The Evolution of the Spiracular Region From Jawless Fishes to Tetrapods
- 1Key Laboratory of Vertebrate Evolution and Human Origins of Chinese Academy of Sciences, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing, China
- 2Center for Excellence in Life and Paleoenvironment, Beijing, China
- 3College of Earth and Planetary Sciences, University of Chinese Academy of Sciences, Beijing, China
- 4Department of Organismal Biology, Uppsala University, Uppsala, Sweden
- 5Bristol Palaeobiology Group, School of Earth Sciences, University of Bristol, Bristol, United Kingdom
The spiracular region, comprising the hyomandibular pouch together with the mandibular and hyoid arches, has a complex evolutionary history. In living vertebrates, the embryonic hyomandibular pouch may disappear in the adult, develop into a small opening between the palatoquadrate and hyomandibula containing a single gill-like pseudobranch, or create a middle ear cavity, but it never develops into a fully formed gill with two hemibranchs. The belief that a complete spiracular gill must be the ancestral condition led some 20th century researchers to search for such a gill between the mandibular and hyoid arches in early jawed vertebrates. This hypothesized ancestral state was named the aphetohyoidean condition, but so far it has not been verified in any fossil; supposed examples, such as in the acanthodian Acanthodes and symmoriid chondrichthyans, have been reinterpreted and discounted. Here we present the first confirmed example of a complete spiracular gill in any vertebrate, in the galeaspid (jawless stem gnathostome) Shuyu. Comparisons with two other groups of jawless stem gnathostomes, osteostracans and heterostracans, indicate that they also probably possessed full-sized spiracular gills and that this condition may thus be primitive for the gnathostome stem group. This contrasts with the living jawless cyclostomes, in which the mandibular and hyoid arches are strongly modified and the hyomandibular pouch is lost in the adult. While no truly aphetohyoidean spiracular gill has been found in any jawed vertebrate, the recently reported presence in acanthodians of two pseudobranchs suggests a two-step evolutionary process whereby initial miniaturization of the spiracular gill was followed, independently in chondrichthyans and osteichthyans, by the loss of the anterior pseudobranch. On the basis of these findings we present an overview of spiracular evolution among vertebrates.
Introduction
The origin of the vertebrate spiracle is a major 100-year-old unresolved mystery in vertebrate evolution since the German morphologist Carl Gegenbaur proposed the classic segmentation theory of the vertebrate head (Gegenbaur, 1872). An external dorsal opening with a tube extending to the oro-pharyngeal cavity, known as the spiracle, exists between the mandibular and hyoid arches in most extant sharks (Figure 1B), all rays except mantas (Figures 1C,D), and in some primitive bony fishes (sturgeons, paddlefishes and bichirs) (Figures 1E–H; Bone and Moore, 2008; Holland and Long, 2009; Kardong, 2012; Graham et al., 2014; Ziermann et al., 2019). It originates in the embryo as a pharyngeal pouch (the hyomandibular pouch) between two visceral arches, very much like the more posterior gill slits, but the adult condition is distinctively different from the normal gills. The spiracle is restricted to the dorsal half of the arches, lodged between the palatoquadrate (mandibular arch) and hyomandibula (hyoid arch), and never extends ventral to the jaw joint. It contains a small gill-like structure known as the pseudobranch, which differs from a normal gill in two respects. Firstly, while a normal gill slit between two gill arches contains two half-gills or hemibranchs, one attached to the anterior gill arch and one to the posterior gill arch, the spiracle contains only a single pseudobranch attached to the posterior wall. Secondly, while the normal gills all receive deoxygenated blood, the pseudobranch receives already oxygenated blood from the anteriormost normal gill. The function of the pseudobranch has been debated extensively but somewhat inconclusively; it may have a role in supplementing the oxygen supply to the eye (Möhlich et al., 2009; Perry et al., 2011; Waser, 2011). Although the spiracle has vanished altogether in some sharks (great whites, makos, hammerheads) and in most living ray-finned bony fishes, they still retain the pseudobranch.
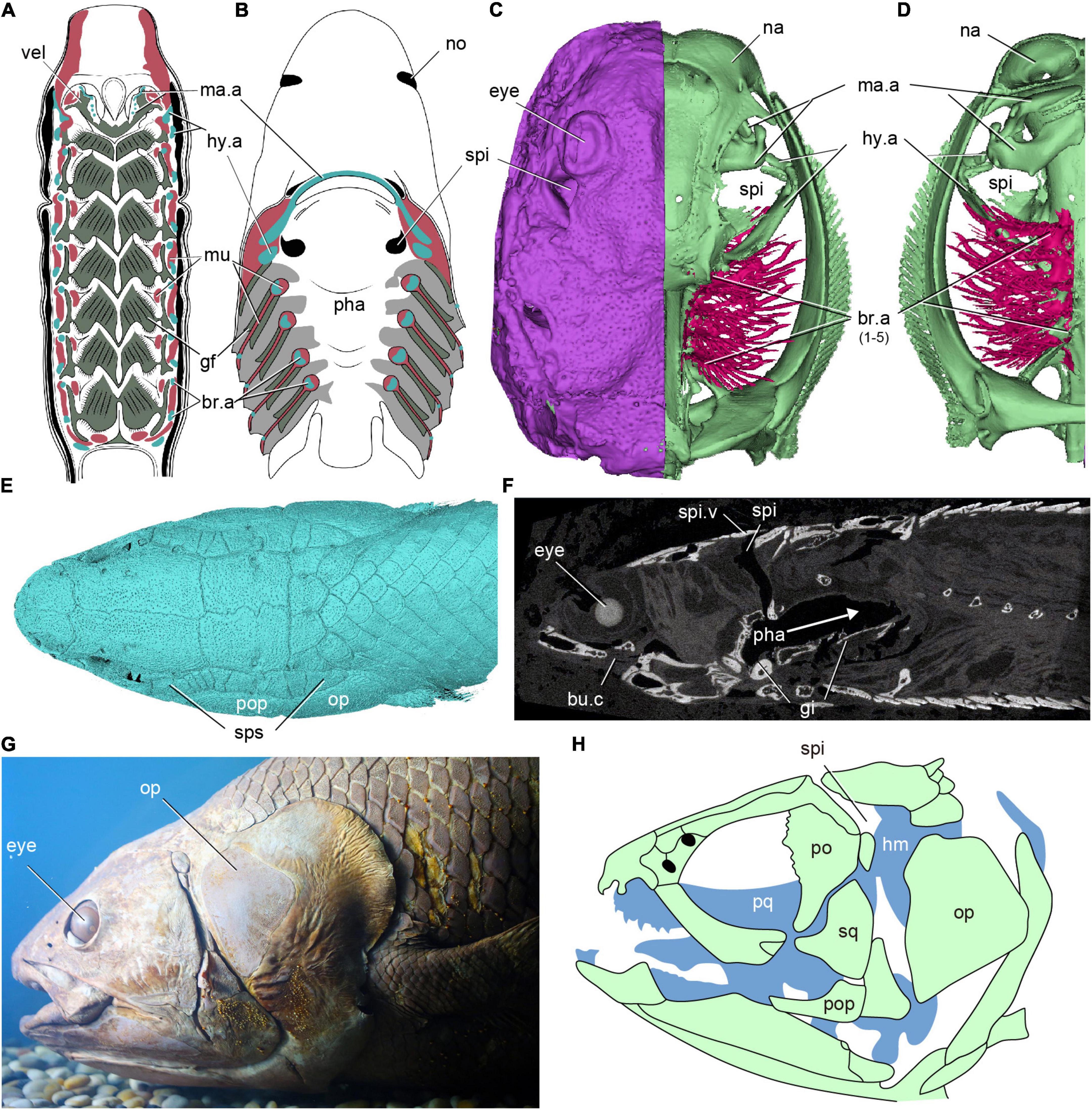
Figure 1. The distribution of spiracles in extant fishes. (A) Dorsal half of a horizontally sectioned lamprey (Petromyzon marinus) ammocoete larva, in ventral view, showing no branchial chambers between mandibular (velum) and hyoid arch. (B) Dorsal half of a horizontally sectioned dogfish (Squalus acanthias), in ventral view, showing a ventral opening of the spiracular tube on the palate between mandibular (jaw) and hyoid arch. (C,D) The 3D reconstruction of a modern ray, showing a spiracle between the mandibular and hyoid arches, (C) in dorsal view, panel (D) in ventral view. (E,F) 3D reconstruction (E) and a sagittal Micro CT image (F) of a polypterid fish Erpetoichthys calabaricus, showing the path (arrows) of air through the spiracles to the buccopharyngeal chamber and lungs. (G,H) Photo (G) and skeleton (H) of the head of an African coelacanth Latimeria chalumnae, in lateral view, showing a non-functional internal spiracular cavity but no external opening. [(A) redraw from Gaskell (1908), (B) redraw from Mallatt (1984), (G) photo from the specimen IVPP OP392, (H) redraw from Forey (1998)] br.a, branchial arch; bu.c, buccal chamber; gi, gill; gf, gill filaments; hy.a, hyoid arch; hm, hyomandubular; ma.a, mandibular arch; mu, muscle; na, nasal sacs; no, nostril; op, opercle; pha, pharynx; po, postorbital; pop, preopercle; pq, palatoquadrate; spi, spiracle; spi.v, spiracle valve; sps, spiracular series; vel, velum.
Compared with the posterior gill slits, the morphology and function of the spiracle are highly specialized. It is an inhalant opening for the influx of water in chondrichthyans (Hughes, 1960; Summers and Ferry-Graham, 2001) and air in Polypterus (Allis, 1922; Graham et al., 2014). In tetrapods, the spiracular pouch of the embryo gives rise to the middle ear cavity and the Eustachian tube, while the dorsal part of the embryonic hyoid arch gives rise to the stapes, which is either the sole ear ossicle of the middle ear (in amphibians, reptiles and birds) or the innermost of three ossicles (in mammals). This suggests that the middle ear arose as a modification of the spiracle plus hyomandibula, but the relationship between the two is complicated and there is evidence that a middle ear adapted for amplifying airborne sound has evolved more than once (Clack, 1993, 2002; Liem et al., 2001; Clack et al., 2003; Brazeau and Ahlberg, 2006).
The origin of the spiracle is a major evolutionary puzzle and cannot be fully resolved on the basis of evidence from living vertebrates. Spiracular pouches are present in all vertebrate embryos. However, in the jawless cyclostomes (hagfishes and lampreys)—which, as the sister group of total-group gnathostomes, might be expected to show an informative and potentially ancestral outgroup condition—the adult morphology never includes a spiracular gill slit. Although a hyomandibular pouch exists between the mandibular and hyoid arches in the lamprey embryo, it disappears early in development and never develops into a normal gill pouch (Janvier, 1996b; Miyashita and Diogo, 2016). In adult lampreys, the hyoid arch is closely associated with the mandibular arch (velar skeleton) without a gill slit between them (Figure 1A; Gaskell, 1908; Janvier, 1996b). The glossopharyngeal nerve is thought to innervate the gill muscles of the first branchial arch, and the vagus nerve innervates those of the remaining arches and viscera (Johnston, 1905), whereas the facial nerve (VII) has no branchial component (Johnston, 1905; Jefferies, 1986; Kuratani et al., 1997). In hagfishes, the hyomandibular pouch and the following two gill pouches, degenerate during embryonic development (Stockard, 1906; Holmgren, 1946). In adult hagfishes, the facial nerve is rather small and does not participate in the sensory innervation, but innervates several muscles associated with the basal cartilage (Wicht and Tusch, 1998). Therefore, none of the cyclostome gill pouches can be regarded as homologous with the gnathostome spiracle.
The adult condition of the anterior pharyngeal region of gnathostomes is arguably less modified from its embryonic beginnings than that of cyclostomes, insofar as the mandibular and (especially) the hyoid arches somewhat resemble branchial arches in shape, and the spiracular pouch often persists in some form, whether as a spiracle or a middle ear. The fact that the mandibular and hyoid arches, and the spiracular pouch between them, appear modified relative to the iterative pattern of the more posterior branchial arches and pouches, led researchers to propose that the ancestral condition for the pharynx was a complete series of gill arches separated by gill slits. The so-called “aphetohyoidean” theory speculated that the hyoid arch did not initially attach to or support the mandibular arch, and that a fully open (i.e., gill-like) cleft existed between the mandibular and hyoid arches in early gnathostomes (Gegenbaur, 1872; Watson, 1937; Janvier, 1996b). However, after a century of careful scrutiny, the search for a prehyoidean gill cleft (aphetohyoidean condition) in early jawed vertebrates such as placoderms, acanthodians, and Chondrichthyes has so far been unsuccessful (Watson, 1937; Zangerl and Williams, 1975; Maisey, 1986; Johanson, 1998; Frey et al., 2020).
In recent years, the impact of molecular phylogenies has resulted in a robust phylogenetic consensus that cyclostomes are a clade (Heimberg et al., 2010), not paraphyletic with hagfishes as the sister group to lampreys plus gnathostomes (Forey, 1995; Janvier, 1996a,2001; Donoghue et al., 2000; Donoghue and Smith, 2001). This new consensus also places all of the extinct bony jawless vertebrates, or “ostracoderms,” with the possible exception of some anaspids, in the jawed vertebrate stem group (Miyashita et al., 2021). This gives them potential to be uniquely informative about the origin of the jawed vertebrate spiracle, as they can be expected not to possess the confusing autapomorphies of cyclostomes. Galeaspids, a 435–370-million-year-old “ostracoderm” group from China and Vietnam, are regarded as the sister group of osteostracans plus jawed vertebrates. Osteostracans have a highly specialized cranial anatomy with an anteriorized pharynx (Kuratani and Ahlberg, 2018), but galeaspids lack these specializations and appear to provide key insights into the reorganization of the vertebrate head before the evolutionary origin of jaws (Gai et al., 2011, 2019; Gai and Zhu, 2012). Using synchrotron radiation X-ray tomographic microscopy of Shuyu and new material of gill filaments in galeaspids, here, we provide the first fossil evidence for a complete gill-functional hyomandibular pouch (aphetohyoidean condition) in stem-gnathostomes, an observation that sheds new light on the origin of the spiracle of jawed vertebrates. Based on our new findings, the evolution of the vertebrate spiracular region is reviewed.
Materials and Methods
The material of Shuyu zhejiangensis investigated in this study includes a total of 28 specimens (IVPP V14334.1-28) collected from the Silurian of Zhejiang Province. Among these specimens, specimens V14334.1-7 are preserved with three-dimensional neurocrania suitable for Synchrotron X-ray Tomographic Microscopy (SRXTM) from which virtual endocasts may be derived, whereas specimens V14334.8-27 represent incomplete headshields or natural endocasts which are suitable for simple visual and optic microscopic examination, illustrated by digital photographs. These specimens are used to complement the description of the SRXTM data. In addition, the specimen of Polybranchiaspis liaojiaoshanensis IVPP V3027 and the new material of Laxaspis-like galeaspids from the Xishancun Formation of the Lower Devonian of Yunnan Province, were also investigated to illustrate the dorsal roof of the oralobranchial chamber and gill filaments in the hyomandibular branchial pouch for the first time. All specimens are housed in the collections of the Institute of Vertebrate Paleontology and Paleoanthropology (IVPP), Chinese Academy of Sciences, Beijing, China.
Our SRXTM investigations were performed at the X02DA TOMCAT beamline of the Swiss Light Source at the Paul Scherrer Institute (Villigen, Switzerland). Slice data derived from the scans were then analyzed and manipulated using AVIZO software1 for computed tomography on a Hewlett Packard Workstation with a 2-GHz Intel processor and 16 GB of RAM. For further details of the materials and methods refer to Gai (2018), Gai et al. (2019, 2011). Following best practice (Davies et al., 2017), the tomographic slice data, AVIZO models, and a stereolithographic model of virtual endocast of Shuyu zhejiangensis have been made openly available from the University of Bristol Data Repository: https://data.bris.ac.uk/data/dataset/p34vnx48p4772ouez5a1sfoqh, published as (Gai et al., 2019). Photographs and micrographs were obtained using a Canon EOS 5D Mark III camera coupled with a Canon EF 100 mm 1:2.8 L macro photo lens for the general morphology as well as a Canon MP-E 65 mm 1:2.8 1–5 × macro photo lens for close-up images of gill filaments in the hyomandibular branchial pouch.
Results
Our new evidence indicates that the so-called interbranchial ridges of galeaspids are actually the dorsal portion of branchial arches (Gai, 2011; Gai et al., 2011; Figures 2A–F, 3A–C, 4A). They are incorporated into the neurocranium to form a massive skull as assumed in osteostracans by Stensiö (Stensiö, 1927, 1964). Compared to osteostracans, the entire branchial apparatus in Shuyu retains a general vertebrate condition, thus, it is easy to identify the mandibular and hyoid arches according to their topological position and nerve innervation (Figures 2A–F, 3A–C, 4A). Like most of gnathostomes, galeaspids primitively possess a mandibular, hyoid, and five gill arches (ma.a, hy.a, br.a1–5, Figure 4A). Topologically, the mandibular arch (ma.a, Figures 2A–F, 3A–C, 4A) is located directly behind the orbital cavity and formed the posterior wall of orbital cavity. There is a broad and shallow fossa (vel.f, Figures 2A,C,E, 3B, 4A) rostral to the mandibular arch. The fossa slants anteriorly in a bigger angle than the posterior six branchial fossae and is obviously differentiated from the posterior six branchial fossae. Gai et al. (2011) interpreted this fossa as containing a velum like that of lampreys, since it is supported by the mandibular arch. The identification of the mandibular arch and velar fossa is also corroborated by its nerve innervation. In specimens V14334.13B (Figure 3A) and V14334.3, 4 (Figures 2B–D), the whole path of trigeminal nerve canal can be traced continuously. The canals for the maxillo-mandibular branch or nasopalatine component of the trigeminal nerve (V2,3) arises from the junction of the mesencephalic and metencephalic division anteroventrally (Figure 2D). It extends forward into a large cavity for the trigeminal chamber and leads to the velar fossa. The presence of a velum in galeaspids is also supported by a large canal separates from the marginal artery and penetrates the roof of velar fossa (vj.v, Figures 2G,H; Gai, 2011; Gai et al., 2019). This indicates that a strong muscle probably for the velum was attached in this fossa. In addition, the whole oralobranchial chamber of galeaspids is closed posteriorly by a complete postbranchial wall (Wang, 1991; Janvier et al., 2009; Gai et al., 2022), which precludes tidal ventilation by the hypobranchial muscle. Therefore, a velum must have been present in galeaspids to provide ventilation from the oronasal cavity to the gill pouches as in osteostracans (Janvier, 1996b).
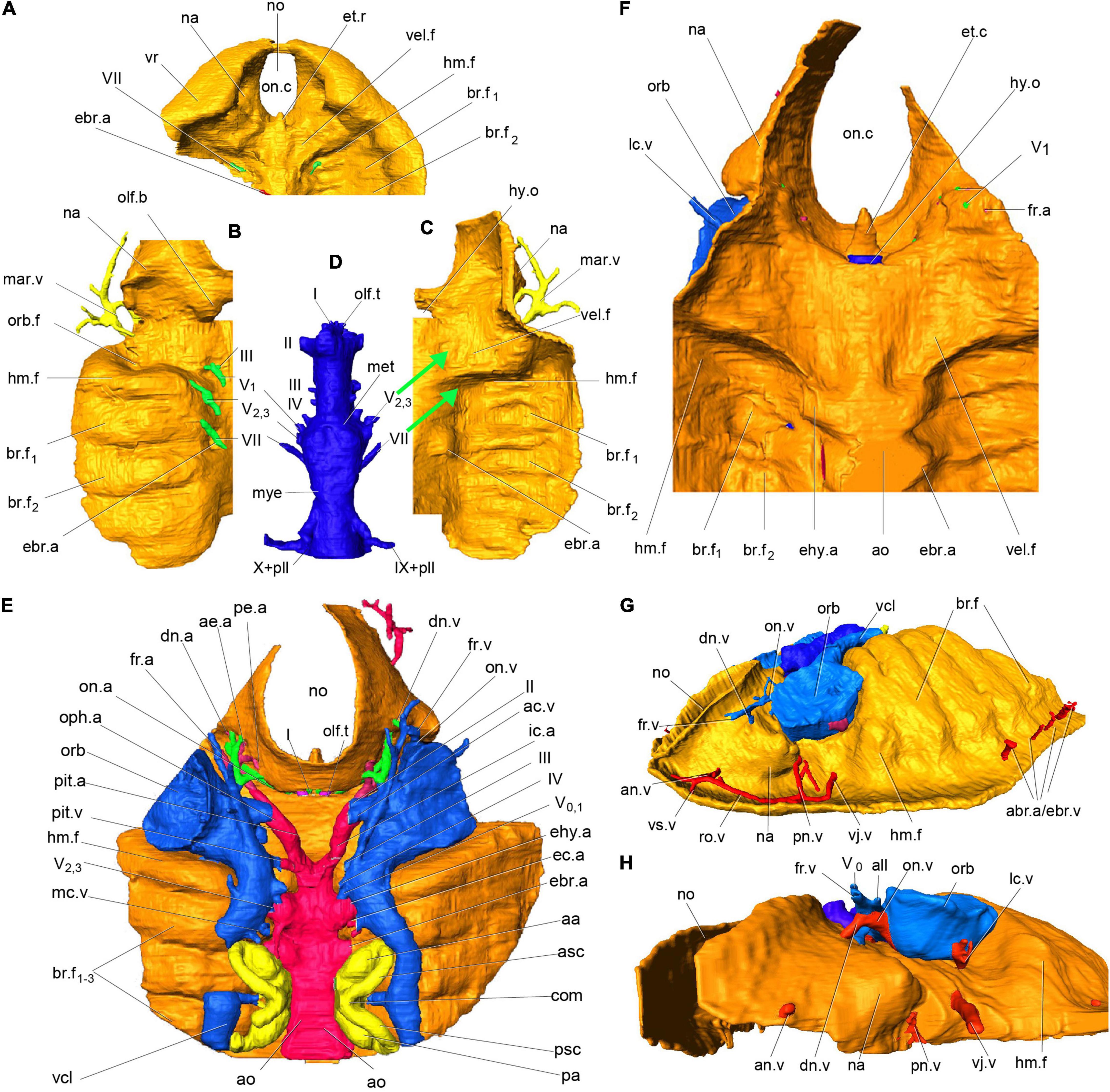
Figure 2. Virtual endocast and oralobranchial chamber of Shuyu zhejiangensis and its associated cranial nerve and blood vessels. (A) 3D reconstruction of oralobranchial chamber, anterior part, in ventral view, V 14334.2. (B,C) 3D reconstruction of an incomplete oralobranchial chamber, left half part, V 14334.4, in dorsal view, showing the nasopalatine component of trigeminal nerve (V2,3) innervates the first interbranchial ridge for the mandibular arch and the facial nerve (VII) innervates the second interbranchial ridge for the hyoid arch, (C) in ventral view, showing a large shallow fossa probably for a velum (vel.f) beneath the orbital cavity which obviously differentiates for the posterior six branchial fossae (br.f). (D–F) Endocast of skull reconstructed from the specimen V14334.3 (E) after removing the brain cavity and cranial nerve (D), in dorsal view; and anterior part of the oralobranchial chamber (F), in ventral view, showing a large shallow fossa probably for a velum (vel.f) beneath the orbital cavity which obviously differentiates for the posterior six branchial fossae (br.f). (G) Virtual endocast of specimen V14334.2, in antero-lateral view from the left side. (H) Virtual endocast of specimen V14334.1, in antero-lateral view from the left side. (A–H) The thickness of golden material is meaningless. aa, anterior ampulla; abr.a, afferent branchial artery; ac.v, anterior cerebral vein; ae.a, anterior ethmoidal artery; al.a, anterior labyrinth artery; all, anterior lateral line nerve; al.v, anterior labyrinth vein; an.v, anterior nasal vein; ao, dorsal aorta; asc, anterior semicircular canal; br.f, branchial fossae; com, commissural division of two vertical semicircular canals; dn.a, dorsal nasal artery; dn.v, dorsal nasal vein; ebr.a, efferent branchial artery; ec.a, external carotid artery; ehy.a, efferent hyoid artery; et.r, ethmoid rod; fr.a, frontal artery; fr.v, frontal vein; hm.f, hyomandibular fossa; hy.o, hypophysial opening; ic.a, internal carotid artery; mar.v, marginal vein; mc.a, middle cerebral artery; mc.v, middle cerebral vein; met, metencephalic division; mye, myelencephalic division; na, nasal sacs; no, nostril; lab.v, labyrinth vein; lc.v, lateral capitis vein; olf.t, olfactory tract; on.a, orbitonasal artery; oph.a, ophthalmic artery; orb, orbital opening; orb.f, orbital fossa; olf.b, olfactory bulb; on.c, oronasal chamber; on.v, orbitonasal vein; pa, posterior ampulla; pe.a, posterior ethmoidal artery; pll, posterior lateral line nerve; pl.v, posterior labyrinth vein; pi, pineal organ; pit.a, pituitary artery; pit.v, pituitary vein; pn.v, posterior nasal vein; psc, posterior semicircular canals; ro.v, rostral vein; vcl, lateral head vein; vel.f, velar fossa; vj.v, ventral jugular vein; vr, ventral rim; I, olfactory nerve; II, optic nerve; III, oculomotor nerve; IV, trochlear nerve; V0, superficial ophthalmic branch of trigeminal nerve; V1, deep ophthalmic or profundus branch of trigeminal nerve; V2,3, nasopalatine component or maxillo-mandibular nerve; VII, facial nerve; VIII, auditory nerve; IX, glossopharyngeal nerve; X, vagus nerve.

Figure 3. Natural casts of oralobranchial chamber in galeaspids. (A) Natural cast of oralobranchial chamber of Shuyu zhejiangensis, specimen V14334.13B, showing a complete hyomandibular fossa (hm.f), four branchial fossae (br.f), paired grooves for dorsal aorta (ao), a middle ridge for the notochord (nt), the nasopalatine component of trigeminal nerve (V2,3) innervates the first interbranchial ridge for the mandibular arch (ma.a) and the facial nerve (VII) innervates the second interbranchial ridge for the hyoid arch. (B) Natural cast of oralobranchial chamber of Polybranchiaspis liaojiaoshanensis, specimen V3027, in ventral view, showing a large shallow differentiated fossa (vel.f) probably for a velum anterior to the first branchial fossa. (C–E) Natural cast of oralobranchial chamber (C) of a Laxaspis-like galeasids from the Lower Devonian of Qujing showing the impression of gill filaments (D,E) in the hyomandibular pouch (D). abbreviations as in Figures 1, 2.
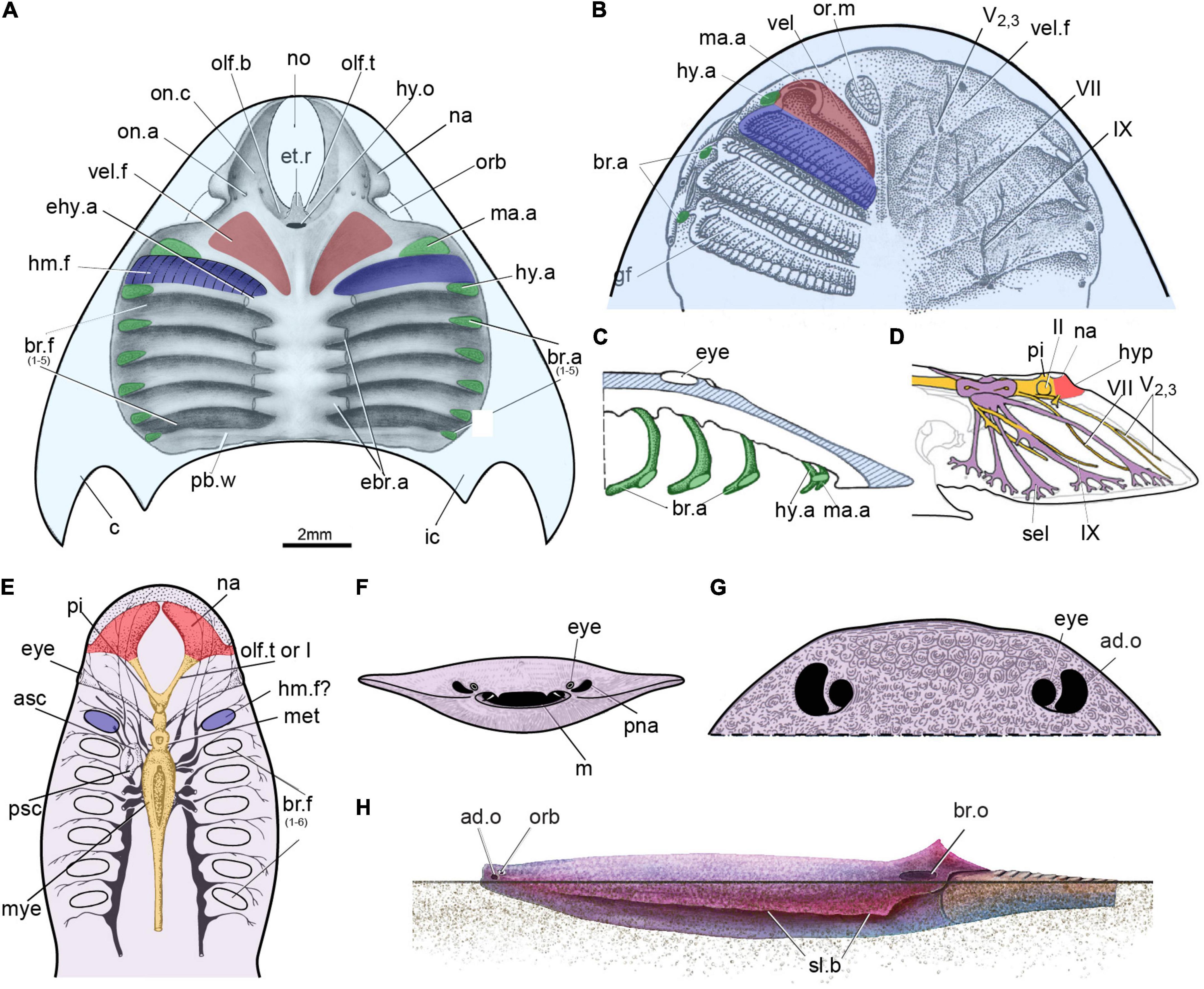
Figure 4. The possible hyomandibular pouch in the stem-gnathostomes. (A) Roof of the oralobranchial cavity of Shuyu from the Silurian of China. (B–D) Roof of the oralobranchial cavity of Scolenaspis from the Early Devonian of Spitsbergen, with velum, gill arch, and gills reconstructed on left-hand side, panel (B) in ventral view, panel (C) in sagittal view with attempted restoration of gill-arch elements; (D) cranial anatomy of osteostracan Norselaspis, composite reconstruction based on Janvier (1985), showing the mandibular branch of trigeminal nerve passed through the foremost canal, together with the maxillary branch. (E) Reconstruction of the internal structure of the heterostracan Poraspis pompeckji Brotzen (redrawn after Novitskaya, 1983). (F,G) An infraorbital opening in a infraorbital position in an amphiaspid heterostracan Gabreyaspis from the Early Devonian of Siberia, was interpreted as a displaced opening of the prenasal sinus (F) (Janvier, 1974), or an adorbital opening (G) (Janvier, 1996b) probably with an inhalent function for intake of clear water. (H) Amphiaspid heterostracan Kureykaspis using its adorbital opening for the intake of respiratory water when buried in sand. ad.o, adorbital opening; br.o, branchial opening; hyp, hypophysis; m, mouth; or.m, oral musculure; pna, prenasal sina; “sel” canal to the lateral field; sl.b, serrated lateral brim; other abbreviations as in Figures 1, 2.
The hyoid arch, which is located just between the mandibular arch and labyrinth cavity (hy.a, Figures 2C, 3A, 4A), did not initially attach to or support the mandibular arch (aphetohyoidean condition). In specimens V1433.13B (Figure 3A), V14334.2 (Figure 2A), V14334.3 (Figures 2D,F), and V14334.4 (Figure 2B), the canal for the facial nerve (VII, Figures 2A,B,D,F, 3A), which can be traced continuously, leaves the myelencephalic cavity through an individual canal ventral to the labyrinth cavity and extends anteroventrally to the hyoid arch. Therefore, the first branchial chamber just posterior to the orbital opening can be interpreted as the hyomandibular pouch (hm.f, Figures 2A–H, 3A–D, 4A) in galeaspids. The dorsal aortic canals collected the oxygenated blood from the branchial chambers through five successive short transverse canals for the efferent branchial arteries (ehy.a, ebr.a1–4, Figures 2C,E,F, 3A,C, 4A), which is just located between the two adjacent branchial fossae (Gai et al., 2019). The first efferent branchial canal is relatively large and leads to the hyomandibular pouch at an anteriorly inclined angle, thus probably contains the efferent hyoid artery (ehy.a, Figures 2C,E,F, 4A). The remaining four efferent branchial canals (ebr.a 1–4, Figures 2C,E,F, 3A,C, 4A) extend transversely to their respective branchial fossae. No canal for the pseudobranchial artery is observed. Our new data indicates that the hyomandibular pouch is complete and indistinguishable from the other five branchial pouches, and probably serves as a normal branchial chamber, which housed the posterior hemibranch of the mandibular arch and the anterior hemibranch of the hyoid arch. This observation has been corroborated by the impressions of the gill filaments (gf, Figures 3C–E) in the first branchial chamber for the hyomandibular pouch (hm.f, Figures 3C–E) just posterior to the orbital opening in our new material of Laxaspis-like galeaspids from the Xishancun Formation of the Lower Devonian of Yunnan Province. So it is apparent that galeaspids possess a fully gill-functioned hyomandibular pouch (aphetohyoidean condition), rather than a reduced spiracle of jawed vertebrates.
Discussion
The Origin of a Functional Spiracular Gill in “Ostracoderms”
The presence of the hyomandibular pouch in other “ostracoderms” still remains controversial (Stensiö, 1927, 1964; Halstead, 1971; Janvier, 1996b,2004; Mallatt, 1996). In heterostracans, the foremost gill compartment impression is always situated behind the eye, and is classically interpreted as the hyomandibular pouch (Figure 4E) (Stensiö, 1964; Novitskaya, 1983, 2015; Mallatt, 1996), though only on the basis of a topological argument (Janvier, 2004). By contrast, Halstead (Halstead, 1971, 1973) thought that a true spiracle has been present in some amphiaspid heterostracans, a group of cyathaspidiforms from the Lower Devonian of Siberia, which possess a pair of bean-shaped openings in the carapace immediately lateral to the eyes (Figures 4G,H), a position moreover exactly as in the living benthonic elasmobranchs. However, most authors think it unlikely that these paired openings are equivalent to the spiracles of gnathostomes (Burrow et al., 2020). They were later interpreted as either displaced openings of the prenasal sina (Figure 4F; Janvier, 1974), or adorbital openings (Figure 4G; Janvier, 1996b), probably with an inhalent function for intake of clear water since these amphiaspids were benthic animals (Janvier, 1996b). As the neurocranial anatomy of heterostracans cannot be reconstructed in detail, they will not be considered further here.
In osteostracans, the entire branchial apparatus uniquely shifted forward so that the first two branchial gill pouches lie anterior to the eyes (Figures 4B,C; Stensiö, 1927, 1964; Jarvik, 1980; Kuratani and Ahlberg, 2018). Therefore, it is difficult to determine the hierarchy of the branchial arches according to their topological positions. Mallatt (1996) considered that the foremost gill compartment was the hyomandibular pouch, whereas Janvier (Janvier, 1985, 1996b, 2007) thought that there is no reason to assume that they possessed a prespiracular or even a spiracular gill-pouch (Figures 4B,C) since the mandibular arch bore a velum as in lampreys. In osteostracans, the canal for the facial nerve fused with the trigeminal chamber in the postero-ventral part of orbital cavity (Janvier, 1981; trig, Figures 13–17). Thus, it still cannot be determined whether the mandibular branch of trigeminal nerve passed through the foremost canal, together with the maxillary branch (Figure 4D), or passed through the second canal, together with the facial nerve (Figure 4B). Janvier preferred the latter interpretation, thus the mandibular arch and the hyoid arch of osteostracans are probably closely associated or perhaps fused into a single arch (Figures 4B,C), and a hyomandibular pouch is absent in osteostracans as that of adult lampreys (Janvier, 1981, 1996b). However, Kuratani and Ahlberg (2018) preferred the first interpretation and the hyomandibular pouch of osteostracans appears to have been developed into a normal full-size gill as in galeaspids. In Shuyu, the canals for the facial nerve and trigeminal nerve have entirely separate courses and there is no evidence for the trigeminal nerve bifurcating into the maxillary and mandibular branches. The condition in Shuyu suggests that the mandibular and the maxillary branch of osteostracans probably always united as the nasopalatine component of trigeminal nerve (V2,3, Figure 4D) and passed through the foremost canal together since there are no differentiated upper and lower jaws developed in these stem groups of gnathostomes. Therefore, osteostracans, probably like galeaspids, possess both the velum and a full-size hyomandibular pouch as well.
Various types of water intake device have evolved with the same function as the spiracles of modern rays in jawless fishes, e.g., the prenasal sinus and nasopharyngeal duct in hagfishes, thelodonts and heterostracans, the adorbital openings in amphiaspid heterostracans and pituriaspids (Figure 4H), and the median dorsal opening in galeaspids. These have been considered analogous, but not homologous, to the gnathostome spiracle (Gai et al., 2018). Thus, the hyomandibular pouch probably has been developed as a normal full-size gill pouch (aphetohyoidean condition) in ostracoderms. It has secondarily been lost in cyclostomes because of the evolution of the rasping tongue, which greatly modifies the mandibular arch. In sum, galeaspids provide the first definitive evidence for the presence of a full-size gill pouch between the hyoid and mandibular arches before it was reduced to a spiracle in jawed vertebrates. This observation is not only based on the number and topology of gill arches compared with that of jawed vertebrates, but also on the innervation of the cranial nerve and the impressions of the gill filaments in the first branchial chamber.
The Evolution of the Spiracle in Early Jawed Vertebrates
While the condition in galeaspids (and probably other ostracoderms) can be described as aphetohyoidean in the sense that a fully developed gill is present between the mandibular and hyoid arches, this does not quite correspond to the “classic” idea of an aphetohyoidean condition in jawed vertebrates, where a fully developed spiracular gill is combined with a mandibular arch developed into jaws. The galeaspid condition would make a perfect evolutionary precursor to the “classic aphetohyoidean” condition, but this would require the retention of the spiracular gill while the mandibular arch was transformed into jaws. Great efforts at finding the aphetohyoidean condition have been made in placoderms, acanthodians and chondrichthyans. Watson (1937) believed that this condition existed in acanthodians (e.g., Acanthodes, Figure 5A), and in placoderms such as arthrodires, petalichthyids and rhenanids, mainly based on the mistaken assumption that their operculum was attached to the mandibular arch, rather than to the hyoid arch as in bony fishes. As the endoskeletal neurocranium and visceral skeleton remain poorly known in early fossil jawed vertebrates, the presence of spiracles has typically been inferred from the spiracular grooves or notches on the cranial bones, as in actinopterygians (Gardiner, 1984; Graham et al., 2014) and sarcopterygians (Jarvik, 1980).
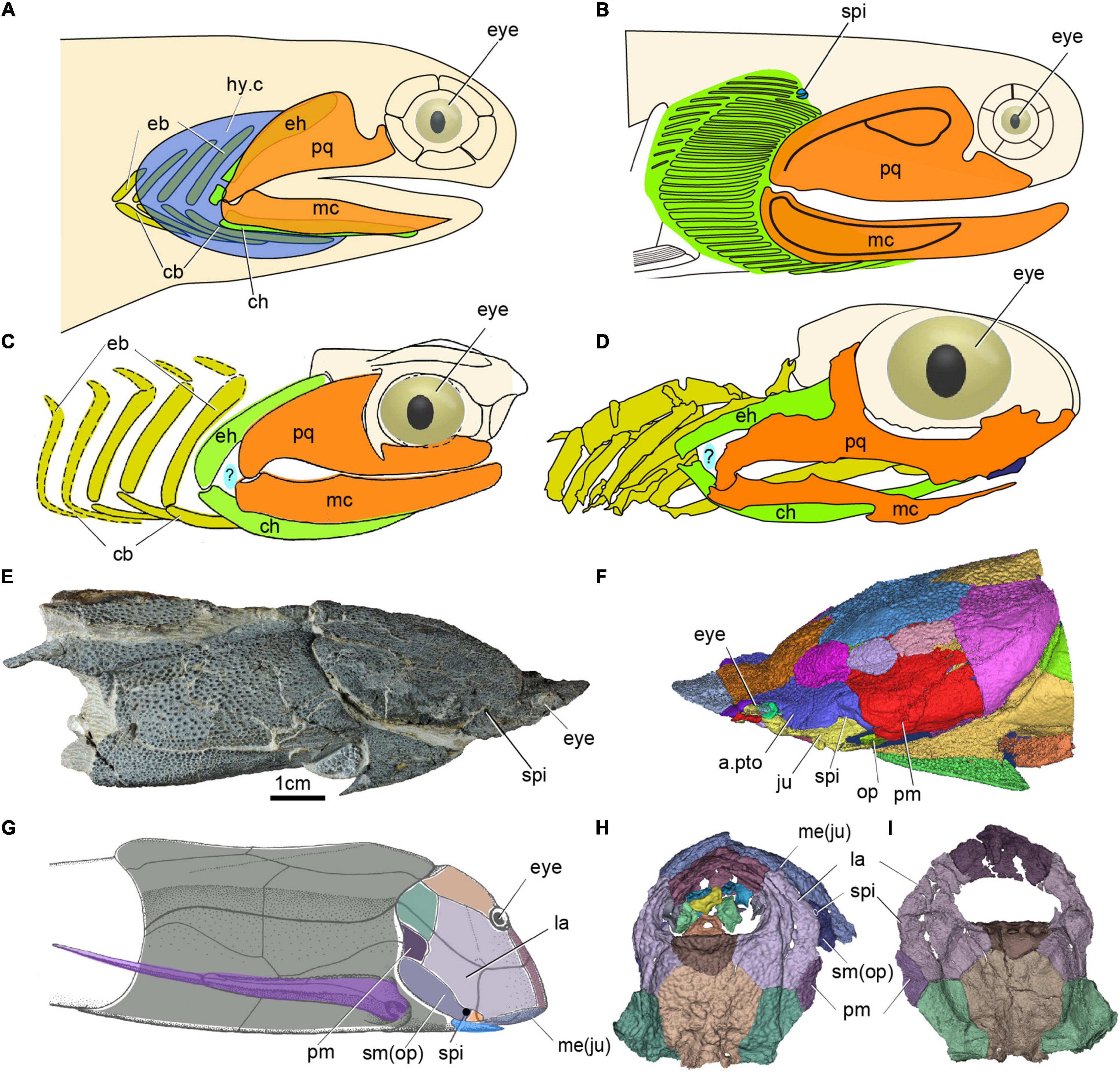
Figure 5. The spiracle in early jawed vertebrates. (A) Head and branchial region of Acanthodes redraw after (DeLaurier, 2018). (B) Head and branchial region of Cheiracanthus showing position of spiracle, redraw after (Burrow et al., 2020). (C) Head and branchial region of Cobelodus, redraw after (Zangerl and Case, 1976). (D) Head and branchial region of Ozarcus, redraw after (Pradel et al., 2014), panels (C,D) showing the possible aphetohyoidean condition, but a respiratory gill pore between its mandibular and hyoid arches is hypothetical (indicated by a question mark). (E,F) Photo (E) and 3D reconstruction (F) of the dermal skeleton of Qilinyu. (G) Dermal skeleton of Bothriolepis redraw after (Arsenault et al., 2004). (H,I) 3D reconstruction of the dermal skeleton of Parayunnanolepis, (E–I) showing a spiracle in placoderms, (A–G) in lateral view, (H) in dorsal view, (I) in ventral view. a.pto, anterior postorbital plate; cb, ceratobranchial; ch, ceratohyal; eb, epibranchial; eh, epihyal; hy.c, hyoid gill cove; hm, hyomandibular; ju, jugal; la, lateral plate; mc, Meckel’s cartilage; me, mental; op, opercular; pm, postmarginal plate; sm, submarginal plate, other abbreviations as in Figures 1, 2.
Among acanthodians, the visceral skeleton is only adequately known in Acanthodes from the Lower Permian of Lebach, Germany, a late representative of this derived genus that had to serve as an endoskeletal proxy for all acanthodians. However, the “aphetohyoidean” condition has been doubted by several authors (Holmgren, 1942; Stensiö, 1947; Denison, 1961). Miles (1964, 1965, 1968, 1973) has studied a large number of well-prepared specimens of Acanthodes bronni and Climatius reticulatus, but found no evidence for such an “aphetohyoidean” condition. By contrast, he found the spiracular grooves on the neurocranium in Acanthodes probably containing the spiracular tube. However, as there is no external spiracle opening observed from these tubes, such openings were presumed to be lacking in Acanthodes, rendering the spiracles non-functional. Recently, a very small external spiracular opening was described from the Middle Devonian acanthodiforms Cheiracanthus (Figure 5B) and Mesacanthus in northern Scotland (Burrow et al., 2020). Remarkably, unlike all living jawed vertebrates, these acanthodians prove to have not one but two spiracular pseudobranchs, represented in the fossils by their cartilage supports (Figure 5B). In addition to the posterior, forward-facing, hyoid arch pseudobranch shared with sharks, rays and bony fishes, there is an anterior, backward-facing pseudobranch that must belong to the mandibular arch (Burrow et al., 2020). The spiracle thus contained a complete, albeit miniaturized, gill. Acanthodians are now generally considered to be a group of stem chondrichthyans (Zhu et al., 2013, 2016; Dupret et al., 2014; Burrow et al., 2016; Maisey et al., 2019), which carries the implication that the mandibular-arch spiracular pseudobranch must have been lost independently in osteichthyans and crown-group chondrichthyans. Nevertheless, this is not a fully aphetohyoidean condition, as the gill is miniaturized and restricted to the dorsal space between the palatoquadrate and hyomandibula.
As for the aphetohyoidean condition in placoderms, fossil evidence since the time of Watson (1937) has convincingly demonstrated that the hyoid arch has been strongly modifìed in many placoderms, e.g., a larger hyomandibula in the Rhenanida (Stensiö, 1963), and suggests that they did not have a complete spiracular gill-slit. One of the evidences in support of Watson’s aphetohyoidean theory is that the postsuborbital plate in placoderms has been interpreted as a mandibular operculum guarding a spiracular slit, because of its relations to the quadrate (Watson, 1937). However, the discovery of Entelognathus, and new phylogenetic frameworks, indicate that the posterior mobile submarginal plate in non-Entelognathus placoderms should be considered homologous with the opercular cover of osteichthyans (Zhu et al., 2013; Gai et al., 2017). The submarginal is primitively free in ptyctodontids and primitive arthrodires, but it is enclosed in the cheek and sutured with surrounding bones in advanced arthrodires (Schultze, 1993). A reduced spiracle has been identified in a hyomandibular position in antiarchs (spi, Figures 5G–I; Stensiö, 1947; Johanson, 1998; Arsenault et al., 2004; Young, 2008; Wang and Zhu, 2020) —the most stemward jawed vertebrate group, and in the maxillate placoderm Qilinyu (spi, Figures 5E,F) — representing the most crownward stem gnathostome group (Zhu et al., 2016). Therefore, a restricted spiracular opening may well have existed in all placoderms, but it would be difficult to distinguish it from a gap between dermal elements in most of them (Miyashita, 2016).
The aphetohyoidean theory was later revived for some symmoriiform chondrichthyans, e.g., Cobelodus from Upper Carboniferous black shales of North America (Zangerl and Williams, 1975; Zangerl and Case, 1976), and Ozarcus from the Lower Carboniferous of Arkansas (Pradel et al., 2014). The Symmoriiformes, which were widely distributed from the Late Devonian through to the early Permian, have been regarded as exemplifying early chondrichthyan, and even generalized gnathostome, conditions (Pradel et al., 2011). The hyoid arch of Ozarcus and Cobelodus gives no support to the jaw, and there appears to be a rather wide space between the mandibular and hyoid arches (Figures 5C,D), which suggests that the prehyoidean gill slit had not been reduced to a spiracle in these sharks (Zangerl and Williams, 1975; Zangerl and Case, 1976; Pradel et al., 2014). However, in the earlier symmoriiform shark Ferromirum from the Late Devonian of the Anti-Atlas in Morocco, the three-dimensionally preserved mandibular and hyoid arches leave no room for a prehyoidean gill slit, implying that the apparent gap between mandibular and hyoid arches in Ozarcus and Cobelodus is most probably a post-mortem artifact (Frey et al., 2020). Recent phylogenetic analyses have resolved symmoriiforms as stem holocephalans (Coates et al., 2017, 2018; Frey et al., 2019, 2020), which suggest that they are too derived to represent the primitive jawed vertebrate condition.
In summary, no placoderm or chondrichthyan has yet been shown convincingly to possess a full, aphetohyoidean, spiracular gill slit. Given that osteichthyans also lack such a gill slit (see below), it seems likely that it had been reduced to a dorsally positioned spiracle in the last common ancestor of living jawed vertebrates, i.e., at the gnathostome crown group node. However, the presence of two pseudobranchs in acanthodians, which are believed to be stem chondrichthyans, suggests that full reduction to the modern spiracular condition was a two-step process: an initial size reduction and shift to a dorsal position in the common ancestor of crown gnathostomes was followed, independently in chondrichthyans and osteichthyans, by the loss of the anterior (mandibular arch) pseudobranch. This interpretation implies that two pseudobranchs were also present in placoderms, where no evidence of their attachment survives.
The Evolution of the Spiracle From Bony Fishes to Tetropods
Among bony fishes, the hyomandibular pouch and its derivatives can be observed directly in extant actinopterygians (polypterids, sturgeons and paddlefishes, gar and bowfin, and teleosts) and sarcoptergian fishes (coelacanths and lungfishes). In addition, reasonably robust inferences about its condition in the adult of extinct groups such as tetrapodomorph fishes can be drawn from the morphology of the surrounding skeletal structures of the fossils. Among actinopterygians, an open spiracle broadly similar to that of sharks is found in polypterids, sturgeons and paddlefishes. In Polypterus (Figure 6A), which has a functional lung and is heavily dependent on air-beathing, the large and dorsally positioned spiracle plays an important role in lung ventilation (Graham et al., 2014). Up to 93% of air inhalation occurs through the spiracle. This has important implications for the interpretation of the morphologically similar spiracular region in tetrapodomorph fishes (see below). By contrast, the small spiracles of sturgeons and paddlefishes seem to have no respiratory function (Burggren, 1978; Burggren and Bemis, 1991). In neopterygians (gars, bowfins and teleosts) the spiracles are vestigial or absent, although the pseudobranch often persists inside the gill chamber. Its function is uncertain; it seems not to regulate the hypoxia response (Perry et al., 2011), but may have a role in supplying oxygen to the retina of the eye (Möhlich et al., 2009; Waser, 2011).
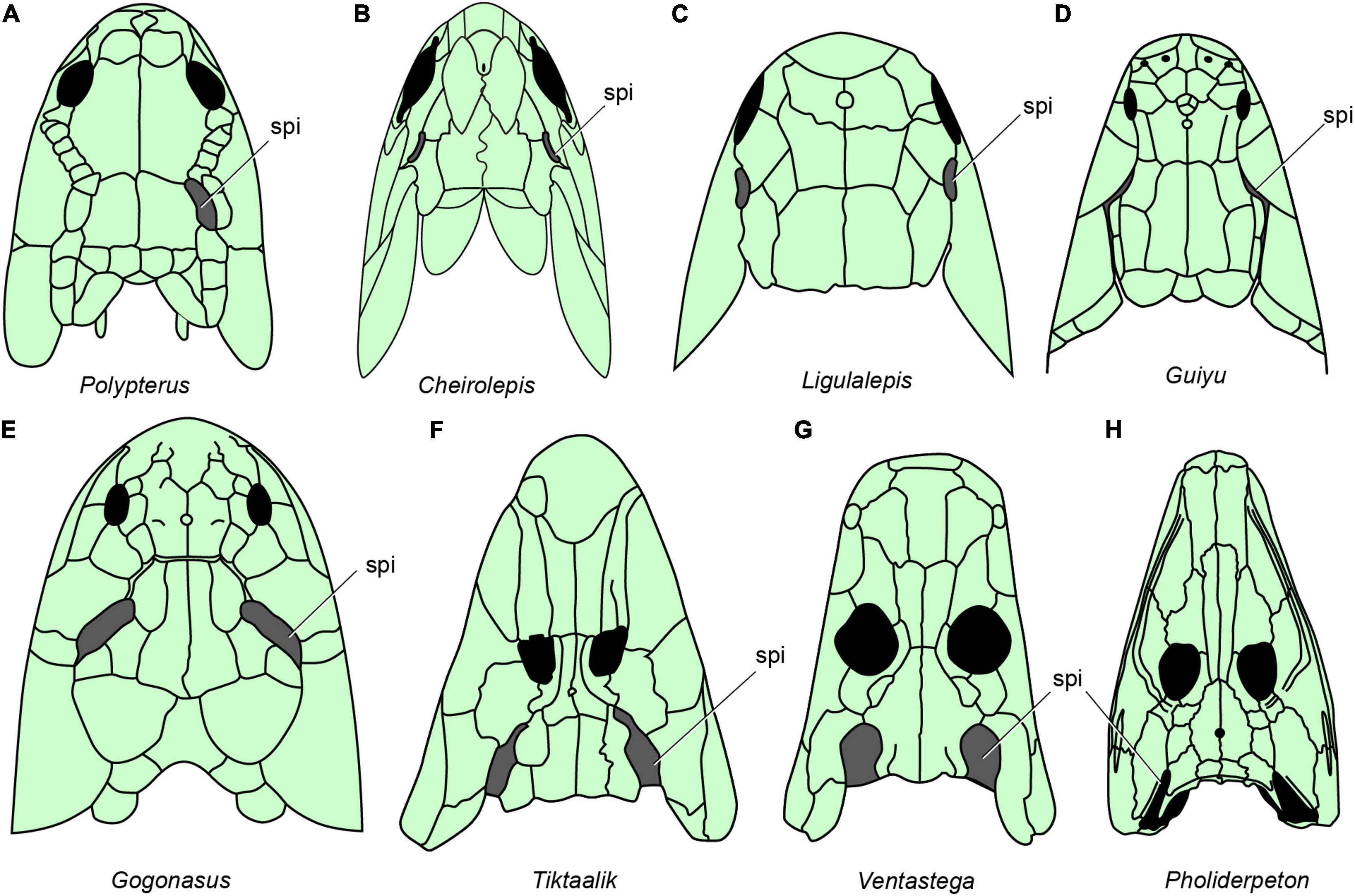
Figure 6. The distribution of spiracles in bony fishes and tetrapods. (A) Polypterus redraw after (Graham et al., 2014). (B) Cheirolepis redraw after (Pearson and Westoll, 1979). (C) Ligulalepis redraw after (Basden and Young, 2001). (D) Guiyu redraw after (Zhu et al., 2009). (E) Gogonasus redraw after (Long et al., 2006). (F) Tiktaalik redraw after (Daeschler et al., 2006). (G) Ventastega redraw after (Ahlberg et al., 2008). (H) Pholiderpeton redraw after (Clack, 2012), (A–H), in dorsal view.
Neither of the two living groups of sarcopterygian fishes has a functional spiracle. In the coelacanth Latimeria there is a spiracular chamber (Figure 1H), but this does not open to the outside (Figure 1G) and the pseudobranch is absent (Forey, 1998). In lungfishes, both spiracle and pseudobranch are lost. The spiracular rudiment consists largely of a solid endodermal cellular strand, which never (or only for short time in ontogeny) opens to the outside and the pharyngeal endoderm, respectively (Bartsch, 1994). By contrast, the early bony fishes such as Cheirolepis (stem actinopterygians, Figure 6B; Pearson and Westoll, 1979), Guiyu (stem sarcopterygian, Figure 6D; Zhu et al., 2009), Ligulalepis (stem osteichthyan, Figure 6C; Basden and Young, 2001), Onychodus (Onychodontiformes, Figure 7C; Long, 2001), Porolepis (porolepiformes, Figure 7B) and tetrapodomorph fishes (fossil fishes belonging to the tetrapod stem group) such as Eusthenopteron (Figures 7A, 8A; Jarvik, 1980) and Gogonasus (Figure 6E; Long et al., 2006) always have an open spiracle (spi, Figures 6, 7), judging by the surrounding skeletal anatomy. In Gogonasus the dorsally positioned spiracle is strikingly large (Figure 6E), suggesting an important respiratory role similar to that of Polypterus.
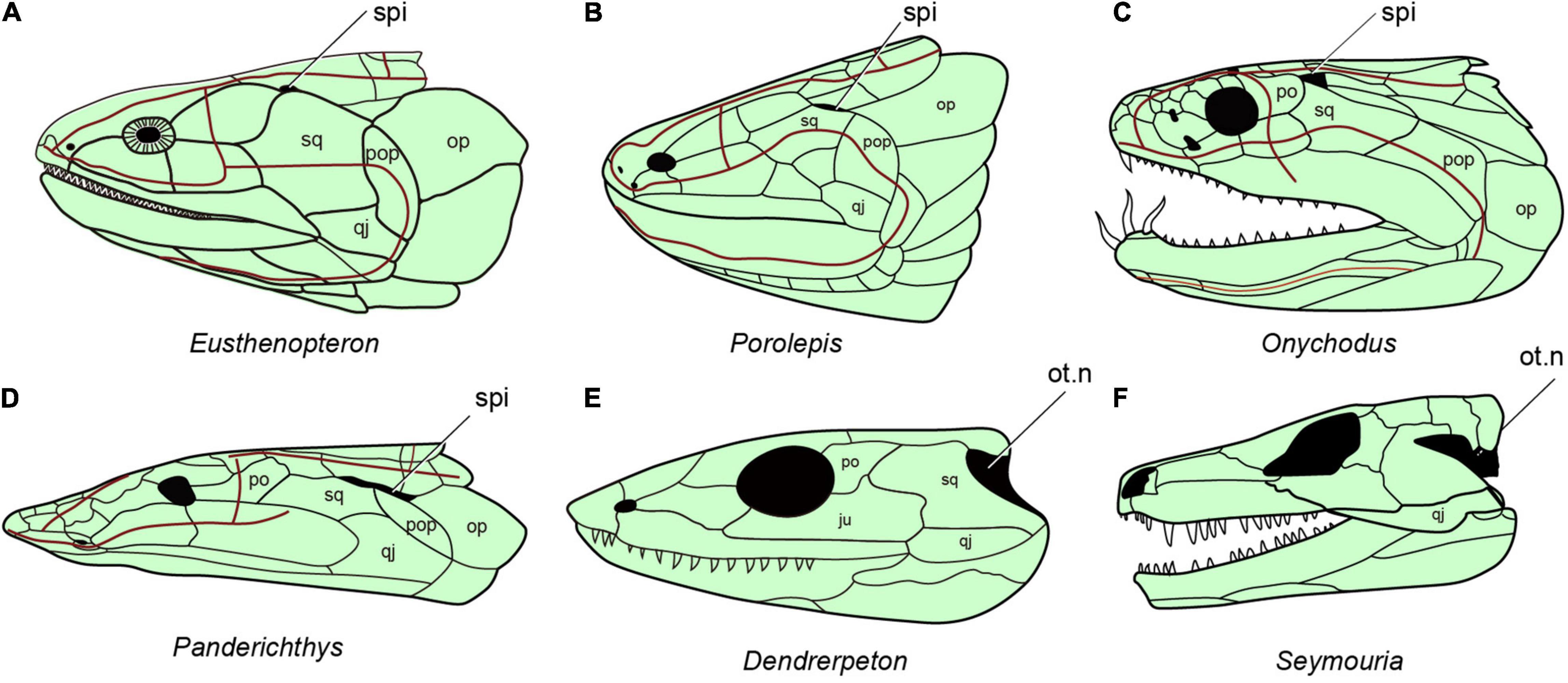
Figure 7. The distribution of spiracles in bony fishes and tetrapods. (A) Eusthenopteron, redraw after (Jarvik, 1980). (B) Porolepis redraw after (Janvier, 1996b). (C) Onychodus redraw after (Long, 2001). (D) Panderichthys redraw after (Vorobyeva and Schultze, 1991). (E) Carboniferous tetrapod Dendrerpeton redraw after (Carroll, 1967). (F) Seymouria redraw after (White, 1939), (A–F), in lateral view.
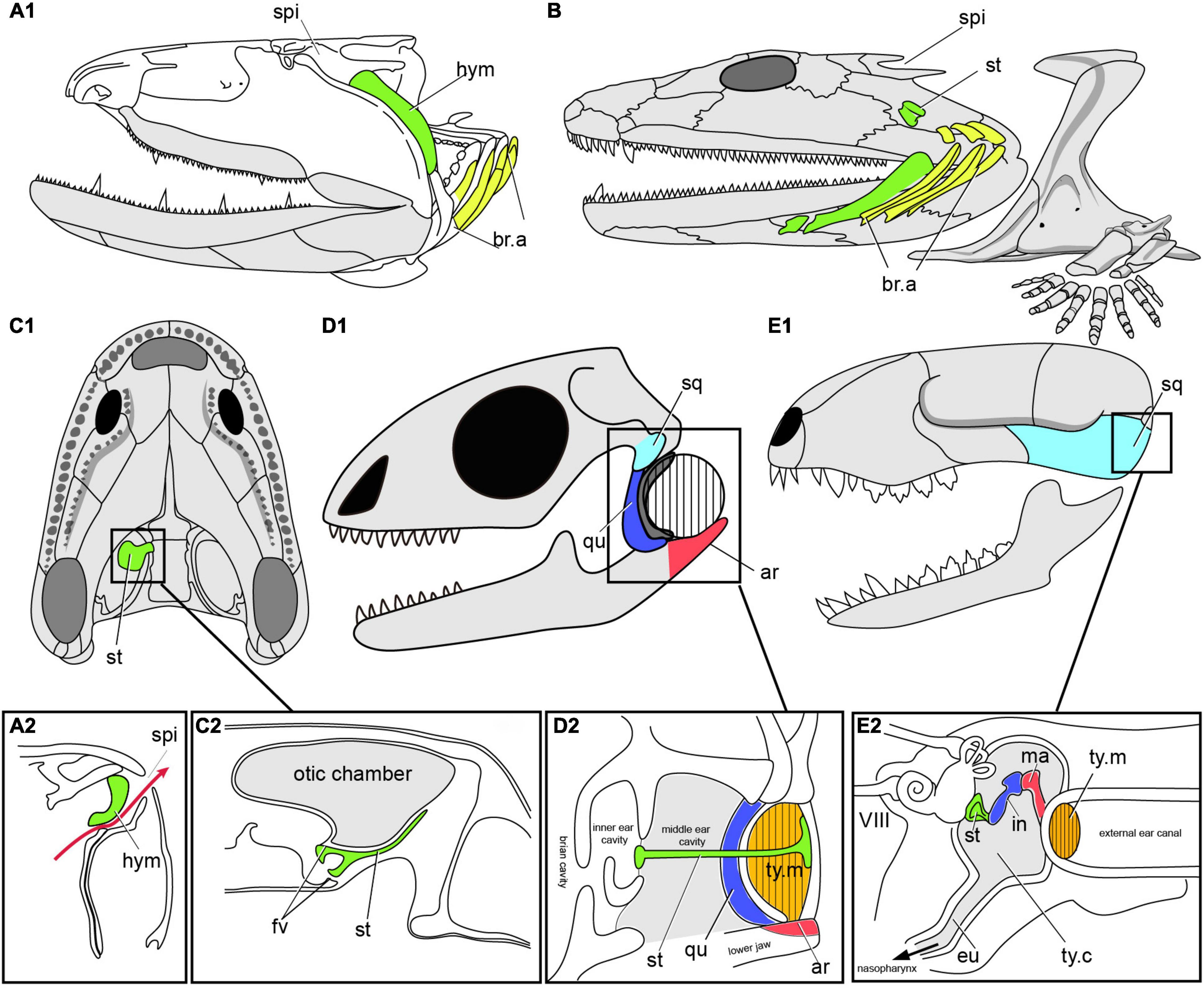
Figure 8. The transition from spiracular pouch of fishes to the middle ear cavity of tetrapods. (A) Fossil osteolepiform Eusthenopteron (A1), Late Devonian, showing the hyomadibular in the spiracular pouch (A2), in lateral view, redrawn after (Janvier, 1996b; Brazeau and Ahlberg, 2006). (B) Early tetrapod Acanthostega, Late Devonian of Greenland, showing stapes and the presumed position of the gill arches, in lateral view, redrawn after (Clack et al., 2003; Clack, 2012). (C) Early tetrapod Ichthyostega (C1), Late Devonian of Greenland, and the reconstructed section (C2) through the air chamber showing its relation to the otic capsule and stapes. (D) Skull of Lizard (D1), showing the section of middle ear cavity, and the tympanic ear (D2) in reptiles, redrawn after (Clack, 2012). (E) Skull of mammals (E1), showing the section of middle ear cavity, and the tympanic ear (E2), redraw after (Farooq et al., 2020). ar, articular; eu, eustachian tube; fv, fenestra vestibuli; hym, hyomandibuar; in, incus; ma. malleus; qu, quadrate; sq, squamosal; st, stapes (columella), ty.c, tympanic cavity; ty.m, tympanic membrane (ear drum); other abbreviations as in Figures 1, 2.
Essentially the same spiracular morphology as in these tetrapodomorphs, but accompanied by a shorter and wider space for the spiracular canal, is found in the elpistostegids Panderichthys (Figure 7D) and Tiktaalik (Figure 6F; Brazeau and Ahlberg, 2006; Downs et al., 2008). Elpistostege is probably similar, though the internal part of the spiracular region is currently unknown (Cloutier et al., 2020). Given the shape of the elpistostegid skull, which suggests a surface-skimming lifestyle, it seems likely that the spiracle was used for air-breathing in a manner similar to Polypterus. This conclusion is further supported by the fact that the external nostril was low on the face, near the upper lip, and would have been submerged in a crocodile-like surface-skimming pose. Interestingly, in both Panderichthys and Tiktaalik the distal part of the hyomandibula has been lost, so that the bone no longer reaches down toward the jaw joint (Brazeau and Ahlberg, 2006; Downs et al., 2008). The corresponding portion of the hyomandibula in Polypterus is the part that lies ventral to the internal opening of the spiracular canal (Datovo and Rizzato, 2018), so it seems highly likely that this truncation of the elpistostegid hyomandibula reflects a change in its relationship to the spiracle, but the exact nature of this change cannot be determined.
Beginning in tetrapods, the hyomandibula ceases to be involved in jaw suspension and instead becomes dedicated eventually to hearing as the stapes (or columella) within the middle ear. Among the earliest Devonian tetrapods, we find the same combination of large, dorsally placed spiracles and small, ventrally facing external nostrils in Parmastega, Ventastega (Figure 6G) and Acanthostega (Figure 8B; Clack, 2003; Ahlberg et al., 2008; Beznosov et al., 2019). This suggests a conserved air-breathing function for the spiracle across the fish-tetrapod transition (Brazeau and Ahlberg, 2006). However, the skeletal morphology of the hyomandibula (henceforth termed “stapes”) and braincase have changed substantially compared to the elpistostegid condition. The most complete example of the earliest tetrapod condition is given by Acanthostega (Figure 6B; Clack, 1998; Clack et al., 2003); the stapes is unknown in Parmastega and Ventastega, though the braincase of Ventastega appears very similar to that of Acanthostega (Ahlberg et al., 2008). In contrast to the fish hyomandibula, which articulates proximally with the lateral commissure (a bony bridge that straddles the jugular vein), the footplate of the tetrapod stapes is lodged in a large opening in the braincase side wall, known as the fenestra vestibuli (fv, Figure 8C2). This puts it in direct contact with the inner ear and suggests that it may have acquired a sound-transducing function even at this very early stage in its evolution. The stapes is also shorter than even the elpistostegid hyomandibula, and is oriented transversely to the long axis of the head.
The stapes of Acanthostega is large, butterfly-shaped and evidently not associated with a tympanum (Figure 8B; Clack, 1998; Clack et al., 2003). Its precise function is difficult to determine, but it was presumably embedded in the posterior wall of the spiracular tract. An essentially similar non-tympanic stapes, usually but not invariably associated with a spiracular notch, is also found in a number of Carboniferous tetrapods including Pederpes, Greererpeton and Pholiderpeton (Figure 6H; Clack et al., 2003). This is thus almost certainly the primitive condition for the tetrapod middle ear. A distinctive variant on the same theme is found in Ichthyostega (Figure 8C1), where the stapes is thin and plate-like, and seems to have formed the floor of a large diverticulum of the spiracular tract (Figure 8C2; Clack et al., 2003). Although it is impossible to fully understand this structure in the absence of the all-important soft tissues, it seems probable that it combined a retained air-breathing function with an aquatic auditory function based on sound waves being picked up by the density contrast between body tissues and the enclosed air space, similar to the use of the swim bladder and Weberian ossicles in ostariophysian teleosts.
Turning to the tetrapod crown group, we find some puzzling patterns that point to multiple origins of the tympanic ear (Figures 8D,E; Clack, 2012). In the temnospondyls, which form the stem group of the extant amphibians, a tympanic ear develops in the same position as the spiracular notch; the transition is documented by the stapes developing a rod-like morphology and the notch acquiring a distinct attachment rim. Unlike Devonian tetrapods, temnospondyls with crocodile-like skulls also have large, dorsally facing external nostrils, which appear to have taken over the air-breathing function previously performed by the spiracles. The earliest well-preserved example of a temnospondyl stapes is that of Dendrerpeton (Figure 7E) from the Late Carboniferous of Canada (Robinson et al., 2005). This type of ear is retained essentially unchanged by modern anurans, whereas urodeles and caecilians have lost the tympanum.
Among crown amniotes, we find that early reptiles such as Captorhinus and early synapsids (stem mammals) such as Ophiacodon lack a tympanic notch and have a large stapes that braces between the quadrate and the braincase. This strongly suggests that the primitive condition for the amniote crown group was non-tympanic, a conclusion that is further supported by the different construction of the middle ears (one ear ossicle or three), and their different position (above the jaw joint or below it), in reptiles (including birds) on the one hand and mammals on the other. The evolution of the mammalian middle ear is well documented in the fossil record (Figure 8E), which shows how it originates at the posteroventral margin of the lower jaw; this is also supported by developmental data (Maier and Ruf, 2016).
Somewhat surprisingly, the stem amniotes Seymouria (Figure 7F) and Diadectes both have tympanic notches that seem to have contained ear drums, and the stapes points toward this notch rather than toward the quadrate; in Diadectes, the tympanum is at least partly ossified (Romer, 1928; Klembara et al., 2020). This type of ear is most similar to that of the temnospondyls, raising the intriguing possibility that a tympanic ear may be primitive for the tetrapod crown group and subsequently lost at the base of the amniote crown group, only to be re-evolved independently in mammals (Figures 8E, 9) on the one hand and reptiles including birds on the other. Critical to answering this question is the phylogenetic position of the anthracosaurs, a Carboniferous to Permian group that includes such genera as Archeria and Pholiderpeton (Figure 6H; Clack, 2012). Anthracosaurs, which are sometimes recovered as stem amniotes and sometimes as stem tetrapods, have a butterfly-shaped stapes and probably had an open spiracle. If they are stem tetrapods, the tympanic ears of stem amniotes and temnospondyls may be homologous, but if anthracosaurs also belong in the amniote stem group these ears are probably convergent. In any case it is clear that tympanic ears evolved at least three times in parallel among the ancestors of the living tetrapods. The persistence of the hyomandibular pouch as an organizing structure in the embryo, coupled with its ability in later development to either break through to the exterior (creating a spiracle), fail to break through but remain separated from the outside world by a thin membrane (creating a tympanum), or recede altogether (creating a non-tympanic ear), provides a mechanistic basis for this remarkable sequence of evolutionary changes.
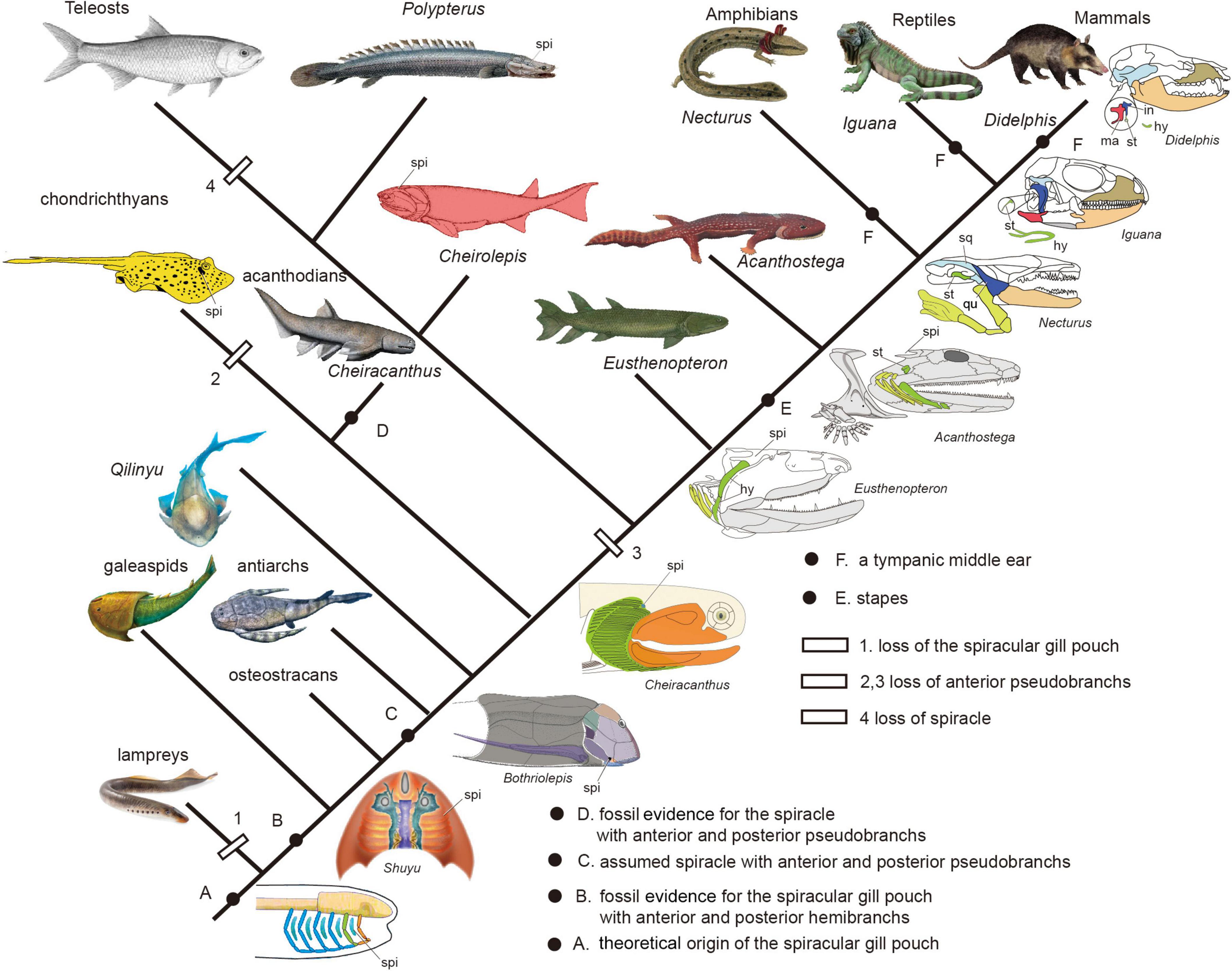
Figure 9. The evolution of spiracular region from jawless fishes to tetrapods (Redrawn after Janvier, 1996b; Arsenault et al., 2004; DeLaurier, 2018; Burrow et al., 2020).
Conclusion
The anatomical triad of mandibular arch, hyomandibular pouch and hyoid arch has had a much more adventurous evolutionary history than the more posterior branchial arches and pouches, reflecting its role as the interface between the mouth and the gill region. While it has been obvious for more than a century that the two arches and intervening pouch may have evolved as modifications of the branchial system, it is only very recently that the combination of a stable phylogeny and new data from fossils have allowed us to begin to understand their early evolution. Critically important has been the recognition that the extant cyclostomes form a clade, not a paraphyletic grade, and that their peculiar adult morphology with a mandibular-arch rasping tongue and an obliterated hyomandibular pouch is thus likely to be a specialization rather than a reflection of the ancestral vertebrate condition.
In contrast to this, the pharyngeal region of jawless stem gnathostomes, the fossil “ostracoderms,” shows conditions likely to be much closer to the ancestral state of crown vertebrates. Most informative among the ostracoderms are the galeaspids, which combine well-preserved three-dimensional anatomy with an absence of the confounding autapomorphies of the other anatomically informative ostracoderm group, the osteostracans. In this paper we present evidence that the galeaspids, exemplified principally by the genus Shuyu, had a fully developed spiracular gill pouch. Less conclusive evidence from osteostracans and heterostracans suggests that these groups also had complete spiracular gills, leading to the conclusion that this condition may be characteristic for the gnathostome total group as a whole.
The discovery of a spiracular gill in galeaspids leads unavoidably to a reconsideration of the “aphetohyoidean” hypothesis of primitive jawed vertebrate anatomy, according to which a complete spiracular gill separated the mandibular and hyoid arches in the earliest jawed vertebrates. In essence, the galeaspid condition would form a plausible evolutionary precursor to this condition, if the gill was retained across the jawless-to-jawed transition. However, at present there is no convincing evidence for a fully aphetohyoidean condition in any group of fossil or living jawed vertebrates. The spiracle, if present, is always a small and dorsally placed opening rather than a complete gill slit. However, in the acanthodians, an extinct group of probable stem chondrichthyans, there is evidence for the presence of a miniaturized but complete spiracular gill, comprising anterior and posterior pseudobranchs (corresponding to the hemibranchs of a normal gill), rather than the single posterior pseudobranch of living jawed vertebrates. The phylogenetic position of acanthodians suggests that the anterior pseudobranch was lost independently in chondrichthyans and osteichthyans.
Among osteichthyans and chondrichthyans, the primitive condition of the spiracle appears to be a small opening as seen in many sharks, but in many representatives of these groups it has undergone one of three modifications: loss, enlargement into an important inhalatory opening, or conversion into a tympanic middle ear for the amplification of airborne sound. Interestingly, all three modifications have happened several times in parallel. The spiracle has been lost in manta rays, requiem sharks, hammerhead sharks, chimaeras, lungfishes, coelacanths and neopterygian ray-finned fishes (bowfins, gars and teleosts). It has become an important inhalatory opening in rays, where it is used for water, and the primitive actinopterygian Polypterus, where it is used for air. A similar spiracular morphology and probable air-breathing function can be inferred for the tetrapod stem group, including tetrapodomorph fishes such as Eusthenopteron, transitionals such as Tiktaalik and stem tetrapods such as Acanthostega. Only in the tetrapod crown group did the spiracle lose this inhalatory function and evolve into a middle ear capped with a tympanum; remarkably, this happened at least three times in parallel, in amphibians, reptiles including birds, and mammals. This extraordinary series of evolutionary transformations bears testimony to the flexibility and potential of the triad of mandibular arch, hyomandibular pouch and hyoid arch, which appears early in the development of every vertebrate embryo.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
ZG, PA, and PD conceived and designed the study. ZG, PD, and MZ performed the 3D segmentation. ZG and PA wrote the first draft of the manuscript. All authors contributed to the interpretation of results, editing of the manuscript, and have approved this final version for re-submission.
Funding
This work was supported by Strategic Priority Research Program of CAS (XDB26000000), the National Natural Science Foundation of China (41972006 and 42072026), National Program for Support of Topnotch Young Professionals, and Key Research Program of Frontier Sciences, CAS (QYZDB-SSW-DQC040). PA acknowledges the support of a Wallenberg Scholarship from the Knut & Alice Wallenberg Foundation. PD was funded by the Natural Environment Research Council (NE/G016623/1 and NE/P013678/1), the Biotechnology and Biological Sciences Research Council (BB/T012773/1) and the Leverhulme Trust (RF-2022-167).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Philippe Janvier and Yajing Wang for constructive discussion and their courtesies allowing us to modify their figures. We thank Neil Gostling, Yemao Hou, Xianghong Lin and Xingdong Cui for assistance in retrieving microCT data, and Mingzhi Kong for assistance in photoing Latimeria. We are especially grateful to Aijuan Shi, Xianghong Lin, Dinghua Yang, Brian Choo, and Nobu Tamura for drawing illustrations.
Footnotes
References
Ahlberg, P. E., Clack, J. A., Lukševičs, E., Blom, H., and Zupiṇš, I. (2008). Ventastega curonica and the origin of tetrapod morphology. Nature 453, 1199–1204. doi: 10.1038/nature06991
Allis, E. P. (1922). The cranial anatomy of Polypterus, with special reference to Polypterus bichir. J. Anat. 56, 190–294.
Arsenault, M., Desbiens, S., Janvier, P., and Kerr, J. (2004). “New data on the soft tissues and external morphology of the antiarch Bothriolepis canadensis (Whiteaves, 1880) from the Upper Devonian of Miguasha, Quebec,” in Recent Advances in the Origin and Early Radiation of Vertebrates, eds G. Arratia, M. V. H. Wilson, and R. Cloutier (München: Verlag), 439–454.
Bartsch, P. (1994). Development of the cranium of Neoceratodus forsteri, with a discussion of the suspensorium and the opercular apparatus in Dipnoi. Zoomorphology 114, 1–31. doi: 10.1007/bf00574911
Basden, A. M., and Young, G. C. (2001). A primitive actinopterygian neurocranium from the Early Devonian of southeastern Australia. J. Vertebr. Paleontol. 21, 754–766. doi: 10.1671/0272-4634(2001)021[0754:apanft]2.0.co;2
Beznosov, P. A., Clack, J. A., Luksevics, E., Ruta, M., and Ahlberg, P. E. (2019). Morphology of the earliest reconstructable tetrapod Parmastega aelidae. Nature 574, 527–531. doi: 10.1038/s41586-019-1636-y
Brazeau, M. D., and Ahlberg, P. E. (2006). Tetrapod-like middle ear architecture in a Devonian fish. Nature 439, 318–321. doi: 10.1038/nature04196
Burggren, W. W. (1978). Gill ventilation in the sturgeon, Acipenser transmontanus: unusual adaptations for bottom dwelling. Respir. Physiol. 34, 153–170. doi: 10.1016/0034-5687(78)90025-7
Burggren, W. W., and Bemis, W. E. (1991). Metabolism and ram gill ventilation in juvenile paddlefish, Polyodon spathula (Chondrostei: Polyodontidae). Physiol. Zool. 65, 515–539.
Burrow, C. J., den Blaauwen, J., Newman, M., and Davidson, R. (2016). The diplacanthid fishes (Acanthodii, Diplacanthiformes, Diplacanthidae) from the Middle Devonian of Scotland. Palaeontol. Electron. 19, 1–83.
Burrow, C. J., Newman, M. J., and den Blaauwen, J. L. (2020). First evidence of a functional spiracle in stem chondrichthyan acanthodians, with the oldest known elastic cartilage. J. Anat. 236, 1154–1159. doi: 10.1111/joa.13170
Clack, J. A. (1993). Homologies in the fossil record: the middle ear as a test case. Acta Biotheor. 41, 391–409. doi: 10.1007/BF00709373
Clack, J. A. (1998). The neurocranium of Acanthostega gunnari Jarvik and the evolution of the otic region in tetrapods. Zool. J. Linn. Soc. 122, 61–97. doi: 10.1111/j.1096-3642.1998.tb02525.x
Clack, J. A. (2002). Patterns and processes in the early evolution of the tetrapod ear. J. Neurobiol. 53, 251–264. doi: 10.1002/neu.10129
Clack, J. A. (2003). A revised reconstruction of the dermal skull roof of Acanthostega gunneri, an early tetrapod from the Late Devonian. Trans. R. Soc. Edinburgh 93, 163–165. doi: 10.1017/s0263593302000111
Clack, J. A. (2012). Gaining Ground: The Origin and Evolution of Tetrapods. Bloomington: Indiana University Press, 1–523.
Clack, J. A., Ahlberg, P. E., Finney, S. M., Alonso, P. D., Robinson, J., and Ketcham, R. A. (2003). A uniquely specialized ear in a very early tetrapod. Nature 425, 65–69. doi: 10.1038/nature01904
Cloutier, R., Clement, A. M., Lee, M. S. Y., Noël, R., Béchard, I., Roy, V., et al. (2020). Elpistostege and the origin of the vertebrate hand. Nature 579, 549–554. doi: 10.1038/s41586-020-2100-8
Coates, M. I., Finarelli, J. A. I, Sansom, J., Andreev, P. S., Criswell, K. E., Tietjen, K., et al. (2018). An early chondrichthyan and the evolutionary assembly of a shark body plan. Proc. R. Soc. B 285:20172418. doi: 10.1098/rspb.2017.2418
Coates, M. I., Gess, R. W., Finarelli, J. A., Criswell, K. E., and Tietjen, K. (2017). A symmoriiform chondrichthyan braincase and the origin of chimaeroid fishes. Nature 541, 208–211. doi: 10.1038/nature20806
Daeschler, E. B., Shubin, N. H., and Jenkins, F. A. (2006). A Devonian tetrapod-like fish and the evolution of the tetrapod body plan. Nature 440, 757–763. doi: 10.1038/nature04639
Datovo, A., and Rizzato, P. P. (2018). Evolution of the facial musculature in basal ray-finned fishes. Front. Zool. 15:40. doi: 10.1186/s12983-018-0285-6
Davies, T. G. I, Rahman, A., Lautenschlager, S., Cunningham, J. A., Asher, R. J., Barrett, P. M., et al. (2017). Open data and digital morphology. Proc. R. Soc. B 284, 1852. doi: 10.1098/rspb.2017.0194
DeLaurier, A. (2018). Evolution and development of the fish jaw skeleton. Wiley Interdiscip. Rev. 8:e337. doi: 10.1002/wdev.337
Denison, R. H. (1961). Feeding mechanisms of agnatha and early gnathostomes. Am. Zool. 1, 177–181. doi: 10.1093/icb/1.2.177
Donoghue, P. C. J., Forey, P. L., and Aldridge, R. J. (2000). Conodont affinity and chordate phylogeny. Biol. Rev. 75, 191–251. doi: 10.1017/s0006323199005472
Donoghue, P. C. J., and Smith, M. P. (2001). The anatomy of Turinia pagei (Powrie), and the phylogenetic status of the Thelodonti. Trans. R. Soc. 92, 15–37. doi: 10.1017/s0263593301000025
Downs, J. P., Daeschler, E. B., Jenkins, F. A., and Shubin, N. H. (2008). The cranial endoskeleton of Tiktaalik roseae. Nature 455, 925–929. doi: 10.1038/nature07189
Dupret, V., Sanchez, S., Goujet, D., Tafforeau, P., and Ahlberg, P. E. (2014). A primitive placoderm sheds light on the origin of the jawed vertebrate face. Nature 507, 500–503. doi: 10.1038/nature12980
Farooq, R., Hussain, K., Tariq, M., Farooq, A., and Mustafa, M. (2020). CRISPR/Cas9: targeted genome editing for the treatment of hereditary hearing loss. J. Appl. Genet. 61, 51–65. doi: 10.1007/s13353-019-00535-6
Forey, P. L. (1995). Agnathans recent and fossil, and the origin of jawed vertebrates. Rev. Fish Biol. Fish. 5, 267–303. doi: 10.1007/bf00043003
Frey, L., Coates, M., Ginter, M., Hairapetian, V., Rucklin, M., Jerjen, I., et al. (2019). The early elasmobranch Phoebodus: phylogenetic relationships, ecomorphology and a new time-scale for shark evolution. Proc. Biol. Sci. 286:20191336.
Frey, L., Coates, M. I., Tietjen, K., Rucklin, M., and Klug, C. (2020). A symmoriiform from the Late Devonian of Morocco demonstrates a derived jaw function in ancient chondrichthyans. Commun. Biol. 3:681. doi: 10.1038/s42003-020-01394-2
Gai, Z., Lu, L., Zhao, W., and Zhu, M. (2018). New polybranchiaspiform fishes (Agnatha: Galeaspida) from the Middle Palaeozoic of China and their ecomorphological implications. PLoS One 13, e0202217. doi: 10.1371/journal.pone.0202217
Gai, Z., and Zhu, M. (2012). The origin of the vertebrate jaw: intersection between developmental biology-based model and fossil evidence. Chin. Sci. Bull. 57, 3819–3828. doi: 10.1007/s11434-012-5372-z
Gai, Z. K. (2011). The Cranial Anatomy of Galeaspida (Agnatha) and the Origin of Jawed Vertebrates. Doctoral dessertation. Bristol: University of Bristol.
Gai, Z. K. (2018). Synchrotron X-ray tomographic microscopy reveals histology and internal structure of Galeaspida (Agnatha). Vert. PalAs. 56, 93–105.
Gai, Z.-K., Donoghue, P. C. J., Zhu, M., Janvier, P., and Stampanoni, M. (2011). Fossil jawless fish from China foreshadows early jawed vertebrate anatomy. Nature 476, 324–327. doi: 10.1038/nature10276
Gai, Z. K., Jiang, W. Y., Zhao, W. J., Li, Q., Shi, X. D., and Zhu, M. (2022). Redescription of the Sanqiaspidae (Galeaspida) from the Lower Devonian of South China and its biostratigraphic significance. Palaeobiodiversity and Palaeoenvironments 102, 173–191. doi: 10.1007/s12549-021-00486-z
Gai, Z. K., Yu, X. B., and Zhu, M. (2017). The evolution of the zygomatic bone from Agnatha to Tetrapoda. Anatom. Rec. 300, 16–29. doi: 10.1002/ar.23512
Gai, Z. K., Zhu, M., and Donoghue, C. J. P. (2019). The circulatory system of Galeaspida (Vertebrata; stem-Gnathostomata) revealed by synchrotron X-ray tomographic microscopy. Palaeoworld 28, 441–460. doi: 10.1016/j.palwor.2019.04.005
Gardiner, B. G. (1984). The relationships of the palaeoniscid fishes, a review based on new specimens of Mimia and Moythomasia from the Upper Devonian of Western Australia. Bull. Br. Museum Geol. 37, 173–428.
Gegenbaur, C. (1872). Untersuchungen zur Vergleichenden Anatomie der Wirbeltiere. 3: Das Kopfskelett der Selachier. Leipzig: Englemann.
Graham, J. B., Wegner, N. C., Miller, L. A., Jew, C. J., Lai, N. C., Berquist, R. M., et al. (2014). Spiracular air breathing in polypterid fishes and its implications for aerial respiration in stem tetrapods. Nat. Commun. 5:3022. doi: 10.1038/ncomms4022
Halstead, L. B. (1971). The presence of a spiracle in the Heterostraci (Agnatha). Zool. J. Linn. Soc. 50, 195–197. doi: 10.1111/j.1096-3642.1971.tb00760.x
Halstead, L. B. (1973). The heterostracan fishes. Biol. Rev. 48, 279–332. doi: 10.1111/j.1469-185x.1973.tb01005.x
Heimberg, A. M., Cowper-Sallari, R., Semon, M., Donoghue, P. C. J., and Peterson, K. J. (2010). MicroRNAs reveal the interrelationships of hagfish, lampreys, and gnathostomes and the nature of the ancestral vertebrate. Proc. Natl. Acad. Sci. U.S.A. 107, 19379–19383. doi: 10.1073/pnas.1010350107
Holland, T., and Long, J. A. (2009). On the phylogenetic position of Gogonasus andrewsae Long 1985, within the Tetrapodomorpha. Acta Zool. 90, 285–296. doi: 10.1111/j.1463-6395.2008.00377.x
Holmgren, N. (1942). Studies on the head of fishes. Part III. Acta Zool. 23, 129–261. doi: 10.1111/j.1463-6395.1942.tb00012.x
Holmgren, N. (1946). On two embryos of Myxine glutinosa. Acta Zool. 27, 1–90. doi: 10.1111/j.1463-6395.1946.tb00019.x
Hughes, G. M. (1960). The mechanism of gill ventilation in the Dogfish and Skate. J. Exp. Biol. 37, 11–27. doi: 10.1016/j.zool.2015.09.002
Janvier, P. (1974). The structure of the naso-hypophysial complex and the mouth in fossil and extant cyclostomes, with remaks on amphiaspiforms. Zool. Scr. 3, 193–200. doi: 10.1111/j.1463-6409.1974.tb00816.x
Janvier, P. (1981). Norselaspis glacialis n.g., n.sp. et les relations phylogénétiques entre les Kiaeraspidiens (Osteostraci) du Dévonien Inférieur du Spitsberg. Palaeovertebrata 11, 19–131.
Janvier, P. (1985). Les Céphalaspides du Spitsberg: Anatomie, Phylogénie et Systématique des Ostéostracés Siluro-Dévoniens; Revisions des Ostéostracés de la Formation de Wood Bay (Dévonien inférieur du Spitsberg). Paris: Centre national de la Recherche scientifique.
Janvier, P. (1996b). The dawn of the vertebrates: characters versus common ascent in the rise of current vertebrate phylogenies. Palaeontology 39, 259–287.
Janvier, P. (2001). “Ostracoderms and the shaping of the gnathostome characters,” in Major Events in Early Vertebrate Evolution: Palaeontology, Phylogeny, Genetics and Development, ed. P. Ahlberg (London: Taylor Francis), 172–186. doi: 10.7717/peerj.5249
Janvier, P. (2004). “Early specializations in the branchial apparatus of jawless vertebrates: a consideration of gill number and size,” in Recent Advances in the Origin and Early Radiation of Vertebrates, eds G. Arratia, M. V. H. Wilson, and R. Cloutier (München: Verlag), 29–52.
Janvier, P. (2007). “Homologies and evolutionary transitions in early vertebrate history,” in Major Transitions in Vertebrate Evolution, eds J. S. Anderson and H.-D. Sues (Bloomington: Indiana University Press), 57–121.
Janvier, P., Thanh, T.-D., Phuong, T. H., Clément, G., and Phong, N. D. (2009). Occurrence of Sanqiaspis, Liu, 1975 (Vertebrata, Galeaspida) in the Lower Devonian of Vietnam, with remarks on the anatomy and systematics of the Sanqiaspididae. C. R. Palevol 8, 59–65. doi: 10.1016/j.crpv.2008.10.008
Johanson, Z. (1998). The upper devonian fish bothriolepis (Placodermi: Antiarchi) from near Canowindra, New South Wales, Australia. Rec. Aust. Museum 50, 315–348. doi: 10.3853/j.0067-1975.50.1998.1289
Johnston, J. B. (1905). The cranial nerve components of Petromyzon. Gegenbaurs Morphol. Jahrb. 34, 149–203.
Klembara, J., Hain, M., Cernansky, A., Berman, D. S., and Henrici, A. C. (2020). Anatomy of the neural endocranium, parasphenoid and stapes of Diadectes absitus (Diadectomorpha) from the early Permian of Germany based on the high-resolution X-ray microcomputed tomography. Anat. Rec. 303, 2977–2999. doi: 10.1002/ar.24376
Kuratani, S., and Ahlberg, P. E. (2018). Evolution of the vertebrate neurocranium: problems of the premandibular domain and the origin of the trabecula. Zool. Lett. 4:1. doi: 10.1186/s40851-017-0083-6
Kuratani, S., Ueki, T., Aizawa, S., and Hirano, S. (1997). Peripheral development of cranial nerves in a cyclostome, Lampetra japonica: morphological distribution of nerve branches and the vertebrate body plan. J. Comp. Neurol. 384, 483–500. doi: 10.1002/(sici)1096-9861(19970811)384:4<483::aid-cne1>3.0.co;2-z
Liem, K., Bemis, W., Walker, W. F. Jr., and Grande, L. (2001). Functional Anatomy of the Vertebrates: an Evolutionary Perspective, 3rd Edn. Philadelphia: Thomson Brooks/Cole.
Long, J. A. (2001). On the relationships of Psarolepis and the onychodontiform fishes. J. Vertebr. Paleontol. 21, 815–820. doi: 10.1671/0272-4634(2001)021[0815:otropa]2.0.co;2
Long, J. A., Young, G. C., Holland, T., Senden, T. J., and Fitzgerald, E. M. G. (2006). An exceptional Devonian fish from Australia sheds light on tetrapod origins. Nature 444, 199–202. doi: 10.1038/nature05243
Maier, W., and Ruf, I. (2016). Evolution of the mammalian middle ear: a historical review. J. Anat. 228, 270–283. doi: 10.1111/joa.12379
Maisey, J. G. (1986). Heads and tails: a chordate phylogeny. Cladistics 2, 201–256. doi: 10.1111/j.1096-0031.1986.tb00462.x
Maisey, J. G., Janvier, P., Pradel, A., Denton, J. S. S., Bronson, A., Miller, R., et al. (2019). “Doliodus and pucapampellids: contrasting perspectives on stem chondrichthyan morphology,” in Evolution and Development of Fishes, eds Z. Johanson, C. Underwood, and M. Richter (Cambridge: Cambridge University Press), 87–109. doi: 10.1017/9781316832172.006
Mallatt, J. (1984). Early vertebrate evolution: pharyngeal structure and the origin of gnathostomes. J. Zool. 204, 169–183. doi: 10.1111/j.1469-7998.1984.tb02368.x
Mallatt, J. (1996). Ventilation and the origin of jawed vertebrates: a new mouth. Zool. J. Linn. Soc. 117, 329–404. doi: 10.1111/j.1096-3642.1996.tb01658.x
Miles, R. S. (1964). A reinterpretation of the visceral skeleton of Acanthodes. Nature 204, 457–459. doi: 10.1038/204457a0
Miles, R. S. (1965). Some features in the cranial morphology of acanthodians and the relationships of the Acanthodii. Acta Zool. 46, 233–255. doi: 10.1111/j.1463-6395.1965.tb00733.x
Miles, R. S. (1968). “Jaw articulation and suspension in Acanthodes and their significance,” in Current Problems of Lower Vertebrate Phylogeney. Nobel Symposium 4, ed. T. Ørvig (Stockholm: Almqvist & Wiksell), 109–127.
Miles, R. S. (1973). Articulated acanthodian fishes from the Old Red Sandstone of England, with a review of the structure and evolution of the acanthodian shoulder-girdle. Bull. Br. Museum Geol. 24, 111–213. doi: 10.5962/p.313823
Miyashita, T. (2016). Fishing for jaws in early vertebrate evolution: a new hypothesis of mandibular confinement. Biol. Rev. 91, 611–657. doi: 10.1111/brv.12187
Miyashita, T., and Diogo, R. (2016). Evolution of serial patterns in the vertebrate pharyngeal apparatus and paired appendages via assimilation of dissimilar units. Front. Ecol. Evol. 4:71. doi: 10.3389/fevo.2016.00071
Miyashita, T., Gess, R. W., and Tietjen, K. E. A. (2021). Non-ammocoete larvae of Palaeozoic stem lampreys. Nature 591, 408–412. doi: 10.1038/s41586-021-03305-9
Möhlich, A., Waser, W., and Heisler, N. (2009). The teleost pseudobranch: a role for preconditioning of ocular blood supply? Fish Physiol. Biochem. 35, 273–286. doi: 10.1007/s10695-008-9207-4
Novitskaya, L. I. (1983). Morfologiya Drevnikh Beschelyustnykh (Heterostraci i problema Svyazi Beschelyustnykh i Chelyustnorotykh Pozvonochnykh. Moscow: Trudi Palaeontologicheskogo Instituta.
Novitskaya, L. I. (2015). Brain of the most ancient vertebrates (Agnatha: Heterostraci) and man: comparison and inferences. Paleontol. J. 49, 79–88. doi: 10.1134/s0031030115010086
Pearson, D. M., and Westoll, T. S. (1979). The Devonian actinopterygian Cheirolepis Agassiz. Trans. R. Soc. Edinburgh 70, 337–399. doi: 10.1017/s0080456800012850
Perry, C. T., Salter, M. A., Harborn, A. R., Crowley, S. F., Jelks, H. L., and Wilson, R. W. (2011). Fish as major carbonate mud producers and missing components of the tropical carbonate factory. Proc. Natl. Acad. Sci. U.S.A. 108, 3865–3869. doi: 10.1073/pnas.1015895108
Pradel, A., Maisey, J. G., Tafforeau, P., Mapes, R. H., and Mallatt, J. (2014). A Palaeozoic shark with osteichthyan-like branchial arches. Nature 509, 608–611. doi: 10.1038/nature13195
Pradel, A., Tafforeau, P., Maisey, J. G., and Janvier, P. (2011). A new paleozoic Symmoriiformes (Chondrichthyes) from the Late Carboniferous of Kansas (USA) and cladistic analysis of early chondrichthyans. PLoS One 6:e24938. doi: 10.1371/journal.pone.0024938
Robinson, J., Ahlberg, P. E., and Koentges, G. (2005). The braincase and middle ear region of Dendrerpeton acadianum (Tetrapoda: Temnospondyli). Zool. J. Linn. Soc. 143, 577–597. doi: 10.1111/j.1096-3642.2005.00156.x
Romer, A. S. (1928). A skeletal model of the primitive reptile Seymouria, and the phylogenetic position of that type. J. Geol. 36, 248–260. doi: 10.1086/623510
Schultze, H.-P. (1993). “Patterns of diversity in the skull of jawed fishes,” in The Skull, Vol. 2, eds J. Janke and B. K. Hall (Chicago: University of Chicago Press), 189–254.
Stensiö, E. A. (1927). The downtonian and devonian vertebrates of spitsbergen. part i. family cephalaspidae. Skrifter Svalbard Nordishavet 12, 1–391.
Stensiö, E. A. (1947). The sensory lines and dermal bones of the cheek in fishes and amphibians. Kungliga Svenska Vetenskapsakademiens Handlingar 24, 1–195.
Stensiö, E. A. (1963). Anatomical studies on the arthrodiran head. Part 1. Preface, geological and geographical distribution, the organization of the head in the Dolichothoraci, Coccosteomorphi and Pachyosteomorphi. Taxonomic appendix. Kungliga Svenska Vetenskapsakademiens Handlingar 9, 1–419. doi: 10.1007/s12105-017-0785-2
Stensiö, E. (1964). “Les Cyclostomes fossiles ou Ostracodermes,” in Traité de Paléontologie, ed. J. Piveteau (Paris: Masson), 96–382.
Stockard, C. R. (1906). The development of the mouth and gills in Bdellostoma stouti. Am. J. Anat. 5, 481–517. doi: 10.1002/aja.1000050405
Summers, A. P., and Ferry-Graham, L. A. (2001). Ventilatory modes and mechanics of the hedgehog skate (Leucoraja erinacea): testing the continuous ?ow model. J. Exp. Biol. 204, 1577–1587. doi: 10.1242/jeb.204.9.1577
Vorobyeva, E. I., and Schultze, H.-P. (1991). “Description and systematics of panderichthyid fishes with comments on their relationship to tetrapods,” in Origins of the Higher Groups of Tetrapods: Controversy and Consensus, eds H.-P. Schultze and L. Trueb (Ithaca: Cornell Publishing Associates), 68–109. doi: 10.7591/9781501718335-005
Wang, N.-Z. (1991). “Two new Silurian galeaspids (jawless craniates) from Zhejiang Province, China, with a discussion of galeaspid-gnathostome relationships,” in Early Vertebrates and Related Problems of Evolutionary Biology, eds M. M. Chang, Y. H. Liu, and G. R. Zhang (Beijing: Science Press), 41–66.
Wang, Y. J., and Zhu, M. (2020). New data on the headshield of Parayunnanolepis xitunensis (Placodermi, Antiarcha), with comments on nasal capsules in antiarchs. J. Vertebr. Paleontol. 40:e1855189. doi: 10.1080/02724634.2020.1855189
Waser, W. (2011). “Transport and exchange of respiratory gases in the blood | root effect: root effect definition, functional role in oxygen delivery to the eye and swimbladder,” in Encyclopedia of Fish Physiology, ed. A. P. Farrell (Cambrige, MA: academic Press).
Watson, D. M. S. (1937). The acanthodian fishes. Philos. Trans. R. Soc. Lond. Ser. B 228, 49–146. doi: 10.1098/rstb.1937.0009
White, T. E. (1939). Osteology of Seymouria baylorensis Broili. Bull. Museum Comp. Zool. 85, 325–409.
Wicht, H., and Tusch, U. (1998). “Ontogeny of the head and nervous system of myxinoids,” in The Biology of Hagfishes, eds J. M. Jørgensen, J. P. Lomholt, R. E. Weber, and H. Malte (London: Chapman & Hall), 431–451. doi: 10.1007/978-94-011-5834-3_28
Young, G. C. (2008). The relationships of antiarchs (Devonian Placoderm Fishes)—evidence supporting placoderm monophyly. J. Vertebr. Paleontol. 28, 626–636. doi: 10.1671/0272-4634(2008)28[626:troadp]2.0.co;2
Zangerl, R., and Case, G. R. (1976). Cobelodus aculeatus (Cope), an snacanthous shark from Pennsylvanian black shales of North America. Palaeontogr. Abt. A 154, 107–157.
Zangerl, R., and Williams, M. E. (1975). New evidence on the nature of the jaw suspension in Palaeozoic anacanthous sharks. Palaeontology 18, 333–341.
Zhu, M., Ahlberg, P. E., Pan, Z., Zhu, Y., Qiao, T., Zhao, W., et al. (2016). A Silurian maxillate placoderm illuminates jaw evolution. Science 354, 334–336. doi: 10.1126/science.aah3764
Zhu, M., Yu, X., Ahlberg, P. E., Choo, B., Lu, J., Qiao, T., et al. (2013). A Silurian placoderm with osteichthyan-like marginal jaw bones. Nature 502, 188–193. doi: 10.1038/nature12617
Zhu, M., Zhao, W.-J., Jia, L.-T., Lu, J., Qiao, T., and Qu, Q.-M. (2009). The oldest articulated osteichthyan reveals mosaic gnathostome characters. Nature 458, 469–474. doi: 10.1038/nature07855
Keywords: spiracle, mandibular arch, hyoid arch, Galeaspida, stem-gnathostome, Shuyu
Citation: Gai Z, Zhu M, Ahlberg PE and Donoghue PCJ (2022) The Evolution of the Spiracular Region From Jawless Fishes to Tetrapods. Front. Ecol. Evol. 10:887172. doi: 10.3389/fevo.2022.887172
Received: 01 March 2022; Accepted: 13 April 2022;
Published: 19 May 2022.
Edited by:
Ingmar Werneburg, University of Tübingen, GermanyReviewed by:
Shigeru Kuratani, Riken Center for Biosystems Dynamics Research (BDR), JapanPhilippe Janvier, Centre National de la Recherche Scientifique (CNRS), France
Copyright © 2022 Gai, Zhu, Ahlberg and Donoghue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Per E. Ahlberg, Per.Ahlberg@ebc.uu.se; Philip C. J. Donoghue, Phil.Donoghue@bristol.ac.uk
 Zhikun Gai
Zhikun Gai Min Zhu
Min Zhu Per E. Ahlberg
Per E. Ahlberg Philip C. J. Donoghue
Philip C. J. Donoghue