Spatiotemporal distribution patterns of soil ciliate communities in the middle reaches of the Yarlung Zangbo River
- School of Ecology and Environment, Tibet University, Lhasa, China
Introduction: Soil ciliates, as protozoa, play a crucial role in biogeochemical cycling and the soil food web, yet they are highly sensitive to environmental fluctuations in soil conditions. The diversity and biogeographic characteristics of soil ciliates in the Tibetan Plateau remain poorly understood. As part of a regional survey focused on soil ciliate diversity, we investigated the composition and spatiotemporal variations of soil ciliate communities along the Yarlung Zangbo River, a representative soil habitat in the Tibetan Plateau.
Methods: A total of 290 soil samples were collected from four habitat types of grassland, shrubland, forestland and wetland in the middle reaches of the Yarlung Zangbo River during the wet and dry seasons, and 138 species of ciliates were identified.
Results: Soil ciliate diversity exhibited greater variation across habitat types than seasons. Moreover, soil ciliate diversity was higher during the wet season compared to the dry season, with the wetland habitat showing the highest diversity and the grassland habitat displaying the lowest. We observed spatiotemporal heterogeneity in the composition of soil ciliate communities across different seasons and habitat types. Notably, Litostomatea, Karyorelictea, and Prostomatea predominated in ciliate communities during the wet season and in grassland habitat. Phyllopharyngers dominated during dry seasons and in forested regions, while Spirotrichea species were prevalent in wetland and forested areas. The co-occurrence network analysis showed that soil ciliate community was more complex in wet season than in dry season, and the stability of soil ciliate community in wet season was higher than that in dry season. The stability of soil ciliate community in wetland was higher than that in forestland, shrubland and grassland, and the anti-interference ability was stronger. Soil temperature (ST), Total nitrogen (TN), Soil organic matter (SOM) and Soil water content (SWC) are important factors affecting the structure of soil ciliate community. By influencing the metabolic rate and nutrient acquisition of soil ciliates, the distribution pattern of soil ciliate community diversity in the middle reaches of Yarlung Zangbo River is shaped.
Discussion: In summary, this study revealed the distribution pattern of soil ciliate community diversity in the Yarlung Zangbo River Basin, and the key factors affecting the spatial and temporal differences and stability of the community, enhancing our understanding of how ciliates adapt to environmental conditions in soil habitats across the Tibetan Plateau.
1 Introduction
Protozoa is one of the main groups of soil microorganisms (Acosta-Mercado and Lynn, 2004; Abraham et al., 2019; 2019). Protozoa, as an important trophic level in microfood web, plays an important role in maintaining ecological balance, energy transport hub and biogeochemical cycle, and is an indispensable part of soil ecosystem (Bonkowski, 2004; Geisen et al., 2018; Xu et al., 2022). As the main group of soil protozoa community, soil ciliates have the characteristics of rich species, short life cycle and rapid community succession (Foissner, 1999), which has an important influence on the assembly, succession and maintenance of the community, and the change of their abundance can drive the direction of community succession (Domonell et al., 2013; Geisen et al., 2018). Soil ciliates are highly sensitive to environmental changes due to the constant interaction between their cell membranes and the external environment (Zheng et al., 2018; Liu et al., 2021). External disturbances promote rapid changes in their community structure and diversity, outpacing the responsiveness of large animals residing in the soil matrix (Bamforth et al., 2005; Foissner, 2005). This rapid response makes soil ciliates sentinel organisms of environmental changes, coupled with ease of culture and observation, can be used as dynamic indicators to assess soil environmental health (Heger et al., 2012; Debastiani et al., 2016). In addition, soil ciliates can decompose organic matter in the soil and maintain the stability of the soil environment (Xiong et al., 2018), a key role that can affect the ecological balance and overall health of the soil (Azam et al., 1983; Geisen et al., 2015). The Qinghai-Tibet Plateau is the region most sensitive to climate change in the world (Tian et al., 2020). The unique properties of soil ciliates make them valuable research objects in the context of ecological resilience of the Qinghai-Tibet Plateau, and have practical application value in assessing soil conditions of different ecosystems or different landscapes on the Qinghai-Tibet Plateau.
The Yarlung Zangbo River Basin is located in the southern part of the Qinghai-Tibet Plateau. With its high average altitude, long sunshine time and low temperature, this region has become a hotspot for studying biodiversity pattern due to its diverse and complex climatic and geographical characteristics (Liu et al., 2018; Shi et al., 2018). In recent years, under the influence of global warming and local human activities, the biodiversity and ecosystem stability of the Brahmaputra River Basin are changing, and the regional ecological risks are increasing (Liu et al., 2018). At present, there are relatively few research reports on protozoa in this region. Researchers Zhang et al. revealed the distribution pattern of protozoa diversity in the upper reaches of the Yarlung Zangbo River (Zhang et al., 2022), and researchers Yang et al. clarified the diversity and composition distribution pattern of eukaryotic microorganisms along the altitude gradient in the middle reaches of the Yarlung Zangbo River (Yang Q. et al., 2023). However, the diversity of soil ciliates in the Yarlung Zangbo River Basin has not been reported, and the soil ecological health status of different ecosystems or different landscapes in the basin is still unclear. The spatial and temporal distribution pattern, community stability, ecological networks among species and main driving factors of soil ciliates in the basin remain to be revealed. In this context, we studied the community composition, spatial and temporal distribution and diversity pattern of soil ciliates in the middle reaches of the Yarlung Zangbo River, analyzed the species association and community stability of soil ciliates in different seasons and different ecosystem types, and discussed the adaptability of soil ciliates in different habitats and the main environmental driving factors.
The purpose of this study was to reveal: (1) the spatial and temporal distribution pattern of soil ciliate communities in the middle reaches of Yarlung Zangbo River; (2) Species association and community stability of soil ciliates in different seasons and different ecosystem types; (3) Adaptability of soil ciliates in different habitats and its main environmental driving factors. This study provided valuable insights for the environmental adaptation of soil ciliates, and provided scientific basis for ecological environmental protection, biogeochemical cycle research and soil ecological risk assessment on the Qinghai-Tibet Plateau.
2 Materials and methods
2.1 Study area overview and sample collection
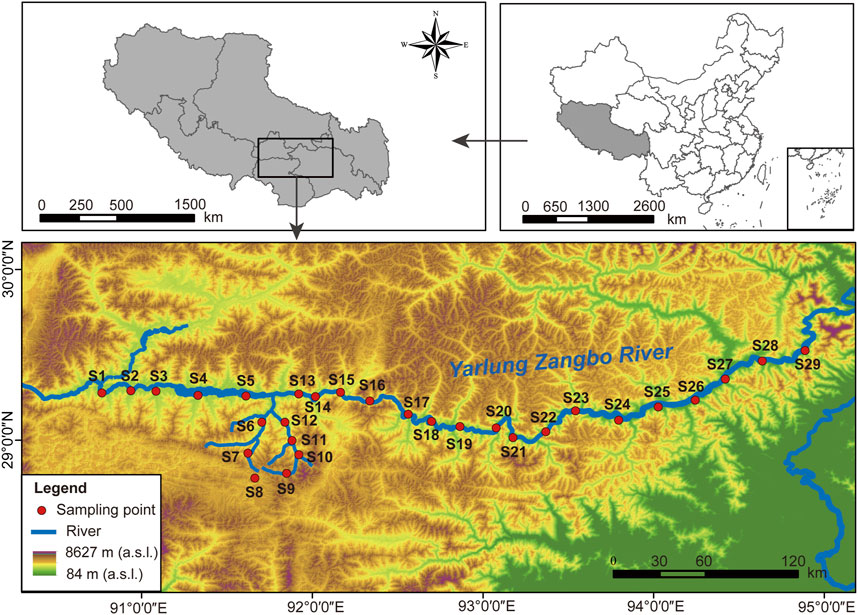
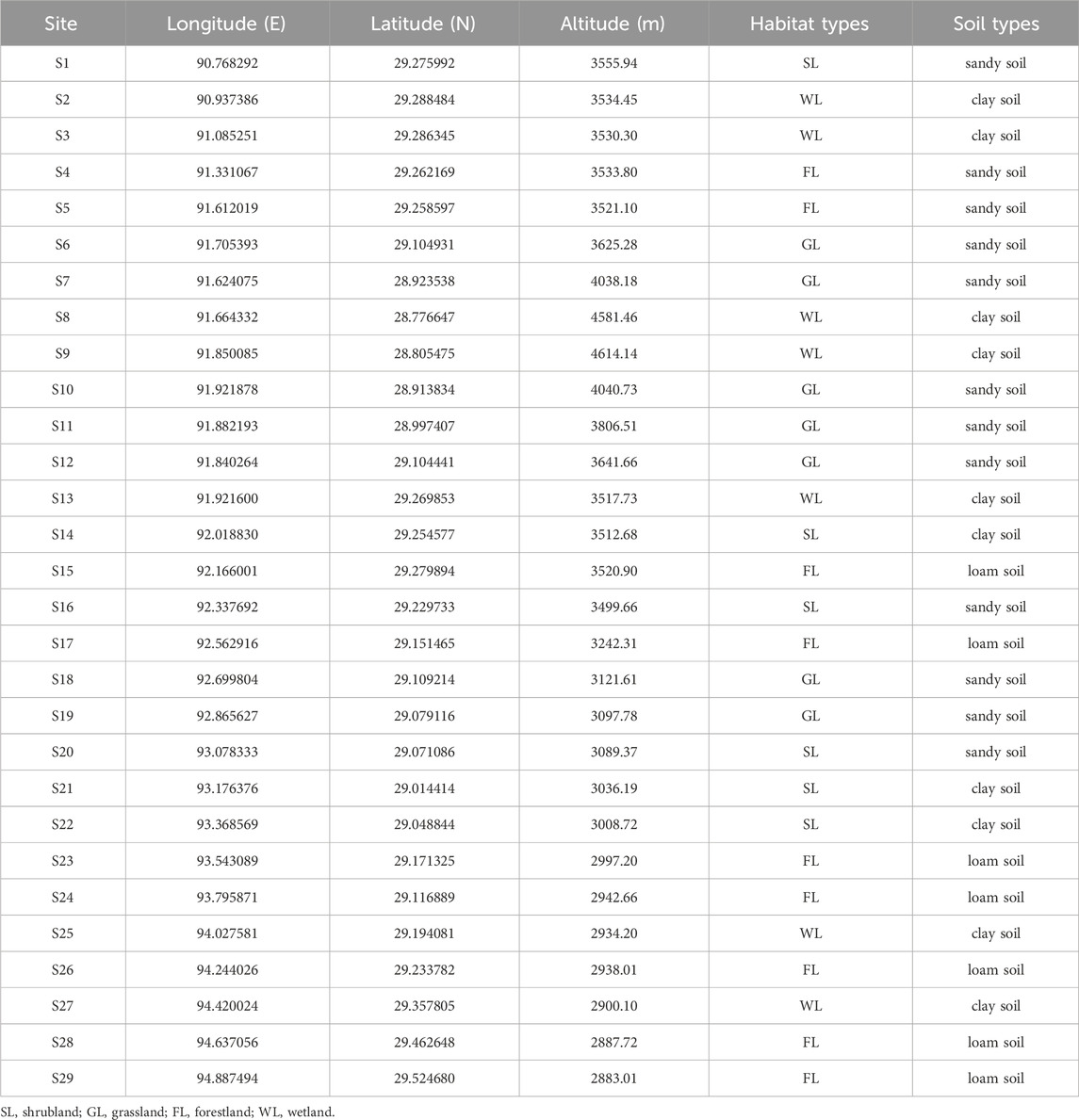
The Yarlung Zangbo River Basin (82°00′—97°07′E, 28°00′—31°16′N) is located between the Gangdis-Nianqing Tanggula Mountain range and the Himalayas (Zhang et al., 2014). It originates from Jemayang Zongqu in Zhongba County, Shigatze City, Tibet Autonomous Region, China. It runs through southern Tibet from east to west, with a high terrain in the west and low in the east. The drainage area is 240,000 km2 (You et al., 2007). The river is divided according to the terrain characteristics and climate types of the basin. The middle reaches of the river are about 1,293 km long and the basin area is 165,000 km2 (Li et al., 1999). The river banks are dominated by floodplains and terraces, and the average annual precipitation is between 300 mm and 600 mm in plateau temperate semi-arid climate (Wu et al., 2021). The complex habitats and vegetation types transition from alpine meadow to alpine scrub and then to forest. The study area covers the middle reaches of the Yarlung Zangbo River. A total of 29 sampling sites were set up in this area (Figure 1), and the sampling sites were divided according to habitat characteristics, including four types of grassland (GL), shrubland (SL), forestland (FL) and wetland (WL) (Table 1). Soil biological sample collection and soil physicochemical index determination will be carried out in August (wet season) and November (dry season) in 2021.
Five sampling sites were set up within an area of about 100 m2 in each sample plot, and the area of each sampling site was 25 m2 (5 m × 5 m). Fresh litter on the soil surface was picked off before sample collection, and relevant data such as soil quality, soil temperature, altitude, geographical coordinates and vegetation status were recorded. Soil sampler (LEICI, JC-802B, China) was used to collect 0–5 cm surface soil samples according to the “five-point pattern of blossom” sampling method (Liu et al., 2022). One soil sample was collected from each sampling point, and a total of five soil samples were collected from each sample plot. The samples were mixed, sealed in bags, and marked. At the same time, the soil ring knife sampler (LEICI, JC-TRCY01A, China) with a volume of 100 cm3 was used to cut the in-situ soil, fill the soil sample with it, seal it and bring it back to the laboratory for the determination of water content. A total of 290 soil samples were collected from 29 sites in two seasons (29 sites × 2 seasons × 5 replicates), and the number of samples was consistent among different habitat types. The soil samples were separately treated after being brought back to the laboratory. The fresh soil samples were used to determine the soil physical and chemical indexes, and the remaining soil samples were naturally air-dried in the laboratory for qualitative observation and quantitative culture of soil ciliates.
2.2 Sample handling and species identification
The “Non-Flooded Petri Dish Method” was employed for qualitative research (Foissner, 1992). Fifty grams of air-dried soil samples were added to Petri dishes with a diameter of 15 cm. Soil leachate was then added until the soil was thoroughly moistened but not submerged. The dishes were placed in a 25°C incubator and continuously cultured for 20 days. Species identification was performed by OLYMPUS CKX53 inverted fluorescence microscope every day after culture, and observations were recorded continuously. Quantitative research was conducted using the direct counting method in cultivation (Ning et al., 2018). Thirty grams of air-dried soil samples were weighed and placed in culture dishes with a diameter of 10 cm, with a water-to-soil ratio of 1:1. These dishes were maintained at a constant liquid level in a 25°C incubator until the maximum counting day (day 9, 10, or 11). On that day, the culture dishes were tilted at a 45° angle and allowed to stand for 5–7 min. The supernatant was then completely drawn off and measured. A drop of the supernatant was taken onto a glass slide, fixed under a microscope for counting, and records were maintained. This process was repeated five times for each soil sample. The obtained counts were converted into ciliate density per 1 mL of water (approximately equal to 22 drops). Using the ciliate density per 1 mL of water, the number of soil ciliates in 30 g of soil samples was calculated.
2.3 Measurement of environmental parameters
Soil temperature (ST) was measured in situ (-10–60°C) with a curved pipe geotherm (NENGH, NHSQ2803,China). Soil pH value (soil: water = 1:2.5) was measured by soil acidity meter (LEICI, TSS-851,China) (Thunjai et al., 2001). The soil moisture content (SWC) was measured by drying method (Davidson et al., 1998). The content of soil available phosphorus (AP) was determined by ultraviolet spectrophotometer (INESA, 752N, China) according to sodium bicarbonate extraction and molybdenum-antimony resistance colorimetric method (Xue et al., 2004). Available potassium (RAK) was determined by ammonium acetate extraction and flame photometer (Chen et al., 2020). The content of total nitrogen (TN) was determined by elemental analyzer (VELP, CN802, Italy) (Bremner, 1960). The content of total potassium (TK) was determined by flame spectrophotometry (Zhang et al., 2013). Soil organic matter (SOM) was determined by potassium dichromate volumetric method using elemental analyzer (VELP, CN802, Italy) (Nóbrega et al., 2015). The content of total phosphorus (TP) was determined by ultraviolet spectrophotometer (INESA, 752N, China) according to sodium hydroxide alkali-molybdenum-antimony reactance colorimetry (Xue et al., 2004).
2.4 Statistical analysis
Alpha diversity indices (including richness index, Shannon-Wiener diversity index, Pielou evenness index, and Simpson dominance index) were calculated using “vegan” package in R (version 4.2.1). One-way analysis of variance was used to determine the significance of associations of alpha diversity indices with seasons or habitat types. Principal coordinate analysis (PCoA) and PERMANOVA analysis were performed based on the Bray–Curtis distance using the “Micoeco” package in R. Mantel tests were used to determine correlations between environmental variables and selected characteristics of ciliates composition. Ciliates co-occurrence patterns were constructed based on Spearman’s rank correlation coefficients. Co-occurrence events were identified as statistically robust correlations (|R| > 0.6, p < 0.05) and the co-occurrence network was visualized in Gephi (version 0.9.7). Sampling sites were mapped in ArcMap 10.6.1.
3 Results
3.1 Environmental factors and ciliates diversity varied
The environmental factors of season and habitat type are presented in Supplementary Figure S1, The average concentrations of SWC and TP in wet season were 36.65% and 0.7 g/kg, respectively, as compared to the 10.31% and 0.54 g/kg in dry season. The Environmental factors SWC and TP contents in wet season were significantly higher than dry season (p < 0.05). In addition, Other environmental factors of average wet than dry season (Supplementary Figure S1A), including TN (1.49 g/kg and 1.35 g/kg, respectively), SOM (29.53 g/kg, 27.77 g/kg), RAK (96.65 mg/kg, 79.59 mg/kg), AP (18.42 mg/kg, 15.29 mg/kg)、pH (7.76, 7.40), TK (20.43 g/kg, 19.99 g/kg), ST (24.20°C, 20.83°C)and VC (71.65%, 67.90%). However, the majority of these environmental factors showed no significant difference between wet and dry season (p > 0.05). In addition, some environmental factors were significantly different among the different habitat types (p < 0.05), such as pH, TK and VC (Supplementary Figure S1B). The pH values of forestland, wetland and grassland were significantly higher than those of shrubland. TK and VC values were higher in forestland and grassland than in shrubland and wetland. The value of TN, SOM and TP in grassland were higher than in shrubland than in wetland than in forestland. The values of RAK, AP, and ST were higher in grassland than in shrubland, forestland, and wetland. The SWC value was higher in wetland than in forestland, shrubland, and grassland. However, there was no significant (p < 0.05) difference in these factors among the different habitat types.
In this study, 58 samples were obtained, of which 29 were wet samples and 29 were dry samples (Supplementary Table S1). In total, we obtained 138 species of ciliates from all samples. These ciliates were belonged to 20 orders, 37 families, 57 genera of wet season, and 20 orders, 37 families, 55 genera of dry season (Supplementary Table S2). The alpha diversity of ciliates varied among different seasons and habitat types for the different indices (Supplementary Table S3). For different seasons, the mean value of richness indices, Shannon indices and Simpson indices in wet was higher than in dry season, but these was no significant (p > 0.05) difference (Figure 2A). Of different habitat types, the mean value of richness and Simpson indices in wetland were higher than in forestland, shrubland and grassland, with significant difference (p < 0.05). The mean value of Shannon indices in wetland were higher than in shrubland and grassland, with significant difference. The mean value of Pielou indices in wetland were higher than in shrubland and grassland, with no significant difference. In general, alpha diversity of ciliates varied in a spatiotemporal manner. In addition, the alpha diversity indices suggested significant differences in ciliates diversity among environment types: wetland samples had the greatest diversity, forestland samples had the lowest Pielou index, and grassland had the lowest diversity (Figure 2B).
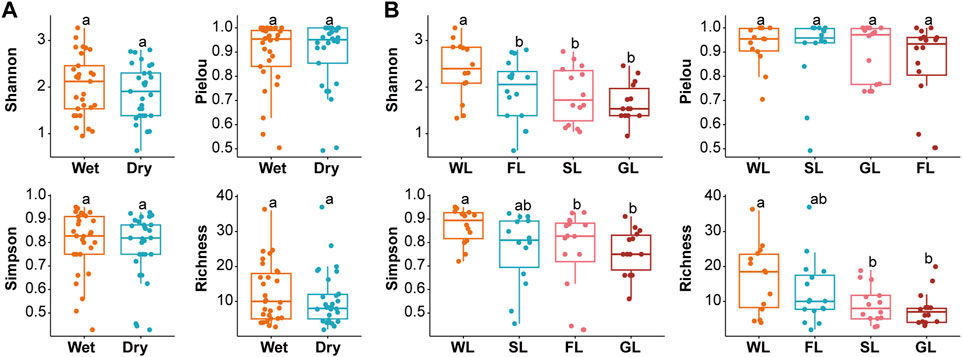
FIGURE 2. Alpha diversity of soil ciliate communities. Seasonal comparisons of alpha diversity estimators for soil ciliate communities (A). Comparisons of alpha diversity estimators for soil ciliate communities in different habitat types (B). Notes: SL: Shrubland; GL: Grassland; FL: Forestland; WL: Wetland.
3.2 Community structure of soil ciliates in the middle reaches of Yarlung Zangbo River
The application of principal coordinate analysis (PCoA) and PERMANOVA analysis unveiled noteworthy dissimilarities in the composition of soil ciliates communities across various seasons and environmental habitat types. The findings indicated that PcoA1 and PcoA2 accounted for 9.0% and 8.2%, respectively, of the overall variation in ciliates communities among different seasons and habitat types (Figures 3A, B). The PERMANOVA analysis provided evidence that the composition of ciliates communities across different habitat types (R2: 0.88, p = 0.001) displayed statistically significant disparities (p < 0.05), whereas variations in different seasons (R2: 0.02, p = 0.22) were not statistically significant. Analysis of the seasonal richness of species revealed that 58.7% (n = 81) of species were present in samples from all two seasons (Figure 3C), 17.4% (n = 24) unique species in the dry season, 23.9% (n = 33) unique species in the wet season. Furthermore, we analyzed the ciliates community composition in different seasons and habitat types based on species abundance information (Figure 3E). In the wet season, the top three ciliate taxa at class levels with their mean relative abundance are as follows: Colpodea (28.2%), Spirotrichea (24.52%) and Oligohymenophorea (14.7%). In the dry season, the top three ciliate taxa at class levels with their mean relative abundance are as follows: Spirotrichea (31.42%), Colpodea (23.75%) and Oligohymenophorea (18.64%) (Supplementary Table S4). In the wet season, the top three ciliates taxa at genus levels with their mean relative abundance are as follows (Supplementary Table S5): Colpoda (21.83%), Plagiocampa (6.31%) and Cyrtolophosis (6.3%). In the dry season, the top three ciliates taxa at genus levels with their mean relative abundance are as follows: Colpoda (14.33%), Cyrtolophosis (9.42%) and Halteria (8.09%).
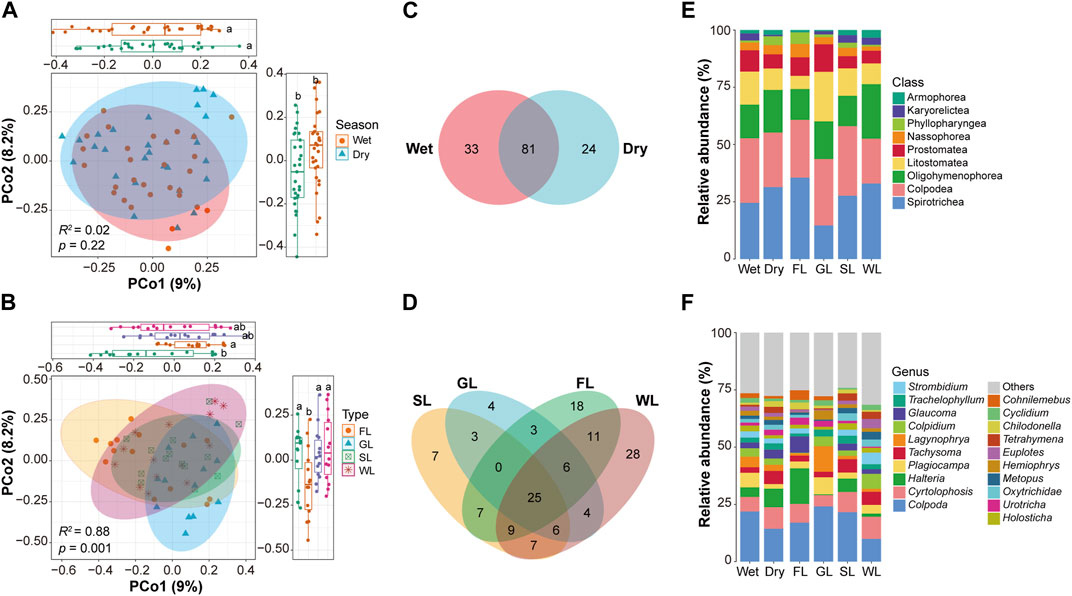
FIGURE 3. Species composition and abundance of soil ciliate communities. Principal coordinate analysis (PcoA) and PERMANOVA analysis of ciliate communities in the two seasons (A) and four habitat types (B). Venn diagram showing the number of shared and specific species of soil ciliates for each season (C) and habitat types (D). Relative abundance of the main phyla of soil ciliates in the two seasons and four habitat types (E). Relative abundance of the main general of soil ciliates in the two seasons and four habitat types (F). Notes: SL: Shrubland; GL: Grassland; FL: Forestland; WL: Wetland.
Analysis of samples from the four different habitats types showed that species from soil samples clustered separately, and the differences were statistically significant (Figure 3B): 18.1% (n = 25) were shared by the four different types (Figure 3D), 2.9% (n = 4) were shared wetland and grassland, 8% (n = 11) were shared by forestland and wetland, 5.1% (n = 7) were shared by wetland and shrubland, 2.2% (n = 3) were shared by forestland and grassland. Furthermore, we conducted an analysis of ciliate community composition in different habitat types based on species abundance information at the class level (Figure 3E). The results revealed the top three ciliate taxa at the class level for each habitat: In grassland: Colpodea (28.96%), Litostomatea (21.64%), and Oligohymenophorea (16.46%). In shrubland: Colpodea (30.37%), Spirotrichea (27.61%), and Oligohymenophorea (13.3%). In forestland: Spirotrichea (35.55%), Colpodea (25.14%), and Oligohymenophorea (13.54%). In wetland: Spirotrichea (32.97%), Oligohymenophorea (23.81%), and Colpodea (19.53%) (Supplementary Table S6). At the genus level, the top three ciliate taxa with their mean relative abundance are as follows: In grassland: Colpoda (24.11%), Lagynophrya (11.14%), and Plagiocampa (7.47%). In shrubland: Colpoda (21.58%), Cyrtolophosis (8.79%), and Halteria (5.89%). In forestland: Colpoda (17.01%), Halteria (15.6%), and Cyrtolophosis (8.14%). In wetland: Colpoda (9.92%), Cyrtolophosis (9.62%), and Colpidium (6.53%) (Figure 3F, Supplementary Table S7).
To unveil the associations driving these spatiotemporal patterns, we focused on characterizing the composition of soil ciliate communities. Specifically, we identified distinct ciliate communities associated with different seasons and environmental habitat types. Subsequently, we employed the LEfSe tool to identify biomarkers ranging from the phylum level down to the species level Figure 4. In the dry season, the ciliate biomarkers included Sporadotrichia, Oxytrichidae, and Spirotrichea. In the wet season, the biomarkers consisted of Loxodidae, Loxodes, Plagiocampa, Plagiocampidae, Operculariidae, Plagiocampa longis, Karyorelictea, and Protostomztida. Regarding habitat types, in shrubland, the ciliate biomarkers were Trachelophyllidaec and Colpodeac. In grassland, they included Litostomateas, Colpoda, Colpodida, Colpodidae, and Colpoda. In forestland, the biomarkers were Halteriidae, Halteria, Grandinellas, Lagyonphrya mucicola, Lagynophrya, Glaucomidae, and Glaucoma. In wetland, they consisted of Spirotrichea, Sporadotrichia, Haptorida, Oligohymenophorea, Hymenostomatida, and Oxytrichidae.
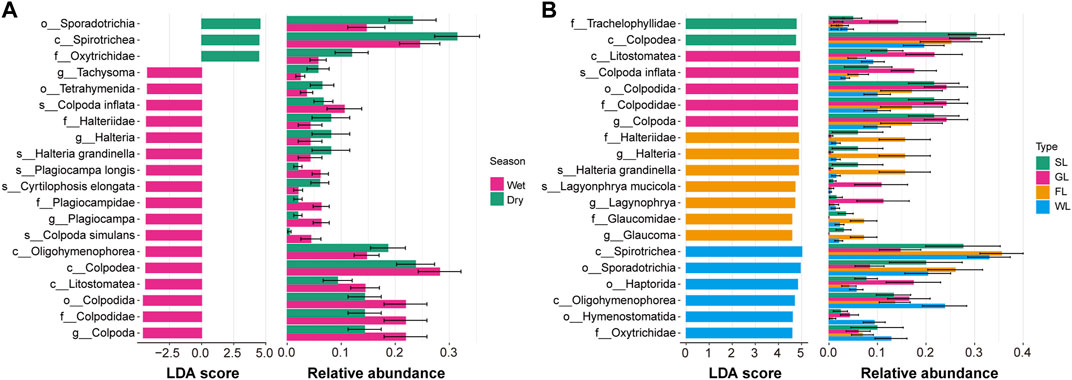
FIGURE 4. Indicator ciliate with LDA scores and relative abundance in soil ciliate communities associated with rhizosphere of different seasons (A) and habitat types (B). Notes: SL: Shrubland; GL: Grassland; FL: Forestland; WL: Wetland.
3.3 Linkages between environmental factors and ciliate composition and diversity
We analyzed the relationship between the alpha diversity of ciliate communities and environmental factors. The Shannon index showed a significant positive correlation with VC and exhibited an initial increase followed by a decrease in relation to SWC. The Pielou index displayed a positive correlation with TN and SWC. The Simpson index was positively correlated with VC and displayed an initial increase followed by a decrease in relation to SWC. These results suggest a restricted alpha diversity of ciliate communities in conditions of extremely high SWC levels. The Richness index showed a positive correlation with VC, TN, and SOM. These findings indicate that alpha diversity was significantly influenced by TN, SOM, VC, and SWC. Particularly, VC and SWC were identified as the most important factors affecting alpha diversity (Figure 5A). To explore the associations underlying these spatiotemporal patterns, we employed the Mantel test to determine which environmental parameters significantly correlated with the abundance of ciliate communities. Ciliate communities exhibited significant correlations with TK values in the wet season, and with SOM and SWC values in the dry season (Figure 5B). The analysis of different habitat types revealed significant correlations between ciliate communities and SOM in forestland and SWC in wetland. In summary, SWC, TK, and SOM were identified as the major factors influencing the turnover in the seasonal and environmental type composition of ciliate communities (Figure 5C).
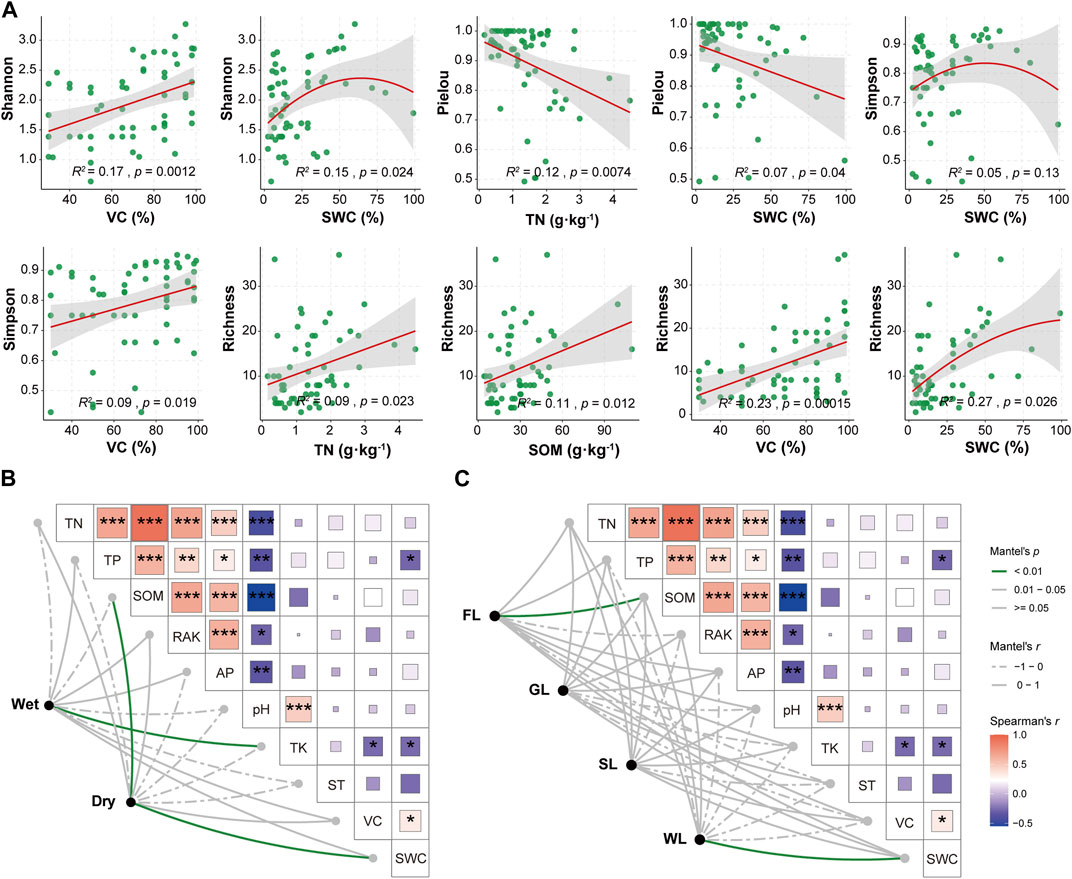
FIGURE 5. Relationship between soil ciliate communities and environmental factors. (A) Relationship between soil ciliate diversity and environmental factors. Environmental drivers of soil ciliate communities, as evaluated by Mantel tests in two seasons (B) and four habitat types (C). Notes: SL: Shrubland; GL: Grassland; FL: Forestland; WL: Wetland; TN: Total nitrogen; TP: Total phosphorus; SOM: Soil organic matter; RAK: Rapidly available potassium; AP: Available phosphorus; TK: Total potassium; ST: Soil temperature; VC: Vegetation coverage; SWC: Soil water content.
3.4 Co-occurrence network analysis of ciliate communities
Co-occurrence network analysis was performed to explore potential relationships between ciliate communities (Figure 6A). The co-occurrence network of ciliate communities followed different patterns on the basis of six network topological parameters (Figure 6B). Modularity coefficients for all co-occurrence networks were >0.4, indicating clear modularity. Different network metrics were found for all two seasons and four habitat types (Supplementary Table S3); particular differences were seen in node and edge numbers, indicating dynamic changes within soil ciliate communities. Analysis of the seasons showed that the co-occurrence network for wet season had the higher total number of edge and total number of nodes. This season also had the lower average path length, network diameter, and modularity than dry season. These results indicate that interactions within the ciliate communities are more complex in wet than in dry. Analysis of the four habitat types (Figure 6B) showed that the co-occurrence network for wetland and forestland types had the highest total number of nodes, edge, and average degree. This environmental type also had the lowest mean clustering coefficient. These results indicate that interactions within the ciliate community are more complex in wetland and forestland than in grassland and shrubland types.
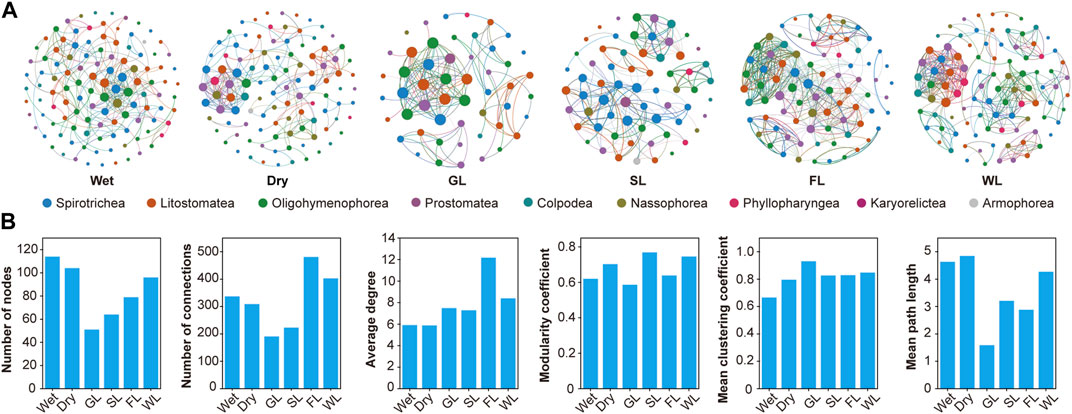
FIGURE 6. Co-occurrence network analysis. Co-occurrence ecological network of ciliates communities in middle of Yarlung Zangbo River (A), and network topological parameters (B). Notes: SL: Shrubland; GL: Grassland; FL: Forestland; WL: Wetland. The nodes in Figure 6A represent the different classes of ciliates.
4 Discussion
4.1 Comprehensive analysis of soil ciliate diversity in the Yarlung Zangbo River
Ciliates, as a major group of protists, play important roles in microorganism communities, exhibiting high diversity in various environments, including rivers, lakes, and soil ecosystems (Bahram et al., 2018; Oshima et al., 2020). In this study, we identified 138 soil ciliate species across two seasons and four habitat types in the middle reaches of the Yarlung Zangbo River. While previous research has reported on the biodiversity of protist in the river environment, there has been relatively limited research on soil ciliates in this region (Zhang et al., 2022; Yang Q. et al., 2023). To our knowledge, this is the first study to investigate protist diversity using morphological methods and to explore the diversity, composition, and seasonal and biogeographic dynamics in the Yarlung Zangbo River. Our findings revealed that soil ciliate diversity was slightly higher in the wet season compared to the dry season, although the differences were not statistically significant, this is consistent with previous studies on soil ciliates communities in the Gannan, which also found higher soil ciliates diversity in wet season than in dry season (Yang C. et al., 2023). The middle reaches of the Yarlung Zangbo River is an arid area with great seasonal temperature variation. Temperature is an important environmental factor regulating the biochemical processes of terrestrial ecosystems (Leavit, 1998). The accumulation and decomposition of litter, soil respiration and CO2 release, and the feeding activities and metabolism of soil animals are all regulated by soil temperature (Kardol et al., 2011; AlSayed et al., 2018). In the seasonal changes, more precipitation in wet season, abundant nutrients available to soil ciliates, frequent feeding activities of soil ciliates, and higher soil temperature in wet season lead to increased metabolic rate of soil ciliates and rapid reproduction, which may be the main reason for the higher diversity of soil ciliates community in wet season than in dry season (Reth et al., 2005). However, this seasonal pattern differs from the biodiversity of protists found in the aquatic environment of the middle reaches of the Yarlung Zangbo River, suggesting significant seasonal variations in the region’s biodiversity (Zhang et al., 2022; Yang Q. et al., 2023). Additionally, our research indicated that soil ciliate diversity varies significantly across different habitat types, with the highest diversity in wetlands, followed by farmland and forestland, and the lowest in grasslands. These findings align with the conclusions of previous studies. Furthermore, we observed substantial variations in soil ciliate diversity among sampling points, indicating the significant environmental variations in the middle reaches of the Yarlung Zangbo River, closely related to the large and dynamic environmental conditions in the region. Despite the relatively high diversity of soil ciliates in the middle reaches of the Yarlung Zangbo River, our use of morphological identification methods may inevitably underestimate their diversity (Li et al., 2021). In today’s era of rapid development in high-throughput sequencing technologies, we propose that future research should comprehensively explore the diversity of soil ciliates in the Yarlung Zangbo River’s middle reaches using environmental DNA (eDNA) techniques such as metagenomics and metabarcoding (Bello et al., 2018; Ke et al., 2022). This research will significantly contribute to our understanding of the biodiversity patterns and maintenance mechanisms of the largest river basin in the Tibetan Plateau—the Yarlung Zangbo River—and hold crucial importance in the context of global biodiversity studies.
4.2 Spatiotemporal patterns of soil ciliate in Yarlung Zangbo River
Ciliates communities form large and highly diverse groups of microbiotas in soil ecosystems (Watson, 1943; Gabilondo, 2018). Our study gives a clear picture of the spatiotemporal patterns of soil ciliates communities in the Yarlung Zangbo River. Significant fluctuations in community composition were found between wet and dry seasons. Similarly, seasonal differences in soil ciliates communities have been reported for other areas (Jousset et al., 2010; Li et al., 2010; Kumar and Foissner, 2016). During the wet season, Colpodea exhibits the highest relative abundance at the class level, whereas in the dry season, Spirotrichea dominates with the highest relative abundance at the class level. This finding aligns with the results observed in Yang et al. ‘s study conducted in the Gannan region (Yang C. et al., 2023). Additionally, we observed that species unique of ciliate community to the wet season outnumber those unique to the dry season, consistent with the findings in Kumar et al.'s research (Kumar and Foissner, 2016). This indicates a higher complexity in the ciliate community during the wet season. These seasonal patterns were also revealed by our analysis of co-occurrence networks. The wet network had the highest node number and network connectivity, indicating that the soil ciliates community is more complex in wet than in dry. Soil ciliates have a complex relationship with their living environment, and they can promote the circulation of nutrients in litter and soil system through their life activities (Schulz-Bohm et al., 2017; Fiore-Donno et al., 2019), especially in the circulation of soil nutrients, especially nitrogen and phosphorus (Adl and Gupta, 2006). In this study, soil TN in wet season was higher than that in dry season (Supplementary Figure S1A). Correlation analysis showed that soil ciliate community richness was significantly positively correlated with TN, while negatively correlated with Pielou index (Figure 5A). The greater the richness and the less the uniformity of the community, the higher the complexity and stability of the co-occurrence network, indicating that the stability of the soil ciliate community in the middle reaches of the Yarlung Zangbo River in the wet season is higher than that in the dry season, and the seasonal difference of TN is the main reason for this phenomenon. In diffident habitat types, significant differences exist in the composition of soil ciliate communities, potentially linked to ecological niche variations among different ciliate taxa in response to soil conditions. The research results of Zhang et al. showed that the species, quantity and biomass of soil ciliates were positively correlated with the content of soil organic matter (Zhang et al., 1999; Abraham et al., 2019). In this study, soil ciliate richness showed a significant positive correlation with SOM (Figure 5A), while SOM showed significant differences in different habitat types (Supplementary Figure S1B), indicating that SOM was a key factor affecting the community structure characteristics of soil ciliates in different habitats. We observed the least divergence in soil ciliate community composition between wetlands and shrubland habitats, while the most significant differences were found between grassland and wetland communities. This divergence may be associated with the heterogeneity of soil physicochemical properties across different habitat types (Bahram et al., 2018; Philippot et al., 2023). For instance, Plagiocampa longis are saprotrophic and exhibit a higher prevalence in wetland environments, whereas Colpoda inflata thrive in dry conditions, leading to a more abundant distribution in grassland habitats (Robinson et al., 2002; Li et al., 2005).
4.3 SWC is an important determinant of soil ciliate composition and diversity
Determining the relationship between soil ciliate composition and diversity and environmental factors is key to understanding the spatiotemporal distribution patterns of the soil ciliate community (Li et al., 2013; Shi et al., 2013; Gabilondo et al., 2015). The diversity of soil ciliate was significantly affected by several water environmental factors, including VC, SWC, SOM and TN. This finding is consistent with the results of previous studies, which showed that SWC is important ecological factor for structuring ciliates community diversity, this is consistent with previous findings in Li et al. and Robinson et al. (Robinson et al., 2002; Li et al., 2013). In addition, we found that SWC seems to limit the diversity of ciliates communities. This discrepancy may be attributed to the predominantly soil moisture conditions in the Yarlung Zangbo River area during the wet season. The combination of low precipitation and high evaporation leads to reduced soil moisture, consequently resulting in decreased diversity (Gui et al., 2023; Roy et al., 2023). This also elucidates the observed pattern of diversity, wherein soil ciliate diversity is highest in wetland habitats and lowest in grassland habitats. Soil water content is the main limiting factor for the survival, reproduction and distribution of soil protozoa (Zou et al., 2009). Most soil organisms live in soil pores and their activities depend on the availability of water (Lee and Foster, 1991; Lavelle and Spain, 2001). In this study, SWC was significantly different in different seasons. Due to more rainfall in the wet season, soil water content was higher, soil ciliates could obtain sufficient water for their own metabolism, and the reproductive ability of the community was enhanced, which may lead to the survival of some species limited by soil water content, and the diversity of the community increased accordingly. Among different habitat types, SWC of wetland was significantly higher than that of the other three types (Supplementary Figure S1B), and the Shannon diversity index, Simpson dominance index and richness index of soil ciliate community of wetland type were higher than those of the other three types (Figure 2B). SWC and soil ciliate abundance and diversity index were significantly positively correlated (Figure 5), indicating that SWC differences affected the spatial and temporal distribution characteristics of soil ciliates and shaped the diversity pattern of soil ciliate community in the middle reaches of Yarlung Zangbo River.
In summary, our study provides a comprehensive examination of the spatiotemporal dynamics of soil ciliates diversity and composition in the Yarlung Zangbo River, enhancing our understanding of the environmental adaptation of ciliates inhabiting soil habitats at the high altitude of the Tibetan Plateau.
5 Conclusion
In conclusion, our study firstly investigated the seasonal and biogeographic dynamics of the soil ciliates community in the Yarlung Zangbo River. The results show that soil ciliates communities and environmental factors exhibited significant seasonal and geographic variations. The alpha diversity of soil ciliates was highest in wet and declined in dry season. Moreover, alpha diversity was much higher in the wetland than in the grassland. The co-occurrence network analysis showed that soil ciliate community was more complex in wet season than in dry season, and the stability of soil ciliate community in wet season was higher than that in dry season. The stability of soil ciliate community in wetland was higher than that in forestland, shrubland and grassland, and the anti-interference ability was stronger. ST, TN, SOM and SWC are important factors affecting the structure of soil ciliate community. By influencing the metabolic rate and nutrient acquisition of soil ciliates, the distribution pattern of soil ciliate community diversity in the middle reaches of Yarlung Zangbo River is shaped. This study may be a valuable contribution to advance the understanding of the global biogeographic diversity of soil ciliates communities.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
QH: Data curation, Writing–original draft, Methodology. ML: Software, Writing–original draft. TL: Resources, Writing–review and editing. SZ: Project administration, Writing–review and editing. WZ: Formal Analysis, Project administration, Writing–review and editing. BP: Conceptualization, Data curation, Methodology, Project administration, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the 2021 Special Funds for the Basic Research and Development Program in the Central Nonprofit Research Institutes of China [Tibetan Finance, Science and Edu-cation Guidance (2021) No. 1].
Acknowledgments
We thank Jinlong Cui and Fuyuan Mai (Tibet University) for their assistance in sample collection. We thank Qing Yang and Peng Zhang (Tibet University) for their assistance in data analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2024.1360015/full#supplementary-material
References
Abraham, J. S., Sripoorna, S., Dagar, J., Jangra, S., Kumar, A., Yadav, K., et al. (2019). Soil ciliates of the Indian Delhi Region: their community characteristics with emphasis on their ecological implications as sensitive bio-indicators for soil quality. Saudi J. Biol. Sci. 26, 1305–1313. doi:10.1016/j.sjbs.2019.04.013
Acosta-Mercado, D., and Lynn, D. H. (2004). Soil ciliate species richness and abundance associated with the rhizosphere of different subtropical plant species. J. Eukaryot. Microbiol. 51, 582–588. doi:10.1111/j.1550-7408.2004.tb00295.x
Adl, M. S., and Gupta, V. S. (2006). Protists in soil ecology and forest nutrient cycling. Can. J. For. Res. 36, 1805–1817. doi:10.1139/x06-056
AlSayed, A., Fergala, A., and Eldyasti, A. (2018). Influence of biomass density and food to microorganisms ratio on the mixed culture type I methanotrophs enriched from activated sludge. J. Environ. Sci. 70, 87–96. doi:10.1016/j.jes.2017.11.017
Azam, F., Fenchel, T., Field, J., Gray, J., Meyer-Reil, L., and Thingstad, F. (1983). The ecological role of water-column microbes in the sea. Mar. Ecol. Prog. Ser. 10, 257–263. doi:10.3354/meps010257
Bahram, M., Hildebrand, F., Forslund, S. K., Anderson, J. L., Soudzilovskaia, N. A., Bodegom, P. M., et al. (2018). Structure and function of the global topsoil microbiome. Nature 560, 233–237. doi:10.1038/s41586-018-0386-6
Bamforth, S. S., Wall, D. H., and Virginia, R. A. (2005). Distribution and diversity of soil protozoa in the McMurdo Dry Valleys of Antarctica. Polar Biol. 28, 756–762. doi:10.1007/s00300-005-0006-4
Bello, M. G. D., Knight, R., Gilbert, J. A., and Blaser, M. J. (2018). Preserving microbial diversity. Science 362, 33–34. doi:10.1126/science.aau8816
Bonkowski, M. (2004). Protozoa and plant growth: the microbial loop in soil revisited. New Phytol. 162, 617–631. doi:10.1111/j.1469-8137.2004.01066.x
Bremner, J. M. (1960). Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 55, 11–33. doi:10.1017/S0021859600021572
Chen, X., Li, T., Lu, D., Cheng, L., Zhou, J., and Wang, H. (2020). Estimation of soil available potassium in Chinese agricultural fields using a modified sodium tetraphenyl boron method. Land Degrad. Dev. 31, 1737–1748. doi:10.1002/ldr.3535
Davidson, E. C. A., Belk, E., and Boone, R. D. (1998). Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob. Change Biol. 4, 217–227. doi:10.1046/j.1365-2486.1998.00128.x
Debastiani, C., Meira, B. R., Lansac-Tôha, F. M., Velho, L. F. M., and Lansac-Tôha, F. A. (2016). Protozoa ciliates community structure in urban streams and their environmental use as indicators. Braz. J. Biol. 76, 1043–1053. doi:10.1590/1519-6984.08615
Domonell, A., Brabender, M., Nitsche, F., Bonkowski, M., and Arndt, H. (2013). Community structure of cultivable protists in different grassland and forest soils of Thuringia. Pedobiologia 56, 1–7. doi:10.1016/j.pedobi.2012.07.001
Fiore-Donno, A. M., Richter-Heitmann, T., Degrune, F., Dumack, K., Regan, K. M., Marhan, S., et al. (2019). Functional traits and spatio-temporal structure of a major group of soil protists (rhizaria: cercozoa) in a temperate grassland. Front. Microbiol. 10, 1332. doi:10.3389/fmicb.2019.01332
Foissner, W. (1992). Estimating the species richness of soil protozoa using the non-flooded petri dish method. Netherlands: Springer.
Foissner, W. (1999). Soil protozoa as bioindicators: pros and cons, methods, diversity, representative examples. Agric. Ecosyst. Environ. 74, 95–112. doi:10.1016/s0167-8809(99)00032-8
Foissner, W. (2005). Two new “flagship” ciliates (Protozoa, Ciliophora) from Venezuela: sleighophrys pustulata and Luporinophrys micelae. Eur. J. Protistology 41, 99–117. doi:10.1016/j.ejop.2004.10.002
Gabilondo, R., Blanco, S., Fernández-Montiel, I., García, D., and Bécares, E. (2018). Ciliates as bioindicators of CO2 in soil. Ecol. Indic. 85, 1192–1203. doi:10.1016/j.ecolind.2017.11.060
Gabilondo, R., Fernández-Montiel, I., García-Barón, I., and Bécares, E. (2015). The effects of experimental increases in underground carbon dioxide on edaphic protozoan communities. Int. J. Greenh. Gas Control 41, 11–19. doi:10.1016/j.ijggc.2015.06.015
Geisen, S., Mitchell, E. A. D., Adl, S., Bonkowski, M., Dunthorn, M., Ekelund, F., et al. (2018). Soil protists: a fertile frontier in soil biology research. FEMS Microbiol. Rev. 42, 293–323. doi:10.1093/femsre/fuy006
Geisen, S., Tveit, A. T., Clark, I. M., Richter, A., Svenning, M. M., Bonkowski, M., et al. (2015). Metatranscriptomic census of active protists in soils. ISME J. 9, 2178–2190. doi:10.1038/ismej.2015.30
Gui, H., Breed, M., Li, Y., Xu, Q., Yang, J., Wanasinghe, D. N., et al. (2023). Continental-scale insights into the soil microbial co-occurrence networks of Australia and their environmental drivers. Soil Biol. Biochem. 186, 109177. doi:10.1016/j.soilbio.2023.109177
Heger, T. J., Straub, F., and Mitchell, E. A. D. (2012). Impact of farming practices on soil diatoms and testate amoebae: a pilot study in the DOK-trial at Therwil, Switzerland. Eur. J. Soil Biol. 49, 31–36. doi:10.1016/j.ejsobi.2011.08.007
Jousset, A., Lara, E., Nikolausz, M., Harms, H., and Chatzinotas, A. (2010). Application of the denaturing gradient gel electrophoresis (DGGE) technique as an efficient diagnostic tool for ciliate communities in soil. Sci. Total Environ. 408, 1221–1225. doi:10.1016/j.scitotenv.2009.09.056
Kardol, P., Reynolds, W. N., Norby, R. J., and Classen, A. T. (2011). Climate change effects on soil microarthropod abundance and community structure. Appl. Soil Ecol. 47, 37–44. doi:10.1016/j.apsoil.2010.11.001
Ke, S., Weiss, S. T., and Liu, Y.-Y. (2022). Dissecting the role of the human microbiome in COVID-19 via metagenome-assembled genomes. Nat. Commun. 13, 5235. doi:10.1038/s41467-022-32991-w
Kumar, S., and Foissner, W. (2016). High cryptic soil ciliate (Ciliophora, Hypotrichida) diversity in Australia. Eur. J. Protistology 53, 61–95. doi:10.1016/j.ejop.2015.10.001
Lavelle, P., and Spain, A. V. (2001). Soil organisms. Soil Ecol., 201–356. doi:10.1007/978-94-017-5279-4_3
Leavit, S. W. (1998). Biogeochemistry, an analysis of global change. Eos, Trans. Am. Geophys. Union 79, 20. doi:10.1029/98EO00015
Lee, K. E., and Foster, R. C. (1991). Soil fauna and soil structure. Soil Res. 29, 745–775. doi:10.1071/sr9910745
Li, F., Xing, Y., Li, J., Al-Rasheid, K. A. S., He, S., and Shao, C. (2013). Morphology, morphogenesis and small subunit rRNA gene sequence of a soil hypotrichous ciliate, P erisincirra paucicirrata (C iliophora, K ahliellidae), from the shoreline of the Y ellow river, north C hina. J. Eukaryot. Microbiol. 60, 247–256. doi:10.1111/jeu.12029
Li, F., Zhang, Y., Altermatt, F., and Zhang, X. (2021). Consideration of multitrophic biodiversity and ecosystem functions improves indices on river ecological status. Environ. Sci. Technol. 55, 16434–16444. doi:10.1021/acs.est.1c05899
Li, J., Liao, Q., Li, M., Zhang, J., Tam, N. F., and Xu, R. (2010). Community structure and biodiversity of soil ciliates at dongzhaigang mangrove forest in hainan island, China. Appl. Environ. Soil Sci. 2010, 1–8. doi:10.1155/2010/103819
Li, Q., Mayzlish, E., Shamir, I., Pen-Mouratov, S., Sternberg, M., and Steinberger, Y. (2005). Impact of grazing on soil biota in a Mediterranean grassland. Land Degrad. Dev. 16, 581–592. doi:10.1002/ldr.680
Li, S., Dong, G., Shen, J., Yang, P., Liu, X., Wang, Y., et al. (1999). Formation mechanism and development pattern of aeolian sand landform in Yarlung Zangbo River valley. Sci. China Ser. D-Earth Sci. 42, 272–284. doi:10.1007/BF02878964
Liu, H., Ning, Y., Yang, Y., Yang, H., Wang, L., Chen, L., et al. (2022). Use of ciliate communities for monitoring ecological restoration of grain for the green in north-western China. Soil Ecol. Lett. 4, 264–275. doi:10.1007/s42832-021-0105-3
Liu, W., Sun, F., Li, Y., Zhang, G., Sang, Y.-F., Lim, W. H., et al. (2018). Investigating water budget dynamics in 18 river basins across the Tibetan Plateau through multiple datasets. Hydrol. Earth Syst. Sci. 22, 351–371. doi:10.5194/hess-22-351-2018
Liu, Y.-X., Qin, Y., Chen, T., Lu, M., Qian, X., Guo, X., et al. (2021). A practical guide to amplicon and metagenomic analysis of microbiome data. Protein Cell 12, 315–330. doi:10.1007/s13238-020-00724-8
Ning, Y. Z., Yang, Y. Q., Dong, W. H., Zhang, H. R., and Ma, J. Y. (2018). Response of soil ciliate community to ecological restoration of different return patterns. Acta Ecol. Sin. 38, 3628–3638. doi:10.5846/stxb201710161852
Nóbrega, G. N., Ferreira, T. O., Artur, A. G., de Mendonça, E. S., Leão, R. A., Teixeira, A. S., et al. (2015). Evaluation of methods for quantifying organic carbon in mangrove soils from semi-arid region. J. Soils Sediments 15, 282–291. doi:10.1007/s11368-014-1019-9
Oshima, T., Shinohara, Y., Asakawa, S., and Murase, J. (2020). Susceptibility and resilience of the soil ciliate community to high temperatures. Soil Sci. Plant Nutr. 66, 870–877. doi:10.1080/00380768.2020.1819148
Philippot, L., Chenu, C., Kappler, A., Rillig, M. C., and Fierer, N. (2023). The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. doi:10.1038/s41579-023-00980-5
Reth, S., Reichstein, M., and Falge, E. (2005). The effect of soil water content, soil temperature, soil pH-value and the root mass on soil CO2 efflux – a modified model. Plant Soil 268, 21–33. doi:10.1007/s11104-005-0175-5
Robinson, B. S., Bamforth, S. S., and Dobson, P. J. (2002). Density and diversity of Protozoa in some arid Australian soils. J. Eukaryot. Microbiol. 49, 449–453. doi:10.1111/j.1550-7408.2002.tb00227.x
Roy, S., Karapurkar, J., Baidya, P., Jose, M., and Bagchi, S. (2023). Community composition, and not species richness, of microbes influences decomposer functional diversity in soil. Soil Biol. Biochem. 187, 109225. doi:10.1016/j.soilbio.2023.109225
Schulz-Bohm, K., Geisen, S., Wubs, E. R. J., Song, C., De Boer, W., and Garbeva, P. (2017). The prey’s scent – volatile organic compound mediated interactions between soil bacteria and their protist predators. ISME J. 11, 817–820. doi:10.1038/ismej.2016.144
Shi, X., Zhang, F., Lu, X., Wang, Z., Gong, T., Wang, G., et al. (2018). Spatiotemporal variations of suspended sediment transport in the upstream and midstream of the Yarlung Tsangpo River (the upper Brahmaputra), China. Earth Surf. Process. Landf. 43, 432–443. doi:10.1002/esp.4258
Shi, Y., Lu, Y., Meng, F., Guo, F., and Zheng, X. (2013). Occurrence of organic chlorinated pesticides and their ecological effects on soil protozoa in the agricultural soils of North Western Beijing, China. Ecotoxicol. Environ. Saf. 92, 123–128. doi:10.1016/j.ecoenv.2013.03.006
Thunjai, T., Boyd, C. E., and Dube, K. (2001). Poind soil pH measurement. J. World Aquac. Soc. 32, 141–152. doi:10.1111/j.1749-7345.2001.tb01089.x
Tian, P., Lu, H., Feng, W., Guan, Y., and Xue, Y. (2020). Large decrease in streamflow and sediment load of Qinghai–Tibetan Plateau driven by future climate change: a case study in Lhasa River Basin. CATENA 187, 104340. doi:10.1016/j.catena.2019.104340
Wu, Y., Fang, H., He, G., Huang, L., and Wang, J. (2021). Climate-driven changes in hydrological and hydrodynamic responses in the Yarlung Tsangpo River. J. Hydrology 598, 126267. doi:10.1016/j.jhydrol.2021.126267
Xiong, W., Jousset, A., Guo, S., Karlsson, I., Zhao, Q., Wu, H., et al. (2018). Soil protist communities form a dynamic hub in the soil microbiome. ISME J. 12, 634–638. doi:10.1038/ismej.2017.171
Xu, R., Zhang, M., Lin, H., Gao, P., Yang, Z., Wang, D., et al. (2022). Response of soil protozoa to acid mine drainage in a contaminated terrace. J. Hazard. Mater. 421, 126790. doi:10.1016/j.jhazmat.2021.126790
Xue, G., Liu, Q., Ren, X., and Han, Y. (2004). Determination of fifteen metal elements in Cynomorium songaricum by flame atomic absorption spectrophotometry (FAAS). Guang Pu Xue Yu Guang Pu Fen Xi 24, 1461–1463. doi:10.1016/j.saa.2004.03.004
Yang, C., Liu, M., and Wang, X. (2023a). Species-abundance distributions of soil ciliates on different aspects in alpine meadows of gannan, China. Eurasian Soil S. C. 56, S325–S336. doi:10.1134/S1064229323602044
Yang, Q., Zhang, P., Li, X., Yang, S., Chao, X., Liu, H., et al. (2023b). Distribution patterns and community assembly processes of eukaryotic microorganisms along an altitudinal gradient in the middle reaches of the Yarlung Zangbo River. Water Res. 239, 120047. doi:10.1016/j.watres.2023.120047
You, Q., Kang, S., Wu, Y., and Yan, Y. (2007). Climate change over the Yarlung Zangbo River Basin during 1961–2005. J. Geogr. Sci. 17, 409–420. doi:10.1007/s11442-007-0409-y
Zhang, G., Yao, T., Xie, H., Zhang, K., and Zhu, F. (2014). Lakes’ state and abundance across the Tibetan Plateau. Chin. Sci. Bull. 59, 3010–3021. doi:10.1007/s11434-014-0258-x
Zhang, P., Xiong, J., Qiao, N., An, R., Da, Z., Miao, W., et al. (2022). Spatiotemporal distribution of protists in the Yarlung Zangbo River, Tibetan plateau. Water Biol. Secur. 1, 100064. doi:10.1016/j.watbs.2022.100064
Zhang, X., Li, C., Yin, X., and Chen, P. (1999). Relation between soil animals and nutrients in the differently used forest lands. Chin. J. Appl. Environ. Biol. 5, 26–31. doi:10.3321/j.issn:1006-687X.1999.01.006
Zhang, Z., Hu, G., and Ni, J. (2013). Effects of topographical and edaphic factors on the distribution of plant communities in two subtropical karst forests, southwestern China. J. Mt. Sci. 10, 95–104. doi:10.1007/s11629-013-2429-7
Zheng, W., Wang, C., Yan, Y., Gao, F., Doak, T. G., and Song, W. (2018). Insights into an extensively fragmented eukaryotic genome: de novo genome sequencing of the multinuclear ciliate uroleptopsis citrina. Genome Biol. Evol. 10, 883–894. doi:10.1093/gbe/evy055
Keywords: protozoa, soil ciliates, community diversity, soil ecology, Yarlung Zangbo River
Citation: Huang Q, Li M, Li T, Zhu S, Wang Z and Pu B (2024) Spatiotemporal distribution patterns of soil ciliate communities in the middle reaches of the Yarlung Zangbo River. Front. Environ. Sci. 12:1360015. doi: 10.3389/fenvs.2024.1360015
Received: 22 December 2023; Accepted: 27 February 2024;
Published: 06 March 2024.
Edited by:
Yan Xing Dou, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Baofeng Chai, Shanxi University, ChinaPan Wan, Northwest A&F University, China
Yang Hu, Xinjiang Agricultural University, China
Copyright © 2024 Huang, Li, Li, Zhu, Wang and Pu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bu Pu, purbu@utibet.edu.cn
 Qian Huang
Qian Huang  Zhuangzhuang Wang
Zhuangzhuang Wang Bu Pu
Bu Pu