Fertilization restructures nematode assemblages by modifying soil pH in croplands of Northeast China
- 1Jilin Province Engineering Laboratory of Plant Genetic Improvement, College of Plant Science, Jilin University, Changchun, China
- 2College of Resources and Environment, Jilin Agricultural University, Changchun, China
- 3Key Laboratory of Plant Nutrition and Agro-environment in Northeast Region, MOA, Institute of Agricultural Resources and Environment Research, Jilin Academy of Agricultural Sciences, Changchun, China
Fertilization is an effective measure to ensure crop yield and food security in modern intensive agriculture. However, the long-term application of mineral fertilizers may lead to soil acidification, consequently affecting soil organisms. Few studies have focused on the effects of mineral fertilizer application on nematode assemblages in various soil types. Soil chemical properties and nematode assemblages were investigated in seven fields at the China Cultivated Land Quality Monitoring Site in Jilin Province, China, to determine the relationship between soil properties (especially soil pH) and soil nematode assemblages and evaluate the effects of mineral fertilizer application on different soil types (e.g., luvisols, fluvisols, gleysols, phaeozems, and chernozems). In all the fields, the soil pH was 0.06–1.00 units lower in the fertilized plots than in the control plots. A total of 8,230 nematode individuals representing 21 nematode genera were identified, with Paraphelenchus being the most abundant genus (relative abundance of 27.93%). Plant parasites were the leading trophic group, accounting for over 50% of the nematode assemblage. For phaeozems, the abundance of total nematodes and the relative abundance of plant parasites were lower in the fertilized plots than in the control plots. Principal component analysis (PCA) was used to distinguish the structures of the nematode assemblages after fertilization in fluvisols and phaeozems but not in the other types of soil. Soil pH was significantly correlated with the nematode assemblage in phaeozems compared to the different soil types. These results demonstrate that the long-term application of mineral fertilizers can lead to soil acidification and negatively affect cropland soil nematode assemblages.
Introduction
Fertilization is crucial in crop yield and food security in modern intensive agriculture (Mei et al., 2021; Hu et al., 2022). However, the long-term application of large amounts mineral of fertilizers has led to ecological and environmental problems in agroecosystems (Guo et al., 2010; Chen et al., 2021; Mei et al., 2021). Soil organic matter and soil carbonates are affected by the long-term application of mineral fertilizers, especially nitrogen fertilizers (Zamanian et al., 2018; Adnan et al., 2021; Zhang et al., 2021a). Meanwhile, the long-term application of mineral fertilizers significantly decreases soil pH in croplands, eventually leading to soil acidification (Guo et al., 2010; Zhou et al., 2015; Mei et al., 2021; Hu et al., 2022). Soil pH In terrestrial ecosystems modulates the content and availability of essential nutrients and toxic substances (Liu et al., 2014; Zhang et al., 2020). Consequently, soil acidification affects the soil quality, fertility, and health of plants and soil organisms (Wang et al., 2018; Kimmel et al., 2020).
Soil acidification, in which soil pH decreases, is a soil degradation type (Guo et al., 2010; Wu et al., 2022). The long-term application of mineral fertilizers, especially nitrogen fertilizers to croplands, can accelerate soil acidification (Raza et al., 2020). After adding nitrogen fertilizer to the soil, the nitrogen-containing substances are transformed into ammonia and nitrate by mineralization and nitrification (Li et al., 2018). During nitrate with base ions such as K+, Na+, Ca2+, and Mg2+ leaching by the moisture, H+ and Al3+ may replace the base ions to adsorb on the soil and release protons into the soil environment, eventually leading to soil acidification (Devries and Breeuwsma, 1987). The soil with a pH > 6.5 contains carbonate, which can neutralize the protons and stabilize the soil pH (Raza et al., 2020). Consequently, soil acidification depends on the characteristics of soil types, agricultural activities, climate, geography, and etc.
Nematodes are the most abundant animals in the soil, play a critical role in terrestrial ecosystems, and are also influenced by soil acidification (Bardgett and van der Patten, 2014; van den Hoogen et al., 2019). Nematodes perform several essential ecological functions related to nutrient cycling and energy transformation. For example, nematodes promote carbon and nitrogen mineralization, stimulate microbial activity, and affect the structure of the food web (Ferris et al., 2001; Zhang et al., 2021b; Wang et al., 2021; Teshita et al., 2023). Moreover, soil nematodes are bioindicators of environmental conditions due to their sensitivity to environmental disturbances including agricultural activities (e.g., tillage, irrigation, and the application of fertilizers and pesticides) (Yeates et al., 1993; Ferris and Matute., 2003; Liang et al., 2009; Hu et al., 2017; Čerevková et al., 2017; Chen et al., 2021; Escalante et al., 2021; Puissant et al., 2021).
Jilin Province, located in the middle of the Northeast China Plain, is a significant cereal production area in China. The main types of soil in Jilin Province include luvisols, fluvisols, gleysols, phaeozems, chernozems (Zhang et al., 2020). These soils are a critical agricultural resource in the northeast region of China and play a crucial role in food security (Gu et al., 2020; Wang et al., 2022; Xia and Zhang, 2022). Many reports have indicated that soil properties have deteriorated in Jilin Province (Wang et al., 2017; Zhang et al., 2020). However, few studies have focused on the effects of mineral fertilizer application on nematode assemblages in various soil types. This study investigated the soil chemical properties and nematode assemblages in five soil types from seven fields at the China Cultivated Land Quality Monitoring Site established by the Ministry of Agriculture and Rural Affairs in Jilin Province, China. The objectives were to determine the relationship between soil nematode assemblages and soil properties, especially soil pH, and to evaluate the effects of mineral fertilizer application on soil properties and soil nematode assemblages in different soil types.
Materials and methods
Study sites
This study was conducted at China Cultivated Land Quality Monitoring Sites in Jilin Province. The China Cultivated Land Quality Monitoring Site was established by the Ministry of Agriculture and Rural Affairs of China, aiming to monitor soil fertility and the environment, protect soil sources, and ensure food production. At each site, a field was selected following respective purposes and requirements, such as soil type. A square plot (10 × 10 m) was separated as the control plot and was no longer fertilized once the plot had been separated in each field. The rest of the field was still fertilized. Except for fertilization, the agricultural activities in control and fertilized plot (e.g., cultivation period and method, crop types, and so on) had no difference.
Seven sites were investigated in this study. These sites were in Tumen, Yongji, Shulan, Yushu, Jiutai, Yitong, and Nong’an in Jilin Province, China (Figure 1). Jilin Province (40°52′-46°18′N, 121°38′-131°19′E) has a temperate continental monsoon climate with a mean annual temperature of 4.2°C and mean annual precipitation of 560 mm (Zhang et al., 2020). Soil types of the fields in the seven sites were classified into luvisols in Tumen, fluvisols in Yongji, gleysols in Shulan, phaeozems in Yushu, and Jiutai, gleysols in Yitong, and chernozems in Nong’an, according to the World Reference Base for Soil Resources (WRB) (FAO, 1998). The fields at seven sites were all fertilized with complex fertilizers before planting in April every year. Table 1 presents establishment dates (the year when the control plot separated and was no longer fertilized), soil types, and fertilization rates in the seven sites. The primary crop in all the fields of the seven sites is monoculture maize (Zea mays L.) without pesticides and herbicides at the growing stage. In 2018, maize was harvested in October.
FIGURE 1. Map of locations of seven fields at China Cultivated Land Quality Monitoring Site in Jilin Province: (A) Tumen, (B) Yongji, (C) Shulan, (D) Yushu, (E) Jiutai, (F) Yitong, and (G) Nong’an. The base map is from the standard map service system of the Map Technology Review Center of the Ministry of Natural Resources of the Ministry of Natural Resources of the People’s Republic of China [review map No. GS (2019) 3266]. http://bzdt.ch.mnr.gov.cn.
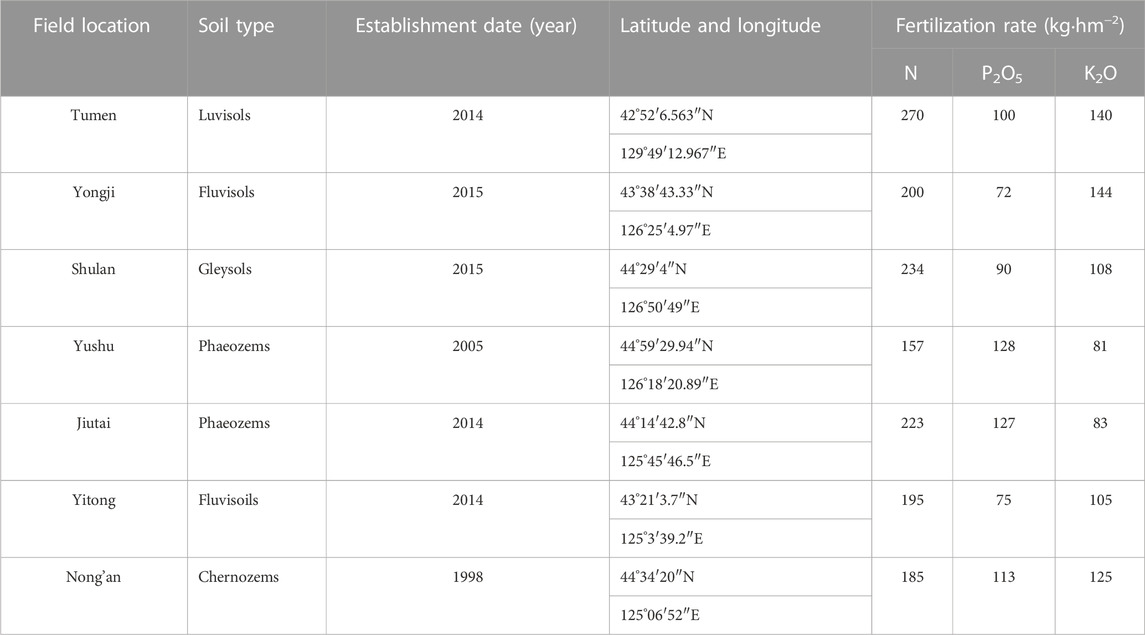
TABLE 1. Field location, soil type, establishment date, latitude and longitude, and fertilization rate of nitrogen (N), phosphorus (P2O5) and potassium (K2O) in fertilized plots from seven fields at China Cultivated Land Quality Monitoring Site site.
Soil sampling
A square plot (10 × 10 m) was selected at each site as the fertilized plot from the fertilized field, which was 10 m away from the control plot. The fertilized plots and control plots were then divided into three identical rectangle plots. Five cores of soil samples were collected and mixed at depths of 0–20 cm on a “W” path from each rectangle plot, both in fertilized and control plots, using a manual auger,. Then, three soil samples were collected in each plot. The mixed soil samples were placed in airproof plastic bags and stored in a cooler box. Then, the soils were immediately stored in a room at 4°C once back to the laboratory. Each site was sampled three times in 2018: April 23 to 27 (before fertilization and planting during the spring), July 30 to August 3 (at the growing stage during the summer), and October 23 to 31 (after harvesting during the autumn). The manual auger was 5 cm in diameter. Each bag of soil samples was divided into two proportions: one for extracting nematodes and one for measuring soil properties.
Nematode isolation and identification
Individual nematodes were extracted from 100 g of fresh soil samples via sugar flotation and centrifugation (Jenkins, 1964; Freckman and Virginia, 1993). After extraction, the nematodes were counted and identified to the genus level under a stereomicroscope (×200). Then, 100 nematodes were randomly selected from each sample for morphological identification, and all the samples with less than 100 nematodes were identified. The nematodes were classified into five trophic groups: plant parasite, bacterivore, fungivore, and omnivore-predator (Yeates et al., 1993).
Soil chemical property analysis
Soil moisture was determined gravimetrically by drying samples at 105°C for 12 h to a constant weight (Zhang et al., 2015). A glass electrode determined soil pH in a 1:2.5 (weight: volume) soil: water solution. A conductivity electrode determined soil conductivity in a 1:5 (weight: volume) soil: water solution. The contents of soil NO3− and NH4+ were determined by extraction with a 1 mol L−1 KCl solution using a Continuous Flow Analyzer (AA3 Continuous-Flow Analysis, Tianjin) (You et al., 2015). The soil available phosphorus content was determined by extraction with a 0.5 mol L−1 NaHCO3 solution, using a sodium hydrogen carbonate solution-Mo-Sb anti-spectrophotometric method (Yan et al., 2015).
Data analysis
Total nematode abundance was calculated as the number of individuals per 100 g of dry soil and were transformed (lg (x+1)) when necessary to meet statistical assumptions. Nematode relative abundance refers to the total number of a trophic group divided by the total number of nematodes in one season. Nematode diversity was assessed using the Shannon−Wiener diversity index (H’), Pielou evenness index (J), and Simpson dominance index (D), as follows (Zhao et al., 2014):
Shannon−Wiener diversity index:
Pielou evenness index:
Simpson dominance index:
Where, “Pi” is the proportion of the individuals of the “ith” group in the community, “S” is the total number of nematode genera in the community.
A one-way analysis of variance (ANOVA) and t-test were used to test the effects of fertilization on nematode abundances, diversity index, and soil chemical properties. Pearson’s correlation was used to test the relationship between nematode abundances and soil pH. Principal component analysis (PCA) was performed using the CANOCO 5.0 to visualize the variations in nematode assemblage structure among the seven fields. The relationships between environmental variables and nematode assemblages were examined by redundancy analysis (RDA) using the same software. Environmental variables included soil pH, electrical conductivity, moisture, NO3− content, NH4+ content, and available phosphorus content. The statistical analysis of nematode abundances, soil properties, diversity index, and Pearson’s correlation was performed using SPSS 16.0. All the figures were generated using SigmaPlot 12.5 software. The difference at p < 0.05 level was considered statistically significant.
Results
Soil chemical properties
Among the seven fields, fertilizer application had the most significant effect on soil pH in Yushu, where the soil pH was 1.00 units lower in the fertilized plots than in the control plots (p < 0.001) (Figure 2). Compared to the control plots, the soil pH in the fertilized plots was 0.49 units lower in Shulan (p < 0.01), 0.47 units lower in Yitong (p < 0.01), 0.39 units lower in Tumen (p < 0.01), 0.37 units lower in Yongji (p < 0.01), and 0.37 units lower in Jiutai (p < 0.05) (Figure 2), respectively. For the plots in Nong’an, fertilizer application did not significantly affect on soil pH. The soil pH in the fertilized plots was 0.06 units lower than in the control plots in Nong’an (Figure 2). Soil moisture varied seasonally, with the highest moisture observed after harvesting and the lowest measured at the growing stage. In Nong’an, soil moisture was lowest compared to the other sites. The contents of NH4+ and available phosphorus with electrical conductivity were higher in the fertilized plots than in the control plots. Remarkably, the content of NO3− was significantly higher in the fertilized plot than that in the control plot at the growing stage in all the sites (p < 0.05) (Supplementary Table S1).
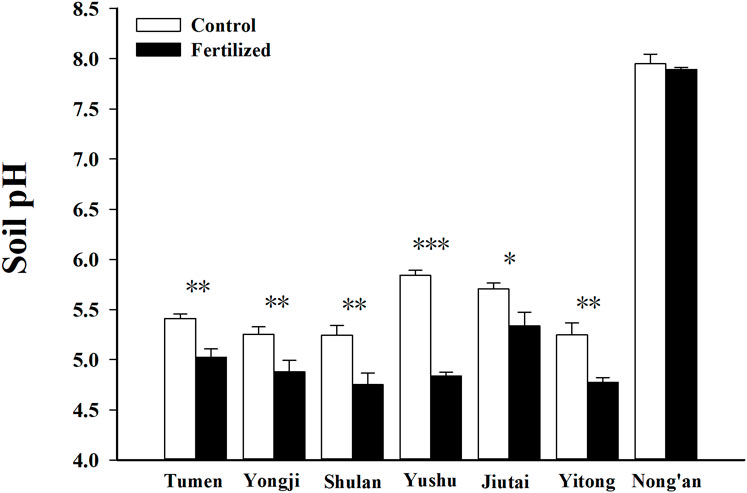
FIGURE 2. Soil pH in fertilized plots and in control plots from seven fields at China Cultivated Land Quality Monitoring Site. “*“, “**“, and “***” indicate differences of soil pH between in fertilized plots and in control plots could be at a significant level of 0.05, 0.01, and 0.001, respectively.
Nematode abundance, composition and diversity
A total of 8,230 individual nematodes representing 21 nematode genera were found in all samples, and Paraphelenchus was the most abundant genus (relative abundance of 27.93%) (Supplementary Table S2). In Yushu, the average abundance of total soil nematodes in the fertilized plots (60.2 individuals per 100 g dry soil) was 50.1% that in the control plots (120.1 individuals per 100 g dry soil). In Jiutai, the average abundance of 89.1 individuals per 100 g dry soil in the fertilized plots was 65.5% that in the control plots (136.1 individuals per 100 g dry soil). In Yitong, the average abundance of 125.1 individuals per 100 g dry soil in the fertilized plots was 130.2% that in the control plots (96.0 individuals per 100 g dry soil). Thus, fertilization significantly decreased nematode abundance in Yushu (p < 0.001) and Jiutai (p < 0.001) but increased nematode abundance considerably in Yitong (p < 0.05). However, in Tumen, Yongji, Shulan, and Nong’an, nematode abundances in fertilized plots had no significant differences from those in control plots (Figure 3).
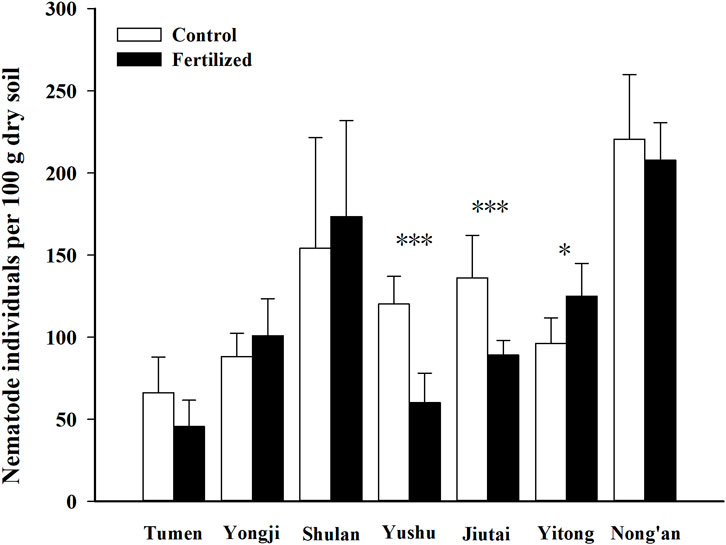
FIGURE 3. Abundances (average individuals/100 per dry soil) of total nematode in fertilized plots and in control plots from seven fields at China Cultivated Land Quality Monitoring Site. “*” and “***” indicate differences of nematode abundances between in fertilized plots and in control plots could be at a significant level of 0.05 and 0.001, respectively.
Plant parasites were the leading trophic group. The relative abundance of plant parasites exceeded 50% in most plots, except in the fertilized plots before fertilization and planting (15.22%) in Yongji, in the control plots (48.88%) at the growing stage in Yongji, both in the fertilized (not found) and in the control plots (5.31%) before fertilization and planting in Shulan, in the fertilized plots at the growing stage (48.23%) in Yitong, and in the control plots after harvesting (26.42%) in Yitong. The relative abundance of omnivores-predators was less than 10% in most plots, except for in the control plots in Tumen at growing stage (14.95%) and in the control plots in Jiutai before fertilization and planting (10.29%) (Table 2).
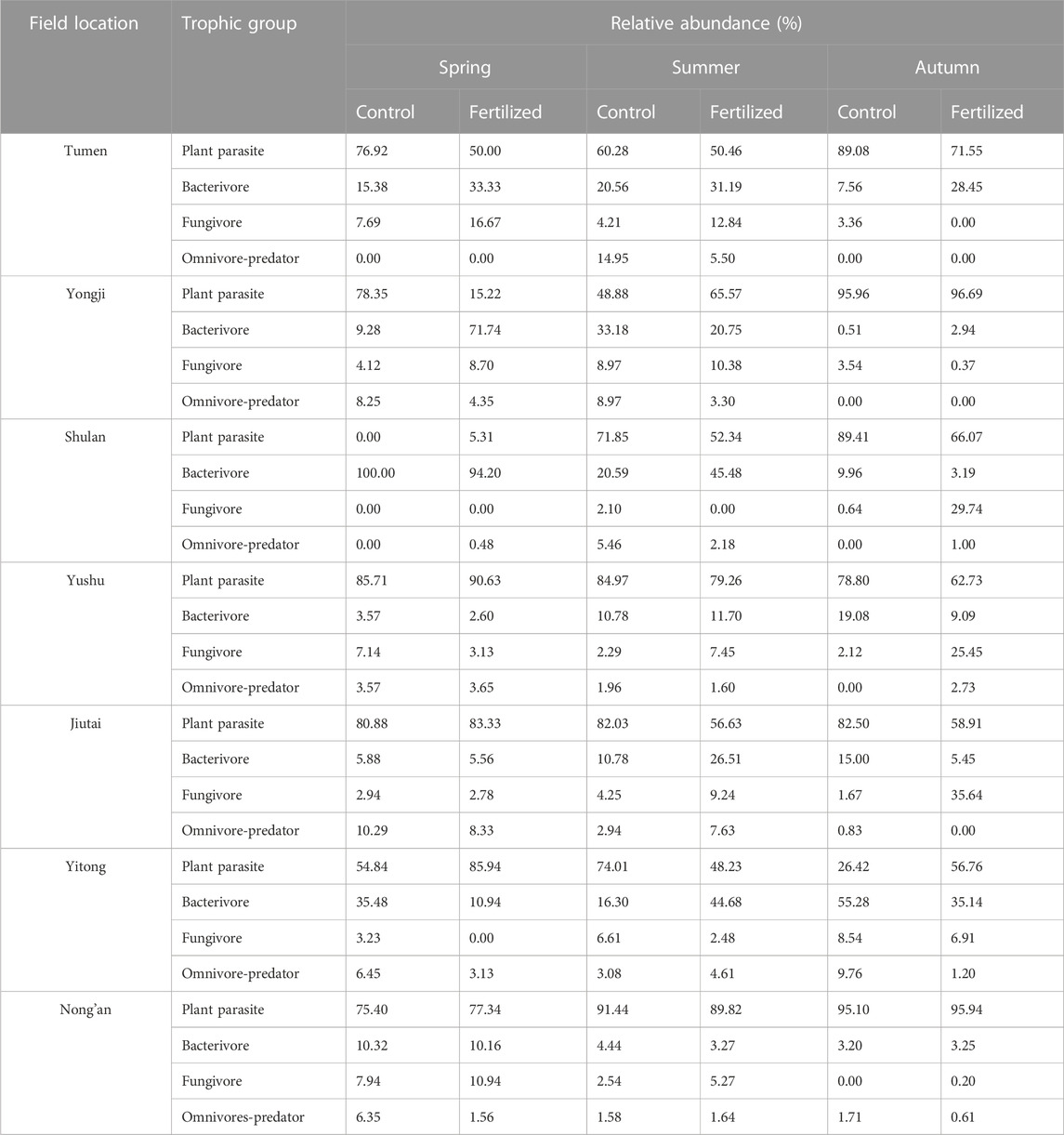
TABLE 2. Relative abundances (%) of plant parasite, bacterivore, fungivore, and omnivore-predator in fertilized plots and in control plots from seven fields at China Cultivated Land Quality Monitoring Site during the spring, Summer, and Autumn, respectively. Spring represents the samples collected before fertilization and planting, summer represents the samples collected at growing stage, and Autumn represents the samples collected after harvesting.
Fertilizer application significantly decreased the Simpson dominance index D in Tumen and Shulan (p < 0.05) but significantly increased D in Yongji (p < 0.05) and Yitong (p < 0.01) (Table 3). Fertilization significantly decreased the Shannon−Wiener diversity index H’ in Yitong (p < 0.05) (Table 3). Fertilizer application significantly decreased the Pielou evenness index J in Yongji (p < 0.05) and Yitong (p < 0.01), whereas it significantly increased J in Tumen (p < 0.01) (Table 3). PCA showed that the composition of the nematode assemblage in chernozems could be distinguished from that in the other soil types; the compositions of nematode assemblages in the fertilized plots could be distinguished from those in the control plots in fluvisols and phaeozems (Figure 4).
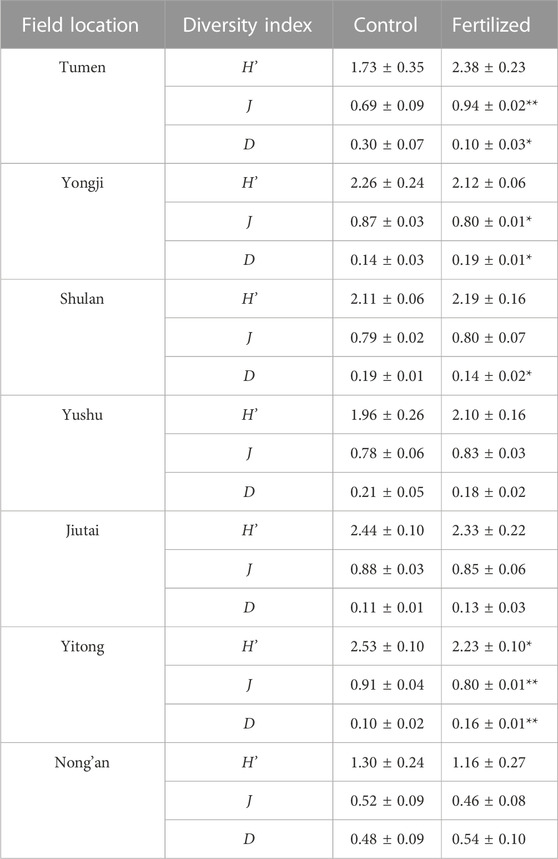
TABLE 3. Shannon-Wiener diversity index (H’), Pielou evenness index (J), and Simpson dominance index (D) in fertilized plots and in control plots from seven fields at China Cultivated Land Quality Monitoring Site. “*” and “**” indicate the differences of diversity index between in fertilized plots and in control plots could be at a significant level of 0.05 and 0.01, respectively.
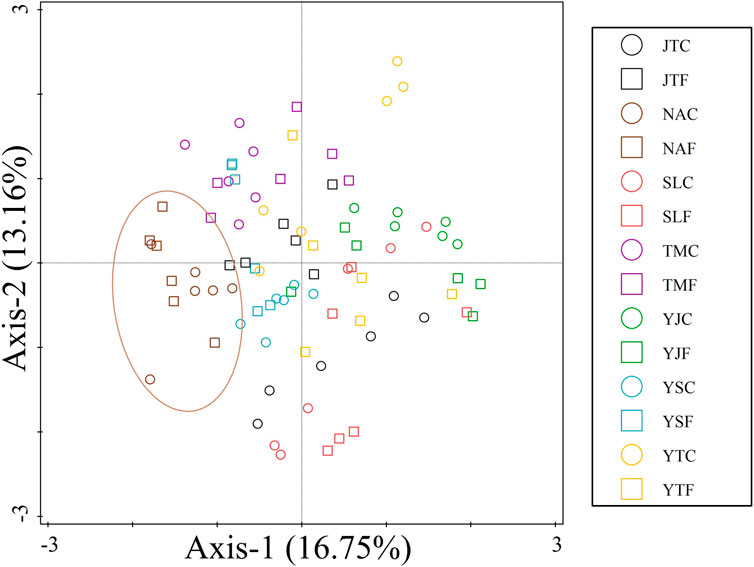
FIGURE 4. Principle component analysis (PCA) of nematode assemblage compositions of seven fields at China Cultivated Land Quality Monitoring Site. Circles represent samples in control plots. Squares represent samples in fertilized plots. Letters in the legend: (1) The first and second letters are the abbreviation of monitoring field locations; JT represents Jiutai, NA represents Nong’an, SL represents Shulan, TM represents Tumen, YJ represents Yongji, YS represents Yushu, and YT represents Yitong, respectively. (2) The last letter represents control plot (C) or fertilized plot (F).
Effects of soil pH on nematodes
In fluvisols, the plant parasite abundance negatively correlated with soil pH (p < 0.05). Moreover, in gleysols, the total nematode abundance negatively correlated with soil pH (p < 0.05). In contrast, the total nematode abundance, plant parasite abundance, and bacterivore abundance had a significantly positive correlation with soil pH (p < 0.01), and the fungivore abundance had a significantly negative correlation with soil pH (p < 0.05) in phaeozems (Table 4).
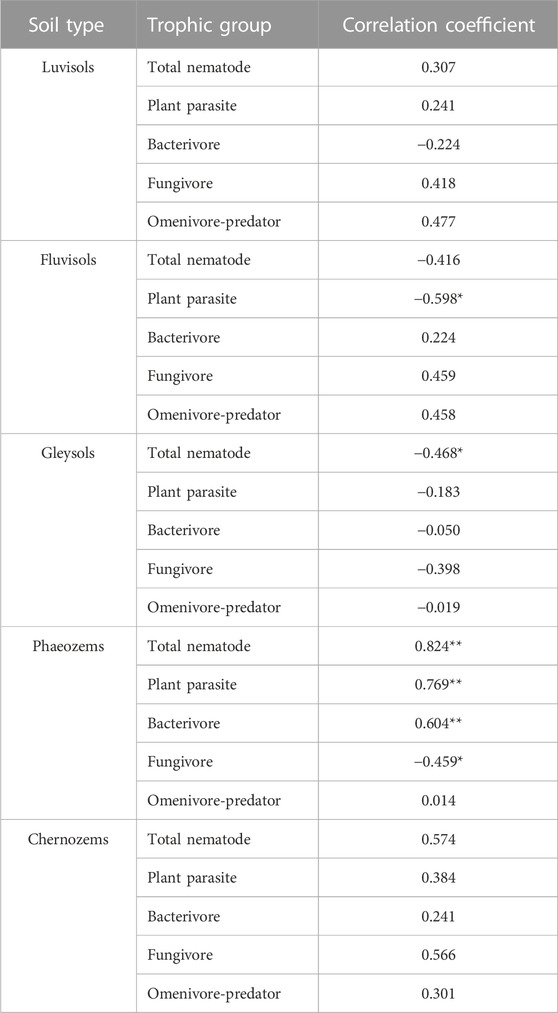
TABLE 4. Pearson’s correlation coefficients between nametode abundances and soil pH in each soil type. Nematode abundances include total nematode abundances, plant parasite abundances, bacterivore abundances, fungivore abundances, and omnivore-predator abundances. Soil types include luvisols, fluvisols, gleysols, phaeozems, and chernozems. “*” and “**” indicate differences between the nametode abundance and soil pH could be at a significant level of 0.05 and 0.01, respectively.
Discussion
Soil acidification has been one of the soil degradation issues affecting agricultural activity in Chinese croplands (Guo et al., 2010; Guo et al., 2018; Li et al., 2019; Wu et al., 2022). Guo et al. (2010) reported that soil pH decreased by 0.13–0.80 units in Chinese croplands from 1980 to 2000. Soil acidification was more severe in cropland used for cash crops (decreased in pH of 0.30–0.80 units) than in cropland where cereal crops were grown (decreased in pH of 0.13–0.76 units) due to the great amount of fertilizer used. Guo et al. (2018) reported that soil pH in the paddy fields decreased by an average of 0.6 units from 1980 to 2010 by excessive fertilizer application in Jiangsu according to the data from soil survey information of Jiangxi Province and Jiangxi soil testing and fertilizer recommendation study. Li et al. (2019) found that in the Sichuan Basin from 1981 to 2012, soil pH decreased significantly by 0.32 units in paddy fields and 0.50 units in orchards but did not change significantly in woodlands and grasslands. In a study in Henan Province, Wu et al. (2022) reported that the topsoil pH of cropland decreased by an average of 0.36 units from 2008 to 2018, with 94% of the cropland showing soil acidification.
The soil pH decreased significantly after fertilization in most soil types, except chernozems, in this study (Figure 2). The most significant decrease in soil pH (1.00 units) was observed in the fertilized phaeozems of Yushu after 14 years of fertilization (Figure 2). In contrast, the soil pH of the chernozems in Nong’an decreased by only 0.06 units after 20 years of fertilization. This non-significant change in pH may be attributed to the presence of carbonates in chernozems. Compared to the phaeozems in Yushu, the soil pH of gleysols in Shulan and Yitong, luvisols in Tumen, fluvisols in Yongji, and phaeozems in Jiutai decreased by less than 0.5 units after just three or 4 years of fertilization (Figure 2). Thus, the soil type affected the change in soil pH. Wang et al. (2017) reported that the changes in topsoil pH in Jilin Province from 2005 to 2013 differed by soil type: the soil pH decreased by an average of 0.3, 0.4, 0.5, and 1.4 units in chernozems, luvisols, phaeozems, and gleysols, respectively, in the croplands of Jilin Province under the soil testing and recommended fertilization program.
Different types of soil may be differently affected by environmental factors. The formation and development of gleysols and fluvisols are related to belowground water and rivers, respectively. The formation and development of phaeozems and luvisols in temperate regions are related to precipitation. Meanwhile, because more precipitation occurs in forest areas than grassland areas, the leaching of base ions differs between phaeozems in grassland areas and luvisols in forest areas. Chernozems appear in temperate semi-arid grassland regions that receive slight precipitation and contain large amounts of carbonate. Therefore, there may be different changes in soil pH after fertilization among different types of soil.
Fertilization can have various effects on nematodes. For example, the content of soil nutrients, particularly nitrogen and phosphorus, is increased by applying of mineral fertilizers. This, in turn, can enhance the feeding of bacterivores and fungivores on soil microorganisms (Zhou et al., 2015; Song et al., 2016). Fertilization also increases crop biomass (Zhou et al., 2015), making more nutrients available for plant parasites in agroecosystems (Liang et al., 2009; Ferris et al., 2012; Song et al., 2015; Teshita et al., 2023). The abundance of omnivores and predators changes along with changes in the other nematode trophic groups (Chen et al., 2015). Thus, fertilization had a positive effect on the nematode assemblage.
Nevertheless, the long-term application of mineral fertilizers deteriorates the soil environment and increases the content of poisonous substances, which inhibit nematode activities (Cole et al., 2008; Zhao et al., 2014). Fertilization improves plant defenses and health to increase plant parasite resistance (Zhou et al., 2021). The soil bacteria and fungi on which nematodes feed are negatively affected by fertilization, which affects bacterivores and fungivores (Bongers et al., 1997; Liang et al., 2009; Zhou et al., 2015; 2016; Grabau and Chen, 2016; Hu et al., 2017; Zhang et al., 2021b; Mei et al., 2021; Teshita et al., 2023). Thus, fertilization has different effects on different nematode trophic groups.
In the current study, plant parasites were the leading trophic group, accounting for over 50% of the nematode assemblage in most plots (Table 2). This may be due to the decrease in the abundance of bacterivores (Zhou et al., 2015; 2016) and the increase in the abundance of plant parasites (Bonger et al., 1997; Ferris et al., 2012) in soils under long-term fertilization. In Shulan, the relative abundances of bacterivores in the fertilized plots and control plots were more than 90% before fertilization and planting in spring. This result may be because the soil properties of gleysols were suitable for bacterivores during this period. Meanwhile, omnivores and predators are sensitive to soil disturbances and environmental changes caused by agricultural activities, such as fertilization and tillage (Liang et al., 2002; Song et al., 2015; 2016; Puissant et al., 2021). In this study, the relative abundance of omnivores and predators was lower than 10% in most plots (Table 2).
Soil properties differ among the different soil types (Supplementary Table S1; Figure 2), resulting in different compositions and structures of nematode assemblages. Soil pH is a crucial environmental factor affecting soil fertility, nutrient availability, and organism activity. Thus, healthy soil usually needs a stable and suitable pH (Zhou et al., 2015; Hu et al., 2017; Zhang et al., 2020). Soil pH may be the main driving force moderating nematode abundance, along with the composition and structure of soil nematode assemblage (Räty and Huhta, 2003; van den Hoogen et al., 2019; Hu et al., 2022). The nematode assemblage in this study was significantly affected by soil pH in phaeozems but not in other soil types. Soil pH had significantly positive effects on plant parasites and bacterivores (p < 0.01) but had significant adverse effects on fungivores (p < 0.05) (Table 4). Meanwhile, RDA showed that the abundances of Coslenchus, Filenchus, Helicotylenchus, Heterodera, Psilenchus, Rotylenchus (classified into plant parasites), Acrobeles, Acrobeloides, and Plectus (classified into bacterivores) were positively correlated with soil pH. In contrast, the abundance of Aphelenchoides (classified into fungivores) showed a significant negative correlation with soil pH (Supplementary Figure S1). Thus, fertilization may significantly decrease the abundance of total nematodes in phaeozems (Figure 3), ultimately leading to a distinguishable nematode assemblage after fertilization in Yushu and Jiutai (Figure 4). The difference in soil pH between chernozems (with a pH > 7.0) and the other types of soil (with a pH < 7.0) may cause the nematode assemblage in chernozems to be distinguished from that in the different soil types (Figure 4). Other studies also found that soil pH affects nematode abundance (Korthals et al., 1996; Räty and Huhta, 2003). Korthals et al. (1996) reported that decreasing the soil pH decreased bacterivore abundance and increased fungivore abundance. Räty and Huhta (2003) also found that total nematode abundance was approximately two to three times greater at pH 6.1 than at pH 4.8.
Due to the addition of nitrogen, fertilization increased the contents of NH4+ and NO3− to different degrees in the different fields (Supplementary Table S1), potentially affecting on nematode assemblages. In phaeozems, the abundance of Coslenchus, Filenchus, Helicotylenchus, Heterodera, Psilenchus, Rotylenchus (classified into plant parasites), Acrobeles, Acrobeloides, and Plectus (classified into bacterivores) showed negative correlations with NO3− content (Supplementary Figure S1). This fact may affect the plant parasite and bacterivores in fertilized plots (Table 2). Similarly, in grasslands enriched with nitrogen for 12 years, Chen et al. (2015) found that nitrogen addition suppressed bacterivores, fungivores, and omnivores-predators due to soil acidification. In contrast, Song et al. (2015; 2016) reported that nitrogen addition enhanced the abundance of total nematodes and bacterivore nematodes but inhibited fungivore nematodes. Chen et al. (2021) indicated that adding nitrogen indirectly reduced nematode abundance and richness by increasing the NO3− content in grazing regimes. Teshita et al. (2023) noted that the total nematode abundance decreased in monoculture maize fields when nitrogen fertilizers were applied. However, in maize fields intercropped with alfalfa, the abundance of total nematodes and omnivore-predator nematodes increased, regardless of the application of nitrogen fertilizers. In conclusion, fertilization may affect the nematode assemblages by altering soil properties.
Conclusion
The soil physiochemical properties and nematode assemblages were investigated in five soil types from seven fields of the China Cultivated Land Quality Monitoring Site in Jilin Province, China. The main conclusions are as follows. (1) Soil pH decreased significantly after long-term fertilization in all soils except chernozems, and the extent of change in pH depended on soil type. (2) The effects of fertilization on nematode abundance, genera, and diversity differed among different types. (3) The structure of the nematode assemblage after fertilization was distinguishable in phaeozems. (4) Soil pH significantly affected on the nematode assemblage in phaeozems, but the other soil types.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
XW, LW, and ZY contributed the conception and design of the study. CL, XW, BC, and JW made soil sampling. XW extracted and identified soil nematodes. CL and ZX performed the soil properties analyses. CL ZX, and JW conducted data statistical analyses. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Project of the Open Fund for the Key Laboratory of Plant Nutrition and Agricultural Environment in Northeast China (KLAE2018502), the Cultivated Project of Interdisciplinary Integration and Innovation of Jilin University (Research on the System of Integrated Innovation of Rural Revitalization Boosting Northeast Black Land Protection, Agricultural Transformation and Income Double Increase, JLUXKJC2020205) and the Special Foundation for National Science and Technology Basic Research Program of China (2018FY100305).
Acknowledgments
The authors would like to thank Huilin Zhang and Qiubin Wang, Jilin Soil Fertilizer General Station for their assistance in soil sampling to complete this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2023.1207379/full#supplementary-material
References
Adnan, M., Xu, H., Muhammad, M. A., Syed, A. A. S., Sun, N., Qudsia, S., et al. (2021). Long-term fertilization enhanced carbon mineralization and maize biomass through physical protection of organic carbon in fractions under continuous maize cropping. Appl. Soil. Ecol. 165, 103971. doi:10.1016/j.apsoil.2021.103971
Bardgett, R. D., and van der Puuten, W. H. (2014). Belowground biodiversity and ecosystem functioning. Nature 515, 505–511. doi:10.1038/nature13855
Bongers, T., van der Meulen, H., and Korthals, G. (1997). Inverse relationship between the nematode maturity index and plant parasite index under enriched nutrient conditions. Appl. Soil. Ecol. 6, 195–199. doi:10.1016/s0929-1393(96)00136-9
Čerevková, A., Miklisová, D., and Cagáň, Ľ. (2017). Effects of experimental insecticide applications and season on soil nematode communities in a maize field. Crop Prot. 92, 1–15. doi:10.1016/j.cropro.2016.10.007
Chen, D. M., Lan, Z. C., Hu, S. J., and Bai, Y. F. (2015). Effects of nitrogen enrichment on belowground communities in grassland: Relative role of soil nitrogen availability vs. soil acidification. Soil Biol. biochem. 89, 99–108. doi:10.1016/j.soilbio.2015.06.028
Chen, Y., Zhang, Y., Nielsen, U. N., Ma, Q. H., Zhang, X., Huang, X. W., et al. (2021). Cattle grazing mitigates the negative impacts of nitrogen addition on soil nematode communities. Ecol. Indic. 129, 107876. doi:10.1016/j.ecolind.2021.107876
Cole, L., Buckland, S. M., and Bardgett, R. D. (2008). Influence of disturbance and nitrogen addition on plant and soil animal diversity in grassland. Soil Biol. biochem. 40, 505–514. doi:10.1016/j.soilbio.2007.09.018
Devries, W., and Breeuwsma, A. (1987). The relation between soil acidification and element cycling. Water Air Soil Poll. 35, 293–310. doi:10.1007/bf00290937
Escalante, L. E., Brye, K. R., and Faske, T. R. (2021). Nematode populations as affected by residue and water management in a long-term wheat-soybean double-crop system in eastern Arkansas. Appl. Soil. Ecol. 157, 103761. doi:10.1016/j.apsoil.2020.103761
Ferris, H., Bonfers, T., and Goede, de R. G. M. (2001). A framework for soil food web diagnostics extension of the nematode faunal analysis concept. Appl. Soil. Ecol. 18, 13–29. doi:10.1016/s0929-1393(01)00152-4
Ferris, H., and Matute, M. M. (2003). Structural and functional succession in the nematode fauna of a soil food web. Appl. Soil. Ecol. 23, 93–110. doi:10.1016/s0929-1393(03)00044-1
Ferris, H., Sánchez-Moreno, S., and Brennan, E. B. (2012). Structure, functions and interguild relationships of the soil nematode assemblage in organic vegetable production. Appl. Soil. Ecol. 61, 16–25. doi:10.1016/j.apsoil.2012.04.006
Freckman, D. H., and Virginia, R. A. (1993). Extraction of nematodes from dry valley antarctic soils. Polar Biol. 13, 483–487. doi:10.1007/bf00233139
Grabau, Z. J., and Chen, S. Y. (2016). Influence of long-term corn-soybean crop sequences on soil ecology as indicated by the nematode community. Appl. Soil Ecol. 100, 172–185. doi:10.1016/j.apsoil.2015.12.016
Gu, S. Y., Wu, S., Guan, Y. P., Zhai, C., Zhang, Z. H., Bello, A., et al. (2020). Arbuscular mycorrhizal fungal community was affected by tillage practices rather than residue management in black soil of northeast China. Soil till. Res. 198, 104552. doi:10.1016/j.still.2019.104552
Guo, J. H., Liu, X. J., Zhang, Y., Shen, J. L., Han, W. X., Zhang, W. F., et al. (2010). Significant acidification in major Chinese croplands. Science 327, 1008–1010. doi:10.1126/science.1182570
Guo, X., Li, H. Y., Yu, H. M., Li, W. F., Ye, Y. C., and Biswas, A. (2018). Drivers of spatio-temporal changes in paddy soil pH in Jiangxi Province, China from 1980 to 2010. Sci. Rep-UK 8, 2702. doi:10.1038/s41598-018-20873-5
Hu, X. J., Gu, H. D., Liu, J. J., Wei, D., Zhu, P., Cui, X. A., et al. (2022). Metagenomics reveals divergent functional profiles of soil carbon and nitrogen cycling under long-term addition of chemical and organic fertilizers in the black soil region. Geoderma 418, 115846. doi:10.1016/j.geoderma.2022.115846
Hu, X. J., Liu, J. J., Wei, D., Zhu, P., Cui, X. A., Zhou, B. K., et al. (2017). Effects of over 30-year of different fertilization regimes on fungal community compositions in the black soils of northeast China. Agr. Ecosyst. Environ. 248, 113–122. doi:10.1016/j.agee.2017.07.031
Jenkins, W. R. (1964). A rapid centrifugal-floatation technique for separating nematodes from soil. Plant Dis. Report. 48, 692.
Kimmel, K., Furey, G. N., Hobbie, S. E., Isbell, F., Tilman, D., and Reich, P. B. (2020). Diversity-dependent soil acidification under nitrogen enrichment constrains biomass productivity. Glob. Change Biol. 26, 6594–6603. doi:10.1111/gcb.15329
Korthals, G. W., Alexiev, A. D., Lexmond, T. M., Kammenga, J. E., and Bongers, T. (1996). Long-term effects of copper and pH on the nematode community in an agroecosystem. Environ. Toxicol. Chem. 15, 979–985. doi:10.1002/etc.5620150621
Li, Q. Q., Li, S., Xiao, Y., Zhao, B., Wang, C. Q., Li, B., et al. (2019). Soil acidification and its influencing factors in the purple hilly area of southwest China from 1981 to 2012. Catena 175, 278–285. doi:10.1016/j.catena.2018.12.025
Li, Y. Y., Chapman, S. J., Nicol, G. W., and Yao, H. Y. (2018). Nitrification and nitrifiers in acidic soils. Soil Biol. biochem. 116, 290–301. doi:10.1016/j.soilbio.2017.10.023
Liang, W. J., Chen, L. J., Li, Q., Wang, P., and Duan, Y. X. (2002). Responses of nematode communities to inorganic fertilizer disturbance in a farmland ecosystem. Pedosphere 12, 193–200.
Liang, W. J., Lou, Y. L., Li, Q., Zhong, S., Zhang, X. K., and Wang, J. K. (2009). Nematode faunal response to long-term application of nitrogen fertilizer and organic manure in Northeast China. Soil Biol. biochem. 41, 883–890. doi:10.1016/j.soilbio.2008.06.018
Liu, J. J., Sui, Y. Y., Yu, Z. H., Shi, Y., Chu, H. Y., Jin, J., et al. (2014). High throughput sequencing analysis of biogeographical distribution of bacterial communities in the black soils of northeast China. Soil Biol. biochem. 70, 113–122. doi:10.1016/j.soilbio.2013.12.014
Mei, N., Zhang, X. Z., Wang, X. Q., Peng, C., Gao, H. J., Zhu, P., et al. (2021). Effects of 40 years applications of inorganic and organic fertilization on soil bacterial community in a maize agroecosystem in northeast China. Eur.J. Agron. 130, 126332. doi:10.1016/j.eja.2021.126332
Puissant, J., Villenave, C., Chauvin, C., Plassard, C., Blanchart, E., and Trap, J. (2021). Quantification of the global impact of agricultural practices on soil nematodes: A meta-analysis. Soil Biol. biochem. 161, 108383. doi:10.1016/j.soilbio.2021.108383
Räty, M., and Huhta, V. (2003). Earthworms and pH affect communities of nematodes and enchytraeids in forest soil. Biol. Fertil. Soils 38, 52–58. doi:10.1007/s00374-003-0614-5
Raza, S., Miao, N., Wang, P. Z., Ju, X. T., Chen, Z. J., Zhou, J. B., et al. (2020). Dramatic loss of inorganic carbon by nitrogen-induced soil acidification in Chinese croplands. Glob. Change Biol. 26, 3738–3751. doi:10.1111/gcb.15101
Song, M., Jing, S. S., Zhou, Y. Q., Hui, Y., Zhu, L. L., Wang, F., et al. (2015). Dynamics of soil nematode communities in wheat fields under different nitrogen management in Northern China Plain. Eur. J. Soil Biol. 71, 13–20. doi:10.1016/j.ejsobi.2015.09.002
Song, M., Li, X. M., Jing, S. S., Lei, L. J., Wang, J. L., and Wan, S. Q. (2016). Responses of soil nematodes to water and nitrogen additions in an old-field grassland. Appl. Soil. Ecol. 102, 53–60. doi:10.1016/j.apsoil.2016.02.011
Teshita, A., Feng, Y. Y., Qian, R., Wang, X. Y., Khan, W., and Gao, Y. Z. (2023). Alfalfa and maize intercropping enhances soil nematode structure and food web complexity in low-nitrogen soils. Appl. Soil. Ecol. 186, 104809. doi:10.1016/j.apsoil.2023.104809
van den Hoogen, J., Geisen, S., Routh, D., Ferris, H., Traunspurger, W., Wardle, D. A., et al. (2019). Soil nematode abundance and functional group composition at a global scale. Nature 572, 194–198. doi:10.1038/s41586-019-1418-6
Wang, J. M., Zhang, Q. R., Chu, H. Y., Shi, Y., and Wang, Q. (2022). Distribution and co-occurrence patterns of antibiotic resistance genes in black soils in Northeast China. J. Environ. Manage. 319, 115640. doi:10.1016/j.jenvman.2022.115640
Wang, Q., Yu, H. Y., Liu, J. F., and Li, F. B. (2018). Attribution of soil acidification in a large-scale region: Artificial intelligence approach application. Soil Sci. Soc. Am. J. 82, 772–782. doi:10.2136/sssaj2017.08.0304
Wang, Y. M., Guan, P. T., Chen, J. W., Li, Z. X., Yang, Y. R., and Wang, P. (2021). A comparison of soil nematode community structure and environmental factors along fen-bush-forest succession in a peatland, northeastern China. Glob. Ecol. Conserv. 28, e01679. doi:10.1016/j.gecco.2021.e01679
Wang, Y., Zhang, X. Y., Gao, Q., Li, C. L., Yan, L., and Feng, G. Z. (2017). Temporal and spatial variability of soil pH in the cropland of Jilin Province. Chin. J. Soil Sci. 48 (2), 387–391.
Wu, Z. F., Sun, X. M., Sun, Y. Q., Yan, J. Y., Zhao, Y. F., and Chen, J. (2022). Soil acidification and factors controlling topsoil pH shift of cropland in central China from 2008 to 2018. Geoderma 408, 115586. doi:10.1016/j.geoderma.2021.115586
Xia, C. Z., and Zhang, Y. (2022). Comparison of the use of Landsat 8, Sentinel-2, and Gaofen-2 images for mapping soil pH in Dehui, northeastern China. Ecol. Inf. 70, 101705. doi:10.1016/j.ecoinf.2022.101705
Yan, L., Wang, Y., Feng, G. Z., and Gao, Q. (2015). Status and change characteristics of farmland soil fertility in Jilin Province. Sci. Agric. Sin. 48, 4800–4810. doi:10.3864/j.issn.0578-1752.2015.23.021
Yeates, G. W., Bongers, T., Goede, de R. G. M., Freckman, D. W., and Georgieva, S. S. (1993). Feeding habits in soil nematode families and genera-an outline for soil ecologists. J. Nematol. 25, 315–331.
You, J. F., Liu, X., Zhang, B., Xie, Z. K., Hou, Z. G., and Yang, Z. M. (2015). Seasonal changes in soil acidity and related properties in ginseng artificial bed soils under a plastic shade. J. GINSENG Res. 39, 81–88. doi:10.1016/j.jgr.2014.08.002
Zamanian, K., Zarebanadkouki, M., and Kuzyakov, Y. (2018). Nitrogen fertilization raises CO2 efflux from inorganic carbon: A global assessment. Glob. Change Biol. 24, 2810–2817. doi:10.1111/gcb.14148
Zhang, J., Wei, D., Zhou, B. K., Zhang, L. J., Hao, X. Y., Zhao, S. C., et al. (2021a). Responses of soil aggregation and aggregate-associated carbon and nitrogen in black soil to different long-term fertilization regimes. Soil till. Res. 213, 105157. doi:10.1016/j.still.2021.105157
Zhang, Y., Ji, L., and Yang, L. X. (2021b). Abundance and diversity of soil nematode community at different altitudes in cold-temperate montane forests in northeast China. Glob. Ecol. Conserv. 29, e01717. doi:10.1016/j.gecco.2021.e01717
Zhang, Y., Shen, H. O., Gao, Q., and Zhao, L. P. (2020). Estimating soil organic carbon and pH in Jilin Province using Landsat and ancillary data. Soil Sci. Soc. Am. J. 84, 556–567. doi:10.1002/saj2.20056
Zhang, Z. Y., Zhang, X. K., Jhao, J. S., Zhang, X. P., and Liang, W. J. (2015). Tillage and rotation effects on community composition and metabolic footprints of soil nematodes in a black soil. Eur. J. SOIL Biol. 66, 40–48. doi:10.1016/j.ejsobi.2014.11.006
Zhao, J., Wang, F. M., Li, J., Zou, B., Wang, X. L., Li, Z. A., et al. (2014). Effects of experimental nitrogen and/or phosphorus additions on soil nematode communities in a secondary tropical forest. Soil Biol. biochem. 75, 1–10. doi:10.1016/j.soilbio.2014.03.019
Zhou, J., Guan, D. W., Zhou, B. K., Zhao, B. S., Ma, M. C., Qin, J., et al. (2015). Influence of 34-years of fertilization on bacterial communities in an intensively cultivated black soil in northeast China. Soil Biol. biochem. 90, 42–51. doi:10.1016/j.soilbio.2015.07.005
Zhou, J., Jiang, X., Zhou, B. K., Zhao, B. S., Ma, M. C., Guan, D. W., et al. (2016). Thirty four years of nitrogen fertilization decreases fungal diversity and alters fungal community composition in black soil in northeast China. Soil Biol. biochem. 95, 135–143. doi:10.1016/j.soilbio.2015.12.012
Keywords: long-term fertilization, soil type, soil acidification, nematode assemblage, Northeast China
Citation: Li C, Wang X, Chen B, Wang L, Xie Z, Wang J and Yang Z (2023) Fertilization restructures nematode assemblages by modifying soil pH in croplands of Northeast China. Front. Environ. Sci. 11:1207379. doi: 10.3389/fenvs.2023.1207379
Received: 17 April 2023; Accepted: 27 June 2023;
Published: 06 July 2023.
Edited by:
Daniela Businelli, University of Perugia, ItalyReviewed by:
Mikhail Semenov, Russian Academy of Agricultural Sciences, RussiaGerhard Du Preez, North-West University, South Africa
Petra Benetkova, Charles University, Czechia
Copyright © 2023 Li, Wang, Chen, Wang, Xie, Wang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Wang, wang_jun@jlu.edu.cn; Zhenming Yang, zmyang@jlu.edu.cn
†These authors have contributed equally to this work
 Chunlin Li
Chunlin Li Xuefeng Wang2†
Xuefeng Wang2†  Lichun Wang
Lichun Wang Zhenming Yang
Zhenming Yang