Understanding Sex Differences in Form and Function of Bird Song: The Importance of Studying Song Learning Processes
- Behavioural Biology, Institute of Biology, Leiden University, Leiden, Netherlands
Birdsong is a culturally transmitted mating signal. Due to historical and geographical biases, song (learning) has been predominantly studied in the temperate zones, where female song is rare. Consequently, mechanisms and function of song learning have been almost exclusively studied in male birds and under the premise that inter- and intra-sexual selection favored larger repertoires and complex songs in males. However, female song is not rare outside the temperate zones and song in both sexes probably is the ancestral state in songbirds. Some song dimorphisms seen today might therefore be manifestations of secondary losses of female song. What selection pressures have favored such losses and other sexual dimorphisms in song? Combined mapping of phylogenetic and ecological correlates of sex differences in song structure and function might provide important clues to the evolution of male and female song. This requires parameterization of the degree of sexual dimorphism. Simple comparison of male-female song might not provide enough resolution, because the same magnitude of difference (e.g., repertoire overlap) could result from different processes: the sexes could differ in how well they learn (“copying fidelity”) or from whom they learn (“model selection”). Different learning mechanisms might provide important pointers toward different selection pressures. Investigating sex-specific learning could therefore help to identify the social and ecological selection pressures contributing to sex differences in adult song. The study of female song learning in particular could be crucial to our understanding of (i) song function in males and females and (ii) the evolution of sex-specific song.
Both Sexes of Songbirds Sing and Learn Their Songs
What is in a name? Song in songbirds (oscines) is so ubiquitous and conspicuous that the songbirds, the most speciouse avian clade (comprising almost half of the ~10,000 extant species), were named after it. Song is typically learned early in life from conspecifics—what and how well young birds learn greatly affects the efficacy of their signals as adults (Catchpole and Slater, 2008; Lachlan et al., 2014; Peters et al., 2014). Song is currently the best-studied and probably most widely accepted animal example of a culturally transmitted mating signal (Slater and Ince, 1979; Mundinger, 1982; Podos and Warren, 2007; Riebel et al., 2015).
In most species song functions both as an armament and as an ornament, serving as a keep-away signal to same-sex competitors in the context of resource defense and as a signal to attract and stimulate mates for breeding (Catchpole and Slater, 2008). These functions tally with the canonical male sex role (Andersson, 1994): males can gain fitness by increased investment into sexual signaling to maximize the number of potential partners and exclude competitors. Male song indeed fulfils both functions (Kroodsma and Byers, 1991).
Social learning (typically from conspecifics) is crucial for the development of fully functional song. Ever since Thorpe's seminal studies on chaffinch song learning in the 1950s kick-started modern birdsong research by introducing spectrographic analyses (Slater, 2003; Riebel et al., 2015), the study of the function of birdsong and vocal learning have gone hand-in-hand. However, this inadvertedly became a tale of male song learning only (Riebel, 2003; Riebel et al., 2005): due to historical and geographic research biases, songbirds were studied for many years predominantly in the Passerida of the temperate zones of Europe and North America, where female song is rare (Morton, 1996; Riebel et al., 2005; Odom et al., 2014). This led to the description of birdsong as a predominantly male trait, despite earlier reports of abundant female song in other biogeographic regions (Robinson, 1949; Morton, 1996). Only since the late 1990's has the mounting evidence of female song in other regions and clades resulted in a revision of this view: female song is now understood to be phylogenetically and geographically widespread (Robinson, 1949; Morton, 1996; Langmore, 1998; Riebel, 2003; Hall, 2004; Slater and Mann, 2004; Riebel et al., 2005; Garamszegi et al., 2007; Price, 2015). A recent phylogenetic analysis and ancestral state reconstruction even indicates song in both sexes as the most probable ancestral state (Odom et al., 2014). Current sex differences are thus likely the outcome of both secondary trait loss and selection pressures on sexually dimorphic song (Kraaijeveld, 2014; Odom et al., 2014; Price, 2015). This raises the question of why females stopped singing in some clades but not in others (Odom et al., 2014) and what selection pressures have led to varying degrees of sexual dimorphism (Price, 2015). One promising approach to tackle these questions that has already proven informative for some clades, is to map sex differences in song structure and function and their ecological correlates onto phylogenetic trees to identify common patterns of diversification and losses (Price, 2009; Odom et al., 2015). However, bird song is a mating signal with a twist: substantial phenotypic variation in this trait can arise from cultural transmission and the underlying social learning networks (Lachlan and Slater, 1999). This means that patterns of sex differences can be misleading if the underlying processes causing them are ignored. In the subsequent sections I shall first briefly highlight what we know about the relationships between song learning—both production learning by males and perception learning by females—and the functions of male song. From there I will move on to the question of how studying song production learning in females might provide important cues for hypothesis development regarding the function and evolution of sex differences in male and female song.
Production and Perception Learning and the Mate Attraction Function of Song
The mate attraction function of song is well supported by a large body of observational and experimental data from lab and field (Kroodsma and Byers, 1991; Andersson, 1994; Searcy and Yasukawa, 1996; Catchpole and Slater, 2008). There is now increasing evidence that female preferences, like male repertoires, are influenced by cultural transmission (Riebel, 2003). For the few species studied experimentally in this respect, the types of songs females experienced when young are generally preferred over unfamiliar songs in adulthood (Riebel, 2003). Learned preferences thus influence which songs within a population are attractive. This influence is not trivial, but guides mate choice (Riebel, 2003, 2009). In extremis, this can lead to preferences for song of another population within just one generation (Freeberg, 1996, 1998) or to preferences for the song of another subspecies (Clayton, 1990) or a preference for males that mimic the song of new host species in brood parasites (Payne et al., 2000). Song preferences affect mating patterns and gene recombination in the next generation, and for this reason learned mating preferences (for learned traits) are no longer seen as non-heritable phenotypic variation but to affect evolutionary dynamics in time and space (Verzijden et al., 2012). This is particularly true for birdsong, in which gene-culture co-evolution processes are driven by behavioral selection for learning the right types of song well (Lachlan and Feldman, 2003; Lachlan et al., 2013, 2014).
But from whom do females learn? Active song model choice has not been systematically studied in either sex (but for promising methods to approach these questions in wild birds see e.g., Lachlan and Slater, 2003; Templeton et al., 2010; Akcay et al., 2014). Experimental data from song tutoring studies in females show that memorization of preferred songs does not merely reflect availability or exposure frequency: female cowbirds, Moluthrus ater, that were raised with controlled exposure to songs preferred as adults those songs that during tutoring had been followed by adult females' “chatter” vocalizations (Freed-Brown and White, 2009). Group-housed young female zebra finches can develop song preferences for their male peers rather than adult tutors (Honarmand et al., 2015). How adult females react to specific variants of male song is thus dependent on their early song experiences (Riebel, 2003), and juvenile social and physical conditions (Holveck and Riebel, 2010; Riebel et al., 2010).
It is likely that in species where males and females sing, similar processes also affect the development of male song preferences. Despite increasing documentation of the potential mate attraction function of female song (Langmore, 1998; Hall, 2004), to the best of my knowledge male song preferences and song based male choice have not been studied, despite empirical evidence for a mate attraction function of female song (Langmore et al., 1996). Males, like females, might also be hormonally stimulated either directly by their partner's or even their own song (Kroodsma, 1976; Cheng, 2003). Interestingly, song can be positively reinforcing even in species with non-singing females, such as zebra finches. Male zebra finches will work for song exposure in operant tasks and prefer to listen to songs of early tutors over unfamiliar songs (Riebel et al., 2002). If mechanisms that combine song memories and behavioral expression of preferences are in place even in species with non-singing females, searching for song preference learning in males might be well worth the while. Some of the methods used for female preference testing, such as phonotaxis paradigms, are likely to work in males as well, because males have been shown to be attracted to and approach playback of female song in species with singing females (Langmore et al., 1996).
Production Learning and the Resource Defence Function of Song
The importance of song in the acquisition and defense of resources (territories, mates, nest sites) is undisputed for male song and male-female duets, and this might also be an important function of female solo song (Cain et al., 2015). But how important is it in this context to sing proper song? Song of male songbirds reared without adult models generally shows impoverished structure but nonetheless contains some species-specific signatures (Marler and Sherman, 1983). Such “isolate” song almost always functions less well (or not at all) in both inter-and intra-sexual contexts (Searcy et al., 1985). However, learning just any species-specific song might not suffice either: In species with clear regional variation, local songs generally elicit stronger territorial responses in playback paradigms where song is used to simulate an intruder (Podos and Warren, 2007; Catchpole and Slater, 2008). Even when learning from the local models only, learning these songs well can be of importance. In swamp sparrows, Melospizia georgiana, song variants that match the most typical regional variants in fine detail best, elicit stronger territorial reactions from territory owners—learning precision thus affects same-sex competition (Lachlan et al., 2014). Similarly, learning repertoires of many different songs might improve both a male's resource defense and mate attraction potential (Searcy, 1992; Beecher and Brenowitz, 2005). For male song, what is learned, from whom and how well can thus affect song function.
The Study of Learning Mechanisms can help Elucidate Function and Evolution of Female Song
If song learning affects the efficacy of male songs in mate attraction and resource defense, this could hold for female song too where it fulfils these functions (examples in Langmore, 1998). But if learning song well is so important, why are there such pronounced sex differences in song (learning)? To date, we have no general explanation for the large interspecific variation in sex differences in song quantity and quality which spans the whole range from species with females that never sing (e.g., the zebra finch, Riebel, 2009) to species where females sing more often and more complex songs than males do (e.g., banded wrens Thryothorus pleurostictus, Illes and Yunes-Jimenez, 2009). Moreover, sex differences in song go far beyond what might be needed to aid sex recognition (which can also be achieved with simple calls, see e.g., Mouterde et al., 2014; Kipper et al., 2015). Identical functions of song and sexual differentiation of song solely for sex recognition therefore seems a poor and unlikely general explanation for the vast differences in quantity, quality and context that can be found between male and female song (Langmore, 1998; Hall, 2004). It is here where the study of song learning mechanisms might provide important clues to understand the function and evolution of female song.
Identifying when and from whom females learn and whom they try to match e.g., whether they learn pre- or postdispersal, from kin from their natal area or from neighbors when establishing territories, from same- or opposite sex individuals, or their future mates provides important clues as to who might be the most important receivers of these songs. This in turn can help to develop testable hypothesis regarding the function of song. For example if song learning takes place only after dispersal and then only from territory neighbors then being able to song type match neighbors during territory defense is likely of (testable) higher relevance than for example kin recognition (in which case song learning should have taken place pre-dispersal and from relatives).
Knowing how females learn their songs should also enable the construction of more informative phylogenies. If song sex differences are scored solely by defining “maleness” of female song by looking at percentage shared song elements, ignoring learning, then we will obtain different trait values than when comparing repertoire size or learning accuracy. The hypothetical examples in Table 1 are intended to illustrate this point: the first column shows schematic spectrograms representing a male and female song in a hypothetical songbird species. The two types of song are roughly of the same length and comparable complexity (both songs contain a 2-note syllable, a whistle note and a buzz note). The second and third column illustrate how male and female song is expected to look in the next generation under each of two different scenarios: (I) sex-specific model choice where males copy selectively from males and females selectively copy from females and (II) sex-specific copying fidelity where both sexes only partially copy their chosen models (some elements are missing, and there is some blending of the different model song types) but overall, one sex (here the male, in line with the classic view) imitates more components and does so more accurately. Below these song examples, I listed four parameters that are often used to score song sex differences. Notably, the two scores that take learning processes into account yield different patterns of scored sex differences than the two scores comparing males and females while ignoring model choice and copying fidelity. Analyses taking song learning into account would score no sex differences in amount and ability of learning under the sex-specific learning strategies in scenario I, but register a sex difference for the songs in scenario II. In contrast, analyses scoring sex differences by looking only at male-female repertoire sharing would arrive at the opposite conclusion: a maximum sex difference score under sex-specific model choice in scenario I and a less pronounced sex difference in scenario II (sex-specific copying fidelity). Song parameter choice thus can affect both the direction and magnitude of sex differences.
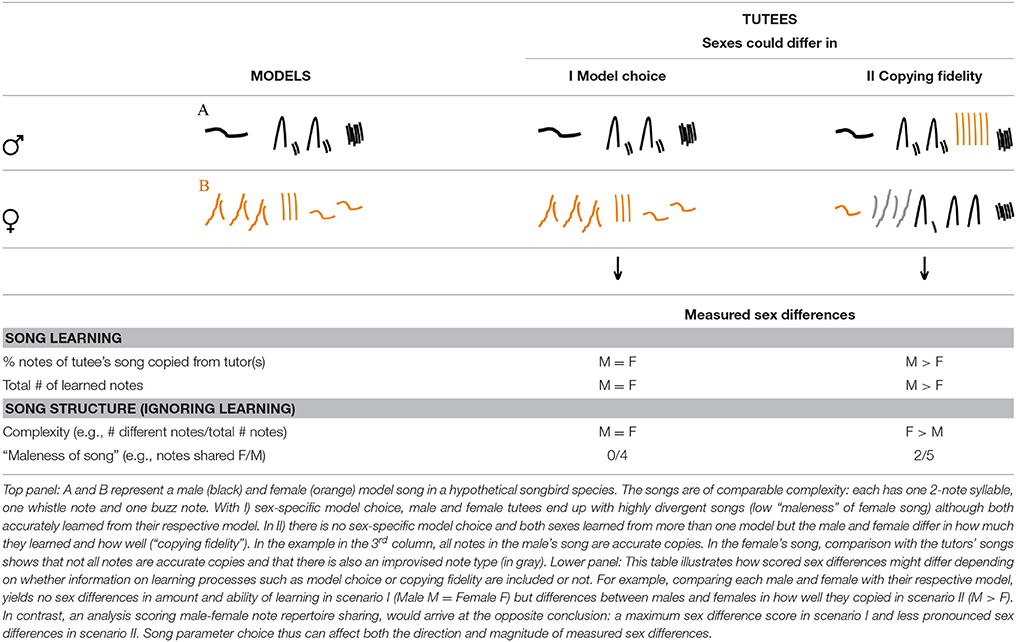
Table 1. Scoring song sex differences with and without taking learning processes into account yields different results.
This is of consequence for our attempts at constructing phylogenies: Evolutionary patterns often can only be discovered when traits are scored continuously rather than dichotomously (Dale et al., 2015; Price, 2015). The above examples illustrate that trait values can yield different results with regard to sex differences in song depending on whether learning processes are ignored or included. It is perhaps too early to speculate which of these measures is the most informative. For now, I hope to raise awareness for (a) that systematically scoring song differences between sexes with one method for all species must be premium for comparative studies and (b) that unraveling the song learning mechanisms and social model choice in combination with study of the interactions between sex-specific contexts and contents of song might provide important hints as to the function of evolution of these sex differences.
Not All Sex Differences in Song are Indicative of Sex Differences in Song Learning Capacity
A final note of caution: sex differences can also be caused by other than social learning processes and not all sex differences in adult song necessarily reflect different learning strategies. Aspects of male and female physiology could differ such that even when both sexes learn the same songs (equally well) their songs sound different (Yamaguchi, 1998) because (1) sex differences in vocal tract anatomy affect vocal output (Ballintijn and ten Cate, 1997) and (2) seasonal and/or sex specific androgen levels could cause sex differences if females do not fully crystallize their song due to lower androgen levels. However, these questions as yet lack systematic study in songbirds (Gahr, 2014) and there are also observations of males and females that show no pronounced sex differences in song despite different steroid levels (Schwabl et al., 2015).
Moreover, physiological mechanisms and learning strategies can interact in multiple ways. And no learning strategy fits all: sex differences could come about because the sexes differ in different aspects of their learning strategies e.g., (1) one sex learns more or more accurately than the other (see Table 1), because (2) there are sex differences in the timing of the sensitive phase (Nelson et al., 1997; Yamaguchi, 1998) or (3) as a side effect of different habitat usage and/or dispersal patterns males and females are exposed to different models or (4) show differences in active model choice, meaning that they either pick different social models (see Table 1) or pick different song models from different tutors (Geberzahn and Gahr, 2013). The most conspicuous variant of the latter strategy would be true sex-specific lineages, where both sexes have specific vocalizations and learning takes place between same-sex individuals only (Price, 1998). This has been hypothesized for a number of species, but there has not yet been systematic study in a single species that was able to exclude all possible alternative explanations (for review and discussion see Riebel, 2003).
Conclusions and Outlook
To summarize and conclude: female songbirds in many species sing and learn which songs to sing. Females of both singing and non-singing species have been documented to also acquire their song preferences through social learning processes (Riebel, 2003). Learning and cultural transmission processes deeply impact the efficacy of the signal and both learned song and preferences are subjected to natural, social, inter- and intra-sexual selection processes. The timing of sensitive phases and mechanisms of model choice but also learning-unrelated behavior such as (sex-specific) dispersal patterns all impact song and eventually fitness. Ideally, the study of how developmental processes contribute to inter-individual variation in traits and preferences should go hand in hand with studies of song function. Questions we might want to ask in future studies investigating sex differences in song (and their costs and benefits) include:
1. Do males and females differ in the timing of song learning?
2. Are there sex differences in model choice (who is learning from whom)?
3. What is learned and how accurately, and does this depend on sex?
4. Is there evidence for sex-specific habitat usage and/or dispersal patterns that will lead to different model availability in males and females?
Identifying sex-specific learning strategies might provide important clues to the selection pressures on sexual differentiation of song. Asking why females in one species needn't learn precisely while in another exact copying (and from selected models) is important might lead us to the social factors selecting for particular learning strategies. There has been no systematic study of these questions in females yet, but systematic comparisons of how learning contributes to inter-individual variation in signaling and signal decoding will provide important steps toward unraveling the function(s) of intra-and inter-sexual song variation.
Author Contributions
The author confirms being the sole contributor of this work and approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I would like to thank the two guest editors of this special issue Michelle Hall and Naomi Langmore for their invitation to contribute to this issue, Pralle Kriengwatana for her comments on an earlier version of this manuscript and the three referees for their much appreciated constructive comments.
References
Akcay, C., Campbell, S. E., Reed, V. A., and Beecher, M. D. (2014). Song sparrows do not learn more songs from aggressive tutors. Anim. Behav. 94, 151–159. doi: 10.1016/j.anbehav.2014.06.003
Ballintijn, M. R., and ten Cate, C. (1997). Vocal development and its differentiation in a non-songbird: the collared dove (Streptopelia decaocto). Behaviour 134, 595–621. doi: 10.1163/156853997X00548
Beecher, M. D., and Brenowitz, E. A. (2005). Functional aspects of song learning in songbirds. Trends Ecol. Evol. 20, 143–149. doi: 10.1016/j.tree.2005.01.004
Cain, K. E., Cockburn, A., and Langmore, N. E. (2015). Female song rates in response to simulated intruder are positively related to reproductive success. Front. Ecol. Evol. 3:119. doi: 10.3389/fevo.2015.00119
Catchpole, C. K., and Slater, P. J. B. (2008). Bird Song: Biological Themes and Variations. Cambridge: Cambridge University Press.
Cheng, M. F. (2003). Vocal self-stimulation: from the ring dove story to emotion-based vocal communication. Adv. Stud. Behav. 33, 309–353. doi: 10.1016/S0065-3454(03)33007-4
Clayton, N. S. (1990). Assortative mating in zebra finch subspecies, Taeniopygia guttata guttata and T. g. castanotis. Philos. Trans. R. Soc. B 330, 351–370. doi: 10.1098/rstb.1990.0205
Dale, J., Dey, C. J., Delhey, K., Kempenaers, B., and Valcu, M. (2015). The effects of life history and sexual selection on male and female plumage colouration. Nature 527, 367–370. doi: 10.1038/nature15509
Freeberg, T. M. (1996). Assortative mating in captive cowbirds is predicted by social experience. Anim. Behav. 52, 1129–1142. doi: 10.1006/anbe.1996.0260
Freeberg, T. M. (1998). The cultural transmission of courtship patterns in cowbirds, Molothrus ater. Anim. Behav. 56, 1063–1073. doi: 10.1006/anbe.1998.0870
Freed-Brown, G., and White, D. J. (2009). Acoustic mate copying: female cowbirds attend to other females' vocalizations to modify their song preferences. Proc. R. Soc. Lond. B 276, 3319–3325. doi: 10.1098/rspb.2009.0580
Gahr, M. (2014). How hormone-sensitive are bird songs and what are the underlying mechanisms? Acta Acustica United Acustica 100, 705–718. doi: 10.3813/AAA.918749
Garamszegi, L. Z., Pavlova, D. Z., Eens, M., and Moller, A. P. (2007). The evolution of song in female birds in Europe. Behav. Ecol. 18, 86–96. doi: 10.1093/beheco/arl047
Geberzahn, N., and Gahr, M. (2013). Song learning in male and female Uraeginthus cyanocephalus, a tropical songbird species. J. Comp. Psychol. 127, 352–364. doi: 10.1037/a0033154
Hall, M. L. (2004). A review of hypotheses for the functions of avian duetting. Behav. Ecol. Sociobiol. 55, 415–430. doi: 10.1007/s00265-003-0741-x
Holveck, M. J., and Riebel, K. (2010). Low-quality females prefer low-quality males when choosing a mate. Proc. R. Soc. Lond. B 277, 153–160. doi: 10.1098/rspb.2009.1222
Honarmand, M., Riebel, K., and Naguib, M. (2015). Nutrition and peer group composition in early adolescence: impacts on male song and female preference in zebra finches. Anim. Behav. 107, 147–158. doi: 10.1016/j.anbehav.2015.06.017
Illes, A. E., and Yunes-Jimenez, L. (2009). A female songbird out-sings male conspecifics during simulated territorial intrusions. Proc. R. Soc. Lond. B 276, 981–986. doi: 10.1098/rspb.2008.1445
Kipper, S., Kiefer, S., Bartsch, C., and Weiss, M. (2015). Female calling? song responses to conspecific call playbacks in nightingales, Luscinia megarhynchos. Anim. Behav. 100, 60–66. doi: 10.1016/j.anbehav.2014.11.011
Kraaijeveld, K. (2014). Reversible trait Loss: the genetic architecture of female ornaments. Ann. Rev. Ecol. Evol. Syst. 45, 159–177. doi: 10.1146/annurev-ecolsys-120213-091550
Kroodsma, D. E. (1976). Reproductive development in a female songbird: differential stimulation by quality of male song. Science 192, 574–575. doi: 10.1126/science.192.4239.574
Kroodsma, D. E., and Byers, B. E. (1991). The function(s) of bird song. Am. Zool. 31, 318–328. doi: 10.1093/icb/31.2.318
Lachlan, R. F., Anderson, R. C., Peters, S., Searcy, W. A., and Nowicki, S. (2014). Typical versions of learned swamp sparrow song types are more effective signals than are less typical versions. Proc. R. Soc. Lond. B 281:20140252. doi: 10.1098/rspb.2014.0252
Lachlan, R. F., and Feldman, M. W. (2003). Evolution of cultural communication systems: the coevolution of cultural signals and genes encoding learning preferences. J. Evol. Biol. 16, 1084–1095. doi: 10.1046/j.1420-9101.2003.00624.x
Lachlan, R. F., and Slater, P. J. B. (1999). The maintenance of vocal learning by gene-culture interaction: the cultural trap hypothesis. Proc. R. Soc. Lond. B 266, 701–706. doi: 10.1098/rspb.1999.0692
Lachlan, R. F., and Slater, P. J. B. (2003). Song learning by chaffinches: how accurate, and from where? Anim. Behav. 65, 957–969. doi: 10.1006/anbe.2003.2091
Lachlan, R. F., Verzijden, M. N., Bernard, C. S., Jonker, P. P., Koese, B., Jaarsma, S., et al. (2013). The progressive loss of syntactical structure in bird song along an island colonization chain. Cur. Biol. 23, 1896–1901. doi: 10.1016/j.cub.2013.07.057
Langmore, N. E. (1998). Functions of duet and solo songs of female birds. Trends Ecol. Evol. 13, 136–140. doi: 10.1016/S0169-5347(97)01241-X
Langmore, N. E., Davies, N. B., Hatchwell, B. J., and Hartley, I. R. (1996). Female song attracts males in the alpine accentor Prunella collaris. Proc. R. Soc. Lond. B 263, 141–146. doi: 10.1098/rspb.1996.0022
Marler, P., and Sherman, V. (1983). Song structure without auditory feedback: emendations of the auditory template hypothesis. J. Neurosci. 3, 517–531.
Morton, E. S. (1996). “A comparison of vocal behavior among tropical and temperate passerine birds,” in Ecology and Evolution of Acoustic Communication in Birds, eds D. E. Kroodsma and E. H. Miller (Ithaca, NY; London: Comstock Publishing Associates), 258–268.
Mouterde, S. C., Theunissen, F. E., Elie, J. E., Vignal, C., and Mathevon, N. (2014). Acoustic communication and sound degradation: how do the individual signatures of male and female zebra finch calls transmit over distance? PLoS ONE 9:e102842. doi: 10.1371/journal.pone.0102842
Mundinger, P. C. (1982). “Microgeographic and macrogeographic variation in the acquired vocalizations of birds,”in Acoustic Communication in Birds, eds D. E. Kroodsma and E. H. Miller (New York, NY: Academic Press), 147–208.
Nelson, D. A., Marler, P., Soha, J. A., and Fullerton, A. L. (1997). The timing of song memorization differs in males and females: a new assay for avian vocal learning. Anim. Behav. 54, 587–597. doi: 10.1006/anbe.1996.0456
Odom, K. J., Hall, M. L., Riebel, K., Omland, K. E., and Langmore, N. E. (2014). Female song is widespread and ancestral in songbirds. Nat. Commun. 5:3379. doi: 10.1038/ncomms4379
Odom, K. J., Omland, K. E., and Price, J. J. (2015). Differentiating the evolution of female song and male-female duets in the New World blackbirds: can tropical natural history traits explain duet evolution? Evolution 69, 839–847. doi: 10.1111/evo.12588
Payne, R. B., Payne, L. L., Woods, J. L., and Sorenson, M. D. (2000). Imprinting and the origin of parasite-host species associations in brood-parasitic indigobirds, Vidua chalybeata. Anim. Behav. 59, 69–81. doi: 10.1006/anbe.1999.1283
Peters, S., Searcy, W. A., and Nowicki, S. (2014). Developmental stress, song-learning, and cognition. Integr. Comp. Biol. 54, 555–567. doi: 10.1093/icb/icu020
Podos, J., and Warren, P. S. (2007). The evolution of geographic variation in birdsong. Adv. Stud. Behav. 37, 403–458. doi: 10.1016/S0065-3454(07)37009-5
Price, J. J. (1998). Family- and sex-specific vocal traditions in a cooperatively breeding song bird. Proc. R. Soc. B 265, 497–502. doi: 10.1098/rspb.1998.0322
Price, J. J. (2009). Evolution and life-history correlates of female song in the New World blackbirds. Behav. Ecol. 20, 967–977. doi: 10.1093/beheco/arp085
Price, J. J. (2015). Rethinking our assumptions about the evolution of bird song and other sexually dimorphic signals. Front. Ecol. Evol. 3:40. doi: 10.3389/fevo.2015.00040
Riebel, K. (2003). The “mute” sex revisited: vocal production and perception learning in female songbirds. Adv. Stud. Behav. 33, 49–86. doi: 10.1016/S0065-3454(03)33002-5
Riebel, K. (2009). Song and female mate choice in zebra finches: a review. Adv. Stud. Behav. 40, 197–238. doi: 10.1016/S0065-3454(09)40006-8
Riebel, K., Hall, M. L., and Langmore, N. E. (2005). Female songbirds still struggling to be heard. Trends Ecol. Evolut. 20, 419–420. doi: 10.1016/j.tree.2005.04.024
Riebel, K., Holveck, M.-J., Verhulst, S., and Fawcett, T. W. (2010). Are high-quality mates always attractive?: state-dependent mate preferences in birds and humans. Commun. Integr. Biol. 3, 271–273. doi: 10.4161/cib.3.3.11557
Riebel, K., Lachlan, R., and Slater, P. (2015). Learning and cultural transmission in chaffinch song. Adv. Stud. Behav. 47, 181–227. doi: 10.1016/bs.asb.2015.01.001
Riebel, K., Smallegange, I. M., Terpstra, N. J., and Bolhuis, J. J. (2002). Sexual equality in zebra finch song preference: evidence for a dissociation between song recognition and production learning. Proc. R. Soc. Lond. B 269, 729–733. doi: 10.1098/rspb.2001.1930
Robinson, A. (1949). The biological significance of bird song in Australia. EMU 48, 291–315. doi: 10.1071/MU948291
Schwabl, H., Dowling, J., Baldassarre, D. T., Gahr, M., Lindsay, W. R., and Webster, M. S. (2015). Variation in song system anatomy and androgen levels does not correspond to song characteristics in a tropical songbird. Anim. Behav. 104, 39–50. doi: 10.1016/j.anbehav.2015.03.006
Searcy, W. A. (1992). Song repertoire and mate choice in birds. Am. Zool. 32, 71–80. doi: 10.1093/icb/32.1.71
Searcy, W. A., Marler, P., and Peters, S. S. (1985). Songs of isolation-reared sparrows function in communication, but are significantly less effective than learned songs. Behav. Ecol. Sociobiol. 17, 223–229. doi: 10.1007/BF00300140
Searcy, W. A., and Yasukawa, K. (1996). “Song and female choice,” in Ecology and Evolution of Acoustic Communication in Birds, eds D. E. Kroodsma and E. H. Miller (Ithaca, NY: Comstock Publishing Associates), 454–473.
Slater, P. J. B. (2003). Fifty years of bird song research: a case study in animal behaviour. Anim. Behav. 65, 633–639. doi: 10.1006/anbe.2003.2051
Slater, P. J. B., and Ince, S. A. (1979). Cultural evolution in chaffinch song. Behaviour 71, 146–166. doi: 10.1163/156853979X00142
Slater, P. J. B., and Mann, N. I. (2004). Why do the females of many bird species sing in the tropics? J. Avian Biol. 35, 289–294. doi: 10.1111/j.0908-8857.2004.03392.x
Templeton, C. N., Akçay, C., Campbell, S. E., and Beecher, M. D. (2010). Juvenile sparrows preferentially eavesdrop on adult song interactions. Proc. R. Soc. Lond. B 277, 447–453. doi: 10.1098/rspb.2009.1491
Verzijden, M. N., ten Cate, C., Servedio, M. R., Kozak, G. M., Boughman, J. W., and Svensson, E. I. (2012). The impact of learning on sexual selection and speciation. Trends Ecol. Evol. 27, 511–519. doi: 10.1016/j.tree.2012.05.007
Keywords: vocal learning, females, oscines, signal evolution, cultural transmission, signal, plasticity
Citation: Riebel K (2016) Understanding Sex Differences in Form and Function of Bird Song: The Importance of Studying Song Learning Processes. Front. Ecol. Evol. 4:62. doi: 10.3389/fevo.2016.00062
Received: 11 December 2015; Accepted: 23 May 2016;
Published: 07 June 2016.
Edited by:
Deseada Parejo, Universidad de Extremadura, SpainReviewed by:
Jordan Price, St. Mary's College of Maryland, USAJill Soha, Duke University, USA
Amélie N. Dreiss, University of Lausanne, Switzerland
Copyright © 2016 Riebel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katharina Riebel, k.riebel@biology.leidenuniv.nl
 Katharina Riebel
Katharina Riebel