- 1Guangdong Provincial Key Laboratory of Animal Nutrition Control, Institute of Subtropical Animal Nutrition and Feed, College of Animal Science, South China Agricultural University, Guangzhou, China
- 2University of Chinese Academy of Sciences, Beijing, China
- 3Laboratory of Animal Nutrition and Health and Key Laboratory of Agro-Ecology, Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha, China
- 4Jiangsu Co-Innovation Center for Important Animal Infectious Diseases and Zoo Noses, Joint International Research Laboratory of Agriculture and Agri-Product Safety of Ministry of Education of China, College of Veterinary Medicine, Yangzhou University, Yangzhou, China
- 5Academics Working Station at The First Affiliated Hospital, Changsha Medical University, Changsha, China
Betaine is a critical nutrient for mammal health, and has been found to alleviate inflammation by lowering interleukin (IL)-1β secretion; however, the underlying mechanisms by which betaine inhibits IL-1β secretion remain to be uncovered. In this review, we summarize the current understanding about the mechanisms of betaine in IL-1β production and release. For IL-1β production, betaine affects canonical and non-canonical inflammasome-mediated processing of IL-1β through signaling pathways, such as NF-κB, NLRP3 and caspase-8/11. For IL-1β release, betaine inhibits IL-1β release through blocking the exocytosis of IL-1β-containing secretory lysosomes, reducing the shedding of IL-1β-containing plasma membrane microvesicles, suppressing the exocytosis of IL-1β-containing exosomes, and attenuating the passive efflux of IL-1β across hyperpermeable plasma membrane during pyroptotic cell death, which are associated with ERK1/2/PLA2 and caspase-8/A-SMase signaling pathways. Collectively, this review highlights the anti-inflammatory property of betaine by inhibiting the production and release of IL-1β, and indicates the potential application of betaine supplementation as an adjuvant therapy in various inflammatory diseases associating with IL-1β secretion.
Introduction
Immune-cell-mediated inflammation is essential for host protection against infections and injuries. The immune system will coordinate the unanimous reaction to eliminate pathogens and restore tissue integrity in response to infections. Innate immune cells (e.g., macrophages) form the first line of defense to identify initial infections and injuries, and then to promote the recruitment of additional immune cells (e.g., T cells) by releasing cytokines and chemokines. The interleukin (IL)-1 family cytokines [for the history of IL-1, refer to the review (1)] are the central mediators of inflammation and play crucial roles in aforementioned processes (2, 3). Notably, IL-1β is the best-characterized and most extensively studied pro-inflammatory cytokine in IL-1 family, and plays a vital role in host defense in response to infections and injuries (4, 5). IL-1β is mainly produced by the activated-inflammatory cells (e.g., monocytes, microglia, and macrophages) with a multistep process involving synthesis of immature pro-IL-1β, proteolytic cleavage to mature IL-1β, and finally release into the extracellular environment (1, 6).
Betaine (trimethylglycine) is a stable and nontoxic natural compound (7) and shows a wide distribution within phylogenetically distant organisms from microorganisms to animals (8). Betaine is the basic biochemical molecule of the methionine/homocysteine cycle (9), and serves as a methyl group donor in transmethylation [a process catalyzed by betaine-homocysteine methyl transferase (BHMT)], and is essential for choline-mediated one-carbon metabolism, cell membrane integrity, signal transduction and neurotransmitter synthesis (10, 11). Besides, betaine is an important osmoprotectant, which modulates cell volume, and protects cells, proteins and enzymes from osmotic/ionic stress (12, 13). Notably, betaine has been proven to be effective against many inflammatory diseases (e.g., diabetes and NAFLD) with its anti-inflammatory functions (14, 15). Interestingly, betaine participates in alleviation of inflammation by lowering secretion of pro-inflammatory cytokines (e.g., IL-1β, TNF-α, IL-6, and IL-23) (16). Increasing studies have reported that betaine dampens activity of nuclear factor kappa B (NF-κB) to block the expression of genes involved in inflammation, such as IL-1β, COX-2, and iNOS (17, 18). Additionally, betaine can restore normal energy metabolism to relieve systemic low-grade inflammation (e.g., obesity and diabetes) (15, 19–22); and for the main metabolic pathways and crucial mediators modulated by betaine in chronic inflammation, refer to the review (23). Since IL-1β is the central mediators of inflammation, it is worthy of lowering IL-1β secretion to alleviate inflammation. Although, betaine inhibits NOD-like receptor (NLRP) 3 inflammasome activation, which highly shapes the pro-IL-β maturation (24–26), the underlying mechanisms by which betaine inhibits IL-1β secretion are still not fully understood.
In this review, we discuss the potential mechanisms by which betaine could inhibit the IL-1β production through canonical and non-canonical inflammasome-mediated processing of IL-1β, and inflammasome-independent sources of IL-1β. Then, we highlight the evidence about the key roles of betaine in inhibition of IL-1β release with special emphasis on the involved mechanisms, including exocytosis of IL-1β-containing secretory lysosomes, shedding of IL-1β-containing plasma membrane microvesicles, exocytosis of IL-1β-containing exosomes, and passive efflux of IL-1β across hyperpermeable plasma membrane during pyroptotic cell death.
Betaine Inhibits IL-1β Production
IL-1β production involves the synthesis of immature pro-IL-1β (31 kD) by the recognition of toll-like receptors (TLRs) (27), and proteolytic cleavage to mature IL-1β (17 kD) by caspase-1 (28). In this section, we summarize the mechanisms by which betaine inhibits IL-1β production, including the canonical and non-canonical inflammasome-mediated processing of IL-1β and inflammasome-independent sources of IL-1β.
Betaine in Canonical Inflammasome-Mediated Processing of IL-1β
As we discussed in the previous part, unprovoked immune cells (when they are under steady-state conditions), like monocytes and macrophages, do not express or just express extremely low level of IL-1β. However, the pro-inflammatory triggers [e.g., (tumor necrosis factor) TNF, IL-1α/6, and TLR-ligands] promote the activation of NF-κB, and expression of IL-1β (29, 30).
Betaine suppresses NF-κB activity and its downstream genes (e.g., IL-1β) expression via inhibiting mitogen-activated protein kinases (MAPKs) and nuclear factor-including kinase/IκB kinase (NIK/IKK) in the aged rats and rat endothelial YPEN-1 cells (18, 31) (Figure 1A). MAPKs include c-Jun NH2-terminal kinase (JNK), protein 38 (p38) and extracelluar signal-regulated kinase (ERK1/2), and are responsible for the expressions of pro-inflammatory cytokines (32); and NIK/IKK relieves the inhibition of IκB, leading to the activation of NF-κB (33). Moreover, betaine also inhibits TLRs which are involved in NF-κB activation (Figure 1B). For instance, in LPS (a TLR4 ligand)-stimulated RAW264.7 cells, betaine suppresses the activation of NF-κB (34). Mechanistically, in high-fat-diet-induced NAFLD rat models, betaine inhibits the mRNA and protein expression of high-mobility group box 1(HMGB1) in liver tissues, which regulates the activation of TLR4 (35) (Figure 1B). Additionally, in fructose-fed rat astrocytes, it is supposed that betaine could suppress the expression of histone deacetylases 3 (HDAC3), which binds to IκBα to activate NF-κB (24) (Figure 1C). Collectively, IL-1β is one of the most important downstream genes of NF-κB; and increasing in vitro and in vivo studies have demonstrated that betaine dampens NF-κB activation. Thus, these findings indicate that betaine inhibits IL-1β production via inhibition of NF-κB signaling pathway.
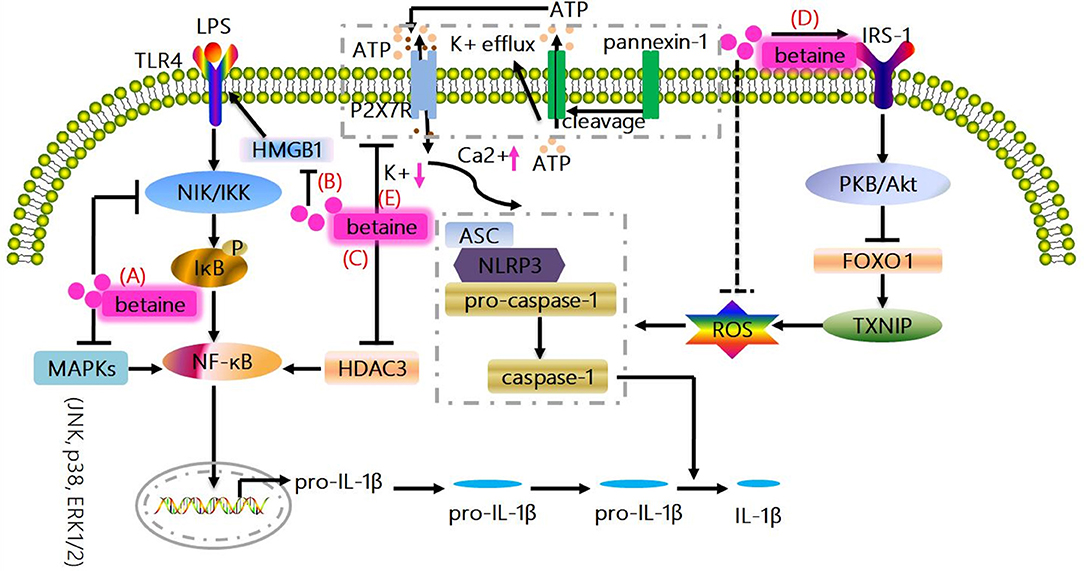
Figure 1. Canonical mechanisms whereby betaine inhibits IL-1β production. (A) MAPKs (JNK, p38, and ERK1/2) are responsible for the expressions of pro-inflammatory cytokines; and NIK/IKK relieves the inhibition of IκB, resulting in the activation of NF-κB to promote the up-regulation of IL-1β. Betaine suppresses NF-κB activity and IL-1β expression via inhibiting MAPKs and NIK/IKK. (B) Betaine inhibits the mRNA and protein expression of HMGB1 which regulates the activation of TLR4, which are involved in NF-κB activation. (C) Betaine suppresses HDAC3 which binds to IκBα to activate NF-κB. (D) NLR-driven ASC recruitment drives pro-caspase-1 activation leading to pro-caspase-1 cleavage and caspase-1 maturation, subsequently caspase-1 cleaves pro-IL-1β to produce the bioactive IL-1β. Betaine enhances IRS-1 phosphorylation to activate PKB/Akt which results in FOXO1 inactivation leading to a FOXO1 inhibition of TXNIP, which functions as the endogenous inhibitor of ROS-scavenging protein, thereby inhibiting the activation of NLRP3 inflammasome. (E) Moreover, the activation of NLRP3 inflammasome is related to the K+ efflux caused by ATP-mediated P2X7R activation. Betaine suppresses NLRP3 activation through maintaining cytosolic normal K+ levels. JNK, c-Jun NH2-terminal kinase; p38, protein 38; ERK1/2, extracelluar signal-regulated kinase; IL-1β, interleukin-1β; NF-κB, nuclear factor kappa B; MAPKs, mitogen-activated protein kinases; NIK/IKK, nuclear factor-including kinase/IκB kinase; HMGB1, high-mobility group box 1; TLR, toll-like receptor; HDAC3, histone deacetylases 3; IRS-1, insulin receptor substrate 1; FOXO1, forkhead box O 1; TXNIP, thioredoxin interacting protein; ROS, reactive oxygen species; P2X7R purinergic; ligand-gated ion channel 7 receptor; NLRP3, NOD-like receptor.
The activated caspase-1 in the canonical inflammasome complex is the most extensively identified mechanism for IL-1β processing. In details, the canonical inflammasomes contain cytosolic sensor molecules [NOD-like receptor (NLR) and absent in melanoma (AIM) 2-like receptor (ALR) families], caspase-1, and adaptor molecule ASC (36, 37). Mechanistically, NLR-driven ASC recruitment drives pro-caspase-1 activation resulting in pro-caspase-1 cleavage and caspase-1 maturation. Then caspase-1 cleaves pro-IL-1β to produce the mature forms of IL-1β (38–40). NLRs include NLRP1, NLRP3 (the main platform for IL-1β processing), NLRP6, NLRP7, NLRP12, and NLRC4, which are all suggested to coordinate inflammasome signaling and induce IL-1β production under specific conditions (41–44), though there exist negative effects (45–48). Indeed, the recognition of pathogen- and/or danger-associated molecular patterns (PAMPs and/or DAMPs) (e.g., bacterial toxins, fungal products, ATP, silica, ceramide, cholesterol crystals, and amyloid β) provokes the inflammasome-mediated IL-1β production, especially via NLRP3 activation (49–51). NLRP3 has a specific disulfide bond between Cys-8 and Cys-108 that may involve in modulation of NLRP3 activation by reactive oxygen species (ROS) based on a high resolution structure analysis (52). Likewise, a study showed that liposomes could induce NLRP3 inflammasome activation by mtROS (53). Extensive studies reveal that the activation of NLRP3 inflammasome is associated with the decreased cytosolic K+ level (called K+ efflux) caused by ATP-mediated purinergic ligand-gated ion channel 7 receptor (P2X7R) activation (39, 54–56). K+ efflux also regulates NLRC4 and NLRP1b activation (57, 58); however, how K+ concentration regulates the assembly of NLRP3 into functional inflammation is unclear. Thus, it is critical to impede IL-1β processing by suppressing the activation of NLRP3 inflammasome.
Increasing studies prove that betaine blocks NLRP3 inflammasome activation in vivo (24–26, 59, 60). Betaine suppresses NLRP3 inflammasome involving a forkhead box O 1(FOXO1) inhibition of thioredoxin interacting protein (TXNIP) which functions as the endogenous inhibitor of ROS-scavenging protein, enhancing ROS to induce NLRP3 inflammasome assembly in macrophages from insulin-resistant obese db/db mice (15, 61). Mechanistically, we suggest that betaine enhances insulin receptor substrate 1 (IRS-1) phosphorylation to indirectly activate PKB/Akt, which results in FOXO1 inactivation through phosphorylating the activated FOXO1 to induce its transfer from the nucleus into the cytoplasm, leading to the inhibition of NLRP3 inflammasome (19, 26) (Figure 1D). Additionally, emerging evidences have demonstrated that betaine enhances/restores Na+-K+-ATPase activity which maintains low Na+ and high K+ cell homeostasis (62, 63), and similarly, reduces K+ efflux (64); therefore, we speculate that betaine suppresses NLRP3 activation through maintaining cytosolic normal K+ levels (Figure 1E). Hence, betaine inhibits IL-1β processing via blocking NLRP3 inflammasome activation directly or through IRS-1/PKB/Akt/FOXO1 signaling pathway to resist the activation of NLRP3 indirectly.
Intriguingly, in macrophages, enhanced pro-IL-1β processing is associated with caspase-1; however, caspase-1-independent mechanism of IL-1β processing accounts for IL-1β secretion in neutrophils (65). Mechanistically, in neutrophils, IKKβ-driven NF-κB positively modulates pro-IL-1β mRNA and serine protease inhibitor genes transcription whose products block the proteinase (PR3) activity, which can process pro-IL-1β (65, 66). Unfortunately, whether betaine affects serine protease inhibitor genes remains largely unexplored. Other mechanisms that beyond the core machinery of the inflammasome complex, are also associated with inflammasome assembly, including NLRP3. For example, double-stranded RNA-dependent protein kinase (PKR) and guanylate binding protein (GBP) 5 both contribute to the NLRP3 oligomerization and activation via physically interacting with certain inflammasome components (67, 68). However, whether betaine inhibits NLRP3 inflammasome activation through influencing their physical interaction with several components (e.g., ASC) still require comprehensive investigation. Various cell types and stimuli determine the activation of NLRP3 inflammasome. For example, the NLRP3 inflammasome can be spontaneously activated by primary stimulation of human monocytes, during which PAMP and DAMP provide ample signals to produce bioactive IL-1β (69). M. tuberculosis still triggers the maturation and production of IL-1β in human monocyte-derived macrophages (70), although it inhibits inflammasome activation (71); however, murine microglia primed with conditioned media from cultures of macrophages infected with M. tuberculosis result in caspase-1 activation and IL-1β production in a NLRP3- and ASC-dependent manner (72). Besides, in unprimed bone marrow-derived macrophages, C. pneumonia infection causes IL-1β maturation and production through NLRP3/ASC/caspase-1 pathway (73); however, Orientia tsutsugamushi triggers IL-1β production in macrophages merely via the activation of ASC inflammasome instead of NLRP3 (74). Overall, these mentioned findings indicate that different cell types and/or stimuli make multitudinous patterns of NLRP3 activation and IL-1β production. Therefore, it would be highly interesting to conduct comparative studies on the effects of betaine on canonical NLRP3 activation and IL-1β production of innate immune cells of different origins (e.g., monocytes, macrophages, and microglia) and/or under different conditions (e.g., unprimed/primed, infection, and injuries).
Betaine in Non-canonical Inflammasome-Mediated Processing of IL-1β
Intriguingly, additional caspases and modulators (e.g., caspase-8/11) have emerging roles in inflammasome-mediated IL-1β maturation. Caspase-8, which regulates extrinsic apoptosis in response to TNF receptor 1 (TNFR1) and Fas activation (75), also modulates pro-IL-1β cleavage at exactly the same site as caspase-1 (76–78). In myeloid cells, Fas engagement triggers caspase-8-dependent IL-1β production through pathway that is fully independent of caspase-1 (79). Although caspase-8 and caspase-1 share the same cleavage site, the caspase-8-dependent IL-1β production does not require caspase-1 participation. CrmA (an inhibitor for caspase-8) inhibits the generation of IL-1β induced by LPS, though the exact mechanism still need to be unraveled (76). Furthermore, an in vitro study reported that both canonical and non-canonical (caspase-11 dependent) inflammasome activation and down-stream IL-1β processing are extremely restrained in RIP3−/−× Caspase-8−/− cells (80). Interestingly, in endoplasmic reticulum (ER) stress, caspase-8-mediated IL-1β maturation does not need ASC expression (81).
Under healthy conditions, caspase-8 is usually present in monomeric form as an inactive enzyme; however, the binding of Fas-associated death domain (FADD) to death receptors facilitates the recruitment of monomeric caspase-8 zymogens, which in turn causes caspase-8 homodimerization and subsequent caspase-8 activation (75). Except for exerting its classic function in apoptosis, caspase-8 also plays a vital role in promoting NF-κB signaling in antigen-stimulated T and B cells (75). Similarly, FADD and caspase-8 control the transcriptional priming of the NLRP3 inflammasome via modulation of the NLRP3 and pro-IL-1β expression. Multiple of reports have been demonstrated that betaine significantly blocks caspase-8 activation and/or reduces caspase-8 activity (59, 82, 83). Thus, betaine may inhibit IL-1β production by preventing the induction of caspase-8 activity/activation (Figure 2A). In fungi- and/or mycobacteria-stimulated dendritic cells (DCs), the triggering of dectin-1 can promote Syk-dependent formation of the CARD9-Bcl-10-MALT1 scaffold that induces NF-κB activation and IL-1β transcription; as well as the formation and activation of a MALT1-caspase-8-ASC complex that mediates the processing of pro-IL-1β (77, 84). As betaine blocks the activation of caspase-8, therefore, we suggest that betaine reduces IL-1β production through suppressing the formation and activation of MALT1-caspase-8-ASC complex (Figure 2B), though there are no current evidence on the effects of betaine on CARD9, Bcl-10, and MALT1, respectively. Summarily, considering caspase-8 controls human macrophage differentiation (85) and human monocyte and microglia activation (86, 87), it is obvious that caspase-8 participates in regulation of cytokines production by these immune cells. Indeed, caspase-8-deficient macrophages and/or DCs are hyperresponsive to TLR activation, and caspase-8 is required for normal M1 macrophage polarization whose markers include IL-1β [Ref. (88)]. Betaine appears to inhibit IL-1β production by reducing caspase-8 activity; however, no specific mechanisms (e.g., RIPK1/caspase-8/RIPK3/MLKL) by which betaine prevents the induction of caspase-8 activation have been found so far (85, 86).
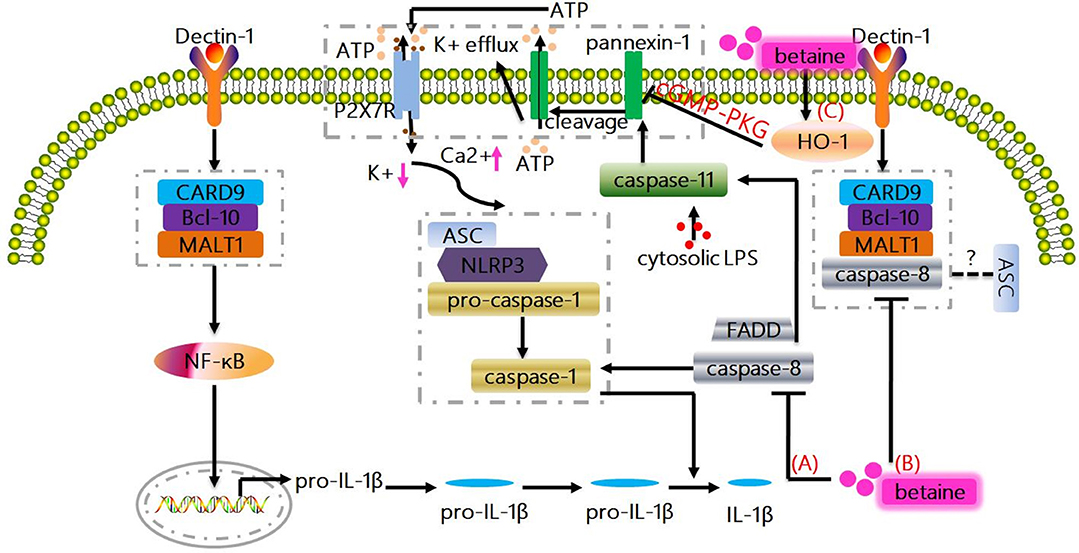
Figure 2. Non-canonical mechanisms whereby betaine inhibits IL-1β production. (A) Caspase-8 plays a vital role in promoting NF-κB signaling, and also modulates pro-IL-1β cleavage at exactly the same site as caspase-1. Actually, FADD and caspase-8 control the transcriptional priming of the NLRP3 inflammasome via modulation of the NLRP3 and pro-IL-1β expression. Betaine inhibits IL-1β production by preventing the induction of caspase-8 activity/activation. (B) The triggering of dectin-1 promotes Syk-dependent formation of the CARD9-Bcl-10-MALT1 scaffold that induces NF-κB activation and IL-1β transcription; as well as the formation and activation of a MALT1-caspase-8-ASC complex that mediates the processing of pro-IL-1β. Betaine reduces IL-1β production through suppressing the formation and activation of MALT1-caspase-8-ASC complex. (C) Caspase-11 is also essential for the NLRP3 inflammasome activation culminating with IL-1β production. Mechanistically, LPS-activated caspase-11 induces plasma membrane channel pannexin-1 cleavage, leading to K+ efflux and ATP release that interacts with P2X7R to promote the activation of NLRP3. Interestingly, betaine reduces IL-1β production by increasing HO-1 expression to activate cGMP-PKG signaling pathway, which ameliorates the pannexin-1 channel activity. HO-1, heme oxygenase-1; PKG, protein kinase G.
Likewise, a study reported that the caspase-1−/− mice is also deficient in caspase-11 (an executioner caspase which promotes pyroptosis or cell death; human orthologues caspase-4/5) expression (89–91). In caspase-1−/− and caspase-11−/− cells, it turned out that caspase-11 is required for caspase-1 activation and IL-1β maturation in response to exogenous stimulus, like E. coli and toxin (89, 92, 93). Mechanistically, in mouse macrophages and/or human myeloid cells, the direct interaction between pro-caspase-11 or pro-caspase-4/5 and LPS by binding of the CARD motif of pro-caspase-11 or pro-caspase-4/5 and the lipid A tail of LPS results in non-canonical inflammasome assembly (Figure not shown) (94–96). Moreover, a non-canonical pathway involving caspase-11 is also essential for the NLRP3 inflammasome activation culminating with IL-1β production. For instance, enterohemorrhagic E. coli (EHEC) infection induces the activation of caspase-11 in NLRP3 inflammasome via TRIF-dependent pathway in bone marrow-derived macrophages (93). In LPS-primed bone marrow-derived macrophages, LPS-activated caspase-11 (functions as a cytosolic LPS sensor) triggers plasma membrane channel pannexin-1 cleavage, resulting in K+ efflux and ATP release that interacts with P2X7R to promote the activation of NLRP3. Notablly, the caspase-11/pannexin-1/NLRP3 is considered as an important mechanism for IL-1β production (97). Indeed, pannexin-1 channel activity can be attenuated by NO and heme oxygenase-1 (HO-1) via activating cGMP-protein kinase G (PKG) signaling pathway (98–100). Interestingly, betaine directly increases the expression levels of HO-1, and this effect may inhibit the NLRP3 inflammasome (101). Taken together, betaine may reduce IL-1β production by increasing HO-1 expression to activate cGMP-PKG signaling pathway, which ameliorates the pannexin-1 channel activity (Figure 2C). Obviously, this potential mechanism needs to be completely elucidated. The delivered intracellular LPS could significantly trigger caspase-11 non-canonical inflammasome activation and IL-1β production in a type I IFN signaling-independent manner (102). Thus, it is interesting to investigate whether betaine can influence gene expression induced by type I IFNs which is responsible for cytoplasmic sensing of LPS by caspase-11 in the future.
Betaine in Inflammasome-Independent Sources of IL-1β
As described in aforementioned sections, inflammasome formation associated with caspase-1 and/or caspase-11 is the most important mechanism for the processing of IL-1β. However, inflammasome-independent ways also affect inflammation and diseases from the observations (uncompleted abrogation of IL-1β production) found in deletion of caspase-1/11 in inflammatory disease models (e.g., osteomyelitis and arthtitis) (103–105). Cathepsin C/G, elastase, chymase, and proteinase-3 are all responsible for cleaving pro-IL-1β into activated IL-1β (106, 107). Mechanistically, in previous mentioned inflammatory diseases, cathepsin C uniquely modulates inflammasome-independent IL-1β production and genetic deletion of cathepsin C significantly lowers IL-1β levels (108). Pharmacological inhibition of elastase and chymase diminish IL-1β production (105). Also, genetic and pharmacological inhibition of proteinase-3 has critical role in mitigating IL-1β-mediated inflammation (107). However, no lines of evidence (direct or indirect) present the effects of betaine in inflammasome-independent sources of IL-1β currently.
Betaine Inhibits IL-1β Release
Release of mature IL-1β into the extracellular environment is essential for IL-1β to exert its host defense function in response to infections and injuries. The mature IL-1β release depends on the non-canonical pathways of export from the cytosol (109–111). As lack of conventional signal peptide, IL-1β cannot target to the conventional ER-Golgi secretory pathway as the same as other cytokines resulting in the accumulation of IL-1β in cytosol (109). In this section, we summarize the influences of betaine in four main possible mechanisms for IL-1β release, including exocytosis via secretory lysosomes, microvesicle shedding from plasma membrane, release of exosomes, and passive efflux across leaky plasma membrane during pyroptotic cell death.
Betaine in Exocytosis of IL-1β-Containing Secretory Lysosomes
A study conducted in 1990 presented the evidence that in activated human monocytes, inhibition of protein transport and secretion through the ER-Golgi formation of endo-membrane system has little effect on IL-1β release (109); however, the IL-1β release is closely related to secretory lysosomes (109). Indeed, the exocytic process can be stimulated by ATP (released from dying cells, etc.), and the subsequent migration of exocytic lysosomes to the plasma membrane allow the content (e.g., IL-1β) trapped in lysosomes, to secret into extracellular compartment (112). Moreover, in human monocytes and mouse macrophages, once ATP-mediated P2X7R activation, the IL-1β and caspase-1 localize to secretory lysosomes and secrete with the lysosomal enzymes (113, 114). Mechanistically, the above process is related to the P2X7R-induced K+ efflux, which leads to the activation of phosphatidylcholine-specific phospholipase C (PLC) to increase intracellular Ca2+ concentration, resulting in Ca2+-dependent phospholipase A2 (PLA2) activation and exocytosis of the IL-1β-containing lysosomes (115). The aforementioned processes can be blocked by using the inhibitors of phosphatidylcholine-specific PLC and/or PLA2. Indeed, in mouse macrophages and/or rat astrocytes, PLA2 activity has been showed to be regulated by phosphorylation by ERK1/2 and ROS (116, 117). Based on previous discussed section, betaine reduces ROS level in stressed cells; and the ERK1/2 signaling pathway could be shut off by betaine in adipogenic-differentiated C2C12 cells (118). Thus, we speculate that betaine may slow down IL-1β release via suppressing exocytosis of the IL-1β-containing lysosomes through reducing the activity of PLA2 by blocking the ERK1/2 signaling pathway (Figure 3A) and/or lowering the ROS level (Figure 3C). However, this needs experimental validation, and the exact mechanisms by which betaine regulates the IL-1β release through inhibiting lysosome exocytosis remain to be revealed.
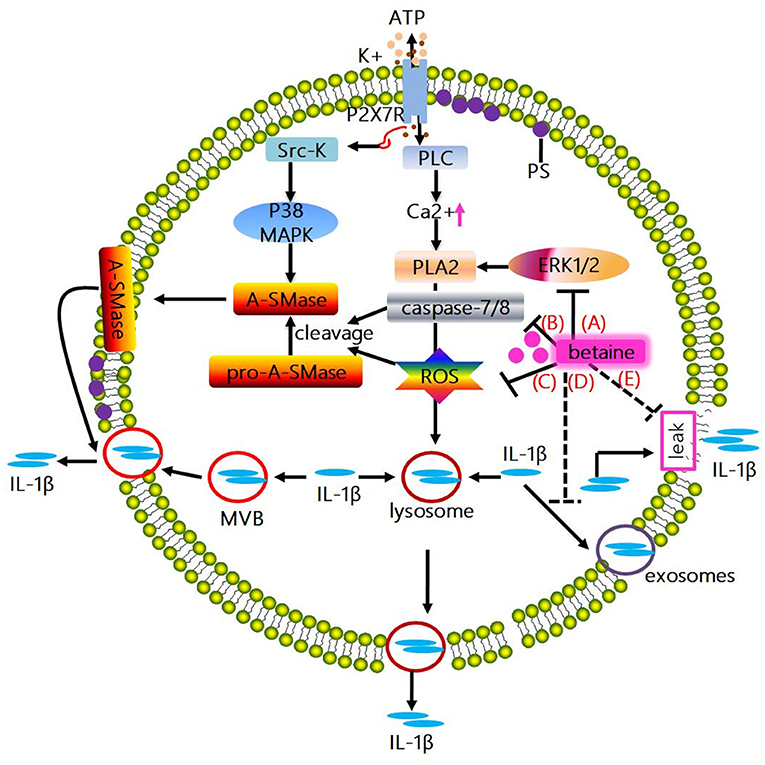
Figure 3. Mechanisms whereby betaine inhibits IL-1β release. (A) The activation of ATP-mediated P2X7R promotes IL-1β and caspase-1 localize to secretory lysosomes together with the lysosomal enzymes secretion. Mechanistically, the above process is associated with the P2X7R-induced K+ efflux, which enhances the activation of PLC, thus increasing intracellular Ca2+ concentration, enabling Ca2+-dependent PLA2 activation and promoting exocytosis of the IL-1β-containing lysosomes. Actually, PLA2 activity has been showed to be regulated by phosphorylation by ERK1/2 and ROS. Betaine inhibits IL-1β release via suppressing exocytosis of the IL-1β-containing lysosomes through reducing the activity of PLA2 by blocking the ERK1/2 signaling pathway and/or lowering the ROS level. (B) ATP-induced P2X7R activation promotes the C-terminal domain interacts with Src-K, phosphorylating the subsequent p38 MAPK, inducing acidic A-SMase delivers from the inner to the outer plasma membrane. Subsequently, A-SMase hydrolyzes sphingomyelin to generate ceramide, altering membrane fluidity, promoting the formation of plasma membrane blebs and resulting in shedding of IL-1β-containing microvesicles. And microvesicle shedding is preceded by flip of PS to the outer leaflet of the plasma membrane. Interestingly, A-SMase can be activated by proteolytic cleavage of pro-A-SMase by caspase-8 and caspase-7 or ROS. Betaine inhibits IL-1β release via blunting the IL-1β-containing microvesicle shedding by blocking the activation of A-SMase through inhibiting caspase-7/8 activation. (C) Betaine inhibits IL-1β release by restraining the IL-1β-containing microvesicle shedding by reducing ROS level. (D) IL-1β release is also involving exocytosis of exosomes; besides, MVBs formation and IL-1β and caspase-1 accumulation can be tightly modulated by inflammasome complex. Betaine lowers the release of IL-1β by inhibiting the formation of MVBs and exosomes via inhibiting the NLRP3 inflammasome activation. (E) Moreover, IL-1β is passively released alongside DAMPs following plasma membrane rupture. Betaine blunts the passive efflux of IL-1β through its effects on protecting cell membrane from external membrane-perturbing compounds-induced rupture. PLA2, phospholipase A2; ERK1/2, extracelluar signal-regulated kinase; A-SMase, acidic sphingomyelinase; ROS, reactive oxygen species; MVBs, multivesicular bodies.
Betaine in Shedding of IL-1β-Containing Plasma Membrane Microvesicles
The in vitro cell models (e.g., THP-1 monocyte, DCs, and microglia) stimulated by P2X7R have demonstrated that a mechanism for IL-1β release depends on shedding of plasma membrane microvesicles (119–121). Mechanistically, ATP-induced P2X7R activation enhances the C-terminal domain interaction with src-protein tyrosine kinase (Src-K) to phosphorylate the subsequent p38 MAP kinase (p38 MAPK), inducing acidic sphingomyelinase (A-SMase) delivery from the inner to the outer plasma membrane. Subsequently, A-SMase hydrolyzes sphingomyelin to generate ceramide, altering membrane fluidity, promoting the formation of plasma membrane blebs and resulting in shedding of IL-1β-containing microvesicles (122). Shed microvesicles possess many phospholipids and proteins [e.g., phosphatidylserine (PS), P2X7R, pro-caspase-1, pro-IL-1β, and IL-1β]. Microvesicle shedding is preceded by flip of PS to the outer leaflet of the plasma membrane; however, the exact mechanism by which IL-1β effluxes out of the microvesicles is still obscure. Interestingly, betaine takes part in the above mentioned processes. A-SMase can be activated by proteolytic cleavage of pro-A-SMase by caspase-8 and caspase-7 (123) or ROS (124, 125). Based on the above discussed section, betaine significantly blocks caspase-8 activation and/or reduces caspase-8 activity (59, 82, 83); and attenuates caspase-7 activation (126), and lowers ROS level. Therefore, betaine seems to inhibit IL-1β release via blunting the IL-1β-containing microvesicle shedding by blocking the activation of A-SMase through inhibiting caspase-7/8 activation (Figure 3B) and/or reducing ROS level (Figure 3C). However, these findings are mainly found in non-immune cells (e.g., PC12 cells) and the possible mechanisms whereby betaine targets microvesicles shed from innate immune cells are not currently available.
Betaine in Exocytosis of IL-1β-Containing Exosomes
Exosomes is the fusion of multivesicular bodies (MVBs) with the cell plasma membrane. A non-canonical pathway for IL-1β release involving exocytosis of exosomes is also found in P2X7R-stimulated macrophages, DCs and B-lymphocytes (127); and pro-IL-1β, pro-caspase-1, bioactive caspase-1, IL-1β, MHCI, and MHCII [a feature of exosomes originated from antigen presenting cells (APCs)] do exist in the exosomes secreted from these cells (127). Interestingly, a study reported that the release of IL-1β and MHCII can be significantly blocked in ASC−/− and NLRP3−/− mice (128). Thus, it seems that MVBs formation and IL-1β and caspase-1 accumulation can be tightly modulated by inflammasome complex. Notably, the aforementioned section indicate that betaine inhibits NLRP3 inflammasome activation, thus we suggest that betaine may lower the release of IL-1β by inhibiting the formation of MVBs and exosomes, though the specific mechanisms still remain to be identified (Figure 3D).
Betaine in Passive Efflux of IL-1β Across Hyperpermeable Plasma Membrane During Pyroptotic Cell Death
IL-1β release is closely related to a loss in membrane integrity during pyroptotic cell death (129–132). Due to the caspase-1/11 drives cell apoptosis and/or pyroptosis and IL-1β cleavage, IL-1β is passively released alongside DAMPs following plasma membrane rupture (133–135). Indeed, ATP-mediated IL-1β release but not its processing can be absolutely blocked by punicalagin which functions as an inhibitor to limit plasma membrane damage induced by external membrane-perturbing compounds (132). Likewise, betaine is essential for maintaining cell membrane integrity and serves as an osmolyte that regulates cell volume and protects cells from environmental stresses (10, 12), and inhibits various hyperosmotic-induced apoptosis-related proteins (e.g., caspase-3/8/9) activity in MDCK cells (83). Therefore, betaine may blunt the passive efflux of IL-1β through its effects on protecting cell membrane from external membrane-perturbing compounds-induced rupture, though the exact mechanism is still not clear (Figure 3E).
Concluding Remarks
IL-1β plays overarching roles in stimulation of innate immune system and inflammatory processes/diseases (136, 137). Various nutrients have proven to be effective in modulation of inflammation and inflammatory diseases by lowering IL-1β secretion (138). Betaine is a stable and nontoxic natural nutrient and has anti-inflammatory effects (16). Mechanistically, betaine inhibits IL-1β production through various pathways, such as NF-κB, canonical NLRP3, and caspase-8/11 (Figures 1, 2). Betaine also inhibits IL-1β release via pathways including ERK1/2/PLA2, caspase-8/A-SMase, MVBs and exosomes (Figure 3). Therefore, it is meaningful to develop betaine as a dietary adjuvant therapy in diverse inflammatory diseases involving in IL-1β secretion (Figure 4). Inflammasome-independent pathway also affects inflammatory process and inflammatory diseases; thus, it is worthy of investigating the effects of betaine in inflammasome-independent sources of IL-1β. Additionally, the P2X7R is responsible for ATP-mediated mature IL-1β release (139); however, whether betaine affects IL-1β release by influencing P2X7R activity remains to be revealed. A study showed that caspase-11 controls IL-1β release through degradation of transient receptor potential channel (TRPC) 1 (140); nevertheless, no current relation between betaine and TRPC1 has been found. Given betaine alters gene expression/function via epigenetic modifications [e.g., miRNAs and DNA methylation (141)], therefore, it is interesting to further study the involvement of epigenetic modification in the effects of betaine in inhibiting IL-1β production and release.
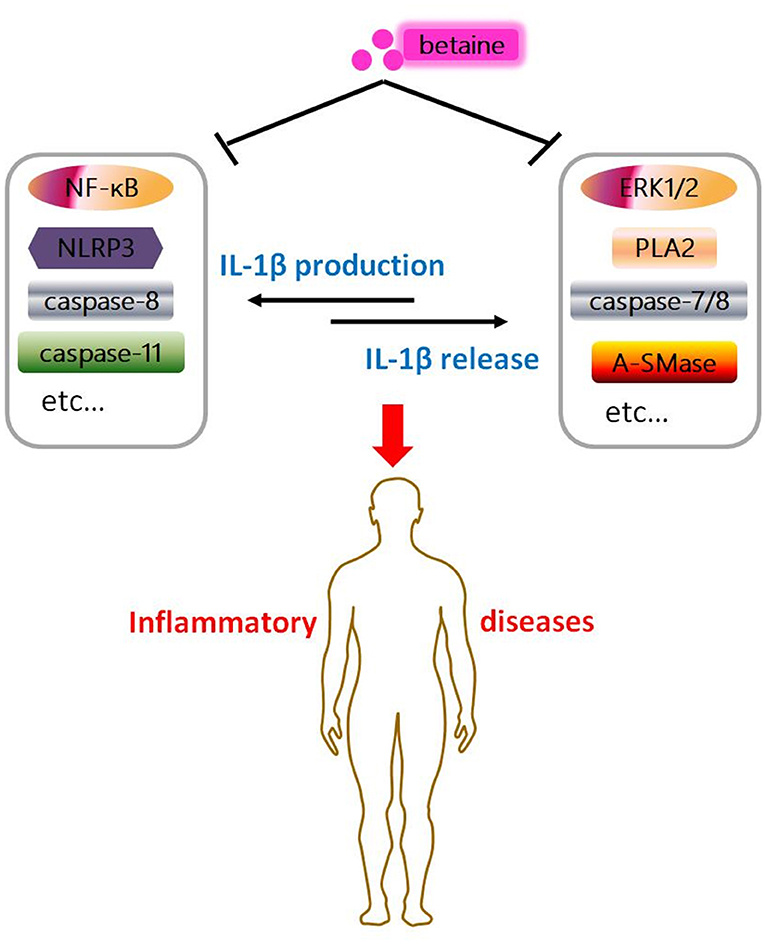
Figure 4. Graphical abstract of betaine in IL-1β secretion. Betaine inhibits IL-1β production and release through various pathways, respectively. And it indicates betaine functions as a dietary adjuvant therapy in diverse inflammatory diseases involving IL-1β secretion.
Author Contributions
YX and WR designed the review article, and YX wrote the review article. RH, YY, and WR revised the review article. YX, SC, and GZ helped with designing figures and finding relevant literature. WR approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Our profound admiration and respect go to researchers in this field and in our laboratories, for their dedication and hard work. We apologize to scientists whose work is in this field if their papers are not cited owing to space limitations. This work was supported by National Key R&D Program of China (No. 2018YFD0500600) and the National Natural Science Foundation of China (Grant Nos. 31872365, 31790411).
Abbreviations
IL, interleukin; BHMT, betaine-homocysteine methyl transferase; NF-κB, nuclear factor kappa B; NLR, NOD-like receptor; TLRs, toll-like receptors; TNF, tumor necrosis factor; MAPKs, mitogen-activated protein kinases; NIK/IKK, nuclear factor-including kinase/IκB kinase; JNK, c-Jun NH2-terminal kinase; p38, protein 38; ERK, extracelluar signal-regulated kinase; HMGB1, high-mobility group box 1; HDAC3, histone deacetylases 3; AIM2, absent in melanoma 2; ALR, AIM2-like receptor; PAMP, pathogen-associated molecular pattern; DAMP, danger-associated molecular pattern; P2X7R, purinergic ligand-gated ion channel 7 receptor; FOXO1, forkhead box O 1; TXNIP, thioredoxin interacting protein; ROS, reactive oxygen species; IRS-1, insulin receptor substrate 1; ER, endoplasmic reticulum; FADD, Fas-associated death domain; PKG, protein kinase G; HO-1, heme oxygenase-1; PLC, phospholipase C; PLA2, phospholipase A2; Src-K, src-protein tyrosine kinase; A-SMase, acidic sphingomyelinase; PS, phosphatidylserine; MVBs, multivesicular bodies; APCs, antigen presenting cells.
References
1. Giuliani AL, Sarti AC, Falzoni S, Di Virgilio F. The P2X7 Receptor-Interleukin-1 Liaison. Front Pharmacol. (2017) 8:123. doi: 10.3389/fphar.2017.00123
2. Lukens JR, Gross JM, Kanneganti TD. IL-1 family cytokines trigger sterile inflammatory disease. Front Immunol. (2012) 3:315. doi: 10.3389/fimmu.2012.00315
3. Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood (2011) 117:3720–32. doi: 10.1182/blood-2010-07-273417
5. Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. (2010) 6:232–41. doi: 10.1038/nrrheum.2010.4
6. Lukens JR, Kanneganti TD. Beyond canonical inflammasomes: emerging pathways in IL-1-mediated autoinflammatory disease. Semin Immunopathol. (2014) 36:595–609. doi: 10.1007/s00281-014-0434-8
7. Craig SA. Betaine in human nutrition. Am J Clin Nutr. (2004) 80:539–49. doi: 10.1093/ajcn/80.3.539
8. Steenge GR, Verhoef P, Katan MB. Betaine supplementation lowers plasma homocysteine in healthy men and women. J Nutr. (2003) 133:1291–5. doi: 10.1093/jn/133.5.1291
9. Ueland PM. Choline and betaine in health and disease. J Inherit Metab Dis. (2011) 34:3–15. doi: 10.1007/s10545-010-9088-4
10. Zeisel SH, Blusztajn JK. Choline and human nutrition. Annu Rev Nutr. (1994) 14:269–96. doi: 10.1146/annurev.nu.14.070194.001413
11. Hoffmann L, Brauers G, Gehrmann T, Haussinger D, Mayatepek E, Schliess F, et al. Osmotic regulation of hepatic betaine metabolism. Am J Physiol Gastrointest Liver Physiol. (2013) 304:G835–46. doi: 10.1152/ajpgi.00332.2012
12. Teixido N, Canamas TP, Usall J, Torres R, Magan N, Vinas I. Accumulation of the compatible solutes, glycine-betaine and ectoine, in osmotic stress adaptation and heat shock cross-protection in the biocontrol agent Pantoea agglomerans CPA-2. Lett Appl Microbiol. (2005) 41:248–52. doi: 10.1111/j.1472-765X.2005.01757.x
13. Kempson SA, Vovor-Dassu K, Day C. Betaine transport in kidney and liver: use of betaine in liver injury. Cell Physiol Biochem. (2013) 32:32–40. doi: 10.1159/000356622
14. Jung YS, Kim SJ, Kwon DY, Ahn CW, Kim YS, Choi DW, et al. Alleviation of alcoholic liver injury by betaine involves an enhancement of antioxidant defense via regulation of sulfur amino acid metabolism. Food Chem Toxicol. (2013) 62:292–8. doi: 10.1016/j.fct.2013.08.049
15. Kim DH, Kim SM, Lee B, Lee EK, Chung KW, Moon KM, et al. Effect of betaine on hepatic insulin resistance through FOXO1-induced NLRP3 inflammasome. J Nutr Biochem. (2017) 45:104–14. doi: 10.1016/j.jnutbio.2017.04.014
16. Slow S, Elmslie J, Lever M. Dietary betaine and inflammation. Am J Clin Nutr. (2008) 88:247–8; author reply 248. doi: 10.1093/ajcn/88.1.247
17. Monaco C, Andreakos E, Kiriakidis S, Mauri C, Bicknell C, Foxwell B, et al. Canonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc Natl Acad Sci USA. (2004) 101:5634–9. doi: 10.1073/pnas.0401060101
18. Go EK, Jung KJ, Kim JY, Yu BP, Chung HY. Betaine suppresses proinflammatory signaling during aging: the involvement of nuclear factor-kappaB via nuclear factor-inducing kinase/IkappaB kinase and mitogen-activated protein kinases. J Gerontol A Biol Sci Med Sci. (2005) 60:1252–64. doi: 10.1093/gerona/60.10.1252
19. Kathirvel E, Morgan K, Nandgiri G, Sandoval BC, Caudill MA, Bottiglieri T, et al. Betaine improves nonalcoholic fatty liver and associated hepatic insulin resistance: a potential mechanism for hepatoprotection by betaine. Am J Physiol Gastrointest Liver Physiol. (2010) 299:G1068–77. doi: 10.1152/ajpgi.00249.2010
20. Li S, Wang H, Wang X, Wang Y, Feng J. Betaine affects muscle lipid metabolism via regulating the fatty acid uptake and oxidation in finishing pig. J Anim Sci Biotechnol. (2017) 8:72. doi: 10.1186/s40104-017-0200-6
21. Xu L, Huang D, Hu Q, Wu J, Wang Y, Feng J. Betaine alleviates hepatic lipid accumulation via enhancing hepatic lipid export and fatty acid oxidation in rats fed with a high-fat diet. Br J Nutr. (2015) 113:1835–43. doi: 10.1017/s0007114515001130
22. Du J, Shen L, Tan Z, Zhang P, Zhao X, Xu Y, et al. Betaine Supplementation Enhances Lipid Metabolism and Improves Insulin Resistance in Mice Fed a High-Fat Diet. Nutrients (2018) 10:131. doi: 10.3390/nu10020131
23. Zhao G, He F, Wu C, Li P, Li N, Deng J, et al. Betaine in Inflammation, Mechanistic Aspects and Applications. Front Immunol. (2018) 9:1070. doi: 10.3389/fimmu.2018.01070
24. Li JM, Ge CX, Xu MX, Wang W, Yu R, Fan CY, et al. Betaine recovers hypothalamic neural injury by inhibiting astrogliosis and inflammation in fructose-fed rats. Mol Nutr Food Res. (2015) 59:189–202. doi: 10.1002/mnfr.201400307
25. Ge CX, Yu R, Xu MX, Li PQ, Fan CY, Li JM, et al. Betaine prevented fructose-induced NAFLD by regulating LXRalpha/PPARalpha pathway and alleviating ER stress in rats. Eur J Pharmacol. (2016) 770:154–64. doi: 10.1016/j.ejphar.2015.11.043
26. Fan CY, Wang MX, Ge CX, Wang X, Li JM, Kong LD. Betaine supplementation protects against high-fructose-induced renal injury in rats. J Nutr Biochem. (2014) 25:353–62. doi: 10.1016/j.jnutbio.2013.11.010
27. Janeway CA. How the immune system works to protect the host from infection, A personal view. Proc Natl Acad Sci USA. (2001) 98:7461–8. doi: 10.1073/pnas.131202998
28. Ainscough JS, Gerberick GF, Zahedi-Nejad M, Lopez-Castejon G, Brough D, Kimber I, et al. Dendritic Cell IL-1 alpha and IL-1 beta are polyubiquitinated and degraded by the proteasome. J Biol Chem. (2014) 289:35582–92. doi: 10.1074/jbc.M114.595686
29. Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, et al. Cutting edge, NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. (2009) 183:787–91. doi: 10.4049/jimmunol.0901363
30. Franchi L, Eigenbrod T, Nunez G. Cutting edge, TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. (2009) 183:792–6. doi: 10.4049/jimmunol.0900173
31. Lee EK, Jang EJ, Jung KJ, Kim DH, Yu BP, Chung HY. Betaine attenuates lysophosphatidylcholine-mediated adhesion molecules in aged rat aorta: modulation of the nuclear factor-kappaB pathway. Exp Gerontol. (2013) 48:517–24. doi: 10.1016/j.exger.2013.02.024
32. Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. (2012) 92:689–737. doi: 10.1152/physrev.00028.2011
33. Je JH, Lee JY, Jung KJ, Sung B, Go EK, Yu BP. NF-kappaB activation mechanism of 4-hydroxyhexenal via NIK/IKK and p38 MAPK pathway. FEBS Lett. (2004) 566:183–9. doi: 10.1016/j.febslet.2004.04.037
34. Kim DH, Sung B, Kang YJ, Jang JY, Hwang SY, Lee Y, et al. Anti-inflammatory effects of betaine on AOM/DSSinduced colon tumorigenesis in ICR male mice. Int J Oncol. (2014) 45:1250–6. doi: 10.3892/ijo.2014.2515
35. Zhang W, Wang LW, Wang LK, Li X, Zhang H, Luo LP, et al. Betaine protects against high-fat-diet-induced liver injury by inhibition of high-mobility group box 1 and Toll-like receptor 4 expression in rats. Dig Dis Sci. (2013) 58:3198–206. doi: 10.1007/s10620-013-2775-x
36. Kanneganti TD. Central roles of NLRs and inflammasomes in viral infection. Nat Rev Immunol. (2010) 10:688–98. doi: 10.1038/nri2851
37. Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. (2006) 440:233–6. doi: 10.1038/nature04517
38. Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. (2016) 16:407–20. doi: 10.1038/nri.2016.58
39. Prochnicki T, Mangan MS, Latz E. Recent insights into the molecular mechanisms of the NLRP3 inflammasome activation. F1000Res (2016) 5:F1000. doi: 10.12688/f1000research.8614.1
40. Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. (2009) 27:229–65. doi: 10.1146/annurev.immunol.021908.132715
41. Qu Y, Misaghi S, Izrael-Tomasevic A, Newton K, Gilmour LL, Lamkanfi M, et al. Phosphorylation of NLRC4 is critical for inflammasome activation. Nature (2012) 490:539–42. doi: 10.1038/nature11429
42. Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature (2011) 477:596–600. doi: 10.1038/nature10510
43. Ataide MA, Andrade WA, Zamboni DS, Wang D, Souza Mdo C, Franklin BS, et al. Malaria-induced NLRP12/NLRP3-dependent caspase-1 activation mediates inflammation and hypersensitivity to bacterial superinfection. PLoS Pathog. (2014) 10:e1003885. doi: 10.1371/journal.ppat.1003885
44. Khare S, Dorfleutner A, Bryan NB, Yun C, Radian AD, de Almeida L, et al. An NLRP7-containing inflammasome mediates recognition of microbial lipopeptides in human macrophages. Immunity (2012) 36:464–76. doi: 10.1016/j.immuni.2012.02.001
45. Anand PK, Malireddi RK, Lukens JR, Vogel P, Bertin J, Lamkanfi M, et al. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature (2012) 488:389–93. doi: 10.1038/nature11250
46. Allen IC, Wilson JE, Schneider M, Lich JD, Roberts RA, Arthur JC, et al. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-kappaB signaling. Immunity (2012) 36:742–54. doi: 10.1016/j.immuni.2012.03.012
47. Normand S, Delanoye-Crespin A, Bressenot A, Huot L, Grandjean T, Peyrin-Biroulet L, et al. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proc Natl Acad Sci USA. (2011) 108:9601–6. doi: 10.1073/pnas.1100981108
48. Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell (2011) 145:745–57. doi: 10.1016/j.cell.2011.04.022
49. Kim YK, Shin JS, Nahm MH. NOD-Like receptors in infection, immunity, diseases. Yonsei Med J. (2016) 57:5–14. doi: 10.3349/ymj.2016.57.1.5
50. Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. (2011) 17:179–88. doi: 10.1038/nm.2279
51. Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. (2008) 9:857–65. doi: 10.1038/ni.1636
52. Bae JY, Park HH. Crystal Structure of NALP3 Protein Pyrin Domain (PYD) and Its Implications in Inflammasome Assembly. J Biol Chem. (2011) 286:39528–36. doi: 10.1074/jbc.M111.278812
53. Zhong Z, Zhai Y, Liang S, Mori Y, Han R, Sutterwala FS, et al. TRPM2 links oxidative stress to NLRP3 inflammasome activation. Nat Commun. (2013) 4:1611. doi: 10.1038/ncomms2608
54. Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM. Nunez G, K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity (2013) 38:1142–53. doi: 10.1016/j.immuni.2013.05.016
55. Alves LA, de Melo Reis RA, de Souza CA, de Freitas MS, Teixeira PC, Neto Moreira Ferreira D, et al. The P2X7 receptor: shifting from a low- to a high-conductance channel - an enigmatic phenomenon? Biochim Biophys Acta (2014) 1838:2578–87. doi: 10.1016/j.bbamem.2014.05.015
56. Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature (2006) 440:228–32. doi: 10.1038/nature04515
57. Fink SL, Bergsbaken T, Cookson BT. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc Natl Acad Sci USA. (2008) 105:4312–7. doi: 10.1073/pnas.0707370105
58. Arlehamn CS, Petrilli V, Gross O, Tschopp J, Evans TJ. The role of potassium in inflammasome activation by bacteria. J Biol Chem. (2010) 285:10508–18. doi: 10.1074/jbc.M109.067298
59. Graf D, Kurz AK, Reinehr R, Fischer R, Kircheis G, Haussinger D. Prevention of bile acid-induced apoptosis by betaine in rat liver. Hepatology (2002) 36(4 Pt 1):829–39. doi: 10.1053/jhep.2002.35536
60. Kim SJ, Lee SM. NLRP3 inflammasome activation in D-galactosamine and lipopolysaccharide-induced acute liver failure: role of heme oxygenase-1. Free Radic Biol Med. (2013) 65:997–1004. doi: 10.1016/j.freeradbiomed.2013.08.178
61. Li X, Rong Y, Zhang M, Wang XL, LeMaire SA, Coselli JS, et al. Up-regulation of thioredoxin interacting protein (Txnip) by p38 MAPK and FOXO1 contributes to the impaired thioredoxin activity and increased ROS in glucose-treated endothelial cells. Biochem Biophys Res Commun. (2009) 381:660–5. doi: 10.1016/j.bbrc.2009.02.132
62. Sehirli AO, Satilmis B, Tetik S, Cetinel S, Yegen B, Aykac A, et al. Protective effect of betaine against burn-induced pulmonary injury in rats. Ulus Travma Acil Cerrahi Derg. (2016) 22:417–22. doi: 10.5505/tjtes.2015.60137
63. Kanbak G, Akyuz F, Inal M. Preventive effect of betaine on ethanol-induced membrane lipid composition and membrane ATPases. Arch Toxicol. (2001) 75:59–61. doi: 10.1007/s002040000179
64. Wei D, Zhang W, Wang C, Meng Q, Li G, Chen THH, et al. Genetic engineering of the biosynthesis of glycinebetaine leads to alleviate salt-induced potassium efflux and enhances salt tolerance in tomato plants. Plant Sci. (2017) 257:74–83. doi: 10.1016/j.plantsci.2017.01.012
65. Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell (2007) 130:918–31. doi: 10.1016/j.cell.2007.07.009
66. Hu Z, Murakami T, Tamura H, Reich J, Kuwahara-Arai K, Iba T, et al. Neutrophil extracellular traps induce IL-1beta production by macrophages in combination with lipopolysaccharide. Int J Mol Med. (2017) 39:549–58. doi: 10.3892/ijmm.2017.2870
67. Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundback P, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature (2012) 488:670–4. doi: 10.1038/nature11290
68. Shenoy AR, Wellington DA, Kumar P, Kassa H, Booth CJ, Cresswell P, et al. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science (2012) 336:481–5. doi: 10.1126/science.1217141
69. Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood (2009) 113:2324–35. doi: 10.1182/blood-2008-03-146720
70. Lee HM, Yuk JM, Kim KH, Jang J, Kang G, Park JB, et al. Mycobacterium abscessus activates the NLRP3 inflammasome via Dectin-1-Syk and p62/SQSTM1. Immunol Cell Biol. (2012) 90:601–10. doi: 10.1038/icb.2011.72
71. Master SS, Rampini SK, Davis AS, Keller C, Ehlers S, Springer B, et al. Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe. (2008) 3:224–32. doi: 10.1016/j.chom.2008.03.003
72. Lee HM, Kang J, Lee SJ, Jo EK. Microglial activation of the NLRP3 inflammasome by the priming signals derived from macrophages infected with mycobacteria. Glia (2013) 61:441–52. doi: 10.1002/glia.22448
73. He X, Mekasha S, Mavrogiorgos N, Fitzgerald KA, Lien E, Ingalls RR. Inflammation and fibrosis during Chlamydia pneumoniae infection is regulated by IL-1 and the NLRP3/ASC inflammasome. J Immunol. (2010) 184:5743–54. doi: 10.4049/jimmunol.0903937
74. Koo JE, Hong HJ, Dearth A, Kobayashi KS, Koh YS. Intracellular invasion of Orientia tsutsugamushi activates inflammasome in asc-dependent manner. PLoS One (2012) 7:e39042. doi: 10.1371/journal.pone.0039042
75. Oberst A, Green DR. It cuts both ways: reconciling the dual roles of caspase 8 in cell death and survival. Nat Rev Mol Cell Biol. (2011) 12:757–63. doi: 10.1038/nrm3214
76. Maelfait J, Vercammen E, Janssens S, Schotte P, Haegman M, Magez S, et al. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J Exp Med. (2008) 205:1967–73. doi: 10.1084/jem.20071632
77. Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, et al. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol. (2012) 13:246–54. doi: 10.1038/ni.2222
78. Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity (2013) 38:27–40. doi: 10.1016/j.immuni.2012.09.015
79. Bossaller L, Chiang PI, Schmidt-Lauber C, Ganesan S, Kaiser WJ, Rathinam VA, et al. Cutting edge: FAS (CD95) mediates noncanonical IL-1beta and IL-18 maturation via caspase-8 in an RIP3-independent manner. J Immunol. (2012) 189:5508–12. doi: 10.4049/jimmunol.1202121
80. Gurung P, Anand PK, Malireddi RK, Vande Walle L, Van Opdenbosch N, Dillon CP, et al. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol. (2014) 192:1835–46. doi: 10.4049/jimmunol.1302839
81. Shenderov K, Riteau N, Yip R, Mayer-Barber KD, Oland S, Hieny S, et al. Cutting edge, Endoplasmic reticulum stress licenses macrophages to produce mature IL-1beta in response to TLR4 stimulation through a caspase-8- and TRIF-dependent pathway. J Immunol. (2014) 192:2029–33. doi: 10.4049/jimmunol.1302549
82. Garrett Q, Khandekar N, Shih S, Flanagan JL, Simmons P, Vehige J, et al. Betaine stabilizes cell volume and protects against apoptosis in human corneal epithelial cells under hyperosmotic stress. Exp Eye Res. (2013) 108:33–41. doi: 10.1016/j.exer.2012.12.001
83. Horio M, Ito A, Matsuoka Y, Moriyama T, Orita Y, Takenaka M, et al. Apoptosis induced by hypertonicity in Madin Darley canine kidney cells: protective effect of betaine. Nephrol Dial Transplant. (2001) 16:483–90. doi: 10.1093/ndt/16.3.483
84. Dupaul-Chicoine J, Saleh M. A new path to IL-1beta production controlled by caspase-8. Nat Immunol. (2012) 13:211–2. doi: 10.1038/ni.2241
85. Buchrieser J, Oliva-Martin MJ, Moore MD, Long JCD, Cowley SA, Perez-Simon JA, et al. RIPK1 is a critical modulator of both tonic and TLR-responsive inflammatory and cell death pathways in human macrophage differentiation. Cell Death Dis. (2018) 9:973. doi: 10.1038/s41419-018-1053-4
86. Oliva-Martin MJ, Sanchez-Abarca LI, Rodhe J, Carrillo-Jimenez A, Vlachos P, Herrera AJ, et al. Caspase-8 inhibition represses initial human monocyte activation in septic shock model. Oncotarget (2016) 7:37456–70. doi: 10.18632/oncotarget.9648
87. Venero JL, Burguillos MA, Brundin P, Joseph B. The executioners sing a new song: killer caspases activate microglia. Cell Death Differ. (2011) 18:1679–91. doi: 10.1038/cdd.2011.107
88. Cuda CM, Pope RM, Perlman H. The inflammatory role of phagocyte apoptotic pathways in rheumatic diseases. Nat Rev Rheumatol. (2016) 12:543–58. doi: 10.1038/nrrheum.2016.132
89. Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, et al. Non-canonical inflammasome activation targets caspase-11. Nature (2011) 479:117–21. doi: 10.1038/nature10558
90. Furuya T, Hayakawa H, Yamada M, Yoshimi K, Hisahara S, Miura M, et al. Caspase-11 mediates inflammatory dopaminergic cell death in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. J Neurosci. (2004) 24:1865–72. doi: 10.1523/jneurosci.3309-03.2004
91. Hisahara S, Yuan J, Momoi T, Okano H, Miura M. Caspase-11 mediates oligodendrocyte cell death and pathogenesis of autoimmune-mediated demyelination. J Exp Med. (2001) 193:111–22. doi: 10.1084/jem.193.1.111
92. Gurung P, Malireddi RK, Anand PK, Demon D, Vande Walle L, Liu Z, et al. Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-beta (TRIF)-mediated caspase-11 protease production integrates Toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens. J Biol Chem. (2012) 287:34474–83. doi: 10.1074/jbc.M112.401406
93. Rathinam VA, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell (2012) 150:606–19. doi: 10.1016/j.cell.2012.07.007
94. Yang J, Zhao Y, Shao F. Non-canonical activation of inflammatory caspases by cytosolic LPS in innate immunity. Curr Opin Immunol. (2015) 32:78–83. doi: 10.1016/j.coi.2015.01.007
95. Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature (2014) 51:87–92. doi: 10.1038/nature13683
96. Vigano E, Diamond CE, Spreafico R, Balachander A, Sobota RM, Mortellaro A. Human caspase-4 and caspase-5 regulate the one-step non-canonical inflammasome activation in monocytes. Nat Commun. (2015) 6:8761. doi: 10.1038/ncomms9761
97. Yang D, He Y, Munoz-Planillo R, Liu Q, Nunez G. Caspase-11 Requires the Pannexin-1 Channel and the Purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity (2015) 43:923–32. doi: 10.1016/j.immuni.2015.10.009
98. Poornima V, Vallabhaneni S, Mukhopadhyay M, Bera AK. Nitric oxide inhibits the pannexin 1 channel through a cGMP-PKG dependent pathway. Nitric oxide (2015) 47:77–84. doi: 10.1016/j.niox.2015.04.005
99. Whyte-Fagundes P, Zoidl G. Mechanisms of pannexin1 channel gating and regulation. Biochim Biophys Acta Biomembranes (2018) 1860:65–71. doi: 10.1016/j.bbamem.2017.07.009
100. Li C, Li LJ, Jin L, Yuan JF. Heme Oxygenase-1 inhibits spring viremia of carp virus replication through carbon monoxide mediated cyclic GMP/Protein kinase G signaling pathway. Fish Shellfish Immunol. (2018) 79:65–72. doi: 10.1016/j.fsi.2018.05.014
101. Lordnejad MR, Schliess F, Wettstein M, Haussinger D. Modulation of the heme oxygenase HO-1 expression by hyperosmolarity and betaine in primary rat hepatocytes. Arch Biochem Biophys. (2001) 388:285–92. doi: 10.1006/abbi.2001.2297
102. Casson CN, Copenhaver AM, Zwack EE, Nguyen HT, Strowig T, Javdan B, et al. Caspase-11 activation in response to bacterial secretion systems that access the host cytosol. PLoS Pathog. (2013) 9:e1003400. doi: 10.1371/journal.ppat.1003400
103. Lukens JR, Gross JM, Calabrese C, Iwakura Y, Lamkanfi M, Vogel P, et al. Critical role for inflammasome-independent IL-1beta production in osteomyelitis. Proc Natl Acad Sci USA. (2014) 111:1066–71. doi: 10.1073/pnas.1318688111
104. Cassel SL, Janczy JR, Bing X, Wilson SP, Olivier AK, Otero JE, et al. Inflammasome-independent IL-1beta mediates autoinflammatory disease in Pstpip2-deficient mice. Proc Natl Acad Sci USA. (2014) 111:1072–7. doi: 10.1073/pnas.1318685111
105. Guma M, Ronacher L, Liu-Bryan R, Takai S, Karin M, Corr M. Caspase 1-independent activation of interleukin-1beta in neutrophil-predominant inflammation. Arthr Rheum. (2009) 60:3642–50. doi: 10.1002/art.24959
106. Fantuzzi G, Ku G, Harding MW, Livingston DJ, Sipe JD, Kuida K, et al. Response to local inflammation of IL-1 beta-converting enzyme- deficient mice. J Immunol. (1997) 158:1818–24.
107. Joosten LA, Netea MG, Fantuzzi G, Koenders MI, Helsen MM, Sparrer H, et al. Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1beta. Arthr Rheum. (2009) 60:3651–62. doi: 10.1002/art.25006
108. Kono H, Orlowski GM, Patel Z, Rock KL. The IL-1-dependent sterile inflammatory response has a substantial caspase-1-independent component that requires cathepsin J Immunol C. (2012) 189:3734–40. doi: 10.4049/jimmunol.1200136
109. Rubartelli A, Cozzolino F, Talio M, Sitia R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. Embo J. (1990) 9:1503–10.
110. Wewers MD. IL-1beta: an endosomal exit. Proc Natl Acad Sci USA. (2004) 101:10241–2. doi: 10.1073/pnas.0403971101
111. Eder C. Mechanisms of interleukin-1beta release. Immunobiology (2009) 214:543–53. doi: 10.1016/j.imbio.2008.11.007
112. Andrei C, Dazzi C, Lotti L, Torrisi MR, Chimini G, Rubartelli A. The secretory route of the leaderless protein interleukin 1beta involves exocytosis of endolysosome-related vesicles. Mol Biol Cell. (1999) 10:1463–75. doi: 10.1091/mbc.10.5.1463
113. Andrei C, Margiocco P, Poggi A, Lotti LV, Torrisi MR, Rubartelli A. Phospholipases C and A2 control lysosome-mediated IL-1 beta secretion, Implications for inflammatory processes. Proc Natl Acad Sci USA. (2004) 101:9745–50. doi: 10.1073/pnas.0308558101
114. Carta S, Tassi S, Semino C, Fossati G, Mascagni P, Dinarello CA, et al. Histone deacetylase inhibitors prevent exocytosis of interleukin-1beta-containing secretory lysosomes: role of microtubules. Blood (2006) 108:1618–26. doi: 10.1182/blood-2006-03-014126
115. Kahlenberg JM, Dubyak GR. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am J Physiol Cell Physiol. (2004) 286:C1100–8. doi: 10.1152/ajpcell.00494.2003
116. Moreira V, Souto P, Vinolo MAR, Lomonte B, Gutierrez JM, Curi R, et al. A catalytically-inactive snake venom Lys49 phospholipase A(2) homolog induces expression of cyclooxygenase-2 and production of prostaglandins through selected signaling pathways in macrophages. Eur J Pharmacol. (2013) 708:68–79. doi: 10.1016/j.ejphar.2013.01.061
117. Liao SL, Ou YC, Chang CY, Chen WY, Kuan YH, Wang WY, et al. Diethylmaleate and iodoacetate in combination caused profound cell death in astrocytes. J Neurochem. (2013) 127:271–82. doi: 10.1111/jnc.12291
118. Wu W, Wang S, Xu Z, Wang X, Feng J, Shan T, et al. Betaine promotes lipid accumulation in adipogenic-differentiated skeletal muscle cells through ERK/PPARgamma signalling pathway. Mol Cell Biochem. (2018) 447:137–49. doi: 10.1007/s11010-018-3299-7
119. MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity (2001) 15:825–35. doi: 10.1016/S1074-7613(01)00229-1
120. Bianco F, Pravettoni E, Colombo A, Schenk U, Moller T, Matteoli M, et al. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol. (2005) 174:7268–77. doi: 10.4049/jimmunol.174.11.7268
121. Pizzirani C, Ferrari D, Chiozzi P, Adinolfi E, Sandona D, Savaglio E, et al. Stimulation of P2 receptors causes release of IL-1beta-loaded microvesicles from human dendritic cells. Blood (2007) 109:3856–64. doi: 10.1182/blood-2005-06-031377
122. Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. Embo J. (2009) 28:1043–54. doi: 10.1038/emboj.2009.45
123. Edelmann B, Bertsch U, Tchikov V, Winoto-Morbach S, Perrotta C, Jakob M, et al. Caspase-8 and caspase-7 sequentially mediate proteolytic activation of acid sphingomyelinase in TNF-R1 receptosomes. Embo J. (2011) 30:379–94. doi: 10.1038/emboj.2010.326
124. Charruyer A, Grazide S, Bezombes C, Muller S, Laurent G, Jaffrezou JP. UV-C light induces raft-associated acid sphingomyelinase and JNK activation and translocation independently on a nuclear signal. J Biol Chem. (2005) 280:19196–204. doi: 10.1074/jbc.M412867200
125. Lang PA, Schenck M, Nicolay JP, Becker JU, Kempe DS, Lupescu A, et al. Liver cell death and anemia in Wilson disease involve acid sphingomyelinase and ceramide. Nature Med (2007) 13:164–70. doi: 10.1038/nm1539
126. Im AR, Kim YH, Uddin MR, Chae S, Lee HW, Kim YH, et al. Betaine protects against rotenone-induced neurotoxicity in PC12 cells. Cell Mol Neurobiol. (2013) 33:625–35. doi: 10.1007/s10571-013-9921-z
127. Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasomeactivation and correlated with exosome release in murine macrophages. J Immunol. (2007) 179:1913–25. doi: 10.4049/jimmunol.179.3.1913
128. Qu Y, Ramachandra L, Mohr S, Franchi L, Harding CV, Nunez G, et al. P2X7 receptor-stimulated secretion of MHC class II-containing exosomes requires the ASC/NLRP3 inflammasome but is independent of caspase-1. J Immunol. (2009) 182:5052–62. doi: 10.4049/jimmunol.0802968
129. Hauser C, Dayer JM, Jaunin F, de Rochemonteix B, Saurat JH. Intracellular epidermal interleukin 1-like factors in the human epidermoid carcinoma cell line A431. Cell Immunol. (1986) 100:89–96.
130. Hogquist KA, Nett MA, Unanue ER, Chaplin DD. Interleukin-1 is processed and released during apoptosis. Proc Natl Acad Sci USA. (1991) 88:8485–9. doi: 10.1073/pnas.88.19.8485
131. Laliberte RE, Eggler J, Gabel CA. ATP treatment of human monocytes promotes caspase-1 maturation and externalization. J Biol Chem. (1999) 274:36944–51.
132. Martin-Sanchez F, Diamond C, Zeitler M, Gomez AI, Baroja-Mazo A, Bagnall J, et al. Inflammasome-dependent IL-1beta release depends upon membrane permeabilisation. Cell Death Differ. (2016) 23:1219–31. doi: 10.1038/cdd.2015.176
133. Vince JE, Silke J. The intersection of cell death and inflammasome activation. Cell Mol Life Sci. (2016) 73:2349–67. doi: 10.1007/s00018-016-2205-2
134. He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. (2015) 25:1285–98. doi: 10.1038/cr.2015.139
135. Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature (2015) 526:660–5. doi: 10.1038/nature15514
136. Mandruppoulsen T, Bendtzen K, Nerup J, Dinarello CA, Svenson M, Nielsen JH Affinity-purified human interleukin-I is cytotoxic to isolated islets of langerhans. Diabetologia (1986) 29:63–7. doi: 10.1007/bf02427283
137. Malozowski S, Sahlroot JT. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. (2007) 357:302–3. doi: 10.1056/NEJMc071324
138. Geng CA, Ma YB, Zhang XM, Yao SY, Xue DQ, Zhang RP, et al. Mulberrofuran G and isomulberrofuran G from Morus alba L.: anti-hepatitis B virus activity and mass spectrometric fragmentation. J Agric Food Chem. (2012) 60:8197–202. doi: 10.1021/jf302639b
139. Ferrari D, Chiozzi P, Falzoni S, DalSusino M, Melchiorri L, Baricordi OR, et al. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. (1997) 159:1451–8.
140. Py BF, Jin M, Desai BN, Penumaka A, Zhu H, Kober M, et al. Caspase-11 controls interleukin-1beta release through degradation of TRPC1. Cell Rep. (2014) 6:1122–8. doi: 10.1016/j.celrep.2014.02.015
Keywords: betaine, caspase-8, IL-1β, inflammation, inflammasome
Citation: Xia Y, Chen S, Zhu G, Huang R, Yin Y and Ren W (2018) Betaine Inhibits Interleukin-1β Production and Release: Potential Mechanisms Front. Immunol. 9:2670. doi: 10.3389/fimmu.2018.02670
Received: 19 August 2018; Accepted: 29 October 2018;
Published: 20 November 2018.
Edited by:
Alexander G. Haslberger, Universität Wien, AustriaReviewed by:
Zhuoran Yin, Harvard Medical School, United StatesHailong Song, University of Missouri, United States
Copyright © 2018 Xia, Chen, Zhu, Huang, Yin and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenkai Ren, renwenkai19@126.com
 Yaoyao Xia1,2,3
Yaoyao Xia1,2,3 Wenkai Ren
Wenkai Ren