Oviposition selection in spotted lanternfly: impact of habitat and substrate on egg mass size and hatchability
- Pennsylvania Department of Conservation and Natural Resources, Harrisburg, PA, United States
Oviposition strategies adopted by insects (e.g., habitat selection, substrate preference, egg size, clutch size, structure, arrangement, parental care) are critical to the survival and development of their eggs. The impact of habitat and oviposition substrate on spotted lanternfly egg mass size and hatchability was studied in Pennsylvania through laboratory observations and field monitoring in 2019 and 2021. Eggs were arranged in single layers of 1–13 columns (1–18 eggs/column) on surfaces of various types of oviposition substrates, with the longest column(s) in the middle of the egg mass. Egg mass size was positively correlated with column number, with a mean of 26.6–35.1 (0–105) eggs/egg mass for different samples. Significant differences in egg mass size were observed between study sites, with larger egg masses found at Wertz (44.8), Sam Lewis (40.6), Pinnacle (39.1), Marsh Creek (37.9), Susquehannock (34.5), and Memorial Lake (33.3) and smaller egg masses at Nolde Forest (25.0), Gordon (24.4), and Antietam (21.0). Significant differences were also detected between types of oviposition substrates with smaller egg masses found on American hornbeam (22.7). In general, more (31.6%–48.0%) eggs hatched in the field compared with the laboratory (10.0%). Egg hatch success was positively correlated with egg mass size, with the highest rates recorded on American beech, American hophornbeam, black birch, black cherry, black locust, hackberry, Norway maple, red maple, and sweet cherry at Wertz, Marsh Creek, Memorial Lake, and Pinnacle. Potential (positive or negative) impacts of tree-of-heaven density, initial infestation, treatment history, and incubation conditions are discussed.
Introduction
Insect eggs are vulnerable to mortality factors such as parasitoids, predators, pathogens, and unfavorable weather conditions in the field (1). Eggshells, oviposition sites, maternal secretions, and other built-in defense mechanisms provide protection to eggs in various environments (1–4). As a life stage which cannot actively defend itself, the escaping strategies for insect eggs include parental care, sociality, concealment, and egg mass formation (1, 5). For example, thick spumaline coating protects egg masses of some caddisfly species from predation by Orthotrichia armata Wells (Trichoptera: Hydroptilidae) larvae (6), whereas egg-stacking and scale-casing are used by Lymantria dispar (L.) (Lepidoptera: Erebidae) and Ochrogaster lunifer Herrich-Schäffer (Lepidoptera: Notodontidae) to prevent inner layer eggs from being parasitized by certain species (7–10). Oviposition strategies have profound impacts on egg survival.
The spotted lanternfly, Lycorma delicatula (White) (Hemiptera: Fulgoridae), a univoltine pest of tree-of-heaven (Ailanthus altissima (Mill.) Swingle [Sapindales: Simaroubaceae]) from China (11), was introduced to Berks County, Pennsylvania, in 2014 (12, 13). It is currently found in 11 states from Massachusetts to Indiana in eastern United States (14). Egg masses are laid on various types of substrates (e.g., surfaces of trees, shrubs, vines, stones, fence posts, and other non-living materials) from mid-September to early November in Pennsylvania, with American beech (Fagus grandifolia Ehrh. [Fagales: Fagaceae]), black birch (Betula lenta L. [Fagales: Betulaceae]), black cherry (Prunus serotina Ehrh. [Rosales: Rosaceae]), grapes (Vitis spp. [Vitales: Vitaceae]), Norway maple (Acer platanoides L. [Sapindales: Sapindaceae]), red maple (Acer rubrum L. [Sapindales: Sapindaceae]), sweet cherry (Prunus avium L. [Rosales: Rosaceae]), tree-of-heaven, and tuliptree (Liriodendron tulipifera L. [Magnoliales: Magnoliaceae]) as favorites (15–17).
The size and hatch success of L. delicatula egg masses have been reported in its native range of Asia (11, 18–20). Limited observations on egg mass characteristics and comparative hatching in the laboratory and the field were also carried out in North America as parts of related studies (16, 21). However, systematic studies in egg mass structure, size, and hatchability are still lacking for the better understanding of their impacts on L. delicatula population dynamics in the field. It is hypothesized that habitat and substrate play an important role in the oviposition selection of L. delicatula. The objectives of this study were therefore to 1) understand the basic structure of L. delicatula egg masses, 2) examine the impact of habitat and oviposition substrate on egg mass size, and 3) compare egg hatch success in the laboratory and the field.
Materials and methods
Study sites
This study was carried out at 11 mixed hardwood sites (~0.5 ha) in Pennsylvania between 16 and 80 km South and West of the initial introduction in Berks County. See Table 1 for location, type, structure, tree-of-heaven density, year of infestation, and treatment history for each study site. Study sites were at least 5 km apart from each other except Gibraltar North and Gibraltar South which were on the opposite sides of the same mountain ridge. Site Antietam was used in both 2019 and 2021. The number of tree-of-heaven trees with a diameter at breast height (DBH) >5 cm was recorded in each study site at the start of the field work. Northern spicebush (Lindera benzoin L. [Laurales: Lauraceae]) and summer grape (Vitis aestivalis Michx. [Vitales: Vitaceae]) were the most common understory species at all study sites.
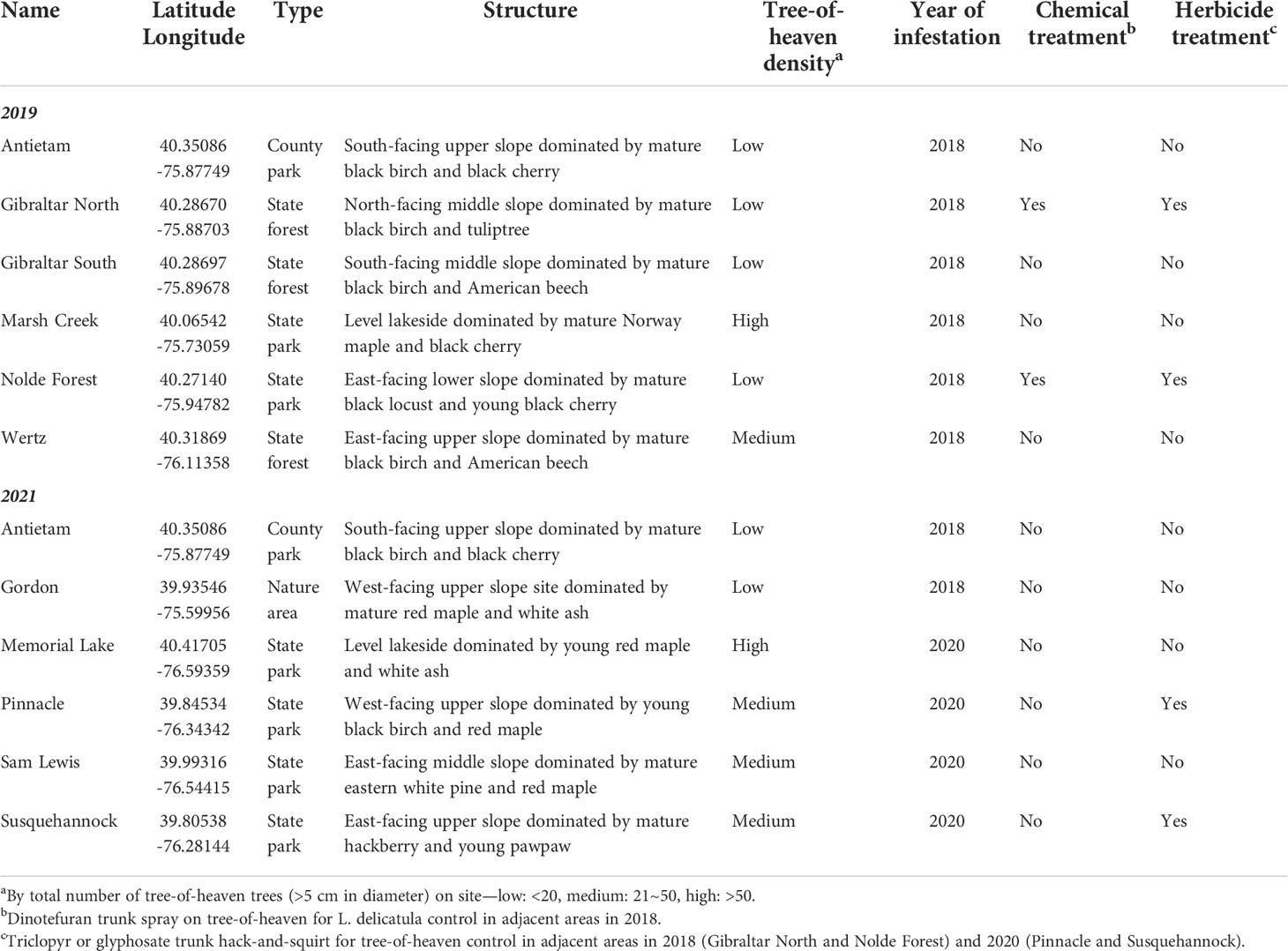
Table 1 Study site location, type, structure, tree-of-heaven density, year of infestation, and treatment history in 2019 and 2021.
Egg mass collection
Egg mass collection was carried out at six study sites (Antietam, Gibraltar North, Gibraltar South, Nolde Forest, Marsh Creek, and Wertz) in late April 2019. Lycorma delicatula egg masses are dark gray in color at the beginning (Figure 1A) and turn grayish white the next spring (Figure 1B). At each study site, the surfaces of live trees, shrubs, and vines were searched for L. delicatula egg masses. Egg masses found on the lower 2-m trunk of the tree (shrub/vine) were collected using a 1.27-cm bench chisel (Buck Brothers, Everett, WA). A rectangle was created first to surround the egg mass by cutting directly into the bark at 0.5 cm away from its outer margins. The egg mass on the surface was then dislodged by gently pushing the chisel under the bark rectangle upward from the lower end. Care was taken to ensure no eggs were accidentally missed, cut, squeezed, or otherwise damaged. Each dislodged egg mass was then held in a 50-ml centrifuge tube (VWR International, Radnor, PA) and labeled by collection date, study site, and type of oviposition substrate before being brought back to the laboratory for examination and incubation.
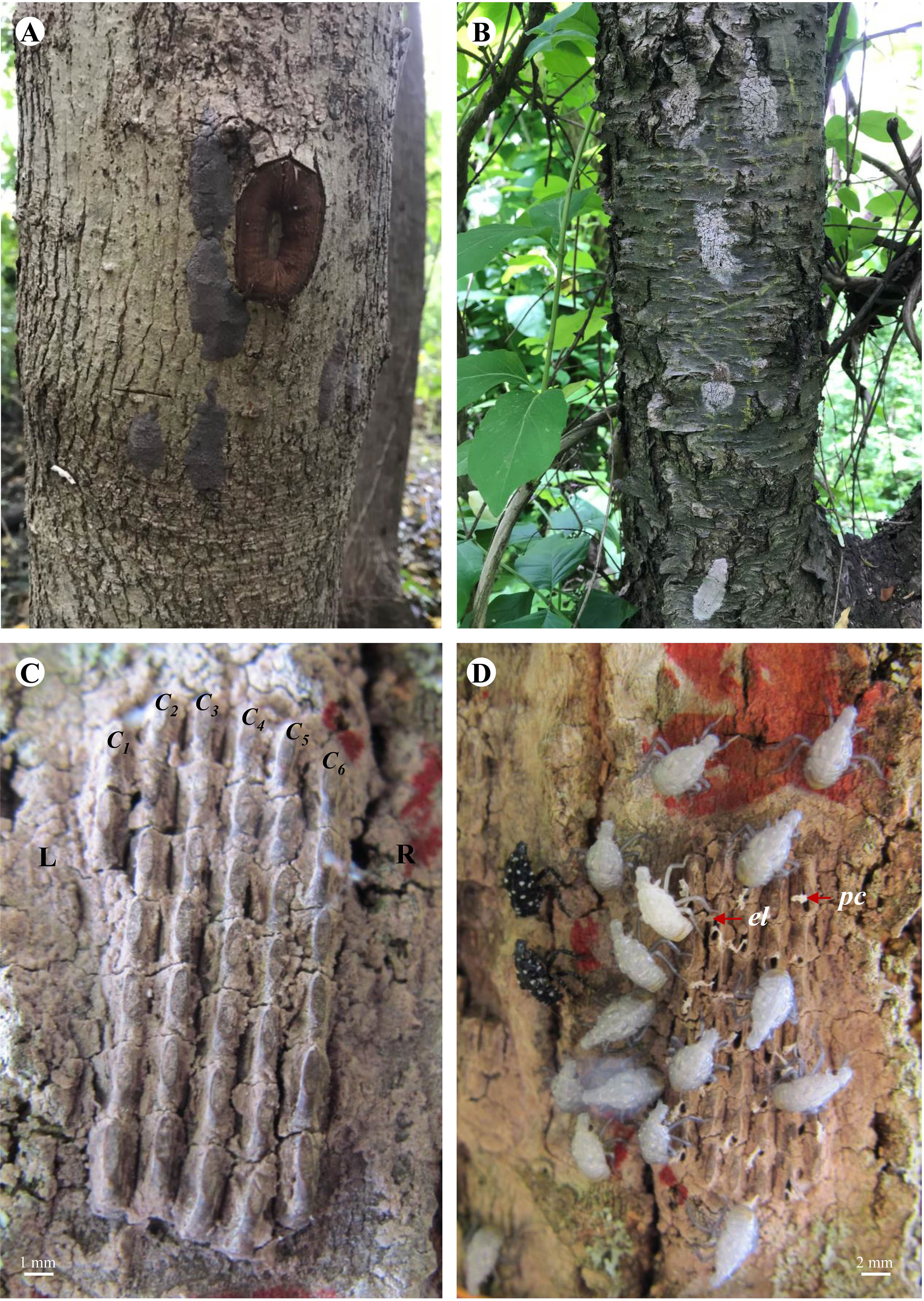
Figure 1 Lycorma delicatula egg masses in the field. (A) Newly laid on red maple. (B) Overwintered on black cherry. (C) Arrangement. (D) Hatching. L—left, R—right, C1—column #1, C2—column #2, C3—column #3, C4—column #4, C5—column #5, C6—column #6, el—egg lid, pc—pronymphal cuticle.
Egg mass marking in the field
Egg mass marking was performed at Antietam, Gibraltar North, Gibraltar South, Nolde Forest, Marsh Creek, and Wertz in 2019 and Antietam, Gordon, Memorial Lake, Pinnacle, Sam Lewis, and Susquehannock in 2021. Current generation egg masses found on the lower 2-m trunk of different types of oviposition substrates near the epicenter at each study site were circled with a yellow timber crayon (Dixon Ticonderoga, Heathrow, FL) in late April for monitoring.
Egg mass structure
Lycorma delicatula eggs are cylindrical with a diameter of 1.5 mm and height of 3.0 mm. They are usually laid in single layer masses with 5–10 columns (10–30 eggs/column) and covered by a layer of gray wax in Asia (11). To characterize the structure of the L. delicatula egg masses in North America, column number (Figure 1C) was recorded from left to right for egg masses collected in 2019 and those marked in the field in 2021, whereas egg number was counted for all egg masses (collected and marked) before hatch. All egg masses were counted again within 2 weeks after hatch completed to ensure accuracy. Direct count was possible for egg masses with little or no waxy cover on the top; however, for those with a thick waxy cover, wax removal with a #2 camel hairbrush (Grumbacher, Leeds, MA) was needed for exact enumeration. This procedure was only carried out after hatch when necessary to avoid potential influence on hatch success. The numbers of eggs and numbers of columns for each egg mass were categorized at intervals of 10 and 1, respectively. Size category, column category, and column size (no. eggs/column) were used in data analysis. Travel restrictions stemming from the COVID-19 pandemic forced cancellations of scheduled field works in 2020 and early 2021.
Egg hatch in the laboratory
Egg masses collected in 2019 were brought back to the laboratory for incubation inside a Percival incubator (model #DR-36VL, Percival Scientific, Perry, IA) at 22 ± 1°C, 40 ± 5% relative humidity (RH), and a 16:8-h photoperiod (light: dark) for 8 weeks (16). Egg mass and egg hatch success were monitored with the number of newly hatched nymphs recorded and removed weekly. Egg mass hatch success was calculated by dividing the number of egg masses with at least one hatched egg by the total number of egg masses at the study site, whereas egg hatch success was calculated by dividing the number of hatched eggs by the total number of eggs in the egg mass.
Egg hatch in the field
Egg hatch in the field was monitored weekly on the marked egg masses for 8 weeks from mid-May to early July in 2019 and 2021. Presence of white 1st-instar nymphs or open egg lids (opercula) and attached whitish/yellowish pronymphal cuticles (Figure 1D) indicates hatch success (20, 22). Hatch success was calculated as described before.
Data analysis
Data analysis was carried out in R (Version 3.3.3) (23). Egg counts per egg mass for field-collected and marked eggs in both years were subjected to Shapiro–Wilk normality test before analysis. If data were overdispersed, a negative binomial generalized linear (nbGLM in R) was used to detect the effect of study site or type of oviposition substrate. Kruskal–Wallis test was used to separate different columns based on eggs per column for egg masses collected or marked in the field. Significant effects were followed by pairwise Wilcoxon rank-sum test with a P-value adjusted by Benjamini–Hochberg method (24). A generalized linear model (GLM) with binomial distribution was used to examine the frequency of size category, frequency of column category, correlation between egg hatch success and egg mass size, or effect of study site or type of oviposition substrate on egg hatch success in each year. A generalized linear model was used to fit egg number with column number in each year.
Results
In total, 300 egg masses were collected from 19 types of oviposition substrates at six study sites (50 egg masses/study site) in 2019, with most found on black birch, red maple, Norway maple, sweet cherry, tuliptree, black locust (Robinia pseudoacacia L. [Fables: Fabaceae]), tree-of-heaven, and black cherry (Table 2). Egg masses were also found on red oak (Quercus rubra L. [Fagales: Fagaceae]), black walnut (Juglans nigra L. [Fagales: Juglandaceae]), black willow (Salix nigra Marshall [Malpighiales: Salicaceae]), American hophornbeam (Ostrya virginiana (Mill.) K. Koch [Fagales: Betulaceae]), autumn olive (Elaeagnus umbellata Thunb. [Rosales: Elaeagnaceae]), northern spicebush, princess tree (Paulownia tomentosa (Thunb.) Steud [Lamiales: Paulowniaceae]), American beech, sassafras (Sassafras albidum (Nutt.) Nees [Laurales: Lauraceae]), shagbark hickory (Carya ovata (Mill.) K. Koch [Fagales: Juglandaceae]), and summer grape (Table 2).
In addition, 212 egg masses were marked in the field, including 120 egg masses on four types of oviposition substrates at six study sites (20 egg masses/study site, two types of oviposition substrates/study site, five plants/type of oviposition substrate, two egg masses/plant) in 2019, and 92 egg masses on 10 types of oviposition substrates at six study sites (4–20 egg masses/study site, 1–4 types of oviposition substrates/study site, 1–5 plants/type of oviposition substrate, 1–5 egg masses/plant) in 2021 (Table 3). The low population density at Antietam and Gordon in 2021 prevented more egg masses from being marked at those study sites. Tree-of-heaven was represented at all study sites in both years except Gordon in 2021, whereas black birch, black locust, and Norway maple were used in 2019, and American hornbeam (Caprinus caroliniana Walter [Fagales: Betulaceae]), black birch, black cherry, boxelder (Acer negundo L. [Sapindales: Sapindaceae]), hackberry (Celtis occidentalis L. [Rosales: Cannabaceae]), pawpaw (Asimina triloba (L.) Dunal [Magnoliales: Annonaceae]), red maple, shagbark hickory, and white ash (Fraxinus americana L. [Lamiales: Oleaceae]) were used in 2021 (Table 3).
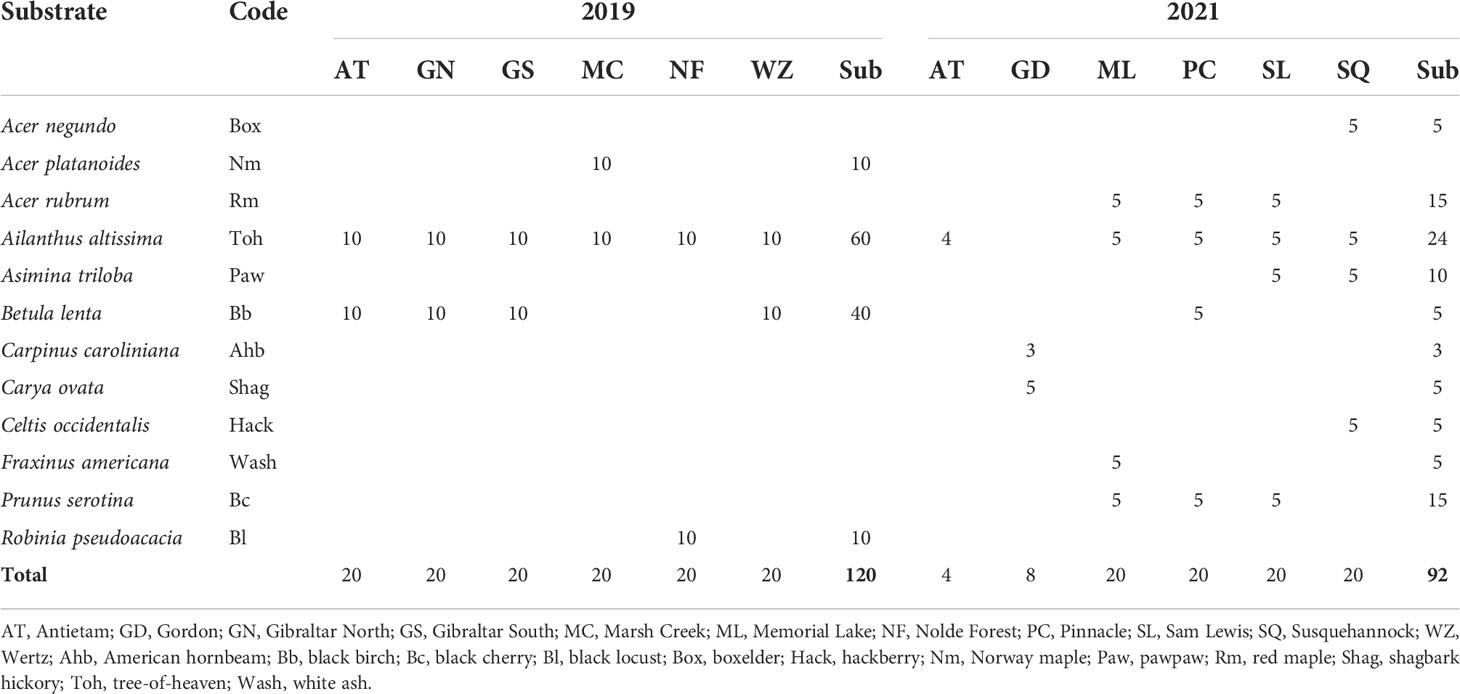
Table 3 Lycorma delicatula egg mass marking in the field by year, study site, and oviposition substrate.
Egg mass structure
Number of eggs in each L. delicatula egg mass ranged from 0 to 105 in Pennsylvania based on 300 egg masses collected from the field in 2019 (Figure 2A). Significant differences in frequency were observed among different size categories (Z-value = -11.820, P < 0.001). Three egg masses (one from Gibraltar North and two from Nolde Forest) contained no eggs. Most egg masses (91.3%) contained <50 eggs, 75.6% had 20–50 eggs, and 15.7% had <20 eggs/egg mass (Figure 2A). Only 1 egg mass had >100 eggs while 2 had >90, 8 with >70, 7 had >60, and 8 had >50 eggs (Figure 2A). Significant differences in frequency were also observed among different column categories (Z-value = -7.141, P < 0.001). Eggs were arranged in 1–13 columns within the egg masses. Most egg masses (88%) contained <7 columns while 77% had 3–7 columns and 11% had <3 columns (Figure 2B). One egg mass had 13 columns while 3 had 12, 2 had 11, 6 had 10, 10 had 9, and 14 had 8 columns (Figure 2B). In total, 10,115 eggs arranged in 1,644 columns were recorded from 297 egg masses with at least 1 egg, including 1,601 eggs (270 columns) at Antietam, 1,447 eggs (239 columns) at Gibraltar North, 1,683 eggs (288 columns) at Gibraltar South, 1,249 eggs (225 columns) at Nolde Forest, 1,893 eggs (286 columns) at Marsh Creek, and 2,242 eggs (336 columns) at Wertz. Column size ranged from 1 to 18 with a mean of 6.2 ± 2.6 eggs/column and differed significantly between columns (χ2 = 179.610, df = 12, P < 0.001). Significant differences were found between columns 2 and 1, 7; 3 and 1, 5, 6, 7, 8, 9; and 4 and 1, 6, 7, 8, 9 (Figure 2C). A significant positive correlation was found between column number and egg number for the egg masses (F = 676.700, df = 1, 298, P < 0.001) (Figure 2D), with total eggs in each egg mass increasing with the increase of columns in it.
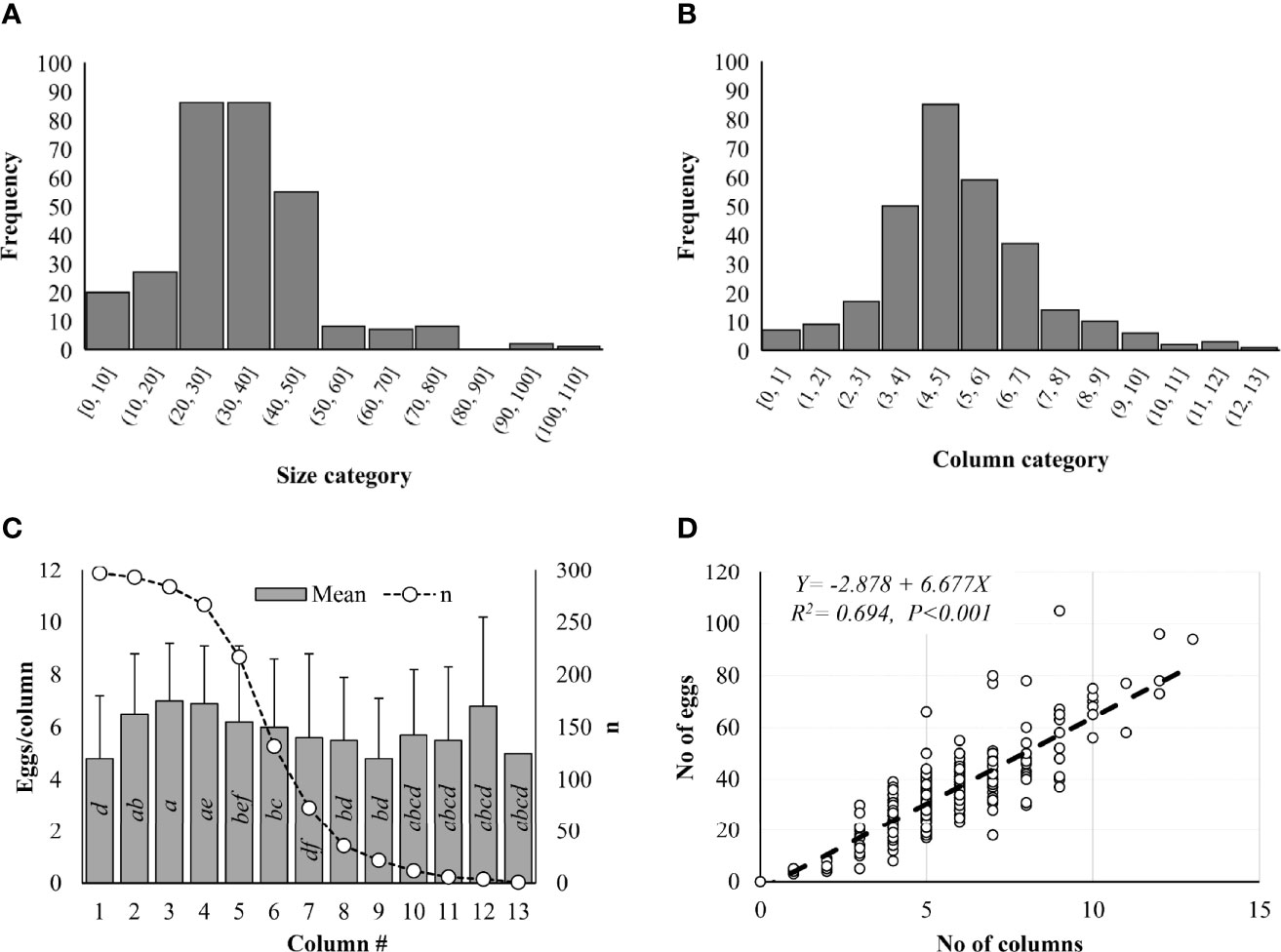
Figure 2 Lycorma delicatula egg mass structure in 2019. (A) Size category. (B) Column category. (C) Mean egg number per column. (D) Correlation between egg number and column number as indicated by the dash line. Means with the same lowercase letters are not significantly different (Wilcoxon rank-sum test, α = 0.05).
Lycorma delicatula egg mass size ranged from 4 to 66 in Pennsylvania based on 92 egg masses marked in the field in 2021 (Figure 3A). No significant difference in frequency was observed among different size categories (Z-value = -0.448, P = 0.654). Most egg masses (93.5%) contained <50 eggs, 81.5% had 20–50 eggs, and 12.0% had <20 eggs/egg mass (Figure 3A). Only one egg mass contained >60 eggs while five had >50 eggs (Figure 3A). No significant difference in frequency was observed among different column categories either (Z-value = 0.932, P = 0.351). Eggs were arranged in 1–10 columns within the egg masses. Most egg masses (90.2%) contained <7 columns, while no egg masses contained <2 columns (Figure 3B). One egg mass had 10 columns while 3 had 9 and 5 had 8 columns (Figure 3B). In total, 3,288 eggs in 535 columns were counted from 92 egg masses, including 84 eggs (16 columns) at Antietam, 195 eggs (39 columns) at Gordon, 666 eggs (108 columns) at Memorial Lake, 781 eggs (132 columns) at Pinnacle, 690 eggs (118 columns) at Sam Lewis, and 812 eggs (122 columns) at Susquehannock. Column size ranged from 1 to 11 with a mean of 6.0 ± 2.4 eggs/column and differed significantly between columns (χ2 = 111.380, df = 9, P < 0.001). Significant differences were found between columns 2 and 1, 7, 8; 3 and 1, 6, 7, 8; and 4 and 1, 6, 7, 8 (Figure 3C). A significant positive correlation was found between column number and egg number for the egg masses (F = 84.820, df = 1, 90, P < 0.001) (Figure 3D), with total eggs in each egg mass increasing with the increase of columns in it.
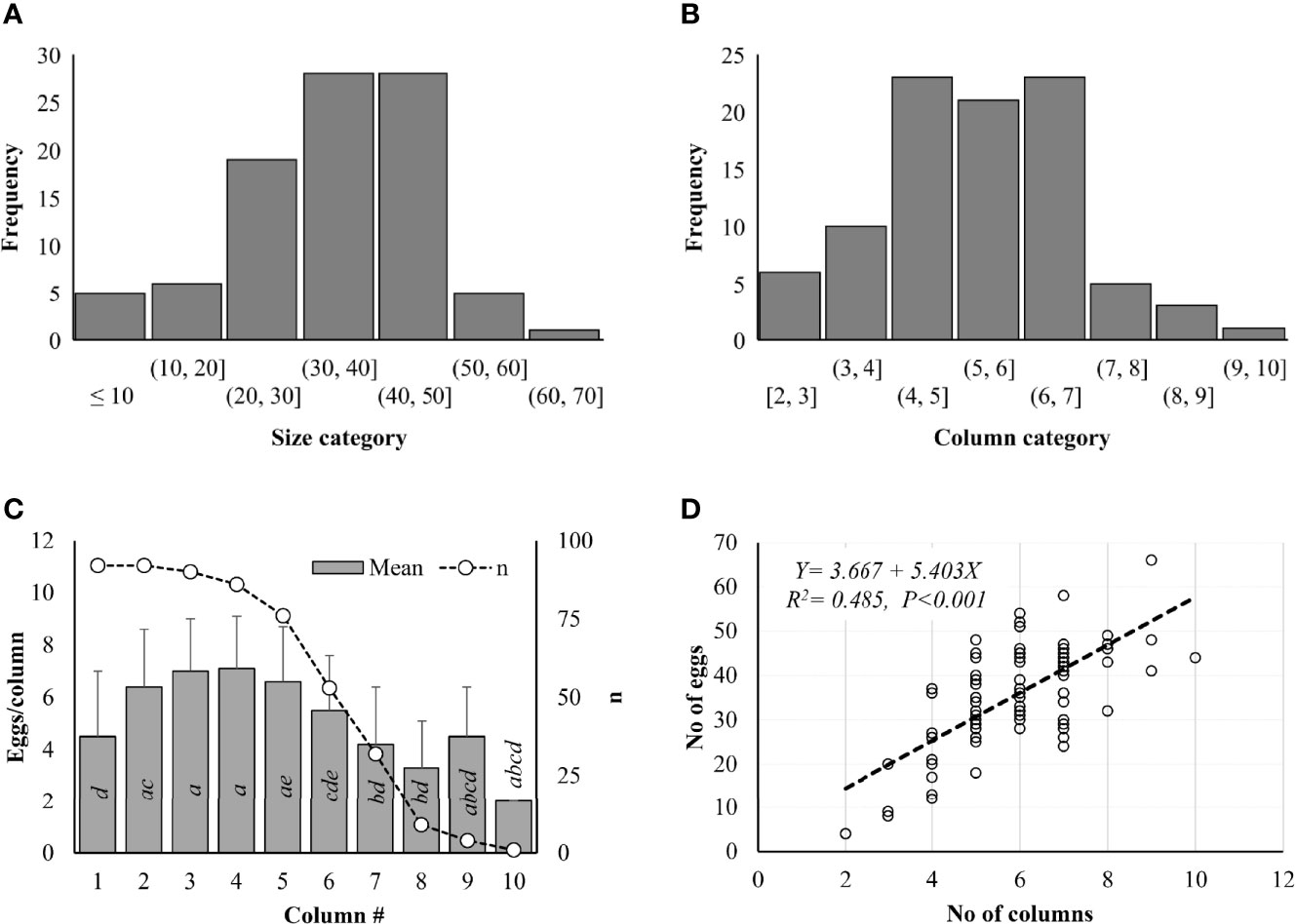
Figure 3 Lycorma delicatula egg mass structure in 2021. (A) Size category. (B) Column category. (C) Mean egg number per column. D) Correlation between egg number and column number as indicated by the dash line. Means with the same lowercase letters are not significantly different (Wilcoxon rank-sum test, α = 0.05).
Egg mass size
The mean (± SD) egg mass size was 33.7 ± 16.0 eggs/egg mass for the 300 egg masses collected in 2019. Significantly larger egg masses were found at Wertz (Z-value = 3.624, P < 0.001) and smaller ones at Nolde Forest (Z-value = -2.619, P = 0.009) (Figure 4A). No significant difference in egg mass size was found between different types of oviposition substrates (α = 0.05) (Figure 4A).
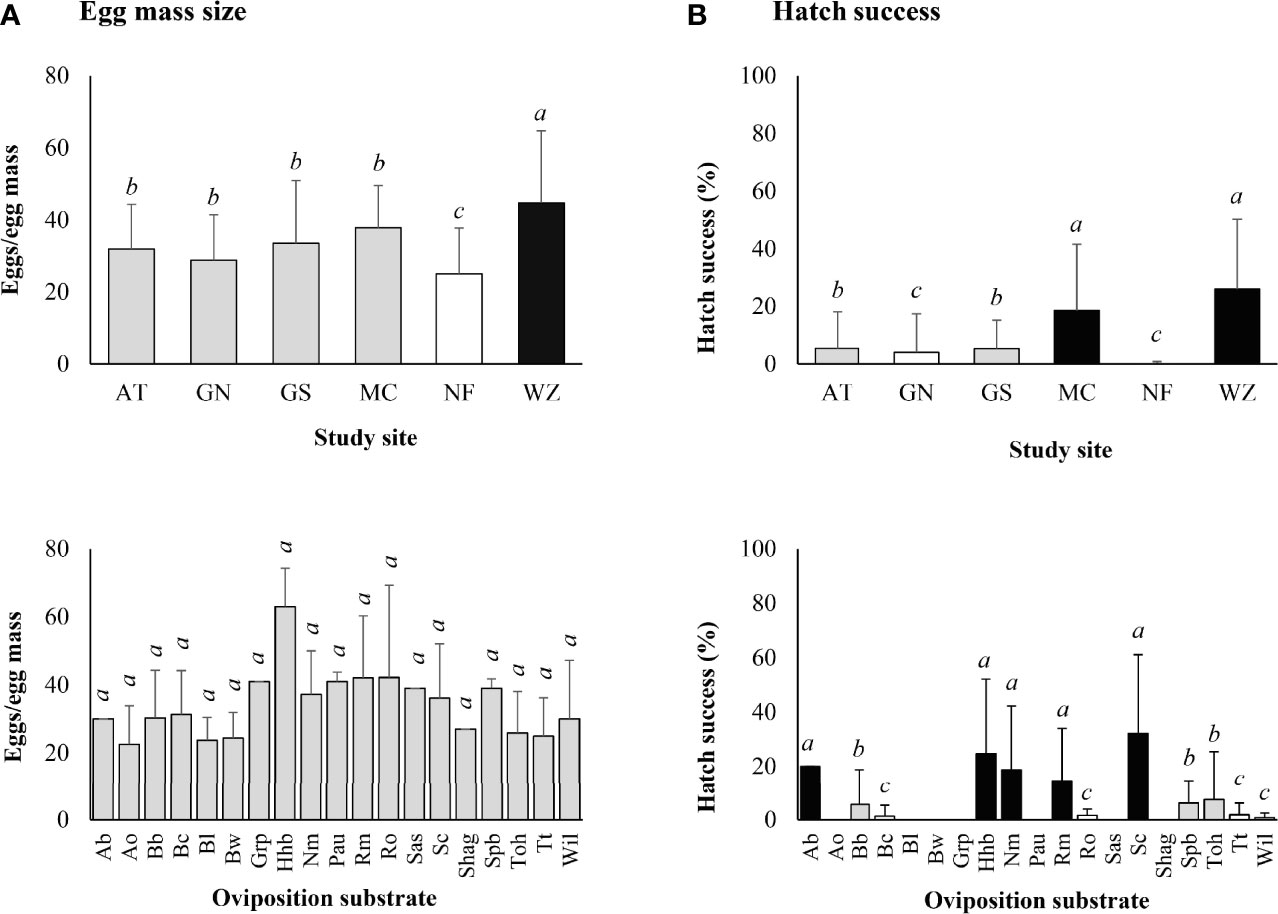
Figure 4 Lycorma delicatula. (A) Egg mass size and (B) egg hatch success by study site and type of oviposition substrate based on laboratory observations in 2019. AT—Antietam, GN—Gibraltar North, GS—Gibraltar South, MC—Marsh Creek, NF—Nolde Forest, WZ—Wertz. Ab—American beech, Ao—autumn olive, Bb—black birch, Bc—black cherry, Bl—black locust, Bw—black walnut, Grp—summer grape, Hhb—American hophornbeam, Nm—Norway maple, Pau—princess tree, Rm—red maple, Ro—red oak, Sas—Sassafras, Sc—sweet cherry, Shag—shagbark hickory, Spb—northern spicebush, Toh—tree-of-heaven, Tt—tuliptree, Wil—black willow. Means with the same lowercase letters are not significantly different (A-negative binomial generalized model, B-generalized linear model with binomial distribution, α = 0.05).
The mean (± SD) egg mass size was 26.6 ± 14.5 eggs/egg mass for the 120 egg masses marked in the field in 2019. Significantly larger egg masses were found at Marsh Creek (Z-value = 2.304, P = 0.021). No significant difference in egg mass size was found between different types of oviposition substrates (α = 0.05) (Figure 5A).
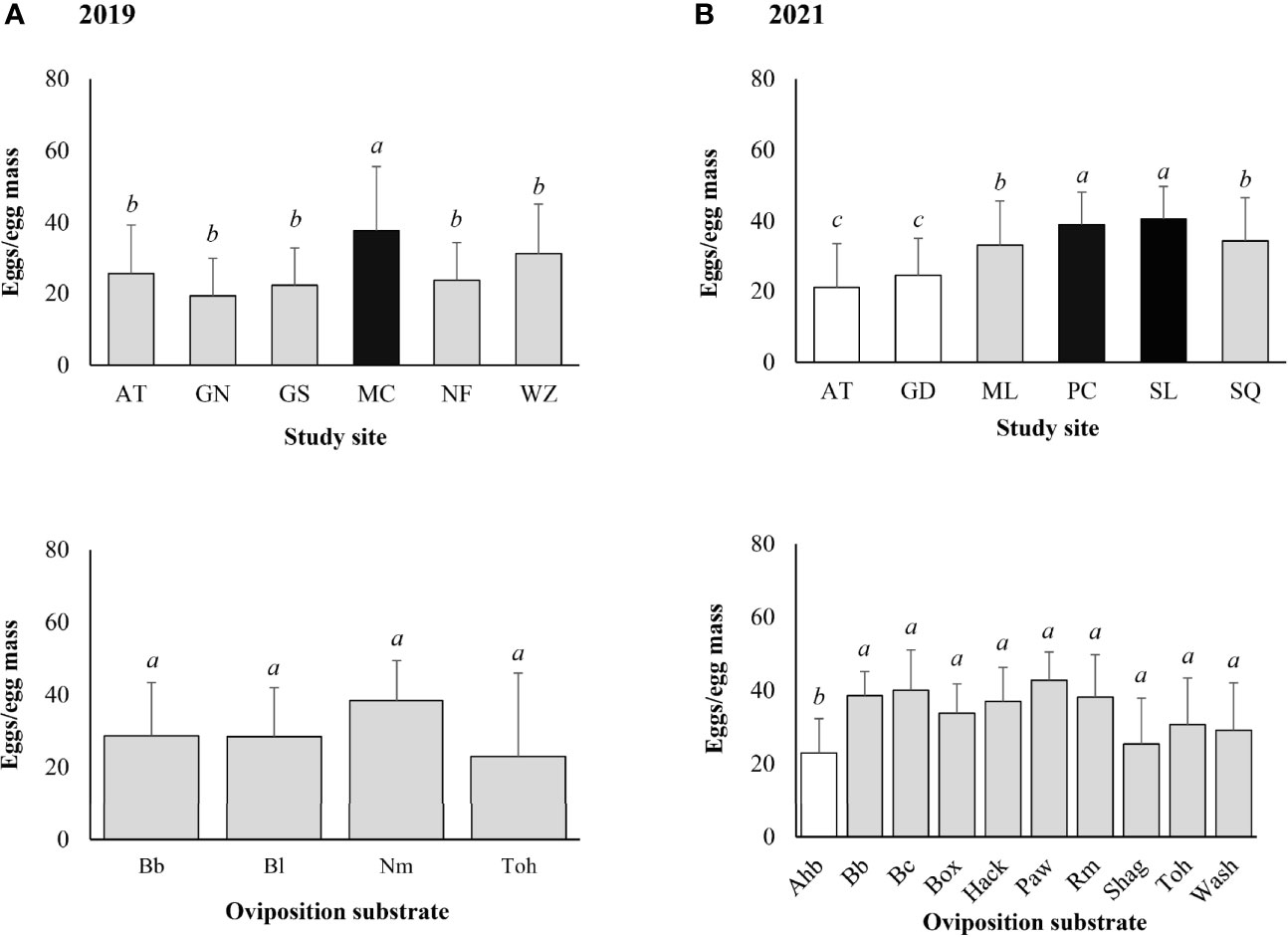
Figure 5 Lycorma delicatula egg mass size by study site and type of oviposition substrate based on field monitoring in (A) 2019 and (B) 2021. AT—Antietam, GD—Gordon, GN—Gibraltar North, GS—Gibraltar South, MC—Marsh Creek, ML—Memorial Lake, NF—Nolde Forest, PC—Pinnacle, SL—Sam Lewis, SQ—Susquehannock, WZ—Wertz. Ahb—American hornbeam, Bb—black birch, Bc—black cherry, Bl—black locust, Box—boxelder, Hack—hackberry, Nm—Norway maple, Paw—pawpaw, Rm—red maple, Shag—shagbark hickory, Toh—tree-of-heaven, Wash—white ash. Means with the same lowercase letters are not significantly different (negative binomial generalized model, α = 0.05).
The mean (± SD) egg mass size was 35.1 ± 11.9 eggs/egg mass for the 92 egg masses marked in the field in 2021. Significantly larger egg masses were found at Sam Lewis (Z-value = 3.275, P = 0.001) and Pinnacle (Z-value = 3.080, P = 0.002). Larger egg masses were also found at Susquehannock (Z-value = 2.460, P = 0.014) and Memorial Lake (Z-value = 2.283, P = 0.022) (Figure 5B). Significantly smaller egg masses were found on American hornbeam (Z-value = -2.023, P = 0.043) compared with other types of oviposition substrates (Figure 5B).
Egg hatch in the laboratory
The three egg masses with no eggs were excluded from laboratory hatch study. Egg hatch started within 2 weeks after incubation and lasted for about 2 weeks for individual egg masses, with peak hatch occurring in the middle of the period. Only 39.7% of the egg masses contained at least one hatched egg in 2019, with the highest egg mass hatch success of 86% at Wertz, followed by Marsh Creek (64%), Gibraltar South (42%), Antietam (28%), Gibraltar North (14.3%), and Nolde Forest (2.1%). The mean (± SD) egg hatch success was 10.0 ± 18.5 (0–90)% for the egg masses, with a positive correlation between hatch success and egg mass size (Z-value = 15.880, P < 0.001).
Significantly higher egg hatch success was found at Wertz (Z-value = 14.967, P < 0.001) and Marsh Creek (Z-value = 10.856, P < 0.001) while significantly lower hatch success was found at Nolde Forest (Z-value = -5.801, P < 0.001) and Gibraltar North (Z-value = -2.063, P = 0.039) (Figure 4B).
Significant differences in egg hatch success were also observed between types of oviposition substrates, with lower rates on red oak (Z-value = -4.353, P<0.001), black cherry (Z-value = -4.262, P < 0.001), tuliptree (Z-value = -4.085, P < 0.001), black willow (Z-value = -3.852, P < 0.001), black birch (Z-value = -2.627, P = 0.009), tree-of-heaven (Z-value = -2.094, P = 0.036), and northern spicebush (Z-value = -1.993, P = 0.046) (Figure 4B). No eggs on autumn olive, black locust, black walnut, princess tree, sassafras, shagbark hickory, and summer grape hatched successfully (Figure 4B).
Egg hatch in the field 2019
In 2019, L. delicatula egg hatch was first observed at Marsh Creek on 21 May, followed by Gibraltar South, Nolde Forest, Wertz, Gibraltar North, and Antietam in the following days in the field. Hatch generally completed within 2–3 weeks for individual egg masses, with the last egg hatch observed on 1 July at Nolde Forest. Overall, 69.2% of the egg masses contained at least one hatched egg, with the highest egg mass hatch success of 90% at Marsh Creek, followed by Gibraltar South (75%), Gibraltar North (70%), Nolde Forest (70%), Antietam (55%), and Wertz (55%). The mean (± SD) egg hatch success was 31.6 ± 30.9 (0–100)% for the egg masses, with a positive correlation between hatch success and egg mass size (Z-value = 4.478, P < 0.001).
Significantly higher egg hatch success was found at Wertz (Z-value = 8.447, P < 0.001), Nolde Forest (Z-value = 5.220, P < 0.001), Marsh Creek (Z-value = 3.784, P < 0.001), and Gibraltar South (Z-value = 2.374, P = 0.018). Significantly lower egg hatch success was found on tree-of-heaven (Z-value = - 8.187, P < 0.001) compared with other types of oviposition substrates (Figure 6A).
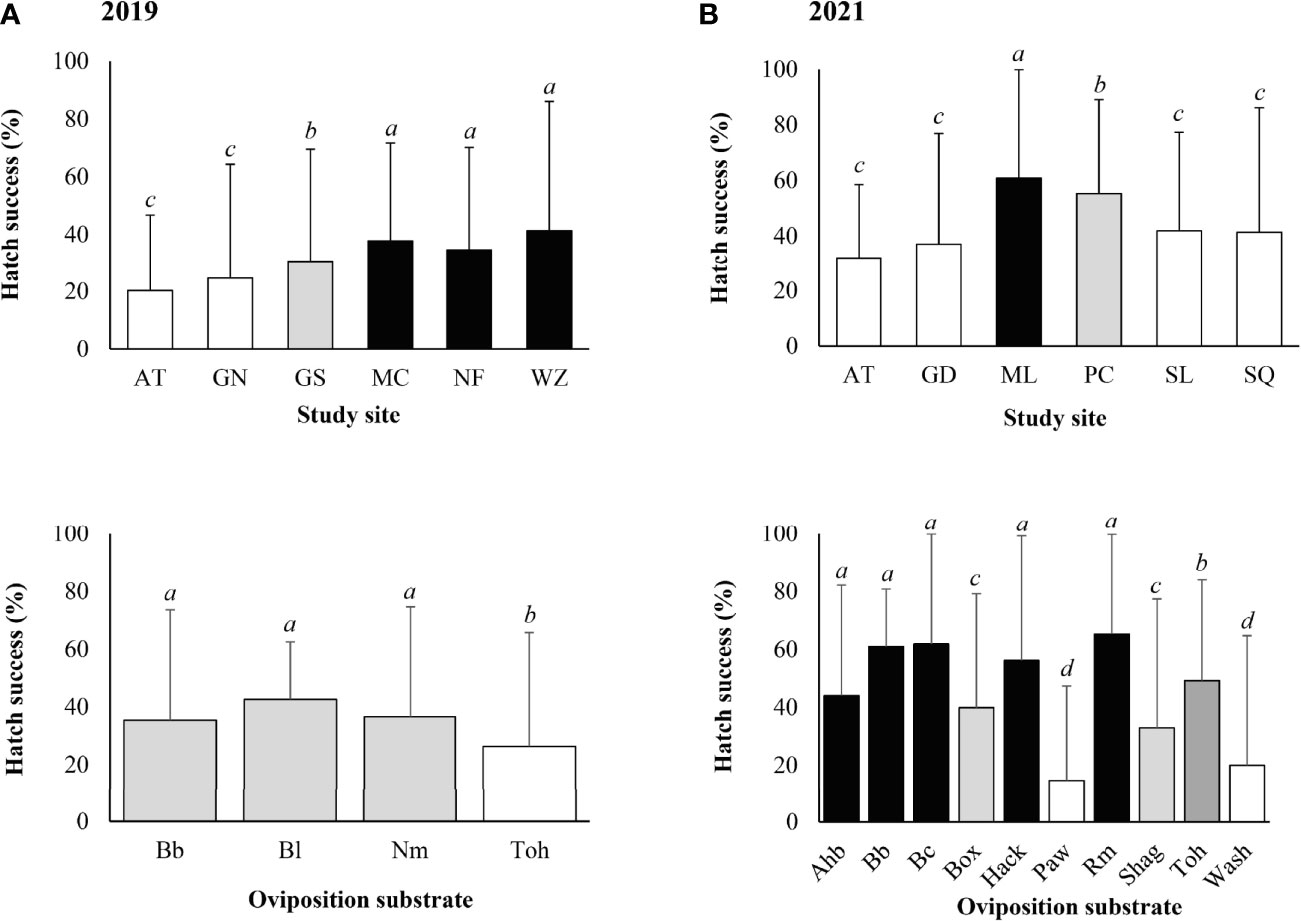
Figure 6 Lycorma delicatula egg hatch success by study site and type of oviposition substrate based on field monitoring in (A) 2019, (B) 2021. AT—Antietam, GD—Gordon, GN—Gibraltar North, GS—Gibraltar South, MC—Marsh Creek, ML—Memorial Lake, NF—Nolde Forest, PC—Pinnacle, SL—Sam Lewis, SQ—Susquehannock, WZ—Wertz. Ahb—American hornbeam, Bb—black birch, Bc—black cherry, Bl—black locust, Box—boxelder, Hack—hackberry, Nm—Norway maple, Paw—pawpaw, Rm—red maple, Shag—Shagbark hickory, Toh—tree-of-heaven, Wash—white ash. Means with the same lowercase letters are not significantly different (generalized linear model with binomial distribution, α = 0.05).
Egg hatch in the field 2021
Lycorma delicatula egg hatch in the field in 2021 was first observed at Pinnacle and Susquehannock on 24 May, followed by Memorial Lake, Sam Lewis, Antietam, and Gordon. Hatch generally completed within 2 weeks for individual egg masses, with the last egg hatch observed on 24 June at Gordon. Overall, 69.6% of the egg masses contained at least one hatched egg, with the highest egg mass hatch success of 80% at Pinnacle, followed by Antietam (75%), Memorial Lake (75%), Sam Lewis (75%), Gordon (62.5%), and Susquehannock (50%). The mean (± SD) egg hatch success was 48.0 ± 38.7 (0–100)% for the egg masses, with a positive correlation between hatch success and egg mass size (Z-value = 3.672, P < 0.001).
Significantly higher egg hatch success was found at Memorial Lake (Z-value = 4.277, P < 0.001) and Pinnacle (Z-value = 2.962, P = 0.003). Significantly lower hatch success was found on pawpaw (Z-value = -11.296, P < 0.001), white ash (Z-value = -7.417, P < 0.001), shagbark hickory (Z-value = -3.161, P = 0.002), boxelder (Z-value = -3.151, P = 0.002), and tree-of-heaven (Z-value = -2.425, P = 0.015) compared with other types of oviposition substrates (Figure 6B).
Discussion
While oviposition substrate played an important role in L. delicatula egg mass structure and hatchability (16), more focus should probably be on habitat structure as those with more tree-of-heaven trees generally supported larger egg masses with more successful egg hatch (Table 1, Figures 4–6). This kind of oviposition selection can be explained by the proximity to suitable habitat for offspring hypothesis (25). The ability to feed on a wild range of hosts and to disperse freely between different host species (16, 26) makes young L. delicatula nymphs nearly independent of the oviposition substrates, rendering the preference–performance hypothesis (27) unlikely as long as tree-of-heaven is available in the habitat for necessary nutrition acquisition and defense sequestration (28). On the other hand, optimal foraging theory (29) should also be explored to shed light on the selective patterns on some tree species (e.g., maples) by late-stage adults as both feeding hosts and oviposition substrates (30, 31). Impacts of habitat and oviposition substrate on egg mass size have also been reported for parallel-banded leafroller moth (Choristoneura parallela (Robinson) [Lepidoptera: Tortricidae]) (32) and beet armyworm (Spodoptera exigua (Hübner) [Lepidoptera: Noctuidae]) (33).
Empty egg masses have been recorded for L. delicatula in a previous study (16). It is not yet clear how females decide to place a certain number of eggs in each column in the egg mass, and why no eggs are laid under the waxy cover in a few of them. In addition to low tree-of-heaven density in the habitats, chemical control of L. delicatula on tree-of-heaven in adjacent areas in the previous year might have a negative impact on the mean egg mass size at Nolde Forest in 2019, whereas a longer infestation history could have contributed to the smaller egg masses observed at Antietam and Gordon in 2021 (Table 1, Figures 4, 5). However, the potential impact of herbicide treatment of tree-of-heaven in adjacent areas at Pinnacle and Susquehannock in 2021 still needs to be examined (Table 1, Figure 5). The largest egg mass ever recorded contained 192 eggs (16). Comparable egg mass sizes (30–50 eggs/egg mass) were also reported before (11, 13, 16, 20, 34).
In general, egg hatch was less successful in the laboratory compared with that in the field (Figures 4,6). However, this may change as laboratory rearing conditions improve in the near future. An egg hatch success of 20.5% was reported in the laboratory compared with 68.2% observed in the field in 2017 (16). In another study, 65.9% egg masses and 58.4% eggs hatched successfully at 15°C in the laboratory (21). On the other hand, egg hatch success dropped to 10.8% when held at 20°C constantly (21). A higher relative humidity and lower than 20°C incubation temperature in the laboratory may be needed to simulate field conditions in late May in southeastern Pennsylvania.
A difference in egg hatch success on different types of oviposition substrates has been reported before. About 80% of eggs on tree-of-heaven hatched whereas only 2%–3% of eggs on Japanese pagoda tree (Styphnolobium japonicum (L.) Schott [Fabales: Fabaceae]) and elms (Ulmus spp. [Rosales: Ulmaceae]) hatched successfully in the field in China (11). On the contrary, only 23% of eggs from tree-of-heaven hatched, whereas 79.6% of eggs from black locust hatched after 2 months of incubation in the laboratory in the United States (16). In Japan, egg hatch success was significantly reduced when wax cover was removed from the surface in the field (20). Oviposition substrates, waxy cover, collection disturbance, incubation conditions, and number of egg masses evaluated all contributed to the reported egg hatch success in the laboratory and the field (11, 16, 18–21).
Oviposition is a critical aspect of the reproductive biology for insects. The decision of when and where and the process of how to lay the eggs have profound impact on the fitness of the species (35). Habitat structure, site conditions, host availability and quality, inter- and intraspecific competition, parasitoids, and predators all play a role in oviposition site selection (1, 4, 36–41). Reproductive success also depends on optimal allocation of available resources by females toward quantity (large clutch size) or quality (large eggs) (42). Lycorma delicatula eggs are relatively well protected from adverse abiotic conditions with thick eggshells and wax cover. No predators rely solely on them while only two species of parasitoids are recorded in the field (43–45). Habitat suitability, tree-of-heaven density, substrate conditions, and intraspecific competition should be the most important factors in oviposition selection for L. delicatula.
Information from this study is beneficial to the understanding of L. delicatula population dynamics and its management in the field in North America. Infestations usually start in suitable habitats with tree-of-heaven trees (11). Egg masses are mostly found on tree-of-heaven and a few neighboring species in the habitats at the beginning (16). Onsite chemical control of L. delicatula, herbicide treatment of tree-of-heaven, and fungal epizootics could interfere with egg mass size and hatchability. Management strategies should therefore focus on newly infested tree-of-heaven trees with egg mass survey extended to preferred substrates in the habitats. Hatch success in the field should be used to evaluate current-generation nymphal populations since those measured in the laboratory were generally lower and more variable depending on incubation conditions. The impact of other key factors (tree-of-heaven health, climatic conditions, natural enemies, and management activities) in L. delicatula population dynamics based on egg mass evaluation should be investigated.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
The author conceptualized the study; conducted the field work; collected and analyzed the data; wrote the draft; and reviewed and edited the final version of the manuscript.
Funding
This project was supported by funding from the Pennsylvania Department of Conservation and Natural Resources.
Acknowledgments
I thank Matthew Hunter for field assistance; Richard Hartlieb, Brent Erb, James Wassell, Brendan Lederer, Nur Ritter, Jennifer Chandler, Courtney Troutman, Nathaniel Brown, and Andrew Leidich for study site access. Comments by the reviewers improved the manuscript.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fatouros NE, Cusumano A, Bin F, Polaszek A, van Lenteren JC. How to escape from insect egg parasitoids: a review of potential factors explaining parasitoid absence across the insecta. Proc R Soc B (2020) 287:20200344. doi: 10.1098/rspb.2020.0344
2. Eisner T, Attygalle AB, Conner WE, Eisner M, MacLeod E, Meinwald J. Chemical egg defense in a green lacewing (Ceraeochrysa smithi). Proc Natl Acad Sci U S A (1996) 93:3280–83. doi: 10.1073/pnas.93.8.3280
3. Senderovich Y, Halpern M. The protective role of endogenous bacterial communities in chironomid egg masses and larvae. ISME J (2013) 7:2147–58. doi: 10.1038/ismej.2013.100
4. Church SH, Donoughe S, de Medeiros BAS, Extavour CG. Insect egg size and shape evolve with ecology but not developmental rate. Nature (2019) 571:58–62. doi: 10.1038/s41586-019-1302-4
5. Goldberg J, Bresseel J, Constant J, Kneubühler B, Leubner F, Michalik P, et al. Extreme convergence in egg-laying strategy across insect orders. Sci Rep (2015) 5:7825. doi: 10.1038/srep07825
6. Bovill WD, Downes BJ, Lancaster J. Caddisfly egg mass morphology mediates egg predation: potential costs to individuals and populations. Freshw Biol (2014) 60:360–72. doi: 10.1111/FWB.12497
7. Crossman SS. Two imported egg parasites of the gypsy moth, Anastatus bifasciatus fonsc. and Schedius kuvanae Howard. J Agric Res (1925) 30:643–75.
8. Brown MW. Literature review of Ooencyrtus kuvanae (Hym.: Encyrtidae), an egg parasite of Lymantria dispar (Lep.: Lymantriidae). Entomophaga (1984) 29:249–65. doi: 10.1007/BF02372112
9. Hofstetter RW, Raffa KF. Endogenous and exogenous factors affecting parasitism of gypsy moth egg masses by Ooencyrtus kuvanae. Entomol Exp Appl (1998) 88:123–35. doi: 10.1046/j.1570-7458.1998.00354.x
10. Uemura M, Perkins L, Battisti A, Zalucki M. Egg mass structure of the processionary caterpillar Ochrogaster lunifer (Lepidoptera: Notodontidae): is the outer egg layer sacrificed for attack by the egg parasitoid Anastatus fuligispina (Hymenoptera: Chalcidoidea: Eupelmidae)? Austral Entomol (2019) 58:810–15. doi: 10.1111/aen.12401
11. Zhou J. Lycorma delicatula (White) (Homoptera: Fulgoridae). In: Xiao G, editor. Forest insects of China. Beijing, China: Chinese Forestry Publishing House (1992)1992. p. 169–70.
12. Barringer LE, Donovall LR, Spichiger S-E, Lynch D, Henry D. The first new world record of Lycorma delicatula (Insecta: Hemiptera: Fulgoridae). Entomol News (2015) 125:20–3. doi: 10.3157/021.125.0105
13. Dara SK, Barringer L, Arthurs SP. Lycorma delicatula (Hemiptera: Fulgoridae): A new invasive pest in the united states. J Integr Pest Manage (2015) 6(1):20. doi: 10.1093/jipm/pmv021
14. New York State Integrated Pest Management. Spotted lanternfly. Available at: https://nysipm.cornell.edu/environment/invasive-species-exotic-pests/spotted-lanternfly/ (Accessed 13 June 2022).
15. Baker TC, Smyers EC, Urban JM, Meng Z, Pagadala Damodaram KJ, Myrick AJ, et al. Progression of seasonal activities of adults of the spotted lanternfly, lycorma delicatula, during the 2017 season of mass flight dispersal behavior in eastern Pennsylvania. J Asia-Pac Entomol (2019) 22:705–13. doi: 10.1016/j.aspen.2019.05.006
16. Liu H. Oviposition substrate selection, egg mass characteristics, host preference, and life history of the spotted lanternfly (Hemiptera: Fulgoridae) in north America. Environ Entomol (2019) 48:1452–68. doi: 10.1093/ee/nvz123
17. Leach H, Leach A. Seasonal phenology and activity of spotted lanternfly (Lycorma delicatula) in eastern US vineyards. J Pest Sci (2020) 93:1215–24. doi: 10.1007/s10340-020-01233-7
18. Lee JS, Kim IK, Koh SH, Cho SJ, Jang SJ, Pyo SH, et al. Impact of minimum winter temperature on Lycorma delicatula (Hemiptera: Fulgoridae) egg mortality. J Asia Pacific Entomol (2011) 14:123–25. doi: 10.1016/j.aspen.2010.09.004
19. Park M. Overwintering ecology and population genetics of lycorma delicatula (Hemiptera: Fulgoridae) in Korea (Ph.D. dissertation). Seoul, South Korea: Seoul National University (2015).
20. Nakashita A, Wang Y, Lu S, Shimada K, Tsuchida T. Ecology and genetic structure of the invasive spotted lanternfly lycorma delicatula in Japan where its distribution is slowly expanding. Sci Rep (2022) 12:1543. doi: 10.1038/s41598-022-05541-z
21. Keena MA, Nielsen AL. Comparison of the hatch of newly laid Lycorma delicatula (Hemiptera: Fulgoridae) eggs from the united states after exposure to different temperatures and durations of low temperature. Environ Entomol (2021) 50:410–17. doi: 10.1093/ee/nvaa177
22. Liu H, Hunter M. Nondestructive sampling for spotted lanternfly (Hemiptera: Fulgoridae) egg masses in woodlands based on fixed-radius plots. J Econ Entomol (2021) 114:1353–61. doi: 10.1093/jee/toab053
23. R Core Team. The r project for statistical computing (2017).Vienna, Austria. R Foundation for Statistical Computing.
24. Habiger JD, Peña EA. Randomized p-values and nonparametric procedures in multiple testing. J Nonparametr Stat (2011) 23:583–604. doi: 10.1080/10485252.2010.482154
25. Lawlor LR, Maynard Smith J. The coevolution and stability of competing species. Am Nat (1976) 110:79–99. doi: 10.1086/283049
26. Barringer LE, Ciafré CM. Worldwide feeding host plants of spotted lanternfly, with significant additions from north America. Environ Entomol (2020) 49:999–1011. doi: 10.1093/ee/nvaa093
27. Jaenike J. On optimal oviposition behavior in phytophagous insects. Theor Popul Biol (1978) 14:350–56. doi: 10.1016/0040-5809(78)90012-6
28. Song S, Kim S, Kwon SW, Lee S-I, Jablonski PG. Defense sequestration associated with narrowing of diet and ontogenetic change to aposematic colours in the spotted lanternfly. Sci Rep (2018) 8:16831. doi: 10.1038/s41598-018-34946-y
29. MacArthur RH, Pianka ER. On optimal use of a patchy environment. Am Nat (1966) 100:603–9. doi: 10.1086/282454
30. Mason CJ, Walsh B, Keller J, Couture JJ, Calvin D, Urban JM. Fidelity and timing of spotted lanternfly (Hemiptera: Fulgoridae) attack patterns on ornamental trees in the suburban landscape. Environ Entomol (2020) 49:1427–36. doi: 10.1093/ee/nvaa109
31. Keller J, Rost J, Hoover K, Urban JM, Leach H, Porras M, et al. Dispersion patterns and sample size estimates for egg masses of spotted lanternfly (Hemiptera: Fulgoridae). Environ Entomol (2020) 49:1462–72. doi: 10.1093/ee/nvaa107
32. Stuart RJ, Polavarapu S. Egg-mass variability and differential parasitism of Choristoneura parallela (Lepidoptera: Tortricidae) by endemic Trichogramma minutum (Hymenoptera: Trichogrammatidae). Ann Entomol Soc Am (2000) 93:1076–84. doi: 10.1603/0013-8746(2000)093[1076:EMVADP]2.0.CO;2
33. Greenberg SM, Sappington TW, Setamou M, Liu TX. Beet armyworm (Lepidoptera: Noctuidae) host plant preferences for oviposition. Environ Entomol (2002) 31:142–48. doi: 10.1603/0046-225X-31.1.142
34. Tomisawa A, Ohimiya S, Fukutomi H, Hayashi K, Ishikawa T. Biological notes on Lycorma delicatula (White) (Hemiptera: Fulgoridae) in ishikawa prefecture, Japan. Jpn J Entomol (2013) 16:3–14.
35. Cury KM, Prud’homme B, Gompel N. A short guide to insect oviposition: when, where, and how to lay an egg. J Neurogenet (2019) 33:75–89. doi: 10.1080/01677063.2019.1586898
36. Wilson K, Hill L. Factors affecting egg maturation in the bean weevil callosobruchus maculatus. Physiol Entomol (1989) 14:115–26. doi: 10.1111/j.1365-3032.1989.tb00943.x
37. Messina FJ, Kemp JL, Dickinson JA. Plasticity in the egg-spacing behavior of a seed beetle: effects of host deprivation and seed patchiness (Coleoptera: Bruchidae). J Insect Behav (1992) 5:609–21. doi: 10.1007/BF01048008
38. Floater GJ, Zalucki MP. Habitat structure and egg distributions in the processionary caterpillar Ochrogaster lunifer: lessons for conservation and pest management. J Appl Ecol (2000) 37:87–99. doi: 10.1046/j.1365-2664.2000.00468.x
39. Gibbs M, Lace LA, Jones MJ, Moore AJ. Egg size-number trade-off and a decline in oviposition site choice quality: female Pararge aegeria butterflies pay a cost of having males present at oviposition. J Insect Sci (2005) 5:39. doi: 10.1093/jis/5.1.39
40. Paukku S, Kotiaho JS. Female oviposition decisions and their impact on progeny life-history traits. J Insect Behav (2008) 21:505–20. doi: 10.1007/s10905-008-9146-z
41. Bertea CM, Casacci LP, Bonelli S, Zampollo A, Barbero F. Chemical, physiological, and molecular responses of host plants to lepidopteran egg-laying. Front Plant Sci (2020) 10:1768. doi: 10.3389/fpls.2019.01768
42. Koch LK, Meunier J. Mother and offspring fitness in an insect with maternal care: phenotypic trade-offs between egg number, egg mass and egg care. BMC Evol Biol (2014) 14:125. doi: 10.1186/1471-2148-14-125
43. Kim IK, Koh SH, Lee JS, Choi WI, Shin SC. Discovery of an egg parasitoid of Lycorma delicatula (Hemiptera: Fulgoridae) an invasive species in south Korea. J Asia Pac Entomol (2011) 14:213–15. doi: 10.1016/j.aspen.2011.01.004
44. Yang Z, Choi WY, Cao L, Wang X, Hou Z. A new species of Anastatus (Hymenoptera: Eupelmidae) from China, parasitizing eggs of Lycorma delicatula (Homoptera: Fulgoridae). Zool Syst (2015) 40:290–302.
Keywords: Lycorma delicatula, egg mass structure, hatch success, Fulgoridae, invasive species
Citation: Liu H (2022) Oviposition selection in spotted lanternfly: impact of habitat and substrate on egg mass size and hatchability. Front. Insect Sci. 2:932433. doi: 10.3389/finsc.2022.932433
Received: 29 April 2022; Accepted: 30 June 2022;
Published: 28 July 2022.
Edited by:
Michael Brewer, Texas A&M AgriLife Research, United StatesReviewed by:
Melody A. Keena, USDA Forest Service, United StatesKier Klepzig, University of Georgia, United States
Copyright © 2022 Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Houping Liu, hliu@pa.gov
 Houping Liu
Houping Liu