- 1Graduate Institute of Medical Sciences, College of Medicine, Taipei Medical University, Taipei, Taiwan
- 2Sleep Center, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan
- 3School of Respiratory Therapy, College of Medicine, Taipei Medical University, Taipei, Taiwan
- 4Division of Pulmonary Medicine, Department of Internal Medicine, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan
- 5School of Biomedical Engineering, College of Biomedical Engineering, Taipei Medical University, New Taipei City, Taiwan
- 6Department of Otolaryngology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
- 7Jockey Club School of Public Health and Primary Care, The Chinese University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 8Key Laboratory of Aerosol Chemistry & Physics, Institute of Earth Environment, Chinese Academy of Sciences, Xi’an, China
- 9School of Public Health, College of Public Health, Taipei Medical University, New Taipei City, Taiwan
- 10Department of Public Health, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
- 11Cell Physiology and Molecular Image Research Center, Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan
Introduction: Obstructive sleep apnea (OSA) is linked to increased cardiovascular and metabolic risks. Although body mass index is a recognized risk factor, the roles of fat mass, muscle mass, and hydration status in influencing OSA severity and sleep architecture remain unclear. This study examined the associations between body composition and apnea-hypopnea index, sleep efficiency, and sleep stages across sex, age, and BMI strata.
Materials and methods: In this cross-sectional study, data from 5,381 adults undergoing overnight polysomnography and bioimpedance analysis were analyzed. Multivariable linear regression, adjusted for age, sex, and BMI were used, with additional analyses stratified by sex, age, and BMI.
Results: A unit increase in fat percentage (FATP) and fat mass (FATM) were associated with increasing AHI, whereas increase in bone mass, and FFM were associated with decreasing AHI, particularly in younger adults. Intracellular and total body water associated with increasing AHI. Stratified analyses revealed stronger associations in females and individuals with obesity. A unit increase in adiposity associated with increasing WASO, while bone mass (BONEM) reduced WASO. A unit increase in BMI and water content were linked to increasing N1 stage, whereas increase in PMM and BONEM associated with increasing deeper stages. Age- and sex-specific analyses showed stronger adiposity-AHI associations in younger adults, while these including hydration parameters, tend to attenuate with age.
Conclusion: Body composition and hydration status were significantly associated with sleep architecture and AHI. These findings support a personalized approach to sleep health and OSA management, emphasizing not only weight control but also muscle preservation and fluid balance optimization.
1 Introduction
Obstructive sleep apnea (OSA) is a common sleep disorder marked by recurrent upper airway collapse during sleep, leading to intermittent oxygen desaturation, disrupted sleep continuity and reduced overall sleep quality. OSA is associated with increased risks of cardiovascular disease, metabolic dysfunction, and neurocognitive decline (1). The apnea-hypopnea index (AHI), which quantifies respiratory events per hour of sleep, is widely used to assess OSA severity. Although obesity has been recognized as a major risk factor for OSA, growing evidence suggests that a more nuanced evaluation of body composition. Body composition (i.e., fat mass (FATM), muscle mass (PMM), bone mass (ONEM), and hydration status) may associate with the pathophysiology and severity of OSA.
Body composition, defined as the relative proportions of fat, muscle, bone, and body water, offers greater specificity than body mass index (BMI) in predicting health outcomes (2–4). Excess adiposity, particularly central and visceral fat, can exacerbate airway obstruction and respiratory instability during sleep (5–8), while greater muscle and bone mass may help preserve upper airway patency (9). Hydration parameters (i.e., total body water (TBW), extracellular water (ECW), and intracellular water (ICW)) may further influence tissue compliance and airway resistance; however, they remain insufficiently studied in the context of OSA.
Despite the well-established association between obesity and OSA (10, 11), limited research has explored specific components of body composition regulates sleep stage architecture. Sleep stages, including light sleep (N1), intermediate (N2), deep sleep (N3), and rapid eye movement (REM) sleep, are increasingly recognized as physiological markers of metabolic and restorative processes. Lighter sleep stages may reflect poor sleep quality and are associated with adverse metabolic outcomes, while deeper sleep stages support tissue recovery and energy balance (12). Together, the effects of fat distribution, muscle mass, and hydration on sleep stages remain unclear.
Sleep disturbances also represent a growing global health concern, with an estimated prevalence of 27% worldwide (13–16). In Taiwan, high rates of sleep dysfunction have been reported across various populations, often coexisting with lifestyle-related and chronic health conditions (17–19). Given the pervasive burden of sleep disorders and their systemic consequences, identifying modifiable physiological contributors such as body composition and fluid balance is essential for effective intervention. The objective of this study is to examine the effects of body composition on AHI, sleep efficiency (SE), and the architecture of sleep stages by sex, age, and BMI strata. The findings may help guide targeted physiology-informed interventions that extend beyond weight management to include strategies focused on muscle preservation, fluid regulation, and sleep stage optimization.
2 Materials and methods
2.1 Study population
This cross-sectional study enrolled 5,381 adult participants (aged 18–80 years) from a sleep center in New Taipei City, Taiwan. The inclusion criteria were subjects ages 18~80 years and diagnosed with OSA (overall AHI ≥ 5 events/hour) by a full-night polysomnography (PSG). We excluded patients with cardiovascular diseases, heart failure, chronic obstructive pneumonia disease, diabetes mellitus, venous insufficiency, renal failure, and patients with hemodialysis. The comorbidity was defined based on clinical diagnosis. The study protocol was reviewed and approved by the Joint Institutional Review Board of Taipei Medical University (TMU-JIRB No. N201912095). As this study employed a retrospective design and involved only routine clinical procedures, the requirement for informed consent was waived.
2.2 Body composition measurements
Body composition was measured before and after overnight PSG using a validated bioelectrical impedance analysis (BIA) device. Parameters recorded included fat mass (FATM), fat percentage (FATP), visceral fat level (VFATL), muscle mass (PMM), fat-free mass (FFM), bone mass (BONEM), and hydration compartments: total body water (TBW), extracellular water (ECW), and intracellular water (ICW). Participants were instructed to fast for 3–4 hours, avoid physical activity for at least 12 hours, and maintain adequate hydration before testing. Bladders were emptied prior to each measurement, and no food or drink was allowed between the pre- and post-sleep assessments.
2.3 Sleep parameter assessment
Sleep parameters were assessed using a digital PSG system (Embla N7000, Medcare, Reykjavik, Iceland), which recorded nasal and oral airflow, thoracic and abdominal respiratory effort, oxygen saturation (SpO2), body movement, sleeping position, and snoring events. All recordings were scored by certified sleep technologists in accordance with the American Academy of Sleep Medicine (AASM) criteria (20, 21). Apnea was defined as a ≥90% reduction in airflow lasting at least 10 seconds, with or without associated desaturation or arousal. Hypopnea was defined as a ≥30% but <90% reduction in airflow lasting at least 10 seconds, accompanied by either ≥3% oxygen desaturation or an arousal. The apnea-hypopnea index (AHI) was computed by dividing the total number of apneas and hypopneas events per hour by the total sleep time (TST) in hours. Sleep architecture was determined by calculating the percentage of TST spent in non-rapid eye movement sleep (N1, N2, N3) and rapid eye movement (REM) sleep. SE was defined as the ratio of TST to time in bed, expressed as a percentage. Wake after sleep onset (WASO) was measured in minutes as the cumulative duration of wakefulness after initial sleep onset.
2.4 Statistical analysis
All statistical analyses were performed using SPSS version 22.0.0.0 for Windows (SPSS Inc., Chicago, IL, USA). Data were first assessed for normality, and extreme values beyond the 1st and 99th percentiles were winsorized to limit the influence of outliers (22). Paired t-tests were applied to compare pre- and post-sleep values of body composition and metabolic parameters. Linear regression analyses were conducted using delta (Δ) values, defined as the difference between post- and pre-sleep measurements. These models examined associations with AHI and other sleep metrics, stratified by sex and body mass index (BMI) categories: normal (<24 kg/m²), overweight (24–27 kg/m²), and obese (≥27 kg/m²). Model 1 adjusted for age and sex, while Model 2 included additional adjustment for BMI. All models controlled for relevant covariates, including waist circumference, neck circumference, FATM, FATP, PMM, VFATL, TBW, ECW, ICW, BONEM, FFM, basal metabolic rate (BMR), metabolic age (METAAGE), impedance (IMP), phase angle (PHASEANGLE), and physical rating (PHYSRATE). Additional regression models were conducted to assess the associations of SE and WASO with body composition and physiological variables, with SE stratified into three categories (≥85%, 70–85%, ≤70%), adjusted for age and sex. Further models examined associations between body composition and sleep stages (N1, N2, N3, REM), adjusted for age and sex. Subgroup analyses included stratification by age group (<50 vs. ≥50 years), adjusted for sex and further stratification by both age and BMI for male and female participants. Regression results were presented as beta coefficients (β) with 95% confidence intervals (CI), and statistical significance was defined as p < 0.05.
3 Results
3.1 Characteristic of study subjects
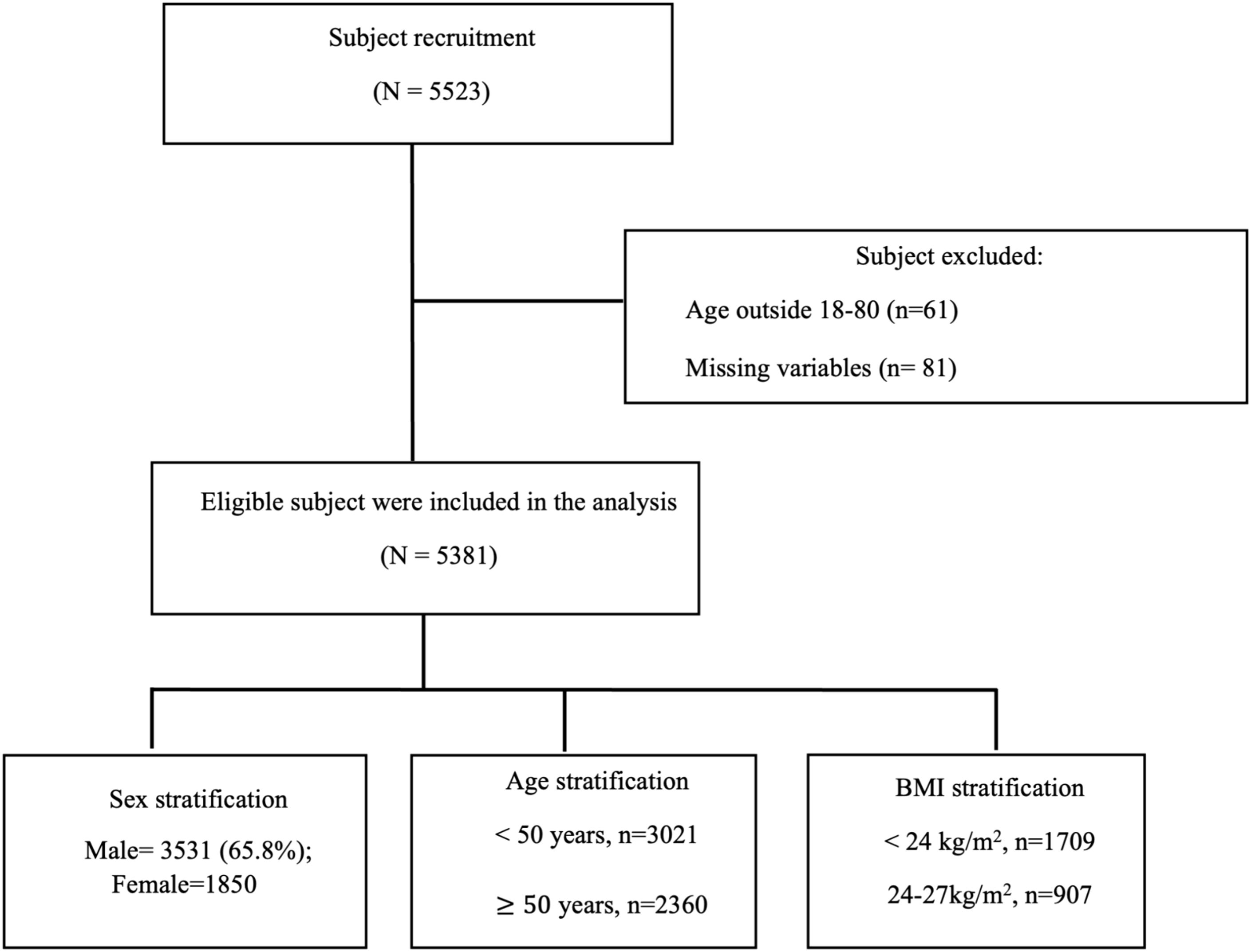
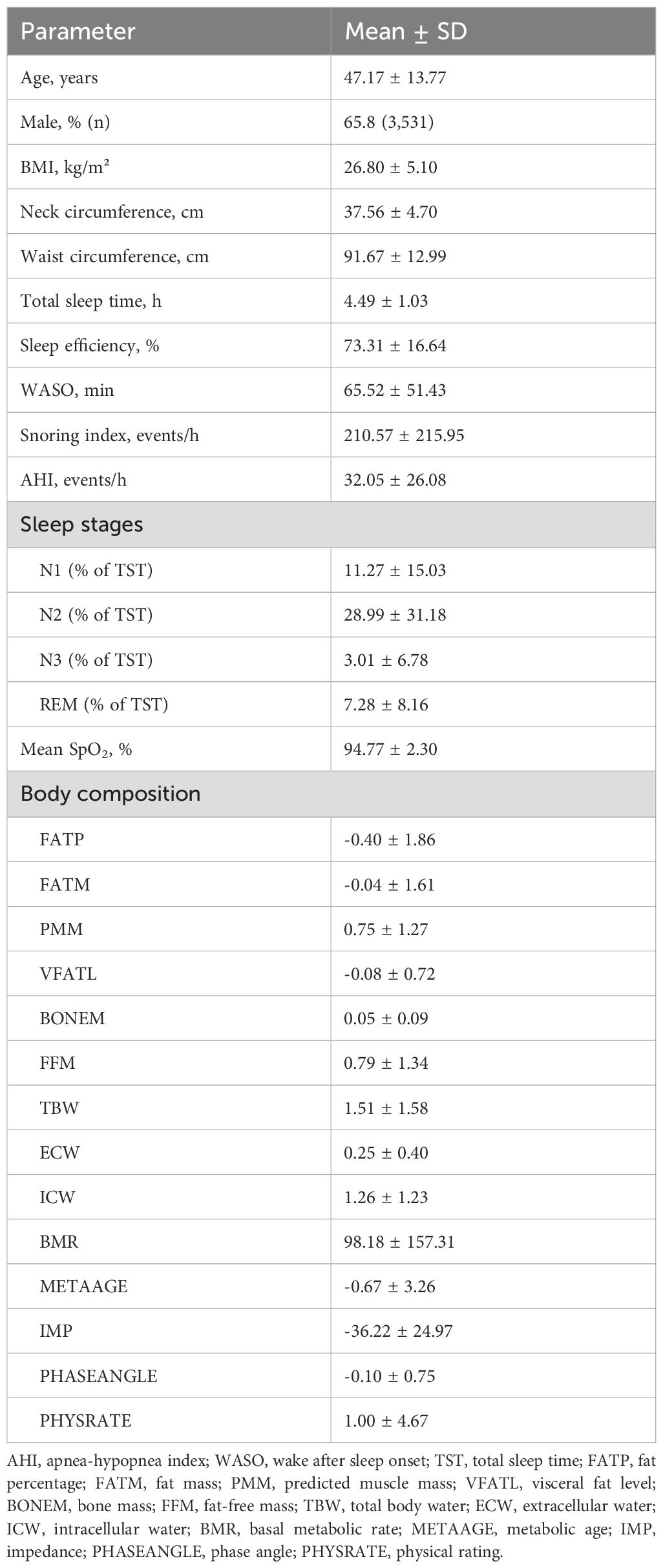
The study subject recruited in this study is shown in Figure 1. The characteristics of the 5,381 study participants are summarized in Table 1. The mean age was 47.17 ± 13.77 years, and 65.8% (n = 3,531) were male. The average BMI was 26.80 ± 5.10 kg/m². The mean neck and waist circumferences were 37.56 ± 4.70 cm and 91.67 ± 12.99 cm, respectively. The average TST was 4.49 ± 1.03 hours, and SE was notably low at 73.31 ± 16.64%. Participants experienced substantial sleep disruption, with an average WASO of 65.52 ± 51.43 minutes and a mean snoring index of 210.57 ± 215.95 events per hour. The AHI was elevated, averaging 32.05 ± 26.08 events per hour. Sleep stage distribution indicated that N1 accounted for 11.27 ± 15.03% of TST, N2 for 28.99 ± 31.18%, N3 for 3.01 ± 6.78%, and REM for 7.28 ± 8.16%. The mean oxygen saturation (SpO2) was reported as 94.77 ± 2.30%. Body composition analysis revealed a FATP of −0.40 ± 1.86 and FATM of −0.04 ± 1.61. The predicted muscle mass (PMM) was 0.75 ± 1.27, and the VFATL was −0.08 ± 0.72. BONEM was 0.05 ± 0.09, while FFM was 0.79 ± 1.34. TBW, ECW, and ICW were 1.51 ± 1.58, 0.25 ± 0.40, and 1.26 ± 1.23, respectively. Metabolic indices showed a BMR of 98.18 ± 157.31 and a METAAGE of −0.67 ± 3.26. IMP was −36.22 ± 24.97, PHASEANGLE was −0.10 ± 0.75, and the PHYSRATE averaged 1.00 ± 4.67.
3.2 Associations between body composition and AHI
Linear regression analyses examining the associations between body composition, hydration status, and metabolic parameters with AHI are presented in Table 2. Unless otherwise specified, all predicators refer to overnight change (△) values. In Model 1, adjusted for age and sex, fat percentage (FATP: β = 1.27, 95% CI: 0.91 to1.63) and fat mass (FATM: β = 1.62, 95% CI: 1.20 to 2.03) were significantly positively associated with AHI (p < 0.05). Conversely, predicted muscle mass (PMM: β = −1.33, 95% CI: −1.87 to −0.80), visceral fat level (VFATL: β = −1.75, 95% CI: −2.67 to −0.82), bone mass (BONEM: β = −20.21, 95% CI: −27.72 to −12.69), and fat-free mass (FFM: β = −1.28, 95% CI: −1.78 to −0.77) showed significant negative associations with AHI (all p < 0.05). Among hydration indices, total body water (TBW: β = 1.17, 95% CI: 0.71 to 1.62) and intracellular water (ICW: β = 1.86, 95% CI: 1.27 to 2.44) were positively correlated with AHI, whereas extracellular water (ECW) was not significantly associated. Metabolic parameters such as basal metabolic rate (BMR: β = −0.01, 95% CI: −0.01 to −0.00) and physical rating (PHYSRATE: β = −0.31, 95% CI: −0.45 to −0.16) were inversely associated with AHI (p < 0.05), while metabolic age (METAAGE), impedance (IMP), and phase angle (PHASEANGLE) showed no significant associations. In Model 2, which additionally adjusted for BMI, the associations remained consistent or became more pronounced. Notably, the positive association between fat mass and AHI increased (β = 1.75, 95% CI: 1.23 to 2.28), while the inverse relationship between muscle mass and AHI strengthened (β = −1.73, 95% CI: −2.28 to −1.17). Similarly, stronger inverse associations were observed for VFATL (β = −3.94), BONEM (β = −25.05), and FFM (β = −1.65). Total body water and intracellular water remained significantly positively associated, while ECW continued to show no significant association.
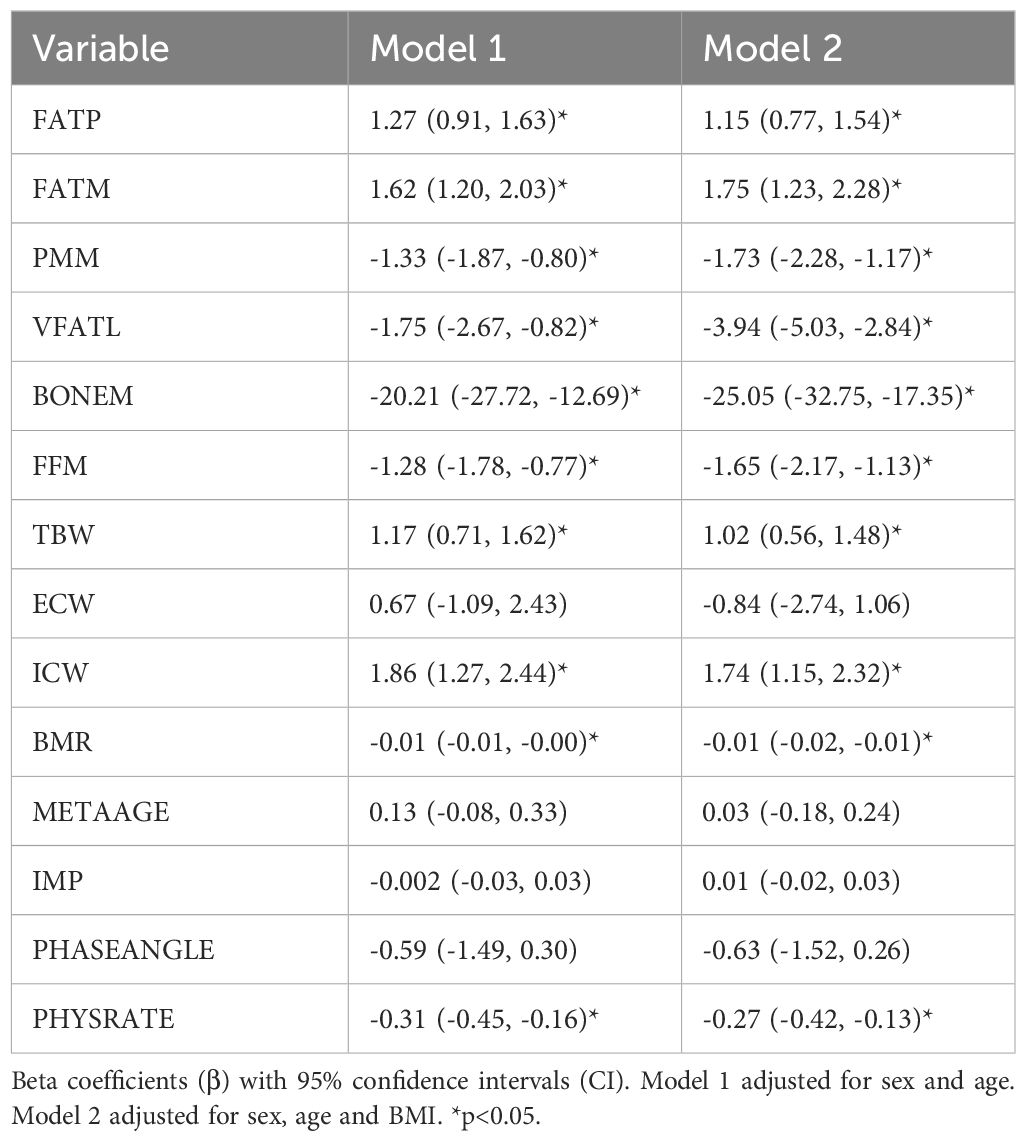
Table 2. Associations of overnight change (△) in body composition, hydration, and metabolic parameters with AHI.
3.3 Associations between physiological parameters, WASO, and sleep efficiency
As shown in Table 3, increased adiposity was significantly associated with longer wake after sleep onset (WASO). Specifically, BMI was positively associated with WASO (β = 4.07, 95% CI: 1.36 to 6.79), as were fat percentage (FATP: β = 1.17, 95% CI: 0.45 to 1.88), fat mass (FATM: β = 1.67, 95% CI: 0.84 to 2.50), and visceral fat level (VFATL: β = 2.72, 95% CI: 0.88 to 4.56). Conversely, bone mass was negatively associated with WASO (BONEM: β = −17.49, 95% CI: −32.46 to −2.52) and was inversely associated with WASO. For sleep efficiency, no adiposity or muscle-related metrics showed significant associations across efficiency categories (≥85%, 70–85%, ≤70%). However, basal metabolic rate (BMR) was significantly associated with lower sleep efficiency in the ≤70% group (β = 4.76, 95% CI: <0.001 to <0.001), and impedance (IMP) was also positively associated with poor sleep efficiency (85-70%) (β = 6.43, 95% CI: <0.001 to <0.001).
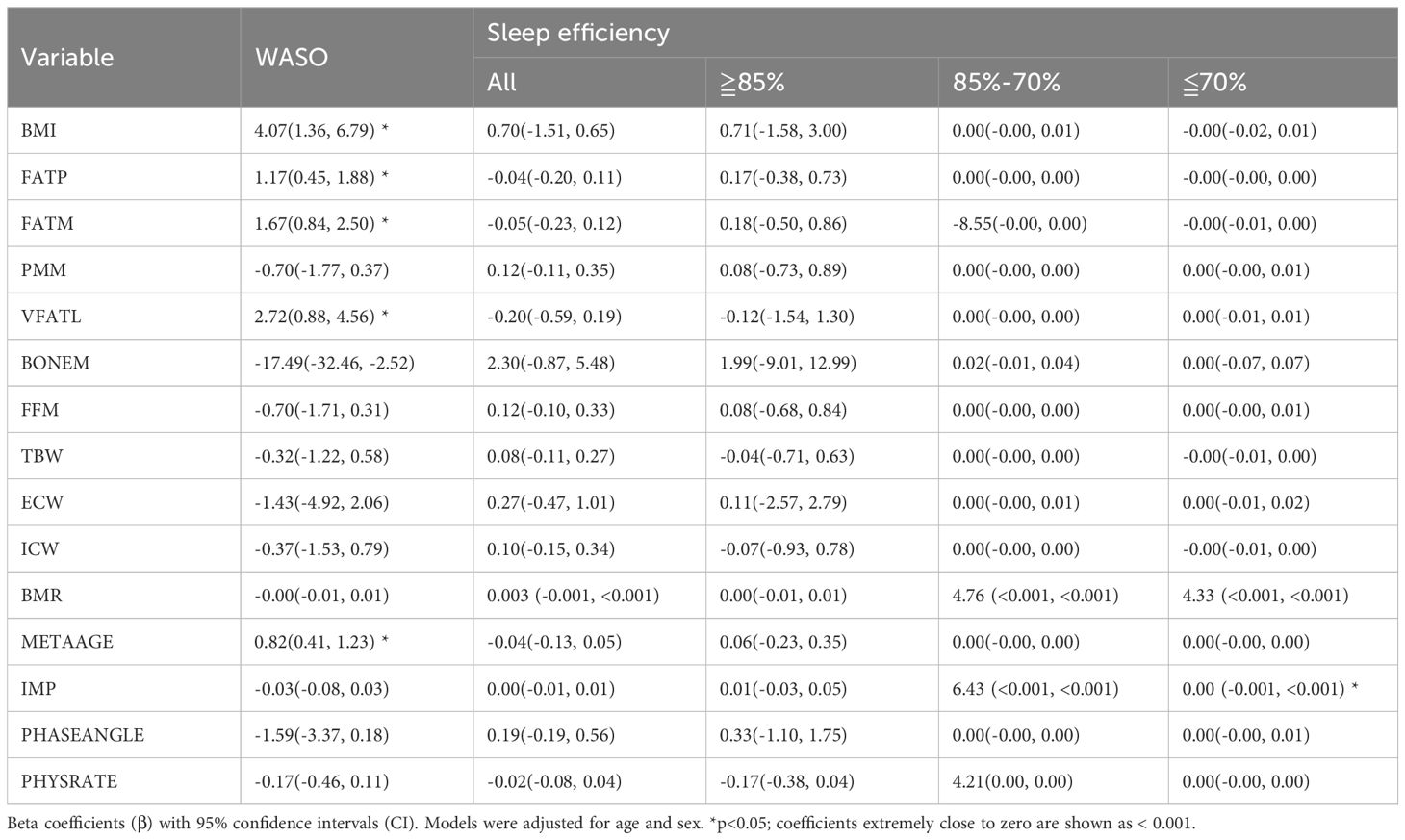
Table 3. Associations of overnight change (△) in body composition, hydration, and metabolic parameters with wake after sleep onset (WASO) and sleep efficiency.
3.4 Associations between physiological parameters and sleep stage
Table 4 presents the regression analysis of physiological parameters with sleep stage architecture. Fat mass (FATM) was positively associated with N1 sleep (β = 0.26, 95% CI: 0.01 to 0.51), while no significant associations were found between adiposity measures and N2, N3, or REM stages. Predicted muscle mass (PMM) showed a marginal association with increased N3 sleep and REM sleep. Bone mass (BONEM) was significantly associated with increased N2 (β = 9.62, 95% CI: 0.21 to 19.04), N3 (β = 2.10, 95% CI: 0.10 to 4.09), and REM sleep (β = 3.50, 95% CI: 1.03 to 5.97). Among hydration metrics, total body water (TBW) was positively associated with N1 (β = 0.53, 95% CI: 0.26 to 0.80), and intracellular water (ICW) was also significantly associated with N1 sleep (β = 0.75, 95% CI: 0.40 to 1.10). Extracellular water (ECW) was positively associated with REM sleep (β = 0.64, 95% CI: 0.06 to 1.22). Impedance (IMP) showed a significant inverse relationship with N2 sleep (β = −0.04, 95% CI: −0.07 to −0.01), while higher physical rating (PHYSRATE) was associated with reduced N1 sleep (β = −0.11, 95% CI: −0.19 to −0.02). Bone and muscle mass were positively associated with higher percentages of N2, N3 and REM, whereas higher impedance and lower physical rating were associated with a higher percentage of N1.
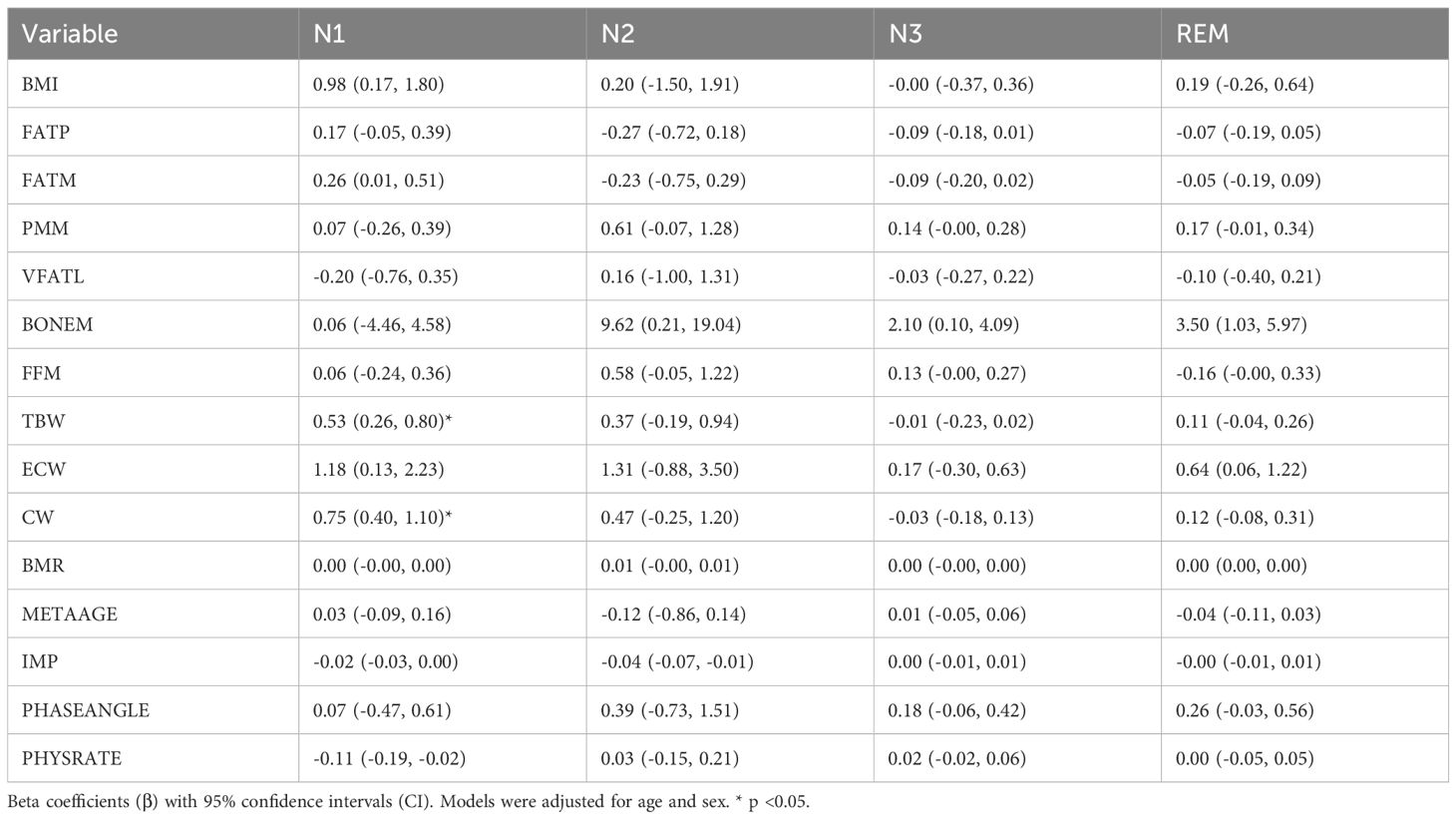
Table 4. Associations of overnight change (△) in body composition, hydration, and metabolic parameters with sleep stage .
3.5 Associations of sex- and BMI-specific body composition with AHI
Table 5 presents the results of sex- and BMI-specific linear regression analyses assessing the associations between body composition, hydration, and metabolic parameters with AHI. In sex-stratified models (adjusted for age and BMI), fat percentage (FATP) and fat mass (FATM) were positively associated with AHI in both males (β = 0.94, 95% CI: 0.45 to 1.43; β = 1.27, 95% CI: 0.64 to 1.90, respectively) and females (β = 1.69, 95% CI: 1.07 to 2.31; β = 3.40, 95% CI: 2.43 to 4.36, respectively) (all p < 0.05). Conversely, muscle-related indices such as predicted muscle mass (PMM), fat-free mass (FFM), and bone mass (BONEM) showed significant negative associations with AHI, with stronger effects observed in females (e.g., PMM: β = −3.48, 95% CI: −4.51 to −2.45) than in males (β = −1.24, 95% CI: −1.91 to −0.58). Hydration parameters showed contrasting associations by sex: total body water (TBW), extracellular water (ECW), and intracellular water (ICW) were positively associated with AHI in males (e.g., TBW: β = 1.61, 95% CI: 1.08 to 2.13), but ECW negatively associated in females (β = −19.21, 95% CI: −22.82 to −15.59). Basal metabolic rate (BMR) was inversely associated with AHI in both sexes, with a stronger effect in females (β = −0.03, 95% CI: −0.04 to −0.02). Physical rating (PHYSRATE) also exhibited a significant negative correlation with AHI in both males and females (p < 0.05). In the BMI-stratified models (adjusted for age and sex), most associations between body composition and AHI were not statistically significant in the normal (BMI < 24) and overweight (BMI 24–27) subgroups. However, in the obese group (BMI ≥ 27), fat mass (β = 0.85, 95% CI: 0.26 to 1.44), muscle mass (β = −0.87, 95% CI: −1.72 to −0.02), bone mass (β = −15.16, 95% CI: −27.45 to −2.86), and total body water (β = 1.08, 95% CI: 0.39 to 1.77) showed significant associations with AHI (p < 0.05). In addition, intracellular water (ICW) was positively associated (β = 1.77, 95% CI: 0.87 to 2.67), while impedance (IMP: β = −0.08) was negatively associated with AHI in the obese subgroup (p < 0.05). In stratified models, the magnitude of associations between body composition, hydration status, and AHI differed by sex and BMI category, with stronger and more consistent relationships observed in females and individuals with obesity. For BMI categories, in the obese group, fat mass was significantly associated with increased AHI (FATM: β=0.85, CI: 0.26 to 1.44). Intracellular water (ICW) also showed a strong positive correlation (β=1.77, CI: 0.87 to 2.67), and total body water (TBW) was positively associated with AHI (β=1.08, CI: 0.39 to 1.77). Additionally, bone mass (BONEM) was negatively correlated with AHI (β=-15.16, CI: -27.45 to -2.86), consistent with higher AHI co-occurring with greater total and intracellular water in individuals with obesity.
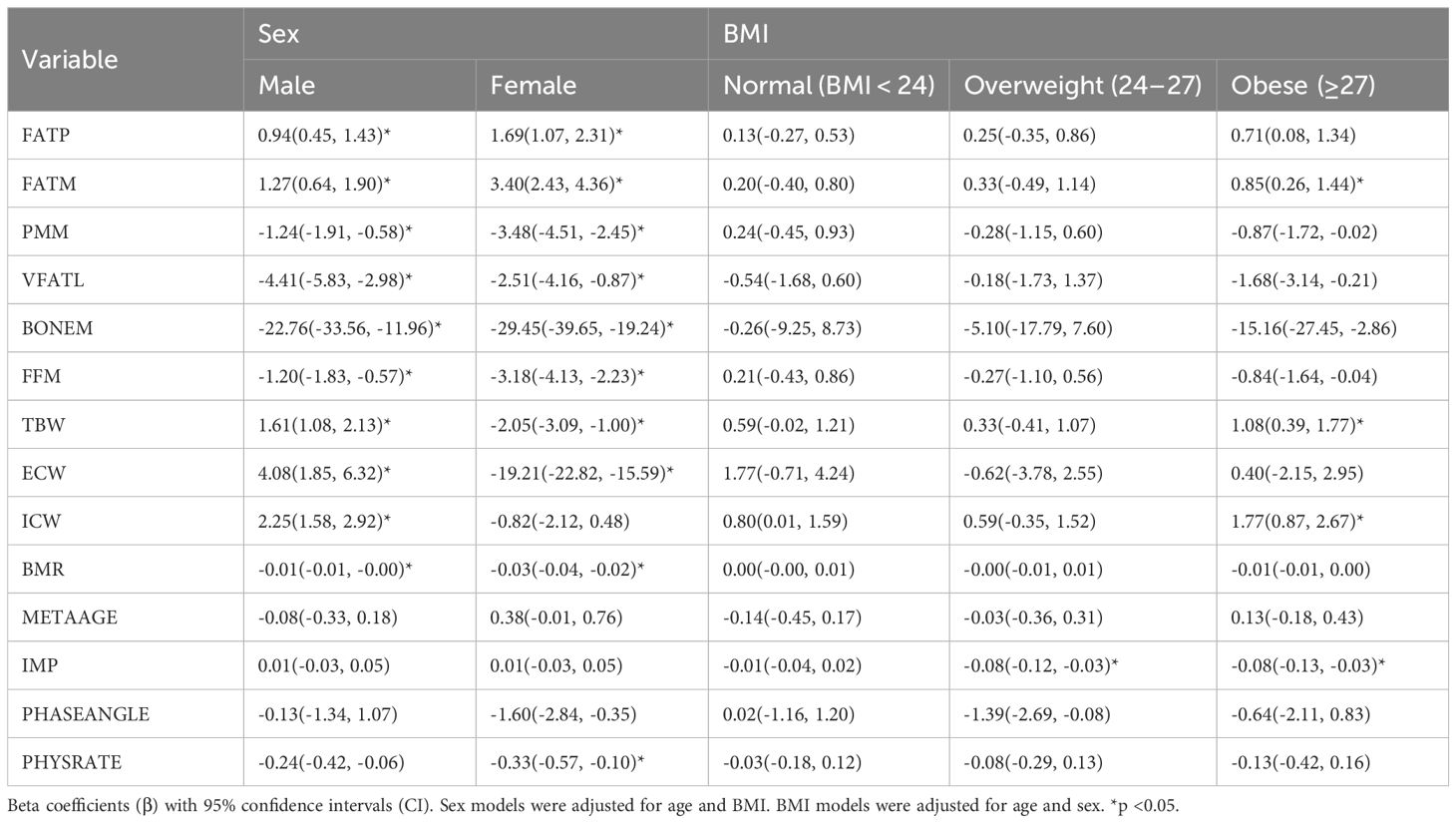
Table 5. Associations of sex- and BMI-specific overnight change (△) in body composition, hydration, and metabolic parameters with the apnea-hypopnea index (AHI).
3.6 Age-stratified analysis of physiological parameters
Table 6 presents a comparative analysis of physiological parameters stratified by age (<50 vs. ≥50 years) with models adjusted for sex. Among participants aged <50 years, body mass index (BMI) (β = 4.03, 95% CI: 2.22 to 5.84), fat percentage (FATP: β = 1.59, 95% CI: 1.03 to 2.15), fat mass (FATM: β = 2.09, 95% CI: 1.49 to 2.69), and hydration markers (i.e. total body water (TBW: β = 1.74, 95% CI: 1.09 to 2.38), and intracellular water (ICW: β = 2.51, 95% CI: 1.68 to 3.33)) were all significantly elevated. In contrast, muscle mass (PMM: β =-1.45, 95% CI:-2.30 to -0.60), visceral fat (VFATL: β = −1.97, 95% CI: −3.31 to −0.63), bone mass (BONEM: β = −20.23, 95% CI: −31.90 to −8.55), and fat free mass (FFM: β =-1.38, 95% CI:-2.18 to -0.59) were significantly reduced in this group. Among participants aged ≥50 years, the associations were less pronounced; however, a significant increases were observed in FATP (β = 0.84, 95% CI: 0.39 to 1.30), FATM (β = 0.95, 95% CI: 0.38 to 1.51), TBW (β = 0.92, 95% CI: 0.30 to 1.53), and ICW (β = 1.60, 95% CI: 0.81 to 2.40). Bone mass was significantly lower in this group (BONEM: β = −17.22, 95% CI: −26.77 to −7.66), as was physical rating (PHYSRATE: β = −0.29, 95% CI: −1.48 to −0.11). These results indicate that while younger adults exhibited broad associations with AHI across adiposity, muscle, bone and hydration parameters, older adults showed significant associations mainly with fat mass, bone mass, hydration, and physical fitness.
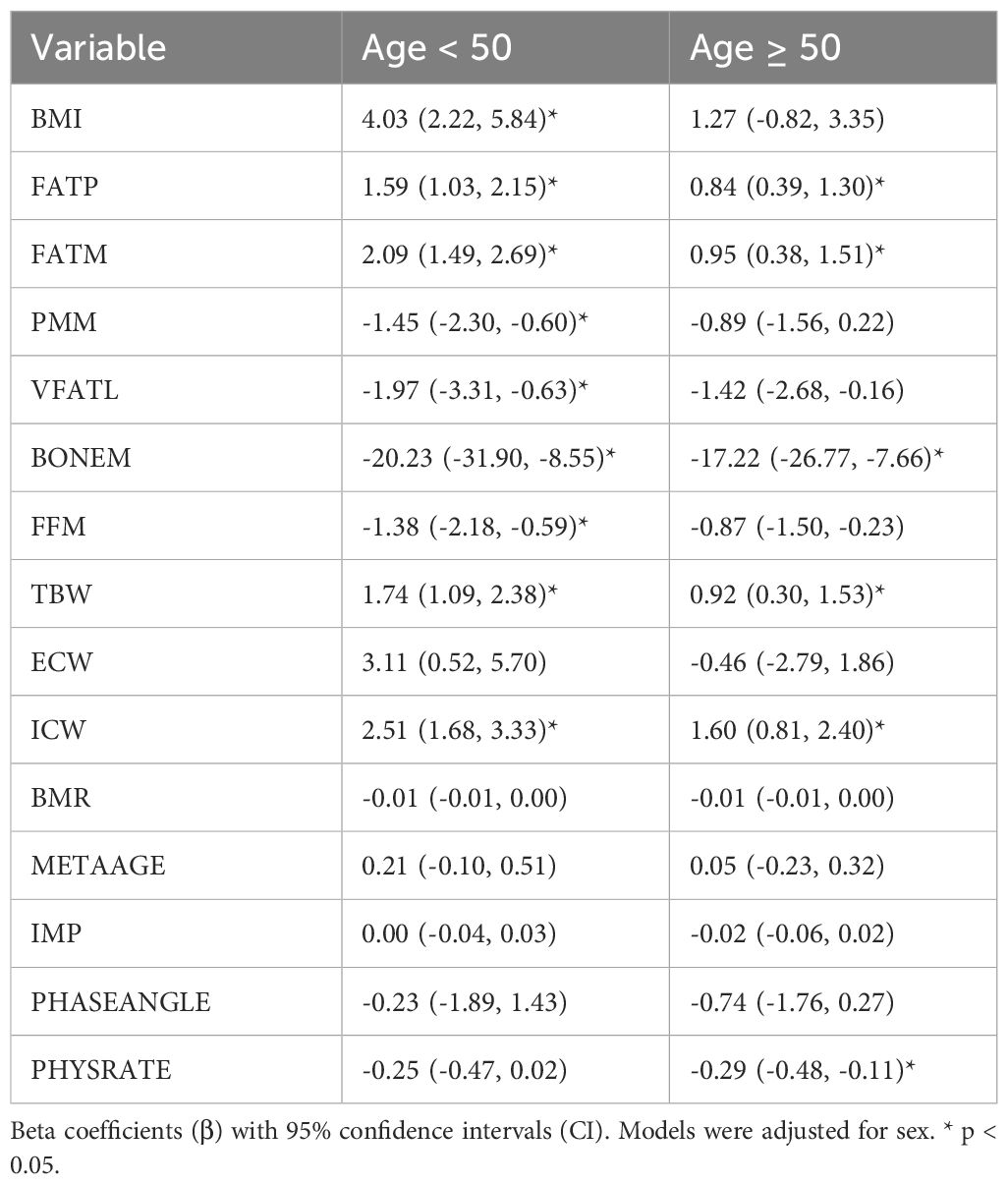
Table 6. Associations of Age-Related Overnight Change (△) in Body Composition, Hydration, and Metabolic Parameters with the Apnea-Hypopnea Index (AHI).
4 Discussion
The findings of this study show the multifactorial relationships between physiological parameters and sleep-disordered breathing, particularly OSA, as measured by the AHI. Notably, significant associations were observed between body composition, hydration status, and AHI, with both muscle and fat metrics playing critical roles. Increased fat mass and reduced muscle mass were significantly associated with higher AHI, aligning with previous evidence suggesting that excess adiposity is linked to greater airway collapsibility and inflammation, whereas lower lean mass may be associated with reduced airway stability (23–25). Recent studies corroborate associations of trunk fat and total body water with OSA severity and imaging work differentiates structural from functional muscle indices (26–28). Similarly, the positive associations between fat metrics and AHI emphasize the role of adiposity in exacerbating OSA severity (29), whereas the inverse relationships observed for muscle and bone mass highlight the potential protective effect of better musculoskeletal health, although the mechanisms remain complex (9). We observed bone mass showed a strong inverse relationship with AHI and WASO. Given that BIA-derived bone mass typically tracks overall physique greater lean and skeletal frame size with lower adiposity and fluid burden rather than a bone-specific effect. This interpretation is consistent with our boarder pattern, higher lean mass, and skeletal frame co-occurred with lower AHI and better sleep continuity, suggesting that the BONEM coefficient largely indexes overall physique rather than a bone-specific airway mechanism. Furthermore, the positive association between ICW and AHI suggests a potential role for fluid retention, may be related to airway narrowing during sleep (30).
In terms of sleep disruption, our findings demonstrated that increased WASO was significantly associated with higher BMI, fat percentage, visceral fat levels, and reduced bone mass. These results are consistent with previous research indicating that disrupted sleep is associated with greater adiposity and lower bone mineral density (31). In this cohort, WASO showed more frequent and stronger associations with body composition variables than sleep efficiency. This pattern highlights sleep continuity as a key phenotypic correlate; whether enhancing continuity improves metabolic or cardiometabolic outcomes requires prospective evaluation. This aligns with prior work showing that simple anthropometric parameters can rapidly and reliably identify cardiometabolic risk in patients with OSA without the need for laboratory tests (32).
When examining sleep stage architecture, we found that lighter sleep stages, particularly N1, were positively associated with BMI, TBW, and ICW, consistent with links between disrupted sleep and fluid retention. These findings align with existing literature indicating that poor sleep quality is associated with metabolic dysregulation, appetite hormone imbalance, and higher adiposity (33–36). In contrast, deeper sleep stages such as N2 and N3 were positively associated with muscle mass and bone indices; mechanistic implications require confirmation. Interestingly, VFATL did not show significant associations with sleep stage distribution, contrasting with some prior studies suggesting disrupted sleep increases visceral adiposity (37, 38). This discrepancy may be due to methodological differences, population variation, or the complexity of fat redistribution, highlighting the need for further mechanistic exploration. The association between REM sleep and bone mass also highlights potential systemic benefits of sleep architecture beyond cognitive function.
Sex- and BMI-stratified analyses further clarified these relationships. Stronger associations between AHI and body composition were observed in females and individuals with obesity, reinforcing findings from previous studies that link BMI with OSA severity (29, 39, 40). Our sex-stratified analyses revealed that hydration indices were positively associated with AHI in men but inversely or non-significantly associated in women, with a particularly strong negative association for ECW in women. This discrepancy may reflect hormonal influence on fluid regulation and airway physiology, as well as sex-specific difference in fat distribution. Women’s greater subcutaneous and peripheral fat depots may buffer extracellular fluid shifts differently from the visceral predominance in men, thereby modifying the effect of rostral fluid redistribution on pharyngeal tissues. Additional contributors, such as vascular compliance, lymphatic drainage or soft-tissue composition, may further account for these sex-specific patterns. In the obese group, increased fat mass and ICW were particularly associated with higher AHI, suggesting that both adiposity and fluid retention may contribute to sleep-disordered breathing (41, 42). These findings emphasize the significance of targeting both weight control and fluid regulation as part of comprehensive OSA management. From a clinical perspective, these subgroup findings suggest that weight control and fluid regulation should be core components of OSA management, especially in high-risk groups. Age-stratified analysis revealed that adiposity-related measures such as fat percentage, and fat mass remained significantly associated with AHI across both age groups, with stronger associations observed in individuals under 50 years. In contrast, older adults exhibited more pronounced reductions in bone mass and physical rating, consistent with aging-related physiological decline (43, 44). The shift from adiposity-driven factors in younger adults to a greater involvement of musculoskeletal and hydration factors in older adults, suggests a need for age-tailored preventive and therapeutic strategies (45–47).
Sex-specific trends were also evident. In younger males, obesity was associated with increased fat mass, TBW, ECW, and ICW, indicating a tendency toward both increased adiposity and fluid retention. In older males, body composition parameters were not significantly associated with AHI, with fat-related variables in particular showing a marked reduction in relevance with age. In contrast, adiposity remained a key factor across all age groups in females, with postmenopausal women particularly affected by increased visceral fat and reduced bone mass (48, 49). These observations support evidence that hormonal changes, particularly estrogen decline, may amplify OSA risk through metabolic and anatomical pathways.
A more granular, hierarchical analysis integrating age, sex, and BMI showed a reduction in predicted muscle mass and fat-free mass among younger obese women, although not statistically significant, suggesting a potential for early sarcopenic obesity (50, 51).
Moreover, ECW levels were significantly lower in younger obese individuals and trended similarly in older age groups, reflecting fluid distribution imbalances possibly related to inflammation and cellular water dysregulation (52, 53). In younger obese males, increased fat mass, TBW, ECW, and ICW were associated with OSA severity, supporting evidence that fluid intake and body composition interact in early adulthood (54–56). In older males, these associations diminished, suggesting a shift in the drivers of sleep-related outcomes from adiposity to lean mass and hydration stability (57). BMR, phase angle, and ECW showed limited associations across groups. Their clinical relevance may require larger datasets or more sensitive methodologies to detect meaningful effects. Nonetheless, these findings contribute to a broader understanding of physiological profiles linked to sleep health.
Our findings provide valuable guidance for tailoring interventions. Enhancing sleep quality, particularly deep sleep stages, may benefit muscle and bone health while reducing AHI. Weight loss, muscle preservation, and hydration management are crucial in younger and obese individuals, whereas older adults may benefit from interventions focused on lean mass retention and fluid balance. Such targeted strategies are essential for improving sleep-disordered breathing and related health outcomes. It is important to note that △ values derived from pre- to post-sleep BIA assessment may not exclusively represent true metabolic changes in fat or muscle compartments. BIA is highly sensitive to body fluid distribution, and an overnight recumbent position is known to induce a rostral fluid shift. Therefore, a proportion of the observed △ values may reflect postural fluid dynamics rather than actual alterations in tissue composition. This study has some limitations. Owing to its cross-sectional design, interpretation is limited to statistical associations between physiological parameters and sleep outcomes; predictive or causal claims are beyond the scope of this work. Furthermore, potential confounders such as lifestyle behavior, diet, physical activity, and comorbidities were not available in our study. In addition, there are more limitations that should be acknowledged. First as a retrospective single-center study, the generalizability of our findings may be limited, and selection bias cannot be excluded. Second, important biochemical markers such as lipid profiles, HbA1c, and inflammatory parameters were not available, which restricts our ability to explore mechanistic links with cardiometabolic risk. Third, we did not have an independent external validation cohort and replication in other populations will be needed to confirm our findings. Finally, although anthropometric and bioimpedance assessments were conducted by trained staff under standardized conditions, potential inter-observer variability cannot be entirely excluded.
5 Conclusion
In conclusion, this study identifies sleep quality, particularly the distribution of sleep stages, as a key factor associated with body composition and hydration status. These findings highlight the potential of targeting sleep architecture, especially enhancing deeper sleep and reducing lighter sleep stages, as a therapeutic approach to support metabolic and physiological health.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the Joint Institutional Review Board of Taipei Medical University (TMU-JIRB No. N201912095). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this study employed a retrospective design and involved only routine clinical procedures. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Y-MC: Conceptualization, Formal Analysis, Methodology, Visualization, Writing – original draft. W-TL: Investigation, Methodology, Resources, Writing – review & editing. C-YT: Investigation, Methodology, Resources, Writing – review & editing. Y-CL: Data curation, Resources, Validation, Writing – review & editing. KH: Formal Analysis, Methodology, Visualization, Writing – review & editing. K-JC: Methodology, Resources, Visualization, Writing – review & editing. H-CC: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing, Data curation, Investigation, Methodology, Resources, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by the National of Science and Technology of Taiwan (111-2314-B-038-079, 112-2314-B-038-013, 112-2628-B-038-010-MY3, and 113-2314-B-038-004).
Acknowledgments
The authors gratefully acknowledge the provision of de-identified polysomnography and body composition data by the Shuang Ho Hospital Sleep Center. All authors carefully reviewed and revised the content following the use of this tool and took full responsibility for the last version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. This manuscript was edited for language clarity using ChatGPT, an AI-based tool developed by OpenAI (San Francisco, CA, USA).
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Alterki A, Abu-Farha M, Al Shawaf E, Al-Mulla F, and Abubaker J. Investigating the relationship between obstructive sleep apnoea, inflammation and cardio-metabolic diseases. Int J Mol Sci. (2023) 24:6807. doi: 10.3390/ijms24076807
2. Carlone Baldino Garcia N, Lopes WA, Locateli JC, Ferraz Simoes C, de Oliveira GH, de Souza Mendes VH, et al. Multidisciplinary obesity treatment program improved health-related quality of life and positively correlated with anthropometric and body composition but not with cardiorespiratory fitness parameters in adolescents. Qual Life Res. (2019) 28:1803–12. doi: 10.1007/s11136-019-02141-9
3. Chen Y, Li C, Cheng S, Pan C, Zhang H, Zhang J, et al. The causal relationships between sleep-related phenotypes and body composition: A mendelian randomized study. J Clin Endocrinol Metab. (2022) 107:e3463–73. doi: 10.1210/clinem/dgac234
4. Uritani D, Matsumoto D, Asano Y, Yoshizaki K, Nishida Y, and Shima M. Effects of regular exercise and nutritional guidance on body composition, blood pressure, muscle strength and health-related quality of life in community-dwelling Japanese women. Obes Res Clin Pract. (2013) 7:e155–63. doi: 10.1016/j.orcp.2011.10.005
5. Kim SR, Lee G, Choi S, Oh YH, Son JS, Park M, et al. Changes in predicted lean body mass, appendicular skeletal muscle mass, and body fat mass and cardiovascular disease. J Cachexia Sarcopenia Muscle. (2022) 13:1113–23. doi: 10.1002/jcsm.12962
6. Kuriyan R. Body composition techniques. Indian J Med Res. (2018) 148:648–58. doi: 10.4103/ijmr.IJMR_1777_18
7. Tung NT, Lin SY, Dung HB, Thuy TPC, Kuan YC, Tsai CY, et al. Associations of overnight changes in body composition with positional obstructive sleep apnea. Sleep Breath. (2023) 27:631–40. doi: 10.1007/s11325-022-02664-5
8. Bonsignore MR. Obesity and obstructive sleep apnea. In: Eckel J and Clément K, editors. From Obesity to Diabetes. Springer International Publishing, Cham (2022). p. 181–201.
9. Matsumoto T, Tanizawa K, Tachikawa R, Murase K, Minami T, Inouchi M, et al. Associations of obstructive sleep apnea with truncal skeletal muscle mass and density. Sci Rep. (2018) 8:6550. doi: 10.1038/s41598-018-24750-z
10. Drager LF, Togeiro SM, Polotsky VY, and Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. (2013) 62:569–76. doi: 10.1016/j.jacc.2013.05.045
11. Ryan S, Arnaud C, Fitzpatrick SF, Gaucher J, Tamisier R, and Pepin JL. Adipose tissue as a key player in obstructive sleep apnoea. Eur Respir Rev. (2019) 28:190006. doi: 10.1183/16000617.0006-2019
12. Balakrishnan G, Burli D, Behbehani K, Burk JR, and Lucas EA. “Comparison of a sleep quality index between normal and obstructive sleep apnea patients,” In: Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, 17–18 Jan 2006. New York, NY, USA: IEEE. (2006). pp. 1154-7.
13. Acquavella J, Mehra R, Bron M, Suomi JM, and Hess GP. Prevalence of narcolepsy and other sleep disorders and frequency of diagnostic tests from 2013–2016 in insured patients actively seeking care. J Clin Sleep Med. (2020) 16:1255–63. doi: 10.5664/jcsm.8482
14. Li CH, Huang KY, Chen WC, Chen CH, Tu CY, Lin CL, et al. Sleep disorders in individuals without sleep apnea increase the risk of peripheral arterial disorder: a nationwide population-based retrospective cohort study. Sleep Med. (2015) 16:966–70. doi: 10.1016/j.sleep.2015.02.538
15. Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. (2002) 6:97–111. doi: 10.1053/smrv.2002.0186
16. Tiseo C, Vacca A, Felbush A, Filimonova T, Gai A, Glazyrina T, et al. Migraine and sleep disorders: a systematic review. J Headache Pain. (2020) 21:126. doi: 10.1186/s10194-020-01192-5
17. Chang YH, Chen YC, Ku LE, Chou YT, Chen HY, Su HC, et al. Association between sleep health and intrinsic capacity among older adults in Taiwan. Sleep Med. (2023) 109:98–103. doi: 10.1016/j.sleep.2023.06.016
18. Chen CM, Kuo CY, Wu MN, Hung JY, Hsu CY, and Tsai MJ. Increased risk of major depressive disorder in sleep apnea patients in Taiwan. Sci Rep. (2021) 11:765. doi: 10.1038/s41598-020-80759-3
19. Lin CE, Chung CH, Chen LF, Chien WC, and Chou PH. The impact of antidepressants on the risk of developing obstructive sleep apnea in posttraumatic stress disorder: A nationwide cohort study in Taiwan. J Clin Sleep Med. (2019) 15:1233–41. doi: 10.5664/jcsm.7910
20. Berry RB, Brooks R, Gamaldo C, Harding SM, Lloyd RM, Quan SF, et al. AASM scoring manual updates for 2017 (Version 2.4). J Clin Sleep Med. (2017) 13:665–6. doi: 10.5664/jcsm.6576
21. Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an american academy of sleep medicine clinical practice guideline. J Clin Sleep Med. (2017) 13:479–504. doi: 10.5664/jcsm.6506
22. Tsai DH, Riediker M, Wuerzner G, Maillard M, Marques-Vidal P, Paccaud F, et al. Short-term increase in particulate matter blunts nocturnal blood pressure dipping and daytime urinary sodium excretion. Hypertension. (2012) 60:1061–9. doi: 10.1161/HYPERTENSIONAHA.112.195370
23. Ekingen T, Sob C, Hartmann C, Ruhli FJ, Matthes KL, Staub K, et al. Associations between hydration status, body composition, sociodemographic and lifestyle factors in the general population: a cross-sectional study. BMC Public Health. (2022) 22:900. doi: 10.1186/s12889-022-13280-z
24. Laja Garcia AI, Morais-Moreno C, Samaniego-Vaesken ML, Puga AM, Varela-Moreiras G, and Partearroyo T. Association between hydration status and body composition in healthy adolescents from Spain. Nutrients. (2019) 11:2692. doi: 10.3390/nu11112692
25. Serra-Prat M, Lorenzo I, Martinez J, Palomera E, Pleguezuelos E, and Ferrer P. Relationship between hydration status and muscle catabolism in the aged population: A cross-sectional study. Nutrients. (2023) 15:4718. doi: 10.3390/nu15224718
26. Beyazal Polat H, Ozyurt S, Tastan M, Beyazal Celiker F, Beyazal M, Sahin U, et al. Muscle and fat composition in OSA: A CT-based study. J Clin Med. (2025) 14:4647. doi: 10.3390/jcm14134647
27. Gao Y, Zhao L, Fan L, Cai W, Rui D, Zhao Z, et al. Decreased muscle percentage and increased fat percentage associated with obstructive sleep apnea syndrome severity in males: Insight from body composition and polysomnogram. Heliyon. (2025) 11:e143131. doi: 10.1016/j.heliyon.2025.e43131
28. Yetim M, Kalcik M, Bekar L, Karavelioglu Y, and Yilmaz YA. Body composition analysis in obstructive sleep apnea: A cross-sectional study using bioelectrical impedance analysis. Clin Respir J. (2025) 19:e70123. doi: 10.1111/crj.70123
29. Bonsignore MR, McNicholas WT, Montserrat JM, and Eckel J. Adipose tissue in obesity and obstructive sleep apnoea. Eur Respir J. (2012) 39:746–67. doi: 10.1183/09031936.00047010
30. Liao Z, Chen Y, Wu L, Huang Y, Li S, Liu J, et al. Associations of obstructive sleep apnea risk with obesity, body composition and metabolic abnormalities in school-aged children and adolescents. Nutrients. (2024) 16:2419. doi: 10.3390/nu16152419
31. Jurado-Fasoli L, Amaro-Gahete FJ, De-la OA, Dote-Montero M, Gutierrez A, and Castillo MJ. Association between sleep quality and body composition in sedentary middle-aged adults. Med (Kaunas). (2018) 54:91. doi: 10.3390/medicina54050091
32. Zorlu D, Boduroğlu Y, and Ertürk A. Echocardiographic evaluation from a different perspective in asthmatic patients. Tuberk Toraks. (2022) 70:166–78. doi: 10.5578/tt.20229807
33. Gangwisch JE. Epidemiological evidence for the links between sleep, circadian rhythms and metabolism. Obes Rev. (2009) 10 Suppl 2:37–45. doi: 10.1111/j.1467-789X.2009.00663.x
34. Gonnissen HK, Mazuy C, Rutters F, Martens EA, Adam TC, and Westerterp-Plantenga MS. Sleep architecture when sleeping at an unusual circadian time and associations with insulin sensitivity. PloS One. (2013) 8:e72877. doi: 10.1371/journal.pone.0072877
35. Gonnissen HK, Rutters F, Mazuy C, Martens EA, Adam TC, and Westerterp-Plantenga MS. Effect of a phase advance and phase delay of the 24-h cycle on energy metabolism, appetite, and related hormones. Am J Clin Nutr. (2012) 96:689–97. doi: 10.3945/ajcn.112.037192
36. Westerterp-Plantenga MS. Sleep, circadian rhythm and body weight: parallel developments. Proc Nutr Soc. (2016) 75:431–9. doi: 10.1017/S0029665116000227
37. Borel AL. Sleep apnea and sleep habits: relationships with metabolic syndrome. Nutrients. (2019) 11:2628. doi: 10.3390/nu11112628
38. Nedeltcheva AV and Scheer FA. Metabolic effects of sleep disruption, links to obesity and diabetes. Curr Opin Endocrinol Diabetes Obes. (2014) 21:293–8. doi: 10.1097/MED.0000000000000082
39. Fattal D, Hester S, and Wendt L. Body weight and obstructive sleep apnea: a mathematical relationship between body mass index and apnea-hypopnea index in veterans. J Clin Sleep Med. (2022) 18:2723–9. doi: 10.5664/jcsm.10190
40. Leppanen T, Kulkas A, Mervaala E, and Toyras J. Increase in body mass index decreases duration of apneas and hypopneas in obstructive sleep apnea. Respir Care. (2019) 64:77–84. doi: 10.4187/respcare.06297
41. Hashiguchi MH, Chubachi S, Yamasawa W, Otsuka K, Harada N, Miyao N, et al. Interaction of BMI and respiratory status in obstructive sleep apnea, a cross-sectional COPD study. NPJ Prim Care Respir Med. (2023) 33:30. doi: 10.1038/s41533-023-00351-w
42. Uzair A, Waseem M, Bin Shahid A, Bhatti NI, Arshad M, Ishaq A, et al. Correlation between body mass index and apnea-hypopnea index or nadir oxygen saturation levels in patients with obstructive sleep apnea. Cureus. (2024) 16:e59066. doi: 10.7759/cureus.59066
43. Moreira VC, Silva CMS, Welker AF, and da Silva ICR. Visceral adipose tissue influence on health problem development and its relationship with serum biochemical parameters in middle-aged and older adults: A literature review. J Aging Res. (2022) 2022:8350527. doi: 10.1155/2022/8350527
44. Briand M, Raffin J, Gonzalez-Bautista E, Ritz P, Abellan Van Kan G, Pillard F, et al. Body composition and aging: cross-sectional results from the INSPIRE study in people 20 to 93 years old. Geroscience. (2025) 47:863–75. doi: 10.1007/s11357-024-01245-6
45. Palmer AK and Jensen MD. Metabolic changes in aging humans: current evidence and therapeutic strategies. J Clin Invest. (2022) 132:e158451. doi: 10.1172/JCI158451
46. Wang Y, Shen S, Han P, Zheng K, Chen C, Wu Y, et al. The association between visceral fat obesity and prefrailty in Chinese older adults: a cross-sectional study. BMC Endocr Disord. (2024) 24:136. doi: 10.1186/s12902-024-01625-1
47. JafariNasabian P, Inglis JE, Reilly W, Kelly OJ, and Ilich JZ. Aging human body: changes in bone, muscle and body fat with consequent changes in nutrient intake. J Endocrinol. (2017) 234:R37–51. doi: 10.1530/JOE-16-0603
48. Bonsignore MR, Saaresranta T, and Riha RL. Sex differences in obstructive sleep apnoea. Eur Respir Rev. (2019) 28:190030. doi: 10.1183/16000617.0030-2019
49. Wang Y, Liu H, Zhou B, Yue W, Wang M, and Hu K. Menopause and obstructive sleep apnea: revealing an independent mediating role of visceral fat beyond body mass index. BMC Endocr Disord. (2025) 25:21. doi: 10.1186/s12902-025-01850-2
50. Palacios S, Chedraui P, Sanchez-Borrego R, Coronado P, and Nappi RE. Obesity and menopause. Gynecol Endocrinol. (2024) 40:2312885. doi: 10.1080/09513590.2024.2312885
51. Woods R, Hess R, Biddington C, and Federico M. Association of lean body mass to menopausal symptoms: The Study of Women’s Health Across the Nation. Womens Midlife Health. (2020) 6:10. doi: 10.1186/s40695-020-00058-9
52. van Marken Lichtenbelt WD and Fogelholm M. Increased extracellular water compartment, relative to intracellular water compartment, after weight reduction. J Appl Physiol. (1999) 87:294–8. doi: 10.1152/jappl.1999.87.1.294
53. Mazariegos M, Kral JG, Wang J, Waki M, Heymsfield SB, Pierson RN, et al. Body composition and surgical treatment of obesity: effects of weight loss on fluid distribution. Ann Surg. (1992) 216:69–73. doi: 10.1097/00000658-199207000-00010
54. Bossingham MJ, Carnell NS, and Campbell WW. Water balance, hydration status, and fat-free mass hydration in younger and older adults. Am J Clin Nutr. (2005) 81:1342–50. doi: 10.1093/ajcn/81.6.1342
55. Laja Garcia AI, Morais-Moreno C, Samaniego-Vaesken ML, Puga AM, Partearroyo T, and Varela-Moreiras G. Influence of water intake and balance on body composition in healthy young adults from Spain. Nutrients. (2019) 11:1923. doi: 10.3390/nu11081923
56. Laja-García A, de Lourdes Samaniego-Vaesken M, Moráis-Moreno C, Partearroyo T, and Varela-Moreiras G. The association between fluid intake, water balance and body composition. Proc Nutr Soc. (2020) 79:E655. doi: 10.1017/S0029665120006047
Keywords: AHI, body composition, hydration status, OSA, polysomnography, sleep architecture
Citation: Chuang Y-M, Liu W-T, Tsai C-Y, Lin Y-C, Ho KF, Chuang K-J and Chuang H-C (2025) Associations between body composition, hydration status, and sleep architecture in obstructive sleep apnea. Front. Endocrinol. 16:1666026. doi: 10.3389/fendo.2025.1666026
Received: 16 July 2025; Accepted: 24 October 2025;
Published: 10 November 2025.
Edited by:
Wei Liu, Huazhong University of Science and Technology, ChinaReviewed by:
Qiao Chen, People’s Liberation Army General Hospital, ChinaAhmet Cemal Pazarlı, Gaziosmanpaşa University, Türkiye
Copyright © 2025 Chuang, Liu, Tsai, Lin, Ho, Chuang and Chuang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hsiao-Chi Chuang, Y2h1YW5naGNAdG11LmVkdS50dw==
 Ya-Min Chuang1
Ya-Min Chuang1 Wen-Te Liu
Wen-Te Liu Yi-Chih Lin
Yi-Chih Lin Kin Fai Ho
Kin Fai Ho Kai-Jen Chuang
Kai-Jen Chuang Hsiao-Chi Chuang
Hsiao-Chi Chuang