The descriptive epidemiology of pre-omicron SARS-CoV-2 breakthrough infections and severe outcomes in Manitoba, Canada
- 1Department of Community Health Sciences, University of Manitoba, Winnipeg, MB, Canada
- 2Department of Medical Microbiology and Infectious Diseases, University of Manitoba, Winnipeg, MB, Canada
- 3Department of Sociology and Criminology, University of Manitoba, Winnipeg, MB, Canada
- 4Winnipeg Regional Health Authority, Winnipeg, MB, Canada
- 5Manitoba Health, Winnipeg, MB, Canada
- 6Cadham Provincial Laboratory, Winnipeg, MB, Canada
Introduction: Vaccination plays a key role in curbing severe outcomes resulting from COVID-19 disease. With the Omicron variant and the relaxing of public health protections breakthrough infections are increasingly common, and certain groups remain at higher risk for severe outcomes from breakthrough infections. We analysed population-based public health data from Manitoba, Canada to understand characteristics of those experiencing breakthrough infections and severe outcomes from breakthrough infections. Data from previous pandemic stages can provide valuable information regarding severe outcomes associated with breakthrough infection in the Omicron and future phases.
Methods: Positive SARS-CoV-2 PCR tests from Cadham Provincial Laboratory were linked to case information from the population-based Public Health Information Management System. A retrospective design was used with time-to-event analyses to examine severe outcomes among those experiencing breakthrough infection.
Results: Breakthrough cases were more likely to have 2 + chronic conditions, compared to age-, sex-, and time-period matched unvaccinated cases (24% vs. 17%), with hypertension (30%), diabetes (17%), and asthma (14%) being the most prevalent chronic conditions amongst breakthrough cases. Severe outcomes resulting from breakthrough infection was associated with age and chronic conditions, with those with 2 + chronic conditions at higher risk of severe outcomes (adjusted hazard ratio: 3.6, 95% confidence intervals: 2.0-6.4). Risk of severe outcomes varied by age group, with those 70 + years at over 13 times the risk of severe outcomes (95% CI: 4.5-39.8), compared to those 18-29 years of age.
Discussion: Our results demonstrate the impact of chronic conditions on the likelihood of, and severity of outcomes from breakthrough infections. These findings underscore the importance of vaccination programs prioritizing vulnerable populations.
Introduction
The speed and scale at which SARS-CoV-2, the virus that causes COVID-19 disease, has impacted citizens globally has been staggering (1), with over half a billion reported infections, and deaths numbering in the millions as of July 2022 (2). Although COVID-19 vaccines have successfully curbed COVID-19 morbidity and mortality, vaccines by themselves have proven to be necessary, but insufficient to eliminate the deleterious impacts of COVID-19 (3). This is especially true in light of waning vaccine effectiveness (4), and the challenges associated with the immune-evading Omicron variant (5, 6, 7). Omicron has rendered breakthrough infections (i.e., infections that occur after a full course of COVID-19 vaccinations) the rule, rather than the exception (7, 8). At the same time, evidence remains of the effectiveness of booster doses in mitigating severe outcomes associated with COVID-19 (9). As most public health protections have been lifted across Canada, the main public policy approach has been through continued vaccination coverage and provision of boosters to targeted segments of the population, with eligibility criteria and uptake heterogeneous across jurisdictions. Moreover, with changing epidemiology and approaches to testing, public health authorities across Canada have been challenged to diagnose and track SARS-CoV-2 infections, limiting knowledge about those most susceptible to breakthrough infections. Although breakthrough infections are associated with reduced risk of severe outcomes, including hospitalization and death (10, 11), a significant proportion of breakthrough cases experience severe outcomes (7, 12–15), necessitating a need to identify those at risk for severe outcomes. In most jurisdictions, populations who are most vulnerable to severe outcomes from COVID-19 have been prioritized in vaccination campaigns, including seniors, and those with comorbid conditions. An important consideration for healthcare planning are understanding the characteristics of vaccinated individuals who experience breakthrough infections. Identification of high-risk populations for breakthrough infections could inform earlier mitigation strategies, such as targeting of public health recommendations, boosters, and therapeutics (12). Thus, although the world is in the Omicron phase of the pandemic, there is much to learn from previous waves.
The province of Manitoba has been one of the hardest struck Canadian provinces (16). At the end of July 2022, Manitoba's cumulative mortality rate, at 149/100,000 population, was second only to the province of Quebec (185/100,000 population) (17), although heterogeneity in how COVID-19 mortality is captured may be contributing to observed regional differences (18). Understanding who is most at risk for breakthrough infections, and how they differ from those who are unvaccinated, as well as characterizing those breakthrough cases most impacted by severe outcomes, can help guide prevention policy, especially in light of more nuanced public health responses being needed as the pandemic enters its third year (19). The main objective of this study was to describe the characteristics of those experiencing pre-Omicron SARS-CoV-2 breakthrough infections, with an emphasis on the association of chronic conditions and breakthrough infections. A secondary objective was to examine the correlates of hospitalizations and ICU admissions among a cohort of Manitobans experiencing breakthrough infections during this period.
Materials and methods
Setting & data sources
In 2021, Manitoba had a population of 1.4 million, with approximately 60% (n = 791,284) of Manitoba's population residing in the city of Winnipeg. Demographic and clinical information from case and contact investigations on all diagnosed COVID-19 cases in Manitoba are maintained in the provincial Public Health Information Management System (PHIMS). Prior to the Omicron wave in December 2022, trained public health nurses were responsible for investigations of all confirmed COVID-19 cases in Manitoba. Vaccination information on all Manitobans is also maintained in PHIMS. Positive SARS-CoV-2 PCR test results from Cadham Provincial Laboratory (CPL) were linked to case information through personal health identification numbers. Prevalent chronic conditions were determined through validated algorithms of the Canadian Chronic Disease Surveillance System (CCDSS), using administrative health records maintained by Manitoba Health, and data linkage performed by Manitoba Health analysts (20). Hospitalizations, ICU admissions, and deaths (including dates) were defined through case investigations. For the purposes of these analyses only information on PCR-positive cases was available.
Analyses
A retrospective cohort design was used for analyses. Cases were considered fully vaccinated if their records indicated having at least two vaccination doses. Epidemiological date was defined as the earliest date of symptom onset or specimen collection date of the laboratory test. Breakthrough infections were defined as fully vaccinated individuals who had a PCR-positive test result >14 days (as measured by epi-date) after their last vaccination dose. Any cases with a COVID-19 infection prior to the study period (i.e., before January 1, 2021) were excluded, as were any cases under the age of 18 at epi-date. Only cases who had an epi-date prior to December 1st, 2021 were included, to address the arrival of the Omicron variant in Manitoba. In addition to summary statistics of the characteristics of COVID-19 cases who had breakthrough infections, we matched breakthrough cases to contemporaneous unvaccinated cases (i.e., cases with no history of vaccination recorded), based on epi-date (within +/− 7 days of the breakthrough case's epi-date), age group, and sex, at a 1:4 ratio. Odds ratios and their 95% confidence intervals (95% CI) from conditional logistic regression models are reported. Chronic conditions captured in the CCDSS included asthma, chronic obstructive pulmonary disease (COPD), diabetes, epilepsy, heart failure, hypertension, ischemic heart disease (IHD), multiple sclerosis, myocardial infarction, osteoarthritis, Parkinson's disease (PD), and stroke.
For the second objective, time-to-event analyses were used to examine the correlates of severe outcomes from breakthrough infections. All reported breakthrough infection cases in Manitoba with an epi-date between January 1 and November 30, 2021 were included. The outcome measure was defined as any evidence of hospitalization or ICU admission up to December 31, 2021, which was determined through case investigation by public health nurses. Only the date of first admission was recorded; subsequent readmissions were not captured, and thus, individuals could only appear once in the analytical databases. Mortality was not examined separately, as mortality outside of hospital settings was not included in our dataset. Epi-date of infection and admission date was used to record time between events. To address nosocomial COVID-19 infections, all individuals with an epi-date after their hospital admission were excluded, as were individuals who were admitted the same day of their epi-date. Sex, age group, dose interval (days between 1st and 2nd doses), RHA, and number of chronic conditions were included in bivariate and multivariable Cox regression models. Crude and adjusted hazard ratios (AHR) and their 95% CI are reported. Proportional hazards assumptions were tested using log-log plots and tests of Schoenfeld residuals. No violation of the proportional hazards assumption was detected. Lastly, the weekly incidence of breakthrough infections was plotted against all other incident infections during the study period. For all analyses, individuals who only had the Johnson & Johnson vaccine (n = 89) were excluded from the analyses. All analyses were performed at CPL using Stata V17 (College Station, TX). Ethics approval was waived by the Human Research Ethics Board at the University of Manitoba, as this was a secondary analysis of routinely-collected public health data.
Results
A total of 3,807 breakthrough infections in adults 18 years and older were reported between January 1st, 2021 and November 30, 2021. Of these, four were excluded because their epi-dates occurred before their first vaccine dose date. Thus, 3,803 individuals with breakthrough infections were included (Table 1). Approximately 55% of reported breakthrough infections were females, and 66% occurring in those <55 years of age; of note, 47% of breakthrough infections occurred in the month of November alone. Compared to age group, sex, and time-matched unvaccinated cases, breakthrough cases were more likely to have 2 + chronic conditions (24% vs. 17%; OR: 2.0, 95% CI: 1.7–2.2). Hypertension, diabetes, and asthma, at 30%, 17%, and 14%, respectively, were the most common chronic conditions reported. Compared to contemporaneous age- and sex-matched unvaccinated cases, breakthrough cases were at 1.5 (95% CI: 1.3–1.6), 1.7 (95% CI: 1.6–1.9), and 1.2 (95% CI: 1.1–1.3) times the odds of having a diagnosis of hypertension, diabetes, and asthma, respectively. Figure 1 is a spider plot showing the prevalence of chronic conditions by the number of chronic conditions (1, 2, 3, and 4+) diagnosed in adult breakthrough cases. Regardless of how many chronic conditions were diagnosed, hypertension was the most prevalent condition reported. Of those with four or more chronic conditions, hypertension was present in 100% of breakthrough cases, IHD in 92%, and diabetes in 90%. The median time from the second dose to breakthrough infection was 131, with an IQR of 94–163 days. Supplementary Figure S1 contains a histogram of the days to infection for those who were fully vaccinated, diagnosed prior to the Omicron period, and were a breakthrough case.

Table 1. Selected characteristics, breakthrough infections in fully vaccinated cases compared to contemporaneous matched unvaccinated cases in Manitoba (January 1–November 30, 2021), and odds ratios (ORs) and 95% confidence intervals (95% CI) from conditional logistic regression models (N = 15,216)a.
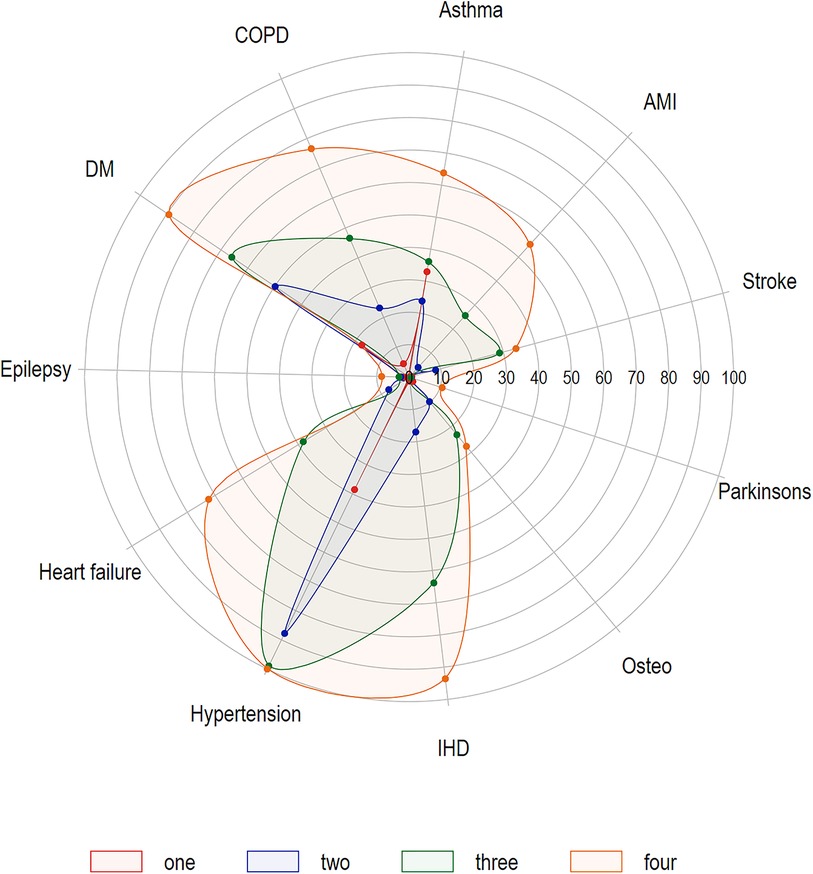
Figure 1. Spider plot of chronic conditions prevalence (%), by number of chronic conditions (one, two, three, four plus), fully-vaccinated adults (18 + years) experiencing SARS-CoV-2 breakthrough infections in Manitoba, January 1–November 30, 2021 (N = 3,803)*.
For Aim 2, and from 3,803 individuals with breakthrough infections, 6.9% experienced a severe outcome (n = 264). Of these 264 individuals, 98 had an epi-date after their hospital admission. These 98 individuals were excluded from analyses, leaving a total of 166 individuals who experienced a severe outcome. Thus, 3,706 individuals were retained in Cox regression models examining correlates of severe outcomes (Table 2). Of these, median time of severe outcomes was 3.5 days (IQR: 1–8 days), while the median age of the breakthrough infection cohort was 45 years (IQR: 33–61), with those experiencing severe outcomes (median: 70, IQR: 59–83) older than those who did not (median: 44, IQR: 33–60). Correspondingly, 52% of the severe outcome group were 70 years or older, compared to 13% of those not experiencing severe outcomes. In fully-adjusted models, no violation of the proportional hazards assumption was detected. Fully-adjusted models did not detect any statistically significant differences due to interval dose, and showed those with 2 + chronic conditions were more likely to experience severe outcomes, compared to those with no recorded chronic condition (AHR: 3.6, 95% CI: 2.0–6.4). Age group was significantly associated with the risk of severe outcomes; relative to those 18–29 years of age, risk for severe outcomes was almost 6-fold higher for those 60–69 years of age (AHR: 5.8, 95% CI: 1.9–17.6), and over 13-fold (95% CI: 4.5–39.8) for those 70 + years of age. Table 3 shows cause-specific AHRs for each chronic condition and the risk of severe outcomes; those with a diagnosis of heart failure (AHR: 3.3, 95% CI: 2.2–4.9) and hypertension (AHR: 2.4, 95% CI: 1.5–3.8) were at the highest risk for severe outcomes.

Table 2. Unadjusted and adjusted hazard ratios (U/AHRs) and 95% confidence intervals (95% CI) from Cox regression models examining determinants of hospitalizations/ICU admissions amongst breakthrough infection cases in Manitoba, January 1–November 30, 2021 (N = 3,706).

Table 3. Adjusted hazard ratios (AHRsa) and 95% confidence intervals (95% CI) from separate Cox regression models, association between specific chronic conditions and hospitalizations/ICU admissions among breakthrough infection cases in Manitoba, January 1–November 30, 2021 (N = 3,706).
Figure 2 shows the weekly summary of reported COVID-19 cases in Manitoba from January 1 to November 30, 2021, based on epi-date. Figures are stratified by number of chronic conditions, and by breakthrough status. Although the majority of infections were from those with no recorded chronic diseases, breakthrough infections were associated with chronic disease status, being especially prevalent in those with 2 + chronic conditions. For example (data not shown), in weeks 45–48, there were a total of 3,316 reported COVID-19 infections among those with no chronic conditions recorded, of which 44% (n = 1,458) were breakthrough infections. During this same time period, there were 567 infections among those with one recorded chronic condition, of which 56% (n = 317) were breakthrough infections; a total of 422 infections were seen amongst those with 2 + chronic conditions, with 67% (n = 282) being breakthrough infections.
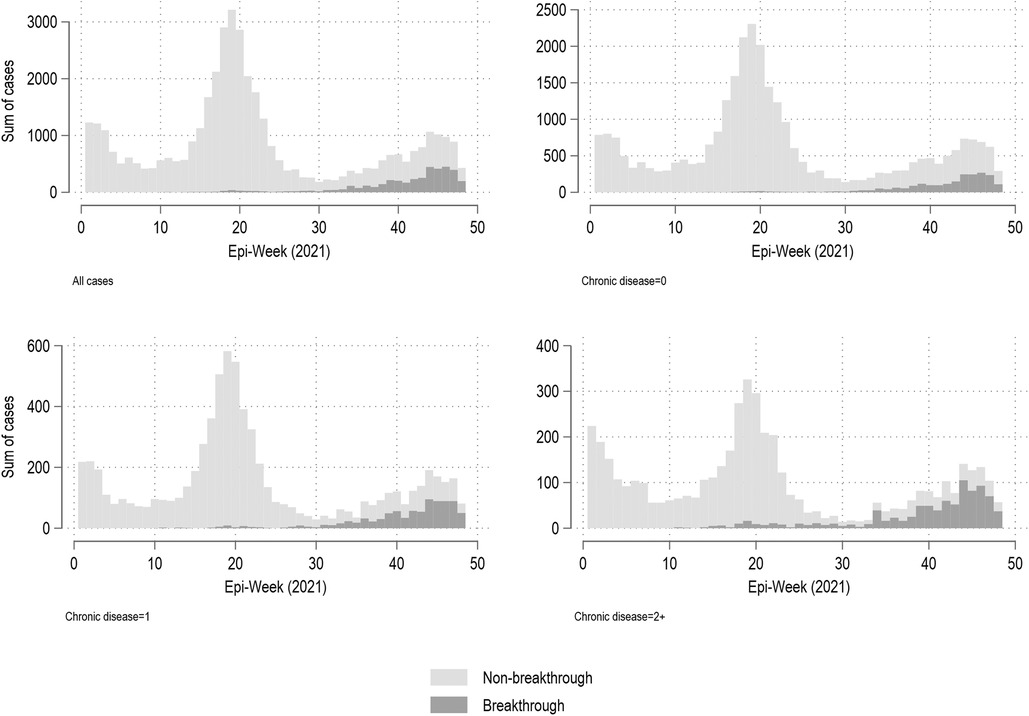
Figure 2. Weekly summary of all reported COVID-19 cases, by breakthrough and chronic disease status, Manitoba (January 1, 2021–November 30, 2021)*. *Note different y-axes based on chronic disease status.
Discussion
Comorbid conditions are a known risk factor for severe outcomes from COVID-19 generally (21), as well as specifically for breakthrough infections (12, 22, 23). At 41% for diabetes, 73% for hypertension, 27% for COPD, and 28% for IHD, chronic disease prevalence among breakthrough cases experiencing severe outcomes in our study mirror what has been published in the literature (15, 22–25). Diabetes prevalence in hospitalized breakthrough cases has ranged from 28% to 48% (15, 22–25), hypertension 64%–71% (22–24), 24% for COPD (24), and 21% for IHD (23). From a policy level, older age groups and those with chronic conditions were prioritized in early vaccination campaigns in Manitoba; our results demonstrate that this prioritization was justified, as evidenced by the high level of chronic conditions in our breakthrough cohort, the higher likelihood of this group to be older and to have chronic conditions, compared to contemporaneous unvaccinated cases, and by the association between chronic conditions and severe outcomes demonstrated in our analyses. Not unexpectedly, a large degree of clustering of chronic conditions was observed, with approximately one out of every four of breakthrough cases having two or more chronic conditions, with the most common chronic conditions being hypertension, ischemic heart disease, and diabetes.
A combination of factors contribute to risk for and from breakthrough infections, including viral evolution, host determinants, immunity characteristics, and vaccination properties (12). Combining epidemiological data with information on immunological profiles would be a valuable future direction to further elucidate risk (26). Future work should also include examination of the determinants of breakthrough infections in the Omicron, and post-Omicron eras, as well as the mediating/moderating role that race/ethnicity may have on vulnerability to breakthrough infections and severe outcomes. As has been established in many settings, COVID-19 has disproportionately impacted Black, Indigenous and people of colour communities (27, 28); in Manitoba Indigenous communities were made a public health priority (16). Our study had a number of strengths, including the availability of population-based data sources, as well as having public health data linked to administrative healthcare databases in order to produce profiles of chronic conditions in an objective manner. Our study also had some limitations. First, we only had data on positive cases of SARS-CoV-2, and could not compare characteristics of breakthrough cases to those vaccinated and did not experience breakthrough infections. Second, the study relied on passive surveillance reporting, and thus the possibility of under-detection of cases amongst vaccinated and unvaccinated individuals exists. However, the reporting of COVID-19 cases was a priority for public health, with significant resources invested in case and contact investigations; however, stigma associated with a COVID-19 infection acting as a barrier for reporting cannot be discounted. It is important to consider as well that in a “post-Omicron” world, loss of public interest in the pandemic and the ubiquity of at-home testing, combined with less public health follow-up of cases (and their contacts) has now obscured the epidemiology of the pandemic. Third, although administrative healthcare algorithms used to define chronic conditions have been validated, the possibility for misclassification remains. However, the prevalence of key chronic conditions in our study, like diabetes and hypertension, was similar to other work. Fourth, we did not include any variables to explore the impact of race and ethnicity. Fifth, our study only captured pre-Omicron infections; however, despite the dynamic immunological landscape, in terms of the impact of previous infection from different variants on population-level immunity, in combination with timing, and dosing of COVID-19 vaccines, and the emergence of new variants, there is still strong evidence that booster shots can reduce risk from severe outcomes from COVID-19 (29). Incidentally, Although we have categorized the study period as pre vs. post-Omicron, it should be noted that a number of variants of concern circulated in Manitoba since the arrival of the wild-type virus, including the Alpha, Beta, and Gamma variants. Notably, the Delta variant arrive in Manitoba in the spring of 2021 and became the most dominant variant by the summer; this variant was responsible for Manitoba's intensive care units being overrun in the spring and summer of 2021. Finally, the association between age and breakthrough infections may be confounded by older adults being more likely to test if symptomatic.
Conclusion
In this retrospective study, we demonstrated the high prevalence of chronic conditions and SARS-CoV-2 breakthrough infections and the risk of severe outcomes related to those infections. Future public health policy should continue to prioritize protection of those most vulnerable to COVID-19 disease.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: data are not available as they cannot be shared outside of the Government of Manitoba. Requests to access these datasets should be directed to Souradet Y. Shaw, souradet.shaw@umanitoba.ca.
Ethics statement
The studies involving humans were approved by Human Research Ethics Board of University of Manitoba. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin because this was a retrospective study of anonymized public health surveillance data.
Author contributions
Designed the study: SYS, JK, LM, DRS. Helped manage and analyze the data: SYS, YJW, DRS. Wrote and edited the article: SYS, JK, LM, JCSB, JNR, DRS. Provided critical review and scientific feedback: CL, YJW, JB, PVC. All authors contributed to the article and approved the submitted version.
Funding
SS is funded by a Canada Research Chair in Program Science and Global Public Health (Tier II, Grant #950-232822). DS is funded by The Canadian COVID-19 Immunity Task Force (Grant #324806) and Canadian Institutes of Health Research Operating Grant (#179430).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) LM, JK declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fepid.2023.1248847/full#supplementary-material
References
1. Koelle K, Martin MA, Antia R, Lopman B, Dean NE. The changing epidemiology of SARS-CoV-2. Science. (2022) 375:1116–21. doi: 10.1126/science.abm4915
2. World Health Organization. WHO coronavirus (COVID-19) dashboard. (2022). Available at: https://covid19.who.int/. (Accessed May 17, 2022, 2022).
3. Webb Hooper M, Nápoles AM, Pérez-Stable EJ. No populations left behind: vaccine hesitancy and equitable diffusion of effective COVID-19 vaccines. J Gen Intern Med. (2021) 36:2130–3. doi: 10.1007/s11606-021-06698-5
4. Andrews N, Tessier E, Stowe J, et al. Duration of protection against mild and severe disease by COVID-19 vaccines. N Engl J Med. (2022) 386:340–50. doi: 10.1056/NEJMoa2115481
5. Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID-19. Science. (2022) 375:1122–7. doi: 10.1126/science.abm8108
6. Gupta RK, Topol EJ. COVID-19 vaccine breakthrough infections. Science. (2021) 374:1561–2. doi: 10.1126/science.abl8487
7. Del Rio C, Omer SB, Malani PN. Winter of omicron-the evolving COVID-19 pandemic. JAMA. (2022) 327:319–20. doi: 10.1001/jama.2021.24315
8. Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and omicron variant predominance—VISION network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:255–63. doi: 10.15585/mmwr.mm7107e2
9. Menni C, May A, Polidori L, et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID study. Lancet Infect Dis. (2022).35405090
10. Lee CJ, Woo W, Kim AY, et al. Clinical manifestations of COVID-19 breakthrough infections: a systematic review and meta-analysis. J Med Virol. (2022) 94:4234–45. doi: 10.1002/jmv.27871
11. Lee JE, Hwang M, Kim YH, et al. Imaging and clinical features of COVID-19 breakthrough infections: a multicenter study. Radiology. (2022) 303:682–92. doi: 10.1148/radiol.213072
12. Amanatidou E, Gkiouliava A, Pella E, et al. Breakthrough infections after COVID-19 vaccination: insights, perspectives and challenges. Metabol Open. (2022) 14:100180. doi: 10.1016/j.metop.2022.100180
13. Butt AA, Nafady-Hego H, Chemaitelly H, et al. Outcomes among patients with breakthrough SARS-CoV-2 infection after vaccination. Int J Infect Dis. (2021) 110:353–8. doi: 10.1016/j.ijid.2021.08.008
14. Leshem E, Nelson K, Lopman BA. Severe breakthrough COVID-19 infections in Scotland-implications for immunisation programmes. Lancet Respir Med. (2021) 9:1354–6. doi: 10.1016/S2213-2600(21)00413-6
15. Wang SY, Juthani PV, Borges KA, et al. Severe breakthrough COVID-19 cases in the SARS-CoV-2 delta (B.1.617.2) variant era. Lancet Microbe. (2022) 3:e4–5. doi: 10.1016/S2666-5247(21)00306-2
16. Government of Manitoba. COVID-19 novel coronavirus: race, ethnicity, indigeneity (REI) analysis wave three. Winnipeg, MB: Manitoba Health & Seniors Care (2021). Available at: https://www.gov.mb.ca/asset_library/en/proactive/20212022/covid19-rei-data-july2021.pdf (Accessed August 20, 2021)
17. Government of Canada. Coronvirus disease (COVID-19): outbreak update. (2021). Available at: https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection.html (Accessed August 20, 2021).
18. Berry I, O’Neill M, Sturrock SL, et al. A sub-national real-time epidemiological and vaccination database for the COVID-19 pandemic in Canada. Sci Data. (2021) 8:173. doi: 10.1038/s41597-021-00955-2
19. Razak F, Shin S, Naylor CD, Slutsky AS. Canada’s response to the initial 2 years of the COVID-19 pandemic: a comparison with peer countries. CMAJ. (2022) 194:E870–7. doi: 10.1503/cmaj.220316
20. Lix LM, Ayles J, Bartholomew S, et al. The Canadian chronic disease surveillance system: a model for collaborative surveillance. Int J Popul Data Sci. (2018) 3:433.32935015
21. Joseph M, Wu Y, Dannebaum R, et al. Global patterns of antigen receptor repertoire disruption across adaptive immune compartments in COVID-19. Proc Natl Acad Sci U S A. (2022) 119:e2201541119. doi: 10.1073/pnas.2201541119
22. Bosch W, Cowart JB, Bhakta S, et al. COVID-19 vaccine-breakthrough infections requiring hospitalization in Mayo Clinic Florida through August 2021. Clin Infect Dis. (2021).
23. Brosh-Nissimov T, Orenbuch-Harroch E, Chowers M, et al. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin Microbiol Infect. (2021).34245907
24. Suleyman G, Fadel R, Brar I, et al. Risk factors associated with hospitalization and death in COVID-19 breakthrough infections. Open Forum Infect Dis. (2022) 9:ofac116. doi: 10.1093/ofid/ofac116
25. Hippisley-Cox J, Coupland CA, Mehta N, et al. Risk prediction of COVID-19 related death and hospital admission in adults after COVID-19 vaccination: national prospective cohort study. Br Med J. (2021) 374:n2244. doi: 10.1136/bmj.n2244
26. Sanghavi DK, Bhakta S, Wadei HM, et al. Low antispike antibody levels correlate with poor outcomes in COVID-19 breakthrough hospitalizations. J Intern Med. (2022) 292:127–35. doi: 10.1111/joim.13471
27. Xia Y, Ma H, Moloney G, et al. Geographic concentration of SARS-CoV-2 cases by social determinants of health in metropolitan areas in Canada: a cross-sectional study. CMAJ. (2022) 194:E195–204. doi: 10.1503/cmaj.211249
28. Garcia MA, Homan PA, Garcia C, Brown TH. The color of COVID-19: structural racism and the disproportionate impact of the pandemic on older black and latinx adults. J Gerontol B Psychol Sci Soc Sci. (2021) 76:e75–80. doi: 10.1093/geronb/gbaa114
Keywords: COVID-19, breakthrough infection, severe outcomes, chronic conditions, vaccination
Citation: Shaw SY, Kindrachuk J, McKinnon L, Biegun JCS, Reimer JN, Loeppky C, Wei YJ, Bullard J, Van Caeseele P and Stein DR (2024) The descriptive epidemiology of pre-omicron SARS-CoV-2 breakthrough infections and severe outcomes in Manitoba, Canada. Front. Epidemiol. 3:1248847. doi: 10.3389/fepid.2023.1248847
Received: 27 June 2023; Accepted: 12 December 2023;
Published: 12 January 2024.
Edited by:
Shailendra Saxena King George’s Medical University, IndiaReviewed by:
Luca Coppeta, University of Rome Tor Vergata, ItalyGregory S. Orf, Abbott, United States
© 2024 Shaw, Kindrachuk, McKinnon, Biegun, Reimer, Loeppky, Wei, Bullard, Van Caeseele and Stein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Souradet Y. Shaw souradet.shaw@umanitoba.ca
 Souradet Y. Shaw
Souradet Y. Shaw Jason Kindrachuk
Jason Kindrachuk Lyle McKinnon
Lyle McKinnon Jeffery C. S. Biegun
Jeffery C. S. Biegun Jocelyn N. Reimer4
Jocelyn N. Reimer4  Jared Bullard
Jared Bullard