Chemical Prevention and Control of the Green Tide and Fouling Organism Ulva: Key Chemicals, Mechanisms, and Applications
- Department of Marine Science, Ocean College, Zhejiang University, Zhoushan, China
The green algae, Ulva spp., have been causing environmental problems worldwide, e.g., green tides and biofoulings. Green tides resulted from bloom floating Ulva have caused substantial economic losses. Ulva foulings increase the maintenance cost of marine facilities and contribute to the biomass of floating algae. Chemical methods are generally very inexpensive and convenient for suppression of Ulva spp. during their early life stages, thus solving the green tide and fouling problem at the source. In this paper, classical chemical methods that have been or are in use and emerging chemical methods under research are systematically reviewed. The advantages, disadvantages, mechanisms, and applications of these methods are also summarized. Highly toxic reagents are used in classical chemical methods, including oxidants, acids, heavy metal compounds, and synthetic biocides directly used or applied in antifouling coatings to kill or inhibit Ulva effectively. However, these toxic reagents have a high risk of resulting in secondary environmental problems. In order to minimize other environmental impacts while solving the current problem, emerging, and environmentally friendly chemical methods have been developed, such as the utilization of degradable natural products (mainly allelochemicals) and semi-natural products for Ulva inhibition and fouling control, and the use of flocculating agents to prevent microscopic propagules from germinating. All these chemical methods provide a promising direction for the prevention and control of Ulva.
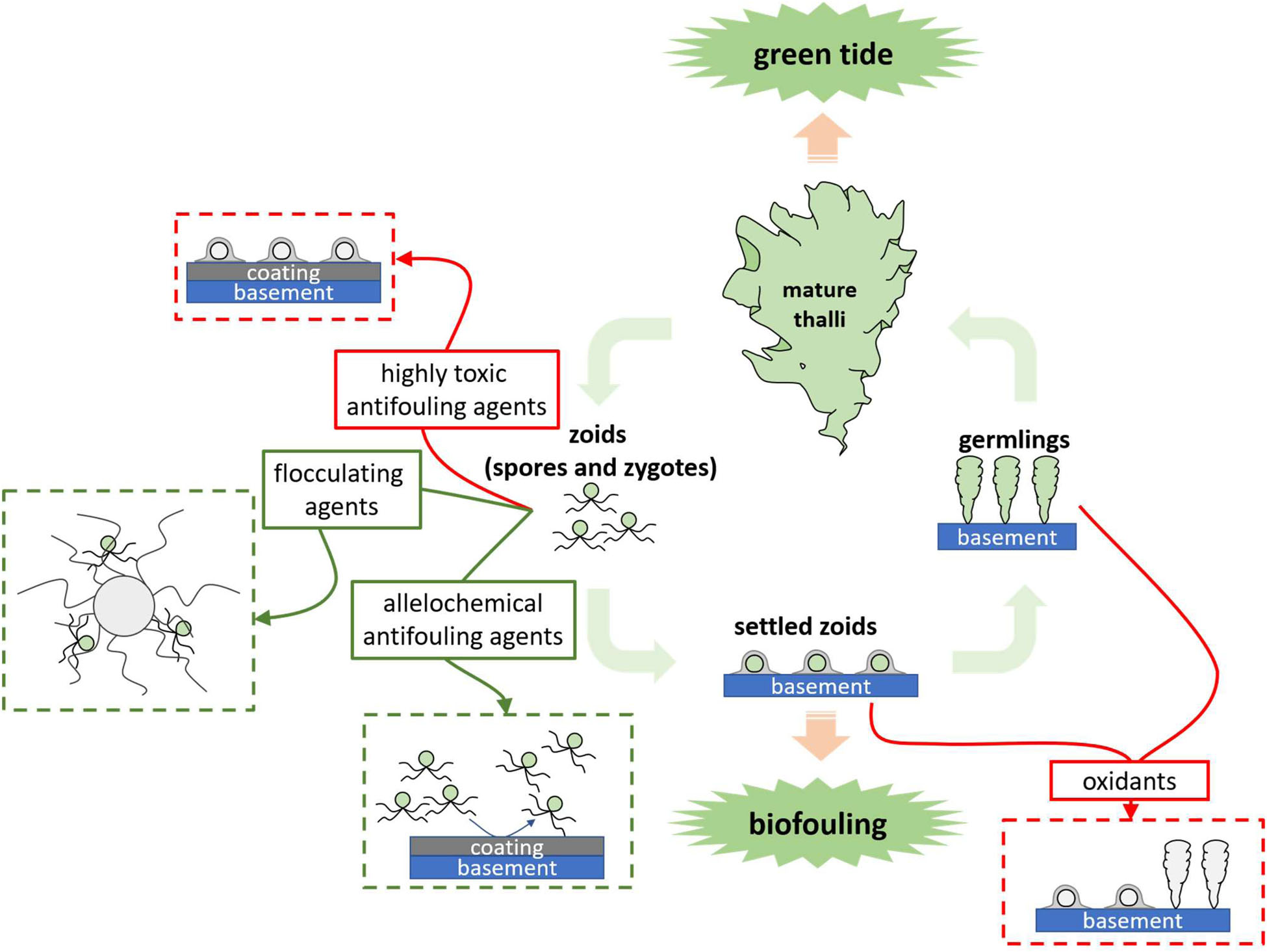
GRAPHICAL ABSTRACT. The problems caused by Ulva in different growth stages and the corresponding control methods.
Emerging Environmental Challenges Raised By Ulva
The Global Expanding Green Tides
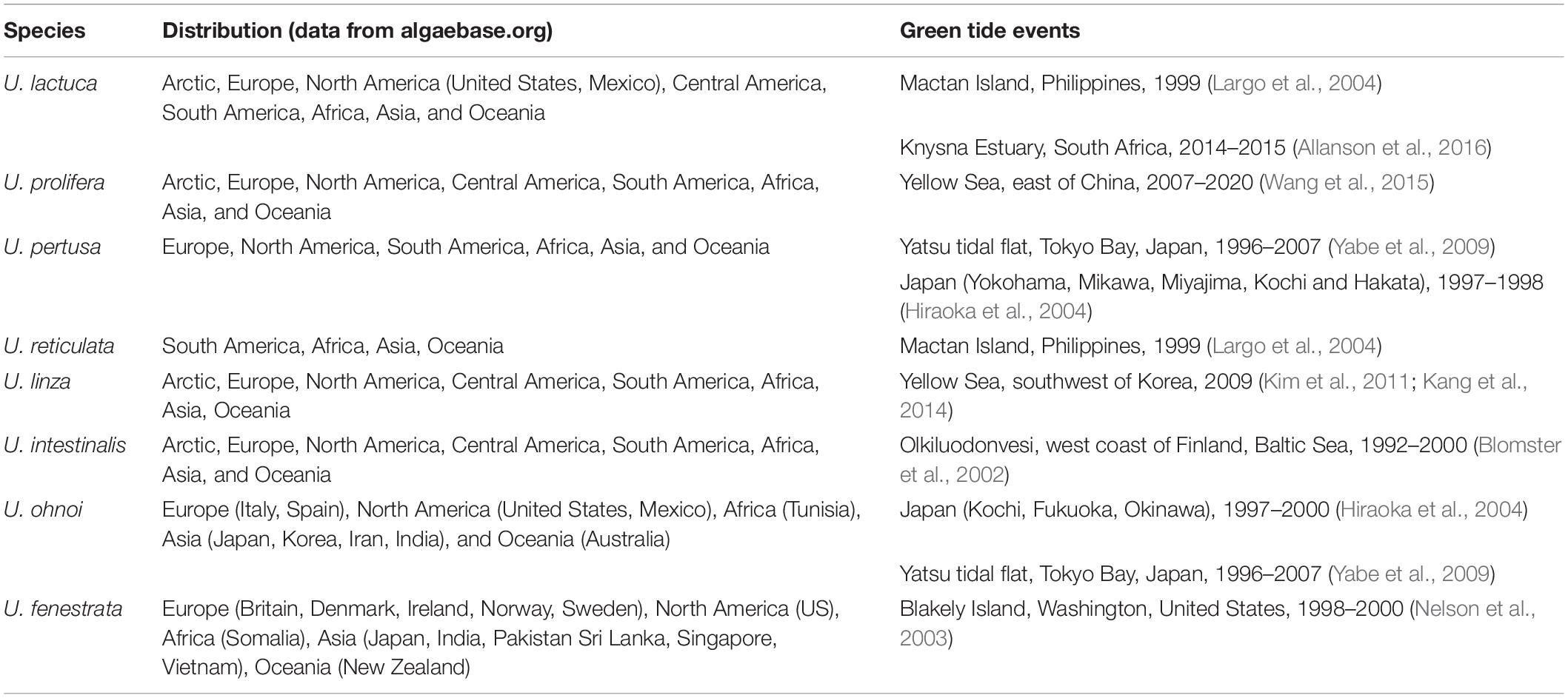
“Green tide” is a phenomenon of green macroalgae (Chlorophyta) blooming in water bodies such as rivers, lagoons, bays, estuaries, and coastal zones (Table 1). Green tides have occurred in a few locations since times immemorial; between 1960 and 1970, green tides have increased in extent and have finally become a global problem (Morand and Merceron, 2005). The most common green tide organisms are various Ulva species, which are distributed on almost all the continents (Fletcher, 1996; Ye et al., 2011; Table 1). Although green tide sometimes breaks out in non-eutrophic waters, it is generally believed that human activities contribute immensely to coastal waters’ enrichment, thereby exacerbating the outbreaks of green tides. Since 2007, green tides have occurred in the Yellow Sea during spring and summer every year. The largest event appeared in 2008 when it covered the Qingdao Olympic Sailing Center just before the match. The government spent about 2 billion CNY (∼300 million USD) to remove more than 100,000 tons of green algae, causing substantial economic losses (Ye et al., 2011). According to molecular biology identification, a kind of Chlorophyte, Ulva prolifera, is responsible for the Yellow Sea green tide (Zhao et al., 2013). The ongoing outbreak of U. prolifera has resulted from its various reproduction methods, including sexual, asexual, and vegetative reproduction (Hiraoka et al., 2003), as well as its rapid growth rate. It is generally considered that these recalcitrant green algae are mainly from Pyropia yezoensis (a kind of laver) aquaculture area. The bamboo poles and the ropes used as laver farming rafts are easily fouled by several green macroalgae (Chlorophyta), including U. prolifera (Song et al., 2018). During rafts withdrawing, a massive amount of green algae attached to the cables are scraped off, washed out into the sea, and become the primary source of floating green algae (Zhang et al., 2018). The floating green algae biomass keeps growing and reproducing and finally forms the green tide dominated by U. prolifera (Han et al., 2019).
The forms of Ulva spp. invisible to the naked eyes, called Ulva microscopic propagules (UMPs), include spores, gametes, zygotes, microscopic germlings, and vegetative thalli fragments. There is a tremendous amount of UMPs distribution in the seawater and sediments along the East China Sea coast (Liu et al., 2012; Song et al., 2015, 2018). The nature of UMPs helps these algae survive the winter; in spring, once the environmental factors such as temperature and light are suitable for growth, these “seed bank” of green tide start activities (Liu et al., 2012).
Fouling
Biofouling is when marine organisms get attached to solid surfaces, such as ship hulls. The problem of fouling caused by Ulva is quite irritating. Ulva is a major ship fouling organism all over the world (Callow, 1986). Fouling on the ship hull increases resistance, causing additional fuel consumption, energy waste and the maintenance costs. Callow et al. (1997) described the detailed settlement process of Ulva spore by high-resolution video microscopy. After being released, the Ulva spores use 2 or 4 flagella to rotate and search for attachments. The spores will first rotate rapidly on the attachment base’s surface like a gyro, meanwhile releasing a small amount of glycoprotein binder for pre-settlement. If the place is not suitable for attachment, the spore will leave only a small amount of adhesive and then swim away to try another base. Once a suitable base is found, the spore will release a large amount of adhesive, then retract the flagella quickly. The spore shape will become round, an amoeboid-like “spreading” and space-filling movement within a minute would be achieved. The cell wall formation will then begin, and within a few hours, germination into a germling will commence. Atomic Force Microscopy showed that the freshly released adhesive was very sticky, then became less sticky and more compressible, assuming a consistency similar to natural rubber (Callow and Callow, 2002).
Prevention and Control Approaches of Ulva
The intensification of the green tide phenomenon in the past few decades is believed to result from eutrophication. Reducing nutrients input is an effective way to mitigate green tides fundamentally, however, it is often in contradiction with regional economies (Ye et al., 2011) and may take up to several decades to reach the goal. There is an urgent need to develop prevention and control methods for Ulva. Presently, various approaches, like physical, biological, and chemical methods, have been developed (Table 2). Physical approaches focus on the treatment of green tides or biofoulings that have developed, while biological and chemical approaches are applicable to the control of the initial biomass of Ulva whereas mitigating the problems at the source.
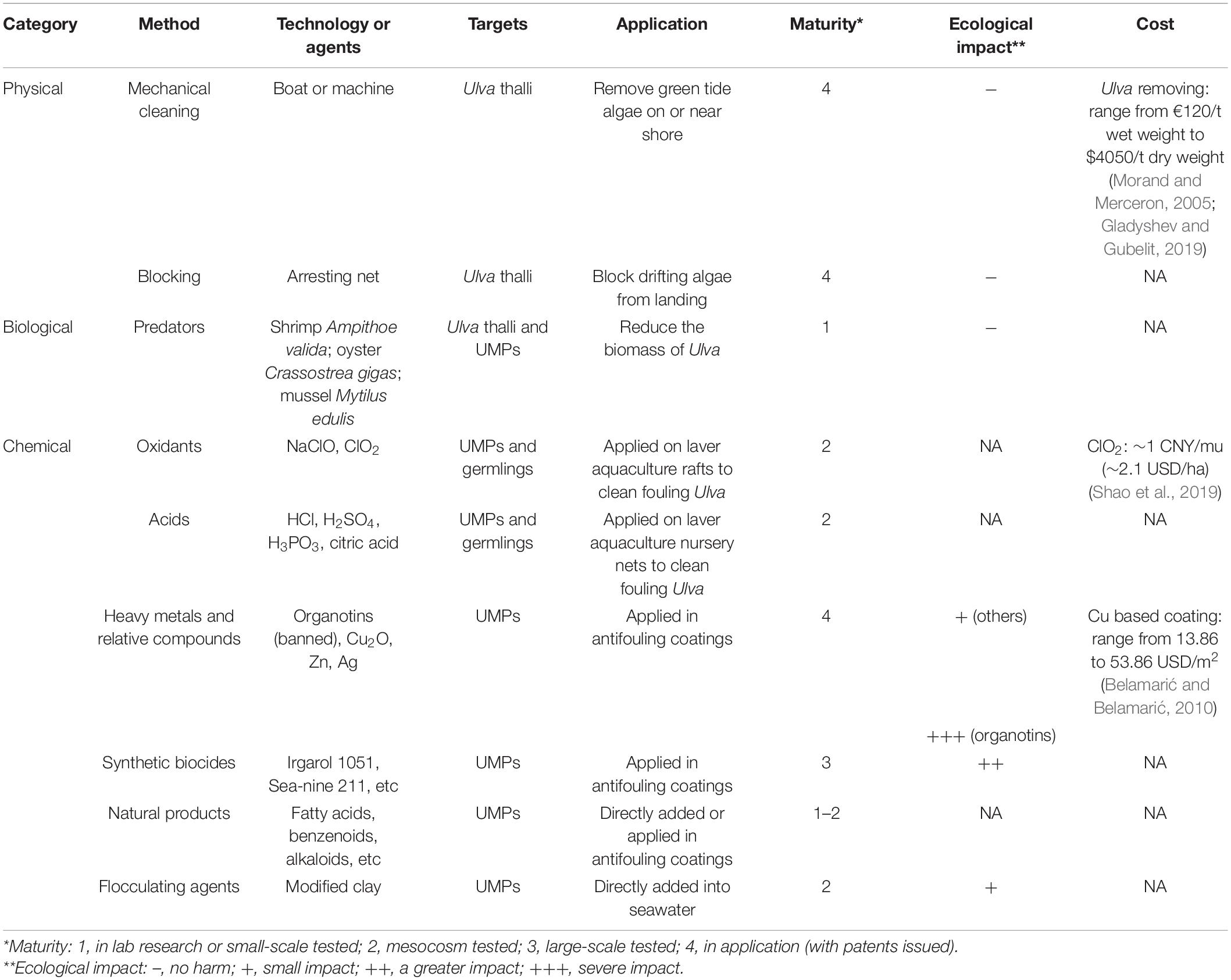
Table 2. Current methods developed for Ulva control, including the targets, application, technology maturity, ecological impact, and cost of these methods.
Physical Approaches
Physical methods have been used to clean the overwhelming Ulva thalli. Removing the floating macroalgae with boats or machines is the most common method (Fletcher, 1996). More recently, offshore intercepting nets have been set up in Qingdao to stop the Ulva from landing, according to the situation of floating green algae monitored by satellite remote sensing and unmanned aerial vehicles. The fouling Ulva can be cleaned by conventional machineries or hydraulic cleaning devices (Holm et al., 2003). Despite the damage to the ship hull when scraping fouling Ulva, these physical methods are natural and harmless. However, it cannot solve the problem from the source, that is, to reduce the settlement of Ulva spores on objects.
Biological Approaches
Limited information is available for the biological treatment in Ulva prevention, which is still in a relatively primary stage. For instance, the grazing effect of shrimp Ampithoe valida was found to reduce the biomass of U. lactuca (Zheng, 2008; Zheng et al., 2014). Oyster Crassostrea gigas and mussel Mytilus edulis could effectively filter out U. prolifera spores in both laboratory experiments and wild tests (Gao et al., 2018), both having the potential to be used in the early prevention of green tide.
Chemical Approaches
Classical Chemical Approaches
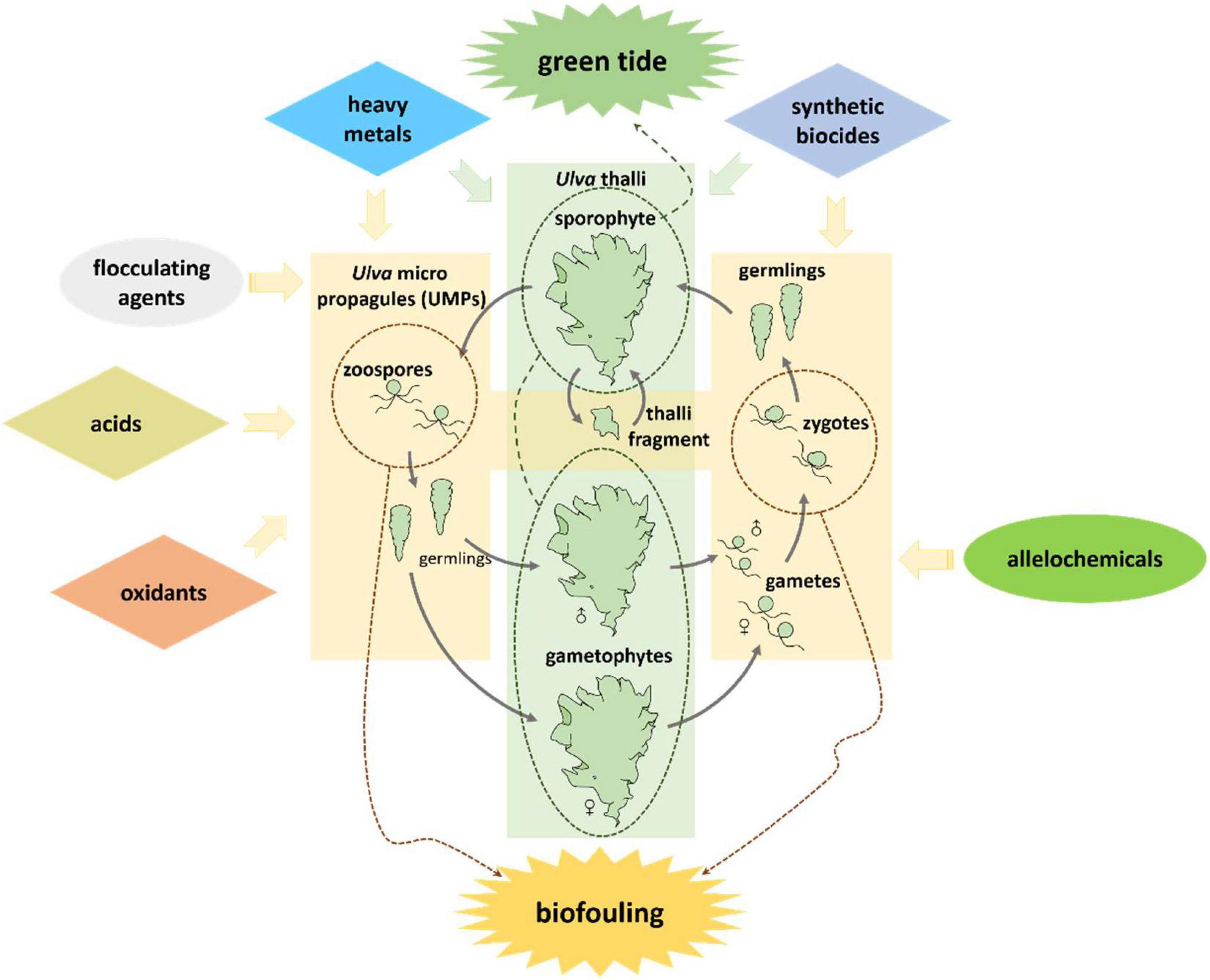
Classical chemical approaches use highly toxic reagents, such as oxidants, acids, heavy metal compounds and synthetic biocides to kill Ulva (Graphical Abstract and Figure 1). They can be added directly into Ulva threatened water bodies, applied on the Ulva fouled surfaces, or used as antifoulants in coatings to prevent the settlement of Ulva spores. Direct use of highly toxic chemicals can be very cheap and convenient; however, they may have substantial environmental issues. For instance, application of CuSO4 may contaminate water bodies with the heavy metal copper. While a classical chemical method is applied, it is necessary to fully consider both the concentration of reagents and the growth state of the target Ulva to minimize environmental pollution.
Emerging Chemical Methods
It is necessary to find solutions for balancing Ulva control and environmental protection. The use of easily degradable or low-toxic chemicals (Graphical Abstract and Figure 1) to replace the highly toxic ones for Ulva inhibition is a good idea. Natural products are metabolites of various organisms, including fatty acids, terpenoids, and alkaloids, etc (DerMarderosian and Beutler, 2002), and they are degradable in the environment. Some natural products act as allelochemicals and are effective on Ulva growth inhibition or fouling control. Another clean approach is using flocculant in precipitating the UMPs and prevent them from germinating (Sun, 2014; Li, 2017; Li et al., 2020).
Fouling-released coatings (FRCs) is a clean and effective antifouling method on vessels. This method utilizes the low surface energy of non-toxic synthetic materials, so that Ulva is not firmly attached and is easily washed down under the action of water flow (Youngblood et al., 2003; Ekin et al., 2007; Hu et al., 2009; Dimitriou et al., 2011). These emerging methods help reduce Ulva problems while minimizing additional environmental challenges.
Classical Chemical Prevention Using Oxidants, Acids, Heavy Metals and Synthetic Biocide
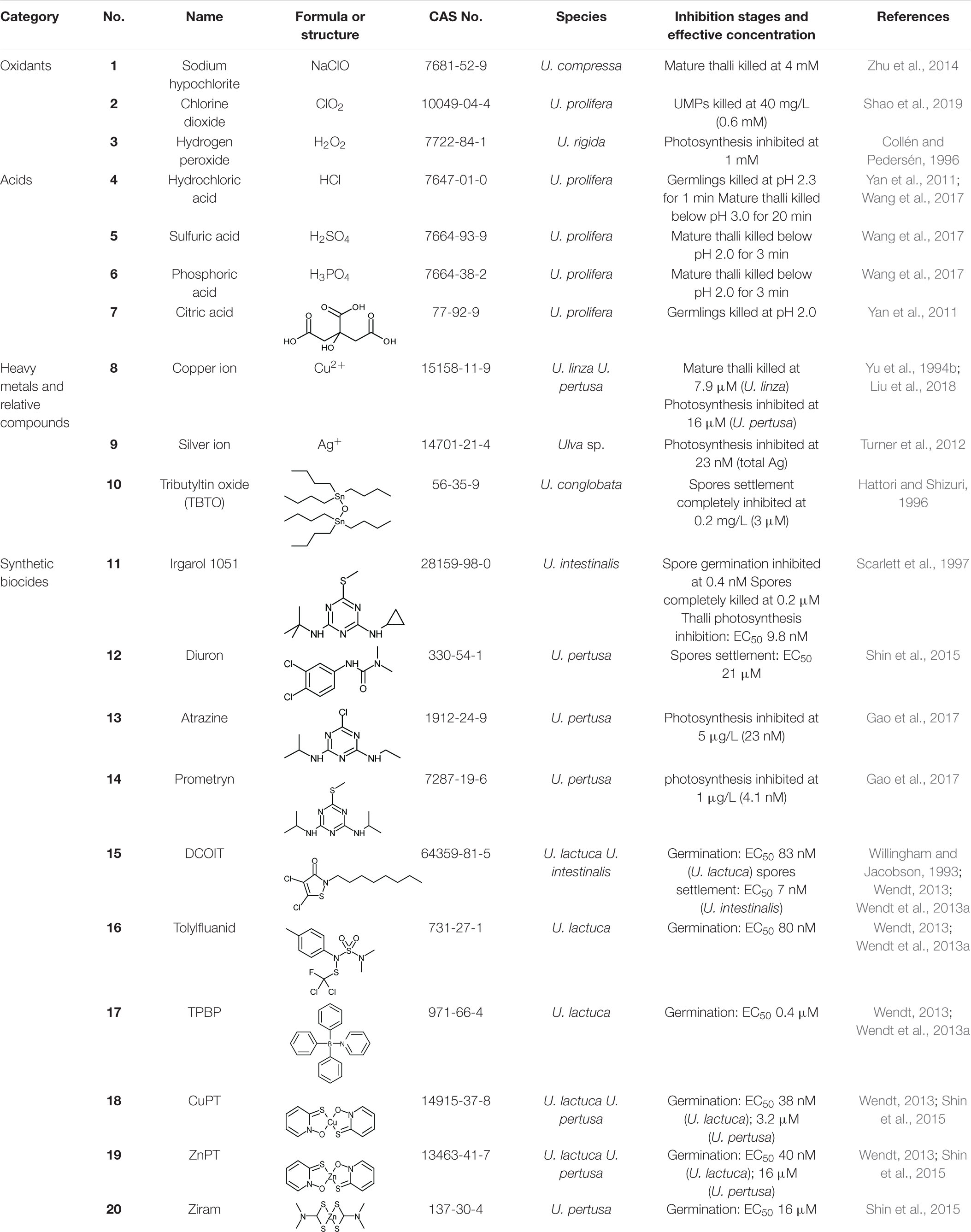
Using compounds with high toxicity to directly kill Ulva has the advantages of high efficiency, thorough removal effect, and are considered as a mature technique with low cost. However, highly toxic compounds tend to non-selectively impact all the organisms including keystone species, causing an imbalance in the ecosystem. Some compounds are difficult to be degraded and may cause continuous pollution in the water body. Commonly used chemicals include oxidants, acids, heavy metals and synthetic biocides (Table 3). Unless field tests are specifically mentioned, the experiments cited in the text were conducted under laboratory; the substrates on which spores settled were mostly glass and plastic.
Oxidants
Oxidants can disturb the normal redox state of cells, damage vital biomolecules including proteins, enzymes, lipids and DNA, destroy the physiological functions of cells, and cause cell damage or death (Drábková et al., 2007; Rezayian et al., 2019). Chlorine-based oxidants such as sodium hypochlorite (NaClO) (1) and chlorine dioxide (ClO2) (2), along with hydrogen peroxide (H2O2) (3) are commonly used as disinfectants due to their strong oxidation activity and broad-spectrum killing or inhibitory effect. They are widely used in sterilization and disinfection of hospitals, pools and homes, and their algicidal effects on Ulva have been tested.
Sodium hypochlorite was reported to kill U. compressa effectively at 4 mM, and suggested to be an ideal reagent for Ulva removal in laver aquaculture area (Zhu et al., 2014). However, a soybean-sized core of U. prolifera was found to remain green in high concentration NaClO solution, while the rest of thalli was bleached out and killed; this inferred a protection mechanism that sacrifices the periphery and preserves the core (Sun et al., 2008). A patent described the method of applying NaClO solution (acidified with citric acid just before use) to the laver culture rafts and ropes at low tide, to kill all fouling green algae including Ulva (Yu et al., 2012). Ministry of Natural Resources of the People’s Republic of China has incorporated the NaClO method into the green tide prevention work plan1. Another patent pointed out that the attached UMPs of U. prolifera on the cables of the laver culture rafts can be effectively killed, by smearing or spraying the solution of commercially available ClO2 effervescent tablets; the cost of ClO2 in this patent is as low as ∼2.1 USD/ha (Shao et al., 2019). H2O2 at 1 mM is enough to reduce the photosynthetic activity of U. rigida significantly (Collén and Pedersén, 1996). However, most of the studies on the effect of H2O2 on Ulva spp. are to investigate the oxidative stress response mechanism of algae (Collén and Pedersén, 1996; Lu et al., 2006), and application research report for Ulva control in field is lacking. All these three oxidants decompose rapidly and do not accumulate in the environment. Disinfection by-products (DBPs) of chlorine-based oxidants have attracted attention. The application of ClO2 in water bodies is more recommended, since the DBPs of NaClO contains carcinogen chloroform. H2O2 is generally considered as a very clean oxidant, as it only decomposes into water and oxygen without DBPs, but it is more expensive than chlorine-based oxidants.
Acids
Acids with a low pH value and a high concentration of hydrogen ions (H+) can damage various cells, such as destroying the structure of cell membranes or denaturing proteins. Therefore, a proper application of these acids can effectively kill UMPs and achieve the purpose of early prevention of Ulva that causes green tide or biofouling.
Hydrochloric acid (HCl) (4) is a strong acid widely used in industries and households. It has been reported that after treatment with dilute HCl with a pH of 2.3 for 1 min, all germlings of U. prolifera died after a period of time, while most germlings of laver Porphyra haitanensis was not affected (Yan et al., 2011). Another common organic acid, citric acid, showed the same algicidal effect against U. prolifera at pH 2.0 for 1 min, without impairing P. haitanensis (Yan et al., 2011). This method to remove the fouling green algae on laver aquaculture nursery nets has been patented (Luo et al., 2010; Wang et al., 2017). Nevertheless, the acids cannot be directly applied to seawater (otherwise it will greatly damage the environment), so the nets need to be collected on the shore or a dedicated boat (Luo et al., 2010) for acid treatment, consuming plenty of labor and resources. Besides, this method cannot prevent the nets from subsequent fouling in seawater. The high volatility of HCl is also easy to impair the health of workers. According to the published patents (Luo et al., 2010; Wang et al., 2017), non-volatile acids such as sulfuric acid (H2SO4) (5), phosphoric acid (H3PO4) (6) and citric acid (7) can also be applied in the same way. These non-volatile acids are more convenient in application than HCl.
Heavy Metals and Relative Compounds
Ulva Control by Direct Toxic Effects of Heavy Metal Ions
Many heavy metal ions can cause protein denaturation or cause cellular oxidative stress (Pinto et al., 2003; Kumar et al., 2010) and are therefore toxic to most organisms, and some of them are commonly used as effective algaecides or antifouling agents.
Copper (Cu) is the most widely used heavy metal in marine antifouling applications. At present, about 80% of antifouling coatings use cuprous oxide (Cu2O) as the main antifouling agent (Wu et al., 2014). Copper ions (Cu2+) (8) can combine with sulfur-containing biomolecules such as proteins in cells, destroy their physiological activity, and poison fouling organisms. Cu2+ can also replace Mg2+ in chlorophyll, thereby inhibiting the photosynthesis of algae (Küpper et al., 2003). It has been reported that Cu2+ could inhibit phosphorus uptake of U. lactuca (Huang et al., 2002) and could subject cells to oxidative stress (Wu, 2009). Copper salts, represented by copper sulfate (CuSO4), are often used as algaecides in various water bodies. Although copper at high concentrations is toxic to most organisms, it is one of the essential trace elements of organisms with a background concentration of about 0.25 μg/L (3.9 nM) in seawater (Blossom, 2007). The copper released by the antifouling agent of ocean-going ships will be quickly diluted in seawater, and the environmental impact can be ignored. However, when the ship is sailing near the coast or staying in the harbor, the released copper is likely to accumulate in these areas, causing some ecological issues (Schiff et al., 2004). Copper-based antifouling agents may not be very effective on Ulva. A report focusing on traditional antifouling agents pointed out that U. linza was commonly found attached on some vessels using Cu2O-based antifouling paints (Callow, 1986). The tolerance of different species and growth states of Ulva to copper ions varied greatly. Yu et al. (1994a) exposed the mature U. linza thalli to CuSO4 solution to obtain its toxic effect; the threshold of Cu2+ was 1.6 μM, and the thalli died soon at 7.9 μM. But according to Sun et al. (2008), mature U. prolifera thalli partially survived even though CuSO4 reached a supersaturated concentration (640 μM). Liu et al. (2018) found that Cu2+ significantly inhibited the photosynthesis of mature U. pertusa thalli at 16 μM.
Silver ion (Ag+) (9) is quite toxic to most biofoulers including Ulva. Ag+ or nanosilver powder has been applied in some laboratory and field antifouling researches (Li et al., 2013; Wu et al., 2014). Total Ag concentration above 23 nM can reduce the photosynthetic efficiency of Ulva thalli (Turner et al., 2012), but Ag+ will combine with Cl– to form a variety of complexes, resulting in free Ag+ accounting for only 0.002% of total Ag (Turner et al., 2012). Silver itself is also a precious metal, therefore it may not be economical to be used on marine algal control. The toxicity of nanosilver powder to marine algae is also due to the released free Ag+, thus nanosilver particles fixed on coatings will also be consumed.
Ulva Control by Self-Polishing Property of Heavy Metal Compounds
Organotins (organic compounds containing Sn) had been used as effective antifouling agents, especially tributyltin (TBT) series compounds represented by tributyltin oxide (TBTO) (10). Polyacrylic resin coatings containing TBT continuously hydrolyze in seawater, revealing a new smooth surface and maintaining a low navigation resistance while releasing TBT. This kind of coatings are named self-polishing coatings (SPCs). TBT does not only enhance the hydrolysis of resin, and it is a highly toxic and highly effective antifoulant killing almost all fouling organisms after release. TBTO has a strong inhibition effect both on settlement and germination of Ulva spores. At a concentration as low as 3 μM, the settlement of U. conglobata spores were completely inhibited (Hattori and Shizuri, 1996). However, TBT is too toxic to non-target organisms, causing sexual aberrations in some shellfish (Jha et al., 2000; Li et al., 2001) and vertebrate immunosuppression (De Vries et al., 1991; Kannan et al., 1998). The International Maritime Organization (IMO) prohibited organotins antifouling paints before 2008 (Bray and Langston, 2006).
Nowadays, zinc (Zn) is also commonly used in the production of SPCs, like zinc acrylate, a replacement of banned organotin SPC (Yonehara et al., 2001). In this kind of coatings, the antifouling agent is not zinc ion itself, but Cu2O or other low-toxic chemicals released in the control of its self-polishing property. To the best of our knowledge, the antifouling effect of zinc itself on Ulva is not clear. A study reported that there are still quite a few Ulva attached to a paint containing zinc powder (Jelic-Mrcelic et al., 2006).
Synthetic Biocides
Since the ban of organotin paints on small boats (<25 m) in the 1980s (Voulvoulis et al., 1999; Tolhurst et al., 2007), compromised alternative antifouling methods have been developed. Synthetic herbicides and algaecides with photosystem II inhibition capability were introduced into copper-based antifouling paints as “booster biocides,” to enhance the toxic effect on fouling macroalgae (especially the copper-tolerant Ulva). Irgarol 1051 (11) and diuron (12) are two of the most important synthetic booster biocides in antifouling applications (Chambers et al., 2006; Konstantinou, 2006; Table 3). The triazine biocide, Irgarol 1051, has a powerful inhibitory effect on Ulva spores. It was reported that Irgarol 1051 killed all U. intestinalis spores at 0.2 μM and significantly inhibited photosynthesis of mature thalli at 0.02 μM (Scarlett et al., 1997). Two other triazine herbicides with similar structures (not registered as booster biocides yet), atrazine (13), and prometryn (14), significantly depressed the photosynthesis efficiency of U. pertusa (Gao et al., 2017). Diuron was proven effective on settlement inhibition on U. pertusa spores, with EC50 of 21 μM (Shin et al., 2015). Triazine herbicides and diuron are difficult to degrade in seawater and sediment (Voulvoulis et al., 1999; Thomas and Brooks, 2010), thus they can be accumulated by marine organisms such as sea cucumbers (Tian et al., 2013; Ren et al., 2014), and eventually threaten human health. Sediments contaminated with antifouling paint particles containing Irgarol 1051 can also inhibit the growth of Ulva when resuspended (Tolhurst et al., 2007). The long-term use of Irgarol 1051 has caused selection pressure on Ulva in some areas, leading to the emergence of Irgarol-resistant Ulva. It was reported that spores of U. lactuca on the west coast of Sweden were not affected by Irgarol 1051 up to 2 μM (Wendt et al., 2013b).
With the awareness of the environmental problems caused by persistent organic pollutants (POPs), the use of these refractory biocides has gradually been reduced in various regions. All triazine herbicides have been banned in the EU, and Irgarol 1051 and diuron have also been banned on small boats in the United Kingdom (Cresswell et al., 2006; Konstantinou, 2006; Tolhurst et al., 2007). A few kinds of easily degradable booster biocides have been introduced (Table 3) as compromises of biofouling control and pollution reduction, before the large-scale applications of “zero-pollution” methods are developed.
DCOIT (4,5-dichloro-2-n-octyl-3(2H)-isothiazolinone, trade name Sea-Nine 211 or Kathon 930) (15), is an isothiazolinone broad-spectrum biocide, which strongly inhibits and kills bacteria, fungi, and algae. Tolylfluanid (16) was initially used as an agricultural fungicide, but can also be used as a booster biocide in antifouling coatings (Thomas and Brooks, 2010). DCOIT and tolylfluanid have more significant germination inhibition effects on U. lactuca spores (EC50 83 and 80 nM, respectively) than Cu2+ (EC50 2 μM) (Wendt, 2013; Wendt et al., 2013a). Another research indicated that DCOIT inhibited U. intestinalis spore settlement with a very low EC50 value of 7 nM (Willingham and Jacobson, 1993). DCOIT has a short half-life between < 1 d and 13 d in the marine environment (Chen and Lam, 2017) and tolylfluanid degrade within 2 weeks in seawater (Lee et al., 2020), but they are highly toxic to most non-target organisms. Therefore, it is necessary to limit their concentration in seawater.
Another booster biocide, triphenylborane pyridine (TPBP) (17), can be photolyzed or hydrolyzed in the environment (Thomas and Brooks, 2010). However, as an antifouling agent to Ulva, a higher concentration is needed (EC50 400 nM, U. lactuca germination inhibition); otherwise the germination will be stimulated in low concentration (∼100 nM) (Wendt, 2013; Wendt et al., 2013a). Therefore, TPBP may not be a good choice for Ulva control.
Two metal complexes, copper pyrithione (CuPT) (18) and zinc pyrithione (ZnPT) (19), are both easily-degradable booster biocides, with a half-life of a few hours in seawater by photodegradation (Konstantinou, 2006). According to the thesis of Wendt (2013), the EC50 values to U. lactuca germination inhibition were 38 and 47 nM, respectively, which were both more effective than DCOIT, tolylfluanid and TPBP mentioned above. Shin et al. (2015) reported the inhibition effects of CuPT and ZnPT on the spore motility and germination of U. pertusa. Another zinc complex fungicide, zinc dimethyldithiocarbamate (ziram) (20), has an inhibitory effect on the germination of U. pertusa spores (Shin et al., 2015), with the half-life less than 1 day (Konstantinou, 2006). It should be noted that the abuse of these heavy metal-containing compounds may exacerbate heavy metal pollution in the estuary and coastal zone.
Ulva Control by Natural Products (Allelochemicals) and Derivatives
Many organisms, including plants, animals, algae and microbes, can produce various compounds to resist biological stress from other species, or help them in niche occupation. These compounds are often called allelochemicals. Marine macroalgae such as Ishige okamurae (Sidharthan et al., 2004) or bacteria like Pseudomonas sp. (Burgess et al., 2003) have been shown to produce antifouling allelochemicals to prevent the settlement of Ulva spores.
Compared with the highly toxic reagents used in traditional chemical methods, allelochemicals are more easily degraded in the environment and have better biological selectivity. However, many allelochemicals have acute toxicity to target or non-target organisms. Qian et al. (2009) suggested that a safe natural antifouling agent should have a therapeutic index [the ratio of 50% lethal concentration (LC50) to 50% effective concentration (EC50)] greater than 50 and an EC50 less than 5 mg/L, but one with relatively high therapeutic index (means relatively more toxic) could also be considered if it is highly degradable in the environment.
Here, natural allelochemicals and some artificial derivatives with Ulva-inhibiting activity with literature records are summarized in Table 4. It should be noted that different species or strains of Ulva have different stress resistance, therefore the EC50 value of these natural products obtained from different laboratories cannot be compared.
Fatty Acids and Relative Compounds
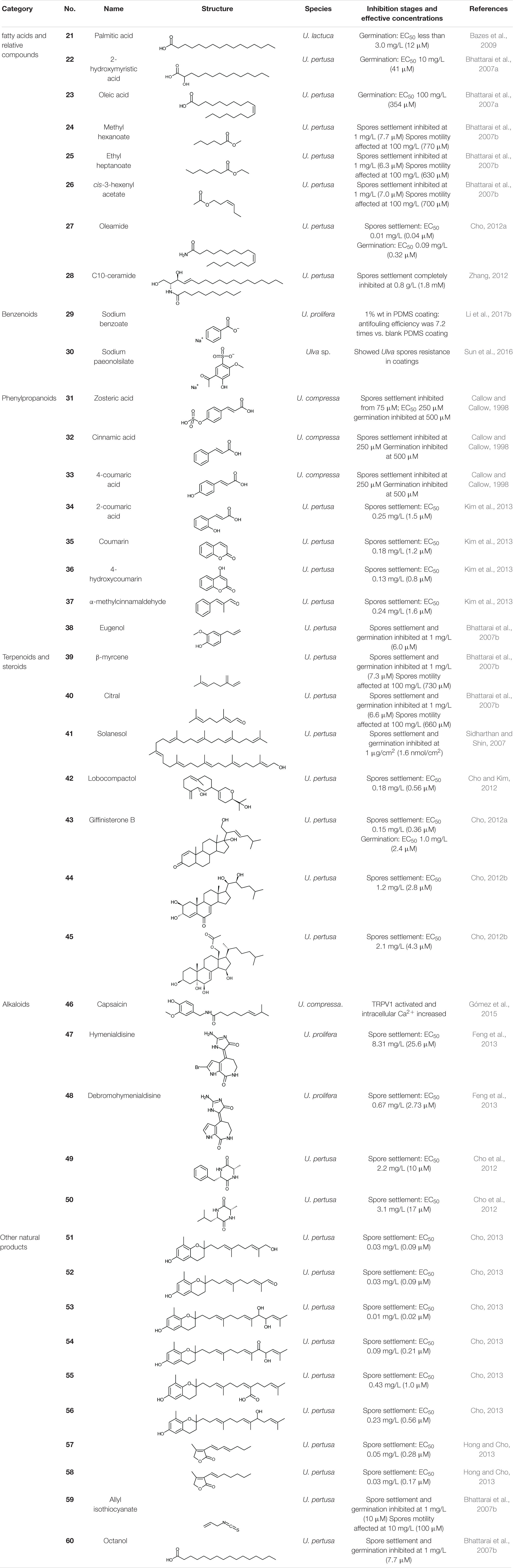
Fatty acids (FAs) are important primary metabolites of organisms and are generally considered as energy storage substances in cells. Palmitic acid, or hexadecanoic acid (21), is the most common saturated FA found in animals, plants and microorganisms. It has a relatively strong inhibition capacity on the germination of U. lactuca spores, with a low EC50 of less than 12 μM (Bazes et al., 2009). Two long-chain fatty acids isolated from the marine bacterium Shewanella oneidensis, 2-hydroxymyristic acid (22), and oleic acid (23), completely inhibited the germination of U. pertusa spores at 41 and 354 μM, respectively (Bhattarai et al., 2007a). The treated plastic plates with coatings containing each of the two FAs (10% wt in dry weight) exhibited excellent antifouling performances in the field test, free from biofouling in 1.5 years, while the untreated controls were covered by various fouling organisms including U. pertusa (Bhattarai et al., 2007a).
Three FA esters, methyl hexanoate (24), ethyl heptanoate (25), and cis-3-hexenyl acetate (26) all inhibited U. pertusa spores according to Bhattarai et al. (2007b), and they all exhibited good antifouling performances in the field test, without any visible fouling organisms attached during 1 year. The long-chain, colorless waxy solid amide, oleamide (27), was also found to be an Ulva inhibition agent, which inhibited U. pertusa spores settlement (EC50 0.01 mg/L, 0.04 μM) and germination (EC50 0.09 mg/L, 0.32 μM) (Cho, 2012a).
Ceramides are a family of waxy lipid molecules commonly found in eukaryotic cell membrane, making up sphingomyelins. Apart from supporting structural molecules, ceramides participate in various cellular signaling pathways, including regulating differentiation, proliferation, and programmed cell death. C10-ceramide (28), extracted from konjac (Amorphophallus konjac), completely inhibited the settlement of U. pertusa at 1.8 mM (Zhang, 2012).
Benzenoids
Benzoic acid, naturally found in gum benzoin from Styrax, is one of the most widely used food preservatives as sodium benzoate (29) form, although industrially used benzoic acid comes from artificial synthesis. Li et al. (2017b) applied polydimethylsiloxane (PDMS) coating with sodium benzoate to nylon ropes; after 24 h, only 3.1% of U. prolifera spores settled and germinated, showing an antifouling efficiency of 7.2 times vs. blank PDMS control.
Paeonol is mainly derived from the root bark of paeony (Paeonia suffruticosa). The artificial sulfonated derivative, sodium paeonolsilate (30), is a semi-natural product and mainly used in medicine. In a study, sodium paeonolsilate was embedded in Zn2Al layered double hydroxides, then dispersed in resin to form controlled release antifouling coating, which effectively reduced the density of attached Ulva spores (Sun et al., 2016).
Phenylpropanoids
The phenylpropanoids are a diverse family of organic compounds synthesized by plants from phenylalanine and tyrosine, with a basic structure of an aromatic phenyl group and a C3 side chain. The phenylpropanoids that have been documented in the literature and have a clear inhibitory effect on Ulva are shown below.
Zosteric acid (31), extracted from the seagrass eelgrass (Zostera marina), is the sulfate ester of 4-coumaric acid. According to Callow and Callow (1998), zosteric acid inhibited the settlement of U. compressa spores from a concentration of 75 μM, surpassing cinnamic acid (32) and 4-coumaric acid (33), which are very similar in structures. Interestingly, zosteric acid did not affect the motility of spores under 250 μM, which indicates that the antifouling activity of zosteric acid at low concentrations is not due to the toxicity to the spores. The authors speculated that the antifouling effect of zosteric acid was due to the combination with the adhesive secreted by the spores, or the attachment to the surface of the glass substrate. The strong hydrophilicity of the sulfuric acid group prevented or reduced the exclusion of the water between the adhesive and the substratum, making it difficult to form the adhesive-substratum interface (Callow and Callow, 1998). In the same year, Shin (1998) reported the antifouling effect of zosteric acid on U. lactuca (formerly known as U. fasciata) spores, which surpassed that of Cu2+ at the same molar concentration. Zosteric acid can be obtained by microbial fermentation (Jendresen and Nielsen, 2019), and this technology will significantly reduce the acquisition cost of zosteric acid.
Besides, 2-coumaric acid (34), coumarin (35), 4-hydroxycoumarin (36), and α-methylcinnamaldehyde (37) from the cinnamon tree (Cinnamomum loureiroi) have an excellent antifouling activity against Ulva, with fairly low EC50 values of 0.8–1.6 μM on U. pertusa spore settlement inhibition (Kim et al., 2013). Eugenol (38), a kind of fragrance substances in many plants, was also found as an antifouling agent against Ulva, inhibited both the settlement and germination of U. pertusa spores (Bhattarai et al., 2007b; Sidharthan and Shin, 2007).
Terpenoids and Steroids
Terpenoids (including terpenes) are biosynthesized by various organisms, especially plants, via mevalonate pathway. Terpenoids have multiple biochemical functions. Some of them have a broad-spectrum antibacterial effect, and some terpenoids act as allelochemicals against other plants (Peng et al., 2002). Studies have found that several terpenoids can significantly inhibit the settlement and germination of Ulva spores, indicating an excellent antifouling effect against Ulva. For instances, the common monoterpene β-myrcene (39) from bay leaf (Laurus nobilis), the monoterpenoid aldehyde citral (lemonal) (40) from lemon (Citrus limon), and the long-chain terpenoid alcohol solanesol (41) from solanaceous plants such as tobacco, potato, and tomato are all antifouling to U. pertusa (Bhattarai et al., 2007b; Sidharthan and Shin, 2007). A novel diterpenoid alcohol produced by a marine actinomycete Streptomyces cinnabarinus, lobocompactol (42), effectively inhibited the germination of U. pertusa spores with a very low EC50 of 0.56 μM (Cho and Kim, 2012).
Steroids are produced by the cyclization of a special triterpene, squalene, followed by a series of biochemical reactions. Three steroids, giffinisterone B (43) and two unnamed compounds (44, 45) extracted from an epiphyte bacterium Leucothrix mucor on red alga, exhibited antifouling effects against U. pertusa spores (Cho, 2012a; b). Giffinisterone B also inhibited germination with an EC50 of 2.4 μM, but the germination inhibition capacity of the two unnamed compounds 44 and 45 were not tested.
Alkaloids
Capsaicin (46) is the main pungent chemical in chili that feels hot and spicy. Capsaicin can repel most fouling organisms and is a broad-spectrum natural antifouling agent (Shi and Wang, 2006). A patent of antifouling coating containing capsaicin was proposed by Watts Water Technologies Inc (Watts, 1995). Capsaicin is degradable in the environment and has low ecological risk (Wang et al., 2014). As to U. compressa, the intracellular calcium level significantly increased while the thalli were exposed to capsaicin (Gómez et al., 2015). According to Thompson et al. (2007), during the settling process of Ulva spores, the cytosolic Ca2+ increased to twice the initial value over a period of time, and then recovered to the initial value. This explains the process of Ulva spore settlement related to cytosolic Ca2+. Besides, the increase of intracellular Ca2+ caused the unicellular green alga Chlamydomonas reinhardtii to lose flagella (deflagellation), loss of exercise ability and other cell function disorders, and the same inhibition effect was confirmed among five species in Chlorophyta (Aiyar et al., 2017). We speculate that the antifouling mechanism of capsaicin on Ulva may be due to the rapid increase of intracellular Ca2+ as the result of the activation of TRPV1 by capsaicin, making the spores hard to settle, or other functional disorders. However, there is no quantitative antifouling research report on capsaicin for Ulva. Natural capsaicin is very expensive, but at present artificial capsaicin and its homologs (capsaicinoids) have become a reality (Peng et al., 2011), which will significantly reduce the cost of using capsaicin as an Ulva inhibitor.
Hymenialdisine (47) and its debrominated derivative debromohymenialdisine (48), two alkaloids extracted from sponges Axinella sp., were confirmed antifouling against U. prolifera spore settlement with EC50 values of 25.6 and 2.73 μM, respectively (Feng et al., 2013), and they are synthesizable (Xu et al., 1997). Two diketopiperazines (DKPs) (49, 50) from a marine bacterium Streptomyces praecox were quite effective in inhibiting the settlement of U. pertusa spores, with EC50 values of 10 and 17 μM, and therapeutic indexes of 18 and 21, respectively (Cho et al., 2012).
Other Natural Products
Six chromanols (51–56) with long side-chain were extracted from the brown alga Sargassum horneri, and they were very effective on the inhibition of U. pertusa spore settlement, with astonishing low EC50 values of 0.02–1.0 μM; the chromanol 53 was the most effective among them (Cho, 2013). Two furanone derivatives (57, 58) were isolated from a seaweed epibiotic bacterium Streptomyces violaceoruber (Hong and Cho, 2013). They both exhibited a strong antifouling performance against U. pertusa spores settlement, with supremely low EC50 values of 0.28 and 0.17 μM, and very high therapeutic indexes of 94 and 140, respectively (Hong and Cho, 2013). Allyl isothiocyanate (59) is the source of the irritating odor of mustard, radish, horseradish and other plants. At a concentration of 1 mM, allyl isothiocyanate affected the motility of U. pertusa spores, and showed a fair antifouling capacity (Bhattarai et al., 2007b) against the spores settlement; it also showed antifouling activity at 10 μM. It was reported that octanol (60) could inhibit U. pertusa spores settlement and germination with a considerable performance at 7.7 μM (Bhattarai et al., 2007b).
Some biomacromolecules such as peptides, proteins, and enzymes have definite antifouling potential against Ulva. However, due to their large molecular weight, their chemical compositions are still not clear. The germination of U. lactuca was reported to be inhibited by an extracellular component of a marine bacterium, Pseudoalteromonas tunicata; the anti-algal component was heat-sensitive, polar and had a molar mass between 3 and 10 kDa, remaining unknown in composition (Egan et al., 2001). Similarly, the extracellular component of another marine bacterium, Alteromonas sp., inhibited the settlement and germination of U. lactuca spores; the active component was filtered to be protein or peptide, with a molecular size ≥ 3500 kDa (Silva-Aciares and Riquelme, 2008).
The adhesive that Ulva spores secrete is a kind of glycoprotein, which can be hydrolyzed by enzymes, such as serine protease, to reduce the adhesion of the spores (Christie et al., 1970; Pettitt et al., 2004). Since enzymes are very expensive and could be easily inactivated in seawater, this method is limited to laboratory research by now, and the application needs to be further optimized.
Chemical Modified Flocculating Agents
Flocculating agents, such as clays have been used for decades to control harmful microalgae (Kojima, 1961). Natural clays (kaolin, montmorillonite, etc.), are mainly composed of various aluminosilicates. Due to their physical and chemical properties, they form colloidal particles after being sprinkled in water bodies, adsorb microalgal cells and then sink to the bottom, enabling the algal blooms to be quickly cleaned. However, the surface of natural clay colloidal particles and microalgae cells are generally negatively charged, which causes electrostatic repulsion resulting in low adsorption efficiency of natural clay to algal cells; if a chemical modifier is added to the clay to make the surface of the colloidal particles positively charged, the electrostatic effect between the colloidal particles and the cells can be changed from repulsion to attraction, which can greatly improve the adsorption efficiency (Yu et al., 1994b). There have been several successful applications in harmful microalgal bloom control using modified clay (Li, 2017). The zoids (spores and zygotes) of Ulva have a similar size to common microalgae in the range of a few to dozen micrometers (Callow et al., 1997), and also negatively charged, therefore can also be effectively adsorbed and cleaned with modified clay (Li et al., 2015).
Polyaluminum chloride (PAC), with a general formula AlnCl(3n–m)(OH)m, is a commonly used clay modifier. In addition to reversing the surface charge of the clay to positive, its long-chain structure can improve the van der Waals interactions to Ulva zoids (Sun, 2014; Li et al., 2015; Li, 2017). Only 0.5 g/L of PAC modified kaolinite can inactivate almost all UMPs in seawater, indicated by a shipboard experiment using the seawater from U. prolifera affected sea area (Li, 2017; Li et al., 2017a). Kaolinite modified with aluminum sulfate [Al2(SO4)3] has a higher effect on UMPs cleaning, and this may be due to the hydrolysis of aluminum ions to form a large amount of amorphous aluminum hydroxide, encasing the UMPs to flocs, removing them from the water body with the precipitation of clay particles (Zhang et al., 2016).
After precipitation with modified clay particles, the growth of UMPs was halted even when they were resuspended (Li et al., 2015, 2017a). The mechanisms of the inhibition effect are not yet fully understood. The possible mechanisms are as follows: (1) The “shading” effect of clay reduces the light intensity received by algal cells (Li et al., 2017a), though, even at a lower light intensity of 32 μmol m–2 s–1, UMPs could still slowly grow (Liu et al., 2012). (2) Damages inside the cells of UMPs. A single-cell model species of Chlorophyta, Chlorella vulgaris, showed oxidative stress response as the up-regulation of relative enzymes when exposed to natural or PAC modified kaolinite; it might indicate disruption to the metabolic balance of the algae, resulting in an accumulation of superoxide free radicals (O2–⋅) (Liu et al., 2016). Whether UMPs have similar cell damage remains to be further studied. (3) Modified clay colloids have a strong adsorption effect on phosphate, which may cause the phosphate in seawater to diffuse into the flocculated UMPs, which deprives it of nutrition and halts germination.
At present, the method of using modified clay to control green tide algae is still in the stage of mesocosm experiments (Li et al., 2020). If this method is to be applied in the prevention and control of green tides, the following issues need to be fully considered: (1) The negative effects of clay minerals on non-target organisms. It was reported that modified clays could affect the filter-feeding behavior of some shellfish even under an Ulva-suppression dosage (Zhang, 2018). (2) Safety issues of modifiers. Aluminum, a metal element that can cause dementia, can be accumulated in some marine organisms, such as shrimps (Sun et al., 2000). (3) The proactive treatment of green tides. For the treatment of microalgae blooms, the modified clay is usually sprayed into the water body to quickly eliminate them after the blooming (Li, 2017). Whereas modified clay application can only be useful before green tides occur, otherwise it will be ineffective to the mature Ulva when green tides have formed. Nevertheless, this method is still a promising method for the early prevention of green tides.
Future Research Perspectives
The green algae, Ulva, multiplies rapidly and has a strong resistance, bringing the problems of green tides and biofouling. Reducing nutrient inputs is indeed an effective solution for macroalgal blooms, however, it may conflict with policies for local economic development (especially in developing countries and regions) and be difficult to achieve. Due to the urgent need for solutions to these Ulva problems, many chemicals have been put into research and application. Classical chemical methods include applying highly toxic compounds such as strong oxidants, heavy metals, and synthetic biocides. The purpose is to quickly kill Ulva when they are in the spore or germling state. Classical chemical methods have the advantages of simple implementation and high efficiency. However, they inevitably bring problems such as environmental pollution and ecological damage. With the gradual attention to environmental and ecological issues, utilization of environmentally friendly methods is the future development direction of Ulva control, including using natural products with better ecological safety to inhibit the settlement and germination of Ulva spores, and using modified clay to inactivate UMPs. These emerging methods have great application prospects, and further research and optimization are needed to promote the progress of application in practical Ulva control.
In suggestion, under the premise of fully evaluating the Ulva removal effect, the application cost and the ecological risks, classical chemical methods can still be applied. For the early control of green tides, it is highly recommended to use easily decomposable oxidants (like NaClO and ClO2) to directly eliminate fouling UMPs on aquaculture rafts. This method has the advantages of high efficiency, low cost, and reliability in practical and ecological safety, as long as properly applied. For the fouling problems of ships and marine facilities, we propose to increase the support for the development of degradable booster biocides and natural antifoulants, and gradually phase out the application of highly polluting and toxic ones. We also encourage in-depth application research and feasibility analysis of emerging environmentally friendly chemical methods for Ulva elimination, and exploration of comprehensive application of emerging methods, for example, the combination of natural antifoulants with non-toxic antifouling coatings.
Author Contributions
XX conceived this review. TT and CL collected data from literatures. JH provided information about physcological physiology. TT, KE, and XX wrote the manuscript, with edits and contributions from all other authors. All authors contributed to the article and approved the submitted version.
Funding
This study was financially supported by the National Key Research and Development Program of China (Grant No. 2016YFC1402104), the National Natural Science Foundation of China (Grant Nos. 21677122 and 21876148), and the Fundamental Research Funds for the Central Universities (Grant No. 2019QNA4051).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Mr. Wenrong Zhu in Xiangshan Xuwen Development of Seaweeds Co., Ltd. and Dr. Naicheng Li in Zhejiang University for valuable discussion about Ulva.
Footnotes
References
Aiyar, P., Schaeme, D., García-Altares, M., Flores, D. C., Dathe, H., Hertweck, C., et al. (2017). Antagonistic bacteria disrupt calcium homeostasis and immobilize algal cells. Nat. Commun. 8:1756.
Allanson, B. R., Human, L. R. D., and Claassens, L. (2016). Observations on the distribution and abundance of a green tide along an intertidal shore, Knysna Estuary. S. Afr. J. Bot. 107, 49–54. doi: 10.1016/j.sajb.2016.02.197
Bazes, A., Silkina, A., Douzenel, P., Faÿ, F., Kervarec, N., Morin, D., et al. (2009). Investigation of the antifouling constituents from the brown alga Sargassum muticum (Yendo) Fensholt. J. Appl. Phycol. 21, 395–403. doi: 10.1007/s10811-008-9382-9
Belamarić, B., and Belamarić, G. (2010). “A ship operation policy strategy optimisation thru multiattribute synthesis procedure based on biocide emissions from antifouling coatings (in Croatian),” in III. SAVJETOVANJE O MORSKOJ TEHNOLOGIJI in memoriam akademiku Zlatku Winkleru, 116–142. Available online at: https://www.bib.irb.hr/517690
Bhattarai, H. D., Ganti, V. S., Paudel, B., Lee, Y. K., Lee, H. K., Hong, Y.-K., et al. (2007a). Isolation of antifouling compounds from the marine bacterium, Shewanella oneidensis SCH0402. World J. Microbiol. Biotechnol. 23, 243–249. doi: 10.1007/s11274-006-9220-7
Bhattarai, H. D., Paudel, B., Park, N., Lee, K. S., and Shin, H. (2007b). Evaluation of antifouling activity of eight commercially available organic chemicals against the early foulers marine bacteria and Ulva spores. J. Environ. Biol. 28:857.
Blomster, J., Bäck, S., Fewer, D. P., Kiirikki, M., Lehvo, A., Maggs, C. A., et al. (2002). Novel morphology in Enteromorpha (Ulvophyceae) forming green tides. Am. J. Bot. 89, 1756–1763. doi: 10.3732/ajb.89.11.1756
Blossom, N. (2007). Copper in the Ocean Environment. Deerfield, IL: American Chemet Corporation, 1–8.
Bray, S., and Langston, W. (2006). Tributyltin Pollution on a Global Scale. An Overview of Relevant and Recent Research: Impacts and Issues. London: World Wildlife Fund UK.
Burgess, J. G., Boyd, K. G., Armstrong, E., Jiang, Z., Yan, L., Berggren, M., et al. (2003). The development of a marine natural product-based antifouling paint. Biofouling 19, 197–205. doi: 10.1080/0892701031000061778
Callow, M. E., and Callow, J. A. (1998). Attachment of zoospores of the fouling alga Enteromorpha in the presence of zosteric acid. Biofouling 13, 87–95. doi: 10.1080/08927019809378373
Callow, M. E., Callow, J. A., Pickett−Heaps, J. D., and Wetherbee, R. (1997). Primary adhesion of Enteromorpha (Chlorophyta, Ulvales) propagules: quantitative settlement studies and video microscopy. J. Phycol. 33, 938–947. doi: 10.1111/j.0022-3646.1997.00938.x
Chambers, L. D., Stokes, K. R., Walsh, F. C., and Wood, R. J. (2006). Modern approaches to marine antifouling coatings. Surf. Coat. Technol. 201, 3642–3652. doi: 10.1016/j.surfcoat.2006.08.129
Chen, L., and Lam, J. C. (2017). SeaNine 211 as antifouling biocide: a coastal pollutant of emerging concern. J. Environ. Sci. 61, 68–79. doi: 10.1016/j.jes.2017.03.040
Cho, J. Y. (2012a). Antifouling activity of giffinisterone B and oleamide isolated from a filamentous bacterium Leucothrix mucor culture against Ulva pertusa. Korean J. Fish. Aquat. Sci. 45, 30–34. doi: 10.5657/kfas.2012.0030
Cho, J. Y. (2012b). Antifouling steroids isolated from red alga epiphyte filamentous bacterium Leucothrix mucor. Fish. Sci. 78, 683–689. doi: 10.1007/s12562-012-0490-8
Cho, J. Y. (2013). Antifouling chromanols isolated from brown alga Sargassum horneri. J. Appl. Phycol. 25, 299–309. doi: 10.1007/s10811-012-9864-7
Cho, J. Y., and Kim, M. S. (2012). Induction of antifouling diterpene production by Streptomyces cinnabarinus PK209 in co-culture with marine-derived Alteromonas sp. KNS-16. Biosci. Biotechnol. Biochem. 76, 1849–1854. doi: 10.1271/bbb.120221
Cho, J. Y., Kang, J. Y., Hong, Y. K., Baek, H. H., Shin, H. W., and Kim, M. S. (2012). Isolation and structural determination of the antifouling diketopiperazines from marine-derived Streptomyces praecox 291-11. Biosci. Biotechnol. Biochem. 76, 1116–1121. doi: 10.1271/bbb.110943
Christie, A., Evans, L., and Shaw, M. (1970). Studies on the ship-fouling alga enteromorpha: II. The effect of certain enzymes on the adhesion of Zoospores. Ann. Bot. 7, 467–482. doi: 10.1093/oxfordjournals.aob.a084383
Collén, J., and Pedersén, M. (1996). Production, scavenging and toxicity of hydrogen peroxide in the green seaweed Ulva rigida. Eur. J. Phycol. 31, 265–271. doi: 10.1080/09670269600651471
Cresswell, T., Richards, J. P., Glegg, G. A., and Readman, J. W. (2006). The impact of legislation on the usage and environmental concentrations of Irgarol 1051 in UK coastal waters. Mar. Pollut. Bull. 52, 1169–1175. doi: 10.1016/j.marpolbul.2006.01.014
De Vries, H., Penninks, A., Snoeij, N., and Seinen, W. (1991). Comparative toxicity of organotin compounds to rainbow trout (Oncorhynchus mykiss) yolk sac fry. Sci. Total Environ. 103, 229–243. doi: 10.1016/0048-9697(91)90148-8
DerMarderosian, A., and Beutler, J. A. (2002). The Review of Natural Products: The Most Complete Source of Natural Product Information, (St. Louis, MO: Facts and Comparisons).
Dimitriou, M. D., Zhou, Z., Yoo, H.-S., Killops, K. L., Finlay, J. A., Cone, G., et al. (2011). A general approach to controlling the surface composition of poly (ethylene oxide)-based block copolymers for antifouling coatings. Langmuir 27, 13762–13772. doi: 10.1021/la202509m
Drábková, M., Admiraal, W., and Maršálek, B. (2007). Combined exposure to hydrogen peroxide and light selective effects on cyanobacteria, green algae, and diatoms. Environ. Sci. Technol. 41, 309–314. doi: 10.1021/es060746i
Egan, S., James, S., Holmström, C., and Kjelleberg, S. (2001). Inhibition of algal spore germination by the marine bacterium Pseudoalteromonas tunicata. FEMS Microbiol. Ecol. 35, 67–73. doi: 10.1111/j.1574-6941.2001.tb00789.x
Ekin, A., Webster, D. C., Daniels, J. W., Stafslien, S. J., Cassé, F., Callow, J. A., et al. (2007). Synthesis, formulation, and characterization of siloxane–polyurethane coatings for underwater marine applications using combinatorial high-throughput experimentation. J. Coat. Technol. Res. 4:435. doi: 10.1007/s11998-007-9039-7
Feng, D., Qiu, Y., Wang, W., Wang, X., Ouyang, P., and Ke, C. (2013). Antifouling activities of hymenialdisine and debromohymenialdisine from the sponge Axinella sp. Int. Biodeterior. Biodegradation 85, 359–364. doi: 10.1016/j.ibiod.2013.08.014
Fletcher, R. (1996). “The occurrence of “green tides”—a review,” in Marine Benthic Vegetation, eds W. Schramm and P. H. Nienhuis, (Berlin: Springer), 7–43. doi: 10.1007/978-3-642-61398-2_2
Gao, Y., Fang, J., Fang, J., Zhao, Y., Ji, H., Li, W., et al. (2018). Biological control feasibility of pacific oyster crassostrea gigas and bule mussel Mytilus edulis on Ulva prolifera microscopic propagules (in Chinese). Oceanol. Limnol. Sin. 49, 1116–1122.
Gao, Y., Jiang, Z., Du, M., Fang, J., Jiang, W., and Fang, J. (2017). Comparison of the herbicide atrazine and prometryn’s toxicity on seagrass and seaweed (in Chinese). Acta Hydrobiol. Sin. 41, 930–934.
Gladyshev, M., and Gubelit, Y. I. (2019). Green tides: new consequences of the eutrophication of natural waters (invited review). Contemp. Probl. Ecol. 12, 109–125. doi: 10.1134/s1995425519020057
Gómez, M., González, A., Sáez, C. A., Morales, B., and Moenne, A. (2015). Copper-induced activation of TRP channels promotes extracellular calcium entry, activation of CaMs and CDPKs, copper entry and membrane depolarization in Ulva compressa. Front. Plant Sci. 6:182. doi: 10.3389/fpls.2015.00182
Han, X., Zhao, T., Miao, H., Sun, B., Zhao, W., Yan, T., et al. (2019). Growth characteristics of green algae from attached to floated growth process in the Subei Shoal (in Chinese). Oceanol. Limnol. Sin. 50, 308–315.
Hattori, T., and Shizuri, Y. (1996). A screening method for antifouling substances using spores of the fouling macroalga Ulva conglobata Kjellman. Fish. Sci. 62, 955–958. doi: 10.2331/fishsci.62.955
Hiraoka, M., Dan, A., Shimada, S., Hagihira, M., Migita, M., and Ohno, M. (2003). Different life histories of Enteromorpha prolifera (Ulvales, Chlorophyta) from four rivers on Shikoku Island, Japan. Phycologia 42, 275–284. doi: 10.2216/i0031-8884-42-3-275.1
Hiraoka, M., Ohno, M., Kawaguchi, S., and Yoshida, G. (2004). Crossing test among floating Ulva thalli forming ‘green tide’ in Japan. Hydrobiologia 512, 239–245. doi: 10.1023/b:hydr.0000020332.12641.a2
Holm, E. R., Haslbeck, E. G., and Horinek, A. A. (2003). Evaluation of brushes for removal of fouling from fouling-release surfaces, using a hydraulic cleaning device. Biofouling 19, 297–305. doi: 10.1080/0892701031000137512
Hong, Y.-K., and Cho, J. Y. (2013). Effect of seaweed epibiotic bacterium Streptomyces violaceoruber SCH-09 on marine fouling organisms. Fish. Sci. 79, 469–475. doi: 10.1007/s12562-013-0604-y
Hu, Z., Finlay, J. A., Chen, L., Betts, D. E., Hillmyer, M. A., Callow, M. E., et al. (2009). Photochemically cross-linked perfluoropolyether-based elastomers: synthesis, physical characterization, and biofouling evaluation. Macromolecules 42, 6999–7007. doi: 10.1021/ma901227k
Huang, Y.-L., Lee, T.-M., Chen, M.-H., and Liao, L.-J. (2002). Effects of Copper on Phosphorus Utilization in Ulva fasciata Delile (Ulvales, Chlorophyta). Rome: Food and Agriculture Organization.
Jelic-Mrcelic, G., Sliskovic, M., and Antolic, B. (2006). Biofouling communities on test panels coated with TBT and TBT-free copper based antifouling paints. Biofouling 22, 293–302. doi: 10.1080/08927010600912291
Jendresen, C. B., and Nielsen, A. T. (2019). Production of zosteric acid and other sulfated phenolic biochemicals in microbial cell factories. Nat. Commun. 10:4071.
Jha, A., Hagger, J., Hill, S., and Depledge, M. (2000). Genotoxic, cytotoxic and developmental effects of tributyltin oxide (TBTO): an integrated approach to the evaluation of the relative sensitivities of two marine species. Mar. Environ. Res. 50, 565–573. doi: 10.1016/s0141-1136(00)00112-4
Kang, E. J., Kim, J.-H., Kim, K., Choi, H.-G., and Kim, K. Y. (2014). Re-evaluation of green tide-forming species in the Yellow Sea. Algae 29, 267–277. doi: 10.4490/algae.2014.29.4.267
Kannan, K., Guruge, K. S., Thomas, N. J., Tanabe, S., and Giesy, J. P. (1998). Butyltin residues in southern sea otters (Enhydra lutris nereis) found dead along California coastal waters. Environ. Sci. Technol. 32, 1169–1175. doi: 10.1021/es970914u
Kim, J.-H., Kang, E. J., Park, M. G., Lee, B.-G., and Kim, K. Y. (2011). Effects of temperature and irradiance on photosynthesis and growth of a green-tide-forming species (Ulva linza) in the Yellow Sea. J. Appl. Phycol. 23, 421–432. doi: 10.1007/s10811-010-9590-y
Kim, Y. D., Shin, H. W., and Cho, J. Y. (2013). Antifouling activity of coumarin and its derivatives isolated from the cinnamon tree Cinnamomum loureiroi. Korean J. Fish. Aquat. Sci. 46, 53–58. doi: 10.5657/kfas.2013.0053
Kojima, S. (1961). Aggregation treatment of plankton algae.(1) (in Japanese). Water Purif. Liq. Wastes Treat. 2, 21–27.
Kumar, M., Kumari, P., Gupta, V., Anisha, P., Reddy, C., and Jha, B. (2010). Differential responses to cadmium induced oxidative stress in marine macroalga Ulva lactuca (Ulvales. Chlorophyta). Biometals 23, 315–325. doi: 10.1007/s10534-010-9290-8
Küpper, H., Šetlík, I., Šetliková, E., Ferimazova, N., Spiller, M., and Küpper, F. C. (2003). Copper-induced inhibition of photosynthesis: limiting steps of in vivo copper chlorophyll formation in Scenedesmus quadricauda. Funct. Plant Biol. 30, 1187–1196. doi: 10.1071/fp03129
Largo, D. B., Sembrano, J., Hiraoka, M., and Ohno, M. (2004). Taxonomic and ecological profile of ‘green tide’ species of Ulva (Ulvales, Chlorophyta) in central Philippines. Hydrobiologia 512, 247–253. doi: 10.1023/b:hydr.0000020333.33039.4b
Lee, H., Depuydt, S., Choi, S., Han, T., and Park, J. (2020). Rapid toxicity assessment of six antifouling booster biocides using a microplate-based chlorophyll fluorescence in Undaria pinnatifida gametophytes. Ecotoxicology 2, 559–570. doi: 10.1007/s10646-020-02207-2
Li, J. (2017). Removal Efficiency of Several HABs Species Using Modified Clay and its Environmental Effects (in Chinese). Beijing: University of Chinese Academy of Sciences.
Li, J., Shao, X., Zhou, Q., Li, M., and Zhang, Q. (2013). The double effects of silver nanoparticles on the PVDF membrane: surface hydrophilicity and antifouling performance. Appl. Surf. Sci. 265, 663–670. doi: 10.1016/j.apsusc.2012.11.072
Li, J., Song, X., Fan, X., and Yu, Z. (2020). Flocculation of Ulva microscopic propagules using modified clay: a mesocosm experiment. J. Oceanol. Limnol. 38, 1283–1291. doi: 10.1007/s00343-020-9348-6
Li, J., Song, X., Zhang, Y., Pan, J., and Yu, Z. (2017a). An investigation of the space distribution of Ulva microscopic propagules and ship-based experiment of mitigation using modified clay. Mar. Pollut. Bull. 117, 247–254. doi: 10.1016/j.marpolbul.2017.01.063
Li, J., Sun, L., Song, X., and Yu, Z. (2015). Removal of microscopic propagule and its germination of Ulva prolifera with modified clay (in Chinese). Oceanol. Limnol. Sin. 15, 345–350.
Li, J., Sun, L., Yu, Z., and Song, X. (2017b). Investigation on the efficiency of a silicone antifouling coating in controlling the adhesion and germination of Ulva prolifera micro-propagules on rafts. Sci. China Earth Sci. 60, 391–396. doi: 10.1007/s11430-016-0018-x
Li, Q., Osada, M., Mori, K., and Wang, R. (2001). Effect of tributyltin oxide on sexual maturation of teh pacific oyster, Crassostrea gigas. J. Ocean Univ. Qingdao 31, 701–706.
Liu, F., Pang, S. J., Zhao, X. B., and Hu, C. M. (2012). Quantitative, molecular and growth analyses of Ulva microscopic propagules in the coastal sediment of Jiangsu province where green tides initially occurred. Mar. Environ. Res. 74, 56–63. doi: 10.1016/j.marenvres.2011.12.004
Liu, Q., Wang, X., and Li, L. (2018). Physiological response of intertidal marine macroalgae Ulva pertusa and Sargassum thunbergii to heavy metal copper stress (in Chinese). Mar. Sci. 5, 35–42.
Liu, S., Yu, Z., Song, X., and Cao, X. (2016). Effect of modified clay flocculation on physiological activity of Chlorella vulgaris (in Chinese). Oceanol. Limnol. Sin. 47, 748–754.
Lu, I.-F., Sung, M.-S., and Lee, T.-M. (2006). Salinity stress and hydrogen peroxide regulation of antioxidant defense system in Ulva fasciata. Mar. Biol. 150, 1–15. doi: 10.1007/s00227-006-0323-3
Luo, Q., Yan, X., Xu, S., Xu, J., Zhou, C., Ma, B., et al. (2010). Acidizing Fluid for Green Algae and Disease Infected Cell in Porphyra haitanensis Cultivation And Treating Method thereof. Chinese Patent, CN101822271A.
Morand, P., and Merceron, M. (2005). Macroalgal population and sustainability. J. Coast. Res. 21, 1009–1020. doi: 10.2112/04-700a.1
Nelson, T., Nelson, A., and Tjoelker, M. (2003). Seasonal and spatial patterns of “green tides”(ulvoid algal blooms) and related water quality parameters in the coastal waters of Washington State, USA. Bot. Mar. 46, 263–275.
Peng, B., Wang, J., Peng, Z., Zhou, S., Wang, F., Ji, Y., et al. (2011). Studies on the synthesis, pungency and anti-biofouling performance of capsaicin analogues (in Chinese). Sci. Sin. Chim. 41, 1646–1654.
Peng, S., Nan, P., and Zhong, Y. (2002). Terpenoids in higher plants and their roles in ecosystems (in Chinese). Chin. J. Ecol. 2, 33–38.
Pettitt, M., Henry, S., Callow, M., Callow, J., and Clare, A. (2004). Activity of commercial enzymes on settlement and adhesion of cypris larvae of the barnacle Balanus amphitrite, spores of the green alga Ulva linza, and the diatom Navicula perminuta. Biofouling 20, 299–311. doi: 10.1080/08927010400027068
Pinto, E., Sigaud−Kutner, T. C., Leitao, M. A., Okamoto, O. K., Morse, D., and Colepicolo, P. (2003). Heavy metal–induced oxidative stress in algae 1. J. Phycol. 39, 1008–1018.
Qian, P.-Y., Xu, Y., and Fusetani, N. (2009). Natural products as antifouling compounds: recent progress and future perspectives. Biofouling 26, 223–234. doi: 10.1080/08927010903470815
Ren, C., Tian, X., Sun, Y., Deng, X., Liu, H., Xue, J., et al. (2014). Residues and risk assessment of 13 triazine herbicides in Apostichopus japonicus (in Chinese). Mod. Food Sci. Technol. 30, 244–249.
Rezayian, M., Niknam, V., and Ebrahimzadeh, H. (2019). Oxidative damage and antioxidative system in algae. Toxicol. Rep. 6, 1309–1313. doi: 10.1016/j.toxrep.2019.10.001
Scarlett, A., Donkin, M., Fileman, T., and Donkin, P. (1997). Occurrence of the marine antifouling agent Irgarol 1051 within the plymouth sound locality: implications for the green macroalga Enteromorpha intestinalis. Mar. Pollut. Bull. 34, 645–651. doi: 10.1016/s0025-326x(96)00187-7
Schiff, K., Diehl, D., and Valkirs, A. (2004). Copper emissions from antifouling paint on recreational vessels. Mar. Pollut. Bull. 48, 371–377. doi: 10.1016/j.marpolbul.2003.08.016
Shao, K., Gong, N., Gu, Y., and Guo, H. (2019). Control of Ulva bloom of Yellow Sea by Elimination of Winter Micropropagule Banks. Chinese Patent, CN109496826A.
Shi, H., and Wang, L. (2006). Nontoxic marine anti-fouling coating containing capsaicin. Mar. Sci. Bull. 8:92.
Shin, H.-W. (1998). Antifouling action of zosteric acid and copper on spores of Ulva fasciata Delile. Algae 13, 271–274.
Shin, H.-W., Kang, S.-G., Son, J.-S., Jeon, J.-H., Lee, H.-J., Jung, S.-M., et al. (2015). Evaluation of antifouling system of new antifouling agents using spores of the green alga, Ulva pertusa and diatom, Nitzschia pungens (in Korean). Korean J. Environ. Ecol. 29, 736–742. doi: 10.13047/kjee.2015.29.5.736
Sidharthan, M., and Shin, H. (2007). Influence of five organic antifouling candidates on spore attachment and germination of a fouling alga Ulva pertusa. J. Environ. Biol. 28:39.
Sidharthan, M., Shin, H., and Joo, J. (2004). Fouling coverage of a green tide alga, Ulva pertusa on some antifouling test surfaces exposed to Ayagin harbor waters, east coast of South Korea. J. Environ. Biol. 25, 39–43.
Silva-Aciares, F., and Riquelme, C. (2008). Inhibition of attachment of some fouling diatoms and settlement of Ulva lactuca zoospores by film-forming bacterium and their extracellular products isolated from biofouled substrata in Northern Chile. Electron. J. Biotechnol. 11, 60–70.
Song, W., Jiang, M., Wang, Z., Wang, H., Zhang, X., and Fu, M. (2018). Source of propagules of the fouling green macroalgae in the Subei Shoal, China. Acta Oceanol. Sin. 37, 102–108. doi: 10.1007/s13131-018-1169-5
Song, W., Li, Y., Fang, S., Wang, Z., Xiao, J., Li, R., et al. (2015). Temporal and spatial distributions of green algae micro-propagules in the coastal waters of the Subei Shoal, China. Estuar. Coast. Shelf Sci. 163, 29–35. doi: 10.1016/j.ecss.2014.08.006
Sun, L. (2014). A Study on the Factors that Influence the Settlement of Ulva prolifera Spores and the Flocculation Effects of Ulva prolifera Spores by Modified Clays (in Chinese). Qingdao: Ocean University of China.
Sun, X., Wang, X., Wang, W., Wang, F., and Liu, Q. (2008). Stress resistance of Enteromorpha prolifera and lethal effect of chemical reagents on this green seaweed (in Chinese). Mar. Fish. Res. 29, 130–136.
Sun, X., Zhang, B., and Yu, Z. (2000). Toxicity study of anti-red tide agents to Penaeus chinensis (in Chinese). Mar. Environ. Sci. 2, 6–8.
Sun, Z., Gu, L., Zheng, J., Zhang, J., Wang, L., Xu, F., et al. (2016). A controlled release strategy of antifouling agent in coating based on intercalated layered double hydroxides. Mater. Lett. 172, 105–108. doi: 10.1016/j.matlet.2016.02.151
Thomas, K. V., and Brooks, S. (2010). The environmental fate and effects of antifouling paint biocides. Biofouling 26, 73–88. doi: 10.1080/08927010903216564
Thompson, S., Callow, J., Callow, M., Wheeler, G., Taylor, A., and Brownlee, C. (2007). Membrane recycling and calcium dynamics during settlement and adhesion of zoospores of the green alga Ulva linza. Plant Cell Environ. 30, 733–744. doi: 10.1111/j.1365-3040.2007.01661.x
Tian, X., Gong, X., Xu, Y., Ren, C., Liu, H., Liu, Y., et al. (2013). Accumulation and elimination of prometryn in Apostichopus japonicus (in Chinese). Mod. Food Sci. Technol. 29, 1580–1585.
Tolhurst, L. E., Barry, J., Dyer, R. A., and Thomas, K. V. (2007). The effect of resuspending sediment contaminated with antifouling paint particles containing Irgarol 1051 on the marine macrophyte Ulva intestinalis. Chemosphere 68, 1519–1524. doi: 10.1016/j.chemosphere.2007.03.005
Turner, A., Brice, D., and Brown, M. T. (2012). Interactions of silver nanoparticles with the marine macroalga, Ulva lactuca. Ecotoxicology 21, 148–154. doi: 10.1007/s10646-011-0774-2
Voulvoulis, N., Scrimshaw, M., and Lester, J. (1999). Alternative antifouling biocides. Appl. Organometal. Chem. 13, 135–143. doi: 10.1002/(sici)1099-0739(199903)13:3<135::aid-aoc831>3.0.co;2-g
Wang, G., Huan, L., Xie, X., and Lu, X. (2017). A Kind of Method for Removing Miscellaneous Algae Enteromorpha on Cultivation Lace Curtaining. Chinese Patent, CN107262439A.
Wang, J., Shi, T., Yang, X., Han, W., and Zhou, Y. (2014). Environmental risk assessment on capsaicin used as active substance for antifouling system on ships. Chemosphere 104, 85–90. doi: 10.1016/j.chemosphere.2013.10.061
Wang, Z., Xiao, J., Fan, S., Li, Y., Liu, X., and Liu, D. (2015). Who made the world’s largest green tide in China?—an integrated study on the initiation and early development of the green tide in Yellow Sea. Limnol. Oceanogr. 60, 1105–1117. doi: 10.1002/lno.10083
Wendt, I. (2013). On the Efficacy and Ecotoxicity of Antifouling Biocides Lethal and Sublethal Effects on Target and Non-target Organisms. Gothenburg: University of Gothenburg.
Wendt, I., Arrhenius, Å, Backhaus, T., Hilvarsson, A., Holm, K., Langford, K., et al. (2013a). Effects of five antifouling biocides on settlement and growth of zoospores from the marine macroalga Ulva lactuca L. Bull. Environ. Contam. Toxicol. 91, 426–432. doi: 10.1007/s00128-013-1057-9
Wendt, I., Arrhenius, Å, Backhaus, T., Hilvarsson, A., Holm, K., Langford, K., et al. (2013b). Extreme irgarol tolerance in an Ulva lactuca L. population on the Swedish west coast. Mar. Pollut. Bull. 76, 360–364. doi: 10.1016/j.marpolbul.2013.08.035
Willingham, G., and Jacobson, A. (1993). “Efficacy and environmental fate of a new isothiazolone antifoulant,” in Proceedings of the PRA Third Asia-Pacific Conf.’Advances in Coatings, Inks and Adhesives Technology, eds G. I. L. I. Willingham and A. H. Jacobson, Singapore.
Wu, T.-M. (2009). Gene Expression in Marine Macroalga Ulva fasciata Delile Against Excess Copper Toxicity. Ph. D. National Sun Yat-sen University, Kaohsiung.
Wu, X., Wang, H., and Zou, J. (2014). Marine biofouling and research progress of environmental friendly antifouling paints for ships (in Chinese). New Chem. Mater. 42, 1–3.
Xu, Y.-Z., Yakushijin, K., and Horne, D. A. (1997). Synthesis of C11N5 marine sponge alkaloids:(±)-hymenin, stevensine, hymenialdisine, and debromohymenialdisine. J. Org. Chem. 62, 456–464. doi: 10.1021/jo9619746
Yabe, T., Ishii, Y., Amano, Y., Koga, T., Hayashi, S., Nohara, S., et al. (2009). Green tide formed by free-floating Ulva spp. at Yatsu tidal flat, Japan. Limnology 10, 239–245. doi: 10.1007/s10201-009-0278-4
Yan, X., Zhong, C., Qi, Q., and Wang, C. (2011). Effects of refrigeration and acid treatment on the survival of the blades in Porphyra haitanensis and Enteromorpha prolifera (in Chinese). J. Shanghai Ocean Univ. 20, 697–704.
Ye, N., Zhang, X., Mao, Y., Liang, C., Xu, D., Zou, J., et al. (2011). ‘Green tides’ are overwhelming the coastline of our blue planet: taking the world’s largest example. Ecol. Res. 26, 477–485. doi: 10.1007/s11284-011-0821-8
Yonehara, Y., Yamashita, H., Kawamura, C., and Itoh, K. (2001). A new antifouling paint based on a zinc acrylate copolymer. Prog. Organ. Coat. 42, 150–158. doi: 10.1016/s0300-9440(01)00157-6
Youngblood, J. P., Andruzzi, L., Ober, C. K., Hexemer, A., Kramer, E. J., Callow, J. A., et al. (2003). Coatings based on side-chain ether-linked poly (ethylene glycol) and fluorocarbon polymers for the control of marine biofouling. Biofouling 19, 91–98. doi: 10.1080/0892701021000053381
Yu, K., Zhang, J., Huo, Y., Zhu, Y., Han, W., Yang, L., et al. (2012). Method for Removing Green Algae in the Middle and Late Culture Stages of Porphyra yezoensis. Chinese Patent, CN102550388B.
Yu, Z., Zhang, J., Wu, Y., Lei, G., Zhang, Y., Shi, D., et al. (1994a). Toxic effect of copper on benthic green algae in the Yellow Sea (in Chinese). Acta Sci. Circumst. 4, 494–500.
Yu, Z., Zou, J., and Ma, X. (1994b). A new method to improve the capability of clays for removing red tide organisms (in Chinese). Oceanol. Limnol. Sin. 28, 226–232.
Zhang, Q., Kong, F., Yan, T., Yu, R., Hu, X., Miao, H., et al. (2018). Green algae detached from aquaculture rafts into seawater resulted in green tide occurrence in the yellow sea (in Chinese). Oceanol. Limnol. Sin. 49, 1014–1020.
Zhang, S. (2012). Studies on the Inhibitory Performance of Ceramide on Marine Fouling Organisms and Their Mechanism of Action (in Chinese). Master thesis, Ocean University of China, Qingdao.
Zhang, Y. (2018). The Effects of Modified Clay on the Typical Harmful Algae and Turbot Embryos (in Chinese). Doctor of Philosophy, University of Chinese Academy of Sciences, Beijing.
Zhang, Y., Song, X., Li, J., Cao, X., and Yu, Z. (2016). Effect of different modified clay on the removal and germination of Ulva prolifera microscopic propagules (in Chinese). Haiyang Xuebao 16, 93–102.
Zhao, J., Jiang, P., Liu, Z., Wei, W., Lin, H., Li, F., et al. (2013). The Yellow Sea green tides were dominated by one species, Ulva (Enteromorpha) prolifera, from 2007 to 2011. Chin. Sci. Bull. 58, 2298–2302. doi: 10.1007/s11434-012-5441-3
Zheng, X. (2008). A Preliminary Study on the Impacts of Amphipods’ Grazing on the Macroalgal Community in Yundang Lagoon (in Chinese). Master Thesis, Xiamen University, Xiamen.
Zheng, X., Huang, L., Wang, Q., and Lin, R. (2014). Amphipods fail to suppress the accumulation of Ulva lactuca biomass in eutrophic Yundang Lagoon. Acta Oceanol. Sin. 33, 155–162. doi: 10.1007/s13131-014-0532-4
Keywords: Ulva, microscopic propagule, green tide, antifouling, heavy metal, allelochemical, natural product, modified clay
Citation: Tang T, Effiong K, Hu J, Li C and Xiao X (2021) Chemical Prevention and Control of the Green Tide and Fouling Organism Ulva: Key Chemicals, Mechanisms, and Applications. Front. Mar. Sci. 8:618950. doi: 10.3389/fmars.2021.618950
Received: 19 October 2020; Accepted: 21 January 2021;
Published: 12 February 2021.
Edited by:
Khuong Van Dinh, Washington State University, United StatesReviewed by:
Xuan-Vy Nguyen, Institute of Oceanography in Nhatrang, VietnamLiam Morrison, National University of Ireland Galway, Ireland
Copyright © 2021 Tang, Effiong, Hu, Li and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xi Xiao, xi@zju.edu.cn; xgshjhxx3@163.com
 Tao Tang
Tao Tang Kokoette Effiong
Kokoette Effiong Jing Hu
Jing Hu Chao Li
Chao Li  Xi Xiao
Xi Xiao