Biodiversity and Ecological Units of the Mesophotic Coral Ecosystems in San Andrés Island, SeaFlower Biosphere Reserve
- Programa Biodiversidad y Ecosistemas Marinos, Instituto de Investigaciones Marinas y Costeras – INVEMAR, Santa Marta, Colombia
Background
Mesophotic Coral Ecosystems (MCE) are usually defined as those located between 30 and 150 m deep, where diverse biological communities with high levels of endemism are being found globally (Sinniger et al., 2016). Those environments have been considered as a kind of lifeboat for shallow coral reefs because its harbor populations of fish, corals and other invertebrates which are uncommon today in shallow waters due to overexploitation and climatic change stressors (Bongaerts et al., 2010).
More than half a thousand studies globally in MCE over the last three decades have yielded an important crop of new species (e.g., Appeldoorn et al., 2016; Pyle et al., 2016; but see Pyle and Copus, 2019), and greatly expanded scientific knowledge about coral reef biota in several aspects, since species richness and distribution in general (e.g., Laverick et al., 2018) until very specific issues such as the genetic diversity of coral holobionts (e.g., Gonzalez-Zapata et al., 2018a). However, such research efforts suffer strong geographic biases, being mostly concentrated in four regions: The Tropical Western Atlantic-TWA, the Hawaiian Archipelago, the Mediterranean Sea and the Northern Red Sea. Most of the MCE research at the TWA has been done in the Caribbean, yielding important information on species richness and abundance, and vertical distribution patterns, mainly on corals and fishes (Pyle and Copus, 2019). However, even in the Caribbean most MCE are unexplored as research has focused on specific locations (i.e., Bahamas, Cayman, Mesoamerican Reef, Jamaica, Puerto Rico, US Virgin Islands, Curacao, and Bonaire; Loya et al., 2019), such that geographic biases remain at the regional scale. For example, only four publications exist on Colombian MCE (Gonzalez-Zapata et al., 2018a,b; Chasqui and González, 2019; Sánchez et al., 2019a), one of such offer data on corals diversity in the fore-reef slope of the eastern side (windward) of San Andrés Island (SAI), which account for 33 species (Sánchez et al., 2019a).
Considering that no previously published works exist related to the ecology of seascape on MCE in Colombia, this work seeks to ignite the topic and contribute with the knowledge on MCE biodiversity of SAI, in the Southwestern Caribbean at the species and habitats level, a relevant information input for the management of the SeaFlower Biosphere Reserve (SFBR) which include SAI. The dataset contains the results of the exploration with Closed-Circuit Rebreather (CCR) technical diving in the upper mesophotic zone (30–70 m deep) at the western side (Leeward) of the Island, where the ecological units Octocorals-Sponges-Mixed corals and Agaricia spp.-Mixed corals have been proposed, and includes taxonomic information for 160 species recorded (algae, invertebrates and fishes), collection codes for several collected specimens (corals), and relative abundances for the observed fish species.
Methods
Study Site and Data Collection
The marine exploration was performed at the western side (leeward) of SAI, in the upper mesophotic zone of a diving spot known as “Nirvana” (12°30′8″ N, 81°44′2″ W, Figures 1A,B). The depth profile of the western side offers a soft slope from coastline until around 20 m depth, then, around 300 m from the coast the bottom suddenly deepens until 50 m depth, giving start to which can be considered the deep fore-reef terrace (Figures 1C–E), then around 50 m depth a structurally complex wall begins (reef slope), which falls until more than 300 m depth. Ledges, undercuts, ridges, and small caves are common characteristics along the slope, which confers structural complexity to the wall and are possibly increasing microhabitats' offer (Figures 1F,G).
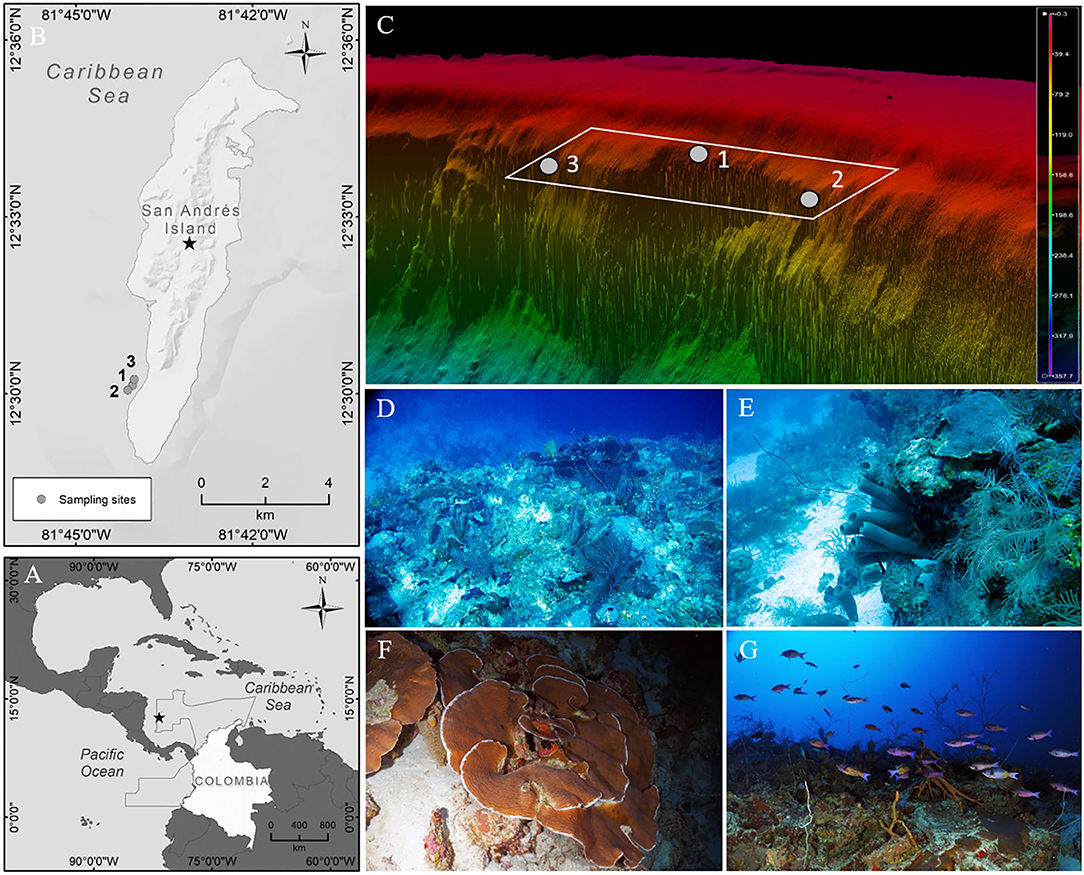
Figure 1. Localization of San Andrés Island in the Caribbean Sea (A), the Nirvana dive spot (B) and the sampling sites between 30 and 70 m deep in a 3D seabed model of the SAI leeward side (C). Ecological units found along the depth gradient: (D,E) Octocorals-Sponges-Mixed corals on the fore reef terrace between 30 and 50 m deep. (F,G) Agaricia spp.-Mixed corals on the upper reef slope between 50 and 70 m deep.
Six dives lasting around 28 min between 30 and 70 m depth with O2ptima CCR and trimix were carried out in three spots at Nirvana (1: 12°30′9″ N, 81°44′2″ W; 2: 12°30′4″ N, 81°44′7″ W; 3: 12°30′15″ N, 81°44′1″ W) between March-April 2018, accounting for a total exploration time of 172 min, during which an area of 2.7 ha (approx.) was covered. The exploration allowed the recognition of the most common and conspicuous biota in the deep fore-reef terrace (30–50 m depth) and the upper reef slope (50–70 m depth) of Nirvana. The sampling involves digital imagery (Panasonic™ DMC-LX10, Nikon™ D7000), collection of typical sessile biota and visual census of fishes using roving diver technique (Schmitt and Sullivan, 1996).
Biota Identification
Sessile biota identification was based on the imagery library and the specimens collected. Each specimen was photographed with and without scale before collection, then was deposited in a tagged “Ziploc” bag for later identification. Fish species were identified in situ.
The octocoral and black coral colonies were air-dried for preservation and identified according to morphological characters of the colonies and characteristics of the sclerites in octocorals and spines in black corals (Bayer, 1961; Opresko and Sánchez, 2005; Sánchez and Wirshing, 2005). Sclerites and spines were recovered from a small bit of tissue dissolved in sodium hypochlorite, then were examined and measured with a microgrid in an optical microscope (Carl Zeiss Primostar). The hard coral colonies were preserved in 70% alcohol and identified according to morphological characters (Veron, 2000; Reyes et al., 2010; Veron et al., 2016). All the specimens collected were deposited in the cnidarian collection (INV CNI) of the Museo de Historia Natural Marina de Colombia (MHNMC) of INVEMAR, the codes are listed in Table 1.

Table 1. Species of sessile benthic biota and fishes recorded in the mesophotic coral ecosystem MCE (30–70 m depth) on the western side (leeward) of San Andrés Island, Colombian Caribbean.
Data Analysis
To assign ecological units to the seascape observed in the sampling site, the guidelines of the book Áreas coralinas de Colombia (Díaz et al., 2000), in which the main aspects are the geoform and the dominant biota, were followed. In Nirvana, two different zones were visually apparent, one between 30 and 50 m depth, and the other one between 50 and 70 m depth. To determine the dominant biota, several video clips made over dives were used. Eight clips lasting 364 s and 5 clips lasting 385 s, for the 30–50 m and 50–70 m depth intervals, respectively, were analyzed. For each interval 17 photo frames from the video clips were obtained using a fixed time interval to avoid possible observer bias. Afterward, data on the conspicuous taxa (octocoral, black coral, sponges, scleractinian at the genus level when possible, algae) and abiotic components of the sea bottom were taken by overlapping an 8 x 8 grid on the frame and choosing 6 quadrants (off 64) with random numbers. To each quadrant only one category was assigned considering the most conspicuous category (frequently just one were seen). A total of 102 quadrants were reviewed for each depth interval, then 2 quadrants randomly chosen were deleted. Finally, with the number of quadrants percentages for each different taxa or abiotic feature (sand, coral rock, gap, undetermined) for each depth interval were obtained.
Dataset Outcomes and Discussion
This first exploration in the upper zone of the MCE in SAI leeward side allowed the recognition of two coral reef ecological units. The analysis of the quadrants show that in the deep fore-reef terrace (30–50 m depth) the octocorals (27%), sponges (13%), several hard coral species (10%), and black corals (9%) were dominant seascape features; the substrate correspond mainly with limestone matrix although some sand patches were also evident, the relief is flat in there, and wavy where corals and sponges are present (Figures 1D,E). The upper reef-slope (50–70 m depth) is a complex wall where the dominant taxa in terms of “conspicuousness” were Agaricia (21%, mainly plate-shaped colonies), sponges (12%, mainly tubular and branching, some barrels), and black corals (7%, mainly sea whips) are the main biotic components (Figures 1F,G).
Supported in this straightforward data analysis the units Octocorals-Sponges-Mixed corals for the 30–50 m range, and Agaricia spp.-Mixed corals (see Díaz et al., 2000) to 50–70 m range were assigned.
In terms of biota, 160 species included in 3 kingdoms (Chromista, Plantae, and Animalia), 7 phyla, 11 classes, 29 orders, 66 families and 103 genera between algae (14 spp.), sponges (34), cnidarians (36), crustaceans (1), ascidians (1), and fishes (74), were recognized (Table 1). The dataset provides information on sample collection for 30 specimens of 15 species, as well as sighting data recorded in situ for the remaining 145 species. Additionally, fish species abundance is presented by categories. In the Figures 2A–M some of the common species recorded in the study are showed.
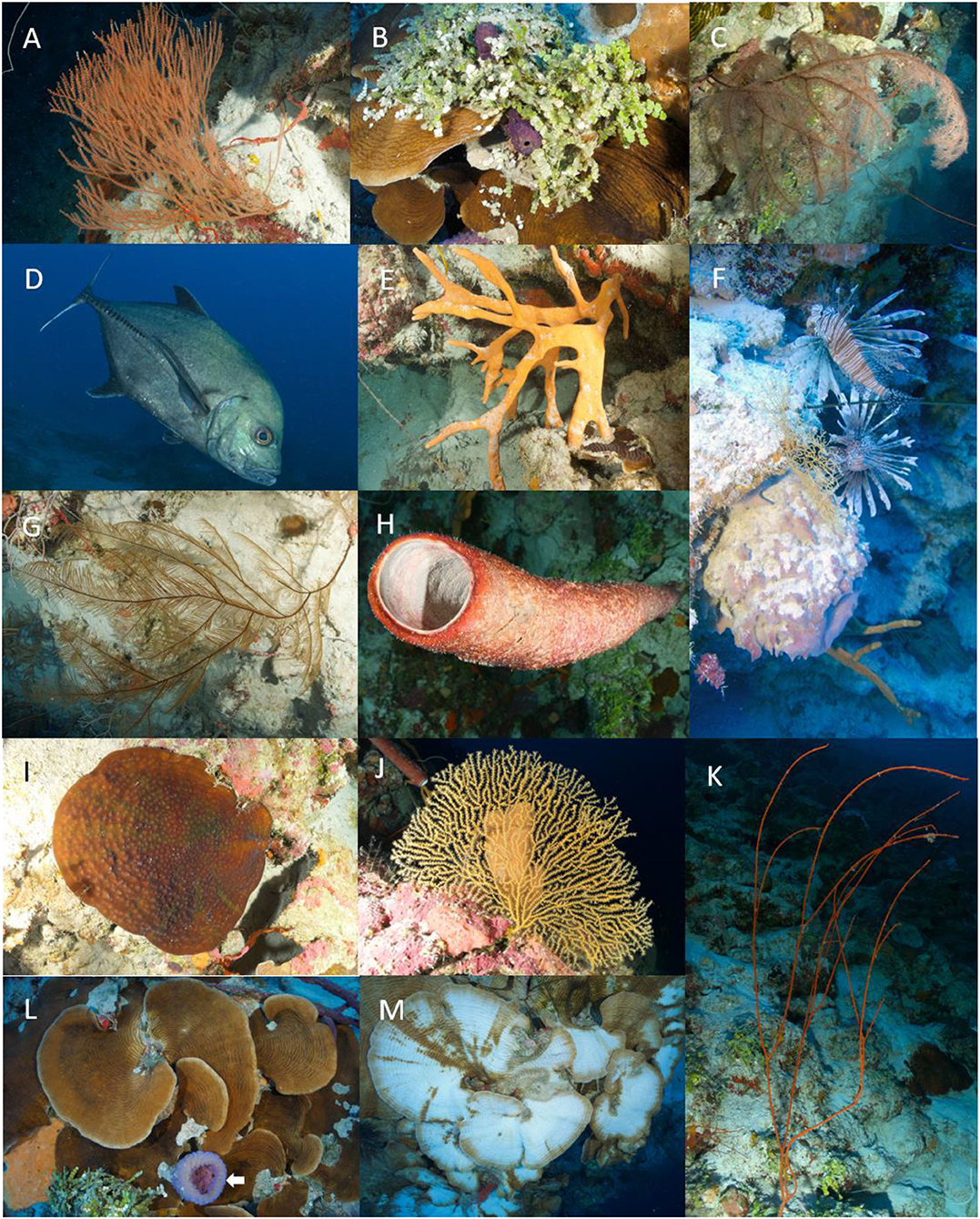
Figure 2. Common biota in the Ecological Units Octocorals-Sponges-Mixed corals and Agaricia spp.-Mixed corals of the mesophotic coral ecosystem (MCE) (30–70 m depth) in the western side (leeward) of San Andrés Island, Colombian Caribbean. (A) Ellisella schmitti; (B) Halimeda copiosa; (C) Tanacetipathes hirta; (D) Caranx lugubris; (E) Agelas sceptrum; (F) Pterois volitans and Xestospongia muta; (G) Plumapathes pennacea; (H) Aplysina archeri; (I) Mycetophyllia reesi; (J) Nicella sp.; (K) Ellisella elongata; (L) Agaricia sp. and Callyspongia sp. (white arrow); (M) Bleaching in Agaricia sp. at 70 m depth.
Until date, 311 species of macroalgae have been reported in the SFBR, including species in the four phyla (Cyanophyta, Rhodophyta, Chlorophyta, and Ochrophyta), but mostly from shallow waters between 0 and 31 m depth (Rincón-Díaz and Ramos-Gallego, 2016; Rincón-Díaz et al., 2018). Here we report the presence of 14 algae species in the mesophotic zone (30 and 70 m depth), most of them already listed for the SFBR except for the genus Rhodymenia (Rhodophyta), which has not been previously reported in the archipelago (nor in the north of Southwestern Caribbean Ecoregion), but recorded on the Colombian mainland coast (Diaz-Pulido and Díaz-Ruíz, 2003), thus expanding the presence of the genus to the north of the ecoregion.
Fifteen species of scleractinian were recorded, five of them were only seen in the deep fore-reef terrace (30–50 m depth: Colpophyllia natans, Mycetophyllia lamarckana, Eusmilia fastigiata, Orbicella franksi, and Porites astreoides), five more were seen exclusively in the reef slope (50–70 m depth: Agaricia grahamae, A. undata, Mycetophyllia reesi, Madracis pharensis, Porites sp.), and five were recorded in both deep strata (Agaricia lamarcki, Scolymia cubensis, Meandrina meandrites, Montastraea cavernosa, and Siderastrea siderea; Table 1). Among these hard coral species, the most conspicuous were those in the genera Agaricia, being frequent to observe big plate-like colonies hanging from the ledges of the wall, some of them showing severe bleaching (Figure 2M). Bleaching in several species of scleractinian corals in MCE (including Agaricia spp.) has been reported in different locations of the Western Atlantic (i.e., Puerto Rico, Bahamas, Cayman Islands, Gulf of Mexico), with the deepest coral bleaching report in the Bahamas at 91 m depth (Weil, 2019). Another striking feature of several Agaricia spp. colonies observed on the leeward reef slope in SAI was the obvious presence of big dark blotches (Figure 2L), which seems very like the Ostreobium endolithic algal association reported by Gonzalez-Zapata et al. (2018b) in colonies of A. undata on MCE at windward reefs in SAI, and other coral areas from the Colombian Caribbean.
The octocoral communities observed between 30 and 50 m depth were similar to those found on shallower zones with a dominance of species like Antillogorgia spp. and Eunicea spp., some of them previously recorded at similar depths in the windward reefs of the island (Sánchez et al., 2019a). The colonization of shallow-water coral species into deeper zones in SAI can be explained by a high light penetration that reaches deep areas in this insular region, which could be promoting an ecological divergence in some species according to Sánchez et al. (2019b). Several octocorals and black coral species which are usually common in Caribbean MCE like Ellisella schmitti, E. elongata (including the synonymous E. barbadensis), Nicella goreaui, Nicella sp., and the unbranched black corals Stichopathes luetkeni and S. occidentalis, were also common below 50 m depth in Nirvana, where shallow-water coral species (e.g., Antillogorgia spp.) begin to be rare. On the reef slope (50–70 m depth) at Nirvana, two new records of corals for the SFBR were found, Iciligorgia schrammi registered by photographs and Muriceopsis petila that was even collected. The black coral Tanacetipathes hirta, a species recently reported in the windward MCE of San Andrés Island by Sánchez et al. (2019a), were also seen in the leeward MCE between 50 and 70 m depth (Figure 2C).
The 34 sponge's species listed in the dataset were recorded mainly by photographs. Ten species were only seen in the shallower zone (30–50 m depth) and 16 were exclusively found on 50–70 m depth; the other eight species were present through the entire depth range (Table 1). The sponge community in the MCE of the leeward face at SAI was very similar to those reported for other Caribbean locations (Pomponi et al., 2019), commonly dominated by tubular and vase sponges like Agelas tubulata, A. sceptrum, Aplysina archeri, and Xestospongia muta (Figures 2F,H). However, in this work just the most conspicuous sponges were recorded—mainly massive ones—, so that sponge richness is probably underestimated, which can be assumed by the unregistered amount of different colored sponges encrusting the hard bottom. This exploration of MCE yielded five new records of sponges for the SFBR (Auletta sp., Dysidea lehnerti, Niphates arenata, Oceanapia peltata, and Xestospongia arenosa), which increase the species richness of the group for this Marine Protected Area, previously estimated in 164 species accounted only for shallow waters (until 30 m depth; Díaz-Sánchez and Zea, 2016).
The fish community observed in the MCE of the leeward side at SAI is very typical of the known ictiofauna in the SFBR (around 653 species; Bolaños-Cubillos et al., 2015) regarding composition, except for the species Carcharhinus longimanus and Decapterus macarellus, which are new records for the island. Conversely, some difference in species abundance between MCE fish community and fish community in shallow waters at Nirvana seems evident (Table 1). For example, several herbivorous fishes that are very common in the Caribbean shallow reefs such as the parrotfishes Scarus iseri, Sparisoma viride, and S. aurofrenatum, became difficult to observe as depth increases. Also, species like the blackcap basslet Gramma melacara which can hardly be observed shallower than 20 m depth, were very common in the fore-reef slope (50–70 m depth). G. melacara is a common species in the deep fore-reef at different sites along the Caribbean, being considered as the most abundant mesophotic reef fish of the Western/Central Caribbean (Dustan and Lang, 2019). Similarly, the longsnout butterflyfish Prognathodes aculeatus, an observed but uncommon fish species in shallow waters of the Caribbean reefs, was one of the common fish species in San Andrés MCE. This species is also one of the common species in the mesophotic fish communities along the Tropical Western Caribbean (Loya et al., 2019), being one of the five species that best characterized the fish assemblage at 70 m depth in Puerto Rico (Appeldoorn et al., 2019).
The lionfish Pterois volitans, maybe the most famous and infamous marine alien invasive species in the Western Atlantic were also seen frequently in this study, to a maximum depth of 70 m. The invader has also been recorded in other localities and ecosystems in the Colombian Caribbean, practically from the surface until 140 m depth (Chasqui et al., 2020; Polanco, unpublished data). In Colombian MCE, there are sightings of lionfish in the National Natural Park Corales de Profundidad (Chasqui and González, 2019), in San Andrés Island (this study, Figure 2I) and the continental platform near Santa Marta city (González, unpublished data). The non-native lionfish have invaded MCE across the Western Atlantic, and several studies in the upper MCE suggest that the species cause negative impacts on native fish communities (Andradi-Brown, 2019), making clear the urgent need to extend lionfish control measures (manual hunting, trapping, etc.) toward mesophotic coral ecosystems.
Reuse Potential
This dataset provides an update of marine organisms recorded in SFBR and specifically SAI with a focus on the mesophotic coral ecosystem, poorly known for the Island. The dataset includes the catalog numbers of the collected biota that was deposited in the Museo de Historia Natural Marina de Colombia—(MHNMC), for any taxonomic verification needed. Additionally, the categories of fish abundance are provided in the dataset for future comparisons. These species records are a valuable source of biodiversity information on MEC in the Southwestern Caribbean, available for future studies and reviews on the topic, a very nascent field of research in this part of the world, particularly in Colombia.
Data Availability Statement
The dataset for this study titled “Biodiversidad de los Ecosistemas Mesofóticos de la isla de San Andrés” can be found through the Integrated Publishing Tool of the OBIS Colombian nodes (SIBM-SIB Colombia), using the link https://doi.org/10.15472/0itxej.
Author Contributions
LC conceived the study. LC and JG collected the data and biological samples. KM-Q identified and processed the collected material. LC, KM-Q, and JG wrote the manuscript. All authors read and accepted the final version of the manuscript.
Funding
This work was supported by a cooperation agreement between Corporación para el Desarrollo Sostenible del Archipiélago de San Andrés, Providencia y Santa Catalina—CORALINA and Instituto de Investigaciones Marinas y Costeras—INVEMAR (Agreement 007- 2017).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank CORALINA staff for support during fieldwork, particularly N. Bolaños for allowing access to his dive tech stuff and secure the funding to MCE research inside CORALINA. To D. Seguro (Tech Bull SAS) for dive logistics, and photo capture. To E. Montoya and N. Rincón (INVEMAR) for tunicates and algae identification. To S. Zea (Universidad Nacional) and P. Parrado (INVEMAR) for help in sponge's identification. E. Montoya and Julio Bohorquez advised on the dataset elaboration. INVEMAR Contribution Number 1281. We thank the two reviewers for their suggestions to improve the manuscript.
References
Andradi-Brown, D. A. (2019). “Invasive Lionfish (Pterois volitans and P. miles): distribution, impact, and management,” (48) in Mesophotic Coral Ecosystems, Coral Reefs of the World, eds Y. Loya, K. A. Puglise, and T. C. L. Bridge (Cham: Springer Nature Switzerland AG), 931–941. doi: 10.1007/978-3-319-92735-0_48
Appeldoorn, R., Ballantine, D., Bejarano, I., Carlo, M., Nemeth, M., Otero, E., et al. (2016). Mesophotic coral ecosystems under anthropogenic stress: a case study at Ponce, Puerto Rico. Coral Reefs 35, 63–75. doi: 10.1007/s00338-015-1360-5
Appeldoorn, R. S., Alfaro, M., Ballantine, D. L., Bejarano, I., Ruíz, H. J., Schizas, N. V., et al. (2019). “Puerto Rico” (7) in Mesophotic Coral Ecosystems, Coral Reefs of the World, eds Y. Loya, K. A. Puglise, and T. C. L. Bridge (Cham: Springer Nature Switzerland AG), 111–129. doi: 10.1007/978-3-319-92735-0_7
Bayer, F. (1961). “The shallow-water Octocorallia of the West Indian Region,” in Martinus Nijoff (La Haya), 373.
Bolaños-Cubillos, N., Abril-Howard, A., Bent-Hooker, H., Caldas, J. P., and Acero, A. (2015). Lista de peces conocidos del archipiélago de San Andrés, Providencia y Santa Catalina, Reserva de Biosfera Seaflower, Caribe occidental colombiano. Bol. Invest. Mar. Cost. 44, 127–162. doi: 10.25268/bimc.invemar.2015.44.1.24
Bongaerts, P., Ridgway, T., Sampayo, E. M., and Hoegh-Guldberg, O. (2010). Assessing the ‘Deep Reef Refugia’ hypothesis: focus on Caribbean reefs. Coral Reefs 29, 309–327. doi: 10.1007/s00338-009-0581-x
Chasqui, L., Rincón-Díaz, N., and Vanegas, M. J. (2020). Abundance of the invasive lionfish Pterois volitans in the coastal coral reefs from Colombian Caribbean. Bol. Invest. Mar. Cost. 49, 157–170. doi: 10.25268/bimc.invemar.2020.49.1.779
Chasqui, L. H., and González, J. D. (2019). Fishes found in mesophotic environments of Bajo Frijol, the shallowest portion of the Corales de Profundidad Natural National Park, using CCR technical diving. Bol. Invest. Mar. Cost. 48, 89–101. doi: 10.25268/bimc.invemar.2019.48.1.759
Díaz, J. M., Barrios, L. M., Cendales, M. H., Garzón-Ferreira, J., Geister, J., López-Victoria, M., et al. (2000). Áreas coralinas de Colombia. Serie Publicaciones Especiales No. 5. Santa Marta: INVEMAR.
Diaz-Pulido, G., and Díaz-Ruíz, M. (2003). Diversity of benthic marine algae of the Colombian Atlantic. Biota Colombiana 4, 203–249.
Díaz-Sánchez, C. M., and Zea, S. (2016). “Esponjas (Porifera) de la Reserva de Biosfera Seaflower”, in Biodiversidad del mar de los siete colores, eds M. Vides, D. Alonso, E. Castro, and N. Bolaños (Instituto de Investigaciones Marinas y Costeras – INVEMAR y Corporación para el Desarrollo Sostenible del Archipiélago de San Andrés, Providencia y Santa Catalina – CORALINA) (Santa Marta: Serie de Publicaciones Generales del INVEMAR No. 84), 72–85.
Dustan, P., and Lang, J. C. (2019). “Discovery bay, Jamaica” (6), in Mesophotic Coral Ecosystems, Coral Reefs of the World, eds Y. Loya, K. A. Puglise, and T. C. L. Bridge (Cham: Springer Nature Switzerland AG), 85–109. doi: 10.1007/978-3-319-92735-0_6
Gonzalez-Zapata, F. L., Bongaerts, P., Ramírez-Portilla, C., Adu-Oppong, B., Walljasper, G., Reyes, A., et al. (2018a). Holobiont diversity in a reef-building coral over its entire depth range in the mesophotic zone. Front. Mar. Sci. 5:29. doi: 10.3389/fmars.2018.00029
Gonzalez-Zapata, F. L., Gómez-Osorio, S., and Sánchez, J. A. (2018b). Conspicuous endolithic algal associations in a mesophotic reef-building coral. Coral Reefs 37, 705–709. doi: 10.1007/s00338-018-1695-9
Laverick, J. H., Piango, S., Andradi-Brown, D. A., Exton, D. A., Bogaerts, P., Bridge, T. C. L., et al. (2018). To what extent do mesophotic coral ecosystems and shallow reefs share species of conservation interest? A systematic review. Environ Evid. 7:15. doi: 10.1186/s13750-018-0127-1
Loya, Y., Puglise, K. A., and Bridge, T. C. L. (2019). Mesophotic Coral Ecosystems, Coral Reefs of the World. (Vol. 12). Cham: Springer Nature Switzerland AG. doi: 10.1007/978-3-319-92735-0
Opresko, D. M., and Sánchez, J. A. (2005). Caribbean shallow-water black corals (Cnidaria: Anthozoa: Antipatharia). Caribb. J. Sci. 41, 492–507.
Pomponi, S. A., Díaz, M. C., van Soest, R. W., Bell, L. J., Busutil, L., Gochfeld, D., et al. (2019). “Sponges” (32), in Mesophotic Coral Ecosystems, Coral Reefs of the World, eds Y. Loya, K. A. Puglise, and T. C. L. Bridge (Cham: Springer Nature Switzerland AG), 563–588. doi: 10.1007/978-3-319-92735-0_32
Pyle, R. L., Boland, R., Bolick, H., Bowen, B. W., Bradley, C. J., Kane, C., et al. (2016). A comprehensive investigation of mesophotic coral ecosystems in the Hawaiian Archipelago. Peer J. 4:e2475. doi: 10.7717/peerj.2475
Pyle, R. L., and Copus, J. M. (2019). “Mesophotic coral ecosystems: introduction and overview” (1), in Mesophotic Coral Ecosystems, Coral Reefs of the World, eds Y. Loya, K. A. Puglise, and T. C. L. Bridge (Cham: Springer Nature Switzerland AG), 3–27. doi: 10.1007/978-3-319-92735-0_1
Reyes, J., Santodomingo, N., and Flórez, P. (2010). Corales Escleractinios de Colombia. Santa Marta: Serie de Publicaciones Especiales del Invemar No. 14.
Rincón-Díaz, M. N., Gavio, B., Wynne, M. J., and Santos-Martínez, A. (2018). Notes on marine algae in the International biosphere reserve seaflower, Caribbean Colombia, VII: additions to the benthic flora of San Andrés Island. Caldasia 40, 97–111. doi: 10.15446/caldasia.v40n1.64597
Rincón-Díaz, M. N., and Ramos-Gallego, F. J. (2016). “Macroalgas marinas. El universo productivo de la Reserva de Biosfera Seaflower”, in Biodiversidad del mar de los siete colores, eds M. Vides, D. Alonso, E. Castro, and N. Bolaños (Instituto de Investigaciones Marinas y Costeras – INVEMAR y Corporación para el Desarrollo Sostenible del Archipiélago de San Andrés, Providencia y Santa Catalina - CORALINA. Serie de Publicaciones Generales del INVEMAR No. 84) (Santa Marta: INVEMAR), 40–55.
Sánchez, J. A., Dueñas, L. F., Rowley, S. J., González, F. L., Vergara, D. C., Montaño-Salazar, S. M., et al. (2019b). “Gorgonian Corals” (39), in Mesophotic Coral Ecosystems, Coral Reefs of the World, eds Y. Loya, K. A. Puglise, and T. C. L. Bridge (Cham: Springer Nature Switzerland AG), 727–745. doi: 10.1007/978-3-319-92735-0_39
Sánchez, J. A., González-Zapata, F. L., Dueñas, L. F., Andrade, J., Pico-Vargas, A. L., Vergara, D. C., et al. (2019a). Corals in the mesophotic zone (40–115 m) at the barrier reef complex from San Andrés Island (Southwestern Caribbean). Front. Mar. Sci. 6:536. doi: 10.3389/fmars.2019.00536
Sánchez, J. A., and Wirshing, H. (2005). A field key to the identification of tropical Western Atlantic zooxanthellate Octocorals (Octocorallia: Cnidaria). Caribb. J. Sci. 41, 508–522.
Schmitt, E. F., and Sullivan, K. M. (1996). Analysis of a volunteer method for collecting fish presence and abundance data in the Florida Keys. Bull. Mar. Sci. 59, 404–416.
Sinniger, F., Ballantine, D. L., Bejarano, I., Colin, P. L., Pochon, X., Pomponi, S. A., et al. (2016). “Biodiversity of mesophotic coral ecosystems,” in Mesophotic Coral Ecosystems – A Lifeboat for Coral Reefs? eds E. K. Baker, K. A. Puglise and P. T. Harris (Nairobi and Arenda: The United Nations Environment Programme and GRID-Arendal), 50–62.
Veron, J. E. N. (2000). Corals of the World, Vol. 1, 2, 3. Townsville, QLD: Australian Institute of Marine Science and CRR Qld Pty Ltd.
Veron, J. E. N., Stafford-Smith, M. G., Turak, E., and deVantier, L. M. (2016). Corals of the World. Available online at: http://www.coralsoftheworld.org/page/authors/ (accessed February 13, 2020).
Keywords: mesophotic coral ecosystems, Southwestern Caribbean MCEs, Colombian Caribbean, SeaFlower Biosphere Reserve, San Andrés Island, ecological units
Citation: Chasqui L, Mejía-Quintero K and González JD (2020) Biodiversity and Ecological Units of the Mesophotic Coral Ecosystems in San Andrés Island, SeaFlower Biosphere Reserve. Front. Mar. Sci. 7:559273. doi: 10.3389/fmars.2020.559273
Received: 05 May 2020; Accepted: 15 October 2020;
Published: 19 November 2020.
Edited by:
Luisa Fernanda Dueñas, National University of Colombia, ColombiaReviewed by:
Frine Cardone, University of Naples Federico II, ItalyLorenzo Angeletti, National Research Council (CNR), Italy
Copyright © 2020 Chasqui, Mejía-Quintero and González. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luis Chasqui, luis.chasqui@invemar.org.co; luischasqui@gmail.com
 Luis Chasqui
Luis Chasqui Katherine Mejía-Quintero
Katherine Mejía-Quintero Juan David González
Juan David González