Interactive Effects of Tree Mixing and Drought on a Primary Forest Pest
- French National Institute for Agricultural Research (INRA), UMR Biogeco, Cestas, France
Climate change and biodiversity erosion are two major threats to the vitality of the world's forests. However, it is difficult to predict the extent to which tree diversity and environmental conditions interact to modify forest health, and in particular resistance and/or tolerance to insect pests. We used a tree diversity experiment with an irrigation treatment to investigate the effect of mixing and diluting host pines (Pinus pinaster) amongst broadleaved trees on pine stem borer (Dioryctria sylvestrella) infestations under contrasting drought conditions. We further tested whether the attack patterns of this primary pest resulted from direct effects of tree diversity and drought, or was indirectly mediated by their combined effects on pine vigor. The total number of stem borer attacks per plot significantly decreased with tree species richness, i.e., increased with the density of pines, being maximum in pure plots. There were more attacks in irrigated plots. Mixing pines with birches resulted in lower plot infestations but only in irrigated plots. The probability of individual tree being attacked was higher in irrigated plots and decreased with increasing pine density. More vigorous trees, i.e., with higher radial growth, were more likely to be attacked. We suggest that the pine stem borer is attracted by the volatile organic compounds released by the resin exuding from bark cracks. Bark fissuring increases with radial growth, which is enhanced by irrigation and reduced intraspecific competition in low-density pine plots. The presence of birch limits plot infestation, probably because it disrupts host-finding behavior through repellence by non-host volatiles. This phenomenon is mainly observed when pines are vigorous, i.e., when they are irrigated, and particularly attractive to the stem borer. Our results confirm that more severe droughts decrease the attacks by primary pests feeding on tree trunks, by reducing host tree vigor, and that focal tree species can obtain protection against specialized insect pests when mixed with non-host species. The functional characteristics of herbivorous insects are therefore to be taken into account in predicting the interactive effects of climate change and loss of diversity on forest health.
Introduction
Climate change and biodiversity loss are two main current disturbances that threaten the vitality of forests on a global scale. Earth has warmed by about 1°C since 1880 (IPCC, 2014) due to increased greenhouse gas emission (Allen et al., 2009). The frequency and intensity of droughts have augmented (Diffenbaugh et al., 2017) and are predicted to increase further in the future (Lehner et al., 2017). Heat waves and severe droughts already had alarming effects on tree mortality worldwide (Allen et al., 2010; Hartmann et al., 2018). Since the end of the twentieth century, the rate of deforestation has accelerated, mainly affecting highly diverse tropical forests (Achard et al., 2002; Taubert et al., 2018). While the global estate of plantation forests, on the contrary, has been expanding (Brockerhoff et al., 2013) over the last decades, this cannot compensate for tree species loss as planted forests are monocultures of trees in the vast majority of cases (Nichols et al., 2006). Yet, general patterns of higher tree mortality and lower productivity have been documented in species-poor forests (Liang et al., 2016; Van de Peer et al., 2016; Grossman et al., 2018).
Beyond their direct effects on forest decline, both climate and tree diversity changes can have indirect effects on forest conditions through consequences on pest population dynamics and damages. Warmer conditions are likely to favor poikilothermic organisms like insects, resulting in more generations per year, higher fecundity and survival, thus leading to increased probability of outbreaks (Pureswaran et al., 2018; Jactel et al., 2019). Water stressed trees are also often more exposed to damage by serious forest pests like bark beetles and insect defoliators (Jactel et al., 2012, 2019). Moreover, it has be shown that tree monocultures are generally more susceptible to herbivore damage than more diverse forests (Castagneyrol et al., 2014; Guyot et al., 2016; Jactel et al., 2017). Several processes can account for associational resistance in diverse forests, i.e., the greater resistance of a tree species when grown in mixture with heterospecific trees. They include the reduction of host plant concentration (Hambäck et al., 2014), chemical or visual host-finding disruption (Jactel et al., 2011; Castagneyrol et al., 2013), lower host tree nutritional quality for herbivores (Moreira et al., 2014; Castagneyrol et al., 2018a) and a better control by natural enemies (Riihimäki et al., 2005; Moreira et al., 2016).
However, how and to which extent climate change and tree diversity erosion will combine their effects on forest health remains an open question (Walter et al., 2012; Castagneyrol et al., 2018a,b). On the one hand, tree diversity has been shown to modify microclimates within forests. Lower daily fluctuations of temperature and drought have been reported in forest stands with higher structural complexity (Lafage et al., 2014; Ehbrecht et al., 2017). Depending on their species composition, mixed forests would improve or worsen the water status of trees (Forrester et al., 2016; Grossiord, 2018). On the other hand, a growing body of evidence supports the view that tree diversity—forest ecosystem functioning relationships are dependent on the local climate (Ratcliffe et al., 2017; Jactel et al., 2018). More particularly in the case of tree diversity effects on forest resistance to herbivores, climatic conditions might differentially affect tree growth of associated species, which would change the effect of neighboring trees on focal host apparency (Castagneyrol et al., 2018b). Host tree nutritional quality could be reduced under drought conditions due to lower leaf water and nitrogen contents (Jactel et al., 2012) but also due to competition for light from heterospecific neighbors (Roberts and Paul, 2006). The semiochemical diversity hypothesis [SDH, (Zhang and Schlyter, 2004)] posits that mixed species forests release a complex mix of host and non-host volatiles, which can disturb olfaction-guided selection of host trees by forest insect herbivores. Yet, it has been shown that the emission of plants semiochemicals can be affected by temperatures (Zhang et al., 1999; Giron et al., 2018). Tree diversity effects on resistance have been shown to vary with herbivore population density (Bognounou et al., 2017; Fernandez-Conradi et al., 2017; Merwin et al., 2017), which can be directly affected by climate or weather conditions. In addition, the results of interactions between site conditions and forest composition on pest damage are likely to depend on the functional characteristics of herbivorous insects. Primary pests (i.e., attacking vigorous trees) feeding on tree foliage and secondary pests (i.e., attacking stressed trees) developing on tree trunks are likely to benefit from drought to increase their damage on stressed trees, principally because of better nutritional quality of leaf tissues (higher nitrogen content) and lower induced defenses in woody tissues (Holopainen et al., 2018). By contrast, primary pests feeding on woody organs would be disadvantaged by drought stress because of lower nutritional quality of woody tissues (lower carbohydrates content) (Jactel et al., 2012). Mixed-species forests would be more resistant to monophagous and oligophagous forest insects than tree monocultures, whereas both types of forests are equally vulnerable to polyphagous pests (Jactel and Brockerhoff, 2007; Castagneyrol et al., 2014). The interactive effects of tree diversity and climate on resulting forest insect damage are thus very difficult to predict on a theoretical basis due to occurrence of both positive and negative feedbacks, which can vary with particular insect-tree associations. A few large-scale analyses using forest diversity gradients or tree diversity trials replicated across a wide range of climates consistently showed that associational effects do vary with abiotic constraints (Guyot et al., 2016; Kambach et al., 2016). However, many confounding factors, including the identity of tree species that vary according to local conditions, make it difficult to understand and predict the response of forest insects to combined changes in tree diversity and climate.
In this study, we used a long-term experiment where both tree diversity and water stress were manipulated to test their interactive effects on forest susceptibility to a pest insect. We focused on maritime pine (Pinus pinaster) infestation by the pine stem borer, Dioryctria sylvestrella (Ratz.), which is a damaging, monophagous pest in Europe. It is considered a primary pest as it mainly attacks vigorous, standing trees (Jactel et al., 1996). In southwest France where the study took place, adult moths emerge in early summer. Female moths are attracted by monoterpenes that originate from the constitutive oleoresin exuding from pruning wounds and bark cracks (Jactel et al., 1996; Kleinhentz et al., 1998, 1999). After mating, eggs are laid individually on the trunk under bark scales. Larvae tunnel through the bark into the phloem where they feed until autumn. They overwinter in their gallery and recommence boring activity in early spring until the pupal stage. While boring their horizontal, subcortical galleries, caterpillars break resin ducts, inducing a resin flow through the entry hole. This results in a distinctive accumulation of yellow to pinkish, coagulated resin mixed with the frass produced by the foraging larva, hereafter called “pralin” (Figure 1A). Pupation occurs within the pralin. The girdling of the stem by the larval gallery causes an interruption of the sap flow, resulting in trunk malformation and increased risk of windbreak [(Baronio and Butturini, 1988); see Figures 1B,C]. Defense reactions of wood tissues around larval galleries, impregnated with resin, also greatly reduce wood quality. It has been shown that the percentage of attacked trees by D. sylvestrella was significantly lower in pine stands bordered by broadleaved forests than in pine stands surrounded by other pine stands, suggesting associational resistance at the landscape level (Jactel et al., 2002). However, the effect of intimate mixing of pine and broadleaved trees has not been yet investigated.

Figure 1. (A) Fresh pralin of resin signaling the presence of a pine stem borer (Dioryctria sylvestrella) attack. (B) Maritime pine tree attacked by the stem borer and then broken by the wind. (C) Close-up on the breakage point where the larval gallery of D. sylvestrella was located (background), and on the distinctive pralin of resin mixed with insect frass (foreground).
In the present study, we investigated the effect of mixing pines with broadleaved species on the probability of D. sylvestrella infestation at the level of both individual pines and forest stands under contrasting drought conditions, and asked whether infestation patterns depended on pine vigor. More particularly, we tested the following hypotheses:
1) Being a primary pest, the pine stem borer exhibits lower infestation in pine stands under water-stressed conditions and when pine trees are less vigorous;
2) Being a monophagous herbivore, the pine stem borer exhibits lower infestation in mixed than in pure pine stands and the number of attacks decreases with increasing proportion of non-host, broadleaved trees (resource concentration hypothesis);
3) There is an interactive effect of drought and tree diversity on stem borer infestation, which is mediated by individual tree vigor.
Materials and Methods
Experimental Design—ORPHEE
The ORPHEE experiment (https://sites.google.com/view/orpheeexperiment/home) was established on a former maritime pine plantation. In total, 25,600 trees belonging to five native species (Maritime pine, P. pinaster; European birch, Betula pendula; Pedunculate oak, Quercus robur; Pyrenean oak, Quercus pyrenaica; Holm oak, Quercus ilex) were planted in 2008. Eight blocks were established with 32 randomly distributed plots in every block corresponding to the 31 possible combinations of 1 to 5 tree species plus an additional replicate of the 5-species combination. Each plot was 400 m2, consisting of 10 rows of 10 trees planted 2 m apart (100 trees per plot). Tree species mixtures were established according to a substitutive design, keeping overall number of trees equal across plots. Within plots, individual trees from different species were planted in a regular alternate pattern, such that a tree from a given species had at least one neighbor from each of the other species within a 3 m radius (Castagneyrol et al., 2013).
The substitutive design of the ORPHEE experiment means that the proportion of pines is inversely proportional to tree species richness in the plots (i.e., pines represent 100% of trees in pure plots to 20% in five species mixtures). We used pine proportion as a predictive variable in our statistical models to directly test for the resource concentration hypothesis.
Previous studies at the same experimental site have shown that birches and pines are much taller than oaks, which are all below the lowest living branches of most pines (Damien et al., 2016). In addition, birch trees mediated associational resistance of pine trees against another oligophagous herbivore, the pine processionary moth (Castagneyrol et al., 2019). We therefore tested more specifically the effect of birch presence in pine plots on stem borer infestation.
Since 2015, four blocks are irrigated each year from May to October, while the other four blocks receive only natural rainfall. The local climate is characterized by a chronic water deficit in summer, hence non-irrigated blocks experience drought during 3 months on average (mid-June to mid-September). Irrigation treatment consists in sprinkling ca 42 m3 per night and per block, corresponding to ca 3 mm per night. This volume is calculated based on regional climatic data (evapotranspiration) and is assumed to avoid soil water deficit in the irrigated blocks during the entire growing season. In August 2016, we confirmed that the irrigation treatment increased soil water content and reduced water stress as compared to non-irrigated (control) blocks (Castagneyrol et al., 2017).
For our study, we selected every plots including pine, which corresponded to 16 different specific compositions (17 plots per block as the 5-species mixture was duplicated, Table 1). Four replicates of each plot type were located in non-irrigated blocks and four other replicates were located in irrigated blocks (except for the plot type with five species, which had 8 replicates for each water treatment). In each plot, we considered only the 36 most central trees (6 inner rows) to avoid edge effects. This represented 1,840 potential pines in 136 plots. Because some pines died or were broken by windthrow or were overlooked, we could only include 1,760 pines from 134 plots into the survey.
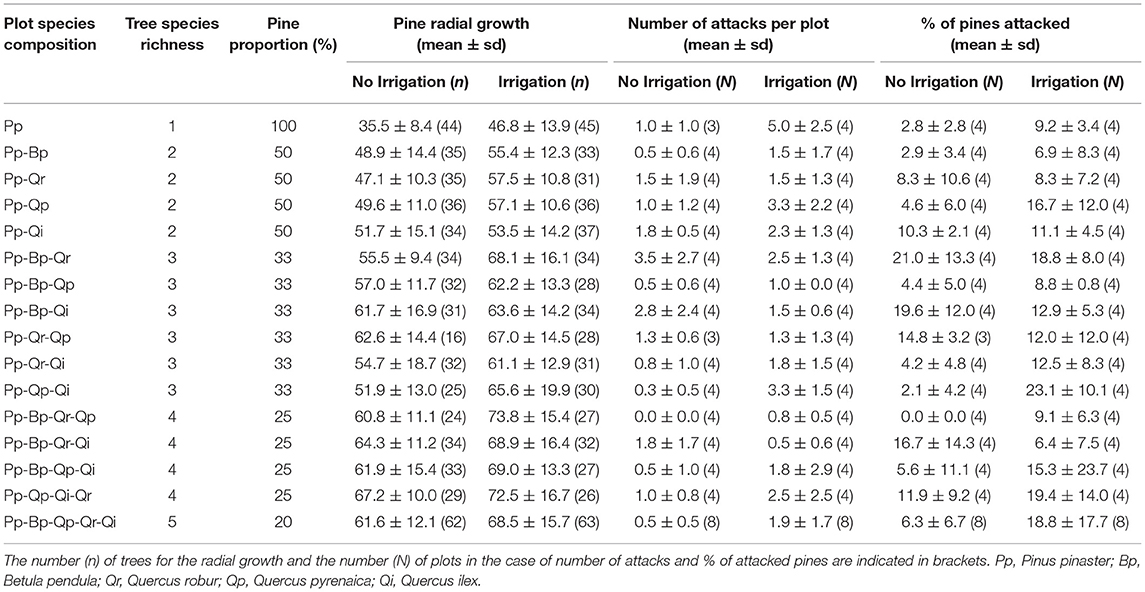
Table 1. Tree species composition of the sampled plots and averaged values (± standard-deviation) of pine radial growth, number of attacks, and percentage of pines attacked by Dioryctria sylvestrella.
We measured the diameter at breast height (DBH) of each of central trees in 2015 and 2016. We then computed the radial growth (DBH increment between the 2 years) of each pine and the mean radial growth of the sampled pines in each plot. Radial growth was used as a proxy for tree vigor, a major driver of pine susceptibility to D. sylvestrella (Jactel et al., 1996).
Assessment of Pine Stem Borer Attacks
Current year attacks by D. sylvestrella can be identified by the presence of fresh pralins on the trunk (Figure 1A). In summer 2016, two observers carefully inspected trunk surface from the ground, using binoculars, and visually counted the number of fresh pralins on each sampled pine. Most attacked pines had only one attack (85%) and the number of attacks per pine never exceeded four attacks (1.20 on average). We thus used the presence/absence of attacks as response variable at the tree level and the total number of attacks at the plot level.
In addition, we surveyed all the pines in the experiment, including those of plot edges (5,120 trees), to record trunk breakage following storms and checked the presence of a D. sylvestrella larval gallery at the point of breakage. Windstorms occur each winter in the study area, with varying degrees of intensity.
Statistical Analyses
We investigated the effects of plot composition (i.e., proportion of pines and presence/absence of birch) and drought on stem borer attacks at both the plot and the tree level, using generalized linear mixed effects models (GLMM). At the plot level, the response variable was the total number of D. sylvestrella attacks per plot, which followed a Poisson distribution. At the tree level, it was the presence/absence of D. sylvestrella attack on a given tree, which followed a binomial distribution. In each case, we first built a full GLMM including the proportion of pines, irrigation, and the presence/absence of birch, as well as all two- and three-ways interactions, as fixed effects. At the tree level, we could indeed test the effect of presence/absence of birch because the substitutive alternate pattern of experimental plots resulted in the presence of at least one birch tree next to each focal pine tree in all pine-birch mixtures. Equations of full models (a) were as follow (fixed effects only):
We then included pine radial growth as an additional predictor in the two first full models to test whether the effects of pine proportion, presence/absence of birch and irrigation on individual pine attack probability and on the total number of attacks per plot were mediated by pine vigor at both plot and tree level. That would be the case if a significant effect of pine proportion, irrigation or presence of birch on D. sylvestrella attacks turned non-significant after including the new predictor. Equations of full models (b) were as follow (fixed effects only):
To investigate further the causes of tree vigor, we also tested the effect of pine proportion, irrigation and the presence/absence of birch (main effects and two- and three-ways interactions) on pine radial growth at plot and tree levels, using linear mixed effect models (LMM) as the residuals were normally distributed.
As the ORPHEE experiment consists in a randomized split-plot design replicated in eight blocks, half of which being irrigated, we adapted the calculation of degrees of freedom and mean sum of squares of residuals (Altman and Krzywinski, 2015) by adding Block × Irrigation as random intercept effect (1|Block:Irrigation in R syntax).
In addition, because trees within a given plot were considered pseudo-replicates (non-independent), we added a random intercept effect with Plot nested within Block [1|Plot:Block in R syntax; (Schielzeth and Nakagawa, 2013)] in analyses carried out at the tree level.
In each case, the full model was simplified following a backward selection approach, which consisted of sequentially dropping the term whose elimination had the lowest impact on model fit, starting with the highest order interactions. This was done by using log-likelihood tests based on a χ2 distribution comparing models with and without the variable of interest, with significance threshold set at α = 0.05.
In all models, quantitative predictors were scaled and centered, which made it possible to compare the magnitude of the effects even when involved in interactions (Schielzeth, 2010). Most-parsimonious (simplified) model parameters were estimated by maximum likelihood estimation (restricted likelihood estimation in the case of LMM) and the significance (α = 0.05) of the effects of predictors was tested through Wald z-tests for GLMM and t-tests with Satterthwaite method for the calculation of degrees of freedom for LMM. We evaluated model fit by calculating the percentage of variance explained by fixed (R2m) and by fixed plus random effects (R2c) (Nakagawa and Schielzeth, 2013).
All analyses were done using the R language programming [version 3.5.1; (R Core Development Team, 2013)] and the lmerTest (Kuznetsova et al., 2017) and MuMIn (Barton, 2018) packages.
Results
Of the 134 plots surveyed, 94 (i.e., 70%) showed at least one attack by D. sylvestrella. The total number of attacks per infested plot varied from 1 to 8 attacks per plot. In the central part of the plots, 176 pines (out of 1,760, i.e., 10%) were attacked by the stem borer (Table 1).
Out of the 5,120 surveyed pines, 2.1% were killed topped during strong windstorms. The presence of at least one stem borer attack at the breakage point confirms causal relationship between initial D. sylvestrella attacks and subsequent breakage during windstorms (Figures 1B,C).
Using a pressure chamber, we found that predawn leaf water potential of maritime pines was significantly lower, i.e., indicating higher water stress, in dry (non-irrigated) blocks than in irrigated blocks, both in 2015 when D. sylvestrella moths attacked trees (ψP = −0.593 ± 0.095 in dry blocks, ψP = −0.359 ± 0.087 in irrigated blocks, n = 32 trees, t-test, p < 0.0001) and in 2016 when D. sylvestrella larvae finished their development (ψP = −1.443 ± 0.170 in dry blocks, ψP = −0.382 ± 0.053 in irrigated blocks, n = 32 trees, t-test, p < 0.0001).
At the plot level, the number of stem borer attacks significantly increased with the proportion of pines, and therefore decreased with tree species richness. The relationship between the number of attacks and pine proportion was independent of the presence of birch and of water regime (no significant Pine proportion × Birch or Pine proportion × Irrigation interactions, Table 2, Model a; Figure 2A).
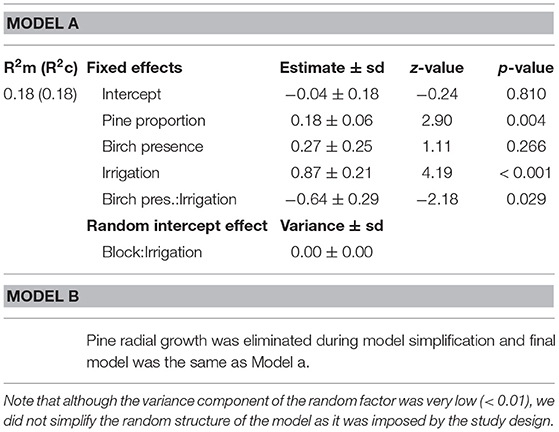
Table 2. Summary of simplified generalized linear mixed effects models (Poisson distribution) of the number of attacks by D. sylvestrella at plot level before (Model a) and after (Model b) introduction of mean pine radial growth as a covariate.
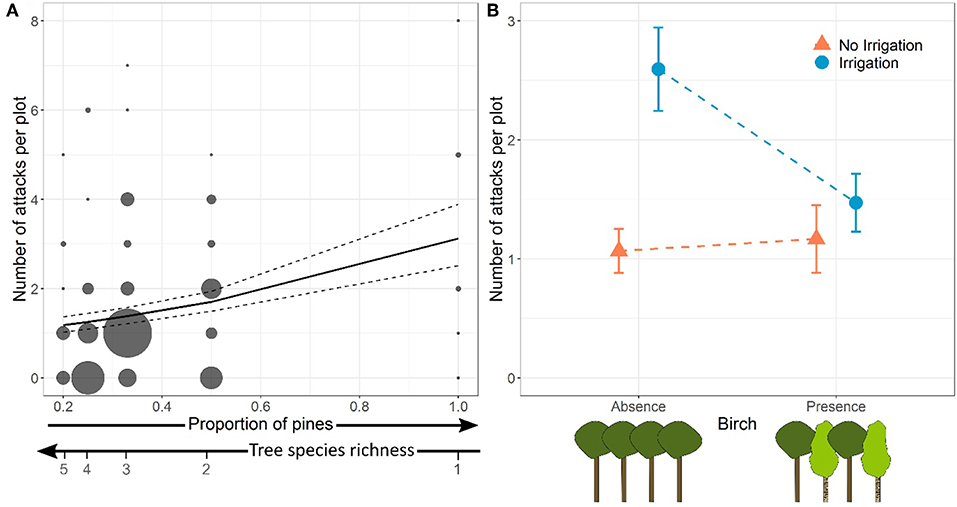
Figure 2. Effects of proportion of pines (inversely proportional to tree species richness) (A) and of the presence of birch in irrigated and non-irrigated plots (B) on the total number of Dioryctria sylvestrella attacks per plot. In (A), dot size is proportional to the number of plots. Model predictions and standard-errors are represented by the solid and dashed lines, respectively. In (B), dots and bars represent means and standard-errors, respectively.
However, there was also a significant interaction between the presence of birch in the plot and the water regime on the number of D. sylvestrella attacks per plot (Table 2). More specifically, the number of attacks in irrigated plots was significantly lower in the presence of birch (Figure 2B; z = −3.22, p = 0.001), whereas the effect of birch was not statistically significant in water stressed plots (z = 0.40, p = 0.690).
We found that the radial growth of pines was significantly higher in irrigated plots and in plots with lower proportion of pines (i.e., higher tree species richness) both factors having additive effects (Table 3; Figure 3). The proportion of pines and irrigation together explained 46% of the variance of average pine radial growth.
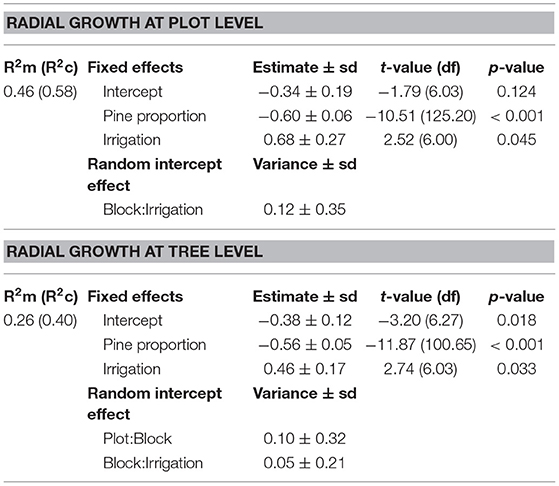
Table 3. Summary of simplified linear mixed effects models of pine radial growth (vigor) at plot and tree level.
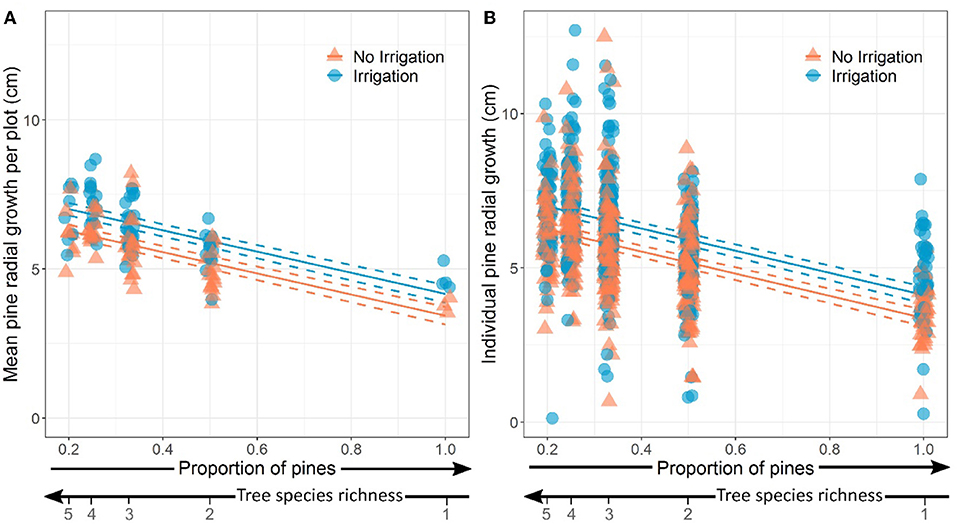
Figure 3. Effects of proportion of pines (inversely proportional to tree species richness) and water treatment in the plots on (A) mean pine radial growth (plot level) and (B) individual pine radial growth. In both panels, dots have been slightly spread around the value of x (proportion of pines) for the sake of visibility. Model predictions and standard-errors are represented by solid and dashed lines, respectively.
When the mean radial growth of pines (average pine vigor) was included as a covariate in the full model of the total number of attacks per plot, it was not kept during model simplification (Table 2, Model b). This suggests that, at the plot scale, the effect of pine proportion on the number of D. sylvestrella attacks was not driven by its effect on pine radial growth. In the final model, the fixed effects accounted for 18% of the variance in number of attacks per plot (Table 2).
At the tree level, the per capita probability of attack decreased significantly with pine proportion (i.e., increased with tree species richness, Table 4, Model a), i.e., there was a dilution of attacks in plots with higher proportion of pines (Figure 4A). The probability of attack on individual pines was significantly higher in irrigated plots than in non-irrigated plots (Figure 4A), irrespective of pine proportion or plot species composition (no significant Irrigation × Pine proportion or Irrigation × Birch presence interaction, Table 4, Model a). We did not detect any effect of birch presence on attack probability at the individual tree level.
Table 4. Summary of generalized linear mixed effects models (Binomial distribution) of individual pine attack probability before (Model a) and after (Model b) introduction of pine radial growth as a covariate.
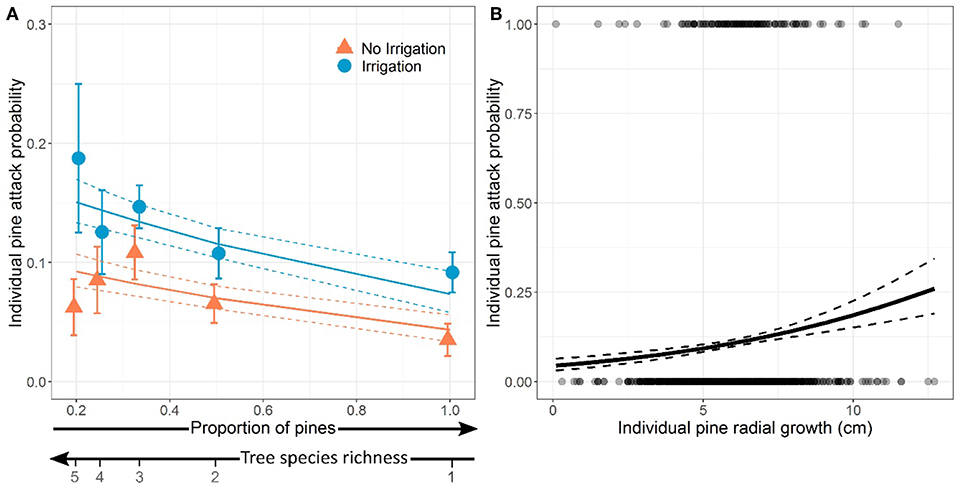
Figure 4. Effects of proportion of pines (inversely proportional to tree species richness) and water treatment in the plot (A) and effect of individual pine vigor (radial growth) (B) on the probability of individual pine being attacked by Dioryctria sylvestrella. Dots and error bars in panel (A) represent the mean probabilities of pine being attacked ± standard-errors for each case of pine proportion. In (B), dots at y = 0 and at y = 1 represent non-attacked and attacked trees, respectively. Model predictions and standard-errors are represented by solid and dashed lines, respectively.
The radial growth of individual pines was also significantly higher in irrigated plots and in plots with lower proportion of pines (i.e., higher tree species richness) (Table 3; Figure 3). The proportion of pines and irrigation had additive effects and explained 26% of the variance at the tree level.
When individual pine vigor (i.e., radial growth) was included in the model, it was retained by model selection instead of both pine proportion and irrigation (Table 4, Model b), suggesting that both effects were mediated by their joint influence on individual tree vigor. The probability of an individual pine being attacked significantly increased with its radial growth (Figure 4B).
Discussion
Using a tree diversity experiment where drought severity was manipulated, we could show that host density as well as the species composition of forest plots influenced maritime pine susceptibility to D. sylvestrella and that these effects were partly the indirect consequences of changes in pine vigor. Importantly, because pine vigor was dependent on irrigation, we further demonstrated that drought reduced pine susceptibility to the stem borer, but that this effect was contingent on the presence of birch, another fast growing tree species, which reduced the total number of D. sylvestrella attacks in pine-birch mixtures.
Pine Vigor Effect
We found that the per capita probability of attack increased with pine vigor, which confirms the status of primary pest of D. sylvestrella. This result is consistent with previous observations that the mean percentage of attacked trees per class of tree diameter significantly increased with mean tree diameter per class (Jactel et al., 1996). In another study, we showed that the pruning of live branches had a positive effect, complementary to that of tree vigor, on the probability of attack (Jactel et al., 1994, 1996). This was interpreted as an olfactory attraction of D. sylvestrella females, prior oviposition, due to the release of terpenic compounds (Kleinhentz et al., 1999) by the resin exuding from pruning wounds or bark cracks. Here we provide new experimental evidence supporting this host selection behavior. Supplying water probably boosted radial growth in summer, which is known to increase exponentially the frequency and depth of bark fissures (MacFarlane and Luo, 2009), leading to higher release of attractive terpenes. Assuming that the dissimilarity in traits related to water capture was greater between species than between pine individuals, it is likely that greater pine vigor in low-pine density plots arises from lower competition for resources at both intra-specific (each pine has a greater space to develop) and inter-specific levels (heterospecific neighbors use resource in a complementary way). Indeed, studies have reported an increase in tree growth in species mixtures (Zhang et al., 2012; Liang et al., 2016; Jactel et al., 2018) and in particular, an increase in biomass production with functional or phylogenetic diversity of plants (Flynn et al., 2011; Paquette and Messier, 2011) due to greater resource use complementarity.
Drought Effects
In our experiment, we observed significantly fewer stem borer attacks and lower probability of individual attack in non-irrigated plots. This finding is consistent with the outcomes of a meta-analysis (Jactel et al., 2012), which revealed that drought does not systematically result in higher biotic damage in forest trees. In particular, we showed that, on average, primary pests feeding on woody organs, like D. sylvestrella, would cause less damage on water stressed trees. Several explanations have been proposed to explain lower damage by primary stem borers on trees in drought conditions. Drought can reduce the nutritional quality of host trees, through reduced concentration of carbohydrates in the bark because of less effective photosynthesis (Speight and Wainhouse, 1989). Water stress is also known to increase nitrogen allocation to the crown at the expense of the stem (Martin et al., 1998), thus reducing the performance of bark feeders (Mattson and Haack, 1987). Induced resistance mechanisms based on accumulation of phenolic compounds in reaction tissues (Franceschi et al., 2000), such as those observed around D. sylvestrella larval galleries, might also be more effective in trees under water-stress because of trade-offs in energy allocation between primary (radial increment) and secondary (defenses) metabolism (Herms and Mattson, 1992). However, both mechanisms, related to antibiosis mechanisms, suppose an adverse effect of the nutritional quality of wood tissues on the survival or performance of the subcortical live stages of the pest. Although never formally tested, those effects are unlikely in the case of stem borer larvae as they have an almost unlimited supply of feeding tissues (one larval gallery per tree trunk) and are completely immune to resin toxicity (they literally crawl in liquid resin and pupate in pralins of resin). Here we rather suggest another mechanism of resistance promoted by water stress, which relates to the non-preference concept [or antixenosis; (Stout, 2013)]. Using monoterpenes as chemical cues to locate their host trees, D. sylvestrella adult females would preferentially choose more vigorous trees as they release a higher amount of attraction signal while ensuring a sustainable feeding resource for the offspring. Because the larvae develop slowly, from summer to the next spring (ca. 10 months), host tree survival is important for the herbivore, which is more likely with vigorous trees. Drought, by limiting radial tree growth, would thus reduce the attractiveness of water-stressed trees, making them less preferred than more vigorous pine trees.
It should be recognized here that, although the irrigation treatment had an obvious positive effect on tree growth, we did not measure the actual water stress on the sampled pines. Our interpretation of the mechanisms behind the positive effect of irrigation on D. sylvestrella attacks is therefore speculative—although well-supported by the literature—until future direct confirmation.
Tree Diversity Effects
Using a tree diversity experiment, we could also investigate the associational resistance to the pine stem borer, i.e., whether the presence of heterospecific neighbors can lead to lower attacks of D. sylvestrella on pine hosts. By specifically comparing D. sylvestrella attacks along a pine proportion (density) gradient in the presence vs. absence of birch, we could decipher the effects of tree species composition and host density on the density of D. sylvestrella at the plot level and on the probability of individual pine being attacked.
At plot level, we found that the total number of stem borer attacks per plot significantly increased with pine proportion, being maximum in pure pine plots. This is consistent with the host concentration hypothesis, which states that specialist herbivores (like D. sylvestrella) show higher abundance in pure, high-density patches of their host plant than in mixtures (Tahvanainen and Root, 1972). This has been found already for several specialist forest insects (e.g., Sholes, 2008; Castagneyrol et al., 2019). A proposed behavioral mechanism for the host concentration hypothesis is that insect herbivores are more likely to find and remain in monocultures where their host plant is at higher density than in mixtures with non-host plants. This mechanism is compatible with our assumption that the pine stem borer uses volatile organic compounds to find its host tree, as more pine trees grouped in the same plot can release a higher quantity of attractive monoterpenes. Due to the substitutive design of the ORPHEE experiment, the concentration of host pines per plot linearly increased with decreasing tree species richness. An alternative, non-exclusive, mechanism to explain the higher number of attacks in plots with higher density of pines is that non-host volatiles would be less abundant or diverse, thus facilitating olfaction-guided selection of host pine trees, consistently with the semiochemical diversity hypothesis (Zhang and Schlyter, 2004).
Looking at the data at the tree level provided another perspective. We found that the probability of individual tree attack decreased with increasing pine proportion. A likely interpretation is that, under conditions of low population level (the overall percentage of attacked trees was 10%), females spread their attacks (on average there was no more than one attack per infested tree) over a smaller number of trees in the less dense plots, leading to higher probability of individual tree attack. This is typical of a process of host-density dependent distribution of attacks within plots, which has already been observed in other forest pests, like the pine processionary moth (Régolini et al., 2014), including in the same experiment (Damien et al., 2016). Another explanation is that in mixed plots, pines suffered less from intraspecific competition and therefore had higher individual growth, leading to an increased risk of attack.
In addition to the effect of host density, we detected a significant protective effect of the presence of birch, a fast growing non-host tree, on D. sylvestrella abundance in mixed plots. This pattern of associational resistance triggered by birch has been already observed, in the same experimental site, for the pine processionary moth (Damien et al., 2016; Castagneyrol et al., 2019). It was interpreted as resulting from a host-finding disruption process mediated by the release of repulsive odors by B. pendula (Jactel et al., 2011). The same mechanism is likely to apply to the pine stem borer, which is known to use pine odors for host selection (Jactel et al., 1996; Kleinhentz et al., 1999), a phenomenon that could be impeded by the presence of masking or repulsive non-host (here birch) odors. We should note that the protective effect of birch was detected at the plot level and not at the tree level. At the time of the study, birch trees were on average 6 m high, i.e., almost as tall as pine trees (7.6 m on average; Castagneyrol et al., 2019). We can thus speculate that a mass effect of many birch trees releasing non-host volatiles is necessary to repel stem borers but that once in the mixed plot, host selection behavior by female moths is not affected by the identity of pine neighbors.
Interactions Between Drought and Tree Diversity
Finally, we could confirm that drought and tree diversity interactively affect stem borer infestation. The total number of attacks was the highest in irrigated plots without birch (2.59 attacks on average) and lowest in non-irrigated (water-stressed) plots without birch (1.07 attacks on average), whereas plots of pines mixed with birches showed intermediate levels of infestation (1.17 and 1.47 attacks on average in non-irrigated and irrigated plots, respectively) (Figure 2B). The Irrigation × Birch presence interaction was negative, meaning that the protective effect of birch presence was only noticeable in irrigated plots. This might be due to the greater abundance of attacks in these plots as a result of higher tree vigor. When the infestations of D. sylvestrella are very low because stem borers are not attracted by host odors, like in water stressed (non-irrigated) plots, any difference between pure and mixed plots are very difficult to detect.
Conclusion
Overall, using an experiment where both tree diversity and water supply were manipulated, we could confirm two theoretical predictions. The first is that more severe drought will negatively affect the population dynamics of a primary pest feeding on tree trunk. The second is that focal tree species can be significantly less damaged by specialist pest insects when mixed with non-host tree species. We could thus verify that species composition of mixed stands is an important driver of associational effects (Jactel and Brockerhoff, 2007; Castagneyrol et al., 2014) as the sole presence of birch could explain lower stem borer infestations in mixed pine plots. Last, we demonstrated that climatic conditions and forest composition significantly interact to drive forest infestation by a pest insect.
At a time when forests have never been so threatened by the huge impact of ongoing deforestation and global warming but have never gained such high profile as a solution to mitigate climate change, through carbon sequestration, there is an urgent need for ambitious afforestation programs worldwide (Verdone and Seidl, 2017; Bastin et al., 2019). However, it remains all the more important to design new forest plantations that are both productive in the long term and sufficiently resistant to biotic and abiotic hazards. The concept of mixed-species plantations, combining few tree species with complementary uses of resources to sustain growth (Baeten et al., 2019) while supporting associational resistance processes (Jactel et al., 2017), therefore appears to be a promising opportunity.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
HJ and BC designed the study. HJ and IH organized the field work. CP analyzed the data. HJ and CP wrote the first version of the manuscript, which was further improved by all co-authors.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the French National Research Agency for the funding of the study under the research project DiPTiCC (ANR-16-CE32-0003). We are grateful to Elodie Le Souchu for helping us count Dioryctria attacks. We acknowledge UEFP 0570, INRA, 69 route d'Arcachon, 33612, Cestas for the management of the ORPHEE experiment and Céline Meredieu for handling tree growth data.
References
Achard, F., Eva, H. D., Stibig, H.-J., Mayaux, P., Gallego, J., Richards, T., et al. (2002). Determination of deforestation rates of the World's Humid Tropical Forests. Science 297, 999–1002. doi: 10.1126/science.1070656
Allen, C. D., Macalady, A. K., Chenchouni, H., Bachelet, D., McDowell, N., Vennetier, M., et al. (2010). A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Eco. Manage. 259, 660–684. doi: 10.1016/j.foreco.2009.09.001
Allen, M. R., Frame, D. J., Huntingford, C., Jones, C. D., Lowe, J. A., Meinshausen, M., et al. (2009). Warming caused by cumulative carbon emissions towards the trillionth tonne. Nature 458, 1163–1166. doi: 10.1038/nature08019
Altman, N., and Krzywinski, M. (2015). Split plot design. Nat. Methods 12, 165–166. doi: 10.1038/nmeth.3293
Baeten, L., Bruelheide, H., Plas, F., van der Kambach, S., Ratcliffe, S., Jucker, T., et al. (2019). Identifying the tree species compositions that maximize ecosystem functioning in European forests. J. Appl. Ecol. 56, 733–744. doi: 10.1111/1365-2664.13308
Baronio, P., and Butturini, A. (1988). Gli insetti nocivi al bosco, Pinus spp. piralidae della corteccia del pino, Dioryctria sylvestrella (Lepidoptera: Phycitidae). Monti e Boschi 6, 3–4.
Barton, K. (2018). MuMIn: Multi-Model Inference. R Package Version 1.42.1. Available online at: https://CRAN.R-project.org/package=MuMIn
Bastin, J.-F., Finegold, Y., Garcia, C., Mollicone, D., Rezende, M., Routh, D., et al. (2019). The global tree restoration potential. Science 365, 76–79. doi: 10.1126/science.aax0848
Bognounou, F., Grandpré, L. D., Pureswaran, D. S., and Kneeshaw, D. (2017). Temporal variation in plant neighborhood effects on the defoliation of primary and secondary hosts by an insect pest. Ecosphere 8:e01759. doi: 10.1002/ecs2.1759
Brockerhoff, E. G., Jactel, H., Parrotta, J. A., and Ferraz, S. F. B. (2013). Role of eucalypt and other planted forests in biodiversity conservation and the provision of biodiversity-related ecosystem services. Forest Ecol. Manage. 301, 43–50. doi: 10.1016/j.foreco.2012.09.018
Castagneyrol, B., Bonal, D., Damien, M., Jactel, H., Meredieu, C., Muiruri, E. W., et al. (2017). Bottom-up and top-down effects of tree species diversity on leaf insect herbivory. Ecol. Evol. 7, 3520–3531. doi: 10.1002/ece3.2950
Castagneyrol, B., Giffard, B., Péré, C., and Jactel, H. (2013). Plant apparency, an overlooked driver of associational resistance to insect herbivory. J. Ecol. 101, 418–429. doi: 10.1111/1365-2745.12055
Castagneyrol, B., Jactel, H., and Moreira, X. (2018a). Anti-herbivore defences and insect herbivory: interactive effects of drought and tree neighbours. J. Ecol. 106, 2043–2057. doi: 10.1111/1365-2745.12956
Castagneyrol, B., Jactel, H., Vacher, C., Brockerhoff, E. G., and Koricheva, J. (2014). Effects of plant phylogenetic diversity on herbivory depend on herbivore specialization. J. Appl. Ecol. 51, 134–141. doi: 10.1111/1365-2664.12175
Castagneyrol, B., Kozlov, M. V., Poeydebat, C., Toïgo, M., and Jactel, H. (2019). Associational resistance to a pest insect fades with time. J. Pest Sci. 1–11. doi: 10.1007/s10340-019-01148-y
Castagneyrol, B., Moreira, X., and Jactel, H. (2018b). Drought and plant neighbourhood interactively determine herbivore consumption and performance. Sci. Rep. 8:5930. doi: 10.1038/s41598-018-24299-x
Damien, M., Jactel, H., Meredieu, C., Regolini, M., van Halder, I., and Castagneyrol, B. (2016). Pest damage in mixed forests: disentangling the effects of neighbor identity, host density and host apparency at different spatial scales. Forest Ecol. Manage. 378, 103–110. doi: 10.1016/j.foreco.2016.07.025
Diffenbaugh, N. S., Singh, D., Mankin, J. S., Horton, D. E., Swain, D. L., Touma, D., et al. (2017). Quantifying the influence of global warming on unprecedented extreme climate events. Proc. Natl. Acad. Sci. U.S.A. 114, 4881–4886. doi: 10.1073/pnas.1618082114
Ehbrecht, M., Schall, P., Ammer, C., and Seidel, D. (2017). Quantifying stand structural complexity and its relationship with forest management, tree species diversity and microclimate. Agric. Forest Meteorol. 242, 1–9. doi: 10.1016/j.agrformet.2017.04.012
Fernandez-Conradi, P., Jactel, H., Hampe, A., Jose Leiva, M., and Castagneyrol, B. (2017). The effect of tree genetic diversity on insect herbivory varies with insect abundance. Ecosphere 8:e01637. doi: 10.1002/ecs2.1637
Flynn, D. F., Mirotchnick, N., Jain, M., Palmer, M. I., and Naeem, S. (2011). Functional and phylogenetic diversity as predictors of biodiversity–ecosystem-function relationships. Ecology 92, 1573–1581. doi: 10.1890/10-1245.1
Forrester, D. I., Bonal, D., Dawud, S., Gessler, A., Granier, A., Pollastrini, M., et al. (2016). Drought responses by individual tree species are not often correlated with tree species diversity in European forests. J. Appl. Ecol. 53, 1725–1734. doi: 10.1111/1365-2664.12745
Franceschi, V. R., Krokene, P., Krekling, T., and Christiansen, E. (2000). Phloem parenchyma cells are involved in local and distant defense responses to fungal inoculation or bark-beetle attack in Norway spruce (Pinaceae). Am. J. Bot. 87, 314–326. doi: 10.2307/2656627
Giron, D., Dubreuil, G., Bennett, A., Dedeine, F., Dicke, M., Dyer, L. A., et al. (2018). Promises and challenges in insect–plant interactions. Entomol. Exp. Appl. 166, 319–343. doi: 10.1111/eea.12679
Grossiord, C. (2018). Having the right neighbors: how tree species diversity modulates drought impacts on forests. N. Phytol. 1–8. doi: 10.1111/nph.15667
Grossman, J. J., Vanhellemont, M., Barsoum, N., Bauhus, J., Bruelheide, H., Castagneyrol, B., et al. (2018). Synthesis and future research directions linking tree diversity to growth, survival, and damage in a global network of tree diversity experiments. Environ. Exp. Bot. 152, 68–89. doi: 10.1016/j.envexpbot.2017.12.015
Guyot, V., Castagneyrol, B., Vialatte, A., Deconchat, M., and Jactel, H. (2016). Tree diversity reduces pest damage in mature forests across Europe. Biol. Lett. 12:20151037. doi: 10.1098/rsbl.2015.1037
Hambäck, P. A., Inouye, B. D., Andersson, P., and Underwood, N. (2014). Effects of plant neighborhoods on plant–herbivore interactions: resource dilution and associational effects. Ecology 95, 1370–1383. doi: 10.1890/13-0793.1
Hartmann, H., Moura, C. F., Anderegg, W. R. L., Ruehr, N. K., Salmon, Y., Allen, C. D., et al. (2018). Research frontiers for improving our understanding of drought-induced tree and forest mortality. N. Phytol. 218, 15–28. doi: 10.1111/nph.15048
Herms, D., and Mattson, W. (1992). The dilemma of plants - to grow or defend. Q. Rev. Biol. 67, 283–335. doi: 10.1086/417659
Holopainen, J. K., Virjamo, V., Ghimire, R. P., Blande, J. D., Julkunen-Tiitto, R., and Kivimäenpää, M. (2018). Climate change effects on secondary compounds of forest trees in the northern hemisphere. Front. Plant. Sci. 9:1445. doi: 10.3389/fpls.2018.01445
IPCC (2014). “Climate change 2014: synthesis report,” in Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds R. K. Pachauri and L. Meyer (Geneva: IPCC). Available at: https://epic.awi.de/id/eprint/37530/ (accessed July 31, 2019).
Jactel, H., Bauhus, J., Boberg, J., Bonal, D., Castagneyrol, B., Gardiner, B., et al. (2017). Tree diversity drives forest stand resistance to natural disturbances. Curr. Forest. Rep. 3, 223–243. doi: 10.1007/s40725-017-0064-1
Jactel, H., Birgersson, G., Andersson, S., and Schlyter, F. (2011). Non-host volatiles mediate associational resistance to the pine processionary moth. Oecologia 166, 703–711. doi: 10.1007/s00442-011-1918-z
Jactel, H., and Brockerhoff, E. G. (2007). Tree diversity reduces herbivory by forest insects. Ecol. Lett. 10, 835–848. doi: 10.1111/j.1461-0248.2007.01073.x
Jactel, H., Goulard, M., Menassieu, P., and Goujon, G. (2002). Habitat diversity in forest plantations reduces infestations of the pine stem borer Dioryctria sylvestrella. J. Appl. Ecol. 39, 618–628. doi: 10.1046/j.1365-2664.2002.00742.x
Jactel, H., Gritti, E. S., Drössler, L., Forrester, D. I., Mason, W. L., Morin, X., et al. (2018). Positive biodiversity–productivity relationships in forests: climate matters. Biol. Lett. 14:20170747. doi: 10.1098/rsbl.2017.0747
Jactel, H., Koricheva, J., and Castagneyrol, B. (2019). Responses of forest insect pests to climate change: not so simple. Curr. Opin. Insect Sci. 35, 103–108. doi: 10.1016/j.cois.2019.07.010
Jactel, H., Menassieu, P., and Raise, G. (1994). Infestation dynamics of Dioryctria sylvestrella (Ratz.) (Lepidoptera: Pyralidae) in pruned maritime pine (Pinus pinaster Ait.). Forest Ecol. Manage. 67, 11–22. doi: 10.1016/0378-1127(94)90003-5
Jactel, H., Menassieu, P., Raise, G., and Burban, C. (1996). Sensitivity of pruned maritime pine (Pinus pinaster Ait) to Dioryctria sylvestrella Ratz. (Lep., Pyralidae) in relation to tree vigour and date of pruning. J. Appl. Entomol. 120, 153–157. doi: 10.1111/j.1439-0418.1996.tb01583.x
Jactel, H., Petit, J., Desprez-Loustau, M.-L., Delzon, S., Piou, D., Battisti, A., et al. (2012). Drought effects on damage by forest insects and pathogens: a meta-analysis. Glob. Change Biol. 18, 267–276. doi: 10.1111/j.1365-2486.2011.02512.x
Kambach, S., Kühn, I., Castagneyrol, B., and Bruelheide, H. (2016). The impact of tree diversity on different aspects of insect herbivory along a global temperature gradient - a meta-analysis. PLoS ONE 11:e0165815. doi: 10.1371/journal.pone.0165815
Kleinhentz, M., Jactel, H., and Menassieu, P. (1999). Terpene attractant candidates of Dioryctria sylvestrella in maritime pine (Pinus pinaster) oleoresin, needles, liber, and headspace samples. J. Chem. Ecol. 25, 2741–2756. doi: 10.1023/A:1020803608406
Kleinhentz, M., Jactel, H., and Raffin, A. (1998). Genetic parameters and gain expected from direct selection for resistance to Dioryctria sylvestrella Ratz. (Lepidoptera: Pyralidae) in Pinus Pinaster Ait., using a full diallel mating design. Int. J. Forest Genet 5, 147–154.
Kuznetsova, A., Brockhoff, P. B., and Christensen, R. (2017). lmerTest pakcage: tests in linear mixed effects models. J. Stat. Softw. 13, 1–26. doi: 10.18637/jss.v082.i13
Lafage, D., Secondi, J., Georges, A., Bouzillé, J.-B., and Pétillon, J. (2014). Satellite-derived vegetation indices as surrogate of species richness and abundance of ground beetles in temperate floodplains. Insect Conserv. Divers. 7, 327–333. doi: 10.1111/icad.12056
Lehner, F., Coats, S., Stocker, T. F., Pendergrass, A. G., Sanderson, B. M., Raible, C. C., et al. (2017). Projected drought risk in 1.5°C and 2°C warmer climates. Geophys. Res. Lett. 44, 7419–7428. doi: 10.1002/2017GL074117
Liang, J., Crowther, T. W., Picard, N., Wiser, S., Zhou, M., Alberti, G., et al. (2016). Positive biodiversity-productivity relationship predominant in global forests. Science 354:aaf8957. doi: 10.1126/science.aaf8957
MacFarlane, D. W., and Luo, A. (2009). Quantifying tree and forest bark structure with a bark-fissure index. Can. J. For. Res. 39, 1859–1870. doi: 10.1139/X09-098
Martin, J. G., Kloeppel, B. D., Schaefer, T. L., Kimbler, D. L., and McNulty, S. G. (1998). Aboveground biomass and nitrogen allocation of ten deciduous southern Appalachian tree species. Can. J. For. Res. 28, 1648–1659. doi: 10.1139/x98-146
Mattson, W. J., and Haack, R. A. (1987). The role of drought in outbreaks of plant-eating insects. Bioscience 37, 110–118. doi: 10.2307/1310365
Merwin, A. C., Underwood, N., and Inouye, B. D. (2017). Increased consumer density reduces the strength of neighborhood effects in a model system. Ecology 98, 2904–2913. doi: 10.1002/ecy.2004
Moreira, X., Abdala-Roberts, L., Parra-Tabla, V., and Mooney, K. A. (2014). Positive effects of plant genotypic and species diversity on anti-herbivore defenses in a tropical tree species. PLoS ONE 9:e105438. doi: 10.1371/journal.pone.0105438
Moreira, X., Abdala-Roberts, L., Rasmann, S., Castagneyrol, B., and Mooney, K. A. (2016). Plant diversity effects on insect herbivores and their natural enemies: current thinking, recent findings, and future directions. Curr. Opin. Insect Sci. 14, 1–7. doi: 10.1016/j.cois.2015.10.003
Nakagawa, S., and Schielzeth, H. (2013). A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. doi: 10.1111/j.2041-210x.2012.00261.x
Nichols, J. D., Bristow, M., and Vanclay, J. K. (2006). Mixed-species plantations: prospects and challenges. Forest Ecol. Manage. 233, 383–390. doi: 10.1016/j.foreco.2006.07.018
Paquette, A., and Messier, C. (2011). The effect of biodiversity on tree productivity: from temperate to boreal forests. Glob. Ecol. Biogeogr. 20, 170–180. doi: 10.1111/j.1466-8238.2010.00592.x
Pureswaran, D. S., Roques, A., and Battisti, A. (2018). Forest insects and climate change. Curr. Forest. Rep. 4, 35–50. doi: 10.1007/s40725-018-0075-6
R Core Development Team (2013). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: http://www.R-project.org/
Ratcliffe, S., Wirth, C., Jucker, T., van der Plas, F., Scherer-Lorenzen, M., Verheyen, K., et al. (2017). Biodiversity and ecosystem functioning relations in European forests depend on environmental context. Ecol. Lett. 20, 1414–1426. doi: 10.1111/ele.12849
Régolini, M., Castagneyrol, B., Dulaurent-Mercadal, A.-M., Piou, D., Samalens, J.-C., and Jactel, H. (2014). Effect of host tree density and apparency on the probability of attack by the pine processionary moth. Forest Ecol. Manage. 334, 185–192. doi: 10.1016/j.foreco.2014.08.038
Riihimäki, J., Kaitaniemi, P., Koricheva, J., and Vehviläinen, H. (2005). Testing the enemy hypothesis in forest stands: the important role of tree species composition. Oecologia 142, 90–97. doi: 10.1007/s00442-004-1696-y
Roberts, M. R., and Paul, N. D. (2006). Seduced by the dark side: integrating molecular and ecological perspectives on the influence of light on plant defence against pests and pathogens. N. Phytol. 170, 677–699. doi: 10.1111/j.1469-8137.2006.01707.x
Schielzeth, H. (2010). Simple means to improve the interpretability of regression coefficients: interpretation of regression coefficients. Methods Ecol. Evol. 1, 103–113. doi: 10.1111/j.2041-210X.2010.00012.x
Schielzeth, H., and Nakagawa, S. (2013). Nested by design: model fitting and interpretation in a mixed model era. Methods Ecol. Evol. 4, 14–24. doi: 10.1111/j.2041-210x.2012.00251.x
Sholes, O. D. (2008). Effects of associational resistance and host density on woodland insect herbivores. J. Anim. Ecol. 77, 16–23. doi: 10.1111/j.1365-2656.2007.01317.x
Speight, M., and Wainhouse, D. (1989). Ecology and Management of Forest Insects. Oxford: Oxford University Press.
Stout, M. J. (2013). Reevaluating the conceptual framework for applied research on host-plant resistance. Insect Sci. 20, 263–272. doi: 10.1111/1744-7917.12011
Tahvanainen, J. O., and Root, R. B. (1972). The influence of vegetational diversity on the population ecology of a specialized herbivore, Phyllotreta cruciferae (Coleoptera: Chrysomelidae). Oecologia 10, 321–346. doi: 10.1007/BF00345736
Taubert, F., Fischer, R., Groeneveld, J., Lehmann, S., Müller, M. S., Rödig, E., et al. (2018). Global patterns of tropical forest fragmentation. Nature 554, 519. doi: 10.1038/nature25508
Van de Peer, T., Verheyen, K., Baeten, L., Ponette, Q., and Muys, B. (2016). Biodiversity as insurance for sapling survival in experimental tree plantations. J. Appl. Ecol. 53, 1777–1786. doi: 10.1111/1365-2664.12721
Verdone, M., and Seidl, A. (2017). Time, space, place, and the Bonn Challenge global forest restoration target. Restorat. Ecol. 25, 903–911. doi: 10.1111/rec.12512
Walter, J., Hein, R., Auge, H., Beierkuhnlein, C., Löffler, S., Reifenrath, K., et al. (2012). How do extreme drought and plant community composition affect host plant metabolites and herbivore performance? Arthropod Plant Interact. 6, 15–25. doi: 10.1007/s11829-011-9157-0
Zhang, Q.-H., and Schlyter, F. (2004). Olfactory recognition and behavioural avoidance of angiosperm nonhost volatiles by conifer-inhabiting bark beetles. Agric. Forest Entomol. 6, 1–20. doi: 10.1111/j.1461-9555.2004.00202.x
Zhang, Q. H., Birgersson, G., Zhu, J., Löfstedt, C., Löfqvist, J., and Schlyter, F. (1999). Leaf volatiles from nonhost deciduous trees: variation by tree species, season and temperature, and electrophysiological activity in Ips typographus. J. Chem. Ecol. 25, 1923–1943. doi: 10.1023/A:1020994119019
Keywords: biodiversity, associational resistance, water stress, Dioryctria, stem borer, pine, climate change
Citation: Jactel H, Poeydebat C, van Halder I and Castagneyrol B (2019) Interactive Effects of Tree Mixing and Drought on a Primary Forest Pest. Front. For. Glob. Change 2:77. doi: 10.3389/ffgc.2019.00077
Received: 01 August 2019; Accepted: 01 November 2019;
Published: 21 November 2019.
Edited by:
Paal Krokene, Norwegian Institute of Bioeconomy Research (NIBIO), NorwayReviewed by:
Fredrik Schlyter, Czech University of Life Sciences Prague, CzechiaBrian Aukema, University of Minnesota Twin Cities, United States
Copyright © 2019 Jactel, Poeydebat, van Halder and Castagneyrol. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hervé Jactel, herve.jactel@inra.fr
 Hervé Jactel
Hervé Jactel Charlotte Poeydebat
Charlotte Poeydebat Inge van Halder
Inge van Halder Bastien Castagneyrol
Bastien Castagneyrol