Ontogeny, Phylotypic Periods, Paedomorphosis, and Ontogenetic Systematics
- 1Zoological Museum, Moscow State University, Moscow, Russia
- 2Gothenburg Natural History Museum, Gothenburg, Sweden
- 3Gothenburg Global Biodiversity Centre, Gothenburg, Sweden
- 4Koltzov Institute of Developmental Biology (RAS), Moscow, Russia
The key terms linking ontogeny and evolution are briefly reviewed. It is shown that their application and usage in the modern biology are often inconsistent and incorrectly understood even within the “evo-devo” field. For instance, the core modern reformulation that ontogeny not merely recapitulates, but produces phylogeny implies that ontogeny and phylogeny are closely interconnected. However, the vast modern phylogenetic and taxonomic fields largely omit ontogeny as a central concept. Instead, the common “clade-” and “tree-thinking” prevail, despite on the all achievements of the evo-devo. This is because the main conceptual basis of the modern biology is fundamentally ontogeny-free. In another words, in the Haeckel’s pair of “ontogeny and phylogeny,” ontogeny is still just a subsidiary for the evolutionary process (and hence, phylogeny), instead as in reality, its main driving force. The phylotypic periods is another important term of the evo-devo and represent a modern reformulation of Haeckel’s recapitulations and biogenetic law. However, surprisingly, this one of the most important biological evidence, based on the natural ontogenetic grounds, in the phylogenetic field that can be alleged as a “non-evolutionary concept.” All these observations clearly imply that a major revision of the main terms which are associated with the “ontogeny and phylogeny/evolution” field is urgently necessarily. Thus, “ontogenetic” is not just an endless addition to the term “systematics,” but instead a crucial term, without it neither systematics, nor biology have sense. To consistently employ the modern ontogenetic and epigenetic achievements, the concept of ontogenetic systematics is hereby refined. Ontogenetic systematics is not merely a “research program” but a key biological discipline which consistently links the enormous biological diversity with underlying fundamental process of ontogeny at both molecular and morphological levels. The paedomorphosis is another widespread ontogenetic-and-evolutionary process that is significantly underestimated or misinterpreted by the current phylogenetics and taxonomy. The term paedomorphosis is refined, as initially proposed to link ontogeny with evolution, whereas “neoteny” and “progenesis” are originally specific, narrow terms without evolutionary context, and should not be used as synonyms of paedomorphosis. Examples of application of the principles of ontogenetic systematics represented by such disparate animal groups as nudibranch molluscs and ophiuroid echinoderms clearly demonstrate that perseverance of the phylotypic periods is based not only on the classic examples in vertebrates, but it is a universal phenomenon in all organisms, including disparate animal phyla.
Introduction
The field of ontogeny is a core biological concept and has enormous applications. Thus, not surprisingly, a significant confusion has arisen during usage and application of the various ontogeny-related terms over centuries. Ontogeny is such a commonly used term, that its meaning has become blurred and is usually referring solely to individual development and also contrasting to phylogeny, with both terms rooted in Haeckel (1866). For Haeckel ontogenesis meant “…die Ontogenie weiter nichts ist als eine kurze Rekapitulation der Phylogenie.” (“= …ontogeny is nothing more than a brief recapitulation of phylogeny, Haeckel, 1866, II, p. 7, our italics). However, evolutionary changes (and hence, a phylogeny) are based on alterations in ontogenetic processes, and this modern understanding is among main general achievements of the evolutionary developmental biology (e.g., Gilbert et al., 1996; Hall, 1999, 2011): “The real Phylogeny of Metazoa has never been direct succession of adult forms, but a succession of ontogenies or life-cycles [thus include both adult and larval periods of ontogeny]” and, the most importantly, “Ontogeny does not recapitulate phylogeny: it creates it.” (Garstang, 1922, p. 82, our italics). Therefore, a further generalization is that the direction of character changes is from ontogeny to evolution, and this has a key meaning for the taxonomy and the entire enormous biodiversity field, since ontogenetic modifications is the basis of the diversity of all organisms (Martynov, 2012a). Without ontogeny and its modifications evolution could not proceed.
Despite this, the incorporation of the ontogeny in the modern taxonomy and biology is only superficial. It is widely acknowledged that there is a modern field of the evolutionary developmental biology (“evo-devo”) in which an apparently exhaustive consideration of the ontogeny has been performed. But in reality, the situation is completely vice versa. There are exceedingly few publications that in some degree discuss biological systematics (taxonomy) and “evo-devo” (e.g., Hawkins, 2002; Minelli, 2007, 2015a,b), and this does not promote the real importance of the ontogeny for taxonomy as a central biological phenomenon. The clearest indication for this, is that while some apparently new terms have been proposed, for instance “phylo-evo-devo” (Minelli, 2009) or “morpho-evo-devo” (Wanninger, 2015), quite contrary these concepts basically rely on the persisting centrality of phylogeny, not on the ontogeny or morphology, per se (see for example the notable comment in Neumann et al., 2021). Compared to the modern time, Haeckel recognized the importance of systematics in the ontogenetic sense: “…die Systematik’ erklärt sich dann einfach aus dem Umstände, dass die individuelle Entwickelungsgeschichte oder die Ontogenie nur eine kurze und gedrungene Wiederholung, gleichsam eine Recapitulation der paläontologischen Entwickelungsgeschichte oder der Phylogenie ist.” = “…systematics’ explained then simply from the fact that the individual evolutionary history or ontogeny is only a short and concise repetition, as it were a recapitulation of the paleontological evolutionary history or phylogeny.” (Haeckel, 1866, II, p. XVIII).
However, in a strong contradiction with the lines above, the fundamental modern neglect of the ontogeny is rooted in the basic works of the major founders of the phylogenetic and ontogenetic thinking in the modern biology: Haeckel (1866), Garstang (1922), and Hennig (1966) as well. Although Haeckel (1866) laid foundation of the evolutionary understanding of ontogeny, his famous aphorism that “ontogeny recapitulates phylogeny” obscured the fact that ontogeny does not mechanistically accumulate evolutionary changes but instead produces them (Figure 1). In a support of this initial Haeckel’s contrasting terms separated ontogeny from phylogeny is the fact that Haeckel equals ontogeny with merely embryology, whereas phylogeny…with paleontology (!): “…dass wir den Begriff der Embryologie (Ontogenie) und der Palaeontologie (Phylogenie) nach Umfang und Inhalt scharf bestimmen.” = “…that we define the concept of embryology (ontogeny) and paleontology (phylogeny) sharply according to scope and content” (Haeckel, 1866, I, p. 53, our italics). Remarkably, this was performed by Haeckel with a very positive intention: to instead make closer exactly ontogeny and phylogeny, “According to the usual biological point of view, however, embryology and paleontology are completely diverse and distant branches of biology, which have nothing in common with one another but the object of the organism” (Haeckel, 1866, I, p. 53). However, more than 150 years after Haeckel’s fundamental insight, the “usual biological point of view” have persisted perfectly, at the level of the scientific publications, educational programs and as “a common comprehension,” represented for example in Wikipedia: “Ontogeny is the developmental history of an organism within its own lifetime, as distinct from phylogeny, which refers to the evolutionary history of a species” (Ontogeny, 2022, Wikipedia, our italics), despite all achievements of the evo-devo! This is not the result which Haeckel would have expected from his scientific descendants. The words and scientific terms are crucial not only for thinking and communication, but also for the proper development of the key scientific fields, such as evolutionary one. The persisting and very strict pre-Haeckelian sharp contrast between “ontogeny and phylogeny” has strongly postponed real development of the entire evolutionary field. Without new terminological (re)formulation furthers steps forward in that ontogenetic and evolutionary field, so strongly perplexed and confused over centuries, will be impossible.
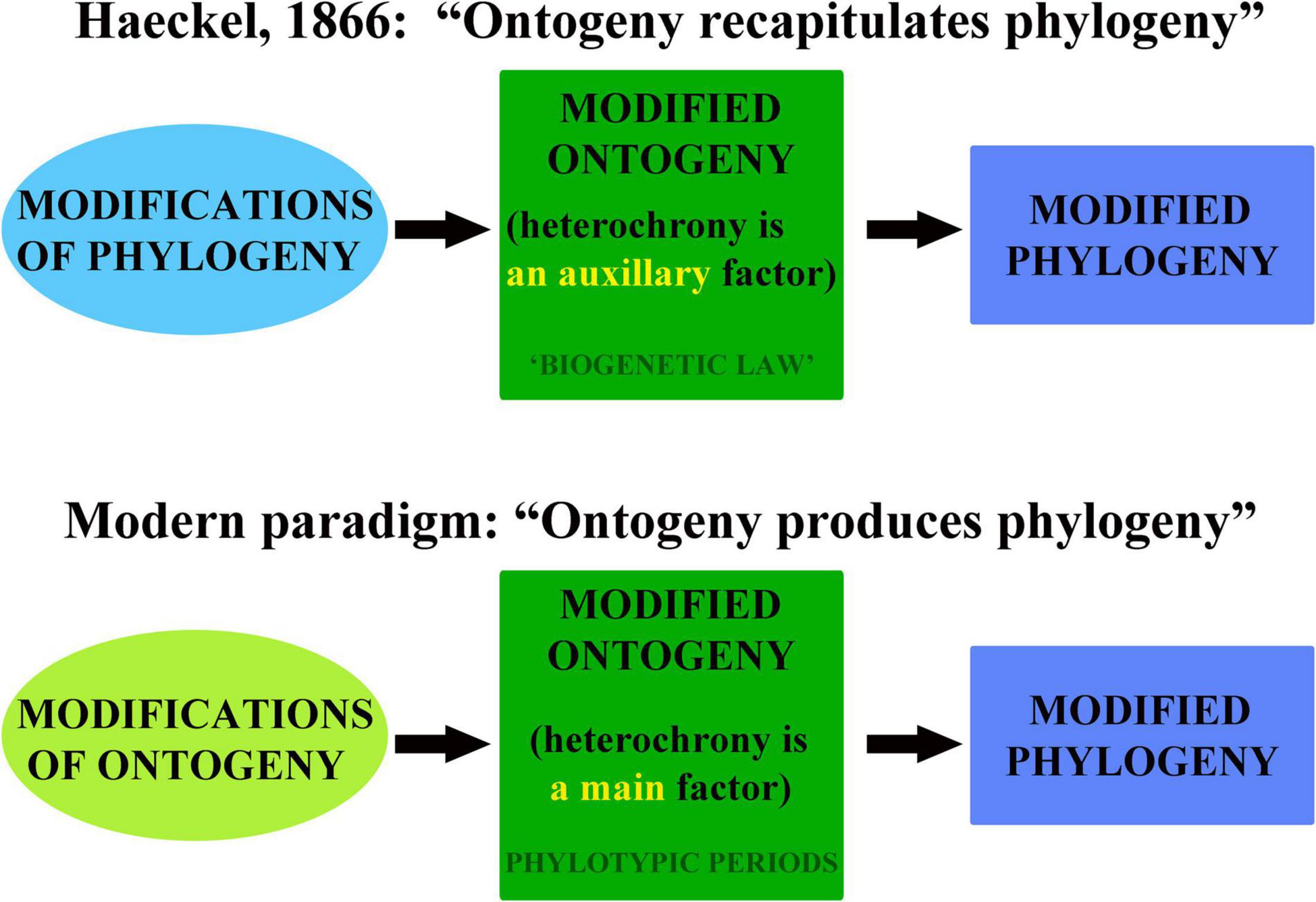
Figure 1. The scheme illustrates changes in the understanding of the “ontogeny and phylogeny” conceptual framework compared with Haeckel’s (1866) and modern paradigm since Garstang (1922) seminal work.
This statement has implications to a much larger extent than currently recognized. Despite that Garstang (1922) subsequently clearly indicated that fundamental deficiency of the Haeckel’s understanding of the ontogeny, in the modern general biological framework, the “ontogeny” and “phylogeny” are usually sufficiently contrasted. This is easy to prove that in majority of the molecular phylogenetic studies, including such crucial as phyla interrelations, ontogeny (or at least a “development”) usually either not mentioned, or if mentioned, but only as a highly subsidiary evidence (e.g., Laumer et al., 2019). The major approach of the phylogenetic inference is a suite of statistical methods based exclusively on the DNA sequences (e.g., Yang, 2014), which cannot be equated with the ontogeny as entire life cycle in all its complexity. The best balanced of the recent studies, which consider the development still make a major focus on the disparity along the clade evolution, than on a common shared ontogenetic patterns among different at adult stages phylum subgroups (Deline et al., 2020). Hennig (1966), despite that discussed ontogeny, concentrated on the phylogenetic aspects from the “ontogeny and phylogeny” pair, and by this, made a significant contribution to the modern persisting theoretical and practical fundamental omission of the ontogeny as a central process, which produced biodiversity.
However, each individual ontogeny is not only a product of the genetic mutations and selection that apparently allow that very formal scheme in Hennig (1966), but interlinks ancestral and descendant ontogenies through epigenetic and other developmental processes (e.g., Danchin et al., 2019; Anastasiadi et al., 2021; Loison, 2021; Yi and Goodisman, 2021; Lemmen et al., 2022). Therefore, the current unprecedented rise of the epigenetic data provides not only strict evidence for the reality of “every day”- ontogenetic modifications, but also some indulgence for a straightforward refusement by Garstang (1922) of the Haeckel’s initial paradigm! Although this is by no means reverse the correct Garstang (1922) conclusion on the major Haeckel’s misconception, that the phylogeny is not only a succession of adults, but alterations of the ontogenetic cycles, in which ontogeny plays the role of a central process. The profound linkage between adult and embryonic/larval parts of an ontogenetic cycles can, however, partly reconcile both Haeckel’s initial variant and Garstang’s reformulation. This is because since the time of Garstang the epigenetic influence of the adult modifications on the gametes has been confirmed (e.g., Anastasiadi et al., 2021). It was also noted that Haeckel adherence with phylogeny and consideration of ontogeny as a dependent process was result of an incorrect formulation, rather than Haeckel’s misunderstanding of the real ontogenetic data (Ezhikov, 1940). Furthermore, and even more paradoxically, Garstang (1922, p. 100, our italics) in turn made too much stress on the disproving of the Haeckel’s “biogenetic law”: “Inevitably there is recapitulation of successive grades of differentiation, but repetition of adult ancestral stages is necessarily and entirely lacking.” While Haeckel’s motto “ontogeny recapitulates phylogeny” masked the fundamental fact that phylogeny is not a separate process, but modification of ontogenetic cycles throughout time, however, Garstang’s definition in turn strongly disrupted the phylogenetic succession of the modified ontogenies, and by refusing of the importance of the linkage between adult and embryonic/larval stages, actually partly returned the ontogeny field to the antievolutionary “types concept” by von Baer (1828/1837). Therefore, in order to formulate true modern paradigm, both Haeckel’s original and Garstang’s subsequent formulations, need to be refined and reunited (Figure 1).
In a great concordance with the main line of the present work, it has been highlighted recently that ontogeny and phylogeny must be considered as a single process—“ontophylogenesis” (Kupiec, 2009)—and this is in turn remarkably mirrored not only the “phylembryogenesis” concept developed more than a century ago using a different argumentation (although still on the ontogeny-based background, Severtsov, 1912), but immediately recalled the basically unsuccessful, although heroic attempt by Haeckel (1866) to make “ontogeny” and “phylogeny” closer. The Severtsov concept, however, during less a century of its further development by its successors, turned to be not a modern reformulation of the Haeckel’s heritage, but became a new dogma, when within the complex and dynamic ontogeny, only three major modes that affects evolutionary modifications have been recognized: “anaboly, deviation and arhallaxis” (e.g., Severtsov, 1939; Ivanova-Kazas, 1995, 2004; see also discussion in Martynov, 2012a), which insufficiency has been already recognized, and limited “phylembryogenesis” was proposed to be substituted exactly with “phylontogenesis” (Vorobyeva, 1991, p. 73). This rigid “three-part” ontogenetic scheme significantly underestimated the real diversity of the ontogenies and phylogenetic results of its modification, and most importantly, did not help to overcome the persisting modern, strong contrasting of “ontogeny and phylogeny.” Obviously, on the immediate contrary to Severtsov (1912, 1939) assert that Haeckel biogenetic law is putatively justified only when ontogeny modified through “an extension of development, anaboly” is based on fundamental underestimation that a descendant ontogeny can be completely free from any ancestral ontogenetic patterns (either adult or larval), which is contradicted by all that is known about real ontogenies (e.g., Martynov and Korshunova, 2015; Korshunova et al., 2020; present work, Figure 2).
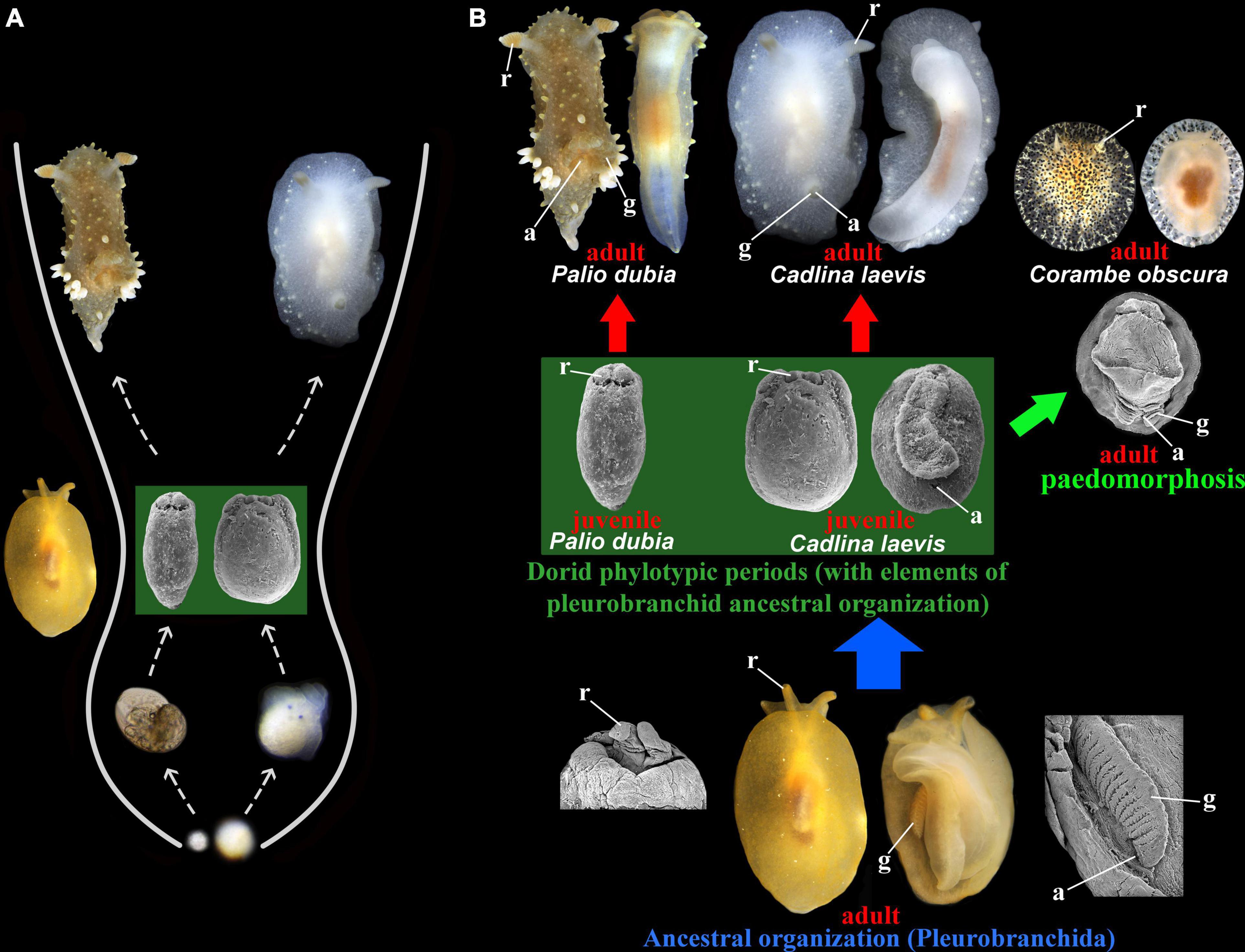
Figure 2. (A) The presentation of phylotypic periods (dark green box) in the disparate at adult phases dorid nudibranch families Polyceridae (represented by Palio dubia) and Cadlinidae (represented by Cadlina laevis) showing essentially similar to the classic vertebrate phylotypic period an irregular hourglass-like earlier ontogenetic patterns. (B) Adult morphological disparity (red arrows) and juvenile conservativeness of the dorid nudibranchs manifesting in the phylotypic periods (dark green box). The dorid phylotypic periods keeps several essential features of the adult pleurobranchid organization (joined rhinophores, ventral anus), which is ancestral for dorids. Thus, evolutionary modifications (blue arrow) of the adult pleurobranchid ontogenetic periods into dorid descendant organization partly remains as some key features in the early ontogenetic dorid phylotypic periods. See Korshunova et al. (2020) for molecular phylogenetic data. Dorid paedomorphic taxa (e.g., Corambe obscura, light green arrow) reveal some key features (including ventral anal opening and gills) which link both adult ancestral organization of pleurobranchids and phylotypic periods of majority of non-paedomorphic dorids. a, anal opening, g, gills, r, rhinophores.
In parallel with the Kupiec’s (2009) remarkable implication that ontogeny and phylogeny represent in reality the same process, it was unequivocally and independently concluded on the paedomorphosis as a process linking individual and historical development (Martynov, 2012a, p. 839). In this respect it is most importantly, that every individual ontogeny incorporates phylotypic periods, which have originated and persisted over a large evolutionary range (Arthur, 2002; Irie and Kuratani, 2011; Martynov, 2014; Martynov and Korshunova, 2015), and therefore ontogeny cannot be easily separated from the “phylogeny,” that was omitted by both Haeckel (1866) and Hennig (1966). However, Garstang (1922) in turn critically omitted the partial perseverance of the adult ancestral organization in form of the Haeckel’s “recapitulations,” and which have been later reformulated as phylotypic stage (Slack et al., 1993) or phylotypic periods (Richardson, 1995, 1999; Arthur, 2002, 2015). This is therefore, although at first glance very surprising, but consistent with these founders of the phylogenetic thinking in biology, when even the core achievement of the “evo-devo,” phylotypic periods, has been disregarded using precisely phylogenetic logic (Hejnol and Dunn, 2016).
Thus, the current situation is truly a most paradoxical one. Everybody understands the importance of ontogeny. This is an open secret at least since Garstang (1922) that the initial Haeckel’s definition of the relation between ontogeny and phylogeny contains a fundamental deficiency. However, the evidence for the phylotypic periods as a key concept of the “evo-devo” (Arthur, 2002; Cridge et al., 2019) and can be considered as partial modern reformulation of the “biogenetic law” (Levit et al., 2022) is almost negligible in the vast “phylogenetic” discipline. There also is an impressive number of the references which since 1866 attempted to discuss the “ontogeny and phylogeny” field under various names and using different aspects (Gould, 1977), or in some way criticize or reformulate Haeckel’s “biogenetic law” and concepts of the Haeckel’s immediate predecessor Müller (1864), and the citations provided here do not pretend to be exhaustive, but to show some punctuated time line (e.g., Gegenbaur, 1888; Hurst, 1893; Bateson, 1894; Sedgwick, 1894; Mehnert, 1897; Keibel, 1898; Morgan, 1908; Smith, 1911; Severtsov, 1912, 1939; Garstang, 1922; de Beer, 1930, 1958; Needham, 1933; Kryzhanovsky, 1939; Schmalhausen, 1942; Ivanov, 1945; Bonner, 1965; Emelyanov, 1968; Mirzoyan, 1974; Gould, 1977; Alberch et al., 1979; Peters, 1980; Raff and Kaufman, 1983; Kluge and Strauss, 1985; McNamara, 1986; McKinney, 1988; Vorobyeva, 1991; Ivanova-Kazas, 1995; Gilbert et al., 1996; Müller, 1997; Bininda-Emonds et al., 2002; Hall, 2003, 2011; Belousov, 2005; Wiens et al., 2005; Minelli, 2007, 2009, 2015a,b; Kupiec, 2009; Martynov, 2009, 2011a,b, 2012a; Wanninger, 2015; Lamsdell, 2020; Martynov et al., 2020; Núñez-León et al., 2021; Levit et al., 2022; Richardson, 2022; Uesaka et al., 2022; and many others). However, despite on all these tremendous efforts, by year 2022, ontogeny remains to be a subsidiary discipline of the broadly phylogenetic studies. The original Haeckel’s definition, Garstang’s reformulation and the apparently modern phylotypic periods concept existed largely separate from each other, despite they are all must be inevitably intersected. Thus, before ontogeny will be a real basis for any biological discipline including taxonomy (and hence a fundamental to immense biodiversity patterns and studies), exactly theoretical basis of the “ontogeny and phylogeny” field must be (one more time) revised. This is a key starting point that can further help to merge the achievements of the evolutionary developmental biology with the true keeper of the worldwide biodiversity—systematics and taxonomy.
Because the ontogenetic field and related evolutionary problems is immense, in the present work we cannot address all arisen questions, but we instead will focus on the clarification of some core concepts which are related to the field of “ontogeny and evolution” and present perspective for the further development of the emerging interdisciplinary field of ontogenetic systematics with an emphasis to the paedomorphosis process linked to the ancestral organization via phylotypic periods.
Terminological Clarification
Ontogeny, Evolution, and Phylogeny
Ontogeny must not be restricted to a developmental stage or a metamorphosis, but it should be explicitly stated that ontogeny is an entire life cycle in all its evolutionary dynamics. The term “evolution” (and therefore, phylogeny) is unfortunately loosely connected with the term “ontogeny,” and the fact that ontogeny is meaningless without invoking ontogeny which produces phylogeny (Figure 1) and it is notable that the original meaning of the term “evolution” was “ontogeny” (Bowler, 1975). Any ontogenetic patterns and processes, among them such central as phylotypic periods and heterochronies, are therefore not solely specific terms of the “evo-devo” field but have direct importance for the origin of the biological diversity and hence is of paramount importance for the biological taxonomy. For example, in a conditional evolutionary-free framework and without consideration of ontogeny as entire life cycle, the juvenile and adult stages can be putatively considered as separate sets of data (Figure 2, red arrows). However, for instance, juveniles of dorid nudibranchs represent from one hand the key features of the pleurobranchid ancestral organization (joined rhinophores and ventral anal opening) and form respective phylotypic periods (Figure 2, dark green box). From the other hand, adult dorid family Corambidae also possess ventral anal opening and gills due to the process of paedomorphosis, which secondarily returns the phylotypic patterns to the adult stages (Figure 2, light green arrow). By these and more examples (Figures 3–5) it became obvious that modifications of ontogeny is a basis for appearing of the biological diversity and that a “phylogenetic” study cannot be performed without consistent incorporation of ontogeny. This is therefore not a surprise that very significant efforts have been done by antievolutionists in attempts to of course unsuccessfully disprove the real existence of the Haeckel’s recapitulations (see e.g., Richards, 2009), which are currently can be partly reformulated as phylotypic periods. This is because exactly phylotypic periods provide direct evidence of the reality of evolution. Modern strict phylogenetists must therefore not attempt to disprove (e.g., Hejnol and Dunn, 2016) the reality of the universal conserved periods in ontogeny of the very different at adult stage organisms (Figures 2–4), but instead highly praise this essential for the understanding of evolution and phylogeny phenomenon.
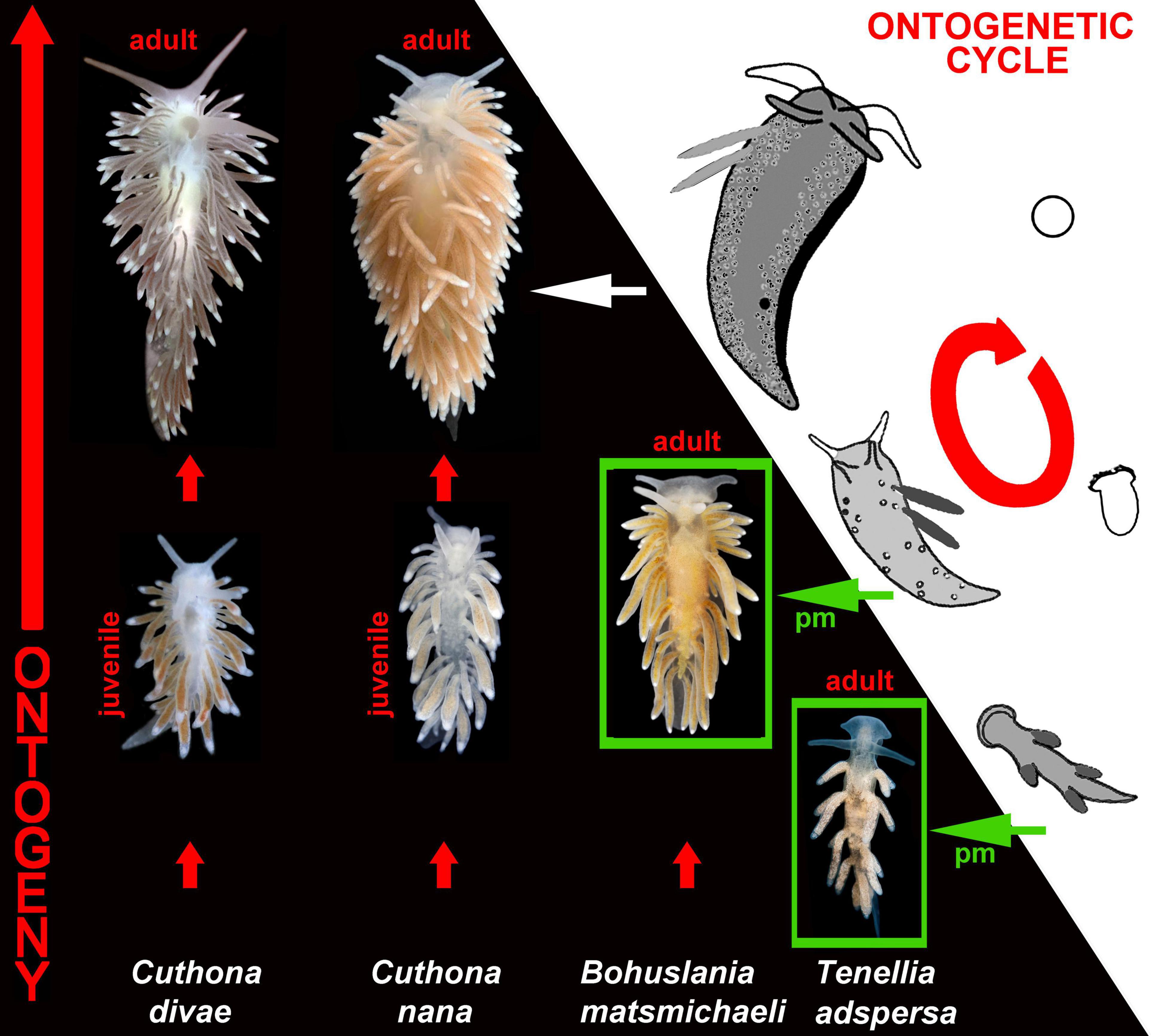
Figure 3. Ontogenetic-and-evolutionary linkage through the modifications of the ontogenetic cycles of aeolidacean nudibranchs. Note significant similarity between aeolidacean ontogenetic periods (hence, phylotypic periods) and adult organization of particular paedomorphic (pm) aeolidacean taxa (e.g., Bohuslania and Tenellia, green boxes). See Korshunova et al. (2018) and Martynov et al. (2020) for molecular phylogenetic data.
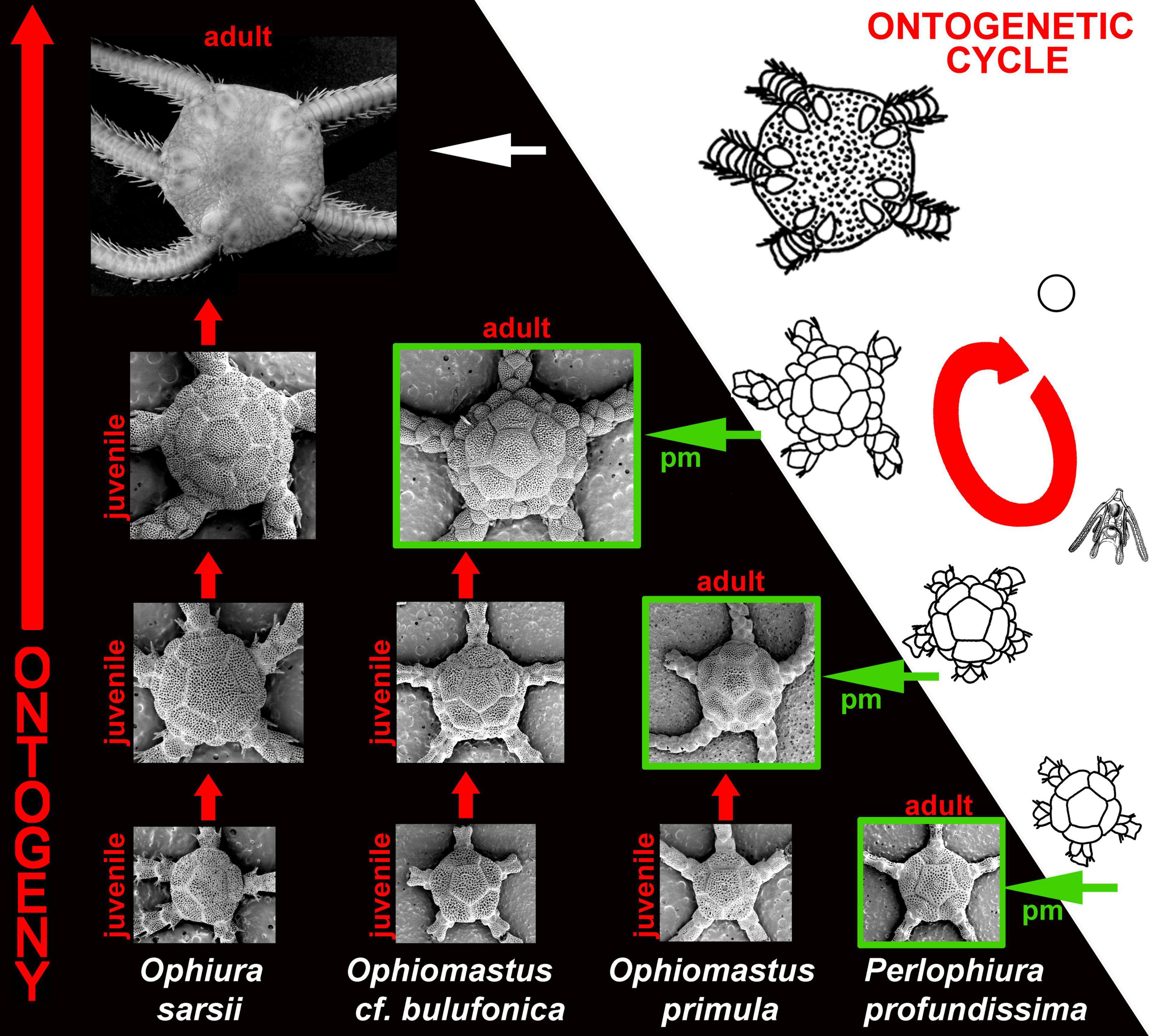
Figure 4. Ontogenetic-and-evolutionary linkage through the modifications of the ontogenetic cycles of ophiuroid echinoderms. Note significant similarity between ophiuroid ontogenetic periods (hence, phylotypic periods) and adult organization of particular paedomorphic (pm) ophiuroid taxa (e.g., Ophiomastus and Perlophiura, green boxes).
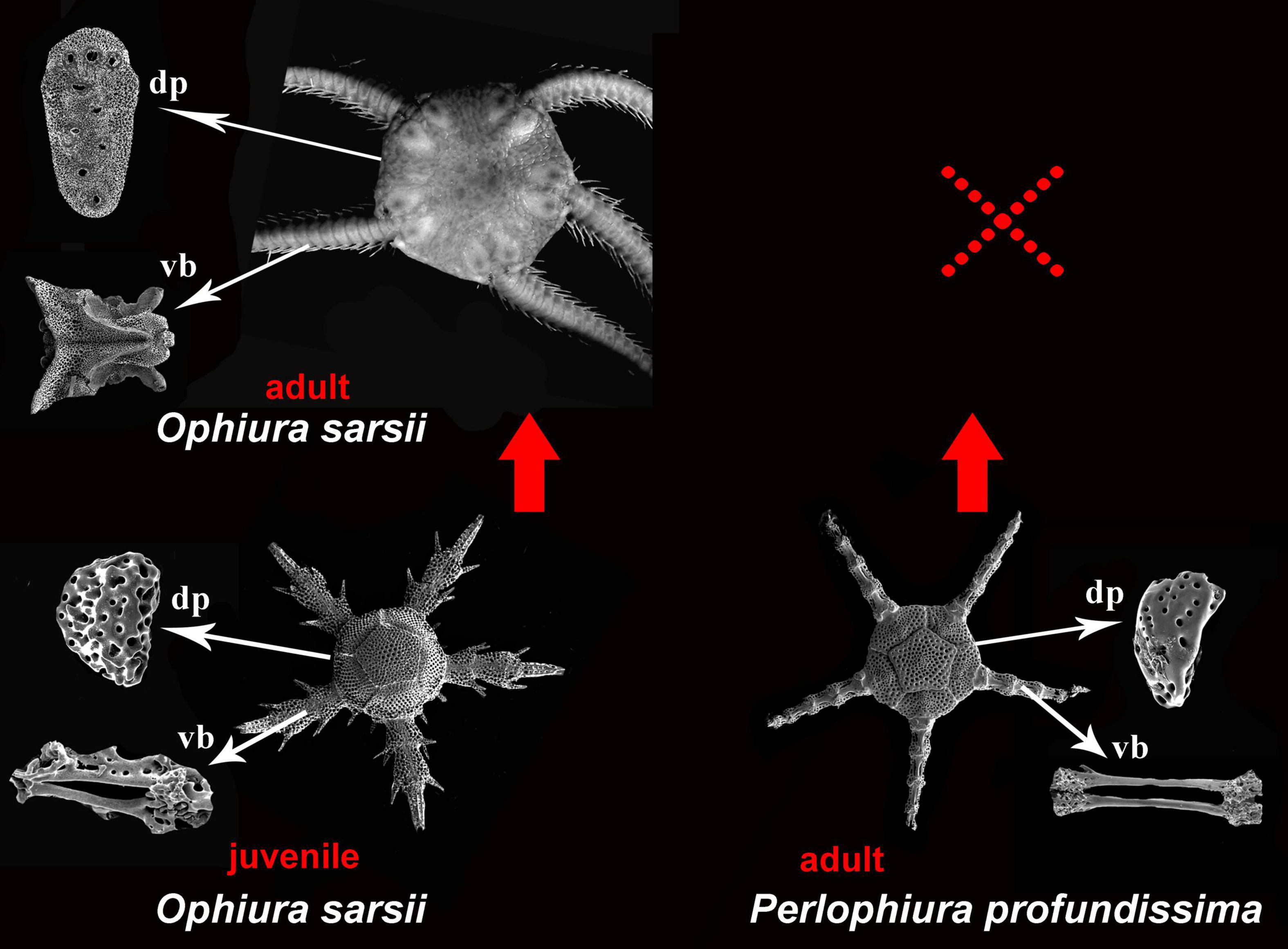
Figure 5. Evidence for essential, unique similarity between respective homologous ophiuroid characters, including external primary disk plates and internal ossicles (dp, dental plate; vb, vertebrae) of the adult strongly paedomorphic ophiuroid Perlophiura profundissima and phylotypic periods of early juveniles (postlarvae) of the complex non-paedomorphic ophiuroid Ophiura sarsii. The considerable reducing of the paedomorphic life cycle, which lacking complex adult stage is indicated by dashed red cross. By this unique similarity between adult paedomorphic taxa and juveniles of the complex taxa makes almost direct ontogenetic-and-evolutionary linkage as impossible to deny.
Phylotypic Periods, Recapitulations, and Biogenetic Law
The concept of phylotypic period implies the presence of similar, homologous and conservative periods of ontogeny shared by various groups with disparate adult morphology (e.g., Arthur, 2002; Martynov and Korshunova, 2015; Cridge et al., 2019; Figure 2). Phylotypic periods and their underlying transcriptomic activity have been documented in disparate metazoan phyla (e.g., Domazet-Lošo and Tautz, 2010; Kalinka et al., 2010; Ninova et al., 2014). The term phylotypic period (sometimes as denounced as a phylotypic stage) largely substituted the term recapitulation (Slack et al., 1993; Richardson, 1995), but this was rather terminological than a biologically founded proposal. Ontogeny, in many cases, preserves major features of an ancestral ontogenetic cycle (which thus can be directly partly observable in form of the phylotypic periods), but not an entire sequence of evolutionary alterations. This is very important to note, that phylotypic periods from one hand do not preserve entire ancestral evolutionary sequence and cannot be considered as completely “uniform” across major subtaxa of a given higher taxonomic group, e.g., vertebrates (Richardson et al., 1997), but this should be not used as a substantiation against its fundamental importance for the inferring of phylogenetic patterns (Richards, 2009; Arthur, 2015). From the other hand, the phylotypic period should be not restricted only to a search for a “single” hourglass-like pattern within an ontogeny that is commonly performed in the “evo-devo” field. Any ontogeny obviously preserve several layers of the ontogeny of ancestors, and therefore several phylotypic periods can be potentially recognized within a given ontogeny of a given group, and every phylotypic periods can potentially roughly corresponds not to single, but several “higher taxa” of traditional taxonomic hierarchy (Martynov and Korshunova, 2015; present work). This novel approach to recognize several phylotypic periods within a given ontogeny is also highlighted here (Figures 2–4).
A very relevant example of the phylotypic periods is dorid nudibranchs, when at early juvenile periods phylogenetically distant and morphologically disparate taxa, such as Cadlina and Palio from different families, see details and molecular phylogeny in Korshunova et al. (2020), show significantly similar morphologies, of course not absolutely identical (Figure 2), exactly as phylotypic periods of birds, although essentially similar to that of mammals are different in some details (Cridge et al., 2019). Such a strong adult divergence and juvenile fundamental similarity essentially conforms to the classic examples of the phylotypic periods in vertebrates (Haeckel, 1866; Slack et al., 1993; Arthur, 2002) and imply direct contributions for the origin of the taxonomic diversity. This is also very important example to show tight linkage between ontogeny and evolution: on the Figure 2 we show that ancestral organization of the order Pleurobranchida (proved also using the molecular phylogenetics, see e.g., Pabst and Kocot, 2018) while modified into disparate dorid descendent organization (Figure 2, blue arrow), however, preserved at the juvenile phylotypic periods some elements of the adult pleurobranchid ancestors, including such key patterns as joined rhinophores and ventral anal opening (Figure 2, dark green box).
This example is very important because demonstrates ontogenetic patterns principally similar to the classic vertebrate “regular or irregular” hourglass-like phylotypic patterns (Cordero et al., 2020) in a completely different animal phylum, Mollusca (Figures 2, 3), as well as within Echinodermata phylum (Figure 4). This also well demonstrates that evolution and resulting phylogeny is not a some theoretical process that needs to be specially proved, but that elements of adult organization of remote ancestors is still integral part of ontogenies of the modern, really existed descendants, and this pattern of the partial preservation of the ancestral morphologies in form of the respective phylotypic periods can be revealed among representatives of such disparate phyla as molluscs (Figure 2) or chordates (e.g., Arthur, 2015). This naturally existed and proved here to be universal ontogenetic preservation of the elements of adult ancestral morphologies (with respective molecular ground, e.g., specific transcriptomic activity) between very different animal phyla (Figures 2–5) was critically omitted by Garstang (1922) in his reformulation of the Haeckel’s “biogenetic law.” It is also possible to recognize several ancestral layers within dorid ontogenies, which represent several phylotypic periods corresponded to the several modifications of ancestral organizations and have been named separately, e.g., as phylotypic periods dp1 and dp2 (see Martynov and Korshunova, 2015). Also, in a remarkable coincidence with partially- or irregularly resembling the famous “hourglass” ontogenetic pattern (with all possible reservations, see Arthur, 2015), because early larval modifications (e.g., planctonic larva in dorid Palio and direct-developed larva in dorid Cadlina, Figure 2) apparently more different than subsequent “middle” phylotypic stage dp. 1 (Figure 2). Therefore, Haeckel’s recapitulations are partly compatible with the both “funnel model” and the hourglass model, the latter dominating in today’s evo-devo. However, as an important comment to this, it is needed to highlight, that despite on the differences, the larval earliest ontogenetic periods in the adult shell-less Palio and Cadlina are still can be considered as phylotypic periods common with the predominantly shelled molluscan class Gastropoda since both Palio and Cadlina bear essentially the same veliger-like structures, although highly reduced in the direct developer Cadlina (Figure 2). Therefore, the “evo-devo” hourglass concept should not disrupt and mask the key ontogenetic consideration, that even within strong “larval adaptations” obvious remnants of the shelled gastropod ancestral organization can be recognized and traced within shell-less at adult stage nudibranchs. By this, it is also relevant to make a special reservation, that although all evidences, including molecular data should be used to confirm evolutionary models and ancestral patterns, we refer to the “molecular phylogenetic data” as a proof not because we cannot provide evidence using ontogenetic data, but because of the obvious dominance of the “phylogenetic thinking” over ontogenetic one, the theory and practice of the ontogenetic systematics were largely not developed. It is now time to explicitly start and reintroduce that “new old” discipline, which consistently encompassed and re-untied “ontogeny and phylogeny” immense field.
It is also necessary to highlight, that manifestation in the ontogeny of descendants only partial, but still key characters of the adult ancestors (e.g., Figure 2) do not disprove recapitulations. Therefore, the past sometimes very harsh and unsubstantiated critic on the entire Haeckel’s fundamental works (e.g., Borzenkov, 1884, p. 130–135) and currently persisted common view that biogenetic law (which is based on the partial recapitulation in ontogeny of descendants of an ancestral organization) has been completely abandoned (e.g., Raff and Kaufman, 1983, p. 19) are in reality profoundly incorrect and does not correspond to the real ontogenetic patterns. Recapitulation in the renewed sense should be understood not as “recapitulation of phylogeny” in strict Haeckel’s sense, but as partial recapitulation of ontogeny of ancestors. This reformulation is important challenge for the contemporary biology since directly influences understanding of the key role of phylotypic periods for the fields of taxonomy and phylogeny. The fact that modern ontogeneses preserve in the phylotypic periods some key features of adult ancestral organization of remote ancestors (for example pharyngeal clefts (arches) in terrestrial mammals) is significantly undervalued by modern evolutionary biologists (e.g., Futuyma and Kirkpatrick, 2017, p. 371), despite clear evidence from the evolutionary developmental biology in presence of various conserved periods (e.g., Irie and Kuratani, 2011; Cridge et al., 2019; Cordero et al., 2020; Hao et al., 2021; Liu et al., 2021; Levit et al., 2022; Uesaka et al., 2022). Therefore, typical modern understanding (e.g., Barnes, 2014) that Haeckel’s core contributions as largely wrong due to the putative failure of the concepts of recapitulations and biogenetic law is in fact fundamentally incorrect and must be no more continue to be mentioned as “wrong” in numerous educational as well as targeted for broad audience sources. We must praise Haeckel for the first consistent application of the evolutionary idea to the ontogeny (e.g., Levit and Hossfeld, 2019), even with respective reformulation of the Haeckel’s key concepts. Especially dangerous sometimes continuing association of Haeckel with the Nazis regime, even in a softened form (e.g., review in Rieppel, 2016), because Haeckel died more than 10 years before that regime has been established, and although Haeckel has made controversial statements of the role of artificial selection in the human society, the potential subsequent malicious usage of his heritage by Nazis by no means should be considered as his guilt. In this respect, Rieppel (2016, p. 78, 79) specially highlighted that “In his own time, at any rate, Haeckel had defend himself not as protagonist of right-wing politics or fascism, but instead of socialism and academic liberalism,” and remarkably, “believe that continuing evolution of the human brain would one time render armed conflicts a thing of the past” (!). Thus, whatever of the Haeckel’s controversies, more than one century later, by the end of February of 2022 we must only conclude that sadly, Haeckel’s desperate call has not been yet implemented by the politicians and the human society.
The tight practical linkage between ontogeny and evolution (instead of the contrasting notion of “ontogeny and phylogeny”) is further reinforced by the widespread phenomenon of paedomorphosis. Paedomorphosis in turn almost directly links juvenile ancestral organization (which is partially kept in ontogeneses in form of phylotypic periods, Figure 2, dark green box) with actual existing adult stages of modern descendants (Figure 2, light green arrow), although it was attempted to deny this (e.g., Mayr, 1963), and with the reinforcement of the solely “phylogeny-part” from the undividable pair of the ontogeny and phylogeny, this “phylogenetic denying” of reality of the universal ontogenetic patterns across distantly related taxa is continuing (e.g., Hejnol and Dunn, 2016). Here, we will present several striking examples of the reality of ontogenetic linkage (see also Martynov et al., 2020) between ancestral juvenile (but at a preceding ancestral level, still representing past adult organization) and descendant adult characters among taxa of very disparate phyla such as molluscs and echinoderms (Figures 2–5).
Phyla Are Real in the Sense of Natural Ontogenetic Properties
Originally, we did not plan to specially emphasize this point, because it is apparently an obvious one. However, a kind comment of one of the reviewers point that situation with the neglecting of the ontogeny in biology (not speaking of taxonomy) is so serious, so we need to specially emphasize this question there. Thus, we find that the phyla can be considered as “not real ones” (with all reservations to the word “real” in a taxonomic context), and at best can be comprehended as a collection of an endless number of phylogenetic clades, those, and not the phyla itself must be a central focus of evolutionary study (e.g., Hejnol and Dunn, 2016). Because in the latter paper even most serious, naturally ontogenetic evidence of the real existence of the ontogenetic phylotypic periods across at adult stages dramatically different taxa (Levin et al., 2016), has received a severe critic from the “phylogeny-centered” colleagues, it will be not very convincing to provide just logical or theoretical arguments in support of the existence of the universal ontogenetic patterns. Instead, we will provide here a very simple and a very practical test. Given a marine location, where we have performed some sampling. In the obtaining samples we may find at a first glance, an endless diversity of invertebrate animals. However, such diversity is endless only putatively. Almost 300 years of long and controversial development of the systematic zoology starting from just a structural comparison (still within a non-evolutionary thinking), through acceptance of the evolution and following by the phylogeny-based boom, do not only disprove, but instead strongly strengthened an obvious and natural fact: in any sample obtaining at any depth and at any environment we will be not able to find more than 40 major structural multicellular animal-related organizations, which the systematic zoology assessed as animal phyla, i.e., any endless number of individuals and subgroups will be encompassed by just less than forty major structural plans (with only minor disagreement since some of the phyla can be treated as subphyla/other subgroups). This is a remarkable scientific achievement that judge from the comments from the phylogeny field (e.g., Hejnol and Dunn, 2016) either do not acknowledge at all, or misleadingly interpreted.
The next test, that even if we have a strong intention, this will be highly unlikely that someone will easily describe a completely new phylum. A description of a new phylum is a rare, exceptional, and obviously not a routine event. Furthermore, even the most recent phylogenies confirmed validity of absolute majority of the animal phyla (e.g., Laumer et al., 2019), and the main alterations rather concern mostly the status of annelid-related phyla (subgroups). Therefore, someone may wish to find more or deny the existence of the very limited number of the basic animal organizations, when you will come across with a real sample from a real environment, it will be an exceedingly rare and lucky chance that you will be able to detect some completely new organization, beyond that less than 40 main animal structural organizations (even it is still possible to somewhat extend or reduce in number). Notably, there is a recent proposal from the “evo-devo” to consider vertebrates as a separate phylum (Irie et al., 2018). However, again, even with possible somewhat extension of the main structural organizations, they will be still very limited in number, and the “phylum-hypothesis” continues to be confirmed on daily basis by practicing biologists. We specially avoid here the term “body-plan” in order not to be aligned with the pre-evolutionary thinking, but for the majority of phyla, even despite on the subgroup diversity and reduction it is possible to provide a diagnosis that will contain a specific for every phylum set of the adult and juvenile (larval) characters.
Which the most important facts evolutionary developmental biology has added to this well established and obviously natural structural pattern of the highest conservatism discovered by the systematic zoology and largely supported by the molecular phylogenetics? Evolutionary developmental biology has concluded that even in the highly disparate at adult stages representatives of an animal phylum, there are some ontogenetic stage(s) of highly conserved period(s), which even with all reservations and subgroups diversity (e.g., Richardson et al., 1997, Richardson, 2022; Richards, 2009; Arthur, 2015; Levin et al., 2016; Deline et al., 2020) are persisted in their inter-taxon conservatism. For every extant phyla, despite on all the class diversity and reductions it is possible to provide a particular, unique enough set of adult and larval patterns, both according to the classic “structural” approach with some evolutionary ground (e.g., Brusca and Brusca, 1990) or framed in apparently very strict “modern phylogenetic framework” (e.g., Schierwater and DeSalle, 2021). The latter book is especially relevant for the main topic of the present contribution. It is titled as “Invertebrate Zoology: A Tree of Life Approach,” despite that in an abstract it is described as “Synthesizing …. classical morphology, sequencing data, and evo-devo studies”! This is a best proof that even if ontogeny in some way is included into modern studies and reviews, it is an important but still an “add-one” to the phylogeny. Therefore, it is absolutely justified to conclude that there is no “ontogeny” as a primary discipline compared to the resulting process of phylogeny in the contemporary biology. The continuous urgent call for the necessity of the independent, primary discipline of the ontogenetic systematics is therefore fully justified.
Thus, the concept of the phylotypic periods (“stages”) instead of being falsely alleged to be an “idealistic discipline,” at a new level synthesizes the achievements of the systematic zoology exactly in the evolutionary sense. The newest data in support of the real existence of the phylotypic periods in the ontogeny of such fundamentally different divisions of organisms as animals and plants are continued to be available (e.g., Liu et al., 2021). If we will discard phylotypic periods from the core concept of the evolutionary developmental biology, then the entire field of the “evo-devo” must be discarded, because otherwise “evo-devo” is just a supplementary to a phylogenetic analysis, which will be always primary to the ontogeny. Indeed, a lot of efforts still need to be done in the recognition of the shared phylotypic periods at many different levels of ontogeny across majority of the animal and plant phyla (divisions), but this do not mean that if we do not have a clearly recognized phylotypic periods for all phyla and for many subgroups, we should refuse this key ontogenetic and evolutionary approach. The inclusion of paleontological data is special challenge, but as we already mentioned above, in every phylogeny there are not just “single phylotypic period,” but several layers of ancestral ontogenies (e.g., Figures 2–4), and therefore shared phylotypic periods can be assessed also for supraphyletic taxa, including extinct phyla and other taxonomic groups. That is why a separate field of ontogenetic systematics which is clearly put forward the fundamental precedence of the ontogeny for the evolution, and hence for any phylogenetic analysis is fully justified and highly necessary. The key proposal of the ontogenetic systematic is that the phylotypic periods are no more “isolate Baer’s entities” but inevitably reflect elements of ancestral organization, and hence indispensable for the reconstruction of the ancestral organizations at any taxonomic levels. That can be a better involvement of truly evolutionary principles and that could be a better antidote against any antievolutionary approaches, than the very phylotypic periods approach? It is therefore really important to present why apparently profoundly evolutionary phylogeneticists deny for the ontogeny field the ability to even more clearly present an ancestral organization that it partially manifested at the phylotypic periods of ontogeny. With the only reservation perhaps that the entire ontogeny itself as entire set of several phylotypic periods. To clearly understand this, we need to more strictly outline the basic principles of the ontogenetic systematics, as we presented in this contribution, respectively.
Paedomorphosis
Paedomorphosis is a next to the phylotypic periods very important evidence for the very tight linkage between ontogeny and phylogeny, as well as between ontogeny and evolution (Martynov, 2012a). This is because while a paedomorphic organism is formed, partial structural patterns which in ancestors persist only at larval or juvenile stage, in paedomorphic adult descendants, become part of adult organization. Although this is partly evident, but in reality this is a highly underestimated consideration. Because in this case, a paedomorphic organism partly became… a functional, adult phylotypic period of ancestral ontogenies! This is not a stretch or a pure theoretical consideration. Adult paedomorphic dorids of the family Corambidae essentially similar externally to the phylotypic periods of the complex non-paedomorphic dorids (Figure 2), paedomorphic cuthonid aeolidacean nudibranch genus Bohuslania essentially similar to the juvenile phylotypic periods of the genus Cuthona (Figure 3), and strongly paedomorphic ophiuroid of the genus Perlophiura fundamentally similar to postlarvae of the non-paedomorphic ophiuroids both externally and internally in such degree (Martynov, 2009, 2011a,b, 2012a; Stöhr and Martynov, 2016), that can be confused with a real postlarva/earlier juvenile (Figures 4, 5). This is in turn, very nicely corroborated the classic example of paedomorphosis, the axolotl and further examples of various obligate or non-obligate paedomoprhosis cases among amphibians (e.g., Wiens et al., 2005), and also partially evokes the “fish-like” ancestral organization (usually kept only as the ontogenetic phylotypic period), but at the adult stage.
In this respect, in relations to the phylotypic periods and adult and juveniles ancestral and descendant organization, paedomorphosis represent several remarkable layers of primary modifications of an adult ancestral phase into juvenile phylotypic periods of descendants, and then, a secondary partial restoration of the primary adult ancestral organization at the descendant secondary adult paedomorphic organization (Figure 2). These consequential and complex interactions between adult, juvenile phases in ontogeny linked by phylotypic periods is fundamentally omitted in the modern taxonomy and biology. To further complicate that picture, some ontogenetic level can be exclusively larval since the most ancestral organization, e.g., sponges-like biphasic adult-larval ontogenetic cycle persisted in the majority of the modern bilaterians, and the larval phases can be only partially involved into formation of the adult organization. Of course, since there is an almost endless number of ancestral ontogenies, the term “primary and secondary” in the given example are applying only to illustrate that general principle. Therefore, that yet unnoticed for Haeckel, and rather exotic modus of phylogeny for Hennig, paedomorphosis is a widespread and in reality the central and one of the most important evidence of the indivisible linkage between ontogeny and phylogeny. And hence paedomorphosis is among also most striking and most “self-evident” strongest evidence of the evolution.
However, currently just at a terminological level, there is incorrect usage around the term “paedomorphosis,” that needs to be clarified here. Particularly, “neoteny” and “progenesis” are still sometimes used interchangeable and as plain synonyms of paedomorphosis, without reference to original meaning. This generates considerable confusion despites on previous attempts to clarify the definition (e.g., Reilly et al., 1997). Therefore, below terminological clarification is given. The term paedomorphosis was initially suggested (Garstang, 1928) and subsequently revised (McNamara, 1986) to encompass various phenomena of the appearance of larval/juvenile characters of ancestors at the adult stages of descendants and to highlight its role in (macro)evolution. It is needed to be noted here, as it clearly implied by the all ontogeny-centered field, the strict distinction between “micro-” and “macro”-evolution can generate significant exaggeration of putatively “separate macroevolutionary” processes instead of universal ontogeny-based evolutionary process, and this distinction must be therefore avoided. The terms “progenesis” and “neoteny” were instead originally proposed for very restricted cases without phylogenetic context. “Neoteny” was proposed by Kollman (1885, p. 391) specifically to indicate the retardation of development in a few amphibian species including the axolotl. Kollman clearly described “neoteny” as an intraspecific process, without link to evolution. However, the term “neoteny” has been incorrectly applied to describe the evolutionary process, particularly in humans (Gould, 1977), and despite on subsequent clarification (Reilly et al., 1997) still is sometimes being wrongly used in this sense (Skulachev et al., 2017). “Progenesis” was first suggested by Giard (1887, p. 23) in a highly specific sense, in reference to precocious maturation in some decapod crustaceans due to parasitic castration (!).
The term “paedomorphosis” is currently universally accepted as a higher-level term encompassing both “neoteny” and “progenesis” (McNamara, 1986; Smirnov, 1991; Martynov et al., 2020), however, especially “progenesis” is sometimes used separately and as a substitute for the term paedomorphosis (e.g., Yushin and Malakhov, 2014). This is wrong because originally the terms “progenesis” and “neoteny” lacked the key evolutionary component and were highly inconsistent with the initial and modern meanings of the term paedomorphosis. It is especially important to highlight the wrong application of “neoteny” and “progenesis” as synonyms of the entire paedomorphosis central concept, because especially in Russia there is a long tradition of the wrong substitution of the paedomorphosis with “neoteny” (e.g., Karavaev, 1934; Ivanova-Kazas, 1936).
Another crucial consideration is that “progenetic” and “neotenic” patterns are just different sides of the same paedomorphic process. In various organismal groups, often a taxon that demonstrates evident juvenile characters at the adult state is difficult to attribute exactly to “progenetic” or “neotenic” ones due to a strong heterochronic mosaicism of delayed and accelerated growth characters (Godfrey and Sutherland, 1996; Rundell and Leander, 2010; Lecointre et al., 2020). It is therefore is of key importance in assessing of the paedomorphic features not to make the strict differences between patterns and processes, between basic ontogenetic (including paedomorphosis) processes and “morphological results,” otherwise the artificial substitution of “neoteny” over paedomorphosis may appear (Gould, 1977; Smirnov, 1991). Because if both retardation of the development of somatic organs or acceleration of maturation may lead to paedomorphosis (Gould, 1977, p. 8), then in the latter case a retardation of somatic development will be also required, otherwise the resulting morphology will be not paedomorphic. In this respect, it is especially relevant to indicate that the original definitions of “progenesis” and “neoteny” did not refer to the evolutionary heterochronic processes, per se, and did not necessarily link shifting maturation time with somatic differentiations. Therefore, the general term paedomorphosis should be used instead of controversial terms “progenesis” and “neoteny” (Reilly et al., 1997). As an important reservation it should be clearly stated, that although paedomorphosis is a very important mode of the evolution (e.g., Gould, 1977; Korshunova et al., 2018; Lamsdell, 2020; present review), this does not imply that ancestral developmental patterns are easily disappeared in a course of an evolutionary modification. Practical confirmed examples of paedomorphosis in nudibranch molluscs show that before a distinct paedomorphic organization has been formed, e.g., in the nudibranch families Corambidae or Okadaiidae (Korshunova et al., 2020), or in aeolidacean genus Bohuslania (Korshunova et al., 2018) a significant amount of gradual modifications of an ancestral organizations (= ancestral ontogenetic cycles) were occurred.
Paedomorphosis and Progress as Integral Parts of Heterochronies, Whereas “Peramorphosis” Is a Redundant Term
Paedomorphosis is a part of broader ontogenetic processes, heterochronies (different timing of character appearance in ontogeny) (e.g., Lamsdell, 2020; Lecointre et al., 2020). While paedomorphosis can be clearly defined in terms of correspondence of the juvenile characters of ancestors to the adult features of descendants, an “opposite term” peramorphosis has been proposed (review in McNamara, 1986) with the main meaning of “development beyond ancestral organization.” Such definition may generate confusion since basically it does not differ from evolution of novelties, i.e., progressive development (or just progress) in a broader sense (see also discussion in Martynov, 2012a). In this respect the term “peramorphosis” appears as redundant and confusing and we recommend avoiding it, and use instead “progress,” “progressive.” However, any real organism, even which represent strong paedomorphic characters is a mixture of paedomorphic and progressive traits. A remarkable example of mixture of paedomorphic and progressive features have been assessed for modern humans, which show general paedomorphic delay of many features of body similar to the juveniles of apes, but in contrast demonstrate a highly progressive development of brain (e.g., Godfrey and Sutherland, 1996). Further notable example of such intricate mixture of the paedomorphic and progressive traits is the dorid nudibranch family Corambidae, which secondarily returned the phylotypic condition of ventral anal opening and gills (the paedomorphic part of corambid evolution, Figure 2, Corambe obscura), but acquired also a special shedding cuticle unique among molluscs (Martynov, 1994; Martynov and Schrödl, 2011) that can be considered as a progressive side of the evolution of the family Corambidae. We also understand that the usage of the progress instead of “peramorphosis” can be partly misleading too, because progress can be also used in the sense of not a progressive increasing of a complexity, but for example in an ecological sense, like morphological over-simplified nematodes are highly abundant and thus can represent a “progress.” However, taking into consideration all that pro and contra we still consider that “peramorphosis” is an obscure and much later rather unnecessary term, compare to the basic term “progress,” which in relations to the ontogeny we propose to clarify and partly re-defined and refer to solely of an evident material increasing of complexity of organization, which is based on addition of a particular new periods in ontogeny including specific number of new characters/elements, which were lacked in the ancestral ontogeny, and not just some vague ecological considerations. In some respect, the progress is a formation of an obvious, new, well recognized phylotypic periods, which have been absent in preceding ancestral ontogenies.
For example, dorid nudibranchs (order Doridida) represent a well-defined progressive development since during modification of its ontogeny has been firmly fixed the specific of increasing of complexity by appearing of the closed gill cavity which is formed by the folding and complete closing of the posterior mantle lobes (Martynov, 2011a,b, 2012a; Martynov and Korshunova, 2015; Figure 2). Such phylotypic period of ontogeny definitely absent in any other gastropod molluscs including the nudibranchs sensu stricto (order Nudibranchia without dorids, see Martynov and Korshunova, 2011; Korshunova et al., 2020). Additional problem may be posed that any ontogeny is not a straightforward row of mechanistic additions (Haeckel, 1866) or rigid modifications (Severtsov, 1912, 1939), but often “a novelty” is arisen from an alterations of several ancestral features, that not easily to align with either progressive or a regressive, paedomorphic ontogenetic processes. All these complications should be carefully considered while step by step a theory of the organismal form evolution, e.g., true theory of the modifications of the ontogenetic cycles over a time, will be finally completed.
Ontogenetic Systematics
Because the organismal diversity is generated during alterations of ontogeneses, hence ontogeny must be central to the theory and practice of taxonomy. Therefore, establishing robust hypotheses of phylogenetic lineages critically omits the underlying dynamics of ontogenetic cycles, including broad array of epigenetic and heterochronic processes (Figures 1–5). However, due to the domination of the almost exclusively phylogenetic, lineage-based thinking throughout the second part of the twentieth century, the organism, per se, and hence its underlying ontogenetic cycles have been largely removed from the central consideration of the evolutionary theory. There are a number of previous and recent attempts to highlight or return importance of the organism (e.g., Godfrey-Smith, 1996; Nicholson, 2014; Baedke, 2019), but still a phylogenetic lineage gains a central position within the evolutionary theory, whereas organism just a subsidiary part of an “endless” evolutionary/phylogenetic flow. To prove the latter statement no particular citation is needed, because all the modern biology and “taxonomy” are just completely “phylogenetic,” and some recent doubts in the absolute importance of the “lineage-thinking” (Freudenstein et al., 2017) do not change that still persisted general picture. Therefore, previous attempts to accommodate ontogeny into taxonomy either are exceedingly scarce, and never gained any broad attention (e.g., Orton, 1955) or were fundamentally based on the phylogenetic thinking, in which ontogeny, like the organism is always auxiliary, either explicitly or implicitly compare to the phylogeny, despite on discussions and proposals (e.g., Kluge and Strauss, 1985). Whereas some ontogenetic traits can be indeed indicated just as part of taxonomic descriptions (e.g., Costa et al., 2021) or the application in some “phylogeny-based” studies with inclusion of ontogenetic elements (e.g., Wolfe and Hegna, 2013; Gee, 2020), the “phylogeny-first” still basically implied. As a best confirmation of the absence (despite on putative claims) of any “ontogeny-first” central concept, is that within the apparent inevitable keeper of the “everything ontogenetic” in the biology, the very evolutionary developmental biology, taxonomy has been mentioned rather as an exception (e.g., Minelli, 2007). Furthermore, a publication remarkably entitled “Ontogenetic systematics, molecular developmental genetics, and the angiosperm petal” (Albert et al., 1998), although was an important attempt to link ontogenetic patterns with character evolution, did not offer challenging theoretical (to return ontogeny as a central place within the evolutionary theory) proposal. Another highly symptomatic feature of the modern understanding of the ontogeny, it is the continuous mentioning of Karl Baer among founders of the modern evolutionary biology (e.g., Futuyma and Kirkpatrick, 2017). It must therefore make very clearly, that Baer was a strong antievolutionist and his misleading concept widely cited as one of his “laws” directly implies that an embryo of an organism only similar to an embryo of another organism (taxon), but not to its adult! (von Baer, 1828/1837). It must be therefore explicitly stated, that Baer’s unequivocal prohibition of the linkage between an embryo of one taxon and an adult form of another taxon is not a basis of the modern evolutionary theory, but, on a complete contrary, the evolutionary blind alley. Therefore, perhaps the most counterintuitive that despite on apparent more than two hundred centuries of “ontogenetic studies,” despites on the Haeckel name and potential large number of references regarding “ontogeny and evolution” topic, but there is a strong suppression of ontogeny as a central phenomenon in the evolutionary theory and taxonomy. Thus, this is not researchers from the evo-devo field who on the obviously strictly evolutionary grounds provide strong evidences for the reality of the common ontogenetic phylotypic periods between very different taxa (e.g., Levin et al., 2016) have applied a “non-scientific idealistic theory of the body plans,” but exactly the entire biological field still lauds the antievolutionist Karl Baer as a founder of the “modern developmental biology.”
Therefore, there is nothing stretch that taxonomy has remained essentially ontogeny-free (Martynov, 2012a; Stöhr and Martynov, 2016), and the few twentieth-century publications failed to evoke the deserved paradigm shift in understanding of the key importance of ontogeny for the classification of the world biological diversity. The term ontogenetic systematics was independently proposed and unambiguously applied to the field of taxonomy (Martynov, 2009; see also Martynov et al., 2020) not as an attempt just to add some theoretical consideration, but exactly as return of the centrality of the ontogeny in the evolutionary studies. Colleagues may clearly feel that obvious shortage of the “ontogeny in phylogeny” (e.g., Minelli, 2015a; Faria et al., 2020), and these attempts are obviously in support of our present approach, and we are very thankful for that. However, still commonly any consideration of ontogeny in a broadly taxonomic/biodiversity field involves a rather basic phylogenetic approach with some addition of “evo-devo” (e.g., Minelli, 2009; Wanninger, 2015), instead of started the real theory of evolutionary processes and modifications (i.e., the evolution), with real underlying process, i.e., the ontogeny. Notably, Kupiec (2009) just radically avoided the Haeckel’s dichotomy of “ontogeny and phylogeny” by introducing of the “ontophylogenesis,” and this concept greatly corroborates the long-term previous achievements of the ontogeny/evolution field (Severtsov, 1912; Garstang, 1922). Unfortunately, since that no real shift in the paradigm of the modern profound misunderstanding of the fundamental role of the ontogeny, and the “tree of life” instead of an “ontogeny of life” remains fashionable in the recent publications (Schierwater and DeSalle, 2021). Kupiec (2009) in the introduction also mentioned that yet several decades ago, he was rather a dissident to the contemporary biology, but currently his contribution in the field of the stochastic understanding of the ontogeny is recognized (e.g., Viñuelas et al., 2012). Remarkably, two of the completely independent reviewers of the present paper asked us why we do not referred in the initial version of our manuscript to the Kupiec’s book! Such coincidence in asking to cite that once almost neglected approach from one side makes us hope that ontogenetic understanding of the phylogeny will finally overcome that unfortunately persisted dichotomy of the “ontogeny and phylogeny,” but from the other hand, the undisrupted integrity of ontogeny and phylogeny was absolutely clear for us yet started our initial works on the ontogenetic systematics (Martynov, 2009, 2011a,b, 2012a,b), when this approach has been developed completely independently from the very supportive for our conception Kupiec’s conceptualizations. However, this did not result in a real paradigm shift, the tree-thinking not only prevails, but colleagues from the phylogeny field continue to allege even the most notable contribution from the evo-devo field, the phylotypic-based approach (e.g., Levin et al., 2016) in an adherence with a “pre-evolutionary body plan thinking.” As a further very important reservation, in Kupiec (2009), neither taxonomy nor phylogenetics itself does not mention, therefore, even in a most rigorous way, the attempt to merge “phylogeny and ontogeny” does not directly relevant for the present approach. Although the discussion on the stochastic understanding of the ontogeny vs. strict “genetic programming” largely beyond of the limits of the present paper, but ontogenesis, despite on the undisputable at least partly stochastic grounds able to keep very complex and essentially similar ancestral morphological traits over a number of generations.
Facing such obvious not just bias toward the phylogeny-centered modern research, but like an indisputable and unequivocal central modern dogma, that only a phylogeny can resolve relationship within organisms, irrelevantly either colleagues are strict phylogeneticists or a morphology-/“evo-devo”-advocates (e.g., Lee and Palci, 2015; Wanninger, 2015; Hejnol and Dunn, 2016). It would be not a surprise, when such approach also attempted to be omitted nowadays, or it is considered as just something secondarily, insignificant, as some “research program” among many others (Pavlinov, 2020). This is instead of promoting help to return the ontogeny as central component of evolutionary theory, and therefore as central component of any biodiversity studies (since all that enormous biodiversity that we observed currently, have originated as evolutionary modifications of ontogenetic cycles) is partly contributing to its further postponing. Thus, one more time, “morpho-evo-devo” and even “evo-devo” at a general scale did no help the ontogenetic-taxonomic and morphology field to stop to be a “secondarily” and scarcely promoted discipline compared to the phylogenetics. First of all this happen because at the main theoretical level there is still no clear understanding that ontogeny (interacting with other ontogenies and environment) is real primary process in evolution, whereas phylogeny is instead is a secondary result. To make this absolutely clear and to make the synthesis between ontogeny and phylogeny broadly understood and irreversible one is a task for the near future development of the ontogenetic systematics.
For Haeckel (1866) importance of ontogeny for the systematics was evident (“systematics’ explained ……ontogeny is only a short and concise repetition…of phylogeny”), however through the following “century of acceptance of the evolution” resulting in the Hennig (1966) phylogenetic systematics (Rieppel, 2016), ontogeny largely vanished from the taxonomy. And, again, most paradoxically Haeckel takes a significant responsibility for that, because instead of initial proposing something like “evolving ontogenies” or “ontogenetic evolution” he instead strictly contrasted “ontogeny” and “phylogeny” at the terminological level. And his successors instead of carefully reversing “evolution” to its original meaning “ontogeny” (Bowler, 1975), and by this strongly highlight the natural unity of the “ontogeny and phylogeny,” instead made strongest accent on “phylogeny” (Hennig, 1966) and by this, the enormous confusion in such a most important biological field is persisted and the problem is growing. This is therefore, one more time to conclude, that it is always possible to find some references in the history of the biological studies that already somehow state the importance of ontogeny in taxonomy (Danser, 1950), which in reality based on that very ancient consideration that living organisms indeed represent a quasicyclical development, and this obvious fact have been indicated both antievolutionists (e.g., Agassiz and Gould, 1857) and apparently strict phylogeneticists (Hennig, 1966). However, this does not help when the “lineage-thinking” obviously engulfed the ontogeny, and the organism, per se. It was remarkably echoed when Lyubischev concluded yet in 1960 (published only in 1982) briefly discussing exactly the Haeckel’s heritage, that toward later works of Haeckel compared to his opus magnum of 1866 year “historical morphology devoured constructional [structural] one” (Lyubischev, 1982, p. 202, our italics). This should not be interpreted as revival of any idealistic shadow, but this is a great metaphor that exaggeration of the solely phylogeny in the reality hardly divisible pair “ontogeny and phylogeny” in course of the last 150 years of the biology development has led to the fundamental negation that ontogeny (and their respective structural patterns, including phylotypic periods) is a basis of any evolutionary processes, whereas phylogeny it is just result of ontogenetic modifications over a time period. Therefore, truly insignificant if there were some attempts “between Haeckel and Hennig” or not, to remind about existence of ontogeny, when it was almost completely shadowed by the “phylogenetic thinking” and with the rise of the molecular phylogenetics, this ontogenetic neglecting was reinforced enormously. This is now time to clearly formulate that profound neglecting and practical steps how to fundamentally improve the current situation. The key implications therefore that this is not “a phylogeny” itself constitutes material basis for evolutionary modifications, but ontogeny. Thus, by removal of the ontogeny as primary evolutionary process and by refusing of the crucial importance of phylotytpic periods for the reconstruction of the ancestral organization we in reality remove phylogenetics (and even more, the entire evolutionary field!) from scientific disciplines.
Therefore, facing such fundamental neglecting of the primacy of the ontogeny, the additional citations of five, ten or more sources will not change that obvious fact: despite on the all achievements of the evo-devo, and despite this year we are celebrating 100th anniversary of the seminal Garstang (1922) publication, ontogeny is still not a central process of understanding of evolution. This is a very easy to prove. Because even the researchers from the evo-devo field, which obviously must be strict advocates of the centrality of ontogeny (and morphology since this is a central part of any ontogeny), however, on a complete contrary argued that “to understand how phenotypic diversity evolved” we need in a phylogeny! Compare Wanninger (2015, p. 12, our italics) “in other words, once all organisms have been sequenced and once we have agreed on the “true” phylogenetic tree, we will still need morphology to understand how phenotypic diversity evolved” and Neumann et al. (2021, p. 1) “The assumption that genomic data will automatically “swamp out” morphological data is not always true for the sister of all metazoan question.” In another words, the question that constitutes a major basis of the understanding the ancestral patterns in the animal and organismal evolution is not necessarily can be correctly addressed exactly by the molecular phylogenetics.
This is a best “litmus probe” (if to rephrase a well-recognized Russian idiom) that while evolutionary developmental biology is claimed to be a separate, just relatively recently emerged discipline (Gilbert et al., 1996), in reality it is just a subsidiary discipline of a (molecular) phylogenetic study. Especially indicative that in the same paper Wanninger (2015, p. 12, citing also Scholtz, 2010, our italics) definitely said that: “…morphologists should further and proactively embrace the evolutionary disciplines that are currently dominated by molecular approaches, especially phylogenetics and EvoDevo, and integrate these into their own research programs, in order to avoid becoming a mere add-on to these and degenerate to a “shrinking or even vanishing field” (!). However, this in reality appears as more an “advertising slogan” than a true program to challenge the “shrinking or even vanishing field” because despite that other colleagues, which since at least 2009 explicitly warned on the true ongoing catastrophe exactly with the evaluation of the importance of the morphology and ontogeny facing of the molecular phylogenetic dominance, have not been cited or acknowledged. Therefore, if ontogeny with all their internal complexity is truly underlying basis of any evolutionary processes (and hence, phylogeny), including the epigenetics which clearly breaks straightforward purely genetic inheritance (the main initial basis for “evo-devo,” e.g., Raff and Kaufman, 1983; Ivanova-Kazas, 1995), then ontogenetic field is of its own importance and developmental evidences must be at least of equal weight with the molecular phylogenetic, and not just by definition as a secondary one. This is one of the most important implications of the present work, since there are number of examples, when obvious developmental data were uncritically and uncarefully discarded in favor of molecular phylogenetic data.
Nobody would of course refuse strong necessity of a “time-scale phylogeny” (e.g., Lee and Palci, 2015). However, the main problem that currently the all “final evidences” still expected and referred to a “molecular phylogenetic study,” a notoriously known cliché of a necessity of a “robust phylogeny.” In this respect, the robustness of the even most expensive and most complicated phylogenomic analyses has been repeatedly contested in that very notable “molecular phylogenetic controversy” of the “porifera-first vs. ctenophora-first.” This is very relevant and one of the most notable example when overhyped contemporary molecular phylogenetics, strongly put forward the “ctenophora-first” story, when evident morphological and developmental data (in another words, ontogenetic in its real sense) of the fundamental similarity of sponges to the choanoflagellate colonial protists have been disregarded, just because a molecular phylogenetic analysis has been published in a high-impacted journal (Moroz et al., 2014) putatively disproved the well-established ontogenetic (in the broad, but real sense) data (e.g., Martynov, 2012b; Nielsen, 2012; Adamska, 2016). And despite that a number of subsequent analysis recovered serious errors in the molecular phylogenetic assessments of the “ctenophora-first” (e.g., Pisani et al., 2015; Simion et al., 2017), the gravity of putative “authority” of the molecular phylogenetic analysis still forced researches to claim and search for a non-existed ambiguity of the “sponges-first” (Li et al., 2021). In spite of the significant amount of the recent data which strongly conclude that the analyses which showed the “ctenophora-first” have been affected by the critical errors (e.g., Juravel et al., 2021; Nejad Kourki, 2021; Redmond and Mclysaght, 2021). However, while still continuing allegation of the evolutionary morphology and ontogeny-based data for an arbitrary approach, we then must placed a similar “disclaimer” to any molecular phylogenetic study, especially dangerous when they presented a putatively challenging “truth” in journals with a high impact. In order the researches from other fields should not be used a molecular phylogeny as an “unequivocal truth,” as it was already regularly happen to make a crucial claim using data of subsequently contested molecular data (e.g., Brown et al., 2008; Dunn et al., 2015).
By this statement we need to specially emphasize that we by no means deny importance of the molecular phylogenetic data, and we themselves are profoundly use it (e.g., Korshunova et al., 2017a,b. Korshunova et al., 2018, 2020, 2021; Martynov et al., 2020). However, it is very important to remove ontogeny from the strong tenets and label of an “outdated” science, and to put at least as equally important to the molecular phylogenetic inference. Symptomatically, although surprisingly, the necessity of the revival of morphological data (which is inevitable part of any ontogeny) has been recently emerged not from the “non-fashionable and arbitrary” evolutionary morphology, but from the very rigorous statistical-based phylogenetics, but with a strong implications, that molecular phylogenetic does not necessary may answer “difficult phylogenetic questions” (Neumann et al., 2021). While this is just a lesser critics to the molecular phylogenetic field, however the fundamental deficiency of the strictly molecular phylogenetic approach is that it unable to reconstruct the ancestral organization, and only considers “sister relationship” that as, practice clearly shown can be very misleading (compare Moroz et al., 2014 vs. Redmond and Mclysaght, 2021, or Cameron et al., 2000 vs. Tassia et al., 2016). Thus, despite on any past and recent claims on importance of ontogeny and “evo-devo” (Haeckel, 1866; Garstang, 1922; Gould, 1977; Minelli, 2009; Wanninger, 2015), the morphology, ontogeny and “evo-devo” field remained to be just a “supplementary” to the molecular phylogenetics.
Therefore, ontogenetic systematics is not just an auxiliary to the “phylogenetic and development” field, but highlights phylotypic periods as a real, and not just “hypothetically inferred” proxy for an ancestral organization. The ontogenetic systematics is therefore a real synthetic discipline that consider all possible evidence, including molecular phylogenetic data, but in a consequential, consistent ontogenetic framework. As another crucial implication that because obviously ontogeny bears a lot of truly evolutionary information about ancestral organization, the reliable framework how to turn ontogenetic field into truly evident discipline, much more theoretical and practical efforts need to be done. The current concept of the ontogenetic systematics is one more step towards to the development of such “ontogeny-evident” theory of the modifications of the organisms (= ontogenetic cycles) in course of the time. Nobody will claim that this will be easy and straightforward. However, without the urgent necessity to form the ontogeny-centered, consistent taxonomic field, any next steps to develop detailed practical applications of the ontogenetic systematics will be impossible.
Thus, the history of the term ontogenetic systematics is itself remarkable since, being actually introduced two times independently in different senses from two different fields: molecular and taxonomic, but both definitions are clearly indicated the obvious necessity of further and real synthesis of the immense modern data on the ontogeny (both at molecular and morphological levels) with astonishing taxonomic diversity of the organisms. Whilst the significance of ontogeny is well understood in the evolutionary developmental biology per se, it is still not applied to the major biological fields of taxonomy and phylogenetics. And there is not any stretch in the conclusion, that a search of publications in the Web of Science (Martynov et al., 2015; Stöhr and Martynov, 2016) confirmed the absence of general ontogenetic principles in modern taxonomy. It is possible to list some works where metamorphic stages are used for taxonomic diagnostic, but the fundamental understanding that not a still strictly pre-evolutionary typological taxonomic diagnosis or some “phylogeny” are primary for the taxonomy, but instead universal for any organisms ontogenetic process (with different complexity) is fundamental to the systematics. Therefore, the main field of biodiversity studies is really still devoid of consideration of ontogeny as the central biological process, that fundamentally encompasses all other biological processes. In that sense, ontogenetic systematics is not merely one of many “research programs” in taxonomy, but a fundamental, core discipline that encompasses “static” taxonomic assessments of biodiversity and dynamic evolutionary aspects. By this, ontogenetic systematics cannot be alleged in any way as a “typological discipline” (Pavlinov, 2020). Our earlier analyses (Martynov, 1994, 2009, 2011a,b, 2012a,b) clearly pointed to the problems of taxonomy if ontogenetic basis would be removed, and this is especially justified because of subsequent recent appearance of proposals in a fundamentally similar direction, i.e., to integrate evolutionary developmental biology, phylogenetics and to point to a shortage of the morphological and ontogenetic studies in the molecular era (e.g., Richter and Wirkner, 2014; Wanninger, 2015; Faria et al., 2020).
The failure to incorporate ontogenetic data into systematic biology inevitably limits the precision of taxonomic representations of natural patterns. The premise that the same genes determine an organism’s identity at the egg and adult stage of an individual, thereby obviating the need to include ontogenetic data in phylogenetic studies (Mayr, 1963), strongly ignores epigenetic and other processes beyond strict inherent genetic control (e.g. Müller, 2017). Therefore, molecular analysis cannot substitute the ontogenetic approach. Yet few decades ago, when “phylogenetic thinking” reached its peak, such a statement would be controversial. However, the currently accumulated developmental evidence instead strongly favor a shift to a further change of the paradigm (Martynov, 2009) and place ontogeny not as “just a possible research program” but as a core of taxonomy: “We argue that evolutionary biologists should return from a purely gene-centric view of evolution and place more focus on analyzing and defining conserved developmental processes and periods” (Ferretti et al., 2020: 1). This is because there is no “biological reality” beyond ontogeneses and all living organisms, even prokaryotes have a complex underlying system of patterns and processes. Ontogenetic systematics instead of drifting toward some “formal-logical” biology-free theories is therefore a most natural integrator of traditional evolutionary-free taxonomy, ontogeny-free phylogenetics and evolutionary developmental biology. Ontogenetic systematics is based not only on formalized molecular genealogies of taxa, but on analysis and description of the major structural modifications and transformations of organisms, particularly in analysis of degree of shared ontogenetic conservativeness (as expressed in phylotypic periods, see e.g., Richardson, 1995; Arthur, 2002; Martynov and Korshunova, 2015; present work) among taxa which are disparate at adult stages, with a careful integration of the molecular data (Korshunova and Martynov, 2020; Korshunova et al., 2020; Martynov et al., 2020), instead of giving an absolute priority to the molecular phylogenetic data. By this ontogenetic systematics is perfectly verifiable science. The major structural modifications of every taxonomic groups are manifested in the phylotypic periods at different taxonomic levels (very importantly: not only at the phylum level, see e.g., Martynov and Korshunova, 2015), and this, compared to misleading comments from the strict phylogeny-based field, not a return to “pre-evolutionary” thinking or “circular arguing,” but instead most natural recognition of the really existed, common between taxonomically disparate organisms ontogenetic periods, as a major basis for the reconstruction of ancestral organization(s). This is an essence of the ontogenetic systematics. That could be least “idealistic” and instead directly evolutionary discipline!
The ontogenetic challenge for taxonomy is a practical call to make systematics of the world biodiversity as science to be really based on the underlying patterns and processes of majority of organisms (i.e., ontogeny). Below are given several examples from different phyla of the ontogenetic systematics approach implying ontogenetic-and-evolutionary linkage of the adult and juvenile features among both closely related and disparate taxa in frames of the modifications of the ontogenetic cycles, carefully aligned (whenever this possible) with molecular phylogenies, but this is an outstanding goal of the ontogenetic systematics for the vast majority of the living organisms. Although previously we outlined several principles in ontogenetic systematics (e.g., Martynov, 2009, 2011a,b, 2012a; Stöhr and Martynov, 2016; Korshunova et al., 2017a,b, 2018, 2021; Martynov et al., 2020), and while more detailed practical principles will be outlined elsewhere, the refined basic principles of the ontogenetic systematics still need to be more rigorously presented here:
1. Ontogeny is the central underlying process of the evolution.
2. Phylogeny is a secondary result of the (evolutionary) modification of ontogeny.
3. Ontogeny and phylogeny must not be strictly separated as they are closely interlinked through epigenetic inheritance and other developmental processes.
4. Biological organisms are not just subsidiary part of an endless evolutionary/phylogenetic flow, but separate entities, which represent most complex ever appeared natural objects, encompassed trillions of interlinked elements, which underlie the basic energy- and external-resources depended organism functioning, such as growth, defense, feeding and reproduction.
5. Organisms do not exist only as a separate either embryonal, or juvenile, or adult stages, but exist only as ontogenetic cycles of various degree of complexity, from very simple in viruses, bacteria and archaea, and most complex in the bilaterian animals.
6. The term ontogeny therefore implies not a developmental stage, but equal to an ontogenetic cycle of any organism. This must be understand very clearly and do not restrict ontogeny only to the “embryonal-juvenile” period which is still commonly used in the current literature.
7. Taxonomy is the multi-level understanding of the biological diversity of the organisms, those emergence result of the evolutionary modifications of ontogeny.
8. Without centrality of the ontogenetic principles it is impossible to correctly classify the organisms (and hence taxonomy is impossible without ontogeny).
9. Taxonomy therefore is not a pure typological or a “service-based” discipline that just technically assign some names to organismal groups to define the enormous diversity and allow study (provides the service—names) to other “proper” scientific fields such as any molecular investigations, but is a central biological discipline that encompass all organismal-related process at all levels.
10. Since any organisms are existed in frames of only a specific ontogenetic process (ontogenetic cycle), therefore ontogeny (by definition) is a real natural object that must be basic and central for any evolutionary and phylogenetic studies.
11. Molecular phylogenetics must not be considered as solely central evidence for the relationships of the organisms (ontogenetic cycles), as it currently widely claimed because modifications of ontogeny are not restricted only to the genetic inheritance, but as shown by numerous studies, also significantly influenced by epigenetic processes.
12. Phylotypic periods are not “flawed heritage” of the pre-evolutionary “idealistic body plan” concepts, but a key object of ontogenetic systematics, the naturally existed inter-taxon nodes that linked at the earlier ontogenetic periods taxa with very different adult morphologies. Beyond classical examples of the vertebrates taxonomy, here we present evidence of the universal occurrence of the phylotypic periods across such both morphologically and phylogenetically very disparate animal phyla as molluscs (nudibranchs) and echinoderms (ophiuroids) (Figures 2–4).
13. Therefore, we cannot just restrict the main target of the all biology—inference of unequivocal order of emergence and modifications (evolution) of consequential ancestral organizations (ancestral ontogenetic cycles) either to the plain “taxonomy/systematics,” which have an exceeding number of negative connotations and still basically non-evolutionary, real typological discipline, or morphological and molecular phylogenetics. The latter has been claimed to be a strictly evolutionary field, but in reality critically removed ontogeny as the central biological process. Instead, a separate term is in a strong need. Such term is proposed as ontogenetic systematics since it is a most general way encompassed both the apparently “static” taxonomic assessments and the developmental (evolutionary aspect), and hence both putatively strictly “individual” and “historical “development” will be conjoined under universal ontogeny-based term.
Practical Examples of Applications of Ontogenetic Systematics
These examples are not just target “to illustrate” using few selected cases, but first of all aim to provide robust evidence that such only putatively well-known central ontogenetic phenomena as phylotypic periods and paedomorphosis by no way restricted merely to the well-recognized vertebrate taxonomic groups, but they also universally occurs in such very different animal phyla as molluscs and echinoderms, and inevitably, similar ontogenetic patterns present in absolute majority of the organisms. The reality and most important predictive power of the universally existed phylotypic periods can be proved by the practices. If for example we will find a new species of a vertebrate mammal, bird, and reptilians, or dorid nudibranch molluscs, or ophiuroid echinoderms, for which earlier ontogenetic patterns are yet completely unknown, we can with a high confidence predict that the basic organization of their respective phylotypic periods will be essentially similar (despite on some inevitable differences). The predictive power is a central attribute of any scientific study, and therefore the ontogenetic systematics reinforced by the concept of the phylotypic periods represents true scientific discipline with its own set of theories, concepts and practical applications. Some of them are briefly explained below.
Nudibranch Molluscs
Dorid Nudibranchs
Cryptobranch and phanerobranch dorids that are morphologically and taxonomically disparate in the adult stage are essentially similar in the early juvenile stages (Martynov and Korshunova, 2015; Zaitseva et al., 2015; Korshunova et al., 2020; Figure 2). The following developmental processes are the same in both cryptobranchs and phanerobranchs: (1) the strictly separated rhinophores form later in development; (2) the ventral anus forms before its dorsal translocation and (3) the gills start to develop significantly later, after juveniles reach more than 1 mm in length. This conserved development conforms to the evolutionary phenomenon of phylotypic periods, i.e., similarity of early developmental patterns across large taxonomic groups with considerably different adult morphology (e.g., presence of the ancestral pharyngeal arches in early ontogeny of all vertebrates, even if they no longer have gills in the adult state). This intersection of development and evolution is well established at the morphological level (Richards, 2009) and has been confirmed using transcriptomic analysis (Irie and Kuratani, 2011). There are several partly or considerably paedomorphic lineages arisen independently among dorids, notably Corambidae (Martynov, 1994; Martynov et al., 2011), but also Okadaiidae and other groups (Korshunova et al., 2020). This is also important to highlight, that not merely ontogenies, but ontogeny-based integration of the molecular phylogenetic data (Korshunova et al., 2020; this study) directly contributes to the evolutionary models and based on it classification of the gastropod nudibranch molluscs. The predictive power of the present dorid ontogenetic model, that recognized phylotypic stages essentially similar with the adult disparate Cadlina and Palio (Martynov and Korshunova, 2015; present study, Figure 2) has been detected by an independent study (Moles et al., 2017) in a very different dorid taxonomic group. As important practical example, from the ontogenetic field must be removed the previously widely promoted, and still sometimes applied in Russia, these Severtsov (1912, 1939) inflexible schemes that all ontogenetic modifications fall to the Procrustean bed of the only three major changes: “anaboly, deviation and archalaksis”. Despite previously we partially praised Severtsov as a conceptual predecessor of Garstang (1922) work (Martynov, 2011a). The real ontogenetic patterns strongly contradict to that theoretical scheme. For example, even highly modified direct developers such as dorid nudibranch Cadlina still preserve major features of the dorid ontogeny representing by the phylotypic periods (Martynov and Korshunova, 2015; Korshunova et al., 2020; Figure 2). Another major misunderstanding, when in an extreme case of strongly modified direct development is difficult to recognize major features of ancestral dorid organization at early ontogenetic stages in the dorid families Bathydorididae (Moles et al., 2017), or Okadaiidae (Martynov and Korshunova, 2011) it is result of long series of reductions proved also by the molecular data (Korshunova et al., 2020), and not only a putative solely drastic “earlier development changes.” In addition, earlier stages of ontogeny was not yet studied and still may contain although reduced but still remnants of the dorid phylotypic periods. By this we also would like to one more time warn, that despite that paedomorphosis (as a very substantial reversal, although only partial to an ancestral organization, via partial “adultization” of phylotypic periods) is a highly important evolutionary concept, it must be applied very carefully, without typical consideration, when a complex ancestral organization is disappeared “to nowhere,” as it is sometimes has been proposed recently (e.g., Nielsen, 2008).
Aeolid Nudibranchs
During the ontogeny of all aeolidacean nudibranchs, a few juvenile ceratal rows (1–2 anterior rows in earlier postlarval stages, about 3–4 anterior rows in more advanced juveniles) precede the adult state with numerous ceratal rows (commonly more than ten in total, more than four in anterior rows) (Figure 3). Therefore, the presence of a smaller number of ceratal rows in the adult stage is a sign of at least partial paedomorphosis, and not just an overall reduction/loss, especially if small-sized taxa with a smaller number of anterior ceratal rows are sister to large-sized taxa with numerous cerata. The recent confirmation of the evidently paedomorphic adult characters of the family Pseudovermidae using both morphological and molecular data (see details in Flammensbeck et al., 2019; Martynov et al., 2020) is exactly in line with the core approach of the ontogenetic systematics outlined above. As a direct practical application of the principles of ontogenetic systematics in the aeolidacean group, morphological and molecular data indicate that brackish water speciation was triggered by paedomorphic evolution among aeolidacean nudibranchs at least two times independently, i.e., in the Cuthonidae for the genus Bohuslania and in the Trinchesiidae for the genus Tenellia (see Korshunova et al., 2018). If to apply instead a strictly molecular phylogenetic approach, without ontogenetic data, the flawed concepts of overlumped “Tenellia” and “Flabellina” will appear (see review in Korshunova et al., 2017a,b).
Ophiuroid Echinoderms
To date, various data have been accumulated on postlarval development for the majority of ophiuroid families (see details in Martynov et al., 2015; Figures 4, 5). The ophiuroid postlarvae have a conserved morphology as follows: 1. The major part of the dorsal disk is occupied by the primary plate rosette comprised of a single central primary plate and usually five radial primary plates; 2. The radial shields and genital plates have yet to appear or are underdeveloped; 3. Each half-jaw is narrow, elongate and bears ventrally a few rudimentary oral papillae, commonly bar-shaped; 4. The dental plate is small, convex and bears a few tooth sockets of unspecific shape; 5. Each adoral shield bears a papilla (spine) of various length and shape; 6. Arm segments are limited in number and considerably elongated proximally; 7. Vertebrae are comprised of two separate, loosely connected elongated parts; 8. The vertebral articulation is generally underdeveloped without a distally well-defined condyle.
Highly relevant for the above outlined evidence of the almost direct linkage between only putatively disrupted ontogenetic phylotypic periods and phylogenetic (evolutionary) patterns, both juvenile of non-paedomorphic ophiuroid taxa and adult specimens of ophiuroid paedomorphic taxa (see Martynov, 2012a; Martynov et al., 2015) have very short concave dental plate with a single tooth, and considerably elongated, only partially fused vertebrae (Figure 5). Therefore, instead to deny of the juvenile—adult ontogenetic (evolutionary) linkage (e.g., Mayr, 1963; Hennig, 1966) there is the remarkable correspondence between the adult shape and placement of primary plates, dental plates and vertebrae of the adult paedomorphic ophiuroids and the homologous structures in early juveniles of related non-paedomorphic taxa (Figures 4–5). This is a very important evidence, not merely for “phylogenetic” or “evolutionary” approaches, but for the approach of ontogenetic systematics.
Furthermore, the manifestation of the paedomorphic processes varies among various ophiuroid taxa from an almost exact correspondence to the early postlarval stages in adult Perlophiura, to the correspondences of the later postlarval stages in paedomorphic Ophiomastus to the complex ancestral ophiuroids (Figure 4). Indeed, any ontogeny is not a plain scheme, and the direct linkage between earlier juvenile ancestral and adult paedomorphic morphologies are certainly not exact because of the influence from various ontogenetic processes, but generally the similarity between ancestral early juvenile and adult paedomorphic characters are striking (Figures 4–5) and the evolutionary linkage through particular periods (stages) of the ontogeny cannot be denied. The independent appearance of paedomorphic taxa within remote ophiuroid families, such as Ophiuridae and Ophiolepididae has been also indicated (Martynov, 2009, 2012a). In some cases, resulting external and internal morphology of adult paedomorphic ophiuroids from remote families is almost indistinguishable. A comprehensive morphological scanning electron study of about 200 species of more than 100 genera of majority of modern ophiuroid families confirmed this. Studying of spine articulations of the lateral arm plates (the shape of which is very conservative at the family level) remains the reliable method to distinguish some taxa of paedomorphic brittle stars (Martynov, 2009, 2010a,b). Recent molecular phylogenomic data on ophiuroids (O’Hara et al., 2017) are generally concordant with the earlier proposed ontogenetic model of ophiuroids (Martynov, 2012a; Figure 4) with several independent paedomorphic lineages.
These practical examples confirm that ontogenetic systematics is a natural integrator of morphological and molecular data. The quest for that is an obvious and significant problem of biodiversity studies, since a few decades ago formal molecular phylogenies started to substitute the real biological organisms, which are essentially represented by the ontogenies. In all these cases from two very different metazoan phyla, representing major traditional bilaterian supraphyletic groupings as protostomiens (Mollusca) and deuterostomiens (Echinodermata), the presence of the conservative ontogenetic periods which are linked at different layers of the both adult and juvenile ancestral organizations via phylotypic periods and paedomoprhosis are confirmed. By this, the main theme of the present contribution, the tight, indivisible linkage between ontogeny as a primary process, and phylogeny as a secondarily result of the evolutionary modifications of the ontogeny is thoroughly confirmed both at the theoretical and practical levels.
Conclusion
1. Ontogeny is apparently old and well-established biological term, however there are still significant contradictions and deficiency in its evolutionary and taxonomic applications.
2. Ontogeny and phylogeny are two sides of fundamental biological properties of organisms.
3. Paedomorphosis (and not “neoteny” or “progenesis”) should solely be used as term to describe cases of retention of juvenile features of ancestors at the adult stages of descendants.
4. Phylotypic periods represent real manifestation of some features of the ancestral ontogenetic cycles in the ontogenies of modern descendants and therefore partial recapitulations of the ancestral organization.
5. Recapitulations and biogenetic law are not abandoned concepts but represents actual existing patterns of ontogenies, denoting persistence of phylotypic periods in the ontogenies of the modern taxa.
6. If to remove phylotypic periods which a natural basis for existence of the phyla concepts, as a core concept of‘evo-devo,” then evolutionary developmental biology will be turned to an auxiliary discipline of the molecular phylogenetics.
7. The evolutionary linkage between juvenile ancestral characters and adult features of descendants (paedomorphosis) is a widespread phenomenon, as evidently showed here using examples from molluscs and echinoderms (Figures 1–5).
8. Ontogenetic systematics is not merely a “research program” in taxonomy, and not an auxiliary subdivision of “evo-devo.” Ontogenetic systematics is an interdisciplinary field which encompasses and further promotes achievements of “evo-devo” and epigenetics to link it to the still separated fields of phylogenetics and taxonomy. Among the main goals of the ontogenetic systematics is to achieve more precise knowledge on the patterns and processes (both universal/conservative and unique ones) in the worldwide biodiversity, that currently is endangered due to ongoing climatic and other challenges.
9. The central paradigm of the ontogenetic systematics and “ontogeny and evolution field,” applying Haeckel’s initial formulation, Garstang’s reformulation, and synthesizing it with the material fact of the universal existence of the phylotypic periods across disparate organisms’ groups, can be now formulated at a new conceptual level as following: ontogeny partially keeps (past) ancestral ontogenies (importantly, at adult and larval phases) and produces (future) ontogenies, and both these patterns in total form the phylogenetic (evolutionary) process—which is nothing but modifications of the interacting ontogenies in course of time.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
This study was supported by research project of MSU Zoological Museum (18-1-21 No. 121032300105-0, AM). The work of TK was conducted under the IDB RAS basic research program in 2021 No. 0088-2021-0008.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Electron Microscopy Laboratory MSU thanked for providing electron microscopy facilities. We are thankful to reviewers for the advices that helped to improve the manuscript.
Dedication
This article is dedicated to the 100th anniversary of the seminal article by Walter Garstang (1922), which for the first time explicitly concluded, that ontogeny produces phylogeny (and hence, evolution), instead to be solely a “storage room” for the evolutionary process; but with a remained elusive for Garstang key addition: Phylotypic periods are partial, dynamic keepers of the adult ancestral organizations within the constantly evolved ontogenetic cycles!
References
Adamska, M. (2016). Sponges as models to study emergence of complex animals. Curr. Opin. Genet. Dev. 39, 21–28. doi: 10.1016/j.gde.2016.05.026
Alberch, P. S., Gould, J., Oster, G. F., and Wake, D. B. (1979). Size and shape in ontogeny and phylogeny. Paleobiology 5, 296–317. doi: 10.1162/artl.2009.15.2.15200
Albert, V. A., Gustafsson, M. H. G., and Di Laurenzio, L. (1998). “Ontogenetic systematics, molecular developmental genetics, and the angiosperm petal,” in Molecular systematics of plants II, eds P. S. Soltis, D. E. Soltis, and J. J. Doyle (Boston, MA: Kluwer Acad. Publ.), 349–374. doi: 10.1007/978-1-4615-5419-6_12
Anastasiadi, D., Venney, C. J., Bernatchez, L., and Wellenreuther, M. (2021). Epigenetic inheritance and reproductive mode in plants and animals. Trends Ecol. Evol. 36, 1124–1140. doi: 10.1016/j.tree.2021.08.006
Arthur, W. (2002). The emerging conceptual framework of evolutionary developmental biology. Nature 415, 757–764. doi: 10.1038/415757a
Arthur, W. (2015). “Internal factors in evolution: the morphogenetic tree, developmental bias, and some thoughts on the conceptual structure of evo-devo,” in Conceptual Change in Biology. Scientific and Philosophical Perspectives on Evolution and Development, ed. A. C. Love (Dordrecht: Springer Science & Business Media), 343–363. doi: 10.1007/978-94-017-9412-1_16
Baedke, J. (2019). “What is a biological individual?,” in Old Questions and Young Approaches to Animal Evolution, Fascinating Life Sciences, eds J. M. Martín-Durán and B. C. Vellutini (Cham: Springer Nature Switzerland AG), 269–284. doi: 10.1007/978-3-030-18202-1_13
Barnes, M. E. (2014). Ernst Haeckel’s Biogenetic Law (1866). Tempe, AZ: Embryo Project Encyclopedia.
Bininda-Emonds, O. R. P., Jeffery, J. E., Coates, M. I., and Richardson, M. K. (2002). From Haeckel to event-pairing: the evolution of developmental sequences. Theory Biosci. 121, 297–320. doi: 10.1007/s12064-002-0016-5
Bonner, J. T. (1965). Size and Cycle: An Essay on the Structure of Biology. Princeton, NJ: Princeton University Press.
Borzenkov, Y. A. (1884). Readings on comparative anatomy. Uch. Zap. Imp. Mosc. Univ. Otd. Estest. Istor. 4, 1–242. doi: 10.1017/9781108869348.006
Bowler, P. J. (1975). The changing meaning of “evolution”. J. Hist. Ideas 36, 95–114. doi: 10.2307/2709013
Brown, F. D., Prendergast, A., and Swalla, B. J. (2008). Man is but a worm: chordate origins. Genes 46, 605–613. doi: 10.1002/dvg.20471
Cameron, C. B., Garey, J. R., and Swalla, B. J. (2000). Evolution of the chordate bodyplan: new insights from phylogenetic analyses of deuterostome phyla. Proc. Natl Acad. Sci. U.S.A. 97, 4469–4474. doi: 10.1073/pnas.97.9.4469
Cordero, G. A., Sánchez-Villagra, M. R., and Werneburg, I. (2020). An irregular hourglass pattern describes the tempo of phenotypic development in placental mammal evolution. Biol. Lett. 16:20200087. doi: 10.1098/rsbl.2020.0087
Costa, S. G. D. S., Welbourn, C., Klimov, P., and Pepato, A. G. (2021). Integrating phylogeny, ontogeny and systematics of the mite family Smarididae (Prostigmata, Parasitengona): classification, identification key, and description of new taxa. Syst. Appl. Acarol. 26, 85–123. doi: 10.11158/saa.26.1.6
Cridge, A. G., Dearden, P. K., and Brownfield, L. R. (2019). “The developmental hourglass in the evolution of embryogenesis,” in Evolutionary Developmental Biology, eds L. Nuño de la Rosa and G. B. Müller (Cham: Springer Nature Switzerland AG).
Danchin, E., Pocheville, A., Rey, O., Pujol, B., and Blanchet, S. (2019). Epigenetically facilitated mutational assimilation: epigenetics as a hub within the inclusive evolutionary synthesis. Biol. Rev. 94, 259–282. doi: 10.1111/brv.12453
Deline, B., Thompson, J. R., Smith, N. S., Zamora, S., Rahman, I. A., Sheffield, S. L., et al. (2020). Evolution and development at the origin of a phylum. Curr. Biol. 30, 1672–1679. doi: 10.1016/j.cub.2020.02.054
Domazet-Lošo, T., and Tautz, D. (2010). A phylogenetically based transcriptome age index mirrors ontogenetic divergence patterns. Nature 468, 815–818. doi: 10.1038/nature09632
Dunn, C. V., Leys, S. P., and Haddock, S. H. D. (2015). The hidden biology of sponges and ctenophores. Trends Ecol. Evol. 30, 282–291. doi: 10.1016/j.tree.2015.03.003
Emelyanov, S.V. (ed.) (1968). Tempo of Individual Development of Animals and Its Evolutionary Modifications. Moscow: Nauka.
Ezhikov, I. I. (1940). “The concept of recapitulation and its critics,” in Basic Biogenetic Law, eds F. Müller and E. Haeckel (Moscow-Leningrad: Academy of Science USSR).
Faria, L. R. R., Pie, M. R., Salles, F. F., and Soares, E. D. G. (2020). The Haeckelian shortfall or the tale of the missing semaphoronts. J. Zool. Syst. Evol. Res. 59, 359–369. doi: 10.1111/jzs.12435
Ferretti, L., Krämer-Eis, A., and Schifferet, P. H. (2020). Conserved patterns in developmental processes and phases, rather than genes, unite the highly divergent Bilateria. Life (Basel) 10:182. doi: 10.3390/life10090182
Flammensbeck, C. K., Haszprunar, G., Korshunova, T. A., Martynov, A. V., Neusser, T. P., and Jörger, K. M. (2019). Pseudovermis paradoxus 2.0—3D microanatomy and ultrastructure of a vermiform, meiofaunal nudibranch (Gastropoda, Heterobranchia). Org. Divers. Evol. 19, 41–62. doi: 10.1007/s13127-018-0386-2
Freudenstein, J. V., Broe, M. B., Folk, R. A., and Sinn, B. T. (2017). Biodiversity and the species concept — lineages are not enough. Syst. Biol. 66, 644–656. doi: 10.1093/sysbio/syw098
Futuyma, D. J., and Kirkpatrick, M. (2017). Evolution, 4th Edn. Sunderland, MA: Sinauer, Associates, Inc., Publishers.
Garstang, W. (1922). The theory of recapitulation: a critical restatement of the biogenetic law. Proc. Linn. Soc. Zool. 35, 81–101. doi: 10.1111/j.1096-3642.1922.tb00464.x
Garstang, W. (1928). The morphology of the Tunicata, and its bearing on the phylogeny of the Chordata. Q. J. Microsc. Sci. 72, 52–187. doi: 10.1186/s12862-014-0214-z
Gee, B. M. (2020). Size matters: the effects of ontogenetic disparity on the phylogeny of Trematopidae (Amphibia: Temnospondyli). Zool. J. Linn. Soc. 190, 79–113. doi: 10.1093/zoolinnean/zlz170
Giard, D. (1887). La castration parasitaire et son influence sur les caracteres exterieurs du sexe male ches les crustaces decapodes. Bull. Sci. Dep. du Nord 18, 1–28.
Gilbert, S. F., Opitz, J. M., and Raff, R. A. (1996). Resynthesizing evolutionary and developmental biology. Dev. Biol. 173, 357–372. doi: 10.1006/dbio.1996.0032
Godfrey, L. R., and Sutherland, M. R. (1996). Paradox of peramorphic paedomorphosis: heterochrony and human evolution. Am. J. Phys. Anthropol. 99, 17–42. doi: 10.1002/ajpa.1330990102
Godfrey-Smith, P. (1996). Complexity and the Function of Mind in Nature. Cambridge: Cambridge University Press.
Hall, B. K. (1999). Evolutionary Developmental Biology, 2nd Edn. Dordrecht: Kluwer Academic Publishers.
Hall, B. K. (2003). Evo–Devo: evolutionary developmental mechanisms. Int. J. Dev. Biol. 47, 491–495.
Hall, B. L. (2011). Ontogeny does not recapitulate phylogeny, it creates phylogeny: a review of The Tragic Sense of Life: Ernst Haeckel and the Struggle Over Evolutionary Thought, by Robert J. Richards. Evol. Dev. 13, 401–404. doi: 10.1111/j.1525-142x.2011.00495.x
Hao, Z., Zhang, Z., Xiang, D., Venglat, P., Chen, J., Gao, P., et al. (2021). Conserved, divergent and heterochronic gene expression during Brachypodium and Arabidopsis embryo development. Plant Reprod. 34, 207–224. doi: 10.1007/s00497-021-00413-4
Hawkins, J. (2002). “Evolutionary developmental biology: Impact on systematic theory and practice, and the contribution of systematics,” in Developmental Genetics and Plant Evolution, eds Q. C. B. Cronk, R. M. Bateman, and J. A. Hawkins (London: Taylor and Francis), 32–51. doi: 10.1201/9781420024982.ch3
Hejnol, A., and Dunn, C. W. (2016). Animal evolution: are phyla real? Curr. Biol. 26, 424–426. doi: 10.1016/j.cub.2016.03.058
Irie, N., and Kuratani, S. (2011). Comparative transcriptome analysis reveals vertebrate phylotypic period during organogenesis. Nat. Commun. 2:248. doi: 10.1038/ncomms1248
Irie, N., Satoh, N., and Kuratani, S. (2018). The phylum Vertebrata: a case for zoological recognition. Zool. Lett. 4:32. doi: 10.1186/s40851-018-0114-y
Ivanov, A. N. (1945). On the questions of so called “prophetic phase” in evolution of Kosmoceratidae. Bull. Mos. Soc. Nat. Geogr. 20, 1–32.
Juravel, K., Porras, L., Höhna, S., Pisani, D., and Wörheide, G. (2021). Improved resolution of recalcitrant nodes in the animal phylogeny through the analysis of genome gene content and morphology. bioRxiv [Preprint] doi: 10.1101/2021.11.19.469253
Kalinka, A. T., Varga, K. M., Gerrard, D. T., Preibisch, S., Corcoran, D. L., Jarrells, J., et al. (2010). Gene expression divergence recapitulates the developmental hourglass model. Nature 468, 811–814. doi: 10.1038/nature09634
Kluge, A. G., and Strauss, R. E. (1985). Ontogeny and systematics. Ann. Rev. Ecol. Syst. 16, 247–268. doi: 10.1146/annurev.es.16.110185.001335
Kollman, J. (1885). Das ueberwintern von europeischen frosch- und tritonlarven und die umwandlung des mexikanischen Axolotl. Verh. Naturfor. Gesell. Basel 7, 387–398.
Korshunova, T., and Martynov, A. (2020). Consolidated data on the phylogeny and evolution of the family Tritoniidae (Gastropoda: Nudibranchia) contribute to genera reassessment and clarify the taxonomic status of the neuroscience models Tritonia and Tochuina. PLoS One 15:e0242103. doi: 10.1371/journal.pone.0242103
Korshunova, T. A., Driessen, F. M. F., Picton, B. E., and Martynov, A. V. (2021). The multilevel organismal diversity approach deciphers difficult to distinguish nudibranch species complex. Sci. Rep. 11:18323. doi: 10.1038/s41598-021-94863-5
Korshunova, T. A., Fletcher, K., Picton, B., Lundin, K., Kashio, S., Sanamyan, N., et al. (2020). The Emperor’s Cadlina, hidden diversity and gill cavity evolution: new insights for taxonomy and phylogeny of dorid nudibranchs (Mollusca: Gastropoda). Zool. J. Linn. Soc. 189, 762–827.
Korshunova, T. A., Lundin, K., Malmberg, K., Picton, B., and Martynov, A. V. (2018). First true brackish water nudibranch mollusc provides new insights for phylogeny and biogeography and reveals paedomorphosis-driven evolution. PLoS One 13:e0192177. doi: 10.1371/journal.pone.0192177
Korshunova, T. A., Martynov, A. V., and Picton, B. E. (2017a). Ontogeny as an important part of integrative taxonomy in tergipedid aeolidaceans (Gastropoda: Nudibranchia) with a description of a new genus and species from the Barents Sea. Zootaxa 4324, 1–22. doi: 10.11646/zootaxa.4324.1.1
Korshunova, T. A., Martynov, A., Bakken, T., Evertsen, J., Fletcher, K., Mudianta, I. W., et al. (2017b). Polyphyly of the traditional family Flabellinidae affects a major group of Nudibranchia: aeolidacean taxonomic reassessment with descriptions of several new families, genera, and species (Mollusca, Gastropoda). Zookeys 717, 1–139. doi: 10.3897/zookeys.717.21885
Kryzhanovsky, S. G. (1939). Das Rekapitulationsprinzip und die Bedingungen der historischen Auffassung der Ontogenese. Acta Zool. 20, 1–87. doi: 10.1111/j.1463-6395.1939.tb00493.x
Lamsdell, J. C. (2020). A new method for quantifying heterochrony in evolutionary lineages. Paleobiology 47, 1–22. doi: 10.1007/978-3-319-33038-9_71-1
Laumer, C. E., Fernández, R., Lemer, S., Combosch, D., Kocot, K., Riesgo, A., et al. (2019). Revisiting metazoan phylogeny with genomic sampling of all phyla. Proc. R. Soc. B 286:20190831. doi: 10.1098/rspb.2019.0831
Lecointre, G., Schnell, N. K., and Teletchea, F. (2020). Hierarchical analysis of ontogenetic time to describe heterochrony and taxonomy of developmental stages. Sci. Rep. 10:19732. doi: 10.1038/s41598-020-76270-4
Lee, M. S. Y., and Palci, A. (2015). Morphological phylogenetics in the genomic age. Curr. Biol. 25, 922–929. doi: 10.1016/j.cub.2015.07.009
Lemmen, K. D., Verhoeven, K. J. F., and Declerck, S. A. J. (2022). Experimental evidence of rapid heritable adaptation in the absence of initial standing genetic variation. Funct. Ecol. 36, 226–238. doi: 10.1111/1365-2435.13943
Levin, M., Anavy, L., Cole, A. G., Winter, E., Mostov, N., Khair, S., et al. (2016). The mid-developmental transition and the evolution of animal body plans. Nature 531, 637–641. doi: 10.1038/nature16994
Levit, G. S., and Hossfeld, U. (2019). Ernst Haeckel in the history of biology. Curr. Biol. 29, 1276–1284. doi: 10.1016/j.cub.2019.10.064
Levit, G. S., Hoßfeld, U., Naumann, B., Lukas, P., and Olsson, L. (2022). The biogenetic law and the gastraea theory: from Ernst Haeckel’s discoveries to contemporary views. J. Exp. Zool. B Mol. Dev. Evol. 338, 13–27. doi: 10.1002/jez.b.23039
Li, Y., Shen, X.-X., Evans, B., Dunn, C. W., and Rokas, A. (2021). Rooting the animal tree of life. Mol. Biol. Evol. 38, 4322–4333. doi: 10.1093/molbev/msab170
Liu, J., Viales, R. R., Khoueiry, P., Reddington, J. P., Girardot, C., Furlong, E., et al. (2021). The hourglass model of evolutionary conservation during embryogenesis extends to developmental enhancers with signatures of positive selection. Genome Res. 31, 1573–1581. doi: 10.1101/gr.275212.121
Loison, L. (2021). Epigenetic inheritance and evolution: a historian’s perspective. Philos. Trans. R. Soc. B 376:20200120. doi: 10.1098/rstb.2020.0120
Lyubischev, A. A. (1982). Problems of the Form of the Systematics and Evolution of Organisms. Moscow: Nauka.
Martynov, A. V. (1994). Materials for revision of nudibranch molluscs of the family Corambidae (Gastropoda, Opisthobranchia). Part II. Origin. Zool. Zh. 73, 36–43.
Martynov, A. V. (2009). From ontogeny to evolution: an expectation for changing current systematic paradigm. Tr. Zool. Muz. Mosk. Gos. Univ. 50, 145–229. ISBN 978-5-87317-617-5.
Martynov, A. V. (2010a). “Structure of the arm spine articulation ridges as a basis for taxonomy of Ophiuroidea (a preliminary report),” in Proceedings of the Twelfth International Echinoderm Conference, Durham, NH, ed. L. Harris (Rotterdam: Balkema), 233–239. doi: 10.1201/9780203869543-c37
Martynov, A. V. (2010b). Reassessment of the classification of the Ophiuroidea (Echinodermata), based on morphological characters: I. General character evaluation and delineation of the families Ophiomyxidae and Ophiacanthidae. Zootaxa 2697, 1–154. doi: 10.11646/zootaxa.2697.1.1
Martynov, A. V. (2011a). Ontogenetic Systematics and a New Model of Evolution of Bilateria. Moscow: KMK.
Martynov, A. V. (2011b). From ‘tree-thinking’ to ‘cycle-thinking’: ontogenetic systematics of nudibranch molluscs. Thalassas 27, 193–224.
Martynov, A. V. (2012a). Ontogenetic systematics: the synthesis of taxonomy, phylogenetics, and evolutionary developmental biology. Paleont. J. 46, 833–864. doi: 10.1134/S003103011208007
Martynov, A. V. (2012b). Ontogeny, systematics, and phylogenetics: perspectives of future synthesis and a new model of the evolution of Bilateria. Biol. Bull. 39, 393–401. doi: 10.1134/s106235901205010x
Martynov, A. V. (2014). “Ontogeny as a central paradigm of biology: declarative importance and practical underestimation,” in Proceedings of the 3rd International Congress on Invertebrate Morphology, Berlin, 128.
Martynov, A. V., Brenzinger, B., Hooker, Y., and Schrödl, M. (2011). 3D Anatomy of a new tropical peruvian nudibranch gastropod species, Corambe mancorensis, and novel hypothesis on dorid gill ontogeny and evolution. J. Molluscan Stud. 77, 129–141. doi: 10.1093/mollus/eyq047
Martynov, A. V., Ishida, Y., Irimura, S., Tajiri, R., O’Hara, T., and Fujita, T. (2015). When ontogeny matters: a new Japanese species of brittle star illustrates importance of considering both adult and juvenile characters in taxonomic practice. PLoS One 10:e0139463. doi: 10.1371/journal.pone.0139463
Martynov, A. V., and Korshunova, T. A. (2011). Opisthobranch Molluscs of the Seas of Russia. A Colour Guide to their Taxonomy and Biology. Moscow: Fiton Press.
Martynov, A. V., and Korshunova, T. A. (2015). A new deep-sea genus of the family Polyceridae (Nudibranchia) possesses a gill cavity, with implications for cryptobranch condition and a ‘Periodic Table’ approach to taxonomy. J. Molluscan Stud. 81, 365–379. doi: 10.1093/mollus/eyv003
Martynov, A. V., Lundin, K., Picton, B., Fletcher, K., Malmberg, K., and Korshunova, T. (2020). Multiple paedomorphic lineages of soft-substrate burrowing invertebrates: parallels in the origin of Xenocratena and Xenoturbella. PLoS One 15:e0227173. doi: 10.1371/journal.pone.0227173
Martynov, A. V., and Schrödl, M. (2011). Phylogeny and evolution of corambid nudibranchs (Mollusca: Gastropoda). Zool. J. Linn. Soc. 163, 585–604. doi: 10.1111/j.1096-3642.2011.00720.x
McKinney, M.L. (ed.) (1988). Heterochrony in Evolution: A Multidisciplinary Approach. New York, NY: Plenum.
McNamara, K. J. (1986). A guide to the nomenclature of heterochrony. J. Paleontol. 60, 4–13. doi: 10.1017/s0022336000021454
Mehnert, E. (1897). Kainogenese. Eine gesetzmässige Abänderung der embryonalen Entfaltung in Folge von erblicher Uebertragung in der Phylogenese erworbener Eigenthümlichkeiten. Morph. Arb 7, 1–156. doi: 10.1159/000393310
Minelli, A. (2007). Invertebrate taxonomy and evolutionary developmental biology. Zootaxa 1668, 55–60. doi: 10.11646/zootaxa.1668.1.7
Minelli, A. (2009). Phylo–evo–devo: combining phylogenetics with evolutionary developmental biology. BMC Biol. 7:36. doi: 10.1186/1741-7007-7-36
Minelli, A. (2015a). Biological systematics in the evo-devo era. Eur. J. Taxon. 125, 1–23. doi: 10.1017/9781108873130.003
Minelli, A. (2015b). “EvoDevo and its significance for animal evolution and phylogeny,” in Evolutionary Developmental Biology of Invertebrates 1, ed. A. Wanninger (Vienna: Springer), 1–23. doi: 10.1007/978-3-7091-1862-7_1
Moles, J., Wägele, H., Cutignano, A., Fontana, A., Ballesteros, M., and Avila, C. (2017). Giant embryos and hatchlings of Antarctic nudibranchs (Mollusca: Gastropoda: Heterobranchia). Mar. Biol. 164, 114. doi: 10.54173/f512114
Moroz, L., Kocot, K. M., Citarella, M. R., Dosung, S., Norekian, T. P., Povolotskaya, I. S., et al. (2014). The ctenophore genome and the evolutionary origins of neural systems. Nature 510, 109–114. doi: 10.1038/nature13400
Müller, W. A. (1997). “Comparative review: the phylotypic stage of vertebrates, common versus distinct features, and aspects of evolution,” in Developmental Biology (New York, NY: Springer). doi: 10.1007/978-1-4612-2248-4_4
Müller, G. B. (2017). Why an extended evolutionary synthesis is necessary. Interface Focus 7:20170015. doi: 10.1098/rsfs.2017.0015
Needham, J. (1933). On the dissociability of the fundamental process in ontogenesis. Biol. Rev. 8, 180–223. doi: 10.1111/j.1469-185x.1933.tb01153.x
Nejad Kourki, A. (2021). Evolution of the Eumetazoan Body Plan. Ph.D. thesis. Bristol: University of Bristol.
Neumann, J. S., Desalle, R., Narechania, A., Schierwater, B., and Tessler, M. (2021). Morphological characters can strongly influence early animal relationships inferred from phylogenomic data sets. Syst. Biol. 10, 360–375. doi: 10.1093/sysbio/syaa038
Nicholson, D. J. (2014). The return of the organism as a fundamental explanatory concept in biology. Philos. Compass. 9, 347–359. doi: 10.1111/phc3.12128
Nielsen, C. (2008). Six major steps in animal evolution: are we derived sponge larvae? Evol. Dev. 10, 241–257. doi: 10.1111/j.1525-142X.2008.00231.x
Nielsen, C. (2012). Animal Evolution: Interrelationships of the Living Phyla, 3rd Edn. Oxford: Oxford University Press.
Ninova, M., Ronshaugen, M., and Griffiths-Jones, S. (2014). Conserved temporal patterns of microRNA expression in Drosophila support a developmental hourglass model. Gen. Biol. Evol. 6, 2459–2467. doi: 10.1093/gbe/evu183
Núñez-León, D., Cordero, G. A., Schlindwein, X., Jensen, P., Stoeckli, E., Sánchez Villagra, M. R., et al. (2021). Shifts in growth, but not differentiation, foreshadow the formation of exaggerated forms under chicken domestication. Proc. R. Soc. B 288:20210392. doi: 10.1098/rspb.2021.0392
O’Hara, T. D., Hugall, A. F., Thuy, B., Stöhr, S., and Martynov, A. V. (2017). Restructuring higher taxonomy using broadscale phylogenomics: the living Ophiuroidea. Mol. Phyl. Evol. 107, 415–430. doi: 10.1016/j.ympev.2016.12.006
Ontogeny (2022). Wikipedia. Available online at: https://en.wikipedia.org/wiki/Ontogeny (accessed February 27, 2022).
Orton, G. R. (1955). The role of ontogeny in systematics and evolution. Evolution 9, 75–83. doi: 10.2307/2405359
Pabst, E. A., and Kocot, K. M. (2018). Phylogenomics confirms monophyly of Nudipleura (Gastropoda: Heterobranchia). J. Molluscan Stud. 84, 259–265. doi: 10.1093/mollus/eyy013
Pavlinov, I. Y. (2020). Multiplicity of research programs in the biological systematics: a case for scientific pluralism. Philosophies 5:7. doi: 10.3390/philosophies5020007
Peters, S. (1980). Das biogenetische grundgesetz – vorgeschichte und folgerungen. Medizinhist. J. 15, 57–69.
Pisani, D., Pett, W., Dohrmann, M., Feuda, R., Rota-Stabelli, O., Philippe, H., et al. (2015). Genomic data do not support comb jellies as the sister group to all other animals. Proc. Nat. Acad. Sci. U.S.A. 112, 15402–15407. doi: 10.1073/pnas.1518127112
Raff, R. A., and Kaufman, T. C. (1983). Embryos, Genes, and Evolution. Bloomington, IN: Indiana University Press.
Redmond, A. K., and Mclysaght, A. (2021). Evidence for sponges as sister to all other animals from partitioned phylogenomics with mixture models and recoding. Nat. Comm. 12:1783. doi: 10.1038/s41467-021-22074-7
Reilly, S. M., Wiley, E. O., and Meinhardt, D. J. (1997). An integrative approach to heterochrony: the distinction between interspecific and intraspecific phenomena. Biol. J. Linn. Soc. 60, 119–143. doi: 10.1006/bijl.1996.0092
Richards, R. J. (2009). Haeckel’s embryos: fraud not proven. Biol. Phil. 24, 147–154. doi: 10.1007/s10539-008-9140-z
Richardson, M. K. (1995). Heterochrony and the phylotypic period. Dev. Biol. 172, 412–421. doi: 10.1006/dbio.1995.8041
Richardson, M. K. (1999). Vertebrate evolution: the developmental origins of adult variation. Bioessays 21, 604–613. doi: 10.1002/(SICI)1521-1878(199907)21:7<604::AID-BIES9>3.0.CO;2-U
Richardson, M. K. (2022). Theories, laws, and models in evo-devo. J. Exp. Zool. 338, 36–61. doi: 10.1002/jez.b.23096
Richardson, M. K., Hanken, J., Gooneratne, M. L., Pieau, C., Raynaud, A., Selwood, L., et al. (1997). There is no highly conserved embryonic stage in the vertebrates: implications for current theories of evolution and development. Anat. Embr. 196, 91–106. doi: 10.1007/s004290050082
Richter, S., and Wirkner, C. S. (2014). A research program for evolutionary morphology. J. Zool. Syst. Evol. Res. 52, 338–350. doi: 10.1111/jzs.12061
Rundell, R. J., and Leander, B. S. (2010). Masters of miniaturization: convergent evolution among interstitial eukaryotes. Bioessays 32, 430–437. doi: 10.1002/bies.200900116
Schierwater, B., and DeSalle, R. (eds). (2021). Invertebrate Zoology: A Tree of Life Approach. Boca Raton, FL: CRC press.
Schmalhausen, I. I. (1942). Organism as a Whole in Individual and Historical Development. Moscow–Leningrad: Akad. Nauk USSR.
Scholtz, G. (2010). Deconstructing morphology. Acta Zool. 91, 44–63. doi: 10.1111/j.1463-6395.2009.00424.x
Sedgwick, A. (1894). On the law of development commonly known as Von Baer’s law. Q. J. Microsc. Sci. 36, 35–52. doi: 10.1242/jcs.s2-36.141.35
Severtsov, A. N. (1912). Essays on the Theory of Evolution: Individual Development and Evolution. Kiev: Imp. Univ. Sv. Vladimira.
Severtsov, A. N. (1939). Morphological Patterns of Evolution. Moscow–Leningrad: Akad. Nauk USSR Publishing house.
Simion, P., Philippe, H., Baurain, D., Jager, M., Richter, D. J., Di Franco, A., et al. (2017). A large and consistent phylogenomic dataset supports sponges as the sister group to all other animals. Curr. Biol. 27, 1–10. doi: 10.1016/j.cub.2017.02.031
Skulachev, V. P., Holtze, S., Vyssokikh, M. Y., Bakeeva, L. E., Skulachev, M. V., Markov, A. V., et al. (2017). Neoteny, prolongation of youth: from naked mole rats to “naked apes” (Humans). Physiol. Rev. 97, 699–720. doi: 10.1152/physrev.00040.2015
Slack, J. M. W., Holland, P. W. H., and Graham, C. F. (1993). The zootype and the phylotypic stage. Nature 361, 490–492. doi: 10.1038/361490a0
Smirnov, A. V. (1991). “Paedomorphosis as mechanism of evolutionary transformations of organisms,” in Modern Evolutionary Morphology, eds E. I. Vorobyeva and A. A. Vronsky (Kiev: Naukova Dumka), 88–103.
Stöhr, S., and Martynov, A. V. (2016). Paedomorphosis as an evolutionary driving force: insights from deep-sea brittle stars. PLoS One 11:e0164562. doi: 10.1371/journal.pone.0164562
Tassia, M. G., Cannon, J. T., Konikoff, C. E., Shenkar, N., Halanych, K. M., and Swalla, B. J. (2016). The global diversity of Hemichordata. PLoS One 11:e0162564. doi: 10.1371/journal.pone.0162564
Uesaka, M., Kuratani, S., and Irie, N. (2022). The developmental hourglass model and recapitulation: an attempt to integrate the two models. J. Exp. Zool. 338, 76–86. doi: 10.1002/jez.b.23027
Viñuelas, J., Kaneko, G., Coulon, A., Beslon, G., and Gandrillon, O. (2012). Towards experimental manipulation of stochasticity in gene expression. Prog. Biophys. Mol. Biol. 110, 44–53. doi: 10.1016/j.pbiomolbio.2012.04.010
von Baer, K. E. (1828/1837). Entwickelungsgeschichte der Thiere: Beobachtung und Reflexion. 2 Bd. Königsberg: Bornträger, 264.
Vorobyeva, E. I. (1991). “Modern problems of the evolution of ontogeny,” in Modern Evolutionary Morphology, eds E. I. Vorobyeva and A. A. Vronsky (Kiev: Naukova Dumka), 72–87.
Wanninger, A. (2015). Morphology is dead – long live morphology! Integrating MorphoEvoDevo into molecular EvoDevo and phylogenomics. Front. Ecol. Evol. 3:54.
Wiens, J. J., Bonnet, R. M., and Chippindale, P. T. (2005). Ontogeny discombobulates phylogeny: paedomorphosis and higher level salamander relationships. Syst. Biol. 54, 91–110. doi: 10.1080/10635150590906037
Wolfe, J. M., and Hegna, T. A. (2013). Testing the phylogenetic position of Cambrian pancrustacean larval fossils by coding ontogenetic stages. Cladistics 30, 366–390. doi: 10.1111/cla.12051
Yi, S. V., and Goodisman, M. A. D. (2021). The impact of epigenetic information on genome evolution. Philos. Trans. R. Soc. B 376:20200114. doi: 10.1098/rstb.2020.0114
Yushin, V. V., and Malakhov, V. V. (2014). The origin of nematode sperm: progenesis at the cellular level. Russ. J. Mar. Biol. 40, 71–81. doi: 10.1134/S1063074014020114
Zaitseva, O. V., Shumeev, A. N., Korshunova, T. A., and Martynov, A. V. (2015). Heterochronies in the formation of the nervous and digestive systems in early postlarval development of opisthobranch mollusks: organization of major organ systems of the Arctic dorid Cadlina laevis. Biol. Bull. 42, 186–195. doi: 10.1134/s1062359015030152
Keywords: ontogeny, evolution, phylogeny, ontogenetic systematics, paedomorphosis
Citation: Martynov A, Lundin K and Korshunova T (2022) Ontogeny, Phylotypic Periods, Paedomorphosis, and Ontogenetic Systematics. Front. Ecol. Evol. 10:806414. doi: 10.3389/fevo.2022.806414
Received: 31 October 2021; Accepted: 14 March 2022;
Published: 13 May 2022.
Edited by:
Pedro Martinez, University of Barcelona, SpainReviewed by:
Alessandro Minelli, University of Padua, ItalyGuillaume Lecointre, Muséum National d’Histoire Naturelle, France
Copyright © 2022 Martynov, Lundin and Korshunova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander Martynov, martynov@zmmu.msu.ru
 Alexander Martynov
Alexander Martynov Kennet Lundin
Kennet Lundin Tatiana Korshunova
Tatiana Korshunova