Sublethal and intergenerational effects of fipronil on Binodoxys communis larvae based on transcriptome sequencing
- 1Zhengzhou Research Base, State Key Laboratory of Cotton Biology, School of Agricultural Sciences, Zhengzhou University, Zhengzhou, China
- 2State Key Laboratory of Cotton Biology, Institute of Cotton Research, Chinese Academy of Agricultural Sciences, Anyang, China
- 3Hubei Insect Resources Utilization and Sustainable Pest Management Key Laboratory, Huazhong Agricultural University, Wuhan, China
Fipronil is widely used in the agricultural world as an efficient phenylpyrazole insecticide to control pests. Binodoxys communis is a key parasitic natural enemy of major homopteran pests and can successfully control the population of pests such as cotton aphids. It has not yet been studied what effects would sublethal doses of fipronil have on Binodoxys communis larvae. Here, this study evaluated the effect of fipronil on Binodoxys communis larvae and analyze the transcriptome results. The results showed that LC10 (1.19 mg/L) and LC25 (1.73 mg/L) had significant negative effects on the survival rate and parasitism rate of F0 generation. Moreover, exposure to high concentrations (LC25) of fipronil still had obvious passive effect on the F1 generation of Binodoxys communis. These results indicated that sublethal doses of fipronil have malignant effects on the biological functions of parasitoids and their offspring. The results of transcriptome analysis showed that differentially expressed genes (DEGs) of Binodoxys communis after LC10 treatment are mainly related to immunity and detoxification. LC25 treatment instead resulted in changes in the expression of genes related to nutrition, energy and metabolism reactions. Seven of the identified DEGs were selected for real-time fluorescence quantitative PCR analysis. To the best of our knowledge, this is the first report to evaluate the sublethal, intergenerational, and transcriptomic side effects of fipronil on larvae of parasitic natural pest enemies. Our findings provide data to accurately assess the risk of fipronil usage on Binodoxys communis larvae, and provide important theoretical support for the comprehensive prevention and control of natural enemies and pesticides.
Introduction
Insects have the ability to regulate and support the ecosystem in which they live, as such they are key members of both farmland and terrestrial ecosystems. In recent years, global insect populations have changed dramatically due to anthropogenic pollution, appearance and migration of invasive species, and climate change (Sánchez-Bayo and Wyckhuys, 2019; Seibold et al., 2019; Wagner, 2020). In order to ensure the yield of food crops, a large number of chemical pesticides are currently used for pest control, inevitably damaging non-target beneficial natural enemies of the pest being targeted (Overton et al., 2021; Sánchez-Bayo, 2021). It is necessary to establish a balance between pesticide usage and the presence of beneficial natural enemies of pests. Studying the impact of pesticides on natural enemies and on other non-target organisms in fields, will also contribute to the achieving of sustainable development in farmland ecology.
Binodoxys communis (Hymenoptera: Braconidae) is a dominant parasitoid widely distributed in fields with a wide range of possible hosts. It mainly parasitizes major homopteran pests such as cotton aphids, aphids and glycine aphids (Wyckhuys et al., 2008; Ghising et al., 2012; Yang et al., 2017; Zhang et al., 2020). There are two main ways for parasitoids to obtain nutrients: the larvae mainly obtain nutrients by feeding on hemolymph and lipids from the host, while in the adult stage these nutrients are supplemented with pollen, nectar or honeydew secreted by the Homoptera (Burger et al., 2004; Shi et al., 2009). In the field environment, parasitic wasps are therefore exposed to different types of pesticides directly and indirectly through the nutrients.
Fipronil (CAS 120068-37-3, MW 437.16) is a strong polar phenylpyrazole insecticide that has high neurotoxicity. It controls pests mainly through stomach toxicity, contact and inhalation, and it does not present any risk in terms of crop safety (Pisa et al., 2015; Wu et al., 2015). Being an efficient pesticide, fipronil degradates slowly in the natural environment (Bobé et al., 1998), having a half-life of up to 7 months (Bonmatin et al., 2015; dos Santos et al., 2016). Since 2009, despite the high effectiveness in controlling pests due to its neurotoxicity, China has limited the use of fipronil in view of its high toxicity to aquatic organisms and bees. Nevertheless fipronil is still being detected in different environments, demonstrating its continued usage in agricultural practices (Yang et al., 2010; Gan et al., 2012; Wu et al., 2015; Wei et al., 2017; Shi et al., 2020; Li et al., 2021; Wu et al., 2021). Previous studies have suggested that fipronil is widely present in soil and water, and in the crops themselves, which greatly increases the risk of non-target beneficial insects coming across the compound. It has been reported that exposure to sublethal doses of fipronil in bees, a major pollinator, affects their motility, fertility, biological morphology and causes changes in their intestinal flora (Roat et al., 2017; Paris et al., 2020; Farder-Gomes et al., 2021).
In view of the ecological advantages of Binodoxys communis and the presence of fipronil in the environment, it is important to assess if parasitoid larvae could be affected by side effects when indirectly exposed to the insecticide. Considering the particularity of the development of Binodoxys communis larvae in the host, we combined transcriptomic results to analyze the effects of fipronil on larvae at the molecular level. To this date, most studies have focused on the effects of pesticides on adults, while little is known about their effects on eggs and larvae. In our study, we evaluated the sublethal and intergenerational effects of fipronil on several biological indicators, including survival rate, parasitic rate and survival time of Binodoxys communis larvae exposed to fipronil. In parallel, we subjected the expression results on the transcriptome to differential gene expression analysis, gene ontology (GO) classification and Kyoto Encyclopedia of Genes and Genomes (KEGG) annotation. Our results fill the gap in the risk assessment of using fipronil taking into consideration its effects on parasitic wasp larvae. In addition, our findings can improve pest management strategies with Binodoxys communis in agricultural systems. This study provides a basis for future research on biodiversity and conservation biology.
Materials and methods
Test organisms and reagents
Binodoxys communis (Hymenoptera: Braconidae) and Aphis gossypii (Hemiptera: Aphidoidea) were collected from the experimental farm (36°5′34.8″ N, 114°31′47.19″ E) of the Cotton Research Institute, Chinese Academy of Agricultural Sciences, and bred for many generations in the laboratory at the Institute of Biotechnology, Institute of Plant Protection, Chinese Academy of Agricultural Sciences. The tested insects were cultured in typical laboratory conditions: temperature 26 ± 1°C, humidity 65 ± 5%, photoperiod 14 L: 10 D. Binodoxys communis were reared in artificial intelligence light incubators, and A. gossypii were reared in gauze cages (30 cm × 30 cm × 30 cm).
The cotton variety Zhongmiansuo 49 used in this experiment was provided by the Institute of Cotton Research, Chinese Academy of Agricultural Sciences. The planting conditions in the artificial climate chamber were 26 ± 1°C, photoperiod 14 L: 10 D, humidity 75 ± 5%. The standard fipronil with a purity of 97% used in the test was purchased from Bayer Crop Science Co., Ltd.
Sublethal bioassay
The concentration of fipronil sublethal to Binodoxys communis (LC10 and LC25) was determined by exposing the parasitoids to the dry residue of the insecticide for 1 h. The results of this experiment have been submitted for publication and they can be summarized as follows: LC10 and LC25 of fipronil were respectively 1.19 (95% confidence interval: 0.16–0.54 mg/L) and 1.73 (95% confidence interval: 0.37–0.91 mg/L) mg/L (Du et al. submitted). 0.1% Triton solution was used as diluent, and three treatment groups were set up: 1) LC10 treatment group, 2) LC25 treatment group, 3) 0.1% Triton solution group. Fresh cotton leaves were fully immersed in the control solution or in the concentrations of fipronil corresponding to the group (LC10 and LC25) for 10 s. The leaves were left to dry completely, 30 healthy second instar cotton aphids were subsequently inoculated in each leaf. After 24 h of full contact, the aphids were moved to clean cotton leaves not treated with pesticide and inoculated with a pair of parasitic wasps (eclosion within 24 h). After 8 h, the parasitized cotton aphids were transferred to fresh cotton leaves for further feeding. The feeding conditions of the intelligent light incubator were set to 26 ± 1°C, humidity 65 ± 5%, and photoperiod 14 L: 10D. The newly emerged F0 Binodoxys communis were transferred to the finger tube of the hole and provided with a amount of 10% honey water. The survival of the parasitoid wasps was evaluated daily until death, and the survival rate, survival time and parasitic rate of the F0 generation were noted for evaluation. Each pair of parasitic wasps was used as a biological replicate, and each treatment was repeated three times.
In order to evaluate whether fipronil has intergenerational effects, we randomly collected three pairs of F0 adults without any treatment and repeated the above operation. The survival rate, survival time and parasitic rate of F1 generation were recorded and evaluated.
We dissected the aphids that were parasitized for 3 days (B. communis larvae hatched after 3 days of parasitism) and collected the parasitoid larvae to examine any molecular changes in B. communis larvae. Immediately after removal, the larvae were transferred to a 1.5 ml non-enzymatic centrifuge tube in liquid nitrogen and stored at -80°C. Three independent biological replicates were set up for each treatment group, and >30 parasitoid larvae were collected for subsequent gene expression analysis.
RNA extraction, cDNA library construction, sequencing
After purification, larval RNA was extracted using TRIzol reagents (Invitrogen, Karlsbad, United States). RNA samples were evaluated for quality using a NanoDrop 2000C spectrophotometer (Thermo, United States) and 1.0% agarose gel electrophoresis. Samples with absorbance between 1.8 and 2.0 at 260/280 nm were analyzed. The library was constructed according to standardized procedures in Biomarker Technology Co., Ltd. (Beijing, China) and sequenced on the company‘s sequencing platform. The original sequencing data of this experiment have been stored in the NCBI Sequence Read Archive (SRA) database (Accession number: PRJNA901131).
RNA sequencing data analysis and gene annotation
The raw data was obtained by Illumina NovaSeq high-throughput sequencing platform based on Sequencing By Synthesis (SBS) technology. Clean Data was obtained by filtering low-quality reads and removing sequencing joints and primer sequences. The filtered and trimmed reads were subjected to a series of assemblies using the short read program Trinity (Trinity - v2.5.1, min_kmer_cov default set to 1, all other parameters set to default), and the longest sequence resulting from the assemblies after removing any redundancy was defined as unigene. Mapped reads from each sample that successfully aligned with the assembled Unigene library sequence were used for subsequent analysis.
The abundance of the reads was estimated using the RNA-seq by Expectation Maximization (RSEM) by using the Bowtie program to align the reads with the de novo assembled transcripts. In order to obtain comprehensive gene information, we set the BLAST parameter E-value≤1e-5 and HMMER parameter E-value≤1e-10, and used DIAMOND, KOBAS, InterProScan and HMMER software to compare unigene sequences with the following databases: 1) Non-Redundant Protein Sequence Database (NR), 2) Swiss-Prot Protein Sequence Database (Swiss-Prot), 3) Clusters of Orthologous Groups (COG), 4) euKaryotic Orthologous Groups (KOG), 5) Non-supervised Orthologous Groups (eggNOG4.5), 6) Kyoto Encyclopedia of Genes and Genomes (KEGG), 7) Protein family database (Pfam) and 8) Gene Ontology database (GO).
We used false discovery rate (FDR) to evaluate the corrected p-value of multiple tests. We required differentially expressed unigenes with FDR<0.01 and | log2FC (fold change) | ≥ 2. GO enrichment analysis of differentially expressed gene functions was performed using the Goatools program. The differentially expressed unigenes were then divided into three ontologies: biological processes, cellular components and molecular functions. KEGG pathway analysis was performed to identify DEGs enriched pathways. When p ≤ 0.05, the pathway was considered significantly enriched.
Validation of transcriptomic data by qRT-PCR
In order to verify the transcriptome data, we randomly selected seven genes from the DEG list and performed real-time fluorescence quantitative PCR with AK and RPL as internal reference genes. The primer sequences for all genes are shown in Supplementary Table S1. Total RNA was transcribed into cDNA using the PrimeScript RT Reagent Kit (product number: RRO37Q). The qPCR was performed using 10 μL of reaction mixture: 5 μL 2 × transgenic top green qPCR super mixture (+DyeI), 0.4 μL forward primer, 0.4 μL reverse primer, 2 μL cDNA, and 2.2 μL DEPC nuclease-free water. The qPCR cycles were: 95 °C for 30 s, 40 cycles at 95 °C for 5 s and at 55°C for 15 s, and 72°C 10 s.
Data processing and statistical analysis
LC10, LC25 and the 95% confidence intervals required for this experiment were calculated by logarithmic probability regression analysis using SPSS v.16.0 (SPSS Inc., Chicago, IL) (Du et al. submitted). One-way analysis of variance was used to evaluate the significance of differences in survival rate, survival time and parasitic rate between control and treatment groups. Duncan’s new multiple range method was used to test the data. When p < 0.05 we considered the difference statistically significant. The relative expression of each gene was calculated by the 2−ΔΔCt method. Graphpad Prism 8.0.2 software was used in this experiment.
Results
Sublethal effects of fipronil on survival rate, survival time and parasitism rate of B. communis
In this study, we identified LC10 (1.19 mg/L) and LC25 (1.73 mg/L) as sublethal doses at which to evaluate the safety of fipronil. The results showed that exposure at both low and high doses of fipronil had a significant negative effect on the survival rate of B. communis (Figure 1A). LC10 and LC25 doses both had significant effects on the survival rate of parasitoids, with respectively only 26.67% (p = 0.002) and 50% (p = 0.035) of the parasitoids surviving, compared with 76.67% of the control group.
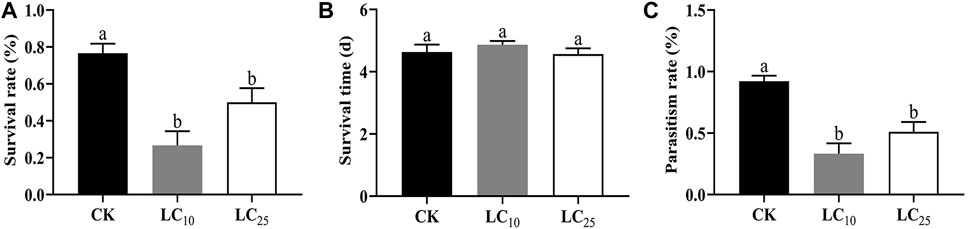
FIGURE 1. Sublethal effects of fipronil on survival rate, survival time and parasitism rate of Binodoxys communis. Note: (A) survival rate of Binodoxys communis; (B) survival days; (C) parasitism rate; Columns (Mean ± SE) with the same letters are not significantly different (Duncan test; p > 0.05).
We observed no difference in survival times between treatment groups (LC10 and LC25) and solvent control group (p > 0.05) (Figure 1B). The average survival time of Binodoxys communis in the control group was 4.63 ± 0.24 d, and the survival time of parasitoids in the LC10 and LC25 treatment groups was respectively 4.87 ± 0.12 d and 4.57 ± 0.19 d (p > 0.05).
At LC10 and LC25, fipronil had a significant negative effect on the parasitism rate of cotton aphids (Figure 1C). Only 33.33% of parasitoids exposed to LC10 (p = 0.003) and 51.11% of those exposed to LC25 (p = 0.016) successfully parasitized aphids, in comparison with 92.22% of the control group.
Transgenerational effects of sublethal doses of fipronil on the offspring of B. communis larvae
In order to determine whether sublethal insecticide treatment has an intergenerational transmission effect on Binodoxys communis, we evaluated the effects of fipronil on the survival rate, survival time and parasitic rate of F1 parasitic wasps (Figure 2). The results showed that 80% of parasitoids in the LC10 treatment group survived in the absence of fipronil, which was not the same rate as observed in the control group (Figure 2A). However, the survival rate of the LC25 treatment group had a significant reduction, and only 61.33% of the parasitoids survived (p = 0.041).
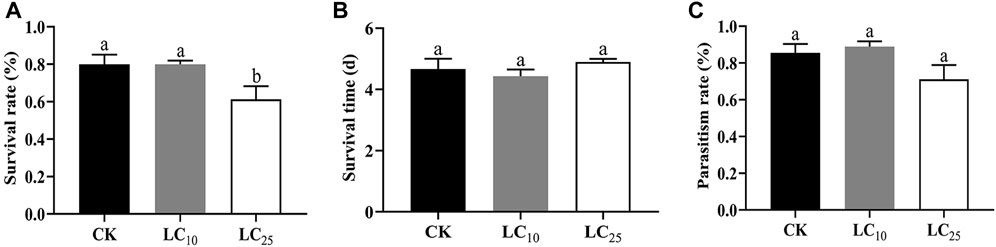
FIGURE 2. Transgenerational effects of sublethal doses of fipronil on offspring of Binodoxys communis. Note, (A) survival rate of Binodoxys communis; (B) survival days; (C) parasitism rate; Columns (Mean ± SE) with the same letters are not significantly different (Duncan test; p > 0.05).
The effects of sublethal doses of fipronil on the survival time of Binodoxys communis progeny are shown in Figure 2B. Compared with the control group (4.67 ± 0.33 d, p = 0.23), there were no significant differences in the survival time of LC10 and LC25 (respectively 4.43 ± 0.22 d and 4.9 ± 0.1 day).
The parasitism rates of the F1 generation are shown in Figure 2C. There were no significant differences between treatment groups and control. The overall parasitic rate of B. communis increased significantly in the F1 generation compared with the F0 generation. The parasitism rates of the LC10 and LC25 B. communis groups were respectively 88.67% and 71%, a decrease compared to the 83.33% of the control group.
Transcriptome sequencing, gene annotation and differential genes (DEGs) analysis
The transcriptome data of the two groups of B. communis larvae treated with sublethal doses of fipronil are shown in Supplementary Table S2. After several steps of quality control on the original data, we obtained a total of 134,139,984 reads. The sequencing of the two treatment groups resulted in 78.53 Gb of clean data. From each sample, we obtained up to 6.05 Gb of clean data, with a proportion of Q30 bases between 90.22% and 93.51%, and a GC percentage above 38.19%. We compared the clean reads of each sample with the reference sequence assembled by Trinity software, and the mapping alignment rate of each sample was between 73.44% and 79.47%. The assembled unigenes were functionally annotated by comparing them with the nine databases. We annotated a total of 13,427 unigenes, accounting for 83.2% of the total unigenes, indicating that the annotation success rate was high (Supplementary Figure S1) and proving the reliability of our transcriptome annotation results.
The differentially expressed genes (DEGs) identified in the LC10 and LC25 treatment groups were analyzed by the FPKM method. We visualized the DEGs on a volcano plot that showed their number was related to the concentration of fipronil (Figure 3). After log2FC > 1 (higher than double expression) and error detection rate (FDR) correction p < 0.01, we found 1404 DEGs in the LC10 exposure group, 968 of which were up-regulated and 436 down-regulated. When the fipronil dose was increased to LC25, the total number of DEGs increased to 1868, 1089 up-regulated and 779 down-regulated.
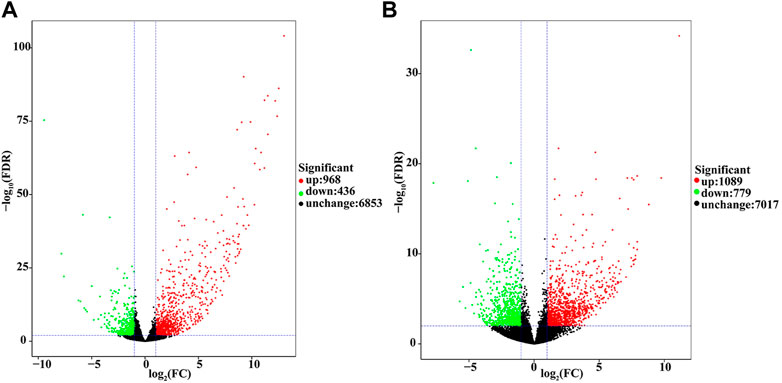
FIGURE 3. DEGs between control and treatment groups are shown in the volcanic map (log10p-value vs. log2FC). Note, Red dots and green dots represent transcripts respectively with up-regulation and down-regulation changes. Black dots represent unchanged transcripts.
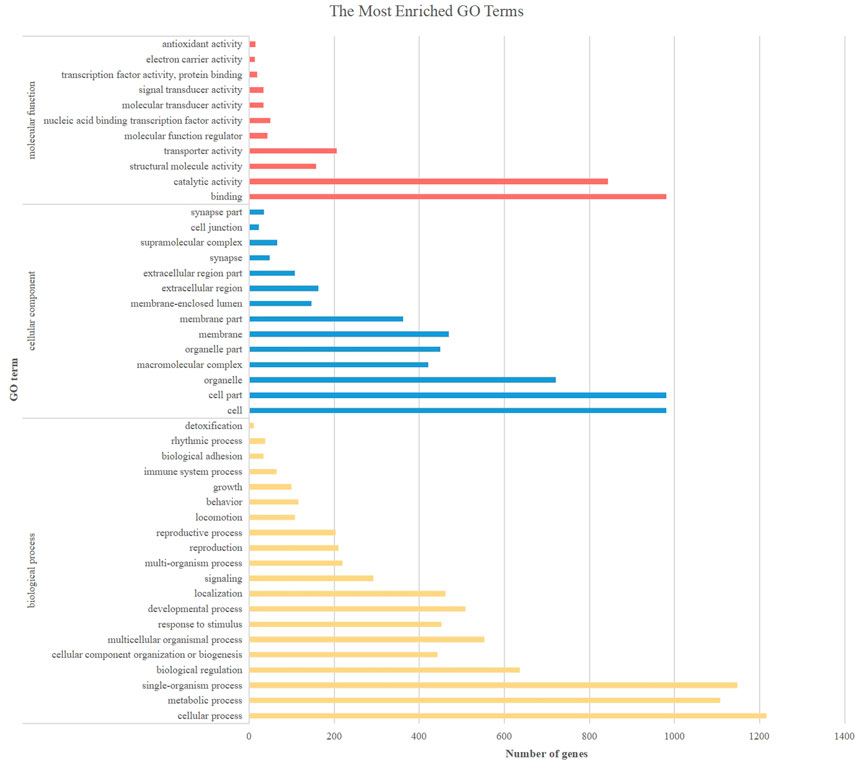
GO functional analysis of DEGs revealed changes in gene expression in Binodoxys communis larvae under fipronil stress (Figure 4). The results showed that a total of 15,284 DEGs were significantly enriched across 50 major subcategories of biological process, cellular component and molecular function. In the biological process, the differential genes were mainly enriched in cellular, metabolic and single-organism processes, and in biological regulation. In terms of cell components, the differential genes were mainly enriched in cell, cell part and organelle. In molecular function, differential genes were mainly enriched in binding and catalytic activity.
In order to clarify the potential pathways of DEGs after fipronil exposure, we performed KEGG ontology analysis on the up-regulated DEGs (Figure 5A). Research showed that starch and sucrose metabolism (ko00500), neuroactive ligand-receptor interaction (ko04080), steroid biosynthesis (ko00100), lysosome (ko04142), valine, leucine and isoleucine degradation (ko00280), glycosaminoglycan degradation (ko00531), pentose and glucuronate interconversions (ko00040) and glyoxylate and dicarboxylate metabolism (ko00630) were the eight most significantly enriched pathways for up-regulated genes after fipronil exposure (qvalue <0.01). 1169 DEGs with down-regulated expression were annotated into 125 pathways, and the top 20 pathways with significant enrichment are shown in Figure 5B, with neuroactive ligand-receptor interaction (ko04080) and ribosome biogenesis in eukaryotes (ko03008) being the two most significant pathways (qvalue <0.01). The up-regulated genes in the neuroactive ligand pathway were annotated, by comparing with the NR database, as chymotrypsin-like protein (CTRL), chymotrypsin-1-like (Chy1) and chymotrypsin-2 (Chy-2), while down-regulated genes were mainly different types of chymotrypsin (Chy), trypsin (Try) and serine protease (Sps). 50 genes related to carbohydrate synthesis and metabolism, such as beta-glucuronidase (GUS) and beta-galactosidase (beta-gal), were expressed at normal levels in theLC10 group, but were significantly up-regulated in the LC25 group. In addition, 14 genes enriched in steroid biosynthesis pathway, including venom carboxylesterase-6 (V carE-6), lipase 3-like (Lip 3) and gastric triacylglycerol lipase (LIPF), were up-regulated in the LC10 treatment group, while most of the up-regulated genes in the LC25 group returned to normal expression levels. 19 genes in the lysosomal pathway were up-regulated in the LC10 treatment group, while 39 were up-regulated in the LC25 group. The number of up-regulated DEGs in the amino acid degradation pathway increased from 9 to 23 with the increasing concentration of fipronil, and included 2-oxoisovalerate dehydrogenase (OgdH), 3-hydroxyacyl-CoA dehydrogenase (HADH) and 3-ketoacyl-CoA thiolase (KAT). There were 14 down-regulated genes enriched in ribosome biogenesis in eukaryotes pathway in the LC10 group, while only six were down-regulated in the LC25 group, includings ribosome biogenesis protein (RBP) and RNA-binding protein.
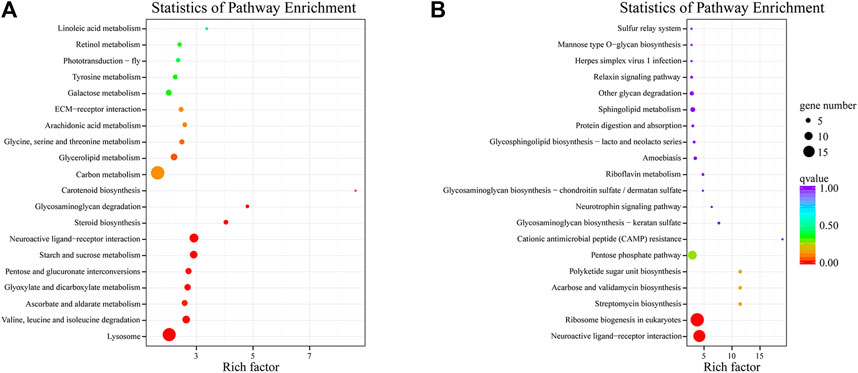
FIGURE 5. KEGG pathway analysis of DEGs. Note, Y-axis represents pathways and X-axis represents the enrichment score. (A) After exposure to fipronil, the KEGG pathway enriched by DEGs was up-regulated. (B) After exposure to fipronil, the KEGG pathway enriched by DEGs was down-regulated.
Validation of transcriptome data by qPCR
The transcriptome data of seven genes chosen at random were verified by real-time fluorescence quantitative PCR. The results of the qRT-PCR verification are shown in Figure 6. The trends in gene expression levels observed were the same as in the transcriptome data, proving the reliability of RNA-Seq results in our study.
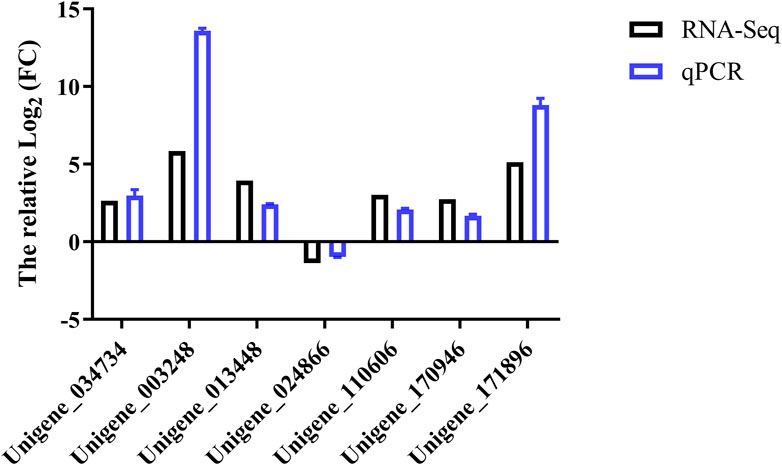
FIGURE 6. qRT-PCR and RNA-Seq analysis results of differentially expressed genes in Binodoxys communis.
Discussion
To this day, fipronil plays a major role in the prevention and control of agricultural pests and is used in agriculture worldwide. Today it is unrealistic to suggest a complete halt in its use. Yet in a field environment, both pests and non-target beneficial insects, such as natural enemies of pests, are exposed to insecticides, which can have a variety of sublethal effects through feeding and contact, and harm offspring and even entire species population (Stark et al., 2007; Biondi et al., 2012; Bueno et al., 2017). It is therefore of the foremost importance to study the sublethal effects of insecticides on non-target species in order to better understand the environmental risks of pesticide use. The effects of pesticides on larvae of parasitic natural enemies have not yet been researched in detail, especially in terms of intergenerational effects. To the best of our knowledge, this is the first study to evaluate the biological effects of exposure to sublethal doses of pesticides on parasitoid larvae and their offspring, and the corresponding alterations in gene expression. In this study, insect larvae exposed to sublethal concentrations of fipronil showed a significant decrease in survival and parasitism rates, effects on the feeding of offspring, and caused multiple changes in gene expression levels in larvae.
Exposing Binodoxys communis larvae to sublethal doses of fipronil leads to decreased parasitic and survival rates. Several studies have reported on the sublethal or lethal effects of fipronil on non-target insects (Pisa et al., 2015; Fontes et al., 2018; Bownik and Szabelak, 2021). It is worth noting that at the LC10 dose, the negative effect of Binodoxys communis larvae was very strong, and the survival rate and parasitism rate were significantly reduced. Compared with the low dose, the decrease of survival rate and parasitic rate of Binodoxys communis larvae at the high dose was not so strong. Here, combined with transcriptome results, we hypothesized that high concentration of fipronil stress caused an increase in the number of differential genes in Binodoxys communis larvae in response to drug stress. In addition, we evaluated the effects of fipronil sublethal dosage on F1 generation. In the presence of high concentration of fipronil, the parasitic rate of F1 parasitoids decreased significantly. The effects of survival rate and parasitism rate on F1 parasitoids were not significant. These findings are in agreement with similar previous reports of intergenerational effects of pesticides on insects (Blanc et al., 2020; Brevik et al., 2021; Tamilselvan et al., 2021). In conclusion, fipronil has obvious intergenerational effects on the larvae of the natural pest enemy Binodoxys communis, which should be carefully considered in future fipronil applications.
Most previous research has focused on the biological and characterization abnormalities of insects caused by sublethal pesticide exposure, but there is a gap in the knowledge of genetic effects at the molecular level (Bovi et al., 2018; Shen et al., 2019). We recommend further genome-wide analysis to fully understand the impact of environmental pollutants on natural enemies of pests. Our transcriptomics analysis found that many DEGs showed concentration effects. At high concentrations of fipronil, the number of both up-regulated and down-regulated genes showed an increasing trend. Similarly, studies have reported that when non-target insects are exposed to sublethal doses of pesticides, pesticide concentrations are positively correlated with the number of differentially expressed genes in insects (Fent et al., 2020; Xu et al., 2021). Our findings can therefore guide and lay down requirements to determine the pesticide concentration appropriate to the parasitic wasp’s living environment.
In groups treated with LC10 and LC25 fipronil concentrations, DEGs were mainly involved in glucose metabolism-related pathways, neuroactive ligand-receptor interactions, steroid biosynthesis, lysosomes, amino acid degradation, and ribosome biogenesis. It is well known that the main larval energy source in parasitic wasps comes from the conversion and absorption of host energy (Horwood and Hales, 1991). Studies have shown that sugar metabolism-related pathways and carbohydrate metabolism pathways provide energy for other metabolic pathways and are necessary to maintain normal growth and development in insects (Wang et al., 2013; Maynard and Kanarek, 2020). As base components of protein, amino acids also provide energy for metabolic pathways in insects (Parkhitko et al., 2016; Parkhitko et al., 2020). HADH and KAT, which we identified as upregulated DEGs, promote fatty acid metabolism, where OgdH is a key enzyme in the tricarboxylic acid cycle (Nakai et al., 1997; Liu et al., 2020). GUS and beta-gal, upregulated in the LC25 group, are essential regulatory enzymes in carbohydrate-related metabolism, and the latter is also involved in lactose hydrolysis, glycoprotein modification and degradation in vivo (Bar et al., 2018; Yi et al., 2021). In this study, we noted how these genes were increasingly up-regulated at higher fipronil concentrations, indicating that high-dose pesticide stress may demand parasitic wasp larvae a large amount of energy in order to reduce damage to development. Similarly to our findings, previous research has reported that sugar-related metabolic pathways in insects are up-regulated in the presence of insecticide stress (Rochford et al., 2018; Zhang et al., 2021). The neuroactive ligand-receptor interaction pathway transduces different signals such as biogenic amines, neuropeptides, and lipoproteins and regulates insect development and homeostasis (Kwon et al., 2016; Dong et al., 2021). We showed that this pathway was dysregulated in the expression of genes essential to physiological processes such as digestion, defense, humoral immunity, and development: CTRL, Chy, Try and Sps expression levels were modified in the LC10 concentration treatment group, while most genes returned to normal expression levels in the LC25 group. These. Extracellular SPs can amplify physiological or pathological signals and activate Toll-mediated responses to microbial infections (Veillard et al., 2016; Cao and Jiang, 2018). Previous studies have found that phenol stress caused significant disorder response of this pathway in chironomid (Cao et al., 2013). V Care-6, a DEG in the steroid biosynthesis pathway, was up-regulated in the LC10 group, but returned to normal expression levels at LC25 dose. Although the role of V Care has not yet been fully understood, it has been reported that it has lipolytic activity and it participates in the distribution of venom by degrading host blood triglycerides in Bombus ignitus (Deng et al., 2021). Lysosomes maintain cell homeostasis by regulating intracellular degradation, circulation, and signal transduction (Miao et al., 2020). Our results showed that high-dose fipronil has an obvious stimulation effect on lysosomes, which is similar to the results of Dong’s study: the uncomfortable environment caused the enrichment of differential genes in the lysosome pathway in Chilo suppressalis (Walker) larvae (Dong et al., 2021). The results of our study showed that many ribosome biosynthetic component genes were down-regulated after exposure to low concentrations of fipronil. The inhibition of the ribosome biosynthesis pathway not only causes the assembly of ribosomes with functional changes, but also modifies their functions in regulating key developmental processes (Xue and Barna, 2012; Sanchez et al., 2016). Transcriptome results showed that, although low concentrations of fipronil exposure resulted in the up-regulated expression of detoxification and immune-related genes in Binodoxys communis larvae, most of these genes had normal expression levels after exposure to high concentrations, while genes related to nutrition and energy response were significantly up-regulated in both the LC10 and the LC25 groups. At the same time, exposure to high-doses of fipronil resulted in a significant decrease in the survival rate of F1 Binodoxys communis and had a negative impact on the parasitic rate of F1 parasitoids. We speculate that Binodoxys communis requires a lot of energy to cope with high-dose fipronil stress, and this stimulates and affects the biological function of parasitoids for a long time.
Conclusion
In conclusion, our study shows that fipronil has significant negative effects on the survival rate and parasitism rate of Binodoxys communis larvae at LC10 and LC25 doses. Fipronil also reduces the survival rate of offspring in the high-dose group and has a potential negative impact on the parasitic rate. These results indicate that exposure to sublethal doses of fipronil has potential long-term risks to Binodoxys communis populations. Transcriptome analysis revealed that fipronil induced considerable gene-level variation, with the expression of many genes involved in nutrition, energy, detoxification, and immune responses changing. To the best of our knowledge, this is the first study to assess the combination of biological characteristics and molecular effects of fipronil usage on parasitic natural enemy larvae. Our results provide a basis to understand the multi-level effects of sublethal doses of fipronil on the growth and development of Binodoxys communis and its offspring and to assess the risk of fipronil usage to larvae of other fields parasitic natural enemies.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
Ethical review and approval was not required for the animal study because Our experiments do not involve any living vertebrates and higher invertebrates and do not require approval.
Author contributions
XG, Conceptualization, Writing- Reviewing and Editing. XZ, Supervision. JC, Methodology, Software. LZ, Investigation. KZ, Data curation. LW, Formal analysis. DL,Validation. LD, Software, Writing - Original. LN, Supervision. JJ, Supervision, Software. JL, Methodology, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Agricultural Science and Technology Innovation Program of Chinese Academy of Agricultural Sciences and China Agriculture Research System.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.1080601/full#supplementary-material
References
Bar, S., Prasad, M., and Datta, R. (2018). Neuromuscular degeneration and locomotor deficit in a Drosophila model of mucopolysaccharidosis VII is attenuated by treatment with resveratrol. Dis. Model. Mech. 11, dmm036954. doi:10.1242/dmm.036954
Biondi, A., Mommaerts, V., Smagghe, G., Viñuela, E., Zappalà, L., and Desneux, N. (2012). The non-target impact of spinosyns on beneficial arthropods. Pest Manag. Sci. 68, 1523–1536. doi:10.1002/ps.3396
Blanc, M., Cormier, B., Hyötyläinen, T., Krauss, M., Scherbak, N., Cousin, X., et al. (2020). Multi- and transgenerational effects following early-life exposure of zebrafish to permethrin and coumarin 47: Impact on growth, fertility, behavior and lipid metabolism. Ecotoxicol. Environ. Saf. 205, 111348. doi:10.1016/j.ecoenv.2020.111348
Bobé, A., Meallier, P., Cooper, J. F., and Coste, C. M. (1998). Kinetics and Mechanisms of Abiotic Degradation of Fipronil (Hydrolysis and Photolysis). J. Agric. Food Chem. 46, 2834–2839. doi:10.1021/jf970874d
Bonmatin, J. M., Giorio, C., Girolami, V., Goulson, D., Kreutzweiser, D. P., Krupke, C., et al. (2015). Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 22, 35–67. doi:10.1007/s11356-014-3332-7
Bovi, T. S., Zaluski, R., and Orsi, R. O. (2018). Toxicity and motor changes in Africanized honey bees (Apis mellifera L.) exposed to fipronil and imidacloprid. An. Acad. Bras. Cienc. 90, 239–245. doi:10.1590/0001-3765201820150191
Bownik, A., and Szabelak, A. (2021). Short-term effects of pesticide fipronil on behavioral and physiological endpoints of Daphnia magna. Environ. Sci. Pollut. Res. 28, 33254–33264. doi:10.1007/s11356-021-13091-6
Brevik, K., Bueno, E. M., McKay, S., Schoville, S. D., and Chen, Y. H. (2021). Insecticide exposure affects intergenerational patterns of DNA methylation in the Colorado potato beetle, Leptinotarsa decemlineata. Evol. Appl. 14, 746–757. doi:10.1111/eva.13153
Bueno, A. D., Carvalho, G. A., dos Santos, A. C., Sosa-Gomez, D. R., and da Silva, D. M. (2017). Pesticide selectivity to natural enemies: Challenges and constraints for research and field recommendation. Cienc. Rural. 47, 10. doi:10.1590/0103-8478cr20160829
Burger, J., Hemerik, L., van Lenteren, J., and Vet, L. (2004). Reproduction now or later: Optimal host-handling strategies in the whitefly parasitoid Encarsia formosa. Oikos 106, 117–130. doi:10.1111/j.0030-1299.2004.12908.x
Cao, C., Wang, Z., Niu, C., Desneux, N., and Gao, X. (2013). Transcriptome profiling of Chironomus kiinensis under phenol stress using Solexa sequencing technology. PloS one 8, e58914. doi:10.1371/journal.pone.0058914
Cao, X., and Jiang, H. (2018). Building a platform for predicting functions of serine protease-related proteins in Drosophila melanogaster and other insects. Insect Biochem. Mol. Biol. 103, 53–69. doi:10.1016/j.ibmb.2018.10.006
Deng, Y., Kim, B. Y., Lee, K. Y., Yoon, H. J., Wan, H., Li, J., et al. (2021). Lipolytic activity of a carboxylesterase from bumblebee (Bombus ignitus) venom. Toxins 13, 239. doi:10.3390/toxins13040239
Dong, C. L., Lu, M. X., and Du, Y. Z. (2021). Transcriptomic analysis of pre-diapause larvae of Chilo suppressalis (walker) (Lepidoptera: Pyralidae) in natural populations. Comp. Biochem. physiology Part D, Genomics & proteomics 40, 100903.
dos Santos, A., Zanetti, R., dos Santos, J. C., Biagiotti, G., Evangelista, A. L., Serrão, J. E., et al. (2016). Persistence of fipronil residues in Eucalyptus seedlings and its concentration in the insecticide solution after treatment in the nursery. Environ. Monit. Assess. 188, 314. doi:10.1007/s10661-016-5304-5
Farder-Gomes, C. F., Fernandes, K. M., Bernardes, R. C., Bastos, D. S. S., Martins, G. F., and Serrão, J. E. (2021). Acute exposure to fipronil induces oxidative stress, apoptosis and impairs epithelial homeostasis in the midgut of the stingless bee Partamona helleri Friese (Hymenoptera: Apidae). Sci. Total Environ. 774, 145679. doi:10.1016/j.scitotenv.2021.145679
Fent, K., Schmid, M., and Christen, V. (2020). Global transcriptome analysis reveals relevant effects at environmental concentrations of cypermethrin in honey bees (Apis mellifera). Environ. Pollut. (Barking, Essex 1987) 259, 113715. doi:10.1016/j.envpol.2019.113715
Fontes, J., Roja, I. S., Tavares, J., and Oliveira, L. (2018). Lethal and sublethal effects of various pesticides on Trichogramma achaeae (hymenoptera: Trichogrammatidae). J. Econ. Entomol. 111, 1219–1226. doi:10.1093/jee/toy064
Gan, J., Bondarenko, S., Oki, L., Haver, D., and Li, J. X. (2012). Occurrence of fipronil and its biologically active derivatives in urban residential runoff. Environ. Sci. Technol. 46, 1489–1495. doi:10.1021/es202904x
Ghising, K., Harmon, J. P., Beauzay, P. B., Prischmann-Voldseth, D. A., Helms, T. C., Ode, P. J., et al. (2012). Impact of <I>Rag1</I> aphid resistant soybeans on <I>Binodoxys communis</I> (hymenoptera: Braconidae), a parasitoid of soybean aphid (Hemiptera: Aphididae). Environ. Entomol. 41, 282–288. doi:10.1603/en11196
Horwood, M. A., and Hales, D. F. (1991). Fat body changes in a locust, Chortoicetes terminifera (Walker) (Orthoptera: Acrididae), parasitized by a nemestrinid fly. Arch. Insect Biochem. Physiol. 17, 53–63. doi:10.1002/arch.940170107
Kwon, H., Ali Agha, M., Smith, R. C., Nachman, R. J., Marion-Poll, F., and Pietrantonio, P. V. (2016). Leucokinin mimetic elicits aversive behavior in mosquito Aedes aegypti (L.) and inhibits the sugar taste neuron. Proc. Natl. Acad. Sci. U. S. A. 113, 6880–6885. doi:10.1073/pnas.1520404113
Li, B. J., Wang, K. K., Chen, D. P., Yan, Y., Cai, X. L., Chen, H. M., et al. (2021). Distinct roles of two RDL GABA receptors in fipronil action in the diamondback moth (Plutella xylostella). Insect Sci. 28, 1721–1733. doi:10.1111/1744-7917.12892
Liu, L., Zhou, S., and Deng, Y. (2020). The 3-ketoacyl-CoA thiolase: An engineered enzyme for carbon chain elongation of chemical compounds. Appl. Microbiol. Biotechnol. 104, 8117–8129. doi:10.1007/s00253-020-10848-w
Maynard, A. G., and Kanarek, N. (2020). NADH ties one-carbon metabolism to cellular respiration. Cell metab. 31, 660–662. doi:10.1016/j.cmet.2020.03.012
Miao, R., Li, M., Zhang, Q., Yang, C., and Wang, X. (2020). An ECM-to-nucleus signaling pathway activates lysosomes for C. elegans larval development. Dev. Cell 52, 21–37.e5. doi:10.1016/j.devcel.2019.10.020
Nakai, N., Collier, G. R., Sato, Y., Oshida, Y., Fujitsuka, N., and Shimomura, Y. (1997). Activities of liver pyruvate dehydrogenase complex and 3-hydroxyacyl-CoA dehydrogenase in sand rat (Psammomys obesus). Life Sci. 60, 51–55. doi:10.1016/s0024-3205(96)00588-7
Overton, K., Hoffmann, A. A., Reynolds, O. L., and Umina, P. A. (2021). Toxicity of Insecticides and Miticides to Natural Enemies in Australian Grains: A Review, 12.Insects
Paris, L., Peghaire, E., Moné, A., Diogon, M., Debroas, D., Delbac, F., et al. (2020). Honeybee gut microbiota dysbiosis in pesticide/parasite co-exposures is mainly induced by Nosema ceranae. J. Invertebr. pathology 172, 107348. doi:10.1016/j.jip.2020.107348
Parkhitko, A. A., Binari, R., Zhang, N., Asara, J. M., Demontis, F., and Perrimon, N. (2016). Tissue-specific down-regulation of S-adenosyl-homocysteine via suppression of dAhcyL1/dAhcyL2 extends health span and life span in Drosophila. Genes Dev. 30, 1409–1422. doi:10.1101/gad.282277.116
Parkhitko, A. A., Ramesh, D., Wang, L., Leshchiner, D., Filine, E., Binari, R., et al. (2020). Downregulation of the tyrosine degradation pathway extends Drosophila lifespan. eLife 9, e58053. doi:10.7554/elife.58053
Pisa, L. W., Amaral-Rogers, V., Belzunces, L. P., Bonmatin, J. M., Downs, C. A., Goulson, D., et al. (2015). Effects of neonicotinoids and fipronil on non-target invertebrates. Environ. Sci. Pollut. Res. 22, 68–102. doi:10.1007/s11356-014-3471-x
Roat, T. C., Carvalho, S. M., Palma, M. S., and Malaspina, O. (2017). Biochemical response of the Africanized honeybee exposed to fipronil. Environ. Toxicol. Chem. 36, 1652–1660. doi:10.1002/etc.3699
Rochford, G., Molphy, Z., Browne, N., Surlis, C., Devereux, M., McCann, M., et al. (2018). In-vivo evaluation of the response of Galleria mellonella larvae to novel copper(II) phenanthroline-phenazine complexes. J. Inorg. Biochem. 186, 135–146. doi:10.1016/j.jinorgbio.2018.05.020
Sanchez, C. G., Teixeira, F. K., Czech, B., Preall, J. B., Zamparini, A. L., Seifert, J. R., et al. (2016). Regulation of ribosome biogenesis and protein synthesis controls germline stem cell differentiation. Cell stem Cell 18, 276–290. doi:10.1016/j.stem.2015.11.004
Sánchez-Bayo, F. (2021). Indirect effect of pesticides on insects and other arthropods. Toxics 9, 177. doi:10.3390/toxics9080177
Sánchez-Bayo, F., and Wyckhuys, K. A. G. (2019). Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 232, 8–27. doi:10.1016/j.biocon.2019.01.020
Seibold, S., Gossner, M. M., Simons, N. K., Blüthgen, N., Müller, J., Ambarlı, D., et al. (2019). Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 574, 671–674. doi:10.1038/s41586-019-1684-3
Shen, M., Zhang, Y., Zhu, Y., Song, B., Zeng, G., Hu, D., et al. (2019). Recent advances in toxicological research of nanoplastics in the environment: A review. Environ. Pollut. (Barking, Essex 1987) 252, 511–521. doi:10.1016/j.envpol.2019.05.102
Shi, L., Chen, L., Wan, Y., Zeng, H., and Xia, W. (2020). Spatial variation of fipronil and its derivatives in tap water and ground water from China and the fate of them during drinking water treatment in Wuhan, central China. Chemosphere 251, 126385. doi:10.1016/j.chemosphere.2020.126385
Shi, S., Zang, L., Liu, T. X., Ruan, C., and Sun, G. (2009). Host-feeding behaviors of parasitoids on hosts and implications for biological control. Acta Entomol. Sin. 52, 424–433.
Stark, J. D., Vargas, R., and Banks, J. E. (2007). Incorporating ecologically relevant measures of pesticide effect for estimating the compatibility of pesticides and biocontrol agents. J. Econ. Entomol. 100, 1027–1032. doi:10.1093/jee/100.4.1027
Tamilselvan, R., Kennedy, J. S., and Suganthi, A. (2021). Sublethal and transgenerational effects of spinetoram on the biological traits of Plutella xylostella (L.) (Lepidoptera: Plutellidae). Ecotoxicol. Lond. Engl. 30, 667–677. doi:10.1007/s10646-021-02385-7
Veillard, F., Troxler, L., and Reichhart, J. M. (2016). Drosophila melanogaster clip-domain serine proteases: Structure, function and regulation. Biochimie 122, 255–269. doi:10.1016/j.biochi.2015.10.007
Wagner, D. L. (2020). Insect declines in the anthropocene. Annu. Rev. Entomol. 65, 457–480. doi:10.1146/annurev-ento-011019-025151
Wang, W., Zhang, L., Chen, H., Wang, J., Zhang, J., and Liu, Y. (2013). Effects of temperature and light on diapause induction in lady beetle Coccinella septempunctata in Beijing, China. Chin. J. Biol. Control 29, 24–30.
Wei, Y., Li, H., Zhang, J., Xiong, J., Yi, X., and You, J. (2017). Legacy and current-use insecticides in agricultural sediments from south China: Impact of application pattern on occurrence and risk. J. Agric. Food Chem. 65, 4247–4254. doi:10.1021/acs.jafc.7b00620
Wu, C. H., Lu, C. W., Hsu, T. H., Wu, W. J., and Wang, S. E. (2021). Neurotoxicity of fipronil affects sensory and motor systems in zebrafish. Pestic. Biochem. Physiol. 177, 104896. doi:10.1016/j.pestbp.2021.104896
Wu, J., Lu, J., Lu, H., Lin, Y., and Wilson, P. C. (2015). Occurrence and ecological risks from fipronil in aquatic environments located within residential landscapes. Sci. Total Environ. 518-519, 139–147. doi:10.1016/j.scitotenv.2014.12.103
Wyckhuys, K. A., Strange-George, J. E., Kulhanek, C. A., Wäckers, F. L., and Heimpel, G. E. (2008). Sugar feeding by the aphid parasitoid binodoxys communis: How does honeydew compare with other sugar sources? J. insect physiology 54, 481–491. doi:10.1016/j.jinsphys.2007.11.007
Xu, L., Zhao, J., Xu, D., Xu, G., Gu, Z., Xiao, Z., et al. (2021). Application of transcriptomic analysis to unveil the toxicity mechanisms of fall armyworm response after exposure to sublethal chlorantraniliprole. Ecotoxicol. Environ. Saf. 230, 113145. doi:10.1016/j.ecoenv.2021.113145
Xue, S., and Barna, M. (2012). Specialized ribosomes: A new frontier in gene regulation and organismal biology. Nat. Rev. Mol. Cell Biol. 13, 355–369. doi:10.1038/nrm3359
Yang, F., Wu, Y. K., Xu, L., Wang, Q., Yao, Z. W., Žikić, V., et al. (2017). Species composition and richness of aphid parasitoid wasps in cotton fields in northern China. Sci. Rep. 7, 9799. doi:10.1038/s41598-017-10345-7
Yang, X. B., Ying, G. G., Peng, P. A., Wang, L., Zhao, J. L., Zhang, L. J., et al. (2010). Influence of biochars on plant uptake and dissipation of two pesticides in an agricultural soil. J. Agric. Food Chem. 58, 7915–7921. doi:10.1021/jf1011352
Yi, Y., Li, J., Zong, Z., Liu, X., Song, H., Wang, H., et al. (2021). Cloning, expression, and characteristic analysis of the novel β-galactosidase from silkworm, Bombyx mori. Genesis (New York, NY) 59, e23446, doi:10.1002/dvg.23446
Zhang, R., Cao, Y. Y., Du, J., Thakur, K., Tang, S. M., Hu, F., et al. (2021). Transcriptome analysis reveals the gene expression changes in the silkworm (Bombyx mori) in response to hydrogen sulfide exposure. Insects 12, 1110. doi:10.3390/insects12121110
Keywords: fipronil, Binodoxys communis, larvae, sublethal, intergenerational effects, transcriptome
Citation: Du L, Zhao L, Zhu X, Wang L, Zhang K, Li D, Ji J, Niu L, Luo J, Cui J and Gao X (2022) Sublethal and intergenerational effects of fipronil on Binodoxys communis larvae based on transcriptome sequencing. Front. Environ. Sci. 10:1080601. doi: 10.3389/fenvs.2022.1080601
Received: 26 October 2022; Accepted: 29 November 2022;
Published: 09 December 2022.
Edited by:
Zhiyuan Meng, Yangzhou University, ChinaCopyright © 2022 Du, Zhao, Zhu, Wang, Zhang, Li, Ji, Niu, Luo, Cui and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junyu Luo, luojunyu1818@126.com; Jinjie Cui, aycuijinjie@163.com; Xueke Gao, 15036138389@163.com
†These authors have contributed equally to this work and share first authorship
 Lingen Du1,2,3†
Lingen Du1,2,3†  Xiangzhen Zhu
Xiangzhen Zhu Jichao Ji
Jichao Ji Xueke Gao
Xueke Gao