Hosts and impacts of elongate hemlock scale (Hemiptera: Diaspididae): A critical review
- 1Northern Research Station, U.S. Department of Agriculture (USDA) Forest Service, St. Paul, MN, United States
- 2Plant Protection Division, Minnesota Department of Agriculture, St. Paul, MN, United States
- 3Department of Entomology, University of Minnesota, St. Paul, MN, United States
- 4Department of Forestry and Environmental Resources, North Carolina State University, Raleigh, NC, United States
- 5Northern Research Station, U.S. Department of Agriculture (USDA) Forest Service, East Lansing, MI, United States
Fiorinia externa Ferris, elongate hemlock scale, was inadvertently introduced to North America from Japan. This insect is particularly problematic on hemlock, Tsuga spp., though it has been reported in association with several other conifers. The evidence that other conifers might be hosts, capable of supporting growing populations of the insect, has not been previously reviewed. Our review confirms that F. externa is an oligophagous pest of members of Pinaceae. Although species of Cupressaceae and Taxaceae have been reported as hosts of F. externa, they seem unable to support population growth of this pest. Evidence of the tree-killing potential of the insect, even on suitable hosts, is remarkably scant. The degree of pest risk posed by F. externa with respect to tree mortality in areas beyond the geographic range of hemlock seems modest, but uncertain.
Introduction
Fiorinia externa Ferris, elongate hemlock scale, is an alien armored scale (Hemiptera: Diaspididae) in North America. It is a primary threat to hemlock, Tsuga spp. (Figure 1A), that also are imperiled by the hemlock wooly adelgid, Adelges tsugae (Annand), another invasive alien insect (1). Fiorinia externa was first detected in 1908 in Queens and New York, NY, USA in association with an unspecified Tsuga sp. and Japanese hemlock, likely Tsuga diversifolia (Maxm.) Mast., respectively (2). By 1958, the insect had spread from New York state to Connecticut, Maryland, Massachusetts, New Jersey, Ohio, and Pennsylvania (3, 4), with no additional states reporting infestation through 1965 (5). By 1980, F. externa occurred as far south as Georgia (6). At about this time, the insect was spreading at 4.8 km/year in Connecticut (7). The insect is now established also in Delaware, Kentucky, Maine, Michigan, New Hampshire, North Carolina, Rhode Island, South Carolina, Tennessee, Vermont, Virginia, and West Virginia, states with significant acreages of Canadian/eastern hemlock, Tsuga canadensis (L.) Carrière (8). This insect has spread more slowly across the United States than many other invasive forest insects (9, 10).

Figure 1 Elongate hemlock scale, Fiorinia externa, on the underside of eastern hemlock, Tsuga canadensis: (A) on the most current year of hemlock growth; and (B) closeup of adult females within tests. Both photographs by F.W. Ravlin, Department of Entomology, Michigan State University.
At the time F. externa was first detected in the United States, the insect was recognized as having been accidentally imported from Japan (2). In his description of the species, Ferris (11) noted: “It is with regret that this species is described as new, since it would seem very probable that it has previously been named from some other part of the world.” Yet, no earlier descriptions of the species have been found. Ironically, confirmed records of the species outside the United States were unknown until Takagi (12) first definitively recovered and reported F. externa from Honshu and Shikoku islands of Japan and characterized Japan as the “cradleland” for the species. Fiorinia externa also has been reported from Fujian, Guangdong, and Sichuan, China (13, 14). Whether the species is native or introduced there is unclear. Van Driesche et al. (15) speculated that reports of F. externa from China might be misidentifications, as infested trees were difficult to locate. Elsewhere globally, F. externa was reported from an “alpine house” in England (16) but was never considered to be established in the wild (17). Kosztarab (18) also listed this scale as being present in Canada without details on the basis for this report. Fiorinia externa has not established in Canada, but future spread from the United States into Canada is probable (D. Pureswaran, personal communication).
Continued spread of F. externa in the United States is a concern to regulatory agencies, Christmas tree growers, and foresters (19–22). Human-mediated spread of this insect has been detected in California, Wisconsin, Minnesota, Oregon, and Florida on cut host material, e.g., Christmas trees, garlands, and wreaths, but the insect is not known to be established in these states (19, 21, 23, 24). The movement of infested nursery stock also may contribute to spread (25). Successful spread is contingent, in part, on the ability of the insect to locate and colonize hosts in its new environment(s) (9, 26). Herein, we briefly review the bionomics of F. externa that are relevant to interactions with conifers and summarize multiple studies on the diversity and quality of conifers as hosts. We conclude with a commentary on other taxa that might be hosts and the potential impacts this insect might have beyond the geographic range of hemlock.
Bionomics
The biology of F. externa has been reviewed extensively elsewhere (e.g., 5, 27–30). The species has one to two generations annually with extensive overlap between generations due to a prolonged period of egg hatch (6, 28, 31, 32). Eggs and adult females are generally considered to be the primary overwintering stages. Adult females are pupillarial, having developed within the exuvium of the second instar (Figure 1B); the enclosure also is known as the test (33). Adults are dimorphic. Only males possess functional wings. The species reproduces sexually. Females mate shortly after completion of the third instar and contract to about one-third of their previous size within the test. Eggs are laid in two rows along the central mid-line within the space of the test. Because only adult males can fly, first instars (i.e., crawlers) are the dispersal stage for females, with movement over short distances achieved by walking and intermediate distances facilitated by wind (34). Colonization of isolated trees is happenstance, affected by the speed and direction of wind, the densities of crawlers on infested trees, and the proximity of source trees to uninfested trees (10). Longer dispersal distances might occur if crawlers could hitchhike on birds and mammals, but phoretic movement of F. externa has not yet been demonstrated.
Fiorinia externa is a piercing-sucking insect. This insect feeds on host plants through a feeding tube made from fused mandibular and maxillary stylets. A crawler creates a feeding site, typically on the underside of the newest needles and occasionally on the surface of developing cones (18), by scraping away the host epidermis with its forelegs and inserting the stylets between epidermal cells or into a stomate (5). The stylets remain inserted once feeding commences (5). The stylets penetrate mesophyll cells and allow the insect to extract photosynthates and other cell contents from the needles and to inject of phytotoxic saliva (29). For adult females, the stylet is 6-7 times the body length (5).
Host range
In the first official U.S. report of the species, Sasscer (2) noted that the insect, then identified as Fiorinia fiorinae japonica, had been collected on Tsuga spp. He also shared anecdotal reports of the scale on other Pinaceae: Veitch fir, Abies veitchi Lindley; an unspecified Pinus sp.; a Japanese five-needled pine, Pinus pentaphylla Mayr [=P. parviflora var. pentaphylla (Mayr) Henry, currently]; Japanese black pine, P. thunbergii Parl.; and southern Japanese hemlock, T. sieboldii Carrière. He included additional hosts among Podocarpaceae: Podocarpus elata [=P. elatus R. Br. Ex Endl., currently], P. chinensis (Roxb.) Wall ex J. Forbes, and P. nageia R. Br. Ex Mirbel [=Naegia nagi (Thunb.) O. Kuntze, currently]. However, Sasscer (2) likely conflated potential hosts for Fiorinia japonica Kuwana, now known to have several hosts within Podocarpaceae, with hosts for F. externa, which has no confirmed hosts in that plant family (27).
The current list of hosts for F. externa was derived primarily from reported associations of the insect with different plants. Frequently, the reports summarized observations of plants growing near infested Tsuga spp. in ornamental plantings including arboreta. Some observations simply recorded the presence of F. externa on a plant. In other cases, observations included F. externa densities on different plants but often with no information about the size of the colonizing population or the time since infestation. If true, differences in densities of F. externa on different plants would reflect differences in host quality with respect to survivorship, developmental, or reproductive rates or chance variation in the number of crawlers that colonized a tree over time. In rare cases (e.g., 35), potential host trees were artificially infested to standardize initial colonization densities, so that differences in scale densities over time likely reflect differences in suitability among trees.
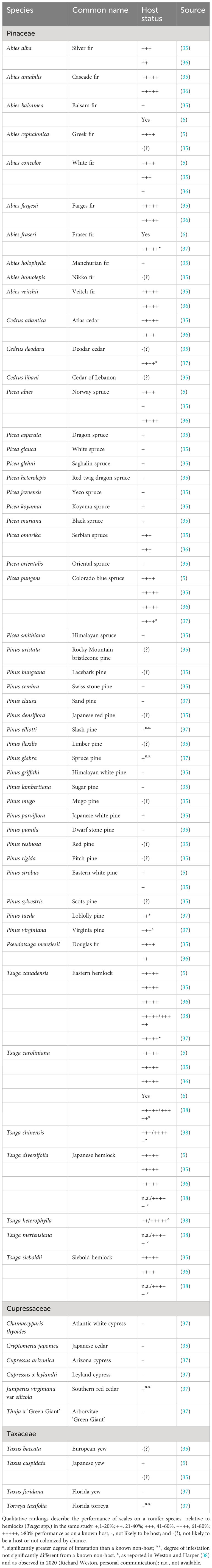
For this review, we assembled literature on potential hosts of F. externa published after Ferris’ (1942) description of the species (Table 1). Ferris (11) considered Tsuga spp. to be the primary hosts for F. externa. Thus, when possible, we compare the performance of F. externa on a plant relative to its performance on eastern/Canadian hemlock, Tsuga canadensis (L.) Carr. or Carolina hemlock, T. caroliniana Engelm. in the same study and location. ‘Performance’ is either the density of scales on individual trees (often expressed as scales/needle) or the proportion of trees that had been colonized. Qualitative ratings were assigned to facilitate comparisons among studies: ‘+++++,’ >80% performance as on a known host; ‘+++,’ ~40-60% performance as on a known host; ‘+,’ 1-20% performance as on a known host; and ‘-,’ not likely to be a host. In cases where non-host status may reflect chance escape from colonization, we use ‘-(?)’. Intermediate categories were applied as needed. Wallner (5) characterized F. externa densities as “heaviest”, “heavy”, and “light,” which we expressed as “+++++”, “++++”, and “+”, respectively. All studies in our review observed F. externa directly.
Pinaceae
Fiorinia externa is an oligophagous pest of Pinaceae, though not all species within this plant family are equally suitable. The insect is regularly characterized as a serious pest of hemlocks, especially eastern/Canadian hemlock, Japanese hemlock, and Carolina hemlock, particularly in ornamental plantings (3, 6, 31, 39–41). In Japan, the primary host appears to be southern Japanese hemlock, Tsuga sieboldii Carrière (12). In addition, early reports of F. externa from North America casually note its association with fir, Abies spp., and spruce, Picea spp., typically without reference to species (3, 39–41). Murakami (42) listed Sakhlin fir, Abies sachalinensis (F. Schmidt) Masters var ‘Mayriana’ as a host. Stimmel (6) observed F. externa on Fraser fir, Abies fraseri (Pursh) Poir. Williams (16) reported F. externa as persisting and likely reproducing on Korean fir, Abies koreana E.H. Wilson.
The highest quality hosts for F. externa include many hemlocks and firs (Table 1). Other high-quality hosts are Atlas cedar, Cedrus atlantica (Endl.) Manetti ex Carrière, Norway spruce, Picea abies (L) Karsten, and Colorado blue spruce, Picea pungens Engelmann. However, other spruces and cedars seem to be of substantially lower quality. Deodar cedar, Cedrus deodara (Roxb. ex D.Don) G.Don, and Douglas fir, Pseudotsuga menziesii (Mirbel) Franco, may be quality hosts for F. externa, but the performance of the insect on these hosts was highly variable among studies (Table 1). Pines, Pinus spp., tend to be intermediate- to low-quality hosts or non-hosts (Table 1).
Cupressaceae
Members of this family are unlikely to be reproductive hosts (i.e., plant species that support population growth of a specified herbivore) for F. externa. Tao (14) lists the arborvitae Biota orientalis (L.) Endl [=Platycladus orientalis (L.) Franco, currently]; Chinese juniper, Juniperus chinensis L., and needle juniper, Juniperus rigida Siebold & Zucc. as hosts for F. externa without further information. No other sources independently corroborate these host associations. In earlier studies, false cypress, Chamaecyparis sp.; Japanese cedar, Cryptomeria japonica (L) Don, and junipers, Juniperus spp., growing next to infested hemlocks were not colonized by F. externa and therefore not considered to be hosts (35). Similarly, after being artificially infested on plants, F. externa failed to persist on the arborvitae Thuja x ‘Green Giant’ (a hybrid of Thuja plicata x Thuja standishii); Arizona cypress ‘Blue Ice’, Cupressus arizonica Greene; Atlantic whitecedar, Chamaecyparis thyoides (L.) B.S. P.; Leyland cypress, Cupressus x leylandii A. B. Jacks. & Dallim.; or southern eastern red cedar, Juniperus virginiana var silicicola (Small) E. Murray (37). Fiorinia externa began to feed on all of these plants except Thuja x ‘Green Giant’ and Cupressus x leylandii; typically, <10% of trees in Cupressaceae - but 57% of J. virginiana var silicicola – had live scales at 19 weeks after infestation (37). By 50 weeks after infestation, no Cupressaceae had live scales, except J. virginiana var silicicola whose 3% frequency of infestation was equivalent to a known non-host (37). Members of Cupressaceae have been confirmed as hosts of the congeneric Fiorinia japonica in multiple studies (27).
Taxaceae
Members of this family are probably not reproductive hosts for F. externa. Yews, Taxus spp., were listed as (secondary) hosts in early publications about the species (3, 5, 30, 35, 39, 40). These reports typically noted the association of F. externa with the plant genus without mentioning species (3, 30, 39). Wallner (5) observed light densities of F. externa on Japanese yew, Taxus cuspidata Siebold et Zuccarini, compared to Tsuga spp. McClure and Fergione (35) documented that F. externa densities were less than one scale per 500 needles on European yew, Taxus baccata L, and Japanese yew. Dale et al. (37) recorded 90% of Florida torreya, Torreya taxifolia Arnott, and 80% of Florida yew, Taxus floridana Nuttall ex Chapman, were still infested at 19 weeks after artificial infestation; by 50 weeks, the frequency of infestation dropped to 3% and 0%, respectively, no different from known non-hosts.
Impact
Feeding by F. externa can cause decline-like symptoms such as needle yellowing, needle cast, branch dieback, stunted growth, and occasionally tree death (4, 5, 39, 41). Feeding likely reduces overall host vigor and may increase tree susceptibility to other abiotic and biotic stresses. Unlike hemlock woolly adelgid, feeding by F. externa has no measurable effect on the phytohormones abscisic acid or salicylic acid (43). The ability of the insect to vector plant pathogens is unknown. Dense infestations give foliage a “whitewashed appearance” from the waxy secretions from male scales (3, 6, 40).
The potential for F. externa to kill trees, even of highly suitable hemlocks, remains unclear. The progression of plant symptoms first was described for ornamental trees as no natural stands or plant nurseries were known to be infested at that time (reviewed in (5)). McClure (44) and McClure and Fergione (35) reaffirmed F. externa as a potential tree-killing insect and casually expanded the scope of potential impacts to include forest hemlocks. These anecdotal descriptions were sufficient for Miller et al. (45) to list F. externa as a “serious pest” of hemlock, arguably among the more threatening species of non-native scale insects in North America. However, the frequency of mortality among infested trees or the extent of mortality at stand scales had/has never been reported for this insect. (Note: Zahradnik (46) did not include F. externa among “the more important or interesting” scales that affect conifers.) More recently, Mech et al. (47) concluded that F. externa is capable, at worst, of killing “individual healthy plants” and, thus, did not consider it to be a high-impact species.
The severity of symptoms is partially related to the density of F. externa. Densities of F. externa are affected by soil texture and moisture (44) and are positively correlated with the nitrogen content of needles, both within and among host species (48). McClure and Fergione (35) posited that a density of >6 scales/100 needles was sufficient to cause substantial hemlock-needle discoloration and drop from the lower crown, but they did not provide the basis for this threshold. At low densities (<1 scale/needle), F. externa did not affect growth or foliar nitrogen content of T. canadensis, but at similar densities, hemlock woolly adelgid occasionally did (49). The intensity, beyond being “high”, or duration of infestation that might be necessary for mortality has not been specified. McClure (50) argued that F. externa could kill hemlocks within 10 years if left uncontrolled but provided no data. Johnson and Lyon (51) suggested that infested hosts may begin to recover (i.e., flush new needles) if scale densities decline, for example, from intraspecific competition.
More recent research suggests that F. externa rarely kills trees and may prove modestly beneficial to hemlocks in the presence of hemlock woolly adelgid. During the late 1990s and early 2000s, the geographic range and density of F. externa substantially increased on Massachusetts and Connecticut (USA) hemlock with little apparent impact to tree health; hemlock mortality correlated more strongly with densities of hemlock woolly adelgid than F. externa (52). When hemlocks in this area were artificially infested with both insect species, densities of each insect were reduced by ~30%, but lateral growth was greater on trees infested with both species than with hemlock woolly adelgid alone (53). Fiorinia externa alone had no measurable impact on branch growth (53) or tree ring formation (54). Subsequent research has demonstrated that hemlocks with F. externa have similar foliar chemistry and growth rates as uninfested trees, unlike trees infested with hemlock woolly adelgid (49). The early presence of F. externa substantially lowered settlement rates for hemlock woolly adelgid crawlers (55); crawlers avoided settling at the base of needles where F. externa was already established (56). Ultimately, Miller-Pierce and Preisser (55) characterized F. externa as “relatively innocuous” to hemlock health in comparison to hemlock woolly adelgid. Preliminary observations from Michigan are consistent with these trends as no relationship was observed between current-year hemlock growth and F. externa densities (20).
Discussion
Fiorinia externa is an oligophagous pest of trees in the Pinaceae. The phylogeny and evolutionary history of genera within this family (57) and patterns of host utilization by F. externa (Table 1) suggest other genera within Pinaceae might be heretofore unreported hosts, particularly Keteleeria, Nothotsuga, and Pseudolarix, all native to portions of eastern Asia. Further research into these potential host associations is warranted and might provide insights into the co-evolutionary history of F. externa with Pinaceae in Asia. Although the insect may attempt to feed on Cupressaceae or Taxaceae, F. externa appears unable to sustain populations on plants from these families.
Fiorinia externa, like other Diaspididae, is recognized as a parasite of its host plants (58). Females are incapable of directed long-distance movements, and directional movements are constrained by the walking capacity of crawlers, potentially <1 m (59). Further, once a crawler establishes a feeding site, it cannot retract its stylets to continue host searching (25). Thus, behavior plays little role in host choice for this species unlike many other oligophagous/polyphagous insects. The limited dispersal abilities of this insect put it under strong evolutionary pressure to attempt feeding on a potential host and not kill it until densities are high. Similarly, limited dispersal has contributed to the rapid micro-evolution of F. externa to different host species (60) and potentially to environmental tolerances (61). Demic adaptations to hosts are not so strong as to preclude colonization of new hosts (59).
The risk that F. externa poses to regions beyond the range of Tsuga spp. is highly uncertain. Pest risk, in this context, is a function of the likelihood that F. externa might invade a new area and the extent of damages it might cause once there. If introduced to new areas on cut foliage, the insect may face significant challenges to locate live host material and withstand local climatic conditions. The arrival of F. externa on propagative hosts would thus seem to be the riskier pathway. Despite being present in the United States for >100 years, the circumstances that might lead F. externa to kill otherwise healthy trees are only coarsely characterized. Widespread tree mortality from Fiorinia externa has never been documented, even on preferred hosts. Evidence from prior pest activity and sentinel trees (62) indicates that although future host mortality from F. externa is possible, extensive host mortality is unlikely.
Author contributions
RV: Writing – original draft, Writing – review & editing. AA: Writing – review & editing. BA: Writing – review & editing. RJ: Writing – review & editing. TP: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding was provided by the Northern Research Station of the U.S. Department of Agriculture, Forest Service for the publication of this article.
Acknowledgments
We thank Pheylan Anderson, Marie Hallinen, Julia Leone, Clarissa Moore, Deepa Pureswaran, and Richard Weston for discussions about the ecology of F. externa. We thank Kevin T. Smith, Elizabeth Larry, and the reviewers for constructive comments on early drafts of this paper. The findings and conclusions in this publication are those of the authors and should not be construed to represent any official USDA or U.S. Government determination or policy.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Preisser EL, Oten KLF, Hain FP. Hemlock wooly adelgid in the eastern United States: what have we learned? Southeastern Nat (2014) 13(Special Issue 6):1–15.
2. Sasscer ER. The genus Fiorinia in the United States. Technical series No. 16. Part V, Howard LO, editors. Washington, D.C: Government Printing Office (1912).
3. Davidson JA, McComb CW. Notes on the biology and control of Fiorinia externa Ferris. J Econ Entomol (1958) 51(3):405–6.
5. Wallner WE. Biology and control of Fiorinia hemlock scale Fiorinia externa Ferris. Ithaca, NY: Cornell University (1965).
6. Stimmel JF. Seasonal history and occurrence of Fiorinia externa in Pennsylvania (Homoptera: Diaspididae). Proc Entomol Soc Wash (1980) 82(4):700–6.
7. McClure MS. Controlling hemlock scales with least environmental impact. Bulletin 844. New Haven, CT: The Connecticut Agricultural Experiment Station (1987). 8 p.
8. USDA. Elongate hemlock scale, Fiorinia externa Ferris: Alien forest pest explorer - species map. USDA Forest Service, Northern Research Station and Forest Health Protection (2019). Available at: https://www.fs.usda.gov/nrs/tools/afpe/maps/pdf/EHS.pdf.
9. Hudgins EJ, Liebhold AM, Leung B. Predicting the spread of all invasive forest pests in the United States. Ecol Letters (2017) 20(4):426–35. doi: 10.1111/ele.12741
10. McClure MS. Importance of weather to the distribution and abundance of introduced adelgid and scale insects. Agric For Meteorol (1989) 47(2-4):291–302. doi: 10.1016/0168-1923(89)90101-9
11. Ferris GF. Atlas of scale insects of North America. Stanford, CA: Stanford University Press (1942).
12. Takagi S. Discovery of Fiorinia externa Ferris in Japan (Homoptera: Coccoidea). Insecta Matsumurana (1963) 26(2):115–7.
13. Wei J, Zhang B, Feng J. Two new species of Fiorinia Targioni-Tozzetti (Hemiptera: Coccoidea: Diaspididae) from China. Zootaxa (2013) 3641(1):092–100. doi: 10.11646/zootaxa.3641.1.10
14. Tao C-c. List of Coccoidea (Homoptera) of China. Wufeng, Taiching, Taiwan R.O.C: Taiwan Agricultural Research Institute Special Publication No. 78 (1999). 176 p.
15. Van Driesche R, Reardon R, Montgomery M, Cowles R, Abell K, Nunn C, et al. Classical biological control of the elongate hemlock scale, Fiorinia externa: 2004 activities. In: Onken B, Reardon R, editors. Third symposium on hemlock wooly adelgid in the eastern United States. Asheville, NC: U.S. Department of Agriculture, Forest Service, Forest Health Technology Enterprise Team (2005). p. 135–44.
16. Williams DJ. Fiorinia externa Ferris (Hemiptera: Diaspididae) found in Surrey infesting Abies koreana. Entomologist’s Gazette (1988) 39(2):151–2.
17. Malumphy C. First incursion of crown scale Fiorinia coronata Williams & Watson (Hemiptera: Diaspididae) in England, with a review of Fiorinia species detected in Britian and a key to their identification. Entomologist’s Gazette. (2013) 64:269–76.
18. Kosztarab M. Scale insects of northeastern North America: Identification, biology, and distribution. Martinsville, VA: Virginia Museum of Natural History (1996). 650 p.
19. Leathers J. California Pest Rating for Fiorinia externa Ferris: elongate hemlock scale (Hemiptera: Diaspididae) Pest Rating A. Sacramento, CA: California Department of Food and Agriculture (2016).
20. Petrice TR, Poland TM, Ravlin FW. Proceedings of the 7th North American Forest Insect Work Conference. Elongate hemlock scale in Michigan: initial assessment of distribution, impacts, and natural enemies. In: Arango RA, Pureswaran DS, editors. Proceedings of the 7th North American Forest Insect Work Conference; Virtual: U.S. Department of Agriculture, Forest Health Assessment and Applied Sciences Team (Washington, DC: U.S. Department of Agriculture, Forest Health Assessment and Applied Sciences Team) (2022). p. 95–7.
21. Ambourn A, Shimek S. Proceedings of the 7th North American Forest Insect Work Conference. The scale that stole Christmas. In: Arango RA, Pureswaran DS, editors. Proceedings of the 7th North American Forest Insect Work Conference; Virtual: U.S. Department of Agriculture, Forest Health Assessment and Applied Sciences Team (2022). p. 136–7.
22. Darr MN, Coyle DR, Jetton RM. Arthropod and disease management in Fraser fir (Pinales: Pinaceae) Christmas trees in the southeastern United States. Washington, D.C., J Integr Pest Manage (2022) 13(1):17. doi: 10.1093/jipm/pmac001
23. Stocks IC. Armored scale (Hemiptera: Diaspididae) pests on Abies fraseri (Pinaceae) Christmas trees imported into Florida. Florida Entomol (2016) 99(4):785–7. doi: 10.1653/024.099.0435
24. Ahmed MZ, Moore MR, Rohrig EA, McKenzie CL, Liu D, Feng J, et al. Taxonomic and identification review of adventive Fiorinia Targioni Tozzetti (Hemiptera, Coccomorpha, Diaspididae) of the United States. ZooKeys (2021) 1065:141–203. doi: 10.3897/zookeys.1065.69171
25. Beardsley JW, Gonzalez RH. Biology and ecology of armored scales. Annu Rev Entomol (1975) 20:47–73. doi: 10.1146/annurev.en.20.010175.000403
26. Koch FH. Considerations regarding species distribution models for forest insects. Agric For Entomol (2021) 23(4):393–9. doi: 10.1111/afe.12458
27. García Morales M, Denno BD, Miller DR, Miller GL, Ben-Dov Y, Hardy NB. ScaleNet: A literature-based model of scale insect biology and systematics (2016). Available at: http://scalenet.info.
28. Garrett WT, Langford GS. Seasonal life cycle of Fiorinia externa in Maryland. J Econ Entomol (1969) 62(5):1221–2. doi: 10.1093/jee/62.5.1221
29. McClure MS. Armored scale insects: Their biology, natural enemies and control (Volume A). Rosen D, editor. New York: Elsevier (1990).
30. Talerico RL, McComb CW, Garrett WT. Forest Pest Leaflet 107: Fiorinia externa Ferris, a scale insect of hemlock. Washington, DC: U.S. Department of Agriculture, Forest Service (1971).
31. McClure MS. Resurgence of scale, Fiorinia externa (Homoptera: Diaspididae), on hemlock following insecticide application. Environ Entomol (1977) 6(3):480–4. doi: 10.1093/ee/6.3.480
32. McClure MS. Population dynamics of Japanese hemlock scales: a comparison of endemic and exotic communities. Ecology (1986) 67(5):1411–21. doi: 10.2307/1938696
33. Stoetzel MB. Scale-cover formation in Diaspididae (Homoptera: Coccoidea). Proc Entomol Soc Wash (1976) 78(3):323–32.
34. McClure MS. Spatial and seasonal distribution of disseminating stages of Fiorinia externa (Homoptera, Diaspididae) and natural enemies in a hemlock forest. Environ Entomol (1979) 8(5):869–73. doi: 10.1093/ee/8.5.869
35. McClure MS, Fergione MB. Fiorinia externa and Tsugaspidiotus tsugae (Homoptera: Diaspididae): distribution, abundance, and new hosts of two destructive scale insects of eastern hemlock in Connecticut. Environ Entomol (1977) 6(6):807–11. doi: 10.1093/ee/6.6.807
36. McClure MS. Foliar nitrogen: a basis for host suitability for elongate hemlock scale, Fiorinia externa (Homoptera: Diaspididae). Ecology (1980) 61(1):72–9. doi: 10.2307/1937157
37. Dale AG, Birdsell T, Sidebottom J. Evaluating the invasive potential of an exotic scale insect associated with annual Christmas tree harvest and distribution in the southeastern U.S. Trees Forests People (2020) 2:100013. doi: 10.1016/j.tfp.2020.100013
38. Weston PA, Harper RW. Potential of Tsuga spp. from western North America and Asia as replacements for eastern hemlock (Tsuga canadensis). Arboricult Urban Forest (2009) 35(1):5–9.
39. Wallner WE. A field test with insecticides to control the scale Fiorinia externa on Canadian hemlock. J Econ Entomol (1962) 55(5):798–9. doi: 10.1093/jee/55.5.798a
40. Garrett WT, Langford GS. Control of Fiorinia externa on hemlock in Maryland. J Econ Entomol (1969) 62:1449–50. doi: 10.1093/jee/62.6.1449
41. Felt EP. Observations on shade tree insects. J Econ Entomol (1933) 26(1):45–51. doi: 10.1093/jee/26.1.45
42. Murakami Y. A review of biology and ecology of diaspine scales in Japan (Homoptera: Coccoidea). Mushi (1970) 43(25):65–114.
43. Schaeffer RN, Wang Z, Thornber CS, Preisser EL, Orians CM. Two invasive herbivores on a shared host: patterns and consequences of phytohormone induction. Oecologia (2018) 186:973–82. doi: 10.1007/s00442-018-4063-0
44. McClure MS. Dispersal of the scale Fiorinia externa (Homoptera: Diaspididae) and effects of edaphic factors on its establishment on hemlock. Environ Entomol (1977) 6(4):539–44. doi: 10.1093/ee/6.4.539
45. Miller DR, Miller GL, Hodges GS, Davidson JA. Introduced scale insects (Hemiptera: Coccoidea) of the United States and their impact on U.S. agriculture. Proc Entomol Soc Wash (2005) 107(1):123–59.
46. Zahradnik J. Forests. In: Rosen D, editor. Armored scale insects: Their biology, natural enemies and control. Volume B. New York: Elsevier (1990). p. 633–7.
47. Mech AM, Thomas KA, Marsico TD, Herms DA, Allen CR, Ayres MP, et al. Evolutionary history predicts high-impact invasions by herbivorous insects. Ecol Evol (2019) 9(21):12216–30. doi: 10.1002/ece3.5709
48. McClure MS. Competition between herbivores and increased resource heterogeneity. In: Denno RF, McClure MS, editors. Variable plants and herbivores in natural and managed systems. New York: Academic Press (1983). p. 125–53.
49. Miller-Pierce MR, Orwig DA, Preisser E. Effects of hemlock woolly adelgid and elongate hemlock scale on eastern hemlock growth and foliar chemistry. Environ Entomol (2010) 39(2):513–9. doi: 10.1603/EN09298
50. McClure MS. The elongate hemlock scale, Fiorinia externa Ferris (Homoptera: Diaspididae): a new look at an old nemesis. In: Oken B,RR, Lashomb J, editors. Symposium on the hemlock woolly adegid in eastern North America. East Brunswick, NJ: New Jersey Agricultural Experiment Station, Rutgers (2002). p. 248–53.
51. Johnson WT, Lyon HH. Insects that feed on trees and shrubs. Ithaca, NY: Cornell University Press (1991). 560 p.
52. Preisser EL, Lodge AG, Orwig DA, Elkinton JS. Range expansion and population dynamics of co-occurring invasive herbivores. Biol Invasions (2008) 10(2):201–13. doi: 10.1007/s10530-007-9123-z
53. Preisser E, Elkinton JS. Exploitative competition between invasive herbivores benefits a native host plant. Ecology (2008) 89(10):2671–7. doi: 10.1890/08-0299.1
54. Gonda-King L, Radville L, Preisser EL. False ring formation in eastern hemlock branches: impacts of hemlock woolly adelgid and elongate hemlock scale. Environ Entomol (2012) 41(3):523–31. doi: 10.1603/EN11227
55. Miller-Pierce MR, Preisser EL. Asymmetric priority effects influence the success of invasive forest insects. Ecol Entomol (2012) 37(5):350–8. doi: 10.1111/j.1365-2311.2012.01371.x
56. Gomez S, Gonda-King L, Orians CM, Preisser EL. Competitor avoidance drives within-host feeding site selection in a passively dispersed herbivore. Ecol Entomol (2014) 39(1):10–6. doi: 10.1111/een.12059
57. Ran JH, Shen TT, Wu H, Gong X, Wang XQ. Phylogeny and evolutionary history of Pinaceae updated by transcriptomic analysis. Mol Phylogenet Evol (2018) 129:106–16. doi: 10.1016/j.ympev.2018.08.011
58. Morse GE, Normark BB. A molecular phylogenetic study of armoured scale insects (Hemiptera: Diaspididae). Syst Entomol (2006) 31(2):338–49. doi: 10.1111/j.1365-3113.2005.00316.x
59. Hanks LM, Denno RF. The role of demic adapatation in colonization and spread of scale insect populations. In: Kim KC, McPheron BA, editors. Evolution of insect pests: Patterns of variation. New York: John Wiley & Sons (1993). p. 393–412.
60. McClure MS. Reproduction and adpations of exotic hemlock scales (Homptera: Diaspididae) on their new and native hosts. Environ Entomol (1983) 12:1811–5. doi: 10.1093/ee/12.6.1811
61. Preisser EL, Elkinton JS, Abell K. Evolution of increased cold tolerance during range expansion of the elongate hemlock scale Fiorinia externa Ferris (Hemiptera: Diaspididae). Ecol Entomol (2008) 33(6):709–15. doi: 10.1111/j.1365-2311.2008.01021.x
Keywords: invasive alien species, Tsuga, Abies, plant-insect interactions, forest health
Citation: Venette RC, Ambourn A, Aukema BH, Jetton RM and Petrice TR (2024) Hosts and impacts of elongate hemlock scale (Hemiptera: Diaspididae): A critical review. Front. Insect Sci. 4:1356036. doi: 10.3389/finsc.2024.1356036
Received: 14 December 2023; Accepted: 22 January 2024;
Published: 12 February 2024.
Edited by:
Leonardo Marianelli, Council for Agricultural and Economics Research (CREA), ItalyReviewed by:
Francesco Paoli, Centro di Ricerca Difesa e Sperimentazione (CREA-DC), ItalyCopyright © 2024 Venette, Ambourn, Aukema, Jetton and Petrice. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert C. Venette, Robert.C.Venette@usda.gov
†These authors have contributed equally to this work
 Robert C. Venette
Robert C. Venette Angie Ambourn2†
Angie Ambourn2†  Brian H. Aukema
Brian H. Aukema Robert M. Jetton
Robert M. Jetton Toby R. Petrice
Toby R. Petrice