Pollen and nectar have different effects on the development and reproduction of noctuid moths
- 1Institute of Urban Agriculture, Chinese Academy of Agricultural Sciences (CAAS), Chengdu, China
- 2State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences (CAAS), Beijing, China
- 3State Key Laboratory of Ecological Pest Control for Fujian and Taiwan Crops, Institute of Applied Ecology, Fujian Agriculture and Forestry University, Fuzhou, China
Although many noctuid insects are agricultural pests that threaten food production, they are also the major nocturnal pollinators of flowering plants. Larval foods of noctuid pest insects have been well studied for developing control strategies, but knowledge on host plants for the adults is rather scarce. Here, the impact of plant-derived foods on adult survival, fecundity and reproductive physiology of four global species of noctuid pests (Mythimna separata Walker, Mythimna loreyi Duponchel, Athetis lepigone Möschler, and Hadula trifolii Hufnagel) was assessed in laboratory experiments. Our results indicated that nectar slowed testis decay and prolonged the oviposition period and lifespan, increasing fecundity. Acacia nectar increased the longevity of male and female adults by 3.2∼10.9 and 2.4∼5.0 days, respectively, and fecundity of females by 1.22∼3.34 times compared to water-fed individuals. The fitness among the different species of noctuid moths differed on specific pollen diets. On pine pollen, the fecundity of female moths of M. separata, A. lepigone and H. trifolii was 10.06, 33.52, and 28.61%, respectively, lower than those of the water-fed females, but the fecundity of female moths of M. loreyi on pine pollen was 2.11 times greater than for the water-fed individuals. This work provides valuable information on the nutritional ecology for noctuid moths, which can aid the development and design of nutritional attractants within noctuid pests-infected cropping systems and provide a basis for effective and targeted management of global noctuid pests.
Introduction
Among the lepidopteran families, Noctuidae is one of the most diverse and abundant lineage in the number of species. Its members have major roles in the function and stability of terrestrial and aquatic ecosystems (Kristensen et al., 2007; Foottit and Adler, 2009). The larval and adult stages of noctuid species are closely associated with cultivated and wild plants. The larvae feed on the roots, stems or leaves of vascular plants using biting-chewing mouthparts and are agricultural and forestry pests (Cass, 1959; Sharma and Davies, 1983; Jiang et al., 2011; Zhang and Yu, 2021; Duan et al., 2022). The adults usually are anthophilous; they visit flowers and suck nectar and/or pollen using a proboscis and are important nocturnal pollinators of many flowering plants (Krenn, 2010; Devoto et al., 2011; Kato and Kawakita, 2017; Ribas-Marquès et al., 2022). In addition, noctuid insects are important food sources for other aquatic and terrestrial organisms including fishes, frogs, spiders, birds, bats, predatory and parasitic insects (García-Navas and Sanz, 2011; Fox, 2013; Chapman et al., 2015). However, there is no accurate published information on the contribution of noctuid insects to ecological function and stability. Similarly, knowledge on host plants used by adults of Noctuidae is rather scarce.
Many noctuid insects make seasonal migrations across the Earth and lead to outbreaks of agricultural and forestry pests, biological invasion, and pollination over large regions (Chapman et al., 2015; Hu et al., 2016; Song et al., 2021b). Migratory noctuid moths usually feed on nectar and/or pollen to meet energy for development of the internal reproductive system, and reproductive and flight activities (Krenn, 2010; Balzan and Wäckers, 2013; Liu et al., 2017a,b; He et al., 2022). The studies revealed the importance of carbohydrate-rich nectar or pollen as food sources to bolster fitness and raise adult lifespan, reproduction and flight performance (Wu and Guo, 1997; Lee and Heimpel, 2008; Lundgren, 2009; Jiang et al., 2015; Solayman et al., 2016; Liu et al., 2017a; He et al., 2021a). Understanding the interaction between adult food sources and noctuid moths is thus necessary for exploring their behavioral ecology, population dynamics and ecological function, as well as population monitoring and regional management of pests.
Mythimna separata Walker, Mythimna loreyi Duponchel, Athetis lepigone Möschler and Hadula trifolii Hufnagel are well-known migratory noctuid pests in agricultural ecosystems and globally distributed in tropical, subtropical, and temperate regions (Table 1 and Figure 1). They have frequently occurred in crop fields in China and other Asian countries in recent years, posing a threat to food production security. Earlier studies have shown that migratory noctuid insects visit flowers of a wide range of herbaceous and woody plants including maize, rapeseed, sunflowers, and various members of Pinaceae and Rosaceae during migration process and exhibit different host preferences (Liu et al., 2016, 2017a; Chang et al., 2018; Guo et al., 2018; He et al., 2022). Floral nectar is primarily composed of sugar, water, amino acids, inorganic ion, alkaloids and phenolics and a high-value diet for numerous noctuid insects including A. lepigone, Helicoverpa armigera Hübner, M. separata, Spodoptera exigua Hübner, S. frugiperda Smith and S. litura Fabricius (Wu and Guo, 1997; Jiang et al., 2015; Solayman et al., 2016; Liu et al., 2017b; He et al., 2021a). Pollen contains carbohydrates, proteins, lipids, amino acids and vitamins and also provides energy and nutrition for many insects (Wäckers et al., 2007; Ares et al., 2018). A previous study reported that sesame flowers had no effect on adult longevities and fecundities of H. armigera, S. exigua, S. litura, M. separate, and Ostrinia furnacalis Guenée but Plutella xyllostella (L.) females laid more eggs when fed on sesame flowers compared to the water (Liu et al., 2017b). Earlier work on S. frugiperda has shown how pollen-containing diets impact adult fecundity and longevity (He et al., 2021a). However, how various plant-derived foods affect reproductive development, fertility and survival of M. separata, M. loreyi, A. lepigone and H. trifolii still remains unclear.
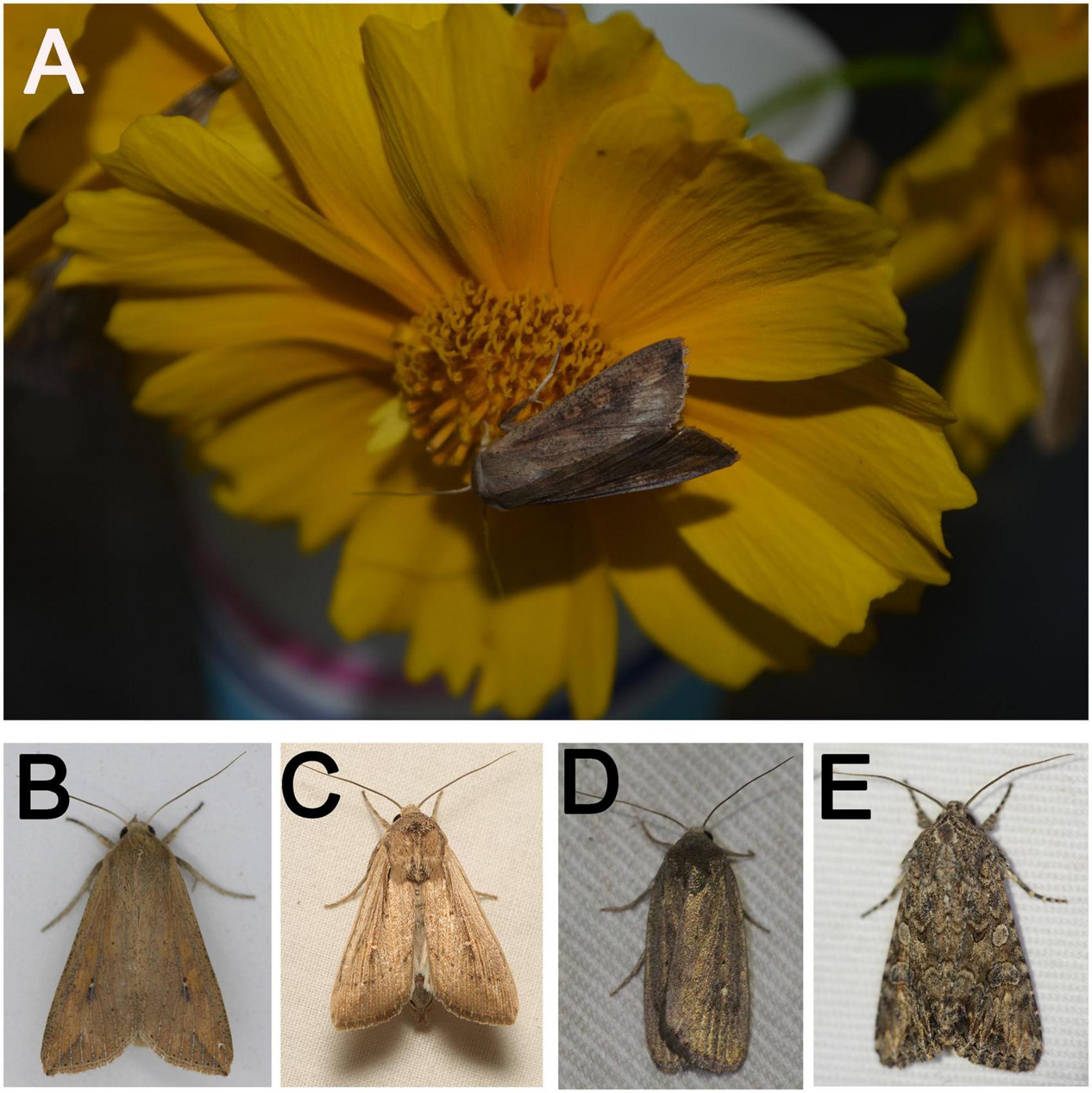
Figure 1. Representative images of noctuid moths used in the study (A): Mythimna separata male adult, visiting flower and sucking nectar. (B): M. separata female adult; (C): Mythimna loreyi male adult; (D): Athetis lepigone adult; (E): Hadula trifolii adult). All images were taken by Limei He and Shengyuan Zhao with Nikon D5100 (A) and Canon EOS 60D (B-E).
In the present study, we thus assessed the life history traits and reproductive physiology of M. separata, M. loreyi, A. lepigone and H. trifolii adults when fed different types, concentrations and mixtures of nectar and pollen to better understand flower visitation and/or population dynamics of noctuid pest insects. We predicted longer longevity and higher fecundity for these moths when they fed on nectar and that nectar and pollen would have different effects on the development and reproduction of the four noctuid moths. We also speculated that adults of each noctuid species would differ in their host preferences and that camellia, maize, rose and motherwort might provide highly suitable floral resources for adults of M. separata, M. loreyi, A. lepigone and H. trifoli, respectively.
Materials and methods
Larval feeding and adult fecundity trials
Trials were done from May to October 2018 and 2019 at the Xinxiang Experimental Station of the Chinese Academy of Agricultural Sciences (CAAS; 35°18′13.71″ N, 113°55′15.05″E) in Henan Province (China). In May 2018, adults of M. separata, M. loreyi, A. lepigone, and H. trifolii were collected using vertically aimed searchlight traps (model JLZ1000BT; Shanghai Yaming Lighting Co., Ltd., Shanghai, China), which were opened at sunset and closed at sunrise. Moths were gathered with a nylon net bag (60 mesh) beneath the trap and reared for three consecutive generations in the laboratory at 24 ± 1°C, 70 ± 5% RH, and 16 h L:8 h D. Larvae of M. separata and M. loreyi were fed Zea mays L. leaves and wheat bran-based artificial diet. A. lepigone larvae were fed soybean and wheat bran-based artificial diet. F1 generation larvae of H. trifolii were fed Chenopodium album L. leaves, and F2 and F3 generation larvae were fed C. album leaves and wheat bran-based artificial diet. The larvae of the four species tested were reared in 22 × 15 × 8 cm plastic boxes, and F3 generation adults were assessed for reproductive output. As soon as larvae were fully developed, they were moved to plastic boxes with vermiculite for pupation. On the 4th-5th day following pupation, the male and female pupae were separated as described previously (Zhao et al., 2011).
Newly emerged adults (♀:♂ = 1:1) were paired and moved to 460-ml plastic cups with a 10-cm-long white nylon binding rope and a 10 × 10 cm sterile gauze stopper. The pairs received one of 11 diets in distilled water: distilled water (water), 5% (v/v) acacia nectar (acacia nectar), 1% (m/v) camellia pollen (camellia pollen), 1% (m/v) maize pollen (maize pollen), 1% (m/v) lotus pollen (lotus pollen), 1% (m/v) motherwort pollen (motherwort pollen), 1% (m/v) pine pollen (pine pollen), 1% (m/v) rapeseed pollen (rapeseed pollen), 1% (m/v) rose pollen (rose pollen), 1% (m/v) schisandra pollen (schisandra pollen) and 1% (m/v) sunflower pollen (sunflower pollen). Water and acacia nectar served as control treatments. Acacia nectar was purchased from Beijing Baihua bee Co., Ltd., Beijing, China. All pollen was purchased from Xinzhou Wutaishan Bee Industry Co., Ltd., Shanxi Province, China, except pine pollen which was purchased from Changbai Mountain Yipin Store, Jilin Province, China. One absorbent cotton ball (1 cm in diameter) that had absorbed 5 mL of the test diet solution was put into each cup and replaced every 2 days. Moths were kept at 24 ± 1°C, 75 ± 5% RH and 16 h L:8 h D. On a daily basis, any eggs on the plastic cup, nylon binding rope or sterile gauze stopper were counted, removed from the cup, and placed in a separate 12 × 17 cm zip-lock bag to determine hatchability and document the pre-oviposition time and survival rate of adults that developed from the eggs. Dead female H. trifolii moths were dissected using a stereomicroscope (TS-63X; Shanghai Shangguang New Optical Technology Co., Ltd., Shanghai, China) to count the number of spermatophores in the spermathecae and thus ascertain mating frequency.
Examination of reproductive system
Testicular or ovarian anatomy was examined to evaluate the effect of different food items on the reproductive system, for unmated male or female M. separata and M. loreyi adults. The effect of diets on ovarian development was determined for female adults of varying age (i.e., M. separata: 1- to 12-days old; M. loreyi: 1- to 11-days old). We measured the major axis length of male testes for adults of varying ages (i.e., M. separata: 1- to 12-days old; M. loreyi: 1- to 11-days old) (Chen et al., 2017; He et al., 2019, 2021a) to assess the effect of the various diets. Larvae were fed Z. mays leaves and wheat bran-based artificial diet, and 1st to 6th instar larvae were reared in 22 × 15 × 8 cm plastic boxes. Once larvae were fully developed, they were transferred to plastic trays with vermiculite for pupation. On the 6th day after pupation, female and male pupae were separated.
After pupal emergence, M. separata and M. loreyi moths were reared on one of nine food items: distilled water, 5% (v/v) acacia nectar (acacia nectar), 5‰ (m/v) rapeseed pollen, 5‰ (m/v) maize pollen, 5‰ (m/v) sunflower pollen, 5‰ (m/v) rose pollen, 5‰ (m/v) pine pollen, 2.5% (v/v) acacia nectar + 2.5‰ (m/v) rapeseed pollen (acacia nectar + rapeseed pollen) and 2.5% (v/v) acacia nectar + 2.5‰ (m/v) pine pollen (acacia nectar + pine pollen) in 50 × 50 × 50 cm cages (200 mesh nylon, 100 moths per cage) and kept at 25 ± 1°C and 70 ± 5% RH. Water, acacia nectar and combinations of pollen and nectar served as control treatments. Before excision of reproductive organs using a stereomicroscope (TS-63X), female moths were held at −20°C for 4 h, and male moths were immersed in 75% ethanol for 30 s (He et al., 2019, 2021a). The ovarian development index (levels 1–5) was classified based on standard practice (Dai et al., 1962). The major axis length of the testis was determined with OLD-SGD show software (Shanghai Shangguang New Optical Technology Co., Ltd., Shanghai, China). Ten male or female M. separata and M. loreyi moths of a given age were similarly treated for each diet with three replicates.
Statistical analyses
The effects of the 11 diets on reproductive variables were analyzed using a one-way analysis of variance (ANOVA) followed by Duncan’s new multiple range test (MRT). Effects of nectar and pollen diets on the longevity ratio (= Mean longevity on nectar or pollen diet/Mean longevity on water) and ratio of oviposition period (= Mean oviposition period on nectar or pollen diet / Mean oviposition period on water) and fecundity ratio (= Mean number of eggs deposited per female on nectar or pollen diet/Mean number of eggs deposited per female on water) for the four noctuid moths were also analyzed using a one-way ANOVA followed by Duncan’s MRT. Pearson linear correlation was used to test for a correlation between longevity and fecundity of female adults. Log rank was used to test for differences in survival curves of male and female adults for the four noctuid moths on the 11 diets. Effects of dietary treatment, sex, access to acacia nectar, and access to pollen on survivorship of M. separata, M. loreyi, A. lepigone and H. trifolii were evaluated using Cox proportional hazards model (Wyckhuys et al., 2008). Differences in male testis size, female ovarian development index of M. separata and M. loreyi reared with different foods or between sexes were analyzed using a two-way ANOVA. Curve Estimation (linear, logarithmic, inverse, quadratic, cubic, power, compound, S-curve, logistic, growth, and exponential) models were used to estimate curves for male testis size and female ovarian development index of M. separata and M. loreyi adults at different ages. Then, based on statistics such as F value, P value and coefficient of determination R2 of the significance test of the regression equation, the cubic model was selected as the optimal model for fitting (Cai, 2014). The equation for the cubic model was y = ax3 + bx2 + cx + d, where a, b, c and d were model parameters, and y was male testis size or female ovarian development index for M. separata or M. loreyi at x days old. SPSS version 20 (IBM, Armonk, NY, USA) was used for all analyses, except for the log rank test, which was done in GraphPad Prism 8 (GraphPad Software Inc., San Diego, CA, USA).
Results
Survival and longevity
Mythimna separata
The survival rate curves of adults after feeding on different foods differed greatly between the two sexes of M. separata (female: χ2 = 89.290, df = 10, p < 0.001; male: χ2 = 170.300, df = 10, p < 0.001; Figures 2A,B). Dietary treatment had a significant effect on M. separata longevity (χ2 = 4.682, df = 1, p = 0.030), as did sex (χ2 = 4.347, df = 1, p = 0.037), access to acacia nectar (χ2 = 40.845, df = 1, p < 0.001), and access to pollen (χ2 = 5.354, df = 1, P = 0.021). Interactions were significant between sex and access to acacia nectar (χ2 = 12.538, df = 1, p < 0.001) and between sex and diet (χ2 = 5.971, df = 1, p = 0.015). Male moths attained the longest lifespan on acacia nectar, followed by maize pollen, and camellia pollen (F10, 554 = 2.528, p = 0.006). For female M. separata, the longest lifespan was achieved on acacia nectar, followed by maize pollen (F10, 813 = 9.196, p < 0.001). However, the third best diet for female M. separata appeared to be schisandra pollen. Longevity for both male and female M. separata was shortest on water. When reared on acacia nectar and maize pollen, males lived longer than females, but females lived longer than males on all other diets (Table 2).
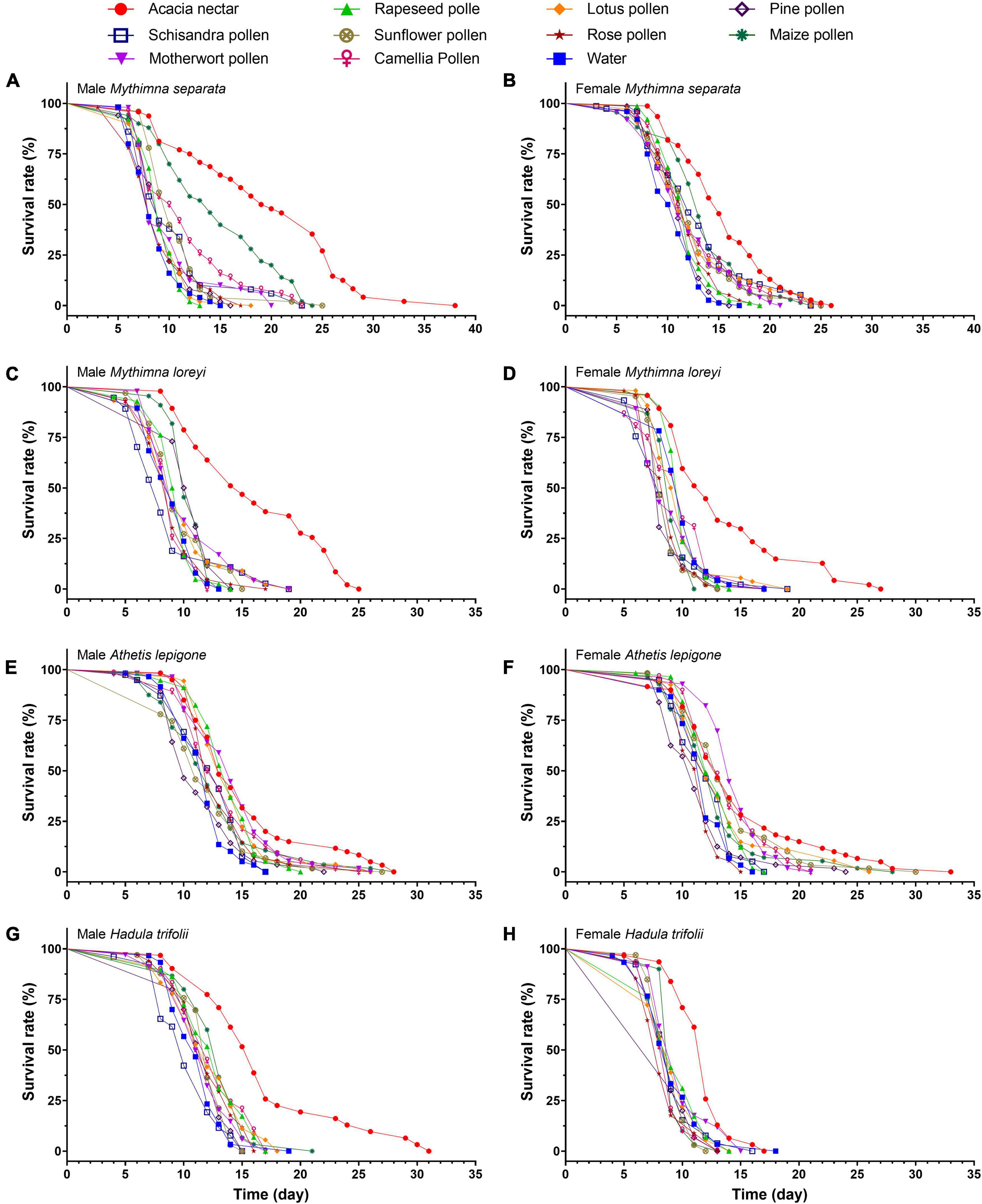
Figure 2. Age-specific survival rate of male and female adults of Mythimna separata (A,B), Mythimna loreyi (C,D), Athetis lepigone (E,F), and Hadula trifolii (G,H) on different diets.
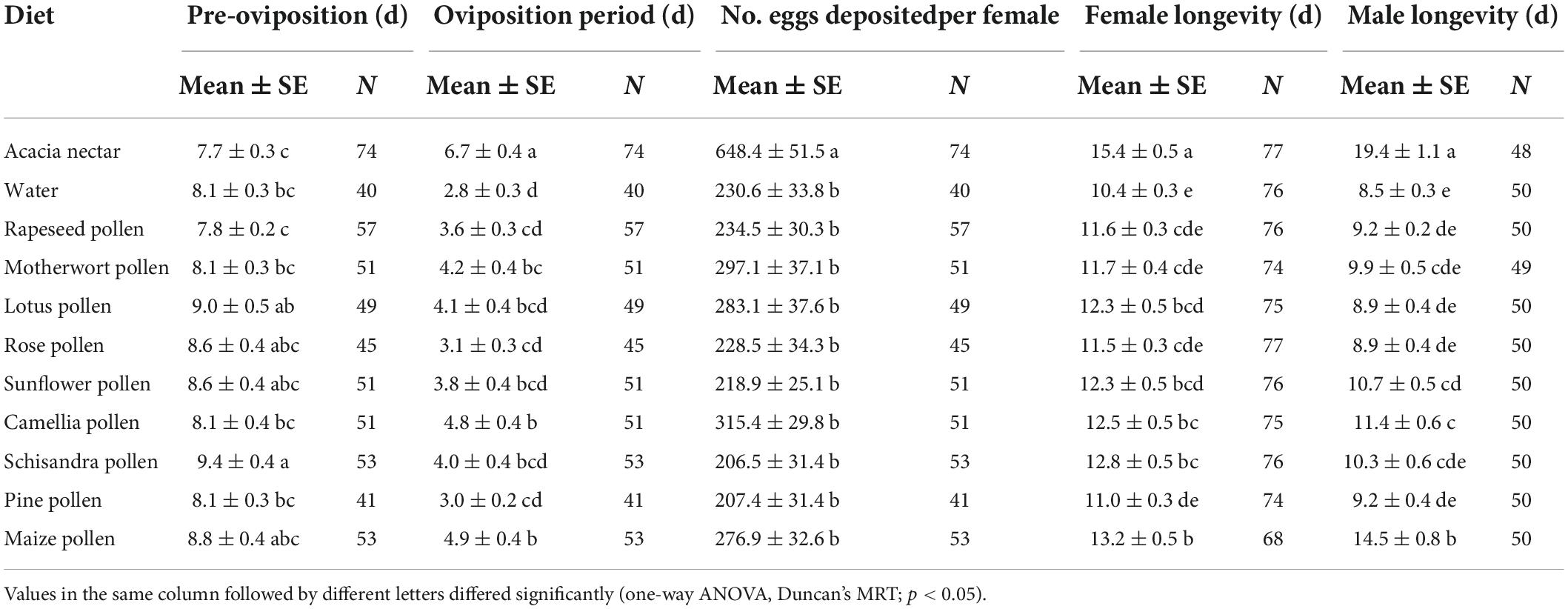
Table 2. Means (± SE) for longevity and fecundity variables for adults of Mythimna separata on different diets.
Mythimna loreyi
The survival rate curves of adults after feeding on different foods differed greatly between the two sexes of M. loreyi (female: χ2 = 92.090, df = 10, p < 0.001; male: χ2 = 120.300, df = 10, p < 0.001; Figures 2C,D). Access to acacia nectar had a significant effect on M. loreyi longevity (χ2 = 102.137, df = 1, p < 0.001), as did the interaction between sex and diet (χ2 = 9.427, df = 1, p = 0.002). Male moths attained the longest lifespan on acacia nectar, followed by maize pollen, and pine pollen (F10, 459 = 23.497, p < 0.001). For female M. loreyi, the longest lifespan was recorded for acacia nectar, followed by rapeseed pollen, and water (F10, 549 = 14.496, p < 0.001). Both male and female M. loreyi obtained the shortest longevity on schisandra pollen. When reared on acacia nectar, motherwort pollen, maize pollen, sunflower pollen, pine pollen and rose pollen, males lived longer than females, but females lived longer on the other diets (Table 3).
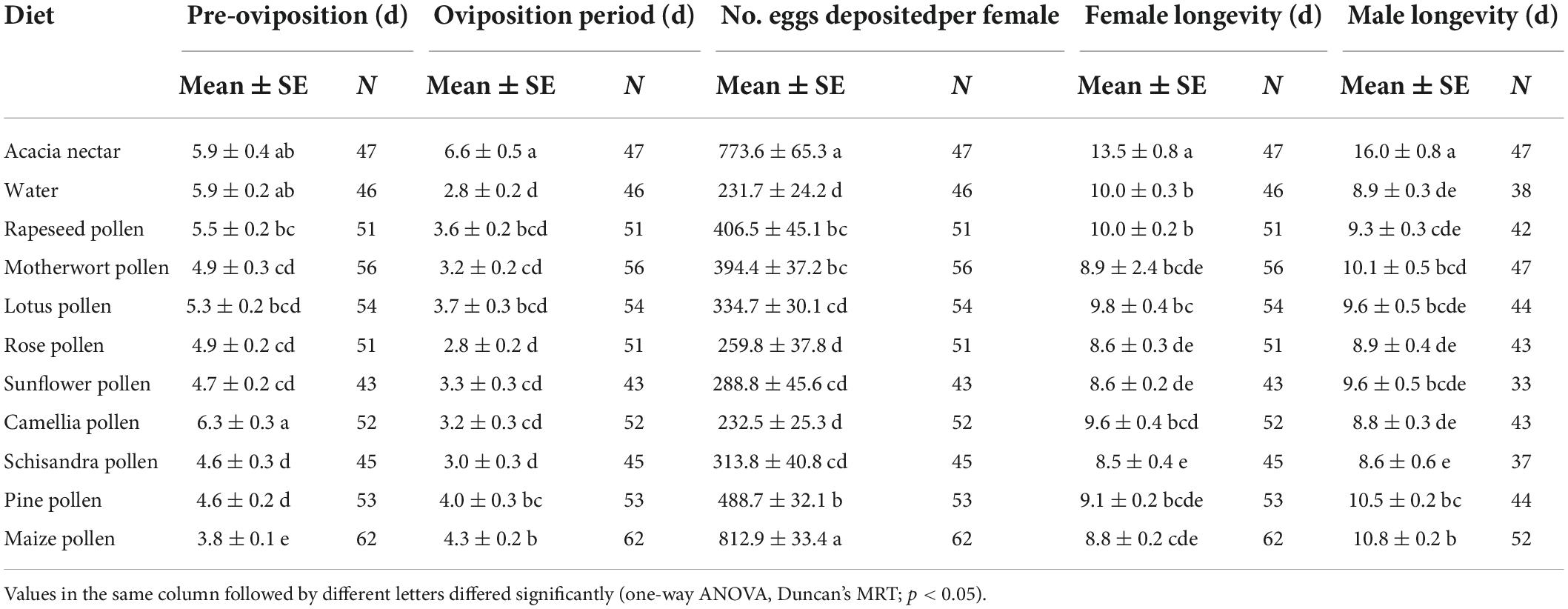
Table 3. Means (± SE) for longevity and fecundity variables for adults of Mythimna loreyi on different diets.
Athetis lepigone
The survival rate curves of adults after feeding on different foods differed greatly between the two sexes of A. lepigone (female: χ2 = 84.510, df = 10, p < 0.001; male: χ2 = 56.570, df = 10, p < 0.001; Figures 2E,F). Dietary treatment had a significant effect on A. lepigone longevity (χ2 = 4.594, df = 1, p = 0.032), as did access to acacia nectar (χ2 = 18.166, df = 1, p < 0.001) and to pollen (χ2 = 13.068, df = 1, p < 0.001). However, no significant effect was recorded for sex (χ2 = 0.085, df = 1, p = 0.770). Male moths lived the longest on acacia nectar, followed by motherwort pollen, and lotus pollen (F10, 497 = 4.997, p < 0.001). Females lived the longest on motherwort pollen and acacia nectar, followed by sunflower pollen (F10, 597 = 5.478, p < 0.001). On acacia nectar, rapeseed pollen, lotus pollen, rose pollen, schisandra pollen and pine pollen, males lived longer than females, but females lived longer on the other diets except for water (Table 4).
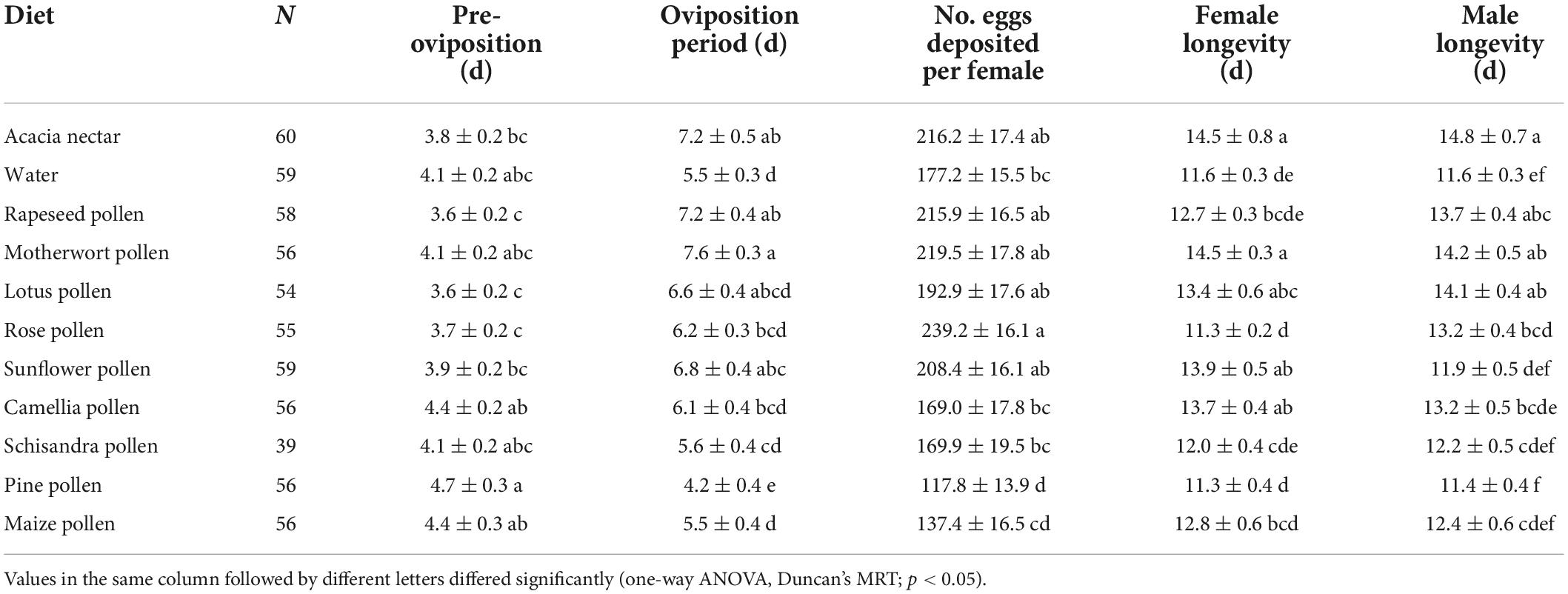
Table 4. Means (± SE) for longevity and fecundity variables for adults of Athetis lepigone on different diets.
Hadula trifolii
The survival rate curves of adults after feeding on different foods differed greatly between the two sexes of H. trifolii (female: χ2 = 38.670, df = 10, p < 0.001; male: χ2 = 60.810, df = 10, p < 0.001; Figures 2G,H). Access to acacia nectar had a significant effect on H. trifolii longevity (χ2 = 6.639, df = 1, p = 0.010), as did sex (χ2 = 4.128, df = 1, p = 0.042). Males lived longest on acacia nectar, followed by maize pollen, rapeseed pollen and camellia pollen, and the shortest on schisandra pollen (F10, 331 = 8.843, p < 0.001). Females lived longest on acacia nectar also, followed by motherwort pollen, pine pollen and rapeseed pollen but the shortest on rose pollen (F10, 331 = 5.565, p < 0.001). Males lived longer than females on all diets (Table 5).
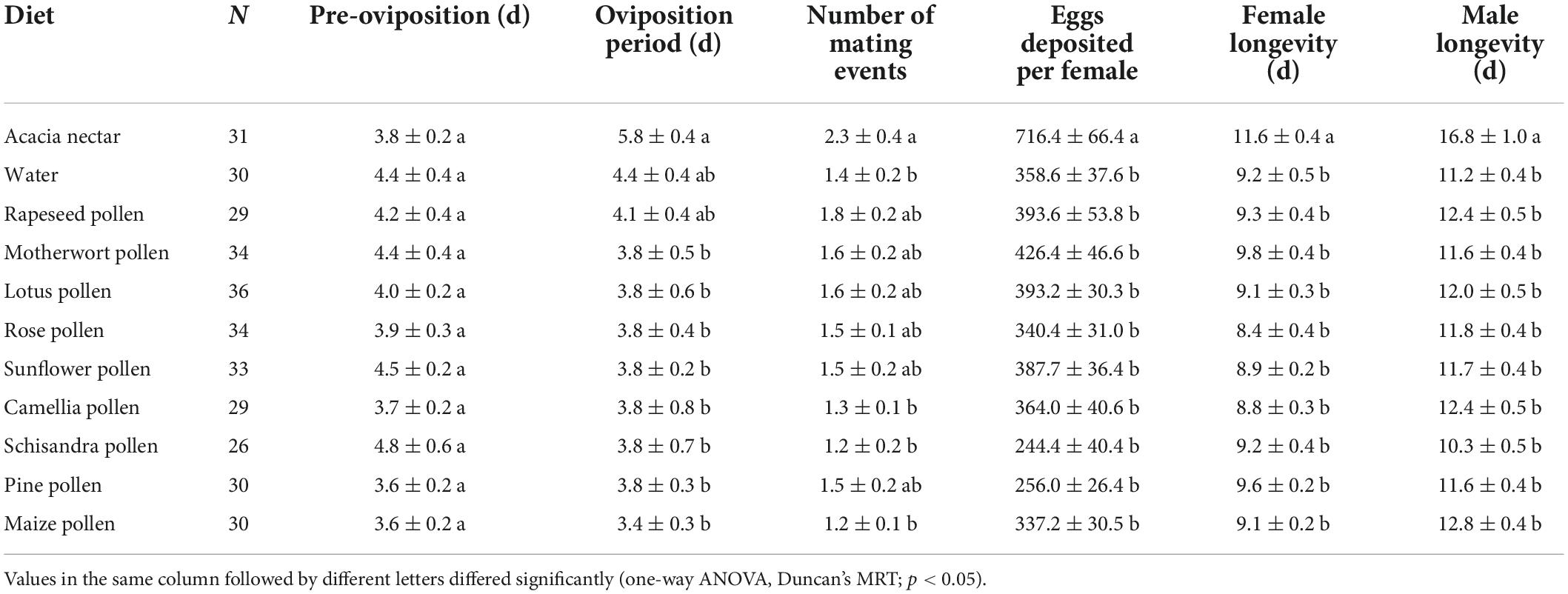
Table 5. Means (± SE) for longevity and fecundity variables for adults of Hadula trifolii on different diets.
Summary
Briefly, nectar and pollen diets had a significant effect on the longevity ratio of males (F9, 30 = 4.648, p = 0.001; Figure 3A) and females (F9, 30 = 2.454, p = 0.031; Figure 3B). Male and female longevity ratios for acacia nectar were significantly higher than for pollen diets (Figure 3).
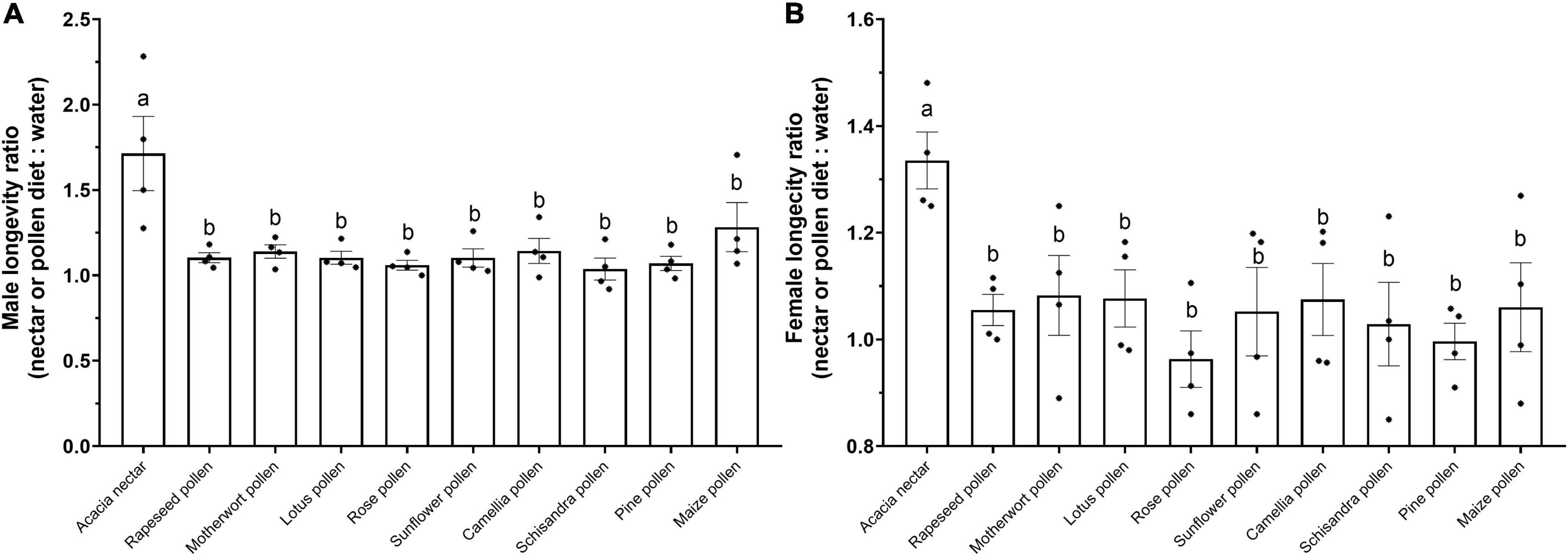
Figure 3. Mean (± SE) male (A) and female (B) longevity ratio of noctuid moths on different nectar or pollen diets. Asterisks for each diet indicate actual ratios for each species of noctuid moths. Different lowercase letters on the top of bar indicates a significant difference in ratios (one-way ANOVA, Duncan’s MRT; p < 0.05).
Fecundity
Mythimna separata
Dietary treatments greatly affected the reproductive characteristics of M. separata adults (Figure 4A), with significant effects on pre-oviposition period (F10, 554 = 2.528, p = 0.006), oviposition period (F10, 554 = 9.917, p < 0.001) and number of eggs deposited per female (F10, 554 = 15.675, p < 0.001) among the 11 food items (Table 2). The pre-oviposition period on acacia nectar and rapeseed pollen were considerably shorter than on other diets, while schisandra pollen yielded the longest pre-oviposition period. Females attained the longest oviposition period on acacia nectar, followed by maize pollen and camellia pollen. While the shortest oviposition period was attained for water-fed adults. Highest female fecundity was recorded on acacia nectar, and fewer eggs were deposited per female on schisandra pollen and pine pollen than on other diets. Compared with the water diet, acacia nectar, rapeseed pollen, motherwort pollen, lotus pollen, camellia pollen and maize pollen prolonged the oviposition period and increased the number of eggs deposited per female of M. separata adults. On the contrary, the pine pollen, schisandra pollen, rose pollen, sunflower pollen and rose pollen diets reduced female fecundity (i.e., oviposition period and eggs deposited per female). Overall, fecundity was positively correlated with the survival of female moths; the longer the longevity, the more eggs were deposited (Pearson’s r = 0.820, P < 0.01).
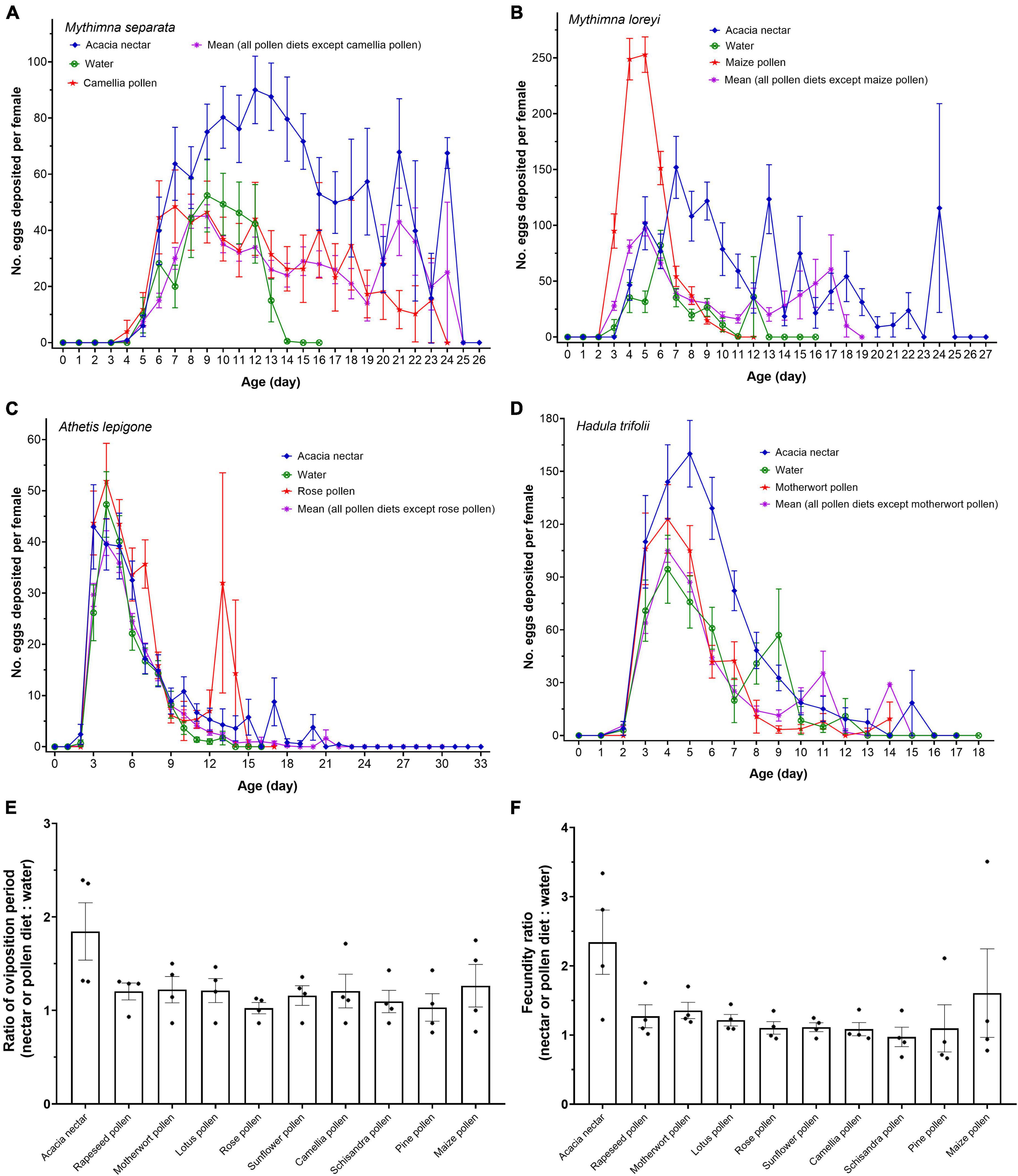
Figure 4. Mean (± SE) daily average fecundity curves of female adults of Mythimna separata (A), Mythimna loreyi (B), Athetis lepigone (C) and Hadula trifolii (D) and the ratio of oviposition period (E) and fecundity (F) for females on different diets. Asterisks for each diet indicate the actual ratio for each species of noctuid moths.
Mythimna loreyi
Food items affected pre-oviposition (F10, 549 = 9.073, p < 0.001), oviposition period (F10, 549 = 12.929, p < 0.001) and number of eggs deposited per female (F10, 549 = 28.974, p < 0.001; Figure 4B and Table 3). The pre-oviposition period on acacia nectar and water were longer than on other diets. Females attained the shortest pre-oviposition period on maize pollen, followed by pine pollen and schisandra pollen. The longest oviposition period was attained for acacia nectar-fed adults, the shortest on water. Significantly more eggs were deposited per female on acacia nectar and maize pollen than on other diets. Nectar and pollen diets all enhanced female fecundity. No significant correlation was recorded between the longevity and fecundity of females (Pearson’s r = 0.468, p > 0.05).
Athetis lepigone
Diets had a significant effect on pre-oviposition (F10, 597 = 3.090, p < 0.001), oviposition period (F10, 597 = 6.667, p < 0.001) and eggs deposited per female (F10, 597 = 6.600, p < 0.001; Figure 4C) of female (Table 4). Compared with water, acacia nectar, rapeseed pollen, motherwort pollen, rose pollen, lotus pollen and sunflower pollen enhanced the fecundity of females, but maize pollen, pine pollen, schisandra pollen and camellia pollen shortened the oviposition period and reduced the number of eggs deposited per female. On motherwort pollen, rapeseed pollen and acacia nectar, the oviposition period was longer than on other diets. Females deposited the most eggs on diets of rose pollen, followed by motherwort pollen, acacia nectar and rapeseed pollen. The shortest oviposition period and the fewest eggs deposited per female were recorded for pine pollen. No significant correlation was recorded between the longevity and fecundity of female A. lepigone moths (Pearson’s r = 0.361, p > 0.05).
Hadula trifolii
Diets also affected oviposition period (F10, 331 = 4.030, p < 0.001), mating frequency (F10, 331 = 2.424, p = 0.009) and number of eggs deposited per female (F10, 331 = 8.760, p < 0.001; Figure 4D and Table 5). On acacia nectar, females attained the longest oviposition period, the highest mating frequency and most eggs deposited per female, while fecundity was the lowest on schisandra pollen. Overall, fecundity was positively correlated with the survival of female moths; the longer the longevity, the more eggs were deposited (Pearson’s r = 0.781, p < 0.01). Diets did not affect the pre-oviposition period (F10, 331 = 1.408, p = 0.175).
Summary
Overall, nectar and pollen diets had no significant effect on the ratio of oviposition period (F9, 30 = 1.972, p = 0.079; Figure 4E) or fecundity (F9, 30 = 1.947, p = 0.083; Figure 4F) for the four species of noctuid moths.
Reproductive physiology
Mythimna separata
Male testis size was significantly affected by diet (F8, 3149 = 29.318, p < 0.001), age (F11, 3149 = 747.698, p < 0.001) and interaction between diet and age (F88, 3149 = 3.199, p < 0.001) (Figure 5A). Male testis size decreased significantly with increasing age (Figure 5C). Compared with 1-day-old males, testis size of 12-day-old males was 42.41% lower. Testes were longest on acacia nectar + pine pollen, followed by acacia nectar + rapeseed pollen, and maize pollen, and shortest on rose pollen. On diets containing nectar and pollen (i.e., acacia nectar + pine pollen, and acacia nectar + rapeseed pollen), testis length was 5.53%, 4.80%, and 3.26∼9.32% longer, respectively, than on water, acacia nectar, or pollen only (Figure 5E).
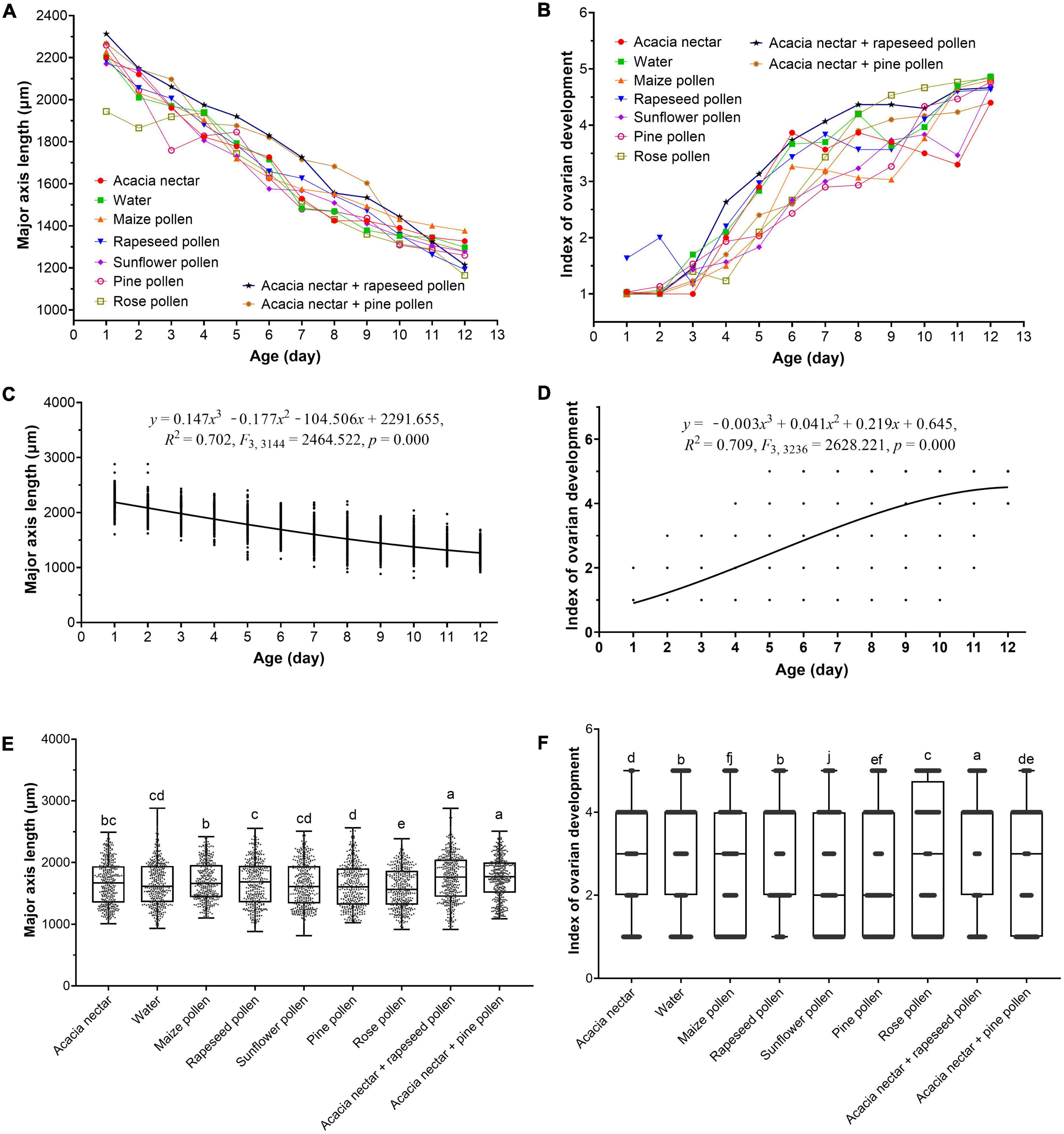
Figure 5. Effect of age and diet on testis size (A,C,E) and ovarian development level (B,D,F) of Mythimna separata. In (C,D), means ± SE are shown. In (E,F), the bottom and top of the black box indicate the first and third quartile values, respectively; the horizontal solid line shows the median for each category; bars indicate the maximum and minimum. Different lowercase letters above the bar indicates a significant difference (two-way ANOVA, Duncan’s MRT; p < 0.05).
Ovarian development also was significantly affected by diet (F8, 3240 = 40.559, p < 0.001), age (F11, 3240 = 1002.519, p < 0.001) and the interaction between diet and age (F88, 3240 = 8.813, p < 0.001) (Figure 5B). Ovarian development level increased significantly with increasing age (Figure 5D). Index of ovarian development was greatest on acacia nectar + rapeseed pollen, followed by rapeseed pollen, then water, and lowest on sunflower pollen (Figure 5F).
Mythimna loreyi
Testis size of M. loreyi was significantly affected by diet (F8, 2901 = 82.064, p < 0.001), age (F10, 2901 = 636.957, p < 0.001) and the interaction between diet and age (F80, 2901 = 6.357, p < 0.001) (Figure 6A). Testis size decreased significantly with increasing age (Figure 6C). Compared with 1-day-old males, 11-day-old males had testes that were 37.33% smaller. The longest testis developed on rose pollen, followed by acacia nectar + rapeseed pollen, and acacia nectar + pine pollen, and the shortest was obtained on water. In diets containing nectar and/or pollen, testis length was 4.00∼9.32% longer than on water (Figure 6E).
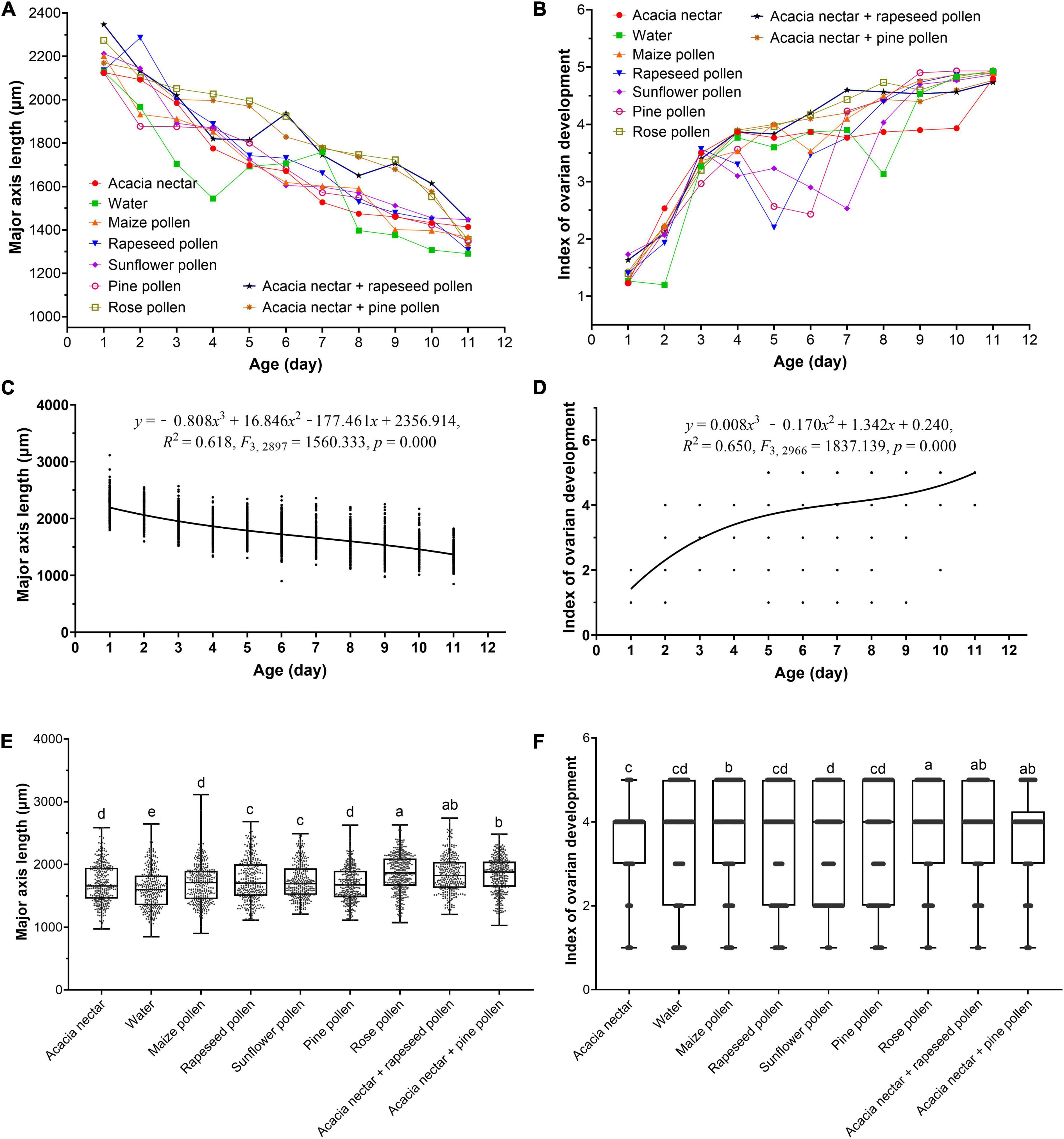
Figure 6. Effect of age and diet on testis size (A,C,E) and ovarian development level (B,D,F) of Mythimna loreyi. In (C,D), means ± SE are showen. In (E,F), the bottom and top of the black box indicate the first and third quartile values, respectively; the horizontal solid line shows the median for each category; bars indicate the maximum and minimum. Different lowercase letters above the bar indicates a significant difference (two-way ANOVA, Duncan’s MRT; p < 0.05).
Female ovarian development also was significantly affected by diet (F8, 2970 = 26.053, p < 0.001), age (F10, 2970 = 877.148, p < 0.001) and the interaction between diet and age (F80, 2970 = 13.353, p < 0.001) (Figure 6B). Ovarian development level increased significantly with increasing age (Figure 6D). Index of ovarian development was greatest on rose pollen, followed by acacia nectar + rapeseed pollen, and acacia nectar + pine pollen and lowest on sunflower pollen, followed by water (Figure 6F).
Discussion
Our previous study found that noctuid insects visit a variety of plants during migration process and feed on their nectar and/or pollen (Liu et al., 2016, 2017a; Chang et al., 2018; He et al., 2022). Many literatures report the effect of nectar or honey on the reproduction and longevity noctuid adults, but knowledge on pollen-diets for the noctuid adults of is rather scarce. In this study, we use laboratory assays to demonstrate how various plant-derived foods enhance development and reproduction of M. separata, M. loreyi, A. lepigone, and H. trifolii. The testes sizes are good indicators of the effect of diets on development of the male reproductive system in noctuid moths. The testis size of M. separata and M. loreyi decreased significantly with increasing age and was further affected by diet. Rapeseed pollen, maize pollen and nectar-containing diets resulted in larger testes for M. separata, and nectar and/or pollen-containing diets resulted in larger testes for M. loreyi. Thus, those food items may help slow testis decay, similar findings were reported for Ostrinia nubilalis Hübner, Cnaphalocrocis medinalis Guenée and S. frugiperda (Milonas and Andow, 2010; Chen et al., 2017; He et al., 2019, 2021a). Meanwhile, ovary status showed that M. separata and M. loreyi continuously develop eggs, which is further enhanced through supplementary nutrition, in line with a previous study for S. frugiperda (He et al., 2021a). Given that M. separata and M. loreyi ovaries were poorly-developed on sunflower pollen, the type of pollen is likely important in ovary maturation.
The type of pollen and nectar also affects the longevity and reproductive and flight abilities of insects (Liu et al., 2017b; He et al., 2021a). Here, we revealed that life history traits and reproductive physiology of M. separata, M. loreyi, A. lepigone and H. trifolii moth were favored by sugar-rich (nectar) or floral-derived (pollen) diets. The lifespan and reproductive traits spanned a range of variabilities among these four species of noctuid moths in our laboratory conditions. Adult feeding on nectar prolonged longevity and enhanced fecundity, which is in line with previous studies for A. lepigone, H. armigera, S. frugiperda, S. exigua and S. litura (Jiang et al., 2015; Liu et al., 2017b; Zhou et al., 2019; He et al., 2021a). However, the four species differed in their fitness on the pollen diets. On 5% acacia nectar, the longevity of female adults for four noctuid moths increased with 2.4 to 5.0 days compared to distilled water. Similarly, on 5% acacia nectar, the longevity of male adults of four noctuid moths increased with 3.2 to 10.9 days compared to distilled water. Pollen solutions slightly increased adult longevity of M. separata and A. lepigone compared to distilled water. Similar findings were made for S. frugiperda and S. exigua (Liu et al., 2017b; He et al., 2021a).
The fecundity of four noctuid moths fed on 5% acacia nectar was 1.22 to 3.34 times as much as water-fed individuals, similar to previous studies for other noctuid moth (Wu and Guo, 1997; Jiang et al., 2015; Liu et al., 2017b; Zhou et al., 2019; He et al., 2021a). While the fecundity of four species for noctuid moth varied in different pollen diets. On suitable pollen diets (e.g., M. separata: motherwort, lotus, camellia and maize pollen solution; M. loreyi: rapeseed, motherwort, lotus, sunflower, schisandra, pine and maize pollen solution; A. lepigone: rapeseed, motherwort, rose and sunflower pollen solution; H. trifolii: rapeseed, sunflower, motherwort and lotus pollen solution), the fecundity of female moths increased with 8.12 to 250.84% as compared to water-fed individuals. On 1% pine pollen, the fecundity of female M. separata, A. lepigone and H. trifolii moths was decreased with 10.06% to 33.52% as compared to water-fed individuals. On the contrary, female fecundity for M. loreyi on 1% pine pollen was 2.11 times greater than for water-fed individuals, similar to previous studies on H. armigera, S. exigua and S. frugiperda (Liu et al., 2017b; He et al., 2021a). Overall, the fecundity of M. separata and H. trifolii moths were positively correlated with the survival of female adults in line with an earlier study that showed that enhanced survival is likely to translate into increased fecundity (Rosenheim, 2011). Similar findings were made for H. armigera and S. frugiperda (Zhou et al., 2019; He et al., 2021a), underlining how sugar-rich (nectar) or floral-derived food items contribute to population build-up, foraging and migration, ultimately enhancing a species’ adaptability to variable or unpredictable environments.
In this study, adult longevity of four noctuid moths was significantly affected by interaction between sex and diet/access to acacia nectar; ovarian development and testis size were significantly affected by interaction between diet and age. These findings indicated that the developmental and reproductive parameters of noctuid moths are not only affected by gender, age, and food diets, but also by the combined effects of gender/age and food diets, which may be due to the different nutritional requirements and/or host preferences of noctuid adults between females and males or among varying age. Previous studies have shown that the host plant species among the different species of noctuid moths differed on their migratory individuals (Liu et al., 2016, 2017a; Chang et al., 2018; He et al., 2022). The larvae of four noctuid moths tested usually feed on angiosperms such as gramineous species (maize and wheat) and exhibit varying fitness performance (Table 1). The reports of noctuid larvae feeding on gymnosperms (Pinus spp.) is rather scarce. While migratory noctuid moths not only feed on flowers of herbs such as maize and rice but also visit pine flowers and consume their nectar and/or pollen (Liu et al., 2016, 2017a; Chang et al., 2018; He et al., 2022). These studies indicate the host plant range of adults differs from that of larvae. Our findings provide preliminary evidence that pollen-diets affects reproduction and longevity of four noctuid moths, as also found for S. frugiperda (He et al., 2021a). As mentioned above, the development and reproduction of insects are affected by a variety of internal or external factors such as age, sex, and diets, thus further study is need to explore host-plant feeding preferences of adult noctuid moths and assess the effects on population dynamics.
Generally, flower-visiting and nectar-feeding insects exhibit marked preferences for pollen or nectar from certain plant species and can be attracted by floral volatile compounds (Andrade et al., 2018; Haber et al., 2018; Kessler et al., 2019). Floral volatiles have been used to trap noctuid moths and monitor and forecast their populations (Tingle and Mitchell, 1992; He et al., 2021b). Migrating moths of M. separata and H. trifolii are often contaminated with pollen of Amaranthaceae, Compositae, Pinaceae, Poaceae and Rosaceae (Liu et al., 2017a; Guo et al., 2018; He et al., 2022). Our findings that rapeseed, maize, motherwort, rose and pine pollen enhanced fitness and fecundity of several species of noctuid moth reveal that particular types of pollen may be highly attractive to M. separata, M. loreyi, A. lepigone and H. trifolii and can be used to develop and design nutritional attractants for eventual incorporation in monitoring, “attract-and-kill” systems and “push-pull” strategy (Miller and Cowles, 1990; Gregg et al., 2018).
Our work demonstrated that the nectar diet commonly slowed testis decay, prolonged the oviposition period and lifespan, and raised fecundity for noctuid pest insects. However, different species of noctuid moths differed in their fitness on the pollen diets, and certain pollen, just like nectar, enhanced fecundity of different moths. The valuable information on nutritional ecology of these noctuid will aid the development and design of nutritional attractants in cropping systems and provide a basis for developing effective, targeted management practices against global noctuid pests.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
LH and KW conceived and designed the experiments and wrote the manuscript. LH performed the experiments and analyzed the data. SZ and WH revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Key R&D Program of Shandong Province (2020CXGC010802) and the Science and Technology Innovation Project of Chinese Academy of Agricultural Sciences (Y2022XK18).
Acknowledgments
We are grateful to Juan Li, Lingmin Zhang, and Yanru Li for their contributions to larval rearing and other assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Andrade, K. A., Aguiar-Menezes, E. L., Gonçalves-Esteves, V., Mendonça, C. B. F., Vieira, G. R. M., Melo, S. J., et al. (2018). Pollen ingestion by Chrysoperla externa (Hagen) adults in a diversified organic agroecosystem. Neotrop. Entomol. 47, 118–130. doi: 10.1007/s13744-017-0537-8
Ares, A. M., Valverde, S., Bernal, J. L., Nozal, M. J., and Bernal, J. (2018). Extraction and determination of bioactive compounds from bee pollen. J. Pharmaceut. Biomed. 147, 110–124. doi: 10.1016/j.jpba.2017.08.009
Balzan, M. V., and Wäckers, F. L. (2013). Flowers to selectively enhance the fitness of a host-feeding parasitoid: adult feeding by Tuta absoluta and its parasitoid Necremnus artynes. Biol. Control 67, 21–31. doi: 10.1016/j.biocontrol.2013.06.006
Cai, Z. Q. (2014). Using SPSS software, cure estimation method to predict the well-off level in developed area—In Guangdong Province as an example. Electr. Test 31–32.
Cass, L. M. (1959). Damage to cabbage by the clover cutworm, Scotogramma trifolii (Rott.) (Lepidoptera: Phalaenidae). Can. Entomol. 91:477.
Chang, H., Guo, J. L., Fu, X. W., Liu, Y. Q., Wyckhuys, K. A. G., Hou, Y. M., et al. (2018). Molecular-assisted pollen grain analysis reveals spatiotemporal origin of long-distance migrants of a noctuid moth. Int. J. Mol. Sci. 19:567.
Chapman, J. W., Reynolds, D. R., and Wilson, K. (2015). Long-range seasonal migration in insects: mechanisms, evolutionary drivers and ecological consequences. Ecol. Lett. 18, 287–302. doi: 10.1111/ele.12407
Chen, Q. H., Zeng, J., Zeng, W., Li, Q., Chen, X. J., and Zou, Y. (2017). Application of the morphological indicators of the male internal reproduction system in forecasting the population dynamics of the rice leaf roller. Cnaphalocrocis medinalis (Lepidoptera: Pyralidae) by sex pheromone trapping. Acta Entomol. Sin. 60, 927–935. doi: 10.16380/j.kcxb.2017.08.010
Dai, Z. L., Jiao, M. Y., and Qian, Y. M. (1962). Study on the reproductive system of armyworm. Shenyang Agric. Univ. 1, 68–74.
Devoto, M., Bailey, S., and Memmott, J. (2011). The ‘night shift’: nocturnal pollen-transport networks in a boreal pine forest. Ecol. Entomol. 36, 25–35. doi: 10.1111/j.1365-2311.2010.01247.x
Duan, Y., Chen, Q., Guo, P., Miao, J., Xia, P. L., Gong, Z. J., et al. (2022). Research progress on the occurrence, damage and control of Mythimna loreyi (Lepidoptera: Noctuidae). Acta Entomol. Sin. 65, 522–532. doi: 10.16380/j.kcxb.2022.04.012
Foottit, R. G., and Adler, P. H. (2009). Insect Biodiversity: Science and Society. Chichester: Wiley-Blackwell, doi: 10.1002/9781444308211
Fox, R. (2013). The decline of moths in Great Britain: a review of possible causes. Insect Conserv. Diver. 6, 5–19. doi: 10.1111/j.1752-4598.2012.00186.x
Fu, X. W., Liu, Y. Q., Li, Y. H., Ali, A., and Wu, K. M. (2014). Does Athetis lepigone moth (Lepidoptera: Noctuidae) take a long-distance migration? J. Econ. Entomol. 107, 995–1002. doi: 10.1603/EC14014
García-Navas, V., and Sanz, J. J. (2011). The importance of a main dish: nestling diet and foraging behaviour in Mediterranean blue tits in relation to prey phenology. Oecologia 165, 639–649. doi: 10.1007/s00442-010-1858-z
Gregg, P. C., Del Socorro, A. P., and Landolt, P. J. (2018). Advances in attract-and-kill for agricultural pests: beyond pheromones. Annu. Rev. Entomol. 63, 453–470. doi: 10.1146/annurev-ento-031616-035040
Guo, P., Wang, G. P., Jin, L. J., Fan, X. Q., He, H. L., Zhou, P. W., et al. (2018). Identification of summer nectar plants contributing to outbreaks of Mythimna separata (Walker) (Lepidoptera: Noctuidae) in North China. J. Integr. Agr. 17, 1516–1526. doi: 10.1016/S2095-3119(17)61840-9
Haber, A. I., Sims, J. W., Mescher, M. C., De Moraes, C. M., Carr, D. E., and Brody, A. (2018). A key floral scent component (β−trans-bergamotene) drives pollinator preferences independently of pollen rewards in seep monkeyflower. Funct. Ecol. 33, 218–228. doi: 10.1111/1365-2435.13246
He, L. M., Fu, X. W., Huang, Y. X., Shen, X. J., Sun, X. T., and Wu, K. M. (2018). Seasonal patterns of Scotogramma trifolii Rottemberg (Lepidoptera: Noctuidae) migration across the Bohai Strait in northern China. Crop Prot. 106, 34–41. doi: 10.1016/j.cropro.2017.12.002
He, L. M., Jiang, S., Chen, Y. C., Wyckhuys, K. A. G., Ge, S. S., He, W., et al. (2021a). Adult nutrition affects reproduction and flight performance of the invasive fall armyworm. Spodoptera frugiperda in China. J. Integr. Agri. 20, 715–726. doi: 10.1016/S2095-3119(20)63198-7
He, W., Zhao, X. C., Ge, S. S., and Wu, K. M. (2021b). Food attractants for field population monitoring of Spodoptera exigua (Hübner). Crop Prot. 145:105616. doi: 10.1016/j.cropro.2021.105616
He, L. M., Liu, Y. Q., Guo, J. L., Chang, H., and Wu, K. M. (2022). Host plants and pollination regions for the long-distance migratory noctuid moth. Hadula trifolii Hufnagel in China. Ecol. Evol. 12:e8819. doi: 10.1002/ece3.8819
He, W., Zhao, S. Y., Ge, S. S., Jiang, Y. Y., Zhao, X. C., and Wu, K. M. (2019). Population prediction method using sexual trapping for Spodoptera frugiperda. Plant Prot. 45, 48–53. doi: 10.16688/j.zwbh.2019317
Ho, H. Y., Tsai, R. S., Hsu, E. L., Chow, Y. S., and Kou, R. (2002). Investigation of possible sex pheromone components of female loreyi leafworm. Acantholeucania loreyi (Duponchel) (Lepidoptera: Noctuidae) in Taiwan. Zool. Stud. 41, 188–193. doi: 10.2108/zsj.19.485
Hu, G., Lim, K. S., Horvitz, N., Clark, S. J., Reynolds, D. R., Sapir, N., et al. (2016). Mass seasonal bioflows of high-flying insect migrants. Science 354, 1584–1587. doi: 10.1126/science.aah4379
Jalaeian, M., Farahpour-Haghani, A., and Esfandiari, M. (2017). First report of damage caused by Leucania loreyi (Lep.: octuidae) on rice in Guilan province. Plant Pest Res. 7, 77–80.
Jiang, J. Y., Li, X. Q., Xu, Y. H., Li, Z. H., Zhang, Z. Y., and Xu, H. (2008). Preliminary studies on Athetis (Proxenus) lepigone. Plant Prot. 34, 123–129.
Jiang, X. F., Luo, L. Z., Zhang, L., Sappington, T. W., and Hu, Y. (2011). Regulation of migration in Mythimna separata (Walker) in China: a review integrating environmental, physiological, hormonal, genetic, and molecular factors. Environ. Entomol. 40, 516–533. doi: 10.1603/EN10199
Jiang, X. F., Yao, R., Zhang, L., Cheng, Y. X., Liu, T. Q., and Luo, L. Z. (2015). Effects of supplementary nutrition on adult reproduction and longevity of Athetis lepigone (Möschler). J. Plant Prot. 42, 1004–1008. doi: 10.13802/j.cnki.zwbhxb.2015.06.021
Kessler, D., Bing, J., Haverkamp, A., Baldwin, I. T., and Manson, J. (2019). The defensive function of a pollinator-attracting floral volatile. Funct. Ecol. 33, 1223–1232. doi: 10.1111/1365-2435.13332
Krenn, H. W. (2010). Feeding mechanisms of adult Lepidoptera: structure, function, and evolution of the mouthparts. Annu. Rev. Entomol. 55, 307–327. doi: 10.1146/annurev-ento-112408-085338
Kristensen, N. P., Scoble, M. J., and Karsholt, O. (2007). Lepidoptera phylogeny and systematics: the state of inventorying moth and butterfly diversity. Zootaxa 1668, 699–747. doi: 10.11646/zootaxa.1668.1.30
Lee, J. C., and Heimpel, G. E. (2008). Effect of floral nectar, water, and feeding frequency on Cotesia glomerata longevity. BioControl 53, 289–294. doi: 10.1007/s10526-007-9070-8
Li, G. B., Wong, H. H., and Woo, W. S. (1964). Route of the seasonal migration of the oriental armyworm moth in the eastern part of China as indicated by a three-year result of releasing and recapturing of marked moths. J. Plant Prot. 3, 101–109.
Li, J. H. (2010). Occurrence law of armyworm in China and its identification and prevention. Plant Dis. Pests 1, 31–36. doi: 10.19579/j.cnki.plant-d.p.2010.03.010
Lindeborg, M. (2008). Remarkable records of Macrolepidoptera in Sweden 2007. Entomol. Tidskr. 129, 43–52.
Liu, Y. Q., Fu, X. W., Mao, L. M., Xing, Z. L., and Wu, K. M. (2016). Host plants identification for adult Agrotis ipsilon, a long-distance migratory insect. Int. J. Mol. Sci. 17:851. doi: 10.3390/ijms17060851
Liu, Y. Q., Fu, X. W., Mao, L. M., Xing, Z. L., and Wu, K. M. (2017a). Identification of host plant use of adults of a long-distance migratory insect, Mythimna separata. PLoS One 12:e0184116. doi: 10.1371/journal.pone.0184116
Liu, K., Zhu, P. Y., Lü, Z. X., Chen, G. H., Zhang, J. M., Lü, Y. B., et al. (2017b). Effects of sesame nectar on longevity and fecundity of seven Lepidoptera and survival of four parasitoid species commonly found in agricultural ecosystems. J. Integr. Agr. 16, 2534–2546. doi: 10.1016/S2095-3119(17)61665-4
Lundgren, J. G. (2009). Relationships of Natural Enemies and Non-prey Foods, Vol. 7. Berlin: Springer, doi: 10.1007/978-1-4020-9235-0
Miller, J. R., and Cowles, R. S. (1990). Stimulo-deterrent diversion: a concept and its possible application to onion maggot control. J. Chem. Ecol. 16, 3197–3212. doi: 10.1007/bf00979619
Milonas, P. G., and Andow, D. A. (2010). Virgin male age and mating success in Ostrinia nubilalis (Lepidoptera: Crambidae). Anim. Behav. 79, 509–514. doi: 10.1016/j.anbehav.2009.12.005
Nam, H. Y., Kwon, M., Kim, H. J., and Kim, J. (2020). Development of a species diagnostic molecular tool for an invasive pest. Mythimna loreyi using LAMP. Insects 11:817. doi: 10.1101/2020.10.01.323089
Nikolaevitch, P. A., and Vjatcheslavovna, I. E. (2002). The Noctuidae (Lepidoptera) of the Daghestan Republic (Russia). Phegea 30, 11–36.
Nowacki, J., Holowinski, M., and Palka, K. (2001). Athetis lepigone (Möschler, 1860) (Lepidoptera. Noctuidae), a noctuid moth new for the Polish fauna. Pol. Pismo Entomol. 70, 271–275.
Poltavsky, A., Matov, A. Y., and Ivliev, P. (2009). Heteroceran moths (Lepidoptera. Heterocera) of the Don River delta. Entomol. Rev. 89, 1072–1081. doi: 10.1134/S0013873809090085
Ren, M., Wu, X. M., and Yang, X. G. (2006). Scotogramma trifolii Rottemberg occurred on a large scale in Tongyu County. China Plant Prot. 26:22.
Ribas-Marquès, E., Díaz-Calafat, J., and Boi, M. (2022). The role of adult noctuid moths (Lepidoptera: Noctuidae) and their food plants in a nocturnal pollen-transport network on a Mediterranean island. J. Insect Conserv. 26, 243–255. doi: 10.1007/s10841-022-00382-7
Rosenheim, J. A. (2011). Stochasticity in reproductive opportunity and the evolution of egg limitation in insects. Evolution 65, 2300–2312. doi: 10.1111/j.1558-5646.2011.01305.x
Sertkaya, E., and Bayram, A. (2005). Parasitoid community of the loreyi leaf worm Mythimna (Acantholeucania) loreyi: novel host-parasitoid associations and their efficiency in the eastern mediterranean region of Turkey. Phytoparasitica 33, 441–449. doi: 10.1007/BF02981393
Sharma, H. C., and Davies, J. C. (1983). The oriental armyworm, Mythimna separata (Wlk.) distribution, biology and control: a literature review. Miscellaneous Report No 59. London: Overseas Development Administration.
Solayman, M., Islam, M. A., Paul, S., Ali, M. Y., Khalil, M. I., Alam, N., et al. (2016). Physicochemical properties, minerals, trace elements, and heavy metals in honey of different origins: a comprehensive review. Compr. Rev. Food Sci. Saf. 15, 219–233. doi: 10.1111/1541-4337.12182
Song, Y. F., Yang, X. M., Zhang, H. W., Zhang, D. D., He, W., Wyckhuys, K. A. G., et al. (2021b). Interference competition and predation between invasive and native herbivores in maize. J. Pest Sci. 94, 1053–1063. doi: 10.1007/s10340-021-01347-6
Song, H. Y., Li, L. L., Zhang, Q. Q., Song, Y. Y., Zhu, Z. G., Lu, Z. B., et al. (2021a). Southward migration routes of insect species in Shandong province. Chin. J. Appl. Entomol. 58, 592–600. doi: 10.7679/j.issn.2095-1353.2021.060
Tingle, F. C., and Mitchell, E. R. (1992). Attraction of Heliothis virescens (F.) (Lepidoptera: Noctuidae) to volatiles from extracts of cotton flowers. J. Chem. Ecol. 18, 907–914. doi: 10.1007/BF00988331
Wäckers, F. L., Romeis, J., and Rijn, P. V. (2007). Nectar and pollen feeding by insect herbivores and implications for multitrophic interactions. Annu. Rev. Entomol. 52, 301–323. doi: 10.1146/annurev.ento.52.110405.091352
Wang, J., Yu, Y., Zhao, N., Zhang, A. S., Zhou, X. H., Zhuang, Q. Y., et al. (2013). The research progress of Proxenus lepigone in China. Biol. Disaster Sci. 36, 95–99. doi: 10.3969/j.issn.20953704.2013.01.023
Wang, Z. Y., Shi, J., and Dong, J. G. (2012). Reason analysis on Proxenus lepigone outbreak of summer corn region in the Yellow River, Huai and Hai Rivers Plain and the countermeasures suggested. J. Maize Sci. 20, 132–134. doi: 10.13597/j.cnki.maize.science.2012.01.002
Wu, K. M., and Guo, Y. Y. (1997). Effects of food quality and larval density on flight capacity of cotton bollworm. Acta Entomol. Sin. 40, 51–57. doi: 10.16380/j.kcxb.1997.01.008
Wyckhuys, K. A. G., Strange-George, J. E., Kulhanek, C. A., Wäckers, F. L., and Heimpel, G. E. (2008). Sugar feeding by the aphid parasitoid Binodoxys communis: How does honeydew compare with other sugar sources? J. Insect Physiol. 54, 481–491. doi: 10.1016/j.jinsphys.2007.11.007
Zhang, J. M., and Yu, G. Y. (2021). Identification and control of Hadula trifolii Hufnagel. Vegetables 82–83.
Zhang, Y. H., Cheng, D. F., Jiang, Y. Y., Zhang, Y. J., and Sun, J. R. (2010). Analysis on the population status of the overwintering generation of the clover cutworm Scotogramma trifolii (Lepidoptera: Noctuidae) in Beijing. Sci. Agric. Sin. 43, 1815–1822. doi: 10.3864/j.issn.0578-1752.2010.09.007
Zhao, Q., Zhang, Y. H., Liu, H., and Cheng, D. F. (2011). A method used for distinguishing between the sexes of Scotogramma trifolii. Chin. J. Appl. Entomol. 48, 1879–1881.
Zhao, Z. J., Chen, E. X., and Zhang, Y. (1992). Study on the biological characteristics of Scotogramma trifolii R. and its control. Sugar Crops China 25–28.
Keywords: fecundity, ovarian anatomy, testis anatomy, nutritional physiology, supplementary nutrition
Citation: He L, Zhao S, He W and Wu K (2022) Pollen and nectar have different effects on the development and reproduction of noctuid moths. Front. Ecol. Evol. 10:976987. doi: 10.3389/fevo.2022.976987
Received: 29 June 2022; Accepted: 31 August 2022;
Published: 29 September 2022.
Edited by:
Xavier Martini, University of Florida, United StatesReviewed by:
Rui Tang, Institute of Zoology, Guangdong Academy of Science (CAS), ChinaChris Stone, University of Illinois at Urbana-Champaign, United States
Bożena Denisow, University of Life Sciences of Lublin, Poland
Copyright © 2022 He, Zhao, He and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kongming Wu, wukongming@caas.cn
 Limei He1
Limei He1  Shengyuan Zhao
Shengyuan Zhao Kongming Wu
Kongming Wu