Nanogenerator-Based Sensors for Energy Harvesting From Cardiac Contraction
- 1Department of Biology, College of Science, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
- 2Basic and Applied Scientific Research Center, College of Science, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
- 3Master Program of Biotechnology, Institute for Research and Medical Consultations, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
- 4Genetics Research Department Institute for Research and Medical Consultations, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
- 5Paediatrics Department (Cardiology Division), Faculty of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
- 6Nano Medicine Research Department, Institute for Research and Medical Consultations, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
Biomedical electric devices provide great assistance for health and life quality. However, their maintainable need remains a serious issue for the restricted duration of energy storage. Therefore, scientists are investigating alternative technologies such as nanogenerators that could harvest the mechanical energy of the human heart to act as the main source of energy for the pacemaker. Cardiac contraction is not a source for circulation; it utilizes body energy as an alternative energy source to recharge pacemaker devices. This is a key biomedical innovation to protect patients’ lives from possible risks resulting from repeated surgery. A batteryless pacemaker is possible via an implantable energy collecting tool, exchanging the restriction of the current batteries for a sustainable self-energy resource technique. In this context, the physiology of heart energy in the preservation of blood distribution pulse generation and the effects of cardiac hormones on the heart’s pacemaker shall be outlined. In this review, we summarized different technologies for the implantable energy harvesters and self-powered implantable medical devices with emphasis on nanogenerator-based sensors for energy harvesting from cardiac contraction. It could conclude that recent hybrid bio-nanogenerator systems of both piezoelectric and triboelectric devices based on biocompatible biomaterials and clean energy are promising biomedical devices for harvesting energy from cardiac and body movement. These implantable and wearable nanogenerators become self-powered biomedical tools with high efficacy, durability, thinness, flexibility, and low cost. Although many studies have proven their safety, there is a need for their long-term biosafety and biocompatibility. A further note on the biocompatibility of bio-generator sensors shall be addressed.
Introduction
Bioelectronic device technology that can be implanted in the body has a life-saving function in organ damage, such as a pacemaker, and early diagnosis and follow-up of many diseases. Primitive electronic devices use batteries that can be controlled externally and are very sophisticated. In addition, performing long-term in vivo studies is not easy and may cause unintended outcomes for the patient who requires multiple high-risk operations repeatedly. For instance, sinoatrial (SA) node dysfunction and high-grade atrioventricular block require permanent pacemaker implantation (Da Costa et al., 2002). Millions of patients today use battery-depended pacemakers, defibrillators, and other life-saving implantable devices that must be replaced every 5 to 10 years. Replacing implants after completing their lifetime requires surgery, which is costly and can cause complications and infection (Dong et al., 2019a). Current cardiac pacemakers utilize a battery power supply to maintain hearts beating regularly. In practice, a couple of problems such as difficulty in charging, insufficient battery life, and risk of restart still await solutions. This is hindered by the restricted duration of energy storage. Thus, a way for the battery-less pacemaker to harvest kinetic energy from the heart to sustainably power the device may be the ultimate solution (Melville, 2021). The body is rich in energy from the mechanical source such as the physical movements of the musculoskeletal system. Electromagnetic, piezoelectric, and triboelectric energy harvesting devices were investigated for converting biomechanical energy into electric type to be used as power for several wearable and implantable electronic devices. However, the complex construction, cost, in addition to challenges of these devices reduce the advancement and commercialization of the technology of bioenergy harvesters (Shi et al., 2019).
At this point, a nanogenerator is an ideal power supply that collects biomechanical energy from the heartbeat and harvests electrical signals for self-charging (Sun et al., 2021)
This review aims to cast some light on implantable energy harvesters and self-powered implantable medical devices, with emphasis on nanogenerator-based sensors for energy harvesting from cardiac contraction. Together with the physiology of pacemakers and the effect of hormones, limitations are comprehensively elucidated.
This review is focused on sustainable energy strategies for cardiac medical applications based on nanoscale devices. The topic covers the physiology of heart energy; pacemakers; key challenges; limitations; transformation of biomechanical activity into energy harvesters; design principles of piezoelectric, triboelectric, and pyroelectric nanogenerator-based devices; and considerations of biosafety and biocompatibility.
Physiology of Natural Pacemaker and the Effect of Hormones
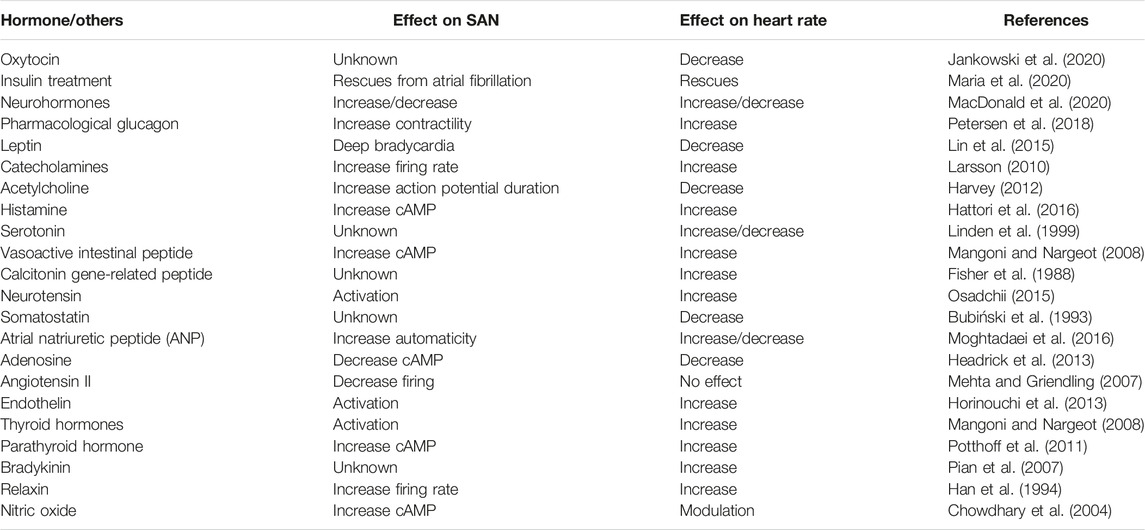
Every heartbeat is controlled and initiated by the sinoatrial (SA) node; it is the natural pacemaker of the heart. This tissue is controlled by circulating and locally released factors in addition to the autonomic nervous system (MacDonald et al., 2020). The SA node is the natural initiator of the heart contraction and regulates the cardiac rhythm. The SA node is a collection of spindle-shaped myocytes located at the sulcus terminalis of the right atrium lateral to the superior vena cava junction. This specialized cluster of pacemaker cells generates 60–100 electrical impulses per minute that propagate through the heart conduction system, the atria, atrioventricular (AV) node, and bundle of His, reaching Purkinje fibers causing subsequent contraction. The activity of the SA node is interpreted via the P wave in the electrocardiogram (ECG). Dysfunctions in the SA node activity include sinus arrest, sinus arrhythmia, and SA node block characterized by an absent or varying P-P wave interval length in the ECG. Unlike other myocardial cells, the electrical activity of pacemaker cells has no resting phase, meaning that they go to the depolarization phase immediately after firing an action potential (Kashou et al., 2022). The autonomic nervous system (ANS) controls the activity of the SA node. For instance, the activation of the sympathetic nervous system increases heart rate (HR), while the parasympathetic nervous system reduces HR through its rest and digest response. This autonomic control allows for proper adaptation to physiological stressors. Neurotransmitters such as catecholamines (epinephrine and norepinephrine), serotonin, histamine, and acetylcholine influence the activity of the SA node (Gordan et al., 2015). In addition, different groups of hormones have a role in the physiology of pacemaker cells. For example, it is known that patients with hyperthyroidism experience an increase in HR (tachycardia), while those with hypothyroidism have a lower HR (bradycardia), which indicates the direct effect of thyroid hormones on the SA node (Valcavi et al., 1992) (Figure 1). Saito et al. found great quantities of Angiotensin II (AGNII) converting enzyme in the SA node, which may indicate its synthesis there (Saito et al., 1987). Another hormone family known as natriuretic peptides (NPs) are produced and stored in the atrial myocytes and have a role in the physiology and pathophysiology of the cardiovascular system. Brain natriuretic peptide (BNP) usually increases in conditions associated with heart failure and congestive heart failure (Cowie and Mendez, 2002). However, their direct effect on the SA node is not fully understood yet (MacDonald et al., 2020). A study conducted by La Villa et al. measured the atrial natriuretic peptide (ANP), brain NP (BNP), and arterial pressure in 15 patients with an implanted dual-chamber pacemaker and unimpaired heart function during ventricular pacing. Patients were ECG-monitored while receiving atrioventricular and ventricular pacing for 30 min with a constant rate of 80 beats/minute. An increase in plasma ANP was observed within 1 h in patients with atrioventricular dissociation with no change in both BNP and arterial pressure, while those with retrograde conduction had a significant increase in both ANP and BNP levels. However, the arterial pressure also remained unchanged. This indicates the presence of a dual NP peptide system composed of ANP in the atria and BNP in the ventricles (La Villa et al., 1994). All in all, the SA node is structurally heterogeneous. Hence, further work is needed for a complete, comprehensive understanding of the complex mechanisms involved in the neurohumoral control of SA node function. The effects of many endocrine factors are summarized in Table 1.
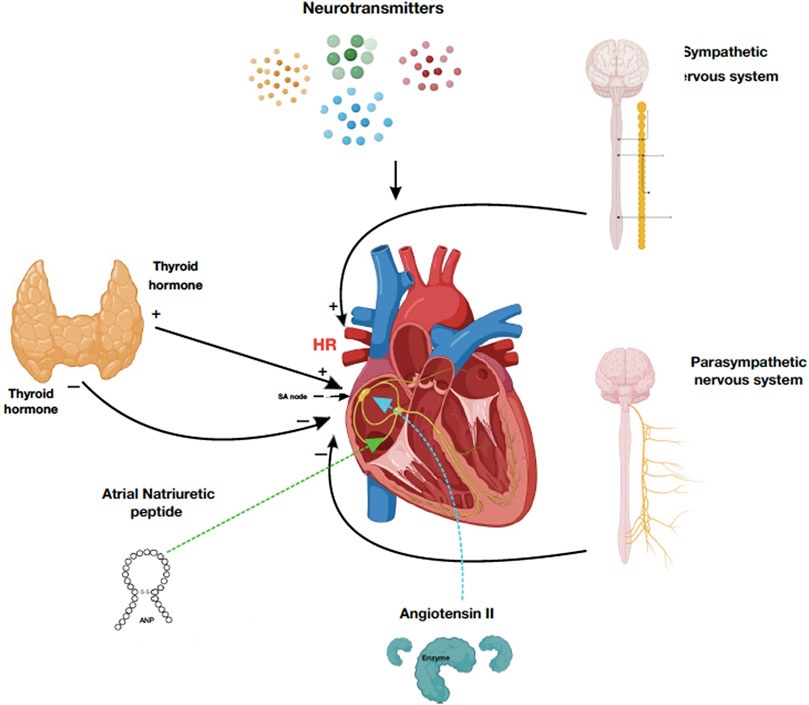
FIGURE 1. Schematic diagrams of TENGs operation modes. (A): vertical contact separation mode, (B): freestanding triboelectric layer mode, (C): single electrode mode (D): lateral sliding mode.
Physiology of Heart Energy
The heart functions as a cyclic energy generator with two phases: internal and external loads. The internal load is the mechanical characteristics of the ventricle, while the external load is the characteristics of the arterial tree. Hydraulic energy is conveyed to blood as blood pressure and flow. Vascular resistance causes energy loss during steady flow. In contrast, the arterial tree adjusts blood pressure and flow through circulation (Muñoz and Sacco, 1997). The generally accepted paradigm of heart function is the pressure generated to maintain blood circulation. However, the heart’s primary function is to generate pulse, not pressure. Blood circulation existed in the original direction even with the severe diseases of the heart valve, which is opposite to the physical law. With a non-functional heart valve, blood would flow from the main arteries toward the veins due to the pressure in the heart chamber (Papp, 2008). Therefore, the circulation seems to be driven not only by the heart but also by a complementary driving mechanism exiting by the vessels. Blood vessels are involved in the driving mechanism for blood circulation, but the precise mechanism regarding how the blood vessels, especially the capillary, pump blood under stander pressure remains unclear (Thudichum, 1855; De Langen, 1951; Manteuffel-Szoege, 1960; Furst, 2014). However, flow mechanisms play an essential part in the vascular system to drive from arteries to veins in the natural direction (Li and Pollack, 2020). Usually, tubular surfaces have a significant role in driving the intratubular flow due to the generated axial chemical concentration gradients (Rohani and Pollack, 2013; Yu et al., 2014; Li and Pollack, 2020). The determination of the surface-induced flow direction correlates with the intratubular concentration gradient direction. Nevertheless, certain factors control the direction of intratubular flow based on the tubular surface removal or induce substance and the change along the tube length of the surface to volume ratio. A vascular network has a group of vessels with the same diameter within each hierarchical level. According to Li and Pollack (2020), the flow can be predicted by assuming identical behavior in all vessel walls within each hierarchical level, in which the surface-to-volume ratio of these blood vessels is inversely proportional to their average diameter. Therefore, blood flow should circulate from narrower to wider vessels. Li and Pollack (2020) discovered a flow mechanism that can operate without compulsory pressure. They used the infrared (IR) energy to verify the driving mechanism in blood vessels using an early chick embryo model. They tested the blood circulation under two states postmortem and in the normal physiological state using infrared (IR) energy. They demonstrated and confirmed the finding of the second circulatory mechanism originating from the blood vessels. Surface-induced flow originating from vessels can be driven by infrared radiation energy, which can appear naturally from metabolic heat and external sources. The metabolic activity in any organism would generate heat that is released in the IR energy form to facilitate circulation and nourish the tissues. Some cardiovascular diseases and novel therapies can be overcome in the future by appreciating this second circulatory existence (Li and Pollack, 2020).
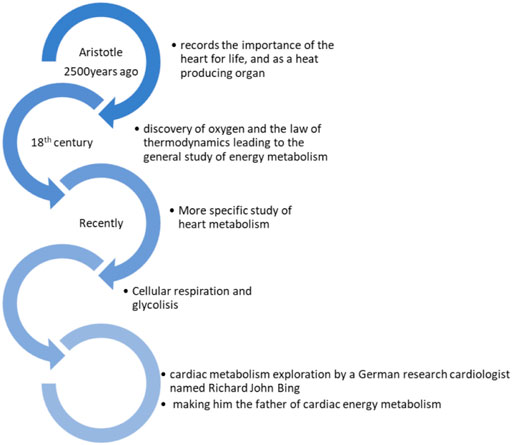
The heart is an aerobic pump, oxidative substrates to generate energy. There is harmonic relation between myocardial oxygen use (MV̇02) and the essential factors of the systolic role: contractile state, heart rate, and wall stress. In a mechanical pump, not all the investing energy is transformed into an external force. In cardiac physiology, mechanical efficiency is the proportion of useful energy released (stroke work [SW]) to the oxygen utilized. In normal cases, this proportion is ≈ 25%, while the remaining energy at most recedes as heat, but in heart failure, mechanical efficiency is decreased, and it is supposed that the elevated expense of energy related to work participates in the progress of the disease. Therapy that enhances mechanical efficiency has evidenced its effectiveness in relation to outcome (Knaapen et al., 2007). The history of the heart discovery goes back to Aristotle’s manuscripts 2,500 years ago, where he records the importance of the heart for life and as a heat-producing organ. In the 18th century, the discovery of oxygen and the idea of energy preservation (or the law of thermodynamics as known now) gave rise to the general study of energy metabolism and, later, a more specific study of heart metabolism. Cellular respiration was understood more clearly with the discovery of glycolysis. Although many scientists researched energy metabolism, a German research cardiologist named Richard John Bing conducted cardiac metabolism exploration, making him the father of cardiac energy metabolism (Beloukas et al., 2013) Figure 2. Cardiometabolic disorders have been found to be linked to energy transduction by the myocardium. When the heart is failing, glycolysis and ketone oxidation occur, trying to meet the high energy demand of the continuously beating heart. The beneficial cardiac effects of some antidiabetic drugs are facilitated partly by modified myocardial metabolism (Honka et al., 2021). Electrical stimulation of large mammalian ventricular heart surfaces was demonstrated at different stimulus strengths. Ventricular surfaces of pig hearts that span large areas were attached to long line electrodes and activated individually. It was found that endocardial stimulation activated more heart tissues than epicardial stimulation, making it a possible futuristic cardiac electrotherapy (Moreno et al., 2019). Batokines are secreted by brown adipose tissue (BAT), found to be the regulatory factors mostly studied in experimental models. Targets of batokines are the liver, heart, and skeletal muscle. In humans, the link between active BAT and a healthy metabolism may be explained by the endocrine role of BAT (Gavaldà-Navarro et al., 2021).
Limitation of Available Cardiac Implantable Electronics
Implantable biomedical tools are commonly used for the treatment and monitoring of some disorders. An implantable heart pacemaker is the main current therapeutic device for remedying bradyarrhythmia, but its replacement surgically is unavoidable each 5–12 years because of the restricted life of its built-in battery (Li et al., 2019). Sensor technology has recorded considerable advances, particularly because of the evolution of materials and the emergence of new technologies such as nanotechnology, thereby necessitating the exploration of novel opportunities. Implantable electronic devices are examples of such explorations in the healthcare sector, largely transforming therapeutic interventions for cardiac arrhythmias (Surendran et al., 2021). However, this technology requires an external power supply, which might pose other problems (Li et al., 2020). These devices are thus associated with several complications, which must be identified and addressed to prevent undesirable outcomes. One of these limitations is associated with the external power supply, particularly the defects in batteries. According to Hauser et al., possible battery longevity issues may also be characterized by the battery’s current capacity and its chemical characteristics (Hauser et al., 2021). Additional factors include housekeeping current drain, percent pacing, and lead impedance. Furthermore, current shunting challenges may also be associated with possible defects of the pulse generator. Another major limitation of cardiac pacemakers includes electromagnetic interferences from cellular devices, security systems, medical equipment, power generators, and wireless chargers. Although this limitation is scantly covered in the literature, available studies argue that electromagnetic fields can influence the performance of pacemakers by inhibiting pacing. According to Huang et al., this issue may be reduced by twisting excess leads (Huang et al., 2020). Unfortunately, even leads are bound to fail, which may aggravate the situation, potentially causing implantable cardioverter-defibrillator (ICD) system-based morbidity and mortality. Consequences of lead failure include pacing inhibition and inappropriate ICD shocks. One way of monitoring this limitation is by manipulating the Lead Integrity Alert, which, unfortunately, is also limited by its algorithm dependence (Kella and Stambler, 2021). Additional limitations are associated with nanotechnology and nanomaterial. For instance, the piezoelectricity and biocompatibility of various nanoparticles, such as ZnO, face electromechanical coupling issues and high rigidity concerns, thereby necessitating only a considerably low electrical output, which prevents further explorations (Li and Wang, 2021). This implies that the number of usable nanocrystals is limited. The impact of cardiac pacemakers cannot be overemphasized, owing to their abilities to prolong patient life. However, access to this vital device is problematic, particularly in regions with few supporting resources. According to Khairy et al., the lack of these supportive systems inhibits re-sterilization and reuse, which in turn affects their performance or viability, mainly because of infection possibilities. According to them, patients who reused their devices had a high likelihood of contracting infections. Pathogens associated with the infections included Staphylococcus aureus and S. epidermidis (Khairy et al., 2020). Patients are also prone to other forms of problems, which are associated with either pacing or sensing. Limitations associated with pacing include output failure and capture failure. Possible consequences include bradyarrhythmias and asystole. Sensing limitations include under-sensing and over-sensing, which can deliver inappropriate shocks. Pseudomalformation limitations include fusion and pseudo-fusion, pacemaker crosstalk, and ventricular safety pacing (Liaquat et al., 2021). The upper rate behavior, pacemaker mediated tachycardia, and runaway pacemakers are additional limitations. Apart from limitations of battery, which reduced life expectancy of cardiac pacemakers, Franzina et al. noted that other concerns of invasive implantation, blood clot risks, single-axis acceleration dependency, and unadaptable size and shape of pacemakers, owing to the heart environments are considerable limitations (Franzina et al., 2020). Figure 3 summarizes these crucial limitations and demands prompt attention to facilitate the future of implantable electronic devices.
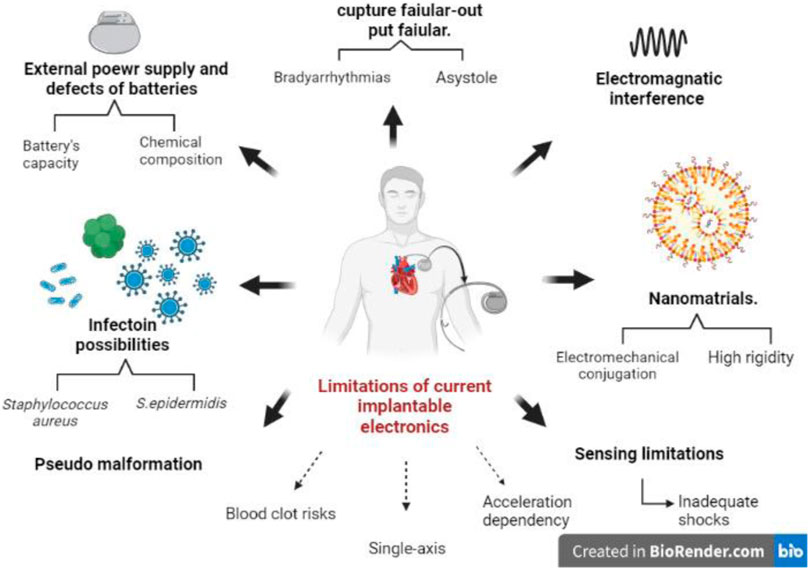
FIGURE 3. The most effective limitations of implantable electronics devices (pacemakers). Starting with the power supply and the longevity of batteries. Current shunting may also defect the pulse generator. Pathological infections of Staphylococcus aureus or S. epidermidis are a limitation faced by patients who reused their pacemakers. Additional concerns associated with pacing are capture failure that may complicate the situations with bradyarrhythmias and asystole. Sensing limitation is a challenge with a possible consequence of inadequately rapid shocks. Electromagnetic interference is related to cardiac pacemaker complications as well. Nanomaterials used in implantable electronic devices could cause electromechanical conjugation, and high rigidity issues resulted in low electrical output. Additional limitations include blood clot risks, single axis, unadaptable size and shape of the pacemaker, and acceleration dependency.
Transformation of Biomechanical Vibration (Movement) Into Nano-Energy Generator
Implantable medical devices (IMDs) are becoming an important tool for enhancing patients’ longevity and quality of life. The latest research on this topic is to overcome those limitations and focus on internal charging using energy produced by body activities. Piezoelectric nanogenerator (PENG) and triboelectric nanogenerator (TENG) energy harvesters with sophisticated structural and material designs have been developed to harvest biomechanical energy efficiently. The human body holds diverse forms of energy in it, including chemical, heat, and mechanical energy. The nanogenerator, a device capable of converting mechanical energy into electric energy, was developed in 2006 and has been used in various ways as a self-powered sensor since then (Li et al., 2020).
Medical devices are powered by energy from body movements, such as contraction and relaxation of muscles, motions of the heart and lungs, and circulation of the blood. More recently, PENGs and TENGs are utilized. Self-powered medical systems are futuristic opportunities for biodegradable, flexible, and intelligent devices that monitor and treat disease (Zheng et al., 2017). The body integrated self-powered system (BISS) is a highly efficient and economic technology to hunt energy from biomechanical energy in the human body, which can be harvested through an electrode tool close to the skin. The main rule of the BISS is enthused by the inclusive function of triboelectrification among soles and ground and human’s electrification. The technical feasibility has been proven to power electronic devices using the BISS either in vitro or in vivo. BISS shows an amazingly simple, cost-effective, and appropriate tactic for producing energy from human movement, with promising applications for wearable and implantable electronic devices (Shi et al., 2019). Although many energy harvesting strategies were discovered in recent years, they were not efficiently applied. Thus, many strategies for straight firing a contemporary and functional cardiac pacemaker have been investigated, which pace the heart of porcine in vivo through harvesting its energy from heartbeats, free from any external energy source. The energy harvester consists of an elastic structure and two piezoelectric compounds that could produce an elevated-output current of 15 μA in vivo with the state-of-the-art function. This strategy resolved the energy shortage of implantable devices using energy harvesting by piezoelectric (PZT) components (Li et al., 2019).
In vivo real-time monitoring of human physiological activities and treatment of diseases are developed to replace the use of large instruments such as the electrocardiogram (ECG) monitor and electroencephalograph (EEG). New devices such as the pulse generator for brain stimulation and other implantable devices are rapidly developed for flexibility and portability (Xia et al., 2020; Jiang et al., 2021). However, one of the challenges of using these devices includes frequent battery replacement due to a short lifespan.
Energy harvesting and vibration sensing may utilize PZT components, which are available and well established, yet alterations are proposed to improve large-scale vibration energy harvesting (Eghbali et al., 2020). In industry, energy harvesting can be accomplished by cantilever oscillators covered by a layer of piezoelectric material (Tommasino et al., 2022). PZT components can convert mechanical pressure applied to them to electrical signals, and the opposite applies as well. This ability may be used to harvest mechanical energy from human motion and vibrations to electrical energy to power small devices (Mahapatra et al., 2021). Devices that harvest energy will avoid patients from additional surgical interventions and the possibility of miniaturizing existing pacemakers and defibrillators. Another study used an automatic wristwatch as an energy harvesting device, converting cardiac wall motion (kinetic energy) to electrical energy. Motion analysis of the left ventricle based on MRI showed that the basal region was the most favorable for energy harvesting. It referes to the researchers of study of (Zurbuchen et al., 2013). We can say: The study also developed a mathematical model to optimize the device’s configuration. An in vitro experiment validated the model as a robot arm reproducing the cardiac motion. Another in vivo experiment also validated the model as the device was attached to a sheep heart for 1 h. Both experiments generated enough energy to power a modern pacemaker (in vitro 30 μW and in vivo 16.7 μW) (Zurbuchen et al., 2013). Piezoelectric energy harvesting may occur through the contractile and relaxation motion of the diaphragm, lungs, and heart. This has been studied in animal models with organ sizes close to human organ size (Dagdeviren et al., 2014). Regular medical follow-ups are required to ensure optimal functioning of cardiac pacemakers. ∼25% of pacemaker replacements are due to battery depletion. In addition, the specialized wire (pacemaker lead) that delivers energy from the pacemaker to the heart muscle is subjected to dislocations and fractures, and harvesting endocardial energy at favorable locations may assist in the drawbacks of typical pacemakers (Zurbuchen et al., 2017). Researchers harvested energy from the cardiac valvular movement to power a wireless sonomicrometry sensor, where piezoelectric sutures were placed close to the valvular regions using the valve movement for self-powering. It was found that the range of harvested energy would be from nano-watts to milli-watts. The highest amount of the harvested energy was from the leaflet plane (Kondapalli et al., 2018). Wearable technologies are increasing, and the demand for novel methods for powering these devices is met through new energy harvesting materials such as the piezoelectric polymer. Mokhtari et al. developed a polyvinylidene fluoride (PVDF)/LiCl electrospun nanofiber, using it in a nanogenerator package that is flexible and lightweight. This nanogenerator could generate voltage and current output of 3 V and 0.5 μA with a power density output of 0.3 μW cm−2 at the frequency of 200 Hz. This energy harvesting material has promising applications for self-power electrical devices (Mokhtari et al., 2020). Figure 4.
Nanogenerator-Based Sensor for Energy Harvesting From Cardiac Contraction
The electric nanogenerator (ENG) is an ideal power supply that extracts and converts the energy from physiological activities such as blood flow, heartbeat, muscle contraction, body movement, respiration, and body heat to produce electrical energy. ENGs possess a promising role in terms of medical applications such as tissue repairing, pathological indicators, neural stimulators, and cardiac pacemakers (Bhatia et al., 2010; Sun et al., 2021). Generally, ENGs can be classified into three categories based on the electrical production mode known piezoelectric nanogenerator (PENG) (Bhatia et al., 2010), triboelectric nanogenerator (TENG) (Wang, 2013), and pyroelectric nanogenerator (PYENG) (Yang et al., 2012).
Piezoelectric Nanogenerator
The invention of the piezoelectric nanogenerator by Z. L. Wang (Li Z. et al., 2020; Zagabathuni and Kanagaraj, 2022) was a development that led to the functioning of biological devices without the need for a battery or external power source. Bio-nanogenerators capture mechanical energy generated from body movement and convert it into electrical energy to drive the function of implanted medical devices (Li Z. et al., 2020; Jiang et al., 2021; Zagabathuni and Kanagaraj, 2022). Recently medical implants, sensors, and wearable devices harvest energy using piezoelectric nanogenerators (Jiang et al., 2020). Inorganic materials such as wurtzite semiconductors are used in piezoelectric materials utilized in different sensors and actuators (Wang R et al., 2021; Dahiya et al., 2018); piezoelectric ceramics with high dielectric and piezoelectric properties (Dudem et al., 2018; Hyeon and Park, 2019); and organic materials, such as PVDF and its copolymer P(VDF-TrFE) (Dong et al., 2019b).
The human body contains energy in different forms, including thermal, chemical, and mechanical energy. Amongst them, mechanical energy is the most abundant form in which PENG can transform into electricity. This was firstly invented by Zhong Lin Wang in 2006 (Wang, 2007). PENG is composed of piezoelectric materials, flexible substrates, and external electrodes. The core component of PENG is piezoelectric material in which the electric current generated due to the crystal deformation in response to the changes in the condition of the body parameters includes blood vessels contractions and other physiological mechanical activities (Bhatia et al., 2010). The mutual transformation between mechanical and electrical energy can be recognized by the piezoelectric effect. Thus, when the piezoelectric material is exposed to external pressure, an electric dipole moment will be produced because of the mutual displacements of cations and anions in the crystal. As a result, the electric potential difference is generated in the tension direction of the material. Therefore, the continuous electrical pulse could be produced in the external circuit as the mechanical force applied to the PENG (Jiang et al., 2020; Li Z. et al., 2020). Typically, inorganic piezoelectric materials such as ZnO (Zhao et al., 2014; Murillo et al., 2017), lead zirconate titanate (PZT) (Dagdeviren et al., 2017), barium titanate (BT) (Jiang et al., 2019), and organic polyvinylidene fluoride (PVDF) (Yu et al., 2020) are the most commonly used in PENG for wearable and implantable medical devices. ZnO and PVDF exhibit lightweight with great flexibility; hence, they are suitable for multidimensional deformation applications, including wearable biosensors. In contrast, PZT- and BT-based devices are suitable for energy harvesting where they possess a high piezoelectric strain constant (Li Z. et al., 2020). In order to obtain good stability and high output, the appropriate structure of PENG has developed from single nanowires to films. Taking ZnO nanowires as an example, it has a wurtzite structure in which Zn2+ and O2- coordinate to form tetrahedral in the crystal structure. The piezoelectric effect of ZnO appears in the lack of central symmetry of the wurtzite structure. In the beginning, the cations and anions centers are coincided with each other due to the absence of the strain. However, when the external force is applied, the crystal structure is deformed and the charge centers are separated from each other, resulting in an electric dipole. The generated electric potential is preserved as long as the mechanical force is applied. Thus, the output current will be driven through the external load when ZnO nanowires are connected to an external circuit. The working mechanism of the other materials is similar to ZnO nanowires (Wang et al., 2010; Li X. et al., 2020; Sun et al., 2021). Cheng et al. reported the usage of piezoelectric thin film for harvesting mechanical energy to electricity through the contraction and expansion of the aorta. They conducted an in vivo ENGs examination of piezoelectric PVDF via wrapping it around the ascending aorta of a pig. The obtained outpower was 40 nW (Cheng et al., 2016). Kondapalli et al. used PENG to harvest energy from cardiac valvular perturbations for wireless powering sonomicrometry sensors (Kondapalli et al., 2018). Han et al. created different three-dimensional piezoelectric microsystems and demonstrated their application in mechanical energy harvesting in vivo (Han et al., 2019). Two and three-component nanohybrids are used for energy harvesting in the form of a bio-based piezoelectric eggshell membrane (ESM). A bio-waste piezo-filler in a piezoelectric polymer matrix was designed using organically modified two-dimensional nanoclay. These nanohybrid materials are appropriate for energy harvesting. The effectiveness of the device was tested using a range of body movements leading to energy storage. The hybrid device is a strong nanogenerator as it is durable and mechanically stable for repeated energy conversion. Cellular studies also found it suitable for powering biomedical devices and implants (Gaur et al., 2019).
Triboelectric Nanogenerator
Triboelectric nanogenerator sensors are highly sensitive detectors of mechanical energy and provide a high-quality amount of electrical energy output. They are easily activated by slight motions of the ribcage during respiration, heart contraction, blood flow, and gastric peristalsis (Ouyang et al., 2019; Xia et al., 2020; Sun et al., 2021). This attribute makes this sensor worthy of in vivo biomedical monitoring (Xia et al., 2020; Sun et al., 2021).
The triboelectrification effect is quite common in our daily life. It appears at the interface between two different contacted materials. The power generation mechanism of triboelectric nanogenerator (TENG) relies on the coupling of two principles: triboelectricity and electrostatic induction (Wang et al., 2015). The electrostatic charges are generated because of the triboelectrification effect, where the material becomes electrically charged after two friction surfaces are brought into contact. The friction layers could carry the opposite signs with the same number of triboelectric charges after separation, forming an electrostatic field and driving the electrons’ movement through the external circuit (Jiang et al., 2020; Sun et al., 2021). TENG exhibited large output power with large corresponding charge transfer when the materials were significantly different in the frictional electric sequences. According to the structure of two friction layers and the connection of the external load, the working modes of TENG were categorized into four groups as shown in Figure 5: lateral sliding mode, vertical contact separation mode, freestanding triboelectric layer mode, and single electrode mode (Jiang et al., 2020; Li X. et al., 2020; Sun et al., 2021). TENG utilized the energy from muscle movement, heartbeat, and respiration as a source of biomechanical energy for disease treatment and physiological signal detection (Sun et al., 2021). In 2019, Ouyang et al. designed a pacemaker based on TENG, which harvested energy from heart motion (Ouyang et al., 2019). Furthermore, Shi et al. designed a body-integrated self-powered system based on TENG for harvesting biomechanical energy from body movements (Shi et al., 2019). Li et al. prepared biodegradable TENG modified by gold nanorods that possess stability for more than 28 days in vivo (Li Z et al., 2018). Moreover, biodegradable TENG has been fabricated by Jiang et al. and implemented in the dorsal subcutaneous region of rats, where the maximum voltage, current, and power density reach up to 55 V, 0.6 μA, and 21.6 mW/m2, respectively.
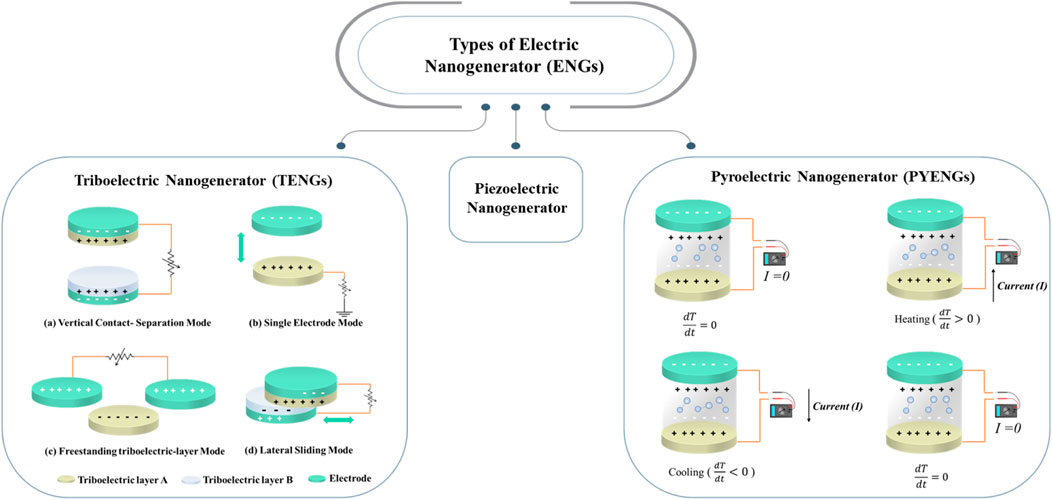
FIGURE 5. Illustrates the types of nanogenerators: Triboelectric (TENGs), Piezoelectric, and Pyroelectric (PYENGs) nanogenerators. Left schematic diagrams show TENGs operation modes. While right schematic diagram illustrates the working mechanism of PYENGs. In TENGs operation modes. (A): vertical contact separation mode, (B): freestanding triboelectric layer mode, (C): single electrode mode).
Pyroelectric Nanogenerator
A pyroelectric nanogenerator (PYENG) is a thermoelectric energy harvesting device that can convert heat energy into electrical energy. The working principle of PYENG is based on the Seebeck effect (Yang et al., 2012), which utilizes a temperature gradient to polarize nanomaterials driving the charge diffusion. The spontaneous polarization of pyroelectric materials changes with the temperature variation when exposed to heat that transforms the surface bounded charge causing current output. In the absence of temperature difference, the spontaneous polarization of pyroelectric material remains unchanged, and no current is generated. However, once the temperature rises with time, the polarization density will decrease and generate the pyroelectric current to an external circuit until reaching the equilibrium state. On the contrary, when the temperature of the medium decreases, such as cooling the material, the polarization density will increase, generating a reverse current in the external circuit until an equilibrium is reached, as illustrated in Figure 5 (Li X. et al., 2020; Sun et al., 2021). Sultana et al. reported the fabrication of PYENG that harvested the waste body heat from the respiration process and body surface. It can detect human heat, work as a temperature sensor, and monitor the respiratory process. The produced output reached 1.5 V and 1.5 µA (Sultana et al., 2018).
Biocompatibility of Bio-Nanogenerator Sensors
Due to the persistent mechanical activities in human bodies, it is expected that materials placed inside the body should have the strength to withstand continuous movements. An implantable cardiac device, for example, should meet certain standards so that it can stand the constant heart contractions without injuring the body through toxicity or stimulation of an adverse immune response is said to be biocompatible (Suvaneeth and Nair, 2018). Biocompatibility was defined as “the ability of a material to perform with an appropriate host response in a specific application.” This suggests that a biocompatible material is not completely non-reactive with the environment but exerts an appropriate reaction. Accordingly, a more acceptable definition of biocompatibility is the natural tendency of a material to carry out the required function, such as health monitoring or treatment, without causing unwanted effects locally or systemically on the individual (Elshahawy, 2011). Therefore, a biocompatible material is expected to react with the environment in a specific way instead of the environment refusing to notice its presence.
The biocompatibility of biomaterial is dependent on factors such as its chemical and physical components, the nature of the environment where the biomaterial will be placed, and the length of time it will remain in that location. One of the challenges of using these devices is the possibility of draining some of their component material into the body or causing undesirable effects. Therefore, it is important to make an in-depth assessment and safety evaluation of such biomaterials. Regulatory bodies and institutions, such as the U.S. Food and Drug Administration (FDA), the International Organization for Standardization (ISO), and the Japanese Ministry of Health and Welfare (JMHW), produced guidelines that made it compulsory for manufacturers of medical devices to conduct pre-clinical and clinical testing of their products before they are approved for use by the public (Suvaneeth and Nair, 2018).
A medical device that may be implanted in the human body for long-term therapy or monitoring should meet the biocompatibility requirement of not causing injury to the tissues it will be in contact with (Williams, 2008). This means that the material biocompatibility (including bio-nanogenerators) should be tested before implantation into human tissues. Ideally, three types of biocompatibility testing should be conducted, including in vitro testing, in vivo animal, and clinical testing. The clinical trials are carried out on a volunteer where the material being assessed is placed into the tissues of such an individual, and they will be observed for any reactions (Elshahawy, 2011). The physical and chemical properties of nanoparticles and their surface characteristics determine their cellular uptake and bio-distribution (Kunzmann et al., 2011; Adabi et al., 2017). Furthermore, the immune reaction caused by the interactions between the nanoparticles and the immune cells, which may have engulfed the nanoparticles, is keenly observed to understand the mechanism (Kunzmann et al., 2011; Adabi et al., 2017).
Ubiquitous mechanical energy is seen as a recent sustainable energy source, as it was successfully harvested by different types of nanogenerators. Biocompatible nanogenerators are of great interest due to their possible biomedical applications (Wang YM et al., 2021).
The most studied energy harvesting mechanisms are based on the use of piezoelectric and triboelectric effects. The biocompatible ferroelectret nanogenerator (FENG) is efficient, flexible, and biocompatible. It uses the active material of polypropylene ferroelectret (PPFE). The mechanism of the mechanical-electrical energy conversion in PPFE films is described by the finite element method (FEM). Each time the device is folded, the generated voltage and current signals are doubled (Li et al., 2016).
Implantable nanogenerators showed high efficacy for self-powered medical devices. The most needed factor is its biosafety and long term. Li J et al. (2018) have surveyed the technology of polydimethylsiloxane (PDMS), and PDMS/Parylene-C embalmed polyvinylidene fluoride (PVDF) NGs embedded in female mice for 6 months. In vitro, the PVDF NG showed a constant output of 0.3 V exposed to 7,200 cycles, while in vivo and under distending, the output was 0.1 V. Advanced methods of imaging studies were applied, such as ultrasound, computed tomography, and photoacoustic to monitor the in vivo performance. NGs displayed excellent adherence to the adjoining muscle and showed steady electric energy within the study. Interestingly, there was no evidence of toxicity or incompatibility with the adjacent tissues and body systems proven by humoral biochemical and pathological examinations. The PDMS complex device displayed effective insulation from the biological environment of the body with minor loss of currents at a pA hierarchy. These multiple in vivo and in vitro examinations prove the biological achievability of applying the innovative technology of mechanical energy harvesting in the biological environment in vivo.
Triboelectric nanogenerators (TENGs) are considered a good choice in the energy source field due to their low cost, high durability, and processability. The addition of biomaterials to TENGs gives rise to biomaterial-based TENGs (bio-TENGs), which are more environmentally friendly, with great potential for applications in wearable and implantable devices (Li Z. et al., 2020).
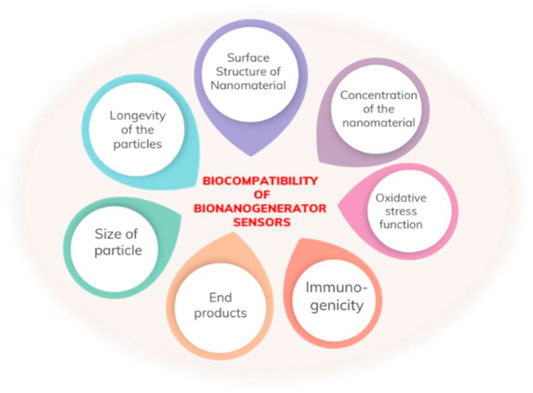
A cost-effective energy harvesting technology emerged in recent years, harvesting energy from renewable and clean energy sources. This technology is a nanogenerator founded on piezoelectric or triboelectric materials. The technology is also used for biomedical wearable or implantable applications, using advances in biocompatible soft materials and nano-structures. Hybrid bio-nanogenerators (HBNGs) are hybrid systems that depend on combining piezoelectric and triboelectric devices based on biocompatible materials aiming to harvest energy from the environment or for biomedical applications (Mariello, 2022). Figure 6 Shows biocompatibility of bio-nanogenerator sensors.
Conclusion
Biomedical electric devices greatly help in managing life’s quality of many patients. However, their sustainability and safety in the human body still represent considerable issues for the limitations, such as restriction of energy storage, contamination, risk of its wastes, harm reaction of chemical and physical composites with the body, and effect of external magnetic, electric sources. All these factors are unknown, and others can arise, which show the importance of considering the full biocompatibility of these devices. This attracted scientists to find out substitute technologies for energy generators, such as nanogenerators, which can utilize the mechanical energy of the human heart and other physical activities to provide a sustainable source of energy for the implantable devices, in which the cardiac pacemaker represents the most important device for maintaining human life. Cardiac systole is a source of small and systematic blood circulation, and it is used to generate pressure and energy, which could be used as identical innovative technology as an energy provider to revive the pacemaker device, to keep the patients away from potential complications of repeated surgery. A battery-less pacemaker became an implantable energy harvesting device, overcoming the limitations of the available battery-dependent pacemaker to a maintainable and friendly self-energy resource medical tool. This review outlined the implantable/wearable energy harvester and self-recharge medical devices, highlighting the importance of nanogenerator-dependent sensors for the electrical energy generated from heart contraction. It could be summarized that recent hybrid bio-nanogenerator systems of both piezoelectric and triboelectric devices based on biocompatible biomaterials and friendly energy are potential biomedical candidates showing in vitro and in vivo self-powered biomedical tools characterized by significant value, tractability, robustness, adjustable size, and cost-effectiveness. Although many studies have proven being body friendly and biocompatible, further clinical studies and critical reviews are needed for its long-term safety, immune reactions, and biocompatibility.
Energy harvesting and self-powered sensing strategies provide practical and promising power solutions for implantable cardiac devices and will reduce the reliance on batteries for powering cardiac medical devices. More human trials will be needed before these devices are clinically employed. Long-term animal studies will also be needed to understand the impact of daily life on the device’s stability and energy storage.
Author Contributions
EA-S designed the idea, drafted the first and final version of the manuscript, and reviewed and edited it. MA drafted, reviewed, and edited the manuscript. AH drafted and reviewed the manuscript. AB reviewed the manuscript critically. The rest of the co-authors contributed to drafting the manuscript, designing the figures, and reviewing the references.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adabi, M., Naghibzadeh, M., Adabi, M., Zarrinfard, M. A., Esnaashari, S. S., Seifalian, A. M., et al. (2017). Biocompatibility and Nanostructured Materials: Applications in Nanomedicine. Artif. Cells Nanomed. Biotechnol. 45 (4), 833–842. doi:10.1080/21691401.2016.1178134
Beloukas, A. I., Magiorkinis, E., Tsoumakas, T. L., Kosma, A. G., and Diamantis, A. (2013). Milestones in the History of Research on Cardiac Energy Metabolism. Can. J. Cardiol. 29 (11), 1504–1511. doi:10.1016/j.cjca.2012.10.008
Bhatia, D., Bairagi, S., Goel, S., and Jangra, M. (2010). Pacemakers Charging Using Body Energy. J. Pharm. Bioall. Sci. 2 (1), 51. doi:10.4103/0975-7406.62713
Bubiński, R., Kuś, W., and Goch, J. (1993). Effect of Somatostatin on the Conduction System of the Heart. Kardiol. Pol. 38 (4), 258–262.
Cheng, X., Xue, X., Ma, Y., Han, M., Zhang, W., Xu, Z., et al. (2016). Implantable and Self-Powered Blood Pressure Monitoring Based on a Piezoelectric Thinfilm: Simulated, In Vitro and In Vivo Studies. Nano Energy 22, 453–460. doi:10.1016/j.nanoen.2016.02.037
Chowdhary, S., Marsh, A. M., Coote, J. H., and Townend, J. N. (2004). Nitric Oxide and Cardiac Muscarinic Control in Humans. Hypertension 43 (5), 1023–1028. doi:10.1161/01.hyp.0000126171.46645.f9
Cowie, M. R., and Mendez, G. F. (2002). BNP and Congestive Heart Failure. Prog. Cardiovasc. Dis. 44 (4), 293–321. doi:10.1053/pcad.2002.24599
Da Costa, D., Brady, W. J., and Edhouse, J. (2002). ABC of Clinical Electrocardiography: Bradycardias and Atrioventricular Conduction Block. BMJ 324 (7336), 535–538. doi:10.1136/bmj.324.7336.535
Dagdeviren, C., Yang, B. D., Su, Y., Tran, P. L., Joe, P., Anderson, E., et al. (2014). Conformal Piezoelectric Energy Harvesting and Storage from Motions of the Heart, Lung, and Diaphragm. Proc. Natl. Acad. Sci. U.S.A. 111 (5), 1927–1932. doi:10.1073/pnas.1317233111
Dagdeviren, C., Javid, F., Joe, P., von Erlach, T., Bensel, T., Wei, Z., et al. (2017). Flexible Piezoelectric Devices for Gastrointestinal Motility Sensing. Nat. Biomed. Eng. 1 (10), 807–817. doi:10.1038/s41551-017-0140-7
Dahiya, A. S., Morini, F., Boubenia, S., Nadaud, K., Alquier, D., and Poulin-Vittrant, G. (2018). Organic/Inorganic Hybrid Stretchable Piezoelectric Nanogenerators for Self-Powered Wearable Electronics. Adv. Mat. Technol. 3 (2), 1700249. doi:10.1002/admt.201700249
De Langen, C. D. (1951). The Vis a Tergo, Capillary Pressure and Capillary Function. Acta Med. Scand. 140 (6), 437–445. doi:10.1111/j.0954-6820.1951.tb10186.x
Dong, L., Han, X., Xu, Z., Closson, A. B., Liu, Y., Wen, C., et al. (2019a). Flexible Porous Piezoelectric Cantilever on a Pacemaker Lead for Compact Energy Harvesting. Adv. Mat. Technol. 4 (1), 1800148. doi:10.1002/admt.201800148
Dong, L., Wen, C., Liu, Y., Xu, Z., Closson, A. B., Han, X., et al. (2019b). Piezoelectric Buckled Beam Array on a Pacemaker Lead for Energy Harvesting. Adv. Mat. Technol. 4 (1), 1800335. doi:10.1002/admt.201800335
Dudem, B., Kim, D. H., Bharat, L. K., and Yu, J. S. (2018). Highly-flexible Piezoelectric Nanogenerators with Silver Nanowires and Barium Titanate Embedded Composite Films for Mechanical Energy Harvesting. Appl. Energy 230, 865–874. doi:10.1016/j.apenergy.2018.09.009
Eghbali, P., Younesian, D., Moayedizadeh, A., and Ranjbar, M. (2020). Study in Circular Auxetic Structures for Efficiency Enhancement in Piezoelectric Vibration Energy Harvesting. Sci. Rep. 10 (1), 16338–16411. doi:10.1038/s41598-020-73425-1
Elshahawy, W. (2011). “Biocompatibility,” in Advances in Ceramics - Electric and Magnetic Ceramics, Bioceramics, Ceramics and Environment. Editor C. Costas Sikalidis (London: IntechOpen). Available at: https://www.intechopen.com/chapters/18280. doi:10.5772/18475
Fisher, R. A., Robertson, S. M., and Olson, M. S. (1988). Stimulation and Homologous Desensitization of Calcitonin Gene-Related Peptide Receptors in Cultured Beating Rat Heart Cells. Endocrinology 123 (1), 106–112. doi:10.1210/endo-123-1-106
Franzina, N., Zurbuchen, A., Zumbrunnen, A., Niederhauser, T., Reichlin, T., Burger, J., et al. (2020). A Miniaturized Endocardial Electromagnetic Energy Harvester for Leadless Cardiac Pacemakers. Plos one 15 (9), e0239667. doi:10.1371/journal.pone.0239667
Furst, B. (2014). “Hemodynamics of the Early Embryo Circulation,” in The Heart and Circulation (London: Springer), 21–29. doi:10.1007/978-1-4471-5277-4_3
Gaur, A., Tiwari, S., Kumar, C., and Maiti, P. (2019). Retracted Article: A Bio-Based Piezoelectric Nanogenerator for Mechanical Energy Harvesting Using Nanohybrid of Poly(vinylidene Fluoride). Nanoscale Adv. 1 (8), 3200–3211. doi:10.1039/c9na00214f
Gavaldà-Navarro, A., Villarroya, J., Cereijo, R., Giralt, M., and Villarroya, F. (2021). The Endocrine Role of Brown Adipose Tissue: An Update on Actors and Actions. Rev. Endocr. Metab. Disord. 23 (1), 1–11. doi:10.1007/s11154-021-09640-6
Gordan, R., Gwathmey, J. K., and Xie, L.-H. (2015). Autonomic and Endocrine Control of Cardiovascular Function. World J. Cardiol. 7 (4), 204. doi:10.4330/wjc.v7.i4.204
Han, X., Habuchi, Y., and Giles, W. R. (1994). Relaxin Increases Heart Rate by Modulating Calcium Current in Cardiac Pacemaker Cells. Circ. Res. 74 (3), 537–541. doi:10.1161/01.res.74.3.537
Han, M., Wang, H., Yang, Y., Liang, C., Bai, W., Yan, Z., et al. (2019). Three-dimensional Piezoelectric Polymer Microsystems for Vibrational Energy Harvesting, Robotic Interfaces and Biomedical Implants. Nat. Electron 2 (1), 26–35. doi:10.1038/s41928-018-0189-7
Harvey, R. D. (2012). Muscarinic Receptor Agonists and Antagonists: Effects on Cardiovascular Function. Muscarinic Recept., 299–316. doi:10.1007/978-3-642-23274-9_13
Hattori, Y., Hattori, K., and Matsuda, N. (2016). “Regulation of the Cardiovascular System by Histamine,” in Histamine and Histamine Receptors in Health and Disease. Editors Y. Hattori, and R. Seifert (Cham: Springer), 241, 239–258. doi:10.1007/164_2016_15
Hauser, R. G., Casey, S. A., Gitter, C. B., Tang, C. Y., Abdelhadi, R. H., Gornick, C. C., et al. (2021). Reliability and Longevity of Implantable Defibrillators. J. Interv. Card. Electrophysiol. 62 (3), 507–518. doi:10.1007/s10840-020-00920-w
Headrick, J. P., Ashton, K. J., Rose'Meyer, R. B., and Peart, J. N. (2013). Cardiovascular Adenosine Receptors: Expression, Actions and Interactions. Pharmacol. Ther. 140 (1), 92–111. doi:10.1016/j.pharmthera.2013.06.002
Honka, H., Solis-Herrera, C., Triplitt, C., Norton, L., Butler, J., and DeFronzo, R. A. (2021). Therapeutic Manipulation of Myocardial Metabolism. J. Am. Coll. Cardiol. 77 (16), 2022–2039. doi:10.1016/j.jacc.2021.02.057
Horinouchi, T., Terada, K., Higashi, T., and Miwa, S. (2013). Endothelin Receptor Signaling: New Insight into its Regulatory Mechanisms. J. Pharmacol. Sci. 123 (2), 85–101. doi:10.1254/jphs.13r02cr
Huang, J., Lin, K., Lu, W., Ding, R., Wu, B., Cai, M., et al. (2020). An In Vitro Evaluation of the Effect of Transient Electromagnetic Fields on Pacemakers and Clinical Mitigation Measures. Front. Cardiovasc. Med., 343. doi:10.3389/fcvm.2020.607604
Hyeon, D. Y., and Park, K.-I. (2019). Piezoelectric Flexible Energy Harvester Based on BaTiO 3 Thin Film Enabled by Exfoliating the Mica Substrate. Energy Technol. 7 (10), 1900638. doi:10.1002/ente.201900638
Jankowski, M., Broderick, T. L., and Gutkowska, J. (2020). The Role of Oxytocin in Cardiovascular Protection. Front. Psychol. 11, 2139. doi:10.3389/fpsyg.2020.02139
Jiang, L., Yang, Y., Chen, R., Lu, G., Li, R., Xing, J., et al. (2019). Ultrasound-Induced Wireless Energy Harvesting for Potential Retinal Electrical Stimulation Application. Adv. Funct. Mat. 29 (33), 1902522. doi:10.1002/adfm.201902522
Jiang, D., Shi, B., Ouyang, H., Fan, Y., Wang, Z. L., and Li, Z. (2020). Emerging Implantable Energy Harvesters and Self-Powered Implantable Medical Electronics. ACS Nano 14 (6), 6436–6448. doi:10.1021/acsnano.9b08268
Jiang, Y., Deng, Y., and Qi, H. (2021). Microstructure Dependence of Output Performance in Flexible PVDF Piezoelectric Nanogenerators. Polymers 13 (19), 3252. doi:10.3390/polym13193252
Kashou, A. H., Basit, H., and Chhabra, L. (2022). “Physiology, Sinoatrial Node,” in StatPearls (Treasure Island, FL: StatPearls Publishing). Available at: https://www.ncbi.nlm.nih.gov/books/NBK459238/.
Kella, D. K., and Stambler, B. S. (2021). Failure of Lead Integrity Alert to Detect Implantable Cardioverter-Defibrillator Lead-System Failure in a Pacemaker-dependent Patient. Hear. Case Rep. 7 (1), 3–7. doi:10.1016/j.hrcr.2020.08.021
Khairy, T. F., Lupien, M.-A., Nava, S., Baez, F. V., Ovalle, F. S., Ochoa, N. E. L., et al. (2020). Infections Associated with Resterilized Pacemakers and Defibrillators. N. Engl. J. Med. 382 (19), 1823–1831. doi:10.1056/nejmoa1813876
Knaapen, P., Germans, T., Knuuti, J., Paulus, W. J., Dijkmans, P. A., Allaart, C. P., et al. (2007). Myocardial Energetics and Efficiency. Circulation 115 (7), 918–927. doi:10.1161/circulationaha.106.660639
Kondapalli, S. H., Alazzawi, Y., Malinowski, M., Timek, T., and Chakrabartty, S. (2018). Feasibility of Self-Powering and Energy Harvesting Using Cardiac Valvular Perturbations. IEEE Trans. Biomed. Circuits Syst. 12 (6), 1392–1400. doi:10.1109/tbcas.2018.2865405
Kunzmann, A., Andersson, B., Thurnherr, T., Krug, H., Scheynius, A., and Fadeel, B. (2011). Toxicology of Engineered Nanomaterials: Focus on Biocompatibility, Biodistribution and Biodegradation. Biochim. Biophys. Acta General Subj. 1810 (3), 361–373. doi:10.1016/j.bbagen.2010.04.007
La Villa, G., Padeletti, L., Lazzeri, C., Salvi, S., Michelucgi, A., Fronzaroli, C., et al. (1994). Plasma Levels of Natriuretic Peptides during Ventricular Pacing in Patients with a Dual Chamber Pacemaker. Pacing Clin. Electro 17 (5), 953–958. doi:10.1111/j.1540-8159.1994.tb01438.x
Larsson, H. P. (2010). How Is the Heart Rate Regulated in the Sinoatrial Node? Another Piece to the Puzzle. J. General Physiol. 136 (3), 237–241. doi:10.1085/jgp.201010506
Li, Z., and Pollack, G. H. (2020). Surface-induced Flow: A Natural Microscopic Engine Using Infrared Energy as Fuel. Sci. Adv. 6 (19), eaba0941. doi:10.1126/sciadv.aba0941
Li, J., and Wang, X. (2021). Materials Perspectives for Self-Powered Cardiac Implantable Electronic Devices toward Clinical Translation. Acc. Mat. Res. 2 (9), 739–750. doi:10.1021/accountsmr.1c00078
Li, W., Torres, D., Wang, T., Wang, C., and Sepúlveda, N. (2016). Flexible and Biocompatible Polypropylene Ferroelectret Nanogenerator (FENG): On the Path toward Wearable Devices Powered by Human Motion. Nano Energy 30, 649–657. doi:10.1016/j.nanoen.2016.10.007
Li, N., Yi, Z., Ma, Y., Xie, F., Huang, Y., Tian, Y., et al. (2019). Direct Powering a Real Cardiac Pacemaker by Natural Energy of a Heartbeat. Acs Nano 13 (3), 2822–2830. doi:10.1021/acsnano.8b08567
Li, J., Kang, L., Yu, Y., Long, Y., Jeffery, J. J., Cai, W., et al. (2018). Study of Long-Term Biocompatibility and Bio-Safety of Implantable Nanogenerators. Nano Energy 51, 728–735. doi:10.1016/j.nanoen.2018.07.008
Li, X., Jiang, C., Ying, Y., and Ping, J. (2020). Biotriboelectric Nanogenerators: Materials, Structures, and Applications. Adv. Energy Mat. 10 (44), 2002001. doi:10.1002/aenm.202002001
Li, Z., Feng, H., Zheng, Q., Li, H., Zhao, C., Ouyang, H., et al. (2018). Photothermally Tunable Biodegradation of Implantable Triboelectric Nanogenerators for Tissue Repairing. Nano Energy 54, 390–399. doi:10.1016/j.nanoen.2018.10.020
Li, Z., Zheng, Q., Wang, Z. L., and Li, Z. (2020). Nanogenerator-based Self-Powered Sensors for Wearable and Implantable Electronics. Research 2020, 1–25. doi:10.34133/2020/8710686
Liaquat, M. T., Ahmed, I., and Alzahrani, T. (2021). “Pacemaker Malfunction,” in StatPearls. Treasure Island (FL): StatPearls Publishing. Available at: https://europepmc.org/article/nbk/nbk553149.
Lin, Y.-C., Huang, J., Hileman, S., Martin, K. H., Hull, R., Davis, M., et al. (2015). Leptin Decreases Heart Rate Associated with Increased Ventricular Repolarization via its Receptor. Am. J. Physiol. Heart Circulatory Physiol. 309 (10), H1731–H1739. doi:10.1152/ajpheart.00623.2015
Linden, A., Desmecht, D., Amory, H., and Lekeux, P. (1999). Cardiovascular Response to Intravenous Administration of 5-hydroxytryptamine after Type-2 Receptor Blockade, by Metrenperone, in Healthy Calves. Veterinary J. 157 (1), 31–37. doi:10.1053/tvjl.1998.0250
MacDonald, E. A., Rose, R. A., and Quinn, T. A. (2020). Neurohumoral Control of Sinoatrial Node Activity and Heart Rate: Insight from Experimental Models and Findings from Humans. Front. Physiol. 11, 170. doi:10.3389/fphys.2020.00170
Mahapatra, S. D., Mohapatra, P. C., Aria, A. I., Christie, G., Mishra, Y. K., Hofmann, S., et al. (2021). Piezoelectric Materials for Energy Harvesting and Sensing Applications: Roadmap for Future Smart Materials. Adv. Sci. 8 (17), 2100864. doi:10.1002/advs.202100864
Mangoni, M. E., and Nargeot, J. (2008). Genesis and Regulation of the Heart Automaticity. Physiol. Rev. 88 (3), 919–982. doi:10.1152/physrev.00018.2007
Manteuffel-Szoege, L. (1960). Energy Sources of Blood Circulation and the Mechanical Action of the Heart. Thorax 15 (1), 47–53. doi:10.1136/thx.15.1.47
Maria, Z., Campolo, A. R., Scherlag, B. J., Ritchey, J. W., and Lacombe, V. A. (2020). Insulin Treatment Reduces Susceptibility to Atrial Fibrillation in Type 1 Diabetic Mice. Front. Cardiovasc. Med. 7, 134. doi:10.3389/fcvm.2020.00134
Mariello, M. (2022). Recent Advances on Hybrid Piezo-Triboelectric Bio-Nanogenerators: Materials, Architectures and Circuitry. Nanoenergy Adv. 2 (1), 64–109. doi:10.3390/nanoenergyadv2010004
Mehta, P. K., and Griendling, K. K. (2007). Angiotensin II Cell Signaling: Physiological and Pathological Effects in the Cardiovascular System. Am. J. Physiol. Cell Physiol. 292 (1), C82–C97. doi:10.1152/ajpcell.00287.2006
Melville, N. Y. (2021). "Cardiac Pacemaker Could be Powered by Kinetic Energy From the Heart," in American Institute Of Physics. AIP.
Moghtadaei, M., Polina, I., and Rose, R. A. (2016). Electrophysiological Effects of Natriuretic Peptides in the Heart Are Mediated by Multiple Receptor Subtypes. Prog. Biophys. Mol. Biol. 120 (1-3), 37–49. doi:10.1016/j.pbiomolbio.2015.12.001
Mokhtari, F., Shamshirsaz, M., Latifi, M., and Foroughi, J. (2020). Nanofibers-based Piezoelectric Energy Harvester for Self-Powered Wearable Technologies. Polymers 12 (11), 2697. doi:10.3390/polym12112697
Moreno, A., Walton, R. D., Constantin, M., Bernus, O., Vigmond, E. J., and Bayer, J. D. (2019). Wide-area Low-Energy Surface Stimulation of Large Mammalian Ventricular Tissue. Sci. Rep. 9 (1), 15863–15911. doi:10.1038/s41598-019-51364-w
Muñoz, H. R., and Sacco, C. M. (1997). Cardiac Mechanical Energy and Effects on the Arterial Tree. J. Cardiothorac. Vasc. Anesth. 11 (3), 289–298.
Murillo, G., Blanquer, A., Vargas-Estevez, C., Barrios, L., Ibáñez, E., Nogués, C., et al. (2017). Electromechanical Nanogenerator-Cell Interaction Modulates Cell Activity. Adv. Mat. 29 (24), 1605048. doi:10.1002/adma.201605048
Osadchii, O. E. (2015). Emerging Role of Neurotensin in Regulation of the Cardiovascular System. Eur. J. Pharmacol. 762, 184–192. doi:10.1016/j.ejphar.2015.05.025
Ouyang, H., Liu, Z., Li, N., Shi, B., Zou, Y., Xie, F., et al. (2019). Symbiotic Cardiac Pacemaker. Nat. Commun. 10 (1), 1821–1910. doi:10.1038/s41467-019-09851-1
Papp, L. (2008). Isn't the Heart the Source of Energy for Blood Circulation? “The Heart Doesn't Know the Basic Laws of Physics". Orvosi Hetil. 149 (31), 1443–1447. doi:10.1556/oh.2008.28366
Petersen, K. M., Bøgevig, S., Holst, J. J., Knop, F. K., and Christensen, M. B. (2018). Hemodynamic Effects of Glucagon: a Literature Review. J. Clin. Endocrinol. Metabol. 103 (5), 1804–1812. doi:10.1210/jc.2018-00050
Pian, P., Bucchi, A., DeCostanzo, A., Robinson, R. B., and Siegelbaum, S. A. (2007). Modulation of Cyclic Nucleotide-Regulated HCN Channels by PIP2 and Receptors Coupled to Phospholipase C. Pflugers Arch. Eur. J. Physiol. 455 (1), 125–145. doi:10.1007/s00424-007-0295-2
Potthoff, S. A., Janus, A., Hoch, H., Frahnert, M., Tossios, P., Reber, D., et al. (2011). PTH-receptors Regulate Norepinephrine Release in Human Heart and Kidney. Regul. Pept. 171 (1-3), 35–42. doi:10.1016/j.regpep.2011.06.002
Rohani, M., and Pollack, G. H. (2013). Flow through Horizontal Tubes Submerged in Water in the Absence of a Pressure Gradient: Mechanistic Considerations. Langmuir 29 (22), 6556–6561. doi:10.1021/la4001945
Saito, K., Gutkind, J. S., and Saavedra, J. M. (1987). Angiotensin II Binding Sites in the Conduction System of Rat Hearts. Am. J. Physiol. Heart Circulatory Physiol. 253 (6), H1618–H1622. doi:10.1152/ajpheart.1987.253.6.h1618
Shi, B., Liu, Z., Zheng, Q., Meng, J., Ouyang, H., Zou, Y., et al. (2019). Body-integrated Self-Powered System for Wearable and Implantable Applications. ACS Nano 13 (5), 6017–6024. doi:10.1021/acsnano.9b02233
Sultana, A., Alam, M. M., Middya, T. R., and Mandal, D. (2018). A Pyroelectric Generator as a Self-Powered Temperature Sensor for Sustainable Thermal Energy Harvesting from Waste Heat and Human Body Heat. Appl. Energy 221, 299–307. doi:10.1016/j.apenergy.2018.04.003
Sun, M., Li, Z., Yang, C., Lv, Y., Yuan, L., Shang, C., et al. (2021). Nanogenerator-based Devices for Biomedical Applications. Nano Energy 89, 106461. doi:10.1016/j.nanoen.2021.106461
Surendran, P. J., Jacob, P., Selvamani, D., Papasavvas, T., Swaminathan, N., Mathew, G., et al. (2021). Upper Extremity Dysfunctions in Patients with Cardiac Implantable Electronic Devices: a Systematic Review. Int. J. Ther. Rehabilitation 28 (7), 1–18. doi:10.12968/ijtr.2020.0160
Suvaneeth, P., and Nair, N. D. (2018). Biomaterials and Biocompatibility. World J. Pharm. Res. 7 (10), 161–171. doi:10.20959/wjpr201810-12253
Thudichum, I. L. W. (1855). On the Cause of the Emptiness of the Arteries after Death. BMJ 3 (110), 122–127. doi:10.1136/bmj.s3-3.110.122
Tommasino, D., Moro, F., Bernay, B., De Lumley Woodyear, T., de Pablo Corona, E., and Doria, A. (2022). Vibration Energy Harvesting by Means of Piezoelectric Patches: Application to Aircrafts. Sensors 22 (1), 363. doi:10.3390/s22010363
Valcavi, R., Menozzi, C., Roti, E., Zini, M., Lolli, G., Roti, S., et al. (1992). Sinus Node Function in Hyperthyroid Patients. J. Clin. Endocrinol. Metabol. 75 (1), 239–242. doi:10.1210/jcem.75.1.1619016
Wang, Z. L., Yang, R., Zhou, J., Qin, Y., Xu, C., Hu, Y., et al. (2010). Lateral Nanowire/nanobelt Based Nanogenerators, Piezotronics and Piezo-Phototronics. Mater. Sci. Eng. R Rep. 70 (3-6), 320–329. doi:10.1016/j.mser.2010.06.015
Wang, S., Lin, L., and Wang, Z. L. (2015). Triboelectric Nanogenerators as Self-Powered Active Sensors. Nano Energy 11, 436–462. doi:10.1016/j.nanoen.2014.10.034
Wang R, R., Liu, S., Liu, C. R., and Wu, W. (2021). Data-driven Learning of Process Property Performance Relation in Laser-Induced Aqueous Manufacturing and Integration of ZnO Piezoelectric Nanogenerator for Self-Powered Nanosensors. Nano Energy 83, 105820. doi:10.1016/j.nanoen.2021.105820
Wang YM, Y. M., Zeng, Q., He, L., Yin, P., Sun, Y., Hu, W., et al. (2021). Fabrication and Application of Biocompatible Nanogenerators. Iscience 24 (4), 102274. doi:10.1016/j.isci.2021.102274
Wang, Z. L. (2007). The New Field of Nanopiezotronics. Mater. Today 10 (5), 20–28. doi:10.1016/s1369-7021(07)70076-7
Wang, Z. L. (2013). Triboelectric Nanogenerators as New Energy Technology for Self-Powered Systems and as Active Mechanical and Chemical Sensors. ACS Nano 7 (11), 9533–9557. doi:10.1021/nn404614z
Williams, D. F. (2008). On the Mechanisms of Biocompatibility. Biomaterials 29 (20), 2941–2953. doi:10.1016/j.biomaterials.2008.04.023
Xia, X., Liu, Q., Zhu, Y., and Zi, Y. (2020). Recent Advances of Triboelectric Nanogenerator Based Applications in Biomedical Systems. EcoMat 2 (4), e12049. doi:10.1002/eom2.12049
Yang, Y., Guo, W., Pradel, K. C., Zhu, G., Zhou, Y., Zhang, Y., et al. (2012). Pyroelectric Nanogenerators for Harvesting Thermoelectric Energy. Nano Lett. 12 (6), 2833–2838. doi:10.1021/nl3003039
Yu, A., Carlson, P., and Pollack, G. H. (2014). Unexpected Axial Flow through Hydrophilic Tubes: Implications for Energetics of Water. Eur. Phys. J. Spec. Top. 223 (5), 947–958. doi:10.1140/epjst/e2013-01837-8
Yu, X., Liang, X., Krishnamoorthy, R., Jiang, W., Zhang, L., Ma, L., et al. (2020). Transparent and Flexible Hybrid Nanogenerator with Welded Silver Nanowire Networks as the Electrodes for Mechanical Energy Harvesting and Physiological Signal Monitoring. Smart Mat. Struct. 29 (4), 045040. doi:10.1088/1361-665x/ab7737
Zagabathuni, A., and Kanagaraj, S. (2022). “Development of Piezoelectric Nanogenerator Based on Micro/Nanofabrication Techniques and its Application on Medical Devices,” in Advanced Micro-and Nano-Manufacturing Technologies (Singapore: Springer), 225–244. doi:10.1007/978-981-16-3645-5_10
Zhao, Y., Deng, P., Nie, Y., Wang, P., Zhang, Y., Xing, L., et al. (2014). Biomolecule-adsorption-dependent Piezoelectric Output of ZnO Nanowire Nanogenerator and its Application as Self-Powered Active Biosensor. Biosens. Bioelectron. 57, 269–275. doi:10.1016/j.bios.2014.02.022
Zheng, Q., Shi, B., Li, Z., and Wang, Z. L. (2017). Recent Progress on Piezoelectric and Triboelectric Energy Harvesters in Biomedical Systems. Adv. Sci. 4 (7), 1700029. doi:10.1002/advs.201700029
Zurbuchen, A., Pfenniger, A., Stahel, A., Stoeck, C. T., Vandenberghe, S., Koch, V. M., et al. (2013). Energy Harvesting from the Beating Heart by a Mass Imbalance Oscillation Generator. Ann. Biomed. Eng. 41 (1), 131–141. doi:10.1007/s10439-012-0623-3
Keywords: pacemaker, heart, hormone, charge, nanogenerators, nanosensors, mechanical, electric energy
Citation: Al-Suhaimi EA, Aljafary MA, Alfareed TM, Alshuyeh HA, Alhamid GM, Sonbol B, Almofleh A, Alkulaifi FM, Altwayan RK, Alharbi JN, Binmahfooz NM, Alhasani ES, Tombuloglu H, Rasdan AS, lardhi AA, Baykal A and Homeida AM (2022) Nanogenerator-Based Sensors for Energy Harvesting From Cardiac Contraction. Front. Energy Res. 10:900534. doi: 10.3389/fenrg.2022.900534
Received: 20 March 2022; Accepted: 13 April 2022;
Published: 17 May 2022.
Edited by:
Ghulam Yasin, Beijing University of Chemical Technology, ChinaCopyright © 2022 Al-Suhaimi, Aljafary, Alfareed, Alshuyeh, Alhamid, Sonbol, Almofleh, Alkulaifi, Altwayan, Alharbi, Binmahfooz, Alhasani, Tombuloglu, Rasdan, lardhi, Baykal and Homeida. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ebtesam Abdullah Al-Suhaimi, ealsuhaimi@iau.edu.sa; Meneerah Abdulrahman Aljafary, maljafary@iau.edu.sa
 Ebtesam Abdullah Al-Suhaimi
Ebtesam Abdullah Al-Suhaimi Meneerah Abdulrahman Aljafary1*
Meneerah Abdulrahman Aljafary1*  Tahani M. Alfareed
Tahani M. Alfareed Galyah Mohammed Alhamid
Galyah Mohammed Alhamid Atheel Almofleh
Atheel Almofleh Reham Khalid Altwayan
Reham Khalid Altwayan Jamilah Naif Alharbi
Jamilah Naif Alharbi Huseyin Tombuloglu
Huseyin Tombuloglu Alia Saeed Rasdan
Alia Saeed Rasdan