Toward New Ecologically Relevant Markers of Health for Cetaceans
- 1School of Biological Sciences, University of Aberdeen, Aberdeen, United Kingdom
- 2Scottish Marine Animal Stranding Scheme, Inverness, United Kingdom
Multiple stressors caused by human-induced disturbances can affect the foraging opportunities of cetaceans, potentially depleting their energy stores, and ultimately impact survival and reproductive success. Currently, blubber thickness and lipid composition is used as measure of health and nutritional status in cetaceans. This assumes that blubber functions in the same manner as adipose tissue in terrestrial mammals. However, cetaceans have evolved to have thickened blubber which serves as thermoregulation, buoyancy and energy store. In addition, blubber is composed of several layers and regions that have different physiological functions. We currently lack a clear understanding of how blubber biology contributes to maintaining energy status in cetaceans and several studies show blubber thickness, and composition in some body regions, is an inappropriate measure of health. Before new markers of health can be identified, we need to understand how environmental stressors influence blubber biology and particularly unravel its complex signaling roles with other organs. Currently, we do not understand how changes in energy status drive changes in health in cetaceans, and eventually population dynamics. This review synthesizes recent developments in cetacean blubber biology to propose potential directions to develop novel cetacean health markers.
Introduction
Cetaceans underwent critical morphological and physiological adaptations to their anatomy and energy metabolism as they transitioned from a terrestrial to an aquatic lifestyle (Parry, 1949; Scholander et al., 1950). This included the loss of hind limbs, sensory adaptations for navigation and foraging, and changes to the respiratory system to allow for prolonged and deep dives (Berta et al., 2015). Blubber thickening provided insulation (Berta et al., 2015) and opened opportunities to store energy and deal with more sporadic foraging opportunities (Williams et al., 1993, 2001; Vasseur and Yodzis, 2004). Fat stores not only play a role in maintaining energy balance, but also play a crucial signaling role to other organs to regulate energy metabolism (Trayhurn and Beattie, 2007; Ye, 2011). There are indications that some of these signaling functions have changed during the evolutionary trajectory of these species (Ball et al., 2017; Derous et al., 2019). Crucially, these adaptations impact the ways in which individuals decide to invest in reproduction and define their abilities to survive under varied environmental pressures.
As the conservation relevance of sub-lethal stressors (e.g., noise, contaminants, prey limitation) caused by anthropogenic disturbances becomes more important for cetaceans, management frameworks are being developed to estimate the cumulative impact of sub-lethal stressors that can affect survival and reproduction and therefore population trajectory (National Academies of Sciences, Engineering, and Medicine, 2017). The Population Consequences of Multiple Stressors (PCoMS) are mediated by the impacts of physiological and behavioral changes associated with exposure to those stressors on the health of individuals (National Academies of Sciences, Engineering, and Medicine, 2017). Health in this framework is seen in an ecological context; that is, it encompasses the physiological systems key to enable individuals to survive and reproduce. Energy metabolism, rather than acute immune responses for example, is therefore key to understanding this ecological health status. The framework uses blubber thickness as a measure of health to relate the cumulative behavioral and physiological impacts of anthropogenic stressors to an individual’s demographic contribution (Pirotta et al., 2015). Ecologically relevant indicators of health are therefore crucial to understand how different stressors affect survival and reproduction and hence population dynamics in cetaceans. Early attempts to use ecological proxies for the notion of health, such as body condition, have had mixed results to inform PCoMS (New et al., 2014; Pirotta et al., 2015). Indeed, it was recently suggested that using blubber thickness alone is providing a limited view of cetacean ecological health (Kershaw et al., 2019).
We need to determine how multiple stressors are integrated in the energy metabolism of the targeted species at a molecular and cellular level to understand how they affect health. For example, response to stress is centrally regulated by the hypothalamic-pituitary-adrenal axis (HPA) and signals to mobilize energy from stores to maintain the exercising muscles and the brain (Romero and Wingfield, 2016). This allows for a rapid response and optimizes survival from immediate threats. However, long-term stress causes structural changes to the adrenal gland (Clark et al., 2006) and can cause deterioration or even death in marine mammals (Cowan and Curry, 2008). Stressors may affect energy metabolism of cetaceans to such a level that it could have detrimental effects, even after the stressor is removed. We are currently facing a knowledge gap between health and energy metabolism in cetaceans, and how they mediate potential metabolic constraints faced during periods of exposure to stressors.
We therefore need to understand the physiological systems involved in energy metabolism in cetaceans. We know that adipose tissue in all mammals, blubber in cetaceans, plays a critical role in energy metabolism not only as an energy store, but also as an emitter of regulatory signals. The majority of cetacean blubber biology studies have focussed on lipid composition (Kershaw et al., 2019), hormone profiling (Boggs et al., 2017, 2019; Kershaw et al., 2017), persistent organic pollution and contamination (Montie et al., 2008; Fair et al., 2010; Kucklick et al., 2011; Pinzone et al., 2015; Zhan et al., 2019), and morphology (Montie et al., 2008). A few have focussed on the role of blubber and its link to health status/reproductive success in cetaceans (Miller et al., 2011; Christiansen et al., 2013, 2014; Irvine et al., 2017). Here, we review how this existing information is relevant to the PCoMS challenge and what future directions are needed to better qualify cetacean health in an ecologically relevant manner.
Terrestrial Mammals’ Energy Stores and Condition of Health
In terrestrial mammals, the size of energy stores is a classical body condition and health proxy (Barnes, 1984; Markusfeld et al., 1997; Morfeld et al., 2016). From studies with species such as rodents and humans, we know that adipose tissue serves as an energy storage and is involved in regulating metabolism by producing several signaling molecules (adipokines) such as leptin (Trayhurn and Beattie, 2007; Ye, 2011). Adipose tissue can be categorized into two main types based on the position: visceral and subcutaneous fat. Excessive visceral fat is associated with numerous metabolic disorders including insulin resistance and cardiovascular diseases in humans, rodents and canine pets (Mathieu et al., 2010; Thengchaisri et al., 2014) while subcutaneous fat does not have such a detrimental effect on health (Gabriely et al., 2002). The secretion of leptin is in proportion to the amount of adipose tissue and is therefore a key peripheral signal to communicate energy status to other organs. Mammalian reproduction is sensitive to the state of energy stores and leptin secretion plays a role in maintaining reproductive function during adulthood (Williams et al., 2002; German, 2006; Sam and Dhillo, 2010; Yan et al., 2011; Nahar et al., 2013; Pantasri and Norman, 2014; Morfeld and Brown, 2016).
Cetaceans’ Energy Stores and Condition of Health
Blubber Thickness and Reproductive Cycle
The amount of adipose tissue in cetaceans has also been used as an indicator of health and reproductive success (Miller et al., 2011; Christiansen et al., 2013, 2014; Irvine et al., 2017). These energy stores seem to vary depending on strategies for financing the costs of reproduction. Stores of income breeders (e.g., bottlenose dolphins, Tursiops truncatus and sea lions, Zalophus californianus) stay relatively constant throughout the year, while those of capital breeders (e.g., humpback whales, Megaptera novaeangliae and elephant seals, Mirounga spp.) vary with reproductive class and migratory stages (Irvine et al., 2017). For example, pregnant humpback whales have higher relative energy stores than non-pregnant females and males (Irvine et al., 2017). In addition, larger pregnant females had relatively larger energy stores compared to smaller pregnant females (Irvine et al., 2017). In North Atlantic right whales (Eubalaena glacialis), blubber thickness depends on reproductive cycle; it is thickest 3 to 6 months prior to pregnancy and thinnest during lactation (Miller et al., 2011). The blubber was thickest in pregnant North Atlantic fin whales (Balaenoptera physalus) compared to other reproductive classes (Williams et al., 2013). This indicates that, like for terrestrial mammals, energy stores are needed for gestation and lactation as these are energetically very costly (Iverson, 2009). In addition, smaller individuals may experience higher levels of energetic stress during the migration fast (Irvine et al., 2017). In minke whales (Balaenoptera acutorostrata) blubber volume was associated with fetal investment (Christiansen et al., 2013). Fetus length was reduced for those female minke whales with a poorer body condition, suggesting that they reduce their energic investment in fetus growth to optimize their own survival (Christiansen et al., 2014). In North Atlantic fin whales, a decline in pregnancy rate in breeding age females is associated with low blubber thickness (Williams et al., 2013). In North Atlantic right whales it has been suggested that during periods of low prey availability, early term abortion could be a mechanism to save energy (Knowlton et al., 1994).
Blubber Thickness and Nutritional Stress
The blubber of North Atlantic right whales is thinner in years with low prey availability (Miller et al., 2011). Inter-annual variability in blubber thickness associated with prey availability was also observed in North Atlantic fin whales (Williams et al., 2013). The blubber mass of harbor porpoises (Phocoena phocoena) was lost quickly when they were disturbed, losing approximately 4% of initial body mass after 24 h disturbance (Kastelein et al., 2019).
Blubber thickness is one of the attributes contributing to insulation against the cold besides the lipid content and lipid composition (Iverson, 2009). Smaller cetacean species would require an increased metabolism for heat production (Iverson, 2009). Hence, smaller cetaceans are therefore more susceptible to changes in their prey availability, and thus starvation, as demonstrated by the rapid decline in body mass of harbor porpoises (Kastelein et al., 2019). The inner most layer of blubber in harbor porpoises has characteristics (both structure of fat cells and gene expression of UCP1) associated with brown adipose tissue, which plays a key role in thermoregulation and heat production in some land mammals (Hashimoto et al., 2015). In bowhead whales (Balaena mysticetus), however, blubber thickness did not vary with seasons or life stages (Ball et al., 2015), and similar results are found in other species including sperm whales (Physeter macrocephalus) (Evans et al., 2003; Ruchonnet et al., 2006; Caon et al., 2007; Koopman, 2007; Gómez-Campos et al., 2011). Interestingly, larger cetaceans such as bowhead and sperm whales seem to lack functional proteins of UCP1, one of the key genes related to heat production (Keane et al., 2015). Hence, the evolution on a species level may have ecological implications of how blubber is used during periods of starvation, the cost of thermoregulation and the deleterious effects it may have on health. Indeed, smaller aquatic animals are constrained for thermoregulatory costs while larger aquatic animals have limitations on feeding efficacy (Gearty et al., 2018). We should therefore place blubber thickness in the context of how it may affect reproduction and if, as an ecological health indicator, it is relevant to demographic contributions of a specie. Using blubber thickness alone is providing a limited view of cetacean ecological health (Kershaw et al., 2019) and thus using blubber thickness as an ecological health proxy might be flawed in many species.
Blubber Thickness and Body Condition
Blubber thickness as a marker of body condition in cetaceans has its limitations (Kershaw et al., 2019). In sperm whales, blubber thickness was positively related to total length but there was a lack of relationship with age, sex or reproductive success (Evans et al., 2003). In addition, blubber lipid content was not related to blubber thickness in these stranded sperm whales. This is in agreement with data from dorsal blubber samples from stranded ziphiids and balaenopterids where the lipid content of the blubber and blubber thickness provided little information on overall body condition (Kershaw et al., 2019). In striped dolphins (Stenella coeruleoalba), blubber thickness did not differ between reproductive classes and nutritional condition (Gómez-Campos et al., 2011). In two mass strandings of rough-toothed dolphins (Steno bredanensis), the dorsal blubber thickness was not different although clear differences were found in body condition measured by body mass index between euthanized and rehabilitated animals (Karns et al., 2019). However, differences were found in the lateral and ventral blubber thickness of these animals (Karns et al., 2019). Depending on biopsy site, the blubber thickness indeed varies in the same animals and this depending on the length and cause of death (Figure 1).
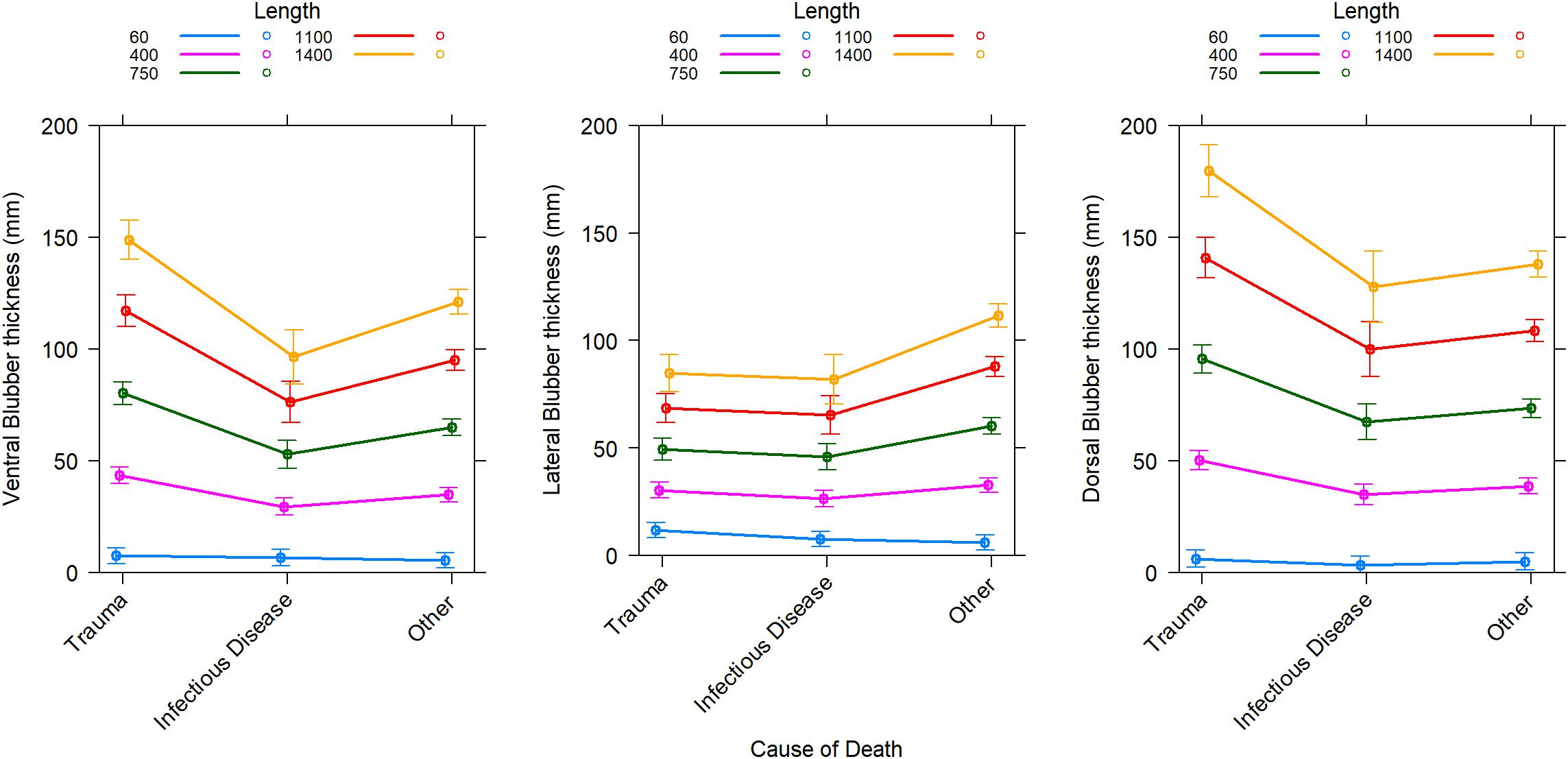
Figure 1. Predicted change in blubber thickness at three locations along the mid-body depending on the cause of death for Odontocetes categorized in 4 taxa occurring more often in strandings in Scotland (bottlenose dolphins, harbor porpoises, pelagic delphinids, sperm and beaked whales). Individuals that died from trauma can be expected to have “typical” blubber thickness (unchallenged) for their population. Predictions are from the best general mixed-effect linear model for each blubber location which includes an effect of body length (Length in cm) depending on the effect of cause of death (Trauma, Infectious disease, or other) and random effects of taxa and year of stranding in taxa. Marginal coefficients of determination are 0.72, 0.65, and 0.78, respectively for the ventral, lateral, and dorsal blubber thickness models. Conditional coefficients of determination are 0.79, 0.75, and 0.83, respectively for the ventral, lateral, and dorsal blubber thickness models. Error bars are 95% confidence intervals. Historical stranding data (since 1991) from the Scottish Marine Animal Stranding Scheme (database available at https://www.strandings.org/cgi-bin/smass/map.pl and previously published by Kershaw et al., 2017).
Hence, using blubber thickness alone in some cetaceans as a health marker may not reflect whether individuals are in negative energy status or not. In addition, in a meta-analysis of stranded male harbor porpoises with different causes of death only 50% of the variation in body mass/length2 ratio could explain the body condition while model validation for ventral blubber thickness was very poor (Kershaw et al., 2017). Therefore, blubber thickness might indeed be a poor index of condition in harbor porpoises as well as other species (Read, 1990; Evans et al., 2003; Ruchonnet et al., 2006; Caon et al., 2007; Koopman, 2007; Gómez-Campos et al., 2011). Kershaw et al. (2017) argued that muscle mass may be used as fuel during periods of starvation as blubber has other functions such as thermoregulation and buoyancy as a consequence of secondary adaptations to life in water. Fat may also be stored within the muscle and although no significant changes were found in blubber between reproductive classes of North Atlantic fin whales, the adult females do appear to store more fat in their tail muscles (Lockyer et al., 1985). Lockyer (2007) also argues that it is critical to understand each species energy metabolism to understand how fat storage is linked to reproductive success and health (Lockyer, 2007). More than a decade later, we still lack a good physiological understanding of how health/reproduction/energy stores are regulated in the majority of cetaceans. We therefore first need to understand (1) how the different layers of blubber function, (2) what triggers lipid deposition, and (3) how this is related to ecological health.
Functionality of the Different Blubber Layers
Blubber can be divided into different layers, with the outer layer mainly used for structural support and inner layer for energy storage. For example, the adipocytes of healthy porpoises in the outer layer of blubber were smaller than inner blubber adipocytes. Starved porpoises, however, had fewer, smaller adipocytes in the inner layer blubber, suggesting a possible combination of adipocyte shrinkage and loss (Koopman et al., 2002). Interestingly, the adipocytes of the deepest layer of the blubber in lactating dolphins were significantly smaller compared to pregnant animals (Montie et al., 2008). This suggests that lactation is energetically more costly for these animals and more stored fat is being used to meets these metabolic demands. In addition, lipid content differed between the blubber layers of fin whales, yet the composition of the top layer was not different between reproductive classes of the whales (Aguilar and Borrell, 1990). Levels of cortisol (i.e., stress hormone) in stranded harbor porpoises showed a clear difference in concentration with the inner layer having a higher concentration compared to the top layer (Kershaw et al., 2017). A study using five parameters (i.e., lipid percent and classes, contaminant concentrations and profiles, fatty acid profiles) to assess body condition found that results varied depending on the layer sampled in beluga (Delphinapterus leucas) and killer whales (Orcinus orca) (Krahn et al., 2004). Hence, classical measures in superficial biopsy samples may not be relevant health proxies.
Regulation of Lipid Deposition
In our classical view of mammalian energy metabolism, hormonally regulated lipid storage/deposition depends on the interaction between an individual’s physiological needs and environmental changes. A key regulatory hormone is leptin, which is involved in regulating food intake and energy metabolism depending on fat mass via key neuropeptides in the hypothalamus region of the brain (Friedman, 2019).
Leptin gene expression changed drastically depending on the migratory stage of bowhead whales (Ball et al., 2017). Leptin expression in mature bowhead whales differed between the outer and inner blubber layers, and for the inner layer leptin changed significantly according to season. Blubber thickness, however, did not vary in bowhead whales with season or life stage. This is likely due to the adipocyte cell size reducing rather than their number combined with an increase in structural fiber density (Ball et al., 2015). As leptin and binding to its receptor seems to be highly conserved, the modification in leptin functions observed in these animals seems to therefore stem on its regulatory mechanism. The results of Ball et al. (2017) indicated cetaceans may be departing from the classical mammalian model of leptin regulation of energy metabolism, and temporary leptin resistance during migration may be a mechanism to reduce the anorexigenic effects of leptin. In addition, Ball et al. (2017) speculates that the unusual large differences in seasonal leptin levels is likely a signal to induce a physiological response to change feeding habits or start migration, rather than a measure of total lipid stores as is the case in typical mammalian energy metabolism.
Therefore, whale foraging mechanisms may have evolved to compensate for large size being limited by prey availability, posing questions about satiation mechanisms in which leptin is key in other mammals. These large animals may face some metabolic constraints on their energy metabolism based on the energy cost for thermoregulation and feeding efficiency (Gearty et al., 2018; Goldbogen et al., 2019).
Genes related to triacylglycerol synthesis and lipolysis processes were positively selected in cetaceans compared to terrestrial mammals (Wang et al., 2015) and indeed, a large portion of positively selected genes in cetaceans are involved in energy metabolism (Nery et al., 2013). Positive gene selection in energy metabolism-related biological pathways suggests these genes may have been positively selected to adapt to a glucose-poor diet and it is unlikely that fat depots signaling function in the same manner as in terrestrial mammals. Specifically adiposity is not likely to have the deleterious health consequences known in terrestrial mammals (Derous et al., 2019). For example, while low blubber thickness negatively impacted pregnancy rate in North Atlantic fin whales, larger than normal blubber thickness was still associated with the same rate of pregnancy as that of typical healthy animals (Williams et al., 2013). This is in stark contrast with land mammals where both reduced and increased fat mass (signaled by leptin) leads to reduced reproductive success (Caprio et al., 2001). Hence, cetaceans may not face a metabolically constrained upper limit of the amount of fat they can store but the evolutionarily increase in body-size of whales does seemed to be constrained by prey availability (Goldbogen et al., 2019). In a land mammal, large volume of adipose tissue triggers inflammatory responses and can lead to metabolic dysfunctions at a physiological level (Mantovani et al., 2002). In cetaceans, key genes related to inflammation are positively selected in such a way that fat deposits signaling may not be as limited by inflammation, metabolic dysfunctions and reproduction (Derous et al., 2019). These phylogenomic studies indicate that due to evolutionary constraints associated with secondary adaptations to life in water, we cannot expect adipose signaling to interact with energy metabolism in cetaceans like it does in the classical mammalian model. We therefore need de novo studies of the role of adipose signaling in cetacean appetite regulation and reproductive investment.
Toward New Markers of Health – Omics Approach
We can now characterize many thousands of genes, metabolites and proteins associated with phenotypic traits and this is key to the discovery of health biomarkers in human diseases (Abu-Asab et al., 2011; Hasin et al., 2017; Karczewski and Snyder, 2018; Peña-Bautista et al., 2018; Blencowe et al., 2019). To date, we still lack a detailed understanding of whole genome dynamics associated with changes in energy status in cetacean blubber and the consequences for cetacean cellular biology (Kershaw et al., 2018; Misra et al., 2019). However, recent attempts have been made to elucidate how metabolism of cetaceans is regulated by using an omics approach (Kershaw et al., 2018; Misra et al., 2019). Proteomics data from full depth blubber subsamples from dead-stranded harbor porpoises identified 295 proteins mostly involved in cell function and metabolism (45%), immune response and inflammation (15%) and lipid metabolism (11%) (Kershaw et al., 2018). Blubber metabolomics analysis of stranded bottlenose dolphins compared to skin biopsies indicated, unsurprisingly, a clear role of blubber in fatty acid metabolism and ketogenic amino acids. In addition both tissues were involved in urea cycle, nucleotide metabolism, amino acid metabolism, glutathione metabolism among others (Misra et al., 2019). Although these studies give us insight into the metabolome/proteome, it is hard to conclude the role of blubber in health status. There is a need to unravel the complex signaling mechanisms in blubber and to understand how environmental stressors influence blubber biology and signaling.
Conclusion
Blubber thickness has previously been used in cetaceans as a measure of their energy stores and thus their health. However, using blubber thickness alone may provide a limited view of cetacean’s ecological health. For example, blubber thickness measured at three locations of varies species and varies causes of death seems to lead to differs in conclusions in terms of how their energy stores are reflective for their health. These conclusions related to blubber thickness is based on the assumptions that cetaceans are using their fat stores in a similar manner as terrestrial mammals. Based on empirical data and the lack of experimental data, it is unclear if this is indeed the case. For example, while a clear decrease in morphometric body condition of juvenile and adult humpback whales was observed at different time points (from 18.8% to −8.1%; and from 8.6% to −3.4%, respectively), this was not reflected by a change in outer layer blubber lipid concentration (Christiansen et al., 2020). We therefore urge to move toward interdisciplinary research to try to understand how these animals are regulating their energy stores (e.g., different positions and different layers) and if we can find novel markers that are more reflective of their energy state. The evolution of metabolic pathways shape mammalian physiology and may impact the ways in which cetaceans “decide” to invest in reproduction and define their abilities to survive under varied environmental pressures. These changes therefore have the scope to alter the way individuals make biological decisions about reproduction investment given their energetic metabolic state. Food availability is more clustered in time and space and thermoregulatory challenges are acute, externalizing some motivational processes. This will have ecological implications of how blubber is used during periods of starvation and the cost of thermoregulation. Relying on prey limitations to guide satiation may have advantages to ensure the maintenance of blubber and reduced stochasticity in its dynamics to help maintain its thermoregulatory advantages. For example, leptin in cetaceans is likely a signal to change feeding habits or start migration rather than a measure of total lipids stores. This has profound implications for the way energy metabolism status influences reproduction and survival, which we do not understand clearly at the moment. In addition, energy metabolism is also regulated by the microbiome and changes in composition could have an effect on signaling molecules related to appetite, energy uptake and storage, and energy expenditure (Heiss and Olofsson, 2018). We need innovative management tools to assess the impact of PCoMS on the conservation of key species like cetaceans. However, to achieve this there is a need to move toward interdisciplinary research as the current knowledge gap requires an integration of ecological insights and de novo molecular biology analyses using systems biology approaches.
Author Contributions
DD and DL contributed to the design and concept of the manuscript. DD wrote the manuscript. DL performed the statistical analysis and made the figure. AB and ND provided the intellectual input. MD organized the database. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abu-Asab, M. S., Chaouchi, M., Alesci, S., Galli, S., Laassri, M., Cheema, A. K., et al. (2011). Biomarkers in the age of omics: time for a systems biology approach. OMICS A J. Integr. Biol. 15, 105–112. doi: 10.1089/omi.2010.0023
Aguilar, A., and Borrell, A. (1990). Patterns of lipid content and stratification in the blubber of fin whales (Balaenoptera physalus). J. Mammal. 71, 544–554. doi: 10.2307/1381793
Ball, H. C., Londraville, R. L., Prokop, J. W., George, J. C., Suydam, R. S., Vinyard, C., et al. (2017). Beyond thermoregulation: metabolic function of cetacean blubber in migrating bowhead and beluga whales. J. Comp. Physiol. B 187, 235–252. doi: 10.1007/s00360-016-1029-6
Ball, H. C., Stavarz, M., Oldaker, J., Usip, S., Londraville, R. L., George, J. C., et al. (2015). Seasonal and ontogenetic variation in subcutaneous adipose of the bowhead whale (Balaena mysticetus). Anatom. Rec. 298, 1416–1423. doi: 10.1002/ar.23125
Barnes, B. M. (1984). Influence of energy stores on activation of reproductive function in male golden-mantled ground squirrels. J. Comp. Physiol. B 154, 421–425. doi: 10.1007/BF00684449
Berta, A., Sumich, J., and Kovacs, K. (2015). Marine Mammals, 3rd Edn. Cambridge, MA: Academic Press.
Blencowe, M., Karunanayake, T., Wier, J., and Hsu, Yang, X. (2019). Network modeling approaches and applications to unravelling non-alcoholic fatty liver disease. Genes 10:966. doi: 10.3390/genes10120966
Boggs, A. S. P., Ragland, J. M., Zolman, E. S., Schock, T. B., Morey, J. S., Galligan, T. M., et al. (2019). Remote blubber sampling paired with liquid chromatography tandem mass spectrometry for steroidal endocrinology in free-ranging bottlenose dolphins (Tursiops truncatus). Gen. Comp. Endocrinol. 281, 164–172. doi: 10.1016/j.ygcen.2019.06.006
Boggs, A. S. P., Schock, T. B., Schwacke, L. H., Galligan, T. M., Morey, J. S., McFee, W. E., et al. (2017). Rapid and reliable steroid hormone profiling in Tursiops truncatus blubber using liquid chromatography tandem mass spectrometry (LC-MS/MS). Anal. Bioanal. Chem. 409, 5019–5029. doi: 10.1007/s00216-017-0446-z
Caon, G., Fialho, C. B., and Danilewicz, D. (2007). Body fat condition in franciscanas (Pontoporia blainvillei) in Rio Grande do Sul, Southern Brazil. J. Mammal. 88, 1335–1341. doi: 10.1644/06-mamm-a-364r.1
Caprio, M., Fabbrini, E., Isidori, A. M., Aversa, A., and Fabbri, A. (2001). Leptin in reproduction. Trends Endocrinol. Metab. TEM 12, 65–72.
Christiansen, F., Sprogis, K. R., Gross, J., Castrillon, J., Warick, H. A., Leunissen, E., et al. (2020). Variation in outer blubber lipid concentration does not reflect morphological body condition in humpback whales. J. Exp. Biol. 223:jeb213769. doi: 10.1242/jeb.213769
Christiansen, F., Víkingsson, G. A., Rasmussen, M. H., and Lusseau, D. (2013). Minke whales maximise energy storage on their feeding grounds. J. Exp. Biol. 216, 427–436. doi: 10.1242/jeb.074518
Christiansen, F., Víkingsson, G. A., Rasmussen, M. H., and Lusseau, D. (2014). Female body condition affects foetal growth in a capital breeding mysticete. Funct. Ecol. 28, 579–588. doi: 10.1111/1365-2435.12200
Clark, L. S., Cowan, D. F., and Pfeiffer, D. C. (2006). Morphological changes in the atlantic bottlenose dolphin (Tursiops truncatus) adrenal gland associated with chronic stress. J. Comp. Pathol. 135, 208–216. doi: 10.1016/j.jcpa.2006.07.005
Cowan, D. F., and Curry, B. E. (2008). Histopathology of the alarm reaction in small odontocetes. J. Comp. Pathol. 139, 24–33. doi: 10.1016/j.jcpa.2007.11.009
Derous, D., Sahu, J., Douglas, A., Lusseau, D., and Wenzel, M. (2019). Adaptations of energy metabolism in cetaceans have consequences for their response to foraging disruption. bioRxiv [Preprint]. doi: 10.1101/709154
Evans, K., Hindell, M. A., and Thiele, D. (2003). Body fat and condition in sperm whales, Physeter macrocephalus, from southern Australian waters. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 134, 847–862. doi: 10.1016/S1095-6433(03)00045-X
Fair, P. A., Adams, J., Mitchum, G., Hulsey, T. C., Reif, J. S., Houde, M., et al. (2010). Contaminant blubber burdens in Atlantic bottlenose dolphins (Tursiops truncatus) from two southeastern US estuarine areas: concentrations and patterns of PCBs, pesticides, PBDEs, PFCs, and PAHs. Sci. Total Environ. 408, 1577–1597. doi: 10.1016/j.scitotenv.2009.12.021
Friedman, J. M. (2019). Leptin and the endocrine control of energy balance. Nat. Metab. 1, 754–764. doi: 10.1038/s42255-019-0095-y
Gabriely, I., Ma, X. H., Yang, X. M., Atzmon, G., Rajala, M. W., Berg, A. H., et al. (2002). Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes 51, 2951–2958. doi: 10.2337/diabetes.51.10.2951
Gearty, W., McClain, C. R., and Payne, J. L. (2018). Energetic tradeoffs control the size distribution of aquatic mammals. Proc. Natl. Acad. Sci. U.S.A. 115:201712629. doi: 10.1073/pnas.1712629115
German, A. J. (2006). The growing problem of obesity in dogs and cats. J. Nutr. 136, 1940S–1946S. doi: 10.1093/jn/136.7.1940s
Goldbogen, J. A., Cade, D. E., Wisniewska, D. M., Potvin, J., Segre, P. S., Savoca, M. S., et al. (2019). Why whales are big but not bigger: physiological drivers and ecological limits in the age of ocean giants. Science 366, 1367–1372. doi: 10.1126/science.aax9044
Gómez-Campos, E., Borrell, A., and Aguilar, A. (2011). Assessment of nutritional condition indices across reproductive states in the striped dolphin (Stenella coeruleoalba). J. Exp. Mar. Biol. Ecol. 405, 18–24. doi: 10.1016/j.jembe.2011.05.013
Hashimoto, O., Ohtsuki, H., Kakizaki, T., Amou, K., Sato, R., Doi, S., et al. (2015). Brown adipose tissue in cetacean blubber. PLoS One 10:e0116734. doi: 10.1371/journal.pone.0116734
Hasin, Y., Seldin, M., and Lusis, A. (2017). Multi-omics approaches to disease. Genome Biol. 18:83. doi: 10.1186/s13059-017-1215-1
Heiss, C. N., and Olofsson, L. E. (2018). Gut microbiota-dependent modulation of energy metabolism. J. Innate Immun. 10, 163–171. doi: 10.1159/000481519
Irvine, L. G., Thums, M., Hanson, C. E., McMahon, C. R., and Hindell, M. A. (2017). Quantifying the energy stores of capital breeding humpback whales and income breeding sperm whales using historical whaling records. R. Soc. Open Sci. 4:160290. doi: 10.1098/rsos.160290
Karczewski, K. J., and Snyder, M. P. (2018). Integrative omics for health and disease. Nat. Rev. Genet. 19, 299–310. doi: 10.1038/nrg.2018.4
Karns, B. L., Ewing, R. Y., and Schaefer, A. M. (2019). Evaluation of body mass index as a prognostic indicator from two rough-toothed dolphin (Steno bredanensis) mass strandings in Florida. Ecol. Evol. 9, 10544–10552. doi: 10.1002/ece3.5574
Kastelein, R. A., Helder-Hoek, L., Jennings, N., van Kester, R., and Huisman, R. (2019). Reduction in body mass and blubber thickness of harbor porpoises (Phocoena phocoena) due to near-fasting for 24 hours in four seasons. Aquat. Mamm. 45, 37–47. doi: 10.1578/AM.45.1.2019.37
Keane, M., Semeiks, J., Webb, A. E., Li, Y. I., Quesada, V., Craig, T., et al. (2015). Insights into the evolution of longevity from the bowhead whale genome. Cell Rep. 10, 112–122. doi: 10.1016/j.celrep.2014.12.008
Kershaw, J. L., Botting, C. H., Brownlow, A., and Hall, A. J. (2018). Not just fat: investigating the proteome of cetacean blubber tissue. Conserv. Physiol. 6:coy003. doi: 10.1093/conphys/coy003
Kershaw, J. L., Brownlow, A., Ramp, C. A., Miller, P. J. O., and Hall, A. J. (2019). Assessing cetacean body condition: is total lipid content in blubber biopsies a useful monitoring tool? Aquat. Conserv. 29, 271–282. doi: 10.1002/aqc.3105
Kershaw, J. L., Sherrill, M., Davison, N. J., Brownlow, A., and Hall, A. J. (2017). Evaluating morphometric and metabolic markers of body condition in a small cetacean, the harbor porpoise (Phocoena phocoena). Ecol. Evol. 7, 3494–3506. doi: 10.1002/ece3.2891
Knowlton, A. R., Kraus, S. D., and Kenney, R. D. (1994). Reproduction in North Atlantic right whales (Eubalaena glacialis). Can. J. Zool. 72, 1297–1305. doi: 10.1139/z94-173
Koopman, H. N. (2007). Phylogenetic, ecological, and ontogenetic factors influencing the biochemical structure of the blubber of odontocetes. Mar. Biol. 151, 277–291. doi: 10.1007/s00227-006-0489-488
Koopman, H. N., Pabst, D. A., McLellan, W. A., Dillaman, R. M., and Read, A. J. (2002). Changes in blubber distribution and morphology associated with starvation in the harbor porpoise (Phocoena phocoena): evidence for regional differences in blubber structure and function. Physiol. Biochem. Zool. 75, 498–512. doi: 10.1086/342799
Krahn, M., Herman, D., Ylitalo, G., Sloan, C., BURROwS, D., Hobbs, R., et al. (2004). Stratification of lipids, fatty acids and organochlorine contaminants in blubber of white whales and killer whales. J. Cetacean Res. Manag. 6, 175–189.
Kucklick, J., Schwacke, L., Wells, R., Hohn, A., Guichard, A., Yordy, J., et al. (2011). Bottlenose dolphins as indicators of persistent organic pollutants in the Western North Atlantic Ocean and Northern Gulf of Mexico. Environ. Sci. Technol. 45, 4270–4277. doi: 10.1021/es1042244
Lockyer, C. (2007). All creatures great and smaller: a study in cetacean life history energetics. J. Mar. Biol. Assoc. U.K. 87:1035. doi: 10.1017/S0025315407054720
Lockyer, C. H., McConnell, L. C., and Waters, T. D. (1985). Body condition in terms of anatomical and biochemical assessment of body fat in North Atlantic fin and sei whales. Can. J. Zool. 63, 2328–2338. doi: 10.1139/z85-345
Mantovani, A., Sozzani, S., Locati, M., Allavena, P., and Sica, A. (2002). Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23, 549–555. doi: 10.1016/S1471-4906(02)02302-5
Markusfeld, O., Galon, N., and Ezra, E. (1997). Body condition score, health, yield and fertility in dairy cows. Vet. Rec. 141, 67–72. doi: 10.1136/vr.141.3.67
Mathieu, P., Lemieux, I., and Després, J. P. (2010). Obesity, inflammation, and cardiovascular risk. Clin. Pharmacol. Ther. 87, 407–416. doi: 10.1038/clpt.2009.311
Miller, C. A., Reeb, D., Best, P. B., Knowlton, A. R., Brown, M. W., and Moore, M. J. (2011). Blubber thickness in right whales Eubalaena glacialis and Eubalaena australis related with reproduction, life history status and prey abundance. Mar. Ecol. Prog. Ser. 438, 267–283. doi: 10.3354/meps09174
Misra, B. B., Ruiz-Hernández, I. M., Hernández-Bolio, G. I., Hernández-Núñez, E., Díaz-Gamboa, R., and Colli-Dula, R. C. (2019). 1H NMR metabolomic analysis of skin and blubber of bottlenose dolphins reveals a functional metabolic dichotomy. Comp. Biochem. Physiol. D Genom. Proteom. 30, 25–32. doi: 10.1016/J.CBD.2019.02.004
Montie, E. W., Garvin, S. R., Fair, P. A., Bossart, G. D., Mitchum, G. B., McFee, W. E., et al. (2008). Blubber morphology in wild bottlenose dolphins (Tursiops truncatus) from the Southeastern United States: influence of geographic location, age class, and reproductive state. J. Morphol. 269, 496–511. doi: 10.1002/jmor.10602
Morfeld, K. A., and Brown, J. L. (2016). Ovarian acyclicity in zoo African elephants (Loxodonta africana) is associated with high body condition scores and elevated serum insulin and leptin. Reprod. Fertil. Dev. 28:640. doi: 10.1071/RD14140
Morfeld, K. A., Meehan, C. L., Hogan, J. N., and Brown, J. L. (2016). Assessment of Body Condition in African (Loxodonta africana) and Asian (Elephas maximus) Elephants in North American zoos and management practices associated with high body condition scores. PLoS One 11:e0155146. doi: 10.1371/journal.pone.0155146
Nahar, A., Maki, S., and Kadokawa, H. (2013). Suppressed expression of granulocyte macrophage colony-stimulating factor in oviduct ampullae of obese cows. Anim. Reprod. Sci. 139, 1–8. doi: 10.1016/j.anireprosci.2013.03.014
National Academies of Sciences, Engineering, and Medicine (2017). Approaches to Understanding the Cumulative Effects of Stressors on Marine Mammals. Washington, DC: The National Academies Press.
Nery, M. F., González, D. J., and Opazo, J. C. (2013). How to make a dolphin: molecular signature of positive selection in cetacean genome. PLoS One 8:e65491. doi: 10.1371/journal.pone.0065491
New, L. F., Clark, J. S., Costa, D. P. D., Fleishman, E., Hindell, M. A., Klanjšèek, T., et al. (2014). Using short-term measures of behaviour to estimate long-term fitness of southern elephant seals. Mar. Ecol. Prog. Ser. 496, 99–108. doi: 10.3354/meps10547
Pantasri, T., and Norman, R. J. (2014). The effects of being overweight and obese on female reproduction: a review. Gynecol. Endocrinol. 30, 90–94. doi: 10.3109/09513590.2013.850660
Parry, D. A. (1949). The structure of whale blubber, and a discussion of its thermal properties. J. Cell Sci. 90, 13–25.
Peña-Bautista, C., Baquero, M., Vento, M., and Cháfer-Pericás, C. (2018). Omics-based biomarkers for the early alzheimer disease diagnosis and reliable therapeutic targets development. Curr. Neuropharmacol. 17, 630–647. doi: 10.2174/1570159x16666180926123722
Pinzone, M., Budzinski, H., Tasciotti, A., Ody, D., Lepoint, G., Schnitzler, J., et al. (2015). POPs in free-ranging pilot whales, sperm whales and fin whales from the Mediterranean Sea: influence of biological and ecological factors. Environ. Res. 142, 185–196. doi: 10.1016/j.envres.2015.06.021
Pirotta, E., Harwood, J., Thompson, P. M., New, L., Cheney, B., Arso, M., et al. (2015). Predicting the effects of human developments on individual dolphins to understand potential long-term population consequences. Proc. R. Soc. B Biol. Sci. 282:20152109. doi: 10.1098/rspb.2015.2109
Read, A. J. (1990). Estimation of body condition in harbour porpoises, Phocoena phocoena. Can. J. Zool. 68, 1962–1966. doi: 10.1139/z90-276
Romero, L. M., and Wingfield, J. C. (2016). Tempests, Poxes, Predators, and People: Stress in Wild Animals and How They Cope. Oxford series in behavioral neuroendocrinology. Oxford: Oxford University Press.
Ruchonnet, D., Boutoute, M., Guinet, C., and Mayzaud, P. (2006). Fatty acid composition of Mediterranean fin whale Balaenoptera physalus blubber with respect to body heterogeneity and trophic interaction. Mar. Ecol. Prog. Ser. 311, 165–174. doi: 10.3354/meps311165
Sam, A. H., and Dhillo, W. S. (2010). Endocrine links between fat and reproduction. Obstet. Gynaecol. 12, 231–236. doi: 10.1576/toag.12.4.231.27614
Scholander, P. F., Walters, V., Hock, R., and Irving, L. (1950). Body insulation of some arctic and tropical mammals and birds. Biol. Bull. 99, 225–236. doi: 10.2307/1538740
Thengchaisri, N., Theerapun, W., Kaewmokul, S., and Sastravaha, A. (2014). Abdominal obesity is associated with heart disease in dogs. BMC Vet. Res. 10:131. doi: 10.1186/1746-6148-10-131
Trayhurn, P., and Beattie, J. H. (2007). Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 60, 329–339. doi: 10.1079/PNS200194
Vasseur, D. A., and Yodzis, P. (2004). The color of environmental noise. Ecology 85, 1146–1152. doi: 10.1890/02-3122
Wang, Z., Chen, Z., Xu, S., Ren, W., Zhou, K., and Yang, G. (2015). ‘Obesity’ is healthy for cetaceans? Evidence from pervasive positive selection in genes related to triacylglycerol metabolism. Sci. Rep. 5:14187. doi: 10.1038/srep14187
Williams, G. L., Amstalden, M., Garcia, M. R., Stanko, R. L., Nizielski, S. E., Morrison, C. D., et al. (2002). Leptin and its role in the central regulation of reproduction in cattle. Domestic Anim. Endocrinol. 339–349. doi: 10.1016/S0739-7240(02)00169-8
Williams, R., Vikingsson, G. A., Gislason, A., Lockyer, C., New, L., Thomas, L., et al. (2013). Evidence for density-dependent changes in body condition and pregnancy rate of North Atlantic fin whales over four decades of varying environmental conditions. ICES J. Mar. Sci. 70, 1273–1280. doi: 10.1093/icesjms/fst059
Williams, T. M., Friedl, W. A., and Haun, J. E. (1993). The physiology of bottlenose dolphins (Tursiops truncatus): heart rate, metabolic rate and plasma lactate concentration during exercise. J. Exp. Biol. 179, 31–46.
Williams, T. M., Haun, J., Davis, R. W., Fuiman, L. A., and Kohin, S. (2001). A killer appetite: metabolic consequences of carnivory in marine mammals. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 129, 785–796. doi: 10.1016/S1095-6433(01)00347-6
Yan, X., Huang, Y., Zhao, J.-X., Long, N. M., Uthlaut, A. B., Zhu, M.-J., et al. (2011). Maternal obesity-impaired insulin signaling in sheep and induced lipid accumulation and fibrosis in skeletal muscle of offspring1. Biol. Reprod. 85, 172–178. doi: 10.1095/biolreprod.110.089649
Ye, J. (2011). Adipose tissue vascularization: its role in chronic inflammation. Curr. Diabetes Rep. 11, 203–210. doi: 10.1007/s11892-011-0183-1
Keywords: cetaceans, blubber thickness, health, PCoMS, PCoD
Citation: Derous D, ten Doeschate M, Brownlow AC, Davison NJ and Lusseau D (2020) Toward New Ecologically Relevant Markers of Health for Cetaceans. Front. Mar. Sci. 7:367. doi: 10.3389/fmars.2020.00367
Received: 27 February 2020; Accepted: 30 April 2020;
Published: 28 May 2020.
Edited by:
Kelly Ortega Cisneros, Rhodes University, South AfricaReviewed by:
Danielle Kreb, Conservation Foundation for Rare Aquatic Species of Indonesia, IndonesiaLaura J. May-Collado, University of Vermont, United States
Minke Witteveen, Nelson Mandela University, South Africa
Copyright © 2020 Derous, ten Doeschate, Brownlow, Davison and Lusseau. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Davina Derous, davina.derous@abdn.ac.uk; David Lusseau, d.lusseau@abdn.ac.uk
 Davina Derous
Davina Derous Mariel ten Doeschate
Mariel ten Doeschate Andrew C. Brownlow
Andrew C. Brownlow Nicholas J. Davison2
Nicholas J. Davison2  David Lusseau
David Lusseau