The Influence of CO2 Enrichment on Net Photosynthesis of Seagrass Zostera marina in a Brackish Water Environment
- Department of Marine Biology, Estonian Marine Institute, University of Tartu, Tallinn, Estonia
Seagrasses are distributed across the globe and their communities may play key roles in the coastal ecosystems. Seagrass meadows are expected to benefit from the increased carbon availability which might be used in photosynthesis in a future high CO2 world. The main aim of this study was to examine the effect of elevated pCO2 on the net photosynthesis of seagrass Zostera marina in a brackish water environment. The short-term mesocosm experiments were conducted in Kõiguste Bay (northern part of Gulf of Riga, the Baltic Sea) in June–July 2013 and 2014. As the levels of pCO2 naturally range from ca. 150 μatm to well above 1000 μatm under summer conditions in Kõiguste Bay we chose to operate in mesocosms with the pCO2 levels of ca. 2000, ca. 1000, and ca. 200 μatm. Additionally, in 2014 the photosynthesis of Z. marina was measured outside of the mesocosm in the natural conditions. In the shallow coastal Baltic Sea seagrass Z. marina lives in a highly variable environment due to seasonality and rapid changes in meteorological conditions. This was demonstrated by the remarkable differences in water temperatures between experimental years of ca. 8°C. Thus, the current study also investigated the effect of elevated pCO2 in combination with short-term natural fluctuations of environmental factors, i.e., temperature and PAR on the photosynthesis of Z. marina. Our results show that elevated pCO2 alone did not enhance the photosynthesis of the seagrass. The photosynthetic response of Z. marina to CO2 enrichment was affected by changes in water temperature and light availability.
Introduction
Over the last 20 years, it has been established that the pH of the world's oceans is decreasing which is caused primarily by the uptake of carbon dioxide (CO2) from the atmosphere, also known as ocean acidification (OA). Since the start of the Industrial Revolution in the late eighteenth century, atmospheric carbon dioxide concentration has increased by ~40% primarily due to the burning of fossil fuels (Raven et al., 2005). The surface-ocean has absorbed ~30% of anthropogenic CO2 released from the atmosphere, which causes fundamental changes in seawater carbonate chemistry (Sabine et al., 2004). The mean surface ocean pH has already fallen by ~0.1 units and if global emissions of CO2 continue to rise, the pH may decrease 0.3–0.4 units by the year 2100 (IPCC, 2013).
The projected range of pH changes are not uniform across the globe and there are regional differences. Models project that the surface water pH in the central Baltic Sea may decrease 0.3–0.4 units by 2100 (Omstedt et al., 2012; Schneider et al., 2015). Moreover, the brackish Baltic Sea is sensitive to a future increase in acidity due to low carbonate buffering capacity, which is related to its low salinity, particularly in the northern parts (Hjalmarsson et al., 2008; Omstedt et al., 2015). However, Hjalmarsson et al. (2008) found high alkalinity in the Gulf of Riga caused by extensive inputs of freshwater from the river runoff. In contrast to the open sea, the shallow coastal waters of the Baltic Sea are characterized by high temporal (diurnal and seasonal) and spatial variation of environmental factors (Feistel et al., 2008). Besides light and water temperature, pH and partial pressure of carbon dioxide (pCO2) show a substantial amplitude of natural variability, especially under summer conditions. These daily pH changes may be of a larger magnitude than the scenario modeling suggests for the surface-water pH decrease in the Baltic Sea by the end of this century (personal measurements, 2013, 2014). However, biological responses to the elevated water CO2 concentrations in combination with these short-term natural fluctuations of environmental factors have generally received little attention.
Seagrass communities play a key role in the coastal ecosystems. They are important primary producers on soft bottoms (Larkum et al., 2006), contribute to sediment stabilization (Duarte, 2002; Orth et al., 2006), carbon sequestration (Duarte et al., 2005), cycling of nutrients and providing shelter and food for the coastal food webs (Duarte, 2002). Seagrasses are distributed across the globe and there are ~60 seagrass species (Orth et al., 2006). Unfortunately, seagrass communities are globally in serious decline (Orth et al., 2006; Waycott et al., 2009) mainly due to eutrophication, habitat destruction and climate change (Duarte, 2002).
Seagrass meadows are expected to be influenced by the increase in seawater acidity in the future. However, the response of seagrasses to elevated pCO2 can vary depending on the seagrass species as well as the habitat (Koch et al., 2013 and references therein). Besides, observed seagrass responses to CO2 enrichment can be affected by the methods used. Numerous studies have been conducted near natural CO2 vents, which allow assessment of the long-term effects of ocean acidification (Hall-Spencer et al., 2008; Fabricius et al., 2011; Porzio et al., 2011; Russell et al., 2013; Takahashi et al., 2015), although it is not always clear in these studies whether observed responses are exclusively driven by changes in CO2 concentration. There are no underwater CO2 vents in the Baltic Sea, so we used mesocosms to investigate the effects of OA in the field.
This study focuses on the seagrass Zostera marina, which is a widely distributed seagrass of the temperate coastal waters in the northern hemisphere (den Hartog, 1970; Moore and Short, 2006). Seagrass Z. marina is one of the most common macrophytes on the species-poor sandy bottoms and is regarded as a key species of this habitat found throughout the Baltic Sea. These plants provide a secondary substrate for a diverse epiflora and fauna (Boström et al., 2014 and references therein). The majority of investigations on the distribution of Z. marina have shown a significant decline of their meadows mainly due to eutrophication in Danish, Swedish, and Polish coastal ecosystems (Boström et al., 2014 and references therein). However, in the Estonian coastal waters (NE Baltic Sea), the effect of eutrophication on their communities is less obvious compared to the other parts of the Baltic Sea (Möller and Martin, 2007).
While the effects of eutrophication on seagrass meadows in the Baltic Sea are well documented, the impacts of the increasing water CO2 concentrations on Z. marina in the Baltic Sea are still mainly unknown. Previous research has shown substantial effect of warming and small positive effect of elevated CO2 on the growth of Z. marina from the Kattegat region of the Baltic (Eklöf et al., 2012). Outside of the Baltic Sea, there are several studies describing the effects of elevated pCO2 on the temperate seagrass species Z. marina. These studies have shown increased growth rate, enhanced photosynthesis, increased reproduction and below-ground biomass of Z. marina under elevated pCO2 (Thom, 1996; Zimmerman et al., 1997; Palacios and Zimmerman, 2007). Brodie et al. (2014) pointed out that Z. marina is likely to thrive at high latitudes although the habitat will be altered considerably due to carbonate undersaturation.
The main goal of this study was to examine the effect of elevated pCO2 on the net photosynthesis of seagrass Z. marina in the brackish water conditions. The second goal of this study was to determine whether elevated pCO2 in combination with short-term natural fluctuations of environmental factors exerts interactive effects on the photosynthetic rate of Z. marina. Our hypotheses were that the photosynthetic rate of Z. marina increases with the elevated pCO2 levels and that the response would vary depending on the surrounding meteorological conditions. We tested these hypotheses in situ in short-term mesocosm experiments during two different experimental years under summer conditions in the north-eastern Baltic Sea.
Materials and Methods
Collection of Seagrass Zostera marina
The specimens of Zostera marina were collected by SCUBA diving at a depth of 3.0 m from Küdema Bay (58.533°N, 22.238°E) in June 2013 and in June 2014. Whole shoots were carefully removed from the sandy sediment to keep shoots with connected bundles and horizontal rhizomes intact. All specimens were placed in coolers containing water collected at the site and transported to the experimental site immediately.
Experimental Design
The mesocosm experiments were conducted in the shallow semi-enclosed Kõiguste Bay, Gulf of Riga, northern Baltic Sea (58.371°N, 22.980°E). The study area is affected by nutrient inputs from the moderately eutrophic brackish Gulf of Riga (Astok et al., 1999; Kotta et al., 2008).
The experimental design used in the current study has been previously used in a number of studies targeting different macrophyte species (e.g., Pajusalu et al., 2013, 2015, 2016). The mesocosm experiments were conducted during two experimental periods in 2013 and 2014 (18 July 2013–27 July 2013 and 16 July 2014–26 July 2014). The experimental design was identical during both years of incubation. Approximately 20 Z. marina specimens were transplanted to each plastic pot containing the mixture of fine gravel and sand (roughly 20:80). In the both experimental years, the plant material was acclimatized at the experimental site for 3 weeks prior the start of the net photosynthesis measurements. Four plastic pots with ca. 80 individual Z. marina specimens were incubated in each mesocosm with manipulated pCO2 levels. In 2014 also four plastic pots were placed outside of the mesocosm in the sea. Open plastic bags (double wall of LDPE foil, 175 μm each) externally supported by metal frames were used as mesocosms. The bags were floating in the sea, fixed to the bottom by anchors at a depth of 0.8 m. The bags were open on the top, so there was free gas exchange with the atmosphere, but not with the surrounding water. Three plastic bag mesocosms, each with dimensions of 1.2 × 1.0 × 1.5 m, and a volume of 400 l were set up: two mesocosms with elevated pCO2 levels ca. 1000 and 2000 μatm and one with the untreated level of ca. 200 μatm (control treatment). The levels of pCO2 in the control treatment was ca. 200 μatm during the photosynthesis measurements. The pCO2 level of 2000 μatm is much higher than the recommended maximum pCO2 level of 1000 μatm predicted by 2100 for seawater (Barry et al., 2010). In our experiment, the high target pCO2 level ca. 2000 μatm was chosen because natural values of pCO2 in Kõiguste Bay were measured prior to the start of the mesocosm experiments, which turned out to be well above the concentration of 1000 μatm in the summer mornings. Water from the sea area adjacent to the mesocosms incubation site was sieved using a 0.25 mm mesh and used for mesocosms. Food grade pure carbon dioxide from CO2 tanks was slowly bubbled into the water in the mesocosms. A custom-made controller was used to maintain elevated pCO2 levels in the treatments. The controller switched on or off the CO2 supply and treatment according to the CO2 level measured by underwater (sensor) automatic CO2 data logger (CONTROS DETECT 2.0, Germany). However, due to the response lag of the used CO2 sensor (15–20 min), the actual CO2 level oscillated around the lower level present by the controller. The pHNBS (National Bureau of Standards scale) values of each treatment were measured during experiments. The measurements of photosynthesis were carried out between 10 a.m. and 4 p.m.
Environmental Variables
In the mesocosm experiments water temperature, oxygen saturation, pHNBS, and salinity were measured continuously using a YSI 6600V2 environmental multiprobe (pH electrode YSI 6589FR). Measurements were performed during a full 24-h cycle with a frequency of 30 s. The irradiance at the incubation depths was measured as photosynthetically active radiation (PAR) using a spherical light sensor (Alec Electronics Co Ltd.). Additionally, in parallel with the photosynthesis measurements, the diurnal fluctuations of water pH, pCO2, and oxygen saturation were measured outside the mesocosms at a depth of 0.8 m in the natural shallow water macroalgal habitat. Water samples were taken from the experimental site daily and frozen immediately until further laboratory analyses. Nutrient concentrations: total nitrogen (TN), total phosphorus (TP), phosphates (P-PO4), and nitrites + nitrates (N-NOx) were measured in a laboratory with a continuous flow automated wet chemistry analyzer Skalar SANplus (Skalar Analytic B.V., De Breda, The Netherlands) using the standard methods EN ISO 11905-11, EN ISO 15681-22, and EN ISO 133953.
Measurements of Net Photosynthesis
The photosynthetic rate of Z. marina leaves at different pCO2 levels (mesocosms) was measured once a day. For this procedure about 0.1 g (dry weight, dw) of plant material (one leaf) was incubated in 600 ml glass bottles. The glass bottles were filled with water from the inside of the mesocosm and placed horizontally on the transparent trays hanging at a depth of 0.5 m. All incubations with Z. marina were performed in six replicates per treatment. Bottles without plant material (in triplicate per treatment) served as controls. The dw of the plant material was determined after drying at 60°C for 48 h. Net primary productivity (NPP) (given as mg O2 gdw−1 h−1) was calculated from the differences in oxygen concentrations in incubation bottles with and without plant material, measured over the incubation period (ca. 1 h) (Paalme, 2005). The dissolved oxygen concentrations were measured with a Marvet Junior dissolved oxygen meter (MJ2000, Elke Sensor, Estonia) using the standard method EN ISO 58144.
Statistical Analyses
A one-factor permutational multivariate analysis of variance (PERMANOVA) with 9999 permutations was used to statistically test single and interactive treatment effects on the net photosynthetic rates of Z. marina: pCO2 was used as the fixed factor with 3 levels in 2013 and 4 levels in 2014; photosynthetic radiation (PAR) and water temperature were treated as covariates. Significant effects were explored when necessary with pairwise post-hoc tests (with 9999 permutations). Statistical analyses were performed using PERMANOVA (PRIMER, PRIMER-E Ltd., Plymouth, UK). For all statistical tests, a probability of 0.05 was used to determine statistical significance.
Results
Environmental Variables
The average values of PAR during the experimental period were 744 ± 248.09 μmol m−2s−1 (± standard deviation, SD) in 2013 and 642 ± 179.13 μmol m−2s−1 in 2014. Remarkable differences in water temperature between the two experimental periods were found: the average water temperature at the experimental site was 13.5 ± 2.56°C in 2013 and 21.9 ± 1.45°C in 2014 (Tables 1, 2). The average salinity measured during the experimental periods was 5.7 ± 0 PSU in 2013 and 5.7 ± 0 PSU in 2014 (Tables 1, 2). Carbonate parameters pCO2 and pHNBS in mesocosms with different pCO2 levels in 2013 and 2014 are presented in Tables 1, 2. Data on nutrient concentrations of the sampling sites for different experimental periods are presented in Table 3.
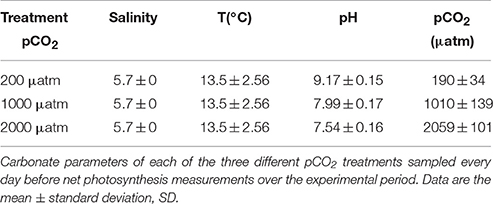
Table 1. Environmental variables (salinity and temperature) and carbonate parameters pCO2 (n = 10) and pHNBS (n = 10) in different treatments measured during the experimental period in 2013.
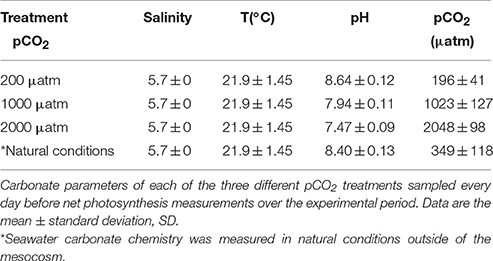
Table 2. Environmental variables (salinity and temperature) and carbonate parameters pCO2 (n = 11) and pHNBS (n = 11) in different treatments measured during the experimental period in 2014.
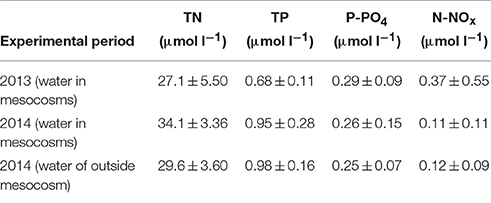
Table 3. Total nitrogen (TN), total phosphorus (TP), phosphates (P-PO4), and nitrites + nitrates (N-NOx) content (mean of the experimental period ± standard deviation, SD) of the water in mesocosm 2013 (n = 30), 2014 (n = 33) and water of outside mesocosm 2014 (n = 11).
Net Photosynthetic Rate of Zostera marina at Different pCO2 Levels
The PERMANOVA analyses (conducted separately with the data obtained in 2013 and 2014) showed that the photosynthetic rate of the Z. marina varied significantly between different pCO2 levels (Figure 1; Tables 4, 5; PERMANOVA: p < 0.05). In 2013 the variations of NPP rates were also affected by one of the tested environmental variables, namely water temperature, while the effect of the PAR was dependent on the level of pCO2 and water temperature (Table 4; PERMANOVA: p < 0.05). The net photosynthetic rates of Z. marina increased with increasing water temperature. In 2014 the NPP rates of Z. marina were affected by interactions of the PAR with water temperature and with the level of pCO2 (Table 5; PERMANOVA: p < 0.05). The results of the NPP measurements in mesocosm experiments are visualized in Figures 2, 3.
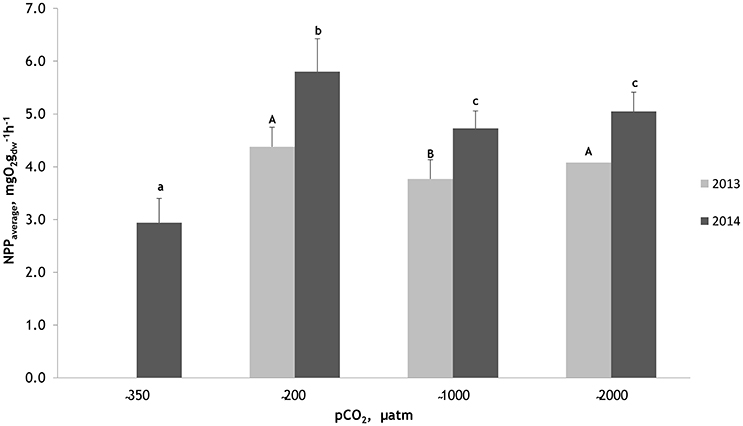
Figure 1. Mean net photosynthetic rates measured during two experimental periods (18 July 2013–27 July 2013 and 16 July 2014–26 July 2014) for seagrass Zostera marina at different water pCO2 levels. Within experimental periods, same uppercase letters in 2013 and lowercase letters in 2014 indicate that net photosynthetic rates are not significantly different from one another (PERMANOVA pair-wise test for factor CO2). Bars represent mean ± deviation (in mesocosm 2013 (n = 30), 2014 (n = 33) and in natural conditions 2014 (n = 11).
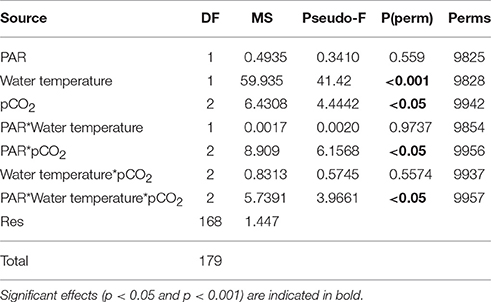
Table 4. Results of PERMANOVA analysis on the separate and interactive effects of pCO2, PAR, and water temperature on the net primary production rate of Zostera marina in 2013.
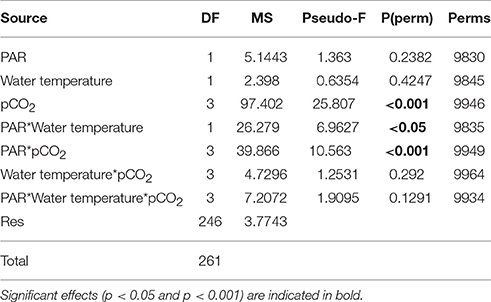
Table 5. Results of PERMANOVA analysis on the separate and interactive effects of pCO2, PAR, and water temperature on the net primary production rate of Zostera marina in 2014.
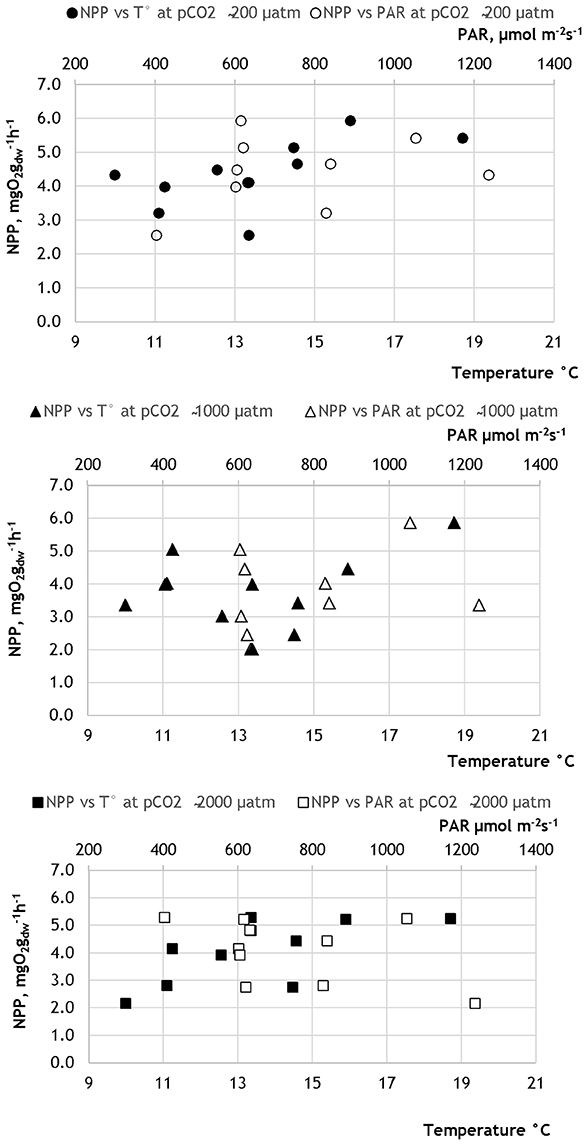
Figure 2. Net photosynthesis rates (NPP) plotted against water temperature (T°) and photosynthetically active radiation (PAR) at different pCO2 levels in 2013.
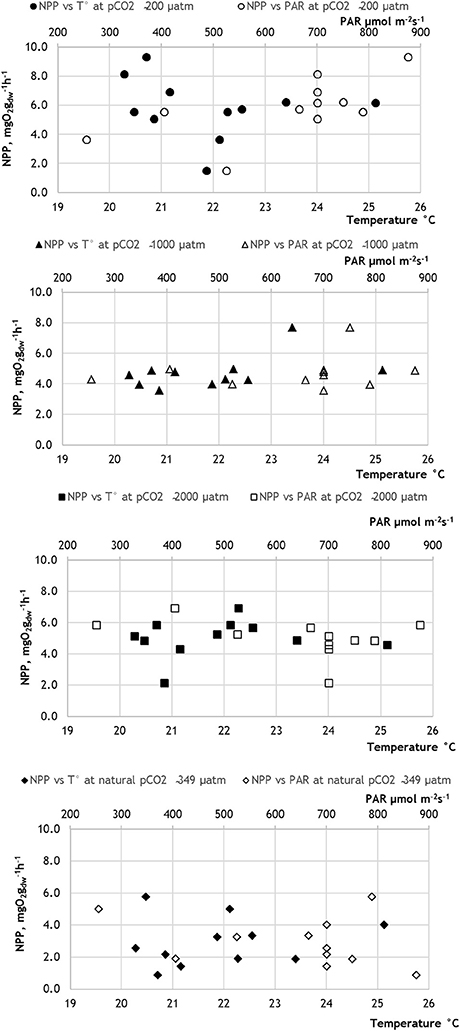
Figure 3. Net photosynthesis rates (NPP) plotted against water temperature (T°) and photosynthetically active radiation (PAR) at different pCO2 levels in 2014.
There were remarkable differences in the NPP rates of Z. marina between the two experimental periods: the average NPP rate was 4.08 mg O2 gdw−1 h−1 in 2013 and 5.19 O2 gdw−1 h−1 in 2014 (Figure 1). However, in both experimental periods, the NPP rates showed similar response patterns to CO2 enrichment in mesocosm experiment. The highest NPP rates for Z. marina were measured in the untreated water (control conditions), while at the intermediate pCO2 level the seagrass had lower rates than at the high pCO2 levels (Figure 1). Based on a PERMANOVA pairwise post-hoc test, the differences in the average NPP rates of Z. marina at the pCO2 levels between 1000 and 2000 μatm were significant (p < 0.01) in 2013. At the same time, NPP rates of Z. marina at the pCO2 levels between 200 and 2000 μatm were not significant, while at the pCO2 level of 200 μatm a significantly higher average NPP rate was measured compared to the elevated pCO2 level of 1000 μatm (p < 0.01) in 2013 (Figure 1). In 2014 the differences in the NPP rates of Z. marina at the pCO2 levels of 1000 and 2000 μatm were not significant but at the pCO2 level of 200 μatm a significantly higher average photosynthetic rate compared to elevated pCO2 levels was measured (PERMANOVA pairwise post-hoc test: p < 0.05; Figure 1). In the natural conditions outside of mesocosm the average photosynthetic rates of Z. marina were significantly lower (PERMANOVA pairwise post-hoc test: p = 0.0001) compared to the NPP values measured in mesocosms in 2014. At the pCO2 levels of 200 μatm (mesocosm control conditions), the average NPP values of Z. marina were 4.38 mg O2 gdw−1 h−1 in 2013 and 5.80 mg O2 gdw−1 h−1 in 2014. At the high pCO2 levels of 2000 μatm the average NPP value was 4.08 mg O2 gdw−1 h−1 in 2013 and 5.05 mg O2 gdw−1 h−1 in 2014, while at the intermediate pCO2 level the average NPP value was 3.77 mg O2 gdw−1 h−1 in 2013 and 4.73 mg O2 gdw−1 h−1 in 2014. The lowest average NPP rate for Z. marina was measured under natural conditions outside of the mesocosm: 2.94 mg O2 gdw−1 h−1 in 2014 (Figure 1).
Discussion
The vast majority of studies have shown that photosynthesis of seagrasses increases with the elevated pCO2 (e.g., Thom, 1996; Zimmerman et al., 1997; Invers et al., 2001; Ow et al., 2015). However, this effect is highly species specific (e.g., Invers et al., 1997, 2001; Ow et al., 2015). For example, Invers et al. (1997) investigated the effect of pH on photosynthesis in the three species of seagrasses: Posidonia oceanica, Cymodocea nodosa, and Zostera noltii in the laboratory conditions. Seagrasses P. oceanica and C. nodosa demonstrated a decrease in photosynthetic rates with an increase of pH and showed a significant reduction in NPP rates at pH 8.8. At the same time, Z. noltii showed high photosynthetic rates up to pH 8.8 and significant reduction in NPP rates only at pH 9.0. On the other hand, several studies have found unaltered response in the growth rate of seagrasses under long-term CO2 enrichment (e.g., Palacios and Zimmerman, 2007; Campbell and Fourqurean, 2013). We hypothesized that in our mesocosm experiments the net photosynthetic rates of Zostera marina would increase with the elevated pCO2 levels. In our mesocosm experiments the highest NPP rate of Z. marina was measured in the untreated water (control conditions) compared to the average values of elevated pCO2 levels in both experimental periods. Cox et al. (2016) investigated the effect of ocean acidification on the seagrass P. oceanica meadows under controlled CO2/pH conditions using a Free Ocean Carbon Dioxide Enrichment (FOCE) system. They found no effect of elevated pCO2 on the photosynthesis of Mediterranean seagrass P. oceanica from the two enclosures, neither pH manipulated nor control.
However, in 2014 our results showed that the NPP rates of Z. marina were significantly lower in the natural conditions outside of the mesocosm as compared to the results from the mesocosm experiment (elevated water pCO2 levels as well as the untreated water). There might be several explanations for that since in natural conditions several stress factors might be affecting the condition of the plants which are eliminated in the mesocosm, e.g., water movement or presence of suspended organic particles consuming part of the oxygen production in an incubation bottle during NPP measurement.
In the shallow coastal Kõiguste Bay, pH, and pCO2 show a substantial amplitude of natural variability under summer conditions. In the early morning pCO2 values were extremely high, being well above the concentration of 1000 μatm in the natural conditions outside of the mesocosm. But during the daytime, when inorganic carbon was used for photosynthesis, pCO2 declined to ~150 μatm. In addition, fluctuation of pH between 8 and 9 are common in shallow water macroalgal habitats under summer conditions. There might be remarkable differences in pH and pCO2 changes between different days. These natural fluctuations of the environmental factors in the shallow coastal waters are driven by direct effects of photosynthesis (increasing pH), respiration (lowering pH) and meteorological conditions. Additionally, in the mesocosm control treatment, during the photosynthesis measurements the average pCO2 was ca. 200 μatm. At the same time (hour) the average pCO2 was ca. 350 μatm in the natural conditions outside of the mesocosm. So, considering above-mentioned natural fluctuations of pCO2, we chose to operate in mesocosms with the pCO2 levels of ca. 2000, ca. 1000, and control ca. 200 μatm. Similarly, Paul et al. (2015) have used the pCO2 levels from ambient (240 μatm) up to 1650 μatm in mesocosm experiments carried out in the Gulf of Finland, northern Baltic Sea.
In the mesocosm experiments some variation between NPP rates within the two experimental periods was found which could be explained by the differences in the weather conditions and other limiting environmental factors. Several studies conducted outside of the Baltic Sea have shown that the capacity of seagrasses to respond to ocean acidification depends on the nutrients content in the water and light levels, and their interactions (e.g., Invers et al., 1997; Palacios and Zimmerman, 2007; Martínez-Crego et al., 2014). Besides seagrasses, Celis-Plá et al. (2015) found that macroalgal responses to elevated pCO2 depend on nutrients content and light availability. Within the Baltic Sea, the environmental factors vary highly regionally and seasonally (Feistel et al., 2008). In the Estonian coastal waters, the seagrass Z. marina grows at its lowest salinity limit (5–7 PSU) and this may cause physiological stress for their communities. Therefore, in the north-eastern Baltic Sea the factors driving the dynamics of Z. marina populations and their physiological processes are expected to be different compared to other regions of the Baltic Sea (Boström et al., 2014; Möller et al., 2014). In this study, variation in NPP rates within the two experimental periods could be explained first of all by the differences in the water temperature. In our experiment the higher NPP values of Z. marina were measured under the higher water temperatures in 2014 (average water temperature 21.9°C and average rate of NPP 5.19 mg O2 gdw−1 h−1) compared to the values of 2013 (average water temperature 13.5°C and average rate of NPP 4.08 mg O2 gdw−1 h−1). Likewise, the favorable effect of the elevated pCO2 on the photosynthesis of Z. marina was more pronounced at higher water temperature in 2014 than in 2013. Therefore, it could be suggested that a future increase in water temperature under climate change may benefit the net photosynthesis of Z. marina and also alter the response of Z. marina to CO2 enrichment. This highlights the fact that in the Baltic Sea, where the temperature is characterized by high seasonal and annual variations, the effects of increasing CO2 and water temperature should be observed together. In the Mediterranean, Baggini et al. (2014) found that macroalgal community responses to increased pCO2 are strongly affected by season and this can alter community composition under future ocean acidification.
Light availability controls sea plants carbon fixation and is therefore often the primary limiting factor for seagrass productivity (Hemminga and Duarte, 2000; Zimmerman, 2006). Several studies have shown that the decline of Z. marina distribution is caused by deteriorating underwater light climate due to eutrophication in the Baltic Sea (Boström et al., 2014 and references therein). In the both experimental years, our results showed that NPP rates of Z. marina were affected by the combined effects of light and pCO2 levels. In the Estonian coastal waters, the main distribution depth of Z. marina is between 2 and 4 m (Möller et al., 2014). This low distribution depth of Z. marina is probably due to poor underwater light climate. Therefore, seagrass responses to the increasing water CO2 concentrations also depend on the light availability in the north-eastern Baltic Sea.
The response of seagrasses to the elevated pCO2 will depend also on the carbon source they use for photosynthesis as well as on the mechanisms of carbon acquisition. Several studies have found that Z. marina is using as external Ci-source for photosynthesis (Sand-Jensen and Gordon, 1984; Beer and Rehnberg, 1997; Koch et al., 2013). Beer and Rehnberg (1997) investigated two different acquisition systems of inorganic carbon for Z. marina and showed that was used as a main source of inorganic carbon at the normal seawater pH of 8.2. Rather recently Hellblom et al. (2001) detected other utilization mechanism for Z. marina when using the buffer tris (hydroxymethyl) aminomethane (TRIS) for keeping a constant pH. The addition of buffering solution (pH 8.1) to natural seawater reduced net photosynthetic rates of Z. marina by some 70% as compared to the values in the non-buffered seawater of the same pH. So, considering above-mentioned results, Z. marina can use different mechanisms of carbon acquisition for photosynthesis under different environmental conditions. It could be speculated that in our experiments under high-pH treatment in the enclosed conditions transport is more efficient for Z. marina. However, this statement needs further verification. Similarly, Axelsson et al. (2000) found that brown macroalgae Laminaria saccharina appears to be able to utilize the of seawater even more efficiently at high pH.
Seagrass meadows are amongst the most productive autotrophic ecosystems on the planet (Duarte and Chiscano, 1999). Several studies have found seagrass meadows to be globally significant as carbonate reservoir (e.g., Duarte et al., 2010; Hendriks et al., 2014; Mazarrasa et al., 2015). Seagrass metabolic activity is likely to buffer ocean acidification in the seagrass meadows, but the magnitude of this effect will depend on their metabolic parameters and vary regionally as well seasonally (Hendriks et al., 2014). We can suggest that also in the Baltic Sea conditions seagrass beds could have a buffering role in the case of elevated pCO2 conditions.
In conclusion, our study shows that elevated pCO2 alone did not enhance the photosynthesis of the seagrass Z. marina. The photosynthetic response of Z. marina to elevated pCO2 levels appears to be the result of interactions with water temperature and light availability in the north-eastern Baltic Sea.
Author Contributions
Original idea: LP and GM. Study design: AP, GM and LP. Performing the experiments: LP and AP. Data analyses: LP, AP and TP. Writing a draft of the article LP and TP.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank hydrochemistry laboratory of Estonian Marine Institute, University of Tartu for analysing water samples. We are also grateful to Teemar Püss for help with the design of the experiments and Birgit Jullinen for her assistance in the field work. The work was funded by Estonian Ministry of Education and Research (grant SF0180005s10). We would also like to thank Katarina Oganjan for revising the text of the manuscript.
Footnotes
1. ^EN ISO 11905-1 Water quality - Determination of nitrogen. Part 1: Method using oxidative digestion with petoxodisulfate.
2. ^EN ISO 15681-2 Water quality - Determination of orthophosphate and total phosphorus contents by flow analysis (FIA and CFA) - Part 2: Method by continuous flow analysis (CFA).
3. ^EN ISO 13395 Water quality - Determination of nitrite nitrogen and nitrate nitrogen and the sum of both by flow analysis (CFA and FIA) and spectrometric detection.
4. ^EN ISO 5814 Water quality - Determination of dissolved oxygen - Electrochemical probe method.
References
Astok, V., Otsmann, M., and Suursaar, Ü. (1999). Water exchange as the main physical process in semi-enclosed marine systems: the Gulf of Riga case. Hydrobiologia 393, 11–18. doi: 10.1023/A:1003517110726
Axelsson, L., Mercado, J. M., and Figueroa, F. L. (2000). Utilization of at high pH by the brown macroalga Laminaria saccharina. Eur. J. Phycol. 35, 53–59. doi: 10.1080/09670260010001735621
Baggini, C., Salomidi, M., Voutsinas, E., Bray, L., Krasakopoulou, E., and Hall-Spencer, J. M. (2014). Seasonality affects macroalgal community response to increases in pCO2. PLoS ONE 9:e106520. doi: 10.1371/journal.pone.0106520
Barry, J. P., Tyrrell, T., Hansson, L., Plattner, G. K., and Gattuso, J. P. (2010). “Atmospheric CO2 targets for ocean acidification perturbation experiments,” in Guide to Best Practices for Ocean Acidification Research and Data Reporting, eds U. Riebesell, V. J. Fabry, L. Hansson, and J.-P. Gattuso (Luxembourg: Publications Office of the European Union), 53–66.
Beer, S., and Rehnberg, J. (1997). The acquisition of inorganic carbon by the seagrass Zostera marina. Aquat. Bot. 56, 277–283. doi: 10.1016/S0304-3770(96)01109-6
Boström, C., Baden, S., Bockelmann, A.-C., Dromph, K., Fredriksen, S., Gustafsson, C., et al. (2014). Distribution, structure and function of Nordic eelgrass (Zostera marina) ecosystems: implications for coastal management and conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 24, 410–434. doi: 10.1002/aqc.2424
Brodie, J., Williamson, C. J., Smale, D. A., Kamenos, N. A., Mieszkowska, N., and Santos, R. (2014). The future of the northeast Atlantic benthic flora in a high CO2 world. Ecol. Evol. 4, 2787–2798. doi: 10.1002/ece3.1105
Campbell, J. E., and Fourqurean, J. W. (2013). Effects of in situ CO2 enrichment on the structural and chemical characteristics of the seagrass Thalassia testudinum. Mar. Biol. 160, 1465–1475. doi: 10.1007/s00227-013-2199-3
Celis-Plá, P. S., Hall-Spencer, J. M., Horta, P. A., Milazzo, M., Korbee, N., Cornwall, C. E., et al. (2015). Macroalgal responses to ocean acidification depend on nutrient and light levels. Front. Mar. Sci. 2:26. doi: 10.3389/fmars.2015.00026
Cox, T. E., Gazeau, F., Alliouane, S., Hendriks, I. E., Mahacek, P., Le Fur, A., et al. (2016). Effects of in situ CO2 enrichment on structural characteristics, photosynthesis, and growth of the Mediterranean seagrass Posidonia oceanica. Biogeosciences 13, 2179–2194. doi: 10.5194/bg-13-2179-2016
Duarte, C. M. (2002). The future of seagrass meadows. Environ. Conserv. 29, 192–206. doi: 10.1017/S0376892902000127
Duarte, C. M., and Chiscano, C. L. (1999). Seagrass biomass and production: a reassessment. Aquat. Bot. 65, 159–174. doi: 10.1016/S0304-3770(99)00038-8
Duarte, C. M., Marbà, N., Gacia, E., Fourqurean, J. W., Beggins, J., Barrón, C., et al. (2010). Seagrass community metabolism: assessing the carbon sink capacity of seagrass meadows. Glob. Biogeochem. Cycles 24, 1–8. doi: 10.1029/2010GB003793
Duarte, C. M., Middelburg, J. J., and Caraco, N. (2005). Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2, 1–8. doi: 10.5194/bg-2-1-2005
Eklöf, J. S., Alsterberg, C., Havenhand, J. N., Sundbäck, K., Wood, H. L., and Gamfeldt, L. (2012). Experimental climate change weakens the insurance effect of biodiversity. Ecol. Lett. 15, 864–872. doi: 10.1111/j.1461-0248.2012.01810.x
Fabricius, K. E., Langdon, C., Uthicke, S., Humphrey, C., Noonan, S., De'ath, G., et al. (2011). Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Change 1, 165–169. doi: 10.1038/nclimate1122
Feistel, R., Nausch, G., and Wasmund, N. (eds.). (2008). State and Evolution of the Baltic Sea, 1952–2005: A Detailed 50-year Survey of Meteorology and Climate, Physics, Chemistry, Biology, and Marine Environment. New Jersey, NJ: John Wiley & Sons.
Hall-Spencer, J. M., Rodolfo-Metalpa, R., Martin, S., Ransome, E., Fine, M., Turner, S. M., et al. (2008). Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454, 96–99. doi: 10.1038/nature07051
Hellblom, F., Beer, S., Björk, M., and Axelsson, L. (2001). A buffer sensitive inorganic carbon utilisation system in Zostera marina. Aquat. Bot. 69, 55–62. doi: 10.1016/S0304-3770(00)00132-7
Hendriks, I. E., Olsen, Y. S., Ramajo, L., Basso, L., Steckbauer, A., Moore, T. S., et al. (2014). Photosynthetic activity buffers ocean acidification in seagrass meadows. Biogeosciences 11, 333–346. doi: 10.5194/bg-11-333-2014
Hjalmarsson, S., Wesslander, K., Anderson, L. G., Omstedt, A., Perttilä, M., and Mintrop, L. (2008). Distribution, long-term development and mass balance calculation of total alkalinity in the Baltic Sea. Cont. Shelf Res. 28, 593–601. doi: 10.1016/j.csr.2007.11.010
Invers, O., Romero, J., and Pérez, M. (1997). Effects of pH on seagrass photosynthesis: a laboratory and field assessment. Aquat. Bot. 59, 185–194. doi: 10.1016/S0304-3770(97)00072-7
Invers, O., Zimmerman, R. C., Alberte, R. S., Pérez, M., and Romero, J. (2001). Inorganic carbon sources for seagrass photosynthesis: an experimental evaluation of bicarbonate use in species inhabiting temperate waters. J. Exp. Mar. Biol. Ecol. 265, 203–217. doi: 10.1016/S0022-0981(01)00332-X
IPCC (2013). “Climate Change 2013: the physical science basis,” in Contribution of Working Group I to the Fifth Assessment Report (AR5) of the Intergovernmental Panel on Climate Change, eds T. F. Stocker, D. Qin, G. K. Plattner, M. Tignor, S. K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex, and P. M. Midgley (Cambridge: Cambridge University Press), 1535.
Koch, M., Bowes, G., Ross, C., and Zhang, X.-H. (2013). Climate change and ocean acidification effects on seagrasses and marine macroalgae. Glob. Chan. Biol. 19, 103–132. doi: 10.1111/j.1365-2486.2012.02791.x
Kotta, J., Lauringson, V., Martin, G., Simm, M., Kotta, I., Herkül, K., et al. (2008). “Gulf of Riga and Pärnu Bay,” in Ecology of Baltic Coastal Waters, ed U. Schiewer (Berlin; Heidelberg: Springer-Verlag), 217–243.
Larkum, A. W. D., Orth, R. J., and Duarte, C. M. (eds.) (2006). Seagrasses: Biology, Ecology and Conservation. Dordrecht: Springer.
Martínez-Crego, B., Olivé, I., and Santos, R. (2014). CO2 and nutrient driven changes across multiple levels of organization in Zostera noltii ecosystems. Biogeosciences 11, 7237–7249. doi: 10.5194/bg-11-7237-2014
Mazarrasa, I., Marbà, N., Lovelock, C. E., Serrano, O., Lavery, P. S., Fourqurean, J. W., et al. (2015). Seagrass meadows as a globally significant carbonate reservoir. Biogeosci. Discuss. 12, 4107–4138. doi: 10.5194/bgd-12-4107-2015
Möller, T., Kotta, J., and Martin, G. (2014). Spatiotemporal variability in the eelgrass Zostera marina L. in the north-eastern Baltic Sea: canopy structure and associated macrophyte and invertebrate communities. Est. J. Ecol. 63, 90–108. doi: 10.3176/eco.2014.2.03
Möller, T., and Martin, G. (2007). Distribution of the eelgrass Zostera marina L. in the coastal waters of Estonia, NE Baltic Sea. Proc. Estonian Acad. Sci. Biol. Ecol. 56, 270–277.
Moore, K. A., and Short, F. T. (2006). “Zostera: biology, ecology, and management,” in Seagrasses: Biology, Ecology and Conservation, eds A. W. D. Larkum, R. J. Orth, and C. Duarte (Dordrecht: Springer), 361–386.
Omstedt, A., Edman, M., Claremar, B., Frodin, P., Gustafsson, E., Humborg, C., et al. (2012). Future changes in the Baltic Sea acid-base (pH) and oxygen balances. Tellus B 64, 1–23. doi: 10.3402/tellusb.v64i0.19586
Omstedt, A., Edman, M., Claremar, B., and Rutgersson, A. (2015). Modelling the contributions to marine acidification from deposited SOx, NOx, and NHx in the Baltic Sea: past and present situations. Cont. Shelf Res. 111, 234–249. doi: 10.1016/j.csr.2015.08.024
Orth, R. J., Carruthers, T. J., Dennison, W. C., Duarte, C. M., Fourqurean, J. W., Heck, K. L., et al. (2006). A global crisis for seagrass ecosystems. Bioscience 56, 987–996. doi: 10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2
Ow, Y. X., Collier, C. J., and Uthicke, S. (2015). Responses of three tropical seagrass species to CO2 enrichment. Mar. Biol. 162, 1005–1017. doi: 10.1007/s00227-015-2644-6
Paalme, T. (2005). Nuisance Brown Macroalga Pilayella Littoralis: Primary Production, Decomposition and Formation of Drifting Algal Mats. Dissertation, Ph.D. thesis 10, University of Tallinn.
Pajusalu, L., Martin, G., Paalme, T., and Põllumäe, A. (2016). The effect of CO2 enrichment on net photosynthesis of the red alga Furcellaria lumbricalis in a brackish water environment. PeerJ 4:e2505. doi: 10.7717/peerj.2505
Pajusalu, L., Martin, G., Põllumäe, A., and Paalme, T. (2013). Results of laboratory and field experiments of the direct effect of increasing CO2 on net primary production of macroalgal species in brackish-water ecosystems. Proc. Estonian Acad. Sci. 62, 148–154. doi: 10.3176/proc.2013.2.09
Pajusalu, L., Martin, G., Põllumäe, A., Torn, K., and Paalme, T. (2015). Direct effects of increased CO2 concentrations in seawater on the net primary production of charophytes in a shallow, coastal, brackish-water ecosystem. Boreal Env. Res. 20, 413–422.
Palacios, S. L., and Zimmerman, R. C. (2007). Response of eelgrass Zostera marina to CO2 enrichment: possible impacts of climate change and potential for remediation of coastal habitats. Mar. Ecol. Prog. Ser. 344, 1–13. doi: 10.3354/meps07084
Paul, A. J., Bach, L. T., Schulz, K. G., Boxhammer, T., Czerny, J., Achterberg, E. P., et al. (2015). Effect of elevated CO2 on organic matter pools and fluxes in a summer Baltic Sea plankton community. Biogeosciences 12, 6181–6203. doi: 10.5194/bg-12-6181-2015
Porzio, L., Buia, M. C., and Hall-Spencer, J. M. (2011). Effects of ocean acidification on macroalgal communities. J. Exp. Mar. Biol. Ecol. 400, 278–287. doi: 10.1016/j.jembe.2011.02.011
Raven, J., Caldeira, K., Elderfield, H., Hoegh-Guldberg, O., Liss, P., Riebesell, U., et al. (2005). Ocean Acidification Due to Increasing Atmospheric Carbon Dioxide. Policy document 12/05. London: The Royal Society.
Russell, B. D., Connell, S. D., Uthicke, S., Muehllehner, N., Fabricius, K. E., and Hall-Spencer, J. M. (2013). Future seagrass beds: can increased productivity lead to increased carbon storage? Mar. Poll. Bull. 73, 463–469. doi: 10.1016/j.marpolbul.2013.01.031
Sabine, C. L., Feely, R. A., Gruber, N., Key, R. M., Lee, K., Bullister, J. L., et al. (2004). The oceanic sink for anthropogenic CO2. Science 305, 367–371. doi: 10.1126/science.1097403
Sand-Jensen, K., and Gordon, D. M. (1984). Differential ability of marine and freshwater macrophytes to utilize and CO2. Mar. Biol. 80, 247–253. doi: 10.1007/BF00392819
Schneider, B., Eilola, K., Lukkari, K., Muller-Karulis, B., and Neumann, T. (2015). “Environmental impacts—marine biogeochemistry,” in Second Assessment of Climate Change for the Baltic Sea Basin, ed BACC II Author Team (Springer: International Publishing), 337–361. doi: 10.1007/978-3-319-16006-1
Takahashi, M., Noonan, S. H. C., Fabricius, K. E., and Collier, C. J. (2015). The effects of long-term in situ CO2 enrichment on tropical seagrass communities at volcanic vents. ICES J. Mar. Sci. 73, 876–886. doi: 10.1093/icesjms/fsv157
Thom, R. M. (1996). CO2-Enrichment effects on eelgrass (Zostera marina L.) and bull kelp (Nereocystis luetkeana (mert.) P & R.). Water Air Soil Pollut. 88, 383–391. doi: 10.1007/BF00294113
Waycott, M., Duarte, C. M., Carruthers, T. J., Orth, R. J., Dennison, W. C., Olyarnik, S., et al. (2009). Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. U.S.A. 106, 12377–12381. doi: 10.1073/pnas.0905620106
Zimmerman, R. C. (2006). “Light and photosynthesis in seagrass meadows,” in Seagrasses: Biology, Ecology and Conservation, eds A. W. D. Larkum, R. J. Orth, and C. Duarte (Dordrecht: Springer), 303–321.
Keywords: carbon dioxide, Zostera marina, Baltic Sea, marine acidification, net photosynthesis, seagrass, brackish water
Citation: Pajusalu L, Martin G, Põllumäe A and Paalme T (2016) The Influence of CO2 Enrichment on Net Photosynthesis of Seagrass Zostera marina in a Brackish Water Environment. Front. Mar. Sci. 3:239. doi: 10.3389/fmars.2016.00239
Received: 15 June 2016; Accepted: 03 November 2016;
Published: 18 November 2016.
Edited by:
Frédéric Gazeau, Laboratoire d'Océanographie de Villefranche (CNRS/UPMC, UMR7093), FranceReviewed by:
Jason Michael Hall-Spencer, Plymouth University, UKIris Eline Hendriks, University of the Balearic Islands, Spain
Traci Erin Cox, Dauphin Island Sea Laboratory, France
Copyright © 2016 Pajusalu, Martin, Põllumäe and Paalme. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liina Pajusalu, liina.pajusalu@ut.ee
 Liina Pajusalu
Liina Pajusalu Georg Martin
Georg Martin Arno Põllumäe
Arno Põllumäe Tiina Paalme
Tiina Paalme