Forest Proximity Positively Affects Natural Enemy Mediated Control of Fall Armyworm in Southern Africa
- 1Department of Forest and Conservation Sciences, Faculty of Forestry, University of British Columbia, Vancouver, BC, Canada
- 2International Maize and Wheat Improvement Center (CIMMYT), Southern Africa Regional Office, Harare, Zimbabwe
The fall armyworm (FAW, Spodoptera frugiperda) is a major crop pest in southern Africa. It threatens the livelihoods and food security of smallholder farmers in the region by negatively impacting maize yield. Although scientific evidence suggests that natural enemy-mediated predation can potentially reduce FAW infestation, the effectiveness of natural enemies such as birds, bats, parasitoids, and generalist predators on FAW is poorly understood. This study reviews existing literature to assess how birds, bats, parasitoids, and generalist predators’ control FAW infestation, as well as the role of forest or tree cover in natural enemy mediated pest control of FAW in maize in southern Africa. We then present a case study to examine the role of forest proximity in reducing FAW infestation in maize in Zimbabwe. We conclude that birds, bats, parasitoids, and generalist predators are likely drivers of the reduced success of FAW near forests in southern Africa. While predators influence FAW survival and development, their role is largely undermined by parasitoids, which are more efficient in affecting FAW populations. Birds, bats, parasitoids, and generalist predators play an important role in controlling FAW on farms in heterogenous landscapes with diverse vegetation and near-forest proximity. The findings of our case study from Zimbabwe suggest that the distance to forest had a much higher impact on FAW incidence than maize variety, planting date, or the rate of nitrogen applied. Lack of enough case studies from maize in southern Africa makes it challenging to assess the mechanism and the effectiveness of bird predation on FAW. For this reason, further research is necessary to examine how predation by birds, bats and arthropods and parasitism impacts maize yield. We discuss research barriers, recommend appropriate methods for experimental studies, and suggest possible management options to control FAW in southern Africa.
Introduction
The fall armyworm (FAW, Spodoptera frugiperda), an agricultural pest native to North and South America, has recently become widespread across Africa and Asia (Assefa and Ayalew, 2019; Sharanabasappa et al., 2019; Sisay et al., 2019). Africa is particularly vulnerable to FAW infestation due to the prevalence of diverse host plants and suitable agro-ecological conditions (Day et al., 2017). Although FAW attacks up to 350 plant species in its native range (Montezano et al., 2018), it favors graminaceous crops, especially maize (Baudron et al., 2019). Maize is the most widely grown staple food crop in Africa, covering 37 million hectares (Hruska, 2019), and it provides food and livelihood for about 208 million smallholder farmers in the region (Sisay et al., 2019; Tambo et al., 2019). FAW infestation reduces maize yield up to 53% (Day et al., 2017; Kumela et al., 2019; De Groote et al., 2020) and can cause up to $US13 billion per annum crop losses across Africa (Day et al., 2017). Thus, FAW infestation on maize poses a serious threat to the food security and livelihood of smallholder farmers in Africa (Day et al., 2017).
FAW control measures range from handpicking of larvae (Tambo et al., 2019) or applying chemical pesticides (Kumela et al., 2019) to diverse agro-ecological practices such as minimum tillage, intercropping, and diversifying the farm environment (Baudron et al., 2019; Harrison et al., 2019). A wide range of natural enemies, such as birds, bats, parasitoids, and pathogens can also control FAW larva (Capinera, 2000; Molina-Ochoa et al., 2003; Assefa and Ayalew, 2019; Sisay et al., 2019). Moreover, generalist predators such as ground beetles (Carabidae), rove beetles (Staphylinidae), ants (Formicidae), earwigs (Labiduridae), social wasps (Hymenoptera), and spiders (Araneae) are known to attack FAW eggs and larvae (Harrison et al., 2019; Rukundo et al., 2020). Natural enemy-mediated predation can be effective in controlling FAW infestation in maize, reducing FAW pupae up to 73% (Capinera, 2000). Previous studies suggest that insectivorous birds are effective in reducing FAW infestation in fields close to hedgerows (Wyckhuys and O’Neil, 2006). Laboratory trials also show that red-winged black birds (Agelaius phoeniceus) are important control agents, as they preferentially feed on relatively larger and non-parasitized FAW larvae (Jones et al., 2005). Similarly, bats can be particularly effective in controlling adult moths (Maine and Boyles, 2015) as evidenced in Texas where 100 million Brazilian free-tailed bats (Tadarida brasiliensis) feed on up to 4 billion noctuid moths every night (Lee and McCracken, 2005). However, the understanding of the effectiveness of natural enemy mediated predation on FAW and the role of forest or tree cover in reducing FAW infestation through natural predation is still limited.
The objective of this study is to first review bat, bird, parasitoid and other generalist predator mediated control of FAW in maize and assess how natural enemy mediated FAW control is influenced by proximity to forest in southern Africa. We then present a case study from Zimbabwe assessing whether proximity to forest reduces FAW infestations in maize fields.
Materials and Methods
For this paper, we followed the Food and Agriculture Organization of the United Nations (FAO) delineation of southern Africa, which comprises the countries of Angola, Botswana, Comoros, Lesotho, Madagascar, Malawi, Mauritius, Mozambique, Namibia, Seychelles, South Africa, Eswatini Tanzania, Zambia, and Zimbabwe.
Description of the Review Method
The literature reviewed for this study was found using a combination of Google Scholar and the UBC Library Search Collection functions. We searched for combinations of natural enemy mediated pest control by birds, bats, parasitoids, and generalist predators on FAW in its natural range; natural enemy mediated pest control on cash crops in southern Africa, and natural enemy mediated pest control on FAW in southern Africa. Papers were selected based on whether the information pertained to bat, bird, parasitoid, or generalist natural enemy mediated pest control either directly on FAW or on other crop pests in southern Africa, on maize crops in the native range of FAW, or cash crops in areas with similar climates to southern Africa. Considering that FAW was only recently introduced to Africa, there were few papers available that examined natural enemy mediated pest control of FAW in Africa. For this reason, we relied on studies that examine natural enemy mediated control on fields near forests elsewhere in the world to examine the likelihood of different mechanisms of pest control occurring in FAW infestations in southern Africa. In addition, we selected papers based on whether the methodology used could be useful for further examining FAW natural enemy mediated pest control in southern Arica, even if the study location in some of the reviewed papers is not in southern Africa. Key words used in the literature search were: natural enemy mediated pest control, FAW in southern Africa, bird predation on crop pests, bat predation on crop pests, parasitoids and FAW; in addition, location-based search terms were used, such as FAW in native range, or FAW in southern Africa.
Study Case in Zimbabwe
The case study was conducted in Chipinge District in Zimbabwe, where the presence of FAW is observed since early 2017. The district is in south-eastern Zimbabwe at an average altitude of 1,134 m above sea level. The district is characterized by a population density of approximately 33 inhabitants per km2, a mean annual rainfall of 1,097 mm (90 years average), a mean annual temperature of 28°C (10 years average). The analysis was conducted at the peak of the rainy season, and thus water sources (mainly in the form of stagnant water) were available throughout the landscape. The district has sandy soils with black and red clays as the major soil types. Maize, cotton, and sorghum are the major crops and cattle, goats, pigs, and chicken as the major livestock species (Baudron et al., 2019).
We surveyed a total of 278 maize fields in Ward 16, Ward 18, and Ward 20 in Chipinge district between the 18th and the 22nd of February 2019 (Figure 1). The manager of each field was interviewed regarding the management of the plot (variety, planting date, fertilization, etc.) and the plot was then scouted for FAW damage. Following this survey, five groups of ten plants were selected following a “W sampling” and the number of plants in each sampling point displaying leaf damage due to FAW was recorded as well as the severity of this damage using a scale ranging from 1 to 9. A GPS point of the center of the plot was also recorded. All data (included GPS point) was recorded with Galaxy Tab A and Galaxy J5 using the application “CommCare”.1 Sentinel-2 Level-2A images for 24th January 2019 (8.44% cloud cover in the area) and 28th February (0% cloud cover) were acquired and an object-based land cover classification was performed (Champagne, 2019). Forest patches of at least 0.5 ha were identified and the distance between the center of each field and the closest forest patch of at least 0.5 ha was calculated. The distance of the sampled maize fields to the identified forest patches ranged from 0 to 4193.12 m. The spatial analysis focused only on forest patches vs. other land covers that included both crops and fallows. Grasses were available throughout the landscape. No negative control group was used in this study.
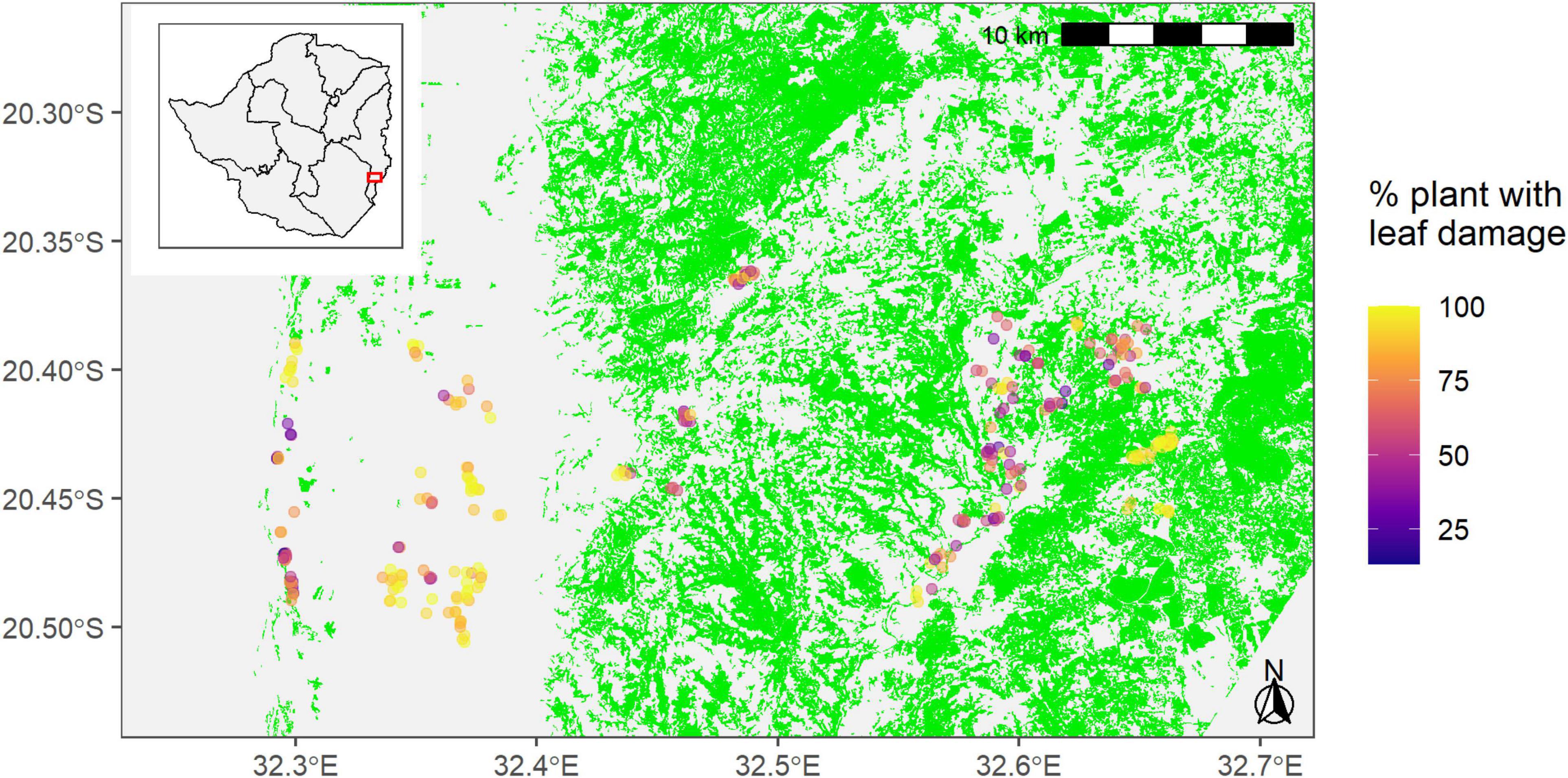
Figure 1. Locations of 278 sampling points in maize fields across Wards 16, 18, and 20 in Chipinge District in Zimbabwe. The green areas are forest patches of at least 0.5 ha. The gray area represents annual crops (mainly maize and sorghum) and grassy fallows. The spatial analysis focused only on forest patches vs. other land covers that included both crops and fallows. Grasses were available throughout the landscape.
The variability of the proportion of plants with leaf damage due to FAW in each sampling point (N = 1,668) was analyzed using generalized linear models with a logit distribution. Variables included in the model were “Ward” (Ward 16, Ward 18, or Ward 20), elevation (m.a.s.l.), planting date (in number of days after November 1st), previous crop (maize or other), soil (lighter soils vs. heavier soils), tillage intensity (conventional vs. minimum), variety (open pollinated, Panar variety, variety PHB30G19, Seedco variety 400 serie, Seedco variety 500 serie, variety ZAP61, or other variety), quantity of N applied (kg ha–1), manure applied or not, intercropping or not, presence of hedgerow or not, weeding frequency (infrequent—0 or 1—and frequent—2 or more), pesticide applied or not, and distance to the closest patch of forest of at least 0.5 ha.
Results
Natural Enemy-Mediated Control of Fall Armyworm in Proximity to Forest
We reviewed a total of 28 studies on natural enemy mediated pest control in various geographical locations and cropping systems to draw conclusions about natural enemy mediated control of FAW in proximity of forest. Among these, 10 examined bird-mediated natural enemy pest control as it pertains to forest proximity to a field and the potential for birds to drive the reduced success of FAW in southern Africa. Similarly, nine other studies examined bat-mediated natural enemy pest control and impact of forest proximity in varying geographical locations and cropping systems, including two studies conducted in southern Africa. Moreover, we reviewed nine studies that assessed parasitoid-mediated pest control and role of forest proximity in varying geographical locations and cropping systems that included three studies from southern Africa.
Birds
Insectivorous birds can consume both FAW larvae and adult moths and thus act as possible biological drivers for the reduced success of FAW. For instance, predation by birds contributed significantly to FAW control on farms in Central America (FAO, 2018). Studies from its native range suggest that birds are more likely to eat the FAW caterpillars than the adult moths, but birds may have difficulty accessing the larvae deeper in the maize plant where larval development takes place (Harrison et al., 2019). However, it is unclear if the FAW larvae consumption by birds from the surface level is sufficient to significantly reduce the success of FAW.
Forests can suppress crop pests by providing habitat for birds that consume crop pests, likely because of an increased abundance of birds in the farm field near forest through the spillover effect (Puckett et al., 2009). Many pest-consuming bird species in tropical areas are forest birds, and the rates of bird predation on crop pests also increases in forested landscapes (Boesing et al., 2017). For instance, increased distance from the forest edge resulted in decreased predation of crop pests by birds in sun-grown and shade-grown coffee plantations in Kenya (Milligan et al., 2016). However, this conclusion relies on the proxy of bird abundance for pest control by birds. Although many studies used bird abundance as a proxy for reduced crop pests, there is not enough evidence in the literature to conclude that using bird abundance as proxy for pest consumption can be generalized across landscapes (Puckett et al., 2009; Boesing et al., 2017).
Pest control by birds depends on the spatial characteristics of the farm such as presence of native plants on the farm, whereas bird abundance is strongly linked to the landscape characteristics such as landscape heterogeneity and proximity to forest habitat (Boesing et al., 2017). High diversity in land-use such as forest, agriculture, or agroforestry, as well as crop mixes supports greater abundance of birds in West Africa (Boesing et al., 2017; Deikumah et al., 2017). For example, in a study by Deikumah et al. (2017) on avian-mediated pest control in Ghana, the mean observed species richness in cocoa farms with large trees integrated is about 50% greater than in a monoculture. Moreover, maintaining landscape connectivity is important in supporting a high abundance of insectivorous birds as is evidenced in tropical montane of Ethiopia (Gove et al., 2013). These findings suggest that farms that are closer to forests will likely have greater abundance of insectivorous birds than farms that are far from forests or contain no forest patches. Nevertheless, the relationship between bird abundance and pest predation on farms also depends on biological factors such as foraging ability in open areas. Birds need to balance the energy costs of traveling to nearby farms for food and therefore may forage only within 20 m of near-forest farmland (Puckett et al., 2009). In addition, pesticide use in farm fields may reduce the number of birds venturing into fields because of potentially lower foraging success (Puckett et al., 2009).
In terms of FAW in southern Africa, there is no empirical evidence pertaining to FAW predation by birds except some anecdotes suggesting that birds are natural predators of FAW. Previous research has focused primarily on cash crops (e.g., coffee, cocoa), that are often grown in agroforestry systems in southern Africa (Williams-Guillén et al., 2008; Maas et al., 2016; Boesing et al., 2017). The few studies that assessed bird-mediated pest control in maize farms in southern Africa suggest that farms in heterogenous landscapes support relatively high bird species richness and diversity, which is a mechanism that could significantly reduce the infestations of FAW in southern Africa (FAO, 2018). The biological mechanisms behind the link between increased landscape heterogeneity and bird-mediated pest control are well supported in general, but the understanding of which bird species provide pest control service and to what degree is limited in Africa (Boesing et al., 2017). There is no evidence of predation by birds on larvae because much of the larval development takes place deep within the maize plant, making the larva inaccessible to birds; for this reason, among others, significant reduction in FAW by birds in southern Africa is unlikely (Harrison et al., 2019).
In conclusion, even though birds are known predators of FAW elsewhere, it is uncertain if they are responsible for the control of FAW in southern Africa. There is not enough evidence in the literature to support the hypothesis that predation by birds is the biological mechanism causing the decline in FAW on near-forest farms (Harrison et al., 2019; Lindell et al., 2018). Based on the limitations of the literature, extensive further research on bird-mediated FAW control is needed.
Bats
Insectivory in bats is widespread and they are effective predators, consuming up to 70% of their body weight in insects per night (Russo et al., 2018). By consuming adult insects, bats can control the mating and spawning success of crop pests. This is evidenced by increased bat activity on the farms during the growing season, which sees increased abundance of crop pests (Weier et al., 2018). Exclusion studies show a direct relationship between the absence of bats and increase in crop pests, as evidenced by Maine and Boyles (2015), who found that excluding bats from their study site led to 59% more corn earworm larvae/ear (Kalka et al., 2008; Maas et al., 2013). For example, exclosure of birds and bats resulted in increased arthropod herbivory in Indonesian cacao agroforestry systems (Maas et al., 2013; Gras et al., 2016). In contrast, the absence of bats resulted in increased crop damage by 56% as compared to controls and increased larval density of the corn ear moth in maize fields in Illinois (Maine and Boyles, 2015). Bats reduced herbivory by arthropods more than birds in tropical forests in Panama, but it is difficult to say if this can scale out to farms and crop plants (Kalka et al., 2008).
Bats can additionally influence their prey by creating a “landscape of fear,” a predator-prey interaction where the prey alters its behavior or habitat use to avoid bat predation (Russo et al., 2018). Bats initiate a trophic cascade in the maize pests by lowering the larval density of the moth and shifting the reproductive behavior of the moths to more self-preservationist actions (Maine and Boyles, 2015). For example, some moths have tympanic organs that allow them to hear the calls that bats make for echolocation of prey. This alters the moth’s behavior to maximize the likelihood of survival such as changing habitat or foraging or spawning behavior (Russo et al., 2018; Cinel and Taylor, 2019). Using paired control plots and nocturnal bat exclosures on maize fields in Illinois, Maine and Boyles (2015) found that even when bats were unable to access corn ear moths to consume them, the echolocation sounds used by the bats to hunt may have had an impact on the reproductive behaviors of the moths.
Bats are known natural predators of FAW in its native range, and consumption of FAW moths by bats is a possible mechanism of FAW control in southern Africa (FAO, 2018; Harrison et al., 2019). An experiment examining the impact of bats on FAW moths showed that FAW moths are equipped with tympanic organs, which in the presence of bats can influence the moths to alter their flight or halt pheromone release (Cinel and Taylor, 2019). This study also found significant changes in the brain tissue of the FAW moths that were exposed to bat sounds, potentially affecting their mating behaviors. This suggests a closely evolved predator-prey relationship between bats and FAW and thus can be an effective mechanism for FAW control in southern Africa. However, it is difficult to determine the scale of the impact of bat predation on moth abundance and behavior due to a paucity of case studies across sites and crop types.
Proximity to forest can influence the rate of bat predation on FAW depending on their feeding guilds (Williams-Guillén and Perfecto, 2011; Weier et al., 2018). For example, cluster-edge feeders, i.e., the bats that feed at the edge, decrease in abundance further into agricultural fields, while open-air feeders increase further into a field when pests are abundant (Weier et al., 2018). Bats are more abundant in heterogenous landscapes with at least some trees in their foraging habitat (Russo et al., 2018). They prefer to feed in areas with forest fragments or with low intensity agriculture in proximity to roosting sites, as is evidenced in a shade-grown coffee site in Mexico (Williams-Guillén and Perfecto, 2011). Feeding activity was reduced in cluster-edge bats, while it remained similar in open-air feeders in more intensely managed agricultural landscapes (Williams-Guillén and Perfecto, 2011).
Although there is clear evidence about the general relationship between the presence of bats and the reduction in crop pests, little information is available on biological control of crop pests by bats in Africa (Kalka et al., 2008; Maas et al., 2013; Maine and Boyles, 2015). African bat species are poorly studied compared to the bat species of Europe, Asia, and North America, so there is little information available about their habitat preferences and foraging behavior. The studies on bat-mediated pest control so far have primarily focussed on cash crops instead of subsistence farms in Africa. For example, bat predation on pests in macadamia plantations in South Africa increased as the abundance of prey increased (Weier et al., 2018). Species richness and bat activity levels were similar between macadamia plantation and riparian area habitat types in macadamia plantations in South Africa (Taylor et al., 2013). As this study was conducted in a forested landscape, the findings suggest that a heterogenous landscape with a variety of habitat types supports high species richness and feeding activity in bats (Taylor et al., 2013). However, it is difficult to draw conclusions about the potential impact of bats on crop yields in Africa due to lack of adequate evidence on bat predation on FAW in maize cultivation (Kunz et al., 2011; Weier et al., 2018).
Given the research on the impact of bats on maize, bats are a likely driver of the reduced success of FAW near forests in southern Africa. However, considerable additional research is necessary to draw any definitive conclusions.
Generalist Predators
Apart from the vertebrate predators, a diverse array of generalist predators of FAW have been reported in the Americas, Africa, and Asia (Wan et al., 2021). Among these, ground beetles (Carabidae), rove beetles (Staphylinidae), ants (Formicidae), earwigs (Labiduridae), social wasps (Hymenoptera), and spiders (Araneae) are known to attack FAW eggs and larvae (Harrison et al., 2019; Rukundo et al., 2020). For example, the earwigs Doru lineare and D. luteipes, that occur throughout the maize crop cycle, lay eggs inside the maize whorl and prey on FAW eggs and larvae (Sueldo et al., 2010; Prasanna et al., 2018). Nymphs and adults of D. luteipes feed on 8–12 and 10–21 FAW larvae daily, respectively (Prasanna et al., 2018). Similarly, predacious bugs such as Picromerus lewisi, Arma chinensis, Eocanthecona furcellata, and Andrallus spinidens prey on FAW larvae (Keerthi et al., 2020; Wan et al., 2021). Moreover, carabid beetles, rove beetles, spiders, and ants predate on FAW in maize fields as evidenced in North America, West Africa, and Asia (Harrison et al., 2019; Sharanabasappa et al., 2019; Dassou et al., 2021).
Generalist predators can play a significant role in controlling FAW population by directly consuming FAW eggs and larvae in maize fields (Prasanna et al., 2018; Harrison et al., 2019). For instance, social wasps extracted FAW larvae from maize whorl and reduced 77% of FAW present in maize in Brazil (Prezoto and Machado, 1999). FAW damage in maize fields increased significantly when these generalist predators were selectively removed from the field (Clark, 1993). Conversely, decreased FAW damage in minimum-tillage maize fields in Florida and Mexico was attributed to higher density of general predators (Clark, 1993; Rivers et al., 2016).
Proximity of maize fields to forest increases the local abundance and diversity of natural enemies, such as predatory solitary wasps (Harrison et al., 2019). For example, increasing distance of maize fields from the forest in Brazil resulted in a decline in predatory solitary wasp abundance and increase in FAW abundance (Sousa et al., 2011). Similarly, spiders and ground beetles were more abundant in maize fields within coffee agroforest landscapes (Wyckhuys and O’Neil, 2006; Harrison et al., 2019). However, the role of forest proximity on other generalist predators and their control of FAW is still poorly understood.
In southern Africa, generalist predators, such as spiders, beetles, earwigs, social wasps, and ants are crucial in controlling lepidopteran pests of cereal crops and these predators attack FAW larvae as well (Harrison et al., 2019). For example, several species of earwigs such as Diaperasticus erythrocephalus have been found in the whorl and ears of maize in Africa (Prasanna et al., 2018). Similarly, many ant species predate on FAW in maize fields as evidenced in Ghana (Koffi et al., 2020), Cameroon (Dassou et al., 2021), and Nicaragua (Perfecto, 1991). Nevertheless, there is limited evidence available regarding the effectiveness of other generalist predators in controlling FAW population and the role of forest proximity in influencing generalist predators.
Parasitoids
Parasitoids are natural enemies of FAW that can reduce feeding capacity and weight gain of FAW larvae in maize fields (Meagher et al., 2016). There are over 150 parasitoids that attack FAW in its native range (Kenis et al., 2019). Some parasitoid species such as Telenomus remus have also been introduced in countries to control FAW outbreak (Kenis et al., 2019). Parasitoid control of FAW is more effective in maize fields with good soil conditions, such as high organic matter and active soil biology, no-till fields, and fields with integrated crop residues such as mulch (Harrison et al., 2019). In contrast, pesticide use and frequent disturbances of soil reduce parasitoid abundance (Letourneau et al., 2012; Quispe et al., 2017). For example, parasitism levels ranged from 1% in commercial maize treated with pesticides to 91.7% in experimental maize fields without any pesticide treatment (Meagher et al., 2016).
Parasitoid abundance on farms near forests depends on a variety of landscape characteristics, including proximity to forest. Landscapes with vegetation cover sustain a greater abundance of parasitoids by providing diversity of potential hosts, stable areas to pupate, and a continuous supply of resources (Letourneau et al., 2012). Refuges or habitats with woody plant species also sustain higher level of parasitoid species richness than habitats with non-woody species; for example, a study by Quispe et al. (2017) surveying parasitoid richness in maize crops observed five species of parasitoids in the control maize field as compared to a total of 89 species across six experimental refuges, with a diversity of woody and herbaceous plants, adjacent to the maize field. Forests can also act as population source for the agricultural land that requires recolonization by parasitoids after disturbance, as evidenced in maize fields near a tropical rainforest (Kankonda et al., 2017). For instance, parasitoid abundance increased closer to the forest or at the edge of the field and decreased toward the middle of the field in Florida (Hay-Roe et al., 2016). Parasitoids particularly in the order Hymenoptera needs access to other resources such as pollen or nectar and move further into the center of a field when supplied with continuous vegetative cover and abundant flowering plants (Quispe et al., 2017). Thus, proximity to permanent vegetation and proximity to forest are important factors determining parasitoid abundance and consequently FAW control by parasitoids on farms.
Unlike birds and bats, several studies directly examined parasitoid-mediated FAW control mechanism in Africa (Kenis et al., 2019; Sisay et al., 2019; Agboyi et al., 2020). Studies suggest that a variety of FAW parasitoids may exist in Africa despite the relatively recent colonization of FAW in the region (Kenis et al., 2019). This is evidenced by observation of T. remus in Kenya in 1988, much earlier than the FAW outbreak in Africa. Recent surveys also observed T. remus in Benin, Niger, Côte d’Ivoire, Kenya, and South Africa (Kenis et al., 2019) and six other species of parasitoids in Ethiopia, Kenya, and Tanzania (Sisay et al., 2019). Similarly, ten species of FAW egg, egg-larval, and larval parasitoids were found in two countries in East Africa (Agboyi et al., 2020). Most of the maize fields that contained FAW also had parasitoids of FAW, suggesting a direct relationship (Sisay et al., 2019). Many of these species of parasitoids of FAW belong to the order hymenoptera, which needs habitats with vegetation cover, suggesting that parasitoids are responsible for the reduced success of FAW on near-forest farms.
Considering that there are over hundred parasitoids of FAW in its native range, it is likely that many potential parasitoid species in Africa that could be crucial for FAW control have not yet been identified (Kenis et al., 2019). Moreover, parasitoid impact on crop yields in Africa is still poorly understood (Kenis et al., 2019; Sisay et al., 2019; Agboyi et al., 2020). This calls for an extensive survey of the presence of parasitoids of FAW and research on the impact of parasitoid-mediated FAW control on crop yields in southern Africa. Nevertheless, there is enough evidence to conclude that parasitoids play an important role in controlling FAW on farms near forests.
Case Study in Zimbabwe: Does Proximity to Forest Reduce Fall Armyworm Infestation?
Incidence of FAW plant damage (% plants displaying leaf damage due to FAW) was found to increase significantly with increasing distance from a forest patch of at least 0.5 ha (Figure 2). The only factors having a significant effect at 5% were “Ward” (with higher infestation in the wetter Wards 16 and 18 than in the drier Ward 20) and “Pesticide application” (with incidence higher in plots where pesticides were applied, pesticide application being no doubt the result of higher infestation and not the other way around) (Figure 2). Thus, in this case, distance to forest had a much higher impact on FAW incidence than maize variety, planting date, or nitrogen applied.
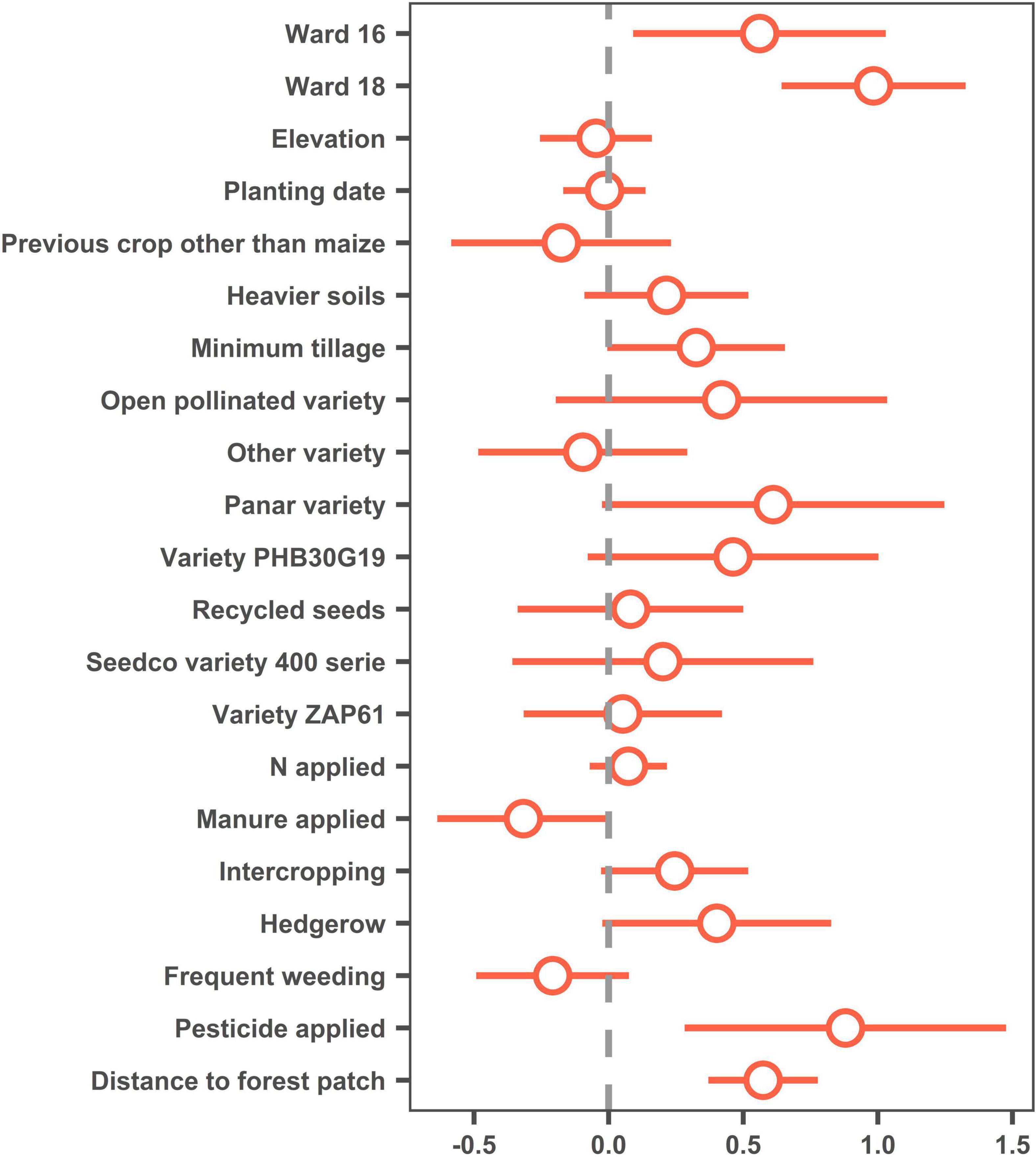
Figure 2. Dotwhisker plot representing estimates (dots) and 95% confidence intervals (whiskers) for a generalized linear models with a logit distribution. Whiskers crossing the y-axis (gray dotted line) indicate non-significance for the corresponding factor. “Ward 20,” “maize as previous crop,” “conventional tillage,” “Seedco variety 500 serie,” “no manure applied,” “no intercropping,” “no hedgerow,” “infrequent weeding (none or one)” and “no pesticide applied” were used as control in the model.
Discussion
The results of this literature review suggests that research on natural enemy mediated control of FAW so far has primarily focused on cash crops and in Europe and North America. One of the drivers for such a bias in research focus could be disproportionate funding availability for economically valuable cash crops in high demand such as coffee, cocoa, and macadamia nuts in Europe and North America. Additionally, more funding may be directed toward studies on farming practices that are associated with an incentive for biodiversity conservation such as agroforestry sites with higher plant diversity. However, it is difficult to tease apart the conservation and economic drivers in the case of high value crops such as coffee that are also agroforestry products. It is also important to note that the results of this study were limited to an extent by the specific databases used for the literature review. The databases we chose, i.e., Google Scholar may provide results based on past search history unique to the researcher and UBC Library is not accessible by all researchers, thus affecting the replicability of this work.
The results of the case study conducted in Zimbabwe support the claim that proximity to a forest patch will reduce the incidence of FAW on maize (Figure 2). This result is consistent with the findings of most of the literature used in this review, which demonstrates that landscape heterogeneity is correlated positively with natural enemy mediated pest control (Harrison et al., 2019). Another recent study by Jordon et al. (2021) conducted in Ghana found that FAW damage increases with increasing distance from a patch of natural habitat, which supports the findings of our case study. Much of the literature included in this review has been primarily focused on maize crops outside southern Africa or on cash crops. Although the case study did not examine mechanisms of the reduced FAW damage, in light of the available literature the relationship could be related to either the decreased reproductive success of FAW, due to bat and parasitoid predation, or the increased predation of caterpillar due generalist predators (Kalka et al., 2008; Quispe et al., 2017). The case study provides a base for additional research, which could attempt to identify the mechanism of the decreased FAW damage as it relates to near-field proximity to a forest patch.
Although the existing literature is inconclusive on the effectiveness of natural enemy mediated control of FAW in southern Africa, several studies suggest experimental methods for assessing predation of FAW by birds, bats, generalist predators and parasitoids, in addition to those demonstrated in the case study. In terms of specific recommendations for future research, predation of FAW by birds and bats can be evaluated using diurnal and nocturnal exclosures, respectively. A diurnal exclosure with increasing proximity to the forest can be useful for examining the foraging success of birds on FAW. A design of sites that are exclosed or open placed in pairs at increasing distance from the forest diurnally or nocturnally can be useful for determining FAW control by birds and bats, respectively. The same researchers can conduct the studies on birds and bats simultaneously on different farms to optimize the use of time, resources, and potentially farmer participation. The experiment would then contain plots with no cover, diurnal cover, nocturnal cover, and constant cover, as suggested in previous studies (Kalka et al., 2008; Karp et al., 2013; Maas et al., 2013). Data on the crop damage can be collected in both sites to determine the efficacy of natural enemy mediated FAW control.
Many of the parasitioids of FAW in Africa are larval parasitoids, but there are some eggs and larval-pupal parasitoids as well. Setting up traps at sites with varying distances from the forest can be useful in determining the percentage of parasitoidism. The percentages of parasitoidism from samples of various life-history stages such as eggs, larvae and/or pupae can be used to measure the rate of parasitoidism within the field. As the larvae are well protected from predators within the corn plant, parasitoids may be the most efficient biological control mechanism for FAW (Hay-Roe et al., 2016). Unfortunately, there are limited examples of methods examining how parasitism rates impacts crop damage. The level of parasitism has been assessed from the number of parasitoids on sampled plants from infested maize fields (Sisay et al., 2019; Agboyi et al., 2020). Similarly, parasitoid abundance is determined by placing traps consisted of PVC cylinders at different points within maize fields and later analyzing in the laboratory (Hay-Roe et al., 2016).
Additional challenges for conducting FAW research exist in considering the priorities and beliefs around natural enemy mediated pest control in southern Africa. The fear surrounding bats could potentially be a significant barrier for discussing bat-mediated pest control for farmers. Bats are often considered to carry disease and farmers may be unwilling to take actions or participate in experiments that would increase the abundance of bats on their farm (Harrison et al., 2019). Furthermore, not all bats and birds are insectivorous; many birds and bats are also considered crop pests. Thus, farmers may not want to participate in activities that could increase the abundance of crop-consuming vertebrates by improving habitat on their farms, even if it comes with the benefit of reducing FAW. For this reason, it is recommended that research on the needs and beliefs of farmers must be conducted before research on ways to improve natural enemy mediated pest control on farms.
In terms of management, there are potential negative implications to identifying a controller vs. identifying suppressors. In this case, the controller is the group that reduces FAW success the most, whereas the suppressors will reduce FAW success only to a certain degree. If a controller is identified, it is possible that research funding and policy would focus mainly on improving the species richness and abundance of that group without fully appreciating the redundancies and suppression that the other groups provide. For example, management activities that support bats such as placing bat boxes in a field would do little to support birds or parasitoids that will not or cannot venture far into a maize field. One possible outcome to focusing on only one group is that the suppression effects of the other groups might be negatively affected. Providing habitat for only one group will also decrease the resilience of natural enemy mediated pest control on the farm. Therefore, another recommendation for further research is to evaluate the interactions between the pest-controlling groups carefully before making management suggestions for FAW control.
Finally, this review and the findings of the case study suggests that increasing landscape diversity has the potential to improve the abundance of natural enemies to FAW. However, convincing farmers to add trees and perennials at the field scale would be easier with the economic justification that damage from pests will be reduced. Trees provide a myriad of other benefits to increase productivity of farms (Isbell et al., 2017) and it is recommended that this evidence can be used to make the case to farmers to improve tree cover on farms. Trees and perennials on a farm can contribute to subsistence or even cash crop production and still provide benefits to wildlife (Jose, 2012). Options for using trees for more productive farms include planting fruit trees, using trees as fodder banks for livestock, or planting trees that grow a cash crop or medicine (Sinclair, 1999). However, trees might also negatively affect crop production and thus require careful consideration to balance trade-offs (Sida et al., 2018). Conducting research to determine which species are the most effective natural enemies to FAW is critical to combat food insecurity in southern Africa. Research on FAW natural enemy control in concert with improving landscape heterogeneity in a way that is acceptable for farmers is the best path forward for organizations seeking to improve livelihoods in southern Africa.
Controlling FAW infestation in maize cultivation in Africa is key to ensuring food security and livelihoods for smallholder farmers. Our review of existing research on natural enemy mediated pest control in the context of the FAW invasion of southern Africa suggests that the most specific and conclusive evidence on natural enemy mediated control of FAW exists for parasitoids followed by bats, whereas the effectiveness of bird predation and generalist predators on FAW control in southern Africa is not well understood. Due to the research gap surrounding natural enemy mediated pest control on subsistence farms, no clear conclusions about its effect on FAW in southern Africa can be drawn. We suggest possible experimental methods for future studies on the relationship between forest proximity and natural enemy mediated pest control. Nevertheless, evidence from previous studies and our case study support the claim that landscape heterogeneity improves natural enemy pest control on farms. Considering that FAW is a highly polyphagous foreign pest, further research is necessary to demonstrate that landscape heterogeneity improves both the habitat for potential natural enemies and the predation level by those enemies on FAW in southern Africa. Therefore, we recommend that future management strategies should focus on improving tree and perennial cover at the field scale to provide habitat for potential natural enemies while simultaneously conducting research on biological mechanisms of natural enemy mediated FAW control and its impact on crop yield.
Author Contributions
JC conducted the literature review and wrote the first draft. FB collected the data and analyzed the case study. JB wrote the subsequent drafts. TS supervised the work. All authors equally contributed in revisions of the manuscript.
Funding
This work was implemented by the University of British Columbia and the International Maize and Wheat Improvement Center (CIMMYT, www.cimmyt.org), GOAL (www.goalglobal.org) and was made possible by the generous support of Irish Aid (www.irishaid.ie), Bakker Brothers (www.bakkerbrothers.nl) and CRP MAIZE (www.maize.org).
Author Disclaimer
Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of Irish Aid, Bakker Brothers and CRP MAIZE.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Agboyi, L. K., Goergen, G., Beseh, P., Mensah, S. A., Clottey, V. A., Glikpo, R., et al. (2020). Parasitoid complex of fall armyworm, spodoptera frugiperda, in ghana and benin. Insects 11:68. doi: 10.3390/insects11020068
Assefa, F., and Ayalew, D. (2019). Status and control measures of fall armyworm (Spodoptera frugiperda) infestations in maize fields in Ethiopia: a review. Cogent Food Agricu. 5, 423–431. doi: 10.1080/23311932.2019.1641902
Baudron, F., Zaman-Allah, M. A., Chaipa, I., Chari, N., and Chinwada, P. (2019). Understanding the factors influencing fall armyworm (Spodoptera frugiperda J.E. Smith) damage in African smallholder maize fields and quantifying its impact on yield. a case study in Eastern Zimbabwe. Crop Prot. 120, 141–150. doi: 10.1016/j.cropro.2019.01.028
Boesing, A. L., Nichols, E., and Metzger, J. P. (2017). Effects of landscape structure on avian-mediated insect pest control services: a review. Landsc. Ecol. 32, 931–944. doi: 10.1007/s10980-017-0503-1
Capinera, J. L. (2000). Fall armyworm, Spodoptera frugiperda (JE Smith)(Insecta: Lepidoptera: Noctuidae). Gainesville: University of Florida IFAS Extension.
Champagne, C. (2019). Detecting Crop Damage using Sentinel-2 Imagery in a Smallholder Agriculture Landscape. Faculté des Bioingénieurs. Defourny: Université Catholique de Louvain.
Cinel, S. D., and Taylor, S. J. (2019). Prolonged bat call exposure induces a broad transcriptional response in the male fall armyworm (Spodoptera frugiperda; lepidoptera: noctuidae) brain. Front. Behav. Neurosci. 13:36. doi: 10.3389/fnbeh.2019.00036
Clark, M. S. (1993). Generalist predators In Reduced-Tillage Corn: Predation On Armyworm, Habitat Preferences, and A Method To Estimate Absolute Densities. Doctoral Dissertation. Virginia: Polytechnic Institute and State University.
Dassou, A. G., Idohou, R., Azandémè-Hounmalon, G. Y., Sabi-Sabi, A., Houndété, J., Silvie, P., et al. (2021). Fall armyworm, Spodoptera frugiperda (J.E. Smith) in maize cropping systems in Benin: abundance, damage, predatory ants and potential control. Int. J. Trop Insect. Sci. 41, 2627–2636. doi: 10.1007/s42690-021-00443-5
Day, R., Abrahams, P., Bateman, M., Beale, T., Clottey, V., Cock, M., et al. (2017). Fall Armyworm: Impacts and Implications for Africa. Outlook pest man. 28, 196–201. doi: 10.1564/v28_oct_02
De Groote, H., Kimenju, S. C., Munyua, B., Palmas, S., Kassie, M., and Bruce, A. (2020). Spread and impact of fall armyworm (Spodoptera frugiperda J.E. Smith) in maize production areas of Kenya. Agricu. Ecosyst. Environ. 292:106804. doi: 10.1016/j.agee.2019.106804
Deikumah, J. P., Kwafo, R., and Konadu, V. A. (2017). Land use types influenced avian assemblage structure in a forest–agriculture landscape in Ghana. Ecol. Evol. 7, 8685–8697. doi: 10.1002/ece3.3355
FAO. (2018). Integrated Management Of the Fall Armyworm On Maize A Guide For Farmer Field Schools In Africa. Rome: Food & Agriculture Org: FAO.
Gove, A. D., Hylander, K., Nemomissa, S., Shimelis, A., and Enkossa, W. (2013). Structurally complex farms support high avian functional diversity in tropical montane Ethiopia. J. Trop. Ecol. 29, 87–97. doi: 10.1017/s0266467413000023
Gras, P., Tscharntke, T., Maas, B., Tjoa, A., Hafsah, A., and Clough, Y. (2016). How ants, birds and bats affect crop yield along shade gradients in tropical cacao agroforestry. J. Appl. Ecol. 53, 953–963. doi: 10.1111/1365-2664.12625
Harrison, R. D., Thierfelder, C., Baudron, F., Chinwada, P., Midega, C., Schaffner, U., et al. (2019). Agro-ecological options for fall armyworm (Spodoptera frugiperda JE Smith) management: Providing low-cost, smallholder friendly solutions to an invasive pest. J. Environ. Manag. 243, 318–330. doi: 10.1016/j.jenvman.2019.05.011
Hay-Roe, M. M., Meagher, R. L., Nagoshi, R. N., and Newman, Y. (2016). Distributional patterns of fall armyworm parasitoids in a corn field and a pasture field in Florida. Biol. Control 96, 48–56. doi: 10.1016/j.biocontrol.2016.02.003
Hruska, A. (2019). Fall armyworm (iSpodoptera frugiperda/i) management by smallholders. CAB Rev. 14, 1–11. doi: 10.1079/PAVSNNR201914043
Isbell, F., Gonzalez, A., Loreau, M., Cowles, J., Díaz, S., Hector, A., et al. (2017). Linking the influence and dependence of people on biodiversity across scales. Nature 546, 65–72. doi: 10.1038/nature22899
Jones, K. L., Barzen, J. A., and Ashley, M. V. (2005). Geographical partitioning of microsatellite variation in the sarus crane. Anim. Conserv. 8, 1–8. doi: 10.1017/S1367943004001842
Jordon, M. W., Hackett, T. D., Aboagye-Antwi, F., Eziah, V. Y., and Lewis, O. T. (2021). Effects of distance from semi-natural habitat on fall armyworm (Spodoptera frugiperda, J. E. Smith) and its potential natural enemies in Ghana. Bull. Entomol. Res. 111, 1–11. doi: 10.1017/S0007485321000894
Jose, S. (2012). Agroforestry for conserving and enhancing biodiversity. Agrofor. Syst. 85, 1–8. doi: 10.1007/s10457-012-9517-5
Kalka, M. B., Smith, A. R., and Kalko, E. K. V. (2008). Bats limit arthropods and herbivory in a tropical forest. Science 320, 71–71. doi: 10.1126/science.1153352
Kankonda, O. M., Akaibe, B. D., Ong’amo, G. O., and Le Ru, B.-P. (2017). Diversity of lepidopteran stemborers and their parasitoids on maize and wild host plants in the rain forest of Kisangani. DR Congo. Phytoparasitica 45, 57–69. doi: 10.1007/s12600-017-0561-6
Karp, D. S., Mendenhall, C. D., Sandí, R. F., Chaumont, N., Ehrlich, P. R., Hadly, E. A., et al. (2013). Forest bolsters bird abundance, pest control and coffee yield. Ecol. Lett. 16, 1339–1347. doi: 10.1111/ele.12173
Keerthi, M. C., Sravika, A., Mahesha, H. S., Gupta, A., Bhargavi, H. A., and Ahmed, S. (2020). Performance of the native predatory bug, Eocanthecona furcellata (wolff) (hemiptera: pentatomidae), on the fall armyworm, Spodoptera frugiperda (J. E. Smith) (lepidoptera: noctuidae), and its limitation under field condition. Egypt. J. Biol. Pest Control. 30:69. doi: 10.1186/s41938-020-00272-7
Kenis, M., Du Plessis, H., Van den Berg, J., Ba, M. N., Goergen, G., Kwadjo, K. E., et al. (2019). Telenomus remus, a candidate parasitoid for the biological control of Spodoptera frugiperda in Africa, is already present on the continent. Insects 10:92. doi: 10.3390/insects10040092
Koffi, D., Kyerematen, R., Eziah, V. Y., Agboka, K., Adom, M., Goergen, G., et al. (2020). Natural enemies of the fall armyworm, Spodoptera frugiperda (J.E. Smith) (lepidoptera: noctuidae) in Ghana. Fla. Entomol. 103:85. doi: 10.1653/024.103.0414
Kumela, T., Simiyu, J., Sisay, B., Likhayo, P., Mendesil, E., Gohole, L., et al. (2019). Farmers’ knowledge, perceptions, and management practices of the new invasive pest, fall armyworm (Spodoptera frugiperda) in Ethiopia and Kenya. Int. J.Pest Manag. 65, 1–9. doi: 10.1080/09670874.2017.1423129
Kunz, T. H., De Torrez, E. B., Bauer, D., Lobova, T., and Fleming, T. H. (2011). Ecosystem services provided by bats. Europe 31:32.
Lee, Y.-F., and McCracken, G. F. (2005). Dietary variation of brazilian free-tailed bats links to migratory populations of pest insects. J.Mammal. 86, 67–76. doi: 10.1644/1545-15422005086<0067:DVOBFB<2.0.CO;2
Letourneau, D. K., Bothwell Allen, S. G., and Stireman, J. O. III (2012). Perennial habitat fragments, parasitoid diversity and parasitism in ephemeral crops. J. Appl. Ecol. 49, 1405–1416. doi: 10.1111/1365-2664.12001
Lindell, C., Eaton, R. A., Howard, P. H., Roels, S. M., and Shave, M. E. (2018). Enhancing agricultural landscapes to increase crop pest reduction by vertebrates. Agric. Ecosyst. Environ. 257, 1–11. doi: 10.1016/j.agee.2018.01.028
Maas, B., Clough, Y., and Tscharntke, T. (2013). Bats and birds increase crop yield in tropical agroforestry landscapes. Ecol. Lett. 16, 1480–1487. doi: 10.1111/ele.12194
Maas, B., Karp, D. S., Bumrungsri, S., Darras, K., Gonthier, D., Huang, J. C.-C., et al. (2016). Bird and bat predation services in tropical forests and agroforestry landscapes: ecosystem services provided by tropical birds and bats. Biol. Rev. 91, 1081–1101. doi: 10.1111/brv.12211
Maine, J. J., and Boyles, J. G. (2015). Bats initiate vital agroecological interactions in corn. Proc. Natl. Acad. Sci. 112, 12438–12443. doi: 10.1073/pnas.1505413112
Meagher, R. L. Jr., Nuessly, G. S., Nagoshi, R. N., and Hay-Roe, M. M. (2016). Parasitoids attacking fall armyworm (lepidoptera: noctuidae) in sweet corn habitats. Biol. Control 95, 66–72. doi: 10.1016/j.biocontrol.2016.01.006
Milligan, M. C., Johnson, M. D., Garfinkel, M., Smith, C. J., and Njoroge, P. (2016). Quantifying pest control services by birds and ants in Kenyan coffee farms. Biol. Conserv. 194, 58–65. doi: 10.1016/j.biocon.2015.11.028
Molina-Ochoa, J., Carpenter, J. E., Heinrichs, E. A., and Foster, J. E. (2003). Parasitoids and parasites of Spodoptera frugiperda (lepidoptera: noctuidae) in the Americas and Caribbean basin: an inventory. Fla. Entomol. 86, 254–289. doi: 10.1653/0015-4040(2003)086[0254:paposf]2.0.co;2
Montezano, D. G., Specht, A., Sosa-Gómez, D. R., Roque-Specht, V. F., Sousa-Silva, J. C., Paula-Moraes, S. V., et al. (2018). Host Plants of Spodoptera frugiperda (lepidoptera: noctuidae) in the Americas. Afr. Entomol. 26, 286–300. doi: 10.4001/003.026.0286
Perfecto, I. (1991). Ants (hymenoptera: formicidae) as natural control agents of pests in irrigated maize in Nicaragua. J. Econ. Entomol. 84, 65–70. doi: 10.1093/jee/84.1.65
Prasanna, B. M., Huesing, J. E., Eddy, R., and Peschke, V. M. (Eds.). (2018). Fall Armyworm in Africa: A Guide for Integrated pest Management, First. Mexico: CDMX CIMMYT.
Prezoto, F., and Machado, V. L. (1999). Ação de Polistes (aphanilopterus) simillimus Zikán (hymenoptera: vespidae) na produtividade de lavoura de milho infestada com Spodoptera frugiperda (smith)(lepidoptera: noctuidae). Rev. Bras. Zoociências 1, 19–30.
Puckett, H. L., Brandle, J. R., Johnson, R. J., and Blankenship, E. E. (2009). Avian foraging patterns in crop field edges adjacent to woody habitat. Agric. Ecosyst. Environ. 131, 9–15. doi: 10.1016/j.agee.2008.08.015
Quispe, R., Mazón, M., and Rodríguez-Berrío, A. (2017). Do refuge plants favour natural pest control in maize crops? Insects 8:71. doi: 10.3390/insects8030071
Rivers, A., Barbercheck, M., Govaerts, B., and Verhulst, N. (2016). Conservation agriculture affects arthropod community composition in a rainfed maize–wheat system in central Mexico. Appl. Soil Ecol. 100, 81–90. doi: 10.1016/j.apsoil.2015.12.004
Rukundo, P., Karangwa, P., Uzayisenga, B., Ingabire, J. P., Waweru, B. W., Kajuga, J., et al. (2020). “Outbreak of fall armyworm (Spodoptera frugiperda) and Its impact in rwanda agriculture production,” in Sustainable Management of Invasive Pests in Africa, Sustainability in Plant and Crop Protection, eds S. Niassy, S. Ekesi, L. Migiro, and W. Otieno (Cham: Springer International Publishing), 139–157. doi: 10.1007/978-3-030-41083-4_12
Russo, D., Bosso, L., and Ancillotto, L. (2018). Novel perspectives on bat insectivory highlight the value of this ecosystem service in farmland: research frontiers and management implications. Agric. Ecosyst. Environ. 266, 31–38. doi: 10.1016/j.agee.2018.07.024
Sharanabasappa, D., Kalleshwaraswamy, C. M., Poorani, J., Maruthi, M. S., Pavithra, H. B., and Diraviam, J. (2019). Natural Enemies of spodoptera frugiperda (J. E. Smith) (lepidoptera: noctuidae), a recent invasive pest on maize in South India. Fla. Entomol. 102:619. doi: 10.1653/024.102.0335
Sida, T. S., Baudron, F., Kim, H., and Giller, K. E. (2018). Climate-smart agroforestry: Faidherbia albida trees buffer wheat against climatic extremes in the central rift valley of Ethiopia. Agric. For. Meteorol. 248, 339–347. doi: 10.1016/j.agrformet.2017.10.013
Sinclair, F. L. (1999). A general classification of agroforestry practice. Agrofor. syst. 46, 161–180.
Sisay, B., Simiyu, J., Mendesil, E., Likhayo, P., Ayalew, G., Mohamed, S., et al. (2019). Fall armyworm, Spodoptera frugiperda Infestations in East Africa: assessment of damage and Parasitism. Insects 10:195. doi: 10.3390/insects10070195
Sousa, E. H. S., Matos, M. C. B., Almeida, R. S., and Teodoro, A. V. (2011). Forest fragments’ contribution to the natural biological control of Spodoptera frugiperda Smith (lepidoptera: noctuidae) in maize. Braz. Arch. Biol. Technol. 54, 755–760. doi: 10.1590/s1516-89132011000400015
Sueldo, M. R., Bruzzone, O. A., and Virla, E. G. (2010). Characterization of the earwig, doru lineare, as a predator of larvae of the fall armyworm, spodoptera frugiperda: a functional response study. J. Insect Sci. 10, 38. doi: 10.1673/031.010.3801
Tambo, J. A., Day, R. K., Lamontagne-Godwin, J., Silvestri, S., Beseh, P. K., Oppong-Mensah, B., et al. (2019). Tackling fall armyworm (Spodoptera frugiperda) outbreak in Africa: an analysis of farmers’ control actions. Int. J. Pest Manag. 66, 1–13. doi: 10.1080/09670874.2019.1646942
Taylor, P. J., Monadjem, A., and Nicolaas Steyn, J. (2013). Seasonal patterns of habitat use by insectivorous bats in a subtropical African agro-ecosystem dominated by macadamia orchards. Afr. J. Ecol. 51, 552–561. doi: 10.1111/aje.12066
Wan, J., Huang, C., Li, C., Zhou, H., Ren, Y., Li, Z., et al. (2021). Biology, invasion and management of the agricultural invader: Fall armyworm, Spodoptera frugiperda (lepidoptera: noctuidae). J. Int. Agricu. 20, 646–663. doi: 10.1016/S2095-3119(20)63367-6
Weier, S. M., Grass, I., Linden, V. M., Tscharntke, T., and Taylor, P. J. (2018). Natural vegetation and bug abundance promote insectivorous bat activity in macadamia orchards, South Africa. Biol. Conserv. 226, 16–23. doi: 10.1016/j.biocon.2018.07.017
Williams-Guillén, K., and Perfecto, I. (2011). Ensemble composition and activity levels of insectivorous bats in response to management intensification in coffee agroforestry systems. PLoS One 6:e16502. doi: 10.1371/journal.pone.0016502
Williams-Guillén, K., Perfecto, I., and Vandermeer, J. (2008). Bats limit insects in a neotropical agroforestry system. Science 320, 70–70. doi: 10.1126/science.1152944
Keywords: predator-prey interaction, biological pest control, insectivorous birds, bat predation on FAW, maize cultivation
Citation: Clarkson J, Borah JR, Baudron F and Sunderland TCH (2022) Forest Proximity Positively Affects Natural Enemy Mediated Control of Fall Armyworm in Southern Africa. Front. For. Glob. Change 5:781574. doi: 10.3389/ffgc.2022.781574
Received: 22 September 2021; Accepted: 08 April 2022;
Published: 06 May 2022.
Edited by:
Marije Schaafsma, University of Southampton, United KingdomReviewed by:
Dora Kilalo, University of Nairobi, KenyaKelli Hoover, The Pennsylvania State University (PSU), United States
Esayas Mendesil, Jimma University, Ethiopia
Maria Gabriela Murua, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina
Copyright © 2022 Clarkson, Borah, Baudron and Sunderland. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joli R. Borah, joliborah@gmail.com
 Juliet Clarkson
Juliet Clarkson Joli R. Borah
Joli R. Borah Frédéric Baudron
Frédéric Baudron Terry C. H. Sunderland
Terry C. H. Sunderland