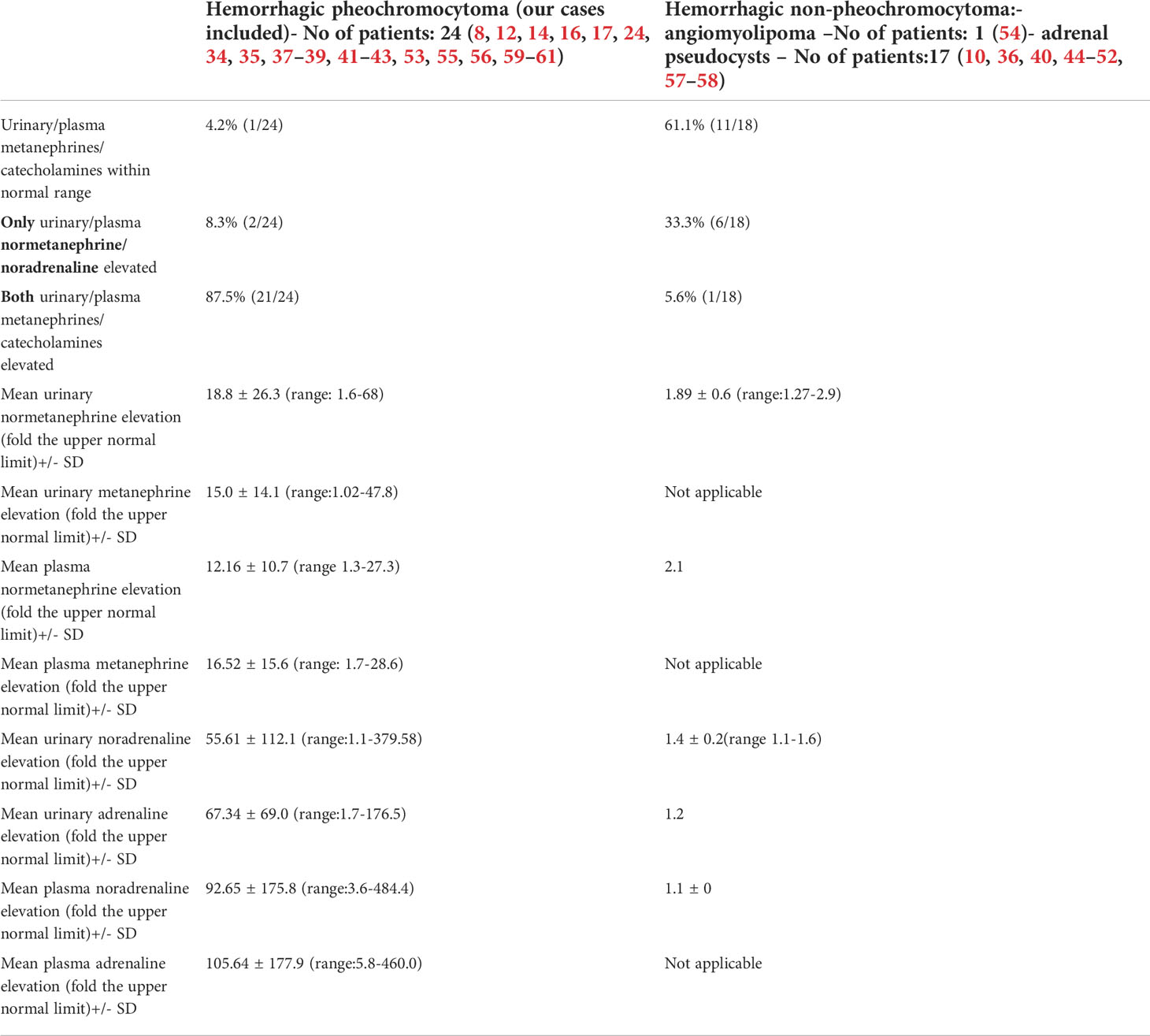
Table 3 Levels of catecholamines/metanephrines in patients with hemorrhagic pheochromocytoma and adrenal bleeding without pheochromocytoma based on the literature (8, 10, 12, 14, 16, 17, 24, 34–61).
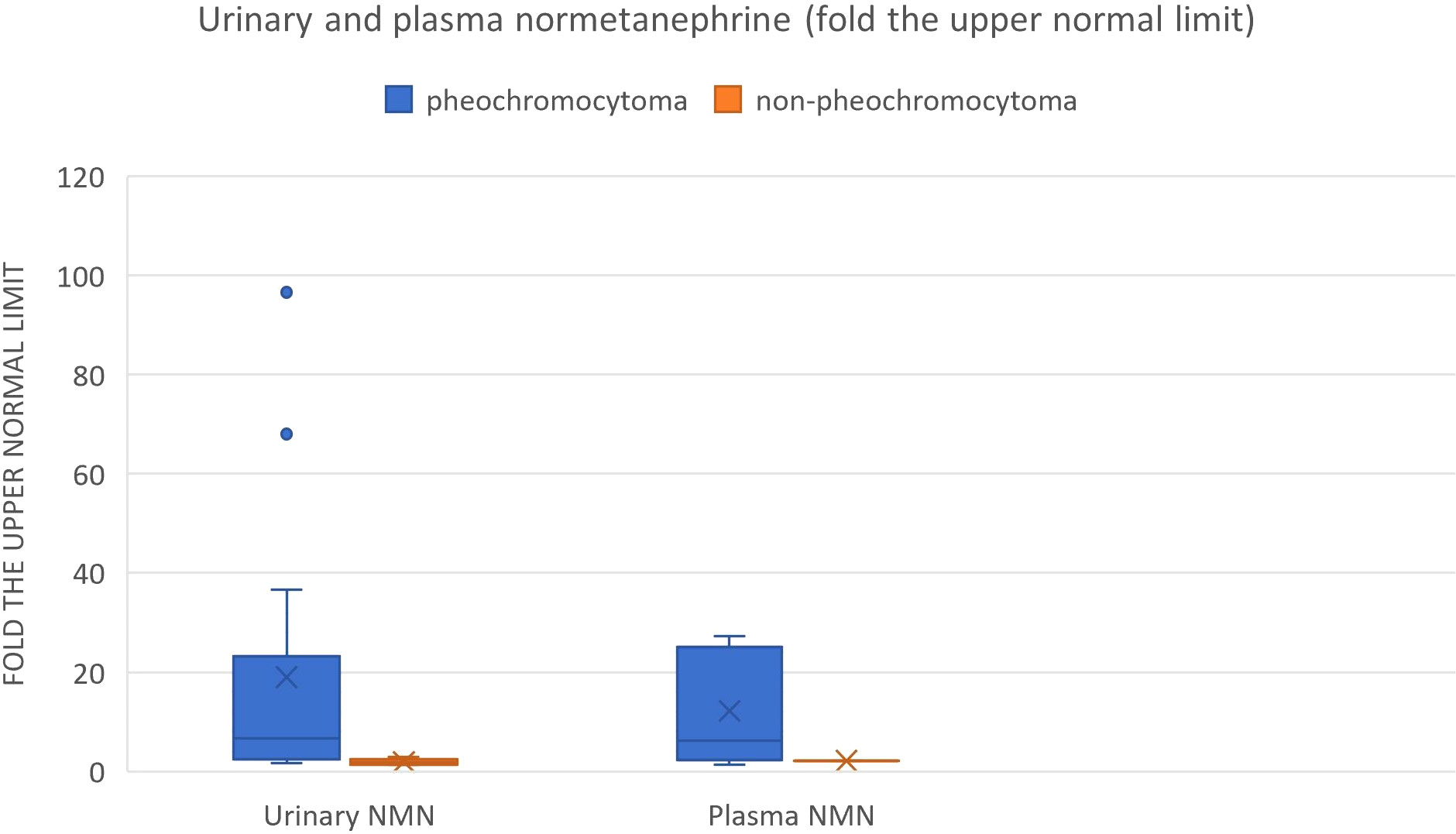
Figure 9 Urinary and plasma normetanephrine levels in patients with hemorrhagic pheochromocytoma and adrenal bleeding without pheochromocytoma based on the literature (3, 10, 12, 14, 20, 25–29, 30–39, 40–49, 50–54.).
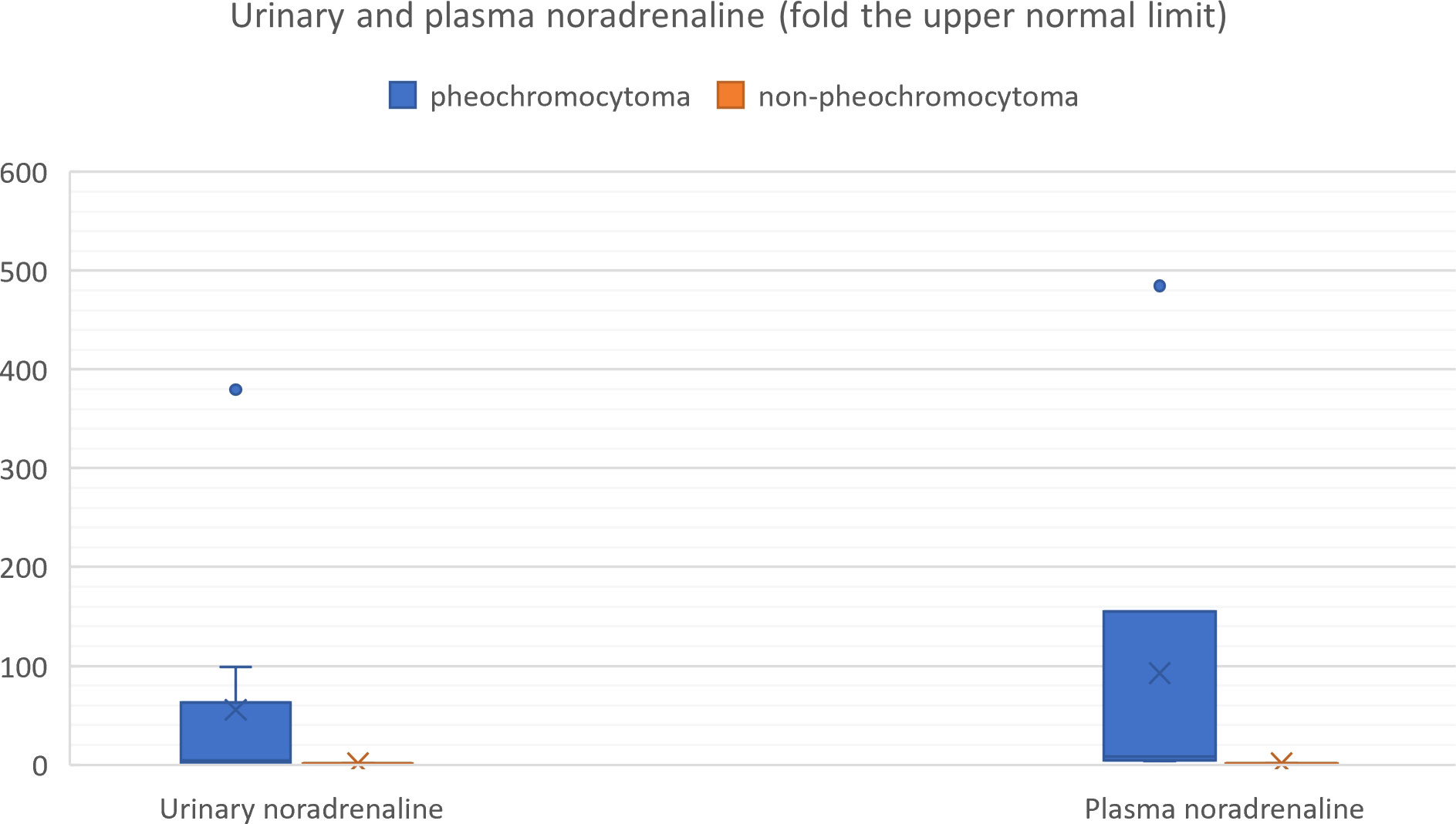
Figure 10 Urinary and plasma noradrenaline levels in patients with hemorrhagic pheochromocytoma and adrenal bleeding without pheochromocytoma based on the literature (3, 10, 12, 14, 20, 25–29, 30–39, 40–49, 50–54).
Emergency surgery with regard to hemorrhagic pheochromocytoma has a high mortality rate of 40-45% (1, 3, 11, 22). Operation without proper pharmacological treatment jeopardizes patient’s safety due to high risk of catecholamine crisis and postoperative hypotension (4, 56). However, in some groups of patients, i.e. with massive intraperitoneal bleeding, ineffective preoperative management or embolization, emergency exploratory laparotomy becomes the only treatment option (2, 4, 11, 59).
Alpha-adrenergic receptor (AR) blockers are the first-choice medications in preoperative management of pheochromocytoma. Phenoxybenzamine and doxazosin are the most commonly used. The main differences between those two drugs concern selectivity and affinity of drug-receptor interactions. Phenoxybenzamine as the non-selective, non-competitive alpha-adrenergic receptor blocker binds irreversibly with both alpha 1 and alpha 2 – AR, which results in strong, long-acting inhibition. Doxazosin competitively inhibits only alpha 1-AR. For this reason, treatment with phenoxybenzamine gives more effective AR-receptors blockade and better control of hypertension, at the cost of higher risk of postoperative hypotension and other side effects, like reflex tachycardia, oedema and nasal congestion, comparing to doxazosin. Selective alpha-AR antagonist is more likely to be used in combination with additional antihypertensive drugs, i.e. calcium channel blockers (62, 63).
In our work, three patients were treated with phenoxybenzamine, in three patients doxazosin was administered , as phenoxybenzamine was no longer broadly available in Poland. In one patient, who had been prepared with 12 milligrams of doxazosin in a maximum dose, administration of urapidil was needed during surgery due to significant hypertension.
According to literature, transcatheter arterial embolization (TAE) preoperatively, particularly in a case of a ruptured pheochromocytoma, in patients who don’t respond to red blood cell transfusions and initial conservative management, might be preferred initial therapy (1, 3, 7, 11, 53, 59). It can help to stabilize the patient’s state, perform biochemical testing and administer pharmacological treatment before undergoing operation, which improves survival (3, 11, 59). Another possible benefit of trans-arterial embolization preceding elective surgery is consolidation of the hematoma and tumor shrinkage (3, 53). There are only three cases evaluating catecholamine levels around TAE – two of them reported post-TAE peak of circulating catecholamines, resulted in hypertension, nausea, epigastric pain or constipation (11, 64). Therefore, careful observation of patient’s vital signs and symptoms is necessary after TAE procedures. Marti et al. included embolization in proposed treatment algorithm for patients with hemorrhagic adrenal neoplasms (1).
In the literature, there are only several studies comprising cases of pheochromocytoma hemorrhage (1–3, 11, 12). There were summarized, together with our work, in Table 4. In comparison to our group, previously reported cases were characterized by higher proportion of emergency surgery (47-58.3% vs 14.3% in our work) and worse survival outcomes (27-41.7% of patients died vs 14.3% in our group). It could be explained by less number of patients with diagnosis of pheochromocytoma stated preoperatively but also more cases of severe, intraperitoneal and retroperitoneal bleeding (21-100% of intraperitoneal bleeding and 50-55% of retroperitoneal bleeding vs both – 14.3% in our work). Kobayashi showed that failed preoperative diagnosis of hemorrhagic pheochromocytoma was an independent factor for poor prognosis. Moreover, there was a strong correlation between correct preoperative diagnosis and elective surgery. Hemodynamic instability had a significant influence on the correct diagnosis of a pheochromocytoma (2). In recent years, improvement of survival rates despite the comparable proportion of more severe hemorrhage can be seen (11). It seems to be connected with better availability of imaging studies, development of new procedures in adrenal bleeding, including TAE and emphasis on preoperative management and preferentially elective type of surgery.
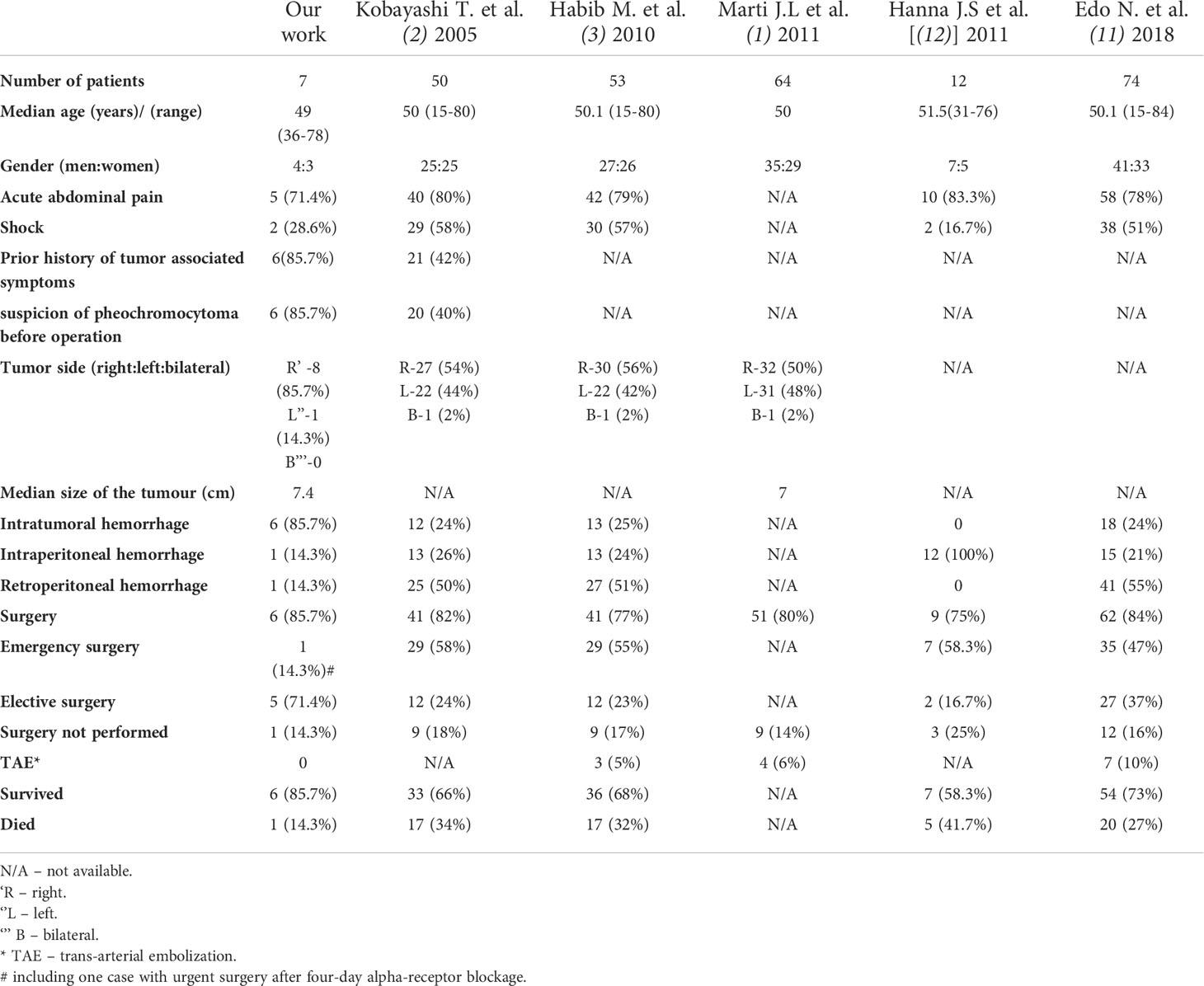
Table 4 Clinicopathological characteristics and treatment results of hemorrhagic pheochromocytoma in our study and previously published series.
However, in the article summarizing 12 cases of intraperitoneal hemorrhage due to pheochromocytoma, conservative management was connected with 100% mortality (3/3 patients), contrarily to the survival rate of 71.4% (5/7) in patients treated by emergency surgery. Those results therefore stressed the need for more aggressive and faster surgical interventions in case of massive intraperitoneal hemorrhage in pheochromocytoma patients (12). Successful hemodynamic stabilization by TAE procedures in patients with ruptured pheochromocytomas accompanied by intraperitoneal hemorrhage has been also described (34, 35).
None of the previous series has focused on histopathological results in a hemorrhagic pheochromocytoma. Nevertheless, in the literature, reported cases of adrenal bleeding in pheochromocytomas with high PASS score, metastases and recurrences can be found (53, 55, 59, 64). Moreover, Xin-Gao et al. showed that intratumoral hemorrhage was abundantly detected in histologically high-graded pheochromocytomas (65). In our group, the PASS score was no higher than 3 in three cases, but it exceeded 3 in two remaining patients. Nevertheless, in two patients it couldn’t be determined. Moreover in four out of five patients with known hormonal status, the level of dopamine metabolite, 3-metoxythyramine was substantially elevated. It is known that dopamine-producing tumors are more likely to present aggressive course of the disease and metastasize (66). Therefore, we suggest that in patients with hemorrhagic pheochromocytoma, careful follow-up is of great importance, since due to a massive bleeding, histopathological assessment is especially difficult and carries a risk of a PASS score underestimation and underprediction of other histopathological factors suggesting malignant course of the disease.
The potential risk factors for the pheochromocytoma hemorrhage are difficult to establish, since the most cases are diagnosed at the moment of ongoing adrenal bleeding, which may affect levels of circulating catecholamines and tumor size. The previous cases with repetitive assessment of catecholamines showed that their levels can fluctuate after the episode of adrenal bleeding within pheochromocytoma, probably because of tumor tissue damage and necrosis (60, 67). In one of our patients, concentration of urinary metanephrines decreased significantly three days after the hemorrhage (see Table 1, Patient Number 5). Catecholamine levels can be also influenced by pharmacological treatment with vasopressors (17). Some authors suggest, that the mechanism of hemorrhage may be explained by excessive amount of circulating catecholamines leading to vasoconstriction of the draining venules and extensive necrosis of pheochromocytoma, with subsequent decrease of catecholamines secretion, increase in blood flow into the tumor and high intratumoral pressure. It can result in rupture of the thin walled adrenal venules and tumor capsule. Moreover, anatomical characteristics of the adrenal gland vessels may also contribute to an increase in intratumoral pressure because the blood outflow vessels in the adrenal glands are much narrower than inflow vessels and the muscle bundles surrounding the central and external adrenal veins are arranged eccentrically, leading to turbulent flow within the vein (1, 3, 18, 23, 38, 59, 60).
In addition, AR-blockers have been reported as a potential cause of tumor rupture in pheochromocytoma (60).
In the group of 64 patients with hemorrhagic pheochromocytoma, described by Marti et al. median tumor size was 7 cm, which is similar to our observations (7.4 cm in our group). Based on the earlier studies (1–3) and our research, adrenal bleeding seems to be predominantly observed in pheochromocytomas localized in right adrenal glands. In two recent series, it was reported more often in men than women (1, 11), but some works, as well as our observations, did not confirm the predominance of men (2, 3, 12). The median age at diagnosis of hemorrhage in reported series, including ours, was about 50 years (1–3, 11, 12).
The major limitations of our study is its retrospective design. Moreover, a non-surgical character of our department, might result in bias in patient selection with respect to mortality, treatment strategies, and timing of surgery. Some patients with more severe bleeding and hemodynamic instability might have be transferred directly to surgical departments, which could explain low rate of intra- and retro-peritoneal bleeding in our patients and high percentage of preoperatively diagnosed pheochromocytoma. It could also lead to an underestimation of a number of hemorrhagic cases among all pheochromocytoma patients.
Based on the current available literature and our own experience, we suggest an algorithm for the diagnosis and treatment of adrenal hemorrhage in the course of pheochromocytoma (1–4, 7, 11, 12, 14, 15, 22, 23, 26, 27, 35, 38, 40, 43, 62, 68). It is shown on Figure 11. We have also summarized the medical management in hemorrhagic pheochromocytoma:
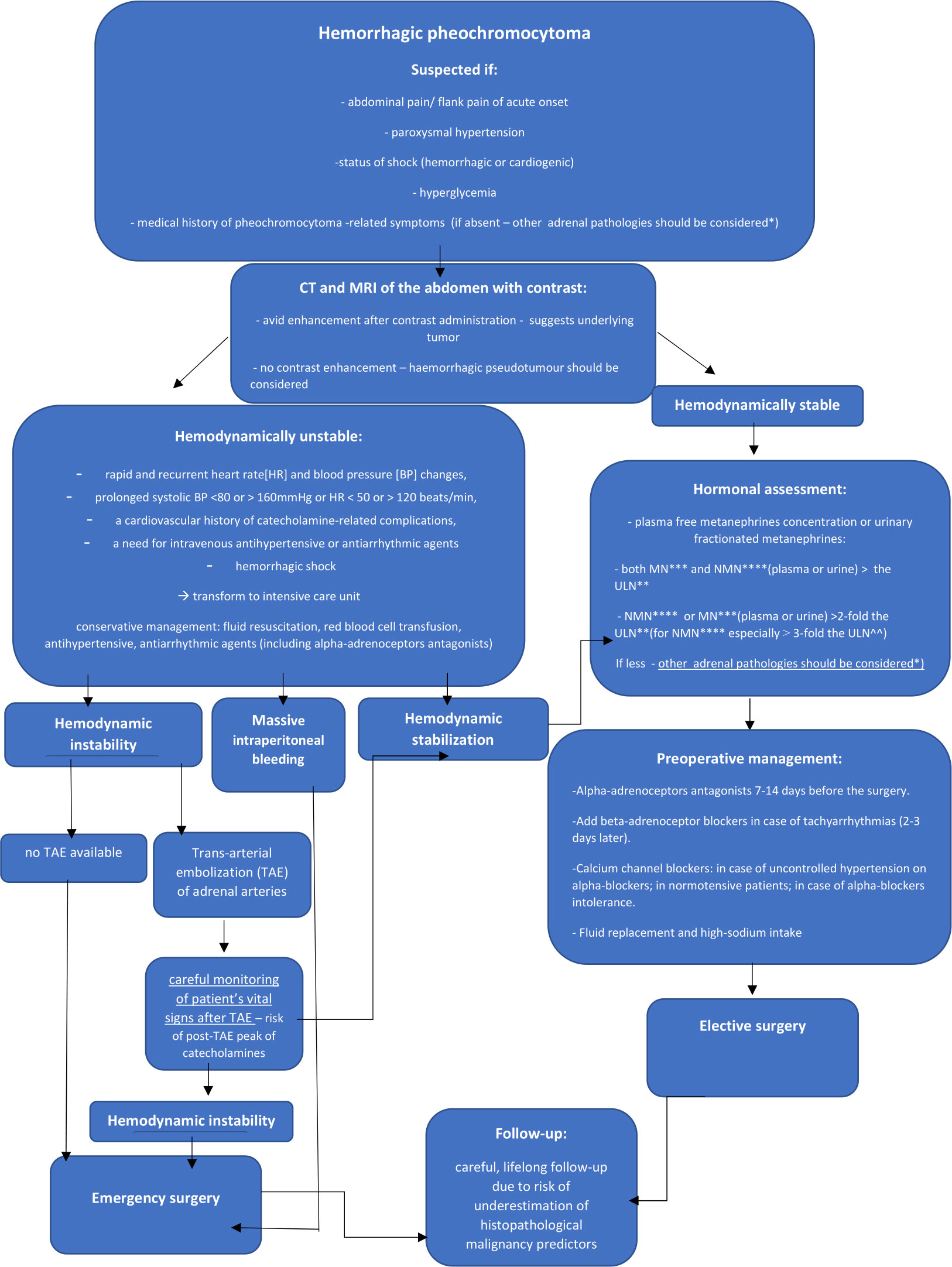
Figure 11 Algorithm for the diagnosis and treatment of adrenal hemorrhage in the course of pheochromocytoma. *mainly hemorrhagic pseudotumour, metastases, myelolipoma, primary adrenocortical carcinoma ** The upper limit of normal *** metanephrine****normetanephrine
If hemorrhagic pheochromocytoma is suspected, perform urgent CT scan or MRI of the abdomen. It can help to assess the severity of bleeding and reveal underlying adrenal mass. Patients with unstable hemodynamic conditions due to catecholamine-induced arrythmias (rapid and recurrent heart rate [HR] and blood pressure [BP] changes, prolonged systolic BP <80 or > 160mmHg or HR < 50 or > 120 beats/min, a cardiovascular history of catecholamine-related complications, a need for intravenous antihypertensive or antiarrhythmic agents) or hemorrhagic shock should be treated in intensive care units (15). For the latter, fluid resuscitation and red blood cell (RBC) transfusion are the mainstay of treatment. In hemodynamically stable cases, perform hormonal assessment of cortisol and catecholamines. Increments in either metanephrine or normetanephrine in excess of twice the upper reference limit (for normetanephrine especially at least 3-fold) or increases in two metabolites indicate a high likelihood of pheochromocytoma (27). All hemodynamically stable patients should be qualified to elective surgery preceded by pharmacological management. Start the preoperative treatment of pheochromocytoma patients with alpha-adrenoceptors antagonists 7-14 days before the surgery. Beta-adrenoceptor blocking agents can be added in case of tachyarrhytmias, only after adequate pretreatment with alpha-adrenoceptors blockers (not earlier than 2-3 days after alpha-blockade). The β -blockers implementation without preceding α-blockers treatment may result in hypertensive crisis due to inhibition of β2-adrenoceptor mediated vasodilatation with coexistence of unopposed α -adrenoceptor-mediated vasoconstriction (27, 68). Cardioselective β1-adrenoceptors blockers are preferable. Labetalol and Carvedilol, with a higher potency for β- than α-adrenergic receptors should be avoided in monotherapy in pheochromocytoma patients (15, 27, 68). Calcium channel blockers are good option for the patients with uncontrolled blood pressure despite α -adrenoceptor blockers use, in case of α-adrenoceptor blockers intolerance and severe side effects or in patients with normal blood pressure or only intermittent hypertension to avoid α -adrenoceptor induced hypotension. (27, 62, 69). In such cases, ivabradine can also be used (15). In case of hypotension, unrelated to blood loss, consider potential β2-receptor overstimulation and administer a non-selective β2-adrenoceptor blocking agents (propranolol) or phenylephrine. For all patients adequate fluid replacement (with intravenous saline infusion in the evening before operation) and high-sodium intake prior to surgery is also important (62). Suggested optimal presurgical heart rate and blood pressure targets are: seated blood pressure <130/80mmHg and an upright systolic blood pressure > 90mmHg; heart rate 60-70 and 70-80 bpm in seated and upright position, respectively (15). For the patients with hypertensive crisis, intravenous administration of phentolamine sodium, nitroprusside, nicardipine or urapidil is recommended (69). In hemodynamically unstable patients despite conservative management, perform trans-arterial embolization (TAE) of adrenal arteries, followed by careful monitoring of patient’s vital signs. Patients unsuccessfully treated by TAE procedures, hemodynamically unstable despite conservative management, in case of unavailable TAE or with massive intraperitoneal bleeding should be qualified to emergency surgery. After the operation of hemorrhagic pheochromocytoma, careful life-long follow-up of the patients seems to be the most appropriate.
Conclusions
Adrenal bleeding is a rare, life-threatening complication of pheochromocytoma, which constitutes a diagnostic and therapeutic challenge. Proper diagnosis is essential for adequate preparation for surgery. Clinical symptoms including abdominal pain, accompanying by hemodynamic shock and previous history of pheochromocytoma-associated symptoms should alert about potential risk of adrenal bleeding. The emergency surgery in patients with adrenal bleeding from ruptured pheochromocytoma can be connected with significantly higher morbidity and mortality. Stabilizing arterial embolization could be an effective initial therapy in patients with hemodynamic instability, giving the opportunity for better preoperative management. In patients with hemorrhagic pheochromocytoma, careful postoperative follow-up is of great importance, since due to a massive bleeding, histopathological assessment may be less credible.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The study was approved by Jagiellonian University Ethical Committee (approval number:1072.6120.218.2020). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
ER, data collection, data analysis, manuscript drafting, manuscript editing and approval, study coordination. JK, data analysis, manuscript editing and approval. AG and MU-B, data collection, analysis, manuscript editing, and approval. ML, data collection, manuscript editing, and approval. MO, manuscript drafting, editing, and approval. EP-M and AH-D, manuscript editing and approval, study coordination. AG-J, data collection, data analysis, manuscript drafting, editing and approval, and study coordination. All authors contributed to the article and approved the submitted version.
Funding
The study is sponsored as the part of the Special Purpose Grant for Young Scientists by the Jagiellonian University, Medical College, Cracow, Poland, SAP N41/DBS/000117).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Marti JL, Millet J, Ann J, Roman SA, Carling T, Udelsman R. Spontaneous adrenal hemorrhage with associated Masses: Etiology and management in 6 cases and a review of 133 reported cases. World J Surg (2012) 36(1):75–82. doi: 10.1007/s00268-011-1338-6
2. Kobayashi T, Iwai A, Takahashi R, Ide Y, Nishizawa K, Mitsumori K. Spontaneous rupture of adrenal pheochromocytoma: Review and analysis of prognostic factors. J Surg Oncol (2005) 90(1):31–5. doi: 10.1002/jso.20234
3. Habib M, Tarazi I, Batta M. Arterial embolization for ruptured adrenal pheochromocytoma. Curr Oncol (2010) 17(6):65–70. doi: 10.3747/co.v17i6.597
4. Scholten A, Cisco RM, Vriens MR, Cohen JK, Mitmaker EJ, Liu C, et al. Pheochromocytoma crisis is not a surgical emergency. J Clin Endocrinol Metab (2013) 98(2):581–91. doi: 10.1210/jc.2012-3020
5. Charalampakis V, Stamatiou D, de Bree E, Christodoulakis M, Zoras O. Spontaneous adrenal hemorrhage. report of two cases and review of pathogenesis , diagnosis and management. J Surg Case Rep (2018) 2018(6):rjy129. doi: 10.1093/jscr/rjy129
6. Sano F, Fujikawa N, Hirai K, Ueki T, Kitami K. Pheochromocytoma manifested by traumatic adrenal hemorrhage. Hinyokika Kiyo (2006) 52(1):15–7.
7. Souiki T, Tekni Z, Laachach H, Bennani A, Zrihni Y, Tadmori A, et al. Catastrophic hemorrhage of adrenal pheochromocytoma following thrombolysis for acute myocardial infarction: Case report and literature review. World J Emerg Surg (2014) 9(1):50. doi: 10.1186/1749-7922-9-50
8. Rebollo-Román A, Alhambra-Expósito MR, Herrera-Martínez Y, Leiva-Cepas F, Alzas C, Muñoz-Jiménez C, et al. Catecholaminergic crisis after a bleeding complication of COVID-19 Infection : A case report. Front Endocrinol (Lausanne) (2021) 12:693004. doi: 10.3389/fendo.2021.693004
9. Kyoda Y, Tanaka T, Maeda T, Masumori N, Tsukamoto T. Adrenal hemorrhagic pseudocyst as the differential diagnosis of pheochromocytoma – a review of the clinical features in cases with radiographically diagnosed pheochromocytoma. J Endocrinol Invest (2013) 36(9):707–11. doi: 10.3275/8928
10. Zheng W, Fung KM, Cheng L, Osunkoya AO. Benign vascular tumors, cysts, and pseudocysts of the adrenal gland: A contemporary multi-institutional clinicopathological analysis. Hum Pathol (2018) 82:95–102. doi: 10.1016/j.humpath.2018.07.013
11. Edo N, Yamamoto T, Takahashi S, Mashimo Y, Morita K, Saito K, et al. Optimizing hemodynamics with transcatheter arterial embolization in adrenal pheochromocytoma rupture. Intern Med (2018) 57(13):1873–8. doi: 10.2169/internalmedicine.9907-17
12. Hanna JS, Spencer PJ, Savopoulou C, Kwasnik E, Askari R. Spontaneous adrenal pheochromocytoma rupture complicated by intraperitoneal hemorrhage and shock. World J Emerg Surg (2011) 6(1):27. doi: 10.1186/1749-7922-6-27
13. Sweeney AT, Malabanan AO, Blake MA, de las Morenas A, Cachecho R, Melby JC. Megacolon as the presenting feature in pheochromocytoma. J Clin Endocrinol Metab (2000) 85(11):396872. doi: 10.1210/jcem.85.11.6947
14. Tanaka K, Noguchi S, Shuin T, Kinoshita Y, Kubota Y, Hosaka M. Spontaneous rupture of adrenal pheochromocytoma: a case report. J Urol (1994) 151(1):120–1. doi: 10.1016/s0022-5347(17)34886-3
15. Nazari MA, Rosenblum JS, Haigney MC, Rosing DR, Pacak K. Pathophysiology and acute management of tachyarrhythmias in pheochromocytoma: JACC review topic of the week. J Am Coll Cardiol (2020) 76(4):451–64. doi: 10.1016/j.jacc.2020.04.080
16. Yuan S, He T, Yang L, Chu Q, Huang W, Dai H. Basal takotsubo syndrome induced by pheochromocytoma rupture. Cardiovasc J Afr (2021) 32(3):171–4. doi: 10.5830/CVJA-2020-039
17. Iio K, Sakurai S, Kato T, Nishiyama S, Hata T, Mawatari E, et al. Endomyocardial biopsy in a patient with hemorrhagic pheochromocytoma presenting as inverted takotsubo cardiomyopathy. Heart Vessels (2013) 28(2):255–63. doi: 10.1007/s00380-012-0247-4
18. Vella A, Nippoldt TB, Morris JC 3rd. Adrenal hemorrhage: a 25-year experience at the Mayo clinic. Mayo Clin Proc (2001) 76(2):161–8. doi: 10.1016/S0025-6196(11)63123-6
19. Gavrilova-Jordan L, Edmister WB, Farrell MA, Watson WJ. Spontaneous adrenal hemorrhage during pregnancy: A review of the literature and a case report of successful conservative management. Obstet Gynecol Surv (2005) 60(3):191–5. doi: 10.1097/01.ogx.0000157357.15401.c3
20. Hoeffel C, Legmann P, Luton JP, Chapuis Y, Fayet-Bonnin P. Spontaneous unilateral adrenal hemorrhage: Computerized tomography and magnetic resonance imaging findings in 8 cases. J Urol (1995) 154(5):1647–51. doi: 10.1016/s0022-5347(01)66738-7
21. Hammond NA, Lostumbo A, Adam SZ, Remer EM, Nikolaidis P, Yaghmai V, et al. Imaging of adrenal and renal hemorrhage. Abdom Imaging (2015) 40(7):2747–60. doi: 10.1007/s00261-015-0453-5
22. Arora S, Vargo S, Lupetin AR. Computed tomography appearance of spontaneous adrenal hemorrhage in a pheochromocytoma. Clin Imaging (2009) 33(4):314–7. doi: 10.1016/j.clinimag.2008.12.008
23. Kawashima A, Sandler CM, Ernst RD, Takahashi N, Roubidoux MA, Goldman SM, et al. Imaging of nontraumatic hemorrhage of the adrenal gland. Radiographics (1999) 19(4):949–63. doi: 10.1148/radiographics.19.4.g99jl13949
24. Baez JC, Jagannathan JP, Krajewski K, O'Regan K, Zukotynski K, Kulke M, et al. Pheochromocytoma and paraganglioma: Imaging characteristics. Cancer Imaging (2012) 12(1):153–62. doi: 10.1102/1470-7330.2012.0016
25. Sanal HT, Kocaoglu M, Yildirim D, Bulakbasi N, Guvenc I, Tayfun C, et al. Imaging features of benign adrenal cysts. Eur J Radiol (2006) 60(3):465–9. doi: 10.1016/j.ejrad.2006.08.005
26. Ali A, Singh G, Balasubramanian SP. Acute non-traumatic adrenal haemorrhage — management, pathology and clinical outcomes. Gland Surg (2018) 7(5):428–32. doi: 10.21037/gs.2018.07.04
27. Lenders JWM, Kerstens MN, Amar L, Prejbisz A, Robledo M, Taieb D, et al. Genetics, diagnosis, management and future directions of research of phaeochromocytoma and paraganglioma: A position statement and consensus of the working group on endocrine hypertension of the European society of hypertension. J Hypertens (2020) 38(8):1443–56. doi: 10.1097/HJH.0000000000002438
28. Ahn J, Park JY, Kim G, Jin SM, Hur KY, Lee SY, et al. Urinary free metanephrines for diagnosis of pheochromocytoma and paraganglioma. Endocrinol Metab (2021) 36(3):697–701. doi: 10.3803/EnM.2020.925
29. Därr R, Kuhn M, Bode C, Bornstein SR, Pacak K, Lenders JWM, et al. Accuracy of recommended sampling and assay methods for the determination of plasma-free and urinary fractionated metanephrines in the diagnosis of pheochromocytoma and paraganglioma: A systematic review. Endocrine (2017) 56(3):495–503. doi: 10.1007/s12020-017-1300-y
30. Grossman A, Pacak K, Sawka A, Lenders JW, Harlander D, Peaston RT, et al. Biochemical diagnosis and localization of pheochromocytoma: Can we reach a consensus? Ann N Y Acad Sci (2006) 1073:332–47. doi: 10.1196/annals.1353.038
31. Eisenhofer G, Peitzsch M, Kaden D, Langton K, Mangelis A, Pamporaki C, et al. Reference intervals for LC-MS/MS measurements of plasma free, urinary free and urinary acid-hydrolyzed deconjugated normetanephrine, metanephrine and methoxytyramine. Clin Chim Acta (2019) 490:46–54. doi: 10.1016/j.cca.2018.12.019
32. de Jong WH, Eisenhofer G, Post WJ, Muskiet FA, de Vries EG, Kema IP. Dietary influences on plasma and urinary metanephrines: Implications for diagnosis of catecholamine-producing tumors. J Clin Endocrinol Metab (2009) 94(8):2841–9. doi: 10.1210/jc.2009-0303
33. Karwacka IM, Obołończyk Ł, Sworczak K. Adrenal hemorrhage : A single center experience and literature review. Adv Clin Exp Med (2018) 27(5):681–7. doi: 10.17219/acem/68897
34. O'Neal PB, Moore FD Jr, Gawande A, Cho NL, Moalem J, Ruan DT. Hemorrhagic shock as the initial manifestation of pheochromocytoma: Report of a sequential management strategy. Endocr Pract (2012) 18(4):e81–4. doi: 10.4158/EP11149.CR
35. Amin A, Biswas S, Baccay F. Traumatic injury causing intraperitoneal hemorrhage of an occult pheochromocytoma. Case Rep Crit Care (2012) 2012:342819. doi: 10.1155/2012/342819
36. Wordsworth S, Thomas B, Agarwal N, Hoddell K, Davies S. Elevated urinary catecholamines and adrenal haemorrhage mimicking phaeochromocytoma. BMJ Case Rep (2010) 2010:bcr0120102612. doi: 10.1136/bcr.01.2010.2612
37. Chiu CC, Chen YC, Teng TH, Yang LH, Chen YP, Siao FY. Sudden cardiac arrest after minor abdominal trauma: A successful resuscitation in a patient with haemorrhagic phaeochromocytoma. Resuscitation (2009) 80(11):1323–4. doi: 10.1016/j.resuscitation.2009.07.012
38. Park JH, Kang KP, Lee SJ, Kim CH, Park TS, Baek HS. A case of a ruptured pheochromocytoma with an intratumoral aneurysm managed by coil embolization. Endocr J (2003) 50(6):653–6. doi: 10.1507/endocrj.50.653
39. Marzano LA, Tauchmanova L, Marzano E, Arienzo R, Guarino R, Ciancia G, et al. Large Idiopathic unilateral adrenal hematoma in a young woman. J Endocrinol Invest (2007) 30(1):52–8. doi: 10.1007/BF03347396
40. Kumar S, Nanjappa B, Kumar S, Prasad S, Pushkarna A, Singh SK. Adrenal artery pseudoaneurysm in pheochromocytoma presenting with catastrophic retroperitoneal haemorrhage. Can Urol Assoc J (2013) 7(3-4):E254–6. doi: 10.5489/cuaj.541
41. Peng CZ, Chen JD, How CK, Yen DH, Huang MS. Catecholamine crisis due to spontaneous ruptured adrenal pheochromocytoma. J Cardiovasc Med (Hagerstown) (2011) 12(7):518–9. doi: 10.2459/JCM.0b013e328346a6ea
42. Orikasa K, Namima T, Ohnuma T, Munakata M, Kimura N, Arai Y. Spontaneous rupture of adrenal pheochromocytoma with capsular invasion. Int J Urol (2004) 11(11):1013–5. doi: 10.1111/j.1442-2042.2004.00937.x
43. Park BK, Kim CK, Kwon GY. Spontaneous rupture of pheochromocytoma: computed tomography-pathologic features and correlation. Acta Radiol (2008) 49(2):230–2. doi: 10.1080/02841850701545805
44. Beltran S, Makdassi R, Robert F, Remond A, Fournier A. Hématome surrénalien unilatéral inaugural d'un syndrome des antiphospholipides [Inaugural unilateral adrenal hematoma of an antiphospholipid syndrome]. Presse Med (2004) 33(6):385–8. doi: 10.1016/s0755-4982(04)98601-0
45. Tan PL, Moore NR. Spontaneous idiopathic bilateral adrenal haemorrhage in adults. Clin Radiol (2003) 58(11):890–2. doi: 10.1016/s0009-9260(03)00338-6
46. Paramythiotis D, Bangeas P, Karakatsanis A, Goulas P, Nikolaou I, Rafailidis V, et al. Surgical management of a giant adrenal Pseudocyst : A case report and review of the literature in the last decade. Case Rep Surg (2018) 2018:8473231. doi: 10.1155/2018/8473231
47. Isono M, Ito K, Seguchi K, Kimura T, Tachi K, Kono T, et al. A case of hemorrhagic adrenal pseudocyst mimicking solid tumor. Am J Case Rep (2017) 18:1034–8. doi: 10.12659/ajcr.905063
48. Cantisani V, Petramala L, Ricci P, Porfiri A, Marinelli C, Panzironi G, et al. A giant hemorragic adrenal pseudocyst: Contrast-enhanced examination ( CEUS ) and computed tomography ( CT ) features. Eur Rev Med Pharmacol Sci (2013) 17(18):2546–50.
49. Bovio S, Porpiglia F, Bollito E, Allasino B, Reimondo G, Rovero E, et al. Adrenal pseudocyst mimicking cancer: A case report. J Endocrinol Invest (2007) 30(3):256–8. doi: 10.1007/BF03347435
50. Suga H, Inagaki A, Ota K, Taguchi S, Kato T, Kakiya S, et al. Adrenal pseudocyst mimicking a pheochromocytoma found after a traffic accident. Intern Med (2003) 42(1):66–71. doi: 10.2169/internalmedicine.42.66
51. Kar M, Pucci E, Brody F. Laparoscopic resection of an adrenal pseudocyst. J Laparoendosc Adv Surg Tech A (2006) 16(5):478–81. doi: 10.1089/lap.2006.16.478
52. Laforga JB, Bordallo A, Ara FI. Vascular adrenal pseudocyst: cytologic and immunohistochemical study. Diagn Cytopathol (2000) 22(2):110–2. doi: 10.1002/(sici)1097-0339(200002)22:2<110::aid-dc11>3.0.co;2-i
53. Ichikawa T, Oyabu C, Minamida M, Ichijo Y, Hashimoto Y, Asano M, et al. Changes in the size of a ruptured pheochromocytoma after transcatheter arterial embolization. Case Rep Med (2021) 2021:5568978. doi: 10.1155/2021/5568978
54. Nerli RB, Ghagane SC, Kadeli V, Nutalpati S, Mohan S, Hiremath MB. Bleeding angiomyolipoma mimiking a ruptured adrenal tumour. Urol Case Rep (2019) 28:101031. doi: 10.1016/j.eucr.2019.101031
55. Gilly O, Brucker-Davis F, Bernard JL, Barlier A, Quintard H, Benisvy D, et al. Unilateral aggressive pheochromocytoma revealed by a massive intraperitoneal hemorrhage five years after an initial presentation suggesting an adrenal hematoma. Ann Endocrinol (Paris) (2018) 79(1):48–52. doi: 10.1016/j.ando.2017.08.003
56. Aggeli C, Nixon AM, Parianos C, Vletsis G, Papanastasiou L, Markou A, et al. Surgery for pheochromocytoma: A 20-year experience of a single institution. Hormones (Athens) (2017) 16(4):388–95. doi: 10.14310/horm.2002.1759
57. Rao N, Burns B Jr, Cobble D. Traumatic adrenal hemorrhage masking as a pseudotumor. Cureus (2020) 12(3):e7256. doi: 10.7759/cureus.7256
58. Karayiannakis AJ, Polychronidis A, Simopoulos C. Giant adrenal pseudocyst presenting with gastric outlet obstruction and hypertension. Urology (2002) 59(6):946. doi: 10.1016/s0090-4295(02)01617-5
59. Elmoheen A, Yousry M, Elmesery A, Bashir K. Ruptured functioning adrenal tumour, atypical presentation with renal colic and hypertension. BMJ Case Rep (2020) 13(12):e236050. doi: 10.1136/bcr-2020-236050
60. Murai N, Azami T, Iida T, Mikura K, Imai H, Kaji M, et al. A case of pheochromocytoma with a marked decrease in catecholamine levels after rupture in which a good outcome was achieved by elective surgery. Endocr J (2018) 65(11):1093–9. doi: 10.1507/endocrj.EJ18-0071
61. Mañas-Martínez AB, Medrano-Navarro AL, Aguillo-Gutiérrez E. Hemorrhagic pheochromocytoma presenting as severe hypertension with myocardial infarction. Ann Endocrinol (Paris) (2017) 78(1):54–6. doi: 10.1016/j.ando.2016.10.004
62. Fang F, Ding L, He Q, Liu M. Preoperative management of pheochromocytoma and paraganglioma. Front Endocrinol (Lausanne) (2020) 11:586795. doi: 10.3389/fendo.2020.586795
63. Zawadzka K, Więckowski K, Małczak P, Wysocki M, Major P, Pędziwiatr M, et al. Selective vs non-selective alpha-blockade prior to adrenalectomy for pheochromocytoma: systematic review and meta-analysis. Eur J Endocrinol (2021) 184(6):751–60. doi: 10.1530/EJE-20-1301
64. Müssig K, Horger M, Häring HU, Wehrmann M. Spontaneous rupture of malignant adrenal phaeochromocytoma. Emerg Med J (2008) 25(4):242. doi: 10.1136/emj.2007.052076
65. Gao X, Yamazaki Y, Pecori A, Tezuka Y, Ono Y, Omata K, et al. Histopathological analysis of tumor microenvironment and angiogenesis in pheochromocytoma. Front Endocrinol (Lausanne) (2020) 11:587779. doi: 10.3389/fendo.2020.587779
66. Eisenhofer G, Lenders JW, Siegert G, Bornstein SR, Friberg P, Milosevic D, et al. Plasma methoxytyramine: A novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer (2012) 48(11):1739–49. doi: 10.1016/j.ejca.2011.07.016
67. Saxena N. Traumatic haemorrhage into an occult phaeochromocytoma : presentation and management in a patient with septic shock. Anaesthesia (2008), 63(4) 428–32. doi: 10.1111/j.1365-2044.2007.05401.x
68. Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab (2007) 92(11):4069–79. doi: 10.1210/jc.2007-1720
Keywords: pheochromocytoma, hemorrhage, adrenal, bleeding, diagnosis, treatment
Citation: Rzepka E, Kokoszka J, Grochowska A, Ulatowska-Białas M, Lech M, Opalińska M, Przybylik-Mazurek E, Gilis-Januszewska A and Hubalewska-Dydejczyk A (2022) Adrenal bleeding due to pheochromocytoma - A call for algorithm. Front. Endocrinol. 13:908967. doi: 10.3389/fendo.2022.908967
Received: 31 March 2022; Accepted: 05 July 2022;
Published: 05 August 2022.
Edited by:
Ricardo Correa, University of Arizona, United StatesReviewed by:
Piotr Glinicki, Centre of Postgraduate Medical Education, PolandJoseph M. Pappachan, Lancashire Teaching Hospitals NHS Foundation Trust, United Kingdom
Copyright © 2022 Rzepka, Kokoszka, Grochowska, Ulatowska-Białas, Lech, Opalińska, Przybylik-Mazurek, Gilis-Januszewska and Hubalewska-Dydejczyk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aleksandra Gilis-Januszewska, myjanusz@cyf-kr.edu.pl
 Ewelina Rzepka
Ewelina Rzepka Joanna Kokoszka
Joanna Kokoszka Anna Grochowska3
Anna Grochowska3 Marta Opalińska
Marta Opalińska Alicja Hubalewska-Dydejczyk
Alicja Hubalewska-Dydejczyk