Impact of Bt corn expressing Bacillus thuringiensis Berliner insecticidal proteins on the growth and survival of Spodoptera frugiperda larvae in Colombia
- 1Crops for Nutrition and Health, The Alliance of Bioversity International and Centro Internacional de Agricultura Tropical (International Center for Tropical Agriculture), Palmira, Colombia
- 2Universidad Nacional de Colombia Sede Palmira, Palmira, Colombia
- 3Resistance Management NorLa-Bayer, Mexico City, Mexico
- 4Bayer – Regulatory, Bogotá, Colombia
Bioassays were conducted under controlled conditions to determine the response of Spodoptera frugiperda (J. E. Smith) larvae fed with corn materials expressing Bacillus thuringiensis (Bt) insecticidal endotoxins: (1) VT Double Pro® (VT2P) expressing Cry1A.105-Cry2Ab2 proteins and (2) VT Triple Pro® (VT3P) expressing Cry1A.105-Cry2Ab2-Cry3Bb1 proteins. The parameters assessed were: (i) mortality rate, and (ii) growth inhibition (GI) with respect to the control. To conduct this study, larvae were collected from commercial non-Bt corn fields, in four agricultural sub-regions in Colombia, between 2018 and 2020. Fifty-two populations were assessed from the field and neonate larvae from each of the populations were used for the bioassays. The study found that mortality rates in the regions for larvae fed with VT2P corn ranged from 95.1 to 100.0%, with a growth inhibition (%GI) higher than 76.0%. Similarly, mortality rate for larvae fed with VT3P corn were between 91.4 and 100.0%, with a %GI above 74.0%. The population collected in Agua Blanca (Espinal, Tolima; Colombia) in 2020, showed the lowest mortality rate of 53.2% and a %GI of 73.5%, with respect to the control. The population that exhibited the lowest %GI was collected in 2018 in Agua Blanca (Espinal, Tolima, Colombia) with a 30.2%, growth inhibition, with respect to the control. In recent years, the use of plant tissue to monitor susceptibility to fall armyworm has proven to be useful in the resistance management program for corn in Colombia determining that the FAW populations are still susceptible to Bt proteins contained in VT2P and VT3P.
Introduction
Fall armyworm, Spodoptera frugiperda, is one of the most serious pests in agricultural production in the Americas (1, 2). It is distributed widely among hosts in the Western Hemisphere (in more than 27 plant families), it can migrate very easily (3–6), and has had a big economic impact on corn, sorghum, rice, and cotton crops (2, 3, 7, 8). S. frugiperda was first reported in West Africa in 2016 (2, 9, 10) and then spread throughout much of sub-Saharan Africa by 2017 (11). In 2018, it was reported in India and China, where it probably migrated from Africa (5, 12, 13).
The inclusion of Bt crops in agricultural systems has brought enormous economic and environmental benefits, such as higher yields, due to less damage to plants, and a significant reduction in the use of insecticides (14, 15). Special academic interest has been shown in strategies that prevent or delay the emergence of resistance by target insects to Bt (16–19).
Although most target populations still show high susceptibility to Bt crops, under field conditions resistance in biotech corn and cotton has been documented for some lepidopteran pests which include: Busseola fusca in South Africa to Cry1Ab protein (20, 21); S. frugiperda in Puerto Rico to Cry1F protein (22); Pectinophora gossypiella, in western India to Cry1Ac; Helicoverpa zea in the southwestern United States (Cry1Ac and Cry2Ab2), and for Helicoverpa punctigera in Australia to (Cry1Ac and Cry2Ab) (21, 23–25). The reported emergence of resistance in the field was demonstrated by evidence of increased damage by the target insect, that is to say, the loss of efficacy of the biotech crop to control B. fusca, S. frugiperda, P. gossypiella and H. zea (20–22, 24–26). The widespread use of Bt crops offers an opportunity to test the hypothesis of how responses to selection pressure are influenced by various genetic and ecological factors (21).
The main purpose of monitoring the susceptibility of target pests in Bt crops, is to be able to detect changes in the response to the action of proteins that allow corrective actions to be taken before the control measure loses its efficacy (27, 28). Therefore, as mentioned above, changes in the susceptibility of target pests based on the LD50 variation do not necessarily imply changes in susceptibility under field conditions (26, 29). This means that regulatory decisions on the continued use of Bt technologies must include information about the relationship between variation in susceptibility and control under field conditions (24).
In Colombia, S. frugiperda is the most damaging pest in corn production. It was first reported in 1917 in the department of Antioquia. It then appeared in Caldas and Valle del Cauca, and then spread to all corn growing areas (30). Losses of up to 60% have been reported in Colombia due to S. frugiperda, which can increase production costs from 5.6 to 10.0% for technified corn (31–33). Blanco et al. (8), reported S. frugiperda to be the most damaging pest for non-Bt corn production in Colombia. Using chemicals to control S. frugiperda (organophosphates and pyrethroids) has become the most widely used method of reducing the impact of the insect in the field (33). These control measures have had a significant impact on the environment and the ability to resist S. frugiperda. This has prompted the need to look for new alternatives, such as the use of pathogens as control agents (34, 35). B. thuringiensis Berliner is one of the biggest species with insecticidal capacity (36) and its results are similar to chemical control (34, 37). Additionally, the adoption of genetically modified crops (such as Bt corn that express Cry and Vip toxins derived from bacterium B. thuringiensis var. kurstaki), have become another alternative for reducing the impact of S. frugiperda (35). Bt crops are an important tool for the control of S. frugiperda (38), that significantly reduce the need for insecticides and ensure protection of the crop throughout its growth (39, 40).
In Colombia, the introduction, release, and commercialization of GM crops is carried out under the Cartagena Protocol on Biosecurity, an international instrument that regulates living modified organisms (LMOs) which are the product of modern biotechnology. Colombia has been sowing genetically modified (GM) crops in its production systems since 2002. 2,000 ha of cotton was the first crop to be planted. In 2007, 6,000 ha of corn was then authorized and sown (41). The number of GM hectares sown in Colombia then increased significantly. By 2021, 31.6% more land had been sown with GM crops than the previous year. That is a total of 150,451 ha, of which 142,975 ha was GM corn, 7,464 GM cotton, and 12 ha blue flowers (cut) (42).
The purpose of this study is to assess the effect of the consumption of corn containing Bt proteins on the mortality and growth of S. frugiperda larvae collected under field conditions within the Insects Resistance Management (IRM) monitoring program in Colombia.
Materials and methods
Larvae collection and establishing colonies
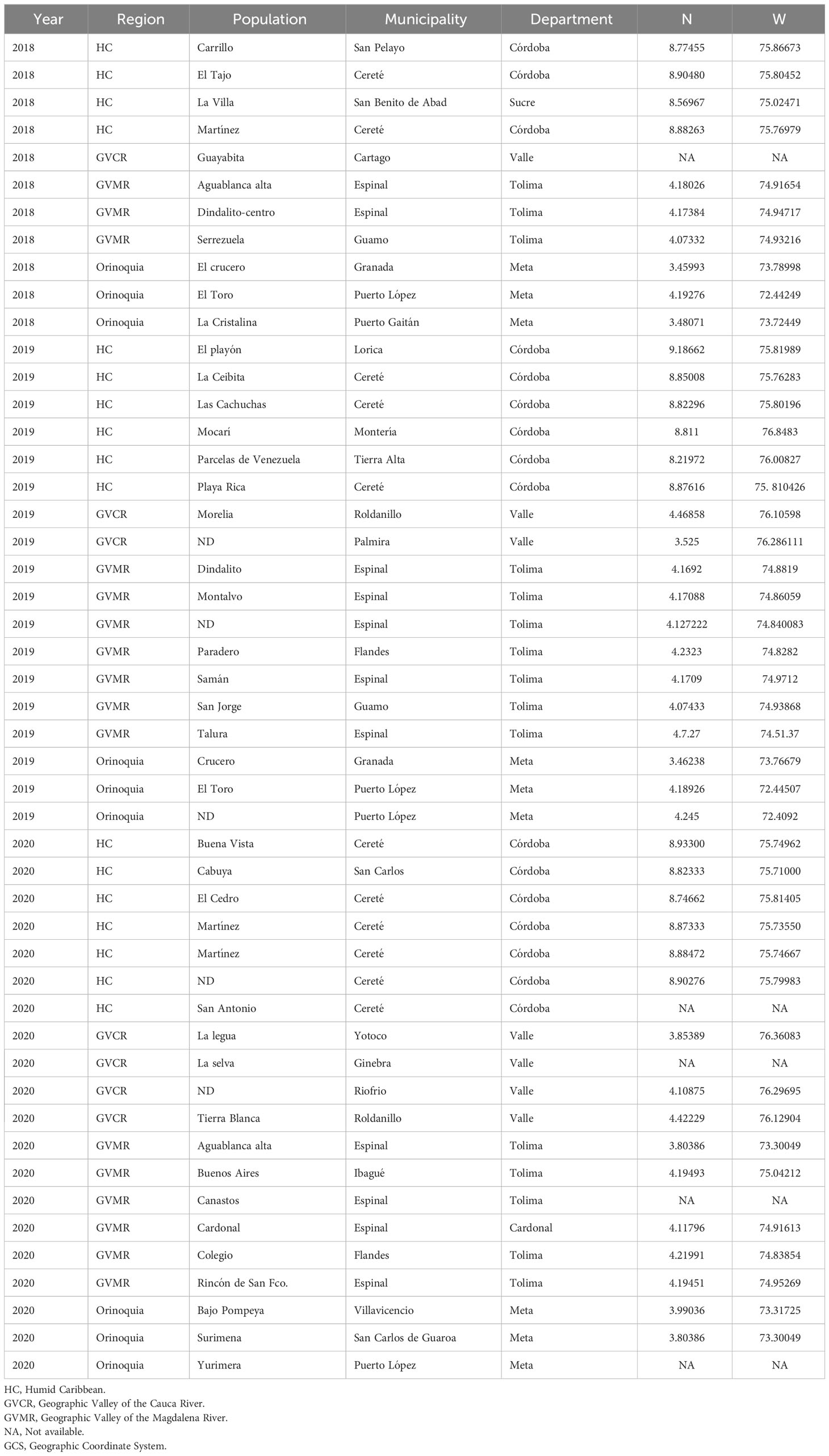
Larvae were collected from 49 locations during the period 2018 to 2020, in commercial plots with conventional corn (non-Bt) from the four agricultural subregions of Colombia (Table 1). In each location, three hundred larvae in different stages of development (F0) were collected and taken to the CIAT (International Center for Tropical Agriculture) facilities, where each collection was handled separately, to obtain the F1 necessary for the assembly of the corresponding bioassays.
Each collection (Table 1) represented an independent colony, which was kept under controlled conditions; 27 ± 2°C, 14:10 (light/dark) artificial photoperiod and 70-75% relative humidity. Adults were housed in cages (35 cm long, 25 cm wide and 25 cm high) and labeled according to where they were collected (Table 1). Adults were fed with a water solution and pasteurized honey (1:1 vol/vol) using absorbent wet cotton that was kept in small containers inside the cage. Egg masses were removed and deposited individually in labeled petri dishes until larvae emerged (F1). Neonate larvae were quarantined for 24 hours prior to setting up bioassays to correct natural mortality.
Feeding bioassays with Bt material
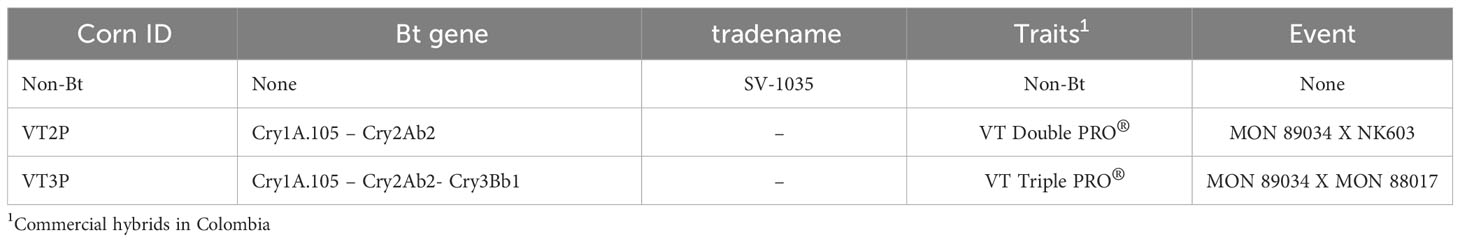
Plant materials used included commercial hybrids SV-1035 (non-Bt) and two hybrids (Bt), VT Double Pro® (VT2P) and VT Triple Pro® (VT3P) (Table 2). About Bt materials, 32 replicates were assessed, each with 16 larval neonates (<24 hours) with a total of 512 larvae per plant material. For the non-Bt material (control), 8 replicates with 16 larvae each were used for a total of 128 larvae.
The materials assessed were sown under semi-controlled conditions in a screen house at the CIAT campus in Palmira, Valle del Cauca, Colombia. When the materials reached vegetative stage V6 - V8, the youngest fully expanded leaves were harvested and taken to the laboratory for bioassays (43). Before starting the bioassays, random samples were taken from the plants, which were analyzed to confirm the presence of the proteins using a strip test. Bioassay trays (White Rearing Tray 32 cells, ref: RT32W, Frontier Agriculture Sciences) were used for the bioassays.
Each cell was inoculated with one neonate larva (24 hours after emerging) and placed in an air-conditioned chamber at 27± 1° C, in 60% relative humidity in darkness for 7 days. Leaf portions (measuring 9.0 cm2) from each of the materials were used as food. To make sure the larvae food was fresh, it was replaced every 48 hours with freshly harvested material from the mesh house. A cotton ball moistened with distilled water was placed in each of the wells, to maintain adequate humidity (44).
The assessed parameters were: (i) mortality rate 7 days after consuming corn (dac) and (ii) growth inhibition (%GI) with respect to the larvae control that managed to survive 7dd (45–48).
With regard to the 7 days after consuming corn (dac) mortality record, it was found that larvae that had not passed the first stage and those that weighed ≤10 mg were considered dead (46, 49).
With reference to the control, growth inhibition (%GI) was calculated by dividing the average weight of surviving larvae in each Bt material (VT2P, VT3P) by the average weight of larvae in the control (non-Bt) and shown as a percentage. In each of the materials, live larvae were individually weighed to obtain the average using a Sartorius Quintix35 -1S precision balance (Sartorius Lab Instruments GmbH & Co) (50).
Data analysis
Mortality was measured 7 days after consuming (dac) Bt corn plant material and understood as the sum of (dead larvae + Larvae L1 and/or weight ≤10 mg) (46). Mortality data were corrected with the Henderson and Tilton (H&T) formula (51). Tukey’s test (P<0.05) and GLM for multiple comparisons (52) were used to compare means.
Results
This section summarizes the information on the response of 49 S. frugiperda populations to the effect of VT2P and VT3P corn consumption from Colombia’s four agricultural subregions between 2018 and 2020 (Table 2, Figure 1).
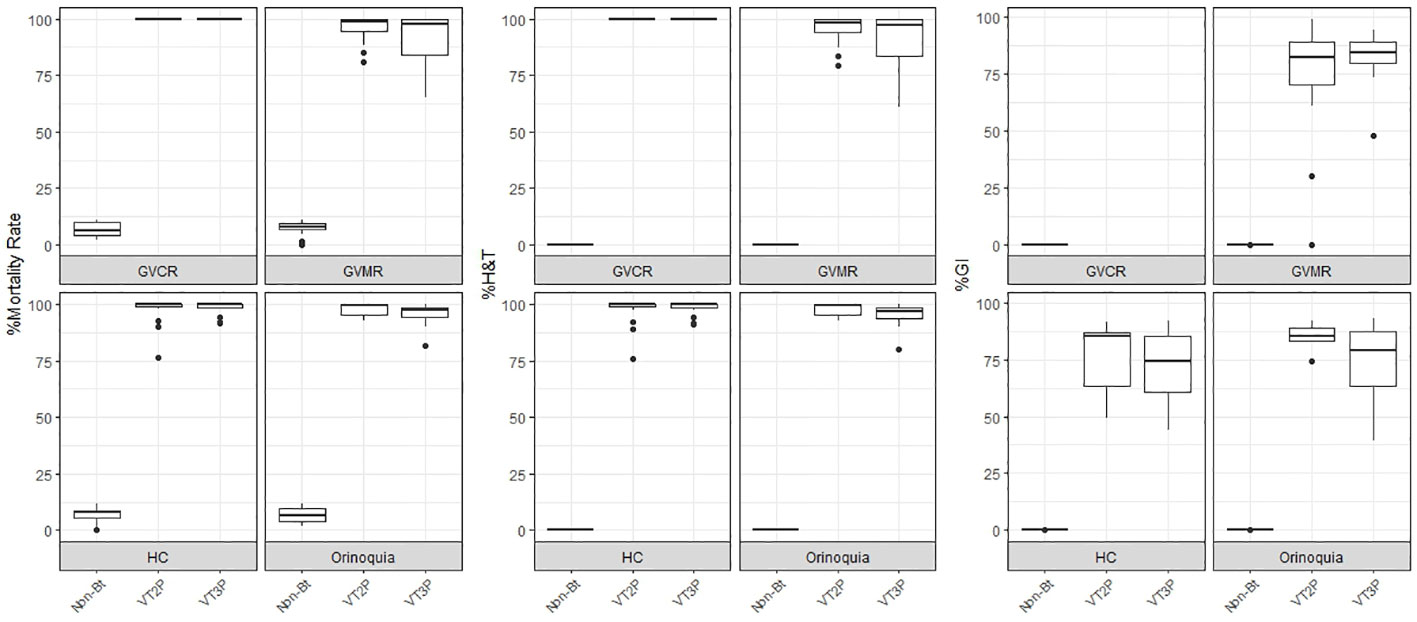
Figure 1 Response of S. frugiperda populations, according to % Mortality Rate, % H&T and Growth Inhibition (%GI), following the consumption of corn materials with VT2P and VT3P technology in Colombia, for collections 2018 to 2020.
With respect to the interaction between corn materials (Non-Bt, VT2P, VT3P) and the year collected, there were significant differences in mortality rates, with values ranging from 8.6% after consuming non-Bt corn to 98.6% after consuming VT3P corn (Table 3). Growth inhibition (%GI) because of consuming Bt corn, showed statistical differences, with values ranging between 56.3 and 86.8% for VT2P (2018) and VT3P (2019), respectively (Table 3, Figure 1).
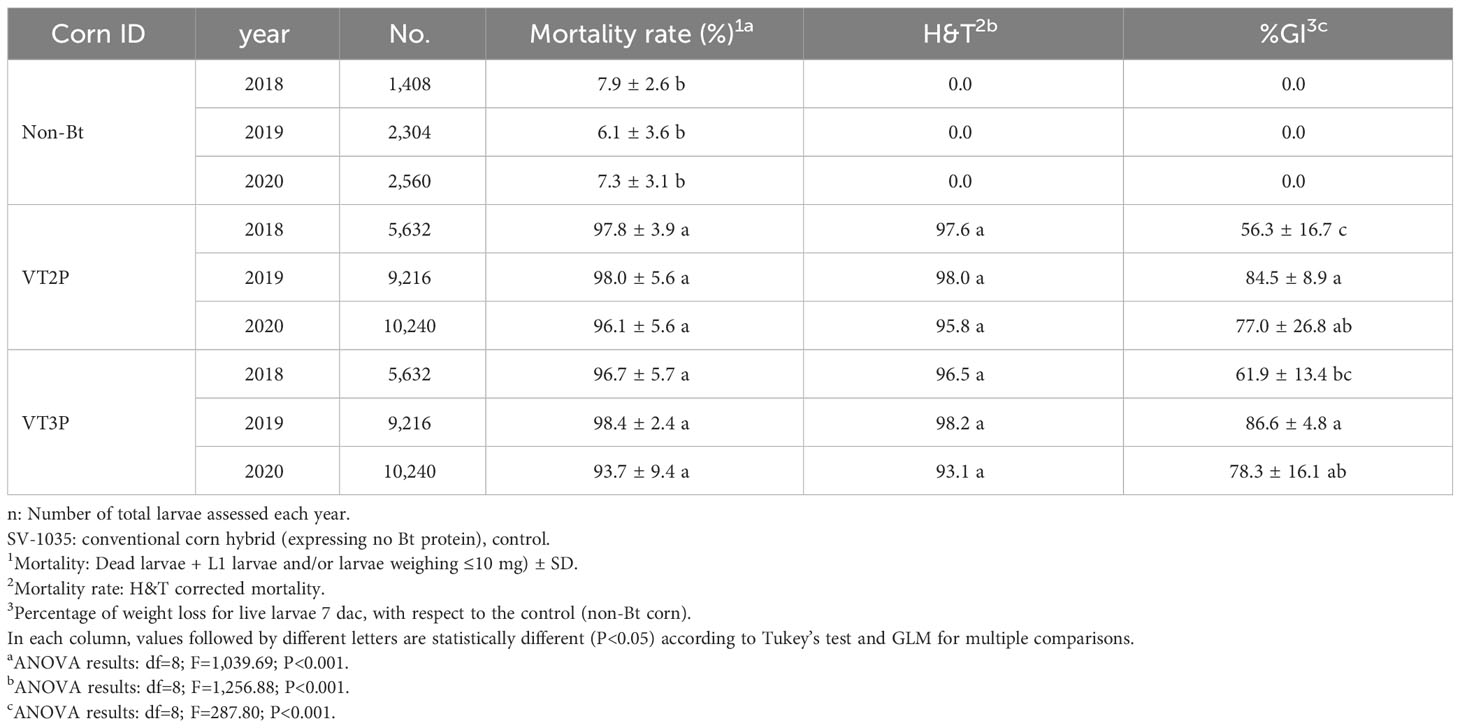
Table 3 Means ± SD of mortality rate and growth inhibition 7 days after consuming (dac) non-Bt, VT2P and VT3P corn for S. frugiperda populations collected between 2018 and 2020.
The response of S. frugiperda populations to the corn material collected from the four agricultural subregions showed statistical differences for mortality rates after consuming Non-Bt and Bt corn (Table 4). Values ranged from 7.1% for the Orinoco populations fed with non-Bt corn to 100.0% for those collected in GVCR and fed with VT2P and VT3P corn. With respect to growth inhibition (GI), there were extreme values in HC collections, with 74.2% and 84.5% recorded for collections from Orinoco, fed with VT3P and VT2P, respectively (Table 4, Figure 2).

Table 4 Means ± SD of mortality rate and growth inhibition 7 days after consuming (dac) non-Bt, VT2P and VT3P corn on S. frugiperda populations collected in the four agricultural subregions.
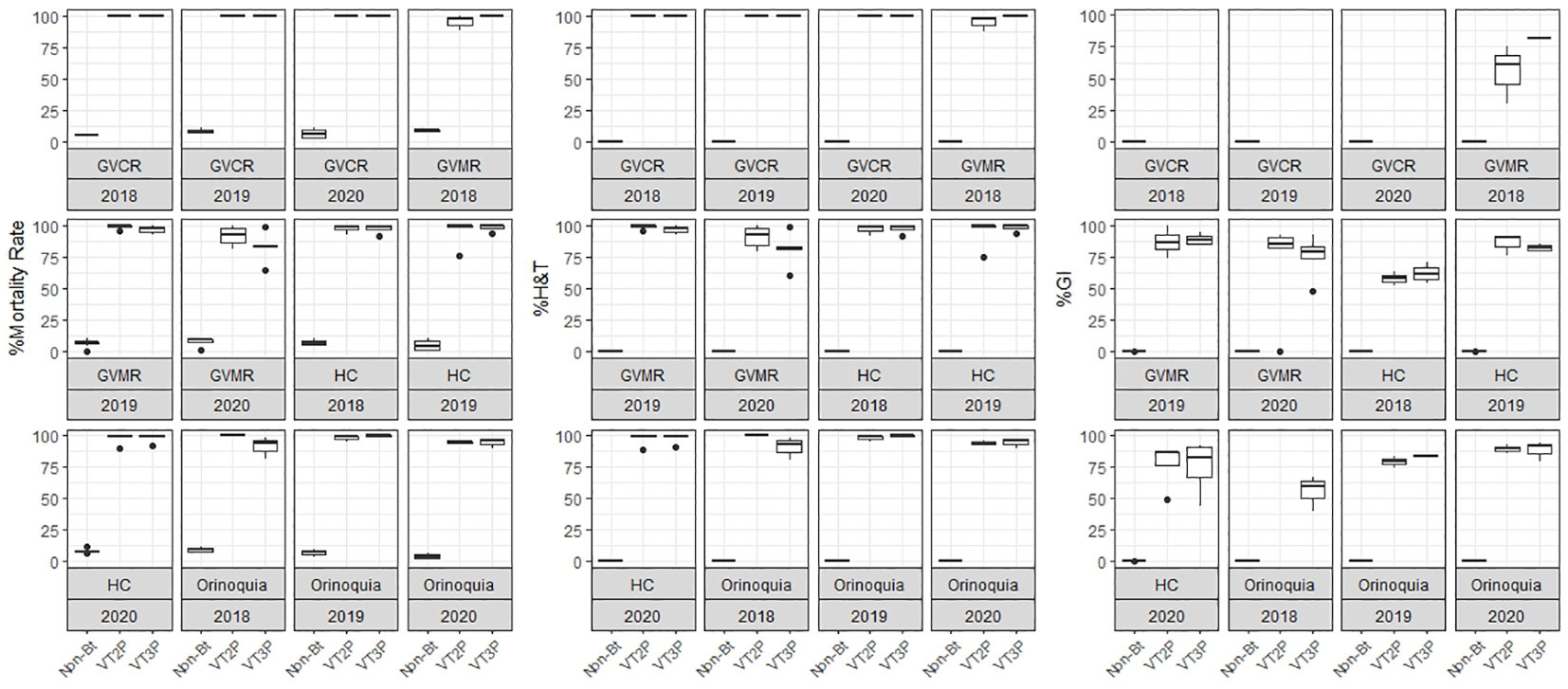
Figure 2 Response of S. frugiperda populations, according to % Mortality Rate, % H&T and Growth Inhibition (%GI), because of consuming maize materials with VT2P and VT3P technology for four agricultural subregions of Colombia for collections 2018 to 2020.
Susceptibility of humid Caribbean populations
The response to consumption for the interaction year and for corn material showed statistical differences between non-Bt and Bt corn (VT2P, VT3P). Values ranging from 4.7 to 98.8% corresponded to the 2019 collections that consumed non-Bt corn and the 2020 collections that consumed VT3P corn, respectively (Table 5, Figure 3).
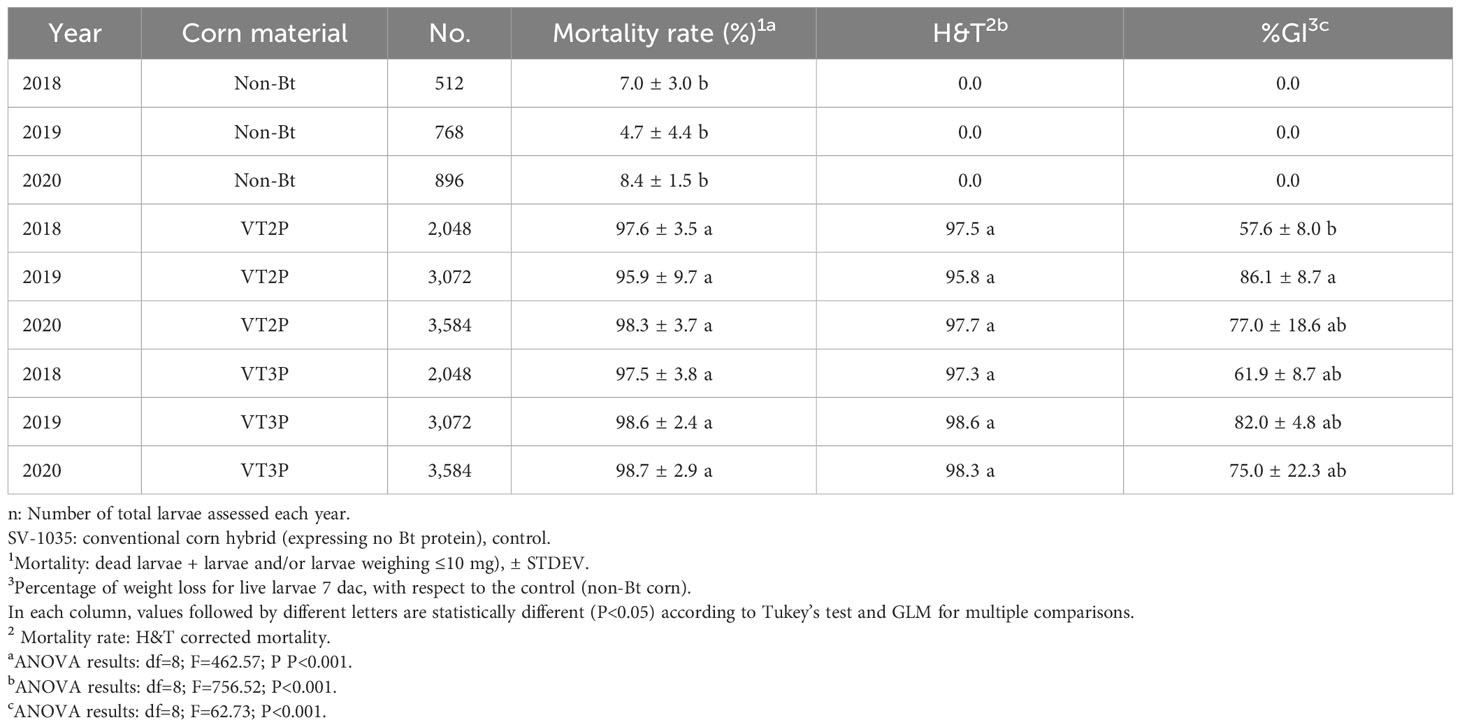
Table 5 Means ± SD of mortality rate and growth inhibition 7 days after consuming (dac) non-Bt, VT2P and VT3P corn on S. frugiperda collected between 2018 and 2020 in HC.
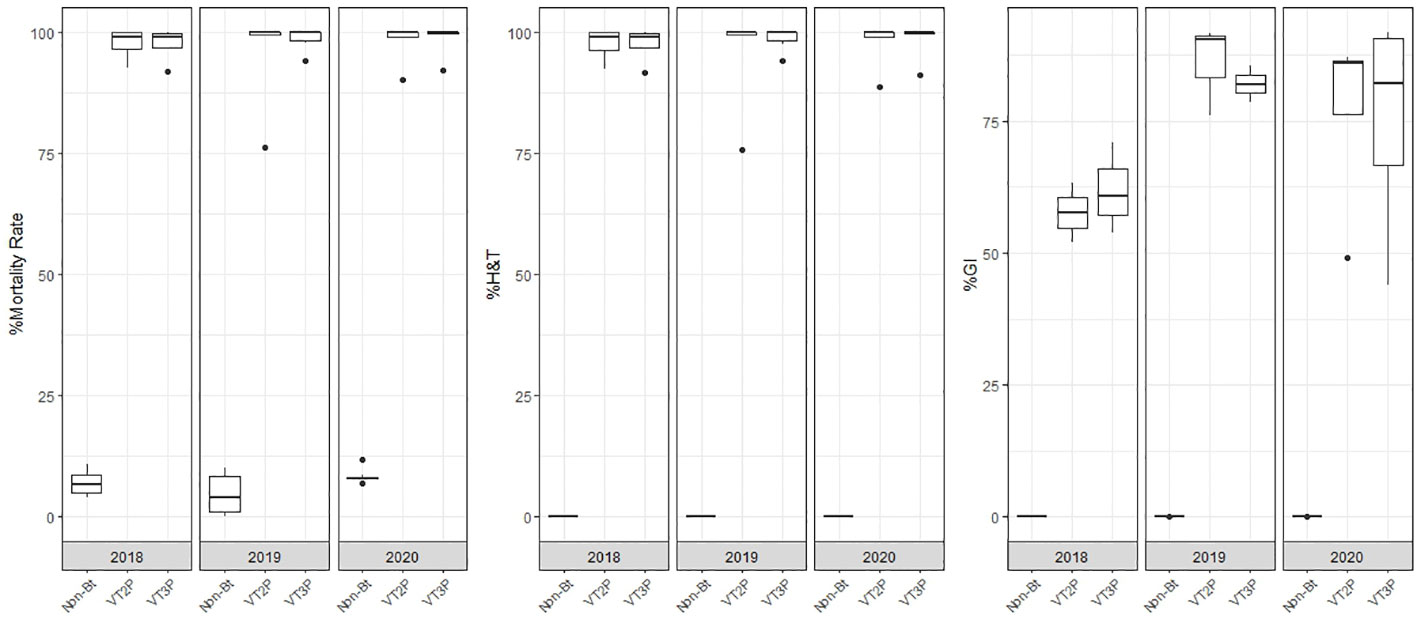
Figure 3 Response of S. frugiperda according to according to % Mortality Rate, % H&T and Growth Inhibition (%GI), due to consuming corn materials with VT2P and VT3P technology in HC (Colombia), 2018-2020.
About collections made between 2018 and 2020, mortality due to consumption of VT2P and VT3P corn material was >95.0% (H&T), there were no statistical differences between materials (Table 5, Figure 4) and values ranged from 57.6 to 86.1% for the %GI parameter. The effect of consuming VT2P was statistically different for populations collected in 2018 compared to the rest (Table 5). The mortality rate (H&T) for the assessed populations was between 75.6 and 100.0%. The lowest mortality rate was for the population collected in La Ceibita (Cereté) in 2019, due to VT2P corn consumption (Table 6, Figure 4). Of the 16 populations that were fed VT3P, seven showed 100.0% mortality while the rest were between 89.4 and 99.6% (Table 6, Figure 5). The lowest growth inhibition percentage corresponded to larvae collected in 2020 in San Antonio (Cereté) and fed with VT3P corn (43.9%). In contrast, the highest value for %GI was for the population collected in Cabuya (San Carlos) in 2020 and fed with VT2P (Table 6, Figure 4).
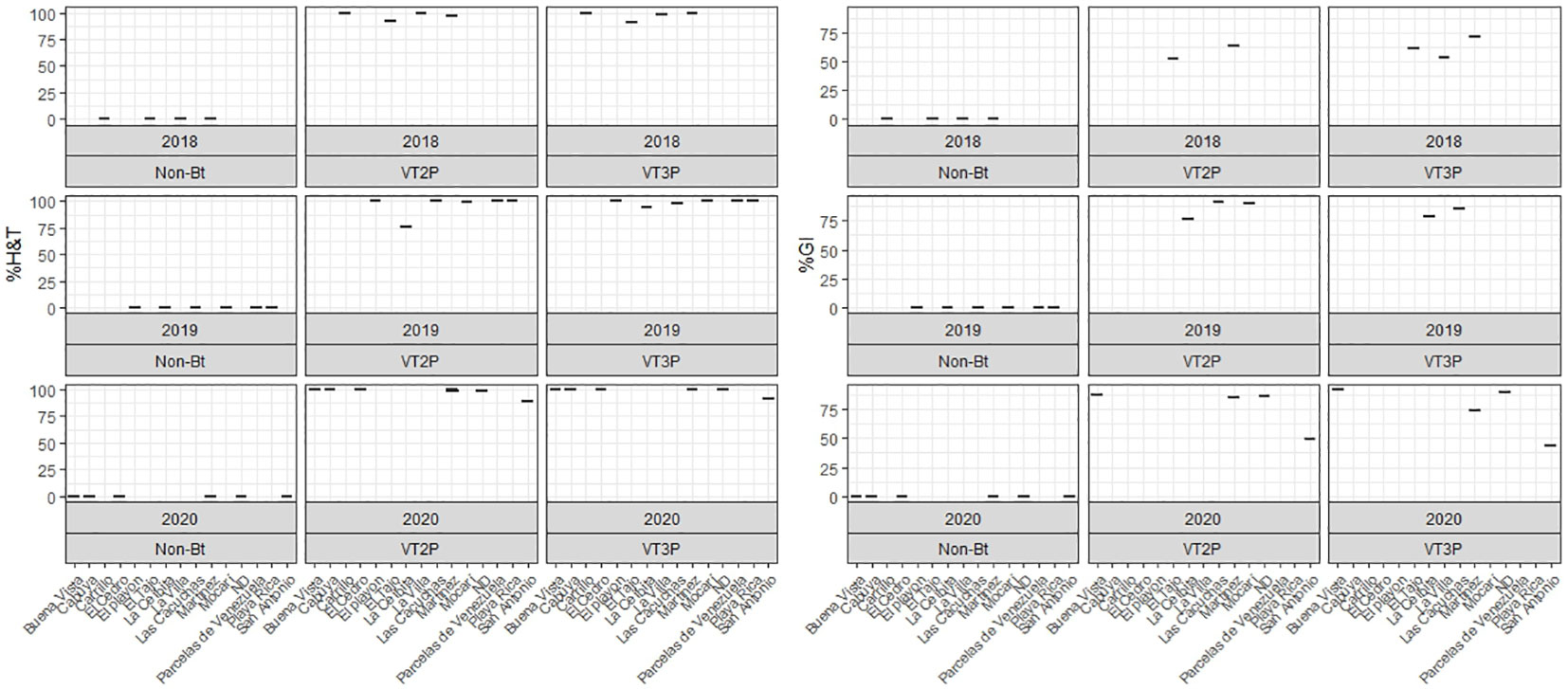
Figure 4 Response of S. frugiperda populations collected in the HC (2018-2020), according to % Mortality Rate, % H&T and Growth Inhibition (%GI), because of consuming of corn materials with VT2P and VT3P technology.
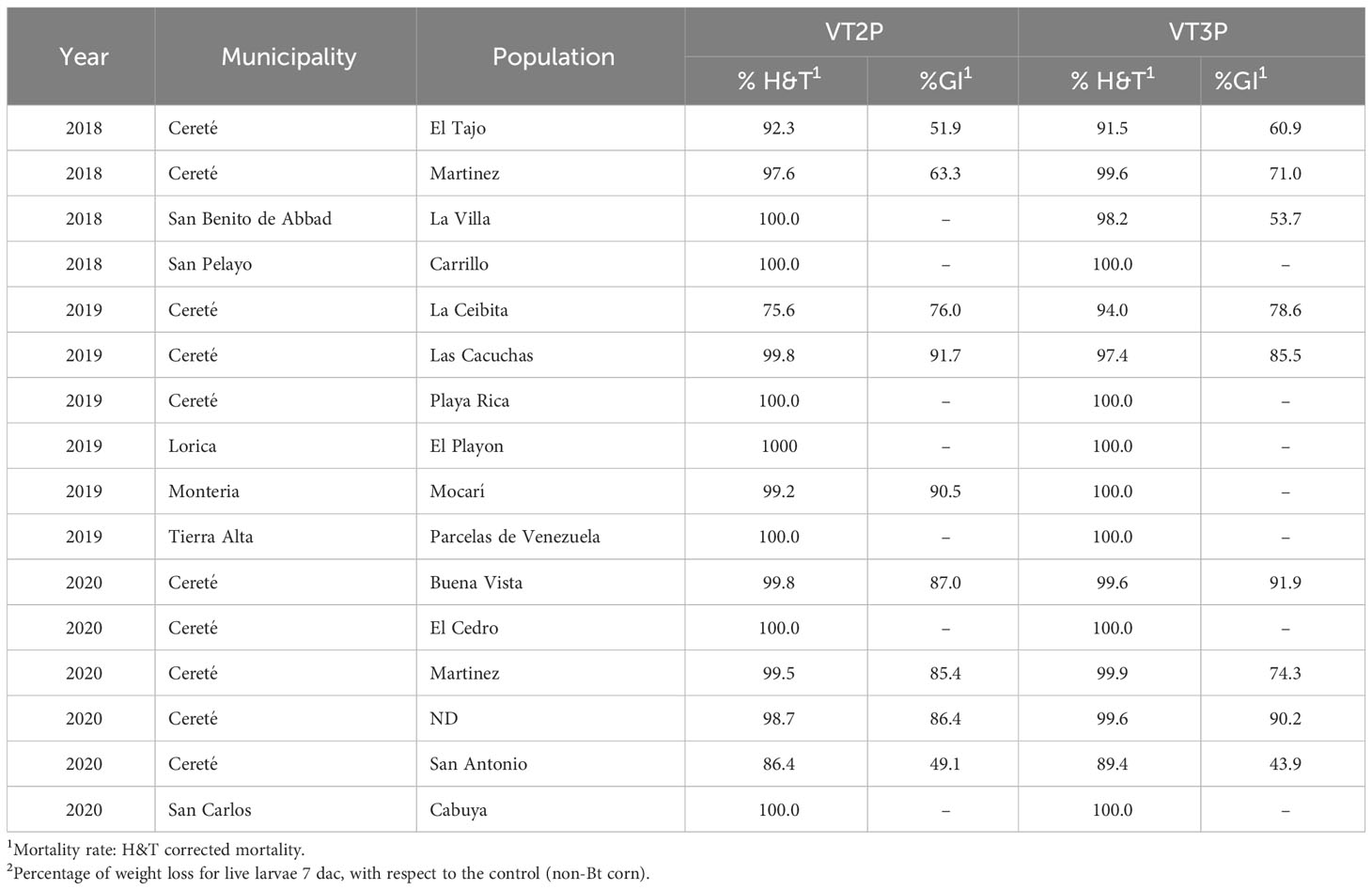
Table 6 Mortality rate (%H&T) and growth inhibition (%) 7 days after consuming (dac) VT2P and VT3P corn on S. frugiperda populations collected between 2018 and 2020 in HC.
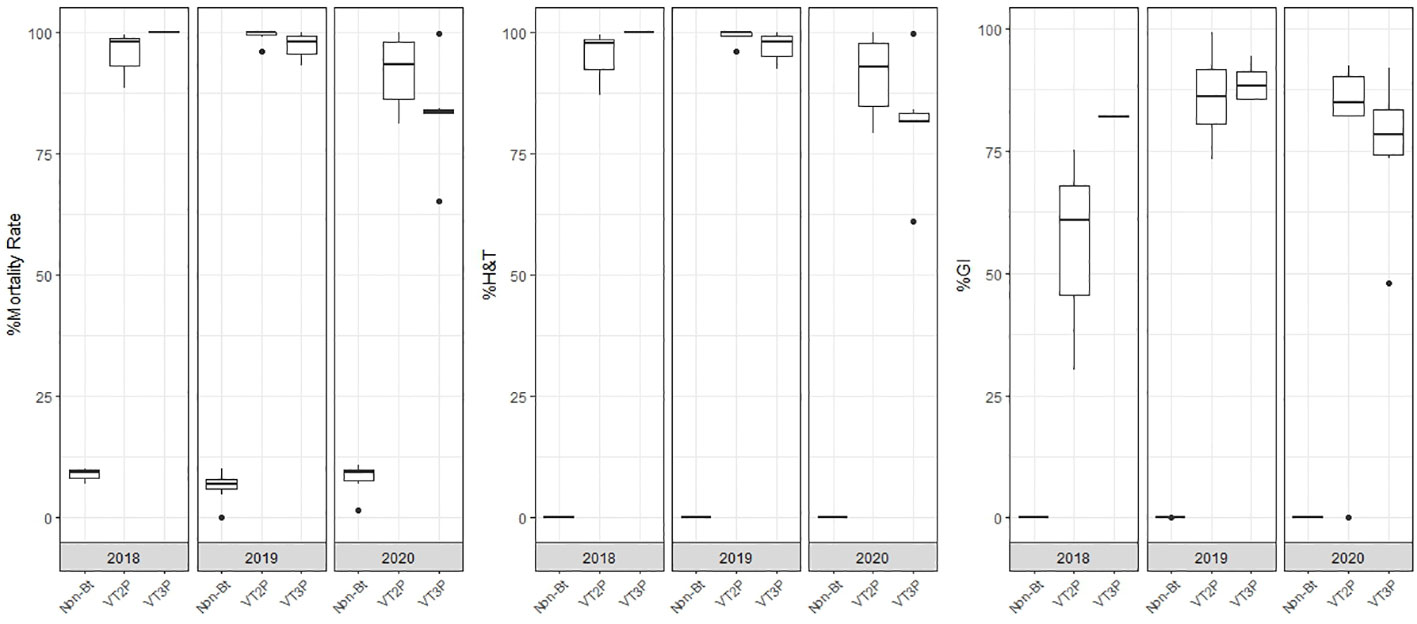
Figure 5 Response of S. frugiperda collected in GVMR (2018-2020), depending on the percentage of mortality rate, H&T and Growth Inhibition (%IG), due to consumption of VT2P and VT3P corn materials.
Susceptibility of geographic valley of the Magdalena river populations
The populations assessed in GVMR from 2018 to 2020, showed mortality rates between 85.6 and 99.9% due to Bt corn consumption, which were statistically different from what had been seen in non-Bt corn (Table 7). With respect to mortality (H&T), values between 82.3 and 99.9% were recorded for S. frugiperda larvae fed with VT3P corn. The lowest value (82.3%) was recorded for populations collected in 2020 (Table 7). With reference to the %GI parameter, observations made in 2018 which were fed with VT2P corn and those made in 2019 fed with VT3P corn, were statistically different recording values of 55.4 and 89.0%, respectively (Table 7, Figure 5).
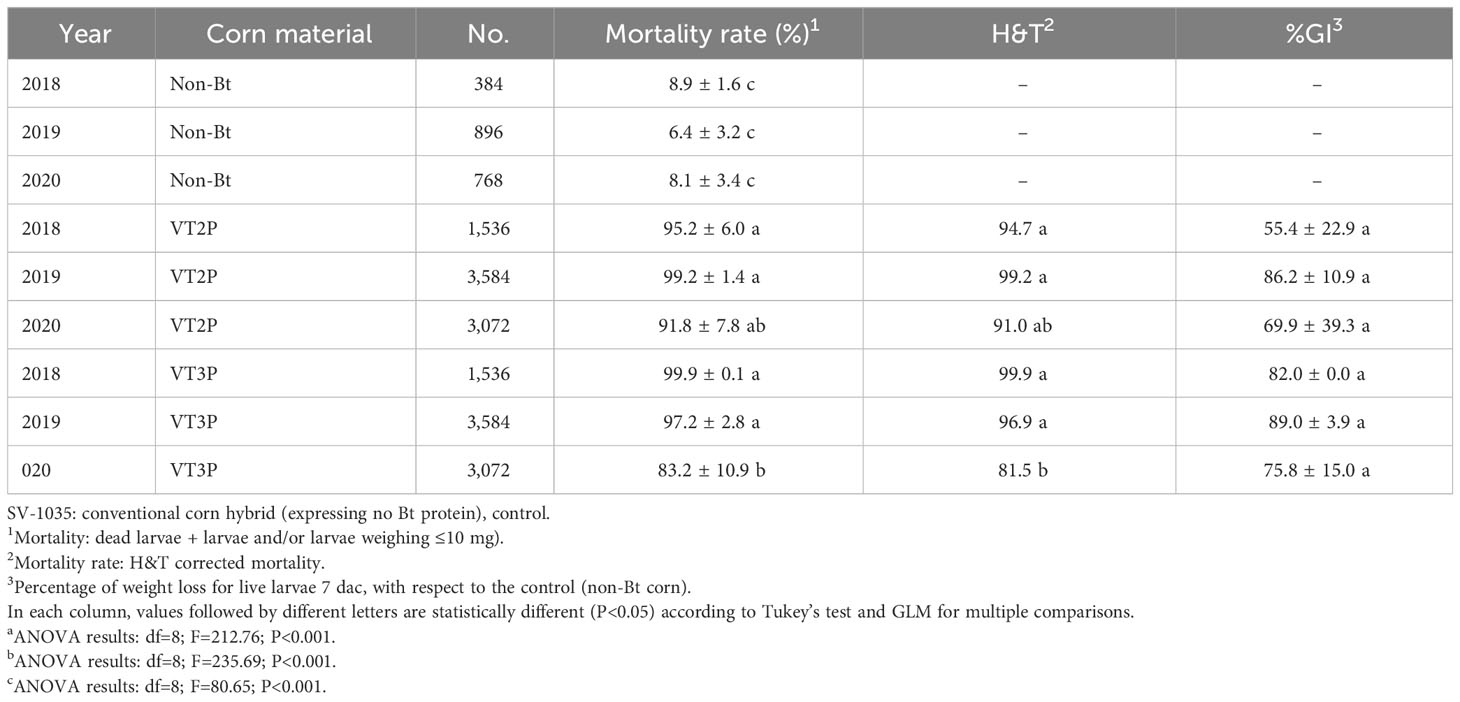
Table 7 Means ± SD of mortality rate and growth inhibition 7 days after consuming (dac) non-Bt, VT2P and VT3P corn on S. frugiperda collected from 2018 to 2020 in GVMR.
The behavior of each of the 16 populations assessed in GVMR for mortality % (H&T) and fed with VT2P varied between 77.5 and 100%. The lowest value was recorded for the population collected in Buenos Aires (Ibagué, Tolima) in 2020, with growth inhibition of >80.0% (Table 8, Figure 6). In the case of larvae fed with VT3P corn, mortality values (H&T) were between 76.6 and 99.6%. Values for %GI between 48.1 and 94.4% were recorded for collections in Cardonal (Espinal, 2020) and Dindalito (Espinal, 2018) (Table 8, Figure 6).
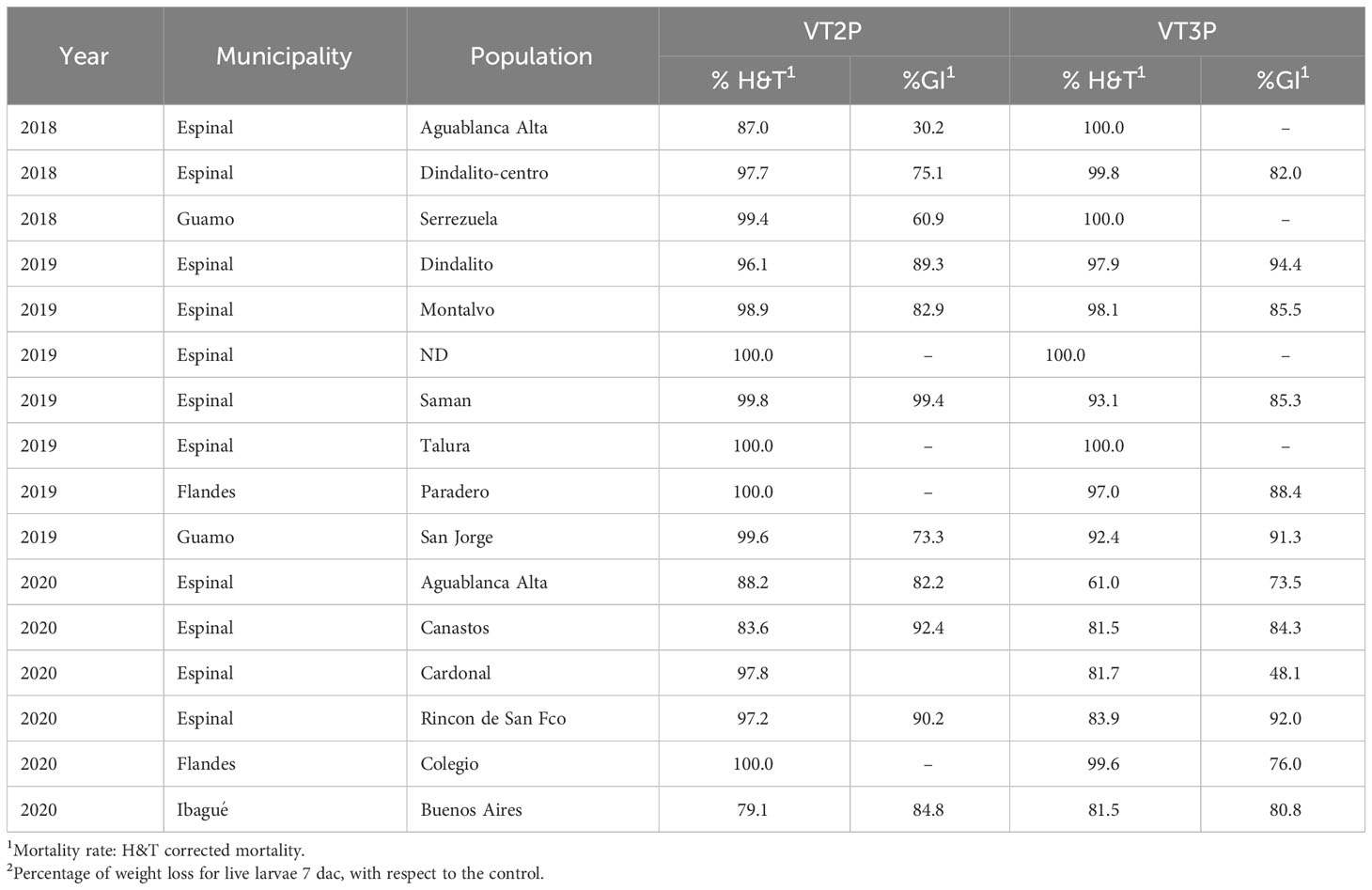
Table 8 Mortality rate and growth inhibition 7 days after consuming (dac) non-Bt, VT2P and VT3P corn on S. frugiperda populations collected from 2018 to 2020 in GVMR.
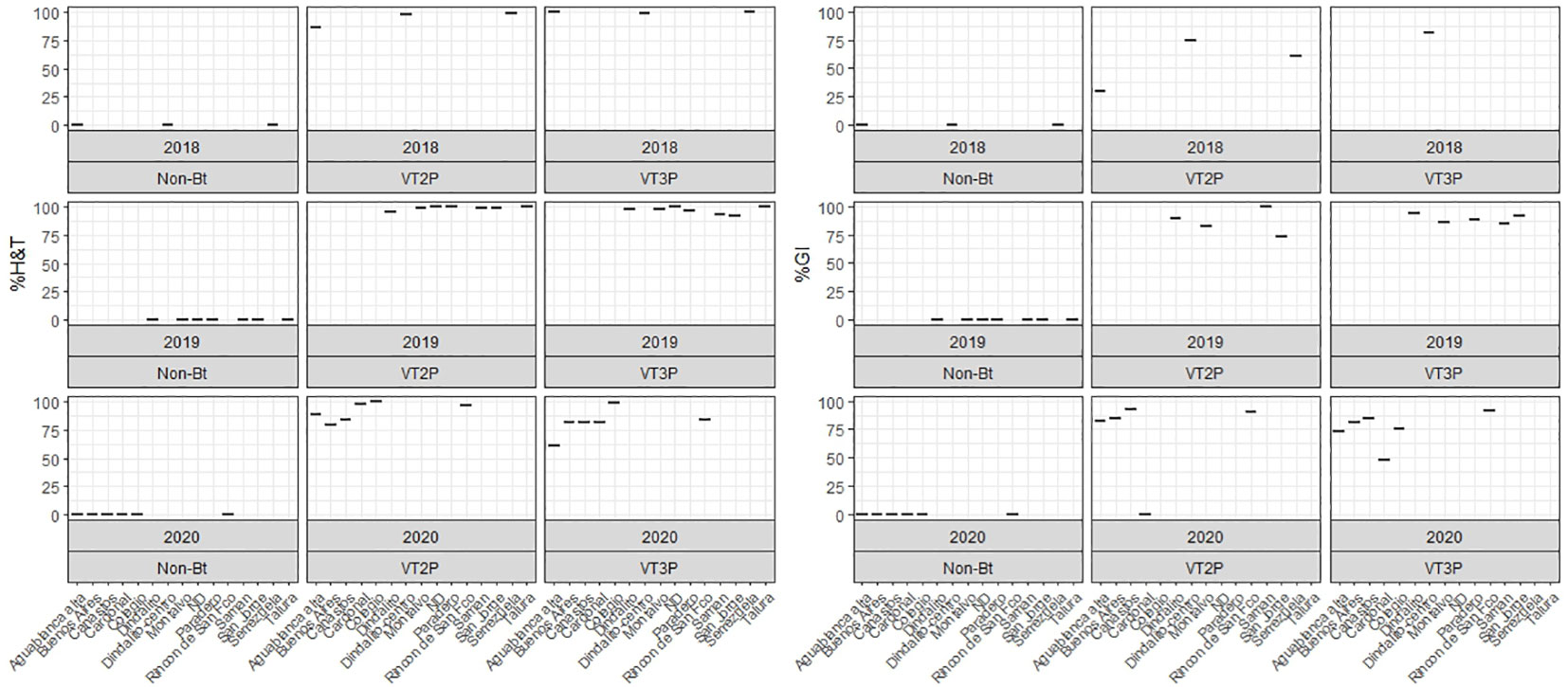
Figure 6 Response of S. frugiperda populations according to % Mortality Rate, % H&T and Growth Inhibition (%GI), due to consuming corn materials with VT2P and VT3P technology in GVMR (Colombia) 2018-2020.
Susceptibility of Orinoquia populations
For the collections made between 2018 and 2020 in the Orinoco, it was possible to establish values for the mortality rate parameter at between 3.9 and 100%, which showed significant differences because of consuming of non-Bt corn and Bt corn (VT2P and VT3P) (Table 9). There was a statistical difference in mortality (H&T) between the larvae collected in 2018 and fed with VT3P (90.7%), compared to those collected in 2018 and larvae fed with VT2P (100.0%) (Table 9, Figure 7). There were also statistical differences for growth inhibition, because of Bt corn consumption, with values ranging from between 55.3 and 88.7%. These corresponded to the 2018 collections which were fed with VT3P, and the larvae collected in 2020 fed with VT2P, respectively. Regarding the nine populations fed with VT2P corn, mortality rate values (H&T) ranged between 92.7 and 100.0%. Values for %GI were 78.8% for larvae collected in 2019 in El Crucero (Granada) and 92.1% for the collections in 2020 in Bajo Pompeya (Villavicencio) (Table 9). The mortality rate (H&T) values were higher than 80.0% because of consuming VT3P corn. The lowest mortality rate (H&T) was for the population collected in 2018 in Toro (Puerto López) (Table 10). In contrast, values for %GI ranged from 39.5 to 93.2%, with the lowest value given to the population collected in El Toro (Puerto Lopez) in 2018 (Table 10, Figure 8).
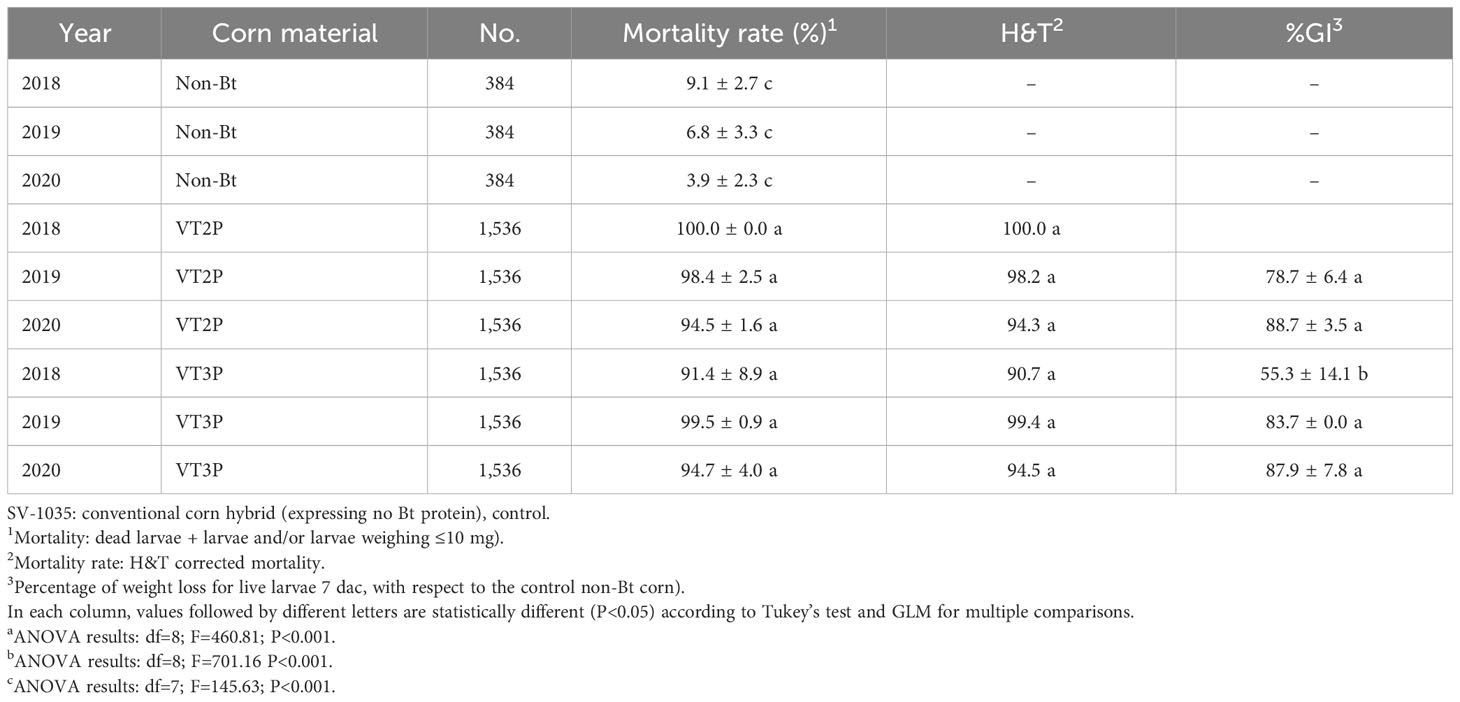
Table 9 Means ± SD of mortality rate and growth inhibition 7 days after consuming (dac) non-Bt, VT2P and VT3P corn, on S. frugiperda collected between 2018 and 2020 in the Orinoquia.

Figure 7 Response of S. frugiperda collected in Orinoquia (2018-2020), depending on the percentage of mortality rate, H&T and Growth Inhibition (%IG), due to consumption of VT2P and VT3P corn materials.
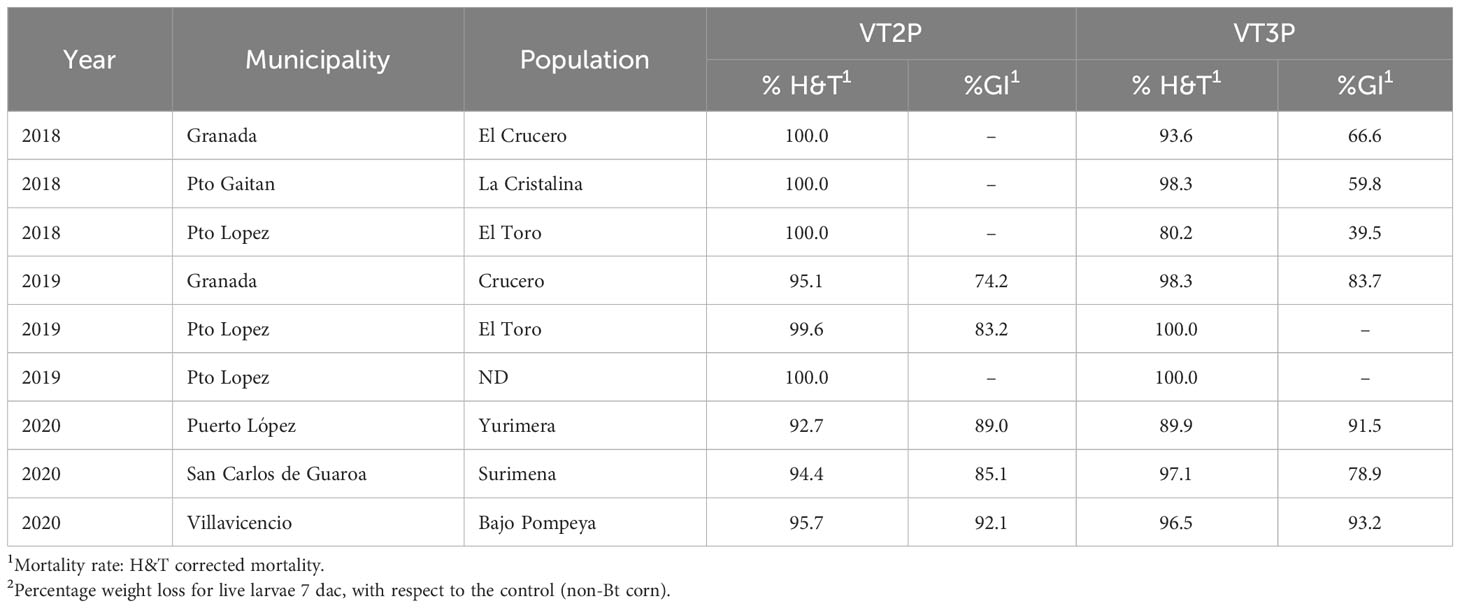
Table 10 Mortality rate and growth inhibition 7 days after consuming (dac) non-Bt, VT2P and VT3P corn on S. frugiperda populations collected from 2018 to 2020 in the Orinoquia.
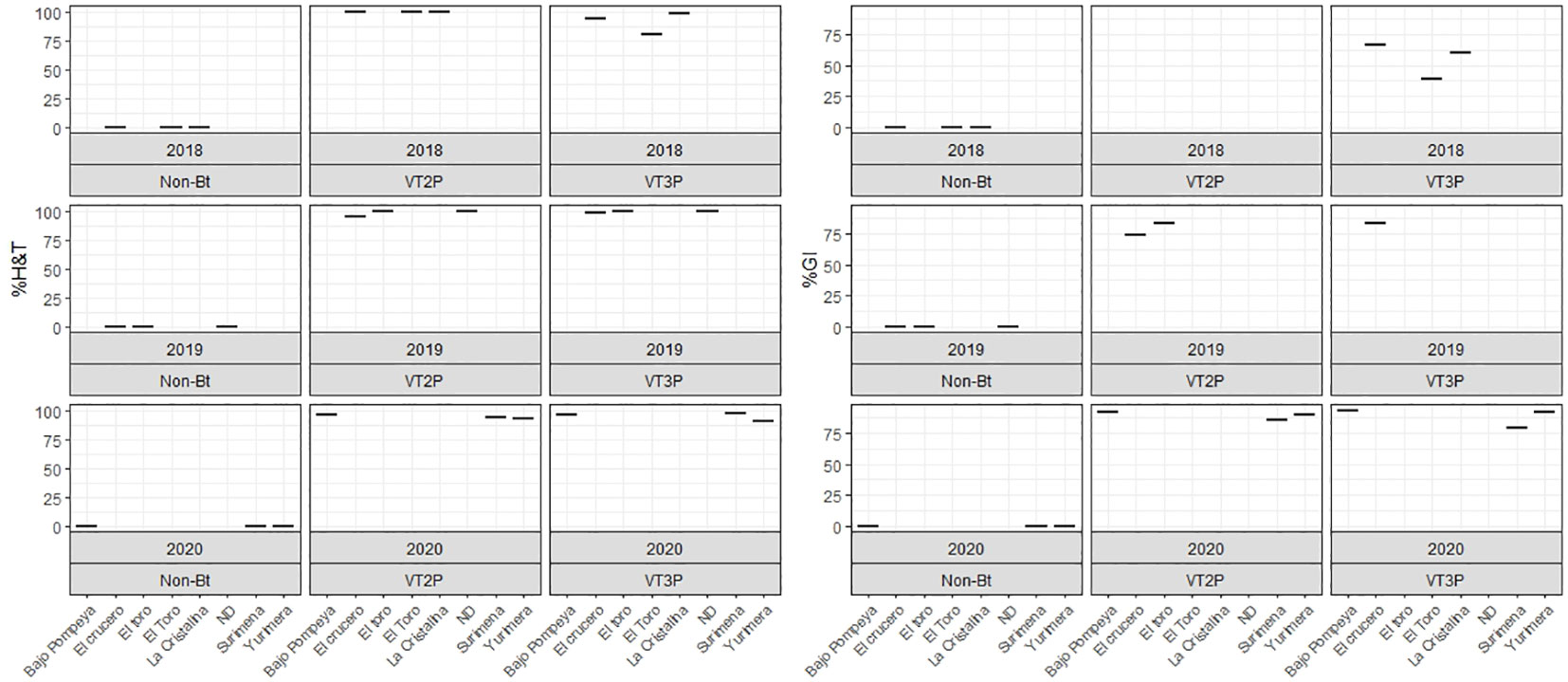
Figure 8 Response of S. frugiperda populations according to % Mortality Rate, % H&T and Growth Inhibition (%GI), due to consuming corn materials with VT2P and VT3P technology in Orinoquia (Colombia) 2018-2020.
Susceptibility of GVCR populations
There were statistical differences for the mortality rate for the interaction year and corn material (Table 11). Mortality rate values ranged from 4.7 to 100.0% due to consuming Non-Bt and Bt corn (VT2P - VT3P), respectively (Table 11). All six populations collected in the GVCR showed 100% mortality in response to consuming plant material with VT2P and VT3P technologies (Table 12).
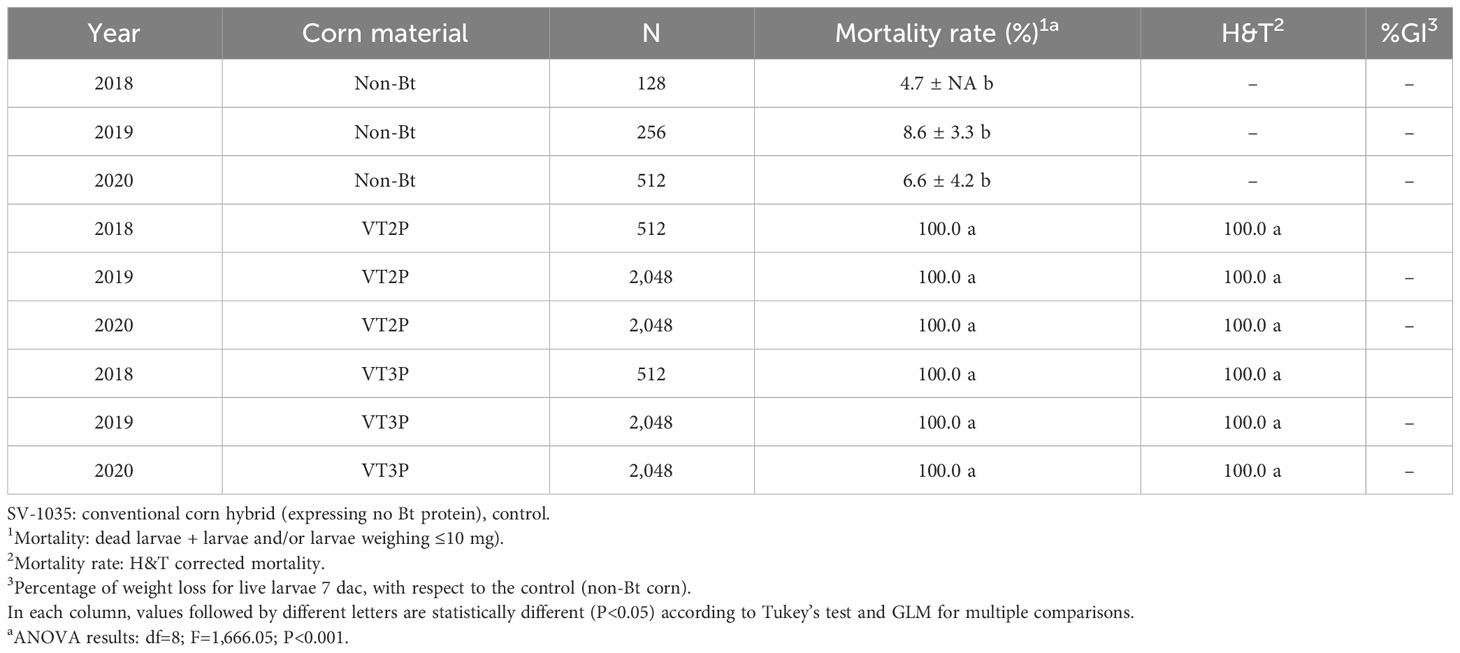
Table 11 Means ± SD of mortality rate and growth inhibition 7 days after consuming (dac) non-Bt, VT2P and VT3P corn on S. frugiperda collected between 2018 and 2020 in GVCR.
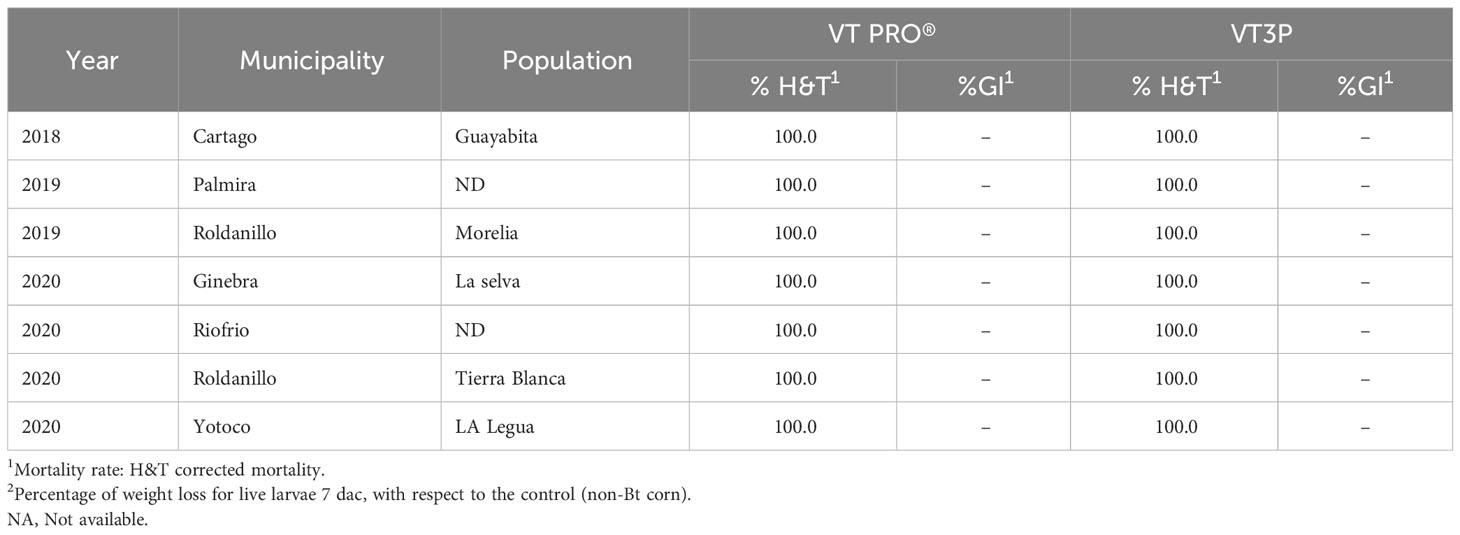
Table 12 Mortality rate and growth inhibition 7 days after consuming (dac) non-Bt, VT2P and VT3P corn on S. frugiperda populations collected from 2018 to 2020 in GVCR.
Discussion
The susceptibility of S. frugiperda to corn materials with B. thuringiensis insecticidal endotoxins can be influenced by multiple biotic and abiotic factors. However, the data reported in this study is about the susceptibility of S. frugiperda populations collected in the four agricultural subregions of Colombia after consuming VT2P (cry1A.105-Cry2Ab2) and VT3P (Cry1A.105-Cry2Ab2-Cry3Bb1) corn by mortality rate and percentage of growth inhibition. These results agree with the results reported under field conditions (53), about the effect of corn materials expressing Cry1A.105 and Cry2Ab2 proteins.
Previous studies on the effect of the consumption of Bt corn expressing Cry1A.105 and Cry2Ab2 proteins on the survival of S. frugiperda larvae indicated mortality rates from 97.0 to 100.0% (54–57). In this study, the mortality rate for S. frugiperda populations assessed from 2018 to 2020 in Colombia (because of consuming corn containing VT2P and VT3P technologies) confirms their susceptibility to these proteins which have produced an effective control for S. frugiperda under this study’s conditions. This contrasts with the results reported by (56) that showed 100% mortality 21 days after consuming corn containing Cry1A.105, Cry2Ab2, Cry3Bb1 proteins. Growth inhibition (GI) values greater than 57.0% denote a sublethal effect on S. frugiperda as a result of the consumption of VT2P and VT3P corn, generating an impact on the population over time, which with the proper implementation of refuge areas, will become a strategy that leads to the susceptibility of S. frugiperda over time (56, 58, 59).
Analyzing the impact of the consumption of Bt corn material on mortality and growth inhibition rates (according to the year collected and the corn material consumed), it was possible to determine the efficacy of Bt proteins on the populations of S. frugiperda assessed from 2018 to 2020 in Colombia. These results confirm the current susceptibility of S. frugiperda to VT2P and VT3P corn materials in the subregions assessed under the conditions in this study.
Assessing the impact of consuming VT2P and VT3P corn (according to four agricultural subregions of Colombia from which the S. frugiperda was collected), it was found that there was no statistical difference in mortality rates between the subregions, and that in regions such as the GVCR, all the populations that were assessed demonstrated high susceptibility to the proteins expressed in VT2P and VT3P corn. Additionally, larvae that managed to survive after seven days of consuming Bt corn, showed values for growth inhibition (%GI) that were higher than 74.0%, compared to larvae fed with non-Bt corn. It was confirmed that (regardless of origin), seven days of consuming corn with Cry1A.105, Cry2Ab2, Cry3Bb1 proteins produces a sublethal effect on live larvae according to their growth inhibition (56, 59).
In the case of the S. frugiperda populations collected in HC, the lowest mortality rates corresponded to the 2020 collections from San Antonio (Cereté) and coincided with the lowest percentages for growth inhibition (GI), after consuming VT2P and VT3P corn for seven days. This information establishes a baseline for future research aimed at detecting possible changes in the susceptibility of S. frugiperda in the HC subregion. (24).
In the case of GVMR, the lowest mortality rate value corresponded to the population collected in 2020 and fed with VT2P and VT3P corn. In contrast, the lowest growth inhibition percentage was recorded for larvae fed with VT2P corn in 2018 and for larvae fed with VT3P in 2020. This confirms the importance of S. frugiperda monitoring programs as a tool for identifying possible changes in susceptibility to commercially released proteins as early as possible (26, 28).
The mortality rate in Orinoquia between 2018 and 2020 shows the current susceptibility of S. frugiperda in that region. Moreover, the effect on larval development according to growth inhibition (with respect to larvae fed with Non-Bt corn) was sublethal (59).
The GVMR is the only region in Colombia where all S. frugiperda populations demonstrated high susceptibility consuming Bt corn expressing Cry1A.105 - Cry2Ab2 (VT2P) and Cry1A.105 - Cry2Ab2 - Cry3Bb1 (VT3P) proteins.
This information highlights the importance of constant monitoring (under field conditions) to detect variations in the susceptibility of S. frugiperda populations, and this is particularly important in tropical conditions. In addition, careful attention needs to be paid to the high genetic variability of S. frugiperda currently reported in Colombia (6, 30, 60). This monitoring also needs to be carried out to compare results obtained in laboratory conditions and the efficacy of technologies used in the field (26, 29).
Finally, these monitoring results should be treated with caution (the conditions of the study should be taken into consideration), when implementing strategies that encourage the susceptibility of S. frugiperda in Colombia.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
JR-C: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft. SV: Investigation, Methodology, Supervision, Writing – review & editing. AR-C: Investigation, Methodology, Writing – review & editing. FS-G: Writing – review & editing, Resources. DR: Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Donor Bayer S.A.; Funding Type: BLR; Region: LAC; Bioversity-CIAT Alliance internal code 471.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rosas-García NM, Herrera-Mayorga V, Rivera G, Mireles-Martínez M, Sánchez FAP, Villegas-Mendoza JM. Spodoptera frugiperda1 biotype identification from maize host plants in different regions of Mexico. SOUTHWESTERN ENTOMOLOGIST (2016) 41:761–70. doi: 10.3958/059.041.0318
2. Nagoshi RN, Dhanani I, Asokan R, Mahadevaswamy HM, Kalleshwaraswamy CM, Sharanabasappa, et al. Genetic characterization of fall armyworm infesting South Africa and India indicate recent introduction from a common source population. PloS One (2019) 14:e0217755. doi: 10.1371/journal.pone.0217755
3. Health, E.P.o.P, Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, et al. Pest categorisation of Spodoptera frugiperda. EFSA J (2017) 15:e04927. doi: 10.2903/j.efsa.2017.4927
4. Nagoshi RN, Fleischer S, Meagher RL, Hay-Roe M, Khan A, Murua MG, et al. Fall armyworm migration across the Lesser Antilles and the potential for genetic exchanges between North and South American populations. PloS One (2017) 12:e0171743. doi: 10.1371/journal.pone.0171743
5. Jing DP, Guo JF, Jiang YY, Zhao JZ, Sethi A, He KL, et al. Initial detections and spread of invasive Spodoptera frugiperda in China and comparisons with other noctuid larvae in cornfields using molecular techniques. Insect Sci (2020) 27:780–90. doi: 10.1111/1744-7917.12700
6. Marin DV, Castillo DK, Lopez-Lavalle LAB, Chalarca JR, Perez CR. An optimized high-quality DNA isolation protocol for spodoptera frugiperda J. E. smith (Lepidoptera: Noctuidae). MethodsX (2021) 8:101255. doi: 10.1016/j.mex.2021.101255
7. Sena DG Jr., Pinto FAC, Queiroz DM, Viana PA. Fall armyworm damaged maize plant identification using digital images. Biosyst Eng (2003) 85:449–54. doi: 10.1016/S1537-5110(03)00098-9
8. Blanco CA, Chiaravalle W, Dalla-Rizza M, Farias JR, Garcia-Degano MF, Gastaminza G, et al. Current situation of pests targeted by Bt crops in Latin America. Curr Opin Insect Sci (2016) 15:131–8. doi: 10.1016/j.cois.2016.04.012
9. Goergen G, Kumar PL, Sankung SB, Togola A, Tamò M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (JE Smith)(Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PloS One (2016) 11(10). doi: 10.1371/journal.pone.0165632
10. Nagoshi RN, Goergen G, Tounou KA, Agboka K, Koffi D, Meagher RL. Analysis of strain distribution, migratory potential, and invasion history of fall armyworm populations in northern Sub-Saharan Africa. Sci Rep (2018) 8 3710. doi: 10.1038/s41598-018-21954-1
12. Guo J, Jing D, Tai H, Zhang A, He K, Wang Z. Morphological characteristics of Spodoptera frugiperda in comparison with three other lepidopteran species with similar injury characteristics and morphology in cornfileds. Plant Prot (2019) 45:7–12.
13. Navasero MV, Navasero MM, Burgonio GAS, Ardez KP, Ebuenga MD, Beltran MJB, et al. Detection of the fall armyworm, Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae) using larval morphological characters, and observations on its current local distribution in the Philippines. (2019).
14. Perlak FJ, Oppenhuizen M, Gustafson K, Voth R, Sivasupramaniam S, Heering D, et al. Development and commercial use of Bollgard cotton in the USA–early promises versus today's reality. Plant J (2001) 27:489–501. doi: 10.1046/j.1365-313x.2001.01120.x
15. Shelton AM, Zhao JZ, Roush RT. Economic, ecological, food safety, and social consequences of the deployment of bt transgenic plants. Annu Rev Entomol (2002) 47:845–81. doi: 10.1146/annurev.ento.47.091201.145309
16. Tabashnik BE. Managing resistance with multiple pesticide tactics: theory, evidence, and recommendations. J Econ Entomol (1989) 82:1263–9. doi: 10.1093/jee/82.5.1263
17. Tabashnik BE, Finson N, Groeters FR, Moar WJ, Johnson MW, Luo K, et al. Reversal of resistance to Bacillus thuringiensis in Plutella xylostella. Proc Natl Acad Sci U.S.A (1994) 91:4120–4. doi: 10.1073/pnas.91.10.4120
18. Lewis WJ, van Lenteren JC, Phatak SC, Tumlinson JH 3rd. A total system approach to sustainable pest management. Proc Natl Acad Sci U.S.A (1997) 94:12243–8. doi: 10.1073/pnas.94.23.12243
19. Andow DA, Olson DM, Hellmich RL, Alstad DN, Hutchison WD. Frequency of resistance to Bacillus thuringiensis toxin Cry1Ab in an Iowa population of European corn borer (Lepidoptera : Crambidae). J Economic Entomology (2000) 93:26–30. doi: 10.1603/0022-0493-93.1.26
20. Kruger M, Van Rensburg JBJ, Van den Berg J. Perspective on the development of stem borer resistance to Bt maize and refuge compliance at the Vaalharts irrigation scheme in South Africa. Crop Prot (2009) 28:684–9. doi: 10.1016/j.cropro.2009.04.001
21. Carriere Y, Crowder DW, Tabashnik BE. Evolutionary ecology of insect adaptation to Bt crops. Evol Appl (2010) 3:561–73. doi: 10.1111/j.1752-4571.2010.00129.x
22. Matten SR, Head GP, Quemada HD. How governmental regulation can help or hinder the integration of Bt crops within IPM programs. In: Integration of Insect-Resistant Genetically Modified Crops within IPM Programs. Springer Netherlands. (2008). p. 27–39.
23. Luttrell R, Ali I, Allen K, Young S III, Szalanski A, Williams K, et al. Resistance to Bt in Arkansas populations of cotton bollworm. (2004).
24. Tabashnik BE, Van Rensburg JB, Carriere Y. Field-evolved insect resistance to Bt crops: definition, theory, and data. J Econ Entomol (2009) 102:2011–25. doi: 10.1603/029.102.0601
25. Bagla P. India. Hardy cotton-munching pests are latest blow to GM crops. Science (2010) 327:1439. doi: 10.1126/science.327.5972.1439
26. Tabashnik BE, Gassman AJ, Crowder DW, Carrière Y. Field-evolved resistance to Bt toxins. Nat Biotechnol (2008) 26:1074–6. doi: 10.1038/nbt1208-1383b
27. Agency., U.E.P. Biopesticides registration action document-Bacillus thuringiensis plant-incorporated protectants. (2001).
28. Siegfried BD, Spencer T, Crespo AL, Storer NP, Head GP, Owens ED, et al. Ten years of bt resistance monitoring in the european corn borer: What we know, what we don't know, and what we can do better. Am Entomologist (2007) 53:208–14. doi: 10.1093/ae/53.4.208
29. National Research Council. Pesticide resistance: strategies and tactics for management. Washington, DC: The National Academies Press (1986).
30. Vélez AM, Arango Isaza RE, Villanueva-Mejía DF, Aguilera G E, Saldamando-Benjumea CI. Identificación de biotipos de Spodoptera frugiperda (Lepidoptera: Noctuidae) mediante marcadores mitocondriales y nucleares. Rev Colombiana Entomología (2008) 34:145–50. doi: 10.25100/socolen.v34i2.9270
31. Zenner DPI, Maldonado HAA, Mejía R, Sánchez JLD. Spodoptera frugiperda: respuesta de distintas poblaciones a la toxina Cry1Ab. Rev Colomb Entomol (2009) 35:34–41. doi: 10.25100/socolen.v35i1.9186
32. Barrera G, Simón O, Villamizar L, Williams T, Caballero P. multiple nucleopolyhedrovirus as a potential biological insecticide: Genetic and phenotypic comparison of field isolates from Colombia. Biol Control (2011) 58:113–20. doi: 10.1016/j.biocontrol.2011.04.009
33. Jaramillo-Barrios CI, Varón-Devia EH, Monje-Andrade B. Economic injury level and action thresholds for Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae) in maize crops. Rev Facultad Nacional Agronomía Medellín (2020) 73:9065–76. doi: 10.15446/rfnam.v73n1.78824
34. Flores Hueso R, Hueso RF. Efecto de la variedad de maíz sobre el desarrollo y susceptibilidad de larvas de spodoptera frugiperda (lepidoptera: noctuidae) a bacillus thuringiensis. (2000).
35. Sauka DH, Benintende GB. Bacillus thuringiensis: generalidades. Un acercamiento a su empleo en el biocontrol de insectos. Rev Argent microbiología (2008) 40:124–40.
36. Niedmann Lolas L, Meza-Basso L. Evaluación de cepas nativas de Bacillus thuringiensis como una alternativa de manejo integrado de la polilla del tomate (Tuta absoluta Meyrick; Lepidoptera: Gelechiidae) en Chile. Agricultura Técnica (2006) 66:235–46.
37. Bernhard K, Jarrett P, Meadows M, Butt J, Ellis D, Roberts G, et al. Natural isolates ofBacillus thuringiensis: worldwide distribution, characterization, and activity against insect pests. J Invertebrate Pathol (1997) 70:59–68. doi: 10.1006/jipa.1997.4669
39. Hossain F, Pray CE, Lu Y, Huang J, Fan C, Hu R. Genetically modified cotton and farmers' health in China. Int J Occup Environ Health (2004) 10:296–303. doi: 10.1179/oeh.2004.10.3.296
40. Casmuz A, Juárez ML, Socías MG, Murúa MG, Prieto S, Medina S, et al. Revisión de los hospederos del gusano cogollero del maíz, Spodoptera frugiperda (Lepidoptera: Noctuidae). Rev la Sociedad Entomológica Argent (2010) 69:209–31.
41. ISAAA. Global status of commercialized Biotech/GM crops: 2016. In: ISAAA briefs 52. ISAAA, Ithaca, NY (2016).
42. AGRO-BIO. Cultivos transgénicos aumentan un 31% en 2021 (2022). Available online at: https://agrobio.org/noticias/transgenicos-en-Colombia-2021#:~:text=Este%20aumento%20del%2031%25%20da,contenedores%20ocasionada%20por%20la%20pandemia (Accessed 10 enero 2023).
43. Farias JR, Andow DA, Horikoshi RJ, Sorgatto RJ, dos Santos AC, Omoto C. Dominance of Cry1F resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae) on TC1507 Bt maize in Brazil. Pest Manag Sci (2016) 72:974–9. doi: 10.1002/ps.4077
44. Valencia-Cataño SJ, Rodríguez-Chalarca J, Blanco CA. Effect of genetically-modified cotton cultivars on demographic parameters of spodoptera frugiperda1 in Colombia. Southwestern Entomologist (2016) 41:963–70. doi: 10.3958/059.041.0426
45. Marçon P, Young LJ, Steffey KL, Siegfried B. Baseline susceptibility of european corn borer (Lepidoptera: crambidae) to bacillus thuringiensis toxins. J OF ECONOMIC ENTOMOLOGY (1999) 92:279–85. doi: 10.1093/jee/92.2.279
46. Siegfried BD, Spencer T, Nearman J. Baseline susceptibility of the corn earworm (Lepidoptera: Noctuidae) to the Cry1Ab toxin from Bacillus thuringiensis. J Econ Entomol (2000) 93:1265–8. doi: 10.1603/0022-0493-93.4.1265
47. Adamczyk JJ, Hardee D, Adams L, Sumerford D. Correlating differences in larval survival and development of bollworm (Lepidoptera: Noctuidae) and fall armyworm (Lepidoptera: Noctuidae) to differential expression of Cry1A (c) δ-endotoxin in various plant parts among commercial cultivars of transgenic Bacillus thuringiensis cotton. J Economic Entomology (2001) 94:284–90. doi: 10.1603/0022-0493-94.1.284
48. Jalali SK, Mohan KS, Singh SP, Manjunath TM, Lalitha Y. Baseline-susceptibility of the old-world bollworm, Helicoverpa armigera (Hubner) (Lepidoptera : Noctuidae) populations from India to Bacillus thuringiensis Cry1Ac insecticidal protein. Crop Prot (2004) 23:53–9. doi: 10.1016/s0261-2194(03)00170-4
49. Guzmán Prada DA, Rodríguez Chalarca J, Valencia Cataño SJ. Identification of Lepidoptera larval stages- a maize pest. CIAT Publication No. 473. International Center for Tropical Agriculture (CIAT), Cali, Colombia. 48. Available at: https://hdl.handle.net/10568/97857.
50. Rivero-Borja M, Rodríguez-Maciel J, Urzúa Gutiérrez J, Silva-Aguayo G, Chandrasena DI, Felix-Bermudez NC, et al. Baseline of susceptibility to the cry1F protein in Mexican Populations of fall armyworm. J economic entomology. (2019). doi: 10.1093/jee/toz280
51. Henderson CF, Tilton EW. Tests with acaricides against the brown wheat mite. J Economic Entomology (1955) 48:157–61. doi: 10.1093/jee/48.2.157
53. Crosariol Netto J. Efeitos de proteinas Cry e vip nos parâmetros biológicos de populações de Spodoptera frugiperda (Smith, 1797) e injúrias nas plantas de milho por Lepidópteros. Universidade Estadual Paulista (Unesp). (2017). Available at: http://hdl.handle.net/11449/148895.
54. Bernardi D, Bernardi O, Horikoshi RJ, Salmeron E, Okuma DM, Omoto C. Biological activity of Bt proteins expressed in different structures of transgenic corn against Spodoptera frugiperda. Ciec Rural (2016) 46:1019–24. doi: 10.1590/0103-8478cr20150047
55. Acharya B, Head GP, Price PA, Huang F. Fitness costs and inheritance of Cry2Ab2 resistance in Spodoptera frugiperda (JE Smith). J invertebrate Pathol (2017) 149:8–14. doi: 10.1016/j.jip.2017.06.014
56. Gomez VA, Villalba GE, Arias OR, Ramírez MB, Gaona EF. Toxicidad sobre Spodoptera frugiperda (Smith)(Lepidoptera: Noctuidae) de la proteína Bt expresada en hojas de diferentes eventos de maíz transgénico liberados en Paraguay. Rev la Sociedad Entomológica Argent (2017) 76:1–10. doi: 10.25085/rsea.761201
57. Botha AS, Erasmus A, du Plessis H, Van den Berg J. Efficacy of bt maize for control of spodoptera frugiperda (Lepidoptera: noctuidae) in South Africa. J Econ Entomol (2019) 112:1260–6. doi: 10.1093/jee/toz048
58. Tabashnik BE, Brevault T, Carriere Y. Insect resistance to Bt crops: lessons from the first billion acres. Nat Biotechnol (2013) 31:510–21. doi: 10.1038/nbt.2597
59. Valencia Cataño SJ, Rodríguez Chalarca J, Mesa Cobo NC. Efecto de variedades de algodón genéticamente modificadas sobre larvas de Spodoptera frugiperda Smith (Lepidoptera: Noctuidae). Acta Agronómica (2014) 63:1–10. doi: 10.15446/acag.v63n1.38356
Keywords: Bt corn, Cry1a.105, Cry2Ab2, Spodoptera frugiperda, growth inhibition
Citation: Rodriguez-Chalarca J, Valencia SJ, Rivas-Cano A, Santos-González F and Romero DP (2024) Impact of Bt corn expressing Bacillus thuringiensis Berliner insecticidal proteins on the growth and survival of Spodoptera frugiperda larvae in Colombia. Front. Insect Sci. 4:1268092. doi: 10.3389/finsc.2024.1268092
Received: 27 July 2023; Accepted: 30 January 2024;
Published: 23 February 2024.
Edited by:
Chrysantus Mbi Tanga, International Centre of Insect Physiology and Ecology (ICIPE), KenyaReviewed by:
Yueping He, Huazhong Agricultural University, ChinaXiaoming Xia, Shandong Agricultural University, China
Copyright © 2024 Rodriguez-Chalarca, Valencia, Rivas-Cano, Santos-González and Romero. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jairo Rodriguez-Chalarca, j.chalarca@cgiar.org
 Jairo Rodriguez-Chalarca
Jairo Rodriguez-Chalarca Sandra J. Valencia
Sandra J. Valencia Alejandra Rivas-Cano1,2
Alejandra Rivas-Cano1,2