- 1Amsterdam Rheumatology and Immunology Center (ARC), Department of Rheumatology and Clinical Immunology, Amsterdam UMC, University of Amsterdam, Amsterdam, Netherlands
- 2Experimental Immunology, Amsterdam Infection and Immunity Institute, Amsterdam UMC, University of Amsterdam, Amsterdam, Netherlands
- 3Department of Molecular Cell Biology and Immunology, Amsterdam Infection and Immunity Institute, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, Netherlands
Lymph nodes (LNs) are crucial for the orchestration of immune responses. LN reactions depend on interactions between incoming and local immune cells, and stromal cells. To mediate these cellular interactions an organized vascular network within the LN exists. In general, the LN vasculature can be divided into two components: blood vessels, which include the specialized high endothelial venules that recruit lymphocytes from the bloodstream, and lymphatic vessels. Signaling via TNF receptor (R) superfamily (SF) members has been implicated as crucial for the development and function of LNs and the LN vasculature. In recent years the role of cell-specific signaling of TNFRSF members in different endothelial cell (EC) subsets and their roles in development and maintenance of lymphoid organs has been elucidated. Here, we discuss recent insights into EC-specific TNFRSF member signaling and highlight its importance in different EC subsets in LN organogenesis and function during health, and in lymphocyte activation and tertiary lymphoid structure formation during inflammation.
Introduction
Lymph nodes (LNs) are positioned at strategic sites throughout the body where they are essential for initiating and shaping immune responses. Via the vascular system soluble factors and cells are transported from peripheral tissues into the LNs, which is crucial for the initiation of (adaptive) immune responses (1, 2). Lymph node reactions are tightly regulated and depend on interactions between incoming immune cells, local immune cells and LN stromal cells (3, 4).
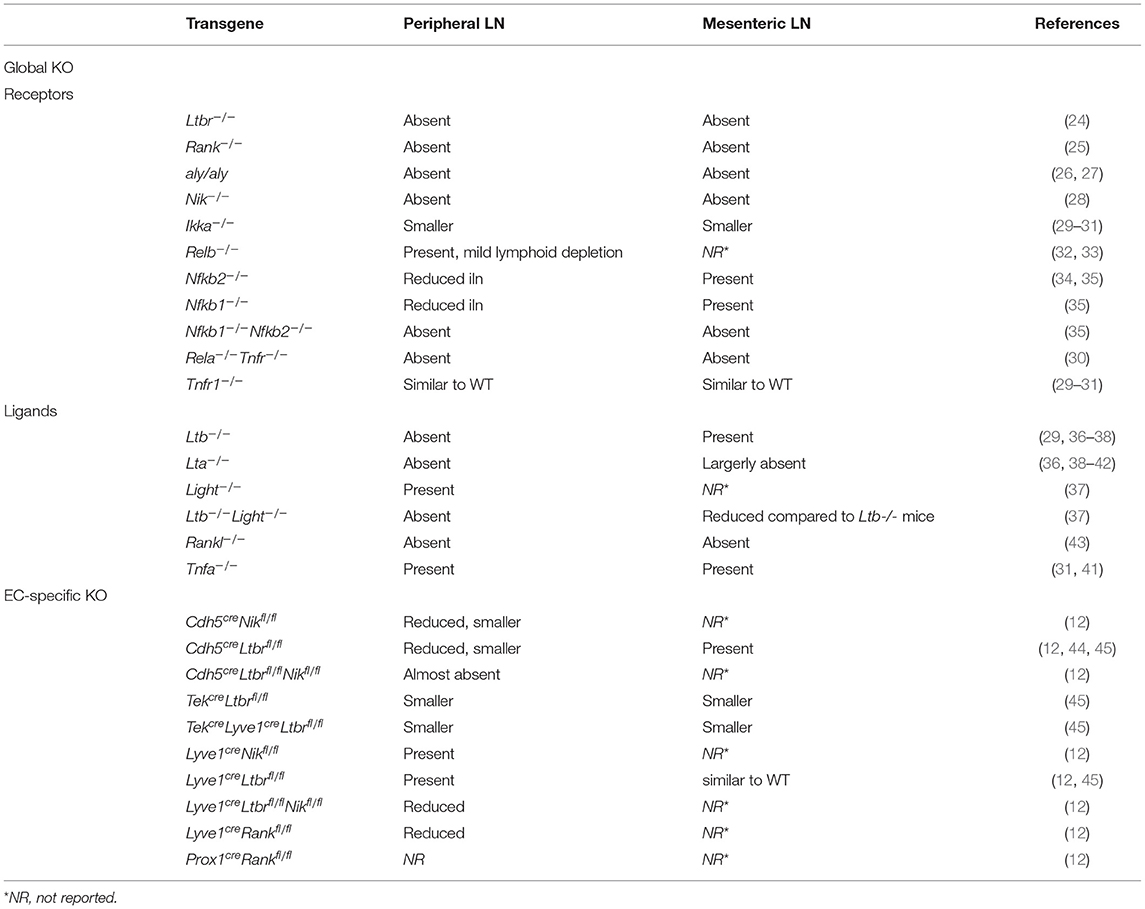
Generally, LNs can be divided into three areas; the cortex containing B cell follicles, the paracortex consisting predominantly of T cells, and the medulla representing the primary maturation site of antibody producing plasmablasts (1, 2). This spatial organization is orchestrated by LN stromal cells, that include distinct fibroblastic reticular cell subsets (FRCs) and endothelial cells (EC) (4, 5). The FRCs generate the highly organized scaffold network and comprises distinct stromal subsets such as T cell zone reticular cells (TRCs), marginal reticular cells (MRCs), follicular dendritic cells (FDCs), medullary reticular cells (MedRCs) and perivascular reticular cells (PRCs), reviewed elsewhere (5). Endothelial stromal cells can be divided into blood endothelial cells (BECs) and lymphatic endothelial cells (LECs). Importantly, the LN blood vessels include two main components: regular BVs and specialized high endothelial venules (HEVs) (Figure 1).
Figure 1. The lymph node vascular structure. Organization of the LN vasculature (right). The LN vasculature consists of BVs, HEVs and LVs. BVs can be found throughout the whole LN, with specialized HEVs located within the T cell areas. Afferent LVs enter the LN where they transit into sinuses that ultimately exit via the efferent LV. HEVs are characterized by cuboidal ECs, pericyte coverage and a network of FRCs. HEV are specialized BVs that orchestrate extravasation of lymphocytes into the LN (top left). In contrast to HEVs, normal BVs are lined by flat ECs and have minimal pericyte coverage (middle left). LVs are characterized by overlapping lymphatic ECs that allow influx of (dendritic) cells and soluble factors into the vessel. Within the LN the LVs transit into sinuses that allow exit of the lymph fluid and its cells and soluble factors into the LN (lower left). LN, lymph node; BV, blood vessel; HEV, high endothelial venule; LV, lymphatic vessels; FRC, fibroblastic reticular cell.
Arterial blood that enters the LN flows into capillaries which proceed into the network of HEVs. High endothelial venules located within the T cell areas are the sites were naïve lymphocytes leave the bloodstream to enter the LN and interact with local cells. After passing through the HEVs venous blood exits the LN via the efferent blood vessels (4, 6). Afferent lymphatic vessels (LVs) enter the LN and proceed into subcapsular sinuses (SCSs) that ultimately exit via the medullary LVs and efferent vessels (3, 7). Via the LVs, lymph fluid containing both soluble factors and cells is distributed by the SCSs to mediate interaction with local immune cells or stromal cells (3). Over the past years increasing attention has been paid to the role of endothelial cells (ECs) in LN development and function. More specifically, knowledge is being gained on EC-specific pathways necessary for LN organogenesis and function.
Among the signaling cascades that have been recognized as essential for LN development is signaling via the tumor necrosis factor receptor (TNFR) superfamily (SF). It is well-established that signaling of TNFRSF members in many cell types is required for proper LN development and function, including LN EC subsets.
Here, we present an overview of the EC-specific TNFRSF member signaling cascades that are important for LN organogenesis and development and maintenance of the different LN EC subsets. In addition, the importance of these cells and signaling pathways during inflammation are discussed, focusing on LN inflammatory reactions and development of tertiary lymphoid structures (TLSs). Lastly, we will discuss whether targeting of EC-specific TNFRSF member signaling may hold potential as a therapeutic target in the treatment of inflammatory diseases.
Development of Lymph Nodes
Development of LNs starts around embryonic day (E) 9 at the same time that ECs start budding from the anterior cardinal vein and begin to express the lymphatic EC (LEC) marker lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1). Between E12.5-14.5, CD45+CD4+RORγt+IL-7Rα+ lymphoid tissue inducer (LTi) cells are recruited into the LN anlagen (8, 9) a process that is dependent on interactions with local stromal cells (10). Recently, it was demonstrated that CD4− pre-LTi cells egress from venous vessels at locations where there is low coverage of smooth muscle cells (SMCs) (11). These pre-LTi cells then mature locally into CD4+ LTi cells that are transported to and retained at the site of LN development by the LVs (11).
Only once enough LTi cells appear and are retained at the site of the LN anlagen, definitive formation of LNs is started (12). Clustering of LTi cells that express both receptor activator of nuclear factor kappa B (RANK, also known as TNF-related activation-induced cytokine (TRANCE) receptor, and TNFRSF11A) and RANK ligand (RANKL, TRANCE, TNFSF11) leads to autocrine production of lymphotoxin α1β2 (LTα1β2, TNF-C) on LTi cells that engages the LTβ receptor (LTβR, TNFRSF3) on the surrounding stromal cells (13). Signaling via the RANKL-RANK and LTα1β2-LTβR axes creates a positive feedback loop, which leads to the recruitment of more LTi cells and expression of adhesion molecules by stromal cells. The differentiation of these mesenchymal lymphoid tissue organizer (LTo) cells is necessary to further support retention of LTi cells at the LN anlage (8, 14, 15). Next, the stromal LTo cells start to produce chemokines and cytokines that are necessary for definitive formation of LNs (8, 9, 15–18). Key molecules expressed by the LTo cells via the LTα1β2-LTβR signaling axis at this point include mucosal addressin cell adhesion molecule (MAdCAM)-1, vascular cell adhesion molecule (VCAM)-1, and intercellular adhesion molecule (ICAM)-1, and the chemokines C-X-C motif ligand (CXCL)13, C-C motif ligand (CCL) 19 and CCL21 (14, 19, 20). As the interactions between incoming (LTi) cells and local stromal (LTo) cells continue, a premature LN is formed that will ultimately develop into a fully functional LN a process that largely relies on TNFRSF member signaling.
TNFR Superfamily Signaling in LN Development
Recently, more insights have been obtained into the importance of TNFRSF member signaling in ECs in the context of LN development. Ligation of TNFRSF members, including TNFRI, LTβR and RANK, predominantly activates nuclear factor kappa B (NF-κB) signaling (Figure 2). This signaling cascade can be divided into two distinct routes, the canonical and noncanonical NF-κB pathways (21). Canonical NF-κB signaling is dependent on activity of the IκB kinase (IKK) complex which is comprised of three subunits; IKKα, IKKβ and the regulatory subunit NF-κB essential modulator (NEMO, IKKγ). Activation of this complex leads to phosphorylation-induced degradation of IκBα resulting in the rapid nuclear translocation of the dimers p65(RelA)/p50 (22). Non-canonical NF-κB signaling is dependent on NF-κB inducing kinase (NIK) activity. Under homeostatic conditions, TNFR associated factor (TRAF) 2 and 3 (for LTβR and RANK signaling) or (for TNFRI signaling) 5 act as negative regulators of NIK by mediating its ubiquitination. Upon receptor ligation TRAF2 and TRAF3/5 are degraded by cellular inhibitor of apoptosis protein 1 and−2 (cIAP1/2), resulting in stabilization and accumulation of NIK, which enables its complex formation with IKKα (23). Activity of the NIK-IKKα complex leads to phosphorylation and degradation of p100 into p52 which can form dimers with RelB that are able to translocate to the nucleus. Although canonical and noncanonical NF-κB signaling are thought to follow two distinct routes, it has been shown that crosstalk exists between the two pathways. Recently, it was demonstrated that NIK can also drive canonical NF-κB signaling in EC (21), indicating that the effects of NIK are not solely restricted to the noncanonical pathway.
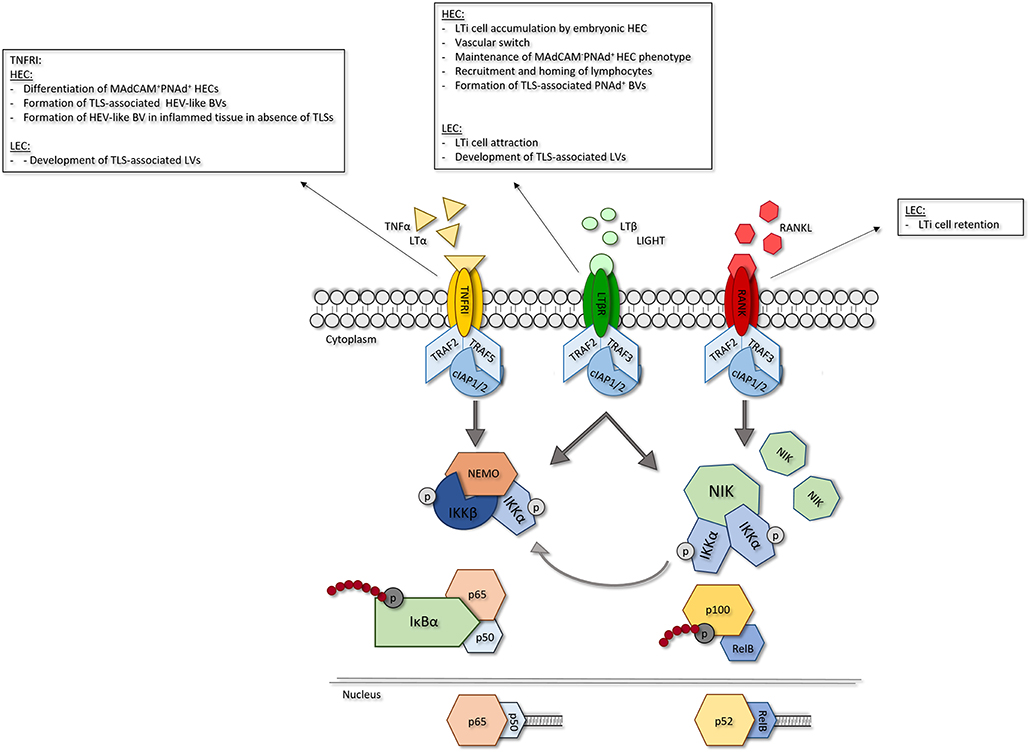
Figure 2. Role of EC-specific TNFRSF member signaling in LN development. Signaling via TNFRSF members leads to NF-κB signaling. In response to receptor ligation TRAF2 and−5 are degraded via activity of ciAPl/2 leading to activation of NF-κB signaling. Canonical NF-κB signaling is dependent on activity of the IKK complex. Activation of IKKleads to phosphorylation-induced degradation of Iκβα, allowing nuclear translocation of p65/p50 dimers and transcription of canonical NF-κB target genes. Non-canonical NF-κB signaling is dependent on accumulation of NIK which forms a complex with IKKα. Activity of the NIK/IKKα complex leads to phosphorylation-induced degradation of plOO into p52 and p52/ReiB dimer translocation to the nucleus resulting in transcription of non-canonical NF-κB target genes. In EC, NIK/IKKα complex activity can also induce canonical NF-κB signaling. For each TNFR its role in the different LN EC subsets is shown. HEC, high endothelial cell; LEC, lymphatic endothelial cell; TNFRI, tumor necrosis factor receptor I; LTβR, lymphotoxin beta receptor; RANK, receptor activator of nuclear factor kappa B; LIGHT, TNFSF 14; TRAF, TNFR associated factor; clap, cellular inhibitor of apoptosis protein; NEMO, NF-κB essential modulator; IKK, IKB kinase; NIK; NF-κB inducing kinase.
The importance of TNFRSF signaling in LN development and function is underlined by many studies in animal models with altered function of different components of the TNFR-NF-κB signaling cascades (Table 1). It has for instance been demonstrated that LTβR signaling is essential for viability, expansion and differentiation of LTo cells, as well as for recruitment of LTi cells (14, 19, 46). In addition, treating pregnant mice with anti-RANKL antibodies blocks LN formation in the offspring (47) and Rankl−/− (43) and Rank−/− (48) mice lack all LNs (25). Although early studies into TNFRSF member signaling were largely facilitated by global knock out (KO) models, the importance of EC-specific signaling via TNFRSF members in LN formation and function has recently been demonstrated with the aid of cell-specific KO models. For example, EC-specific Ltbr or Nik ablation impairs LN formation and function (12, 44, 45) and LEC-specific RANK signaling is shown to be essential for interaction with LTi cells (12). Thus, EC-specific signaling via members of the TNFRSF is crucial for LN development and function.
TNFR Superfamily Members in High Endothelial Venule Development and Function
Recruitment of lymphocytes from the blood into the LNs occurs via a network of post-capillary venules, the HEVs (6, 49). These specialized vessels that are largely restricted to lymphoid organs differ from regular BVs in that they are lined by cuboidal ECs, that express molecules critical for recruitment of immune cells (6, 50). Many of these key HEV markers are controlled via NF-κB signaling, including peripheral node addressin (PNAd), MadCAM-1, and other adhesion molecules (i.e., VCAM-1 and ICAM-1) and chemokines (i.e., CCL19 and CCL21) (51). In PLN, the most defining HEV marker is the adhesion molecule PNAd, which is a ligand for L-selectin+ lymphocytes. PNAd mediates rolling and tethering of lymphocytes on HEVs thereby allowing interaction of CCR7, expressed by lymphocytes, with CCL21 on HEVs (52). PNAd can be detected by binding of the MECA-79 antibody that binds to 6-sulpho sialyl Lewisx on extended core-1 branched O-linked sugars on CD34, glycosylation-dependent adhesion molecule (GlyCAM)-1, podocalyxin, endomucin and nepmucin (53, 54). Expression of PNAd is considered to be an exclusive feature of HEVs, whereas other markers expressed by HEV, such as MAdCAM-1 and CCL21, can also be expressed by other stromal cells, including LECs (55). Interestingly, it has recently been shown that expression of these markers by HEV can be relatively heterogeneous during LN homeostasis (56).
True HEVs are not present until birth when EC begin to form a network in the T cell areas surrounding the B cell follicles, which is critical for completion of the LN infrastructure and function (12). Initiation of HEV development occurs by ligation of the TNFRs on blood ECs (BECs) leading to vasculature growth and HEV formation, followed by entry of lymphocytes into the LN (44, 57, 58) and completion of the HEV network around postnatal day (P) 4 (19). It has been proposed that initial canonical NF-κB signaling via LTα (TNF-β)-TNFRI interaction generates MadCAM-1+PNAd+ flat HEVs, and that sustained LTβR and downstream non-canonical NF-κB signaling induces development of MadCAM-1−PNAd+ cuboidal HECs (6). A key event in the maturation of HEVs is the simultaneous upregulation of PNAd and downregulation of MadCAM-1 a process known as the vascular addressin switch (9, 50, 59). Until time of birth HEVs express both MAdCAM-1 and PNAd (59). Next, over a period of 4 weeks there is a change in addressing expression, eventually leading to MAdCAM-1−PNAd+ HEV (59). It is suggested that this switch is induced by LTα1β2 expressing DCs originating from the gut (60, 61). These DCs engage the LTβR on immature HEVs resulting in a decrease of MAdCAM-1 expression and an increase in PNAd expression and subsequent homing of lymphocytes (60, 61). Of note, while MAdCAM-1 expression is completely downregulated in PLN HEVs, HEVs in LN associated with mucosal tissues continue to express MAdCAM-1 alongside PNAd (6, 51). Once the switch from MadCAM-1+PNAd− to MadCAM-1−PNAd+ HEVs is completed, the LTβR needs to be frequently, if not constantly, engaged in order to maintain a mature HEV phenotype (44, 57, 62). It has been shown that CD11c+ DC can fulfill this function as they make frequent contact with the HEVs to establish the LTα1β2-LTβR interaction and downstream NF-κB signaling that is essential to control PNAd and MAdCAM-1 levels (57, 60, 63). It is thought that LTβR signaling is the dominant receptor in maintaining the HEV phenotype since LTβR, but not TNFRI, blockade leads to the loss of several HEV-specific markers (57). For instance, interfering with LTβR signaling during PLN homeostasis influences expression of several genes involved in cell adhesion and expression of HEV markers such as Glycam-1 while having no effect on others, including CCL21 (56).
Although true HEVs only start to develop after birth, a recent study identified a small subset of EC with characteristics of HECs already present during embryonic development (45). These ECs express genes, including Madcam-1, Cxcl13, and Ccl21, which are also expressed by HECs present after birth (45). Targeting these embryonic HECs in a TekcreLtbrfl/fl model resulted in a reduction of LTi cell accumulation and subsequent defects in LN maturation suggesting that LTβR signaling in embryonic HECs may play a role in LN formation during embryogenesis (45).
TNFR Superfamily Members in Lymphatic Vasculature Development and Function
Lymphatic vessels are blind ending, thin walled, vessels that are the first entry points for antigen and antigen presenting cells (APC) from tissues into the LNs (64). Characteristic LV markers include LYVE-1, prospero homeobox protein 1 (PROX-1), podoplanin (PDPN), CCL21 and vascular endothelial growth factor (VEGF) receptors (R)−2 and−3 (55, 65). Via extensions into the T and B cell areas LVs are able to centralize antigen presentation, as well as lymphocyte distribution and migration within the LN, either by simply delivering soluble factors or cells, or by acting as APCs themselves (1, 66–69).
Afferent LVs originating from the peripheral tissue branch into the SCSs located directly underneath the LN capsule, extend into the T and B cell areas, and exit as efferent vessels (7, 70). Via these extensions LVs are able to centralize antigen presentation, as well as lymphocyte distribution and migration within the LN, either by simply delivering soluble factors or cells, or by acting as APCs themselves (1, 66–69).
Unlike formation of HEVs, LV formation is already initiated within the same timeframe as LN formation (8, 9, 11). Details for LV formation have mostly been studied in inguinal (i) LN as these can already be found prenatally. In iLN the first event in the development of LVs is the formation of a capillary-like plexus (11, 71) which matures into LYVE-1lowVEGFR+ collecting LVs between E15.5-E16.5 (11, 72) ultimately forming a lymphatic cup that surrounds the developing LN anlagen by E20.5 (11). Remodeling of initial LVs is dependent on engagement of VEGFR-3 on LECs by VEGF-C produced by surrounding stromal cells in a LTβR-dependent manner (20, 73). While the mechanisms underlying VEGFR-3 expression by LECs are not fully understood, at least one study identified VEGFR-3 as downstream target of canonical NF-κB signaling (74).
Recently, the details of the sequence of events and the importance of LECs during iLN development have become clear (11). Although starting within the same timeframe, initial formation of the LN anlagen is independent of LEC differentiation (11, 75). Differentiation of LECs into collecting LVs is important for uptake and transport of mature CD4+ LTi cells into the iLN anlagen. In addition, iLN size is also defined by the number of cells that can be retained a process that depends on CXCR5-CXCL13 mediated interaction between LT LTi cells and LTβR expressing LTo cells (10, 11). CXCL13 expression by LTo cells is known to be indispensable for LTi cell retention and it is now clear that LTβR signaling together with interstitial fluid flow regulated by collecting LVs can induce LTo cell CXCL13 expression (11).
Recently, the functions of LECs in LN development have become more clear, aided by studies focusing on the role of LEC-specific TNFRSF member signaling (12, 45, 76). It was shown that more than half of LYVE-1CreLtbrfl/flNikfl/fl mice have a loss of PLNs due to incapacity to attract sufficient LTi cells to expand the LN anlagen (12). Interestingly, single deletion of either Ltbr or Nik in LECs does not affect the number of PLN formed (12, 45), indicating that compensatory mechanisms may take over when either LTβR or NIK is not functional. In addition, it was shown that LEC-specific NIK deletion impairs the recruitment of B cells into the PLN and it is suggested that this might be due to reduced CXCL13 expression (77). Consequently, LTβR-NIK signaling in LEC may be crucial for the expansion and maturation of fully functional LNs. In addition to LTβR signaling, LEC-specific RANK signaling is involved in LN formation. It is suggested that interfering with RANK signaling reduces expression of ICAM-1 and VCAM-1 on LECs, leading to impaired LTi cell retention in the developing LN anlagen (12).
For a long time, the exact role for LECs in LN development was not completely clear. A recent study using LYVE-1creRankfl/fl mice suggests that recruitment of LTi cells by LECs is the first step leading to formation of the LN anlagen (12, 78), whereas it has also been shown that formation of the LN anlagen can be initiated in absence of LECs (11, 75). The recent findings by Bovay et al. provide evidence that although LTi cells may form an initial LN anlage independently of LECs, LECs are crucial for the formation of definitive LNs by transporting CD4+ LTi cells that have egressed from venous locations to the LN anlagen (11).
The Lymph Node Vasculature During Inflammation
Blood Vessels
During inflammation soluble factors, antigens, DCs and/or lymphocytes are drained from the site of insult into the LNs (79). The inflammatory response that follows leads to an increase in LN size which mostly relies on remodeling of the blood vasculature (80–82) and expansion of the LV (83). Once inflammation is resolved, the vasculature and with that the complete LN, returns to its homeostatic condition (79).
Under inflammatory conditions the number and characteristics of the immune cells infiltrating the draining LN is altered, which triggers changes in the local vasculature. These changes include general expansion of the LN blood vasculature to increase blood supply and influx of lymphocytes. Expansion of the HEV network is regulated by DCs producing VEGF-A, which directly stimulates HEV growth, and LTα1β2 which ligates LTβR fibroblastic stromal cells leading to production of more VEGF-A (57, 81, 84). In addition to alterations in the volume of lymphocyte influx, the composition of the influx is also changed which is predominantly caused by a change in the expression of homing associated molecules and inflammatory chemokines, that enable recruitment of (activated) immune cells that are not recruited under homeostatic conditions (50, 85).
Several studies showed that phenotypic changes occur in HEVs during inflammation without compromising their ability to recruit naïve lymphocytes (56, 82, 86). These changes suggest a reversal toward a more immature state, where downregulation of mature HEVs genes coincides with temporary upregulation of more immature HEVs genes like MAdCAM-1 (56, 57, 62, 87). It is proposed that this change in gene expression aids the entry of LTi and other innate lymphoid cells (ILC) which are necessary for eventual restoration of the homeostatic architecture (87). More recently, using single-cell analysis, detailed insights into the temporary changes occurring in PLN HECs during inflammation were obtained. Generally, it was shown that temporary changes include downregulation of several mature HEV genes like Glycam-1 and Ccl21 during the first few days after immunization, with a restoration in expression by day 7 post immunization. However, expression of other HEV markers, including MECA-79 is maintained, most likely because these are essential for recruitment of naïve lymphocytes into PLNs. In addition, there is a temporal upregulation of other adhesion molecules, like MAdCAM-1 and E- and P-selectin (56).
This remodeling of HEVs and thereby the entire composition of the LN during inflammatory responses is largely dependent on engagement of LTβR mediated by LTα1β2-expressing B cells, among others, and to a lesser extent on classical growth factors like VEGF-A (57, 81, 88).
Importantly, during inflammation LTα1β2-LTβR mediated crosstalk between HEVs and LVs exists: HEVs are demonstrated to locate around LV, and in some cases HECs that express LYVE-1 are observed, indicating that during inflammation HEVs might adopt certain features of LVs (56, 62). These double positive vessels are mainly found during the first few days after immunization and eventually disappear. Interestingly, changes in the LV network seem to occur in parallel with changes in HEVs, since during the first days following immunization there is a temporary decrease in LV function, which is restored in parallel with HEV recovery (62).
Lymphatic Vessels
Lymphatic ECs are among the first cells to react to inflammatory insults and expansion of the local LVs via lymphangiogenesis plays an important role during inflammation. Lymphangiogenesis enables increased transport of fluid, immune cells and APCs from the site of inflammation into the LN to initiate the first immune response (62, 89). Lymphatic vessel remodeling is not only apparent at the site of insult, but also in the draining LNs (90).
Interestingly, during inflammation there seems to be a strict spatial and temporal regulation of LN lymphangiogenesis that is thought to regulate the sequential regulation of DCs and T cells (90, 91); lymphangiogenesis is first observed in the SCSs, and only later in the cortical and medullary sinuses (91). In addition to morphological changes, LECs also alter gene expression during inflammatory responses including upregulation of many NF-κB related genes including Icam-1, Vcam-1, Cxcl12 and Ccl21 (55). Of these chemokines, CCL21 is best characterized: it is upregulated under inflammatory conditions and known to mediate migration of CCR7+ immune cells (92). Together, this suggests that LVs are important for initiation and shaping of immune responses within the LN. Similar to HEV remodeling, lymphangiogenesis can be reduced by interfering with LTβR signaling or by depleting (LTα1β2-expressing) B cells, suggesting concerted action of the LTβR and B cells in lymphangiogenesis and HEVs plasticity (62, 93).
Once inflammation is resolved there is a return to normal LN homeostasis, including reversal of the vasculature. However, if inflammation persists, the inflammatory phenotype of the LN is maintained, contributing to persistence of inflammation (94, 95).
Tertiary Lymphoid Structure Formation
In addition to changes in LNs, persistent inflammation and antigen stimulation can lead to local formation of lymphoid like tissues, which can vary from unorganized cellular infiltrates to highly organized tertiary lymphoid structures (TLSs) (96). TLSs resemble LNs, in that they are characterized by a network of activated stromal cells and FDCs, as well as the presence of PNAd+ ECs that represent the HECs in LN. In addition, TLSs are composed of distinct B and T cell areas. But in contrast to LNs, TLSs lack a capsule and independent afferent LVs (96, 97). Emerging evidence suggests that, in addition to structural similarities, there is also overlap between the cells and molecules directing LN and TLS development. TLSs have ectopic expression of lymphoid chemokines and cytokines, including LTα, LTβ, CXCL13, CXCL12, CCL21, and CCL19 (98–100). The development and function of TLSs, as well as the cellular players involved have been extensively reviewed elsewhere (96, 97, 99–101) and therefore we will mainly focus on the role of TNFRSF members in TLSs and their associated vessels.
Many animal studies have shown that overexpression of a single inflammatory cytokine or homeostatic chemokine is sufficient to initiate TLS development and TNFRSF members and their ligands are among the main players [reviewed in (97)]. More than 20 years ago, the group of Ruddle showed that mice overexpressing LTα1β2 or TNFα under the rat insulin promotor (RIP-LTα1β2 and RIP-TNFα transgenic (tg) mice, respectively) spontaneously develop infiltrates consisting of T and B cells in the pancreas (102). Additional studies further investigated the role of TNFRSF members in TLS development and function. Overall, these studies demonstrated that signaling through TNFRSF members plays a critical role in the development of TLSs. Signaling through the LTα/TNF-TNFRI axis is sufficient to initiate TLS formation, but LTα1β2-LTβR signaling leads to larger and more organized TLS with higher expression of TLS associated cytokines and chemokines (i.e., CXCL13, CCL19, CCL21) (98, 102, 103). It is thought that LTα1β2 and other cytokines and chemokines produced by immune cells acting like LTi cells, ligate receptors on local stromal cells which are then stimulated to differentiate into a lymphoid stromal cell-like phenotype that can drive TLS formation (97, 104–106). So, interaction between immune cells acting like LTi cells and stromal cells acting like LTo is crucial for the development of TLSs. Together, this suggests that the signaling pathways and players involved in TLS and LN development largely overlap.
Tertiary Lymphoid Structure Associated High Endothelial Venules
As already mentioned, one of the characteristics of TLSs is the presence of PNAd+ HEC-like cells. During chronic inflammation the blood vasculature undergoes remodeling in order to recruit immune cells into the inflamed tissue. This remodeling involves upregulation of adhesion molecules and production of chemoattractants by BECs (107–109). Ultimately, as inflammation persists, these BECs can differentiate into PNAd+ HEC-like cells that orchestrate extravasation of L-selectin+ and CCR7+ immune cells into the TLSs (97).
In general, TLS associated PNAd+ BVs have the same function as LN HEVs, namely recruitment of (naïve) immune cells into the tissue to mediate interaction with cognate antigens leading to immune cell activation and memory cell formation (98, 102, 110–112). Interestingly, in TLSs the consequences of these interactions may be different depending on the pathology. For example, in autoimmune diseases like rheumatoid arthritis, recruitment and local activation of autoreactive B and plasma cells is likely to exacerbate disease (113, 114). In contrast, in infections recruitment of immune cells to the site of infection might aid in its resolution by limiting spreading of the microorganisms and confining the immune reaction (6, 96). In the case of cancer, PNAd+ BVs may act anti-tumoral via recruitment of naïve and memory T cells (115–117), but they can also facilitate immune evasion by recruitment of immunosuppressive cells, like regulatory T cells (50).
Similar to LN HEVs, formation of PNAd+ BVs in TLSs seems to be LTβR dependent. It has been shown that initiation of TLS-PNAd+ BVs occurs via the LTα-TNFRI-canonical NF-κB axis leading to MAdCAM-1+ BVs and that LTα1β2-LTβR-noncanonical NF-κB signaling generates PNAd+ BVs (29, 98, 118). This is underlined by the finding that TLS PNAd+ BVs located in the synovial tissue of rheumatoid arthritis patients are NIK+ comparable to LN HEVs (119). In addition, in the synovial tissue of these patients ILC3s were found, and although present in very small numbers, they might act similar to LTi cells in the formation of PNAd+ BVs (119). Although LTβR signaling is typically required for the formation of mature HEVs, when PNAd+ BVs are formed in absence of real TLSs, PNAd+ BVs can also develop via LTα-TNFRI signaling, independent of LTβR signaling (120). Together, these data suggest that the same mechanisms that lead to formation of LN HEVs are involved in the formation of TLS associated PNAd+ BVs. However, the function of these vessels is shown to vary between pathologies, making it hard to predict the outcome of targeting HEVs during disease.
Tertiary Lymphoid Structure Associated Lymphatic Vessels
The role of LVs in inflammation has long been recognized; i.e., LVs play an immunoregulatory role in inflammation via fluid drainage, scavenging of inflammatory chemokines and suppression of DC maturation (121, 122). During chronic inflammation, it is thought that the lymphangiogenic process is altered and that the amount of infiltrating immune cells exceeds the draining capacity of LVs. Lack of efficient draining might lead to local persistence of antigens and immune cells, and non-functional LVs might compete with functional LVs leading to trapping of immune cells at the site of insult, favoring development or maintenance of TLSs (123, 124).
Although LYVE-1+ Prox-1+, PDPN+CCL21+ vessels are found in TLSs of many pathologies, their exact role and function is not well-understood (125, 126). They often contain cells, implying that they have a role as transporters. On the other hand, these vessels can be so crowded with cells that their drainage and efferent functions are likely impaired (127). In contrast to LN LVs, it seems less probable that TLS LVs are necessary for antigen transport since TLSs are already characterized by local presence of antigens. The source of these antigens may be the LVs itself, since it has been shown that LECs are capable of antigen archiving and –presentation (69). It has also been proposed that within TLS, LECs might act as APCs to induce tolerance or T cell activation (125).
In mice lacking LTβR, TNFRI or LTα, the development of LVs in the inflamed area is impaired, while the surrounding LVs remain largely unaffected (128, 129). It was shown that LTα is sufficient to induce lymphangiogenesis even before the onset of organized TLSs, whereas LTα1β2 is not required (129). Of note, it was shown that LTα1β2 might even negatively regulate lymphangiogenesis since Ltb−/− mice exhibit increased lymphangiogenesis, which may be due to the fact that mice lacking LTα1β2 have more LTα available to form trimers that bind to TNFRI (129). Together this indicates that TNFRSF member signaling is involved in inflammatory development of LVs, but not for the maintenance of the existing lymphatic vasculature.
The role of RANK signaling in TLS associated LVs has not yet been fully addressed, but with the recent evidence demonstrating an important role for that RANK signaling in LECs in the development of mature functional LNs (12), it will be of great interest to investigate the importance of RANK signaling in the formation and function of TLS.
Concluding Remarks and Future Perspectives
It is clear that signaling through TNFRSF members is crucial for development and functioning of LNs and TLSs, both in health and disease. Here, we emphasized the importance of EC-specific TNFRSF member signaling cascades in these processes.
It is clear that TNFRSF signaling, mainly via the LTβR, in BECs is crucial for their differentiation into HECs, which subsequently regulate recruitment of lymphocytes into the LNs. In addition, the same TNFRSF-mediated mechanisms are involved in formation of TLS associated PNAd+ BVs.
Although considerably less is known about TNFRSF member signaling in LVs, recent data does point toward an important role for these pathways in LEC function in LN development and function. A prominent role seems to be reserved for signaling via RANK, although LTβR signaling is also likely to be involved. Interestingly, different mechanisms may be involved in functioning of LECs in LNs and TLSs.
The currently available studies aimed at unraveling the role of signaling pathways in LN EC subsets already prompt possible refinements of the existing models for LN development and the role of TNFRSF member signaling in ECs within this process, but there are still several outstanding questions. One of these questions is to what extent the timing of TNFRSF signaling is important, since it is not fully understood at what exact time point signaling events in specific EC subsets are crucial for the development of fully functional lymphoid organs. In this respect, the development of EC-specific conditional KO mice is very promising and aid tremendously in addressing this issue.
In line with this, it will also be interesting to see if interfering with specific signaling pathways in EC subsets holds therapeutic potential in treatment of chronic inflammatory diseases and other pathologies. Several clues point toward this, for example the finding that NIK can be detected in TLS associated PNAd+ BVs and other blood vessels in inflamed tissues, but not in healthy tissues (130). Here, conditional EC-specific KO models in combination with disease models will allow to dissect the importance of TNFRSF members and their ligands in EC both in LN and target tissues before onset and during active disease.
It is likely to assume that in the near future many outstanding questions will be answered. With the current possibilities in animal models and advanced techniques to compare patients with healthy individuals, it is only a matter of time until deeper insights into the cell specific signaling pathways in development and maintenance of LNs and TLSs are gained. More importantly, it will be of great interest to uncover how these pathways contribute to disease and whether they hold therapeutic potential.
Author Contributions
KJ: conceived the general idea, wrote the manuscript, and created the figures. JK and RM: provided expert opinion/knowledge input, and co-wrote the manuscript. ST: conceived the general idea, provided expert opinion/knowledge input, and co-wrote the manuscript.
Funding
This work was supported by the Dutch Arthritis Foundation grant to ST (RF16-1-302) and by a Netherlands Organization for Scientific Research grant to JK (ALWOP.271).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Junt T, Scandella E, Ludewig B. Form follows function: lymphoid tissue microarchitecture in antimicrobial immune defence. Nat Rev Immunol. (2008) 8:764–75. doi: 10.1038/nri2414
2. Willard-Mack CL. Normal structure, function, and histology of lymph nodes. Toxicol Pathol. (2006) 34:409–24. doi: 10.1080/01926230600867727
3. Malhotra D, Fletcher AL, Turley SJ. Stromal and hematopoietic cells in secondary lymphoid organs: partners in immunity. Immunol Rev. (2013) 251:17. doi: 10.1111/imr.12023
4. Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol. (2009) 9:618–29. doi: 10.1038/nri2588
5. Perez-Shibayama C, Gil-Cruz C, Ludewig B. Fibroblastic reticular cells at the nexus of innate and adaptive immune responses. Immunol Rev. (2019) 289:31–41. doi: 10.1111/imr.12748
6. Ager A. High endothelial venules and other blood vessels: critical regulators of lymphoid organ development and function. Front Immunol. (2017) 8:45. doi: 10.3389/fimmu.2017.00045
7. Petrova TV, Koh GY. Organ-specific lymphatic vasculature: from development to pathophysiology. J Exp Med. (2018) 215:35–49. doi: 10.1084/jem.20171868
8. van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. (2010) 10:664–74. doi: 10.1038/nri2832
9. Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. (2003) 3:292–303. doi: 10.1038/nri1054
10. van de Pavert SA, Olivier BJ, Goverse G, Vondenhoff MF, Greuter M, Beke P, et al. Chemokine CXCL13 is essential for lymph node initiation and is induced by retinoic acid and neuronal stimulation. Nat Immunol. (2009) 10:1193–9. doi: 10.1038/ni.1789
11. Bovay E, Sabine A, Prat-Luri B, Kim S, Son K, Willrodt AH, et al. Multiple roles of lymphatic vessels in peripheral lymph node development. J Exp Med. (2018) 215:2760–77. doi: 10.1084/jem.20180217
12. Onder L, Morbe U, Pikor N, Novkovic M, Cheng HW, Hehlgans T, et al. Lymphatic endothelial cells control initiation of lymph node organogenesis. Immunity. (2017) 47:80–92 e4. doi: 10.1016/j.immuni.2017.05.008
13. Yoshida H, Naito A, Inoue JI, Satoh M, Santee-Cooper SM, Ware CF, et al. Different cytokines induce surface lymphotoxin-αβ on IL-7 receptor-α cells that differentially engender lymph nodes and Peyer's Patches. Immunity. (2001) 17:11. doi: 10.1016/S1074-7613(02)00479-X
14. Benezech C, White A, Mader E, Serre K, Parnell S, Pfeffer K, et al. Ontogeny of stromal organizer cells during lymph node development. J Immunol. (2010) 184:4521–30. doi: 10.4049/jimmunol.0903113
15. Vondenhoff MF, Greuter M, Goverse G, Elewaut D, Dewint P, Ware CF, et al. LTβR signaling induces cytokine expression and up-regulates lymphangiogenic factors in lymph node anlagen. J Immunol. (2009) 182:5439–45. doi: 10.4049/jimmunol.0801165
16. Cupedo T, Mebius RE. Cellular interactions in lymph node development. J Immunol. (2005) 174:21–5. doi: 10.4049/jimmunol.174.1.21
17. Cupedo T, Kraal G, Mebius RE. The role of CD45+CD4+CD3- cells in lymphoid organ development. Immunol Rev. (2002) 189:10. doi: 10.1034/j.1600-065X.2002.18905.x
18. Mueller CG, Hess E. Emerging functions of RANKL in lymphoid tissues. Front Immunol. (2012) 3:261. doi: 10.3389/fimmu.2012.00261
19. White A, Carragher D, Parnell S, Msaki A, Perkins N, Lane P, et al. Lymphotoxin a-dependent and -independent signals regulate stromal organizer cell homeostasis during lymph node organogenesis. Blood. (2007) 110:1950–9. doi: 10.1182/blood-2007-01-070003
20. Cupedo T, Lund FE, Ngo VN, Randall TD, Jansen W, Greuter MJ, et al. Initiation of cellular organization in lymph nodes is regulated by non-B cell-derived signals and is not dependent on CXC chemokine ligand 13. J Immunol. (2004) 173:4889–96. doi: 10.4049/jimmunol.173.8.4889
21. Kucharzewska P, Maracle CX, Jeucken KCM, van Hamburg JP, Israelsson E, Furber M, et al. NIK-IKK complex interaction controls NF-kappaB-dependent inflammatory activation of endothelium in response to LTbetaR ligation. J Cell Sci. (2019) 132:13. doi: 10.1242/jcs.225615
22. Sun SC. The noncanonical NF-κB pathway. Immunol Rev. (2012) 246:125–40. doi: 10.1111/j.1600-065X.2011.01088.x
23. Sun SC. The non-canonical NF-κB pathway in immunity and inflammation. Nat Rev Immunol. (2017) 17:545–58. doi: 10.1038/nri.2017.52
24. Fütterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin β receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. (1998) 9:12. doi: 10.1016/S1074-7613(00)80588-9
25. Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, et al. RANK is essential for osteoclast and LN development. Genes Dev. (1999) 13:13. doi: 10.1101/gad.13.18.2412
26. Shinkura R, Kitada K, Matsuda F, Tashiro K, Ikuta K, Suzuki M, et al. Alymphoplasia is caused by a point mutation in the mouse gene encoding NF-κB-inducing kinase. Nat Genet. (1999) 22:4. doi: 10.1038/8780
27. Miyawaki S, Nakamura Y, Suzuka H, Koba M, Yasumizu R, Ikehara S, et al. A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur J Immunol. (1994) 24:16. doi: 10.1002/eji.1830240224
28. Yin L, Wu L, Wesche H, Arthur CD, White JM, Goeddel DV, et al. Defective lymphotoxin-β receptor-induced NF-κB transcriptional activity in NIK-deficient mice. Science. (2001) 291:5. doi: 10.1126/science.1058453
29. Drayton DL, Bonizzi G, Ying X, Liao S, Karin M, Ruddle NH. I kappa B kinase complex alpha kinase activity controls chemokine and high endothelial venule gene expression in lymph nodes and nasal-associated lymphoid tissue. J Immunol. (2004) 173:6161–8. doi: 10.4049/jimmunol.173.10.6161
30. Alcamo E, Hacohen N, Schulte LC, Rennert PD, Hynes RO, Baltimore D. Requirement for the NF-kappaB family member RelA in the development of secondary lymphoid organs. J Exp Med. (2002) 195:233–44. doi: 10.1084/jem.20011885
31. Pasparakis M, Alexopoulou L, Grell M, Pfizenmaier K, Bluethmann H, Kollias G. Peyer's patch organogenesis is intect yet formation of B lymphocyte follicles is defective in peripheral lymphoid organs of mice deficient for tumor necrosis factor and its 55-kDa receptor. Proc Natl Acad Sci USA. (1997) 94:5. doi: 10.1073/pnas.94.12.6319
32. Weih F, Carrasco D, Durham SK, Barton DS, Rizzo CA, Ryseck R, et al. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-κB/Rel family Cell. (1995) 80:10. doi: 10.1016/0092-8674(95)90416-6
33. Wu L, D'Amico A, Winkel KD, Suter M, Lo D, Shortman K. RelB is essential for the development of myeloid-related CD8α- dendritic cells but not of lymphoid-related CD8α- dendritic cells. Immunity. (1998) 9:9. doi: 10.1016/S1074-7613(00)80649-4
34. Franzoso G, Carlson L, Poljak L, Shores EW, Epstein S, Leonardi A, et al. Mice deficient in nuclear factor (NF)-kappa B/p52 present with defects in humoral responses, germinal center reactions, and splenic microarchitecture. J Exp Med. (1998) 187:147–59. doi: 10.1084/jem.187.2.147
35. Lo JC, Basak S, James ES, Quiambo RS, Kinsella MC, Alegre ML, et al. Coordination between NF-kappaB family members p50 and p52 is essential for mediating LTbetaR signals in the development and organization of secondary lymphoid tissues. Blood. (2006) 107:1048–55. doi: 10.1182/blood-2005-06-2452
36. Koni PA, Sacca R, Lawton P, Browning JL, Ruddle NH, Flavell RA. Distinct roles in lymphoid organogenesis for lymphotoxins α and β revealed in lymphotoxin β-deficient mice. Immunity. (1997) 6:10. doi: 10.1016/S1074-7613(00)80292-7
37. Scheu S, Alferink J, Potzel T, Barchet W, Kalinke U, Pfeffer K. Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin beta in mesenteric lymph node genesis. J Exp Med. (2002) 195:1613–24. doi: 10.1084/jem.20020215
38. Alimzhanov MB, Kuprash DV, Kosco-Vilbois MH, Luz A, Turetskaya RL, Tarakhovsky A, et al. Abnormal development of secondary lymphoid tissues in lymphotoxin β-deficient mice. Proc Natl Acad Sci USA. (1997) 94:6. doi: 10.1073/pnas.94.17.9302
39. De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. (1994) 264:703–7. doi: 10.1126/science.8171322
40. Banks TA, Rouse BT, Kerley MK, Blair PJ, Godfrey VL, Kuklin NA, et al. Lymphotoxin-alpha-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol. (1995) 155:9.
41. Körner H, Cook M, Riminton DS, Lemckert FA, Hoek RM, Ledermann B, et al. Distict roles for lymphotoxin-α and tumor necrosis factor in organogenesis and spatial organization of lymphoid tissue. Eur J Immunol. (1997) 27:10. doi: 10.1002/eji.1830271020
42. Matsumoto M, Mariathasan S, Nahm MH, Baranyay F, Peschon JJ, Chaplin DD. Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science. (1996) 271:9. doi: 10.1126/science.271.5253.1289
43. Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte develoment and lymph-node organogenesis. Nature. (1999) 397:9. doi: 10.1038/16852
44. Onder L, Danuser R, Scandella E, Firner S, Chai Q, Hehlgans T, et al. Endothelial cell–specific lymphotoxin-β receptor signaling is critical for lymph node and high endothelial venule formation. J Exp Med. (2013) 210:465–73. doi: 10.1084/jem.20121462
45. Wang Z, Chai Q, Zhu M. Differential Roles of LTβR in endothelial cell subsets for lymph node organogenesis and maturation. J Immunol. (2018) 201:69–76. doi: 10.4049/jimmunol.1701080
46. Rennert PD, Browning JL, Mebius R, Mackay F, Hochman PS. Surface lymphotoxin alpha/beta complex is required for the development of peripheral lymphoid organs. J Exp Med. (1996) 184:1999–2006. doi: 10.1084/jem.184.5.1999
47. Sugiyama M, Nakato G, Jinnohara T, Akiba H, Okumura K, Ohno H, et al. Expression pattern changes and function of RANKL during mouse lymph node microarchitecture development. Int Immunol. (2012) 24:369–78. doi: 10.1093/intimm/dxs002
48. Kim D, Mebius RE, MacMicking JD, Jung S, Cupedo T, Castellanos Y, et al. Regulation of peripheral lymph node genesis by the tumor necrosis factor family member TRANCE. J Exp Med. (2000) 192:1467–78. doi: 10.1084/jem.192.10.1467
49. Kawashima H, Fukuda M. Sulfated glycans control lymphocyte homing. Ann N Y Acad Sci. (2012) 1253:112–21. doi: 10.1111/j.1749-6632.2011.06356.x
50. Ager A, May MJ. Understanding high endothelial venules: Lessons for cancer immunology. Oncoimmunology. (2015) 4:14. doi: 10.1080/2162402X.2015.1008791
51. Lee M, Kiefel H, LaJevic MD, Macauley MS, Kawashima H, O'Hara E, et al. Transcriptional programs of lymphoid tissue capillary and high endothelium reveal control mechanisms for lymphocyte homing. Nat Immunol. (2014) 15:982–95. doi: 10.1038/ni.2983
52. Nolz JC, Starbeck-Miller GR, Harty JT. Naive, effector and memory CD8 T-cell trafficking: parallels and distinctions. Immunotherapy. (2011) 3:1223–33. doi: 10.2217/imt.11.100
53. Mitoma J, Bao X, Petryanik B, Schaerli P, Gauguet J-M, Yu S-Y, et al. Critical functions of N-glycans in L-selectin-mediated lymphocyte homing and recruitment. Nat Immunol. (2007) 8:409–18. doi: 10.1038/ni1442
54. Hirakawa J, Tsuboi K, Sato K, Kobayashi M, Watanabe S, Takakura A, et al. Novel anti-carbohydrate antibodies reveal the cooperative function of sulfated N- and O-glycans in lymphocyte homing. J Biol Chem. (2010) 285:40864–78. doi: 10.1074/jbc.M110.167296
55. Malhotra D, Fletcher AL, Astarita J, Lukacs-Kornek V, Tayalia P, Gonzalez SF, et al. Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat Immunol. (2012) 13:12. doi: 10.1038/ni.2262
56. Veerman K, Tardiveau C, Martins F, Coudert J, Girard JP. Single-cell analysis reveals heterogeneity of high endothelial venules and different regulation of genes controlling lymphocyte entry to lymph nodes. Cell Rep. (2019) 26:3116–31 e5. doi: 10.1016/j.celrep.2019.02.042
57. Browning JL, Allaire N, Ngam-Ek A, Notidis E, Hunt J, Perrin S, et al. Lymphotoxin-beta receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity. (2005) 23:539–50. doi: 10.1016/j.immuni.2005.10.002
58. Carragher D, Johal R, Button A, White A, Eliopoulos A, Jenkinson E, et al. A stroma-derived defect in NF-kappaB2-/- mice causes impaired lymph node development and lymphocyte recruitment. J Immunol. (2004) 173:2271–9. doi: 10.4049/jimmunol.173.4.2271
59. Mebius RE, Streeter PR, Michie S, Butcher EC, Weissman IL. A developmental switch in lymphocyte homing receptor and endothelial vascular addressin expression regulates lympocyte homing and permits CD4+CD3- cells to colonize lymph nodes. Proc Natl Acad Sci USA. (1996) 93:6. doi: 10.1073/pnas.93.20.11019
60. Moussion C, Girard JP. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature. (2011) 479:542–6. doi: 10.1038/nature10540
61. Zhang Z, Li J, Zheng W, Zhao G, Zhang H, Wang X, et al. Peripheral lymphoid volume expansion and maintenance are controlled by gut microbiota via RALDH+ dendritic cells. Immunity. (2016) 44:330–42. doi: 10.1016/j.immuni.2016.01.004
62. Liao S, Ruddle NH. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J Immunol. (2006) 177:3369–79. doi: 10.4049/jimmunol.177.5.3369
63. Wendland M, Willenzon S, Kocks J, Davalos-Misslitz AC, Hammerschmidt SI, Schumann K, et al. Lymph node T cell homeostasis relies on steady state homing of dendritic cells. Immunity. (2011) 35:945–57. doi: 10.1016/j.immuni.2011.10.017
64. Kerjaschki D. The lymphatic vasculature revisited. J Clin Invest. (2014) 124:874–7. doi: 10.1172/JCI74854
65. Wang P, Cheng Y. Gene expression profile of lymphatic endothelial cells. Cell Biol Int. (2011) 35:1177–87. doi: 10.1042/CBI20100871
66. Randolph GJ, Ivanov S, Zinselmeyer BH, Scallan JP. The lymphatic system: integral roles in immunity. Annu Rev Immunol. (2017) 35:31–52. doi: 10.1146/annurev-immunol-041015-055354
67. Rouhani SJ, Eccles JD, Riccardi P, Peske JD, Tewalt EF, Cohen JN, et al. Roles of lymphatic endothelial cells expressing peripheral tissue antigens in CD4 T-cell tolerance induction. Nat Commun. (2015) 6:13. doi: 10.1038/ncomms7771
68. Dubrot J, Duraes FV, Potin L, Capotosti F, Brighouse D, Suter T, et al. Lymph node stromal cells acquire peptide-MHCII complexes from dendritic cells and induce antigen-specific CD4(+) T cell tolerance. J Exp Med. (2014) 211:1153–66. doi: 10.1084/jem.20132000
69. Al-Soudi A, Kaaij MH, Tas SW. Endothelial cells: From innocent bystanders to active participants in immune responses. Autoimmun Rev. (2017) 16:951–62. doi: 10.1016/j.autrev.2017.07.008
70. Forster R, Braun A, Worbs T. Lymph node homing of T cells and dendritic cells via afferent lymphatics. Trends Immunol. (2012) 33:271–80. doi: 10.1016/j.it.2012.02.007
71. Escobedo N, Oliver G. Lymphangiogenesis: origin, specification, and cell fate determination. Annu Rev Cell Dev Biol. (2016) 32:677–91. doi: 10.1146/annurev-cellbio-111315-124944
72. Schulte-Merker S, Sabine A, Petrova TV. Lymphatic vascular morphogenesis in development, physiology, and disease. J Cell Biol. (2011) 193:607–18. doi: 10.1083/jcb.201012094
73. Lee YG, Koh GY. Coordinated lymphangiogenesis is critical in lymph node development and maturation. Dev Dyn. (2016) 245:9. doi: 10.1002/dvdy.24456
74. Flister MJ, Wilber A, Hall KL, Iwata C, Miyazono K, Nisato RE, et al. Inflammation induces lymphangiogenesis through up-regulation of VEGFR-3 mediated by NF-kappaB and Prox1. Blood. (2009) 115:418–29. doi: 10.1182/blood-2008-12-196840
75. Vondenhoff MF, van de Pavert SA, Dillard ME, Greuter M, Goverse G, Oliver G, et al. Lymph sacs are not required for the initiation of lymph node formation. Development. (2008) 136:29–34. doi: 10.1242/dev.028456
76. Gale NW, Prevo R, Espinosa J, Ferguson DJ, Dominguez MG, Yancopoulos GD, et al. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol Cell Biol. (2007) 27:595–604. doi: 10.1128/MCB.01503-06
77. Yang J, Zhang S, Zhang L, Xie X, Wang H, Jie Z, et al. Lymphatic endothelial cells regulate B-cell homing to lymph nodes via a NIK-dependent mechanism. Cell Mol Immunol. (2019) 16:165–77. doi: 10.1038/cmi.2017.167
78. Onder L, Ludewig B. A fresh view on lymph node organogenesis. Trends Immunol. (2018) 39:775–87. doi: 10.1016/j.it.2018.08.003
79. Gentek R, Bajenoff M. Lymph node stroma dynamics and approaches for their visualization. Trends Immunol. (2017) 38:236–47. doi: 10.1016/j.it.2017.01.005
80. Kumar V, Chyou S, Stein JV, Lu TT. Optical projection tomography reveals dynamics of HEV growth after immunization with protein plus CFA and features shared with HEVs in acute autoinflammatory lymphadenopathy. Front Immunol. (2012) 3:282. doi: 10.3389/fimmu.2012.00282
81. Dasoveanu DC, Shipman WD, Chia JJ, Chyou S, Lu TT. Regulation of lymph node vascular-stromal compartment by dendritic cells. Trends Immunol. (2016) 37:764–77. doi: 10.1016/j.it.2016.08.013
82. Mondor I, Jorquera A, Sene C, Adriouch S, Adams RH, Zhou B, et al. Clonal proliferation and stochastic pruning orchestrate lymph node vasculature remodeling. Immunity. (2016) 45:877–88. doi: 10.1016/j.immuni.2016.09.017
83. Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, Skobe M, et al. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. (2006) 24:203–15. doi: 10.1016/j.immuni.2006.01.003
84. Chyou S, Benahmed F, Chen J, Kumar V, Tian S, Lipp M, et al. Coordinated regulation of lymph node vascular–stromal growth first by CD11c+ cells and then by T and B cells. J Immunol. (2011) 187:5558–67. doi: 10.4049/jimmunol.1101724
85. Pruenster M, Mudde L, Bombosi P, Dimitrova S, Zsak M, Middleton J, et al. The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat Immunol. (2009) 10:101–8. doi: 10.1038/ni.1675
86. Hoke D, Mebius RE, Dybdal N, Dowbenko D, Gribling P, Kyle C, et al. Selective modulation of the expression of L-selectin ligands by an immune response. Curr Biol. (1995) 5:9. doi: 10.1016/S0960-9822(95)00132-1
87. Scandella E, Bolinger B, Lattmann E, Miller S, Favre S, Littman DR, et al. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat Immunol. (2008) 9:667–75. doi: 10.1038/ni.1605
88. Kumar V, Scandella E, Danuser R, Onder L, Nitschke M, Fukui Y, et al. Global lymphoid tissue remodeling during a viral infection is orchestrated by a B cell-lymphotoxin-dependent pathway. Blood. (2010) 115:4725–33. doi: 10.1182/blood-2009-10-250118
89. Zgraggen S, Ochsenbein AM, Detmar M. An important role of blood and lymphatic vessels in inflammation and allergy. J Allergy. (2013) 2013:9. doi: 10.1155/2013/672381
90. Vranova M. Lymphatic vessels in inflammation. J Clin Cell Immunol. (2014) 5:11. doi: 10.4172/2155-9899.1000250
91. Tan KW, Yeo KP, Wong FH, Lim HY, Khoo KL, Abastado JP, et al. Expansion of cortical and medullary sinuses restrains lymph node hypertrophy during prolonged inflammation. J Immunol. (2012) 188:16. doi: 10.4049/jimmunol.1101854
92. Johnson LA, Jackson DG. Inflammation-induced secretion of CCL21 in lymphatic endothelium is a key regulator of integrin-mediated dendritic cell transmigration. Int Immunol. (2010) 22:839–49. doi: 10.1093/intimm/dxq435
93. Shrestha B, Hashiguchi T, Ito T, Miura N, Takenouchi K, Oyama Y, et al. B cell-derived vascular endothelial growth factor A promotes lymphangiogenesis and high endothelial venule expansion in lymph nodes. J Immunol. (2010) 184:4819–26. doi: 10.4049/jimmunol.0903063
94. Manzo A, Pitzalis C. Lymphoid tissue reactions in rheumatoid arthritis. Autoimmun Rev. (2007) 7:30–4. doi: 10.1016/j.autrev.2007.03.006
95. Manzo A, Bombardieri M, Humby F, Pitzalis C. Secondary and ectopic lymphoid tissue responses in rheumatoid arthritis: from inflammation to autoimmunity and tissue damage/remodeling. Immunol Rev. (2010) 233:19. doi: 10.1111/j.0105-2896.2009.00861.x
96. Pitzalis C, Jones GW, Bombardieri M, Jones SA. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol. (2014) 14:447–62. doi: 10.1038/nri3700
97. Buckley CD, Barone F, Nayar S, Benezech C, Caamano J. Stromal cells in chronic inflammation and tertiary lymphoid organ formation. Annu Rev Immunol. (2015) 33:715–45. doi: 10.1146/annurev-immunol-032713-120252
98. Drayton DL, Ying X, Lee J, Lesslauer W, Ruddle NH. Ectopic LT alpha beta directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J Exp Med. (2003) 197:1153–63. doi: 10.1084/jem.20021761
99. Nerviani A, Pitzalis C. Role of chemokines in ectopic lymphoid structures formation in autoimmunity and cancer. J Leukoc Biol. (2018) 104:333–41. doi: 10.1002/JLB.3MR0218-062R
100. Corsiero E, Bombardieri M, Manzo A, Bugatti S, Uguccioni M, Pitzalis C. Role of lymphoid chemokines in the development of functional ectopic lymphoid structures in rheumatic autoimmune diseases. Immunol Lett. (2012) 145:62–7. doi: 10.1016/j.imlet.2012.04.013
101. Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. (2012) 33:297–305. doi: 10.1016/j.it.2012.04.006
102. Kratz A, Campos-Neto A, Hanson MS, Ruddle NH. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J Exp Med. (1996) 183:12. doi: 10.1084/jem.183.4.1461
103. Picarella DE, Kratz A, Li CB, Ruddle NH, Flavell RA. Transgenic tumor necrosis factor (TNF)-alpha production in pancreatic islets leads to insulitis, not diabetes. Distinct patterns of inflammation in TNF-alpha and TNF-beta mice. J Immunol. (1993) 150:15.
104. Barone F, Nayar S, Campos J, Cloake T, Withers DR, Toellner KM, et al. IL-22 regulates lymphoid chemokine production and assembly of tertiary lymphoid organs. Proc Natl Acad Sci USA. (2015) 112:11024–9. doi: 10.1073/pnas.1503315112
105. Fleige H, Ravens S, Moschovakis GL, Bolter J, Willenzon S, Sutter G, et al. IL-17-induced CXCL12 recruits B cells and induces follicle formation in BALT in the absence of differentiated FDCs. J Exp Med. (2014) 211:643–51. doi: 10.1084/jem.20131737
106. Lochner M, Ohnmacht C, Presley L, Bruhns P, Si-Tahar M, Sawa S, et al. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORgamma t and LTi cells. J Exp Med. (2011) 208:125–34. doi: 10.1084/jem.20100052
107. Clark PR, Manes TD, Pober JS, Kluger MS. Increased ICAM-1 expression causes endothelial cell leakiness, cytoskeletal reorganization and junctional alterations. J Invest Dermatol. (2007) 127:762–74. doi: 10.1038/sj.jid.5700670
108. Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. (2007) 7:803–15. doi: 10.1038/nri2171
109. Adams DH, Shaw S. Leucocyte-endothelial interactions and regulation of leucocyte migration. Lancet. (1994) 343:6. doi: 10.1016/S0140-6736(94)92029-X
110. Nasr IW, Reel M, Oberbarnscheidt MH, Mounzer RH, Baddoura FK, Ruddle NH, et al. Tertiary lymphoid tissues generate effector and memory T cells that lead to allograft rejection. Am J Transplant. (2007) 7:1071–9. doi: 10.1111/j.1600-6143.2007.01756.x
111. Hu D, Mohanta SK, Yin C, Peng L, Ma Z, Srikakulapu P, et al. Artery tertiary lymphoid organs control aorta immunity and protect against atherosclerosis via vascular smooth muscle cell lymphotoxin beta receptors. Immunity. (2015) 42:1100–15. doi: 10.1016/j.immuni.2015.05.015
112. Joshi NS, Akama-Garren EH, Lu Y, Lee DY, Chang GP, Li A, et al. Regulatory T Cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity. (2015) 43:579–90. doi: 10.1016/j.immuni.2015.08.006
113. Humby F, Bombardieri M, Manzo A, Kelly S, Blades MC, Kirkham B, et al. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med. (2009) 6:e1. doi: 10.1371/journal.pmed.0060001
114. Scheel T, Gursche A, Zacher J, Haupl T, Berek C. V-region gene analysis of locally defined synovial B and plasma cells reveals selected B cell expansion and accumulation of plasma cell clones in rheumatoid arthritis. Arthritis Rheum. (2011) 63:63–72. doi: 10.1002/art.27767
115. Hindley JP, Jones E, Smart K, Bridgeman H, Lauder SN, Ondondo B, et al. T-cell trafficking facilitated by high endothelial venules is required for tumor control after regulatory T-cell depletion. Cancer Res. (2012) 72:11. doi: 10.1158/0008-5472.CAN-12-1912
116. Schrama D, thor Straten P, Fischer WH, McLellan AD, Bröcker EB, Reisfeld RA, et al. Targeting of lymphotoxin-α to the tumor elicits an efficient immune response associated with induction of peripheral lymphoid-like tissue. Immunity. (2001) 14:11. doi: 10.1016/S1074-7613(01)00094-2
117. Yu P, Lee Y, Liu W, Chin RK, Wang J, Wang Y, et al. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol. (2004) 5:141–9. doi: 10.1038/ni1029
118. Cuff CA, Schwartz J, Bergman CM, Russell KS, Bender JF, Ruddle NH. Lymphotoxin α3 induces chemokines and adhesion molecules: insight into the role of LTα in inflammation and lymphoid organ development. J Immunol. (1998) 161:8.
119. Noort AR, van Zoest KP, van Baarsen LG, Maracle CX, Helder B, Papazian N, et al. Tertiary lymphoid structures in rheumatoid arthritis: NF-kappaB-inducing kinase-positive endothelial cells as central players. Am J Pathol. (2015) 185:1935–43. doi: 10.1016/j.ajpath.2015.03.012
120. Peske JD, Thompson ED, Gemta L, Baylis RA, Fu YX, Engelhard VH. Effector lymphocyte-induced lymph node-like vasculature enables naive T-cell entry into tumours and enhanced anti-tumour immunity. Nat Commun. (2015) 6:7114. doi: 10.1038/ncomms8114
121. Jamieson T, Cook DN, Nibbs RJ, Rot A, Nixon C, McLean P, et al. The chemokine receptor D6 limits the inflammatory response in vivo. Nat Immunol. (2005) 6:403–11. doi: 10.1038/ni1182
122. Podgrabinska S, Kamalu O, Mayer L, Shimaoka M, Snoeck H, Randolph GJ, et al. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J Immunol. (2009) 183:1767–79. doi: 10.4049/jimmunol.0802167
123. von der Weid PY, Rehal S, Ferraz JG. Role of the lymphatic system in the pathogenesis of Crohn's disease. Curr Opin Gastroenterol. (2011) 27:335–41. doi: 10.1097/MOG.0b013e3283476e8f
124. Kajiya K, Detmar M. An important role of lymphatic vessels in the control of UVB-induced edema formation and inflammation. J Invest Dermatol. (2006) 126:919–21. doi: 10.1038/sj.jid.5700126
125. Ruddle NH. High endothelial venules and lymphatic vessels in tertiary lymphoid organs: characteristics, functions, and regulation. Front Immunol. (2016) 7:491. doi: 10.3389/fimmu.2016.00491
126. Ruddle NH. Lymphatic vessels and tertiary lymphoid organs. J Clin Invest. (2014) 124:953–9. doi: 10.1172/JCI71611
127. Huang Y, Caputo CR, Noordmans GA, Yazdani S, Monteiro LH, van den Born J, et al. Identification of novel genes associated with renal tertiary lymphoid organ formation in aging mice. PLoS ONE. (2014) 9:e91850. doi: 10.1371/journal.pone.0091850
128. Furtado GC, Marinkovic T, Martin AP, Garin A, Hoch B, Hubner W, et al. Lymphotoxin β receptor signaling is required for inflammatory lymphangiogenesis in the thyroid. Proc Natl Acad Sci USA. (2007) 104:6. doi: 10.1073/pnas.0606697104
129. Mounzer RH, Svendsen OS, Baluk P, Bergman CM, Padera TP, Wiig H, et al. Lymphotoxin-alpha contributes to lymphangiogenesis. Blood. (2010) 116:2173–82. doi: 10.1182/blood-2009-12-256065
Keywords: LN development, TLS, inflammation, LN vasculature, endothelial cell, TNFR superfamily, NF-κB signaling
Citation: Jeucken KCM, Koning JJ, Mebius RE and Tas SW (2019) The Role of Endothelial Cells and TNF-Receptor Superfamily Members in Lymphoid Organogenesis and Function During Health and Inflammation. Front. Immunol. 10:2700. doi: 10.3389/fimmu.2019.02700
Received: 20 August 2019; Accepted: 04 November 2019;
Published: 20 November 2019.
Edited by:
Marcus Thelen, Institute for Research in Biomedicine (IRB), SwitzerlandReviewed by:
Burkhard Ludewig, Kantonsspital St. Gallen, SwitzerlandCinzia Fionda, Sapienza University of Rome, Italy
Copyright © 2019 Jeucken, Koning, Mebius and Tas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sander W. Tas, s.w.tas@amsterdamumc.nl
 Kim C. M. Jeucken
Kim C. M. Jeucken Jasper J. Koning
Jasper J. Koning Reina E. Mebius
Reina E. Mebius Sander W. Tas
Sander W. Tas