Ecosystem Functioning Under the Influence of Bottom-Trawling Disturbance: An Experimental Approach and Field Observations From a Continental Slope Area in the West Iberian Margin
- 1Centre for Environmental and Marine Studies, Department of Biology, Universidade de Aveiro, Aveiro, Portugal
- 2Marine Biology Research Group, Department of Biology, Ghent University, Ghent, Belgium
- 3NIOZ Royal Netherlands Institute for Sea Research, Department of Estuarine and Delta Systems, Utrecht University, Utrecht, Netherlands
- 4Hellenic Centre for Marine Research, Institute of Oceanography, Heraklion, Greece
Understanding the effects of bottom-trawling induced changes in benthic community structure, diversity and ecosystem functioning across different benthic-size components is imperative to determine the future sustainability of bottom-trawling fisheries in deep-sea regions. In this study, we combined field sampling observations with a pulse-chase experiment on sediments obtained from two stations of interest along the West Iberian Margin (WIM) distinguished by different trawling pressures. We compared these two stations in terms of meio- and macrofauna (infauna) standing stocks, biodiversity and several ecosystem function proxies. These proxies included: (i) 13C uptake by bacterial communities, (ii) infauna respiration rates, (iii) penetration of 13C in the sediment, and (iv) sediment pore-water nutrient concentrations. The pulse-chase experimental results were complemented with a larger biological dataset partially compiled from previous studies in the area, to investigate structural and functional diversity ecosystem functioning (respiration) patterns across the WIM. Our observations indicated that different regimes of trawling pressure influenced both macrofaunal respiration rates with disturbed sediments predominantly composed of deposit-/detritus-feeding smaller-sized macrofauna species. Moreover, sediment biogeochemical functioning (ammonium profiles) and 13C bacterial uptake showed differences among the two disturbance regimes. On the contrary, the biomass of small-sized biota, including bacteria and meiofauna, did not show marked differences between stations. The general depletion in macrofauna species richness across impacted areas of the study region was also correlated with a reduction in total biomass and respiration, suggesting that the long history of trawling disturbance at the WIM may affect regulatory ecosystem functions. These preliminary findings alert for the impacts of trawling on crucial functions of benthic ecosystems that may be imperceptible to the current tools used in monitoring programs.
Introduction
There is cumulative evidence on the influence of anthropogenic activities on marine biodiversity (Ramirez-Llodra et al., 2011). This includes the deep sea, where exploitation of marine resources has been frequently associated with changes in benthic structure and biodiversity loss (Ramirez-Llodra et al., 2011 and references therein; Vanreusel et al., 2016; Stratmann et al., 2018).
Since biodiversity is potentially linked to ecosystem functions and services (Strong et al., 2015), the increasing pressure of human-induced disturbance raises serious concerns about the deterioration of ecosystem functioning and its integrity (Worm et al., 2006; Danovaro et al., 2008). In marine sediments, benthic organisms are responsible for supporting various ecosystem functions in the sediment, which varies according to their traits (e.g., size, mobility capacity, and feeding strategies). These functions can be either represented by sediment reworking, feeding and respiration activities, that directly/indirectly affect organic material mineralization and by other biogeochemical processes (Aller, 1982; Lohrer et al., 2004; Braeckman et al., 2010). The macrofauna size fraction in particular (animals generally ≥ 250 μm to few centimeters) plays a fundamental role in sustaining sediment biogeochemistry fluxes, as well as diversity and efficiency of microbial communities, either through bioturbation (particle mixing), bio-irrigation (solute transfer and sediment permeability; Aller, 1982; Lohrer et al., 2004; Braeckman et al., 2010), and/or through biological interactions (e.g., carbon transfer by predation). Similarly, certain meiofaunal taxa (i.e., Foraminifera, nematodes) also contribute to sediment processes via micro-bioturbation, particularly in the absence of diverse macrofaunal assemblages (Rysgaard et al., 2000; Bonaglia et al., 2014). As such, even small alterations in infauna standing stocks, community structure, diversity and functional traits, may result in changes in oxygen and nutrient penetration depth in the sediment, and affect microbial-mediated processes such as carbon remineralization and nutrient cycling (Aller, 1982; Lohrer et al., 2004; Braeckman et al., 2010).
Among the most destructive anthropogenic activities in the deep sea, bottom-trawling fisheries severely affect benthic organisms and may consequently have an impact on ecosystem functioning (Martín et al., 2014a; Clark et al., 2016; Sciberras et al., 2018). High faunal damage and mortality rates and marked alterations of seabed habitats have been reported in both shelf and deep-sea studies (National Research Council [NRC], 2002; Hiddink et al., 2006; Clark et al., 2016 and references therein). In soft sediments, trawl nets typically homogenize the sediment surface and, depending on trawling frequency and intensity, these may also modify sediment biogeochemistry (Sañé et al., 2013; Oberle et al., 2016). Sediment removal and remixing by trawl gears causes thick nepheloid layers, reduces sediment surface organic matter concentrations and increases sediment sorting and porosity, which inevitably weakens water-sediment nutrient fluxes (Sañé et al., 2013; Martín et al., 2014a, b,c; Oberle et al., 2016). In extreme cases, repeated bottom-trawl fisheries have physically modified entire benthic basins in terms of its composition, texture and morphology (Martín et al., 2014a). Moreover, the induced faunal mortality and alteration of habitat can modify faunal interactions and benthic community structure, and induce biodiversity loss of functionally important benthic components (National Research Council [NRC], 2002; Clark et al., 2016; Ramalho et al., 2018). Noteworthy is that effects of trawling on the benthos appear to vary depending on their size and position in relation to the seabed (infauna/epifauna), with larger-sized fauna, such as mega-epifauna and macro-infauna typically more susceptible to removal or damage by trawl gears (Jennings et al., 2001a, b; Queirós et al., 2006; Clark et al., 2016) by comparison to small-sized biota (e.g., bacteria and meiofauna; Jennings et al., 2001b; Schratzberger et al., 2002; Queirós et al., 2006).
So far, few studies have addressed the structure and diversity of infauna communities in parallel with ecosystem functions in areas affected by bottom trawling (Duplisea et al., 2001; Hiddink et al., 2006; Sciberras et al., 2016, 2017; Hale et al., 2017) and even less so in the deep sea (Pusceddu et al., 2014; Leduc et al., 2016). The study of Ramalho et al. (2017) have examined, the changes in mega-epibenthic assemblages associated with trawling, while the study of Ramalho et al. (2018), investigated changes in diversity in the infaunal component of the sediment, the macrobenthos. Both studies were carried in a continental slope area in the Western Iberian Margin (WIM) that has been subjected to intensive bottom-trawling fisheries for several decades (Leocádio et al., 2012). These studies showed that the main fishing ground area was subject to a decrease of species richness and presented important taxonomic and trophic changes in comparison to undisturbed areas, with a higher prevalence of opportunistic taxa. Moreover, macrofaunal communities in the fishing grounds showed lower trophic redundancy, which likely makes these assemblages functionally vulnerable to further increases in disturbance, as functional (trophic) complexity was sustained by fewer species when compared to undisturbed locations (Ramalho et al., 2018).
Following these studies, the present work aimed to further explore the disturbance influence on the two major components of infauna (macro- and meiofaunal) standing stocks and diversity patterns in concert with several ecosystem functions. Specifically, the first part of the study we aim to determine the existence of a biodiversity-ecosystem functioning relationships [BEF; in the sense of Solan et al. (2004)] across different locations in the SW Iberian margin. The assessment of how biodiversity relates with ecosystem functioning can assist predicting the efficiency and stability of an ecosystem under (anthropogenic) disturbance (Strong et al., 2015). In the second part of the study, we investigated in detail, using an experimental approach, several faunal parameters in relation to bioturbation activity and ecosystem function proxies (i.e., bacterial production and biogeochemical functioning) in two locations fishing ground (FG) and an adjacent area under low trawl pressure (AA).
Overall, we hypothesized that changes in infaunal standing stocks, functional traits (e.g., feeding strategies and mean size) and diversity associated with different trawling disturbance regimes, observed in previous studies, will (i) reduce bioturbation and organic matter processing in the sediment; (ii) lead to a reduction of nutrient fluxes in the sediment, and (iii) these responses will be primarily linked with changes in macrobenthic assemblages rather by meiofauna. Finally, we hypothesize that the decrease of biological diversity across the region will negatively affect secondary productivity in the fishing ground.
Materials and Methods
Study Area
The West Iberian continental margin (WIM) presents complex and diverse geomorphological features (Relvas et al., 2007; Maestro et al., 2013), such as submarine canyons and rocky outcrops. These features interact with several water masses and fronts, determining its spatial and temporal variability in salinity, temperature and oxygen content (Relvas et al., 2007).
Under the influence of the Iberian upwelling system, the high seasonal primary production along the WIM (associated with upwelling) determines productive fisheries’ conditions (Santos, 2001; Picado et al., 2014; Kämpf and Chapman, 2016). Specifically, the south and southwest regions off Portugal are among the most disturbed in Europe by bottom-contact trawling fisheries (Eigaard et al., 2016), where the majority (93.6%) of the seabed between 200 and 1000 m water depth being disturbed at least once a year. Furthermore, these fisheries are also associated with an enormous footprint per unit of landings (ca. 17 km–2 t–1; Eigaard et al., 2016), and high by-catch and discard rates (ca. 40 − 70%; Borges et al., 2001; Monteiro et al., 2001).
Sampling Strategy and Onboard Sample Processing
During the RV Belgica cruises B2013/17 (10/06/2013–18/06/2013) and B2014/15 (02/06/2014–10/06/2014), a total of seven stations were sampled along the upper continental slope off Sines and near the Setúbal Canyon (ca. 250 − 550 m depth) for the analysis of sediment environmental parameters, and meiofauna and macrofauna assemblages, in areas subjected to varying trawling pressure (Figure 1 and Supplementary Tables 1, 2). Sampling stations were initially selected based on trawling pressure information obtained from Vessel monitoring systems (VMS) data compiled by the Direção Geral de Recursos Marinhos − DGRM (MAMAOT, 2012) and as in Bueno-Pardo et al. (2017). Estimation of the pressure induced by trawling fisheries to the seabed surface is here expressed as surface swept area ratio (grid cell 0.05 × 0.05 degrees). Swept area ratio values were obtained from the OSPAR Data & Information Management system database for both 2013 and 2014 (Figure 1; OSPAR Data & Information Management System database, 2020). This, together with the visual assessment of the seabed conditions (i.e., presence and condition of the trawl scars; Ramalho et al., 2017) allowed us to confidently allocate each station to one of the following categories: not trawled (NT), an adjacent area to the main fishing ground (AA) and the fishing ground (FG). The NT label was only assigned to the stations safeguarded by current legal restrictions and where trawling has not occurred for the past decades (st. 9 and st. 10 in the vicinity of the Setúbal canyon head; null swept area ratio). The adjacent area (AA) stations correspond to those that have been either undisturbed or subjected to very few trawl passages in time and space (st. 2 and st. 6; swept area ratio ranging between 0.32 and 0.93), but are adjacent to the main fishing ground (Figures 1B,C). This AA area presented overall very few and mostly eroded trawl scars during ROV video surveys (Ramalho et al., 2017). Fishing ground (FG) stations were located in the area where crustacean otter trawlers are typically fishing (st. 1, st. 4, and st. 7; swept area ratio ≥ 1.5) and video surveys detected a high number of apparently recent trawl scars on the seabed (Figures 1B,C; Ramalho et al., 2017).
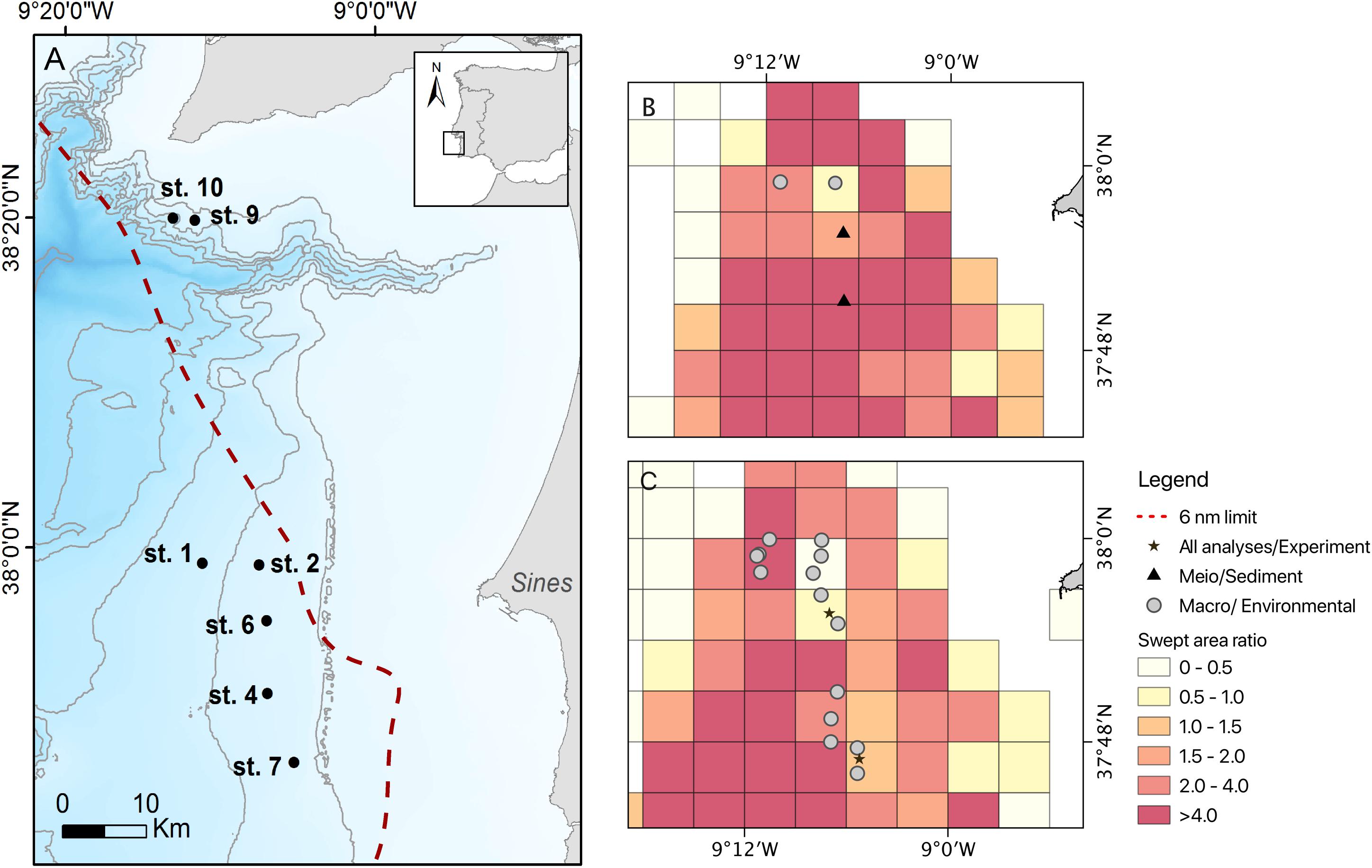
Figure 1. (A) Study area with an indication of all sampled stations and their position in relation to bottom trawling surface swept area ratio for panels (B) 2013 and (C) 2014, with the corresponding analysis: meiofauna, macrofauna and environmental parameters (sediment) or pulse-chase experiment. The Setúbal canyon area (st. 9 and st. 10) was only sampled for macrofauna and environmental parameters; bottom trawling surface swept area ratio for these stations are not shown here due to null trawling pressure values (NT). Surface swept area data was compiled from the OSPAR Data & Information Management system database (grid cell 0.05 × 0.05 degrees). Bathymetry data obtained from EMODNet Bathymetry database (2017). The red dashed line denotes the six nautical miles limit from the coastline, where trawling activities are legally prohibited.
In each station three replicate samples were collected for environmental and meiofauna analysis with a multicorer (MUC, ∅ 10 cm), whereas those for macrofauna analysis were collected with a NIOZ box corer (∅ 32 cm; Supplementary Tables 1, 2). Meiofauna and environmental samples were sliced every centimeter down to 10 cm depth and preserved in borax-buffered 4% formalin and frozen at −20°C, respectively. Macrofauna samples were initially processed by collecting the small fraction of water on the surface of each core and sieve it through a 250 μm mesh. Then the sediment was subsampled at three depth layers (0−1, 1−5, and 5−15 cm). To facilitate sediment washing and sorting procedure, each layer was washed onboard through a set of 1 mm, 500 μm and 250 μm sieves and fixed with 96% ethanol. Amongst the seven stations surveyed, two (st. 6 and st. 7) were selected for the collection of additional MUC cores in order to perform an onboard pulse-chase experiment to explore ecosystem function aspects in comparable sites at different levels of trawling pressure (see section “Time-Series Isotope Enrichment Experiment”). This selection was determined by the fact that these were the only two stations with a similar environmental setting [depth and sediment composition, being muddy-sand sediments (Instituto Hidrográfico, 2005a, b)], but different trawling pressure regimes (with swept area ratio of 0.9 and 1.5 for the st. 6 and st. 7, respectively, for the year of 2014). Even though we recognize the importance of including a true control (NT) in our experimental set up, due to differences in depth and grain-size differences with the main fishing ground/adjacent areas, this undisturbed location could not be included.
Field Sample Analyses
Environmental Variables
Sediment environmental characterization analyses included sediment grain size, total organic carbon and total nitrogen content, obtained from Lins et al. (2017); Ramalho et al. (2018) for all sampled stations (Supplementary Tables 1, 2). Grain-size distribution was determined using a particle size analyzer Malvern Mastersizer 2000, with a particle size range of 0.02–2000 μm and then classified into five categories following the Wenthworth (1922) scale: silt + clay (<63 μm), very fine sand (63 – 125 μm), fine sand (125 − 250 μm), medium sand (250 – 500 μm), and coarse sand (500 μm − 2 mm). Total organic carbon (TOC) and total nitrogen (TN), expressed as percentage of sediment dry weight, were measured using a Carlo Erba25 elemental analyzer, after acidification with 1% HCl to eliminate carbonates.
Macro- and Meiofaunal Community Analyses
Both meiofauna and macrofauna composition data were collected from the Lins et al. (2017), Ramalho et al. (2018), respectively.
In summary, sediments retained in between 32 and 1000-μm sieves, were centrifuged using colloidal silica polymer LUDOX HS-40 (specific gravity 1.19) for extraction of meiofauna organisms. This dataset, included total abundances of exclusively metazoan meiobenthic organisms classified following Higgins and Thiel (1988), Giere (2009). In addition, a list of the nematode genera abundance was also provided from the sediment surface slice (0−1 cm), estimated from a randomly picked subset of 100 to 120 nematodes mounted on permanent slides (or all nematodes when abundances were lower than 120 per sample). Deeper sediment layer were not analyzed in terms of nematode genera identification due to laboratory time restrictions. The nematodes were identified to genus level using the pictorial keys provided by Platt and Warwick (1983, 1988), online identification keys and other relevant literature available in the Nemys Database (Guilini et al., 20161). Each nematode genus was allocated to a matching trophic group, following the Wieser (1953) classification: selective deposit feeders (1A), non-selective deposit feeders (1B), epistratum feeders (2A), and predators/scavengers (2B).
Macrofauna (≥250 μm; Gage et al., 2002) individuals were sorted and identified to the lowest taxonomical level possible, and in the cases where a match with a species name was not possible; each taxon was ascribed with a consistent code across all sampled stations. To avoid overlap in the faunal size-groups investigated, “typical” meiofauna taxa, i.e., Nematoda, Copepoda and Ostracoda, were excluded from this dataset. Each taxon was assigned to a matching trophic guild according to its food source (or foraging behavior), feeding mode and food type/size, following the classification proposed by Macdonald et al. (2010) and other relevant literature available (e.g., Fauchald and Jumars, 1979; Jumars et al., 2015). The following categories were considered for: (a) food source: epibenthic (EP), sediment surface (SR), and sediment subsurface (SS); (b) feeding mode: omnivorous (Om), deposit feeders (De), detritus feeders (Dt), grazers (Gr), scavengers (Sc), predators (Pr), suspension/filter feeders (Su), mixotrophs (Mx) and suctorial parasites (Sp); and (c) food type/size: sediment (sed), particulate organic matter (poc) microfauna (mic), meiofauna (mei), macrofauna (mac), zooplankton (zoo), and fish (fis).
Biomass
Nematode biomass measured in this study was determined for a subsample of 100−120 individuals per sediment layer. Individual nematode length [excluding filiform tail tips; L (μm)] and maximum body width [W (μm)] was measured under the compound microscope (Olympus BX-50) with Olympus Cell^D software, and body volume estimated by applying Andrassy’s formula (wet weight; Andrassy, 1956; Wieser, 1960). A ratio of a 0.124 was assumed to convert nematode wet weight into carbon weight (μgC; Baguley et al., 2004).
Macrofauna biomass data obtained from Ramalho et al. (2018), as wet weight (mg) grouped by specimens of the same family for sample and each sediment layer (0−1; 1−5; and 5−15 cm), was converted into carbon weight (mgC) following the taxa-specific conversion factors of Rowe (1983). Due to their small values, macrofaunal wet weights were measured by transferring all individuals belonging to the same family in each sub-sample to previously weighed microtubes containing 96% ethanol that were then weighed again to obtain the wet weight of the lot.
Individual mean biomass was calculated as the weight of the taxon group divided by the number of individuals counted, while total biomass was calculated as the sum of the products of individual biomass and abundance of each taxon. Total biomass for meio- and macrofauna were expressed as mg C.m–2.
Allometric Respiration Rates
Allometric respiration estimates were calculated for both nematode (meiofauna) and macrofauna assemblages following Mahaut’s formula (Mahaut et al., 1995). The mass dependent respiration rate (R, d–1) was calculated as:
where W is the mean individual biomass (in mg C), and the constant α=7.4×10−3 and b = −0.24. Total community respiration of both meiofaunal and macrofaunal assemblages was calculated as the product of the mass-dependent respiration rate (R) and total biomass (in mgC.m–2), expressed as mgC.m–2.d–1.
Time-Series Isotope Enrichment Experiment
Experimental Set-Up
In addition to field sampling, a pulse-chase experiment was performed during the B2014/15 cruise, aiming to measure bioturbation and different proxies of ecosystem functioning at three distinct time points: at the start of the experiment (T0), after 3 (T3), and 5 (T5) days. The end of the experiment (T5) was determined by the ship time available, and T3 was considered as a mid-point observation. Sediments were collected from two 300-m deep stations that exhibited distinct trawling disturbance regimes, i.e., st. 6 (AA) and st. 7 (FG; Figure 1), but similar environmental setting (muddy-sand sediments), initially established from detailed sediment charts from Instituto Hidrográfico (2005a,b). By choosing two areas with similar environmental characteristics we attempted to attenuate the influence of important environmental conditions on our observations (e.g., grain size, permeability and food availability), known to strongly shape deep-sea infauna assemblages, as well as oxygen and nutrient fluxes in the sediment (Levin et al., 2001; Glud, 2008). Furthermore, even though we recognize the importance of including an area close to pristine conditions and legally protected (NT) in the experimental set up, due to differences in depth and grain-size differences with the main fishing ground, this could not be included in the experimental set-up.
In total, 18 MUC cores were collected: nine at each station (AA and FG) with three replicates at each time point (Supplementary Table 2). The cores were maintained in a cold room in the dark for 24h at in situ water temperature, i.e., 12°C, and constant oxygen flow provided by aquarium pumps. After acclimatization, each core was randomly assigned to a distinct sampling time step (n = 3 for T0, T3, and T5) and, except for the cores assigned to T0 that were used as controls, a suspension of 13C labeled algae (Skeletonema costatum) was added homogeneously to the sediment surface of each core with a long pipette (ca. 2.6 mg C per core; 26% of 13C enrichment). S. costatum was chosen because it is a common diatom species in phytoplankton assemblages throughout the year along the Iberian Margin (Silva et al., 2009). At each time step, the selected cores from each trawl pressure group were sliced per centimeter down to 10 cm, and subsampled for the analysis of: 13C uptake by sedimentary total organic carbon (ca. 2 ml), 13C uptake by bacteria-specific phospholipid-derived fatty acids (PLFAs) (ca. 10 ml), and pore-water nutrients concentrations (remaining sediment for both ammonium and nitrate concentrations). Sub-samples for pore-water nutrient concentrations were stored at −20°C, while the remaining sub-samples were stored at −80°C for further laboratory analysis. Bacterial biomass from the T0 samples was used in conjunction with meio- and macrofaunal biomass to compare infaunal standing stocks between AA and FG (only possible for the surface layer 0−1 cm).
Assessment of Biogeochemical Functioning, Bioturbation and Bacterial Biomass and Production
The pore-water dissolved inorganic nitrogen concentrations, specifically ammonium and nitrate concentrations (expressed as μmol.l–1), were investigated along the vertical sediment profile (down to 10 cm), as a proxy for biogeochemical functioning. Changes in these nutrients concentrations within the sediment column can indirectly indicate changes in bioirrigation and bioturbation, as well as carbon remineralization processes (Lohrer et al., 2004; Volkenborn et al., 2007). The pore-water was extracted from each sub-sample through Whatman GF/C filters and analyzed using a continuous flow analyzer the SKALAR SAN.
Bioturbation was inferred from 13C incorporation in the sediment. Each sediment sub-sample was first freeze-dried and grinded. Quantification of organic carbon content and isotopic ratios were then carried out using a Thermo Flash EA 1112 element analyzer, coupled with a Thermo Delta V Advantage Isotopic mass spectrometer (Thermo Fisher scientific). Due to laboratory and analysis constraints, 13C labeled algae content in the sediment and corresponding total organic carbon and total nitrogen values in the experimental cores were only measured down to 5 cm depth.
Bacteria 13C algae uptake (production) and biomass were derived from the concentrations of bacteria-specific phospholipid-derived fatty acids PLFA’s, for the layers 0−1 cm and 4−5 cm as described by van Oevelen et al. (2006). The polar lipid fraction was extracted from the freeze-dried and grinded sediments and derivatized using the mild alkaline methanolysis to yield fatty acid methyl esters (FAMEs), following the Bligh and Dyer method (Bligh and Dyer, 1959; Boschker, 2004). 13C concentrations of this component were analyzed with a gas chromatography combustion interface isotope-ratio mass spectrometer (GC-C-IRMS). Due to laboratory and analysis constraints we analyzed only 0−1 and 4−5 cm for comparison. The bacteria-specific PLFAs used included the i14:0 and ai15:0, present in all of our samples, and accounted roughly for 8% of all bacterial PLFAs (Middelburg et al., 2000) and 5.6% of the total carbon content in bacterial cells (Brinch-Iversen and King, 1990), allowing for the estimation of total bacterial biomass.
Data Analyses
BEF Relationships Under Different Trawling Regimes at the WIM
Correlations between structural diversity and ecosystem functions (i.e., respiration rate and total respiration) for the whole meio- and macrofauna field dataset (both B2013/17 and B2014/15 campaigns), was explored by means of non-parametric Spearman rank correlations using the software GraphPad PRISM v6. Similar procedure was applied for analyzing the relationship between ecosystem functions (i.e., respiration rate and total respiration) and trawling pressure (swept area ratio). Significant p-Values were adjusted for multiple testing using the Bonferroni correction (Shaffer, 1995), by dividing the significance value of each test by the number of hypotheses tested. Biodiversity indices for all seven stations were calculated using the software PRIMER v6 (Clarke and Gorley, 2006).
Comparison Between the Highly Disturbed and Low Disturbed Stations (FG and AA)
The biological and environmental data from the field samples collected at the same time and location as the samples for the pulse-chase experiment (B2014/15; st. 6 and st. 7, from here on designated as the AA and FG stations, respectively), were tested for differences by means of non-parametric Mann-Whitney U-tests, after rejection of normality and homogeneity of dispersion (Quinn and Keough, 2002), using the software GraphPad PRISM v6. The environmental parameters tested for the surface (0−1 cm) and sub-surface (4−5 cm) by means of non-parametric Mann-Whitney U-tests included: grain-size, porosity, total organic carbon (TOC) and total nitrogen (TN). Biological parameters were only compared from the surface layer (0−1 cm), and included total bacterial biomass, meio- and macrofaunal abundance, meio- and macrofaunal mean individual biomass/total biomass and meio- and macrofaunal respiration rate/total respiration. Note that comparisons for the deeper layers (>1 cm) were not done here due to the absence of consistent data for all benthic size-groups for the different sediment depth layers. Nematodes were the dominant meiofaunal taxon (68−90%), and thus with “meiofauna” diversity and biomass, we are only referring to the nematodes. Taxonomic and functional (trophic) biodiversity patterns were also analyzed for meio- and macrofauna for both stations (AA and FG) using several diversity indices, namely: species or genus richness/trophic guilds richness (S/TG), Shannon-Wiener diversity (H’), evenness (J’) (Pielou, 1966) and Hurlbert’s expected number of taxa or trophic guilds (ES(n)/ETG(n)) for 20 individuals (Hurlbert, 1971). These biodiversity indices were calculated using the software PRIMER v6 (Clarke and Gorley, 2006), and were also tested for differences between stations by means of non-parametric Mann-Whitney U-tests, using GraphPad PRISM v6.
Ecosystem functions investigated during the enrichment experiment included: biogeochemical functioning (by assessing vertical profiles of pore-water ammonium and nitrate), bioturbation (13C sediment uptake) and bacterial production (13C bacterial uptake). These variables were tested separately for differences between stations subjected to distinct trawling pressures [“Station(TP)”] over time (“Time”) and accounting for sediment depth dependency (“Sediment depth”), by means of a permutational multivariance analysis of variance (PERMANOVA) using PRIMER v6 and the PERMANOVA + add-on (Clarke and Gorley, 2006; Anderson et al., 2008). These tests were applied on a Euclidean distance matrix after normalization of the dataset. The PERMANOVA design followed a 4-factor layout, with “Station (TP)” as a fixed factor (levels: AA and FG); “Time” as a fixed factor [levels: T0 (only for ammonium/nitrate concentrations), T3, T5]; “Sediment depth” as a fixed factor (levels: every cm down to 10 cm for biogeochemical functioning, and 0−1 and 4−5 cm for the other variables), and “replicate core” as a random factor nested in “Station (TP) × Time.” This design allowed to account for the dependency of the depth layers within each replicate. When a statistically significant effect (p ≤ 0.05) was found for any of the factors investigated in the PERMANOVA main test, pair-wise pseudo-t-tests were subsequently performed. Homogeneity of multivariate dispersions was also tested using the PERMDISP routine, but none of the factors identified by the PERMANOVA tests showed a significant dispersion effect. Corresponding total organic carbon and nitrogen content in the experimental cores down to 5 cm were also investigated using similar statistical analyses.
Results
BEF Relationships Under Different Trawling Regimes at the WIM
We identified significant negative correlations, after Bonferroni correction, between trawling pressure and macrofauna total respiration (R = −0.512; p ≤ 0.01; Figure 2F), and total macrofauna biomass (R = −0.514; p ≤ 0.01; Figure 2B), while respiration rates were positively correlated with trawling pressure (R = 0.363; p ≤ 0.01; Figure 2D). Note that between trawling pressure and different measures of meiofauna/nematode abundance and diversity no significant correlations were detected (Supplementary Figure 1), nor between trawling pressure and nematode respiration rates and total respiration (Figures 2A,C,E).
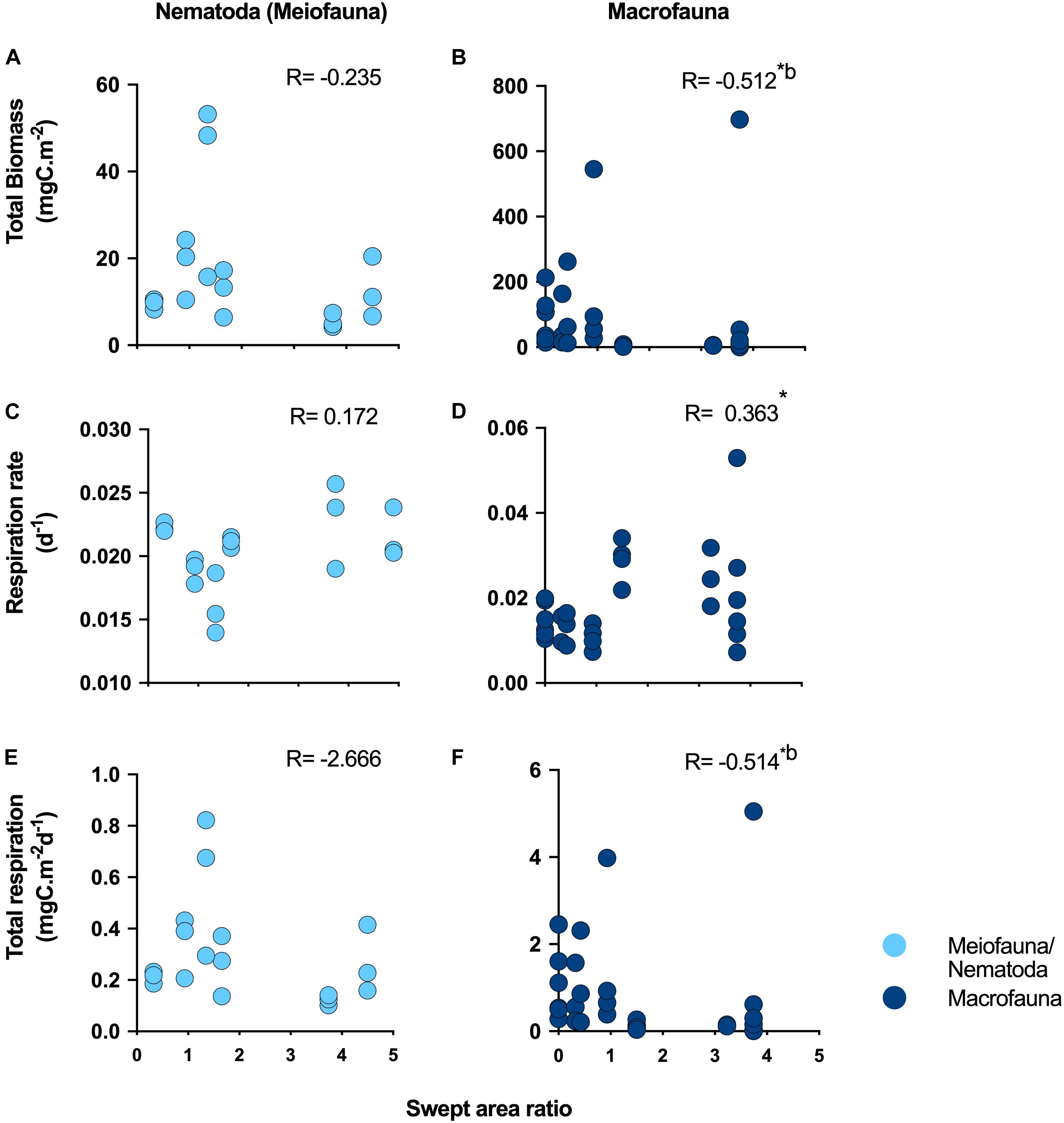
Figure 2. Ecosystem functioning relationship with bottom trawling surface swept area ratio. Ecosystem functions investigated included (A,B) total biomass; (C,D) respiration rate and (E,F) total respiration on the sediment surface (0–1 cm) for Nematoda (meiofauna) and macrofauna, respectively. *Indicates statistically significant correlations; bindicates statistical significance after Bonferroni correction.
Macrofauna BEF relationships investigated through correlations between species richness and ecosystem metabolism proxies (respiration rates and total respiration) were only significant (positive) for total respiration (R = 0.433; p ≤ 0.05) (Table 1). Significant positive correlations were also identified between macrofauna species richness and biomass (R = 0.030; p ≤ 0.05; Table 1). (Negative) BEF correlations for meiofauna were identified between nematode genus richness and respiration rates (R = −0.683, p ≤ 0.01), however, only within FG stations (Supplementary Table 3).
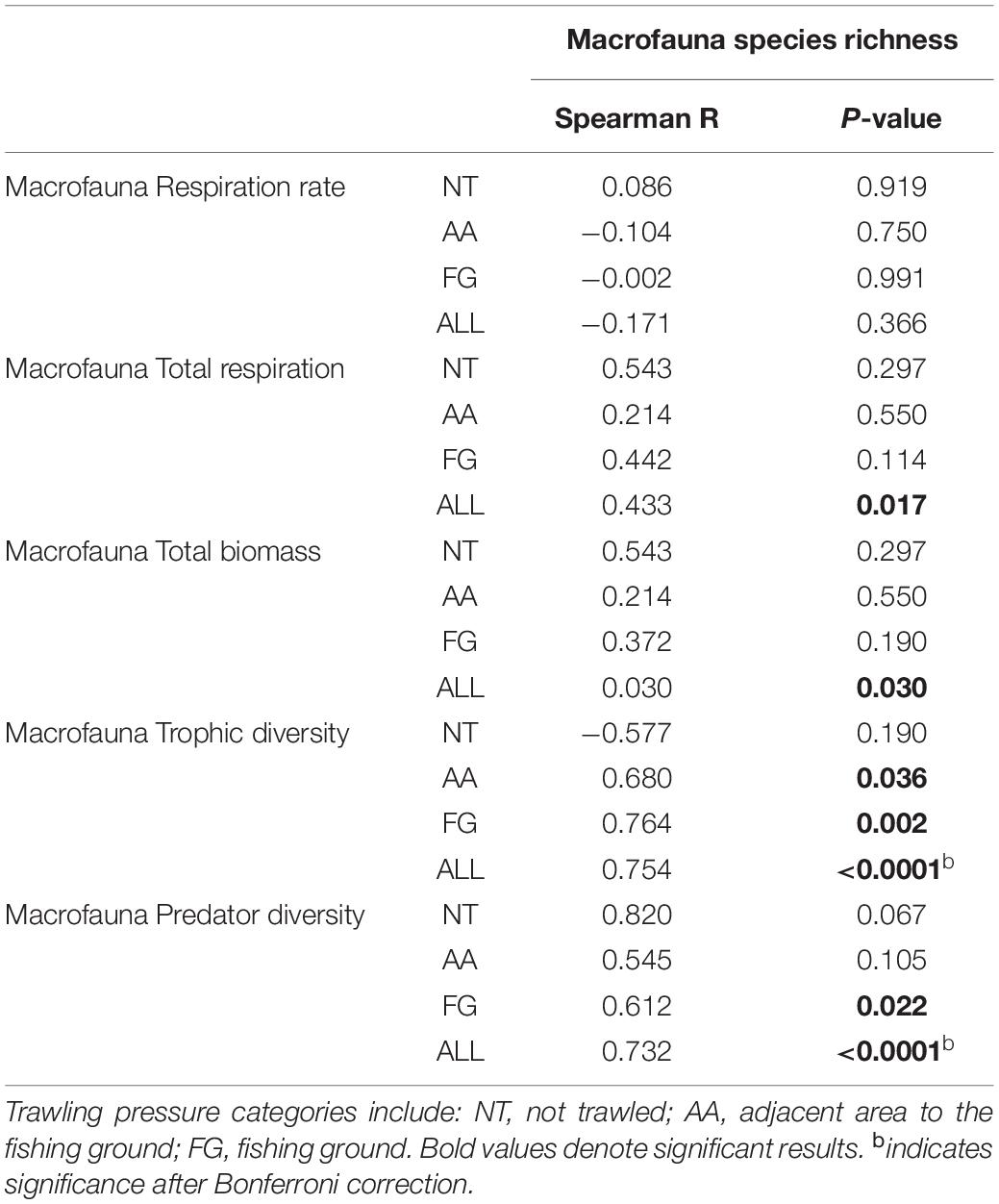
Table 1. Overview of the non-parametric Spearman-rank correlations results for macrofauna species richness and macrofauna biomass, macrofauna associated ecosystem function (respiration), and functional (trophic) diversity.
Significant positive relationships after Bonferroni corrections were identified between macrofauna species richness and trophic (functional) diversity (R = 0.754; p ≤ 0.01; Table 1). Specifically, predator-feeding guilds were positively linked with species richness (R = 0.732; p ≤ 0.01; Table 1), despite the comparable relative contribution of these feeding guilds to the macrofauna trophic structure among all stations (Table 1).
Comparison Between the Highly Disturbed and Low Disturbed Stations (FG and AA)
Environmental Parameters
Generally, similar environmental conditions were observed at the two stations sampled for the pulse-chase experiment (AA and FG) in both the surface (0−1 cm) and sub- surface layers (4−5 cm; Table 2). Overall, sediments were characterized as muddy-sand (silt + clay content > 10%) composed of high proportions of both very fine and fine sand content (ca. 40−55%), with no significant differences in terms of sediment porosity for either the surface layer (U = 3; p > 0.99) and sub-surface (U = 2; p > 0.80). TOC and TN concentrations were also similar in both stations, both at surface (U = 2; p > 0.80 and U = 2; p > 0.80) and sub-surface layers (U = 0; p > 0.20 and U = 1; p > 0.40).
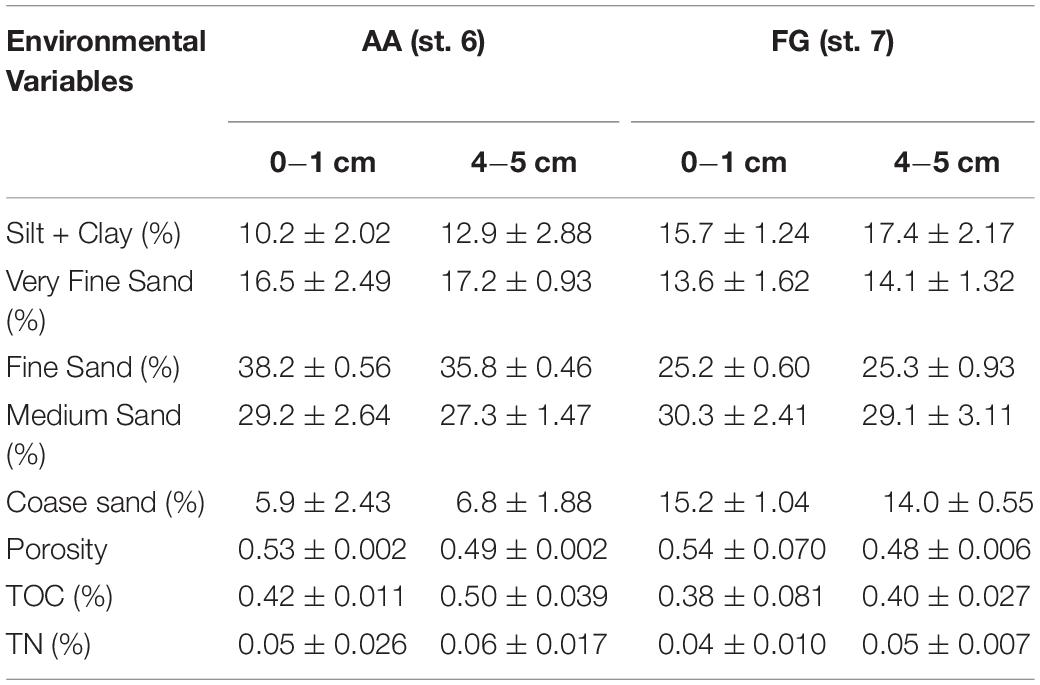
Table 2. Overview of the sediment environmental characteristics (average ± standard error) at the fishing ground (FG) and its adjacent area (AA) stations in the sediment surface (0−1) and subsurface layers (4−5) cm.
Infaunal Standing Stock, Diversity, and Trophic Composition
The infauna (meio- and macrofauna combined) showed consistently higher abundances in the 0−1 cm layer at FG (st. 7) than at AA (st. 6; Figure 3A). Total macrofauna abundances were 672 ± 194.7 and 1035 ± 145.9 ind.m–2 at the AA and FG station, respectively, and differed significantly (U = 0; p ≤ 0.05). Meiofauna was typified by the dominance of nematodes (68−90%) and total abundances found in the AA and FG station, respectively, were 39367 ± 5948.8 and 49070 ± 7656.6 ind.m–2. No significant differences among stations were found for meiofauna total abundances (U = 1; p > 0.05).
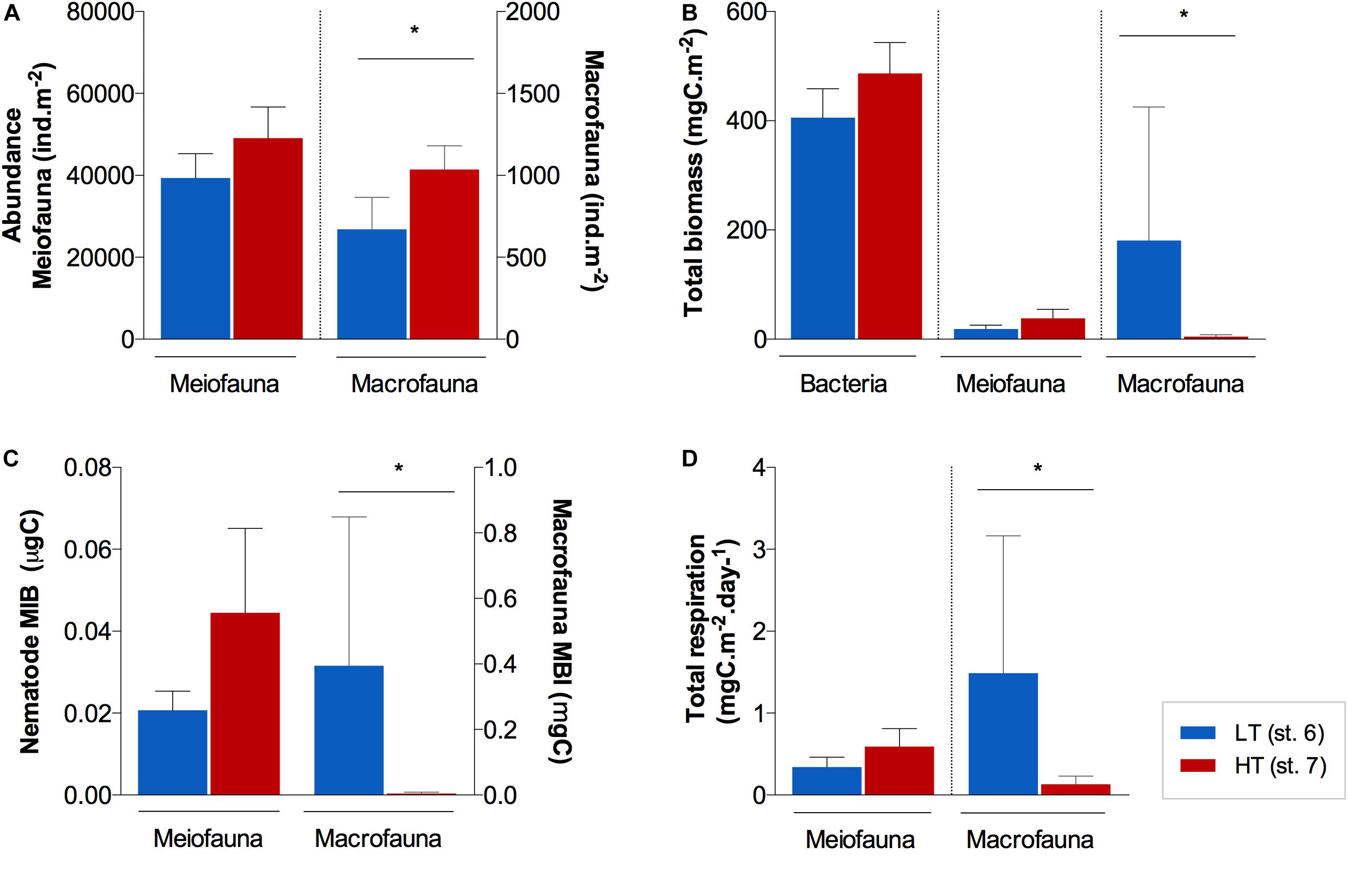
Figure 3. Average (±standard error) benthic (A) abundances, (B) biomass, (C) mean individual biomass (MIB), and (D) total respiration for all size groups (bacteria, meiofauna and macrofauna) at the surface of the sediments (0–1 cm) of station AA and FG. Note that for bacteria, only biomass measurements were available. *Indicates significant differences between stations (p ≤ 0.05).
Unlike abundance, benthic biomass showed contrasting trends between stations, dependent of the size group. Overall, bacteria were the main contributor to total biomass at both stations (Figure 3B), with a higher average contribution at FG (91%) than at AA (67%), although not significantly different (U = 1; p > 0.05). Nematoda (meiofauna) was identified as the second most important contributor to total benthic biomass at FG with larger nematode genera, while macrofauna relative contribution prevailed over meiofauna at AA (macrofauna ca. 30% at AA vs. 1% at FG). Macrofauna biomass was significantly different between these stations (U = 0; p ≤ 0.05; Figure 3B), associated with a much higher mean individual weight at the sediment surface (0−1 cm) of AA (st. 6; 0.34 ± 0.227 mgC; Figure 3C).
Macrofauna and nematode biodiversity indices did not differ significantly between the AA and FG stations (p > 0.05), with the exception of macrofauna ETG(20) (U = 0; p ≤ 0.05), which indicated a higher trophic (functional) diversity at AA when compared to FG (Table 1). Macrofauna trophic structure was more complex (Supplementary Figure 2B) in the AA sediments. This resulted from relatively even contributions of the various trophic groups that comprised the macrofaunal assemblages at AA station. At the AA station, the relative contribution of deposit and detritus feeders (37%) was highest, followed of predators (23%), suspension feeders (16%), omnivores (8%), and gazers (5%; Supplementary Figure 2B). The FG station was characterized by a larger contribution of both surface and subsurface deposit and detritus feeders (56%). Nematode trophic composition at AA was also composed by lower contributions of Wieser (1953) equivalent to deposit/detritus feeding guilds (1A + 1B; 41%) when compared to FG (53%; Supplementary Figure 2A).
Ecosystem Functions
Total nematode respiration estimates varied between 0.34 ± 0.069 and 0.59 ± 0.111 mgC.10 m–2d–1 in AA and FG sediments, respectively, not differing significantly (U = 2; p > 0.05; Figure 3D). Total respiration estimates for the macrofauna assemblages showed significantly higher values (U = 0; p ≤ 0.05) in AA (1.49 ± 1.676 mgC.m–2d–1) than in FG sediments (0.13 ± 0.098 mgC.m–2d–1) (Figure 3D).
Biogeochemical functioning, investigated through pore-water nutrient concentrations, showed marginally significant higher concentration of ammonium in FG (F = 5.3926; p = 0.0485). Ammonium concentrations increased with depth (F = 27.609; p ≤ 0.01); with the increase in ammonium concentrations in sediment layers deeper than 5 cm relative to the surface and subsurface layers (0 – 4 cm; Supplementary Tables 4, 5), within the first 3 days of experiment (Figures 4A,B). Ammonium profiles were similar after 5 days in both AA and FG stations (Figure 4C). Pore-water nitrate concentrations significantly decreased below 3 cm depth, but no significant differences were detected between the profiles of the two studied stations for the whole experiment duration (Figures 4D–F and Supplementary Tables 6, 7).
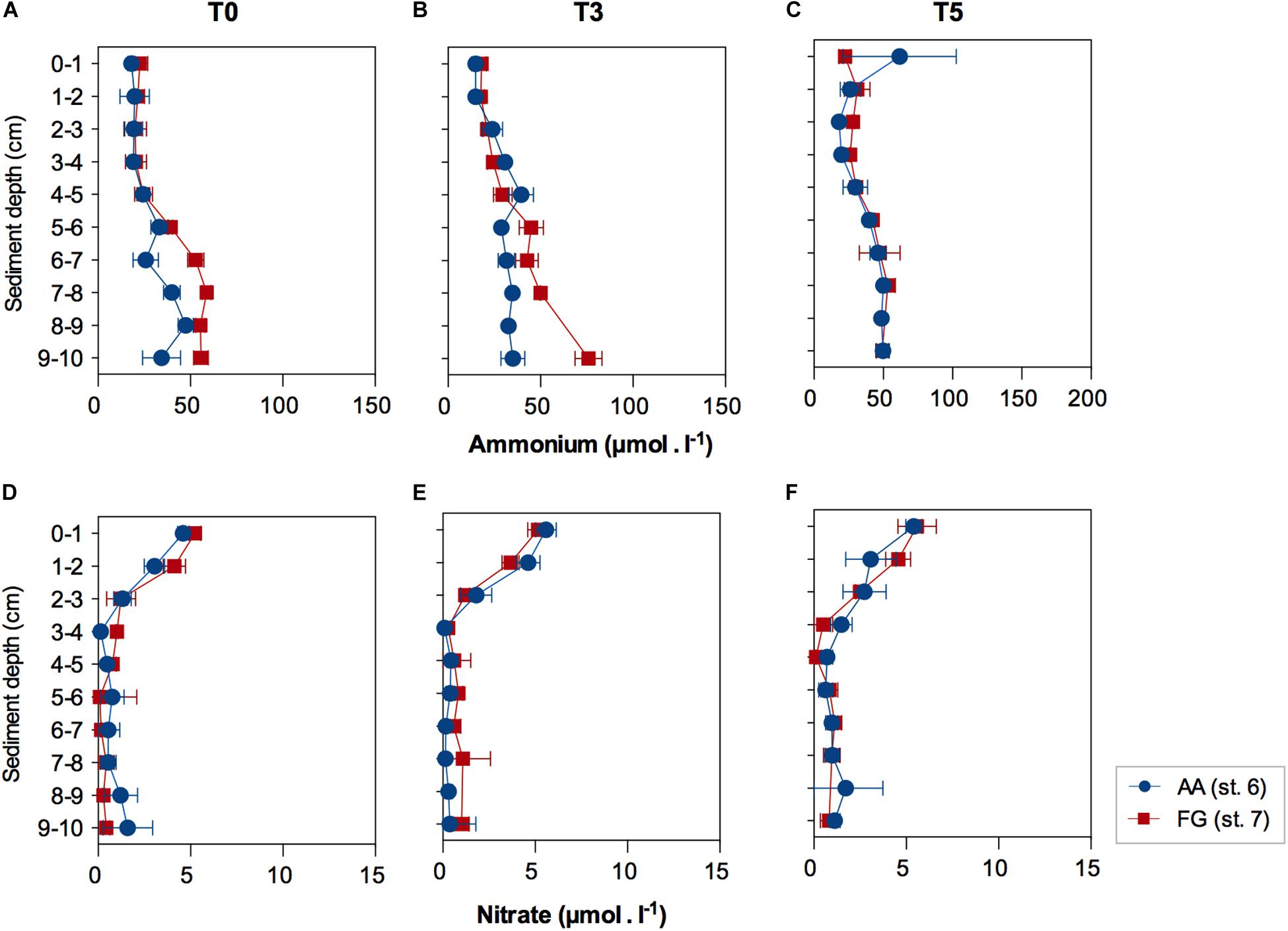
Figure 4. Pore-water ammonium and nitrate concentrations (average ± standard error) in function of sediment depth at the AA and FG stations, after (A,D) acclimatization (T0), (B,E) three (T3) and (C,F) five (T5) days. Values expressed as average ± standard error.
The 13C labeled algae added were detected within the first 3 days of the experiment down to 3−4 cm sediment depth. After 5 days, the 13C labeled algae signal was detected in both AA and FG down to the deepest sediment layer investigated (4−5 cm), yet no significant differences were detected between the two stations (F = 0.505; p > 0.05). Significant differences were found between the sediment depth layers (F = 68.702; p ≤ 0.01) over time (F = 5.549; p ≤ 0.05) associated with evident transport of the 13C labeled algae to the deeper layer over the course of the experiment (Figures 5A,B and Supplementary Tables 8, 9). FG sediments showed overall significantly higher concentrations of organic carbon (%) in the sediment surface layers over the course of the experiment (Supplementary Figure 3 and Supplementary Tables 10, 11), while nitrogen concentrations were more variable among replicates (Supplementary Tables 12, 13).
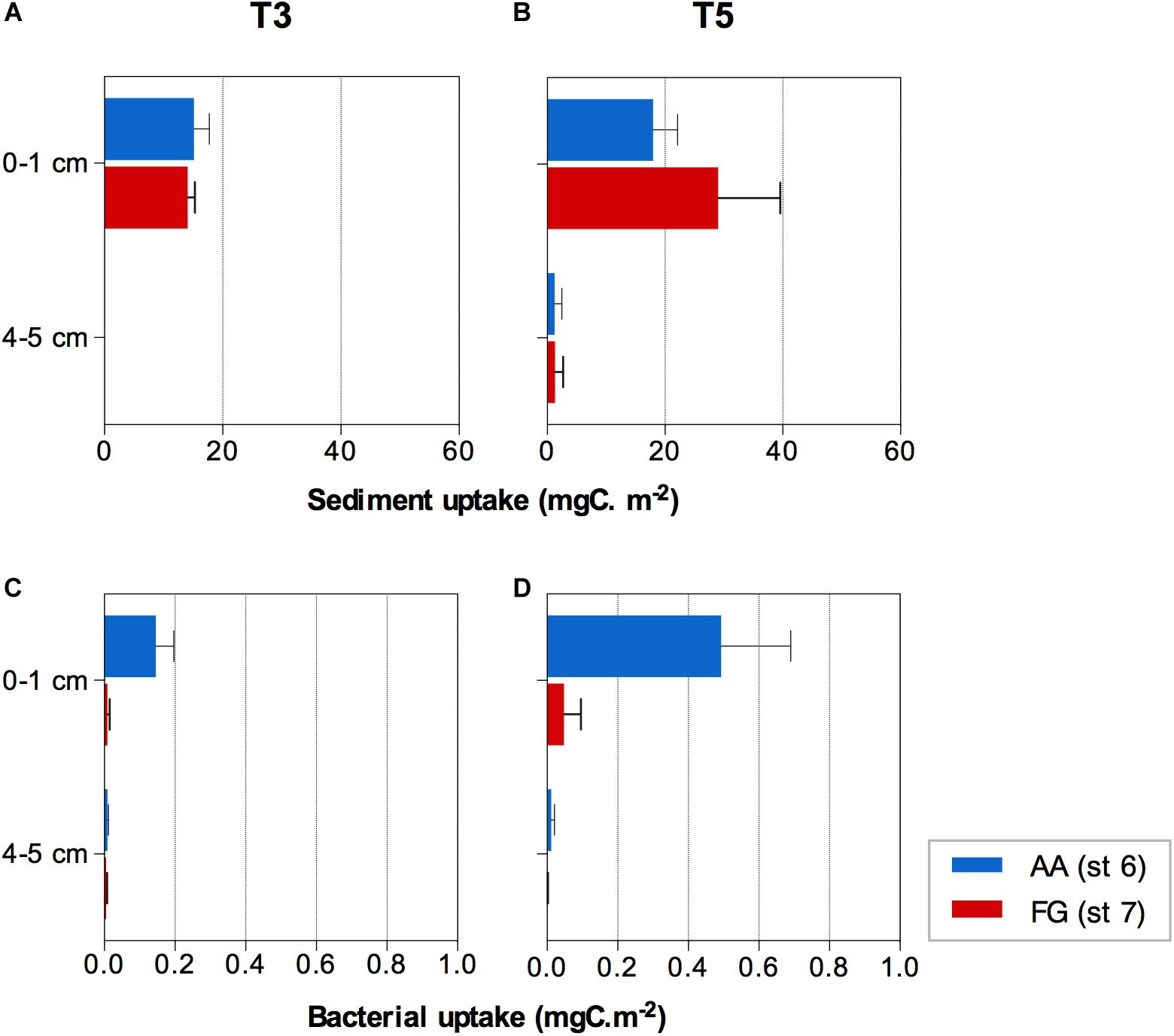
Figure 5. 13C algae uptake by the (A,B) sediment and (C,D) bacterial communities at the AA and FG station after three (T3) and five (T5) days, respectively. Values expressed as average ± standard error.
The average uptake of the 13C labeled algae by bacteria (bacterial production) showed significant differences between stations with different trawling pressure and sediment depths (interaction of both factors; F = 9.777; p ≤ 0.05; Supplementary Table 14), mostly due to higher uptake in the surface layer (0−1 cm; pair-wise test t = 2.957; p ≤ 0.05; Supplementary Table 15). The higher bacterial uptake was consistently observed at the AA station after both 3 and 5 days (Figures 5C,D).
Discussion
Bottom trawling activities are effectively associated with the deterioration of the seabed integrity, not only by altering topography, substrate structure, but also by causing both direct and indirect changes on the benthic assemblages (Martín et al., 2014a; Clark et al., 2016). As a consequence of this activity, changes in taxonomic and functional diversity (e.g., Duplisea et al., 2001; National Research Council [NRC], 2002; Lohrer et al., 2004) may occur, leading to altered ecosystem functions in the sediment. Our primary goal in this study was to investigate how changes in both meio- and macrofauna standing stocks diversity and traits (feeding preferences, size-spectra) are related to sediment ecosystem functions in areas subjected to different trawling pressure regimes. To our knowledge, trawling pressure on benthic processes has received little attention in the deep sea, such as the Western Iberian margin (WIM), where trawling is known to impose an enormous pressure on benthic habitats (Eigaard et al., 2016). In the context of the European Union’s Marine Strategy Framework Directive 2008/56/EC (MSFD, European Commission, 2008) the existing assessment of Good Environmental Status (GES) have a low degree of confidence, and is hindered by the limited availability of data (MAMAOT, 2012), including for key descriptors, such as descriptor 1 (biodiversity is maintained) and descriptor 6 (seafloor integrity insures functioning of the ecosystems) (European Commission, 2008).
Diversity and Ecosystem Function Trends Across the WIM
General diversity trends investigated in field samples (Lins et al., 2017; Ramalho et al., 2018) allowed us to account for the spatial heterogeneity beyond the two stations investigated during the pulse-chase experiment. Overall there was a general decrease in species richness with increasing trawling pressure for macrofaunal assemblages (Ramalho et al., 2018), but not for meiofaunal genus richness. This cannot be interpreted as a non-detrimental influence of trawling pressure on nematode diversity, since unlike the macrofauna, nematodes were not identified down to species level, and also because the metrics investigated may not be sensitive enough to detect effects on nematode assemblages. However, as nematode biodiversity showed no correlation with increasing trawling pressure, further interpretation of the BEF relationship (even if absent) cannot assist predicting nematode-related ecosystem functioning alterations under trawling disturbance conditions in the present study.
Regarding macrofauna assemblages, the highly disturbed locations showed overall a decrease in biomass with increasing trawling pressure, which suggests that the shift in the benthos size structure under conditions of high disturbance may be consistent across the study region. Furthermore, we identified significant positive correlations between macrofauna species richness and total biomass and respiration, and with trophic (functional) diversity, where both NT and AA displayed consistently the highest functional diversity, including predator richness. Baldrighi and Manini (2015), Baldrighi et al. (2017), found similar positive correlations (both linear and exponential) between biodiversity and functions related to macrofaunal assemblages (i.e., biomass, trophic diversity, predator richness), despite larger sample size, differences in geographical areas, depth ranges and biodiversity (species richness) ranges investigated in their studies. The observed alterations of the trophic structure, respiration rates and benthic secondary production (indirectly assessed by biomass), in relation to trawling disturbance, is an indication of its negative influence on nutrient and energy fluxes across the food web. As energy transfer in marine systems (across the food web) is predominantly determined by biotic interactions among organisms (e.g., predation, but also competition, facilitation; Strong et al., 2015; Spiers et al., 2016), the capture and conversion of primary production into secondary production by consumers is a key function of the benthos (Strong et al., 2015). Moreover, there is increased evidence that loss of species at higher trophic levels would have more severe effects on the stability of food webs through top-down control, and thus groups such as predators can have a crucial role in carbon and energy cycling (Atwood et al., 2015; Spiers et al., 2016).
Despite the indication of detrimental effects suggested by our results, they need to be interpreted with caution owing to the poor replication in relation to spatial environmental heterogeneity and trawling disturbance intensity. Additional observations are needed to determine if the observed significant BEF relations can be extrapolated across depth-ranges and along the WIM. This will be key to predict, with high confidence, how disturbance patterns (e.g., exploitation of new fishing grounds) may affect ecosystem functions that are facilitated by the infaunal communities in the region.
Changes in Benthic Infauna and Associated Ecosystem Functions
Different benthic faunal compartments (e.g., macrofauna and meiofauna) have different capacities to sustain, recolonize and re-establish after one or several disturbance events. Their distinct responses will depend on both the assemblage traits, turnover rates and faunal interactions (e.g., prey-predator relations, facilitation processes; Sciberras et al., 2017), as well as on the post-disturbance habitat conditions (Clark et al., 2016 and references therein; Yesson et al., 2016). Post-disturbance environmental conditions in soft sediments habitats will be determined by direct changes in sediment structure (e.g., porosity and permeability), but also by alterations of the biotic and abiotic processes that follow.
Our results suggest that some of ecosystem functions investigated may be impaired, under conditions of varying disturbance history. These differences were primarily perceived by an increase of total macrofaunal abundances in the FG station, which was in fact mostly due to an increase of the smaller sized taxa (lower MIB) inhabiting disturbed sediments. This was also associated with differences in trophic structure in the two investigated stations (larger proportion of surface and subsurface deposit and detritus feeders in the fishing ground; Ramalho et al., 2018). The influence of trawling disturbance on macrofauna was also evident in the lower total respiration for macrofauna in FG (st. 7), but not in nematode (meiofauna) assemblages.
The shift in species size and traits toward smaller opportunistic taxa under conditions of disturbance also observed in mega-epibenthic taxa by Ramalho et al. (2017) has been associated with a lower recovery capacity of sediment biogeochemical conditions, as mediation of macronutrients and carbon cycling was decoupled (Hale et al., 2017). In the present study, in addition to the differences in terms of traits of macrobenthos (both trophic, mean size, and respiration rates) between the two FG and AA stations, we have also observed significant differences in bacterial uptake rates and biogeochemical functions based on ammonium concentrations in sediments collected in these stations. Specifically, bacterial uptake rates associated with the carbon transformation processes were lower in FG sediments, while an increase in ammonium concentrations was observed in FG sediments in deeper and typically anoxic layers during the first 3 days of the experiment. Increases in ammonium concentration in anoxic layers can occur under alterations of the denitrifying bacterial communities and/or depleted oxygen concentrations required to convert ammonium into N2 via anaerobic ammonium oxidation (anammox) (Laverock et al., 2011). Similar rises in ammonium concentrations were found by Hale et al. (2017), while also similarly to this study, nitrate profiles showed no differences between sediments of low to high fishing disturbance. The high ammonium concentrations found here, together with the presence of smaller-sized macrofaunal individuals (lower MIB) in the FG sediments compared to the AA sediments could point to inefficient bioturbation activity in the disturbed area that could indirectly affect biogeochemical functions and bacterial productivity. Smaller macrofauna individuals (lower MIB), but conversely larger nematode genera in FG sediments when compared to AA sediments, are congruent with a deprived oxygen provision in the deeper layers of the sediments in highly disturbed seafloor areas. A decrease in macrofauna standing stock (Levin, 2002) and the prevalence of larger-sized nematodes (larger MIB observed in FG than in AA sediments) has been observed in oxygen-deprived deep-sea areas. The latter has been considered an adaption to maximize oxygen absorption under oxygen-deprived conditions (Jensen, 1986). Although the present results give some indication of changes in ecosystem function depending on trawling disturbance, a direct link between changes in macrofauna size-spectra and a depletion of biogeochemical functions and bacterial productivity cannot be effectively established. The investigated faunal variables (e.g., trophic preferences, biomass, and diversity) were not assessed on the same sediment cores as the biogeochemical function proxies and therefore spatial variability cannot be excluded as a factor in the observed differences. Furthermore, the hypothesis that changes in faunal assemblages will cause altered bioturbation potential of the sediments remains unanswered (no significant differences observed among treatments) probably owing to the low number of replicates used. Finally, even though we recognize the importance of including an area close to pristine conditions and legally protected (NT) in the experimental set up, due to differences in depth and grain-size differences between the only close NT area and the main fishing ground/adjacent areas, this undisturbed location could not be included, and thus biogeochemical functions and 13C bacterial uptake in undisturbed conditions at the WIM remain untested.
Noteworthy is that, in opposition to what was observed for macrofauna, the absence of a significant effect of trawling pressure on meiofaunal standing stocks (both abundance and biomass), community composition (Lins et al., 2017) and respiration, advocates for an overall absence of a detrimental effect on the measured meiofauna/nematode variables. These results contradict the results obtained by Pusceddu et al., 2014. The contrasting results may be related with the differences in organic matter concentrations under different conditions of trawling pressure. Here at the start of the experiment, the first 5 cm of sediment showed similar environmental conditions in both AA and FG, including in terms of organic carbon and nitrogen, while Pusceddu et al., 2014 showed decreased organic carbon concentrations with increased trawling pressure. Generally, meiofaunal standing stocks are positively related with food availability and quality in deep-sea sediments (e.g., Ingels et al., 2009; Pape et al., 2013; Lins et al., 2017), so this may partially explain the decreased in meiobenthos with increasing trawling pressure in the study of Pusceddu et al. (2014). Other differences identified between these two studies were varying habitat conditions (e.g., depths, canyon vs. slope) and/or differences in sample size (c.a. 50% more samples analyzed by Pusceddu et al., 2014). Nevertheless, while meiofauna can contribute to ecosystem processes via micro-bioturbation (Rysgaard et al., 2000; Bonaglia et al., 2014), in high diversity systems such as the study area (Ramalho et al., 2018), the influence of strong interactions with the macrofauna (competition and predation), may mask or decrease the relevance of the meiofauna to sediment biogeochemical functioning comparatively to macrofauna (Rysgaard et al., 2000; Bonaglia et al., 2014).
Conclusion
The present study suggested a negative influence of trawling disturbance on the benthos and related ecosystem functions. The most evident effects were detected for the macrofauna assemblages, with a prevalence of small-sized opportunistic species under high physical disturbance conditions (fishing grounds). Moreover, the general decline in macrofauna species richness, functional (trophic) diversity and total respiration, suggests that the long history of trawling disturbance along the WIM may be affecting the integrity of the seafloor and the capacity of the benthos to ensure fundamental ecosystem functions and services. In contrast, the biomass of the small-sized biota (meiofauna and bacteria) showed no marked differences associated with trawling regimes, although bacterial production (13C uptake) was reduced in the FG sediments investigated during the pulse-chase experiment. Trawling-induced changes in macrofauna traits and size structure may result in an inefficient bioturbation and low bioirrigation potential affecting both biogeochemical functioning and productivity in marine sediments. Further investigation is required to substantiate the observed function impairment across the study area, including larger sampling effort within AA and FG areas and a comparison with pristine locations.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
MC, AV, EP, and NL generated the study idea and funding. MC, AV, SR, EP, and LL were responsible for sample collection and experimental set-up, further analyzed by SR, EP and LL. KS was the main responsible for 13C and PLFA sample processing analyses. SR and EP wrote the manuscript with significant contribution from all authors. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the CESAM (UIDP/50017/2020 + UIDB/50017/2020) funds, granted by FCT/MCTES through national funds. SR work was funded through a MARES Grant (FPA 2011-0016) and is currently supported by national funds (OE), through the FCT – Fundação para a Ciência e a Tecnologia, I.P., in the scope of the “CEEC Individual 2017” contract (CEECIND/00758/2017). LL work as funded by the BOF (12/DOS/006) and CAPES (BEX 11595/13-2) grants. EP was financed by the FWO project “Unraveling the enigma of nematode success in the deep sea” (G083512N).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all scientific parties, the captain, and the crew, for their excellent logistical support during the RV Belgica 2013/2017 and RV Belgica 2014/2015 cruises. We are thankful to Pieter Van Rijswijck (NIOZ Yerseke) for his work with the 13C and PLFA sample processing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.00457/full#supplementary-material
Footnotes
References
Aller, R. C. (1982). “The effects of macrobenthos on chemical properties of marine sediment and overlying water,” in Animal-Sediment Relations. Topics in Geobiology, Vol. 100, eds P. L. McCall and M. J. S. Tevesz (Boston, MA: Springer), 53–102. doi: 10.1007/978-1-4757-1317-6_2
Anderson, M. J., Gorley, R. N., and Clarke, R. K. (2008). Permanova+ for Primer: Guide to Software and Statistical Methods. Plymouth: PRIMER-E Ltd.
Andrassy, I. (1956). Die rauminhalts- und gewichtsbestimmung der fadenwurmer (Nematoden). Acta Zool. 2, 1–15.
Atwood, T. B., Connolly, R. M., Ritchie, E. G., Lovelock, C. E., Heithaus, M. R., Hays, G. C., et al. (2015). Predators help protect carbon stocks in blue carbon ecosystems. Nat. Clim. Chang 5, 1038–1045. doi: 10.1038/nclimate2763
Baguley, J. G., Hyde, L. J., and Montagna, P. A. (2004). A semi-automated digital microphotographic approach to measure meiofaunal biomass. Limnol. Oceangr. 2, 181–190. doi: 10.4319/lom.2004.2.181
Baldrighi, E., Giovannelli, D., D’Errico, G., Lavaleye, M., and Manini, E. (2017). Exploring the relationship between macrofaunal biodiversity and ecosystem functioning in the deep sea. Front. Mar. Sci. 4:198. doi: 10.3389/fmars.2017.00198
Baldrighi, E., and Manini, E. (2015). Deep-sea meiofauna and macrofauna diversity and functional diversity: are they related? Mar. Biodivers 45, 469–488. doi: 10.1007/s12526-015-0333-9
Bligh, E. G., and Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917. doi: 10.1139/o59-099
Bonaglia, S., Nascimento, F. J. A., Bartoli, M., Klawonn, I., and Br¨chert, V. (2014). Meiofauna increases bacterial denitrification in marine sediments. Nat. Commun. 5:5133. doi: 10.1038/ncomms6133
Borges, T. C., Erzini, K., Bentes, L., Costa, M. E., Goncalves, J., Lino, P. G., et al. (2001). By-catch and discarding practices in five Algarve (southern Portugal) metiers. J. Appl. Ichthyol. 17, 104–114. doi: 10.1111/j.1439-0426.2001.00283.x
Boschker, H. T. S. (2004). “Linking microbial community structure and functioning: stable istope (13C) labeling in combination with PLFA analysis,” in Molecular Microbial Ecology Manual, 2nd Edn, ed. G. A. Kolwalchuk (Dordrecht: Kluwer Academic Publishers), 1673–1688.
Braeckman, U., Provoost, P., Gribsholt, B., Van Gansbeke, D., Middelburg, J. J., Soetaert, K., et al. (2010). Role of macrofauna functional traits and density in biogeochemical fluxes and bioturbation. Mar. Ecol. Prog. Ser. 399, 173–186. doi: 10.3354/meps08336
Brinch-Iversen, J., and King, G. M. (1990). Effects of substrate concentration, growth-state, and oxygen availability on relationships among bacterial carbon, nitrogen and phospholipid phosphorus-content. FEMS Microbiol. Ecol. 74, 345–355. doi: 10.1016/0378-1097(90)90687-L
Bueno-Pardo, J., Ramalho, S. P., García-Alegre, A., Morgado, M., Vieira, R. P., Cunha, M. R., et al. (2017). Deep-sea crustacean trawling fisheries in portugal: quantification of effort and assessment of landings per unit effort using a vessel monitoring system (VMS). Sci. Rep. 7:40795. doi: 10.1038/srep40795
Clark, M. R., Althaus, F., Schlacher, T. A., Williams, A., Bowden, D. A., and Rowden, A. A. (2016). The impacts of deep-sea fisheries on benthic communities: a review. ICES J. Mar. Sci. 73:fsv123. doi: 10.1093/icesjms/fsv123
Clarke, K. R., and Gorley, R. N. (2006). PRIMER Version 6: User Manual/Tutorial. Plymouth: PRIMER-E Ltd.
Danovaro, R. C., Gambi, M. C., Dell’Anno, A., Corinaldesi, C., Fraschetti, S., Vanreusel, A., et al. (2008). Exponential decline of deep-sea ecosystem functioning linked to benthic biodiversity loss. Curr. Biol. 18, 1–8. doi: 10.1016/j.cub.2007.11.056
Duplisea, D. E., Jennings, S., Malcolm, S. J., Parker, R., and Sivyer, D. B. (2001). Modelling potential impacts of bottom trawl fisheries on soft sediment biogeochemistry in the North Sea. Geochem. Trans. 2:112. doi: 10.1039/b108342b
Eigaard, O. R., Bastardie, F., Hintzen, N. T., Buhl-Mortensen, L., Buhl-Mortensen, P., Catarino, R., et al. (2016). The footprint of bottom trawling in European waters: distribution, intensity, and seabed integrity. ICES J. Mar. Sci. 74, 847–865. doi: 10.1093/icesjms/fsw194
European Commission. (2008). Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008. Brussels: Official Journal of the European Union.
Fauchald, K., and Jumars, P. A. (1979). The diet of worms: a study of polychaete feeding guilds. Oceanogr. Mar. Biol. Annu. Rev. 17, 193–284.
Gage, J. D., Hughes, D. J., and Vecino, J. L. G. (2002). Sieve size influence in estimating biomass, abundance and diversity in samples of deep-sea macrobenthos. Mar. Ecol. Prog. Ser. 225, 97–107. doi: 10.3354/meps225097
Giere, O. (2009). Meiobenthology: The Microscopic Motile Fauna of Aquatic Sediments. Berlin: Springer-Verlag.
Glud, R. N. (2008). Oxygen dynamics of marine sediments. Mar. Biol. Res. 4, 243–289. doi: 10.1080/17451000801888726
Guilini, K., Bezerra, T. N., Deprez, T., and Fonseca, G. F. C. (2016). NeMys: World Database of Free-Living Marine Nematodes. Avaliable at: https://nemys.ugent.be/. (accessed December 3, 2015)Google Scholar
Hale, R., Godbold, J. A., Sciberras, M., Dwight, J., Wood, C., Hiddink, J. G., et al. (2017). Mediation of macronutrients and carbon by post-disturbance shelf sea sediment communities. Biogeochemistry 135, 121–133. doi: 10.1007/s10533-017-0350-9
Hiddink, J. G., Jennings, S., Kaiser, M. J., Queirós, A. M., Duplisea, D. E., and Piet, G. J. (2006). Cumulative impacts of seabed trawl disturbance on benthic biomass, production, and species richness in different habitats. Can. J. Fish. Aquat. Sci. 63, 721–736. doi: 10.1139/f05-266
Higgins, R. P., and Thiel, H. (1988). Introduction to the Study of Meiofauna. Washington D.C: Smithsonian Institution Press.
Hurlbert, S. H. (1971). The nonconcept of species diversity: a critique and alternative parameters. Ecology 52, 577–586. doi: 10.2307/1934145
Ingels, J., Kiriakoulakis, K., Wolff, G. A., and Vanreusel, A. (2009). Nematode diversity and its relation to the quantity and quality of sedimentary organic matter in the deep Nazaré Canyon. Western Iberian Margin. Deep-Sea Res. Part I 56, 1521–1539. doi: 10.1016/j.dsr.2009.04.010
Instituto Hidrográfico (2005a). Carta dos Sedimentos Superficiais da Plataforma Continental Portuguesa - Folha 5 - Escala 1, 150 000. Portugal: Instituto Hidrográfico.
Instituto Hidrográfico (2005b). Carta dos Sedimentos Superficiais da Plataforma Continental Portuguesa - Folha 6A - Escala 1, 150 000. Portugal: Instituto Hidrográfico.
Jennings, S., Dinmore, T. A., Duplisea, D. E., Warr, K. J., and Lancaster, J. E. (2001a). Trawling disturbance can modify benthic production processes. J. Anim. Ecol. 70, 459–475. doi: 10.1046/j.1365-2656.2001.00504.x
Jennings, S., Pinnegar, J. K., Polunin, N. V., and Warr, K. J. (2001b). Impacts of trawling disturbance on the trophic structure of benthic invertebrate communities. Mar. Ecol. Prog. Ser. 213, 127–142. doi: 10.3354/meps213127
Jensen, P. (1986). Nematode fauna in the sulphide-rich brine seep and adjacent bottoms of the East Flower Garden. NW Gulf of Mexico. Mar. Biol. 92, 489–503. doi: 10.1007/BF00392509
Jumars, P. A., Dorgan, K. M., and Lindsay, S. M. (2015). Diet of worms emended: an update of polychaete feeding guilds. Ann. Rev. Mar. Sci. 7, 497–520. doi: 10.1146/annurev-marine-010814-020007
Kämpf, J., and Chapman, P. (2016). Upwelling Systems of the World. Switzerland: Springer International Publishing.
Laverock, B., Gilbert, J. A., Tait, K., Osborn, A. M., and Widdicombe, S. (2011). Bioturbation: impact on the marine nitrogen cycle. Biochem. Soc. Trans. 39, 315–320. doi: 10.1042/BST0390315
Leduc, D., Pilditch, C. A., and Nodder, S. D. (2016). Partitioning the contributions of mega-, macro- and meiofauna to benthic metabolism on the upper continental slope of New Zealand: potential links with environmental factors and trawling intensity. Deep-Sea Res. Part I 108, 1–12. doi: 10.1016/j.dsr.2015.12.003
Leocádio, A. M., Whitmarsh, D., and Castro, M. (2012). Comparing trawl and creel fishing for norway lobster (Nephrops norvegicus): biological and economic considerations. PLoS One 7:e39567. doi: 10.1371/journal.pone.0039567.t006
Levin, L. A. (2002). Deep-ocean life where oxygen is scarce. Am. Sci. 90, 436–444. doi: 10.1511/2002.5.436
Levin, L. A., Etter, R. J., Rex, M. A., Gooday, A. J., Smith, C. R., Pineda, J., et al. (2001). Environmental Influences on regional deep-sea species diversity. Annu. Rev. Ecol. Syst. 32, 51–93. doi: 10.1146/annurev.ecolsys.32.081501.114002
Lins, L., Leliaert, F., Riehl, T., Ramalho, S. P., Cordova, E. A., Esteves, A. M., et al. (2017). Evaluating environmental drivers of spatial variability in free-living nematode assemblages along the Portuguese margin. Biogeosciences 14, 651–669. doi: 10.5194/bg-14-651-2017
Lohrer, A. M., Thrush, S. F., and Gibbs, M. M. (2004). Bioturbators enhance ecosystem function through complex biogeochemical interactions. Nature 431, 1092–1095. doi: 10.1038/nature03042
Macdonald, T. A., Burd, B. J., Macdonald, V. I., and van Roodselaar, A. (2010). Taxonomic and Feeding Guild Classification for the Marine Benthic Macroinvertebrates of the Strait of Georgia, British Columbia. Sidney, BC: Fisheries and Oceans Canada.
Maestro, A., López-Martínez, J., Llave, E., Bohoyo, F., Acosta, J., Hernández-Molina, F. J., et al. (2013). Geomorphology of the Iberian Continental Margin. Geomorphol. 196, 13–35. doi: 10.1016/j.geomorph.2012.08.022
Mahaut, M. L., Sibuet, M., and Shirayama, Y. (1995). Weight-dependent respiration rates in deep-sea organisms. Deep Sea Res. Part I 42, 1575–1582. doi: 10.1016/0967-0637(95)00070-M
MAMAOT (2012). Estratégia Marinha Para a subdivisão do Continente. Diretiva Quadro Estratégia Marinha. Lisbon: Ministério da Agricultura, do Mar.
Martín, J., Puig, P., Palanques, A., and Giamportone, A. (2014a). Commercial bottom trawling as a driver of sediment dynamics and deep seascape evolution in the. Anthropocene 7, 1–15. doi: 10.1016/j.ancene.2015.01.002
Martín, J., Puig, P., Palanques, A., and Ribó, M. (2014b). Trawling-induced daily sediment resuspension in the flank of a mediterranean submarine canyon. Deep Sea Res. Part II 104, 174–183. doi: 10.1016/j.dsr2.2013.05.036
Martín, J., Puig, P., Masqué, P., Palanques, A., and Sánchez-Gómez, A. (2014c). Impact of bottom trawling on deep-sea sediment properties along the flanks of a submarine canyon. PLoS One 9:e104536. doi: 10.1371/journal.pone.0104536
Middelburg, J. J., Barranguet, C., Boschker, H. T. S., Herman, P. M., Moens, T., and Heip, C. H. (2000). The fate of intertidal microphytobenthos carbon: an in situ 13C-labeling study. Limnol. Oceangr. 45, 1224–1234. doi: 10.4319/lo.2000.45.6.1224
Monteiro, P., Araújo, A., Erzini, K., and Castro, M. (2001). Discards of the Algarve (southern Portugal) crustacean trawl fishery. Hydrobiologia 449, 267–277. doi: 10.1023/A:1017575429808
National Research Council [NRC] (2002). Effects of Trawling and Dredging on Seafloor Habitat. Washington, DC: The National Academies Press.
Oberle, F. K. J., Storlazzi, C. D., and Hanebuth, T. J. J. (2016). What a drag: quantifying the global impact of chronic bottom trawling on continental shelf sediment. J. Mar. Syst. 159, 109–119. doi: 10.1016/j.jmarsys.2015.12.007
Pape, E., Jones, D. O., Manini, E., Bezerra, T. N., and Vanreusel, A. (2013). Benthic-pelagic coupling: effects on nematode communities along southern European continental margins. PLoS One 8:e59954. doi: 10.1371/journal.pone.0059954
Picado, A., Alvarez, I., Vaz, N., Varela, R., Gomez-Gesteira, M., and Dias, J. M. (2014). Assessment of chlorophyll variability along the northwestern coast of Iberian Peninsula. J. Sea Res. 93, 2–11. doi: 10.1016/j.seares.2014.01.008
Pielou, E. C. (1966). The measurement of diversity in different types of biological collections. J. Theor. Biol. 13, 131–144. doi: 10.1016/0022-5193(66)90013-0
Platt, H. M., and Warwick, R. M. (1983). Free Living Marine Nematodes. Part 1: British Enoplids. Pictorial key to World Genera and Notes for the Identification of British Species. Cambridge: Cambridge University Press.
Platt, H. M., and Warwick, R. M. (1988). Free-Living Marine Nematodes. Part II: British Chromadorids. Cambridge: Cambridge University Press.
Pusceddu, A., Bianchelli, S., Martín, J., Puig, P., Palanques, A., Masqué, P., et al. (2014). Chronic and intensive bottom trawling impairs deep-sea biodiversity and ecosystem functioning. Proc. Nat. Acad. Sci. U.S.A. 111, 8861–8866. doi: 10.1073/pnas.1405454111
Queirós, A. M., Hiddink, J. G., Kaiser, M. J., and Hinz, H. (2006). Effects of chronic bottom trawling disturbance on benthic biomass, production and size spectra in different habitats. J. Exp. Mar. Bio. Ecol. 335, 91–103. doi: 10.1016/j.jembe.2006.03.001
Quinn, G. P., and Keough, M. J. (2002). Experimental Design and Data Analysis for Biologists. Cambridge: Cambridge University Press.
Ramalho, S. P., Almeida, M. D., Esquete, P., Génio, L., Ravara, A., Rodrigues, C. F., et al. (2018). Bottom trawling fisheries influence on macrofauna standing stocks, community composition and diversity from the West Iberian Margin. Deep Sea Res. Part I 138, 131–145. doi: 10.1016/j.dsr.2018.06.004
Ramalho, S. P., Lins, L., Bueno-Pardo, J., Cordova, E. A., Amisi, J. M., Lampadariou, N., et al. (2017). Deep-sea mega-epibenthic assemblages from the SW Portuguese margin (NE Atlantic) subjected to bottom trawling fisheries. Front. Mar. Sci. 4:350. doi: 10.3389/fmars.2017.00350
Ramirez-Llodra, E., Tyler, P. A., Baker, M. C., Bergstad, O. A., Clark, M. R., Escobar, E., et al. (2011). Man and the last great wilderness: human impact on the deep sea. PLoS One 6:e22588. doi: 10.1371/journal.pone.0022588
Relvas, P., Barton, E. D., Dubert, J., Oliveira, P. B., Peliz, Á, da Silva, J. C. B., et al. (2007). Physical oceanography of the western Iberia ecosystem: latest views and challenges. Prog. Oceanogr 74, 149–173. doi: 10.1016/j.pocean.2007.04.021
Rowe, G. T. (1983). “Biomass and production of the deep-sea macrobenthos,” in Deep-Sea Biology - The Sea, ed. G. T. Rowe (New York, NY: John Wiley and Sons), 97–121.
Rysgaard, S., Christensen, P. B., Sorensen, M. V., Funch, P., and Berg, P. (2000). Marine meiofauna, carbon and nitrogen mineralization in sandy and soft sediments of Disko Bay. West Greenland. Aquat. Microb. Ecol. 21, 59–71. doi: 10.3354/ame021059
Sañé, E., Martín, J., Puig, P., and Palanques, A. (2013). Organic biomarkers in deep-sea regions affected by bottom trawling: pigments, fatty acids, amino acids and carbohydrates in surface sediments from the La Fonera (Palamós) Canyon. NW Mediterranean Sea. Biogeosciences 10:8093. doi: 10.5194/bg-10-8093-2013
Santos, A. (2001). Sardine and horse mackerel recruitment and upwelling off Portugal. ICES J. Mar. Sci. 58, 589–596. doi: 10.1006/jmsc.2001.1060
Schratzberger, M., Dinmore, T. A., and Jennings, S. (2002). Impacts of trawling on the diversity, biomass and structure of meiofauna assemblages. Mar. Biol. 140, 83–93. doi: 10.1007/s002270100688
Sciberras, M., Hiddink, J. G., Jennings, S., Szostek, C. L., Hughes, K. M., Kneafsey, B., et al. (2018). Response of benthic fauna to experimental bottom fishing: a global meta-analysis. Fish. Fish. 19, 698–715. doi: 10.1111/faf.12283
Sciberras, M., Parker, R., Powell, C., Robertson, C., Kröger, S., Bolam, S. G., et al. (2016). Impacts of bottom fishing on the sediment infaunal community and biogeochemistry of cohesive and non-cohesive sediments. Limnol. Oceangr. 61, 2076–2089. doi: 10.1002/lno.10354
Sciberras, M., Tait, K., Brochain, G., Hiddink, J. G., Hale, R., Godbold, J. A., et al. (2017). Mediation of nitrogen by post-disturbance shelf communities experiencing organic matter enrichment. Biogeochemistry 135, 135–153. doi: 10.1007/s10533-017-0370-5
Shaffer, J. P. (1995). Multiple hypothesis-testing. Ann. Rev. Psychol. 46, 561–584. doi: 10.1146/annurev.ps.46.020195.003021
Silva, A., Palma, S., Oliveira, P. B., and Moita, M. T. (2009). Composition and interannual variability of phytoplankton in a coastal upwelling region (Lisbon Bay. Portugal). J. Sea Res. 62, 238–249. doi: 10.1016/j.seares.2009.05.001
Solan, M., Cardinale, B. J., Downing, A. L., Engelhardt, K. A. M., Ruesink, J. L., and Srivastava, D. S. (2004). Extinction and ecosystem function in the marine benthos. Science 306, 1177–1180. doi: 10.1126/science.1103960
Spiers, E. K. A., Stafford, R., Ramirez, M., Izurieta, D. F. V., Cornejo, M., and Chavarria, J. (2016). Potential role of predators on carbon dynamics of marine ecosystems as assessed by a Bayesian belief network. Ecol. Inform. 36, 77–83. doi: 10.1016/j.ecoinf.2016.10.003
Stratmann, T., Lins, L., Purser, A., Marcon, Y., Rodrigues, C. F., Ravara, A., et al. (2018). Abyssal plain faunal carbon flows remain depressed 26 years after a simulated deep-sea mining disturbance. Biogeosciences 15, 4131–4145. doi: 10.5194/bg-15-4131-2018
Strong, J. A., Andonegi, E., Bizsel, K. C., Danovaro, R. C., Elliott, M., Franco, A., et al. (2015). Marine biodiversity and ecosystem function relationships: the potential for practical monitoring applications. Estuar. Coast. Shelf Sci. 161, 46–64. doi: 10.1016/j.ecss.2015.04.008
van Oevelen, D., Moodley, L., Soetaert, K., and Middelburg, J. J. (2006). The trophic significance of bacterial carbon in a marine intertidal sediment: results of an in situ stable isotope labeling study. Limnol. Oceangr. 51, 2349–2359. doi: 10.4319/lo.2006.51.5.2349
Vanreusel, A., Hilario, A., Ribeiro, P. A., Menot, L., and Arbizu, P. M. (2016). Threatened by mining, polymetallic nodules are required to preserve abyssal epifauna. Sci. Rep. 6:26808. doi: 10.1038/srep26808
Volkenborn, N., Hedtkamp, S. I. C., van Beusekom, J. E. E., and Reise, K. (2007). Effects of bioturbation and bioirrigation by lugworms (Arenicola marina) on physical and chemical sediment properties and implications for intertidal habitat succession. Estuar. Coast. Shelf Sci. 74, 331–343. doi: 10.1016/j.ecss.2007.05.001
Wenthworth, C. K. (1922). The Wenthworth scale of grain size for sediments. J. Geol. 30, 377–392. doi: 10.1086/622910
Wieser, W. (1953). Die beziehung zwischen mundhöhlengestalt, ernährungsweise und vorkommen bei freilebenden marinen nematoden. Ark. Zool. 4, 439–484.
Wieser, W. (1960). Benthic studies in buzzards bay II: the meiofauna. Limnol. Oceanogr. 5, 121–137. doi: 10.4319/lo.1960.5.2.0121
Worm, B., Barbier, E. B., Beaumont, N., Duffy, J. E., Folke, C., Halpern, B. S., et al. (2006). Impacts of biodiversity loss on ocean ecosystem services. Science 314, 787–790. doi: 10.1126/science.1132294
Keywords: bacteria, meiofauna, macrofauna, pulse-chase experiment, bottom-trawling disturbance, deep sea
Citation: Ramalho SP, Lins L, Soetaert K, Lampadariou N, Cunha MR, Vanreusel A and Pape E (2020) Ecosystem Functioning Under the Influence of Bottom-Trawling Disturbance: An Experimental Approach and Field Observations From a Continental Slope Area in the West Iberian Margin. Front. Mar. Sci. 7:457. doi: 10.3389/fmars.2020.00457
Received: 17 September 2019; Accepted: 22 May 2020;
Published: 23 June 2020.
Edited by:
Les Watling, University of Hawai‘i at Mᾱnoa, United StatesReviewed by:
Ian David Tuck, National Institute of Water and Atmospheric Research, New ZealandJacobo Martin, Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina
Copyright © 2020 Ramalho, Lins, Soetaert, Lampadariou, Cunha, Vanreusel and Pape. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sofia P. Ramalho, sramalho@ua.pt
 Sofia P. Ramalho
Sofia P. Ramalho Lidia Lins
Lidia Lins Karline Soetaert3
Karline Soetaert3  Nikolaos Lampadariou
Nikolaos Lampadariou Marina R. Cunha
Marina R. Cunha Ann Vanreusel
Ann Vanreusel Ellen Pape
Ellen Pape