Anthropogenic Contaminants and Histopathological Findings in Stranded Cetaceans in the Southeastern United States, 2012–2018
- 1Marine Wildlife Veterinary Medicine and Research Program, Harbor Branch Oceanographic Institute, Florida Atlantic University, Fort Pierce, FL, United States
- 2Infectious Diseases Laboratory, College of Veterinary Medicine, University of Georgia, Athens, GA, United States
- 3College of Veterinary Medicine and Center for Marine Science and Technology, North Carolina State University, Morehead City, NC, United States
- 4Marine Mammal Pathology Services, Olney, MD, United States
- 5College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins, CO, United States
- 6Center for Applied Isotope Studies, University of Georgia, Athens, GA, United States
- 7Toxicology Section, Veterinary Diagnostic Laboratory, Michigan State University, East Lansing, MI, United States
- 8Marine Mammal Stranding Network of the Central North Carolina Coast, North Carolina Division of Marine Fisheries, Morehead City, NC, United States
- 9North Carolina Aquariums, Raleigh, NC, United States
- 10Loggerhead Marinelife Center, Juno Beach, FL, United States
Anthropogenic contaminants in the marine environment often biodegrade slowly, bioaccumulate in organisms, and can have deleterious effects on wildlife immunity, health, reproduction, and development. In this study, we evaluated tissue toxicant concentrations and pathology data from 83 odontocetes that stranded in the southeastern United States during 2012–2018. Mass spectrometry was used to analyze blubber samples for five organic toxicants (atrazine, bisphenol-A, diethyl phthalates, nonylphenol monoethoxylate [NPE], triclosan), and liver samples were analyzed for five non-essential elements (arsenic, cadmium, lead, mercury, thallium), six essential elements (cobalt, copper, manganese, iron, selenium, zinc) and one toxicant mixture class (Aroclor1268). Resultant data considerably improve upon the existing knowledge base regarding toxicant concentrations in stranded odontocetes. Toxicant and element concentrations varied based on animal demographic factors including species, sex, age, and location. Samples from bottlenose dolphins had significantly higher average concentrations of lead, manganese, mercury, selenium, thallium, and zinc, and lower average concentrations of NPE, arsenic, cadmium, cobalt, and iron than samples from pygmy sperm whales. In adult female bottlenose dolphins, average arsenic concentrations were significantly higher and iron concentrations were significantly lower than in adult males. Adult bottlenose dolphins had significantly higher average concentrations of lead, mercury, and selenium, and significantly lower average manganese concentrations compared to juveniles. Dolphins that stranded in Florida had significantly higher average concentrations of lead, mercury, and selenium, and lower concentrations of iron than dolphins that stranded in North Carolina. Histopathological data are presented for 72 animals, including microscopic evidence of Campula spp. and Sarcocystis spp. infections, and results of Morbillivirus and Brucella spp. molecular diagnostic testing. Sublethal cellular changes related to toxicant exposure in free-ranging odontocetes may lead to health declines and, in combination with other factors, may contribute to stranding.
Introduction
Anthropogenic toxicants are released into marine ecosystems through a number of different sources, biodegrade at variable rates, and many can persist for decades or even centuries (Godfray et al., 2019). The amount of manufactured waste released into the environment has grown exponentially over time, in line with the rampant mass production of consumer products catering to a rapidly growing human population (Cole et al., 2011). Plastics are a particularly harmful form of marine debris, because they are durable, slow to biodegrade, and susceptible to indiscriminate disposal, making environmental accumulation a major concern (Barnes et al., 2009; Cole et al., 2011). In addition to the sheer physical accumulation of plastics in the oceans and the ingestion of plastic materials by all kinds of organisms, plastics and other waste contain and attract harmful contaminants that can bioaccumulate in organisms and may pose a threat to their health, including reproduction, development, and immunity (Mato et al., 2001; Gregory, 2009; Worm et al., 2017). Many plastic components and plasticizers (substances added to synthetic resins to produce or promote plasticity) include phthalates, bisphenol-A, nonylphenol ethoxylates, and polychlorinated biphenyls (PCBs); although typically associated with their use as electrical insulators in capacitators and transformers, PCBs were also used as plasticizers and were shown by early researchers to be associated with plastic ingestion in marine animals (Gregory, 1978; Ryan et al., 1998). Plasticizers are known or suspected endocrine disrupting chemicals (EDCs), a structurally and functionally active group of xenobiotics that can have adverse effects on multiple organ systems of wildlife species (Staples et al., 1997; Barnes et al., 2009; Oehlmann et al., 2009; Muncke, 2011; Frouin et al., 2012; Mathieu-Denoncourt et al., 2015). Previously demonstrated adverse effects of EDCs in live animals include malformed reproductive organs, disruption of spermatogenesis, gonadal dysgenesis, reduced metamorphosis, apoptosis in liver and gonads, hormonal imbalances, and renal dysfunction (Bustamante et al., 2003; Hayes et al., 2010; Park et al., 2010; Muncke, 2011; Canesi and Fabbri, 2015; Mathieu-Denoncourt et al., 2015; Li et al., 2017).
Many other toxic chemicals including heavy metals can be sourced to anthropogenic products, tend to biomagnify within the marine food web, and bioaccumulate within bodily tissues of higher trophic organisms, including cetaceans (Bryan et al., 2007; Stavros et al., 2007; Bryan et al., 2012; Aubail et al., 2013; Monteiro et al., 2016a). Elements are grouped as essential (cobalt [Co], copper [Cu], iron [Fe], manganese [Mn], selenium [Se], zinc [Zn]) – those that have a biological function in organisms but can become toxic at high concentrations – and non-essential (arsenic [As], cadmium [Cd], mercury [Hg], lead [Pb], thallium [Tl]), those with no known biological function (Chang et al., 1996). Previous studies have shown that exposure to certain elements can have adverse effects in marine animals including renal damage, immunosuppression, and neurological, developmental, and reproductive impairments (Stavros et al., 2007; Jakimska et al., 2011; Frouin et al., 2012). Inorganic element concentrations in odontocetes have been documented in many tissues including blubber, liver, kidney, skin, and blood (Bustamante et al., 2003; Santos et al., 2006; Stavros et al., 2007; Kunito et al., 2008; Schaefer et al., 2011, 2015; Stavros et al., 2011; Bryan et al., 2012; Aubail et al., 2013; Monteiro et al., 2016a, b; Titcomb et al., 2017). As apex predators with relatively long lifespans, odontocetes are sentinel species that can readily reflect anthropogenic threats through their health (Wells et al., 2004, 2005; Bossart, 2011).
In addition to the numerous pollutants already present in the biosphere, hundreds of new compounds with incomplete toxicity testing enter the consumer market and therefore the oceans every year; as a result a considerable information lag arises between the initial creation and use of these chemicals and the time at which researchers and advocates understand the extent to which they affect the health of individuals, populations, and ecosystems (Weijs and Zaccaroni, 2016). Exposure to multiple toxicants and/or EDCs often happens simultaneously and can severely impact organismal homeostasis and function, since these substances can exert additive or synergistic effects which could lead to decreased growth rates and deleterious effects on long-term functional population viability (Crews et al., 2003; Beck et al., 2013; Worm et al., 2017). Due to a paucity of data on how EDCs and lesser-studied essential and non-essential elements affect marine mammals, inferences regarding the biological effects of potentially toxic compounds are primarily based on studies that used laboratory-reared animals in controlled experiments (Ferzand et al., 2008; Posnack et al., 2012; Gear and Belcher, 2017). Thus, as part of ongoing efforts to catch up with the output of the chemical industry and to provide more information on existing anthropogenic contaminants in free-ranging odontocetes, the objectives of this study were to: (1) establish concentrations of specific toxicants and elements (atrazine, bisphenol-A [BPA], diethyl phthalate [DEP], polychlorinated biphenyl mixture 1268 [Aroclor1268], nonylphenol monoethoxylate [NPE, as a representative of the class of nonylphenol ethoxylates], triclosan, arsenic, cadmium, cobalt, copper, iron, lead, manganese, mercury, selenium, thallium, and zinc) in liver and blubber of stranded odontocetes from the southeastern United States, (2) evaluate relationships between contaminants and demographic parameters (species, sex, age class, and stranding location), and (3) describe histopathologic lesions observed in these cases. This study provides baseline concentrations of several widely used and dispersed anthropogenic contaminants in biological samples taken from cetaceans that stranded in the southeastern United States during 2012–2018. Relating these concentrations to animal demographic and histopathology data allows us to gain insight into secondary (biotic or abiotic) factors that may influence exposure to and biological effects of certain toxicants.
Materials and Methods
Sample and Data Collection
Blubber and liver samples were collected at necropsy from odontocetes that stranded along the Atlantic coast of Florida (northern extent: Sebastian Inlet; southern extent: Biscayne Bay) and North Carolina (Albemarle, central coast, Pamlico Sound, northern and southern coasts) during 2012–2018. Stranding response and necropsies were conducted by regional marine mammal stranding network partners including Florida Atlantic University Harbor Branch Oceanographic Institute’s (FAU-HBOI) Stranding, Health, and Rehabilitation Program, and the Marine Mammal Stranding Network of the North Carolina Central Coast. Euthanasia decisions were made by attending veterinarians based on prognosis and in accordance with protocols issued by the National Marine Fisheries Service for cetacean stranding response and euthanasia (Whaley and Borkowski, 2009). One blubber sample was collected from a live dolphin during dorsal fin tagging following live capture for disentanglement, using local anesthesia and aseptic technique. Body condition was determined subjectively just prior to necropsy/sampling, based on gross visibility of the ribs, scapulae, degree of indentation lateral to the dorsal fin, and degree of post-nuchal indentation. Each animal’s body condition was thus categorized as either emaciated, thin, good, or robust.
For all animals, post-mortem external and internal examinations were performed and tissue samples were collected according to standard protocols for cetacean necropsy (Pugliares et al., 2007). Representative tissue samples of all major organs and any grossly observed lesions were collected and placed into 10% neutral buffered formalin. Depending on the circumstances of the necropsy, variable tissues were available for histological review and included brain, skin, heart, lung, liver, kidney, spleen, lymph nodes, adrenal, stomach, intestines, thyroid, thymus, and pancreas. Formalin-fixed tissues were embedded in paraffin, sectioned at 4–7 μm, stained with hematoxylin and eosin, and examined microscopically by a board-certified veterinary pathologist. Depending on the circumstances, tissue samples were submitted for infectious disease testing using polymerase chain reaction (PCR) assays. Specifically, Morbillivirus testing was performed using the universal Morbillivirus primers directed against the phosphoprotein gene (after Barrett et al., 1993); and testing for Brucella spp. was performed using a real-time PCR assay for Brucella spp. DNA (after Wu et al., 2014). Representative blubber (2–5 g each) and liver (0.25–0.5 g each) samples were collected from each animal, stored in unlined tinfoil or cryogenic vials, and frozen at –80°C for up to 5 years prior to further analysis.
Some of the bottlenose dolphin (Tursiops truncatus) cases from Florida were known inhabitants of the Indian River Lagoon (IRL), representing a well-studied coastal population with historically high body toxicant concentrations (Fair et al., 2009; Schaefer et al., 2011, 2015). For the majority of these IRL dolphins, extensive photographic documentation exists, often encompassing individual dolphin identification based on dorsal fin characteristics, preferred geographic location(s) and behaviors, family lineage, and approximate age. The FAU-HBOI Dolphin Photo ID Program has been systematically collecting these data on IRL-resident dolphins since 1996.
Case Review
Case data analyzed included: species, date and location stranded, morphometric measurements including total body length (cm) and weight (kg, when available), and necropsy results including histopathologic and ancillary diagnostics data. Data were reviewed for the frequency and severity of histopathologic lesions, with particular attention paid to lesions in the hepatobiliary, lymphoreticular, cardiovascular, nervous, endocrine, and genitourinary systems, as these organ systems may be more likely to be affected by acute and/or chronic exposure to toxicants (Haschek et al., 2010).
Organic Toxicant Analyses
Twenty-seven liver samples and 36 blubber samples were shipped overnight on dry ice to the University of Georgia Center for Applied Isotope Studies in Athens, GA, United States, where they were analyzed for concentrations of Aroclor1268 (liver), and atrazine, BPA, DEP, NPE, and triclosan (blubber) using gas chromatography-tandem mass spectrometry (GC-MS/MS). The majority of these samples were stored in foil; to circumvent plastic contamination of any samples that were stored in cryovials, the central portion of the tissue sample that did not touch the cryovial was used. Furthermore, ‘blank’ extracts from cryovials were used to determine the detection limit for that GC-MS/MS run. All samples were analyzed dry and extracted using HPLC-grade solvents. Lipid content was not analyzed in the samples, therefore toxicant and nutritional element concentrations were not normalized to lipid content. To prepare the samples, about 2 g of each dried tissue was weighed in a 50 mL centrifuge tube and then treated with 20 mL of a 1:1 mixture containing acetone/dichloromethane (DCM) (Fisher Scientific, Hampton, NH, United States). The samples were then placed in an ultrasonic bath for 30 min, followed by centrifugation for 15 min at ∼1,900 g (3,000 rpm). The clear supernatant layers were then transferred into evaporation tubes while the residues were re-extracted with another 20 mL of the acetone/DCM mixture, centrifuged and their supernatant layers were combined with the previous extracts in the relevant evaporation tubes. The combined extracts were evaporated under nitrogen at 55°C. The residues left were dissolved by adding 2 separate aliquots each of 0.5 mL of methanol and vortex shaken for 1 min. Both methanol aliquots were combined in 1 mL volumetric tubes and made up to volume with methanol. Internal standard solution (50 μl of 40 μg mL–1 Pd10) was added and the contents of the volumetric tubes were mixed thoroughly then transferred into 2 mL gas chromatography vials, capped and store refrigerated (4°C) until analysis. Standards for atrazine, BPA, DEP, and triclosan were acquired as 1,000 μg/mL ampoules from Absolute Standards, Inc. (Hamden, CT, United States). The standard for NPE was acquired from Fisher Scientific Co. (Suwanee, GA, United States). The Aroclor standard was obtained as 1,000 μg/mL in iso-octane from Supelco (Bellefonte, PA, United States). Organic contaminants were analyzed using GC-MS/MS with a CP-3800 oven, CP-8400 autosampler with CP-8410 auto-injector, and the 4000 Ion Trap Mass Spectrometer (Agilent Technologies, Santa Clara, CA, United States). Electronic ionization was run under the MS Workstation software version 6.9 SP1. Separation of target compounds was accomplished using a capillary column VF-5 ms 30 m × 0.25 mm ID and 0.25 μm internal coat thickness from Agilent Technologies, and a helium mobile phase with a flow rate of 1 mL min–1. The oven temperature for analysis of the four contaminants was held at 80°C for 1 min and increased at a rate of 6°C min–1 until it reached 280°C and held at this temperature for 10 min. For Aroclor1268, the oven temperature was held at 80°C for 2 min, then increased to 200°C at 20°C min–1, then increased again at 10°C min–1 to 300°C and held for 5 min.
Supplementary Table S1 shows the chromatographic time segments of mass spectrometer acquisition parameters of the four toxicants in blubber extracts as optimized using the Selected Ion Storage (SIS) technique pertinent to Ion Trap mass spectrometers, and the SIS parameters for the Aroclor1268 extract in the liver samples. All concentrations are reported in μg g–1 dry weight (dw). Detection limits in μg g–1 were as follows: atrazine, BPA, DEP, NPE: 0.01; triclosan: 0.02; Aroclor1268: 0.1. Laboratory blanks were analyzed during each analytical run to ensure that there was minimal laboratory contamination. Two blubber samples were analyzed in duplicate to ensure reproducibility of results. The average coefficients of variation (CV) for the blubber duplicate samples were as follows: atrazine, 2.88%; BPA, 0%; DEP, 0.53%; NPE, 7.75%; triclosan, 3.79%.
Essential and Non-essential Element Analyses
Fifty-four liver samples were shipped overnight on dry ice to Michigan State University’s Veterinary Diagnostic Laboratory (MSU VDL) in Lansing, MI, United States, where they were analyzed for essential and non-essential elements (arsenic, cadmium, cobalt, copper, iron, manganese, lead, mercury, selenium, thallium, zinc) using inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7900 ICP-MS, Agilent Technologies, Santa Clara, CA, United States) in accordance with manufacturer’s instructions. Samples were weighed and wet liver samples (∼2 g) were dried at 75°C overnight in a drying oven to generate a dry weight fraction. Dried samples (∼1 g) were weighed and digested with 1–2 mL concentrated 69–70% nitric acid (Avantor Performance Materials, Center Valley, PA, United States, type J. T. Baker ACS reagent grade) in 15 mL PFA digestion vessels (Savillex, Eden Prairie, MN, United States) in a 95°C oven overnight. The amount of acid to be added was made relative to the weight of sample. Overnight digests were then diluted 1:100 in Millipore Filter (Burlington, MA, United States) deionized water prior to analysis. Standard reference materials (SRMs) that were used as quality control (QC) for this process included National Institute of Standards and Technology (NIST; Gaithersburg, MD, United States) SRM 1577c Bovine Liver, NIST SRM 2976 Mussel and an in-house maintained QC-160 tissue spike control as well as a digest blank. Details for average QC results (average result [ng g–1]/expected result [ng g–1]/standard deviation) and average digest blanks (ng g–1) during the month are shown in Supplementary Table S2. Average coefficient of variation (CV) for the individual elements over the various QC materials were as follows: arsenic, 4.69%; cadmium, 6.64%; cobalt, 3.42%; copper, 5.27%; iron, 4.65%; lead, 6.08%; manganese, 4.29%; mercury, 4.10% selenium, 4.37%, thallium, 5.80%; and zinc, 5.18%. The limits of quantitation (LOQ) in μg g–1 were as follows: arsenic, lead, thallium, cadmium, cobalt, 0.1; mercury, 0.5; selenium, 0.02; iron, zinc, 5.0; copper, 2.0; manganese, 0.05.
A subset of 28 liver samples were analyzed at the University of Georgia Infectious Diseases Laboratory in Athens, GA, United States. Using a comparable protocol to that of MSU VDL, liver samples were analyzed for concentrations of 11 essential and non-essential elements (arsenic, cadmium, cobalt, copper, manganese, mercury, lead, selenium, thallium, zinc) with ICP-MS. All solutions were prepared with analytical reagent-grade chemicals and ultra-pure (18MΩ) water. Commercially available trace-metal grade HNO3 (67% v v–1; Thermo Fisher Scientific, Waltham, MA, United States) and standard stock solutions (SPEX CertiPrep, Metuchen, NJ, United States) were used. Multi-element and individual standard solutions (SPEX CertiPrep) were used to prepare a tuning solution containing cobalt, indium, lithium, thallium, cesium, barium, and uranium. Quality control standards and internal standards were purchased from Inorganic Ventures (Christiansburg, VA, United States). Liver samples were freeze-dried overnight. The dried samples were then predigested for 24 h with trace-metal grade HNO3. After pre-digestion, the samples were treated with trace-metal grade H2O2 and digested for 2 h at 90°C. Samples were diluted with 18 MΩ water and analyzed by ICP-MS. Procedural blanks were performed following the same protocol. Bovine liver 1577c (NIST) SRM was used as the QC for digestions. ICP-MS measurements were performed at the Plasma Chemistry Lab, Center for Applied Isotope Studies on a Thermo Scientific X Series II instrument equipped with hexapole Collision Cell Technology. The sample solutions were pumped by peristaltic pump using a Cetac ASX 520 auto-sampler (Cetac, Omaha, NE, United States). The internal standard was added in-line using a Trident Internal Standard Kit (Glass Expansion, Pocasset, MA, United States). Sample introduction into the plasma was performed using a MicroMist EzyFit nebulizer (Glass Expansion, Pocasset, MA, United States), which reduces oxide formation, has a high total dissolved solids tolerance, and has reduced sample uptake rates. The cyclonic spray chamber was maintained at 3°C to further minimize oxide formation. Ion lens voltages, nebulizer flow, and stage positioning were optimized using tuning solution to maximize ion signal and stability while minimizing oxide levels (CeO+/Ce+) and doubly charged ions (Ba2+/Ba+). A multi-elemental analysis was performed in standard mode for all elements. Calibration check standards were analyzed following initial calibration at the end of the sample run, and, at most, after every 12 samples. Quality control check standards were accepted as passing if the measured concentration of each element was found to be within ±10% of the certified concentration. Detailed instrument settings are shown in Supplementary Table S3.
Statistical Analyses
Descriptive statistics (average ± standard deviation (SD) and/or median, minimum, maximum) were calculated for each toxicant analyzed. Median concentrations were calculated for all parameters that included samples that tested below the detection limit (BDL) for that assay. For all other statistical analyses, BDL toxicant concentrations were assigned values one-half their respective detection limit (Helsel, 2006). Atrazine, Aroclor1268, and thallium concentrations were not used in statistical analyses because most samples were BDL and therefore only median, minimum, and maximum were calculated for these variables.
Based on stranding data, cases were grouped into categories according to several selected demographic parameters: genus, age class (fetus/neonate, juvenile, adult, assigned based on total body length and morphologic features such as tooth eruption, fetal folds, etc.), sex, and location stranded (Florida versus North Carolina). Bottlenose dolphins and pygmy sperm whales (Kogia breviceps) were chosen for further statistical analysis because they had the greatest number of samples of the species examined. Spearman rank correlation coefficients (rs) were calculated to evaluate the strength and direction of correlations between toxicant and essential and non-essential element analytes. All data were tested for normality using the Shapiro–Wilk test and variables found to have non-Gaussian distribution were log-transformed to meet the assumptions of normality. Student’s t-tests were then used to compare the average toxicant, essential element, and non-essential element concentrations between adult bottlenose dolphins and adult pygmy sperm whales. Essential and non-essential element data from bottlenose dolphin liver samples were analyzed in more detail, since the relatively larger sample size (N = 46) for this species allowed us to compare various groups with adequate statistical power. Data were tested for normality using the Shapiro–Wilk test and variables found to have non-Gaussian distribution were log-transformed to meet the assumptions of normality. Student’s t-tests were used to compare the average essential and non-essential element concentrations between adult male and female dolphins, adult and juvenile dolphins, dolphins that stranded in Florida versus those that stranded in North Carolina, dolphins infected with Morbillivirus and uninfected dolphins, and dolphins that were known IRL inhabitants (based on dolphin photo identification data) versus Florida-stranded dolphins that were not known IRL inhabitants (Bossart et al., 1985; Read et al., 1993). All statistical analyses were conducted using SPSS statistical software (IBM Corp, 2017).
Ethics Statement
All animal procedures were conducted with approval from the FAU-HBOI and NCSU animal care and use committees under protocols #A18-03 and #15-001-O. FAU-HBOI and NCSU handle cetacean stranding response and recovery under Stranding Agreements issued by the NOAA-NMFS Southeast Regional Office (FAU-HBOI: permit #932-1905-01/MA-009526-01). VT is authorized under Section 109(h) within the North Carolina Division of Marine Fisheries.
Results
Animals and Samples
Samples were analyzed from 83 odontocetes that stranded along the Atlantic coast of North Carolina and Florida during 2012–2018, including T. truncatus (common bottlenose dolphin, N = 46), K. breviceps (pygmy sperm whale, N = 21), Stenella frontalis (Atlantic spotted dolphin, N = 4), Peponocephala electra (melon-headed whale, N = 3), Mesoplodon europaeus (Gervais’ beaked whale, N = 2), Mesoplodon densirostris (Blainville’s beaked whale, N = 2), Kogia sima (dwarf sperm whale, N = 1), Stenella attenuata (pantropical spotted dolphin, N = 1), Lagenorhynchus albirostris (white-beaked dolphin, N = 1), Grampus griseus (Risso’s dolphin, N = 1), and Ziphius cavirostris (Cuvier’s beaked whale, N = 1) (Table 1). Thirty-three of these animals were encountered alive, euthanized, and necropsied shortly afterward; 23 were freshly dead/minimally decomposed at necropsy, and 26 were moderately decomposed at necropsy (Geraci and Lounsbury, 2005). Subjective body condition scoring revealed that 31 animals were emaciated, nine were thin (but not emaciated), 34 were in good or “normal” body condition, and nine were robust. From these animals, we analyzed a total of 111 samples, including 36 blubber and 75 liver samples. Average (or median, for variables with samples that tested BDL) concentrations of these analytes are presented in Table 2 according to species. There were three adult male S. frontalis that were part of a mass stranding event in Hatteras, North Carolina in 2012, and four mother-fetus/calf pairs (three K. breviceps, one M. europaeus, Figure 1); all of the other animals included in this study were single stranding events.
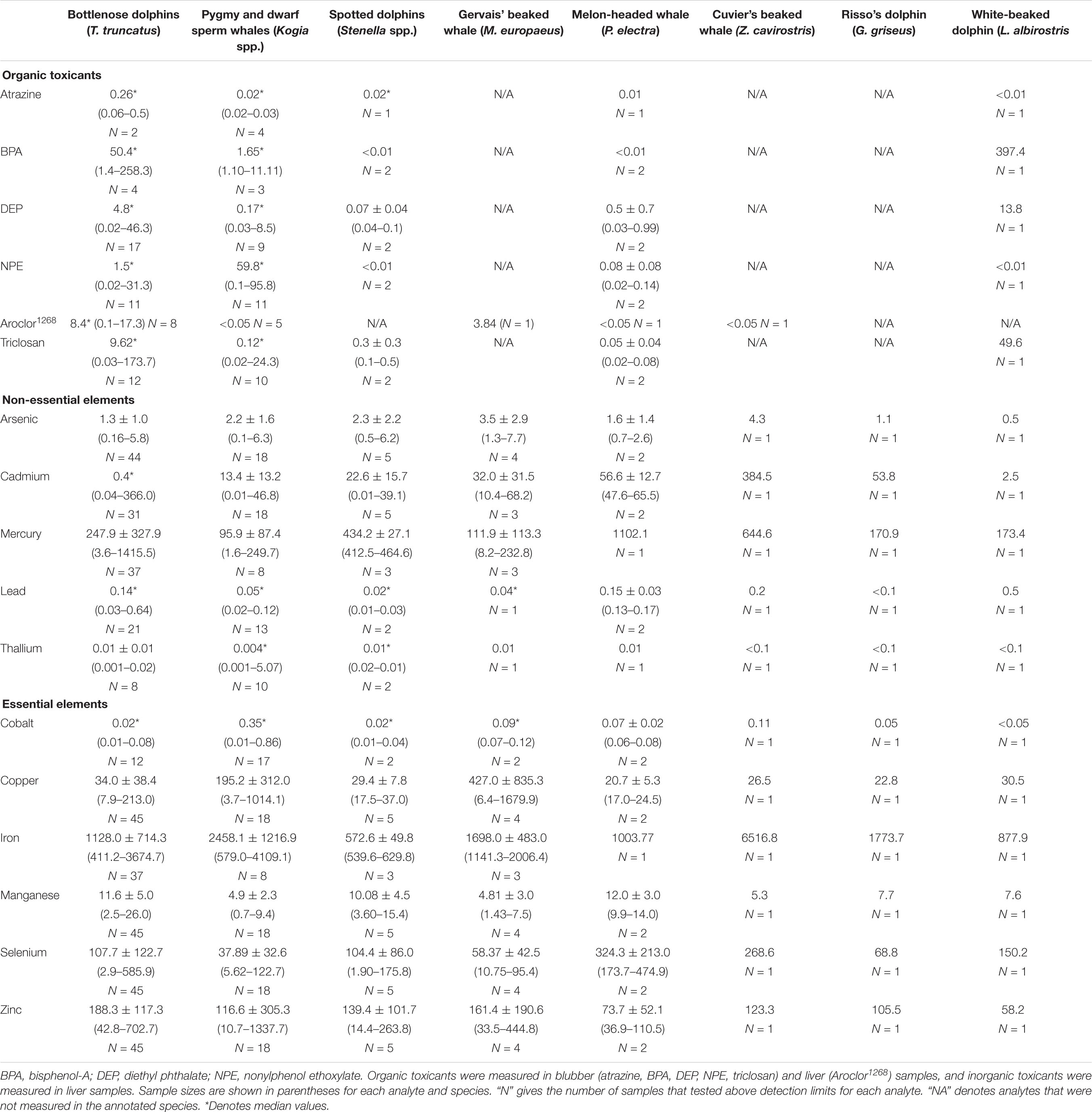
Table 2. Average (*median) concentrations (μg g–1 dw) and ranges for organic and inorganic toxicants and essential and non-essential elements in blubber and liver samples taken from 10 species of odontocetes that stranded in the southeastern United States during 2012–2018.
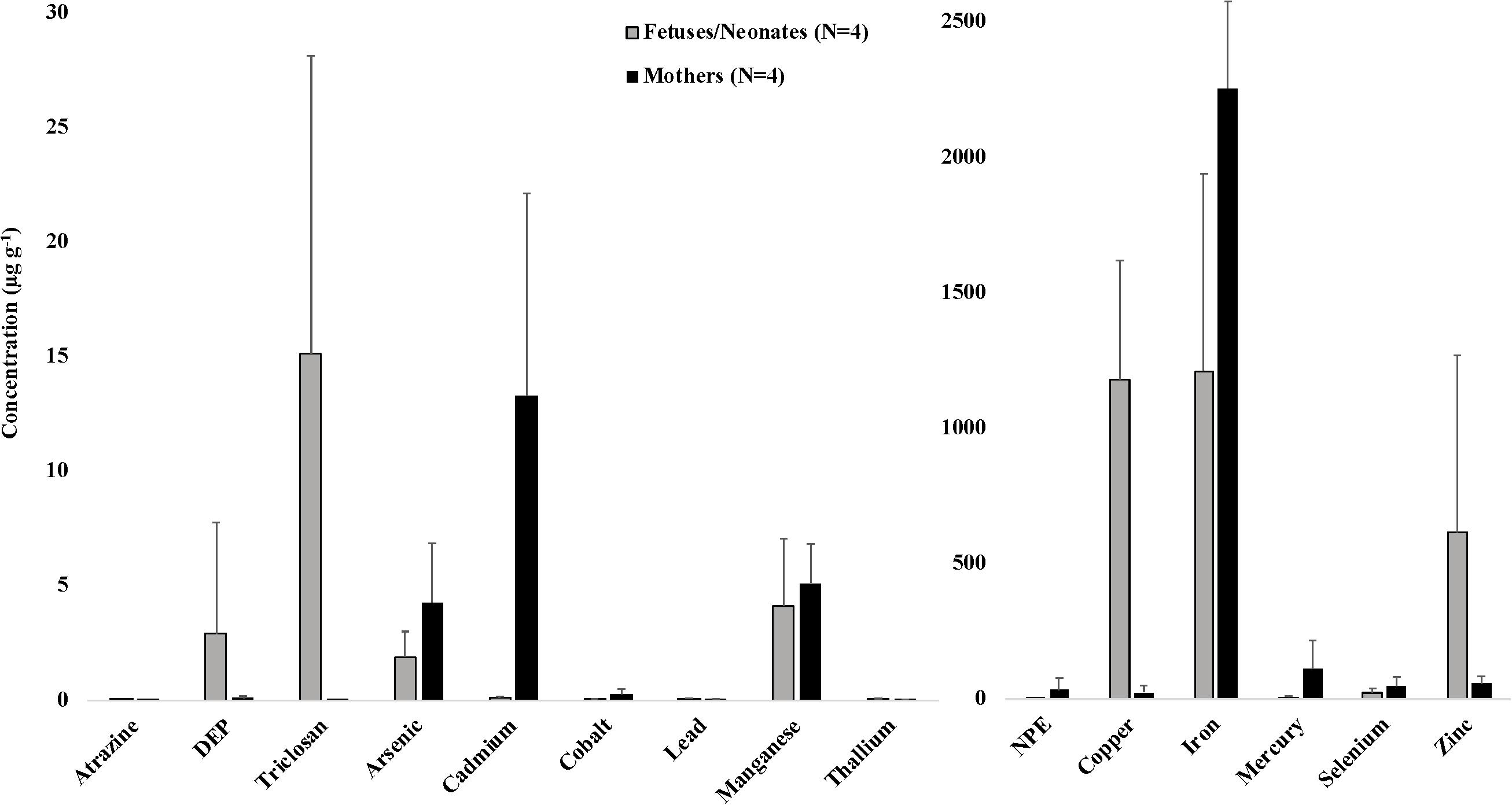
Figure 1. Average (±SD) concentrations (μg g–1 dw) of toxicants and essential and non-essential elements for four mother-fetus/neonate pairs of stranded odontocetes. Includes three pairs of pygmy sperm whales (two fetuses and one neonate) and one pair of Gervais’ beaked whales (fetus) that stranded in the southeastern United States, 2012–2018. DEP, diethyl phthalate; NPE, nonylphenol ethoxylate. Organic toxicants were measured in blubber samples. Essential and non-essential elements were measured in liver samples.
In descending order, the median concentrations of organic toxicants in blubber samples from bottlenose dolphins were in the order: BPA > triclosan > DEP > NPE > atrazine, whereas in pygmy sperm whales the order was NPE > BPA > DEP > triclosan > atrazine. The highest median concentrations of essential and non-essential elements in liver samples from bottlenose dolphins and pygmy sperm whales were in iron and mercury, and the lowest median concentrations were in lead and thallium.
Liver samples from 29 adult bottlenose dolphins had significantly higher average concentrations of lead (0.10 μg g–1, t = 3.19, P < 0.01), manganese (10.60 μg g–1, t = 4.91, P < 0.01), mercury (246.67 μg g–1, t = 2.10, P = 0.02), selenium (111.43 μg g–1, t = 3.94, P < 0.01), thallium (0.03 μg g–1, t = 88, P < 0.01), and zinc (144.22 μg g–1, t = 5.97, P < 0.01), and significantly lower average concentrations of NPE (0.15 μg g–1, t = –3.89, P < 0.01), arsenic (0.94 μg g–1, t = 1.80, P = 0.04), cadmium (0.35 μg g–1, t = –4.66, P < 0.01), cobalt (0.03 μg g–1, t = –7.66, P < 0.01), and iron (1,120.40 μg g–1, t = –3.88, P < 0.01) than liver samples from 12 adult pygmy sperm whales (lead: 0.05 μg g–1, manganese: 4.62 μg g–1, mercury: 104.85 μg g–1, selenium: 32.87 μg g–1, thallium: 0.01 μg g–1, zinc: 41.79 μg g–1, NPE: 30.09 μg g−1, arsenic: 1.69 μg g–1, cadmium: 10.61 μg g–1, cobalt: 0.18 μg g–1, iron: 2,922.46 μg g–1).
In bottlenose dolphins, Spearman rank correlation coefficient tests revealed statistically significant, positive correlations between mercury and selenium (rs = 0.99, P < 0.001), cadmium (rs = 0.43, P < 0.001), and iron (rs = 0.37, P < 0.05); selenium and cadmium (rs = 0.42, P < 0.01), lead (rs = 0.42, P < 0.01), and iron (rs = 0.35, P < 0.05); arsenic and copper (rs = 0.31, P < 0.05), cadmium (rs = 0.43, P < 0.001), and manganese (rs = 0.39, P < 0.01); zinc and thallium (rs = 0.49, P < 0.01), cobalt (rs = 0.35, P < 0.05), and manganese (rs = 0.54, P < 0.01); cobalt and thallium (rs = 0.80, P < 0.01); iron and lead (rs = 0.40, P < 0.05); and BPA and atrazine (rs = 0.71, P < 0.05). Statistically significant, negative correlations were found between selenium and zinc (rs = –0.37, P < 0.05); cadmium and NPE (rs = –0.51, P < 0.05); lead and NPE (rs = –0.51, P < 0.05); and lead and DEP (rs = –0.51, P < 0.05).
In pygmy sperm whales, Spearman rank correlation coefficient tests revealed statistically significant, positive correlations between selenium and mercury (rs = 0.81, P < 0.05), cadmium (rs = 0.68, P < 0.001), cobalt (rs = 0.71, P < 0.01), and zinc (rs = 0.53, P < 0.05); cobalt and arsenic (rs = 0.65, P < 0.01); copper and triclosan (rs = 0.89, P < 0.001); mercury and iron (rs = 0.76, P < 0.05); cadmium and arsenic (rs = 0.48, P < 0.05); cadmium and cobalt (rs = 0.80, P < 0.001); zinc and thallium (rs = 0.74, P < 0.01); zinc and atrazine (rs = 0.80, P < 0.05); BPA and NPE (rs = 0.78, P < 0.01); BPA and atrazine (rs = 0.71, P < 0.05); and thallium and manganese (rs = 0.50, P < 0.05). Statistically significant, negative correlations were found between mercury and lead (rs = –0.86, P < 0.05); and cadmium and copper (rs = –0.52, P < 0.05).
Bottlenose Dolphins
The results of the t-tests showed that in 11 adult bottlenose dolphin females, arsenic concentrations were significantly higher (1.35 μg g–1, t = –1.82, P = 0.04) and iron concentrations were significantly lower (862.46 μg g–1, t = 2.13, P = 0.02) than in 18 adult males (arsenic: 0.75 μg g–1, iron: 1,559.83 μg g–1). Thirty-one adult bottlenose dolphins had significantly higher concentrations of lead (0.10 μg g–1, t = 2.67, P = 0.01), mercury (244.39 μg g–1, t = 4.67, P < 0.01), and selenium (111.43 μg g–1, t = 6.09, P < 0.01), and significantly lower concentrations of manganese (10.60 μg g–1, t = –3.05, P < 0.01) compared to 12 juvenile dolphins (lead: 0.05 μg g–1, mercury: 34.82 μg g–1, selenium: 14.25 μg g–1, manganese: 15.22 μg g–1).
Eighteen adult bottlenose dolphins that stranded in North Carolina had significantly higher concentrations of iron (1,489.43 μg g–1, t = 1.81, P = 0.04), and significantly lower concentrations of lead (0.08 μg g–1, t = –2.29, P = 0.02), mercury (191.19 μg g–1, t = –2.32, P = 0.02), and selenium (73.47 μg g–1, t = –3.67, P < 0.01) compared to 12 adult dolphins that stranded in Florida (iron: 829.49 μg g–1, lead: 0.14 μg g–1, mercury: 490.02 μg g–1, selenium: 201.03 μg g–1). Eleven dolphins that stranded in Florida were identified as known IRL inhabitants based on dorsal fin photo identification data. Average essential and non-essential element concentrations in liver samples were compared between these 11 IRL dolphins and six non-IRL dolphins that stranded elsewhere in Florida. Non-IRL Florida dolphins had significantly higher average liver concentrations of arsenic (1.85 μg g–1, t = 2.99, P = 0.01), cadmium (3.52 μg g–1, t = 3.56, P < 0.01), and selenium (260.54 μg g–1, t = 1.92, P = 0.04) than IRL dolphins (arsenic: 0.44 μg g–1, cadmium: 0.10 μg g–1, selenium: 123.37 μg g–1). No other analytes significantly differed between sexes, age classes, or locations based on the t-test results.
Mercury, Cadmium, and Selenium
There were two bottlenose dolphins – an adult male that stranded in Waves, North Carolina in 2012, and an adult female that stranded in North Palm Beach, Florida in 2018 – that had liver mercury concentrations of 1,402 μg g–1 and 1,416 μg g–1, respectively – an order of magnitude higher than liver mercury concentrations observed in other bottlenose dolphins in this study. These two dolphins also had relatively high concentrations of cadmium, iron, lead, and selenium, likely indicating chronic or repeated exposures to these elements. The average mercury:selenium molar ratios for bottlenose dolphin neonates (N = 3), juveniles (N = 12), and adults (N = 23) were 0.41, 0.67, and 1.01, respectively, and differed significantly between juveniles and adults (t = 4.60, P < 0.01).
Relevant Histopathological Data
Overall, histopathological data were available for 72 individual animals, including 41 bottlenose dolphins, 15 pygmy sperm whales, four Atlantic spotted dolphins, three melon-headed whales, two Blainville’s beaked whales, two Gervais’ beaked whales, one dwarf sperm whale, one pantropical spotted dolphin, one white-beaked dolphin, one Risso’s dolphin, and one Cuvier’s beaked whale (detailed in Supplementary Table S5). Table 3 presents the number of cases with histopathological changes per organ system. Of the 72 cases, 59 (82%) had at least one of the histopathological changes listed in Table 3, while 13 (18%) did not.
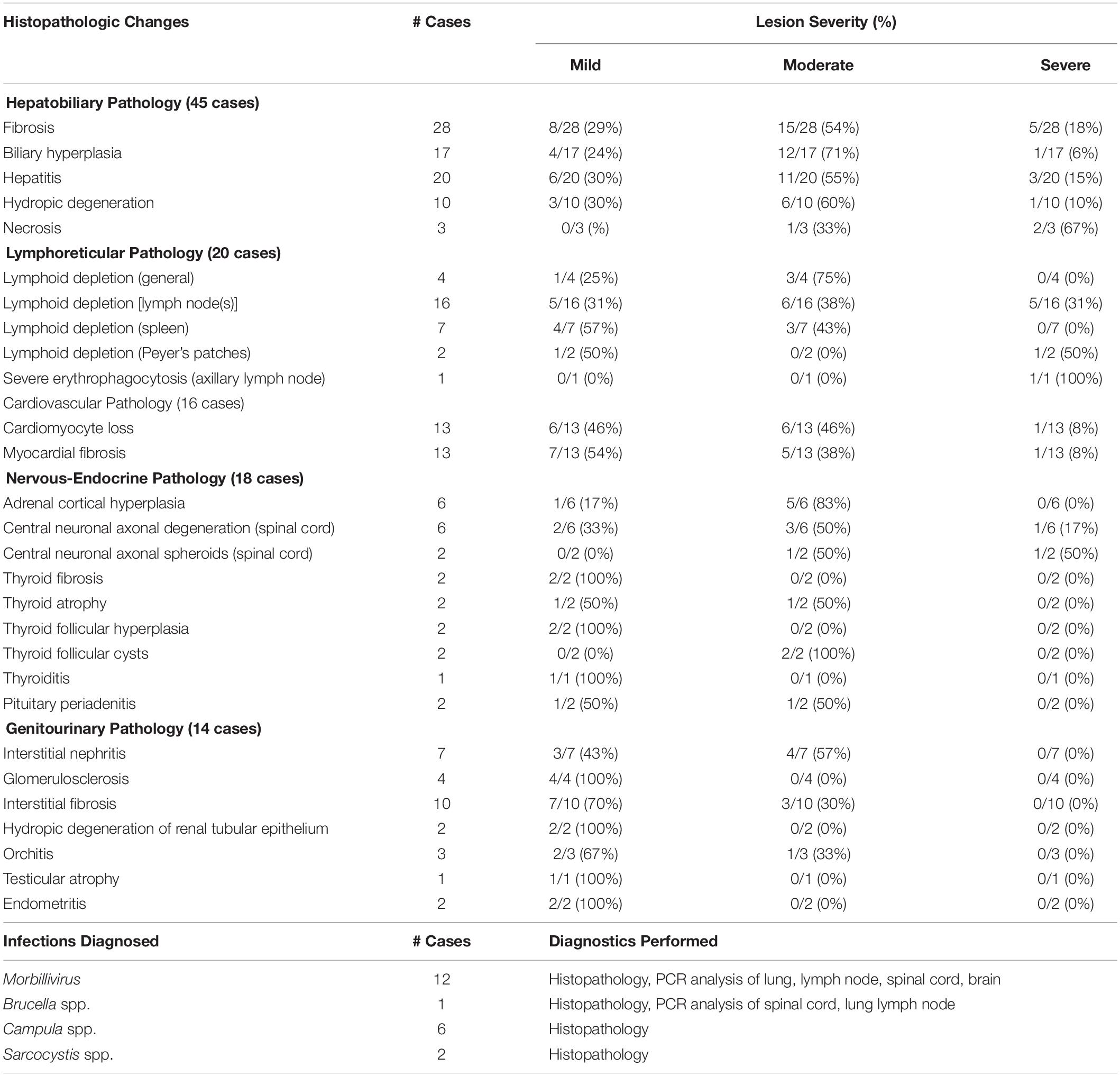
Table 3. Histopathological lesions observed and infections diagnosed in 72 cases of stranded odontocetes that had a full suite of microscopic data available for review.
Of the 72 cases with histopathology data, 47 (65%) stranded during the Morbillivirus-related Atlantic bottlenose dolphin unusual mortality event of 2013–2016. Samples from 18 animals (25%) were tested for Morbillivirus DNA using polymerase chain reaction (PCR) analysis of lung, pulmonary lymph node, spinal cord, and/or brain tissues that were analyzed at the University of California, Davis Marine Ecosystem Health Diagnostic & Surveillance Laboratory. In the other cases, ancillary diagnostics were not used due to lack of clinical signs consistent with Morbillivirus infection, along with budgetary and/or logistical constraints. Morbillivirus infections were diagnosed in 12 bottlenose dolphins (17%), including six adults and five subadults that stranded along the central coast of North Carolina, and one subadult that stranded in Vero Beach, Florida. Morbillivirus was diagnosed based on pathology data (i.e., lymphoid depletion, bronchointerstitial pneumonia, pleural fibrosis, respiratory and lymphoid syncytia (myelo/meningo)encephalitis, myelin sheath expansion, demyelination, meningeal fibrosis) (N = 7), or based on pathology data combined with PCR results (N = 5) (Di Guardo and Mazzariol, 2016). In 11 of the dolphins that tested positive for Morbillivirus, significantly lower concentrations of lead (0.09 μg g–1, t = –1.97, P = 0.03) and selenium (29.85 μg g–1, t = –2.36, P = 0.01) were observed compared to average concentrations in 25 bottlenose dolphins that did not have Morbillivirus infections (lead: 0.12 μg g–1, selenium: 88.15 μg g–1) (Supplementary Table S4). None of the other essential or non-essential element concentrations significantly differed between dolphins with/without Morbillivirus infections.
There were nine bottlenose dolphins, one Risso’s dolphin, and one Cuvier’s beaked whale suspected to have Brucella spp. infection based on histopathology including scapulohumeral joint arthritis, meningoencephalitis, mastitis, and orchitis. However, confirmatory PCR analysis of affected tissues was only performed in one bottlenose dolphin. There were five bottlenose dolphins and one melon-headed whale diagnosed with hepatic trematodiasis (e.g., Campula spp.); two of those dolphins also had Morbillivirus co-infections. Protozoal cysts consistent with Sarcocystis spp. were observed within the skeletal muscle of one bottlenose dolphin and within cardiac muscle of one pygmy sperm whale.
Five different types of neoplasia were observed in 4/72 (6%) cases (Table 4), including a testicular seminoma and a pheochromocytoma in an adult male white-beaked dolphin (Thayer et al., 2018). This dolphin had mild to moderate hepatobiliary fibrosis, mild lymphoid depletion in the spleen, thymus, and the tracheobronchial, pulmonary, prescapular, and mesenteric lymph nodes, mild to moderate cardiomyocyte loss (possibly related to the pheochromocytoma), nodular thyroid hyperplasia, and mild renal interstitial fibrosis with glomerulosclerosis. This animal also had the highest concentration of BPA measured in this study (397.4 μg g–1), and had relatively high (compared to other animals in this study) concentrations of DEP (13.8 μg g–1), lead (0.52 μg g–1), and triclosan (49.6 μg g–1). The other three animals with neoplastic lesions did not have relatively high concentrations of any of the toxicants measured.
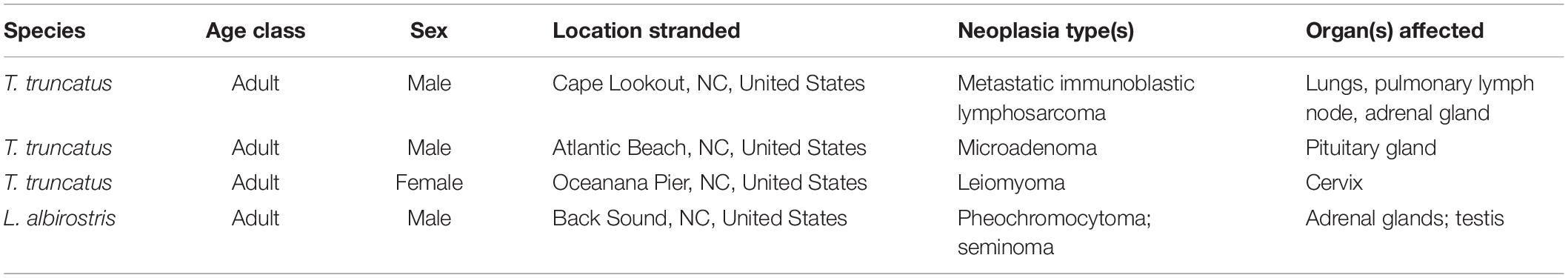
Table 4. Five types of neoplasia were observed in 4 of 72 (6%) cases of stranded odontocetes that had a full suite of pathology data available for review.
Discussion
The toxicant concentration data presented here considerably improve upon the existing knowledge base regarding toxicant concentrations in stranded odontocetes. Several previous studies have focused on the accumulation of more well-known EDCs in marine mammal tissues including BPA, PCBs, organochlorines, and triclosan (Tilbury et al., 1999; Fair et al., 2009; Jepson et al., 2015; Xue and Kannan, 2016; Damseaux et al., 2017). Additionally, previous studies measuring toxicants and essential and non-essential elements in free-ranging odontocete species have been conducted globally, in a variety of tissues including blood, skin, blubber, kidney, liver, muscle, and brain (Kemper et al., 1994; Bryan et al., 2007; Durden et al., 2007; Stavros et al., 2011; Schaefer et al., 2011, 2015; Beck et al., 2013; Monteiro et al., 2016a, b). To date, however, this is the first published report examining concentrations of atrazine, DEP, NPE, and triclosan in blubber tissues of stranded cetaceans. Our study is also the first to report concentrations of toxicants in a white-beaked dolphin and in Gervais’ beaked whales, species for which the scientific literature remains sparse. Documenting toxicants in cetaceans is a critical step in tracing chemical contaminants within the marine food web and understanding their effects on biological systems (Hermabessiere et al., 2017).
Demographic Factors Influence Toxicant Exposure and Accumulation: Species Comparisons
Despite geographic and food source overlap between species, the examined specimens disclosed significant differences in predominance of organic contaminants and essential and non-essential elements. Tissue contaminant concentrations are thought to be influenced by many factors such as an animal’s age, sex, nutritional status, reproductive status, foraging habitat(s), and location (Durden et al., 2007; Schaefer et al., 2011; Stavros et al., 2011; Beck et al., 2013; Hansen et al., 2016; Genov et al., 2019). The main sources of toxicant exposure in cetaceans are through their diet and via maternal transfer during gestation and/or lactation (Kajiwara et al., 2008; Bossart, 2011; Alonso et al., 2015). Differences in prey selection could explain the species-specific differences in analyte concentrations seen here, since previous evidence suggests that animals that primarily prey on cephalopods, including pygmy sperm whales, can be exposed to higher levels of certain heavy metals such as cadmium, which can accumulate in squid, compared to primarily piscivorous species such as dolphins (Monaci et al., 1998; Bustamante et al., 2003; Bloodworth and Odell, 2008). Spearman rank correlation coefficient tests showed statistically significant correlations between concentrations of multiple toxicants and nutritional elements in bottlenose dolphins and pygmy sperm whales. Positive correlations demonstrate similarities in exposure, transport mechanisms, detoxification mechanisms, and bodily distribution of certain analytes; while negative correlations indicate possible antagonistic effects of certain analytes (Monaci et al., 1998; Hansen et al., 2016).
The organic toxicant concentrations presented here were not normalized to lipid concentration, since lipid concentrations were not measured in the samples due to cost constraints. This presents some challenges to interpreting and comparing concentrations of lipophilic toxicants, which can fluctuate in cetacean fatty tissues depending on body condition. For example, in fasting or emaciated animals, increased lipolysis may be accompanied by concomitant release of lipophilic toxicants into circulation, followed by redistribution within remaining lipid stores (Bengtson Nash et al., 2013). Thus, results of species comparisons presented here for concentrations of Aroclor1268, atrazine, BPA, DEP, NPE, and triclosan in bottlenose dolphins versus pygmy sperm whales were likely influenced by the animals’ body condition at the time of stranding. Specifically, of 46 bottlenose dolphins, half were in ‘good’ (41%) or ‘robust’ (9%) body condition at the time of stranding, while 6 (13%) were ‘thin,’ and 17 (37%) were ‘emaciated.’ In comparison, of 21 pygmy sperm whales, none (0%) were considered ‘robust’ at the time of stranding, 9 (43%) were in ‘good’ body condition, 3 (14%) were ‘thin,’ and 9 (43%) were ‘emaciated’ (Supplementary Table S5).
We also present data on toxicant and essential and non-essential element concentrations in liver and blubber samples from four mother-fetus/neonate pairs, including three pygmy sperm whale pairs and one Gervais’ beaked whale pair (Figure 1). Although a low sample size precluded statistical analysis, some interesting trends were observed. Average concentrations of certain analytes were consistently higher in all maternal samples compared to their offspring, including arsenic (4.29 μg g–1 in mothers versus 1.90 μg g–1 in offspring), cadmium (13.30 μg g–1 in mothers versus 0.12 μg g–1 in offspring), cobalt (0.28 μg g–1 in mothers versus 0.04 μg g–1 in offspring), mercury (113.08 μg g–1 in mothers versus 6.82 μg g–1 in offspring), and selenium (48.15 μg g–1 in mothers versus 23.67 μg g–1 in offspring). Average concentrations of other analytes were consistently higher in all offspring samples compared to their mothers, including copper (1,181.03 μg g–1 in offspring versus 26.28 μg g–1 in mothers), lead (0.07 μg g–1 in offspring versus 0.05 μg g–1 in mothers), thallium (0.07 μg g–1 in offspring versus 0.04 μg g–1 in mothers), and zinc (615.44 μg g–1 in offspring versus 57.55 μg g–1 in mothers). These data reflect patterns of in utero (and in the case of the mother-neonate pair, also via lactation) maternal transfer of toxicants, and are presumed to vary depending on whether a toxicant is lipophilic and whether it tends to bioaccumulate with age, among other factors (Krahn et al., 2009; Alonso et al., 2015). In mother-fetus pairs of short-beaked common dolphins (Delphinus delphis) that stranded along the French coasts, fetal liver tissues exhibited consistently low concentrations of cadmium and mercury, and high concentrations of copper (Lahaye et al., 2007). Other previous studies have focused on maternal transfer of lipophilic toxicants in odontocetes. For example, in long-finned pilot whales (Globicephala melas), the average gestational transfer rates of PCBs and DDTs (calculated as the ratios between newborn loads and maternal loads) were estimated to be ∼7% and ∼8%, respectively (Borrell et al., 1995). In harbor porpoises (Phocoena phocoena), a gestational transfer rate of 15% was observed for organochlorine compounds (Duinker and Hillebrand, 1979). Transplacental transfer rates also depend on the ratio of the body weight of the fetus to that of the pregnant female (Tanabe et al., 1982). Offloaded amounts of contaminants tend to decrease with a mother’s age and are consequently much higher in primiparous females than in those that have already given birth (Aguilar and Borrell, 1994a, b; Borrell et al., 1995). Maternal transfer of toxicants during gestation and lactation to rapidly developing offspring may put young cetaceans at greater risk for adverse health effects, including immune and endocrine system dysfunction (Krahn et al., 2009).
Arsenic concentrations from this study can be compared to data previously reported, including one study that presented arsenic concentrations in liver tissues of seven cetacean species, ranging from 0.20 to 5.96 μg g–1 dw and varying widely among species (Wells et al., 2004; Mazumder, 2005; Kajiwara et al., 2008). Average arsenic concentrations reported here for all species fell within a relatively wider range (0.07–7.73 μg g–1), and maximum values reported here for pygmy and dwarf sperm whales, Stenella spp. dolphins, and Gervais’ beaked whales were higher than previously reported maximum arsenic concentrations in similar species (Tu et al., 2015). One animal in our study, an adult female Gervais’ beaked whale that stranded in Sebastian, Florida in 2017, had the highest liver concentration of arsenic (7.73 μg g–1) reported for any marine mammal to date (to the authors’ knowledge). Arsenic is widespread in the marine environment, is highly toxic in its inorganic forms, and the liver is the main site of arsenic storage and metabolism (Kubota et al., 2001; Tu et al., 2015; Li et al., 2016). Feeding habit strongly influenced marine mammal liver arsenic concentrations in one study, as species feeding on cephalopods and crustaceans tended to have higher arsenic concentrations than those feeding on fish (Kubota et al., 2001). This was upheld in our study, as pygmy sperm whales had significantly higher arsenic concentrations than bottlenose dolphins. Another explanation for high arsenic concentrations in tissues of animals that strand in Florida may be effluent from natural phosphate mineral deposits in the area that contain high levels of arsenic (Neff, 1997; Perry et al., 2015).
Demographic Factors Influence Toxicant Exposure and Accumulation: Bottlenose Dolphins
As long-lived, apex predators, dolphins show increased accumulation of some toxicants with age, particularly in males (Hickie et al., 2013; Gui et al., 2014). For trace elements, tissue accumulation patterns vary by element, and differences in distribution of different analytes between males and females, and juveniles and adults are often attributable to differences in metabolism and storage of these elements (Cardellicchio et al., 2002; Carvalho et al., 2002; Finlayson et al., 2019). Results of Student’s t-tests showed that in adult male bottlenose dolphins, average iron concentrations were significantly higher and average arsenic concentrations were significantly lower than those found in adult females. We also found that in adult bottlenose dolphins, average lead, mercury, and selenium concentrations were significantly higher, and average manganese concentration was significantly lower than average concentrations of these elements in juveniles. One possible explanation for these results is that adults bioaccumulate lead, mercury, and selenium after exposure through their fish-based diet, and females offload iron stores during gestation and through lactation (Houde et al., 2006; Hickie et al., 2013; Fisher and Nemeth, 2017).
Toxicant exposure dynamics also vary by location, depending on land use, toxicant source, and means by which industrial, urban, and agricultural chemicals enter coastal habitats. Inshore dolphins and those inhabiting areas adjacent to human activity are particularly susceptible to exposure to high concentrations of these contaminants as they enter the marine ecosystem via runoff and/or direct discharge (Hansen et al., 2004, 2016; Stavros et al., 2007; Finlayson et al., 2019). In this study we found that bottlenose dolphins that stranded in Florida had significantly higher average concentrations of lead, mercury, and selenium than dolphins that stranded in North Carolina, and significantly lower average concentrations of iron. This is similar to findings from a previous study, which found that liver samples from dolphins that stranded in Florida were significantly higher in lead, mercury, and selenium than those found in dolphins that stranded in South Carolina, United States (Stavros et al., 2011). Since dolphins are primarily exposed to toxicants via ingestion of prey items, their patterns of contamination often closely match those of their preferred prey species (Senthilkumar et al., 1999; Yeung et al., 2009). Because many of their prey species are also preferred food fish by humans, monitoring concentrations of these and other contaminants in stranded dolphins can provide a relatively low-cost snapshot of the potential exposure risks in humans and other organisms that feed at the upper trophic levels (Bossart, 2011; Reif et al., 2015).
Mercury and Selenium
Compared to data from several other studies, liver mercury concentrations reported here were high, including some extremely high concentrations that are comparable to the highest ever reported (Endo et al., 2002; Bustamante et al., 2003; Stavros et al., 2011; Bryan et al., 2012; Genov et al., 2019). Specifically, there were two bottlenose dolphins, an adult male that stranded in Waves, North Carolina in 2012, and an adult female that stranded in North Palm Beach, Florida in 2018, that had liver mercury concentrations that were an order of magnitude higher than liver mercury concentrations observed in other bottlenose dolphins in this study. These two dolphins also had relatively high concentrations of cadmium, iron, lead, and selenium.
Both methylmercury and inorganic mercury tend to biomagnify in marine mammal species, and bottlenose dolphins from Florida are known to carry very high mercury burdens in their tissues (Schaefer et al., 2011, 2015; Gui et al., 2014; Seixas et al., 2014; Damseaux et al., 2017). Bottlenose dolphins and pygmy sperm whales, along with some other odontocete species, seem able to tolerate high levels of mercury, cadmium, and certain other metals, and detoxify them through several physiological processes including binding with selenium or metallothioneins to mitigate the toxic effects of exposure (Meador et al., 1999; Klaassen et al., 2009; Bryan et al., 2012; Hansen et al., 2016). These protective mechanisms in odontocetes likely reduce some of the direct effects that can be seen with heavy metal toxicity, such as oxidative stress, inhibition of lysosomal digestive enzymes, damage to subcellular membranes, and deregulation of apoptotic pathways (Kershaw and Hall, 2019). Here, we found evidence to support this hypothesis, since liver selenium concentrations were positively correlated with mercury, cadmium, and lead in adult bottlenose dolphins, and positively correlated with mercury and cadmium in pygmy sperm whales. This is in alignment with findings from many other studies, which confirm mercury:selenium molar ratios close to 1 in adult cetacean liver and kidney samples (Capelli et al., 2000; Bustamante et al., 2003; Yang et al., 2007; Caceres-Saez et al., 2013; Hansen et al., 2016). The detoxifying effects of selenium on mercury are only thought to occur above a minimum mercury concentration in the liver (100 μg kg–1), thus juveniles tend to have a lower mercury:selenium ratio than adults that have been accumulating mercury over a longer period of time (Palmisano et al., 1995; Storelli et al., 1998). This trend was also upheld in our study, as median mercury:selenium ratios in bottlenose dolphins incrementally increased with age in fetuses/neonates, juveniles, and adults, and differed significantly between juveniles and adults. While selenium binding may effectively function to detoxify mercury and other toxicants, it can also leave the animal in a selenium-deficient state, leading to neurotoxic effects and potentially other sequelae such as nutritional myodegeneration (Cullen, 2007; Kehrig et al., 2013). Therefore, adequate dietary selenium must be maintained to prevent indirect effects of selenium detoxification, which can be a problem for top predators like odontocetes because mercury biomagnifies up the food chain at a higher rate (5.4 times) than selenium (2.4 times) (Kunito et al., 2002).
Histopathological and Diagnostic Data
Although infectious disease testing was not performed in all cases, data resulting from cases examined using molecular diagnostics provide information on disease status and allow for comparisons between groups. Because contaminant exposure is thought to have immunotoxic effects in cetaceans, the potential synergistic effects of co-exposure to multiple pollutants may modulate the pathogenic and pathogenetic activity of marine mammal Morbilliviruses (Desforges et al., 2016, 2017). Previous studies suggest that high contaminant loads may synergistically interact to increase Morbillivirus disease severity and favor transmission between cetacean species (Aguilar and Raga, 1993; Aguilar and Borrell, 1994a; Aznar et al., 2005; Fossi et al., 2007). This pattern was not observed in this study for non-essential elements; however low sample sizes precluded comparisons of lipophilic toxicants for animals with and without Morbillivirus infections.
Many histological lesions observed in the tissues of stranded marine mammals are non-specific changes that can be associated with the acute physiological and physical derangements that accompany live stranding, including sequelae of catecholaminergic and neurogenic shock (e.g., pulmonary edema/hemorrhage, acute venous congestion of shock organs), trauma (e.g., sunlight-thermal burn, sand impaction/ingestion, superficial epithelial abrasions, corneal damage), hyperthermia, acute skeletal and cardiac myodegeneration, and multiorgan failure (Geraci and Lounsbury, 2005; Bogomolni et al., 2010; Diaz-Delgado et al., 2018). Other non-specific lesions (e.g., fibrotic or hydropic changes of the hepatic, renal, respiratory, and gastrointestinal systems; lymphoid depletion; inflammation) are often more chronic in nature and can be attributable to various causes, including stress, starvation, infection(s), and/or prolonged or repeated exposure to certain toxicants such mercury, cadmium, and lead (Goyer, 1989; Johri et al., 2010; Branco et al., 2012). Neoplasia in free-ranging wildlife can occur naturally at low prevalence; however, contaminant exposure should be considered as a differential in wild animals that present with neoplastic lesions (McAloose and Newton, 2009). While it is difficult to confirm whether any of the histopathological lesions observed in these cases were caused or exacerbated by toxicant exposure, toxicants should not be ruled out or overlooked in cases with certain life-history characteristics and pathological findings. Collecting and archiving fatty tissues and organ samples (e.g., liver, kidney, brain, etc.) from stranded animals is valuable in that it allows us to conduct retrospective toxicological studies such as this one. By examining toxicant concentrations alongside sublethal histopathologic changes in specific tissues, we can begin to better understand some of the potential health impacts that exposure to these compounds can have on vulnerable and understudied species like cetaceans.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by the Florida Atlantic University-HBOI and North Carolina State University Animal Care and Use Committees under protocols #A18-03 and #15-001-O.
Author Contributions
AP-K, CL, BR, CH, and JP: study design. AP-K, CH, DR, SH, SMH, AL, JB, VT, JS, and EC: sample analysis. AP-K, CL, and JP: data analysis. All authors: manuscript preparation.
Funding
Funding for this work was provided by the Florida State License Program ‘Protect Wild Dolphins’ and ‘Protect Florida Whales’ grants (administered by the Harbor Branch Oceanographic Institute Foundation), and the John H. Prescott Grant #’s NA14NMF4390181, NA11NMF4390065, NA17NMF4390103, NA12NMF4390165, and NA16NMF4390141.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the members of the FAU-HBOI Marine Mammal Stranding Response Team, including Wendy Marks, Steve Burton, Adam Schaefer, Jeffrey Cartzendafner, Anne Sleeman, Tyler Harrington, and Luke Yrastorza. We thank the members of the Marine Mammal Stranding Response Team of the North Carolina Coast, including Maria Serrano, Karen Clark, Paul Doshkov, William McLellan, Keith Rittmaster, D. Ann Pabst, Josh Summers, Cape Lookout and Cape Hatteras National Seashores, North Carolina Division of Marine Fisheries Marine Patrol, and University of North Carolina Wilmington for necropsy assistance and aid in transporting carcasses, and transport to stranded animals. Also thanks to the FAU-HBOI Marine Mammal Photo ID team for assistance with identifying known IRL inhabitants.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.00630/full#supplementary-material
Abbreviations
Arorclor1268, polychlorinated biphenyl isomer 1268; BPA, bisphenol A; DEP, diethyl phthalate; EDCs, endocrine-disrupting contaminants; FAU-HBOI, Florida Atlantic University, Harbor Branch Oceanographic Institute; GC-MS/MS, gas chromatography-tandem mass spectrometry; ICP-MS, inductively-coupled plasma mass spectrometry; IRL, Indian River Lagoon; NPE, nonylphenol ethoxylate.
References
Aguilar, A., and Borrell, A. (1994a). Abnormally high polychlorinated biphenyl levels in striped dolphins (Stenella coeruleoalba) affected by the 1990-1992 Mediterranean epizootic. Sci. Total Environ. 154, 237–247. doi: 10.1016/0048-9697(94)90091-4
Aguilar, A., and Borrell, A. (1994b). Reproductive transfer and variation of body load of organochlorine pollutants with age in fin whales (Belaenoptera physalus). Arch. Environ. Contam. Toxicol. 27, 546–554. doi: 10.1007/BF00214848
Aguilar, A., and Raga, J. A. (1993). The striped dolphin epizootic in the Mediterranean Sea. Ambio 22, 524–528.
Alonso, M. B., Feo, M. L., Corcellas, C., Gago-Ferrero, P., Bertozzi, C. P., Marigo, J., et al. (2015). Toxic heritage: maternal transfer of pyrethroid insecticides and sunscreen agents in dolphins from Brazil. Environ. Pollut. 207, 391–402. doi: 10.1016/j.envpol.2015.09.039
Aubail, A., Mendez-Fernandez, J., Bustamante, P., Churlaud, C., Ferreira, M., Vingada, J., et al. (2013). Use of skin and blubber tissues of small cetaceans to assess the trace element content of internal organs. Mar. Pollut. Bull. 76, 158–169. doi: 10.1016/j.marpolbul.2013.09.008
Aznar, F. J., Perdiguero, D., Pérez del Olmo, A., Repullés, A., Agustí, C., and Raga, J. A. (2005). Changes in epizoic crustacean infestations during cetacean die-offs: the mass mortality of Mediterranean striped dolphins Stenella coeruleoalba revisited. Dis. Aquat. Org. 67, 239–247. doi: 10.3354/dao067239
Barnes, D. K. A., Galgani, F., Thompson, R. C., and Barlaz, M. (2009). Accumulation and fragmentation of plastic debris in global environments. Phil. Trans. R. Soc. B 364, 1985–1998. doi: 10.1098/rstb.2008.0205
Barrett, T., Visser, I. K., Mamaev, L., Goatley, L., van Bressem, M. F., and Osterhaust, A. D. (1993). Dolphin and porpoise morbilliviruses are genetically distinct from phocine distemper virus. Virol. 193, 1010–1012. doi: 10.1006/viro.1993.1217
Beck, S., Foote, A. D., Kotter, S., Harries, O., Mandleberg, L., Stevick, P. T., et al. (2013). Using opportunistic photo-identifications to detect a population decline of killer whales (Orcinus orca) in British and Irish waters. J. Mar. Biol. Assoc. U.K. 94, 1327–1333. doi: 10.1017/S0025315413001124
Bengtson Nash, S. M., Waugh, C. A., and Schlabach, M. (2013). Metabolic concentration of lipid soluble organochlorine burdens in the blubber of southern hemisphere humpback whales through migration and fasting. Env. Sci. Tech. 47, 9404–9413. doi: 10.1021/es401441n
Bloodworth, B. E., and Odell, D. K. (2008). Kogia breviceps (Cetacea: Kogiidae). Mammalian Spec. 819, 1–12. doi: 10.1644/819.1
Bogomolni, A. L., Pugliares, K. R., Sharp, S. M., Patchett, K., Harry, C. T., LaRocque, J. M., et al. (2010). Mortality trends of stranded marine mammals on Cape Cod and southeastern Massachusetts, USA, 2000 to 2006. Dis. Aquat. Org. 88, 143–155. doi: 10.3354/dao02146
Borrell, A., Bloch, D., and Desportes, G. (1995). Age trends and reproductive transfer of organochlorine compounds in long-finned pilot whales from the Faroe Islands. Environ. Pollut. 88, 283–292. doi: 10.1016/0269-7491(95)93441-2
Bossart, G. D. (2011). Marine mammals as sentinel species for oceans and human health. Vet. Pathol. 43, 676–690. doi: 10.1177/0300985810388525
Bossart, G. D., Odell, D. K., and Altman, N. H. (1985). Cardiomyopathy in stranded and dwarf sperm whales. J. Am. Vet. Med. Assoc. 187, 1137–1140.
Branco, V., Ramos, P., Canario, J., Lu, J., Holmgren, A., and Carvalho, C. (2012). Biomarkers of adverse response to mercury: histopathology versus Thioredoxin reductase activity. J. Biomed. Biotechnol. 2012:359879. doi: 10.1155/2012/359879
Bryan, C. E., Christopher, S. J., Balmer, B. C., and Wells, R. S. (2007). Establishing baseline levels of trace elements in blood and skin of bottlenose dolphins in Sarasota Bay, Florida: implications for non-invasive monitoring. Sci. Total Environ. 388, 325–342. doi: 10.1016/j.scitotenv.2007.07.046
Bryan, C. E., Davis, W. C., McFee, W. E., Neumann, C. A., Schulte, J., Bossart, G. D., et al. (2012). Influence of mercury and selenium chemistries on the progression of cardiomyopathy in pygmy sperm whales. Kogia breviceps. Chemosphere 89, 556–562. doi: 10.1016/j.chemosphere.2012.05.051
Bustamante, P., Garrigue, C., Breau, L., Caurant, F., Dabin, W., Greaves, J., et al. (2003). Trace elements in two odontocete species (Kogida breviceps and Globicephala macrorhynchus) stranded in New Caledonia (South Pacific). Environ. Pollut. 124, 263–271. doi: 10.1016/s0269-7491(02)00480-3
Caceres-Saez, I., Dellabianca, N. A., Goodall, R. N. P., Cappozzo, H. L., and Ribeiro Geuvara, S. (2013). Mercury and selenium in subantarctic Commerson’s dolphins (Cephalorhynchus c. commersonii). Biol. Trace Elem. Res. 151, 195–208. doi: 10.1007/s12011-012-9555-x
Canesi, L., and Fabbri, E. (2015). Environmental effects of BPA: focus on aquatic species. Dose Resp. 13, 1–14. doi: 10.1177/1559325815598304
Capelli, R., Drava, G., De Pellegrini, R., Minganti, V., and Poggi, R. (2000). Study of trace elements in organs and tissues of striped dolphins (Stenella coeruleoalba) found dead along the Ligurian coasts (Italy). Adv. Environ. Res. 4, 31–43. doi: 10.1016/S1093-0191(00)00005-8
Cardellicchio, N., Decataldo, A., Di Leo, A., and Giandomenico, S. (2002). Trace elements in organs and tissues of striped dolphins (Stenella coeruleoalba) from the Mediterranean Sea (Southern Italy). Chemosphere 49, 85–90. doi: 10.1016/S0045-6535(02)00170-4
Carvalho, M. L., Pereira, R. A., and Brito, J. (2002). Heavy metals in soft tissues of Tursiops truncatus and Delphinus delphis from west Atlantic Ocean by X-ray spectrometry. Sci. Total Environ. 292, 247–254. doi: 10.1016/s0048-9697(01)01131-7
Cole, M., Lindeque, P., Halsband, C., and Galloway, T. S. (2011). Microplastics as contaminants in the marine environment: a review. Mar. Pollut. Bull. 62, 2588–2597. doi: 10.1016/j.marpolbul.2011.09.025
Crews, D., Putz, O., Thomas, P., Hayes, T., and Howdeshell, K. (2003). Wildlife as models for the study of how mixtures, low doses, and the embryonic environment modulate the action of endocrine-disrupting chemicals. Pure Appl. Chem. 75, 2305–2320. doi: 10.1351/pac200375112305
Cullen, J. M. (2007). “Liver, biliary system and exocrine pancreas,” in Pathologic Basis of Veterinary Disease, eds M. D. McGavin and J. F. Zachary (St. Louis, MO: Elsevier Inc), 393–461.
Damseaux, F., Kiszka, J. J., Heithaus, M. R., Scholl, G., Eppe, G., Thome, J.-P., et al. (2017). Spatial variation in the accumulation of POPs and mercury in bottlenose dolphins of the Lower Florida Keys and the coastal Everglades (South Florida). Environ. Pollut. 220(Part A), 577–587. doi: 10.1016/j.enpol.2016.10.005
Desforges, J. P., Levin, M., Jasperse, L., De Guise, S., Eulaers, I., Letcher, R. J., et al. (2017). Effects of polar bear and killer whale derived contaminant cocktails on marine mammal immunity. Environ. Sci. Technol. 51, 11431–11439. doi: 10.1021/acs.est.7b03532
Desforges, J. P., Sonne, C., Levin, M., Siebert, U., De Guise, S., and Dietz, R. (2016). Immunotoxic effects of environmental pollutants in marine mammals. Environ. Int. 86, 126–139. doi: 10.1016/j.envint.2015.10.007
Di Guardo, G., and Mazzariol, S. (2016). Cetacean Morbillivirus-associated pathology: knowns and unknowns. Frontiers Microbiol. 7:112. doi: 10.3389/fmicb.2016.00112
Diaz-Delgado, J., Fernandez, A., Sierra, E., Sacchini, S., Andrada, M., Vela, A. I., et al. (2018). Pathologic findings and causes of death of stranded cetaceans in the Canary Islands (2006-2012). PLoS One 13:e0204444. doi: 10.1371/journal/pone.0204444
Duinker, J. C., and Hillebrand, M. T. (1979). Mobilization of organochlorines from female lipid tissue and transplacental transfer to fetus in a harbor porpoise (Phocoena phocoena) in a contaminated area. Environ. Contam. Toxicol. 23, 728–732. doi: 10.1007/BF01770032
Durden, W. N., Stolen, M. K., Adams, D. H., and Stolen, E. D. (2007). Mercury and selenium concentrations in stranded bottlenose dolphins from the Indian River Lagoon system. Florida. B. Mar. Sci. 81, 37–54.
Endo, T., Haraguchi, K., and Sakata, M. (2002). Mercury and selenium concentrations in the internal organs of toothed whales and dolphins marketed for human consumption in Japan. Sci. Total Environ. 300, 15–22. doi: 10.1016/s0048-9697(02)00137-7
Fair, P. A., Lee, H.-B., Adams, J., Darling, C., Pacepavicius, G., Alae, M., et al. (2009). Occurrence of triclosan in plasma of wild Atlantic bottlenose dolphins (Tursiops truncatus) and in their environment. Environ. Pollut. 157, 2248–2254. doi: 10.1016/j.envpol.2009.04.002
Ferzand, R., Gadahi, J. A., Saleh, S., and Ali, Q. (2008). Histological and haematological disturbance caused by arsenic toxicity in mice model. Pakistan J. Biol. Sci. 11, 1405–1413. doi: 10.3923/pjbs.2008.1405.1413
Finlayson, K., Weijs, L., and van de Merwe, J. (2019). Health Impacts Of Organochlorines And Trace Elements In Humpback And Snubfin Dolphins In The Port Of Gladstone. Gladstone: Gladstone Ports Corporation.
Fisher, A. L., and Nemeth, E. (2017). Iron homeostasis during pregnancy. Am. J. Clin. Nutr. 106, (Suppl. 6), 1567S–1574S. doi: 10.3945/ajcn.117.155812
Fossi, M. C., Casini, S., and Marsili, L. (2007). Potential toxicological hazard due to endocrine-disrupting chemicals on Mediterranean top predators: state of art, gender differences and methodological tools. Environ. Res. 104, 174–182. doi: 10.1016/j.envres.2006.06.014
Frouin, H., Loseto, L. L., Stern, G. A., Haulena, M., and Ross, P. S. (2012). Mercury toxicity in beluga whale lymphocytes: limited effects of selenium protection. Aquat. Toxicol. 109, 185–193. doi: 10.1016/j.aquatox.2011.09.021
Gear, R. B., and Belcher, S. M. (2017). Impacts of bisphenol A and ethinyl estradiol on male and female CD-1 mouse spleen. Sci. Rep. 7, 1–12. doi: 10.1038/s41598-017-00961-8
Genov, T., Jepson, P. D., Barber, J. L., Hace, A., Gaspari, S., Centrih, T., et al. (2019). Linking organochlorine contaminants with demographic parameters in free-ranging common bottlenose dolphins from the northern Adriatic Sea. Sci. Total Environ. 657, 200–212. doi: 10.1016/j.scitotenv.2018.12.025
Geraci, J. R., and Lounsbury, V. J. (2005). Marine Mammals Ashore: A Field Guide for Strandings. Baltimore: National Aquarium in Baltimore.
Godfray, H. C. J., Stephens, A. E. A., Jepson, P. D., Jobling, S., Johnson, A. C., Matthiessen, P., et al. (2019). A restatement of the natural science evidence base on the effects of endocrine disrupting chemicals on wildlife. Proc. R. Soc. B 286:20182416. doi: 10.1098/rspb.2018.2416
Goyer, R. A. (1989). Mechanisms of lead and cadmium nephrotoxicitry. Toxicol. Lett. 46, 153–162. doi: 10.1016/0378-4274(89)90124-0
Gregory, M. R. (1978). Accumulation and distribution of virgin plastic granules on New Zealand beaches. N. Zeal. J. Mar. Freshw. Res. 12, 399–414. doi: 10.1080/00288330.1978.9515768
Gregory, M. R. (2009). Environmental implications of plastic debris in marine settings—entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philos. Trans. R. Soc. Lond. Biol. Sci. 364, 2013–2025. doi: 10.1098/rstb.2008.0265
Gui, D., Riqing, Yu, Xuan, H., Qin, T., Laiguo, C., and Wu, Y. (2014). Bioaccumulation and biomagnification of persistent organic pollutants in Indo-Pacific humpback dolphins (Sousa chinensis) from the Pearl River Estuary, China. Chemosphere 114, 106–113. doi: 10.1016/j.chemosphere.2014.04.028
Hansen, A. M. K., Bryan, C. E., West, K., and Jensen, B. A. (2016). Trace element concentrations in liver of 16 species of cetaceans stranded on Pacific Islands from 1997 through 2013. Arch. Environ. Contam. Toxicol. 70, 75–95. doi: 10.1007/s00244-015-0204-1
Hansen, L. J., Schwacke, L. H., Mitchum, G. B., Hohn, A. A., Wells, R. S., Zolman, E. S., et al. (2004). Geographic variation in polychlorinated biphenyl and organochlorine pesticide concentration in the blubber of bottlenose dolphins from the US Atlantic coast. Sci. Total Environ. 319, 147–172. doi: 10.1016/S0048-9697(03)00371-1
Haschek, W. M., Rousseaux, C. G., and Wallig, M. A. (2010). Fundamentals of Toxicologic Pathology, 2nd Edn, London: Elsevier Inc.
Hayes, T. B., Khoury, V., Narayan, A., Nazir, M., Park, A., Brown, T., et al. (2010). Atrazine induces complete feminization and chemical castration in male African clawed frogs (Xenopus laevis). Proc. Natl. Acad. Sci. U.S.A. 107, 4612–4617. doi: 10.1073/pnas.0909519107
Helsel, D. R. (2006). Fabricating data: how substituting values for non-detects can ruin results, and what can be done about it. Chemosphere 65, 2434–2439. doi: 10.1016/j.chemosphere.2006.04.051
Hermabessiere, L., Dehaut, A., Paul-Pont, I., Lacroix, C., Jezequel, R., Soudant, P., et al. (2017). Occurrence and effects of plastic additives on marine environments and organisms: a review. Chemosphere 182, 781–793. doi: 10.1016/j.chemosphere.2017.05.096
Hickie, B., Cadieus, M. A., Riehl, K. N., Bossart, G. D., Alava, J. J., and Fair, P. (2013). Modeling PCB-bioaccumulation in the bottlenose dolphin (Tursiops truncatus): estimating a dietary threshold concentration. Environ. Sci. Tech. 47, 12314–12324. doi: 10.1021/es403166b
Houde, M., Pacepavicius, G., Wells, R. S., Fair, P. A., Letcher, R. J., Alaee, M., et al. (2006). Polychlorinated biphenyls and hydroxylated polychlorinated biphenyls in plasma of bottlenose dolphins (Tursiops truncatus) from the Western Atlantic and the Gulf of Mexico. Environ. Sci. Technol. 40, 5860–5866. doi: 10.1021/es060629n
Jakimska, A., Konieczka, P., Skora, K., and Namiesnik, J. (2011). Bioaccumulation of metals in tissues of marine animals, Part I: the role and impact of heavy metals on organisms. Pol. J. Environ. Stud. 20, 1117–1125.
Jepson, P. D., Deaville, R., Barber, J. L., Aguilar, A., Borrell, A., Murphy, S., et al. (2015). PCB pollution continues to impact populations of orcas and other dolphins in European waters. Sci. Reports 6:18573. doi: 10.1038/srep18573
Johri, N., Jacquillet, G., and Unwin, R. (2010). Heavy metal poisoning: the effects of cadmium on the kidney. Biometals 23, 783–792. doi: 10.1007/s10534-010-9328-y
Kajiwara, N., Kamikawa, S., Amano, M., Hayano, A., Yamada, T. K., Miyazaki, N., et al. (2008). Polybrominated diphenyl ethers (PBDES) and organochlorines in melon-headed whales, Peponocephala electra, mass stranded along the Japanese coasts: maternal transfer and temporal trend. Environ. Pollut. 156, 106–114. doi: 10.1016/j.envpol.2007.12.034
Kehrig, H. A., Seixas, T. G., Malm, O., Di Beneditto, A. P. M., and Rezende, C. E. (2013). Mercury and selenium biomagnification in a Brazilian coastal food web using nitrogen stable isotope analysis: a case study in an area under the influence of the Paraiba do Sul River plume. Mar. Pollut. Bull. 75, 283–290. doi: 10.1016/j.marpolbul.2013.06.046
Kemper, C., Gibbs, P., Obendorf, D., Marvanek, S., and Lenghaus, C. (1994). A review of heavy metal and organochlorine levels in marine mammals in Australia. Sci. Total Environ. 154, 129–139. doi: 10.1016/0048-9697(94)90083-3
Kershaw, J. L., and Hall, A. J. (2019). Mercury in cetaceans: exposure, bioaccumulation and toxicity. Sci. Total Env. 694:133683. doi: 10.1016/j.scitotenv.2019.133683
Klaassen, C. D., Liu, J., and Diwan, B. A. (2009). Metallothionein protection of cadmium toxicity. Toxicol. Appl. Pharmacol. 238, 215–220. doi: 10.1016/j.taap.2009.03.026
Krahn, M. M., Hanson, M. B., Schorr, G. S., Emmons, C. K., Burrows, D. G., Bolton, J. L., et al. (2009). Effects of age, sex, and reproductive status on persistent organic pollutant concentrations in “Southern Resident” killer whales. Mar. Pollut. Bull. 58, 1522–1529. doi: 10.1016/j.marpolbul.2009.05.014
Kubota, R., Kunito, T., and Tanabe, S. (2001). Arsenic accumulation in the liver tissue of marine mammals. Environ. Pollut. 115, 303–312. doi: 10.1016/S0269-7491(01)00099-9
Kunito, T., Kubota, R., Fujihara, J., Agusa, T., and Tanabe, S. (2008). Arsenic in marine mammals, seabirds, and sea turtles. Rev. Environ. Contam. Toxicol. 195, 31–69. doi: 10.1007/978-0-387-77030-7-2
Kunito, T., Watanabe, I., Yasunaga, G., Fujise, Y., and Tanabe, S. (2002). Using trace elements in skin to discriminate the populations of minke whales in southern hemisphere. Mar. Environ. Res. 53, 175–197. doi: 10.1016/s0141-1136(01)00119-2
Lahaye, V., Bustamante, P., Dabin, W., Churlaud, C., and Caurant, C. (2007). Trace element levels in foetus-mother pairs of short-beaked common dolphins (Delphinus delphis) stranded along the French coasts. Environ. Int. 33, 1021–1028. doi: 10.1016/j.envint.2007.05.008
Li, C., Li, P., Min Tan, Y., Hong Lam, S., Chan, E. C. Y., and Gong, Z. (2016). Metabolomic characterizations of liver injury caused by acute arsenic toxicity in zebrafish. PLoS One 11:e0151225. doi: 10.1371/journal.pone.0151225
Li, F., Yao, L., Sun, W., Jiang, Y., Li, Z., and Zhai, Y. (2017). Histopathological liver and testis alterations in male half-smooth tongue sole (Cynoglossus semilaevis) exposed to endocrine disruptors. J. Coast. Res. 33, 678–683. doi: 10.2112/jcoastres-D-15-00244.1
Mathieu-Denoncourt, J., Wallance, S. J., de Solla, S. R., and Langlois, V. S. (2015). Plasticizer endocrine disruption: highlighting developmental and reproductive effects in mammals and non-mammalian aquatic species. Gen. Comp. Endocrinol. 219, 74–88. doi: 10.1016/j.ygcen.2014.11.003
Mato, Y., Isobe, T., Takada, H., Kanehiro, H., Ohtake, C., and Kaminuma, T. (2001). Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 35, 318–324. doi: 10.1021/es0010498
Mazumder, D. N. (2005). Effect of chronic intake of arsenic-contaminated water on liver. Toxicol. Appl. Pharmacol. 206, 169–175. doi: 10.1016/j.taap.2004.08.025
McAloose, D., and Newton, A. L. (2009). Wildlife cancer: a conservation perspective. Nat. Rev. Cancer 9, 517–526. doi: 10.1038/nrc2665
Meador, J. P., Ernest, D., Hohn, A. A., Tilbury, K., Gorzelany, J., Worthy, G., et al. (1999). Comparison of elements in bottlenose dolphins stranded on the beaches of Texas and Florida in the Gulf of Mexico over a one-year period. Arch. Environ. Contam. Toxicol. 36, 87–98. doi: 10.1007/s002449900446
Monaci, F., Borrel, A., Leonzio, C., Marsili, L., and Calzada, N. (1998). Trace elements in striped dolphins (Stenella coeruleoalba) from the western Mediterranean. Environ. Pollut. 99, 61–68. doi: 10.1016/S0269-7491(97)00174-7
Monteiro, S. S., Pereira, A. T., Costa, E., Torres, J., Oliveira, I., Bastos-Santos, J., et al. (2016a). Bioaccumulation of trace element concentrations in common dolphins (Delphinus delphis) from Portugal. Mar. Pollut. Bull. 113, 400–407. doi: 10.1016/j.marpolbul.2016.10.033
Monteiro, S. S., Torres, J., Ferreira, M., Marçalo, A., Nicolau, L., Vingada, J. V., et al. (2016b). Ecological variables influencing trace element concentrations in bottlenose dolphins (Tursiops truncatus, Montagu 1821) stranded in continental Portugal. Sci. Total Environ. 544, 837–844. doi: 10.1016/j.scitotenv.2015.12.037
Muncke, J. (2011). Endocrine disrupting chemicals and other substances of concern in food contact materials: an updated review of exposure, effect and risk assessment. J. Steroid Biochem. Mol. Biol. 127, 118–127. doi: 10.1016/jsbmb.2010.10.004
Neff, J. M. (1997). Ecotoxicology of arsenic in the marine environment. Env. Toxicol. Chem. 16, 917–927.
Oehlmann, J., Schulte-Oehlmann, U., Kloas, W., Jagnytsch, O., Lutz, I., Kusk, K. O., et al. (2009). A critical analysis of the biological impacts of plasticizers on wildlife. Phil. Trans. R. Soc. B 364, 2047–2062. doi: 10.1098/rstb.2008.0242
Palmisano, F., Cardellichio, N., and Zambonin, P. G. (1995). Speciation of mercury in dolphin liver: a two-stage mechanism for the demethylation accumulation process and role of selenium. Mar. Environ. Res. 40, 109–121. doi: 10.1016/0141-1136(94)00142-C
Park, C. J., Kang, H. S., and Gye, M. C. (2010). Effects of nonylphenol on early embryonic development, pigmentation and 3,5,3’-triiodothyronine-induced metamorphosis in Bombina orientalis (Amphibia: Anura). Chemosphere 81, 1292–1300. doi: 10.1016/j.chemosphere.2010.08.039
Perry, H., Isphording, W., Trigg, C., and Riedel, R. (2015). Heavy metals in red crabs Chaceon quinquedens, from the Gulf of Mexico. Mar. Poll. Bull. 101, 845–851. doi: 10.1016/j.marpolbul.2015.11.020
Posnack, N. G., Swift, L. M., Kay, M. W., Lee, N. H., and Sarvazyan, N. (2012). Phthalate exposure changes the metabolic profile of cardiac muscle cells. Environ. Health Perspect. 120, 1243–1251. doi: 10.1289/ehp.1205056
Pugliares, K. R., Bogomolni, A., Touhey, K. M., Herzig, S. M., Harry, C. T., and Moore, M. J. (2007). Marine Mammal Necropsy: An Introductory Guide For Stranding Responders And Field Biologists. Woods Hole: Woods Hole Oceanographic Institution.
Read, A. J., Wells, R. S., Hohn, A. A., and Scott, M. D. (1993). Patterns of growth in wild bottlenose dolphins, Tursiops truncatus. J. Zool. Lond. 231, 107–123. doi: 10.1111/j.1469-7998.1993.tb05356.x
Reif, J. S., Schaefer, A. M., and Bossart, G. D. (2015). Atlantic bottlenose dolphins (Tursiops truncatus) as a sentinel for exposure to mercury in humans: closing the loop. Vet. Sci. 2, 407–422. doi: 10.3390/vetsci2040407
Ryan, P. G., Connell, A. D., and Gardner, B. D. (1998). Plastic ingestion and PCBs in seabirds: is there a relationship? Mar. Pollut. Bull. 19, 174–176. doi: 10.1016/0025-326X(88)90674-1
Santos, M. B., Pierce, G. J., Lopex, A., Reid, R. J., Ridoux, V., and Mente, E. (2006). Pygmy sperm whales Kogia breviceps in the Northeast Atlantic: new information on stomach contents and strandings. Mar. Mammal Sci. 22, 600–616. doi: 10.1111/j.1748-7692.2006.00038.x
Schaefer, A. M., Stavros, H.-C. W., Bossart, G. D., Fair, P. A., Goldstein, J. D., and Reif, J. S. (2011). Association between mercury and hepatic, renal, endocrine, and hematological parameters in Atlantic bottlenose dolphins (Turiops truncatus) along the eastern coast of Florida and South Carolina. Arch. Environ. Contam. Toxicol. 61, 688–695. doi: 10.1007/s00244-011-9651-5
Schaefer, A. M., Titcomb, E. M., Fair, P. A., Stavros, H.-C. W., Mazzoil, M., Bossart, G. D., et al. (2015). Mercury concentrations in Atlantic bottlenose dolphins (Tursiops truncatus) inhabiting the Indian River Lagoon, Florida: patterns of spatial and temporal distribution. Mar. Pollut. Bull. 97, 544–547. doi: 10.1016/j.marpolbul.2015.05.007
Seixas, T. G., Moreira, I., Siciliano, S., Malm, O., and Kehrig, H. A. (2014). Differences in methylmercury and inorganic mercury biomagnification in a tropical marine food web. Bull. Environ. Contam. Toxicol. 92, 274–278. doi: 10.1007/s00128-014-1208-7
Senthilkumar, K., Kannan, K., Sinha, R., Tanabe, S., and Giesy, J. P. (1999). Bioaccumulation profiles of polychlorinated biphenyl congeners and organochlorine pesticides in Ganges River dolphins. Environ. Toxicol. Chem. 18, 1511–1520. doi: 10.1002/etc.5620180725
Staples, C. A., Peterson, D. R., Parkerton, T. F., and Adams, W. J. (1997). The environmental fate of phthalate esters: a literature review. Chemosphere 35, 667–749. doi: 10.1016/S0045-6535(97)00195-1
Stavros, H.-C. W., Bossart, G. D., Hulsey, T. C., and Fair, P. A. (2007). Trace element concentrations in skin of free-ranging bottlenose dolphins (Tursiops truncatus) from the southeast Atlantic coast. Sci. Total Environ. 388, 300–315. doi: 10.1016/j.scitotenv.2007.07.030
Stavros, H.-C. W., Stolen, M., Durden, W. N., McFee, W., Bossart, G. D., and Fair, P. A. (2011). Correlation and toxicological inference of trace elements in tissues from stranded and free-ranging bottlenose dolphins (Tursiops truncatus). Chemosphere 82, 1649–1661. doi: 10.1016/j.chemospere.2010.11.019
Storelli, M. M., Ceci, E., and Marcotrigiano, G. O. (1998). Comparison of total mercury, methylmercury, and selenium in muscle tissues and in the liver of Stenella coueruleoalba (Meyen) and Caretta caretta (Linnaeus). Bull. Environ. Contam. Toxicol. 61, 541–547. doi: 10.1007/s001289900796
Tanabe, S., Tatsukawa, R., Maruyama, K., and Miyazaki, N. (1982). Transplacental transfer of PCBs and chlorinated hydrocarbon pesticides from the pregnant striped dolphin (Stenella coeruleoalba) to her fetus. Agric. Biol. Chem. 46, 1249–1254. doi: 10.1080/00021369.1982.10865248
Thayer, V. G., Harms, C. A., Rittmaster, K. A., Rotstein, D. S., and Hairr, J. E. (2018). A North Carolina stranding of a white-beaked dolphin (Lagenorhynchus albirostris), Family Delphinidae: a new southerly record. Aquat. Mammals 44, 32–38. doi: 10.1578/AM.44.1.2018.32
Tilbury, K. L., Adams, N. G., Krone, C. A., Meador, J. P., Early, G., and Varanasi, U. (1999). Organochlorines in stranded pilot whales (Globicephala melaena) from the coast of Massachusetts. Arch. Environ. Contam. Toxicol. 37, 125–134. doi: 10.1007/s002449900497
Titcomb, E. M., Reif, J. S., Fair, P. A., Stavros, H.-C. W., Mazzoil, M., Bossart, G. D., et al. (2017). Blood mercury concentrations in common bottlenose dolphins from the Indian River Lagoon, Florida: Patterns of social distribution. Mar. Mammal Sci. 33, 771–784. doi: 10.1111/mms.12390
Tu, T., Calabro, S. R., Lee, A., Maczurek, A. E., Budzinska, M. A., Warner, J., et al. (2015). Hepatocytes in liver injury: victim, bystander, or accomplice in progressive fibrosis? J. Gastroenterol. Hepatol. 30, 1696–1704. doi: 10.1111/jgh.13065
Weijs, L., and Zaccaroni, A. (2016). Toxicology of marine mammals: new developments and opportunities. Arch. Environ. Contam. Toxicol. 70, 1–8. doi: 10.1007/s00244-015-0233-9
Wells, R. S., Rhinehart, H. L., Hansen, L. J., Sweeney, J. C., Townsend, F. I., Stone, R., et al. (2004). Bottlenose dolphins as marine ecosystem sentinels: developing a health monitoring system. EcoHealth 1, 246–254. doi: 10.1007/s10393-004-0094-6
Wells, R. S., Tornero, V., Borrell, A., Aguilar, A., Rowles, T. K., Rhinehart, H. L., et al. (2005). Integrating life-history and reproductive success data to examine potential relationships with organochlorine compounds for bottlenose dolphins (Tursiops truncatus) in Sarasota Bay. Florida. Sci. Total Environ. 349, 106–109. doi: 10.1016/j.scitotenv.2005.01.010
Whaley, J. E., and Borkowski, R. (2009). Policies and Best Practices, Marine Mammal Stranding Response, Rehabilitation, and Release. Portland: NOAA National Marine Fisheries Service, 114.
Worm, B., Lotze, H. K., Jubinville, I., Wilcox, C., and Jambeck, J. (2017). Plastic as a persistent marine pollutant. Ann. Rev. Environ. Resour. 42, 1–26. doi: 10.1146/annurev-environ-102016-060700
Wu, Q., McFee, W. E., Goldstein, T., Tiller, R. V., and Schwacke, L. (2014). Real-time PCR assays for detection of Brucella spp. and the identification of genotype ST27 in bottlenose dolphins (Tursiops truncatus). Med. Epidemiol. 100, 99–104. doi: 10.1016/j.mimet.2014.03.001
Xue, J., and Kannan, K. (2016). Novel finding of widespread occurrence and accumulation of bisphenol a diglycidyl ethers (BADGEs) and novolac glycidyl ethers (NOGEs) in marine mammals from the United States coastal waters. Environ. Sci. Technol. 50, 1703–1710. doi: 10.1021/acs.est.5b04650
Yang, J., Kunito, T., Tanabe, S., and Mayazaki, N. (2007). Mercury and its relation with selenium in the liver of Dall’s porpoises (Phocoenoides dalli) off the Sanriku coast of Japan. Environ. Pollut. 148, 669–673. doi: 10.1016/j.envpol.2006.11.008
Yeung, L. W. Y., Yamashita, N., Taniyasu, S., Lam, K. S., Sinha, R. K., Borole, D. V., et al. (2009). A survey of perfluorinated compounds in surface water and biota including dolphins from the Ganges River and in other water bodies in India. Chemosphere 76, 55–62. doi: 10.1016/j.chemosphere.2009.02.055
Keywords: dolphins, endocrine disrupting contaminants, EDCs, heavy metals, mercury, odontocete, pathology, toxicity
Citation: Page-Karjian A, Lo CF, Ritchie B, Harms CA, Rotstein DS, Han S, Hassan SM, Lehner AF, Buchweitz JP, Thayer VG, Sullivan JM, Christiansen EF and Perrault JR (2020) Anthropogenic Contaminants and Histopathological Findings in Stranded Cetaceans in the Southeastern United States, 2012–2018. Front. Mar. Sci. 7:630. doi: 10.3389/fmars.2020.00630
Received: 10 February 2020; Accepted: 09 July 2020;
Published: 05 August 2020.
Edited by:
Debra Lee Miller, The University of Tennessee, Knoxville, United StatesReviewed by:
Elizabeth McHuron, University of California, Santa Cruz, United StatesAntonio Fernandez, University of Las Palmas de Gran Canaria, Spain
Copyright © 2020 Page-Karjian, Lo, Ritchie, Harms, Rotstein, Han, Hassan, Lehner, Buchweitz, Thayer, Sullivan, Christiansen and Perrault. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Annie Page-Karjian, cpagekarjian@fau.edu
 Annie Page-Karjian
Annie Page-Karjian Catherine F. Lo
Catherine F. Lo Branson Ritchie2
Branson Ritchie2  Craig A. Harms
Craig A. Harms David S. Rotstein
David S. Rotstein Sayed M. Hassan
Sayed M. Hassan Andreas F. Lehner
Andreas F. Lehner John P. Buchweitz
John P. Buchweitz Victoria G. Thayer
Victoria G. Thayer Justin R. Perrault
Justin R. Perrault