Population and Nesting Site Evidence for Diamondback Terrapins, Malaclemys terrapin, in Northeast Florida
- 1Department of Biology, University of North Florida, Jacksonville, FL, United States
- 2Department of Economics and Geography, University of North Florida, Jacksonville, FL, United States
- 3Department of Mathematics and Statistics, University of North Florida, Jacksonville, FL, United States
Diamondback terrapins (Malaclemys terrapin) are listed as Vulnerable by the IUCN Red List Index of Threatened Species. Among the challenges terrapins encounter are habitat loss due to coastal development and sea level rise, mortality at all life stages by mammalian and avian predators, road mortality, boat strikes, harvest for the pet trade, and drowning in crab traps. The primary objective of this study was to locate populations and nesting areas of diamondback terrapins in the four northeastern-most counties of Florida (Nassau, Duval, St. Johns, and Flagler). We conducted head counts and performed land surveys of shorelines and high spots for evidence of terrapin presence. During the land surveys we searched for crawls, intact and depredated nests, dead terrapins, and terrapin bones. To evaluate whether woody plant presence affected nest site choices, we recorded the occurrence of 10 common woody plant species during each land survey and compared areas where nesting did and did not occur. We collected 404 records of terrapin activity in 2013 and 2014. Most were from Nassau County (277) and only one was from Flagler County. Most data were in the form of depredated nests (205) and terrapin remains (147). The woody plant data suggest that terrapins were significantly more likely to nest when Christmas berry (Lycium carolinianum) was present, and nesting was less likely when either wax myrtle (Myrica cerifera) or oak (Quercus spp.) were present.
Introduction
Diamondback terrapins (Malaclemys terrapin) are listed as Vulnerable by the IUCN Red List Index of Threatened Species (Roosenburg et al., 2019). Among the challenges terrapins encounter are habitat loss due to coastal development and sea level rise, mortality at all life stages by mammalian and avian predators, road mortality (Maerz et al., 2018), boat strikes (Lester et al., 2018), harvest for the pet trade, and drowning in crab traps (Chambers and Maerz, 2018).
Commonly used references concerning reptile natural history (e.g., Ernst and Lovich, 2009; Powell et al., 2016) describe the distribution of diamondback terrapins to be from Cape Cod, Massachusetts to Corpus Christi, Texas. They provide range maps with a continuous line drawn along the Atlantic and Gulf coastlines between those two places. While these maps are useful for general information, terrapin distribution along those lines is not truly continuous due to natural or anthropogenic habitat interruptions. It would be helpful for researchers seeking to study local populations to know where terrapin concentrations exist. Accurate local distribution information can also inform governmental decisions concerning allocation of conservation resources and protection of essential habitats. To this end, one of the major objectives of the Diamondback Terrapin Working Group1 has been to create a “living” map of historical and current terrapin populations throughout the range (Butler et al., 2006). Historical populations can be identified from existing literature or museum collections, while the distribution and abundance of contemporary populations is determined by surveys.
In Florida, terrapin populations are known from Merritt Island National Wildlife Refuge (Seigel, 1980a,b, c, 1984, 1993), the Keys (Wood, 1992; Baldwin et al., 2005), the western part of Everglades National Park (Hart and McIvor, 2008), Sanibel Island (C. Lechowicz, pers. comm.), Tampa Bay and St. Martins Key (C. Boykin, pers. comm), the Big Bend region (Butler and Heinrich, 2013), and the extreme western Panhandle (R. O’Conner, pers. comm.). In northeast Florida, several discrete terrapin populations in Nassau and Duval counties have been studied (Butler, 2000, 2002; Butler et al., 2004), but no systematic surveys were performed there. In a review of 58 museums only six terrapin records are listed for Florida’s four northeastern-most counties (Krysko et al., 2011): one from Nassau, four from Duval, one from St. Johns, and none from Flagler. The primary objective of this study was to identify terrapin populations and nesting sites in these four northeastern Florida counties.
Some oviparous reptiles, including diamondback terrapins, exhibit temperature-dependent sex determination (TSD) where offspring sex is a function of incubation temperature (Bull, 1980; Jeyasuria et al., 1994; Burke and Calichio, 2014; Wibbels et al., 2018). Incubation temperature depends on variables such as nest depth and shading, and in some cases shading by overstory vegetation affects nest depth or nest choice (Kolbe and Janzen, 2002; Czaja et al., 2020). Diamondback terrapins prefer to nest in sandy areas above the high tide line (Palmer and Cordes, 1988; Roosenburg, 1994). These areas often support some woody shrubs and trees that require such soils and do not withstand extensive flooding. Butler and Heinrich (2013) noted that several woody plant species were frequently associated with terrapin nesting areas, and we hypothesized that terrapins may use this vegetation as a distant visual signal that an area is appropriate for nesting. Thus, another objective was to determine if certain woody plant species could be indicators of terrapin nesting sites.
Materials and Methods
Study Site
The study area encompassed shorelines, marsh islands, tidal creeks and rivers associated with the Intracoastal Waterway (ICW) of the four northeastern-most coastal Florida counties of Nassau, Duval, St. Johns, and Flagler (Figure 1). The northern boundary was the St. Mary’s River and we surveyed southward nearly 160 km to the southern border of Flagler County. The area includes inlets at the St. Mary’s, Nassau, St. Johns, Fort George rivers, the city of St. Augustine, and the Matanzas Inlet. Most of the habitat adjacent to the ICW in Nassau, Duval and St. Johns counties is described as salt marsh by Montague and Wiegert (1990) with smooth cordgrass (Spartina alterniflora) dominating and intermittent stands of needlerush (Juncus roemerianus). About midway through St. Johns County, near the Guana-Tolomato-Matanzas National Estuarine Research Reserve (GTM), the northernmost black mangroves (Avicennia germinans) occur (Williams et al., 2014), but not until further south in Flagler County do mangroves dominate shorelines.
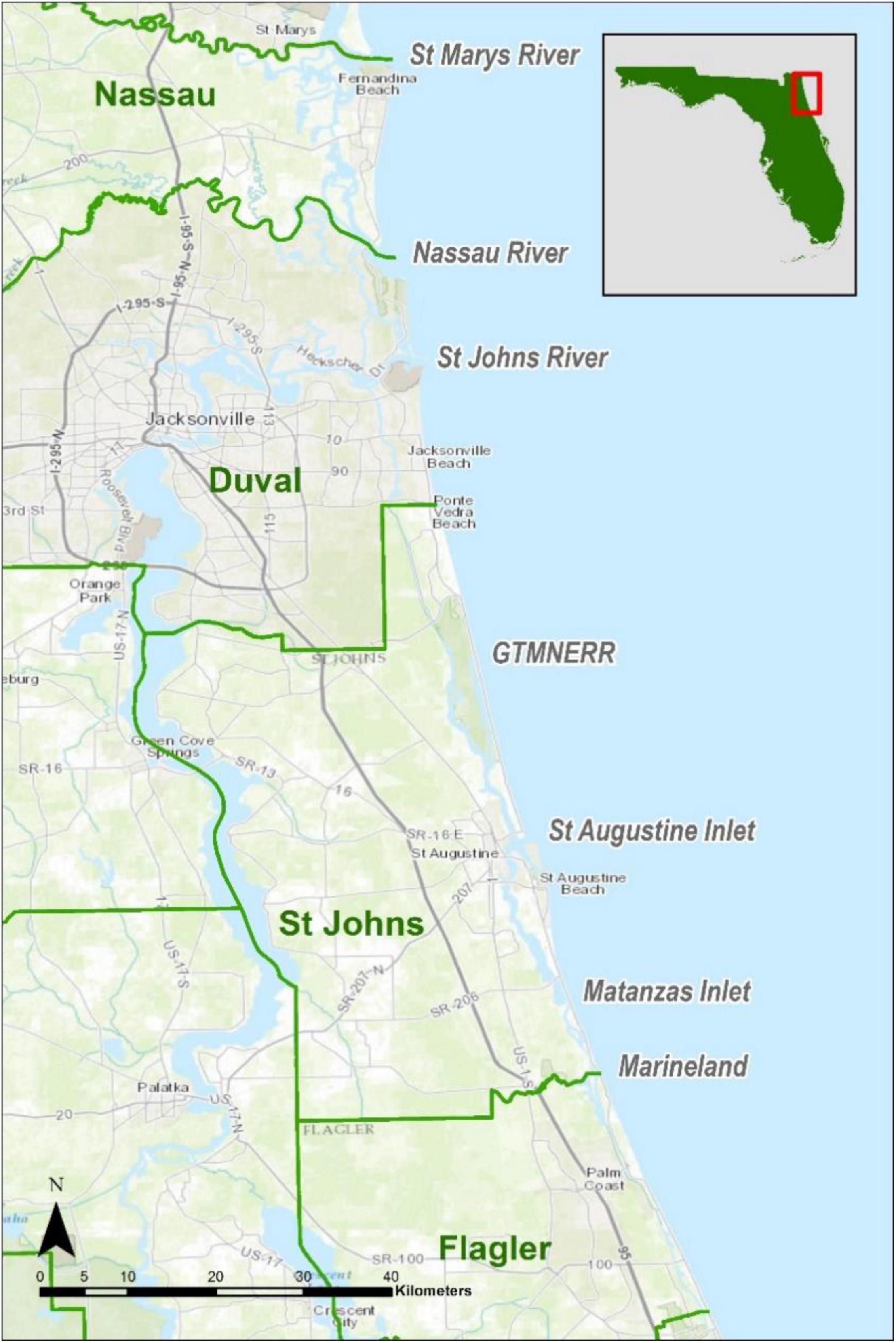
Figure 1. The northeast Florida Diamondback Terrapin study area showing boundaries of the four counties along with major inlets to the ICW.
From May 7 through August 6, 2013, three researchers surveyed 1 day per week for terrapins employing head counts and land surveys (Butler and Heinrich, 2013) in St. Johns County between GTM and Marineland. In 2014, from May 1, through August 1, three researchers surveyed for diamondback terrapins 3–4 days per week in all four of Florida’s northeastern-most counties. Some data collected between 1995 and 2002 from all but Flagler County during earlier studies by JB are included in our maps in the interest of completeness, but they are not counted as new data for this study.
Head Counts
Harden et al. (2009) compared terrapin head counts to population estimates derived from mark-recapture studies. Head counts, while less accurate for quantifying population levels, allow researchers to determine occupancy of an area without needing to engage in time-consuming capture techniques. We counted terrapin heads from our boat as we traveled at idle speed in adjacent tidal creeks, rivers, and occasionally the ICW. For each sighting we recorded GPS locations using a hand-held unit (Garmin GPSMAP 78 SC).
Land Surveys
We conducted walking surveys of all shorelines and dredge spoil or natural marsh islands exhibiting potential terrapin nesting habitat (i.e., above the high tide line with soil composed mostly of sand or sand-shell mix, Palmer and Cordes, 1988; Roosenburg, 1994). At such sites we detected terrapin presence by recording depredated terrapin nests, terrapin remains (carcasses, bones, or scutes), intact nests, or crawls (Butler and Heinrich, 2013; Roosenburg and Burke, 2018). Raccoons (Procyon lotor) are major predators of diamondback terrapin nests throughout their range (Burger, 1977; Roosenburg, 1992; Feinberg and Burke, 2003). When raccoons excavate nests, they usually eat the egg contents at the nest site, leaving the eggshells behind. Therefore, we identified depredated nests by finding the eggshells associated with exhumed nests. Further, raccoons often kill female terrapins before they have time to nest (Seigel, 1980a), which was the source of most of the terrapin remnants we found. Occasionally, substrate conditions were such that tracks left in the sand by nesting terrapins could be identified indicating terrapin usage (crawls, Butler, 2002).
Other
Several of our data points resulted from encounters with live terrapins that were not from head counts, not anticipated, and defied placement within our categories. These will be explained below.
Maps
We used Geographic Information System software (ArcGIS 10.3, ESRI, Redlands, CA, United States) to create a geodatabase for all field observations using the GPS locations and field notes. Although vegetation survey locations were included in the geodatabase, maps included here focus on the locations of terrapin evidence and waypoints that indicate the areas surveyed.
Statistical Analysis
We recorded our time spent in the field on most days allowing calculation or the number of data points recorded over time (CPUE as # of records/time).
To establish whether terrapin evidence data points were distributed randomly, we applied an optimized hot spot analysis (Getis-Ord Gi*) in ArcGIS Pro version 2.9.0 using all terrapin evidence points with the total number of observations as the analysis field.
To determine whether vegetation composition affected nest site choice by terrapins, during the land surveys in 2014, we recorded the presence/absence of 10 plant species associated with Florida salt marshes. We limited our records to woody species because most are sensitive to extensive flooding and therefore occur above the high tide line where terrapins normally nest. We did not record the number of each species, only presence/absence. The shrub and tree species we documented are: marsh elder (Iva frutescens), saltbush (Batis halimifolia), Christmas berry (Lycium carolinianum), southern red cedar (Juniperus silicicola), wax myrtle (Myrica cerifera), cabbage palm (Sabal palmetto), saw palmetto (Serenoa repens), yaupon (Ilex vomitoria), oak (Quercus spp.), and pine (Pinus spp., elliotii or palustris).
We compared the presence/absence of the 10 woody species at sites where nesting was detected and locations lacking nests using specialized matched-pairs t-testing, to determine if plant species composition differences between nesting and non-nesting sites were statistically significant. We then used decision tree analysis and a generalized ordinal logistic fit regression to model the odds of a nesting or non-nesting event using SAS 9.4 and JMP 15.0.
Results
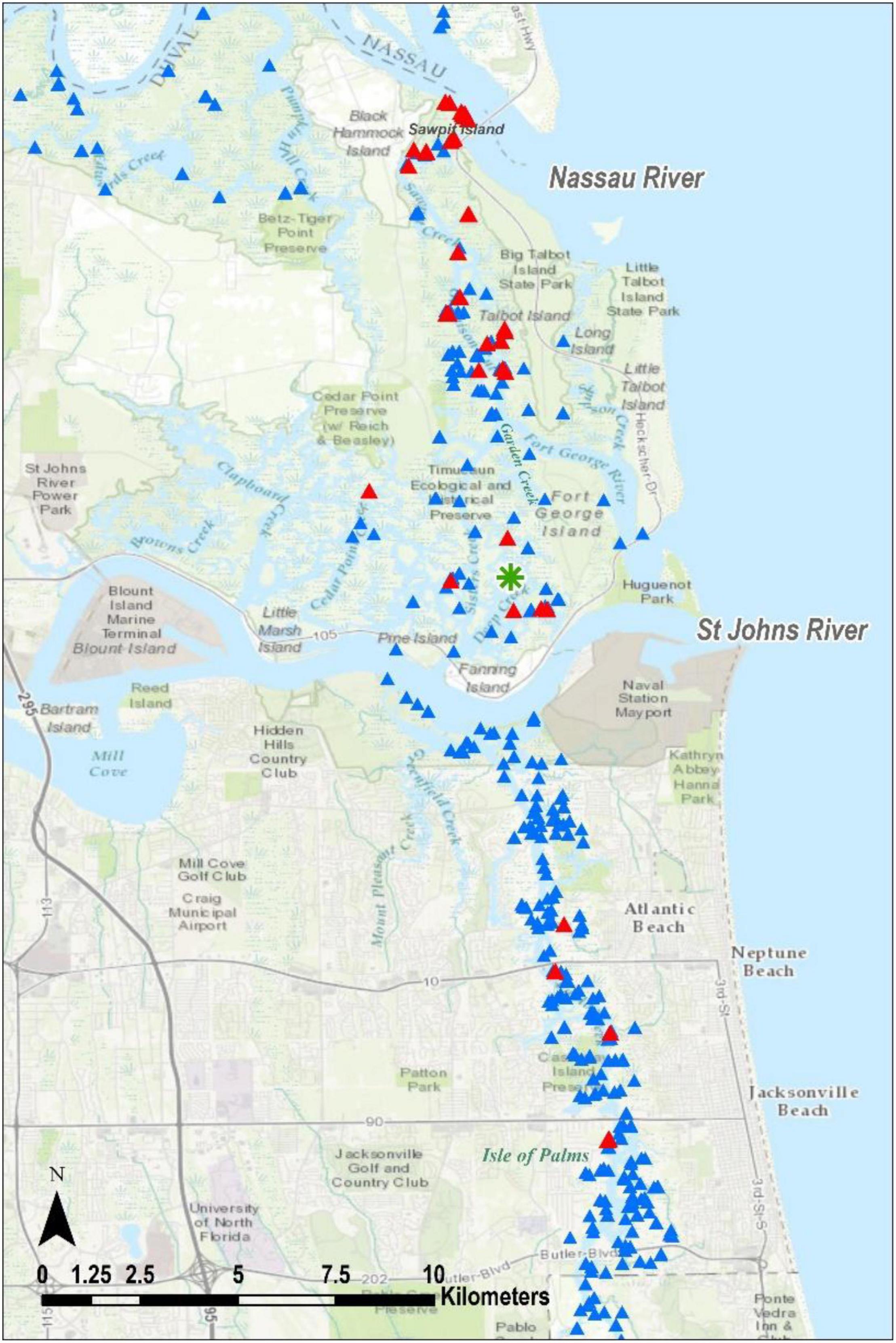
We documented 404 records of terrapin evidence in northeast Florida (Table 1 and Figure 2). For ease of analysis, we bundled the data into three groups referred to herein as the Nassau evidence (Figures 3A,B), the Duval evidence (Figure 4), and the St. Johns evidence (Figure 5). Sixty-eight percent of our records were from Nassau County, with another 20% from Duval County. St. Johns County records were from several discrete areas, and we had only one record from Flagler County.

Table 1. Records of Carolina Diamondback Terrapins in the four northeastern-most Florida counties of Nassau, Duval, St. Johns, and Flagler in 2013 and 2014.
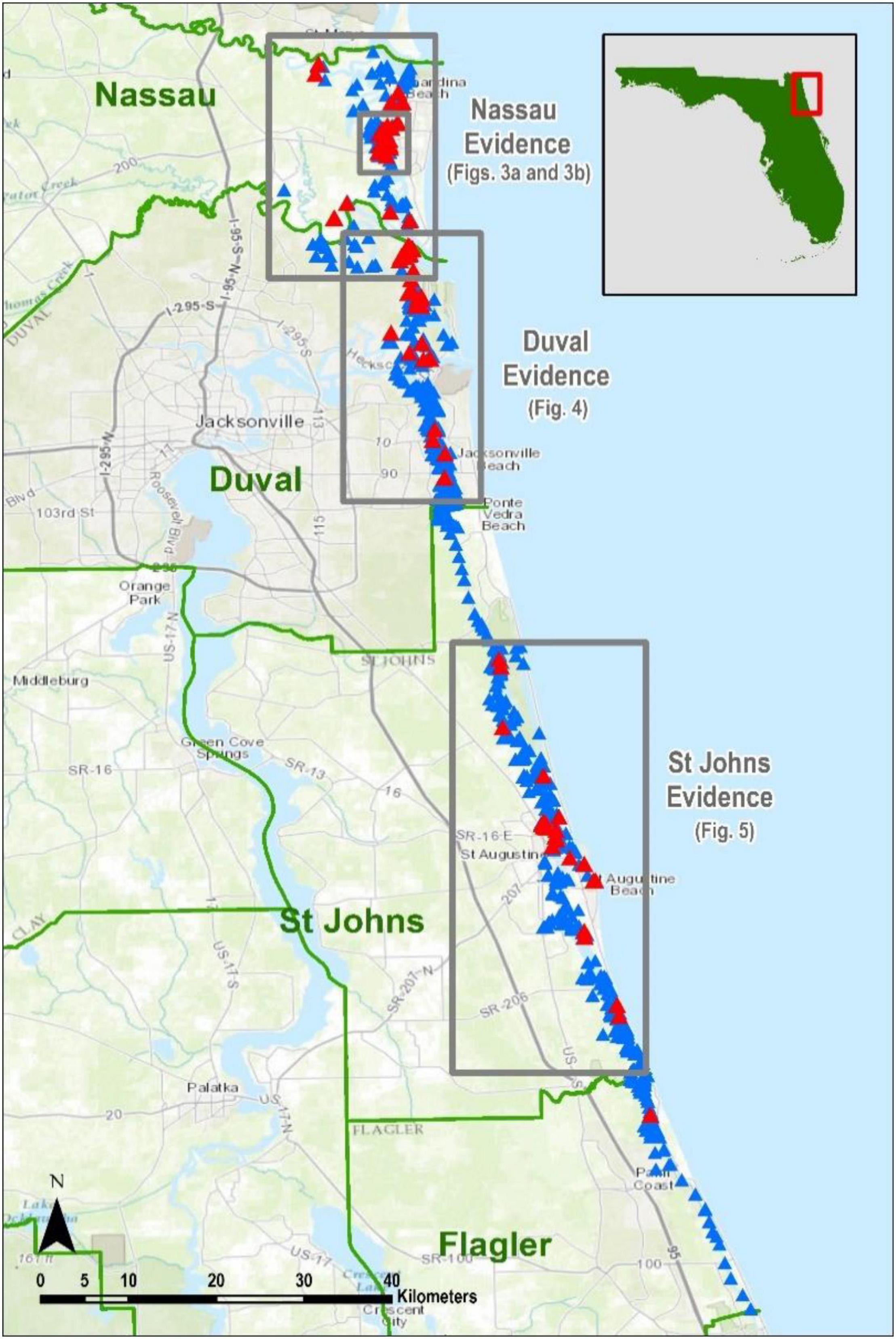
Figure 2. Northeast Florida study area with the three evidence centers outlined and labeled as Nassau, Duval, and St. Johns evidence, with an important Terrapin concentration at Jackson Creek (Figure 3B). Blue triangles depict all places where we conducted surveys. Red triangles indicate locations where evidence of Terrapins was found, and each represents between 1 and 102 findings.
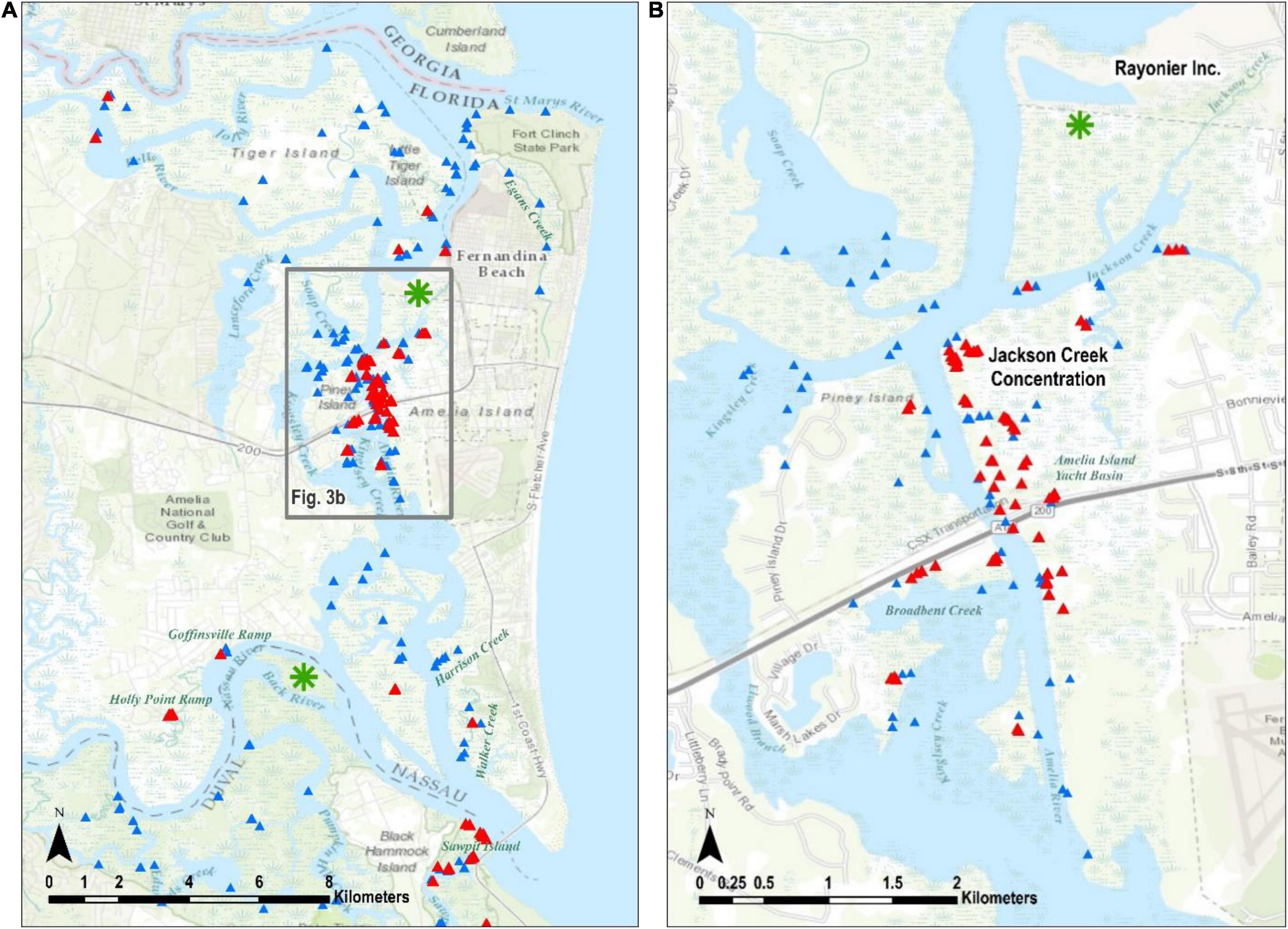
Figure 3. The Nassau Terrapin evidence. All records are indicated in (A) along with the Jackson Creek concentration in the box. The Jackson Creek concentration is highlighted in (B). Green asterisks are places where Terrapin evidence was recorded before the current study, and they are discussed in the body of the paper. Triangles as defined in Figure 2.
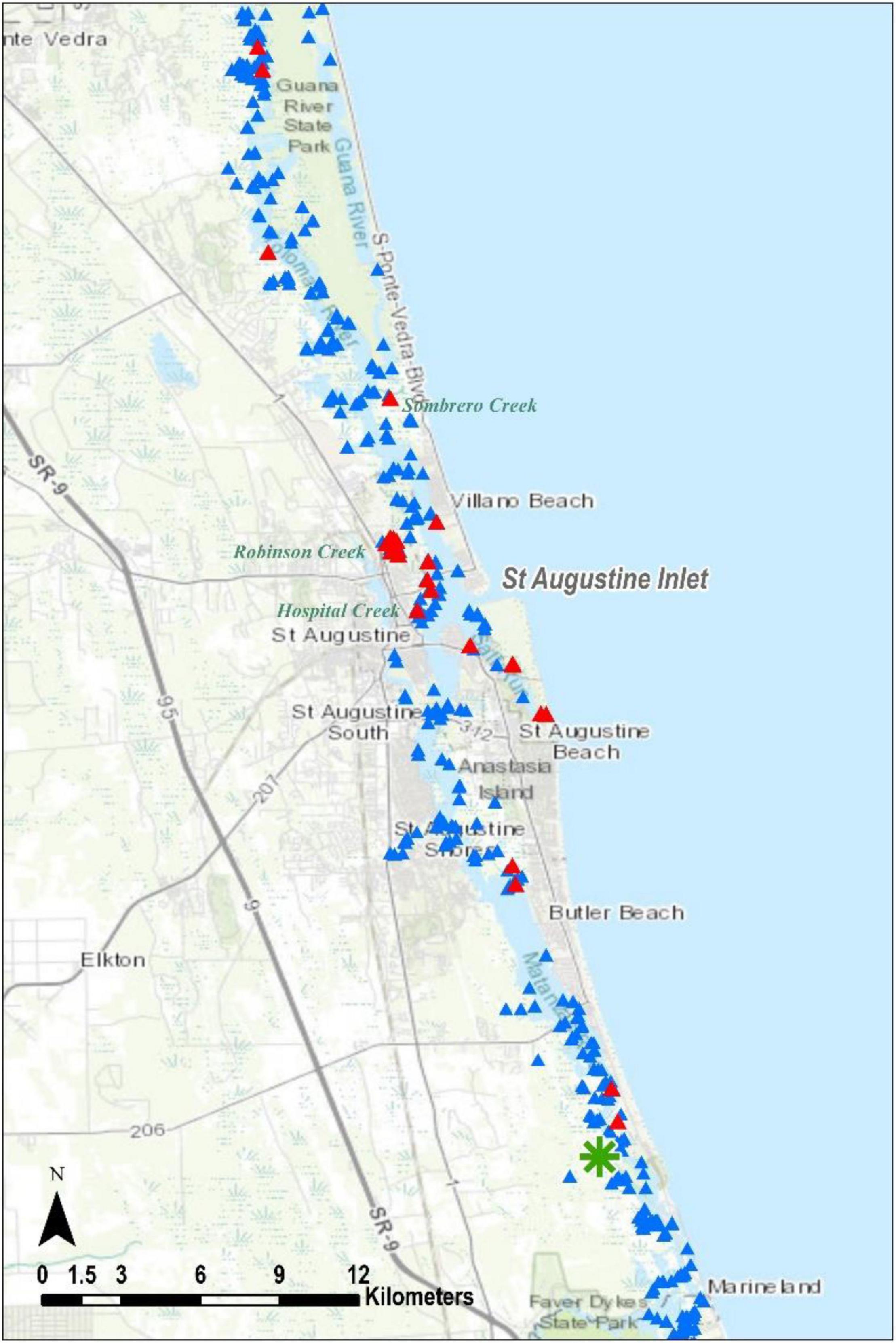
Figure 5. The St. Johns Terrapin evidence. Triangles and asterisks as in Figures 2, 3, respectively.
Head Counts
Of our 404 observations, 31 (7.7%) were from head counts (Table 1). The largest single head count (8) was in Jackson Creek (Figure 3B) in Nassau County and most of our head count records occurred in May 2014 (24), which is when we surveyed most of Nassau and Duval counties; we counted only six heads in St. Johns County and none in Flagler.
Land Surveys
Most of our terrapin evidence records were of depredated nests (51%) and again most were in Nassau County (Table 1). Among our observations in that county were 88 depredated nests at boat ramps (Figure 3A, Holly Point Ramp 58, Goffinsville Ramp 30), 19 on a spoil island in Broadbent Creek, and another 10 along railroad tracks beneath the SR 200 Bridge (Figure 3B). In Duval County, we found 12 depredated nests on islands within the marsh between the ICW (called Sisters Creek in this area) and Fort George Island (Figure 4), but most often we found fewer than five at a time. In St. Johns County, we recorded 17 depredated nests at a shoreline upstream in Robinson Creek, three on an island in Sombrero Creek, and other observations were of single raided nests (Figure 5). Finally, in Flagler County we located a single depredated nest on a spoil island just south of Marineland. Burke et al. (2009) determined that in Jamaica Bay Wildlife Refuge, New York, raccoons shifted their behavior later in the nesting season such that they devoured eggshells, rather than leaving them near the nest site. If this is the case in northeast Florida, our depredated nest counts would be undercounted.
We discovered most of the terrapin remains in Nassau County (Table 1), and it is notable that 87 of these were collected on 1 day from a site near the mouth of Jackson Creek (Figure 3B, where we also recorded the most heads). We recorded all 11 crawls on 1 day at Sawpit Island in Duval County (Figure 4), a known terrapin nesting site (Butler et al., 2004; Munscher et al., 2012). A terrapin deposited a nest near the Fernandina Harbor Marina (Nassau County, Figure 3A) on July 11, 2014 (C. Hoblin, Fernandina resident, pers. comm.). We dug to verify the presence of eggs (not clutch size) and recorded this as one of our intact nests. We discovered a nesting terrapin in St. John’s County on 16 July 2013 as it deposited a nest of three eggs on an island adjacent to Hospital Creek in St. Johns County (Figure 5). We recorded this as another intact nest (Table 1).
In Duval County, we sighted one terrapin abandoning a basking site in the marsh between Sisters Creek and Fort George Island, and we captured two others as they swam at the creek-mouth leading to the Isle of Palms community (Table 1, Other; Figure 4). Six terrapins were sighted swimming and photographed in Salt Run near a marina and restaurant in St. Johns County (S. Eastman, Florida DEP, and T. Dodson, St. Johns County Govt., pers. comm.). These nine individuals compose the “Other” column in Table 1.
Statistical Results
Of the 62 possible field days, we recorded time spent on 56 of them for a total of 263 h and 18 min. During that period, we recorded 276 terrapin evidence data points, so CPUE = 1.05 records/h. We did not include terrapin records obtained from sources other than our own methods in the calculation of CPUE. Further, we did not consider the number of researchers in the calculation, so the hours are not man-hours.
The optimized hot spot analysis calculated optimal neighborhood size as 5003.5 m, so we rounded to 5 k. There is a high level of confidence (95 to 99%) that the only collection of non-random terrapin evidence data points occurs at the “Jackson Creek Concentration” in Nassau County (Figures 3A,B).
We documented vegetation data during 184 land surveys: 147 were from non-nesting sites and 37 were from nesting sites which resulted in 911 woody species observations. The most commonly occurring woody species in both nesting and non-nesting areas was southern red cedar (J. silicicola), followed closely by marsh elder (Iva frutescens), then yaupon (Ilex vomitoria) and cabbage palm (S. palmetto, Table 2).
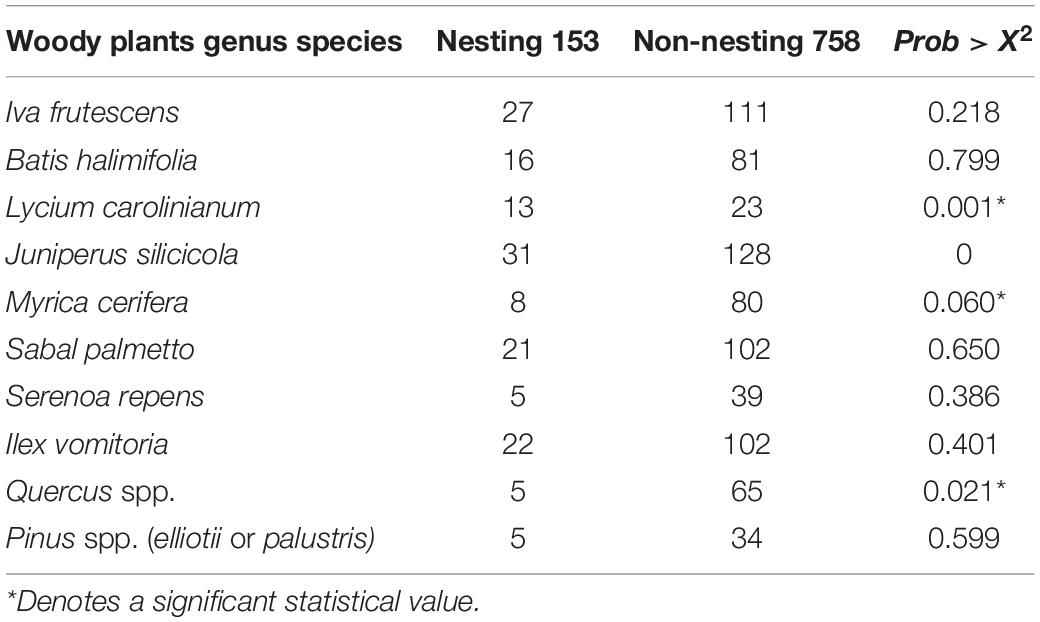
Table 2. Records of presence of 10 species of woody plants observed during land surveys of Diamondback Terrapin nesting and non-nesting habitats in northeast Florida.
A specialized matched-pairs t-test resulted in a mean difference of 52.3 (i.e., an average difference of 52.3 more non-nesting than nesting sites). The | t| ratio provided strong evidence that the difference between non-nesting and nesting was significant (Figure 6). In addition, the specialized matched-pairs t-test confirmed there are no outliers, indicating that decision tree analysis was an appropriate next step.
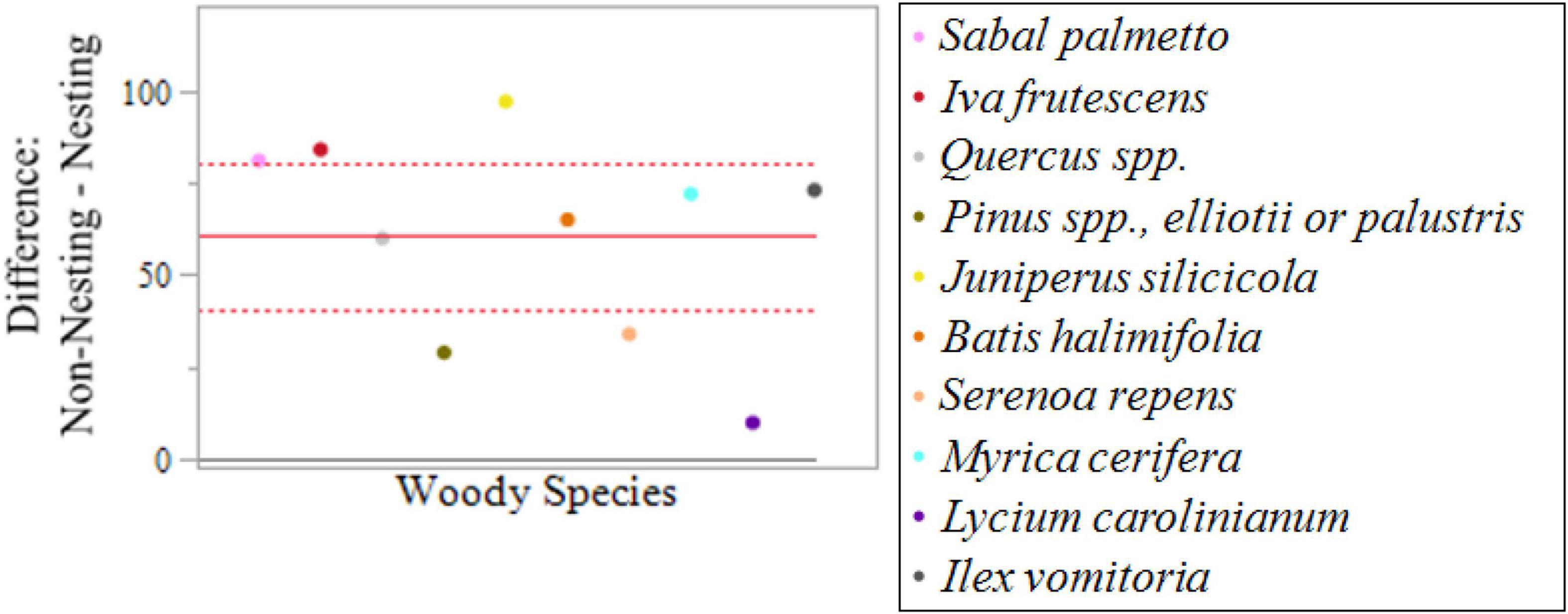
Figure 6. Specialized matched-pairs t-testing comparing woody species composition of nesting and non-nesting sites with a |t| ratio of 6.93 with |t| < 0.0001. A specialized matched-pairs t-test resulted in a mean difference of 52.3 represented by the solid red line and a 95% confidence interval of (40.8, 80.2) represented by the dotted red lines.
Decision tree analysis allowed us to predict terrapin activity based on available choices such as the presence of woody species at potential nesting sites (Stiglic et al., 2012). These results indicate that terrapins prefer to nest when Christmas berry (L. carolinianum) is present amongst the other available woody species, with the choices being equally weighted except for saw palmetto and pine, and with oak (Q. spp.) and wax myrtle (M. cerifera) following closely behind (Figures 7A,B). This conclusion is supported as R2 = 0.025 in the least squares regression with four splits using a Classification and Regression Trees (CART) algorithm and a Whole Model Effects test outcome with |Prob > χ2| = 0.012 which is statistically significant.
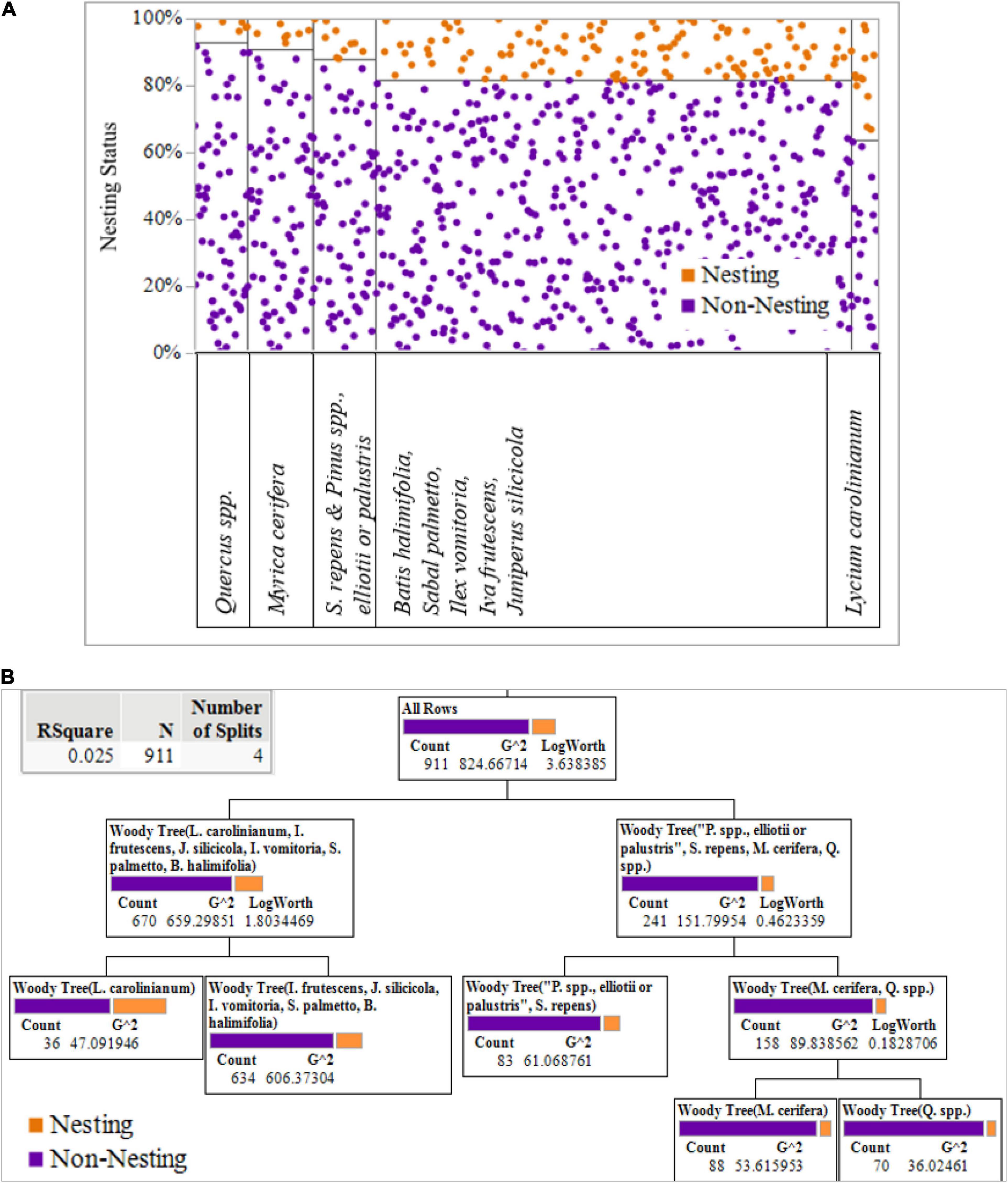
Figure 7. (A) Partition via Classification and Regression Trees (CART) analysis supporting the conclusion that woody plant data suggest that significantly more nesting occurred when Christmas Berry (Lycium carolinianum) was present, and nesting was less likely when either Wax Myrtle (Myrica cerifera) or Oak (Quercus spp.) were present. (B) Decision tree with R2 = 0.025 in the least squares regression with four splits using the CART algorithm.
Finally, we used a generalized linear binomial distribution fit model (i.e., GLM with binomial logit and maximum likelihood estimation) to determine whether the results from the decision tree were supported by the overall parameter estimates. Generalized linear binomial distribution fit produced a Whole Model test outcome showing that sites where Christmas berry was present exhibited a significantly higher proportion of terrapin nesting (|Prob > χ2| = 0.001) than those without (Table 2). Conversely, nesting was significantly less likely when either wax myrtle (|Prob > χ2| = 0.060) or oak (|Prob > χ2| = 0.021) were present. The presence of the other plant species had no significant statistical effect (Table 2). Thus, both tests produced statistically significant results which indicate that terrapins were likely to nest at sites where Christmas berry is present and tended to avoid nesting when oak and/or wax myrtle are present (Figure 6).
Discussion
Based upon the evidence, the most active area for terrapins in northeast Florida is in Nassau County. In the northern part of Nassau County, we recorded evidence of terrapins in the Bells River, Little Tiger Island, and in and around the Fernandina Harbor Marina (Figure 3A). We found no evidence in the Jolly River, Lanceford Creek, the southern shoreline of the St. Mary’s River including that of Ft. Clinch State Park, or in Egan’s Creek. We visited most places only once during the survey, therefore not finding evidence is not conclusive; common sense would dictate that terrapins are likely present throughout areas where the habitat is appropriate and that are bordered by areas where terrapins have been observed and documented.
In the southern part of Nassau County, the Holly Point and Goffinsville boat ramps had numerous depredated nests, and their shorelines offer the only apparent nesting habitat in the area, even though both are of anthropogenic origin (Figure 3A). The unnamed marsh island adjacent to Back River was identified by Butler (2002) as a place where at least some terrapins that nest on Sawpit Island spend the rest of their seasons (Figure 3A, green asterisk); we did not visit that marsh during this survey. Further east, we found no terrapin evidence in Harrison Creek and counted only one head in Walker Creek, even though both produced numerous head counts in previous years (Butler, unpubl. data).
The area around Jackson Creek (Figure 3B) yielded a variety of terrapin evidence including head counts, depredated nests, and many remains. The marsh area north of Jackson Creek abuts property owned by Rayonier Incorporated, which specializes in pulp, paper, and other cellulose products. Numerous depredated nests were recorded in 1995 on the southern banks of the Rayonier settling pond (Butler, unpubl. data, green asterisk on Figure 3B), but we were not granted permission to search the property for the current survey. Jackson Creek and its adjoining tributaries have been intensively trapped for blue crabs (Callinectes sapidus) for decades (P. Leary, resident of Jackson Creek, pers. comm.) and many terrapins enter and drown in crab traps in that area (Butler and Heinrich, 2007). Although most crab trappers would likely leave drowned terrapins in their traps as bait, we wonder if the extensive terrapin remains at the mouth of Jackson Creek are related to crab trapping. Conversely, these remains could be the result of extensive raccoon predation. Much of the habitat between Jackson Creek and SR 200 is owned by the Amelia Island Yacht Basin and considerable nesting occurs along the shoreline and some other high spots on the property. We found numerous depredated nests on the shoulders of the railroad tracks just northeast of SR 200.
Some of the Nassau evidence indicates that terrapins are not averse to exploiting habitat opportunities created anthropogenically. Most alterations of habitat by humans (i.e., channel dredging, bulkheading, building roads through salt marshes) are considered threats to terrapin survival (Maerz et al., 2018). However, Seigel (1980c) found terrapins using man-made lagoons and nesting on adjacent dyke roads. Feinberg and Burke (2003) recorded highest depredated terrapin nest densities on man-made trails. Roosenburg et al. (2014) demonstrated that terrapins will sometimes use artificially produced habitats for nesting with some success. The nesting activity associated with the Goffinsville and Holly Point boat ramps would not be possible without the construction associated with the ramps. Further, the construction of the SR 200 bridge and associated railroad tracks created more nesting opportunities at its base.
Within Duval County (Figure 4) the most notable area for terrapins is the nesting beach of Sawpit Island, which has been studied and monitored intermittently since 1996 (Butler, 2000, 2002; Butler et al., 2004; Munscher et al., 2012). In recent years, currents at the mouth of the Nassau River have led to extensive beach erosion, and part of the island has been breached to the extent that human passage is difficult or impossible during high tide (M. Simmons, Florida Fish and Wildlife, pers. comm.). Sand loss has decreased the area available for nesting, thus facilitating nest detection by predators. If the shoreline is destroyed, the fate of the terrapins that depend on this beach is unclear, as appropriate nesting habitat is scarce in the area.
Between Sawpit Island and the Ft. George River, terrapin evidence was present on most marsh islands and tributaries of the ICW. The area between the Ft. George and St. Johns rivers was also studied extensively during the late 1990s with head counts recorded in Cedar and Deep creeks nearly daily and radio telemetry studies done in Deep and Garden creeks (Butler, 2000, 2002). The current survey is notable for its paucity of terrapin evidence in all three of those creeks. Butler (2002) reported numerous terrapin heads in Deep Creek nearly every day during the nesting seasons over a three-year period (Figure 4, green asterisk). In 2014, despite traveling the entire length of Deep Creek on two separate days in May, we recorded no heads; and we saw no heads in Garden Creek. All creeks in that area were periodic sites of commercial crab trapping in the 1990s, and we fear the terrapin population could have been depleted. From the St. John’s River south to the Duval/St. John’s County line we found little evidence of terrapins.
In parts of northern St. John’s County, we were unable to perform land surveys because both east and west shorelines were privately owned (Figure 2); from the boat neither appeared to be appropriate nesting habitat. One locale in that county worthy of further research is the St. Augustine Inlet. On the western shoreline, both Hospital and Robinson creeks produced a variety of terrapin evidence. Further, the numerous adult terrapin sightings in Salt Run, to the southeast, signal what may be a notable population, and it is important to know where they nest so those areas can be protected. We recorded several depredated nests on the adjacent Conch Island, but these were sparse and do not appear to reflect a significant nesting site. Finally, in a May 2002 survey in St. Johns County, 15 swimming terrapins were counted while researchers waded an unnamed creek on the west shoreline of the Matanzas River, directly across from the north end of Rattlesnake Island, which is the home of the Ft. Matanzas National Monument (Butler and Heinrich, unpubl. data; green asterisk, Figure 4). We visited this creek during this survey in 2014 and found no evidence of terrapins.
Diamondback terrapins were more likely to nest in areas where Christmas berry was present. We do not suggest that terrapins seek out or even recognize Christmas berry, rather that it is present at most nest sites and can be a useful marker for researchers. This shrub is common in coastal marshes throughout Florida and the southeastern United States and is resistant to high salt concentrations, periodic flooding, and drought (Nelson, 1996). When present, Christmas berry is usually the first woody vegetation encountered as one proceeds inland from the water’s edge, and likely affords some cover for nesting terrapins and desirable thermal conditions for egg development. Conversely, terrapins were less likely to nest when oak and/or wax myrtle were present, and both species, when present in coastal regions, are typically found further inland (Johnson and Barbour, 1990). These areas may be unappealing to terrapins because they provide cover to mammalian nest predators (Burger and Montevecchi, 1975). Further, thicker canopies lead to lower nest temperatures (Jeyasuria et al., 1994) which would result in overproduction of male hatchlings, and Roosenburg (1996) suggested that female terrapins choose nesting sites that will produce both sexes. Christmas berry and marsh elder were often associated with terrapin nesting sites in the Big Bend area of Florida and their presence was used by the researchers as a possible nest site indicator (Hackney, 2010; Butler and Heinrich, 2013) found many depredated terrapin nests under wax myrtle in Virginia, and we report that in northeast Florida terrapins are less likely to nest in areas where this species is present. Future research on woody species as terrapin nest site markers could include comparing relative concentrations and canopy cover of each; Mitchell and Walls (2013) looked at this but not at the species level.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee – UNF.
Author Contributions
JB contributed as the principal investigator, wrote grants for field study, managed students, and wrote most of the verbiage concerning terrapins. JL oversaw all terrapin data, produced maps for Figures 1–5, and provided interpretations when necessary. MD ran all vegetation statistics and provided interpretation. DM did fieldwork with JB and did some lab studies associated with the study. All authors contributed to the article and approved the submitted version.
Funding
We received funding from the UNF Coastal Biology Program, UNF Transformational Learning Opportunities Scholarships, UNF Student Summer Research Scholarship, a UNF Environmental Center Seed Grant, and a grant from the Diamondback Terrapin Working Group. We had a Florida Fish and Wildlife Conservation Commission Scientific Collecting Permit (LSSC-11-00080C) and project approval from the UNF Institutional Animal Care and Use Committee (IACUC 13-003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We appreciate field assistance from UNF Biology majors Sofia Herrera, Lili Kolluri, Mary Jo Monk, Lisa Parnell, Sam Shannon, and Dylan Van Pelt. Alice Bard, Scott Eastman, and Marie Melnechuk (all of FL DEP), and Tara Dodson (St. Johns County Environmental Supervisor) provided several reliable terrapin sightings from St, Johns County. Charlie Hoblin (resident of Fernandina Beach) alerted us to an intact terrapin nest in Nassau County and Mike Simmons (FL DEP) informed us of changing conditions on Sawpit Island. We thank the following people and organizations who allowed us to store/dock the boat at various times during the project: Talbot Islands State Park, GTM, Joe Springer at Fernandina Harbor Marina, Beach Marine, Camachee Cove Yacht Harbor, and Eric Ziecheck and Chris Kelly of Ripple Effect Ecotours at Marineland Marina. We thank Eric Johnson of the UNF Biology Department for assistance with statistical analysis and David Wilson of the UNF Center for Instruction and Research Technology for help constructing the maps.
Footnotes
References
Baldwin, J. D., Latino, L. A., Mealey, B. K., Parks, G. M., and Forstner, M. R. J. (2005). “The Diamondback Terrapin in Florida Bay and the Florida Keys: insights into turtle conservation and ecology,” in Amphibians and Reptiles: status and Conservation in Florida, eds W. E. Meshaka Jr. and K. J. Babbitt (Malabar, Florida, USA: Krieger Publishing Company), 180–186.
Burger, J. (1977). Determinants of hatching success in the Diamondback Terrapin, Malaclemys terrapin. Am. Midl. Nat. 97, 444–464.
Burger, J., and Montevecchi, W. A. (1975). Nest site selection in the terrapin Malaclemys terrapin. Copeia 1975, 113–119. doi: 10.2307/1442413
Burke, R. L., and Calichio, A. M. (2014). Temperature-dependent sex determination in the diamond-backed terrapin (Malaclemys terrapin). J. Herpetol. 48, 466–470. doi: 10.1670/13-188
Burke, R. L., Felice, S. M., and Sobel, S. G. (2009). Changes in Raccoon (Procyon lotor) predation behavior affects turtle (Malaclemys terrapin) nest census. Chelonian Conserv. Biol. 8, 208–211.
Butler, J. A. (2000). Status and distribution of the Carolina Diamondback Terrapin, Malaclemys terrapin centrata, in Duval County. Final Report. Tallahassee, Florida, USA: Florida Fish and Wildlife Conservation Commission.
Butler, J. A. (2002). Population ecology, home range, and seasonal movements of the Carolina Diamondback Terrapin, Malaclemys terrapin centrata, in northeastern Florida. Final Report. Tallahassee, Florida, USA: Florida Fish and Wildlife Conservation Commission.
Butler, J. A., Broadhurst, C., Green, M., and Mullin, Z. (2004). Nesting, nest predation, and hatchling emergence of the Carolina Diamondback Terrapin, Malaclemys terrapin centrata, in northeastern Florida. Am. Midl. Nat. 152, 145–155.
Butler, J. A., and Heinrich, G. H. (2007). The effectiveness of bycatch reduction devices on crab pots at reducing capture and mortality of Diamondback Terrapins (Malaclemys terrapin) in Florida. Estuaries Coasts 30, 179–185. doi: 10.1007/bf02782978
Butler, J. A., and Heinrich, G. H. (2013). Distribution of the Ornate Diamondback Terrapin (Malaclemys terrapin macrospilota) in the Big Bend Region of Florida. Southeast. Nat. 12, 552–567. doi: 10.1656/058.012.0309
Butler, J. A., Heinrich, G. H., and Seigel, R. A. (2006). Third workshop on the ecology, status, and conservation of Diamondback Terrapins (Malaclemys terrapin): results and recommendations. Chelonian Conserv. Biol. 5, 331–334.
Chambers, R. M., and Maerz, J. C. (2018). “Bycatch in blue crab fisheries,” in Ecology and Conservation of the Diamond-backed Terrapin, eds W. M. Roosenburg and V. S. Kennedy (Baltimore, USA: Johns Hopkins University Press), 231–244.
Czaja, R. A., Scholz, A. L., Figueras, M. P., and Burke, R. L. (2020). The role of nest depth and site choice in mitigating the effects of climate change on an oviparous reptile. Diversity 12:151. doi: 10.3390/d12040151
Ernst, C. H., and Lovich, J. E. (2009). Turtles of the United States and Canada, 2nd Edn. Baltimore, Maryland, USA: Johns Hopkins University Press.
Feinberg, J. A., and Burke, R. L. (2003). Nesting ecology and predation of Diamondback Terrapins, Malaclemys terrapin, at Gateway National Recreation Area, New York. J. Herpetol. 37, 517–526. doi: 10.1670/207-02a
Hackney, A. D. (2010). Conservation biology of the Diamondback Terrapin in North America: policy status, nest predation, and managing island populations. Ph.D. thesis. South Carolina, USA: Clemson University, 69
Harden, L. A., Pittman, S. E., Whitfield, J. W., and Dorcas, M. E. (2009). Development of a rapid assessment technique for Diamondback Terrapin (Malaclemys terrapin) populations using head-count surveys. Appl. Herpetol. 6, 237–245.
Hart, K. M., and McIvor, C. C. (2008). Demography and ecology of Mangrove Diamondback Terrapins in a wilderness area of Everglades National Park, Florida. Copeia 2008, 200–208.
Jeyasuria, P., Roosenburg, W. M., and Place, A. R. (1994). Role of P-450 aromatase in sex determination of the Diamondback Terrapin, Malaclemys terrapin. J. Exp. Zool. 270, 95–111. doi: 10.1002/jez.1402700111
Johnson, A. F., and Barbour, M. G. (1990). “Dunes and maritime forests,” in Ecosystems of Florida, eds R. L. Myers and J. J. Ewel (Gainesville, USA: University Presses of Florida), 429–480.
Kolbe, J. L., and Janzen, F. J. (2002). Impact of nest site selection on nest success and nest temperature in natural and disturbed habitats. Ecology 83, 269–281. doi: 10.1002/jez.2198
Krysko, K. L., Enge, K. M., and Moler, P. E. (2011). Atlas of amphibians and reptiles in Florida. Final Report, Project Agreement 08013. Tallahassee, FL, USA: Florida Fish and Wildlife Conservation Commission.
Lester, L. A., Avery, H. W., Harrison, A. S., and Standora, E. A. (2018). “Interactions with motorboats,” in Ecology and Conservation of the Diamond-backed Terrapin, eds W. M. Roosenburg and V. S. Kennedy (Baltimore, USA: Johns Hopkins University Press), 221–230.
Maerz, J. C., Seigel, R. A., and Crawford, B. A. (2018). “Conservation in terrestrial habitats: mitigating habitat loss, road mortality, and subsidized predators,” in Ecology and Conservation of the Diamond-backed Terrapin, eds W. M. Roosenburg and V. S. Kennedy (Baltimore, USA: Johns Hopkins University Press), 201–220.
Mitchell, J. C., and Walls, S. C. (2013). Nest site selection by Diamondback Terrapins (Malaclemys terrapin) on a mid-Atlantic barrier island. Chelonian Conserv. Biol. 12, 303–308. doi: 10.2744/ccb-1041.1
Montague, C. L., and Wiegert, R. G. (1990). “Salt marshes,” in Ecosystems of Florida, eds R. L. Myers and J. J. Ewel (Gainesville, USA: University Presses of Florida), 481–516.
Munscher, E. C., Kuhns, E. H., Cox, C. A., and Butler, J. A. (2012). Decreased nest mortality for the Carolina Diamondback Terrapin (Malaclemys terrapin centrata) following removal of Raccoons (Procyon lotor) from a nesting beach in northeastern Florida. Herpetol. Conserv. Biol. 7, 167–184.
Palmer, W. M., and Cordes, C. L. (1988). Habitat suitability index models: diamondback Terrapin (nesting)-Atlantic coast. U.S. Fish and Wildlife Service Biological Report 82 (10.151). Washington, DC: U.S. Fish and Wildlife Service, 8.
Powell, R., Conant, R., and Collins, J. T. (2016). Peterson Field Guide to Reptiles and Amphibians, Eastern and Central North America, 4rd Edn. New York, New York, USA: Houghton Mifflin Harcourt Publishing Company.
Roosenburg, W. M. (1992). Life history consequences of nest site choice by the Diamondback Terrapin, Malaclemys terrapin. Ph.D. thesis. Philadelphia USA: University of Pennsylvania
Roosenburg, W. M. (1994). Nesting requirements of the Diamondback Terrapin: a geographic comparison. Wetl. Issues 6, 8–11.
Roosenburg, W. M. (1996). Maternal condition and nest site choice: an alternative for the maintenance of environmental sex determination? Am. Zool. 36, 157–168. doi: 10.1093/icb/36.2.157
Roosenburg, W. M., Baker, P. J., Burke, R., Dorcas, M. E., and Wood, R. C. (2019). Malaclemys terrapin. The IUCN Red List of Threatened Species 2019: e.T12695A507698. Available Online at: https://dx.doi.org/10.2305/IUCN.UK.2019-1.RLTS.T12695A507698.en (accessed on 14 February 2022)
Roosenburg, W. M., and Burke, R. L. (2018). “Capture, measurement, and field techniques,” in Ecology and Conservation of the Diamond-backed Terrapin, eds W. M. Roosenburg and V. S. Kennedy (Maryland, USA: Johns Hopkins University Press), 7–25.
Roosenburg, W. M., Spontak, D. M., Sullivan, S. P., Mathews, E. L., Heckman, M. L., Trimbath, R. J., et al. (2014). Nesting habitat creation enhances recruitment in a predator-free environment: malaclemys nesting at the Paul S. Sarbanes Ecosystem Restoration Project. Restor. Ecol. 22, 815–823. doi: 10.1111/rec.12147
Seigel, R. A. (1980a). Predation by Raccoons on Diamondback Terrapins, Malaclemys terrapin tequesta. J. Herpetol. 14, 87–89. doi: 10.2307/1563885
Seigel, R. A. (1980b). Courtship and mating behavior of the Diamondback Terrapin Malaclemys terrapin tequesta. J. Herpetol. 14, 420–421. doi: 10.2307/1563703
Seigel, R. A. (1980c). Nesting habits of Diamondback Terrapins (Malaclemys terrapin) on the Atlantic coast of Florida. Trans. Kans. Acad. Sci. 83, 239–246. doi: 10.2307/3628414
Seigel, R. A. (1984). “Parameters of two populations of Diamondback Terrapins (Malaclemys terrapin) on the Atlantic coast of Florida,” in Vertebrate Ecology and Systematics: a Tribute to Henry, eds S. Fitch, R. A. Seigel, L. E. Hunt, J. L. Knight, L. Malaret, and N. L. Zuschlag (Lawrence, Kansas, USA: Museum of Natural History, The University of Kansas), 77–87.
Seigel, R. A. (1993). Apparent long-term decline in Diamondback Terrapin populations at the Kennedy Space Center, Florida. Herpetol. Rev. 24, 102–103.
Stiglic, G., Kocbek, S., Pernek, I., and Kokol, P. (2012). Comprehensive decision tree models in bioinformatics. PLoS One 7:e33812. doi: 10.1371/journal.pone.0033812
Wibbels, T., Roberge, T., and Place, A. R. (2018). “Temperature-dependent sex determination,” in Ecology and Conservation of the Diamond-backed Terrapin, eds W. M. Roosenburg and V. S. Kennedy (Baltimore, USA: Johns Hopkins University Press), 127–145.
Williams, A. A., Eastman, S. F., Eash-Loucks, W. E., Kimball, M. E., Lehmann, M. L., and Parker, J. D. (2014). Record northernmost endemic mangroves on the United States Atlantic coast with a note on latitudinal migration. Southeast. Nat. 13, 56–63. doi: 10.1656/058.013.0104
Keywords: Malaclemys terrapin, nesting, nest predation, population, predators, terrapin, turtle
Citation: Butler JA, Lambert JD, DeDeo M and Murphy DP (2022) Population and Nesting Site Evidence for Diamondback Terrapins, Malaclemys terrapin, in Northeast Florida. Front. Ecol. Evol. 10:833199. doi: 10.3389/fevo.2022.833199
Received: 10 December 2021; Accepted: 21 February 2022;
Published: 15 March 2022.
Edited by:
J. Sean Doody, University of South Florida, United StatesReviewed by:
Russell L. Burke, Hofstra University, United StatesKristen Marie Hart, United States Geological Survey (USGS), United States
Copyright © 2022 Butler, Lambert, DeDeo and Murphy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joseph A. Butler, jbutler@unf.edu
 Joseph A. Butler
Joseph A. Butler J. David Lambert2
J. David Lambert2  Michelle DeDeo
Michelle DeDeo Daniel P. Murphy
Daniel P. Murphy