Mesozoic Notocupes revealed as the sister group of Cupedidae (Coleoptera: Archostemata)
- 1State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing, China
- 2Bristol Palaeobiology Group, School of Earth Sciences, University of Bristol, Bristol, United Kingdom
- 3Hokkaido University Museum, Hokkaido University, Sapporo, Japan
- 4Integrative Research Center, Field Museum of Natural History, Chicago, IL, United States
- 5Lingpoge Amber Museum, Shanghai, China
- 6Paleo-diary Museum of Natural History, Beijing, China
- 7Fujian Paleo-diary Bioresearch Centre, Fuzhou, China
Despite encompassing only about 50 extant species, beetles of the suborder Archostemata have a rich fossil history, being known from the Permian and dominating coleopteran assemblages in the Mesozoic before declining in richness towards the Late Cretaceous. Given the limited diversity of extant archostematans, fossils provide a valuable line of evidence for reconstructing the relationships among its constituent families. Here we re-evaluate the phylogenetic position of the Triassic–Cretaceous genus Notocupes, the most species-rich archostematan taxon in the fossil record. Exquisitely preserved fossils from the Middle Jurassic Haifanggou Formation (Daohugou; ~165 Ma) and mid-Cretaceous amber (~99 Ma) reveal critical differences from Ommatidae in the presence of separated procoxae and overlapping abdominal ventrites. Our analyses confirm that Notocupes is not a member of Ommatidae, but is closely related to Cupedidae. Our fossils reveal that Notocupes possessed unique adaptations for protecting their appendages, such as unusual dorsal pronotal grooves for the reception of antennae and epipleural grooves for the reception of legs, shedding light on ecological interactions in Mesozoic saproxylic habitats. The high similarity between Jurassic and Cretaceous Notocupes provides an exceptional example of long-term morphological stasis, suggesting a consistent microhabitat for the group.
1 Introduction
Archostemata is one of the four extant beetle suborders. While just around 50 extant archostematan species are known in 15 genera (Hörnschemeyer, 2016), the group was considerably diverse in the geological past. Archostematans dominated early Mesozoic fossil beetle assemblages and over 200 extinct species have been described across the world (Ślipiński et al., 2011). In some analyses, Archostemata (sensu stricto, not including stem-group beetles such as Tshekardocoleidae and Permocupedidae) is recovered as the earliest-diverging clade within Coleoptera (Friedrich et al., 2009; Bocak et al., 2014), highlighting its importance for understanding the origin of the present-day diversity of beetles. Because they superficially resemble Permian stem-group beetles (Ponomarenko et al., 2014), archostematans have often been regarded as “living fossils” (Cai and Huang, 2017; Jarzembowski et al., 2020a). This is particularly the case for the families Cupedidae (reticulated beetles) and Ommatidae, which possess elytra with regularly arranged rows of window punctures resembling those found in early beetles from the Permian. Owing to their similar body plans, Cupedidae and Ommatidae have been at times regarded as a single family, Cupedidae sensu lato (Ponomarenko, 2000; Kirejtshuk, 2020). However, recent molecular phylogenetic studies indicate that Ommatidae is more closely related to Micromalthidae, rather than Cupedidae sensu stricto, and thus “Cupedidae s.l.” is not a monophyletic group (Mckenna et al., 2015; McKenna et al., 2019). Therefore, in this paper we treat Ommatidae and Cupedidae as separate families. The interrelationships of Cupedidae, Ommatidae, Micromalthidae and the two remaining archostematan families, Crowsoniellidae and the enigmatic Jurodidae, have been historically difficult to elucidate based on morphology, and no molecular data have been available for the latter two. Both Crowsoniellidae and Jurodidae are rare and species-poor in the recent fauna, with Crowsoniellidae known from only three specimens collected in 1973 in Italy (Pace, 1975; Kirejtshuk et al., 2010; Ge et al., 2011), and Jurodidae known from a single Recent specimen found in Far Eastern Russia and scarce Jurassic fossils (Lafer, 1996; Kirejtshuk, 1999; Yan et al., 2014). Since Jurodidae combines characters found in Adephaga, Archostemata and Polyphaga, some authors do not include it within Archostemata and treat it as a taxon of uncertain affinities (Lawrence, 2016).
Given the limited diversity of extant archostematan beetles, fossils provide crucial evidence for reconstructing the phylogeny of the group (Tan et al., 2012; Li et al., 2019). The fossil record of archostematans is also important for understanding biotic change in Mesozoic terrestrial ecosystems, namely the conversion from an archostematan-dominated beetle fauna to a polyphagan-dominated one in the late Mesozoic–early Cenozoic (Soriano and Delclòs, 2006; Friedrich et al., 2009).
Notocupes Ponomarenko is the most abundant genus of archostematans in Mesozoic deposits. With over 50 extinct species known from the Triassic–Cretaceous it is also one of the most species-rich insect genera in the fossil record (Strelnikova and Yan, 2023). Additional species are assigned to the form genus Zygadenia Handlirsch that is reserved for isolated elytra likely belonging to representatives of Notocupes. Since the first discovery of a Zygadenia elytron by Giebel (1856) from the Cretaceous Purbeck Limestone Group of southern England, the Notocupes–Zygadenia complex has been reported from Europe, Asia, South America, and Australia (Kirejtshuk, 2020). Notocupes has been historically placed into the family Ommatidae (or Ommatinae in Cupedidae s.l.), and into the tribe Notocupedini erected by Ponomarenko (Ponomarenko, 1966). Despite its wide distribution, the morphology of Notocupes remains insufficiently known. Most Notocupes specimens were described based on compressions from Mesozoic strata, and thus many morphological characters are difficult to interpret or not preserved at all. The morphology of the three Notocupes species reported from mid-Cretaceous Burmese amber (Tihelka et al., 2019; Jarzembowski et al., 2020b; Jiang et al., 2020) remains insufficiently described, owing to the challenging optical properties of the amber matrix. Here we report four exquisitely preserved Notocupes fossils from Middle–Late Jurassic Daohugou Biota and mid-Cretaceous Burmese amber. With the aid of a range of imaging techniques, including confocal laser scanning microscopy, scanning electron microscopy, and microtomography, we aim to clarify the external morphology of Notocupes and evaluate the systematic position of this genus within Archostemata.
2 Materials and methods
2.1 Fossils and imaging
The three compression fossils photographed herein (Figures 1A–F, 2) originated from Daohugou Village, Ningcheng County, Inner Mongolia, China (~165 Ma). An additional compression fossil (Supplementary Figure 2) originated from Huangbanjigou Village, Shangyuan Township, Beipiao City, Liaoning Province, China (~125 Ma). These specimens are deposited in the Nanjing Institute of Geology and Palaeontology (NIGP), Chinese Academy of Sciences, Nanjing, China. The Burmese amber specimen BA202101 (Figures 1G, H, 3) originated from amber mines near Noije Bum (26°20’ N, 96°36’ E), Hukawng Valley, Kachin State, northern Myanmar (~99 Ma), and is deposited in the Lingpoge Amber Museum, Shanghai, China.
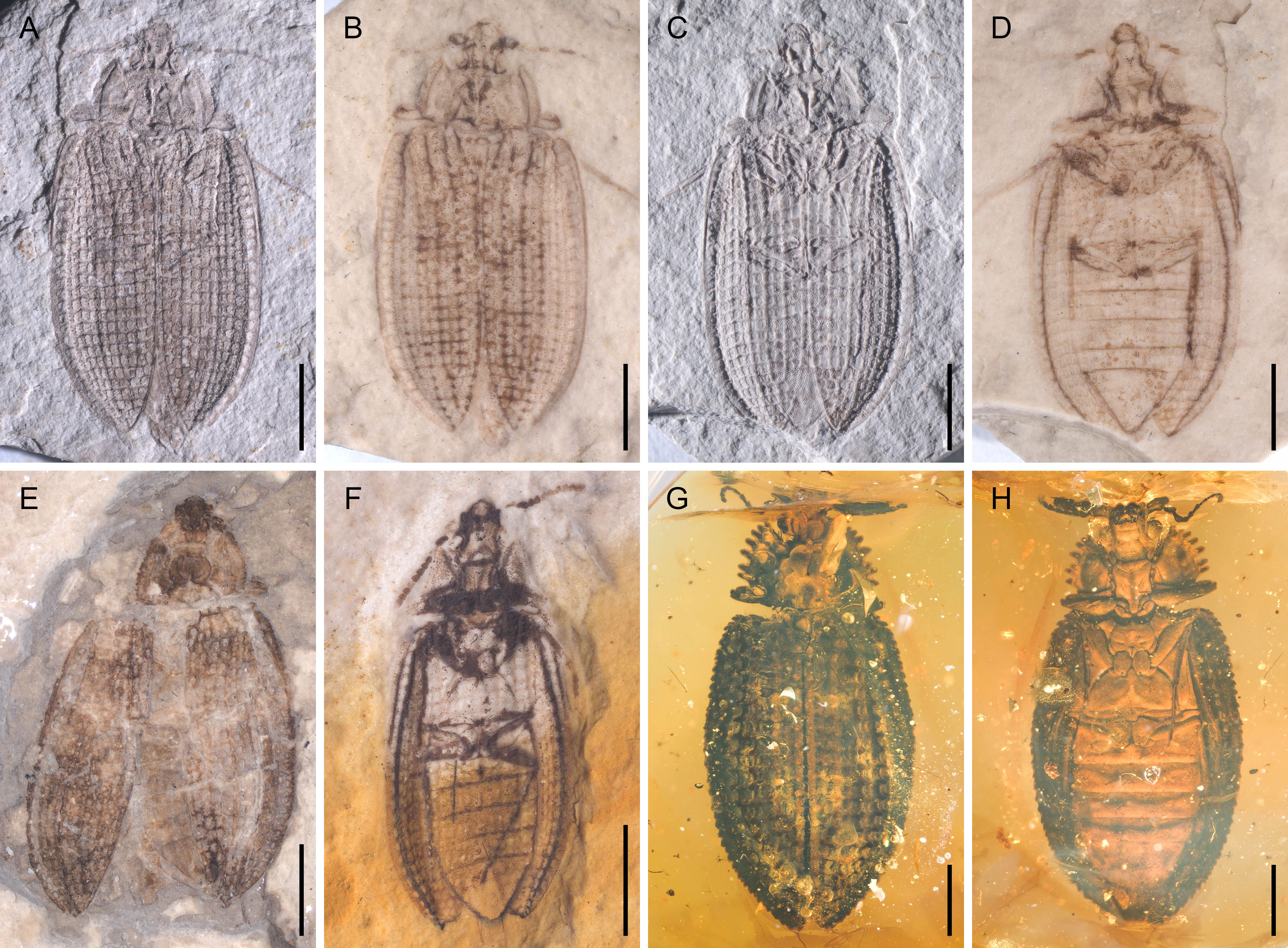
Figure 1 General habitus of Notocupes spp., under incident light, dry (A, C) or moistened with 70% ethanol (B, D–F). (A, B) NIGP174673a. (C, D) NIGP174673b. (E) NIGP174674. (F) NIGP174675. (G) BA202101, dorsal view. (H) BA202101, ventral view. Scale bars: 5 mm in (E), 3 mm in (A–D), 2 mm in (F–H).
Photographs under incident light were taken with a Zeiss Discovery V20 stereo microscope. Where necessary, compression fossils were moistened with 70% ethanol to improve contrast of morphological characters. Widefield fluorescence images were captured with a Zeiss Axio Imager 2 light microscope combined with a fluorescence imaging system. Confocal images were obtained with a Zeiss LSM710 confocal laser scanning microscope, using the 488 nm Argon laser excitation line (Fu et al., 2021). Images under incident light and widefield fluorescence were stacked in Helicon Focus 7.0.2 or Zerene Stacker 1.04. Confocal images were stacked with Helicon Focus 7.0.2. Scanning electron microscopic (SEM) images were obtained with a Hitachi SU 3500 scanning electron microscope, operating with an accelerating voltage of 18 kV and a pressure of 60 Pa. Microtomographic data for BA202101 were obtained with a Zeiss Xradia 520 Versa 3D X-ray microscope at the micro-CT laboratory of NIGP, and analyzed in VGStudio MAX 3.0. Scanning parameters were as follows: isotropic voxel size, 14.096 μm; power, 3 W; acceleration voltage, 40 kV; exposure time, 4 s; projections, 2001. Images were further processed in Adobe Photoshop CC to adjust brightness and contrast.
The full set of descriptions and figures, along with the new taxonomic acts, will be presented in a separate paper.
2.2 Phylogenetic analysis
To evaluate the systematic placement of Notocupes, morphological phylogenetic analyses were performed. The data matrix (Supplementary Data 1, 2) was derived from a previously published dataset (Beutel et al., 2008). The original matrix consists of 90 morphological characters of recent and fossil beetles, including representatives of all archostematan families. Several changes to the scoring of the characters were made. Firstly, head protuberances are important for generic-level identification of Cupedidae (Hörnschemeyer, 2009). However, the definition of these protuberances is sometimes unclear for other archostematans. For example, the protuberances P3 were defined as “between P2, on both sides of the median line of the head” in (Hörnschemeyer et al., 2006), but in Tetraphalerus Waterhouse, the posterior protuberances are situated roughly posteriorly to P2 (Figure 10D in Beutel et al., 2008), making it difficult to determine if they should be coded as P3 or P4. Besides, there might be some miscoding for head protuberances in Beutel et al. (2008). For example, the protuberances P2 for Omma Newman were coded as strongly pronounced, but we failed to detect any strongly pronounced protuberances on the head of Omma (Figure 16 in Escalona et al., 2020). We therefore excluded characters 6–9 in Beutel et al. (2008) from our analysis. Secondly, Beutel et al. (2008) coded the propleuron as reaching the anterior margin of prothorax in Tetraphalerus and Crowsoniella Pace (their character 42). In fact, the propleuron of Tetraphalerus does not reach the anterior prothoracic margin (Friedrich et al., 2009; Li et al., 2021), and no separate propleuron is present in Crowsoniella at all (Figure 16 in Kirejtshuk et al., 2010); we adapted our character matrix accordingly. Lastly, character 31 in Beutel et al. (2008) was coded as (2) for Crowsoniella, which is a non-existent character state and was therefore corrected in our matrix. Thus, our decisive matrix included 86 characters in total.
The problematic family Jurodidae has been considered as a member of Adephaga, Archostemata, or Polyphaga (Ponomarenko, 1985; Lafer, 1996; Kirejtshuk, 1999; Beutel et al., 2008; Hörnschemeyer, 2009). Its puzzling combination of characters, seemingly combining states found in three coleopteran suborders, represents a potential source of incongruence in phylogenetic analyses. Hence, we prepared two matrices, one including Jurodidae (represented by the extant Sikhotealinia Lafer and the extinct Jurodes Ponomarenko), and one excluding the family.
Parsimony analyses were conducted in the program TNT 1.5 (Goloboff et al., 2008; Goloboff and Catalano, 2016). We experimented with the use of equal and implied weighting. Parsimony analyses have been shown to achieve higher accuracy under a moderate weighting scheme (e.g., when concavity constants, K, are between 5 and 20) (Goloboff et al., 2018; Smith, 2019). Therefore, we set the concavity constant to 12 in our analyses with implied weighting, as suggested by Goloboff et al. (2018). Most parameters were set as default in the “new technology search”, while the value for “find min. length” was changed from 1 to 100. When multiple most parsimonious trees were obtained, a strict consensus tree was calculated, and a standard bootstrap analysis was implemented with 1,000 pseudoreplicates, where the support values were shown as frequency differences (Goloboff et al., 2003). Settings for the equal weighting approach were identical, employing default parameters. Character states were mapped onto the tree with WinClada 1.00.08. The tree was graphically edited with Adobe Illustrator CC 2017.
3 Systematic paleontology
Order Coleoptera Linnaeus, 1758
Suborder Archostemata Kolbe, 1908
3.1 Genus Notocupes Ponomarenko, 1964
3.1.1 Type species
Notocupes picturatus Ponomarenko, 1964
3.1.2 Revised diagnosis
Head (Figure 2E) prognathous, nearly as long as wide, narrowing behind eyes and forming distinct neck region. Compound eyes hemispherical, distinctly protruding. Antennae (Figure 2H) moderately long, slightly serrate, extending at most to posterior pronotal apices. Mandibles with horizontal cutting edge, lacking vertically arranged mandibular teeth (Figure 3E). Suture separating mentum and gulamentum present. Weakly impressed grooves on dorsal surface of pronotum for housing antennae at least sometimes present (Figures 2C, G). Pronotal disc broad, often with produced anterior angles, with lateral margins straight or jagged. Prosternum in front of coxae subquadrate, with tarsal grooves along pleurosternal sutures (Figures 2A, 3B, G). Prosternal process well-developed, extending beyond middle of procoxae (Figures 2J, 3B, F). Procoxae narrowly separated by prosternal process. Elytra elongate, with ten longitudinal rows of window punctures on disc and one row of window punctures on explanate epipleuron, sometimes with raised veins with coniform protuberances (elytral spines; Figures 2I, 3H), veins A1 and CuA fused before elytral apex. Anterior portion of explanate elytral epipleura at least sometimes with grooves for housing mesotibiae and mesotarsi (Figures 2B, K, 3C, D). Tarsi 5-segmented, elongate; tarsomeres not emarginate or ventrally lobed (Figures 3D, G). Abdominal ventrites overlapping (Figures 1H, 2D, L).

Figure 2 Details of Notocupes spp. from the Middle Jurassic Daohugou biota, under incident light (A–D) or scanning electron microscopy (E–L). (A) NIGP174673a, head and prothorax, showing the protarsal groove along pleurosternal suture (arrowhead). (B) NIGP174673b, groove on the elytral epipleuron for housing mesotibia and -tarsus (arrowhead). (C) NIGP174674, antenna in the prothoracic antennal groove (arrowhead). (D) NIGP174675, abdomen with overlapping ventrites. (E) NIGP174673a, head. (F) NIGP174673b, mouthparts. (G) NIGP174673a, prothoracic antennal groove (arrowhead). (H) NIGP174673a, antenna. (I) NIGP174673a, scale-covered coniform protuberances on elytron (arrowhead). (J) NIGP174675, prosternum, showing the complete prosternal process (arrowhead). (K) NIGP174675, groove on the elytral epipleuron (arrowhead). (L) NIGP174674, abdomen with overlapping ventrites. an4–7, antennomeres 4–7; hd, head; pn, pronotum; ps, prosternum; v2–5, ventrites 2–5. Scale bars: 1 mm in (A–D, L), 500 μm in (E–H, J–K), 200 μm in (I).
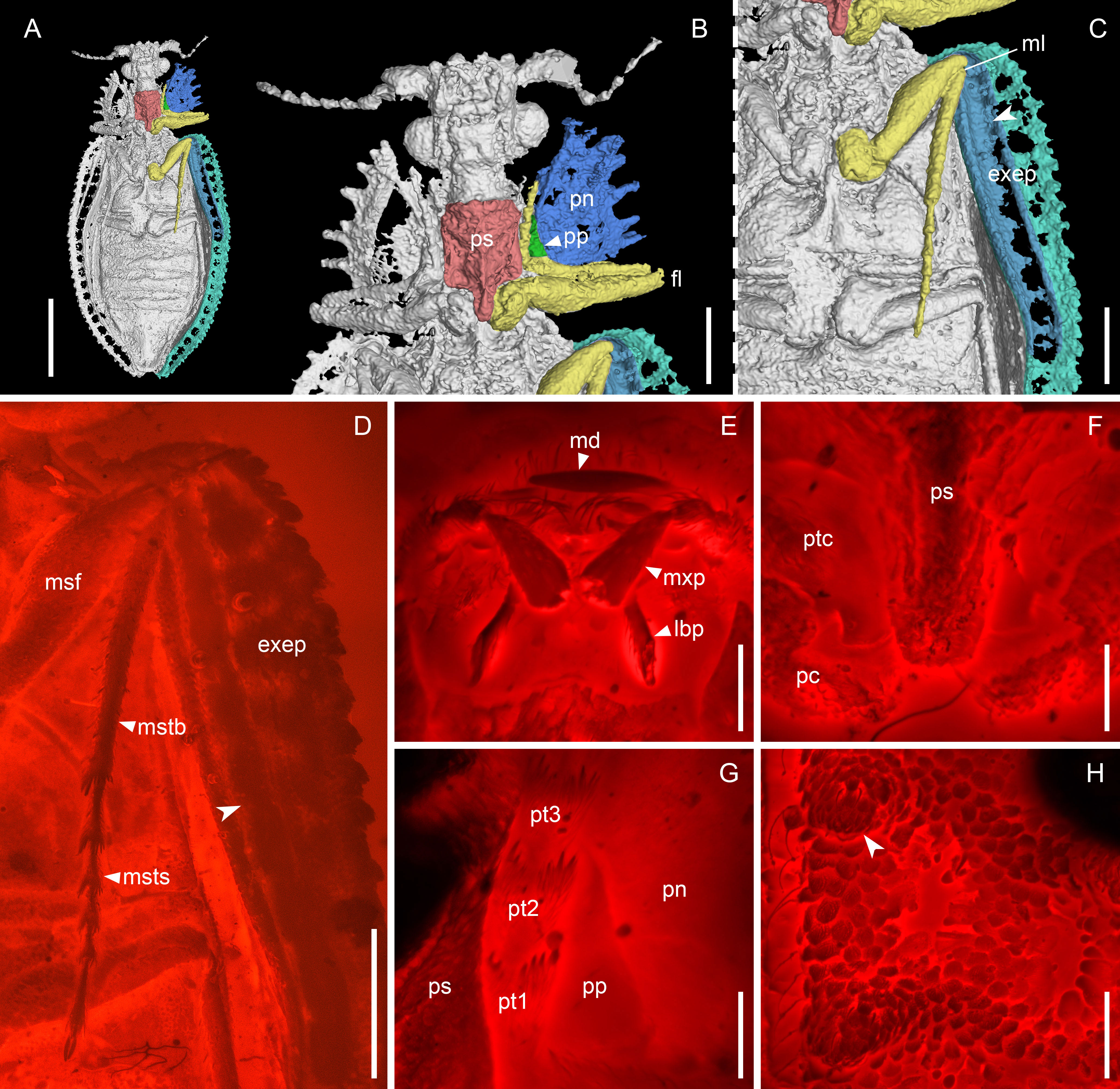
Figure 3 Notocupes sp., BA202101, in mid-Cretaceous amber from Myanmar. (A–C), X-ray microtomographic reconstruction, ventral view, with groove on the elytral epipleuron for housing mesotibia and -tarsus highted in (C) (arrowhead). (D) Groove on the elytral epipleuron, ventral view, under widefield fluorescence. (E–H) Confocal images. (E) Mouthparts, ventral view. (F) Prothorax, ventral view. (G) Protarsus in the protarsal groove, ventral view. (H) Elytron, dorsal view, showing the scale-covered coniform protuberances (arrowhead). exep, explanate epipleuron; fl, fore leg; lbp, labial palp; md, mandible; ml, mid leg; msf, mesofemur; mstb, mesotibia; msts, mesotarsus; mxp, maxillary palp; pc, procoxa; pn, pronotum; pp, propleuron; ps, prosternum; pt1–3, protarsomeres 1–3; ptc, protrochanter. Scale bars: 3 mm in (A), 1 mm in (B–D), 200 μm in (E–H).
3.1.3 Remarks
Notocupes has sometimes been treated as a junior synonym of Zygadenia (e.g., Ponomarenko, 2000; Kirejtshuk, 2020). The name Zygadenia Handlirsch was proposed based on an isolated elytron. As elytra with similar morphology may belong to different taxa (Strelnikova and Yan, 2021), we here reserve Notocupes for complete body fossils of unambiguous systematic assignment, following the practice of Ponomarenko and Ren (2010).
Notocupes differs from Ommatidae primarily by its horizontal cutting edge of mandibles, separated procoxae and overlapping abdominal ventrites, and differs from Cupedidae primarily by its relatively short prosternal process (not reaching posterior end of procoxae) and simple tarsomeres. Notocupes may deserve a new familial status. However, the morphology and phylogenetic placement of the genera historically associated with Notocupes, including Notocupoides Ponomarenko, Rhabdocupes Ponomarenko, and Eurydicton Ponomarenko, are not currently clear. Thus, Notocupes, along with these genera, is temporarily left in Archostemata, without a familial attribution.
4 Discussion
4.1 Exceptional fossils illuminate the morphology of Notocupes
4.1.1 Compression fossils and amber inclusions provide a complementary view of Notocupes morphology
Traditionally, Notocupes has been placed in the family Ommatidae (or Ommatinae in Cupedidae s.l.), primarily based on the length of its antennae and contiguous procoxae (Ponomarenko, 1964). However, our examination of exceptionally preserved Notocupes compressions from Daohugou and amber from northern Myanmar revealed a suite of morphological characters that are not diagnostic for Ommatidae, but correspond well to Cupedidae s. s.
Some beetles possess a transverse suture between the posterior tentorial pits, separating the submentum and gula, while in others (including all extant Ommatidae) this suture is reduced, and the submentum and gula are fused into a single gulamentum. Though this suture was not explicitly described, the line drawings by Ponomarenko (Ponomarenko, 1969) suggested the presence of a distinct suture separating the submentum and gula in Notocupes and closely related genera. Our observations, in contrast, showed no suture between the posterior tentorial pits, but a suture separating the mentum and gulamentum (Figure 1H).
The Ommatidae + Micromalthidae clade is characterized by vertically arranged mandibular teeth (Hörnschemeyer, 2009; Li et al., 2020b; Tihelka et al., 2020b), while in Cupedidae the mandibles have a horizontal cutting edge. Similar to cupedids, Notocupes also possesses a horizontal mandibular cutting edge (Figure 3E).
In Ommatidae, the procoxae are contiguous (except for the aberrant genus Stegocoleus Jarzembowski & Wang which gained this character independently; Jarzembowski and Wang, 2016; Li et al., 2020a; Tihelka et al., 2020a), and the prosternal process is reduced, not reaching the posterior half of the procoxae. In the Notocupes specimen from Burmese amber we examined, the prosternal process is relatively well-developed, extending beyond the middle of procoxae, though not reaching the posterior end of procoxae (Figures 2J, 3B, F). The procoxae are completely separated by the prosternal process, which are similar to Cupedidae and contradictory to previous descriptions of this genus based on compression fossils (Ponomarenko, 1964; Tan and Ren, 2009). We suppose that the contiguous procoxae reported by previous researchers could have been a taphonomic artefact caused by distortion during the fossilization processes. Indeed, the elongate prosternal process has also been inexplicitly noted by Ponomarenko (in Jarzembowski et al., 2015), and recently reported by Lee et al. (2022).
In most ommatids, the abdominal ventrites are coplanar and separated by wide grooves (Beutel et al., 2008) (Figure 4A in Yamamoto, 2017; Figures 71–74 in Escalona et al., 2020). In most cupedids, the abdominal ventrites are overlapping (Beutel et al., 2008; but see Kirejtshuk et al., 2016). Notocupes has overlapping abdominal ventrites (Figures 1H, 2D, L) which are discordant with a placement in Ommatidae (Ponomarenko, 1969; Ponomarenko, 2006; Ponomarenko and Ren, 2010; Tan et al., 2012; Strelnikova, 2019). Notably, Kirejtshuk (2020) transferred Ovatocupes alienus Tan & Ren, a species reported from the Yixian Formation, into Notocupes. However, it was originally placed in Cupedidae based on its separated procoxal cavities and overlapping abdominal ventrites (Tan and Ren, 2006).
4.1.2 New potential apomorphies of Notocupes
Curiously, Notocupes possesses a pair of weak grooves on the dorsal surface of the pronotum. In NIGP174674 and the holotype of N. denticollis (likely also in the holotype of N. ohmkuhnlei), the antennae are positioned within these grooves (Figure 2C). This character is unusual, as most beetles with antennal grooves have ventral ones, not dorsal ones (Lawrence and Ślipiński, 2013). This character represents a potential apomorphy of Notocupes, provided its presence can be confirmed in other early members of the genus.
There is a distinct groove along the propleurosternal suture in Notocupes, which functions for housing the protarsi, as clearly shown in the amber specimen BA202101 and other compression fossils (Figures 2A, 3B, G). This protarsal groove is also preserved in some previously noted amber and compression fossils of Notocupes (e.g., Plate II, Figure 2 in Tan et al., 2006; Figure 1 in Jarzembowski et al., 2020b). Grooves for housing the protarsi are also present in the majority of extant Cupedidae (except for Priacma LeConte and Paracupes Kolbe) and Crowsoniellidae. However, in Cupedidae, the protarsal groove runs along the notopleural and notosternal sutures; and in Crowsoniella, the propleuron is reduced or fused with other sclerites, and the protarsal groove runs along the apparent notosternal suture (Figure 16 in Kirejtshuk et al., 2010). Notably, such prothoracic grooves for housing tarsi are absent in Ommatidae (Lawrence, 1999).
A groove is also present in the anterior portion of the elytral epipleura of Notocupes, most clearly shown by the amber specimen BA202101 (Figures 3C, D). The position and length of this groove suggest that it housed the mesotibia and mesotarsus. Similar structures are also preserved in our newly discovered compression Notocupes fossils from Daohugou (Figures 2B, K). However, it would be hard to correctly interpret them without the aid of amber fossils. The groove for housing legs in elytral epipleura is, to our knowledge, reported in beetles for the first time, and may represent a further apomorphy of Notocupes.
4.2 Systematic placement of Notocupes and the evolution of Archostemata
4.2.1 Phylogeny of Archostemata
We integrated our updated understanding of Notocupes morphology into a formal phylogenetic analysis to evaluate the placement of the genus within Archostemata. The result was generally consistent with Beutel et al. (2008), except for the position of Ademosynidae and Schizophoridae. Our analyses have consistently recovered a monophyletic Archostemata, including the extinct family Catiniidae, albeit with low support (Figure 4). The monophyly of Archostemata including Catiniidae was unaffected by the exclusion of Jurodidae (Supplementary Figure 1). The relationships among archostematan families remained almost the same regardless of the analytical approach used or the dataset analyzed. Ommatidae appears to be the earliest-diverging archostematan family in the present analyses, though with extremely low support. Our analyses, regardless of the weighting used or the dataset analysed, supported three archostematan clades, (i) Ommatidae, (ii) Crowsoniellidae, Micromalthidae, and Catiniidae, and (ii) Notocupes and Cupedidae, although the support values were not high (bootstrap values = 23–52).

Figure 4 Strict consensus tree of three equally shortest trees from TNT implied weighting parsimony analysis, with all taxa included. The equal weighting analysis produced exactly the same topology. Black circles indicate nonhomoplasious changes; white circles indicate homoplasious characters; numbers above the branches of the strict consensus tree indicate character numbers. The insets show representatives of Archostemata from Daohugou and Burmese amber.
4.2.2 Systematic placement of Notocupes
Regardless of the analytical method used or the inclusion of Jurodidae, Notocupes was always recovered as sister to Cupedidae (bootstrap values = 34–52). Cupedidae excluding Notocupes was strongly supported as monophyletic (bootstrap values = 88–92). Notocupes shares with Cupedidae the apomorphic arrangement of the abdominal sterna with both taxa possessing overlapping ventrites (character 62: 1). Both taxa also share the presence of scale-like setae (3: 1). Notocupes differs from Cupedidae in possessing a distinctly developed mentum: (35: 0) and possessing prosternal grooves for tarsomeres (40: 1). Besides these characters, Notocupes differs from Cupedidae by its relatively short prosternal process not reaching posterior end of procoxae and simple tarsomeres.
Based on the previous discussions, we conclude that Notocupes differs substantially from Ommatidae in morphology. Since a potential inclusion of Notocupes in Cupedidae would necessitate a dramatic revision of the latter’s diagnosis, we prefer to temporarily leave Notocupes without familial attribution, before more information is available for the possibly associated Notocupoides, Rhabdocupes and Eurydicton.
4.3 Paleobiology and evolutionary significance
Extant members of Cupedidae and Ommatidae are associated with decaying wood, although some adults have been reported to feed on pollen (Crowson, 1962; Atkins, 1963; Evans, 2014; Escalona et al., 2020). A saproxylic mode of life may also be expected in Notocupes. The relatively flattened habitus of Notocupes suggests that the beetles may have occupied narrow spaces such as crevices under bark, while the presence of sharp spines in some species suggest the beetles also occurred in open habitats, such as on tree trunks. Species from Burmese amber, fossils previously placed into the genus Amblomma, and NIGP174673, possessed dentate lateral edges of the prothorax or (and) sharp spines on elytra (Tan and Ren, 2009; Tihelka et al., 2019; Jiang et al., 2020) that may have fulfilled a defensive function or alternatively played a role in bark mimesis. Color patterns preserved in some Cretaceous Notocupes/Zygadenia elytra (Jarzembowski et al., 2015; Strelnikova and Yan, 2021) (Supplementary Figure 2) may have served as disruptive camouflage, breaking up the beetle’s outline and concealing them from visual predators. Grooves along the pleurosternal suture for housing the protarsi, grooves on the dorsal surface of the pronotum for housing the antennae, and epipleural grooves represent further morphological adaptations for life in confined space or may have served a protective function.
The more than 80 Myr range of the Notocupes–Zygadenia complex in the fossil record, from the Middle Triassic (Bathonian) to the Late Cretaceous (Coniacian), makes Notocupes a prime example of morphological, and probably also ecological, conservatism in Mesozoic archostematans. The morphology of Middle Jurassic Notocupes from Daohugou corresponds astonishingly well to that of the mid-Cretaceous one from Burmese amber. The prothoracic protarsal grooves and the epipleural mesotibial and -tarsal grooves remained almost unchanged for at least 66 Myr. Even some fine structures, such as the scale-covered coniform protuberances on elytra, persisted at least in some of the lineages. This high-level of morphological stability might suggest the group managed to track an almost consistent microhabitat (Marín et al., 2018; Cerca et al., 2020), in spite of the dramatic climatic changes over geological timescale. Nevertheless, some other pressures may have also played a role in conserving some characters.
Notocupes highlights ancestral character states with respect to Cupedidae, facilitating future comparative work on the latter family. Our study highlights the importance of examining fossils representing different types of preservation, such as compressions and amber inclusions, to shed light on controversial characters that may be distorted by taphonomic processes and build a more accurate evolutionary picture of extinct insect groups.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
Y-DL and C-YC conceived the study. D-YH, C-YC, F-YX and YL acquired and processed the fossils. Y-DL acquired and processed the photomicrograph data. Y-DL, ET and C-YC drafted the manuscript, to which SY and AFN contributed. All authors commented on the manuscript and gave final approval for publication.
Funding
Financial support was provided by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB26000000 and XDB18000000), the National Natural Science Foundation of China (42222201 and 42288201), the Second Tibetan Plateau Scientific Expedition and Research project (2019QZKK0706), and Grant-in-Aid for JSPS Fellows (20J00159) from the Japan Society for the Promotion of Science.
Acknowledgments
We are grateful to Margaret K. Thayer for helpful discussion, Su-Ping Wu for technical help in micro-CT reconstruction, Yan Fang for technical help in confocal imaging, and Chun-Zhao Wang for technical help in SEM imaging.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1015627/full#supplementary-material
References
Atkins M. (1963). The Cupedidae of the world. Can. Entomol. 95 (2), 140–162. doi: 10.4039/Ent95140-2
Beutel R. G., Ge S.-Q., Hörnschemeyer T. (2008). On the head morphology of Tetraphalerus, the phylogeny of Archostemata and the basal branching events in Coleoptera. Cladistics 24 (3), 270–298. doi: 10.1111/j.1096-0031.2007.00186.x
Bocak L., Barton C., Crampton-Platt A., Chesters D., Ahrens D., Vogler A. P. (2014). Building the Coleoptera tree-of-life for >8000 species: composition of public DNA data and fit with Linnaean classification. Syst. Entomol. 39 (1), 97–110. doi: 10.1111/syen.12037
Cai C., Huang D. (2017). Omma daxishanense sp. nov., a fossil representative of an extant Australian endemic genus recorded from the Late Jurassic of China (Coleoptera: Ommatidae). Alcheringa 41 (2), 277–283. doi: 10.1080/03115518.2016.1225251
Cerca J., Meyer C., Stateczny D., Siemon D., Wegbrod J., Purschke G., et al. (2020). Deceleration of morphological evolution in a cryptic species complex and its link to paleontological stasis. Evolution 74 (1), 116–131. doi: 10.1111/evo.13884
Crowson R. (1962). Observations on the beetle family Cupedidae, with descriptions of two new fossil forms and a key to the recent genera. Ann. Mag. Nat. Hist. 5 (51), 147–157. doi: 10.1080/00222936208651227
Escalona H. E., Lawrence J. F., Ślipiński A. (2020). The extant species of the genus Omma Newman and description of Beutelius gen. nov. (Coleoptera: Archostemata: Ommatidae: Ommatinae). Zootaxa 4728 (4), 547–574. doi: 10.11646/zootaxa.4728.4.11
Evans A. V. (2014). “Reticulated beetles (Cupedidae),” in Beetles of Eastern North America (Princeton, NJ: Princeton University Press), 60.
Friedrich F., Farrell B. D., Beutel R. G. (2009). The thoracic morphology of Archostemata and the relationships of the extant suborders of Coleoptera (Hexapoda). Cladistics 25 (1), 1–37. doi: 10.1111/j.1096-0031.2008.00233.x
Fu Y.-Z., Li Y.-D., Su Y.-T., Cai C.-Y., Huang D.-Y. (2021). Application of confocal laser scanning microscopy to the study of amber bioinclusions. Palaeoentomology 4 (3), 266–278. doi: 10.11646/palaeoentomology.4.3.14
Ge S.-Q., Hörnschemeyer T., Friedrich F., Beutel R. G. (2011). Is Crowsoniella relicta really a cucujiform beetle? Syst. Entomol. 36 (1), 175–179. doi: 10.1111/j.1365-3113.2010.00552.x
Giebel C. (1856). Die Insecten und Spinnen der Vorwelt mit steter Berücksichtigung der lebenden Insekten und Spinnen. Die Fauna der Vorwelt 2, 1–511.
Goloboff P. A., Catalano S. A. (2016). TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 32 (3), 221–238. doi: 10.1111/cla.12160
Goloboff P. A., Farris J. S., Källersjö M., Oxelman B., Ramírez M. J., Szumik C. A. (2003). Improvements to resampling measures of group support. Cladistics 19 (4), 324–332. doi: 10.1111/j.1096-0031.2003.tb00376.x
Goloboff P. A., Farris J. S., Nixon K. C. (2008). TNT, a free program for phylogenetic analysis. Cladistics 24 (5), 774–786. doi: 10.1111/j.1096-0031.2008.00217.x
Goloboff P. A., Torres A., Arias J. S. (2018). Weighted parsimony outperforms other methods of phylogenetic inference under models appropriate for morphology. Cladistics 34 (4), 407–437. doi: 10.1111/cla.12205
Hörnschemeyer T. (2009). The species-level phylogeny of archostematan beetles—where do Micromalthus debilis and Crowsoniella relicta belong? Syst. Entomol. 34 (3), 533–558. doi: 10.1111/j.1365-3113.2009.00476.x
Hörnschemeyer T. (2016). “Introduction and phylogeny. Archostemata Kolbe, 1908,” in Handbook of Zoology, Arthropoda: Insecta, Coleoptera, beetles, Vol. 1: morphology and systematics (Archostemata, Adephaga, Myxophaga, Polyphaga partim), 2nd Edition. Eds. Beutel R. G., Leschen R. A. (Berlin: Walter de Gruyter), 41–43.
Hörnschemeyer T., Goebbels J., Weidemann G., Faber C., Haase A. (2006). The head morphology of Ascioplaga mimeta (Coleoptera: Archostemata) and the phylogeny of Archostemata. Eur. J. Entomol. 103 (2), 409. doi: 10.14411/eje.2006.055
Jarzembowski E. A., Wang B. (2016). An unusual basal beetle from Myanmar (Coleoptera: Archostemata). Alcheringa 40 (2), 297–302. doi: 10.1080/03115518.2016.1132493
Jarzembowski E. A., Wang B., Zhang H., Fang Y. (2015). Boring beetles are not necessarily dull: new notocupedins (Insecta: Coleoptera) from the Mesozoic of Eurasia and East Gondwana. Cretac. Res. 52, 431–439. doi: 10.1016/j.cretres.2014.03.006
Jarzembowski E. A., Wang B., Zheng D. (2020a). An archaic-beetle ‘Jaws’ from mid-Cretaceous Burmese amber (Coleoptera: Archostemata). Proc. Geo. Assoc. 131 (2), 155–159. doi: 10.1016/j.pgeola.2020.02.003
Jarzembowski E. A., Wang B., Zheng D. (2020b). The first notocupedin beetle in mid-Cretaceous amber of northern Myanmar (Insecta: Coleoptera: Archostemata). Cretac. Res. 106, 104225. doi: 10.1016/j.cretres.2019.104225
Jiang Z., Li Y., Song C., Shi H., Liu Y., Chen R., et al. (2020). A new species of the genus Notocupes from mid-Cretaceous Burmese amber (Coleoptera: Archostemata: Ommatidae). Cretac. Res. 108, 104335. doi: 10.1016/j.cretres.2019.104335
Kirejtshuk A. G. (1999). Sikhotealinia zhiltzovae (Lafer 1996)–Recent representative of the Jurassic coleopterous fauna (Coleoptera, Archostemata, Jurodidae). Proc. Zool. Inst. Russ. Acad. Sci. 281, 21–26.
Kirejtshuk A. G. (2020). Taxonomic review of fossil coleopterous families (Insecta, Coleoptera). Suborder Archostemata: superfamilies Coleopseoidea and Cupedoidea. Geosciences 10 (2), 73. doi: 10.3390/geosciences10020073
Kirejtshuk A. G., Nel A., Collomb F.-M. (2010). New Archostemata (Insecta: Coleoptera) from the French Paleocene and Early Eocene, with a note on the composition of the suborder. Ann. Soc. Entomol. Fr. 46 (1-2), 216–227. doi: 10.1080/00379271.2010.10697661
Kirejtshuk A., Nel A., Kirejtshuk P. (2016). Taxonomy of the reticulate beetles of the subfamily Cupedinae (Coleoptera: Archostemata), with a review of the historical development. Invertebr. Zool. 13 (2), 61–190. doi: 10.15298/invertzool.13.2.01
Lafer G. S. (1996). “Fam. Sikhotealiniidae Lafer,” in Key to the Insects of the Russian Far East, vol. 3, part 3. Ed. Lafer P. (Vladivostok: Dal’nauka), 298–302.
Lawrence J. F., Ślipiński A. (2013). Australian Beetles. Volume 1: Morphology, Classification and Keys (Clayton: CSIRO Publishing).
Lawrence J. F. (1999). The Australian Ommatidae (Coleoptera: Archostemata): new species, larva and discussion of relationships. Invertebr. Syst. 13 (3), 369–390. doi: 10.1071/IT99008
Lawrence J. F. (2016). “Classification (families & subfamilies),” in Handbook of Zoology, Arthropoda: Insecta, Coleoptera, beetles, Vol. 1: morphology and systematics (Archostemata, Adephaga, Myxophaga, Polyphaga partim), 2nd Edition. Eds. Beutel R. G., Leschen R. A. (Berlin: Walter de Gruyter), 13–22.
Lee S. B., Nam G. S., Li Y.-D. (2022). A new species of Notocupes (Coleoptera: Archostemata) from the Lower Cretaceous (Albian) Jinju Formation in South Korea. Cretac. Res. 140, 105357. doi: 10.1016/j.cretres.2022.105357
Li Y.-D., Huang D.-Y., Cai C.-Y. (2021). Revisiting the morphology and systematic placement of the enigmatic Cretaceous ommatid beetle Bukhkalius lindae (Coleoptera: Archostemata: Ommatidae). Pap. Avulsos Zool. 61, e20206063. doi: 10.11606/1807-0205/2021.61.28
Li Y.-D., Liu Z.-H., Jarzembowski E. A., Yin Z.-W., Huang D.-Y., Cai C.-Y. (2019). Early evolution of Cupedidae revealed by a mid-Cretaceous reticulated beetle from Myanmar (Coleoptera: Archostemata). Syst. Entomol. 44 (4), 777–786. doi: 10.1111/syen.12355
Li Y.-D., Tihelka E., Yamamoto S., Huang D.-Y., Cai C.-Y. (2020a). A close affinity of the enigmatic genus Stegocoleus with Lepidomma revealed by new fossil evidence (Coleoptera: Archostemata: Ommatidae). Palaeoentomology 3 (6), 632–640. doi: 10.11646/palaeoentomology.3.6.15
Li Y.-D., Yamamoto S., Huang D.-Y., Cai C.-Y. (2020b). A miniaturized ommatid beetle in mid-Cretaceous Burmese amber (Coleoptera: Archostemata: Ommatidae). Pap. Avulsos Zool. 60, e20206063. doi: 10.11606/1807-0205/2020.60.63
Marín A. G., Olave M., Avila L. J., Sites J. W. Jr., Morando M. (2018). Evidence of body size and shape stasis driven by selection in Patagonian lizards of the Phymaturus patagonicus clade (Squamata: Liolaemini). Mol. Phylogenet. Evol. 129, 226–241. doi: 10.1016/j.ympev.2018.08.019
McKenna D. D., Shin S., Ahrens D., Balke M., Beza-Beza C., Clarke D. J., et al. (2019). The evolution and genomic basis of beetle diversity. Proc. Natl. Acad. Sci. 116 (49), 24729–24737. doi: 10.1073/pnas.1909655116
Mckenna D. D., Wild A. L., Kanda K., Bellamy C. L., Beutel R. G., Caterino M. S., et al. (2015). The beetle tree of life reveals that Coleoptera survived end-Permian mass extinction to diversify during the Cretaceous terrestrial revolution. Syst. Entomol. 40 (4), 835–880. doi: 10.1111/syen.12132
Pace R. (1975). An exceptional endogeous beetle: Crowsoniella relicta n. gen. n. sp. of Archostemata Tetraphaleridae from Central Italy. Boll. Mus. civ. stor. nat. Verona 2, 445–458.
Ponomarenko A. (1964). New beetles of of the family Cupedidae from the Jurassic of Karatau. Paleontol. Zh. 2, 49–62.
Ponomarenko A. (1966). Beetles of the family Cupedidae, Lower Triassic of Soviet. Paleontol. Zh. 4, 47–68.
Ponomarenko A. (1969). Historical development of archostomatan beetles. Trudy Paleontologicheskogo Instituta Akademii Nauk SSSR 125, 1–240.
Ponomarenko A. (1985). “Coleoptera,” in Jurassic Insects of Siberia and Mongolia. Trudy Paleontologicheskogo Instituta Akademii Nauk SSSR. Ed. Rasnitsyn A. (Moscow: Nauka), 47–87.
Ponomarenko A. (2000). Beetles of the family Cupedidae from the Lower Cretaceous locality of Semen, Transbaikalia. Paleontol. J. 34 (SUPP/3), S317–S322.
Ponomarenko A. (2006). On the types of Mesozoic archostematan beetles (Insecta, Coleoptera, Archostemata) in the Natural History Museum, London. Paleontol. J. 40 (1), 90–99. doi: 10.1134/S0031030106010102
Ponomarenko A., Ren D. (2010). First record of Notocupes (Coleoptera: Cupedidae) in locality Daohugou, Middle Jurassic of Inner Mongolia, China. Ann. Zool. 60 (2), 169–171. doi: 10.3161/000345410X516812
Ponomarenko A., Yan E., Huang D.-Y. (2014). New beetles (Coleoptera) from the terminal Middle Permian of China. Paleontol. J. 48 (2), 191–200. doi: 10.1134/S0031030114010109
Ślipiński S. A., Leschen R. A. B., Lawrence J. F. (2011). Order Coleoptera Linnaeus, 1758. In: Zhang, Z.-Q. (Ed.) Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa 3148 (1), 203–208. doi: 10.11646/zootaxa.3148.1.39
Smith M. R. (2019). Bayesian and parsimony approaches reconstruct informative trees from simulated morphological datasets. Biol. Lett. 15 (2), 20180632. doi: 10.1098/rsbl.2018.0632
Soriano C., Delclòs X. (2006). New cupedid beetles from the Lower Cretaceous of Spain and the palaeogeography of the family. Acta Palaeontol. Pol. 51, 185–200.
Strelnikova O. D. (2019). New Cupedidae (Insecta: Coleoptera, Cupedidae) from the Lower Cretaceous of Buryatia. Paleontol. J. 53 (3), 292–299. doi: 10.1134/S0031030119030146
Strelnikova O. D., Yan E. V. (2021). Redescriptions of beetles of the Notocupes generic complex (Coleoptera: Archostemata: Ommatidae) from the Lower Cretaceous of Buryatia. Palaeoentomology 4 (5), 499–514. doi: 10.11646/palaeoentomology.4.5.15
Strelnikova O. D., Yan E. V. (2023). Redescriptions of the Triassic Notocupes beetles (Archostemata: Ommatidae) from Kyrgyzstan and South Kazakhstan. Palaeoentomology 6 (25), 174–190. doi: 10.11646/palaeoentomology.6.2.9
Tan J.-J., Ren D. (2006). Ovatocupes: a new cupedid genus (Coleoptera: Archostemata: Cupedidae) from the Jehol Biota (Late Jurassic) of western Liaoning, China. Entomol. News 117 (2), 223–232. doi: 10.3157/0013-872X(2006)117[223:OANCGC]2.0.CO;2
Tan J., Ren D., Shih C., Ge S. (2006). New fossil beetles of the family Ommatidae (Coleoptera: Archostemata) from the Jehol Biota of China. Acta Geol. Sin. 80 (4), 474–485. doi: 10.1111/j.1755-6724.2006.tb00266.x
Tan J., Wang Y., Ren D., Yang X. (2012). New fossil species of ommatids (Coleoptera: Archostemata) from the Middle Mesozoic of China illuminating the phylogeny of Ommatidae. BMC Evol. Biol. 12 (1), 113. doi: 10.1186/1471-2148-12-113
Tihelka E., Huang D., Cai C. (2019). New notocupedin beetle in Cretaceous Burmese amber (Coleoptera: Archostemata: Ommatidae). Palaeoentomology 2 (6), 570–575. doi: 10.11646/palaeoentomology.2.6.5
Tihelka E., Huang D., Cai C. (2020a). New data on Ommatidae (Coleoptera) from mid-Cretaceous Burmese amber. Cretac. Res. 106, 104253. doi: 10.1016/j.cretres.2019.104253
Tihelka E., Huang D., Cai C. (2020b). A new genus and species of Micromalthidae from Burmese amber (Coleoptera: Archostemata). Earth Environ. Sci. Trans. R. Soc. Edinb. 111 (1), 39–46. doi: 10.1017/S1755691019000185
Yamamoto S. (2017). A new genus of Brochocoleini beetle in Upper Cretaceous Burmese amber (Coleoptera: Archostemata: Ommatidae). Cretac. Res. 76, 34–39. doi: 10.1016/j.cretres.2017.04.008
Keywords: Archostemata, Notocupes, Cupedidae, compression fossils, amber
Citation: Li Y-D, Tihelka E, Yamamoto S, Newton AF, Xia F-Y, Liu Y, Huang D-Y and Cai C-Y (2023) Mesozoic Notocupes revealed as the sister group of Cupedidae (Coleoptera: Archostemata). Front. Ecol. Evol. 11:1015627. doi: 10.3389/fevo.2023.1015627
Received: 09 August 2022; Accepted: 21 July 2023;
Published: 22 August 2023.
Edited by:
Xing Xu, Yunnan University, ChinaReviewed by:
Yongjie Wang, Guangdong Academy of Sciences, ChinaYanhong Pan, Nanjing University, China
Copyright © 2023 Li, Tihelka, Yamamoto, Newton, Xia, Liu, Huang and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen-Yang Cai, cycai@nigpas.ac.cn
 Yan-Da Li
Yan-Da Li Erik Tihelka
Erik Tihelka Shûhei Yamamoto
Shûhei Yamamoto Alfred F. Newton4
Alfred F. Newton4  Chen-Yang Cai
Chen-Yang Cai