Land Use Impacts on Coral Reef Health: A Ridge-to-Reef Perspective
- 1Center for Global Discovery and Conservation Science, Arizona State University, Tempe, AZ, United States
- 2Emmett Interdisciplinary Program in Environment and Resources, Stanford University, Stanford, CA, United States
Over 60% of the world’s reefs experience damage from local activities such as overfishing, coastal development, and watershed pollution. Land-based sources of pollution are a critical threat to coral reefs, and understanding “ridge-to-reef” changes is urgently needed to improve management and coral survival in the Anthropocene. We review existing literature on spatial-ecological connections between land use and coral health, specifically examining vegetative, agricultural, urban, and other land-use types. In general, forested land use is positively related to metrics of coral condition, while anthropogenic land uses like urban development and agriculture drive a decline in coral cover, diversity, colony size, and structural complexity. However, land-use and land-cover impacts vary across time and space, and small portions of the landscape (e.g., discrete segments of unpaved roads, grazed and scalded hillsides) may have an outsized effect on reef pollution, presenting opportunities for targeted conservation. Some coral species show resilience under land-use and land-cover change, and the impact of land use on coral recovery from bleaching remains an active area of research. Finally, a spatial bibliography of existing literature reveals that most ridge-to-reef studies focus on a handful of regional hotspots, surface water, and watershed-scale dynamics; more research is needed to address groundwater connectivity and to compare land-use impacts across multiple regions and scales. Approaches from landscape ecology that assess spatial patterns of, and synergies between, interlocking land cover may assist conservation managers in designing more resilient reefscapes.
Introduction
Land-use planning is fundamental to coral protection in the Anthropocene. Coral reefs downstream of land disturbance are often degraded by disease; low larval recruitment and survival; low rates of calcification and photosynthesis; and mortality from hypoxia, tissue degradation, and macroalgal competition (Fabricius, 2005; Weber et al., 2012; Amato et al., 2016). As a result, marine regulations that do not integrate land-based controls can be prone to failure (Allison et al., 1998; Álvarez-Romero et al., 2011; Halpern et al., 2013; Bégin et al., 2016).
The need to couple land-sea planning is widely acknowledged, however, executing ridge-to-reef conservation is often complex. Historically, conservation managers have delineated marine reserves based on static measures of site composition (e.g., hotspots for marine biodiversity) rather than the time-variant dynamics that define land-sea processes, such as animal migration or contaminant flows (Stoms et al., 2005; Gonzalez et al., 2017). An added complication is that the cost-effectiveness of land conservation for marine protection varies by location: in one study, one unit of forest conservation was roughly 500 times more cost effective than an equal conservation area in a different, lower-impact watershed (Klein et al., 2012). It is often difficult for managers to quantify the relative benefits of different land-use scenarios, particularly in data-poor regions with scarce technical resources (Roberts et al., 2017). In addition, land conservation invites a new suite of socioeconomic interests and stakeholder groups into the marine planning process, and terrestrial and marine values may prioritize different spaces for conservation (Klein et al., 2014). Nonetheless, in recent years, a community of practice surrounding ridge-to-reef protection has emerged, introducing new methods and advances to land-sea planning (Beger et al., 2010; Álvarez-Romero et al., 2015; Jupiter et al., 2017; Oleson et al., 2017; Saunders et al., 2017; Delevaux et al., 2018a,b,c).
This review summarizes existing literature on how land use affects coral health. Existing studies have addressed various facets of ridge-to-reef impacts. Extensive research documents coral physiology and community responses to land-based pollutants (Fabricius, 2005; Erftemeijer et al., 2012), the hydrologic fate and transport of pollutants from land to reef (Brodie et al., 2010; Knee et al., 2016; Margvelashvili et al., 2018), and oceanographic processes affecting sediment and nutrient dispersal (Storlazzi et al., 2004, 2009; Carter et al., 2009; Fabricius et al., 2014). Applied conservation research also addresses barriers to joint land-sea policymaking (Jupiter et al., 2017), social-ecological systems underpinning sustainable ridge-to-reef use (Delevaux et al., 2018c; Winter et al., 2018), and, while this literature is still nascent, quantitative tradeoff analyses to select conservation areas across land and sea (Tallis et al., 2008; Makino et al., 2013; Álvarez-Romero et al., 2015; Oleson et al., 2017). However, to date, no study has explicitly reviewed the state of current knowledge on the relationship between land use and coral condition.
To address this gap, we examine the major impacts of forest vegetation, agriculture, urban, and other land-use types on coral reefs. We define these land uses as follows:
• Forest vegetation: Conserved land use, i.e., non-cultivated vegetated lands, including deciduous, coniferous, herbaceous, and wetland land cover. Most ridge-to-reef studies do not differentiate between vegetative cover types, and we therefore refer to all vegetative lands as “forest.”
• Agriculture: The commercial harvest of natural resources, including pastureland and hay, and cultivated crops. Common agriculture types include beef/cattle ranching, sugarcane, bananas, horticulture, and oil palm.
• Urban: The anthropogenic built environment: impervious urban cover, ports, residences, recreational development (e.g., golf courses), and wastewater infrastructure like septic tanks and sewage outfalls.
• Other: Diverse land use types not captured by the previous categories. Includes extractive facilities like mines and quarries, and rural roads.
While these categories are broad and may encompass numerous classes of land cover (e.g., urban land use may consist of both residential lawn and impervious cover), many studies focus on human use events like the growth of a city or agriculture expansion, and thus, we organize this review by general use categories while addressing the biophysical nuances of land cover where relevant. We start with background information on vectors of land-reef connectivity, as informed by the hydrological literature. We then review each land-use type, and in turn, summarize existing literature on the relationship between land-use change and coral health. We then present a “spatial bibliography” comprised of (1) an overview of the geographic distribution and breadth of current literature and (2) a concise summary of spatial considerations in land-use and land-cover research, including the role of scale, orientation, and land-cover pattern (e.g., patch density) in land-reef interactions. We conclude by identifying opportunities for further research.
We identified literature using a “Web of Science” v5.32 (Clarivate Analytics, 2019) search of the keywords “land use + coral reef,” “land cover + coral reef,” and “ridge-to-reef.” From the approximately 600 articles returned and their references, we selected studies that explicitly measured variation in both land use (or land cover) and coral condition. We excluded laboratory experiments that did not measure land use change, and sediment or nutrient budgets that did not measure coral health, though we reviewed these studies for context. We included studies that surveyed corals at increasing distances from shore as a proxy for land-use pressure. In addition, we included existing reviews on region-specific topics (e.g., sedimentation in the Great Barrier Reef), and case studies that examine the long-term effects of land use change on coral condition in places of interest, such as major cities and bays. This review is focused on coral species and we therefore do not comprehensively review studies of fish, algae, bioeroders, and other organisms. However, given the importance of these taxa to coral health, most sections offer brief contextual background on their role in ridge-to-reef processes. Where studies reference fish and algae as mediators of coral outcomes, we note these details.
Background: Land-Reef Interactions
Land-use and land-cover change affects coral reefs through several key ecological processes, in particular:
• Freshwater regulation: Land and reefs primarily interact through hydrologic channels: either surface flow or submarine groundwater discharge. Land cover modulates the amount of freshwater that reaches coral reefs, which can alter reef salinity and temperature (Prouty et al., 2009; Kroon et al., 2014; Tarya et al., 2018). Freshwater particularly impacts corals’ early life history stages. For example, a 20% decrease in salinity can cause an 86% decrease in fertilization success during coral spawning and a 50% reduction in larval developmental success (Richmond, 1995; Richmond et al., 2019).
• Contaminant retention: Land spaces may either serve as sources or sinks for surface and groundwater contaminants, particularly nutrients, sediment, herbicides like diuron and atrazine, and novel pathogens like fecal bacteria (Jones et al., 2003; McKergow et al., 2005; Dinsdale et al., 2008; Goh et al., 2017). Terrigenous contaminants pose a major threat to coral growth, reproduction, and post-disturbance recovery (Wolanski et al., 2004; Fabricius, 2005) and, depending on ocean hydrodynamics, can persist and undergo periodic resuspension on reefs for many years (Tribble et al., 2015; Teneva et al., 2016).
• Land-sea biological exchange: Land-use and land-cover change can increase the abundance of invasive species on coral reefs (Lapointe and Bedford, 2011) and degrade upstream habitat for native anadromous species, which are more abundant in high tropical islands than larger continents (Jenkins et al., 2010).
Table 1 summarizes how specific land-use and land-cover regimes affect these processes. Strong similarities exist between anthropogenic land covers: in general, developed and denuded landscapes lead to heavy freshwater and contaminant flux at the coast. Human-dominated land uses, however, differ in several key respects. Agriculture maintains permeable soils that receive intensive doses of nutrients and are thus prone to groundwater leaching, whereas urban areas create impermeable surfaces (e.g., pavement) that funnel large volumes of water and contaminants to discharge points (Han et al., 2017; Hall, 2018). Urban pollutants reflect the influence of large groups of people, for example, flame retardants, pharmaceuticals, detergents, personal care products, heavy metals, and petroleum-based compounds (Wear and Thurber, 2015; Breckwoldt et al., 2016; Takesue and Storlazzi, 2017). Urbanization has also been specifically linked to novel biota like fecal or fungal pathogens on reefs (Bonkosky et al., 2009; Norat-Ramírez et al., 2019). Historically, however, few studies have compared land uses directly: if diverse human activities affect a common water body, it is difficult to isolate their individual or unique contributions to the problem. Exceptions include parcel-based sediment export models (Ramos-Scharrón and MacDonald, 2007), comparisons of watersheds with highly dissimilar land-use regimes (Cox et al., 2006), and coral core or algal tissue sampling where land uses have a distinct chemical tracer (Fallon et al., 2002; Lewis et al., 2007; Umezawa et al., 2012; Amato et al., 2016). A summary of methods for assessing ridge-to-reef interactions can be found in Oleson et al. (2018).
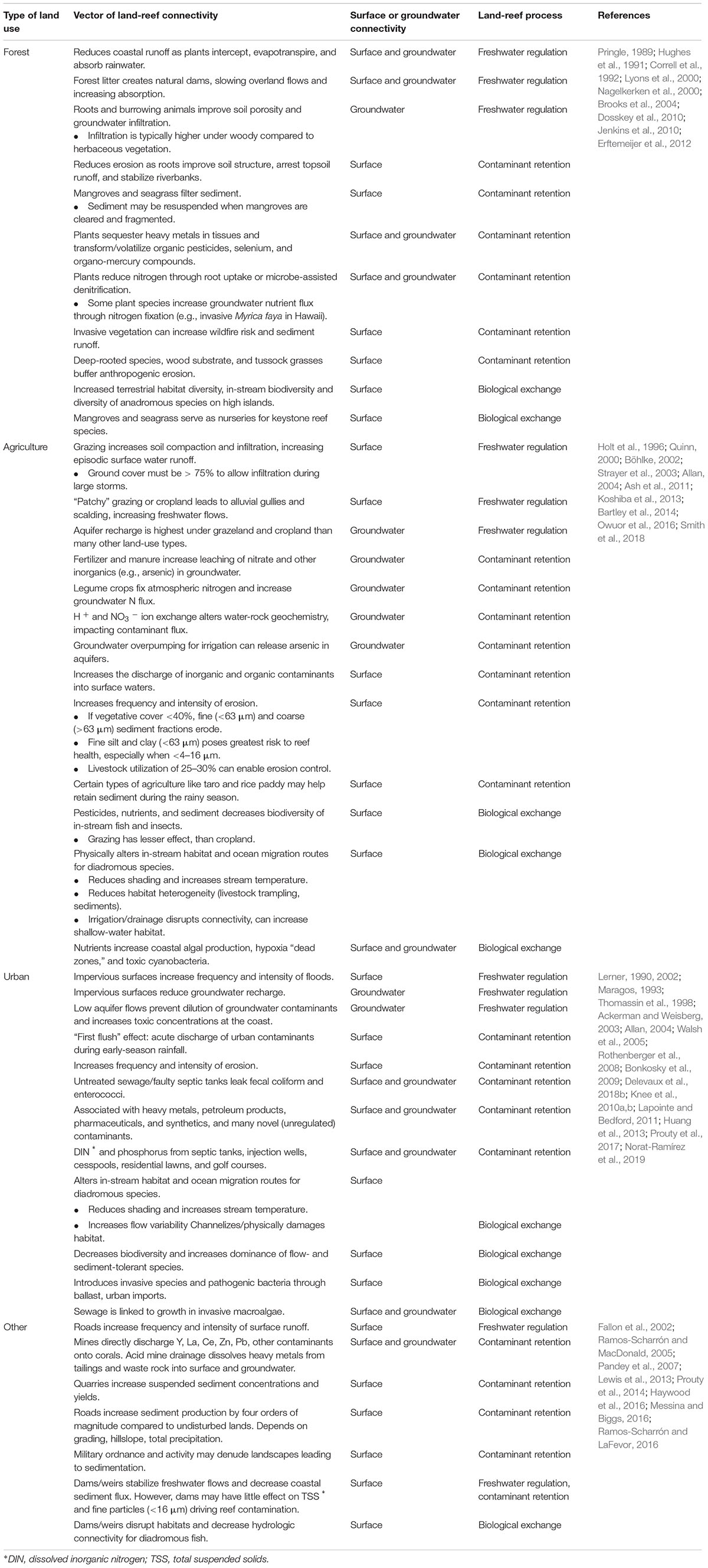
Table 1. Description of land use impacts on coral reefs through freshwater regulation, contaminant retention, and land-sea biological exchange. Processes are categorized by impact on surface water and/or groundwater.
Land-sea connectivity is particularly strong on high volcanic islands due to several unique features of island ecology. First, islands contain erosive andesite soils and small, steep watersheds, where each unit of land-use change can heavily impact coastal water quality (Verbist et al., 2010; Jupiter et al., 2017). In addition, a temporal mismatch between tropical rainfall patterns and wave dynamics can prevent sediment from dispersing offshore. For example, in Hanalei Bay, Kaua‘i, heavy rains occurred during multiple seasons (late winter, spring, and summer of 2006), yet wave energy was highest in early winter, creating a time lag between sediment deposition and ocean advection that increased sediment time on reefs (Draut et al., 2009). Finally, marine species of island ecosystems are uniquely sensitive to land-based change. More fish and invertebrate species are diadromous on islands compared to continental landmasses, perhaps as an adaptive response to ephemeral streams, and therefore, island biodiversity relies heavily on terrestrial habitats (Jenkins et al., 2010). In sum, the unique properties of islands make them a high priority of ongoing research.
Impacts of Land Use on Coral Reefs
Forest or Vegetative Land Use
Vegetated landscapes generally affect coral reefs by (1) absorbing rainfall and reducing freshwater inputs to marine habitats, (2) stabilizing soils to reduce bankside erosion and sediment flux, (3) absorbing and fixing soil nutrients and mineralizing heavy metals in soils and groundwater, and (4) providing habitat for anadromous fishes and other species connecting land and sea (Hefting et al., 2005; Dosskey et al., 2010; Arthington et al., 2016). Plant physiological traits and landscape features that amplify these processes can have a strong effect on coastal environments (see Table 1).
Studies consistently show that forest cover has a positive relationship to metrics of coral condition. In Madagascar, simulated forest loss of 25–50% increased predictions of sediment export to coral reefs by up to 200%, and sediment loss projections were most sensitive to change in wet, tropical forests on the northern side of the island (Maina et al., 2013). Delevaux et al. (2018a) used scenario-based models in Fiji to show that 573.8 ha of forest restoration across eight watersheds decreased sediment delivery to the coast by 24.2 tons yr–1, resulting in a 1.1% mean increase in coral cover across 577 ha of benthic habitat, and 13.9 kg ha–1 increase in fish biomass. Importantly, this study defined restoration as the conversion of commercial pine to native forest, underlining the relatively low capacity of commercial forest for sediment retention (Delevaux et al., 2018a). In the Caribbean island of Bonaire, mean percent cover of vegetative land predicted a significant increase in deep (>10 m) coral cover, but in the same study, plant biomass showed a negative impact on coral cover, perhaps because Bonaire’s deep-rooted trees are less effective than herbaceous species at arresting shallow topsoil runoff in an arid climate (Roberts et al., 2017). Overall, these studies indicate that forest-reef interactions are mediated by the condition and functional traits of vegetation as well as environmental factors like climate and topography. Different forest types do not have equivalent ecological and hydrologic roles, and therefore, future research that compares forest genera, ecoregions, and scales can help managers identify more specific opportunities for reef-safe landscapes.
Case studies on deforestation show similar associations between forest cover and coral health. At two sites in Guam (Fouha Bay and coastline of the Asan-Piti watershed), declines in coral growth rates, richness, and cover were linked to timelines of forest loss by military activity in the Battle of Guam (Prouty et al., 2014), and forest burning by pig and deer hunters (Richmond et al., 2007). In the Solomon Islands, extensive logging led to a loss of reef habitat from 3528 to 1941 ha, which decreased populations of inshore Acroporid corals and associated parrotfish species (Hamilton et al., 2017), and led to only 9.3% coral cover inshore in 2008, compared to 41.6% in an offshore MPA (Albert et al., 2014). These trends are less visible on Borneo, where the deforestation “hotspot” of Sarawak retains 3% of its forest cover but shows healthy coral cover of 23–39% and low coral disease, bleaching, and bioerosion - though bioerosion increased fivefold during the 2017 wet season, and diversity is low relative to the coral triangle region (Brown et al., 2018).
Mangroves are a specific class of forest cover that have received considerable attention at the land-reef interface. Mangroves thrive in inter-tidal, high-sediment environments, and thus serve as frontline sediment filters along coral reefs of southeast Asia, Australia, the Caribbean, and some Pacific Islands (Duke and Wolanski, 2000). In central Java, fringing reefs near mangroves experienced lower carbonate destruction (loss of 0.54 kg CaCO3 m–2 yr–1) compared to sites farther from mangroves (maximum loss of 8.77 kg CaCO3 kg m–2 yr–1), though coral growth rates were lowest near mangroves (Edinger and Risk, 2000). Corals located near fringing, sediment-trapping mangroves in Moloka‘i exhibited higher growth rates (11.87 mm/year) than unprotected sites (minimum rate of 8.37 mm/year), likely due to mangroves serving as sediment reservoirs (Prouty et al., 2010). In two estuaries in Palau, mangroves covering 3.8% of each catchment trapped 15–44% of fine sediment inflow; nevertheless, mangroves in one estuary (Ngerikiil) that received high sediment due to basin agriculture and construction were unable to prevent large volumes of sediment (peak of 50 mg L–1) from reaching coral reefs (Golbuu et al., 2003; Victor et al., 2004). In Pohnpei, 40% of riverine sediment settled in mangroves and 40% settled in a lagoon reef habitat where residents attribute coral die-offs to sedimentation (Victor et al., 2006). In both Palau and Pohnpei, coral damage is exacerbated by lack of swell exposure within a protected bay and lagoon, where sediment settled quickly on substrate without resuspension. Therefore, mangroves are critical but occasionally insufficient barriers against coral sedimentation, particularly where land uses like construction or agriculture drive excessive erosion.
In a few emergent cases, mangroves serve as sanctuary niches for corals themselves. For example, in the United States Virgin Islands (USVI), Yates et al. (2014) observed 33 of the region’s 40 scleractinian coral species growing on mangrove roots after a bleaching event, an unusual phenomenon given coral aversion to silty substrates and freshwater. Mangrove habitats may offer variable water temperatures, which can increase coral resilience to thermal stress (Oliver and Palumbi, 2011); shade corals from excessive visible and ultraviolet radiation; and create biochemical buffers against ocean acidification (Yates et al., 2014). Nonetheless, examples of mangrove-coral refugia are rare, and in some cases, mangroves proliferate under high sediment and nutrient flows and can therefore foretell coral degradation (Duke and Wolanski, 2000). Mangrove services vary across each river basin: Ewel et al. (1998) developed a functional classification of mangrove forests, pointing out that mangrove zonation—whether mangroves are “fringe” (tide-dominated), “riverine” (river-dominated), or “basin” (catchment interior) stands—dictates plant productivity, nutrient cycling, sediment trapping, and the export of organic matter. It is necessary to account for this functional diversity when planning ridge-to-reef conservation. In addition, much remains unknown about the biogeochemical connectivity between mangroves and coral reefs, and further research is needed to quantify nitrogen and phosphorus exchange between these ecosystems (Davis et al., 2009), and to examine mangrove-coral interactions in higher order watersheds with high discharge and low residence times.
Examples of paired Terrestrial and Marine Protected Areas also demonstrate forest-reef interactions. Locations where forest and marine spaces are jointly protected may yield better outcomes for coral health. A full review of land-sea protection strategies is beyond the scope of this paper; however, several examples are worth noting. Gilby et al. (2016) found in a scenario-based simulation that sediment reduction through catchment rehabilitation paired with Marine Protected Area (MPA) expansion produced the most favorable outcomes for coral health in Moreton Bay, Australia, while actions to reduce fishing (limits on size or total catch per day) and sewage reduction resulted in smaller effects. In Saint Lucia, an analysis of two well-enforced MPAs showed that MPA status had no effect on coral cover, though high-sediment sites exhibited lower coral cover (Bégin et al., 2016). Likewise, MPA and fished sites in a case study from the Solomon Islands showed no differences in percent coral cover, likely due to excessive sedimentation at MPAs from destructive logging practices (Halpern et al., 2013). Analyses from Mexico and the Great Barrier Reef are more equivocal: MPAs can create resilience to short-term flood events (Olds et al., 2014), however, chronic or above-average flood exposure and human development ultimately outweigh MPA benefits, at times driving over 50% coral loss (Lamb et al., 2016; Wenger et al., 2016; Suchley and Alvarez-Filip, 2018). In sum, MPAs without forest conservation may be ineffective, and marrying vegetative land use with MPAs can improve cross-ecosystem outcomes.
Agricultural Land Use
At least 25% of coral reefs worldwide are threatened by pollutants from agriculture (Burke et al., 2011; Kroon et al., 2014). Intensive agriculture is a highly erosive process that transmits sediment, inorganic and organic nutrients (especially nitrogen), and other contaminants to waterways, aquifers, and vulnerable reefs. On an aquifer-dominated island in Japan, nutrient pollution due to major increases in grazing lands (+597%) between 1977 and 2005 corresponded to a ∼10% decrease in coral cover (Dadhich et al., 2017). On the Great Barrier Reef and Fouha Bay, Guam, a model for river plume and cyclone disturbances suggested that, with agricultural expansion, corals show poor recovery as heavy sedimentation prevents new larvae and propagates from seeding damaged reefs, and deters herbivorous fish from grazing down algal mats (Wolanski et al., 2004). On the island of Maui, sugarcane and pineapple plantations were the most significant drivers of coastal nitrogen flux from groundwater, contributing more coastal nitrogen than any other land use including developed areas with high densities of septic tanks and cesspools (Bishop et al., 2017), though additional research also indicated wastewater as a source of coastal nitrogen (Amato et al., 2016).
A large body of research focuses on agricultural impacts on the Great Barrier Reef, where some watersheds are covered by ∼95% agriculture (Fabricius et al., 2005; Hunter and Walton, 2008; Drewry et al., 2009; Brodie J. et al., 2012; Bartley et al., 2014; Star et al., 2018). Studies across 35 river basins show that higher-rainfall basins with intensive cropland (sugar cane, bananas, and horticulture) are primarily responsible for dissolved nutrient and pesticide/herbicide runoff into the Great Barrier Reef, while drier savanna/woodland catchments with heavy grazing contribute the majority of sediment and particulate nutrients, though sources vary by watershed (Lewis et al., 2009; Kroon et al., 2012; Waterhouse et al., 2012; Thorburn and Wilkinson, 2013; Bainbridge et al., 2018).
Exposure to sediment and nutrients on the Great Barrier Reef follows an inshore-offshore gradient (Wolff et al., 2018), as nutrient-rich fine silt and clay (<16 μm) from hillside pastures and gullies travel farther (3–5+ km) than larger particles (>63 μm), which typically settle close to river deltas but can be resuspended with wave and tidal energy (Wilkinson et al., 2013; Bartley et al., 2014). Dissolved nutrients can travel over 10–200 km north from the river mouth over primarily inner shelf reefs <20 km from coast, intensifying coral bleaching (Devlin and Brodie, 2005; Wooldridge and Done, 2009) and contributing to Crown-of-Thorns outbreaks (Wolanski and De’ath, 2005). Importantly, some turbid, nearshore areas of the reef show high diversity, cover, and growth rates and exhibit few signs of bleaching, indicating possible refugia (Browne et al., 2010; Browne, 2012; Perry et al., 2012; Morgan et al., 2016, 2017). Many studies exist on the sources and distribution of sediment in the Great Barrier Reef (Larcombe and Woolfe, 1999; McCulloch et al., 2003; Bainbridge et al., 2009; Hughes et al., 2009; Brodie J. E. et al., 2012; Liu et al., 2018), policy measures to curb pollution (Carroll et al., 2012; Kroon et al., 2016; Reside et al., 2017), and farm-based mitigation strategies (Thorburn and Wilkinson, 2013), and several reviews address agriculture in the Great Barrier Reef (e.g., Brodie et al., 2011; Brodie J. et al., 2012; Bartley et al., 2014).
Agricultural impacts vary significantly in space and time based on soil erodibility, rainfall, oceanic resuspension, and other biophysical factors. On the south coast of Moloka‘i, 50% of the sediment on reefs originated from only 1% of a watershed, and only 1–3 large storm events (or 8–10 h of flow) per year drove overland runoff (Bothner et al., 2006; Stock et al., 2010, 2011; Risk, 2014; Tribble et al., 2015). These findings are consistent with those from the Great Barrier Reef, where nitrogen models for 16 rivers indicated that the total area of reef exposed to Dissolved Inorganic Nitrogen was more than four times greater in a high-flooding year (2010–2011) than a drier year (2012–2013), and a single watershed, the Burdekin, affected over half of the reef area (Wolff et al., 2018). Agricultural risk factors noted throughout the literature include landscape topography (slope and surface roughness), heavy or drought-breaking rainfall, size of catchment or watershed, aquifer heterogeneity and geomorphology, erosive volcanic or basaltic soils, filtering by mangroves and seagrass, land-use proximity to a water body, and oceanographic conditions that resuspend legacy pollutants (Schaffelke et al., 2012; Bartley et al., 2014; Gallo et al., 2015; Chaplin-Kramer et al., 2016; Tulloch et al., 2016).
A small number of studies explore variation in the effects of agriculture on reef ecosystems and options for sustainable agriculture. Research on the Johnstone River in Australia compared banana, sugar cane, beef, and dairy pastureland, revealing that sugarcane was responsible for 60% of nitrate flux in nearby waterways, even though this crop covered only 12% of the landscape (Hunter and Walton, 2008). In Papua New Guinea, researchers simulated coral reef response to three palm oil expansion scenarios: (1) unchecked plantings, (2) plantings following lower-impact Roundtable on Sustainable Palm Oil (RSPO) guidelines, or (3) plantings following “enhanced” RSPO measures (plantings limited to <15° slopes, prohibited near the coast, increased riparian buffers). After 10 years of simulated plantation growth, RSPO and “enhanced” RSPO scenarios retained, respectively, ∼33 and 60% of reefs in good condition (>75% of current reef) (Tulloch et al., 2016). Yamano and Watanabe (2016) suggest that rice paddies can serve as sediment reservoirs, and conversion of paddies to erosive sugarcane in Ishigaki Island, Japan in the 1980s led to a decline in coral cover from 2.8 to 0.8 ha over 10 years. However, converting sugarcane to pasture can achieve a sediment removal efficiency of 86.1%, while practices like mulching and “spring planting” of seedlings after harvest achieve 62.5–75% sediment removal upstream of coral reefs (Sith et al., 2019). Finally, Beher et al. (2016) evaluated the cost-effectiveness of diverse agricultural projects aimed at sediment reduction in two Australian watersheds (Fitzroy and Mackay–Whitsunday). Among 123 managed grazing and 172 sugarcane projects, no single project type emerged as universally cost effective for sediment reduction, but rather, cost-effectiveness varied based on catchment, slope, plot size, and other contextual factors.
With the exception of these studies and analyses of the Great Barrier Reef, most ridge-to-reef research evaluates agriculture as a general category. This is because most studies focus on the watershed or local scale, where there is a limited portfolio of agro-products. Future research may assess the potential impacts of subsistence and intensive agriculture on reef systems, and evaluate the prospective benefits of sustainable agriculture strategies like rotational grazing, terraced cropland, low-till, cover crops, and fertilizer reduction (Thorburn and Wilkinson, 2013) across multiple regions.
Urban Land Use
Urban land use includes industrial hubs, residences, major infrastructure such as ports, and other uses designed to support concentrated human settlement. While these activities are heterogeneous, they often spatially covary and are evaluated together under proxies like Total Impervious Area or the Landscape Development Intensity Index (Brabec et al., 2002; Brown and Vivas, 2005; Dadhich et al., 2017). Urban land use affects corals through a number of pathways, including (1) habitat loss from nearshore earthmoving, (2) industrial pollution from factories and power plants, (3) untreated sewage from both sewer outfalls and underground storage tanks, (4) stormwater runoff from impervious pavement, (5) marine debris and artificial substrates, and (6) impacts of artificial light on reef organisms like zooplankton, polychaete worms, and reef fish (Aubrecht et al., 2008; DeGeorges et al., 2010; Suchley and Alvarez-Filip, 2018). Urbanization also affects reefs indirectly, i.e., by aggregating human populations close to reefs, thereby concentrating ocean uses like fishing and recreation in road-accessible locales.
Numerous studies attribute a reduction in coral diversity, cover, colony size, rugosity, and density to various types of urban land use and land cover. In Hawai‘i, Delevaux et al. (2018b) used land-cover analysis and groundwater modeling to identify golf courses and residential areas as human nutrient sources affecting two reefs. By combining groundwater monitoring data with local maps of wastewater systems and fertilizer estimates, these authors (1) identified golf courses, injection wells, and residential cesspools as major nutrient sources in two aquifers, and (2) linked land-based nutrients to lower fish biomass and higher turf algae cover. Similarly, numerous spatial analyses of urban reefs have shown that urban sewage, industrial waste, seaside development, household litter, and/or heavy metals reduce indicators of coral health along an inshore-offshore gradient. These studies document nearshore decreases in coral cover (Edinger et al., 2000; Cleary et al., 2006; Smith et al., 2008; Abaya et al., 2018), diversity (Cleary et al., 2016; Ennis et al., 2016), number of coral recruits, colony diameter (Dutra et al., 2006), and structural complexity (Cleary et al., 2016), and increases in coral bleaching (Nemeth and Nowlis, 2001) and coral disease/growth anomalies (Becker et al., 2013; Redding et al., 2013; Yoshioka et al., 2016). Exceptions exist where water circulation clears pollutants from reefs close to shore (Leão and Kikuchi, 2005).
Findings may diverge based on metrics of coral health and spatial extent of analysis: in Jakarta, Cleary et al. (2006) found that coral cover increased but taxon richness did not vary along an inshore-offshore gradient, while Rees et al. (1999) found increases in both diversity and cover at sites further from Jakarta, though the latter study used a smaller spatial extent of 72 km offshore. In contrast, Baum et al. (2015) found that, while Jakarta Bay showed heavy urban impact, some offshore sites in close proximity varied widely in coral health and water quality as a result of local drivers, such as nearby pollution. In a study of 13 sites across 5 regions in Indonesia, coral extension rate did not differ between onshore/offshore sites near nutrient sources like urban sewage and agriculture, but onshore sites showed lower coral cover and diversity as well as higher cover by fleshy algae and bioeroders (Edinger et al., 2000). These findings illustrate the importance of reconciling spatial extent and coral health indicators when comparing multiple studies of urban impact in the same region.
On the island of St. Croix, USVI, two metrics of urban cover showed different relationships to coral health. Impervious surface was correlated with a decline in three-dimensional (3D) stony coral cover (a measure of topographic complexity), while the Landscape Development Index, which accounts for both urban and agricultural classes of land cover, was negatively related to 3D cover, colony density, taxa richness, and colony size (Oliver et al., 2011). These findings perhaps indicate the specific impacts of urban runoff on corals with high complexity, and the additive effects of multiple land-cover types in determining overall coral health. Finally, case studies in Hawai‘i and Mexico describe how large built or “gray” infrastructure—for example, navigation channels, levees, breakwaters, marinas, and ports—deform reef habitats either directly, or by dredging estuarine and dune ecosystems that maintain the reef-sediment equilibrium, a dynamic process that can be difficult to reverse (Wolanski et al., 2009; Martinez et al., 2017). However, in certain locations, infrastructural retrofits like sewage diversion allow corals to regrow (Birkeland et al., 2013; Stimson, 2018). Kane‘ohe Bay, O‘ahu is the site of a phase-shift reversal: sewage diversion in 1978 decreased sewage discharge on the reef from 19,400 to 1,100 m3 day–1 (Smith et al., 1981), driving long-term nitrate reduction and doubling coral cover from 12% in 1971 to 26% in 1983 (Hunter and Evans, 1995; Bahr et al., 2015).
Urban land use is also associated with spatial habitat restriction. For example, in turbid reefs near Singapore, coral abundance and post-bleaching recovery is highest in a narrow depth band (3–4 m) due to the dual mechanisms of (1) light limitations below 6 m (Guest et al., 2016) and (2) macroalgal competition in upper reef flats (0–2 m) (Low, 2015; Heery et al., 2018). Shallow corals (<5 m) may be particularly affected by urban land use because urban areas are associated with boat traffic, contact by snorkelers, and anchor damage that impact shallow reefs more heavily (Roberts et al., 2017). Intuitively, depth restriction depends on dominant urban activities: in Indonesia, mechanical damage caused greater reduction in coral diversity in shallow (3 m) water, but industrial and sewage effluent reduced diversity at all depths (Edinger et al., 1998). Conversely, urban land use may generate novel habitats through artificial substrates like large debris, which are common near major ports (Burt et al., 2009a, b; Ng et al., 2015) but may also serve as rafts for exotic and invasive species (Hoeksema et al., 2012; Santos and Reimer, 2018).
Other Land-Use Types
Additional land uses commonly found in the ridge-to-reef literature include mines, quarries, and roads. These activities are often aberrations on the landscape: they are surrounded by rural or natural areas but exert unique impacts on the coast. Roads have been particularly well studied in the Caribbean and Hawai‘i (MacDonald et al., 1997, 2001; Ramos-Scharrón and MacDonald, 2005, 2007; Ramos-Scharrón and LaFevor, 2016; Oleson et al., 2017). On the island of Saint Lucia, coral core analysis revealed that both terrigenous sediment and calcareous sediment (indicating coral death) increased 2- to 3-fold over the past ∼50 years, and that unpaved roads were responsible for 83–95% of terrigenous sediment export in 2010, while agricultural (banana) expansion had negligible effects (Bégin et al., 2014). Likewise, in the USVI, MacDonald et al. (1997) showed that sediment export from watersheds across the island have increased by 400% compared to historic levels due largely to unpaved roads, which outweigh other anthropogenic factors like plantation agriculture. Small road areas are responsible for the majority of pollution: in Saint Lucia, <20% of the road network contributed about 50% of estimated coastal sediment yields (Bégin et al., 2014). Findings are similar for mines and quarries: a quarry in the Faga’alu watershed of American Samoa covered only 1.1% of the watershed, but contributed 36% of event-based watershed sediment—49 times more sediment than an upstream, forested catchment (Messina and Biggs, 2016). In Papua New Guinea, mines discharging heavy metals caused a decline in coral density, extension, calcification, and tissue layer thickness (Barnes and Lough, 1999; Fallon et al., 2002), and some metals like Zn and Pb remain in reefs many years after mining ceases (Fallon et al., 2002). Nickel mining in New Caledonia discharges heavy metals (Ni, Cr, Zn, and Co), and is linked to low coral abundance close to shore (Adjeroud et al., 2019). In sum, granular landscape features like point sources can occupy small areas but have outsized impacts on reefs and should thus be carefully identified during landscape analysis.
Species-Specific Effects
While many ridge-to-reef studies estimate coral health using general metrics like coral species richness or percent coral cover, some research highlights species-specific effects. Coral species differ in sediment tolerance, light requirements, and salinity preferences and therefore react variably to land-based inputs like freshwater and sediments (Erftemeijer et al., 2012). Coral traits that affect sediment rejection include coral colony shape (convexity) and orientation, calyx size, polyp extensibility, and number of septa (Todd, 2008) as well as symbiont preference (Innis et al., 2018).
Coral species assemblages shift under increasing sediment exposure, e.g., from urban development over time. Reef surveys within 20 km of eleven Asian cities revealed that many urban-adjacent reefs harbor higher relative abundances of massive, submassive, and encrusting species of merulinids, Porites, and Montipora, and fewer branching corals like Acropora, leading to lower habitat complexities that support fewer fish and invertebrate taxa (Heery et al., 2018). Similarly, in the USVI, coral communities near developed watersheds were dominated by the “weedy” species Porites astreoides and Siderastrea siderea, which are small and dispersive but provide less habitat complexity than branching or massive corals (Shaish et al., 2010; Oliver et al., 2018). Coral surveys in Hong Kong between 1980 and 1986 revealed that small-polyped species (e.g., Porites lobata) decreased by 26% as the human urban population increased by 0.5 million, perhaps because larger polyps are more effective at removing sediment than small polyps (Scott, 1990). Similarly, Todd et al. (2001) used a photographic technique to measure polyp morphology in Favia speciosa off the coast of Singapore, finding distinctly large polyps at a site with heaviest sedimentation. Additional studies in Japan (West and van Woesik, 2001), Panama (Aronson et al., 2014), Indonesia (Edinger and Risk, 2000; Cleary et al., 2006; Baum et al., 2015), Thailand (Reopanichkul et al., 2009), Malaysia (Brown et al., 2018), Hong Kong (Fabricius and McCorry, 2006), Jamaica (Mallela et al., 2004), Zanzibar (Herrán et al., 2017), Australia (Fabricius et al., 2005; De’ath and Fabricius, 2010; Moustaka et al., 2019), and the Solomon Islands (Brown and Hamilton, 2018) document shifts in coral species at increasing distances from urban and agricultural/logging lands.
Effects of land use on coral species assemblages may vary between recruitment, juvenile, and adult stages. For example, van Woesik et al. (1999) found that assemblages of coral recruits were significantly different than adult species, suggesting that post-settlement mortality near land was the primary structuring force for adult communities. A study in Palau refined this perspective: Acropora transplants (at least one year old) survived equally well near and far from land, yet naturally occurring adult Acropora were rare near land, suggesting that the first year of life may be the most vulnerable for Acropora (Golbuu et al., 2011). Juvenile assemblages differed but adults did not after high flooding in the Great Barrier Reef (Thompson et al., 2014), while reefs in Singapore demonstrate an opposite pattern (Dikou and van Woesik, 2006). Additional research may reconcile these findings and assist restoration efforts by suggesting species and life-history stages when outplants are most likely to survive nearshore (Ng et al., 2016).
Certain coral species can shift their trophic mode in low water quality (Grottoli et al., 2006), feeding upon nutrient-rich organic matter (Tomascik and Sander, 1985; Anthony, 2000; Edinger et al., 2000). Turbid waters favor heterotrophic and mixotrophic species, which maintain high diversity and cover when exposed to land-based pollution (De’ath and Fabricius, 2010; Morgan et al., 2016). For example, coral autotrophy/heterotrophy may support unusually high coral cover and diversity in nearshore, turbid waters of the Great Barrier Reef (coral cover of 38 ± 24% in Paluma Shoals compared to 12% average for the Reef) (Morgan et al., 2016). Several other studies attribute high coral cover, diversity, and growth rates nearshore to heterotrophic feeding (Isdale, 1983; Lough and Barnes, 1992; Edinger et al., 2000; Lirman and Fong, 2007; Loiola et al., 2019). Importantly, this mechanism may help corals survive bleaching events by increasing zooxanthellae density and chlorophyll concentration through heterotrophy, while benefiting from UV attenuation by sediment (Guest et al., 2016; Morgan et al., 2017).
Overall, coral species that are “generalists” and exhibit massive and encrusting growth forms, low-light tolerance, heterotrophy, and/or fast growth appear to dominate urban or agricultural habitats. Species that appear to survive well under land-use pressure include soft corals as well as Porites, Merulina, Pectinia, Montipora, Turbinaria, and Echinopora (Guest et al., 2016; Ng et al., 2016; Heery et al., 2018). However, species assemblages differ by region and depend on site-specific interactions between hydrodynamics, bathymetry, and selective pressures like sediment stress and bleaching (Darling et al., 2013; Wiedenmann et al., 2013; MacNeil et al., 2019). Taxa like Acropora that may suffer under pollution stress in regions like Palau and Singapore can thrive in turbid areas of the Great Barrier Reef (Browne et al., 2010; Morgan et al., 2017). Most research addresses species composition at surface water outflows, in which nutrients, sediment, and salinity levels covary. Research that disentangles water quality stressors (e.g., in locations that experience high groundwater flows with low concentrations of nutrients and sediment) may reveal more specific mechanisms for coral tolerance.
Spatial Bibliography
Land-reef impacts depend not only on land-use and land-cover change, but on geophysical factors like slope and elevation, lithology, climate, and ocean resuspension, the influences of which vary by region (Gallo et al., 2015). It is therefore necessary to acknowledge the geographic distribution of existing ridge-to-reef studies in order to identify the specific ecosystems and processes they represent. Figure 1 displays the regions covered by current ridge-to-reef literature. This map highlights research explicitly linking land use and coral health, and therefore excludes research that focuses solely on land-based processes (e.g., sediment budgets in high island states), or on coral response to ambient or unsourced contaminants.
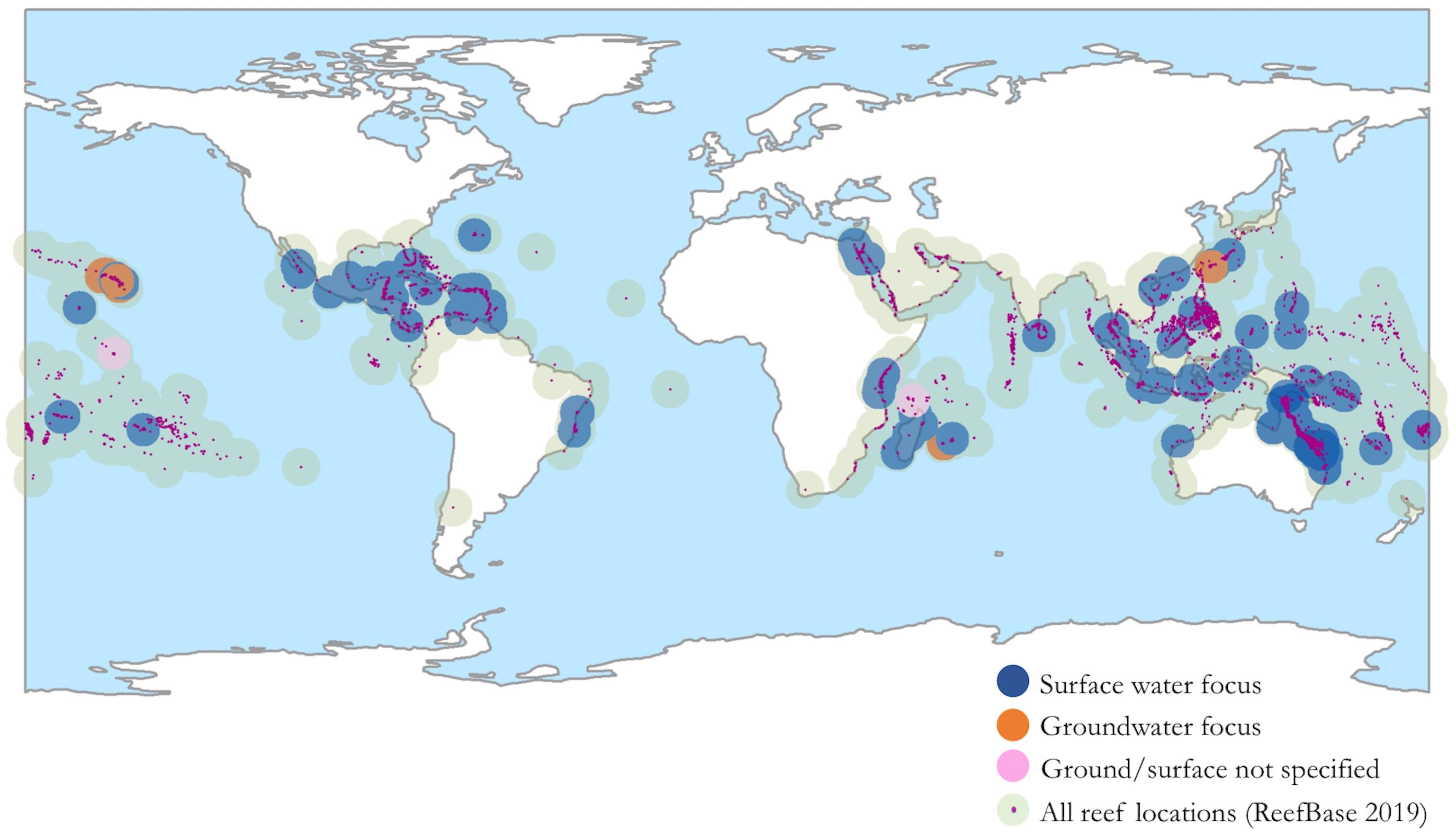
Figure 1. A map of current ridge-to-reef studies delineated by primary land-reef vector: surface flow or groundwater discharge. References for mapped studies can be found in Supplementary Material.
Several focus regions in the current literature include Australia and certain Caribbean islands (Puerto Rico, St. Croix, Saint Lucia), with increasing research emerging in Hawai‘i and Fiji (e.g., Brown et al., 2017; Delevaux et al., 2018a,b,c). Areas that seldom appear in ridge-to-reef literature include East Africa (with some exceptions in Kenya and Madagascar, e.g., McClanahan and Obura, 1997; Maina et al., 2012), several countries in Asia, and many small islands across the Indo-Pacific, perhaps due to low technical resources (Rude et al., 2016; Brown et al., 2017). As noted, land-sea connectivity is particularly strong on high volcanic islands, and therefore expanding research in island communities is particularly important. Numerous island communities in Hawai‘i, Fiji, Palau, and the Solomon Islands have exercised customary “ridge-to-reef” tenure systems for centuries (Clarke and Jupiter, 2010; Winter et al., 2018), and local experts in these systems offer valuable contributions and perspectives to island research.
The majority of studies address surface water (overland runoff and sedimentation) as the primary land-reef vector and comparatively few researchers have explored groundwater discharge, despite its importance to reefs worldwide (Paytan et al., 2006; Sawyer et al., 2016; Prouty et al., 2017; Haßler et al., 2019; Shuler et al., 2019). Existing study regions are primarily are watersheds, evaluated at either catchment or watershed scales. Additional research is needed to examine land-use impacts via “aquifer sheds,” or recharge areas, and the relative impact of land use, climate, and oceanographic variables at multiple spatial and temporal scales.
Future research may also consider the influence of land-cover pattern and orientation on coral ecosystems. Studies in landscape ecology demonstrate that land-cover fragmentation and hydrologic connectivity, and not simply percent cover, mediates water quality. For example, in South Korea, urban and forested lands with higher patch density and edge density resulted in higher reservoir Biochemical Oxygen Demand, Chemical Oxygen Demand, total nitrogen, and total phosphorus (Lee et al., 2009). Gardner et al. (2011) demonstrated that spatially diffuse point sources (e.g., septic tanks) had a smaller influence on in-stream nitrate compared to hydrologically connected, concentrated sources. Martínez-Rendis et al. (2015) linked a 250% urban expansion and forest fragmentation in coastal Mexico to a 50% decline in coral area, as well as a decrease in coral connectivity (patch density, edge density, and other metrics). Land cover cannot be evaluated in isolation, but rather as a matrix of interlocking features whose pattern, scale, and spatial structure dictate energetic and particle flows across ecosystems. Further research may consider, for example, how the urban form (e.g., concentrated versus diffuse impervious surface) affects coral health, or include spatial fragmentation and connectivity as future coral health metrics.
Furthermore, the scale of analysis may moderate the relationship between land cover and reef health. Reefs may respond differently to land-based inputs at colony, reef, and regional scales (Prouty et al., 2008; Reopanichkul et al., 2009). In the Great Barrier Reef, Fabricius et al. (2005) examined the effect of water quality on coral richness at the region and site (within-region) scales: taxonomic richness differed significantly between regions (twice as high at a higher water quality region), but within regions, richness was not significantly related to water quality. In Okinawa, Japan, a study of reefs at several distances from the mouth of the Hija River showed distinct “spikes” in coral community structure up to 400 meters offshore, and therefore an iterative, moving window analysis was required to identify the appropriate scale for coral surveys (West and van Woesik, 2001). Coral core analysis in Honduras and Belize showed that, among 18 trace metals linked to land-based activities, five metals varied at reef and colony scales while five varied by region, highlighting the influence of both land-use pressure and individual growth and metabolism (Prouty et al., 2008). In the Fitzroy River Basin, cropland predicted pollutant concentrations at basin and sub-catchment scales, but grazelands—the dominant land use in the basin—contributed to the majority of long-term annual pollutants (Packett et al., 2009). Research that identifies the influence of scale on land-sea processes can help conservationists in scoping new policies and interventions.
Conclusion and Future Directions
Over 60% of reefs worldwide are threatened by local activities like destructive land use practices and coastal population growth (Burke et al., 2011), underscoring opportunities to protect reefs through direct, local action. Traditional conservation measures typically address marine and terrestrial ecosystems in isolation, irrespective of the fundamental linkages between land and sea. However, anthropogenic land uses like intensive agriculture and urban development can drive significant declines in coral cover, species richness, colony size, and structural complexity while altering fundamental ecological functions like disturbance response and depth-related niche partitioning.
Land use and land cover can affect reefs through a number of processes, including (1) regulating coastal freshwater through surface flows and submarine groundwater discharge, (2) providing sources or sinks for contaminants, and (3) altering biological exchange between land and sea. Forests are positively related to metrics of coral health, as plants stabilize soils or create natural “dams” against erosion and can absorb or sequester groundwater contaminants while providing habitat for anadromous species. Agriculture leads to marine nutrient discharge through groundwater, particularly from high-fertilizer crops like sugarcane, and grazeland is the dominant source of sediment plumes on the Great Barrier Reef, though only the fine sediment fraction (<16 μm) affects wide reef areas. Agriculture impacts vary across time and space, as erosion can arise from small portions of the landscape and occurs primarily during sporadic storms, which also resuspend legacy sediment. Urban land cover is heterogeneous but is linked to declines in coral health along inshore-offshore and depth gradients, though certain species (e.g., large-polyp and generalist species) show resilience to urban stress. Finally, unpaved roads and point sources like mines and quarries can rank among the most significant contributors to reef sediment and heavy metals, despite their small signature on the landscape.
A review of existing literature highlights several opportunities for future research. Current studies focus on a handful of study regions, such as the Great Barrier Reef, Fiji, and certain Caribbean and Asian islands, and primarily assess surface water contamination. Very few studies address groundwater discharge or compare multiple regions, though land-reef interactions vary by ecoregion (Maina et al., 2013) and groundwater is a critical vector of nitrogen pollution in coral reefs around the world (Paytan et al., 2006; Knee et al., 2010a, b; Amato et al., 2016). In addition, while most ridge-to-reef research is conducted at a watershed scale, land-use and land-cover impacts may be scale dependent (Tu, 2011; Huang et al., 2015; Shi et al., 2017). Further research is needed to (1) assess groundwater-driven relationships between land cover and coral health, and (2) compare multiple regions and scales to better define opportunities and limitations for land-based interventions. This work may be assisted by advances in remote sensing, which enables researchers to track land-cover change and sediment plumes in high resolution (Chérubin et al., 2008; Jupiter and Marion, 2008; Álvarez-Romero et al., 2013; Restrepo et al., 2016), measure erosion rates on hillsides (Stock et al., 2011), identify granular but problematic features of the landscape (Zreda et al., 2012; Bartley et al., 2014), and disentangle spatial relationships between multiple stressors (Wedding et al., 2018).
Significant opportunities exist to explore ecological interactions between land and reefs. For example, plant species vary in their effectiveness at preventing erosion and nutrient leaching (Dosskey et al., 2010), and future research is needed to examine how various hydraulic and structural traits in vegetation modulate coral condition. In addition, the sporadic nature of land-sea pollution events in regions like the Great Barrier Reef invites comparison of coral response to acute versus chronic land-based disturbance (following, for example, Castorani et al., 2018). Terrigenous pollutants have specific impacts on the early life histories of corals, and additional research is needed to examine how land conservation may bolster “enabling conditions” for reef recovery after bleaching. Finally, corals display unique community responses to urban activities, and further research may address the impacts of artificial substrates and depth restriction on coral niche space and inter-species interactions.
A broad examination of ridge-to-reef research offers several insights into the state of existing literature. Many studies survey reefs along one or several “pour points” of pollution discharges from a location of interest, therefore aggregating the effects of a heterogeneous patchwork of land uses. However, strong advances have been made in our ability to attribute in-water pollutants to highly specific sources including septic tanks and land parcels, model the fate and transport of contaminants within reef habitats, and anticipate possible taxon-specific tolerances for pollution loading. Integrating these approaches—coral health assessments, sediment/pollution budgets, and source identification—would help target incentives and policy measures (e.g., city planning ordinances or Clean Water Act Section 319 funding for agricultural Best Management Practices) toward areas of highest conservation value.
Ridge-to-reef research is frequently led by marine researchers, perhaps because corals are considered the “downstream” endpoints of land-use byproducts. However, land-sea connectivity is bidirectional, with reefs providing storm protection and myriad social-economic benefits to coastal communities. Customary management units like the Hawaiian moku system, designed to protect highly coupled ecosystems, can offer lessons for crafting sustainable rules of use. Research into diverse stakeholder perspectives on land-sea conservation (e.g., Ureta et al., 2016; Smith et al., 2017) can be utilized to build mutually beneficial partnerships across ecosystems. Integrated research frameworks such as that proposed by Yamano et al. (2015) can build dialogue between experts in terrestrial, marine, and agricultural fields. Many studies treat land uses as static categories (population centers, impervious surface); however, the urban form varies by location and over time, as do forest ecosystems and grazing/cropping practices. Agriculture, urbanization, and other human uses are not inherently destructive, as demonstrated by several studies on sustainable farming and harvesting (Koshiba et al., 2013; Yamano and Watanabe, 2016; Winter et al., 2018). Future research may look to disciplines such as sustainable development, agribusiness, urban planning, and the social sciences for tools to mitigate land-use impacts on environmental outcomes—e.g., Low Impact Development and ecosystems services models.
The type and scale of land use directly impacts the health of coral reefs, and thus integrated ridge-to-reef planning is a crucial element of coral conservation worldwide. Management strategies that account for spatially explicit ecosystem connectivity, variable pollution tolerances of coral species, paired conservation benefits to terrestrial and marine ecosystems, interactions between land use and climate change, and interdisciplinary tools from sustainable development will promote higher outcomes for coral health.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2019.00562/full#supplementary-material
References
Abaya, L. M., Wiegner, T. N., Beets, J. P., Colbert, S. L., Carlson, K. M., and Kramer, L. K. (2018). Spatial distribution of sewage pollution on a Hawaiian coral reef. Mar. Pollut. Bull. 130, 335–347. doi: 10.1016/j.marpolbul.2018.03.028
Ackerman, D., and Weisberg, S. B. (2003). Relationship between rainfall and beach bacterial concentrations on Santa Monica Bay beaches. J. Water Health 1, 85–89. doi: 10.2166/wh.2003.0010
Adjeroud, M., Poisson, E., Peignon, C., Penin, L., and Kayal, M. (2019). Spatial patterns and short-term changes of coral assemblages along a cross-shelf gradient in the Southwestern Lagoon of New Caledonia. Diversity 11:21. doi: 10.3390/d11020021
Albert, S., Grinham, A., Gibbes, B., Tibbetts, I., and Udy, J. (2014). Indicators of coral reef ecosystem recovery following reduction in logging and implementation of community-based management schemes in the Solomon Islands. Pac. Conserv. Biol. 20, 75–85.
Allan, J. D. (2004). Influence of land use and landscape setting on the ecological status of rivers. Annu. Rev. Ecol. Evol. Syst. 35, 257–284. doi: 10.1146/annurev.ecolsys.35.120202.110122
Allison, G. W., Lubchenco, J., and Carr, M. H. (1998). Marine reserves are necessary but not sufficient for marine conservation. Ecol. Appl. 8, S79–S92. doi: 10.2307/2641365
Álvarez-Romero, J. G., Devlin, M., Teixeira da Silva, E., Petus, C., Ban, N. C., Pressey, R. L., et al. (2013). A novel approach to model exposure of coastal-marine ecosystems to riverine flood plumes based on remote sensing techniques. J. Environ. Manag. 119, 194–207. doi: 10.1016/j.jenvman.2013.01.036
Álvarez-Romero, J. G., Pressey, R. L., Ban, N. C., and Brodie, J. (2015). Advancing land-sea conservation planning: integrating modelling of catchments, land-use change, and river plumes to prioritise catchment management and protection. PLoS One 10:e0145574. doi: 10.1371/journal.pone.0145574
Álvarez-Romero, J. G., Pressey, R. L., Ban, N. C., Vance-Borland, K., Willer, C., Klein, C. J., et al. (2011). Integrated land-sea conservation planning: the missing links. Annu. Rev. Ecol. Evol. Syst. 42, 381–409. doi: 10.1146/annurev-ecolsys-102209-144702
Amato, D. W., Bishop, J. M., Glenn, C. R., Dulai, H., and Smith, C. M. (2016). Impact of submarine groundwater discharge on marine water quality and reef biota of Maui. PLoS One 11:e0165825. doi: 10.1371/journal.pone.0165825
Anthony, K. R. N. (2000). Enhanced particle-feeding capacity of corals on turbid reefs (Great Barrier Reef, Australia). Coral Reefs 19, 59–67. doi: 10.1007/s003380050227
Aronson, R. B., Hilbun, N. L., Bianchi, T. S., Filley, T. R., and McKee, B. A. (2014). Land use, water quality, and the history of coral assemblages at Bocas del Toro, Panamá. Mar. Ecol. Prog. Ser. 504, 159–170. doi: 10.3354/meps10765
Arthington, A. H., Dulvy, N. K., Gladstone, W., and Winfield, I. A. N. J. (2016). Fish conservation in freshwater and marine realms: status, threats and management. Aquat. Conserv. Mar. Freshw. Ecosyst. 26, 838–857. doi: 10.1002/aqc.2712
Ash, A. J., Corfield, J. P., McIvor, J. G., and Ksiksi, T. S. (2011). Grazing management in tropical savannas: utilization and rest strategies to manipulate rangeland condition. Rangeland Ecol. Manag. 64, 223–239. doi: 10.2111/rem-d-09-00111.1
Aubrecht, C., Elvidge, C. D., Longcore, T., Rich, C., Safran, J., Strong, A. E., et al. (2008). A global inventory of coral reef stressors based on satellite observed nighttime lights. Geocarto Int. 23, 467–479. doi: 10.1080/10106040802185940
Bahr, K. D., Jokiel, P. L., and Toonen, R. J. (2015). The unnatural history of Kāne‘ohe Bay: coral reef resilience in the face of centuries of anthropogenic impacts. PeerJ 3:e950. doi: 10.7717/peerj.950
Bainbridge, Z., Lewis, S., Bartley, R., Fabricius, K., Collier, C., Waterhouse, J., et al. (2018). Fine sediment and particulate organic matter: a review and case study on ridge-to-reef transport, transformations, fates, and impacts on marine ecosystems. Mar. Pollut. Bull. 135, 1205–1220. doi: 10.1016/j.marpolbul.2018.08.002
Bainbridge, Z. T., Brodie, J. E., Faithful, J. W., Sydes, D. A., and Lewis, S. E. (2009). Identifying the land-based sources of suspended sediments, nutrients and pesticides discharged to the Great Barrier Reef from the Tully - Murray Basin, Queensland, Australia. Mar. Freshw. Res. 60, 1081–1090. doi: 10.1071/MF08333
Barnes, D. J., and Lough, J. M. (1999). Porites growth characteristics in a changed environment: Misima Island, Papua New Guinea. Coral Reefs 18, 213–218. doi: 10.1007/s003380050185
Bartley, R., Bainbridge, Z. T., Lewis, S. E., Kroon, F. J., Wilkinson, S. N., Brodie, J. E., et al. (2014). Relating sediment impacts on coral reefs to watershed sources, processes and management: a review. Sci. Total Environ. 468–469, 1138–1153. doi: 10.1016/j.scitotenv.2013.09.030
Baum, G., Januar, H. I., Ferse, S. C. A., and Kunzmann, A. (2015). Local and regional impacts of pollution on coral reefs along the thousand islands north of the megacity Jakarta, Indonesia. PLoS One 10:e0138271. doi: 10.1371/journal.pone.0138271
Becker, G. C., Dalziel, B. D., Kersch-Becker, M. F., Park, M. G., and Mouchka, M. (2013). Indirect effects of human development along the coast on coral health. Biotropica 45, 401–407. doi: 10.1111/btp.12019
Beger, M., Grantham, H. S., Pressey, R. L., Wilson, K. A., Peterson, E. L., Dorfman, D., et al. (2010). Conservation planning for connectivity across marine, freshwater, and terrestrial realms. Biol. Conserv. 143, 565–575. doi: 10.1016/j.biocon.2009.11.006
Bégin, C., Brooks, G., Larson, R. A., Dragicevic, S., Ramos-Scharrón, C. E., and Côté, I. M. (2014). Increased sediment loads over coral reefs in Saint Lucia in relation to land use change in contributing watersheds. Ocean Coast. Manag. 95, 35–45. doi: 10.1016/j.ocecoaman.2014.03.018
Bégin, C., Schelten, C. K., Nugues, M. M., Hawkins, J., Roberts, C., and Côté, I. M. (2016). Effects of protection and sediment stress on coral reefs in Saint Lucia. PLoS One 11:e0146855. doi: 10.1371/journal.pone.0146855
Beher, J., Possingham, H. P., Hoobin, S., Dougall, C., and Klein, C. (2016). Prioritising catchment management projects to improve marine water quality. Environ. Sci. Policy 59, 35–43. doi: 10.1016/j.envsci.2016.02.005
Birkeland, C. E., Green, A., Fenner, D., Squair, C., and Dahl, A. L. (2013). Substratum stability and coral reef resilience: insights from 90 years of disturbances to reefs in American Samoa. Micronesica 6, 1–16.
Bishop, J. M., Glenn, C. R., Amato, D. W., and Dulai, H. (2017). Effect of land use and groundwater flow path on submarine groundwater discharge nutrient flux. J. Hydrol. Reg. Stud. 11, 194–218. doi: 10.1016/j.ejrh.2015.10.008
Böhlke, J.-K. (2002). Groundwater recharge and agricultural contamination. Hydrogeol. J. 10, 153–179. doi: 10.1007/s10040-001-0183-3
Bonkosky, M., Hernández-Delgado, E. A., Sandoz, B., Robledo, I. E., Norat-Ramírez, J., and Mattei, H. (2009). Detection of spatial fluctuations of non-point source fecal pollution in coral reef surrounding waters in southwestern Puerto Rico using PCR-based assays. Mar. Pollut. Bull. 58, 45–54. doi: 10.1016/j.marpolbul.2008.09.008
Bothner, M. H., Reynolds, R. L., Casso, M. A., Storlazzi, C. D., and Field, M. E. (2006). Quantity, composition, and source of sediment collected in sediment traps along the fringing coral reef off Molokai, Hawaii. Mar. Pollut. Bull. 52, 1034–1047. doi: 10.1016/j.marpolbul.2006.01.008
Brabec, E., Schulte, S., and Richards, P. L. (2002). Impervious surfaces and water quality: a review of current literature and its implications. J. Plann. 16, 499–514. doi: 10.1177/088541202400903563
Breckwoldt, A., Dsikowitzky, L., Baum, G., Ferse, S. C. A., van der Wulp, S., Kusumanti, I., et al. (2016). A review of stressors, uses and management perspectives for the larger Jakarta Bay Area, Indonesia. Mar. Pollut. Bull. 110, 790–794. doi: 10.1016/j.marpolbul.2016.08.040
Brodie, J., Schroeder, T., Rohde, K., Faithful, J., Masters, B., Dekker, A., et al. (2010). Dispersal of suspended sediments and nutrients in the Great Barrier Reef lagoon during river-discharge events: conclusions from satellite remote sensing and concurrent flood-plume sampling. Mar. Freshw. Res. 61, 651–664. doi: 10.1071/MF08030
Brodie, J. E., Devlin, M., Haynes, D., and Waterhouse, J. (2011). Assessment of the eutrophication status of the Great Barrier Reef lagoon (Australia). Biogeochemistry 106, 281–302. doi: 10.1007/s10533-010-9542-2
Brodie, J. E., Kroon, F. J., Schaffelke, B., Wolanski, E. C., Lewis, S. E., Devlin, M. J., et al. (2012). Terrestrial pollutant runoff to the Great Barrier Reef: an update of issues, priorities and management responses. Mar. Pollut. Bull. 65, 81–100. doi: 10.1016/j.marpolbul.2011.12.012
Brodie, J., Wolanski, E., Lewis, S., and Bainbridge, Z. (2012). An assessment of residence times of land-sourced contaminants in the Great Barrier Reef lagoon and the implications for management and reef recovery. Mar. Pollut. Bull. 65, 267–279. doi: 10.1016/j.marpolbul.2011.12.011
Brooks, M., D’Antonio, C., Richardson, D., Grace, J., Keeley, J., DiTomaso, J., et al. (2004). Effects of invasive alien plants on fire regimes. BioScience 54, 677–688.
Brown, C., Browne, N., McIlwain, J. L., and Zinke, J. (2018). Inshore, turbid coral reefs from northwest Borneo exhibiting low diversity, but high cover show evidence of resilience to various environmental stressors. PeerJ Preprints 6:e27422v1.
Brown, C. J., and Hamilton, R. J. (2018). Estimating the footprint of pollution on coral reefs with models of species turnover. Conserv. Biol. 32, 949–958. doi: 10.1111/cobi.13079
Brown, C. J., Jupiter, S. D., Albert, S., Klein, C. J., Mangubhai, S., Maina, J. M., et al. (2017). Tracing the influence of land-use change on water quality and coral reefs using a Bayesian model. Nat. Sci. Rep. 7:4740. doi: 10.1038/s41598-017-05031-7
Brown, M. T., and Vivas, B. M. (2005). Landscape development intensity index. Environ. Monitor. Assess. 101, 289–309. doi: 10.1002/cssc.201701648
Browne, N. K. (2012). Spatial and temporal variations in coral growth on an inshore turbid reef subjected to multiple disturbances. Mar. Environ. Res. 77, 71–83. doi: 10.1016/j.marenvres.2012.02.005
Browne, N. K., Smithers, S. G., and Perry, C. T. (2010). Geomorphology and community structure of Middle Reef, central Great Barrier Reef, Australia: an inner-shelf turbid zone reef subject to episodic mortality events. Coral Reefs 29, 683–689. doi: 10.1007/s00338-010-0640-3
Burke, L., Reytar, K., Spalding, M., and Perry, A. (2011). Reefs at Risk Revisited. Washington, DC: World Resources Institute.
Burt, J., Bartholomew, A., Usseglio, P., Bauman, A., and Sale, P. F. (2009b). Are artificial reefs surrogates of natural habitats for corals and fish in Dubai, United Arab Emirates? Coral Reefs 28, 663–675. doi: 10.1007/s00338-009-0500-1
Burt, J., Bartholomew, A., Bauman, A., Saif, A., and Sale, P. F. (2009a). Coral recruitment and early benthic community development on several materials used in the construction of artificial reefs and breakwaters. J. Exp. Mar. Biol. Ecol. 373, 72–78. doi: 10.1016/j.jembe.2009.03.009
Carroll, C., Waters, D., Vardy, S., Silburn, D. M., Attard, S., Thorburn, P. J., et al. (2012). A Paddock to reef monitoring and modelling framework for the Great Barrier Reef: Paddock and catchment component. Mar. Pollut. Bull. 65, 136–149. doi: 10.1016/j.marpolbul.2011.11.022
Carter, R. M., Larcombe, P., Dye, J. E., Gagan, M. K., and Johnson, D. P. (2009). Long-shelf sediment transport and storm-bed formation by Cyclone Winifred, central Great Barrier Reef, Australia. Mar. Geol. 267, 101–113. doi: 10.1016/j.margeo.2009.08.009
Castorani, M. C. N., Reed, D. C., and Miller, R. J. (2018). Loss of foundation species: disturbance frequency outweighs severity in structuring kelp forest communities. Ecology 99, 2442–2454. doi: 10.1002/ecy.2485
Chaplin-Kramer, R., Hamel, P., Sharp, R., Kowal, V., Wolny, S., Sim, S., et al. (2016). Landscape configuration is the primary driver of impacts on water quality associated with agricultural expansion. Environ. Res. Lett. 11, 1–11.
Chérubin, L. M., Kuchinke, C. P., and Paris, C. B. (2008). Ocean circulation and terrestrial runoff dynamics in the Mesoamerican region from spectral optimization of SeaWiFS data and a high resolution simulation. Coral Reefs 27, 503–519. doi: 10.1007/s00338-007-0348-1
Clarivate Analytics, (2019). Web of Science v5.32. Available at: www.webofknowledge.com (accessed November 15, 2019).
Clarke, P., and Jupiter, S. D. (2010). Law, custom and community-based natural resource management in Kubulau District (Fiji). Environ. Conserv. 37, 98–106. doi: 10.1017/S0376892910000354
Cleary, D. F. R., Polónia, A. R., Renema, W., Hoeksema, B. W., Rachello-Dolmen, P. G., Moolenbeek, R. G., et al. (2016). Variation in the composition of corals, fishes, sponges, echinoderms, ascidians, molluscs, foraminifera and macroalgae across a pronounced in-to-offshore environmental gradient in the Jakarta Bay–Thousand Islands coral reef complex. Mar. Pollut. Bull. 110, 701–717. doi: 10.1016/j.marpolbul.2016.04.042
Cleary, D. F. R., Suharsono, S., and Hoeksema, B. W. (2006). Coral diversity across a disturbance gradient in the Pulau Seribu reef complex off Jakarta, Indonesia. Biodivers. Conserv. 15, 3653–3674. doi: 10.1007/s10531-004-4692-y
Correll, D., Jordan, T. E., and Weller, D. E. (1992). Nutrient flux in a landscape: effects of coastal land use and terrestrial community mosaic on nutrient transport to coastal waters. Estuaries 15, 431–442.
Cox, C. A., Sarangi, A., and Madramootoo, C. A. (2006). Effect of land management on runoff and soil losses from two small watersheds in St. Lucia. Land Degrad. Dev. 17, 55–72. doi: 10.1002/ldr.694
Dadhich, A. P., Nadaoka, K., Motomura, Y., and Watanabe, A. (2017). Potential impacts of land use change dynamics and submarine groundwater discharge on fringing reefs of Kuroshima Island, Japan. J. Coast. Conserv. 21, 245–254. doi: 10.1007/s11852-017-0495-7
Darling, E. S., McClanahan, T. R., and Côté, I. M. (2013). Life histories predict coral community disassembly under multiple stressors. Glob. Change Biol. 19, 1930–1940. doi: 10.1111/gcb.12191
Davis, S. E., Lirman, D., and Wozniak, J. R. (2009). “Nitrogen and phosphorus exchange among tropical coastal ecosystems,” in Ecological Connectivity Among Tropical Coastal Ecosystems, ed. I. Nagelkerken, (Dordrecht: Springer), 9–43. doi: 10.1007/978-90-481-2406-0_2
De’ath, G., and Fabricius, K. (2010). Water quality as a regional driver of coral biodiversity and macroalgae on the Great Barrier Reef. Ecol. Appl. 20, 840–850. doi: 10.1890/08-2023.1
DeGeorges, A., Goreau, T. J., and Reilly, B. (2010). Land-sourced pollution with an emphasis on domestic sewage: lessons from the Caribbean and implications for coastal development on indian ocean and pacific coral reefs. Sustainability 2, 2919–2949. doi: 10.3390/su2092919
Delevaux, J. M. S., Whittier, R., Stamoulis, K. A., Bremer, L. L., Jupiter, S., Friedlander, A. M., et al. (2018b). A linked land-sea modeling framework to inform ridge-to-reef management in high oceanic islands. PLoS One 13:e0193230. doi: 10.1371/journal.pone.0193230
Delevaux, J. M. S., Jupiter, S. D., Stamoulis, K. A., Bremer, L. L., Wenger, A. S., Dacks, R., et al. (2018a). Scenario planning with linked land-sea models inform where forest conservation actions will promote coral reef resilience. Nat. Sci. Rep. 8, 1–21. doi: 10.1038/s41598-018-29951-0
Delevaux, J. M. S., Winter, K. B., Jupiter, S. D., Blaich-Vaughan, M., Stamoulis, K. A., Bremer, L. L., et al. (2018c). Linking land and sea through collaborative research to inform contemporary applications of traditional resource management in Hawai‘i. Sustainability 10, 1–19. doi: 10.3390/su10093147
Devlin, M. J., and Brodie, J. (2005). Terrestrial discharge into the Great Barrier Reef Lagoon: nutrient behavior in coastal waters. Mar. Pollut. Bull. 51, 9–22. doi: 10.1016/j.marpolbul.2004.10.037
Dikou, A., and van Woesik, R. (2006). Survival under chronic stress from sediment load: spatial patterns of hard coral communities in the southern islands of Singapore. Mar. Pollut. Bull. 52, 1340–1354. doi: 10.1016/j.marpolbul.2006.02.011
Dinsdale, E. A., Pantos, O., Smriga, S., Edwards, R. A., Angly, F., Wegley, L., et al. (2008). Microbial ecology of four coral atolls in the Northern Line Islands. PLoS One 3:e1584. doi: 10.1371/journal.pone.0001584
Dosskey, M. G., Vidon, P., Gurwick, N. P., Allan, C. J., Duval, T. P., and Lowrance, R. (2010). The Role of Riparian vegetation in protecting and improving chemical water quality in streams. J. Am. Water Resourc. Assoc. 46, 261–277. doi: 10.1007/978-3-319-04414-9_25
Draut, A. E., Bothner, M. H., Field, M. E., Reynolds, R. L., Cochran, S. A., Logan, J. B., et al. (2009). Supply and dispersal of flood sediment from a steep, tropical watershed: Hanalei Bay, Kaua’i, Hawai‘i, USA. Bull. Geol. Soc. Am. 121, 574–585. doi: 10.1130/B26367.1
Drewry, J. J., Higham, W., and Mitchell, C. (2009). “Water quality objectives and targets in the Mackay Whitsunday region to protect water quality to the Great Barrier Reef,” in Proceedings of the 18th World IMCAS/MODSIM Congress, Cairns. 3308–3314.
Duke, N. C., and Wolanski, E. (2000). “Muddy coastal waters and depleted mangrove coastlines - depleted seagrass and coral reefs,” in Oceanographic Processes of Coral Reefs: Physical and Biological Links in the Great Barrier Reef, ed. E. Wolanski, (Washington, DC: CRC Press), doi: 10.1201/9781420041675
Dutra, L. X. C., Kikuchi, R. K. P., and Leão, Z. M. A. N. (2006). Effects of sediment accumulation on reef corals from Abrolhos, Bahia, Brazil. J. Coast. Res. 2, 633–638.
Edinger, E. N., Jompa, J., Limmon, G., Widjatmoko, W., and Risk, M. (1998). Reef degradation and coral biodiversity in Indonesia: effects of land-based pollution, destructive fishing practice and changes over time. Mar. Pollut. Bull. 36, 617–630. doi: 10.1016/s0025-326x(98)00047-2
Edinger, E. N., Limmon, G. V., Jompa, J., Widjatmoko, W., Heikoop, J. M., and Risk, M. J. (2000). Normal coral growth rates on dying reefs: are coral growth rates good indicators of reef health? Mar. Pollut. Bull. 40, 404–425. doi: 10.1016/s0025-326x(99)00237-4
Edinger, E. N., and Risk, M. J. (2000). Effect of land-based pollution on central Java coral reefs. J. Coast. Dev. 3, 593–613.
Ennis, R. S., Brandt, M. E., Wilson Grimes, K. R., and Smith, T. B. (2016). Coral reef health response to chronic and acute changes in water quality in St. Thomas, United States Virgin Islands. Mar. Pollut. Bull. 111, 418–427. doi: 10.1016/j.marpolbul.2016.07.033
Erftemeijer, P. L. A., Riegl, B., Hoeksema, B. W., and Todd, P. A. (2012). Environmental impacts of dredging and other sediment disturbances on corals: a review. Mar. Pollut. Bull. 64, 1737–1765. doi: 10.1016/j.marpolbul.2012.05.008
Ewel, K. C., Twilley, R. R., and Ong, J. E. (1998). Different kinds of Mangrove forests provide different goods and services. Glob. Ecol. Biogeogr. Lett. 7, 83–94. doi: 10.2307/2997700
Fabricius, K., De’ath, G., McCook, L., and Williams, D. M. (2005). Changes in algal, coral and fish assemblages along water quality gradients on the inshore Great Barrier Reef. Mar. Pollut. Bull. 51, 384–398. doi: 10.1016/j.marpolbul.2004.10.041
Fabricius, K. E. (2005). Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar. Pollut. Bull. 50, 125–146. doi: 10.1016/j.marpolbul.2004.11.028
Fabricius, K. E., Logan, M., Weeks, S., and Brodie, J. (2014). The effects of river run-off on water clarity across the central Great Barrier Reef. Mar. Pollut. Bull. 84, 191–200. doi: 10.1016/j.marpolbul.2014.05.012
Fabricius, K. E., and McCorry, D. (2006). Changes in octocoral communities and benthic cover along a water quality gradient in the reefs of Hong Kong. Mar. Pollut. Bull. 52, 22–33. doi: 10.1016/j.marpolbul.2005.08.004
Fallon, S. J., White, J. C., and McCulloch, M. T. (2002). Porites corals as recorders of mining and environmental impacts: Misima Island, Papua New Guinea. Geochim. Cosmochim. Acta 66, 45–62. doi: 10.1016/s0016-7037(01)00715-3
Gallo, E. L., Meixner, T., Aoubid, H., Lohse, K., and Brooks, P. D. (2015). Combined impact of catchment size, land cover, and precipitation on streamflow and total dissolved nitrogen: a global comparative analysis. Glob. Biogeochem. Cycles 29, 1109–1121. doi: 10.1111/1462-2920.13280
Gardner, K. K., Mcglynn, B. L., and Marshall, L. A. (2011). Quantifying watershed sensitivity to spatially variable N loading and the relative importance of watershed N retention mechanisms. Water Resour. Res. 47, 1–21.
Gilby, B. L., Olds, A. D., Connolly, R. M., Stevens, T., Henderson, C. J., Maxwell, P. S., et al. (2016). Optimising land-sea management for inshore coral reefs. PLoS One 11:e0164934. doi: 10.1371/journal.pone.0164934
Goh, S. G., Bayen, S., Burger, D., Kelly, B. C., Han, P., Babovic, V., et al. (2017). Occurrence and distribution of bacteria indicators, chemical tracers and pathogenic vibrios in Singapore coastal waters. Mar. Pollut. Bull. 114, 627–634. doi: 10.1016/j.marpolbul.2016.09.036
Golbuu, Y., van Woesik, R., Richmond, R. H., Harrison, P., and Fabricius, K. E. (2011). River discharge reduces reef coral diversity in Palau. Mar. Pollut. Bull. 62, 824–831. doi: 10.1016/j.marpolbul.2010.12.015
Golbuu, Y., Victor, S., Wolanski, E., and Richmond, R. H. (2003). Trapping of fine sediment in a semi-enclosed bay, Palau, Micronesia. Estuar. Coast. Shelf Sci. 57, 941–949. doi: 10.1016/S0272-7714(02)00424-9
Gonzalez, A., Thompson, P., and Loreau, M. (2017). Spatial ecological networks: planning for sustainability in the long-term. Curr. Opin. Environ. Sustain. 29, 187–197. doi: 10.1016/j.cosust.2018.03.012
Grottoli, A. G., Rodrigues, L. J., and Palardy, J. E. (2006). Heterotrophic plasticity and resilience in bleached corals. Nature 440, 1186–1189. doi: 10.1038/nature04565
Guest, J. R., Tun, K., Low, J., Vergés, A., Marzinelli, E. M., Campbell, A. H., et al. (2016). 27 years of benthic and coral community dynamics on turbid, highly urbanised reefs off Singapore. Nat. Sci. Rep. 6:36260. doi: 10.1038/srep36260
Hall, B. (2018). Influence of Urban Expansion on Groundwater Recharge in Southeast Melbourne, Australia. Melbourne VIC: RMIT University.
Halpern, B. S., Selkoe, K. A., White, C., Albert, S., Aswani, S., and Lauer, M. (2013). Marine protected areas and resilience to sedimentation in the Solomon Islands. Coral Reefs 32, 61–69. doi: 10.1007/s00338-012-0981-1
Hamilton, R. J., Almany, G. R., Brown, C. J., Pita, J., Peterson, N. A., and Howard Choat, J. (2017). Logging degrades nursery habitat for an iconic coral reef fish. Biol. Conserv. 210, 273–280. doi: 10.1016/j.biocon.2017.04.024
Han, D., Currell, M. J., Cao, G., and Hall, B. (2017). Alterations to groundwater recharge due to anthropogenic landscape change. J. Hydrol. 554, 545–557. doi: 10.1016/j.jhydrol.2017.09.018
Haywood, M. D. E., Dennis, D., Thomson, D. P., and Pillans, R. D. (2016). Mine waste disposal leads to lower coral cover, reduced species richness and a predominance of simple coral growth forms on a fringing coral reef in Papua New Guinea. Mar. Environ. Res. 115, 36–48. doi: 10.1016/j.marenvres.2016.02.003
Haßler, K., Dähnke, K., Kölling, M., Sichoix, L., Nickl, A. L., and Moosdorf, N. (2019). Provenance of nutrients in submarine fresh groundwater discharge on Tahiti and Moorea, French Polynesia. Appl. Geochem. 100, 181–189. doi: 10.1016/j.apgeochem.2018.11.020
Heery, E. C., Hoeksema, B. W., Browne, N. K., Reimer, J. D., Ang, P. O., Huang, D., et al. (2018). Urban coral reefs: degradation and resilience of hard coral assemblages in coastal cities of East and Southeast Asia. Mar. Pollut. Bull. 135, 654–681. doi: 10.1016/j.marpolbul.2018.07.041
Hefting, M. M., Clement, J.-C., Bienkowski, P., Dowrick, D., Guenat, C., Butturini, A., et al. (2005). The role of vegetation and litter in the nitrogen dynamics of riparian buffer zones in Europe. Ecol. Eng. 24, 465–482. doi: 10.1016/j.ecoleng.2005.01.003
Herrán, N., Narayan, G. R., Reymond, C. E., and Westphal, H. (2017). Calcium carbonate production, coral cover and diversity along a distance gradient from stone town: a case study from Zanzibar, Tanzania. Front. Mar. Sci. 4:412. doi: 10.3389/fmars.2017.00412
Hoeksema, B. W., Roos, P. J., and Cadée, G. C. (2012). Trans-Atlantic rafting by the brooding reef coral Favia fragum on man-made flotsam. Mar. Ecol. Prog. Ser. 445, 209–218. doi: 10.3354/meps09460
Holt, J. A., Bristow, K. L., and Mcivor, J. G. (1996). The effects of grazing pressure on soil animals and hydraulic properties of two soils in semi-arid tropical Queensland. Aust. J. Soil Res. 1, 69–79.
Huang, H., Li, X. B., Titlyanov, E. A., Ye, C., Titlyanova, T. V., Guo, Y. P., et al. (2013). Linking macroalgal δ15N-values to nitrogen sources and effects of nutrient stress on coral condition in an upwelling region. Bot. Mar. 56, 471–480. doi: 10.1515/bot-2012-0223
Huang, J., Huang, Y., Pontius, R. G., and Zhang, Z. (2015). Geographically weighted regression to measure spatial variations in correlations between water pollution versus land use in a coastal watershed. Ocean Coast. Manag. 103, 14–24. doi: 10.1016/j.ocecoaman.2014.10.007
Hughes, A. O., Olley, J. M., Croke, J. C., and McKergow, L. A. (2009). Sediment source changes over the last 250 years in a dry-tropical catchment, central Queensland, Australia. Geomorphology 104, 262–275. doi: 10.1016/j.geomorph.2008.09.003
Hughes, F., Vitousek, P. M., and Tunison, T. (1991). Alien grass invasion and fire in the seasonal submontane zone of Hawai‘i. Ecology 72, 743–747. doi: 10.2307/2937215
Hunter, C. L., and Evans, C. W. (1995). Coral reefs in Kaneohe Bay, Hawaii: two centuries of western influence and two decades of data. Bull. Mar. Sci. 57, 501–515.
Hunter, H. M., and Walton, R. S. (2008). Land-use effects on fluxes of suspended sediment, nitrogen and phosphorus from a river catchment of the Great Barrier Reef, Australia. J. Hydrol. 356, 131–146. doi: 10.1016/j.jhydrol.2008.04.003
Innis, T., Cunning, R., Wall, C. B., and Gates, R. D. (2018). Coral color and depth drive symbiosis ecology of Montipora capitata in Kāne‘ohe Bay, O’ahu, Hawai‘i. Coral Reefs 37, 423–430. doi: 10.1007/s00338-018-1667-0
Isdale, P. P. (1983). “Geographical patterns in coral growth rates on the Great Barrier Reef,” in Proceedings of the Inaugural Great Barrier Reef Conference, (Townsville: James Cook University Press).
Jenkins, A. P., Jupiter, S. D., QauQau, I., and Atherton, J. (2010). The importance of ecosystem-based management for conserving aquatic migratory pathways on tropical high islands: a case study from Fiji. Aquat. Conserv. Mar. Freshw. Ecosyst. 20, 224–238. doi: 10.1002/aqc
Jones, R. J., Muller, J., Haynes, D., and Schreiber, U. (2003). Effects of herbicides diuron and atrazine on corals of the Great Barrier Reef, Australia. Mar. Ecol. Prog. Ser. 251, 153–167. doi: 10.1371/journal.pone.0075798
Jupiter, S. D., and Marion, G. S. (2008). Changes in forest area along stream networks in an agricultural catchment of the Great Barrier Reef lagoon. Environ. Manag. 42, 66–79. doi: 10.1007/s00267-008-9117-3
Jupiter, S. D., Wenger, A., Klein, C. J., Albert, S., Mangubhai, S., Nelson, J., et al. (2017). Opportunities and constraints for implementing integrated land-sea management on Islands. Environ. Conserv. 44, 254–266. doi: 10.1017/S0376892917000091
Klein, C. J., Jupiter, S. D., Selig, E. R., Watts, M. E., Halpern, B. S., Kamal, M., et al. (2012). Forest conservation delivers highly variable coral reef conservation outcomes. Ecol. Appl. 22, 1246–1256. doi: 10.1890/11-1718.1
Klein, C. J., Jupiter, S. D., Watts, M., and Possingham, H. P. (2014). Evaluating the influence of candidate terrestrial protected areas on coral reef condition in Fiji. Mar. Policy 44, 360–365. doi: 10.1016/j.marpol.2013.10.001
Knee, K. L., Crook, E. D., Hench, J. L., Leichter, J. J., and Paytan, A. (2016). Assessment of submarine groundwater discharge (SGD) as a source of dissolved radium and nutrients to Moorea (French Polynesia) coastal waters. Estuaries and Coasts 39, 1651–1668. doi: 10.1007/s12237-016-0108-y
Knee, K. L., Gossett, R., Boehm, A. B., and Paytan, A. (2010a). Caffeine and agricultural pesticide concentrations in surface water and groundwater on the north shore of Kauai (Hawaii, USA). Mar. Pollut. Bull. 60, 1376–1382. doi: 10.1016/j.marpolbul.2010.04.019
Knee, K. L., Street, J. H., Grossman, E. E., Boehm, A. B., and Paytan, A. (2010b). Nutrient inputs to the coastal ocean from submarine groundwater discharge in a groundwater-dominated system: relation to land use (Kona coast, Hawaii, U.S.A.). Limnol. Oceanogr. 55, 1105–1122. doi: 10.4319/lo.2010.55.3.1105
Koshiba, S., Besebes, M., Soaladaob, K., Isechal, A. L., Victor, S., and Golbuu, Y. (2013). Palau’s taro fields and mangroves protect the coral reefs by trapping eroded fine sediment. Wetlands Ecol. Manag. 21, 157–164. doi: 10.1007/s11273-013-9288-4
Kroon, F. J., Kuhnert, P. M., Henderson, B. L., Wilkinson, S. N., Kinsey-Henderson, A., Abbott, B., et al. (2012). River loads of suspended solids, nitrogen, phosphorus and herbicides delivered to the Great Barrier Reef lagoon. Mar. Pollut. Bull. 65, 167–181. doi: 10.1016/j.marpolbul.2011.10.018
Kroon, F. J., Schaffelke, B., and Bartley, R. (2014). Informing policy to protect coastal coral reefs: insight from a global review of reducing agricultural pollution to coastal ecosystems. Mar. Pollut. Bull. 85, 33–41. doi: 10.1016/j.marpolbul.2014.06.003
Kroon, F. J., Thorburn, P., Schaffelke, B., and Whitten, S. (2016). Towards protecting the Great Barrier Reef from land-based pollution. Glob. Change Biol. 22, 1985–2002. doi: 10.1111/gcb.13262
Lamb, J. B., Wenger, A. S., Devlin, M. J., Ceccarelli, D. M., Williamson, D. H., and Willis, B. L. (2016). Reserves as tools for alleviating impacts of marine disease. Philos. Trans. R. Soc. B Biol. Sci. 371:20150210. doi: 10.1098/rstb.2015.0210
Lapointe, B. E., and Bedford, B. J. (2011). Stormwater nutrient inputs favor growth of non-native macroalgae (Rhodophyta) on O’ahu, Hawaiian Islands. Harmful Algae 10, 310–318. doi: 10.1016/j.hal.2010.11.004
Larcombe, P., and Woolfe, K. J. (1999). Increased sediment supply to the Great Barrier Reef will not increase sediment accumulation at most coral reefs. Coral Reefs 18, 163–169. doi: 10.1016/j.scitotenv.2013.09.030
Leão, Z. M. A. N., and Kikuchi, R. K. P. (2005). A relic coral fauna threatened by global changes and human activities, Eastern Brazil. Mar. Pollut. Bull. 51, 599–611. doi: 10.1016/j.marpolbul.2005.04.024
Lee, S.-W., Hwang, S.-J., Lee, S.-B., Hwang, H.-S., and Sung, H.-C. (2009). Landscape ecological approach to the relationships of land use patterns in watersheds to water quality characteristics. Landsc. Urban Plann. 92, 80–89. doi: 10.1016/j.landurbplan.2009.02.008
Lerner, D. N. (1990). “Groundwater recharge in urban Areas,” in Hydrological Processes and Water Management in Urban Areas, eds H. Massing, J. Packman, and F. C. Zuidema, (Wallingford: International Association of Hydrological Sciences).
Lerner, D. N. (2002). Identifying and quantifying urban recharge: a review. Hydrogeol. J. 10, 143–152. doi: 10.1007/s10040-001-0177-1
Lewis, S. E., Bainbridge, Z. T., Kuhnert, P. M., Sherman, B. S., Henderson, B., Dougall, C., et al. (2013). Calculating sediment trapping efficiencies for reservoirs in tropical settings: a case study from the Burdekin Falls Dam, NE Australia. Water Resour. Res. 49, 1017–1029. doi: 10.1002/wrcr.20117
Lewis, S. E., Brodie, J. E., Bainbridge, Z. T., Rohde, K. W., Davis, A. M., Masters, B. L., et al. (2009). Herbicides: a new threat to the Great Barrier Reef. Environ. Pollut. 157, 2470–2484. doi: 10.1016/j.envpol.2009.03.006
Lewis, S. E., Shields, G. A., Kamber, B. S., and Lough, J. M. (2007). A multi-trace element coral record of land-use changes in the Burdekin River catchment, NE Australia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 246, 471–487. doi: 10.1016/j.palaeo.2006.10.021
Lirman, D., and Fong, P. (2007). Is proximity to land-based sources of coral stressors an appropriate measure of risk to coral reefs? An example from the Florida Reef Tract. Mar. Pollut. Bull. 54, 779–791. doi: 10.1016/j.marpolbul.2006.12.014
Liu, S., Ryu, D., Webb, J. A., Lintern, A., Waters, D., Guo, D., et al. (2018). Characterisation of spatial variability in water quality in the Great Barrier Reef catchments using multivariate statistical analysis. Mar. Pollut. Bull. 137, 137–151. doi: 10.1016/j.marpolbul.2018.10.019
Loiola, M., Cruz, I. C. S., Lisboa, D. S., Mariano-Neto, E., Leão, Z. M. A. N., Oliveira, M. D. M., et al. (2019). Structure of marginal coral reef assemblages under different turbidity regime. Mar. Environ. Res. 147, 138–148. doi: 10.1016/j.marenvres.2019.03.013
Lough, J. M., and Barnes, D. J. (1992). Comparisons of skeletal density variations in Porites from the central Great Barrier Reef. J. Exp. Mar. Biol. Ecol. 155, 1–25. doi: 10.1016/0022-0981(92)90024-5
Low, J. K. Y. (2015). Sargassum on Singapore’s Reefs. PhD Thesis, National University of Singapore: Singapore:.
Lyons, J., Thimble, S. W., and Paine, L. K. (2000). Grass versus trees: managing riparian areas to benefit streams of central north america1. JAWRA J. Am. Water Resour. Assoc. 36, 919–930. doi: 10.1111/j.1752-1688.2000.tb04317.x
MacDonald, L. H., Anderson, D. M., and Dietrich, W. E. (1997). Paradise threatened: land use and erosion on St. John, US Virgin Islands. Environ. Manag. 21, 851–863. doi: 10.1007/s002679900072
MacDonald, L. H., Sampson, R. W., and Anderson, D. M. (2001). Runoff and road erosion at the plot and road segment scales, St. John, US Virgin Islands. Earth Surf. Process. Landf. 26, 251–272. doi: 10.1002/1096-9837(200103)26:3<251::aid-esp173>3.3.co;2-o
MacNeil, M. A., Mellin, C., Matthews, S., Wolff, N. H., McClanahan, T. R., Devlin, M., et al. (2019). Water quality mediated resilience on the Great Barrier Reef. Nat. Ecol. Evol. 2l, 620–627. doi: 10.1038/s41559-019-0832-3
Maina, J., de Moel, H., Vermaat, J. E., Bruggemann, J. H., Guillaume, M. M. M., Grove, C. A., et al. (2012). Linking coral river runoff proxies with climate variability, hydrology and land-use in Madagascar catchments. Mar. Pollut. Bull. 64, 2047–2059. doi: 10.1016/j.marpolbul.2012.06.027
Maina, J., De Moel, H., Zinke, J., Madin, J., McClanahan, T., and Vermaat, J. E. (2013). Human deforestation outweighs future climate change impacts of sedimentation on coral reefs. Nat. Commun. 4:1986. doi: 10.1038/ncomms2986
Makino, A., Beger, M., Klein, C. J., Jupiter, S. D., and Possingham, H. P. (2013). Integrated planning for land-sea ecosystem connectivity to protect coral reefs. Biol. Conserv. 165, 35–42. doi: 10.1016/j.biocon.2013.05.027
Mallela, J., Perry, C. T., and Haley, M. P. (2004). Reef morphology and community structure along a fluvial gradient, Rio Bueno, Jamaica. Caribbean J. Sci. 40, 299–311.
Maragos, J. E. (1993). Impact of coastal construction on coral reefs in the U.S.-affiliated pacific Islands. Coast. Manag. 21, 235–269. doi: 10.1371/journal.pone.0050719
Margvelashvili, N., Andrewartha, J., Baird, M., Herzfeld, M., Jones, E., Mongin, M., et al. (2018). Simulated fate of catchment-derived sediment on the Great Barrier Reef shelf. Mar. Pollut. Bull. 135, 954–962. doi: 10.1016/j.marpolbul.2018.08.018
Martinez, M. L., Silva, R., Lithgow, D., Mendoza, E., Flores, P., Martinez, R., et al. (2017). Human impact on coastal resilience along the coast of Veracruz, Mexico. J. Coast. Res. 77, 143–154.
Martínez-Rendis, A., González, G. A., Hernández-Stefanoni, J. L., and González, J. E. A. (2015). Quantifying the reefscape transformation of a coastal Caribbean coral reef during a phase shift and the associated coastal landscape change. Mar. Ecol. 37, 697–710. doi: 10.1111/maec.12334
McClanahan, T. R., and Obura, D. (1997). Sedimentation effects on shallow coral communities Kenya. J. Exp. Mar. Biol. Ecol. 209, 103–122. doi: 10.1016/s0022-0981(96)02663-9
McCulloch, M., Fallon, S., Wyndham, T., Hendy, E., Lough, J., and Barnes, D. (2003). Coral record of increased sediment flux to the inner Great Barrier Reef since European settlement. Nature 421, 727–730. doi: 10.1038/nature01361
McKergow, L. A., Prosser, I. P., Hughes, A. O., and Brodie, J. (2005). Sources of sediment to the Great Barrier Reef World Heritage Area. Mar. Pollut. Bull. 51, 200–211. doi: 10.1016/j.marpolbul.2004.11.029
Messina, A. M., and Biggs, T. W. (2016). Contributions of human activities to suspended sediment yield during storm events from a small, steep, tropical watershed. J. Hydrol. 538, 726–742. doi: 10.1016/j.jhydrol.2016.03.053
Morgan, K. M., Perry, C. T., Johnson, J. A., and Smithers, S. G. (2017). Nearshore turbid-zone corals exhibit high bleaching tolerance on the great barrier reef following the 2016 ocean warming event. Front. Mar. Sci. 4:224. doi: 10.3389/fmars.2017.00224
Morgan, K. M., Perry, C. T., Smithers, S. G., Johnson, J. A., and Daniell, J. J. (2016). Evidence of extensive reef development and high coral cover in nearshore environments: implications for understanding coral adaptation in turbid settings. Sci. Rep. 6, 1–10. doi: 10.1038/srep29616
Moustaka, M., Mohring, M. B., Holmes, T., Evans, R. D., Thomson, D., Nutt, C., et al. (2019). Cross-shelf heterogeneity of coral assemblages in Northwest Australia. Diversity 11, 1–19. doi: 10.3390/d11020015
Nagelkerken, I., van der Velde, G., Gorissen, M. W., Meijer, G. J., Van’t Hof, T., and den Hartog, C. (2000). Importance of mangroves, seagrass beds and the shallow coral reef as a nursery for important coral reef fishes, using a visual census technique. Estuar. Coast. Shelf Sci. 51, 31–44. doi: 10.1006/ecss.2000.0617
Nemeth, R. S., and Nowlis, J. S. (2001). Monitoring the effects of land development on the near-shore reef environment of St. Thomas, USVI. Bull. Mar. Sci. 69, 759–775.
Ng, C. S. L., Lim, S. C., Yong, J. Y., Teo, L. M. S., Chou, L. Mi, Chua, K. E., et al. (2015). Enhancing the biodiversity of coastal defence structures: transplantation of nursery-reared reef biota onto intertidal seawalls. Ecol. Eng. 82, 480–486. doi: 10.1016/j.ecoleng.2015.05.016
Ng, C. S. L., Toh, T. C., and Chou, L. M. (2016). Coral restoration in Singapore’s sediment-challenged sea. Reg. Stud. Mar. Sci. 8, 422–429. doi: 10.1016/j.rsma.2016.05.005
Norat-Ramírez, J., Méndez-Lázaro, P., Hernández-Delgado, E. A., Mattei-Torres, H., and Cordero-Rivera, L. (2019). A septic waste index model to measure the impact of septic tanks on coastal water quality and coral reef communities in Rincon, Puerto Rico. Ocean Coast. Manag. 169, 201–213. doi: 10.1016/j.ocecoaman.2018.12.016
Olds, A. D., Pitt, K. A., Maxwell, P. S., Babcock, R. C., Rissik, D., and Connolly, R. M. (2014). Marine reserves help coastal ecosystems cope with extreme weather. Glob. Change Biol. 20, 3050–3058. doi: 10.1111/gcb.12606
Oleson, K. L. L., Falinski, K. A., Audas, D., Coccia-Schillo, S., Groves, P., Teneva, L., et al. (2018). “Linking landscape and seascape conditions: science, tools, and management,” in Seascape Ecology, ed. S. J. Pittman, (Oxford: Wiley-Blackwell), 319–364.
Oleson, K. L. L., Falinski, K. A., Lecky, J., Rowe, C., Kappel, C. V., Selkoe, K. A., et al. (2017). Upstream solutions to coral reef conservation: the payoffs of smart and cooperative decision-making. J. Environ. Manag. 191, 8–18. doi: 10.1016/j.jenvman.2016.12.067
Oliver, L. M., Fisher, W. S., Fore, L., Smith, A., and Bradley, P. (2018). Assessing land use, sedimentation, and water quality stressors as predictors of coral reef condition in St. Thomas, U.S. Virgin Islands. Environ. Monitor. Assess. 190, 1–16. doi: 10.1007/s10661-018-6562-1
Oliver, L. M., Lehrter, J. C., and Fisher, W. S. (2011). Relating landscape development intensity to coral reef condition in the watersheds of St. Croix, US Virgin Islands. Mar. Ecol. Prog. Ser. 427, 293–302. doi: 10.3354/meps09087
Oliver, T. A., and Palumbi, S. R. (2011). Do fluctuating temperature environments elevate coral thermal tolerance? Coral Reefs 30, 429–440. doi: 10.1007/s00338-011-0721-y
Owuor, S. O., Butterbach-Bahl, K., Guzha, A. C., Rufino, M. C., Pelster, D. E., Díaz-Pinés, E., et al. (2016). Groundwater recharge rates and surface runoff response to land use and land cover changes in semi-arid environments. Ecol. Process. 5, 1–21. doi: 10.1186/s13717-016-0060-6
Packett, R., Dougall, C., Rohde, K., and Noble, R. (2009). Agricultural lands are hot-spots for annual runoff polluting the southern Great Barrier Reef lagoon. Mar. Pollut. Bull. 58, 976–986. doi: 10.1016/j.marpolbul.2009.02.017
Pandey, P. K., Sharma, R., Roy, M., and Pandey, M. (2007). Toxic mine drainage from Asia’s biggest copper mine at Malanjkhand, India. Environ. Geochem. Health 29, 237–248. doi: 10.1007/s10653-006-9079-4
Paytan, A., Shellenbarger, G. G., Street, J. H., Gonneea, M. E., Davis, K., Young, M. B., et al. (2006). Submarine groundwater discharge: an important source of new inorganic nitrogen to coral reef ecosystems. Limnol. Oceanogr. 51, 343–348. doi: 10.4319/lo.2006.51.1.0343
Perry, C. T., Smithers, S. G., Gulliver, P., and Browne, N. K. (2012). Evidence of very rapid reef accretion and reef growth under high turbidity and terrigenous sedimentation. Geology 40, 719–722. doi: 10.1130/G33261.1
Pringle, A. W. (1989). The History of Dredging in Cleveland Bay, Queensland and its Effect on Sediment Movement and on the Growth of Mangroves, Corals and Seagrass. Townsville, QLD: Great Barrier Reef Marine Park Authority.
Prouty, N. G., Field, M. E., Stock, J. D., Jupiter, S. D., and McCulloch, M. (2010). Coral Ba/Ca records of sediment input to the fringing reef of the southshore of Moloka‘i, Hawai‘i over the last several decades. Mar. Pollut. Bull. 60, 1822–1835. doi: 10.1016/j.marpolbul.2010.05.024
Prouty, N. G., Hughen, K. A., and Carilli, J. (2008). Geochemical signature of land-based activities in Caribbean coral surface samples. Coral Reefs 27, 727–742. doi: 10.1007/s00338-008-0413-4
Prouty, N. G., Jupiter, S. D., Field, M. E., and McCulloch, M. T. (2009). Coral proxy record of decadal-scale reduction in base flow from Moloka‘i, Hawaii. Geochem. Geophys. Geosyst. 10, 1–18. doi: 10.1029/2009GC002714
Prouty, N. G., Storlazzi, C. D., McCutcheon, A. L., and Jenson, J. W. (2014). Historic impact of watershed change and sedimentation to reefs along west-central Guam. Coral Reefs 33, 733–749. doi: 10.1007/s00338-014-1166-x
Prouty, N. G., Swarzenski, P. W., Fackrell, J. K., Johannesson, K., and Palmore, C. D. (2017). Groundwater-derived nutrient and trace element transport to a nearshore Kona coral ecosystem: experimental mixing model results. J. Hydrol. Reg. Stud. 11, 166–177. doi: 10.1016/j.ejrh.2015.12.058
Quinn, J. M. (2000). “Effects of pastoral development,” in New Zealand Stream Invertebrates: Ecology and Implications for Management, eds K. J. Collier, and M. J. Winterbourn, (Christchurch, NZ: New Zealand Limnological Society with the assistance of the National Institute of Water and Atmospheric Research), 208–229.
Ramos-Scharrón, C. E., and LaFevor, M. C. (2016). The role of unpaved roads as active source areas of precipitation excess in small watersheds drained by ephemeral streams in the Northeastern Caribbean. J. Hydrol. 533, 168–179. doi: 10.1016/j.jhydrol.2015.11.051
Ramos-Scharrón, C. E., and MacDonald, L. H. (2005). Measurement and prediction of sediment production from unpaved roads, St John, US Virgin Islands. Earth Surf. Process. Landf. 30, 1283–1304. doi: 10.1002/esp.1201
Ramos-Scharrón, C. E., and MacDonald, L. H. (2007). Measurement and prediction of natural and anthropogenic sediment sources, St. John, U.S. Virgin Islands. Catena 71, 250–266. doi: 10.1016/j.catena.2007.03.009
Redding, J. E., Myers-Miller, R. L., Baker, D. M., Fogel, M., Raymundo, L. J., and Kim, K. (2013). Link between sewage-derived nitrogen pollution and coral disease severity in Guam. Mar. Pollut. Bull. 73, 57–63. doi: 10.1016/j.marpolbul.2013.06.002
ReefBase (2019). ReefBase: A Global Information System for Coral Reefs. Available at: http://www.reefbase.org
Rees, J. G., Setiapermana, D., Sharp, V. A., Weeks, J. M., and Williams, T. M. (1999). Evaluation of the impacts of land-based contaminants on the benthic faunas of Jakarta Bay, Indonesia. Oceanol. Acta 22, 627–640. doi: 10.1016/s0399-1784(00)88954-9
Reopanichkul, P., Schlacher, T. A., Carter, R. W., and Worachananant, S. (2009). Sewage impacts coral reefs at multiple levels of ecological organization. Mar. Pollut. Bull. 58, 1356–1362. doi: 10.1016/j.marpolbul.2009.04.024
Reside, A. E., Beher, J., Cosgrove, A. J., Evans, M. C., Seabrook, L., Silcock, J. L., et al. (2017). Ecological consequences of land clearing and policy reform in Queensland. Pac. Conserv. Biol. 23, 219–230. doi: 10.1071/pc17001
Restrepo, J. D., Park, E., Aquino, S., and Latrubesse, E. M. (2016). Coral reefs chronically exposed to river sediment plumes in the southwestern Caribbean: Rosario Islands, Colombia. Sci. Total Environ. 553, 316–329. doi: 10.1016/j.scitotenv.2016.02.140
Richmond, R. H. (1995). Effects of coastal runoff on coral reproduction. Oceanogr. Literature Rev. 9:776.
Richmond, R. H., Golbuu, Y., and Shelton, A. J. III (2019). “Successful management of coral reef-watershed networks,” in Coasts and Estuaries, eds E. Wolanski, J. Day, M. Elliott, and R. Ramesh, (Amsterdam: Elsevier Inc.), doi: 10.1016/B978-0-12-814003-1.00026-5
Richmond, R. H., Rongo, T., Golbuu, Y., Victor, S., Idechong, N., Davis, G., et al. (2007). Watersheds and coral reefs: conservation science, policy, and implementation. BioScience 57, 598–607. doi: 10.1641/b570710
Risk, M. J. (2014). Assessing the effects of sediments and nutrients on coral reefs. Curr. Opin. Environ. Sustain. 7, 108–117. doi: 10.1016/j.cosust.2014.01.003
Roberts, M., Hanley, N., Williams, S., and Cresswell, W. (2017). Terrestrial degradation impacts on coral reef health: evidence from the Caribbean. Ocean Coast. Manag. 149, 52–68. doi: 10.1016/j.ocecoaman.2017.09.005
Rothenberger, P., Blondeau, J., Cox, C., Curtis, S., Fisher, W. S., Garrison, V., et al. (2008). “The state of coral reef ecosystems of the U.S. Virgin Islands,” in The State of Coral Reef Ecosystems of the United States and Pacific Freely Associated States: 2008, eds J. E. Wadell and A. M. Clarke, (Silver Spring, MD: NOAA Center for Coastal Monitoring and Assessment’s Biogeography Team).
Rude, J., Minks, A., Doheny, B., Tyner, M., Maher, K., Huffard, C., et al. (2016). Ridge to reef modelling for use within land – sea planning under data-limited conditions. Aquat. Conserv. Mar. Freshw. Ecosyst. 26, 251–264. doi: 10.1002/aqc.2548
Santos, M. E. A., and Reimer, J. D. (2018). Rafting in Zoantharia: a hitchhiker’s guide to dispersal? Mar. Pollut. Bull. 130, 307–310. doi: 10.1016/j.marpolbul.2018.03.041
Saunders, M. I., Bode, M., Atkinson, S., Klein, C. J., Metaxas, A., Beher, J., et al. (2017). Simple rules can guide whether land- or ocean-based conservation will best benefit marine ecosystems. PLoS Biol. 15:e2001886. doi: 10.1371/journal.pbio.2001886
Sawyer, A. H., Michael, H. A., and Schroth, A. W. (2016). From soil to sea: the role of groundwater in coastal critical zone processes. Wiley Interdiscip. Rev. Water 3, 706–726. doi: 10.1002/wat2.1157
Schaffelke, B., Carleton, J., Skuza, M., Zagorskis, I., and Furnas, M. J. (2012). Water quality in the inshore Great Barrier Reef lagoon: implications for long-term monitoring and management. Mar. Pollut. Bull. 65, 249–260. doi: 10.1016/j.marpolbul.2011.10.031
Scott, P. J. B. (1990). Chronic pollution recorded in coral skeletons in Hong Kong. J. Exp. Mar. Biol. Ecol. 139, 51–64. doi: 10.1016/0022-0981(90)90038-e
Shaish, L., Levy, G., Katzir, G., and Rinkevich, B. (2010). Employing a highly fragmented, weedy coral species in reef restoration. Ecol. Eng. 36, 1424–1432. doi: 10.1016/j.ecoleng.2010.06.022
Shi, P., Zhang, Y., Li, Z., Li, P., and Xu, G. (2017). Influence of land use and land cover patterns on seasonal water quality at multi-spatial scales. Catena 151, 182–190. doi: 10.1016/j.catena.2016.12.017
Shuler, C. K., Amato, D. W., Gibson, V., Baker, L., Olguin, A. N., Dulai, H., et al. (2019). Assessment of terrigenous nutrient loading to coastal ecosystems along a human land-use gradient, Tutuila, American Samoa. Hydrology 6, 1–27. doi: 10.3390/hydrology6010018
Sith, R., Watanabe, A., Nakamura, T., Yamamoto, T., and Nadaoka, K. (2019). Assessment of water quality and evaluation of best management practices in a small agricultural watershed adjacent to Coral Reef area in Japan. Agric. Water Manag. 213, 659–673. doi: 10.1016/j.agwat.2018.11.014
Smith, A., Yee, S. H., Russell, M., Awkerman, J., and Fisher, W. S. (2017). Linking ecosystem service supply to stakeholder concerns on both land and sea: an example from Guánica Bay watershed, Puerto Rico. Ecol. Indic. 74, 371–383. doi: 10.1016/j.ecolind.2016.11.036
Smith, R., Knight, R., and Fendorf, S. (2018). Overpumping leads to California groundwater arsenic threat. Nat. Commun. 9, 1–6. doi: 10.1038/s41467-018-04475-3
Smith, S. V., Kimmerer, W. J., Laws, E. A., Brock, R. E., and Walsh, T. W. (1981). Kaneohe Bay sewage diversion experiment: perspectives on ecosystem responses to nutritional perturbation. Pac. Sci. 35, 279–395.
Smith, T. B., Nemeth, R. S., Blondeau, J., Calnan, J. M., Kadison, E., and Herzlieb, S. (2008). Assessing coral reef health across onshore to offshore stress gradients in the US Virgin Islands. Mar. Pollut. Bull. 56, 1983–1991. doi: 10.1016/j.marpolbul.2008.08.015
Star, M., Rolfe, J., McCosker, K., Smith, R., Ellis, R., Waters, D., et al. (2018). Targeting for pollutant reductions in the Great Barrier Reef river catchments. Environ. Sci. Policy 89, 365–377. doi: 10.1016/j.envsci.2018.09.005
Stimson, J. (2018). Recovery of coral cover in records spanning 44 yr for reefs in Kāne‘ohe Bay, Oa‘hu, Hawai‘i. Coral Reefs 37, 55–69. doi: 10.1007/s00338-017-1633-2
Stock, J. D., Cochran, S. A., Field, M. E., Jacobi, J. D., and Tribble, G. (2011). From Ridge to Reef: Linking Erosion and Changing Watersheds to Impacts on the Coral Reef Ecosystems of Hawai‘i and the Pacific Ocean. Reston, VA: United States Geological Survey.
Stock, J. D., Rosener, M., Schmidt, K. M., Hanshaw, M. N., Brooks, B. A., Tribble, G., et al. (2010). Sediment Budget for a Polluted Hawaiian Reef Using Hillslope Monitoring and Process Mapping (Invited). Washington, DC: American Geophysical Union.
Stoms, D. M., Davis, F. W., Andelman, S. J., Carr, M. H., Gaines, S. D., Halpern, B. S., et al. (2005). Integrated coastal reserve planning: making the land–sea connection. Front. Ecol. Environ. 3:429–436. doi: 10.1890/1540-92952005003[0429:ICRPMT]2.0.CO;2
Storlazzi, C. D., Field, M. E., Bothner, M. H., Presto, M. K., and Draut, A. E. (2009). Sedimentation processes in a coral reef embayment: Hanalei Bay, Kauai. Mar. Geol. 264, 140–151. doi: 10.1016/j.margeo.2009.05.002
Storlazzi, C. D., Ogston, A. S., Bothner, M. H., Field, M. E., and Presto, M. K. (2004). Wave- and tidally-driven flow and sediment flux across a fringing coral reef: Southern Molokai, Hawaii. Cont. Shelf Res. 24, 1397–1419. doi: 10.1016/j.csr.2004.02.010
Strayer, D. L., Beighley, R. E., Thompson, L. C., Brooks, S., Nilsson, C., Pinay, G., et al. (2003). Effects of land cover on stream ecosystems: roles of empirical models and scaling issues. Ecosystems 6, 407–423. doi: 10.1007/s10021-002-0170-0
Suchley, A., and Alvarez-Filip, L. (2018). Local human activities limit marine protection efficacy on Caribbean coral reefs. Conserv. Lett. 11, 1–9. doi: 10.1111/conl.12571
Takesue, R. K., and Storlazzi, C. D. (2017). Sources and dispersal of land-based runoff from small Hawaiian drainages to a coral reef: insights from geochemical signatures. Estuar. Coast. Shelf Sci. 188, 69–80. doi: 10.1016/j.ecss.2017.02.013
Tallis, H., Ferdaña, Z., and Gray, E. (2008). Linking terrestrial and marine conservation planning and threats analysis. Conserv. Biol. 22, 120–130. doi: 10.1111/j.1523-1739.2007.00861.x
Tarya, A., Hoitink, A. J. F., Van der Vegt, M., van Katwijk, M. M., Hoeksema, B. W., Bouma, T. J., et al. (2018). Exposure of coastal ecosystems to river plume spreading across a near-equatorial continental shelf. Cont. Shelf Res. 153, 1–15. doi: 10.1016/j.csr.2017.12.003
Teneva, L. T., McManus, M. A., Jerolmon, C., Neuheimer, A. B., Clark, S. J., Walker, G., et al. (2016). Understanding reef flat sediment regimes and hydrodynamics can inform erosion mitigation on land. Collabra 2, 1–12. doi: 10.1525/collabra.25
Thomassin, B. A., Gourbesville, P., Gout, B., and Arnoux, A. (1998). “Impact of an industrial and urban sewage off a coral fringing reef at Mauritius (Indian ocean): modeling of plumes, distribution of trace metals in sediments and effects of the eutrophisation on coral reef communities,” in Proceedings of the Conference on IEEE Oceanic Engineering Society. OCEANS (Cat. No.98), (Nice).
Thompson, A., Schroeder, T., Brando, V. E., and Schaffelke, B. (2014). Coral community responses to declining water quality: Whitsunday Islands, Great Barrier Reef, Australia. Coral Reefs 33, 923–938. doi: 10.1007/s00338-014-1201-y
Thorburn, P. J., and Wilkinson, S. N. (2013). Conceptual frameworks for estimating the water quality benefits of improved agricultural management practices in large catchments. Agric. Ecosyst. Environ. 180, 192–209. doi: 10.1016/j.agee.2011.12.021
Todd, P. A. (2008). Morphological plasticity in scleractinian corals. Biol. Rev. 83, 315–337. doi: 10.1111/j.1469-185X.2008.00045.x
Todd, P. A., Sanderson, P. G., and Chou, L. M. (2001). Morphological variation in the polyps of the scleractinian coral Favia speciosa around Singapore. Hydrobiologia 444, 227–235.
Tomascik, T., and Sander, F. (1985). Effects of eutrophication on reef-building corals. Mar. Biol. 87, 143–155. doi: 10.1007/bf00539422
Tribble, G., Stock, J., and Jacobi, J. (2015). “Watershed processes from ridge to reef: consequences of feral ungulates for coral reef and effects of watershed management,” in Proceedings of the 5th Interagency Conference on Research in the Watersheds and Headwaters to Estuaries: Advances in Watershed Science and Management, eds S. E. Christina, K. W. Ken, and L. S. James, (Asheville, NC: U.S. Department of Agriculture Forest Service), 194.
Tu, J. (2011). Spatially varying relationships between land use and water quality across an urbanization gradient explored by geographically weighted regression. Appl. Geogr. 31, 376–392. doi: 10.1016/j.apgeog.2010.08.001
Tulloch, V. J. D., Brown, C. J., Possingham, H. P., Jupiter, S. D., Maina, J. M., and Klein, C. (2016). Improving conservation outcomes for coral reefs affected by future oil palm development in Papua New Guinea. Biol. Conserv. 203, 43–54. doi: 10.1016/j.biocon.2016.08.013
Umezawa, Y., Miyajima, T., Yamamuro, M., Kayanne, H., and Koike, I. (2012). Fine-scale mapping of land-derived nitrogen in coral reefs by 15 N in macroalgae. Limnol. Oceanogr. 47, 1405–1416. doi: 10.4319/lo.2002.47.5.1405
Ureta, J. C. P., Lasco, R. D., Sajise, A. J. U., and Calderon, M. M. (2016). A ridge-to-reef ecosystem-based valuation approach to biodiversity conservation in Layawan Watershed, Misamis Occidental, Philippines. J. Environ. Sci. Manag. 19, 64–75.
van Woesik, R., Tomascik, T., and Blake, S. (1999). Coral assemblages and physico-chemical characteristics of the Whitsunday Islands: evidence of recent community changes. Mar. Freshw. Res. 50, 427–440. doi: 10.1071/MF97046
Verbist, B., Poesen, J., van Noordwijk, M., Widianto, Suprayogo, D., Agus, F., et al. (2010). Factors affecting soil loss at plot scale and sediment yield at catchment scale in a tropical volcanic agroforestry landscape. Catena 80, 34–46. doi: 10.1016/j.catena.2009.08.007
Victor, S., Golbuu, Y., Wolanski, E., and Richmond, R. H. (2004). Fine sediment trapping in two mangrove-fringed estuaries exposed to contrasting land-use intensity, Palau, Micronesia. Wetlands Ecol. Manag. 12, 277–283. doi: 10.1007/s11273-005-8319-1
Victor, S., Neth, L., Golbuu, Y., Wolanski, E., and Richmond, R. H. (2006). Sedimentation in mangroves and coral reefs in a wet tropical island, Pohnpei, Micronesia. Estuar. Coast. Shelf Sci. 66, 409–416. doi: 10.1016/j.ecss.2005.07.025
Walsh, C. J., Roy, A. H., Feminella, J. W., Cottingham, P. D., Groffman, P. M., and Morgan, R. P. (2005). The urban stream syndrome: current knowledge and the search for a cure. J. North Am. Benthol. Soc. 24, 706–723. doi: 10.1899/04-028.1
Waterhouse, J., Brodie, J., Lewis, S., and Mitchell, A. (2012). Quantifying the sources of pollutants in the Great Barrier Reef catchments and the relative risk to reef ecosystems. Mar. Pollut. Bull. 65, 394–406. doi: 10.1016/j.marpolbul.2011.09.031
Wear, S. L., and Thurber, R. V. (2015). Sewage pollution: mitigation is key for coral reef stewardship. Ann. N. Y. Acad. Sci. 1355, 15–30. doi: 10.1111/nyas.12785
Weber, M., de Beer, D., Lott, C., Polerecky, L., Kohls, K., Abed, R. M. M., et al. (2012). Mechanisms of damage to corals exposed to sedimentation. PNAS 109, E1558–E1567. doi: 10.1073/pnas.1100715109
Wedding, L. M., Lecky, J., Gove, J. M., Walecka, H. R., Donovan, M. K., Williams, G. J., et al. (2018). Advancing the integration of spatial data to map human and natural drivers on coral reefs. PLoS One 13:e0189792. doi: 10.1371/journal.pone.0189792
Wenger, A. S., Williamson, D. H., da Silva, E. T., Ceccarelli, D. M., Browne, N. K., Petus, C., et al. (2016). Effects of reduced water quality on coral reefs in and out of no-take marine reserves. Conserv. Biol. 30, 142–153. doi: 10.1111/cobi.12576
West, K., and van Woesik, R. (2001). Spatial and temporal variance of river discharge on Okinawa (Japan): inferring the temporal impact on adjacent coral reefs. Mar. Pollut. Bull. 42, 864–872. doi: 10.1016/S0025-326X(01)00040-6
Wiedenmann, J., D’Angelo, C., Smith, E. G., Hunt, A. N., Legiret, F. E., Postle, A. D., et al. (2013). Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat. Clim. Change 3, 160–164. doi: 10.1038/nclimate1661
Wilkinson, S. N., Hancock, G. J., Bartley, R., Hawdon, A. A., and Keen, R. J. (2013). Using sediment tracing to assess processes and spatial patterns of erosion in grazed rangelands, Burdekin River basin, Australia. Agric. Ecosyst. Environ. 180, 90–102. doi: 10.1016/j.agee.2012.02.002
Winter, K. B., Beamer, K., Vaughan, M. B., Friedlander, A. M., and Kido, M. H. (2018). The Moku system: managing biocultural resources for abundance within social-ecological regions in Hawai‘i. Sustainability 10:3554. doi: 10.3390/su10103554
Wolanski, E., and De’ath, G. (2005). Predicting the impact of present and future human land-use on the Great Barrier Reef. Estuar. Coast. Shelf Sci. 64, 504–508. doi: 10.1016/j.ecss.2005.03.017
Wolanski, E., Martinez, J. A., and Richmond, R. H. (2009). Quantifying the impact of watershed urbanization on a coral reef: Maunalua Bay, Hawaii. Estuar. Coast. Shelf Sci. 84, 259–268. doi: 10.1016/j.ecss.2009.06.029
Wolanski, E., Richmond, R. H., and McCook, L. (2004). A model of the effects of land-based, human activities on the health of coral reefs in the Great Barrier Reef and in Fouha Bay, Guam, Micronesia. J. Mar. Syst. 46, 133–144. doi: 10.1016/j.jmarsys.2003.11.018
Wolff, N. H., da Silva, E. T., Devlin, M., Anthony, K. R. N., Lewis, S., Tonin, H., et al. (2018). Contribution of individual rivers to Great Barrier Reef nitrogen exposure with implications for management prioritization. Mar. Pollut. Bull. 133, 30–43. doi: 10.1016/j.marpolbul.2018.04.069
Wooldridge, S. A., and Done, T. J. (2009). Improved water quality can ameliorate effects of climate change on corals. Ecol. Appl. 19, 1492–1499. doi: 10.1890/08-0963.1
Yamano, H., Satake, K., Inoue, T., Kadoya, T., Hayashi, S., Kinjo, K., et al. (2015). An integrated approach to tropical and subtropical island conservation. J. Ecol. Environ. 38, 271–279. doi: 10.5141/ecoenv.2015.028
Yamano, H., and Watanabe, T. (2016). “Coupling remote sensing and coral annual band data to investigate the history of catchment land use and coral reef status,” in Coral Reef Science, ed. H. Kayanne, (Tokyo: Springer), 47–53. doi: 10.1007/978-4-431-54364-0_3
Yates, K. K., Rogers, C. S., Herlan, J. J., Brooks, G. R., Smiley, N. A., and Larson, R. A. (2014). Diverse coral communities in mangrove habitats suggest a novel refuge from climate change. Biogeosciences 11, 4321–4337. doi: 10.5194/bg-11-4321-2014
Yoshioka, R. M., Kim, C. J. S., Tracy, A. M., Most, R., and Harvell, C. D. (2016). Linking sewage pollution and water quality to spatial patterns of Porites lobata growth anomalies in Puako, Hawaii. Mar. Pollut. Bull. 104, 313–321. doi: 10.1016/j.marpolbul.2016.01.002
Keywords: land use, ridge-to-reef, coral reefs, nutrients, sediment, watersheds, groundwater, spatial planning
Citation: Carlson RR, Foo SA and Asner GP (2019) Land Use Impacts on Coral Reef Health: A Ridge-to-Reef Perspective. Front. Mar. Sci. 6:562. doi: 10.3389/fmars.2019.00562
Received: 02 April 2019; Accepted: 27 August 2019;
Published: 18 September 2019.
Edited by:
Annette Breckwoldt, Leibniz Centre for Tropical Marine Research (LG), GermanyReviewed by:
Amelia Wenger, The University of Queensland, AustraliaJon Brodie, James Cook University, Australia
Copyright © 2019 Carlson, Foo and Asner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachel R. Carlson, rrcarl@stanford.edu
 Rachel R. Carlson
Rachel R. Carlson Shawna A. Foo
Shawna A. Foo Gregory P. Asner
Gregory P. Asner