Marine Natural Products: New Avenue in Treatment of Osteoporosis
- 1Central Research Laboratory, D. G. Ruparel College, Mumbai, India
- 2Advanced Centre for Treatment, Research and Education in Cancer, Tata Memorial Centre, Kharghar, India
- 3Homi Bhabha National Institute, Mumbai, India
Bone metabolism is a physiological process that maintains the skeletal integrity and bone functions. Skeletal integrity is always balanced by two key cell types—bone resorbing osteoclasts and bone-forming osteoblasts. Imbalance between generation and function of osteoclasts and osteoblasts often leads to pathological conditions such as osteoporosis, osteopetrosis, Paget's disease. Osteoporosis is one of the most common age-related diseases characterized by decreased bone mineral density and microarchitectural deterioration. Current therapies are indeed effective in preventing bone loss but are also followed by side effects. Since many years, marine organisms have been considered as a good source of bioactive molecules or compounds with potential pharmaceutical properties. Marine Natural Products (MNPs) derived from various marine resources such as marine cyanobacteria, dinoflagellates, algae, sponges, soft corals, molluscs, fishes, and mangroves had shown profound effect on bone metabolism through inhibiting osteoclastogenesis and up-regulating osteoblastogenesis via modulating RANK/RANKL/OPG pathway. Amongst the pre-clinically investigated MNPs for management of osteoporosis, very few are under phase I clinical trials. This review discusses the currently available pharmacological drugs and there major health concern in osteoporosis treatment. It further gives an insight into various marine resources and marine-derived bioactive products, depicting their mechanism of action, functional role, and how these can be exploited for the treatment of osteoporosis.
Introduction
Osteoporosis is a silent disease in humans which leads to increased mortality and poor quality of life in aged people, particularly in post-menopausal women due to estrogen deficiency. Microarchitectural deterioration and low bone mass are the salient features of osteoporosis that trigger susceptibility to fractures (Randell et al., 1995; Raisz, 2005; Leboime et al., 2010). The balance between bone resorption and bone formation is a crucial aspect of maintaining bone integrity. Bone consists of organic protein matrix and inorganic hydroxyapatite. Throughout adult life, continuous bone remodeling brings about a removal of old bone followed by its replacement with new bone. Bone biology is significantly defined by two types of cells, osteoblasts, and osteoclasts. Osteoblasts are derived from multipotent mesenchymal stem cells that play a major role in mineralization and matrix deposition by expressing alkaline phosphatase, osteocalcin, and osteopontin. Osteoclasts are large multinucleated cells, derived from myeloid precursors and are capable of demineralizing bone, dentine and calcified cartilage (Cooper et al., 1992; Clarke, 2008). Formation and fusion of multinucleated osteoclasts rely on receptor activator of nuclear factor-κB (RANK) ligand and macrophage colony-stimulating factor (M-CSF). The balance between these two factors is essential to maintain the skeletal integrity and calcium metabolism (Das and Crockett, 2013). Overall, the process of bone formation and resorption are paired such that there is no net change in bone mass. The balance between bone resorption and bone formation is a crucial aspect of maintaining bone integrity. This metabolic bone turnover is also called as “Bone remodeling” measurably regulated by immune cells, pro-inflammatory cytokines, and number of other factors (Lorenzo et al., 2008; Murthy, 2011). Several research studies indicate that chronic heavy alcohol drinking, early menopause, and estrogen deficiency and other lifestyle factors like tobacco use, nutrition, strenuous exercise, low calcium intake, Vitamin D deficiency, increased body weight affects bone development, and genetic predisposition can lead to osteoporosis (Sampson, 2002).
Prevalence of Osteoporosis
Osteoporosis is regarded as a global health issue in elderly people especially in postmenopausal women. According to estimations, 200 million people worldwide are said to suffer from osteoporosis. Further, one in three women and one in five men aged over 50 will be diagnosed with osteoporotic fractures (Randell et al., 1995). In the United States and Europe, nearly 30% of the postmenopausal women have osteoporosis (Melton et al., 1992; Reginster and Burlet, 2006). Approximately 50 million people are affected by osteoporosis in Europe, Japan, and USA (Guglielmi, 2013). National Health and Nutrition Examination survey conducted in the United States revealed that a number of adults aged over 50 with low bone mass or osteoporosis will rise to approximately 54 million to 64.4 million by 2020 and 71.2 million by 2030 (Wright et al., 2014). Similarly, according to the multinational study conducted in Asian countries, the incidence of hip fractures has increased by 2–3 folds in elderly population due to major health problem like osteoporosis (Lau, 2009). India is the most affected country in the world where every one out of eight males and one out of three females suffer from this condition (Cosman et al., 2014).
Present Pharmacological Treatment Over Osteoporosis
An increased pathophysiological understanding of osteoporosis has led to the development of new treatment approaches targeting Bone Mineral Density (BMD), fractures, and bone turnover. Reports suggest only moderate effect of anti-osteoporotic drugs (e.g., Alendronate) in reducing the risk of vertebral and hip fractures while their effect on non-vertebral fractures remains further inadequate (Ro and Cooper, 2013). The treatment of osteoporosis involves two extensively used agents, anti-resorptive agents, and anabolic agents. Anti-resorptive agents mainly include bisphosphonates, Hormone Replacement Therapy (HRT), estrogen agonists, calcitonin, while parathyroid hormone (PTH), denosumab and strontium ranelate comprise the anabolic agents.
Bone Resorptive Agents
Bisphosphonates
Bisphosphonates are class of pyrophosphates which have a strong affinity with hydroxyapatite crystals. Because of this property, bisphosphonates possess high affinity for bone calcium, thus get localized to bone within a few minutes of administration and can remain there for years. Bisphosphonates induce apoptosis in osteoclast by inhibiting ATP dependent enzymes (1st and 2nd generation bisphosphonates) or by inhibiting cholesterol pathway (3rd generation nitrogen containing bisphosphonates, also known as aminobisphosphontes) (Wynn, 2005; Drake et al., 2008). Oral bisphosphonates are most prescribed first-line therapy for prevention of osteoporotic fractures throughout the world (Green et al., 2010). Bisphosphonates registered for osteoporosis treatment generally include alendronate, risedronate, ibandronate, and zoledronic acid. Bisphosphonates reduce vertebral fractures risk and increase BMD. Non-vertebral hip fractures are also effectively reduced by some of these bisphosphonates (Wynn, 2005; Caplan et al., 2010). Alendronate, being associated with increased BMD and fracture risk reduction, is extensively recommended as a drug for treatment of postmenopausal osteoporosis (Black et al., 2000). There is trepidation on prescription of oral bisphosphonates as it shows adverse effects on long term use which includes osteonecrosis of the jaw, severe musculoskeletal pain, and atrial fibrillation. On the basis of evidence published by world health organization, there is a double risk of developing esophageal cancer, associated with long-term consumption of oral bisphosphonates. Currently, Zoledronate is used as only treatment for metastasis associated increased bone resorption (Black et al., 2007; Abrahamsen et al., 2009; Reid and Cornish, 2011; Wolfe et al., 2013).
Hormone Replacement Therapy (HRT)
Hormone replacement therapy (HRT) is one amongst the effective treatments for postmenopausal osteoporosis and offers good protection for bone. HRT has been considered as a rational approach in postmenopausal women as it preserves and even increases BMD in lumbar spine, femoral neck, and forearm (Gambacciani and Levancini, 2014). HRT showed significant decrease in the number of hip and spine fractures compared with placebo (Cauley et al., 2003; The Women's Health Initiative Steering, 2004). Even in low doses, estrogen alone or along with other steroids (androgenic or estrogenic properties) has been shown to increase bone mass, but long term HRT has always been controversial and debatable issue. However, prolonged utilization of HRT may show side effects like, risks of cardiovascular events and thromboembolism, the development of breast, ovarian, and endometrial cancer. Therefore, HRT is no longer suggested for the prevention or treatment of postmenopausal osteoporosis (Bowring and Francis, 2011).
Estrogen Agonists
Selective estrogen receptor modulators (SERMs) are synthetic steroidal molecules that act as estrogen agonists and bind to estrogen receptors. These SERMs have a favorable effect on bone function (Lewis and Jordan, 2005). Raloxifene and tamoxifen are the representatives of SERMs (Lewis and Jordan, 2005). Daily consumption of raloxifene not only increases BMD in lumbar vertebrae and femur bone but also prevents estrogen receptor-positive breast cancer risk (Agnusdei and Iori, 2000). Major concern rose against the side effects of SERMs. Along with increased risk of fatal stroke and venous thromboembolic disease in postmenopausal women there is also an increase in thrombotic and thromboembolic events. However, SERMs should not be prescribed or used in bedridden patients who are at risk for thromboembolism (Grady et al., 2000; Barrett-Connor et al., 2006).
Calcitonin
Calcitonin, a polypeptide, binds to osteoclasts and prevents bone resorption. It essentially reduces blood calcium and inhibits resorption activity by suppressing osteoclast formation and attachment in bone (Kallio et al., 1972; de Paula and Rosen, 2010). Since 1980's, calcitonin from salmon having similarity with human calcitonin, is used for the treatment of osteoporosis. It is secreted by thyroid like gland of salmon having much higher intrinsic potency than human calcitonin. It has been approved by FDA for treatment of postmenopausal osteoporosis, Paget's disease as well as an adjunctive treatment for hypercalcemia (Sondergaard et al., 2010). Calcitonin is rapidly metabolized by kidney, blood, and peripheral tissues into inactive metabolites and destroyed by gastric acid. Adverse effects associated with calcitonin products are specific to the formulation, wherein injectable formulation may cause systemic hypersensitivity (Overgaard et al., 1995; Karsdal et al., 2007).
Bone Anabolic Agents
PTH is classically considered as an anabolic agent for the treatment of osteoporosis. It increases BMD by restoring trabecular microarchitecture. This anabolic group comprises of Human recombinant parathyroid hormone (hrPTH 1–84) and human recombinant PTH peptide 1–34 (teriparatide) (Blick et al., 2008). Teriparatide has been proven to have beneficial effect through reduction of vertebral and non-vertebral fractures as well as increased lumbar and femoral BMD. PTH basically stimulates pre-osteoblasts into mature bone-forming osteoblasts which eventually initiated collagen secretion and mineralization. Teriparatide treatment significantly increases lumbar spine BMD (6.6%) as compared to the control group (1.06%) (Senn et al., 2014). Previous findings suggest that Teriparatide induces at least 75% increase in bone forming biochemical markers like Type I collagen C-terminal propeptide, osteocalcin, and bone alkaline phosphatase, after treatment (Glover et al., 2009; Pleiner-Duxneuner et al., 2009). It has been observed that around 11% of the patients administered with teriparatide everyday developed hypercalcemia. Teriparatide increased the bone turnover, which might have mobilized stores of skeletal calcium and elevated renal tubular calcium resorption (Cheng and Gupta, 2012). Reports by public citizen's health research group USA, suggests that, 45% of the teriparatide treated rats expressed aggressive form of bone cancer, raising the risk of osteosarcoma in patients. In accordance with the side effect profile, hrPTH treatment leads to considerable incidences of hypercalcemia, hypercalciuria, and nausea (Verhaar and Lems, 2009).
Denosumab
Denosumab, a Food and Drug Administration (FDA) approved human Ig2 monoclonal antibody, is used to treat osteoporosis in postmenopausal women who are at the risk of fractures it functions similar to osteopterigin (OPG). Denosumab's mechanism of action is similar to that of osteopterigin (OPG). Both prevent the interaction of RANK ligand (RANKL) with its receptor present on surface of osteoclasts by binding with high affinity to RANKL. Therefore, Denosumab inhibits osteoclast differentiation and activity, resulting in rapid decrease in bone resorption (Cummings et al., 2009; Gehret, 2010). Kostenuik et al. have stated that administration of high doses of Denosumab in adult ovariectomized cynomolgus monkeys (up to 16 months) significantly improved the structural bone strength and bone mass (Kostenuik et al., 2011). In a randomized placebo-controlled and FREEDOM trial, Denosumab treatment to postmenopausal women with osteoporosis, caused significant reduction in risk of non-vertebral, vertebral, and hip fractures by 20, 68, and 40% (P < 0.001), respectively, however it increased the risk of cellulitis (Cummings et al., 2009; Bell and Bell, 2011; Törring, 2015). According to the literature, Denosumab worked better than risedronate, raloxifene, and alendronate, at diminishing the occurrence of vertebral fractures (Freemantle et al., 2013). Besides this, Teriparatide in combination with Denosumab enhanced BMD to a greater extent than either drug alone (Tsai et al., 2013). Research proves that postmenopausal women having low bone mass can be treated more effectively with Denosumab than with oral bisphosphonates (Miller et al., 2016; McClung, 2017).
Strontium Ranelate
Strontium ranelate is an antiresorptive and bone anabolic agent which has been approved for postmenopausal osteoporosis treatment in Europe (Cianferotti et al., 2013). Preliminary studies showed that strontium ranelate decreases osteoclast differentiation and function simultaneously promoting osteoblastogenesis and osteoblast activity. Strontium ranelate directly targets osteoblasts by inducing preosteoblast proliferation and augmenting osteoblast activity. This in turn increases bone matrix mineralization by expressing of several osteoblastic markers like type I collagen, alkaline phosphatase, bone sialoprotein and osteocalcin (Marie et al., 2011). It has recently been reported that strontium enhances osteogenesis both in multipotent C3H10T1/2 cells and in bone marrow stromal cells while inhibiting adipogenesis (Fournier et al., 2012; Li et al., 2012). Studies by Reginster et al. showed that postmenopausal women treated by strontium ranelate, exhibited higher OPG levels in serum, within a period of 3 months to 3 years after treatment initiation (Reginster et al., 2012) Collectively, strontium ranelate increases bone mass, mineral content, cortical thickness, and also improves trabecular and cortical micro-architecture, thereby increasing bone quality and strength in rats, ovariectomized rodents, and immobility-induced osteoporotic animal models (Ammann et al., 2004).
Marine Environment as a Source of Marine Natural Products
Ocean covers 70% of earth's surface and contains extraordinary diversified marine life. Because of the extreme conditions and complex habitats of marine organisms, wide varieties of biologically active secondary metabolites can be produced which are unique in structure and functions. Several compounds with promising biological and pharmacological activities derived from Marine Natural Products (MNPs) have been investigated in the past two decades. The ocean contains rich resources of novel compounds with enormous prospective pharmaceuticals, nutraceuticals, cosmetics, agrochemicals, and enzymes (Faulkner, 2002). Marine plants, algae, microorganisms and invertebrates such as sponges, tunicates, bryozoans, molluscs, and fishes are possible sources of bioactive compounds. MNP's such as steroids, terpenoids, isoprenoids, nonisoprenoids, quinones, brominated compounds, nitrogen heterocyclics, and nitrogen sulfur heterocyclics have potential to be bioactive compounds. These compounds are being used for treating several diseases like cancer, tuberculosis, malaria, osteoporosis, Alzheimer's disease, neurological disorders, inflammatory diseases, and HIV (Datta et al., 2015). Over the past decade, beyond 13,000 molecules have already been described out of which 5,000 exhibited good bioactive properties.
Marine Resources, Derived Marine Natural Products, and Bone Metabolism
Nowadays, finding the suitable treatment for bone-related diseases is a matter of great concern. Current therapies are indeed effective in preventing bone loss but with adverse side effects. Since natural products show fewer side effects and are more suitable for long-term use, they are rapidly replacing synthetic drugs. It has been found that several marine extracts or marine-derived bioactive compounds have a profound effect on regulation of bone metabolism through decreasing trabecular separation and enhancing bone mass, trabecular bone volume, number, and thickness (Senthilkumar et al., 2014). In this context, MNPs could be beneficial for the treatment of bone-related diseases and regulate bone metabolism.
Marine Cyanobacteria
Biselyngbyaside (Figure 1A) is a glycosylated 18 membered macrolide compound conjugated with 1,3 diene, two olefins and a side chain at C 17 derived from marine cyanobacteria Lyngbya spp. It has been reported that Biselyngbyaside is a potent inhibitor of Ca2+ pump with unique binding capacity (Teruya et al., 2009; Morita et al., 2015). It has an inhibitory effect on primary bone marrow macrophages as well as RANKL induced osteoclastogenesis in murine RAW 264.7 cells at low concentrations and also suppresses osteoblast-mediated osteoclast differentiation in cocultures. Biselyngbyaside induces apoptosis by caspase 3 activation and nuclear condensation and also inhibits expression of c-fos and NFATc1 (Yonezawa et al., 2012). A cyclic depsipeptide, largazole (Figure 1B) isolated by Luesch and co-workers from a marine cyanobacterium Symploca spp. exhibits in vitro and in vivo osteogenic activity by its histone deacetylases (HDACs) inhibition. Largazole is a 16 membered macrocycle possessing unusual structural features, includes a substituted 4-methylthiazoline linearly fused to a thiazole and a 3-hydroxy-7-mercaptohept-4-enoic acid unit (Bowers et al., 2008). Biological evaluation suggests that largazole and its key analogs are class I HDAC inhibitor. Due to the inhibitory effect of largazole on HDAC's the increased Runx2 activation triggers osteoblast differentiation which in turn increases bone formation, repair and regeneration (Lee et al., 2011). Due to its dual action of stimulating bone formation and inhibiting bone resorption, largazole has shown significant bone forming efficacy in in-vivo mouse calvarial bone formation assay and rabbit calvarial bone fracture healing model (Lee et al., 2011).
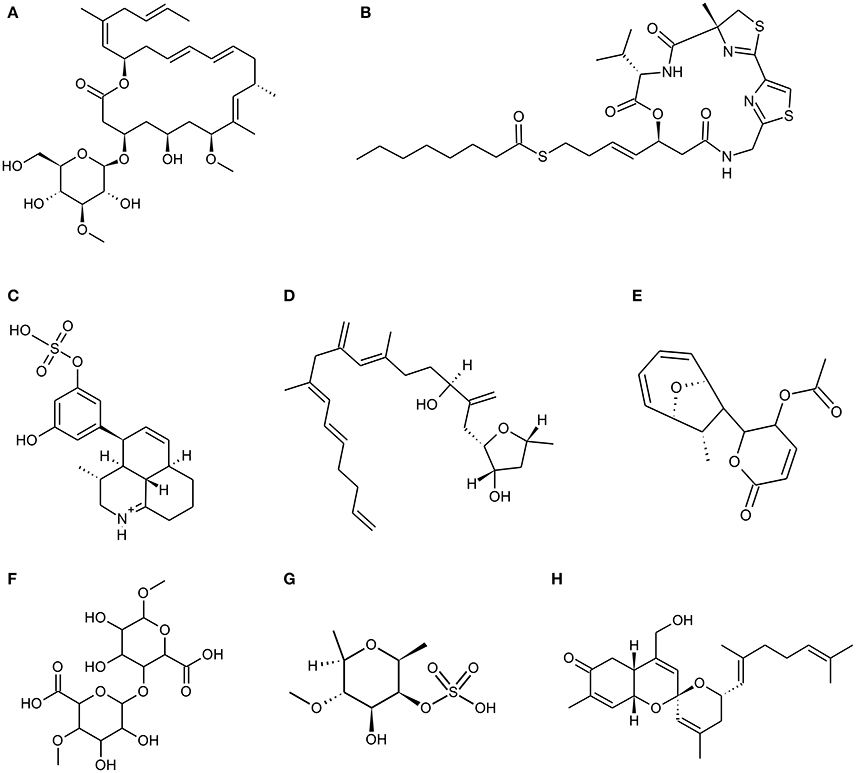
Figure 1. Chemical Structure of marine natural compounds isolated from marine resources. (A) Biselyngbyaside; (B) Largazole; (C) Symbioimine; (D) Amphirionin-4; (E) Mycoepoxydiene; (F) Alginate (homopolymeric blocks of (1–4)-linked β-D-mannuronate); (G) Fucoidan (sulphated fucan); (H) Phorbaketal A.
Marine Dinoflagellates
An amphoteric metabolite, Symbioimine (Figure 1C) having characteristic 6,6,6-tricyclic iminium ring structure and an aryl sulfate moiety was isolated from symbiotic marine dinoflagellate Symbiodinium spp. It inhibited the differentiation of RAW 264.7 cells into osteoclasts thus considered as potent anti-resorptive drug for the treatment and prevention of post-menopausal osteoporosis (Kita et al., 2004). The escalation in proliferation rate was observed in murine osteoblastic MC3T3-E1 cells and murine bone-marrow derived stromal ST-2 cells due to a novel linear polyketide Amphirionin-4 (Figure 1D) derived from a cultured marine dinoflagellate Amphidinium species (Akakabe et al., 2014). Amphirionin-4 (Figure 1D) consists of a linear C22 carbon chain with four C1 branches (Minamida et al., 2014).
Marine Fungi
Various marine fungal strains are known to produce polyketides which show various biological activities. A polyketide Mycoepoxydiene (MED) (Figure 1E) has been isolated from the mangrove associated fungal strain Diaporthe spp. HLY-1. Mycoepoxydiene (MED), is composed of a α, β-unsaturated δ-lactone moiety and polyketide backbone with an oxygen-bridged cyclooctadiene core (Wang et al., 2010). MED has shown anti-microbial, anti-cancer and anti-inflammatory activities (Takao et al., 2002; Lin et al., 2005). MED also helps in reducing bone loss by suppressing receptor activator of nuclear factor kappa-B ligand (RANKL)-induced osteoclast differentiation and NF-κB activation in mice. It is also observed that MED shows its effect at early stage of osteoclast differentiation (Asagiri and Takayanagi, 2007; Zhu et al., 2013).
Marine Algae
Padina pavonica extract (EPP) is the key ingredient of dictyol, which is used as a dietary supplement against osteoporosis. EPP increases the uptake and fixation of calcium by osteoblasts in vitro, simultaneously acting as a potent phytoestrogen increasing bone collagen status and bone density (Galea et al., 2000). An anabolic agent from Sargassum horneri has shown osteoblastic bone formation properties and inhibitory effects on bone resorption. The anabolic agents from the extract are basically a peptide and a polysaccharide having bone formation and anti-resorption properties respectively thus, making it an ideal extract for drug development (Uchiyama and Yamaguchi, 2002; Yamaguchi, 2013). S. horneri extract has a preventive effect on bone loss in ovariectomized animal models and in the healthy human. It also prevents bone loss associated with aging and pathologic condition (Yamaguchi, 2013). Chitin, chitosan, agar, alginate, carrageenan, and fucoidan are some of the medically significant marine algal derived polysaccharides. Alginate (Figure 1F) and fucoidan (Figure 1G) has an important role in bone tissue engineering. Alginate is a family of linear copolymers containing blocks of (1,4)-linked β-D-mannuronate (M) and α-L-guluronate (G) residues and potential biomaterial in the field of tissue engineering, drug delivery, wound healing, and cell culture (Lee and Mooney, 2012). Alginate gels are preferred over other materials for cartilage and bone regeneration since, it can be introduced into the body in a minimally invasive manner and its ease of chemical modification with adhesion ligands and controlled release of tissue induction factors such as bone morphogenetic protein (BMP), Tra TGF-β (transforming growth factor beta) (Lee and Mooney, 2012). In recent years, stem cell therapy has been considered as one of the approaches to solve bone repair or bone defect. Alginate scaffolds with stem cells were wildly checked for bone tissue regeneration (Lee and Mooney, 2012; Sun and Tan, 2013; Kim and Lee, 2016). Brown algae like mozuku, kombu, limumoui, bladderwrack, and wakame mainly show presence of a sulfated polysaccharide called Fucoidan (Figure 1G) (Senthilkumar et al., 2013). Low molecular weight, fucoidan induces proliferation of bone cells and influences alkaline phosphatase activity (ALP), collagen type 1 expression and mineral deposition (Jin and Kim, 2011; Hwang et al., 2016). Fucoidan derived from Undaria pinnatifida significantly induced factors involved in osteoblastic differentiation such as ALP, osteocalcin, and BMP-2 for bone formation without any cytotoxic effects on osteoblast cells (Cho et al., 2009).
Marine Sponges
Sponges are considered to be a prospective source of pharmacologically active compounds among the marine invertebrates. In past decades, marine sponges have been positioned at the top with regard to their wide diversity of chemical and structural metabolites with unique activities. Numerous experimental studies suggest that bioactive compounds derived from sponges have therapeutic properties such as antimicrobial, immune modulatory, antioxidant, antihypertensive, anti-coagulant, anticancer, anti-inflammatory (Narsinh and Müller, 2004; Perdicaris et al., 2013). Research conducted by Wang and his colleagues reveal that biopolymers viz. biogenic silica (bio-silica) and biogenic polyphosphate (bio-polyP) produced by deep sea sponge's aid biomineralization and also prevent osteoclastogenesis (Wang et al., 2013). Marine sponges display fibrillar, non-fibrillar, and filamentous collagen type, soft in structure mimicking the cancellous architecture of bone tissue. Sponges contain a complex canal system that builds a porous environment ideal for cellular integration when coalesce with cells for tissue engineering. Marine sponge skeletons are increasingly being used as a source of Scaffold's development for tissue engineering and regeneration because of their potential as cell conductive and inductive framework (Lin et al., 2011; Nandi et al., 2015). Nandi and colleagues investigated that the impregnation of converted sponge scaffold by IGF-1 and BMP-2, promoted excellent osseous tissue formation. This implies that, the marine sponge alone and in combination with these growth factors, forms a promising biomaterial for bone repair and augmentation (Nandi et al., 2015). Likewise, Zen et al. has shown that marine sponges are potential bioscaffolds for osteogenesis. Processed marine sponge Cally spongiidae skeleton consisted of a collagenous fibrous network having porous structure and interconnecting channels. This collagenous fibrous network provides a good framework that supports cellular aggregation, adhesion and expansion of osteoblast cells with expression of osteoblast markers (Lin et al., 2011; Clarke et al., 2016). Folmer et al. concludes that marine algae, bacteria, and invertebrates including marine sponges are a source of NF-κB inhibitors which inhibit TNF-α induced Nuclear factor kappa-light chain enhancer of activated B cells (NF-κB) activation (Folmer et al., 2009). Phorbaketal A (Figure 1H) is a tricyclic skeleton sesterterpenoid having a spiroketal of the hydrobenzopyran moiety; isolated from the Korean marine sponge Phorbas spp. (Joung et al., 2017). Phorbaketal A showed significant increase in calcium deposition activity and also stimulated osteoblast differentiation. It not only escalated the expression of transcriptional regulator, TAZ (Transcriptional co-activator with PDZ-binding motif) which resulted in osteoblast differentiation, but also intensified its interaction with Runx2 leading to ERK signal mediated osteogenic stimulation (Byun et al., 2012). Recently, Seo et al. studied the effect on phorbaketal A on inflammatory mediators such as nitric oxide (NO) and prostaglandin (PGE) in LPS stimulated RAW 264.7 cells. Their study demonstrated that phorbaketal A inhibits NO production but not PGE in these cells. It also causes downregulation of NF-κB pathway and up-regulation of heme oxygenase-1 (HO-1) pathway (Seo et al., 2015). Salicylihalamides A (Figure 2A) is a salicylic acid moiety incorporated into a 12-membered macrolide ring, which in turn had a 7-carbon unsaturated fatty acid attached through an enamide linkage isolated from marine sponge Haliclona spp. (Newman and Cragg, 2004). Salicylihalamides A is a first marine Vacuolar-ATPase (V-ATPase) inhibitor which has been proposed in drug designing to target cell components. V-ATPase presents on osteoclasts plasma membrane which acidifies the bone surface and activates osteoclast-secreted acid hydrolases. Therefore, Inhibitory activity of Salicylihalamides A for V-ATPase is useful in treatment of osteoporosis (Beutler and McKee, 2003).
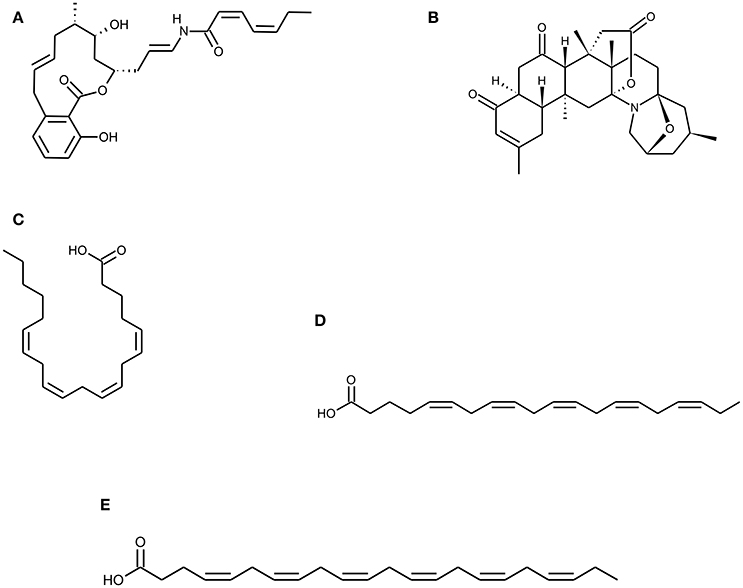
Figure 2. Chemical Structure of marine natural compounds isolated from marine resources. (A) Salicylihalamide A, (B) Norzoanthamine, (C) Arachidonic acid (ARA), (D) Eicosapentaenoic acid (EPA), and (E) Docosahexaenoic acid (DHA).
Marine Soft Corals
Marine soft corals are a rich source of structurally and biologically active intriguing compounds. It has been reported that compounds isolated from soft corals possess potent anticancer as well as chemopreventive activity (Hegazy et al., 2012; Suarez-Jimenez et al., 2012). Diterpenoids are a group of biologically active compounds derived from marine soft corals that have shown an anti-inflammatory, antioxidant antiosteoporotic activity (Takaki et al., 2003; Kim et al., 2008; Wanzola et al., 2010). Recently, Nguyen Phuong Thao et al. reported that diterpenoids (Figures 3A–D) derived from Vietnamese soft corals Sinularia maxima and Lobophytum crassum exhibited anti-osteoclastogenic, radical scavenging and reducing capacity. Considering the structure–activity relationship (SAR) of these diterpenes indicates that the presence of an epoxy group at C-7/C-8 and/or C-5/C-8 is necessary for antiosteoclastogenic activity. Diterpenes remarkably stimulate the differentiation of pre-osteoblastic MC3T3-E1 cells into mature osteoblasts that increase collagen synthesis and mineralization. It has also shown inhibitory effect on the differentiation of pre-osteoclastic RAW 264.7 cells while exhibiting intracellular antioxidant activity (Thao et al., 2015).
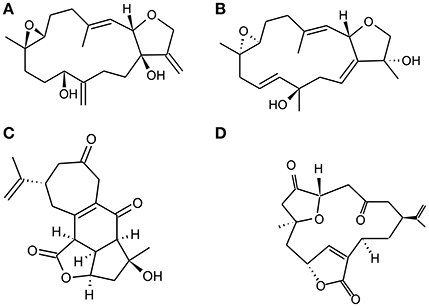
Figure 3. Chemical Structure of representative diterpenoids isolated from Vietnamese soft corals S. maxima and L. Crassum. (A). Sinumaximol D, (B) Sinumaximol F, (C) Scabrolide A, and (D) 5-epinorcembrene.
Zoanthus spp., another type of marine soft coral has been used to derive Marine alkaloids, Norzoanthamine, and its homologs (Figure 2B). Norzoanthamine and norzoanthamine hydrochloride inhibit or suppress IL6 secretion, a known cytokine for osteoclast differentiation which in turn is effective in the prevention of osteoporosis. Therefore, these marine alkaloids counteract to reduce bone weight and strength in the ovariectomized mouse model (Yamaguchi et al., 1999). According to Kinugawa et al. norzoanthamine suppressed the proteolysis of collagen and elastin, which protect the degradation of extracellular matrix proteins. It also preserves and enhances survival of skeletal proteins from external stress. They further demonstrated that the formation of collagen–hydroxyapatite composite, an essential bone tissue structure was significantly accelerated by norzoanthamine and also improved collagen release from immobilized matrix vesicles. This increased collagen concentration promotes calcification without affecting hydroxyapatite crystallization (Kinugawa et al., 2009).
Marine Molluscs
Molluscs are wildly distributed invertebrate group having various representatives in estuarine and marine ecosystem worldwide. Molluscs are considered to be a major group in context with potential compounds with pharmaceutical applications. Studies on Molluscs derived bioactive compounds manifested antibacterial, antiviral, antitumor, and antileukemic activities (Kamiya et al., 1984; Anand et al., 1997; Rajaganapathi et al., 2000). It is interesting to know that 98% of bioactive compounds are derived either from gastropods or bivalves. Natural product chemists highly prefer soft-bodied molluscs since the chances of finding bioactive chemicals in these are expected to be higher. Molluscan shells contain organic matrix which has the ability to regulate calcium deposition and mineralization. Biomineralization is a highly orchestrated biological process in the mollusc. The organic matrix of the mollusc directs crystallization, mineral deposition (Marin and Luquet, 2004).
Bivalves, gastropods, and cephalopods produce a calcium carbonate structure as an internal shell coating which is known as Nacre. As a result of its mechanical properties, chemical complexity, and optical effects, nacre had evolved as a highly organized structure (Rousseau and Rollion-Bard, 2012). Nacre has been best studied for its calcium carbonate biomineralization. Concurrent treatment of Cicer arietinum seeds extract (CAE) and powder of Coelatura aegyptiaca shell (CES) may be effective in treating osteoporosis, as evident by their amelioration on estrogen sensitive organs (Fahmy et al., 2015). Previous studies have shown the functional nature of nacre as a biomaterial for bone repair after its successful in vivo implantation in sheep femur, where it triggered bone remodeling at the interphase between bone and nacre implant (Atlan et al., 1999). Fragments of nacre from the bivalve Pinctada maxima when implanted into the bone of rats, sheep, dogs, and humans elicited a biological response including local osteogenic activity and incorporation of the nacre, without any inflammatory reaction (Duplat et al., 2007; Benkendorff, 2010). Studies conducted by Fujita et al. suggest that a bioavailable form of calcium carbonate from Oyster shell lysate of Ostrea edulis can be used for the treatment of osteoporosis patients (Fujita et al., 1993). The water-soluble fraction of nacre extract reduced the osteoclastic resorption activity by inhibiting cathepsin K without reducing the osteoclast number, adhesion, and survival (Duplat et al., 2007). N16, derived from the nacreous layer of a pearl oyster Pinctada fucata, is a protein that promotes biomineralization, however; it inhibits RANKL induced osteoclast differentiation. N16 effectively up-regulates alkaline phosphatase activity and mRNA expression of osteopontin and osteocalcin, which in turn increases differentiation of osteoblast and mineralized nodule formation. Ma et al. investigated the anabolic and antiresorptive effects of N16 on bone and concluded that it is a potentially useful for treating osteoporosis (Ma et al., 2016). Our research group has focused on marine gastropods such as Euchelus Asper and Turbo brunneus. Studies demonstrated that methanolic extract of E. asper displayed immunosuppressive as well as antiosteoporotic effect. E. asper methanolic extract (EAME) reduced the rapid bone loss in ovariectomized mice leading to an imbalance in bone metabolism using bilaterally ovariectomized mouse model. Administration of EAME in ovariectomized mice lowered the elevated levels of bone turnover markers such as Serum Acid Phosphatase (ACP), serum calcium, and Serum Alkaline Phosphatase (ALP). On the other hand, restoration of trabecular separation and increase in bone trabeculation by EAME treatment was observed in the histomorphometric analysis. Similarly, in this context, our recent study has proved that Turbo Methanol Extract (TME) derived from T. brunneus has beneficial effects on ovariectomized mice having estrogen deficiency-induced bone resorption. We evaluated the action of TME through lymphocytes-bone interaction and inhibition of bone resorption by regulating the T cell function. Oral administration of crude TME exhibited the potential to regulate osteoclastogenesis by reducing TNFα level. Currently, we are investigating the role of active compounds or molecules from purified fractions of TME/EAME and their functions on osteoclastogenesis and osteoblast differentiation (Ponkshe and Indap, 2002; Akerkar et al., 2009; Balakrishnan et al., 2014a,b).
Marine Fishes
Besides, food consumption or food industry marine fishes have drawn much attention toward their bioactive natural products and their natural abundance. Fish protein hydrolysates and fish oils are basic sources of bioactive compounds which have pharmaceutical applications. Fish bones, skin, fins, trimmed-off muscles, and gills, that are seafood processing wastes, have been considered as potential sources of bioactive peptides, omega fatty acids, etc. Therefore, they are deemed to be a useful resource for pharmaceutical, nutraceutical, and food industries (Koyama et al., 2001). Gelatin or collagen hydrolysate is one of the marine collagen products that are promoted as supplements to improve bone strength and density. Animal studies have proved the positive role of fish collagen hydrolysates on osteoporosis. Mice and rats which were previously fed with calcium or protein deficient diet showed improved bone collagen metabolism and BMD after being fed with hydrolyzed collagen enriched diet. Similarly, increased production of newly synthesized type I collagen and proteoglycan was found in the bone matrix of the ovariectomized rat after the oral administration of collagen hydrolysate from shark skin (Wu et al., 2004; Nomura et al., 2005). Guillerminet et al. administered a fish and bovine-derived collagen hydrolysate enriched diet to ovariectomized mice for 12 weeks and observed that there was an increase in the osteoblast activity along with the reduction in differentiation and maturation of osteoclasts (Guillerminet et al., 2010). Studies have shown that cod bone gelatine may prevent bone loss by decreasing bone resorption in ovariectomized rats (Han et al., 2009). In osteoporosis patients, particularly in postmenopausal women, a collagen protein-rich diet may enhance and prolong the therapeutic effect of calcitonin (Adam et al., 1996; Cuneo et al., 2010). Furthermore, fish collagen peptides initiate the gene expression for osteoblastic cells differentiation and posttranscriptional modification for collagen maturation. Besides promoting hard tissue formation, fish collagen is also used as a natural polymer for scaffolds which provide structural integrity to cells for growth (Yamada et al., 2014).
Marine fishes are considered as the main source of n-6 and n-3, long-chain polyunsaturated fatty acids (PUFAs). Linolenic acid belongs to α-linolenic and g-linolenic acid, n-3 and n-6 series respectively while linoleic acid belongs to only n-6 series. Both of these can be converted to other PUFA's like arachidonic acid, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) (Figures 2C–E) within the body (Blunt et al., 2003; Simopoulos, 2008). PUFAs are responsible for the synthesis of prostaglandin (prostaglandin E2) involved in bone metabolism whereas bone formation is stimulated by arachidonic acid in low concentrations; however, it is inhibited in high concentrations. Pro-inflammatory cytokines (TNF α and IL1) that are considered as mediators of post-menopausal bone loss are inhibited by n-3 PUFAs. n-3 PUFA supplementation in animal models also had an advantageous effect on calcium absorption and balance (James et al., 2000; Mundy, 2007; Poulsen et al., 2007). Thus, diet supplemented with fatty acids from marine fishes may promote bone health, aid in the development of the healthy skeleton and reduce the risk of osteoporosis.
Marine Mangroves
Throughout past decades, it has been observed that halophytes are a source of novel bioactive compounds. Halophytes are known to inhabit harsh environmental conditions which make them produce therapeutic compounds like polyphenols, flavonoids, and saponins. Considering potential health benefits of halophytes such as Salicornia herbacea, Spergularia marina etc. were studied for their novel bioactive substances (Patra and Thatoi, 2011). Fatih et al. have reported that S. herbaceais is a potential source of an anti-osteoporotic agent that promotes osteoblastogenesis while inhibiting adipogenesis. Similarly, extract of S. herbacea in MC3T3-E1 pre-osteoblasts enhances bone formation by elevating the expression of osteoblastogenesis indicators, osteocalcin, BMP-2, and collagen type I (collagen-I) and alkaline phosphatase activity (Karadeniz et al., 2014b). Another halophyte, S. marina extract possesses anti-osteoporotic activity by simultaneously blocking adipogenesis and enhancing osteoblastogenesis thus making it a promising source of bioactive compounds. Marine plant derived saponins and flavonoids were described to have anti-adipogenic and pro-osteoblastogenic properties (Karadeniz et al., 2014a).
Mechanism of Action of Marine Natural Products on Bone Metabolism
Bone is specialized tissue in vertebrate skeleton which not only supports its structure but also provides shape and protection by undergoing continuous remodeling. Under normal conditions; bone remodeling is maintained by a balance between osteoblast and osteoclast cells (Datta et al., 2008). The bone's mechanical properties comprise the size and structure, its geometry and density, collagen matrix, and its turnover rate. Defects in any of these mentioned parameters lead to bone-related disease like osteoporosis, osteopetrosis, osteogenesis imperfecta and Paget's disease (Datta et al., 2008). Local factors, cytokines, chemokines, transcription factors, mechanical forces, and bone turnover hormones bring about the tight regulation of bone homeostasis. The expression of a key osteoblast differentiation transcription factor, Runt-related transcription factor 2 (Runx2) is essential for Mesenchymal stem cells differentiation into the osteoblastic lineage (Liu and Lee, 2013). Runx2 is also called as core-binding factor subunit alpha-1(CBF-alpha-1) protein encoded by the Runx2 gene. The Runx2 expression is triggered by BMPs and Wnt pathway collectively (Wu et al., 2016). Research on human and animal models prove that the complex spatiotemporally regulated molecular interaction between transforming growth factor-b (TGF-b)/ BMP signaling and canonical Wnt pathway along with Runx2 (CBFA1/AML3) play an important role in osteoblast differentiation, skeletal development, and bone formation (Pinson et al., 2000; Brault et al., 2001; Logan and Nusse, 2004; Day et al., 2005). The TGF-β superfamily comprises of around 30 different types of BMPs, which not only regulate differentiation of MSCs into components of cartilage, bone, or adipose tissue but also possess osteoinductive properties (Ducy et al., 1997; Komori et al., 1997; Kundu et al., 2002; Day et al., 2005). The differentiation of pre-osteoblasts into immature osteoblasts is directed by osterix and beta-catenin. Immature osteoblasts express various proteins such as osterix, BMP-2, β-catenin, bone sialoprotein, bone matrix protein, osteopontin and develop into mature osteoblasts (Komori, 2006). Inhibition of collagen matrix degradation along with stimulation of osteoblast activity is brought about by circulating insulin-like growth factor (IGF-I) (Varanasi and Datta, 2005). Osteoclastogenesis is crucially dependent on two cytokines viz. RANKL and M-CSF (Monocyte-Colony Stimulation Factor). RANKL is also called as TRANCE (TNF-related activation-inducing cytokine) which directly regulates the process of differentiation by activation of RANK while MCSF is vital for osteoclast progenitor proliferation. These cytokines also stimulate osteoclast-specific gene expression including Tartrate-Resistant Acid Phosphatase (TRAP), Cathepsin K (CATK), β 3-integrin and Calcitonin (Wiktor-Jedrzejczak et al., 1990; Lacey et al., 1998; Yasuda et al., 1998). In bone remodeling process, TRAP and pro-CATK are secreted by osteoclast, which degrades the bone minerals and collagen matrices. RANK–RANKL interaction, with the help of an alternate pathway involving TRAF-6, c-Fos c-Jun, and p38 activates NFATc1 (Boyce and Xing, 2008; Kim and Kim, 2016). NFATc1 is important for osteoclastogenesis since its up-regulation eliminates the RANKL requirement for osteoclastogenesis and NFATc1 (−/−) monocytes fail to produce osteoclasts. Intracellular Ca2+ oscillations directed by a G-protein are induced by RANK-RANKL signaling leading to calmodulin and calcineurin-dependent NFATc1 activation (Huang et al., 2006; Zhao et al., 2010). The number of cytokines, cell surface receptors, and signaling pathways plays a major role in the interaction between immune system and skeletal system. Activated T cells are able to regulate osteoclast differentiation and activity, owing to their property of upregulating RANKL surface expression (Kong et al., 1999; Kotake et al., 2001). T cells produce many cytokines including RANKL, TNF-α, IL-6, and IL-17 that stimulate and modulate osteoclast activity while IL-4, IL-13, and IL-10 show inhibitory properties (Kotake et al., 1999; Moreno et al., 2003; Grcevic et al., 2006; Gillespie, 2007). TNFα and IL-1 activate NF-κB which augments RANKL-mediated osteoclastogenesis and their survival. On the contrary, cytokines such as IL-4, IL-10, and IL-13 suppress osteoclast formation and activation. Interestingly, recent reports indicate the possible important role of IL-4 in the suppression of osteoclastogenesis with the help of CD4+ CD25+ Foxp3+ T cell regulation (Takayanagi et al., 2002; Kim et al., 2007). In vitro and in vivo studies have shown that IFN-γ (type II IFN) strongly suppresses bone resorption through direct inhibition of RANK-RANKL signaling, which is essential for osteoclast differentiation (Zhao and Ivashkiv, 2011).
The marine-derived compounds exhibit profound effects on bone through downregulation of osteoclastogenesis and up-regulation of osteoblastogenesis as represented in Figure 4. These marine-derived compounds under preclinical and clinical trials are summarized in Table 1. Marine algal derived alginate and fucoidan induce the release of BMP and TGF-beta while influencing the activity of ALP and osteocalcin secretion whereas Mycoepoxydiene suppresses RANKL-induced osteoclastogenesis by down regulating ERK1/2 and NF-κB activity via blocking Transforming growth factor beta-activated kinas 1 (TAK1) activation which is an upstream activator of NF-κB and ERK (Smidsrod and Skjak-Braek, 1990; Asagiri and Takayanagi, 2007; Lee and Mooney, 2012; Zhu et al., 2013). Norzoanthamine and its homologs inhibit IL6 secretion which in turn prevent osteoclastogenesis and also induce the formation of a collagen-hydroxyapatite composite (Yamaguchi et al., 1999; Kinugawa et al., 2009). Recently it was found that sponge derived bioscaffolds are potential constructs for osteogenesis (Clarke et al., 2016). However, sponge derived compounds like phorbaketal A helps in regulating osteoblastic gene expression including ALP, Dlx5, and osteocalcin and also increases the levels of TAZ and Runx2 for osteoblast differentiation (Lin et al., 2011; Byun et al., 2012). Biselyngbyaside inhibits osteoclastogenesis by suppressing RANKL and NFATc1 expression whereas largazole mediates osteogenic activity through the increased expression of Runx2 and BMPs (Taori et al., 2008; Yonezawa et al., 2012). Mollusc derived nacre implants or nacre soluble proteins have shown to promote biomineralization while reducing bone resorptive activity (Ma et al., 2016). Mollusc extracts also possess T cell regulatory activity which in turn regulates osteoclastogenesis in ovariectomized mice model (Balakrishnan et al., 2014a,b; Ma et al., 2016). Similarly, marine fishes derived collagen hydrolysates, peptides, omega unsaturated fatty acids stimulate bone formation, and mineralization, osteoblastic cell differentiation and inhibition of proinflammatory cytokines respectively (James et al., 2000; Mundy, 2007; Poulsen et al., 2007; Guillerminet et al., 2010; Yamada et al., 2014).
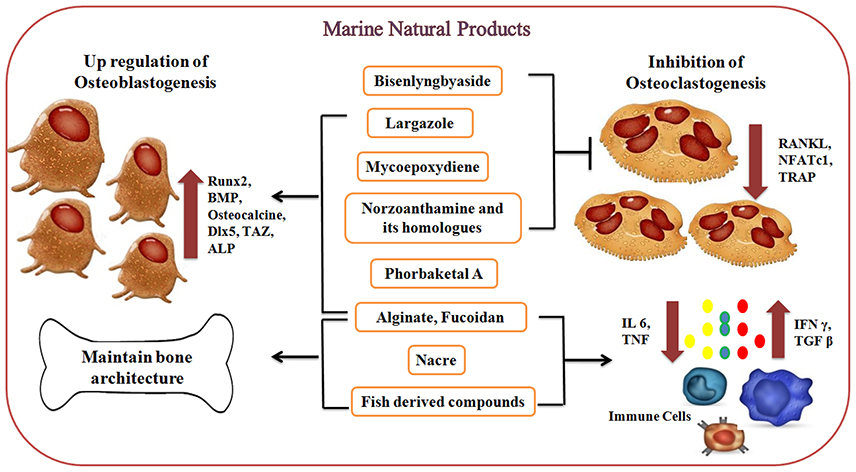
Figure 4. Effect of marine-derived compounds on bone metabolism via up-regulation of osteoblastogenesis and downregulation of osteoclastogenesis. Marine alginate, fucoidan, phorbaketal A, largazole, and Norzoanthamine and its homologs are directly involved in osteoblastogenesis by increasing the expression of Runx2, BMPs, osteocalcin, and other transcription factors and thereby regulating the levels of osteoblastic markers. Mollusc derived nacre proteins and fish-derived compounds have been shown to promote biomineralization while reducing bone resorptive activity via regulating cytokine production (IL6 and TNF). On the other side, marine-derived compounds like Mycoepoxydiene, Norzoanthamine, and its homologs, Biselyngbyaside, largazole suppresses RANKL-induced osteoclastogenesis via downregulating the expression of osteoclast differentiating factors (RANKL, NFATc1, and TRAP).
Future Scope with Respect to Marine Natural Product and Osteoporosis
In the last few decades, natural products have proven to be a promising source for various drugs. Marine ecosystem as compared with terrestrial ecosystem provides numerous diversified resources for human nutrition and health. Similarly, MNPs and marine nutraceuticals have particularly gained immense popularity for their significant bioactivities. Marine resources have seldom been investigated for their anti-osteoporotic compounds responsible for prevention and reversal of osteoporosis. Hence, there is a need to identify bioactive molecules from marine resources that possess anti-osteoporotic activity. These bioactive molecules should also be screened for their effectiveness in regulating bone resorption by modulation of immune mediators such as transcription factors, immune receptors and key factors like RANKL/OPG. These novel metabolites or compounds may have important implication in the therapeutic management of skeletal diseases.
Author Contributions
SC, MI, and SVC were responsible for literature review, interpretation, figure illustration, and drafting of this manuscript. All authors have contributed to critical revision and final approval of this manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the Department of Biotechnology, DBT, India, BT/PR11563/AAQ/3/676/2014 for providing financial support.
References
Abrahamsen, B., Eiken, P., and Brixen, K. (2009). Atrial fibrillation in fracture patients treated with oral bisphosphonates. J. Intern. Med. 265, 581–592. doi: 10.1111/j.1365-2796.2008.02065.x
Adam, M., Spacek, P., Hulejová, H., Galiánová, A., and Blahos, J. (1996). [Postmenopausal osteoporosis. Treatment with calcitonin and a diet rich in collagen proteins]. Cas Lek Cesk 135, 74–78.
Agnusdei, D., and Iori, N. (2000). Raloxifene: results from the MORE study. J. Musculoskelet. Neuronal Interact. 1, 127–132.
Akakabe, M., Kumagai, K., Tsudaa, M., Konishia, Y., Tominagab, A., Tsudac, M., et al. (2014). Amphirionin-5, a novel linear polyketide from a cultured marine dinoflagellate Amphidinium species with a potent cell proliferation-promoting activity. Tetrahedron Lett. 55, 3491–3494. doi: 10.1016/j.tetlet.2014.04.086
Akerkar, A. S., Ponkshe, C. A., and Indap, M. M. (2009). Evaluation of immunomodulatory activity of extracts from marine animals. Indian J. Mar. Sci. 38, 22–27.
Ammann, P., Shen, V., Robin, B., Mauras, Y., Bonjour, J. P., and Rizzoli, R. (2004). Strontium ranelate improves bone resistance by increasing bone mass and improving architecture in intact female rats. J. Bone Miner. Res. 19, 2012–2020. doi: 10.1359/jbmr.040906
Anand, P. T., Rajaganapathy, J., and Edward, P. (1997). Antibacterial activity of marine mollusks from Porto Nova region. Indian J Mar Sci. 26, 206–208.
Asagiri, M., and Takayanagi, H. (2007). The molecular understanding of osteoclast differentiation. Bone 40, 251–264. doi: 10.1016/j.bone.2006.09.023
Atlan, G., Delattre, O., Berland, S., LeFaou, A., Nabias, G., Cot, D., et al. (1999). Interface between bone and nacre implants in sheep. Biomaterials 20, 1017–1022. doi: 10.1016/S0142-9612(98)90212-5
Balakrishnan, B., Chiplunkar, S. V., and Indap, M. M. (2014a). Methanol extract of Euchelus asper prevents bone resorption in ovariectomised mice model. J. Osteoporos. 2014:348189.
Balakrishnan, B., Indap, M. M., Singh, S. P., Krishna, C. M., and Chiplunkar, S. V. (2014b). Turbo methanol extract inhibits bone resorption through regulation of T cell function. Bone 58, 114–125. doi: 10.1016/j.bone.2013.10.008
Barrett-Connor, E., Mosca, L., Collins, P., Geiger, M. J., Grady, D., Kornitzer, M., et al. (2006). Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N. Engl. J. Med. 355, 125–137. doi: 10.1056/NEJMoa062462
Bell, A. D., and Bell, B. R. (2011). The FREEDOM trial: is family medicine ready for biologic therapies? Can. Fam. Phys. 57, 438–441.
Benkendorff, K. (2010). Molluscan biological and chemical diversity: secondary metabolites and medicinal resources produced by marine molluscs. Biol. Rev. Camb. Philos. Soc. 85, 757–775. doi: 10.1111/j.1469-185X.2010.00124.x
Beutler, J. A., and McKee, T. C. (2003). Novel marine and microbial natural product inhibitors of vacuolar ATPase. Curr. Med. Chem. 10, 787–796. doi: 10.2174/0929867033457827
Black, D. M., Delmas, P. D., Eastell, R., Reid, I. R., Boonen, S., Cauley, J. A., et al. (2007). Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N. Engl. J. Med. 356, 1809–1822. doi: 10.1056/NEJMoa067312
Black, D. M., Thompson, D. E., Bauer, D. C., Ensrud, K., Musliner, T., Hochberg, M. C., et al. (2000). Fracture risk reduction with alendronate in women with osteoporosis: the fracture intervention trial. FIT Research Group. J. Clin. Endocrinol. Metab. 85, 4118–4124. doi: 10.1210/jcem.85.11.6953
Blick, S. K., Dhillon, S., and Keam, S. J. (2008). Teriparatide: a review of its use in osteoporosis. Drugs 68, 2709–2737. doi: 10.2165/0003495-200868180-00012
Blunt, J. W., Copp, B. R., Keyzers, R. A., Munro, M. H., and Prinsep, M. R. (2003). Marine natural products. Nat. Prod. Rep. 20, 1–48. doi: 10.1039/b207130b
Bowers, A., West, N., Taunton, J., Schreiber, S. L., Bradner, J. E., and Williams, R. M. (2008). Total synthesis and biological mode of action of largazole: a potent class I histone deacetylase inhibitor. J. Am. Chem. Soc. 130, 11219–11222. doi: 10.1021/ja8033763
Bowring, C. E., and Francis, R. M. (2011). National osteoporosis society's position statement on hormone replacement therapy in the prevention and treatment of osteoporosis. Menopause Int. 17, 63–65. doi: 10.1258/mi.2011.011012
Boyce, B. F., and Xing, L. (2008). Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 473, 139–146. doi: 10.1016/j.abb.2008.03.018
Brault, V., Moore, R., Kutsch, S., Ishibashi, M., Rowitch, D. H., McMahon, A. P., et al. (2001). Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253–1264.
Byun, M. R., Kim, A. R., Hwang, J. H., Sung, M. K., Lee, Y. K., Hwang, B. S., et al. (2012). Phorbaketal A stimulates osteoblast differentiation through TAZ mediated Runx2 activation. FEBS Lett. 586, 1086–1092. doi: 10.1016/j.febslet.2012.03.008
Caplan, L., Pittman, C. B., Zeringue, A. L., Scherrer, J. F., Wehmeier, K. R., Cunningham, F. E., et al. (2010). An observational study of musculoskeletal pain among patients receiving bisphosphonate therapy. Mayo Clin. Proc. 85, 341–348. doi: 10.4065/mcp.2009.0492
Cauley, J. A., Robbins, J., Chen, Z., Cummings, S. R., Jackson, R. D., LaCroix, A. Z., et al. (2003). Effects of estrogen plus progestin on risk of fracture and bone mineral density: the womens health initiative randomized trial. JAMA 290, 1729–1738. doi: 10.1001/jama.290.13.1729
Cheng, M. L., and Gupta, V. (2012). Teriparatide - indications beyond osteoporosis. Indian J. Endocrinol. Metab. 16, 343–348. doi: 10.4103/2230-8210.95661
Cho, Y.-S., Jung, W.-K., Kim, J-A., Choi, I-W., and Kima, S-K. (2009). Beneficial effects of fucoidan on osteoblastic MG-63 cell differentiation. Food Chem. 116, 990–994. doi: 10.1016/j.foodchem.2009.03.051
Cianferotti, L., D'Asta, F., and Brandi, M. L. (2013). A review on strontium ranelate long-term antifracture efficacy in the treatment of postmenopausal osteoporosis. Ther. Adv. Musculoskelet. Dis. 5, 127–139. doi: 10.1177/1759720X13483187
Clarke, B. (2008). Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 3(Suppl. 3), S131–S139. doi: 10.2215/CJN.04151206
Clarke, S. A., Choi, S. Y., McKechnie, M., Burke, G., Dunne, N., Walker, G., et al. (2016). Osteogenic cell response to 3-D hydroxyapatite scaffolds developed via replication of natural marine sponges. J. Mater. Sci. Mater. Med. 27:22. doi: 10.1007/s10856-015-5630-0
Cooper, C., Campion, G., and Melton, L. J. (1992). Hip fractures in the elderly: a world-wide projection. Osteoporos. Int. 2, 285–289. doi: 10.1007/BF01623184
Cosman, F., de Beur, S. J., LeBoff, M. S., Lewiecki, E. M., Tanner, B., Randall, S., et al. (2014). Clinician's guide to prevention and treatment of osteoporosis. Osteoporos. Int. 25, 2359–2381. doi: 10.1007/s00198-014-2794-2
Cummings, S. R., San Martin, J., McClung, M. R., Siris, E. S., Eastell, R., Reid, I. R., et al. (2009). Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 361, 756–765. doi: 10.1056/NEJMoa0809493
Cuneo, F., Costa-Paiva, L., Pinto-Neto, A. M., Morais, S. S., and Amaya-Farfan, J. (2010). Effect of dietary supplementation with collagen hydrolysates on bone metabolism of postmenopausal women with low mineral density. Maturitas 65, 253–257. doi: 10.1016/j.maturitas.2009.10.002
Das, S., and Crockett, J. C. (2013). Osteoporosis - a current view of pharmacological prevention and treatment. Drug Des. Devel. Ther. 7, 435–448. doi: 10.2147/DDDT.S31504
Datta, D., Talapatra, S. N., and Swarnakar, S. (2015). Bioactive compounds from marine invertebrates for potential medicines - an overview. Int. Lett. Nat. Sci. 7, 42–61. doi: 10.18052/www.scipress.com/ILNS.34.42
Datta, H. K., Ng, W. F., Walker, J. A., Tuck, S. P., and Varanasi, S. S. (2008). The cell biology of bone metabolism. J. Clin. Pathol. 61, 577–587. doi: 10.1136/jcp.2007.048868
Day, T. F., Guo, X., Garrett-Beal, L., and Yang, Y. (2005). Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell 8, 739–750. doi: 10.1016/j.devcel.2005.03.016
de Paula, F. J., and Rosen, C. J. (2010). Back to the future: revisiting parathyroid hormone and calcitonin control of bone remodeling. Horm. Metab. Res. 42, 299–306. doi: 10.1055/s-0030-1248255
Drake, M. T., Clarke, B. L., and Khosla, S. (2008). Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin. Proc. 83, 1032–1045. doi: 10.4065/83.9.1032
Ducy, P., Zhang, R., Geoffroy, V., Ridall, A. L., and Karsenty, G. (1997). Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89, 747–754. doi: 10.1016/S0092-8674(00)80257-3
Duplat, D., Gallet, M., Berland, S., Marie, A., Dubost, L., Rousseau, M., et al. (2007). The effect of molecules in mother-of-pearl on the decrease in bone resorption through the inhibition of osteoclast cathepsin K. Biomaterials 28, 4769–4778. doi: 10.1016/j.biomaterials.2007.07.036
Fahmy, S. R., Soliman, A. M., Sayed, A. A., and Marzouk, M. (2015). Possible antiosteoporotic mechanism of Cicer arietinum extract in ovariectomized rats. Int. J. Clin. Exp. Pathol. 8, 3477–3490.
Folmer, F., Jaspars, M., Solano, G., Cristofanon, S., Henry, E., Tabudravu, J., et al. (2009). The inhibition of TNF-α induced NF-κB activation by marine natural products. Biochem. Pharmacol. 78:592. doi: 10.1016/j.bcp.2009.05.009
Fournier, C., Perrier, A., Thomas, M., Laroche, N., Dumas, V., Rattner, A., et al. (2012). Reduction by strontium of the bone marrow adiposity in mice and repression of the adipogenic commitment of multipotent C3H10T1/2 cells. Bone 50, 499–509. doi: 10.1016/j.bone.2011.07.038
Freemantle, N., Cooper, C., Diez-Perez, A., Gitlin, M., Radcliffe, H., Shepherd, S., et al. (2013). Results of Indirect and mixed treatment comparison of fracture efficacy for osteoporosis treatments: a meta-analysis. Osteoporos. Int. 24, 209–217. doi: 10.1007/s00198-012-2068-9
Fujita, T., Fujii, Y., Kitagawa, R., and Fukase, M. (1993). Calcium supplementation in osteoporosis. Osteoporosis Int. 3, 159–162. doi: 10.1007/BF01621895
Galea, R., Montalto, A., Brincat, M., Saliba, C., Serrar, M., Gutierrez, G., et al. (2000). Phytoestrogen/SERM like activity from a marine alga derived molecule on bone density and collagen markers in postmenopausalwomen. Int. J. Gynecol. Obst. 70:A31. doi: 10.1016/S0020-7292(00)82056-1
Gambacciani, M., and Levancini, M. (2014). Hormone replacement therapy and the prevention of postmenopausal osteoporosis. Prz Menopauzalny. 13, 213–220. doi: 10.5114/pm.2014.44996
Gehret, C. January/February. (2010). Denosumab: a new therapy for osteoporosis. Pharmacotherapy update XIII(I).
Gillespie, M. T. (2007). Impact of cytokines and T lymphocytes upon osteoclast differentiation and function. Arthritis Res. Ther. 9:103. doi: 10.1186/ar2141
Glover, S. J., Eastell, R., McCloskey, E. V., Rogers, A., Garnero, P., Lowery, J., et al. (2009). Rapid and robust response of biochemical markers of bone formation to teriparatide therapy. Bone 45, 1053–1058. doi: 10.1016/j.bone.2009.07.091
Grady, D., Wenger, N. K., Herrington, D., Khan, S., Furberg, C., Hunninghake, D., et al. (2000). Postmenopausal hormone therapy increases risk for venous thromboembolic disease. The heart and estrogen/progestin replacement study. Ann. Intern. Med. 132, 689–696. doi: 10.7326/0003-4819-132-9-200005020-00002
Grcevic, D., Lukic, I. K., Kovacić, N., Ivcević, S., Katavić, V., and Marusić, A. (2006). Activated T lymphocytes suppress osteoclastogenesis by diverting early monocyte/macrophage progenitor lineage commitment towards dendritic cell differentiation through down-regulation of receptor activator of nuclear factor-kappaB and c-Fos. Clin. Exp. Immunol. 146, 146–158. doi: 10.1111/j.1365-2249.2006.03181.x
Green, J., Czanner, G., Reeves, G., Watson, J., Wise, L., and Beral, V. (2010). Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case-control analysis within a UK primary care cohort. BMJ 341:c4444. doi: 10.1136/bmj.c4444
Guglielmi, G. (ed.). (2013). Osteoporosis and Bone Densitometry Medical Rediology, Diagnostic Imaging. Berlin; Heidelberg: Springer Verlag. doi: 10.1007/978-3-642-27884-6
Guillerminet, F., Beaupied, H., Fabien-Soulé, V., Tomé, D., Benhamou, C. L., Roux, C., et al. (2010). Hydrolyzed collagen improves bone metabolism and biomechanical parameters in ovariectomized mice: an in vitro and in vivo study. Bone 46, 827–834. doi: 10.1016/j.bone.2009.10.035
Han, X., Xu, Y., Wang, J., Pei, X., Yang, R., Li, N., et al. (2009). Effects of cod bone gelatin on bone metabolism and bone microarchitecture in ovariectomized rats. Bone 44, 942–947. doi: 10.1016/j.bone.2008.12.005
Hegazy, M.-E. F., Eldeen, A. M. G., Shahat, A. A., Abdel-Latif, F. F., Mohamed, T. A., Whittlesey, B. R., et al. (2012). Bioactive Hydroperoxyl Cembranoids from the Red Sea Soft Coral Sarcophyton glaucum. Mar. Drugs 10:209. doi: 10.3390/md10010209
Huang, H., Chang, E. J., Ryu, J., Lee, Z. H., Lee, Y., and Kim, H. H. (2006). Induction of c-Fos and NFATc1 during RANKL-stimulated osteoclast differentiation is mediated by the p38 signaling pathway. Biochem. Biophys. Res. Commun. 351, 99–105. doi: 10.1016/j.bbrc.2006.10.011
Hwang, P. A., Hung, Y. L., Phan, N. N., Hieu, B. T., Chang, P. M., Li, K. L., et al. (2016). The in vitro and in vivo effects of the low molecular weight fucoidan on the bone osteogenic differentiation properties. Cytotechnology 68, 1349–1359. doi: 10.1007/s10616-015-9894-5
James, M. J., Gibson, R. A., and Cleland, L. G. (2000). Dietary polyunsaturated fatty acids and inflammatory mediator production. Am. J. Clin. Nutr. 71(1 Suppl.), 343S−348S.
Jin, G., and Kim, G. H. (2011). Rapid-prototyped PCL/fucoidan composite scaffolds for bone tissue regeneration: design, fabrication, and physical/biological properties. J. Mater. Chem. 21, 17710–17718. doi: 10.1039/c1jm12915e
Joung, S., Kim, R., and Lee, H. Y. (2017). Total synthesis of (-)-phorbaketal A. Org. Lett. 19, 3903–3906. doi: 10.1021/acs.orglett.7b01797
Kallio, D. M., Garant, P. R., and Minkin, C. (1972). Ultrastructural effects of calcitonin on osteoclasts in tissue culture. J. Ultrastruct. Res. 39, 205–216. doi: 10.1016/S0022-5320(72)90017-2
Kamiya, H., Muramoto, K., and Ogata, K. (1984). Antibacterial activity in the egg mass of a sea hare. Cell. Mol. Life Sci. 40, 947–949. doi: 10.1007/BF01946452
Karadeniz, F., Kim, J. A., Ahn, B. N., Kim, M., and Kong, C. S. (2014a). Anti-adipogenic and Pro-osteoblastogenic Activities of Spergularia marina Extract. Prev. Nutr. Food Sci. 19, 187–193. doi: 10.3746/pnf.2014.19.3.187
Karadeniz, F., Kim, J. A., Ahn, B. N., Kwon, M. S., and Kong, C. S. (2014b). Effect of Salicornia herbacea on osteoblastogenesis and adipogenesis in vitro. Mar. Drugs 12, 5132–5147. doi: 10.3390/md12105132
Karsdal, M. A., Sondergaard, B. C., Arnold, M., and Christiansen, C. (2007). Calcitonin affects both bone and cartilage: a dual action treatment for osteoarthritis? Ann. N. Y. Acad. Sci. 1117, 181–195. doi: 10.1196/annals.1402.041
Kim, H. K., Woo, E. R., Lee, H. W., Park, H. R., Kim, H. N., Jung, Y. K., et al. (2008). The correlation of Salvia miltiorrhiza extract-induced regulation of osteoclastogenesis with the amount of components tanshinone I, tanshinone IIA, cryptotanshinone, and dihydrotanshinone. Immunopharmacol. Immunotoxicol. 30, 347–364. doi: 10.1080/08923970801949133
Kim, H., and Lee, J. (2016). Strategies to maximize the potential of marine biomaterials as a platform for cell therapy. Mar. Drugs 14:29. doi: 10.3390/md14020029
Kim, J. H., and Kim, N. (2016). Signaling pathways in osteoclast differentiation. Chonnam Med. J. 52, 12–17. doi: 10.4068/cmj.2016.52.1.12
Kim, Y. G., Lee, C. K., Nah, S. S., Mun, S. H., Yoo, B., and Moon, H. B. (2007). Human CD4+CD25+ regulatory T cells inhibit the differentiation of osteoclasts from peripheral blood mononuclear cells. Biochem. Biophys. Res. Commun. 357, 1046–1052. doi: 10.1016/j.bbrc.2007.04.042
Kinugawa, M., Fukuzawa, S., and Tachibana, K. (2009). Skeletal protein protection: the mode of action of an anti-osteoporotic marine alkaloid, norzoanthamine. J. Bone Miner. Metab. 27, 303–314. doi: 10.1007/s00774-009-0049-7
Kita, M., Kondo, M., Koyama, T., Yamada, K., Matsumoto, T., Lee, K. H., et al. (2004). Symbioimine exhibiting inhibitory effect of osteoclast differentiation, from the symbiotic marine dinoflagellate Symbiodinium sp. J. Am. Chem. Soc. 126, 4794–4795. doi: 10.1021/ja049277f
Komori, T. (2006). Regulation of osteoblast differentiation by transcription factors. J. Cell. Biochem. 99, 1233–1239. doi: 10.1002/jcb.20958
Komori, T., Yagi, H., Nomura, S., Yamaguchi, A., Sasaki, K., Deguchi, K., et al. (1997). Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89, 755–764. doi: 10.1016/S0092-8674(00)80258-5
Kong, Y. Y., Feige, U., Sarosi, I., Bolon, B., Tafuri, A., Morony, S., et al. (1999). Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402, 304–309. doi: 10.1038/46303
Kostenuik, P. J., Smith, S. Y., Jolette, J., Schroeder, J., Pyrah, I., and Ominsky, M. S. (2011). Decreased bone remodeling and porosity are associated with improved bone strength in ovariectomized cynomolgus monkeys treated with denosumab, a fully human RANKL antibody. Bone 49, 151–161. doi: 10.1016/j.bone.2011.03.769
Kotake, S., Udagawa, N., Takahashi, N., Matsuzaki, K., Itoh, K., Ishiyama, S., et al. (1999). IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Invest. 103, 1345–1352. doi: 10.1172/JCI5703
Kotake, S., Udagawa, N., Hakoda, M., Mogi, M., Yano, K., Tsuda, E., et al. (2001). Activated human T cells directly induce osteoclastogenesis from human monocytes: possible role of T cells in bone destruction in rheumatoid arthritis patients. Arthritis Rheum. 44, 1003–1012. doi: 10.1002/1529-0131(200105)44:5<1003::AID-ANR179>3.0.CO;2-#
Koyama, Y., Hirota, A., Mori, H., Takahara, H., Kuwaba, K., Kusubata, M., et al. (2001). Ingestion of gelatin has differential effect on bone mineral density and body weight in protein undernutrition. J. Nutr. Sci. Vitaminol. 47, 84–86. doi: 10.3177/jnsv.47.84
Kundu, M., Javed, A., Jeon, J. P., Horner, A., Shum, L., Eckhaus, M., et al. (2002). Cbfbeta interacts with Runx2 and has a critical role in bone development. Nat. Genet. 32, 639–644. doi: 10.1038/ng1050
Lacey, D. L., Timms, E., Tan, H. L., Kelley, M. J., Dunstan, C. R., Burgess, T., et al. (1998). Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176. doi: 10.1016/S0092-8674(00)81569-X
Lau, E. M. (2009). The epidemiology of osteoporosis in Asia. IBMS Bonekey 6, 190–193. doi: 10.1138/20090378
Leboime, A., Confavreux, C. B., Mehsen, N., Paccou, J., David, C., and Roux, C. (2010). Osteoporosis and mortality. Joint Bone Spine 77, S107–S112. doi: 10.1016/S1297-319X(10)70004-X
Lee, K. Y., and Mooney, D. J. (2012). Alginate: properties and biomedical applications. Prog. Polym. Sci. 37, 106–126. doi: 10.1016/j.progpolymsci.2011.06.003
Lee, S. U., Kwak, H. B., Pi, S. H., You, H. K., Byeon, S. R., Ying, Y., et al. (2011). In vitro and in vivo osteogenic activity of largazole. ACS Med. Chem. Lett. 2, 248–251. doi: 10.1021/ml1002794
Lewis, J. S., and Jordan, V. C. (2005). Selective estrogen receptor modulators (SERMs): mechanisms of anticarcinogenesis and drug resistance. Mutat. Res. 591, 247–263. doi: 10.1016/j.mrfmmm.2005.02.028
Li, Y., Li, J., Zhu, S., Luo, E., Feng, G., Chen, Q., et al. (2012). Effects of strontium on proliferation and differentiation of rat bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 418, 725–730. doi: 10.1016/j.bbrc.2012.01.088
Lin, X., Huang, Y., Fang, M., Wang, J., Zheng, Z., and Su, W. (2005). Cytotoxic and antimicrobial metabolites from marine lignicolous fungi, Diaporthe sp. FEMS Microbiol. Lett. 251, 53–58. doi: 10.1016/j.femsle.2005.07.025
Lin, Z., Solomon, K. L., Zhang, X., Pavlos, N. J., Abel, T., Willers, C., et al. (2011). In vitro evaluation of natural marine sponge collagen as a scaffold for bone tissue engineering. Int. J. Biol. Sci. 7, 968–977. doi: 10.7150/ijbs.7.968
Liu, T. M., and Lee, E. H. (2013). Transcriptional regulatory cascades in Runx2-dependent bone development. Tissue Eng. B Rev. 19, 254–263. doi: 10.1089/ten.teb.2012.0527
Logan, C. Y., and Nusse, R. (2004). The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810. doi: 10.1146/annurev.cellbio.20.010403.113126
Lorenzo, J., Horowitz, M., and Choi, Y. (2008). Osteoimmunology: interactions of the bone and immune system. Endocr. Rev. 29, 403–440. doi: 10.1210/er.2007-0038
Ma, J.-Y., Wong, K-L., Xu, Z. Y., Au, K. Y., Lee, N. L., Su, C., et al. (2016). N16, a nacreous protein, inhibits osteoclast differentiation and enhances osteogenesis. J. Nat. Prod. 79, 204–212. doi: 10.1021/acs.jnatprod.5b00854
Marie, P. J., Felsenberg, D., and Brandi, M. L. (2011). How strontium ranelate, via opposite effects on bone resorption and formation, prevents osteoporosis. Osteoporos. Int. 22, 1659–1667. doi: 10.1007/s00198-010-1369-0
Marin, F., and Luquet, G. (2004). Molluscan shell proteins. Comptes Rendus Palevol. 3, 469–492. doi: 10.1016/j.crpv.2004.07.009
McClung, M. R. (2017). Denosumab for the treatment of osteoporosis. Osteoporosis Sarcopenia 3, 8–17. doi: 10.1016/j.afos.2017.01.002
Melton, L. J. III., Chrischilles, E. A., Cooper, C., Lane, A. W., and Riggs, B. L. (1992). Perspective. How many women have osteoporosis? J. Bone Miner. Res. 7, 1005–1010. doi: 10.1002/jbmr.5650070902
Miller, P. D., Pannacciulli, N., Brown, J. P., Czerwinski, E., Nedergaard, B. S., Bolognese, M. A., et al. (2016). Denosumab or zoledronic acid in postmenopausal women with osteoporosis previously treated with oral bisphosphonates. J. Clin. Endocrinol. Metab. 101, 3163–3170. doi: 10.1210/jc.2016-1801
Minamida, M., Kumagai, K., Ulanova, D., Akakabe, M., Konishi, Y., Tominaga, A., et al. (2014). Amphirionin-4 with potent proliferation-promoting activity on bone marrow stromal cells from a marine dinoflagellate amphidinium species. Org. Lett. 16, 4858–4861. doi: 10.1021/ol5023504
Moreno, J. L., Kaczmarek, M., Keegan, A. D., and Tondravi, M. (2003). IL-4 suppresses osteoclast development and mature osteoclast function by a STAT6-dependent mechanism: irreversible inhibition of the differentiation program activated by RANKL. Blood 102, 1078–1086. doi: 10.1182/blood-2002-11-3437
Morita, M., Ogawa, H., Ohno, O., Yamori, T., Suenaga, K., and Toyoshima, C. (2015). Biselyngbyasides, cytotoxic marine macrolides, are novel and potent inhibitors of the Ca2+ pumps with a unique mode of binding. FEBS Lett. 589, 1406–1411. doi: 10.1016/j.febslet.2015.04.056
Mundy, G. R. (2007). Osteoporosis and inflammation. Nutr. Rev. 65(12 Pt. 2), S147–S151. doi: 10.1301/nr.2007.dec.S147-S151
Murthy, M. B. (2011). Osteoimmunology - unleashing the concepts. J. Indian Soc. Periodontol. 15, 190–198. doi: 10.4103/0972-124X.85659
Nandi, S. K., Kundu, B., Mahato, A., Thakur, N. L., Joardar, S. N., and Mandal, B. B. (2015). In vitro and in vivo evaluation of the marine sponge skeleton as a bone mimicking biomaterial. Integr. Biol. 7, 250–262. doi: 10.1039/C4IB00289J
Narsinh, L. T., and Müller, W. E. G. (2004). Biotechnological potential of marine sponges. Curr. Sci. 86, 1506–1512.
Newman, D. J., and Cragg, G. M. (2004). Advanced preclinical and clinical trials of natural products and related compounds from marine sources. Curr. Med. Chem. 11, 1693–1713. doi: 10.2174/0929867043364982
Nomura, Y., Oohashi, K., Watanabe, M., and Kasugai, S. (2005). Increase in bone mineral density through oral administration of shark gelatin to ovariectomized rats. Nutrition 21, 1120–1126. doi: 10.1016/j.nut.2005.03.007
Overgaard, K., Lindsay, R., and Christiansen, C. (1995). Patient responsiveness to calcitonin salmon nasal spray: a subanalysis of a 2-year study. Clin. Ther. 17, 680–685. doi: 10.1016/0149-2918(95)80044-1
Patra, J. K., and Thatoi, H. N. (2011). Metabolic diversity and bioactivity screening of mangrove plants: a review. Acta Physiol. Plant. 33, 1051–1061. doi: 10.1007/s11738-010-0667-7
Perdicaris, S., Vlachogianni, T., and Valavanidis, A. (2013). Bioactive natural substances from marine sponges: new developments and prospects for future pharmaceuticals. Nat. Prod. Chem. Res. 1:114. doi: 10.4172/2329-6836.1000114
Pinson, K. I., Brennan, J., Monkley, S., Avery, B. J., and Skarnes, W. C. (2000). An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 407, 535–538. doi: 10.1038/35035124
Pleiner-Duxneuner, J., Zwettler, E., Paschalis, E., Roschger, P., Nell-Duxneuner, V., and Klaushofer, K. (2009). Treatment of osteoporosis with parathyroid hormone and teriparatide. Calcif. Tissue Int. 84, 159–170. doi: 10.1007/s00223-009-9218-x
Ponkshe, C. A., and Indap, M. M. (2002). In vivo and in vitro evaluation for immunomodulatory activity of three marine animal extracts with reference to phagocytosis. Indian J. Exp. Biol. 40, 1399–1402.
Poulsen, R. C., Moughan, P. J., and Kruger, M. C. (2007). Long-chain polyunsaturated fatty acids and the regulation of bone metabolism. Exp. Biol. Med. 232, 1275–1288. doi: 10.3181/0704-MR-100
Raisz, L. G. (2005). Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J. Clin. Invest. 115, 3318–3325. doi: 10.1172/JCI27071
Rajaganapathi, J., Thyagarajan, S. P., and Edward, J. K. (2000). Study on cephalopod's ink for anti-retroviral activity. Indian J. Exp. Biol. 38, 519–520.
Randell, A., Sambrook, P. N., Nguyen, T. V., Lapsley, H., Jones, G., Kelly, P. J., et al. (1995). Direct clinical and welfare costs of osteoporotic fractures in elderly men and women. Osteoporos. Int. 5, 427–432. doi: 10.1007/BF01626603
Reginster, J. Y., and Burlet, N. (2006). Osteoporosis: a still increasing prevalence. Bone 38(2 Suppl. 1), S4–S9. doi: 10.1016/j.bone.2005.11.024
Reginster, J. Y., Bruyere, O., and Collette, J. (2012). Strontium ranelate treatment increases osteoprotegerin serum levels in postmenopausal osteoporotic women. Bone 50, 1201–1202. doi: 10.1016/j.bone.2011.12.024
Reid, I. R., and Cornish, J. (2011). Epidemiology and pathogenesis of osteonecrosis of the jaw. Nat. Rev. Rheumatol. 8, 90–96. doi: 10.1038/nrrheum.2011.181
Ro, C., and Cooper, O. (2013). Bisphosphonate drug holiday: choosing appropriate candidates. Curr. Osteoporos. Rep. 11, 45–51. doi: 10.1007/s11914-012-0129-9
Rousseau, M., and Rollion-Bard, C. (2012). Influence of the depth on the shape and thickness of nacre tablets of pinctada margaritifera pearl oyster, and on oxygen isotopic composition. Minerals 2:55. doi: 10.3390/min2010055
Sampson, H. W. (2002). Alcohol and other factors affecting osteoporosis risk in women. Alcohol Res. Health 26, 292–298.
Senn, C., Gunther, B., Popp, A. W., Perrelet, R., Hans, D., and Lippuner, K. (2014). Comparative effects of teriparatide and ibandronate on spine bone mineral density (BMD) and microarchitecture (TBS) in postmenopausal women with osteoporosis: a 2-year open-label study. Osteoporos. Int. 25, 1945–1951. doi: 10.1007/s00198-014-2703-8
Senthilkumar, K., Manivasagan, P., Venkatesan, J., and Kim, S. K. (2013). Brown seaweed fucoidan: biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 60, 366–374. doi: 10.1016/j.ijbiomac.2013.06.030
Senthilkumar, K., Venkatesan, J., and Kim, S-K. (2014). Marine derived natural products for osteoporosis. Biomed. Prev. Nutr. 4, 1–7. doi: 10.1016/j.bionut.2013.12.005
Seo, Y. J., Lee, K. T., Rho, J. R., and Choi, J. H. (2015). Phorbaketal A, Isolated from the marine sponge Phorbas sp., exerts its anti-inflammatory effects via NF-kappaB inhibition and heme oxygenase-1 activation in lipopolysaccharide-stimulated macrophages. Mar. Drugs 13, 7005–7019. doi: 10.3390/md13117005
Simopoulos, A. P. (2008). The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 233, 674–688. doi: 10.3181/0711-MR-311
Smidsrod, O., and Skjak-Braek, G. (1990). Alginate as immobilization matrix for cells. Trends Biotechnol. 8, 71–78. doi: 10.1016/0167-7799(90)90139-O
Sondergaard, B. C., Madsen, S. H., Segovia-Silvestre, T., Paulsen, S. J., Christiansen, T., Pedersen, C., et al. (2010). Investigation of the direct effects of salmon calcitonin on human osteoarthritic chondrocytes. BMC Musculoskelet. Disord. 11, 1471–2474. doi: 10.1186/1471-2474-11-62
Suarez-Jimenez, G.-M., Burgos-Hernandez, A., and Ezquerra-Brauer, J. M. (2012). Bioactive peptides and depsipeptides with anticancer potential: sources from marine animals. Mar. Drugs 10:963. doi: 10.3390/md10050963
Sun, J., and Tan, H. (2013). Alginate-based biomaterials for regenerative medicine applications. Materials 6:1285. doi: 10.3390/ma6041285
Takaki, H., Koganemaru, R., Iwakawa, Y., Higuchi, R., and Miyamoto, T. (2003). Inhibitory effect of norditerpenes on LPS-induced TNF-alpha production from the Okinawan soft coral, Sinularia sp. Biol. Pharm. Bull. 26, 380–382. doi: 10.1248/bpb.26.380
Takao, K.-I., Watanabe, G., Yasui, H., and Tadano, K. (2002). Total Synthesis of (±)-mycoepoxydiene, a novel fungal metabolite having an oxygen-bridged cyclooctadiene skeleton. Org. Lett. 4, 2941–2943. doi: 10.1021/ol026338a
Takayanagi, H., Kim, S., Matsuo, K., Suzuki, H., Suzuki, T., Sato, K., et al. (2002). RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature 416, 744–749. doi: 10.1038/416744a
Taori, K., Paul, V. J., and Luesch, H. (2008). Structure and activity of largazole, a potent antiproliferative agent from the Floridian marine cyanobacterium Symploca sp. J. Am. Chem. Soc. 130, 1806–1807. doi: 10.1021/ja7110064
Teruya, T., Sasaki, H., Kitamura, K., Nakayama, T., and Suenaga, K. (2009). Biselyngbyaside, a macrolide glycoside from the marine cyanobacterium Lyngbya sp. Org. Lett. 11, 2421–2424. doi: 10.1021/ol900579k
Thao, N. P., Luyen, B. T. T., Lee, S. H., Jang, H. D., Van Kiem, P., Van Minh, C., et al. (2015). Antiosteoporotic and antioxidant activities of diterpenoids from the Vietnamese soft corals Sinularia maxima and Lobophytum crassum. Med. Chem. Res. 24, 3551–3560. doi: 10.1007/s00044-015-1395-8
The Women's Health Initiative Steering, C. (2004). Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the women's health initiative randomized controlled trial. JAMA 291, 1701–1712. doi: 10.1001/jama.291.14.1701
Törring, O. (2015). Effects of denosumab on bone density, mass and strength in women with postmenopausal osteoporosis. Ther. Adv. Musculoskelet. Dis. 7, 88–102. doi: 10.1177/1759720X15579189
Tsai, J. N., Uihlein, A. V., Lee, H., Kumbhani, R., Siwila-Sackman, E., McKay, E. A., et al. (2013). Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet 382, 50–56. doi: 10.1016/S0140-6736(13)60856-9
Uchiyama, S., and Yamaguchi, M. (2002). Anabolic effect of marine alga Sargassum horneri extract on bone components in the femoral-diaphyseal and -metaphyseal tissues of young and aged rats in vivo. J. Health Sci. 48, 325–330. doi: 10.1248/jhs.48.325
Varanasi, S. S., and Datta, H. K. (2005). Characterisation of cytosolic FK506 binding protein 12 and its role in modulating expression of Cbfa1 and osterix in ROS 17/2.8 cells. Bone 36, 243–253. doi: 10.1016/j.bone.2004.09.021
Verhaar, H. J., and Lems, W. F. (2009). PTH-analogs: comparable or different? Arch. Gerontol. Geriatr. 49, e130–e132. doi: 10.1016/j.archger.2008.11.004
Wang, J., Zhao, B., Zhang, W., Wu, X., Wang, R., Huang, Y., et al. (2010). Mycoepoxydiene, a fungal polyketide, induces cell cycle arrest at the G2/M phase and apoptosis in HeLa cells. Bioorg. Med. Chem. Lett. 20, 7054–7058. doi: 10.1016/j.bmcl.2010.09.105
Wang, X., Schroder, H. C., Feng, Q., Draenert, F., and Müller, W. E. (2013). The deep-sea natural products, biogenic polyphosphate (Bio-PolyP) and biogenic silica (Bio-Silica), as biomimetic scaffolds for bone tissue engineering: fabrication of a morphogenetically-active polymer. Mar. Drugs 11, 718–746. doi: 10.3390/md11030718
Wanzola, M., Furuta, T., Kohno, Y., Fukumitsu, S., Yasukochi, S., Watari, K., et al. (2010). Four new cembrane diterpenes isolated from an Okinawan soft coral Lobophytum crassum with inhibitory effects on nitric oxide production. Chem. Pharm. Bull. 58, 1203–1209. doi: 10.1248/cpb.58.1203
Wiktor-Jedrzejczak, W., Bartocci, A., Ferrante, A. W., Ahmed-Ansari, A., Sell, K. W., Pollard, J. W., et al. (1990). Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc. Natl. Acad. Sci. U.S.A. 87, 4828–4832. doi: 10.1073/pnas.87.12.4828
Wolfe, F., Bolster, M. B., O'Connor, C. M., Michaud, K., Lyles, K. W., and Colón-Emeric, C. S. (2013). Bisphosphonate use is associated with reduced risk of myocardial infarction in patients with rheumatoid arthritis. J. Bone Miner. Res. 28, 984–991. doi: 10.1002/jbmr.1792
Wright, N. C., Looker, A. C., Saag, K. G., Curtis, J. R., Delzell, E. S., Randall, S., et al. (2014). The recent prevalence of osteoporosis and low bone mass in the united states based on bone mineral density at the femoral neck or lumbar spine. J. Bone Miner. Res. 29, 2520–2526. doi: 10.1002/jbmr.2269
Wu, J., Fujioka, M., Sugimoto, K., Mu, G., and Ishimi, Y. (2004). Assessment of effectiveness of oral administration of collagen peptide on bone metabolism in growing and mature rats. J. Bone Miner. Metab. 22, 547–553. doi: 10.1007/s00774-004-0522-2
Wu, M., Chen, G., and Li, Y. P. (2016). TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res 4:16009. doi: 10.1038/boneres.2016.9
Wynn, R. L. (2005). Bisphosphonates, hypercalcemia of malignancy, and osteonecrosis of the jaw. Gen. Dent. 53, 392–395.
Yamada, S., Yamamoto, K., Ikeda, T., Yanagiguchi, K., and Hayashi, Y. (2014). Potency of fish collagen as a scaffold for regenerative medicine. Biomed Res. Int. 2014:302932. doi: 10.1155/2014/302932
Yamaguchi, K., Yada, M., Tsuji, T., Kuramoto, M., and Uemura, D. (1999). Suppressive effect of norzoanthamine hydrochloride on experimental osteoporosis in ovariectomized mice. Biol. Pharm. Bull. 22, 920–924. doi: 10.1248/bpb.22.920
Yamaguchi, M. (2013). Marine alga sargassum horneri component and bone homeostasis: role in osteoporosis prevention. Int. J. Food Sci. Nutr. Diet. 2, 9–14. doi: 10.19070/2326-3350-130003
Yasuda, H., Shima, N., Nakagawa, N., Yamaguchi, K., Kinosaki, M., and Mochizuki, S. (1998). Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. U.S.A. 95, 3597–3602. doi: 10.1073/pnas.95.7.3597
Yonezawa, T., Mase, N., Sasaki, H., Teruya, T., Hasegawa, S., Cha, B. Y., et al. (2012). Biselyngbyaside, isolated from marine cyanobacteria, inhibits osteoclastogenesis and induces apoptosis in mature osteoclasts. J. Cell. Biochem. 113, 440–448. doi: 10.1002/jcb.23213
Zhao, B., and Ivashkiv, L. B. (2011). Negative regulation of osteoclastogenesis and bone resorption by cytokines and transcriptional repressors. Arthritis Res. Ther. 13:234. doi: 10.1186/ar3379
Zhao, Q., Wang, X., Liu, Y., He, A., and Jia, R. (2010). NFATc1: functions in osteoclasts. Int. J. Biochem. Cell Biol. 42, 576–579. doi: 10.1016/j.biocel.2009.12.018
Keywords: marine resources, marine natural products, bone, osteoporsis, marine molluscs
Citation: Chaugule SR, Indap MM and Chiplunkar SV (2017) Marine Natural Products: New Avenue in Treatment of Osteoporosis. Front. Mar. Sci. 4:384. doi: 10.3389/fmars.2017.00384
Received: 11 May 2017; Accepted: 14 November 2017;
Published: 29 November 2017.
Edited by:
Roland Wohlgemuth, Sigma-Aldrich (Switzerland), SwitzerlandReviewed by:
Vitor Hugo Pomin, Universidade Federal do Rio de Janeiro, BrazilJong-Ki Kim, Catholic University of Daegu, South Korea
Copyright © 2017 Chaugule, Indap and Chiplunkar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Madhavi M. Indap, madhaviindap@yahoo.com
Shubhada V. Chiplunkar, schiplunkar@actrec.gov.in
 Sachin R. Chaugule
Sachin R. Chaugule Madhavi M. Indap
Madhavi M. Indap Shubhada V. Chiplunkar
Shubhada V. Chiplunkar