Review of the Diversity, Ecology, and Conservation of Elasmobranchs in the Azores Region, Mid-North Atlantic
- 1Marine and Environmental Sciences Centre (MARE), Department of Oceanography and Fisheries, University of the Azores, Horta, Portugal
- 2Institute of Marine Research (IMAR), University of the Azores, Horta, Portugal
A vulnerable species group, such as, the elasmobranchs, in a data-deficient context presents a complicated management problem. Evidence suggests that the Azores islands, a remote archipelago on the Mid-Atlantic Ridge, serve essential functions in the life-history of species across taxa. The diversity of marine resources within its EEZ are exploited by local to international fleets, and the full extent of fishing pressure can often be underestimated. Although sharks and rays appear to be of minor importance in the fishery, the possibilities of illegal, unreported, and unregulated fishing raises concerns about these threatened species. However, this group has failed to attract management attention, visible in the lack of regional studies focused on biodiversity, ecology, or threats of elasmobranchs. Our work attempts to review and update the information on elasmobranchs of the Azores and identify potential threats, mainly by the local fisheries. We aim to highlight knowledge gaps that require further research and conservation actions. We (1) update the annotated checklist of elasmobranch species, (2) compare species distribution across a biogeographically similar section of the North Atlantic, and (3) analyze the interaction of elasmobranch species with local fisheries. We confirm 61 chondrichthyan species for the Azores (39 sharks, 17 rays, and 5 chimaeras), adding 19 species to the previous annotated checklist of 1997. The Azores elasmobranch species assemblage most resembles Madeira, the neighboring Macaronesian archipelago. Biogeographic affinities between the chosen regions of the North Atlantic are reflected in the taxonomic structure of families. Although underestimated in the local fisheries, elasmobranchs constitute a regular but highly variable portion of total landings. Misreporting and misidentification is perhaps the greatest concern in the local fisheries records, further aggravated by few existing catch regulations for elasmobranchs. Local knowledge indicates that the Azores serves as essential habitat for at least a few species in coastal areas and shallow seamounts, and potentially so for a number of deep-sea elasmobranchs. The intersection of fishery threats and local essential habitat functions around the archipelago warrants greater research effort and studies.
Introduction
The isolation of oceanic islands from continental masses often creates a unique gathering of species (Sandin et al., 2008; Kier et al., 2009; Kulbicki et al., 2013). Higher productivity regimes that are typically found around oceanic islands result in greater marine biodiversity compared with the surrounding open ocean (Doty and Oguri, 1956; Gove et al., 2016). Like for terrestrial fauna and flora (Patiño et al., 2015), islands can connect marine populations by forming ecological “stepping stones” across ocean basins (Joyeux et al., 2001; Hobbs et al., 2010). They often function as critical stops on the ontogenetic or annual migratory route of species, serving as important breeding (Carr et al., 1974; Olavarría et al., 2007) or feeding (Ashmole and Ashmole, 1967; Engel and Martin, 2009) grounds.
The unique traits of marine ecosystems around oceanic islands render them more vulnerable, deserving superior conservation and management. For instance, the populations and function of predatory species that play an essential role in maintaining ecological balance and ecosystem vitality. The top-down control of lower trophic-level species and ecosystem functions can be seriously disrupted in case of declines in predator populations (Pace et al., 1999). Elasmobranchs (sharks and rays) are a key group of marine predators, suspected to mediate trophic cascades as top or meso- predatory species (Myers et al., 2007; Baum and Worm, 2009; Heithaus et al., 2012). In recent years, these species have attracted increasing scientific concern due to the large declines in their population abundances (FAO, 1999; Baum and Myers, 2004; Ferretti et al., 2008, 2010) and a greater understanding of their ecological importance (Heupel et al., 2014) and high vulnerability to extinction risk (Camhi et al., 2008; García et al., 2008; Dulvy et al., 2014).
The main direct threat to elasmobranch species is fisheries exploitation, leading in some cases to extirpation and shifts in local species assemblages (Ward and Myers, 2005; Sguotti et al., 2016). These species exhibit density-dependent recruitment and K-strategy life history traits (Compagno, 1990; Hoenig and Gruber, 1990; Frisk et al., 2001) expressed in greater longevity, slower maturation, and lower fecundity. This makes them considerably more vulnerable to exploitation pressure than teleost counterparts. Elasmobranch species are often weakly regulated by fisheries management since they have little commercial significance, both in catch volume and monetary value (Bonfil, 1994; Stevens, 2000; Dulvy et al., 2008; Ferretti et al., 2010; Techera and Klein, 2011). Expansion and mechanization of fisheries over the last half-century marked a remarkable increase in fish catches (Swartz et al., 2010), sharks, and rays being no exception (Barker and Schluessel, 2005; Ferretti et al., 2010). Yet, elasmobranch catches and landings are not recorded in detail (Ferretti et al., 2010; Musick and Musick, 2011) obscuring the extent of threat to these species (Stevens, 2000; Dulvy et al., 2014).
The Azores are a group of remote oceanic islands situated on the Mid-Atlantic Ridge surrounded by depths regularly exceeding 1,500 m (Santos et al., 1995). These islands function as essential habitats for a variety of marine life. The dynamic bathymetry around the islands includes distinctive features such as, seamounts and ridges that harbor vulnerable marine ecosystems such as, cold-water coral gardens, hydrothermal vents, and deep-sea sponge aggregations (Abecasis et al., 2015; Pham et al., 2015). Evidence suggests that several charismatic species like marine birds (León et al., 2005), cetaceans (Silva et al., 2014), oceanic elasmobranchs (Afonso et al., 2014; Thorrold et al., 2014; Vandeperre et al., 2014b, 2016; Queiroz et al., 2016), and fishes (Druon et al., 2016) use the archipelago as a feeding, mating, or breeding ground. Large cetaceans (Silva et al., 2014; Tobeña et al., 2016) and fishes (Kohler et al., 1998; Hilborn et al., 2010) alike frequent these waters during their seasonal migrations.
The exclusive economic zone (EEZ) of the archipelago occupies an expansive one million square kilometers, where a 100 nautical miles (NM) buffer from the coast is currently reserved for the regional and national fleet beyond which other European fleets are authorized to operate (European Council, 2003). International and regional legislation also regulate the use of fishing nets in the area (European Council, 2005), banning the use of bottom and pelagic trawls within an area roughly coinciding with the EEZ (European Council, 1995). The local fishery is typically artisanal and essentially composed of small (<15 m) vessels using hooks and lines (longlines, handlines, pole-and-line; Pinho and Menezes, 2009; Carvalho et al., 2011; Morato, 2012). Gillnets and traps are limited to inshore coastal fishes and crustaceans. Despite the cautious nature of the local fishery, foreign pelagic longline vessels from Portuguese mainland and the EU operate unsupervised within the EEZ. These vessels land directly in mainland ports, without reporting Azorean catches to regional authorities (Pham et al., 2013). At the local scale, fishing activity is mostly concentrated around island slopes and seamounts (Pinho and Menezes, 2009), exposing non-target species with higher vulnerability to fisheries pressure (Morato et al., 2006). The true extent of fisheries extraction and its potential impact within the EEZ is thus greatly underestimated.
This situation raises conservation and management concerns for local shark and ray populations that are not always protected by catch regulations but are particularly susceptible owning to their life-history strategies (Afonso et al., 2014). Despite these concerns, a comprehensive overview of the elasmobranch assemblage and current threats, and the importance of this region for their populations has not been attempted, until now. Literature on elasmobranchs from the Azores is limited to a handful of species-specific accounts. Studies on ecology or biodiversity often overlook elasmobranchs as their primary focus. To address this knowledge gap, the current work has three main objectives: first, to provide an updated and annotated checklist of chondrichthyan species from the Azores region; second, to verify the uniqueness of the Azorean elasmobranch assemblage and resolve the biogeographical affinities with other comparable regions in the North Atlantic; and finally, to present an overview of potential vulnerabilities using information from local fisheries, regional extinction risk indices, and local ecological scientific knowledge.
Methods
Checklist
Using the last complete annotated checklist from the region (Santos et al., 1997) as our starting point, we reviewed the list of Chondrichthyes (Elasmobranchii and Holocephali) found in the Azores EEZ. Additions and deletions were made strictly using primary scientific data, where the species were identified directly by its authors. This included results from experimental fisheries surveys, underwater visual surveys and other published literature describing the biodiversity within the Azores EEZ. The results were compared with the FAO Fisheries Catalogue for the region (Ebert and Stehmann, 2013) as a measure of global knowledge about the region. Clarifications regarding taxonomy and species occurrence were sought from local experts where necessary.
The primary sources used were:
• Scientific fishing surveys using bottom longlines carried out by the Department of Oceanography and Fisheries, University of Azores (DOP/UAz), Horta (Menezes et al., 2006, 2012; Menezes and Giacomello, 2013; Menezes, 2014) around the nine islands and important seamounts in the Azores EEZ;
• The MAR-ECO project by the Census of Marine Life (Fossen et al., 2008; Wenneck et al., 2008), using vertical and bottom longlines deployed within the regional EEZ limits;
• An exploratory fishing survey of orange roughy (Hoplostethus atlanticus) in the Azores using trawl fishing (Melo and Menezes, 2002), important for species not caught using longlines;
• Underwater visual census conducted by researchers of the Institute of Marine Research (IMAR—University of the Azores) each year in the shallow-water areas around the islands and offshore seamounts (Afonso et al., 2013; Schmiing et al., 2013; Afonso, unpublished data).
Species were classified into four classes to designate their presumed frequency of occurrence. The different classes were differentiated by color codes as follows:
• Commonly encountered species were designated green—these species have multiple records and no known taxonomic conflicts;
• Species that occur occasionally around the Azores were yellow —they are rarely recorded despite susceptibility to commonly used fishing gears;
• Species with scant records, probably due to observational constraints were orange—these species only appear in fishing surveys using trawl gears (Melo and Menezes, 2002; Kukuev, 2006; Kukuev and Pavlov, 2008) or were captured in gillnets before the fishery was regulated (Azevedo et al., 2003; Fergusson et al., 2008);
• Species with uncertain identification were designated red.
Note on Systematic Arrangement
Elasmobranch taxonomy is a work in progress, with changes in nomenclature almost every year (Weigmann, 2016). Two classification schemes have predominated elasmobranch taxonomy (Naylor et al., 2005; Nelson, 2006). One places batoids (rays) as a sister group of dorsoventrally flattened sharks (sawsharks and angelsharks), which together are placed within the superorder Squalimorphii along with Squaliformes and Hexanchiformes (Compagno et al., 2005). The other, which considers Batoidea separated from the Selachii (all sharks; Naylor et al., 2005) and supported by recent molecular evidence (Heinicke et al., 2009; Naylor et al., 2012) is adopted here (see Weigmann, 2016 for details). Subclass Holocephali was included to provide a complete overview. The sequence of orders and families adopted here follows Nelson et al. (2016).
Elasmobranch Assemblage and Biogeography
The updated species list was then used to analyze the biogeographical relationship of the Azorean elasmobranch assemblage within the North Atlantic. In order to maintain analytical coherence through a broad comparable environmental envelope, we focused our analysis on the section of the Atlantic bounded by the 42°N (northern extent of Azores EEZ) and 26°N (south of the Canary Islands) latitudes, thus including the Macaronesian archipelagos of Madeira and the Canaries, and the continental margins of the Atlantic to the east (Iberian Peninsula) and west (United States east coast). This section of the North Atlantic was assumed to represent the highest potential biogeographic affinity of species present in the Azores. Tropical regions such as, the archipelago of Cape Verde and Caribbean islands were excluded being separate biogeographic regions (Floeter et al., 2008) and owing to the few highly-migratory species shared between these sections of the North Atlantic.
Species lists of the other regions were adapted from regional annotated checklists (Brito et al., 2002; Wirtz et al., 2008; Wirtz, 2011) and recently published literature [Freitas and Biscoito, 2007; Ferreira et al., 2008; Freitas et al., 2011; Kyne et al., 2012 (only species from NWA); Ebert and Stehmann, 2013; Delgado et al., 2017], augmented by local expert knowledge where possible (P. Pascual IEO/Canaries, pers. comm. 2017; M. Freitas OOM/Madeira, pers. comm. 2017). Updated, comprehensive, region-specific annotated checklists were often not available, leading to a certain probability of erroneous species records. However, these were not considered to significantly affect the overall biogeographic pattern.
First, we focused on the elasmobranch species occurring in the Azores and how they are shared with the study areas across the North Atlantic. We added current knowledge on migratory habits of these species (Fowler, 2014) to overlay the observed biogeographic patterns. Next, we built a presence-absence table of all species by region, and analyzed biogeographic similarities using cluster analysis. The dissimilarity matrix was constructed with binary distances, and unweighted agglomerative method (UPGMA) used to build the dendrogram. All calculations were executed in RStudio® software environment using the “base” package. The structure of elasmobranch assemblages divided by family was used to understand which species groups influence similarities and differences between regions.
Possible Threats and Vulnerabilities
To determine threats to elasmobranchs at the regional level, we focused on the landing records of local fisheries; since there is no clear evidence of habitat destruction, pollution, or other factors threatening the local elasmobranch assemblage. The goal was to identify gear types more likely to land elasmobranchs and the species landed by these gears. Analysis of the landing records also provides the opportunity to recognize potential inaccuracies in the registration process itself. Local fisheries began recording elasmobranch landings in greater taxonomic resolution in official statistics since the mid-1990s. Thus, we chose a 19-year period from 1996 until 2014 to analyze how the local fishery interacts with elasmobranchs, using temporally aggregated landing information.
Landing records are collected from the auction houses (lotas) or point-of-first sale from all nine islands of the archipelago. It is maintained by Lotaçor SA in collaboration with the Department of Oceanography and Fisheries of the University of the Azores. The dataset used in this study was extracted from the Lotaçor database, structured by year, month, gear type (“métier”), and weight landed (kilograms) of each landing within the study period. A subset of this data included the weight of elasmobranch species per landing. Elasmobranchs appeared in the database under their local names with corresponding documentation on scientific names (Lotaçor, 2015).
Having no direct information on catch or effort, we scaled the impact of different métiers using two indicators. The first indicator was the weight of elasmobranchs landed proportional to other species by each métier. The weight of non-elasmobranch species was used as proxy for effort since they are often the target or more lucrative species. Thus, métiers with higher proportional weight landed of elasmobranchs, i.e., weight per unit effort (WPUE), could be preferentially landing these species. The second indicator was the frequency of elasmobranch landings per métier, measured as a percentage of total landings. Using the total number of landings per métier as a proxy for effort, métiers with higher percentage of landings with elasmobranchs, or landings per unit effort (LPUE), would be those more partial to these species.
WPUE was calculated by aggregating the total weight and elasmobranch weight landed by each métier for every month in the study period, separately. The weight of other species was obtained by subtracting weight of elasmobranchs from the total weight, and the ratio obtained for landed weight of elasmobranchs to other species. The arithmetic mean of the ratios was used to reduce the influence of disproportionately large values, and standard deviation used as an indication of variability. Similarly for LPUE values, the total number of landings per métier and the number of landings with elasmobranchs were aggregated to obtain the frequency of elasmobranch landings. All calculations and data analysis was executed in RStudio®.
The métiers were then separated into two categories to facilitate meaningful comparisons. One category featured métiers with higher elasmobranch LPUE (both proportional weight and landing frequency), thus greater tendency to catch and land these species, and a second category for métiers that land less elasmobranchs. The species composition of the two groups was analyzed separately. We used average weight of species per landing to identify species that are landed infrequently but could require greater management attention.
The species landed by the local fisheries were then related to their regional IUCN Red List (Nieto et al., 2015) status as a proxy for potential conservation concern. This analysis was then complemented with an evaluation of information collected opportunistically and mostly available through gray literature or unpublished data on known and putative essential fish habitats (EFH), to preliminarily identify potential areas of greater overlap with fisheries.
Results
Annotated Species Checklist
Our literature search confirmed 61 chondrichthyans from the Azores EEZ (39 sharks, 17 batoids, and 5 chimaeras; Table 1), of which four species still require taxonomic clarification. The previous annotated checklist (Santos et al., 1997) included 44 species identified from the Azores. Thus, our study adds 19 species (40%) to that list and removes two species following taxonomic corrections. Six species presumed to commonly occur in the Azores did not feature or were considered uncertain in the earlier checklist. We compiled an annotated checklist of the species that do not appear in the main checklist of Santos et al. (1997), which is presented here. The species for which a declared first record could not be found were marked with an asterisk (*).
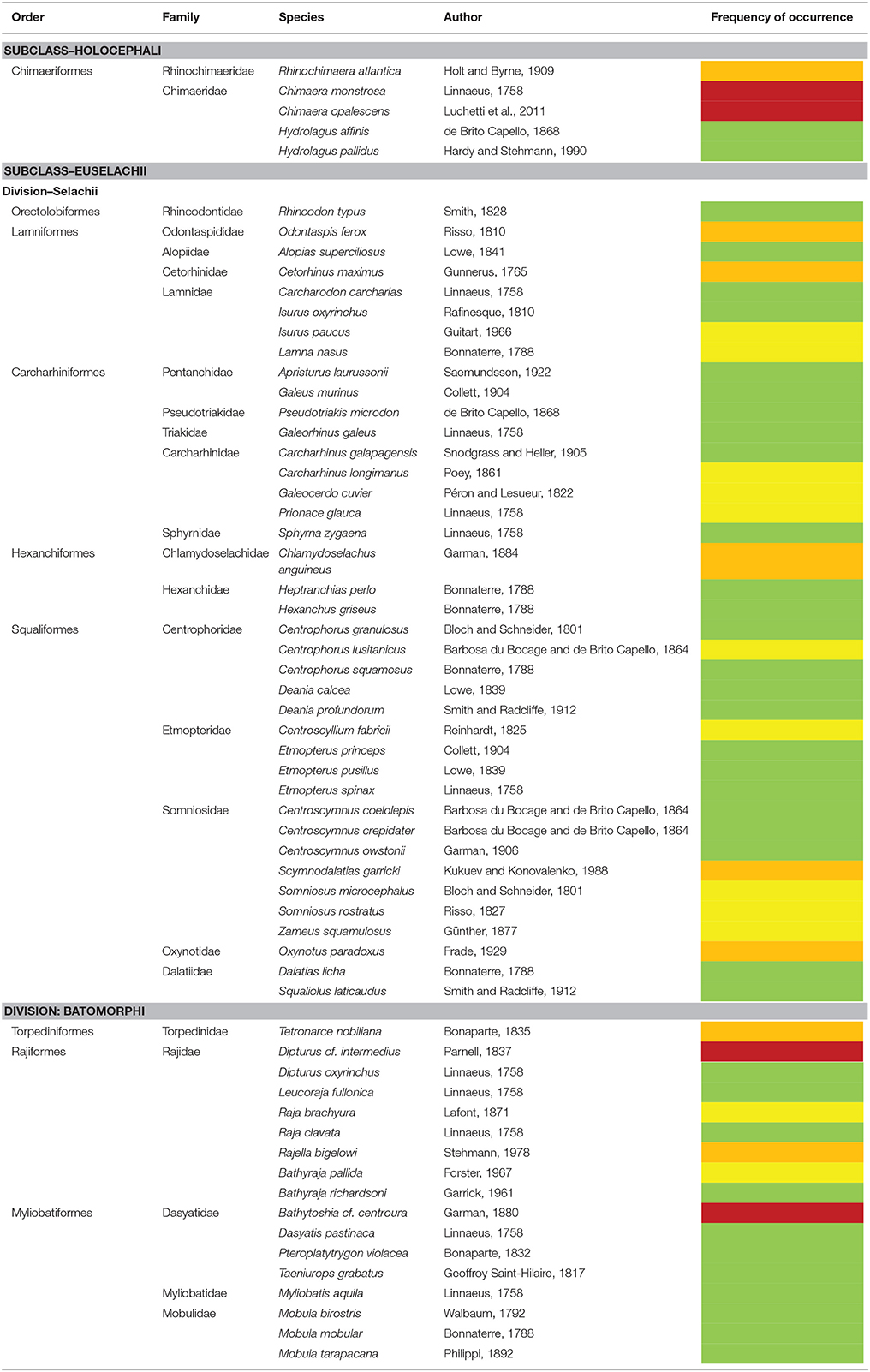
Table 1. Final species checklist with color codes; green: common, yellow: rare, orange: rare due to gear constraints, red: uncertain identification.
Elasmobranch Species Appearing in the Appendix to the Checklist in Santos et al. (1997) that are Confirmed in the Current Checklist
NB: The appendix in Santos et al. (1997) included: (i) species whose occurrence in the Azores needs further confirmation besides the references already found in the literature; (ii) species whose identification criteria are doubtful; (iii) littoral species whose occurrences are presumably exceptional; and/or (iv) species recorded at the outer limits of the Azorean EEZ.
Lamniformes
Alopiidae
Alopias superciliosus Lowe, 1841
Santos et al. (1997) report one specimen of this species recorded from south of the Azores EEZ, which is considered a first record of the species from the region. A 1999 study on shark bycatch from pelagic longline fisheries mentions the presence of this species based on the author's personal observations (Simões, 1999). The occurrence of this species in the region has since been confirmed by experimental pelagic longlines (Martins, 2013), and observer logbook data from commercial longlines operating in the region (Fernandez-Carvalho et al., 2015).
Frequency of occurrence: Green (common)
Record for Azores in FAO Fish Finder (Ebert and Stehmann, 2013): Yes
Depth range (Ebert and Stehmann, 2013): 300-500 m (day); 10-100 m (night)
Local gear susceptibility: Pelagic longlines
Local primary literature where species appears: (Simões, 1999; Martins, 2013).
Carcharhiniformes
Pentanchidae
Apristurus laurussonii (Saemundsson, 1922)
The first published record of A. maderensis, now synonymous with A. laurussonii (Eschmeyer et al., 2017), appears in an in-situ study by Saldanha and Biscoito (1997). This record was considered provisional by the authors due to difficulties in identifying members of genus Apristurus (Saldanha and Biscoito, 1997). The presence of this species was confirmed in Azores EEZ was during an experimental fishing expedition of Atlantic orange roughy (H. atlanticus) (Melo and Menezes, 2002; Menezes et al., 2012). Apristurus manis is also suspected to occur here (Melo and Menezes, 2002), however remains unconfirmed owing to the difficulty in identifying members of this genus (Gui Menezes IMAR/Azores, pers. comm. 2016).
Frequency of occurrence: Green (common)
Record for Azores in FAO Fish Finder (Ebert and Stehmann, 2013): No
Depth range (Ebert and Stehmann, 2013): 560–2,060 m
Local gear susceptibility: Bottom trawls
Local primary literature where species appears: Appears as A. maderensis or A. laurussonii (Saldanha and Biscoito, 1997; Melo and Menezes, 2002; Menezes et al., 2012).
Rajiformes
Rajidae
Dipturus cf. intermedius (Parnell, 1837)
Dipturus batis appears in the appendix of Santos et al. (1997) as requiring further documentation from the region. The presence of D. batis has since been confirmed by experimental fisheries studies (Menezes et al., 2006; Menezes and Giacomello, 2013; Menezes, 2014), and is regarded as “common” in the Azores (Menezes et al., 2006, suppl. mat.). In 2011, this species was revealed to be a cryptic species complex of two separate species D. flossada and D. intermedius (Iglésias et al., 2010). It is still uncertain which of the two species is present in Azores. Preliminary morphological studies indicate the species is probably Dipturus intermedius (G. Menezes IMAR/Azores, pers. comm. 2016). However, genetic studies are required to confirm this hypothesis.
Frequency of occurrence: Red (species identification uncertain)
Record for Azores in FAO Fish Finder (Ebert and Stehmann, 2013): Possible
Depth range (Ebert and Stehmann, 2013): 200–600 m (~1,500 m)
Local gear susceptibility: Bottom longlines
Local primary literature where species appears: Appears as D. batis (Menezes et al., 2006; Menezes and Giacomello, 2013)
Dipturus oxyrinchus (Linnaeus, 1758)
D. oxyrinchus featured in the appendix of Santos et al. (1997) owing to the lack of primary references from the Azores and only one dried specimen deposited at a regional museum. Experimental fishing and longline surveys confirm the presence of this species in the region.
Frequency of occurrence: Green (common)
Record for Azores in FAO Fish Finder (Ebert and Stehmann, 2013): No
Depth range (Ebert and Stehmann, 2013): 90–900 m
Local gear susceptibility: Bottom longlines
Local primary literature where species appears: (Menezes, 2003; Menezes et al., 2006; Menezes and Giacomello, 2013).
Myliobatiformes
Dasyatidae
Bathytoshia cf. centroura (Mitchill, 1815)
Two specimens of Dasyatis centroura are recorded in Santos et al. (1997), leading the authors to recommend further investigation of the species from the region. The presence of this species was confirmed as occurring frequently in the region (Afonso et al., 2013). This species was recently moved to the resurrected genus Bathytoshia (Last et al., 2016a,b). B. centroura is supposed to be present on the western margin of the Atlantic, while the species in north-east Atlantic are the brown stingray B. lata (Last P. et al., 2016). Considering the central position of the Azores, further investigation is required to ascertain whether B. lata or B. centroura is present in the region.
Frequency of occurrence: Red (species identification uncertain)
Record for Azores in FAO Fish Finder (Ebert and Stehmann, 2013): No (for D. centroura) (NB: No records of either B. lata or B. centroura from the region in Last P. et al., 2016)
Depth range (Ebert and Stehmann, 2013): Not Available
Local gear susceptibility: Bottom longlines?
Local primary literature where species appears: Confirmed for D. centroura (Afonso et al., 2013).
Elasmobranch Species That Do Not Appear in the Checklist Santos et al. (1997) That Are Confirmed in the Current Checklist
Chimaeriformes
Rhinochimaeridae
Rhinochimaera atlantica Holt and Byrne, 1909
The first published record of this species occurs in Melo and Menezes (2002) from an experimental bottom-trawl fishery survey.
Frequency of occurrence: Orange (observation constraint)
Record for Azores in FAO Fish Finder (Ebert and Stehmann, 2013): No
Depth range (Ebert and Stehmann, 2013): 400–1,500 m
Local gear susceptibility: Bottom trawls
Local primary literature where species appears: (Melo and Menezes, 2002; Menezes et al., 2012)
Chimaeridae
Chimaera opalescens* (Luchetti et al., 2011)
A recently described Holocephalan that is known only since 2011 (Luchetti et al., 2011). It is suspected that records of Chimaera monstrosa from the region might be misidentified C. opalescens (Diana Catarino IMAR/Azores, pers. comm. 2016). C. opalescens has been observed near seamounts in North-East Atlantic (Vieira and Cunha, 2014) but the presence of this species in the region is not yet confirmed in published literature.
Frequency of occurrence: Red (species identification uncertain)
Record for Azores in FAO Fish Finder (Ebert and Stehmann, 2013): Not available
Depth range (Ebert and Stehmann, 2013): Not available
Local gear susceptibility: ?
Local source: D. Catarino (IMAR/Azores) pers. comm. 2016.
Lamniformes
Lamnidae
Isurus paucus Guitart, 1966
The first published record of this species from the Mid-Atlantic Ridge is based on two specimens caught north-west of the Azores (Queiroz et al., 2008). This is a naturally rare species, with one study reporting catch ratio of one long-fin mako shark I. paucus caught for 364 short-fin mako sharks I. oxyrinchus (Mucientes et al., 2013) based on logbook data of commercial longliners. Within Azores EEZ, a regional study using pelagic longlines reports two specimens (Martins, 2013).
Frequency of occurrence: Yellow (rare)
Record for Azores in FAO Fish Finder (Ebert and Stehmann, 2013): Possible
Depth range (Ebert and Stehmann, 2013): 120–240 m
Local gear susceptibility: Pelagic longlines
Local primary literature where species appears: (Martins, 2013).
Carcharhiniformes
Pentanchidae
Galeus murinus (Collett, 1904)
First published record of this species in the Azores EEZ was a single specimen caught at a depth of around 1,000 m by longline surveys (Menezes et al., 2006). This species seems to have increased in occurrence in recent years (G. Menezes IMAR/Azores, pers. comm. 2016), and is well documented in local primary literature.
Frequency of occurrence: Green (common)
Record for Azores in FAO Fish Finder (Ebert and Stehmann, 2013): No
Depth range (Ebert and Stehmann, 2013): 380–1,250 m
Gear susceptibility: Bottom longlines
Local primary literature where species appears: (Menezes et al., 2006, 2012; Fossen et al., 2008; Menezes and Giacomello, 2013).
Hexanchiformes
Chlamydoselachidae
Chlamydoselachus anguineus Garman, 1884
This rare deepsea species was first recorded from the Azores when an experimental fishing expedition of Atlantic orange roughy (H. atlanticus) caught one individual (Melo and Menezes, 2002; Menezes et al., 2012). Another experimental fishing study reported a catch of 34 specimens in one haul from a seamount north of the Azores (Kukuev and Pavlov, 2008) confirming their occurrence on the MAR.
Frequency of occurrence: Orange (observation constraint)
Record for Azores in FAO Fish Finder (Ebert and Stehmann, 2013): No (yes for MAR)
Depth range (Ebert and Stehmann, 2013): 20–1,500 m
Local gear susceptibility: Bottom trawls
Local primary literature where species appears: (Melo and Menezes, 2002).
Squaliformes
Centrophoridae
Centrophorus lusitanicus* du Bocage and de Brito Capello, 1864 Barbosa
This is a poorly known and rarely sampled species, often confused with its congener C. granulosus (Veríssimo et al., 2014). A scientific survey of landing centers in mainland Portugal does not record the occurrence of C. lusitanicus (Veríssimo et al., 2014). Locally this species has not been caught in the scientific demersal fisheries surveys, but was recorded extensively in the local fisheries from 2009 to 2014. The presence of this species has only recently been confirmed from the Azores (F. Porteiro DRAM/Azores, pers. comm. 2017) but is presumably rare.
Frequency of occurrence: Yellow (rare)
Record for Azores in FAO Fish Finder (Ebert and Stehmann, 2013): No
Depth range (Ebert and Stehmann, 2013): 300–1,400 m
Local gear susceptibility: Bottom longlines
Local primary literature where species appears: Not available
Etmopteridae
Centroscyllium fabricii* (Reinhardt, 1825)
This species is rarely recorded in regional demersal fisheries surveys, but is confirmed to occur in the region.
Frequency of occurrence: Yellow (rare)
Record for Azores in FAO Fish Finder (Ebert and Stehmann, 2013): No
Depth range (Ebert and Stehmann, 2013): 300–1,400 m
Local gear susceptibility: Bottom longlines
Local primary literature where species appears: (Menezes and Giacomello, 2013; Menezes, 2014).
Somniosidae
Somniosus microcephalus* (Bloch and Schneider, 1801)
This cold-water species occurs infrequently in the Azores EEZ, with 2 individuals reported from bottom longline surveys north of the Azores (Fossen et al., 2008) and one individual captured in a bottom trawl survey (Menezes et al., 2012).
Frequency of occurrence: Yellow (rare)
Record for Azores in FAO Fish Finder (Ebert and Stehmann, 2013): No
Depth range (Ebert and Stehmann, 2013): < 1,400 m
Gear susceptibility: Bottom longlines and trawls
Local primary literature where species appears: (Fossen et al., 2008; Menezes et al., 2012).
Somniosus rostratus (Risso, 1827)
First published record from the Azores of one individual in Menezes et al. (2006). Rarely occurs in the region but confirmed by experimental fishing.
Frequency of occurrence: Yellow (rare)
Record for Azores in FAO Fish Finder (Ebert and Stehmann, 2013): No
Depth range (Ebert and Stehmann, 2013): 180–2,200 m
Local gear susceptibility: Bottom longlines
Local primary literature where species appears: (Wenneck et al., 2008; Menezes, 2014)
Zameus squamulosus* (Günther, 1877)
Synonymous with Scymnodon obscurus (Eschmeyer et al., 2017) which appears in regional demersal fisheries survey records.
Frequency of occurrence: Yellow (rare)
Record for Azores in FAO Fish Finder (Ebert and Stehmann, 2013): No
Depth range (Ebert and Stehmann, 2013): 300–1,400 m
Gear susceptibility: Bottom longlines and trawls
Local primary literature where species appears: Appears as S. obscurus (Menezes et al., 2012; Menezes, 2014).
Rajiformes
Arhynchobatidae
Bathyraja pallida (Forster, 1967)
This species is captured infrequently, known from only 12 specimens (Orlov et al., 2006). The first published record for the Mid-Atlantic Ridge appears in the MAR-ECO expedition, with two individuals captured north of the Azores (Fossen et al., 2008). It is confirmed to occur within the Azores EEZ (G. Menezes IMAR/Azores, pers. comm. 2016).
Frequency of occurrence: Yellow (rare)
Record for Azores in FAO Fish Finder (Ebert and Stehmann, 2013): Possible
Depth range (Ebert and Stehmann, 2013): 1,879–2,950 m
Local gear susceptibility: Bottom longlines
Local primary literature where species appears: (Fossen et al., 2008).
Bathyraja richardsoni (Garrick, 1961)
First published record from the region appears in bottom trawl survey for Atlantic orange roughy (Menezes et al., 2012). Very deep sea species (recorded between 2,050 and 2,200 m in Menezes et al. (2012), but appears to be common in the MAR region (Orlov et al., 2006).
Frequency of occurrence: Green (common)
Record for Azores in FAO Fish Finder (Ebert and Stehmann, 2013): Yes (for MAR)
Depth range (Ebert and Stehmann, 2013): 526–2,951 m (for MAR)
Local gear susceptibility: Bottom longlines
Local primary literature where species appears: (Fossen et al., 2008; Menezes et al., 2012).
Myliobatiformes
Myliobatidae
Mobula tarapacana (Philippi, 1892)
Mobula mobular was regularly recorded in the Azores until Sobral and Afonso (2014) rectified most of these sightings to actually be sickle-fin mobula ray (Mobula tarapacana) using photographic data. The authors found that this warm-water species is frequent during summer aggregations over shallow Azorean seamounts, whereas M. mobular in fact rarely occurs in the EEZ. The same authors noted that a clarification of sightings of the latter was needed given that M. mobular is thought to be restricted to the Mediterranean, while the closely-related M. japanica is circumglobal (Poortvliet et al., 2015). This was recently resolved to be a single species with the designation M. mobular based on molecular data (White and Last, 2012; White et al., 2017), and is thus here retained as the valid species for the region together with M. tarapacana.
Frequency of occurrence: Green (common)
Record for Azores in FAO Fish Finder (Ebert and Stehmann, 2013): Not available [NB: No records for the region for M. tarapanca and M. mobular (Last P. et al., 2016)]
Depth range (Ebert and Stehmann, 2013): Not Available
Gear susceptibility: ?
Local primary literature where species appears: (Sobral and Afonso, 2014).
Elasmobranch Species That Appear in the Checklist Santos et al. (1997) but Were Not Confirmed in the Current Checklist
Alopias vulpinus (Bonnaterre, 1788)
Records of Alopias superciliosus were quite probably misidentified as A. vulpinus. A shark bycatch study from pelagic longline fisheries states A. vulpinus reported in landings data, while the author personally records A. superciliosus (Simões, 1999). This species that is known to occur much closer to continental landmass has not been recorded in observer data or in pelagic longline experiments (P. Afonso, unpublished data).
Raja maderensis Lowe, 1838
Raja maderensis is reported to occur in the Azores (Santos et al., 1997; Weigmann, 2016) but genetic studies show that a morphotype of R. clavata, a species known to exhibit phenotypic plasticity (Aloncle, 1966; Chevolot et al., 2006), is present in these waters (Ball et al., 2016). Hence, records of R. madarensis are now considered R. clavata (G. Menezes IMAR/Azores, pers. comm. 2016).
Biogeographic Similarities
Since the distribution of C. opalescens, B. cf. centroura and D. cf. intermedius are still uncertain, the remaining 58 species were used to compare biogeographic affinities of the Azores with the rest of the North Atlantic. About one-third of elasmobranch species of the United States east coast occur in the Azores, while the archipelago shares more than half of the species of continental Europe (Figure 1). Elasmobranch species composition in the Azores was dominated by non-migratory species, majority of which were same as eastern North Atlantic. Migratory species that occur in the Azores were evenly present across the North Atlantic, with a slightly lower number of these species occurring in the Canaries.
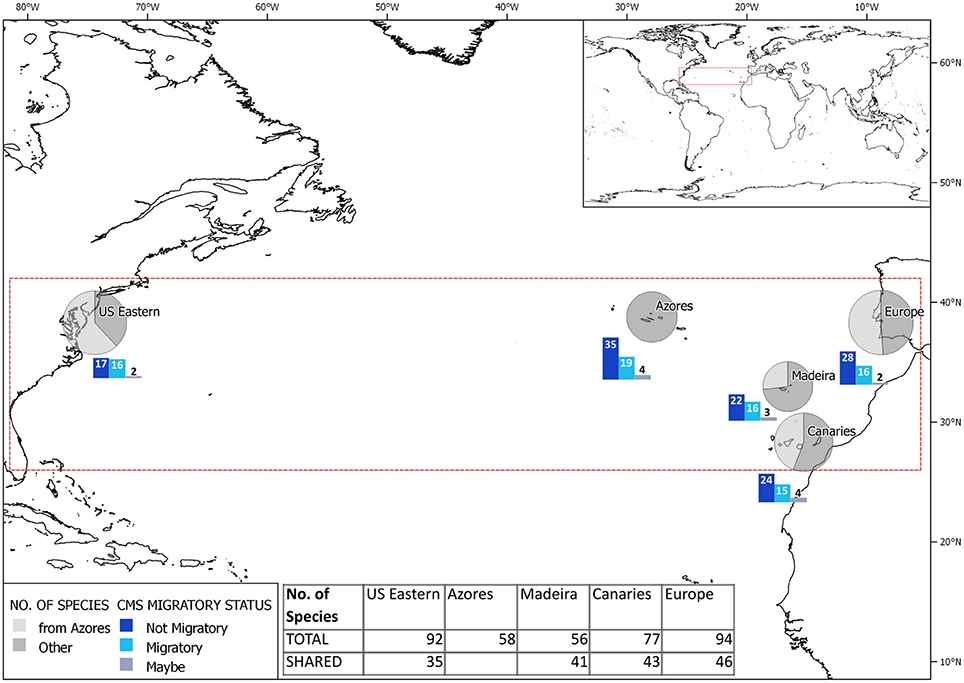
Figure 1. Distribution of species shared between the Azores and the study regions in the North Atlantic, and their currently known migratory status. The size of the circles are relative to total species diversity.
The elasmobranch assemblage was most similar between the Azores archipelago and Madeira (Figure 2), with Madeira sharing almost three-quarters of the species with the Azores (Figure 1). The diversity of elasmobranch families of these two regions was comparable as well (Figure 3). Deepwater Squaliformes dominated in species diversity in both regions. However, Madeira had a greater number of carcharhinids while Azores had a higher count of lamniforms.
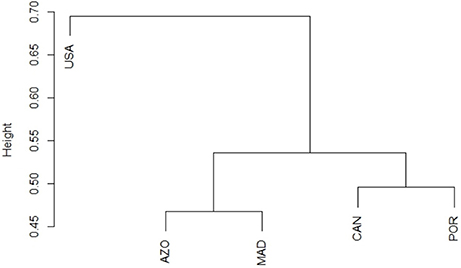
Figure 2. Similarity between biogeographic regions by cluster analysis; y-axis represents binary distances. USA, United States east coast; AZO, Azores; MAD, Madeira; CAN, Canaries; POR, Portugal continental shelf.
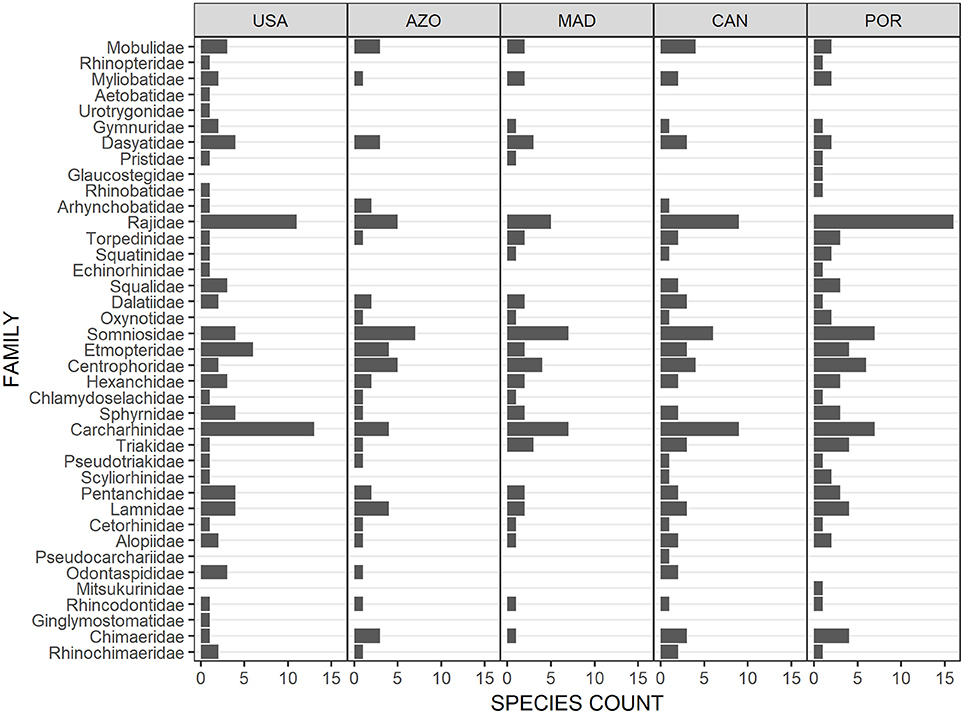
Figure 3. Configuration of elasmobranch diversity by families across the North Atlantic. USA, United States east coast; AZO, Azores; MAD, Madeira; CAN, Canaries; POR, Portugal continental shelf.
Not all archipelagos of the Macaronesian biogeographic unit clustered together; the Canaries and Portugal continental shelf formed a separate group (Figure 2). The species diversity (Figure 1) and composition of elasmobranch families (Figure 3) of the Canaries was more similar to mainland Portugal than to Madeira and Azores, even if family diversity was lower than the continental margin. Other evident points of difference were the greater diversity of families in the order Carcharhinidae and dominance of rajids on the continental margin as compared to the Canaries.
There was a clear separation between the elasmobranch species composition of the western and eastern halves of the North Atlantic (Figure 2). Elasmobranch assemblage structure by family indicated that the two continental margins had high, but contrasting species diversity. The western margin was dominated by requiem sharks (Carcharhinids) and a high diversity of Myliobatiformes (Dasyatidae to Urotrygonidae) (Figure 3). On the contrary, the eastern continental species assemblage was dominated by Rajiformes. Deepwater species also differ visibly between the eastern and western halves of the North Atlantic. In particular, Squaliformes (Centrophoridae to Squalidae) had strikingly low species diversity in the west compared to the central and eastern parts.
Local Threats and Vulnerabilities
Interactions with Local Fisheries
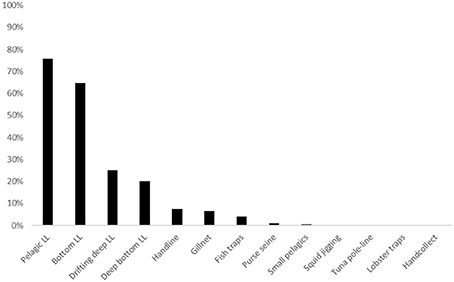
Local vessels operating within the Azores EEZ landed elasmobranchs regularly, but landings were highly variable within and across métiers. All longline gears had greater landings of elasmobranchs per unit effort (proportional weight and frequency), even when compared to other métiers using hooks such as handlines (Figure 4). The tuna pole and line fishery, also using hooks, had no elasmobranch landings.
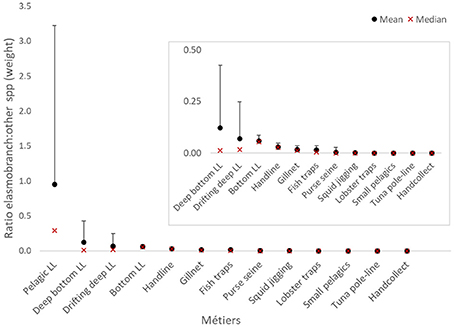
Figure 4. Proportional weight landed of elasmobranchs per unit weight of other species, by fishery métier. INSET, After removing métier pelagic longline.
Pelagic longlines landed by far the largest quantity, proportion (average 0.95 kg elasmobranch WPUE, Figure 4) and frequency (75.6% LPUE, Figure 5) of elasmobranchs. This proportion varied substantially with year, the maximum reaching 20.6 kg elasmobranch per kg of other species. Bottom longlines showed a comparable frequency of elasmobranch landings (64.7% LPUE) but landed smaller proportion weight (<0.1 kg elasmobranch WPUE). In comparison, both the deeper bottom and drifting deep longlines recorded only a quarter or less of the landings that included elasmobranchs.
Handlines, gillnets, and fish traps landed between 0.05 and 0.01 kg elasmobranch WPUE with less than 10% elasmobranch LPUE. Six of the 13 métiers recorded in the local fisheries database had minimal interaction with elasmobranchs (<0.01 WPUE, Figure 4; and <1% LPUE, Figure 5) and were excluded from further analyses.
Species Composition by Métier and Related Red List Status
The longline gears were analyzed separate from the other métiers for species composition by average weight per landing. The average weight per landing of most species was associated with a great degree of variability, deep bottom longline recorded the highest average weight (>1,500 kg/landing) for a species appearing as Centrophorus lusitanicus, categorized as Endangered in the European Red List (Nieto et al., 2015). The other species with high average weight per landing were Dalatias licha (611.5 kg/landing), Deania calcea (272.6 kg/landing), and Centrophorus granulosus (141.6 kg/landing; Figure 6A). The first two species have been categorized as Endangered by the regional Red List, while C. granulosus is listed as Critically Endangered. The landings of the pelagic longline was singularly dominated by blue shark Prionace glauca (Figure 6B; 753.4 kg/landing). However, this amount varied considerably along the study period. Other species like short-fin mako shark Isurus oxyrinchus were landed in much smaller quantities (147.3 kg/landing). The circumglobal big-eyed thresher shark Alopias superciliosus registered only one landing (154.3 kg). P. glauca is regionally assessed as Near Threatened, while I. oxyrinchus and A. superciliosus are assessed Data Deficient and Endangered, respectively. The bottom longline recorded the highest diversity of species landed (Figure 6C), with high but variable landing of C. lusitanicus (625.9 kg/landing). This was followed by a species recorded as Deania hystricosa (83.4 kg/landing) that does not appear in our regional species checklist and is listed as Data Deficient in the regional Red List. Elasmobranch landings of the drifting deep longline had lower average weight than the other longline gear, with maximum average weight of C. granulosus (398.9 kg/landing). Four of the eight species landed by this métier are recorded only once (Figure 6D).
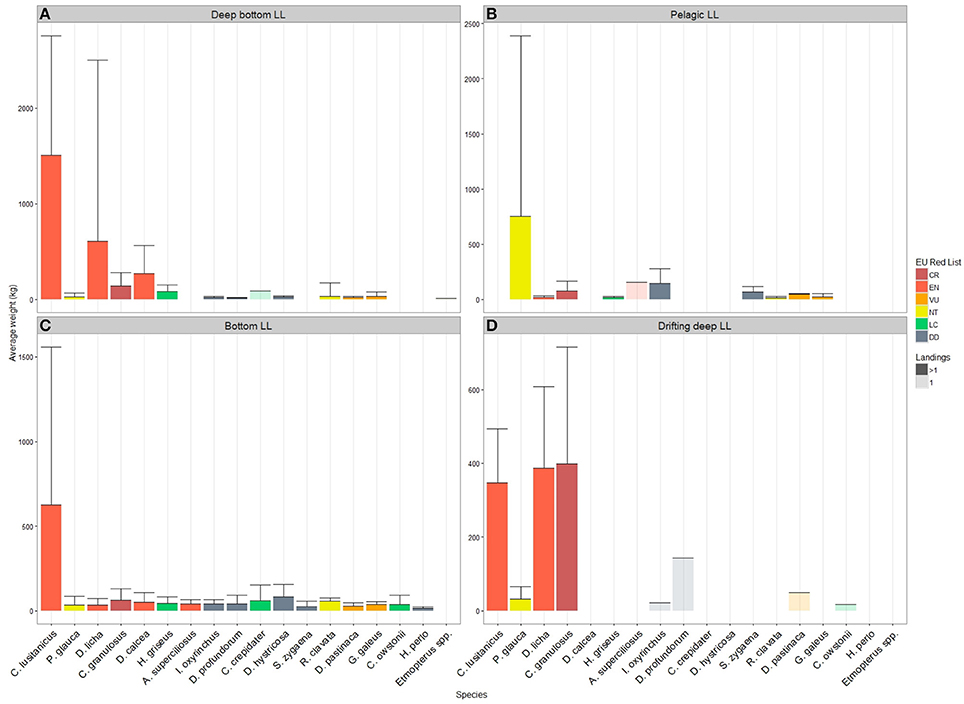
Figure 6. Species composition, by regional Red List status, and average weight per landing of métiers using longlines; semi-transparent bars for species with only one landing record. NB, Y-axis different for each panel. (A) Deep bottom longline, (B) Pelagic longline, (C) Bottom longline, (D) Drifting bottom longline.
The elasmobranch landings of the remaining métiers visibly differed in species composition from the longline métiers. Instead of a few species dominating landings, multiple species were landed in comparable quantities. Handlines landed the highest diversity of species, with slope species (e.g., genus Deania and Centrophorus) landed in greater average weight than coastal species (e.g., Dasyatis pastinaca, Raja clavata, Galeorhinus galeus). A one-time landing of a species with no corresponding Latin name (local name “sapata quilha,” tentatively designated as Deania spp.) was the maximum average weight of this métier (Figure 7A). The apex predator sixgill shark Hexanchus griseus had high average elasmobranch weight in handlines (174.5 kg/landing) and gillnets (53.9 kg/landing), but with substantial variance. Other species landed by gillnets included the coastal G. galeus, regionally Vulnerable (30.1 kg/landing) but also oceanic I. oxyrinchus (30.1 kg/landing), P. glauca (22.3 kg/landing) and Sphyrna zygaena, assessed DD, (15.3 kg/landing) (Figure 7B). Species such as tope shark G. galeus and thornback ray R. clavata, as well as mako shark I. oxyrinchus, were more important in average weight per elasmobranch landing of fish traps (Figure 7C).
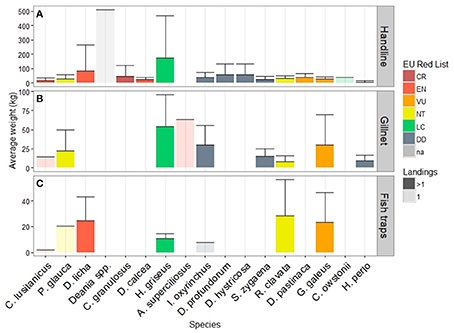
Figure 7. Species composition, by regional Red List status, and average weight per landing of remaing métiers (considering only landings with elasmobranch); semi-transparent bars for species with only one landing record. NB, Y-axis different for each panel. (A) Handline, (B) Gillnets, (C) Fish traps.
Vulnerability and EFHs
Azorean fisheries broadly target all the three main marine habitat types in the region: coastal (gillnets and handlines), island slopes and seamounts (handlines, drifting deep, and fixed bottom/deep bottom longlines) and pelagic (surface longlines). The sparse empirical information available suggests the presence of EFH for elasmobranchs in each of these three habitats, including nurseries and adult aggregation sites inshore and in the open-ocean, and deepwater ray egg deposition sites (J. Pichazek, pers comm. 2017). Blue sharks use the region's pelagic environment as a nursery for small juveniles that remain in the area for up to 2 years, and maybe even as a pupping ground (Vandeperre et al., 2014a,b, 2016). Sightings and long-term acoustic tagging data also show that smooth hammerhead shark S. zygaena uses the inshore coastal areas around some of the islands as nursery habitats (PA, unpublished data). Occasional sightings and incidental catches of pregnant females in the summer suggest that these nurseries are most possibly pupping grounds as well (PA, unpublished data). Similarly, juvenile tope sharks also aggregate in discrete coastal areas as revealed by occasional high catches in experimental fishing sets (G. Menezes pers. comm. 2016). Certain shallow seamounts are well-known aggregating sites for adult devil rays (M. tarapacana) that are observed over consecutive years (Sobral and Afonso, 2014). The function of these aggregations is yet unknown, but these could well be mating sites (Sobral, 2013). The deepwater frilled shark Chlamydoselachus anguineus is speculated to use seamounts in the Azores EEZ as a mating site (Kukuev and Pavlov, 2008), though this hypothesis is based on a single fishing haul.
Discussion
A Diverse Elasmobranch Assemblage Around a Remote Archipelago
The species composition of the chondrichthyan assemblage around the Azores is dominated by species that are either oceanic or deep-sea, reflecting the dominant marine habitat surrounding the archipelago. The fact that they are underwhelmingly known becomes evident in the lack or misrepresentation of species occurrence records in regional and global species catalogs. However, the Azorean elasmobranch assemblage emerges as more diverse than expected. Most of the new species additions are a result of nearly two decades of additional sampling effort and the use of different sampling gears. Species like the frilled shark C. anguineus and broadnose chimera Rhinochimaera atlantica, for example, were only obtained in experimental deep-water trawl nets (Melo and Menezes, 2002). More importantly, technological advancements over the last two decades and an increasing interest in the deep ocean (Costello et al., 2010) have allowed sampling at greater depths in more remote locations. Nevertheless, better known and accessible areas like coasts and nearby seamounts also revealed new additions. Evidence shows that morphologically similar species have been misidentified due to prevalent assumptions. For example, coastal stingrays are often identified as the common stingray D. pastinaca. A similar looking species, until now considered to be roughtail stingray D. centroura (Afonso et al., 2013), also occurs in the region. Recently, this species has undergone a reclassification to two different species (Last et al., 2016b), prompting the need to re-identify and reconsider the relative abundance of the two morphologically similar species present in the Azores EEZ. A similar story occurred for the devil rays M. tarapacana and M. mobular, and was resolved to yield unexpected results (Sobral and Afonso, 2014).
Continental margins are often richer in biodiversity because of their greater productivity and older geological age. It is thus not surprising that the elasmobranch species on the Azores archipelago are less diverse compared to the continental margins of the Atlantic, a trend common to bony fishes as well (Santos et al., 1995, 1997; e.g., Almada et al., 2013). Despite the low diversity, the Azores elasmobranch species assemblage have a wider provenance, especially when compared with the other Macaronesian archipelagos. This result may be explained by the fact that the Azores region is considered to represent a transition zone (ecotone) between cold and warm temperate waters, where the Gulf Stream provides a warmer input than would be normal at this latitude. This transitional signature explains why half the demersal fish (sensu lato, including elasmobranchs) assemblage is composed of species of subtropical origin (Menezes et al., 2006), and which also account for nearly 90% of the more rare fish occurrences (Afonso et al., 2013). Our results provide renewed support to this scenario by showing that the region falls in the boundary between the southern limit of cold-water species like deepwater skates (genus Bathyraja) and Greenland shark Somniosus microcephalus, and the northern limit of tropical and sub-tropical species like whale shark Rhincodon typus, sicklefin devil-ray M. tarapacana or round sting ray Taeniura grabata. The importance of this region as a fringe habitat is bound to increase with possible shifts in global distribution (Afonso et al., 2013), thus highlighting the need for continued monitoring of the elasmobranch species assemblage around the Azores EEZ.
Biogeographic studies showed that the Azores have greater proportion of coastal fish species from outside north-east Atlantic compared to the entire Lusitanian province (Almada et al., 2013). Shallow water fauna of the Azores also constitutes species with origins in the east as well as the west of the Atlantic basin (Santos et al., 1995). Similarly, the elasmobranch diversity in the Azores also claims different regional affiliations in the North Atlantic. Highly migratory sharks like blue and mako sharks travel from the eastern coast of North America to the Mid-Atlantic Ridge, close to the Azores (Vandeperre et al., 2014a, 2016; Queiroz et al., 2016). The Azores and associated Mid-Atlantic Ridge maybe an important migratory corridor and feeding ground for some pelagic elasmobranch species (Gore et al., 2008; Wögerbauer et al., 2015; Doherty et al., 2017). Conversely, the biogeographic pattern of non-migratory deep-sea sharks and rays seems to highlight the potential barrier posed by abyssal plains, as well as the possible role of seamounts as dispersal “stepping stones.” Comprehensive genetic and telemetry studies can shed light on the role of the wider Azores region in connecting both sides of the Atlantic basin for species of different habitat ecology.
Essential Fish Habitats and Interaction with Local Fisheries
Local fisheries in the Azores operate at a much smaller scale compared to mainland Portugal and other European fleets, where elasmobranch landings can be almost 20 times greater by weight (Correia et al., 2016). However, the interaction between elasmobranchs and the fishery is far from simple. A recent study of elasmobranch fisheries in the Azores identified four main species–tope shark, thornback ray, blue shark, and short-fin mako shark–to be important to the local fishery (Torres et al., 2016). This study overlooked other elasmobranch species that do not dominate official landing records by weight. Our results show that the local fishery in fact involves other species, landed sporadically but in considerable volume. These less frequent species demand greater management attention due to their higher extinction risk and, perhaps more importantly, high discard potential.
The Demersal Fisheries
The kite-fin shark D. licha was the only deep-sea elasmobranch commercially targeted by the local fishery, with landings to the tune of 900 t in the 1980s (ICES, 2015). The fishery has since declined, while it is still not certain whether the decrease in landings was due to falling population abundance or market demand. Within our study period, landings of species C. lusitanicus dominate by average weight, even though this species is known to be rare and is seldom encountered in landing centers (Veríssimo et al., 2014). The implementation of zero total allowable catch (TAC) of C. squamosus and C. granulosus by the European Commission in 2012 led to masking the landings of these species as C. lusitanicus in mainland Portugal (ICES, 2015; Correia et al., 2016). Our results suggest that similar misreporting occurred in the Azores as well.
Records from the deepwater drifting longline fishery targeting black scabbardfish are similarly riddled with inconsistencies. Observer reports from the experimental fishery in the Azores (Machete et al., 2011) allow a direct comparison of catch and reported landings between 1999 and 2005. The catch of elasmobranchs from the experimental fishery was dominated by C. squamosus (Machete et al., 2011, suppl. mat.), which does not even appear in the official landings. Instead, the largest landings of a deep-sea shark species from this fishery was attributed to C. lusitanicus.
Regionally abundant species such as the demersal lantern sharks Etmopterus spp. also do not appear in the landing records, however is locally the most abundant elasmobranch genus in fishing surveys (Wenneck et al., 2008; Menezes, 2014). Current discard estimates calculate that around 300 mt of this species group is discarded for every 10 kg recorded in landings (Pham et al., 2013). Evidence suggests that lanternshark species are declining due to commercial fishing in Southern Portugal, where they are frequently discarded as well (Coelho, 2007). The extent of unreported and unregulated fishing could seriously affect the population of these two species, assessed as near threatened (E. spinax) and data deficient (E. pussilus) for European populations (Nieto et al., 2015).
The landings of deepwater sharks present an interesting case influenced strongly by legislation as well as commercial value. Misreporting and unreported discards are notorious problems in landing records from deep-sea fisheries (Musick and Musick, 2011; ICES, 2015). This highlights the immediate need to account for the unreported and discarded catch from the demersal fisheries for deep-water sharks, in light of the planned implementation of the EU Landings Obligation act.
Oceanic Pelagic Fisheries
In the Azores EEZ, the fisheries pressure on blue shark is severely underestimated in local records, as a majority of the catches are landed in ports outside the Azores. Our results show that, within the local fishery, reported landings of blue shark can be 20 times higher than landings of other species by weight. These results do not consider the discard or unreported landings, estimated to be up to 80% more than reported landings (Pham et al., 2013).
Blue shark catch by surface longlines has generally not been considered a grave threat to the population status (Cortés et al., 2010; ICCAT, 2015). However, catch analysis from surface longlines in north-west Atlantic suggests that including the discard estimates in the assessments could “substantially change the perception of the population health” (Campana et al., 2006). This sentiment is echoed in the recent stock assessment by the International Commission on Conservation of Atlantic Tunas (ICCAT) that stressed the need to reconstruct discard estimates for a more reliable indication of the stock status (ICCAT, 2015). The known essential function of the wider Azores region as a nursery and juvenile habitat for blue shark (Vandeperre et al., 2014a,b, 2016) further exacerbates the problem, given that demographic analyses show that the productivity of this species is heavily dependent on the survival of juveniles (0–4 years; Aires-da-Silva and Gallucci, 2007). The intersection of these three facts emphasize the need to further examine susceptibility of the species within the EEZ to inform management actions.
Of the other species appearing in the pelagic fishery landings, short-fin mako is ranked second most vulnerable out of the 11 pelagic elasmobranch species in the North Atlantic, suggesting it is at high risk of overexploitation (Cortés et al., 2010). Additionally, both short-fin mako (Morato et al., 2010) and big-eye thresher A. superciliosus sharks (Litvinov, 2008) are known to associate with seamounts. The highly probable occurrence of oceanic essential fish habitat for these species and other oceanic sharks (e.g., the adult phase of the smooth hammerhead shark) around the Azores and MAR seamounts demands urgent studies.
Coastal Fisheries
Regional fisheries legislation prohibits the use of any longline gear within a buffer of 6 NM from the coast, but handlines are allowed to operate within buffer limits. This métier seems to opportunistically land elasmobranchs that constitute a regular, though small fraction of their total landings. However, landings from the gillnet fishery, though much smaller in quantity, require special attention. The gillnet fishery in the Azores is regulated by catch limits for both target and non-target species and only operates inshore since it is prohibited below 30 m deep (Portaria n.91, 2005). The “nearshore netting of questionable legality recently observed for tope shark, with small hammerhead sharks also being caught” reported Santos et al. (1995, p. 325) essentially continues with small quantities of tope and hammerhead sharks regularly reported in recent landings. The exclusively coastal nature of the fishery implies that they might overlap with suspected pupping and nursery areas of these sharks. This possibility urgently requires further study, and highlights the need to closely monitor this fishery, including discard practices. Hammerhead and tope sharks were recently assessed as Data Deficient and Vulnerable to extinction risk in the regional Red List, respectively. Both species are highly migratory and seen as having single stocks in the North or north-east Atlantic. This leads us to hypothesize that the putative essential fish habitats for both species in the Azores could have a significant contribution to the larger population.
Future Possibilities and Recommendations
Based on our results we propose three broad areas of further research that can help to further determine the importance of the wider Azores region for elasmobranchs.
The first data-gap that requires closer inspection are errors and omissions in local fishery data. Robust estimates on the extent of unreported/misreported and discarded catch are required to gauge the actual impact of the local fishery on regional stocks of sharks and rays. Since landings cannot provide a holistic overview of local population status, sentinel or fishery-independent surveys are also imperative to monitor the local elasmobranch populations. Some of these could use non-invasive techniques such as, baited remote cameras for coastal nurseries and deepwater sharks on slopes and seamount summits. Additionally, fisheries studies would benefit from an analysis of socio-economic incentives for landing or discarding of different species. This could provide clues regarding future exploitation trends. The second major gap is the lack of region-specific studies focused on the biology and ecology of local elasmobranchs. Finally, and perhaps most importantly, the essential habitat function of the Azores as nursery, mating, migration, feeding needs to be studied in detail, and across species and habitats. Resolving the importance of the Azores in the wider context of the Atlantic will need many more studies on connectivity of shark and ray populations with, or isolation from, other parts of the species distribution ranges. Population dynamics and tagging studies using electronic, genetic or chemical markers, for example, together with the continued monitoring of species occurrence, will further elucidate the importance of the region as an ontogenetic, permanent, or transitionary habitat, and help pinpoint critical habitats for eventual protection as well as future species range shifts in light of current climatic change scenarios.
Author Contributions
PA and DD have equally contributed to: conception and design of this study, data acquisition, analysis, and interpretation, drafting the manuscript and revising it critically for important intellectual content, final approval of the version to be published. Both authors agree to be fully accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of this study are appropriately investigated and resolved.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are very grateful to Dr. Gui Menezes (IMAR/DRAM, Azores, Portugal), Dr. Pedro Pascual (IEO, Canaries, Spain) and Dr. Mafalda Freitas (OOM/MARE, Madeira, Portugal) for contributing their time to correct the species list from their respective study areas. The authors would like to thank Ms. Dalia Reis (DOP/IMAR) for her help with obtaining the fisheries data, and Dr. Diana Catarino (DOP/IMAR) for pointing out the taxonomic conflict between C. monstrosa and C. opalescens. The authors also offer thanks to Dr. Eva Giacomello (DOP/IMAR) for her patience and help with finalizing the species checklist. Finally, we would like to thank the two reviewers who have shared their time and advice to critically improve this manuscript. This study had the support of Fundação para a Ciência e Tecnologia (FCT) through the strategic project UID/MAR/04292/2013 granted to MARE and the contract awarded to PA (SFRH/IF/01640/2015).
References
Abecasis, R. C., Afonso, P., Colaço, A., Longnecker, N., Clifton, J., Schmidt, L., et al. (2015). Marine conservation in the azores: evaluating marine protected area development in a remote Island context. Front. Mar. Sci. 2:104. doi: 10.3389/fmars.2015.00104
Afonso, P., Porteiro, F. M., Fontes, J., Tempera, F., Morato, T., Cardigos, F., et al. (2013). New and rare coastal fishes in the azores islands: occasional events or tropicalization process? J. Fish Biol. 83, 272–294. doi: 10.1111/jfb.12162
Afonso, P., Vandeperre, F., Fontes, J., and Porteiro, F. (2014). “Conservation of pelagic elasmobranchs in the Azores,” in The Sea of Azores: Scientific Forum for Decision Support, eds G. P. Carreira, R. M. Higgins, F. Cardigos and F. M. Porteiro (Azores: Arquipelago - Life and Marine Sciences. Supplement 8), 25–30.
Aires-da-Silva, A. M., and Gallucci, V. F. (2007). Demographic and risk analyses applied to management and conservation of the blue shark (Prionace glauca) in the North Atlantic Ocean. Mar. Freshw. Res. 58, 570–580. doi: 10.1071/MF06156
Almada, V. C., Toledo, J. F., Brito, A., Levy, A., Floeter, S. R., Robalo, J. I., et al. (2013). Complex origins of the Lusitania biogeographic province and northeastern Atlantic fishes. Front. Biogeogr. 5, 20–28. Available online at: http://escholarship.org/uc/item/8r69r6f6
Aloncle, H. (1966). A propos d'un caractère anatomique intéressant dans la détermination des Rajidae. Bulletin de l'Institut des Pêches Maritimes du Maroc. 14, 42–50.
Ashmole, N. P., and Ashmole, M. J. (1967). Comparative feeding ecology of sea birds of a tropical oceanic Island. Connecticut: Peabody Museum Nat. Hist. Bull. 24, 131.
Azevedo, J. M. N., Sousa, F. L., and Brum, J. M. M. (2003). Dermal denticles and morphometrics of the sailfin roughshark Oxynotus paradoxus (Elasmobranchii, Oxynotidae), with comments on its geographic distribution. Cybium 27, 117–122. Available online at: http://sfi.mnhn.fr/cybium/numeros/2003/272/06-Azevedo%20109.pdf
Ball, R. E., Serra-Pereira, B., Ellis, J., Genner, M. J., Iglésias, S., Johnson, A. F., et al. (2016). Resolving taxonomic uncertainty in vulnerable elasmobranchs: are the Madeira skate (Raja maderensis) and the thornback ray (Raja clavata) distinct species? Conserv. Genet. 17, 565–576. doi: 10.1007/s10592-015-0806-1
Barker, M. J., and Schluessel, V. (2005). Managing global shark fisheries: suggestions for prioritizing management strategies. Aquat. Conserv. Mar. Freshw. Ecosyst. 15, 325–347. doi: 10.1002/aqc.660
Baum, J. K., and Myers, R. (2004). Shifting baselines and the decline of pelagic sharks in the Gulf of Mexico. Ecol. Lett. 7, 135–145. doi: 10.1111/j.1461-0248.2003.00564.x
Baum, J. K., and Worm, B. (2009). Cascading top-down effects of changing oceanic predator abundances. J. Anim. Ecol. 78, 699–714. doi: 10.1111/j.1365-2656.2009.01531.x
Bonfil, R. (1994). Overview of World Elasmobranch Fisheries. FAO Fisheries Technical Paper. Available online at: http://www.fao.org/3/a-v3210e/index.html%5Cn; http://www.fao.org/documents/card/en/c/a1f94b92-d9a6-5971-9062-34dd9eb153f7/
Brito, A., Pascual, P. J., Falcón, J. M., Sancho, A., and González, G. (2002). Peces de las Islas Canarias: Catálogo Comentado e Ilustrado., ed F. LEMUS La Laguna: Francisco Lemus. Available online at: https://catalog.hathitrust.org/Record/008336765
Camhi, M. D., Pikitch, E. K., and Babcock, E. A. (2008). Sharks of the Open Ocean: Biolog, Fisheries and Conservation. Fisheries. Blackwell Publishing Ltd. Available online at: https://books.google.com/books?id=lc9MyMaXHgEC&pgis=1. doi: 10.1002/9781444302516
Campana, S., Marks, L., Joyce, W., and Kohler, N. (2006). Effects of recreational and commercial fishing on blue sharks (Prionace glauca) in Atlantic Canada, with inferences on the North Atlantic population. Can. J. Fish. Aquat. Sci. 63, 670–682. doi: 10.1139/f05-251
Carr, A., Ross, P., and Carr, S. (1974). Internesting behavior of the Green Turtle, Chelonia mydas, at a Mid-Ocean Island breeding ground. Copeia 1974, 703–706. doi: 10.2307/1442684
Carvalho, N., Edwards-Jones, G., and Isidro, E. (2011). Defining scale in fisheries: small versus large-scale fishing operations in the Azores. Fish. Res. 109, 360–369. doi: 10.1016/j.fishres.2011.03.006
Chevolot, M., Hoarau, G., Rijnsdorp, A. D., Stam, W. T., and Olsen, J. L. (2006). Phylogeography and population structure of thornback rays (Raja clavata L., Rajidae). Mol. Ecol. 15, 3693–3705. doi: 10.1111/j.1365-294X.2006.03043.x
Coelho, R. (2007). Biology, Population Dynamics, Management and Conservation of Deep Water Lantern Sharks, Etmopterus spinax and Etmopterus pusillus (Chondrichthyes: Etmopteridae) in Southern Portugal (Northeast Atlantic). Dissertation, Faculty of Marine Sciences and Environment. Faro: University of the Algarve.
Compagno, L. J. V., Didier, D. A., and Burgess, G. H. (2005). “Classification of chondrichthyan fish,” in Sharks, Rays and Chimaeras: The Status of the Chondrichthyan Fishes, eds S. L. Fowler, R. D. Cavanagh, M. Camhi, G. H. Burgess, G. M. Cailliet, S. V. Fordham, et al. (Gland: IUCN SSC Shark Specialist Group), 4–11.
Compagno, L. J. V. (1990). Alternative life-history styles of cartilaginous fishes in time and space. Environ. Biol. Fishes 28, 33–75. doi: 10.1007/978-94-009-2065-1_3
Correia, J. P., Morgado, F., Erzini, K., and Soares, A. M. V. M. (2016). Elasmobranch landings in the portuguese commercial fishery from 1986 to 2009. Arquipel. Life Mar. Sci. 33, 81–109. Available online at: http://hdl.handle.net/10400.3/3983
Cortés, E., Arocha, F., Beerkircher, L., Carvalho, F., Domingo, A., Heupel, M., et al. (2010). Ecological risk assessment of pelagic sharks caught in Atlantic pelagic longline fisheries. Aquat. Living Resour. 23, 25–34. doi: 10.1051/alr/2009044
Costello, M. J., Coll, M., Danovaro, R., Halpin, P., Ojaveer, H., and Miloslavich, P. (2010). A census of marine biodiversity knowledge, resources, and future challenges. PLoS ONE 5:e12110. doi: 10.1371/journal.pone.0012110
Delgado, J., Carvalho, D., Freitas, M., Biscoito, M., and De Gouveia, E. P. (2017). Records of some rare deep-sea fishes caught in the Lion, Susan, and Unicorn seamounts, off the archipelago of Madeira (East-central Atlantic). Acta Ichthyol. Piscat. 47, 91–96. doi: 10.3750/AIEP/02088
Doherty, P. D., Baxter, J. M., Gell, F. R., Godley, B. J., Graham, R. T., Hall, G., et al. (2017). Long-term satellite tracking reveals variable seasonal migration strategies of basking sharks in the north-east Atlantic. Sci. Rep. 7:42837. doi: 10.1038/srep42837
Doty, M. S., and Oguri, M. (1956). The Island mass effect. ICES J. Mar. Sci. 22, 33–37. doi: 10.1093/icesjms/22.1.33
Druon, J. N., Fromentin, J. M., Hanke, A. R., Arrizabalaga, H., Damalas, D., Ticina, V., et al. (2016). Habitat suitability of the Atlantic bluefin tuna by size class: an ecological niche approach. Prog. Oceanogr. 142, 30–46. doi: 10.1016/j.pocean.2016.01.002
Dulvy, N. K., Baum, J. K., Clarke, S., Compagno, L. J. V., Cortés, E., Domingo, A., et al. (2008). You can swim but you can't hide: the global status and conservation of oceanic pelagic sharks and rays. Aquat. Conserv. Mar. Freshw. Ecosyst. 18, 459–482. doi: 10.1002/aqc.975
Dulvy, N. K., Fowler, S. L., Musick, J. A., Cavanagh, R. D., Kyne, P. M., Harrison, L. R., et al. (2014). Extinction risk and conservation of the world's sharks and rays. Elife 3:e00590. doi: 10.7554/eLife.00590
Ebert, D. A., and Stehmann, M. F. W. (2013). Sharks, Batoids, and Chimaeras of the North Atlantic. FAO Species Catalogue for Fishery Purposes, 7. Rome: FAO, 523. Available online at: http://www.fao.org/docrep/017/i3178e/i3178e.pdf
Engel, M. H., and Martin, A. R. (2009). Feeding grounds of the western South Atlantic humpback whale population. Mar. Mammal Sci. 25, 964–969. doi: 10.1111/j.1748-7692.2009.00301.x
Eschmeyer, W., Fricke, R., and van der Laan, R. (2017). Catalog of Fishes: Genera, Species, References. Available online at: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (Accessed February 15, 2016).
European Council (1995). Council Regulation (EC) No 2027/95 of 15 June 1995 Establishing a System for the Management of Fishing Effort Relating to Certain Community Fishing Areas and Resources. European Council.
European Council (2003). Council Regulation (EC) No 1954/2003 of 4 November 2003 on the Management of the Fishing Effort Relating to Certain Community Fishing Areas and Resources and Modifying Regulation (EC) No 2847/93 and Repealing Regulations (EC) No 685/95 and (EC) No 2027/95. European Council.
European Council (2005). Council Regulation (EC) No 1568/2005 of 20 September 2005 Amending Regulation (EC) No 850/98 as Regards the Protection of Deep-Water Coral Reefs from the Effects of Fishing in Certain Areas of the Atlantic Ocean. Available online at: http://faolex.fao.org/docs/pdf/eur53886.pdf
FAO (1999). International Plan of Action for the Conservation and Management of Sharks. Rome: Food and Agriculture Organization of the United Nations
Fergusson, I. K., Graham, K. J., and Compagno, L. J. V. (2008). Distribution, abundance and biology of the smalltooth sandtiger shark Odontaspis ferox (Risso, 1810) (Lamniformes: Odontaspididae). Environ. Biol. Fishes 81, 207–228. doi: 10.1007/s10641-007-9193-x
Fernandez-Carvalho, J., Coelho, R., Mejuto, J., Cortés, E., Domingo, A., Yokawa, K., et al. (2015). Pan-Atlantic distribution patterns and reproductive biology of the bigeye thresher, Alopias superciliosus. Rev. Fish Biol. Fish. 25, 551–568. doi: 10.1007/s11160-015-9389-7
Ferreira, S., Sousa, R., Delgado, J., Carvalho, D., and Chada, T. (2008). Weight-length relationships for demersal fish species caught off the Madeira archipelago (eastern-central Atlantic). J. Appl. Ichthyol. 24, 93–95. doi: 10.1111/j.1439-0426.2007.01027.x
Ferretti, F., Myers, R., Serena, F., and Lotze, H. K. (2008). Loss of large predatory sharks from the Mediterranean Sea. Conserv. Biol. 22, 952–964. doi: 10.1111/j.1523-1739.2008.00938.x
Ferretti, F., Worm, B., Britten, G. L., Heithaus, M. R., and Lotze, H. K. (2010). Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 13, 1055–1071. doi: 10.1111/j.1461-0248.2010.01489.x
Floeter, S. R., Rocha, L. A., Robertson, D. R., Joyeux, J. C., Smith-Vaniz, W. F., Wirtz, P., et al. (2008). Atlantic reef fish biogeography and evolution. J. Biogeogr. 35, 22–47. doi: 10.1111/j.1365-2699.2007.01790.x
Fossen, I., Cotton, C. F., Bergstad, O. A., and Dyb, J. E. (2008). Species composition and distribution patterns of fishes captured by longlines on the Mid-Atlantic Ridge. Deep. Res. II Top. Stud. Oceanogr. 55, 203–217. doi: 10.1016/j.dsr2.2007.09.004
Freitas, M., Almeida, A. J., Delgado, J., González, J. A., Santana, J. I., and Biscoito, M. (2011). First record of Hydrolagus affinis (Holocephali: Chimaeriformes: Chimaeridae) from Madeira and the seine seamount (North Atlantic Ocean). Acta Ichthyol. Piscat. 41, 255–257. doi: 10.3750/AIP2011.41.3.14
Freitas, M., and Biscoito, M. (2007). Four Chondrichthyes New for the Archipelago of Madeira and Adjacent Seamounts. Bocagiana - Mus. Munic. do Funchal História Nat. 7. Available online at: http://dspace.cm-funchal.pt/bitstream/100/1483/1/Boc221-2007.pdf.
Frisk, M. G., Miller, T. J., and Fogarty, M. J. (2001). Estimation and analysis of biological parameters in elasmobranch fishes: a comparative life history study. Can. J. Fish. Aquat. Sci. 58, 969–981. doi: 10.1139/f01-051
García, V. B., Lucifora, L. O., and Myers, R. A. (2008). The importance of habitat and life history to extinction risk in sharks, skates, rays and chimaeras. Proc. R. Soc. B 275, 83–89. doi: 10.1098/rspb.2007.1295
Gore, M. A., Rowat, D., Hall, J., Gell, F. R., and Ormond, R. F. (2008). Transatlantic migration and deep mid-ocean diving by basking shark. Biol. Lett. 4, 395–398. doi: 10.1098/rsbl.2008.0147
Gove, J. M., McManus, M. A., Neuheimer, A. B., Polovina, J. J., Drazen, J. C., Smith, C. R., et al. (2016). Near-island biological hotspots in barren ocean basins. Nat. Commun. 7, 1–8. doi: 10.1038/ncomms10581
Heinicke, M. P., Naylor, G. J. P., and Hedges, S. B. (2009). “Cartilaginous fishes (Chondrichthyes),” in The Timetree of Life, eds S. B. Hedges and S. Kumar (Oxford University Press), 320–327. Available online at: http://php.scripts.psu.edu/dept/evobio/hedgeslab/pubs/216.pdf
Heithaus, M. R., Wirsing, A. J., and Dill, L. M. (2012). The ecological importance of intact top-predator populations: a synthesis of 15 years of research in a seagrass ecosystem. Mar. Freshw. Res. 63, 1039–1050. doi: 10.1071/MF12024
Heupel, M. R., Knip, D. M., Simpfendorfer, C. A., and Dulvy, N. K. (2014). Sizing up the ecological role of sharks as predators. Mar. Ecol. Prog. Ser. 495, 291–298. doi: 10.3354/meps10597
Hilborn, R., Galuardi, B., Royer, F., Golet, W., Logan, J., Neilson, J., et al. (2010). Complex migration routes of Atlantic bluefin tuna (Thunnus thynnus) question current population structure paradigm. Can. J. Fish. Aquat. Sci. 67, 966–976. doi: 10.1139/F10-033
Hobbs, J. P. A., Ayling, A. M., Choat, J. H., Gilligan, J. J., McDonald, C. A., Neilson, J., et al. (2010). New records of marine fishes illustrate the biogeographic importance of Christmas Island, Indian Ocean. Zootaxa 2422, 63–68. Available online at: http://www.mapress.com/j/zt/article/view/8072
Hoenig, J. M., and Gruber, S. H. (1990). “Life-History patterns in the elasmobranchs: implications for fisheries management,” in Elasmobranchs as Living Resources: Advances in the Biology, Ecology, Systematics, and the Status of the Fisheries, eds H. L. Pratt, S. H. Gruber, and T. Taniuchi (Springfield, VA: NOAA Technical Report NMFS 90), 1–15.
Iglésias, S. P., Toulhoat, L., and Sellos, D. Y. (2010). Taxonomic confusion and market mislabelling of threatened skates: important consequences for their conservation status. Aquat. Conserv. Mar. Freshw. Ecosyst. 20, 319–333. doi: 10.1002/aqc.1083
Joyeux, J., Floeter, S. R., Ferreira, C. E. L., and De, J. L. G. (2001). Biogeography of tropical reef fishes : the South Atlantic puzzle. J. Biogeogr. 28, 831–841. doi: 10.1046/j.1365-2699.2001.00602.x
Kier, G., Kreft, H., Lee, T. M., Jetz, W., Ibisch, P. L., Nowicki, C., et al. (2009). A global assessment of endemism and species richness across island and mainland regions. Proc. Natl. Acad. Sci. U.S.A. 106, 9322–9327. doi: 10.1073/pnas.0810306106
Kohler, N. E., Casey, J. G., and Turner, P. A. (1998). NMFS cooperative shark tagging program, 1962-93: an atlas of shark tag and recapture data. Mar. Fish. Rev. 60, 1–87.
Kukuev, E. I. (2006). The second capture of a rare shark Scymnodalatias garricki (Dalatiidae) on a rise of the South Azores Complex and additional data on the distribution of S. albicauda. J. Ichthyol. 46, 811–814. doi: 10.1134/S0032945206090153
Kukuev, E. I., and Pavlov, V. P. (2008). The first case of mass catch of a rare frill shark Chlamydoselachus anguineus over a seamount of the Mid-Atlantic Ridge. J. Ichthyol. 48, 676–678. doi: 10.1134/S0032945208080158
Kulbicki, M., Parravicini, V., Bellwood, D. R., Arias-Gonzàlez, E., Chabanet, P., Floeter, S. R., et al. (2013). Global biogeography of reef fishes: a hierarchical quantitative delineation of regions. PLoS ONE 8:e81847. doi: 10.1371/journal.pone.0081847
Kyne, P. M., Carlson, J. K., Ebert, D. A., Fordham, S. V., Bizzarro, J. J., Graham, R. T., et al. (2012). The Conservation Status of North American, Central American, and Caribbean Chondrichthyans. Vancouver, BC: IUCN Species Survival Commission Shark Specialist Group.
Last, P. R., de Carvalho, M. R., Corrigan, S., Naylor, G., Séret, B., and Yang, L. (2016a). “The rays of the world project - an explanation of nomenclatural decisions,” in Rays of the World: Supplementary Information, eds P. R. Last and G. K. Yearsley (Hobart, TAS: CSIRO Australian National Fish Collection), 1–10.
Last, P. R., Naylor, G. J. P., and Manjaji-Matsumoto, B. M. (2016b). A revised classification of the family Dasyatidae (Chondrichthyes: Myliobatiformes) based on new morphological and molecular insights. Zootaxa 4139, 345–368. doi: 10.11646/zootaxa.4139.3.2
Last, P., White, W. T., de Carvalho, M., Séret, B., Stehmann, M., and Naylor, G. (2016). Rays of the World. 1st Edn. Ithaca, NY: Cornell University Press.
León, A. D., Mínguez, E., and Aplicada, D. B. (2005). Factors affecting breeding distribution and seabird richness within the Azores archipelago. Atl. Seabirds 7, 15–22.
Litvinov, F. (2008). “Fish visitors to seamounts: aggregations of large pelagic sharks above seamounts,” in Seamounts: Ecology, Fisheries & Conservation, eds T. J. Pitche, T. Morato, P. J. B. Hart, M. R. Clark, N. Haggan, and R. S. Santos (Oxford, UK: Blackwell Publishing Ltd), 202–206.
Lotaçor, S. A. (2015). Lista espécies marinhas capturadas nos Açores e transacionadas na Lotaçor SA. 146. Available online at: http://www.lotacor.pt/docs/lista_especies_acores_lotacor.pdf (Accessed October 20, 2016).
Luchetti, E. A., Iglésias, S. P., and Sellos, D. Y. (2011). Chimaera opalescens n. sp., a new chimaeroid (Chondrichthyes: Holocephali) from the north-eastern Atlantic Ocean. J. Fish Biol. 79, 399–417. doi: 10.1111/j.1095-8649.2011.03027.x
Machete, M., Morato, T., and Menezes, G. (2011). Experimental fisheries for black scabbardfish (Aphanopus carbo) in the Azores, Northeast Atlantic. ICES J. Mar. Sci. 68, 302–308. doi: 10.1093/icesjms/fsq087
Martins, L. M. A. (2013). By-Catch de Espadarte (Xiphias gladius) e Tintureira (Prionace glauca) Juvenil no Palangre de Superfície. Master's thesis, Department of Oceanography and Fisheries. Horta: University of the Azores.
Melo, O., and Menezes, G. (2002). Exploratory Fishing of the Orange Roughy (Hoplostethus atlanticus) in Some Seamounts of the Azores archipelago. ICES C. 2002 M26 Theme Sess Oceanogr. Ecol. Seamounts Indic. Unique Ecosyst. 26, 11. Available online at: http://www.ices.dk/products/CMdocs/2002/M/M2602.PDF
Menezes, G. (2014). Demersais Survey in the Azores Between 1996 and 2013. Eur. Node Ocean Biogeogr. Inf. Syst. Available online at: https://doi.org/10.14284/22 [Accessed March 22, 2017].
Menezes, G. M. M. (2003). Demersal Fish Assemblages in the Atlantic Archipelagos of the Azores, Madeira, and Cape Verde Demersal Fish Assemblages in the Atlantic Archipelagos of the Azores. Dissertation, Department of Oceanography and Fisheries. Horta: University of the Azores.
Menezes, G. M., and Giacomello, E. (2013). Spatial and temporal variability of demersal fishes at condor seamount (Northeast Atlantic). Deep. Res. Part Top. Stud. Oceanogr. 98, 101–113. doi: 10.1016/j.dsr2.2013.08.010
Menezes, G. M., Rosa, A., Melo, O., and Porteiro, F. (2012). Annotated list of demersal fishes occurring at Sedlo Seamount, Azores north-east central Atlantic Ocean. J. Fish Biol. 81, 1003–1018. doi: 10.1111/j.1095-8649.2012.03355.x
Menezes, G. M., Sigler, M. F., Silva, H. M., and Pinho, M. R. (2006). Structure and zonation of demersal fish assemblages off the Azores Archipelago (mid-Atlantic). Mar. Ecol. Prog. Ser. 324, 241–260. doi: 10.3354/meps324241
Morato, T. (2012). Description of Environmental Issues, Fish Stocks and Fisheries in the EEZs Around the Azores and Madeira. Report for the European Commission, Directorate-General Maritime Affairs and Fisheries, B-1049 Brussels, Belgium. Available online at: https://stecf.jrc.ec.europa.eu/documents/43805/465474/Item+6.2+Report+Morato_Azores_Madeira.pdf
Morato, T., Cheung, W. W. L., and Pitcher, T. J. (2006). Vulnerability of seamount fish to fishing: fuzzy analysis of life-history attributes. J. Fish Biol. 68, 209–221. doi: 10.1111/j.0022-1112.2006.00894.x
Morato, T., Hoyle, S. D., Allain, V., and Nicol, S. J. (2010). Seamounts are hotspots of pelagic biodiversity in the open ocean. Proc. Natl. Acad. Sci. U.S.A. 107, 9707–9711. doi: 10.1073/pnas.0910290107
Mucientes, G., Ba-ón, R., and Queiroz, N. (2013). Updated distribution range of longfin mako Isurus paucus (Lamniformes: Lamnidae) in the North Atlantic. J. Appl. Ichthyol. 29, 1163–1165. doi: 10.1111/jai.12203
Musick, J. A., and Musick, S. (2011). Sharks. FAO Fisheries and Aquaculture Reviews and Studies. Rome: FAO. Available online at: ftp://ftp.fao.org/fi/document/reviews&studies/sharks.pdf
Myers, R. A., Baum, J. K., Shepherd, T. D., Powers, S. P., and Peterson, C. H. (2007). Cascading effects of the loss of apex predatory sharks from a Coastal Ocean. Science 315, 1846–1850. doi: 10.1126/science.1138657
Naylor, G. J. P., Ryburn, J. A., Fedrigo, O., and Lopez, A. (2005). “Phylogenetic relationships among the major lineages of modern Elasmobranchs,” in Reproductive Biology and Phylogeny of Chondrichthyes: Sharks, Batoids, and Chimaeras, Vol. 3, ed W. C. Hamlett (Enfield, NH: CRC Press), 1–25.
Naylor, G. J. P., Caira, J. N., Jensen, K., Rosana, K. A. M., Straube, N., and Lakner, C. (2012). “Elasmobranch phylogeny: a mitochondrial estimate based on 595 species,” in Biology of Sharks and Their Relatives, 2nd End., eds J. C. Carrier, J. A. Musick, and M. R. Heithaus (Boca Raton, FL: CRC Press), 31–56. doi: 10.1201/b11867-4
Nelson, J. S. (2006). Fishes of the World 4th Edn. Hoboken, NJ: John Wiley & Sons. Available online at: http://www.worldcat.org/title/fishes-of-the-world/oclc/28965588&referer=brief_results
Nelson, J., Grande, T., and Wilson, M. (2016). Classification of Fishes from Fishes of the World 5th Edn. Hoboken, NJ: John Wiley & Sons.
Nieto, A., Ralph, G. M., Comeros-Raynal, M. T., Kemp, J., Criado, M. G., Allen, D. J., et al. (2015). European Red List of Marine Fishes. IUCN European Regional Office IUCN Global Species Programme.
Olavarría, C., Baker, C. S., Garrigue, C., Poole, M., Hauser, N., Caballero, S., et al. (2007). Population structure of South Pacific humpback whales and the origin of the eastern Polynesian breeding grounds. Mar. Ecol. Prog. Ser. 330, 257–268. doi: 10.3354/meps330257
Orlov, A., Cotton, C., and Byrkjedal, I. (2006). Deepwater skates (Rajidae) collected during the 2004 cruises of R.V. “G.O. Sars” and M.S. “Loran” in the Mid-Atlantic Ridge area. Cybium 30, 35–48. Available online at: http://sfi.mnhn.fr/cybium/numeros/2006/304%20EEA/05.Orlov1.pdf
Pace, M. L., Cole, J. J., Carpenter, S. R., and Kitchell, J. F. (1999). Trophic cascades revealed in diverse ecosystems. Trends Ecol. Evol. 14, 483–488. doi: 10.1016/S0169-5347(99)01723-1
Patiño, J., Carine, M., Mardulyn, P., Devos, N., Mateo, R. G., González-Mancebo, J. M., et al. (2015). Approximate Bayesian computation reveals the Crucial role of Oceanic Islands for the assembly of continental biodiversity. Syst. Biol. 64, 579–589. doi: 10.1093/sysbio/syv013
Pham, C. K., Canha, A., Diogo, H., Pereira, J. G., Prieto, R., and Morato, T. (2013). Total marine fishery catch for the Azores (1950–2010). ICES J. Mar. Sci. 70, 564–577. doi: 10.1093/icesjms/fst024
Pham, C. K., Vandeperre, F., Menezes, G., Porteiro, F., Isidro, E., and Morato, T. (2015). The importance of deep-sea vulnerable marine ecosystems for demersal fish in the Azores. Deep. Res. Part Oceanogr. Res. Pap. 96, 80–88. doi: 10.1016/j.dsr.2014.11.004
Pinho, M. R., and Menezes, G. (2009). Pescaria de Demersais dos Açores (Demersal Fisheries of the Azores). Bol. do Núcleo Cult. da Horta 18, 85–102. Available online at: http://www.nch.pt/biblioteca-virtual/bol-nch18/Boletim_2009-p85.pdf
Poortvliet, M., Olsen, J. L., Croll, D. A., Bernardi, G., Newton, K., Kollias, S., et al. (2015). A dated molecular phylogeny of manta and devil rays (Mobulidae) based on mitogenome and nuclear sequences. Mol. Phylogenet. Evol. 83, 72–85. doi: 10.1016/j.ympev.2014.10.012
Portaria n.91 (2005). Regulamenta, na Região Autónoma dos Açores, a Pesca com Redes de Emalhar. Portaria n.91/2005, Série, N.° 51, de 22 de Dezembro de 2005. Horta: Secretária Regional da Agricultura e Pescas, Subsecretário das Pescas.
Queiroz, N., Araújo, S., Ribeiro, P. A., Tarroso, P., Xavier, R., and Santos, A. M. (2008). A first record of longfin mako, Isurus paucus, in the mid-North Atlantic. Mar. Biodivers. Rec. 1, 1–2. doi: 10.1017/S1755267206003484
Queiroz, N., Humphries, N. E., Mucientes, G., Hammerschlag, N., Lima, F. P., Scales, K. L., et al. (2016). Ocean-wide tracking of pelagic sharks reveals extent of overlap with longline fishing hotspots. Proc. Natl. Acad. Sci. U.S.A. 113, 1582–1587. doi: 10.1073/pnas.1510090113
Saldanha, L., and Biscoito, M. (1997). Fishes from the Lucky Strike and Menez Gwen hydrothermal vent sites (Mid-Atlantic Ridge). Bol. do Mus. Munic. do Funchal 49, 189–206.
Sandin, S. A., Vermeij, M. J. A., and Hurlbert, A. H. (2008). Island biogeography of Caribbean coral reef fish. Glob. Ecol. Biogeogr. 17, 770–777. doi: 10.1111/j.1466-8238.2008.00418.x
Santos, R. S., Porteiro, F. M., and Barreiros, J. P. (1997). Marine Fishes of the Azores - Annoted Checklist and Bibliography. Horta: Arquipélago - Life Mar. Sci., University of the Azores.
Santos, R., Hawkins, S., Monteiro, L. R., Alves, M., and Isidro, E. J. (1995). Marine research, resources and conservation in the Azores. Aquat. Conserv. Mar. Freshw. Ecosyst. 5, 311–354. doi: 10.1002/aqc.3270050406
Schmiing, M., Afonso, P., Tempera, F., and Santos, R. S. (2013). Predictive habitat modelling of reef fishes with contrasting trophic ecologies. Mar. Ecol. Prog. Ser. 474, 201–216. doi: 10.3354/meps10099
Sguotti, C., Lynam, C. P., García-Carreras, B., Ellis, J. R., and Engelhard, G. H. (2016). Distribution of skates and sharks in the North Sea: 112 years of change. Glob. Change Biol. 22, 2729–2743. doi: 10.1111/gcb.13316
Silva, M. A., Prieto, R., Cascão, I., Seabra, M. I., Machete, M., Baumgartner, M. F., et al. (2014). Spatial and temporal distribution of cetaceans in the mid-Atlantic waters around the Azores. Mar. Biol. Res. 10, 123–137. doi: 10.1080/17451000.2013.793814
Simões, P. R. (1999). By-catch of Swordfish fishery in the Azores from 1987-96; an annotation on shortfin Mako Shark and Blue Shark. Collect. Vol. Sci. Pap. ICCAT 49, 283–287.
Sobral, A. F. L. (2013). Biology, ecology and conservation of Mobulid rays in the Azores. Available online at: http://hdl.handle.net/10400.3/3081
Sobral, A. F. L., and Afonso, P. (2014). Occurrence of mobulids in the Azores, central North Atlantic. J. Mar. Biol. Assoc. U.K. 94, 1671–1675. doi: 10.1017/S0025315414000964
Stevens, J. (2000). The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J. Mar. Sci. 57, 476–494. doi: 10.1006/jmsc.2000.0724
Swartz, W., Sala, E., Tracey, S., Watson, R., and Pauly, D. (2010). The spatial expansion and ecological footprint of fisheries (1950 to present). PLoS ONE 5:e15143. doi: 10.1371/journal.pone.0015143
Techera, E. J., and Klein, N. (2011). Fragmented governance: reconciling legal strategies for shark conservation and management. Mar. Policy 35, 73–78. doi: 10.1016/j.marpol.2010.08.003
Thorrold, S. R., Afonso, P., Fontes, J., Braun, C. D., Santos, R. S., Skomal, G. B., et al. (2014). Extreme diving behaviour in devil rays links surface waters and the deep ocean. Nat. Commun. 5:4274. doi: 10.1038/ncomms5274
Tobeña, M., Prieto, R., Machete, M., and Silva, M. A. (2016). Modeling the potential distribution and richness of Cetaceans in the Azores from fisheries observer program Data. Front. Mar. Sci. 3:202. doi: 10.3389/FMARS.2016.00202
Torres, P., Tristão da Cunha, R., and Santos Rodrigues, A. dos (2016). The elasmobranch fisheries of the Azores. Mar. Policy 73, 108–118. doi: 10.1016/j.marpol.2016.07.027
Vandeperre, F., Aires-da-Silva, A., Fontes, J., Santos, M., Serrão Santos, R., and Afonso, P. (2014a). Movements of blue sharks (Prionace glauca) across their life history. PLoS ONE 9:e103538. doi: 10.1371/journal.pone.0103538
Vandeperre, F., Aires-da-Silva, A., Lennert-Cody, C., Serrão Santos, R., and Afonso, P. (2016). Essential pelagic habitat of juvenile blue shark (Prionace glauca) inferred from telemetry data. Limnol. Oceanogr. 61, 1605–1625. doi: 10.1002/lno.10321
Vandeperre, F., Aires-da-Silva, A., Santos, M., Ferreira, R., Bolten, A. B., Serrao Santos, R., et al. (2014b). Demography and ecology of blue shark (Prionace glauca) in the central North Atlantic. Fish Res. 153, 89–102. doi: 10.1016/j.fishres.2014.01.006
Veríssimo, A., Cotton, C. F., Buch, R. H., Guallart, J., and Burgess, G. H. (2014). Species diversity of the deep-water gulper sharks (Squaliformes: Centrophoridae: Centrophorus) in North Atlantic waters - current status and taxonomic issues. Zool. J. Linn. Soc. 172, 803–830. doi: 10.1111/zoj.12194
Vieira, R. P., and Cunha, M. R. (2014). In situ observation of chimaerid species in the Gorringe Bank: new distribution records for the north-east Atlantic Ocean. J. Fish Biol. 85, 927–932. doi: 10.1111/jfb.12444
Ward, P., and Myers, R. A. (2005). Shifts in open-ocean fish communities coinciding with the commencement of commercial fishing. Ecology 86, 835–847. doi: 10.1890/03-0746
Weigmann, S. (2016). Annotated checklist of the living sharks, batoids and chimaeras (Chondrichthyes) of the world, with a focus on biogeographical diversity. J. Fish Biol. 88, 837–1037. doi: 10.1111/jfb.12874
Wenneck, T. D. L., Falkenhaug, T., and Bergstad, O. A. (2008). Strategies, methods, and technologies adopted on the R.V. G.O. Sars MAR-ECO expedition to the mid-Atlantic Ridge in 2004. Deep-Sea Res. II. 55, 6–28. doi: 10.1016/j.dsr2.2007.09.017
White, W. T., and Last, P. R. (2012). A review of the taxonomy of chondrichthyan fishes: a modern perspective. J. Fish Biol. 80, 901–917. doi: 10.1111/j.1095-8649.2011.03192.x
White, W. T., Corrigan, S., Yang, L., Henderson, A. C., Bazinet, A. L., Swofford, D. L., et al. (2017). Phylogeny of the manta and devilrays (Chondrichthyes: mobulidae), with an updated taxonomic arrangement for the family. Zool. J. Linn. Soc. zlx018,1–26. doi: 10.1093/zoolinnean/zlx018
Wirtz, P., Fricke, R., and Biscoito, M. J. (2008). The coastal fishes of Madeira Islands – new records and an annotated check-list. Zootaxa 1715, 1–26. Available online at: http://www.mapress.com/j/zt/article/view/4562
Wögerbauer, C., O'Reilly, S., Doody, C., Green, P., and Roche, W. (2015). Recent data (2007-2013) from the Irish blue shark recreational fishery. Rep. ICCAT Blue Shark Stock Assess. Sess. 72, 1150–1166. Available online at: https://www.iccat.int/Documents/CVSP/CV072_2016/n_5/CV072051150.pdf
Keywords: Mid-Atlantic Ridge, vulnerable species, Elasmobranchii, annotated species checklist, fisheries, essential fish habitat
Citation: Das D and Afonso P (2017) Review of the Diversity, Ecology, and Conservation of Elasmobranchs in the Azores Region, Mid-North Atlantic. Front. Mar. Sci. 4:354. doi: 10.3389/fmars.2017.00354
Received: 07 November 2016; Accepted: 20 October 2017;
Published: 06 November 2017.
Edited by:
Maria Lourdes D. Palomares, FishBase Information and Research Group, PhilippinesReviewed by:
Bernard Seret, ICHTYO CONSULT, FranceJoão Pedro Santos Correia, Polytechnic Institute of Leiria, Portugal
Copyright © 2017 Das and Afonso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diya Das, diyadas.d@gmail.com
 Diya Das
Diya Das Pedro Afonso
Pedro Afonso