Dental topography and dietary specialization in Papionini primates
- 1Departament de Biologia Evolutiva, Ecologia i Ciències Ambientals (BEECA), La Secció de Zoologia i Antropologia Biològica, Facultat de Biologia, Universitat de Barcelona (UB), Barcelona, Spain
- 2Departament de Biologia Animal, Vegetal i Ecologia, Facultat de Biociències, Universitat Autònoma de Barcelona, Barcelona, Spain
- 3Departamento de Biotecnología, Facultad de Ciencias, Universidad de Alicante, Alicante, Spain
- 4Instituto Universitario de Investigación en Arqueología y Patrimonio Histórico (INAPH), Universidad de Alicante, Alicante, Spain
- 5Archaeology of Social Dynamics, Institución Milá y Fontanals de Investigación en Humanidades (CSIC), Barcelona, Spain
- 6Institut d’Arqueologia de la Universitat de Barcelona (IAUB), Barcelona, Spain
Our understanding of primate adaptive evolution depends on appreciating the way in which dental functional morphology affects food processing. The Papionini tribe of Cercopithecoidea primates shows great dietary versatility and ecological adaptations to resource seasonality across the African and Asian ecosystems, however, there are few studies focusing on the occlusal topography of the bilophodont teeth and the effect of tooth wear in the crown shape. Here, we explore the relationship between wear-related dental functional morphology and dietary ecological constraints within the Papionini. Three-dimensional (3D) polygonal meshes of the upper permanent molar row (M1-3) were obtained in a large papionine sample (838 specimens) of known dietary preferences including species from six genera (Cercocebus, Lophocebus, Macaca, Mandrillus, Papio, and Theropithecus). All the sample was classified in four diet categories and four topographic metrics (orientation patch count rotated, OPCR; Dirichlet normal energy, DNE; occlusal relief, OR; and ambient occlusion, portion de ciel visible, PCV) were measured for each tooth-type according to wear stage (lightly and moderately worn) to determine diet-related interspecific morphological changes with long-term functionality. The results indicate that hard-object feeders (Cercocebus and Lophocebus) and grass eaters (Theropithecus gelada) exhibit a pattern of occlusal complexity (OPCR), surface curvature (DNE), relief (OR), and morphological wear resistance (PCV) that is significantly different from the omnivores and folivore-frugivore species (Mandrillus and Macaca) despite the overall homogeneity of the bilophodont dentition. A multifactorial ANOVA showed that the topographic metrics were sensitive to tooth wear as expected. The results also indicate that the interspecific variability of dental topography of the upper molars reflects dietary specializations rather than phylogenetic proximity. These findings support the hypothesis that evolutionary convergence processes could have affected the Papionini, clustering the hard-object feeders (Lophocebus and Cercocebus) together in the morphospace, and clearly discriminating this group from the graminivorous and frugivores-folivores.
Introduction
The Papionini tribe (Family Cercopithecidae; Subfamily Cercopithecinae) has the greatest geographical range of extant non-human primates across Africa and Asia, demonstrating broad adaptability and evolutionary success (Perelman et al., 2011; Monson and Hlusko, 2014). Extant Papionini primates include the mangabeys (genera Lophocebus and Cercocebus), which occupy regions of eastern and central Africa (Daegling and McGraw, 2007), the macaques (Macaca), which inhabit Asia and some regions in North Africa (Ménard et al., 2014), the mandrills and drills (Mandrillus) from West Africa (Tutin et al., 1997), the baboons (Papio), distributed throughout the sub-Saharan savannahs (Kamilar, 2006), and the geladas (Theropithecus), from the highlands of Ethiopia (Jablonski, 1993). This wide ecological distribution reflects a great dietary diversity and different behavioral responses to resource seasonality, including the intake of foods with different mechanical and physical properties that vary according to microhabitats (Brugiere et al., 2002; Swindler, 2002).
The species of the Papionini tribe show great morphological diversity according to its wide range of ecological exploitation. Notable morphological similarities previously lead researchers to conclude that the small-bodied mangabeys (Cercocebus and Lophocebus) and the large-bodied taxa such as Papio, Theropithecus and Mandrillus formed potential subclades in the Papionini (Jolly, 1972; Szalay and Delson, 1979; Strasser and Delson, 1987). This morphological phenetic classification prevailed until molecular studies showed that the mangabeys might not constitute a monophyletic group (Disotell et al., 1992; Disotell, 1994, 2000; Harris and Disotell, 1998; Harris, 2000). Within the mangabeys, Lophocebus may be more closely related to the savannah (Papio) and the gelada (Theropithecus) baboons, whereas Cercocebus was more closely related to the mandrills (Figure 1). Dental and postcranial similarities between the Cercocebus/Mandrillus and Lophocebus/Papio groups were shown to support the hypothesis of paraphyly for this clade (Fleagle and McGraw, 1999, 2002; McGraw and Fleagle, 2006; Gilbert, 2007). Reappraisals of allometry-corrected cranial anatomy suggest that the craniodental anatomy is a valuable source of phylogenetic information (Gilbert, 2013). There is not yet a consensus, however, about the relationships among Theropithecus, Papio and Lophocebus (Perelman et al., 2011; Gilbert, 2013; Zinner et al., 2013; Pugh and Gilbert, 2018), since high levels of craniomorphic homoplasy are present (Fleagle and McGraw, 1999; Lockwood and Fleagle, 1999; Collard and O’Higgins, 2002; Gilbert and Rossie, 2007; Gilbert et al., 2009). Convergence and divergence in morphological traits are likely to have hindered determination of the actual phylogenetic proximity within the Papionini, resulting in the ambiguous evolutionary scenario of morphological and genetic signatures (Strasser and Delson, 1987; Harris and Disotell, 1998; Harris, 2000; Gilbert, 2007).
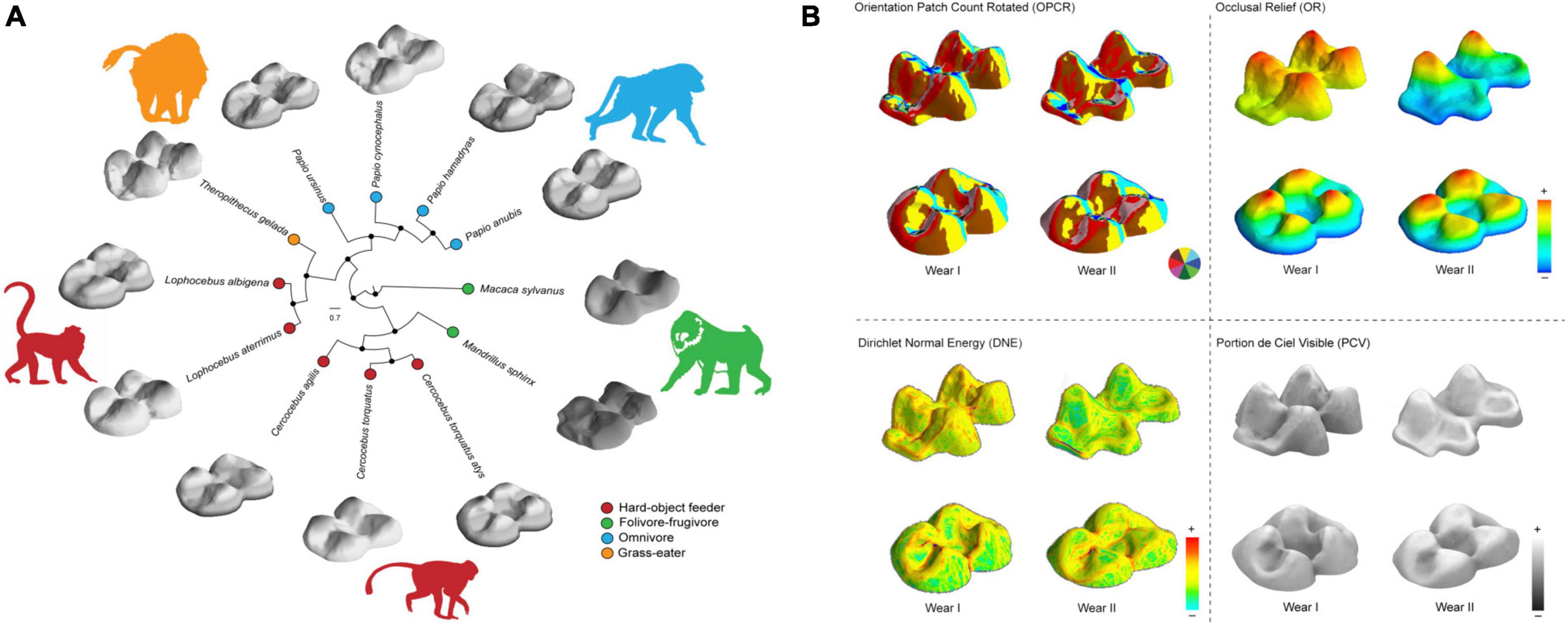
Figure 1. (A) Polar tree showing the Bayesian inference of phylogenetic relationships for the papionins analyzed (10kTrees; https://10ktrees.nunn-lab.org/). Species were grouped by dietary ecology. The comparative upper second molar (M2) morphology between species is shown. (B) Occlusal-lingual view of M2 triangulated meshes for species Theropithecus gelada (above) and Cercocebus atys displaying morphometric maps at different stages of dental wear (Wear I and II) for topographic metrics including: Orientation patch count rotated (OPCR), the complexity maps indicate surface orientation patches (see color wheel); Dirichlet Normal Energy (DNE) for the variability in surface curvature; occlusal relief (OR) for the measurement of relative crown height and, portion de ciel visible (PCV) to quantify morphological wear resistance. Warmer and cooler colors on DNE and elevation (OR) maps indicate higher and lower curvature and crown height, respectively; brighter areas indicate higher PCV values. Teeth are not to scale. Lingual: right; distal: left.
The Cercopithecidae share an ancestral bilophodont molar occlusal cusp pattern (Swindler, 1976; Lucas, 2004) consisting of four marginal cusps connected by two transverse lophs or ridges that define three foveas with a reduced lingual cingulum (Delson, 1975). The occlusal bite contact between the maxillary and mandibular bilophodont molars allows both shearing and tooth-to-tooth grinding contacts, which may represent an adaptation to increased seed consumption and folivory (Delson, 1975; Ungar, 2010). The Papionini primates show a common, although greatly variable, morphology of molar teeth (Figure 1) reflecting species-specific evolutionary adaptations to food processing (Swindler, 2002). They widen outwards, laterally from the cusp apexes to the cervix, especially on the buccal face of the lower and on the lingual face of the upper molars (Delson, 1975). This morphology results in differences between the cervical and apical width dimensions, and varies among the Papionini. It is least pronounced in Cercocebus and Papio and least in Theropithecus (Swindler, 2002). Theropithecus differs among the Papionini, with a higher molar crown relief, longer mediodistal distance, and a wider central basin, which is deeper than the other two somewhat elongated basins (Delson, 1975).
This morphological dental variability may reflect specific dietary adaptations within the different taxa of the Papionini group. Although Cercopithecoidea primates are mostly fruit eaters that inhabit seasonal ecosystems, their dietary spectra vary from leaves, bark, grass, nuts and flowers to mammals and lizards (Swindler, 2002). For instance, Theropithecus gelada from the Ethiopian Highlands is specialized in the exploitation of a graminivorous niche (Dunbar and Bose, 1991), with slight variation in diet composition between dry and wet seasons (Mau et al., 2009; Moges and Balakrishnan, 2014). A multi-year study at Guassa Plateau –an intact, tall-grass ecosystem in the Ethiopian Central highlands– has shown that specimens from Guassa rely heavily on forbs and invertebrates, however, and less on graminoids, expanding the documented diversity of gelada diets (Fashing et al., 2014). Occlusal microwear texture analyses have also shown a wide range of ecological adaptations to diet and feeding in Theropithecus, reflecting population-specific dietary variability (Fashing et al., 2014; Shapiro et al., 2016).
The highly variable, species- and season-specific dietary regimes observed in the Papionini require primary diets, consisting of the preferred and most abundant foods consumed year-round, to be differentiated from secondary diets (i.e., fallback foods). Derived dental morphology specializations, such as thick enamel and well-developed marginal crests, are likely to suggest the consumption of mechanically demanding fallback foods, at least for a short period when the preferred, year-round, less demanding foods are scarce. For instance, the two species of mangabeys, the terrestrial Cercocebus and the arboreal Lophocebus, adapted to feed on hard objects, both showing very thick-enameled molar teeth (Kay, 1981), large incisors (Hylander, 1975), powerful jaws (Hylander, 1979), and a facial configuration that provides a mechanical advantage for producing large occlusal bite forces (Singleton, 2005). Although various species of both genera can be described as seed eaters and frugivores with a strong dependence on hard foods, numerous studies have shown significant differences in food preference (Lambert et al., 2004; McGraw et al., 2014; Ungar et al., 2018). Cercocebus atys depends strongly on fruits and seeds with very hard covers, such as the nut of Sacoglotis gabonensis, which they consume all-year round in different proportions (Daegling et al., 2011). Both the primary and secondary diets of Cercocebus atys are based on this type of fruit as a key resource. The Lophocebus species are all highly frugivorous, however, mainly consuming fleshy fruits, while hard fallback foods, such as seeds and bark, are consumed when there are shortages of preferred foods (Lambert et al., 2004). Although the enamel of both genera of mangabeys is some of the hardest enamel within the order of primates (McGraw et al., 2012), Lophocebus relies only on dry hard seeds and barks during the dry season or extreme El Niño events (Lambert et al., 2004; Daegling et al., 2011). Some authors argue that preferred foods have the greatest selective effect and are responsible for these morphological differences (Kay, 1975), however, it has also been suggested that the dependence on fallback foods during times of shortage can place much stronger selective pressure on dental anatomy (Fleagle and McGraw, 2002; Ungar et al., 2018). Other authors have suggested that preferred foods may be associated with adaptations for food procurement (efficient locomotion and food detection), while fallback foods, which are more abundant during dry seasons, involve challenging mechanical properties (hardness of food items) that require prolonged processing in the mouth, which promotes the development of food processing capacities that facilitate the efficient chewing and digestion of these foods (Marshall and Wrangham, 2007).
Analyses of dental topography and the shape of surface features in primates have explored the dynamic relationship between occlusal morphology, diet, and dental occlusal wear (M’Kirera and Ungar, 2003; Ungar and M’Kirera, 2003; Dennis et al., 2004; Ungar et al., 2018; Berthaume et al., 2020), including some Cercopithecoidea primates (Bunn and Ungar, 2009; Thiery et al., 2017a). During mastication the biomechanical action of teeth is based on the relationship between the mechanical properties of the foods and the dental action, defined as how teeth are used to access or fragment food (Kay, 1981; Lucas, 2004; Daegling et al., 2011; McGraw et al., 2014; Berthaume, 2016). In primates, these mechanical properties include toughness (resistance to crack propagation) and hardness (local resistance to elastic deformation) (Berthaume, 2016). Dental actions may vary among species, despite eating similar foods, due to the use of different motions or loads. Topographic variables thus quantify different aspects of tooth shape in relation to diet. For instance, frugivores with low-cusped crowns have a lower relief index, computed as the ratio between the 3D tooth surface area and its 2D projection area on the occlusal plane, than folivores, which have high cusped molars (Teaford and Ungar, 2000; Allen et al., 2015; Pampush et al., 2018). The curvature of the crown, however, calculated as Dirichlet normal energy (DNE; Bunn et al., 2011), is expected to be higher in folivores. DNE measures surface variability, meaning teeth with curvy surfaces, such as taller cusps, are generally sharper and have a higher DNE. Therefore, we may expect that frugivores and hard fruit eaters show a lower curvature and relief index than folivores or omnivores. Tooth occlusal complexity, which corresponds to the average number of dental elements, has been correlated with the significance of herbivory (Evans et al., 2007). These dental elements or triangles can be computed as the sum of the changes in triangle patch directions (OPCR; orientation patch count). Occlusal surfaces with greater patch counts have been connected to the enamel edges involved in shearing fibrous food (Pineda-Muñoz et al., 2017), however, large overlapping levels of OPCR values have been documented in primate species with distinct diets (Berthaume et al., 2020). Ambient occlusion (PCV; portion de ciel visible), has recently been introduced as a dental topographic metric (Berthaume et al., 2019a,2020), that measures the exposure of a surface to ambient lighting when it come from the occlusal direction. Cusp tips, crest and blade edges tend to be more exposed to light and have higher PCV values, while the foveae, or sides of enamel caps, tend to be less exposed and have lower PCV values. PCV has been correlated with the efficiency of hard object feeding; primates with more fibrous diets (i.e., folivores) will have lower PCV values than frugivores or hard-object feeders that have low cusps highly exposed to light (Berthaume, 2016; Berthaume et al., 2020).
Although worn teeth are underrepresented in functional analysis, selective pressures are active throughout the entire functional life of teeth, modifying the morphology of the crowns. Some authors have suggested that wear, and the subsequent dentine exposure, may contribute to maintaining the functional efficiency of dental mastication (M’Kirera and Ungar, 2003; Ulhaas et al., 2004; King et al., 2005; Ungar, 2015; Glowacka et al., 2016), and, worn teeth could therefore be used to discriminate dietary specializations. Topographic variables change throughout wear stages. For instance, occlusal surface curvature (DNE) shows greater values in unworn molars with sharp cusps than in molar caps showing worn cusps and flatter occlusal surfaces (Bunn et al., 2011). Primates have flat dental crowns (non-hypsodont) compared to some other mammals, and dental wear significantly changes tooth shape, from the initial appearance of wear facets to a complete dentine exposure in occlusal surfaces, which may affect food chewing, and compromise structural integrity. The relationship between enamel and the underlying dentine also plays a role in the tooth’s functional efficiency that it is retained as tooth wears (Ungar, 2015). Natural selection shapes animal teeth to cope with wear and to retain dental functionality to fracture foods throughout a lifetime. In this context, some studies have found that wear patterns within primate taxa tend to maintain relatively high values of relief on occlusal surfaces, for example in folivorous compared to frugivorous species (M’Kirera and Ungar, 2003; Ungar, 2004; Bunn and Ungar, 2009; Ungar et al., 2018). This implies that teeth continue to maintain differences in occlusal morphologies with wear, or change their shapes in different ways as they wear (Ungar and M’Kirera, 2003). Diverse aspects of tooth shape can change throughout the wear process, such as cusp relief and slope, both of which can be measured by using topographic metrics. Examining topographic parameters is therefore a good approach to studying gross occlusal form, as teeth wear distinctively among primates in different species in relation to diet (Ungar, 2015). Several authors agree, however, that additional studies are needed to explore the dietary implications of dental sculpting in primates, and especially in the bilophodont teeth of the Papionini, which exploited a wide range of resources (Ulhaas et al., 2004; Bunn and Ungar, 2009; Pampush et al., 2018).
The present study focuses on the association between molar crown topography of the three upper molars (M1, M2, and M3) and the known dietary specializations of the Papionini specimens, which are analyzed to test the relationship between crown shape with different stage of wear and diet. If natural selection shapes primate teeth and retains their functionality as tooth wears, then the occlusal morphology of worn teeth will maintain these differences in order to also retain chewing efficiency throughout lifetime. Dental studies have shown significant levels of homoplasy in dental morphology in mammal lineages (Evans et al., 2007), as well as within the Papionini (Lockwood and Fleagle, 1999), and, so as a proxy of molar functional morphology, molar topography would be expected to reflect feeding strategies regarding the mechanical properties of food, rather than the phylogenetic proximity derived from molecular data alone. If confirmed, this could offer an important insight into the potential use of molar topography when studying the evolution of primate teeth.
Materials and methods
Studied samples
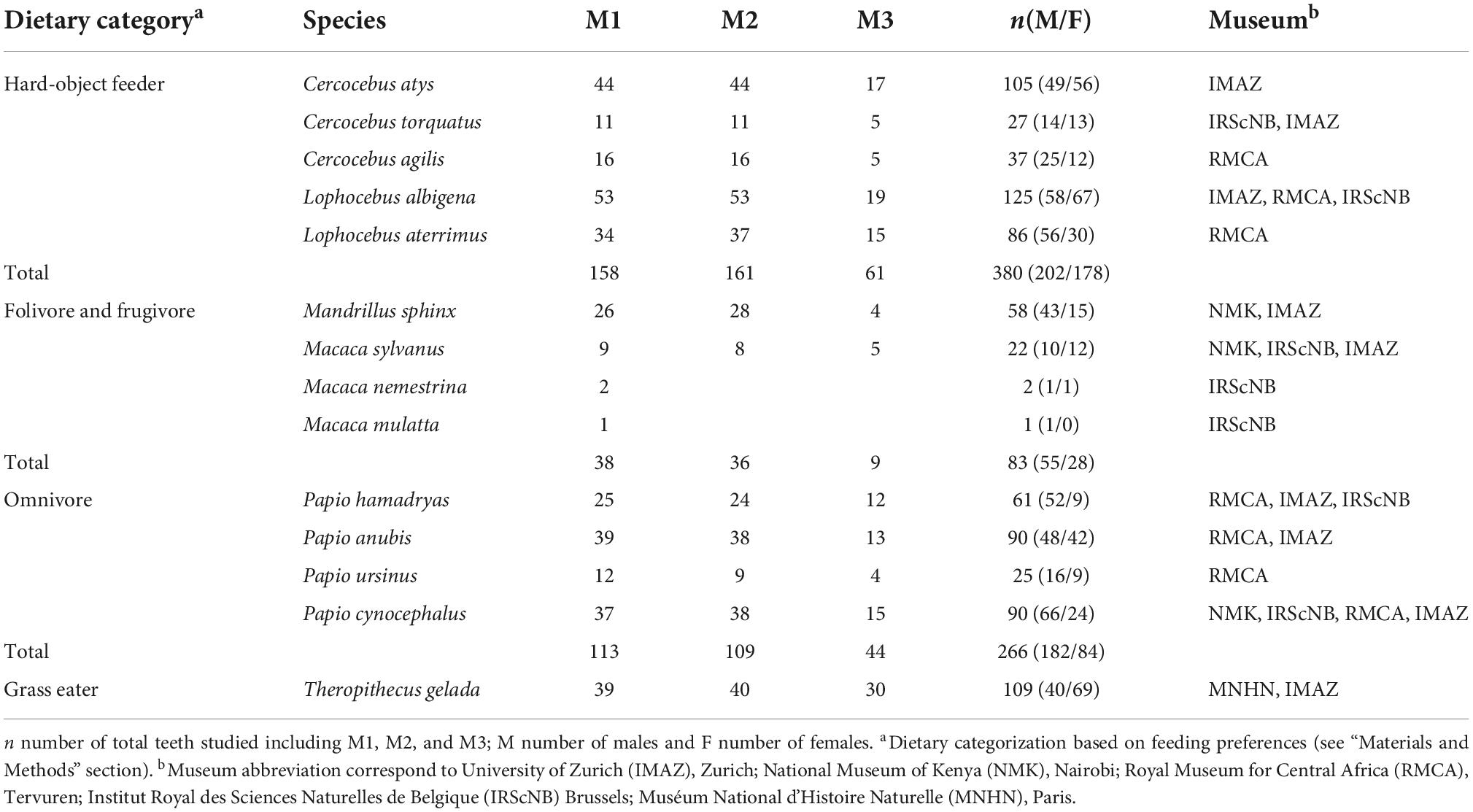
A total of 838 well-preserved, in situ (only molars that were placed in the maxillary row) first (M1; n = 345), second (M2; n = 347), and third (M3; n = 146) permanent upper molars were selected from maxillary tooth rows from the dry skulls of adult (third molar erupted and in full occlusion) specimens belonging to the Papionini tribe (Table 1), including six genera and fourteen species: Cercocebus (C. agilis, C. atys, C. torquatus), Lophocebus (L. albigena, L.aterrimus), Macaca (M. sylvanus, M.mulatta and M. nemestrina), Mandrillus (Ma. sphinx), Papio (P. anubis, P. cynocephalus, P. hamadryas, P. ursinus), and Theropithecus (T. gelada). The specimens were sexed according to available museum records. Maxillary molars were selected for analyses because they exhibit a stable bilophodont cusp pattern of four main cusps, with minor size differences compared to mandibular molars, and also lack the distinct morphology of the extended talonid of the lower third molars (Delson, 1975).
The species considered were grouped into four primary diet categories (hard-object feeders, folivore-frugivores, omnivores, and grass-eaters) on the basis of the percentage of fruits and seeds vs. leaves and underground storage organs (USOs) consumed (Quris, 1975; Hoshino, 1985; Lahm, 1986; Mitani, 1989; Maisels et al., 1994; Tutin et al., 1997; Hill and Dunbar, 2002; Shah, 2003; Thierry, 2007; McGraw et al., 2011; Fashing et al., 2014; Jarvey et al., 2018). The Lophocebus (L. albigena, L. aterrimus) and Cercocebus mangabeys (C. agilis, C. t. torquatus, C. torquatus atys) species are hard-object feeders, mainly consuming hard fruits and seeds, and sharing habitats and dietary preferences (Quris, 1975; Waser, 1975; Mitani, 1989; Olupot et al., 1997; Shah, 2003; McGraw et al., 2011; DeCasien et al., 2017). Mandrillus sphinx and Macaca sp. are folivore-frugivore taxa, mainly consuming leaves, and fruits, but also seeds and herbs during the fruit-scarce season (Hoshino, 1985; Ménard and Vallet, 1986; Lucas and Corlett, 1991; Tutin et al., 1997: DeCasien et al., 2017; Powell et al., 2017). Papio species (P. anubis, P. cynocephalus, P. hamadryas, P. ursinus) are generalized omnivores with greatly variable diets dominated by fruits, leaves, and underground items (Hill and Dunbar, 2002), and they also consume invertebrates and small mammals. Finally, Theropithecus gelada has been traditionally described as a graminivorous specialist (Dunbar and Bose, 1991), although their diet might be significantly broader (Fashing et al., 2014; Shapiro et al., 2016; Souron, 2018).
Dental wear scoring
Both unworn and lightly worn-out teeth were studied to test for the effect of occlusal wear on dental topography (Berthaume et al., 2018; Pampush et al., 2018). All the teeth were scored and classified using a five-stages dental wear template for cercopithecid species (Meikle, 1977) as follow: unworn (Grade 1), in erupted, though not yet full occlusion, molars; lightly worn (Grade 2), showing wear facets at or toward the top of the cusps but no part of the crown worn enough to expose the underlying dentine; moderate wear (Grade 3), circles of dentine exposure at the cusp apices, the protocone cusp and the lingual cusps being affected before other cusps, lacking connections between them; medium wear (Grade 4), buccal and lingual areas of dentine start to merge along the lophs, although dentine exposure is still greater on the lingual half of the lophs, and dentine contact between the mesial and distal areas is still lacking; marked wear (Grade 5), with larger and similar dentine exposure areas on the buccal and lingual halves, with an incipient mesio-distal dentine contact affecting the talon. These occlusal wear scores were then grouped into two gross-wear categories following previous procedures (Berthaume et al., 2018): Wear I (Meikle’s Grades 1 and 2), unworn or lightly worn molars without visible dentine exposure in the lingual cusps; Wear II (Meikle’s Grade 3, 4), molars with moderate cusp removal with exposed dentine and a coalescence of dentine areas (Supplementary Figure 1). Molars in the advanced wear stage (Grade 5) were excluded of the sample in this study.
Sample processing
Maxillary in situ tooth rows of original specimens were cleaned with cotton swabs moistened with pure ethanol prior to molding. Silicone-base molds were made using Affinis® Regular body (Coltène-Whaledent™) polyvinylsiloxane, and high-resolution casts were produced from the molds using the two-base component polyurethane Feropur PR-55 (Feroca® Composites, Spain) following standardized procedures (Galbany et al., 2006). Three-dimensional (3D) polygonal meshes of the dental casts were generated, using a structured light scanner (DAVID™ SLS-2) at a resolution of 0.05 mm, and were exported to polygon file format files (PLY). Meshes were post-processed using Geomagic™ Studio 2014 (3D Systems, Morrisville, USA) to isolate molar crowns by cropping the dental crown along the cervical and the interproximal rims. The meshes were slightly smoothed to reduce noise and cropped to the lowest point on the occlusal basin (BCO method; see Berthaume et al., 2019b), and finally simplified down to 10,000 polygons (Winchester, 2016). The processed polygonal models were imported into Meshlab (ISTI—CNR Research Centre, University of Pisa) and oriented to maximize the crown-base projection by placing the occlusal surfaces perpendicular to the Z-axis (Pampush et al., 2016).
Acquisition of three-dimensional dental topography
Dental topographic metrics were recorded from 3D polygonal meshes (Berthaume et al., 2018; Pampush et al., 2018) including the complexity (OPCR; Orientation Patch Count Rotated), the curvature (DNE; Dirichlet Normal Energy), and the occlusal relief (OR), using the MorphoTester software (Winchester, 2016), as well as the ambient occlusion (PCV; Percentage du Ciel Visible) using the CloudCompare 3D software following the outlined procedures (Berthaume et al., 2019a). Briefly, occlusal complexity (OPCR) quantifies the number of enamel patches with distinct surface orientation (Evans et al., 2007). It was calculated using a minimum patch size of five polygons and averaging the oriented patch values of eight rotations around the Z-axis, with a 45° mesh rotation of each iteration (Winchester, 2016). DNE was calculated as the sum of energy values across a polygonal mesh surface, and the algorithm was invariant to mesh size and orientation (Bunn et al., 2011; Winchester, 2016). Surface bending was proportional to the number of mesh polygons, however, thus requiring the 10,000-polygon standardization (Winchester, 2016; Berthaume et al., 2018); a 0.1% (99.9th percentile) energy × area outlier removal was used to prevent changes in energy output due to polygon vertices (Winchester, 2016). The occlusal relief (OR) was estimated as the ratio of the 3D surface area of the cropped mesh to the projected 2D planimetric surface area (Berthaume et al., 2019b). Finally, the average PCV was calculated for each tooth using the PCV command in the Portion of Visible Sky plugin following Berthaume et al. (2019a,2020) procedure.
Statistical analyses
Dental topographic variables were non-normally distributed by species (Shapiro–Wilk test; p < 0.01). Rank-transformed data was, thus, used for all the statistical analyses. A factorial ANOVA was run to test for the interaction effects of tooth-type (M1, M2, M3), sex (female, male), wear stages (I, II), and diet (hard-object feeders, folivore-frugivores, omnivores, grass-eaters) on crown shape descriptors (OPCR, DNE, OR and PCV). One-way ANOVAs on each variable by tooth-type and dietary groups were used to determine sources of significant variation by wear stages using Tukey’s honestly significance test (HSD) for the paired comparisons. Finally, a principal component analysis (PCA) was performed on the correlation matrix to determine patterns of topographic variation that accounted for variation in dietary groups. Descriptive and statistical procedures were performed in SPSS™ Statistics 22.0 (IBM, Armonk, NY, USA) and PAST 4.02. The significance level was set at α = 0.05.
Results
The multifactorial ANOVA showed that the effects of tooth-type, occlusal wear stage, and diet were highly significant (p < 0.001) for all the topographic metrics (Table 2), while the sex factor only showed a significant effect for OR (p = 0.018). Accordingly, sex was not considered for subsequent analyses. Significant interactions between factors (tooth, wear and diet) were observed only when the diet factor was included, which suggest that differences between dietary categories remained when tooth and wear are considered. The interactions of the model showed that there were significant differences for all the topographic variables for each tooth and dietary category tested and there were significant differences for complexity (p < 0.001) and OR (p = 0.021) when both wear stages and dietary categories were considered.
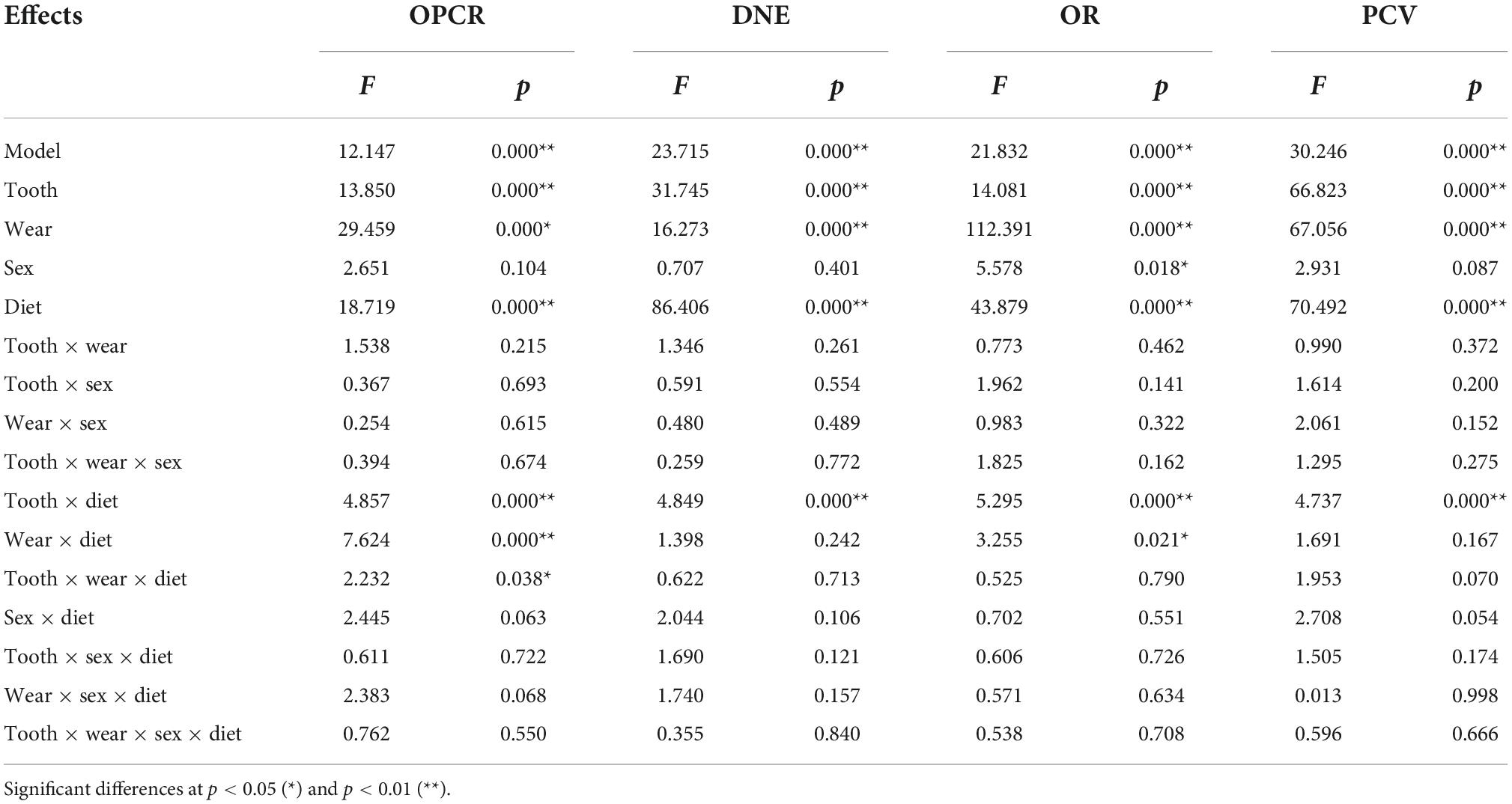
Table 2. Factorial ANOVA model for the effects of tooth-type, wear stage, sex, and diet on topographic metrics.
Effect of wear on dental shape
Overall, occlusal wear was shown to be a highly significant factor affecting dietary-specific dental shape descriptors (Tables 3–6). One-way ANOVA comparisons of OPCR by tooth-type and dietary groups showed that complexity increased with wear stages (I to II) except for the higher-crowned grass eater T. gelada. There were significant differences in complexity values between wear stages in all dietary groups for the first molar (M1), and for hard-object feeders and folivore-frugivores for the second (M2) and third (M3) molars (Table 3). DNE significantly decreased with wear for M1(r = –0.343; p < 0.01) and M2 (r = –0.408; p < 0.01) in hard-object feeders, and for M2 (r = –0.219; p = 0.021) and M3 (r = –0.475; p < 0.01) in omnivorous primates (Table 4). Relative cusp height loss (OR) was associated with tooth wear resistance (PCV) as a general trend. OR significantly decreased with wear for all the teeth in all the four dietary groups (Table 5), while PCV significantly increased with wear in all the dietary groups except for T. gelada for M1, and folivore-frugivore for M3, despite the fact that some sample sizes were small (Table 6). Overall, wear caused an increase in crown complexity (OPCR), not related to dentin exposure and probably due to the formation of wear facets, a reduction in crown curvature (DNE), due to increased flatness of occlusal enamel surfaces, a reduction of crown relief (OR) due to the loss of cusp heights, and an increase in ambient occlusion (PCV) as horizontal surfaces increase with wear.
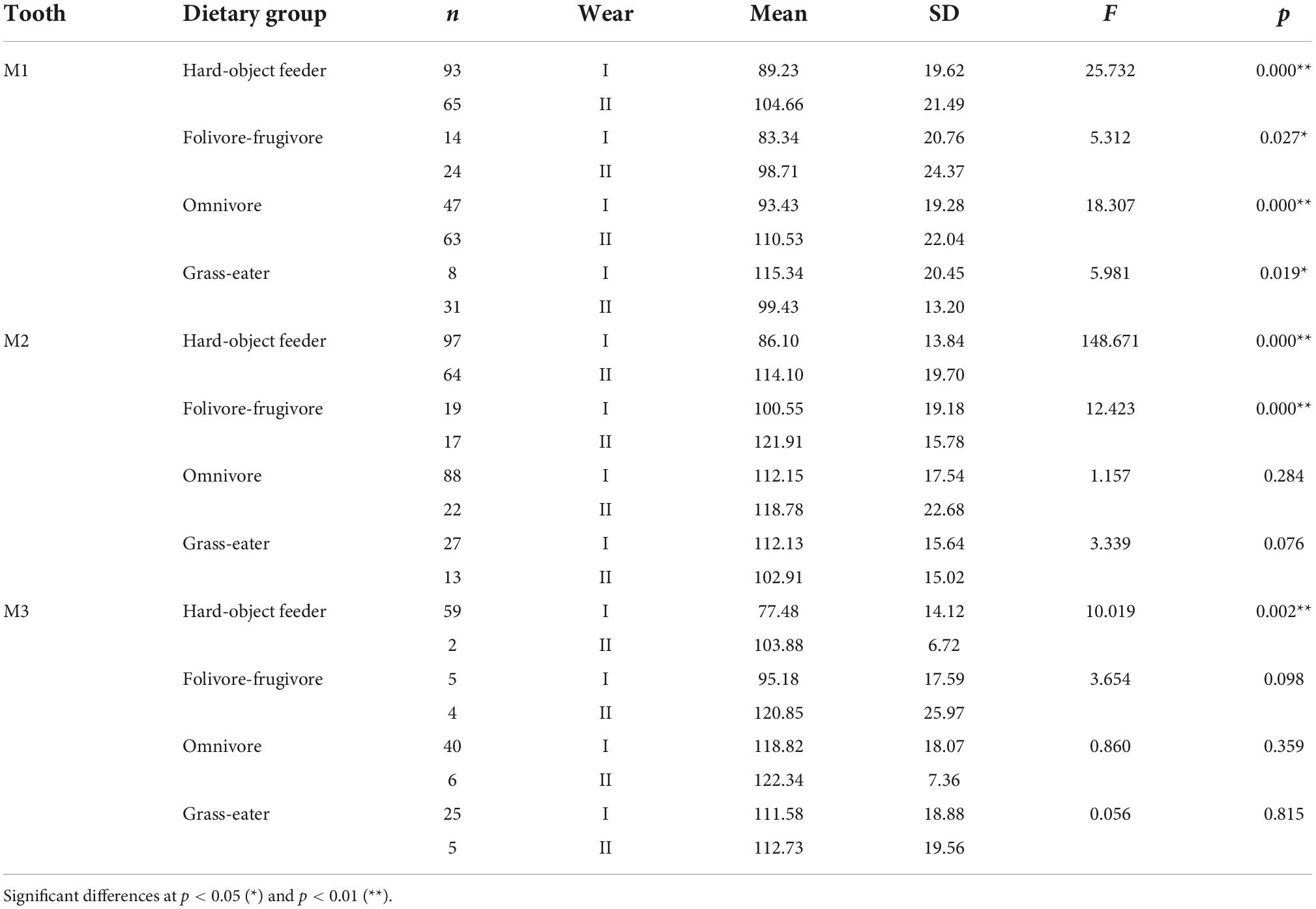
Table 3. Descriptive statistics and differences (one-factor ANOVA) for orientation patch count rotated (OPCR) among dietary grouping species by tooth-type and wear stage.
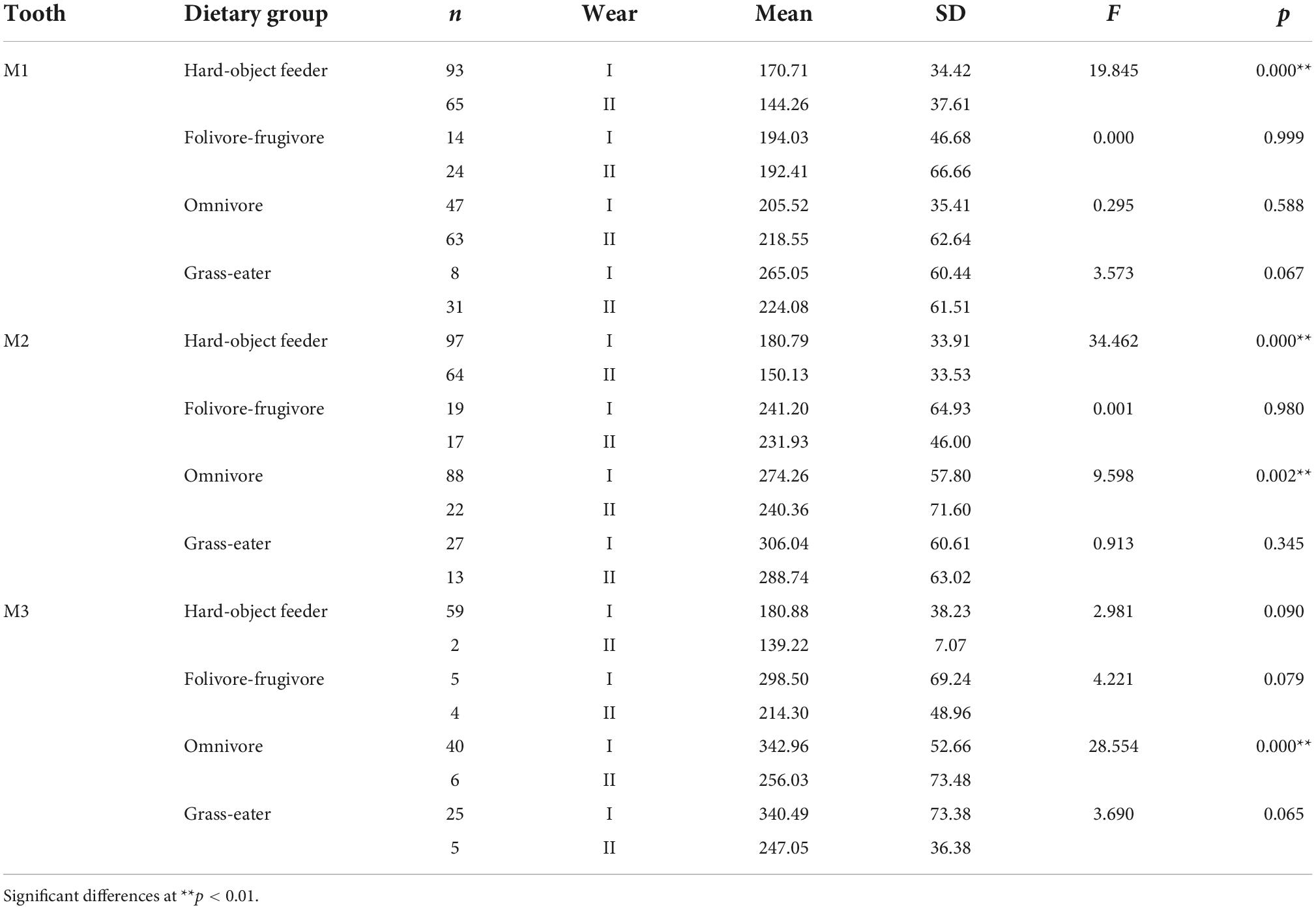
Table 4. Descriptive statistics and differences (one-factor ANOVA) for Dirichlet normal energy (DNE) among dietary grouping species by tooth-type and wear stage.
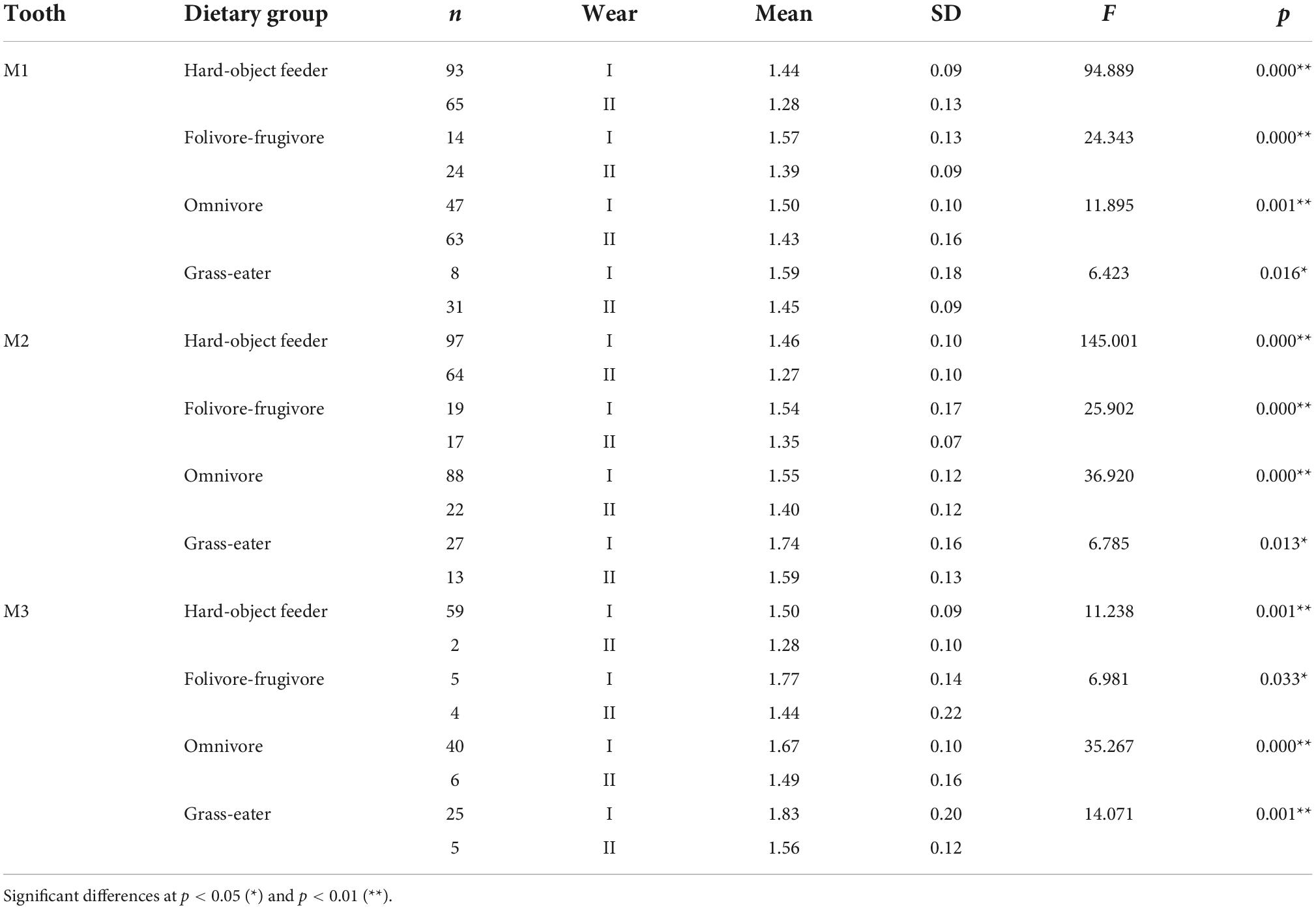
Table 5. Descriptive statistics and differences (one-factor ANOVA) for occlusal relief (OR) among dietary grouping species by tooth-type and wear stage.
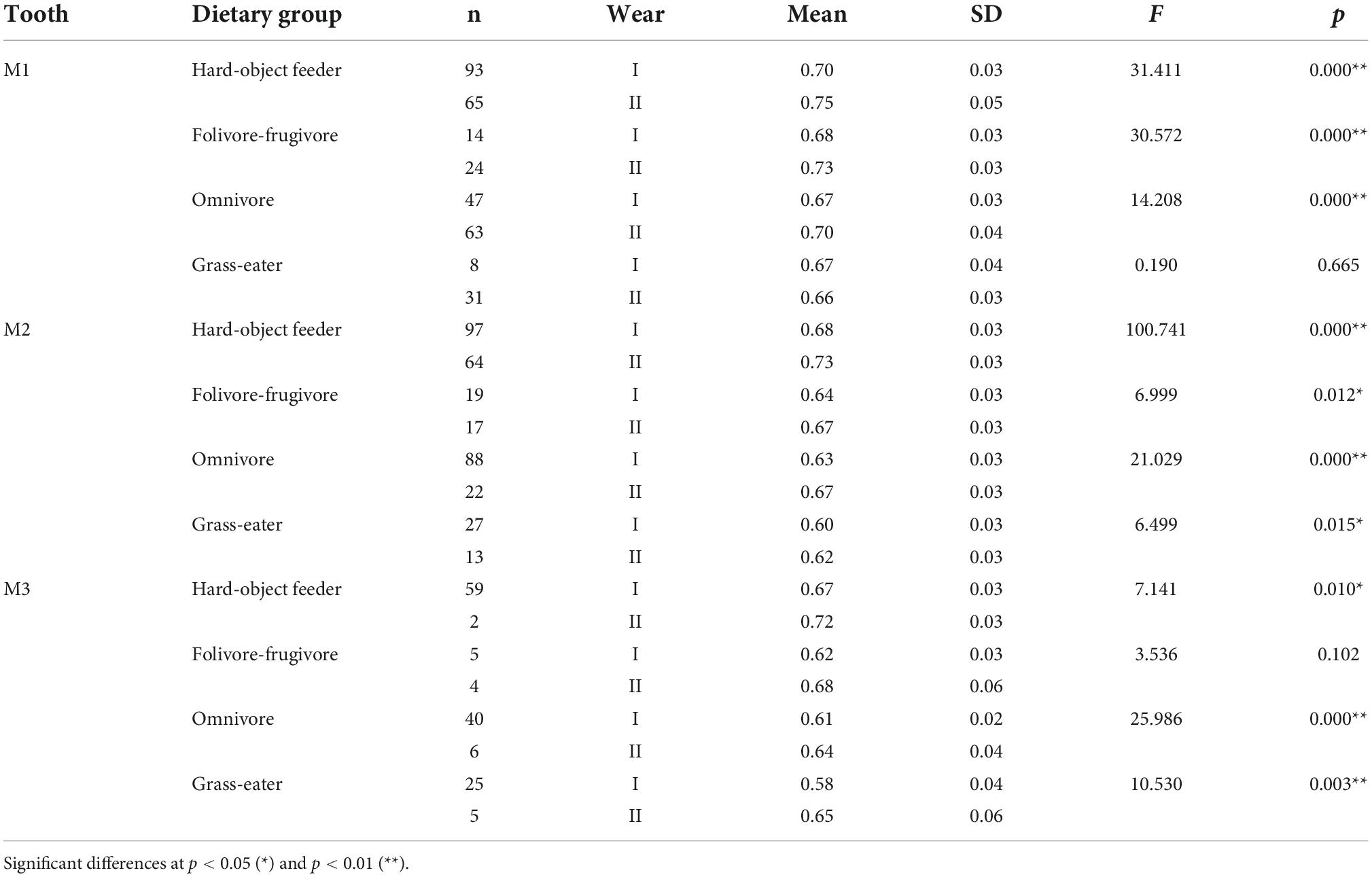
Table 6. Descriptive statistics and differences (one-factor ANOVA) for portion de ciel visible (PCV) among dietary grouping species by tooth-type and wear stage.
Dental shape patterns by dietary groups
The dental shape metrics showed significant differences among the four dietary groups. More differences in tooth shape existed between hard-object feeders and other dietary groups than between other dietary groups for both unworn and worn teeth (Tukey’s HSD; p < 0.05; Tables 6, 7). The topographic variables that most differed in hard-object feeders were PCV, OR and DNE, and their crowns showed the lowest curvature and OR values, and the highest PCV, characteristic of low rounded cusps. OPCR was also significant lower in this group (p < 0.01).
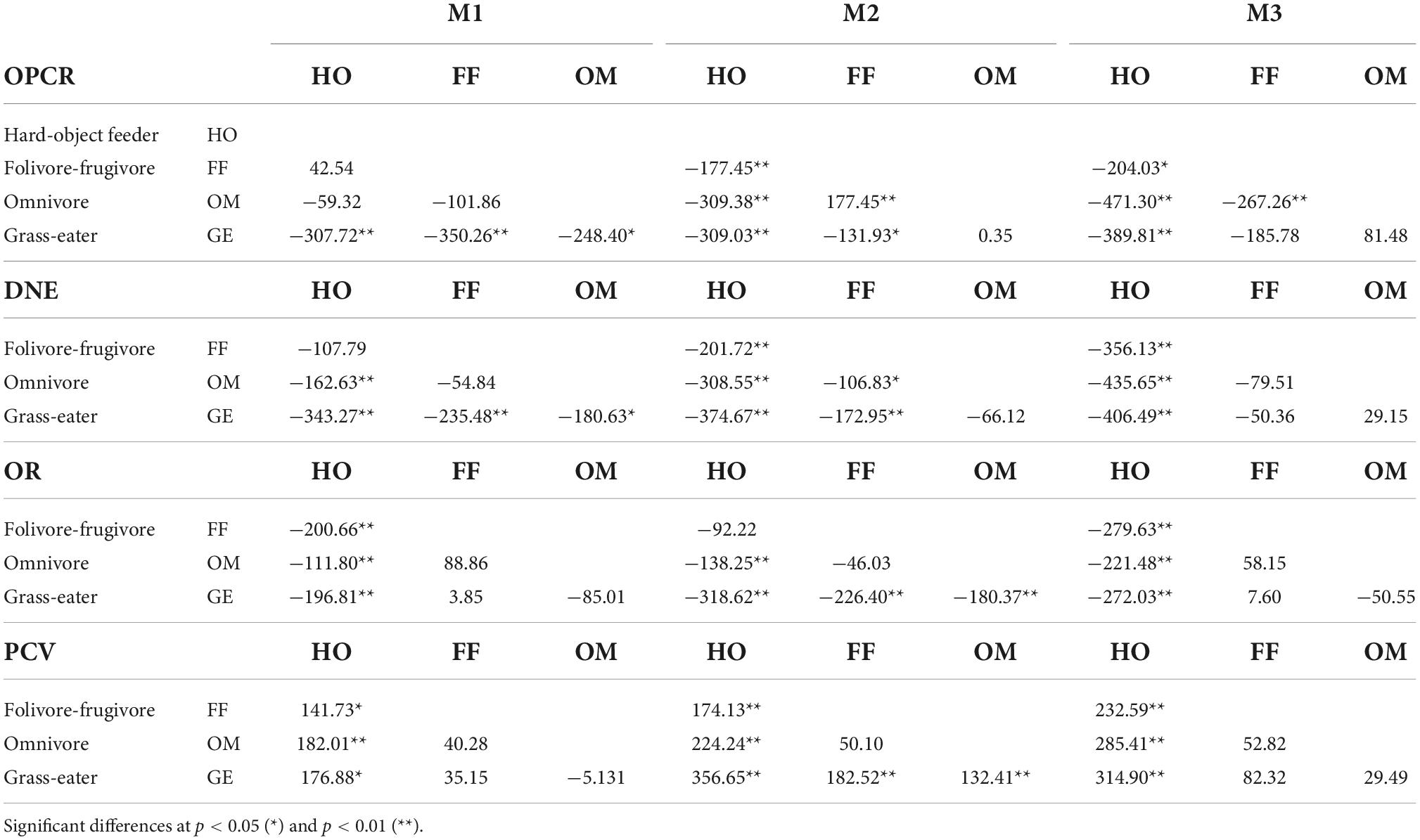
Table 7. Tukey matrices of pairwise mean differences between dietary groups for topographic metrics by tooth-type (wear I).
Graminivorous T. gelada showed the highest DNE and OR values and the lowest PCV values for all three teeth (Tables 3–6), a trend demonstrated by the paired, post hoc comparisons (Table 7). The post hoc pairwise differences (Tukey’s HSD; p < 0.05; Table 7) for the wear score I, showed that T. gelada differed significantly from all other diet groups in exhibiting higher OPCR and DNE values for M1, and differed significantly for OR and PCV from the hard-object feeders. The DNE and OPCR of the second lower molars of the graminivorous group also differed significantly from the frugivore/folivores, the former having a higher curvature and complexity, when the tooth crown was unworn or slightly worn. Greater OR values from graminivorous species denoted high-crowned molars and an expected inverse trend for PCV which differed significantly from the other dietary groups (Table 7).
The omnivores showed significantly higher complexities in all tooth crowns, but especially so in the second upper molar, which differed from the hard-object feeders and the folivore/frugivores groups (p < 0.01; Table 7). The DNE of the omnivores was significantly lower than that of the folivore/frugivore and hard-object feeders’ groups for the unworn M2.
Lastly, the folivore/frugivore group showed few significant differences from the rest of the groups. The topographic parameters that differed significantly in this group compared to the hard-object feeders for the M1, M2 and M3, were OR and PCV, a trend similar to that observed between the omnivores and graminivorous groups.
In general, the between-diet comparisons of the worn teeth (wear score II) showed less inter-group differences, although they were similar in the M1 (Table 8). Dental complexity decreased as wear increased and, non-significant differences between diet groups were therefore observed for OPCR for the three teeth. DNE, OR and PCV showed similar trends to those seen in the unworn (wear score I) teeth, however T. gelada preserved the highest DNE and OR values, albeit smaller than those of the unworn teeth (Tables 3–6). Hard-object feeders showed the lowest values of DNE and OR compared to the rest of the groups while the PCV maintained the highest value despite the wear. These differences in M2 were significant for the hard-object eater group compared to the rest of the groups considered (except for the PCV comparison between folivore-frugivores, which was not significantly different).
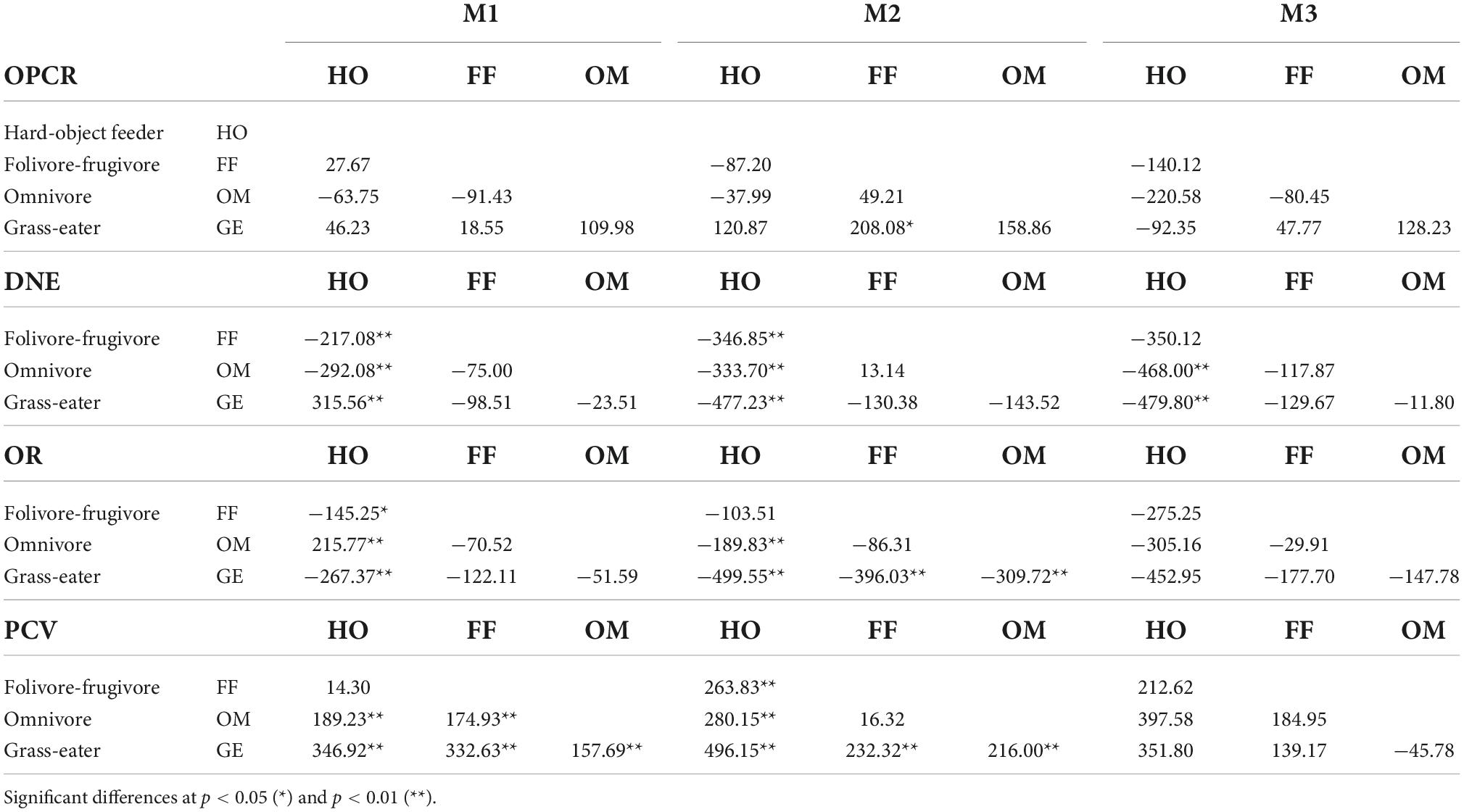
Table 8. Tukey matrices of pairwise mean differences between dietary groups for topographic metrics by tooth-type (wear II).
The PCA summarized the trends in the topographic metrics of the dietary defined groups (Table 9 and Figure 2). The first two principal components (PC1-2) derived for each tooth (M1, M2, and M3) explained > 85% of the total variance in molar topography of the Papionini for the dietary preferences factor (Table 9). DNE and OR loaded positively (r > 0.6; p < 0.01) onto the PC1 (> 45% of total variance), while PCV showed strong negative loading (r < −0.88; p < 0.01) with PC1. This component clearly separated the hard-object feeders (negative PC1 values) from the omnivores and T. gelada (positive PC1 values) for all three molars, and a greater extent in the wear score I group compared to the II group (Figure 2). PC2 explained < 45% of total variance and was strongly and positively correlated with OPCR and (r = 0.971; p < 0.01) and DNE (r = 0.735; p < 0.01) for M1 (Table 9). As expected, the PCV for PC2 showed positive loadings with PC2 while OR showed negative loadings for all three teeth and the two wear groups (I and II) smaller OR values result in larger PCV values as the tooth wears down. Similar loadings were obtained between the topographic metrics and the PCs for all the teeth considered, and for both wear categories (Figure 2), which is consistent with the hypothesis that wear tends to decrease crown shape among dietary groups.
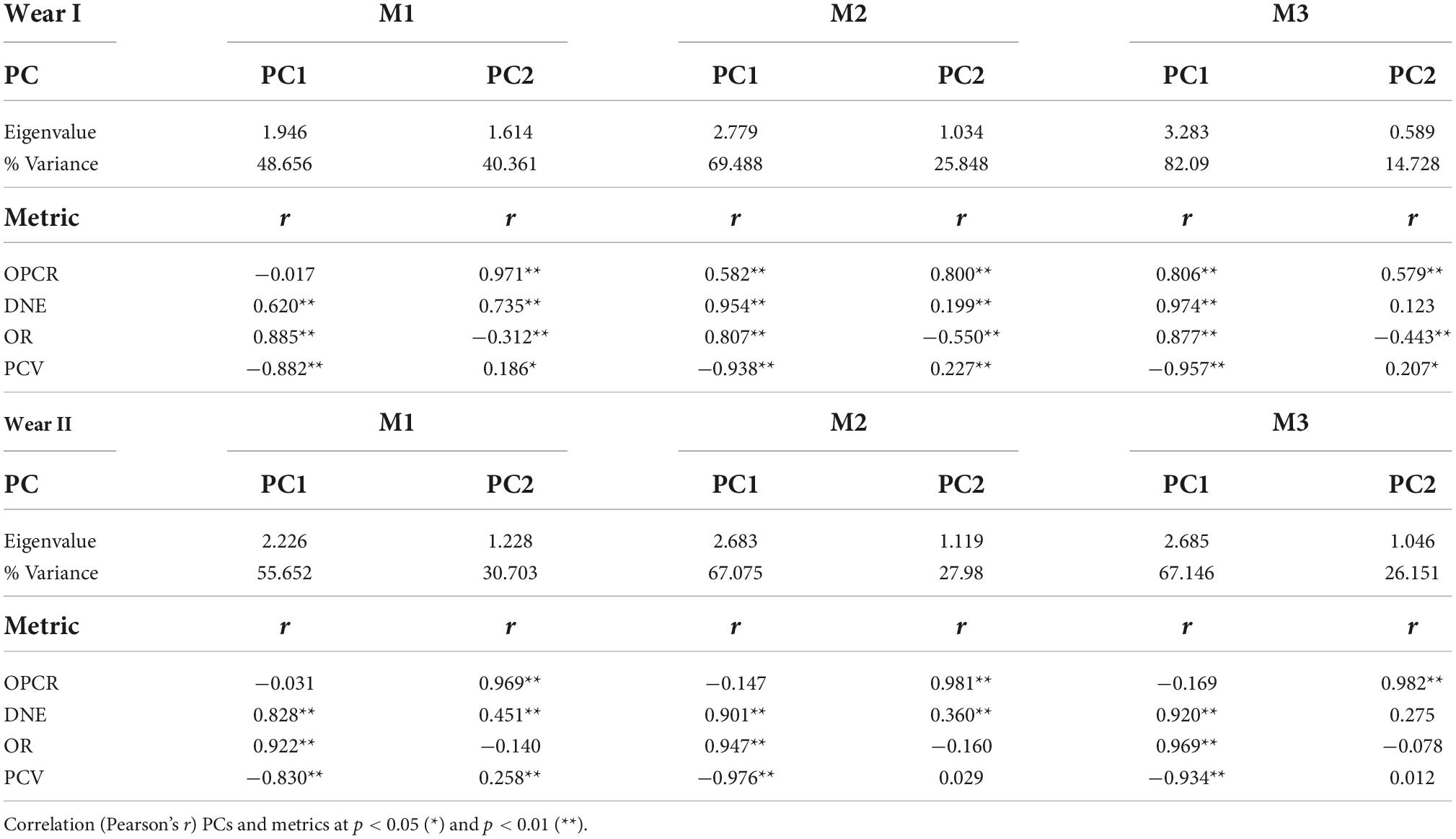
Table 9. Factor loadings of the first two principal components (PC1-2) on dental topographic metrics for the teeth analyzed by wear stage.
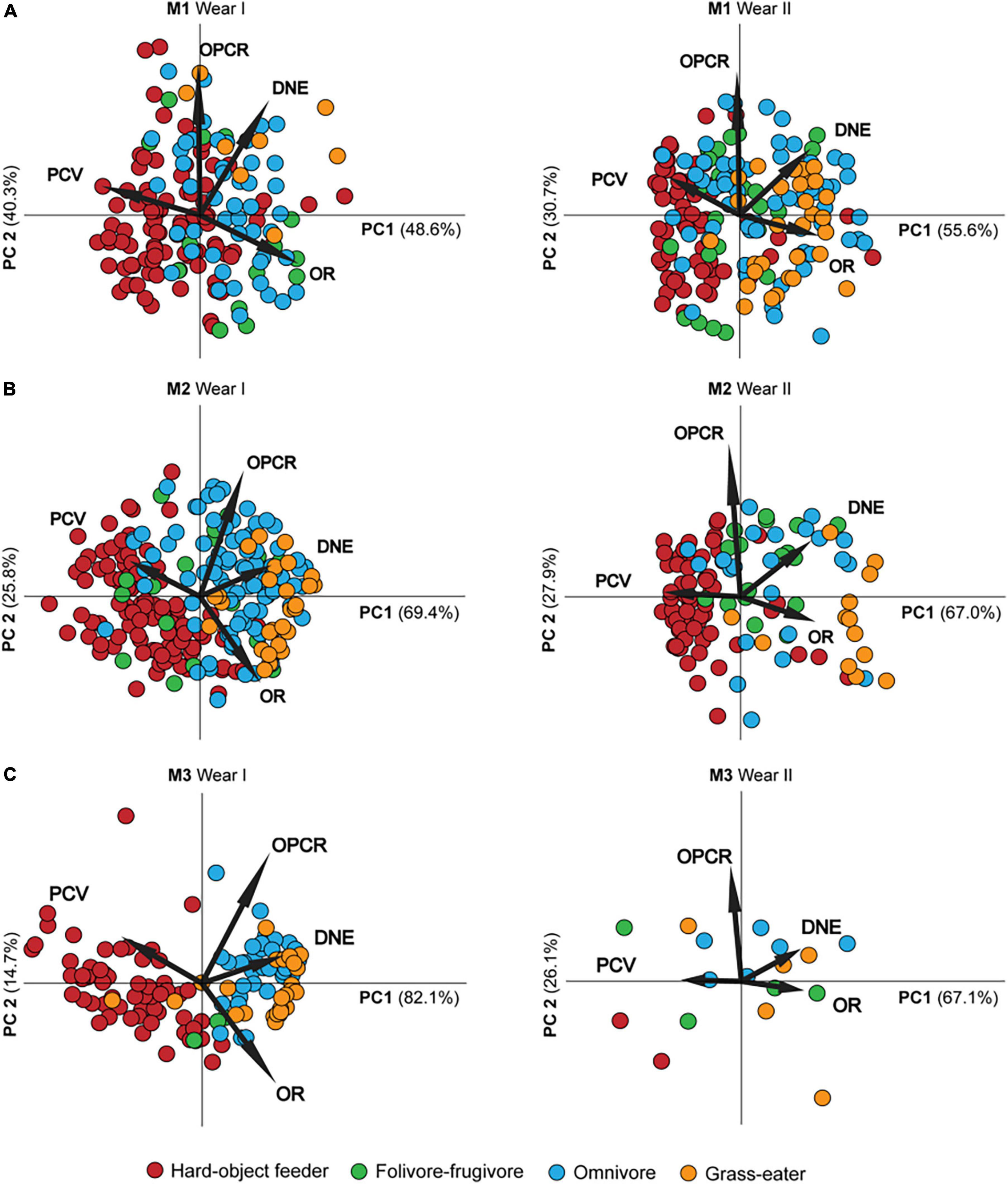
Figure 2. Scatter plot of the first two principal components (PC1-2) for first (A), second (B) and third (C) permanent maxillary molars by wear stage (Wear I and II) accounting for > 85% of the shape descriptor metrics variance between dietary groups. The labeled rays show the loadings of the topographic metrics onto PCs. Note the differences in molar topographic patterns despite wear clearly reflecting the adaptive variation of feeding habits.
Discussion
The 3D crown topography of the upper molars (M1-3) of the studied Papionini shows a clear diet-related signal despite the overall homogeneity of the bilophodont dentition. The observed, dietary related among groups differences in crown topography reflect distinct adaptations to resource exploitation. The unworn dental crowns show a stronger dietary signal than the worn teeth, but the analyzed worn teeth still retained an overall dietary signal, which may allow to gather larger samples than if only unworn teeth are considered.
Tooth shape and diet
The dental crowns of hard-object feeders tend to have lower and less complex cusps with more horizontally oriented enamel surfaces (Berthaume et al., 2019a) as an adaptation for breaking and grinding hard and brittle foods, such as seeds and nuts (Lucas, 2004; Ungar, 2004). Within the Papionini, the bilophodont morphology varies from hard object consumers to grass-eaters in relation to the amount of hard, brittle food particles chewed. Although OPCR has been applied to both extant and fossil taxa, Pineda-Muñoz et al. (2017) have suggested that it may have a reliable discriminating power only when applied to disparate tooth morphologies. We found, however, significant differences for the first molar (M1) between the high-crowned teeth of grass eaters and the rest of dietary groups. Moreover, most of the dietary groups showed significant differences in complexity for the second (M2) and third (M3) upper molar, suggesting that complexity varied between tooth type and the diet considered.
Among sympatric African apes, the DNE of the second lower molar failed to differentiate folivores from frugivores except when dietary competition (between large bodied primates) was considered due to character displacement (Berthaume and Schroer, 2017). We showed that the curvature, complexity and morphological wear resistance of the upper molars (M1-3) of hard-object feeders differs among the Papionini from the other dietary groups considered. The multivariate analyses shown clearly differentiates the hard-object feeders Papionini from the omnivores and grass eaters, represented here by Papio and T. gelada, respectively. Theropithecus have dental crowns with high cusps and deep valleys, as shown by their high crown curvatures and reliefs which reflects an adaptation for increased chewing surface areas to facilitate the food processing activity of digestive enzymes (Szalay and Delson, 1979; Dunbar and Bose, 1991). Among Papio species, the dietary habits of P. ursinus, who inhabit heterogeneous ecosystems in Southern Africa, include the consumption of up to 30–50% of grasses in their diet, which is a higher value than that reported for baboons from African savanna environments (Codron et al., 2005). A 56% intake of graminoid grasses has been reported for T. gelada from Guassa (Fashing et al., 2014). These similarities in DNE values between omnivores and grass-eaters could be related to similar dietary preferences, although Papio diet is more diverse than Theropithecus diet (Hill and Dunbar, 2002; Souron, 2018). Moreover, high crown cap curvatures have also been related to the consumption of fibers, leaves and foods of low digestibility, as well as insects, both in platyrrhine and in Hominoidea primates (Bunn et al., 2011; Winchester et al., 2014; Berthaume and Schroer, 2017).
Shape and wear in relation to diet
All the topographic metrics studied showed significant differences in relation to both wear and diet factors, suggesting that dental crown shape differences remain between dietary types despite an increased loss of dental crown through wear, at least in the upper bilophodont teeth analyzed. The OPCR, PCV and OR were the topographic metrics most sensitive to wear in all dietary groups considered. Overall, dental crown shape varies as cusps begin to decrease in height. Dentine is exposed in bilophodont teeth from the cusp tip, gradually developing an enamel ridge around the dentine pool. Unworn or slightly worn teeth show lower morphological wear resistance (PCV), as has already been suggested for bunodont teeth (Berthaume et al., 2018), while DNE and OPCR tend to show lower values in worn teeth. It has been suggested that DNE increases with macrowear (Pampush et al., 2016), especially in folivorous primates (Pampush et al., 2018), which is consistent with the dental sculpting hypothesis, suggesting that DNE could be used to study wear-related variably in different primate groups, including cercopithecoids. Overall, our results show that DNE decreases with wear, but significant differences were only observed in the hard-object feeders and the omnivores, which suggests that the dental sculpting hypothesis is not evident in these dietary groups for the first stages of wear, in line with the hypothesis proposed by Bunn et al. (2011) and Winchester et al. (2014).
On the other hand, OPCR and OR showed a significant interaction with wear. OR demonstrated significant interaction for M1 and M2 in all dietary groups, and for M2. These results suggest that there were differences in complexity along wear stages specifically for the M1 of all dietary groups, and for the M2 in hard-object feeders and folivore-frugivore. The occlusal relief decreased significantly with wear, which was an expected association since tooth height decreases as enamel and dentine tissue is lost (M’Kirera and Ungar, 2003; Ungar, 2015; Ungar et al., 2018; Romero et al., 2022). High OPCR values, indicative of a complex surface topography, may be indicative of a great ability to shear fibrous foods (Evans et al., 2007; Evans and Janis, 2014). OPCR showed increased values along wear stages in the upper bilophodont molars in all the dietary groups except in the grass-eater Theropithecus. This suggests that T. gelada maintains the functional complexity of occlusal morphology with wear. Although a dental sculpting hypothesis was initially described for hypsodont taxa (Fortelius, 1985; Jablonski, 1994) proposed a similar ungulate-like secondary morphology for Theropithecus, which has high-crowned molars with deep and widely separated basins showing long shearing blades apparently adapted to a predominantly grass-eating diet (Jolly, 1970; Teaford, 1993). Our results, however, denote that the crown cap of Theropithecus did not show significantly increased OPCR values in worn out teeth consistent with the dental sculpting hypothesis. On the contrary, the complexity tended to decrease between wear stages in the upper first and second molars. Occlusal relief (OR) tends to be maintained regardless of wear patterns in both folivore and hard-object feeder primates, the folivores showing a higher relief, sharp edges and sloping surfaces at a given stage of wear (M’Kirera and Ungar, 2003; Ungar and M’Kirera, 2003; Bunn and Ungar, 2009), while omnivores and hard object eaters show lower shearing crests, similar to frugivores (Winchester et al., 2014; Allen et al., 2015). The distribution of both the unworn and worn tooth crowns caps in the morphospace was practically the same in the present study, and hard-object and grass feeders were clearly distinct from the other dietary groups. Although topographic metrics might differ between wear stages, the differences in cap topography among dietary groups remained for DNE, PCV and OR, suggesting that dental shape changes due to wear did not obscure the distinct dietary-related topographies, at least for the lightly worn teeth considered in this study.
Tooth shape and phylogeny
The dental topography of the upper molars (M1-3) seems to better reflect dietary specializations than phylogenetic proximity. Lophocebus and Cercocebus, which both have a hard-object dietary regime, cluster together in our topographic morphospace, and genetic evidence shows that Lophocebus is more closely related to baboons (Disotell et al., 1992; Disotell, 1994, 2000; Harris and Disotell, 1998; Harris, 2000). As some authors have suggested, this is consistent with processes of evolutionary convergence affecting the Papionini (Lockwood and Fleagle, 1999; Lycett and Collard, 2005; Collard and Wood, 2007), as also shown by inconsistencies between molecular and morphological inferences (Guevara and Steiper, 2014; Liedigk et al., 2014; Pugh et al., 2018). Skeletal structures that are subject to great biomechanical stress are prone to homoplasies compared to regions with reduced stress (Lycett and Collard, 2005; Collard and Wood, 2007). The masticatory apparatus in the skull has great biomechanical constraints related to craniofacial bone tensions during the chewing cycle (Wall, 1999; Vinyard et al., 2003), resulting in a greater phenotypic plasticity that means the associated structures are less reliable indicators of phylogeny (Lieberman, 1995) compared to a dietary-related proxy.
The dietary classifications used in the present study represent year-round resource exploitation groups, however, seasonal fallback foods may play a more important role in shaping tooth morphology when favored items are inaccessible for primate species (Thiery et al., 2017b; Ungar et al., 2018). In order to use dental topography as a proxy for functional morphology to infer dietary strategies, it is important to understand such ecological and dietary determinants in the Papionini clade. Our findings may contribute to understanding the evolution of dental crown morphology in relation with environmental changes and dietary resources in the Papionini lineage along the Plio-Pleistocene, and thus to the study of the isolated teeth, both unworn and worn, that are abundant in the fossil record. The Papionini model may be an analogous model with which to interpret the evolutionary trend of the Hominini lineage, as both the Papionini and Homini lineages emerged during the Pliocene in African sites, and, therefore, confronted the same ecological constraints and seasonal shifts in resource acquisition. Understanding functional adaptations to different ecological strategies may be a clue to allowing us to infer diet in fossil taxa.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LM, AR, FE-S, and AP-P conceived the research and approach and led the manuscript writing. YA, AR, and LM conducted the analyses. AP-P and AR acquired the funding. YA, LM, FE-S, and EC-T collected the material. All authors contributed to the writing of the manuscript and approved it for publication.
Funding
Grants PID2020-112963GB-I00 to APP and PID2020-114517GB-I00 to AR, funded by MCIN/AEI/ 10.13039/501100011033 and by “ERDF A way of making Europe”, by the “European Union”. www.paleobaboonproject.science.
Acknowledgments
We are grateful to all the curators and technical personnel of the different institutions where the specimens were molded. We thank all the work of the reviewers and the editor that help to improve the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.969007/full#supplementary-material
References
Allen, K. L., Cooke, S. B., Gonzales, L. A., and Kay, R. F. (2015). Dietary inference from upper and lower molar morphology in platyrrhine primates. PLoS One 10:e0118732. doi: 10.1371/journal.pone.0118732
Berthaume, M. A. (2016). On the relationship between tooth shape and masticatory efficiency: a finite element study. Anat. Rec. 299, 679–687. doi: 10.1002/ar.23328
Berthaume, M. A., Delezene, L. K., and Kupczik, K. (2018). Dental topography and the diet of Homo naledi. J. Hum. Evol. 118, 14–26. doi: 10.1016/j.jhevol.2018.02.006
Berthaume, M. A., Lazzari, V., and Guy, F. (2020). The landscape of tooth shape: over 20 years of dental topography in primates. Evol. Anthropol. 29, 245–262. doi: 10.1002/evan.21856
Berthaume, M. A., and Schroer, K. (2017). Extant ape dental topography and its implications for reconstructing the emergence of early Homo. J. Hum. Evol. 112, 15–29. doi: 10.1016/j.jhevol.2017.09.001
Berthaume, M. A., Winchester, J., and Kupczik, K. (2019a). Ambient occlusion and PCV (portion de ciel visible): a new dental topographic metric and proxy of morphological wear resistance. PLoS One 14:e0215436. doi: 10.1371/journal.pone.0215436
Berthaume, M. A., Winchester, J., and Kupczik, K. (2019b). Effects of cropping, smoothing, triangle count, and mesh resolution on 6 dental topographic metrics. PLoS One 14:e0216229. doi: 10.1371/journal.pone.0216229
Brugiere, D., Gautier, J. P., Moungazi, A., and Gautier-Hion, A. (2002). Primate diet and biomass in relation to vegetation composition and fruiting phenology in a rain forest in Gabon. Am. J. Primatol. 23, 999–1024.
Bunn, J. M., Boyer, D. M., Lipman, Y., St. Clair, E. M., Jernvall, J., and Daubechies, I. (2011). Comparing Dirichlet normal surface energy of tooth crowns, a new technique of molar shape quantification for dietary inference, with previous methods in isolation and in combination. Am. J. Phys. Anthropol. 145, 247–261. doi: 10.1002/ajpa.21489
Bunn, J. M., and Ungar, P. S. (2009). Dental topography and diets of four old world monkey species. Am. J. Primatol. 71, 466–477. doi: 10.1002/ajp.20676
Codron, J., Codron, D., Lee-Thorp, J. A., Sponheimer, M., Bond, W. J., de Ruiter, D., et al. (2005). Taxonomic, anatomical, and spatio-temporal variations in the stable carbon and nitrogen isotopic compositions of plants from an African savanna. J. Archaeol. Sci. 32, 1757–1772. doi: 10.5167/uzh-25350
Collard, M., and O’Higgins, P. (2002). Ontogeny and homoplasy in the papionin monkey face. Evol. Dev. 3, 322–331. doi: 10.1046/j.1525-142x.2001.01042.x
Collard, M., and Wood, B. (2007). “Defining the genus homo,” in Handbook of Paleoanthropology, eds W. Henke and I. Tattersall (New York, NY: Springer), 1575–1610. doi: 10.1007/978-3-642-39979-4_51
Daegling, D. J., and McGraw, W. S. (2007). Functional morphology of the mangabey mandibular corpus: relationship to dental specializations and feeding behavior. Am. J. Phys. Anthropol. 134, 50–62. doi: 10.1002/ajpa.20621
Daegling, D. J., McGraw, W. S., Ungar, P. S., Pampush, J. D., Vick, A. E., and Bitty, E. A. (2011). Hard-Object feeding in sooty mangabeys (Cercocebus atys) and interpretation of early hominin feeding ecology. PLoS One 6:e23095. doi: 10.1371/journal.pone.0023095
DeCasien, A. R., Williams, S. A., and Higham, J. P. (2017). Primate brain size is predicted by diet but not sociality. Nat. Ecol. Evol. 1, 1–7.
Delson, E. (1975). “Evolutionary history of the Cercopithecidae,” in Approaches to Primate Paleobiology: Contributions to Primatology, ed. F. Szalay (Basel: S. Karger).
Dennis, J. C., Ungar, P. S., Teaford, M. F., and Glander, K. E. (2004). Dental topography and molar wear in Alouatta palliata from Costa Rica. Am. J. Phys. Anthropol. 125, 152–161. doi: 10.1002/ajpa.10379
Disotell, T. R. (1994). Generic level relationships of the Papionini (Cercopithecoidea). Am. J. Phys. Anthropol. 94, 47–57. doi: 10.1002/ajpa.1330940105
Disotell, T. R. (2000). “Molecular systematics of the Cercopithecidae,” in Old World Monkeys, eds P. F. Whitehead and C. J. Jolly (New York, NY: Cambridge University Press), doi: 10.1111/j.1096-3642.2006.00253.x
Disotell, T. R., Honeycutt, R. L., and Ruvulo, M. (1992). Mitochondrial DNA phylogeny of the Old-World monkey tribe Papionini. Mol. Biol. Evol. 9, 1–13. doi: 10.1093/oxfordjournals.molbev.a040700
Dunbar, R. I. M., and Bose, U. (1991). Adaptation to grass-eating in gelada baboons. Primates 32, 1–7. doi: 10.1007/BF02381596
Evans, A. R., and Janis, C. M. (2014). The evolution of high dental complexity in the horse lineage. Ann. Zool. Fenn. 51, 73–79. doi: 10.5735/086.051.0209
Evans, A. R., Wilson, G. P., Fortelius, M., and Jernvall, J. (2007). High-level similarity of dentitions in carnivorans and rodents. Nature 445, 78–81. doi: 10.1038/nature05433
Fashing, P. J., Nguyen, N., Venkataraman, V. V., and Kerby, J. T. (2014). Gelada feeding ecology in an intact ecosystem at Guassa, Ethiopia: variability over time and implications for theropith and hominin dietary evolution. Am. J. Phys. Anthropol. 155, 1–16. doi: 10.1002/ajpa.22559
Fleagle, J. G., and McGraw, W. S. (1999). Skeletal and dental morphology supports diphyletic origin of baboons and mandrills. PNAS 96, 1157–1161. doi: 10.1073/pnas.96.3.1157
Fleagle, J. G., and McGraw, W. S. (2002). Skeletal and dental morphology of African papionins: unmasking a cryptic clade. J. Hum. Evol. 42, 267–292. doi: 10.1006/jhev.2001.0526
Fortelius, M. (1985). Ungulate cheek teeth: developmental, functional, and evolutionary interrelations. Acta Zool. Fenn. 180, 1–76.
Galbany, J., Estebaranz, F., Martínez, L. M., Romero, A., De Juan, J., Turbón, D., et al. (2006). Comparative analysis of dental enamel polyvinylsiloxane impression and polyurethane casting methods for SEM research. Microsc. Res. Tech. 69, 246–252. doi: 10.1002/jemt.20296
Gilbert, C. C. (2007). Identification and description of the first Theropithecus (Primates: Cercopithecidae) material from Bolt’s Farm, South Africa. Ann. Transvaal Mus. 44, 1–10.
Gilbert, C. C. (2013). Cladistic analysis of extant and fossil African papionins using craniodental data. J. Hum. Evol. 64, 399–433. doi: 10.1016/j.jhevol.2013.01.013
Gilbert, C. C., Frost, S. R., and Strait, D. S. (2009). Allometry, sexual dimorphism, and phylogeny: a cladistic analysis of extant African papionins using craniodental data. J. Hum. Evol. 57, 298–232. doi: 10.1016/j.jhevol.2009.05.013
Gilbert, C. C., and Rossie, J. B. (2007). Congruence of molecules and morphology using a narrow allometric approach. PNAS 104, 11910–11914. doi: 10.1073/pnas.0702174104
Glowacka, H., McFarlin, S. C., Catlett, K. K., Mudakikwa, A., Bromage, T. G., Cranfield, M. R., et al. (2016). Age-related changes in molar topography and shearing crest length in a wild population of mountain gorillas from Volcanoes National Park, Rwanda. Am. J. Phys. Anthropol 160, 3–15. doi: 10.1002/ajpa.22943
Guevara, E. E., and Steiper, M. E. (2014). Molecular phylogenetic analysis of the Papionina using concatenation and species tree methods. J. Hum. Evol. 66, 18–28. doi: 10.1016/j.jhevol.2013.09.003
Harris, E. E. (2000). Molecular systematics of the Old World monkey tribe Papionini: analysis of the total available genetic sequences. J. Hum. Evol. 38, 235–256. doi: 10.1006/jhev.1999.0318
Harris, E. E., and Disotell, T. R. (1998). Nuclear gene trees and the phylogenetic relationships of the mangabeys (Primates: Papionini). Mol. Biol. Evol. 15, 892–900. doi: 10.1093/oxfordjournals.molbev.a025993
Hill, R. A., and Dunbar, R. I. M. (2002). Climatic determinants of diet and foraging behaviour in baboons. Evol. Ecol. 16, 579–593. doi: 10.1023/A1021625003597
Hoshino, J. (1985). Feeding ecology of mandrills (Mandrillus sphinx) in campo animal reserve, cameroon. Primates 26, 248–273. doi: 10.1007/BF02382401
Hylander, W. L. (1975). Incisor size and diet in anthropoids with special reference to Cercopithecidae. Science 189, 1095–1098. doi: 10.1126/science.808855
Hylander, W. L. (1979). Mandibular function in Galago crassicaudatus and Macaca fascicularis, an in vivo approach to stress analysis of the mandible. J. Morphol. 159, 253–296. doi: 10.1002/jmor.1051590208
Jablonski, N. G. (1993). “Evolution of the masticatory apparatus in Theropithecus”, in Theropithecus: the Rise and Fall of a Primate Genus, ed N. G. Jablonski (Cambridge: Cambridge University Press).
Jablonski, N. G. (1994). Convergent evolution in the dentitions of grazing macropodine marsupials and the grass-eating cercopithecine primate Theropithecus gelada. J. R. Soc. West. Aust. 77, 37–43.
Jarvey, J. C., Low, B. S., Pappano, D. J., Bergman, T. J., and Beehner, J. C. (2018). Graminivory and fallback foods: annual diet profile of geladas (Theropithecus gelada) living in the simien Mountains National Park. Ethiopia. Int. J. Primatol. 39, 105–126. doi: 10.1007/s10764-018-0018-x
Jolly, C. J. (1970). The seed-eaters: a new model of hominid differentiation based on a baboon analogy. Man 5, 5–26.
Jolly, C. J. (1972). The classification and natural history of Theropithecus (Simopithecus) (Andrews, 1916), baboons of the African Plio-pleistocene. Bull. Br. Mus. Nat. 22, 1–123.
Kamilar, J. M. (2006). “Geographic variation in savanna baboon (Papio) ecology and its taxonomic and evolutionary implications,” in Primate Biogeography, eds S. M. Lehman and J. G. Fleagle (New York, NY: Springer Press), 169–200.
Kay, R. F. (1981). The nut-crackers-. a new theory of the adaptations of the Ramapithecinae. Am. J. Phys. Anthropol. 55, 141–151. doi: 10.1002/ajpa.1330550202
King, S. J., Arrigo-Nelson, S. J., Pochron, S. T., Semprebon, G. M., Godfrey, L. R., Wright, P. C., et al. (2005). Dental senescence in a long-lived primate links infant survival to rainfall. PNAS 102, 16579–16583. doi: 10.1073/pnas.0508377102
Lahm, S. A. (1986). Diet and habitat preference of Mandrillus sphinx in gabon: implications of foraging strategy. Am. J. Primatol. 11, 9–26. doi: 10.1002/ajp.1350110103
Lambert, J. E., Chapman, C. A., Wrangham, R. W., and Conklin-Brittain, N. L. (2004). Hardness of cercopithecine foods: implications for the critical function of enamel thickness in exploiting fallback foods. Am. J. Phys. Anthropol. 125, 363–368. doi: 10.1002/ajpa.10403
Lieberman, D. E. (1995). Testing hypotheses about recent human evolution from skulls: integrating morphology, function, development, and phylogeny. Curr. Anthropol. 36, 159–197.
Liedigk, R., Roos, C., Brameier, M., and Zinner, D. (2014). Mitogenomics of the old world monkey tribe Papionini. BMC Evol. Biol. 14:176. doi: 10.1186/s12862-014-0176-1
Lockwood, C. A., and Fleagle, J. G. (1999). The recognition and evaluation of homoplasy in primate and human evolution. Yearb. Phys. Anthropol. 42, 189–232.
Lucas, P. (2004). Dental Functional Morphology. How Teeth Work. Cambridge: Cambridge University Pres.
Lucas, P. W., and Corlett, R. T. (1991). Relationship between the diet of Macaca fascicularis and forest phenology. Folia Primatol. 57, 201–215. doi: 10.1159/000156587
Lycett, S. J., and Collard, M. (2005). Do homologies impede phylogenetic analyses of the fossil hominids? an assessment based on extant papionin craniodental morphology. J. Hum. Evol. 49, 618–642. doi: 10.1016/j.jhevol.2005.07.004
Maisels, F., Gautier-Hion, A., and Gautier, J.-P. (1994). Diets of two sympatric colobines in Zaire: more evidence on seed-eating in forests on poor soils. Int. J. Primatol. 15, 681–701. doi: 10.1007/BF02737427
Marshall, A. J., and Wrangham, R. W. (2007). Evolutionary consequences of fallback foods. Int. J. Primatol. 28, 1219–1235. doi: 10.1007/s10764-007-9218-5
Mau, M., Südekum, K.-H., Johann, A., Sliwa, A., and Kaiser, T. M. (2009). Saliva of the graminivorous Theropithecus gelada lacks proline-rich proteins and tannin-binding capacity. Am. J. Primatol. 71, 663–669. doi: 10.1002/ajp.20701
McGraw, W. S., and Fleagle, J. G. (2006). “Biogeography of cercocebus-mandrillus clade: evidence from the face,” in Primate Biogeography, eds S. Lehman and J. G. Fleagle (New York, NY: Springer).
McGraw, W. S., Pampush, J. D., and Daegling, D. J. (2012). Brief communication: Enamel thickness and durophagy in mangabeys revisited. Am. J. Phys. Anthropol. 147, 326–333.
McGraw, W. S., Vick, A. E., and Daegling, D. J. (2011). Sex and age differences in the diet and ingestive behaviors of sooty mangabeys (Cercocebus atys) in the Tai Forest, Ivory Coast. Am. J. Phys. Anthropol. 144, 140–1153. doi: 10.1002/ajpa.21402
McGraw, W. S., Vick, A. E., and Daegling, D. J. (2014). Dietary variation and food hardness in sooty mangabeys (Cercocebus atys): implications for fallback foods and dental adaptation. Am. J. Phys. Anthropol. 154, 413–423. doi: 10.1002/ajpa.22525
Meikle, W. E. (1977). Molar Wear Stages in Theropithecus gelada. Pap. Kroeber Anthropol. Soc. 50, 21–26.
Ménard, N., Rantier, Y., Foulquier, A., Qarro, M., Chillasse, L., Vallet, D., et al. (2014). Impact of human pressure and forest fragmentation on Moroccan Barbary macaque (Macaca sylvanus) populations. Oryx 48, 276–284. doi: 10.1017/S0030605312000312
Ménard, N., and Vallet, D. (1986). Le régime alimentaire de Macaca sylvanus dans différents habitats d’Algérie: régime en forêt sempervirente et sur les sommets rocheux. Rev. Ecol-Terre Vie 41, 173–192.
Mitani, M. (1989). Cercocebus torquatus: adaptive feeding and ranging behaviors related to seasonal fluctuations of food resources in the tropical rain forest of Southwestern Cameroon. Primates 30, 307–323. doi: 10.1007/BF02381257
M’Kirera, F., and Ungar, P. S. (2003). Occlusal relief changes with molar wear in Pan troglodytes and Gorilla. Am. J. Primatol. 60, 31–41. doi: 10.1002/ajp.10077
Moges, E., and Balakrishnan, M. (2014). Nutritional composition of food plants of geladas (Theropithecus gelada) in Guassa Community Protected Area. Ethiopia. J. Biol. Agric. Healthc. 4, 38–45.
Monson, T. A., and Hlusko, L. J. (2014). Identification of a derived dental trait in the papionini relative to other old world monkeys. Am. J. Phys. Anthropol. 155, 422–429. doi: 10.1002/ajpa.22586
Olupot, W., Chapman, C. A., Waser, P. M., and Isabirye-Basuta, G. (1997). Mangabey (Cercocebus albigena) ranging patterns in relation to fruit availability and the risk of parasite infection in Kibale National Park, Uganda. Am. J. Primatol. 43, 65–78. doi: 10.1002/(SICI)1098-2345(1997)43:1<65::AID-AJP5>3.0.CO;2-W
Pampush, J., Spradley, J., Morse, P., Griffith, D., Gladman, J., Gonzales, L., et al. (2018). Adaptive wear-based changes in dental topography associated with atelid (Mammalia: Primates) diets. Biol. J. Linn. Soc. 124, 84–606. doi: 10.1093/biolinnean/bly069
Pampush, J. D., Spradley, J. P., Morse, P. E., Harrington, A. R., Allen, K. L., Boyer, D. M., et al. (2016). Wear and its effects on dental topography measures in howling monkeys (Alouatta palliata). Am. J. Phys. Anthropol. 161, 705–721. doi: 10.1002/ajpa.23077
Perelman, P., Johnson, W. E., Roos, C., Seuánez, H. N., Horvath, J. E., Moreira, M. A. M., et al. (2011). A molecular phylogeny of living primates. PLoS Genetics 7:e1001342. doi: 10.1371/journal.pgen.1001342
Pineda-Muñoz, S., Lazagabaster, I. A., Alroy, J., and Evans, A. R. (2017). Inferring diet from dental morphology in terrestrial mammals. Methods Ecol. Evol. 8, 481–491. doi: 10.1111/2041-210X.12691
Powell, L. E., Isler, K., and Barton, R. A. (2017). Re-evaluating the link between brain size and behavioural ecology in primates. Proc. R. Soc. B: Biol. Sci. 284:20171765. doi: 10.1098/rspb.2017.1765
Pugh, K. D., and Gilbert, C. C. (2018). Phylogenetic relationships of living and fossil African papionins: combined evidence from morphology and molecules. J. Hum. Evol. 123, 35–51. doi: 10.1016/j.jhevol.2018.06.002
Pugh, K. D., Gilbert, C. C., Patel, B. A., Campisano, C. J., Singh, N. P., Singleton, M., et al. (2018). A small-bodied catarrhine from the Miocene of India: additional evidence of an undersampled radiation. Am. J. Phys. Anthropol. 165, S66–S215.
Quris, R. (1975). Ecologie et organisation sociale de Cercocebus galeritus agilis dans le nord-est du Gabon. Rev. Ecol-Terre Vie 3, 337–398.
Romero, A., Pérez-Pérez, A., García Atiénzar, G., Martínez, L. M., and Macho, G. A. (2022). Do rates of dental wear in extant African great apes inform the time of weaning? J. Hum. Evol. 163:103126. doi: 10.1016/j.jhevol.2021.103126
Shah, N. (2003). Foraging Strategies in Two Sympatric Mangabey Species (Cercocebus agilis and Lophocebus albigena). PhD dissertation, New York, NY: State University of New York.
Shapiro, A. E., Venkataraman, V. V., Nguyen, N., and Fashing, P. J. (2016). Dietary ecology of fossil Theropithecus: inferences from dental microwear textures of extant geladas from ecologically diverse sites. J. Hum. Evol. 99, 1–9. doi: 10.1016/j.jhevol.2016.05.010
Singleton, M. (2005). “Functional shape variation in the cercopithecine masticatory complex,” in Modern Morphometrics In Physical Anthropology, ed. D. E. Slice (Boston: Springer), doi: 10.1007/0-387-27614-9
Souron, A. (2018). Morphology, diet, and stable carbon isotopes: on the diet of Theropithecus and some limits of uniformitarianism in paleoecology. Am. J. Phys. Anthropol. 166, 261–267. doi: 10.1002/ajpa.23414
Strasser, E., and Delson, E. (1987). Cladistic analysis of cercopithecid relationships. J. Hum. Evol. 16, 81–99. doi: 10.1016/0047-2484(87)90061-3
Swindler, D. R. (2002). Primate Dentition: An Introduction to the Teeth of Non-Human Primates. Cambridge: Cambridge University Press.
Szalay, F. S., and Delson, E. (1979). Evolutionary History of the Primates. New York, NY: Academic Press, doi: 10.1002/ajpa.1330600122
Teaford, M. (1993). “Dental microwear and diet in extant and extinct Theropithecus: preliminary analyses,” in Theropithecus: The Rise and Fall of a Primate Genus, ed. N. G. Jablonski (Cambridge: Cambridge University Press), 331–349.
Teaford, M. F., and Ungar, P. S. (2000). Diet and the evolution of the earliest human ancestors. PNAS 97, 13506–13511. doi: 10.1073/pnas.26036889
Thierry, B. (2007). “The macaques: a double-layered social organization,” in Primates in Perspective, eds C. J. Campbell, A. Fuentes, K. C. MacKinnon, M. Panger, and S. K. Bearder (New York, NY: Oxford University Press), 224–239.
Thiery, G., Gillet, G., Lazzari, V., Merceron, G., and Guy, F. (2017a). Was Mesopithecus a seed eating colobine? assessment of cracking, grinding and shearing ability using dental topography. J. Hum. Evol. 112, 79–92. doi: 10.1016/j.jhevol.2017.09.002
Thiery, G., Guy, F., and Lazzari, V. (2017b). Investigating the dental toolkit of primates based on food mechanical properties: feeding action does matter. Am. J. Primatol. 79:e22640. doi: 10.1002/ajp.22640
Tutin, C. E. G., Ham, R. M., White, L. J. T., and Harrison, M. J. S. (1997). The primate community of the Lope Reserve, Gabon: diets, responses to fruit scarcity, and effects on biomass. Am. J. Primatol. 42, 1–24. doi: 10.1002/(SICI)1098-2345(1997)42:1<1::AID-AJP1>3.0.CO;2-0
Ulhaas, L., Kullmer, O., Schrenk, F., and Henke, W. (2004). A new 3D approach to determine functional morphology of cercopithecoid molars. Ann. Anat. 186, 487–493. doi: 10.1016/S0940-9602(04)80090-6
Ungar, P. S. (2004). Dental topography and diets of Australopithecus afarensis and early Homo. J. Hum. Evol. 46, 605–622. doi: 10.1016/j.jhevol.2004.03.004
Ungar, P. S. (2010). Mammal Teeth: Origin, Evolution, and Diversity. Baltimore, MD: Johns Hopkins University Press.
Ungar, P. S. (2015). Mammalian dental function and wear: a review. Biosurf. Biotribol. 1, 25–41. doi: 10.1016/j.bsbt.2014.12.001
Ungar, P. S., Healy, C., Karme, A., Teaford, M. F., and Fortelius, M. (2018). Dental topography and diets of platyrrhine primates. Hist. Biol. 30, 64–75. doi: 10.1080/08912963.2016.1255737
Ungar, P. S., and M’Kirera, F. (2003). A solution to the worn tooth conundrum in primate functional anatomy. PNAS 100, 3874–3877. doi: 10.1073/pnas.0637016100
Vinyard, C. J., Wall, C. E., Williams, S. H., and Hylander, W. L. (2003). Comparative functional analysis of skull morphology of tree-gouging primates. Am. J. Phys. Anthropol. 120, 153–170. doi: 10.1002/ajpa.10129
Wall, C. E. (1999). A model of temporomandibular joint function in anthropoid primates based on condylar movements during mastication. Am. J. Phys. Anthropol. 109, 67–88. doi: 10.1002/(SICI)1096-8644(199905)109:1<67::AID-AJPA7>3.0.CO;2-F
Waser, P. M. (1975). Monthly variations in feeding and activity patterns of the mangabey, Cercocebus albigena (Lydekker). East Afr. Wildl. J. 13, 249–263. doi: 10.1111/j.1365-2028.1975.tb00138.x
Winchester, J. M. (2016). MorphoTester: an open source application for morphological topographic analysis. PLoS One 11:e0147649. doi: 10.1371/journal.pone.0147649
Winchester, J. M., Boyer, D. M., St Clair, E. M., Gosselin-Ildari, A. D., Cooke, S. B., and Ledogar, J. A. (2014). Dental topography of platyrrhines and prosimians: convergence and contrasts. Am. J. Phys. Anthropol. 153, 29–44. doi: 10.1002/ajpa.22398
Keywords: dental topography, diet, wear, Cercopithecoidea, Papionini
Citation: Avià Y, Romero A, Estebaranz-Sánchez F, Pérez-Pérez A, Cuesta-Torralvo E and Martínez LM (2022) Dental topography and dietary specialization in Papionini primates. Front. Ecol. Evol. 10:969007. doi: 10.3389/fevo.2022.969007
Received: 14 June 2022; Accepted: 28 October 2022;
Published: 11 November 2022.
Edited by:
Michael Anthony Berthaume, London South Bank University, United KingdomReviewed by:
Marina Melchionna, University of Naples Federico II, ItalyElizabeth St. Clair, Johns Hopkins University, United States
Copyright © 2022 Avià, Romero, Estebaranz-Sánchez, Pérez-Pérez, Cuesta-Torralvo and Martínez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Mónica Martínez, lmartinez@ub.edu
 Yasmina Avià1,2
Yasmina Avià1,2  Alejandro Romero
Alejandro Romero Ferran Estebaranz-Sánchez
Ferran Estebaranz-Sánchez Laura Mónica Martínez
Laura Mónica Martínez