Intraspecific Variation in Responses of a Montane Grass, Festuca thurberi, to Simulated Biological Invasion
- 1Department of Environmental Conservation, University of Massachusetts, Amherst, MA, United States
- 2Rocky Mountain Biological Laboratory, Crested Butte, CO, United States
High elevation plant populations, such as those found in sub-alpine meadows, are at the forefront of climate change and likely to experience novel interactions with migrating plants from lower elevations, including non-native species. Some of these non-native plants, particularly members of the Brassicaceae, produce secondary metabolites that have been shown to inhibit root fungi in other ecosystems. We conducted a growth experiment with plant leachates in order to evaluate the degree to which the dominant high elevation grass species, Festuca thurberi would be affected by future novel interactions with the non-native mustard, Thlaspi arvense, relative to a native mustard (Noccaea fendleri). We assessed growth, chlorophyll content, biomass, mortality, and percent colonization of arbuscular mycorrhizal fungi (AMF) and dark septate endophytes (DSE) in different genotypes of F. thurberi exposed to leachates from native and non-native mustards as well as F. thurberi leachate and a deionized (DI) water control. New growth and mortality varied more by genotype than by treatment with leachate of F. thurberi, T. arvense, and N. fendleri. Treatment, genotype, and the treatment × genotype interaction all had significant effects on chlorophyll content, with N. fendleri treatments demonstrating higher relative greenness levels than control treatments. Percent of fine roots with dark septate endophytes was significantly affected by individual genotype and treatment × genotype interaction, but there were no effects of treatment, genotype, or their interaction on percent root colonization by arbuscules or vesicles. Overall, we show that performance of a dominant high-altitude grass species varies in its response to the presence of an expanding, non-native plant, which may become increasingly common due to climate change.
Introduction
Plant invasions have historically been less common in high elevation ecosystems relative to low elevation ecosystems, due to the harsh climate and low human population densities (Pauchard et al., 2009). However, over the next 100 years, temperatures in the Rocky Mountains may increase by as much as 5.5°C (Funk et al., 2014). Several studies tracking plant range over elevation have found upward shifts in range over time across North America (Kelly and Goulden, 2008; Kopp and Cleland, 2014; Savage and Vellend, 2015), with species’ mean elevation shifting upwards in our study area in the Gunnison basin of the Rocky Mountains, CO., United States (Zorio et al., 2016). Using elevation-for-time substitutions, Wasserman et al. (2012) found a consistent relationship between increases in temperature and change in elevation. Longer stretches of time between early summer snow melt and summer monsoons may result in extended periods of drought-like conditions (Sloat et al., 2015). In addition, using data from direct manipulation studies some researchers have theorized that plant communities will continue to shift under future climate conditions (Harte et al., 2015). The combined effects of shifts in temperature, snowmelt, and precipitation are likely to impact plant community composition in the Rocky Mountains. Given these trends in plant range expansion over elevation, research must incorporate the effects of lowland invasive and introduced plants on high-elevation naïve ecosystem archetypes to determine what effects, if any, these novel interactions are likely to produce.
Festuca thurberi is a clonal dominant perennial, high-elevation grass typically found in dense stands in sub-alpine meadows (Moir, 1967). In the southwestern United States, it is endemic across the Rocky Mountain range from Wyoming to New Mexico (Smith, 2019). Festuca thurberi stands consist of dispersed individual ramets as well as many clumps, each consisting of multiple individual clonal ramets. In experiments investigating the potential effects of climate change on high-elevation communities, F. thurberi flowering time showed an extended duration of flowering in response to warmer soil, decreased soil degree-days, and later snowmelt date in contrast to all other plants studied (Dunne et al., 2003). Thus, in future climate change scenarios, F. thurberi may become an even more dominant component of the sub-alpine meadow landscape. Festuca plants are also important reservoirs of fungal diversity. A previous survey of high elevation foliar fungal associates (Kivlin et al., 2019) found that the highest number of indicator species belong to F. thurberi including Clonostachys rosea, a potential beneficial endophyte, as well as Aspergillus niger and Phaeosphaeria caricis, a generalist and saprotroph, respectively. Festuca species are typically considered to be highly colonized by arbuscular mycorrhizal fungi (AMF) compared to co-occurring plant species in the Gunnison Basin (Molina et al., 1978). Intraspecific variation in plant-microbe interactions (Rudgers et al., 2020) and in plant responses to global change factors, including plant invasions, is increasingly well documented (e.g., Gibson et al., 2014) but has not been extensively studied in F. thurberi.
Members of the Brassicaceae (mustard family) are known to produce a variety of secondary metabolites that protect against disease and herbivory and may also facilitate invasion (Ahuja et al., 2010; Schranz et al., 2011; Brolsma, 2014). Thlaspi arvense is a Eurasian weed in the mustard family that has become widely distributed in the United States and Canada and is known to readily establish on disturbed soils (Best and McIntyre, 1975; Warwick et al., 2002). Thlaspi arvense produces the alkenyl-glucosinolate, sinigrin (Tolra et al., 2006), the same secondary metabolite produced by the highly invasive, close relative Alliaria petiolata. Sinigrin has been demonstrated to have allelopathic effects (Vaughn and Berhow, 1999) and to suppress native plant growth and their mycorrhizal fungi in deciduous forest ecosystems of North America, but not in A. petiolata’s European home range (Vierheilig and Ocampo, 1990; Schreiner and Koide, 1993). Another close relative to T. arvense, Noccaea fendleri, is native to sub-alpine meadows in the Gunnison Basin and widely distributed across the western US (Reeves et al., 1983). Contrary to T. arvense, N. fendleri predominantly produces isopropyl glucosinolate (Chew, 1979), occurs at low densities across its native range, and has co-existed through evolutionary time with our study species F. thurberi. Due to the recent encroachment of T. arvense from lower to higher elevations in the Gunnison Valley and the ecological dominance of F. thurberi at higher elevations, we aimed to quantify the results of this novel interaction. We hypothesized that secondary metabolites produced by T. arvense would: (1) reduce new growth; (2) increase mortality; (3) reduce chlorophyll content; (4) reduce plant biomass and; (5) reduce root colonization by mycorrhizal fungi of F. thurberi in a potting study. We also tested the hypothesis that (6) there would be intraspecific variation in the impacts of. T. arvense on the growth, survival, and fungal symbioses of F. thurberi.
Materials and Methods
Experimental Design
Festuca thurberi plants were grown in a weatherport on site at the Rocky Mountain Biological Laboratory (N 38.95807°, W 106.98853°; 2,889 m). The experiment tested the effects of leachate from an introduced member of the Brassicaceae (Thlaspi arvense), a native member of the Brassicaceae that shares habitat with our study species (Noccaea fendleri), a positive control with leachate from additional Festuca thurberi, and a negative control treatment [deionized (DI) water] on plant growth and mortality of F. thurberi over time. We included the co-occurring native mustard N. fendleri in this experiment to ensure that any treatment effect observed by T. arvense was due to the novel interaction with an introduced mustard and not simply the effect of phytochemical interactions with any member of the Brassicaceae.
Experimental plants were collected as individual ramets from eight distinct clumps of F. thurberi and an arbitrary selection of dispersed individuals at the beginning of the growing season on 6/24/2018 from a sub-alpine meadow (∼3,826 m; Supplementary Table 1). This collection site was selected due to the absence of the introduced mustard T. arvense and the presence of N. fendleri. For the purposes of this study, we assumed that ramets from the same clump were replicates of the same genotype, given the clonal growth form of the species. We assigned a separate category (D) to replicates of dispersed ramets growing individually in the same meadow. Following soaking, each ramet was manually sorted and separated from the larger root mass. Ramets, composed of an individual shoot and root mass, were harvested from each individual clump (genotype) and yielded between 18 and 44 clones. Ramets were then cleaned, air-dried, weighed and planted into 38-cell propagation trays (Greenhouse Megastore; item CN-PRT 38-10) within 24-h. Ramets of each clone were planted in peat moss mixture, augmented with field soil collected from beneath mature F. thurberi individuals at a 6:1 ratio, in order to expose clones to microbial communities associated with F. thurberi. One ramet of F. thurberi (N = 276; Supplementary Table 1) was grown in each cell of 10 38-cell propagation trays in a randomized experimental array. Experimental plants were watered with tap water for 7 days and allowed to settle in propagation sheets to minimize transplant stress. Each plant was then randomly assigned a treatment (leacheate of T. arvense, N. fendleri, F. thurberi, or a water control) with approximately 70 Festuca plants in each treatment (Supplementary Table 2).
Treatment plants (T. arvense, N. fendleri, and F. thurberi) were also collected at the beginning of the experiment, with three replicates of three plants per pot transplanted into 5 L pots for each treatment. Treatment plants were collected from a similar elevation to the field station (∼2,889 m), with the exception of F. thurberi for which treatment plants were collected from the same site as experimental plants (∼3,826 m). Leachate was collected separately for each replicate of each species, following design by Hagan et al. (2013) by running 1.3 L of DI water through a pot until roughly 1 L of leachate had been collected. In order to determine if root exudates are responsible for any treatment effect, as has been established in A. petiolata (Stinson et al., 2006), we used leachate rather than entire plants. Each of the three, 5 L treatment plant pots, was used to extract leachate successively to water the experimental plants.
Experimental plants were watered with treatment leachate or control DI until liquid started to pool at the surface of individual cells every other day or every third day depending on how long plants took to dry out. Plants were harvested over the course of 5 days, 51 days after transplant into the experimental array. Root sub-samples (5 fine roots) were collected from a subset of experimental plants (n = 106) and stored in 70% ethanol and whole individual plants were stored in brown paper bags until processing for biomass at the University of Massachusetts, Amherst.
Data Collection
New growth was assessed on every experimental plant in the array every 10 days starting 10 days after the first leachate treatment was applied (7/10/2018, 7/20/2018, and 7/30/2018) and marked as either new growth (i.e., new sprouts from the base of the plant) present (1) or absent (0). Similarly, mortality was evaluated twice, 15 and 30 days after transplant (7/15/2018 and 7/30/2018) and marked as either above ground green biomass absent (1) or present (0). Relative measure of greenness linked to chlorophyll content was measured using Spad technology (Spad 502; Minolta) every 2 days from 7/10/2018–7/20/2018. Only plants that produced new growth since the start of treatment application as assessed on 7/10/2018 were evaluated for relative greenness. Once harvested, plants were separated into above ground biomass (shoots and blades) and belowground biomass (roots).
Colonization by arbuscules, vesicles, and dark septate endophytes (DSE) were quantified in fine roots using staining followed protocols established in Ranelli et al. (2015). Samples were rinsed in tap water to remove ethanol and then partitioned into tissue cassettes for clearing and staining. Cassettes were cleared in a 10% KOH solution for 5–7 days, with variations of up to 2 days in clearing time due to individual thickness of roots. Potassium hydroxide was rinsed from cassettes and transferred to acidified water. Roots were then stained with a 5% Schaffer ink/white vinegar solution for 15–20 min and then de-stained in 10% KOH for several days. Quantification was performed using the magnified intersection method at 450 × magnification, spanning the upper and lower limit of each root piece in order to satisfy the requirements of the method (McGonigle et al., 1990).
Statistical Analysis
All analyses were conducted in R version 4.0.2. (R Core Team, 2020) with the significant p-value set to below or equal to 0.05.
To confirm that plants were effectively randomized across the treatments we ran an ANOVA with treatment as the main effect and fresh biomass measured prior to transplant into the experimental array as the response variable.
Because we were interested in the role of intraspecific variation in plant responses to invasion, we adapted a standard quantitative genetics modeling approach (Falconer, 1960) to assess the relative contributions of the leachate treatments (environment) and intraspecific variation among F. thurberi clones (genotype). Our models included main effects of leachate, genotype, and the leachate × genotype interaction. We use “genotype” in this study in the broadest sense, in that ramets within individual clumps of the clonal study species, F. thurberi are assumed to be genetic replicates of one another and that clonal clumps separated by several meters may be genetically distinct. For the purposes of this study, we interpret a leachate effect as evidence of allelopathic effects of native and non-native mustard plants on F. thurberi phenotypic traits (i.e., differences in the mean response of F. thurberi plants to the leachate treatments), a genotype effect as a test for evidence of intraspecific variation in F. thurberi phenotypic responses to the leachate treatment (visualized as the mean response of the genotypes in the figures), and a significant GXE interaction as evidence for variance among genotypes in the direction of the phenotypic response to the treatments (visualized as lines that intersect in the figures).
To test our hypotheses that T. arvense leachate would affect growth through time, we constructed binomial regression models with treatment, genotype, and a treatment × genotype interaction term as the main effects and observations of new growth [0 (absent) or 1 (present)] as the response variable. We included experiment days (i.e., days since experiment began) as a covariate to account for changes in growth over time. We also constructed linear models using treatment, genotype, and a treatment × genotype interaction as the main effects with mean growth as the response variable. For mortality, we used a binomial regression with the same predictive variables and mortality (live or dead) as the response variable.
To determine if T. arvense leachate reduced general greenness linked to chlorophyll content we constructed linear models using experiment days, treatment, genotype, and a treatment × genotype interaction term as predictive variables and Spad reading as the response variable. We also constructed linear models using treatment, genotype, and a treatment × genotype interaction as the main effects with Spad reading as the response variable.
To determine if T. arvense leachate affected F. thurberi growth we constructed linear models using treatment, genotype, and a treatment × genotype interaction for the predictive variable related to final size (response variable): whole plant dry biomass, above ground biomass, belowground biomass, and root:shoot ratio. Dry biomass was normalized through log transformation. A constant was added to all values prior to log transformation to correct for negative values resulting from the transformation.
To determine the effects of T. arvense leachate on fungal root colonization we constructed a linear model with genotype, treatment, and a treatment × genotype interaction as the predictive variable and colonization of fine roots by arbuscules, vesicles, and DSE as the response variable. These response variables were normalized through an arcsin transformation. Dark septate endophytes were analyzed with the same effects in the model but with a Poisson distribution.
To visualize effects of the environmental treatments and the potential for underlying genetic variation in each response variable, we plotted reaction norms (Falconer, 1960; Via and Lande, 1985) of the mean trait value for each genotype.
Results
Fresh weight biomass was not significantly affected by treatment (p = 0.652), indicating that we sufficiently randomized the subject plants at the onset of the study.
New growth was significantly affected by individual genotype and experiment days (p = 0.015, p < 0.001, respectively) in the binomial regressions and mean growth was significantly affected by genotype in the general linear model (Figure 1 and Table 1). There were also significant effects of genotype and the genotype × treatment interaction on mortality (Figure 2 and Table 1). Relative greenness was significantly affected by experiment days, genotype, and treatment (p = 0.010, p < 0.001, p < 0.001, respectively; Figure 3). Effects of genotype, treatment and the genotype × treatment interaction were also present in the general linear model (Table 1). Dry weight biomass and root:shoot biomass ratio were significantly affected by genotype (Figures 4A,B and Table 1).
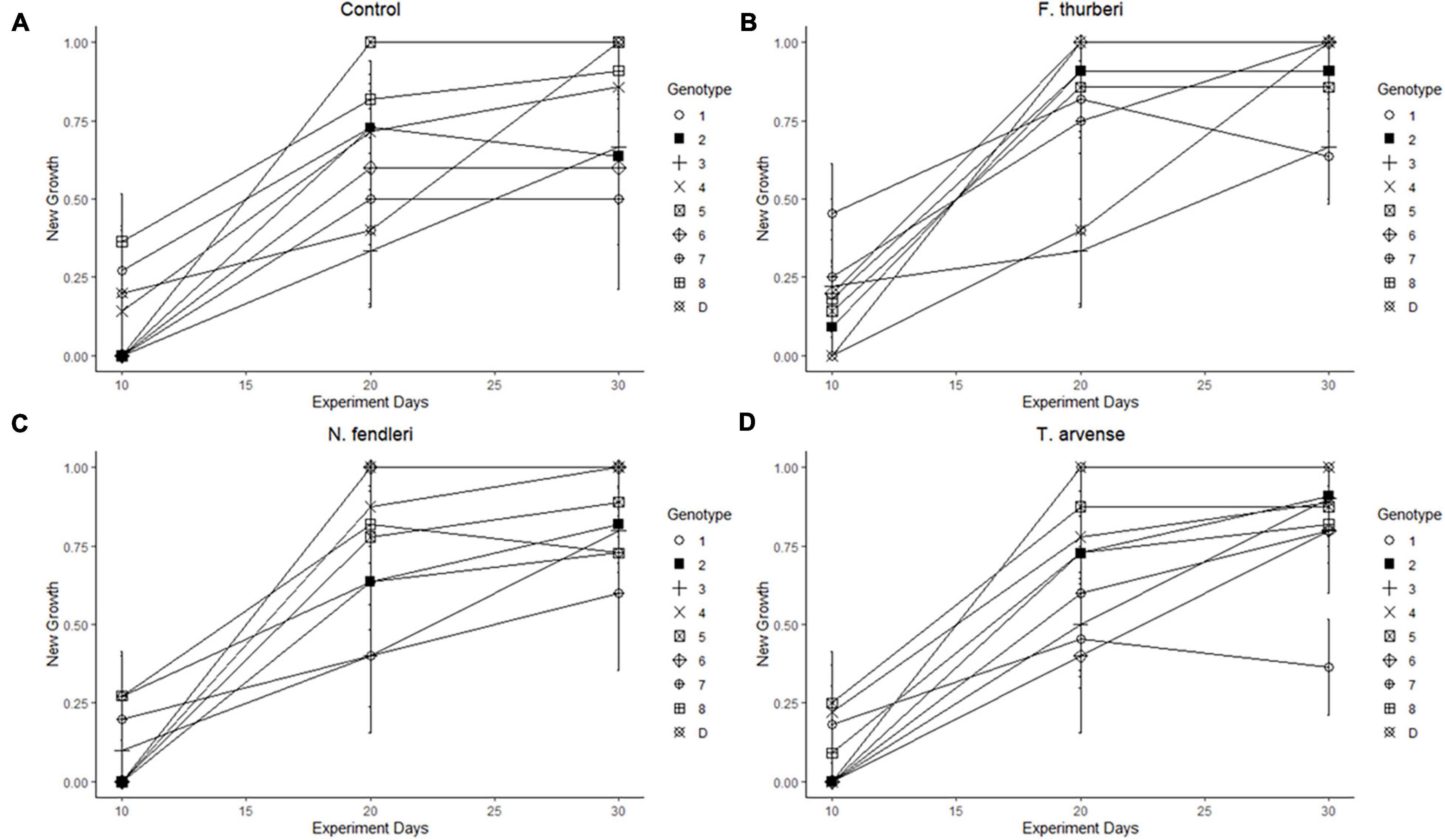
Figure 1. Reaction norms are presented from new growth of Festuca thurberi genotypes separated out by treatment: (A) control, (B) F. thurberi, (C) N. fendleri, and (D) T. arvense; to show differential effect of treatment on genotype over time. Data points represent average number of plants in each genotype with new growth present (1) or absent (0) during three points during the experiment 10 days apart. Error bars represent standard error.
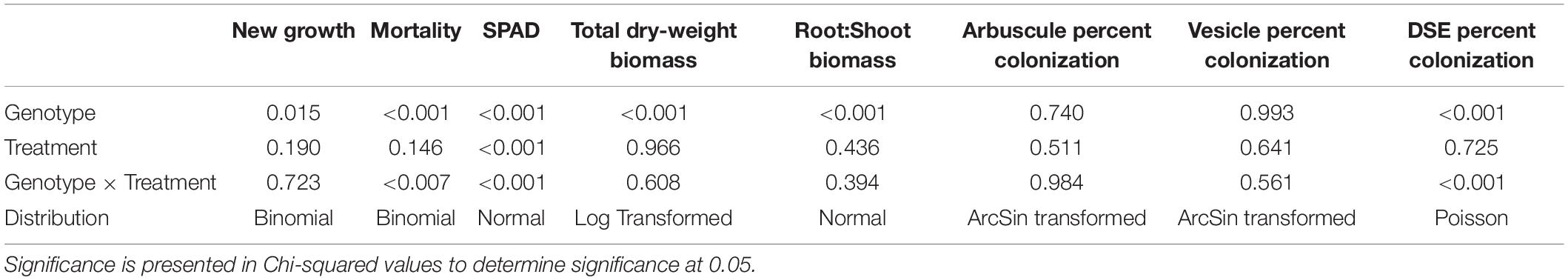
Table 1. Results of generalized linear models are presented alongside distributions for each of the response variables (top row) and main effects (first column).
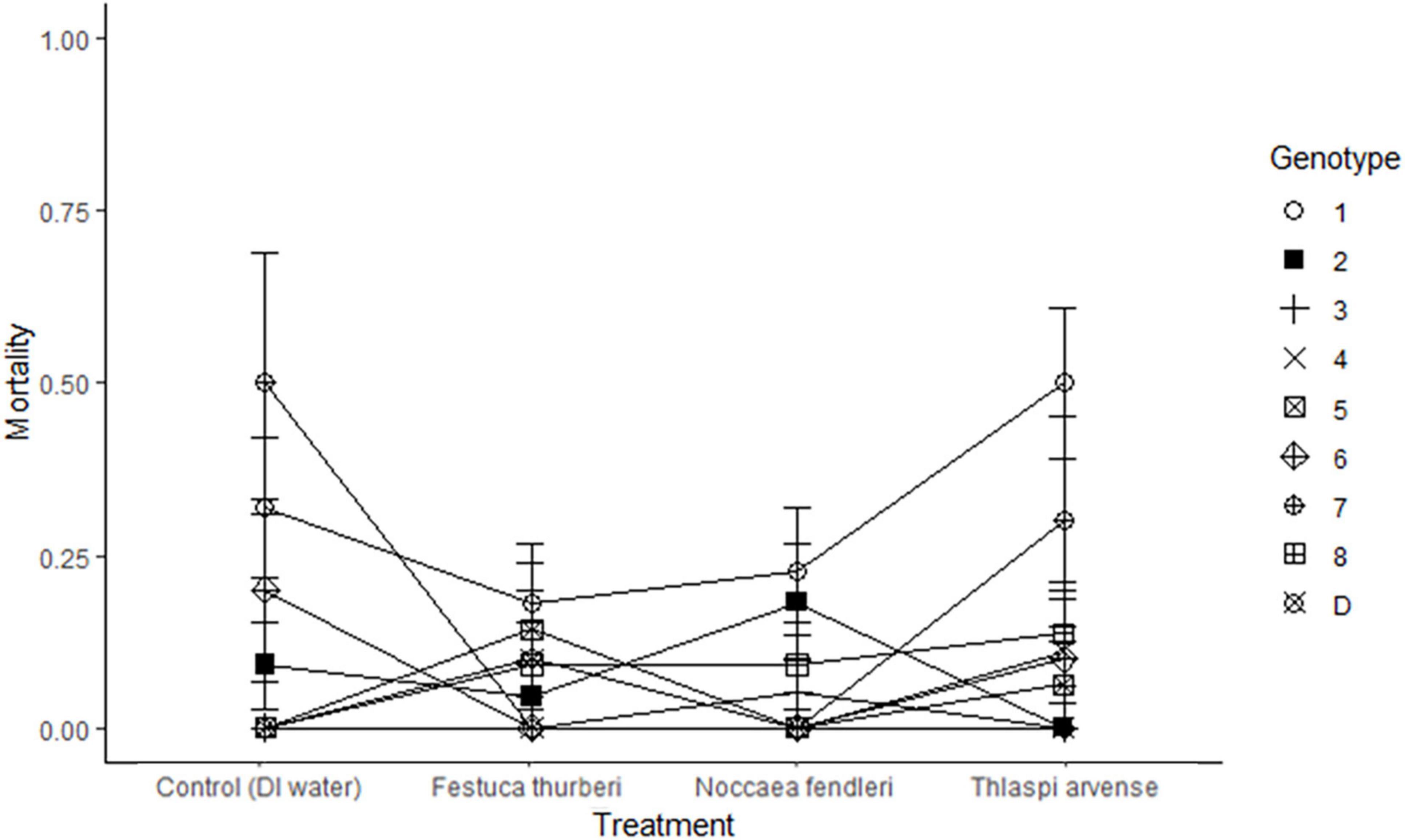
Figure 2. Rate of mortality in Festuca thurberi is shown in a reaction norm averaged across treatment. Mortality was inferred from either: no above ground green plant biomass (1); or plant surviving (0). Average mortality lines crossing indicate a different response, as measured by mortality, by a genotypes between treatments. Error bars represent standard error.
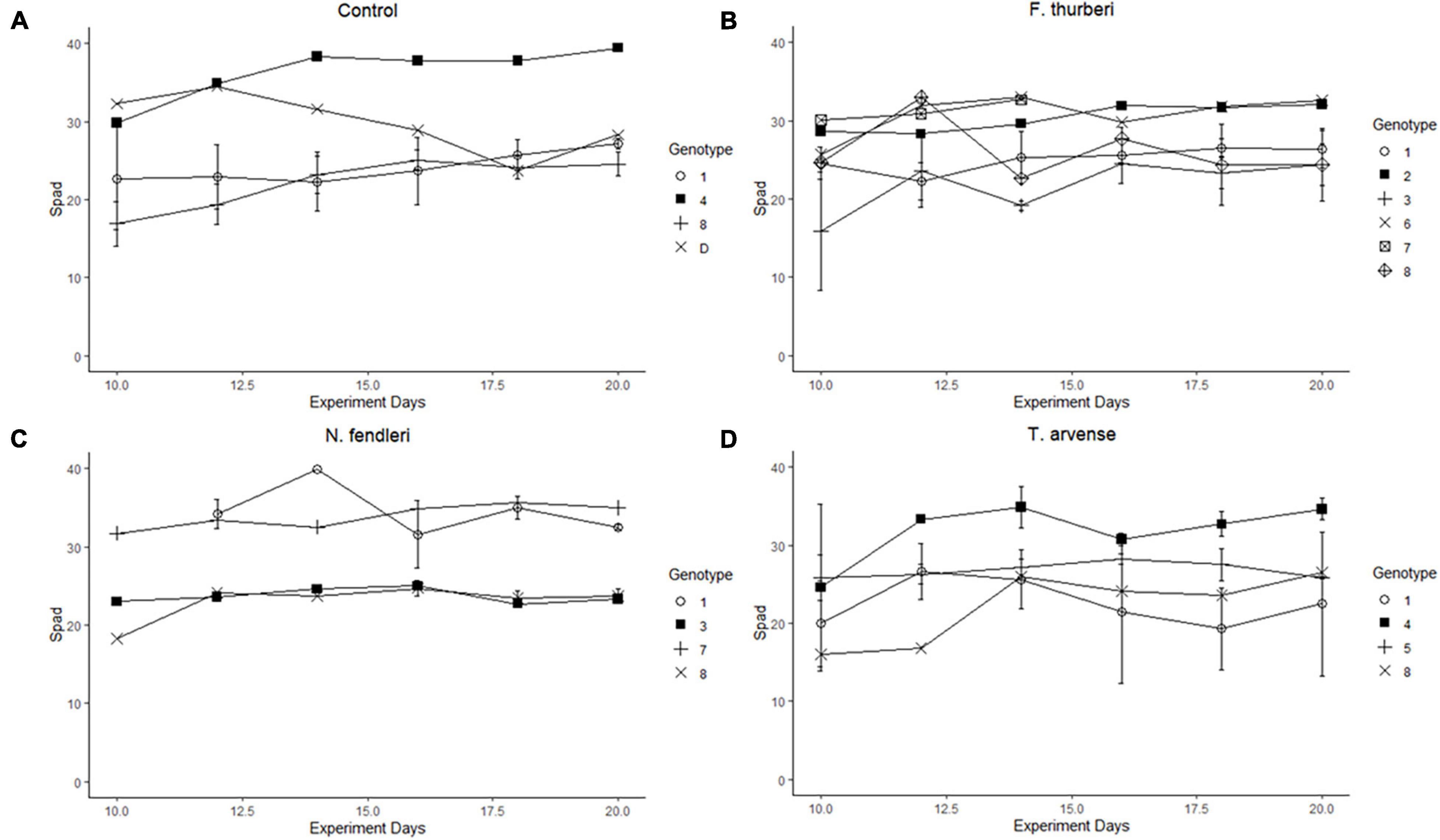
Figure 3. Reaction norms were constructed from relative greenness surveys (Spad) of Festuca thurberi individuals. Treatments: (A) control, (B) F. thurberi, (C) N. fendleri, (D) T. arvense; were separated to show differential effect of treatment on genotype over time. Relative greenness, linked to chlorophyll content, was measured 6 times between the 10th and 20th day of treatment with only plants displaying new growth being measured. Error bars represent standard error.
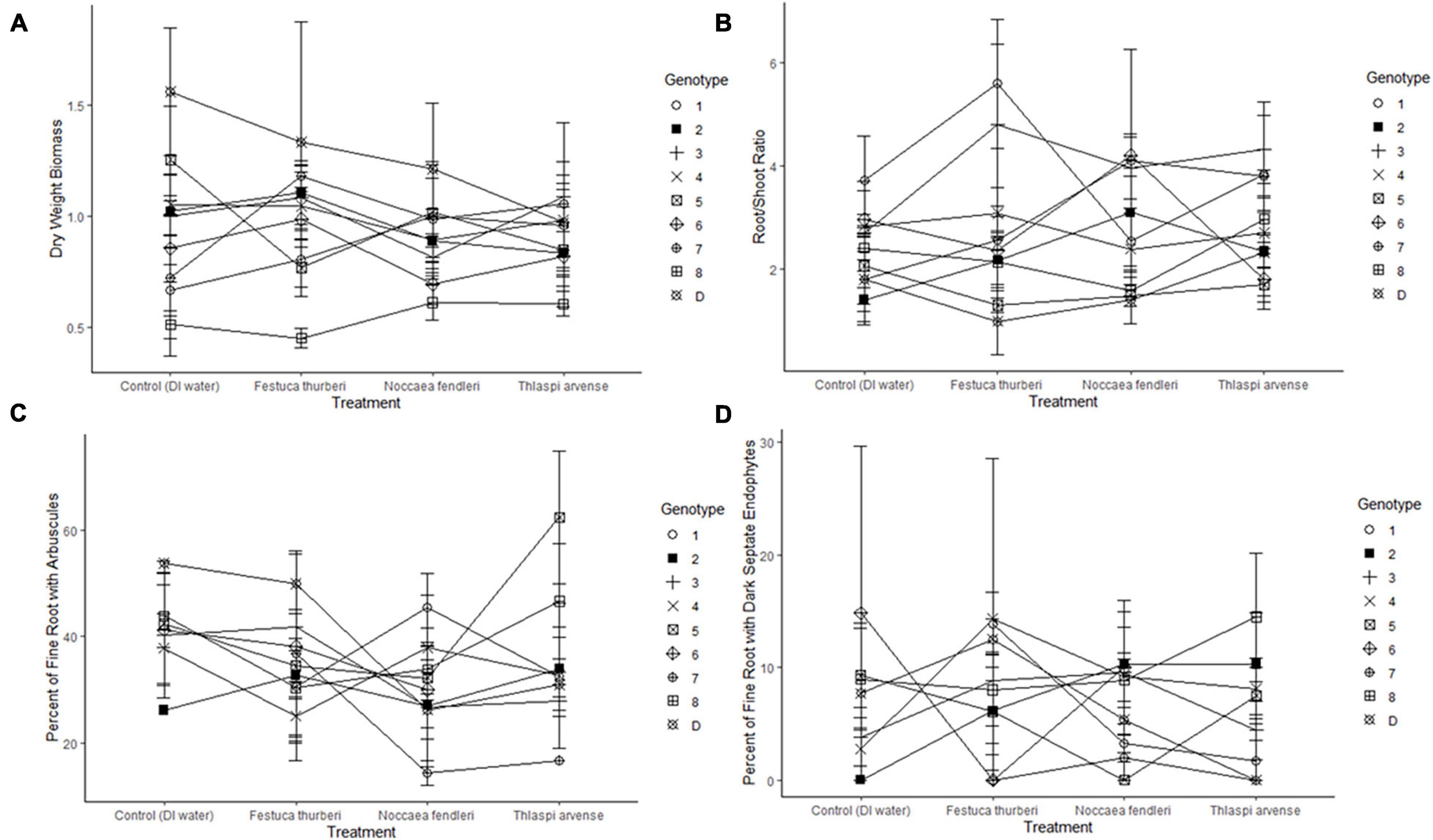
Figure 4. Reaction norms, constructed by treatment for each genotype across (A) dry weight biomass, (B) root:shoot ratio, (C) percent of fine root with arbuscules present, (D) percent of fine root with dark septate endophytes. Average value lines crossing indicate a different response by a genotypes between treatments. Error bars represent standard error.
Treatment, genotype, and a treatment by genotype interaction did not have a significant effect on percent of fine root with arbuscules present (Figure 4C). No significant effects were present for percent of fine roots with arbuscules or vesicles present (Figure 4C and Table 1). Treatment and treatment × genotype interaction had significant effects on percent of fine root with DSE present (Figure 4D and Table 1).
Discussion
The main goal of our study was to test the hypothesis that secondary metabolites in leachate of the non-native plant, Thlaspi arvense, affects the growth, mortality, and root colonization by fungal associates of the sub-alpine grass Festuca thurberi. given that T. arvense’s close relative, A. petiolata is known to alter mycorrhizal association and growth of North American tree seedlings (Stinson et al., 2006; Strauss et al., 2006; Wolfe et al., 2008). We also tested for potential intraspecific variation in F. thurberi responses to T. arvense, given evidence in the literature that other species demonstrate variation within populations in their responses to the presence of invasives (e.g., Strauss et al., 2006). To differentiate potential effects of the introduced mustard T. arvense from those of co-occurring native mustard species, we also included leachates of the native member of the Brassicaceae, N. fendleri. We found evidence for leachate effects on relative greenness and genotype × treatment effects on mortality, greenness, and DSE colonization. There was also evidence for genotype effects on most of the observed traits. We thus conclude that there is intraspecific variation in the responses of F. thurberi to T. arvense as well as the native mustard species. Overall, our study suggests that invasion by the non-native T. arvense may have patchy effects on local populations of this native grass species.
Examining the Effects of Intraspecific Variation and Simulated Invasion on Festuca thurberi
Effects of biological invasion on the recruitment and mortality of native plant species are likely to affect the future composition of vegetation communities around the world (Alvarez and Cushman, 2002). Recruitment in clonal plants such as F. thurberi is linked to new growth of ramets (Shumway, 1995). Here we found that new growth of individual ramets varied across genotypes regardless of exposure to leachates from native and non-native mustards or to the control treatments. Several genotypes lagged in new growth early after experiment establishment but had new growth present 20 or 30 days into the experiment. We also noted an interaction between genotype and time, as indicated by the intersecting trendlines in Figure 1. Such plastic responses are likely to play an important role in the persistence of plant populations as global change introduces novel pressures, including the incursion of non-native plants (Frei et al., 2014). This variation in recruitment may be amplified in natural settings due to the natural fragmentation of high elevation populations, similar to island habitats (Burkey, 1995). Mortality in F. thurberi was similarly affected by individual genotypes with some genotypes having little to no mortality and others experiencing higher levels of mortality across the different leachate treatments (1.3–30.7%; Figure 2). The interaction effect of genotype and leachate treatment on mortality further suggests genotypes within a population of F. thurberi may vary widely in their ability to survive in the presence of leachate from mustard species.
Contrary to our hypotheses, simulated invasion by T. arvense with leachate application resulted in no significant effects on mean phenotypic values for new growth, biomass, or percent colonization with arbuscular mycorrhizal fungi of F. thurberi, and the treatment effect on relative greenness was attributable to the native mustard, N. fendleri as discussed below. The lack of an effect of treatment on these traits was surprising, given that leachates from T. arvense’s close relative, the invasive plant Alliaria petiolata, were shown to prevent germination of AMF, inhibit AMF symbioses, and reduce root lengths in native North American tree seedlings (Roberts and Anderson, 2001). Moreover, lower levels of AMF colonization in three native tree species were observed in soils invaded by A. petiolata (Stinson et al., 2006). Growth of AMF in pure culture was also completely inhibited by application of an isothiocyanate associated with sinigrin, one of the main glucosinolates found in both T. arvense and A. petiolata (Wolfe et al., 2008). Despite the lack of effects of T. arvense on F. thurberi in this experiment, further study may be warranted to determine if future interactions between T. arvense and endemic plants and soil microorganisms will result in more significant disruption to normal ecosystem function. The fact that we found evidence for genotype effects in many F. thurberi traits and responses to our experimental treatments underscores the importance of understanding intraspecific variation in plant species’ responses to biological invasion and other global change factors (Gibson et al., 2014; Johnson et al., 2015). In addition to recruitment, mortality, and relative greenness, dry-weight biomass allocation to roots and shoots had a high degree of intraspecific variability (Figures 4A,B). Although heterogeneity of the environment has been shown to be an important component of invasive plant dynamics (Pyšek et al., 2012), genetic diversity of endemic populations, as well as species richness, is also an important variable in evaluating the effects associated with novel interactions in a changing climate.
AMF colonization did not vary significantly between genotypes or respond to synthetic invasion by T. arvense. Dark septate endophytes were found less frequently than either arbuscules or vesicles, but percent of fine roots with DSE present varied in distribution across genotypes and showed a genotype × treatment interaction (Figure 4C). Dark septate endophytes are an enigmatic group of fungi that may play ecological roles similar to mycorrhizal fungi. Additionally, DSE have been shown to be more abundant in stressful environments (Mandyam and Jumpponen, 2005). Mycorrhizal association is generally understood to be a mutualistic symbiosis where plant C is exchanged soil P, N, and water (Smith and Read, 2008), although direct mineral cycling has recently been questioned and further and tight coupling of AMF and decomposers may play a more important role than historically understood (Bunn et al., 2019). Abundance in our experiment, as indicated by the percentage of fine roots with arbuscules present, also varied significant across treatment when controlling for genotype (Figure 4D). Arbuscules are specialized interfaces across which nutrients are exchanged between a plant and fungal partner (Smith and Read, 2008), and therefore critical to understanding how F. thurberi may respond to novel interactions. Future research on high elevation populations of F. thurberi should take into account questions regarding the consistency of mycorrhizal response to non-native plants across the spectrum of life histories as well as whether these responses will be consistent under future climate change scenarios.
Comparison of the Native, High-Elevation Mustard to the Introduced, Low-Elevation Mustard
Interestingly, we found a significant effect of N. fendleri leachate, but not T. arvense leachate on relative greenness (Figure 3). Treatment with leachate from N. fendleri resulted in elevated relative greenness that was not significantly different from T. arvense but was significantly different from control and F. thurberi treatments. While N. fendleri is a native mustard present at the elevation of the source F. thurberi, T. arvense is a non-native mustard that has become cosmopolitan at lower elevations. We chose to include N. fendleri in this study in order to compare the effects of a locally native Brassicaceae species to that of the encroaching non-native, the latter of which contains phytochemicals known to relax constraints on arbuscular mycorrhizal fungal phylogenetic diversity (Trautwig et al., 2021).
Mycorrhizal fungi have been shown to interact with plant hosts along a mutualism/parasitism continuum (Johnson et al., 1997). No significant effects were found in our study between final dry-weight biomass, root:shoot ratio, or percent of fine root colonization with arbuscules by treatment. However, a significant interaction between genotype and treatment on mortality, general greenness linked to chlorophyll content, and percent of fine root colonized by DSE indicated that evidence for variance among genotypes in the direction of the phenotypic response to the treatments is present (Figures 2, 3, 4D and Table 1). Further research could explore whether both members of the Brassicaceae filter microorganisms (including carbon hungry AMF whose abundance inversely correlates with plant biomass; Lynn et al., 2019) out of a community. In addition, novel microbes associated with T. arvense at lower elevations may disrupt previously existing dynamics in high elevation meadows (Trautwig et al., 2021). A co-culturing experiment that examines prolonged plant-plant and plant-microbe interactions may add additional context to our experiment.
One reason for the interactions we observed may have been that novel microorganism communities associated with T. arvense may be more adapted to disturbance than microorganisms associated with N. fendleri and therefore recovered more quickly following transplanting. Arbuscular mycorrhizal communities have been shown to be highly influenced by mechanical disturbance (Schnoor et al., 2010) and the presence of T. arvense may have selected for more disturbance-tolerant species.
Conclusion
We found evidence that both native and non-native mustard species can impact the biology and performance of a dominant native grass in high altitude ecosystems, where the impacts of global change are ongoing but biological invasions have thus far been less common than in lowland ecosystems. We tested whether allelopathy from a non-native mustard, T. arvense, differentially impacts clones of the native grass, Festuca thurberi. Our experiment demonstrated intraspecific variation in F. thurberi’s responses to simulated invasion with leachate, with evidence for variation among clones in the effects of T. arvense leachate on mortality, relative greenness, and percent colonization of roots by DSE. There was also significant variation among clones in a number of phenotypic traits regardless of leachate treatment. Interestingly, leachate from the related native mustard, N. fendleri, increased relative greenness in F. thurberi regardless of genotype, suggesting that the presence of native mustards may have a positive rather than negative effect on performance. Our research contributes to the growing understanding that intraspecific variation is an important variable to consider as climate change continues to alter high-elevation ecosystems and facilitates invasions where they have historically been absent.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
AT and KS conceived of the research question and experimental methodology. AT conducted the field research with assistance from EC. AT performed statistical analysis and wrote the manuscript. AT, EC, and KS edited the manuscript. KS secured the funding. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Jeff Chieppa provided insight to statistical analysis. Natashia Djuric provided crucial field support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2022.768557/full#supplementary-material
References
Ahuja, I., Rohloff, J., and Bones, A. M. (2010). Defence mechanisms of brassicaceae: implications for plant-insect interactions and potential for integrated pest management. a review. Agron. Sustain. Dev. 30, 311–348.
Alvarez, M. E., and Cushman, J. H. (2002). Community-level consequences of a plant invasion: effects on three habitats in coastal California. Ecol. Appl. 12, 1434–1444.
Best, K. F., and McIntyre, G. I. (1975). The biology of canadian weeds: 9. Thlaspi arvense L. Can. J. Plant Sci. 55, 279–292. doi: 10.4141/cjps75-039
Brolsma, K. M. (2014). Linkages Between Plant Traits and Soil Ecology in the Rhizosphere and Through Litter Decomposition. Wageningen: Wageningen University.
Bunn, R. A., Simpson, D. T., Bullington, L. S., Lekberg, Y., and Janos, D. P. (2019). Revisiting the ‘direct mineral cycling’hypothesis: arbuscular mycorrhizal fungi colonize leaf litter, but why? ISME J. 13, 1891–1898. doi: 10.1038/s41396-019-0403-2
Burkey, T. V. (1995). Extinction rates in archipelagoes: implications for populations in fragmented habitats. Conserv. Biol. 9, 527–541.
Chew, F. S. (1979). Community ecology and Pieris: crucifer coevolution. J. N. Y. Entomol. Soc. 87, 128–134.
Dunne, J. A., Harte, J., and Taylor, K. J. (2003). Subalpine meadow flowering phenology responses to climate change: integrating experimental and gradient methods. Ecol. Monogr. 73, 69–86.
Frei, E. R., Ghazoul, J., and Pluess, A. R. (2014). Plastic responses to elevated temperature in low and high elevation populations of three grassland species. PLoS One 9:e98677. doi: 10.1371/journal.pone.0098677
Funk, J., Saunders, S., Sanford, T., Easley, T., and Markham, A. (2014). Rocky Mountain Forests at Risk: Confronting Climate-Driven Impacts From Insects, Wildfires, Heat, and Drought. Report From the Union of Concerned Scientists and the Rocky Mountain Climate Organization. Cambridge, MA: Union of Concerned Scientists.
Gibson, D. J., Dewey, J., Goossens, H., and Dodd, M. M. (2014). Intraspecific variation among clones of a naïve rare grass affects competition with a nonnative, invasive forb. Ecol. Evol. 4, 186–199. doi: 10.1002/ece3.919
Hagan, D. L., Jose, S., and Lin, C. H. (2013). Allelopathic exudates of cogongrass (imperata cylindrica): implications for the performance of native pine savanna plant species in the southeastern us. J. Chem. Ecol. 39, 312–322. doi: 10.1007/s10886-013-0241-z
Harte, J., Saleska, S. R., and Levy, C. (2015). Convergent ecosystem responses to 23-year ambient and manipulated warming link advancing snowmelt and shrub encroachment to transient and long-term climate–soil carbon feedback. Glob. Change Biol. 21, 2349–2356. doi: 10.1111/gcb.12831
Johnson, L. C., Olsen, J. T., Tetreault, H., DeLaCruz, A., Bryant, J., and Morgan, T. J. (2015). Intraspecific variation of a dominant grass and local adaptation in reciprocal garden communities along a US Great Plains’ precipitation gradient: implications for grassland restoration with climate change. Evol. Appl. 8, 705–723. doi: 10.1111/eva.12281
Johnson, N. C., Graham, J. H., and Smith, F. A. (1997). Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytol. 135, 575–585. doi: 10.3389/fpls.2016.01465
Kelly, A. E., and Goulden, M. L. (2008). Rapid shifts in plant distribution with recent climate change. Proc. Natl. Acad. Sci. U.S.A. 105, 11823–11826. doi: 10.1073/pnas.0802891105
Kivlin, S. N., Kazenel, M. R., Lynn, J. S., Taylor, D. L., and Rudgers, J. A. (2019). Plant identity influences foliar fungal symbionts more than elevation in the Colorado Rocky Mountains. Microb. Ecol. 78, 688–698. doi: 10.1007/s00248-019-01336-4
Kopp, C. W., and Cleland, E. E. (2014). Shifts in plant species elevational range limits and abundances observed over nearly five decades in a western north america mountain range. J. Veg. Sci. 25, 135–146.
Lynn, J. S., Kazenel, M. R., Kivlin, S. N., and Rudgers, J. A. (2019). Context-dependent biotic interactions control plant abundance across altitudinal environmental gradients. Ecography 42, 1600–1612.
Mandyam, K., and Jumpponen, A. (2005). Seeking the elusive function of the root-colonising dark septate endophytic fungi. Stud. Mycol. 53, 173–189. doi: 10.3114/sim.53.1.173
McGonigle, T. P., Miller, M. H., Evans, D. G., Fairchild, G. L., and Swan, J. A. (1990). A new method which gives an objective measure of colonization of roots by vesicular—arbuscular mycorrhizal fungi. New Phytol. 115, 495–501. doi: 10.1111/j.1469-8137.1990.tb00476.x
Moir, W. H. (1967). The subalpine tall grass, festuca thurberi, community of sierra blanca, new mexico. Southwestern Nat. 12, 321–328.
Molina, R. J., Trappe, J. M., and Strickler, G. S. (1978). Mycorrhizal fungi associated with festuca in the western united states and canada. Can. J. Bot. 56, 1691–1695.
Pauchard, A., Kueffer, C., Dietz, H., Daehler, C. C., Alexander, J., Edwards, P. J., et al. (2009). Ain’t no mountain high enough: plant invasions reaching new elevations. Front. Ecol. Environ. 7:479–486. doi: 10.1890/080072
Pyšek, P., Jarošík, V., Hulme, P. E., Pergl, J., Hejda, M., Schaffner, U., et al. (2012). A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species’ traits and environment. Glob. Change Biol. 18, 1725–1737. doi: 10.1111/j.1365-2486.2011.02636.x
R Core Team (2020). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Ranelli, L. B., Hendricks, W. Q., Lynn, J. S., Kivlin, S. N., and Rudgers, J. A. (2015). Biotic and abiotic predictors of fungal colonization in grasses of the colorado rockies. Divers. Distrib. 21, 962–976. doi: 10.1111/ddi.12310
Reeves, R. D., Macfarlane, R. M., and Brooks, R. R. (1983). Accumulation of nickel and zinc by western North American genera containing serpentine-tolerant species. Am. J. Bot. 70, 1297–1303. doi: 10.2307/2443420
Roberts, K. J., and Anderson, R. C. (2001). Effect of garlic mustard [alliaria petiolata (beib. cavara & grande)] extracts on plants and arbuscular mycorrhizal (am) fungi. Am. Midland Nat. 146, 146–152. doi: 10.1674/0003-0031(2001)146[0146:eogmap]2.0.co;2
Rudgers, J. A., Afkhami, M. E., Bell-Dereske, L., Chung, Y. A., Crawford, K. M., Kivlin, S. N., et al. (2020). Climate disruption of plant-microbe interactions. Annu. Rev. Ecol. Evol. Syst. 51, 561–586.
Savage, J., and Vellend, M. (2015). Elevational shifts, biotic homogenization and time lags in vegetation change during 40 years of climate warming. Ecography 38, 546–555. doi: 10.1111/ecog.01131
Schnoor, T. K., Lekberg, Y., Rosendahl, S., and Olsson, P. A. (2010). Mechanical soil disturbance as a determinant of arbuscular mycorrhizal fungal communities in semi-natural grassland. Mycorrhiza 21, 211–220. doi: 10.1007/s00572-010-0325-3
Schranz, M. E., Edger, P. R., Pires, J. C., van Dam, N. M., Wheat, C. W., Edwards, D., et al. (2011). “Comparative genomics in the brassicales: ancient genome duplications, glucosinolate diversification and pierinae herbivore radiation,” in Genetics, Genomics and Breeding of Oilseed Brassicas, eds D. Edwards, J. Batley, I. Parkin, and C. Kole (Boca Raton, FL: CRC Press), 206–218.
Schreiner, P. R., and Koide, R. T. (1993). Antifungal compounds from the roots of mycotrophic and non-mycotrophic plant species. New Phytol. 123, 99–105. doi: 10.1111/j.1469-8137.1993.tb04535.x
Shumway, S. W. (1995). Physiological integration among clonal ramets during invasion of disturbance patches in a New England salt marsh. Ann. Bot. 76, 225–233. doi: 10.1006/anbo.1995.1091
Sloat, L. L., Henderson, A. N., Lamanna, C., and Enquist, B. J. (2015). The effect of the foresummer drought on carbon exchange in subalpine meadows. Ecosystems 18, 533–545. doi: 10.1007/s10021-015-9845-1
Stinson, K. A., Campbell, S. A., Powell, J. R., Wolfe, B. E., Callaway, R. M., Thelen, G. C., et al. (2006). Invasive plant suppresses the growth of native tree seedlings by disrupting belowground mutualisms. PLoS Biol. 4:e140. doi: 10.1371/journal.pbio.0040140
Strauss, S. Y., Lau, J. A., and Carroll, S. P. (2006). Evolutionary responses of natives to introduced species: what do introductions tell us about natural communities? Ecol. Lett. 9, 357–374. doi: 10.1111/j.1461-0248.2005.00874.x
Tolra, R., Pongrac, P., Poschenrieder, C., Vogel-Mikuš, K., Regvar, M., and Barcelo, J. (2006). Distinctive effects of cadmium on glucosinolate profiles in cd hyperaccumulator thlaspi praecox and non-hyperaccumulator thlaspi arvense. Plant Soil 288, 333–341. doi: 10.1007/s11104-006-9124-1
Trautwig, A. N., Anthony, M. A., Frey, S. D., and Stinson, K. A. (2021). Effects of an introduced mustard, thlaspi arvense, on soil fungal communities in subalpine meadows. Fungal Ecol. 56:101135. doi: 10.1016/j.funeco.2021.101135
Vaughn, S. F., and Berhow, M. A. (1999). Allelochemicals isolated from tissues of the invasive weed garlic mustard (alliaria petiolata). J. Chem. Ecol. 25, 2495–2504.
Via, S., and Lande, R. (1985). Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution 39, 505–522. doi: 10.1111/j.1558-5646.1985.tb00391.x
Vierheilig, H., and Ocampo, J. A. (1990). Role of root extract and volatile substances of non-host plants on vesicular-arbuscular mycorrhizal spore germination. Symbiosis 9, 199–202.
Warwick, S. I., Francis, A., and Susko, D. J. (2002). The biology of canadian weeds. 9. Thlaspi arvense L.(updated). Can. J. Plant Sci. 82, 803–823. doi: 10.4141/p01-159
Wasserman, T. N., Cushman, S. A., Shirk, A. S., Landguth, E. L., and Littell, J. S. (2012). Simulating the effects of climate change on population connectivity of american marten (martes americana) in the northern rocky mountains, USA. Landscape Ecol. 27, 211–225. doi: 10.1007/s10980-011-9653-8
Wolfe, B. E., Rodgers, V. L., Stinson, K. A., and Pringle, A. (2008). The invasive plant alliaria petiolata (garlic mustard) inhibits ectomycorrhizal fungi in its introduced range. J. Ecol. 96, 777–783. doi: 10.1111/j.1365-2745.2008.01389.x
Keywords: Festuca thurberi, Thlaspi arvense, Noccaea fendleri, sub-alpine meadows, arbuscular mycorrhizal fungi, dark septate endophytes, intraspecific variation
Citation: Trautwig AN, Cousins EA and Stinson KA (2022) Intraspecific Variation in Responses of a Montane Grass, Festuca thurberi, to Simulated Biological Invasion. Front. For. Glob. Change 5:768557. doi: 10.3389/ffgc.2022.768557
Received: 31 August 2021; Accepted: 13 January 2022;
Published: 04 February 2022.
Edited by:
Camille Truong, Royal Botanic Gardens Victoria, AustraliaReviewed by:
Patricia Silva-Flores, Universidad Católica de Maule, ChileAlan Feest, University of Bristol, United Kingdom
Copyright © 2022 Trautwig, Cousins and Stinson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adam N. Trautwig, antrautw@gmail.com
 Adam N. Trautwig
Adam N. Trautwig Elsa A. Cousins1,2
Elsa A. Cousins1,2