Distribution and Habitat Suitability of Ross Seals in a Warming Ocean
- 1Alfred-Wegener-Institut, Helmholtz-Zentrum für Polar- und Meeresforschung, Bremerhaven, Germany
- 2Mammal Research Institute, Department of Zoology & Entomology, University of Pretoria, Pretoria, South Africa
- 3Gateway Antarctica, School of Earth and Environment, University of Canterbury, Christchurch, New Zealand
- 4Department of Arctic and Marine Biology, UiT The Arctic University of Norway, Tromsø, Norway
- 5Department of Marine Sciences, University of Gothenburg, Gothenburg, Sweden
Understanding the determinants of poorly studied species’ spatial ecology is fundamental to understanding climate change impacts on those species and how to effectively prioritise their conservation. Ross seals (Ommatophoca rossii) are the least studied of the Antarctic pinnipeds with a limited knowledge of their spatial ecology. We present the largest tracking study for this species to date, create the first habitat models, and discuss the potential impacts of climate change on their preferred habitat and the implications for conservation. We combined newly collected satellite tracking data (2016–2019: n = 11) with previously published data (2001: n = 8) from the Weddell, King Haakon VII and Lazarev seas, Antarctica, and used 16 remotely sensed environmental variables to model Ross seal habitat suitability by means of boosted regression trees for summer and winter, respectively. Five of the top environmental predictors were relevant in both summer and winter (sea-surface temperature, distance to the ice edge, ice concentration standard deviation, mixed-layer depth, and sea-surface height anomalies). Ross seals preferred to forage in waters ranging between −1 and 2°C, where the mixed-layer depth was shallower in summer and deeper in winter, where current speeds were slower, and away from the ice edge in the open ocean. Receding ice edge and shoaling of the mixed layer induced by climate change may reduce swimming distances and diving depths, thereby reducing foraging costs. However, predicted increased current speeds and sea-surface temperatures may reduce habitat suitability in these regions. We suggest that the response of Ross seals to climate change will be regionally specific, their future success will ultimately depend on how their prey responds to regional climate effects and their own behavioural plasticity.
Introduction
Climate change and extreme weather events are increasing and as a consequence are influencing the distribution and movements of species globally (Karl and Trenberth, 2003; Meehl et al., 2007). Species are shifting higher in altitude or poleward, but most species will not be able to shift their distributions or adapt their movement behaviours fast enough to keep up with climate shifts (Chen et al., 2011; Schloss et al., 2012; Pinsky et al., 2013). In the Southern Ocean, rapid climate change is expressed through various pathways (Meredith et al., 2019; Rogers et al., 2020); e.g., sea-surface temperatures are increasing and sea-surface temperature isotherms previously associated with oceanic fronts, are shifting toward the pole, the sea-ice extent is decreasing, and the Southern Annular Mode has a tendency toward a positive phase, which coincides with the poleward shift and strengthening of the westerlies (White and Peterson, 1996; de la Mare, 1997; Tynan, 1998; Sallée et al., 2010; Young et al., 2011; Bracegirdle et al., 2013; Meijers et al., 2019; Hindell et al., 2020). These substantial changes, associated with anthropogenically driven climate change (Turner et al., 2014; Meredith et al., 2019) increasingly impact Southern Ocean predator species’ distribution, diet, behaviour, and life-history (Rodríguez et al., 2019; Ropert-Coudert et al., 2019; Bestley et al., 2020; Hindell et al., 2020; Hückstädt et al., 2020; Rogers et al., 2020).
Changing sea-ice has large impacts on ecosystem processes (e.g., Convey and Peck, 2019). Pagophilic seal and penguin species depend on the extent and of sea-ice and fast-ice stability for resting and breeding, whereas the extent of sea-ice also controls accessibility to areas of foraging (Barbraud and Weimerskirch, 2001; Labrousse et al., 2015, 2019). The loss of sea-ice and fast-ice is strongly linked to sea temperature anomalies and reduced survival of individuals (Barbraud et al., 2000; McMahon and Burton, 2005; Jenouvrier et al., 2006). King penguins (Aptenodytes patagonicus) breeding on the sub-Antarctic Crozet islands, migrate toward the ice and Antarctic Polar Front and are forced to travel further to reach known prey fields due to shifting foraging grounds (Péron et al., 2012; Cristofari et al., 2018). Population decline of the southern elephant seal (Mirounga leonina) at Macquarie Island is driven by changing sea-ice conditions off the Victoria Land Coast and in the Ross Sea. An increase in dense sea-ice along the Victoria Land Coast negatively affects access to foraging areas for female elephant seals that forage in this area. Whereas decreasing ice extent in the Ross Sea, causes females that forage in this region to increase the distances travelled between breeding and feeding sites. Both scenarios of changing sea-ice conditions likely forces southern elephant seals to leave their foraging grounds earlier (Van den Hoff et al., 2014; Hindell et al., 2016, 2017; Younger et al., 2016).
Southern Ocean higher-order predators (whales, seals, seabirds, and penguins) are relatively well studied compared to other components within this environment although, knowledge of these mobile consumers is biassed by life-history phase (lack of data on juveniles and non-breeders), timing of expeditions (paucity in winter data), and ease of access to species [pack-ice breeding species are relatively poorly studied (McIntyre, 2014; Bestley et al., 2020)]. Additionally, knowledge of pagophilic seal species’ habitat and distributions are biassed toward haul-out data because most of the available data and understandings of distribution and habitat use of ice breeding seals are based on observations made during surveys from helicopters and ships (Southwell et al., 2012).
The Ross seal (Ommatophoca rossii) is the rarest and most elusive of the Antarctic seals (e.g., Würsig et al., 2018). They are considered to comprise only 1% of all Antarctic pack-ice seals and are perhaps, together with the leopard seal, the most understudied and underrepresented marine predators in terms of tracking and biologging studies in Antarctica. Currently, only two studies describe the at-sea movements of Ross seals: eight individuals tracked in the Weddell Sea (Blix and Nordøy, 2007) and four individuals tracked in the Amundsen Sea (Arcalís-Planas et al., 2015). Ross seals also behave differently compared to other well-studied Antarctic marine predators because they travel away from the Antarctic pack-ice and forage pelagically most of the year (Blix and Nordøy, 2007; Arcalís-Planas et al., 2015).
Given their pelagic foraging behaviour (Blix and Nordøy, 2007; Arcalís-Planas et al., 2015), Ross seals are expected to respond differently to climatic shifts and extreme weather events compared to other Southern Ocean predators. Because Ross seals spend most of their lives in the open ocean (Blix and Nordøy, 2007; Arcalís-Planas et al., 2015), it is suggested that their foraging behaviour is least likely to be negatively affected by changes in sea-ice coverage, unless the preferred prey species respond by shifting their distributional range (Siniff et al., 2008). A reduction in winter sea-ice extent may result in open pelagic waters that are closer to pack-ice areas where Ross seals haul out to breed and moult, which reduces swimming distance to their pelagic foraging grounds after breeding and moulting.
The lack of data for this species restricts the extent of our potential predictions of Ross seal behaviour in response to climate change. The limiting factor is baseline data on the oceanographic conditions that characterise the foraging habitats and availability of prey of Ross seals. Here, we combine recently collected tracking data (2016, 2018, 2019) from Ross seals in the eastern Weddell Sea and the area to the east, off Queen Maud Land (including the King Haakon VII and Lazarev seas) with the only other movement data of Ross seals from the same area (Blix and Nordøy, 2007). We build seasonal habitat suitability models using remotely sensed oceanographic variables to (1) identify oceanographic conditions that characterise summer and winter foraging habitat of Ross seals in the Weddell Sea and adjoining areas to the east; and (2) to predict suitable foraging habitat for them during summer and winter, respectively in that area. We further discuss the implications of Ross seals’ foraging habitat in response to predicted climate change effects.
Materials and Methods
Study Area
Ross seals were captured, instrumented and sampled in the King Haakon VII Sea off Queen Maud Land (6954′S–7220′S and 200′W–1746′W) and in two cases in the marginal sea-ice zone, north of the Lazarev Sea around ∼59°S and 5–21°E in spring 2019 (Supplementary Table 1). We combined tracking data of Ross seals collected within this region during South African National Antarctic Expedition S55 (December 2015–February 2016), the German Antarctic Expedition PS111 (January 2018–March 2018), and the South African Southern Ocean Seasonal Experiment a.k.a. SCALE (October–November 2019), with published data from Norwegian Antarctic Research Expedition (NARE 2000/01) from 2000 to 2001 (Blix and Nordøy, 2007). For brevity, hereafter we refer to the King Haakon VII Sea, Lazarev Sea, Weddell Sea (predicted to in habitat models–see below), and the rest of the area bounded by 40°S to 80°S; 80°W to 80°E as “the Weddell Sea and adjoining areas.”
Capturing and Tag Deployment
Ross seals were captured and physically restrained on pack-ice floes in austral summers of 2016 (n = 11), 2018 (n = 2), and spring of 2019 (n = 2) as the animals were encountered along the cruise track of the MV SA Agulhas II (2016, 2019) and RV Polarstern (2018, Alfred-Wegener-Institut Helmholtz-Zentrum für Polar- und Meeresforschung, 2017). An A-frame net, consisting of two stainless steel poles, hinged at the front end, with a nylon net in between, were used to capture the seals. After restraining the seal, a small hole was cut into the net where the top of the seal’s head was located, to place the satellite transmitter. Quick-setting Araldite Epoxy resin (AW2101/HW2951) was used to glue the transmitter to the seals’ heads. The capture and tagging procedure followed Arcalís-Planas et al. (2015) and animals were restrained for a maximum of 1 h. We determined the sex of the animals, and took standard length and girth measurements (Bonner and Laws, 1993). The age of individuals is unknown. The standard measures are not exactly comparable to the NARE 2000/01 data where animals were anaesthetized and therefore fully relaxed and extended (Blix and Nordøy, 2007), but are comparable to those of Arcalís-Planas et al. (2015). During the 2015/2016 summer voyage (hereafter S55) six SPOT6 and five Splash-series tags (Wildlife Computers, Redmond, WA, United States) Argos-linked (CLS, Toulouse, France) satellite trackers were deployed. During summer 2018 (hereafter PS111) two SPLASH10-309A tags, and during SCALE in spring 2019, two SPOT6 tags, were deployed. Satellite transmissions from the satellite tags were not duty cycled and were allowed data to be transmitted every day for all hours of the day, and were restricted to 500 accumulated transmissions per day. Transmissions were paused when the animal was hauled out for longer than 12 h, but allowed to transmit again if the haul out lasts longer than 8 days. Transmissions relayed data that were collected the previous 2 days. Methods on the capturing, immobilisation and deployment of satellite trackers during the Norwegian Ross seal study from 2001 to 2002 are in Blix and Nordøy (2007). The Norwegian dataset consists of Argos-collected satellite tracking data from 10 individuals fitted with SDR-T16 trackers (Wildlife Computers, Redmond, WA, United States).
State-Space Models for Basic Filtering
Location data collected through the ARGOS satellite system contain intrinsic errors. To account for this, we fitted a two-state, behaviourally switching, state-space model to individual tracks (Jonsen et al., 2005; Jonsen, 2016). This procedure filtered erroneous location estimates and provided interpolated tracks with estimated locations at 3 h time intervals. This time step was based on a combination of the median number of Argos location points per day (∼30 points per day) and following logic on available temporal resolution of the environmental covariates (i.e., it does not make sense to have 30 points per day compared with, for example, only two values per day of sea-surface temperatures). Bayesian State-space models were fitted using Markov chain Monte Carlo in “rjags” (Plummer, 2016), via the “bsam” library (Jonsen et al., 2005; Jonsen, 2016) implemented in R (R Core Team, 2020). Two Markov chains were run in parallel, each of 55,000 iterations, only using every 50th value, while the first 10,000 values (i.e., burn-in) were excluded. Diagnostic plots were used to assess converging and appropriate mixing of the two Markov chains (Jonsen et al., 2013). Apart from filtering and interpolating the tracking data, state-space models also identify two hidden states in the movement data and classify location estimates into either “travelling” and “area-restricted search” behaviour, which is based on the animal’s swimming speed and turning angle (Jonsen et al., 2013). Animals travelling between points are assumed to swim faster and in a straight line to cover a large area in low detail, whereas animals searching, hunting or foraging, are expected to swim slower and turn more often to cover a small area in detail, making area-restricted search a proxy for prey searching or hunting behaviour (e.g., Fauchald and Tveraa, 2003; Patterson et al., 2008).
Kernel Density Estimation
We calculated 50% kernel utilisation distributions (i.e., core habitat use; Worton, 1989) for each individual split between the austral seasons (Summer: October–March; Winter: April–September) using the R library “adehabitatHR” (Calenge, 2006). H-values were selected using the ad hoc method (Silverman, 1986). Kernel densities were plotted for illustrative purposes and data exploration.
Environmental Correlates
We used 16 remotely sensed environmental covariates to describe the habitat use of Ross seals (Table 1 and Supplementary Table 2). Environmental variables chosen for the habitat modelling are known to affect marine predator foraging behaviour and are commonly used to model their habitat use (Raymond et al., 2015; Reisinger et al., 2018; Wege et al., 2019; Hindell et al., 2020). Variables were extracted from the Australian Antarctic Data Centre using the R library “raadtools” (Sumner, 2015) and processed further if needed using R library “raster” (Hijmans, 2016). We matched the date and time of each seal location to the nearest environmental data in space and time for the dynamic environmental covariates (Supplementary Table 2). To avoid the inherent collinearity among environmental variables, we filtered for the most informative set by calculating variance inflation factors for the entire Weddell Sea region and immediately adjoining waters to the east and west (i.e., 40°S to 80°S; 80°W to 80°E) using the R library “fmsb” (Nakazawa, 2018). Variables with a variance inflation factor larger than 10 were excluded, because this is a good indication of strong collinearity (Nakazawa, 2018).
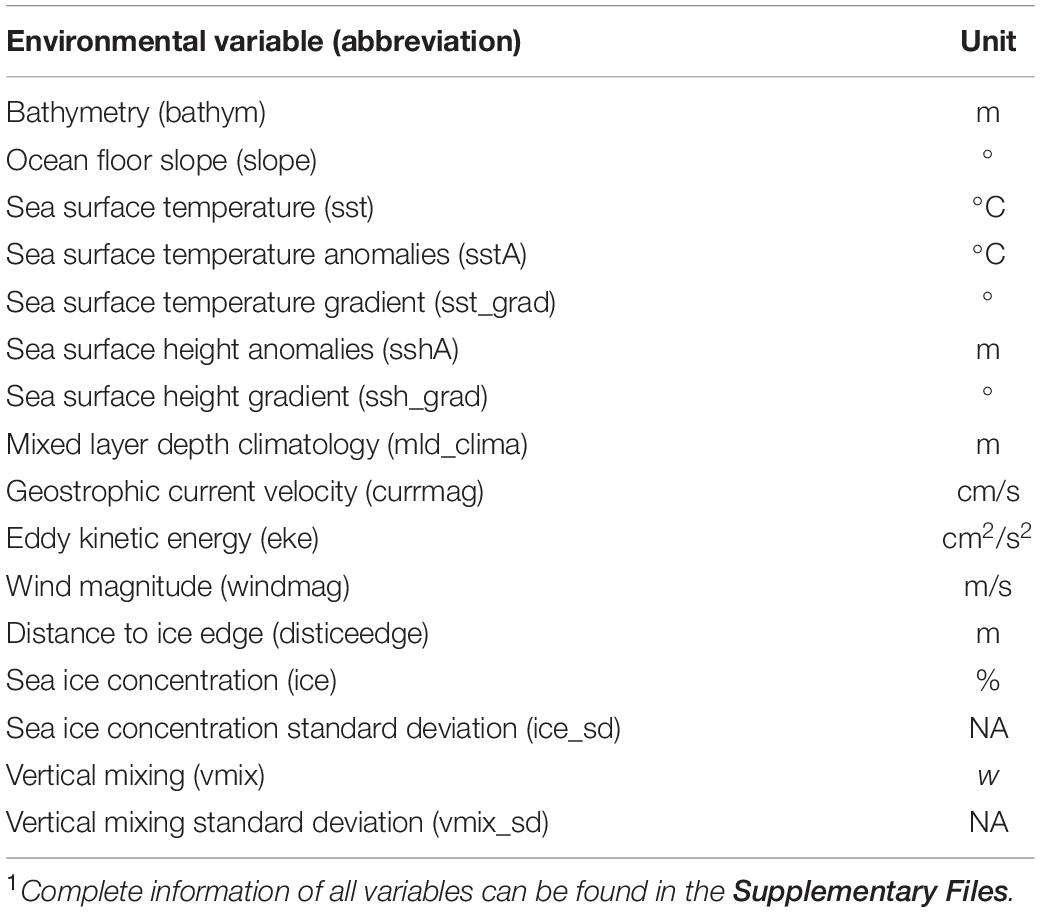
Table 1. Environmental variables used as covariates in habitat models for Ross seals (Ommatophoca rossii)1.
Species Distribution Modelling
Species distribution models were created using a supervised machine learning technique, boosted regression trees. We separated the summer and winter portions (see Kernel density estimation above) of the tracks to create a species distribution model for each season. Boosted regression trees were chosen because they are known to perform well in predicting species’ distributions (Reisinger et al., 2018; Wege et al., 2019; Hindell et al., 2020). The state-space modelled “area restricted search” or “travelling” estimates were used as the binary response variable inherently turning these models into classification tree ensembles with a Bernoulli distribution. Given that there are not enough tracks in different years, we did not consider any annual variations. We trained models using the “caret” library in R, which employs functions from the R library “gbm” (Ridgeway, 2015; Kuhn et al., 2019). For summer tracking data we held out 30% of the data (test data, nsummer = 3,241; nwinter = 4,784) with an equal number of area-restricted search and travelling points in each test data set. The remaining training data (nsummer = 11,669; nwinter = 11,163) was unbalanced between the number of “area-restricted search” and “travelling” points (area-restricted searchsummer = 7,835 vs. travellingsummer = 3,834; area-restricted searchwinter = 11,524 vs. travellingwinter = 2,384). We made use of a bootstrapped modelling approach. The bootstraps involved running 500 boosted regression tree models independently, using random sub-sample of 2000 area-restricted search and travelling points, respectively, which was repeated for summer and winter data. This method balanced the data sets, which reduces overfitting of models and the amount of spatial-autocorrelation that is inherent in tracking data (Hijmans, 2012). Each bootstrap model was tuned respectively using the “tuneGrid” function in the “caret” library, through compiling a range of candidate models and choosing the best model and optimal set of hyperparameters based on the lowest Area Under the Curve (AUC) value from the Random Operator Curve (ROC). Candidate models for each bootstrap made use of a 10-fold cross-validation approach. We calculated the out-of-bag AUC value as the goodness-of-fit measure for each of the bootstrapped models using the 30% hold-out test data.
To generate a prediction map of potential foraging habitat of Ross seals we calculated a seasonal mean value per grid square at the same spatiotemporal resolution applied to each environmental variable used in each of the model bootstraps (Reisinger et al., 2018; Wege et al., 2020). We predicted potential foraging habitat of Ross seals for the entire Weddell and adjacent seas and further to 80° easterly and westerly longitudes and from the coastline to 50°S Latitude and beyond for each bootstrap for summer and winter data, respectively using the “predict” function in the R library “raster” (Hijmans, 2016). Using the out-of-bag AUC value of each bootstrap as the weight of each bootstrap, we calculated a weighted mean average probability of area-restricted search behaviour of the 500 bootstraps using the “weighted mean” function in R library “raster” (Hijmans, 2016). The relative influence (%) of each environmental predictor variable was calculated for each bootstrap for the summer and winter ensembles and averaged across bootstraps. Partial dependence plots of the predictor variables, for each of the bootstraps were created in R library “pdp” (Greenwell, 2017) and averaged to create a mean ± standard deviation partial dependence plot.
Results
Of the 25 satellite tags deployed across the four expeditions, 19 satellite tags successfully transmitted data for >5 days and produced 32,377 location estimates (Supplementary Table 1). Ross seals immediately travelled north after deployment and completing their annual moult (February and March) during minimum ice extent, and remained in open water just south of the Polar Front (defined by Orsi et al. (1995); Figure 1). In the winter, Ross seals migrated south toward the Marginal Ice Zone (i.e., the areas directly inward and outward from the ice boundary. They spent most of the winter in the open ocean within 500 km of the ice-edge and moved northward with the expanding sea-ice edge (Figure 1). Between September and November, assumed breeding individuals (n = 6 females) entered the consolidated pack-ice further south again to pup and mate, after which they travelled north again in December. In January, Ross seals travelled back into the remaining consolidated pack-ice against, but within 50 km of the Antarctic ice shelf, to moult (Figure 1). In summer, Ross seals spent on average 68.8% (sd: ±23.2%) in the open ocean (range: 35.0–100%), and in winter Ross seals spent on average 65.7% (sd: ±27.9%) in the open ocean (range: 23.3–100%).
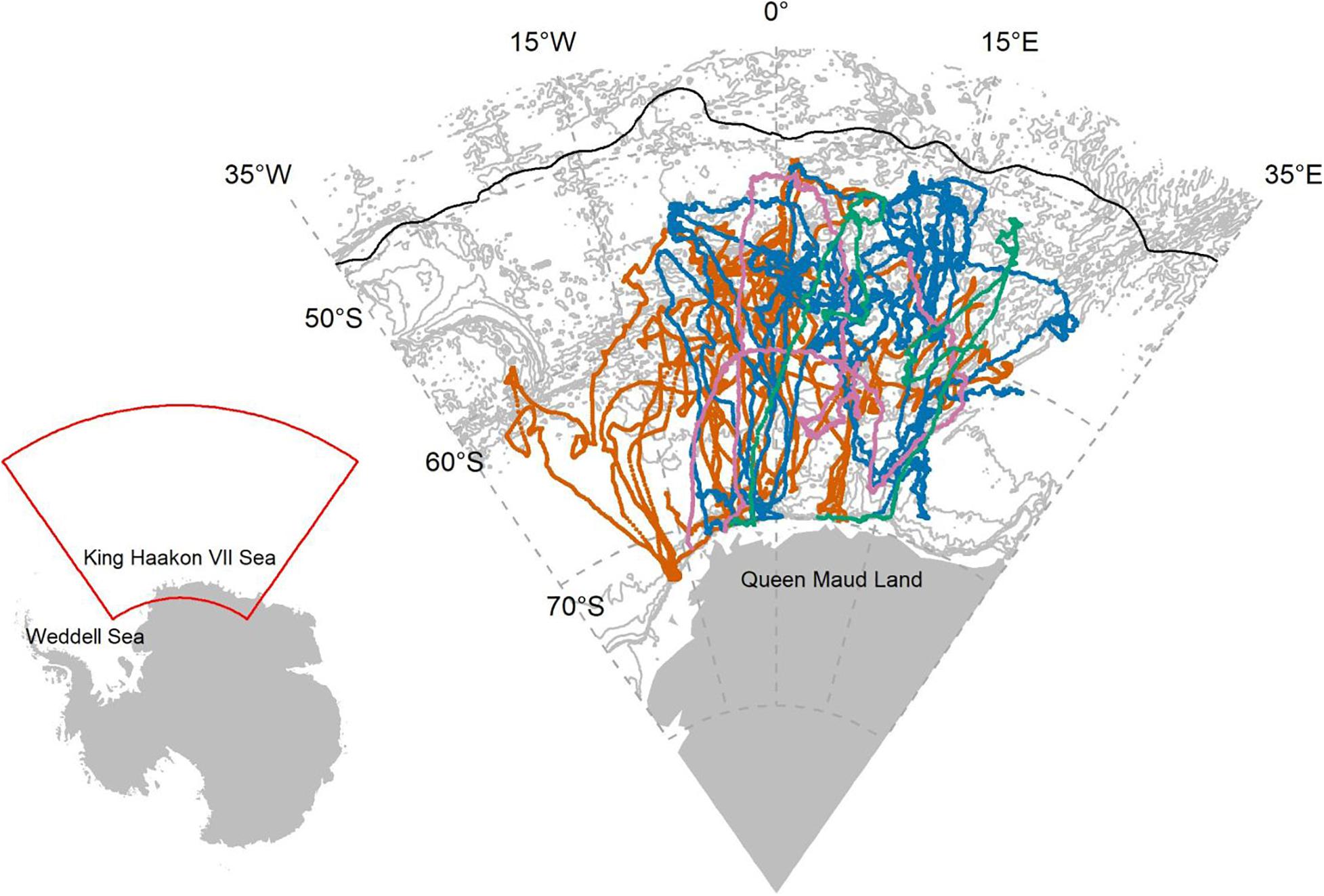
Figure 1. State-space modelled location estimates for each of the 19 Ross seals in the King Haakon VII Sea that were used to model important foraging habitat in the Weddell Sea and surrounding areas. Colours represent the four different expeditions: orange: NARE 2000/01; blue: S55 2015/16; pink: PS111 2018; green: Scale 2019. The black line indicates the position of the Polar Front (Orsi et al., 1995) and the grey lines are GEBCO 1 arc-min bathymetrical contours (IOC et al., 2003). Inset: The position of this map in relation to the Antarctic continent.
Highest habitat use, from the kernel density analyses, was largely the same in summer and winter, in the open ocean and away from the Antarctic continent, but south of the Polar Front (Figure 2). The large area of the kernel is a product of their south–north movements throughout the year. The winter core kernel area shifted eastward by ∼15°. The most noticeable seasonal difference in core habitat use was the summer moult haul-out close to the Antarctic continent (Figure 2).
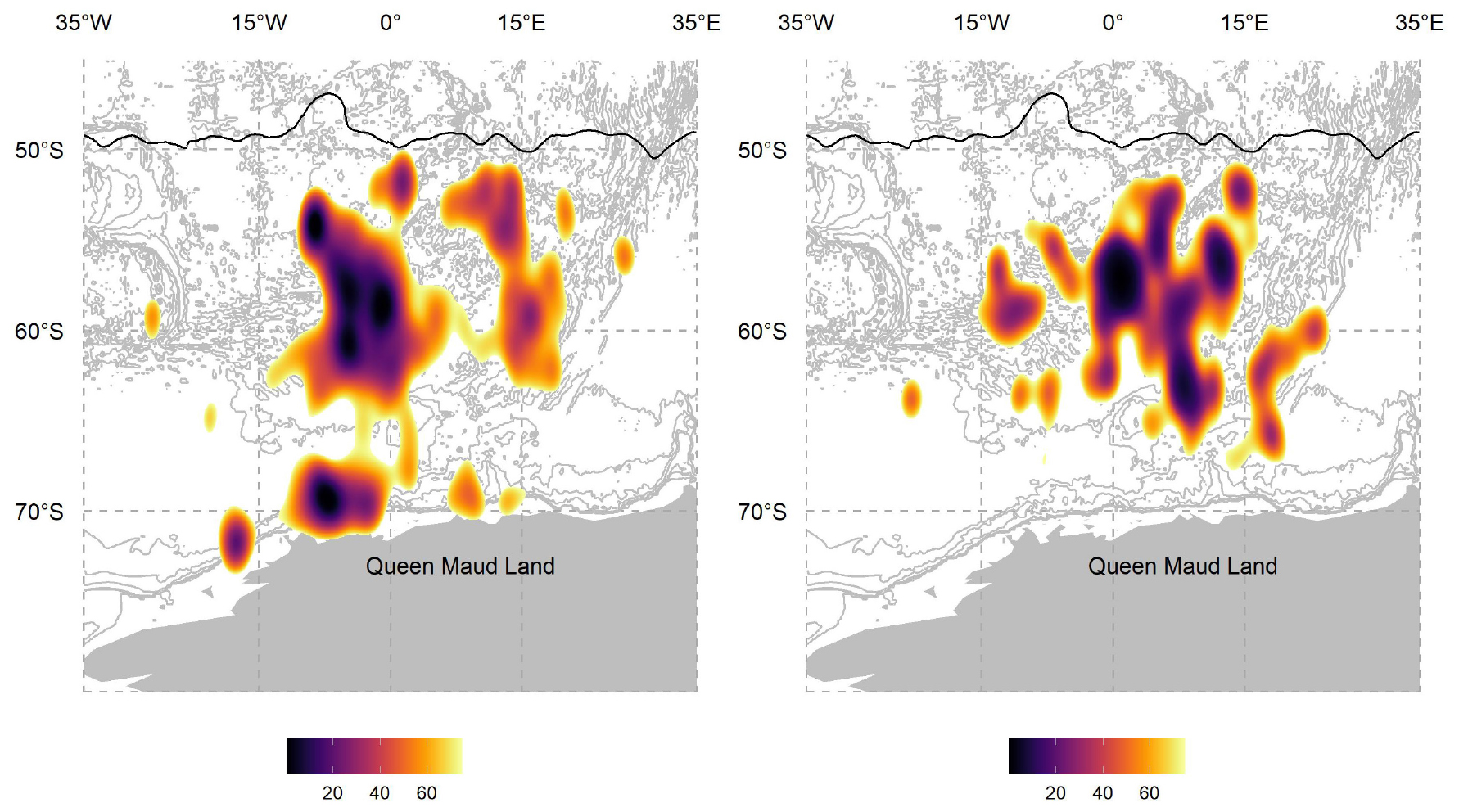
Figure 2. The summer (left) and winter (right) percentage utilisation distributions for 19 Ross seals are shown in relation to the Polar Front (black line) (Orsi et al., 1995) and the Antarctic Continent. The grey lines are GEBCO 1 arc-min bathymetrical contours (IOC et al., 2003).
Boosted regression tree model performance (AUC) was high among bootstraps, with summer models’ mean ± standard deviation AUC = 0.90 ± 0.002 (range: 0.89–0.90) and winter models’ mean ± standard deviation AUC = 0.93 ± 0.003 (range: 0.92–0.94). The predicted area-restricted search habitat suitability maps for summer (Figures 3A,B) and winter (Figures 3C,D) illustrate the seasonal differences between Ross seal potential foraging habitat and how this expands in summer and contracts in winter. Mean importance of predictor variables changed between seasons (Figure 4). During summer the top six variables based on mean relative influence (%) were sea surface temperature (sst; °C), ice concentration standard deviation (ice_sd), mixed-layer depth summer climatology (mld_clima; m), bathymetry (bathym; m), distance to the ice edge (dist_iceedge; m), and sea-surface height anomalies (sshA; m) (Figure 4A). The top six variables in winter based on mean relative influence (%) were the distance to the ice edge (dist_iceedge; m), standard deviation of ice concentration (ice_sd), sea surface temperature (sst;°C), sea surface height anomalies (sshA; m), mixed-layer depth winter climatology (mld_clima; m), and current magnitude (currmag; cm.s–1) listed in order of decreasing importance.
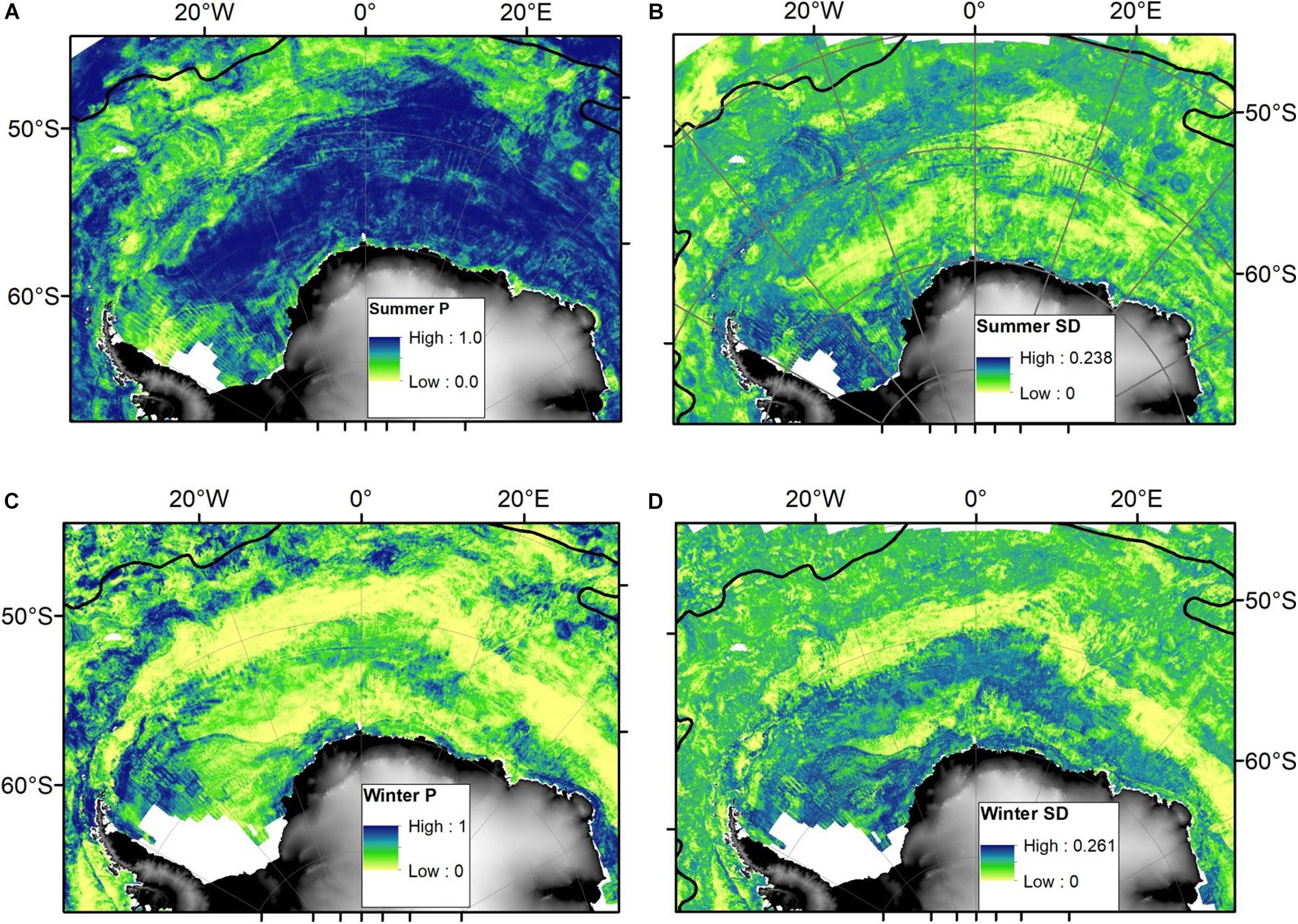
Figure 3. Predicted Ross seal area-restricted search habitat for summer (A,B) and winter (C,D), respectively illustrating the probability (P) and standard deviation (sd) of a Ross seal making use of area-restricted search behaviour across the entire Weddell Sea and adjacent waters. The black line indicates the Polar Front (Orsi et al., 1995).
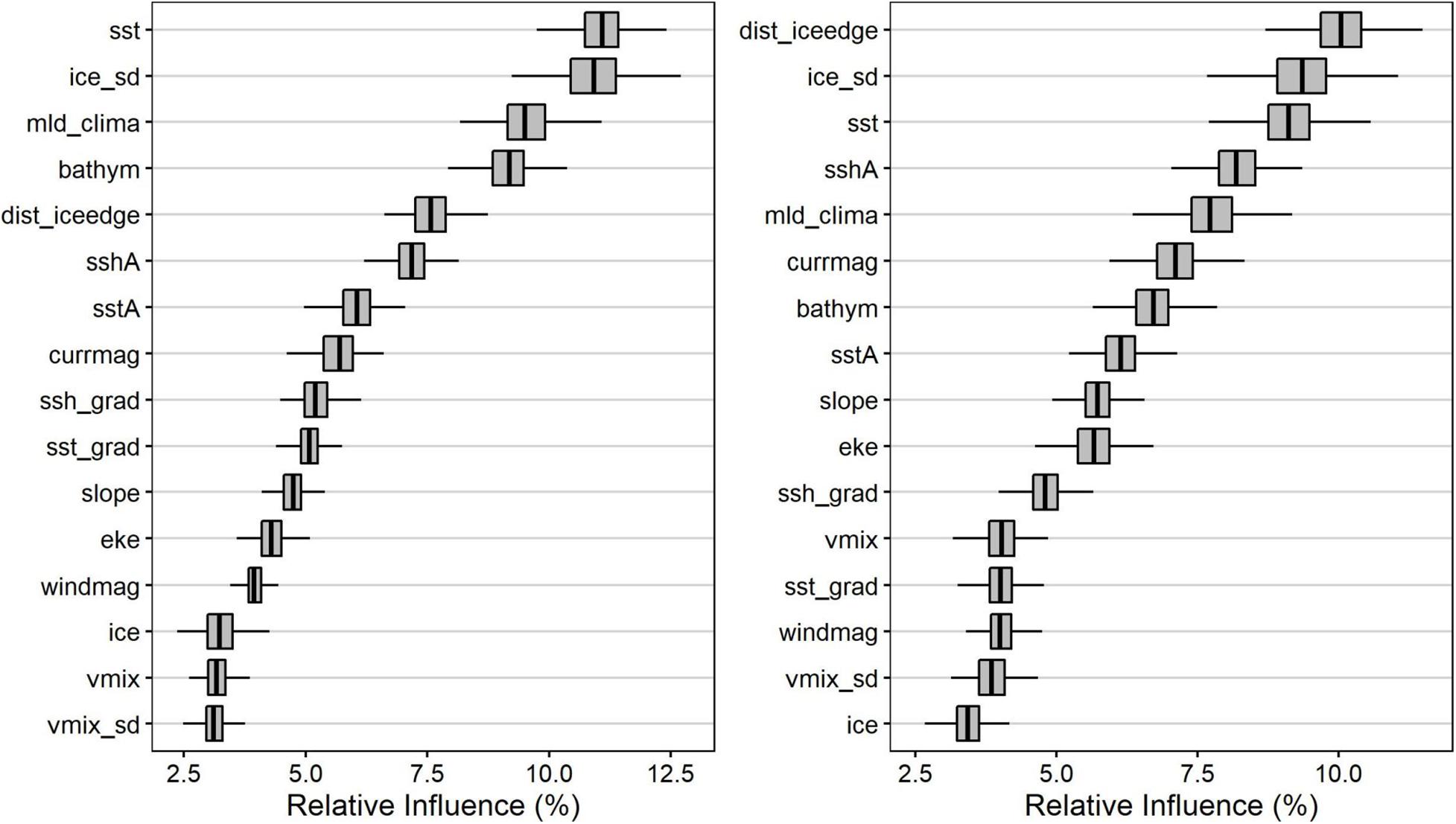
Figure 4. Relative influence (%) for the 500 bootstraps of the environmental variables used in the habitat models during summer (A) and winter (B) of Ross seals tracked within the eastern Weddell Sea. Bathym, bathymetry; slope, slope of the sea floor; dist_iceedge, distance from the ice edge; eke, eddy kinetic energy; ice, sea ice concentration; ice_sd, ice concentration standard deviation; mld_clima, mixed layer depth climatology; sst, sea surface temperature; sshA, sea surface height anomaly; sstA, sea surface temperature anomaly; ssh_grad, sea surface height gradient; sst_grad, sea surface temperature gradient; vmix, vertical mixing speed; vmix_sd, vertical mixing speed standard deviation; currmag, current magnitude; windmag, wind magnitude.
Partial dependence plots show that in summer, area-restricted search probability peaked between 0 and 1.8°C and increased almost linearly with an increasing ice concentration standard deviation (Figure 5). Area-restricted search probability was highest at a mixed-layer depth of 20–40 m, increasing again at mld_clima above 80 m (Figure 5), while area-restricted search probability decreases as ocean depth became shallower up to 2000 m, but then peaked again between 1000 and 1800 m deep–just off the continental slope. Area-restricted search probability decreased the further seals moved away from the ice edge, with highest probabilities at 1500–2000 km away from the ice edge, whereas area-restricted search probability was highest between 0.025 and 0.075 m sshA, but was highly variable for values below 0.05 m (Figure 5). In winter, the relationship between area-restricted search and the distance to the ice edge was bimodal, peaking at 400 m and >1500 km away (Figure 6). Similar to summer, area-restricted search probability increased with increasing ice_sd and was highest at sea-surface temperatures between −1 and 2°C (Figure 6). At sshA values smaller than 0.04 m and larger than 0.08 m seals were more likely to display area-restricted search behaviour but the likelihood was also more variable within these ranges. The mixed-layer depths where area-restricted search was likely was deeper than in summer (100–130 m), and was highest where current magnitude was 0 and decreases with increasing current speeds (Figure 6).
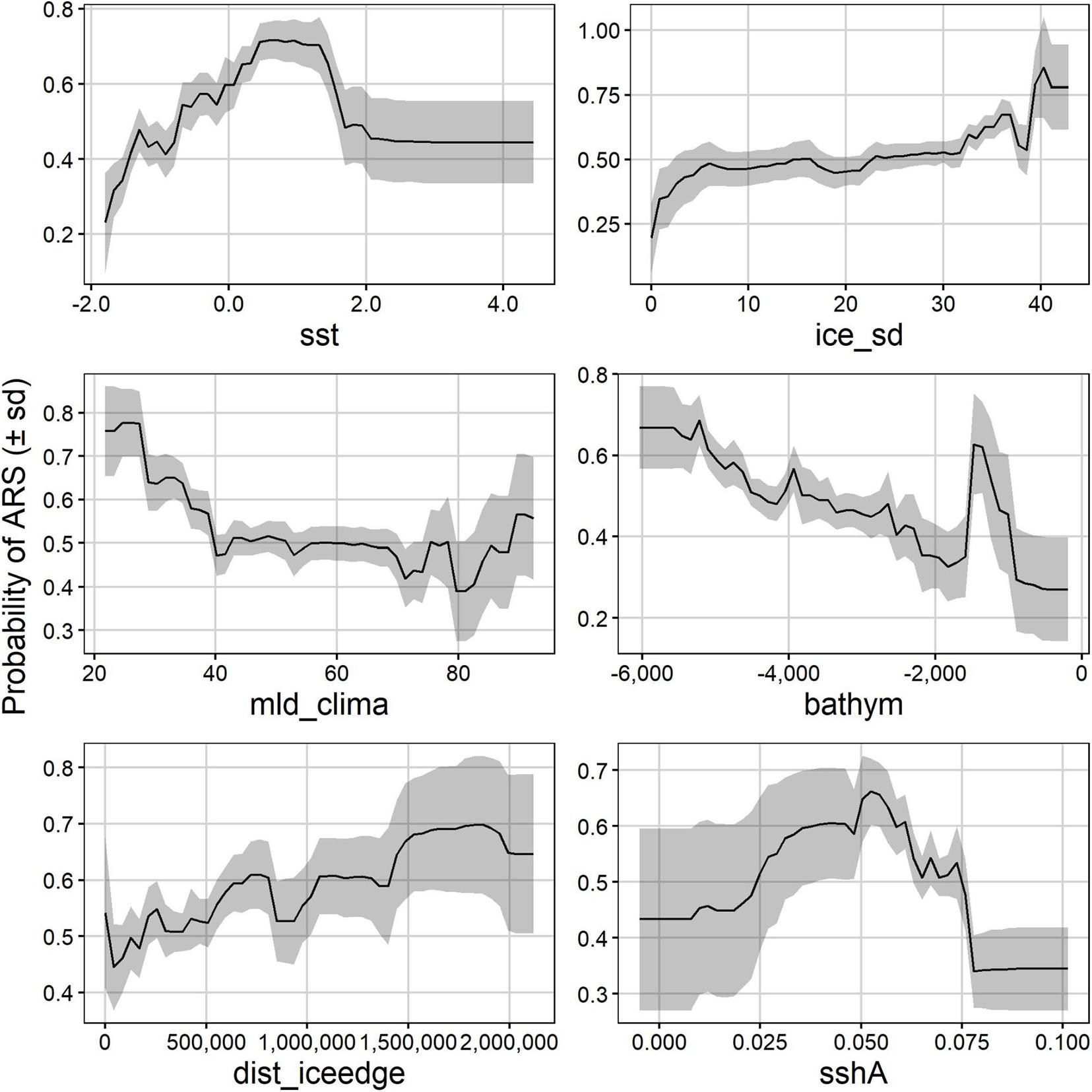
Figure 5. Summer partial dependence plots showing how the probability of area restricted search (ARS) is influenced by the top six environmental variables. Mean probability of ARS (solid line) ± sd (grey shaded) across the 500 bootstraps are shown. Bathym, bathymetry (m); dist_iceedge, distance from the ice edge (m); ice_sd, ice concentration standard deviation; mld_clima, mixed layer depth climatology (m); sst, sea surface temperature (°C); sshA, sea surface height anomaly (m).
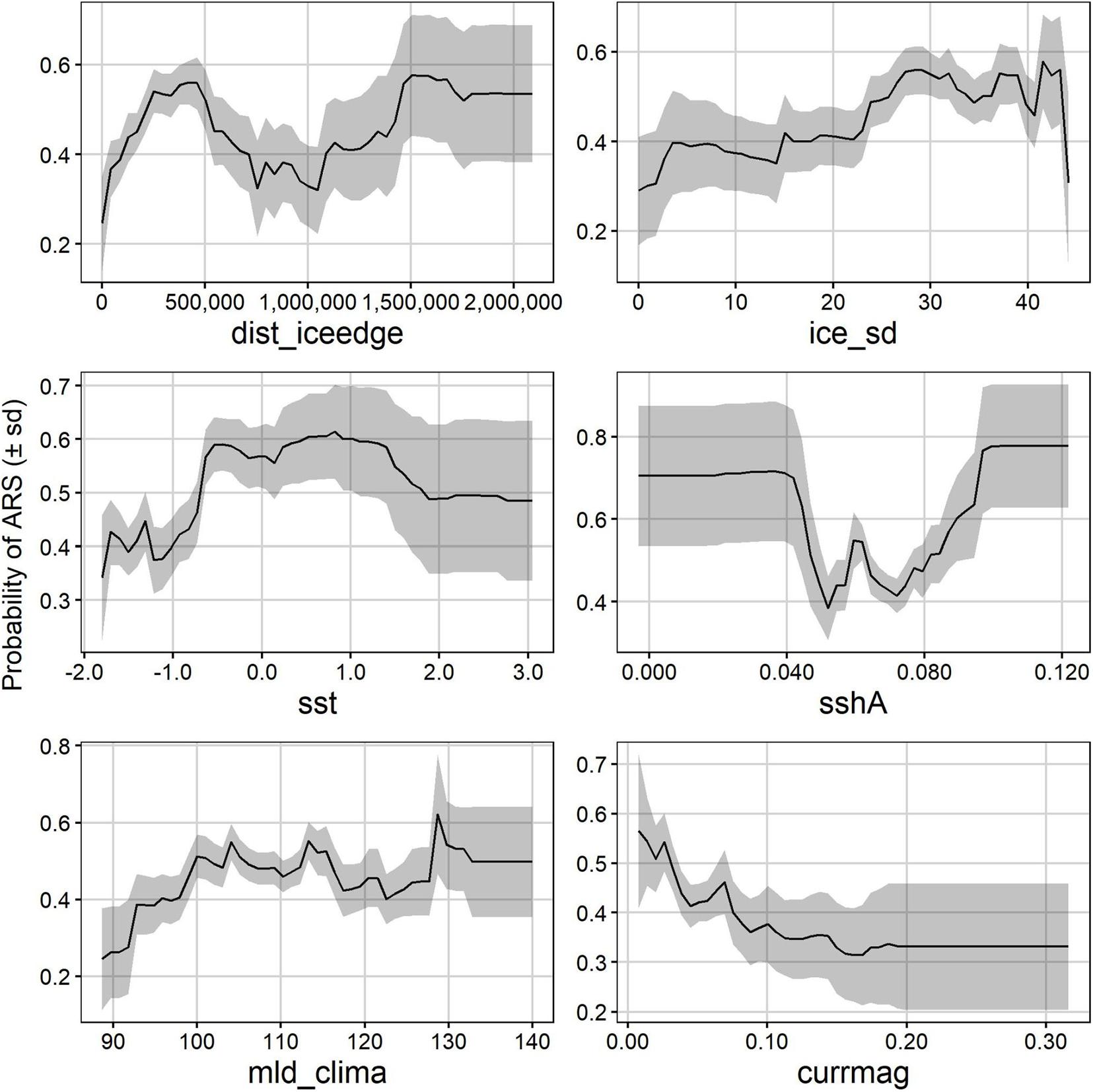
Figure 6. Winter partial dependence plots showing how the probability of area restricted search (ARS) is influenced by the top six environmental variables in the winter models. Mean probability of ARS (solid line) ± sd (grey shaded) across the 500 bootstraps are shown. dist_iceedge, distance from the ice edge (m); ice_sd, ice concentration standard deviation; mld_clima, mixed layer depth climatology (m); sst, sea surface temperature (°C); sshA, sea surface height anomaly; currmag, current magnitude (m.s− 1).
Discussion
Conservation practices skewed toward certain species, age-classes and sexes can result in bias and ignore underrepresented species (e.g., McIntyre, 2014). The Ross seal is arguably one of the most data deficient species in the Southern Ocean. In this study, we combine all the available data for Ross seals tracked in the eastern Weddell Sea and adjoining waters (n = 19) to create the first ever species distribution model. Environmental variables associated with Ross seal foraging areas in the summer and winter are used to identify the drivers of their movements during the unique pelagic phase. Habitat modelling provides novel insight into the year-round and large-scale distribution of the species and the potential impacts of climate change.
Distribution and Diet
Instrumented Ross seals travelled away from the pack-ice and spend most of their time in the open ocean (Blix and Nordøy, 2007; Arcalís-Planas et al., 2015, this study) and traversed the Atlantic sector of the Southern Ocean within a very narrow longitudinal band four times a year (Figure 1 and Supplementary Animations). The likelihood of area-restricted search behaviour in summer and winter covaried with ice concentration variability (Figures 5, 6), which serves as a proxy for sea-ice edge zones and accessibility of ice-covered areas (Wege et al., 2020). The sea-ice edge zones are productive areas due to the input of nutrients in the water column as the sea ice melts, which promote phytoplankton blooms which in turn trigger grazers and other mid-level and higher-order predators to aggregate to forage (Nicol, 2006; Arrigo et al., 2008; Riekkola et al., 2019). The thick multi-year pack-ice present in the Weddell Sea and an increased swimming distance to the open ocean likely makes the southern Weddell Sea an unsuitable habitat for Ross seals, which would explain the low habitat suitability within the Weddell Sea embayment year-round (Figure 3). Bester et al. (2020) reviewed all observational data of Ross seals and found that during their annual moult (late summer, early autumn), they are absent within the inner reaches of the Weddell Sea south of 73°S and west of 30°W. Our habitat model showed that within the Weddell Sea embayment, a small area is still likely foraging habitat (Figure 3). Bester et al. (2020) used only observational data (i.e., Ross seals hauled out to moult/rest), while in contrast our study predicts potential foraging habitat based on environmental variables. It is also important to consider that a habitat model only predicts on the variables given to it, and that there are potentially unknown or currently immeasurable variables that dictate Ross seal foraging habitat.
In summer, the foraging behaviour of Ross seals can be split into two stages: a period close to the narrow continental shelf off Queen Maud Land during the annual moult and open ocean foraging between 55° and 65°S, south of the Polar Front. Open ocean foraging happens either post-moult when seals travel north for the winter, post-breeding (November–December) when breeding individuals return from the ice, and for non-breeders that remained at their northern range throughout the summer (Figures 2, 3). This explains why Ross seal foraging probability increases as the waters become deeper (negatively correlated) and preferred to forage over abyssal water (>4000 m deep; Figure 5). However, there is a peak in the probability of area-restricted search behaviour between 1000 and 1500 m depth (Figure 5), which is considered the continental slope and where Ross seals forage during their annual moult (Southwell, 2005; Blix and Nordøy, 2007; Arcalís-Planas et al., 2015).
Stomach content analyses from earlier investigations suggested that Ross seals sampled in January off Queen Maud Land predominantly prey on Antarctic silverfish and squid (Skinner and Klages, 1994). Antarctic silverfish are pelagic and only occur close to the Antarctic continent, on the continental shelf, or on the shallow slope areas in cold water temperatures ranging between (−1.75 to −2°C) (DeWitt, 1970; Hubold, 1984; DeWitt et al., 1990; Ekau, 1990; Eastman, 1993; White and Piatkowski, 1993; Kellerman, 1996; Trunov, 2001; Donnelly et al., 2004; Davis et al., 2017; La Mesa et al., 2019), and higher temperatures of up to 4°C around islands of the Scotia Arc (Mintenbeck and Torres, 2017). Ross seals are likely only forage on Antarctic silverfish during their annual moult, while over the continental shelf and slope. Based on our understanding of Ross seal movements outside of the moult, these diet samples collected in January are not a reflection of the year-round diet. This agrees with stable isotope results (Rau et al., 1992; Zhao et al., 2004; Brault et al., 2019) and diving behaviour analyses, which also suggest that Ross seals are open ocean foragers that prey on mesopelagic fish and squid (Blix and Nordøy, 2007). Fisheries in the Southern Ocean primarily focus on krill (Euphausia spp.), toothfish (Dissostichus spp.) and squid (Agnew et al., 2005; Kock et al., 2007; Nicol et al., 2012; Chown and Brooks, 2019), which means Ross seals are not in direct competition with krill and toothfish fisheries. Currently, it is unknown what percentage of the Ross seal diet is comprised of cephalopods, but it is not their dominant prey species (Rau et al., 1992; Skinner and Klages, 1994; Zhao et al., 2004). Potential for conflict with cephalopod fisheries are also unknown, given the scarcity of data on cephalopod fishing in the open ocean around Antarctica (Agnew et al., 2005; Chown and Brooks, 2019).
Climate Change Implications
Siniff et al. (2008) suggested that the pelagic foraging behaviour of Ross seals makes them least susceptible to climate change compared to other Antarctic seals. They may even be considered as potential climate change winners, because the shrinking distance from the continent to the sea-ice edge in theory would require Ross seals not to swim as far to reach potential foraging grounds.
Five out of the six top variables influencing Ross seal probability of area-restricted search behaviour were the same between summer and winter, i.e., sea-surface temperature, distance to the ice edge, ice concentration variability, the mixed layer depth and wind-driven sea-surface height anomalies. Ross seals preferred to spend time in waters between −1 and 2°C (Figures 5, 6) and displayed less area-restricted search behaviour in warm sea-surface temperature anomalies (Supplementary Figures 1, 2) during both seasons. Sea-surface temperature is a dominant predictor in most Antarctic predators’ movement behaviour (Hindell et al., 2020), and coincides with productive areas in the ocean, marine predator abundance (Block et al., 2011), and subsequent prey distribution and availability (Reisinger et al., 2018; Bestley et al., 2020).
During the summer, Ross seal area-restricted search probability decreased with an increased mixed-layer depth, while in winter area-restricted search probability increased with an increase in mixed-layer depth. The mixed-layer depth influences depth of prey aggregations and any fluctuations in the mixed-layer depth will influence how deep seals have to dive (and the amount of energy to expend) to find prey. The difference between the correlations of area-restricted search probability and mixed-layer depth across the seasons is likely linked to location of dives (continental shelf vs. off-shelf) and a general expected increase in mixed-layer depths during winter months (e.g., Sallée et al., 2010). In areas of the ocean covered in sea-ice, melting ice causes freshening of the water column and the mixed-layer depth to shoal (Sallée et al., 2013; Meijers, 2014). However, how the mixed-layer depth is likely to respond to climate change is not reliably known because of the inability of climate models to accurately represent stratification in the winter months, and are often biassed toward shallower mixed layers (Sallée et al., 2013), which means climate models cannot always accurately predict what will happen to mixed-layer depths throughout the Southern Ocean. The interactions between processes that can affect stratification–primarily through momentum and buoyancy fluxes–are complex, and so how the mixed-layer depth will be affected by climate change differs by region and proximity to continental shelf. If, as suggested by climate models, the mixed layer depth shoals, it could potentially be beneficial to diving Ross seals and reduce the amount of energy expended while foraging at depth. In summer, Ross seals displayed more area-restricted search behaviour where ocean currents were weaker (Figure 5), which would also typically increase in strength due to higher wind speeds under future climate change scenarios (Young et al., 2011; Young and Ribal, 2019), and therefore could be disadvantageous to their foraging habitat.
In summer, probability for area-restricted search behaviour and distance from the ice-edge covaried, which coincides with the Ross seals’ northward migration away from the continent after the moult, and post-breeding when breeders also travelled out of the ice again. Post-moult in February, when the Antarctic sea-ice extent is at its minimum, changes in Antarctic sea ice extent would have minimal effect on Ross seal foraging. However, November–December, post-breeding Ross seals return to the open ocean and forage pelagically. Expanding sea-ice extent would mean Ross seals have to swim further north to reach open water post-breeding, which could potentially incur higher energetic costs to individuals. In winter, the relationship between Ross seal area-restricted search behaviour and distance from the ice edge was bi-modal, with peaks in area-restricted search behaviour around 500 and 1500 km away from the ice edge. This likely results from the seasonality in the expanding ice-edge: during the early winter months (April and May) Ross seals are still foraging at sea at their maximal range away from Antarctica and as the ice-edge expands, they move southward toward the ice edge, then following the growing seasonal ice (Animations in the Supplementary Files). If the ice edge recedes, this would mean that Ross seals will have to swim smaller distances to reach the open ocean and to return to the ice edge, which could potentially be beneficial to them and reduce the amount of energy expended swimming to foraging areas (Siniff et al., 2008). In summer, there was more predicted suitable foraging habitat available compared to winter for Ross seals (Figure 3). This is because of the seasonal pack-ice growth in winter and Ross seal’s preference for open ocean foraging, further indicating that receding ice edge under future climate scenarios will reduce the swimming distance to available foraging habitat, and that potentially more foraging habitat will become available. However, in the Weddell Sea and Ross Sea regions of the Southern Ocean the seasonal ice edge was expanding and sea-surface temperatures decreasing [but summer ice extent recently recorded to decrease (Turner et al., 2020)]; while the Amundsen Sea ice losses are increasing along with rising sea-surface temperatures (Zhang, 2007; Bintanja et al., 2013; Meehl et al., 2016). These contrasting ice-dynamics will result in different responses in foraging behaviour by Ross seals to climate change.
Despite the impacts on their energy expenditures (swimming distance and diving depth), their preferred foraging grounds are also influenced through changing sea-surface temperatures, increased wind and current magnitudes, increased wave height, and poleward shifting wind (Constable et al., 2014). How these changes will affect the distribution of prey is critical to understand how Ross seals will respond. Myctophids in the Southern Ocean are predicted to undergo species-specific shifts in their distribution, but overall most species shift toward the poles (Freer et al., 2019), which would require sub-Antarctic predators to travel south from their sub-Antarctic islands toward the ice to forage (Labrousse et al., 2017; Reisinger et al., 2018). Ross seals travel northward (this study), and a southward migration of prey would reduce travel distances and could be advantageous. Cephalopods, which also form a component of the Ross seal diet (Skinner and Klages, 1994), are unlikely to be affected by sea-ice changes, but they are vulnerable to mesoscale fluctuations and ocean acidification that are affecting entire ecosystems (Rodhouse, 2013; Xavier et al., 2018). How fish and squid species will respond to climate change and increased fishing pressures within mesopelagic ecosystems is largely still unknown and most studies are focussed on Antarctic krill (Euphausia superba) (Murphy et al., 2007, 2013).
Crabeater seals (Lobodon carcinophaga) rely on Antarctic krill for 90% of its diet (Hückstädt et al., 2012) while Antarctic fur seals (Arctocephalus gazella) breeding in the south Atlantic also predominantly prey on krill (Reid and Arnould, 1996). Dietary specialisation makes both species susceptible to any variances and shifts in prey distribution and abundance and are not able to buffer climate fluctuations as well as other Antarctic seals and seabirds (Forcada et al., 2008; Hückstädt et al., 2020). Generalist predators are more likely to switch between prey species are likely to fair better than specialist foragers (e.g., Angermeier, 1995; Terraube et al., 2011), like Weddell seals (Leptonychotes weddellii) for example, whose diet varies among many species of fishes, cephalopods, and crustaceans (Burns et al., 1998; Lake et al., 2003). But, due to Weddell seals’ association with fast-ice for breeding, they are likely to lose breeding habitat and increase in competition with emperor penguins (Aptenodytes forsteri) (LaRue et al., 2019; Jenouvrier et al., 2020; Trathan et al., 2020). Here, we only considered Ross seals foraging movements and potential energy expenditure in line with climate shifts. Like southern elephant seals, Ross seals are generalists and are free-roaming pelagic foragers, who can travel great distances to find prey, which is why elephant seals are also suggested to likely benefit from climate change (Costa et al., 2010). Unlike elephant seals who breed on sub-Antarctic islands, Ross seals breed on the pack-ice like crabeater and leopard (Hydrurga leptonyx) seals. Despite the energetic gains from swimming shorter distances to foraging locations, they are most likely to suffer breeding habitat loss, which might increasing breeding substrate competition with other pack-ice breeders, similar to Weddell seals and emperor penguins (LaRue et al., 2019). Southern elephant seals, who also prey on squid and fish (Daneri and Carlini, 2002; Van den Hoff et al., 2003) and can dive to depths of over 2000 m, beyond the diving reach of the Ross seal (Blix and Nordøy, 2007; McIntyre et al., 2010), are likely to compete with Ross seals for prey resources.
Conclusion
Overall, these results suggest that climate change will likely alter Ross seals’ foraging behaviour, but it is not clear whether it will be for the better. They will expend less energy while diving due to shoaling mixed-layers, and swimming shorter distances in the winter due to the contracting sea-ice, but in summer post-breeding individuals might swim further distances to reach open water. Their responses will be regionally specific to climatic shifts, rather than uniform. However, toward the end of the century under the “business as usual” models, sea-ice is predicted to decline around the entire Antarctic continent (Gutt et al., 2015) and temperatures of all water masses are predicted to increase (Meijers, 2014).
From a conservation perspective, currently an entire ecosystem assessment of the overall ecosystem effects of climate change in the Southern Ocean is still lacking and has been identified as one of the priority research questions for future research (Kennicutt et al., 2014; Gutt et al., 2015), especially given the planned marine protected area in the Weddell Sea (Teschke et al., 2020). It is therefore unknown how low trophic levels through to top predators will collectively respond to climate shifts and cumulatively affect each other. With expanding fisheries (Brooks, 2013) future conflict and competition between Ross seals and fisheries cannot be ruled out. Even so, it appears that the Ross seal will be the seal species whose foraging habitat is least affected by climate change. We only considered Ross seal foraging habitat and no other aspects of their life-history and their dependence on seasonal pack-ice for breeding substrate makes them vulnerable to climate change induced breeding habitat-loss (see above). Ultimately, several aspects of their life-histories remain unknown, which makes effective conservation planning for this data deficient species exceedingly difficult.
Data Availability Statement
The original contributions presented in the study are publicly available. This data can be found here: https://doi.pangaea.de/10.1594/PANGAEA.929949.
Ethics Statement
The animal study was reviewed and approved by the University of Pretoria Animal Ethics Committee (Number EC082–15). The study was cleared under South African Department of Environmental Affairs Permit 04/2015–2019, pursuant to the provisions of Article 3 of the Protocol on Environmental Protection to the Antarctic Treaty, and Annex II and Annex V (Article 10(2)).
Author Contributions
MB, HB, and MW contributed funding and collected the new data. AB and EN contributed previously collected data. MW analysed the data and wrote the manuscript. LB provided invaluable expertise on the oceanographic interpretations. All authors edited and contributed intellectual input to the final manuscript.
Funding
The National Research Foundation (NRF) South African National Antarctic Programme (SANAP) Grant Number 93088. The Alfred Wegener Institute for Polar and Marine Research through the Helmholtz Association Research Programme Polar Regions and Coasts in the changing Earth System II (PACES II), Topic 1, WP 6. The work on board RV Polarstern refers to Grant-No. AWI_PS111_00. The Norwegian Research Council and the Norwegian Polar Research Institute as part of the NARE 2000/2001 expedition for financial and logistical support. The Department of Environment, Forestry and Fisheries for logistical support within South African National Antarctic Programme (SANAP) and the Department of Science and Technology, through the National Research Foundation (NRF), for funding this project.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Dr. Martin Postma, Dr. Nico Lübcker, and Wiam Haddad are thanked for their assistance in the field. The Officers and Crew of the MV SA Agulhas II and RV Polarstern extended every possible courtesy to us in support of our research objectives. Chief Scientists of the respective voyages, Dr. Thato Mtshali, Dr. Mike Schröder, and Dr. Tommy Ryan-Keogh are thanked for their support and the Department of Environment Affairs’ Co-ordinating Officer (DCO) and Deputy DCO, for facilitation. We acknowledge support by the Open Access Publication Funds of Alfred-Wegener-Institut Helmholtz-Zentrum für Polar- und Meeresforschung.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.659430/full#supplementary-material
References
Alfred-Wegener-Institut Helmholtz-Zentrum für Polar- und Meeresforschung. (2017). Polar research and supply vessel POLARSTERN operated by the alfred-wegener-institute. J. Large Scale Res. Facil. 3:A119. doi: 10.17815/jlsrf-3-163
Agnew, D. J., Hill, S. L., Beddington, J. R., Purchase, L. V., and Wakeford, R. C. (2005). Sustainability and management of Southwest Atlantic squid fisheries. Bull. Mar. Sci. 76, 579–593.
Angermeier, P. L. (1995). Ecological attributes of extinction prone species: loss of freshwater species of fishes of Virginia. Conserv. Biol. 9, 143–158. doi: 10.1046/j.1523-1739.1995.09010143.x
Arcalís-Planas, A., Sveegaard, S., Karlsson, O., Harding, K. C., Wåhlin, A., Harkonen, T., et al. (2015). Limited use of sea ice by the Ross seal (Ommatophoca rossii), in Amundsen Sea, Antarctica, using telemetry and remote sensing data. Polar Biol. 38, 445–461. doi: 10.1007/s00300-014-1602-y
Arrigo, K. R., van Dijken, G. L., and Bushinsky, S. (2008). Primary production in the Southern Ocean, 1997-2006. J. Geophys. Res. Ocean 113:C08004.
Barbraud, C., and Weimerskirch, H. (2001). Emperor penguins and climate change. Nature 411, 183–186. doi: 10.1038/35075554
Barbraud, C., Weimerskirch, H., Guinet, C., and Jouventin, P. (2000). Effect of sea-ice extent on adult survival of an Antarctic top predator: the snow petrel Pagodroma nivea. Oecologia 125, 483–488. doi: 10.1007/s004420000481
Bester, M. N., Wege, M., Oosthuizen, W. C., and Bornemann, H. (2020). Ross seal distribution in the weddell sea: fact and fallacy. Polar Biol. 43, 35–41. doi: 10.1007/s00300-019-02610-4
Bestley, S., Ropert-Coudert, Y., Bengston Nash, S., Brooks, C. M., Cotté, C., Dewar, M., et al. (2020). Marine ecosystem assessment for the southern Ocean: birds and marine mammals in a changing climate. Front. Ecol. Environ. 8:566936. doi: 10.3389/fevo.2020.566936
Bintanja, R., Van Oldenborgh, G. J., Drijfhout, S. S., Wouters, B., and Katsman, C. A. (2013). Important role for ocean warming and increased ice-shelf melt in Antarctic sea-ice expansion. Nat. Geosci. 6, 376–379. doi: 10.1038/ngeo1767
Blix, A. S., and Nordøy, E. S. (2007). Ross seal (Ommatophoca rossii) annual distribution, diving behaviour, breeding and moulting, off Queen Maud Land. Antarctica. Polar Biol. 30, 1449–1458. doi: 10.1007/s00300-007-0306-y
Block, B. A., Jonsen, I. D., Jorgensen, S. J., Winship, A. J., Shaffer, S. A., Bograd, S. J., et al. (2011). Tracking apex marine predator movements in a dynamic ocean. Nature 475, 86–90. doi: 10.1038/nature10082
Bonner, W. N., and Laws, R. M. (1993). “Morphometrics, specimen collection and preservation,” in Antarctic Seals: Research Methods and Techniques, ed. R. M. Laws (Cambridge: Cambridge University Press), 161–171. doi: 10.1017/cbo9780511565281.010
Bracegirdle, T. J., Shuckburgh, E., Sallée, J. B., Wang, Z., Meijers, A. J. S., Bruneau, N., et al. (2013). Assessment of surface winds over the Atlantic, Indian, and Pacific Ocean sectors of the Southern Ocean in CMIP5 models: historical bias, forcing response, and state dependence. J. Geophys. Res. Atmos. 118, 547–562. doi: 10.1002/jgrd.50153
Brault, E. K., Koch, P. L., Costa, D. P., McCarthy, M. D., Hückstädt, L. A., Goetz, K. T., et al. (2019). Trophic position and foraging ecology of Ross, Weddell, and crabeater seals revealed by compound-specific isotope analysis. Mar. Ecol. Prog. Ser. 611, 1–18. doi: 10.3354/meps12856
Brooks, C. M. (2013). Competing values on the antarctic high seas: CCAMLR and the challenge of marine-protected areas. Polar J. 3, 277–300. doi: 10.1080/2154896X.2013.854597
Burns, J. M., Trumble, S. J., Castellini, M. A., and Testa, J. W. (1998). The diet of Weddell seals in McMurdo sound, antarctica as determined from scat collections and stable isotope analysis. Polar Biol. 19, 272–282. doi: 10.1007/s003000050245
Calenge, C. (2006). The package adehabitat for the R software: a tool for the analysis of space and habitat use by animals. Ecol. Modell. 197, 516–519. doi: 10.1016/j.ecolmodel.2006.03.017
Chen, I.-C., Hill, J. K., Ohlemuller, R., Roy, D. B., and Thomas, C. D. (2011). Rapid range shifts of species associated with high levels of Climate Change. Science 333, 1024–1026. doi: 10.1126/science.1206432
Chown, S. L., and Brooks, C. M. (2019). The state and future of antarctic environments in a global context. Annu. Rev. Environ. Resour. 44, 1–30. doi: 10.1146/annurev-environ-101718-033236
Constable, A. J., Melbourne-Thomas, J., Corney, S. P., Arrigo, K. R., Barbraud, C., Barnes, D. K. A., et al. (2014). Climate change and Southern Ocean ecosystems I: how changes in physical habitats directly affect marine biota. Glob. Chang. Biol. 20, 3004–3025. doi: 10.1111/gcb.12623
Convey, P., and Peck, L. S. (2019). Antarctic environmental change and biological responses. Sci. Adv. 11:eaaz0888. doi: 10.1126/sciadv.aaz0888
Costa, D. P., Hückstädt, L. A., Crocker, D. E., McDonald, B. I., Goebel, M. E., and Fedak, M. A. (2010). Approaches to studying climatic change and its role on the habitat selection of antarctic pinnipeds. Integr. Comp. Biol. 50, 1018–1030. doi: 10.1093/icb/icq054
Cristofari, R., Liu, X., Bonadonna, F., Cherel, Y., Pistorius, P. A., Le Maho, Y., et al. (2018). Climate-driven range shifts of the king penguin in a fragmented ecosystem. Nat. Clim. Chang. 8, 245–251. doi: 10.1038/s41558-018-0084-2
Daneri, G. A., and Carlini, A. R. (2002). Fish prey of southern elephant seals, Mirounga leonina, at King George Island. Polar Biol. 25, 739–743. doi: 10.1007/s00300-002-0408-5
Davis, L. B., Hofmann, E. E., Klinck, J. M., Piñones, A., and Dinniman, M. S. (2017). Distributions of krill and Antarctic silverfish and correlations with environmental variables in the western Ross Sea, Antarctica. Mar. Ecol. Prog. Ser. 584, 45–65. doi: 10.3354/meps12347
de la Mare, W. K. (1997). Abrupt mid-twentieth century decline in Antarctic sea-ice extent from whaling records. Nature 389, 57–60. doi: 10.1038/37956
DeWitt, H. (1970). “The character of the midwater fish fauna of the ross sea, antarctica,” in Antarctic Ecology, ed. M. W. Holdgate (London: Academic), 305–314.
DeWitt, H., Heemstra, P., and Gon, O. (1990). “Nototheniidae,” in Fishes of the Southern Ocean, eds O. Gon and P. C. Heemstra (Grahamstown: JLB Smith Institute of Ichthyology), 279–331.
Donnelly, J., Torres, J. J., Sutton, T. T., and Simoniello, C. (2004). Fishes of the eastern Ross Sea. Antarctica. Polar Biol. 27, 637–650. doi: 10.1007/s00300-004-0632-2
Eastman, J. T. (1993). Antarctic Fish Biology: Evolution in a Unique Environment. San Diego, CA: Academic Press.
Ekau, W. (1990). Demersal fish fauna of the Weddell Sea. Antarctica. Antarct. Sci. 2, 129–137. doi: 10.1017/S0954102090000165
Fauchald, P., and Tveraa, T. (2003). Using first-passage time in the analysis of area-restricted search and habitat selection. Ecology 84, 282–288. doi: 10.1890/0012-9658(2003)084[0282:ufptit]2.0.co;2
Forcada, J., Trathan, P. N., and Murphy, E. J. (2008). Life history buffering in Antarctic mammals and birds against changing patterns of climate and environmental variation. Glob. Chang. Biol. 14, 2473–2488. doi: 10.1111/j.1365-2486.2008.01678.x
Freer, J. J., Genner, M. J., Tarling, G. A., Collins, M. A., and Partridge, J. C. (2019). Predicting future distributions of lanternfish, a significant ecological resource within the Southern Ocean. Divers. Distrib. 25, 1259–1272. doi: 10.1111/ddi.12934
Greenwell, B. M. (2017). pdp: an r package for constructing partial dependence plots. R J. 9, 421–436. doi: 10.32614/rj-2017-016
Gutt, J., Bertler, N., Bracegirdle, T. J., Buschmann, A., Comiso, J., Hosie, G., et al. (2015). The Southern Ocean ecosystem under multiple climate change stresses - an integrated circumpolar assessment. Glob. Chang. Biol. 21, 1434–1453. doi: 10.1111/gcb.12794
Hijmans, R. J. (2012). Cross-validation of species distribution models: removing spatial sorting bias and calibration with a null model. Ecology 93, 679–688. doi: 10.1890/11-0826.1
Hijmans, R. J. (2016). raster: Geographic Data Analysis and Modeling. R Package Version 2.5-8. Avaliable at: https://CRAN.R-project.org/package=raster (accessed February 1, 2020).
Hindell, M. A., McMahon, C. R., Bester, M. N., Boehme, L., Costa, D. P., Fedak, M. A., et al. (2016). Circumpolar habitat use in the southern elephant seal: implications for foraging success and population trajectories. Ecosphere 7:e01213.
Hindell, M. A., Reisinger, R. R., Ropert-Coudert, Y., Hückstädt, L. A., Trathan, P. N., Bornemann, H., et al. (2020). Tracking of marine predators to protect Southern Ocean ecosystems. Nature 580, 87–92. doi: 10.1038/s41586-020-2126-y
Hindell, M. A., Sumner, M., Bestley, S., Wotherspoon, S., Harcourt, R. G., Lea, M. A., et al. (2017). Decadal changes in habitat characteristics influence population trajectories of southern elephant seals. Glob. Chang. Biol. 23, 5136–5150. doi: 10.1111/gcb.13776
Hubold, G. (1984). Spatial distribution of Pleuragramma antarcticum (Pisces: Nototheniidae) near the Filchner- and Larsen Ice Shelves (Weddell Sea/Antarctica). Polar Biol. 3, 231–236. doi: 10.1007/bf00292628
Hückstädt, L. A., Burns, J. M., Koch, P. L., McDonald, B. I., Crocker, D. E., and Costa, D. P. (2012). Diet of a specialist in a changing environment: the crabeater seal along the western Antarctic Peninsula. Mar. Ecol. Prog. Ser. 455, 287–301. doi: 10.3354/meps09601
Hückstädt, L. A., Piñones, A., Palacios, D. M., McDonald, B. I., Dinniman, M. S., Hofmann, E. E., et al. (2020). Projected shifts in the foraging habitat of crabeater seals along the Antarctic Peninsula. Nat. Clim. Chang 10, 472–477. doi: 10.1038/s41558-020-0745-9
IOC, IHO, and BODC (2003). “GEBCO digital atlas, centenary edn”, in Published on CD-ROM on Behalf of the Intergovernmental Oceanographic Commission and the International Hydrographic Organisation as Part of the General Bathymetric Chart of the Oceans, Liverpool: British Oceanographic Data Centre.
Jenouvrier, S., Barbraud, C., and Weimerskirch, H. (2006). Sea ice affects the population dynamics of Adelie penguins in Terre Adélie. Polar Biol. 29, 413–423. doi: 10.1007/s00300-005-0073-6
Jenouvrier, S., Holland, M., Iles, D., Labrousse, S., Landrum, L., Garnier, J., et al. (2020). The Paris Agreement objectives will likely halt future declines of emperor penguins. Glob. Chang. Biol. 26, 1170–1184. doi: 10.1111/gcb.14864
Jonsen, I. D. (2016). Joint estimation over multiple individuals improves behavioural state inference from animal movement data. Sci. Rep. 6:20625. doi: 10.1038/srep20625
Jonsen, I. D., Basson, M., Bestley, S., Bravington, M. V., Patterson, T. A., Pedersen, M. W., et al. (2013). State-space models for bio-loggers: a methodological road map. Deep Sea Res. Part II Top. Stud. Oceanogr. 88–89, 34–46. doi: 10.1016/j.dsr2.2012.07.008
Jonsen, I. D., Flemming, J. M., and Myers, R. A. (2005). Robust state-space modeling of animal movement data. Ecology 86, 2874–2880. doi: 10.1890/04-1852
Kellerman, A. K. (1996). “Midwater fish ecology,” in Foundations for Ecological Research West of the Antarctic Peninsula, Antarctic Research Series, Vol. 70, eds R. M. Ross, E. E. Hofman, and L. N. Quetin (Washington DC: American Geophysical Union), 231–156. doi: 10.1029/ar070p0231
Kennicutt, M. C. II, Chown, S. L., and Cassano, J. (2014). Six priorities for Antarctic science. Nature 512, 23–25. doi: 10.1038/512023a
Kock, K.-H., Reid, K., Croxall, J. P., and Nicol, S. (2007). Fisheries in the Southern Ocean: an ecosystem approach. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 362, 2333–2349. doi: 10.1098/rstb.2006.1954
Kuhn, M., Wing, J., Weston, S., Williams, A., Keefer, C., Engelhardt, A., et al. (2019). caret: Classification and Regression Training. R Package Version 6.0-83. Available online at: https://cran.r-project.org/package=caret (accessed February 1, 2020).
La Mesa, M., Piepenburg, D., Pineda-Metz, S. E. A., Riginella, E., and Eastman, J. T. (2019). Spatial distribution and habitat preferences of demersal fish assemblages in the southeastern Weddell Sea (Southern Ocean). Polar Biol. 42, 1025–1040. doi: 10.1007/s00300-019-02495-3
Labrousse, S., Orgeret, F., Solow, A. R., Barbraud, C., Bost, C. A., Sallée, J. B., et al. (2019). First odyssey beneath the sea ice of juvenile emperor penguins in East Antarctica. Mar. Ecol. Prog. Ser. 609, 1–16. doi: 10.3354/meps12831
Labrousse, S., Sallée, J. B., Fraser, A. D., Massom, R. A., Reid, P., Hobbs, W., et al. (2017). Variability in sea ice cover and climate elicit sex specific responses in an Antarctic predator. Sci. Rep. 7:43236. doi: 10.1038/srep43236
Labrousse, S., Vacquié-Garcia, J., Heerah, K., Guinet, C., Sallée, J. B., Authier, M., et al. (2015). Winter use of sea ice and ocean water mass habitat by southern elephant seals: the length and breadth of the mystery. Prog. Oceanogr. 137, 52–68. doi: 10.1016/j.pocean.2015.05.023
Lake, S., Burton, H., and van den Hoff, J. (2003). Regional, temporal and fine-scale spatial variation in Weddell seal diet at four coastal locations in east Antarctica. Mar. Ecol. Prog. Ser. 254, 293–305. doi: 10.3354/meps254293
LaRue, M. A., Salas, L., Nur, N., Ainley, D. G., Stammerjohn, S. E., Barrington, L., et al. (2019). Physical and ecological factors explain the distribution of Ross Sea Weddell seals during the breeding season. Mar. Ecol. Prog. Ser. 612, 193–208. doi: 10.3354/meps12877
McIntyre, T. (2014). Trends in tagging of marine mammals: a review of marine mammal biologging studies. African J. Mar. Sci. 36, 409–422. doi: 10.2989/1814232X.2014.976655
McIntyre, T., de Bruyn, P. J. N., Ansorge, I. J., Bester, M. N., Bornemann, H., Plötz, J., et al. (2010). A lifetime at depth: vertical distribution of southern elephant seals in the water column. Polar Biol. 33, 1037–1048. doi: 10.1007/s00300-010-0782-3
McMahon, C. R., and Burton, H. R. (2005). Climate change and seal survival: evidence for environmentally mediated changes in elephant seal. Mirounga leonina, pup survival. Proc. R. Soc. Lond. B 272, 923–928. doi: 10.1098/rspb.2004.3038
Meehl, G. A., Arblaster, J. M., Bitz, C. M., Chung, C. T. Y., and Teng, H. (2016). Antarctic sea-ice expansion between 2000 and 2014 driven by tropical Pacific decadal climate variability. Nat. Geosci. 9, 590–595. doi: 10.1038/ngeo2751
Meehl, G. A., Stocker, T. F., Collins, W. D., Friedlingstein, P., Gaye, T., Gregory, J. M., et al. (2007). “Global climate projections,” in IPCC, 2007: Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, eds S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K. B. Averyt, et al. (Cambridge: Cambridge University Press), 747–846.
Meijers, A. J. S. (2014). The Southern ocean in the coupled model intercomparison project phase 5. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 372:20130296. doi: 10.1098/rsta.2013.0296
Meijers, A. J. S., Meredith, M. P., Murphy, E. J., Chambers, D. P., Belchier, M., and Young, E. F. (2019). The role of ocean dynamics in king penguin range estimation. Nat. Clim. Chang. 9, 120–121. doi: 10.1038/s41558-018-0388-2
Meredith, M., Sommerkorn, M., Cassotta, S., Derksen, C., Ekaykin, A., Hollowed, A., et al. (2019). “International panel of climate change scientific report: ocean and cryosphere,” in IPCC Spec. Rep. Ocean Cryosph. a Chang. Clim. - Summ. Policymakers, Vol. 173. Available online at: https://report.ipcc.ch/srocc/pdf/SROCC_FinalDraft_Chapter3.pdf (accessed February 1, 2020).
Mintenbeck, K., and Torres, J. J. (2017). “Impact of climate change on the antarctic silverfish and its consequences for the antarctic ecosystem,” in The Antarctic Silverfish: A Keystone Species in a Changing Ecosystem, Advances in Polar Ecology 3, eds M. Vacchi and L. Ghigliotti (Cham: Springer), 253–286. doi: 10.1007/978-3-319-55893-6_12
Murphy, E. J., Hofmann, E. E., Watkins, J. L., Johnston, N. M., Piñones, A., Ballerini, T., et al. (2013). Comparison of the structure and function of Southern Ocean regional ecosystems: the Antarctic Peninsula and South Georgia. J. Mar. Syst. 109–110, 22–42. doi: 10.1016/j.jmarsys.2012.03.011
Murphy, E. J., Trathan, P. N., Watkins, J. L., Reid, K., Meredith, M. P., Forcada, J., et al. (2007). Climatically driven fluctuations in Southern Ocean ecosystems. Proc. R. Soc. B 274, 3057–3067. doi: 10.1098/rspb.2007.1180
Nakazawa, M. (2018). fmsb: Functions for Medical Statistics Book With Some Demographic Data. R Packag. Version 0.6.3. Available online at: https://cran.r-project.org/package=fmsb.
Nicol, S. (2006). Krill, currents, and sea ice: Euphausia superba and its changing environment. Bioscience 56, 111–120. doi: 10.1641/0006-3568(2006)056[0111:kcasie]2.0.co;2
Nicol, S., Foster, J., and Kawaguchi, S. (2012). The fishery for Antarctic krill - recent developments. Fish Fish. 13, 30–40. doi: 10.1111/j.1467-2979.2011.00406.x
Orsi, H., Whitworth, T. III, and Nowlin, W. D. Jr. (1995). On the meridional extent and fronts of the antarctic circumpolar current. Deep Sea Res. 1 Oceanogr. Res. Pap. 42, 641–673. doi: 10.1016/0967-0637(95)00021-W
Patterson, T. A., Thomas, L., Wilcox, C., Ovaskainen, O., and Matthiopoulos, J. (2008). State-space models of individual animal movement. Trends Ecol. Evol. 23, 87–94. doi: 10.1016/j.tree.2007.10.009
Péron, C., Weimerskirch, H., and Bost, C.-A. (2012). Projected poleward shift of king penguins’ (Aptenodytes patagonicus) foraging range at the Crozet Islands, southern Indian Ocean. Proc. R. Soc. B Biol. Sci. 279, 2515–2523. doi: 10.1098/rspb.2011.2705
Pinsky, M. L., Worm, B., Fogarty, M. J., Sarmiento, J. L., and Levin, S. A. (2013). Marine taxa track local climate velocities. Science 341, 1239–1243. doi: 10.1126/science.1239352
Plummer, M. (2016). rjags: Bayesian Graphical Models using MCMC. R package version 4-6. Available online at: https://cran.r-project.org/package=rjags (accessed February 1, 2020).
R Core Team (2020). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rau, G. H., Ainley, D. G., Bengtson, J. L., Torres, J. J., and Hopkins, T. L. (1992). 15N/14N and 13C/12C in Weddell Sea birds, seals, and fish: implications for diet and trophic structure. Mar. Ecol. Prog. Ser. 84, 1–8. doi: 10.3354/meps084001
Raymond, B., Lea, M. A., Patterson, T. A., Andrews-Goff, V., Sharples, R., Charrassin, J., et al. (2015). Important marine habitat off east Antarctica revealed by two decades of multi-species predator tracking. Ecography 38, 121–129. doi: 10.1111/ecog.01021
Reid, K., and Arnould, J. P. Y. (1996). The diet of Antarctic fur seals Arctocephalus gazella during the breeding season at South Georgia. Polar Biol. 16, 105–114. doi: 10.1007/s003000050035
Reisinger, R. R., Raymond, B., Hindell, M. A., Bester, M. N., Crawford, R. J. M., Davies, D., et al. (2018). Habitat modelling of tracking data from multiple marine predators identifies important areas in the Southern Indian Ocean. Divers. Distrib. 24, 535–550. doi: 10.1111/ddi.12702
Ridgeway, G. (2015). gbm: Generalized Boosted Regression Models. R Package Version 2.1.1. Available online at: https://cran.r-project.org/package=gbm (accessed February 1, 2020).
Riekkola, L., Andrews-Goff, V., Friedlaender, A., Constantine, R., and Zerbini, A. N. (2019). Environmental drivers of humpback whale foraging behavior in the remote Southern Ocean. J. Exp. Mar. Bio. Ecol. 517, 1–12. doi: 10.1016/j.jembe.2019.05.008
Rodhouse, P. G. K. (2013). Role of squid in the Southern Ocean pelagic ecosystem and the possible consequences of climate change. Deep. Res. Part II Top. Stud. Oceanogr. 95, 129–138. doi: 10.1016/j.dsr2.2012.07.001
Rodríguez, A., Arcos, J. M., Bretagnolle, V., Dias, M. P., Holmes, N. D., Louzao, M., et al. (2019). Future directions in conservation research on petrels and shearwaters. Front. Mar. Sci. 6:94. doi: 10.3389/fmars.2019.00094
Rogers, A. D., Frinault, B. A. V. A. V., Barnes, D. K. A. K. A., Bindoff, N. L. L., Downie, R., Ducklow, H. W. W., et al. (2020). Antarctic futures: an assessment of climate-driven changes in ecosystem structure, function, and service provisioning in the Southern Ocean. Ann. Rev. Mar. Sci. 12, 87–120. doi: 10.1146/annurev-marine-010419-011028
Ropert-Coudert, Y., Chiaradia, A., Ainley, D. G., Barbosa, A., Boersma, P. D., Brasso, R., et al. (2019). Happy feet in a hostile world? The future of penguins depends on proactive management of current and expected threats. Front. Mar. Sci. 6:248. doi: 10.3389/fmars.2019.00248
Sallée, J. B., Shuckburgh, E., Bruneau, N., Meijers, A. J. S., Bracegirdle, T. J., and Wang, Z. (2013). Assessment of Southern Ocean mixed-layer depths in CMIP5 models: historical bias and forcing response. J. Geophys. Res. Ocean 118, 1845–1862. doi: 10.1002/jgrc.20157
Sallée, J. B., Speer, K. G., and Rintoul, S. R. (2010). Zonally asymmetric response of the Southern Ocean mixed-layer depth to the Southern Annular Mode. Nat. Geosci. 3, 273–279. doi: 10.1038/ngeo812
Schloss, C. A., Nunez, T. A., and Lawler, J. J. (2012). Dispersal will limit ability of mammals to track climate change in the Western Hemisphere. Proc. Natl. Acad. Sci. U.S.A. 109, 8606–8611. doi: 10.1073/pnas.1116791109
Silverman, B. W. (1986). Density Estimation for Statistics and Data Analysis. London: Chapman and Hall.
Siniff, D. B., Garrott, R. A., Rotella, J. J., Fraser, W. R., and Ainley, D. G. (2008). Opinion: projecting the effects of environmental change on Antarctic seals. Antarct. Sci. 20, 425–435. doi: 10.1017/S0954102008001351
Skinner, J. D., and Klages, N. T. W. (1994). On some aspects of the biology of the Ross seal Ommatophoca rossii from King Haakon VII Sea. Antarctica. Polar Biol. 14, 467–472.
Southwell, C. J. (2005). Diving behaviour of two ross seals off East Antarctica. Wildl. Res. 32, 63–65. doi: 10.1071/wr03090
Southwell, C. J., Bengtson, J. L., Bester, M. N., Blix, A. S., Bornemann, H., Boveng, P. L., et al. (2012). A review of data on abundance, trends in abundance, habitat use and diet of ice-breeding seals in the Southern Ocean. CCAMLR Sci. 19, 1–26.
Sumner, M. D. (2015). raadtools: TOOLS for Synoptic Environmental Spatial Data. Available online at: https://github.com/AustralianAntarcticDivision/raadtools (accessed February 1, 2020).
Terraube, J., Arroyo, B., Madders, M., and Mougeot, F. (2011). Diet specialisation and foraging efficiency under fluctuating vole abundance: a comparison between generalist and specialist avian predators. Oikos 120, 234–244. doi: 10.1111/j.1600-0706.2010.18554.x
Teschke, K., Pehlke, H., Siegel, V., Bornemann, H., Knust, R., and Brey, T. (2020). An integrated data compilation for the development of a marine protected area in the Weddell Sea Earth Syst. Sci. Data Discuss 12, 1003–1023. doi: 10.5194/essd-2019-86
Trathan, P. N., Wienecke, B., Barbraud, C., Jenouvrier, S., Kooyman, G. L., Le Bohec, C., et al. (2020). The emperor penguin - Vulnerable to projected rates of warming and sea ice loss. Biol. Conserv. 241:108216. doi: 10.1016/j.biocon.2019.108216
Trunov, I. A. (2001). Occurrence of Pleuragramma antarcticum (Nototheniidae) off South Georgia Island and the South Sandwich Islands (Antarctica). J. Ichthyol. 41, 549–550.
Turner, J., Barrand, N. E., Bracegirdle, T. J., Convey, P., Hodgson, D. A., Jarvis, M., et al. (2014). Antarctic climate change and the environment: an update. Polar Rec. 50, 237–259.
Turner, J., Guarino, M. V., Arnatt, J., Jena, B., Marshall, G. J., Phillips, T., et al. (2020). Recent Decrease of Summer Sea Ice in the Weddell Sea, Antarctica. Geophys. Res. Lett. 47:e2020GL087127. doi: 10.1029/2020GL087127
Tynan, C. T. (1998). Ecological importance of the Southern Boundary of the Antarctic Circumpolar Current. Nature 392, 708–710. doi: 10.1038/33675
Van den Hoff, J., Burton, H., and Davies, R. (2003). Diet of male southern elephant seals (Mirounga leonina L.) hauled out at Vincennes Bay, East Antarctica. Polar Biol. 26, 27–31. doi: 10.1007/s00300-002-0447-y
Van den Hoff, J., McMahon, C. R., Simpkins, G. R., Hindell, M. A., Alderman, R., and Burton, H. R. (2014). Bottom-up regulation of a pole-ward migratory predator population. Proc. R. Soc. B Biol. Sci. 281:20132842. doi: 10.1098/rspb.2013.2842
Wege, M., de Bruyn, P. J. N., Hindell, M. A., Lea, M. A., and Bester, M. N. (2019). Preferred, small-scale foraging areas of two Southern Ocean fur seal species are not determined by habitat characteristics. BMC Ecol. 19:36. doi: 10.1186/s12898-019-0252-x
Wege, M., Salas, L., and LaRue, M. A. (2020). Citizen science and habitat modelling facilitates conservation planning for crabeater seals in the Weddell Sea. Divers. Distrib. 26, 1291–1304. doi: 10.1111/ddi.13120
White, M. G., and Piatkowski, U. (1993). Abundance, horizontal and vertical distribution of fish in eastern Weddell Sea micronekton. Polar Biol. 13, 41–53.
White, W. B., and Peterson, R. G. (1996). An Antarctic circumpolar wave in surface pressure, wind, temperature and sea-ice extent. Nature 380, 699–702. doi: 10.1038/380699a0
Worton, B. J. (1989). Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70, 164–168. doi: 10.2307/1938423
Würsig, B., Thewissen, J. G. M., and Kovacs, K. M. (2018). Encyclopedia of Marine Mammals, 3rd Edit Edn. San Diego, CA: Elsevier Press.
Xavier, J. C., Cherel, Y., Allcock, L., Rosa, R., Sabirov, R. M., Blicher, M. E., et al. (2018). A review on the biodiversity, distribution and trophic role of cephalopods in the Arctic and Antarctic marine ecosystems under a changing ocean. Mar. Biol. 165, 1–26. doi: 10.1007/s00227-018-3352-9
Young, I. R., and Ribal, A. (2019). Multiplatform evaluation of global trends in wind speed and wave height. Science 364, 548–552. doi: 10.1126/science.aav9527
Young, I. R., Zieger, S., and Babanin, A. V. (2011). Global trends in wind speed and wave height. Science 332, 451–455. doi: 10.1126/science.1197219
Younger, J. L., van den Hoff, J., Wienecke, B., Hindell, M. A., and Miller, K. J. (2016). Contrasting responses to a climate regime change by sympatric, ice-dependent predators. BMC Evol. Biol. 16:61. doi: 10.1186/s12862-016-0630-3
Zhang, J. (2007). Increasing Antarctic sea ice under warming atmospheric and oceanic conditions. J. Clim. 20, 2515–2529. doi: 10.1175/JCLI4136.1
Keywords: Antarctica, biologging, boosted regression trees models, climate change, habitat utilisation, species distribution model, remote sensing, Weddell Sea
Citation: Wege M, Bornemann H, Blix AS, NordØy ES, Biddle L and Bester MN (2021) Distribution and Habitat Suitability of Ross Seals in a Warming Ocean. Front. Mar. Sci. 8:659430. doi: 10.3389/fmars.2021.659430
Received: 27 January 2021; Accepted: 08 April 2021;
Published: 13 May 2021.
Edited by:
Alastair Martin Mitri Baylis, South Atlantic Environmental Research Institute, Falkland IslandsReviewed by:
Daniel Paul Costa, University of California, Santa Cruz, United StatesCarey Kuhn, Alaska Fisheries Science Center (NOAA), United States
Copyright © 2021 Wege, Bornemann, Blix, Nordøy, Biddle and Bester. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mia Wege, mia.wege@gmail.com; orcid.org/0000-0002-9022-3069
 Mia Wege
Mia Wege Horst Bornemann1
Horst Bornemann1  Arnoldus Schytte Blix
Arnoldus Schytte Blix