Integrating Multiple Techniques to Estimate Population Size of an Impacted Dusky Dolphin’s Population in Patagonia, Argentina
- 1Marine Mammal Laboratory, Center for the Study of Marine Systems, CENPAT-CONICET, Puerto Madryn, Argentina
- 2Universidad Nacional de la Patagonia San Juan Bosco, Puerto Madryn, Argentina
Successful conservation strategies require an accurate estimation of population size, and this parameter is one of the most important factors that determine the rate of various evolutionary processes. In this study, we integrated information from genetic, photo-identification, and aerial surveys of dusky dolphins to determine the effective population size and the number of dolphins that inhabit the gulfs of Península Valdés [Golfo Nuevo (GN) and Golfo San José (GSJ)] and are exposed to tourism vessel traffic. Photo-identification sessions and biopsies samples were collected from a research vessel between 2004 and 2013 to estimate the number of dolphins in the area by capture–recapture methods and the effective population size using mtDNA data. In addition, four aerial surveys were carried out using line transects to estimate density via distance sampling. The long-term effective size estimated was 992.87–1,103.19 individuals. During the photo-identification period and by applying a mark rate of 0.416, the total estimated individuals in GN was 374 (95% CI: 237–511). For GSJ, the estimated individuals in the area were 55 (95% CI: 2–108, mark rate 0.692). From aerial surveys, the overall density estimate was D⌢ = 0.13 dolphins/km2 (95% CI: 0.052–0.35; CV: 49.60). The estimation for GN was 325 (95% CI: 130–875), whereas for GSJ, it was 104 (95% CI: 42–280) individuals. These are the first estimations of the number and the effective population size for dusky dolphins in a small-scale area along north Patagonia. Dusky dolphins groups are impacted by anthropogenic activities; therefore, the information reported here contributes population information to help to the species management and conservation.
Introduction
Information on population status of a species is essential to implement correct management actions (Sveegaard et al., 2015). Such information is not always available for wild animal populations; and for marine mammals, especially cetaceans, it is even more difficult to obtain their often extensive geographic ranges and their ability to travel long distances. Successful conservation strategies require an accurate estimation of population size, and this parameter is one of the most important factors that determine the rate of various evolutionary processes, for example, the loss of genetic diversity and ability to evolve in response to environmental change and the deleterious effects of inbreeding on reproduction and survival (Frankham, 2003; Ansmann et al., 2013). However, knowledge of the total number of individuals in a population is not sufficient for an accurate description of these evolutionary processes. Effective population size (Ne) is the number of reproductive adults that will produce the next generation and determines the amount of genetic variability retained in a population. Thus, the effective population size is normally substantially lower, being around 1/3 to 1/10 of the census sizes (Frankham, 1995).
Several studies in marine mammals estimated Ne based on mitochondrial DNA, reflecting female effective population size, Nef (Harlin et al., 2003; Curtis et al., 2011; Oliveira et al., 2016). Mitochondrial DNA is maternally transmitted, without recombination or conversion events between molecules from different female lines (Avise et al., 1988); regardless, an estimate of Ne based on nuclear markers should be approximately equal to twice that based on mitochondrial markers in a panmictic species with a 1:1 sex ratio (Avise et al., 1988; Curtis et al., 2011). Deviations in the sex ratio may act as an enhancer of inbreeding effects on extinction rate, owing to the effect of sex ratio on effective population size (Mills and Smouse, 1994). For free-ranging cetaceans, the identification of the sex of an individual is difficult, because usually there is no sexual dimorphism. Therefore, sex identification through analyses of molecular markers is a commonly used tool in the study of wild populations of cetaceans (e.g., Shaw et al., 2003; Oliveira et al., 2009, 2016).
The dusky dolphin Lagenorhynchus obscurus are found in temperate and cold-temperate waters in the Southern Hemisphere. In the Argentine Sea, dusky dolphins are distributed from 38°00′S to 56°00′S (Figure 1). Genetic analysis for dusky dolphins along the Argentina coast using both microsatellites and mtDNA suggests the existence of one panmictic population where gene flow is mediated by males (Loizaga de Castro, 2013). Nonetheless, on the basis of maternally inherited markers, mtDNA, an additional partition, was identified, where the three northern Patagonian gulfs [Golfo San Matías, Golfo San José (GSJ), and Golfo Nuevo (GN)] are isolated from southern gulf (Golfo San Jorge) and insular population at Tierra del Fuego. Moreover, the analyses performed on the complete data set show strong patterns of phylogeographic structuring at a local scale (Loizaga de Castro et al., 2016). Loizaga de Castro (2013) identified a total of 33 haplotypes among Argentinean dusky dolphins. Eight unique matrilines (24% private haplotypes) were found among dolphins sampled in GN, whereas only four unique matrilines (12%) were reported in Golfo San Jose.
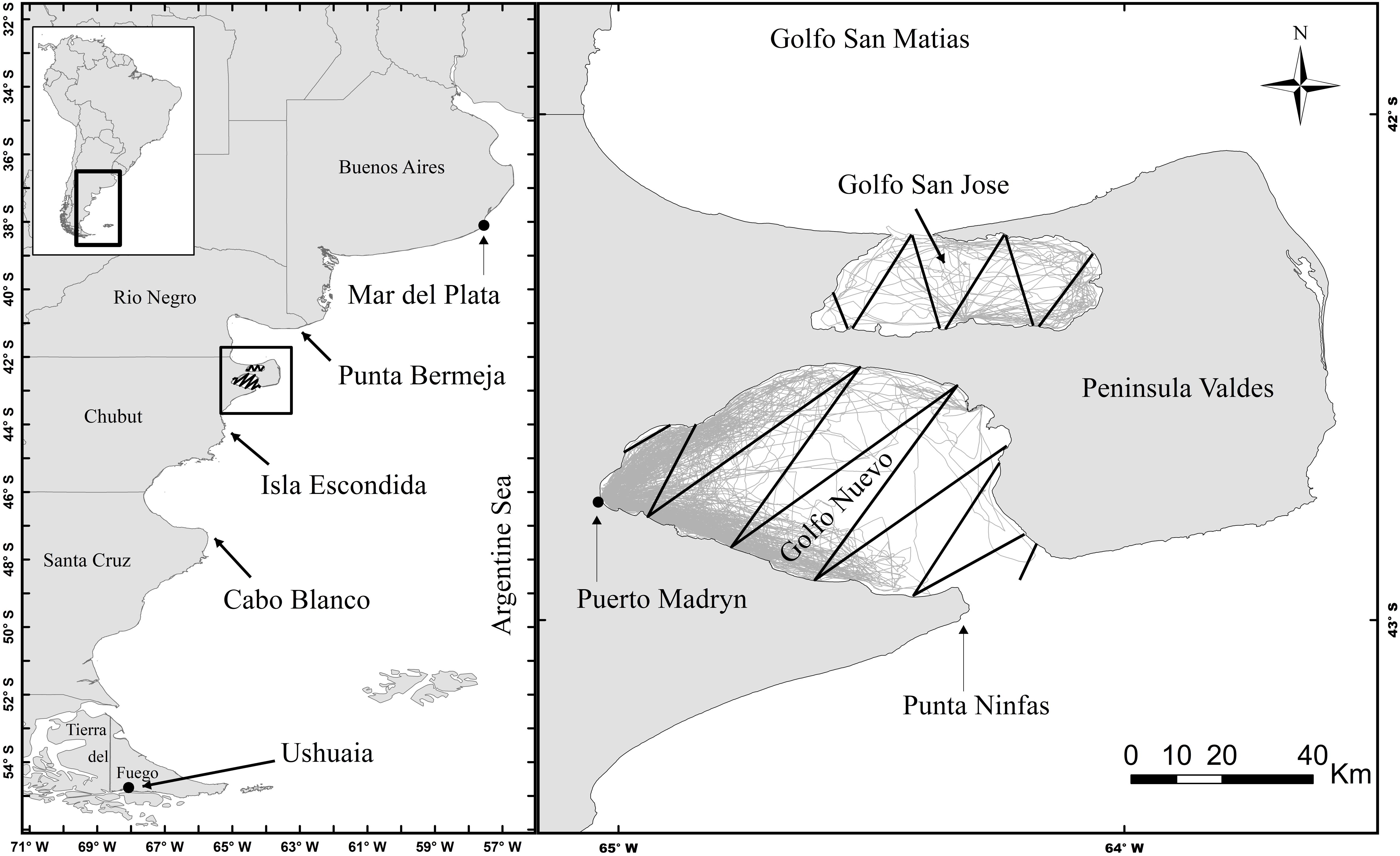
Figure 1. Study area with the line transect covered by aerial surveys in black and trackline of the vessel-based surveys in gray, in Golfo Nuevo and Golfo San José.
Dusky dolphins are subject to multiple anthropogenic impacts along its distribution (Crespo et al., 1997a; Bastida and Rodríguez, 2003). The main threat to this specie is incidental mortality (Crespo et al., 1997b, 2000), and high levels were reported during the 1990s (Corcuera et al., 1994; Crespo et al., 1997b; Dans et al., 2003). Since 1999, dolphin watching has increased in popularity among tourists in GN. Coscarella et al. (2003), and Dans et al. (2008) evaluated the effect of tour vessels on the behavioral budget of dusky dolphins. These studies showed that feeding behavior and energy budget are affected by presence of tour boats. These effects on feeding behavior could add energetic costs and have long-term effect like reduction in individual’s survival and reproductive capacity (Dans et al., 2012). In addition, the presence of private haplotypes in this area indicates that only a specific part of the population is being affected by this activity (Loizaga de Castro, 2013; Loizaga de Castro et al., 2016). Therefore, the potential for several different sources of disturbance to be impacting dusky dolphins at different geographic scales should be considered when designing and implementing conservation and management strategies for dusky dolphins in Argentine waters.
The only published population size estimate for dusky dolphins in Argentine waters was 6,628 individuals (0.148 individuals/km2) in 1995 in central Patagonia (Schiavini et al., 1999) from Punta Ninfas (42°58′S–64°19′W) to Cabo Blanco (47°12′S–65°44′W, Figure 1). This estimate came from aerial surveys and should be considered as a lower density value (Schiavini et al., 1999). This estimation was done for a large area, and abundance estimates at smaller spatial scales are not published yet. Recently, an estimation for the North of Golfo San Matías (North of Península Valdés, Figure 1) was performed with a result of 0.7 dolphins/km2, in an area of 1,550 km2 (Curcio, 2019).
Although the International Union for Conservation of Nature (IUCN) Red List status for this species is “Least Concern” (Alafaro-Shiguieto et al., 2019; Degrati et al., 2019a), population structure and abundance have been little studied in most areas. The absence of abundance data precludes any assessment of trends for dusky dolphins, and intensive photo-identification, genetic, and survey studies are recommended by the IUCN in all areas (Alafaro-Shiguieto et al., 2019). In this study, we integrated information from genetic, photo-identification, and aerial surveys of dusky dolphins to determine the effective population size and the number of dolphins that inhabit the gulfs of Península Valdés and are exposed to the tourism vessels in GN. On the one hand, the estimation of the long-term effective population size based on mtDNA provides the number of reproductive adults that are found all along the northern Patagonian gulfs (Golfo San Matías, GSJ, and GN), although through the capture–recapture and aerial line transect methodology, the number of dolphins that inhabit the gulfs of Península Valdés (GSJ and GN) was estimated. These results will increase knowledge of the dusky dolphins in Argentinean waters and will provide information essential to monitoring this population, which is subject to different anthropogenic pressures throughout their distribution.
Materials and Methods
The study area includes GN and GSJ. These gulfs surround Península Valdes (42°30′S, 64°40′W, Figure 1), a protected area declared a World Heritage Site by the United Nations Educational, Scientific and Cultural Organization (UNESCO) in 1999. GN represents the area where dolphin watching is conducted during the austral summer.
Sampling Procedures
Boat Surveys
Photo-identification data and biopsy samples were collected from a research vessel. Two research vessels were used: a 7-m fiberglass boat with a 105-hp outboard engine and an inflatable boat of 6 m with a 60-hp outboard engine. A non-systematic search method was used to locate dolphins. A group of dolphins was defined as any collection of individuals located in close proximity (<10 m) to one another (Smolker et al., 1992; Degrati et al., 2008), moving together and displaying a similar activity. Surveys were completed during the daytime and in good weather conditions (Beaufort Sea state ≤ 4). The research vessel maintained a distance of <50 m from the focal group. Groups were followed until they were lost from view or sighting conditions became too poor to continue the observation. Each trip lasted in average 05:20 h (min 01:25 h, max 09:00 h). Sampling days, number of dusky dolphin photo-identification groups, and number of dolphins biopsied during the vessel-based surveys are summarized in Table 1.
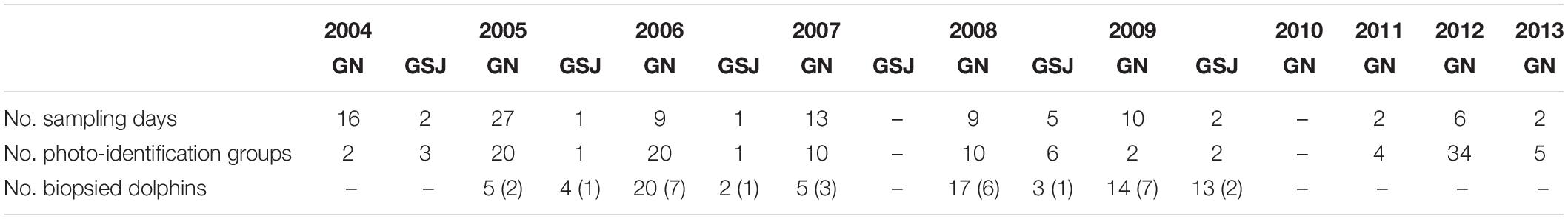
Table 1. Sampling days, number of dusky dolphin photo-identification groups, and number of dolphins biopsied during the nautical surveys; the numbers in brackets indicate the number of groups the biopsies were derived from.
Skin biopsies were collected from June 2005 to May 2009 with a biopsy pole, following Loizaga de Castro et al. (2013). Dusky dolphins that were traveling parallel and close to the vessel (bow-riding dolphins) were sampled. During a typical sampling event, the person taking the biopsy pole stood at the front of the vessel, with another observer close behind recording the biopsy sampling response and taking notes on the sampled individual (e.g., color pattern, scars on the dorsal fin, and sex if possible). Most dolphins returned to the research vessel after being sampled and continued bow-riding. It was easy to observe the round, white biopsy wound in the dolphins, helping to avoid these animals for further sampling. Collection of samples was approved by Secretaría de Turismo and Dirección de Fauna y Flora Silvestre de la Provincia del Chubut (Research permit no. 23/07). Skin samples were exported under CITES permit no. 032423, 31374/75/76, extended by Ministerio de Salud y Ambiente Secretaria de Ambiente y Desarrollo Sustentable, Buenos Aires, Argentina.
For purposes of individual photo-identification, digital images were obtained from November 2004 to February 2013, using Nikon digital cameras (D80 and D2H) with 70–300 and 80–200 mm lenses. As many photographs of dolphins as possible were collected during group observations (Würsig and Jefferson, 1990). Identification of dusky dolphin individuals was based on identifiable mark(s) on the dorsal fin (Würsig and Jefferson, 1990). For each photo-identification session, the researcher attempted to photograph every individual in a group, and preferentially photographing any particular individual was avoided. Only adult and juvenile dolphins with a pattern of nicks and notches along the trailing edge of a dorsal fin to allow future re-identification were included in the photo-identification data set (Degrati et al., 2019b).
Aerial Surveys
Four flights were carried out on fixed, predetermined (designed with the DISTANCE 3.5) routes used as line transects to estimate density via distance sampling (Figure 1; Burnham and Anderson, 2002). Between 2003 and 2015 during spring or summer, each flight covered all tracklines present in both gulfs, consistently flying at an altitude of 500 ft while average speed of the aircraft remained constant at 90 knots (Crespo et al., 2015, 2019). The surveys were carried out using high-wing, double-engine aircraft and flat windows with a blind angle of 30° (Cessna B-337). Each survey involved a crew of four: one pilot, one recorder, and one observer on each side of the plane. The data recorded were species, number of animals, location and time (obtained from a GPS), observer making the sighting, angle between the horizon and the target when perpendicular to the aircraft (estimated using a hand-held clinometer), and environmental conditions, including the Beaufort Sea state (≤3), sun glare, percent cloud cover, and visibility (Gómez de Segura et al., 2006).
Data Analysis
Estimation of the Effective Population Size–DNA Analysis
DNA was extracted from tissue samples preserved in salt saturated with 20% dimethyl sulfoxide (DMSO; Amos and Hoezel, 1991) by a standard phenol/chloroform extraction method (Hoelzel, 1992). The mitochondrial DNA control region was amplified with the primers MTCRf (5′-TTCCCCGGTGTAAACC) and MTCRr (5′-ATTTTCAGTGTCTTGCTTT; Hoelzel and Green, 1998) with the following PCR profile: 5 min at 95°C; then 35 cycles of 1 min at 51°C, 1:30 min at 72°C and 45 s at 94°C; and then 1 min at 51°C at last a final extension of 8 min at 72°C. All PCRs (25 μl, final volume) contained 1 μl of DNA template (approximately 10 ng), 10 mM of Tris-HCl (pH 8.4), 2 mM of MgCl2, 200 μM OF each dNTP, 0.3 μM of each primer, and 0.5–1 unit of Taq polymerase. PCR products were purified with QIAGEN PCR purification columns. Individuals were sexed by amplifying portions of the genes ZFX and ZFY as described in Bérubé and Palsbøll (1996). The χ2 test with α = 0.05 was used to verify if the resulting sex ratio deviates significantly from expected ratio of 1:1.
Long-term female effective population size (Nef) based on genetic diversity from the mtDNA control region was estimated as follows:
where μ = mutational rate per generation and θ = genetic diversity (estimated here by π; Avise et al., 1988). Generation time (g) estimated for dusky dolphin and used for calculation was 12.82 (Marine Mammal Laboratory, unpublished data) with a mutation rate of 6.3 × 10–7 to 7.0 × 10–7 (Harlin et al., 2003). The generation time g was calculated from survivorship and fecundity schedules from a sample of dusky dolphins incidentally caught by fisheries and stranded dolphins in Patagonia (Dans, 1999), which is the average length of time between the birth of an individual and the birth of one of its own offspring. This, being an average, is the sum of all these birth-to-birth times, divided by the total number of offspring (Begon et al., 2006):
where x = age (years); lx = survivorship [proportion of original cohort surviving to the cohort dying during age (years) beginning of age-class x]; mx = the age-specific fecundity (the fecundity per surviving individual).
Estimation of the Number of Individuals in the Area of Península Valdés by Capture–Recapture (Photo-Identification Analysis)
Cataloging of photographic records was accomplished using the program DARWIN 2.22 (Digital Analysis and Recognition of Whales Images on a Network) PhotoId Software. Photographs were ranked based on focus, angle, and fin exposure in bad (Q0), ordinary (Q1), good (Q2), and very good quality (Q3), with Q3 being the highest-quality photographs, in focus with the dorsal fin perpendicular to the photographer. Q2 photographs are of lower quality than Q3 but contained sufficient information to identify animals and permit recapture between years (Hammond et al., 1990; Würsig and Jefferson, 1990; Gowans et al., 2000). Only Q2 and Q3 photographs were included in the catalog. In some cases, additional body markings like scars and rake marks were used as secondary characteristics to identify some individuals. Final matching decisions were made by MD.
The number of animals with long-lasting marks identified in the area (Nm) was estimated using the population analysis module of SOCPROG v.2.7 (Whitehead, 2009). A 1-year sampling interval was used to obtain an inter-year abundance estimate during the period 2004–2013 in GN and 2004–2009 in GSJ. The following models were run: (1) “Closed Schnabel,” which assumes a closed population; (2) “mortality,” which assumes a population of constant size where mortality (which may include permanent emigration) is balanced by birth (which may include immigration); (3) “mortality-trend,” which assumes a population growing or declining at a constant rate; (4) “reimmigration,” in this model, members of a closed population move from (emigration rate) and into (reimmigration rate) a study area; and (5) “reimmigration-mortality,” which is the reimmigration model with the exception that mortality (which may include permanent emigration from the total population) is balanced by birth (which may include immigration; Whitehead, 1990; Gowans et al., 2000; Pearson et al., 2012; Podestà et al., 2016). Akaike information criterion (AIC) values were used to select the most appropriate model (Burnham and Anderson, 2002). If models’ difference in AIC was less than 2, they were both considered as plausible models (Burnham and Anderson, 2002). Non-parametric bootstrap estimates (n = 100) of the standards errors (SEs) and 95% confidence intervals of the parameters were used (Buckland and Garthwaite, 1991).
The number of individuals with and without long-lasting marks was determined by taking photographs of all dolphins at random for each survey. Then the number of animals that use the area (N) was estimated by applying the mark rate (θ), which was obtained by calculating the ratio of number of photographs of animals with marks to the total number of dolphins photographed (both with and without marks; Williams et al., 1993; Wilson et al., 1999).
where Nm is the mark-recapture estimate of the number of animals with long-lasting mark identified in the area. The variance and standard error were calculated as follows:
Variance was estimated using the delta method (Wilson et al., 1999), where n is the total number of animals from which θ was estimated. The final confidence intervals assume the same error distribution as the estimate of the distinctive population estimate (Read et al., 2003; Ansmann et al., 2013).
Estimation of the Number of Individuals in the Area of Península Valdés by Aerial Line Transect (Distance Sampling)
Data were analyzed using DISTANCE 6.2 software (Thomas et al., 2010). Perpendicular sighting distance of each dusky dolphin group sighting was estimated by converting the recorded angle to distance in meters using standard trigonometry. Observations were used to estimate dolphin density and dolphin abundance as the product of dusky dolphin density and the survey area.
Each transect was treated as a replicate unit of sampling effort. The effective strip half-width was determined using the computer program DISTANCE 6.2 to fit the PSD data to a uniform distribution function, a half-normal, and the hazard-rate model and evaluating the probability density function model at the transect (Lerczak and Hobbs, 1998; Buckland et al., 2001), where each transect is treated as a replicate sample Buckland et al. (2001) that defined density as follows:
where
i = i, …, k, for k transects;
n = the number of dusky dolphin groups sightings on transect i;
= the expected group size; and
l = the length of transect i.
Results
Estimation of the Effective Population Size–DNA Analysis
A total of 84 sequences were obtained from biopsied dusky dolphin individuals. Two sequences belonging to GN were removed because of bad quality. All sequences were confirmed to represent different animals on the basis of the microsatellite comparisons described in Loizaga de Castro (2013). As GN and GSJ are not genetically isolated, all samples were pooled together for further genetic analysis. Nucleotide diversity π was estimated using DnaSP 4.00 (Rozas et al., 2003) as π = 0.00891. The female effective population size was estimated to be between 496.43 and 551.59 individuals. No significant difference from a 1:1 sex ratio was detected for sampled individuals at any of the two sampling locations. At GN, 63 individuals were sexed (nfemales = 36, nmales = 27, χ2 Yates = 0.39, df = 1, and p = 0.534). On the other hand, for GSJ, 22 individuals were sexed (nfemales = 11, nmales = 11, χ2 Yates = 0.09, df = 1, and p = 0.763). Considering that no sex bias was detected, a total long-term effective size (Ne) was estimated for dusky dolphins of 992.87–1,103.19, by doubling the females’ estimate.
Estimation of the Number of Individuals in the Area of Península Valdés by Capture–Recapture (Photo-Identification Analysis)
During the photo-identification period, a total of 190 individuals were identified based on 2,466 dorsal fin records in GN. From GSJ, 80 individuals were identified based on 282 dorsal fin records. Between 40% and 50% dolphins were sighted more than one time in GSJ and GN, respectively. Any given individual was observed during a mean of two sampling sessions (with a maximum of 6 for GSJ and 8 for GN, Figure 2). Seven dusky dolphins were sighted in both areas. The minimum interval between resightings was 1 day, and the maximum was 7.5 years.
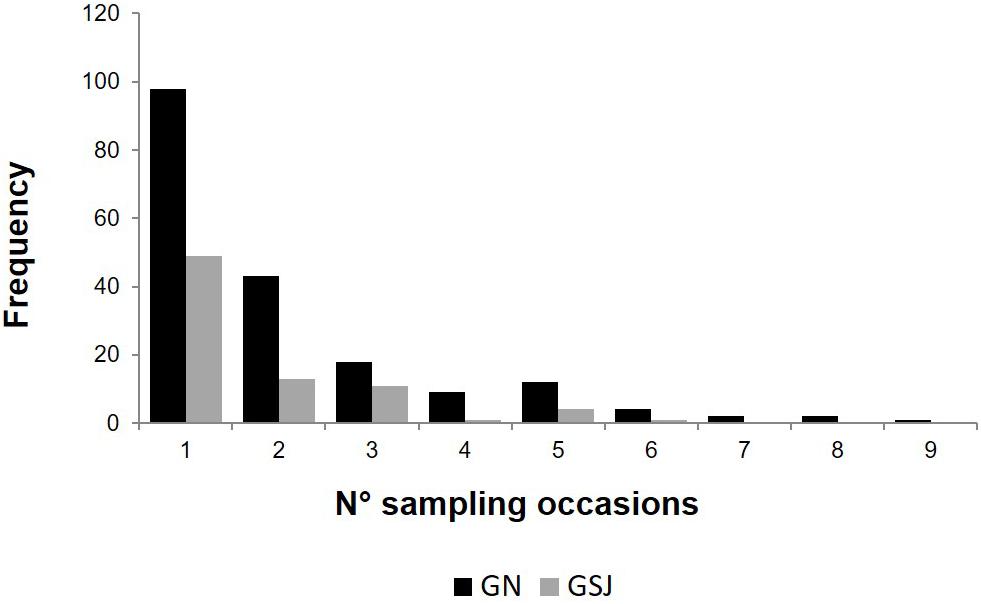
Figure 2. Histogram of dusky dolphin sightings by sampling period in Golfo Nuevo (GN) and Golfo San José (GSJ).
With the use of a 1-year sampling interval, the estimated number of marked dusky dolphins found in GN was 156 (mortality model, 95% CI: 119–212); by applying a mark rate of 0.416, the total estimated individuals in the area was 374 (95% CI: 237–511, Table 2). For GSJ, the estimated number of marked dolphins was 38 (Mortality + Trend model, 95% CI: 24–75). By applying a mark rate of 0.692, the total estimated individuals in the area was 55 (95% CI: 2–108, Table 2).
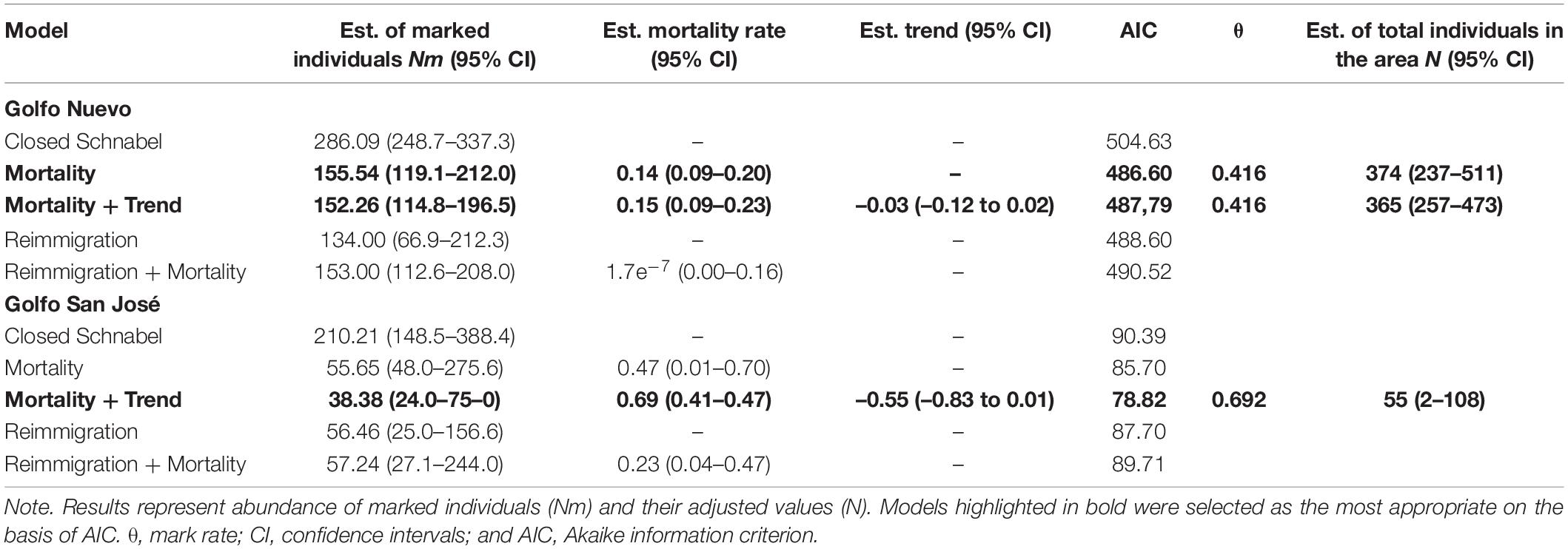
Table 2. Inter-year population abundance model results for dusky dolphins observed in Golfo Nuevo and Golfo San José.
Mortality + Trend model has a difference in AIC of less than two units with respect to the Mortality model; therefore, it was considered to fit the data equally well (view Table 2 for estimations). The Mortality model assumes a population of constant size, with mortality and/or permanent emigration balanced by birth and/or permanent immigration, whereas the Mortality + Trend model assumes a population growing, or declining at a constant rate.
Estimation of the Number of Individuals in the Area of Península Valdés by Aerial Line Transect (Distance Sampling)
The estimation by means of aerial line transects was made by adjusting to a half-normal model without expansion series. The overall density estimate was dolphins/km2 (95% CI: 0.052–0.35; CV 49.60) for aerial surveys covering the surface area of both gulfs. The estimation for GN was 325 (95% CI: 130–875) individuals, considering an area of 2,500 km2, whereas for GSJ, it was 104 (95% CI: 42–280) individuals, and considering an area of 800 km2.
Discussion
These are the first estimates of abundance, effective population size, and sex ratio for the Patagonian population of dusky dolphins surrounding Península Valdés. The only published abundance estimate for dusky dolphins along Patagonia corresponded to a bigger area (Schiavini et al., 1999) and used aerial surveys as sampling platform. In the present study, different methodological approaches were used to assess the Ne and the number of dolphins that inhabit the gulfs of Península Valdés and are target of dolphin-watching activities.
The estimates derived from both techniques, capture–recapture and aerial line transects, were similar for dolphins in both gulfs that surround Península Valdés. However, the capture–recapture estimate had higher precision (smaller confidence intervals). The same was observed for an abundance estimation of Hector’s dolphins (Cephalorhynchus hectori) in New Zealand (Gormley et al., 2005) and for humpback whales (Megaptera novaeangliae) in the West Coast of the USA and Mexico (Calambokidis and Barlow, 2004). In our study, aerial survey data represent the number of dolphins using the areas during the warm season (spring and summer). On the other hand, the photo-identification-based mark recapture estimates pertain to all animals that use the area, whether or not they were present during the time of the surveys.
Using two different methods to estimate abundance was useful to evaluate the relative merits and limitations of both during the data analysis. One of the advantages of aerial line transects surveys is that it uses all the observations. Capture–recapture surveys of species do not, because not all of the individuals are marked, and also it is necessary to estimate the mark rate. In addition, aerial line transects surveys take less time, because animals only need to be seen once, whereas capture–recapture methods needs many more sampling days. In this sense, aerial line transects surveys are largely favored. However, the photo-identification data used in capture–recapture estimates of abundance are taken over a much longer period, including animals that stay only occasionally in the sampling area, whether or not all individuals are present at any one time (Gormley et al., 2005; Whitehead, 2009). In addition, capture–recapture methods provide a lot of other information including home range, movement, survival rate, calving interval, longevity, and associations (Hammond et al., 1990; Gormley et al., 2005). Therefore, aerial line transects are the method of choice when the objective is to know the number of dolphins in a given area and are particularly valuable when a rapid assessment of population size is needed to aid in the conservation and management of the population. Nonetheless, if long-term population parameters are needed in order to detect possible population changes, including small-scale distribution shifts or declines in fertility rates that could lead to a population decrease, the capture–recapture method is recommended.
Effective population size is the number of reproductive adults that will produce the next generation and is substantially lower than census sizes, with Ne usually around 1/3 to 1/10 of the census sizes (Frankham, 1995). Given the small geographic scale of this study area, the number of individuals (N) estimated by capture–recapture and aerial line transects is lower than the long-term Ne estimated by using mtDNA nucleotide diversity, reinforcing the hypothesis of a high gene flow and a high rate of renewal of animals between the groups inside and outside the gulfs (Loizaga de Castro, 2013; Loizaga de Castro et al., 2016). However, considering the estimation made by Schiavini et al. (1999), with 6,628 dolphins, and another estimation made in 2004–2005 (Marine Mammal Lab, unpublished data) of 13,438 dolphins in the Patagonian region (between Isla Escondida, 43°50′S 65°20′W and Punta Bermeja 41°10′S 63°00′W, Figure 1), the Ne calculated in the present study, is around the expected value for the dusky dolphin population in Patagonia, Argentina.
Many species of mammals are faithful to their natal and breeding sites or their social groups, and it is usually males that disperse more than females (Greenwood, 1980; Avise, 1995). When a species shows female philopatry, conservation and management efforts should place a stronger emphasis on this sex. For instance, our calculations of Ne only from female nucleotide diversity suggest an effective female population of 496–551. Another Nef estimation of 15,700–17,500 individuals was reported for New Zealand dusky dolphins, using the same mutation rates used in the present study (Harlin et al., 2003).
Wild populations need to be about an order of magnitude 500–5,000 individuals to maintain their evolutionary potential (Franklin and Frankham, 1998; Gautschi et al., 2003). Therefore, dusky dolphin’s effective population size is large enough to cope with environmental changes. However, dolphin-watching activities affect only a small portion of the entire population. Exclusive matrilines have been identified among dolphins sampled in GN (Loizaga de Castro et al., 2016), suggesting female philopatry to this area. To avoid the loss of these females over time, this philopatry should be taken into account when designing conservation and management strategies.
Despite several studies available for dusky dolphins, very little is known about sex ratio. Harlin et al. (2003) at New Zealand waters found that only in Kaikoura and from samples collected in all seasons did the sex ratio equal the expected 1:1. However, in others regions such as Otago and the West Coast, the ratio of females to males was 13: 1 and 7:3, respectively. On the other hand, in Admiralty Bay, all individuals examined were males (Würsig et al., 1997; Harlin et al., 2003), and suggesting that New Zealand dusky dolphin are segregated in time and space.
The dusky dolphin’s effective population size and the number of dolphins found at Patagonia northern gulfs, estimated in the present study, must be taken into consideration when designing and regulating the dolphin-watching activity. All in all, the information reported here contributes population information that is important to the management and conservation of Argentine dusky dolphins. The integration of molecular techniques with behavioral, demographic, and environmental information has proven to be extremely powerful as a conservation tool (Caro, 2007). In order to effectively conserve the dusky dolphins of Argentina, regular monitoring of populations is recommended, and long-term abundance, social structure, behavioral information, and genetic data from several surveys are required to reliably assess populations’ trends.
Data Availability Statement
The datasets generated for this study can be found in the GenBank Accession no. KJ207081–KJ207199.
Ethics Statement
This study was carried out in accordance with the ethical principles approved by Secretaría de Turismo and Dirección de Fauna y Flora Silvestre de la Provincia del Chubut, Argentina (Research permit No. 23/07). Skin samples were exported under CITES permit No. 032423, 31374/75/76, extended by the Ministerio de Salud y Ambiente Secretaria de Ambiente y Desarrollo Sustentable, Buenos Aires, Argentina.
Author Contributions
MD, RL, and SD performed boat surveys/data collection. MD and RL analyzed photo-identification and genetic data. MD, MC, NS, EC, and SD performed aerial surveys. MC and NS analyzed line transect data and prepared the figure. All authors listed have made a substantial, direct and intellectual contribution to the work and approved it for publication.
Funding
This research received logistic and institutional support from Centro Nacional Patagónico (CONICET) and the University of Patagonia (UNPSJB). Funds were received from Agencia Nacional de Promoción Científica y Tecnológica (PICT Nos. 01-4030, PICT No. 11679 and 33934), CONICET (PID 320/99, PIP 2015-2017-112 201501-00615CO), Fundación BBVA BIOCON 04 (2005–2008), Fundación BBVA BIOCON 08 (2009–2012), Project PNUD ARG 02/018 (B-B27), Fundación Vida Silvestre Argentina, and The Mohamed Bin Zayed species Conservation Fund (2011–2014). Nautical fieldwork was assisted by Hydrosport SRL, J. Owen, members of Marine Mammals Laboratory, and personnel of the nautical service of CESIMAR-CENPAT-CONICET.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We mainly acknowledge G. Garaffo for her help during field work. This work was carried out under permits of the Dirección de Fauna y Flora Silvestre and Secretaría de Turismo de la Provincia de Chubut and complies with the current laws of Argentina.
References
Alafaro-Shiguieto, J., Crespo, E., Elwen, S., Lundquist, D., and Mangel, J. (2019). Lagenorhynchus obscurus (amended version of 2019 assessment). The IUCN Red List of Threatened Species 2019: e.T11146A160698904. Available at: https://dx.doi.org/10.2305/IUCN.UK.2019-1.RLTS.T11146A160698904.en (accessed April 27, 2020).
Amos, W., and Hoezel, A. R. (1991). Long-term preservation of whale skin for DNA analysis. Rep. Int. Whal. Commiss. Spec. Issue 13, 99–103.
Ansmann, I. C., Lanyon, J. M., Seddon, J. M., and Parra, G. J. (2013). Monitoring dolphins in an urban marine system: total and effective population size estimates of Indo-Pacific Bottlenose Dolphins in Moreton Bay, Australia. PLoS One 8:e65239. doi: 10.1371/journal.pone.0065239
Avise, J. C. (1995). Mitochondrial DNA polymorphism and a connection between genetics and demography of relevance to conservation. Conserv. Biol. 9, 686–690.
Avise, J. C., Ball, R. M., and Arnold, J. (1988). Current versus historical population sizes in vertebrate species with a high gene flow: a comparison based on mitochondrial DNA lineages and inbreeding theory for neutral mutations. Mol. Biol. Evol. 5, 331–344. doi: 10.1093/oxfordjournals.molbev.a040504
Bastida, R., and Rodríguez, D. (2003). Mamíferos Marinos de Patagonia y Antártida, 1st Edn. Argentina: Vázquez Maziini eds Buenos Aires.
Begon, M., Townsend, C. R., and Harper, J. L. (2006). “Life, death and lifes histories,” in Ecology: From Individuals to Ecosystems, 4th Edn, Ed. J. L. Harper, (New York, NY: Blackwell Publication).
Bérubé, M., and Palsbøll, P. J. (1996). Identification of sex in cetaceans by multiplexing with three ZFX and ZFY specific primers. Mol. Ecol. 5, 283–287. doi: 10.1111/j.1365-294x.1996.tb00315.x
Buckland, S. T., Anderson, D. R., Burnham, K. P., Laake, J. L., Borchers, D. L., and Thomas, L. (2001). Introduction to Distance Sampling. Oxford: Oxford University Press.
Buckland, S. T., and Garthwaite, P. H. (1991). Quantifying precision of mark-recapture estimates using the bootstrap and related methods. Biometrics 47, 255–268.
Burnham, K. P., and Anderson, D. R. (2002). Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd Edn. New York, NY: Springer-Verlag.
Calambokidis, J., and Barlow, J. (2004). Abundance of blue and humpback whales in the eastern north Pacific estimated by capture-recapture and line-transect methods. Mar. Mamm. Sci. 20, 63–85.
Caro, T. M. (2007). Behavior and conservation: a bridge too far? Trends Ecol. Evol. 22, 394–400. doi: 10.1016/j.tree.2007.06.003
Corcuera, J., Monzon, F., Crespo, E. A., Aguilar, A., and Raga, J. A. (1994). Interactions between marine mammals and the coastal fisheries of Necochea and Claromecó (Buenos Aires Province, Argentina). Gillnets and Cetaceans. Proceedings of the Symposium and Workshop on Mortality of Cetaceans in Passive Fishing Nets and Traps. Rep. Int. Whal.Commiss. Spec. Issue 15, 283–290.
Coscarella, M. A., Dans, S. L., Crespo, E. A., and Pedraza, S. N. (2003). Potential Impact of dolphin watching unregulated activities in Patagonia. J. Cetacean Res. Manag. 1, 77–84.
Crespo, E. A., Koen-Alonso, M., Dans, S. L., García, N. A., Pedraza, S. N., Coscarella, M. A., et al. (2000). Incidental catch of dolphins in mid-water trawls for southern anchovy off Patagonia. J. Cetacean Res. Manag. 2, 11–16.
Crespo, E. A., Pedraza, S. N., Coscarella, M., Garcia, N. A., Dans, S. L., Iñiguez, M., et al. (1997a). Distribution Of Dusky Dolphins (Lagenorhynchus Obscurus (Gray, 1828)), In The Southwestern Atlantic Ocean With Notes On The Size Of Herds. Rep. Int. Whal. Comm. 47, 693–698.
Crespo, E. A., Pedraza, S. N., Dans, S. L., Koen Alonso, M., Reyes, L. M., Garcia, N. A., et al. (1997b). Direct and indirect effects of the highseas fisheries on the marine mammal populations in the northern and central patagonian coast. J. Northwest Atl. Fish. Sci. 22, 189–207.
Crespo, E. A., Pedraza, S. N., Dans, S. L., Coscarella, M. A., Svendsen, G. M., Degrati, M., et al. (2015). More Whales Eubalaena Australis And Decreasing Trend. San Diego, CA: Report of International Whaling Commission.
Crespo, E. A., Pedraza, S. N., Dans, S. L., Svendsen, G. M., Degrati, M., and Coscarella, M. A. (2019). Southern Right Whales Eubalaena australis still growing but at a decelerated speed. Mar. Mamm. Sci. 35, 93–107. doi: 10.1111/mms.12526
Curcio, N. (2019). Distribución y uso del hábitat de dos especies de delfines simpátricos en Golfo San Matías: relaciones con los factores abióticos y la disponibilidad de presas. PhD Thesis, Universidad Nacional de Buenos Aires, Argentina.
Curtis, C., Stewart, B. S., and Karl, S. A. (2011). Genetically effective population sizes of Antarctic seals estimated from nuclear genes. Conserv. Genet. 12, 1435–1446. doi: 10.1007/s10592-011-0241-x
Dans, S. L. (1999). Ecología poblacional del delfín oscuro Lagenorhynchus obscurus (Gray, 1828) en el litoral patagónico, Atlántico Sudoccidental. PhD Thesis, Universidad de Buenos Aires, Argentina
Dans, S. L., Crespo, E. A., Koen Alonso, M., and Pedraza, S. N. (2003). Incidental catch of dolphins in trawling fisheries off Patagonia, Argentina: can populations persist? Ecol. Appl. 13, 754–762.
Dans, S. L., Crespo, E. A., Pedraza, S. N., Degrati, M., and Garaffo, G. V. (2008). Dusky dolphins and tourist interaction: effect on diurnal feeding behavior. Mar. Ecol. Progr. Ser. 369, 287–296.
Dans, S. L., Degrati, M., Pedraza, S. N., and Crespo, E. A. (2012). Tour boats effects on dolphins behavior: a sensitivity analysis applied to Markov chains. Conserv. Biol. 26, 708–716. doi: 10.1111/j.1523-1739.2012.01844.x
Degrati, M., Dans, S. L., Pedraza, S. N., Crespo, E. A., and Garaffo, G. V. (2008). Diurnal behaviour of dusky dolphins. Lagenorhynchus obscurus, in Golfo Nuevo. J. Mamm. 89, 1241–1247.
Degrati, M., Dellabianca, N. A., García, N. A., Loizaga de Castro, R., Mandiola, A., and Romero, M. A. (2019a). “Lagenorhynchus obscurus,” in Categorización 2019 de los mamíferos de Argentina según su riesgo de Extinción, eds SAyDS and SAREM. Lista Roja de los mamíferos de Argentina. Digital version: http://cma.sarem.org.ar
Degrati, M., Coscarella, M., Crespo, E., and Dans, S. (2019b). Dusky dolphins group dynamics and association patterns in Península Valdés, Argentina. Mar. Mamm. Sci. 35, 416–433. doi: 10.1111/mms.12536
Frankham, R. (1995). Effective population size/adult population size ratios in wildlife: a review. Genet. Res. 66, 95–107. doi: 10.1017/S0016672308009695
Franklin, I. R., and Frankham, R. (1998). How large must populations be to retain evolutionary potential. Anim. Conserv. 1, 69–73.
Gautschi, B., Müller, J., and Schmid, B. (2003). Effective number of breeders and maintenance of genetic diversity in the captive bearded vulture population. Heredity 91, 9–16. doi: 10.1038/sj.hdy.6800278
Gómez de Segura, A., Crespo, E. A., Pedraza, S. N., Hammond, P. S., and Raga, J. A. (2006). Abundance of small cetaceans in waters of the central Spanish Mediterranean. Mar. Biol. 150, 149–160.
Gormley, A., Dawson, S. M., Slooten, E., and Bräger, S. (2005). Mark-recapture estimates of Hector’s dolphin abundance at Banks Peninsula. New Zealand. Mar. Mamm. Sci. 21, 204–216.
Gowans, S., Whitehead, H., Arch, J. K., and Hooker, S. K. (2000). Population size and residency patterns of northern bottlenose whales (Hyperoodon ampullatus) using the Gully. Nova Scotia. J. Cetacean Res. Manag. 2, 201–210.
Greenwood, P. J. (1980). Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 28, 1140–1162.
Hammond, P. S., Mizroch, S. A., and Donovan, G. P. (1990). Individual Recognition of cetaceans: Use of photo-identification and other techniques to estimate population parameters. Rep. Int. Whal. Comm. Special Issue 12, 1–448. doi: 10.1371/journal.pone.0198167
Harlin, A. D., Markowitz, T., Baker, C. S., Würsig, B., and Honeycutt, R. L. (2003). Genetic structure, diversity, and historical demography of New Zealand’s dusky dolphin (Lagenorhynchus obscurus). J. Mamm. 84, 702–717.
Hoelzel, A. R. (1992). Molecular Genetic Analysis of Populations: A Practical Approach. Oxford: Oxford University Press.
Hoelzel, A. R., and Green, A. (1998). “PCR protocols and popu- lation analysis by direct DNA sequencing and PCR-based fingerprinting,” in Molecular Genetic Analysis Of Population, A Practical Approach, 2nd Edn, ed. A. R. Hoelzel, (Oxford: Oxford University Press).
Lerczak, J. A., and Hobbs, R. C. (1998). Calculating sighting distances from angular readings during shipboard, aerial and shore-based marine mammal surveys. Mar. Mammal Sci. 14, 590–598.
Loizaga de Castro, R. (2013). Genética de Poblaciones del delfín oscuro, Lagenorhynchus obscurus, en la costa de Argentina. PhD Thesis, Universidad Nacional del Comahue, Bariloche.
Loizaga de Castro, R., Dans, S. L., and Crespo, E. A. (2016). Spatial genetic structure of dusky dolphin, Lagenorhynchus obscurus, along the argentine coast: preserve what scale? Aquatic Conserv: Mar. Freshw. Ecosys. 26, 173–183.
Loizaga de Castro, R., Hoelzel, A. R., and Crespo, E. A. (2013). Behavioral responses of argentine coastal dusky dolphins, Lagenorhynchus obscurus, to a biopsy pole system. Anim. Welf. 22, 13–23.
Mills, L. S., and Smouse, P. E. (1994). Demographic consequences of inbreeding in remnant populations. Am. Nat. 144, 412–431. doi: 10.1086/285684
Oliveira, L. R., Ott, P. H., Flores, P. A., Siciliano, S., Almeida, R., and Bonatto, S. L. (2009). First molecular estimate of sex ratio of southern right whale calves. Eubalaena australis, for Brazilian waters. J. Mar. Biol. Assoc. U.K. 89, 1003–1007.
Oliveira, L. R., Ott, P. H., Moreno, I. B., Tavares, M., Siciliano, S., and Bonatto, S. L. (2016). Effective population size of an offshore population of bottlenose dolphins, Tursiops truncatus, from the São Pedro and São Paulo Archipelago, Brazil. Latin Am. J. Aquat. Mamm. 11, 162–169. doi: 10.5597/lajam00225
Pearson, H. C., Vaughn-Hirshorn, R. L., Srinivasan, M., and Würsig, B. (2012). Avoidance of mussel farms by dusky dolphins (Lagenorhynchus obscurus) in New Zealand. New. Zeal. J. Mar. Fresh. 46, 567–574. doi: 10.1080/00288330.2012.712977
Podestà, M., Azzellino, A., Cañadas, A., Frantzis, A., Moulins, A., Rosso, M., et al. (2016). “Chapter Four - Cuvier’s Beaked Whale, Ziphius cavirostris, distribution and occurrence in the mediterranean sea: high-use areas and conservation threats,” in Advances in Marine Biology, Vol. 75, eds G. Notarbartolo Di Sciara, M. Podestà, and B. E. Curry, (Cambridge, MA: Academic Press), 103–140.
Read, A. J., Urian, K. W., Wilson, B., and Waples, D. M. (2003). Abundance of bottlenose dolphins in the bays, sounds, and estuaries of North Carolina. Mar. Mamm. Sci. 1, 59–073.
Rozas, J., Sánchez-Del Barrio, J. C., Messeguer, X., and Rozas, R. (2003). DNASP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19, 2496–2497.
Schiavini, A., Pedraza, S. N., Crespo, E. A., González, R., and Dans, S. L. (1999). The abundance of dusky dolphins (Lagenorhynchus obscurus) in spring, off North and Central Patagonia, Argentina, and a comparison with the incidental catch in fisheries. Mar. Mamm. Sci. 3, 828–840.
Shaw, C., Wilson, P., and White, B. N. (2003). A reliable molecular method of gender determination for mammals. J. Mamm. 84, 123–128.
Smolker, R. A., Richards, A. F., Connor, R. C., and Pepper, J. W. (1992). Sex differences in patterns of association among Indian Ocean bottlenose dolphins. Behaviour 123, 38–69.
Sveegaard, S., Galatius, A., Dietz, R., Kyhn, L., Koblitz, J. C., Amundin, M., et al. (2015). Defining management units for cetaceans by combining genetics, morphology, acoustics and satellite tracking. Glob. Ecol. Conserv. 3, 839–850.
Thomas, L., Buckland, S. T., Rexstad, E. A., Laake, J. L., Strindberg, S., Hedley, S. L., et al. (2010). Distance software: design and analysis of distance sampling surveys for estimating population size. J. Appl. Ecol. 47, 5–14.
Whitehead, H. (1990). Mark-recapture estimates with emigration and re-immigration. Biometrics 46, 473–479.
Whitehead, H. (2009). SOCPROG programs: Analyzing animal social structures. Behav. Ecol. Sociobiol. 63, 765–778.
Williams, J. A., Dawson, S. M., and Slooten, E. (1993). The abundance of and distribution of bottlenose dolphins (Tursiops truncatus) in Doubtful Sound. New Zealand. Can. J. Zool. 71, 2080–2088.
Wilson, B., Hammond, P. S., and Thompson, P. M. (1999). Estimating size and assessing trends in a coastal bottlenose dolphin population. Ecol. Appl. 1, 288–300.
Würsig, B., Cipriano, F., Slooten, E., Barr, K., Yin, S., and Constantine, R. (1997). Dusky dolphins (Lagenorhynchus obscurus) off New Zealand: status of present knowledge. Rep. Inter. Whal. Comm. 47, 715–722.
Keywords: effective population size, abundance, genetic, photo-identification, dusky dolphins, conservation
Citation: Degrati M, Loizaga R, Coscarella MA, Sueyro N, Crespo EA and Dans SL (2020) Integrating Multiple Techniques to Estimate Population Size of an Impacted Dusky Dolphin’s Population in Patagonia, Argentina. Front. Mar. Sci. 7:289. doi: 10.3389/fmars.2020.00289
Received: 18 December 2019; Accepted: 09 April 2020;
Published: 19 May 2020.
Edited by:
Jeremy Kiszka, Florida International University, United StatesReviewed by:
Phil J. Bouchet, University of St. Andrews, United KingdomAimee R. Lang, Southwest Fisheries Science Center (NOAA), United States
Copyright © 2020 Degrati, Loizaga, Coscarella, Sueyro, Crespo and Dans. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mariana Degrati, degrati@cenpat-conicet.gob.ar
 Mariana Degrati
Mariana Degrati Rocio Loizaga
Rocio Loizaga Mariano A. Coscarella
Mariano A. Coscarella Nicolás Sueyro
Nicolás Sueyro Enrique A. Crespo1,2
Enrique A. Crespo1,2