Feeding Ecology of Elusive Caribbean Killer Whales Inferred From Bayesian Stable Isotope Mixing Models and Whalers’ Ecological Knowledge
- 1Department of Biological Sciences, Institute of Environment, Florida International University, North Miami, FL, United States
- 2Department of Zoology and Entomology, Rhodes University, Grahamstown, South Africa
- 3UMS Pelagis, La Rochelle, France
- 4HTC Honors College and Center for Interdisciplinary Studies, Coastal Carolina University, Conway, SC, United States
Investigating the feeding ecology of marine predators is critical for understanding their roles and functional importance in ecosystems. However, assessing the diet of large and wide-ranging predators can be challenging, particularly in the case of rare and elusive species. Killer whales (Orcinus orca) are large apex predators in global oceans, but very little is known about their feeding ecology in tropical ecosystems, particularly in the Caribbean Sea. Killer whales are distributed throughout the Caribbean, and are a regular target of artisanal whalers in St. Vincent and the Grenadines (West Indies). Here we investigate the feeding ecology of Caribbean killer whales using a combination of stable isotope analysis (δ15N, δ13C) and traditional ecological knowledge (TEK) data from whalers operating from St. Vincent. Potential prey data from the Caribbean region included large pelagic teleosts, sea turtles, oceanic sharks, cetaceans, and oceanic cephalopods. Mixing models suggest that the contribution of odontocete cetaceans to the diet of killer whales is the most important (overall mean contribution: 60.4%), particularly mesopelagic delphinids (Lagenodelphis hosei; 26.4%, SD = 0.14), large teuthophageous odontocetes (Physeter macrocephalus, Globicephala macrorhynchus; 20.0%, SD = 0.14), and epipelagic delphinids (Stenella attenuata; 14%, SD = 0.14). Oceanic sharks (Carcharhinus longimanus) had a contribution of 17.0% of the diet of killer whales (SD = 0.13), and other potential prey had a relatively marginal contribution. TEK data suggest that whalers believe killer whales feed almost exclusively on cetaceans, particularly odontocete species that they hunt (e.g., G. macrorhynchus). Stable isotope and TEK data were consistent to some extent, although TEK data are qualitative and based on a limited number of observations. Despite some limitations (including sample size for both methods), this study highlights the value of combining independent data sources and methodologies to investigate the ecological roles of marine predators in data-poor regions.
Introduction
Investigating the feeding ecology of marine predators is critical to assess their functional roles in food webs, and to understand their ecological importance (Bowen, 1997; Heithaus et al., 2008; Kiszka et al., 2014; Estes et al., 2016). Due to their high metabolic rates and high abundance in some regions, marine mammals, such as cetaceans, have the potential to affect communities and ecosystems via consumptive and non-consumptive effects, and other processes such as nutrient translocation and recycling (Katona and Whitehead, 1988; Heithaus et al., 2008; Roman et al., 2014; Estes et al., 2016). Understanding the trophic interactions of these predators can be used in modeling ecosystem dynamics, and predict ecosystem consequences of their decline or recovery (Kiszka et al., 2015). It is critical to quantify the importance of prey sources for these predators, particularly to predict how prey will respond to predation in space and time.
Investigating the trophic interactions and diet of marine mammals, such as cetaceans, remains challenging. Some species are elusive and rarely observed, and therefore research methods, such as focal follows and biopsy sampling, can rarely be carried out (Kiszka et al., 2014; Trites and Spitz, 2018). The most limiting factor to dietary studies is, however, the lack of samples available, including hard remains from stomach contents or scats, or any soft and hard tissues for both predators and prey, to carry out stable isotope or fatty acid analysis (e.g., see Trites and Spitz, 2018; for a review). Stable carbon and nitrogen isotope analysis has increasingly been used to investigate the trophic interactions within marine predator communities (including marine mammals), as well as spatial, ontogenetic, and sex variations of the diet of predators across a diversity of marine ecosystems (Hobson et al., 1996; Walker and Macko, 1999; Lesage et al., 2001; Kiszka et al., 2014). Stable isotopes integrate diet over multiple temporal scales depending on turnover rates of tissues used (Lesage et al., 2002; Vander Zanden et al., 2015). A major limitation of stable isotope analysis is that it does not provide a full description of the diet of consumers, typically provided through the analysis of stomach contents (Spitz et al., 2011; Jansen et al., 2013). However, mass-balanced isotopic models potentially address this problem by estimating the dietary composition of a consumer based on isotope data of candidate prey (Moore and Semmens, 2008; Parnell et al., 2013; Stock et al., 2018). Stable isotope mixing models incorporate uncertainty for each parameter and employ specific diet-tissue discrimination factors (or trophic enrichment factors, TEFs), which account for the changes in isotopes through the food chain. Over the past decade, stable isotope mixing models have improved our understanding of the feeding ecology of a range of cetacean species (Marcoux et al., 2012; Kiszka et al., 2014; Ryan et al., 2014; Warlick et al., 2020). However, it has been acknowledged that combining stable isotope mixing models with other independent sources of data, including other dietary indicators such as stomach contents (Jansen et al., 2013) or behavioral observations (Kiszka et al., 2014), would reinforce the credibility of these models.
Traditional ecological knowledge (TEK) has emerged since the late 20th century as a valuable source of ecological data (Freeman, 1992). TEK (or what is also commonly referred to as local ecological knowledge, LEK) has been extensively used to assess the occurrence, distribution, and conservation status of a diversity of species, including in marine ecosystems (Gray et al., 2017; Temple et al., 2018; Ayala et al., 2019). While TEK has in the past been discounted owing to its “anecdotal, non-quantitative, and unscientific” nature (Hobson, 1992), more recent analyses have highlighted the value of TEK data, particularly to study the spatiotemporal distribution, foraging ecology, trends in abundance, and threats to elusive and charismatic marine megafauna (Ferguson et al., 2012; Leeney and Poncelet, 2015; Early-Capistrán et al., 2018, 2020; Ayala et al., 2019). For example, TEK data collected from eastern Nunavut communities in the Canadian Arctic has contributed to document the feeding ecology of killer whales in this region, including prey preferences and behavior, distribution of predation events, and prey capture techniques (Ferguson et al., 2012). Therefore, the combination of TEK and biological data has the potential to provide a holistic view on the ecological role of elusive marine predators.
Killer whales (Orcinus orca) are distributed globally and feed on a wide variety of prey, including marine mammals (Jefferson et al., 1991; Baird and Dill, 1995; Pitman et al., 2001, 2015), teleosts (Similä et al., 1996), marine reptiles (Pitman and Dutton, 2004; Fertl and Fulling, 2007), and elasmobranchs (Fertl et al., 1996; Visser, 2005). However, many populations specialize on a narrow range of prey, and can be morphologically and genetically distinct (Pitman and Ensor, 2003; Foote et al., 2009; de Bruyn et al., 2013). In the Caribbean region, killer whales are not abundant but occur regularly throughout the region on a year-round basis. The population identity of Caribbean killer whales is unclear. They share some of their morphological features with several known coastal and inshore populations from other regions around the globe (Bolaños-Jiménez et al., 2014), but no genetic information is available. Off the archipelagic nation of St. Vincent and the Grenadines, killer whales (and other small cetaceans) are regularly targeted by artisanal whalers based in the village of Barrouallie, along the leeward coast of St. Vincent (Caldwell and Caldwell, 1975; Fielding, 2018). An average of six individual killer whales are taken per year, particularly since the late 1990s (2007–2012; Fielding, 2018). Previous knowledge on the feeding ecology of killer whales in the Caribbean region shows that these animals have been observed feeding on other cetaceans as well as some other species. These include pygmy sperm whales (Kogia breviceps), dwarf sperm whales (Kogia sima), pantropical spotted dolphins (Stenella attenuata), sperm whales (Physeter macrocephalus), Bryde’s whales (Balaenoptera edeni), and possibly large pelagic teleosts (Bolaños-Jiménez et al., 2014). Early whaling records in St. Vincent also suggest that killer whales have been found with sea turtles (particularly Dermochelys coriacea) in their stomachs (Caldwell and Caldwell, 1969). However, knowledge of the diet and foraging behavior of killer whales in the Caribbean is primarily based on anecdotal records. Here, we combine multiple methods, particularly stable isotope analysis and the ecological knowledge of whalers from the village of Barrouallie (St. Vincent) to assess the feeding ecology of killer whales from the southern Caribbean.
Materials and Methods
Sample Collection
Killer whale muscle tissues were collected in August 2015 (n = 3), April 2016 (n = 2) and June 2016 (n = 3) from individuals caught for human consumption by whalers operating in the waters of St. Vincent and the Grenadines (13°10′N, 61°14′W). Carcasses were landed on the beach of the village of Barrouallie (13°14′09″ N, 61°16′19″ W), and tissue samples were collected by a local assistant (see McCormack et al., 2020, for details on sampling procedure). Permission was obtained from the vendor, who possessed and sold cetacean products. Approximately 20 g of muscle tissue was systematically collected at the same location on the body (abdominal area) and stored individually in a plastic bag at −20°C. Samples were shipped to the University of the South (Sewanee, TN) in December 2016, and stored at −80°C until processing and stable isotope analysis. Potential prey species were selected based on previous studies on the feeding ecology of killer whales in temperate and tropical waters (e.g., Jefferson et al., 1991; Fertl et al., 1996; Pitman et al., 2001, 2003; Visser, 2005), particularly the Caribbean region (see Bolaños-Jiménez et al., 2014, for a review), and based on availability of stable isotope data. These potential killer whale prey data were compiled from the literature (Table 1) or collected in the field.
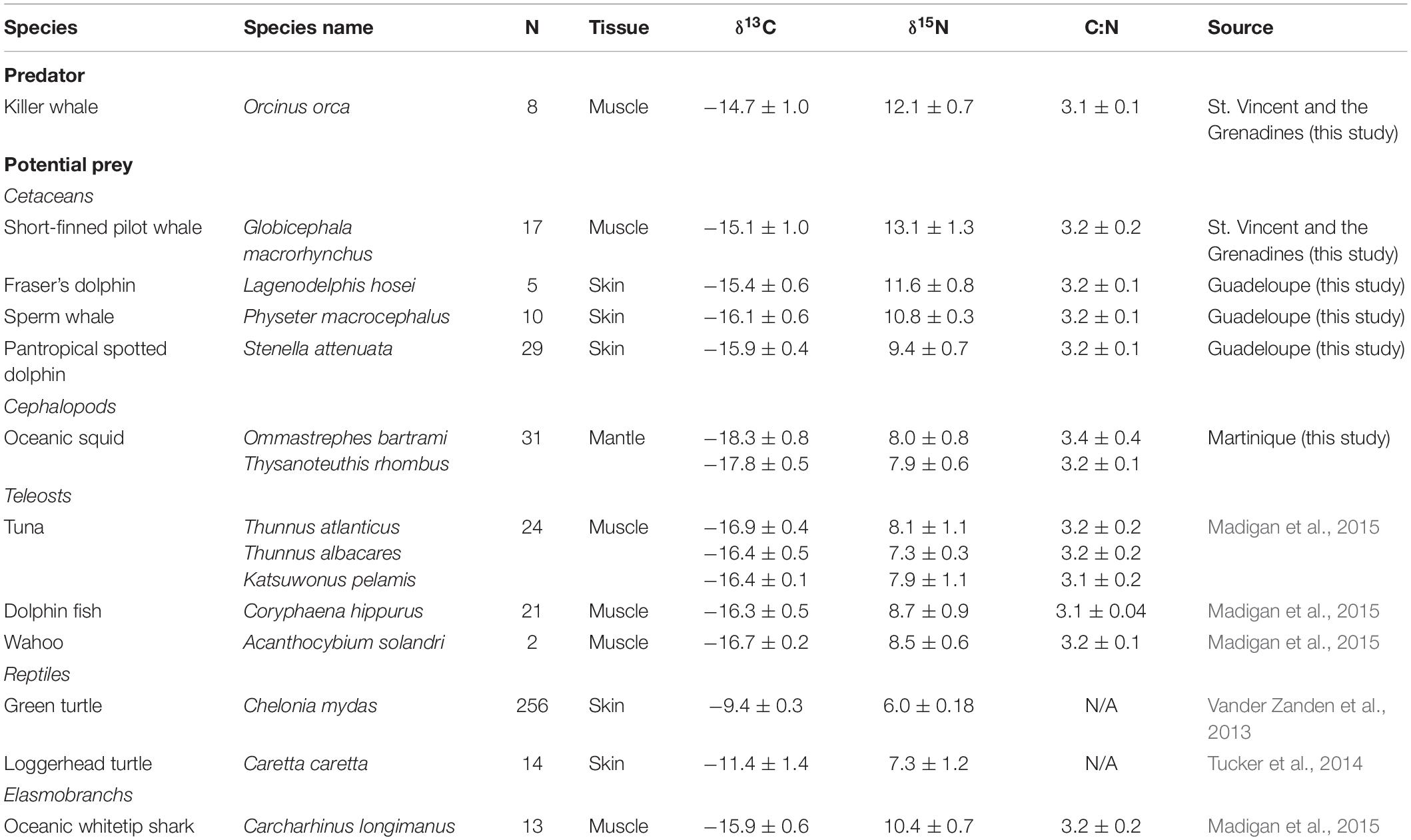
Table 1. Carbon (δ13C) and nitrogen (δ15N) isotope value (mean ± SD, ‰), sample sizes (number of individuals, n) and C:N ratios (mean ± SD, ‰, when available) of killer whales (Orcinus orca) and their potential prey species in the Caribbean.
Short-finned pilot whale (Globicephala macrorhynchus) samples (muscle) were collected similarly to killer whale samples, off the coast of St. Vincent. Other cetaceans that were potential prey were collected from the French West Indies (Guadeloupe, 16°15′N, 61°34′W), in April 2015, 300 km north of St. Vincent (Table 1). Skin and blubber biopsy samples were obtained during coastal boat-based cetacean surveys. When cetaceans were encountered, animals were sampled using a crossbow (BARNETT Velocity-Speed® Class, 68-kg draw weight) with Finn Larsen (Ceta-Dart, Copenhagen, Denmark) bolts and tips (dart 25 and 40-mm long). The animals were hit below the dorsal fin when sufficiently close (5–15 m) to the research boat (see Méndez-Fernandez et al., 2018 for details). Biopsy sampling was conducted under scientific permit delivered by DEAL Guadeloupe (February 12th, 2015, Autorisation Préfectorale de Dérogation pour la Perturbation Intentionnelle de Spécimens d’Espèces Animales Protégées). Sampling for neon flying squids (Ommastrephes bartramii) and diamondback squids (Thysanoteuthis rhombus) took place around the island of Martinique (14.6415°N, 61.0242°W) between December 2016 and February 2017, and between October 2017 and March 2018. Fishing surveys were carried out as part of an exploratory study led by the Regional Committee for Maritime Fisheries and Marine Farming. Stable isotope data from other species were gathered from the literature, including sea turtles (Vander Zanden et al., 2013; Tucker et al., 2014) and range of teleost species and oceanic sharks (Madigan et al., 2015; Table 1).
Stable Isotope Analysis
Stable isotope analysis was completed at the Center for Aquatic Chemistry and Environment in the Institute of Environment (Florida International University, North Miami, FL, United States). Muscle and skin samples were dried, homogenized into a fine powder, and lipid extracted prior to analysis because lipids are 13C depleted (DeNiro and Epstein, 1978). Lipids were extracted by agitating muscle and skin tissues in a 2:1 chloroform:methanol mixture for 1 min with a solvent volume 5-times greater than the sample, after which the samples were left at room temperature for 1 h, centrifuged and the supernatant was removed. After repeating this procedure two more times, each sample was rinsed in deionized water, dried, and 0.4–0.5 mg of sample added to a 4 mm × 6 mm tin capsule for stable isotope analysis using a ThermoFinnigan Delta V isotope ratio mass spectrometer (IRMS) coupled with a NA 1500 Ne elemental analyzer. Analytical reproducibility was based on replicates of internal standards including bovine liver (NBS standard reference material) and glycine (Alfa Aesar); variation among standards was 0.07‰ and 0.08‰ for δ13C and δ15N, respectively. The mean C:N values from analyzed tissues were less than 4, indicating an adequate lipid extraction (Lesage et al., 2010). Isotopic ratios (R) are reported in the standard delta (δ) notation relative to the international standards of Vienna Pee Dee belemnite (δ13C) and atmospheric nitrogen (δ15N) using the following equation:
Where X is 13C or 15N and R is the isotope ratio 13C/12C or 15N/14N.
Stable Isotope Mixing Models
Bayesian mass-balance stable isotope mixing models were built using the “MixSIAR” package in R (Stock et al., 2018) to estimate the relative contribution of potential prey to the diet of killer whales from St. Vincent. Mixing models were run with three Markov chain Monte Carlo chains of 300,000 draws and a burn-in rate of 200,000 draws. Convergence of the models was checked using Gelmen-Rubin and Geweke diagnostics. The appropriateness of prey groups for killer whale diet and the TEF used here was evaluated by determining the likelihood that prey groups were included in a simulated killer whale mixing polygon (Smith et al., 2013). Mean (±SD) isotope values from muscle (tuna and other large pelagic fish, including sharks; Madigan et al., 2015), mantle (oceanic cephalopods: this study), skin (sea turtles: Vander Zanden et al., 2013; Tucker et al., 2014; cetaceans: this study) were calculated from potential prey species in the Caribbean region (Table 1). Stable isotopes are incorporated into tissues based on species and tissue-type specific turnover rates. Since no specific TEF is available on killer whales, we used TEFs from the skin of bottlenose dolphin (Tursiops truncatus) which were based on feeding experiments which took place over 350 days, ensuring isotopes stabilized in the samples before calculating TEFs (Giménez et al., 2016). These have been used in previous research on killer whales (e.g., Reisinger et al., 2016), as taxonomically close species are known to have similar TEF values (Giménez et al., 2016). The TEFs used here were 1.01 ± 0.37‰ for δ13C and 1.57 ± 0.52‰ for δ15N. All statistical analyses were performed in R v.4.0.0.
Ecological Knowledge From Whalers
Ethnographic data and information about the ecological knowledge held by Vincentian whalers were recorded during fieldwork conducted between 2008 and 2020 (under IRB oversight at Louisiana State University, University of Denver, and University of the South, United States). Throughout most of this period, four whaling boats operated from Barrouallie, each with a rotating crew of approximately twelve whalers. Seven whalers were interviewed repeatedly during this 12-year period and whaling activities were observed primarily during a 3-month period of participant observation during the fall of 2009, and augmented by other regular interviews afterward. Informal interviews with whalers mainly consisted of lengthy, casual conversations, occurring during periods of downtime when the weather precluded going to sea, or with whalers who stayed ashore on days when their colleagues were at sea. Regardless of the formal or informal nature of the interviews, meticulous field notes were taken and transcribed immediately afterward. Discussions were not solely focused on the foraging ecology of killer whales, but on their whaling practices and any aspects of the behavior and ecology of cetaceans in St. Vincent and the Grenadines. When absent from the field site, one of the authors (RF) was in regular communication with whalers via email and online messaging.
Results
Sampling
In St. Vincent, eight killer whale and 20 short-finned pilot whale samples were collected in 2015 and 2016. In addition, skin and blubber biopsy samples were also collected for three additional species as potential prey in April 2015 in the waters of Guadeloupe, in the French West Indies. These were sperm whale (Physeter macrocephalus, n = 10), Fraser’s dolphin (Lagenodelphis hosei, n = 5), and pantropical spotted dolphin (Stenella attenuata, n = 29; Table 1). Other prey collected from fishing surveys include oceanic squids from Martinique (FWI), including neon flying squid (Ommastrephes bartramii, n = 7) and diamondback squid (Thysanoteuthis rhombus, n = 24). All potential prey data collected from the literature are provided in Table 1.
Trophic Interactions and Mixing Model
A one-way ANOVA revealed that potential prey included in the analysis had significantly different δ13C (F6, 16 = 69.14, p < 0.0001) and δ15N values (F6, 37 = 66.82, p < 0.0001, Figure 1). However, Tukey’s post-hoc pairwise comparisons were used to determine which prey species could be grouped for the model. As C. hippurus and A. solandri were not significantly different for either δ13C and δ15N values (p > 0.05), they were grouped as “other large pelagic fish.” Similarities for either isotope were also found within tuna species (T. atlanticus, T. albacares, and K. pelamis, Table 1), so these were grouped as “tuna” in the mixing model. A similar approach was adopted for oceanic cephalopods (O. bartrami and T. rhombus, grouped as “oceanic squids”) and for the largest sampled teuthophageous odontocetes (P. macrocephalus and G. macrorhynchus, grouped as “whales”). All killer whales fell within the mixing polygon, indicating that the TEF and prey selection was appropriate (Smith et al., 2013). Mixing models of dietary contributions identified odontocete cetaceans as the main prey for killer whales (Figure 2), particularly the Fraser’s dolphins (L. hosei; 26.4%, SD = 0.14), large teuthophageous odontocetes (P. macrocephalus, G. macrorhynchus; 20%, SD = 0.14), the oceanic whitetip shark (C. longimanus, 17%, SD = 0.13), and the pantropical spotted dolphin (S. attenuata; 14%, SD = 0.14). Other potential prey had a negligible contribution, including tuna (0.8%, SD = 0.07), other large teleosts (0.9%, SD = 0.08), and oceanic squids (0.9%, SD = 0.05; Figure 2).
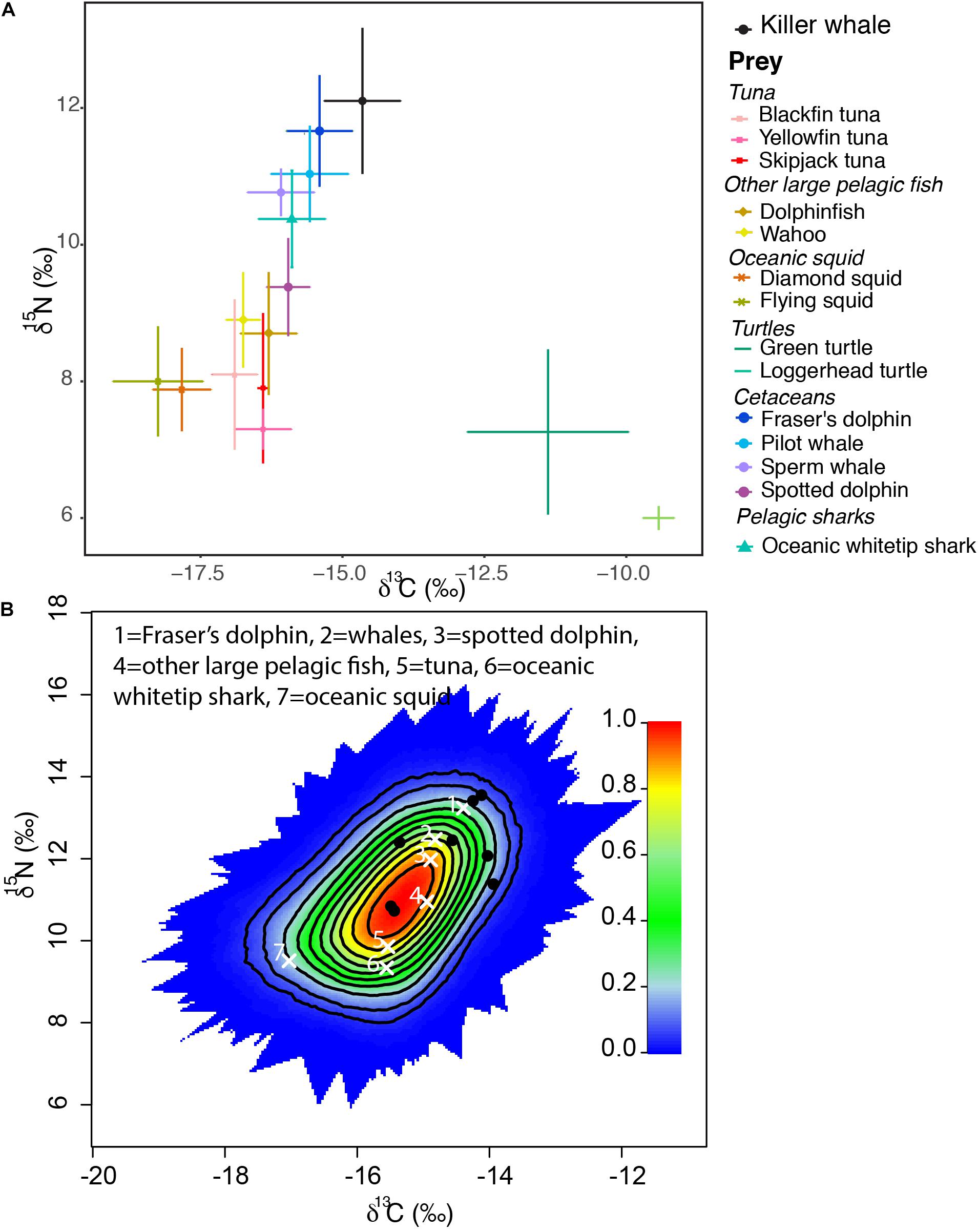
Figure 1. (A) Mean carbon (δ13C) and nitrogen (δ15N) isotope values (± SD) of killer whales and their potential prey in the Caribbean Sea and; (B) a simulated mixing polygon of the biplot in panel (A), including the average source signatures (white crosses) and position of the killer whale consumers (black dots). Probability contours start at the 5% level and every 10% level subsequently.
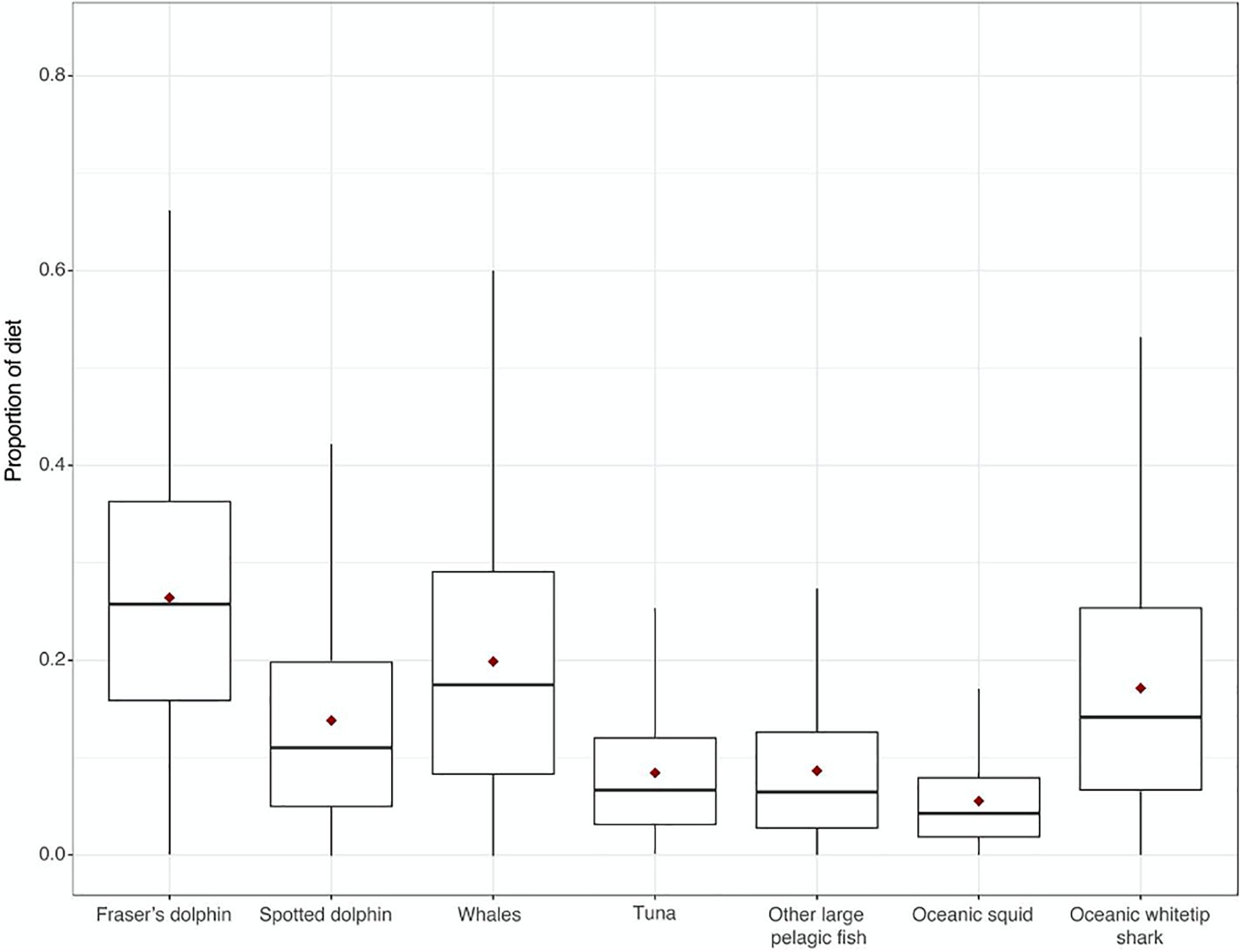
Figure 2. Proportion of dietary contribution of eight different potential prey items to killer whale diets using Bayesian mixing model analysis with trophic enrichment factors (Giménez et al., 2016) of stable isotope samples taken in the Caribbean Sea. Median proportion is indicated as a thick black line, and mean with a red diamond.
Whalers’ Ecological Knowledge
All whalers consistently reported that killer whales in St. Vincent and the Grenadines prey upon other odontocetes. They all mention that several species are particularly targeted by killer whales, including G. macrorhynchus, L. hosei, several species of smaller delphinids (particularly S. attenuata, S. longirostris). Whalers consistently reported that killer whales feed on the species that whalers target, particularly G. macrorhynchus. A perception of interspecific “competition” (between humans and killer whales) is used as one of the justifications for the hunting of killer whales, according to all whalers. We did not encounter whalers who claimed to have actually witnessed killer whale predation upon other odontocetes. Rather, whalers base this conclusion on their assessment that the presence of killer whales in the waters surrounding St. Vincent coincides with the absence or change of habitat of other species of odontocetes, as well as their occasional, non-systematic examination of killer whale stomach contents. No direct predation of killer whales on other species has been reported by any interviewed whalers, although it is acknowledged that killer whales can also feed on other prey, such as sea turtles, based on the opportunistic examination of stomach contents. The lead whaler (as identified by his social status in the whaling community as well as the dominance of his boat in the catch records) also stated that the number of killer whales present in the waters surrounding St. Vincent has been increasing in recent years. He mentioned it remains important to his livelihood that whalers are permitted to take killer whales, both for the meat and blubber they provide and to reduce the competition for other odontocetes.
Discussion
This study is the first to describe the feeding ecology of Caribbean killer whales using stable isotope mixing models. In addition, TEK data from artisanal whalers on the ecology and behavior of killer whales off St. Vincent and the Grenadines allows us to expand our understanding of the foraging ecology of this species. Due to the informal and qualitative nature of information collected from whalers, we mostly attempted to use TEK data to compare them with stable isotope mixing model outputs. We found some consistency between isotopic and TEK data, particularly with regards to prey preferences of killer whales. Overall, the results of this preliminary study suggest that killer whales sampled off the island of St Vincent mostly feed on cetaceans, particularly small (delphinids) and larger teuthophageous odontocetes such as short-finned pilot whales and sperm whales. Large pelagic teleosts (tuna and tuna-like species) and sea turtles might be rarely consumed by killer whales.
A number of limitations can hinder the interpretation of the results we obtained. Firstly, our sample size was limited to eight individuals, mostly due to the fact that the number of killer whales brought to shore by whalers and sampled is small. Our access to samples is also opportunistic in nature, and we rely strongly on local sample collectors that are not necessarily available when cetaceans are landed by whalers. In addition, some prey sources included in the study were collected in regions that are relatively distant from St. Vincent (e.g., tuna, large pelagic fish and oceanic sharks from the Bahamas; Madigan et al., 2015). Prey sources from the Bahamas may not be representative of carbon and nitrogen isotope values in tuna and other large pelagic fishes in the eastern Caribbean. However, these published prey data were the closest available to the location where killer whales were sampled. Also, killer whales are suspected to move considerably throughout the entire Caribbean region (Bolaños-Jiménez et al., 2014). Consequently, using prey data from the wider Caribbean region could still be a reasonable approach. The contribution of other potential prey previously documented in the diet of killer whales in various tropical regions, including the Caribbean, have not been included since isotope data for these species were unavailable (large baleen whales such as M. novaeangliae or Balaenoptera edeni; Flórez-González et al., 1994; Pitman et al., 2015 or leatherback turtles Dermochelys coriacea; Pitman and Dutton, 2004; Bolaños-Jiménez et al., 2014, for example). Therefore, the breadth of the diet of Caribbean killer whales may have been underestimated. On the other hand, the relative contribution of prey species included within prey groups (e.g., “whales”) could have been overestimated. Thus, within a group of several preys that have similar stable carbon and nitrogen isotope values, only one or a few of them might actually be consumed by killer whales. Finally, stable isotope mixing models can be challenging to interpret when predators investigated have a generalized diet, as the relative contribution of potential sources may not reveal clear patterns in prey selection (Di Beneditto et al., 2011). It is therefore critical to carefully consider how potential prey is selected, and incorporate potential sources using a priori knowledge on the system and species considered (Phillips et al., 2014).
Despite some limitations, the results of stable isotope mixing models strongly suggest that killer whales have a significant preference for odontocete cetaceans in the eastern Caribbean region, with a contribution of more than 60% to their diet (including Fraser’s dolphins, spotted dolphins, and teuthophageous whales). However, the contribution of other prey types, such as oceanic sharks, suggests that killer whales in the Caribbean could feed upon both cetaceans and predatory sharks. Consequently, unlike a number of coastal populations, Caribbean killer whales could have a relatively broad diet (e.g., Jefferson et al., 1991; Similä et al., 1996; Ford et al., 1998). Thus, a number of killer whale populations exhibit a relatively broad diet that could reflect the availability of prey species (Reisinger et al., 2016; Tixier et al., 2019). The predominance of odontocete cetaceans in the diet of killer whales in the Caribbean could be due to the widespread distribution and relatively high abundance of several species in the region, particularly in peri-insular waters (e.g., Mannocci et al., 2013; Gero et al., 2014; Luksenburg, 2014).
Killer whale foraging on odontocete cetaceans has already been documented across a number of tropical and subtropical regions around the globe (e.g., Jefferson et al., 1991; Dunn and Claridge, 2014). Killer whales are known to attack and feed on sperm whales (Reeves et al., 2006), small and medium-sized cetaceans such as delphinids and kogiid whales (Dunphy-Daly et al., 2008; Dunn and Claridge, 2014), and several species of large baleen whales (Jefferson et al., 1991; Baird and Dill, 1995; Pitman et al., 2015). The results of our model suggest that the Fraser’s dolphin is the most commonly consumed species. This species has already been recorded as a prey for killer whales, including in the Caribbean region (Dunn and Claridge, 2014), but nowhere else in tropical waters. The occurrence of short-finned pilot whales in the diet of killer whales in the Caribbean has rarely been documented (Jefferson et al., 1991), but both stable isotope and TEK data strongly suggests it is the case off St. Vincent. The consumption of sperm whales by killer whales is less clear from our isotope data, and not confirmed by whalers’ records. However, a number of anecdotal observations and photographic records show that some species of cetaceans, such as sperm whales, experience predation attempts from killer whales in the eastern Caribbean, such as off the coast of Guadeloupe (Figure 3). However, the magnitude of these interactions is not well documented. Predation attempts on other cetaceans could potentially be investigated from photographic records collected throughout the region, particularly on species that are resident in the Caribbean.
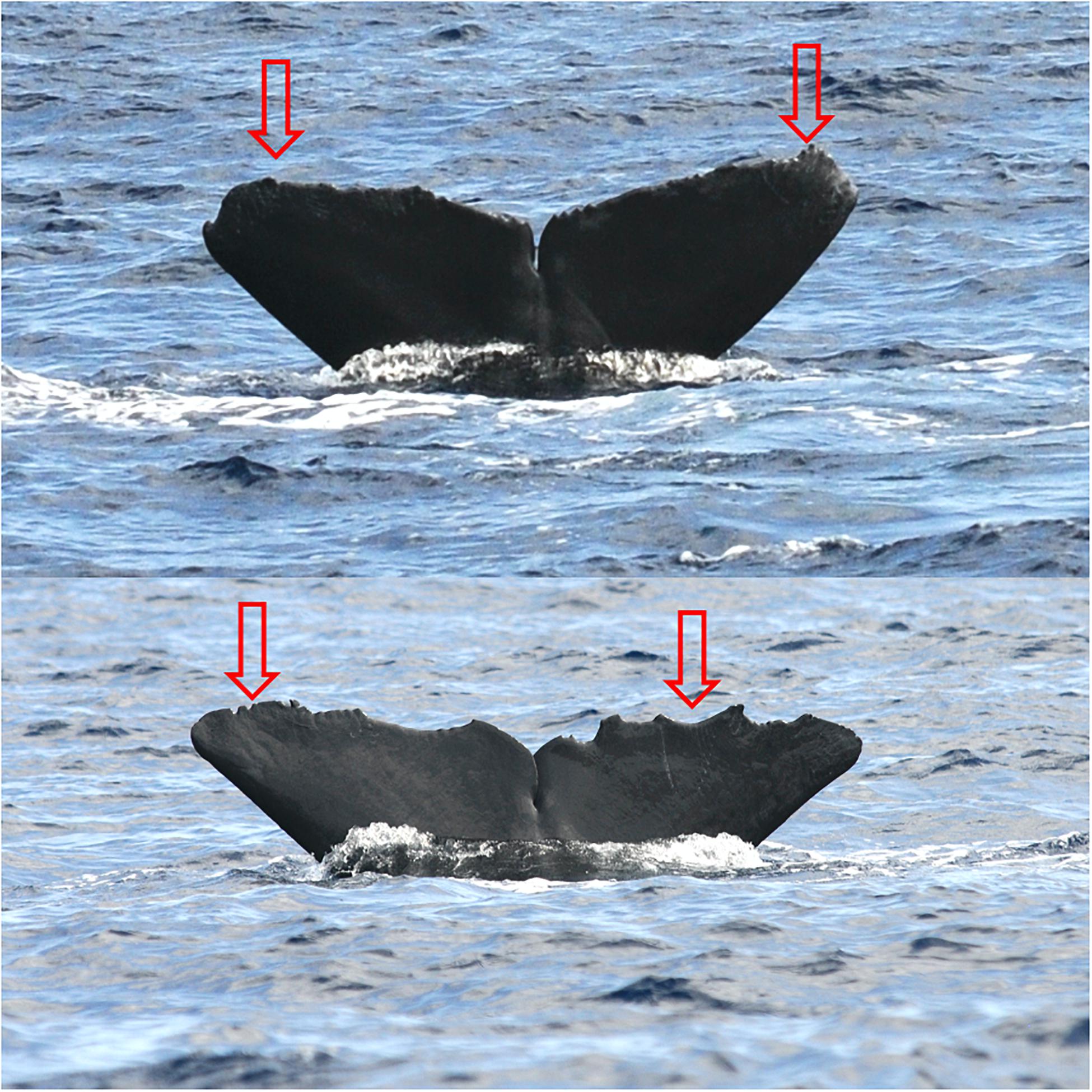
Figure 3. Tooth rake marks possibly caused by killer whales (Orcinus orca) on adult sperm whales (Physeter macrocephalus) photographed off the west coast of Guadeloupe (French West Indies) in 2014 and 2015 (Photographs: Laurent Bouveret], OMMAG).
The interpretation of stable isotope mixing models can be facilitated when patterns identified can be directly compared with other independent sources of information, such as behavioral observations (Kiszka et al., 2014) or other dietary indicators such as stomach content analysis (Jansen et al., 2013). Interestingly, although sample size was limited to seven individuals, TEK data collected from whalers also suggest that killer whales mostly feed on odontocete cetaceans off St. Vincent and the Grenadines. Whalers base their perceptions on occasional stomach content observations when processing killer whales. However, the differential digestion rate of prey may lead to over or underestimation of the occurrence of some prey. In addition, whalers believe that when killer whales are present along the leeward coast of St. Vincent, they strongly affect the behavior of other species of odontocetes by changing their habitat and behavior. Whalers believe that when killer whales are sighted over a period of several days, no other cetacean species are usually observed. Predation events on any prey type have not been reported by any interviewed whaler, and most whalers’ perceptions seem to be based on the behavioral response of several cetacean species that constitute potential prey for killer whales. Moreover, whalers also perceive killer whales as competitors, which creates an additional incentive to hunt them, despite high mercury levels found in a range of tissues consumed by the local population (McCormack et al., 2020). Overall, TEK data are qualitative, and should be considered with caution. However, TEK data clearly support isotope data suggesting that killer whales in the eastern Caribbean mostly feed on odontocete cetaceans.
Conclusion
Investigating the ecology and behavior of rare marine predators is a major challenge in many regions around the globe, particularly where research efforts are limited such as in the Caribbean. Multi-disciplinary approaches, including those incorporating social science data, are particularly relevant, but have rarely been considered by biologists and ecologists (but see Ferguson et al., 2012). Here, we strongly recommend that marine ecologists consider the combination of multiple methods when studying the ecology and behavior of coastal cetaceans and potentially other charismatic species, particularly the ecological knowledge that certain stakeholders might have. Collaborations between cetacean ecologists and social scientists can greatly improve our understanding of the ecology and feeding behavior of rare and elusive species, such as killer whales, in marine ecosystems.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by IRB Louisiana State University, University of Denver, and University of the South. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. The animal study was reviewed and approved by IACUC (Florida International University).
Author Contributions
JK conceived the study, led the analysis, collected and analyzed the samples, and wrote the manuscript. MC was involved in the data analysis and participated in the redaction of the manuscript. PM-F participated in the collection and analysis of samples, and participated in the redaction of the manuscript. RF collected and analyzed the interview survey data, participated in the redaction of the manuscript. All authors were highly involved in the preparation of this manuscript and reviewed and approved its submission.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Emma Smith, of the University of the West Indies-Cave Hill, who assisted with storage and preparation of the samples. We would also like to thank the whaling community of Barrouallie (St. Vincent) for allowing us to collect cetacean tissue samples, and specifically Diallo Boyea for managing the collection program. We would further like to thank Krishna Das (University of Liège, Belgium) for running the stable isotope analyses for cephalopods, and Cédric Pau (Regional Committee for Maritime Fisheries and Marine Farming) for collecting the sampled squids off Martinique. We would also like to thank Valeria Paz and Tristan Normand for preparing samples for stable isotope analysis. This is contribution #243 of the Coastal and Oceans Division in the Institute of Environment at Florida International University.
References
Ayala, L., Ortiz, M., and Gelcich, S. (2019). Exploring the role of fishers’ knowledge in assessing marine megafauna bycatch: insights from the Peruvian longline artisanal fishery. Anim. Cons. 22, 251–261. doi: 10.1111/acv.12460
Baird, R. W., and Dill, L. M. (1995). Occurrence and behaviour of transient killer whales: seasonal and pod-specific variability, foraging behaviour, and prey handling. Can. J. Zool. 73, 1300–1311. doi: 10.1139/z95-154
Bolaños-Jiménez, J., Mignucci-Giannoni, A. A., Blumenthal, J., Bogomolni, A., Casas, J. J., Henríquez, A., et al. (2014). Distribution, feeding habits and morphology of killer whales Orcinus orca in the Caribbean Sea. Mamm. Rev. 44, 177–189. doi: 10.1111/mam.12021
Bowen, W. D. (1997). Role of marine mammals in aquatic ecosystems. Mar. Ecol. Progr. Ser. 158, 267–274. doi: 10.3354/meps158267
Caldwell, D. K., and Caldwell, M. C. (1969). Addition of the leatherback sea turtle to the known prey of the killer whale, Orcinus orca. J. Mamm. 50, 636–636. doi: 10.2307/1378803
Caldwell, D. K., and Caldwell, M. C. (1975). Dolphin and small whale fisheries of the Caribbean and West Indies: occurrence, history, and catch statistics—with special reference to the Lesser Antillean Island of St. Vincent. J. Fish. Board Can. 32, 1105–1110. doi: 10.1139/f75-132
de Bruyn, P. J. N., Tos, C. A., and Terauds, A. (2013). Killer whale ecotypes: is there a global model? Biol. Rev. 88, 62–80. doi: 10.1111/j.1469-185x.2012.00239.x
DeNiro, M. J., and Epstein, S. (1978). Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 42, 495–506. doi: 10.1016/0016-7037(78)90199-0
Di Beneditto, A. P. M., de Souza, C. M. M., Kehrig, H. A., and Rezende, C. E. (2011). Use of multiple tools to assess the feeding preference of coastal dolphins. Mar. Biol. 158, 2209–2217. doi: 10.1007/s00227-011-1726-3
Dunn, C., and Claridge, D. (2014). Killer whale (Orcinus orca) occurrence and predation in the Bahamas. J. Mar. Biolog. U.K. 94, 1305–1309. doi: 10.1017/S0025315413000908
Dunphy-Daly, M. M., Heithaus, M. R., and Claridge, D. E. (2008). Temporal variation in dwarf sperm whale (Kogia sima) habitat use and group size off Great Abaco Island, Bahamas. Mar. Mamm. Sci. 24, 171–182. doi: 10.1111/j.1748-7692.2007.00183.x
Early-Capistrán, M. M., Sáenz-Arroyo, A., Cardoso-Mohedano, J. G., Garibay-Melo, G., Peckham, S. H., and Koch, V. (2018). Reconstructing 290 years of a data-poor fishery through ethnographic and archival research: the East Pacific green turtle (Chelonia mydas) in Baja California, Mexico. Fish. Fish. 19, 57–77. doi: 10.1111/faf.12236
Early-Capistrán, M.-M., Solana-Arellano, E., Abreu-Grobois, F. A., Narchi, N. E., Garibay-Melo, G., Seminoff, J. A., et al. (2020). Quantifying local ecological knowledge to model historical abundance of long-lived, heavily-exploited fauna. Peer J. 8:e9494. doi: 10.7717/peerj.9494
Estes, J. A., Heithaus, M., McCauley, D. J., Rasher, D. B., and Worm, B. (2016). Megafaunal impacts on structure and function of ocean ecosystems. Ann. Rev. Env. Res. 41, 83–116. doi: 10.1146/annurev-environ-110615-085622
Ferguson, S. H., Higdon, J. W., and Westdal, K. H. (2012). Prey items and predation behavior of killer whales (Orcinus orca) in Nunavut, Canada based on Inuit hunter interviews. Aquat. Biosyst. 8:3. doi: 10.1186/2046-9063-8-3
Fertl, D., Acevedo-Gutierrez, A., and Darby, F. L. (1996). A report of killer whales (Orcinus orca) feeding on a carcharhinid shark in Costa Rica. Mar. Mamm. Sci. 12, 606–611. doi: 10.1111/j.1748-7692.1996.tb00075.x
Fertl, D., and Fulling, G. L. (2007). Interactions between marine mammals and turtles. Mar. Turt. Newsletter 115, 4–8.
Fielding, R. (2018). The Wake of the Whale: Hunter Societies in the Caribbean and North Atlantic. Cambridge MA: Harvard University Press.
Flórez-González, L., Capella, J. J., and Rosenbaum, H. C. (1994). Attack of killer whales (Orcinus orca) on humpback whales (Megaptera novaeangliae) on a South American Pacific breeding ground. Mar. Mamm. Sci. 10, 218–222. doi: 10.1111/j.1748-7692.1994.tb00264.x
Foote, A. D., Newton, J., Piertney, S. B., Willerslev, E., and Gilbert, M. T. P. (2009). Ecological, morphological and genetic divergence of sympatric North Atlantic killer whale populations. Mol. Ecol. 18, 5207–5217. doi: 10.1111/j.1365-294X.2009.04407.x
Ford, J. K. B., Ellis, G. M., Barrett-Lennard, L. G., Morton, A. B., Palm, R. S., and Balcomb, K. C. III (1998). Dietary specialization in two sympatric populations of killer whales (Orcinus orca) in coastal British Columbia and adjacent waters. Can. J. Zool. 76, 1456–1471. doi: 10.1139/z98-089
Freeman, M. M. (1992). The nature and utility of traditional ecological knowledge. North. Perspect. 20, 9–12.
Gero, S., Milligan, M., Rinaldi, C., Francis, P., Gordon, J., Carlson, C., et al. (2014). Behavior and social structure of the sperm whales of Dominica, West Indies. Mar. Mamm. Sci. 30, 905–922. doi: 10.1111/mms.12086
Giménez, J., Ramírez, F., Almunia, J., Forero, M. G., and de Stephanis, R. (2016). From the pool to the sea: applicable isotope turnover rates and diet to skin discrimination factors for bottlenose dolphins (Tursiops truncatus). J. Exp. Mar. Biol. Ecol. 475, 54–61. doi: 10.1016/j.jembe.2015.11.001
Gray, T. N., Phommachak, A., Vannachomchan, K., and Guegan, F. (2017). Using local ecological knowledge to monitor threatened Mekong megafauna in Lao PDR. PLoS One 12:e0183247. doi: 10.1371/journal.pone.0183247
Heithaus, M. R., Frid, A., Wirsing, A. J., and Worm, B. (2008). Predicting ecological consequences of marine top predator declines. Trends Ecol. Evol. 23, 202–210. doi: 10.1016/j.tree.2008.01.003
Hobson, K. A., Schell, D. M., Renouf, D., and Noseworthy, E. (1996). Stable carbon and nitrogen isotopic fractionation between diet and tissues of captive seals: implications for dietary reconstructions involving marine mammals. Can. J. Fish. Aquat. Sci. 53, 528–533. doi: 10.1139/f95-209
Jansen, O. E., Michel, L., Lepoint, G., Das, K., Couperus, A. S., and Reijnders, P. J. (2013). Diet of harbor porpoises along the Dutch coast: a combined stable isotope and stomach contents approach. Mar. Mamm. Sci. 29, E295–E311.
Jefferson, T. A., Stacey, P. J., and Baird, R. W. (1991). A review of killer whale interactions with other marine mammals: predation to co-existence. Mamm. Rev. 21, 151–180. doi: 10.1111/j.1365-2907.1991.tb00291.x
Katona, S., and Whitehead, H. (1988). Are cetacea ecologically important. Oceanogr. Mar. Biol. Annu. Rev. 26, 553–568.
Kiszka, J. J., Heithaus, M. R., and Wirsing, A. J. (2015). Behavioural drivers of the ecological roles and importance of marine mammals. Mar. Ecol. Progr. Ser. 523, 267–281. doi: 10.3354/meps11180
Kiszka, J. J., Méndez-Fernandez, P., Heithaus, M. R., and Ridoux, V. (2014). The foraging ecology of coastal bottlenose dolphins based on stable isotope mixing models and behavioural sampling. Mar. Biol. 161, 953–961. doi: 10.1007/s00227-014-2395-9
Leeney, R. H., and Poncelet, P. (2015). Using fishers’ ecological knowledge to assess the status and cultural importance of sawfish in Guinea-Bissau. Aquat. Cons. Mar. Freshw. Ecosyst. 25, 411–430. doi: 10.1002/aqc.2419
Lesage, V., Hammill, M. O., and Kovacs, K. M. (2001). Marine mammals and the community structure of the Estuary and Gulf of St Lawrence, Canada: evidence from stable isotope analysis. Mar. Ecol. Progr. Ser. 210, 203–221. doi: 10.3354/meps210203
Lesage, V., Hammill, M. O., and Kovacs, K. M. (2002). Diet-tissue fractionation of stable carbon and nitrogen isotopes in phocid seals. Mar. Mamm. Sci. 18, 182–193. doi: 10.1111/j.1748-7692.2002.tb01027.x
Lesage, V., Morin, Y., Rioux, È, Pomerleau, C., Ferguson, S. H., and Pelletier, É (2010). Stable isotopes and trace elements as indicators of diet and habitat use in cetaceans: predicting errors related to preservation, lipid extraction, and lipid normalization. Mar. Ecol. Progr. Ser. 419, 249–265. doi: 10.3354/meps08825
Luksenburg, J. A. (2014). The cetaceans of Aruba, southern Caribbean. J. Mar. Biol. Assoc. U.K. 94, 1161–1174. doi: 10.1017/s0025315413000337
Madigan, D. J., Brooks, E. J., Bond, M. E., Gelsleichter, J., Howey, L. A., Abercrombie, D. L., et al. (2015). Diet shift and site-fidelity of oceanic whitetip sharks Carcharhinus longimanus along the Great Bahama Bank. Mar. Ecol. Prog. Ser. 529, 185–197. doi: 10.3354/meps11302
Mannocci, L., Monestiez, P., Bolaños-Jiménez, J., Dorémus, G., Jeremie, S., Laran, S., et al. (2013). Megavertebrate communities from two contrasting ecosystems in the western tropical Atlantic. J. Mar. Syst. 111, 208–222. doi: 10.1016/j.jmarsys.2012.11.002
Marcoux, M., McMeans, B. C., Fisk, A. T., and Ferguson, S. H. (2012). Composition and temporal variation in the diet of beluga whales, derived from stable isotopes. Mar. Ecol. Prog. Ser. 471, 283–291. doi: 10.3354/meps10029
McCormack, M. A., Fielding, R., Kiszka, J. J., Paz, V., Jackson, B. P., Bergfelt, D. R., et al. (2020). Mercury and selenium concentrations, and selenium: mercury molar ratios in small cetaceans taken off St. Vincent, West Indies. Env. Res. 181:108908. doi: 10.1016/j.envres.2019.108908
Méndez-Fernandez, P., Kiszka, J. J., Heithaus, M. R., Beal, A., Vandersarren, G., Caurant, F., et al. (2018). From banana fields to the deep blue: assessment of chlordecone contamination of oceanic cetaceans in the eastern Caribbean. Mar. Poll. Bull. 137, 56–60. doi: 10.1016/j.marpolbul.2018.10.012
Moore, J. W., and Semmens, B. X. (2008). Incorporating uncertainty and prior information into stable isotope mixing models. Ecol. Lett. 11, 470–480. doi: 10.1111/j.1461-0248.2008.01163.x
Parnell, A. C., Phillips, D. L., Bearhop, S., Semmens, B. X., Ward, E. J., Moore, J. W., et al. (2013). Bayesian stable isotope mixing models. Environmetrics 14, 387–399.
Phillips, D. L., Inger, R., Bearhop, S., Jackson, A. L., Moore, J. W., Parnell, A. C., et al. (2014). Best practices for use of stable isotope mixing models in food-web studies. Can. J. Zool. 92, 823–835. doi: 10.1139/cjz-2014-0127
Pitman, R. L., and Dutton, P. H. (2004). Killer whale predation on a leatherback turtle in the northeast Pacific. Pacific Sci. 58, 497–498. doi: 10.1353/psc.2004.0034
Pitman, R. L., and Ensor, P. (2003). Three forms of killer whales (Orcinus orca) in Antarctic waters. J. Cet. Res. Manag. 5, 131–140.
Pitman, R. L., Ballance, L. T., Mesnick, S. I., and Chivers, S. J. (2001). Killer whale predation on sperm whales: observations and implications. Mar. Mamm. Sci. 17, 494–507. doi: 10.1111/j.1748-7692.2001.tb01000.x
Pitman, R. L., OSulivan, S., and Mase, B. (2003). Killer whales (Orcinus orca) attack a school of pantropical spotted dolphins (Stenella attenuata) in the Gulf of Mexico. Aquat. Mamm. 39, 321–324. doi: 10.1578/01675420360736488
Pitman, R. L., Totterdell, J. A., Fearnbach, H., Ballance, L. T., Durban, J. W., and Kemps, H. (2015). Whale killers: prevalence and ecological implications of killer whale predation on humpback whale calves off Western Australia. Mar. Mamm. Sci. 31, 629–657. doi: 10.1111/mms.12182
Reeves, R. R., Berger, J., and Clapham, P. J. (2006). “Killer whales as predators of large baleen whales and sperm whales,” in Whales, Whaling and Ocean Ecosystems, eds J. A. Estes, D. P. Demaster, D. F. Doak, T. M. Williams, and R. L. Brownell Jr. (London: University of California Press), 174–187. doi: 10.1525/california/9780520248847.003.0014
Reisinger, R. R., Gröcke, D. R., Lübcker, E. L., McClymont, E. L., Hoelzel, A. R., and de Bruyn, P. J. N. (2016). Variation in the diet of killer whales Orcinus orca at Marion Island, Southern Ocean. Mar. Ecol. Prog. Ser. 549, 263–274. doi: 10.3354/meps11676
Roman, J., Estes, J. A., Morissette, L., Smith, C., Costa, D., McCarthy, J., et al. (2014). Whales as marine ecosystem engineers. Front. Ecol. Environ. 12:377–385. doi: 10.1890/130220
Ryan, C., Berrow, S. D., McHugh, B., O’Donnell, C., Trueman, C. N., and O’Connor, I. (2014). Prey preferences of sympatric fin (Balaenoptera physalus) and humpback (Megaptera novaeangliae) whales revealed by stable isotope mixing models. Mar. Mamm. Sci. 30, 242–258. doi: 10.1111/mms.12034
Similä, T., Holst, J. C., and Christensen, I. (1996). Occurrence and diet of killer whales in northern Norway: seasonal patterns relative to the distribution and abundance of Norwegian spring-spawning herring. Can. J. Fish. Aquat. Sci. 53, 769–779. doi: 10.1139/f95-253
Smith, J. A., Mazumder, D., Suthers, I. M., and Taylor, D. (2013). To fit or not to fit: evaluating stable isotope mixing models using simulated mixing polygons. Methods Ecol. Evol. 4, 612–618. doi: 10.1111/2041-210x.12048
Spitz, J., Cherel, Y., Bertin, S., Kiszka, J., Dewez, A., and Ridoux, V. (2011). Prey preferences among the community of deep-diving odontocetes from the Bay of Biscay, Northeast Atlantic. Deep Sea Res. 58, 273–282. doi: 10.1016/j.dsr.2010.12.009
Stock, B. C., Jackson, A. L., Ward, E. J., Parnell, A. C., Phillips, D. L., and Semmens, B. X. (2018). Analyzing mixing systems using a new generation of Bayesian tracer mixing models. PeerJ 6:e5096. doi: 10.7717/peerj.5096
Temple, A. J., Kiszka, J. J., Stead, S. M., Wambiji, N., Brito, A., Poonian, C. N., et al. (2018). Marine megafauna interactions with small-scale fisheries in the southwestern Indian Ocean: a review of status and challenges for research and management. Rev. Fish Biol. Fish. 28, 89–115. doi: 10.1007/s11160-017-9494-x
Tixier, P., Giménez, J., Reisinger, R. R., Méndez-Fernandez, P., Arnould, J. P., Cherel, Y., et al. (2019). Importance of toothfish in the diet of generalist subantarctic killer whales: implications for fisheries interactions. Mar. Ecol. Progr. Ser. 613, 197–210. doi: 10.3354/meps12894
Trites, A. W., and Spitz, J. (2018). “Diet,” in Encyclopedia of Marine Mammals, eds B. Würsig, J. G. M. Thewissen, and K. M. Kovacs (Cambridge, MA: Academic Press), 255–259.
Tucker, A. D., MacDonald, B. D., and Seminoff, J. A. (2014). Foraging site fidelity and stable isotope values of loggerhead turtles tracked in the Gulf of Mexico and northwest Caribbean. Mar. Ecol. Prog. Ser. 502, 267–279. doi: 10.3354/meps10655
Vander Zanden, H. B., Arthur, K. E., Bolten, A. B., Popp, B. N., Lagueux, C. J., Harrison, E., et al. (2013). Trophic ecology of a green turtle breeding population. Mar. Ecol. Prog. Ser. 476, 237–249. doi: 10.3354/meps10185
Vander Zanden, M. J., Clayton, M. K., Moody, E. K., Solomon, C. T., and Weidel, B. C. (2015). Stable isotope turnover and half-life in animal tissues: a literature synthesis. PLoS One 10:e0116182. doi: 10.1371/journal.pone.0116182
Visser, I. N. (2005). First observations of feeding on thresher (Alopias vulpinus) and hammerhead (Sphyrna zygaena) sharks by killer whales (Orcinus orca), which specialise on elasmobranchs as prey. Aquat. Mamm. 31, 83–88. doi: 10.1578/AM.31.1.2005.83
Walker, J. L., and Macko, S. A. (1999). Dietary studies of marine mammals using stable carbon and nitrogen isotopic ratios of teeth. Mar. Mamm. Sci. 15, 314–334. doi: 10.1111/j.1748-7692.1999.tb00804.x
Keywords: diet, mixing models, cetaceans, interview surveys, Lesser Antilles, δ15N and δ13C, Orcinus orca
Citation: Kiszka JJ, Caputo M, Méndez-Fernandez P and Fielding R (2021) Feeding Ecology of Elusive Caribbean Killer Whales Inferred From Bayesian Stable Isotope Mixing Models and Whalers’ Ecological Knowledge. Front. Mar. Sci. 8:648421. doi: 10.3389/fmars.2021.648421
Received: 31 December 2020; Accepted: 30 March 2021;
Published: 30 April 2021.
Edited by:
Adrian C. Gleiss, Murdoch University, AustraliaReviewed by:
Ryan Rudolf Reisinger, University of California, Santa Cruz, United StatesPhil J. Bouchet, University of St Andrews, United Kingdom
Copyright © 2021 Kiszka, Caputo, Méndez-Fernandez and Fielding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeremy J. Kiszka, jkiszka@fiu.edu
 Jeremy J. Kiszka
Jeremy J. Kiszka Michelle Caputo
Michelle Caputo Paula Méndez-Fernandez
Paula Méndez-Fernandez Russell Fielding
Russell Fielding