Species, Spatial-Temporal Distribution, and Contamination Assessment of Trace Metals in Typical Mariculture Area of North China
- 1CAS Key Laboratory of Coastal Environmental Processes and Ecological Remediation, Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, Yantai, China
- 2Shandong Key Laboratory of Coastal Environmental Processes, Research Center for Coastal Environment Engineering Technology of Shandong Province, Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, Yantai, China
- 3Center for Ocean Mega-Science, wChinese Academy of Sciences, Qingdao, China
- 4University of Chinese Academy of Sciences, Beijing, China
The species, spatial-temporal distribution, sources, correlations with physiochemical factors, and the contamination of trace metals (Cu, Pb, Cd, and Zn) were investigated in a typical mariculture area of North China. The concentrations of three species of trace metals, including total dissolved, dissolved reactive, and dissolved inert, were obtained. The total dissolved concentrations of Cu, Pb, Cd, and Zn ranged within 1.75–8.08, 0.43–2.75, 0.07–0.29, and 2.58–30.28 μg/L, respectively. These ranges were measured over 5 months (March, May, July, September, and November) and their concentrations decreased in the following order: Zn > Cu > Pb > Cd. The concentrations of Cu, Pb, Cd, and Zn decreased from nearshore to offshore, showing a distinctly regional variation. All concentrations of trace metals over the whole mariculture area were lower than the grade-II seawater quality standard of China. The relationships between trace metals with micronutrient metal (Fe) and other environmental factors (i.e., temperature, chlorophyll a, salinity, dissolved oxygen, and conductivity) were studied in detail. Discharged industrial and aquaculture effluents, uptake by organisms, and atmospheric deposition were found to be possible sources of trace metals in the studied area. Sea current and physicochemical parameters were factors that possibly influenced the spatial-temporal distribution of trace metals.
Introduction
Trace metals (TMs) are important micronutrients in marine systems and are essential for the growth of organisms (Morel et al., 2003; Li et al., 2017; Posacka et al., 2017). Whether TMs are essential or toxic mainly depends on their concentrations (Herrero et al., 2014). On the one hand, a number of TMs can be limiting factors to the primary production of an ecosystem if their concentrations are too low (Martin et al., 1994; Maldonado et al., 2006). On the other hand, if their concentrations exceed a certain threshold, they may become toxic for marine organisms (Sunda et al., 1987; Moffett et al., 1997; Hook and Fisher, 2001; Rivera-Duarte et al., 2005; Dong et al., 2015). Although many TMs can be found at extremely low concentrations in seawater, through biological accumulation, TM concentrations in seafood can be hundreds of times greater than in seawater (Maity et al., 2017). Once excess TM enters the body through the food chain, it will be detrimental for human health.
Marine metal pollution has become the focus of many scholars (Ellwood and Van den Berg, 2000; Fitzwater et al., 2000; Ellwood, 2008; Croot et al., 2011; Zhang et al., 2014; Heller and Croot, 2015). TM pollutants not only gradually deteriorate the seawater quality, but also cause serious pollution of aquatic products. With the continuing industrial development and population growth, human activities release large amounts of TMs into the marine environment, which seriously harms marine ecosystems (Maity et al., 2017). As a result, the determination and analysis of TMs in seawater are urgently needed. In seawater, only dissolved TMs can be directly absorbed and utilized by organisms. These dissolved TMs can be subdivided into total dissolved TMs, dissolved reactive TMs, and dissolved inert TMs. The total dissolved TM concentration is the sum of the dissolved reactive TM and the dissolved inert TM (Han et al., 2018). So far, environmental measurements have only established the total concentration of target metals in samples (Kot and Namiesńik, 2000), which was proved to be inadequate for assessing their bioavailability, behavior, and toxicity (Huang et al., 2003; Adnívia et al., 2016). In the water body, the degree of TM contamination and its effect on both their biological activity and toxicity are directly related to the TM species present. Hence, it is essential to determine the species of TMs in marine environments. In this experiment, the concentrations of total dissolved TM, dissolved reactive TM, and dissolved inert TM were determined by at least three measurements.
There are many techniques for separation and detection of TMs, including gas chromatography (Wang and Cui, 2016), capillary zone electrophoresis (Kubáň and Timerbaev, 2014), supercritical fluid chromatography (Henry and Yonker, 2006), atomic absorption spectrometry (Ribeiro et al., 2017), inductively coupled plasma mass spectrometry (Zhu et al., 2017), and atomic emission spectrometry (Vidal et al., 2016). Among the analytical techniques, electroanalytical techniques are the most universal analytical tool due to the simultaneous quantitative and qualitative determinations of TMs in various matrices with high levels of convenience and rapid and straightforward analytical processes.
Yantai Sishili Bay is a typical mariculture area of North China and is located in Shandong Province. Its area of about 87 km2 include a shallow sea aquaculture area of about 53 km2, and mariculture activities are pursued throughout the year. It is an important breeding ground for fish, shrimp, crab, and shellfish, and mainly cultures seafood for the local area, especially scallops. As Sishili Bay is a semi-closed sea area, its water exchange capacity is poor, cargo throughput increases, and mariculture, industrial, agricultural, and domestic sewage discharges exert great pressure on the marine ecosystem of Sishili Bay (Wang et al., 1995; Tang, 2004; Liu et al., 2009; Zhang et al., 2012). Effluent discharge from the sewage treatment plant of Xinan River and Zhifu Island, aquatic animal feed residues and excrement, as well as domestic waste from workers of aquatic farms all contain a certain level of TMs, which pollute the marine environment of Sishili Bay. Chemical drugs are often used in mariculture to control pests, and the residues of these drugs also cause TM pollution of both the marine environment and the marine ecosystem. For example, CuSO4 is widely used to treat shrimp diseases along the Pearl River Delta, resulting in severe Cu pollution in the aquatic environment of this region (Hu et al., 2007). As a mariculture area, it is very important to investigate the pollution level of seawater, especially the metal levels. Although various studies investigated TMs in seawater (Nimmo et al., 1989; Meng et al., 2008; Mao et al., 2009; Tian et al., 2009; Wang et al., 2012; Su et al., 2015, 2016, 2017; Han et al., 2018), little information is available on the spatial-temporal distributions and species of TMs. However, studying the spatial-temporal distributions and species of TM is helpful to achieve a better understanding of the TM pollution degree in seawater and provides effective data support for aquaculture.
Although Cu, Pb, Cd, and Zn are essential metals for the growth of organisms in marine environments, they are also the main elements that cause marine metal pollution. Once these metal elements occur excess, they cause serious harm to aquatic animals, aquatic plants, and the human body. Excessive Cu and Zn can harm the normal reproduction and growth of aquatic animals and plants, excessive Cu and Pb are teratogenic and carcinogenic, and excessive Cd can lead to kidney damage (Xie et al., 2014). Therefore, the pollutions of Cu, Pb, Cd, and Zn must not be ignored. This study investigated species, spatial-temporal distribution, sources, correlations with physiochemical factors, and contamination of specific TMs (Cu, Pb, Cd, and Zn) in the typical mariculture area of North China. Different species of Cu, Pb, Cd, and Zn were simultaneously analyzed by anodic stripping voltammetry (ASV) with different procedures. The relationships between different species of metals with the micronutrient metal Fe, chlorophyll a, salinity, dissolved oxygen (DO), conductivity, pH, and temperature were investigated in detail.
Materials and Methods
Sample Collection
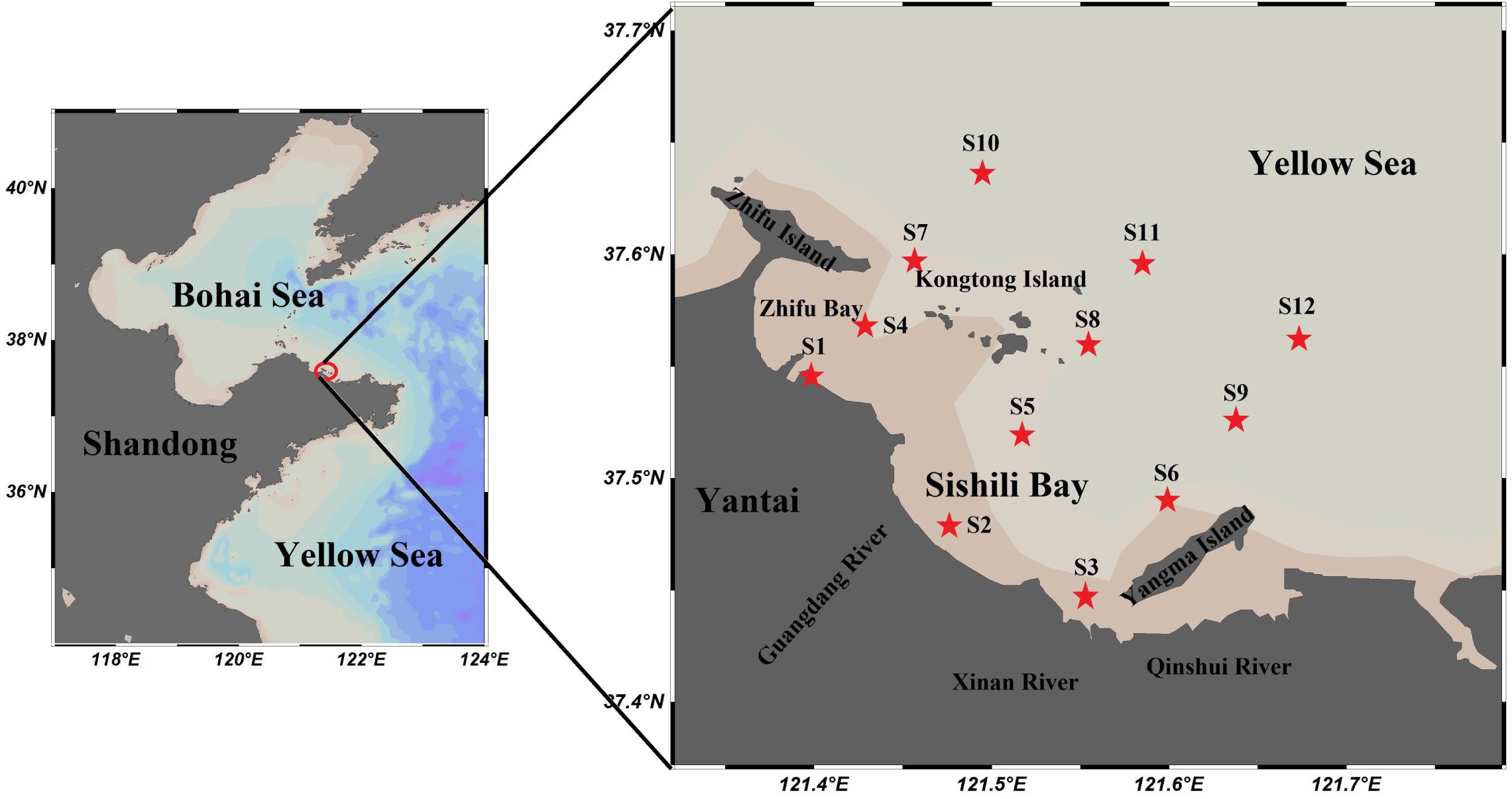
Samples were collected from the surface seawater of Sishili Bay (Yellow Sea, China, see Figure 1), covering 12 stations and 5 months of 2018 (March, May, July, September, and November). Cleaning procedures are strictly followed with GEOTRACES manual when collecting water samples. All seawater samples were collected with a 2.5-L sample collector (Taiheng Plastics Corporation, Henan, China) into individual polypropylene bottles (Xiangyun Plastic Products Factory, Hebei, China). These polypropylene bottles had been cleaned with 1 M HCI (analytical grade) and thoroughly rinsed with deionized water (18.2 MΩ cm specific resistance). Following collection, they were filtered through 0.45-μm cellulose acetate membranes (Shanghai Xingya Purification Material Factory, Shanghai, China) and were stored in polypropylene bottles at 4°C until further analysis. Chlorophyll a (Chl a) was collected from all seawater samples and stored in 0.45-μm cellulose acetate membranes wrapped with aluminum foil (Xuhuacheng Plastic Co., Ltd., Shanghai, China), and stored at −20°C until use. The temperature, salinity, DO, conductivity, and pH in surface seawater were measured by an YSI MI-parameter Meter (ProPlus, United States).
All containers used in the experiment were soaked in nitric acid (HNO3, 5 wt%, analytical grade) for at least 24 h before use, and rinsed thoroughly with deionized water before starting the experiment. All reagents used were of analytical grade and purchased from Shanghai Sinopharm Chemical Reagent Co., China. All deionized water was sourced from the Pall Cascada’s laboratory water system (Port Washington, New York, United States).
Sampling Stations
The semi-closed Sishili Bay is located on the eastern shore of Yantai city and is one of the most intensive scallop aquaculture areas of North China (Dong et al., 2019). The average water depth is less than 15 m. The quality of the marine environment is not only affected by mariculture, but also by industrial, agricultural, and domestic sewage discharges by local Zhifu and Laishan districts. Studies showed that a large quantity of industrial materials and wastewater are discharged by the Zhifu Island sewage treatment plant every year (Sheng et al., 2012). In this experiment, all sampling stations were located in Sishili Bay within the area from 37°26′49.44″N to 37°38′10.14″N and 121°24′0.00″E to 121°40′27.85″E. The details can be found in Supplementary Table S1. The locations of the 12 sampling stations (S1–S12) in this study are shown in Figure 1. These were situated in the nearshore areas (S1–S3, where S3 is near the Xinan River sewage treatment plant), port areas (S4 is near Yantai port), mariculture areas (S5, S6, S8, and S9), sewage outfall areas (S7 is near the Zhifu Island sewage treatment plant), and offshore areas (S10–S12). These locations are severely affected by human activities (Li et al., 2013; Dong et al., 2019).
Determination of Different Species of Cu, Pb, Cd, and Zn
In this study, all voltammetric measurements were carried out using the 797 VA Computrace system with software (version 1.3) from Metrohm (Beijing, China). A three-electrode system was used, consisting of a working electrode, a reference electrode, and a counter electrode. A standard addition method (Illuminati et al., 2010) was adopted for all TM detections.
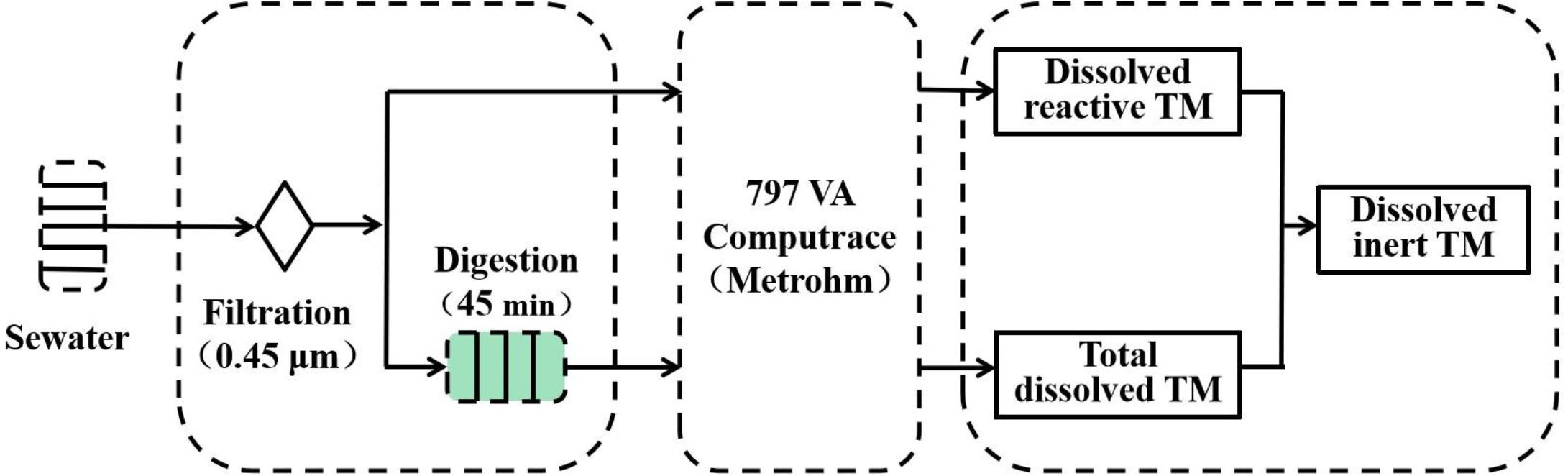
The concentrations of different species of Cu, Pb, Cd, and Zn in seawater samples were determined by ASV, which imposes detection limits of 28.87, 1.06, 2.97, and 12.70 ng L–1, respectively (Han et al., 2018). Seawater samples were pretreated, as shown in Figure 2. The filtered samples were transferred to quartz tubes, and then, UV digested for 45 min. When the sample had cooled, 10 mL were transferred into the voltammetric cell and DO was removed with high-purity nitrogen for 5 min. Finally, the total dissolved TM concentration was obtained by ASV (Achterberg and van den Berg, 1994). The UV digestion apparatus was provided by van den Berg’s research group (Oceanography Laboratories, University of Liverpool, United Kingdom). Dissolved reactive TM concentrations were obtained via direct electrochemical detection of the filtered samples. Filtered samples (10 mL) were placed into the voltammetric cell and DO was removed with high-purity nitrogen for 5 min; then, the dissolved reactive TM concentration was obtained by ASV (Han et al., 2018). Dissolved inert TMs were obtained via the difference between total dissolved TMs and dissolved reactive TMs (Florence, 1986).
The detailed parameters of the differential pulse voltammetry were as follows: deposition time was 60 s, deposition potential was −1.15 V, initial potential was −1.15, termination potential was 0.02 V, sweep rate of 0.03 Vs–1, and the peak positions of Cu, Pb, Cd, and Zn were −0.17, −0.41, −0.58, and −1.0 V, respectively (Han et al., 2018). The accuracy and reliability of the method have been verified in certified reference materials (CASS-6 and NASS-6) (Han et al., 2018).
Determination of Total Dissolved Fe
Cathode stripping voltammetry (CSV) was used to determine the total dissolved Fe concentration in a 797 VA Computrace system, which imposes a detection limit of 0.84 nM (Lin et al., 2016). Detection details followed were described in Lin et al. (2016). Seawater samples were pretreated, as shown in Supplementary Figure S1. First, concentrated nitric acid was added to maintain the pH value of the water sample to less than 2.0, and added H2O2. Next, NaOH was added to maintain the pH at 8.0 after 90 min of UV digestion. 10 mL seawater samples, 100 μL 1 M HEPPS/0.5 M NaOH (Buffer solution, pH value of the system was about 8.0), 10 μL 0.02 M DHN (Complexing ligand) and 500 μL 0.4 M KBrO3 (Oxidant) were added successively in the voltammetric cell and then remove dissolved oxygen with high-purity nitrogen for 5 min (Lin et al., 2016). The detailed parameters of DPV were as follows: the deposition potential was −0.1 V, deposition time was 60 s, initial potential was −0.1 V, termination potential was −1.1 V, with a sweep rate of 0.024 Vs–1 (Lin et al., 2016). The accuracy and reliability of the method have been verified in certified reference materials (CASS-5 and NASS-6) (Lin et al., 2016).
Determination of Chl a
The concentration of Chl a was determined by using a Turner Designs Trilogy fluorometer (George et al., 2015). The filter membrane was cut into pieces and extracted with a 90% acetone solution at 4°C for 24 h, then centrifuged at 4000 r speed for 10 min. The supernatant was taken for the concentration determination of Chl a (Zhang et al., 2016). The whole process was done in dark light.
Evaluation of the Trace Metal Contamination Level
In this study, both the contamination factor (Cf) and the contamination degree (Cd) were used to evaluate the surface seawater quality (Hakanson, 1980; Lü et al., 2015). Cf and Cd reflect the contamination level of a single TM and overall TMs in the medium, respectively (Wang et al., 2018).
where Cc represents the concentration of TM, and Cb represents the background value of corresponding TM in the seawater. The background concentrations used the grade-I seawater quality standard for China (5 μg/L Cu, 1 μg/L Pb, 1 μg/L Cd, and 20 μg/L Zn; GB 3097–1997) (Aqsiq, 1997). Cf and Cd values were interpreted as recommended by Hakanson (1980).
Results and Discussion
Species of TMs
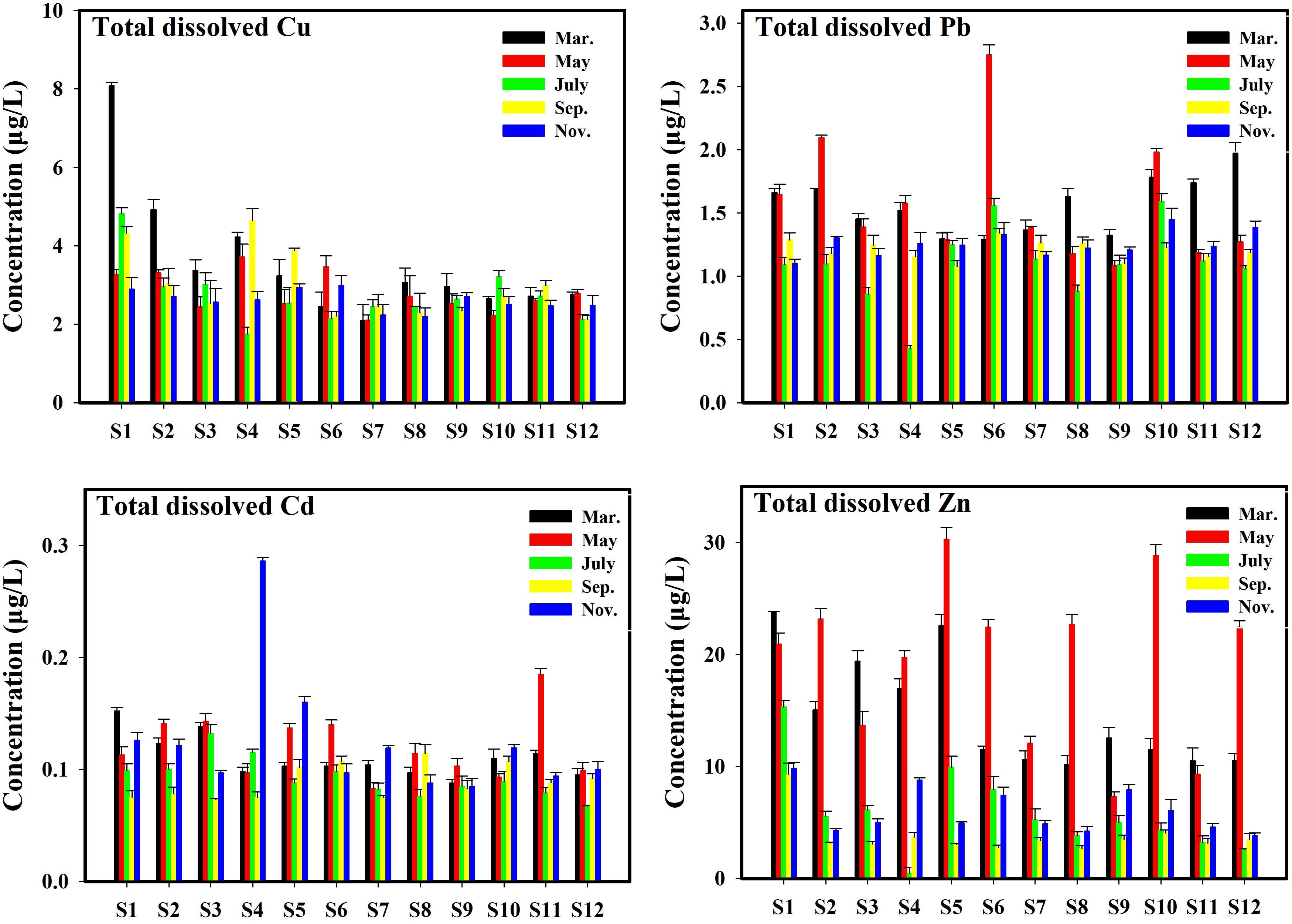
The concentrations of TMs (Cu, Pb, Cd, and Zn) in different species in the surface seawater of Sishili Bay during the five investigated months are listed in Supplementary Table S2. The total dissolved Cu, Pb, Cd, and Zn concentrations ranged within 1.75–8.08, 0.43–2.75, 0.07–0.29, and 2.58–30.28 μg/L, respectively. The dissolved reactive Cu, Pb, Cd, and Zn concentrations ranged within 1.05–5.55, 0.15–1.59, 0.04–0.23, and 1.62–20.14 μg/L, respectively. The dissolved inert Cu, Pb, Cd, and Zn concentrations have ranges of 0.22–2.94, 0.02–1.56, 0.01–0.07, and 0.43–16.96 μg/L, respectively. The concentrations of different species of TMs followed (in decreasing order): Zn > Cu > Pb > Cd.
The concentrations of total dissolved Cu, Pb, Cd, and Zn in all samples for different months are shown in Figure 3. The statistical results identified significant differences in TM concentrations for all stations in different months. In March, the highest values of total dissolved Cu, Cd, and Zn were found at station S1, and the highest value of total dissolved Pb was found at station S12. In May, the highest values of total dissolved Cu, Pb, Cd, and Zn were found at stations S4, S6, S11, and S5, respectively. In July, the highest value of total dissolved Cu and Zn was found at station S1, and the highest values of total dissolved Pb and Cd were found at stations S10 and S3, respectively. In September, the highest values of total dissolved Cu, Pb, Cd, and Zn were found at stations S4, S6, S8, and S1, respectively. In November, the highest values of total dissolved Cu, Pb, Cd, and Zn were found at stations S6, S10, S4, and S1, respectively. In summary, the maximum values of dissolved Cu, Pb, Cd, and Zn were 8.08 (station S1 in March), 2.75 (station S6 in May), 0.29 (station S4 in November), and 30.28 μg/L (station S5 in May), respectively.
The annual mean concentrations of total dissolved Cu, Pb, Cd, and Zn in Sishili Bay were compared with those from other marine areas (Table 1). The results for all dissolved concentrations of Cu, Pb, Cd, and Zn found values of the same order of magnitude as those reported for Bohai Bay (Meng et al., 2008; Mao et al., 2009), Jinzhou Bay (Wang et al., 2012), the North Yellow Sea (Tian et al., 2009), the San Francisco Bay (Flegal et al., 1991), Boston Harbor (Li et al., 2010), and the Weser estuary (Turner et al., 1992). The concentrations of Cu in Sishili Bay were comparable with those in Bohai Bay, Jinzhou Bay, and the Weser estuary but higher than concentrations in the North Yellow Sea, the San Francisco Bay, and Boston Harbor. The concentrations of Pb in Sishili Bay were higher than in other sea areas, but lower than in Bohai Bay. The concentrations of Cd in Sishili Bay were lower than in Jinzhou Bay but higher than in Boston Harbor and San Francisco Bay. The concentrations of Zn were lower than in Bohai Bay but higher than in the North Yellow Sea, Boston Harbor, and San Francisco Bay.
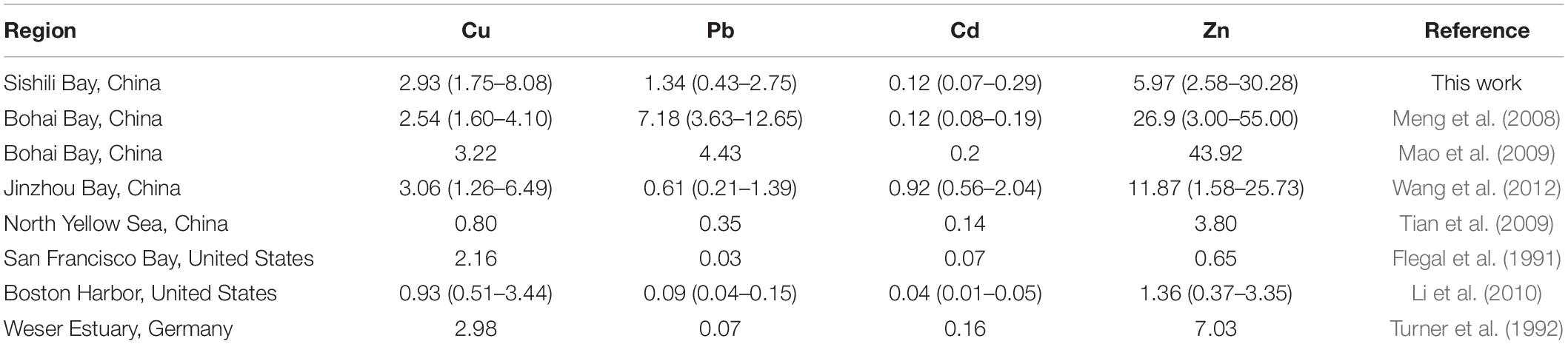
Table 1. Comparison of the annual mean concentrations (μg/L) of dissolved Cu, Pb, Cd, and Zn in the seawater of other regions of the world [parentheses present the range of trace metals (TMs)].
The annual mean percentages of dissolved reactive and dissolved inert concentrations of Cu, Pb, Cd, and Zn in the total dissolved concentrations are presented in Figure 4. The proportions of dissolved reactive TMs are higher, which is consistent with previous observations by Han et al. (2018). The mean percentages of the dissolved reactive concentrations of Cu, Pb, Cd, and Zn were 65.67, 59.92, 72.56, and 64.29%, respectively, following (in decreasing order): Cd > Cu > Zn > Pb. The dissolved reactive TMs (detected by ASV) were classified as free ions, inorganic complexes, and weak organic complexes (Achterberg et al., 2003). Moreover, only free ions, a number of inorganic complexes, and a small number of organic complexes are responsible for the uptake processes by the cells of organisms (Huang et al., 2003). These results indicate that the dissolved reactive TMs had high bioavailability and toxicity for marine organisms. The lowest annual mean percentages of the dissolved reactive Cu, Pb, Cd, and Zn were found at stations S1, S10, S2, and S5, respectively. Considering that the chlorophyll a level was the highest at station S1, uptake by phytoplankton could explain the low proportion of the dissolved reactive Cu (Su et al., 2015). The low proportion of the dissolved reactive Pb at station S10 may have been caused by pollutions from the sewage outfall area, influenced by tidal currents that contained a large amount of dissolved inert Pb. At station S2, higher biological activity may have been the cause for the low proportion of dissolved reactive Cd. Station S5 was an intensive scallop mariculture, and uptake from scallops could be an explanation for the low proportion of dissolved reactive Zn (Zhang et al., 2012).
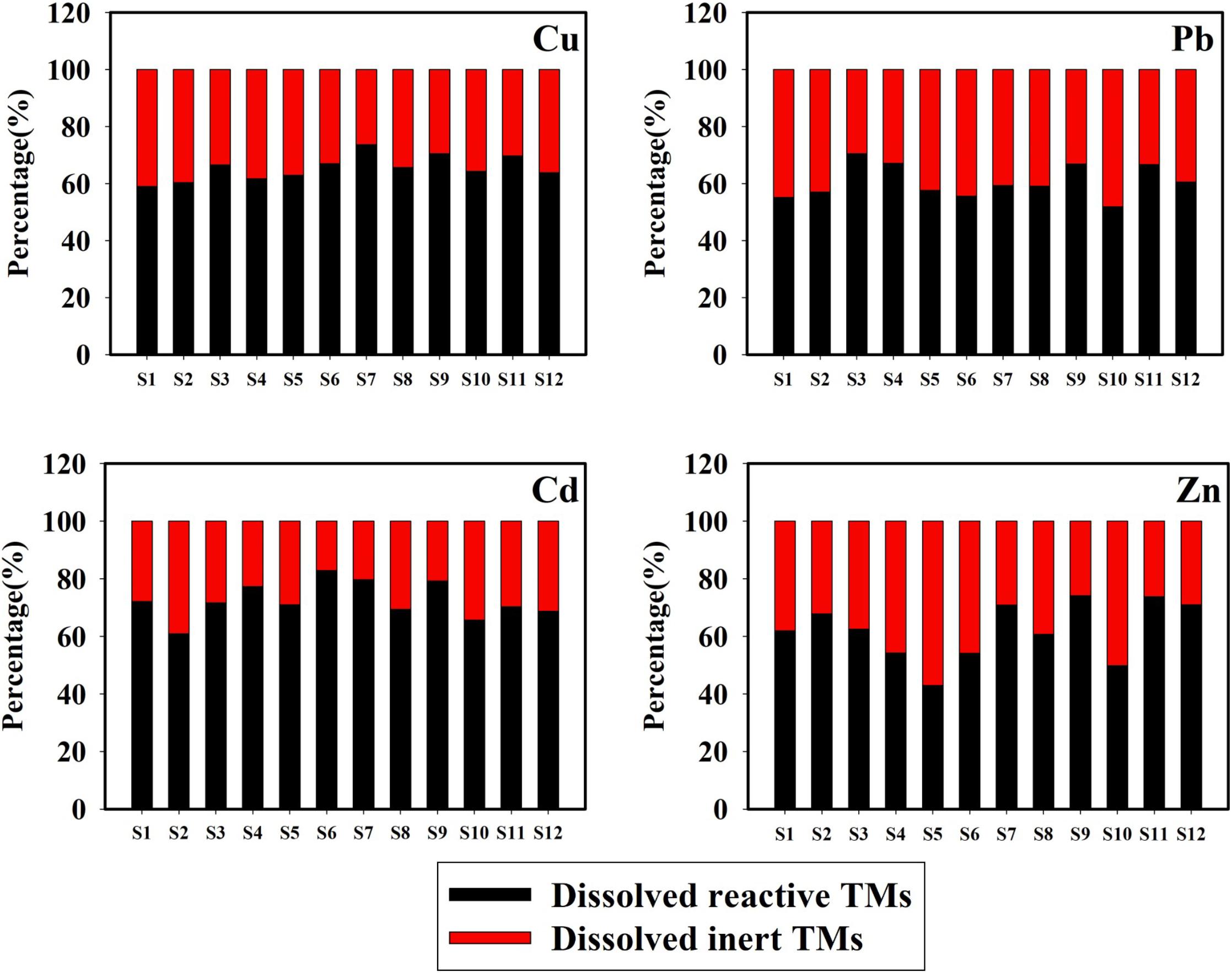
Figure 4. Annual mean percentage of the dissolved reactive and inert concentrations of Cu, Pb, Cd, and Zn in surface seawater of Sishili Bay.
Spatial-Temporal Distribution Characteristics of TMs
Spatial Distribution Characteristics and Sources of TMs
The spatial distributions of TMs (annual average values) in the surface seawater of Sishili Bay are presented in Figure 5. The spatial distributions of total dissolved Cu, Cd, and Zn were similar, indicating that they might have the same or similar input sources. The concentrations decreased from nearshore to offshore and were higher in the west than in the east. The total dissolved Pb concentrations were also higher at nearshore stations than at offshore stations, but lower in the west than in the east. The spatial distribution patterns of the tested metals implied that they may have been affected by anthropogenic pollution and coastal sources. The highest concentrations of total dissolved Cu and Zn were found at station S1, near nearshore areas, where Zhifu Bay coastal industrial and agricultural activities may cause higher Cu and Zn concentrations. The highest total dissolved Cd concentration was found at station S4, near Yantai port, suggesting vessel traffic and vessel sewage as the main reasons for the high concentration of Cd (Liu et al., 2012; Han et al., 2018). Pb is a atmophile-type metal, and its atmospheric deposition is widely considered to be an important source of Pb in seawater (Stumm and Morgan, 1996; Bruland and Lohan, 2003). Therefore, the higher Pb concentration at offshore stations may have been caused by atmospheric deposition. The highest Pb concentration was found at station S6, near mariculture areas, suggesting that feed residues, chemical drug residues, and household waste from aquaculture workers may contribute to the high levels of Pb. Pb at S10 station was also relatively high, which may have been caused by pollutants from sewage outfall areas, which were carried by oceanic currents (Liu et al., 2012).

Figure 5. Spatial distribution of the concentrations of total dissolved and dissolved reactive TMs (annual average values) in the surface seawater of Sishili Bay. (A) Total dissolved Cu (μg/L), (B) dissolved reactive Cu (μg/L), (C) total dissolved Pb (μg/L), (D) dissolved reactive Pb (μg/L), (E) total dissolved Cd (μg/L), (F) dissolved reactive Cd (μg/L), (G) total dissolved Zn (μg/L), and (H) dissolved reactive Zn (μg/L).
Further study showed that the spatial distributions of dissolved reactive TMs and total dissolved TMs were similar (Figure 5). The highest concentrations of dissolved reactive Cu, Pb, Cd, and Zn were found at stations S1, S6, S4, and S1, respectively. These results indicated that the factors and sources of dissolved reactive TMs and total dissolved TMs may be similar. At station S2, the concentration of dissolved reactive Cd was low. According to the data results, the percentage of dissolved inert Cd at station S2 was highest (Figure 4), which can explain the lower dissolved reactive Cd.
Temporal Distribution Characteristics
The temporal variation of TMs (monthly average values) in Sishili Bay were significant (as presented in Figure 6). The temporal variations of total dissolved TMs and dissolved reactive TMs were similar, indicating that they might have the same or similar input sources. Total dissolved TM and dissolved reactive TM concentrations were highest in March, May, and November, and lowest in July and September. Aquaculture exerts a certain influence on the TM concentration, which adds complexity to the study of TM. The highest values of chlorophyll a were found in July, followed by September, which explains the lower concentrations of TMs in July and September (Su et al., 2015). Bioactivity decreased in March, May, and November, which may be a reason for the higher concentrations of TMs.
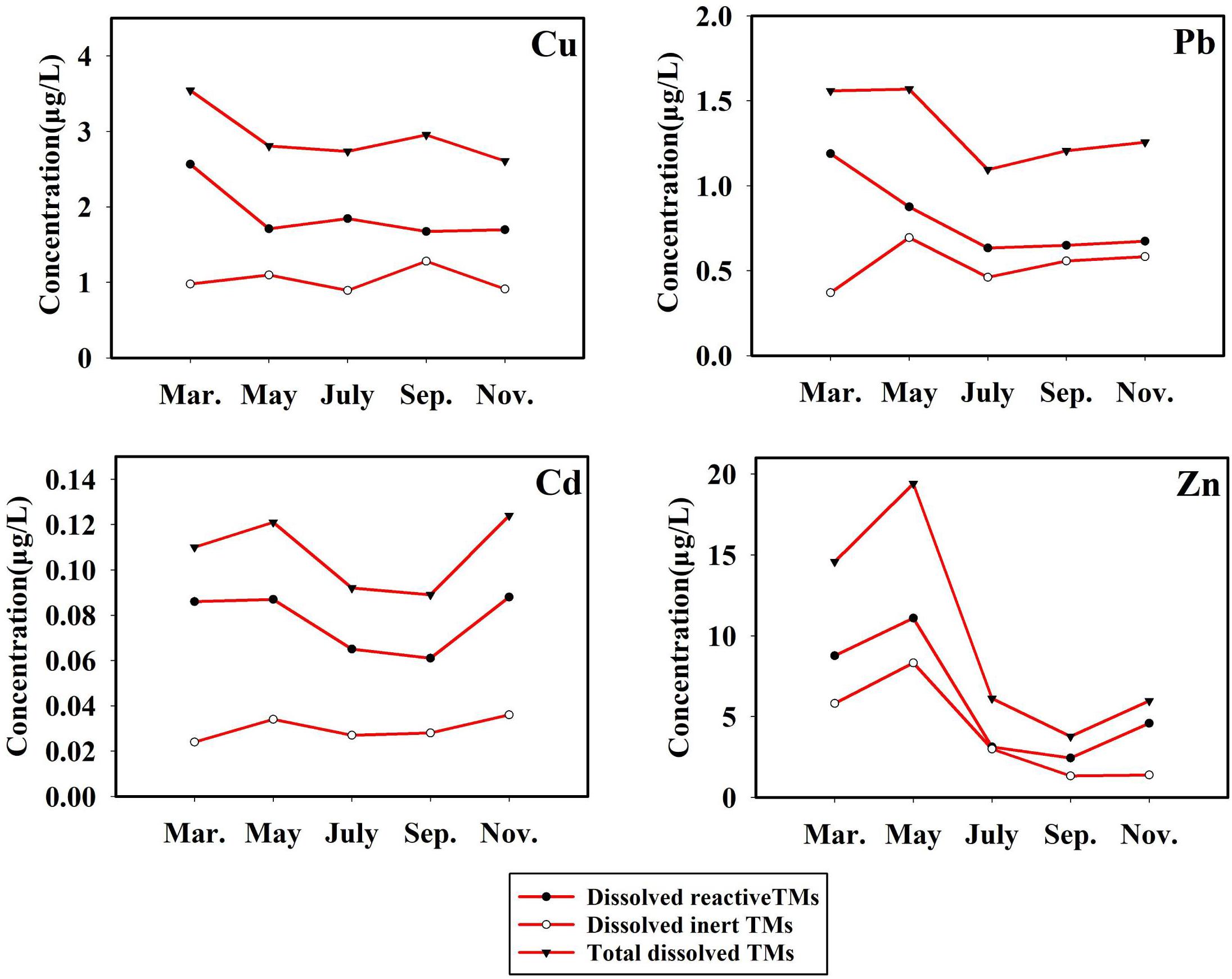
Figure 6. Temporal variation of different species of Cu, Pb, Cd, and Zn (μg/L, monthly average values) in the surface seawater of Sishili Bay.
Contamination Level of TMs
The total dissolved TM concentrations were assessed according to the seawater quality standards of China (Table 2). The concentrations of total dissolved Cd in the present study remained far below the grade-I seawater quality standard of China. In March, May, September, and November, the Pb concentrations in all locations exceeded the grade-I seawater quality of China. However, each TM level in the present study remained lower than the grade-II seawater quality standard of China. The concentrations of total dissolved TMs (annual mean values) of all 5 months were used to calculate Cf and Cd (Figure 7). The contamination levels of the total dissolved TMs were assessed according to Cf and Cd value standards (Table 3). The Cf values of Cu, Pb, Cd, and Zn ranged within 0.35–1.62, 0.43–2.75, 0.07–0.29, and 0.13–1.51, respectively. Cf values of Cd were all lower than 1, indicating that Cd is at a low contamination level in Sishili Bay. Pb had the highest Cf value, followed by Cu, Zn, and Cd, indicating low to moderate contamination levels. These results indicate that the major contamination pressure in Sishili Bay originates from Pb, Cu, and Zn. Cd values ranged from 1.11 to 4.70, which indicated a low contamination level of Sishili Bay. If the grade-I seawater quality standard is used as background data, contamination levels may rank differently. The actual pollution level might be higher than identified in the present study. In general, TM contamination in the seawater of Sishili Bay is light.
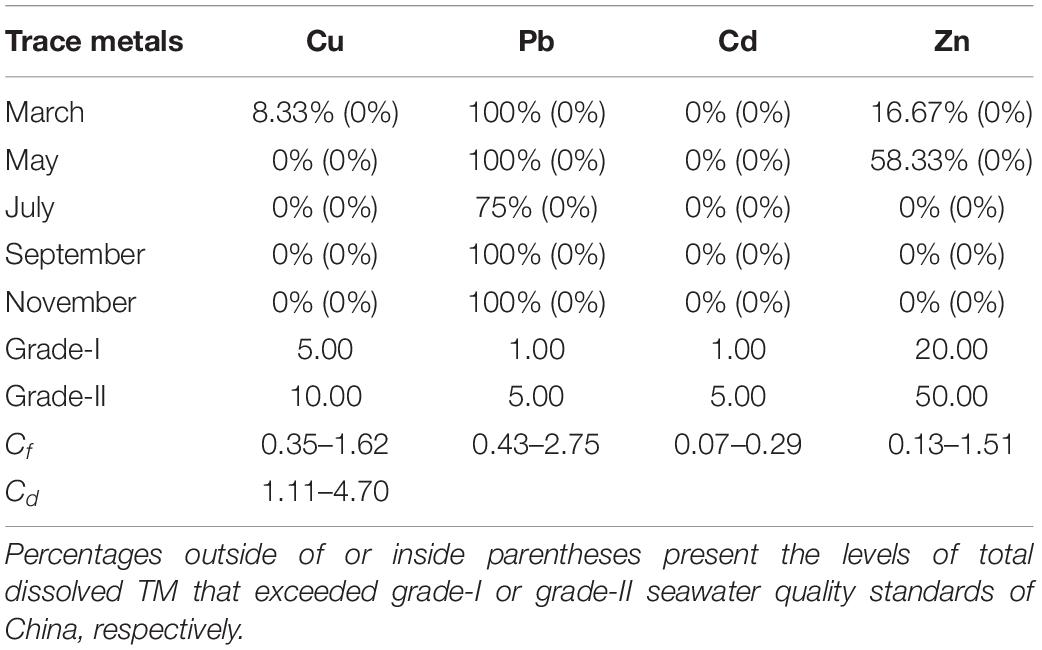
Table 2. Rates of total dissolved TMs exceeding seawater quality standards of China among sampling locations.
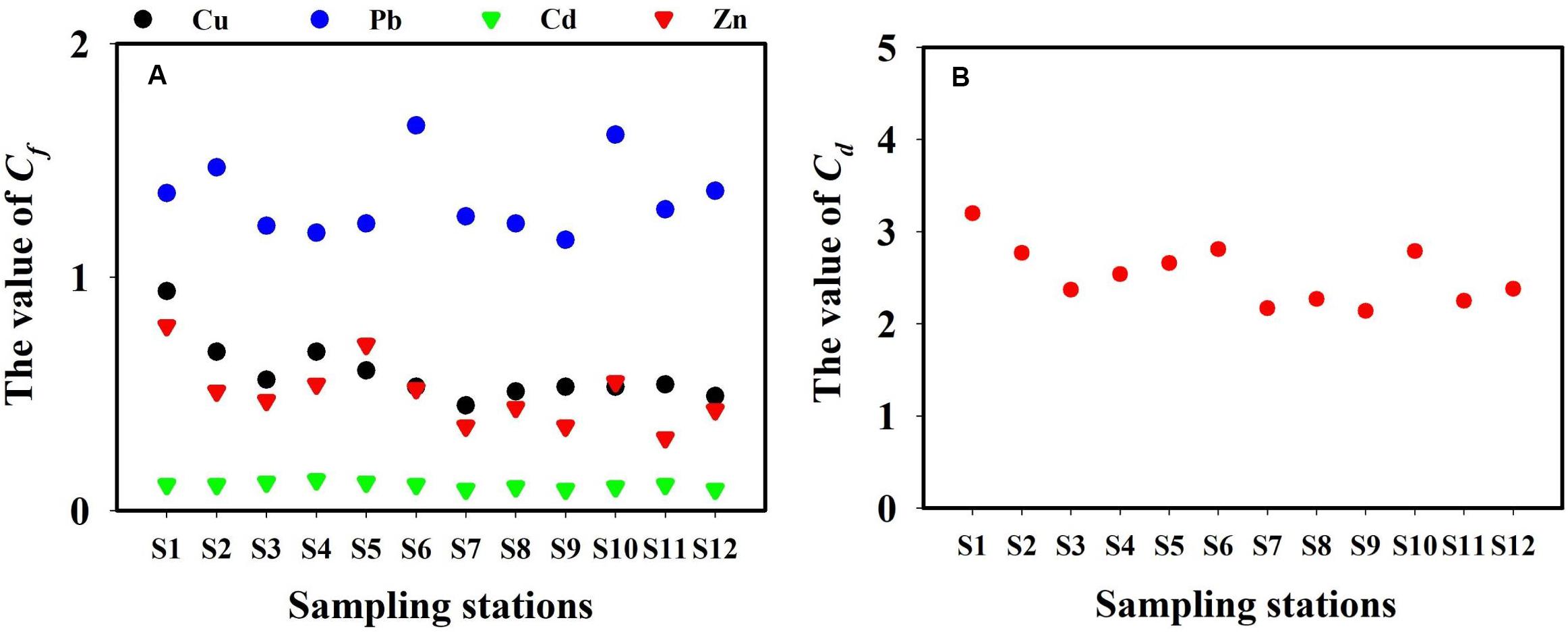
Figure 7. Cf and Cd for total dissolved TMs (annual mean concentrations) in the surface seawater of Sishili Bay. (A) Value of Cf and (B) the value of Cd.
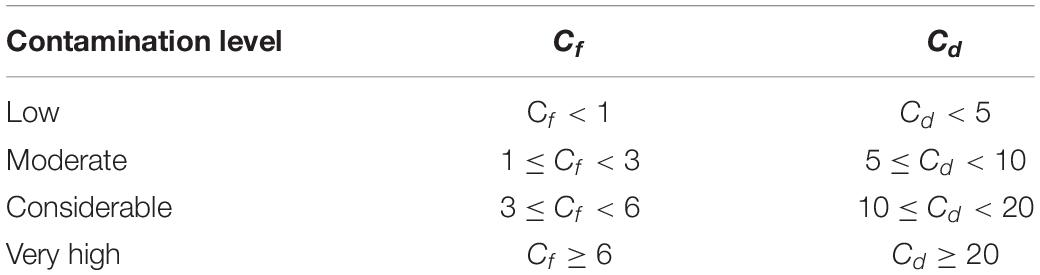
Table 3. Cf and Cd standards (Hakanson, 1980; Lü et al., 2015).
Relationship of TMs With Micronutrient Metal (Fe)
Fe is ubiquitous in the natural environment, an essential nutrient for almost all organisms (Kustka et al., 2002), and key to limiting phytoplankton growth (Martin et al., 1991). To obtain more valuable experimental data, the total dissolved Fe concentration was determined and the relationship between Fe and TMs was further analyzed. The results showed that Fe concentrations were higher in Sishili Bay than in other areas (Nimmo et al., 1989; Su et al., 2016, 2015, 2017). The spatial distribution of the total dissolved Fe (annual average values) in the surface seawater of Sishili Bay is presented in Figure 8A. Unlike total dissolved Cu, Pb, Cd, and Zn, a reverse distribution pattern was found for total dissolved Fe, indicating that the source of Fe might differ from the sources of Cu, Pb, Cd, and Zn. The concentrations were lower at nearshore stations than at offshore stations. The highest concentration of total dissolved Fe was found at station S12. Station S12 was located near offshore areas, where the dry-wet deposition of atmospheric aerosols was the main external source of Fe (Johnson et al., 1994; Yuan et al., 2003). This indicates that atmospheric deposition may be the cause for the higher Fe levels. However, station S12 was close to the aquaculture zone, suggesting that sewage from the aquaculture area carried by ocean currents may be responsible for the higher Fe. The temporal variation of the total dissolved Fe (monthly average values) in Sishili Bay is presented in Figure 8B. Significant temporal variations in total dissolved Fe were found. The total dissolved Fe concentrations were highest in March and lowest in May. The variation trends of total dissolved Cu, Pb, Cd, Zn, and Fe are shown in Figure 8C. From March to May, the concentration of Fe and Cu decreased obviously, while the concentrations of Pb, Cd, and Zn increased slightly. March to May is when phytoplankton begins to grow, which suggests that during this time, Fe and Cu are more readily absorbed by phytoplankton. Then, Fe and Cu started to increase, but Pb, Cd, and Zn started to decrease and did not start to increase until July. From May to July, phytoplankton began to bloom, which may be the reason why Pb, Cd, and Zn decreased rapidly. From July to September, Cd and Zn still showed a slight decrease. From September to November, only Cu followed a slight decreasing trend. Overall, except for November, Cu presented a similar tendency than Fe.
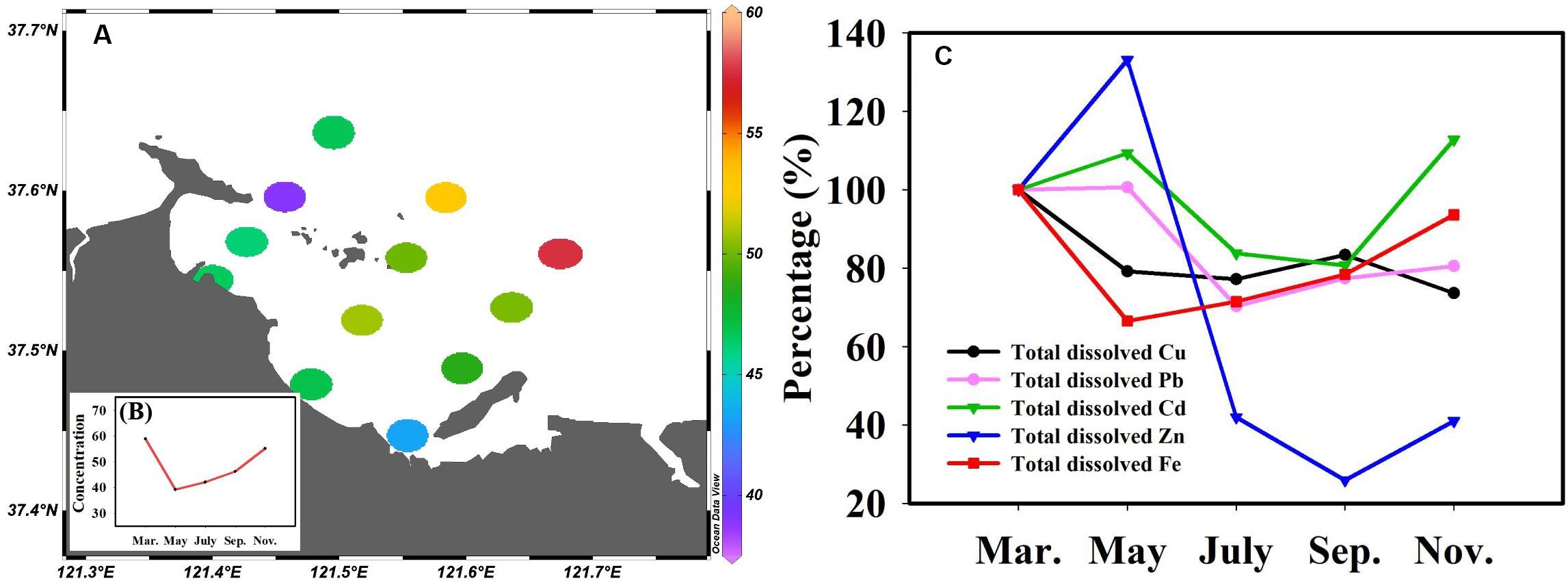
Figure 8. Spatial-temporal distribution of total dissolved Fe concentrations in surface seawater of Sishili Bay and the change trend of Cu, Pb, Cd, Zn, and Fe. (A) Spatial distribution (nmol/L, annual average values), (B) temporal variation (nmol/L, monthly average values). (C) Change trend of Cu, Pb, Cd, Zn, and Fe. Percentage values indicate the change trend of the metal concentrations in other months compared with the first months (where the first month’s concentration is equal to 100%).
Correlations With Physiochemical Factors of TMs
The values of the physiochemical parameters in Sishili Bay during the five investigated months are listed in Supplementary Table S2. Water temperature, pH, salinity, DO, conductivity, and chlorophyll a ranged within 3.5–25.2°C, 7.78–8.40, 28.89–32.4, 3.92–17.26 mg/L, 45.22–50.89 μS/cm, and 0.02–21.19 μg/L, respectively. The spatial distributions of the physiochemical parameters (annual average values) for Sishili Bay are presented in Figure 9. Significant spatial variation was found for chlorophyll a. The values decreased from nearshore to offshore stations, and were higher in the west than in the east, which was similar to the previously observed results by Shen et al. (2014). The highest values of temperature, pH, salinity, DO, conductivity, and chlorophyll a were found at stations S10, S6, S9, S11, S9, and S1, respectively. The temporal variations of physicochemical parameters (monthly average values) in Sishili Bay are presented in Figure 10. The temporal variations of temperature and chlorophyll a were similar, and were significantly positively correlated (Yu et al., 2009). Water temperature and chlorophyll a values differed significantly among the 5 months, where the highest values were found in July, which was followed by September. The salinity and pH values were ∼31 and 8.1, respectively. DO and conductivity were much higher in March than in the other 4 months.
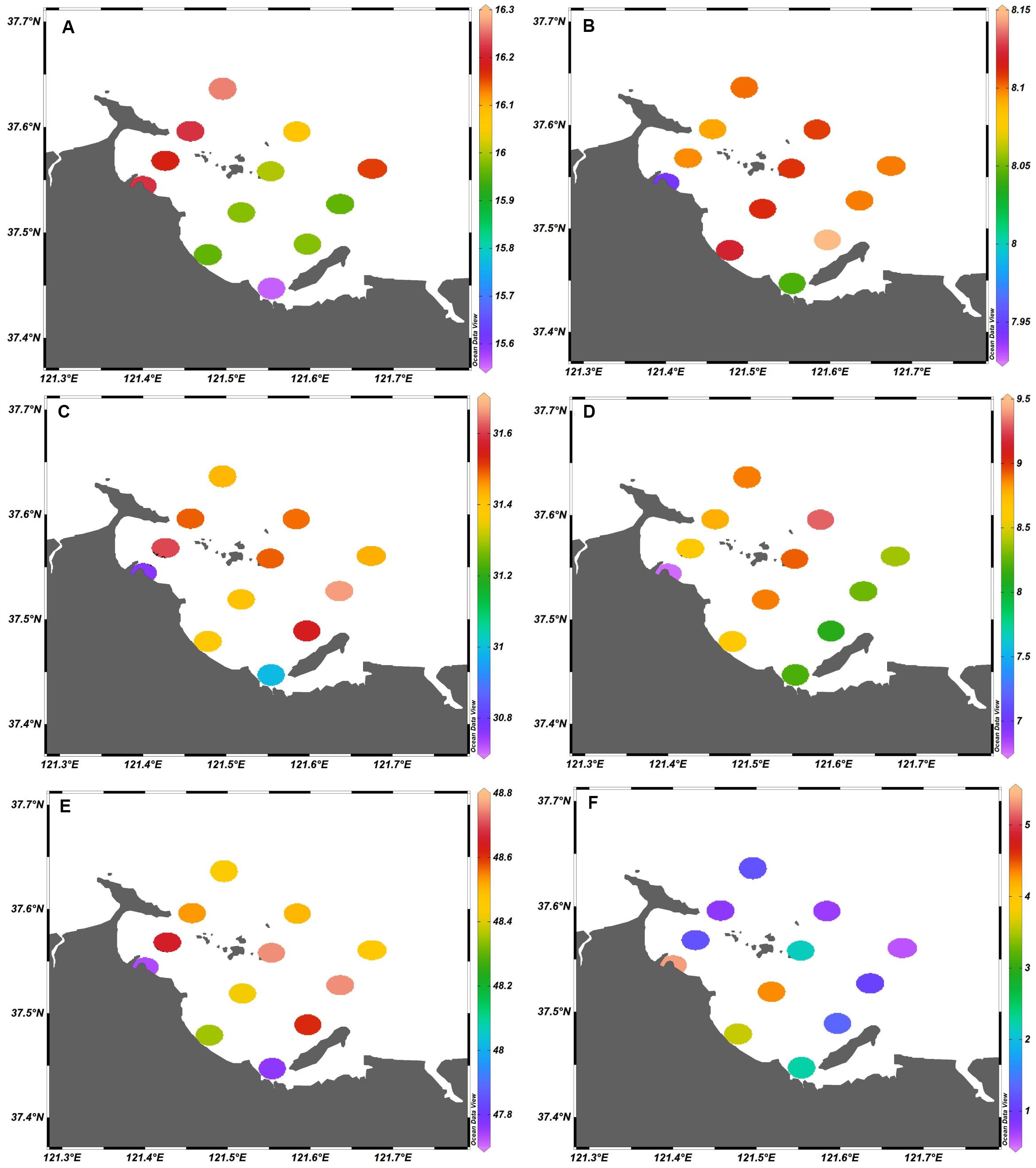
Figure 9. Spatial distribution of physicochemical parameters (annual average values) in surface seawater of Sishili Bay. (A) Temperature (°C), (B) pH, (C) salinity, (D) dissolved oxygen (DO; mg/L), (E) conductivity (μS/cm), and (F) chlorophyll a (μg/L).

Figure 10. Temporal variation of physicochemical parameters (monthly average values) in the surface seawater of Sishili Bay. (A) Temperature (°C), (B) pH, (C) salinity, (D) DO (mg/L), (E) conductivity (μS/cm), and (F) chlorophyll a (μg/L).
The Pearson correlation test was used to determine the correlation coefficients among dissolved reactive TM concentrations and physicochemical parameters. The results are shown in Table 4 (monthly average values). Statistical analysis showed that the concentrations of Cu, Pb, Cd, and Zn were positively correlated with each other, indicating that these four TMs have certain homology and compound contamination. The concentrations of Cu, Pb, Cd, and Zn were negatively related to temperature and chlorophyll a, and positively correlated to salinity, DO, and conductivity. The concentrations of dissolved reactive TMs were significantly positively correlated with salinity, indicating that the existing species of TMs were affected by salinity. This might be because increasing salinity intensifies the adsorption competition between ions, and the electrical properties of the adsorbent will also change; this leads to the desorption of TMs and the conversion of numerous granular TMs into dissolved states (Wu, 2012). The concentrations of Cu, Pb, Cd, and Zn were negatively correlated to pH, and the correlation was weak.
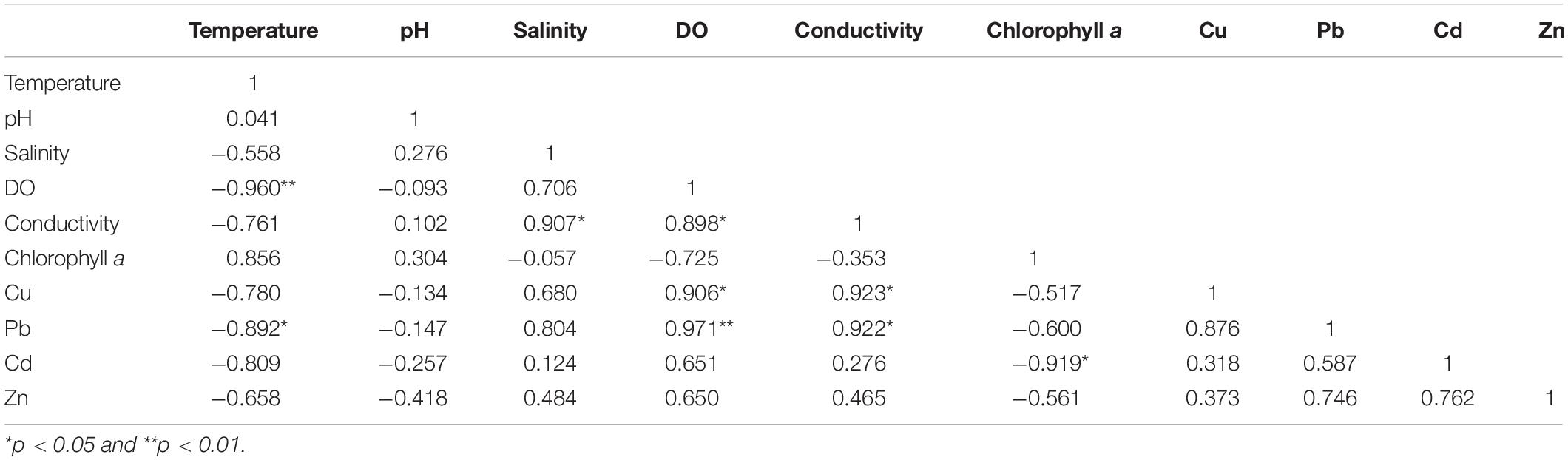
Table 4. Pearson correlation matrix for the monthly average concentrations of dissolved reactive Cu, Pb, Cd, and Zn and the physicochemical parameters in the surface seawater of Sishili Bay.
Conclusion
The species, spatial-temporal distributions, sources, correlations with physiochemical factors, and the contamination assessments of TMs (Cu, Pb, Cd, and Zn) were investigated in a typical mariculture area of North China. Significant spatial-temporal distributions of TMs were identified. The spatial distribution patterns of these metals implied that they may be affected by anthropogenic pollution, discharged effluent, industrial and aquaculture pollution, uptake by organisms, atmospheric deposition, and other coastal sources. The dissolved reactive TMs and total dissolved TMs have similar spatial-temporal distributions, indicating that they also have similar factors and sources. Significant differences were found in TM concentrations at all stations in different months. The mean percentages of the dissolved reactive concentrations of Cu, Pb, Cd, and Zn were 65.67, 59.92, 72.56, and 64.29%, respectively. These values indicated that the dissolved reactive TMs have high bioavailability and toxicity for marine organisms. All TMs in the surface water were below the grade-II seawater quality standard of China. The single contaminator factors of the elements follow the order: Pb > Cu > Zn > Cd. This showed that the identified contamination degree of the mariculture area was at a low pollution level.
Data Availability Statement
All datasets presented in this study are included in the article/Supplementary Material.
Author Contributions
DP and HH designed the experiments. XD performed the experiments and analyses. SZ and CW helped the data analyses. DP and XD wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was financially supported by the National Key R&D Program of China (2019YFD0901103), the Original Innovation Project of Chinese Academy of Sciences (ZDBS-LY-DQC009), and the Key Research and Development Plan of Shandong Province (2017GHY215002).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.552893/full#supplementary-material
References
Achterberg, E. P., Herzl, V. M., Braungardt, C. B., and Millward, G. E. (2003). Metal behaviour in an estuary polluted by acid mine drainage: the role of particulate matter. Environ. Pollut. 121, 283–292. doi: 10.1016/S0269-7491(02)00216-6
Achterberg, E. P., and van den Berg, C. M. G. (1994). In-line ultraviolet-digestion of natural water samples for trace metal determination using an automated voltammetric system. Anal. Chim. Acta. 291, 213–232. doi: 10.1016/0003-2670(94)80017-0
Adnívia, S. C. M., Corinne, P., André, H. R., and José, P. P. (2016). Towards field trace metal speciation using electroanalytical techniques and tangential ultrafiltration. Talanta 152, 112–118. doi: 10.1016/j.talanta.2016.01.053
Aqsiq. (1997). Sea Water Quality Standard (GB 3097-1997). Administration of Quality Supervision, Inspection and Quarantine. Beijing: Standard Press of China.
Bruland, K. W., and Lohan, M. C. (2003). “Controls of trace metals in seawater,” in Treatise on Geochemistry, ed. H. Elderfield (Amsterdam: Elsevier), 23–47. doi: 10.1016/B0-08-043751-6/06105-3
Croot, P. L., Baars, O., and Streu, P. (2011). The distribution of dissolved zinc in the Atlantic sector of the Southern Ocean. Deep Sea Res. II Top. Stud. Oceanogr. 58, 2707–2719. doi: 10.1016/j.dsr2.2010.10.041
Dong, Y., Rosenbaum, R. K., and Hauschild, M. Z. (2015). Assessment of metal toxicity in marine ecosystems: comparative toxicity potentials for nine cationic metals in coastal seawater. Environ. Sci. Technol. 50, 269–278. doi: 10.1021/acs.est.5b01625
Dong, Z. J., Liu, D. Y., Wang, Y. J., and Di, B. P. (2019). Temporal and spatial variations of coastal water quality in Sishili Bay, northern Yellow Sea of China. Aquat. Ecosyst. Health Mgmt. 22, 30–39. doi: 10.1080/14634988.2018.1525264
Ellwood, M. J. (2008). Wintertime trace metal (Zn, Cu, Ni, Cd, Pb and Co) and nutrient distributions in the subantarctic zone between 40–52°S; 155–160°E. Mar. Chem. 112:107. doi: 10.1016/j.marchem.2008.07.008
Ellwood, M. J., and Van den Berg, C. M. G. (2000). Zinc speciation in the northeastern Atlantic Ocean. Mar. Chem. 68, 295–306. doi: 10.1016/S0304-4203(99)00085-7
Fitzwater, S. E., Johnson, K. S., Gordon, R. M., Coale, K. H., and Smith, W. O. (2000). Trace metal concentrations in the ross sea and their relationship with nutrients and phytoplank-ton growth. Deep Sea Res. II. 47, 3159–3179. doi: 10.1016/S0967-0645(00)00063-1
Flegal, A. R., Smith, G. J., Gill, G. A., Sanudo-Wilhelmy, S. A., and Anderson, L. C. D. (1991). Dissolved trace element cycles in the San Francisco Bay estuary. Mar. Chem. 36, 329–363. doi: 10.1016/S0304-4203(09)90070-6
Florence, T. M. (1986). Electrochemical approaches to trace element speciation in waters. A review. Analyst 111, 489–505. doi: 10.1039/AN9861100489
George, J. A., Lonsdale, D. J., Merlo, L. R., and Gobler, C. J. (2015). The interactive roles of temperature, nutrients, and zooplankton grazing in controlling the winter–spring phytoplankton bloom in a temperate, coastal ecosystem, long Island sound. Limnol. Oceanogr. 60, 110–126. doi: 10.1002/lno.10020
Hakanson, L. (1980). An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 14, 975–1001. doi: 10.1016/0043-1354(80)90143-8
Han, H. T., Pan, D. W., Zhang, S. H., Wang, C. C., Hu, X. P., Wang, Y. C., et al. (2018). Simultaneous speciation analysis of trace heavy metals (Cu, Pb, Cd and Zn) in seawater from Sishili Bay, North Yellow Sea, China. Bull. Environ. Contam. Toxicol. 101, 486–493. doi: 10.1007/s00128-018-2431-4
Heller, M. I., and Croot, P. L. (2015). Copper speciation and distribution in the Atlantic sector of the Southern Ocean. Mar. Chem. 173, 253–268. doi: 10.1016/j.marchem.2014.09.017
Henry, M. C., and Yonker, C. R. (2006). Supercritical fluid chromatography, pressurized liquid extraction, and supercritical fluid extraction. Anal. Chem. 78, 3909–3915. doi: 10.1021/ac0605703
Herrero, E., Arancibia, V., and Rojas-Romo, C. (2014). Simultaneous determination of Pb2+, Cd2+ and Zn2+ by adsorptive stripping voltammetry using Clioquinol as a chelating-adsorbent agent. J. Electroanal. Chem. 729, 9–14. doi: 10.1016/j.jelechem.2014.06.039
Hook, S. E., and Fisher, N. S. (2001). Sublethal effects of silver in zooplankton: importance of exposure pathways and implications for toxicity testing. Environ. Toxicol. Chem. 20, 568–574. doi: 10.1002/etc.5620200316
Hu, W. J., Yang, S. Y., and Zhu, X. M. (2007). The impact of mariculture on the marine ecosystem and studies on bioremediation. Nat. Sci. S 1, 197–202.
Huang, S., Wang, Z., and Ma, M. (2003). Measuring the bioavailable/toxic concentration of copper in natural water by using anodic stripping voltammetry and Vibrio qinghaiensis sp. Nov. Q67 bioassay. Chem. Spec. Bioavailab. 15, 37–45. doi: 10.3184/095422903782775226
Illuminati, S., Truzzi, C., Annibaldi, A., Migliarini, B., Carnevali, O., and Scarponi, G. (2010). Cadmium bioaccumulation and metallothionein induction in the liver of the Antarctic teleost Trematomus bernacchii during an on-site short-term exposure to the metal via seawater. Toxicol. Environ. Chem. 92, 617–640. doi: 10.1080/02772240902902349
Johnson, K. S., Coale, K. H., and Elrod, V. A. (1994). Iron Photochemistry in seawater from the equatorial pacific. Mar. Chem. 46, 319–334. doi: 10.1016/0304-4203(94)90029-9
Kot, A., and Namiesńik, J. (2000). The role of speciation in analytical chemistry. Trends Anal. Chem. 19, 69–79. doi: 10.1016/S0165-9936(99)00195-8
Kubáň, P., and Timerbaev, A. R. (2014). Inorganic analysis using CE: advanced methodologies to face old challenges. Electrophoresis 35, 225–233. doi: 10.1002/elps.201300302
Kustka, A., Carpenter, E. J., and Sañudo-Wilhelmy, S. A. (2002). Iron and marine nitrogen fixation: progress and future directions. Res. Microbiol. 153, 255–262. doi: 10.1016/S0923-2508(02)01325-6
Li, B. Q., Keesing, J. K., Liu, D. Y., Han, Q. X., Wang, Y. J., Dong, Z. J., et al. (2013). Anthropogenic impacts on hyperbenthos in the coastal waters of Sishili Bay, Yellow Sea. Chin. J. Oceanol. Limnol 31, 1257–1267. doi: 10.1007/s00343-013-2173-4
Li, L., Pala, F., Jiang, M., Krahforst, C., and Wallace, G. (2010). Three-dimensional modeling of Cu and Pb distributions in Boston Harbor, Massachusetts and Cape Cod Bays. Estuar. Coast. Shelf Sci. 88, 450–463. doi: 10.1016/j.ecss.2010.05.003
Li, L., Wang, X. J., Liu, J. H., and Shi, X. F. (2017). Dissolved trace metal (Cu, Cd, Co, Ni, and Ag) distribution and Cu speciation in the southern Yellow Sea and Bohai Sea, China. J. Geophys. Res. Oceans. 122, 1190–1205. doi: 10.1002/2016JC012500
Lin, M. Y., Pan, D. W., Hu, X. P., Zhu, Y., Han, H. T., Li, F., et al. (2016). Speciation analysis of iron in Yantai coastal waters. J. Environ. Chem 35, 297–304.
Liu, D., Keesing, J. K., Xing, Q., and Shi, P. (2009). World’s largest macroalgal bloom caused by expansion of seaweed aquaculture in China. Mar. Pollut. Bull. 58, 888–895. doi: 10.1016/j.marpolbul.2009.01.013
Liu, D. Y., Shi, Y. J., Di, B. P., Sun, Q. L., Wang, Y. J., Dong, Z. J., et al. (2012). The impact of different pollution sources on modern dinoflagellate cysts in Sishili Bay, Yellow Sea, China. Mar. Micropaleontol. 84, 1–13. doi: 10.1016/j.marmicro.2011.11.001
Lü, D., Zheng, B., Fang, Y., Shen, G., and Liu, H. (2015). Distribution and pollution assessment of trace metals in seawater and sediment in Laizhou Bay. Chin. J. Oceanol. Limnol. 33, 1053–1061. doi: 10.1007/s00343-015-4226-3
Maity, S., Sahu, S. K., and Pandit, G. G. (2017). Determination of traces of Pb, Cu and Cd in seawater around Thane Creek by anodic stripping voltammetry method. Bull. Environ. Contam. Toxicol. 98, 534–538. doi: 10.1007/s00128-016-2025-y
Maldonado, M. T., Allen, A. E., Chong, J. S., Lin, K., Leus, D., Karpenko, N., et al. (2006). Copper-dependent iron transport in coastal and oceanic diatoms. Limnol. Oceanogr. 51, 1729–1743. doi: 10.4319/lo.2006.51.4.1729
Mao, T. Y., Dai, M. X., Peng, S. T., and Li, G. L. (2009). Temporal-spatial variation trend analysis of heavy metals (Cu, Zn, Pb, Cd, Hg) in Bohai Bay in 10 years. J. Tianjin Univ. 42, 817–825.
Martin, J. H., Coale, K. H., Johnson, K. S., Fitzwater, S. E., Gordon, R. M., Tanner, S. J., et al. (1994). Testing the iron hypothesis in ecosystems of the equatorial Pacifific-Ocean. Nature 371, 123–129. doi: 10.1038/371123a0
Martin, J. H., Gordon, M., and Fitzwater, S. E. (1991). The case for iron. Limnol. Oceanogr. 36, 1793–1802. doi: 10.4319/lo.1991.36.8.1793
Meng, W., Qin, Y., Zheng, B., and Zhang, L. (2008). Heavy metal pollution in Tianjin Bohai bay. China. J. Environ. Sci. 20, 814–819. doi: 10.1016/S1001-0742(08)62131-2
Moffett, J. W., Brand, L. E., Croot, P. L., and Barbeau, K. A. (1997). Cu speciation and cyanobacterial distribution in harbors subject to anthro pogenic Cu inputs. Limnol. Oceanogr. 42, 789–799. doi: 10.4319/lo.1997.42.5.0789
Morel, F. M. M., Milligan, A. J., and Saito, M. A. (2003). “Marine bioinorganic chemistry: The role of trace metals in the oceanic cycles of major nutrients,” in Treatise Geochemistry, eds H. Elderfield, H. D. Holland, and K. K. Turekian (Cambridge: Elsevier), 113–143. doi: 10.1016/B978-0-08-095975-7.00605-7
Nimmo, M., van den Berg, C. M. G., and Brown, J. (1989). The chemical speciation of dissolved nickel, copper, vanadium and iron in Liverpool Bay, Irish Sea. Estuar. Coast. Shelf Sci. 29, 57–74. doi: 10.1016/0272-7714(89)90073-5
Posacka, A. M., Semeniuk, D. M., Whitby, H., van den Berg, C. M. G., Cullen, J. T., Orians, K., et al. (2017). Dissolved copper (dCu) biogeochemical cycling in the subarctic Northeast Pacific and a call for improving methodologies. Mar. Chem. 196, 47–61. doi: 10.1016/j.marchem.2017.05.007
Ribeiro, D. R. G., Faccin, H., Molin, T. R. D., de Carvalho, L. M., and Amado, L. L. (2017). Metal and metalloid distribution in different environmental compartments of the middle Xingu river in the Amazon, Brazil. Sci. Total Environ. 605, 66–74. doi: 10.1016/j.scitotenv.2017.06.143
Rivera-Duarte, I., Rosen, G., Lapota, D., Chadwick, D. B., Kear-Padilla, L., and Zirino, A. (2005). Copper toxicity to larval stages of three marine invertebrates and copper complexation capacity in San Diego Bay, California. Environ. Sci. Technol. 39, 1542–1546. doi: 10.1021/es040545j
Shen, C. Y., Shi, P., and Zhao, H. (2014). Spatial-temporal distribution characteristics of chorophyll a and the controlling facters in the Sishili Bay of Yantai. Mar. Sci. 38, 33–38. doi: 10.11759/hykx20130925002
Sheng, Y. Q., Sun, Q. Y., Bottrell, S. H., Mortimer, R. J. G., and Shi, W. J. (2012). Anthropogenic impacts on reduced inorganic sulfur and heavy metals in coastal surface sediments, north Yellow Sea. Environ. Earth Sci. 68, 1367–1374. doi: 10.1007/s12665-012-1835-4
Stumm, W., and Morgan, J. J. (1996). Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters. New York, NY: John wiley and Sons Inc, doi: 10.5860/choice.33-6312
Su, H., Yang, R. J., Pižeta, I., Omanović, D., Wang, S., and Li, Y. (2016). Distribution and speciation of dissolved iron in Jiaozhou Bay (Yellow Sea, China). Front. Mar. Sci. 3:99. doi: 10.3389/fmars.2016.00099
Su, H., Yang, R. J., Zhang, A. B., and Li, Y. (2015). Dissolved iron distribution and organic complexation in the coastal waters of the East China Sea. Mar. Chem. 173, 208–221. doi: 10.1016/j.marchem.2015.03.007
Su, H., Yang, R. J., Zhang, A. B., Li, Y., Qu, S. L., and Wang, X. C. (2017). Characteristics of trace metals and phosphorus in seawaters offshore the Yangtze River. Mar. Pollut. Bull. 124, 1020–1032. doi: 10.1016/j.marpolbul.2017.01.022
Sunda, W. G., Tester, P. A., and Huntsman, S. A. (1987). Effects of cupric ion and zinc ion activities on the survival and reproduction of marine copecods. Mar. Biol. 94, 203–210. doi: 10.1007/BF00392932
Tang, Q. (2004). Study on Ecosystem Dynamics in Coastal Ocean. III, Atlas of the Resources and Environment in the East China Sea and the Yellow Sea Ecosystem. Beijing: Science Press.
Tian, L., Chen, H. T., Du, J. T., and Wang, X. H. (2009). Factors influencing distribution of soluble heavy metals in north yellow sea surface seawaters. Period. Ocean. Univ. Chin. 39, 617–621. doi: 10.16441/j.cnki.hdxb.2009.04.011
Turner, A., Millward, G. E., Schuchardt, B., Schirmer, M., and Prange, A. (1992). Trace metal distribution coefficients in the Weser estuary (Germany). Continent. Shelf Res. 12, 1277–1292. doi: 10.1016/0278-4343(92)90064-Q
Vidal, L., Silva, S. G., Canals, A., and Nóbrega, J. A. (2016). Tungsten coil atomic emission spectrometry combined with dispersive liquid–liquid microextraction: a synergistic association for chromium determination in water samples. Talanta 148, 602–608. doi: 10.1016/j.talanta.2015.04.023
Wang, J., Liu, R. H., Yu, P., Tang, A. K., Xu, L. Q., and Wang, J. Y. (2012). Study on the pollution characteristics of heavy metals in seawater of Jinzhou Bay. Proc. Environ. Sci. 13, 1507–1516. doi: 10.1016/j.proenv.2012.01.143
Wang, X. E., Xu, Z. F., and Zhou, X. J. (1995). Animal survey in Yantai inshores. Chin. J. Ecol. 14, 6–10. doi: 10.13292/j.1000-4890.1995.0002
Wang, X. Y., Zhao, L. L., Xu, H. Z., and Zhang, X. M. (2018). Spatial and seasonal characteristics of dissolved heavy metals in the surface seawater of the Yellow River Estuary, China. Mar. Pollut. Bull. 137, 465–473. doi: 10.1016/j.marpolbul.2018.10.052
Wang, Z. F., and Cui, Z. J. (2016). Determination of arsenic species in solid matrices utilizing supercritical fluid extraction coupled with gas chromatography after derivatization with thioglycolic acidn-butyl ester. J. Sep. Sci. 39, 4568–4576. doi: 10.1002/jssc.201600510
Wu, Y. F. (2012). Distribution of heavy metals in the surface water of Luoyuan Bay. J. Fujian Fish. 5, 387–391. doi: 10.14012/j.cnki.fjsc.2012.05.005
Xie, W. P., Zhu, X. P., Zheng, G. M., and Ma, L. S. (2014). Residues and health risk assessment of HCHs, DDTs and heavy metals in water and tilapias from fish ponds of guangdong. Environ. Sci. 35, 4663–4670. doi: 10.13227/j.hjkx.2014.12.033
Yu, L., Hao, Y. J., and Cai, Y. Y. (2009). Annual variation of nutrient and Chla during HABs’ periods in Sishili Bay. Mar. Environ. Sci. 5, 558–562.
Yuan, Z., Qi, J. H., and Zhang, M. P. (2003). The origin of iron in seawater and its relationship with phytoplankton. Trans.Oceanol.Limnol. 4, 38–48. doi: 10.13984/j.cnki.cn37-1141.2003.04.005
Zhang, G. S., Liu, D. Y., Wu, H. F., Chen, L. L., and Han, Q. X. (2012). Heavy metal contamination in the marine organisms in Yantai coast, northern Yellow Sea of China. Ecotoxicology 21, 1726–1733. doi: 10.1007/s10646-012-0958-4
Zhang, H. Y., Lin, M. Y., Zhang, Q., Han, H. T., and Pan, D. W. (2014). Bismuth as internal standard for reliable detection of trace lead at screen-printed electrode. Asian J. Chem. 26, 5217–5222. doi: 10.14233/ajchem.2014.16740
Zhang, Y., Wang, Y. J., Wang, Y. Q., and Liu, D. Y. (2016). Spatial distribution and correlation of environmental factors and chlorophyll a concentrations in the Bohai Sea during the summer of 2013. Mar. Sci. Bull. 5, 571–578. doi: 10.11840/j.issn.1001-6392.2016.05.011
Keywords: metal species, spatial-temporal distribution, contamination, trace metals, mariculture
Citation: Pan D, Ding X, Han H, Zhang S and Wang C (2020) Species, Spatial-Temporal Distribution, and Contamination Assessment of Trace Metals in Typical Mariculture Area of North China. Front. Mar. Sci. 7:552893. doi: 10.3389/fmars.2020.552893
Received: 17 April 2020; Accepted: 24 August 2020;
Published: 23 September 2020.
Edited by:
Dragana S. Ðorđević, University of Belgrade, SerbiaReviewed by:
Periyadan K. Krishnakumar, King Fahd University of Petroleum and Minerals, Saudi ArabiaHuamao Yuan, Institute of Oceanology (CAS), China
Copyright © 2020 Pan, Ding, Han, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dawei Pan, dwpan@yic.ac.cn
 Dawei Pan
Dawei Pan Xiaoyan Ding
Xiaoyan Ding Haitao Han
Haitao Han Shenghui Zhang
Shenghui Zhang Chenchen Wang1,2
Chenchen Wang1,2