Skin Deep: An Assessment of New Zealand Blue Whale Skin Condition
- Geospatial Ecology of Marine Megafauna Lab, Marine Mammal Institute, Department of Fisheries and Wildlife, Oregon State University, Newport, OR, United States
Skin condition assessment of wildlife can provide insight into individual and population health. Yet, logistics can limit skin condition assessment of large whales. We developed a standardized, quantitative protocol using photographs to assess skin condition of blue whales in New Zealand, and demonstrate the value gained by testing hypotheses, documenting new morphologies, and establishing baselines that can be monitored for change. We reviewed a photo-identification catalog to compile common markings, categorized markings according to existing definitions, and described markings not previously documented. Photographs of blue whale skin (n = 1,466) were assessed to quantify marking prevalence, severity, and co-occurrence patterns. Of the whales assessed (n = 148), 96.6% had cookie cutter shark bites, 80.4% had blister lesions, 56.0% had pigmentation blazes on the dorsal fin, and 33.7% had holes in the dorsal fin. Additionally, 35.8% had “starburst” lesions, a previously undocumented marking. Blister and cookie cutter shark bite severity did not accumulate linearly, indicating that the two marking types are unrelated. There was a positive relationship between blister severity and number of starbursts, indicating that the two could be related; based on morphological similarities, starburst lesions may derive from ruptured blisters. Whales with holes in their dorsal fin had significantly higher blister severity than those without, indicating that these markings could be related; this is supported by observed blisters on dorsal fins of blue whales. There was a significantly higher probability of fresher cookie cutter shark bites on whales observed at more northerly latitudes, but no relationship between blister severity or number of starbursts and latitude. These latitudinal patterns indicate that blue whales in New Zealand accumulate cookie cutter shark bites at more northerly latitudes; this finding is supported by the known range of cookie cutter sharks in New Zealand waters. Of the eight individual whales re-sighted across multiple years, there was no uniform pattern in lesion change over time, however, individual cases revealed lesion healing over a multi-year timeframe. Our protocol for quantifying skin condition can be applied to any cetacean photo-identification catalog, and can be used to compare across individuals and populations, and explore causal links between skin condition and cetacean health.
Introduction
Wildlife population health can be difficult to quantify non-invasively. Yet, visual assessment of external morphology including skin condition has been used as an indicator of underlying individual and population health in several species including wolves (Oleaga et al., 2011), lemurs (Berg et al., 2009), and elephants (Wemmer et al., 2006). Markings on cetacean skin have long been used to identify individuals, and more recently studies have used photographs to assess and monitor cetacean health (Thompson and Hammond, 1992; Wilson et al., 2000; Van Bressem et al., 2003; Hamilton and Marx, 2005). Baseline information on population health is important for monitoring changes over time. While skin condition of wild cetaceans is difficult to study due to the logistical constraints of data collection, it can be done non-invasively via photographs and used to monitor health, as well as impacts of environmental changes and anthropogenic pressures. Population-level assessments of baleen whale skin lesion prevalence have proven useful to understand changes over time and infer population health (Hamilton and Marx, 2005).
A new population of blue whales was recently documented in New Zealand (Torres, 2013; Barlow et al., 2018). These blue whales are genetically distinct from all other documented populations in the southern hemisphere, are present in New Zealand waters year-round, and have an estimated population size of 718 individuals (95% CI = 279–1,926) (Barlow et al., 2018). However, despite evidence for a primary foraging ground in an industrial region of New Zealand known as the South Taranaki Bight (STB), little is known about this blue whale population’s ecology, migration and residency patterns, reproductive rates, exposure to anthropogenic threats, or health. As a newly documented population, efforts to establish baseline information about their biology and ecology are important to support conservation management. A study of 31 photo-identified blue whales in New Zealand conducted by Olson et al. (2015) qualitatively described the whales as exhibiting “poor skin condition, with numerous scars from lesions and cookie cutter shark bites,” comparable in appearance to blue whales observed in Australia and better in appearance than blue whales observed in Antarctica. However, no quantitative assessment of skin condition has been conducted to-date for New Zealand blue whales. In this study we maximize the value of blue whale photographs captured in the field for identification purposes to evaluate skin condition health of this New Zealand blue whale population.
Blue whales caught by commercial whaling operations in the Southern Hemisphere were described as having characteristic “pits” on their sides and flanks (Mackintosh and Wheeler, 1929). The wounds were subsequently attributed to a small squaloid shark of the genus Isistius, commonly known as cookie cutter shark (Jones, 1971). The characteristic round cookie cutter shark bite wounds have also been observed on swordfish (Papastamatiou et al., 2010), large sharks (Hoyos-Padilla et al., 2013), and other marine mammal species including baleen whales (Mackintosh and Wheeler, 1929; Best and Photopoulou, 2016), dolphins (Heithaus, 2001), porpoises (Van Utrecht, 1959), and pinnipeds (Le Boeuf et al., 1987; Gallo-Reynoso and Figueroa-Carranza, 1992). Although knowledge on the global distribution of cookie cutter sharks is limited, it is believed that their geographic range is circumpolar, in tropical and subtropical waters (Jones, 1971; Jahn and Haedrich, 1987; Nakano and Tabuchi, 1990). It has therefore been suggested that cookie cutter shark bite wounds on marine mammals can be indicative of the migration routes and geographic range of large baleen whales (Best and Photopoulou, 2016). In addition to cookie cutter shark bites, other markings on blue whale skin have been documented worldwide, including blister-like lesions, a tattoo-like skin disease, and scars from killer whale (Orcinus orca) predation (Brownell et al., 2007; Martinez-Levasseur et al., 2011; Olson et al., 2015).
Understanding the type, prevalence, and occurrence patterns of skin markings on cetaceans can provide insight into individual and population health, healing rates, vulnerability to infections, energetic costs, and ecological associations (e.g., oceanographic conditions, movement patterns) that would otherwise require logistically difficult or invasive data collection methods. The objectives of this study are to assess markings on the skin of New Zealand blue whales in a quantitative, repeatable manner, to describe skin condition patterns. It is imperative that any population assessment be conducted in a rigorous manner, so that the methodology can be repeated for other time periods or populations, and linkages can be made between data streams to infer causal links. We reviewed our existing photo-identification catalog to compile commonly observed markings. We conducted a thorough literature review to categorize observed markings according to prior descriptions when possible, and described markings not previously documented in the literature. We used our compiled data on blue whale skin condition in New Zealand to develop and test a series of hypotheses about marking prevalence, severity, and co-occurrence patterns. This comprehensive skin condition assessment establishes a baseline understanding of New Zealand blue whale skin condition and sheds light on their biology and ecology.
Materials and Methods
Data Sources
Photographs of blue whales were obtained from dedicated vessel-based surveys of blue whales in the STB region, which lies between the north and south islands of New Zealand, and from opportunistic photographs taken around New Zealand (data detailed in Barlow et al., 2018). These data included over 1,900 photographs of blue whales collected between 2004 and 2018. Using standard photo-identification methods (Sears et al., 1990) 162 individual blue whales were identified in this dataset. Age was unknown for all whales. Calves were identified if accompanied by an adult whale, and all other whales were classified as adults as it was not possible to distinguish juveniles from adults. All photos of each individual whale were pooled by calendar year for further assessment.
Skin Condition Definitions
Photographs were first reviewed to obtain reference images of markings frequently seen on blue whales in New Zealand. Next, a thorough literature review was conducted to determine whether these markings had been previously identified in any cetacean population. Markings not described in the existing literature were given a definition, otherwise previously published definitions were applied.
Markings on blue whales were classified into three categories: lesions, blazes, and holes (Table 1). Lesions were defined as a class of marks that leave a depression or white scar (Hamilton and Marx, 2005). Blazes were considered to be an area of light-colored pigmentation in a swath on the leading edge of the dorsal fin. Holes were classified as any circular, hollow punctures through the dorsal fin, excluding notches or tears (Table 1).

Table 1. Definitions and reference images for each class and subclass of skin condition markings evaluated.
Lesions were further categorized into sub-classes: cookie cutter shark bites, blister-like vesicles (hereafter referred to as “blisters”), and starbursts. Cookie cutter shark bites were defined as small, crater-like wounds of variable depth that are round, oval, or crescent in shape (Jones, 1971; Dwyer and Visser, 2011; Best and Photopoulou, 2016). Cookie cutter shark bites were subsequently further classified by phase of healing based on bite morphology and pigmentation (Table 1). Blisters were defined as air or fluid-filled elevations of the epidermis, observed either as a single lesion or a cluster, distinguishable from mottled skin pigmentation by their three-dimensionality (Hamilton and Marx, 2005; Brownell et al., 2007). Blisters were of comparable size to cookie cutter shark bites or slightly larger, but rounder in shape than the typically oval bite marks (Brownell et al., 2007). Starbursts were not previously described in the literature, yet we noted their presence on the epidermis of many blue whales; hence, we introduce this new skin lesion type, defined as light-colored markings with a clear central origin and spindly tendrils that extend outward, away from the origin (Table 1).
When lesion presence was determined, the photographs were evaluated for lesion severity. Reference images for each severity category were obtained and agreed upon by all analysts prior to the assessment process. Each severity category for cookie cutter shark bite and blister lesions was assigned a numerical score (Table 2).

Table 2. Definitions and reference images for each severity category for cookie cutter shark bite and blister lesions.
Data Processing
To minimize bias arising from how much of the whale’s body was visible in the photographs, we designated an “assessment area” of the body (Hamilton and Marx, 2005). The assessment area was defined by creating a gridded box and overlaying it on the photo(s) in which the greatest amount of the whale’s body was visible for each identified individual, using the dorsal fin as a visual control. The box was aligned so that the upper bound crossed the intersection of the trailing edge of the dorsal fin and the dorsal ridge, and resized so that 25% of the box was caudal to the dorsal fin and 75% was cranial to the dorsal fin (Figure 1). For each whale assessed, the percentage of the assessment area visible above the water was recorded for each side of the whale. Images of each assessed whale with the assessment area illustrated (e.g., Figure 1) were compiled for skin condition assessment by analysts.
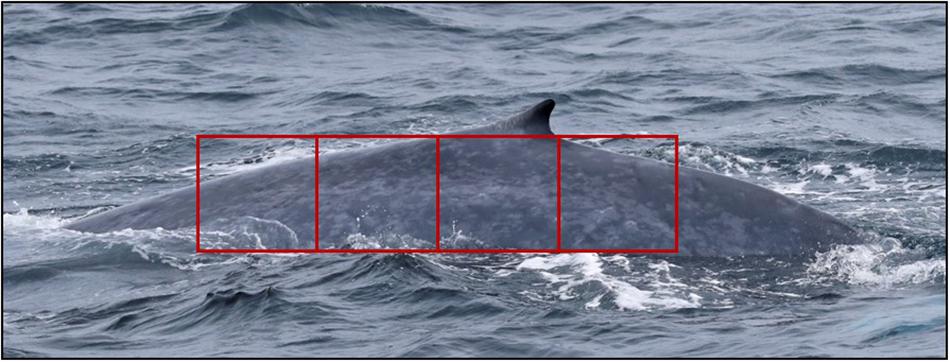
Figure 1. Standardized area for all skin condition assessments. The rectangular assessment area is aligned such that 25% of the total area is caudal to the dorsal fin, and 75% is cranial to the dorsal fin. In this image, 100% of the assessment area is visible.
Photo brightness, sharpness, contrast, and saturation were manipulated in the Windows Photos application to increase visibility of markings. Each assessment was also given an overall photo quality score of poor, fair, good, or excellent based on distance, clarity, brightness, and focus of the images (Supplementary Table S1). The skin condition assessment was conducted for the side with poorer quality photos first to minimize observer bias from assumptions based on prior knowledge of markings. All individuals photographed in each calendar year were evaluated for the presence or absence of lesions, blazes, and holes within the assessment area. For cookie cutter shark bites and blisters, the overall severity score was also recorded (Table 2). For cookie cutter shark bites, the presence or absence of each phase (Table 1) was also determined. For starbursts, the number of starburst lesions within the assessment area was counted for each side of the whale. Where presence or severity could not be determined (CBD) due to photo quality it was scored as “could not be determined.”
Two analysts (DRB and ALP) conducted the skin condition assessment for all blue whales independently. The reference images demarking the assessment area for each individual ensured that the two analysts evaluated exactly the same area on the whale. Subsequently, the two independent assessments were reconciled and in instances where there was a difference (n = 19, 12.8%) a third, independent analyst (LGT) assessed the photographs for which there was a discrepancy to assign the final classifications and scoring. This process was meant to minimize bias from any one observer and ensure that our assessment scoring was robust (Bradford et al., 2009; Maldini et al., 2010; Yang et al., 2013).
Statistical Analysis
Once the data were processed, statistical analyses were conducted to test a series of six hypotheses (Table 3). A portion of the photo-identification dataset only included images of one side of the whale. Therefore, we tested the hypothesis that (H1) skin condition on one side of a whale was representative of the other side. We compared markings on both sides of whales where data were available and photo quality was rated as fair or better. Paired t-tests were used to compare cookie cutter shark bite and blister severity scores, the number of starbursts, and blaze presence between the left and right sides.
The occurrence rate of skin lesions was assessed by calculating the proportions of whales with no, mild, medium, and severe cookie cutter shark bites and blisters. This assessment was conducted separately for adults and calves to address the hypothesis that (H2) these lesions accumulate with age, and therefore could potentially be used as a proxy for age class. To test the hypothesis that (H3) whales accumulate cookie cutter shark bite and blister severity at a similar rate, a confusion matrix was generated to examine their co-occurrence pattern. A heatmap was produced from the confusion matrix to visualize this co-occurrence pattern.
We hypothesized that (H4) starburst lesions derive from blisters, and evaluated this theory by assessing the relationship between starbursts and blister severity, and starbursts and cookie cutter shark bite severity. The total number of starbursts counted per whale were first standardized by the proportion of the assessment area evaluated (left-side count + right-side count × proportion of total assessment area visible on both sides). The standardized number of starbursts metric was log-transformed for further analysis to account for skew toward zero. Linear regression was used to examine the relationship between the standardized number of starbursts and both blister severity score and cookie cutter shark bite severity score, using the relationship with cookie cutter shark bites to test the null hypothesis that starburst are not related to cookie cutter shark bites.
Furthermore, we also hypothesized that (H5) holes in the dorsal fin of blue whales derive from ruptured blisters on the dorsal fin. T-tests were used to test for any difference in blister severity score, cookie cutter shark bite severity score, and standardized number of starbursts between whales with and without holes in their dorsal fin.
Like other studies (Jones, 1971; Dwyer and Visser, 2011; Best and Photopoulou, 2016) we hypothesize (H6) that the phase (freshness) of cookie cutter shark bites on blue whales is related to their latitudinal occurrence. We compared the proportion of whales with phase 1 or 2 cookie cutter shark bites north of −39° to those south of −39°, because the southernmost record of a cookie cutter shark in New Zealand waters is ∼−39° latitude (Dwyer and Visser, 2011). To further quantify whether there was a relationship between cookie cutter shark bite freshness and latitude, the presence or absence of phase 1 and 2 bites as a function of latitude was tested using a logistic regression. Null hypotheses of relationships between latitude and lesions were also tested using linear regression between blister severity score and latitude, and standardized number of starbursts and latitude.
For the individuals photographed in multiple years, the change in cookie cutter shark bite and blister severity between sightings was evaluated. Where possible, healing progression of individual lesions over time was assessed for each individual whale.
All statistical analyses and plotting were conducted using R, version 3.5.0.
Results
Of all the photographs reviewed (n > 1,900), the assessment area of the whale was visible in 1,466 images. After removing poor-quality photographs, 148 photo-identified whales were retained in the dataset for further analysis. Of these 148, there were eight individuals sighted in multiple years, one of which was sighted in four separate years (2010, 2014, 2016, and 2017). Therefore, our final dataset contained 138 unique individual blue whales photographed around New Zealand.
Using individuals with fair or better quality photographs for both sides (n = 23), we determined no significant difference in cookie cutter shark bite severity score, blister severity score, presence or absence of blazes, or the number of starbursts between the left- and right-hand sides (paired t-tests, t < 1.2, p > 0.2 for all). With this support for H1 we justifiably assumed the same skin markings were applicable for both sides of whales where only one side was photographed or photo quality was poor for one side.
Marking Rates and Severity
Of the whales assessed (n = 148), 96.6% had cookie cutter shark bites, 80.4% had blisters, 35.8% had starbursts, 56.0% had blazes, and 33.7% had holes in their dorsal fin. The proportion of whales with mild, medium, and severe cookie cutter shark bite severity scores were about equal, and this was true for both adults (n = 137) and calves (n = 11; Figure 2). No blistering was observed for 16.0% of adults, and blister severity was scored as medium or severe for 50.3% of adult whales. In contrast, 63.6% of calves had no blistering present, 18.1% had mild blister severity, and no calves had medium or severe blistering (Figure 2), lending support for H2. More whales were given a score of CBD for blister severity than for cookie cutter shark bite severity, indicating that once presence of the lesion was determined, severity was more difficult to ascertain for blisters than for cookie cutter shark bites (Figure 2).
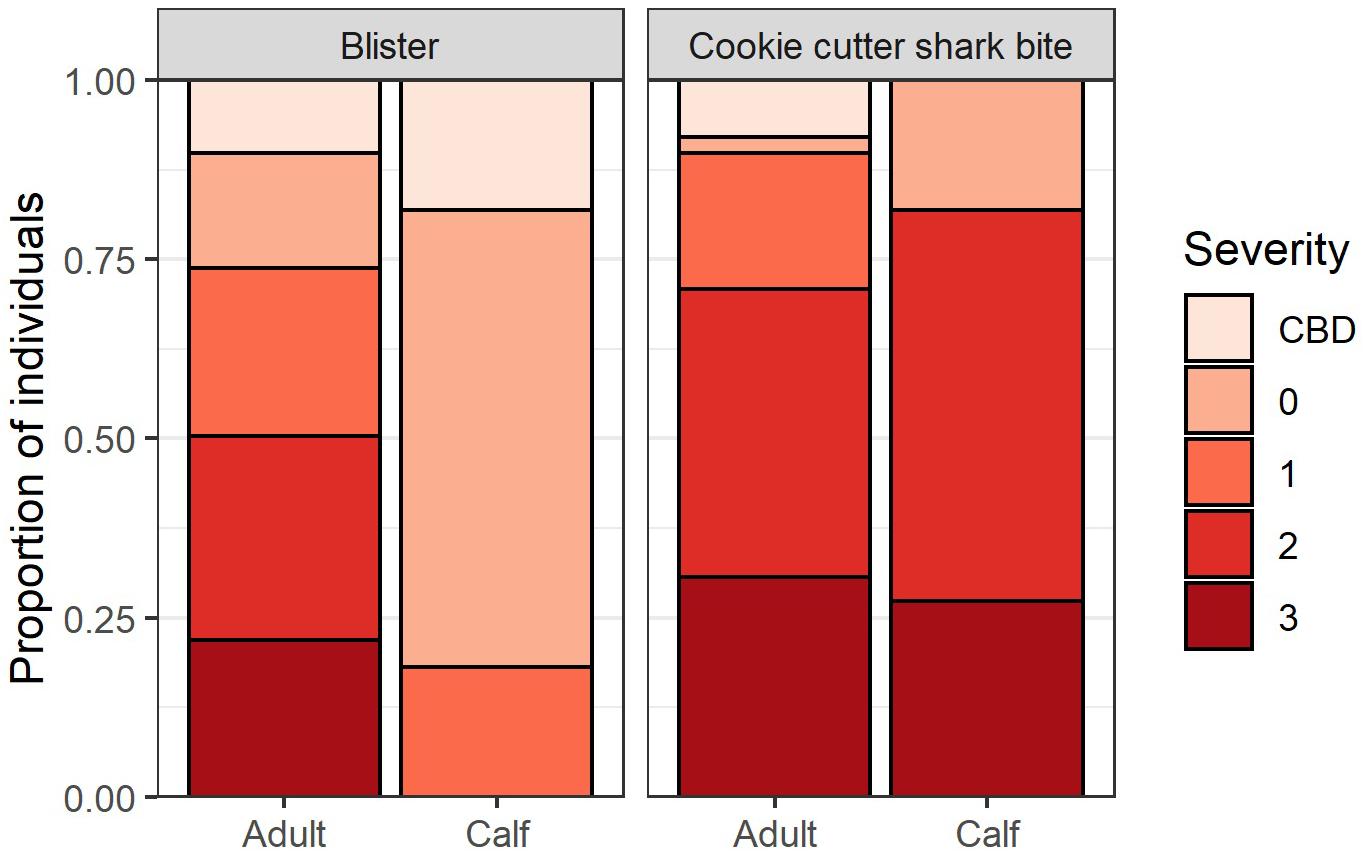
Figure 2. Blister lesion and cookie cutter shark bite severity for adults (n = 137) and calves (n = 11). A score of 0 = no lesions present, 1 = mild, 2 = medium, 3 = severe, and CBD = severity could not be determined.
Co-occurrence of Marking Types
According to the confusion matrix and heat map (Figure 3) blister severity and cookie cutter shark bite severity do not accumulate linearly, thus rejecting H3. The most common co-occurrence pattern was medium cookie cutter shark bites with medium blistering, followed by severe cookie cutter shark bites with no or mild blistering, and mild cookie cutter shark bites with severe blistering (Figure 3).
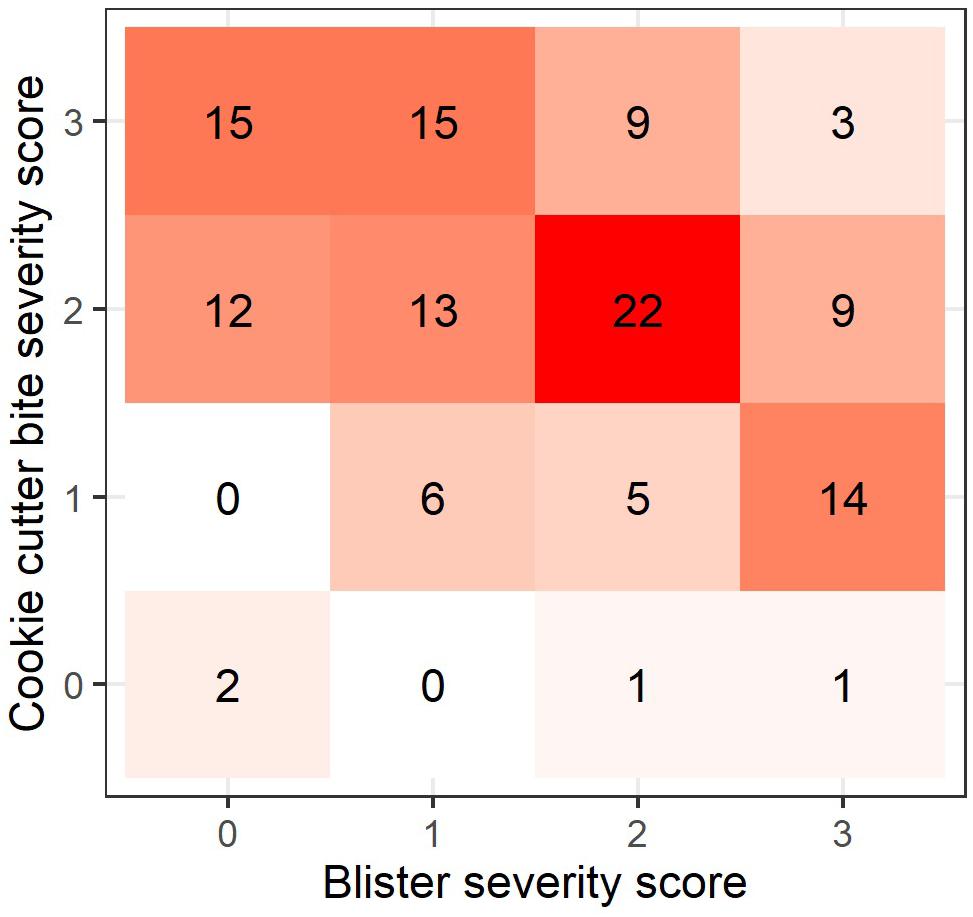
Figure 3. Heatmap showing the pattern of co-occurrence in blister and cookie cutter shark bite severity (n = 127). Numbers and colors within each square represent the number of cases for each co-occurrence pattern.
Support for H4 is provided by the significant positive linear relationship between the standardized number of starbursts and blister severity score (linear regression, R2 = 0.124, F(1,115) = 16.32, p < 0.001; Figure 4A). There was a weaker negative linear relationship between standardized number of starbursts and cookie cutter shark bite severity score (linear regression, R2 = 0.041, F(1,113) = 4.865, p = 0.029; Figure 4B).
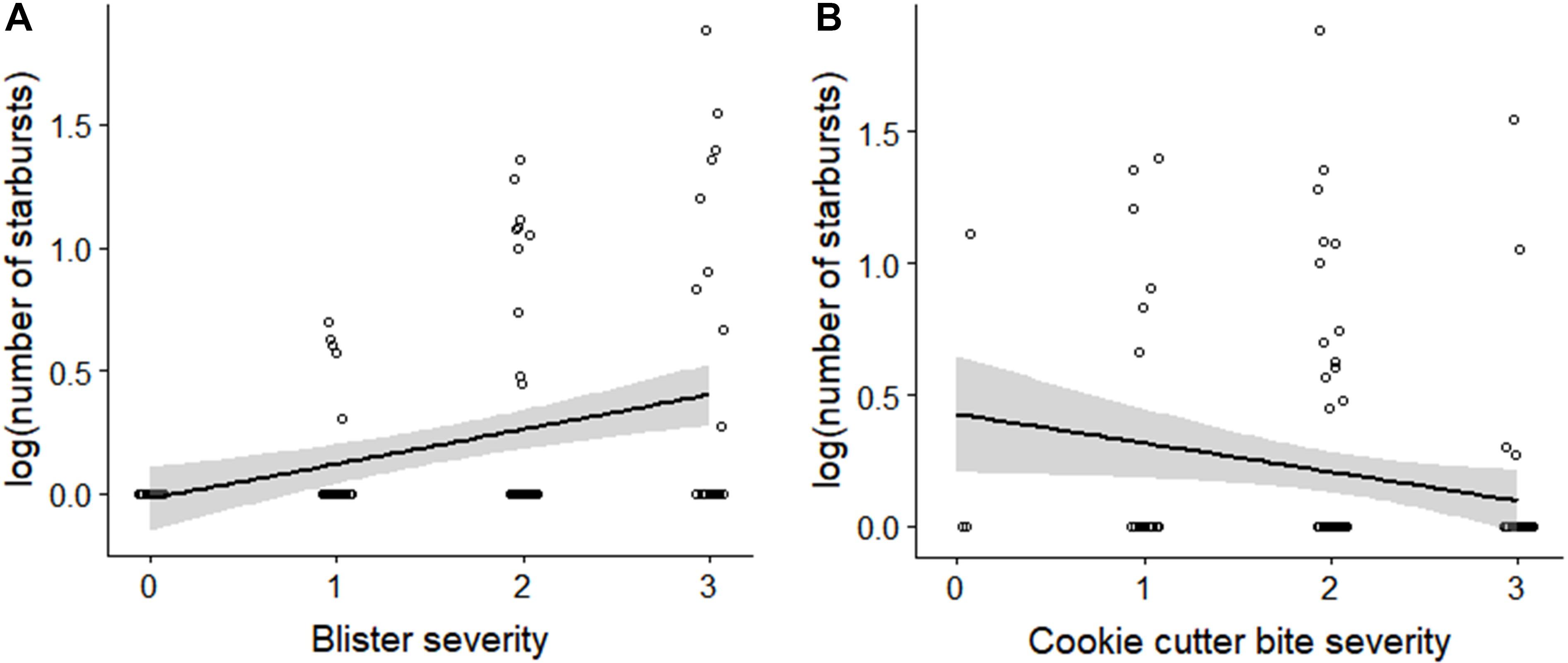
Figure 4. Number of starburst lesions relative to (A) blister severity score, and (B) cookie cutter shark bite severity score. The number of starbursts represents the total count of starbursts within the assessment area for both sides, standardized by the percentage of the assessment area that was visible. Number of starbursts was log-transformed to account for skew toward zero.
Whales with holes in their dorsal fin had higher blister severity scores (n = 50, mean score = 2.5) than those without (n = 134, mean score = 1.3), and this difference was statistically significant (t-test, t = −6.477, df = 31.751, p < 0.001). In contrast, there was no significant difference in cookie shark cutter bite severity score between whales with and without holes in their dorsal fin (t-test, t = −0.012, df = 15.07, p = 0.99). These results support H5.
Regional Patterns in Lesion Severity and Phase
Of the whales photographed north of −39° (n = 46), 76% had phase 1 or 2 cookie cutter shark bites present. In contrast, 57.1% of whales photographed south of −39° (n = 133) had phase 1 or 2 cookie cutter shark bites. A logistic regression of the presence of phase 1 or 2 cookie cutter shark bites as a function of latitude showed a significantly higher probability of fresher bites at the more northerly latitudes (odds ratio = 1.332, z = 2.535, df = 115, p = 0.011). There was no significant relationship between blister severity score and latitude (linear regression, R2 = 0.035, F(1,103) = 3.793, p = 0.054). Similarly, there was no relationship between the standardized number of starbursts and latitude (linear regression, R2 = 0.002, F(1,91) = 0.201, p = 0.654). These results support H6 that New Zealand blue whales accumulate fresh cookie cutter shark bites more often when in more northerly latitudes.
Changes in Lesion Severity Over Time
Of the eight individual whales re-sighted across multiple years, there was no uniform pattern in lesion change over time. Of the re-sighted whales for which blister severity could be assessed in multiple sightings (n = 6), severity declined for one individual (1 to 0 over a 5-year period), increased for one individual (2 to 3 over a 7-year period), and remained unchanged for four individuals (Supplementary Figure S1).
Cookie cutter shark bite severity increased for two individuals (0 to 1 over a 1-year period and 1 to 2 over a 3-year period, respectively), declined for one individual (3 to 2 over a 7-year period), and did not change for five individuals (Supplementary Figure S2). However, examination of healing of individual cookie cutter shark bites over time for one individual whale revealed progression from phase 2 to 3 over a 38-month period, from phase 2 to 4 over a 38- to 69-month period, and from phase 3 to 4 over a 31- to 69-month period (Figure 5). It should be noted that these healing times represent a maximum amount of time between healing phases considering the gaps between photographic records.
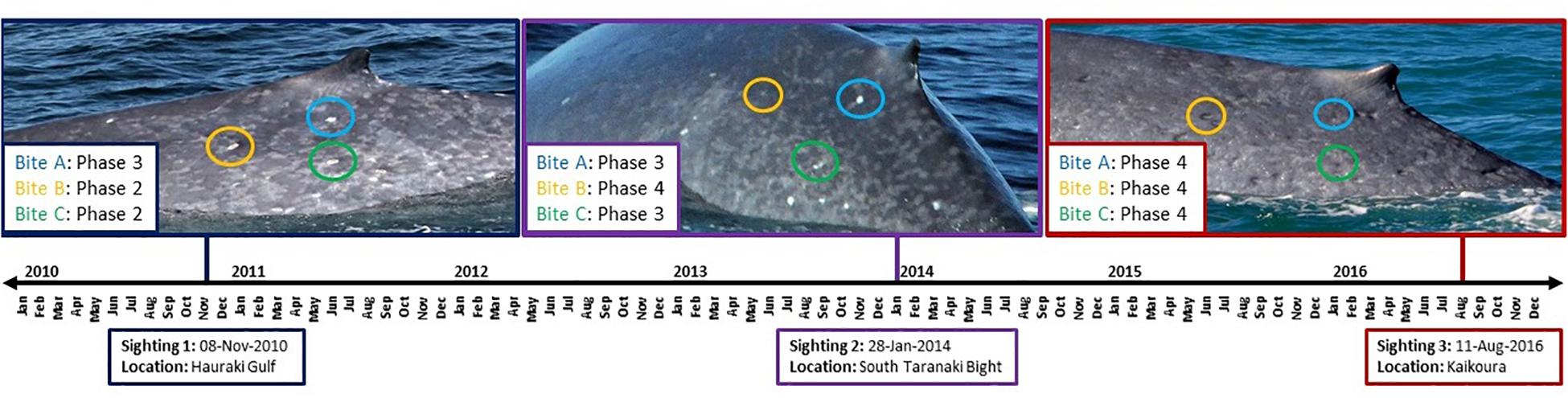
Figure 5. Timeline for cookie cutter shark bite healing for one individual blue whale that was re-sighted three times over a 7-year period. Example bites are circled in each image, where corresponding color denotes the same marking at each sighting over time.
Discussion
In this study, we developed and applied a standardized skin condition assessment protocol to successfully test multiple hypotheses about the skin condition of blue whales in New Zealand (Table 3). We document high rates of both cookie cutter shark bites and blister lesions, pigmentation blazes on over half of the population, describe and quantify a new lesions type – starburst – and demonstrate its association with blisters, present evidence that holes in dorsal fins may derive from blisters, demonstrate that fresher cookie cutter shark bites are more prevalent in lower latitudes, and provide the first description of lesion healing patterns in individual blue whales over time. These methods can be applied widely to any cetacean photo-identification catalog containing dorsal fin images, which would allow for standardized comparative studies across populations and time periods.
New Zealand blue whales have a very high rate of cookie cutter shark bite presence, with 96.6% of the photographed individuals exhibiting cookie cutter shark bites. In comparison, 69.2% of blue whales photo-identified in Chile had cookie cutter shark bites present (Brownell et al., 2007). The calves examined in this study also exhibited severe cookie cutter shark bites despite their young age. In the case of sei whales caught by commercial whaling operations off the coast of South Africa, it was determined that cookie cutter shark bite accumulation was higher for lactating females, suggesting that increased time spent near the surface and slow movement of mother-calf pairs may leave them more vulnerable to cookie cutter shark attacks (Best and Photopoulou, 2016).
The distribution of cookie cutter sharks is thought to be circumpolar in tropical and subtropical waters, with a geographic range that is limited by water temperature (Jones, 1971; Jahn and Haedrich, 1987; Nakano and Tabuchi, 1990). The southernmost record of a cookie cutter shark is −41°40′, near Tasmania (Best and Photopoulou, 2016), and a cookie cutter shark specimen has been caught as far south as −39° in New Zealand waters (Dwyer and Visser, 2011). The finding from this study that the probability of fresher bites is greater at more northerly latitudes supports the notion that in New Zealand, the geographic range of blue whales overlaps partially with the geographic range of cookie cutter sharks. Similarly, killer whales photographed in Antarctic waters frequently exhibited scars from healed cookie cutter shark bite wounds, whereas open bite wounds were more commonly observed on killer whales sighted in northern New Zealand waters (Dwyer and Visser, 2011). Furthermore, the presence of severe, fresh cookie cutter shark bites on blue whale calves may indicate time spent in warmer, lower latitude waters during calving and nursing. A preliminary evaluation of cookie cutter shark bite freshness (phase) between seasons from the data included in this study revealed no clear temporal trends, however, differences in sample size between different times of year prevented a more rigorous statistical examination of temporal trends in cookie cutter shark bite freshness (Supplementary Table S2).
We determined that New Zealand blue whales also have a high rate of blister presence (80.4%). Blister lesions have been reported previously on blue whale skin in New Zealand (Olson et al., 2015), Chile (Brownell et al., 2007), and the Gulf of California, Mexico (Martinez-Levasseur et al., 2011). In Chile, blister lesions were observed on 76.4% of photo-identified blue whales (Brownell et al., 2007). The rate of blister presence for blue whales in the Gulf of California was reported to be between 60–80%, which is substantially higher than the prevalence of blisters for sperm whales (Physeter macrocephalus, 40–60%), and fin whales (Balaenoptera physalus, 0–10%) in the same study region (Martinez-Levasseur et al., 2011). Blisters have also been reported for 17.3% of North Atlantic right whales (Eubalaena glacialis) photographed in the Cape Cod Bay and Bay of Fundy regions of the northeast coast of North America (Hamilton and Marx, 2005). It therefore appears that blue whales may exhibit higher blister prevalence than other large whale species, and that among blue whale populations, the New Zealand population may exhibit comparable or somewhat elevated rates of blister presence.
The hypothesis that cookie cutter shark bite and blister severity accumulate at a similar rate (H3) was rejected (Figure 3 and Table 3), indicating that the causes for these two lesion types are distinct and unrelated. There was a positive relationship between blister severity and the number of starburst lesions, indicating that these two lesion types could be related (H4). Based on morphological characteristics, it is possible that ruptured blisters could lead to starburst lesions, however, this causal link is purely speculative. Blister severity was also significantly higher for whales with a hole in the dorsal fin, once again indicating a relationship between the two skin morphologies (H6). Blisters were observed on the dorsal fins of blue whales in several cases (Figure 6), lending support to this hypothesis. While the cause of blistering on cetacean skin is not definitively known or linked to a single causal factor, ultraviolet (UV) radiation from sun exposure has been suggested as a possible cause (Martinez-Levasseur et al., 2011). Light pigmentation may make blue whales particularly vulnerable to UV radiation and consequently increased blistering. In the STB of New Zealand, blue whale prey is surface oriented leading to the frequent observation of surface feeding during the austral summer (Torres et al., unpublished), which may also result in more UV exposure during summer foraging. Although not quantified in this study, analysts noted that blistering appeared common on the caudal peduncle of blue whales, which may be a region of the whale subjected to increased UV radiation, lending support to this hypothesis.
The hypothesis that skin lesions accumulate over time and can be used as a proxy for age class (H2) could not be fully explored in this study due to a low sample size of individual re-sightings and no known-age individuals (other than calves). It would therefore be valuable for this skin condition assessment method to be applied to cetacean photo-identification catalogs for populations with more re-sightings and known-age individuals. In addition to accumulating markings over time, some lesions heal over time, as evidenced by the example in Figure 5. A skin condition assessment with more re-sighted individuals than available for this study would therefore also be useful in elucidating rates of lesion accumulation and healing over time.
In this study, we did not observe the tattoo-like skin disease documented on a blue whale in Chile (Brownell et al., 2007) or rake mark scars from killer whale predation that were described for blue whales in Gulf of California (Martinez-Levasseur et al., 2011). The tattoo-like skin disease observed on blue whales Chilean waters (Brownell et al., 2007) is caused by poxviruses, and has been observed on marine mammal species worldwide, including small odontocetes in New Zealand (Van Bressem et al., 2009). Based on our assessment, poxvirus appears to be absent from the New Zealand blue whale population at the time of this study. Killer whales do not prey primarily on marine mammals in New Zealand waters; rather they specialize on feeding on elasmobranchs such as stingrays (Visser, 1999, 2005). The absence of killer whale rake mark scars in our study implies that blue whales are not a target of killer whale predation in New Zealand.
In order to infer causes and health implications of skin markings on cetaceans, future studies would benefit from pairing skin condition with other data streams such as stress and reproductive hormone levels and body condition. While we have documented seemingly high rates of blister lesions and cookie cutter shark bites in the New Zealand blue whale population, it remains to be seen whether there are associated health implications such as increased stress levels, energetic costs, or infection risk. The comprehensive protocol for quantifying skin condition from any cetacean photo-identification catalog established in this study sets the stage for pairing skin condition information with other data streams to explore causal links between skin condition and underlying health of cetacean populations. Precautions taken to minimize bias included a standardized assessment area, reviewing poor-quality photographs first, and assessment by multiple observers. The methods are repeatable and can be utilized to obtain baseline information, test hypotheses (Table 3), and track changes in skin condition over time, all at both the individual and population level.
Cetacean health and physiology is difficult to study and monitor due to the logistical limitations of studying wild cetaceans, particularly large baleen whales (e.g., they cannot be held in captivity, skin tissue samples are difficult to collect non-invasively, and repeated samples of individuals can be difficult to obtain). Skin condition is variable within and between populations, and is likely indicative of both extrinsic factors such as sun exposure, prey availability and quality, and pollution, as well as intrinsic factors such as stress hormone levels, body condition, nutritional state, and immune system status. Skin condition can be assessed non-invasively via photographs, and many extensive photographic catalogs exist for wild cetacean populations worldwide. These catalogs allow for comparison of individuals and populations over time, as well as comparison between populations and species. We recommend that such photographic archives be used to explore biological questions about skin marking sources and implications, and ultimately be used to evaluate individual and population health for cetaceans.
Data Availability Statement
The skin condition assessment dataset generated in this study is available upon request to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by the New Zealand Department of Conservation.
Author Contributions
DB contributed to the project development, data collection, and data processing, and led the data analysis, manuscript writing. AP led the skin condition assessment protocol development, data processing, and contributed to the manuscript preparation. LT conceived the project, acquired funding for fieldwork, oversaw the data collection and analysis, and contributed to the manuscript preparation.
Funding
This research was supported by the Aotearoa Foundation, the New Zealand Department of Conservation, the Marine Mammal Institute at Oregon State University, Greenpeace New Zealand, OceanCare, Kiwis against Seabed Mining, the International Fund for Animal Welfare, the Thorpe Foundation, and an anonymous donor.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The project was accomplished through the dedicated work and support of many individuals including the crew of the RV Star Keys (Western Work Boats, Ltd.) and the RV Ikatere (National Institute of Water and Atmospheric Research, Ltd.), Kathy Minta and Minda Stiles from Oregon State University, Mike Ogle, Callum Lilley, Ian Angus, Laura Boren, Hannah Hendriks, Andrew Lamason, and Dave Lundquist from the New Zealand Department of Conservation, and Kristin Hodge and Chris Tessaglia-Hymes from the Cornell University Bioacoustics Research Program. Blue whale photo contribution is also recognized from OMV, Ltd., Petroleum Geo-Services, the Australian Antarctic Division, The University of Auckland, Massey University, Auckland Whale and Dolphin Safari, Whale Watch Kaikoura, and the following individuals: Olive Andrews, Haley Baxter, Aneke Bowker, Jaime Brown, Deanna Clement, Sonja Clemens, Rochelle Constantine, Tony Crocker, Eric de Boer, Nico de la Brosse, Mike Double, Sarah Dwyer, Deanna Elvines, Viraj Gamage, Sarah Gardner, Pete Gill, Dan Govier, Theresa Kirchner, Krista Hupman, Catherine Lea, Helen McConnell, Don Neale, Paula Olson, Catherine Peters, Karen Stockin, Terry Visser, Jody Weir, and Roger Williams.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2019.00757/full#supplementary-material
References
Barlow, D. R., Torres, L. G., Hodge, K. B., Steel, D., Baker, C. S., Chandler, T. E., et al. (2018). Documentation of a New Zealand blue whale population based on multiple lines of evidence. Endanger. Species Res. 36, 27–40. doi: 10.3354/esr00891
Berg, W., Jolly, A., Rambeloarivony, H., Andrianome, V., and Rasamimanana, H. (2009). A scoring system for coat and tail condition in ringtailed lemurs. Lemur catta. Am. J. Primatol. 71, 183–190. doi: 10.1002/ajp.20652
Best, P. B., and Photopoulou, T. (2016). Identifying the “demon whale-biter”: patterns of scarring on large whales attributed to a Cookie-Cutter Shark Isistius sp. PLoS One 11:e0152643. doi: 10.1371/journal.pone.0152643
Bradford, A. L., Weller, D. W., Ivashchenko, Y. V., Burdin, A. M., and Brownell, R. L. (2009). Anthropogenic scarring of western gray whales (Eschrichtius robustus). Mar. Mamm. Sci. 25, 161–175. doi: 10.1111/j.1748-7692.2008.00253.x
Brownell, R. L., Carlson, C. A., Galletti Vernazzani, B., and Cabrera, E. (2007). Skin Lesions on Blue Whales off Southern Chile: Possible Conservation Implications? Washington DC: U.S. Department of Commerce.
Dwyer, S. L., and Visser, I. N. (2011). Cookie cutter shark (Isistius sp.) bites on cetaceans, with particular reference to killer whales (Orca) (Orcinus orca). Aquat. Mamm. 37, 111–138. doi: 10.1578/AM.37.2.2011.111
Gallo−Reynoso, J. −P., and Figueroa−Carranza, A. −L. (1992). A Cookiecutter Shark wound on a guadalupe fur seal male. Mar. Mamm. Sci. 8, 428–430. doi: 10.1111/j.1748-7692.1992.tb00060.x
Hamilton, P. K., and Marx, M. K. (2005). Skin lesions on North Atlantic right whales: categories, prevalence and change in occurrence in the 1990s. Dis. Aquat. Organ. 68, 71–82. doi: 10.3354/dao068071
Heithaus, M. R. (2001). Predator-prey and competitive interactions between sharks (order Selachii) and dolphins (suborder Odontoceti): a review. J. Zool. 253, 53–68. doi: 10.1017/S0952836901000061
Hoyos-Padilla, M., Papastamatiou, Y. P., O’Sullivan, J., and Lowe, C. G. (2013). Observation of an attack by a Cookiecutter Shark (Isistius brasiliensis) on a White Shark (Carcharodon carcharias). Pacific Sci. 67, 129–134. doi: 10.2984/67.1.10
Jahn, A. E., and Haedrich, R. L. (1987). Notes on the pelagic squaloid shark Isistius brasiliensis. Biol. Oceanogr. 5, 297–309. doi: 10.1080/01965581.1987.10749519
Jones, E. C. (1971). Isistius brasiliensis, a squaloid shark, the probable cause of crater wounds on fishes and cetaceans. Fish Bull. 69, 791–798.
Le Boeuf, B. J., McCosker, J. E., and Hewitt, J. (1987). Crater wounds on NOrthern elephant seals: the cookiecutter shark strikes again. fish. Bulletin 85, 387–392.
Mackintosh, N. A., and Wheeler, J. F. G. (1929). Southern blue and fin whales. Dis. Rep. 1, 257–540.
Maldini, D., Riggin, J., Cecchetti, A., and Cotter, M. P. (2010). Prevalence of epidermal conditions in California coastal bottlenose dolphins (Tursiops truncatus) in monterey bay. Ambio 39, 455–462. doi: 10.1007/s13280-010-0066-8
Martinez-Levasseur, L. M., Gendron, D., Knell, R. J., O’Toole, E. A., Singh, M., and Acevedo-Whitehouse, K. (2011). Acute sun damage and photoprotective responses in whales. Proc. R. Soc. B Biol. Sci. 278, 1581–1586. doi: 10.1098/rspb.2010.1903
Nakano, H., and Tabuchi, M. (1990). Occurrence of the Cookiecutter Shark Isistius brasiliensis in surface waters of the North Pacific Ocean. Japanese J. Ichthyol. 37, 60–63.
Oleaga, Á, Casais, R., Balseiro, A., Espí, A., Llaneza, L., Hartasánchez, A., et al. (2011). New techniques for an old disease: sarcoptic mange in the Iberian wolf. Vet. Parasitol. 181, 255–266. doi: 10.1016/j.vetpar.2011.04.036
Olson, P. A., Ensor, P., Olavarria, C., Bott, N., Constantine, R., Weir, J., et al. (2015). New Zealand blue whales: residency, morphology, and feeding behavior of a little-known population. Pacific Sci. 69, 477–485. doi: 10.2984/69.4.4
Papastamatiou, Y. P., Wetherbee, B. M., O’Sullivan, J., Goodmanlowe, G. D., and Lowe, C. G. (2010). Foraging ecology of Cookiecutter sharks (Isistius brasiliensis) on pelagic fishes in Hawaii, inferred from prey bite wounds. Environ. Biol. Fish. 88, 361–368.
Sears, R., Williamson, M. J., Wenzel, F. W., Bérubé, M., Gendron, D., and Jones, P. (1990). Photographic identification of the Blue Whale (Balaenoptera musculus) in the Gulf of St. Lawrence, Canada. Rep. Int. Whal. Comm. Spec. Issue 12, 335–342.
Thompson, P., and Hammond, P. (1992). The Use of photography to monitor dermal disease in wild bottlenose dolphins (Tursiops truncatus). Ambio 21, 135–137.
Torres, L. G. (2013). Evidence for an unrecognised blue whale foraging ground in New Zealand. New Zeal. J. Mar. Freshw. Res. 47, 235–248. doi: 10.1080/00288330.2013.773919
Van Bressem, M. F., Gaspar, R., and Aznar, F. J. (2003). Epidemiology of tattoo skin disease in bottlenose dolphins tursiops truncatus from the Sado estuary, Portugal. Dis. Aquat. Organ. 56, 171–179. doi: 10.3354/dao056171
Van Bressem, M. F., Van Waerebeek, K., Aznar, F. J., Raga, J. A., Jepson, P. D., Duignan, P., et al. (2009). Epidemiological pattern of tattoo skin disease: a potential general health indicator for cetaceans. Dis. Aquat. Organ. 85, 225–237. doi: 10.3354/dao02080
Van Utrecht, W. L. (1959). Wounds and scars in the skin of the common porpoise, phocaena phocaena (L.). Mammalia 23, 100–122. doi: 10.1515/mamm.1959.23.1.100
Visser, I. (1999). Benthic foraging on stingrays by killer whales (.Orcinus orca) In New Zealand waters. Mar. Mamm. Sci. 15, 220–227. doi: 10.1111/j.1748-7692.1999.tb00793.x
Visser, I. N. (2005). First observations of feeding on thresher alopias vulpinus and hammerhead (Sphyrna zygaena Sharks by Killer Whales (Orcinus orca), Aquat. Mamm. 31, 83–88. doi: 10.1578/am.31.1.2005.83
Wemmer, C., Krishnamurthy, V., Shrestha, S., Hayek, L. A., Thant, M., and Nanjappa, K. A. (2006). Assessment of body condition in Asian elephants (Elephas maximus). Zoo Biol. 25, 187–200. doi: 10.1002/zoo.20099
Wilson, B., Grellier, K., Hammond, P. S., Brown, G., and Thompson, P. M. (2000). Changing occurrence of epidermal lesions in wild bottlenose dolphins. Mar. Ecol. Prog. Ser. 205, 283–290. doi: 10.3354/meps205283
Keywords: skin condition, blue whale, New Zealand, cetacean, skin lesions, cookie cutter shark, health
Citation: Barlow DR, Pepper AL and Torres LG (2019) Skin Deep: An Assessment of New Zealand Blue Whale Skin Condition. Front. Mar. Sci. 6:757. doi: 10.3389/fmars.2019.00757
Received: 31 July 2019; Accepted: 21 November 2019;
Published: 05 December 2019.
Edited by:
Lyne Morissette, M – Expertise Marine, CanadaReviewed by:
Marc Girondot, Université Paris-Sud, FranceSimon David Berrow, Galway-Mayo Institute of Technology, Ireland
Copyright © 2019 Barlow, Pepper and Torres. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dawn R. Barlow, dawn.barlow@oregonstate.edu
 Dawn R. Barlow
Dawn R. Barlow Acacia L. Pepper
Acacia L. Pepper Leigh G. Torres
Leigh G. Torres