Seagrass Structural Traits Drive Fish Assemblages in Small-Scale Fisheries
- 1Department of Ecology, Environment and Plant Sciences, Stockholm University, Stockholm, Sweden
- 2Project Seagrass, Bridgend, United Kingdom
- 3Department of Earth Sciences, Natural Resources and Sustainable Development, Uppsala University, Uppsala, Sweden
- 4Department of Biosciences, Swansea University, Swansea, United Kingdom
- 5Institute of Fisheries Research, Ministry of Agriculture, Natural Resources, Livestock and Fisheries, Zanzibar, Tanzania
Seagrasses – a group of foundation species in coastal ecosystems – provide key habitat for diverse and abundant faunal assemblages and support numerous ecosystem functions and services. However, whether the habitat role of seagrasses is influenced by seagrass diversity, by dominant species or both, remains unclear. To that end, we sought to investigate the specific seagrass characteristics (e.g., species diversity, seagrass traits) that influence tropical fish assemblages, and place this in the context of small-scale fishery use. We surveyed seagrass variables at 55 plots, nested within 12 sites around Zanzibar (Tanzania) in the Western Indian Ocean, and used Baited Remote Underwater Video (BRUV) systems to assess fish assemblages across plots. Using linear mixed models, we reveal that seagrass structural complexity and depth were the best predictors of fish abundance, with higher abundance occurring in deeper meadows or meadows with high canopy, leaf length and number of leaves per shoot. Moreover, an interaction between seagrass cover and land-use was the best predictor of fish species richness, where sites closer to human impacts were less affected by cover than sites with lower human impact. Overall, models with seagrass species richness or functional diversity as predictors poorly explained fish assemblages. Fish taxa that were important for small-scale fishery sectors (e.g., emperors, snappers, rabbitfish, and parrotfish) were primarily driven by seagrass structural complexity. Our results provide a unique analysis of the relationship between seagrass habitat and its associated fish assemblages in that we show that seagrass species diversity had little effect on seagrass fish assemblages, which instead appear driven by specific seagrass traits and seagrass cover. If conserving high value species that support adjacent fisheries is the priority for protecting seagrass meadows, then seagrass areas should be chosen with high cover and structural complexity that are in deeper waters. Any conservation measures also need to balance the needs of fishers that use the resources supported by seagrasses.
Introduction
Foundation species like trees, corals and seagrasses play a pivotal role in driving ecosystem functions and services globally (Angelini et al., 2011), notably by facilitating the creation of habitats. Therefore, loss of biodiversity, particularly habitat biodiversity, as well as the homogenization of ecosystems is a global threat (Hoag, 2010; Oliver, 2016). Two not mutually exclusive ecological hypotheses are proposed to influence the effects of biodiversity on ecosystem function. First, the ‘mass ratio’ hypothesis proposes that ecosystem functions, like complex habitats favoring high biodiversity, are primarily determined by the functional traits of dominant species within the community (Grime, 1998). In contrast, the ‘complementarity hypothesis’ proposes that the taxonomic and/or functional diversity within the community are instead the key drivers of ecosystem functions (Tilman et al., 1997). In order to manage ecosystems for the services they provide, it is vital to understand how biodiversity drives ecosystem functions (e.g., whether it is species composition or diversity, or both), especially in the context of global change (Benkwitt et al., 2020). While the topic has become a heated debate, especially with regards to terrestrial ecosystems (Picasso, 2018), meta-analyses of hundreds of experiments in terrestrial (Cardinale et al., 2011) and marine ecosystems (Gamfeldt et al., 2015) suggest that both the species composition and diversity can jointly influence ecosystem functions.
In the marine environment, structure-forming foundation species can strongly influence fish assemblage organization (Gorham and Alevizon, 1989; Caley and St John, 1996; Beukers and Jones, 1998; Harding and Mann, 2001). Indeed, many small-scale fishers have their own opinions on where the best place to fish is, be it a complex structure or a specific area defined by certain species traits, which might also shift over time (Katikiro, 2014). Yet in general, we value the role of marine foundation species either in isolation (e.g., kelp) or by grouping species into much broader functional entities (e.g., coral reefs). As a result, we see a focus on protecting flagship and iconic foundation species or habitats, regardless of their state, qualities or characteristics (Caveen et al., 2014). However, this overlooks the fact that most ecosystems are structured by co-occurring foundation species, which can range greatly in their structural and functional attributes (Bruno and Bertness, 2001). One example is seagrasses, a group of habitat-forming marine plants occurring along the world’s coastlines (McKenzie et al., 2020). Pooling seagrass assemblages into bioregions reveal the highest species richness in the tropical Indo-Pacific (Short et al., 2007), consistent with the latitudinal diversity gradient (Willig et al., 2003; Jablonski et al., 2006). Up to 14 seagrass species co-occur with each other in the region (Miguel et al., 2018), yet many of these have very different functional attributes (e.g., low-high root biomass, short-long leaf length, low-high nutrient content). With reported seagrass loss across the region being high (Waycott et al., 2009), and some species being more at risk than others (Short et al., 2011), we need to urgently unpack this broad functional entity of seagrass meadows to understand how specific seagrass species losses could influence ecosystem service provision, and target management accordingly.
Given the Indo-Pacific region is one of the most densely populated by humans (Williams, 2013), sustaining the important ecosystem services that biodiversity provides is vital. Human dependence on seafood is substantial (Donner and Potere, 2007), but the foundation habitats that contribute to this provision are being degraded (Burke et al., 2011; Coles et al., 2011; Giri et al., 2011). In response, much research and conservation funding in the region has been directed toward coral reefs (Unsworth et al., 2019a), partly due to a failure to recognize that coastal fishers utilize multiple habitats, like seagrass meadows (Nordlund et al., 2018b), and partly because coral reefs are much more well-known and researched than other coastal habitats (UN Environment et al., 2018). Yet we now know the vital role seagrass plays globally in supporting fisheries and food supply (Unsworth et al., 2019b), which in the Western Indian Ocean can provide economic gains that locally may be four times greater than coral reefs (de la Torre-Castro et al., 2014). Previous research shows that tropical fish assemblages are influenced by variations in the structure of seagrass habitats (Heck and Orth, 1980), by seagrass canopy complexity (Bell and Westoby, 1986a, b; Nakamura and Sano, 2004) and by seagrass landscapes (Salita et al., 2003). Yet, the extent to which this is driven by seagrass diversity and/or by traits of dominant species remains unclear. The UN Decade of Ocean Science for Sustainable Development mandates for solution orientated science and management that balances biodiversity conservation with the needs of local people (von Schuckmann et al., 2020). The aspects of seagrass diversity that are key to sustaining biodiversity, and thus ecosystem services, remain unclear in general and for the (relatively speaking) ‘hyper-diverse’ seagrass meadows within the Indo-Pacific Ocean in particular (Nordlund et al., 2018a). This is especially pressing when we consider the multiple threats facing seagrass (Unsworth et al., 2018).
The aim of this study was to assess to what extent the fish habitat function of seagrass meadows is explained by the diversity and/or composition of seagrasses, from both a taxonomic and traits perspective. We surveyed seagrass meadows across a gradient of fishing pressure and anthropogenic impact around Zanzibar, Tanzania. We sought to determine (i) which seagrass meadow attributes best influenced seagrass meadow fish assemblages, and (ii) investigate the relationships between seagrass meadow attributes and the presence of fish important to small-scale fisheries. In the context of documented seagrass loss across the Indo-Pacific (Harcourt et al., 2018; Unsworth et al., 2018; Tin et al., 2020), we seek to indicate which attributes are key for managing seagrass for the maintenance of important ecosystem functions.
Methods
Study Design
We used standard Baited Remote Underwater Video (Cappo et al., 2007) and benthic quadrat assessment methods (McKenzie et al., 2001) to survey seagrass flora and motile fauna at 55 distinct plots nested within 12 sites around Zanzibar (Unguja Island), Tanzania, between October and November 2018 (Figure 1). Seagrass meadows around Zanzibar provide a suitable setting to examine the influence of seagrass species composition, diversity and structure on faunal productivity in that they are complex and comprised of a number of species in varying densities (Gullström et al., 2002). These are characteristically comprised of mixed, or monospecific Halodule uninervis, Halophila ovalis, and Cymodocea rotundata areas toward the upper intertidal limits of the meadow, shifting to both mixed and monospecific H. uninervis, C. rotundata, Thalassia hemprichii and H. ovalis areas in the lower intertidal limits of the meadow. The upper subtidal areas are comprised of C. serrulata, T. hemprichii, H. ovalis and Syringodium isoetifolium, shifting to C. serrulata, T. hemprichii, H. ovalis, S. isoetifolium and Thalassodendron ciliatum before being often dominated by T. ciliatum and Enhalus acoroides, growing in monospecific or mixed strands, in deeper areas. While one site was adjacent to a no-take marine reserve (but not within), all sites were typically fished. Plots were selected non-randomly to encapsulate variability in both seagrass species composition and species richness; we wanted to include mixed and monospecific plots for multiple seagrass genera. However, not all sites had the same seagrass species which ultimately led to the unbalanced nature of number of plots within each site.
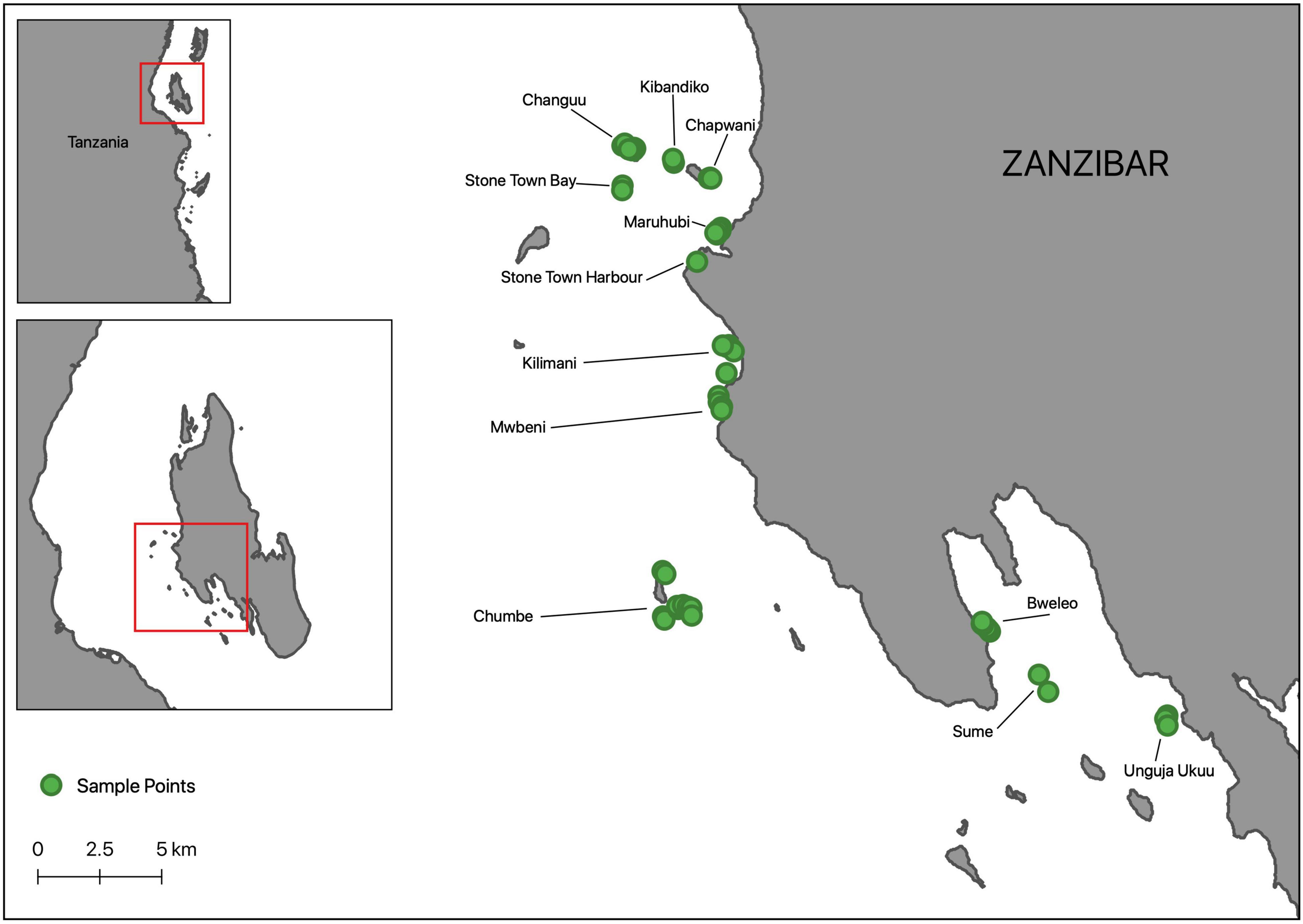
Figure 1. Map of Zanzibar, Tanzania showing the location of 55 plots, nested within 12 distinct sites (identifiable by names), where fish assemblage and seagrass surveys were conducted during October-November 2018.
Seagrass Community Structure
At each of the 55 plots, two 50 m transects were placed in a cross, centered around the location of the fish sampling point (Figure 2). Within twenty-two 0.25 m2 quadrats, placed at 5 m intervals along transects (0-50 m), we collected data on various seagrass meadow attributes pertinent to our hypothesis (structure, cover, diversity and composition; Table 1). We estimated seagrass shoot density (0.0225 m2), total percentage cover (0-100%) and floral species composition (McKenzie et al., 2001). Canopy height (cm), leaf length (cm), leaf width (cm) and number of leaves per shoot were recorded from three random shoots within in each quadrat, and the mean was later used in the statistical analyses (McKenzie et al., 2001). Seagrass species were sorted into two structural trait categories - leaf growth form (strap, branched, paddle, and cylindrical) and canopy type (low, middle and high) - to calculate community weighted functional trait indices (functional dispersion) and functional richness (see data analysis). Prior to data analysis, the seagrass trait characteristics (n = 1210) were averaged for each plot (n = 55) to match the scale of the fish sampling (see site averages in Supplementary Table 1).
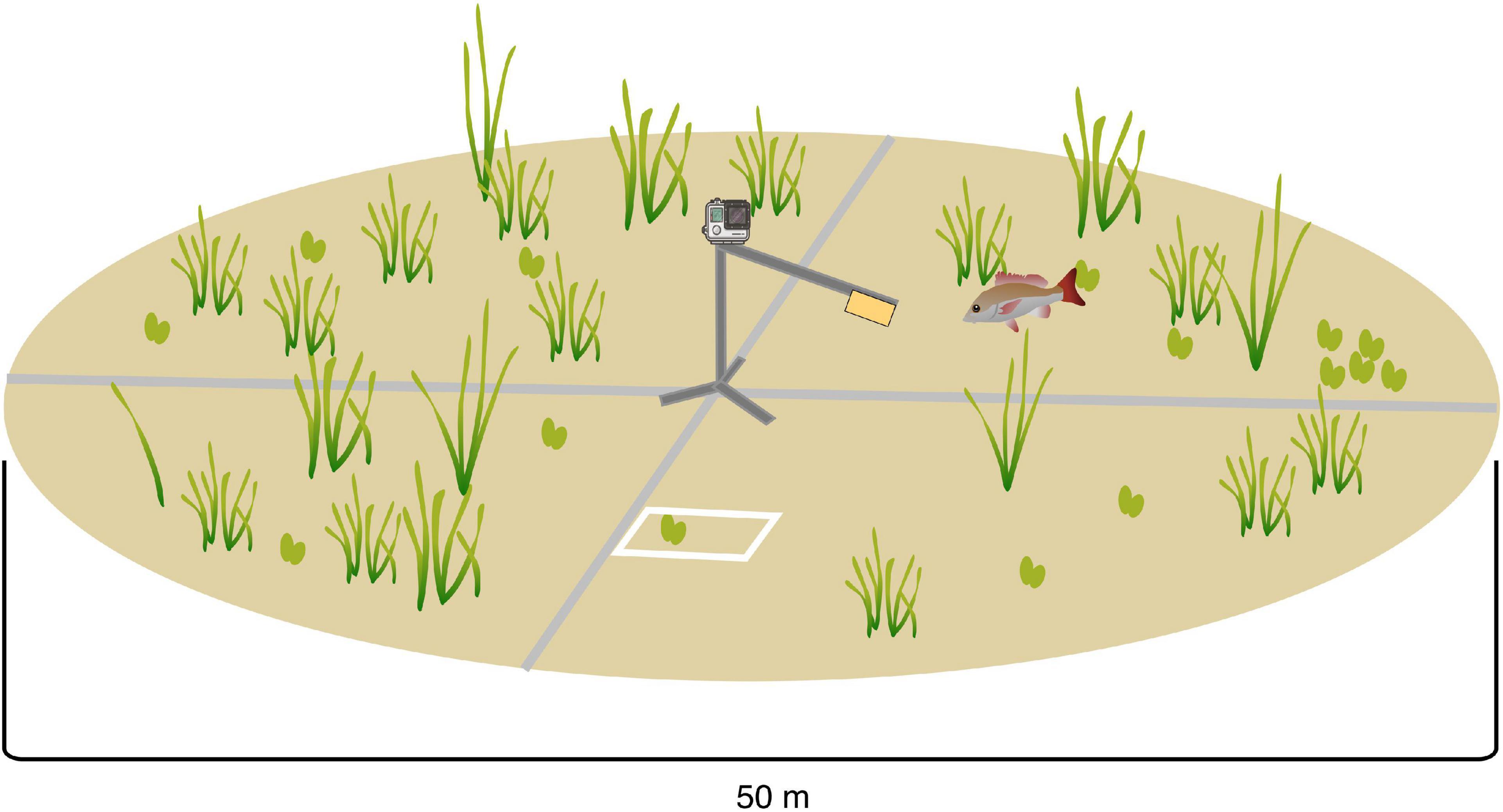
Figure 2. Conceptual diagram of survey setup at each sample location, showing the location of the Baited Remote Underwater Video (BRUV) system toward the center of the sample plot and location of the two 50m transects (not to scale).
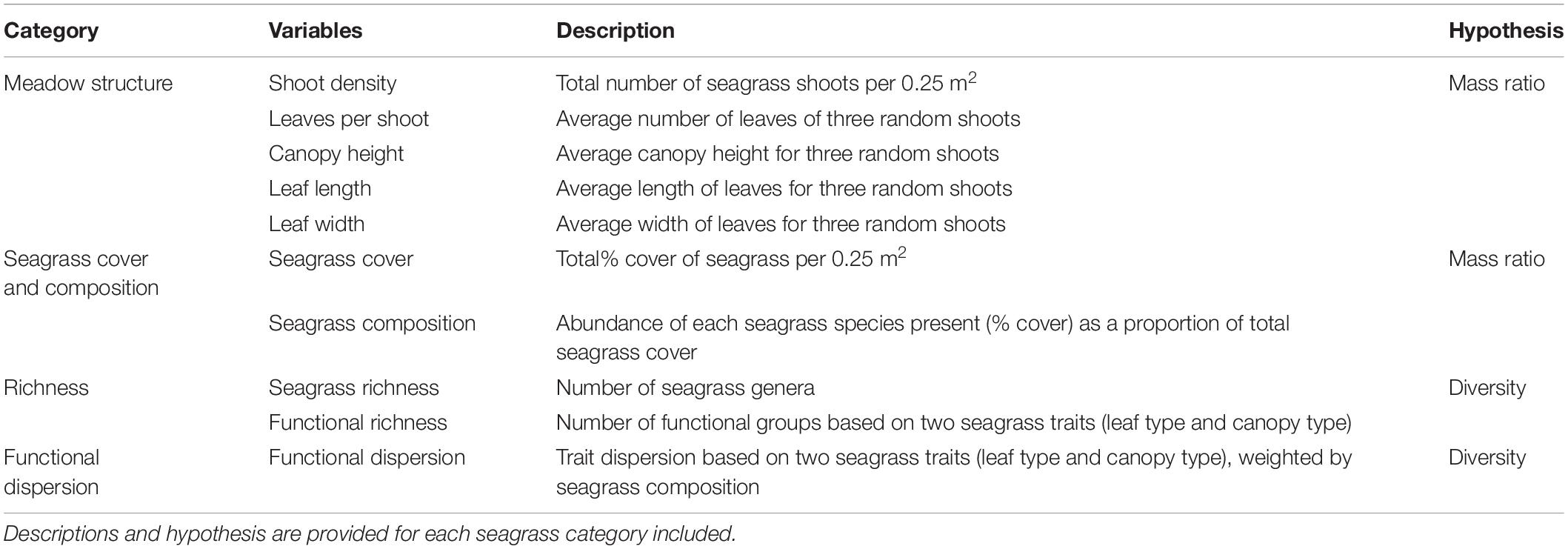
Table 1. Seagrass meadow structure, cover, composition, richness and community-weighted trait values investigated as predictors of fish abundance and species richness in the present study.
Environmental Characteristics and Anthropogenic Impact
For each plot, we then calculated several seascape variables that have been previously shown to structure fish assemblages (see site averages in Supplementary Table 2), including distance to nearest reef or area of coral bommies (Campbell et al., 2011) and nearest mangrove area (Dorenbosch et al., 2007). Individual mangrove trees were not considered and only areas with sufficient mangrove cover were included. Water depth, which ranged from 0.6 to 6.3 m, was recorded at time of fish sampling for each plot (Pogoreutz et al., 2012).
Human land-use intensity can strongly affect seagrass state (Quiros et al., 2017). Therefore, we utilized a similar method to that used by Quiros et al. (2017) to assess land-use characteristics at a site level. We estimated human land-use characteristics within a 2 km radius from each site (using Google Earth Pro v. 7.3) and calculated the proportion (km2) of land utilized in this 2 km radius by six categories. These were bare ground (exposed soil, shrub), human development (houses, villages, industry, and roads), aquaculture, farmland, vegetation (forest, mangrove) and water (ocean, river). We also recorded distance to nearest catchment.
Fishing pressure is also a well-known driver of seagrass fish assemblages, including those around Zanzibar (Alonso Aller et al., 2014). Even though the influence of fishing was not a main factor in our analysis, we sought to account for its influence as a covariate. First, distance to fishery landing site was calculated for each plot based on landing sites reported by the Department of Fisheries Development (2016). Second, fishing effort was approximated using a semi-quantitative two-level scale (low, high). This was developed based on discussions with local fishers and resource users (McCluskey and Lewison, 2008) and further quantified using field observations of ongoing fishery activity during Oct-Dec 2018 (Table 2). With observations, we considered both the number of fishers and the fishing gear used. For example, observations of multiple spear and trap fishermen operating from non-motorized boats were considered to be lower intensity than a single > 10 person drag net team operating from a larger motorized boat since drag nets catch more fish (Jiddawi and Öhman, 2002) and are less selective (McClanahan and Mangi, 2004).
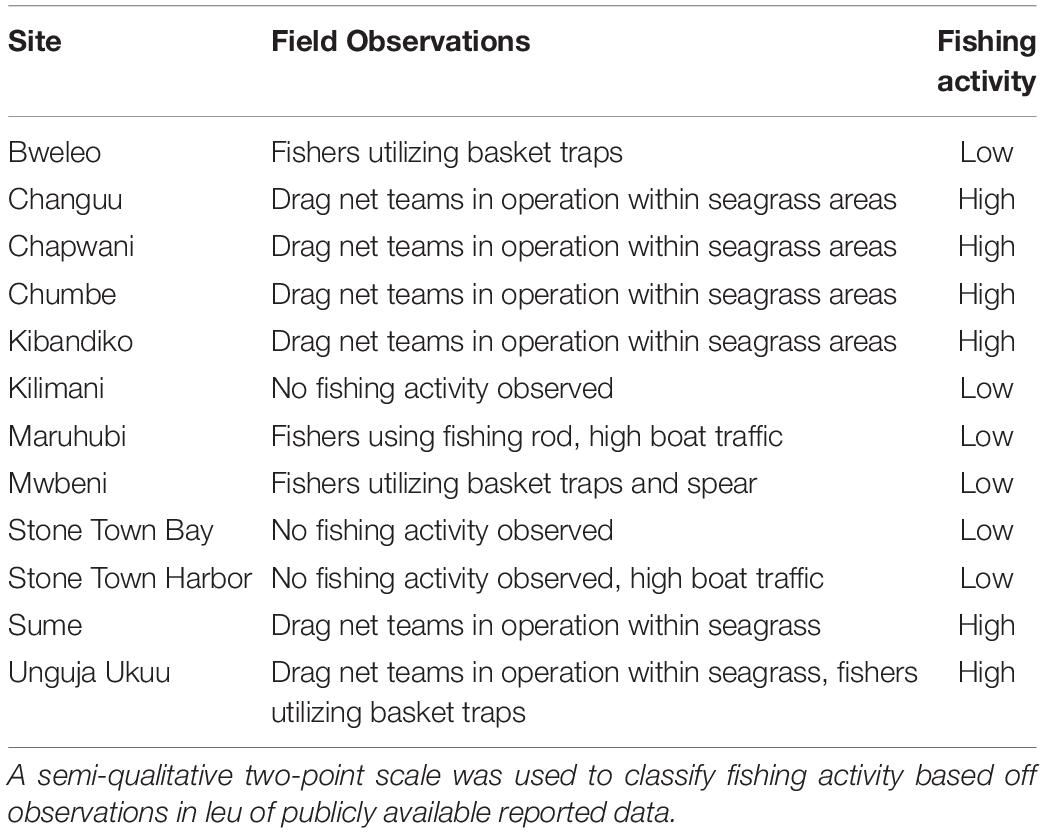
Table 2. Observations of ongoing fishing activity between October and December 2018 at 12 sites in Zanzibar, Tanzania.
Surveys of Seagrass Fish Assemblages
The abundance and diversity of seagrass fish assemblages were evaluated at each of the 55 plots using a single Baited Remote Underwater Video system (BRUVs) (Jones et al., 2018). BRUVs are a widely used and established method for understanding motile fauna in marine environments. Examples include their use in understanding the effects of marine reserves and protected areas (e.g., Whitmarsh et al., 2014; Bornt et al., 2015; Coleman et al., 2015; Gilby et al., 2017), assessing the faunal communities of reef systems (e.g., Lindfield et al., 2016), quantifying shark populations (e.g., MacNeil et al., 2020) and investigating demersal fish populations in different habitat types (e.g., Furness and Unsworth, 2020). Such assessments focus on fish diversity as a response and not only focus on top predators, but numerous demersal fish species. Moreover, the use of different types of video methods is becoming increasingly common for investigations into seagrass habitat and fish interactions (e.g., Smith et al., 2011; Whitmarsh et al., 2014; Díaz-Gil et al., 2017; Henderson et al., 2017; Kiggins et al., 2018). We chose to use a BRUV system for several reasons: they have been shown to be more effective for describing seagrass fish assemblages given the “diver effect” caused by traditional methods (Edgar et al., 2004; Zarco-Perello and Enríquez, 2019) have greater statistical power at low sample numbers and also attract herbivores (e.g., Harvey et al., 2007; Langlois et al., 2010; Watson et al., 2010; Andradi-Brown et al., 2016; Schramm et al., 2020). While this choice may result in fewer cryptic species being recorded (Watson et al., 2005), we considered traditional methods inappropriate for use in such intensely fished areas (Lindfield et al., 2014). The ability to ensure a consistent methodology between observations (Jones et al., 2021) was considered of greater importance than greater species resolution afforded by other methods.
The mono-BRUV system was constructed based on designs by Cappo et al. (2004). An aluminium tripod (Smatree X1S; 50 cm high) was used as a mount for a GoPro Hero 5 camera, with 2 kg lead weight placed at the base for stability underwater. A bait arm (20 mm ∅ PVC) extending 1 m from the camera supported a plastic bait container (Trappy Betesbox; 112 cm3), which was filled with standardized oily bait (Sardinella sp. purchased locally) before all deployments. Oily fish were used as they are considered the most effective bait for use in BRUVs (Bond et al., 2012; Dorman et al., 2012).
BRUVs were deployed for 35 min, with the first 5 min considered a buffer time to allow fauna to respond to disturbance. The remaining 30 min were used for analysis which is considered adequate time to assess fish assemblages (Wraith et al., 2013). All BRUVs were deployed from a boat during daylight hours on an incoming tide, around one hour after low tide in order to reduce variability in bait plume area caused by current velocity (e.g., Taylor et al., 2013; Piggott et al., 2020). All deployments were conducted within 5 days of a low spring tide and therefore the majority of plots were placed in seagrass areas that were almost always subtidal.
Each of the 55 plot videos was analyzed to determine the MaxN of each fish species and fish species richness; a metric commonly used for the quantification of the relative abundance of fish observed in underwater video (Cappo et al., 2004; Unsworth et al., 2014). MaxN is equal to the maximum number of fish recorded at any one time (single video frame) and therefore removes concerns associated with double counting of individual fish (Priede et al., 1994). MaxN was determined for each species in every video frame throughout the 30 min of footage. The highest MaxN for each species at the end of each 30 min was used in further analysis.
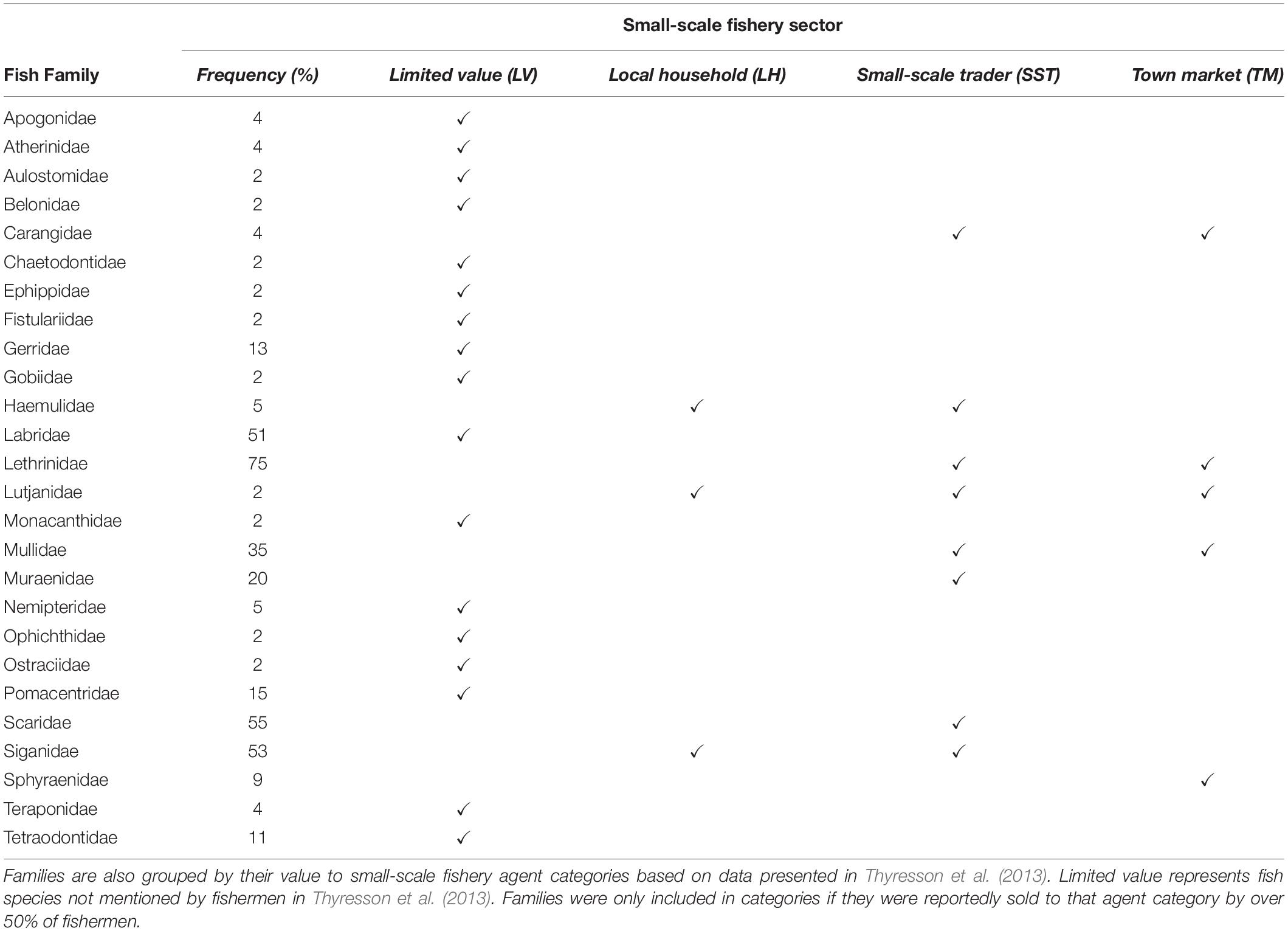
Fish were identified to species level where possible using FishBase (Froese and Pauly, 2015) and numerous identification guides (Richmond, 2011; Smith and Heemstra, 2012; Taquet and Alain, 2012). All fish species identified were then categorized into four categories based on perceived value to small-scale fisheries sectors [based on Thyresson et al. (2013)]; (i) Low Value [species not mentioned by Thyresson et al. (2013)], (ii) Local Household, (iii) Small-Scale Trader and (iv) Town Market (Table 3).
Data Analysis
All statistical analyses were conducted using R version 3.6.3 (R Development Core Team, 2020). We first calculated community weighted functional trait indices (functional dispersion) and functional richness using the FD package in R (Laliberté and Legendre, 2010). Using a trait matrix based on leaf growth form and canopy type (see seagrass community structure), we calculated functional dispersion by using species trait scores and species abundance (based on species composition). Functional dispersion, a measure of functional diversity, is defined as the mean distance in multidimensional trait space of individual species to the centroid of all species, weighted by abundance (Laliberté and Legendre, 2010); in other words how morphologically different each seagrass species is compared to the average seagrass morphology in the local community. The same approach was used for functional richness, but negated species abundance and instead used species presence to account for richness. Seagrass community variables were grouped into four distinct categories pertinent to the hypotheses: meadow structure (shoot density, canopy height, leaf length, leaf width, and number of leaves per shoot), cover and composition (seagrass cover, seagrass composition), richness (species richness, functional richness) and functional dispersion (Table 1). Given that multiple variables were included within the category meadow structure, a Principle Component Analysis (PCA) was performed using the prcomp() function in R and the values for PC1 decomposed and exported to create a single variable accounting for the majority of variance (James and McCulloch, 1990; Harrison et al., 2018). We accounted for dispersion in meadow structure (e.g., whether meadows are similar in their structural properties) by computing a PCA on all structural quadrat variables (n = 1210) and calculated the coefficient of variation (mean/standard deviation) for the PC1 values for each of the 55 plots. We called this variable meadow structure variability. We also used the PCA approach for seagrass composition (% composition of each species present as a proportion of total cover) and for land-use (% area of bare ground, human development, aquaculture, farmland, vegetation, ocean within a 2 km radius and distance to catchment).
In the PCA of meadow structure, PC1 accounted for 58.02% of the variance. All variables had substantial factor loadings on PC1 which was negatively correlated with canopy height, leaf length, leaf width and number of leaves per shoot and positively correlated with shoot density (Supplementary Figure 1). We inverted this axis so that high PC1 values represent high seagrass structural complexity (e.g., higher canopy, longer and wider leaves, greater number of leaves per shoot) and low values represent low seagrass structural complexity. For seagrass composition, PC1 accounted for 53.35% of variation and was positively associated with higher abundance of canopy-forming species (e.g., T. ciliatum) and negatively associated with shorter, meadow-forming species (e.g., C. serrulata, T. hemprichi; Supplementary Figure 2). In the PCA for land-use, PC1 was responsible for 58.14% of variance and negatively associated with area of human development and farmland, and positively associated with distance from catchment and area of ocean (Supplementary Figure 3). We also inverted this axis so that low values represent low impact sites, and higher values represent high impact sites.
We then used mixed-effects linear models with maximum likelihood estimation to evaluate the influence of our seagrass community variables on seagrass fish assemblages. We determined the maximum complexity of models by using the square root of N (√55 = 7.4), and the most complex model included 6 parameters. We specifically examined whether response and predictor variables were normally distributed and whether there was multicollinearity between any of the predictors by calculating the variance inflation factor (VIF). We used log (MaxN, seagrass species richness, community composition, functional richness and functional dispersion, depth, distance to coral reef) and cube root transformations (meadow structure variability) to transform predictors and response variables, and used Pearson’s correlation tests to test for multicollinearity (r > 0.7) (Dormann et al., 2013). Based on these tests we excluded distance to mangrove, distance to coral reef, distance to landing site and fishing pressure from the final variable set, as all were strongly correlated with land-use (Supplementary Figure 4). Our final variable set consisted of 9 predictors (seven seagrass community predictors, two environmental predictors), all with VIF values < 3 (Zuur et al., 2010).
Using linear mixed-effects models (Zuur et al., 2009), we explored the relative importance of seven seagrass variables (meadow structure, meadow structure variability, seagrass cover, seagrass species richness, community composition, functional richness and functional dispersion), depth and land-use on two response variables: fish abundance and fish species richness. We also computed models to evaluate the effects of seagrass variables on the abundance of fish within the four value categories. Linear mixed-effects models were fitted with the lmer() function in the lme4 package for R (Bates et al., 2015). Each of the seagrass predictors was tested independently with an interaction with land-use and depth as fixed predictors, given that land-use influences seagrass condition (Quiros et al., 2017) and depth influences fish abundance (Alonso Aller et al., 2014). Given that the 55 plots were nested within 12 sites, all models included a random effect of site to account for biogeographic differences in environmental conditions and potential differences in fishing pressure (given we excluded it). Based on Akaike’s Information Criterion (AIC) scores (Akaike, 1974), we then pruned each model without removing the seagrass variable itself. We also included a null model that included land-use, depth and the random factor of site to compare against seagrass predictors. We report the fit of models using R2GLMM which was calculated using the rsquared() function from the piecewiseSEM package for R (Lefcheck, 2016).
Results
General Description of Fish Assemblages
A total of 1,676 individual fish, representing 65 species and 26 families, were recorded. Many of the species were rare (<10 observed individuals) and five fish families (Siganidae, Lethrinidae, Lutjanidae, Scaridae, and Labridae) were responsible for over 85% of the total abundance pooled across sites. In addition, fish families important for human consumption in the region were the most common, with emperors (Lethrinidae) occurring in 75% of samples, followed by parrotfish (Scaridae, 55%), rabbitfish (Siganidae, 53%), wrasses (Labridae, 51%), goatfish (Mullidae, 35%) and moray eels (Muraenidae, 20%). For six of the samples, no fish species were recorded and only two sites recorded greater than 100 individuals.
Seagrass Meadow Variables Driving Fish Abundance and Diversity
We compared seven models testing the influence of seagrass meadow variables on fish abundance (MaxN), along with a null model (land-use + depth). The best-fitting model (lowest AIC) included the additive effects of meadow structure and depth, with an AICc weight of 0.99 (R2 = 0.73; Table 4). Meadow structure had a positive effect on fish abundance; meadows with taller canopies, longer, broader and more numerous leaves and lower shoot density had higher fish abundances (Figure 3). All other models had a Δ AIC > 10, and only seagrass cover, functional dispersion and meadow structure variability were better predictors of fish abundance than the null model (Table 4).
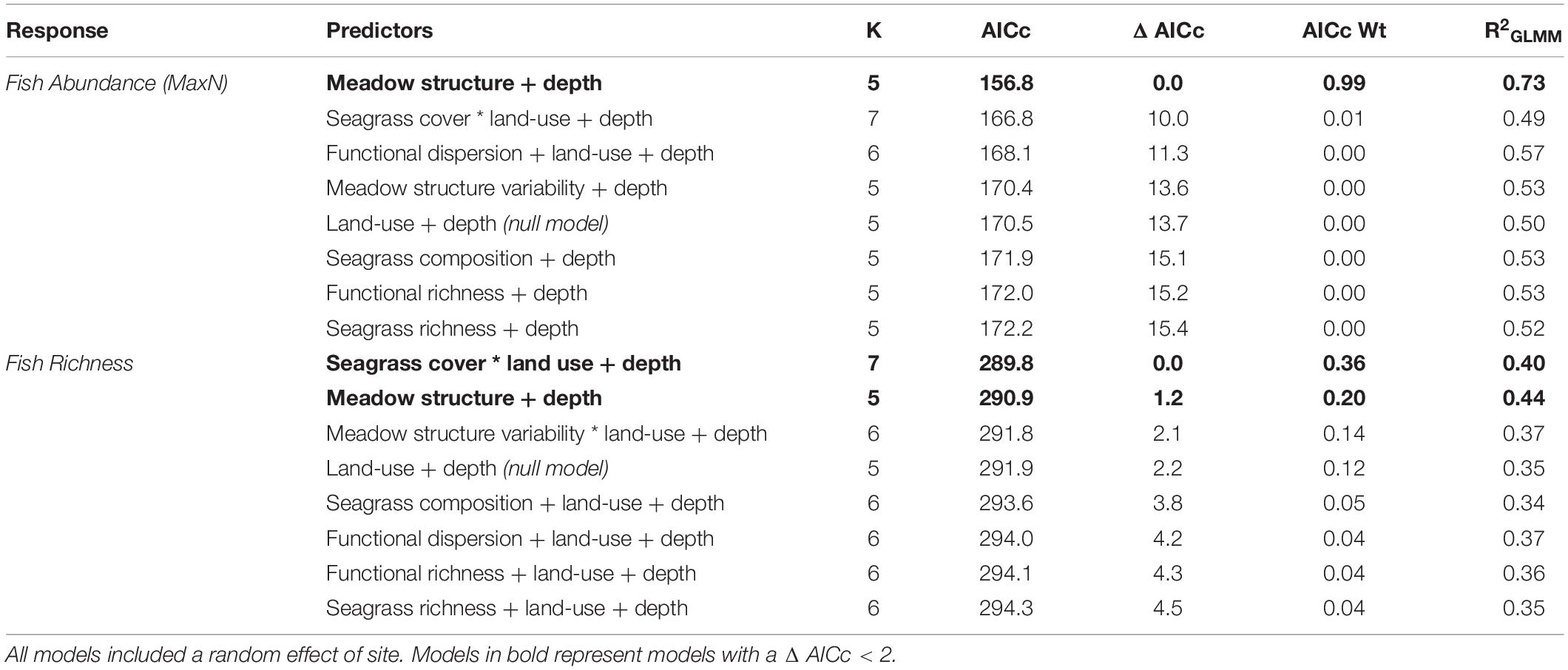
Table 4. Candidate models for fish abundance (MaxN) and richness, sorted by AIC corrected for small sample sizes.
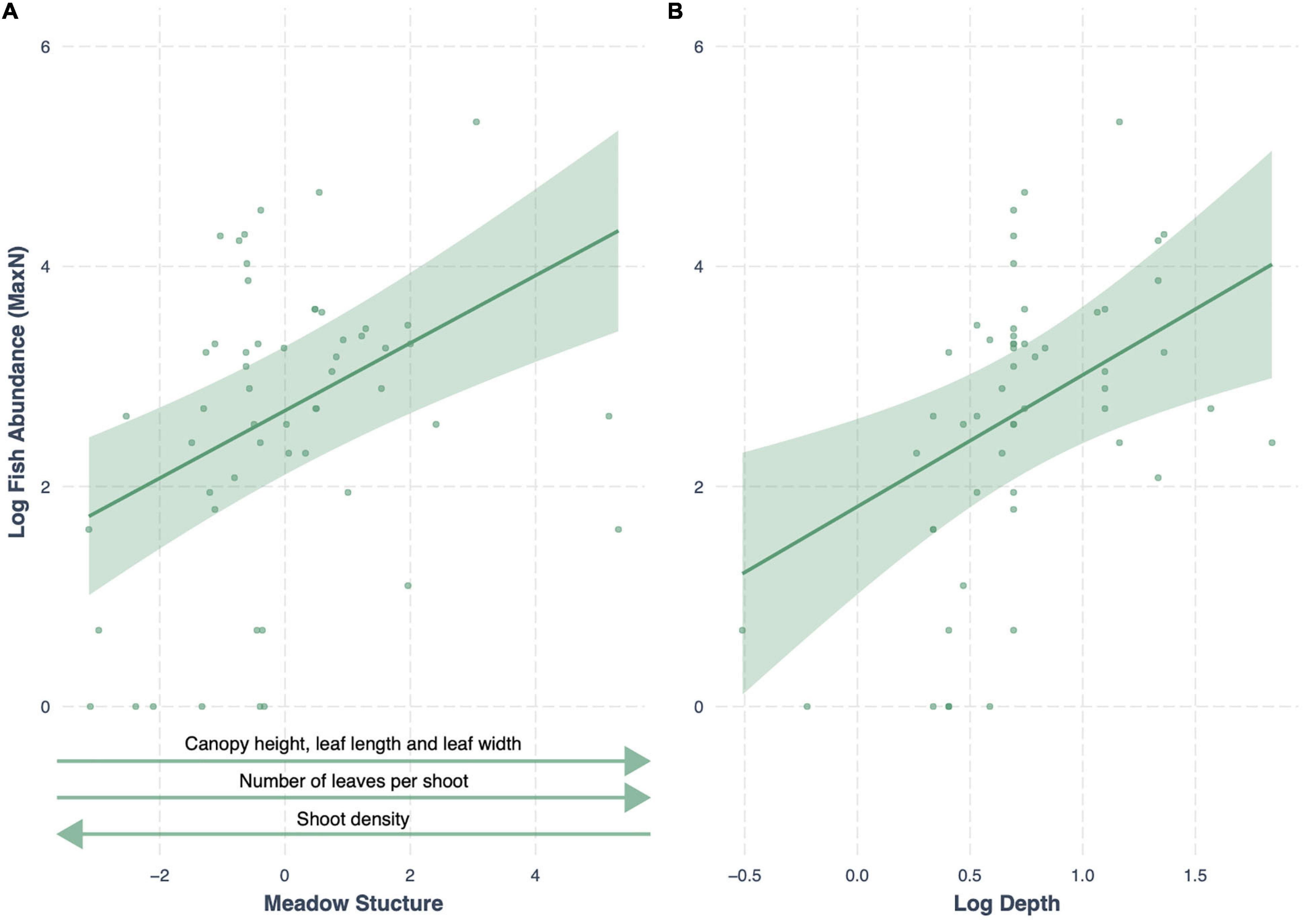
Figure 3. Conditional effect plots for the model best predicting log fish abundance (R2 = 0.73) in tropical seagrass meadows across 12 sites. Predictor variables include (A) meadow structure (inverse of PC1) and (B) log depth (m). Shaded areas represent 95% confidence intervals, reflecting the variance of the fixed effects.
In terms of fish richness, the best model included an interaction between seagrass cover and land-use, as well as the effect of depth, and had an AICc weight of 0.36 (R2 = 0.40; Table 4). Seagrass cover positively influenced fish richness at sites with low levels of human development. This influence declined with increasing human development, with no effect at the highest levels of development (e.g., sites close to large human populations; Figure 4). However, while seagrass cover had little effect at sites with the highest levels of human development, it was at these sites where we recorded high fish species richness – regardless of whether seagrass cover was low or high. Meanwhile, fishing pressure was negatively associated with human development (see Supplementary Material Appendix 1 and Figure 1). Consequently, sites with higher fish richness were also sites with lower fishing pressure. Finally, depth influenced fish assemblages, with generally greater richness observed in deeper seagrass areas.
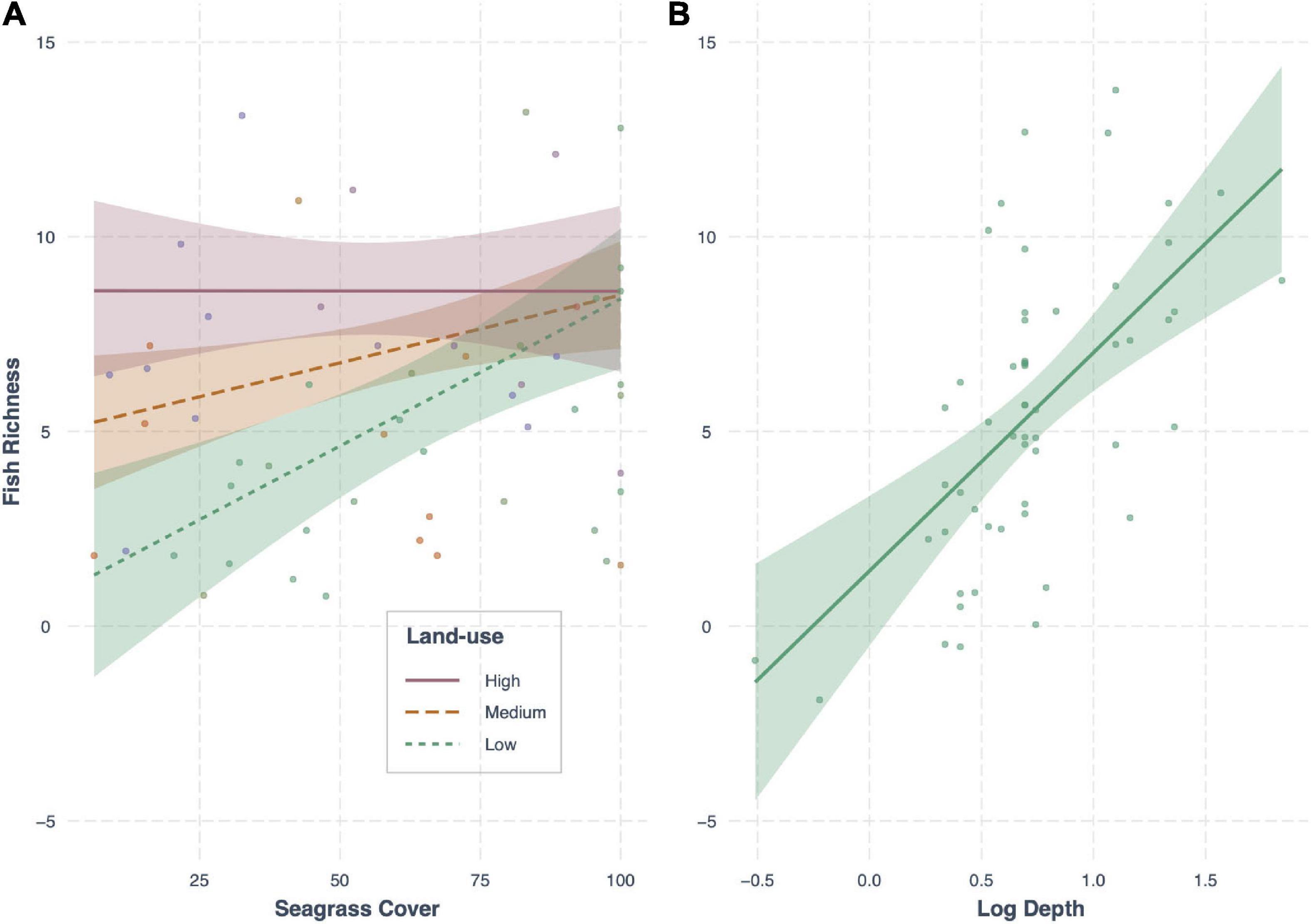
Figure 4. Conditional effect plots for the model best predicting fish richness (R2 = 0.47) in tropical seagrass meadows across 12 sites. Predictor variables include (A) the interaction between land-use (low, medium, high) and seagrass cover (%), and (B) log depth (m). Shaded areas represent 95% confidence intervals, reflecting the variance of the fixed effects.
Meadow structure was also a strong predictor of seagrass fish richness, with an AICc weight of 0.20 (R2 = 0.44), with an additive effect of depth (Δ AIC < 2). As with fish abundance, meadow structure and depth had a positive effect on fish richness; generally deeper seagrass meadows with complex canopy structures (high canopy, long and more numerous leaves, but low shoot density) had greater fish richness (Figure 5). All other models had a Δ AIC score > 2, and only meadow structure variability was a better predictor than the null model (Table 3).
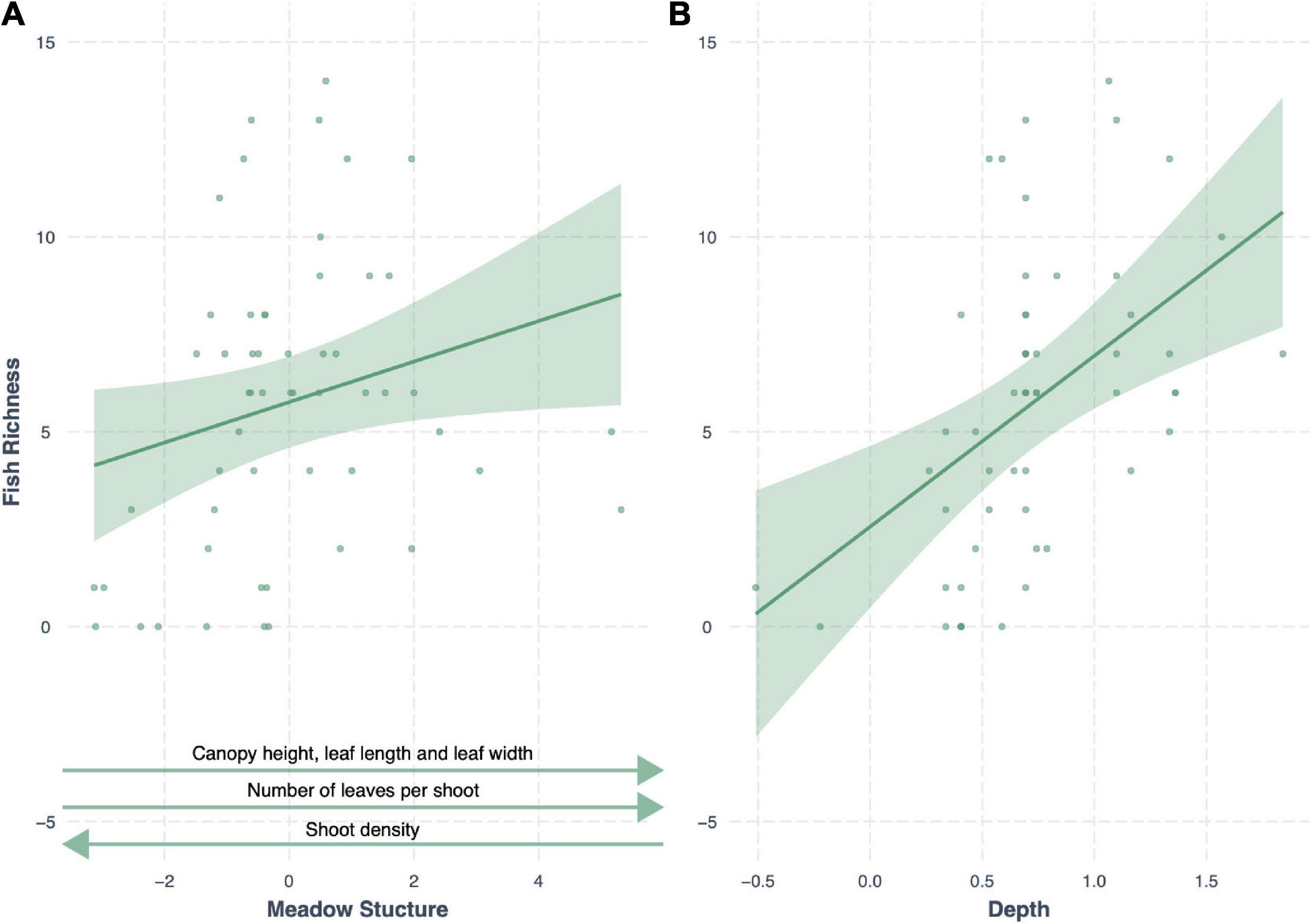
Figure 5. Conditional effect plots for the second-best model predicting fish richness (R2 = 0.42) in tropical seagrass meadows across 12 sites. Predictor variables include (A) meadow structure (inverse of PC1) and (B) log depth (m). Shaded areas represent 95% confidence intervals, reflecting the variance of the fixed effects.
For both response variables tested (fish abundance and richness), seagrass richness, functional richness and seagrass composition were poor predictors of fish richness, all with AICc weights of less than 0.05 for both response variables (Table 4) and performing worse than the null model.
Seagrass Meadow Variables Driving Abundance of Fish Important to Small-Scale Fishery Sectors
Three models with a cumulative AICc weight of over 0.83, were important for driving the abundance of fish important for sale at town markets (e.g., Carangidae, Sphyraenidae, and Mullidae; Table 5). These were seagrass meadow structure (R2 = 0.47), functional dispersion (R2 = 0.44) and seagrass cover (R2 = 0.34; Table 5). Meadow structure and depth had positive effects on abundance for this fish group (Figure 6), as did seagrass cover, which included the additive effects of land-use and depth (both of which were positive for abundance). However, functional dispersion had a negative effect on abundance, with more fish present when certain functional traits are present.
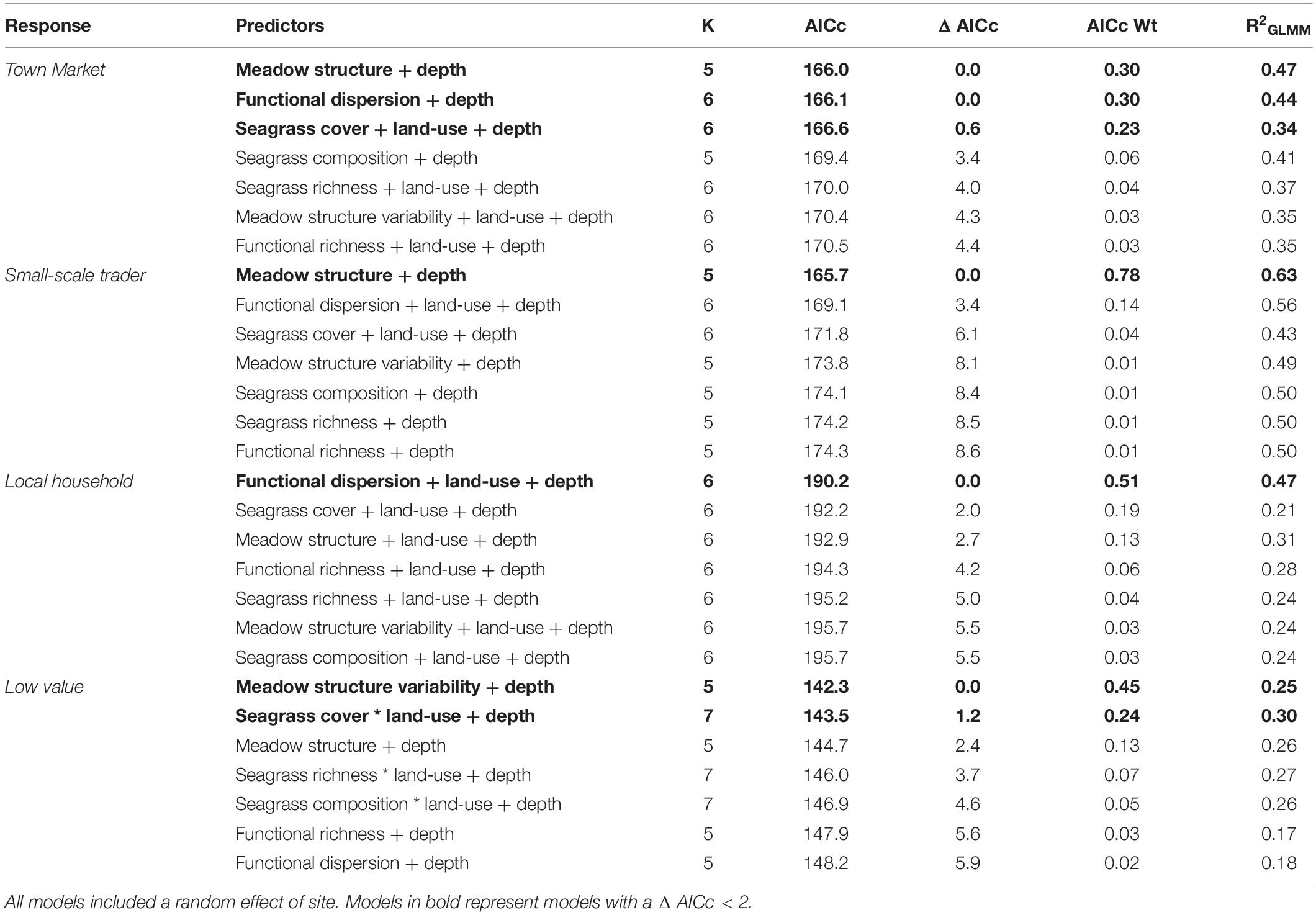
Table 5. Candidate models for fish abundance across small-scale fishery sectors, sorted by AIC corrected for small sample sizes.
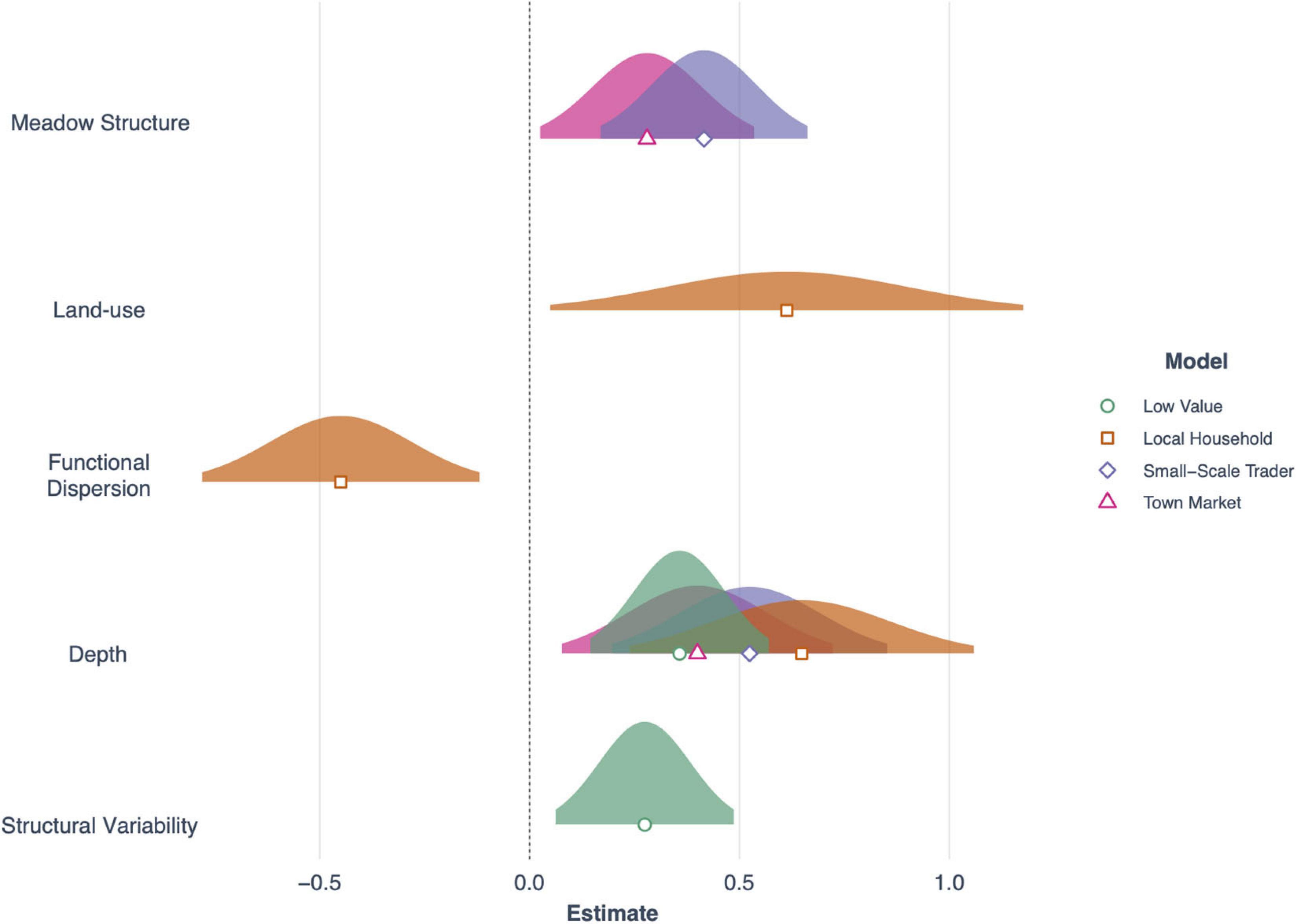
Figure 6. Forest plot showing the effects of seagrass and environmental predictors across the four fish value categories used in this study (Low value, Local household, Small-scale trader and Town market). Plotted are normal distributions of 95% confidence intervals.
The abundance of fish important for sales to small-scale traders was primarily explained by seagrass meadow structure (Figure 6), with an AICc weight of 0.78 (R2 = 0.63). Meadow structure and depth influenced fish abundance (Table 5), with deeper and more complex meadows (e.g., high canopy height, leaf length) characteristic of higher abundance of fish from this fishery sector. All other models had a ΔAIC of > 2.
The abundance of fish important to local household consumers was best predicted by two candidate models with a cumulative AICc weight of 0.70 (Table 5). Functional dispersion was the best predictor (R2 = 0.47) followed by seagrass structure (R2 = 0.21). In the top model, functional dispersion and land-use both had a negative effect on fish abundance, suggesting more fish are present when certain functional traits are in high abundance, situated away from areas with high human development. Seagrass meadow structure was positively associated to fish abundance.
Finally, the abundance of fish species with a limited or low value to small-scale fisheries sectors was best explained by two models, with a cumulative AICc weight of 0.69. These were meadow structure variability (R2 = 0.25) and seagrass cover (R2 = 0.30; Table 5). Both models included an effect of depth, and the seagrass cover model also included an interaction between seagrass cover and land-use. As with total fish richness, the models suggested that seagrass cover was important for driving fish abundance in areas of low human development but not in high. Meadow structure variability had a negative effect on the abundance of fish with limited or low value; sites with higher more variable structural traits had lower abundance of fish species.
Discussion
The present study provides a unique analysis of the relationship between different features of seagrass habitats and their associated fish assemblages. We show that specific seagrass traits and seagrass cover drive seagrass fish assemblages, and that seagrass species diversity (both taxonomic and functional) had little effect. Understanding how biodiversity influences the structure and function of faunal assemblages is key (Morin et al., 2018), and a necessity for more effective marine protection. While trait-based approaches have been utilized to gain a greater understanding of the mechanisms that drive fish assemblages in coral reefs (Darling et al., 2017), this study is one of few to use such an approach for seagrass meadows.
In our study, seagrass meadow structure consistently drove two fish assemblage indicators; abundance (MaxN) and richness. Specifically, seagrass meadows with greater structure (e.g., higher canopy, longer and wider leaves, greater numbers of leaves per shoot, and lower overall shoot density) harbored faunal assemblages with greater abundance and richness (Figure 7). We stress here the importance of structural complexity in influencing seagrass fish assemblages (Gullstrom et al., 2008), given that more structurally complex meadows increase habitat complexity and the availability of food resources, and reduce predation pressure (Hovel et al., 2002; Vonk et al., 2010). However, in our analysis, we compressed numerous seagrass trait characteristics into a single variable, meadow structure, which accounted for 58% of variability across sites. Therefore, in our attempt to create an index for seagrass structural complexity, we advertently lost variability which is important to note when interpreting these findings.
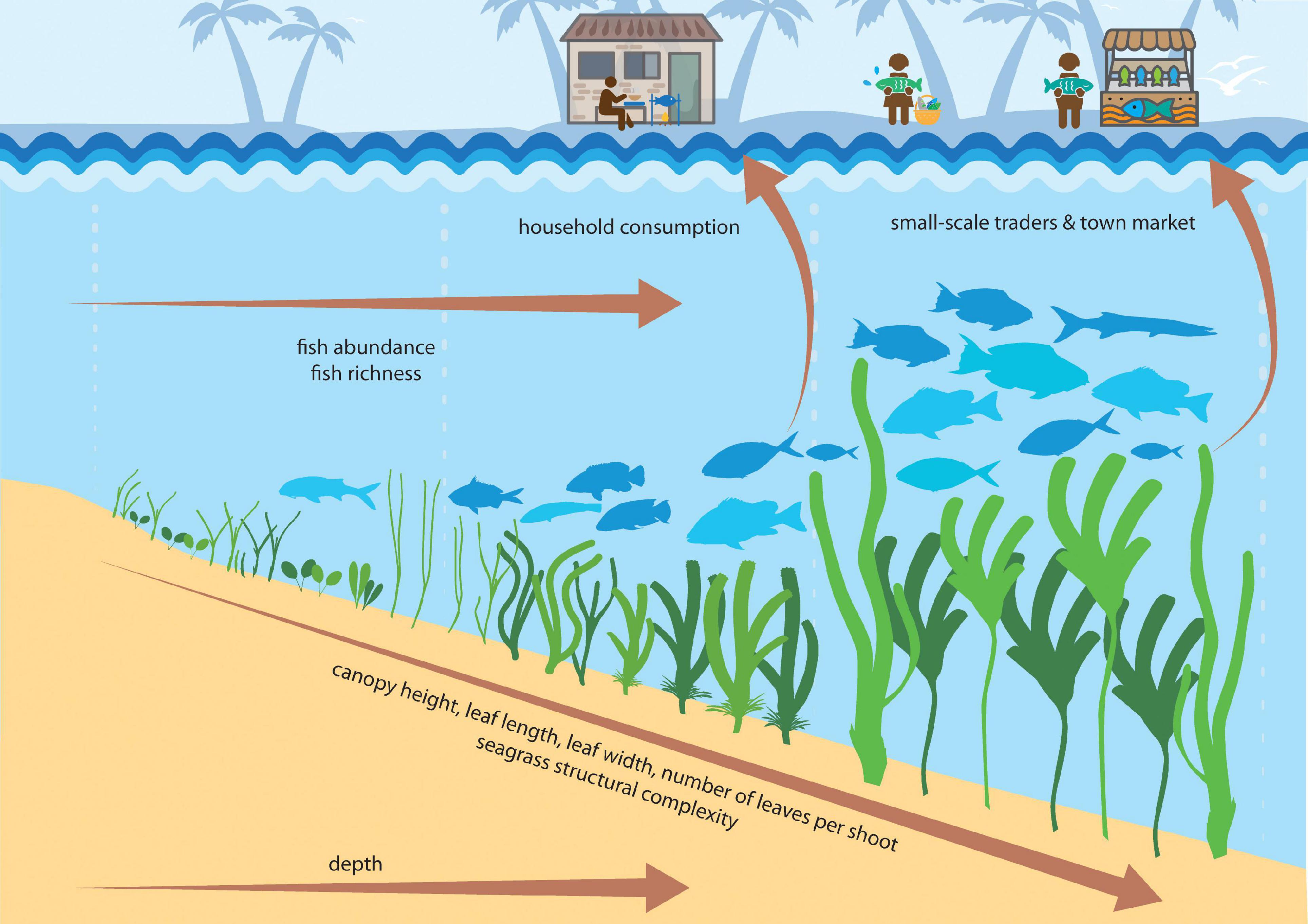
Figure 7. Conceptual diagram showing how seagrass structural complexity influences seagrass social-ecological systems. Seagrass meadows with taller canopies, longer and wider leaves and more numerous leaves per shoot have greater fish abundance and richness, and in particular support species of value to small-scale fisheries.
Unlike any previous work (which focus on one or two seagrass species), our study tested the relative importance of all seagrass genera found within the tropical seascape and can suggest that the important traits (e.g., long leaves, high canopy) were primarily driven by dominant seagrass species rather than high seagrass species diversity (e.g., mass ratio: Grime, 1998; Díaz et al., 2007). So far we can only speculate on the reason(s) behind the lack of a seagrass diversity (richness, functional dispersion, etc.) effect. One potential reason could be that the diversity gradient is relatively short (1-6 co-occurring species in plots) compared to that in e.g., terrestrial grasslands, or that co-occurring seagrass species may compete more than complement each other (e.g., many are functionally similar in terms of structure). It should also be noted that our results do not exclude the possibility of stronger seagrass diversity effects at larger spatial and/or temporal scales (Bracken et al., 2017) or for other processes/functions or ‘ecosystem multifunctionality’ (Lefcheck et al., 2015).
Depth was also a factor driving fish assemblages, supported by previous studies conducted in Zanzibar (Gullstrom et al., 2008; Alonso Aller et al., 2014), as well as others in the tropics that show that deep seagrass meadows are important for species such as emperors and rabbitfish (Hayes et al., 2020). Given both these fish groups accounted for a large proportion of overall fish abundance, this may explain these findings. Using a novel technique to locate seagrass meadows within the region, Esteban et al. (2018) showed that deep seagrass meadows, comprised of a single dominant species (T. ciliatum), supported diverse and near-pristine fish assemblages. The broadly positive effects that we see here may be due to several factors such as their likely position within the seascape and the increased likelihood of being in closer proximity to adjacent coral habitat (Gullstrom et al., 2008).
Seagrass fish richness was best explained by an interaction between seagrass cover and land-use intensity; a variable negatively correlated with fishing pressure. Conforming to previous studies in the region (e.g., Alonso Aller et al., 2014), the positive effect of seagrass cover on fish richness decreased with increasing human impact (higher land-use), with no effect where human populations were highest. However, in our study, we surprisingly found that human impacts had no single effect on fish richness. Where there was greatest human development, fish richness remained high regardless of whether seagrass cover was low or high. One possible explanation for this lack of effect may be that high human development in fact acts to buffer local fishing pressure and benefit local fish populations, because of the high frequency of boat traffic but also potential concerns over water quality in these areas. Such sites were Stone Town Harbor and Maruhubi, situated just outside the Port of Zanzibar (Figure 1). The area is well-known as being polluted (De Wolf and Rashid, 2008) and highly disturbed (Khamis et al., 2019), but still harbored high abundances of both fish and perceivably healthy seagrass communities. In these areas, seagrass cover (or meadow structure) had no effect on fish richness; the simple presence of seagrass was enough to support high fish richness.
In general, the strong effects of seagrass meadow structure, seagrass cover and depth were also observed when looking at the abundance of fish species valuable to town markets and small-scale traders. The fish species included in these value categories were primarily predators (fish and invertebrate feeders), with exception of herbivorous parrotfish and rabbitfish. For the predators, the positive effects of complexity are likely driven by increased food availability (Hovel et al., 2002; Vonk et al., 2010); more complex structures provide a greater range of habitats for fish and invertebrates. However, these predators are in turn also eaten by larger predators occasionally feeding in seagrass areas (e.g., sharks), so they may also benefit from seagrasses for shelter. The key role of depth here is specifically important for herbivores given commercially valuable rabbitfish species use networked habitats (coral and seagrass), but have a small home range of <200 m (Ebrahim et al., 2020a). Deeper seagrass meadows, with higher structural complexity, are generally closer to adjacent coral structures. Given that rabbitfish can dominate catches across the Western Indian Ocean (Hicks and McClanahan, 2012) and are a source of protein that sustain humans populations (Grandcourt and Cesar, 2003), our findings provide evidence that high structure seagrass meadows are key in supporting this provision. Moreover, meadows of this type support the fish species of value to fish traders (so called ‘middlemen’); a stakeholder group instrumental in shaping social-ecological small-scale fishery systems (Crona et al., 2010; Fröcklin et al., 2013).
Three families key in the household consumption category – grunts, snappers and rabbitfish (Thyresson et al., 2013) – are reportedly declining in the region (Benansio and Jiddawi, 2016). Their abundance was negatively influenced by functional dispersion – seagrass meadows with similar traits were more important – but positively influenced by depth and land use. Given that seagrass structure was not a driver, these effects were likely driven by meadows comprised of intermediate genera like Thalassia and Cymodocea which have similar traits, that were closer to areas of human populations with greater epiphyte coverage. Epiphytic material is an important food source for many small invertebrate grazers in tropical seagrass meadows (Belicka et al., 2012), which can be a primary food source for juvenile grunts and snappers (de la Moriniere et al., 2003). Likewise, epiphytes constitute an important food source for generalist herbivores like rabbitfish (Ebrahim et al., 2020b).
Fish categorized as low value were primarily small bodied omnivores, and while the effect size was low, the local abundance of these species was driven by high variability in seagrass structural traits (i.e., high coefficient of variation for seagrass structure). Omnivores select food based on a range of characteristics including nutrition, shape and texture. Meanwhile, Prado and Heck (2011) found that variability in seagrass structural traits — different seagrass shapes and sizes — were a dominant driver of food choice. Specifically, this is because narrow (e.g., Halodule, Syringodium) and paddle shaped (e.g., Halophila) seagrass leaves are easier to manipulate for smaller bodied fish (Prado and Heck, 2011). This likely explains why the abundance of this fish category increased with trait variability but was not influenced to the same extent by meadow structure (e.g., dominant traits). Finally, it should be noted that while these species were low value in fishery terms, they can contribute to important ecological functions such as regulating epiphyte loads (Gilby et al., 2016).
Conservation is generally underpinned by protecting key species or habitats (Caveen et al., 2014). This has led to the creation of numerous well intentioned yet misplaced marine protected area’s (MPA’s), which fail to meet the function they were intended for Jantke et al. (2018). In the wake of calls to acknowledge seagrass meadows within the marine conservation agenda (e.g., Unsworth et al., 2019a), we are increasingly seeing seagrass meadows protected or highlighted as priority areas for protection (e.g., as nursery areas or for ‘blue carbon’). However, a failure to align policy with science, means these protected seagrass areas could have a limited function for the reasons that they have been protected. For example, Cambodia’s first MPA designates seagrass for its nursery function (Boon et al., 2014), yet the meadow in question is dominated by sparse growing Halodule and Halophila spp. (Phalla et al., 2014), which based on our analysis here could provide very limited function as a fish habitat due to their trait characteristics (e.g., low canopy, small leaves, low number of leaves per shoot). BRUV surveys conducted within the MPA in Cambodia support this, with very low fish abundance (Gourlay, 2017).
It is prudent to acknowledge that traits associated with fish assemblages may not be the only important variables to consider when thinking about ecosystem services more broadly. For example, the aboveground traits that were important for driving fish assemblages in this study, such as high canopy and increased seagrass cover, have little effect on sedimentary carbon trapping, which is instead driven by belowground traits and sediment type (Gullström et al., 2018). Therefore, for effective management of seagrass meadows across the Indo-Pacific, we stress the importance of pairing reason for protection with ecological functions. This is fundamental given calls for community seagrass conservation based on payments for ecosystem services (UNEP, 2020). As seagrass ecosystem provision is non-linear among seagrass taxa it is not economically, ecologically or socially efficient and sustainable to protect seagrass meadows if the seagrass species present do not provide the ecosystem services in question (Nordlund et al., 2016).
In conclusion, we show that fish assemblages in tropical seagrass meadows are driven by characteristics provided by dominant species, such as high structural complexity and cover, as more broadly by depth. Consequently, we show that seagrass diversity (both functional and species) had little effect on fish assemblages. While this study was conducted in Zanzibar Island, Tanzania, the seagrass species studied here are common across the Indo-Pacific in similar densities (e.g., Short et al., 2007; Ambo-Rappe et al., 2013; Jones et al., 2018; Jinks et al., 2019), provide habitat for the same groups of fish families (Unsworth et al., 2008; Pogoreutz et al., 2012; Honda et al., 2013) and are subsequently targeted by small-scale fishers (e.g., Cullen-Unsworth et al., 2014; de la Torre-Castro et al., 2014; Quiros et al., 2018). Our findings are therefore not just useful for Zanzibar, but across the Indo-Pacific and show that different types of seagrass meadows have different values for both ecology and society. Placing the results of this study in a social-ecological systems context, focusing on fisheries, then leaves us in a predicament. From an ecological perspective, priority areas for seagrass conservation are high cover and high canopy, as these are areas with both the highest abundance and richness of fish species. Yet from a social perspective, these areas are likely the best and most favorable fishing grounds. On the one hand, protecting these habitats either as an MPA or no take area would therefore force fishers to increase fishing effort in other areas, with implications for livelihoods and food security. On the other hand, protecting seagrass areas with the most abundant fish assemblages may, in theory, generate enough ‘spillover’ to enhance fisheries in those nearby areas (McClanahan and Mangi, 2000; Januchowski-Hartley et al., 2013). Our study revealed that seagrass sites in Zanzibar close to large human populations have high fish richness and abundance, and low relative fishing intensity. While the mechanisms underpinning this need further study, qualitative observations suggest that fishers perceive these areas as either too ‘dirty’ (polluted) or inaccessible due to the high frequency of boat traffic, to utilize as fishing grounds. These findings somewhat flip the so called human ‘gravity’ hypothesis that exists for coral reefs (Cinner et al., 2018), i.e., that with increasing human population size and accessibility to coral reefs conservation gains are diminished (increased fish biomass and predators). Whether this is specific to Zanzibar or a wider case for seagrass warrants further investigation. If this is indeed the case, then these relatively unfished areas adjacent to areas of high land-use could become a priority, or an easy win, for fisheries management strategies, that could help fuel recruitment for nearby and fished areas.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: All data and R code is available from https://github.com/BoardshortsBen/SeagrassFish.
Author Contributions
BJ, JE, and LN conceived the ideas and designed the study with methodological input from RU and site choice input from NJ. BJ collected and analyzed all data and led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Funding
This research was supported by the Swedish Research Council (Grant Numbers 2017-05535 and 2017-05411). Open Access fees were provided by Stockholm University.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
The authors would like to thank local boat operators in Zanzibar, and in particular Mwinyi Mwaka of Unguja Ukuu for providing valuable local ecological knowledge.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.640528/full#supplementary-material
References
Akaike, H. (1974). A new look at the statistical model identification. IEEE Trans. Autom. Control 19, 716–723. doi: 10.1109/tac.1974.1100705
Alonso Aller, E., Gullström, M., Eveleens Maarse, F. K. J., Gren, M., Nordlund, L. M., Jiddawi, N., et al. (2014). Single and joint effects of regional- and local-scale variables on tropical seagrass fish assemblages. Mar. Biol. 161, 2395–2405. doi: 10.1007/s00227-014-2514-7
Ambo-Rappe, R., Nessa, M. N., Latuconsina, H., and Lajus, D. L. (2013). Relationship between the tropical seagrass bed characteristics and the structure of the associated fish community. Open J. Ecol. 3, 331–342. doi: 10.4236/oje.2013.35038
Andradi-Brown, D. A., Macaya-Solis, C., Exton, D. A., Gress, E., Wright, G., and Rogers, A. D. (2016). Assessing caribbean shallow and mesophotic reef fish communities using baited-remote underwater video (BRUV) and diver-operated video (DOV) survey techniques. PLoS One 11:e0168235. doi: 10.1371/journal.pone.0168235
Angelini, C., Altieri, A. H., Silliman, B. R., and Bertness, M. D. (2011). Interactions among foundation species and their consequences for community organization, biodiversity, and conservation. BioScience 61, 782–789. doi: 10.1525/bio.2011.61.10.8
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models usinglme4. J. Stat. Softw. 67:48.
Belicka, L. L., Burkholder, D., Fourqurean, J. W., Heithaus, M. R., Macko, S. A., and Jaffé, R. (2012). Stable isotope and fatty acid biomarkers of seagrass, epiphytic, and algal organic matter to consumers in a pristine seagrass ecosystem. Mar. Freshw. Res. 63, 1085–1097. doi: 10.1071/mf12027
Bell, J. D., and Westoby, M. (1986a). Importance of local changes in leaf height and density to fish and decapods associated with seagrasses. J. Exp. Mar. Biol. Ecol. 104, 249–274. doi: 10.1016/0022-0981(86)90109-7
Bell, J. D., and Westoby, M. (1986b). Variation in seagrass height and density over a wide spatial scale - effects on common fish and decapods. J. Exp. Mar. Biol. Ecol. 104, 275–295. doi: 10.1016/0022-0981(86)90110-3
Benansio, J. S., and Jiddawi, N. (2016). Investigating changes in fish biodiversity in coastal villages of Zanzibar Island, Tanzania. Int. J. Fish. Aquac. 8, 117–125.
Benkwitt, C. E., Wilson, S. K., and Graham, N. A. J. (2020). Biodiversity increases ecosystem functions despite multiple stressors on coral reefs. Nat. Ecol. Evol. 4, 919–926. doi: 10.1038/s41559-020-1203-9
Beukers, J. S., and Jones, G. P. (1998). Habitat complexity modifies the impact of piscivores on a coral reef fish population. Oecologia 114, 50–59. doi: 10.1007/s004420050419
Bond, M. E., Babcock, E. A., Pikitch, E. K., Abercrombie, D. L., Lamb, N. F., and Chapman, D. D. (2012). Reef sharks exhibit site-fidelity and higher relative abundance in marine reserves on the Mesoamerican Barrier Reef. PLoS One 7:e32983. doi: 10.1371/journal.pone.0032983
Boon, P. Y., Mulligan, B., Benbow, S., Thorne, B., Phalla, L., and Longhurst, K. (2014). Zoning cambodia’s first marine fisheries management area. Cambodian J. Nat. Hist. 2014, 55–65.
Bornt, K. R., Mclean, D. L., Langlois, T. J., Harvey, E. S., Bellchambers, L. M., Evans, S. N., et al. (2015). Targeted demersal fish species exhibit variable responses to long-term protection from fishing at the Houtman Abrolhos Islands. Coral Reefs 34, 1297–1312. doi: 10.1007/s00338-015-1336-5
Bracken, M. E. S., Douglass, J. G., Perini, V., and Trussell, G. C. (2017). Spatial scale mediates the effects of biodiversity on marine primary producers. Ecology 98, 1434–1443. doi: 10.1002/ecy.1812
Bruno, J. F., and Bertness, M. D. (2001). “Habitat modification and facilitation in benthic marine communities,” in Marine Community Ecology, eds M. D. Bertness, S. D. Gaines, and M. E. Hay (Sunderland, MA: Sinauer Associates), 201–216.
Burke, L., Reytar, K., Spalding, M., and Perry, A. (2011). Reefs at Risk Revisited. Washington, DC: World Resources Institute.
Caley, M. J., and St John, J. (1996). Refuge availability structures assemblages of tropical reef fishes. J. Anim. Ecol. 43, 414–428. doi: 10.2307/5777
Campbell, S. J., Kartawijaya, T., and Sabarini, E. K. (2011). Connectivity in reef fish assemblages between seagrass and coral reef habitats. Aquat. Biol. 13, 65–77. doi: 10.3354/ab00352
Cappo, M., De’ath, G., and Speare, P. (2007). Inter-reef vertebrate communities of the Great Barrier Reef Marine Park determined by baited remote underwater video stations. Mar. Ecol. Prog. Ser. 350, 209–221. doi: 10.3354/meps07189
Cappo, M., Speare, P., and De’ath, G. (2004). Comparison of baited remote underwater video stations (BRUVS) and prawn (shrimp) trawls for assessments of fish biodiversity in inter-reefal areas of the Great Barrier Reef Marine Park. J. Exp. Mar. Biol. Ecol. 302, 123–152. doi: 10.1016/j.jembe.2003.10.006
Cardinale, B. J., Matulich, K. L., Hooper, D. U., Byrnes, J. E., Duffy, E., Gamfeldt, L., et al. (2011). The functional role of producer diversity in ecosystems. Am. J. Bot. 98, 572–592. doi: 10.3732/ajb.1000364
Caveen, A., Polunin, N., Gray, T., and Stead, S. M. (2014). The Controversy Over Marine Protected Areas: Science Meets Policy. Berlin: Springer.
Cinner, J. E., Maire, E., Huchery, C., MacNeil, M. A., Graham, N. A. J., Mora, C., et al. (2018). Gravity of human impacts mediates coral reef conservation gains. Proc. Natl. Acad. Sci. U.S.A. 115, E6116–E6125.
Coleman, M., Bates, A., Stuart-Smith, R., Malcolm, H., Harasti, D., Jordan, A., et al. (2015). Functional traits reveal early responses in marine reserves following protection from fishing. Divers. Distrib. 21, 876–887. doi: 10.1111/ddi.12309
Coles, R. G., Grech, A., Rasheed, M. A., McKenzie, L. J., Unsworth, R. K. F., and Short, F. (2011). “Seagrass ecology and threats in the tropical Indo-Pacific bioregion,” in Seagrass: Ecology, Uses and Threats, ed. R. S. Pirog (Hauppauge, NY: Nova Science Publishers, Inc).
Crona, B., Nyström, M., Folke, C., and Jiddawi, N. (2010). Middlemen, a critical social-ecological link in coastal communities of Kenya and Zanzibar. Mar. Policy 34, 761–771. doi: 10.1016/j.marpol.2010.01.023
Cullen-Unsworth, L. C., Nordlund, L. M., Paddock, J., Baker, S., McKenzie, L. J., and Unsworth, R. K. (2014). Seagrass meadows globally as a coupled social-ecological system: implications for human wellbeing. Mar. Pollut. Bull. 83, 387–397. doi: 10.1016/j.marpolbul.2013.06.001
Darling, E. S., Graham, N. A. J., Januchowski-Hartley, F. A., Nash, K. L., Pratchett, M. S., and Wilson, S. K. (2017). Relationships between structural complexity, coral traits, and reef fish assemblages. Coral Reefs 36, 561–575. doi: 10.1007/s00338-017-1539-z
de la Moriniere, E. C., Pollux, B. J. A., Nagelkerken, I., and Van Der Velde, G. (2003). Diet shifts of Caribbean grunts (Haemulidae) and snappers (Lutjanidae) and the relation with nursery-to-coral reef migrations. Estuar. Coast. Shelf Sci. 57, 1079–1089. doi: 10.1016/s0272-7714(03)00011-8
de la Torre-Castro, M., Di Carlo, G., and Jiddawi, N. S. (2014). Seagrass importance for a small-scale fishery in the tropics: the need for seascape management. Mar. Pollut. Bull. 83, 398–407. doi: 10.1016/j.marpolbul.2014.03.034
De Wolf, H., and Rashid, R. (2008). Heavy metal accumulation in Littoraria scabra along polluted and pristine mangrove areas of Tanzania. Environ. Pollut. 152, 636–643. doi: 10.1016/j.envpol.2007.06.064
Department of Fisheries Development (2016). Zanzibar Fisheries Frame Survey 2016 Report. Zanzibar: Revolutionary Government Of Zanzibar, Ministry of Agriculture, Natural Resources, Livestock and Fisheries Zanzibar.
Díaz, S., Lavorel, S., De Bello, F., Quétier, F., Grigulis, K., and Robson, T. M. (2007). Incorporating plant functional diversity effects in ecosystem service assessments. Proc. Natl. Acad. Sci. U.S.A. 104, 20684–20689. doi: 10.1073/pnas.0704716104
Díaz-Gil, C., Smee, S. L., Cotgrove, L., Follana-Berná, G., Hinz, H., Marti-Puig, P., et al. (2017). Using stereoscopic video cameras to evaluate seagrass meadows nursery function in the Mediterranean. Mar. Biol. 164:137.
Donner, S. D., and Potere, D. (2007). The inequity of the global threat to coral reefs. Bioscience 57, 214–215. doi: 10.1641/b570302
Dorenbosch, M., Grol, M. G. G., Nagelkerken, I., Lugendo, B. R., and Van Der Velde, G. (2007). Different fish composition in seagrass beds adjacent to extensive mangrove areas as opposed to coral reefs. Bull. Mar. Sci. 80, 919–919.
Dorman, S. R., Harvey, E. S., and Newman, S. J. (2012). Bait effects in sampling coral reef fish assemblages with stereo-BRUVs. PLoS One 7:e41538. doi: 10.1371/journal.pone.0041538
Dormann, C. F., Elith, J., Bacher, S., Buchmann, C., Carl, G., Carré, G., et al. (2013). Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36, 27–46. doi: 10.1111/j.1600-0587.2012.07348.x
Ebrahim, A., Bijoux, J. P., Mumby, P. J., and Tibbetts, I. R. (2020a). The commercially important shoemaker spinefoot, Siganus sutor, connects coral reefs to neighbouring seagrass meadows. J. Fish Biol. 96, 1034–1044. doi: 10.1111/jfb.14297
Ebrahim, A., Martin, T. S. H., Mumby, P. J., Olds, A. D., and Tibbetts, I. R. (2020b). Differences in diet and foraging behaviour of commercially important rabbitfish species on coral reefs in the Indian Ocean. Coral Reefs 39, 977–988. doi: 10.1007/s00338-020-01918-6
Edgar, G. J., Barrett, N. S., and Morton, A. J. (2004). Biases associated with the use of underwater visual census techniques to quantify the density and size-structure of fish populations. J. Exp. Mar. Biol. Ecol. 308, 269–290. doi: 10.1016/j.jembe.2004.03.004
Esteban, N., Unsworth, R. K. F., Gourlay, J. B. Q., and Hays, G. C. (2018). The discovery of deep-water seagrass meadows in a pristine Indian Ocean wilderness revealed by tracking green turtles. Mar. Pollut. Bull. 134, 99–105. doi: 10.1016/j.marpolbul.2018.03.018
Fröcklin, S., de la Torre-Castro, M., Lindström, L., and Jiddawi, N. S. (2013). Fish traders as key actors in fisheries: gender and adaptive management. AMBIO 42, 951–962. doi: 10.1007/s13280-013-0451-1
Furness, E., and Unsworth, R. K. (2020). Demersal fish assemblages in NE atlantic seagrass and kelp. Diversity 12:366. doi: 10.3390/d12100366
Gamfeldt, L., Lefcheck, J. S., Byrnes, J. E. K., Cardinale, B. J., Duffy, J. E., and Griffin, J. N. (2015). Marine biodiversity and ecosystem functioning: what’s known and what’s next? Oikos 124, 252–265. doi: 10.1111/oik.01549
Gilby, B., Henderson, C. J., Tibbetts, I., and Burfeind, D. (2016). Quantifying the influence of small omnivorous fishes on seagrass epiphyte load. J. Fish Biol. 89, 1905–1912. doi: 10.1111/jfb.13096
Gilby, B. L., Tibbetts, I. R., and Stevens, T. (2017). Low functional redundancy and high variability in Sargassum browsing fish populations in a subtropical reef system. Mar. Freshw. Res. 68, 331–341. doi: 10.1071/mf15386
Giri, C., Ochieng, E., Tieszen, L. L., Zhu, Z., Singh, A., Loveland, T., et al. (2011). Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 20, 154–159. doi: 10.1111/j.1466-8238.2010.00584.x
Gorham, J. C., and Alevizon, W. S. (1989). Habitat complexity and the abundance of juvenile fishes residing on small scale artificial reefs. Bull. Mar. Sci. 44, 662–665.
Gourlay, J. B. Q. (2017). Local and Regional Variability of Indo-Pacific Seagrass Fish Assemblages. Sketty: Swansea University.
Grandcourt, E. M., and Cesar, H. S. J. (2003). The bio-economic impact of mass coral mortality on the coastal reef fisheries of the Seychelles. Fish. Res. 60, 539–550. doi: 10.1016/s0165-7836(02)00173-x
Grime, J. P. (1998). Benefits of plant diversity to ecosystems: immediate, filter and founder effects. J. Ecol. 86, 902–910. doi: 10.1046/j.1365-2745.1998.00306.x
Gullstrom, M., Bodin, M., Nilsson, P. G., and Ohman, M. C. (2008). Seagrass structural complexity and landscape configuration as determinants of tropical fish assemblage composition. Mar. Ecol. Prog. Ser. 363, 241–255. doi: 10.3354/meps07427
Gullström, M., de la Torre Castro, M., Bandeira, S. O., Björk, M., Dahlberg, M., Kautsky, N., et al. (2002). Seagrass ecosystems in the western Indian Ocean. AMBIO 31, 588–597. doi: 10.1579/0044-7447-31.7.588
Gullström, M., Lyimo, L. D., Dahl, M., Samuelsson, G. S., Eggertsen, M., Anderberg, E., et al. (2018). Blue carbon storage in tropical seagrass meadows relates to carbonate stock dynamics, plant–sediment processes, and landscape context: insights from the western Indian Ocean. Ecosystems 21, 551–566. doi: 10.1007/s10021-017-0170-8
Harcourt, W. D., Briers, R. A., and Huxham, M. (2018). The thin(ning) green line? Investigating changes in Kenya’s seagrass coverage. Biol. Lett. 14:20180227. doi: 10.1098/rsbl.2018.0227
Harding, J. M., and Mann, R. (2001). Oyster reefs as fish habitat: opportunistic use of restored reefs by transient fishes. J. Shellfish Res. 20:951.
Harrison, X. A., Donaldson, L., Correa-Cano, M. E., Evans, J., Fisher, D. N., Goodwin, C. E. D., et al. (2018). A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 6:e4794.
Harvey, E. S., Cappo, M., Butler, J. J., Hall, N., and Kendrick, G. A. (2007). Bait attraction affects the performance of remote underwater video stations in assessment of demersal fish community structure. Mar. Ecol. Prog. Ser. 350, 245–254. doi: 10.3354/meps07192
Hayes, M. A., Mcclure, E. C., York, P. H., Jinks, K. I., Rasheed, M. A., Sheaves, M., et al. (2020). The differential importance of deep and shallow seagrass to nekton assemblages of the great barrier reef. Diversity 12:292. doi: 10.3390/d12080292
Heck, K. L., and Orth, R. J. (1980). “Seagrass habitats: the roles of habitat complexity. Competition and predation in structuring associated fish and motile macroinvertebrate assemblages,” in Esturine Perspectives, ed. V. S. Kennedy (Sydney: Academic Press), 449–464. doi: 10.1016/b978-0-12-404060-1.50043-5
Henderson, C. J., Gilby, B. L., Lee, S. Y., and Stevens, T. (2017). Contrasting effects of habitat complexity and connectivity on biodiversity in seagrass meadows. Mar. Biol. 164:117.
Hicks, C. C., and McClanahan, T. R. (2012). Assessing gear modifications needed to optimize yields in a heavily exploited, multi-species, seagrass and coral reef fishery. PLoS One 7:e36022. doi: 10.1371/journal.pone.0036022
Hoag, H. (2010). Confronting the biodiversity crisis. Nat. Rep. Clim. Change 1, 51–54. doi: 10.1038/climate.2010.38
Honda, K., Nakamura, Y., Nakaoka, M., Uy, W. H., and Fortes, M. D. (2013). Habitat use by fishes in coral reefs, seagrass beds and mangrove habitats in the Philippines. PLoS One 8:e65735. doi: 10.1371/journal.pone.0065735
Hovel, K. A., Fonseca, M. S., Myer, D. L., Kenworthy, W. J., and Whitfield, P. E. (2002). Effects of seagrass landscape structure, structural complexity and hydrodynamic regime on macrofaunal densities in North Carolina seagrass beds. Mar. Ecol. Prog. Ser. 243, 11–24. doi: 10.3354/meps243011
Jablonski, D., Roy, K., and Valentine, J. W. (2006). Out of the tropics: evolutionary dynamics of the latitudinal diversity gradient. Science 314, 102–106. doi: 10.1126/science.1130880
James, F. C., and McCulloch, C. E. (1990). Multivariate analysis in ecology and systematics: panacea or Pandora’s box? Annu. Rev. Ecol. Syst. 21, 129–166. doi: 10.1146/annurev.es.21.110190.001021
Jantke, K., Jones, K. R., Allan, J. R., Chauvenet, A. L. M., Watson, J. E. M., and Possingham, H. P. (2018). Poor ecological representation by an expensive reserve system: evaluating 35 years of marine protected area expansion. Conserv. Lett. 11:e12584. doi: 10.1111/conl.12584
Januchowski-Hartley, F. A., Graham, N. A. J., Cinner, J. E., and Russ, G. R. (2013). Spillover of fish naivete from marine reserves. Ecol. Lett. 16, 191–197. doi: 10.1111/ele.12028
Jinks, K. I., Brown, C. J., Rasheed, M. A., Scott, A. L., Sheaves, M., York, P. H., et al. (2019). Habitat complexity influences the structure of food webs in Great Barrier Reef seagrass meadows. Ecosphere 10:e02928.
Jones, B. L., Cullen-Unsworth, L. C., Howard, R., and Unsworth, R. K. (2018). Complex yet fauna-deficient seagrass ecosystems at risk in southern Myanmar. Bot. Mar. 61, 193–203. doi: 10.1515/bot-2017-0082
Jones, R. E., Griffin, R. A., Herbert, R. J. H., and Unsworth, R. K. F. (2021). Consistency is critical for the effective use of baited remote video. Oceans 2, 215–232. doi: 10.3390/oceans2010013
Katikiro, R. E. (2014). Perceptions on the shifting baseline among coastal fishers of Tanga, Northeast Tanzania. Ocean Coast. Manag. 91, 23–31. doi: 10.1016/j.ocecoaman.2014.01.009
Khamis, Z., Kalliola, R., and Käyhkö, N. (2019). Spatial modelling of cumulative human pressure in the tropical coastscape of Zanzibar, Tanzania. Afr. J. Mar. Sci. 41, 337–352. doi: 10.2989/1814232x.2019.1667436
Kiggins, R. S., Knott, N. A., and Davis, A. R. (2018). Miniature baited remote underwater video (mini-BRUV) reveals the response of cryptic fishes to seagrass cover. Environ. Biol. Fish. 101, 1717–1722. doi: 10.1007/s10641-018-0823-2
Laliberté, E., and Legendre, P. (2010). A distance-based framework for measuring functional diversity from multiple traits. Ecology 91, 299–305. doi: 10.1890/08-2244.1
Langlois, T. J., Harvey, E. S., Fitzpatrick, B., Meeuwig, J. J., Shedrawi, G., and Watson, D. L. (2010). Cost-efficient sampling of fish assemblages: comparison of baited video stations and diver video transects. Aquat. Biol. 9, 155–168. doi: 10.3354/ab00235
Lefcheck, J. S. (2016). piecewiseSEM: piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol. Evol. 7, 573–579. doi: 10.1111/2041-210x.12512
Lefcheck, J. S., Byrnes, J. E. K., Isbell, F., Gamfeldt, L., Griffin, J. N., Eisenhauer, N., et al. (2015). Biodiversity enhances ecosystem multifunctionality across trophic levels and habitats. Nat. Commun. 6:6936.
Lindfield, S. J., Harvey, E. S., Halford, A. R., and Mcilwain, J. L. (2016). Mesophotic depths as refuge areas for fishery-targeted species on coral reefs. Coral Reefs 35, 125–137. doi: 10.1007/s00338-015-1386-8
Lindfield, S. J., Harvey, E. S., Mcilwain, J. L., and Halford, A. R. (2014). Silent fish surveys: bubble-free diving highlights inaccuracies associated with SCUBA-based surveys in heavily fished areas. Methods Ecol. Evol. 5, 1061–1069. doi: 10.1111/2041-210x.12262
MacNeil, M. A., Chapman, D. D., Heupel, M., Simpfendorfer, C. A., Heithaus, M., Meekan, M., et al. (2020). Global status and conservation potential of reef sharks. Nature 583, 801–806.
McClanahan, T. R., and Mangi, S. (2000). Spillover of exploitable fishes from a marine park and its effect on the adjacent fishery. Ecol. Appl. 10, 1792–1805. doi: 10.1890/1051-0761(2000)010[1792:soeffa]2.0.co;2
McClanahan, T. R., and Mangi, S. C. (2004). Gear-based management of a tropical artisanal fishery based on species selectivity and capture size. Fish. Manag. Ecol. 11, 51–60. doi: 10.1111/j.1365-2400.2004.00358.x
McCluskey, S. M., and Lewison, R. L. (2008). Quantifying fishing effort: a synthesis of current methods and their applications. Fish Fish. 9, 188–200. doi: 10.1111/j.1467-2979.2008.00283.x
McKenzie, L. J., Campbell, S. J., and Roder, C. A. (2001). Seagrass-Watch: Manual for Mapping & Monitoring Seagrass Resources by Community (citizen) Volunteers. Cairns: Queensland Fisheries Service, Department of Primary Industries.
McKenzie, L. J., Nordlund, L. M., Jones, B. L., Cullen-Unsworth, L., Roelfsema, C. M., and Unsworth, R. K. F. (2020). The global distribution of seagrass meadows. Environ. Res. Lett. 15:074041. doi: 10.1088/1748-9326/ab7d06
Miguel, D. F., Jillian Lean Sim, O., Yi Mei, T., Anchana, P., Japar Sidik, B., and Siti Maryam, Y. (2018). Seagrass in Southeast Asia: a review of status and knowledge gaps, and a road map for conservation. Bot. Mar. 61, 269–288. doi: 10.1515/bot-2018-0008
Morin, X., Fahse, L., Jactel, H., Scherer-Lorenzen, M., García-Valdés, R., and Bugmann, H. (2018). Long-term response of forest productivity to climate change is mostly driven by change in tree species composition. Sci. Rep. 8:5627.
Nakamura, Y., and Sano, M. (2004). Comparison between community structures of fishes in Enhalus acoroides- and Thalassia hemprichii-dominated seagrass beds on fringing coral reefs in the Ryukyu Islands, Japan. Ichthyol. Res. 51, 38–45. doi: 10.1007/s10228-003-0191-5
Nordlund, L. M., Jackson, E. L., Nakaoka, M., Samper-Villarreal, J., Beca-Carretero, P., and Creed, J. C. (2018a). Seagrass ecosystem services – What’s next? Mar. Pollut. Bull. 134, 145–151. doi: 10.1016/j.marpolbul.2017.09.014
Nordlund, L. M., Unsworth, R. K. F., Gullström, M., and Cullen-Unsworth, L. C. (2018b). Global significance of seagrass fishery activity. Fish Fish. 19, 399–412. doi: 10.1111/faf.12259
Nordlund, L. M., Koch, E. W., Barbier, E. B., and Creed, J. C. (2016). Seagrass ecosystem services and their variability across genera and geographical regions. PLoS One 11:e0163091. doi: 10.1371/journal.pone.0163091
Oliver, T. H. (2016). How much biodiversity loss is too much? Science 353, 220–221. doi: 10.1126/science.aag1712
Phalla, L., Benbow, S., and Mulligan, B. (2014). Seagrass diversity and distribution in the Koh Rong Archipelago, Preah Sihanouk Province, Cambodia. Camb. J. Nat. Hist. 2014, 37–46.
Picasso, V. D. (2018). The “biodiversity–ecosystem function debate”: an interdisciplinary dialogue between ecology, agricultural science, and agroecology. Agroecol. Sustain. Food Syst. 42, 264–273. doi: 10.1080/21683565.2017.1359806
Piggott, C. V. H., Depczynski, M., Gagliano, M., and Langlois, T. J. (2020). Remote video methods for studying juvenile fish populations in challenging environments. J. Exp. Mar. Biol. Ecol. 532:151454. doi: 10.1016/j.jembe.2020.151454
Pogoreutz, C., Kneer, D., Litaay, M., Asmus, H., and Ahnelt, H. (2012). The influence of canopy structure and tidal level on fish assemblages in tropical Southeast Asian seagrass meadows. Estuar. Coast. Shelf Sci. 107, 58–68. doi: 10.1016/j.ecss.2012.04.022
Prado, P., and Heck, K. L. Jr. (2011). Seagrass selection by omnivorous and herbivorous consumers: determining factors. Mar. Ecol. Prog. Ser. 429, 45–55. doi: 10.3354/meps09076
Priede, I. G., Bagley, P. M., Smith, A., Creasey, S., and Merrett, N. R. (1994). Scavenging deep demersal fishes of the porcupine seabight, northeast atlantic - observations by baited camera, trap and trawl. J. Mar. Biol. Assoc. U.K. 74, 481–498. doi: 10.1017/s0025315400047615
Quiros, T. A. L., Beck, M. W., Araw, A., Croll, D. A., and Tershy, B. (2018). Small-scale seagrass fisheries can reduce social vulnerability: a comparative case study. Ocean Coast. Manag. 157, 56–67. doi: 10.1016/j.ocecoaman.2018.02.003
Quiros, T. A. L., Croll, D., Tershy, B., Fortes, M. D., and Raimondi, P. (2017). Land use is a better predictor of tropical seagrass condition than marine protection. Biol. Conserv. 209, 454–463. doi: 10.1016/j.biocon.2017.03.011
R Development Core Team (2020). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Richmond, M. D. (2011). A Field Guide to the Seashores of Eastern Africa and the Western Indian Ocean. Zanzibar: JSTORSida/WIOMSA.
Salita, J. T., Ekau, W., and Saint-Paul, U. (2003). Field evidence on the influence of seagrass landscapes on fish abundance in Bolinao, northern Philippines. Mar. Ecol. Prog. Ser. 247, 183–195. doi: 10.3354/meps247183
Schramm, K. D., Harvey, E. S., Goetze, J. S., Travers, M. J., Warnock, B., and Saunders, B. J. (2020). A comparison of stereo-BRUV, diver operated and remote stereo-video transects for assessing reef fish assemblages. J. Exp. Mar. Biol. Ecol. 524:151273. doi: 10.1016/j.jembe.2019.151273
Short, F., Carruthers, T., Dennison, W., and Waycott, M. (2007). Global seagrass distribution and diversity: a bioregional model. J. Exp. Mar. Biol. Ecol. 350, 3–20. doi: 10.1016/j.jembe.2007.06.012
Short, F. T., Polidoro, B., Livingstone, S. R., Carpenter, K. E., Bandeira, S., Bujang, J. S., et al. (2011). Extinction risk assessment of the world’s seagrass species. Biol. Conserv. 144, 1961–1971.
Smith, T. M., Hindell, J. S., Jenkins, G. P., Connolly, R. M., and Keough, M. J. (2011). Edge effects in patchy seagrass landscapes: the role of predation in determining fish distribution. J. Exp. Mar. Biol. Ecol. 399, 8–16. doi: 10.1016/j.jembe.2011.01.010
Taylor, M. D., Baker, J., and Suthers, I. M. (2013). Tidal currents, sampling effort and baited remote underwater video (BRUV) surveys: are we drawing the right conclusions? Fish. Res. 140, 96–104. doi: 10.1016/j.fishres.2012.12.013
Thyresson, M., Crona, B., Nyström, M., de la Torre-Castro, M., and Jiddawi, N. (2013). Tracing value chains to understand effects of trade on coral reef fish in Zanzibar, Tanzania. Mar. Policy 38, 246–256. doi: 10.1016/j.marpol.2012.05.041
Tilman, D., Knops, J., Wedin, D., Reich, P., Ritchie, M., and Siemann, E. (1997). The influence of functional diversity and composition on ecosystem processes. Science 277, 1300–1302. doi: 10.1126/science.277.5330.1300
Tin, H. C., Phung, B. T., and Hieu, D. V. (2020). Species biodiversity and distribution of seagrass beds in several coastal areas of central Vietnam. Region. Stud. Mar. Sci. 61:101531. doi: 10.1016/j.rsma.2020.101531
UN Environment, International Coral Reef Initiative, and World Conservation Monitoring Centre (2018). Analysis of International Funding for the Sustainable Management of Coral Reefs and Associated Coastal Ecosystems. Nairobi: UN Environment.
Unsworth, R. K. F., Ambo-Rappe, R., Jones, B. L., La Nafie, Y. A., Irawan, A., Hernawan, U. E., et al. (2018). Indonesia’s globally significant seagrass meadows are under widespread threat. Sci. Total Environ. 634, 279–286. doi: 10.1016/j.scitotenv.2018.03.315
Unsworth, R. K. F., McKenzie, L. J., Collier, C. J., Cullen-Unsworth, L. C., Duarte, C. M., Eklof, J. S., et al. (2019a). Global challenges for seagrass conservation. Ambio 48, 801–815.
Unsworth, R. K. F., Nordlund, L. M., and Cullen-Unsworth, L. C. (2019b). Seagrass meadows support global fisheries production. Conserv. Lett. 12:e12566. doi: 10.1111/conl.12566
Unsworth, R. K. F., Peters, J. R., Mccloskey, R. M., and Hinder, S. L. (2014). Optimising stereo baited underwater video for sampling fish and invertebrates in temperate coastal habitats. Estuar. Coast. Shelf Sci. 150, 281–287. doi: 10.1016/j.ecss.2014.03.020
Unsworth, R. K. F., Salinas De Leon, P., Garrard, S., Jompa, J., Smith, D. J., and Bell, J. J. (2008). High connectivity of Indo-Pacific seagrass fish assemblages with mangrove and coral reef habitats. Mar. Ecol. Prog. Ser. 353, 213–224. doi: 10.3354/meps07199
von Schuckmann, K., Holland, E., Haugan, P., and Thomson, P. (2020). Ocean science, data, and services for the UN 2030 sustainable development goals. Mar. Policy 121:104154. doi: 10.1016/j.marpol.2020.104154
Vonk, J. A., Christianen, M. J. A., and Stapel, J. (2010). Abundance, edge effect, and seasonality of fauna in mixed-species seagrass meadows in southwest Sulawesi, Indonesia. Mar. Biol. Res. 6, 282–291. doi: 10.1080/17451000903233789
Watson, D., Harvey, E., Anderson, M., and Kendrick, G. (2005). A comparison of temperate reef fish assemblages recorded by three underwater stereo-video techniques. Mar. Biol. 148, 415–425. doi: 10.1007/s00227-005-0090-6
Watson, D. L., Harvey, E. S., Fitzpatrick, B. M., Langlois, T. J., and Shedrawi, G. (2010). Assessing reef fish assemblage structure: how do different stereo-video techniques compare? Mar. Biol. 157, 1237–1250. doi: 10.1007/s00227-010-1404-x
Waycott, M., Duarte, C. M., Carruthers, T. J. B., Orth, R. J., Dennison, W. C., Olyarnik, S., et al. (2009). Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. U.S.A. 106, 12377–12381. doi: 10.1073/pnas.0905620106
Whitmarsh, S. K., Fairweather, P. G., Brock, D. J., and Miller, D. (2014). Nektonic assemblages determined from baited underwater video in protected versus unprotected shallow seagrass meadows on Kangaroo Island, South Australia. Mar. Ecol. Prog. Ser. 503, 205–218. doi: 10.3354/meps10733
Williams, J. N. (2013). Humans and biodiversity: population and demographic trends in the hotspots. Popul. Environ. 34, 510–523. doi: 10.1007/s11111-012-0175-3
Willig, M. R., Kaufman, D. M., and Stevens, R. D. (2003). Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 34, 273–309. doi: 10.1146/annurev.ecolsys.34.012103.144032
Wraith, J., Lynch, T., Minchinton, T. E., Broad, A., and Davis, A. R. (2013). Bait type affects fish assemblages and feeding guilds observed at baited remote underwater video stations. Mar. Ecol. Prog. Ser. 477, 189–199. doi: 10.3354/meps10137
Zarco-Perello, S., and Enríquez, S. (2019). Remote underwater video reveals higher fish diversity and abundance in seagrass meadows, and habitat differences in trophic interactions. Sci. Rep. 9:6596.
Zuur, A., Ieno, E. N., Walker, N., Saveliev, A. A., and Smith, G. M. (2009). Mixed Effects Models and Extensions in Ecology with R. Berlin: Springer.
Keywords: seagrass meadows, fish assemblages, species diversity, small-scale fisheries, habitat structure, functional ecology
Citation: Jones BL, Nordlund LM, Unsworth RKF, Jiddawi NS and Eklöf JS (2021) Seagrass Structural Traits Drive Fish Assemblages in Small-Scale Fisheries. Front. Mar. Sci. 8:640528. doi: 10.3389/fmars.2021.640528
Received: 11 December 2020; Accepted: 19 March 2021;
Published: 12 April 2021.
Edited by:
Brett W. Molony, Oceans and Atmosphere (CSIRO), AustraliaReviewed by:
Catherine Jane Collier, James Cook University, AustraliaW. Judson Kenworthy, Independent Researcher, Beaufort, United States
Copyright © 2021 Jones, Nordlund, Unsworth, Jiddawi and Eklöf. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benjamin L. Jones, Benjamin.Jones@su.se
 Benjamin L. Jones
Benjamin L. Jones Lina M. Nordlund
Lina M. Nordlund Richard K. F. Unsworth
Richard K. F. Unsworth Narriman S. Jiddawi
Narriman S. Jiddawi Johan S. Eklöf
Johan S. Eklöf