Male chicks play more than females – sex differences in chicken play ontogeny
- AVIAN Behavior Genomics and Physiology Group, Division of Biology, Department of Physics Chemistry and Biology, Linköping University, Linköping, Sweden
Play indicates positive affective states and can therefore potentially be used as an indicator of positive welfare. Sex differences in play has been reported in many mammalian species, but in birds, this is still to be explored. It is known that young chickens perform play behavior during their early ontogeny, but potential sex differences have not previously been addressed. Therefore, we aimed to investigate potential sex effects on play ontogeny in young chickens, by comparing play occurrence in young males and females of a commercial hybrid of White Leghorn. Eighteen chicks of each sex were hatched in the same incubator and then housed in sex-separated groups. Six groups of three chicks each were randomly created for each sex, and the same three chicks were then moved to enriched play arenas twice per week, from day 6 until day 53 post hatch. The frequency of different play behaviors, categorized as locomotor play, social play and object play were recorded during 30 min on each observation day. Each group of three birds constituted the independent statistical replicate. Males played significantly more than females, due to more social and object play, whereas for locomotor play, no difference was found between the sexes. In conclusion, clear sex differences in play in chickens was demonstrated, and this may be linked to the highly sexually dimorphic behavior of adult cockerels and hens. Further research is needed to elucidate the relationships between type and frequency of play in chicks and later behavior as adults.
1 Introduction
Play behavior is a widespread phenomenon among animals, particularly in mammals and birds (Smaldino et al., 2019). Despite this, many questions related to, for instance, its origin, evolution, and purpose are still unanswered. Furthermore, to describe and define play has proven to be a great challenge (Held, 2017). The challenge is two-fold: (1) to cover the huge behavioral variation that the term “play” entails; (2) to keep it separate from descriptions of other types of behaviors (Bekoff and Allen, 2011). In the absence of a widely accepted definition, it has been suggested that behaviors should meet five criteria to be considered play (Burghardt, 2005): (1) the behavior appears to lack immediate function in the occurring context; (2) it is rewarding and/or pleasurable to the performer; (3) it diverges in structure or temporal organization from functional expression of behavior; (4) it is performed repeatedly throughout the ontogeny in a non-stereotypic nature; (5) it occurs mainly when the animal is in good health and free from stress. Three main categories of play are often identified, i.e., locomotor play, social play, and object play (Held and Špinka, 2011). However, to fully separate them can be challenging, since play behaviors often entail elements of more than one of the categories.
One factor that can potentially affect how and how much an animal plays is its sex. In an extensive systematic review, Marley et al. (2022) noted sex differences in rough and tumble play (RTP, a type of social play) across non-human mammals. Males largely perform more RTP than females, but in many mammalian species, no sex differences have been found, and in rare cases, females play more than males. The authors emphasize how the methodology, i.e. test conditions and the way play is measured (duration of play bouts, total time spent playing, play initiations), affects the outcome. For rats and hamsters, findings regarding sex differences in social play (RTP) varies, and testing conditions seem to have a considerable impact (Cooper et al., 2023). In rats, differences have been found in the ontogeny and structure of social play (Pellis, 2002; Paul et al., 2014). Furthermore, male rats have been found to play socially more than females (Olioff and Stewart, 1978; Meaney et al., 1983). However, this was not supported by Northcutt and Nwankwo (2018) who tested rats of different strains in two conditions: with a cage mate and with an unfamiliar sibling. No sex differences were found in any strain when cage mates were tested together, but for one out of three strains, when tested with an unfamiliar sibling, females played more than males. In hamsters (Mesocricetus auratus), males have been found to play more than females (Vieira et al., 2005), while other studies found no sex differences (Guerra et al., 1992). In tufted capuchin monkeys (Cebus apella) and white‐faced capuchins (Cebus capucinus), males have been found to engage more in social play than females, however, for solitary play (object and locomotor), no sex differences were found (tufted capuchin monkeys: Paukner and Suomi, 2008; white‐faced capuchins: Winkler and Perry, 2022). In lowland gorillas, no sex differences were found in type of play, nonetheless, play partner preferences were observed (Brown, 1988). Males played fairly equal with other males and females, while females played more with males than other females.
Sex difference in play frequency and structure may be particularly pronounced in species showing a large sexual dimorphism. Winkler and Perry (2022) suggest that the sex with greater fitness benefits from social bonding would have a higher play frequency, or that the sex with greater need for skills in physical agility and tactics should play more. The chicken is therefore an interesting species to study in this respect. Domesticated chickens descend from the Red Junglefowl (Wang et al., 2020), and findings suggests that domestication occurred around 8000 years ago (West and Zhoub, 1988). The Red Junglefowl is native to South and South-East Asia (Tixier-Boichard et al., 2011; Wang et al., 2020). They are social birds, typically living in groups of several females and males, where females, most often, outnumber the males (Collias and Collias, 1996). Red Junglefowl is a highly sexually dimorphic species, where males are richly colored and ornamented, and considerably larger than females (Desta, 2019). A high male–male competition over mating and intense sexual selection has likely contributed to the sexual dimorphism in the species.
We have previously shown that although domesticated chicks play in the same way and with the same ontogenetic development as ancestral Red Junglefowl, the total amount of play behaviors is larger in the domesticates (Lundén et al., 2022). However, in that study, sex differences were not included as a factor. Other studies have been conducted on broilers, with an animal welfare perspective. Environmental enrichment has been found to either decrease or not affect the amount of play performed by broiler chickens (Baxter et al., 2019; Vasdal et al., 2019; Liu et al., 2020), but although both sexes were included in all these studies, sex differences were not investigated. Moreover, how different rearing treatments affects play (among other factors), has also been studied in female laying hen chicks (Campbell et al., 2022). Rearing treatment was not found to affect play behavior, but since only females were included, sex differences were not addressed. To the best of our knowledge, no systematic comparison of sex differences in play behavior has been conducted on any avian species.
The aim of this project was to study sex differences in chicken play ontogeny. Considering the large sexual dimorphism of the species, we predicted that there should be sex differences in type of play as well as frequency of play behavior.
2 Materials and methods
2.1 Animals
The aim of the experiment was to describe sex differences in the ontogeny of play. The birds used were commercial White Leghorn (WL) chicks (n = 36, 18 males and 18 females) of the layer hybrid Lohmann LSL-LITE. As it was crucial to know the sex of the chicks straight before composing experimental groups, males and females of a commercial laying hen hybrid were used, since it is possible to wing-sex them right after hatch.
The eggs were retrieved from a commercial hatchery in Sweden, and thereafter incubated and hatched at Linköping University. The incubation occurred in darkness, in the same small incubator (Masalles 25-I HLC) with settings; 38.5°C, 65% relative humidity and hourly rotation. After hatch, the chicks were vaccinated, wing tagged, and feather sexed through the appearance of the wing coverts and primaries.
2.2 Housing
The birds were housed in groups of 6 individuals throughout the experimental period, where males and females were kept separate. The pens were solid floor cages (W×L×H: 0.685×0.51×0.52 m) and were provided with sawdust, a heat-roof, and ad lib access to food and water. At four weeks of age, the heat-roofs were exchanged for perches.
2.3 Experimental set-up and procedure
As the chicks were introduced to their home pens, random groups of three birds were created within each cage, using unique codes of colored leg rings. Each of these groups then constituted the test unit and were always tested together. The chicks were tested at the following days of age: 6, 8, 11, 15, 18, 22, 25, 29, 32, 36, 39, 43, 46, 50 and 53. Previous studies have shown that access to more space and objects to play with can stimulate play in chicks (Baxter et al., 2019; Liu et al., 2020; Lundén et al., 2022). Therefore, and because we wanted to use the same method as in a previous study (Lundén et al., 2022), the tests were conducted in fully enclosed, sound insulated arenas considerably larger than the home pens (L×W×H: 1.17×0.8×1 m), allowing to eliminate visual stimuli and most sound from outside. Each arena had permanently mounted overhead video cameras and lights that were remotely operated. The arenas were set up with sawdust, a small pile of hay along one long end, a perch along one short end, and a chain hanging from the ceiling. The home pens and the test arenas were in the same lab room.
For each of the test sessions, the chicks were gently caught and put in enclosed transport boxes, and then placed in the arenas within five minutes of catching. The lights were kept off until the recording started within two minutes of introducing the chicks to the arenas. Four identical arenas were used, allowing four groups to be tested at the same time. In total, three test rounds per observation day were necessary, as there were 12 groups in total. Recordings were started simultaneously in all four arenas. Each test session was 30 min. To stimulate play further, 10 min into the test, a fake rubber worm was inserted through a small opening with a lid, ensuring that the birds could only see the hand of the person adding the object. For the first three test days, the fake worm measured 2×60 mm, and this was then exchanged with a larger one (3×165 mm) used for the rest of the experimental period. Furthermore, twenty minutes into the test, a black rubber strip measuring 5×95 mm for the first three tests, and thereafter 10×115 mm, was added to the arena via a small opening in the opposite corner to the first one used.
For a complete ethogram and video footage of different play behaviors, see the Supplementary Table S1 and the Supplementary Videos S3–S5 in Lundén et al. (2022). From the videos, the occurrence of 12 play behaviors was scored (same as in Lundén et al., 2022, minus spinning and worm running, which were not observed in the present study), and subsequently, these were grouped into the three categories: locomotor play, social play and object play. Furthermore, we summed all occurrences of play into the overall category “total play”. Locomotor play included running, frolicking, wing flapping, and spinning while wing flapping. Object play included object running, object chasing, object exchange and worm (object) pecking. Social play included sparring jumping with or without contact, sparring stand-off with or without contact.
2.4 Sampling and data analysis
Throughout the 30 min, the behaviors were recorded in 15 s segments. During each time segment, 1/0 sampling was used for each chick, i.e., for every segment of 15 s, it was recorded how many of the three individuals that performed each behavior at least one time. Accordingly, in every segment, each behavior got a score from 0 to 3, and for the entire 30 min test, the total value of each behavior could vary from 0 (implying that the behavior was never observed) to 360 (implying that all three birds performed the behavior at least once during all 15 s segments). All videos were scored by one and the same observer (the first author).
Each group of three birds constituted an independent statistical replicate. We used Generalized Linear Mixed Models with a repeated measures design to analyze the effects of age, sex, and their interactions. The interactions were not significant for any of the four play categories (total play, locomotor play, social play and object play), and were therefore removed and the model re-run with only the main factors. The model was fitted for negative binomial distribution with log-link function since there was high variance in the data. Additionally, we performed independent samples t-tests to compare the overall difference in frequency of play between the sexes. The data are presented as mean number of observations per group with standard error of the mean. The statistical analyses were computed in SPSS 29.0.0.0.
3 Results
All 12 play behaviors were observed in both sexes. The frequency of total play (all play behaviors summed) showed a significant change with age in both sexes, with a steady increase until a peak at 43 days in males and 36 days in females, thereafter, followed by a gradual decrease (Figure 1A; effects of age: F14, 164 = 13.250, P < 0.001). Males showed significantly more total play than females (Figure 1A (line graph); effects of sex: F1, 164 = 121.719, P < 0.001), also when all ages were considered simultaneously (Figure 1A (boxplot); t(6.613) = 3.608, p = 0.010).
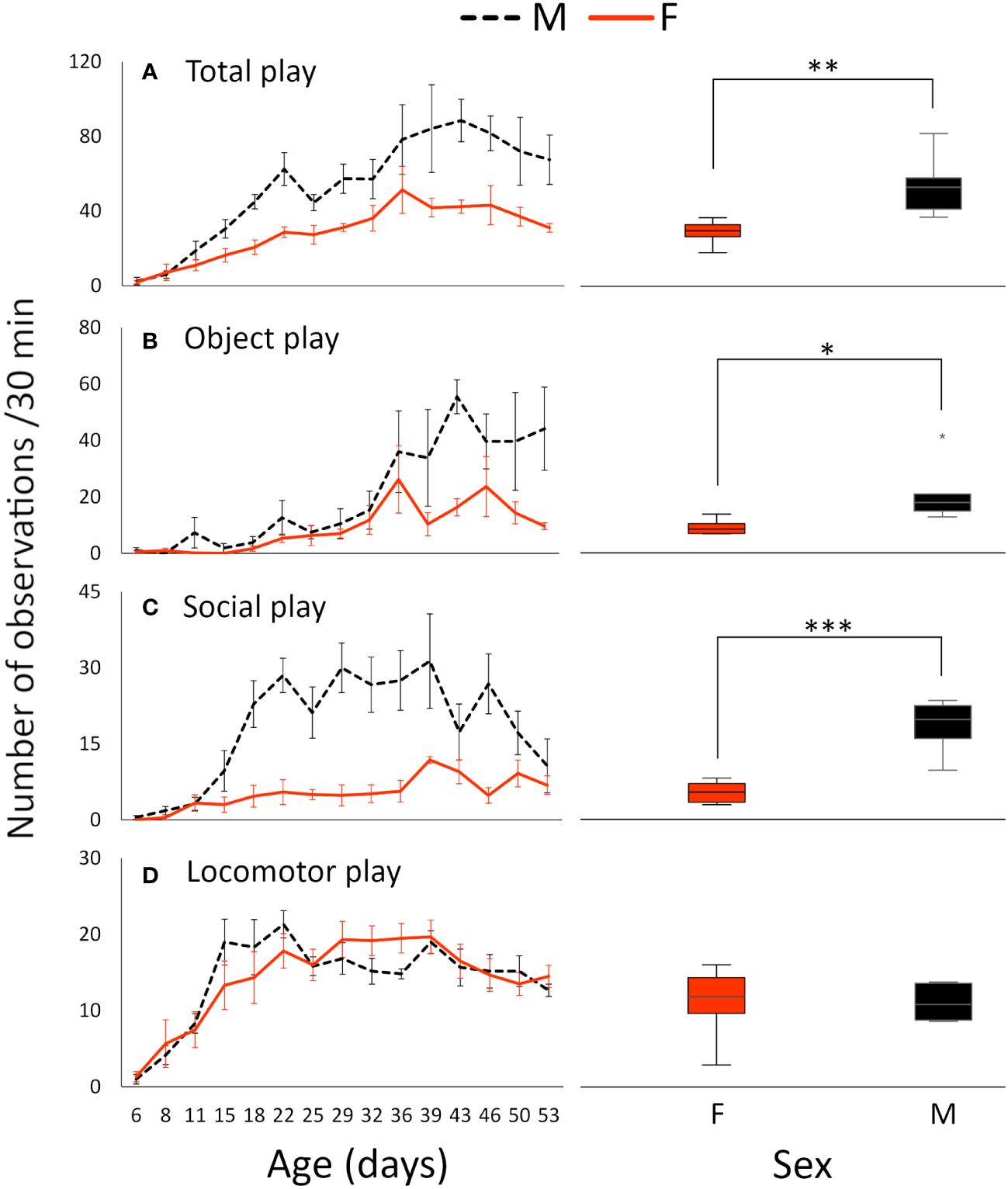
Figure 1 Mean number of observations ( ± standard error) per group per 30 min of (A) total play, (B) object play, (C) social play, and (D) locomotor play at different ages (line graphs), and overall, regardless of age (boxplots), in male and female White Leghorn chicks. Note that the scales differ between the graphs. * ≤ 0.05; ** ≤ 0.01; *** ≤ 0.001.
Object play was the most common of the subtypes (Figure 1B), followed by social play (Figure 1C) and locomotor play (Figure 1D). Object and social play were more frequent in males (Object play (line graph): F1, 164 = 54.167, P < 0.001, (boxplot); t(5.665) = 2.689, p = 0.038; social play (line graph): F1, 164 = 144.030, P < 0.001, (boxplot); t(6.533) = 5.796, p < 0.001), whereas there was no difference in the frequency of locomotor play ((line graph); F1, 164 = 1.375, P = 0.243, (boxplot); t(7.205) = −0.013, p = 0.990). All subcategories showed significant age effects (object play: F14, 164 = 10.207, P < 0.001; locomotor play: F14, 164 = 7.785, P < 0.001; social play: F14, 164 = 8.239, P < 0.001).
4 Discussion
This study aimed to investigate sex differences in chicken play ontogeny. No qualitative differences in play behavior were found between the sexes, but males played significantly more than females in total, caused by more social and object play, while the frequency of locomotor play did not differ. As expected, based on our previous results (Lundén et al., 2022), the total play frequency, as well as all three sub-categories of play, showed a significant change with age in both sexes. This was due to the observed increase, peak and decrease of play frequency with increasing age, that is characteristic for play behavior during early ontogeny.
The results support our hypothesis that males would engage more in play than females, which may be due to the greater fitness benefit from practicing various skills related to physical ability and social tactics in males. In the ancestors, roosters and hens have clearly different social roles within the groups, where males are competing for pairings, are more vigilant and provide food for females (Pizzari, 2003). The males are also victims to predation to a much larger degree than females, probably because of their conspicuous appearance and behavior (Collias and Collias, 1996). Assuming that one function of play is to prepare animals for future challenges, it would be reasonable for natural selection to favor more play in the sex where the fitness variance is greater and depending on social and physical skills. As suggested by Kruijt (1964), worm-running (the second most prevalent type of object play) may be the ontogenetic precursor of tidbitting, a series of courtship behaviors performed by males and directed toward females, which is in line with this reasoning. However, it is noteworthy that we found no sex differences in the amount of locomotory play, suggesting that this may be less related to any sexually dimorphic aspects of functions in relating to behavior in adults. When testing this hypothesis in future research, it would be important to relate fitness related variation in adult traits, such as social and physical differences, to the previously performed amount and type of play on an individual level.
In a small study in young spotted hyenas (Crocuta crocuta), females were found to play more frequently than males, but the social context during testing affected which type of play was elevated (Pedersen et al., 1990). In same-sex groups, females engaged considerably more in social play, while in mixed-sex groups, no difference in social play occurred. Compared to chickens and most mammals, the sexual dimorphism is reversed in spotted hyenas, and females are known to display more territory defense, aggression, and social dominance (McCormick et al., 2022). This strengthens our speculations regarding sex differences in play behavior being linked to sexual dimorphism. However, even though one would predict sex differences in play in any species where there is a large sexual dimorphism in the adults, Marley et al. (2022) found less consistency across species regarding male bias in social play than expected. The authors argue that this could indicate that the reason for the male bias often found in social play is not as straightforward as previously thought, but furthermore conclude that factors such as methodology, environment and sample size are likely to impact the outcome, and that these aspects should be considered carefully in future comparative studies. However, since there is no generally agreed common function of play behavior (Zosh et al., 2018), it is also possible that any observed sex differences are in fact caused by other factors than the one suggested above. For example, the sex differences found in the present study could have been influenced by differences in fearfulness. Female chickens, domesticated and non-domesticated, have been reported as more fearful than male chickens (Campler et al., 2009), and since play mainly occurs in the absence of stress, this could have influenced how the chicks behaved in the play arenas. Hence, we cannot exclude that the lower overall frequency of play observed in the females was related to them being more fearful.
In previous studies of play in chickens, for different reasons, sex differences have not been investigated (Baxter et al., 2019; Vasdal et al., 2019; Liu et al., 2020; Campbell et al., 2022; Lundén et al., 2022). Our results suggest that, when studying play in chickens, sex should be considered wherever possible. Furthermore, except for our previous comparison of domesticated and non-domesticated birds (Lundén et al., 2022), breed differences in play behavior have not yet been studied in chickens. Considering that sex differences in play seem to vary with strain in rats (Northcutt and Nwankwo, 2018), it is reasonable to explore possible breed differences in chickens. It should also be noted that we have used a commercial egg-laying hybrid for the present experiment, which has been heavily selected mainly for female traits (egg production). It is possible that this has affected the sex differences observed, which further calls for including different breeds in future studies. For a more comprehensive view of how sex differences in play are expressed, mixed-sex groups should also be studied.
To our knowledge, this is the first study to examine the occurrence of sex differences in chicken play ontogeny. We found clear sex differences, where males performed play behaviors more than females, due to higher frequencies of social and object play. We suggest that these differences may be related to the high sexual dimorphism in adult chickens.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Linköping Committee for Ethical Approval of Animal Experiments. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
RO: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. PJ: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding was obtained through a grant from the Swedish Research Council, grant no. 2019-04869 and faculty support from Linköping University, both to PJ.
Acknowledgments
We thank our technician, Austeja Rutkauskaite, for valuable assistance throughout the experimental period.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fetho.2024.1392378/full#supplementary-material
References
Baxter M., Bailie C. L., O’Connell N. E. (2019). Play behaviour, fear responses and activity levels in commercial broiler chickens provided with preferred environmental enrichments. Animal 13, 171–179. doi: 10.1017/S1751731118001118
Bekoff M., Allen C. (2011). “Intentional communication and social play: How and why animals negotiate and agree to play,” in Readings in zoosemiotics. Berlin, Boston, MA: De Gruyter Mouton.
Brown S. G. (1988). Play behaviour in lowland gorillas: age differences, sex differences, and possible functions*. Primates 29, 219–228. doi: 10.1007/BF02381123
Burghardt G. M. (2005). The genesis of animal play: testing the limits (Cambridge, MA: MIT Press). doi: 10.7551/mitpress/3229.001.0001
Campbell D. L. M., Belson S., Dyall T. R., Lea J. M., Lee C. (2022). Impacts of rearing enrichments on pullets’ and free-range hens’ Positive behaviors across the flock cycle. Animals 12 (3), 280. doi: 10.3390/ani12030280
Campler M., Jongren M., Jensen P. (2009). Fearfulness in red junglefowl and domesticated White Leghorn chickens. Behav. Process. 81, 39–43. doi: 10.1016/j.beproc.2008.12.018
Collias N. E., Collias E. C. (1996). Social organization of a red junglefowl, Gallus gallus, population related to evolution theory. Anim. Behav. 51, 1337–1354. doi: 10.1006/anbe.1996.0137
Cooper M. A., Grizzell J. A., Whitten C. J., Burghardt G. M. (2023). Comparing the ontogeny, neurobiology, and function of social play in hamsters and rats. Neurosci. Biobehav. Rev. 147, 105102. doi: 10.1016/j.neubiorev.2023.105102
Desta T. T. (2019). Phenotypic characteristic of junglefowl and chicken. World’s Poult. Sci. J. 75, 69–82. doi: 10.1017/S0043933918000752
Guerra R. F., Vieira M. L., Takase E., Gasparetto S. (1992). Sex differences in the play fighting activity of golden hamster infants. Physiol. Behav. 52, 1–5. doi: 10.1016/0031-9384(92)90424-Z
Held S. D. E., Špinka M. (2011). Animal play and animal welfare. Anim. Behav. 81, 891–899. doi: 10.1016/j.anbehav.2011.01.007
Held S. D. E. (2017). Play behaviour. In P. Jensen (Ed.), The ethology of domestic animals: an introductory text. Wallingford: CABI.
Kruijt J. P. (1964). Ontogeny of social behaviour in Burmese red jungle fowl (Gallus gallus spadiceus). Behaviour 12, 1–201. doi: 10.1163/9789004631205
Liu Z., Torrey S., Newberry R. C., Widowski T. (2020). Play behaviour reduced by environmental enrichment in fast-growing broiler chickens. Appl. Anim. Behav. Sci. 232. doi: 10.1016/j.applanim.2020.105098
Lundén G., Oscarsson R., Hedlund L., Gjøen J., Jensen P. (2022). Play ontogeny in young chickens is affected by domestication and early stress. Sci. Rep. 12, 13576. doi: 10.1038/s41598-022-17617-x
Marley C. L., Pollard T. M., Barton R. A., Street S. E. (2022). A systematic review of sex differences in rough and tumble play across non-human mammals. Behav. Ecol. Sociobiol. 76 (12). doi: 10.1007/s00265-022-03260-z
McCormick S. K., Holekamp K. E., Smale L., Weldele M. L., Glickman S. E., Place N. J. (2022). Sex differences in spotted hyenas. Cold Spring Harb. Perspect. Biol. doi: 10.1101/cshperspect.a039180
Meaney M. J., Stewart J., Poulin P., McEwen B. S. (1983). Sexual differentiation of social play in rat pups is mediated by the neonatal androgen-receptor system. Neuroendocrinology 37, 85–90. doi: 10.1159/000123524
Northcutt K. V., Nwankwo V. C. (2018). Sex differences in juvenile play behavior differ among rat strains. Dev. Psychobiol. 60, 903–912. doi: 10.1002/dev.21760
Olioff M., Stewart J. (1978). Sex differences in the play behavior of prepubescent rats. Physiol. Behav. 20, 113–115. doi: 10.1016/0031-9384(78)90060-4
Paukner A., Suomi S. J. (2008). Sex differences in play behavior in juvenile tufted capuchin monkeys (Cebus apella). Primates 49, 288–291. doi: 10.1007/s10329-008-0095-0
Paul M. J., Terranova J. I., Probst C. K., Murray E. K., Ismail N. I., de Vries G. J. (2014). Sexually dimorphic role for vasopressin in the development of social play. Front. Behav. Neurosci. 8. doi: 10.3389/fnbeh.2014.00058
Pedersen J. M., Glickman S. E., Frank L. G., Beach F. A. (1990). Sex differences in the play behavior of immature spotted hyenas, crocuta crocuta. Horm. Behav 24, 403–20. doi: 10.1016/0018-506X(90)90018-S
Pellis S. M. (2002). Sex differences in play fighting revisited: traditional and nontraditional mechanisms of sexual differentiation in rats. Arch. Sex Behav. 31, 17–26. doi: 10.1023/A:1014070916047
Pizzari T. (2003). Food, vigilance, and sperm: the role of male direct benefits in the evolution of female preference in a polygamous bird. Behav. Ecol. 14, 593–601. doi: 10.1093/beheco/arg048
Smaldino P. E., Palagi E., Burghardt G. M., Pellis S. M., Quinn J. (2019). The evolution of two types of play. Behav. Ecol. 30, 1388–1397. doi: 10.1093/beheco/arz090
Tixier-Boichard M., Bed’hom B., Rognon X. (2011). Chicken domestication: from archeology to genomics. C. R. Biol. 334, 197–204. doi: 10.1016/j.crvi.2010.12.012
Vasdal G., Vas J., Newberry R. C., Moe R. O. (2019). Effects of environmental enrichment on activity and lameness in commercial broiler production. J. Appl. Anim. Welf. Sci. 22, 197–205. doi: 10.1080/10888705.2018.1456339
Vieira M. L., Garcia M. P., Rau D. D., Prado A. B. (2005). Effects of different opportunities for social interaction on the play fighting behavior in male and female golden hamsters (Mesocricetus auratus). Dev. Psychobiol. 47, 345–353. doi: 10.1002/dev.20101
Wang M. S., Thakur M., Peng M. S., Jiang Y., Frantz L. A. F., Li M., et al. (2020). 863 genomes reveal the origin and domestication of chicken. Cell Res. 30, 693–701. doi: 10.1038/s41422-020-0349-y
West B., Zhoub B.-X. (1988). Did chickens go north? New evidence for domestication. J. Archaeol. Sci. 15, 515–533. doi: 10.1016/0305-4403(88)90080-5
Winkler S. L., Perry S. E. (2022). The development of sex differences in play in wild white-faced capuchins (Cebus capucinus). Am. J. Primatol. 84, e23434. doi: 10.1002/ajp.23434
Keywords: play behavior, sex differences, sexual dimorphism, ontogeny, chickens
Citation: Oscarsson R and Jensen P (2024) Male chicks play more than females – sex differences in chicken play ontogeny. Front. Ethol. 3:1392378. doi: 10.3389/fetho.2024.1392378
Received: 27 February 2024; Accepted: 25 March 2024;
Published: 02 May 2024.
Edited by:
Daniela Alberghina, University of Messina, ItalyReviewed by:
Dana L. M. Campbell, Commonwealth Scientific and Industrial Research Organisation (CSIRO), AustraliaAubrey Kelly, Emory University, United States
Copyright © 2024 Oscarsson and Jensen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Per Jensen, per.jensen@liu.se
 Rebecca Oscarsson
Rebecca Oscarsson  Per Jensen
Per Jensen