Seasonal Photophysiological Performance of Adult Western Baltic Fucus vesiculosus (Phaeophyceae) Under Ocean Warming and Acidification
- 1Institute of Biological Sciences, Applied Ecology and Phycology, University of Rostock, Rostock, Germany
- 2Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, Germany
Shallow coastal marine ecosystems are exposed to intensive warming events in the last decade, threatening keystone macroalgal species such as the bladder wrack (Fucus vesiculosus, Phaeophyceae) in the Baltic Sea. Herein, we experimentally tested in four consecutive benthic mesocosm experiments, if the single and combined impact of elevated seawater temperature (Δ + 5°C) and pCO2 (1100 ppm) under natural irradiance conditions seasonally affected the photophysiological performance (i.e., oxygen production, in vivo chlorophyll a fluorescence, energy dissipation pathways and chlorophyll concentration) of Baltic Sea Fucus. Photosynthesis was highest in spring/early summer when water temperature and solar irradiance increases naturally, and was lowest in winter (December to January/February). Temperature had a stronger effect than pCO2 on photosynthetic performance of Fucus in all seasons. In contrast to the expectation that warmer winter conditions might be beneficial, elevated temperature conditions and sub-optimal low winter light conditions decreased photophysiological performance of Fucus. In summer, western Baltic Sea Fucus already lives close to its upper thermal tolerance limit and future warming of the Baltic Sea during summer may probably become deleterious for this species. However, our results indicate that over most of the year a combination of future ocean warming and increased pCO2 will have slightly positive effects for Fucus photophysiological performance.
Introduction
Recent hot weather conditions in Central Europe, such as the weeks of record-breaking air temperatures during the summer of 2003 and 2018 (Schär et al., 2004; Imbery et al., 2018), pose a significant threat to macrophyte keystone species and their associated organisms, especially in shallow waters (Roth et al., 2010; Winters et al., 2011; Gouvêa et al., 2017). In the Baltic Sea on hard bottoms the bladder wrack Fucus vesiculosus L. (hereafter Fucus) is the most common canopy-forming and biomass dominating brown macroalga (Kautsky et al., 1992; Torn et al., 2006; Rönnbäck et al., 2007). The complex habitat formed by Fucus provides various ecosystem goods and services such as carbon storage and preservation of biodiversity in the coastal zone (Bokn et al., 2002; Rönnbäck et al., 2007; Schagerström et al., 2014).
Photosynthesis, growth and hence biomass production of algae are limited by biotic and abiotic factors if they exceed thresholds for optimal organismal function. Especially, shallow subtidal habitats of the temperate regions like in the Baltic Sea are a highly variable environment where organisms are regularly exposed to strong fluctuations in temperature, pH, irradiance, salinity and nutrient availability. The Baltic Fucus tolerates highly variable environmental conditions, especially annual and seasonal fluctuations in pH (7.4–8.5) and temperature (<0–20/25°C) (Wahl et al., 2010; Wahl et al., 2011). For instance, the Fucus system seems to react very sensitive to environmental change, which is suggested by the sharp decline of Baltic Fucus populations over the past decades (Wahl et al., 2011; Wahl et al., 2015b; Takolander et al., 2017). The decline of perennial Fucus populations is associated with the shift to a predominance of annual filamentous algae (Torn et al., 2006).
In temperate regions, high environmental stress occurs, for example, during summer on clear, calm days (Helmuth et al., 2002) as these conditions lead to harsh thermal stress, particularly in shallow coastal waters (Davison and Pearson, 1996; Collén and Davison, 2001). The ongoing warming trend and its intensification of seasonal fluctuations is stated to be a severe further challenge for Fucus (Jueterbock et al., 2013). Within the natural distribution range of Fucus 20°C is considered as the highest water temperature occurring over longer than weekly periods (Lüning, 1990). In the shallow coastal zone of the Baltic Sea the summer sea surface temperature is already close to this limit and is predicted to rise by 3–6°C till the end of the century (Elken et al., 2015). In addition to decadal-scale, gradual warming trends, short-term extreme warming events (e.g., “marine heat-waves” sensu Hobday et al., 2016) have increased in the Baltic Sea (HELCOM, 2013). In the western Baltic Sea shallow water temperatures may reach >30°C for short periods during summer (Wahl et al., 2010). Thus, Fucus communities populating shallow coastal areas may be one of the first to experience the effects of global warming and heat-waves.
As seaweeds have the capacity to optimize photosynthesis over a wide range of temperatures, they are able to physiologically acclimatize to temperature changes within hours and days (Davison et al., 1991; Kübler and Davison, 1995; Eggert et al., 2006). The potential for seasonal thermal acclimation of photosynthesis has been described for different seaweed species (Mathieson and Norall, 1975; Davison et al., 1991; Pfetzing et al., 2000). Whereas seasonal changes in temperature are usually anticipated and compensated by acclimation processes and/or physiological plasticity of individual organisms (Davison and Pearson, 1996; Kingsolver and Huey, 1998), the sporadic occurrence of extreme temperature conditions can seriously increase mortality (Roth et al., 2010; Winters et al., 2011). Additionally, physiological repair mechanisms may be weakened or the accumulation of harmful intermediates such as reactive oxygen species (ROS) may be induced by stressful, but sub-lethal conditions (Weidner and Ziemens, 1975; Davison and Pearson, 1996).
Photosynthetic activity is a sensitive indicator for thermal tolerance in seaweeds, because the photosynthetic apparatus is particularly sensitive to high temperature stress (Berry and Björkman, 1980; Dutta et al., 2009). The oxygen-evolving complex photosystem II (PSII) can be strongly affected by temperature, as high temperatures negatively influence the stability of PSII (e.g., damage and rapid turnover of the D1 protein) and by impairment of recovery processes due to ROS formation (Berry and Björkman, 1980; Allakhverdiev et al., 2008). The extent of PS II damage due to, for example, heat stress depends on the balance between damage and repair processes during such conditions. This balance provides the basis for acclimation and photosynthetic recovery processes (Adir et al., 2003; Mohanty et al., 2007; Murata et al., 2007). In Fucus, optimal temperature for photosynthesis has a broad range varying between 5 and 25°C in eastern Baltic Sea, North Sea or Atlantic populations, respectively (Niemeck and Mathieson, 1978; Russell, 1987; Nygård and Dring, 2008). For Fucus from the western Baltic Sea optimal temperature for highest photosynthetic performance under controlled laboratory experiments ranged between 20 and 26°C (Graiff et al., 2015b). However, Fucus was unable to photophysiologically acclimate to temperatures higher than 26°C over 3 weeks of incubation (Graiff et al., 2015b).
Future CO2-induced acidification of the surface waters of the Baltic Sea is difficult to predict (Müller et al., 2016). However, oceanographic models for the Baltic Proper project a long-term decrease in surface pH (Omstedt et al., 2012). It was demonstrated that increased pCO2 conditions and/or dissolved inorganic carbon (DIC) enhanced photosynthetic rates of Fucus species and other non-calcifying seaweeds (Gordillo et al., 2001; Nygård and Dring, 2008; Wu et al., 2008; Saderne, 2012; Koch et al., 2013; Olischläger et al., 2013, 2017). The assumed explanation for a positive CO2 effect on non-calcifying seaweeds is the down-regulation of energy-consuming carbon-concentrating mechanisms (CCMs) by facilitating their access to carbon (in the form of CO2) under these conditions (Beardall and Giordano, 2002; Wu et al., 2008). The outcome of ocean acidification effects on photosynthesis seems to be dependent on the interaction with other environmental factors, such as sub-optimal temperatures (Fu et al., 2007; Feng et al., 2008; Olischläger and Wiencke, 2013; Sarker et al., 2013), limited nutrient availability (Gordillo et al., 2001; Xu et al., 2010) and light conditions (Rokitta and Rost, 2012; Sarker et al., 2013).
Due to anthropogenic global change, ocean warming and acidification may singly or interactively affect photophysiological performance of Fucus during the different seasons. Fucus was exposed to single and combined treatments of elevated seawater temperature (Δ + 5°C) and pCO2 (1100 ppm) in all four seasons as predicted for shallow coastal Baltic habitats for the year 2100 (Elken et al., 2015; Schneider et al., 2015; Müller et al., 2016). These scenarios, including natural fluctuations forced by diurnal and seasonal changes, atmospheric and hydrographic conditions, were simulated using benthic mesocosms [Kiel Outdoor Benthocosms (KOBs), Wahl et al., 2015a]. We hypothesized that warming and increased pCO2 (singly and combined) would have positive effects on the photophysiology of Fucus (i.e., oxygen production, in vivo chlorophyll a fluorescence and chlorophyll concentration) during winter, under low irradiances.
Materials and Methods
Collection of Algae and Sampling Site
Fucus vesiculosus L. specimens were sampled, in each season (spring: April 2, 2013; summer: July 2, 2013; autumn: 8 October; winter: January 14, 2014) from a depth of 0.2–1 m in the Kiel Fjord (Bülk), western Baltic Sea, Germany (54°27′N; 10°11,5′E). In the atidal Kiel Fjord, F. vesiculosus forms almost monospecific dense stands on stones between 0.3 and 3 m depth. Fucus individuals still attached to stones were randomly collected from the field resulting in a variety of different sizes, growth forms and maturity levels. All collected individuals were taller than 15 cm and considered as “adults.” After sampling, the algae were directly placed in water-filled buckets and brought to the experimental site at the GEOMAR Helmholtz Centre for Ocean Research. For later identification all specimens were labeled.
Experimental Design and Treatments
The mesocosm experiments were performed in the KOB infrastructure at GEOMAR in the inner Kiel Fjord (54°20′N; 10°09′E). In Wahl et al. (2015a) the technical details of this facility, the experimental setup and monitoring, are described in detail. The KOB system consists of six flow-through tanks (ca. 1800 L per day) divided into 12 completely autonomous experimental units with a water volume of 1470 L each and covered with gas-tight, translucent foils. The KOB facility is exposed to ambient light (irradiance and photoperiod) conditions year-round. Photosynthetically active radiation (PAR) data were obtained from the German Weather Service (DWD, for details see Graiff et al., 2020). Water conditions were kept as close as possible to the real ambient conditions of the Kiel Fjord, including their fluctuations. For this the experimental units were supplied with non-filtered seawater pumped from 1 m depth near the KOB facility and inside each tank a pump circulated the water continuously. Twenty individuals of Fucus growing on their natural substrata were established in each experimental unit. The rock substratum of each Fucus was placed in a small plastic dish (Ø = 14 cm, h = 4 cm) attached to a grating at a water depth of 40 cm in the tank.
The single and combined effects of ocean warming and acidification on Fucus photophysiology were tested by crossing two temperatures (in situ Kiel Fjord temperature vs. elevated temperature by +5°C) and two CO2 levels (ca. 400 ppm vs. ca. 1100 ppm in the headspace above the mesocosm) in four seasonal experiments. Thus, four treatments were surveyed: (1) the ambient in situ Kiel Fjord temperature and CO2 conditions (Ambient), (2) ambient temperature with elevated pCO2 (+CO2), (3) elevated temperature with ambient pCO2 (+Temp), and (4) elevated temperature with elevated pCO2 (+Temp +CO2). Each treatment was replicated in three independent experimental units. The treatments were superimposed on the natural fluctuations of all environmental variables. Elevated levels of both factors were chosen according to climate change forecasts for shallow coastal Baltic habitats for the year 2100 (Elken et al., 2015; Schneider et al., 2015; Müller et al., 2016). Before the experiments, the Fucus individuals were acclimated to the KOB conditions for 2 days under ambient conditions. The temperature in the warming treatments was elevated by 2°C on the second day and by 3°C on the third day to reach a 5°C higher temperature compared to the natural Kiel Fjord temperature on the fourth day. Under computer control, CO2 was injected from the second day onward into the headspace above the elevated pCO2 treatment tanks in order to maintain the headspace pCO2 close to 1100 ppm.
The investigation of seasonal variations of single and combined effects of simulated ocean warming and acidification on Fucus photophysiological performance was restricted by the need to maintain the technical equipment and sensors every 4 months. Thus, the study was divided into four consecutive experiments rather than 1 year-round study. The experiments ran from April 4, to June 19, 2013 (spring), from July 4, to September 17, 2013 (summer), from October 10, to December 18, 2013 (autumn), and from January 16, to April 1, 2014 (winter), each lasting for at least 10 weeks.
Manipulation of Temperature and pCO2
In each experimental unit the water temperature was constantly logged by sensors and automatically adjusted by heat exchangers and internal heating elements to a temperature rise of 5°C. The Kiel Fjord water temperature is subjected to the typical temperate seasonal pattern. In spring and early summer (April to June) mean water temperature increased and reached maximum values (24.1–24.8°C) in July to August. During autumn and early winter (September to December) the water temperature declined and reached a minimum of 4.2°C in January. Subsequently, the temperature increased from February to March again (Supplementary Table 1).
The manipulation of the seawater pCO2 was achieved by computer-controlled injection of pure CO2 into the headspace atmosphere above each experimental unit and was maintained at approximately 1100 ppm CO2. Inside the tanks the pH was continuously monitored by sensors (gel-electrolyte filled glass electrode, GHL Advanced Technology, Kaiserslautern, Germany) and was additionally measured daily using hand-held pH-meter to permit post hoc correction of sensor drift (Seven Multi + InLab Expert Pro, Mettler Toledo GmbH, Giessen, Germany). Salinity was continuously logged at the institute pier (<100 m distant) by GEOMAR, and dissolved inorganic nitrogen (DIN) data were obtained from the State Agency for Agriculture, Environment and Rural Areas Schleswig-Holstein (LLUR). Variations in total alkalinity (TA) and DIC were measured regularly and are presented in detail in Wahl et al. (2015a). The analyses followed the procedures recommended by Dickson et al. (2007). The water pCO2 in the four different treatments was calculated from regular measurements of TA, DIC, pH, salinity, and temperature using the CO2SYS Excel Macro spreadsheet developed by Pierrot et al. (2006). Water motion was regularly induced by a wave generator and thereby promoted diffusion of CO2 from the headspace into the water column.
In situ pH in the Kiel Fjord was measured close to the inlet of the flow-through. Kiel Fjord surface water pH was high (8.5) in spring (April to June) and low (7.7) in autumn (October to November). Seawater carbonate chemistry parameters (pH, pCO2, TA, and DIC) differed among the four different treatments and seasons (Supplementary Table 2). The overall mean effect of head-space enrichment with CO2 from ambient (380–450 ppm) to 1050–1100 ppm decreased the tank water pH by 0.18 ± 0.08 pH units (Supplementary Figure 1). Mean difference between the ambient and increased CO2 treatments was 340 ppm CO2 at ambient temperature and 460 ppm CO2 at elevated temperature (M. Böttcher and V. Winde, pers. comm.). Nevertheless, the seasonal amplitude of pH and pCO2 in the water of the KOBs was large and sometimes exceeded the treatment size. Additionally, the diurnal metabolic activity of Fucus altered the in situ seasonal fluctuations. All abiotic variables assessed in the KOB experiments are available at the PANGAEA data platform1.
Photophysiological Responses
Photosynthetic performance was measured with two different methods, one based on in vivo chlorophyll a fluorescence measurements of photosystem II (PSII), the other one based on oxygen production. For each experiment and treatment, three F. vesiculosus specimens 15–25 cm long with 91 ± 30 total apices and apparently equal vigor were chosen, each individually growing on a stone (10–15 cm in diameter) from a single holdfast. These individuals were visually free of macroscopic epiphytes and epizoobenthos.
For the in vivo chlorophyll fluorescence measurements a portable pulse-amplitude-modulated fluorometer (PAM 2000, Walz, Effeltrich, Germany) was used. Before measurement, the tips were carefully cleaned from visible epiphytes with seawater. The vegetative tip of the longest thalli per Fucus individual was measured at the beginning (spring: April 4, 2013; summer: July 4, 2013; autumn: October 10, 2013; winter: January 16, 2014) and constituted the “initial state.” Every 2–4 weeks during the experiments (spring: 2 and 30 May 2013; summer: July 18, and August 15, 2013; autumn: 7 and 21 November 2013; winter: February 13 and March 13, 2014) and at the end of each experiment (spring: June 19, 2013; summer: September 17, 2013; autumn: December 18, 2013; winter: April 1, 2014) this assessment was repeated at in situ KOB conditions.
The determination of the potential maximum quantum yield (Fv/Fm) was performed according to Hanelt (1998). The protocol was modified as follows: after 5 min of dark adaptation and a 5-s far red light pulse, the minimal fluorescence F0 was recorded with a pulsed measuring light (650 nm, 0.3 μmol photons m–2 s–1), followed by short pulses of completely saturating white light (0.4–0.8 s, 1,000–5,000 μmol photons m–2 s–1) to record Fm (Fv = Fm–F0). The maximum quantum yield indicates the possible photosynthetic efficiency or stress level of the seaweed.
To determine changes in the photosynthetic performance, photosynthesis vs. irradiance curves (PI-curve) were calculated. Algae were exposed to eleven increasing photon flux densities (PFD) of actinic red light (6, 15, 27, 36, 53, 73, 108, 154, 227, 332, and 490 μmol photons m–2 s–1, LED 650 nm), each for 2 min. The distance between the fiber optic and thallus surface was always kept constant at 1 mm. After each light step, a saturating white light pulse was applied to define the effective PSII quantum efficiency (ΔF/Fm′) followed by a far-red pulse for 3 s to re-oxidize the electron transport chain (Ihnken et al., 2010). Accordingly, the relative electron transport rate (rETR) through PSII was calculated by multiplying ΔF/Fm′ with the appropriate PFD values (rETR = ΔF/Fm′ × PFD) (Genty et al., 1989; Schreiber et al., 1995). PI-curves with rETR as a function of PFD were fitted after Webb et al. (1974) due to the absence of photoinhibition. From each particular curve, the maximum relative electron transport rates (rETRmax) as well as the initial slope alpha (α) were calculated. The light saturation coefficient of the curves (Ik) was calculated as a quotient of rETRmax and α (Henley, 1993). In addition, the non-photochemical quenching (NPQ) for each PFD was recorded. For a quantitative description of NPQ as a function of PFD, NPQ vs. PFD curves were fitted after Serôdio and Lavaud (2011). Depending on this model NPQmax as the maximum NPQ value of the NPQ vs. PFD curve was calculated. NPQmax was used as estimator of the photoprotection capacity.
Similarly, complementary energy dissipation pathways of Fucus individuals were investigated by using PAM measurements. For this reason, the regulated non-photochemical quenching of light energy (Y(NPQ) = F/Fm′-F/Fm) was calculated for each Fucus individual. Y(NPQ) is used as a measure of the overall photoprotective capacity of the photosynthetic apparatus (e.g., Lavaud et al., 2007; Goss and Lepetit, 2015). In addition, non-regulated non-photochemical quenching (Y(NO) = F/Fm) was determined. This energy dissipation pathway reveals by increasing Y(NO) values changes at the PSII antenna complex, PSII damage or impacts on the thylakoid. The third energy dissipation pathway is via photochemistry and was calculated as the effective quantum yield Y(PSII) = (Fm′-F)/Fm′. The Y(PSII) was measured at ambient light. Importantly, the total energy is conserved whereby Y(PSII) + Y (NPQ) + Y(NO) = 1. Photoprotection mechanisms are applying if Y(NPQ) increases relative to Y(NO) and Y(PSII). Conversely, if Y(NO) increases at the expense of Y(PSII) and Y(NPQ), this suggests photoinhibition or permanent destruction of the photosynthetic apparatus (Klughammer and Schreiber, 2008). Therefore, it was hypothesized that Fucus individuals show greater Y(NPQ) and Y(NO) at the cost of photochemistry (Y(PSII)) under elevated temperatures, especially in the summer months, as a means of dissipating excessive energy.
As the in vivo chlorophyll a fluorescence measurements by the PAM do not allow quantifying respiration, PI-curves were also measured using oxygen optodes (PreSens GmbH, Regensburg, Germany) to detect oxygen consumption in the dark and oxygen formation under increasing PFDs. The oxygen optode, as a sensor device, optically measures oxygen concentration by means of a fluorescence dye. During preliminary experiments the saturating irradiance for Fucus vegetative apical tips was identified by recording PI-curves with oxygen production as a function of PFD at 10°C, 15°C, and 20 ± 0.1°C. The vegetative apical thallus tips (2 ± 0.1 cm) obtained from Fucus individuals grown in their native habitat in the Kiel Fjord (June 2013) were kept in dimmed light for wound healing overnight in ambient water. Closed cylindrical acryl glass chambers (25 mL) were used for measuring the production-light response of the vegetative tips and a water bath which was tempered by a thermostat provided constant temperature (DC10 K10; Thermo Haake GmbH, Karlsruhe, Germany). Each apical tip was fixed horizontally on a coarse plastic mesh in the chamber, and a magnetic stirrer assured good mixing of the chamber water. Before measurement, a two-point calibration (0 and 100% oxygen saturation) was carried out. Moreover, the medium of the samples in the chambers was enriched with 2 mM NaHCO3 (final concentration) to avoid carbon limitation during the incubation and purged with nitrogen to start measurements at oxygen saturation levels between 50 and 70%. Respiration was recorded for at least 30 min before exposure to the increasing PFD levels. Incident light on the thallus surface was supplied from 150 W halogen lamps (HLX 64634, OSRAM GmbH, Bad Homburg, Germany) provided by flexible fiber optics (KL 1500, SCHOTT AG, Mainz, Germany). Subsequently, oxygen exchange rates were calculated over 10-min intervals after acclimation (5 min) to the respective PFD. The maximum photon flux density was set at 1400 μmol photon m–2 s–1, and variable photon fluxes (0, 26, 145, 273, 474, 682, 777, and 988 μmol photon m–2 s–1) were obtained by inserting neutral density filters (Hahne Lichttechnik, Düsseldorf, Germany) between the light source (150 W halogen lamps, HLX 64634, OSRAM GmbH, Bad Homburg, Germany) and the chamber. Respiration and photosynthetic production were measured as oxygen concentration changes by using planar SP-PSt3-PSUP-YOP-D5 oxygen sensor spots (PreSens GmbH, Regensburg, Germany) inside the chambers in combination with fiber optics connecting the outside of the chambers with a fiber optic oxygen meter (OXY-4 Mini-Sensor, PreSens GmbH, Regensburg, Germany) according to Warkentin et al. (2007). A least-squares regression was fit to each of the dissolved oxygen concentrations with respect to time, and the slope was used as the photosynthesis rate. PI-curves were calculated using the photosynthesis model of Walsby (1997). From these curves different photosynthetic parameters e.g., maximum photosynthesis (Pmax), respiration (R), light utilization coefficient (α), the light compensation point (Ic), and the light saturation point (Ik) were calculated. These curves were measured for F. vesiculosus to determine the PFD for light saturation of the photosynthesis in relation to fresh biomass, which showed no significant difference in Ik among the three temperatures tested.
After the determination of Ik, the PFD was kept constant at 200 μmol m–2 s–1 and the temperature at 15 ± 0.2°C in a constant climate room for measuring the oxygen production of F. vesiculosus at the end of each KOB experiment in every season. For these measurements ambient water with the ion and gas composition of the KOBs was used. Therefore, any production of oxygen by microalgae or consumption by microbial organisms in ambient water is accounted for in blanks. The samples were incubated for 30 min in the dark and subsequently for 10 min in light (200 μmol m–2 s–1; 150 W halogen lamps, HLX 64634, OSRAM GmbH, Bad Homburg, Germany). The net oxygen production was referred to the water volume of each acryl glass chamber and the fresh weight per sample.
Additionally, chlorophyll a and c (chl a and c) content was extracted with 100% ethanol from lyophilized, powdered samples of each individual (ca. 100 mg) and photometrically quantified according to Ritchie (2006) at the end of each KOB experiment.
Statistical Analyses
Before statistical analyses, all data were tested for normality with the Kolmogorov–Smirnov or Shapiro–Wilk’s test and for homogeneity with the Levene’s test or Fligner–Killeen test to comply with requirements. Differences in the maximum quantum yield, rETRmax and the complementary energy dissipation pathway parameters [Y(PSII), Y(NO), and Y(NPQ)] of Fucus were analyzed with repeated-measures analysis of variance (rm ANOVA), with the within-subject factor time (day) and the between-subject factors pCO2 and temperature for each experiment separately. If the assumption of sphericity (Mauchly test) was not met, the univariate approach with Greenhouse–Geisser adjusted degrees of freedom and p-values for the F-test was applied. In order to evaluate the interactive effect of temperature and pCO2 on all photosynthetic parameters (rETRmax, alpha, Ik, NPQmax, oxygen production, and chlorophyll content) at the end of every KOB experiment, two-way ANOVAs were used with temperature and pCO2 as fixed factors. However, the two-way ANOVAs did not show significant interactions, therefore one-way ANOVAs were carried out for each factor separately. Furthermore, to assess seasonal differences of the initial photophysiological status of Fucus in its native habitat one-way ANOVAs were applied. When the analyses revealed significant differences, pairwise comparisons between means were further explored using a post hoc Tukey’s honest significant difference test. Differences between sampling dates of the different seasonal experiments cannot be compared because abiotic and biotic conditions were too different between the four consecutive experiments. Data were analyzed using SPSS Statistics 22 (IBM, Armonk, NY, United States) and the R software (Version 4.0.2, R Development Core Team, 2020).
Results
Photophysiological Characteristics
The photosynthetic performance measured as in vivo chlorophyll a fluorescence, oxygen production and chlorophyll concentrations of F. vesiculosus vegetative apical tissue varied over the four seasons and the experimental treatment. At elevated temperatures in summer (>26°C) Fucus individuals died and thus results from this time point are missing.
In vivo chl a Fluorescence Parameters (PI-Curves and Non-photochemical Quenching)
Seasonal variations in the light utilization coefficient (α), relative maximal electron transport rates (rETRmax), and saturation irradiance for photosynthesis (Ik) were evident for Fucus grown in its native habitat (Table 1 and Supplementary Table 3). The light utilization coefficient of vegetative Fucus tips was significantly lower in spring (0.68 electrons photons–1) compared to the other seasons which ranged between 0.96, 1.01, and 0.93 electrons photons–1 in July, October, and January, respectively (Supplementary Table 3). In addition, rETRmax of field grown Fucus in spring and autumn varied around 55 μmol e m–2 s–1 which was almost half of summer and winter where values varied around 100 μmol e m–2 s–1 (Table 1). Light saturation coefficient of Fucus was lowest in autumn with 56 μmol photons m–2 s–1 compared to the other seasons, which ranged between 79, 101, and 112 μmol photons m–2 s–1 (Table 1 and Supplementary Table 3). Maximum non-photochemical quenching (NPQmax) of Fucus in its native habitat varied significantly with season (Supplementary Table 3). In spring and winter mean NPQmax of Fucus was approx. 4.4 and thereby was significantly lower than in summer and autumn where values varied around 6.1 (Table 1).
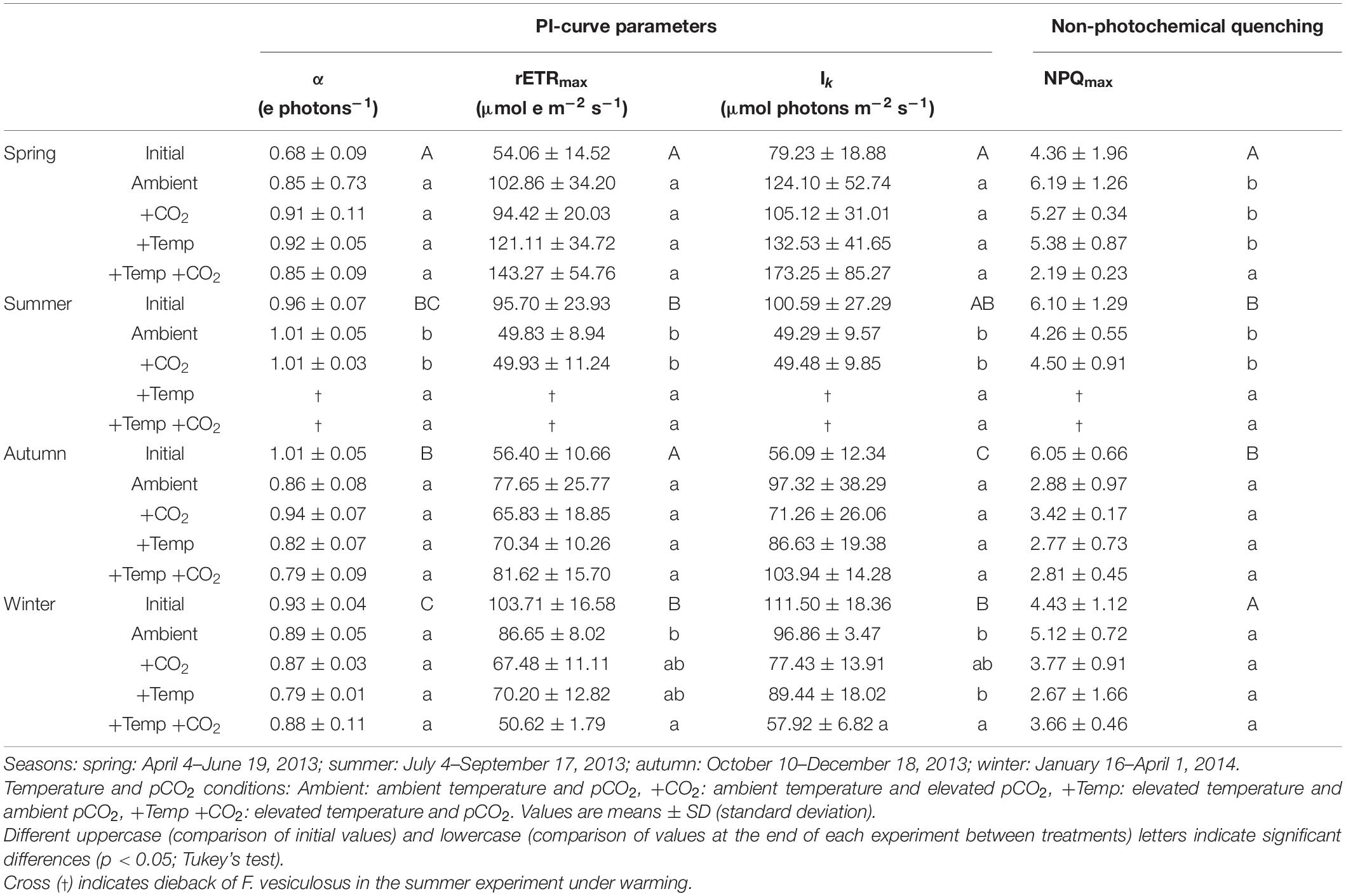
Table 1. Photophysiological responses of initial Fucus vesiculosus individuals growing in its native habitat (n = 12) and at the end of the experiments in the Kiel Outdoor Benthocosms (n = 3), with manipulated temperature and pCO2 conditions over different seasons.
The relative change of Fucus rETRmax during the four different experiments revealed seasonal acclimation of photosynthetic performance (Figure 1A and Table 2). Fucus photosynthesis grown in the KOBs showed a differential temperature optimum and/or tolerance during the different seasons. Fucus revealed increasing rETRmax during the spring experiment, when there were adequate temperatures (8–16°C) for photosynthetic activity (adequate temperature range: 10–24°C, Graiff et al., 2015b) even under elevated temperature conditions (11–21°C). Although rETRmax of Fucus under combined warming and acidification was slightly enhanced (26%) at the end of the spring experiment compared to the other treatments, this enhancement was not significant (Table 1). In the course of the summer experiment, an unexpected natural heat-wave in the Kiel Fjord produced peak temperatures of 27–30°C over a period of 30 days in the experimental warming treatments (Supplementary Table 4). This period of high water temperatures resulted in a dieback of the Fucus individuals under increased temperature conditions. During summer, rETRmax of Fucus decreased drastically from 70 to 15 μmol e m–2 s–1 with the increasing water temperatures and PAR (Supplementary Table 5). In the autumn experiment, rETRmax was low under all treatments revealing no influence of experimental warming (Table 2). During the winter experiment, rETRmax of Fucus was slightly higher under ambient conditions, but declined under pCO2 enriched conditions (Figure 1A and Table 1).
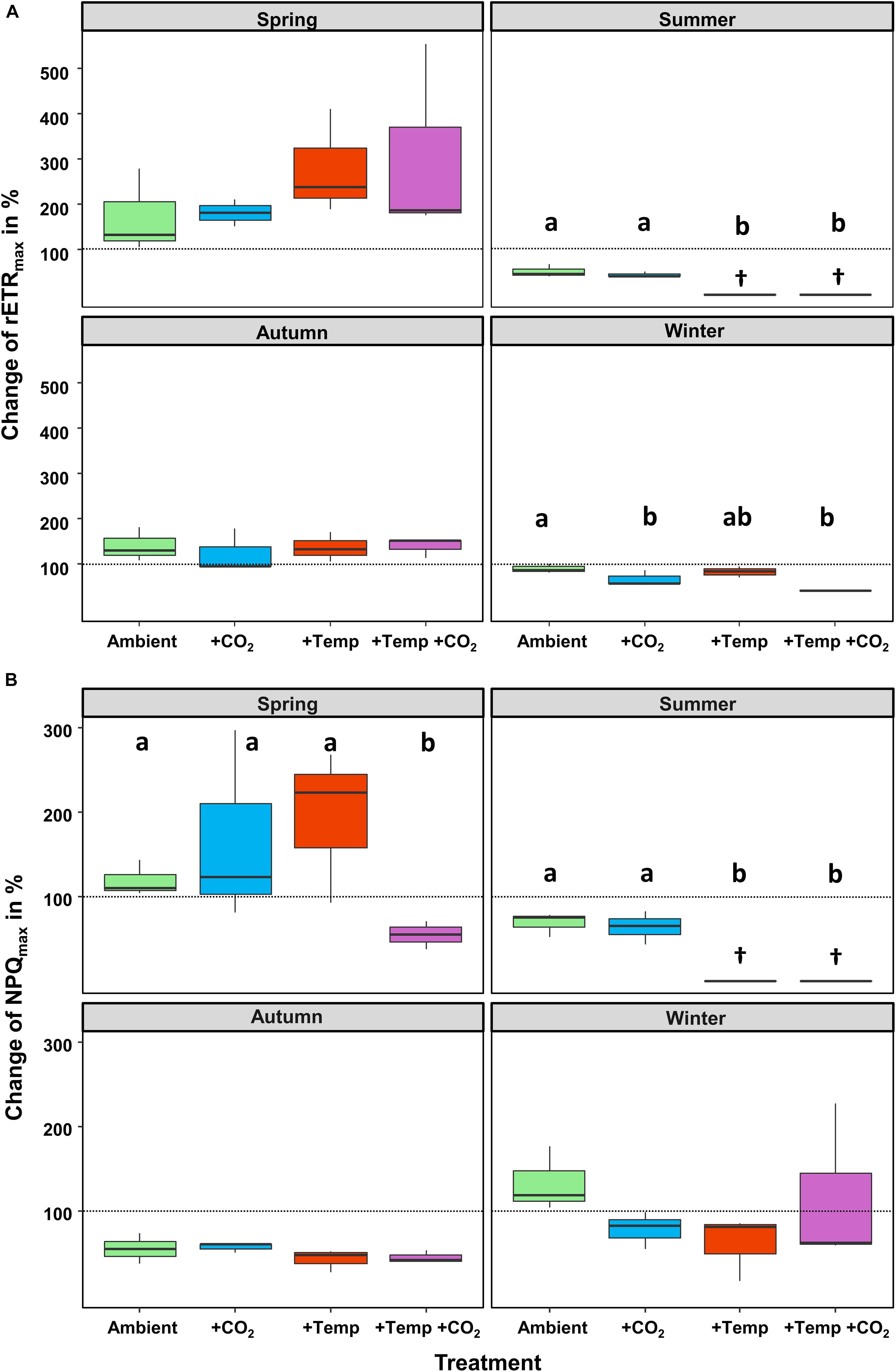
Figure 1. Relative changes (as %) between the initial and final state of each experiment. (A) Maximum relative electron transport rate (rETRmax) and (B) maximum non-photochemical quenching (NPQmax) of Fucus vesiculosus during experiments in the Kiel Outdoor Benthocosms with various temperature and pCO2 conditions over different seasons; dashed line represent no change, values above 100% mean higher rETR or NPQ values at the end of the experiment. Seasons: spring: April 4–June 19, 2013; summer: July 4–September 17, 2013; autumn: October 10–December 18, 2013; winter: January 16–April 1, 2014. Temperature and pCO2 conditions: Ambient: ambient temperature and pCO2, +CO2: ambient temperature and elevated pCO2, +Temp: elevated temperature and ambient pCO2, +Temp +CO2: elevated temperature and pCO2 (n = 3). Cross (†) indicates dieback of F. vesiculosus in the summer experiment under warming. Horizontal lines represent the median; boxes, the interquartile range; whiskers, 1.5× of inter-quartile range. Different lowercase letters indicate significant differences (p < 0.05; Tukey’s test).
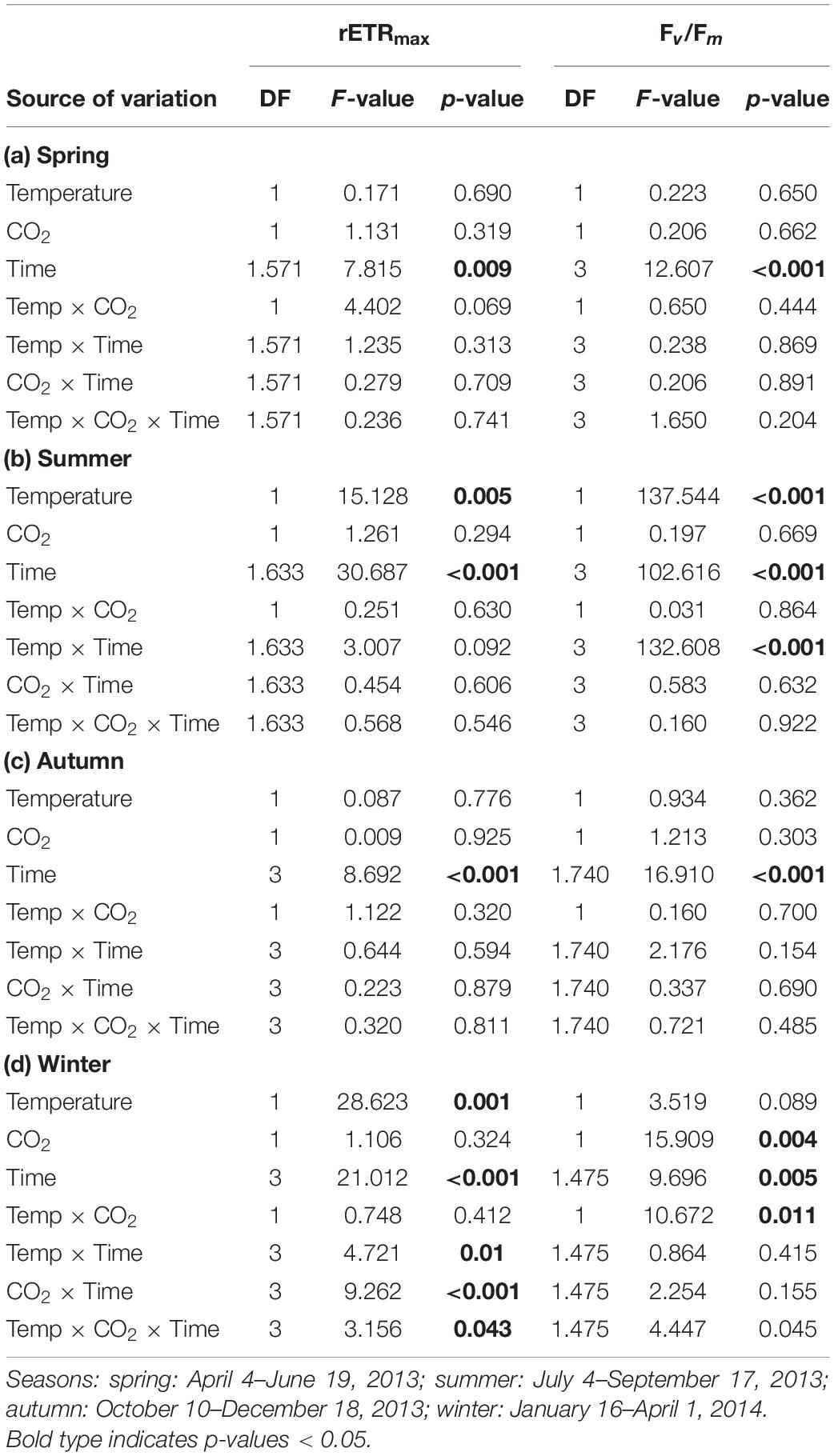
Table 2. Results of repeated-measures ANOVA for effects of temperature, CO2 and time during each experiment on maximum relative electron transport rates (rETRmax) and maximum quantum yields (Fv/Fm) in Fucus vesiculosus in different seasons.
Thus, after growing in the KOBs for 3 months at the different experimental conditions during spring, autumn and winter α of the Fucus individuals did not significantly differ between the applied treatments (Table 1, for statistics see Supplementary Table 6). Correspondingly, rETRmax and Ik were not significantly different between the treatments at the end of the spring and autumn experiment (Supplementary Table 6). In contrast, NPQmax of Fucus was significantly decreased by 60% under combined warming and acidification in the spring experiment (Figure 1B and Supplementary Table 7). At the end of the winter experiment, combined warming and acidification decreased rETRmax and Ik significantly by 40% (Table 1 and Supplementary Table 6). In addition, elevated temperature levels decreased NPQmax during the winter experiment, but this reduction was not significant (Figure 1B and Supplementary Table 7).
Maximum Quantum Yield
During the spring experiment, the maximum quantum yield (Fv/Fm) of vegetative Fucus apices increased until the end of the season under all treatments (Figure 2 and Table 2). In the summer experiment, the Fv/Fm of Fucus under warming remained at a high level after 2 weeks of incubation in the KOBs (Supplementary Figure 2). However, in the subsequent weeks warming reduced the Fv/Fm of Fucus significantly by 40% until mid-August (Supplementary Figure 2 and Table 2). When the Fjord water temperature exhibited a natural heat-wave in the following weeks, water temperatures in the warming treatment reached maximum levels which clearly exceeded the thermal tolerance of Fucus (Graiff et al., 2015b). Thus, the increasing destructive influence of warming on the photochemical apparatus of Fucus was also indicated by significantly reduced Fv/Fm values among the measurement dates (Supplementary Figure 2 and Table 2). There was neither a stimulating nor a detrimental effect of increased pCO2 on Fv/Fm of Fucus during the summer experiment (Figure 2). During autumn, the Fv/Fm of Fucus slightly decreased until the end of the experiment under all treatments (Figure 2). In the winter experiment, Fv/Fm of Fucus was significantly reduced among measurement dates and under warming (Supplementary Figure 2 and Table 2). However, this effect of warming on Fucus was slightly mitigated at increased pCO2 conditions during the winter experiment, which kept the Fv/Fm at the control level (Figure 2 and Table 3).
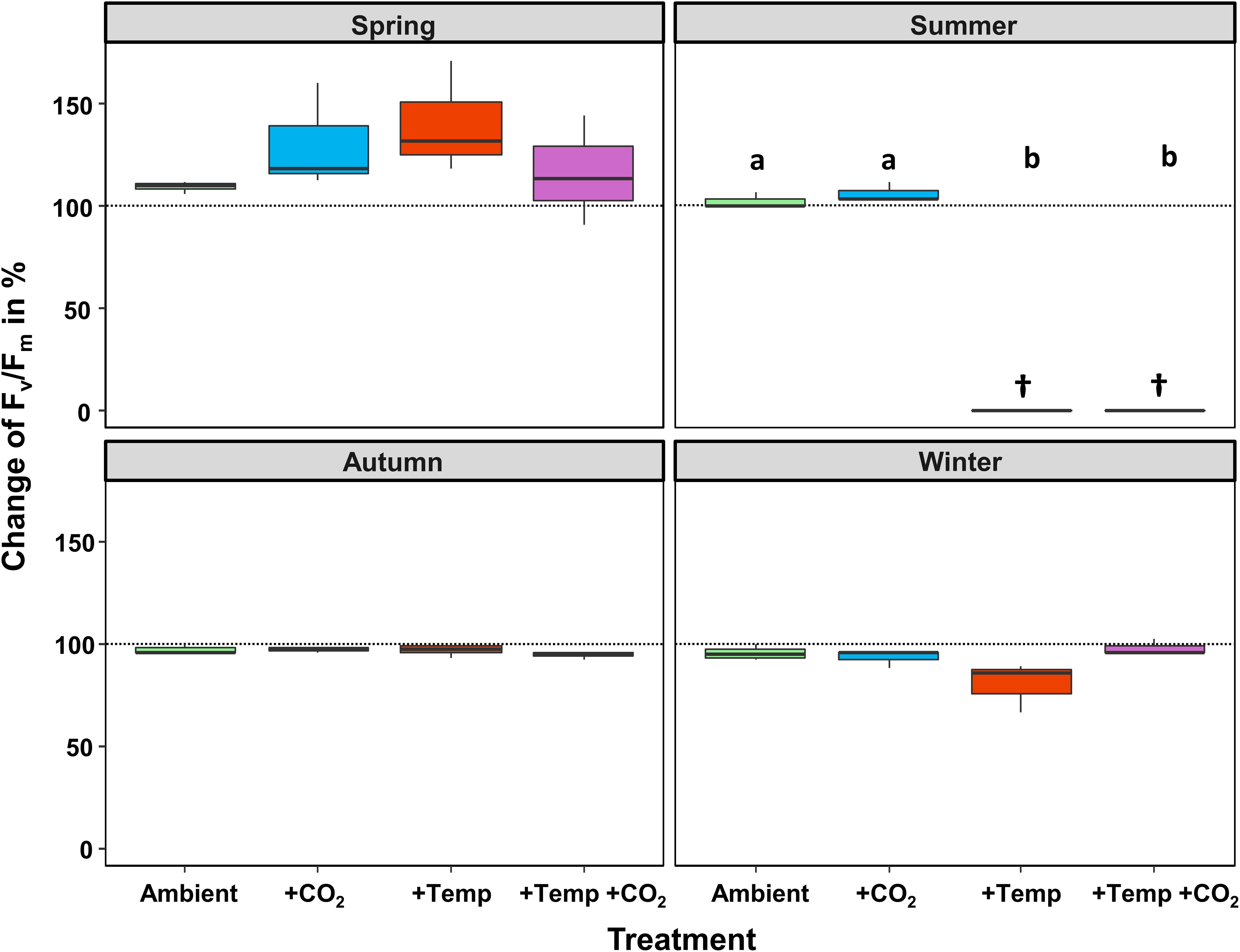
Figure 2. Relative change (as %) between the initial and final state of maximum quantum yield (Fv/Fm) of Fucus vesiculosus during experiments in the Kiel Outdoor Benthocosms with various temperature and pCO2 conditions over different seasons; dashed line represent no change, values above 100% mean higher Fv/Fm values at the end of the experiment. Seasons: spring: April 4–June 19, 2013; summer: July 4–September 17, 2013; autumn: October 10–December 18, 2013; winter: January 16–April 1, 2014. Temperature and pCO2 conditions: Ambient: ambient temperature and pCO2, +CO2: ambient temperature and elevated pCO2, +Temp: elevated temperature and ambient pCO2, +Temp +CO2: elevated temperature and pCO2 (n = 3). Cross (†) indicates dieback of F. vesiculosus in the summer experiment under warming. Horizontal lines represent the median; boxes, the interquartile range; whiskers, 1.5× of inter-quartile range. Different lowercase letters indicate significant differences (p < 0.05; Tukey’s test).
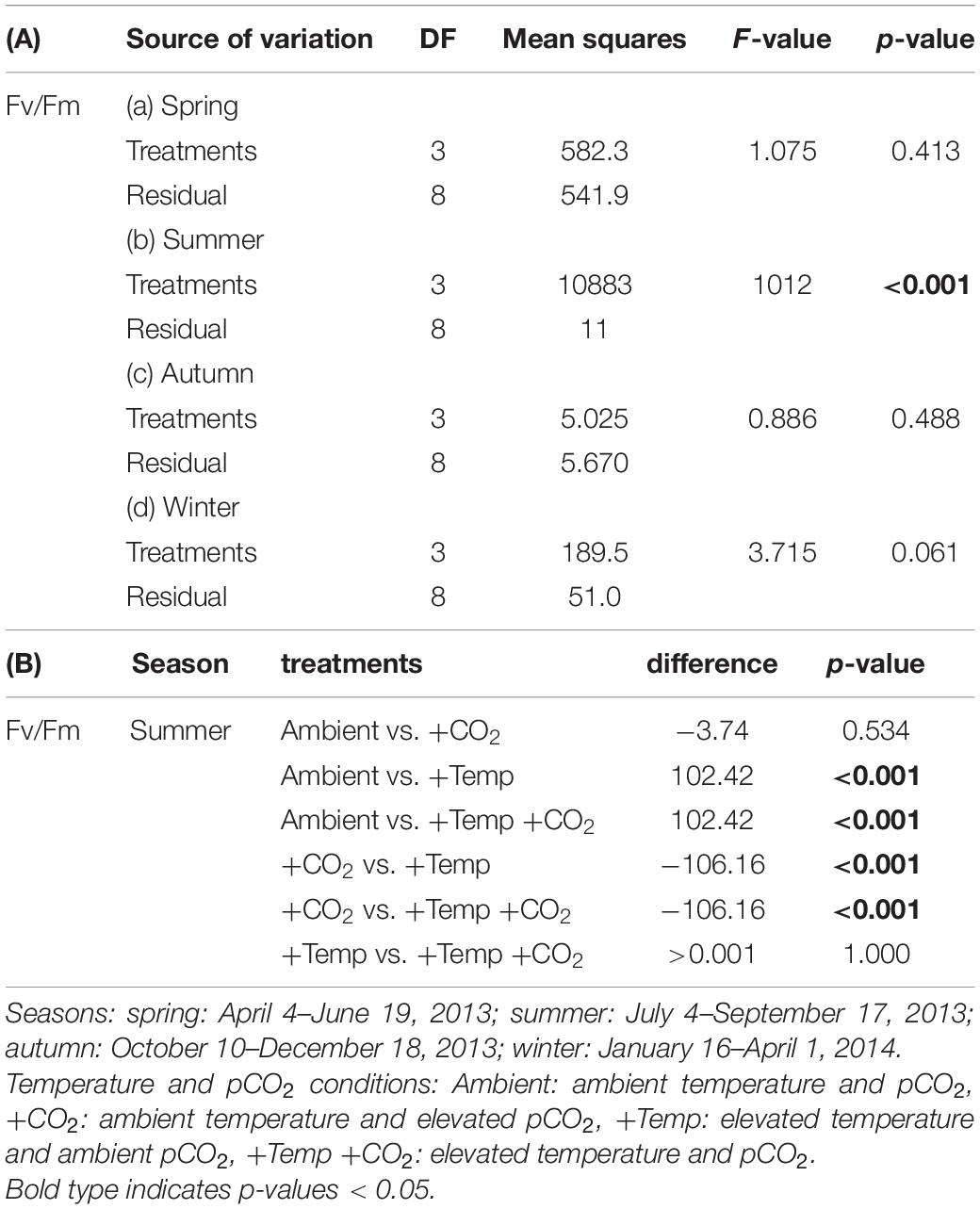
Table 3. Results of (A) one-way ANOVAs and (B) Tukey’s test for effects of manipulated temperature and CO2 conditions on maximum quantum yields (Fv/Fm) in Fucus vesiculosus over different seasons.
Energy Dissipation Pathways
Fucus apical tips showed variable energy distribution among the complementary energy dissipation pathways under natural ambient light: photochemistry Y(PSII), non-regulated non-photochemical quenching Y(NO) and regulated non-photochemical quenching Y(NPQ) at PS II centers during the different seasons and treatments (Figure 3). During spring Y(PSII) slightly and Y(NPQ) significantly increased as Y(NO) significantly decreased until the end of the experiment, emphasizing acclimation processes of Fucus (for statistics see Supplementary Tables 8–10). The regulated non-photochemical quenching Y(NPQ) was significantly reduced under warming in the spring experiment (Supplementary Table 10).
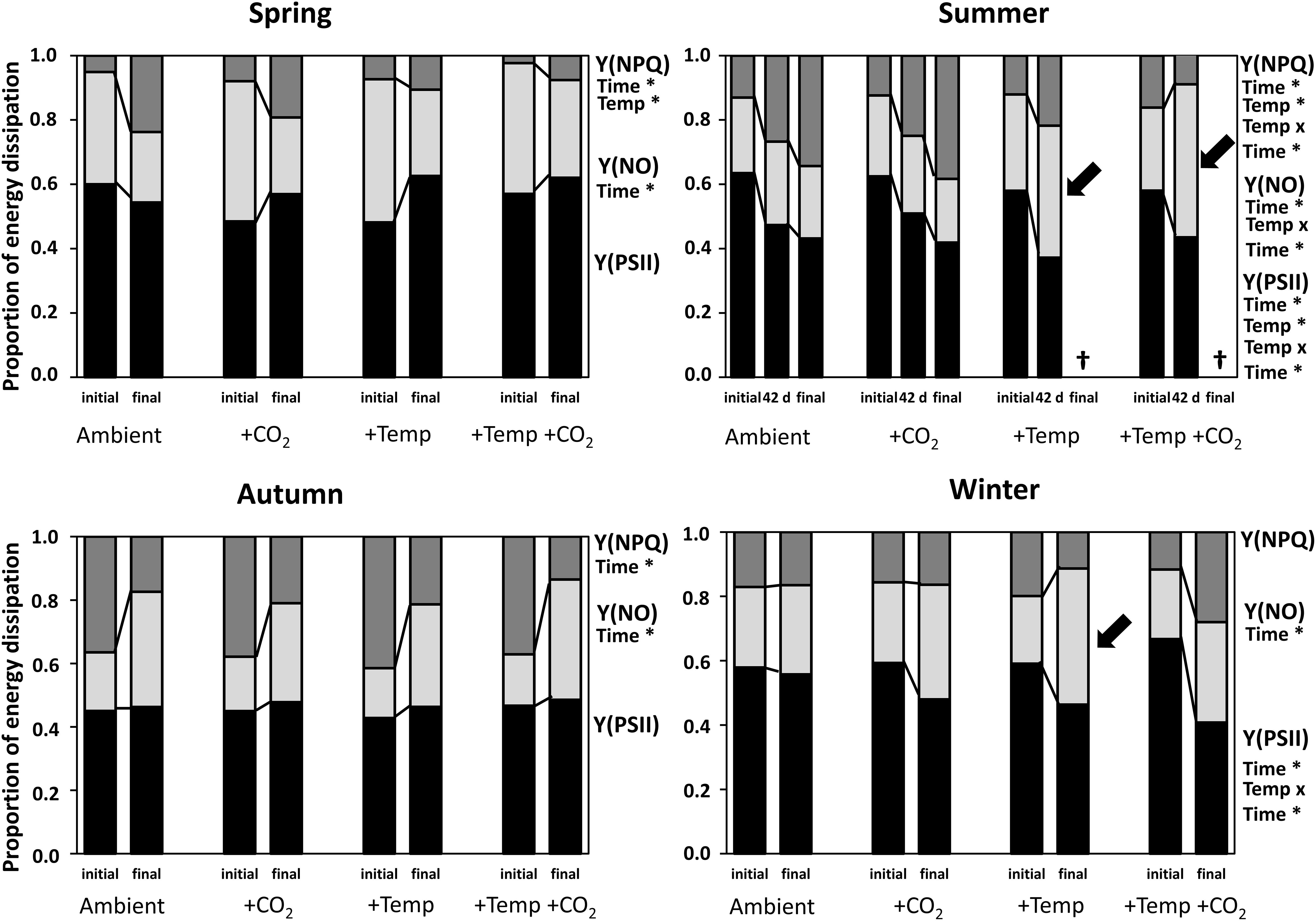
Figure 3. Initial and final complementary energy dissipation pathways of Fucus vesiculosus during experiments in the Kiel Outdoor Benthocosms with various temperature and pCO2 conditions over different seasons. Seasons: spring: April 4–June 19, 2013; summer: July 4–September 17, 2013; autumn: October 10–December 18, 2013; winter: January 16–April 1, 2014. Temperature and pCO2 conditions: Ambient: ambient temperature and pCO2, +CO2: ambient temperature and elevated pCO2, +Temp: elevated temperature and ambient pCO2, +Temp +CO2: elevated temperature and pCO2. Y(PSII) = photochemistry (black bars); Y(NO) = energy dissipation (non-regulated non-photochemical quenching; light gray bars); Y(NPQ) = xanthophyll cycling (regulated non-photochemical quenching; dark gray bars). Values are means (n = 3). Factors listed with an asterisk for each season and parameter are those shown by repeated-measures ANOVA to have significant effects. The statistics are related to the initial vs. final values (Supplementary Tables 9–11). Cross (†) indicates dieback of F. vesiculosus in the summer experiment under warming. Black arrows indicate main increases in Y(NO) during summer and winter experiments.
In summer, effective quantum yield of PSII (photosynthetic efficiency in the light) of Fucus was significantly decreased by warming and time (Supplementary Table 8). Also, regulated non-photochemical quenching Y(NPQ) was decreased by warming (Supplementary Table 10). However, under ambient summer conditions Y(NPQ) increased significantly until the end of the experiment (Supplementary Table 10), reflecting down-regulation of PS II as a protective mechanism against excess photon fluence rates. Non-regulated non-photochemical quenching Y(NO) was enhanced by warming and time (Supplementary Table 9). Thus, photoinhibition or permanent destruction of the photosynthetic apparatus was obvious under elevated temperature levels on day 42 (Figure 3, see black arrows).
Non-regulated non-photochemical quenching of Fucus increased (Supplementary Table 9) at the expense of regulated non-photochemical quenching (Supplementary Table 10), but effective quantum yield was kept constant under all treatments during the autumn experiment (Figure 3). In winter Y(PSII) was significantly decreased by warming and time until the end of the experiment (Supplementary Table 8). Especially, under elevated temperature levels in winter non-regulated non-photochemical quenching Y(NO) increased significantly among the measurement dates (Figure 3, see black arrow and Supplementary Table 9), suggesting that both photochemical energy conversion and protective regulatory mechanisms were inefficient.
Net Oxygen Production
Net oxygen production of Fucus showed a clear seasonal pattern, with highest oxygen production in spring (50–170 μmol O2 g–1 FM h–1) compared to the other seasons which ranged between 29 and 105 μmol O2 g–1 FM h–1 (Figure 4). In spring, increased CO2 slightly enhanced oxygen production of Fucus under ambient as well as increased temperature conditions, however this tendency was not significant. During autumn and winter there were no differences between the treatments (Figure 4 and Supplementary Table 11).
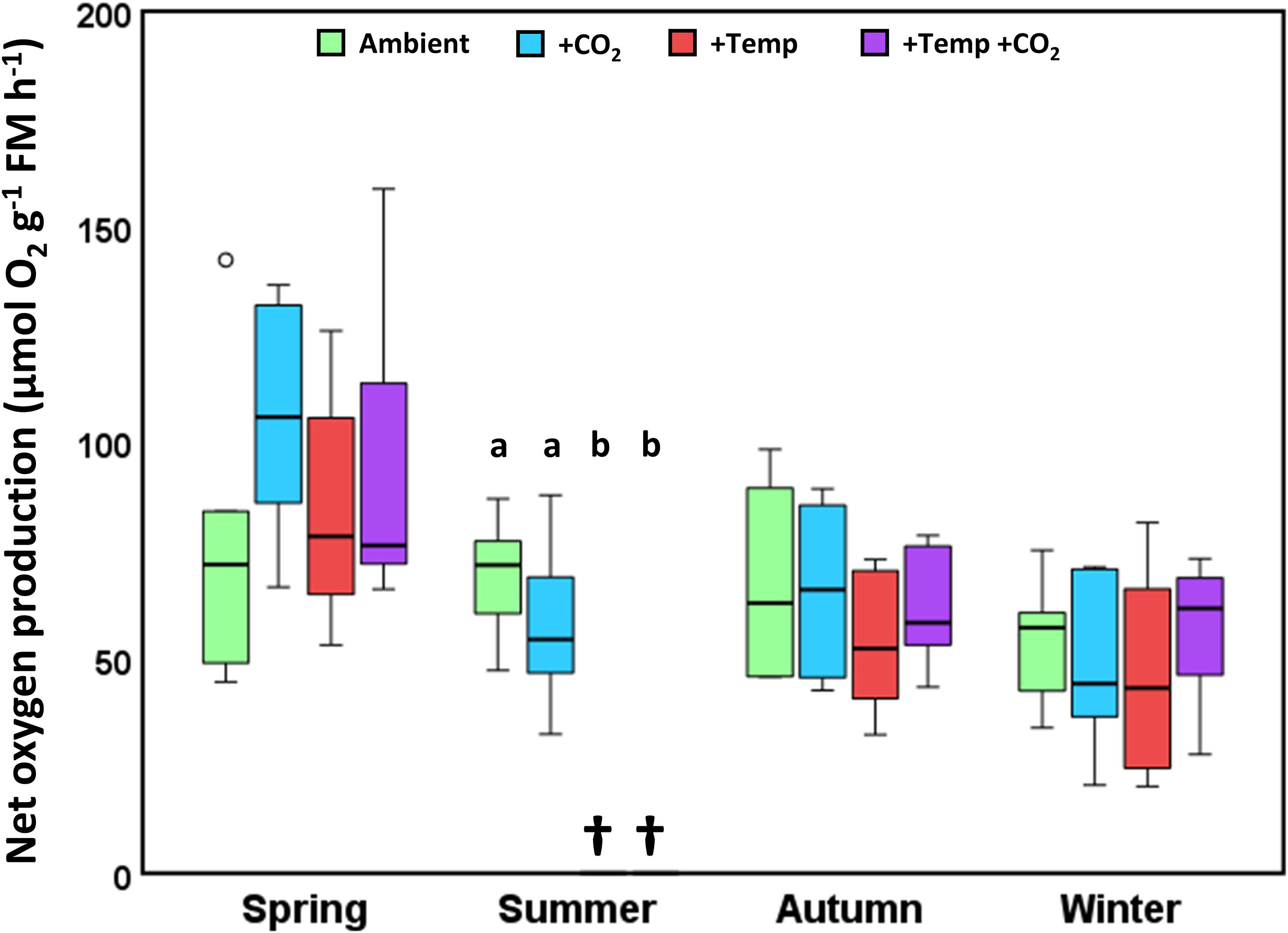
Figure 4. Net oxygen production of Fucus vesiculosus individuals at the end of the experiments in the Kiel Outdoor Benthocosms (KOBs), with various temperature and pCO2 conditions over different seasons. Experimental individuals were measured at 15°C and 200 μmol photons m–2 s–1 after 3 months of incubation in the KOBs. Seasons: spring: April 4–June 19, 2013; summer: July 4–September 17, 2013; autumn: October 10–December 18, 2013; winter: January 16–April 1, 2014. Temperature and pCO2 conditions: Ambient: ambient temperature and pCO2, +CO2: ambient temperature and elevated pCO2, +Temp: elevated temperature and ambient pCO2, +Temp +CO2: elevated temperature and pCO2 (n = 3). Different lowercase letters indicate significant differences (p < 0.05; Tukey’s test). Horizontal lines represent the median; boxes, the interquartile range; whiskers, 1.5× of inter-quartile range; circle, the outlier. Cross (†) indicates dieback of F. vesiculosus in the summer experiment under warming.
Chlorophyll a and c
Chlorophyll a and c contents were high at the end of the spring experiment and decreased during summer to minimal values (Table 4). In the subsequent autumn experiment chlorophyll content of Fucus increased again and reached highest values at the end of the winter experiment. However, chl a and c content did not significantly differ between the treatments of each seasonal experiment (Table 4).
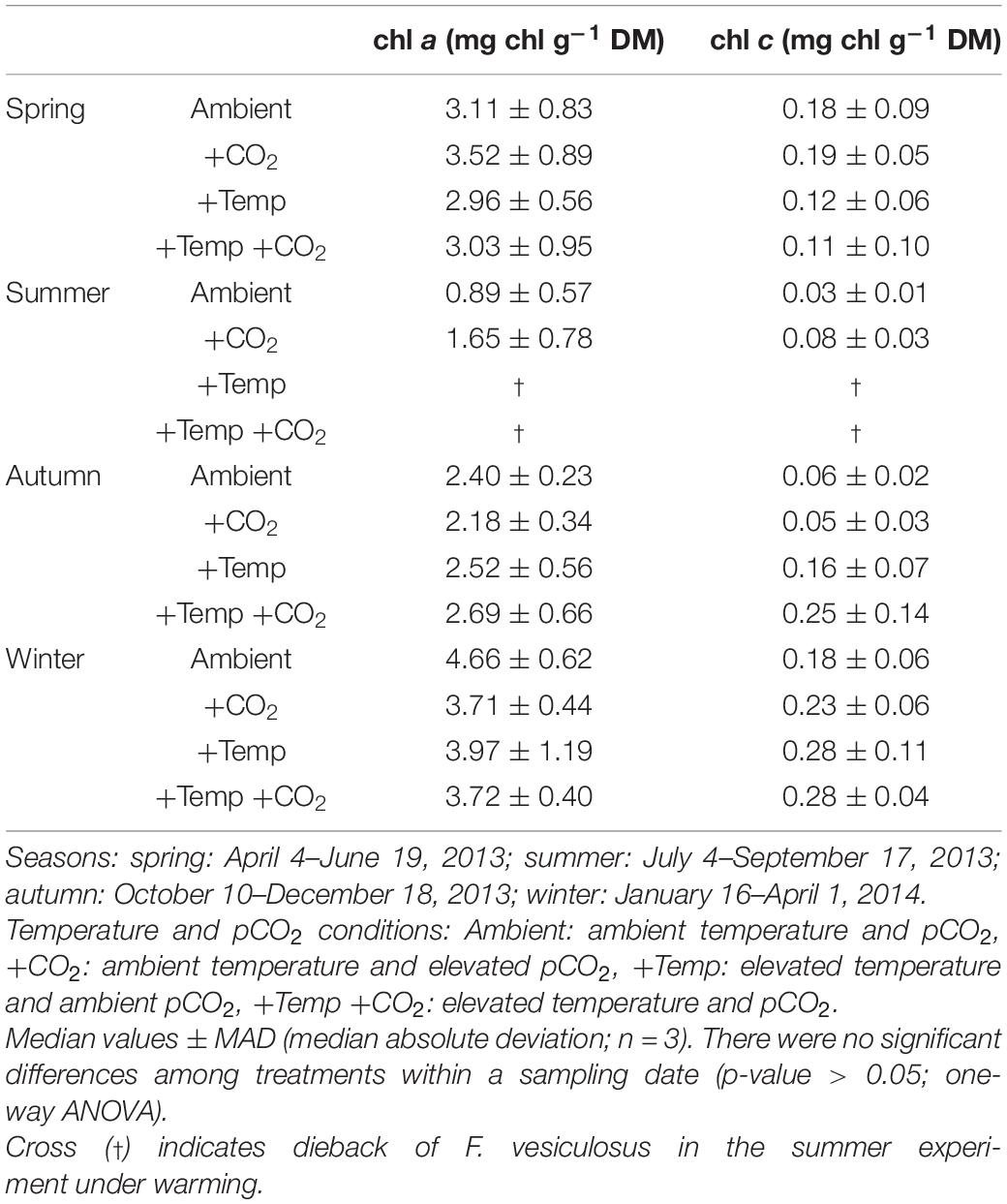
Table 4. Chlorophyll a and c contents (mg chl g–1 dry mass) of Fucus vesiculosus individuals at the end of the experiments in the Kiel Outdoor Benthocosms, with various temperature and pCO2 conditions over different seasons.
Discussion
Photosynthetic performance and efficiency (measured as net oxygen production, rETRmax and Fv/Fm) of Fucus in the KOBs under ambient conditions was highest in spring/early summer when annual growth activity of the species commenced and water temperature as well as solar irradiance increased naturally, while it was lowest in winter (December to January/February). Temperature had a stronger effect than pCO2 on photosynthetic performance of Fucus in all seasons. Photophysiological responses were generally positive during the cooler spring months, but strongly negatively affected during summer (due to a marine heat-wave). Especially, future summer temperatures exceeded the thermal tolerance threshold of western Baltic Sea Fucus and had a deleterious impact overall. In contrast to the expectation that warmer winter conditions might be beneficial for Fucus, increased temperature conditions in combination with sub-optimal low winter light conditions decreased photophysiological performance. Overall, the results of this study suggest potential benefits of the combination of future ocean warming and increased pCO2 over most of the year for Fucus photophysiological performance, but not during summer peak temperatures.
Seasonal Photosynthetic Acclimation
Photosynthetic parameters indicate that Fucus metabolism under ambient conditions became up-regulated during spring and down-regulated during winter (December to January/February). Net oxygen production, rETRmax and chlorophyll content of Fucus declined from high values at the end of the spring experiment (June) to minimal values at the end of the summer experiment (September). As a consequence, Fucus individuals accumulated high quantities of mannitol as main photosynthetic product over spring until June and exhibited low quantities in December (Graiff et al., 2015a). Fucus had lowest rETRmax and light saturation coefficients (Ik) as well as a very low chlorophyll content in summer under high irradiances compared to all other seasons. Concordantly, Rothäusler et al. (2018) stated that Fucus needs low to moderate irradiances to reach maximum photosynthesis. For most seaweeds growing in the shallow subtidal, the available PAR during summer is often far exceeding values needed to saturate photosynthesis (Franklin and Forster, 1997). Excess irradiance may induce photo-oxidative damage via increased production of ROS, causing pigment bleaching and death in extreme cases (Müller et al., 2001). In order to avoid irradiance stress, seaweeds must acclimate to changes in irradiance by optimizing photosynthesis.
The different energy dissipation pathways including regulated non-photochemical quenching (NPQ) of Fucus, are subjected to a seasonal cycle, with spring/summer maxima and a minimum in December. NPQ is an important, rapidly inducible, photoregulation mechanism to optimize light utilization, serving to prevent or reduce chronic photoinhibition and maximize photosynthetic efficiency under high irradiances (Müller et al., 2001; Wilhelm and Selmar, 2011; Lavaud and Lepetit, 2013). NPQ is a proxy of xanthophyll cycling processes which dissipate excess irradiance energy as heat to protect the photosynthetic apparatus from over-excitation (Hanelt et al., 1993; Franklin and Forster, 1997; Lavaud et al., 2002a, b; Ruban et al., 2007; Lavaud and Lepetit, 2013). Therefore, a reduced rETRmax and NPQmax during summer indicated that seasonal acclimation of Fucus photochemistry was limited, probably due to the natural heat-wave in the Kiel Fjord in combination with excess summer irradiance.
In winter during low-light conditions, a reversed acclimation pattern is expected in order to allow efficient light harvesting at reduced irradiance levels. Photoacclimation can be obtained through either an alteration in the size or number of photosynthetic units (Richardson et al., 1983; Falkowski and LaRoche, 1991; Beer et al., 2014). Under low-light conditions like in winter, an increase in photosynthetic unit sizes is reflected by an increase in light utilization efficiency (α), but a decline in maximal photosynthesis (Pmax or rETRmax) (Richardson et al., 1983; Beer et al., 2014). Higher light-harvesting capability in winter months was indicated for Fucus through lowered rETRmax and Ik but higher chlorophyll content compared to the other seasons under ambient conditions. In conclusion, seasonal acclimation of photochemistry was apparent in Fucus. Data indicate that photoacclimation under ambient conditions maximizes both photoprotection via NPQ during high-light at the end of spring and up-regulation of light-harvesting capability during low-light winter months (December to January). These photoacclimation processes reflect an important physiological trait of Fucus to maintain photosynthesis over a seasonal cycle of highly fluctuating environmental conditions.
Photosynthesis Under Ocean Warming and Acidification
Relative maximum electron transport rate and Fv/Fm of Fucus increased within a temperature range of 13–24°C and thereby experimental warming was even beneficial for photosynthesis in spring as it was still in the range of optimum temperatures for photosynthesis. This positive effect of warm temperatures >20°C on photosynthetic performance is attributed to its role in carbon fixation (Davison et al., 1991). Warm temperatures positively affect the availability of inorganic carbon (Surif and Raven, 1990) and the rate of carbon fixation by RuBisCO (Sukenik et al., 1987).
Similarly, in the first 2 weeks of the summer experiment (early July) rETRmax and Fv/Fm of Fucus remained at similarly high levels under the warming scenario as under ambient temperature conditions. In contrast, growth of Fucus under the same warming scenario stopped when temperatures increased above 24°C (Graiff et al., 2015a). This temperature was identified as a critical thermal threshold in the laboratory (Graiff et al., 2015b). Optimal temperature for photosynthetic performance (24°C) is higher and the optimal temperature range of photosynthesis much narrower compared to that for growth (15–20°C) in Fucus (Graiff et al., 2015b) as both physiological processes are not directly coupled (Kübler et al., 1991; Eggert and Wiencke, 2000; Eggert, 2012).
With rising temperatures, up to the thermal threshold of 26°C, Fucus exhibited increasing electron transport rates, followed by a rapid decline (Graiff et al., 2015b). A similar pattern was also found by Figueroa et al. (2019) for Fucus serratus. This sudden reduction in photosynthetic capacity at temperatures >26°C can be attributed to several factors such as temperature sensitivity of enzymes for carbon fixation, photophosphorylation and/or the thermal stability of PSII (Lynch and Thompson, 1984; Davison et al., 1991; Wahid et al., 2007). The damaging influence of elevated temperature levels on the photochemical apparatus of Fucus became apparent by significantly reduced Fv/Fm and the increase of non-regulated non-photochemical quenching Y(NO). When Kiel Fjord temperatures reached 27–30°C under the warming scenario during a natural heat-wave (late July to early August) (Supplementary Table 5) the thermal range exceeded the tolerance or lethal limit of Fucus (Graiff et al., 2015b) and this seaweed died. In conclusion, at temperatures exceeding 26°C Fucus from the western Baltic Sea was unable to acclimate.
Despite the fact that Fucus is already at risk to decline in abundance or vanish over summer in the shallow subtidal of the Baltic Sea, it may theoretically benefit from mild winter temperatures (Bartsch et al., 2012) as the duration of favorable temperatures for growth may increase (BACC II Author Team, 2015). In contrast to these expectations, during the winter experiment warming decreased Fv/Fm, and NPQmax, and the mannitol content of Fucus was significantly lowered (Graiff et al., 2015a). This situation together with an increased non-regulated non-photochemical quenching Y(NO) under increased temperature conditions in winter, clearly indicates a reduced functionality of the photosynthetic apparatus. This negative impact of elevated winter temperature on Fucus performance seems conflicting, as the simulated warming increased temperatures from ambient 4–7°C to 8–12°C (December to January) and thereby shifting toward the optimum temperature range of Fucus growth (Graiff et al., 2015b). There might be a mismatch between limited photosynthesis due to low-light conditions in winter and enhancement of general metabolic activity during warmer winters that might explain this imbalance (Rohde et al., 2008). This response may also indicate that Fucus has seasonally varying temperature optima for photosynthesis which is known e.g., for subtidal red algae (Mathieson and Norall, 1975). Seaweeds sampled during winter exhibited higher rates of apparent photosynthesis at low water temperature, lower temperature optima, and a reduced tolerance to high temperatures than specimens sampled during summer (Mathieson and Norall, 1975).
Net oxygen production and chlorophyll a fluorescence parameters obtained from apical tissue of Fucus were not significantly enhanced under acidification in the KOB experiments during all seasonal experiments. The lack of significant effects of acidification on Fucus photophysiological performance in the present study may be due to F. vesiculosus already being adapted to natural high and strongly fluctuating pCO2 and pH conditions in their native habitat in the Kiel Fjord (Thomsen et al., 2010; Saderne, 2012; Melzner et al., 2013). In the spring experiment, however, acidification slightly enhanced net oxygen production of Fucus, which is in accordance with Olischläger et al. (2012), who found a tendency of increased net photosynthesis of young vegetative sporophytes of Laminaria hyperborea at elevated pCO2. The effect of stimulating photosynthesis and relative electron transport rates under increasing external DIC was described in different brown algae (Forster and Dring, 1992; Nygård and Dring, 2008; Johnson et al., 2012). Especially, the kelps Laminaria digitata and Saccharina latissima as well as Baltic F. vesiculosus reacted to moderately increased DIC with enhanced rates of photosynthesis and carbon acquisition (Schmid et al., 1996; Klenell et al., 2004; Nygård and Dring, 2008). Under elevated pCO2, it is presumed, that photorespiration is reduced and less energy is needed for restoring the internal carbon storage after periods of high photosynthetic activity. Increased pCO2 can also trigger changes in pigment composition and chlorophyll content which are a frequently observed responses in different algal species as part of an acclimation mechanism (García-Sánchez et al., 1994; Gordillo et al., 2003). However, F. serratus (Johnston and Raven, 1990) and F. vesiculosus (Takolander et al., 2019 and the present study) did not show any changes in their pigment concentration under increased pCO2.
The present study reveals new details about the combined effects of ocean warming and acidification on the seasonal regulation of the photophysiological performance of Fucus (i.e., net oxygen production, chlorophyll a fluorescence parameters, energy dissipation pathways and chlorophyll content) which are otherwise not well studied and understood (Raven et al., 2011; Kübler and Dudgeon, 2015; Ji and Gao, 2020). It was predicted that under light saturation like in late spring and early summer, enhanced pCO2 in combination with warming may have positive synergistic effects on seaweed performance (Kübler and Dudgeon, 2015), as previously reported for filamentous turf algae (Connell and Russell, 2010). In the KOBs photosynthetic performance of Fucus under warming was not synergistically enhanced by acidification in spring and summer which might be a consequence of photorespiration. The latter physiological process is interactively regulated by increasing pCO2 and rising temperatures (Sage and Kubien, 2007). The pCO2 treatments were quite variable in the KOBs due to natural pCO2 fluctuations in the Kiel Fjord water; this may have masked physiological responses of Fucus, but better represent their ecological in situ response pattern.
Conclusion
Global warming will elevate maximum temperatures during all seasons, such that sub-lethal and lethal conditions may become more frequent and sustained, especially over summer (Meehl and Tebaldi, 2004; Vasseur et al., 2014). Understanding the mechanisms of tolerance and species acclimation to these extremes and also to prolonged sub-lethal conditions or altered optimum is crucial for predicting climate change impacts, particularly for species growing close to their thermal limits (Collier et al., 2011; Brodie et al., 2014). Fucus in the Baltic already lives close to its upper thermal tolerance limit in summer and probably cannot cope with such elevated average temperatures and expected increasing heat-wave frequency in the shallow subtidal of the future Baltic Sea. The results of the current study and of Graiff et al. (2015a, b) as well as Wahl et al. (2019) indicate that future ocean warming effects on Fucus will be strongest during summer. The expected combined increase in warming and pCO2 will have slightly positive effects in spring, autumn and winter for Fucus as revealed by photophysiological performance (this study), growth (Graiff et al., 2015a) and fertility (Graiff et al., 2017). This suggests that Fucus populations may have a better start into the next growing season under the tested global change conditions. Although frequency and intensity of summer heat-waves approaching lethal temperatures may probably be the limiting factor for overall Fucus population’s persistence.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: PANGAEA https://doi.org/10.1594/PANGAEA.842719.
Author Contributions
AG, UK, and IB designed the study. AG performed the experiments and wrote the manuscript, with UK, IB, and KG providing substantial contributions. AG and KG analyzed the data. UK and IB provided funding and intellectual input into the analysis. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the Project BIOACID Phase III of the German Federal Ministry of Education and Research (BMBF; FKZ 03F0728K) and the DFG project (GR5088/2-1).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We gratefully thank Björn Buchholz for the maintenance of the Kiel Outdoor Benthocosms, Lars Gutow for helpful comments in planning the sampling procedures and Romina Bläsner, Jascha Berberich, and Henrike Pfefferkorn for their support during sampling. We also gratefully acknowledge all members of the BIOACID Phase II consortium “Benthic assemblages” for their cooperation and support. We thank the reviewers for their helpful critiques and suggestions.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.666493/full#supplementary-material
Abbreviations
α, light utilization efficiency; CCM, carbon-concentrating mechanism; Fv/Fm, maximum quantum yield of PSII; Ik, saturation irradiance for photosynthesis; KOB, Kiel Outdoor Benthocosm; NO, non-regulated non-photochemical quenching, NPQ, regulated non-photochemical quenching, rETRmax, relative maximum electron transport rate; PAR, photosynthetically active radiation; ROS, reactive oxygen species.
Footnotes
References
Adir, N., Zer, H., Shochat, S., and Ohad, I. (2003). Photoinhibition – a historical perspective. Photosynth. Res. 76, 343–370.
Allakhverdiev, S. I., Kreslavski, V. D., Klimov, V. V., Los, D. A., Carpentier, R., and Mohanty, P. (2008). Heat stress: an overview of molecular responses in photosynthesis. Photosynth. Res. 98, 541–550. doi: 10.1007/s11120-008-9331-0
BACC II Author Team (2015). Second Assessment of Climate Change for the Baltic Sea Basin. Berlin: Springer.
Bartsch, I., Wiencke, C., and Laepple, T. (2012). “Global seaweed biogeography under a changing climate: the prospected effects of temperature,” in Seaweed Biology, eds C. Wiencke and K. Bischof (Berlin Heidelberg: Springer-Verlag), 383–406. doi: 10.1007/978-3-642-28451-9_18
Beardall, J., and Giordano, M. (2002). Ecological implications of microalgal and cyanobacterial CO2 concentrating mechanisms, and their regulation. Funct. Plant Biol. 29, 335–347. doi: 10.1071/pp01195
Beer, S., Björk, M., and Beardall, J. (2014). Photosynthesis in the Marine Environment. Oxford, UK: Wiley Blackwell.
Berry, J., and Björkman, O. (1980). Photosynthetic response and adaption to temperature in higher plants. Annu. Rev. Plant Physiol. 31, 491–543. doi: 10.1146/annurev.pp.31.060180.002423
Bokn, T. L., Moy, F. E., Christie, H., Engelbert, S., Karez, R., Kersting, K., et al. (2002). Are rocky shore ecosystems affected by nutrient-enriched seawater? some preliminary results from a mesocosm experiment. Hydrobiologia 484, 167–175. doi: 10.1007/978-94-017-3190-4_14
Brodie, J., Williamson, C. J., Smale, D. A., Kamenos, N. A., Mieszkowska, N., Santos, R., et al. (2014). The future of the northeast Atlantic benthic flora in a high CO2 world. Ecol. Evol. 4, 2787–2798.
Collén, J., and Davison, I. R. (2001). Seasonality and thermal acclimation of reactive oxygen metabolism in Fucus vesiculosus (Phaeophyceae). J. Phycol. 37, 474–481. doi: 10.1046/j.1529-8817.2001.037004474.x
Collier, C. J., Uthicke, S., and Waycott, M. (2011). Thermal tolerance of two seagrass species at contrasting light levels: implications for future distribution in the Great Barrier Reef. Limnol. Oceanogr. 56, 2200–2210. doi: 10.4319/lo.2011.56.6.2200
Connell, S. D., and Russell, B. D. (2010). The direct effects of increasing CO2 and temperature on non-calcifying organisms: increasing the potential for phase shifts in kelp forests. Proc. R. Soc. 277, 1409–1415. doi: 10.1098/rspb.2009.2069
Davison, I. R., and Pearson, G. A. (1996). Stress tolerance in intertidal seaweeds. J. Phycol. 32, 197–211. doi: 10.1111/j.0022-3646.1996.00197.x
Davison, I. R., Greene, R. M., and Podolak, E. J. (1991). Temperature acclimation of respiration and photosynthesis in the brown alga Laminaria saccharina. Mar. Biol. 110, 449–454. doi: 10.1007/bf01344363
Dickson, A., Sabine, C., and Christian, J. (2007). Guide to best Practices for Ocean CO2 Measurements. Columbia: PICES Special Publication
Dutta, S., Mohanty, S., and Tripathy, B. C. (2009). Role of temperature stress on chloroplast biogenesis and protein import in pea. Plant Physiol. 150, 1050–1061. doi: 10.1104/pp.109.137265
Eggert, A. (2012). “Seaweed responses to temperature,” in Seaweed Biology, eds C. Wiencke and K. Bischof (Berlin Heidelberg: Springer-Verlag), 47–66. doi: 10.1007/978-3-642-28451-9_3
Eggert, A., and Wiencke, C. (2000). Adaptation and acclimation of growth and photosynthesis of five Antarctic red algae to low temperatures. Polar Biol. 23, 609–618. doi: 10.1007/s003000000130
Eggert, A., Visser, R. J. W., Van Hasselt, P. R., and Breeman, A. M. (2006). Differences in acclimation potential of photosynthesis in seven isolates of the tropical to warm temperate macrophyte Valonia utricularis (Chlorophyta). Phycologia 45, 546–556. doi: 10.2216/05-03.1
Elken, J., Lehmann, A., and Myrberg, K. (2015). “Recent change-marine circulation and stratification,” in Second Assessment of Climate Change for the Baltic Sea Basin, ed. T. B. I. A. Team (Berlin: Springer), 131–144. doi: 10.1007/978-3-319-16006-1_7
Falkowski, P. G., and LaRoche, J. (1991). Acclimation to spectral irradiance in algae. J. Phycol. 27, 8–14. doi: 10.1111/j.0022-3646.1991.00008.x
Feng, Y., Warner, M. E., Zhang, Y., Sun, J., Fu, F. X., Rose, J. M., et al. (2008). Interactive effects of increased pCO2, temperature and irradiance on the marine coccolithophore Emiliania huxleyi (Prymnesiophyceae). Eur. J. Phycol. 43, 87–98. doi: 10.1080/09670260701664674
Figueroa, F. L., Celis-Plá, P. S. M., Martínez, B., Korbee, N., Trilla, A., and Arenas, F. (2019). Yield losses and electron transport rate as indicators of thermal stress in Fucus serratus (Ochrophyta). Algal Res. 41:101560. doi: 10.1016/j.algal.2019.101560
Forster, R. M., and Dring, M. J. (1992). Interactions of blue light and inorganic carbon supply in the control of light-saturated photosynthesis in brown algae. Plant Cell Environ. 15, 241–247. doi: 10.1111/j.1365-3040.1992.tb01478.x
Franklin, L. A., and Forster, R. M. (1997). The changing irradiance environment: consequences for marine macrophyte physiology, productivity and ecology. Eur. J. Phycol. 32, 207–232. doi: 10.1080/09670269710001737149
Fu, F. X., Warner, M. E., Zhang, Y., Feng, Y., and Hutchins, D. A. (2007). Effects of increased temperature and CO2 on photosynthesis, growth, and elemental ratios in marine Synechococcus and Prochlorococcus (Cyanobacteria). J. Phycol. 43, 485–496. doi: 10.1111/j.1529-8817.2007.00355.x
García-Sánchez, M. J., Fernández, J. A., and Niell, X. (1994). Effect of inorganic carbon supply on the photosynthetic physiology of Gracilaria tenuistipitata. Planta 194, 55–61.
Genty, B., Briantais, J. M., and Baker, N. R. (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 990, 87–92. doi: 10.1016/s0304-4165(89)80016-9
Gordillo, F. J. L., Figueroa, F. L., and Niell, F. X. (2003). Photon- and carbon-use efficiency in Ulva rigida at different CO2 and N levels. Planta 218, 315–322. doi: 10.1007/s00425-003-1087-3
Gordillo, F. J., Niell, F. X., and Figueroa, F. L. (2001). Non-photosynthetic enhancement of growth by high CO2 level in the nitrophilic seaweed Ulva rigida C. Agardh (Chlorophyta). Planta 213, 64–70. doi: 10.1007/s004250000468
Goss, R., and Lepetit, B. (2015). Biodiversity of NPQ. J. Plant Physiol. 172, 13–32. doi: 10.1016/j.jplph.2014.03.004
Gouvêa, L. P., Schubert, N., Martins, C. D. L., Sissini, M., Ramlov, F., Rodrigues, E. R. O., et al. (2017). Interactive effects of marine heatwaves and eutrophication on the ecophysiology of a widespread and ecologically important macroalga. Limnol. Oceanogr. 62, 2056–2075. doi: 10.1002/lno.10551
Graiff, A., Bartsch, I., Ruth, W., Wahl, M., and Karsten, U. (2015a). Season exerts differential effects of ocean acidification and warming on growth and carbon metabolism of the seaweed Fucus vesiculosus in the western Baltic Sea. Front. Mar. Sci. 2:112.
Graiff, A., Dankworth, M., Wahl, M., Karsten, U., and Bartsch, I. (2017). Seasonal variations of Fucus vesiculosus fertility under ocean acidification and warming in the western Baltic Sea. Bot. Mar. 60, 239–255.
Graiff, A., Karsten, U., Radtke, H., Wahl, M., and Eggert, A. (2020). Model simulation of seasonal growth of Fucus vesiculosus in its benthic community. Limnol. Oceanogr. Methods 18, 89–115. doi: 10.1002/lom3.10351
Graiff, A., Liesner, D., Karsten, U., and Bartsch, I. (2015b). Temperature tolerance of western Baltic Sea Fucus vesiculosus – growth, photosynthesis and survival. J. Exp. Mar. Bio. Ecol. 471, 8–16. doi: 10.1016/j.jembe.2015.05.009
Hanelt, D. (1998). Capability of dynamic photoinhibition in Arctic macroalgae is related to their depth distribution. Mar. Biol. 131, 361–369. doi: 10.1007/s002270050329
Hanelt, D., Huppertz, K., and Nultsch, W. (1993). Daily course of photosynthesis and photoinhibition in marine macroalgae investigated in the laboratory and field. Mar. Ecol. Prog. Ser. 97, 31–37. doi: 10.3354/meps097031
HELCOM (2013). Climate change in the baltic sea area. HELCOM thematic assessment in 2013. Balt. Sea Environ. Proc. 137, 1–66.
Helmuth, B., Harley, C. D., Halpin, P. M., O’Donnell, M., Hofmann, G. E., and Blanchette, C. A. (2002). Climate change and latitudinal patterns of intertidal thermal stress. Science 298, 1015–1017. doi: 10.1126/science.1076814
Henley, W. J. (1993). Measurement and interpretation of photosynthetic light-response curves in algae in the context of photoinhibition and diel changes. J. Phycol. 29, 729–739. doi: 10.1111/j.0022-3646.1993.00729.x
Hobday, A. J., Alexander, L. V., Perkins, S. E., Smale, D. A., Straub, S. C., Oliver, E. C. J., et al. (2016). A hierarchical approach to defining marine heatwaves. Prog. Oceanogr. 141, 227–238. doi: 10.1016/j.pocean.2015.12.014
Ihnken, S., Eggert, A., and Beardall, J. (2010). Exposure times in rapid light curves affect photosynthetic parameters in algae. Aquat. Bot. 93, 185–194. doi: 10.1016/j.aquabot.2010.07.002
Imbery, F., Friedrich, K., Haeseler, S., Koppe, C., Janssen, W., and Bissolli, P. (2018). Vorläufiger Rückblick auf den Sommer 2018 – eine Bilanz extremer Wetterereignisse. Germany: Deutscher Wetterdienst
Ji, Y., and Gao, K. (2020). Effects of Climate Change Factors on Marine Macroalgae: a Review, 1st Edn. Amsterdam: Elsevier Ltd.
Johnson, V. R., Russell, B. D., Fabricius, K. E., Brownlee, C., and Hall-Spencer, J. M. (2012). Temperate and tropical brown macroalgae thrive, despite decalcification, along natural CO2 gradients. Glob. Chang. Biol. 18, 2792–2803. doi: 10.1111/j.1365-2486.2012.02716.x
Johnston, A. M., and Raven, J. A. (1990). Effects of culture in high CO2 on the photosynthetic physiology of Fucus serratus. Eur. J. Phycol. 25, 75–82. doi: 10.1080/00071619000650071
Jueterbock, A., Tyberghein, L., Verbruggen, H., Coyer, J. A., Olsen, J. L., and Hoarau, G. (2013). Climate change impact on seaweed meadow distribution in the North Atlantic rocky intertidal. Ecol. Evol. 3, 1356–1373. doi: 10.1002/ece3.541
Kautsky, H., Kautsky, L., Kautsky, N., Kautsky, U., and Lindblad, C. (1992). “Studies on the Fucus vesiculosus community in the Baltic Sea,” in Phycological Studies of Nordic Coastal Waters, eds I. Wallentinus and P. Snoeijs (Uppsala), 33–48.
Kingsolver, J. G., and Huey, R. B. (1998). Evolutionary analyses of morphological and physiological plasticity in thermally variable environments. Am. Zool. 545, 1–15. doi: 10.1086/381466
Klenell, M., Snoeijs, P., and Pedersén, M. (2004). Active carbon uptake in Laminaria digitata and L. saccharina (Phaeophyta) is driven by a proton pump in the plasma membrane. Hydrobiologia 514, 41–53. doi: 10.1007/978-94-017-0920-0_4
Klughammer, C., and Schreiber, U. (2008). Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 1, 27–35.
Koch, M., Bowes, G., Ross, C., and Zhang, X.-H. (2013). Climate change and ocean acidification effects on seagrasses and marine macroalgae. Glob. Chang. Biol. 19, 103–132. doi: 10.1111/j.1365-2486.2012.02791.x
Kübler, J. E., and Davison, I. R. (1995). Thermal acclimation of light-use characteristics of Chondrus crispus (Rhodophyta). Eur. J. Phycol. 30, 189–195. doi: 10.1080/09670269500650971
Kübler, J. E., and Dudgeon, S. R. (2015). Predicting effects of ocean acidification and warming on algae lacking carbon concentrating mechanisms. PLoS One 10:e0132806. doi: 10.1371/journal.pone.0132806
Kübler, J. E., Davison, I. R., and Yarish, C. (1991). Photosynthetic adaption to temperature in the red algae Lomentaria baileyana and Lomentaria orcadensis. Br. Phycol. J. 26, 9–19. doi: 10.1080/00071619100650021
Lavaud, J., and Lepetit, B. (2013). An explanation for the inter-species variability of the photoprotective non-photochemical chlorophyll fluorescence quenching in diatoms. Biochim. Biophys. Acta 1827, 294–302. doi: 10.1016/j.bbabio.2012.11.012
Lavaud, J., Rousseau, B., and Etienne, A. L. (2002a). In diatoms, a transthylakoid proton gradient alone is not sufficient to induce a non-photochemical fluorescence quenching. FEBS Lett. 523, 163–166. doi: 10.1016/s0014-5793(02)02979-4
Lavaud, J., Rousseau, B., van Gorkom, H. J., and Etienne, A.-L. (2002b). Influence of the diadinoxanthin pool size on photoprotection in the marine planktonic diatom Phaeodactylum tricornutum. Plant Physiol. 129, 1398–1406. doi: 10.1104/pp.002014
Lavaud, J., Strzepek, R. F., and Kroth, P. G. (2007). Photoprotection capacity differs among diatoms: possible consequences on the spatial distribution of diatoms related to fluctuations in the underwater light climate. Limnol. Oceanogr. 52, 1188–1194. doi: 10.4319/lo.2007.52.3.1188
Lüning, K. (1990). Seaweeds: their Environment, Biogeography and Ecophysiology. New York, NY: JohnWiley and Sons, Inc.
Lynch, D. V., and Thompson, G. A. (1984). Chloroplast phospholipid molecular species alterations during low temperature acclimation in Dunaliella. Plant Physiol. 74, 198–203. doi: 10.1104/pp.74.2.198
Mathieson, A. C., and Norall, T. L. (1975). Photosynthetic studies of Chondrus crispus. Mar. Biol. 33, 207–213. doi: 10.1007/bf00390924
Meehl, G. A., and Tebaldi, C. (2004). More intense, more frequent, and longer lasting heat waves in the 21st century. Science 305, 994–997. doi: 10.1126/science.1098704
Melzner, F., Thomsen, J., Koeve, W., Oschlies, A., Gutowska, M. A., Bange, H. W., et al. (2013). Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar. Biol. 160, 1875–1888. doi: 10.1007/s00227-012-1954-1
Mohanty, P., Allakhverdiev, S. I., and Murata, N. (2007). Application of low temperatures during photoinhibition allows characterization of individual steps in photodamage and the repair of photosystem II. Photosynth. Res. 94, 217–224. doi: 10.1007/s11120-007-9184-y
Müller, J. D., Schneider, B., and Rehder, G. (2016). Long-term alkalinity trends in the Baltic sea and their implications for CO2-induced acidification. Limnol. Oceanogr. 61, 1984–2002. doi: 10.1002/lno.10349
Müller, P., Li, X.-P., and Niyogi, K. K. (2001). Non-photochemical quenching. a response to excess light energy. Plant Physiol. 125, 1558–1566. doi: 10.1104/pp.125.4.1558
Murata, N., Takahashi, S., Nishiyama, Y., and Allakhverdiev, S. I. (2007). Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta 1767, 414–421. doi: 10.1016/j.bbabio.2006.11.019
Niemeck, R. A., and Mathieson, A. C. (1978). Physiological studies of intertidal fucoid algae. Bot. Mar. 21, 221–227.
Nygård, C. A., and Dring, M. J. (2008). Influence of salinity, temperature, dissolved inorganic carbon and nutrient concentration on the photosynthesis and growth of Fucus vesiculosus from the Baltic and Irish Seas. Eur. J. Phycol. 43, 253–262. doi: 10.1080/09670260802172627
Olischläger, M., and Wiencke, C. (2013). Ocean acidification alleviates low-temperature effects on growth and photosynthesis of the red alga Neosiphonia harveyi (Rhodophyta). J. Exp. Bot. 64, 5587–5597. doi: 10.1093/jxb/ert329
Olischläger, M., Bartsch, I., Gutow, L., and Wiencke, C. (2012). Effects of ocean acidification on different life-cycle stages of the kelp Laminaria hyperborea (Phaeophyceae). Bot. Mar. 55, 511–525.
Olischläger, M., Bartsch, I., Gutow, L., and Wiencke, C. (2013). Effects of ocean acidification on growth and physiology of Ulva lactuca (Chlorophyta) in a rockpool-scenario. Phycol. Res. 61, 180–190. doi: 10.1111/pre.12006
Olischläger, M., Iñiguez, C., Koch, K., Wiencke, C., and Gordillo, F. J. L. (2017). Increased pCO2 and temperature reveal ecotypic differences in growth and photosynthetic performance of temperate and Arctic populations of Saccharina latissima. Planta 245, 119–136. doi: 10.1007/s00425-016-2594-3
Omstedt, A., Edman, M. K., Claremar, B., Frodin, P., Gustafsson, E., Humborg, C., et al. (2012). Future changes in the Baltic Sea acid base (pH) and oxygen balances. Tellus 64:19586. doi: 10.3402/tellusb.v64i0.19586
Pfetzing, J., Stengel, D. B., Cuffe, M. M., Savage, A. V., and Guiry, M. D. (2000). Effects of temperature and prolonged emersion on photosynthesis, carbohydrate content and growth of the brown intertidal alga Pelvetia canaliculata. Bot. Mar. 43, 399–407.
Pierrot, D., Lewis, E., and Wallace, D. W. R. (2006). “MS Excel program developed for CO2 system calculations,” in ORNL/CDIAC-105a. OakRidge: Oak Ridge National Laboratory.
R Development Core Team (2020). R: a Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Raven, J. A., Giordano, M., Beardall, J., and Maberly, S. C. (2011). Algal and aquatic plant carbon concentrating mechanisms in relation to environmental change. Photosynth. Res. 109, 281–296. doi: 10.1007/s11120-011-9632-6
Richardson, K., Beardall, J., and Raven, J. A. (1983). Adaptation of unicellular algae to irradiance: an analysis of strategies. New Phytol. 93, 157–191. doi: 10.1111/j.1469-8137.1983.tb03422.x
Ritchie, R. J. (2006). Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth. Res. 89, 27–41. doi: 10.1007/s11120-006-9065-9
Rohde, S., Hiebenthal, C., Wahl, M., Karez, R., and Bischof, K. (2008). Decreased depth distribution of Fucus vesiculosus (Phaeophyceae) in the Western Baltic: effects of light deficiency and epibionts on growth and photosynthesis. Eur. J. Phycol. 43, 143–150. doi: 10.1080/09670260801901018
Rokitta, S. D., and Rost, B. (2012). Effects of CO2 and their modulation by light in the life-cycle stages of the coccolithophore Emiliania huxleyi. Limnol. Oceanogr. 57, 607–618. doi: 10.4319/lo.2012.57.2.0607
Rönnbäck, P., Kautsky, N., Pihl, L., Troell, M., Söderqvist, T., and Wennhage, H. (2007). Ecosystem goods and services from Swedish coastal habitats: identification, valuation, and implications of ecosystem shifts. Ambio 36, 534–544. doi: 10.1579/0044-7447(2007)36[534:egasfs]2.0.co;2
Roth, O., Kurtz, J., and Reusch, T. B. H. (2010). A summer heat wave decreases the immunocompetence of the mesograzer, Idotea baltica. Mar. Biol. 157, 1605–1611. doi: 10.1007/s00227-010-1433-5
Rothäusler, E., Rugiu, L., and Jormalainen, V. (2018). Forecast climate change conditions sustain growth and physiology but hamper reproduction in range-margin populations of a foundation rockweed species. Mar. Environ. Res. 141, 205–213. doi: 10.1016/j.marenvres.2018.09.014
Ruban, A. V., Berera, R., Ilioaia, C., van Stokkum, I. H. M., Kennis, J. T. M., Pascal, A. A., et al. (2007). Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature 450, 575–578. doi: 10.1038/nature06262
Russell, G. (1987). Spatial and environmental components of evolutionary change: interactive effects of salinity and temperature on Fucus vesiculosus as an example. Helgoländer Meeresuntersuchungen 41, 371–376. doi: 10.1007/bf02366199
Saderne, V. (2012). The Ecological Effect of CO2 on the Brown Algae Fucus serratus and its Epibionts: From the Habitat to the Organismic Scale. Ph.D. dissertation. Kiel: Christian-Albrechts-University Kiel.
Sage, R. F., and Kubien, D. S. (2007). The temperature response of C3 and C4 photosynthesis. Plant Cell Environ. 30, 1086–1106.
Sarker, M. Y., Bartsch, I., Olischläger, M., Gutow, L., and Wiencke, C. (2013). Combined effects of CO2, temperature, irradiance and time on the physiological performance of Chondrus crispus (Rhodophyta). Bot. Mar. 56, 63–74.
Schagerström, E., Forslund, H., Kautsky, L., Pärnoja, M., and Kotta, J. (2014). Does thalli complexity and biomass affect the associated flora and fauna of two co-occurring Fucus species in the Baltic Sea? Estuar. Coast. Shelf Sci. 149, 187–193. doi: 10.1016/j.ecss.2014.08.022
Schär, C., Vidale, P. L., Lüthi, D., Frei, C., Häberli, C., Liniger, M. A., et al. (2004). The role of increasing temperature variability in European summer heatwaves. Nature 427, 332–336. doi: 10.1038/nature02300
Schmid, R., Mills, J., and Dring, M. (1996). Influence of carbon supply on the stimulation of light-saturated photosynthesis by blue light in Laminaria saccharina: implications for the mechanism of carbon acquisition in higher brown algae. Plant Cell Environ. 19, 383–391. doi: 10.1111/j.1365-3040.1996.tb00330.x
Schneider, B., Eilola, K., Lukkari, K., Müller-Karulis, B., and Neumann, T. (2015). “Environmental impacts—marine biogeochemistry,” in Second Assessment of Climate Change for the Baltic Sea Basin, ed. BACC II Author Team (Berlin: Springer), 337–361. doi: 10.1007/978-3-319-16006-1_18
Schreiber, U., Bilger, W., and Neubauer, C. (1995). “Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis,” in Ecophysiology of Photosynthesis. Springer Study Edition, Vol 100, eds E. D. Schulze and M. M. Caldwell (Berlin, Germany: Springer).
Serôdio, J., and Lavaud, J. (2011). A model for describing the light response of the nonphotochemical quenching of chlorophyll fluorescence. Photosynth. Res. 108, 61–76. doi: 10.1007/s11120-011-9654-0
Sukenik, A., Bennett, J., and Falkowski, P. (1987). Light-saturated photosynthesis — limitation by electron transport or carbon fixation? Biochim. Biophys. Acta 891, 205–215. doi: 10.1016/0005-2728(87)90216-7
Surif, M. B., and Raven, J. A. (1990). Photosynthetic gas exchange under emersed conditions in eulittoral and normally submersed members of the Fucales and the Laminariales: interpretation in relation to C isotope ratio and N and water use efficiency. Oecologia 82, 68–80. doi: 10.1007/bf00318535
Takolander, A., Cabeza, M., and Leskinen, E. (2017). Climate change can cause complex responses in Baltic Sea macroalgae: a systematic review. J. Sea Res. 123, 16–29. doi: 10.1016/j.seares.2017.03.007
Takolander, A., Cabeza, M., and Leskinen, E. (2019). Seasonal interactive effects of pCO2 and irradiance on the ecophysiology of brown macroalga Fucus vesiculosus L. Eur. J. Phycol. 54, 380–392. doi: 10.1080/09670262.2019.1572226
Thomsen, J., Gutowska, M. A., Saphörster, J., Heinemann, A., Trübenbach, K., Fietzke, J., et al. (2010). Calcifying invertebrates succeed in a naturally CO2- rich coastal habitat but are threatened by high levels of future acidification. Biogeosciences 7, 3879–3891. doi: 10.5194/bg-7-3879-2010
Torn, K., Krause-Jensen, D., and Martin, G. (2006). Present and past depth distribution of bladderwrack (Fucus vesiculosus) in the Baltic Sea. Aquat. Bot. 84, 53–62. doi: 10.1016/j.aquabot.2005.07.011
Vasseur, D. A., Delong, J. P., Gilbert, B., Greig, H. S., Harley, C. D. G., McCann, K. S., et al. (2014). Increased temperature variation poses a greater risk to species than climate warming. Proc. R. Soc. 281, 1–8.
Wahid, A., Gelani, S., Ashraf, M., and Foolad, M. (2007). Heat tolerance in plants: an overview. Environ. Exp. Bot. 61, 199–223. doi: 10.1016/j.envexpbot.2007.05.011
Wahl, M., Buchholz, B., Winde, V., Golomb, D., Guy-Haim, T., Müller, J., et al. (2015a). A mesocosm concept for the simulation of near-natural shallow underwater climates: the kiel outdoor benthocosms (KOB). Limnol. Oceanogr. Methods 13, 651–663. doi: 10.1002/lom3.10055
Wahl, M., Jormalainen, V., Eriksson, B. K., Coyer, J. A., Molis, M., Schubert, H., et al. (2011). “Stress ecology in Fucus: abiotic, biotic and genetic interactions,” in Advances in Marine Biology, ed. M. Lesser (Oxford: Elsevier Ltd), 37–105. doi: 10.1016/b978-0-12-385536-7.00002-9
Wahl, M., Molis, M., Hobday, A. J., Dudgeon, S., Neumann, R., Steinberg, P., et al. (2015b). The responses of brown macroalgae to environmental change from local to global scales: direct versus ecologically mediated effects. Perspect. Phycol. 2, 11–30. doi: 10.1127/pip/2015/0019
Wahl, M., Shahnaz, L., Dobretsov, S., Saha, M., Symanowski, F., David, K., et al. (2010). Ecology of antifouling resistance in the bladder wrack Fucus vesiculosus: patterns of microfouling and antimicrobial protection. Mar. Ecol. Prog. Ser. 411, 33–48. doi: 10.3354/meps08644
Wahl, M., Werner, F. J., Buchholz, B., Raddatz, S., Graiff, A., Matthiessen, B., et al. (2019). Season affects strength and direction of the interactive impacts of ocean warming and biotic stress in a coastal seaweed ecosystem. Limnol. Oceanogr. 65, 807–827. doi: 10.1002/lno.11350
Walsby, A. E. (1997). Modelling the daily integral of photosynthesis by phytoplankton: its dependence on the mean depth of the population. Hydrobiologia 349, 65–74.
Warkentin, M., Freese, H. M., Karsten, U., and Schumann, R. (2007). New and fast method to quantify respiration rates of bacterial and plankton communities in freshwater ecosystems by using optical oxygen sensor spots. Appl. Environ. Microbiol. 73, 6722–6729. doi: 10.1128/aem.00405-07
Webb, W. L., Newton, M., and Starr, D. (1974). Carbon dioxide exchange of Alnus rubra - a mathematical model. Oecologia 17, 281–291. doi: 10.1007/bf00345747
Weidner, M., and Ziemens, C. (1975). Preadaptation of protein synthesis in wheat seedlings to high temperature. Plant Physiol. 56, 590–594. doi: 10.1104/pp.56.5.590
Wilhelm, C., and Selmar, D. (2011). Energy dissipation is an essential mechanism to sustain the viability of plants: the physiological limits of improved photosynthesis. J. Plant Physiol. 168, 79–87. doi: 10.1016/j.jplph.2010.07.012
Winters, G., Nelle, P., Fricke, B., Rauch, G., and Reusch, T. (2011). Effects of a simulated heat wave on photophysiology and gene expression of high- and low-latitude populations of Zostera marina. Mar. Ecol. Prog. Ser. 435, 83–95. doi: 10.3354/meps09213
Wu, H., Zou, D., and Gao, K. (2008). Impacts of increased atmospheric CO2 concentration on photosynthesis and growth of micro- and macro-algae. Sci. China Ser. C Life Sci. 51, 1144–1150. doi: 10.1007/s11427-008-0142-5
Keywords: bladder wrack, chlorophyll fluorescence, mesocosm, multi-factorial change, photoacclimation, photosynthesis, seasonal pattern
Citation: Graiff A, Bartsch I, Glaser K and Karsten U (2021) Seasonal Photophysiological Performance of Adult Western Baltic Fucus vesiculosus (Phaeophyceae) Under Ocean Warming and Acidification. Front. Mar. Sci. 8:666493. doi: 10.3389/fmars.2021.666493
Received: 10 February 2021; Accepted: 09 April 2021;
Published: 29 April 2021.
Edited by:
Christopher Edward Cornwall, Victoria University of Wellington, New ZealandCopyright © 2021 Graiff, Bartsch, Glaser and Karsten. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angelika Graiff, angelika.graiff@uni-rostock.de
 Angelika Graiff
Angelika Graiff Inka Bartsch
Inka Bartsch Karin Glaser
Karin Glaser Ulf Karsten
Ulf Karsten