Costs of Persisting Unreliable Memory: Reduced Foraging Efficiency for Free-Flying Parasitic Wasps in a Wind Tunnel
- 1Laboratory of Entomology, Plant Sciences Group, Wageningen University, Wageningen, Netherlands
- 2Department of Terrestrial Ecology, Netherlands Institute of Ecology (NIOO-KNAW), Wageningen, Netherlands
Parasitic wasps are known to improve their foraging efficiency after learning of herbivore-induced plant volatiles (HIPVs) upon encountering their hosts on these plants. However, due to spatial and temporal variation of herbivore communities, learned HIPV cues can become unreliable, no longer correctly predicting host presence. Little is known about the potential fitness costs when memories holding such unreliable information persist. Here we studied how persistent memory, containing unreliable information, affects the foraging efficiency for hosts in Cotesia glomerata. Wasps were conditioned to associate one of two types of HIPVs with either P. brassicae frass, 1 single oviposition in P. brassicae, 3 ovipositions in P. brassicae spaced in time or they were kept unconditioned. The following day, wasps were allowed to forage in a wind tunnel, in an environment that either conflicted or was congruent with their learned plant experience. The foraging environment consisted of host (P. brassicae) and non-host (Mamestra brassicae) infested plants. The conflicting environment had non-hosts on the conditioned plant species and hosts on the non-conditioned plant species, whereas the congruent environment had hosts on the conditioned plant species and non-hosts on the unconditioned plant species. Wasps had to navigate through five non-host infested plants to reach the host-infested plant. Since C. glomerata wasps do not distinguish between HIPVs induced by host and non-host caterpillars, the conflicting foraging situation caused a prediction error, by guiding wasps to non-host infested plants. Especially wasps given 3 spaced oviposition experiences, tested in a conflicting situation, spent significantly more time on non-host infested plants and showed a high tendency to oviposit in the non-hosts. As a result, they took significantly more time to find their hosts. Conditioned wasps, which were tested in a congruent situation, were more responsive than unconditioned wasps, but there was no difference in foraging efficiency between these two groups in the wasps that showed a response. We conclude that persistent memories, such as formed after 3 experiences spaced in time, can lead to maladaptive foraging behavior if the contained information becomes unreliable.
Introduction
A high degree of spatial and temporal variation exists in herbivore communities, which makes it challenging for predators to find suitable prey. The way parasitoids use environmental cues to find resources such as hosts is of great importance for their realized lifetime reproductive success, a measure of fitness (Van Baalen and Hemerik, 2007). An inexperienced female parasitic wasp is attracted by a range of environmental cues, which have proven their reliability for host finding over generations through natural selection (Stephens, 1993; Van Alphen and Bernstein, 2008; Hoedjes et al., 2011). Due to the high degree of both spatial and temporal variation within and between generations in the availability, distribution and abundance of both host and host plant species, these cues can be insufficient to guide parasitoids to their hosts (Stephens, 1993; Vet, 2001). Parasitoids can, however, acquire and process information as they forage, thereby learning how to become more efficient foragers. Parasitoids are known to use a wide variety of olfactory, visual, auditory and tactile cues to obtain and store information on local host presence, distribution and abundance (Vet and Dicke, 1992; Turlings et al., 1993; Van Alphen and Bernstein, 2008; Ishii and Shimada, 2009). Acquisition of this information can be achieved through learning, in particular through associative ovipositional learning, where an oviposition in a host becomes associated with various environmental cues, such as herbivore-induced plant volatiles (HIPVs), resulting in associative memory (Bleeker et al., 2006). Even an encounter with host traces, such as silk and feces (frass), without the host themselves, results in learning of HIPV's (Geervliet et al., 1998), albeit that such memories are generally less persistent than after an oviposition experience (Lewis and Martin, 1990; Takasu and Lewis, 2003). With these memories, parasitic wasps can temporarily adapt their foraging strategy to current local host and host-plant availability.
In general, only when multiple learning events occur spaced in time, the learned information is considered reliable enough to adapt foraging behavior accordingly for a prolonged time. It is then stored in robust long-term memory (LTM), which can last for days (Menzel, 1999; Hoedjes et al., 2011). Moreover, LTM formation is costly in terms of energy expenditure (Menzel, 1999; Mery and Kawecki, 2004), because it depends on protein synthesis (Tully et al., 1994), which is another reason why single learning events usually results in the formation of energetically inexpensive, short lasting memory, naturally decaying within minutes to hours (Menzel, 1999; Hoedjes et al., 2011).
The generalist larval parasitoid Cotesia glomerata, is well known for its ability to learn in both laboratory and (semi-)field studies (Geervliet et al., 1998; Perfecto and Vet, 2003; Smid et al., 2007; De Rijk et al., 2018; Vosteen et al., manuscript in preparation). Unlike general theory, it consolidates LTM for oviposition events on certain host plants within 4 h after only a single oviposition in its host Pieris brassicae (Smid et al., 2007). This direct LTM induction is most likely due to the spatial distribution and gregarious nature of this host, since a single encounter with a gregarious host reliably predicts many oviposition opportunities. Indeed, when this wasp species encounters a solitary host, P. rapae, it does not form LTM, but a less persistent memory type, anesthesia-resistant memory (Kruidhof et al., 2012). While LTM of a single oviposition wanes over 5 days, spaced conditioning with 3 ovipositions leads to even more persistent LTM, lasting for more than 5 days (Van Vugt et al., 2015). Thus, experiences with only frass, a single oviposition or three ovipositions spaced in time each induce different memories with increasing levels of persistence.
This memory guides C. glomerata to subsequent host patches, but due to the high similarity of HIPV of host and non-host species, these wasps are often unable to discriminate between them (Geervliet et al., 1996; Vos et al., 2001; Bukovinszky et al., 2012), even after oviposition experience (Vosteen et al., manuscript in preparation). The presence of non-host on host plant species has been found to lead to reduced foraging efficiency (Vos et al., 2001; Bukovinszky et al., 2012; De Rijk et al., 2016b; Desurmont et al., 2018; Vosteen et al., manuscript in preparation).
Since environments keep changing, assessment of the reliability of the learned information is a continuous process. Non-hosts might occur on plants previously associated with hosts. Encountering non-hosts on plants that emit HIPVs previously associated with host presence, leads to a predication error; the learned cues do not predict host presence, they have become unreliable. To optimize foraging efficiency, information needs to be processed in an adaptive and integrative way (Hilker and Mcneil, 2008), continuously updating memories and acting according to the most reliable information available.
The different levels of memory persistence described above make these wasps an ideal model to study the risk of maladaptive foraging behavior due to persistent unreliable information. Here we conducted a wind tunnel experiment to study how foraging efficiency is affected in the parasitic wasp C. glomerata, when foraging in an environment, which was either conflicting or congruent with previously learnt information varying in persistence. We confronted the wasps with non-hosts on the plant species on which had they previously found their hosts, and hosts on the plant species not encountered before (conflicting) or vice versa (congruent). We expect that with higher levels of memory persistence, wasps will increasingly suffer from reduced foraging efficiency in the conflicting foraging situation, and benefit on the other hand from improved foraging efficiency in the congruent foraging situation.
Materials and Methods
Insects
Pieris brassicae (Lepidoptera: Pieridae) and Mamestra brassicae (Lepidoptra: Noctuidae) caterpillars were reared on Brussels sprouts plants (Brassicae oleracea L. var. gemmifera cultivar Cyrus). Females of the parasitic wasps C. glomerata (Hymenoptera: Braconidae) were obtained from a yearly re-established culture, and reared on P. brassicae caterpillars, to maintain natural foraging behavior. All insect cultures were maintained at the Laboratory of Entomology, Wageningen University and were reared under the same conditions in a climate-controlled greenhouse with natural light conditions, 21 ± 1°C and 50–70% humidity. First instar P. brassicae caterpillars were used for parasitoid rearing. Upon emergence of the parasitoid larvae, cocoons were collected and kept in Petri dishes which were put in a climate cabinet (21 ± 1°C, L16:D8 photoperiod and 50–70% humidity). Just prior to emergence the cocoons were transferred to cages (40 × 303 × 30 cm, Bugdorm-1 Insect rearing cage, type DP1000, Megaview Science, Taiwan) with honey and water. Two-day-old females were selected from these cages and kept with honey and water until the start of experiments, when females were 3–5 days old.
Plants
For experiments 3–4 weeks old Brassicae nigra L. and Sinapis arvensis L. plants were used. Plants were watered daily and were supported by a small green wooden stick and a metal ring to ensure upright growth. Induction of both plant species was accomplished by placing 2 batches of 5 M. brassicae 48 h prior to experiments, or 2 batches of 5 P. brassicae caterpillars 24 h prior to experiments, on the fourth true leaf of a plant with clip cages. Clip cages were kept upright by attaching each of them to a small green wooden stick (30 cm long, 4 mm diameter) to prevent the leaf from breaking due to the weight of the clip cage. Besides the clip cages, some cotton wool was wrapped around the base of the leaf to prevent the spread of caterpillars to other leaves once the clip cages were removed. Early first instar P. brassicae and late first instar M. brassicae caterpillars were used to infest plants. The difference in age was to obtain similar caterpillar body sizes. M. brassicae, however, caused less feeding damage and the induced plants were less attractive to parasitoids after 24 h induction (personal observation), therefore M. brassicae was kept on the plant 24 h longer than P. brassicae to obtain similar damage and attractiveness of plants. After every 3 h of experiments plants were replaced.
Parasitoid Conditioning
A day before conditioning a B. nigra and a S. arvensis plant were induced with approximately 200–300 P. brassicae caterpillars spread in batches of approximately 50 caterpillars over the plant leaves. A classical (Pavlovian) conditioning procedure was used, which excludes the host-searching phase, adopted from Bleeker et al. (2006), to give wasps an associative learning experience (Figure 1). This procedure consists of giving wasps oviposition experience on a plant leaf, where wasps learned to associate plant odors as the conditioned stimulus (CS) with suitable hosts as the unconditioned stimulus (US). This type of conditioning is considered a form of classical (Pavlovian) conditioning, where the host-searching phase is excluded.
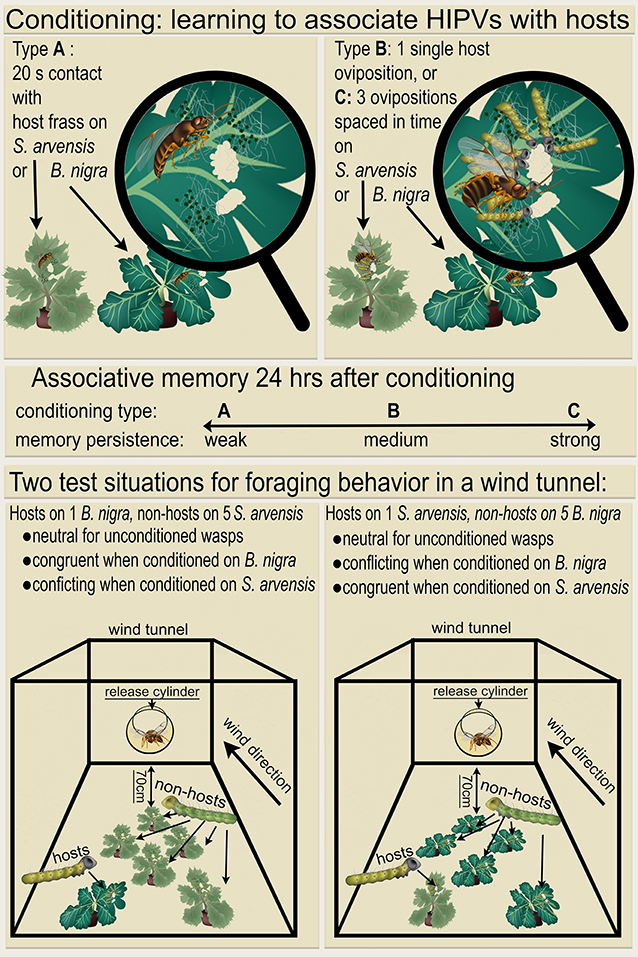
Figure 1. Overview of the conditioning and test procedures. Wasps were given a learning experience (Top), in which they learned to associate herbivore-induced plant volatiles (HIPVs) of Brassicae nigra (dark green plants) or Sinapis arvensis (light green plants) with either a 20 s host-frass (Pieris brassicae) exposure, 1 single host oviposition, or 3 host ovipositions spaced in time. These conditioning treatments resulted in increasing levels of memory persistence (mid panel, indicated with weak, medium or strong), for either B. nigra or S. arvensis as predictor for the presence of P. brassicae hosts. The next day these conditioned wasps were tested in foraging situations created in a wind tunnel (Bottom), which were either congruent or conflicting with their memory. A conflicting situation consisted of non-hosts (Mamestra brassicae) on five of the plant of the species on which the wasps previously experienced hosts or host-frass, and hosts on only one plant of the alternative plant species, located most upwind from the release point. The congruent situation had the same array of 5 plants with non-hosts and one plant with hosts, but in this case the hosts were present on the same plant species on which the previously were conditioned. Unconditioned wasps were also tested, for which both foraging situations were considered as neutral. Altogether, this results in 14 different treatments.
A total of 7 different conditioning treatments were conducted; wasps were kept unconditioned or were given conditioning experience on either the induced B. nigra or S. arvensis plant. Conditioning on these leaves consisted of (A) a single leaf damage experience where a wasp was transferred from a glass vial to a leaf with host feeding damage. The first instar P. brassicae host caterpillars had been removed, but their frass was still present. The wasp was allowed to contact the host frass for 20 s, after which it was gently removed with the glass vial. (B) A single oviposition in a first instar P. brassicae caterpillar, which was performed as under (A), but now with host caterpillars present. After a single oviposition, the wasp was removed with a glass vial. (C) Spaced conditioning consisting of 3 ovipositions spaced in time. It was performed as 3 sequences of single ovipositions, as described for (B), spaced by intervals of 10 min, during which the wasp remained in the glass vial. Wasps were conditioned individually and only ovipositions lasting longer than 2 s were considered successful (Coleman et al., 1999). Figure 1 shows an overview of these conditioning procedures. While both 1 and 3 ovipositions are expected to induce LTM, spaced conditioning with 3 ovipositions leads to longer lasting, more robust LTM (Smid et al., 2007; Van Vugt et al., 2015), with stronger memory persistence (Figure 1). After conditioning, wasps from all treatment groups were placed in small cages (17 × 17 × 17 cm, Bugdorm type 41515, Megaview Science, Taiwan) supplied with water and honey till testing in the wind tunnel the next day.
Wind Tunnel Set-Up
The experiment was conducted in a wind tunnel as described in Geervliet et al. (1994) with wind speed set to 10 cm/s, a temperature of 24 ± 1°C and a relative humidity fluctuating between 50% and 70%. A glass cylinder (30 cm long, diameter 15 cm) was used as release site and was placed 70 cm upwind from the first plant. Six plants were placed 15 cm apart and 10 cm from the walls of the wind tunnel, five plants infested with the non-host M. brassicae and one with host P. brassicae, the latter being placed upwind from the five non-host infested plants. Two different foraging situations were created, with either the non-host M. brassicae on B. nigra and the host P. brassicae on S. arvensis, or vice versa. On a single experimental day both foraging situations were used, each running for 3 h. Both the order of the foraging situations and the position of the P. brassicae plant were alternated daily. The order of the 7 conditioning treatments was randomized, on each experimental day 2 wasps were tested per treatment. The 7 conditioning treatments and the 2 foraging situations lead to a total of 14 treatments, each treatment was replicated 15 times. An overview of the conditioning and test procedure of these various treatments is shown in Figure 1.
The wind tunnel was turned on 1–1.5 h prior to experiments to create stable temperature and humidity values. Just prior to the start of the experiment plants were positioned in the wind tunnel, clip cages and their supporting sticks were removed. Caterpillar movement was restricted to the leaf due to the cotton wool wrapped around the base and caterpillars were counted to make sure 10 live caterpillar would be available. Dead caterpillars were replaced by caterpillars of the same size and age.
Upon the start of the experiment a single wasp was transferred to a glass test tube (12 × 75 mm), from its cage into the glass release cylinder in the wind tunnel. Each wasp was given 5 min to initiate flight and leave the cylinder. Those that did not fly out of the glass cylinder were taken out of the experiment. Wasps which directly flew to the ceiling of the wind tunnel were re-released once.
Behavioral Observations
Wasp behavior was recorded on a hand-held computer with The Observer XT 10 software (Noldus Information Technology B.V., Wageningen, The Netherlands) for 15 min or until first host oviposition. We used the following behavioral parameters for statistical analysis: foraging time (total time of the behavioral recording), time on non-host patches, number of non-host patch visits and non-host oviposition occurrences. Only behavior on the actual infested leaves was considered. Furthermore, direct flight (the percentage of wasps which only landed on the host plant after flight initiation) and wasp response (the percentage of wasps initiating flight and orientation to the HIPVs) were also used for statistical analysis.
Statistics
All statistical analyses were done in R version 3.4.3 (R Development Core Team 2017). Foraging time was analyzed using survival analysis with a cox regression analysis (coxph from the survival package, (Therneau and Lumley, 2015)), where censored data consisted of wasps not finding their host within 900 s. Data on time on non-host patches and number of non-host patch visits were analyzed using linear mixed models (lme from the nlme package, Pinheiro et al., 2014) with experimental day as a random factor. Data on the number of non-host patch visits was log transformed to account for equal variance, time on non-host patches was square root transformed. Presence/absence data on non-host oviposition, direct flight and response was analyzed with a Bernoulli glmm (glmer from the lme4 package, Bates et al., 2014) with day as a random factor.
The statistical models used foraging situation, test plant species and conditioning treatment as fixed factors. Due to an incomplete factorial design, models including unconditioned wasps/neutral foraging situation were run without conditioning treatment and vice versa. Differences between groups were analyzed with a least-square means post-hoc comparison with error correction (lsmeans from the lsmeans package, Lenth, 2016).
Results
The Effect of Foraging Situation and Conditioning Treatment on Foraging Behavior
Wasps given conflicting information had more difficulty finding hosts, than wasps given congruent information as can be seen by the clear divergence of their survival curves in Figure 2. While conditioning treatment did not have a strong effect on its own, the combination of foraging situation and the conditioning treatment shows clear effects of spaced conditioning with 3 ovipositions (Figure 3). While 3 ovipositions with congruent information made them the fastest group to find the host, 3 ovipositions with conflicting information resulted in wasps being the slowest group to find the host. Since the congruent and conflicting survival curves of frass and a single oviposition show a high degree of overlap, the overall difference between congruent and conflicting foraging situations is mainly explained by the effect of spaced conditioning with 3 ovipositions (Figure 3).
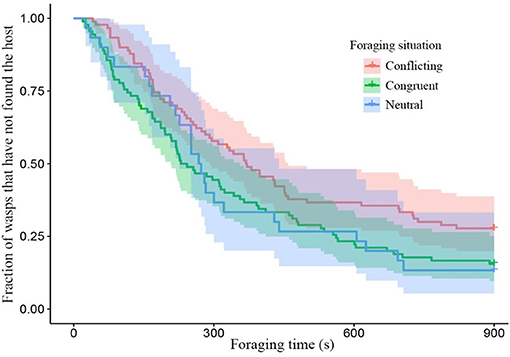
Figure 2. Survival plot of the fraction of wasps which have not found the host with foraging time in different foraging situations. Colored areas around the lines show the 95% confidence interval (neutral n = 30, conflicting and congruent n = 90). Conflicting and congruent survival curves were significantly different (z = −2.38, p = 0.046).
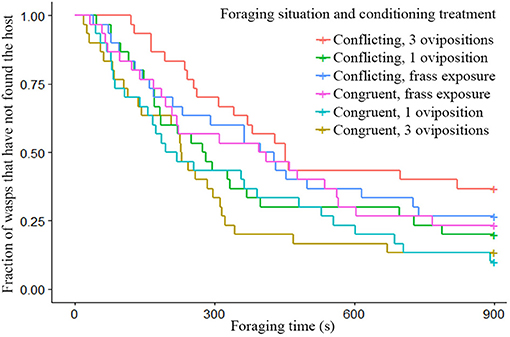
Figure 3. Survival plot of the fraction of wasps which have not found the host within the 900 s foraging time, given different conditioning treatments and foraging situations (n = 30). Wasps given 3 spaced ovipositions in combination with conflicting information were significantly different from wasps given 3 spaced oviposition in combination with congruent information (z = −2.96, p = 0.036).
Assessment of the underlying behavioral components during the foraging period revealed significant differences in the time wasps spent on non-host patches. Wasps given spaced conditioning with conflicting information stayed more than twice as long on non-host patches, than wasps given spaced conditioning with congruent information (Figure 4). The same pattern was observed for non-host oviposition, where wasps given spaced conditioning with conflicting information oviposited 3 times as often in non-hosts, but here the difference between the two spaced conditioning groups had a p-value of 0.063 (Figure 5).
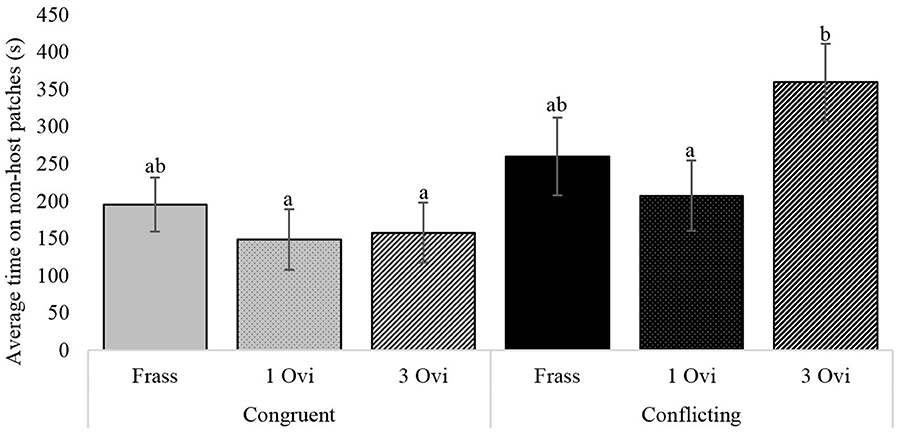
Figure 4. The influence of foraging situation and conditioning treatment on the average time wasps spent on non-host patches. Conditioning treatments consisted of; a 20 s host frass exposure (Frass), 1 oviposition (1 Ovi), or 3 spaced ovipositions (3 Ovi). Bars with different letters are significantly different (n = 30, α = 0.05), error bars show the s.e.
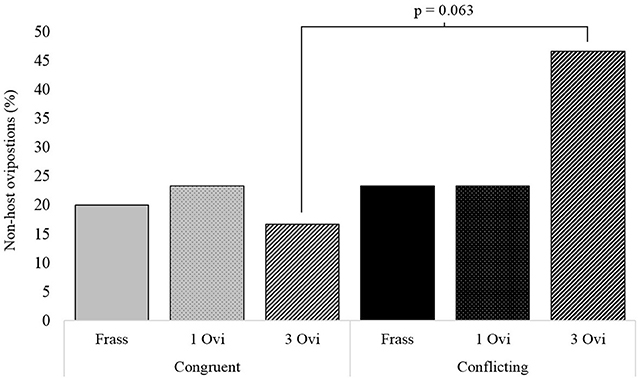
Figure 5. The percentage of wasps oviposting in non-host when given either conflicting or congruent experience with either a 20 s frass experience (Frass), a single oviposition (1 Ovi), or 3 spaced ovipositions (3 Ovi) (n = 30).
Survival analysis of unconditioned wasps, foraging in a neutral situation, switched between the congruent and conflicting conditioned wasps within the first 250 s (Figure 2). Thereafter, the unconditioned wasps behaved very similar to congruently conditioned wasps. Overall, wasps foraging in a neutral situation did not behave significantly different from wasps foraging in a conflicting (z = 1.76, p = 0.183) or congruent situation (z = 0.031, p = 1.000), due to high behavioral variation show in the 95% confidence interval in Figure 2. Unconditioned wasps did make fewer visits to non-host patches, than wasps given a conflicting experience (f = 3.04, p = 0.049, Figure 6A). Furthermore, fewer unconditioned, than conditioned wasps responded to HIPVs in the wind tunnel (z = −5.19, p = 0.000, Figure 6B).
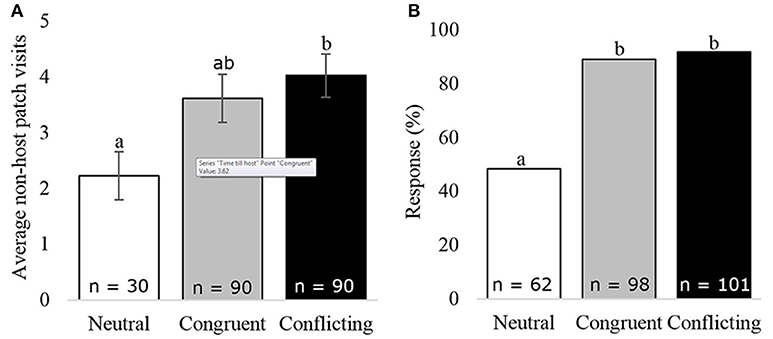
Figure 6. Wasp behavior under different foraging situations: (A) average non-host patch visits, (B) the percentage of wasps responding to the HIPVs in the wind tunnel. Bars with different letters are significantly different (α = 0.05), error bars show the s.e.
Test Plant Species Effects on Foraging Behavior
The plants species offered during the foraging trail also greatly influenced foraging behavior. In foraging situations when non-hosts were present on B. nigra and the hosts on S. arvensis, wasps took longer to find the host (Chi2 = 4.87, p = 0.027), they spent more time on non-host leaves (t = −3.38, p = 0.001, Figure 7A) and visited non-host leaves more often (t = −2.61, p = 0.010, Figure 7B), than when the host was on B. nigra and the non-host on S. arvensis. Furthermore, wasps performed more direct flights when the host was found on B. nigra, than when it was on S. arvensis (z = 2.79, p = 0.005, Figure 7C).
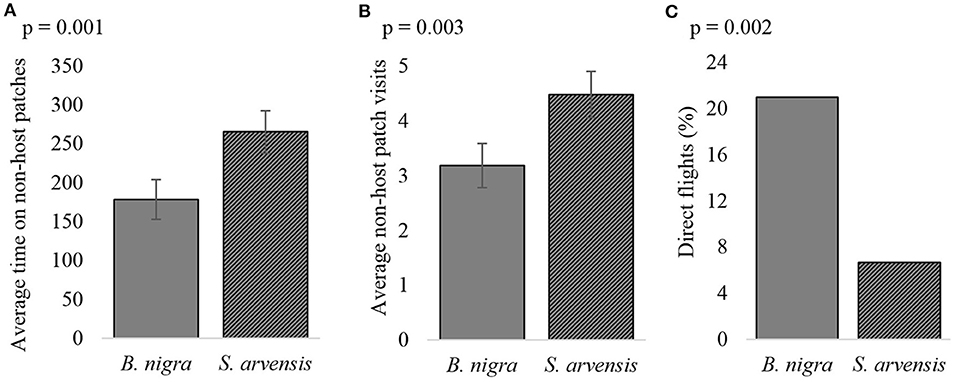
Figure 7. The effect of different host plant species on (A) the average amount of time wasps spent on non-host patches, (B) the average number of visits to non-host patches and (C) the number of direct flights, when the host is found on either Brassica nigra or Sinapis arvensis. Error bars show the s.e. (n = 105).
Discussion
Natural environments are ever changing, and as a consequence learned information can become outdated and should be forgotten. Since most parasitoids are time-limited, they should utilize their time as efficiently as possible, since the way they exploit their environment directly determines their realized fitness (Van Baalen and Hemerik, 2007). While most studies focusing on the effect of learning on foraging efficiency provide the wasp with a foraging situation highly similar to what they have been trained in Geervliet et al. (1998), Takasu and Lewis (2003), Bleeker et al. (2006), Smid et al. (2007), Kruidhof et al. (2015), De Rijk et al. (2018), and Desurmont et al. (2018), we tested how both reliable and unreliable information affects foraging efficiency in a foraging situation with attractive odor plumes of both hosts and non-hosts. As expected, we found maladaptive foraging behavior after providing wasps with conflicting information, especially when the information has previously proven to be reliable through spaced conditioning. It seems that a 3 spaced oviposition experience does not only result in longer lasting memory (Smid et al., 2007; Van Vugt et al., 2015), but also results in a stronger focus on the memory content during foraging as the information is considered more reliable. This was reflected in wasps taking more time to find hosts and spending more time on non-host patches.
Though learning is generally expected to result in an increase in foraging efficiency, finding more hosts and increasing realized fitness, this most likely only applies if the obtained information is correct. Learning is known to be costly in various ways (Mery and Kawecki, 2004) and our study confirms that persistent unreliable memory involves costs primarily associated with time. However, it is still unclear how the wasp will overcome long-term negative effects of this unreliable information. The encounter of a non-host, upon the response to HIPV's previously associated with a host, causes a prediction error and can be considered as a memory extinction event. This event might trigger the formation of additional memory traces, which will diminish the response to the learned cues faster than by natural memory decay (Exton-Mcguinness et al., 2015).
Hoedjes et al. (2011) suggested that high cue variability and low cue reliability within a generation should favor the formation of short-lasting memory forms such as STM and ARM rather than LTM. Since LTM is formed after a single oviposition in P. brassicae it seems likely to assume that the HIPVs cue learned in this experiment are considered to be of high cue reliability under natural conditions. However, P. brassicae and M. brassicae have overlapping host-plant species ranges and share the same habitats. Co-occurrence of these species occurs under natural conditions, on plants in close proximity, but also on the same plant and even the same leaf (Vos et al., 2001). Therefore, it seems likely that non-hosts such as M. brassicae are regularly encountered and the cue reliability would be rather low. However, a single encounter with the gregarious P. brassicae caterpillars consists of such a high reward value, due to multiple oviposition opportunities, that this might outweigh potential negative effects of cue variability and still facilitates LTM formation after a single oviposition. As mentioned in Koops (2004), if the benefit of correct information is high relative to the cost of the information being unreliable, then the wasps should still respond, even if the reliability of the information is relatively low.
The observed foraging behavior also varied with plant species. When hosts were present on B. nigra and non-host on S. arvensis, wasps found the host-infested plants faster, performed more direct flights and spent less time on non-host-infested plants compared with the reciprocal situation. Sinapis arvensis and Brassica nigra are considered sister species (Agerbirk et al., 2008), yet they are apparently different enough to cause substantial differences in foraging behavior, depending on which plant species contained the hosts or non-hosts. Possibly, B. nigra HIPVs are easier to detect, or are more attractive, than HIPVs of S. arvensis, making it easier for wasps to find the attractive B. nigra host-infested plant among the less attractive HIPVs of S. arvensis, than vice versa.
The observation that C. glomerata is less efficient at finding host in the presence of non-hosts has already been shown in various studies (Bukovinszky et al., 2012; De Rijk et al., 2016a), yet so far there has been no mentioning of non-host acceptance under (semi-) natural foraging conditions. Under laboratory conditions, however, Vosteen et al. (manuscript in preparation) and Bukovinszky et al. (2012) found occasional non-host oviposition by C. glomerata in M. brassicae with flight assays. Vosteen et al. (manuscript in preparation) found non-host acceptance levels up to 27%, which seems comparable with our findings. Currently we are investigating to which extent M. brassicae is truly a non-host, if these findings are a side-effect of the test setup and which circumstances favor non-host acceptance.
In contrast to what we expected, we found that congruently conditioned wasps behaved very similar to unconditioned wasps. While the study of Kruidhof et al. (2015) showed higher foraging efficiency after associative learning of HIPVs with C. glomerata, we did not find this in this study. The main reason why we do not find this positive effect of associative learning is most likely since we discarded wasps which did not respond within 5 min. While response levels of the conditioned wasps were around 90%, only 48% of the unconditioned wasps responded within 5 min. Oviposition experienced wasps are known to be more responsive to HIPVs in general. Giving wasps oviposition experience or exposing them to host frass prior to testing is a general way to increase the responsiveness of parasitoid to HIPVs (Geervliet et al., 1998; Takasu and Lewis, 2003; Bleeker et al., 2006; Peñaflor et al., 2017).
Overall we conclude that learning unreliable information causes maladapted foraging behavior, which reduces foraging efficiency under the conflicting test conditions, compared to the congruent test situation. However, parasitoids do not only learn to associate environmental cues with host while foraging, but also with food (Tertuliano et al., 2004; Wäckers et al., 2006). Hungry parasitoid will primarily respond to cues associated with food, while fed parasitoids will primarily respond to cues associated with hosts (Lewis and Takasu, 1990; Luo et al., 2013). Their environment in combination with their physiological state will determine their foraging behavior and the way they use learned cues. The effect of unreliable memory in relation to food learning and foraging behavior has not been researched in parasitoids so far, but has been researched in honeybees with color learning with a food reward. Similar negative effects of persistent unreliable memory were found; 3 learning events led to longer lasting memory than 1 learning event (Menzel, 1968), and bees with 3 learning events returned more often to the previously rewarding color, which now only supplied tap water, than wasps given 1 learning event (Couvillon and Bitterman, 1980).
By learning how parasitoids integrate different kinds of information from their environment to optimize foraging efficiency, we can greatly advance spatial movement models and biological control efforts (Van Alphen and Bernstein, 2008; Ishii and Shimada, 2009; Wajnberg et al., 2016). Furthermore, the higher response of parasitoids to local HIPVs after learning is interesting for biological control practices (Prokopy and Lewis, 1993; Giunti et al., 2015).
Author Contributions
JdB, HS, and LV designed the study. JdB conducted the experiments and analyzed the data. JdB, HS, and LV interpreted the data. JdB prepared the figures and drafted the MS. HS prepared Figure 1. JdB wrote the final version based on comments of HS and LV.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by NWO open competition grant nr. 824.14.023 to LV.
References
Agerbirk, N., Warwick, S. I., Hansen, P. R., and Olsen, C. E. (2008). Sinapis phylogeny and evolution of glucosinolates and specific nitrile degrading enzymes. Phytochemistry 69, 2937–2949. doi: 10.1016/j.phytochem.2008.08.014
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2014). lme4: Linear Mixed-Effects Models Using Eigen and S4. R package version 1, 1–23.
Bleeker, M. A. K., Smid, H. M., Steidle, J. L. M., Kruidhof, H. M., Van Loon, J. J. A., and Vet, L. E. M. (2006). Differences in memory dynamics between two closely related parasitoid wasp species. Anim. Behav. 71, 1343–1350. doi: 10.1016/j.anbehav.2005.09.016
Bukovinszky, T., Poelman, E. H., Kamp, A., Hemerik, L., Prekatsakis, G., and Dicke, M. (2012). Plants under multiple herbivory: consequences for parasitoid search behaviour and foraging efficiency. Anim. Behav. 83, 501–509. doi: 10.1016/j.anbehav.2011.11.027
Coleman, R., Barker, A., and Fenner, M. (1999). Parasitism of the herbivore Pieris brassicae L.(Lep., Pieridae) by Cotesia glomerata L.(Hym., Braconidae) does not benefit the host plant by reduction of herbivory. J. Appl. Entomol. 123, 171–177. doi: 10.1046/j.1439-0418.1999.00334.x
Couvillon, P., and Bitterman, M. (1980). Some phenomena of associative learning in honeybees. J. Comp. Physiol. Psychol. 94:878. doi: 10.1037/h0077808
De Rijk, M., Cegarra Sánchez, V., Smid, H. M., Engel, B., Vet, L. E., and Poelman, E. H. (2018). Associative learning of host presence in non-host environments influences parasitoid foraging. Ecol. Entomol. 43, 318–325. doi: 10.1111/een.12504
De Rijk, M., Wang, Q., Papagiannaki, E., Dicke, M., and Poelman, E. H. (2016a). Herbivore species identity rather than diversity of the non-host community determines foraging behaviour of the parasitoid wasp Cotesia glomerata. Entomol. Exp. Appl. 161, 20–30. doi: 10.1111/eea.12493
De Rijk, M., Zhang, X., Van Der Loo, J. A., Engel, B., Dicke, M., and Poelman, E. H. (2016b). Density-mediated indirect interactions alter host foraging behaviour of parasitoids without altering foraging efficiency. Ecol. Entomol. 41, 562–571. doi: 10.1111/een.12325
Desurmont, G. A., Guiguet, A., and Turlings, T. C. (2018). Invasive insect herbivores as disrupters of chemically-mediated tritrophic interactions: effects of herbivore density and parasitoid learning. Biol. Invasions 20, 195–206. doi: 10.1007/s10530-017-1526-x
Exton-Mcguinness, M. T. J., Lee, J. L. C., and Reichelt, A. C. (2015). Updating memories—The role of prediction errors in memory reconsolidation. Behav. Brain Res. 278, 375–384. doi: 10.1016/j.bbr.2014.10.011
Geervliet, J. B. F., Vet, L. E. M., and Dicke, M. (1994). Volatiles from damaged plants as major cues in long-range host-searching by the specialist parasitoid Cotesia rubecula. Entomol. Exp. Appl. 73, 289–297. doi: 10.1111/j.1570-7458.1994.tb01866.x
Geervliet, J. B. F., Vet, L. E. M., and Dicke, M. (1996). Innate responses of the parasitoids Cotesia glomerata and C. rubecula (Hymenoptera: Braconidae) to volatiles from different plant-herbivore complexes. J. Insect Behav. 9, 525–538. doi: 10.1007/BF02213877
Geervliet, J. B. F., Vreugdenhil, A. I., Dicke, M., and Vet, L. E. M. (1998). Learning to discriminate between infochemicals from different plant-host complexes by the parasitoids Cotesia glomerata and C. rubecula. Entomol. Exp. Appl. 86, 241–252. doi: 10.1046/j.1570-7458.1998.00286.x
Giunti, G., Canale, A., Messing, R. H., Donati, E., Stefanini, C., Michaud, J. P., et al. (2015). Parasitoid learning: current knowledge and implications for biological control. Biol. Control 90, 208–219. doi: 10.1016/j.biocontrol.2015.06.007
Hilker, M., and McNeil, J. (2008). “Chemical and behavioral ecology in insect parasitoids: how to behave optimally in a complex odor-ous environment,” in Behavioral Ecology of Insect Parasitoids, eds E. Wajnberg, C. Bernstein, and J. van Alphen (Oxford: Blackwell Publishing Ltd.), 31–50. doi: 10.1002/9780470696200.ch5
Hoedjes, K. M., Kruidhof, H. M., Huigens, M. E., Dicke, M., Vet, L. E., and Smid, H. M. (2011). Natural variation in learning rate and memory dynamics in parasitoid wasps: opportunities for converging ecology and neuroscience. Proc. Biol. Sci. 278, 889–897. doi: 10.1098/rspb.2010.2199
Ishii, Y., and Shimada, M. (2009). The effect of learning and search images on predator–prey interactions. Popul. Ecol. 52:27. doi: 10.1007/s10144-009-0185-x
Koops, M. A. (2004). Reliability and the value of information. Anim. Behav. 67, 103–111. doi: 10.1016/j.anbehav.2003.02.008
Kruidhof, H. M., Pashalidou, F. G., Fatouros, N. E., Figueroa, I. A., Vet, L. E., Smid, H. M., et al. (2012). Reward value determines memory consolidation in parasitic wasps. PLoS ONE 7:e39615. doi: 10.1371/journal.pone.0039615
Kruidhof, H. M., Roberts, A. L., Magdaraog, P., Munoz, D., Gols, R., Vet, L. E., et al. (2015). Habitat complexity reduces parasitoid foraging efficiency, but does not prevent orientation towards learned host plant odours. Oecologia 179, 353–361. doi: 10.1007/s00442-015-3346-y
Lenth, R. V. (2016). Least-squares means: the R package lsmeans. J. Stat. Softw. 69, 1–33. doi: 10.18637/jss.v069.i01
Lewis, W., and Takasu, K. (1990). Use of learned odours by a parasitic wasp in accordance with host and food needs. 348, 635–636. doi: 10.1038/348635a0
Lewis, W. J., and Martin, W. R. (1990). Semiochemicals for use with parasitoids: status and future. J. Chem. Ecol. 16, 3067–3089. doi: 10.1007/BF00979613
Luo, S., Michaud, J. P., Li, J., Liu, X., and Zhang, Q. (2013). Odor learning in Microplitis mediator (Hymenoptera: Braconidae) is mediated by sugar type and physiological state. Biol. Control 65, 207–211. doi: 10.1016/j.biocontrol.2013.02.010
Menzel, R. (1968). Das gedächtnis der honigbiene für spektralfarben. Zeitschr. Vergleic. Physiol. 60, 82–102. doi: 10.1007/BF00737097
Menzel, R. (1999). Memory dynamics in the honeybee. J. Comp. Physiol. A 185, 323–340. doi: 10.1007/s003590050392
Mery, F., and Kawecki, T. J. (2004). An operating cost of learning in Drosophila melanogaster. Anim. Behav. 68, 589–598. doi: 10.1016/j.anbehav.2003.12.005
Peñaflor, M. F. G., Gonçalves, F. G., Colepicolo, C., Sanches, P. A., and Bento, J. M. S. (2017). Effects of single and multiple herbivory by host and non-host caterpillars on the attractiveness of herbivore-induced volatiles of sugarcane to the generalist parasitoid Cotesia flavipes. Entomol. Exp. Appl. 165, 83–93. doi: 10.1111/eea.12623
Perfecto, I., and Vet, L. E. M. (2003). Effect of a nonhost plant on the location behavior of two parasitoids: the tritrophic system of Cotesia spp. (Hymenoptera: Braconidae), Pieris rapae (Lepidoptera: Pieridae), and Brassica oleraceae. Environ. Entomol. 32, 163–174. doi: 10.1603/0046-225X-32.1.163
Pinheiro, J., Bates, D., Debroy, S., and Sarkar, D. (2014). R Core Team (2014) Nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-117. Available online at: http://CRAN.R-project.org/package=nlme
Prokopy, R. J., and Lewis, W. J. (1993). “Application of learning to pest management,” in Insect Learning: Ecology and Evolutionary Perspectives, eds D. R. Papaj and A. C. Lewis (Boston, MA: Springer US), 308–342.
Smid, H. M., Wang, G., Bukovinszky, T., Steidle, J. L., Bleeker, M. A., Van Loon, J. J., et al. (2007). Species-specific acquisition and consolidation of long-term memory in parasitic wasps. Proc. Biol. Sci. 274, 1539–1546. doi: 10.1098/rspb.2007.0305
Stephens, D. W. (1993). “Learning and behavioral ecology: incomplete information and environmental predictability,” in Insect Learning (Springer), 195–218.
Takasu, K., and Lewis, W. J. (2003). Learning of host searching cues by the larval parasitoid Microplitis croceipes. Entomol. Exp. Appl. 108, 77–86. doi: 10.1046/j.1570-7458.2003.00070.x
Tertuliano, M., Olson, D. M., Rains, G. C., and Lewis, W. J. (2004). Influence of handling and conditioning protocol on learning and memory of Microplitis croceipes. Entomol. Exp. Appl. 110, 165–172. doi: 10.1111/j.0013-8703.2004.00132.x
Therneau, T. M., and Lumley, T. (2015). Package “Survival.” R Top Doc, 128. Available online at: ftp://164.41.45.44/pub/plan/R/web/packages/survival/survival.pdf
Tully, T., Preat, T., Boynton, S. C., and Del Vecchio, M. (1994). Genetic dissection of consolidated memory in Drosophila. Cell 79, 35–47. doi: 10.1016/0092-8674(94)90398-0
Turlings, T. C. L., Wëckers, F. L., Vet, L. E. M., Lewis, W. J., and Tumlinson, J. H. (1993). “Learning of host-finding cues by hymenopterous parasitoids,” in Insect Learning, eds D. R. Papaj and A. C. Lewis (Boston, MA: Springer).
Van Alphen, J. J., and Bernstein, C. (2008). “Information acquisition, information processing, and patch time allocation in insect parasitoids,” in Behav. Ecol. Insect Parasitoids, 172–192. doi: 10.1002/9780470696200.ch8
Van Baalen, M., and Hemerik, L. (2007). “Parasitoid fitness: from a simple idea to an intricate concept,” in Behavioral Ecology of Insect Parasitoids: From Theoretical Approaches to Field Applications (Oxford: Blackwell Publishing Ltd.), 31–50. doi: 10.1002/9780470696200.ch2
Van Vugt, J. J., Hoedjes, K. M., Van De Geest, H. C., Schijlen, E. W., Vet, L. E., and Smid, H. M. (2015). Differentially expressed genes linked to natural variation in long-term memory formation in Cotesia parasitic wasps. Front. Behav. Neurosci. 9:255. doi: 10.3389/fnbeh.2015.00255
Vet, L. (2001). Parasitoid searching efficiency links behaviour to population processes. 36, 399–408. doi: 10.1303/aez.2001.399
Vet, L. E., and Dicke, M. (1992). Ecology of infochemical use by natural enemies in a tritrophic context. Annu. Rev. Entomol. 37, 141–172. doi: 10.1146/annurev.en.37.010192.001041
Vos, M., Berrocal, S. M., Karamaouna, F., Hemerik, L., and Vet, L. (2001). Plant-mediated indirect effects and the persistence of parasitoid–herbivore communities. Ecol. Lett. 4, 38–45. doi: 10.1046/j.1461-0248.2001.00191.x
Wäckers, F., Bonifay, C., Vet, L., and Lewis, J. (2006). Gustatory response and appetitive learning in Microplitis croceipes in relation to sugar type and concentration. Anim. Biol. 56, 193–203. doi: 10.1163/157075606777304230
Keywords: Cotesia glomerata, learning, foraging efficiency, unreliable information, non-host, oviposition, prediction error, memory
Citation: de Bruijn JAC, Vet LEM and Smid HM (2018) Costs of Persisting Unreliable Memory: Reduced Foraging Efficiency for Free-Flying Parasitic Wasps in a Wind Tunnel. Front. Ecol. Evol. 6:160. doi: 10.3389/fevo.2018.00160
Received: 31 July 2018; Accepted: 25 September 2018;
Published: 16 October 2018.
Edited by:
Paul-andré Calatayud, Institut de Recherche pour le Développement (IRD), FranceReviewed by:
Pasquale Trematerra, University of Molise, ItalyMoukaram Tertuliano, University of Georgia, United States
Copyright © 2018 de Bruijn, Vet and Smid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jessica A. C. de Bruijn, jessica.debruijn@wur.nl
 Jessica A. C. de Bruijn
Jessica A. C. de Bruijn Louise E. M. Vet
Louise E. M. Vet Hans M. Smid
Hans M. Smid