Ammonium Uptake Rates in a Seagrass Bed under Combined Waves and Currents
- 1Mangrove Ecology Group, Ecology Department, Leibniz-Zentrum für Marine Tropenökologie, Bremen, Germany
- 2Landscape Ecology and Environmental Systems Analysis, Institute of Geoecology, Technische Universität Braunschweig, Braunschweig, Germany
- 3Department of Estuarine and Delta Systems, Royal Netherlands Institute for Sea Research, Yerseke, Netherlands
In coastal locations seagrass beds are exposed to various hydrodynamic forces that can include waves and/or unidirectional currents. Differences in these forces may be expected to alter nutrient (such as phosphorus and nitrogen compounds e.g., ammonium) uptake rates by seagrass leaves. We investigated in a laboratory flume how high and low velocities with the absence or presence of waves control ammonium absorption. Our results showed that low currents with waves had the highest nutrient uptake compared to all other treatments. This result was ascribed to a combination of mechanisms. The waves may have influenced turbulence and thereby the water movement around the leaf surface, whilst the low current enabled the canopy to remain upright with an open structure, thereby allowing leaves to be exposed to a greater exchange of ammonium rich water. Although, higher currents with waves might have increased turbulence, bending under the high current squeezed the canopy into a compact closed structure. This study indicates that there are broader implications of the observed mechanisms of nutrient uptake, for instance how they depend on the plant morphology such as the leaf area, length and flexibility.
Introduction
Inter-tidal and shallow sub-tidal areas typically form the habitat of seagrass species such as Zostera noltii and Zostera marina in temperate zones and Halodule wrightii and Halophila ovalis in tropical zones (Larkum et al., 2006; Short et al., 2007). In such regions, hydrodynamic forcing can vary greatly by combining different levels of wave action and tidal currents (Charles et al., 2004). The seagrass canopy and leaf structure can significantly alter hydrodynamic forcing via reflection from the leaves (Charles et al., 2004) and absorption of energy through drag and motion (Dijkstra and Uittenbogaard, 2010; Paul et al., 2012). Altered hydrodynamics within seagrass beds also affect the nutrient availability for example phosphorus and nitrogen, and thereby nutrient uptake rates of the plants (Cornelisen and Thomas, 2002; Morris et al., 2008; Weitzman et al., 2013). A greater understanding of the fundamental hydrodynamic processes which control nutrient uptake in seagrass beds is urgently needed especially due to nutrient enrichment pressures on many seagrass beds globally (Burkholder et al., 2007). As eutrophic seagrass beds (nitrogen and phosphorous enriched) alter their allocation of nutrients to their leaves rather than their roots (Lee and Dunton, 1999), which will change processes within the seagrass ecosystems as a whole.
Extensive work has been completed for labeled (dissolved) nitrogen uptake by seagrass plants in flumes with various hydrodynamic regimes (Cornelisen and Thomas, 2002; Morris et al., 2008; Weitzman et al., 2013). Whilst there are some studies on phosphorus uptake with relation to translocation between roots, rhizomes and leaves, these have not considered hydrodynamic conditions (Pérez-Lloréns et al., 1993; Pérez-Lloréns and Niell, 1995). The ammonium () studies identify specific processes, which promote this specific nutrient uptake. For instance, shoot movement under waves opens up the canopy, therefore enhancing uptake (Thomas and Cornelisen, 2003) as the latter is related to the rate of its delivery to seagrass leaves (Cornelisen and Thomas, 2004) and hence to bulk velocity (Cornelisen and Thomas, 2006). More recently, Morris et al. (2008) demonstrated that uptake within a vegetation patch has clear spatial patterns, with uptake rates related to the local canopy flux. As a result, the “wake” from the current separation at the edge of the bed was responsible for enhanced nitrogen uptake at the leading edge (x = 0 m). Weitzman et al. (2013) found that blade motion and boundary layer redevelopment caused by wave movement affect uptake rates. The major conclusions of these studies were that leaves, which experience a reduced boundary layer thickness on their surface due to hydrodynamics (such as waves) would have a greater uptake regardless of the flow type (Cornelisen and Thomas, 2002, 2004, 2006; Thomas and Cornelisen, 2003; Morris et al., 2008; Weitzman et al., 2013).
None of the previous studies investigated uptake with combined effects of currents and waves compared with nutrient uptake with currents or waves only. In a natural situation, however, the major hydrodynamic condition in shallow water seagrass beds is the superposition of small waves on top of a tidal current (O'Callaghan et al., 2010). Waves and currents can have opposing effects on the movement of the seagrass leaves in the water column. Thus, the combined effect of currents and waves in uptake for seagrass patches cannot be easily predicted from previous studies. Hence we investigated this aspect focusing on the leaf uptake rates by Z. noltii as example. To achieve this, unidirectional high (i.e., 0.3 m s−1) and low (i.e., 0.05 m s−1) velocities in presence and absence of small (0.04 m wave height, 1 s wave period) waves were applied to real seagrass beds in a laboratory flume. We aimed to answer how uptake rates by seagrass leaves are affected by contrasting high and low current velocities with and without superimposed waves.
Methodology
Plant Material
The temperate seagrass Z. noltii (leaves: 0.5–1.5 mm wide and 6 ± 0.5 cm long) was used as a model to study how the interaction of vegetation with waves and currents affects the leaf uptake. This species was specifically chosen as it colonizes the intertidal zone between high and low water neaps where the combination of waves and tidal currents is most pronounced. On the 26th and 27th June 2013, healthy and undisturbed sections of Z. noltii (60 × 22.5 cm) were collected from the Oosterschelde tidal lagoon (51°27.987′N. 4°4.512′E) and directly transferred to the Netherlands Institute for Sea Research (NIOZ) in Yerseke, the Netherlands. The seagrass bed sections were then stored in standalone large outdoor holding tanks filled with seawater pumped directly from the Oosterschelde, to allow for recovery from transportation. The conditions in the tanks were the same as found at the collection site (salinity 31 and temperature 16°C). As the holding tanks were located outside, plants were exposed to normal light conditions for the time of year. The tanks had a flow through system in addition to one medium sized pump which aerated the water. Seagrass plants can respond physiologically to culture conditions so the plants were allowed 4 days to acclimatize. The mean 15N values of the seagrass plants were 0.37 ± 0.03% and this did not change over time because no was added to the holding tanks. After acclimation the plants were moved from the separate holding tanks into the flume to build a continuous seagrass bed of 135 cm length across the width of the flume (60 cm) with a density of 1,369 shoots m−2. The flume was filled with natural seawater from the adjacent Oosterschelde (salinity 31 and temperature 16°C) and the water was changed every 4 days to ensure sufficient nutrient and oxygen availability. Additionally, the flume was illuminated at night (~10 h) by 14 Son-T lamps (400 W each) and during weekends (~48 h) to allow for photosynthesis.
Flume Set-Up and Hydrodynamic Conditions
Experiments were carried out under controlled conditions in a racetrack wave flume at NIOZ (Figure 1). The oval flume is 0.6 m wide and has a straight working section of 10.8 m in which a constant water depth of 0.3 m was maintained (Figure 1). A conveyor belt at the long side opposite the working section generates unidirectional currents; regular waves of varying heights and periods can be generated with a wave paddle in front of the working section (Figure 1). The waves can be superimposed onto a current. Velocity profiles were obtained inside the meadow at distances 0, 0.45, 0.9, and 1.35 m from the leading bed edge along the central axis of the patch. At these distances wave and current evolution, canopy height and motion were monitored as well as seagrass plant nutrient uptake. The three components of velocity were recorded at 13 regularly spaced positions along each location with an Acoustic Doppler Velocimeter (ADV) at 100 Hz. Each recording lasted 2 min. Two different current conditions (0.05 and 0.3 m s−1, set as the free stream velocity 0.5 m in front of the bed's edge and 0.2 m above the bed) were generated. These current conditions were chosen because uptake has been shown to be limited at velocities outside of this range and these represented the typical range of current velocities that may be encountered during a normal tide, with 0.05 m s−1 being the lower limit, and 0.3 m s−1 being the upper limit (Koch and Gust, 1999; Cornelisen and Thomas, 2002, 2004; Morris et al., 2008). The bed was exposed to these current conditions once without and once with superimposed regular waves with a wave height of 0.04 m and a period of ~1 s (de los Santos et al., 2010; Paul et al., 2012). For each hydrodynamic condition three replicate runs were performed for uptake of leaves, a total of 12 runs. In addition, the position and motion of 10 plant leaves were traced onto paper through the transparent flume wall and analyzed for canopy height for each condition.
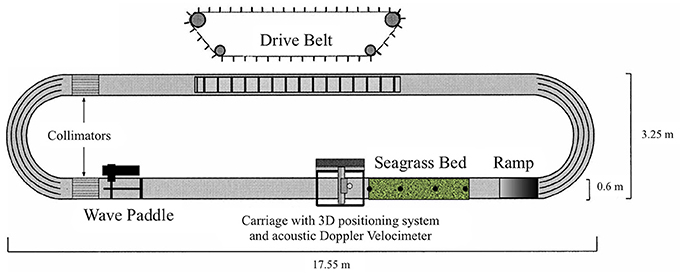
Figure 1. Schematic diagram of the flume and seagrass bed (adapted from Paul et al., 2012). Filled circles in the seagrass bed represent the pots, which were inserted with three seagrass shoots in it, each pot representing a plant sample. These pots were placed at distances 0, 0.45, 0.9, and 1.35 m from the leading bed edge along the central axis of the patch.
Analysis of Velocity Data
Velocity data was cleaned firstly for outliers using a despiking algorithm (Mori et al., 2007) and secondly for low correlation (<85%). Instrument ringing was also found to cause insufficient data for further processing; therefore, some time series were removed. From the resulting time series, the time-averaged components (, , ), the components due to wave presence (uw, vw, ww) and the fluctuating component due to turbulence (u′, v′, w′) were derived in downstream (x), cross-stream (y), and vertical (z) direction, respectively. The turbulence components were used to calculate turbulent kinetic energy TKE (Jonsson et al., 2006):
The mean values over the whole profile for TKE were used.
Ammonium Uptake
At each profile position (0, 0.45, 0.9, and 1.35 m) along the meadow one pot was inserted with three seagrass shoots in it, each pot representing a plant sample (Figure 1). The pots were inserted into the sediment (depth of 5 cm, sediment collected from the Oosterschelde) so only the plants were above the sediment surface; this meant that no other structure perturbing from the sediment altered the current. For each hydrodynamic treatment, 4 new pots were placed into the seagrass bed structure. Three replicates were taken making a total of 48 plant samples with a corresponding velocity profile each. Ammonium chloride (15 CL ~ 10 μmol l−1) was added at the beginning of each day close to the wave paddle to ensure good mixing (Morris et al., 2008). Control plant samples were also set-up in a holding tank where the same physical conditions (temperature, salinity, light conditions) were kept as in the flume. These controls were not exposed to hydrodynamic forcing, but the same concentration of 15 CL was added. Water samples (n = 3) were taken from the flume and controls at the beginning of each day and throughout the day, to establish background nutrient conditions.
Calculation of Ammonium Uptake
The pots were taken from the flume after each treatment and plants were removed from the substrate. Above and below ground tissue were separated. From the above ground tissue, epiphytes were removed by gently brushing the leaves with a fingernail, and then they were washed and frozen before being freeze dried. The plant samples were ground until they were homogeneous and analyzed at NIOZ analytical laboratory with an elemental analysis isotope ratio mass spectrometry (EA-IRMS) using a Thermo Finnigan Flash 1112.
The corrected uptake rate (V) was determined using
where as is the atom % 15N for the component tissue (seagrass leaves), aw is the atom % 15N for the enriched substrate (water) and t is the duration of the treatment (h). aw was calculated from the amount of 99.1% 15 added to the flume and initial water column [], (Δas/Δt) was calculated as the difference Δas in atom % 15N between non-labeled samples and the samples collected at the end of each hydrodynamic treatment divided by the duration of the run (Δt = 1 h). The mean 15N values of the seagrass plants were 0.37 ± 0.03%. α is the correction term following Cornelisen and Thomas's (2006) compensation for changes in [] over time:
where k is the first-order rate of decline in concentration and t (h) is duration of the treatment. Finally, V was normalized to the N content (% N) of the tissue to express the uptake rate as mg N (g DM)−1 h−1. Mean weight of the seagrass leaves was 0.21 ± 0.06 g.
Statistics
One-way ANOVA was used for analyzing the data for canopy height vs. waves and canopy height vs. currents, uptake rates vs. currents, and uptake rates vs. waves. An ANCOVA was applied for the dependent variable ( uptake values) vs. the independent variables (treatments: currents ± waves and TKE), as both uptake and TKE values were continuous. Prior to testing, normality in the data was assessed using a D'Agostino-Pearson test. Differences were considered significant at the level of 0.05 and slightly significant at the level of 0.1. All statistical testing was completed using the R programming platform (R Core Development Team, 2012).
Results
Hydrodynamic Effects on Canopy Height and Motion
The seagrass leaves bowed under hydrodynamic forcing causing a reduction in canopy height. The canopy height showed a significant difference between the different velocities (0.05 and 0.3 m s−1) where the canopy reduced by more than half at the higher velocities compared to lower velocities (p < 0.05, 1-way ANOVA, Figure 2 and Table 1). Mean canopy heights in the presence of waves were slightly lower than for treatments without waves and this difference was slightly significant (p < 0.1, 1-way ANOVA, Figure 2 and Table 1). Treatments with waves caused an undulating motion of the leaves because the canopy was compressed below the wave trough and then elevated again under the wave crest. This undulation was small when the waves were overlaid on the high currents, and resulted in a flapping motion with almost vertical leaves at the most upright position when waves co-occurred with the lower currents.
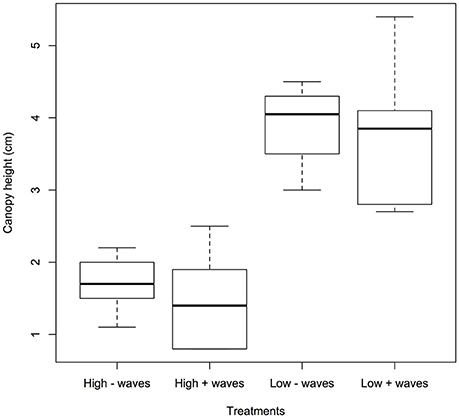
Figure 2. Showing seagrass canopy height (cm) under different treatments: high (0.3 m s−1) and low (0.05 m s−1) currents without/with waves. To obtain each replicate for canopy height, the position and motion of 10 plant leaves were traced onto paper through the transparent flume wall. A significant difference was seen for the two velocities (p < 0.05) and with and without waves (p < 0.1).
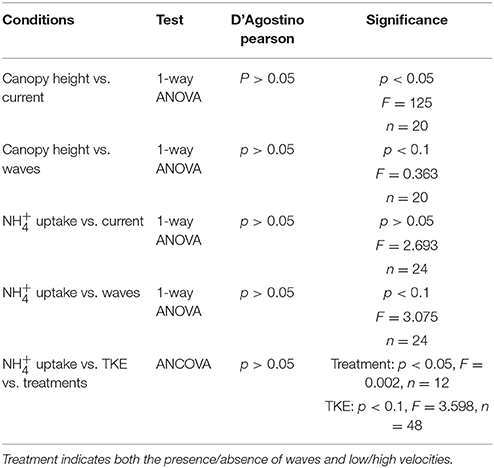
Table 1. Statistical output of the ANOVA (canopy height vs. current, canopy height vs. waves, uptake vs. current, and uptake vs. waves) and the ANCOVA ( uptake vs. TKE vs. treatments).
Effect of Hydrodynamics on Uptake
No significant difference was seen for uptake in Z. noltii leaves between high and low currents (p > 0.05, 1-way ANOVA, Table 1). However, the addition of waves showed slightly significantly enhanced leaf uptake with waves (p < 0.1, 1-way ANOVA, Table 1). Leaf uptake with respect to TKE was slightly significantly different (p < 0.1, ANCOVA, Figure 3 and Table 1). For high currents ± waves TKE values were within a small range whilst treatments with 0.05 m s−1 ± waves showed higher variation (Figure 3). TKE reached maximum values for the low currents and waves (Figure 3). A significant difference was seen for uptake vs. treatments (p < 0.05, ANCOVA, Figure 3 and Table 1) at low velocity with waves.
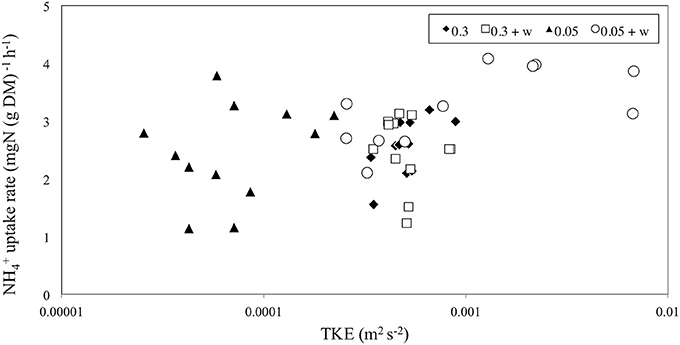
Figure 3. The rate of ammonium [; mg N (g DM)−1 h−1] uptake for seagrass leaves vs. turbulent kinetic energy TKE (m2 s−2). The x-axis is on a logarithmic scale. uptake was significant for the different treatments (p < 0.05, Table 1) and slightly significant for TKE (p < 0.1, Table 1).
Discussion
In agreement with earlier studies (Thomas and Cornelisen, 2003; Morris et al., 2008; Weitzman et al., 2013), we found that leaf uptake by seagrass Z. noltii was determined by the hydrodynamic conditions. We showed the importance of waves in combination with tidal currents, which represent the most commonly occurring field conditions. We found that ammonium uptake at low velocities with waves was enhanced compared to higher velocities with waves. TKE also showed higher values during the low currents plus wave runs where uptake was highest, indicating that higher movement of leaves and turbulence in the water column enhance uptake.
Leaf Movement
Seagrass plant uptake with low velocities without waves was comparable to uptake rates with higher velocities with and without waves. We expect this might be related to the leaves being pushed in only one direction in the water column thereby causing a decrease in leaf surface exposure to the nutrient rich water (Figure 2). Previous work has shown that higher velocities equate to increased uptake (0–0.25 ms−1) due to the greater supply of nutrient rich water and the decrease of the boundary layer on the leaf surface (Thomas and Cornelisen, 2003; Cornelisen and Thomas, 2004; Morris et al., 2008). We found that if the current is too high (0.3 m s−1) it may cause the plants to bend toward the bottom and thereby “close” the canopy so that leaves are no longer exposed to nutrient rich water (Figure 2; Koch et al., 2006). The addition of small waves to high velocities did not fully open up the canopy; rather it caused an undulating up and down motion (Paul and Gillis, 2015). In the presence of low velocities with waves we found the greatest uptake. This was linked to the waves opening the canopy, causing the leaves to have an omnidirectional movement in the water column (Ackerman and Okubo, 1993; Koch et al., 2006). However, another factor could also be the movement of the seagrass leaves, causing acceleration of the flow adjacent to the leaf surface. This would mean that the effect of advection would be greater than diffusion. It has been shown that such conditions lead to an increase in the Sherwood number indicating an increase in nutrient uptake (Magar et al., 2003).
The Diffusion Factor
Morris et al. (2008) showed that within-canopy water currents are a major driver of uptake, which might explain up to two-fold differences in uptake between species of different morphology. It is hypothesized that TKE causes omnidirectional currents within canopies, which decrease the boundary layer surrounding the leaves and hence allowing for greater diffusion of (Thomas and Cornelisen, 2003; Weitzman et al., 2013). Our data found evidence for this theory in that higher TKE was slightly correlated with increased uptake, and omnidirectional currents (0.05 m s−1 and waves) were associated with elevated TKE levels compared to unidirectional currents (0.05 m s−1 no waves; Figure 3). In addition, wave-induced oscillatory currents and/or large scale turbulent eddies can also disrupt the diffusion boundary layer allowing for a greater supply of nutrients to the leaf surface, which we confirmed at low velocities with waves but not at high velocities with waves (Koch et al., 2006; Weitzman et al., 2013). Thomas et al. (2000) found that uptake of in seagrass communities is diffusion limited. This mechanism has been found in other benthic environments notably coral reefs (Hearn et al., 2001). Previous research has found that water current velocity (0.03–0.2 m s−1) pushes the turbulent mixing layer into the canopy reducing the boundary layer, and thus allowing for greater diffusion of into leaves (Cornelisen and Thomas, 2004). In contrast, our results showed that at treatments with 0.3 m s−1 currents the strong bending of the canopy prevents the turbulent mixing layer from entering the canopy. As a result, the boundary layer is not reduced at such high velocities. Consequently, the seagrass leaf flexibility will be an important parameter to consider when investigating uptake (Weitzman et al., 2013). Longer, thinner or more flexible leaves may be moved more easily by a unidirectional current thus closing the canopy and decreasing their ability to uptake nutrients. In return, such leaves will respond much more easily to wave forcing allowing plants with flexible leaves to benefit even from small waves with respect to uptake.
Implications
Our results indicate that uptake from the water column is strongly dependent on flow characteristics. Thus, exposure (e.g., hydrodynamic regime, water depth) will be an important factor when investigating nutrient uptake in-situ, in addition further work should be completed in flumes to investigate a wider variety of wave heights and current flows. Previous research has emphasized that leaf flexibility in seagrass species is an important ecosystem engineering property (Bouma et al., 2005) as well as relevant for nutrient uptake (Morris et al., 2008). In fact Morris et al. (2008) found that Cymodocea nodosa, which has much longer, wider and sparser leaves than Z. noltii, has a two-fold higher leaf uptake for unidirectional currents. Hence leaf morphology and density may have strong implications for the establishment and expansion of different seagrass species under various hydrodynamic conditions. In addition, different seagrass species and consequently seagrass morphologies will have distinct patch dynamics, which will also affect uptake rates. Further research should also focus on how other nutrients such as phosphorus uptake are affected by hydrodynamics.
On a global scale, seagrass beds are greatly affected by high levels of nutrients such as phosphorous or nitrogen exported into the system (eutrophic) (Burkholder et al., 2007). Hydrodynamic processes such as waves and turbulence will obviously be important in delivering vital nutrient requirements via leaves to nutrient limited seagrass beds (oligotrophic). However, it should also be considered that seagrass beds high in nitrogen (eutrophic) have been found to obtain a greater allocation (52%) of their nitrogen requirements via their leaves than nitrogen poor seagrass beds (28%) (Lee and Dunton, 1999). Therefore, understanding the role of hydrodynamics in nutrient delivery including phosphorus and nitrogen will also be important in eutrophic beds for translocation of nutrients from roots/rhizomes to leaves. Further research is required to clarify how hydrodynamics interact with nutrient concentrations and how these two factors affect biomechanics and morphology of the plants (La Nafie et al., 2012) and the spatial characteristics of the beds. The interaction with hydrodynamics will vary between seagrass species, but whether these effects are positive or negative is not yet known. Biogeochemical models such as those used by the US environmental Protection Agency (the Three dimensional Hydrodynamic Eutrophication model; Park et al., 2005) and others used for the Everglades (Everglades Wetland Hydrodynamic Model; Moustafa and Hamrick, 2000) could be used in this instance, by extrapolating from species to a larger scale i.e., beds. These models combine hydrodynamics, eutrophication processes, and diagenetic mechanisms, to determine nutrients loads (Moustafa and Hamrick, 2000; Park et al., 2005), additional flume data obtained from species nutrient uptake could make these models more specific and identify seagrass beds which may be at risk from eutrophication.
Author Contributions
LG designed the experiment, completed the experiment, analyzed the data, and wrote the MS. MP completed the experiment, analyzed the data, and provided extensive feedback for the MS. TB helped design the experiment, supported the analyses of the data, and provided valuable feedback for the MS.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank NIOZ-Yerseke for access to the flume, and the technical staff for supporting the flume work. Particular gratitude is extended to Bert Sinke and Jos van Soelen. MP acknowledges funding by the German Science Foundation (grant no. PA 2547/1-1).
References
Ackerman, J. D., and Okubo, A. (1993). Reduced mixing in a marine macrophyte canopy. Funct. Ecol. 7, 305–309. doi: 10.2307/2390209
Bouma, T. J., De Vries, M. B., Low, E., Peralta, G., Tanczos, C., Van de Koppel, J., et al. (2005). Trade-offs related to ecosystem engineering: a case study on stiffness of emerging macrophytes. Ecology 86, 2187–2199. doi: 10.1890/04-1588
Burkholder, J. M., Tomasko, D. A., and Touchette, B. W. (2007). Seagrasses and eutrophication. J. Exp. Mar. Biol. Ecol. 350, 46–72. doi: 10.1016/j.jembe.2007.06.024
Charles, H. P., Richard, A. L. Jr., Fiorenza, M., and Gregory, A. S. (2004). Attenuation of water flow inside seagrass canopies of differing structure. Mar. Ecol. Prog. Ser. 268, 81–92. doi: 10.3354/meps268081
Cornelisen, C. D., and Thomas, F. I. (2006). Water flow enhances ammonium and nitrate uptake in a seagrass community. Mar. Ecol. Prog. Ser. 312, 1–13. doi: 10.3354/meps312001
Cornelisen, C. D., and Thomas, F. I. M. (2002). Ammonium uptake by seagrass epiphytes: isolation of the effects of water velocity using an isotope label. Limnol. Oceanogr. 47, 1223–1229. doi: 10.4319/lo.2002.47.4.1223
Cornelisen, C. D., and Thomas, F. I. M. (2004). Ammonium and nitrate uptake by leaves of the seagrass Thalassia testudinum: impact of hydrodynamic regime and epiphyte cover on uptake rates. J. Mar. Syst. 49, 177–194. doi: 10.1016/j.jmarsys.2003.05.008
de los Santos, C. B., Brun, F. G., Bouma, T. J., Vergara, J. J., and Pérez-Lloréns, J. L. (2010). Acclimation of seagrass Zostera noltii to co-occurring hydrodynamic and light stresses. Mar. Ecol. Prog. Ser. 398, 127–135. doi: 10.3354/meps08343
Dijkstra, J. T., and Uittenbogaard, R. E. (2010). Modeling the interaction between flow and highly flexible aquatic vegetation. Water Resour. Res. 46:W12547. doi: 10.1029/2010WR009246
Hearn, C., Atkinson, M., and Falter, J. (2001). A physical derivation of nutrient-uptake rates in coral reefs: effects of roughness and waves. Coral Reefs 20, 347–356. doi: 10.1007/s00338-001-0185-6
Jonsson, P. R., van Duren, L. A., Amielh, M., Asmus, R., Aspden, R. J., Daunys, D., et al. (2006). Making water flow: a comparison of the hydrodynamic characteristics of 12 different benthic biological flumes. Aquat. Ecol. 40, 409–438. doi: 10.1007/s10452-006-9049-z
Koch, E., Ackerman, J., Verduin, J., and Keulen, M. (2006). “Fluid dynamics in seagrass ecology—from molecules to ecosystems,” in Seagrasses: Biology, Ecology, and Conservation, eds A. Larkum, R. J. Orth, and C. Duarte (Dordrecht: Springer), 193–225.
Koch, E. W., and Gust, G. (1999). Water flow in tide- and wave-dominated beds of the seagrass Thalassia testudinum. Mar. Ecol. Prog. Ser. 184, 63–72. doi: 10.3354/meps184063
La Nafie, Y. A., de los Santos, C. B., Brun, F. G., van Katwijk, M. M., and Bouma, T. J. (2012). Waves and high nutrient loads jointly decrease survival and separately affect morphological and biomechanical properties in the seagrass Zostera noltii. Limnol. Oceanogr. 57, 1664–1672. doi: 10.4319/lo.2012.57.6.1664
Larkum, A. W. D., Orth, R. J., and Duarte, C. M. (2006). Seagrasses: Biology, Ecology, and Conservation. Dordrecht: Springer.
Lee, K.-S., and Dunton, K. H. (1999). Inorganic nitrogen acquisition in the seagrass Thalassia testudinum: development of a whole-plant nitrogen budget. Limnol. Oceanogr. 44, 1204–1215. doi: 10.4319/lo.1999.44.5.1204
Magar, V., Goto, T., and Pedley, T. J. (2003). Nutrient uptake by a self-propelled steady squirmer. Q. J. Mechanics Appl. Math. 56, 65–91. doi: 10.1093/qjmam/56.1.65
Mori, N., Suzuki, T., and Kakuno, S. (2007). Noise of acoustic doppler velocimeter data in bubbly flows. J. Eng. Mech. 133, 122–125. doi: 10.1061/(ASCE)0733-9399(2007)133:1(122)
Morris, E. P., Peralta, G., Brun, F. G., van Duren, L., Bouma, T. J., and Perez-Llorens, J. L. (2008). Interaction between hydrodynamics and seagrass canopy structure: spatially explicit effects on ammonium uptake rates. Limnol. Oceanogr. 53, 1531–1539. doi: 10.4319/lo.2008.53.4.1531
Moustafa, M. Z., and Hamrick, J. M. (2000). Calibration of the wetland hydrodynamic model to the everglades nutrient removal project. Water Qual. Ecosyst. Model. 1, 141–167. doi: 10.1023/A:1013938700446
O'Callaghan, J. M., Pattiaratchi, C. B., and Hamilton, D. P. (2010). The role of intratidal oscillations in sediment resuspension in a diurnal, partially mixed estuary. J. Geophys. Res. 115:C07018. doi: 10.1029/2009JC005760
Park, K., Jung, H.-S., Kim, H.-S., and Ahn, S.-M. (2005). Three-dimensional hydrodynamic-eutrophication model (HEM-3D): application to Kwang-Yang Bay, Korea. Mar. Environ. Res. 60, 171–193. doi: 10.1016/j.marenvres.2004.10.003
Paul, M., Bouma, T. J., and Amos, C. L. (2012). Wave attenuation by submerged vegetation: combining the effect of organism traits and tidal current. Mar. Ecol. Prog. Ser. 444, 31–41. doi: 10.3354/meps09489
Paul, M., and Gillis, L. G. (2015). Let it flow: how does an underlying current affect wave propagation over a natural seagrass meadow? Mar. Ecol. Prog. Ser. 523, 57–70. doi: 10.3354/meps11162
Pérez-Lloréns, J. L., and Niell, F. X. (1995). Short-term phosphate uptake kinetics in Zostera noltii Hornem: a comparison between excised leaves and sediment-rooted plants. Hydrobiologia 297, 17–27. doi: 10.1007/BF00033498
Pérez-Lloréns, J. L., Visscher, P. D., Nienhuis, P. H., and Niell, F. X. (1993). Light-dependent uptake, translocation and foliar release of phosphorus by the intertidal seagrass Zostera noltii Hornem. J. Exp. Mar. Biol. Ecol. 166, 165–174. doi: 10.1016/0022-0981(93)90216-B
R Core Development Team (2012). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing.
Short, F., Carruthers, T., Dennison, W., and Waycott, M. (2007). Global seagrass distribution and diversity: a bioregional model. J. Exp. Mar. Biol. Ecol. 350, 3–20. doi: 10.1016/j.jembe.2007.06.012
Thomas, F. I., and Cornelisen, C. D. (2003). Ammonium uptake by seagrass communities: effects of oscillatory versus unidirectional flow. Mar. Ecol. Prog. Ser. 247, 51–57. doi: 10.3354/meps247051
Thomas, F. I. M., Cornelisen, C. D., and Zande, J. M. (2000). Effects of water velocity and canopy morphology on ammonium uptake by seagrass communities. Ecology 81, 2704–2713. doi: 10.1890/0012-9658(2000)081[2704:EOWVAC]2.0.CO;2
Keywords: seagrass bed, Zostera noltii, nutrient uptake, flume, currents, waves
Citation: Gillis LG, Paul M and Bouma T (2017) Ammonium Uptake Rates in a Seagrass Bed under Combined Waves and Currents. Front. Mar. Sci. 4:207. doi: 10.3389/fmars.2017.00207
Received: 24 March 2017; Accepted: 15 June 2017;
Published: 28 June 2017.
Edited by:
Sandro Carniel, Consiglio Nazionale Delle Ricerche (CNR), ItalyReviewed by:
Ivica Vilibic, Institute of Oceanography and Fisheries, CroatiaVanesa Magar, Centro de Investigacion Cientifica y Educacion Superior de Ensenada (CICESE), Mexico
Copyright © 2017 Gillis, Paul and Bouma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lucy G. Gillis, lucy.gillis@leibniz-zmt.de
Maike Paul, m.paul@tu-braunschweig.de
 Lucy G. Gillis
Lucy G. Gillis Maike Paul2*
Maike Paul2*