Species conflict at Earth’s edges – Contests, climate, and coveted resources
- 1Department of Fish, Wildlife, and Conservation Biology, Colorado State University, Fort Collins, CO, United States
- 2Wildlife Conservation Society–Global Program, The Bronx, NY, United States
- 3Glacier National Park, West Glacier, MT, United States
Direct conflict between species is an infrequently witnessed biological phenomenon. Potential drivers of such contests can include climate change, especially at Earth’s high elevation and latitudinal extremes where temperatures warm 2–5 times faster than elsewhere and hydro-geomorphic processes such as glacial recession and soil erosion affect species access to abiotic resources. We addressed a component of this broader issue by empirical assessments of mammalian conflict over access to four abiotic resources – minerals, water, snow, and shade – by annotation of past studies and by empirical data collection. Evidence for Nearctic and Palearctic mammals indicates that when desert waters are in short supply, contests intensify, generally favoring larger species regardless of their status as native or exotic. Our empirical data indicate that contests between two large and approximately similarly-sized mammals – mountain goats (Oreamnos americanus) and bighorn sheep (Ovis canadensis) – along a 2,500 km gradient at three high-altitude (above tree-line) sites in the Rocky Mountains of North America, result in striking asymmetries; goats dominated > 95% of interactions. Despite far fewer observations of encounters to access shade or snow patches, an increasingly prominent dialog needs to be held about rarely explored biological phenomena where less is known than we might otherwise presume, whether induced by climate or increasing anthropological alteration because of underpinnings to understand community structure and conservation planning. Observations on the frequency and intensity by which individuals escalate behavior to access abiotic resources remains an underappreciated arena to help identify the proximate importance of scarcity in the natural environment. Notwithstanding Darwin’s prediction some 165 years ago that populations in extreme environments (high-latitude, high-altitude) are more likely to be impacted by abiotic variables than biotic, conflict between species may be reflective of climate degradation coupled with the changing nature of coveted resources.
Introduction
As humans (Homo sapiens) continue the unabated colonization of Earth’s terrestrial regions (Bradshaw and Brook, 2014), potential for conflict over access to rare resources inevitably increases. In extreme environments – such as high latitudes, the loftiest of elevations and areas of scant rainfall – abiotic forces may dictate survival more directly than species interactions. As early as 1859 this was predicted – “When we reach the Arctic regions, or snowcapped summits, or absolute deserts, the struggle for life is almost exclusively with the elements” (Darwin, 1859), and such limitations have been amply confirmed among mammals in Arctic, high mountain, and desert biomes (Anthony, 1976; Caughley and Gunn, 1993; Dale et al., 1994; Hansen et al., 2019). Nonetheless, persistence under exceptionally harsh conditions is about more than abiotic challenge as individuals must still meet nutrient requirements and configure interactions with other species (Krebs et al., 2003; Gauthier et al., 2004; Festa-Bianchet et al., 2011). While neither common nor frequently witnessed, overt contests between species do ensue and must be examined through the broader lens of global change where interactions may be unmasked particularly as landscapes change. Although competition may take different forms as noted long ago (Elton, 1946), conflict in extreme environments should not be discounted where warming temperatures exacerbate survival challenges (Berger, 2018; Mills et al., 2018).
Along the planet’s most northern and southern edges and at the highest altitudes temperatures warm 2–5 times faster than elsewhere (Pörtner et al., 2021). Consequently, the world’s mountains are experiencing mass glacial losses fomented by an accentuation in the timing and intensity of water flow (Barnett et al., 2005; Lee et al., 2021; Smith, 2021). Such physical alterations create additional cryospheric and geomorphic change through the redistribution of inorganic inert materials by leaching (Butler, 2012; Yang et al., 2021), conversions which in turn have consequence for soil development, minerals, and plants (Dixon and Thorn, 2005; Lambert et al., 2020; Zimmer et al., 2022). By example, phosphorus or other bio-metals can become concentrated at depositional sites in mineral licks, which offer essential micro-nutrients to geophagous mammals (Link et al., 2011; Pebsworth et al., 2019). Sodium, in particular, is a prominent cation in such sites and it plays a prominent role in several body functions, including lactation, though a singular universal role of sodium in ungulate salt licks may not exist (Kreulen, 1985; Robbins, 1993; Ayotte et al., 2006).
Still, the overarching importance of access to a restricted abiotic resource was noted as early as 1741. John Bartman commented on the arduous journey of white-tailed deer (Odocoileus virginianus): “… the soil, I suppose contains some saline particles agreeable to the deer who come many miles to one of these places” (Seton, 1927). Empirical documentation is now widespread from environs more extreme than the New England’s temperate forests of Bartman’s explorations. In the exceptionally arid Namib Desert, elephants (Loxodonta africana) travel up to 70 km to access water (Shoshani and Viljoen, 1992). On the comparatively dry Tibetan Plateau above 4,500 m, female wild yaks (Bos grunniens mutus) seek remnant snow patches to sustain milk production for nursing offspring during winter when every other source of water is frozen solid (Berger et al., 2015). Mountain goats (Oreamnos americanus), a species not known for broad locomotor travel efficiency (Côté and Festa-Bianchet, 2003), may cover up to 29 km in geophagous pursuits (Rice, 2010), where mineralized sites vary from caves to roadsides, outcrops and eroding mountain slopes, some because of glacial attrition, and soils below trees (Cowan and Brink, 1949). At least a dozen mammalian orders including Primates, Rodentia, Lagomorpha, Carnivora, Chiroptera, Perissodactyla and Artiodactlya go to great length seeking minerals (Kreulen, 1985; Link et al., 2011; Pebsworth et al., 2019; see also above).
Despite these abbreviated descriptors of onerous travel, we know little about how, when, or where contests resulting in interspecific competition occur between species, particularly over access to four abiotic resources – minerals, shade, water, and snow. Such deficiencies arise simply because overt interspecific interactions are rarely witnessed. Improving knowledge in this mostly uncharted arena is undermined by logistics of data acquisition in difficult-to-reach environments and, assuredly has been exacerbated by a global decline in field-oriented scientific inquiry (Ríos-Saldaña et al., 2018). Yet, understanding how species contest for access to abiotic products remains a fertile avenue for future study.
Here we report on outcomes of species conflict in mammals from extreme landscapes concentrating on priority of access to those abiotic resources most likely to be affected by climate challenge and anthropogenic modification. Specifically, we mobilize disparate evidence as to how hydrogeological and other alterations conflate to shape attainment of abiotic rewards (see schematic in Figure 1). We adopt two approaches: (1) contextualization of the case for conflict at a coarse scale through previously reported aggressive encounters across a range of geographies, and (2) presentation of empirical data on overt conflict at high elevation (above tree-line) sites along a 2,500 km gradient across the Rocky Mountain cordillera (North America). Our study sites were concentrated in areas of relatively recent glacial recession where two large and approximately similarly-sized mammals – mountain goats and bighorn sheep (Ovis canadensis) contested for access to minerals. Moreover, given the magnitude of rapid change in global ecological communities due to anthropogenic-induced impacts, many of which favor invasive species from plants to fish, and birds to mammals, we included in our assessment of conflict exotics (i.e., horse [Equus caballus], yak), especially because of an increasingly prominent dialog about current and future biodiversity conservation using ecological surrogates (Lundgren et al., 2021). We find that when abiotic resources are in short supply, not only do interspecific contests intensify, but larger dominate species regardless of their status as native or exotic.
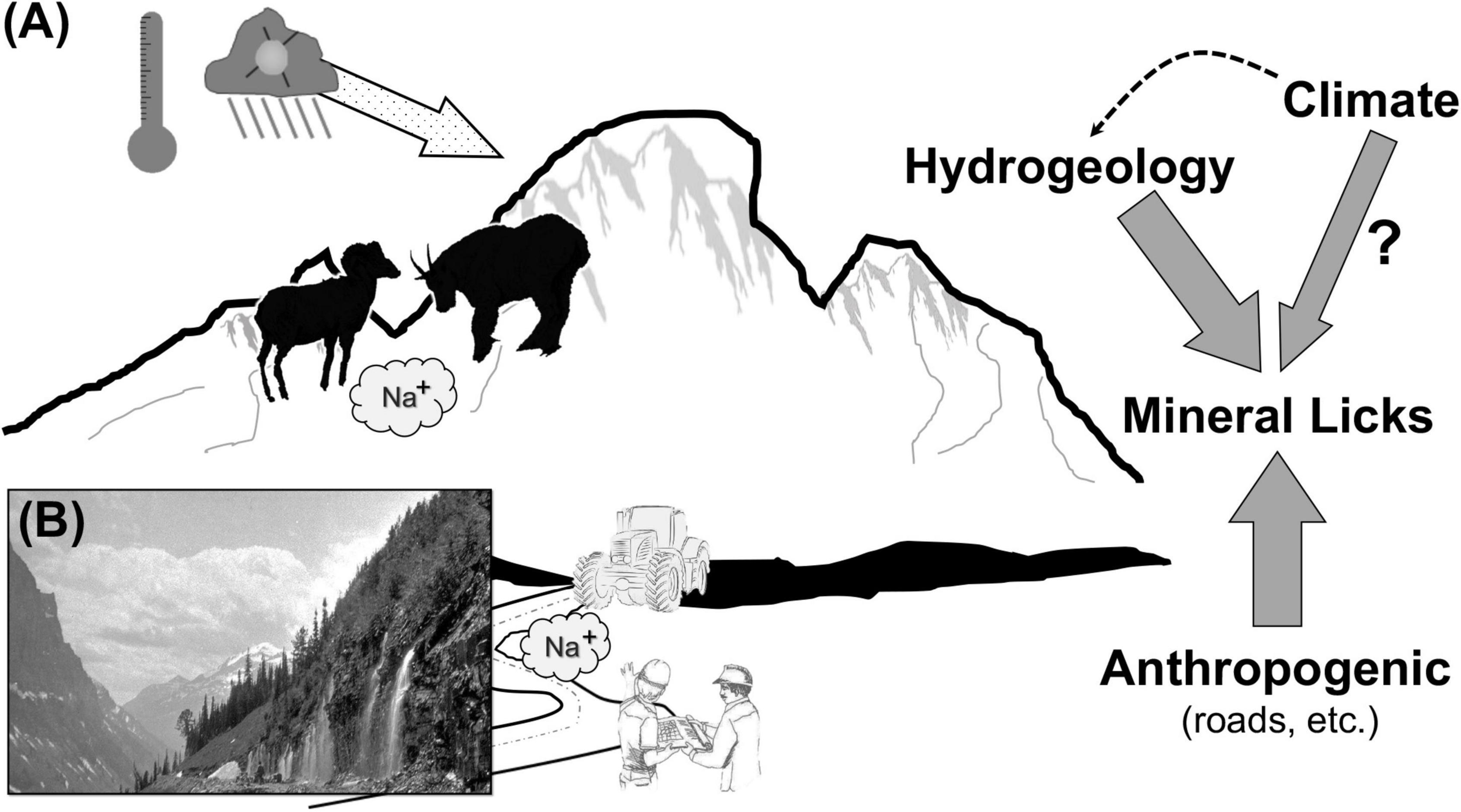
Figure 1. Representation of hypothesized pathways leading to high elevation conflict. (A) Temperature-based melting of glaciers facilitates availability of mineral licks coupled with their loss to geophagous ungulates by highways construction; (right) hydro-geomorphic changes to mineral availability, and (B) 1927 photograph of excessive subterranean-to-surface water flow (Glacier National Park archive photo).
Assumptions, rationale, and framework
Identifying limited abiotic resources
Not all resources are of equivalent value but, by inference as judged by an animal’s behavior, they may be classified as to desirability. Consider something inanimate, an abiotic resource like shade. Most of us will have witnessed a dog or cat seek thermal relief on a hot day, perhaps situating itself under a rocky overhang or a tree. Humans, other primates, and individuals of many species do this of course. In such scenarios where shade is not limited, competition for these spots will be minimal, yet the scientific literature on competition, when shade is limited for wild mammals, is scant. Not only are displacement events rarely observed – or at least not reported – but as our anthropogenic grasp tightens, a focus on its consequent impacts to species and how they interact is useful to understand components of global change.
Of fundamental interest is when a resource is scarce and different species seek to utilize it at the same time. While overt interspecific encounters may be frequently circumvented by temporal separation (Valeix et al., 2007), or by a tendency to avoid conflict through self or opponent assessment (Parker and Rubenstein, 1981; Chapin et al., 2019), the few papers that describe active displacements support the assumption that abiotic resources are at times in short supply.
Desert waters offer a case in point (Table 1). Nearly 300 discrete interspecies encounters involving African elephants, rhinos (Diceros bicornis) and other mammals at drinking points at the same time underwent a level of forced or subtle displacements (Berger and Cunningham, 1998). Aggressive assertations included rushes (or charges), head thrusts or singularly- directed walk-approaches toward interspecifics (Figure 2), all of which resulted in rapid displacements (Table 1). We operationally characterized these sorts of abiotic resources as coveted if they were sites for which species contested priority of access.
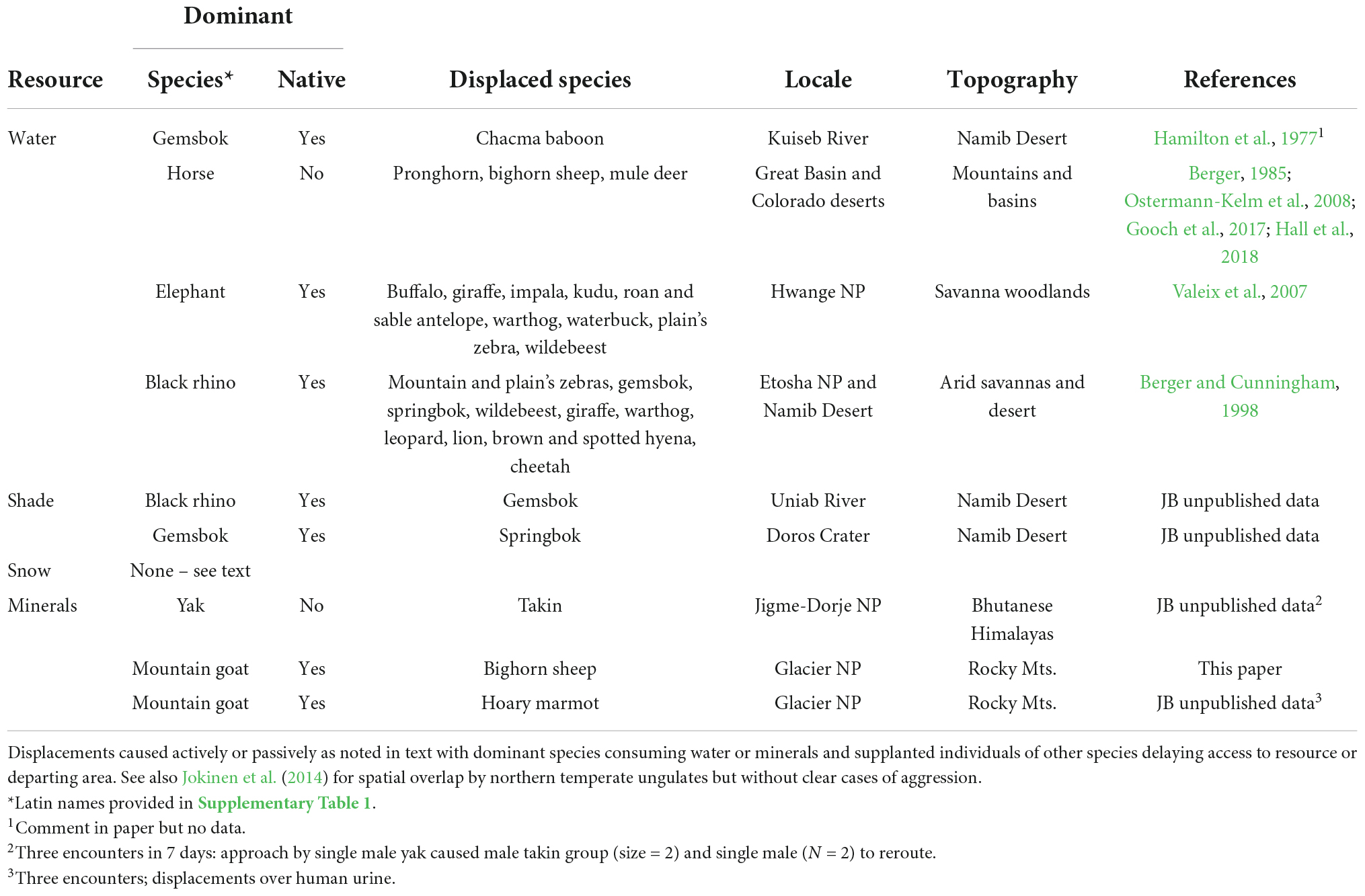
Table 1. Examples of interspecies conflict, displacement in five mammalian orders (Artiodactlya, Perissodactyla, Carnivora, Rodentia, and Primates) over access to water and minerals with notation on potential for contests for additional products (shade, snow) including (feral) horses and (domestic) yaks as exotic species.
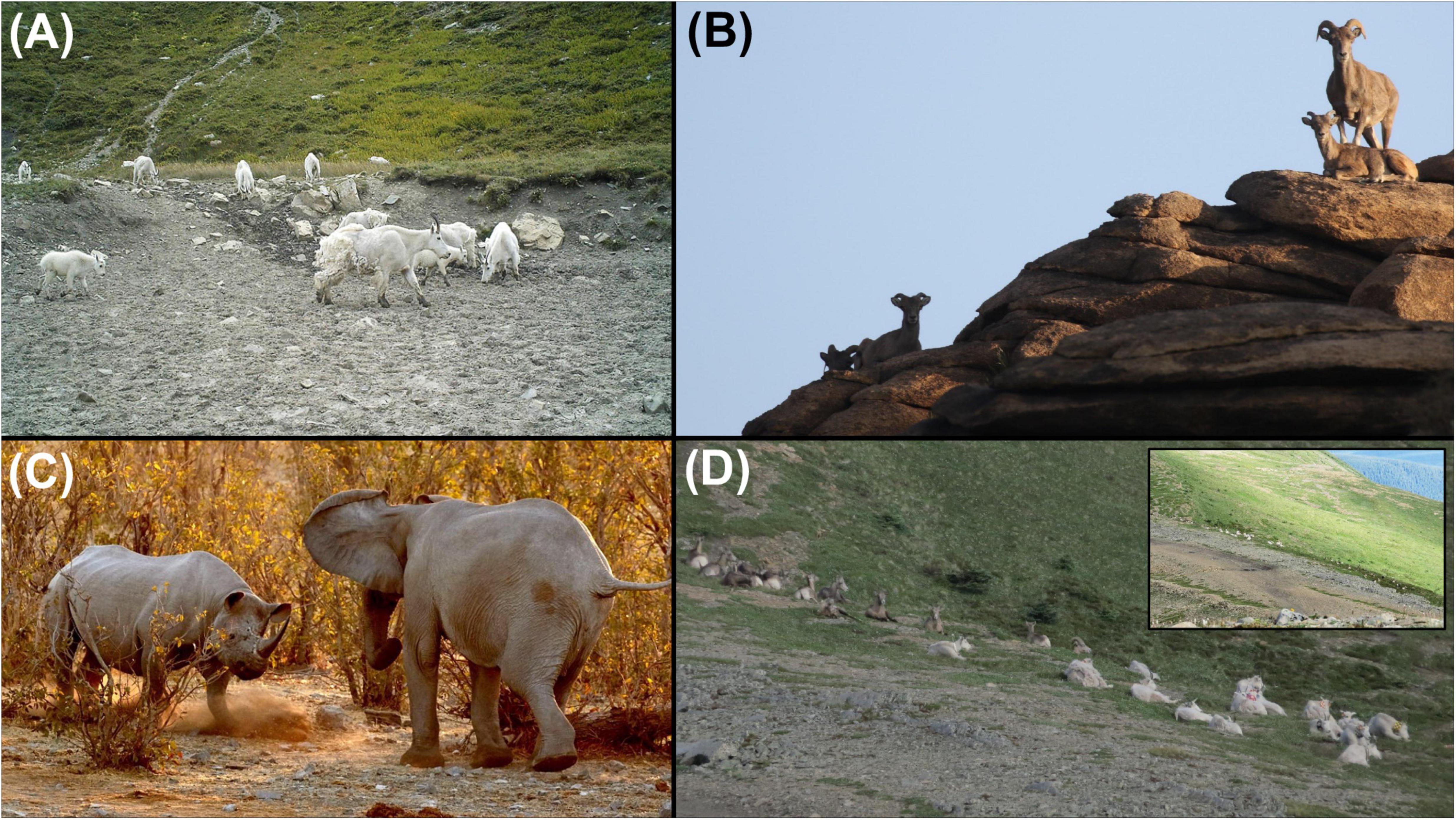
Figure 2. Examples of conflict and tolerance. (A) Mountain goats at a mineral lick in Glacier National Park, MT (W. Sarmento). (B) Closely related taxa of mountain goats and bighorn sheep, herein – female and young Siberian ibex (left) and argali on same rocky outcrops in the Gobi Desert of Mongolia (R. Reading). (C) Black rhino and elephant in Etosha National Park, Namibia (A. Forsyth). (D) Sizeable groups of mountain goats and bighorn sheep at rest sympatrically (and inset of them in broader landscape) on Caw Ridge, Alberta, Canada (F. Dulude-de Broin).
Identifying glacial loss and anthropogenic alteration of mineralized sites
Changes within our study spheres along Rocky Mountain cordillera (Figure 1) include those induced directly by warming temperatures (Martin-Mikle and Fagre, 2019) and by more immediate by human destruction of habitat. Higher temperatures at our three study areas (see below) are strongly associated with the phenology of snow melt and plant growth, and an upslope range shift of shrubs and trees into historical alpine tundra habitats. In northern Montana, specifically, Glacier National Park, 85% of the ice/glacial fields have been lost since the park’s creation in 1916 (Hall and Fagre, 2003).
The extent to which hydrological changes caused by warming has affected mineral licks used by ungulates in the Rocky Mountains is less certain. Yet, across segments of this broad region construction, modification of highways have resulted in massive loss of previously-available habitat and mineralized sites used by elk (Cervus elaphus), moose (Alces alces), mountain goats, and, undoubtedly, other species (Cowan and Brink, 1949; Table 2). The degree that losses of these low elevation sites because of human construction promoted access to high elevation minerals is not clear but access to such mineralized sites is now possible in some areas because ice sheets no longer exist (Hall and Fagre, 2003) as we describe (Figures 1, 3).
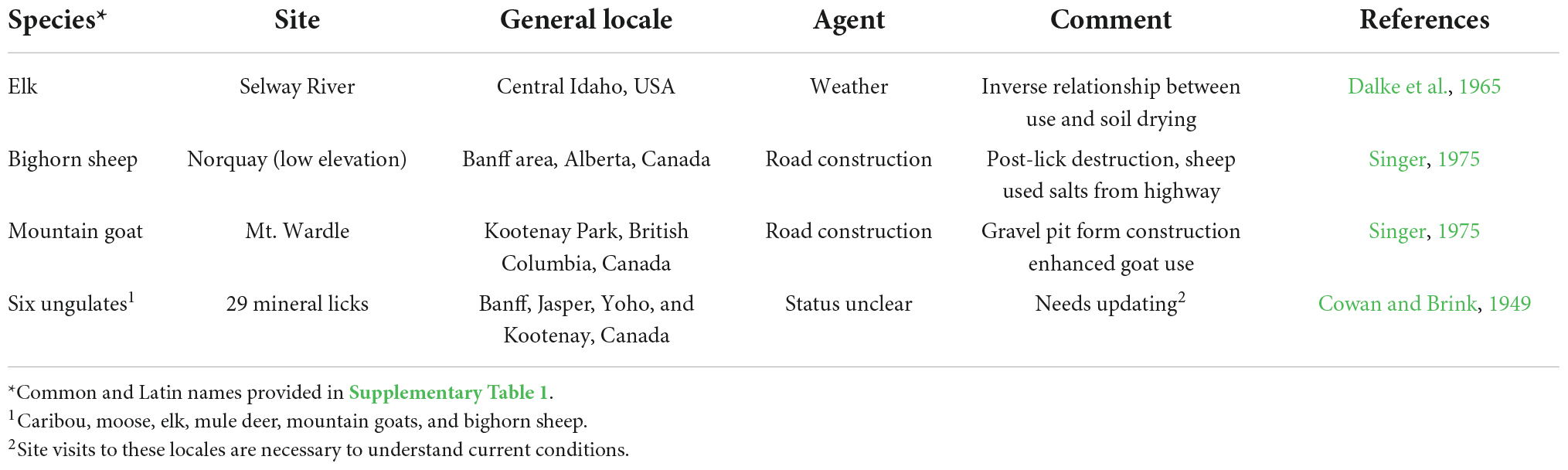
Table 2. Examples of mineral lick usage and change in lick usage due to anthropogenic alteration or local weather conditions.
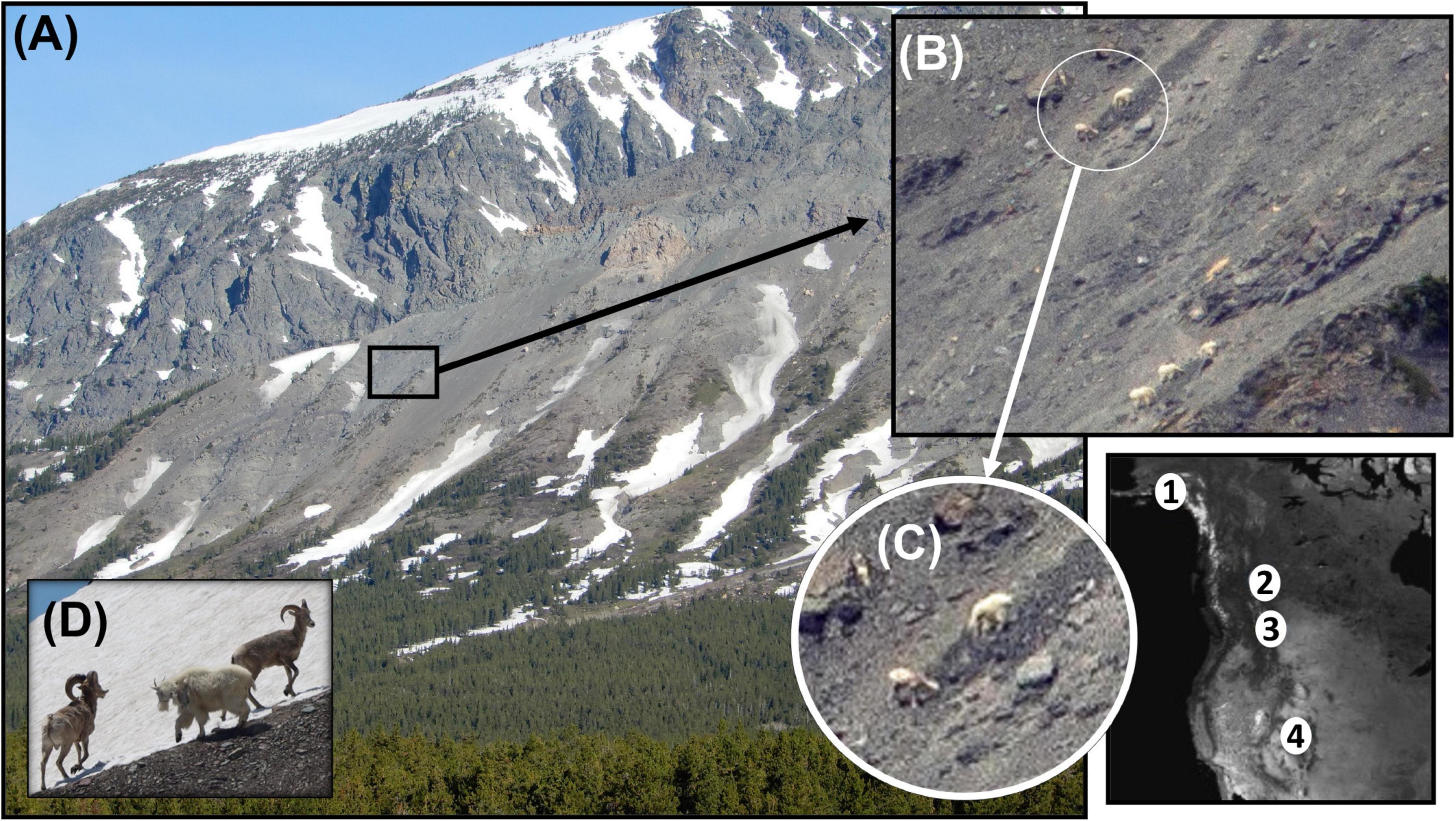
Figure 3. Mountain goat-bighorn sheep contests at alpine sites in Glacier National Park, MT. (A) Remnant snowfield and mineral lick with (B) three goats (lower right central) and single goat and single female bighorn top left at water-saturated mineral lick, (C) sympatric feeding prior to goat displacement of bighorn (enlarged), and (D) male goat with actionable horn threat displacing two male bighorn. Inset reflects four sites of known displacements between mountains goats and (1) Dall sheep (Ovis dalli) in Alaska (Klein, 2019), and bighorn sheep at (2) Caw Ridge, Alberta, Canada (3) Glacier National Park, MT, and (4) Mt. Evans, CO.
Materials and methods
We used two approaches to appraise species interactions for access to four abiotic resources – water, snow, shade and minerals: (1) a synthesis of peer-reviewed studies coupled with gray literature and opportunistic observations, and (2) field work across three high elevation sites.
Assessing conflict and access to three abiotic resources – Water, shade, and snow
We based our assessment of dominance interactions primarily on displacement or obvious cases of avoidance when members of two species approached a discrete abiotic resource. Although numerous accounts are published, we excluded those unless involvement was for an obvious coveted abiotic resource. By way of example, mule deer (Odocoileus hemionus) crossing an alpine meadow, which caused yellow bellied marmots (Marmota flaviventris) to flee (Armitage, 2003), was not included since there was no indication that resources used by the marmots were then usurped by the passing deer. Cases of conflict over resources, mostly water, are tabulated in Table 1.
Shade, another abiotic resource, is notably important as a thermal refuge for a variety of terrestrial vertebrates including that provided by caves, rocky overhangs, and trees (Barrett et al., 2004; Pruetz, 2007; Cain et al., 2008). We were unable to find formal reports of contests between species over access to shade but include our limited observations (Table 1).
Assessing conflict and access to minerals
Our empirically-based fieldwork concentrated at three sites – the Mount Evan region of Colorado (39.5882, –105.6437), the Marias Pass area of Glacier National Park, Montana (48.3166, –113.3548; Figure 3), and Caw Ridge in west-central Alberta (Canada, ∼ 54.8000, –119.8000). Observations were conducted at Glacier National Park (Montana) in late May–early June in 2020–2021, at Mt. Evans, Colorado June–July (2020) and June–August (2021), and at Caw Ridge in 2017–2018 (Dulude-de Broin et al., 2020).
Mountain goats are native to the Montana and Alberta sites and bighorn sheep to all three (Festa-Bianchet and Côté, 2008). In Colorado, however, mountain goats are not native. They were introduced there in 1947 and have increased greatly, as has also been the case (also as introduced species) in Wyoming, Utah, and elsewhere (Côté and Festa-Bianchet, 2003).
In Glacier, our observations concentrated at natural mineral licks. At Mt. Evans, we focused on a 3,600 m site with effluent and salt sought by both goats and sheep (Clay, 2019). At Caw Ridge conflicts were either for access to resting sites or for a few plant morsels but not abiotic products (see Supplementary videos 1, 2). Despite variation in elevation and latitude, the use of three high elevation sites in the Rocky Mountains offered an opportunity to gauge whether the direction of dominance and displacement between bighorn sheep and mountain goats was consistent.
Among the variables we considered to have a possible impact on the outcome of encounters were group sizes. Generally, it is easy to enumerate assembly size but as individuals become more dispersed, evaluations grow more complex. For instance, a half dozen clustered moose can easily be counted as a group but if each individual is spread across several semi-distant willow patches it’s less obvious if this is to be considered a group (Molvar and Bowyer, 1994). Different versions of group metrics (Bowyer, 1987) have been reported for decades; these include inter-individual distances of separation, cohesiveness, or behaviors such as coordinated feeding or resting; little consensus exists (Elgar, 1989; Treves, 2000).
We operationally defined a group as a cluster of individuals in which the behavior of one is likely to affect that of others. Our snapshot approach was obviously a judgment because at times there was certainty of response but not at other times. For example, the five pictured animals (a total of four goats and one sheep) in Figure 3B might be considered one group of five, or two groups, respectively of one and four. At an intraspecific level, which is typically the way groups are defined, perspective and scale matter, but as we note below, group sizes had trivial, if any effect, on outcomes over access to minerals.
Results
Most reports about species conflict at abiotic resources emanate from interactions observed at desert waters. Body size is noted as a major determinant of outcome. Elephants, for instance, are not only the typical victor, but they show little tolerance for other species (Table 1). Agonism, dominance, and directionality is likely to vary by site, history, and necessity. Among feral or otherwise introduced species, native mammals may be delayed or denied access (Hall et al., 2018; Ferretti and Mori, 2020; see also Table 1).
Other spottily distributed abiotic products like shade and snow have received much less scrutiny as resources for which species compete despite their known biological relevance (Rosvold, 2016). Beyond water, however, the only evidence for competition to access shade stems from observations in the Namib Desert or Kalahari Sands where black rhinos displaced ungulates from shade trees they subsequently used (Table 1). With respect to snow patches, we witnessed sympatry between mountain goats and bighorn sheep, but they remained distal without antagonism. Likewise, at about 4,900 m on the Tibetan Plateau wild yaks and chiru (Pantholops hodgsonii) moved to snow patches within 200 m of the other without apparent contests.
Unlike snow and shade which are strongly seasonal features of local weather, mineralized sites are less transient although their availability to animal foragers varies with insolation, local hydrology, and soil. At mineral licks, mountain goats dominated bighorn sheep in more than 95% of observed displacements (Figure 4).
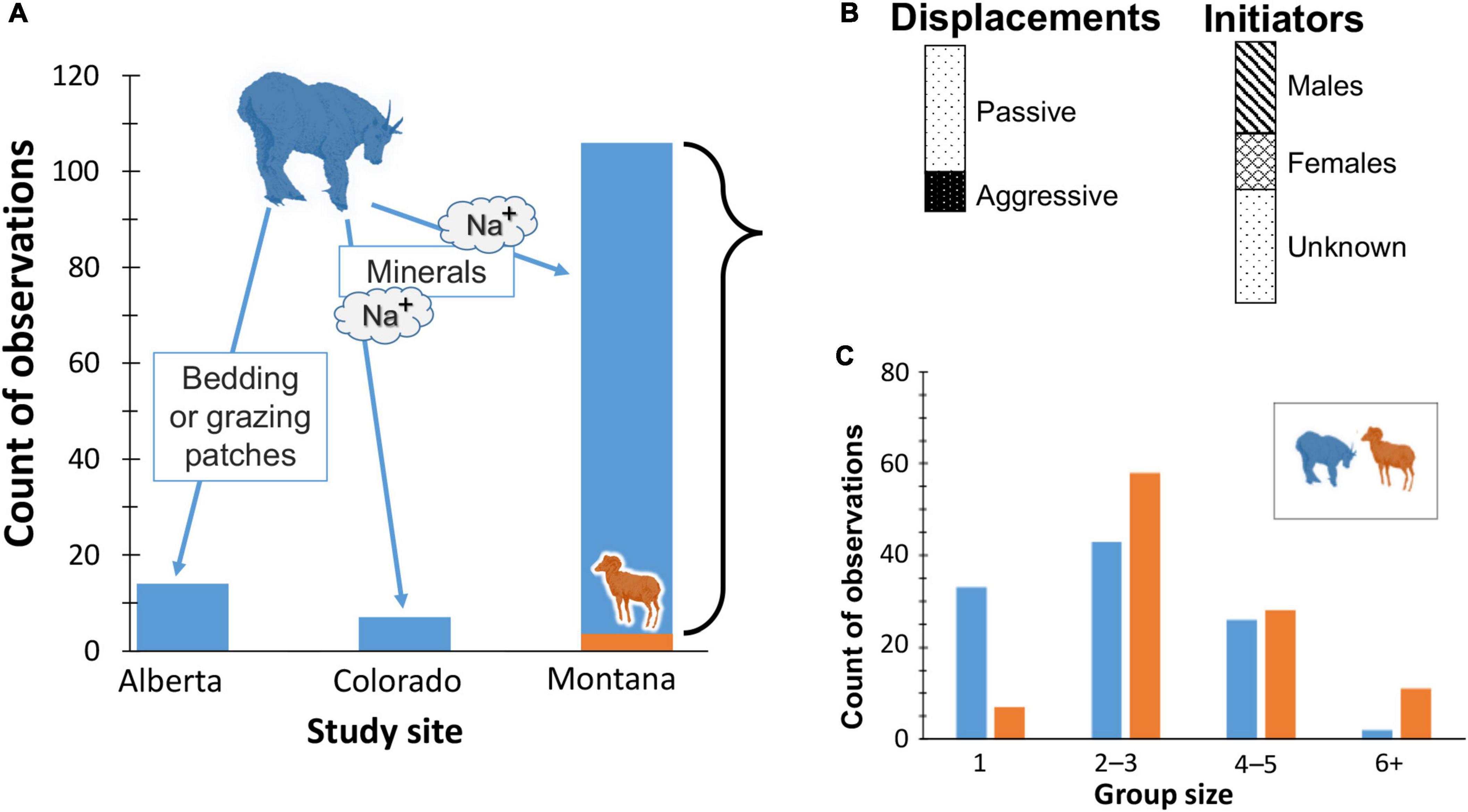
Figure 4. Outcomes of dominance assertion between mountain goats and bighorn sheep. (A) Histogram in which blue column (goats) is % of encounters won relative to orange (sheep), by study site with those in Alberta, Canada for space or food, and in Colorado for micro-nutrients. (B) Relative proportions of goat-initiated displacements of sheep by type (as defined in Methods), and of known sex; (C) distribution of goat and sheep group sizes.
Regardless of site, goats initiated every interaction, and most involved passive approaches (73% of 106) whereby subordinate sheep walked or skipped away. In 12% of the total cases neither goats nor sheep had perceptible responses. Rapid approaches or horn threats (Figure 3D) resulted in flight (< 5%); the longest distance fled was ∼75−100 m. Male goats accounted for 68% of the known encounters (Figure 4). Mean group size differed statistically between species (bighorn sheep , SD = 1.44; mountain goats , SD = 1.43;t test, p < 0.01) but is unlikely biologically relevant given the frequency of overwhelming dominance by goats.
Discussion
Interspecies conflict is not a commonly documented nor well-studied phenomenon, but it obviously occurs as competition for patchily scattered abiotic resources. Like other rarely observed phenomena, such as infanticide or tool use, further discovery among wild species, awaits.
Both interspecific and intraspecific competition create known strong selection pressures that sculpt morphology, behavior, and ecology via evolutionary pathways (Mayr, 1982; Bowyer, 2022). In contemporary settings, the nature of conflict to access resources may or may not be changing, an uncertainty that exists because we lack ecological baselines. Consequently, we know little about whether direct anthropogenic alterations on landscapes have facilitated animal movements into realms where the frequency of interactions has changed. While recent climate alterations are creating hydro-geological change including of soils and the loss of glacial ice that affect the distribution of abiotic resources (Barnett et al., 2005; Lee et al., 2021), due to a literal dearth of information we know little about if or how the immediacy of climate challenge affects interspecies contests.
At an intraspecific level, active competition for abiotic resources is known for reptiles, as up to 128 Aldabra giant tortoises (Aldabrachelys gigantea) are known to pile under a single shade tree (Swingland and Lessells, 1979). Also associated with thermal refugia, are cases involving different genera (Egernia and Eulamprus) of skinks which compete for access to high elevation crevasses (Langkilde and Shine, 2004).
Nonetheless, the extent to which mobile mammals relocate to alternative sites and encounter possible greater competition is highly uncertain because of the aforementioned issue of no ecological baseline. Just as it is often difficult to gauge patterns of trend in disease when monitoring has been insufficient, similar issues confront the immediacy of knowing whether direct interspecific interactions have changed in frequency across time.
Our data on contests for above tree line minerals is a case in point. We do not know if these mineral licks are a newly discovered resource. Perhaps they have increased in availability due recent hydro-geomorphic climate-induced high elevation alterations, as known for the Himalayas (Lee et al., 2021), or something else. Importantly and regardless of whether the mediating forces are directly human such as local habitat destruction, or broader and slower like warming temperatures, given the scarcity and patchiness of abiotic sources (e.g., mineral licks, desert waters) opportunities for interspecies conflict arise over access. Clearly, human alterations of remote desert waters heighten the accessibility challenge (Braithwaite and Muller, 1997; Simpson et al., 2011; Larsen et al., 2012), as has road construction where species have changed their behavior to access mineral licks (Kroesen et al., 2020).
Among life history variables that serve as an arbiter of dominance during interspecies conflict is body size (Berger and Cunningham, 1998). With anthropogenic change, alien-mediated displacement of native fauna occurs (Berger, 1985; Hall et al., 2018) despite scientifically-astute positions about nativity or appropriate ecological surrogates for ecological restoration (Lundgren et al., 2021). Nevertheless, conservation efforts progress in both protected areas and further afield by broadening the distribution of arid-lands water to enhance biodiversity and tourism (Simpson et al., 2011; Larsen et al., 2012).
Conflict between species remains not only of broad ecological interest but harkens to the roots of scientific curiosity about escalated aggression. In the case of high elevation mountain goats, the species occupies a basal position in the Caprini clade (Shafer and Hall, 2010) with stereotypical canalized behavior associated with primitive traits and a propensity for aggression (Geist, 1971; Festa-Bianchet and Côté, 2008). This, in turn, may explain antagonism and dominance over bighorn sheep (Figure 4), a situation with immediate conservation relevance given recent controversy over introduced mountain goats.
As a cold-adapted species, mountain goats symbolize climate alteration (White et al., 2018), are sought by visitors in places like Glacier National Park, and remain emblematical for the Great Northern Pacific Railway. The species was introduced to southern locales as previously noted, and public and scientific opinions are often divisive about sanctity in many areas including Grand Teton and Yellowstone national parks. Recently, nearly 60 goats were removed from the former, actions with a mix of support (National Park Service, 2018) whereas in Yellowstone no controls are in place. Knowledge about species dominance to access rare resources, such as presented in Figure 4, should help agencies deliberate about best conservation paths forward.
More globally, information about interspecies contests among mammals remains sparse, as does understanding whether competitive interactions have changed spatiotemporally. Anthropogenic alterations of the physical environment continue as habitats are erased and as invasive species rework food webs (Berger et al., 2020). While shade may be less apt to function as a coveted resource, snow patches at high elevation disappear with indeterminate consequence (Rosvold, 2016). By contrast, water and minerals are clearly sought where species engage for access. That we understand little of the process complexity that undermines production and change in availability of many abiotic products, other than the heighted pace of human-wreaked landscape modifications, suggests a fertile ground for future in situ field inquiries about species interactions.
We began this narrative pointing to Darwin’s prescience that abiotic processes may be potentially more demographically limiting than biotic factors in extreme areas. We now know high latitude and high elevations sites are changing more rapidly than elsewhere due to climate, but more populated areas are losing habitats more rapidly (Caro et al., 2022). How these factors affect communities as species are more often brought together, resulting in increased competition for abiotic resources, is unclear. If conservation practitioners consider that changes in abiotic resource availability can increase competition, preemptive management decisions can be improved upon to facilitate desirable outcomes. For example, just as limited waters can be managed to benefit biodiversity, proactive recognition, and management of existing and emerging mineral licks may facilitate conservation of geophagous species. Moreover, observations of interspecific contests offer a useful method to better understand the limiting nature of important biological elements. If – which is an important caveat – under global change, contests for abiotic variables increase, this could be an additional unforeseen consequence of climate change with impacts to biodiversity. If, however, curiosity about nature coupled with field studies continue their decline, we will never know.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by IACUC, Colorado State University.
Author contributions
JB conceived the ideas of this manuscript through prolonged conversations with FPH and MB. All authors contributed to the field work, as was the writing.
Funding
Financial support provided by the Wildlife Conservation Society, Colorado State University, Glacier National Park, and Glacier National Park Conservancy.
Acknowledgments
We appreciate the whole-hearted flexibility of our funders to develop insights into how nature works and consider future conservation avenues. We are indebted to F. Dulude-de Broin for providing observational data, sharing biological insights, and providing feedback on this manuscript based on the long-term project at Caw Ridge, Alberta, Canada. We are grateful to J. Lambert for critical questions and strong editing, and to S. Ekernas and Denver Zoological Society for insights on competitive interactions from Mt Evans, Colorado. We further appreciate Denver Mountain Parks and Glacier National Park Conservancy for facilitating field research and providing partial funding. We are thankful to J. Harrington for providing two additional observations of interactions at Mt Evans, and the gracious use of photos by F. Dulude-de Broin, R. Reading and A. Forsyth. We also thank R. T. Bowyer, J. C. Whiting, and K. White for providing valuable suggestions to improve this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.991714/full#supplementary-material
References
Anthony, R. G. (1976). Influence of Drought on Diets and Numbers of Desert Deer. J. Wildl. Manag. 40:140. doi: 10.2307/3800168
Armitage, K. (2003). Behavioral responses of yellow-bellied marmots to birds and mammals. Oecologia Montana 12, 15–20. doi: 10.1073/pnas.97.4.1630
Ayotte, J. B., Parker, K. L., Arocena, J. M., and Gillingham, M. P. (2006). Chemical composition of lick soils: Functions of soil ingestion by four ungulate species. J. Mammal. 87, 878–888. doi: 10.1644/06-MAMM-A-055R1.1
Barnett, T. P., Adam, J. C., and Lettenmaier, D. P. (2005). Potential impacts of a warming climate on water availability in snow-dominated regions. Nature 438, 303–309.
Barrett, L., Gaynor, D., Rendall, D., Mitchell, D., and Henzi, S. P. (2004). Habitual cave use and thermoregulation in chacma baboons (Papio hamadryas ursinus). J. Hum. Evol. 46, 215–222. doi: 10.1016/j.jhevol.2003.11.005
Berger, J. (1985). Interspecific interactions and dominance among wild Great Basin ungulates. J. Mammal. 66, 571–573. doi: 10.2307/1380939
Berger, J. (2018). Extreme Conservation: Life At The Edges Of The World. Chicago: The University of Chicago Press.
Berger, J., and Cunningham, C. (1998). Behavioural ecology in managed reserves: Gender-based asymmetries in interspecific dominance in African elephants and rhinos. Anim. Conserv. 1, 33–38. doi: 10.1111/j.1469-1795.1998.tb00223.x
Berger, J., Schaller, G. B., Cheng, E., Kang, A., Krebs, M., Li, L., et al. (2015). Legacies of past exploitation and climate affect mammalian sexes differently on the roof of the world - the case of wild yaks. Sci. Rep. 5:8676. doi: 10.1038/srep08676
Berger, J., Wangchuk, T., Briceño, C., Vila, A., and Lambert, J. E. (2020). Disassembled food webs and messy projections: Modern ungulate communities in the face of unabating human population growth. Front. Ecol. Evol. 8:128. doi: 10.3389/fevo.2020.00128
Bowyer, R. T. (1987). Coyote group size relative to predation on mule deer. Mammalia 51, 515–526. doi: 10.1515/mamm.1987.51.4.515
Bowyer, R. T. (2022). Sexual Segregation In Ungulates: Ecology, Behavior, And Conservation. Baltimore: Johns Hopkins University Press.
Bradshaw, C. J. A., and Brook, B. W. (2014). Human population reduction is not a quick fix for environmental problems. Proc. Natl. Acad. Sci. U.S.A. 111, 16610–16615. doi: 10.1073/pnas.1410465111
Braithwaite, R. W., and Muller, W. J. (1997). Rainfall, groundwater and refuges: Predicting extinctions of Australian tropical mammal species. Aust. Ecol. 22, 57–67. doi: 10.1111/j.1442-9993.1997.tb00641.x
Butler, D. R. (2012). The impact of climate change on patterns of zoogeomorphological influence: Examples from the Rocky Mountains of the Western U.S.A. Geomorphology 157–158, 183–191. doi: 10.1016/j.geomorph.2011.10.019
Cain, J. W., Jansen, B. D., Wilson, R. R., and Krausman, P. R. (2008). Potential thermoregulatory advantages of shade use by desert bighorn sheep. J. Arid Environ. 72, 1518–1525.
Caro, T., Rowe, Z., Berger, J., Wholey, P., and Dobson, A. (2022). An inconvenient misconception: Climate change is not the principal driver of biodiversity loss. Conserv. Lett. 15:e12868. doi: 10.1111/conl.12868
Caughley, G., and Gunn, A. (1993). Dynamics of large herbivores in deserts: Kangaroos and caribou. Oikos 67:47. doi: 10.2307/3545094
Chapin, K. J., Peixoto, P. E. C., and Briffa, M. (2019). Further mismeasures of animal contests: A new framework for assessment strategies. Behav. Ecol. 30, 1177–1185. doi: 10.1093/beheco/arz081
Clay, J. (2019). Keeping Wildlife Wild: Mountain Goats. Available Online at: https://coloradooutdoorsmag.com/2019/07/09/mountain-goats/ (accessed Sep 3, 2022).
Côté, S. D., and Festa-Bianchet, M. (2003). “Mountain Goats,” in Wild mammals of North America: biology, management, and conservation, eds G. A. Feldhamer and B. C. Thompson (Baltimore, MD: Johns Hopkins University Press), 1061–1075.
Cowan, I. M., and Brink, V. C. (1949). Natural game licks in the Rocky Mountain National Parks of Canada. J. Mammal. 30, 379–387. doi: 10.2307/1375213
Dale, B. W., Adams, L. G., and Bowyer, R. T. (1994). Functional response of wolves preying on barren-ground caribou in a multiple-prey ecosystem. J. Anim. Ecol. 63:644. doi: 10.2307/5230
Dalke, P. D., Beeman, R. D., Kindel, F. J., Robel, R. J., and Williams, T. R. (1965). Use of salt by elk in Idaho. J. Wildl. Manag. 29:319. doi: 10.2307/3798437
Dixon, J. C., and Thorn, C. E. (2005). Chemical weathering and landscape development in mid-latitude alpine environments. Geomorphology 67, 127–145. doi: 10.1016/j.geomorph.2004.07.009
Dulude-de Broin, F., Hamel, S., Mastromonaco, G. F., and Côté, S. D. (2020). Predation risk and mountain goat reproduction: Evidence for stress-induced breeding suppression in a wild ungulate. Funct. Ecol. 34, 1003–1014. doi: 10.1111/1365-2435.13514
Elgar, M. A. (1989). Predator vigilance and group size in mammals and birds: A critical review of the empirical evidence. Biol. Rev. 64, 13–33. doi: 10.1111/j.1469-185X.1989.tb00636.x
Elton, C. (1946). Competition and the structure of ecological communities. J. Anim. Ecol. 15:54. doi: 10.2307/1625
Ferretti, F., and Mori, E. (2020). Displacement interference between wild ungulate species: Does it occur? Ethol. Ecol. Evol. 32, 2–15. doi: 10.1080/03949370.2019.1680447
Festa-Bianchet, M., and Côté, S. D. (2008). Mountain Goats: Ecology, Behavior, And Conservation Of An Alpine Ungulate. Washington, DC: Island Press.
Festa-Bianchet, M., Ray, J. C., Boutin, S., Côté, S. D., and Gunn, A. (2011). Conservation of caribou (Rangifer tarandus) in Canada: An uncertain future. Can. J. Zool. 89, 419–434. doi: 10.1139/z11-025
Gauthier, G., Bêty, J., Giroux, J. F., and Rochefort, L. (2004). Trophic Interactions in a High Arctic Snow Goose Colony. Integr. Comp. Biol. 44, 119–129. doi: 10.1093/icb/44.2.119
Geist, V. (1971). Mountain Sheep: A Study In Behavior And Evolution. Chicago: The Chicago University Press.
Gooch, A. M. J., Petersen, S. L., Collins, G. H., Smith, T. S., McMillan, B. R., and Eggett, D. L. (2017). The impact of feral horses on pronghorn behavior at water sources. J. Arid Environ. 138, 38–43. doi: 10.1016/j.jaridenv.2016.11.012
Hall, L. K., Larsen, R. T., Knight, R. N., and McMillan, B. R. (2018). Feral horses influence both spatial and temporal patterns of water use by native ungulates in a semi-arid environment. Ecosphere 9:e02096. doi: 10.1002/ecs2.2096
Hall, M. H. P., and Fagre, D. B. (2003). Modeled climate-induced glacier change in Glacier National Park, 1850–2100. BioScience 53, 131–140. doi: 10.1641/0006-35682003053[0131:MCIGCI]2.0.CO;2
Hamilton, W. J. III, Buskirk, R., and Buskirk, W. H. (1977). Intersexual dominance and differential mortality of gemsbok Oryx gazella at Namib Desert waterholes. Madoqua 10, 5–19.
Hansen, B. B., Pedersen, ÅØ, Peeters, B., Le Moullec, M., Albon, S. D., Herfindal, I., et al. (2019). Spatial heterogeneity in climate change effects decouples the long-term dynamics of wild reindeer populations in the high Arctic. Glob. Change Biol. 25, 3656–3668. doi: 10.1111/gcb.14761
Jokinen, M., Verhage, M., Anderson, R., and Manzer, D. (2014). Observational description of alpine ungulate use at mineral licks in southwest Alberta, Canada. Bienn. Symp. North. Wild Sheep Goat Counc. 19, 42–63.
Klein, D. R. (2019). The making of an ecologist: My career in Alaska wildlife management and conservation, ed. K Brewster (Fairbanks, AK: University of Alaska Press).
Krebs, C. J., Danell, K., Angerbjörn, A., Agrell, J., Berteaux, D., Bråthen, K. A., et al. (2003). Terrestrial trophic dynamics in the Canadian Arctic. Can. J. Zool. 81, 827–843. doi: 10.1139/z03-061
Kreulen, D. A. (1985). Lick use by large herbivores: A review of benefits and banes of soil consumption. Mamm. Rev. 15, 107–123. doi: 10.1111/j.1365-2907.1985.tb00391.x
Kroesen, L. P., Hik, D. S., and Cherry, S. G. (2020). Patterns of decadal, seasonal and daily visitation to mineral licks, a critical resource hotspot for mountain goats Oreamnos americanus in the Rocky Mountains. Wildl. Biol. 2020:wlb.00736. doi: 10.2981/wlb.00736
Lambert, C. B., Resler, L. M., Shao, Y., and Butler, D. R. (2020). Vegetation change as related to terrain factors at two glacier forefronts, Glacier National Park, Montana, U.S.A. J. Mt. Sci. 17, 1–15. doi: 10.1007/s11629-019-5603-8
Langkilde, T., and Shine, R. (2004). Competing for crevices: Interspecific conflict influences retreat-site selection in montane lizards. Oecologia 140, 684–691. doi: 10.1007/s00442-004-1640-1
Larsen, R. T., Bissonette, J. A., Flinders, J. T., and Whiting, J. C. (2012). Framework for understanding the influences of wildlife water developments in the western United States. Calif. Fish Game 98, 148–163.
Lee, E., Carrivick, J. L., Quincey, D. J., Cook, S. J., James, W. H. M., and Brown, L. E. (2021). Accelerated mass loss of Himalayan glaciers since the Little Ice Age. Sci. Rep. 11:24284. doi: 10.1038/s41598-021-03805-8
Link, A., Galvis, N., Fleming, E., and Di Fiore, A. (2011). Patterns of mineral lick visitation by spider monkeys and howler monkeys in Amazonia: Are licks perceived as risky areas? Am. J. Primatol. 73, 386–396. doi: 10.1002/ajp.20910
Lundgren, E. J., Ramp, D., Stromberg, J. C., Wu, J., Nieto, N. C., Sluk, M., et al. (2021). Equids engineer desert water availability. Science 372, 491–495. doi: 10.1126/science.abd6775
Martin-Mikle, C. J., and Fagre, D. B. (2019). Glacier recession since the Little Ice Age: Implications for water storage in a Rocky Mountain landscape. Arct. Antarct. Alp. Res. 51, 280–289. doi: 10.1080/15230430.2019.1634443
Mayr, E. (1982). The Growth Of Biological Thought: Diversity, Evolution, And Inheritance. Cambridge, Mass: Harvard University Press.
Mills, L. S., Bragina, E. V., Kumar, A. V., Zimova, M., Lafferty, D. J. R., Feltner, J., et al. (2018). Winter color polymorphisms identify global hot spots for evolutionary rescue from climate change. Science 359, 1033–1036. doi: 10.1126/science.aan8097
Molvar, E. M., and Bowyer, R. T. (1994). Costs and benefits of group living in a recently social ungulate: The Alaskan moose. J. Mammal. 75, 621–630. doi: 10.2307/1382509
National Park Service (2018). Grand Teton National Park. Mountain Goat Management Plan Environmental Assessment December 2018. Washington, DC: National Park Service.
Ostermann-Kelm, S., Atwill, E. R., Rubin, E. S., Jorgensen, M. C., and Boyce, W. M. (2008). Interactions between feral horses and desert bighorn sheep at water. J. Mammal. 89, 459–466. doi: 10.1644/07-MAMM-A-075R1.1
Parker, G. A., and Rubenstein, D. I. (1981). Role assessment, reserve strategy, and acquisition of information in asymmetric animal conflicts. Anim. Behav. 29, 221–240. doi: 10.1016/S0003-3472(81)80170-4
Pebsworth, P. A., Huffman, M. A., Lambert, J. E., and Young, S. L. (2019). Geophagy among nonhuman primates: A systematic review of current knowledge and suggestions for future directions. Am. J. Phys. Anthropol. 168, 164–194. doi: 10.1002/ajpa.23724
Pörtner, H. O., Scholes, R. J., Agard, J., Archer, E., Arneth, A., Bai, X., et al. (2021). Scientific Outcome Of The Ipbes-Ipcc Co-Sponsored Workshop On Biodiversity And Climate Change. Honolulu: Zenodo.
Pruetz, J. D. (2007). Evidence of cave use by savanna chimpanzees (Pan troglodytes verus) at Fongoli, Senegal: Implications for thermoregulatory behavior. Primates 48, 316–319. doi: 10.1007/s10329-007-0038-1
Rice, C. G. (2010). Mineral Lick Visitation by Mountain Goats, Oreamnos americanus. Can. Field Nat. 124, 225. doi: 10.22621/cfn.v124i3.1078
Ríos-Saldaña, C. A., Delibes-Mateos, M., and Ferreira, C. C. (2018). Are fieldwork studies being relegated to second place in conservation science? Glob. Ecol. Conserv. 14:e00389. doi: 10.1016/j.gecco.2018.e00389
Rosvold, J. (2016). Perennial ice and snow-covered land as important ecosystems for birds and mammals. J. Biogeogr. 43, 3–12. doi: 10.1111/jbi.12609
Shafer, A. B. A., and Hall, J. C. (2010). Placing the mountain goat: A total evidence approach to testing alternative hypotheses. Mol. Phylogenet. Evol. 55, 18–25. doi: 10.1016/j.ympev.2010.01.015
Shoshani, J., and Viljoen, P. (1992). “The desert elephants of Namibia,” in Elephants: Majestic creatures of the wild, ed. J. Shoshani (Emmaus, PA: Rodale Press), 131–133.
Simpson, N. O., Stewart, K. M., and Bleich, V. C. (2011). What have we learned about water developments for wildlife? Not enough! Calif. Fish Game 97, 190–209.
Singer, F. J. (1975). Behavior of Mountain Goats, elk and Other Wildlife in Relation to US Highway 2, Glacier National Park. Washington, DC: National Park Service.
Smith, K. (2021). Mass balance, accumulation dynamics and high-altitude warfare: The Siachen Glacier as a battlefield. Small Wars Insur. 32, 1193–1220. doi: 10.1080/09592318.2021.1877023
Swingland, I. R., and Lessells, C. M. (1979). The natural regulation of giant tortoise populations on Aldabra Atoll. Movement polymorphism, reproductive success and mortality. J. Anim. Ecol. 48:639. doi: 10.2307/4184
Treves, A. (2000). Theory and method in studies of vigilance and aggregation. Anim. Behav. 60, 711–722. doi: 10.1006/anbe.2000.1528
Valeix, M., Chamaillé-Jammes, S., and Fritz, H. (2007). Interference competition and temporal niche shifts: Elephants and herbivore communities at waterholes. Oecologia 153, 739–748. doi: 10.1007/s00442-007-0764-5
White, K. S., Gregovich, D. P., and Levi, T. (2018). Projecting the future of an alpine ungulate under climate change scenarios. Glob. Change Biol. 24, 1136–1149. doi: 10.1111/gcb.13919
Yang, Y., Weng, B., Yan, D., Niu, Y., Dai, Y., Li, M., et al. (2021). Partitioning the contributions of cryospheric change to the increase of streamflow on the Nu River. J. Hydrol. 598:126330. doi: 10.1016/j.jhydrol.2021.126330
Keywords: competition, climate change, extremes, species interactions, conflict, abiotic resources, mammals, ungulates
Citation: Berger J, Biel M and Hayes FP (2022) Species conflict at Earth’s edges – Contests, climate, and coveted resources. Front. Ecol. Evol. 10:991714. doi: 10.3389/fevo.2022.991714
Received: 11 July 2022; Accepted: 09 September 2022;
Published: 17 October 2022.
Edited by:
R. Terry Bowyer, University of Alaska Fairbanks, United StatesReviewed by:
Jericho C. Whiting, Brigham Young University-Idaho, United StatesKevin White, Retired, Haines, AK, United States
Copyright © 2022 Berger, Biel and Hayes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joel Berger, joel.berger@wcs.org
 Joel Berger
Joel Berger Mark Biel3
Mark Biel3  Forest P. Hayes
Forest P. Hayes