Altering Neighborhood Relatedness and Species Composition Affects Interior Douglas-Fir Size and Morphological Traits With Context-Dependent Responses
- 1Department of Forest and Conservation Sciences, The University of British Columbia, Vancouver, BC, Canada
- 2Department of Biology, McMaster University, Hamilton, ON, Canada
Trees often exist in a complex ecological system with many biological interactions. Here we examine kin interactions of Pseudotsuga menziesii var. glauca (interior Douglas-fir) both in the context of pure kin stands, in accordance with established plant kin selection and recognition studies, but also in combination with inter and intraspecific neighbors in order to observe how interactions may differ in a more complex system. Seedlings grown with kin neighbors (i.e., in stands that contained only kin) were significantly larger (biomass, height, and root length) than those grown with any type of unrelated neighbor. However, of those with an unrelated neighbor, performance was better if that neighbor was interspecific (lodgepole pine rather than a stranger, or non-kin, Douglas-fir neighbor). Interestingly when Douglas-fir was grown in mixed stands, the four growth and four morphological traits of the seedlings examined paralleled neither pure stranger nor pure kin stands. This suggests that a mixed stand environment yielded cues that were uniquely different than either type of pure stand and that these seedlings are able to integrate that information and respond in a different way; for example, with increased early mycorrhizal fungal colonization. The morphological traits fine: coarse root allocation and slenderness (height relative to diameter) closely paralleled the seeding-size results, with the greatest values in pure kin stands. Whereas, fine root: needle allocation showed a kin response of less fine root allocation relative to needle mass compared to strangers, but kin seedlings had more fine root allocation when grown with a pine compared to a stranger Douglas-fir neighbor. We have demonstrated that the kin response in Douglas-fir is influenced by the complexity of the environment in which it grows, and this has significant effects on growth, morphology and mycorrhizal fungal colonization that may affect the success and resiliency of regeneration.
Introduction
In Douglas-fir forests of interior British Columbia, an individual of Pseudotsuga menziesii var. glauca [interior Douglas-fir (Fdi); hereafter referred to as Douglas-fir] interacts with a complex arrangement of neighbors that can include other Douglas-fir individuals that are conspecifics of either kin or stranger relation, different tree species, or many other plant species present in the environment. Seedling growth is commonly affected by the presence and density of neighboring plants (Harper, 1977; Goldberg and Werner, 1983) through their influences on light, water and nutrient availability (Murison, 1960; Williams et al., 1999; Restaino et al., 2016). They can also affect the microclimate, mutualists such as mycorrhizal fungi, and the cues they provide. In past studies, we have found that some Douglas-fir families demonstrate the ability to sense and respond to such cues about the relatedness of conspecific neighbors (Asay, 2013; Gorzelak, 2017; Pickles et al., 2017). Kin recognition and kin selection studies often isolate a species from others it may naturally co-occur with and kin are compared with strangers grown separately in pairs or groups (Cheplick and Kane, 2004; Dudley and File, 2007; Murphy and Dudley, 2009; Biedrzycki and Bais, 2010; Biedrzycki et al., 2010; Bhatt et al., 2011; File et al., 2012b; Asay, 2013; Gorzelak, 2017; Pickles et al., 2017). However, in nature, trees such as Douglas-fir often interact with kin and strangers simultaneously, and with different species, which creates a more complex environment.
Interior Douglas-fir is a pioneer species in the wet climatic regions of interior British Columbia, Canada, but in arid regions, it is present through all stages of secondary succession. It is the climatic climax species in the arid subzones of the interior Douglas-fir (IDF) biogeoclimatic (BEC) zone, and is commonly associated with lodgepole pine (Pinus contorta var. latifolia), a post-fire pioneer, at higher elevations and in northerly latitudes (Williams et al., 1999). Fdi is moderately shade tolerant and is able to persist in the understory of conspecifics or heterospecifics much better than shade-intolerant lodgepole pine (Lavender and Hermann, 2013). When established together, lodgepole pine typically grows faster than Fdi (Vyse et al., 2006).
When Fdi establishes with neighbors, its growth, particularly regarding crown morphology, is plastic (Chen et al., 1996; Williams et al., 1999). The plasticity of Douglas-fir depends not only on the presence but also the size of close neighbors, and neighborhood effects can be species-specific. Douglas-fir had lower diameter when grown with intraspecific (not specifically kin or stranger) neighbors compared to when it was grown with interspecific neighbors (noble fir or grand fir), or when grown in the absence of neighbors, respectively (Devine and Harrington, 2011). These results are consistent with the competitive exclusion principle or niche partitioning hypothesis, which states that interspecific neighbors have distinct abilities to access different resource pools and therefore both species can benefit from the lack of direct competition for those pools (Walter, 1991; Devine and Harrington, 2011; File et al., 2012b).
Previous studies in Douglas-fir and in other plant species have demonstrated differences in performance, adaptive traits, resource transfer and mycorrhizal colonization depending on the relatedness of neighbors, indicative of kin recognition and/or kin selection (Reviewed in File et al., 2012a, b; Asay, 2013; Gorzelak, 2017; Pickles et al., 2017). However, it is not known whether these differences will be measurable in Douglas-fir under conditions that better emulate the composition of natural forests where more factors that potentially influence growth and behavior are present. The response of individuals to the presence of both kin and strangers simultaneously has not been explored in relatedness studies thus far. Groups of kin are commonly compared to groups of strangers, where there is different relatedness between groups, but within a group the degree of relatedness between individuals is the same; either all related or all unrelated (defined as pure stands here). In many plant communities in nature, however, there may be individuals that neighbor both kin and strangers simultaneously (defined as mixed stands here). Plant neighborhoods can vary in relatedness of intraspecific neighbors, whether the individuals in the neighborhood have the same degree of relatedness (pure stands) or not (mixed stands) depending on dispersal patterns, as well as having a variety of interspecific neighbors. This degree of complexity is not commonly included in relatedness studies. A better understanding of within- and between-species interactions will help reveal what is important to the success of seedlings in natural, complex environments and ideally help shape forest regeneration methods that promote resilience in the face of changing systems.
In a greenhouse study, Douglas-fir seedlings were grown in pots in pure kin and stranger stands, mixed kin and stranger stands, in kin stands with an interspecific neighbor, lodgepole pine, and as an individual Douglas-fir with two pine neighbors. We measured plant functional traits and mycorrhizal fungal association with roots. We ask the following questions: Does neighborhood complexity affect the kin recognition responses in seedlings? Which, if any, traits will show a relatedness response: morphology, growth or association with mycorrhizal fungi? How will the kin recognition response in a complex neighborhood, i.e., kin mixed with strangers or mixed with pine, differ from the response in pure stands?
Materials and Methods
Experimental Design and Treatments
A replacement series design was used to test the effects of neighborhood composition on Douglas-fir size and morphological traits. We tested whether the size, morphology or mycorrhizal colonization of Douglas-fir seedlings was responsive to whether neighbors were kin, stranger Douglas-firs, or lodgepole pine, or a combination thereof. Neighborhood effects on size of lodgepole pine seedlings were analyzed separately. The neighborhood treatments, shown in the top row of Figure 1A, included: (1) two kin Douglas-fir seedlings and one stranger Douglas-fir from a different family (Kin/Stranger), this included a related pair neighboring each of other two families (e.g., AAB and AAC; Supplementary Figure 1); (2) three kin Douglas-fir seedlings, all from the same family (Kin); (3) three stranger Douglas-fir seedlings from three different families (Stranger); (4) three lodgepole pine seedlings (Pine); (5)two kin Douglas-fir seedlings and one lodgepole pine (Kin/Pine) and (6) one Douglas-fir seedling and two lodgepole pine seedlings (Douglas-fir/Pine). These neighborhood treatments were applied to three distinct full-sib Douglas-fir families (A, B, and C), and each neighborhood treatment × family combination was replicated five times in a completely randomized design that included 95 pots with 225 Douglas-fir seedlings and 60 lodgepole pine seedlings (Supplementary Figure 1). Five replicates of individual seedlings from the three Douglas-fir families as well as lodgepole pine were grown alone in pots for comparison of families grown in the absence of neighbors (15 individual Douglas-fir and five individual pine seedlings in 20 pots).
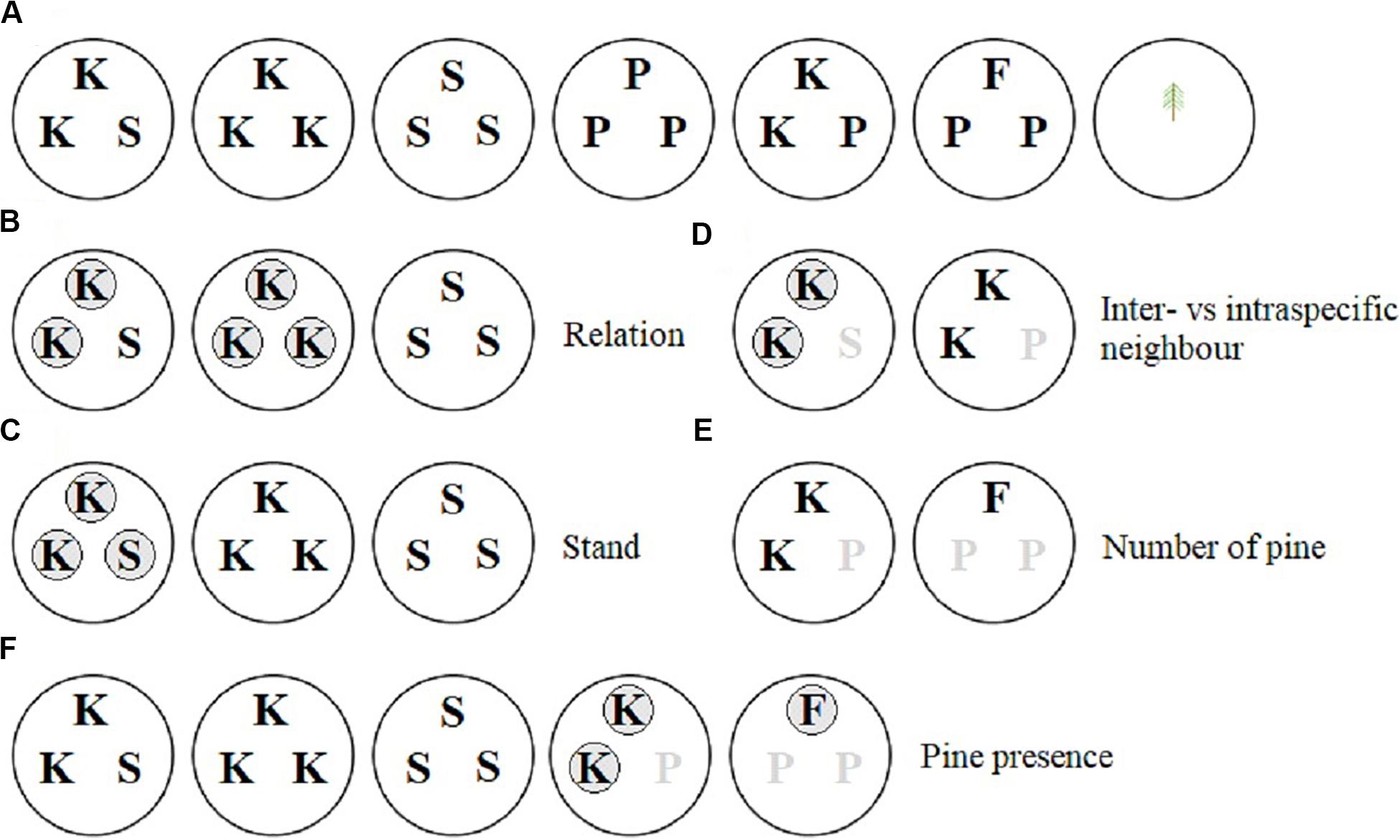
Figure 1. Greenhouse pot organization to examine neighbor effects on interior Douglas-fir. P = lodgepole pine, S = stranger Douglas-fir, K = kin Douglas-fir, F = Douglas-fir (in the Douglas-fir/pine neighbor treatment). Each of three Douglas-fir families were equally represented and the lodgepole pine seedlings were replicated five times in panel (A). The pure pine and single seedling pots were not used in the analyses of Douglas-fir seedlings. The pure pine was used in the pine seedling analyses (as well as Kin/Pine and Douglas-fir/Pine) and all pots were used in the family analysis. Panels (B–F) represent the contrast set ups. Unshaded seedlings were compared with shaded seedlings of the neighbor treatments shown. When seedlings present in a pot were omitted from the contrast they are indicated by a gray letter.
The experiment took place at the University of British Columbia (UBC) Horticulture Greenhouse in Vancouver, BC, Canada over a 5-month period (December 2016 to May 2017). The seed for the three full-sib families of Douglas-fir and lodgepole pine were sourced from the BC Ministry of Forests, Lands and Natural Resources Operations, Kalamalka Research Station, located near Vernon, BC, Canada. Seeds were a product of controlled cross pollinations. Trees were from three seed planning zones (Mica, West Kootenay Low, and East Kootenay), however, all parent trees fell within one BC forest district (Selkirk in the Kootenay-Boundary region). One parent was located in the Montane Spruce (MS) biogeoclimatic zone (maternal, Family B), and all others came from the Interior Cedar-Hemlock (ICH) biogeoclimatic zone (Supplementary Table 1). The elevation of parent tree locations ranged from 585 to 1255 m. Mean annual temperature ranged from 2.2 to 6.8°C and mean annual precipitation ranged from 478 to 1060 mm among the six parent trees (Supplementary Table 2) based on provided GPS coordinates and elevation per parent tree and using data obtained from 1981 to 2010 historical climate normals through ClimateNA (Wang et al., 2016). All trees were located within a 100 km radius. As geographic and climatic conditions varied between parents, some variation due to family was expected and accounted for during the statistical analysis.
Stratified seeds of the three Fdi families and lodgepole pine were sown at a density of three seedlings per pot on December 9, 2016 in the treatments described above. A total of 115 standard two-gallon pots (approximately 7.5 L with a surface area of approximately 346 cm2) were filled with a mixture that was 50% potting mix (75% peat and 25% perlite from West Creek Farms, Fort Langley, BC, Canada) and 50% field soil, which was collected from a clearcut 7 km southeast of Revelstoke, BC, Canada. Extra seedlings were grown in individual trays containing potting mix to replace seeds that did not emerge within 30 days or seedlings that died within 60 days of sowing. The last transplantation occurred on February 8, 2017, and all seedlings grew together for at least 3 months post-transplant. Transplanting did not have a distinctly positive or negative effect on performance. The pots were monitored daily and the surface soil was kept moist for the first month to promote germination, then watered by hand twice per week for the duration of the experiment. This watering schedule allowed for close monitoring of the moisture level. The soil was never allowed to dry out completely, but surface dryness did occur. The greenhouse was kept at a minimum of 16°C. No additional light, shading or fertilization was applied to the seedlings.
Sample Processing
All seedlings were harvested in May 2017. Roots of each individual were separated from above ground biomass at soil level and stored separately in sealable plastic bags at 4°C until further processing. Fresh above-ground height and root collar diameter were measured prior to needles and branches being removed. Needles, branches and stems were then separated, and the shoot tissues were dried at 70°C for a minimum of 48 h and weighed. Roots were cleaned of adhering soil, washed with tap water, scanned, and the morphology data was uploaded into WinRHIZO© Pro software. Up to 50 root tips per individual were randomly selected and examined under a dissecting microscope to determine the proportion of root tips colonized by mycorrhizal fungi (Asay, 2013). Root systems were then dried and weighed as with above-ground biomass.
Data Analysis
Size (biomass, height, diameter, and root length) and mycorrhizal fungal colonization were measured for each seedling. Biomass (mg) was the sum of below and above ground dry biomass. Height (cm) was measured from the base of the seedling at soil level to the apical bud or highest woody stem material (if apical bud had burst). Diameter (mm) was root collar diameter at soil level. Root length was a cumulative measure of the entire fresh root system determined with WinRHIZOTM Pro. Mycorrhizal fungal colonization (referred to as colonization in analysis) was the number of fresh root tips positively identified as colonized by a mycorrhizal fungus divided by the total number of root tips examined (equal to 50, unless the root system had fewer than 50 root tips present) and expressed as a percent. Four morphological traits relating to the allocation of growth within a seedling were analyzed through analysis of covariance, e.g., effects of treatments on root: shoot allocation were measured in an analysis of covariance (ANCOVA) with root mass as the dependent variable and shoot mass as the covariate, and then the root: shoot allocation was estimated for a treatment combination as the estimated square mean. Root: shoot allocation was the total dry root biomass relative to the total dry above ground biomass. Fine: coarse root allocation was the cumulative length of fine roots (defined as any roots with a diameter less than or equal to 2 mm using the traditional fine root classification) relative to the cumulative length of coarse roots (defined as any roots greater than 2 mm in diameter) of fresh samples (McCormack et al., 2015). This calculation was derived from the WinRHIZO Pro© analysis of length per root diameter class. Fine root: needle allocation was the cumulative length of fine roots relative to the total dry mass of all needles. Slenderness was the height relative to the diameter of each seedling.
Statistical analysis was performed using R version 3.5.1 (R Core Team, 2018). Linear models (Model 1) were fit using the “lm” function for each of the four size and colonization response variables for Douglas-fir and lodgepole pine separately, where response variables included biomass, height, diameter and root length. The significance level was α = 0.05.
Model 1 – ANOVA (size):
Linear models (Model 2) were also fit using the “lm” function but with the use of a covariate for each of the four morphological trait response variables for Douglas-fir only. For example, slenderness was the height divided by the diameter of each seedling, but was analyzed through analysis of covariance as follows:
Model 2 – ANCOVA (morphological traits):
Square-root (−1/2) or natural log (loge) data transformations were used when required to obtain near-normal data distributions. Outliers greater than 1.5 times the interquartile range were removed. The number amount of data removed was not significant (less than 1% of all data) and removing the outliers did not change the results described below. Type II analysis of variance (model 1) or covariance (model 2) was conducted to determine the overall effects of the neighborhood treatment and family on each of the size and morphology traits of the seedlings. Pre-planned contrasts were used within the Douglas-fir neighborhood treatments to test the effects of relation, stand, relation by stand interaction, species of neighbor to a kin pair (inter vs. intraspecific neighbor), presence of pine in the pot and, when present, the number of pine seedlings (Figures 1B–F). Relationships that were contrasted included: kin vs. stranger Douglas-fir seedlings (relation; Figure 1B), pure vs. mixed Douglas-fir neighborhoods (stand; Figure 1C), kin pairs vs. either an intra- or interspecific neighbor (Figure 1D), presence vs. absence of an interspecific neighbor (Figure 1F), and the proportion of intra- to interspecific neighbors (Figure 1E). Estimated marginal means were calculated using R package “emmeans” (Russell Lenth, 2018) and contrasts performed using the “contrast” function.
Pine seedlings were compared between the three neighborhood treatments in which they were present (Pine: three pines; Kin/Pine: two related Douglas-fir and one pine; and Douglas-fir/Pine: one Douglas-fir and two pines). A pairwise comparison of least square means was performed with a Bonferroni p value adjustment for multiple testing using “lsmeans” function in R package “emmeans” (Russell Lenth, 2018).
Simple linear models as described above in Models 1 and 2 were also fit to analyze biomass, colonization and fine: coarse root allocation of Douglas-fir with regard to family (A, B, C) and lodgepole pine seedlings as a main effect in addition to neighborhood, but used data from all pots including the single seedling pots grown with no immediate neighbors (305 seedlings in 115 plots, 20 of which were single seedling pots; Figure 1A, far right). The data was not transformed, and no outliers were removed.
Results
Kin Recognition Responses in Morphology, Performance and Mycorrhizal Fungi Associations
Effects of Complex Neighborhoods on the Kin Recognition Response
Neighborhood had a significant effect on all size and allocation response variables in Douglas-fir seedlings except root: shoot allocation (Tables 1, 2).
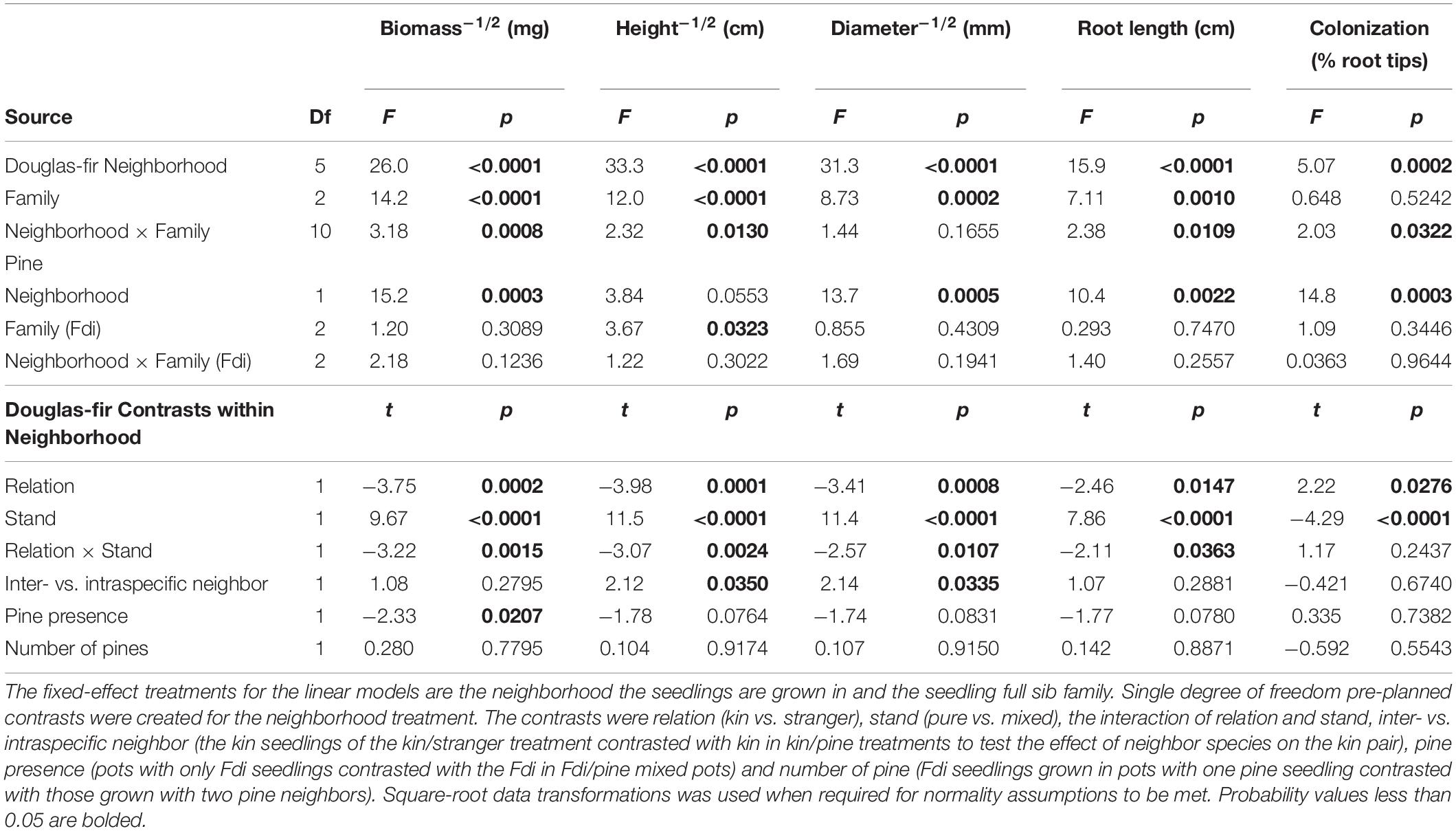
Table 1. Analyses of variance for size measures and mycorrhizal colonization for greenhouse-grown interior Douglas-fir (Fdi).
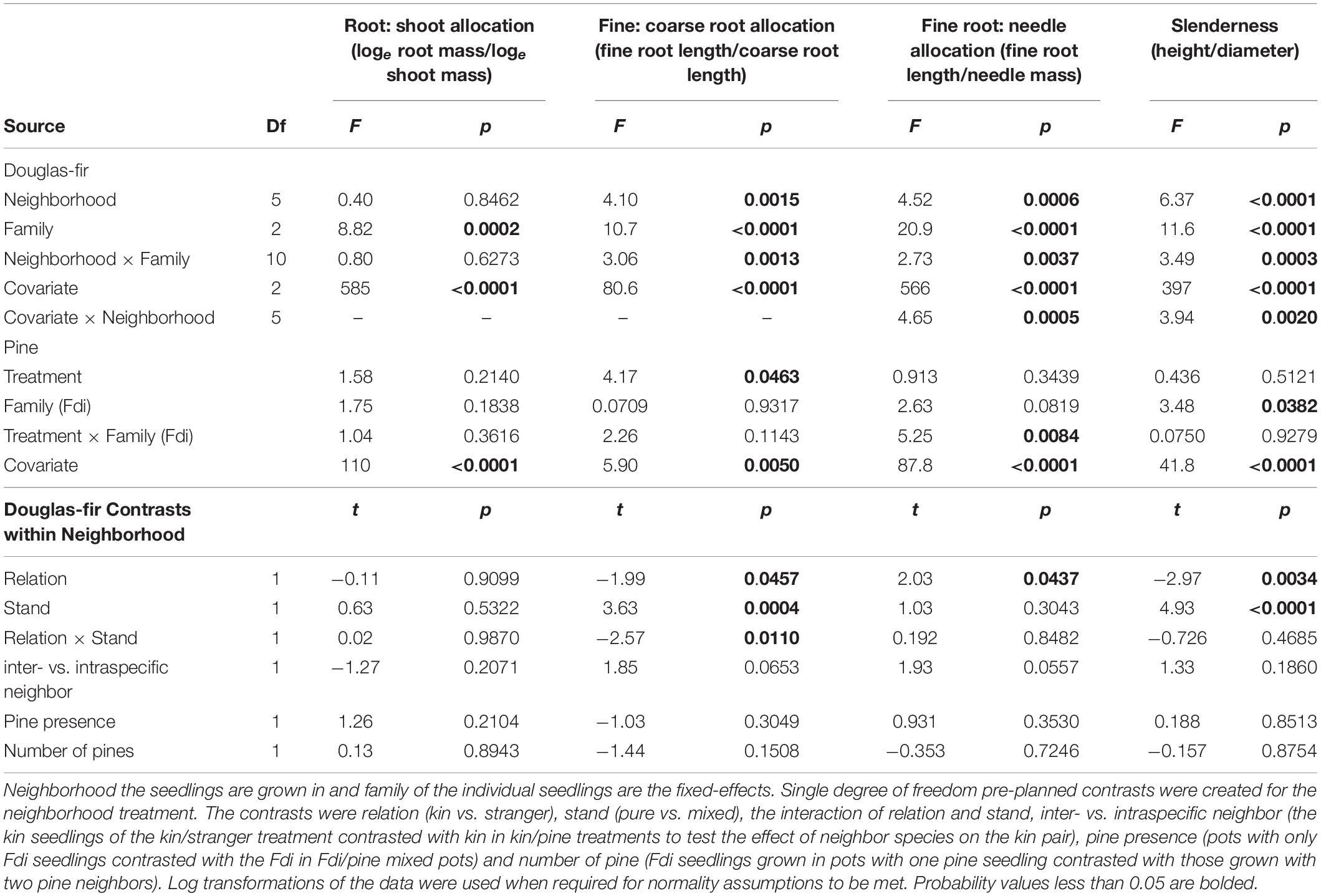
Table 2. Allocation and complex morphological trait analyses for groups of interior Douglas-fir (Fdi) grown in pots, taken from equivalent analyses of covariance (e.g., root: shoot ratio is analyzed with root mass as the dependent variable and shoot mass as the covariate).
Effects of Relatedness and Stand on Performance
When grown with only Douglas-fir neighbors, kin seedlings were consistently larger than strangers with respect to all size-related response variables (Table 1: Planned Contrast -Relation; Figures 2–4). Douglas-fir grown in pure stands (either exclusively kin or strangers) were also consistently larger compared to seedlings grown in mixed Douglas-fir stands (both kin and stranger in the same pot) (Planned Contrast–Stand). Additionally, there was a significant interaction between relation and stand that followed a consistent pattern in the estimated marginal means (Planned Contrast–Relation × Stand). Mean seedling size was largest in pure stands of kin seedlings, then decreased in the order: strangers in pure pots, kin in mixed stands, and strangers in mixed stands, although the difference between kin and stranger within the mixed stands was not significant.
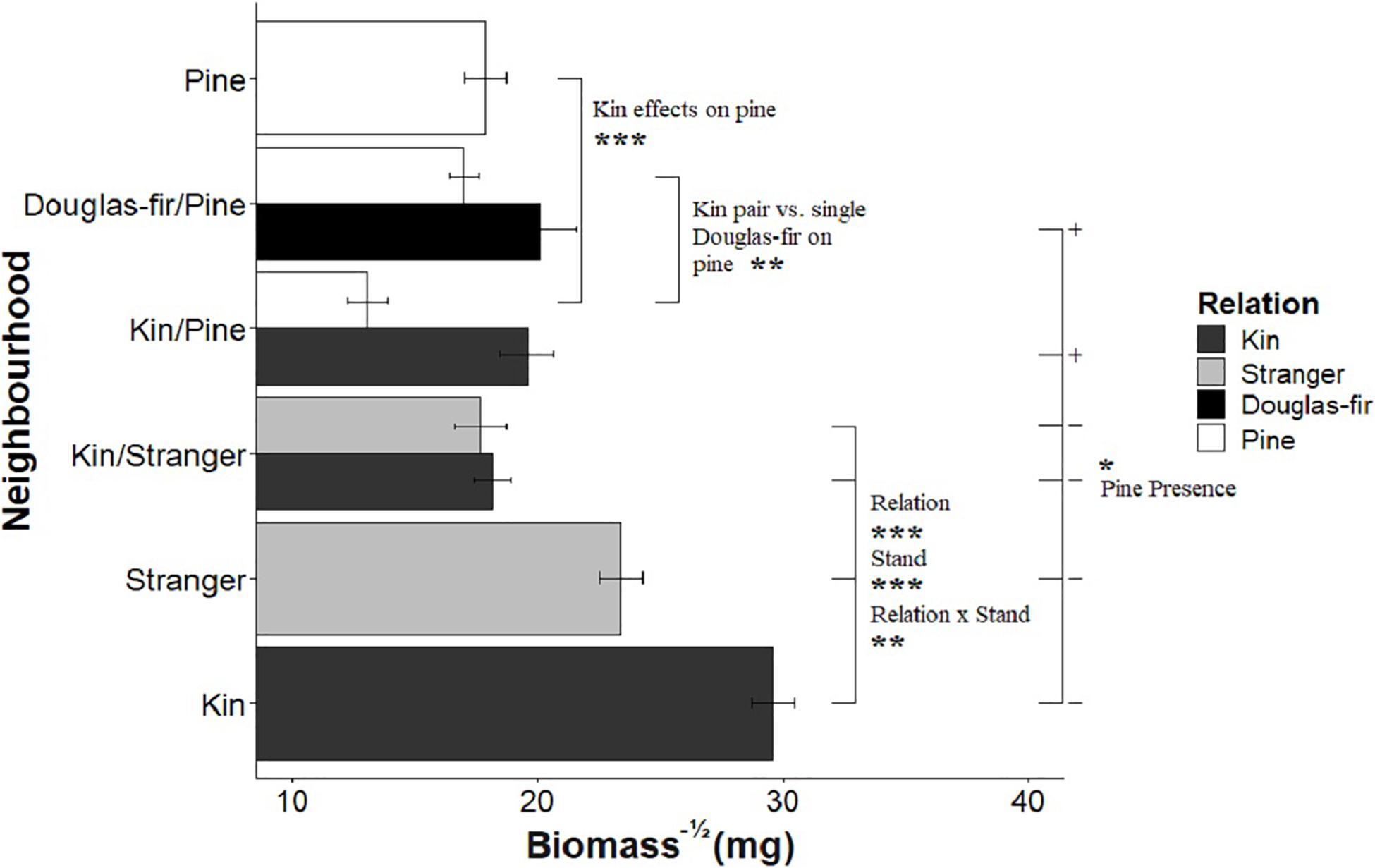
Figure 2. The effect of neighborhood treatments on the square-root of biomass for Douglas-fir seedlings (shaded) and pine seedlings (white). Significant planned contrasts are shown. Relation contrasted kin with stranger seedlings (estimate = –6.68, t ratio = –3.76, p = 0.0002, Df = 206). Stand contrasted seedlings grown in pure stand with those in mixed stands (estimate = 17.2, t ratio = 9.67, p < 0.0001, Df = 206). Relation × stand interaction was also significant (estimate = –5.73, t ratio = –3.22, p = 0.0015, Df = 206). The pine presence contrasted Douglas-fir seedlings grown with a pine neighbor vs. without a pine present in the pot (estimate = –4.77, t ratio = –2.33, p = 0.0207, Df = 206). Pine seedlings grown with a pair of kin had less biomass than those grown in pairs of pine with a single Douglas-fir and less than pine grown in pure pine stands. *p < 0.05, **p < 0.01, and ***p < 0.001.
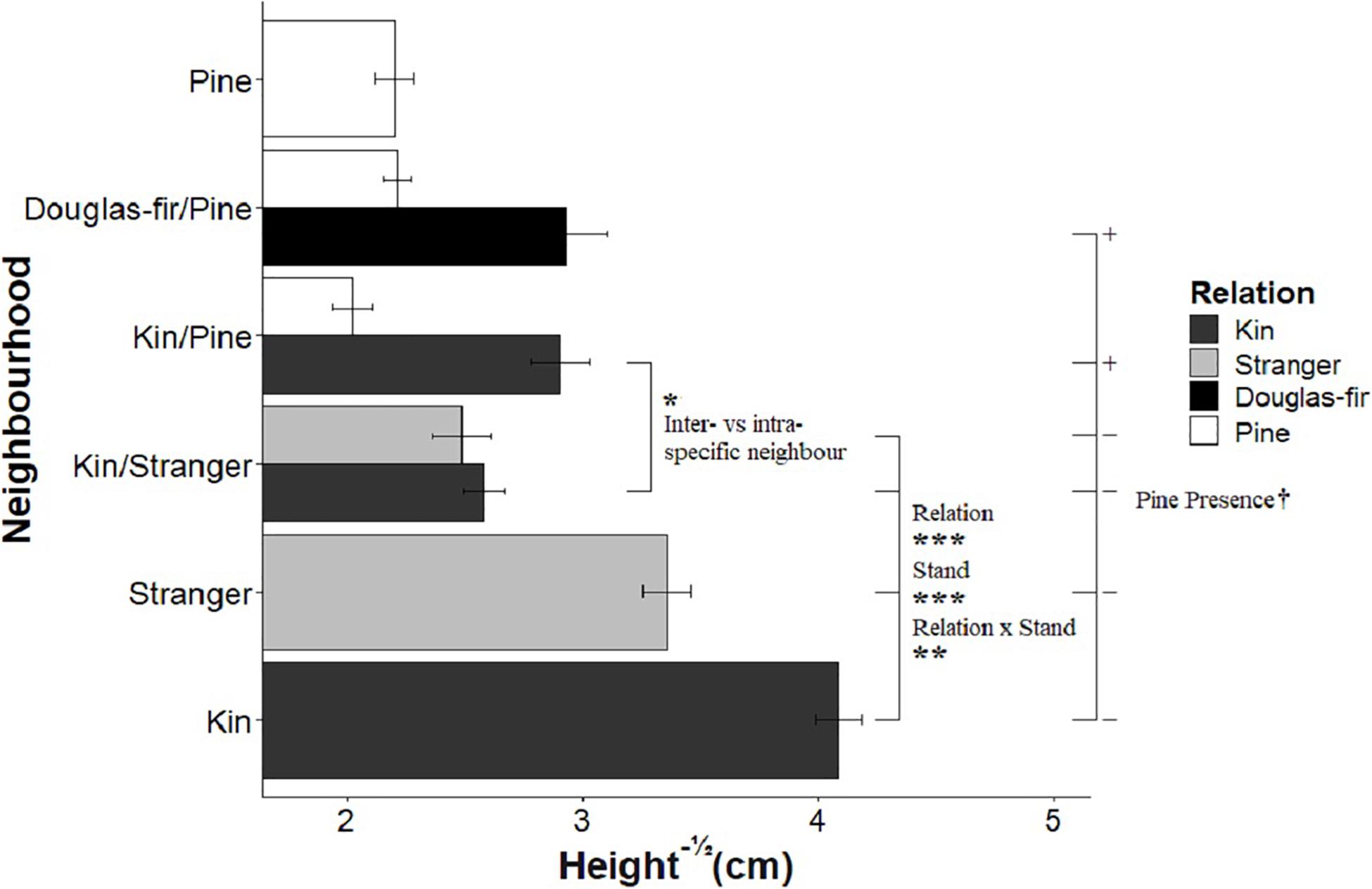
Figure 3. The effect of neighborhood treatments on the square-root of height for Douglas-fir seedlings (shaded) and pine seedlings (white). Significant planned contrasts are shown. Relation contrasted kin with stranger seedlings (estimate = –0.825, t ratio = –3.98, p = 0.0001, Df = 206). Stand contrasted seedlings grown in pure stand with those in mixed stands (estimate = 2.38, t ratio = 11.5, p < 0.0001, Df = 206). Relation × stand interaction was also significant (estimate = –0.637, t ratio = –3.07, p = 0.0024, Df = 206). The inter- vs. intraspecific neighbor contrasted kin seedlings neighbored by stranger Douglas-fir with pine seedlings (estimate = 0.324, t ratio = 2.12, p = 0.0350, Df = 206). The pine presence contrasted Douglas-fir seedlings grown with a pine neighbor vs. without a pine present in the pot (estimate = –0.425, t ratio = –1.78, p = 0.0764, Df = 206). Pine seedlings grown with a pair of kin had less biomass than those grown in pairs of pine with a single Douglas-fir and less than pine grown in pure pine stands. *p < 0.05, **p < 0.01, ***p < 0.001, and †p < 0.1.
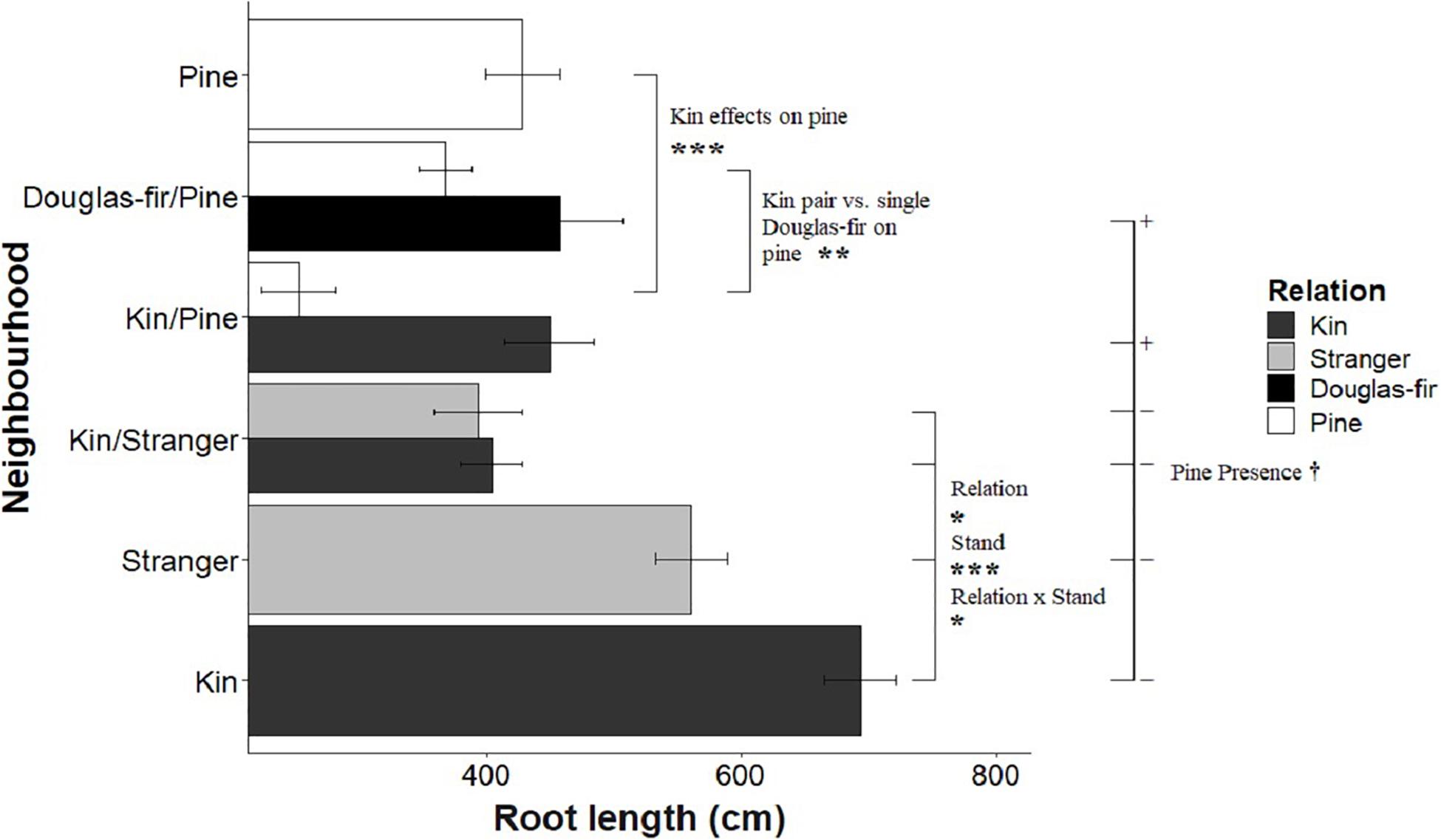
Figure 4. The effect of neighborhood treatments on the root length for Douglas-fir seedlings (shaded) and pine seedlings (white). Significant planned contrasts are shown. Relation contrasted kin with stranger seedlings (estimate = –143, t ratio = –2.46, p = 0.0147, Df = 206). Stand contrasted seedlings grown in pure stand with those in mixed stands (estimate = 457, t ratio = 7.86, p < 0.0001, Df = 206). Relation × stand interaction was also significant (estimate = –122, t ratio = –2.11, p = 0.0363, Df = 206). The pine presence contrasted Douglas-fir seedlings grown with a pine neighbor vs. without a pine present in the pot (estimate = –118, t ratio = –1.77, p = 0.0780, Df = 206). Pine seedlings grown with a pairs of kin had less biomass than those grown in pairs of pine with a single Douglas-fir and less than pine grown in pure pine stands. *p < 0.05, **p < 0.01, ***p < 0.001, and †p < 0.1.
Effects of Relatedness and Stand on Mycorrhizal Fungal Colonization
Relatedness and stand had a significant effect on mycorrhizal fungal colonization (Table 1). Strangers had root systems with a greater percent of root tips colonized by mycorrhizal fungi than kin seedlings (Table 1; Planned Contrast–Relation, Figure 5). Mixed stands had greater colonization than pure stands (Table 1; Planned Contrast–Stand, Figure 5). In contrasts that included interspecific comparisons (Table 1; Planned Contrasts–inter- vs. intraspecific neighbor, Pine presence and Number of pines), there were no significant differences in colonization.
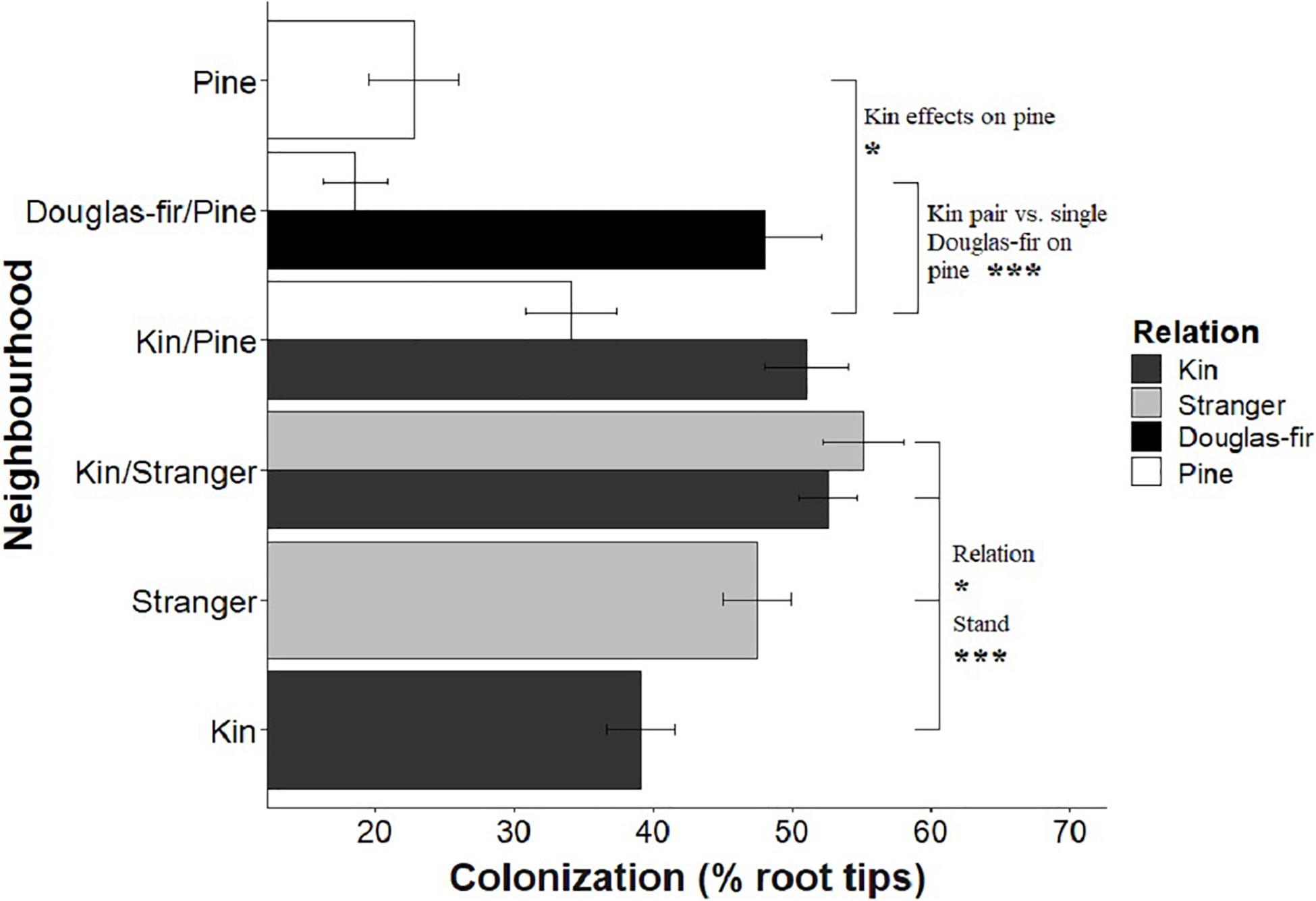
Figure 5. The effect of neighborhood treatments on the colonization for Douglas-fir seedlings (shaded) and pine seedlings (white). Significant planned contrasts are shown. Relation contrasted kin with stranger seedlings (estimate = 10.9, t ratio = 2.22, p = 0.0276, Df = 206). Stand contrasted seedlings grown in pure stand with those in mixed stands (estimate = –21.1, t ratio = –4.29, p < 0.0001, Df = 206). Pine seedlings grown with a pair of kin had less biomass than those grown in pairs of pine with a single Douglas-fir and less than pine grown in pure pine stands. *p < 0.05 and ***p < 0.001
Effects of Relatedness and Stand on Morphology
Morphological traits were also affected by relation, with the exception of root: shoot allocation, which did not vary with neighborhood (Table 2 and Figure 6). Kin seedlings had greater relative allocation to fine roots (fine: coarse root allocation) than stranger seedlings (Figure 7), but kin had lower relative fine root length to needle mass than strangers (Figure 8). Kin seedlings tended to be more slender with a greater height to diameter ratio than strangers (Figure 9). Fine: coarse root allocation and slenderness were also affected by stand, with pure stands having greater fine root allocation than mixed stands (Table 2 and Figure 7). Seedlings in pure stands tended to be more slender (greater height: diameter) and were larger overall than those in mixed stands (Figure 9). The effects of relatedness were influenced by stand for fine: coarse root allocation with a significant relation × stand interaction, but this effect was driven by higher fine root allocation relative to coarse root length among kin seedlings grown in pure stands compared to all other treatments (Figure 7). When examining the distribution of fine root length relative to coarse root length, of the seedlings in pure stands, kin seedlings had higher fine root allocation than strangers with the difference in fine root (between kin and stranger) increasing with more coarse root length.
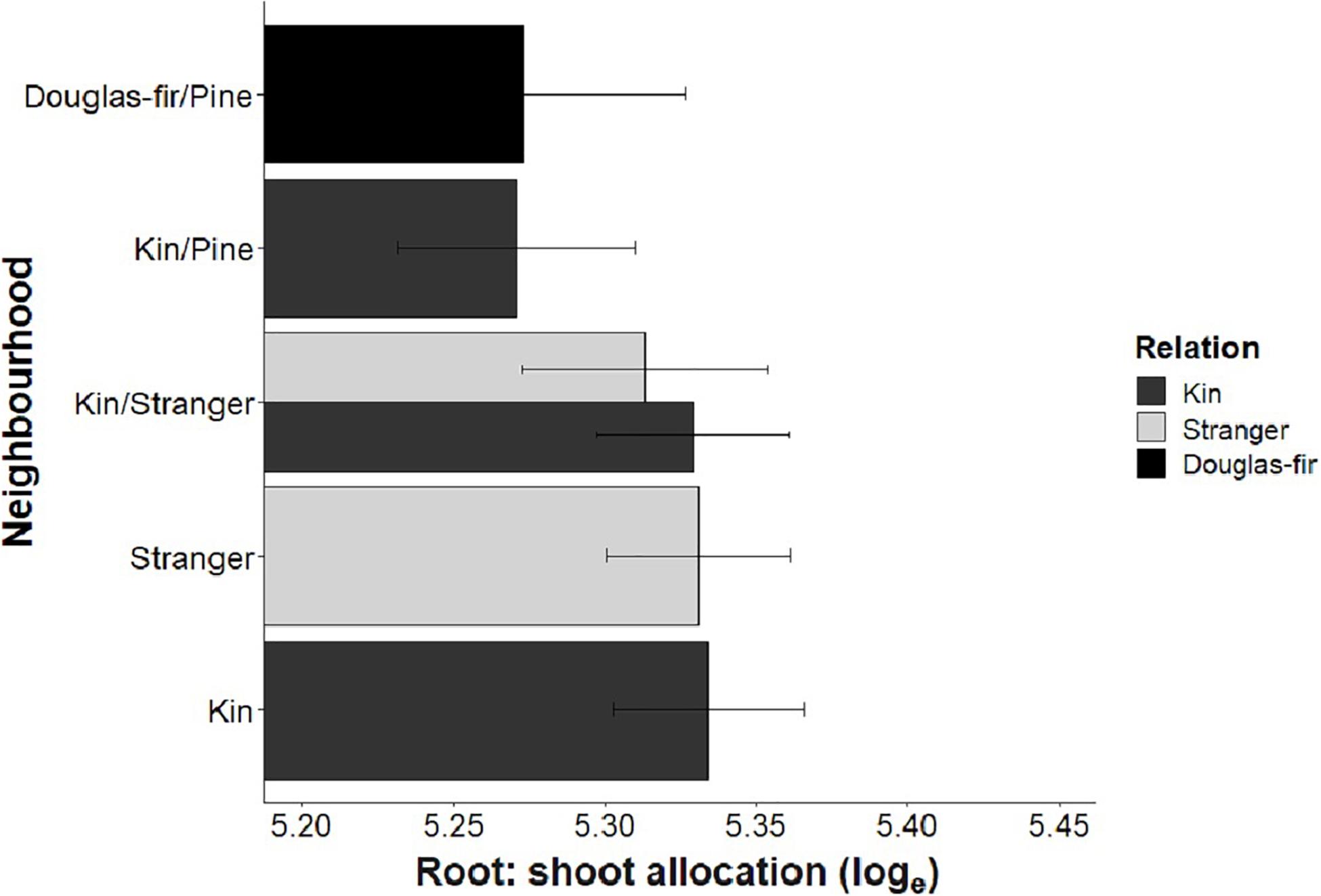
Figure 6. Estimated marginal means from planned contrasts (Table 2) relation, stand, inter- vs. intraspecific neighbor (kin pair with infra- or interspecific as the neighbor) and presence and number of pine seedling on the linear model fine root allocation: loge root ∼ loge shoot + Neighborhood × Family. No contrasts were significant probability values less than 0.05.
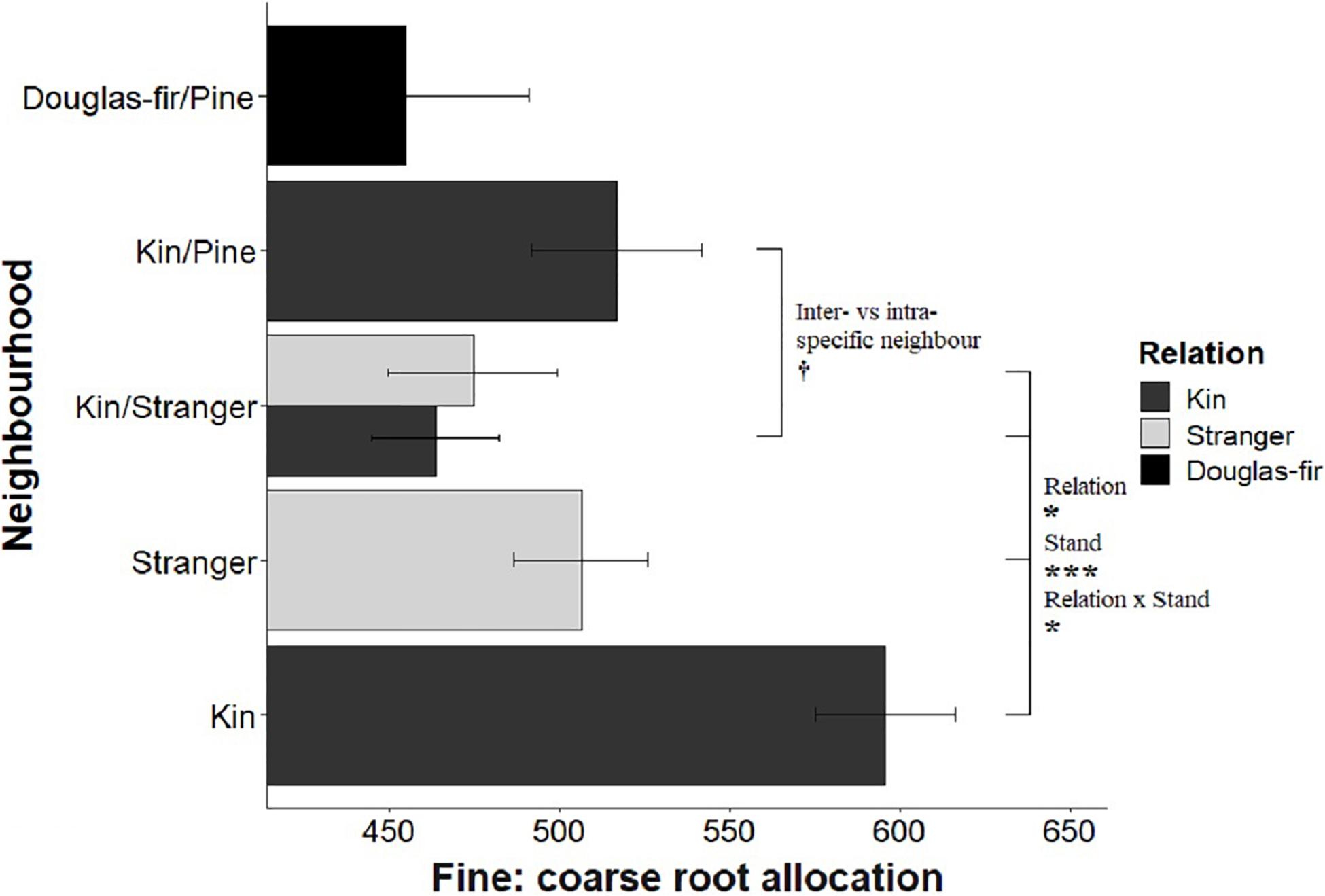
Figure 7. Estimated marginal means from planned contrasts (Table 2) relation (estimate = –78.6, t ratio = –1.99, p = 0.0475, Df = 196), stand (estimate = 164, t ratio = 3.63, p = 0.0004, Df = 196), inter- vs. intraspecific neighbor (kin pair with intra- or interspecific as the neighbor) (estimate = 53.3, t ratio = 1.85, p = 0.0653, Df = 196) and presence and number of pine seedlings (not significant) on the linear model fine root allocation: fine ∼ poly(coarse, 2) + Neighborhood × Family. *p < 0.05, ***p < 0.001, and †p < 0.1.
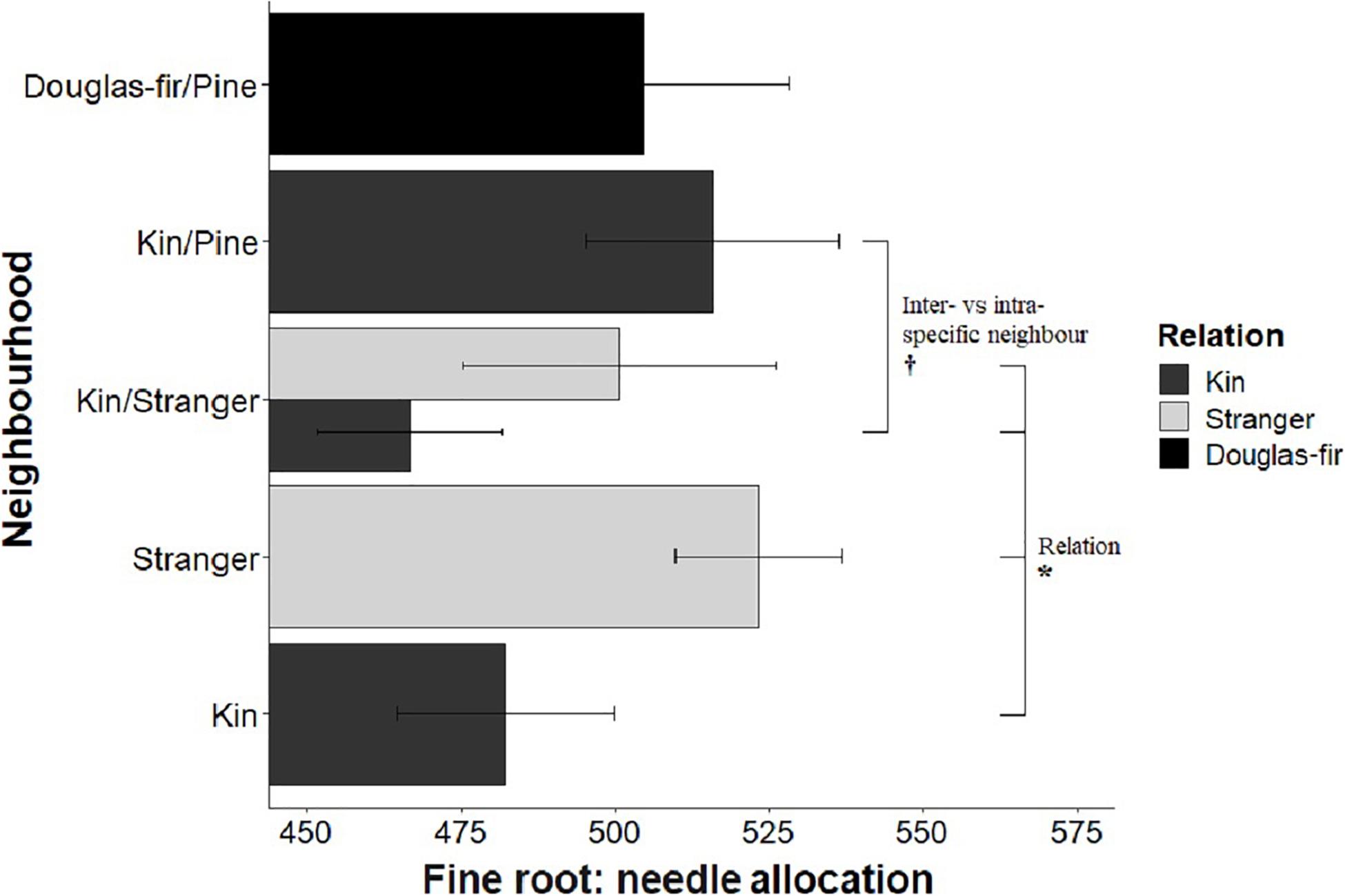
Figure 8. Estimated marginal means from planned contrasts (Table 2) relation (estimate = 74.9, t ratio = 2.03, p = 0.0437, Df = 193), stand (not significant), inter- vs. intraspecific neighbor (kin pair with intra- or interspecific as the neighbor) (estimate = 49.0, t ratio = 1.93, p = 0.0557, Df = 193) and presence and number of pine seedlings (not significant) on the different slopes linear model fine root: needle mass allocation: fine ∼ needle + Neighborhood × Family + needle: Neighborhood. *p < 0.05 and †p < 0.1.
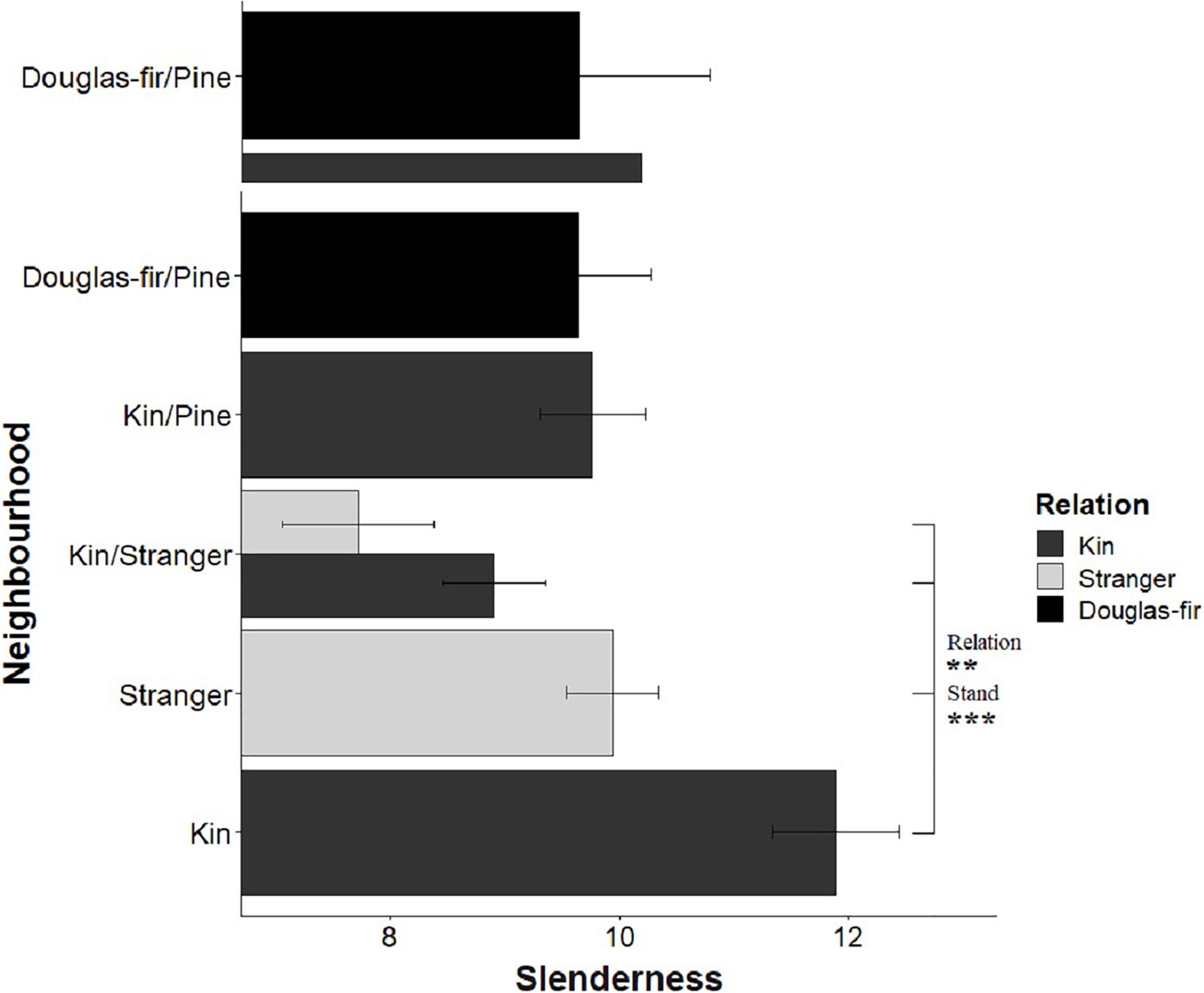
Figure 9. Estimated marginal means from planned contrasts (Table 2) relation (estimate = –3.13, t ratio = –2.97, p = 0.0034. Df = 192), stand (estimate = 5.20, t ratio = 4.93, p < 0.0001, Df = 192), inter- vs. intraspecific neighbor (kin pair with intra- or interspecific as the neighbor) (not significant) and presence and number of pine seedlings (not significant) on the different slopes linear model slenderness: height ∼ diameter + Neighborhood × Family + diameter: Neighborhood. **p < 0.01 and ***p < 0.001.
Kin pairs growing with a pine neighbor were taller than kin pairs grown with a stranger Douglas-fir (Table 1; inter- vs. intraspecific neighbor contrast, Figure 3). Kin pairs also tended to allocate relatively more to fine roots over needles with a pine neighbor than a stranger fir neighbor (Table 1; inter- vs. intraspecific neighbor contrast, Figure 8), and for the majority of kin seedlings grown with pine, there was also greater fine: coarse root allocation (excluding two outlying seedlings with much higher coarse root length) than those grown with strangers (Figure 7). Comparing seedlings grown in Douglas-fir-only pots to those where a pine seedling was present in the neighborhood, only biomass was significantly affected by the presence of pine (Tables 1, 2, Pine presence contrast). Mean biomass of all Douglas-fir seedlings with a pine neighbor was lower than that of all Douglas-fir seedlings grown only among Douglas-fir neighbors (Table 1; Pine presence contrast, Figure 2). There was also a tendency for height and root length to be lower among Douglas-fir seedlings when a pine neighbor was present (p < 0.10 Table 1: Planned Contrast–Pine presence, Figures 3, 4). Morphological traits did not vary according to the presence vs. absence of pine. Neither size nor morphological traits of Douglas-fir seedlings responded to the number (one vs. two) of pine seedlings in the neighborhood.
All pine size variables except height were negatively affected by the presence of a kin pair compared to where one or no Douglas-fir neighbors were present (p < 0.05, except height p = 0.0553, Table 1 and Figures 2–4). However, mycorrhizal fungal colonization of pine grown with a pair of related Douglas-fir was greater than those grown with none or a single Douglas-fir (Figure 5). There was no difference in size or colonization of pine grown in pure pine stands vs. with one Douglas-fir neighbor. Family of the Douglas-fir affected only one pine response variable, height (Table 1). Pine seedlings grown with Douglas-fir family C had greater mean square-root height of approximately 0.3 cm–1/2 (or 1.09 cm, more than 25% taller) than those grown with A or B (5.29 cm compared to 4.20 and 4.04 cm, respectively).
Family Responses in Morphology, Performance and Mycorrhizal Fungi Associations
Douglas-fir family was also a significant main effect for all response variables except colonization, and significant interactions between neighborhood treatment and family were found for all variables except root: shoot allocation; therefore, we investigated the interaction terms (Table 1). Not all contrasts for all variables were significant for all families, but in families with contrasts that were significant, the direction of difference of the estimated marginal means for every variable was consistent; only magnitude differed among families. For this reason, as well as the limited number of families tested, the estimated marginal means were averaged across families, and shown as contrasts within neighborhood treatments. Given that the neighborhood × family interaction was significant for many Douglas-fir response variables, interactions were examined more closely for a subset variables: biomass (Figure 10 bars), fine: coarse root allocation (Figure 10 points) and colonization (Figure 10 percentages). Families had variable mean biomass and fine root allocation relative to coarse root length among kin in both pure and mixed neighborhoods. As kin in both the pure and mixed treatments, family C was greater than A and B (which were not significantly different from each other). Within each family, neighborhood treatment effects on biomass followed similar patterns to pooled data. With respect to the neighborhood × family interaction for fine: coarse root allocation, there was no effect of families within neighborhoods or neighborhood comparisons within families. Colonization did not appear to correlate strongly with either biomass or fine root allocation when examined by family.
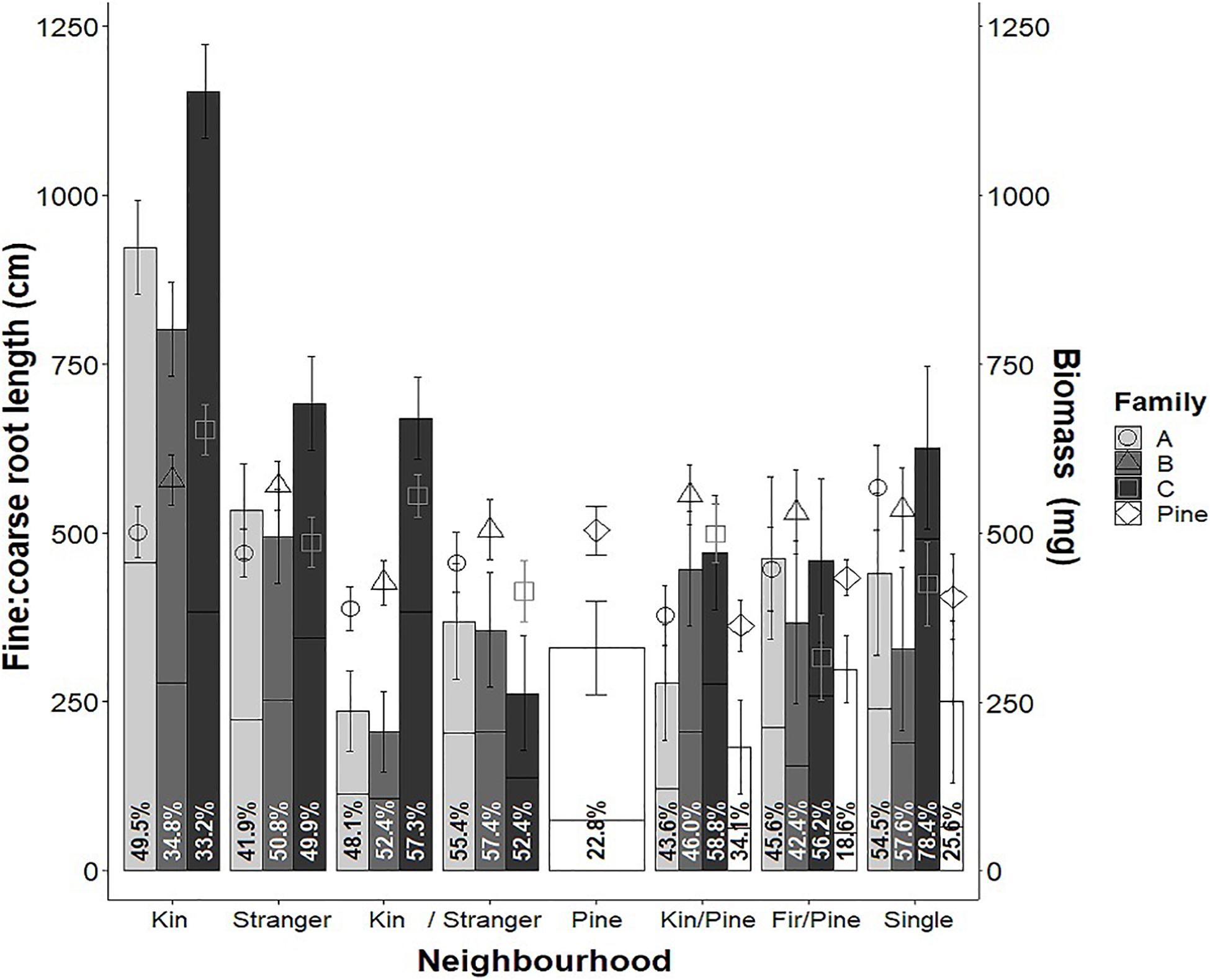
Figure 10. Mean biomass (bars), fine: coarse root allocation (points) and colonization (shown as a percent of biomass in lines and percentages in text) of families A, B, and C of greenhouse-grown interior Douglas-fir (Fdi) and lodgepole pine (Pine) in all neighborhood treatments as well as single seedlings grown in plots with no immediate neighbors. Estimated marginal means are from a linear model of y ∼ Family × Relation × Treatment (y = biomass and colonization) and fine root length ∼ coarse root length + Family × Stand × Relation. Error bars shown as estimated marginal means ± one standard error.
Discussion
To observe how a complex environment influences Fdi kin vs. stranger effects on seedling morphology and size, the neighborhood experienced by the seedlings was manipulated. This was done by creating neighborhoods of three seedlings that comprised pure and mixed stands of kin and strangers (Douglas-fir only), a Douglas-fir kin pair with a lodgepole pine neighbor, a pair of lodgepole pines with a Douglas-fir neighbor, or pure lodgepole pine. This manipulation had a significant effect on most of traits measured regarding both the morphology and size (Tables 1, 2). Relatedness, stand, and species composition all affected performance (biomass, height, diameter, root length; Table 1), morphological adaptation (fine: coarse allocation, fine root: needle allocation and slenderness; Table 2) as well as mycorrhizal fungal colonization (Table 1). The responses of Douglas-fir to lodgepole pine, and pine to Douglas-fir are most consistent with differences in competitive ability, not cued responses. However, Douglas-fir showed strong responses to the relatedness of neighbors, and surprisingly, to stand composition.
The effects that relation and stand had on morphological traits and colonization in this study reflected some of the complexity encountered in natural systems. The environment a plant is growing in can affect the expression of a trait if there is phenotypic plasticity in that trait (Scheiner, 1993). In a complex environment, however, some traits may respond to one aspect of the environment and not another; there may be a response to light but not nutrient availability, for example. Other traits have responses to nutrient but not light availability and still others may respond in different ways to both. Responses may also differ depending on growth strategy (Pigliucci et al., 1995). Kin plants here tended to have more foliage (absolutely and relatively; lower fine root: needle allocation; Figure 11) than strangers, suggesting greater photosynthetic capacity compared to belowground resource acquisition. Kin plants also tended to be more slender supporting allocation in favor of light acquisition or shade avoidance. Kin plants also tended to have lower mycorrhizal colonization, which we would expect to be high among seedlings in need of efficient belowground resource foraging. The lower mycorrhizal fungal colonization in kin seedlings coincided with higher fine: coarse root allocation. We posit that because these patterns, that often suggest higher competition (both above and belowground), occur in conjunction with greater performance of kin seedlings in pure stands, it suggests competition for belowground resources was lower among kin than strangers, and therefore kin seedlings these morphological characteristics were not indicative of high belowground competition (not the determent of related neighbors), but rather high performance among kin neighbors.
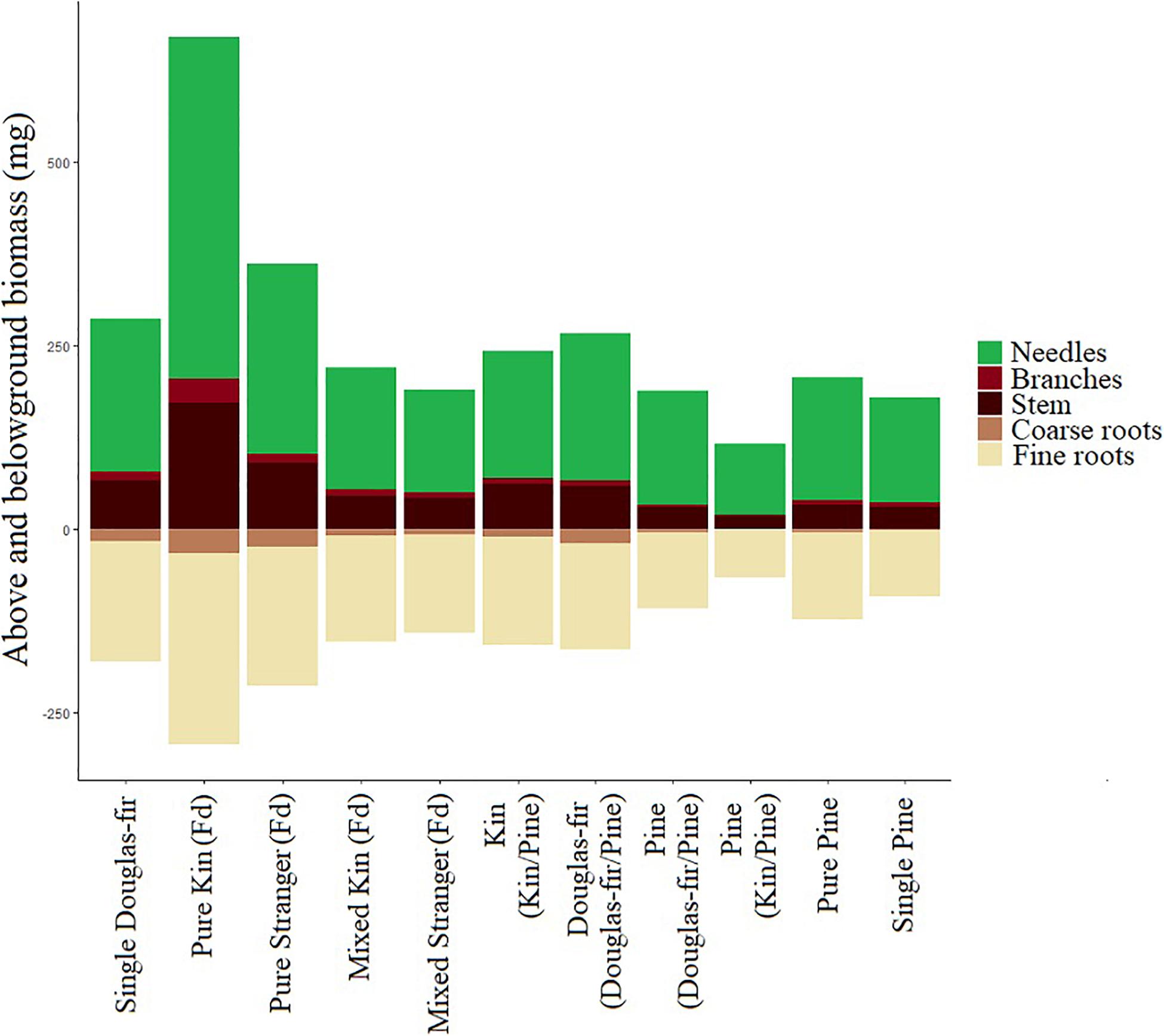
Figure 11. Mean mass (mg) of seedling needles, branches, stem, coarse roots, and fine roots by neighborhood type. Aboveground biomass is presented as mg above zero and belowground as mg below zero. Aboveground mass is the mean dry weight and belowground mass was estimated by total root weight multiplied by the proportion of the root system by volume that was coarse or fine roots per seedling and then averaged.
Many of the differences both in morphological and performance traits between kin and strangers in pure stands were not present when kin and strangers were grown together in the same pot, with the exceptions being fine root: needle allocation and slenderness (kin/stranger neighborhood treatment, Figures 2–9). The Douglas-fir seedlings in the mixed stands (kin/stranger neighborhood treatment) did not behave or perform in a similar manner to seedlings that were in pure stands of either kin or strangers. This indicates that the response signals or cues in pots where both kin and strangers are present are more complex than anticipated. If the recognition response was associated with the detection of a stranger neighbor, we would have expected the presence of any stranger seedlings to result in seedlings responses similar to those in pure stranger stands. However, if recognition was associated with the detection of a kin neighbor, we would have expected responses that were either similar to pure kin stands or, alternatively, intermediate between the kin and stranger responses. In the latter case, the kin response would have been in the same direction as observed between pure stands, but as it was not exclusively a kin or stranger signal present, a weaker kin signal with the presence of stranger cues could have been predicted. Instead, a unique response was observed when kin and strangers were grown in the same pot. Although there was no difference between kin and stranger within mixed stands, seedlings in mixed stands had greater colonization by mycorrhizal fungi (Figure 5), less allocation to fine roots relative to coarse roots (Figure 7), were less slender and were smaller (Figures 2–4) than seedlings in either pure kin or pure stranger stands. This may indicate a complex, non-additive response to receiving both kin and stranger cues. Plants can show complex responses, as demonstrated in the non-additive response to neighbor and nutrient cues in Abutilon theophrasti (Cahill et al., 2010).
Higher mycorrhizal fungal colonization tended to be correlated with smaller seedlings, suggesting a cost or trade-off to early colonization in greenhouse-grown Douglas-fir. During the establishment and early stages of seedling development, colonization of roots by mycorrhizal fungi has a carbon demand and may reduce seedling growth (Johnson et al., 1997; File et al., 2012a). In greenhouse pots, soil nutrients available to the plant and fungi would have been in closer proximity and readily exploited due to the limited space of the pot compared to a natural environment where root exploration is not restricted; however, soil-root contact and soil-hyphae contact would be expected to be similar. With only three seedlings present in the 7.5 L pots, soil nutrients were unlikely limiting to seedling growth, and thus the benefits of associating with fungi may not have been worth the energetic cost (Johnson et al., 1997). The idea that benefits of mycorrhizal association may have failed to outweigh the costs at this early life stage is supported by Barker et al. (2013), who found that field-sown Fdi seedlings remained uncolonized for approximately 4 months following germination, and by Ronsheim (2012) with a herbaceous species, who found that low biomass of Allium vineale was associated with mycorrhizal colonization after 1 month but by 15 months mycorrhizae presence was associated with high biomass. Colonization rates of the 5-month-old seedlings in this study were similar to those of Barker et al. (2013), who found that colonization rose from 0% when seedlings were three months old, to 47 and 100% by the time they were 4 and 12 months old, respectively. By 5 months in this study, higher mycorrhizal colonization occurred in neighborhood treatments where plants were also smaller in size. For example, stranger seedlings tended to have higher colonization and were smaller in size than kin (Figures 2–5). It is impossible to determine if this relationship was correlative or causal (and if so in which direction) in the present study. Seedlings with only kin neighbors may be better able to minimize colonization at this life stage, when colonization is not beneficial, through negative feedback between other kin seedlings and soil organisms (Johnson, 2010). On the other hand, seedlings in mixed stands, or the fungal partner, may associate with one another more quickly given additional, or a different set of signals present when kin and strangers are grown together. Further research and longer experimental timeframes are needed to more fully understand the relationship between seedling performance and mycorrhizal colonization, and how it is influenced by relatedness.
Mycorrhizal fungal colonization between related Douglas-fir seedlings in this study contrasted with Asay (2013), where kin plants had higher rates of mycorrhizal colonization. The difference is likely explained by differences in the design and objectives of each study. In Asay (2013), Douglas-fir seedlings were grown in uneven aged pairs, and when the younger seedling was related to the older pre-established seedling, it had significantly higher rates of colonization; over two times greater percent of total root weight colonized. The higher colonization rate when grown next to a related neighbor was considered to result from an advantage, such as improved root physiology, associated with access to the established mycorrhizal network of the older seedling. With uneven aged pairs, the network may have allowed carbon to flow along a source-sink gradient (Simard, 2009) from the larger, older seedling to the smaller, younger seedling, as demonstrated by Pickles et al. (2017). This carbon subsidy could have decreased the cost of mycorrhizal colonization to the young, establishing seedling, relative to that experienced by same-aged seedlings in the current study. Moreover, the roots of the younger seedlings in Asay (2013) were confined to a mesh bag which prevented root penetration but allowed or prevented fungal hyphae penetration depending on the pore size and were therefore unable to fully explore the full soil volume for nutrients, increasing the benefits of the mycorrhizal fungal association. In contrast, the even-aged seedlings in this study did not have the opportunity to link into an existing network, but rather only associate with the fungal inoculum in the soil to potentially form a new network with neighbors.
Douglas-fir kin pairs benefited from growing with a pine neighbor rather than a stranger Douglas-fir. Kin in the kin/pine neighborhood treatment had greater fine root allocation relative to both coarse root length (Figure 7) and needle mass (Figure 9), which coincided with taller seedlings (Figure 3). These results are consistent with differences in competitive ability between pine and Douglas-fir. Pine seedlings were significantly smaller than Douglas-fir not only in overall biomass but also height and root length (Douglas-fir was approximately twice the size of the lodgepole pine). The smaller size of the pine neighbor, both above and belowground, alone may account for the apparent advantage to growing with an interspecific neighbor. Though the species differed in morphology and size, they did not differ in mycorrhizal fungal colonization in this case; mean colonization was within 2.5% when kin with a pine neighbor were compared to kin with a stranger Douglas-fir neighbor. Niche partitioning is unlikely to be responsible for the differences in fitness (assuming biomass is an appropriate proxy for fitness; Younginger et al., 2017) between species, even though the niches of the two species would not be expected to directly overlap. Lodgepole pine is shade-intolerant with a wide ecological amplitude and Fdi is moderately shade tolerant [though quite shade tolerate in the seedling stage (Lavender and Hermann, 2013)] and grows on a narrower range of site conditions. In contrast to what the niche partitioning theory would predict, the individual with the least overlap in niche space, the single lodgepole pine in this case, was not the most successful; instead the pine was always outperformed by Douglas-fir, consistent with a difference in competitive ability.
The difference in competitive ability between the species also likely explained why increasing the number of pine neighbors had no effect on Douglas-fir, and why increasing numbers of Douglas-fir neighbors had a negative effect on the performance of pine seedlings. Pines were smaller when they were grown with a pair of kin Douglas-fir neighbors (kin/pine) compared to when grown with one (Douglas-fir/pine) or no Douglas-fir (pine). This suggests that the number of smaller pine seedlings (one or two) did not affect Douglas-fir performance but the pine, compared to the typically larger stranger Douglas-fir seedlings in mixed stands (kin/stranger with no pine present), provided less direct competition as a neighbor to a Douglas-fir kin pair. Higher colonization was observed in pine seedlings in the same neighborhood treatments that corresponded with lower performance, consistent with either a cost of early mycorrhizal colonization or a difference in strategy dependent on the neighborhood composition.
Douglas-fir families demonstrated genetic variance for their responses to neighborhood [Douglas-fir family × neighborhood interaction for all response variables (Tables 1, 2)]. While differences in size between families were expected due to genetic and geographic variation, there were also differences in morphological response traits. Families appeared to respond to neighborhood environment differently. Family C exhibited a stronger kin response than the other two families, with greater biomass and higher kin fine root allocation in mixed stands than families A and B; this was mediated by the insignificantly higher stranger fine root allocation in families A and B. The same pattern was observed in biomass, a performance trait (Figure 10). Colonization also varied among families and appeared correlated with fine root allocation and biomass. Arguments have been made in the past that the response attributed to relatedness is explained by genetic variation between families. However, this study was designed with a balanced representation each genotype in each treatment combination equally. In addition, when examining each family, the majority of responses were consistent in their direction, differing only in magnitude, supporting our interpretation that relation effects result in a consistent response. In a previous relatedness study using Fdi, the relatedness effect commonly differed among families. In Pickles et al. (2017), only two of four families demonstrated higher carbon transfer from donor seedlings to related recipients compared to unrelated recipients.
As evidence for kin recognition and selection in plants grows, it is important to examine systems with increasing complexity, closer to natural conditions. The seedlings in this study were examined in a variety of neighborhood treatments attempting to mimic different and complex environments, including mixtures of kin and strangers grown together. While this study does not capture the full complexity of a naturally regenerating stand in the field, it provides interesting insight into how a seedling responds to relatedness and species composition in a variety of contexts and how that translates into performance. At this early stage in development, there was a significant increase in fine root allocation relative to coarse root length in kin vs. stranger seedlings, particularly when grown exclusively with other kin, that corresponded to greater performance. There appeared to be a cost associated with early mycorrhizal fungal colonization and a difference in response to neighbor identity among families (genotypes). This was, however, the result of only one growing season which a limiting factor as to how generally these results can be applied. Understanding these trends progress or change throughout the development and growth of these trees would be of great interest and long-term studies on this topic would be of great value. Would higher rates of early mycorrhizal fungal colonization translate into better performance, fitness or resilience as the tree matures? As resources either above or belowground become scarcer, would the adaptive morphology among kin and strangers change and how would that affect fitness? Over time, would the interaction of genotype and neighborhood composition begin to fade or become stronger or become more complex? This study added system complexity to determine if relatedness can be detected under more realistic contexts, but our understanding of the importance of relatedness in the complexity of a natural forest stand still requires study. The findings have novel implications for current forest regeneration practices. In particular, the potential management of natural regeneration of Douglas-fir to promote the naturally occurring level of relatedness in stands based of these results may impact stand success rather than the introduction of planted seedlings of improved stock chosen for growth and form over relatedness. The retention of natural seed sources in the harvesting plan could also promote stand relatedness. The mixing of natural and planted seedlings, and the complexity of interactions we uncovered may be relevant to the growth and success of Fdi in naturally regenerated and managed forests.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
AA and SS designed and planned the experiment with guidance and advice from SD. AA collected the data and analyzed the data. SD and AA planned the statistical analysis. AA performed the statistical analysis. AA led the writing of the manuscript with significant contributions from SS and SD. All authors contributed to the article and approved the submitted version.
Funding
Funding was provided by the Natural Sciences and Engineering Research Council of Canada Strategic Grant (STPGP 478832-15) to SS and the Forest Enhancement Society of British Columbia (FESBC) grant to Jean Roach and SS.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Sally Aitken for valuable guidance and advice in designing the experiment and the analysis of the data. Dr. Monika Gorzelak was essential in carrying out the experiment providing support and collaboration. Melina Biron, the greenhouse research manager at the UBC Horticulture Greenhouse also lent her support, advice and care taking. We would also like to acknowledge Dan Malvin, Alexia Constantinou, Rachel Green, Sophie Vanderbanck, and Alyx Hough for assistance in seedling harvesting, sample storage and processing as well as for many undergraduate students working with the Belowground Ecosystems Group at UBC.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2020.578524/full#supplementary-material
References
Asay, A. K. (2013). Mycorrhizal Facilitation of kin Recognition in Interior Douglas-fir (Pseudotsuga menziesii var. glauca). Master’s thesis, University of British Columbia, Vancouver, BC.
Barker, J. S., Simard, S. W., Jones, M. D., and Durall, D. M. (2013). Ectomycorrhizal fungal community assembly on regenerating Douglas-fir after wildfire and clearcut harvesting. Oecologia 172, 1179–1189. doi: 10.1007/s00442-012-2562-y
Bhatt, M., Khandelwal, A., and Dudley, S. A. (2011). Kin recognition, not competitive interactions, predicts root allocation in young Cakile edentula seedling pairs. New Phytol. 189, 1135–1142. doi: 10.1111/j.1469-8137.2010.03548.x
Biedrzycki, M. L., and Bais, H. P. (2010). Kin recognition: another biological function for root secretions. Plant Signal. Behav. 5, 401–402. doi: 10.4161/psb.5.4.10795
Biedrzycki, M. L., Jilany, T. A., Dudley, S. A., and Bais, H. P. (2010). Root exudates mediate kin recognition in plants. Commun. Integr. Biol. 3, 28–35. doi: 10.4161/cib.3.1.10118
Cahill, J. F., McNickle, G. G., Haag, J. J., Lamb, E. G., Nyanumba, S. M., and St Clair, C. C. (2010). Plants integrate information about nutrients and neighbors. Science 328:1657. doi: 10.1126/science.1189736
Chen, H. Y. H., Klinka, K., and Kayahara, G. J. (1996). Effects of light on growth, crown architecture, and specific needle area for naturally established Pinus contorta var. latifolia and Pseudotsuga menziesii var. glauca saplings. Can. J. For. Res. 26, 1149–1157. doi: 10.1139/x26-128
Cheplick, G. P., and Kane, K. H. (2004). Genetic relatedness and competition in Triplasis purpurea (Poaceae): resource partitioning or kin selection? Int. J. Plant Sci. 165, 623–630. doi: 10.1086/386556
Devine, W. D., and Harrington, T. B. (2011). Aboveground growth interactions of paired conifer seedlings in close proximity. New For. 41, 163–178. doi: 10.1007/s11056-010-9218-8
Dudley, S. A., and File, A. L. (2007). Kin recognition in an annual plant. Biol. Let. 3, 435–438. doi: 10.1098/rsbl.2007.0232
File, A. L., Klironomos, J., Maherali, H., and Dudley, S. A. (2012a). Plant kin recognition enhances abundance of symbiotic microbial partner. PLoS One 7:e45648. doi: 10.1371/journal.pone.0045648
File, A. L., Murphy, G. P., and Dudley, S. A. (2012b). Fitness consequences of plants growing with siblings: reconciling kin selection, niche partitioning and competitive ability. Proc. R. Soc. B 279, 209–218. doi: 10.1098/rspb.2011.1995
Goldberg, D. E., and Werner, P. A. (1983). Equivalence of competitors in plant communities: a null hypothesis and a field experimental approach. Ame. J. Bot. 70, 1098–1104. doi: 10.1002/j.1537-2197.1983.tb07912.x
Gorzelak, M. A. (2017). Kin-selected Signal Transfer Through Mycorrhizal Networks in Douglas-Fir. dissertation., University of British Columbia, Vancouver, BC.
Johnson, N. (2010). Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc. Natl. Acad. Sci. U.S.A. 107, 2093–2098. doi: 10.1073/pnas.0906710107
Johnson, N., Graham, J., and Smith, F. (1997). Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol. 135, 575–585. doi: 10.1046/j.1469-8137.1997.00729.x
Lavender, D. P., and Hermann, R. K. (2013). Douglas-fir: The Genus Pseudotsuga. Corvallis, OR: Oregon State University.
McCormack, M. L., Dickie, I. A., Eissenstat, D. M., Fahey, T. J., Fernandez, C. W., Guo, D., et al. (2015). Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytolo. 207, 505–518. doi: 10.1111/nph.13363
Murison, W. (1960). ). Macronutrient Deficiency and its Effects on Coniferous Growth. Dissertation, University of British Columbia, Vancouver, BC.
Murphy, G. P., and Dudley, S. A. (2009). Kin recognition: competition and cooperation in Impatiens (Balsaminaceae). Am. J. Bot. 96, 1990–1996. doi: 10.3732/ajb.0900006
Pickles, B. J., Wilhelm, R., Asay, A. K., Hahn, A. S., Simard, S. W., and Mohn, W. W. (2017). Transfer of 13C between paired Douglas-fir seedlings reveals plant kinship effects and uptake of exudates by ectomycorrhizas. New Phytol. 214, 400–411. doi: 10.1111/nph.14325
Pigliucci, M., Whitton, J., and Schlichting, C. D. (1995). Reaction norms of Arabidopsis. I. Plasticity of characters and correlations across water, nutrient and light gradients. J. Evol. Biol. 8, 421–438. doi: 10.1046/j.1420-9101.1995.8040421.x
R Core Team (2018). R: A Language and Environment for Statistical Computing. Vienna: R foundation for Statisitical Computing.
Restaino, C. M., Peterson, D. L., and Littell, J. (2016). Increased water deficit decreases Douglas fir growth throughout western US forests. Proc. Natl. Acad. Sci. U.S.A. 113, 9557–9562. doi: 10.1073/pnas.1602384113
Ronsheim, M. L. (2012). The Effect of Mycorrhizae on Plant Growth and Reproduction Varies with Soil Phosphorus and Developmental Stage. Am. Midland Naturalist. 167, 28–39. doi: 10.1674/0003-0031-167.1.28
Russell Lenth (2018). emmeans: Estimated Marginal Means, Aka Least-Squares Means. R package Version 1.3.0. Avaliable at: http://CRAN.R-project.org/package=emmeans (accessed August 18, 2020).
Scheiner, S. M. (1993). Genetics and evolution of phenotypic plasticity. Annu. Rev. Ecol. Syst. 24, 35–68. doi: 10.1146/annurev.es.24.110193.000343
Simard, S. W. (2009). The foundational role of mycorrhizal networks in self-organization of interior Douglas-fir forests. For. Ecol. Manag. 258, S95–S107. doi: 10.1016/j.foreco.2009.05.001
Wang, T., Hamann, A., Spittlehouse, D. L., and Carroll, C. (2016). Locally downscaled and spatially customizable climate data for historical and future periods for north america. PloS one 11: e0156720. doi: 10.1371/journal.pone.0156720
Vyse, A., Ferguson, C., Simard, S. W., Kano, T., and Puttonen, P. (2006). Growth of Douglas-fir, lodgepole pine, and ponderosa pine seedlings underplanted in a partially-cut, dry Douglas-fir stand in south-central British Columbia. For. Chron. 82, 723–732. doi: 10.5558/tfc82723-5
Walter, G. H. (1991). What is resource partitioning? J. Theor. Biol. 150, 137–143. doi: 10.1016/s0022-5193(05)80327-3
Williams, H., Messier, C., and Kneeshaw, D. D. (1999). Effects of light availability and sapling size on the growth and crown morphology of understory Douglas-fir and lodgepole pine. Can. J. For. Res. 29, 222–231. doi: 10.1139/x98-189
Keywords: kin selection, kin recognition, morphology, plant behavior, information integration, mycorrhizal colonization, interior Douglas-fir
Citation: Asay AK, Simard SW and Dudley SA (2020) Altering Neighborhood Relatedness and Species Composition Affects Interior Douglas-Fir Size and Morphological Traits With Context-Dependent Responses. Front. Ecol. Evol. 8:578524. doi: 10.3389/fevo.2020.578524
Received: 30 June 2020; Accepted: 31 August 2020;
Published: 15 October 2020.
Edited by:
Qinfeng Guo, United States Forest Service (USDA), United StatesReviewed by:
Aaron Weiskittel, The University of Maine, United StatesPhilip Comeau, University of Alberta, Canada
Copyright © 2020 Asay, Simard and Dudley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amanda K. Asay, Amanda.k.Asay@gmail.com; amanda.asay@gov.bc.ca
 Amanda K. Asay
Amanda K. Asay Suzanne W. Simard
Suzanne W. Simard Susan A. Dudley
Susan A. Dudley