Biological Invasions in Conservation Planning: A Global Systematic Review
- 1Institute of Marine Biology, University of Montenegro, Kotor, Montenegro
- 2Department of Palaeontology, University of Vienna, Vienna, Austria
- 3Department of Ecology, School of Biology, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 4National Center for Scientific Research, PSL Université Paris, CRIOBE, USR 3278 CNRS-EPHE_UPVD, Paris, France
- 5Institute of Marine Sciences (ICM-CSIC), Barcelona, Spain
- 6Division of Conservation Biology, Vegetation and Landscape Ecology, University of Vienna, Rennweg, Austria
- 7Department of Marine Sciences, University of the Aegean, Mytilene, Greece
- 8iSea, Environmental Organization for the Preservation of the Aquatic Ecosystems, Thessaloniki, Greece
- 9Enalia Physis Environmental Research Centre, Nicosia, Cyprus
- 10The Biodiversity Research Group, The School of Biological Sciences, ARC Centre of Excellence for Environmental Decisions and NESP Threatened Species Hub, Centre for Biodiversity and Conservation Science, University of Queensland, Brisbane, QLD, Australia
- 11Research Centre of the Westfjords, University of Iceland, Bolungarvík, Iceland
- 12Marine Biology and Ecology Department, Institute of Oceanology – BAS, Varna, Bulgaria
- 13Department of Biodiversity and Nature Conservation, Environment Agency Austria, Vienna, Austria
- 14Department of Zoology and Animal Ecology, Institut Scientifique, University Mohamed V of Rabat, Rabat, Morocco
- 15Marine Biology Department, National Institute of Oceanography, Israel Oceanographic and Limnological Research, Haifa, Israel
- 16Marine Biology Department, Charney School of Marine Science, University of Haifa, Haifa, Israel
- 17Dipartimento di Biologia, Università degli Studi di Firenze, Florence, Italy
- 18National Research Council – Institute of Coastal Marine Environment (IAMC), Naples, Italy
Biological invasions threaten biodiversity in terrestrial, freshwater and marine ecosystems, requiring substantial conservation and management efforts. To examine how the conservation planning literature addresses biological invasions and if planning in the marine environment could benefit from experiences in the freshwater and terrestrial systems, we conducted a global systematic review. Out of 1,149 scientific articles mentioning both “conservation planning” and “alien” or any of its alternative terms, 70 articles met our selection criteria. Most of the studies were related to the terrestrial environment, while only 10% focused on the marine environment. The main conservation targets were species (mostly vertebrates) rather than habitats or ecosystems. Apart from being mentioned, alien species were considered of concern for conservation in only 46% of the cases, while mitigation measures were proposed in only 13% of the cases. The vast majority of the studies (73%) ignored alien species in conservation planning even if their negative impacts were recognized. In 20% of the studies, highly invaded areas were avoided in the planning, while in 6% of the cases such areas were prioritized for conservation. In the latter case, two opposing approaches led to the selection of invaded areas: either alien and native biodiversity were treated equally in setting conservation targets, i.e., alien species were also considered as ecological features requiring protection, or more commonly invaded sites were prioritized for the implementation of management actions to control or eradicate invasive alien species. When the “avoid” approach was followed, in most of the cases highly impacted areas were either excluded or invasive alien species were included in the estimation of a cost function to be minimized. Most of the studies that followed a “protect” or “avoid” approach dealt with terrestrial or freshwater features but in most cases the followed approach could be transferred to the marine environment. Gaps and needs for further research are discussed and we propose an 11-step framework to account for biological invasions into the systematic conservation planning design.
Introduction
Conservation planning is “the process of locating, configuring, implementing and maintaining areas that are managed to promote the persistence of biodiversity and other natural values” (Pressey et al., 2007). To achieve conservation goals, strategies for designing networks of protected areas are needed and should be based on the principles of biodiversity representativeness and persistence, the latter referring to the long-term survival of protected biodiversity by maintaining ecological functions and viable populations and by minimizing threats (Margules and Pressey, 2000). Hence, it is of utmost importance to apply a systematic approach to conservation planning, considering not only the distribution of biodiversity but also of threats (Tallis et al., 2008) and the implementation costs (Naidoo et al., 2006; Carwardine et al., 2008). Such an approach can substantially improve the cost-effectiveness of conservation prioritization for mitigating cumulative threats (Wilson et al., 2006; Auerbach et al., 2014).
Biological invasions threaten biodiversity in terrestrial, freshwater, and marine ecosystems, challenging conservation efforts (Simberloff et al., 2013). Invasive alien species can modify community composition, may cause local extinctions and the loss of native genotypes, modify habitats, and affect food-web properties, ecosystem processes and functioning (Vilà et al., 2010; Katsanevakis et al., 2014; Bellard et al., 2016). Their ecological impacts can be so severe that they are considered as one of the major drivers of biodiversity loss across the globe (CBD, 2016). Invasive alien species can also have detrimental socio-economic impacts, affecting ecosystem services and human well-being (Vilà and Hulme, 2018). However, alien species can sometimes have also positive impacts, for example, through provision of food and shelter or by securing ecosystem processes and functions, especially in ecosystems greatly affected by cumulative human impacts and climate change (Schlaepfer et al., 2011; Katsanevakis et al., 2014).
Biological invasions and their impacts need to be carefully considered in conservation planning, as they can greatly affect the effectiveness of conservation efforts. Nevertheless, various approaches can be taken to consider biological invasions in conservation planning. One approach is to “avoid” including highly invaded areas in conservation planning and invest efforts in the least invaded areas. An alternative approach is to “protect” highly invaded areas, aiming to restore them to a better state through adequate management actions (Giakoumi et al., 2016). However, in the absence of sufficient knowledge on how to deal with biological invasions in the planning phase, alien species are commonly ignored altogether. The selected approach (“avoid,” “protect,” or “ignore”) has important implications on spatial conservation priorities and can lead to very different outcomes (Giakoumi et al., 2016).
Regardless of the selected approach, the negative impacts of invasive alien species need to be mitigated through specific conservation actions. Efforts to eradicate or control terrestrial or freshwater invasive populations at levels below which native biodiversity is adversely affected are commonly undertaken, often with substantial conservation gains (Simberloff, 2009; Jones et al., 2016). In the marine environment, due to the nature of the medium and modes of organism dispersal, such efforts are much more challenging. However, there are also examples of alien species contributing to the achievement of conservation goals (D'Antonio and Meyerson, 2002; Gozlan, 2008; Gleditsch, 2017), and the existence of endangered species that have alien populations may even create a conservation paradox, in which eradication efforts in the invaded ecosystem are in conflict with efforts to protect the endangered species (Marchetti and Engstrom, 2016).
Accounting for biological invasions in conservation planning and prioritizing conservation actions for mitigation is not a simple topic and there is no single widely applicable approach for all cases. Here, to provide guidance to scientists and planners on this issue, we conducted a global review on how the conservation planning literature addressed biological invasions. The review covered all terrestrial, freshwater, transitional, and marine environments with the aim to highlight current practices and their cross-environment variations, to summarize the main methods applied, and to investigate if approaches applied in one environment are transferable to the others. A particular focus was put on methods applied in terrestrial or freshwater environments that are transferable to the marine environment, where alien species most often are ignored in conservation planning (Giakoumi et al., 2016).
Methods
A systematic literature review was performed, applying the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) approach (Moher et al., 2010). The bibliographic search was performed with the Scopus database (www.scopus.com) on peer-reviewed literature published between 1950 and October 2016, with “conservation planning” in the title, abstract or keywords, and at least one among “alien,” “non-native,” “exotic,” “allochthonous,” or “invasive” in all searchable items, including the whole text. Non-English publications were not considered. This process resulted in 1,128 publication records. We included 35 additional papers relevant to the topic, and meeting the above criteria that did not show up in the search but were identified by the authors during the review process. After the removal of duplicates, the resulting database for further analysis consisted of 1,149 publications (Figure S1). The full list of identified publications was managed with the open-source reference management software Zotero (www.zotero.org).
We performed two rounds of paper selection using as the main selection criteria the fact that the papers needed to include a conservation planning case study. First, papers were screened based on their titles, keywords and abstract, and 371 out of 1,149 were selected. Second, the full text was examined and 301 articles were excluded, while 70 articles remained for full analysis (Table S1). Most of the excluded papers simply mentioned the term “conservation planning” for justification/discussion but did not actually include a conservation planning case study.
Data collected from the 70 reviewed papers included information on the geographic area covered by the case study, planning tools utilized, conservation targets, and if and how alien species were taken into consideration. Specifically, the following information was retrieved from each paper: (1) year of publication; (2) environment (marine, transitional, freshwater, terrestrial, combination); (3) scale (subnational, national, supranational, high seas, continental, global); (4) continent (Europe; Asia; Africa; North America; South America; Oceania; Antarctica; several continents; global); (5) location; (6) marine biogeographic realms, if relevant, according to Spalding et al. (2007) (Arctic; Temperate Northern Atlantic; Temperate Northern Pacific; Tropical Atlantic; Western Indo-Pacific; Central Indo-Pacific; Eastern Indo-Pacific; Tropical Eastern Pacific; Temperate South America; Temperate Southern Africa; Temperate Australasia; Southern Ocean; several marine realms) and terrestrial biogeographic realms, if relevant, in line with Olson et al. (2001) (Oceania; Neartic; Neotropic; Palearctic; Afrotropic; Indo-Malay; Australasia; Antarctic); (7) type of environment (river, lake, lagoon, marine offshore, marine coastal, mainland, island); (8) scope of the study (theoretical, applied); (9) if the study referred to a protected area already established; (10) type of protected area; (11) the conservation planning tool used (e.g., MARXAN, ZONATION); (12) if proximity to high risk areas was taken into account; (13) whether the targeted conservation features were species, habitats or ecosystems (i.e., combinations of species, habitats, processes); (14) details on conservation targets; (15) if alien species were considered of concern; (16) if any introduction pathway was mentioned; (17) the specific introduction pathway; (18) if any alien species were considered to have positive or negative impacts; (19) if any positive impacts of alien species were described; (20) whether the approach was to ignore, protect, or avoid invasive alien species in conservation planning; (21) the method used when either the “protect” or “avoid” approach was followed; (22) if the method could be implemented in another environment; (23) if any measures for mitigating invasive alien species' impacts were proposed and (24) which ones specifically; (25) if there were plans for restoration of degraded habitats; (26) if any external drivers such as climate change, pollution, or fisheries were taken into account, and if yes (27) which specifically.
In addition to descriptive statistics to present the review results, cross-tabulation analysis was conducted to quantify the degree of association between selected pairs of variables. The hypothesis of independence between such pairs was tested with chi-square tests.
Results
Overview of the Systematic Review Findings
By excluding biological invasions related keywords from our search in Scopus (i.e., by keeping only “conservation planning” in the title, keywords or abstract) results would have increased 8-fold. Papers addressing invasive alien species in conservation planning started to appear in the late 1990s. Their number increased rapidly after 2007, and at a much greater rate than that of papers exclusively addressing “conservation planning,” “non-native species,” or “marine conservation” (Figure 1), reflecting increased interest in incorporating mitigation strategies on invasive alien species in conservation plans in the last decade.
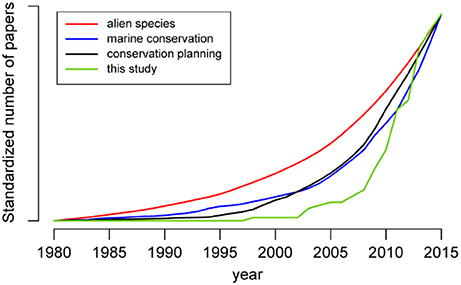
Figure 1. Cumulative number of scientific papers reviewed in this study compared with the results appearing in Scopus by only using the keywords “non-native species,” “marine conservation,” or “conservation planning” (all standardized between 0 and 1). Source: Scopus (October 2016).
Only 10% of the reviewed papers focused on the marine environment (Figure 2A) and most (74%) were at national or subnational scale (Figure 2B). All realms, except the Antarctic ecozone and Oceania, were well represented in terrestrial studies (Figure 2C). In the marine environment, studies were more unevenly distributed; almost half of them had been conducted in the temperate Northern Atlantic (Figure 2D), and of those, more than half in the Mediterranean Sea. Most of the targeted conservation features were species (in 56% of the cases), and 84% of these studies targeted vertebrates (with a few also considering other non-vertebrate taxa), 11% plants and only 5% invertebrates, fungi and unicellular organisms. Habitats were targeted in 15% of studies, whereas ecosystems in 29%. In 12 studies (17%), plans for restoration of degraded habitats were mentioned (Figure 2E).
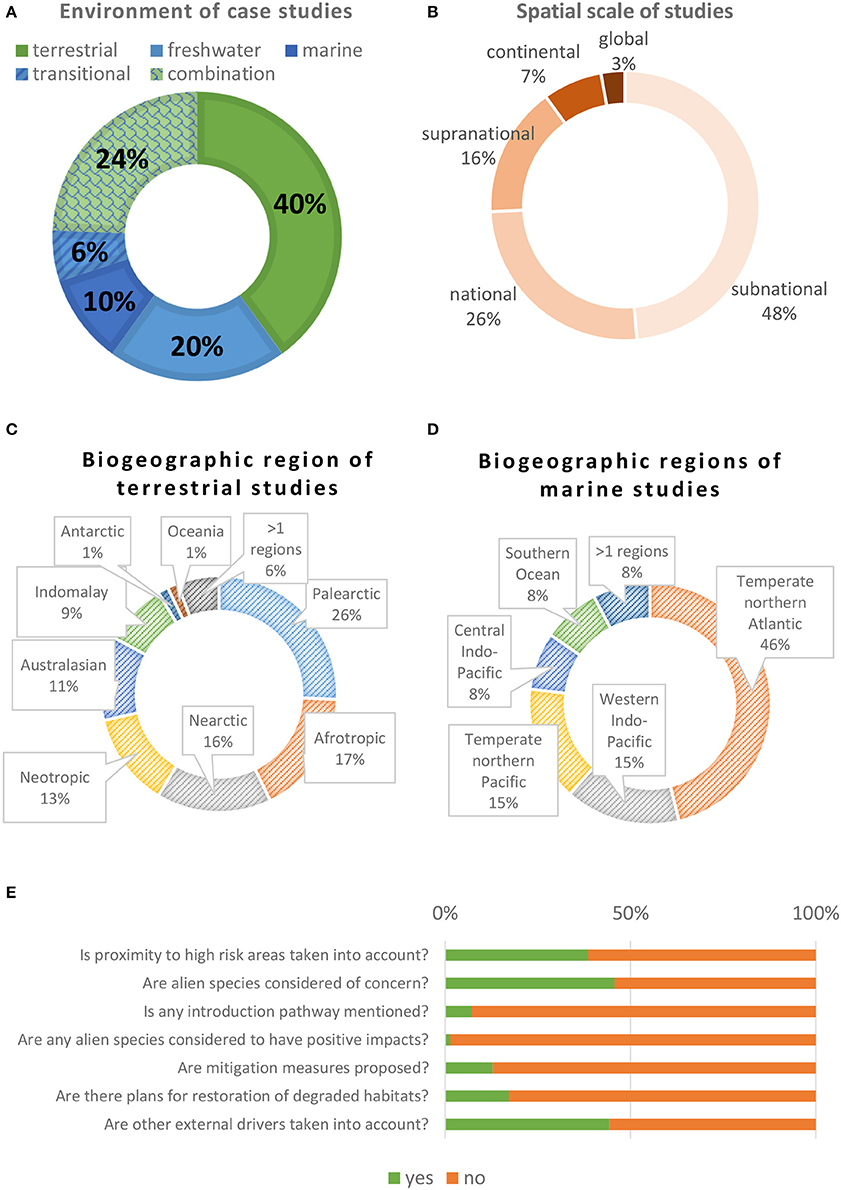
Figure 2. Quantitative results of the reviewed papers. (A) Share of studies by environment. (B) Spatial scale of studies. (C) Share of studies per terrestrial biogeographic region. (D) Share of studies per marine biogeographic region. (E) Proportion of binary outcomes (yes or no) to some questions on the content of the articles.
Alien species were considered of concern in conservation planning in 46% of the studies in both marine and non-marine environments (Figure 2E), with non-significant differences among environments (χ2 test, p = 0.69) (Figure 3A). Among those that did consider them as an important issue, 56% did not follow the “ignore/do not care” approach. Hence, overall among all conservation planning articles published (8 times more papers than those initially considered in this review), it is estimated that only 3.2% (i.e., 1/8 × 0.46 × 0.56) considered alien species in shaping their conservation plans.
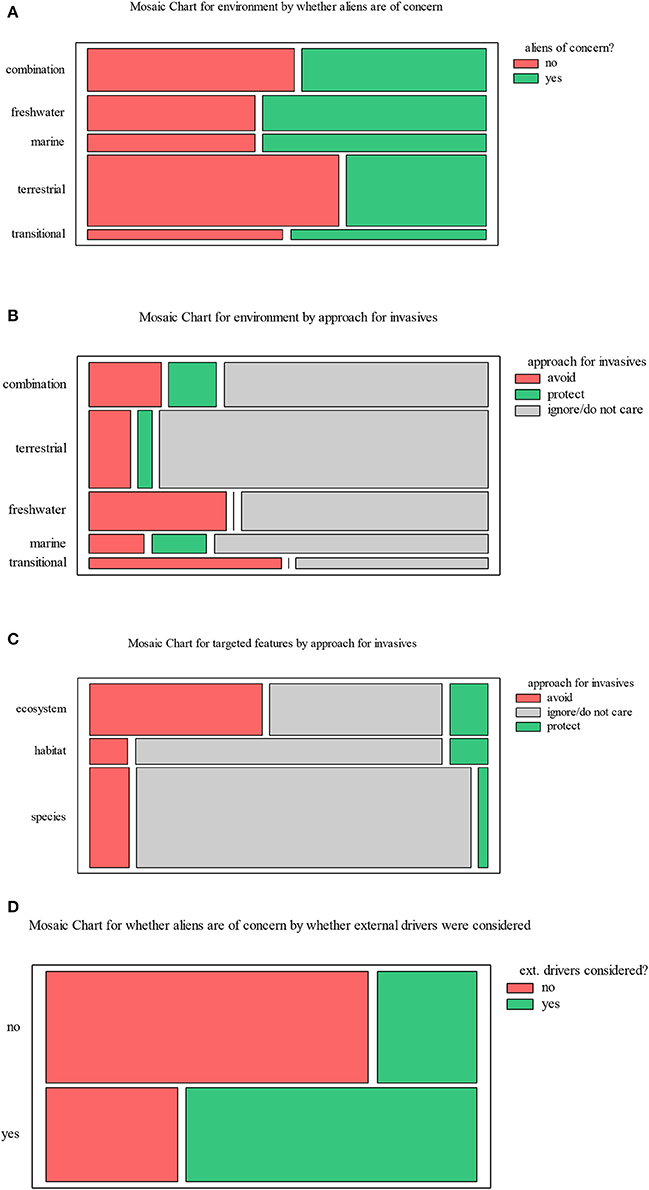
Figure 3. Mosaic charts showing (A) if alien species were considered of concern in the various realms, (B) which approach (“protect,” “avoid,” or “ignore”) was adopted in conservation plans concerning alien species by environment, (C) which approach was adopted in conservation plans concerning alien species in relation to the targeted features, and (D) if other drivers impacting conservation efforts were taken into account in addition to biological invasions.
In most studies (73%), alien species were ignored in the planning process, even if they were considered of concern; in fewer studies (20%), areas impacted by alien species were avoided, while in only 4 studies (6%) the impacted areas were targeted for protection (Figure 3B). Thus, those studies that did not ignore alien species mostly followed the “avoid” approach (Figure 3B); no significant differences were detected on the approach to alien species among environments (χ2 test, p = 0.32). Conservation planning studies focusing on ecosystems (i.e., combinations of species, habitats, processes) were more likely to follow an approach other than “ignore” in comparison to studies targeting species or habitats (χ2 test, p = 0.002) (Figure 3C). Other drivers of impact on biodiversity were more likely to also be considered when alien species were considered of concern than when they were not (χ2 test, p = 0.0002) (Figure 3D).
Most studies that considered alien species of concern were theoretical (57%) rather than applied (43%), and 53% were related to already-established networks of protected areas. Most studies did not specify an introduction pathway (93%) (Figure 2E); among those that did, escape from confinement and intentional release in nature were the most common ones. Positive impacts of alien species have been reported by only one terrestrial and freshwater study (1.4%) (Figure 2E), which mentioned the use of feral animals as food resource for indigenous people (Adams et al., 2014).
In nine studies (13%), of which only one was exclusively marine (targeting marine birds), mitigation measures for the impacts of alien species were proposed. These measures included the removal of invasive alien plants (Cowling et al., 2003; Rouget et al., 2003); the re-establishment of native vegetation on agricultural landscapes (Cowling et al., 2003); shooting, trapping or baiting invasive alien animals (Schüttler et al., 2009; Adams et al., 2014; Spatz et al., 2014; Tulloch et al., 2014). Only one study proposed to undertake pro-active, preventive management measures to minimize vectors of spreading (Reid et al., 2012).
Approaches Applied to Address Biological Invasions in Conservation Planning
Among the 70 reviewed papers, 18 studies followed the “avoid” or “protect” approach (Table 1). Most of these studies were relatively new (six papers were published between 2003 and 2010, and 12 after 2010). All of these studies but one were conducted at national and subnational scales, and most of them were related to the terrestrial (4) and freshwater (5) environments or a combination of environments (5), while only two were completely marine studies and two were conducted in transitional waters.
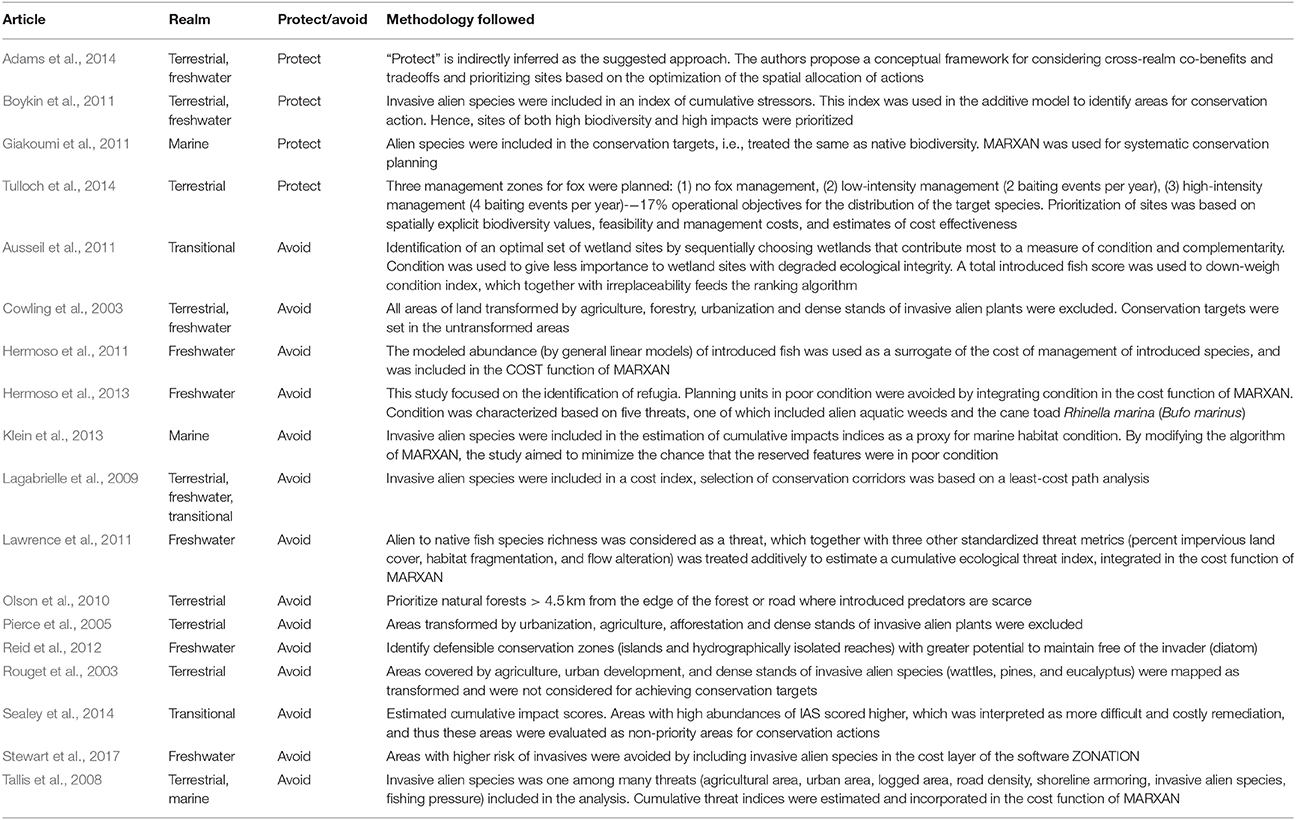
Table 1. Followed methodology by studies either avoiding areas impacted by biological invasions (“avoid” approach) or targeting such areas for conservation (“protect” approach).
The four studies that adopted the “protect” approach essentially followed two opposing concepts. Under the first concept, alien biodiversity was treated the same as native biodiversity, and conservation targets were set for both alien and native species. This approach was applied by one marine study that set conservation targets for all fish species, including the invasive grazer, dusky spinefoot Siganus luridus (Giakoumi et al., 2011). Under the second concept, sites impacted by invasive alien species were prioritized for management actions to control or eradicate the invasive alien species. This concept was applied by Boykin et al. (2011) who included invasive alien species in an index of cumulative stressors and prioritized sites of both high biodiversity and high values of the index, and Tulloch et al. (2014) who prioritized locations to control the invasive alien red fox. In the latter study, prioritization was based on spatially explicit biodiversity values, feasibility and management costs, and estimates of cost effectiveness on each planning unit.
A more complex approach was suggested by Adams et al. (2014) who provided a conceptual framework considering cross-environment co-benefits and tradeoffs, and prioritizing sites based on the optimization of the spatial allocation of actions. Such actions were aimed to mitigate a large variety of stressors, e.g., urbanization, agriculture, grazing, mining, transport networks, exploitation of natural resources, including invasive alien species (e.g., weed management, invasive animal control). In that context, invaded sites might be selected for conservation actions if the benefits of such actions are ranked high in terms of conservation outcomes and cost-efficiency. The creation of action-response curves for alternative actions under consideration was proposed as a visualization tool to facilitate such ranking.
Fourteen of the eighteen reviewed studies followed the “avoid” approach. The simplest way to avoid areas impacted by invasive alien species was to exclude them from the prioritization process. Rouget et al. (2003) and Cowling et al. (2003) mapped terrestrial areas covered by agriculture, urban development, and dense stands of invasive alien species (wattles, pines, and eucalyptus) as transformed and did not consider them for achieving conservation targets. Similarly, Pierce et al. (2005) excluded from the planning process areas transformed by urbanization, agriculture, afforestation and dense stands of invasive alien plants. Olson et al. (2010) prioritized natural forests that were >4.5 km away from the edge of the forest or roads; at such distances introduced predators such as rats, mongoose, cats and dogs were scarce. Reid et al. (2012) attempted to identify defensible conservation zones in freshwater systems with greater potential to remain free of a highly invasive diatom. Another strategy was followed by Sealey et al. (2014), who estimated cumulative impact scores in coastal ecosystems due to physical restructuring, destructive uses, coastal development, and alien plant invasions, evaluating high-score areas as non-priority for conservation actions.
One approach followed by some studies was to integrate alien species in the cost function of MARXAN (Hermoso et al., 2011, 2013; Lawrence et al., 2011) or ZONATION (Stewart et al., 2017), which was aimed to be minimized in the prioritization process. Specifically, Hermoso et al. (2011) modeled the distribution of alien freshwater fish in the study area and used the modeled abundance as a surrogate of the cost of management actions to control invasive alien species. Hermoso et al. (2013) focused on the identification of refugia for freshwater fish biodiversity conservation, and avoided planning units in poor condition by integrating condition in the cost function of MARXAN. Condition was characterized based on five threats, one of which included invasive alien species. Lawrence et al. (2011) estimated a metric of species invasiveness as the ratio of alien to native species richness. This metric was rescaled ranging from 0 to 1 and, together with three other standardized threat metrics (percent of impervious land cover, habitat fragmentation, and flow alteration), was treated additively to estimate a cumulative ecological threat index. The latter was treated as cost in MARXAN for the prioritization of sites. Tallis et al. (2008) estimated cumulative threat indices that included invasive alien species among a number of both terrestrial and marine threats, and incorporated them in the cost function of MARXAN to prioritize terrestrial and marine sites in a cross- environment conservation planning case study. Stewart et al. (2017), in addition to prioritizing watersheds based on species presence, developed cost layers representing human threats (land use) and the presence of alien fish. The cost layers were used to down-weigh native species occurrences.
A novel approach was proposed by Klein et al. (2013) for designing marine reserves that trade off habitat condition, habitat representation and socioeconomic costs. Cumulative impacts indices were used as proxies for marine habitat condition. Four possible scenarios of the marine reserve design were compared, varying only in the number of impacts considered. Invasive alien species were included in two such scenarios. To solve the problem of minimizing the chance that the reserved features were in poor condition, Klein et al. (2013) modified the algorithm of MARXAN by imposing an additional constraint. Hence, invasive alien species were avoided through the selection of habitats that were in good condition, i.e., less affected by cumulative impacts.
Ausseil et al. (2011) developed a systematic conservation routine in R for the identification of an optimal set of wetland sites by sequentially choosing wetlands that contribute most to the preservation of national wetland biodiversity. The selection criteria were: (1) naturalness condition; (2) complementarity with already selected sites; and (3) irreplaceability. A total introduced fish score was used to down-weigh condition index. A single score was later calculated to obtain an overall classification of candidate sites deserving conservation measures within the study area. Condition was used to give less importance to wetland sites with degraded ecological integrity, thus generally avoiding highly invaded sites unless they were irreplaceable.
Lagabrielle et al. (2009) designed conservation corridors through a least-cost path analysis by optimizing both ecological and socio-economic constraints expressed in a cost matrix. Invasive plants were included in this cost matrix through a threats score that combined urbanization, agriculture and plant invasion. Potential extents of the 20 most invasive plants were estimated by climatic envelop modeling and summed to derive a map of invasion threat probability. The latter contributed to the threat score, derived by calculating a mean score among the three threats (urbanization, agriculture, alien plants).
Overall, none of these approaches was environment-specific and they could all be transferred to other environments. Specifically in the marine environment, the approaches that have been followed so far were to either protect alien biodiversity by setting conservation targets in MARXAN for both native and alien species (Giakoumi et al., 2011), or by estimating cumulative impact indices that included invasive alien species and either incorporate them in the cost function (Tallis et al., 2008), or adequately modify the optimization algorithm of MARXAN (Klein et al., 2013). All the other approaches applied in the freshwater or terrestrial environments can be transferred to the marine environment as well, i.e., exclusion of highly impacted areas (Cowling et al., 2003; Rouget et al., 2003; Pierce et al., 2005; Olson et al., 2010; Reid et al., 2012; Sealey et al., 2014), maximizing contribution to measures of condition and complementarity (Ausseil et al., 2011), or applying a least-cost path analysis including invasive alien species in the cost matrix (Lagabrielle et al., 2009).
Discussion
Addressing Biological Invasions in Conservation Planning
Our systematic review revealed that biological invasions have been largely overlooked in systematic conservation planning. We estimated that only 3.2% of conservation planning papers considered alien species in shaping their conservation plans. This troubling result is consistent with that of Giakoumi et al. (2016), whose analysis at the global scale found that only 2.5% of papers on marine spatial planning took into account invasive alien species in an explicit way.
It is evident that there is a need for more thorough consideration of biological invasions in conservation planning overall, in particular as the siting of protected areas may be greatly affected by the approach followed to account for alien species (Giakoumi et al., 2016). Currently, all reviewed studies that considered biological invasions, except Giakoumi et al. (2011), considered alien species as having only negative impacts to biodiversity and either protected impacted sites to apply eradication or control actions or more often avoided highly impacted areas for inclusion in their proposed networks of protected areas. Despite the fact that some alien species might contribute to the achievement of conservation goals or provide socio-economic benefits to parts of society and the prediction by some scientists of an increasing contribution of alien species to conservation objectives in the future (Schlaepfer et al., 2011), we are far from that happening in practice. The specific positive impacts of some alien species on specific objectives have been mentioned in only one study (Adams et al., 2014), which did not set conservation targets for alien species.
Biological Invasions and Conservation Planning in the Marine Environment
In the marine environment, environmental connectivity facilitates dispersal of organisms over large distances, with hydrographic barriers occurring at much larger spatial scales than in terrestrial realms, while connectivity of freshwater environments takes place at intermediate scales between the previous ones (Levin, 1992). As a consequence, the eradication of established invasive alien species, which is sometimes a sensible option in terrestrial systems, becomes difficult in freshwater systems and seems almost impossible in marine ones. While several successful eradication attempts in the terrestrial and the freshwater realms (especially in isolated or confined areas) have been made, marine attempts have mostly failed (Ojaveer et al., 2015), unless at a very initial stage of invasion, e.g., the eradication of Caulerpa taxifolia in California (Anderson, 2005) or of the black-striped mussel Mytilopsis sallei in Australia (Willan et al., 2000). Marine alien species are an unavoidable new component of the ecosystems and cannot be ignored. However, to adequately consider biological invasions in conservation planning, a deep understanding of their role in ecosystem functioning and in the consequent provision of ecosystem services is needed to guide strategies for their treatment. This is a challenging task, especially in a global change context, when local and regional environmental conditions are shifting.
A few marine alien species can provide conservation benefits and some contribute to the flow of ecosystem services (Katsanevakis et al., 2014). For example, in the eastern Mediterranean, the world's most invaded marine region because of the opening of the Suez Canal, a substantial percentage of the commercial fisheries catch is now composed of alien species (Edelist et al., 2013). Furthermore, multi-species collapses of native invertebrate species in the Levantine Sea have been mainly attributed to climate change (Yeruham et al., 2015; Rilov, 2016; Givan et al., 2018), although in some cases the negative impact of specific invasive alien species is also a contributing factor (Galil, 2007). Some alien groups are highly abundant even in well protected MPAs (Rilov et al., 2017). From the ecological standpoint, it is possible that in the context of global warming, ecosystem functioning and services in the Levantine Sea would be substantially impeded without the thermophilic invaders from the Red Sea through the Suez Canal, which are filling the niche of temperature-sensitive native species that are declining or going locally extinct. The contribution of alien species to ecosystem functioning and socio-economy, especially under climate change scenarios and within land-locked seas such as the Mediterranean, the Baltic or the Black Sea, need to be further investigated and taken into account for conservation planning. Shifting from a species-based to an ecological functions-or socioeconomic-based approach, might be an option.
Further research is needed to better understand the impacts of invasive alien species on marine ecosystems and to adopt management strategies accordingly (Ojaveer et al., 2015). Relevant experimental studies are lacking for most invasive alien species (Katsanevakis et al., 2014), while scientific effort is unevenly distributed (as also found in our review, i.e., a bias toward the areas with the greatest concentration of research institutions in Europe and North America) with important gaps in many regions and taxonomic groups. Impact and risk assessments of invasive alien species suffer from substantial uncertainties related to insufficient data, type of ecosystem responses, multiple species interactions and resolution of spatial data (Katsanevakis and Moustakas, 2018).
Marine protected areas are often advocated as a management tool that could be used to increase the resilience of ecosystems in face of biological invasions. It is expected that restored trophic structure could control the spread of invasive alien species and mitigate their impacts (e.g., Mumby et al., 2011). However, some controversial results exist and marine protected areas could also facilitate the spread of alien species populations (Burfeind et al., 2013), which often thrive inside well protected reserves (Rilov et al., 2017). Improving our understanding on the role of biological invasions upon ecosystem structure and functionality would provide the basis for adopting a “protect to control” or a “protect to conserve,” or an “avoid” decision in conservation planning. Still, any such decision and proposed measure are scale-dependent, and generalizations on the expected effects of biological invasions should be avoided.
Experimental work, case-studies in the field, and modeling techniques may offer extremely useful tools for understanding impacts of invasive alien species and the interactions among native and alien species. The development of modeling approaches, especially dynamic ecological models, has become essential to assess and predict impacts of invasive alien species, understand ecological processes and identify and evaluate management options (Wonham and Lewis, 2009), thus effectively inform conservation planning. Despite this, models assessing impacts of alien species are rarely used to provide management recommendations.
An 11-Step Framework to Account for Alien Species in Conservation Planning
The process of biological invasions is highly dynamic and context-dependent, thus very challenging for conservation planning efforts, especially in combination with climate change. Once arriving in a new region, the establishment of an alien species will depend on a multitude of factors, such as: (1) the availability of suitable habitat, (2) its mode of natural or human-assisted further spread, (3) the native community (and other alien species if present) and its ability to resist invasions, and (4) changes in environmental conditions in the potential range of expansion due to increased stress induced by human activities and climate change, which could specifically facilitate the spread of thermophilic species.
In this context, the process of systematic and other conservation planning usually focuses on native biodiversity, and very often ignores or avoids dealing with alien species (either as a threat or as a benefit). In marine environments, invasive alien species usually cannot be completely eradicated and are there to stay. Thus, conservation scientists, managers and decision makers should incorporate biotic invasions in a range of ways and at different phases of the conservation planning process. We propose that biological invasions are incorporated into the systematic conservation planning design suggested by Pressey and Bottrill (2009), which includes 11 steps (Figure 4).
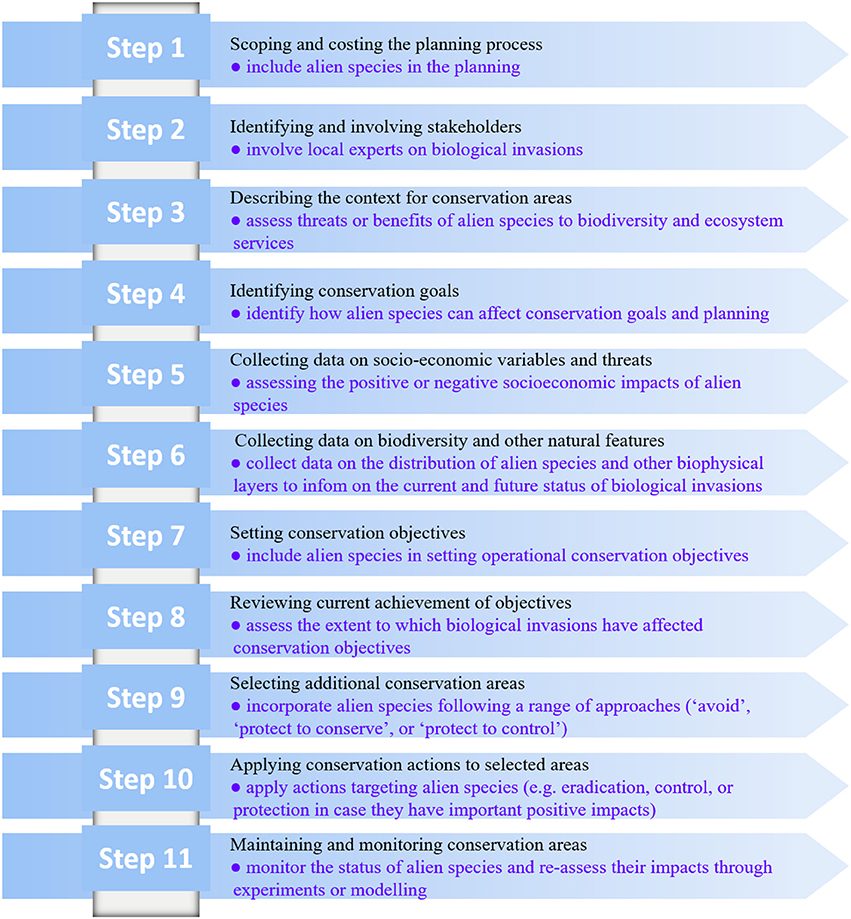
Figure 4. Incorporating biological invasions in the 11-step process of conservation planning, as suggested by Pressey and Bottrill (2009). The original titles of the 11 steps are given in black, and our proposed inclusion of biological invasions at each step is given bulleted in blue.
The first step of the systematic conservation planning (scoping and costing the planning process) can already address alien (including invasive) species, by recognizing their presence in the area of concern and their current ecological role and status. In the second step, where stakeholders are identified and involved, those with expertise and experience related to invasive alien species and biotic invasions should be engaged. In steps 3 and 4, where the context is defined and the conservation goals are identified (respectively), invasive alien species can again be included. For example, one can aim to avoid attempting to protect ecosystems that are highly invaded or on the contrary to protect them as focal areas (for example in an effort to reduce alien species impacts if they are deleterious and can be controlled) for further management actions. In step 5, where socioeconomic data or data on threats is collated, information on the positive and/or negative socioeconomic impacts of invasive alien species should be assessed (or the need to assess it should be recommended if this information does not exist). In step 6 where the biophysical data is collected and assembled, the planning can include a range of biophysical data layers that will inform what conditions are potentially favorable for invasions and could further facilitate invasions to the ecosystem, particularly under climate change. In steps 7 and 8, where conservation objectives are set and the achievements of the objectives are reviewed, respectively, the outcomes of actions relating to biotic invasions, such as eradication or control efforts, can be incorporated. In step 9 that selects additional conservation areas, invasive alien species and invaded habitats can be incorporated in a range of approaches including “avoid,” “protect to control,” or “protect to conserve” alien species. Step 10 that applies conservation actions can include actions that specifically address invasive alien species, and step 11 that maintains and monitors conservation areas is key because invasive alien species ranges are very often dynamic, and can change over space and time especially as new vectors might develop and environmental conditions change. In that context, step 5 that collects data on threats should also be updated and revised.
Biological invasions should be incorporated more often into conservation planning processes in a transparent and practical way. Ignoring biological invasions can lead to failures of conservation plans' expected outcomes. Defining clear targets and objectives that address biological invasions may lead to much better conservation outcomes and to the saving of the limited conservation funds. Incorporating uncertainties arising from climate change and other external drivers of change in management can also help to cope with upcoming biological invasions.
Author Contributions
VM, PGA, and SK coordinated the systematic review, prepared the figures and conducted the statistical analyses. All co-authors equally participated in the systematic review (design and implementation) and contributed to the final draft.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This article was undertaken in the framework of COST Action 15121 “Advancing marine conservation in the European and contiguous seas” (MarCons; http://www.marcons-cost.eu; Katsanevakis et al., 2017)—supported by COST (European Cooperation in Science and Technology, CA15121). We thank the participants of the MarCons workshop in Zagreb on 13-14 October 2016 for sharing their ideas.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2018.00178/full#supplementary-material
References
Adams, V. M., Álvarez-Romero, J. G., Carwardine, J., Cattarino, L., Hermoso, V., Kennard, M. J., et al. (2014). Planning across freshwater and terrestrial realms: cobenefits and tradeoffs between conservation actions. Conserv. Lett. 7, 425–440. doi: 10.1111/conl.12080
Anderson, L. W. J. (2005). California's reaction to Caulerpa taxifolia: a model for invasive species rapid response. Biol. Invasions 7, 1003–1016. doi: 10.1007/s10530-004-3123-z
Auerbach, N. A., Tulloch, A. I. T., and Possingham, H. P. (2014). Informed actions: where to cost effectively manage multiple threats to species to maximize return on investment. Ecol. Appl. 24, 1357–1373 doi: 10.1890/13-0711.1
Ausseil, A. G. E., Chadderton, L. W., Gerbeaux, P., Stephens, R. T., and Leathwick, J. R. (2011). Applying systematic conservation planning principles to palustrine and inland saline wetlands of New Zealand. Freshw. Biol. 56, 142–161. doi: 10.1111/j.1365-2427.2010.02412.x
Bellard, C., Cassey, P., and Blackburn, T. M. (2016). Alien species as a driver of recent extinctions. Biol. Lett. 12:20150623. doi: 10.1098/rsbl.2015.0623
Boykin, K. G., Young, K. E., and Guy, R. K. (2011). Spatial identification of statewide areas for conservation focus in New Mexico: implications for State conservation efforts. Diversity 3, 275–295. doi: 10.3390/d3020275
Burfeind, D. D., Pitt, K. A., Connolly, R. M., and Byers, J. E. (2013). Performance of non-native species within marine reserves. Biol. Invasions 15, 17–28. doi: 10.1007/s10530-012-0265-2
Carwardine, J., Wilson, K. A., Watts, M., Etter, A., Klein, C. J., and Possingham, H. P. (2008). Avoiding costly conservation mistakes: the importance of defining actions and costs in spatial priority setting. PLoS ONE 3:e2586. doi: 10.1371/journal.pone.0002586
CBD (2016). Convention on Biological Diversity, Programme of Work on Invasive Alien Species. Available online at: https://www.cbd.int/invasive (Accessed November 13, 2017)
Cowling, R. M., Pressey, R. L., Sims-Castley, R., le Roux, A., Baard, E., Burgers, C. J., et al. (2003). The expert or the algorithm?—comparison of priority conservation areas in the Cape Floristic Region identified by park managers and reserve selection software. Biol. Conserv. 112, 147–167. doi: 10.1016/S0006-3207(02)00397-X
D'Antonio, C., and Meyerson, L. A. (2002). Exotic plant species as problems and solutions in ecological restoration: a synthesis. Restor. Ecol. 10, 703–713. doi: 10.1046/j.1526-100X.2002.01051.x
Edelist, D., Rilov, G., Golani, D., Carlton, J. T., and Spanier, E. (2013). Restructuring the sea: profound shifts in the world's most invaded marine ecosystem. Divers. Distrib. 19, 69–77. doi: 10.1111/ddi.12002
Galil, B. D., (2007). Loss or gain? Invasive aliens and biodiversity in the Mediterranean Sea. Mar. Pollut. Bull. 55, 314–322. doi: 10.1016/j.marpolbul.2006.11.008
Giakoumi, S., Grantham, H. S., Kokkoris, G. D., and Possingham, H. P. (2011). Designing a network of marine reserves in the Mediterranean Sea with limited socio-economic data. Biol. Conserv. 144, 753–763. doi: 10.1016/j.biocon.2010.11.006
Giakoumi, S., Guilhaumon, F., Kark, S., Terlizzi, A., Claudet, J., Felline, S., et al. (2016). Space invaders; biological invasions in marine conservation planning. Divers. Distrib. 22, 1220–1231. doi: 10.1111/ddi.12491
Givan, O., Edelist, D., Sonin, O., and Belmaker, J. (2018). Thermal affinity as the dominant factor changing Mediterranean fish abundances. Glob. Change Biol. 24, e80–e89. doi: 10.1111/gcb.13835
Gleditsch, J. M. (2017). “The role of invasive plant species in urban avian conservation,” in Ecology and Conservation of Birds in Urban Environments, eds. E. Murgui and M. Hedblom (Cham, Springer), 413–424.
Gozlan, R. E. (2008). Introduction of non-native freshwater fish: is it all bad? Fish Fish. 9, 106–115. doi: 10.1111/j.1467-2979.2007.00267.x
Hermoso, V., Januchowski-Hartley, S., Linke, S., and Possingham, H. P. (2011). Reference vs. present-day condition: early planning decisions influence the achievement of conservation objectives. Aquat. Conserv. 21, 500–509. doi: 10.1002/aqc.1211
Hermoso, V., Ward, D. P., and Kennard, M. J. (2013). Prioritizing refugia for freshwater biodiversity conservation in highly seasonal ecosystems. Divers. Distrib. 19, 1031–1042. doi: 10.1111/ddi.12082
Jones, H. P., Holmes, N. D., Butchart, S. H., Teshy, B. R., Kappes, P. J., Corkery, I., et al. (2016). Invasive mammal eradication on islands results in substantial conservation gains. Proc. Natl. Acad. Sci. U.S.A. 113, 4033–4038. doi: 10.1073/pnas.1521179113
Katsanevakis, S., and Moustakas, A. (2018). Uncertainty in marine invasion science. Fron. Mar. Sci. 5:38. doi: 10.3389/fmars.2018.00038
Katsanevakis, S., Mackelworth, P., Coll, M., Fraschetti, S., Mačić, V., Giakoumi, S., et al. (2017). Advancing marine conservation in European and contiguous seas with the MarCons Action. Res. Ideas Outcomes 3:e11884. doi: 10.3897/rio.3.e11884
Katsanevakis, S., Wallentinus, I., Zenetos, A., Leppäkoski, E., Çinar, M. E., Oztürk, B., et al. (2014). Impacts of marine invasive alien species on ecosystem services and biodiversity: a pan-European review. Aquat. Invasions 9, 391–423. doi: 10.3391/ai.2014.9.4.01
Klein, C. J., Tulloch, V. J., Halpern, B. S., Selkoe, K. A., Watts, M. E., Steinback, C., et al. (2013). Tradeoffs in marine reserve design: habitat condition, representation, and socioeconomic costs. Conserv. Lett. 6, 324–332. doi: 10.1111/conl.12005
Lagabrielle, E., Rouget, M., Payet, K., Wistebaar, N., Durieux, L., Baret, S., et al. (2009). Identifying and mapping biodiversity processes for conservation planning in islands: a case study in Re'union Island (Western Indian Ocean). Biol. Conserv. 142, 1523–1535. doi: 10.1016/j.biocon.2009.02.022
Lawrence, D. J., Larson, E. R., Liermann, C. A. R., Mims, M. C., Pool, T. K., and Olden, J. D. (2011). National parks as protected areas for U.S. freshwater fish diversity. Conserv. Lett. 4, 364–371. doi: 10.1111/j.1755-263X.2011.00185.x
Levin, S. A. (1992). The problem of pattern and scale in ecology: the Robert, H. MacArthur award lecture. Ecology 73, 1943–1967. doi: 10.1007/978-1-4615-1769-6_15
Marchetti, M. P., and Engstrom, T. (2016). The conservation paradox of endangered and invasive species. Conserv. Biol. 30, 434–437. doi: 10.1111/cobi.12642
Margules, C. R., and Pressey, R. L. (2000). Systematic conservation planning. Nature 405, 243–253. doi: 10.1038/35012251
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2010). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 8, 336–341. doi: 10.1016/j.ijsu.2010.02.007
Mumby, P. J., Harborne, A. R., and Brumbaugh, D. R. (2011). Grouper as a natural biocontrol of invasive lionfish. PLoS ONE 6:e21510. doi: 10.1371/journal.pone.0021510
Naidoo, R., Balmford, A., Ferraro, P. J., Polasky, S., Ricketts, T. H., and Rouget, M. (2006). Integrating economic costs into conservation planning. Trends Ecol. Evol. 21, 681–687. doi: 10.1016/j.tree.2006.10.003
Ojaveer, H., Galil, B. S., Campbell, M. L., Carlton, J. T., Canning-Clode, J., Cook, E. J., et al. (2015). Classification of non-indigenous species based on their impacts: considerations for application in marine management. PLoS Biol. 13:e1002130. doi: 10.1371/journal.pbio.1002130
Olson, D. M., Dinerstein, E., Wikramanayake, E. D., Burgess, N. D., Powell, G. V., Underwood, et al. (2001). Terrestrial ecoregions of the world: a new map of life on earth. Bioscience 51, 933–938. doi: 10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2
Olson, D., Farley, L., Patrick, A., Watling, D., Tuiwawa, M., Masibalavu, V., et al. (2010). Priority forests for conservation in Fiji: landscapes, hotspots and ecological processes. Oryx 44, 57–70. doi: 10.1017/S0030605309990688
Pierce, S. M., Cowling, R. M., Knight, A. T., Lombard, A. T., Rouget, M., and Wolf, T. (2005). Systematic conservation planning products for land-use planning: interpretation for implementation. Biol. Conserv. 125, 441–458. doi: 10.1016/j.biocon.2005.04.019
Pressey, R. L., and Bottrill, M. C. (2009). Approaches to landscape-and seascape-scale conservation planning: convergence, contrasts and challenges. Oryx 43, 464–475. doi: 10.1017/S0030605309990500
Pressey, R. L., Cabeza, M., Watts, M. E., Cowling, R. M., and Wilson, K. A. (2007). Conservation planning in a changing world. Trends Ecol. Evol. 22, 583–892. doi: 10.1016/j.tree.2007.10.001
Reid, B. L., Hernández, K. L., Frangópulos, M., Bauer, G., Lorca, M., Kilroy, C., et al. (2012). The invasion of the freshwater diatom Didymosphenia geminata in Patagonia: prospects, strategies, and implications for biosecurity of invasive microorganisms in continental waters. Conserv. Lett. 5, 432–440. doi: 10.1111/j.1755-263X.2012.00264.x
Rilov, G. (2016). Multi-species collapses at the warm edge of a warming sea. Sci. Rep. 6:36897. doi: 10.1038/srep36897
Rilov, G., Peleg, O., Yeruham, E., Garval, T., Vichik, A., and Raveh, O. (2017). Alien turf: overfishing, overgrazing and invader domination in southeastern Levant reef ecosystems. Aquat. Conserv. 28, 351–369. doi: 10.1002/aqc.2862
Rouget, M., Cowling, R. M., Pressey, R. L., and Richardson, D. M. (2003). Identifying spatial components of ecological and evolutionary processes for regional conservation planning in the Cape Floristic Region, South Africa. Divers. Distrib. 9, 191–210. doi: 10.1046/j.1472-4642.2003.00025.x
Schlaepfer, M. A., Sax, D. F., and Olden, J. D. (2011). The potential conservation value of non-native species. Conserv. Biol. 25, 428–437. doi: 10.1111/j.1523-1739.2010.01646.x
Schüttler, E., Klenke, R., McGehee, S., Rozzi, R., and Jax, K. (2009). Vulnerability of ground-nesting waterbirds to predation by invasive American mink in the Cape Horn Biosphere Reserve, Chile. Biol. Conserv. 142, 1450–1460. doi: 10.1016/j.biocon.2009.02.013
Sealey, K. S., McDonough, V. N., and Lunz, K. S. (2014). Coastal impact ranking of small islands for conservation, restoration and tourism development: a case study of The Bahamas. Ocean Coast. Manage. 91, 1–14. doi: 10.1016/j.ocecoaman.2014.01.010
Simberloff, D. (2009). We can eliminate invasions or live with them. Successful management projects. Biol. Invasions 11, 149–157. doi: 10.1007/s10530-008-9317-z
Simberloff, D., Martin, J. L., Genovesi, P., Maris, V., Wardle, D. A., Aronson, J., et al. (2013). Impacts of biological invasions: what's what and the way forward. Trends Ecol. Evol. 28, 58–66. doi: 10.1016/j.tree.2012.07.013
Spalding, M. D., Fox, H. E., Allen, G. R., Davidson, N., Ferdana, Z. A., Finlayson, M., et al. (2007). Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience 57, 573–583. doi: 10.1641/B570707
Spatz, D. R., Newton, K. M., Heinz, R., Tershy, B., Holmes, N. D., Butchart, S. H., et al. (2014). The biogeography of globally threatened seabirds and island conservation opportunities. Conserv. Biol. 28, 1282–1290. doi: 10.1111/cobi.12279
Stewart, D. R., Underwood, Z. E., Rahel, F. J., and Walters, A. W. (2017). The effectiveness of surrogate taxa to conserve freshwater biodiversity. Conserv. Biol. 32, 183–194. doi: 10.1111/cobi.12967
Tallis, H., Ferdaña, Z., and Gray, E. (2008). Linking terrestrial and marine conservation planning and threats analysis. Conserv. Biol. 22, 120–130. doi: 10.1111/j.1523-1739.2007.00861.x
Tulloch, A. I. T., Tulloch, V. J. D., Evans, M. C., and Mills, M. (2014). The value of using feasibility models in systematic conservation planning to predict landholder management uptake. Conserv. Biol. 6, 1462–1473. doi: 10.1111/cobi.12403
Vilà, M., Basnou, C., Pyšek, P., Josefsson, M., Genovesi, P., Gollasch, S., et al. (2010). How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Front. Ecol. Environ. 8, 135–144. doi: 10.1890/080083
Vilà, M., and Hulme, P. E. (2018). Impact of Biological Invasions on Ecosystem Services. Berlin: Springer
Willan, R. C., Russell, B. C., Murfet, N. B., Moore, K. L., McEnnulty, F. R., Horner, S. K., et al. (2000). Outbreak of Mytilopsis sallei (Recluz, 1849) (Bivalvia: Dreissenidae) in Australia. Molluscan Res. 20, 25–30. doi: 10.1080/13235818.2000.10673730
Wilson, K. A., McBride, M. F., Bode, M., and Possingham, H. P. (2006). Prioritizing global conservation efforts. Nature 440, 337–340. doi: 10.1038/nature04366
Wonham, M. J., and Lewis, M. A. (2009). “Modeling marine invasions: current and future approaches,” in Biological Invasions in Marine Ecosystems, eds G. Rilov, J. A. Crooks (Berlin: Springer), 71–105.
Keywords: invasive alien species, management actions, mitigation, non-indigenous species, systematic conservation planning
Citation: Mačić V, Albano PG, Almpanidou V, Claudet J, Corrales X, Essl F, Evagelopoulos A, Giovos I, Jimenez C, Kark S, Marković O, Mazaris AD, Ólafsdóttir GÁ, Panayotova M, Petović S, Rabitsch W, Ramdani M, Rilov G, Tricarico E, Vega Fernández T, Sini M, Trygonis V and Katsanevakis S (2018) Biological Invasions in Conservation Planning: A Global Systematic Review. Front. Mar. Sci. 5:178. doi: 10.3389/fmars.2018.00178
Received: 18 March 2018; Accepted: 04 May 2018;
Published: 25 May 2018.
Edited by:
Agnese Marchini, University of Pavia, ItalyReviewed by:
José Lino Vieira De Oliveira Costa, Universidade de Lisboa, PortugalRebecca Louise Morris, University of Melbourne, Australia
Copyright © 2018 Mačić, Albano, Almpanidou, Claudet, Corrales, Essl, Evagelopoulos, Giovos, Jimenez, Kark, Marković, Mazaris, Ólafsdóttir, Panayotova, Petović, Rabitsch, Ramdani, Rilov, Tricarico, Vega Fernández, Sini, Trygonis and Katsanevakis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vesna Mačić, macic.v@ac.me
†Present Address: Tomás Vega Fernández, Stazione Zoologica Anton Dohrn, Naples, Italy
 Vesna Mačić
Vesna Mačić Paolo G. Albano
Paolo G. Albano Vasiliki Almpanidou
Vasiliki Almpanidou Joachim Claudet
Joachim Claudet Xavier Corrales5
Xavier Corrales5  Franz Essl
Franz Essl Athanasios Evagelopoulos
Athanasios Evagelopoulos Ioannis Giovos
Ioannis Giovos Carlos Jimenez
Carlos Jimenez Salit Kark
Salit Kark Olivera Marković
Olivera Marković Antonios D. Mazaris
Antonios D. Mazaris Guðbjörg Á. Ólafsdóttir
Guðbjörg Á. Ólafsdóttir Marina Panayotova
Marina Panayotova Gil Rilov
Gil Rilov Elena Tricarico
Elena Tricarico Maria Sini
Maria Sini Vasilis Trygonis
Vasilis Trygonis Stelios Katsanevakis
Stelios Katsanevakis