Hurricane Frequency and Intensity May Decrease Dispersal of Kemp’s Ridley Sea Turtle Hatchlings in the Gulf of Mexico
- 1Biology Department, University of West Florida, Pensacola, FL, United States
- 2LGL Ecological Research Associates, Bryan, TX, United States
Environmental variability can be an important factor in the population dynamics of many species. In marine systems, for instance, whether environmental conditions facilitate or impede the movements of juvenile animals to nursery habitat can have a large influence on subsequent population abundance. Both subtle differences in the position of oceanographic features (such as meandering currents) and major disturbances (such as hurricanes) can greatly alter dispersal outcomes. Here, we use an ocean circulation model to explore seasonal and annual variation in the dispersal of post-hatchling Kemp’s ridley sea turtles (Lepidochelys kempii). We simulated the transport of 24 cohorts of young-of-the-year Kemp’s ridley sea turtles dispersing from the three primary nesting areas in the western Gulf of Mexico to describe variability in transport during the main hatching season and across years. We examined whether differences in transport distance among Kemp’s ridley cohorts could be explained by hurricane events. We found that years with high numbers of hurricanes corresponded to shorter dispersal distances and less variance within the first 6 months. Our findings suggest that differences in dispersal among sites and the impact of hurricane frequency and intensity could influence the survivorship and somatic growth rates of turtles from different nesting sites and hatching cohorts, either improving survival by encouraging retention in optimal pelagic habitat or decreasing survival by pushing hatchlings into dangerous shallow habitats. Considering such factors in future population assessments may aid in predicting how the potential for increasing tropical storms, a phenomenon linked to climate change, could affect Kemp’s ridley and other populations of sea turtles in the Atlantic Ocean.
Introduction
Many marine species move across widely separated habitats to seek conditions that are favorable for the development, growth, and survival of different life-stages (Harden Jones, 1968; Putman, 2018). These periods of habitat transition are considered “critical periods” for understanding population abundance and may be closely linked to dynamic ocean circulation processes that either facilitate or impede movement into favorable locations (Hjort, 1914; Secor, 2015). In particular, temporal fluctuations in population abundance are hypothesized to be driven by variability in the oceanic transport of juveniles to nursery grounds (Sagarese et al., 2015; Liu et al., 2016). Sea turtles are iconic examples of the life-history strategy in which juveniles disperse long-distances from their natal site to reach nursery habitat (Carr, 1987). Hatchling turtles dig out from nests deposited on sandy beaches, and upon entering the water for the first time, undertake a 1 to 2 day “swimming frenzy” to move offshore as quickly as possible, but hatchlings are small and cannot swim very quickly (Wyneken and Salmon, 1992; Lutz and Musick, 1996). The initial entrance into the water is the most dangerous, and it is estimated that an average of 30% of hatchlings do not make it through the initial gauntlet of nearshore predators (Witherington and Salmon, 1992; Gyuris, 1994). Ocean currents push hatchlings along and assist in dispersal to pelagic habitat where they remain in the open ocean traveling with the major current systems and, at a smaller scale, associating with habitats such as pelagic Sargassum (Carr, 1987; Collard and Ogren, 1990; Witherington et al., 2012). Upon reaching the large juvenile life stage, they depart for coastal waters and their neritic habitat (Witherington et al., 2012; Wildermann et al., 2018). Upon reaching maturity (perhaps a decade or more later), they return to the vicinity of their hatching site to reproduce (Lohmann et al., 2008). If currents move hatchlings offshore faster, they spend less time in the coastal zone, where they are presumed to be most vulnerable to predators (Witherington and Salmon, 1992; Gyuris, 1994). Owing to natal homing, locations that produce more surviving hatchings might also have higher numbers of turtles returning to nest (Putman et al., 2010a).
The role of hatchling dispersal on regional variation in population abundance is well-established: beaches positioned closer to ocean circulation features that consistently aid in the transport of hatchlings to offshore habitats host larger sea turtle populations than beaches further away (Putman et al., 2010a, b; Okuyama et al., 2011; Shillinger et al., 2012; Ascani et al., 2016; Putman, 2018). There are also indications that temporal variability in ocean circulation could influence population dynamics (Ascani et al., 2016; Scott et al., 2017). Intuitively, periods when ocean currents are more favorable for transport should result in higher survival and subsequently higher recruitment into the adult age classes, but a direct link has been challenging to demonstrate. In part, insufficient data has been collected on temporal variability in the dispersal of hatchlings to rigorously test the hypothesis (Arendt et al., 2013).
As a step toward understanding the population-level implications of temporal variability in hatchling sea turtle dispersal, we used an ocean circulation model to simulate the post-hatchling movements of Kemp’s ridley sea turtles (Lepidochelys kempii) from their nesting beaches in the western Gulf of Mexico. Kemp’s ridleys are the smallest sea turtles in both size and abundance (Lutz and Musick, 1996). Kemp’s ridley sea turtles are also somewhat unique in that they often reproduce in large aggregations known as arribadas which occur only a few times in a season (Bevan et al., 2016). They are listed as critically endangered by the IUCN and as endangered by the United States. Endangered Species Act (NMFS, 2015; IUCN, 2019). Their three primary nesting beaches are Rancho Nuevo in Tamaulipas, Mexico, where >90% of the population nests, several beaches in Veracruz, Mexico, and a head-started population established in 1978 at Padre Island, TX, United States (Putman et al., 2013; Putman, 2018). Kemp’s ridley sea turtles spend their first 2 years in oceanic habitats before recruiting to coastal waters along the Gulf of Mexico and the East Coast of the United States (Collard and Ogren, 1990; Putman et al., 2010b). Kemp’s ridley sea turtles reach maturity within 10 to 15 years (Avens et al., 2017) and return to the vicinity of their natal site to reproduce, thus contributing to different demographic and genetic trajectories for distant nesting aggregations (Putman and Lohmann, 2008; Shaver et al., 2016). The Kemp’s ridley sea turtle reached dangerously low numbers in the late 1900s, but responded well to intensive conservation efforts that led to a considerable population increase (Marquez et al., 2005; Bevan et al., 2016). However, since 2010, population growth appears to have abruptly stopped and has been in decline or fluctuation, and intensive management is still necessary to protect this species (NMFS, 2015; Caillouet et al., 2018). Ultimately, understanding how environmental factors influence hatchling dispersal may help increase the accuracy of population assessments that assist conservation managers in decision making for this critically endangered species.
Here, we used “hindcasts” of historical ocean conditions from the Global Hybrid Coordinate Ocean Model (HYCOM) paired with virtual particle-tracking to describe daily variability in transport during the main hatching season (June through July) and annual variability over the past quarter century (Putman et al., 2020). In these simulations, we did not attempt to simulate swimming behavior, as our aim was simply to produce indices that reflect variability in environmental conditions that influence dispersal. To better understand the processes contributing to variation in Kemp’s ridley sea turtle dispersal, we examined whether tropical storm frequency and intensity contributed to yearly differences in transport predictions (Monzón-Argüello et al., 2012). Though hurricanes often have catastrophic effects on biological communities, they also may aid in the dispersal of various species both native and invasive (Eggleston et al., 2010; Johnston and Purkis, 2015; Smith et al., 2017). As hurricane season coincides with nesting and hatching seasons for many populations of sea turtles, it is plausible that hurricanes have acute impacts on nest survival, hatchling survival and hatchling dispersal (Monzón-Argüello et al., 2012). Finally, we discuss how this work to identify environmental factors that influence hatchling dispersal may help increase the accuracy of population assessments.
Methods
To simulate the initial dispersal of hatchling Kemp’s ridley sea turtles during their first year of life, we used the particle tracking software Ichthyop (v. 2.2) and velocity field outputs from the Global Hybrid Coordinate Ocean Model (HYCOM 3.1) (Chassignet et al., 2007; Lett et al., 2008). HYCOM is an eddy-resolving model that assimilates in situ and satellite observations to depict oceanic conditions that occurred at specific times in the past (Chassignet et al., 2007). Global HYCOM portrays ocean circulation features, such as fronts, filaments and mesoscale eddies, which are relevant to the transport of marine organisms (Chassignet et al., 2007; Putman and He, 2013). We obtained HYCOM data for the years 1993 through 2017 from Reanalysis and Hindcast Experiments 19.0, 19.1, 91.0, 91.1, and 91.21.
Using Ichthyop, we released particles from defined release polygons close to the shore of the three main nesting regions of Kemp’s ridley sea turtles. The central points of these polygons are as follows: Padre Island, Texas (27° 14′ 9.78″N, 97° 20′ 42″W), Rancho Nuevo (23° 22′ 12″N, 97° 45′ 20.88″W), Mexico, and Veracruz, Mexico (20° 28′ 49.44″N, 97° 0′ 44.64″W, and 19° 1′ 20.28″N, 95° 58′ 30″W) and correspond to those used in Putman et al. (2019). These release locations encompass the beaches used by the majority of females in the species (Putman et al., 2013; Putman, 2018). To simulate dispersal throughout the height of the hatching season, we released 350 particles day–1 from June 1 to July 31, the primary hatching period for this species (Rostal et al., 1998), and repeated simulations yearly between 1993 and 2016. This 24-year period encompasses a wide range of variability in environmental conditions within the Gulf of Mexico. Particle tracking took place for 12 months after release to model the initial dispersal into the Gulf of Mexico. Ichthyop simulates movement using a Runge-Kutta 4th-order time step method with 30 min time steps and saves the particle locations daily. These simulations of sea turtle dispersal use the same methods as were applied in Putman et al. (2016) that were shown to account for variability in observed turtle distributions. Specifically, dispersal simulations from Rancho Nuevo and Veracruz predicted temporal variation in the number of small juvenile Kemp’s ridley sea turtles that stranded along the west coast of Florida and dispersal simulations from Texas predicted temporal variation in Kemp’s ridley strandings along the Texas coast (Putman et al., 2020). The agreement between model predictions and Kemp’s ridley strandings suggests that these dispersal simulations can be used to examine the role of ocean circulation dynamics on temporal changes in sea turtle movement and distribution (Putman et al., 2020).
In this present study, we calculated the net straight-line distance from each particle’s starting location to its position after 10 days, 6, and 12 months of drift. We measured the straight-line distance (km) using Python (v2.2) and geospatial data from the particle tracking simulation. Thus, for each release day, we calculated the mean and standard deviation of dispersal distance for each of the 350 particles released from a given site. With this data, our first aim was to describe temporal variation in dispersal distance for turtles hatching at different dates during the hatching season and whether differences exist across years. We then sought to explore whether annual variation in dispersal could be accounted for by major storm events (Monzón-Argüello et al., 2012).
To examine if hurricane frequency and severity affect dispersal distance and variability in dispersal distance, we compiled a list of Atlantic hurricanes, including the total number for the entire season, the number in the Gulf of Mexico each year, and the number occurring during the hatching season. We considered storms entering the ocean between the bounds of 16°N and 78°W to be near enough to the Gulf of Mexico to potentially impact Kemp’s ridley dispersal. Due to the wide distribution of hatchlings throughout the Gulf of Mexico as they disperse and the wandering nature of hurricanes, we did not divide the study area further but rather sought a holistic analysis of hurricane impact. We quantified the severity of the hurricanes by two indices. First, the average maximum wind speed of storms in the entire Atlantic season (kph) (NOAA, 2019). Second, we calculated the average maximum wind speed of hurricanes that occurred in the Gulf of Mexico (kph) (NOAA, 2019). These covariates were used to determine the severity of hurricanes as a potential source of environmental variability during neonate dispersal.
We statistically analyzed if dispersal distances at 6 months varied across the three nesting sites and over time. We selected 6 months of drift to statistically evaluate for hurricane impacts, as the first 6 months of life is a critical period for hatchlings and we wanted to investigate the longer term impacts of hurricane frequency and severity on hatchling dispersal, rather than evaluate the short-term effects on early stage (<3 months) neonates. In addition, we tested whether the number of hurricanes that occurred in the Atlantic Ocean and the Gulf of Mexico annually and during the hatching season, the total average peak wind speed in the Atlantic Ocean, and the average maximum wind speed in the Gulf of Mexico resulted in a net lower dispersal distance and smaller standard deviation. All continuous explanatory variables were standardized to be centered at 0 by taking the value minus the mean divided by the standard deviation using the scale function in R, so that effects across variables could be more easily compared. The dispersal distance did not meet the assumptions of a normal distribution, based on a Shapiro-Wilkes test and visual inspection of qq plots. We tested for collinearity of the explanatory variables using variance inflation factors, and all variables were <3, our a priori threshold, and thus all were included in the global model (Zuur et al., 2009). Initial exploratory analyses of the simple model of hatchling dispersal over 6 months regressed with the number of Gulf of Mexico hurricanes and year suggested a degree of temporal autocorrelation, based on ACF plots, and including an autocorrelation structure substantially increased the model fit. As the sites were reasonably far apart (the two closest sites, Padre Island, Texas and Rancho Nuevo, Mexico are 561 km apart), we did not account for spatial autocorrelation (and including this as a model term did not improve fit). Thus, we used a generalized linear mixed effects model (GLMM), with year as random effect (due to the temporal autocorrelation), and a gamma distribution with a log link.
We used the information theoretic approach to evaluate if hurricane frequency and severity were predictors of hatchling dispersal, using the global model Dispersal distancei,j = β0 + β1 ⋅Hurr_gulf + β2 ⋅Hurr_season + β3 ⋅Hurr_hatch + β4⋅Total_avg_wind + β5⋅Peak_wind_gulf + Sitei + ai + εi,j, where ai ∼ N(0,σ2year), and εi,j ∼ N(0, σ2), of site i and year j. We ranked candidate models using Akaike Information Criterion correction for small sample sizes (AICc; Burnham and Anderson, 2002; Hobbs and Hilborn, 2006). AICc, as an index of model fit to the data, balances the maximum log-likelihood and model complexity (i.e., number of model parameters; Burnham and Anderson, 2002; Johnson and Omland, 2004). As such, AICc is a superior metric to assess model fit than traditional adjusted-R2 goodness-of-fit tests commonly used in frequentist statistical approaches (Johnson and Omland, 2004). Models with ΔAICc ≤ 2 from the top ranked model were considered to have comparable fit to the data while balancing parsimony in the number of explanatory variables and were included in the confidence model set. We used evidence ratios to quantify the probability of the top-ranked model compared to the null model, with an intercept only (Burnham and Anderson, 2002).
Results
Temporal Variability in Dispersal Across the Nesting Beach Sites
There was considerable variation in the dispersal distance across time and sites within the first 10 days (Figures 2B,D), a crucial time when the turtles are small and vulnerable (Wyneken and Salmon, 1992). The first 10 days of post-hatching dispersal across all releases from all years had an absolute minimum mean dispersal distance of 22 km and an absolute maximum mean distance of 378 km (Figure 2B). The ocean currents at the three nesting beaches are different due to their positioning within the Gulf of Mexico (Figure 1A). Rancho Nuevo sits at an optimal location for hatchlings as they are transported directly into a large gyre with a western boundary current that takes them into the deep waters of the Gulf of Mexico while allowing them to avoid both inward coastal currents and the Atlantic bound Loop Current in the eastern Gulf of Mexico (Sturges and Blaha, 1976). As such, there is a consistent pattern of dispersal where Rancho Nuevo has the highest dispersal distance, followed by Veracruz, with Padre Island having the lowest yearly and seasonal dispersal (Figures 2B,D).
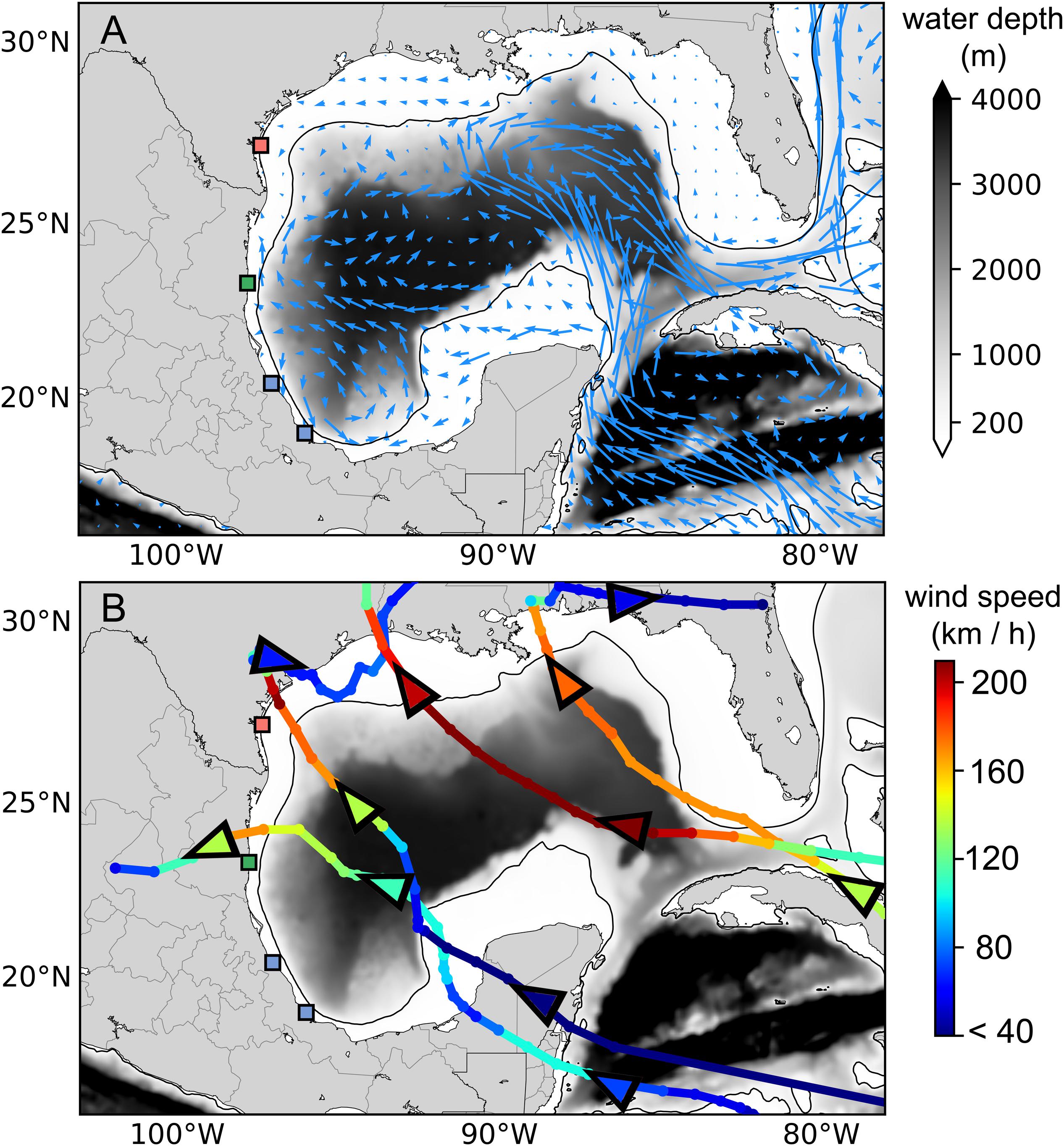
Figure 1. (A) Map of the Gulf of Mexico with major circulation patterns and bathymetry. Nesting beaches of the Kemp’s ridley sea turtle are indicated by colored markers. Sites from top to bottom are Padre Island, TX, United States (Red), Rancho Nuevo, Tamaulipas, Mexico (Green) and Veracruz, Tamaulipas, Mexico (Blue). (B) Map of Gulf of Mexico and Kemp’s ridley sea turtle nesting beaches with the paths of selected hurricanes, Georges (1998), Rita (2005), Alex (2010), and Harvey (2017). Colors of tracks indicate wind speed of the storm, triangles indicate direction of travel.
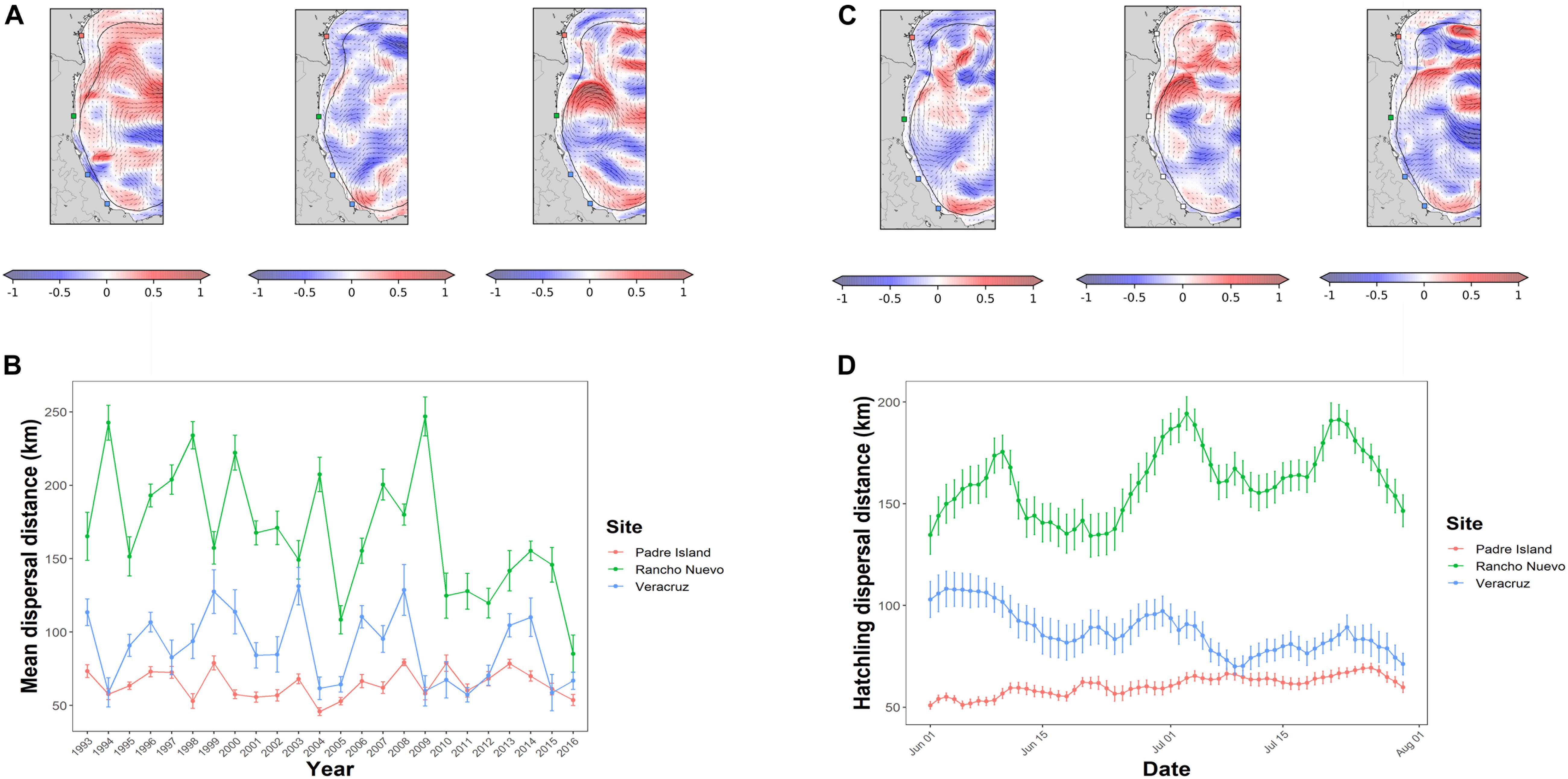
Figure 2. Seasonal and annual variation in hatchling dispersal at 10 days post-hatching. (A) Annual variation in East/West currents (small arrows) on July 1, 2000, July 1, 2013, and July 1, 2016. Red coloration indicates stronger eastward currents, blue coloration indicates stronger westward currents (speed units are m/s). (B) Mean annual dispersal distance after 10 days post-hatching. Error bars indicate the 95% CI. Red corresponds to results for Padre Island, green for Rancho Nuevo, and blue for Veracruz. (C) Seasonal variation in surface currents in the western Gulf of Mexico on June 1, 2015, July 1, 2015, and July 30, 2015. Other conventions as in panel (A). (D) Mean seasonal dispersal distance after 10 days based on the year of hatching. Other conventions as in panel (B).
There were considerable differences in dispersal across years with a range of 45–246 km across the sites (Figures 2A,B). Rancho Nuevo, Veracruz, and Padre Island had average yearly distances of 165 (±54 SD) km, 89 (±47 SD) km, and 64 (±18 SD) km (Figure 2B). There are visible differences in the intensity and placement of currents during different years (Figure 2A). Rancho Nuevo and Veracruz followed a pattern of years with high and low dispersal distance. Padre Island also matched the pattern in several years. In general, and across all three sites, there was a decrease in dispersal distance between 1993 and 2016, which was most evident in Rancho Nuevo (Figure 2A).
In Rancho Nuevo and Veracruz, there were also distinct seasonal peaks in dispersal at 10 days based on what day the turtles hatched. A turtle hatching in early June, early July, and late July traveled much farther and much faster than hatchings in the middle of the month (Figure 2D). The seasonal range was from 50 to 194 km across the sites. Rancho Nuevo, Veracruz, and Padre Island had seasonal average distances of 161 (±67 SD) km, 87 (±54 SD) km, and 60 (±20 SD) km. The Veracruz beaches had smaller within-season peaks than Rancho Nuevo; dispersal started relatively higher and decreased over time with peaks in early June, early July, and late July (Figure 2D). Padre Island lacked within season variability, but instead dispersal distance tended to increase over the season (Figure 2D).
Hurricane Frequency and Intensity as Predictors of Hatchling Dispersal Distance
Across 1993–2016, there were 378 Atlantic hurricanes, with 147 of them passing through or near the Gulf of Mexico, and 64 of them taking place during the hatching season for Kemp’s ridley sea turtles (NOAA, 2019; Supplementary Table S1, see Supplementary Material). 2005 had the highest number of storms (n = 31), while 2014 and 1997 had the lowest (n = 9). The average yearly peak wind speed of the storms, used as a proxy for storm severity, ranged from 54 mph in 1994 to 91 mph in 2004.
We analyzed models predicting mean dispersal distance over 6 and 12 months. We included the candidate explanatory variables of (i) the number of hurricanes in the Gulf of Mexico, (ii) number of hurricanes during the hatchling season only, (iii) the number of hurricanes for the entire season in in the Atlantic and Gulf of Mexico, (iv) peak wind speed, (v) average wind speed, and (vi) nesting beach site (Texas, Rancho Nuevo, and Veracruz). As there was significant temporal autocorrelation in the dataset, we also included year as a repeated effect in the GLMM. Model output for dispersal distance at 12 months was similar, but did not show as strong of relationships as 6 months, so we do not discuss it in the main body (but see Table 1 and Supplementary Material for model output).
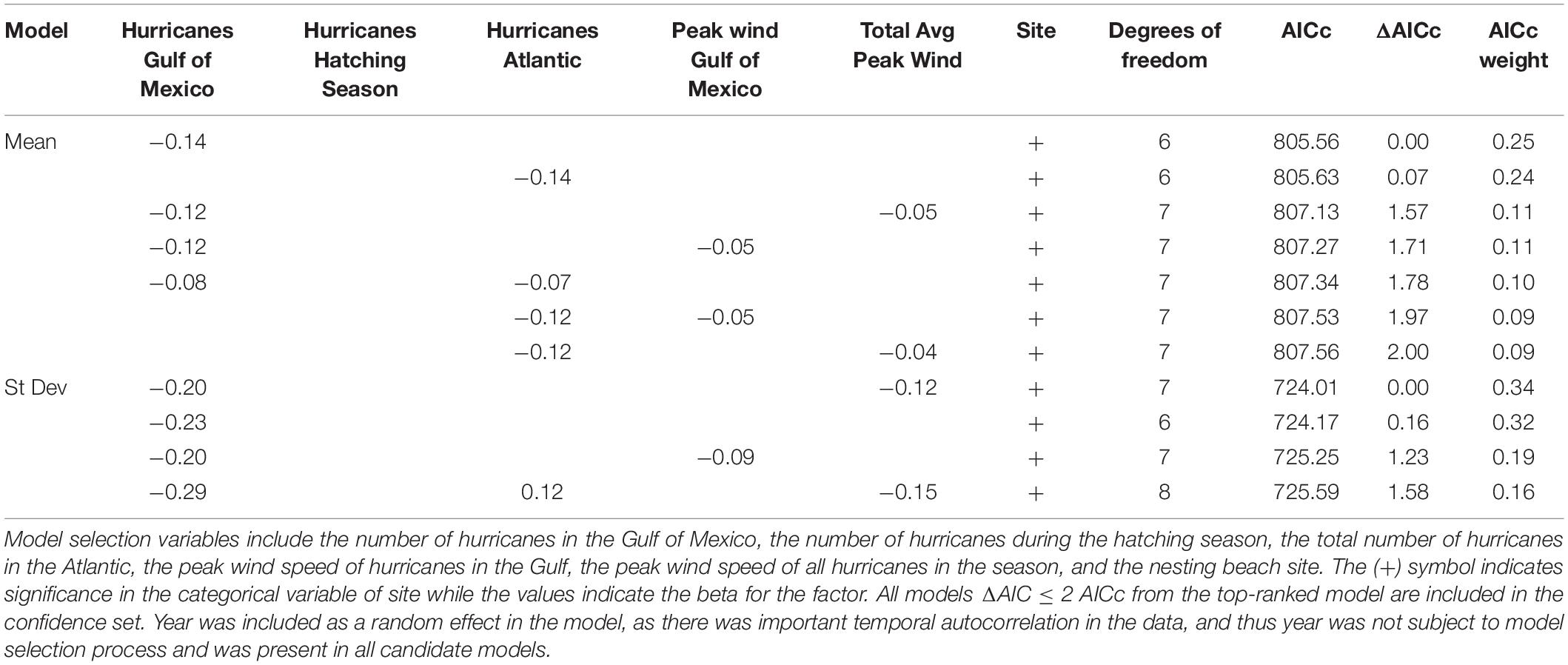
Table 1. Confidence set for models describing mean dispersal distance and standard deviation of dispersal distance at 6 months.
For the mean dispersal distance for 6 months, all models in the confidence set (ΔAICc < 2) included nesting beach site as an explanatory variable, while hurricanes in the Gulf of Mexico, hurricanes in the Atlantic, peak wind speed, or average wind speed were included in some of the models as well (Table 1). Interestingly, the number of hurricanes during the hatching season was not included as an important variable in the confidence set. All models in the confidence set included one of the hurricane frequency variables (hurricanes in the Gulf of Mexico, or hurricanes in the Atlantic). In general, mean dispersal distance at 6 months decreased with increasing hurricanes in the Gulf of Mexico or throughout the Atlantic Ocean (Figures 3A,B). There were strong differences in dispersal distance across the three nesting beach sites, but the negative relationship between dispersal distance and hurricane frequency was conserved across the sites. As the evidence ratio of the top-ranked model, relative to the null model (Dispersal distancei,j = β0 + ai + εi,j) was 1.02. 1025, there is strong support for the effects of hurricane frequency and nesting location on dispersal outcome. The two indices of hurricane severity (total average peak wind speed for the season and the peak wind speed in the Gulf of Mexico) were also important explanatory variables and figured in the confidence model set (Figures 3C,D). Increasing storm severity in a season was correlated with decreased mean dispersal distance, albeit this relationship was not quite as strong as the number of hurricanes (Figure 3).
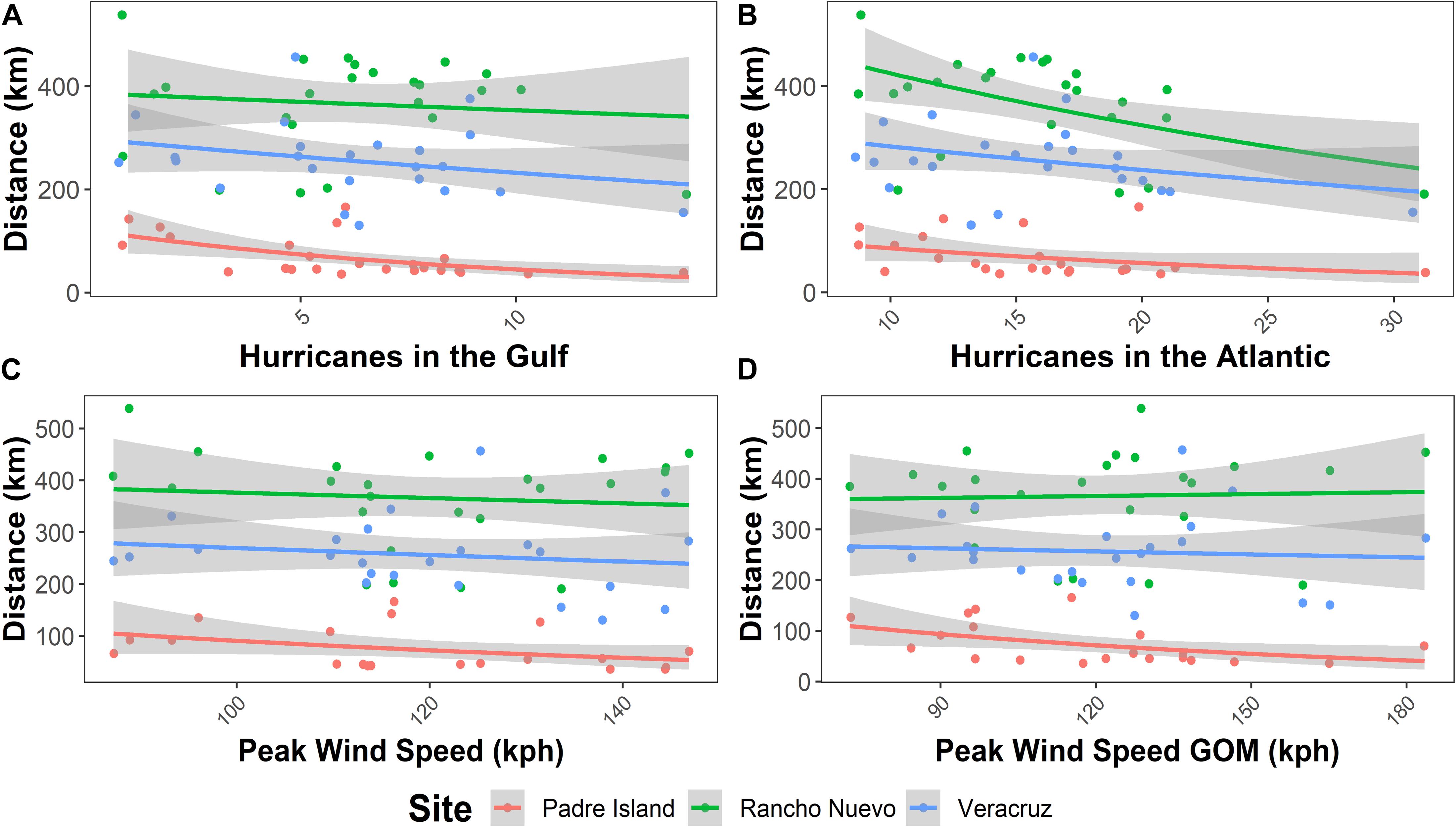
Figure 3. Relationships between mean 6-month dispersal distance and (A) number of hurricanes in the Gulf of Mexico, (B) number of hurricanes in the Atlantic, (C) average peak wind speed for all hurricanes each year (kph), and (D) average peak wind speed for hurricanes in the Gulf of Mexico (kph) from 1993 to 2016. Red corresponds to results for Padre Island, green for Rancho Nuevo, and blue for Veracruz. To improve visualization of the relationships with the explanatory variables we plot unscaled variables though in the statistical analysis we standardized the variables.
To assist in visualizing the effect of hurricanes on hatchling dispersal, we created maps of the years with the greatest and least numbers of storms to illustrate the positions of the released particles after 6 months (Figure 4). In 2005, the year with the highest number of storms, virtually no particles exited the Gulf of Mexico after 6 months - only 0.0047% of particles from Rancho Nuevo and none from Veracruz or Padre Island. In contrast, for years with the lowest numbers of hurricanes, 1997 and 2014, 3.35 and 1.16% of particles from Rancho Nuevo, 0.02 and 0.34% of particles from Veracruz entered the Atlantic within each year, respectively (but still none from Padre Island). The number of hurricanes in the Atlantic was inversely correlated with the percentage of particles entering the Atlantic from Rancho Nuevo and Veracruz within 6 months (Pearson’s r = −0.43, p = 0.036, n = 24 for both), but less so for Padre Island (Pearson’s r = −0.28, p = 0.185, n = 24). In contrast, there was no relationship between the percentage of particles that beached (i.e., advected into the model coastline) and hurricane frequency for Rancho Nuevo and Veracruz (Pearson’s r > −0.10, p > 0.641, n = 24, for both), but a positive relationship was apparent for Padre Island (Pearson’s r = 0.45, p = 0.027, n = 24). Thus, hurricanes appear to differentially impact the dispersal potential from different nesting areas, increasing retention in the Gulf of Mexico for Rancho Nuevo and Veracruz (with little impact on beaching) and increasing beaching for Padre Island (with little impact on transport to the Atlantic).
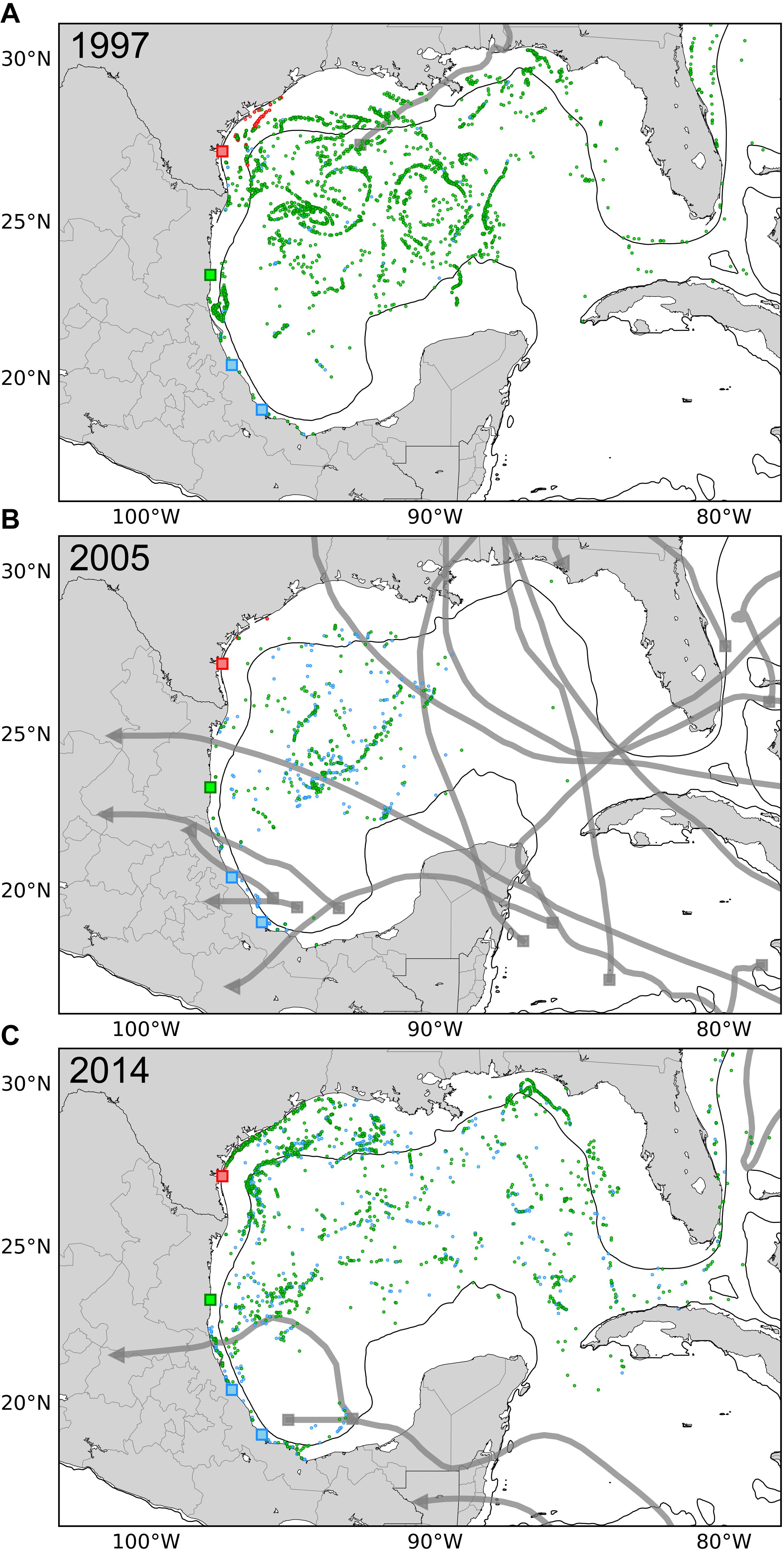
Figure 4. Distribution of virtual particles released from Padre Island (red), Rancho Nuevo (green) and Veracruz (blue). Particles were released from each site (colored squares) and tracked for 6 months (n = 21,000 per year, per site). The thin black line delineates the continental shelf (200 m water depth) and the thicker gray lines indicate paths of hurricanes and tropical storms for a given year. Starting locations of storms are marked by a square, the end location is shown with a triangle. Results are shown for (A) 1997, a year with few hurricanes (9 in the Atlantic, 1 in the Gulf of Mexico); (B) 2005, a year with many hurricanes (31 in the Atlantic, 14 in the Gulf of Mexico); and (C) 2014, a year with few hurricanes (9 in the Atlantic, 2 in the Gulf of Mexico). Differences in the numbers of virtual particles shown among panels primarily result from “beaching” within the model, but also from overlap among particles and exit from the Gulf of Mexico. Annual differences in the percentage of particles that beach and exit the Atlantic from each nesting site and year (1993 through 2016) are shown in the Supplementary Material.
Standard Deviation in Dispersal Distance
As with mean dispersal distance, the magnitude of the standard deviation of dispersal distance typically followed the pattern of Rancho Nuevo having the highest values and Padre Island having the lowest, though the standard deviation lacked the consistency seen in the values of mean dispersal distance. Variability in dispersal distance tended to decrease with increasing hurricane frequency and storm severity (Supplementary Figure S1 and see Supplementary Material). The standard deviation of dispersal distance at 6 months was most heavily influenced by both site and the number of hurricanes in the Gulf of Mexico, and all models in the confidence set included both variables (Table 1). Hurricane severity was also an important predictor, and all but one model included either the peak wind speed in the Gulf of Mexico or average peak wind speed. One model included two measures of hurricane frequency (seasonal total and Gulf of Mexico total) and total average wind speed. As with dispersal distance and the 6-month dispersal observations, there was a noticeable difference between the three nesting sites, where Rancho Nuevo had the highest and Padre Island had the lowest standard deviation of dispersal distance. Hurricane severity (peak wind speed or total average wind speed) also occurred in the confidence model set, and greater storm intensity was correlated with decreased standard deviation of dispersal distance across all three nesting beaches (Supplementary Figure S3).
Discussion
Variation in Dispersal Distance Across Nesting Beaches and Time
As shown in previous modeling studies for Kemp’s ridley sea turtles and other sea turtle species, ocean currents near populous nesting sites optimize hatchling movement to safe, productive nursery areas (Hays et al., 2010; Putman et al., 2010a, b, 2012a; Okuyama et al., 2011; Shillinger et al., 2012; Casale and Mariani, 2014; Putman, 2018). It is possible that better dispersal dynamics lowers hatchling mortality (Witherington and Salmon, 1992; Gyuris, 1994), causing more turtles to survive to adulthood and to lay nests at their natal beach (Putman et al., 2010a). Dispersal distance from Rancho Nuevo was consistently highest, followed by Veracruz, and then Padre Island, which coincides with the rank order of nesting population size (Putman et al., 2013). While the Padre Island nesting beach, in general, had the lowest dispersal distance nesting here was previously supplemented by translocation of eggs/hatchlings from Rancho Nuevo, and a head-starting program, making it difficult to draw conclusions about the effect of low dispersal on nesting at this location at this time (Shaver and Caillouet, 2015). Due to the arribada nesting strategy of Kemp’s ridley sea turtles, understanding the dispersal dynamics specifically when the nests are hatching en masse could help us predict the impact of ocean currents during those specifically timed events (Bevan et al., 2016). For instance, dispersal distance from both Rancho Nuevo and Veracruz peaked at approximately the beginning of each month during the hatching season. Relating the seasonal variability in dispersal conditions to nesting events and subsequent hatching may provide further insight into the environmental drivers of nesting phenology (Bézy et al., 2020). Similarly, individual years also have clear distinctions in dispersal distance that could provide some insight into the overall survival of all turtles hatching by year.
Hurricanes
Our analysis revealed that there are considerable temporal differences in hatchling dispersal distance across sites and among years. Hurricane frequency and intensity appear to decrease dispersal distance and variability in that distance for Kemp’s ridley hatchlings. Many hurricanes enter the Gulf of Mexico from the south and move westward (Figures 1B, 4). When this occurs, hurricanes most likely push hatchlings back into the Gulf of Mexico, perhaps even back onto the continental shelf, counter to prevailing currents, and reduce the distance traveled (Monzón-Argüello et al., 2012). Hurricanes do not necessarily have to be in the vicinity of the nesting beaches or occur during the hatching season to decrease dispersal distance. Hurricane severity, as measured by wind speed, also decreased hatchling dispersal distance. A year with many hurricanes in a season pushes the turtles back many times, and a year with strong storms likely pushes them with more intensity.
It is unlikely that there is an optimal dispersal distance to ensure the greatest survival rate for hatchlings. Generally, after the frenzy swimming period ends, Kemp’s ridley sea turtles aim to associate with floating mats of pelagic Sargassum (Witherington et al., 2012). This habitat provides food sources and critical protection from oceanic predators. Thus, it seems likely that dispersal distances that result in reaching these offshore habitats would be ideal. However, these offshore Sargassum mats often become entrained in the Loop Current, which exits the Gulf of Mexico and coalesces into the Gulf Stream. There is evidence that older, oceanic-stage juvenile Kemp’s ridleys orient themselves to remain in the Gulf of Mexico (Putman and Mansfield, 2015). Staying in the Gulf of Mexico keeps young turtles in comparatively warmer waters than they might encounter within the Atlantic Ocean, lessening the chance of cold stunning and keeping them out of sub-optimal habitats (Coleman et al., 2017; Avens and Dell’Amico, 2018). Thus, if hurricanes do indeed act as a retention mechanism for Kemp’s ridley sea turtles in the Gulf of Mexico, there may be some benefit for juveniles. Alternatively, when hurricanes push small hatchlings back onto continental shelf habitats where predation is more likely (Witherington and Salmon, 1992; Gyuris, 1994), this could be potentially harmful. Interestingly, hurricanes seem to result in more favorable dispersal outcomes for post-hatchlings from Rancho Nuevo and Veracruz (decreasing transport into the Atlantic) than those from Texas (increasing beaching). This factor may also contribute to large population sizes at the Mexican nesting beaches (Fuentes et al., 2011).
However, survival could be impacted when hatchlings experience rough seas during hurricanes, so any purported benefit of retention in the Gulf of Mexico may be outweighed by additional losses due to physical injury (Monzón-Argüello et al., 2012). Our preliminary work using Ichthyop particles to estimate beaching, exiting the Gulf of Mexico, and approximate position at 6 months shows that there is likely a combination of both outcomes at play (Figure 4 and Supplementary Figures S2, S3). The survival of turtles depends upon the circumstances of their final position in the Gulf of Mexico, as well as their natal beach and the severity of hurricanes in a given year. Though it is already known that dispersal from the Rancho Nuevo site is better at achieving transport to Gulf of Mexico foraging grounds (Putman et al., 2010b), future research addressing spatio-temporal dynamics in neonate survival and empirical estimates of hatchling migratory pathways would be beneficial for extending our understanding of population dynamics during the first year of life for this species (Scott et al., 2014).
Model Caveats/Limitations and Future Research Directions
Our simulations suggest that hatchling dispersal (and thus, potentially, survival) broadly varies over time and across nesting beaches. An important caveat is that, unlike their representatives in Ichthyop, turtles do exhibit active swimming (Wyneken and Salmon, 1992; Putman and Mansfield, 2015). Dispersal distances from our particle tracking model represent how the oceanic currents are acting upon the hatchlings and are not a direct prediction of location, but rather an index of whether environmental conditions are more or less favorable for dispersal. Simulating swimming behavior in sea turtles can certainly alter modeled survivorship, dispersal routes, and the proportion of a population that encounters particular environmental conditions (Gaspar et al., 2012; Putman et al., 2012a, b, 2015; Scott et al., 2012; Lalire and Gaspar, 2019). Empirical movement data in turtles also indicates that they are not “passive drifters” during their post-hatchling and oceanic dispersal stage (Putman and Mansfield, 2015; Christiansen et al., 2016; Mansfield et al., 2017) and actively orient their movements using a suite of guidance mechanisms ranging from a large-scale geomagnetic map (Lohmann et al., 2001; Putman et al., 2011) to fine-scale movements toward pelagic Sargassum mats (Smith and Salmon, 2009). Swimming behavior in small-bodied marine animals appears to be relatively consistent through time and function to move animals toward regions of the ocean that are typically favorable (Putman et al., 2012a, 2020; Putman, 2015, 2018; Naisbett-Jones et al., 2017). Thus, ocean dynamics are likely to be the primary source of variability in the movements in these animals and, indeed, can account for much of the spatial and temporal variability in the distributions of many species (Putman and Naro-Maciel, 2013; Baltazar-Soares et al., 2014; Hays, 2017; Putman et al., 2020). While it is likely that the relative seasonal, annual, and site differences detected in our model are representative of actual conditions, the magnitude of these differences might differ substantially (e.g., dispersal distance from Rancho Nuevo would likely always exceed dispersal distances from Padre Island, but by how much will depend upon aspects of swimming behavior that we do not have information to parameterize) (Putman et al., 2012a, b). Thus, the dispersal metrics we present here are better suited as an index of less to more favorable dispersal conditions, rather than to determine actual survival (Putman et al., 2013).
While much work has focused on the contribution of large juvenile and sub-adult age classes for population recovery (Crouse et al., 1987; Heppell et al., 1996, 2005), our work suggests that hatchling productivity is mutable and the ability of those younger age classes to eventually recruit to the older, more demographically valuable, age classes is extremely variable. While the older age classes are more sensitive to small changes in survival and result in large increases in population growth, our research suggests that there may be large changes in neonate survival rate that may ultimately contribute to extreme variability in recruitment to the more sensitive age classes (Caillouet et al., 2018). Notably, empirical estimates of survival for neonate Kemp’s ridley sea turtles do not exist (National Research and Council, 2010; Wildermann et al., 2018). So, it is not yet possible to parse out how variability in dispersal distance may influence hatchling survival, though it seems likely that spatio-temporal variability in dispersal would indeed influence individual survival rates. Variation in environmental conditions affect survival and reproduction across all species of sea turtles. Earlier work in sea turtles indicates that climate conditions and their influence on resource abundance are correlated with several reproductive factors, including the frequency of reproductive events, the number of offspring produced, offspring sex ratios, and offspring survival (Mrosovsky and Yntema, 1980; Lutz and Musick, 1996; Solow et al., 2002; Vincenzo et al., 2005; Pike and Stiner, 2007; Saba et al., 2007; Piacenza et al., 2016). As such, environmental stochasticity is an important factor that influences life histories and, consequently, their representation in many population models (Lande, 1993; Legault and Melbourne, 2019). As increasingly detailed and global environmental data become available, such as we have generated with these analyses, a promising avenue for further work is to mechanistically link environmental change and population dynamics. Sources of variation such as the seasonal and yearly shifts in ocean currents and the acute disturbances caused by hurricanes impact geographically dispersed sea turtle nesting sites differently (Figures 2, 3) and may be important to consider when designing management strategies and setting conservation goals.
Kemp’s ridley sea turtles are endangered and in need of careful monitoring and conservation (Marquez et al., 2005; NMFS, 2015; Bevan et al., 2016; IUCN, 2019). Currently, conservation management for sea turtles implements beach protections, head-starting hatchlings, and protecting large juveniles and adults from bycatch, among other strategies (Crowder et al., 1994; Garcıía et al., 2003; Shaver and Caillouet, 2015). Given that climate change is predicted to increase hurricane frequency and severity, this species’ ability to be resilient to storms is of utmost concern (Goldenberg et al., 2001; Knutson et al., 2010). A vital part of conservation management is continual monitoring and population assessment to estimate population trends and abundance. Many models integrate environmental stochasticity, but incorporation of environmental indices to improve predictive skill is rare, and variation in dispersal distance has yet to be explored and parameterized for use in these models (Crouse et al., 1987; Heppell et al., 1996; Heppell, 1998; Piacenza et al., 2017). Given the wide variability in hatchling dispersal, and potentially survival rates, it may be imprudent to parameterize hatchling survival in a population model with a static value or an internally estimated value. Our work suggests that hurricane frequency and severity could be a useful environmental index related to hatchling survival. A particular benefit of including hurricane frequency in population and stock assessment models for Kemp’s ridley sea turtles is that it may also be indicative of habitat changes that are relevant to oceanic-stage turtles, such as the distribution of pelagic Sargassum (Witherington et al., 2012; Hardy et al., 2018). A key future step in this effort would be to relate this and other indices that are potentially associated with hatchling dispersal and survival to time-lagged indices of adult Kemp’s ridley abundance, based on age-at-maturity (Caillouet et al., 2016). Detecting strong relationships would indicate the need to then empirically study the relationship between hatchling dispersal and survival rate as it could provide critical information about the early life history of Kemp’s ridley sea turtles that will improve their conservation.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
All authors contributed to writing and editing the manuscript, approved the submitted version and contributed the figures. MD and NP conceived the study and conducted the HYCOM/Ichthyop modeling. SP and MD compiled hurricane data and conducted the statistical analyses.
Funding
MD is supported by the University of West Florida Department of Biology assistantship, the University of West Florida PACE Scholarship, and the University of West Florida Hal Marcus College of Science and Engineering grant. Additionally, funding to present this study has been provided by the International Sea Turtle Symposium travel grant and the University of West Florida Student Government Association. SP and MD were partially supported under startup funding from the University of West Florida. Simulations of hatchling dispersal were funded with support from the Gulf Research Program of the National Academy of Sciences under award number 2000006434 (to NP). The content is the sole responsibility of the authors and does not necessarily reflect the views of the Gulf Research Program, the National Academy of Sciences.
Conflict of Interest
NP was employed by the company LGL Ecological Research Associates.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the HYCOM Consortium and Ichthyop for making their data and software freely available.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.00301/full#supplementary-material
References
Arendt, M. D., Schwenter, J. A., Witherington, B. E., Meylan, A. B., and Saba, V. S. (2013). Historical versus contemporary climate forcing on the annual nesting variability of loggerhead sea turtles in the Northwest Atlantic ocean. PLoS One 8:e81097. doi: 10.1371/journal.pone.0081097
Ascani, F., Van Houtan, K. S., Di Lorenzo, E., Polovina, J. J., and Jones, T. T. (2016). Juvenile recruitment in loggerhead sea turtles linked to decadal changes in ocean circulation. Glob. Chang. Biol. 22, 3529–3538. doi: 10.1111/gcb.13331
Avens, L., and Dell’Amico, F. (2018). Evaluating viability of sea turtle foraging populations at high latitudes: age and growth of juveniles along the French Atlantic coast. Endanger. Species Res. 37, 25–36.
Avens, L., Goshe, L. R., Coggins, L., Shaver, D. J., Higgins, B., and Landry, A. M. Jr., et al. (2017). Variability in age and size at maturation, reproductive longevity, and long-term growth dynamics for Kemp’s ridley sea turtles in the Gulf of Mexico. PLoS One 12:e0173999. doi: 10.1371/journal.pone.0173999
Baltazar-Soares, M., Biastoch, A., Harrod, C., Hanel, R., Marohn, L., Prigge, E., et al. (2014). Recruitment collapse and population structure of the European eel shaped by local ocean current dynamics. Curr. Biol. 24, 104–108. doi: 10.1016/j.cub.2013.11.031
Bevan, E., Wibbels, T., Najera, B. M. Z., Sarti, L., Martinez, F. I., Cuevas, J. M., et al. (2016). Estimating the historic size and current status of the Kemp’s ridley sea turtle (Lepidochelys kempii) population. Ecosphere 7:e01244.
Bézy, V. S., Putman, N. F., Umbanhowar, J. A., Orrego, C. M., Fonseca, L. G., Quirós-Pereira, W. M., et al. (2020). Mass-nesting events in olive ridley sea turtles: environmental predictors of timing and size. Anim. Behav. 163, 85–94.
Burnham, K. P., and Anderson, D. R. (2002). Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York, NY: Springer-Verlag.
Caillouet, C. W., Gallaway, B. J., and Putman, N. F. (2016). Kemp’s ridley sea turtle saga and setback: novel analyses of cumulative hatchlings released and time-lagged annual nests in Tamaulipas, Mexico. Chelonian Conserv. Biol. 15, 115–131.
Caillouet, C. W., Raborn, S. W., Shaver, D. J., Putman, N. F., Gallaway, B. J., and Mansfield, K. L. (2018). Did declining carrying capacity for the Kemp’s ridley sea turtle population within the Gulf of Mexico contribute to the nesting setback in 2010-2017 Chelonian Conserv. Biol. 17, 123–133.
Carr, A. (1987). New perspectives on the pelagic stage of sea turtle development. Conserv. Biol. 1, 103–121.
Casale, P., and Mariani, P. (2014). The first ‘lost year’ of Mediterranean sea turtles: dispersal patterns indicate subregional management units for conservation. Mar. Ecol. Prog. Ser. 498, 263–274.
Chassignet, E. P., Hurlburt, H. E., Smedstad, O. M., Halliwell, G. R., Hogan, P. J., Wallcraft, A. J., et al. (2007). The HYCOM (hybrid coordinate ocean model) data assimilative system. J. Mar. Syst. 65, 60–83.
Christiansen, F., Putman, N. F., Farman, R., Parker, D. M., Rice, M. R., Polovina, J. J., et al. (2016). Spatial variation in directional swimming enables juvenile sea turtles to reach and remain in productive waters. Mar. Ecol. Prog. Ser. 557, 247–259.
Coleman, A. T., Pitchford, J. L., Bailey, H., and Solangi, M. (2017). Seasonal movements of immature Kemp’s ridley sea turtles (Lepidochelys kempii) in the northern gulf of Mexico. Aqua. Conserv. Mar. Freshw. Ecosyst. 27, 253–267.
Collard, S. B., and Ogren, L. H. (1990). Dispersal scenarios for pelagic post-hatchling sea turtles. Bull. Mar. Sci. 47, 233–243.
Crouse, D. T., Crowder, L. B., and Caswell, H. (1987). A stage-based population model for loggerhead sea turtles and implications for conservation. Ecology 68, 1412–1423.
Crowder, L. B., Crouse, D. T., Heppell, S. S., and Martin, T. H. (1994). Predicting the impact of turtle excluder devices on loggerhead sea turtle populations. Ecol. Appl. 4, 437–445.
Eggleston, D. B., Reyns, N. B., Etherington, L. L., Plaia, G. R., and Xie, L. (2010). Tropical storm and environmental forcing on regional blue crab (Callinectes sapidus) settlement. Fish. Oceanogr. 19, 89–106.
Fuentes, M. M. P. B., Bateman, B. L., and Hamann, M. (2011). Relationship between tropical cyclones and the distribution of sea turtle nesting grounds. J. Biogeogr. 38, 1886–1896.
Garcıía, A., Ceballos, G., and Adaya, R. (2003). Intensive beach management as an improved sea turtle conservation strategy in Mexico. Biol. Conserv. 111, 253–261.
Gaspar, P., Benson, S. R., Dutton, P. H., Réveillére, A., Jacob, G., Meetoo, C., et al. (2012). Oceanic dispersal of juvenile leatherback turtles: going beyond passive drift modeling. Mar. Ecol. Prog. Ser. 457, 265–284. doi: 10.3354/meps09689
Goldenberg, S. B., Landsea, C. W., Mestas-Nuñez, A. M., and Gray, W. M. (2001). The recent increase in Atlantic hurricane activity: causes and implications. Science 293, 474–479. doi: 10.1126/science.1060040
Gyuris, E. (1994). The rate of predation by fishes on hatchlings of the green turtle (Chelonia mydas). Coral Reefs 13, 137–144.
Hardy, R. F., Hu, C., Witherington, B., Lapointe, B., Meylan, A., Peebles, E., et al. (2018). Characterizing a sea turtle developmental habitat using landsat observations of surface-pelagic drift communities in the Eastern Gulf of Mexico. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 11, 3646–3659. doi: 10.1109/JSTARS.2018.2863194
Hays, G. C., Fossette, S., Katselidis, K. A., Mariani, P., and Schofield, G. (2010). Ontogenetic development of migration: lagrangian drift trajectories suggest a new paradigm for sea turtles. J. R. Soc. Interf. 7, 1319–1327. doi: 10.1098/rsif.2010.0009
Heppell, S. S. (1998). Application of life-history theory and population model analysis to turtle conservation. Copeia 1998, 367–375.
Heppell, S. S., Crouse, D. T., Crowder, L. B., Epperly, S. P., Gabriel, W., Henwood, T., et al. (2005). A population model to estimate recovery time, population size, and management impacts on Kemp’s ridley sea turtles. Chelonian Conserv. Biol. 4, 767–773.
Heppell, S. S., Crowder, L. B., and Crouse, D. T. (1996). Models to evaluate headstarting as a management tool for long-lived turtles. Ecol. Appl. 6, 556–565.
Hjort, J. (1914). Fluctuations in the great fisheries of northern Europe viewed in the light of biological research. Rapp. Proces Verbaux Com. Int. Pour L’exploration Sci. 20, 1–228.
Hobbs, N. T., and Hilborn, R. (2006). Alternatives to statistical hypothesis testing in ecology: a guide to self teaching. Ecol. Appl. 16, 5–19. doi: 10.1890/04-0645
IUCN (2019). The IUCN Red List of Threatened Species. Version 2019-1. Available online at: www.iucnredlist.org (accessed July 17, 2019).
Johnson, J. B., and Omland, K. S. (2004). Model selection in ecology and evolution. Trends Ecol. Evol. 19, 101–108.
Johnston, M. W., and Purkis, S. J. (2015). Hurricanes accelerated the Florida–Bahamas lionfish invasion. Glob. Chang. Biol. 21, 2249–2260. doi: 10.1111/gcb.12874
Knutson, T. R., McBride, J. L., Chan, J., Emanuel, K., Holland, G., Landsea, C., et al. (2010). Tropical cyclones and climate change. Nat. Geosci. 3, 157–163.
Lalire, M., and Gaspar, P. (2019). Modeling the active dispersal of juvenile leatherback turtles in the North Atlantic Ocean. Mov. Ecol. 7:7. doi: 10.1186/s40462-019-0149-5
Lande, R. (1993). Risks of population extinction from demographic and environmental stochasticity and random catastrophes. Am. Nat. 142, 911–927. doi: 10.1086/285580
Legault, G., and Melbourne, B. A. (2019). Accounting for environmental change in continuous-time stochastic population models. Theor. Ecol. 12, 31–48.
Lett, C., Verley, P., Mullon, C., Parada, C., Brochier, T., Penven, P., et al. (2008). A Lagrangian tool for modelling ichthyoplankton dynamics. Environ. Model. Softw. 23, 1210–1214.
Liu, H., Karnauskas, M., Zhang, X., Linton, B., and Porch, C. (2016). Forecasting dynamics of red snapper (Lutjanus campechanus) in the U.S, Gulf of Mexico. Fish. Res. 187, 31–40.
Lohmann, K. J., Cain, S. D., Dodge, S. A., and Lohmann, C. M. (2001). Regional magnetic fields as navigational markers for sea turtles. Science 294, 364–366. doi: 10.1126/science.1064557
Lohmann, K. J., Putman, N. F., and Lohmann, C. M. F. (2008). Geomagnetic imprinting: a unifying hypothesis of long-distance natal homing in salmon and sea turtles. Proc. Natl. Acad. Sci. U.S.A. 105, 19096–19101. doi: 10.1073/pnas.0801859105
Mansfield, K. L., Mendilaharsu, M. L., Putman, N. F., dei Marcovaldi, M. A., Sacco, A. E., Lopez, G., et al. (2017). First satellite tracks of South Atlantic sea turtle ‘lost years’: seasonal variation in trans-equatorial movement. Proc. R. Soc. B Biol. Sci. 284:20171730. doi: 10.1098/rspb.2017.1730
Marquez, R., Burchfield, P. M., Diaz, J., Sanchez, M., Carrasco, M., Jimenez, C., et al. (2005). Status of the Kemp’s ridley sea turtle, Lepidochelys kempii. Chelonian Conserv. Biol. 4, 761–766.
Monzón-Argüello, C., Dell’Amico, F., Morinière, P., Marco, A., ópez-Jurado, L. F. L., Hays, G. C., et al. (2012). Lost at sea: genetic, oceanographic and meteorological evidence for storm-forced dispersal. J. R. Soc. Interf. 9, 1725–1732. doi: 10.1098/rsif.2011.0788
Mrosovsky, N., and Yntema, C. L. (1980). Temperature dependence of sexual differentiation in sea turtles: implications for conservation practices. Biol. Conserv. 18, 271–280.
Naisbett-Jones, L. C., Putman, N. F., Stephenson, J. F., Ladak, S., and Young, K. A. (2017). A magnetic map leads juvenile European eels to the Gulf Stream. Curr. Biol. 27, 1236–1240. doi: 10.1016/j.cub.2017.03.015
National Research and Council (2010). Assessment of Sea-Turtle Status and Trends: Integrating Demography and Abundance, 1st Edn. Washington, DC: The National Academies Press.
NMFS (2015). Kemp’s Ridley Sea Turtle (Lepidochelys Kempii) 5-Year Review: Summary and Evaluation. Silver Spring, MD: National Marine Fisheries Service.
NOAA (2019). National Hurricane Center and Central Pacific Hurricane Center. Available online at: https://www.nhc.noaa.gov/
Okuyama, J., Kitagawa, T., Zenimoto, K., Kimura, S., Arai, N., Sasai, Y., et al. (2011). Trans-Pacific dispersal of loggerhead turtle hatchlings inferred from numerical simulation modeling. Mar. Biol. 158, 2055–2063.
Piacenza, S., Balazs, G., Hargrove, S., Richards, P., and Heppell, S. (2016). Trends and variability in demographic indicators of a recovering population of green sea turtles Chelonia mydas. Endanger. Species Res. 31, 103–117.
Piacenza, S. E., Richards, P. M., and Heppell, S. S. (2017). An agent-based model to evaluate recovery times and monitoring strategies to increase accuracy of sea turtle population assessments. Ecol. Model. 358, 25–39.
Pike, D. A., and Stiner, J. C. (2007). Fluctuating reproductive output and environmental stochasticity: do years with more reproducing females result in more offspring? Can. J. Zool. 85, 737–742.
Putman, N., Seney, E. E., Verley, P., Shaver, D. J., López-Castro, M., Cook, M., et al. (2019). Predicted distributions and abundances of the sea turtle ‘lost years’ in the western North Atlantic Ocean. Ecography 43, 506–517.
Putman, N. F. (2015). Inherited magnetic maps in salmon and the role of geomagnetic change. Integr. Comp. Biol. 55, 396–405. doi: 10.1093/icb/icv020
Putman, N. F., Bane, J. M., and Lohmann, K. J. (2010a). Sea turtle nesting distributions and oceanographic constraints on hatchling migration. Proc. R. Soc. B Biol. Sci. 277, 3631–3637. doi: 10.1098/rspb.2010.1088
Putman, N. F., Endres, C. S., Lohmann, C. M., and Lohmann, K. J. (2011). Longitude perception and bicoordinate magnetic maps in sea turtles. Curr. Biol. 21, 463–466. doi: 10.1016/j.cub.2011.01.057
Putman, N. F., and He, R. (2013). Tracking the long-distance dispersal of marine organisms: sensitivity to ocean model resolution. J. R. Soc. Interf. 10:20120979. doi: 10.1098/rsif.2012.0979
Putman, N. F., and Lohmann, K. J. (2008). Compatibility of magnetic imprinting and secular variation. Curr. Biol. 18, R596–R597. doi: 10.1016/j.cub.2008.05.008
Putman, N. F., Lumpkin, R., Sacco, A. E., and Mansfield, K. L. (2016). Passive drift or active swimming in marine organisms? Proc. R. Soc. B Biol. Sci. 283:20161689. doi: 10.1098/rspb.2016.1689
Putman, N. F., and Mansfield, K. L. (2015). Direct evidence of swimming demonstrates active dispersal in the sea turtle “Lost Years”. Curr. Biol. 25, 1221–1227. doi: 10.1016/j.cub.2015.03.014
Putman, N. F., Mansfield, K. L., He, R., Shaver, D. J., and Verley, P. (2013). Predicting the distribution of oceanic-stage Kemp’s ridley sea turtles. Biol. Lett. 9:20130345. doi: 10.1098/rsbl.2013.0345
Putman, N. F., and Naro-Maciel, E. (2013). Finding the ‘lost years’ in green turtles: insights from ocean circulation models and genetic analysis. Proc. Biol. Sci. 280:20131468. doi: 10.1098/rspb.2013.1468
Putman, N. F., Scott, R., Verley, P., Marsh, R., and Hays, G. C. (2012a). Natala site and offshore swimming influence fitness and long-distance ocean transport in young sea turtles. Mar. Biol. 159, 2117–2126.
Putman, N. F., Shay, T. J., and Lohmann, K. J. (2010b). Is the geographic distribution of nesting in the Kemp’s ridley turtle shaped by the migratory needs of offspring? Integr. Comp. Biol. 50, 305–314. doi: 10.1093/icb/icq041
Putman, N. F., Verley, P., Endres, C. S., and Lohmann, K. J. (2015). Magnetic navigation behavior and the oceanic ecology of young loggerhead sea turtles. J. Exp. Biol. 218, 1044–1050. doi: 10.1242/jeb.109975
Putman, N. F., Verley, P., Shay, T. J., and Lohmann, K. J. (2012b). Simulating transoceanic migrations of young loggerhead sea turtles: merging magnetic navigation behavior with an ocean circulation model. J. Exp. Biol. 215, 1863–1870. doi: 10.1242/jeb.067587
Putman, N. F., Williams, C. R., Gallagher, E. P., and Dittman, A. H. (2020). A sense of place: pink salmon use a magnetic map for orientation. J. Exp. Biol. 223(Pt 4):jeb218735. doi: 10.1242/jeb.218735
Rostal, D. C., Owens, D. W., Grumbles, J. S., MacKenzie, D. S., and Amoss, M. S. (1998). Seasonal reproductive cycle of the Kemp’s ridley sea turtle (Lepidochelys kempi). Gen. Comp. Endocrinol. 109, 232–243.
Saba, V., Tomillo, P., Reina, R., Spotila, J., Musick, J., Evans, D., et al. (2007). The effect of the El Nino Southern Oscillation on the reproductive frequency of eastern Pacific leatherback turtles. J. Appl. Ecol. 44, 395–404.
Sagarese, S., Bryan, M., Walter, J., Schirripa, M., Grüss, A., and Karnauskas, M. (2015). Incorporating Ecosystem Considerations Within the Stock Synthesis Integrated Assessment Model for Gulf of Mexico Red Grouper (Epinephelus morio). SEDAR42-RW-01. North Charleston, SC: SEDAR.
Scott, R., Biastoch, A., Agamboue, P. D., Bayer, T., Boussamba, F. L., Formia, A., et al. (2017). Spatio-temporal variation in ocean current-driven hatchling dispersion: implications for the world’s largest leatherback sea turtle nesting region. Divers. Distrib. 23, 604–614.
Scott, R., Biastoch, A., Roder, C., Stiebens, V. A., and Eizaguirre, C. (2014). Nano-tags for neonates and ocean-mediated swimming behaviours linked to rapid dispersal of hatchling sea turtles. Proc. Biol. Sci. 281:20141209. doi: 10.1098/rspb.2014.1209
Scott, R., Marsh, R., and Hays, G. C. (2012). A little movement orientated to the geomagnetic field makes a big difference in strong flows. Mar. Biol. 159, 481–488.
Shaver, D., and Caillouet, C. (2015). Reintroduction of Kemp’s ridley (Lepidochelys kempii) sea turtle to Padre Island National Seashore, Texas and its connection to headstarting. Herpetol. Conserv. Biol. 10, 378–435.
Shaver, D. J., Hart, K. M., Fujisaki, I., Rubio, C., Sartain-Iverson, A. R., Peña, J., et al. (2016). Migratory corridors of adult female Kemp’s ridley turtles in the Gulf of Mexico. Biol. Conserv. 194, 158–167.
Shillinger, G. L., Di Lorenzo, E., Luo, H., Bograd, S. J., Hazen, E. L., Bailey, H., et al. (2012). On the dispersal of leatherback turtle hatchlings from Mesoamerican nesting beaches. Proc. Biol. Sci. 279, 2391–2395. doi: 10.1098/rspb.2011.2348
Smith, M. M., and Salmon, M. (2009). A comparison between the habitat choices made by hatchling and juvenile green turtles (Chelonia mydas) and loggerheads (Caretta caretta). Mar. Turtle Newslett. 126, 9–13.
Smith, N. S., Green, S. J., Akins, J. L., Miller, S., and Côté, I. M. (2017). Density-dependent colonization and natural disturbance limit the effectiveness of invasive lionfish culling efforts. Biol. Invasions 19, 2385–2399.
Solow, A. R., Bjorndal, K. A., and Bolten, A. B. (2002). Annual variation in nesting numbers of marine turtles: the effect of sea surface temperature on re-migration intervals. Ecol. Lett. 5, 742–746.
Sturges, W., and Blaha, J. P. (1976). A western boundary current in the Gulf of Mexico. Science 192, 367–369. doi: 10.1126/science.192.4237.367
Vincenzo, P., Otalora, F., Fabrizio, S., and Miguel, F. (2005). Environmental stochasticity in dispersal areas can explain the ‘mysterious’ disappearance of breeding populations. Proc. R. Soc. B Biol. Sci. 272, 1265–1269. doi: 10.1098/rspb.2005.3075
Wildermann, N. E., Gredzens, C., Avens, L., Barrios-Garrido, H. A., Bell, I., Blumenthal, J., et al. (2018). Informing research priorities for immature sea turtles through expert elicitation. Endanger. Species Res. 37, 55–76.
Witherington, B., Hirama, S., and Hardy, R. (2012). Young sea turtles of the pelagic Sargassum-dominated drift community: habitat use, population density, and threats. Mar. Ecol. Prog. Ser. 463, 1–22.
Witherington, B. E., and Salmon, M. (1992). Predation on loggerhead turtle hatchlings after entering the sea. J. Herpetol. 26, 226–228.
Wyneken, J., and Salmon, M. (1992). Frenzy and postfrenzy swimming activity in loggerhead. green, and leatherback hatchling sea turtles. Copeia 1992, 478–484. doi: 10.1242/jeb.061747
Keywords: dispersal, hurricane, sea turtle, ocean circulation model, movement ecology, spatial ecology
Citation: DuBois MJ, Putman NF and Piacenza SE (2020) Hurricane Frequency and Intensity May Decrease Dispersal of Kemp’s Ridley Sea Turtle Hatchlings in the Gulf of Mexico. Front. Mar. Sci. 7:301. doi: 10.3389/fmars.2020.00301
Received: 19 December 2019; Accepted: 15 April 2020;
Published: 12 May 2020.
Edited by:
Donna Jill Shaver, National Park Service (United States), United StatesReviewed by:
Luis Cardona, University of Barcelona, SpainFederico Alberto Abreu-Grobois, National Autonomous University of Mexico, Mexico
Robert Hardy, National Marine Fisheries Service (NOAA), United States
Copyright © 2020 DuBois, Putman and Piacenza. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Morgan J. DuBois, mjdubois01@gmail.com
 Morgan J. DuBois
Morgan J. DuBois Nathan F. Putman
Nathan F. Putman Susan E. Piacenza
Susan E. Piacenza